Long-Term Health Effects of COVID-19: Disability and Function Following SARS-CoV-2 Infection (2024)
Chapter: 3 Selected Long-Term Health Effects Stemming from COVID-19 and Functional Implications
3
Selected Long-Term Health Effects Stemming from COVID-19 and Functional Implications
Long COVID is associated with a wide range of new or worsening health conditions and encompasses more than 200 symptoms involving many different organ systems (Davis et al., 2021; Lubell, 2022). Given the extensive range of symptoms, there have been attempts to cluster these health effects, but to date no consensus in this regard has been reached.
This chapter begins with an overview of the full range of symptoms and health effects associated with Long COVID and a summary of the condition’s epidemiology. The chapter then focuses on three health effects of Long COVID that may not be captured in SSA’s Listing of Impairments but can significantly affect one’s ability to participate in work or school: chronic fatigue and post-exertional malaise (PEM), post–COVID-19 cognitive impairment (PCCI), and autonomic dysfunction. Although a great number of Long COVID health effects could impact function, the committee thinks it will be useful for SSA to become familiar with these three, as they are particularly challenging to treat because of their multisystem nature. (See Chapter 5 for an overview of similar multisystem chronic conditions.) These are the novel conditions for which people seek out Long COVID clinics. For each of these three health effects, the chapter provides an overview of
- the frequency and distribution of their severity and duration in the general population, as well as any differences along racial, ethnic, sex, gender, geographic, or socioeconomic dimensions, or differences specific to populations with particular preexisting or comorbid conditions;
- clinical standards for diagnosis and measurement of each of these health effects;
- any special considerations regarding identification and management of these health effects in special populations, including pregnant people and those with underlying health conditions;
- best practices for quantifying the functional impacts of these health effects; and
- identified challenges for clinicians in evaluating persons with these health effects.
The symptoms and health effects discussed in detail in this chapter do not represent the full range of effects experienced by patients with Long COVID. In an effort to be inclusive, the committee includes at the end of this chapter annex tables organized by body system of selected health effects associated with Long COVID, along with their potential functional impacts and selected management guidelines.
OVERVIEW OF HEALTH EFFECTS ASSOCIATED WITH LONG COVID
Epidemiology of the Long-Term Health Effects of SARS-CoV-2 Infection
Data from the U.S. Centers for Disease Control and Prevention’s (CDC’s) National Health Interview Survey show that in 2022, 6.9 percent of U.S. adults and 1.3 percent of children had Long COVID at some point, while 3.4 percent of adults and 0.5 percent of children had Long COVID at the time of interview (Adjaye-Gbewonyo et al., 2023; Vahratian et al., 2023). Based on these surveys, it is estimated that approximately 8.9 million adults and 362,000 children reported Long COVID symptoms in the United States in 2022 (Adjaye-Gbewonyo et al., 2023; Vahratian et al., 2023). Among adults in the United States, data from the CDC’s Household Pulse Survey show that the prevalence of Long COVID declined from 7.5 percent in June 2022 to 5.9 percent reported in January 2023, then increased to 6.8 percent in January 2024 (NCHS, 2024). Despite an overall decline in prevalence since June 2022, Long COVID’s disease burden remains substantial. In January of 2024, approximately 22 percent of adults with Long COVID reported significant activity limitations (NCHS, 2024).
The body of epidemiological research shows great variation in the incidence and prevalence of the long-term effects of SARS-CoV-2 infection. These variations reflect the dynamic changes in the pandemic itself, as the virus has evolved and spawned many variants and subvariants throughout the pandemic’s course; the effect of vaccines, which were introduced in December 2020 and later shown to reduce the risk of long-term health effects; and the effect of treatments for acute infection (e.g., steroids,
antivirals), which may reduce the risk of long-term health effects. In addition, since awareness about Long COVID in the medical community and the public is still lacking, reported prevalence may be an underestimate.
Adding to this complexity is the broad multisystem nature of the long-term health effects of SARS-CoV-2 and the fact that these effects are expressed differently in different age groups and sexes and by baseline health (Maglietta et al., 2022; Rayner et al., 2023; Tsampasian et al., 2023; Wong et al., 2023). Variation in incidence and prevalence estimates also stems from heterogeneities in study designs, including choice of control groups (e.g., whether studies included people with negative SARS-CoV-2 tests or no known SARS-CoV-2 as controls); methods used to account for the effect of baseline health in ascertaining whether the emergence of specific health effects following infection represents new disease; specification of outcomes; and other methodological differences.
Because of the considerable variation in estimates of the long-term health effects seen in Long COVID, the committee presents average estimates for different body systems based on the published literature. Among people who had COVID-19, most estimates of long-term cardiovascular health effects, which comprise a broad array of sequelae, regress around 4 percent (Xie et al., 2022b). Neurological and psychiatric conditions are also common among people who had COVID-19, with estimates of around 6 percent (Harrison and Taquet, 2023; Ley et al., 2023; Taquet et al., 2021, 2022; Wulf Hanson et al., 2022; Xie et al., 2022a; Xu et al., 2022). The reported incidence of gastrointestinal disorders post–COVID-19 is highly variable, but estimates suggest 6 percent (Xu et al., 2023). Respiratory problems persist in some people following SARS-CoV-2 infection, and prevalence studies at 6 months to 2 years suggest estimates of 2–4 percent (Wulf Hanson et al., 2022). Endocrine conditions are estimated to affect 1–2 percent of people previously infected with SARS-CoV-2 (Ssentongo et al., 2022). Similarly, estimates of the prevalence of genitourinary disorders is around 1 percent (Kayaaslan et al., 2021).
Terminology
The committee considers a health condition to be a state, including injury, illness, or physical or mental diagnosis, that adversely affects a person’s physical and/or mental health and well-being. A symptom is a subjective manifestation of disease experienced and reported by a patient, while a sign is an objective manifestation of disease that an examining practitioner can observe or measure (King, 1968; NIH, n.d.). In the context of this study, the committee uses health effects as an umbrella term that includes symptoms, health conditions, and other sequelae caused by or associated with prior infection with SARS-CoV-2.
Full Range of Health Effects
SARS-CoV-2 infection can lead to post-acute and long-term health effects in nearly every organ system (see Figure 3-1). As described in Chapter 1, the International Classification of Functioning, Disability and Health model of disability identifies three domains of functioning: body functions and structures (i.e., physiological functions of the body, including psychological functions, and functioning of body structures), activity (i.e., actions or tasks), and participation (i.e., performance of tasks in a social context, such as school or work), all of which are mediated by personal and environmental factors that can either enhance or diminish an individual’s activity and participation (WHO, 2023b). Health effects associated with Long COVID may manifest as impairments in body structures and
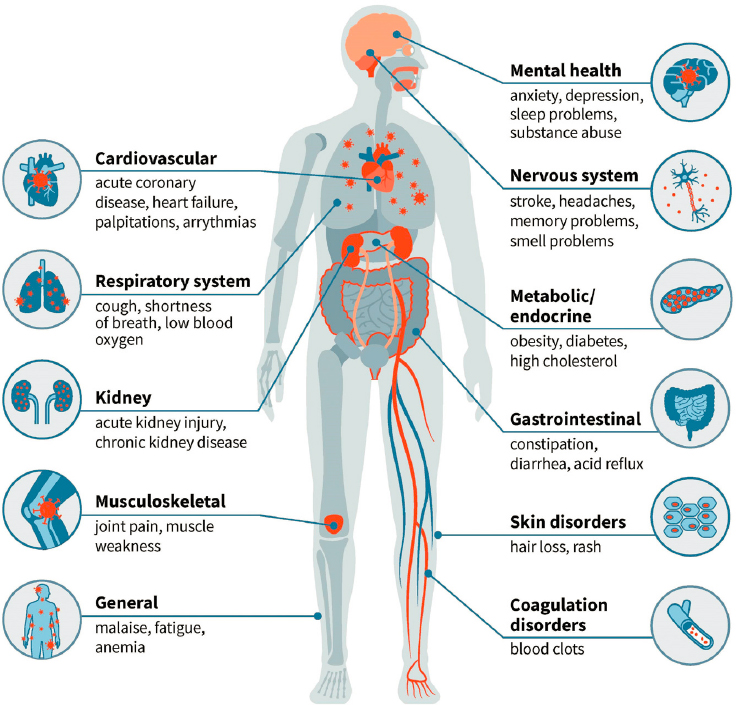
SOURCE: Washington University School of Medicine in St. Louis.
physiological functions, with resulting activity limitations and restrictions on participation. The impairments associated with Long COVID may affect mental (e.g., cognitive, psychosocial, emotional) functioning as well as physical functioning. In addition, individuals with Long COVID may experience multiple and potentially overlapping symptoms and conditions, including PEM, PCCI, and autonomic dysfunction. A number of preexisting conditions (e.g., diabetes, heart failure, chronic obstructive pulmonary disease, dementia) can increase the risk of adverse outcomes from SARS-CoV-2 infection, both during and following acute infection (Awatade et al., 2023; Dubey et al., 2023; Núñez-Gil et al., 2023; Steenblock et al., 2022; Treskova-Schwarzbach et al., 2021). Although persistent health effects associated with SARS-CoV-2 infection may include worsening of preexisting conditions, this chapter focuses primarily on newly acquired conditions. Health care providers and patients need to be aware of potential worsening of preexisting conditions and continue to monitor and treat them as needed. Additionally, the variable health effects of Long COVID may have different impacts on different patients depending on the burden of the preexisting condition, further emphasizing the need for a patient-specific approach for monitoring and treatment of health conditions.
Annex Tables 3-1 through 3-11 at the end of this chapter list selected health conditions associated with Long COVID in adults, organized by body system. Annex Table 3-12 lists selected health effects that are not organ system–specific, including chronic fatigue and PEM, ME/CFS, and fever. These tables include a summary of the potential functional implications of the conditions, selected clinical guidelines for their diagnosis and management, and potentially relevant SSA Listings for adults, where applicable. For the functional implications, the committee chose to focus on the kind of information that SSA collects about functioning in adults, which comes from a variety of sources, including the applicant, medical providers, employers, and other third parties with knowledge of the applicant. Information collected about physical functioning encompasses such activities as sitting, standing, walking, lifting, carrying, reaching, gross manipulation (e.g., handling large objects), fine manipulation (e.g., handling small objects, writing, typing), climbing, and low work (e.g., stooping, crouching, kneeling, crawling) (SSA, 2020). Annex Table 3-13 lists the physical; vison, hearing, and speaking; and mental activities the committee considered in populating the tables, along with their definitions. The committee populated the column on potential functional limitations based on the members’ collective expertise. SSA also collects information about the applicant’s ability to perform various daily activities, such as dressing, bathing, self-feeding, and using the toilet, as well as preparing meals, doing house- and yardwork, getting around, and shopping.
The long-term health effects associated with Long COVID can affect people across race, ethnicity, sex, gender, and age groups. Generally, the risk—on the relative scale—of these long-term health effects increases according to the severity of acute infection: the risk is 2–3 times greater in people who were versus were not hospitalized, and greatest in people who required intensive care (Núñez-Seisdedos et al., 2022; Xie et al., 2022b). However, because most people infected with SARS-CoV-2 experience mild or moderate disease that does not require hospitalization, those with mild or moderate cases constitute the majority of individuals with long-term adverse health effects of SARS-CoV-2 (Lai et al., 2023; Spagnuolo et al., 2020). Rates of Long COVID among pregnant women who had COVID-19 during pregnancy are similar to those of the general population (Kandemir et al., 2024). In addition, among pregnant women with COVID-19 at delivery, rates of caesarean delivery and frequency of maternal complications increased (Knight et al., 2020; Prabhu et al., 2020). The risk of Long COVID among adult females is about twice that among adult males (Munblit et al., 2021; Perlis et al., 2022; Sudre et al., 2021). Some pediatric studies also report a higher prevalence of Long COVID in female compared to male children and adolescents, although the exact risk is still undefined (Vahratian et al., 2023; Zheng et al., 2023).
Some of the long-term health effects of acute SARS-CoV-2 infection are chronic and can negatively impact individuals’ quality of life; ability to participate in the labor market or school; and, in some cases, life expectancy. The extent to which the long-term health effects of infection will have a functional impact on a person’s life and ability to work or participate in school can depend on health and functional status prior to COVID-19 and the severity of the medical condition(s). In addition, symptoms and related functional limitations may fluctuate, waxing and waning over time.
Clustering of Health Effects
Given the vast number of symptoms and health effects associated with Long COVID, several research groups have attempted to cluster patients with similar effects to better understand the disease (see Table 3-1).
The committee found that the evidence on clustering of the post-acute and long-term health effects of SARS-CoV-2 remains inconsistent across studies as a result of differences in study designs, populations studied, enrollment criteria, the era in which the study was undertaken (reflecting the possible differential effect of variants, changes in clinical care, and vaccination on phenotypic clusters), specification of post-acute and long-term health effects of SARS-CoV-2 infection, methodological approaches to clustering, and other factors. These differences yielded inconsistent results
TABLE 3-1 Research on Clusters of Long COVID Health Effects
| Reference | Population and Study Type | Time after Initial COVID-19 Infection | Clustering Methodology | Proposed Long COVID Symptom Clusters | Notes |
|---|---|---|---|---|---|
| Canas et al. (2023) | Prospective cohort study, 9,804 UK-based adults | 84 days | Unsupervised clustering analysis of time-series data, additional testing using data from Covid Symptom Study Biobank |
|
Subclusters determined for wild-type variant in unvaccinated people, alpha variant in unvaccinated people, and delta variant in vaccinated people. |
| Evans et al. (2021) | PHOSP-COVID cohort, 1,077 UK adults | 2 and 7 months | Clustering large applications k-medoids approach |
|
46% in the mild cluster. Full recovery reached in 3% of the very severe cluster, 7% of the severe cluster, 36% of the moderate cluster, and 43% of the mild cluster. Elevated serum C-reactive protein was positively associated with cluster severity. |
| Fischer et al. (2022) | Predi-COVID cohort in Luxembourg, 288 participants | 12 months | Hierarchical ascendant classification |
|
48% in the mild cluster. Severe cluster had a higher proportion of women and smokers. |
| Reference | Population and Study Type | Time after Initial COVID-19 Infection | Clustering Methodology | Proposed Long COVID Symptom Clusters | Notes |
|---|---|---|---|---|---|
| Frontera et al. (2022) | 242 patients hospitalized with COVID-19 | 12 months | Unsupervised hierarchical cluster analysis |
|
The study administered psychological therapy and medications to cluster 2 and PT or OT to cluster 3. They found 100% of those who received psychiatric therapy, 97% who received PT/OT, and 83% who received few interventions improved over time |
| Goldhaber et al. (2022) | UC San Diego health system, 999 respondents | Unspecified | Exploratory factor analysis |
|
Neurocognitive burden associated with depression and anxiety. Musculoskeletal burden associated with older age |
| Kenny et al. (2022) | Multicenter prospective cohort, 233 individuals, 77% mild initial illness | 4 weeks or more | Multiple correspondence analysis on the most common self-reported symptoms and hierarchical clustering |
|
Clusters 1 and 2 had greater functional impairment, longer work absence |
| Kisiel et al. (2023) | 506 patients from 3 Swedish cohorts, mostly hospitalized | 12 weeks or more | K-means cluster analysis and ordinal logistic regression were used to create PCS scores |
|
59% in the mild cluster. Cluster 3 had the most reduced work ability. Smoking, high BMI, diabetes, and COVID-19 onset severity were predictors of cluster 3. |
| Thaweethai et al. (2023) | Prospective cohort study of 9,764 adults at 85 enrolling sites in 33 states plus Washington, DC, and Puerto Rico | 6 months or more | Unsupervised machine learning |
|
Using least absolute shrinkage and selection operator (LASSO), found most representative Long COVID symptoms to be smell/taste, PEM, chronic cough, brain fog, and thirst. |
| Tsuchida et al. (2023) | Adolescents and adults in an outpatient clinic in Japan | 2 months or more | Cluster analysis was performed using CLUSTER (SAS Ver 9.4, SAS Institute Inc., Cary, NC, USA), and cluster classification was performed. |
|
Clusters 2 and 3 had higher proportions of autonomic nervous system disorders and leave of absence from work and school |
| Wulf Hanson et al. (2022) | Pooled 54 studies and 2 medical record databases with data for 1.2 million individuals from 22 countries | 3 months | Selected based on reporting frequency in published studies and availability of health state in Global Burden of Disease study |
|
Among individuals with COVID-19, 6.2% had long COVID at 3 months: 10.6% for female adults, 5.4% for male adults, 2.8% for children and young people. |
| Yong and Liu (2022) | Review of 43 studies on Long COVID | 3 months or more | “narrative review” of the literature (not systematic) |
|
Among individuals with Long COVID symptoms 3 months after symptomatic SARS-CoV-2 infection, an estimated 15% continued to experience symptoms at 12 months. |
across studies, making it challenging to weave the evidence into a coherent narrative to inform policy discussions and clinical care. The heterogeneity of results reflects both the nascency of the field (less than 3.5 years old) and the complexity of Long COVID itself. The generation of better-quality and more consistent evidence will require consensus on terms, definitions, and methodological approaches.
SELECTED MULTISYSTEM HEALTH EFFECTS ASSOCIATED WITH LONG COVID
Three health effects associated with Long COVID that have a significant effect on functioning and are particularly challenging to manage—chronic fatigue and PEM, PCCI, and autonomic dysfunction—are reviewed in this section. There is significant overlap among the symptoms associated with these conditions.
Chronic Fatigue and Post-Exertional Malaise
PEM, also called post-exertional symptom exacerbation, is characterized by a severe worsening of fatigue and other symptoms following physical, mental, social, or emotional exertion that would not typically cause such a reaction in healthy individuals. This exacerbation of symptoms can occur immediately after the stressor or can be somewhat delayed (hours to days). In addition, an episode of PEM can last for days or even weeks. The specific symptoms that worsen vary among individuals, but often can go beyond fatigue to include muscle or joint pain, cognitive difficulties, sleep disturbances, headaches, flu-like symptoms, and/or gastrointestinal disturbances (Vernon et al., 2023).
Fatigue, broadly defined as a distressing or persistent tiredness that is neither proportional to recent activity nor alleviated by rest (Sandler et al., 2021; Twomey et al., 2022), is the most dominant symptom of Long COVID in several studies, ranging from 19 percent to 76.3 percent of patients (Cheung et al., 2023; Hartung et al., 2022; Ho et al., 2023; Kayaaslan et al., 2021; Líška et al., 2022; Sánchez-García et al., 2023; Štſpánek et al., 2023). Of the many descriptive studies that have captured fatigue and other symptoms seen in Long COVID, only a small proportion make a point of measuring PEM. In a large cross-sectional sample of adults who were selected from post–COVID-19 online groups, PEM was reported by 89.1 percent (95% confidence interval [CI] 88–90%); for most participants, PEM lasted a few days (Davis et al., 2021). A recent analysis of the National Institutes of Health Researching COVID to Enhance Recovery (RECOVER) longitudinal study was aimed at developing and validating a quantitative definition of Long COVID using multiple symptoms. In that study, PEM was reported in 87 percent of patients identified as having Long COVID (Thaweethai et al., 2023).
Diagnosis
Both post-COVID fatigue and PEM are generally diagnosed based on patient-reported symptoms and a detailed medical history. Typically, a thorough medical evaluation—which may include blood tests, imaging studies, and other diagnostic tests—is conducted to rule out other possible causes or contributing factors.
In clinical settings, health care providers typically evaluate chronic fatigue and PEM by asking patients to describe their symptoms in detail, including by inquiring about the type of exertion (physical, emotional, or mental) that leads to worsening of symptoms; the duration and severity of the symptom worsening that follows those stressors; how long it takes for the symptoms to return to baseline; what symptoms are experienced; and whether there is a prodrome related to the worsening of the symptoms. Multiple validated questionnaires can be used to measure a person’s perception and severity of fatigue and PEM (Davenport et al., 2023; FACIT, 2021), such as the DePaul Symptom Questionnaire–Post-Exertional Malaise (DSQ-PEM), which captures the frequency and severity of PEM symptoms (Bedree et al., 2019; Cotler et al., 2018; Davenport et al., 2023; FACIT, 2021).
One approach to diagnosing PEM, investigated in myalgic encephalitis/chronic fatigue syndrome (ME/CFS) but not yet in Long COVID, involves two consecutive days of cardiopulmonary exercise testing (CPET) (Stevens et al., 2018). Deconditioned adults and those with other chronic conditions show minimal variation between the first and second day of CPET measurement. In ME/CFS patients with PEM, multiple studies reveal a significant decline in oxygen consumption (V∙ O2) at peak performance and the ventilatory anaerobic threshold on the second day of CPET testing (Davenport et al., 2019; Stevens et al., 2018). The ventilatory anaerobic threshold reflects a person’s ability to sustain continuous work and is more related to everyday exertion. This drop in V∙ O2 between the first- and second-day CPET measurements suggests that patients with PEM risk entering anaerobic metabolism during activities they could have completed without it just the day before (Stevens et al., 2018). In ME/CFS, symptoms most associated with PEM after exercise include cognitive dysfunction, reduced self-reported daily functioning, and mood disturbances (Chu et al., 2018). Although 2-day CPET helps with understanding the physiologic response to exercise in patients with PEM generally, there are health system and patient-level barriers that potentially limit the broad implementation of 2-day CPET in diagnosing PEM in Long COVID and ME/CFS. The health system barriers include limited or inequitable access to CPET, with even fewer trained clinicians who can apply and interpret 2-day CPET to diagnose PEM. Patient-level barriers include prolonged recovery from testing and the prohibitive cost of testing without adequate insurance coverage. Several studies have explored the role of CPET in Long COVID, but not all
have used PEM as an inclusion criterion (Baratto et al., 2021; Durstenfeld et al., 2022; Evers et al., 2022; Wernhart et al., 2023). Although much of the existing research on 2-day CPET in PEM has been in patients with ME/CFS, the findings should be expected to generalize, regardless of suspected onset. Nevertheless, additional research is needed to compare 2-day CPET results in Long COVID versus ME/CFS.
Functional Impacts
Chronic fatigue symptoms in Long COVID impact a person’s ability to work and perform activities of daily living, which makes clinical care and rehabilitation a priority for these patients (Walker et al., 2023). They can also impact productivity by preventing a return to pre-COVID functional levels, thereby affecting the social and economic health of the impacted individual. A cross-sectional observational study on the impact of fatigue on function in 3,754 Long COVID patients, conducted at 31 post-COVID clinics in the United Kingdom, found that 94 percent (3,541) were of working age (18–65 years). Half (n = 1,321/2,600, 50.8 percent) of those who completed the “working days lost” questionnaire reported the loss of at least 1 day of work in the last month. Approximately 20 percent (20.3 percent) reported losing 20–28 days of work, and 20 percent also reported the inability to work completely. These results were associated mainly with fatigue (Walker et al., 2023). Symptoms of chronic fatigue in Long COVID may also influence a person’s attitudes toward leisure time, thereby impacting mental health functioning and perceived stress.
Since PEM is considered a hallmark symptom for diagnosing ME/CFS (CDC, 2021b; IOM, 2015; NICE, 2021d), Long COVID patients with PEM may also fit criteria for ME/CFS. Understanding the functional impact of PEM is made more challenging by the fluctuating nature of the symptom complex and the fact that often people can compensate for limitations in function by first making compensatory changes in the time, effort, and resources they ascribe to certain tasks or by making compensatory changes in their social or leisure activity patterns (NICE, 2021d).
One recent study comparing PEM symptoms in Long COVID with those in ME/CFS found that the PEM symptoms were more likely to improve over 1 year in individuals with Long COVID, while there was no improvement in those with ME/CFS. However, 51 percent of the individuals with ME/CFS in this study had already had symptoms for >4 years, while 82 percent of those with Long COVID had had symptoms for <1 year (Oliveira et al., 2023). In a large cross-sectional sample of adults selected from post–COVID-19 online groups, those participants who had versus those who did not have PEM at 6 months or more after their COVID-19
illness had a significantly higher average number of Long COVID symptoms that persisted beyond 6 months (Davis et al., 2021). In a smaller cross-sectional analysis of more than 200 adults with at least 4 weeks of persistent symptoms after acute COVID-19 illness, PEM was associated with more severe fatigue and a higher risk of work status limitations, no or low physical activity, lower general health, and lower social functioning (Twomey et al., 2022). In a population-based longitudinal cohort study, PEM was associated with increased risk of other symptoms, such as insomnia, cognitive impairment, headache, and generalized pain, compared with those with fatigue without PEM. The presence of PEM was also associated with increased risk of functional impairment, reduced work capacity, and reduced physical activity (Nehme et al., 2023). Similarly, another population-based longitudinal study conducted in Switzerland describing the long-term trajectory of post-COVID symptoms in adults at 1, 2, 6, 9, 12, 18, and 24 months after SARS-CoV-2 infection found that PEM was more likely in those who had worsening of or no change in their perceived health status compared with those who reported improvement or showed continued recovery (Ballouz et al., 2023).
Treatment
When PEM is identified, the focus of treatment turns to comprehensively assessing and managing the functional impact of these symptoms, along with other Long COVID–related symptoms. When PEM is present, rehabilitation interventions and other treatment approaches may need to be personalized to enhance patient safety (Herrera et al., 2021; WHO, 2023a). For physical activity, the anerobic threshold can be used as the physical activity ceiling for safe activity to avoid PEM, recognizing that the nature of PEM is that the anerobic threshold may vary based on the patient’s previous activities and other recent stressors (Davenport et al., 2010).
In addition to using self-report questionnaires to capture the severity of patients’ fatigue and functional limitations, clinicians may ask patients to track their activities in detailed diaries to better capture the types of activities that are most likely to trigger PEM for each individual. Preventing PEM or lessening its severity may require identifying those triggering factors, such as physical, mental, and emotional stressors; orthostatic intolerance; hormonal factors in women; environmental factors (humidity and extreme temperatures); sensory stimuli (light, noise, and smells); certain foods; and infections, including reinfections with SARS-CoV-2. Teaching patients to respect their physiological limits is an important aspect of managing PEM. An important aspect of rehabilitation for people living with PEM is the provision and use of assistive products and environmental modifications to
prevent PEM and its functional impact (WHO, 2023a). Therefore, another important aspect of management is facilitating work or school accommodations that may be necessary to prevent PEM (e.g., flexible hours, telecommuting options, specialized equipment to reduce physical or cognitive exertion).
Selected Populations
To date, few research studies have explored differences in PEM’s prevalence, severity, and functional impact by race/ethnicity, rurality, or other social factors. A study using the TriNetX database to look at the use of outpatient rehabilitation for a post-COVID condition did find that Hispanic individuals had a significantly higher incidence of fatigue (Hentschel et al., 2022). There also is a scarcity of information on Long COVID fatigue and PEM and the impact of the condition in pregnant and lactating women, even though women appear to be at higher risk for the condition (Pagen et al., 2023).
Post-COVID-19 Cognitive Impairment
Cognitive impairment has emerged as one of the most commonly reported health effects associated with Long COVID, potentially portending significant consequences for patient functioning and quality of life. Cognitive impairment is defined as difficulties with thinking processes that can impact various cognitive domains, including memory, attention, processing speed, language, visuospatial, and executive functions (e.g., multitasking, judgment, problem solving). In patients with Long COVID, cognitive impairment has been characterized primarily by deficits in executive functioning (Becker et al., 2023). Cognitive impairment associated with Long COVID can vary in severity, from mild to severe, and often has an impact on instrumental activities of daily living.
Studies have reported varied rates of PCCI ranging from 8 to 80 percent (Becker et al., 2021), likely the result of varying measurement of cognition, including self-reported versus objective neuropsychological measures; use of cognitive screeners (which are insensitive to milder forms of impairment) (Lynch et al., 2022); and telephonic or online administration of measures versus validated, in-person assessments. However, the most robust studies have found PCCI to occur in approximately 24 percent of patients post COVID-19, across the spectrum of acute COVID severity (Becker et al., 2021). These cognitive deficits have been found to persist several months following SARS-CoV-2 infection (Becker et al., 2021, 2023), with some studies reporting long-term persistence beyond 1 year (Cavaco et al., 2023). While the severity of PCCI is relatively mild in comparison to the deficits
seen in neurodegenerative diseases and severe traumatic brain injuries, it can still contribute to significant functional disability in those impacted (Delgado-Alonso et al., 2022).
PCCI can occur regardless of age, preexisting comorbidities, vaccination status, and COVID-19 variant. However, vulnerability to the condition appears to be greater in Black, Hispanic, female, and older-aged populations (Jacobs et al., 2023; Valdes et al., 2022), a finding that has substantial implications for occupational and social functioning. Female sex has also been associated with a greater probability of PCCI (Jacobs et al., 2023; Valdes et al., 2022). Differences have been found among racial and ethnic groups, such that Black and Hispanic individuals may be more likely to experience Long COVID and PCCI compared with non-Hispanic White individuals (Jacobs et al., 2023). Fewer years of education, Black race, and unemployment with baseline disability have likewise been found to confer a greater risk of PCCI (Valdes et al., 2022). Finally, preexisting conditions, including headaches (Jacobs et al., 2023), cognitive impairment (Valdes et al., 2022), neurological disease (Hartung et al., 2022), and renal disease (Bucholc et al., 2022), all appear to confer greater risk of PCCI. Conversely, some data suggest that the COVID-19 vaccine may reduce the risk of PCCI (Gao et al., 2022).
While the term “brain fog” has been deemed synonymous with cognitive impairment in the Long COVID literature, the two may be distinct clinical entities (McWhirter et al., 2023; Orfei et al., 2022). Brain fog is not a recognized medical diagnosis in itself, but rather a debilitating symptom that is usually seen in association with other factors, such as fatigue, psychosocial stress, and both physical and mental conditions (Jennings et al., 2022). Brain fog seen in patients following SARS-CoV-2 infection has anecdotally been described as inattention, forgetfulness, difficulty concentrating, and difficulty finding words (McWhirter et al., 2023). While these difficulties are often observed in formal neuropsychological assessments, it is well known that patients’ subjective cognitive reports may sometimes be discrepant with objective neuropsychological findings (Schild et al., 2023). Nevertheless, it is important to note that individuals with cognitive impairment may report brain fog as a symptom, and that brain fog and PCCI may be equally functionally disabling in patients with Long COVID.
Diagnosis
The prevalence of cognitive impairment, including mild cognitive impairment (MCI) and more severe forms of cognitive impairment (e.g., dementia), increases with age. It is generally uncommon (i.e., less than 3 percent of the population) before age 65 and increases dramatically
after age 75 (Casagrande et al., 2022; U.S. Preventive Services Task Force, 2020). The prevalence of MCI can be difficult to estimate due to several factors. There are varying diagnostic criteria for MCI. Additionally, as the population ages, some cases of MCI return to normal cognition with ongoing follow-up while others progress to dementia (Casagrande et al., 2022; U.S. Preventive Services Task Force, 2020). In addition to a comprehensive medical history, the clinical standard for diagnosis of cognitive impairment includes a comprehensive neuropsychological evaluation consisting of standardized tests that have well-established normative data (Becker et al., 2023; Casaletto and Heaton, 2017). These tests allow a clinician to compare an individual’s scores with those of a normative population, adjusted for age; education; and sometimes other factors, such as sex. An individual’s performance on these tests is often categorized according to standard deviations (SDs) below the mean of the normative sample. Scores within 1 SD below the mean are considered “average,” whereas scores between 1 and 1.5 SDs below the mean are considered “mildly impaired” or “below average,” and those more than 2 SDs below the mean are considered “moderately to severely impaired.” A neuropsychological evaluation often tests all cognitive domains, including attention, working memory, processing speed, executive functions, language, visuospatial abilities, and learning and memory (Becker et al., 2023).
Many clinical challenges are involved in the evaluation and diagnosis of individuals with PCCI. As noted above, some individuals with Long COVID may report brain fog or other cognitive concerns and experience functional impairment, while not necessarily meeting the clinical diagnostic threshold for cognitive impairment (Davis et al., 2023). This may occur for several reasons.
First, most neuropsychological measures were developed to assess individuals with neurodegenerative disorders or traumatic brain injury (Casaletto and Heaton, 2017; Harvey, 2012) and may not adequately capture the often subtle impairment that can result from COVID-19. Similarly, many caveats apply to the normative data with respect to the populations that are considered “normal,” as norms may not always account adequately for such factors as education, cultural background, language proficiency, and other individual differences.
Second, the theory of cognitive reserve posits that individual differences in the ability to cope with brain pathology or damage are influenced by the brain’s resilience and adaptability, which is often related to such factors as educational attainment, occupational complexity, lifelong learning and stimulation, and social engagement (Stern et al., 2019). Individuals with a high cognitive reserve may not immediately show
impairment on neuropsychological tests for several reasons: (1) they may be adept at using alternative cognitive strategies or recruiting additional brain resources to compensate for those areas that are impaired; (2) they may have started with a higher baseline of cognitive abilities, and thus will still score within the “normal” range on tests even if they have experienced some decline; and (3) their brain may be better able to cope with or resist damage because of its adaptability (Stern et al., 2019). At the same time, however, by the time these individuals’ cognitive symptoms become apparent or manifest on neuropsychological tests, the underlying brain pathology may be quite advanced. In some cases, individuals with high cognitive reserve may report subjective cognitive decline; they feel that their cognitive abilities have diminished even if they still score well on formal testing. This subjective feeling may be attributable to patients’ awareness of subtle changes or difficulties that are not yet detectable with standardized tests, a phenomenon that may be consistent with the concept of brain fog. This phenomenon underscores the importance of considering a comprehensive clinical picture, including subjective reports, daily functioning, and other factors, in conjunction with neuropsychological test scores. Without prior neuropsychological testing, it can be challenging to determine the degree of decline or change from an individual’s baseline cognitive abilities, especially if those baseline abilities were above-average.
Third, performance validity can sometimes be an issue in the clinical evaluation of PCCI. That is, an individual’s performance on neuropsychological tests can be influenced by various factors unrelated to PCCI, such as anxiety, depression, fatigue, or even the specific circumstances of the testing day. Such conditions as sleep disorders or chronic pain, medications, or other medical issues can influence cognitive performance and may lead to inconsistent test results, complicating their interpretation. In some cases, an individual’s effort on a test may be called into question; that is, some individuals may have difficulty fully engaging with the evaluation, usually because of psychological or situational factors. It is also possible that individuals may purposely underperform (e.g., for secondary gain, such as disability claims). Fortunately, neuropsychologists have performance validity measures that can detect suboptimal effort.
Finally, it is important to note that other conditions can mimic the symptoms of PCCI. For example, depression may increase one’s perception of brain fog and contribute to poor attention and concentration (Cristillo et al., 2022). Identifying whether cognitive deficits are due to PCCI or other factors can therefore be challenging. For this reason, a thorough medical history can be extremely useful in ruling in or out other potentially contributing factors.
Functional Impacts
Quantifying the functional impacts of PCCI involves integrating a comprehensive medical history, results of objective neuropsychological tests, subjective symptom reports, and real-world observations. A neuropsychological evaluation can help in identifying specific areas of cognitive strength and weakness and in predicting where an individual may have the most difficulty from day to day.
Several instruments (e.g., Instrumental Activities of Daily Living scale) can be used to gauge how PCCI may be impacting daily tasks, such as managing finances, following instructions, or planning activities. Other self-report tools can help capture individuals’ perceptions of their cognitive and functional challenges. For example, numerous self-report instruments have been shown to adequately capture the severity and functional impact of symptoms associated with self-reported brain fog, such as the Neuro-QOL Scale (Shirley Ryan AbilityLab, 2019), the Cogstate (Maruff et al., 2009), or the Everyday Cognition scale (ECog) (Farias et al., 2008). Feedback from family members, coworkers, or educators can also provide a comprehensive view of an individual’s functioning by offering insight into observed challenges in task completion, time management, or problem solving in real-world settings. Similarly, review of work performance evaluations or school assessments can be helpful in delineating where an individual may be struggling. Especially in complex cases, a neuropsychologist may work closely with other professionals (e.g., occupational therapists, speech therapists, educators, vocational counselors) to provide a holistic understanding of the individual’s functional challenges.
Selected Populations
Research on PCCI in selected populations is limited. As described above, several studies have found racial and ethnic differences in the incidence of PCCI, whereby minoritized populations may be disproportionately impacted (Jacobs et al., 2023). While a neuropsychological evaluation can be extremely valuable in quantifying PCCI and its functional impact, it may not always be accessible to all individuals, and there are many barriers to care. First, because of high demand and a limited number of trained neuropsychologists, there can be extended wait times for an evaluation. Second, many neuropsychologists practice in urban areas or academic medical centers. Therefore, people living in rural or remote areas may not have easy access to a neuropsychologist and may have to travel significant distances for an evaluation. Third, neuropsychological assessments can be costly, and not all insurance plans cover them adequately; individuals without insurance may not be able to afford the evaluation. Finally, in certain cultures
or communities, stigma may be associated with seeking psychological or neuropsychological services, preventing some individuals from pursuing an evaluation.
In addition, certain populations may not derive the same benefit from an evaluation. Several cultural nuances come into play here. First, individuals who speak languages other than English and those whose cultural background differs from that of the majority U.S. population may not have access to appropriate and culturally sensitive evaluations or even to neuropsychologists or interpreters with whom they can communicate, which can lead to misinterpretation of results. Second, most neuropsychological tests were developed and standardized on English-speaking populations, and appropriate normative data may not be available for the individual in question. In this case, an individual’s performance can be over- or underestimated. Finally, in some cultures, cognitive challenges may be expressed more in somatic or physical terms, which can affect self-report measures, clinical interviews, and even effort on the neuropsychological tests. Thus, data derived from neuropsychological evaluations in such populations must often be interpreted with some caution.
Autonomic Dysfunction
Orthostatic intolerance and autonomic dysfunction have emerged as a distinct symptom cluster in Long COVID (El-Rhermoul et al., 2023). Autonomic dysfunction is any disturbance of the autonomic nervous system, inclusive of postural orthostatic tachycardia syndrome (POTS). POTS has increasingly been observed in patients following SARS-CoV-2 infection (Amekran et al., 2022). Other, less frequent types of autonomic dysfunction observed in patients with Long COVID include neurocardiogenic syncope (NCS) and orthostatic hypotension (OH) (Blitshteyn and Whitelaw, 2021). When objective tests do not confirm an established autonomic disorder (i.e., POTS, NCS, or OH), but autonomic symptoms arise upon assuming an upright posture and are relieved by being supine, then the diagnosis of orthostatic intolerance can be given. Some symptoms of autonomic dysfunction, such as lightheadedness, improve quickly upon lying down, but other symptoms, such as fatigue and brain fog, can persist for hours or days at a time and can impact activities of daily living (Fedorowski, 2019; Vernino et al., 2021).
Although POTS is itself a diagnosable multisystem disorder, it also has emerged as a distinct phenotype of Long COVID (El-Rhermoul et al., 2023). POTS is characterized by a sustained heart rate of 30 beats per minute or more in the absence of orthostatic hypotension (Amekran et al., 2022). Like Long COVID, POTS is a multisystem disorder; common symptoms include
fatigue, nausea, dizziness, palpitations, chest pain, and exercise intolerance. POTS, and autonomic dysfunction generally, often present secondary to viral infections. Some studies have suggested that POTS could contribute to the pathophysiology of Long COVID, explaining the persistence of symptoms such as fatigue and cognitive issues in Long COVID patients, although the evidence for this hypothesis is limited (Amekran et al., 2022; Barizien et al., 2021; El-Rhermoul et al., 2023; Isaac et al., 2023). Mechanisms of action may include direct tissue damage, immune dysregulation, hormonal disturbances, elevated cytokine levels, and persistent low-grade infection (Carmona-Torre et al., 2022). Autonomic dysfunction appears to play a significant role in Long COVID and its potential neurological complications (Buoite Stella et al., 2022; Diekman and Chung, 2023).
The prevalence of POTS in the general U.S. population varies, with estimates ranging from 0.1 to 1 percent and a higher incidence among females, although in the general population is likely significantly under-diagnosed (Arnold et al., 2018; Bhatia et al., 2016; Shaw et al., 2019). POTS occurs most frequently in females aged 12–50 and is less common in young children (Amekran et al., 2022). Among people with Long COVID, one study reports 4.1 percent of respondents had received a diagnosis of POTS by the time of the survey, and 33.9 percent of those who reported tachycardia had symptoms suggestive of POTS (Davis et al., 2021). Another study reported 12 percent of individuals with Long COVID who underwent standard autonomic testing had results consistent with POTS (Bryarly et al., 2022). Studies indicate that 25–66 percent of Long COVID patients report autonomic dysfunction (Ladlow et al., 2022; Larsen et al., 2022). One study found orthostatic hypotension in 14 percent of subjects with Long COVID symptoms (Buoite Stella et al., 2022). Kavi (2022) cites unpublished data indicating that Long COVID clinics report 15–50 percent of patients having postural symptoms. These numbers should be taken as preliminary estimates given that most of the data to date came from small retrospective studies that vary in timing after initial SARS-COV-2 infection, definitions of POTS and autonomic dysfunction, and testing protocols.
Diagnosis
The diagnostic criteria for POTS are
- a sustained increase in heart rate upon assuming an upright position of ≥30 beats per minute in adults or ≥40 beats per minute in adolescents aged 12–19,
- the presence of chronic symptoms of orthostatic intolerance for at least 3 months, and
- the absence of orthostatic hypotension (Kavi, 2022; Raj et al., 2021, 2022).
In addition to a detailed medical history and physical examination, evaluating for orthostatic intolerance includes autonomic function tests and questionnaires. Autoantibody testing can provide supporting evidence.
Two forms of standing tests are used to diagnose POTS in Long COVID patients. These tests assess heart rate and blood pressure changes upon assuming an upright position, providing insight into autonomic dysfunction and orthostatic intolerance. In the active stand test, patients rest supine for 5 minutes, then immediately stand up, and their blood pressure and heart rate are measured at 2, 5, and 10 minutes. This test captures the immediate response to standing and helps identify orthostatic tachycardia and associated symptoms (Espinosa-Gonzalez et al., 2023). Another potentially useful test is the National Aeronautics and Space Administration’s (NASA’s) lean test, which involves the patient leaning against a wall after resting supine, with blood pressure and heart rate measured every minute for 10 minutes. This posture minimizes the impact of skeletal muscle pump effects on the cardiovascular system (Espinosa-Gonzalez et al., 2023; Kavi, 2022). These standing tests can be conducted in the primary care setting and may provide valuable information for diagnosing POTS without the need for specialist consultation or specialized equipment (Kavi, 2022).
The head-up tilt test is a specialized assessment used in secondary or tertiary health care settings to investigate autonomic dysfunction (Espinosa-Gonzalez et al., 2023). Head-up tilt table testing is usually performed with a motorized table with a foot board for weight bearing (Benditt et al., 1996). The patient lies supine, loosely restrained by safety straps to prevent injury if loss of consciousness occurs. After a variable period of supine rest, usually 15 minutes but in some studies up to 60 minutes, the tilt table is brought upright, usually to 60–70 degrees. This test, designed to explore the underlying causes of loss of consciousness, is the accepted method for investigating fainting in controlled laboratory conditions. During the test, the patient is positioned on a table equipped with motorized tilting capability. Blood pressure and heart rate measurements are taken while the patient is in a supine position and then gradually tilted upward to approximately 60 degrees for a duration of up to 45 minutes (Espinosa-Gonzalez et al., 2023). If syncope or presyncope occurs, the patient is promptly returned to the supine position. While this specialized test is not essential for a straightforward diagnosis of orthostatic tachycardia, it is useful in investigating specific symptoms, such as unexplained syncope. Not all individuals with orthostatic symptoms require head-up tilt testing.
COMPASS-31 is a standardized, easy-to-complete autonomic questionnaire used to screen for autonomic dysfunction and track symptom changes over time (Larsen et al., 2022). Questions fall into one of six domains: orthostatic intolerance, vasomotor, secretomotor, gastrointestinal, bladder, and pupillomotor function. The questionnaire generates a weighted score from 0 to 100, with a score of ≥20 suggesting moderate to severe autonomic
dysfunction (Larsen et al., 2022). COMPASS-31 is frequently used to assess symptoms of autonomic dysfunction in Long COVID research (Bryarly et al., 2022; Buoite Stella et al., 2022; Seeley et al., 2023). Individuals with Long COVID could benefit from screening for symptoms of autonomic dysfunction (e.g., through use of the COMPASS-31 questionnaire), and those experiencing symptoms of orthostatic intolerance would benefit from further evaluation for disorders such as POTS or orthostatic hypotension (Larsen, Stiles, and Miglis, 2021).
Autoantibody testing can provide supporting evidence for diagnosis, although it is currently not particularly sensitive or specific (Carmona-Torre et al., 2022). A feature of POTS following SARS-CoV-2 infection is a high prevalence of specific circulating autoantibodies, including G-protein-coupled receptor (GPCR) antibodies (such as adrenergic, muscarinic, and angiotensin II type-1 receptors) and the ganglionic neuronal nicotinic acetylcholine receptor (g-AChR). Other recognized autoantibodies in POTS include circulating antinuclear, antithyroid, anti-NMDA-type glutamate receptor, anticardiac protein, anti-phospholipid, and Sjögren’s antibodies (Carmona-Torre et al., 2022).
Functional Impacts
Symptoms resulting from autonomic dysfunction following SARS-CoV-2 infection have a substantial impact on individuals’ functioning and quality of life in the short, medium, and long terms (Carmona-Torre et al., 2022). Symptoms are associated with loss of school and work participation, especially in young women (Bourne et al., 2021). In a study involving 20 adult Long COVID patients (70 percent female), residual autonomic symptoms persisted in 85 percent of participants 6–8 months after SARS-CoV-2 infection, with 60 percent being unable to return to work (Blitshteyn and Whitelaw, 2021). Haloot and colleagues (2022) investigated a sample of 40 Long COVID patients who were diagnosed with POTS and found that disabling symptoms persisted in 100 percent of previously high-functioning participants even after 6 months, indicating the enduring impact of the condition. McDonald and colleagues (2014) assert that young adults with POTS experience a degree of functional impairment comparable to that reported in congestive heart failure and chronic obstructive pulmonary disease, leading to a notably low quality of life. A prospective study of 99 participants—including those with post-acute sequelae of COVID-19 (PASC) (another term used for Long COVID), those with POTS, and healthy controls—revealed a high burden of autonomic dysfunction in those with PASC, leading to poor health-related quality of life and high health disutility (Seeley et al., 2023).
Approximately 50 percent of patients with POTS recover within 1–3 years, with lifestyle measures aiding recovery (Fedorowski, 2019). Exercise therapy, including rowing or cycling, has been shown to be effective in improving functioning in individuals with POTS following COVID-19. A minimum of 3 months of exercise therapy has been recommended, and symptoms often worsen before improving (Fu and Levine, 2018; Shibata et al., 2012). The course of recovery for symptoms associated with autonomic dysfunction in Long COVID entails remitting and relapsing as a result of various factors, such as comorbid conditions, stress, and overexertion (Barizien et al., 2021; Seeley et al., 2023); therefore exercise therapy must be individualized and closely monitored.
Assessing the ability to work in individuals experiencing orthostatic intolerance is challenging because of the unpredictable postexertional increase in symptoms for several days after prolonged periods of upright posture. This intolerance can significantly contribute to disability, and the ability to quantify the extent of functional impairments is limited. Self-reported symptom severity is critical in evaluating disability in individuals dealing with both orthostatic intolerance and Long COVID.
Overall functioning in chronic illnesses is often assessed in adults through self-report health-related quality of life questionnaires, such as the 36-Item Short Form Survey (SF-36), the EuroQOL, or the Patient-Reported Outcomes Measurement Information System (PROMIS) questionnaires (Cook et al., 2012; EuroQol Group, 1990; Ware and Sherbourne, 1992). A newly developed self-report questionnaire, the Malmö POTS symptom score, has shown promise for assessing symptom burden and measuring disease progression in adults with POTS (Spahic et al., 2023). For pediatric patients, age-specific instruments, such as the Functional Disability Inventory or Pediatric Quality of Life (PedsQL), are used into young adulthood, effectively distinguishing between healthy and chronically ill individuals (Claar and Walker, 2006; Varni et al., 2001; Walker and Greene, 1991). Self-reported measures of general or cognitive fatigue include the PedsQL, the Multidimensional Fatigue Inventory (MFI), the Wood Mental Fatigue Inventory, the Fatigue Severity Scale, and others (Bentall et al., 1993; Krupp et al., 1989; Varni and Limbers, 2008; Wood et al., 1991).
Selected Populations
Research on Long COVID-associated POTS in selected populations is limited. In a recent study of pregnant women with POTS not specific to Long COVID (8,941 female patients, 40 percent pregnant), the authors found that the severity of pregnancy symptoms in the first trimester could predict the severity of symptoms in the second and third trimesters
(Bourne et al., 2023). If symptoms improved in the first trimester, they were more likely to continue to improve in the later trimesters, while if symptoms worsened in the first trimester, they were likely to continue to worsen in the second and third trimesters. Other selected populations that may experience autonomic dysfunction from Long COVID and thus require health equity considerations include people with certain conditions and/or disabilities, racial and ethnic minorities, people diagnosed as overweight or obese, and uninsured or underinsured individuals. One example of clinical considerations regarding health equity is those with impaired mobility or severe orthostatic intolerance. Some of these patients may be unable to perform standard testing for autonomic dysfunction, such as a 10-minute stand test, requiring testing modifications (Blitshteyn et al., 2022).
HEALTH EFFECTS OF LONG COVID IN CHILDREN AND ADOLESCENTS
While there are various definitions of children, adolescents, and young people, for the purposes of this report “children” or “pediatrics” will refer to the entire pediatric age range and “adolescents” to children in the older end of the spectrum (i.e., ages ~11 to 18 years). Although rates vary by age, children infected with SARS-CoV-2 usually have mild disease (COVID-19) with low rates of hospitalization (<2 percent) or death (<0.01 percent) (Bhopal et al., 2020, 2021). Nonetheless, persistent health effects following SARS-CoV-2 infection have been reported in children, with multisystem inflammatory syndrome in children (MIS-C) and Long COVID being commonly reported (Lopez-Leon et al., 2022). Additionally, Kompaniyets and colleagues (2022) found selected health effects for which children with SARS-CoV-2 infection are at increased risk: pulmonary embolism (adjusted hazard ratio [aHR] 2.01), myocarditis and cardiomyopathy (aHR 1.99), venous thromboembolic events (aHR 1.87), acute and unspecified renal failure (aHR 1.32), type 1 diabetes mellitus (aHR 1.23), coagulation and hemorrhagic disorders (aHR 1.18), smell and taste disturbances (aHR 1.17), type 2 diabetes (aHR 1.17), and cardiac dysrhythmias (aHR 1.16). Vaccination has been shown to reduce the risk of infection and protect against COVID-19–associated illness, including MIS-C, in children (Fowlkes et al., 2022; Tannis et al., 2023; Yousaf et al., 2023; Zambrano et al., 2022), although the majority of children in the United States remain unvaccinated against COVID-19 (AAP, 2023; CDC, 2021a; Zambrano et al., 2024).
Among the long-term health effects of COVID-19, type 1 diabetes has garnered specific attention, showing a higher incidence rate among children in the first year of the pandemic compared with a prepandemic period
(incidence rate ratio [IRR], 1.14; 95% CI, 1.08–1.21) (D’Souza et al., 2023). This finding was mirrored in the months 13–24 of the pandemic, as shown in a meta-analysis (IRR, 1.27; 95% CI, 1.18–1.37) (D’Souza et al., 2023). The increased risk of type 1 diabetes in children after SARS-CoV-2 infection was found even when compared with diagnosis of acute respiratory infection unrelated to COVID-19 (Barrett et al., 2022; Rahmati et al., 2023; D’Souza et al., 2023). Additional studies are needed to further delineate this phenomenon.
MIS-C presents within 2–6 weeks of acute SARSs-CoV-2 infection as a systemic inflammatory illness. Criteria for case definition include severity necessitating hospitalization, fever above 100.4 degrees Fahrenheit, laboratory evidence of systemic inflammation, and multisystemic organ involvement that cannot be explained by another diagnosis (CDC, 2023a). Organ systems affected by MIS-C include gastrointestinal (80–90 percent), mucocutaneous (74–83 percent), cardiovascular (66.7–86.5 percent), hematologic (47.5 percent), respiratory (36.5 percent), and neurologic (12.2 percent) (Blatz and Randolph, 2022). Reassuringly, with MIS-C–directed treatment, the mortality rate has been 1–2 percent (Blatz and Randolph, 2022; Feldstein et al., 2021).
Although children with MIS-C present as critically ill, most inflammatory and cardiac manifestations resolve rapidly (Rao et al., 2022; Penner et al., 2021). However, some children do experience more long-lasting effects (Penner et al., 2021). One study tested children 6 months after hospitalization on the 6-minute-walk test and found that 45 percent of the patients scored below the 3rd percentile for age, demonstrating functional impairment (Penner et al., 2021). In this same study, 98 percent of the children were able to return to full-time education, but formal neuropsychological testing was not conducted to assess school performance (Penner et al., 2021). More research is needed to characterize the potential long-term effects of MIS-C.
Pediatric Long COVID is a separate entity from MIS-C. The true prevalence of Long COVID symptoms and health effects remains unclear for multiple reasons and varies widely in the literature, including limited studies and heterogeneous samples (Pellegrino et al., 2022). However, rates of pediatric Long COVID are lower than the rates of adult Long COVID (Behnood et al., 2022; CDC, 2023b; Jiang et al., 2023; Rao et al., 2022; Zheng et al., 2023). The risk of Long COVID may be associated with severe symptoms during initial infection, hospitalization, the number of organ systems involved, the number of symptoms at presentation, lack of vaccination against COVID-19, medical complexity, and body mass index ≥85th percentile for age and sex (Bygdell et al., 2023; Funk et al., 2022; Rao et al., 2022). Children with MIS-C may also develop Long COVID (Maddux et al., 2022).
Children with Long COVID may have health effects across body systems (Table 3-2). Commonly reported symptoms include fatigue (sometimes with PEM), weakness, headache, sleep disturbance, muscle and joint pain, respiratory problems, palpitations, altered sense of smell or taste, dizziness, and autonomic dysfunction (Morrow et al., 2022; Rao et al., 2022). Guidelines specific to pediatric Long COVID can aid in the diagnosis and management of the condition in children (Malone et al., 2022).
A systematic review of 22 studies (n = 23,141) from 12 countries identified fatigue (47 percent) as the most dominant Long COVID symptom in children and young people (age ≤ 19 years old), followed by headache (35 percent) (Behnood et al., 2022); these findings are consistent with those of several adult studies (Cheung et al., 2023; Líška et al., 2022; Sánchez-García et al., 2023; Štěpánek et al., 2023). In another systematic review and meta-analysis of 21 pediatric studies (n = 80,071), the prevalence of Long COVID was reported as 25.24 percent, with fatigue (9.66 percent) being the second most dominant clinical manifestation, behind mood symptoms (16.50 percent). Sleep disorders (8.42 percent), headache (7.84 percent), and respiratory symptoms (7.62 percent) were among the top-ranked Long COVID manifestations (Lopez-Leon et al., 2022). It is important to note that for this systematic review and meta-analysis, Long COVID criteria included symptoms lasting for at least 4 weeks, which likely contributed to the higher reported prevalence compared with findings of other studies. Studies have shown that isolation, increased stress, and loss of parents and caregivers significantly impacted children’s development during the pandemic and may lead to a future rise in mental illnesses (Lopez-Leon et al., 2022) and other Long COVID sequelae.
Fatigue and PEM present similarly in pediatric populations compared to adults and are common in children with Long COVID; however, data from pediatric populations with ME/CFS indicate better long-term recovery compared with adults (Carruthers et al., 2011). In a cohort observational study of nearly 800 children, ME/CFS had a mean duration of 5 years, with 54 percent of patients reporting recovery between 5–10 years, and 68 percent of patients reporting recovery after more than 10 years (Rowe, 2019); this issue has not been explicitly studied in children with Long COVID.
POTS and other forms of autonomic dysfunction have also been reported in children with Long COVID (Morrow et al., 2022). Presentations and symptomatology, including orthostatic dizziness, palpitations, chest pain, diaphoresis, and nausea, are similar to those in adults. However, the diagnostic criteria for POTS are different in children and in adults: for individuals aged 12–19, the required heart rate increment is an increase of at least 40 beats/minute on a standing tolerance test, compared with 30 beats/minute in adults (Morrow et al., 2022; Vernino et al., 2021). One study compared 29 adolescents with Long COVID who developed
TABLE 3-2 Common Long COVID Symptoms in Children and Adolescents by Body System
| Respiratory | Shortness of breath or dyspnea |
| Chest (thoracic) pain or tightness | |
| Cough | |
| Difficulty with activity/exercise intolerance | |
| Cardiovascular | Palpitations or tachycardia |
| Dizziness/lightheadedness | |
| Syncope | |
| Chest pain | |
| Difficulty with activity/exercise intolerance | |
| Neurological | Headache |
| Dizziness/lightheadedness or vertigo | |
| Orthostatic intolerance | |
| Syncope or presyncope | |
| Tremulousness | |
| Paresthesia or numbness | |
| Difficulty with attention/concentration | |
| Difficulty with memory | |
| Cognitive impairment or brain fog | |
| Special Senses | Abnormal or loss of smell or taste |
| Musculoskeletal | Weakness |
| Muscle, bone, or joint pain | |
| Gastrointestinal | Nausea |
| Vomiting/reflux | |
| Abdominal pain | |
| Bowel irregularities (constipation/diarrhea) | |
| Weight loss | |
| Lack of appetite | |
| Mental Health | Anxiety |
| Depression/low mood | |
| Increased somatic symptoms | |
| School avoidance | |
| Regression of academic or social milestones | |
| No specific organ system | Fatigue (general); Post-exertional malaise |
| Exercise intolerance | |
| Sleep disturbances | |
| Fever |
SOURCES: Behnood et al., 2022; Borch et al., 2022; Drogalis-Kim et al., 2022; Funk et al., 2022; Jiang et al., 2023; Kompaniyets et al., 2022; Kostev et al., 2022; Leftin Dobkin et al., 2021; Lopez-Leon et al., 2022; Malone et al., 2022; Mariani et al., 2023; Morrow et al., 2022; Pellegrino et al., 2022; Radtke et al., 2021; Riera-Canales and Llanos-Chea, 2023; Roessler et al., 2022; Sansone et al., 2023.
an inappropriate sinus tachycardia or POTS with 64 adolescents who developed autonomic dysfunction prior to the COVID-19 pandemic. The authors concluded that, at least with regard to heart-rate variability, adolescents with POTS diagnosed following SARS-CoV-2 infection were not significantly different from the prepandemic controls (Buchhorn, 2023). Management of POTS is similar for children and for adults, with lifestyle interventions and physical therapy protocols being first line, augmented by medications when indicated.
Post–COVID-19 cognitive impairment (PCCI) or brain fog is reported in children with Long COVID and may manifest in behavioral changes and declining school performance. Limited research has focused on the chronic cognitive effects of COVID-19 in children. Objective neuropsychological data in pediatric patients have shown increased attention deficits in these patients and elevated mood/anxiety concerns (Luedke et al., 2024; Morrow et al., 2021; Ng et al., 2022; Tarantino et al., 2022), although these data came from small, single-center studies. In a survey of 510 children with ages ranging from 1–18 years, 61 percent had poor concentration, 46 percent had difficulty remembering information, 40 percent struggled with completing everyday tasks, and 33 percent had difficulties with information processing (Buonsenso et al., 2022). Importantly, other factors, such as fatigue and mental health problems, should be evaluated when cognitive problems are suspected, as these and other factors can contribute to poor attention and other cognitive difficulties.
With respect to management, a cognitive rehabilitation approach should be considered to improve attention regulation and help the child develop compensatory strategies. Whenever possible, support and accommodations should be offered in school settings to prevent the child from falling further behind academically. Periodic reassessment of cognitive functions is also prudent to monitor progress over time. Finally, screening for mental health problems is critical in children with post-COVID cognitive impairment, as PCCI can greatly impact quality of life.
Children with Long COVID may experience new or worsening mental health conditions, such as anxiety and depression. One study with 236 pediatric Long COVID patients found that irritability, mood changes, and anxiety or depression were found in 24.3 percent, 23.3 percent, and 13.1 percent of the cohort, respectively, suggesting a high prevalence of persistent psychiatric symptoms (Roge et al., 2021). Children may experience anxiety and depression differently from adults. Signs of depression can include behavioral problems at school, changes in eating or sleeping habits, and lack of interest in fun activities, while signs of anxiety in children can include fear of being away from a parent, physical symptoms of panic, and refusal to go to school. Diagnosis involves ruling out other conditions that may affect mood, and often includes interviews with the
child and their caregivers (Cleveland Clinic, 2023). Management of mood disorders for pediatric patients with Long COVID is the same as management for those without Long COVID (e.g., psychotherapy, medications); however, special consideration is warranted for the other comorbid Long COVID symptoms and how they may interact with mood disorders or their treatment. The most common antidepressants for children are selective serotonin reuptake inhibitors (SSRIs), which increase the level of serotonin in the brain.
Although there may be overlap between pediatric and adult presentations and intervention options, particularly among adolescents, pediatric management of Long COVID entails specific considerations: developmentally, some young children and those with developmental disabilities may have difficulty describing their symptoms, and patient histories may come from parents and others outside the home, such as caregivers, coaches, or teachers. Compared with adults, children are healthier, with fewer preexisting chronic health conditions. Conditions that may increase the risk of Long COVID in adults, such as type 2 diabetes, are rarely seen in pediatrics, and Long COVID may therefore represent a large change from baseline for previously healthy children (Malone et al., 2022).
In addition to adverse effects of pediatric Long COVID on participation and performance in school, sports, and other activities (Morello et al., 2023; Pellegrino et al., 2022), it also has been shown to negatively affect family functioning as a whole (Chen et al. 2023). Management of pediatric Long COVID is focused on symptomatic support and from a functional perspective, on activities of daily living, such as participation in school and in activities and hobbies. Additionally, the American Heart Association and American Academy of Pediatrics recommend that those treating pediatric patients with moderate or severe acute SARS-CoV-2 infection or with any prolonged cardiac symptoms (e.g., fatigue, syncope, palpitations) use a screen to assess for the possibility of cardiac complications before recommending a return to physical activity (AAP, 2022a).
While the trajectory for children affected by Long COVID is more favorable than that for adults, additional studies are needed to better understand and characterize the diversity of presentations in children and long-term implications related to quality of life and function. The National Institutes of Health’s (NIH’s) RECOVER initiative currently has ongoing clinical trials and studies under way aimed at further understanding how to treat and prevent Long COVID in children in addition to adults (NIH, 2023). However, more research is still needed (Long COVID and kids: More research is urgently needed, 2022).
As detailed in Chapter 1, SSA’s definition and determination of disability in children and adolescents (<18 years) differs from those in adults. “Disability” for adults in the SSA context centers on work disability or an
inability to perform work-related activities (see, e.g., Annex Table 3-13 at the end of this chapter) and to participate in work “in an ordinary work setting, on a regular and continuing basis, and for 8 hours per day, 5 days per week, or an equivalent work schedule” (SSA, 2021). In contrast, the definition and determination of disability in children incorporates the concept of functioning more broadly (i.e., a child’s qualifying impairment(s) must cause “marked and severe functional limitations”1). When evaluating the effects of an impairment or combination of impairments on a child’s functioning, SSA considers “how appropriately, effectively, and independently” the child performs their activities (everything they do at home, at school, and in the community) “compared to the performance of other children [their] age who do not have impairments.”2 In particular, SSA considers functioning in six domains: “(i) acquiring and using information, (ii) attending and completing tasks, (iii) interacting and relating with others, (iv) moving about and manipulating objects, (v) caring for [oneself], and (vi) health and physical well-being.”3
SELECTED GUIDANCE STATEMENTS SPECIFIC TO LONG COVID
In addition to the diagnosis and management guidelines included in Annex Tables 3-1 through 3-12, selected guidance statements specific to Long COVID are listed in Table 3-3. The CDC (2024) maintains a webpage with information on Long COVID (which the CDC refers to as Post-COVID conditions) for health care providers and the general public, covering the topics of assessment and testing, management, documentation, research, and tools and resources. The latter includes a list of “medical professional organization expert opinion and consensus statements.” The World Health Organization (WHO) (2023a) also maintains a webpage on “clinical management of COVID-19,” which includes a link to the most recent version of its COVID-19 Clinical Management: Living Guidance document. This publication contains “the most up-to-date recommendations for the clinical management of people with COVID-19” and includes a section on the “care of COVID-19 patients after acute illness” (WHO, 2023a).
In the United Kingdom, the National Institute for Health and Care Excellence, the Scottish Intercollegiate Guidelines Network, and the Royal College of General Practitioners jointly developed a guideline addressing identification, assessment, and management of the long-term effects of COVID-19 and offering “recommendations about care in all healthcare settings for adults, children and young people who have new or ongoing
___________________
1 20 CFR 416.906.
2 20 CFR 416.926a.
3 20 CFR 416.926a.
TABLE 3-3 Selected Guidance Statements on Long COVID
| Author | Title | Last revised | Source |
|---|---|---|---|
| U.S. Centers for Disease Control and Prevention | Post-COVID conditions: Information for healthcare providers | February 6, 2024 | CDC (2024) |
| World Health Organization | Clinical management of COVID-19: Living guideline, 18 August 2023 | August 18, 2023 | WHO (2023a) |
| National Institute for Health and Care Excellence, Scottish Intercollegiate Guidelines Network, and Royal College of General Practitioners | COVID-19 rapid guideline: Managing the long-term effects of COVID-19 | November 11, 2021 | NICE (2021a) |
| Catalan Society of Family and Community Medicine Long COVID-19 Study Group | Long Covid-19: Proposed primary care clinical guidelines for diagnosis and disease management | April 20, 2021 | Sisó-Almirall et al. (2021) |
| American Academy of Pediatrics | Post-COVID-19 conditions in children and adolescents | September 2, 2022 | AAP (2022b) |
| American Academy of Physical Medicine and Rehabilitation (AAPMR) PASC Multi-Disciplinary Collaborative Pediatric Work Group | Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of postacute sequelae of SARS-CoV-2 infection (PASC) in children and adolescents | August 11, 2022 | Malone et al. (2022) |
| AAPMR PASC Multi-Disciplinary Collaborative Writing Group | Multidisciplinary collaborative consensus guidance statement on the assessment and treatment of fatigue in postacute sequelae of SARS-CoV-2 infection (PASC) patients | July 26, 2021 | Herrera et al. (2021) |
| AAPMR PASC Multi-Disciplinary Collaborative Writing Group | Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of breathing discomfort and respiratory sequelae in patients with post-acute sequelae of SARS-CoV-2 infection (PASC) | November 29, 2021 | Maley et al. (2022) |
| AAPMR PASC Multi-Disciplinary Collaborative Writing Group | Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of cognitive symptoms in patients with post-acute sequelae of SARS-CoV-2 infection (PASC) | December 1, 2021 | Fine et al. (2022) |
| Author | Title | Last revised | Source |
|---|---|---|---|
| AAPMR PASC Multi-Disciplinary Collaborative Writing Group | Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of cardiovascular complications in patients with post-acute sequelae of SARS-CoV-2 infection (PASC) | May 27, 2022 | Whiteson et al. (2022) |
| AAPMR PASC Multi-Disciplinary Collaborative Writing Group | Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of autonomic dysfunction in patients with post-acute sequelae of SARS-CoV-2 infection (PASC) | June 3, 2022 | Blitshteyn et al. (2022) |
| AAPMR PASC Multi-Disciplinary Collaborative Writing Group | Multidisciplinary collaborative consensus guidance statement on the assessment and treatment of neurologic sequelae in patients with postacute sequelae of SARS-CoV-2 infection (PASC) | March 29, 2023 | Melamed et al. (2023) |
| AAPMR PASC Multi-Disciplinary Collaborative Writing Group | Multidisciplinary collaborative consensus guidance statement on the assessment and treatment of mental health symptoms in patients with post-acute sequelae of SARS-CoV-2 infection (PASC) | November 8, 2023 | Cheng et al. (2023) |
symptoms 4 weeks or more after the start of acute COVID-19” (NICE, 2021a). Likewise, a Long COVID study group of the Catalan Society of Family and Community Medicine (Spain) issued proposed primary care clinical guidelines for the diagnosis and management of the condition in 2021 (Sisó-Almirall et al., 2021). The stated primary objective of the guidelines is the identification of individuals “with signs and symptoms of long COVID-19 . . . through a protocolized diagnostic process that studies possible etiologies and establishes an accurate differential diagnosis” (Sisó-Almirall et al., 2021, p. 4350).
Pediatric guidance on Long COVID in the United States includes statements from the American Academy of Pediatrics (AAP, 2022b) and a pediatric work group in alliance with the American Academy of Physical Medicine and Rehabilitation (AAPMR) PASC Multi-Disciplinary Collaborative (Malone et al., 2022). The AAP guidance provides information on
Long COVID in children and adolescents and evaluation and management of some of the potential ongoing symptoms. To assist primary care physicians and assist in initial specialty evaluations for children and adolescents with Long COVID, the AAPMR pediatric guidance statement addresses the initial evaluation of children and adolescents with Long COVID and potential health effects in different body systems, as well as return to play or activity and accommodations for school and activities (Malone et al., 2022). Work groups of the AAPMR PASC Multi-Disciplinary Collaborative have also issued guidance statements on the diagnosis and management of health effects of Long COVID in specific areas: fatigue, breathing discomfort and respiratory sequelae, cognitive symptoms, cardiovascular complications, autonomic dysfunction, neurologic sequelae, and mental health (Blitshteyn et al., 2022; Cheng et al., 2023; Fine et al., 2022; Herrera et al., 2021; Maley et al., 2022; Melamed et al., 2023; Whiteson et al., 2022).
OVERVIEW OF BODY SYSTEMS POTENTIALLY AFFECTED IN LONG COVID
In addition to the information in Annex Tables 3-1 through 3-12 on selected health effects associated with Long COVID, the following narratives, organized by body system, provide a high-level overview of diagnostic, management, and other information potentially relevant to adults with Long COVID. It is important to note that symptoms and health conditions may cross two or more body systems.
Respiratory System
Persistent respiratory symptoms and conditions are common in patients with Long COVID (Evans et al., 2023). The underlying pathophysiology for these symptoms and conditions is complex and multifactorial, encompassing persistent cardiac, pulmonary, or vascular dysfunction. The diagnostic approach to patients with these symptoms and conditions in Long COVID involves a clinical history and physical examination, and may include blood, radiologic, and other diagnostic tests, such as the 1-minute sit-to-stand test, chest X-ray, and pulmonary function testing (Daines et al., 2022). Computed tomography pulmonary angiography (CTPA) or ventilation perfusion (VQ) scintigraphy and VQ single-photon emission computed tomography (Daines et al., 2022; Kim et al., 2022) may be used to identify pulmonary vascular manifestations after acute SARS-CoV-2 infection. In general, the management approaches for all these respiratory symptoms and conditions in Long COVID are not specific to Long COVID, although some studies indicate the use of steroids to improve radiological, subjective, and functional outcomes in selected patients with post-COVID-19 pulmonary involvement (Dhooria
et al., 2022; Goel et al., 2021; Mizera et al., 2024; Myall et al., 2021). Management of respiratory symptoms and conditions associated with Long COVID varies based on the severity of the symptoms, the level of functional impairment, comorbid illnesses, and other clinical factors.
Cardiovascular System
Cardiovascular symptoms and conditions are common following acute SARS-CoV-2 infection, and can include cardiopulmonary symptoms (e.g., chest pain, dyspnea, or palpitations), cardio-neuro symptoms, cardiac inflammatory diseases (e.g., myocarditis and pericarditis), and collateral damage from acute coronary syndromes (DePace and Colombo, 2022; Satterfield et al., 2022). Diagnostic approaches for cardiovascular symptoms and conditions related to Long COVID include electrocardiogram (ECG); transthoracic echocardiography; echocardiography; stress testing; event monitoring; and laboratory assessments such as cardiac biomarkers, cell counts, inflammatory markers, and coagulation markers. Functional tests, such as the 3-minute active stand test, 10-minute POTS assessment, and 6-minute walking test, may be administered. Cardiac magnetic resonance (CMR) can be used to determine abnormalities in ventricular and systolic function; pericardial effusion; and such abnormalities as myocarditis, epicarditis, pericarditis, and endocarditis (Satterfield et al., 2022; Von Knobelsdorff-Brenkenhoff and Schulz-Menger, 2023). Cardiopulmonary exercise testing can be used to better understand the relevant factors contributing to post-COVID diminished exercise capacity. As mentioned in the earlier discussion of PEM, two consecutive days of CPET can help diagnose PEM in individuals with ME/CS (Davenport et al., 2019; Stevens et al., 2018). Long COVID patients may exhibit various ECG abnormalities, including sinus tachycardia, ST changes, T-wave abnormalities, prolonged QT interval, low voltage, and bundle branch block.
Management of cardiovascular issues related to Long COVID currently involves symptom management and rehabilitation. Although there are no specific guidelines for cardiac inflammatory diseases such as myocarditis, ongoing research is exploring the use of antivirals, steroids, and nonsteroidal anti-inflammatory drugs in this condition (Raman et al., 2022). In general, the management approaches for all the cardiovascular symptoms and conditions in Long COVID, are not specific to Long COVID and treatment approaches generally follow the routine guidelines for the underlying condition.
Neurological System
A growing body of evidence indicates that Long COVID can manifest as neurological sequelae, affecting the central and/or peripheral nervous system. Some manifestations occur during the acute phase in hospitalized
patients or as sequelae of a major neurological event, such as a stroke, while other relevant symptoms and conditions either continue or emerge weeks after infection. The neurological abnormalities observed in Long COVID likely arise from a multifaceted interplay of direct viral effects, autoimmunity, inflammation, mitochondrial dysfunction, vascular changes and thrombosis, hormonal imbalances, and persistent viral presence. A comprehensive understanding of these pathophysiological mechanisms is essential for the development of targeted therapeutic strategies for managing and alleviating the neurological sequelae of Long COVID. Management can include pharmacological interventions; physical therapy; pacing; occupational therapy, including lifestyle modifications; and psychological therapy (Tana et al., 2022).
Special Senses
Research on the specific abnormalities of the special senses such as vision, hearing, balance, taste, and smell in Long COVID is still emerging, and understanding of these abnormalities is evolving. Some individuals with Long COVID have reported persistent alterations in taste and smell, conditions known as anosmia (loss of smell) and ageusia (loss of taste). Additionally, studies have found visual and auditory disturbances, such as blurry vision, tinnitus, or hearing loss, in some Long COVID patients. These sensory changes can impact quality of life and may persist after acute SARS-CoV-2 infection even after other COVID-19 symptoms have resolved. In addition to diminishing quality of life, impaired or loss of smell and taste may lead to changes in eating habits (Ferrulli et al., 2022), potentially resulting in weight loss and worsening of other symptoms, including fatigue. Abnormalities of the special senses should be assessed in patients suspected of having Long COVID; however, a thorough medical history and physical examination are always advisable.
There are no well-established treatments for dysfunctions of the special senses due to Long COVID. The authors of a systematic review (Khani et al., 2021) propose several potential medications for treating Long COVID anosmia and ageusia, including pentoxifylline, zinc, omega-3, intranasal fluticasone, intranasal insulin, statins, and melatonin.
Musculoskeletal System
Individuals with Long COVID frequently report musculoskeletal and pain conditions alongside a range of other symptoms, complicating their pain experience and impacting their function and quality of life. Skeletal muscle abnormalities have been associated with lower exercise capacity, and exercise-induced myopathy has been noted with PEM (Appelman et al., 2024).
Diagnosis of musculoskeletal manifestations of Long COVID requires a thorough workup that ranges from noninvasive techniques (e.g., radiology/imaging) to invasive maneuvers (e.g., biopsy). A detailed medical history and physical examination is the first step, before any diagnostic test. Evidence is currently insufficient to recommend or advise against tests including creatine kinase, lactate dehydrogenase, c-reactive protein (CRP), rheumatologic factor, and antinuclear antibody in patients with arthralgia and myalgia lasting more than 12 weeks after COVID-19 (Kim et al., 2022).
There currently is no specific treatment for the musculoskeletal manifestations of Long COVID; patient-centered symptomatic management aimed at improving the quality of life is the goal by default (Calabrese et al., 2022). In a systematic review, Balcom and colleagues (2021) report that Long COVID myositis showed a favorable response to steroids, intravenous immunoglobulin, and tocilizumab. Baricitinib, an anti-inflammatory drug used to treat rheumatoid arthritis, has shown promising results; however, its association with increased risk for the development of malignancy has warranted further investigation (Assadiasl et al., 2021). Physical therapy (low-intensity aerobic training exercises) and psychological measures have proven to be effective in improving muscle strength (Nambi et al., 2022). Of note, people with Long COVID can exhibit changes in skeletal muscle mitochondrial function and metabolism that can further deteriorate with PEM; therefore, engaging in intense exercise is not advisable for some individuals with Long COVID (Appelman et al., 2024).
Endocrine System
Several conditions consistent with endocrine dysfunction, including diabetes mellitus (DM), dyslipidemia, thyroid dysfunction, adrenal dysfunction, and reproductive hormone dysfunction, have been noted in patients with Long COVID. The interplay between the endocrine system and Long COVID can be bidirectional: studies have shown a higher risk of developing Long COVID in patients with DM or those who are overweight, but have also shown that treatments, such as corticosteroids, potentially used both for acute SARS-CoV-2 infection and Long COVID may increase the risk of developing endocrine and metabolic disease (Bornstein et al., 2022).
Thyroid dysfunction has also been reported in Long COVID. Findings are mixed, however, as to the true role played by thyroid dysfunction and even the presence of antithyroid antibodies in Long COVID patients (Lui et al., 2023; Sunada et al., 2022).
Adrenal dysfunction has also been postulated as a relevant feature in patients with Long COVID. Of note, lower levels of serum cortisol have been described in patients with Long COVID compared with non–Long COVID healthy controls (Klein et al., 2023).
Finally, the presence of reproductive hormonal dysfunction in patients with Long COVID has been hypothesized, due mainly to epidemiological factors and to a high incidence (74 percent of females) of patient-reported changes in menstrual cycle as part of the Long COVID complex of symptoms. However, no clear entity has been identified to define a clear diagnostic pathway (Lott et al., 2023).
The diagnosis and treatment of endocrine dysfunction is similar for individuals with and without Long COVID.
Immune System
The immune system is an integral part of the response to SARS-CoV-2 infection itself, as its proper function is critical for the ability to contain and eliminate the infection prior to any additional therapeutic support. Given its role in the control and containment of the primary infection, it is not surprising that dysregulation of the immune system has been reported by patients with Long COVID.
Different levels of immune dysregulation have been identified in patients with Long COVID, including differences in the levels of circulating myeloid and lymphocyte populations compared with control patients without Long COVID; increased levels of antibody response to SARS-CoV-2; and increased levels of antibody response to other pathogens, especially Epstein-Barr virus (Klein et al., 2023). Notably, these abnormalities are not diagnostic of Long COVID, but are observations that support immune dysregulation among Long COVID patients.
Immune dysregulation is a broad theme. The immune system overlaps with all the other body systems and functions, and so the dysregulation noted in patients with Long COVID is hypothesized to potentially affect viral persistence and replication and gut microbiota dysbiosis, and to lead to autoimmunity and immune priming, blood clotting and endothelial abnormalities, and dysfunctional neurological signaling (Bellanti et al., 2023).
Patients with Long COVID frequently report symptoms compatible with mast cell activation syndrome (MCAS). Several of the symptoms frequently seen in Long COVID overlap with those seen in MCAS, which can lead to diagnostic confusion. The diagnostic criteria for MCAS are well established by international societies and are applied similarly in Long COVID patients (Valent et al., 2021).
Treatment of MCAS depends on the individual’s symptoms. It involves, first, guaranteeing anaphylaxis management ability through education and permanent availability of an epinephrine auto-injector and additionally controlling the different mast cell mediators. Treatment is standardized by society guidelines and applies to all patients with MCAS, regardless of the presence of Long COVID (Valent et al., 2020).
Gastrointestinal System
Individuals with Long COVID frequently report gastrointestinal symptoms, in isolation or combined with other symptoms. These symptoms may be compatible with such diagnoses as gastroesophageal reflux disease and irritable bowel syndrome, with similar presentations and management.
Genitourinary System
The relationship between chronic kidney disease and COVID-19 is bidirectional: chronic kidney disease increases susceptibility to COVID-19 infection and risk for severe disease, and Long COVID increases the risk of chronic kidney disease, even independently of acute kidney injury (Schiffl and Lang, 2023). The risk of renal dysfunction increases proportional to the severity of the acute COVID-19 infection (nonhospitalized, hospitalized, or ICU), although the risk extends to all patients, including those with milder cases of COVID-19 (Bowe et al., 2021). Kidney dysfunction is often a silent problem that can progress without being discovered. It is therefore important to monitor kidney function in Long COVID patients who are at higher risk of kidney disease. Diagnosis involves a combination of medical history, physical examination, laboratory tests, and imaging studies, as used in the general population.
Kidney care is an integral part of post-acute care for COVID-19. Treatment and management depend on the disease (i.e., chronic kidney disease or acute kidney injury) and stage, and those protocols are also applied to patients with and without Long COVID according to standardized guidelines.
Integumentary System
Cutaneous manifestations of Long COVID are relatively mild and last an average of 7 to 15 days (Freeman et al., 2023; Martora et al., 2023 Mass General, 2022; Polly and Fernandez, 2022). These conditions would most likely not rise to the level of a disabling impairment.
Mental Health
Because SARS-CoV-2 infection can affect the brain, some of its postacute sequelae may manifest in the form of neuropsychiatric disorders, including depression, anxiety, and adjustment and stress disorders. These disorders are likely driven by structural and/or functional brain alterations following infection and should not be misconstrued as evidence of a psychological basis for Long COVID. That said, mental health problems can also arise as a secondary or tertiary effect of Long COVID, as well as of
the global pandemic more broadly. That is, with the rapid spread of SARS-CoV-2 arose the strict implementation of widespread social restrictions (e.g., quarantine, social distancing measures), often coupled with occupational loss and economic hardships. These factors should also be considered, as they could certainly play a role in initiating and/or exacerbating mental health problems in a subset of individuals. Along those lines, having Long COVID may predispose individuals to mental health problems by virtue of their new onset limitations. Finally, preexisting mental health problems may also be exacerbated following SARS-CoV-2 infection. Overall, determining whether mental health problems in those infected by SARS-CoV-2 differ from those not infected but merely affected by the global pandemic remains an ongoing challenge.
Regardless of their precise etiology, it is advisable for all clinicians to screen for new-onset anxiety and depression in Long COVID patients as they can have profound functional implications. Diagnosis of mental health problems may range from brief screening assessments in medical settings to more thorough clinical assessments by mental health professionals. Thorough assessments involve a review of symptoms, medical history, and psychosocial context. Criteria for diagnosis of mental health disorders are outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) (APA, 2022) or the International Classification of Diseases, Eleventh Revision (ICD-11) (WHO, 2023b). These criteria are used to diagnose anxiety, depression, posttraumatic stress disorder (PTSD), and other metal health disorders that may be pertinent in Long COVID.
The treatment of mental health disorders in patients with Long COVID can vary widely depending on the specific disorder, its severity, and the individual’s unique needs and comorbid symptoms. The most common, evidence-based approaches include psychotherapy (e.g., cognitive-behavioral therapy), medications (e.g., antidepressants, antipsychotics), or a combination of the two (Cleveland Clinic, 2023). Importantly, given the multimorbidity of Long COVID, it is critical for clinicians to consider the potential interaction effects of certain medications, as well as their impact on other Long COVID symptoms.
SUMMARY AND CONCLUSIONS
Long COVID comprises hundreds of health effects caused by or associated with acute SARS-CoV-2 infection that manifest in many different body systems. Evidence on clustering of the post-acute and long-term health effects of SARS-CoV-2 remains inconsistent across studies, and consensus is needed on terms, definitions, and methodological approaches for generating better-quality and more consistent evidence. Health effects associated with Long COVID may manifest as impairments in body structures and
physiological functions, potentially affecting mental (e.g., cognitive, psychosocial, emotional) functioning as well as physical functioning. In addition, individuals with Long COVID may experience multiple and potentially overlapping symptoms and conditions, including chronic fatigue and PEM, PCCI, and autonomic dysfunction. Some health effects can be sufficiently severe to interfere with an individual’s day-to-day functioning, including participation and performance in work and school activities. There is some overlap between SSA’s current Listing of Impairments (Listings) and health effects associated with Long COVID, such as impaired lung and heart function. However, it is likely that most individuals with Long COVID applying for Social Security disability benefits will do so on the basis of health effects not covered in the Listings. Three frequently reported health effects that can significantly interfere with the ability to perform work or school activities and may not be captured in SSA’s Listings are chronic fatigue and PEM, PCCI, and autonomic dysfunction, all of which can be difficult to assess clinically in terms of their severity and effects on a person’s functioning.
Children with Long COVID also may experience health effects across many body systems. Commonly reported symptoms include fatigue, weakness, headache, sleep disturbance, muscle and joint pain, respiratory problems, palpitations, altered sense of smell or taste, dizziness, and autonomic dysfunction. Although pediatric presentations and intervention options may overlap with those in adults, particularly among adolescents, who may be more likely to mimic adult presentation and trajectories, pediatric management of Long COVID entails specific considerations related to developmental age and/or disabilities and history gathering. In general, children have fewer preexisting chronic health conditions than adults; thus, Long COVID may represent a substantial change from their baseline, particularly for those who were previously healthy. Management of pediatric Long COVID, as with adults, is focused on symptomatic support and, from a functional perspective, on activities of daily living, such as participation in school and in activities and hobbies.
REFERENCES
AAP (American Academy of Pediatrics). 2022a. COVID-19 interim guidance: Return to sports and physcial activity. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/covid-19-interim-guidance-return-to-sports/#:~:text=The%20AAP%20recommends%20not%20returning,physician%20evaluation%20has%20been%20completed (accessed January 12, 2024).
AAP. 2022b. Post-COVID-19 conditions in children and adolescents https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/post-covid-19-conditions-in-children-and-adolescents/ (accessed January 17, 2024).
AAP. 2023. Children and COVID-19 vaccination trends. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-vaccination-trends/ (accessed January 16, 2024).
AAPM (American Academy of Pain Medicine). 2023. Clinical guidelines. https://painmed.org/clinical-guidelines/ (accessed July 5, 2024).
ACOG (American College of Obstetricians and Gynecologists). 2018. Dysmenorrhea and endometriosis in the adolescent–Committee opinion number 760. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2018/12/dysmenorrhea-and-endometriosis-in-the-adolescent (accessed March 26, 2024).
ACR (American College of Rheumatology). 2021. Vasculitis clinical practice guidelines https://rheumatology.org/vasculitis-guideline (accessed November 9, 2023).
Adjaye-Gbewonyo, D., A. Vahratian, C. G. Perrine, and J. Bertolli. 2023. Long COVID in adults: United States, 2022. NCHS Data Brief (480):1–8.
Amekran, Y., N. Damoun, and A. J. El Hangouche. 2022. Postural orthostatic tachycardia syndrome and post-acute COVID-19. Global Cardiology Science and Practice 2022(1-2): e202213.
American College of Gastroenterology. 2023. ACG’s gastroenterology guidelines. https://gi.org/guidelines/ (accessed November 14, 2023).
American Epilepsy Society. 2019. AES clinical practice guideline development manual. https://aesnet.org/clinical-care/clinical-guidance/guidelines (accessed April 4, 2024).
Ammirati, E., M. Frigerio, E. D. Adler, C. Basso, D. H. Birnie, M. Brambatti, M. G. Friedrich, K. Klingel, J. Lehtonen, J. J. Moslehi, P. Pedrotti, O. E. Rimoldi, H.-P. Schultheiss, C. Tschöpe, L. T. Cooper, and P. G. Camici. 2020. Management of acute myocarditis and chronic inflammatory cardiomyopathy: An expert consensus document. Circulation: Heart Failure 13(11):e007405.
Angeli, P., M. Bernardi, C. Villanueva, C. Francoz, R. P. Mookerjee, J. Trebicka, A. Krag, W. Laleman, and P. Gines. 2018. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. Journal of Hepatology 69(2):406–460.
APA (American Psychiatric Association). 2022. Diagnostic and statistical manual of mental disorders, Fifth Edition, Text Revision (DSM-5-TR). Washington, DC: APA.
Appelman, B., B. T. Charlton, R. P. Goulding, T. J. Kerkhoff, E. A. Breedveld, W. Noort, C. Offringa, F. W. Bloemers, M. Van Weeghel, B. V. Schomakers, P. Coelho, J. J. Posthuma, E. Aronica, W. Joost Wiersinga, M. Van Vugt, and R. C. I. Wüst. 2024. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nature Communications 15(1):17.
Arnold, A. C., J. Ng, and S. R. Raj. 2018. Postural tachycardia syndrome–Diagnosis, physiology, and prognosis. Autonomic Neuroscience 215(December):3–11.
ASHA (American Speech-Language-Hearing Association). 2016. Diagnosis and management of balance vestibular disorder https://www.asha.org/articles/diagnosis-and-management-of-balance-vestibular-disorder/ (accessed November 10, 2023).
Assadiasl, S., Y. Fatahi, B. Mosharmovahed, B. Mohebbi, and M. H. Nicknam. 2021. Baricitinib: From rheumatoid arthritis to COVID-19. Journal of Clinical Pharmacology 61(10):1274–1285.
Avasthi, A., S. Grover, and T. S. Sathyanarayana Rao. 2017. Clinical practice guidelines for management of sexual dysfunction. Indian Journal of Psychiatry 59(Suppl 1):S91–S115.
Awatade, N. T., P. A. B. Wark, A. S. L. Chan, S. Mamun, N. Y. Mohd Esa, K. Matsunaga, C. K. Rhee, P. M. Hansbro, and S. S. Sohal,. 2023. The complex association between COPD and COVID-19. Journal of Clinical Medicine 12(11):3791.
Balcom, E. F., A. Nath, and C. Power. 2021. Acute and chronic neurological disorders in COVID-19: Potential mechanisms of disease. Brain 144(12):3576–3588.
Ballouz, T., D. Menges, A. Anagnostopoulos, A. Domenghino, H. E. Aschmann, A. Frei, J. S. Fehr, and M. A. Puhan. 2023. Recovery and symptom trajectories up to two years after SARS-CoV-2 infection: Population based, longitudinal cohort study. BMJ 381:e074425.
Baratto, C., S. Caravita, A. Faini, G. B. Perego, M. Senni, L. P. Badano, and G. Parati. 2021. Impact of COVID-19 on exercise pathophysiology: A combined cardiopulmonary and echocardiographic exercise study. Journal of Applied Physiology (1985) 130(5):1470–1478.
Barizien, N., M. Le Guen, S. Russel, P. Touche, F. Huang, and A. Vallée. 2021. Clinical characterization of dysautonomia in long COVID-19 patients. Scientific Reports 11(1):14042.
Barrett, C. E., A. K. Koyama, P. Alvarez, W. Chow, E. A. Lundeen, C. G. Perrine, M. E. Pavkov, D. B. Rolka, J. L. Wiltz, L. Bull-Otterson, S. Gray, T. K. Boehmer, A. V. Gundlapalli, D. A. Siegel, L. Kompaniyets, A. B. Goodman, B. E. Mahon, R. V. Tauxe, K. Remley, and S. Saydah. 2022. Risk for newly diagnosed diabetes >30 days after SARS-CoV-2 infection among persons aged <18 years–United States, March 1, 2020–June 28, 2021. MMWR Morbidity and Mortality Weekly Report 71(2):59–65.
Bateman, L., A. C. Bested, H. F. Bonilla, B. V. Chheda, L. Chu, J. M. Curtin, T. T. Dempsey, M. E. Dimmock, T. G. Dowell, D. Felsenstein, D. L. Kaufman, N. G. Klimas, A. L. Komaroff, C. W. Lapp, S. M. Levine, J. G. Montoya, B. H. Natelson, D. L. Peterson, R. N. Podell, I. R. Rey, I. S. Ruhoy, M. A. Vera-Nunez, and B. P. Yellman. 2021. Myalgic encephalomyelitis/chronic fatigue syndrome: Essentials of diagnosis and management. Mayo Clinic Proceedings 96(11):2861–2878.
Bates, D., B. C. Schultheis, M. C. Hanes, S. M. Jolly, K. V. Chakravarthy, T. R. Deer, R. M. Levy, and C. W. Hunter. 2019. A comprehensive algorithm for management of neuropathic pain. Pain Medicine 20(Supplement_1):S2–S12.
Baugh, R. F., G. J. Basura, L. E. Ishii, S. R. Schwartz, C. M. Drumheller, R. Burkholder, N. A. Deckard, C. Dawson, C. Driscoll, M. B. Gillespie, R. K. Gurgel, J. Halperin, A. N. Khalid, K. A. Kumar, A. Micco, D. Munsell, S. Rosenbaum, and W. Vaughan. 2013. Clinical practice guideline: Bell’s palsy. Otolaryngology–Head and Neck Surgery 149(S3):S1–S27.
Becker, J. H., J. J. Lin, M. Doernberg, K. Stone, A. Navis, J. R. Festa, and J. P. Wisnivesky. 2021. Assessment of cognitive function in patients after COVID-19 infection. JAMA Network Open 4(10):e2130645–e2130645.
Becker, J. H., J. J. Lin, A. Twumasi, R. Goswami, F. Carnavali, K. Stone, M. Rivera-Mindt, M. S. Kale, G. Naasan, J. R. Festa, and J. P. Wisnivesky. 2023. Greater executive dysfunction in patients post-COVID-19 compared to those not infected. Brain, Behavior, and Immunity 114:111–117.
Bedree, H., M. Sunnquist, and L. A. Jason. 2019. The Depaul Symptom Questionnaire-2: A validation study. Fatigue: Biomedicine, Health & Behavior 7(3):166–179.
Behnood, S. A., R. Shafran, S. D. Bennett, A. X. D. Zhang, L. L. O’Mahoney, T. J. Stephenson, S. N. Ladhani, B. L. De Stavola, R. M. Viner, and O. V. Swann. 2022. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: A meta-analysis of controlled and uncontrolled studies. Journal of Infection 84(2):158–170.
Bellanti, J. A., P. Novak, Y. Faitelson, J. A. Bernstein, and M. C. Castells. 2023. The long road of long COVID: Specific considerations for the allergist/immunologist. Journal of Allergy and Clinical Immunology Practice 11(11):3335–3345.
Benditt, D. G., D. W. Ferguson, B. P. Grubb, W. N. Kapoor, J. Kugler, B. B. Lerman, J. D. Maloney, A. Raviele, B. Ross, R. Sutton, M. J. Wolk, and D. L. Wood. 1996. Tilt table testing for assessing syncope. Journal of the American College of Cardiology 28(1):263–275.
Bentall, R. P., G. C. Wood, T. Marrinan, C. Deans, and R. H. Edwards. 1993. A brief mental fatigue questionnaire. British Journal of Clinical Psychology 32(3):375–379.
Bernstein, J. A., D. M. Lang, D. A. Khan, T. Craig, D. Dreyfus, F. Hsieh, J. Sheikh, D. Weldon, B. Zuraw, D. I. Bernstein, J. Blessing-Moore, L. Cox, R. A. Nicklas, J. Oppenheimer, J. M. Portnoy, C. R. Randolph, D. E. Schuller, S. L. Spector, S. A. Tilles, and D. Wallace. 2014. The diagnosis and management of acute and chronic urticaria: 2014 update. Journal of Allergy and Clinical Immunology 133(5):1270–1277.
Bhatia, R., S. J. Kizilbash, S. P. Ahrens, J. M. Killian, S. A. Kimmes, E. E. Knoebel, P. Muppa, A. L. Weaver, and P. R. Fischer. 2016. Outcomes of adolescent-onset postural orthostatic tachycardia syndrome. The Journal of Pediatrics 173:149–153.
Bhopal, S., J. Bagaria, and R. Bhopal. 2020. Children’s mortality from COVID-19 compared with all-deaths and other relevant causes of death: Epidemiological information for decision-making by parents, teachers, clinicians and policymakers. Public Health 185:19–20.
Bhopal, S. S., J. Bagaria, B. Olabi, and R. Bhopal. 2021. Children and young people remain at low risk of COVID-19 mortality. The Lancet Child & Adolescent Health 5(5):e12–e13.
Blatz, A. M., and A. G. Randolph. 2022. Severe COVID-19 and multisystem inflammatory syndrome in children in children and adolescents. Critical Care Clinics 38(3):571–586.
Blitshteyn, S., and S. Whitelaw. 2021. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: A case series of 20 patients. Journal of Immunologic Research 69(2):205–211.
Blitshteyn, S., J. H. Whiteson, B. Abramoff, A. Azola, M. N. Bartels, R. Bhavaraju-Sanka, T. Chung, T. K. Fleming, E. Henning, and M. G. Miglis. 2022. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of autonomic dysfunction in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM&R 14(10):1270–1291.
BLS (Bureau of Labor Statistics). 2020. ORS collection manual. https://www.bls.gov/ors/information-for-survey-participants/pdf/occupational-requirements-survey-collection-manual-082020.pdf (accessed March 26, 2024).
Borch, L., M. Holm, M. Knudsen, S. Ellermann-Eriksen, and S. Hagstroem. 2022. Long COVID symptoms and duration in SARS-CoV-2 positive children—A nationwide cohort study. European Journal of Pediatrics 181(4):1597–1607.
Bornstein, S. R., B. Allolio, W. Arlt, A. Barthel, A. Don-Wauchope, G. D. Hammer, E. S. Husebye, D. P. Merke, M. H. Murad, C. A. Stratakis, and D. J. Torpy. 2016. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 101(2):364–389.
Bornstein, S. R., D. Cozma, M. Kamel, M. Hamad, M. G. Mohammad, N. A. Khan, M. M. Saber, M. H. Semreen, and C. Steenblock. 2022. Long-COVID, metabolic and endocrine disease. Hormone and Metabolic Research 54(8):562–566.
Bourne, K. M., D. S. Chew, L. E. Stiles, B. H. Shaw, C. A. Shibao, L. E. Okamoto, E. M. Garland, A. Gamboa, A. Peltier, A. Diedrich, I. Biaggioni, R. S. Sheldon, D. Robertson, and S. R. Raj. 2021. Postural orthostatic tachycardia syndrome is associated with significant employment and economic loss. Journal of Internal Medicine 290(1):203–212.
Bourne, K. M., K. A. Nerenberg, L. E. Stiles, C. A. Shibao, L. E. Okamoto, E. M. Garland, A. Gamboa, A. Peltier, A. Diedrich, I. Biaggioni, R. S. Sheldon, P. S. Gibson, A. J. Kealey, and S. R. Raj. 2023. Symptoms of postural orthostatic tachycardia syndrome in pregnancy: A cross-sectional, community-based survey. BJOG 130(9):1120–1127.
Bowe, B., Y. Xie, E. Xu, and Z. Al-Aly. 2021. Kidney outcomes in long COVID. Journal of the American Society of Nephrology 32(11):2851–2862.
Brandt, L. J., P. Feuerstadt, G. F. Longstreth, and S. J. Boley. 2015. ACG clinical guideline: Epidemiology, risk factors, patterns of presentation, diagnosis, and management of colon ischemia (CI). American Journal of Gastroenterology 110(1):18–44.
Bryarly, M., J. Cabrera, K. Tarpara, S. Barshikar, and S. Vernino. 2022. Minimal objective autonomic dysfunction in long-COVID. Clinical Autonomic Research 32(5):362.
Buchhorn, R. 2023. Therapeutic approaches to dysautonomia in childhood, with a special focus on long COVID. Children (Basel) 10(2):316.
Bucholc, M., D. Bradley, D. Bennett, L. Patterson, R. Spiers, D. Gibson, H. Van Woerden, and A. J. Bjourson. 2022. Identifying pre-existing conditions and multimorbidity patterns associated with in-hospital mortality in patients with COVID-19. Scientific Reports 12(1):17313.
Budhwar, N., and Z. Syed. 2020. Chronic dyspnea: Diagnosis and evaluation. American Family Physician 101(9):542–548.
Buoite Stella, A., G. Furlanis, N. A. Frezza, R. Valentinotti, M. Ajcevic, and P. Manganotti. 2022. Autonomic dysfunction in post-COVID patients with and without neurological symptoms: A prospective multidomain observational study. Journal of Neurology 269(2):587–596.
Buonsenso, D., F. E. Pujol, D. Munblit, D. Pata, S. McFarland, and F. K. Simpson. 2022. Clinical characteristics, activity levels and mental health problems in children with long coronavirus disease: A survey of 510 children. Future Microbiology 17(8):577–588.
Bygdell, M., J. M. Kindblom, J. Martikainen, H. Li, and F. Nyberg. 2023. Incidence and characteristics in children with post–COVID-19 condition in Sweden. JAMA Network Open 6(7):e2324246–e2324246.
Calabrese, C., E. Kirchner, and L. H. Calabrese. 2022. Long COVID and rheumatology: Clinical, diagnostic, and therapeutic implications. Best Practice & Research Clinical Rheumatology 36(4):101794.
Canas, L. S., E. Molteni, J. Deng, C. H. Sudre, B. Murray, E. Kerfoot, M. Antonelli, K. Rjoob, J. Capdevila Pujol, L. Polidori, A. May, M. F. Österdahl, R. Whiston, N. J. Cheetham, V. Bowyer, T. D. Spector, A. Hammers, E. L. Duncan, S. Ourselin, C. J. Steves, and M. Modat. 2023. Profiling post-COVID-19 condition across different variants of SARS-CoV-2: A prospective longitudinal study in unvaccinated wild-type, unvaccinated Alpha-variant, and vaccinated Delta-variant populations. The Lancet Digital Health 5(7):e421–e434.
Cappel, J. A., and D. A. Wetter. 2014. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clinic Proceedings 89(2):207–215.
Carmona-Torre, F., A. Mínguez-Olaondo, A. López-Bravo, B. Tijero, V. Grozeva, M. Walcker, H. Azkune-Galparsoro, A. López de Munain, A. B. Alcaide, J. Quiroga, J. L. Del Pozo, and J. C. Gómez-Esteban. 2022. Dysautonomia in COVID-19 patients: A narrative review on clinical course, diagnostic and therapeutic strategies. Frontiers in Neurology 13:886609.
Carruthers, B. M., M. I. Van De Sande, K. L. De Meirleir, N. G. Klimas, G. Broderick, T. Mitchell, D. Staines, A. C. P. Powles, N. Speight, R. Vallings, L. Bateman, B. Baumgarten-Austrheim, D. S. Bell, N. Carlo-Stella, J. Chia, A. Darragh, D. Jo, D. Lewis, A. R. Light, S. MarshallGradisbik, I. Mena, J. A. Mikovits, K. Miwa, M. Murovska, M. L. Pall, and S. Stevens. 2011. Myalgic encephalomyelitis: International consensus criteria. Journal of Internal Medicine 270(4):327–338.
Cartwright, S. L., and M. P. Knudson. 2008. Evaluation of acute abdominal pain in adults. American Family Physician 77(7):971–978.
Casagrande, M., G. Marselli, F. Agostini, G. Forte, F. Favieri, and A. Guarino. 2022. The complex burden of determining prevalence rates of mild cognitive impairment: A systematic review. Frontiers in Psychiatry 13:960648.
Casaletto, K. B., and R. K. Heaton. 2017. Neuropsychological assessment: Past and future. Journal of the International Neuropsychological Society 23(9-10):778–790.
Castro, C., and M. Gourley. 2010. Diagnostic testing and interpretation of tests for autoimmunity. Journal of Allergy and Clinical Immunology 125(2 Suppl 2):S238–S247.
Cavaco, S., G. Sousa, A. Gonçalves, A. Dias, C. Andrade, D. Pereira, E. A. Aires, J. Moura, L. Silva, R. Varela, S. Malheiro, V. Oliveira, A. Teixeira-Pinto, L. F. Maia, and M. Correia. 2023. Predictors of cognitive dysfunction one-year post COVID-19. Neuropsychology 37(5):557–567.
CDC (U.S. Centers for Disease Control and Prevention). 2021a. COVID-19 vaccination coverage and vaccine confidence among children. https://www.cdc.gov/vaccines/imz-managers/coverage/covidvaxview/interactive/children.html (accessed January 12, 2024).
CDC. 2021b. IOM 2015 diagnostic criteria. https://www.cdc.gov/me-cfs/healthcareproviders/diagnosis/iom-2015-diagnostic-criteria.html (accessed March 7, 2024).
CDC. 2021c. Sexually transmitted infections treatment guidelines, 2021. https://www.cdc.gov/std/treatment-guidelines/epididymitis.htm (accessed November 14, 2023).
CDC. 2023a. Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C). https://www.cdc.gov/mis/mis-c/hcp_cstecdc/index.html (accessed January 12, 2024).
CDC. 2023b. Information for pediatric healthcare providers. https://www.cdc.gov/coronavirus/2019-ncov/hcp/pediatric-hcp.html2021#PCCs (accessed January 12, 2024).
CDC. 2024. Post-COVID conditions: Information for healthcare providers. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html (accessed January 16, 2024).
Chang, R., T. Yen-Ting Chen, S. I. Wang, Y. M. Hung, H. Y. Chen, and C. J. Wei. 2023. Risk of autoimmune diseases in patients with COVID-19: A retrospective cohort study. EClinicalMedicine 56:101783.
Chen, E. Y., A. K. Morrow, and L. A. Malone. 2023. Exploring the influence of pre-existing conditions and infection factors on pediatric long COVID symptoms and quality of life. American Journal of Physical Medicine and Rehabilitation 103(7):567–574.
Cheng, A. L., J. Anderson, N. Didehbani, J. S. Fine, T. K. Fleming, R. Karnik, M. Longo, R. Ng, Y. Re’em, S. Sampsel, J. Shulman, J. K. Silver, J. Twaite, M. Verduzco-Gutierrez, and M. Kurylo. 2023. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of mental health symptoms in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM&R 15(12):1588–1604.
Cheung, J., K. Nordmeier, S. Kelland, M. Harrington, J. Williman, M. Storer, B. Beaglehole, L. Beckert, S. T. Chambers, M. J. Epton, J. Freeman, D. R. Murdoch, A. M. Werno, and M. J. Maze. 2023. Symptom persistence and recovery among COVID-19 survivors during a limited outbreak in Canterbury, New Zealand: A prospective cohort study. Internal Medicine Journal 53(1):37–45.
Chiabrando, J. G., A. Bonaventura, A. Vecchié, G. F. Wohlford, A. G. Mauro, J. H. Jordan, J. D. Grizzard, F. Montecucco, D. H. Berrocal, A. Brucato, M. Imazio, and A. Abbate. 2020. Management of acute and recurrent pericarditis. Journal of the American College of Cardiology 75(1):76–92.
Chu, L., I. J. Valencia, D. W. Garvert, and J. G. Montoya. 2018. Deconstructing post-exertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: A patient-centered, cross-sectional survey. PLoS ONE 13(6):e0197811.
Claar, R. L., and L. S. Walker. 2006. Functional assessment of pediatric pain patients: Psychometric properties of the functional disability inventory. Pain 121(1-2):77–84.
Cleveland Clinic. 2023. Depression in children. https://my.clevelandclinic.org/health/diseases/14938-depression-in-children (accessed October 10, 2023).
Cook, K. F., A. M. Bamer, D. Amtmann, I. R. Molton, and M. P. Jensen. 2012. Six Patient-Reported Outcome Measurement Information System short form measures have negligible age- or diagnosis-related differential item functioning in individuals with disabilities. Archives of Physical Medicine and Rehabilitation 93(7):1289–1291.
Cotler, J., C. Holtzman, C. Dudun, and L. A. Jason. 2018. A brief questionnaire to assess postexertional malaise. Diagnostics 8(3):66.
Crawford, P., and E. E. Zimmerman. 2011. Differentiation and diagnosis of tremor. American Family Physician 83(6):697–702.
Cristillo, V., A. Pilotto, S. C. Piccinelli, S. Gipponi, M. Leonardi, M. Bezzi, and A. Padovani. 2022. Predictors of “brain fog” 1 year after COVID-19 disease. Neurological Sciences 43(10):5795–5797.
Crockett, S. D., S. Wani, T. B. Gardner, Y. Falck-Ytter, A. N. Barkun, S. Crockett, Y. Falck-Ytter, J. Feuerstein, S. Flamm, Z. Gellad, L. Gerson, S. Gupta, I. Hirano, J. Inadomi, G. C. Nguyen, J. H. Rubenstein, S. Singh, W. E. Smalley, N. Stollman, S. Street, S. Sultan, S. S. Vege, S. B. Wani, and D. Weinberg. 2018. American Gastroenterological Association institute guideline on initial management of acute pancreatitis. Gastroenterology 154(4):1096–1101.
D’Souza, D., J. Empringham, P. Pechlivanoglou, E. M. Uleryk, E. Cohen, and R. Shulman. 2023. Incidence of diabetes in children and adolescents during the COVID-19 pandemic: A systematic review and meta-analysis. JAMA Network Open 6(6): e2321281–e2321281.
Daines, L., B. Zheng, P. Pfeffer, J. R. Hurst, and A. Sheikh. 2022. A clinical review of long-COVID with a focus on the respiratory system. Current Opinion in Pulmonary Medicine 28(3):174–179.
Dalrymple, S. N., J. H. Row, and J. Gazewood. 2023. Bell palsy: Rapid evidence review. American Family Physician 107(4):415–420.
Davenport, T. E., S. R. Stevens, M. J. VanNess, C. R. Snell, and T. Little. 2010. Conceptual model for physical therapist management of chronic fatigue syndrome/myalgic encephalomyelitis. Physical Therapy 90(4):602–614.
Davenport, T. E., M. Lehnen, S. R. Stevens, J. M. VanNess, J. Stevens, and C. R. Snell. 2019. Chronotropic intolerance: An overlooked determinant of symptoms and activity limitation in myalgic encephalomyelitis/chronic fatigue syndrome? Frontiers in Pediatrics 7:82.
Davenport, T. E., S. R. Stevens, J. Stevens, C. R. Snell, and J. M. Van Ness. 2023. Development and measurement properties of the PEM/PESE Activity Questionnaire (PAQ). Work 74(4):1187–1197.
Davis, H. E., G. S. Assaf, L. McCorkell, H. Wei, R. J. Low, Y. Re’em, S. Redfield, J. P. Austin, and A. Akrami. 2021. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 38:101019.
Davis, H. E., L. McCorkell, J. M. Vogel, and E. J. Topol. 2023. Long COVID: Major findings, mechanisms and recommendations. Nature Reviews Microbiology 21(3):133–146.
Day, G. S., N. Scarmeas, R. Dubinsky, K. Coerver, A. Mostacero, B. West, S. R. Wessels, and M. J. Armstrong. 2022. Aducanumab use in symptomatic Alzheimer disease evidence in focus: A report of the AAN Guidelines Subcommittee. Neurology 98(15):619–631.
Degen, C. V., M. Mikuteit, J. Niewolik, D. Schröder, K. Vahldiek, U. Mücke, S. Heinemann, F. Müller, G. M. N. Behrens, and F. Klawonn. 2022. Self-reported tinnitus and vertigo or dizziness in a cohort of adult long COVID patients. Frontiers in Neurology 13:884002.
Delgado-Alonso, C., C. Cuevas, S. Oliver-Mas, M. Díez-Cirarda, A. Delgado-Álvarez, M. J. Gil-Moreno, J. Matías-Guiu, and J. A. Matias-Guiu. 2022. Fatigue and cognitive dysfunction are associated with occupational status in post-COVID syndrome. International Journal of Environmental Research and Public Health 19(20):13368.
Dent, E., J. E. Morley, A. J. Cruz-Jentoft, H. Arai, S. B. Kritchevsky, J. Guralnik, J. M. Bauer, M. Pahor, B. C. Clark, M. Cesari, J. Ruiz, C. C. Sieber, M. Aubertin-Leheudre, D. L. Waters, R. Visvanathan, F. Landi, D. T. Villareal, R. Fielding, C. W. Won, O. Theou, F. C. Martin, B. Dong, J. Woo, L. Flicker, L. Ferrucci, R. A. Merchant, L. Cao, T. Cederholm, S. M. L. Ribeiro, L. Rodríguez-Mañas, S. D. Anker, J. Lundy, L. M. Gutiérrez Robledo, I. Bautmans, I. Aprahamian, J. Schols, M. Izquierdo, and B. Vellas. 2018. International clinical practice guidelines for sarcopenia (ICFSR): Screening, diagnosis and management. Journal of Health and Aging 22(10):1148–1161.
DePace, N. L., and J. Colombo. 2022. Long-COVID syndrome and the cardiovascular system: A review of neurocardiologic effects on multiple systems. Current Cardiology Reports 24(11):1711–1726.
Dhooria, S., S. Chaudhary, I. S. Sehgal, R. Agarwal, S. Arora, M. Garg, N. Prabhakar, G. D. Puri, A. Bhalla, V. Suri, L. N. Yaddanapudi, V. Muthu, K. T. Prasad, and A. N. Aggarwal. 2022. High-dose versus low-dose prednisolone in symptomatic patients with post-COVID-19 diffuse parenchymal lung abnormalities: An open-label, randomised trial (the COLDSTER trial). European Respiratory Journal 59(2):2102930.
Diekman, S., and T. Chung. 2023. Post-acute sequelae of SARS-CoV-2 syndrome presenting as postural orthostatic tachycardia syndrome. Clinical and Experimental Emergency Medicine 10(1):18–25.
Drogalis-Kim, D., C. Kramer, and S. Duran. 2022. Ongoing dizziness following acute COVID-19 infection: A single center pediatric case series. Pediatrics 150(2):e2022056860.
Dubey, S., S. Das, R. Ghosh, M. J. Dubey, A. P. Chakraborty, D. Roy, G. Das, A. Dutta, A. Santra, S. Sengupta, and J. Benito-León. 2023. The effects of SARS-CoV-2 infection on the cognitive functioning of patients with pre-existing dementia. Journal of Alzheimers Disease Reports 7(1):119–128.
Durstenfeld, M. S., K. Sun, P. Tahir, M. J. Peluso, S. G. Deeks, M. A. Aras, D. J. Grandis, C. S. Long, A. Beatty, and P. Y. Hsue. 2022. Use of cardiopulmonary exercise testing to evaluate long COVID-19 symptoms in adults: A systematic review and meta-analysis. JAMA Network Open 5(10):e2236057.
EASL (European Association for the Study of the Liver). 2009. EASL clinical practice guidelines: Management of cholestatic liver diseases. Journal of Hepatology 51(2):237–267.
Elmunzer, B. J., J. L. Maranki, V. Gómez, A. Tavakkoli, B. G. Sauer, B. N. Limketkai, E. A. Brennan, E. M. Attridge, T. J. Brigham, and A. Y. Wang. 2023. ACG clinical guideline: Diagnosis and management of biliary strictures. American Journal of Gastroenterology 118(3):405–426.
El-Rhermoul, F. Z., A. Fedorowski, P. Eardley, P. Taraborrelli, D. Panagopoulos, R. Sutton, P. B. Lim, and M. Dani. 2023. Autoimmunity in long COVID and POTS. Oxford Open Immunology 4(1):iqad002.
ElSayed, N. A., G. Aleppo, V. R. Aroda, R. R. Bannuru, F. M. Brown, D. Bruemmer, B. S. Collins, J. L. Gaglia, M. E. Hilliard, D. Isaacs, E. L. Johnson, S. Kahan, K. Khunti, J. Leon, S. K. Lyons, M. L. Perry, P. Prahalad, R. E. Pratley, J. J. Seley, R. C. Stanton, and R. A. Gabbay, on behalf of the American Diabetes Association. 2023. 2. Classification and diagnosis of diabetes: Standards of care in diabetes—2023. Diabetes Care 46(Supplement 1):S19–S40.
ENTHealth. 2020. Dysgeusia. https://www.enthealth.org/conditions/dysgeusia/ (accessed December 5, 2023).
Espinosa-Gonzalez, A. B., H. Master, N. Gall, S. Halpin, N. Rogers, and T. Greenhalgh. 2023. Orthostatic tachycardia after COVID-19. BMJ 380:e073488.
EuroQol Group. 1990. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 16(3):199–208.
Evans, R. A., H. McAuley, E. M. Harrison, A. Shikotra, A. Singapuri, M. Sereno, O. Elneima, A. B. Docherty, N. I. Lone, O. C. Leavy, L. Daines, J. K. Baillie, J. S. Brown, T. Chalder, A. De Soyza, N. Diar Bakerly, N. Easom, J. R. Geddes, N. J. Greening, N. Hart, L. G. Heaney, S. Heller, L. Howard, J. R. Hurst, J. Jacob, R. G. Jenkins, C. Jolley, S. Kerr, O. M. Kon, K. Lewis, J. M. Lord, G. P. McCann, S. Neubauer, P. J. M. Openshaw, D. Parekh, P. Pfeffer, N. M. Rahman, B. Raman, M. Richardson, M. Rowland, M. G. Semple, A. M. Shah, S. J. Singh, A. Sheikh, D. Thomas, M. Toshner, J. D. Chalmers, L.-P. Ho, A. Horsley, M. Marks, K. Poinasamy, L. V. Wain, C. E. Brightling, on behalf of the PHOSP-COVID Collaborative Group. 2021. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): A UK multicentre, prospective cohort study. The Lancet Respiratory Medicine 9(11):1275–1287.
Evans, R., A. Pick, R. Lardner, V. Masey, N. Smith, and T. Greenhalgh. 2023. Breathing difficulties after COVID-19: A guide for primary care. BMJ 381:e074937.
Evers, G., A. B. Schulze, I. Osiaevi, K. Harmening, R. Vollenberg, R. Wiewrodt, R. Pistulli, M. Boentert, P.-R. Tepasse, and J. R. Sindermann. 2022. Sustained impairment in cardiopulmonary exercise capacity testing in patients after COVID-19: A single center experience. Canadian Respiratory Journal 2022:2466789.
Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), M. M. Cloutier, A. P. Baptist, K. V. Blake, E. G. Brooks, T. Bryant-Stephens, E. DiMango, A. E. Dixon, K. S. Elward, T. Hartert, and J. A. Krishnan. 2020. 2020 focused updates to the asthma management guidelines: A report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. Journal of Allergy and Clinical Immunology 146(6):1217–1270.
FACIT Group. 2021. Functional assessment of chronic illness therapy—Fatigue. https://www.facit.org/measures/facit-f (accessed March 22, 2024).
Farias, S. T., D. Mungas, B. R. Reed, D. Cahn-Weiner, W. Jagust, K. Baynes, and C. Decarli. 2008. The measurement of everyday cognition (ECog): Scale development and psychometric properties. Neuropsychology 22(4):531–544.
Fedorowski, A. 2019. Postural orthostatic tachycardia syndrome: Clinical presentation, aetiology and management. Journal of Internal Medicine 285(4):352–366.
Feldstein, L. R., M. W. Tenforde, K. G. Friedman, M. Newhams, E. B. Rose, H. Dapul, V. L. Soma, A. B. Maddux, P. M. Mourani, and C. Bowens. 2021. Characteristics and outcomes of U.S. children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 325(11):1074–1087.
Ferreira, J. J., T. A. Mestre, K. E. Lyons, J. Benito-León, E. K. Tan, G. Abbruzzese, M. Hallett, D. Haubenberger, R. Elble, and G. Deuschl. 2019. MDS evidence-based review of treatments for essential tremor. Movement Disorders 34(7):950–958.
Ferrulli, A., P. Senesi, I. Terruzzi, and L. Luzi. 2022. Eating habits and body weight changes induced by variation in smell and taste in patients with previous SARS-CoV-2 infection. Nutrients 14(23):5068.
Fine, J. S., A. F. Ambrose, N. Didehbani, T. K. Fleming, L. Glashan, M. Longo, A. Merlino, R. Ng, G. J. Nora, and S. Rolin. 2022. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of cognitive symptoms in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM&R 14(1):96–111.
Fischer, A., N. Badier, L. Zhang, A. Elbéji, P. Wilmes, P. Oustric, C. Benoy, M. Ollert, and G. Fagherazzi. 2022. Long COVID classification: Findings from a clustering analysis in the Predi-COVID cohort study. International Journal of Environmental Research Public Health 19(23):16018.
Ford, B., M. Dore, and E. Harris. 2021. Outpatient Primary Care Management of Headaches: Guidelines from the VA/DoD. American Family Physician 104(3):316–320.
Fowlkes, A. L., S. K. Yoon, K. Lutrick, L. Gwynn, J. Burns, L. Grant, A. L. Phillips, K. Ellingson, M. V. Ferraris, and L. B. LeClair. 2022. Effectiveness of 2-dose BNT162b2 (Pfizer BioNTech) mRNA vaccine in preventing SARS-CoV-2 infection among children aged 5–11 years and adolescents aged 12–15 years—Protect cohort, July 2021–February 2022. MMWR Morbidity and Mortality Weekly Report 71(11):422.
Fraenkel, L., J. M. Bathon, B. R. England, E. W. St Clair, T. Arayssi, K. Carandang, K. D. Deane, M. Genovese, K. K. Huston, G. Kerr, J. Kremer, M. C. Nakamura, L. A. Russell, J. A. Singh, B. J. Smith, J. A. Sparks, S. Venkatachalam, M. E. Weinblatt, M. Al-Gibbawi, J. F. Baker, K. E. Barbour, J. L. Barton, L. Cappelli, F. Chamseddine, M. George, S. R. Johnson, L. Kahale, B. S. Karam, A. M. Khamis, I. Navarro-Millán, R. Mirza, P. Schwab, N. Singh, M. Turgunbaev, A. S. Turner, S. Yaacoub, and E. A. Akl. 2021. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care and Research 73(7):924–939.
Freeman, E. E., I. Garcia-Doval, L. Naldi, and R. J. Hay. 2023. Three years on, COVID-19 and the skin: Long-term impacts, emerging trends and clinical practice. British Journal of Dermatology 189(1):1–3.
Frontera, J. A., L. E. Thorpe, N. M. Simon, A. de Havenon, S. Yaghi, S. B. Sabadia, D. Yang, A. Lewis, K. Melmed, L. J. Balcer, T. Wisniewski, and S. L. Galetta. 2022. Post-acute sequelae of COVID-19 symptom phenotypes and therapeutic strategies: A prospective, observational study. PLoS ONE 17(9):e0275274.
Funk, A. L., N. Kuppermann, T. A. Florin, D. J. Tancredi, J. Xie, K. Kim, Y. Finkelstein, M. I. Neuman, M. I. Salvadori, A. Yock-Corrales, K. A. Breslin, L. Ambroggio, P. P. Chaudhari, K. R. Bergmann, M. A. Gardiner, J. R. Nebhrajani, C. Campos, F. A. Ahmad, L. F. Sartori, N. Navanandan, N. Kannikeswaran, K. Caperell, C. R. Morris, S. Mintegi, I. Gangoiti,
V. J. Sabhaney, A. C. Plint, T. P. Klassen, U. R. Avva, N. P. Shah, A. C. Dixon, M. M. Lunoe, S. M. Becker, A. J. Rogers, V. Pavlicich, S. R. Dalziel, D. C. Payne, R. Malley, M. L. Borland, A. K. Morrison, M. Bhatt, P. B. Rino, I. Beneyto Ferre, M. Eckerle, A. J. Kam, S. L. Chong, L. Palumbo, M. Y. Kwok, J. C. Cherry, N. Poonai, M. Waseem, N. J. Simon, and S. B. Freedman, for the Pediatric Emergency Research Network–COVID-19 Study Team. 2022. Post-COVID-19 conditions among children 90 days after SARS-CoV-2 infection. JAMA Network Open 5(7):e2223253.
Fu, Q., and Levine, B. D. Exercise and non-pharmacological treatment of POTS. Autonomic Neuroscience 215:20–27.
Gao, P., J. Liu, and M. Liu. 2022. Effect of COVID-19 vaccines on reducing the risk of long COVID in the real world: A systematic review and meta-analysis. International Journal of Environmental Research and Public Health 19(19):12422.
Geerts, M., J. G. J. Hoeijmakers, C. M. L. Gorissen-Brouwers, C. G. Faber, and I. S. J. Merkies. 2023. Small fiber neuropathy: A clinical and practical approach. Journal for Nurse Practitioners 19(4):104547.
Georgesen, C., L. P. Fox, and J. Harp. 2020. Retiform purpura: A diagnostic approach. Journal of the American Academy of Dermatology 82(4):783–796.
Gibbs, M. B., J. C. English, and M. J. Zirwas. 2005. Livedo reticularis: An update. Journal of the American Academy of Dermatology 52(6):1009–1019.
Goel, N., N. Goyal, R. Nagaraja, and R. Kumar. 2021. Systemic corticosteroids for management of “long-COVID”: An evaluation after 3 months of treatment. Monaldi Archives for Chest Disease 92(2). https://doi.org/10.4081/monaldi.2021.1981 (accessed April 29, 2024).
Goldhaber, N. H., J. N. Kohn, W. S. Ogan, A. Sitapati, C. A. Longhurst, A. Wang, S. Lee, S. Hong, and L. E. Horton. 2022. Deep dive into the long haul: Analysis of symptom clusters and risk factors for post-acute sequelae of COVID-19 to inform clinical care. International Journal of Environmental Research and Public Health 19(24):16841.
Gole, S., and A. Anand. 2023. Autoimmune encephalitis. In StatPearls. Treasure Island, FL: StatPearls Publishing.
Grover, A., F. Choi, and S. Pei-Wang. 2022. 33624 Long-term cutaneous manifestations in COVID-19 patients: A systematic review. Journal of the American Academy of Dermatology 87(3):AB76.
Grach, S. L., J. Seltzer, T. Y. Chon, and R. Ganesh. 2023. Diagnosis and management of myalgic encephalomyelitis/chronic fatigue syndrome. Mayo Clinic Proceedings 98(10): 1544–1551.
Grundy, S. M., N. J. Stone, A. L. Bailey, C. Beam, K. K. Birtcher, R. S. Blumenthal, L. T. Braun, S. De Ferranti, J. Faiella-Tommasino, D. E. Forman, R. Goldberg, P. A. Heidenreich, M. A. Hlatky, D. W. Jones, D. Lloyd-Jones, N. Lopez-Pajares, C. E. Ndumele, C. E. Orringer, C. A. Peralta, J. J. Saseen, S. C. Smith, L. Sperling, S. S. Virani, and J. Yeboah. 2019. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APHA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 139:e1082–e1143.
Gülen, T., C. Akin, P. Bonadonna, F. Siebenhaar, S. Broesby-Olsen, K. Brockow, M. Niedoszytko, B. Nedoszytko, H. N. G. Oude Elberink, J. H. Butterfield, W. R. Sperr, I. Alvarez-Twose, H.-P. Horny, K. Sotlar, J. Schwaab, M. Jawhar, R. Zanotti, G. Nilsson, J. J. Lyons, M. C. Carter, T. I. George, O. Hermine, J. Gotlib, A. Orfao, M. Triggiani, A. Reiter, K. Hartmann, M. Castells, M. Arock, L. B. Schwartz, D. D. Metcalfe, and P. Valent. 2021. Selecting the right criteria and proper classification to diagnose mast cell activation syndromes: A critical review. Journal of Allergy and Clinical Immunology: In Practice 9(11):3918–3928.
Haloot, J., M. Kabbani, M. Verduzco-Gutierrez, R. Bhavaraju-Sanka, and J. Pillarisetti. 2022. CE-541-02 post-COVID and postural orthostatic tachycardia syndrome. Heart Rhythm 19(5):S54.
Harrison, P. J., and M. Taquet. 2023. Neuropsychiatric disorders following SARS-CoV-2 infection. Brain 146(6):2241–2247.
Hartung, T. J., C. Neumann, T. Bahmer, I. Chaplinskaya-Sobol, M. Endres, J. Geritz, K. G. Haeusler, P. U. Heuschmann, H. Hildesheim, A. Hinz, S. Hopff, A. Horn, M. Krawczak, L. Krist, J. Kudelka, W. Lieb, C. Maetzler, A. Mehnert-Theuerkauf, F. A. Montellano, C. Morbach, S. Schmidt, S. Schreiber, F. Steigerwald, S. Störk, W. Maetzler, and C. Finke. 2022. Fatigue and cognitive impairment after COVID-19: A prospective multicentre study. EClinicalMedicine 53:101651.
Harvey, P. D. 2012. Clinical applications of neuropsychological assessment. Dialogues in Clinical Neuroscience 14(1):91–99.
Heidenreich, P. A., B. Bozkurt, D. Aguilar, L. A. Allen, J. J. Byun, M. M. Colvin, A. Deswal, M. H. Drazner, S. M. Dunlay, L. R. Evers, J. C. Fang, S. E. Fedson, G. C. Fonarow, S. S. Hayek, A. F. Hernandez, P. Khazanie, M. M. Kittleson, C. S. Lee, M. S. Link, C. A. Milano, L. C. Nnacheta, A. T. Sandhu, L. W. Stevenson, O. Vardeny, A. R. Vest, and C. W. Yancy. 2022. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 145:e876–e894.
Hentschel, C. B., B. A. Abramoff, T. R. Dillingham, and L. E. Pezzin. 2022. Race, ethnicity, and utilization of outpatient rehabilitation for treatment of post COVID-19 condition. PM&R 14(11):1315–1324.
Herrera, J. E., W. N. Niehaus, J. Whiteson, A. Azola, J. M. Baratta, T. K. Fleming, S. Y. Kim, H. Naqvi, S. Sampsel, J. K. Silver, M. Verduzco-Gutierrez, J. Maley, E. Herman, and B. Abramoff. 2021. Multidisciplinary collaborative consensus guidance statement on the assessment and treatment of fatigue in postacute sequelae of SARS-CoV-2 infection (PASC) patients. PM&R 13(9):1027–1043.
Ho, F. F., S. Xu, T. M. H. Kwong, A. S. Li, E. H. Ha, H. Hua, C. Liong, K. C. Leung, T. H. Leung, Z. Lin, S. Y. Wong, F. Pan, and V. C. H. Chung. 2023. Prevalence, patterns, and clinical severity of long COVID among Chinese medicine telemedicine service users: Preliminary results from a cross-sectional study. International Journal of Environmental Research and Public Health 20(3):1827.
Holguin, F., J. C. Cardet, K. F. Chung, S. Diver, D. S. Ferreira, A. Fitzpatrick, M. Gaga, L. Kellermeyer, S. Khurana, S. Knight, V. M. McDonald, R. L. Morgan, V. E. Ortega, D. Rigau, P. Subbarao, T. Tonia, I. M. Adcock, E. R. Bleecker, C. Brightling, L.-P. Boulet, M. Cabana, M. Castro, P. Chanez, A. Custovic, R. Djukanovic, U. Frey, B. Frankemölle, P. Gibson, D. Hamerlijnck, N. Jarjour, S. Konno, H. Shen, C. Vitary, and A. Bush. 2020. Management of severe asthma: A European Respiratory Society/American Thoracic Society guideline. European Respiratory Journal 55(1):1900588.
Hopkins, C., M. Alanin, C. Philpott, P. Harries, K. Whitcroft, A. Qureishi, S. Anari, Y. Ramakrishnan, A. Sama, E. Davies, B. Stew, S. Gane, S. Carrie, I. Hathorn, R. Bhalla, C. Kelly, N. Hill, D. Boak, and B. Nirmal Kumar. 2021. Management of new onset loss of sense of smell during the COVID-19 pandemic—BRS consensus guidelines. Clinical Otolaryngology 46(1):16–22.
Hughes, R., E. Wijdicks, R. Barohn, E. Benson, D. Cornblath, A. F. Hahn, J. Meythaler, R. Miller, J. Sladky, and J. Stevens. 2003. Practice parameter: Immunotherapy for Guillain-Barré syndrome: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 61(6):736–740.
Humbert, M., G. Kovacs, M. M. Hoeper, R. Badagliacca, R. M. F. Berger, M. Brida, J. Carlsen, A. J. S. Coats, P. Escribano-Subias, P. Ferrari, D. S. Ferreira, H. A. Ghofrani, G. Giannakoulas, D. G. Kiely, E. Mayer, G. Meszaros, B. Nagavci, K. M. Olsson, J. Pepke-Zaba, J. K.
Quint, G. Rådegran, G. Simonneau, O. Sitbon, T. Tonia, M. Toshner, J. L. Vachiery, A. Vonk Noordegraaf, M. Delcroix, S. Rosenkranz, on behalf of ESC/ERS Scientific Document Group. 2022. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. European Heart Journal 43(38):3618–3731.
IOM (Institute of Medicine). 2015. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: Redefining an illness. Washington, DC: The National Academies Press.
Isaac, R. O., J. Corrado, and M. Sivan. 2023. Detecting orthostatic intolerance in long COVID in a clinic setting. International Journal of Environmental Research and Public Health 20(10):5804.
Jacobs, M. M., E. Evans, and C. Ellis. 2023. Racial, ethnic, and sex disparities in the incidence and cognitive symptomology of long COVID-19. Journal of the National Medical Association 115(2):233–243.
Jafari, Z., B. E. Kolb, and M. H. Mohajerani. 2022. Hearing loss, tinnitus, and dizziness in COVID-19: A systematic review and meta-analysis. Canadian Journal of Neurological Sciences 49(2):184–195.
Jennings, G., A. Monaghan, F. Xue, E. Duggan, and R. Romero-Ortuño. 2022. Comprehensive clinical characterisation of brain fog in adults reporting long COVID symptoms. Journal of Clinical Medicine 11(12):3440.
Jiang, L., X. Li, J. Nie, K. Tang, and Z. A. Bhutta. 2023. A systematic review of persistent clinical features after SARS-CoV-2 in the pediatric population. Pediatrics 152(2):e2022060351.
Kandemir, H., G. A. Bülbül, E. Kirtis’, S. Güney, C. Y. Sanhal, and İ. Mendilcioğlu. 2024. Evaluation of long-COVID symptoms in women infected with SARS-CoV-2 during pregnancy. International Journal of Gynaecology and Obstetrics 164(1):148–156.
Kavi, L. 2022. Postural tachycardia syndrome and long COVID: An update. British Journal of General Practice 72(714):8–9.
Kayaaslan, B., F. Eser, A. K. Kalem, G. Kaya, B. Kaplan, D. Kacar, I. Hasanoglu, B. Coskun, and R. Guner. 2021. Post-COVID syndrome: A single-center questionnaire study on 1007 participants recovered from COVID-19. Journal of Medical Virology 93(12):6566–6574.
KDIGO (Kidney Disease Improving Global Outcomes). 2012. KDIGO clinical practice guideline for acute kidney injury. Official Journal of the International Society of Nephrology 2(1).
KDIGO. 2013. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Supplements 3:1–150.
Kenny, G., K. McCann, C. O’Brien, S. Savinelli, W. Tinago, O. Yousif, J. S. Lambert, C. O’Broin, E. R. Feeney, E. De Barra, P. Doran, and P. W. G. Mallon. 2022. Identification of distinct long COVID clinical phenotypes through cluster analysis of self-reported symptoms. Open Forum of Infectious Diseases 9(4):ofac060.
Khani, E., S. Khiali, S. Beheshtirouy, and T. Entezari-Maleki. 2021. Potential pharmacologic treatments for COVID-19 smell and taste loss: A comprehensive review. European Journal of Pharmacology 912:174582.
Kim, Y., S. E. Kim, T. Kim, K. W. Yun, S. H. Lee, E. Lee, J.-W. Seo, Y. H. Jung, and Y. P. Chong. 2022. Preliminary guidelines for the clinical evaluation and management of long COVID. Infection & Chemotherapy 54(3):566.
King, L. S. 1968. Signs and symptoms. JAMA 206(5):1063–1065.
Kiriyama, S., K. Kozaka, T. Takada, S. M. Strasberg, H. A. Pitt, T. Gabata, J. Hata, K. H. Liau, F. Miura, A. Horiguchi, K. H. Liu, C. H. Su, K. Wada, P. Jagannath, T. Itoi, D. J. Gouma, Y. Mori, S. Mukai, M. E. Giménez, W. S. W. Huang, M. H. Kim, K. Okamoto, G. Belli, C. Dervenis, A. C. W. Chan, W. Y. Lau, I. Endo, H. Gomi, M. Yoshida, T. Mayumi, T. H. Baron, E. De Santibañes, A. Y. B. Teoh, T. L. Hwang, C. G. Ker, M. F. Chen, H. S. Han, Y. S. Yoon, I. S. Choi, D. S. Yoon, R. Higuchi, S. Kitano, M. Inomata, D. J. Deziel, E. Jonas, K. Hirata, Y. Sumiyama, K. Inui, and M. Yamamoto. 2018. Tokyo guidelines 2018: Diagnostic criteria and severity grading of acute cholangitis (with videos). Journal of Hepato-Biliary-Pancreatic Sciences 25(1):17–30.
Kisiel, M. A., S. Lee, S. Malmquist, O. Rykatkin, S. Holgert, H. Janols, C. Janson, and X. Zhou. 2023. Clustering analysis identified three long COVID phenotypes and their association with general health status and working ability. Journal of Clinical Medicine 12(11):3617.
Klein, J., J. Wood, J. R. Jaycox, R. M. Dhodapkar, P. Lu, J. R. Gehlhausen, A. Tabachnikova, K. Greene, L. Tabacof, A. A. Malik, V. Silva Monteiro, J. Silva, K. Kamath, M. Zhang, A. Dhal, I. M. Ott, G. Valle, M. Peña-Hernández, T. Mao, B. Bhattacharjee, T. Takahashi, C. Lucas, E. Song, D. McCarthy, E. Breyman, J. Tosto-Mancuso, Y. Dai, E. Perotti, K. Akduman, T. J. Tzeng, L. Xu, A. C. Geraghty, M. Monje, I. Yildirim, J. Shon, R. Medzhitov, D. Lutchmansingh, J. D. Possick, N. Kaminski, S. B. Omer, H. M. Krumholz, L. Guan, C. S. Dela Cruz, D. van Dijk, A. M. Ring, D. Putrino, and A. Iwasaki. 2023. Distinguishing features of long COVID identified through immune profiling. Nature 623(7985):139–148.
Knight, M., K. Bunch, N. Vousden, E. Morris, N. Simpson, C. Gale, P. O’Brien, M. Quigley, P. Brocklehurst, and J. J. Kurinczuk, on behalf of the UK Obstetric Surveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group. 2020. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: National population based cohort study. BMJ 369:m2107.
Kolasinski, S. L., T. Neogi, M. C. Hochberg, C. Oatis, G. Guyatt, J. Block, L. Callahan, C. Copenhaver, C. Dodge, D. Felson, K. Gellar, W. F. Harvey, G. Hawker, E. Herzig, C. K. Kwoh, A. E. Nelson, J. Samuels, C. Scanzello, D. White, B. Wise, R. D. Altman, D. DiRenzo, J. Fontanarosa, G. Giradi, M. Ishimori, D. Misra, A. A. Shah, A. K. Shmagel, L. M. Thoma, M. Turgunbaev, A. S. Turner, and J. Reston. 2020. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care and Research 72(2):149–162.
Kompaniyets, L., L. Bull-Otterson, T. K. Boehmer, S. Baca, P. Alvarez, K. Hong, J. Hsu, A. M. Harris, A. V. Gundlapalli, and S. Saydah. 2022. Post-COVID-19 symptoms and conditions among children and adolescents–United States, March 1, 2020–January 31, 2022. MMWR Morbidity & Mortality Weekly Report 71(31):993–999.
Kostev, K., L. Smith, A. Koyanagi, M. Konrad, and L. Jacob. 2022. Post-COVID-19 conditions in children and adolescents diagnosed with COVID-19. Pediatric Research 95(1):182–187.
Krupp, L. B., N. G. LaRocca, J. Muir-Nash, and A. D. Steinberg. 1989. The Fatigue Severity Scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology 46(10):1121–1123.
Kusumoto, F. M., M. H. Schoenfeld, C. Barrett, J. R. Edgerton, K. A. Ellenbogen, M. R. Gold, N. F. Goldschlager, R. M. Hamilton, J. A. Joglar, and R. J. Kim. 2019. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology 74(7):e51–e156.
Kwo, P. Y., S. M. Cohen, and J. K. Lim. 2017. ACG clinical guideline: Evaluation of abnormal liver chemistries. Official Journal of the American College of Gastroenterology 112(1):18–35.
Lacy, B. E., M. Pimentel, D. M. Brenner, W. D. Chey, L. A. Keefer, M. D. Long, and B. Moshiree. 2021. ACG clinical guideline: Management of irritable bowel syndrome. Official Journal of the American College of Gastroenterology 116(1):17–44.
Lacy, B. E., J. Tack, and C. P. Gyawali. 2022. AGA clinical practice update on management of medically refractory gastroparesis: Expert review. Clinical Gastroenterology and Hepatology 20(3):491–500.
Ladlow, P., O. O’Sullivan, A. Houston, R. Barker-Davies, S. May, D. Mills, D. Dewson, R. Chamley, J. Naylor, J. Mulae, A. N. Bennett, E. D. Nicol, and D. A. Holdsworth. 2022. Dysautonomia following COVID-19 is not associated with subjective limitations or symptoms but is associated with objective functional limitations. Heart Rhythm 19(4):613–620.
Lai, C. C., C. K. Hsu, M. Y. Yen, P. I. Lee, W. C. Ko, and P. R. Hsueh. 2023. Long COVID: An inevitable sequela of SARS-CoV-2 infection. Journal of Microbiology, Immunology, and Infection 56(1):1–9.
Larsen, N. W., L. E. Stiles, and M. G. Miglis. 2021. Preparing for the long-haul: Autonomic complications of COVID-19. Autonomic Neuroscience 235:102841.
Larsen, N. W., L. E. Stiles, R. Shaik, L. Schneider, S. Muppidi, C. T. Tsui, L. N. Geng, H. Bonilla, and M. G. Miglis. 2022. Characterization of autonomic symptom burden in long COVID: A global survey of 2,314 adults. Frontiers in Neurology 13:1012668.
Larson, S. T., and J. Wilbur. 2020. Muscle weakness in adults: Evaluation and differential diagnosis. American Family Physician 101(2):95–108.
Leftin Dobkin, S. C., J. M. Collaco, and S. A. McGrath-Morrow. 2021. Protracted respiratory findings in children post-SARS-CoV-2 infection. Pediatric Pulmonology 56(12): 3682–3687.
Ley, H., Z. Skorniewska, P. J. Harrison, and M. Taquet. 2023. Risks of neurological and psychiatric sequelae 2 years after hospitalisation or intensive care admission with COVID-19 compared to admissions for other causes. Brain, Behavior, and Immunity 112:85–95.
Li, R., M. Shen, Q. Yang, C. K. Fairley, Z. Chai, R. McIntyre, J. J. Ong, H. Liu, P. Lu, W. Hu, Z. Zou, Z. Li, S. He, G. Zhuang, and L. Zhang. 2023. Global diabetes prevalence in COVID-19 patients and contribution to COVID-19-related severity and mortality: A systematic review and meta-analysis. Diabetes Care 46(4):890–897.
Lightner, D. J., A. Gomelsky, L. Souter, and S. P. Vasavada. 2019. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment 2019. Journal of Urology 202(3):558–563.
Lim, S. H., H. J. Ju, J. H. Han, J. H. Lee, W.-S. Lee, J. M. Bae, and S. Lee. 2023. Autoimmune and autoinflammatory connective tissue disorders following COVID-19. JAMA Network Open 6(10):e2336120.
Lindor, K. D., C. L. Bowlus, J. Boyer, C. Levy, and M. Mayo. 2022. Primary biliary cholangitis: 2021 practice guidance update from the American Association for the Study of Liver Diseases. Hepatology 75(4):1012–1013.
Líška, D., E. Liptaková, A. Babičová, L. Batalik, P. S. Baňárová, and S. Dobrodenková. 2022. What is the quality of life in patients with long COVID compared to a healthy control group? Frontiers in Public Health 10:975992.
Liu, L. W. C., C. N. Andrews, D. Armstrong, N. Diamant, N. Jaffer, A. Lazarescu, M. Li, R. Martino, W. Paterson, G. I. Leontiadis, and F. Tse. 2018. Clinical practice guidelines for the assessment of uninvestigated esophageal dysphagia. Journal of the Canadian Association of Gastroenterology 1(1):5–19.
Long COVID and kids: More research is urgently needed. 2022. Nature 602(7896):183–183.
Lopez-Leon, S., T. Wegman-Ostrosky, N. C. Ayuzo del Valle, C. Perelman, R. Sepulveda, P. A. Rebolledo, A. Cuapio, and S. Villapol. 2022. Long-COVID in children and adolescents: A systematic review and meta-analyses. Scientific Reports 12(1):9950.
Lott, N., C. E. Gebhard, S. Bengs, A. Haider, G. M. Kuster, V. Regitz-Zagrosek, and C. Gebhard. 2023. Sex hormones in SARS-CoV-2 susceptibility: Key players or confounders? Nature Review Endocrinology 19(4):217–231.
Lu, L. 2022. Guidelines for the management of cholestatic liver diseases (2021). Journal of Clinical and Translational Hepatology 10(4):757–769.
Lubell, J. 2022. Long COVID: Over 200 symptoms, and a search for guidance. American Medical Association. https://www.ama-assn.org/delivering-care/public-health/long-covid-over-200-symptoms-and-search-guidance (accessed September 6, 2023).
Luedke, J. C., G. Vargas, D. T. Jashar, A. Morrow, L. A. Malone, and R. Ng. 2024. Cognitive disengagement syndrome in pediatric patients with long COVID: Associations with mood, anxiety, and functional impairment. Child Neuropsychology 30(4):652–672.
Lui, D. T. W., K. H. Tsoi, C. H. Lee, C. Y. Y. Cheung, C. H. Y. Fong, A. C. H. Lee, A. R. Tam, P. Pang, T. Y. Ho, C. Y. Law, C. W. Lam, K. K. W. To, W. S. Chow, Y. C. Woo, I. F. N. Hung, K. C. B. Tan, and K. S. L. Lam. 2023. A prospective follow-up on thyroid function, thyroid autoimmunity and long COVID among 250 COVID-19 survivors. Endocrine 80(2):380–391.
Lynch, S., S. J. Ferrando, R. Dornbush, S. Shahar, A. Smiley, and L. Klepacz. 2022. Screening for brain fog: Is the Montreal Cognitive Assessment an effective screening tool for neurocognitive complaints post-COVID-19? General Hospital Psychiatry 78:80–86.
MacDonald Hull, S. P., M. L. Wood, P. E. Hutchinson, M. Sladden, and A. G. Messenger. 2003. Guidelines for the management of alopecia areata. British Journal of Dermatology 149(4):692–699.
Maddux, A. B., L. Berbert, C. C. Young, L. R. Feldstein, L. D. Zambrano, S. Kucukak, M. M. Newhams, K. Miller, M. M. Fitzgerald, J. He, N. B. Halasa, N. Z. Cvijanovich, L. L. Loftis, T. C. Walker, S. P. Schwartz, S. J. Gertz, K. M. Tarquinio, J. C. Fitzgerald, M. Kong, J. E. Schuster, E. H. Mack, C. V. Hobbs, C. M. Rowan, M. A. Staat, M. S. Zinter, K. Irby, H. Crandall, H. Flori, M. L. Cullimore, R. A. Nofziger, S. L. Shein, M. G. Gaspers, J. R. Hume, E. R. Levy, S. R. Chen, M. M. Patel, M. W. Tenforde, E. Weller, A. P. Campbell, and A. G. Randolph. 2022. Health impairments in children and adolescents after hospitalization for acute COVID-19 or MIS-C. Pediatrics 150(3):e2022057798.
Maglietta, G., F. Diodati, M. Puntoni, S. Lazzarelli, B. Marcomini, L. Patrizi, and C. Caminiti. 2022. Prognostic factors for post-COVID-19 syndrome: A systematic review and meta-analysis. Journal of Clinical Medicine 11(6):1541.
Maley, J. H., G. A. Alba, J. T. Barry, M. N. Bartels, T. K. Fleming, C. V. Oleson, L. Rydberg, S. Sampsel, J. K. Silver, S. Sipes, M. Verduzco-Gutierrez, J. Wood, J. Zibrak, and J. Whiteson 2022. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of breathing discomfort and respiratory sequelae in patients with postacute sequelae of SARS-CoV-2 infection (PASC). PM&R 14(1):77–95.
Malone, L. A., A. Morrow, Y. Chen, D. Curtis, S. D. de Ferranti, M. Desai, T. K. Fleming, T. M. Giglia, T. A. Hall, E. Henning, S. Jadhav, A. M. Johnston, D. R. C. Kathirithamby, C. Kokorelis, C. Lachenauer, L. Li, H. C. Lin, T. Locke, C. MacArthur, M. Mann, S. A. McGrath-Morrow, R. Ng, L. Ohlms, S. Risen, S. C. Sadreameli, S. Sampsel, S. K. S. Tejtel, J. K. Silver, T. Simoneau, R. Srouji, S. Swami, S. Torbey, M. V. Gutierrez, C. N. Williams, L. A. Zimmerman, and L. E. Vaz. 2022. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of postacute sequelae of SARS-CoV-2 infection (PASC) in children and adolescents. PM&R 14(10):1241–1269.
Mariani, F., R. Morello, D. O. Traini, A. La Rocca, C. De Rose, P. Valentini, and D. Buonsenso. 2023. Risk factors for persistent anosmia and dysgeusia in children with SARS-CoV-2 infection: A retrospective study. Children 10(3):597.
Martora, F., A. Villani, G. Fabbrocini, and T. Battista. 2023. COVID-19 and cutaneous manifestations: A review of the published literature. Journal of Cosmetic Dermatology 22(1):4–10.
Maruff, P., E. Thomas, L. Cysique, B. Brew, A. Collie, P. Snyder, and R. H. Pietrzak. 2009. Validity of the CogState brief battery: Relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Archives of Clinical Neuropsychology 24(2):165–178.
Mass General. 2022. Dermatologic manifestations of COVID-19 can become “long-hauler” symptoms. Massachusettts General Hospital Advances in Motion. https://advances.massgeneral.org/research-and-innovation/journal.aspx?id=1868 (accessed March 15, 2024).
Mattei, A., B. Amy de la Bretèque, S. Crestani, L. Crevier-Buchman, C. Galant, S. Hans, A. Julien-Laferrière, A. Lagier, C. Lobryeau, F. Marmouset, D. Robert, V. Woisard, and A. Giovanni. 2020. Guidelines of clinical practice for the management of swallowing disorders and recent dysphonia in the context of the COVID-19 pandemic. European Annals of Otorhinolaryngology, Head and Neck Diseases 137(3):173–175.
Mayo Clinic. 2022. Fever. https://www.mayoclinic.org/diseases-conditions/fever/diagnosis-treatment/drc-20352764 (accessed January 16, 2024).
McDonald, C., S. Koshi, L. Busner, L. Kavi, and J. L. Newton. 2014. Postural tachycardia syndrome is associated with significant symptoms and functional impairment predominantly affecting young women: A UK perspective. BMJ Open 4(6):e004127.
McWhirter, L., H. Smyth, I. Hoeritzauer, A. Couturier, J. Stone, and A. J. Carson. 2023. What is brain fog? Journal of Neuroloy, Neurosurgery, and Psychiatry 94(4):321–325.
Melamed, E., L. Rydberg, A. F. Ambrose, R. Bhavaraju-Sanka, J. S. Fine, T. K. Fleming, E. Herman, J. L. Phipps Johnson, J. R. Kucera, M. Longo, W. Niehaus, C. V. Oleson, S. Sampsel, J. K. Silver, M. M. Smith, and M. Verduzco-Gutierrez. 2023. Multidisciplinary collaborative consensus guidance statement on the assessment and treatment of neurologic sequelae in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM&R 15(5):640–662.
Michael, B. D., D. Walton, E. Westenberg, D. García-Azorín, B. Singh, A. A. Tamborska, M. Netravathi, M. Chomba, G. K. Wood, and A. Easton. 2023. Consensus clinical guidance for diagnosis and management of adult COVID-19 encephalopathy patients. Journal of Neuropsychiatry and Clinical Neurosciences 35(1):12–27.
Michaudet, C., and J. Malaty. 2017. Chronic cough: Evaluation and management. American Family Physician 96(9):575–580.
Mizera, J., S. Genzor, M. Sova, L. Stanke, R. Burget, P. Jakubec, M. Vykopal, P. Pobeha, and J. Zapletalova. 2024. The effectiveness of glucocorticoid treatment in post-COVID-19 pulmonary involvement. Pneumonia (Nathan) 16(1):2.
Morello, R., L. Martino, and D. Buonsenso. 2023. Diagnosis and management of post-COVID (long COVID) in children: A moving target. Current Opinion in Pediatrics 35(2):184–192.
Morice, A. H., E. Millqvist, K. Bieksiene, S. S. Birring, P. Dicpinigaitis, C. Domingo Ribas, M. Hilton Boon, A. Kantar, K. Lai, L. McGarvey, D. Rigau, I. Satia, J. Smith, W.-J. Song, T. Tonia, J. W. K. Van Den Berg, M. J. G. Van Manen, and A. Zacharasiewicz. 2020. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. European Respiratory Journal 55(1):1901136.
Morrow, A. K., R. Ng, G. Vargas, D. T. Jashar, E. Henning, N. Stinson, and L. A. Malone. 2021. Postacute/Long COVID in pediatrics: Development of a multidisciplinary rehabilitation clinic and preliminary case series. American Journal of Physical Medicine & Rehabilitation 100(12):1140.
Morrow, A. K., L. A. Malone, C. Kokorelis, L. S. Petracek, E. F. Eastin, K. L. Lobner, L. Neuendorff, and P. C. Rowe. 2022. Long-term COVID-19 sequelae in adolescents: The overlap with orthostatic intolerance and ME/CFS. Current Pediatrics Reports 10(2):31–44.
Munblit, D., P. Bobkova, E. Spiridonova, A. Shikhaleva, A. Gamirova, O. Blyuss, N. Nekliudov, P. Bugaeva, M. Andreeva, A. DunnGalvin, P. Comberiati, C. Apfelbacher, J. Genuneit, S. Avdeev, V. Kapustina, A. Guekht, V. Fomin, A. A. Svistunov, P. Timashev, V. S. Subbot, V. V. Royuk, T. M. Drake, S. W. Hanson, L. Merson, G. Carson, P. Horby, L. Sigfrid, J. T. Scott, M. G. Semple, J. O. Warner, T. Vos, P. Olliaro, P. Glybochko, and D. Butnaru. 2021. Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clinical & Experimental Allergy 51(9):1107–1120.
Myall, K. J., B. Mukherjee, A. M. Castanheira, J. L. Lam, G. Benedetti, S. M. Mak, R. Preston, M. Thillai, A. Dewar, P. L. Molyneaux, and A. G. West. 2021. Persistent post-COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Annals of the American Thoracic Society 18(5):799–806.
Nambi, G., W. K. Abdelbasset, S. M. Alrawaili, S. H. Elsayed, A. Verma, A. Vellaiyan, M. M. Eid, O. R. Aldhafian, N. B. Nwihadh, and A. K. Saleh. 2022. Comparative effectiveness study of low versus high-intensity aerobic training with resistance training in community-dwelling older men with post-COVID-19 sarcopenia: A randomized controlled trial. Clinical Rehabilitation 36(1):59–68.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2022. Selected heritable disorders of connective tissue and disability. Washington, DC: The National Academies Press.
NCHS (National Center for Health Statistics). 2024. Long COVID household pulse survey. https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm (accessed March 15, 2024).
Nehme, M., F. Chappuis, L. Kaiser, F. Assal, and I. Guessous. 2023. The prevalence, severity, and impact of post-COVID persistent fatigue, post-exertional malaise, and chronic fatigue syndrome. Journal of General Internal Medicine 38(3):835–839.
Ng, R., G. Vargas, D. T. Jashar, A. Morrow, and L. A. Malone. 2022. Neurocognitive and psychosocial characteristics of pediatric patients with post-acute/long-COVID: A retrospective clinical case series. Archives of Clinical Neuropsychology 37(8):1633–1643.
NIAMSD (National Institute of Arthritis and Musculskeletal and Skin Diseases). 2021. Raynaud’s phenomenon. https://www.niams.nih.gov/health-topics/raynauds-phenomenon. (accessed March 13, 2024).
NICE (National Institute for Health and Care Excellence). 2017. Idiopathic pulmonary fibrosis in adults: Diagnosis and management. London, UK: NICE.
NICE. 2020. Tinnitus: Assessment and management. London, UK: NICE
NICE. 2021a. COVID-19 rapid guideline: Managing the long-term effects of COVID-19. London, UK: NICE.
NICE. 2021b. Headaches in over 12s: Diagnosis and management. London, UK: NICE.
NICE. 2021c. Myalgic encephalomyelitis (or encephalopathy)/chronic fatigue syndrome: Diagnosis and management. https://www.nice.org.uk/guidance/ng206 (accessed March 7, 2024).
NICE. 2021d. NICE ME/CFS diagnostic criteria 2021. https://me-pedia.org/wiki/NICE_ME/CFS_diagnostic_criteria_2021#Diagnostic_criteria (accessed March 7, 2024).
NICE. 2023. Thyroid disease: Assessment and management. London, UK: NICE.
NICE. 2023. Venous thromboembolic diseases: Diagnosis, management and thrombophilia testing: NICE Clinical Guidelines, no. 158. London, UK: NICE.
NIH (National Institutes of Health). n.d. Sign or symptom (Concept ID: C3540840). MedG-gen. National Library of Medicine, National Center for Biotechnology Information.
NIH. 2023. RECOVER: Researching COVID to enhance recovery. https://recovercovid.org/ (accessed October 13, 2023).
Núñez-Gil, I. J., G. Feltes, M. C. Viana-Llamas, S. Raposeiras-Roubin, R. Romero, E. Alfonso-Rodríguez, A. Uribarri, F. Santoro, V. Becerra-Muñoz, M. Pepe, A. F. Castro-Mejía, J. Signes-Costa, A. Gonzalez, F. Marín, J. Lopez-País, E. Cerrato, O. Vázquez-Cancela, C. Espejo-Paeres, Á. López Masjuan, L. Velicki, I. El-Battrawy, H. Ramakrishna, A. Fernandez-Ortiz, and J. Perez-Villacastín, on behalf of HOPE-2 Investigators. 2023. Post-COVID-19 symptoms and heart disease: Incidence, prognostic factors, outcomes and vaccination: Results from a multi-center international prospective registry (HOPE 2). Journal of Clinical Medicine 12(2):706.
Núñez-Seisdedos, M. N., I. Lázaro-Navas, L. López-González, and L. López-Aguilera. 2022. Intensive care unit-acquired weakness and hospital functional mobility outcomes following invasive mechanical ventilation in patients with COVID-19: A single-centre prospective cohort study. Journal of Intensive Care Medicine 37(8):1005.
Oldroyd, A. G. S., J. B. Lilleker, T. Amin, O. Aragon, K. Bechman, V. Cuthbert, J. Galloway, P. Gordon, W. J. Gregory, H. Gunawardena, M. G. Hanna, D. Isenberg, J. Jackman, P. D. W. Kiely, P. Livermore, P. M. Machado, S. Maillard, N. McHugh, R. Murphy, C. Pilkington, A. Prabu, P. Rushe, S. Spinty, J. Swan, H. Tahir, S. L. Tansley, P. Truepenny, Y. Truepenny, K. Warrier, M. Yates, C. Papadopoulou, N. Martin, L. McCann, and H. Chinoy. 2022. British society for rheumatology guideline on management of paediatric, adolescent and adult patients with idiopathic inflammatory myopathy. Rheumatology 61(5):1760–1768.
Oliveira, C. R., L. A. Jason, D. Unutmaz, L. Bateman, and S. D. Vernon. 2023. Improvement of long COVID symptoms over one year. Frontiers in Medicine 9:1065620.
Orfei, M. D., D. E. Porcari, S. D’Arcangelo, F. Maggi, D. Russignaga, and E. Ricciardi. 2022. A new look on long-COVID effects: The functional brain fog syndrome. Journal of Clinical Medicine 11(19).
Ortel, T. L., I. Neumann, W. Ageno, R. Beyth, N. P. Clark, A. Cuker, B. A. Hutten, M. R. Jaff, V. Manja, S. Schulman, C. Thurston, S. Vedantham, P. Verhamme, D. M. Witt, I. D. Florez, A. Izcovich, R. Nieuwlaat, S. Ross, H. J. Schünemann, W. Wiercioch, Y. Zhang, and Y. Q. Zhang. 2020. American Society of Hematology 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Advances 4(19):4693–4738.
Pagen, D. M. E., M. Van Herck, C. J. A. van Bilsen, S. Brinkhues, K. Konings, C. D. J. den Heijer, M. A. Spruit, C. Hoebe, and N. Dukers-Muijrers. 2023. High proportions of post-exertional malaise and orthostatic intolerance in people living with post-COVID-19 condition: The prime post-COVID study. Frontiers in Medicine 10:1292446.
PCDS (Primary Care Dermatology Society). 2023. Livedo reticularis and livedoid vasculopathy https://www.pcds.org.uk/clinical-guidance/livedo-reticularis (accessed November 14, 2023).
Pellegrino, R., E. Chiappini, A. Licari, L. Galli, and G. L. Marseglia. 2022. Prevalence and clinical presentation of long COVID in children: A systematic review. European Journal of Pediatrics 181(12):3995–4009.
Penner, J., O. Abdel-Mannan, K. Grant, S. Maillard, F. Kucera, J. Hassell, M. Eyre, Z. Berger, Y. Hacohen, and K. Moshal. 2021. 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: A retrospective cohort study. The Lancet Child & Adolescent Health 5(7):473–482.
Perlis, R. H., M. Santillana, K. Ognyanova, A. Safarpour, K. Lunz Trujillo, M. D. Simonson, J. Green, A. Quintana, J. Druckman, M. A. Baum, and D. Lazer. 2022. Prevalence and correlates of long COVID symptoms among us adults. JAMA Network Open 5(10):e2238804
Petersen, R. C., O. Lopez, M. J. Armstrong, T. S. Getchius, M. Ganguli, D. Gloss, G. S. Gronseth, D. Marson, T. Pringsheim, and G. S. Day. 2018. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 90(3):126–135.
Phillips, T. G., W. P. Slomiany, and R. Allison. 2017. Hair loss: Common causes and treatment. American Family Physician 96(6):371–378.
Polly, S., and A. P. Fernandez. 2022. Common skin signs of COVID-19 in adults: An update. Cleveland Clinic Journal of Medicine 89(3):161–167.
Powers, W. J., A. A. Rabinstein, T. Ackerson, O. M. Adeoye, N. C. Bambakidis, K. Becker, J. Biller, M. Brown, B. M. Demaerschalk, B. Hoh, E. C. Jauch, C. S. Kidwell, T. M. LeslieMazwi, B. Ovbiagele, P. A. Scott, K. N. Sheth, A. M. Southerland, D. V. Summers, and D. L. Tirschwell. 2019. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50(12):e440–e441.
Prabhu, M., K. Cagino, K. C. Matthews, R. L. Friedlander, S. M. Glynn, J. M. Kubiak, Y. J. Yang, Z. Zhao, R. N. Baergen, J. I. DiPace, A. S. Razavi, D. W. Skupski, J. R. Snyder, H. K. Singh, R. B. Kalish, C. M. Oxford, and L. E. Riley. 2020. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: A prospective cohort study. BJOG 127(12):1548–1556.
Premraj, L., N. V. Kannapadi, J. Briggs, S. M. Seal, D. Battaglini, J. Fanning, J. Suen, C. Robba, J. Fraser, and S.-M. Cho. 2022. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. Journal of the Neurological Sciences 434:120162.
Pringsheim, T., G. S. Day, D. B. Smith, A. Rae-Grant, N. Licking, M. J. Armstrong, R. M. de Bie, E. Roze, J. M. Miyasaki, R. A. Hauser, A. J. Espay, J. P. Martello, J. A. Gurwell, L. Billinghurst, K. Sullivan, M. S. Fitts, N. Cothros, D. A. Hall, M. Rafferty, L. Hagerbrant, T. Hastings, M. D. O’Brien, H. Silsbee, G. Gronseth, and A. E. Lang, on behalf of the Guideline Subcommittee of the AAN. 2021. Dopaminergic therapy for motor symptoms in early parkinson disease practice guideline summary: A report of the AAN Guideline Subcommittee. Neurology 97(20):942–957.
Qaseem, A., R. M. McLean, D. O’Gurek, P. Batur, K. Lin, D. L. Kansagara, Clinical Guidelines Committee of the American College of Physicians, Commission on Health of the Public and Science of the American Academy of Family Physicians. 2020. Nonpharmacologic and pharmacologic management of acute pain from non-low back, musculoskeletal injuries in adults: A clinical guideline from the American College of Physicians and American Academy of Family Physicians. Annals of Internal Medicine 173(9):739–748.
Raasing, L. R. M., O. J. M. Vogels, M. Veltkamp, C. F. P. van Swol, and J. C. Grutters. 2021. Current view of diagnosing small fiber neuropathy. Journal of Neuromuscular Diseases 8(2):185–207.
Radtke, T., A. Ulyte, M. A. Puhan, and S. Kriemler. 2021. Long-term symptoms after SARS-CoV-2 infection in children and adolescents. JAMA 326(9):869–871.
Raghu, G., M. Remy-Jardin, C. J. Ryerson, J. L. Myers, M. Kreuter, M. Vasakova, E. Bargagli, J. H. Chung, B. F. Collins, E. Bendstrup, H. A. Chami, A. T. Chua, T. J. Corte, J.-C. Dalphin, S. K. Danoff, J. Diaz-Mendoza, A. Duggal, R. Egashira, T. Ewing, M. Gulati, Y. Inoue, A. R. Jenkins, K. A. Johannson, T. Johkoh, M. Tamae-Kakazu, M. Kitaichi, S. L. Knight, D. Koschel, D. J. Lederer, Y. Mageto, L. A. Maier, C. Matiz, F. Morell, A. G. Nicholson, S. Patolia, C. A. Pereira, E. A. Renzoni, M. L. Salisbury, M. Selman, S. L. F. Walsh, W. A. Wuyts, and K. C. Wilson. 2020. Diagnosis of hypersensitivity pneumonitis in adults: An official ATS/JRS/ALAT clinical practice guideline. American Journal of Respiratory and Critical Care Medicine 202(3):e36–e69.
Raghu, G., M. Remy-Jardin, L. Richeldi, C. C. Thomson, Y. Inoue, T. Johkoh, M. Kreuter, D. A. Lynch, T. M. Maher, F. J. Martinez, M. Molina-Molina, J. L. Myers, A. G. Nicholson, C. J. Ryerson, M. E. Strek, L. K. Troy, M. Wijsenbeek, M. J. Mammen, T. Hossain, B. D. Bissell, D. D. Herman, S. M. Hon, F. Kheir, Y. H. Khor, M. Macrea, K. M. Antoniou, D. Bouros, I. Buendia-Roldan, F. Caro, B. Crestani, L. Ho, J. Morisset, A. L. Olson, A. Podolanczuk, V. Poletti, M. Selman, T. Ewing, S. Jones, S. L. Knight, M. Ghazipura, and K. C. Wilson. 2022. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: An official ATS/ERS/JRS/ALAT clinical practice guideline. American Journal of Respiratory and Critical Care Medicine 205(9):e18–e47.
Rahmati, M., D. K. Yon, S. W. Lee, R. Udeh, M. McEVoy, M. S. Kim, R. M. Gyasi, H. Oh, G. F. López Sánchez, and L. Jacob. 2023. New-onset type 1 diabetes in children and adolescents as postacute sequelae of SARS-CoV-2 infection: A systematic review and meta-analysis of cohort studies. Journal of Medical Virology 95(6):e28833.
Raj, S. R., A. C. Arnold, A. Barboi, V. E. Claydon, J. K. Limberg, V. M. Lucci, M. Numan, A. Peltier, H. Snapper, and S. Vernino. 2021. Long-COVID postural tachycardia syndrome: An American Autonomic Society statement. Clinical Autonomic Research 31(3):365–368.
Raj, S. R., A. Fedorowski, and R. S. Sheldon. 2022. Diagnosis and management of postural orthostatic tachycardia syndrome. Canadian Medical Association Journal 194(10):E378–E385.
Raman, B., D. A. Bluemke, T. F. Lüscher, and S. Neubauer. 2022. Long COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. European Heart Journal 43(11): 1157–1172.
Rao, S., G. M. Lee, H. Razzaghi, V. Lorman, A. Mejias, N. M. Pajor, D. Thacker, R. Webb, K. Dickinson, L. C. Bailey, R. Jhaveri, D. A. Christakis, T. D. Bennett, Y. Chen, and C. B. Forrest. 2022. Clinical features and burden of postacute sequelae of SARS-CoV-2 infection in children and adolescents. JAMA Pediatrics 176(10):1000.
Rayner, D. G., E. Wang, C. Su, O. D. Patel, S. Aleluya, A. Giglia, E. Zhu, and M. Siddique. 2023. Risk factors for long COVID in children and adolescents: A systematic review and meta-analysis. World Journal of Pediatrics 20(2):133–142.
RCOG (Royal College of Obstetricians and Gynaecologists). 2012. The initial management of chronic pelvic pain: Green-top Guideline 41. https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/the-initial-management-of-chronic-pelvic-pain-green-top-guideline-no-41/ (accessed March 26, 2024).
Riera-Canales, C., and A. Llanos-Chea. 2023. COVID-19 and the gastrointestinal tract in children. Current Opinion in Pediatrics 35(5):585–589.
Rodriguez, B., L. Larsson, and W. J. Z’Graggen. 2022. Critical illness myopathy: Diagnostic approach and resulting therapeutic implications. Current Treatment Options in Neurology 24(4):173–182.
Roessler, M., F. Tesch, M. Batram, J. Jacob, F. Loser, O. Weidinger, D. Wende, A. Vivirito, N. Toepfner, F. Ehm, M. Seifert, O. Nagel, C. König, R. Jucknewitz, J. P. Armann, R. Berner, M. Treskova-Schwarzbach, D. Hertle, S. Scholz, S. Stern, P. Ballesteros, S. Baßler, B. Bertele, U. Repschläger, N. Richter, C. Riederer, F. Sobik, A. Schramm, C. Schulte, L. Wieler, J. Walker, C. Scheidt-Nave, and J. Schmitt. 2022. Post-COVID-19-associated morbidity in children, adolescents, and adults: A matched cohort study including more than 157,000 individuals with COVID-19 in Germany. PLoS Medicine 19(11):e1004122.
Roge, I., L. Smane, A. Kivite-Urtane, Z. Pucuka, I. Racko, L. Klavina, and J. Pavare. 2021. Comparison of persistent symptoms after COVID-19 and other non-SARS-CoV-2 infections in children. Frontiers in Pediatrics 9:752385.
Rogers, T. S., M. A. Noel, and B. Garcia. 2023. Dizziness: Evaluation and management. American Family Physician 107(5):514–523.
Rome Foundation. n.d. Rome IV criteria. https://theromefoundation.org/rome-iv/rome-iv-criteria/ (accessed November 14, 2023).
Rovin, B. H., S. G. Adler, J. Barratt, F. Bridoux, K. A. Burdge, T. M. Chan, H. T. Cook, F. C. Fervenza, K. L. Gibson, R. J. Glassock, D. R. W. Jayne, V. Jha, A. Liew, Z.-H. Liu, J. M. Mejía-Vilet, C. M. Nester, J. Radhakrishnan, E. M. Rave, H. N. Reich, P. Ronco, J.-S. F. Sanders, S. Sethi, Y. Suzuki, S. C. W. Tang, V. Tesar, M. Vivarelli, J. F. M. Wetzels, and J. Floege. 2021. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney International 100(4):S1–S276.
Rowe, K. S. 2019. Long term follow up of young people with chronic fatigue syndrome attending a pediatric outpatient service. Frontiers in Pediatrics 7:21.
Sánchez-García, A. M., P. Martínez-López, A. M. Gómez-González, J. Rodriguez-Capitán, F. J. Pavón-Morón, R. J. Jiménez-López, J. M. García-Almeida, E. Avanesi-Molina, N. Zamboschi, C. Rueda-Molina, V. Doncel-Abad, A. I. Molina-Ramos, E. Cabrera-César, I. Ben-Abdellatif, M. Gordillo-Resina, E. Pérez-Mesa, M. Nieto-González, P. NuevoOrtega, C. Reina-Artacho, P. L. Sánchez-Fernández, M. F. Jiménez-Navarro, and M. A. Estecha-Foncea. 2023. Post-intensive care unit multidisciplinary approach in patients with severe bilateral SARS-CoV-2 pneumonia. International Journal of Medical Sciences 20(1):1–10.
Sandler, C. X., V. B. B. Wyller, R. Moss-Morris, D. Buchwald, E. Crawley, J. Hautvast, B. Z. Katz, H. Knoop, P. Little, R. Taylor, K. A. Wensaas, and A. R. Lloyd. 2021. Long COVID and post-infective fatigue syndrome: A review. Open Forum Infectious Diseases 8(10):ofab440.
Sansone, F., G. M. Pellegrino, A. Caronni, F. Bonazza, E. Vegni, A. Lué, T. Bocci, C. Pipolo, G. Giusti, P. Di Filippo, S. Di Pillo, F. Chiarelli, G. F. Sferrazza Papa, and M. Attanasi. 2023. Long COVID in children: A multidisciplinary review. Diagnostics 13(12):1990.
Satterfield, B. A., D. L. Bhatt, and B. J. Gersh. 2022. Cardiac involvement in the long-term implications of COVID-19. Nature Review Cardiology 19(5):332–341.
Schiffl, H., and S. M. Lang. 2023. Long-term interplay between COVID-19 and chronic kidney disease. International Urology and Nephrology 55(8):1977–1984.
Schild, A.-K., D. Scharfenberg, L. Kirchner, K. Klein, A. Regorius, Y. Goereci, D. Meiberth, L. Sannemann, J. Lülling, F. Schweitzer, G. R. Fink, F. Jessen, C. Franke, Ö. Onur, S. Jost, C. Warnke, and F. Maier. 2023. Subjective and objective cognitive deficits in patients with post-COVID syndrome. Zeitschrift für Neuropsychologie 34(2):99–110.
Schlegel, P. N., M. Sigman, B. Collura, C. De Jonge, M. L. Eisenberg, D. J. Lamb, J. P. Mulhall, C. Niederberger, J. I. Sandlow, R. Z. Sokol, S. D. Spandorfer, C. Tanrikut, J. T. Treadwell, J. T. Oristaglio, and A. Zini. 2020. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part I. Fertility and Sterility 115(1):54–61.
Schmidt, J. 2018. Current classification and management of inflammatory myopathies. Journal of Neuromuscular Diseases 5(2):109–129.
Schneider, S. A., S. Desai, O. Phokaewvarangkul, E. C. Rosca, J. Sringean, P. Anand, G. Bravo, F. Cardoso, A. M. Cervantes-Arslanian, H. Chovatiya, D. Crosiers, F. Dijkstra, C. Fearon, F. Grandas, E. Guedj, A. Méndez-Guerrero, M. Hassan, J. Jankovic, A. E. Lang, K. Makhoul, L. Muccioli, S. A. O’Shea, V. R. Ostovan, J. R. Perez-Sanchez, R. Ramdhani, V. Ros-Castelló, C. Schulte, P. Shah, L. Wojtecki, and P. K. Pal. 2023. COVID-19-associated new-onset movement disorders: A follow-up study. Journal of Neurology 270(5):2409–2415.
Seeley, M. C., C. Gallagher, E. Ong, A. Langdon, J. Chieng, D. Bailey, A. Page, H. S. Lim, and D. H. Lau. 2023. High incidence of autonomic dysfunction and postural orthostatic tachycardia syndrome in patients with long COVID: Implications for management and health care planning. American Journal of Medicine (June 29): S0002-9343(23)00402-3.
Shah, S. C., M. B. Piazuelo, E. J. Kuipers, and D. Li. 2021. AGA clinical practice update on the diagnosis and management of atrophic gastritis: Expert review. Gastroenterology 161(4):1325–1332.e7.
Shaw, B. H., L. E. Stiles, K. Bourne, E. A. Green, C. A. Shibao, L. E. Okamoto, E. M. Garland, A. Gamboa, A. Diedrich, V. Raj, R. S. Sheldon, I. Biaggioni, D. Robertson, and S. R. Raj. 2019. The face of postural tachycardia syndrome—Insights from a large cross-sectional online community- based survey. Journal of Internal Medicine 286(4):438–448.
Shibata, S., Q. Fu, T. B. Bivens, J. L. Hastings, W. Wang, and B. D. Levine. 2012. Short-term exercise training improves the cardiovascular response to exercise in the postural orthostatic tachycardia syndrome. Journal of Physiology 590(15):3495–3505.
Shirley Ryan AbilityLab. 2019. Neuro-QOL. https://www.sralab.org/rehabilitation-measures/neuro-qol (accessed March 6, 2024).
Simone, C. G., and P. D. Emmady. 2020. Transverse myelitis. In StatPearls. Treasure Island, FL: StatPearls Publishing.
Sisó-Almirall, A., P. Brito-Zerón, L. Conangla Ferrín, B. Kostov, A. Moragas Moreno, J. Mestres, J. Sellarès, G. Galindo, R. Morera, J. Basora, A. Trilla, and M. Ramos-Casals, on behalf of the CAMFiC Long COVID-19 Study Group. 2021. Long COVID-19: Proposed primary care clinical guidelines for diagnosis and disease management. International Journal of Environmental Research and Public Health 18(8):4350.
Spagnuolo, R., T. Larussa, C. Iannelli, C. Cosco, E. Nisticò, E. Manduci, A. Bruno, L. Boccuto, L. Abenavoli, F. Luzza, and P. Doldo. 2020. COVID-19 and inflammatory bowel disease: Patient knowledge and perceptions in a single center survey. Medicina 56(8):407.
Spahic, J. M., V. Hamrefors, M. Johansson, F. Ricci, O. Melander, R. Sutton, and A. Fedorowski. 2023. Malmö POTS symptom score: Assessing symptom burden in postural orthostatic tachycardia syndrome. Journal of Internal Medicine 293(1):91–99.
SSA (Social Security Administration). 2020. Disability Report - Adult: Form SSA-3368-BK. Woodlawn, MD: SSA. https://www.ssa.gov/forms/ssa-3368-bk.pdf (accessed February 28, 2024).
SSA. 2021. DI 24510.057 Sustainability and the residual functional capacity (RFC) assessment. https://secure.ssa.gov/poms.nsf/lnx/0424510057 (accessed February 28, 2024).
SSA. n.d.-a. Disability evaluation under Social Security: 12.00 Mental disorders—Adult. https://www.ssa.gov/disability/professionals/bluebook/12.00-MentalDisorders-Adult.htm (accessed March 26, 2024).
SSA. n.d.-b. Disability evaluation under Social Security: 112.00 Mental disorders—Childhood. https://www.ssa.gov/disability/professionals/bluebook/112.00-MentalDisorders-Childhood.htm (accessed March 26, 2024).
Ssentongo, P., Y. Zhang, L. Witmer, V. M. Chinchilli, and D. M. Ba. 2022. Association of COVID-19 with diabetes: A systematic review and meta-analysis. Scientific Reports 12(1):20191.
Stand. n.d. The Britannica dictionary. https://www.britannica.com/dictionary/stand (accessed April 2, 2024).
Steenblock, C., M. Hassanein, E. G. Khan, M. Yaman, M. Kamel, M. Barbir, D. E. Lorke, J. A. Rock, D. Everett, S. Bejtullah, A. Heimerer, E. Tahirukaj, P. Beqiri, and S. R. Bornstein. 2022. Diabetes and COVID-19: Short- and long-term consequences. Hormone and Metabolic Research 54(8):503–509.
Štěpánek, L., M. Nakládalová, M. Janošíková, L. Štěpánek, K. Kabrhelová, and A. Boriková. 2023. Predictors and characteristics of post-acute COVID-19 syndrome in healthcare workers. Infectious Diseases 55(2):125–131.
Stern, Y., C. A. Barnes, C. Grady, R. N. Jones, and N. Raz. 2019. Brain reserve, cognitive reserve, compensation, and maintenance: Operationalization, validity, and mechanisms of cognitive resilience. Neurobiology of Aging 83:124–129.
Stevens, S., C. Snell, J. Stevens, B. Keller, and J. M. VanNess. 2018. Cardiopulmonary exercise test methodology for assessing exertion intolerance in myalgic encephalomyelitis/chronic fatigue syndrome. Frontiers in Pediatrics 6:242
Sudre, C. H., B. Murray, T. Varsavsky, M. S. Graham, R. S. Penfold, R. C. Bowyer, J. C. Pujol, K. Klaser, M. Antonelli, L. S. Canas, E. Molteni, M. Modat, M. Jorge Cardoso, A. May, S. Ganesh, R. Davies, L. H. Nguyen, D. A. Drew, C. M. Astley, A. D. Joshi, J. Merino, N. Tsereteli, T. Fall, M. F. Gomez, E. L. Duncan, C. Menni, F. M. K. Williams, P. W. Franks, A. T. Chan, J. Wolf, S. Ourselin, T. Spector, and C. J. Steves. 2021. Attributes and predictors of long COVID. Nature Medicine 27(4):626–631.
Sumantri, S., and I. Rengganis. 2023. Immunological dysfunction and mast cell activation syndrome in long COVID. Asia Pacific Allergy 13(1):50–53.
Sunada, N., H. Honda, Y. Nakano, K. Yamamoto, K. Tokumasu, Y. Sakurada, Y. Matsuda, T. Hasegawa, Y. Otsuka, M. Obika, Y. Hanayama, H. Hagiya, K. Ueda, H. Kataoka, and F. Otsuka. 2022. Hormonal trends in patients suffering from long COVID symptoms. Endocrine Journal 69(10):1173–1181.
Swarnakar, R., S. Jenifa, and S. Wadhwa. 2022. Musculoskeletal complications in long COVID-19: A systematic review. World Journal of Virology 11(6):485.
Tana, C., E. Bentivegna, S.-J. Cho, A. M. Harriott, D. García-Azorín, A. Labastida-Ramirez, R. Ornello, B. Raffaelli, E. R. Beltrán, R. Ruscheweyh, and P. Martelletti. 2022. Long COVID headache. Journal of Headache and Pain 23(1):93.
Tannis, A., J. A. Englund, A. Perez, E. J. Harker, M. A. Staat, E. P. Schlaudecker, N. B. Halasa, L. S. Stewart, J. V. Williams, M. G. Michaels, R. Selvarangan, J. E. Schuster, L. A. Sahni, J. A. Boom, G. Weinberg, P. G. Szilagyi, B. R. Clopper, Y. Zhou, M. L. McMorrow, E. J. Klein, and H. L. Moline. 2023. SARS-CoV-2 epidemiology and COVID-19 mRNA vaccine effectiveness among infants and children aged 6 months–4 years—New Vaccine Surveillance Network, United States, July 2022–September 2023. MMWR Morbidity and Mortality Weekly Report 72(48):1300–1306.
Taquet, M., J. R. Geddes, M. Husain, S. Luciano, and P. J. Harrison. 2021. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. The Lancet Psychiatry 8(5):416–427.
Taquet, M., R. Sillett, L. Zhu, J. Mendel, I. Camplisson, Q. Dercon, and P. J. Harrison. 2022. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: An analysis of 2-year retrospective cohort studies including 1,284,437 patients. The Lancet Psychiatry 9(10):815–827.
Tarantino, S., S. Graziano, C. Carducci, R. Giampaolo, and T. Grimaldi Capitello. 2022. Cognitive difficulties, psychological symptoms, and long lasting somatic complaints in adolescents with previous SARS-CoV-2 infection: A telehealth cross-sectional pilot study. Brain Sciences 12(8):969.
Tenner, S., J. Baillie, J. DeWitt, and S. S. Vege. 2013. American College of Gastroenterology guideline: Management of acute pancreatitis. Official Journal of the American College of Gastroenterology 108(9):1400–1415, 1416.
Thaweethai, T., S. E. Jolley, E. W. Karlson, E. B. Levitan, B. Levy, G. A. McComsey, L. McCorkell, G. N. Nadkarni, S. Parthasarathy, U. Singh, T. A. Walker, C. A. Selvaggi, D. J. Shinnick, C. C. M. Schulte, R. Atchley-Challenner, L. I. Horwitz, A. S. Foulkes, and RECOVER Consortium. 2023. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA 329(22):1934–1946.
The College of Optometrists. 2022. Clinical management guidelines. https://www.college-optometrists.org/clinical-guidance/clinical-management-guidelines/dryeye_keratoconjunctivitissicca_kcs (accessed November 10, 2023).
Treskova-Schwarzbach, M., L. Haas, S. Reda, A. Pilic, A. Borodova, K. Karimi, J. Koch, T. Nygren, S. Scholz, V. Schönfeld, S. Vygen-Bonnet, O. Wichmann, and T. Harder. 2021. Pre-existing health conditions and severe COVID-19 outcomes: An umbrella review approach and meta-analysis of global evidence. BMC Medicine 19(1):212.
Tsampasian, V., H. Elghazaly, R. Chattopadhyay, M. Debski, T. K. P. Naing, P. Garg, A. Clark, E. Ntatsaki, and V. S. Vassiliou. 2023. Risk factors associated with post-COVID-19 condition: A systematic review and meta-analysis. JAMA Internal Medicine 183(6):566–580.
Tsuchida, T., N. Yoshimura, K. Ishizuka, K. Katayama, Y. Inoue, M. Hirose, Y. Nakagama, Y. Kido, H. Sugimori, T. Matsuda, and Y. Ohira. 2023. Five cluster classifications of long COVID and their background factors: A cross-sectional study in Japan. Clinical and Experimental Medicine 23(7):3663–3670.
Tunkel, D. E., C. A. Bauer, G. H. Sun, R. M. Rosenfeld, S. S. Chandrasekhar, E. R. Cunningham, S. M. Archer, B. W. Blakley, J. M. Carter, E. C. Granieri, J. A. Henry, D. Hollingsworth, F. A. Khan, S. Mitchell, A. Monfared, C. W. Newman, F. S. Omole, C. D. Phillips, S. K. Robinson, M. B. Taw, R. S. Tyler, R. Waguespack, and E. J. Whamond. 2014. Clinical practice guideline: Tinnitus. Otolaryngology–Head and Neck Surgery 151(S2): S1–S40.
Twomey, R., J. DeMars, K. Franklin, S. N. Culos-Reed, J. Weatherald, and J. G. Wrightson. 2022. Chronic fatigue and postexertional malaise in people living with long COVID: An observational study. Physical Therapy 102(4):pzac005.
U.S. Preventive Services Task Force. 2020. Screening for cognitive impairment in older adults: U.S. Preventive Services Task Force recommendation statement. JAMA 323(8):757–763.
Vahratian, A., D. Adjaye-Gbewonyo, J.-M. S. Lin, and S. Saydah. 2023. Long COVID in children: United States, 2022. NCHS Data Brief 479:1–6.
Valdes, E., B. Fuchs, C. Morrison, L. Charvet, A. Lewis, S. Thawani, L. Balcer, S. L. Galetta, T. Wisniewski, and J. A. Frontera. 2022. Demographic and social determinants of cognitive dysfunction following hospitalization for COVID-19. Journal of the Neurological Sciences 438:120146.
Valent, P., C. Akin, B. Nedoszytko, P. Bonadonna, K. Hartmann, M. Niedoszytko, K. Brockow, F. Siebenhaar, M. Triggiani, M. Arock, J. Romantowski, A. Górska, L. B. Schwartz, and D. D. Metcalfe. 2020. Diagnosis, classification and management of Mast Cell Activation Syndromes (MCAS) in the era of personalized medicine. International Journal of Molecular Sciences 21(23):9030.
Valent, P., C. Akin, K. Hartmann, I. Alvarez-Twose, K. Brockow, O. Hermine, M. Niedoszytko, J. Schwaab, J. J. Lyons, M. C. Carter, H. O. Elberink, J. H. Butterfield, T. I. George, G. Greiner, C. Ustun, P. Bonadonna, K. Sotlar, G. Nilsson, M. Jawhar, F. Siebenhaar, S. Broesby-Olsen, S. Yavuz, R. Zanotti, M. Lange, B. Nedoszytko, G. Hoermann, M. Castells, D. H. Radia, J. I. Muñoz-Gonzalez, W. R. Sperr, M. Triggiani, H. C. Kluin-Nelemans, S. J. Galli, L. B. Schwartz, A. Reiter, A. Orfao, J. Gotlib, M. Arock, H. P. Horny, and D. D. Metcalfe. 2021. Updated diagnostic criteria and classification of mast cell disorders: A consensus proposal. Hemasphere 5(11):e646.
Van Doorn, P. A., P. Y. K. Van Den Bergh, R. D. M. Hadden, B. Avau, P. Vankrunkelsven, S. Attarian, P. H. Blomkwist-Markens, D. R. Cornblath, H. S. Goedee, T. Harbo, B. C. Jacobs, S. Kusunoki, H. C. Lehmann, R. A. Lewis, M. P. Lunn, E. Nobile-Orazio, L. Querol, Y. A. Rajabally, T. Umapathi, H. A. Topaloglu, and H. J. Willison. 2023. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of Guillain-Barré syndrome. Journal of the Peripheral Nervous System 28(4):535–563.
Varni, J. W., and C. A. Limbers. 2008. The PedsQL multidimensional fatigue scale in young adults: Feasibility, reliability and validity in a university student population. Quality of Life Research 17(1):105–114.
Varni, J. W., M. Seid, and P. S. Kurtin. 2001. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical Care 39(8):800–812.
Venkatesan, A., and R. G. Geocadin. 2014. Diagnosis and management of acute encephalitis: A practical approach. Neurology Clinical Practice 4(3):206–215.
Vernino, S., K. M. Bourne, L. E. Stiles, B. P. Grubb, A. Fedorowski, J. M. Stewart, A. C. Arnold, L. A. Pace, J. Axelsson, J. R. Boris, J. P. Moak, B. P. Goodman, K. R. Chemali, T. H. Chung, D. S. Goldstein, A. Diedrich, M. G. Miglis, M. M. Cortez, A. J. Miller, R. Freeman, I. Biaggioni, P. C. Rowe, R. S. Sheldon, C. A. Shibao, D. M. Systrom, G. A. Cook, T. A. Doherty, H. I. Abdallah, A. Darbari, and S. R. Raj. 2021. Postural orthostatic tachycardia syndrome (POTS): State of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting—Part 1. Autonomic Neuroscience 235:102828.
Vernon, S. D., M. Hartle, K. Sullivan, J. Bell, S. Abbaszadeh, D. Unutmaz, and L. Bateman. 2023. Post-exertional malaise among people with long COVID compared to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Work 74(4):1179–1186.
Virani, S. S., L. K. Newby, S. V. Arnold, V. Bittner, L. C. Brewer, S. H. Demeter, D. L. Dixon, W. F. Fearon, B. Hess, H. M. Johnson, D. S. Kazi, D. Kolte, D. J. Kumbhani, J. Lofaso, D. Mahtta, D. B. Mark, M. Minissian, A. M. Navar, A. R. Patel, M. R. Piano, F. Rodriguez, A. W. Talbot, V. R. Taqueti, R. J. Thomas, S. Van Diepen, B. Wiggins, and M. S. Williams; Peer Review Committee Members. 2023. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: A report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 148(9):e9–e119.
Von Knobelsdorff-Brenkenhoff, F., and J. Schulz-Menger. 2023. Cardiovascular magnetic resonance in the guidelines of the European Society of Cardiology: A comprehensive summary and update. Journal of Cardiovascular Magnetic Resonance 25(1):42.
Walker, L. S., and J. W. Greene. 1991. The functional disability inventory: Measuring a neglected dimension of child health status. Journal of Pediatric Psychology 16(1):39-58.
Walker, S., H. Goodfellow, P. Pookarnjanamorakot, E. Murray, J. Bindman, A. Blandford, K. Bradbury, B. Cooper, F. L. Hamilton, J. R. Hurst, H. Hylton, S. Linke, P. Pfeffer, W. Ricketts, C. Robson, F. A. Stevenson, D. Sunkersing, J. Wang, M. Gomes, W. Henley, and Living with COVID Recovery Collaboration. 2023. Impact of fatigue as the primary determinant of functional limitations among patients with post-COVID-19 syndrome: A cross-sectional observational study. BMJ Open 13(6):e069217.
Ware, J. E., Jr., and C. D. Sherbourne. 1992. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 30(6):473–483.
Weinstock, L. B., J. B. Brook, A. S. Walters, A. Goris, L. B. Afrin, and G. J. Molderings. 2021. Mast cell activation symptoms are prevalent in long-COVID. International Journal of Infectious Diseases 112:217–226.
Wernhart, S., E. Weihe, M. Totzeck, B. Balcer, T. Rassaf, and P. Luedike. 2023. Cardiopulmonary profiling of athletes with post-exertional malaise after COVID-19 infection—A single-center experience. Journal of Clinical Medicine 12(13):4348.
Whitcroft, K. L., and T. Hummel. 2019. Clinical diagnosis and current management strategies for olfactory dysfunction: A review. JAMA Otolaryngology–Head & Neck Surgery 145(9):846–853.
Whiteson, J. H., A. Azola, J. T. Barry, M. N. Bartels, S. Blitshteyn, T. K. Fleming, M. D. McCauley, J. D. Neal, J. Pillarisetti, and S. Sampsel. 2022. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of cardiovascular complications in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM&R 14(7):855878.
WHO (World Health Organization). 2023a. Clinical management of COVID-19: Living guideline. Geneva, Switzerland: WHO.
WHO. 2023b. International statistical classification of diseases and related health problems, 11th revision. Geneva, Switzerland: WHO.
Wilkinson, J. M., D. C. Codipilly, and R. P. Wilfahrt. 2021. Dysphagia: Evaluation and collaborative management. American Family Physician 103(2):97-106.
Winstein, C. J., J. Stein, R. Arena, B. Bates, L. R. Cherney, S. C. Cramer, F. Deruyter, J. J. Eng, B. Fisher, and R. L. Harvey. 2016. Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 47(6):e98–e169.
Wong, M. C., J. Huang, Y. Y. Wong, G. L. Wong, T. C. Yip, R. N. Chan, S. W. Chau, S. C. Ng, Y. K. Wing, and F. K. Chan. 2023. Epidemiology, symptomatology, and risk factors for long COVID symptoms: Population-based, multicenter study. JMIR Public Health and Surveillance 9:e42315.
Wood, G. C., R. P. Bentall, M. Gopfert, and R. H. Edwards. 1991. A comparative psychiatric assessment of patients with chronic fatigue syndrome and muscle disease. Psychological Medicine 21(3):619–628.
Wulf Hanson, S., C. Abbafati, J. G. Aerts, Z. Al-Aly, C. Ashbaugh, T. Ballouz, O. Blyuss, P. Bobkova, G. Bonsel, S. Borzakova, D. Buonsenso, D. Butnaru, A. Carter, H. Chu, C. De Rose, M. M. Diab, E. Ekbom, M. El Tantawi, V. Fomin, R. Frithiof, A. Gamirova, P. V. Glybochko, J. A. Haagsma, S. Haghjooy Javanmard, E. B. Hamilton, G. Harris, M. H. Heijenbrok-Kal, R. Helbok, M. E. Hellemons, D. Hillus, S. M. Huijts, M. Hultström, W. Jassat, F. Kurth, I. M. Larsson, M. Lipcsey, C. Liu, C. D. Loflin, A. Malinovschi, W. Mao, L. Mazankova, D. McCulloch, D. Menges, N. Mohammadifard, D. Munblit, N. A. Nekliudov, O. Ogbuoji, I. M. Osmanov, J. L. Peñalvo, M. S. Petersen, M. A. Puhan, M. Rahman, V. Rass, N. Reinig, G. M. Ribbers, A. Ricchiuto, S. Rubertsson, E. Samitova, N. Sarrafzadegan, A. Shikhaleva, K. E. Simpson, D. Sinatti, J. B. Soriano, E. Spiridonova, F. Steinbeis, A. A. Svistunov, P. Valentini, B. J. van de Water, R. van den Berg-Emons, E. Wallin, M. Witzenrath, Y. Wu, H. Xu, T. Zoller, C. Adolph, J. Albright, J. O. Amlag, A. Y. Aravkin, B. L. Bang-Jensen, C. Bisignano, R. Castellano, E. Castro, S. Chakrabarti, J. K. Collins, X. Dai, F. Daoud, C. Dapper, A. Deen, B. B. Duncan, M. Erickson, S. B. Ewald, A. J. Ferrari, A. D. Flaxman, N. Fullman, A. Gamkrelidze, J. R. Giles, G. Guo, S. I. Hay, J. He, M. Helak, E. N. Hulland, M. Kereselidze, K. J. Krohn, A. Lazzar-Atwood, A. Lindstrom, R. Lozano, D. C. Malta, J. Månsson, A. M. Mantilla Herrera, A. H. Mokdad, L. Monasta, S. Nomura, M. Pasovic, D. M. Pigott, R. C. Reiner, Jr., G. Reinke, A. L. P. Ribeiro, D. F. Santomauro, A. Sholokhov, E. E. Spurlock, R. Walcott, A. Walker, C. S. Wiysonge, P. Zheng, J. P. Bettger, C. J. L. Murray, and T. Vos; Global Burden of Disease Long COVID Collaborators. 2022. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 328(16):1604–1615.
Xie, Y., and Z. Al-Aly. 2022. Risks and burdens of incident diabetes in long COVID: a cohort study. The Lancet Diabetes & Endocrinology 10(5):311–321.
Xie, Y., E. Xu, and Z. Al-Aly. 2022a. Risks of mental health outcomes in people with COVID-19: Cohort study. BMJ:e068993.
Xie, Y., E. Xu, B. Bowe, and Z. Al-Aly. 2022b. Long-term cardiovascular outcomes of COVID-19. Nature Medicine 28(3):583–590.
Xu, E., Y. Xie, and Z. Al-Aly. 2022. Long-term neurologic outcomes of COVID-19. Nature Medicine 28(11):2406–2415.
Xu, E., Y. Xie, and Z. Al-Aly. 2023. Long-term gastrointestinal outcomes of COVID-19. Nature Communications 14(1):983.
Yong, S. J., and S. Liu. 2022. Proposed subtypes of post-COVID-19 syndrome (or long-COVID) and their respective potential therapies. Reviews in Medical Virology 32(4):e2315.
Yousaf, A. R., J. Mak, L. Gwynn, R. Bloodworth, R. Rai, Z. Jeddy, L. B. Leclair, L. Edwards, L. E. W. Olsho, G. Newes-Adeyi, A. F. Dalton, M. Gaglani, S. K. Yoon, K. Hegmann, K. Ellingson, L. R. Feldstein, A. P. Campbell, A. Britton, and S. Saydah. 2023. 1935. COVID-19 mRNA vaccination reduces the occurrence of post-COVID conditions in U.S. children aged 5–17 years following Omicron SARS-CoV-2 infection, July 2021–Septem-ber 2022. Open Forum Infectious Diseases 10(Supplement 2):ofad500.2466.
Zambrano, L. D., M. M. Newhams, S. M. Olson, N. B. Halasa, A. M. Price, J. A. Boom, L. C. Sahni, S. Kamidani, K. M. Tarquinio, A. B. Maddux, S. M. Heidemann, S. S. Bhumbra, K. E. Bline, R. A. Nofziger, C. V. Hobbs, T. T. Bradford, N. Z. Cvijanovich, K. Irby, E. H. Mack, M. L. Cullimore, P. S. Pannaraj, M. Kong, T. C. Walker, S. J. Gertz, K. N. Michelson, M. A. Cameron, K. Chiotos, M. Maamari, J. E. Schuster, A. O. Orzel, M. M. Patel, A. P. Campbell, and A. G. Randolph, for the Overcoming COVID-19 Investigators. 2022. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12–18 years — United States, July–December 2021. MMWR Morbidity and Mortality Weekly Report 71(2):52–58.
Zambrano, L. D., M. M. Newhams, R. M. Simeone, K. E. Fleming-Dutra, N. Halasa, M. Wu, A. O. Orzel-Lockwood, S. Kamidani, P. S. Pannaraj, K. Chiotos, M. A. Cameron, A. B. Maddux, J. E. Schuster, H. Crandall, M. Kong, R. A. Nofziger, M. A. Staat, S. S. Bhumbra, K. Irby, J. A. Boom, L. C. Sahni, J. R. Hume, S. J. Gertz, M. Maamari, C. Bowens, E. R. Levy, T. T. Bradford, T. C. Walker, S. P. Schwartz, E. H. Mack, J. A. Guzman-Cottrill, C. V. Hobbs, M. S. Zinter, N. Z. Cvijanovich, K. E. Bline, S. R. Hymes, A. P. Campbell, and A. G. Randolph, for the Overcoming COVID-19 Investigators. 2024. Characteristics and clinical outcomes of vaccine-eligible US children under-5 years hospitalized for acute COVID-19 in a national network. Pediatric Infectious Disease Journal 43(3):242–249.
Zang, C., Y. Zhang, J. Xu, J. Bian, D. Morozyuk, E. J. Schenck, D. Khullar, A. S. Nordvig, E. A. Shenkman, R. L. Rothman, J. P. Block, K. Lyman, M. G. Weiner, T. W. Carton, F. Wang, and R. Kaushal. 2023. Data-driven analysis to understand long COVID using electronic health records from the recover initiative. Nature Communications 14(1):1948.
Zesiewicz, T. A., R. J. Elble, E. D. Louis, G. S. Gronseth, W. G. Ondo, R. B. Dewey, M. S. Okun, K. L. Sullivan, and W. J. Weiner. 2011. Evidence-based guideline update: Treatment of essential tremor: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 77(19):1752–1755.
Zheng, Y. B., N. Zeng, K. Yuan, S. S. Tian, Y. B. Yang, N. Gao, X. Chen, A. Y. Zhang, A. L. Kondratiuk, P. P. Shi, F. Zhang, J. Sun, J. L. Yue, X. Lin, L. Shi, A. Lalvani, J. Shi, Y. P. Bao, and L. Lu. 2023. Prevalence and risk factor for long COVID in children and adolescents: A meta-analysis and systematic review. Journal of Infection and Public Health 16(5):660–672.
ANNEX TABLE 3-1 Selected Respiratory Conditions Associated with Long COVID
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Pulmonary fibrosis | Standing, walking, strenuous physical activity, lifting, carrying, pushing/pulling, climbing, low work, speaking |
|
3.02 |
| Hypoxemia | Standing; walking; strenuous physical activity; lifting; carrying; pushing/pulling; overhead reaching; climbing; low work; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace | N/A | None |
| Pneumonitis | Walking, strenuous physical activity, lifting, carrying, pushing/pulling, overhead reaching, climbing, low work |
|
None |
| Chronic cough | Strenuous physical activity, speaking |
|
None |
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Chronic pulmonary hypertension | Standing; walking; strenuous physical activity; lifting; carrying; pushing/pulling; overhead reaching; climbing; low work; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace |
|
3.09 |
| Asthma | Walking, strenuous physical activity, carrying, pushing/pulling, overhead reaching, climbing, low work, speaking |
|
None |
| Dyspnea | Walking, strenuous physical activity, carrying, pushing/pulling, overhead reaching, climbing, low work, speaking |
|
None |
SOURCES: Maley et al., 2022; Zang et al., 2023.
ANNEX TABLE 3-2 Selected Cardiovascular Conditions Associated with Long COVID
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Heart failure | Standing, walking, strenuous physical activity, lifting, carrying, pushing/pulling, reaching, foot/leg controls, climbing, low work, speaking |
|
4.02 |
| Coronary disease | Walking, strenuous physical activity, lifting, carrying, pushing/pulling, reaching, climbing, low work |
|
4.04 |
| Dysrhythmia (tachycardia and bradyarrhythmia) | Standing, walking, strenuous physical activity, lifting, carrying, pushing/pulling, reaching, climbing, low work |
|
4.05 |
| Pulmonary embolism | Walking, strenuous physical activity, lifting, carrying, pushing/pulling, overhead reaching, climbing, low work |
|
7.08 |
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Venous thrombosis | Standing, walking, strenuous physical activity, foot/leg controls |
|
4.11 |
| Vasculitis | Strenuous physical activity, lifting, carrying, pushing/pulling, reaching, overhead reaching |
|
14.03 |
| Cardiac inflammatory disease (myocarditis and/or pericarditis) | Walking, strenuous physical activity, lifting, carrying, pushing/pulling, reaching, foot/leg controls, climbing, low work |
|
None |
SOURCES: Whiteson et al., 2022; Xie et al., 2022b; Zang et al., 2023.
ANNEX TABLE 3-3 Selected Neurological Conditions Associated with Long COVID
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Autonomic dysfunction/Postural orthostatic tachycardia syndrome | Sitting; standing; walking; strenuous physical activity; lifting; carrying; pushing/pulling; reaching; climbing; low work; near visual acuity; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace; problem solving |
|
None |
| Dizziness (vestibular/orthostatic) | Sitting; standing; walking; strenuous physical activity; lifting; carrying; pushing/pulling; reaching; climbing; low work; near visual acuity; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace; problem solving |
|
2.07 |
| Encephalitis/Encephalopathy | Sitting; standing; walking; strenuous physical activity; lifting; carrying; pushing/pulling; reaching; gross and fine manipulation; foot/leg controls; climbing; low work; vision; hearing; speaking; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace; problem solving; adapting or managing oneself; interacting with others |
|
None |
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Neurodegenerative disease | Sitting; standing; walking; strenuous physical activity; lifting; climbing; low work; speaking; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace; problem solving; adapting or managing oneself; interacting with others |
|
11.17 |
| Neurocognitive disorders/post-COVID cognitive impairment | Speaking; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace; problem solving; adapting or managing oneself; interacting with others |
|
None |
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Abnormal movements/Tremors | Standing, walking, strenuous physical activity, lifting, carrying, reaching, gross and fine manipulation, foot/leg controls, climbing, low work, speaking |
|
12.07 |
| Dysphagia | Eating, drinking, swallowing |
|
None |
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Guillain-Barré | Sitting, standing, walking, lifting, low work, reaching, gross and fine manipulation, climbing, foot/leg controls, strenuous physical activity, speaking, vision |
|
11.14 |
| Stroke | Sitting; standing; walking; strenuous physical activity; lifting; carrying; pushing/pulling; reaching; gross and fine manipulation; foot/leg controls; climbing; low work; vision; hearing; speaking; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace; problem solving; adapting or managing oneself; interacting with others |
|
11.04 |
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Spinal cord infarction | Standing, sitting, walking, strenuous physical activity, lifting, carrying, pushing/pulling, reaching, gross and fine manipulation, foot/leg controls, climbing, low work | N/A | None |
| Peripheral/small fiber neuropathy | Standing, walking, strenuous physical activity, carrying, reaching, gross and fine manipulation, foot/leg controls, climbing |
|
11.14 |
| Bell’s palsy/cranial neuropathies | Vision, hearing, speaking |
|
11.14 |
| Migraine and other chronic headaches | Strenuous physical activity; vision; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace; interacting with others |
|
None |
| Pain | Standing; walking; strenuous physical activity; lifting; carrying; pushing/pulling; climbing; low work; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace |
|
None |
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Proprioception and balance disorders | Standing, walking, lifting, carrying, pushing/pulling, reaching, gross and fine manipulation, foot/leg controls, climbing, low work |
|
None |
| Seizure | Climbing; strenuous physical activity; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace |
|
11.02 |
| Transverse myelitis or other demyelinating disease | Sitting, standing, walking, strenuous physical activity, lifting, carrying, pushing/pulling, reaching, gross and fine manipulation, foot/leg controls, climbing, low work, vision |
|
11.08 |
| Raynaud’s | Fine manipulation |
|
114.04 |
| Paresthesia | Fine manipulation, foot/leg controls |
|
None |
| Critical illness myopathy and/or neuropathy | Standing, walking, strenuous physical activity, lifting, carrying, pushing/pulling, reaching, gross and fine manipulation, foot/leg controls, low work |
|
None |
SOURCES: Melamed et al., 2023; Premraj et al., 2022; Xu et al., 2022; Zang et al., 2023.
ANNEX TABLE 3-4 Selected Special Senses and Speech Conditions Associated with Long COVID
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Anosmia/dysnosmia | N/A |
|
None |
| Ageusia/dysgeusia | N/A |
|
None |
| Dysarthria/dysphonia | Speaking |
|
2.09 |
| Visual, ocular symptoms (changes in vision/dry eye) | Near or far visual acuity, peripheral vision |
|
2.02, 2.03, 2.04 |
| Tinnitus | Hearing |
|
2.07 |
SOURCES: Melamed et al., 2023; Premraj et al., 2022; Xu et al., 2022.
ANNEX TABLE 3-5 Selected Musculoskeletal Conditions Associated with Long COVID
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Myopathy | Standing, walking, strenuous physical activity, lifting, pushing/pulling, overhead reaching, gross and fine manipulation, climbing, low work, vision |
|
None |
| Muscle weakness | Standing, walking, strenuous physical activity, lifting, pushing/pulling, reaching, gross and fine manipulation, climbing, low work |
|
None |
| Sarcopenia | Standing, walking, strenuous physical activity, lifting, pushing/pulling, reaching, gross and fine manipulation, climbing, low work |
|
None |
| Myositis | Standing, walking, strenuous physical activity, lifting, pushing/pulling, reaching, gross and fine manipulation, climbing, low work |
|
14.05 |
| Myalgia | Standing, walking, strenuous physical activity, lifting, pushing/pulling, reaching, gross and fine manipulation, climbing, low work |
|
None |
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Arthralgia | Standing, walking, strenuous physical activity, lifting, pushing/pulling, reaching, gross and fine manipulation, climbing, low work |
|
None |
| Arthritis | Sitting, standing, walking, strenuous physical activity, lifting, pushing/pulling, reaching, gross and fine manipulation, climbing, low work |
|
14.09 |
SOURCES: Swarnakar et al., 2022; Zang et al., 2023.
ANNEX TABLE 3-6 Selected Endocrine Conditions Associated with Long COVID
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Diabetes type 1 | Standing; walking; strenuous physical activity; lifting; pushing/pulling; reaching; gross and fine manipulation; climbing; low work; vision; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace |
|
None (diabetes complications are included under organ-specific listings) |
| Diabetes type 2 | Standing; walking; strenuous physical activity; lifting; pushing/pulling; reaching; gross and fine manipulation; climbing; low work; vision; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace |
|
None (diabetes complications are included under organ-specific listings) |
| Dyslipidemia | Vision |
|
None |
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Thyroid gland disorders | Standing; walking; strenuous physical activity; lifting; pushing/pulling; reaching; gross and fine manipulation; climbing; low work; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace; problem solving; interacting with others |
|
None |
| Hypothalamic, pituitary, adrenal axis dysfunction | Standing; walking; strenuous physical activity; lifting; pushing/pulling; reaching; gross and fine manipulation; climbing; low work; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace; problem solving; interacting with others |
|
None |
| Reproductive hormone dysfunction | Low testosterone: Understanding, remembering, and applying information; concentrating, persisting, or maintaining pace; problem solving |
|
None |
SOURCES: Xie and Al-Aly, 2022; Xu et al., 2023; Zang et al., 2023.
ANNEX TABLE 3-7 Selected Immune Conditions Associated with Long COVID
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Mast cell activation syndrome | Strenuous physical activity; low work; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace; problem solving; adapting or managing oneself |
|
None |
| Autoimmune disorders (including rheumatoid arthritis, ankylosing spondylitis, systemic lupus erythematosus, myositis, systemic sclerosis, Sjögren’s syndrome, mixed connective tissue disease, and inflammatory bowel disease) | Sitting; standing; walking; strenuous physical activity; lifting; carrying; pushing/pulling; reaching; gross and fine manipulation; foot/leg controls; climbing; low work; vision; hearing; speaking; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace; problem solving; interacting with others; adapting or managing oneself |
|
14.00D |
NOTE: Myositis and rheumatoid arthritis are discussed in Annex Table 3-5 (musculoskeletal conditions).
SOURCES: Chang et al., 2023; Lim et al., 2023; Sumantri and Rengganis, 2023; Weinstock et al., 2021.
ANNEX TABLE 3-8 Selected Gastrointestinal Conditions Associated with Long COVID
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Gastroesophageal reflux and peptic ulcer disease | N/A |
|
None |
| Gastritis/enteritis | Strenuous physical activity |
|
None |
| Ischemic colitis | Strenuous physical activity |
|
None |
| Irritable bowel syndrome/motility disorders | Strenuous physical activity; lifting; carrying; pushing/pulling; reaching; gross and fine manipulation; foot/leg controls; climbing; low work; vision; hearing; speaking; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace; problem solving; interacting with others; adapting or managing oneself |
|
None |
| Gut dysbiosis | N/A | N/A | None |
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Cholestasis | N/A |
|
5.05 |
| Chronic liver disease | Standing, walking, strenuous physical activity |
|
5.05 |
| Cholangitis | N/A |
|
None |
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Pancreatitis | N/A |
|
None |
| Weight loss due to gastrointestinal disease | Strenuous physical activity | N/A | 5.08 |
| Chronic abdominal pain | Strenuous physical activity, overhead reaching |
|
None |
| Nausea or vomiting | Strenuous physical activity, climbing |
|
None |
SOURCES: Xu et al., 2023; Zang et al., 2023.
ANNEX TABLE 3-9 Selected Genitourinary Conditions Associated with Long COVID
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Sexual dysfunction | N/A |
|
None |
| Dysmenorrhea/menstrual irregularities (with moderate to severe pain) | Standing, walking, strenuous physical activity, lifting, carrying, pushing/pulling, foot/leg controls, climbing, low work |
|
None |
| Oligospermia | N/A |
|
None |
| Orchitis/epididymitis (with moderate to severe pain) | Standing, walking, strenuous physical activity, lifting, carrying, pushing/pulling, climbing, low work |
|
None |
| Acute kidney injury (<12 months) | N/A |
|
None |
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Chronic kidney disease (>12 months) | Standing; walking; strenuous physical activity; lifting; carrying; pushing/pulling; reaching; fine manipulation; foot/leg controls; climbing; low work; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace; problem solving |
|
6.05 |
| Overactive bladder syndrome | Sitting; standing; walking; strenuous physical activity; lifting; carrying; pushing/pulling; climbing; low work; concentrating, persisting, or maintaining pace; adapting or managing oneself |
|
None |
SOURCES: Bowe et al., 2021; Schiffl et al., 2023; Zang et al., 2023.
ANNEX TABLE 3-10 Selected Skin Conditions Associated with Long COVID
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Hair loss | N/A |
|
None |
| Livedo reticularis | N/A |
|
None |
| Pernio | Sitting, strenuous physical activity, lifting, carrying, pushing/pulling, reaching, gross and fine manipulation, foot/leg controls, low work |
|
8.05 |
| Retiform purpura | Sitting, strenuous physical activity, lifting, carrying, pushing/pulling, reaching, gross and fine manipulation, foot/leg controls, low work |
|
8.05 |
| Chronic urticaria | Sitting, strenuous physical activity, lifting, carrying, pushing/pulling, reaching, gross and fine manipulation, foot/leg controls, low work |
|
None |
SOURCES: Grover et al., 2022; Zang et al., 2023.
ANNEX TABLE 3-11 Selected Neuropsychiatric Conditions Associated with Long COVID
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Loss of sexual desire | N/A |
|
None |
| Attention deficit/hyperactivity disorder | Sitting; concentrating, persisting, or maintaining pace; interacting with others; adapting or managing oneself |
|
None |
| Anxiety/panic disorders | Speaking; concentrating, persisting, or maintaining pace; interacting with others; adapting or managing oneself |
|
12.06 |
| Depression | Speaking; concentrating, persisting, or maintaining pace; interacting with others; adapting or managing oneself |
|
12.04 |
| Post-traumatic stress disorder | Speaking; concentrating, persisting, or maintaining pace; interacting with others; adapting or managing oneself |
|
12.15 |
| Psychotic disorder | Speaking; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace; problem solving; interacting with others; adapting or managing oneself |
|
12.03 |
| Stress | Concentrating, persisting, or maintaining pace; interacting with others; adapting or managing oneself |
|
12.06 |
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Adjustment disorder | Concentrating, persisting, or maintaining pace; interacting with others; adapting or managing oneself |
|
12.15 |
| Eating disorder | Standing; strenuous physical activity; climbing; concentrating, persisting, or maintaining pace; interacting with others; adapting or managing oneself |
|
None |
| Obsessive compulsive disorder | Concentrating, persisting, or maintaining pace; interacting with others; adapting or managing oneself |
|
None |
| Disordered sleep | Understanding, remembering, and applying information; concentrating, persisting, or maintaining pace; problem solving; interacting with others; adapting or managing oneself |
|
None |
SOURCES: Cheng et al., 2023; Premraj et al., 2022; Xie et al., 2022a; Zang et al., 2023.
ANNEX TABLE 3-12 Multisystem Conditions Associated with Long COVID
| Health Effects | Potential Functional Limitations | Selected Diagnostic and Management Guidelines | Potential Social Security Administration Listings |
|---|---|---|---|
| Chronic fatigue/post-exertional malaise | Walking; strenuous physical activity; lifting; carrying; pushing/pulling; reaching; climbing; low work; understanding, remembering, and applying information; adapting or managing oneself |
|
None |
| Myalgic encephalitis/chronic fatigue syndrome | Sitting; standing; walking; strenuous physical activity; lifting; carrying; pushing/pulling; reaching; gross and fine manipulation; foot/leg controls; climbing; low work; vision; hearing; speaking; understanding, remembering, and applying information; concentrating, persisting, or maintaining pace; problem solving; interacting with others; adapting or managing oneself |
|
None |
| Fever | N/A |
|
None |
SOURCE: Zang et al., 2023.
ANNEX TABLE 3-13 Physical Activities; Vision, Hearing, and Speech; and Mental Activities
| Activity | Definition |
|---|---|
| Physical Activities | |
| Sitting |
For the purpose of collecting occupational data, the U.S. Bureau of Labor Statistics considers sitting to be present when any of the following conditions exists:
|
| From a functional perspective, however, sitting as a physical activity involves resting one’s lower body (buttocks) on a seat or the ground, while maintaining one’s upper body (torso, neck, head) in an upright position. In addition to strong neck, shoulder, and core muscles, sitting requires balance and good proprioception. Although lying on a raised surface (e.g., a bed) may be grouped with sitting, sitting is distinct from lying down on the ground (e.g., lying on a dolly underneath a vehicle), which this report groups under low work. | |
| Standing | For the purpose of collecting occupational data, the Occupational Requirements Survey distinguishes only between sitting (as defined previously) and standing/walking defined as “whenever workers are not sitting or lying down,” including “time spent stooping, crawling, kneeling, crouching, or climbing” (BLS, 2020, p. 112). In other words, “a worker is always either sitting or standing/walking” (BLS, 2020, p. 112). |
| From a functional perspective, standing is distinct from walking, which in turn is distinct from low work (stooping, crawling, kneeling, crouching), or climbing. For the purpose of this report, standing is defined as being “in an upright position with all of [one’s] weight on [one’s] feet” (Stand, n.d.). | |
| Walking | Moving along on foot or advancing by steps, with one foot always on the ground. |
| Distance (long or short) and surface type (uneven, rough) can affect an individual’s ability to walk. | |
| Strenuous physical activity | Strenuous physical activity captures activities that require exertion and stamina—for example, running, jumping, swimming, throwing, catching, and the like. It potentially includes all other physical activities, in addition to running and other impact activities. |
| Lifting (floor to waist and overhead) | Use of upper and/or lower extremities to raise or lower an object from one level to another, including upward pulling (BLS, 2020, p. 118). |
| Activity | Definition |
|---|---|
| Carrying | “Transporting an object, usually by holding it in the hands or arms, or wearing it on the body, usually around the waist or upper torso” (BLS, 2020, p.118). |
| Carrying usually also requires the ability to stand, lift, and walk. | |
| Pushing/pulling | Use of upper and/or lower extremities to exert force upon an object so that the object moves away from or toward the origin of the force (BLS, 2020, p. 125). |
| Reaching | “Extending the hand(s) and arm(s) in any direction, requiring the straightening and extending of the arm(s) and elbow(s) and the engagement of the shoulder(s)” (BLS, 2020, p. 130). |
| Reaching may require standing. | |
| Overhead reaching | Extending the arm(s) with the hand(s) higher than the head and (1) the elbow is bent and the angle at the shoulders is about 90 degrees or more or (2) the elbow is extended and the angle at the shoulder is about 120 degrees or more (BLS, 2020, p. 130). |
| Overhead reaching requires neck extension and may require standing. | |
| At/below the shoulder reaching | Reaching that does not meet the threshold for overhead reaching described above (BLS, 2020, p. 130). |
| At/below the shoulder reaching may require standing. | |
| Gross manipulation | Gross manipulation involves “seizing, holding, grasping, turning, or otherwise working with the hand(s). Fingers are involved only to the extent that they are an extension of the hand to hold or operate an object or tool, such as hammer” (BLS, 2020, p. 187). It includes handling of large objects. |
| Fine manipulation | Fine manipulation involves “touching, picking, pinching, or otherwise working primarily with fingers rather than with the whole hand or arm” (BLS, 2020, p. 133). It includes writing, typing, or handling small objects (fingering). |
| Foot/leg controls | Refers to the “use of one or both feet or legs to move controls on machinery or equipment. Controls include, but are not limited to, pedals, buttons, levers, and cranks” (BLS, 2020, p. 133). |
| Climbing | “The act of ascending or descending stairs, ramps, ladders, ropes or scaffolding and similar structures using feet, legs, hands, and/or arms” (BLS, 2020, p. 142). |
| Activity | Definition |
|---|---|
| Low work |
Low work is a group of activities that includes stooping, crouching, kneeling, crawling, and lying on the ground.
Stooping is the act of “bending the body forward and down while bending the spine at the waist 45 degrees or more either over something below waist level or down towards an object on or near the ground” (BLS, 2020, p. 193). Must be performed standing. Crouching is “bending the body downward and forward by bending the legs and spine” (BLS, 2020, p. 138). Kneeling is “bending the legs at the knees to come to rest on the knee or knees” (BLS, 2020, p. 139). Crawling is “moving about on hands and knees or hands and feet” (BLS, 2020, p. 139). Lying on the ground includes the need to get down and up from the ground (e.g., lying down on a trolley on the ground). Clustering the low work activities is appropriate because one generally has to be able to stoop, crouch, and kneel to be able to crawl. There might be an occasion when someone only has to kneel momentarily (e.g., to lift a child) that might be less difficult for some people, but most of the difficulties are shared among these activities. From a functional perspective, lying on the ground has more in common with other low work activities in that it includes the need to get up and down from the ground and potentially squirming around to do work while on the ground. These are difficult tasks that are equivalent to the other low work activities. |
| Vision, Hearing, and Speaking Activities | |
| Near visual acuity | “Clarity of vision at approximately 20 inches or less, as when working with small objects or reading small print” (BLS, 2020, p. 154), including the use of a computer in support of a critical job function, regardless of distance. |
| Far visual acuity | “Clarity of vision at a distance of 20 feet or more, involving the ability to distinguish features of a person or objects at a distance” (BLS, 2020, p. 154). |
| Peripheral vision | “What is seen above, below, to the left or right by the eye while staring straight ahead” (BLS, 2020, p. 154). |
| Hearing | “Ability to hear, understand, and distinguish speech and/or other sounds” (BLS, 2020, p. 149). Includes hearing in-person one-on-one and group or conference communication; telephones and similar devices, such as radios, walkie-talkies, intercoms, and public address systems; and other such sounds as machinery alarms and equipment sounds. Passing a hearing test may be required for certain jobs. |
| Speaking | “Expressing or exchanging ideas by means of the spoken word to impart oral information to clients or the public and to convey detailed spoken instructions to other workers accurately, loudly, or quickly” (BLS, 2020, p. 149). |
| Activity | Definition |
|---|---|
| Mental Activities | |
| Understand, remember, and apply information | The abilities to learn, recall, and use (apply) information (SSA, n.d.-a, n.d.-b). |
| Concentrate, persist, or maintain pace | The abilities to focus attention on work/school activities and stay on task at a sustained rate (SSA, n.d.-a, n.d.-b). |
| Problem solve | “Analyze issues and make decisions that have a moderate to significant level of difficulty (e.g., the full extent of issues may not be readily apparent and requires independent judgment and research or investigation). The defining characteristics of problem solving are that there is no obvious, immediate solution to a problem or issue, and the worker must identify and weigh alternatives to arrive at a solution” (BLS, 2020, p. 99). |
| Interact with others | The abilities to relate to and work with supervisors, coworkers, the public, teachers, peers, and others—for example, |
| cooperating with others; asking for help when needed; handling conflicts with others; stating [one’s] point of view; initiating or sustaining conversation; understanding and responding to social cues (physical, verbal, emotional); responding to requests, suggestions, criticism, correction, and challenges; and keeping social interactions free of excessive irritability, sensitivity, argumentativeness, or suspiciousness. (SSA, n.d.-a; see also SSA, n.d.-b) | |
| Adapt or manage oneself | The abilities to “regulate emotions, control behavior, and maintain well-being” in a work or school setting—for example, |
| responding to demands; adapting to changes; managing [one’s] psychologically based symptoms; distinguishing between acceptable and unacceptable work performance; setting realistic goals; making plans for [oneself] independently of others; maintaining personal hygiene and [appropriate attire]; and being aware of normal hazards and taking appropriate precautions. (SSA, n.d.-a; see also SSA, n.d.-b) | |
SOURCES: BLS, 2020; SSA, n.d.-a, n.d.-b; table reprinted from NASEM, 2022, p. 197.





























































































