Defining and Evaluating In-Home Drug Disposal Systems For Opioid Analgesics: Proceedings of a Workshop (2024)
Chapter: 6 Regulatory Landscape for Household Opioid Disposal
6
Regulatory Landscape for Household Opioid Disposal
Highlights of Key Points Made by Individual Speakers*
- The Food and Drug Administration (FDA) has required a risk evaluation and mitigation strategy for all opioid analgesics dispensed in outpatient settings. (Raulerson)
- The recent removal of the non-retrievable standard from the SUPPORT Act allows FDA to consider a wider range of opioid disposal options that could be dispensed to patients. (Raulerson)
- In-home disposal systems can provide an option for people who cannot or do not want to bring their unused drugs to a take-back day or community kiosk. (Dhillon)
- The Environmental Protection Agency (EPA) cannot regulate household hazardous waste disposal, but the agency recommends avoiding disposal of hazardous waste by methods that lead to local landfills. EPA recommends incineration of medication over flushing or landfill disposal. (K. Fitzgerald)
- States and localities may regulate the disposal of household hazardous waste, and some prohibit the disposal of medication in solid waste systems. (Atia, K. Fitzgerald, Kellington)
- Extended producer responsibility laws in some states could present barriers to in-home opioid disposal methods. (Atia)
* This list is the rapporteurs’ summary of points made by the individual speakers identified, and the statements have not been endorsed or verified by the National Academies of Sciences, Engineering, and Medicine. They are not intended to reflect a consensus among workshop participants.
PRODUCT STEWARDSHIP
An overview of product stewardship and extended producer responsibility (EPR) laws was provided by Hanz Atia of PSI. PSI is a non-profit organization that “advocat[es] for sustainable practices and responsible management of the full life cycle of products,” working with stakeholders to develop EPR policies, programs, and laws. Atia’s work focuses on promoting programs that expand the safe disposal of pharmaceuticals and sharps.
PSI developed the first principles of product stewardship in 2001, Atia said, and the organization’s work over the past two decades has led to EPR programs in more than 20 product categories. This includes the passage of 133 EPR laws in 33 states, across 17 product categories.
Atia discussed the following:
- “Product stewardship is the act of minimizing the health, safety, environmental, and social impacts of a product and its packaging throughout all life cycle stages while also maximizing economic benefits. Product stewardship can be voluntary or required by law.”
- “Extended producer responsibility is a mandatory type of product stewardship required by law … [EPR] extends both upstream to product design and downstream to postconsumer reuse, recycling, or safe disposal.” Upstream, EPR programs “provid[e] incentives for manufacturers to incorporate environmental considerations in the design of their packaging and products.” Downstream, EPR shifts financial and management responsibility from the public sector back to the manufacturer (with government oversight).
Pharmaceutical EPR
PSI began its push to establish convenient drug take-back programs in 2008 by convening stakeholder meetings that called for new laws and regulations. Atia highlighted subsequent legislative and regulatory actions that have led to the passage of 8 state and 23 local pharmaceutical EPR laws to date (Figure 6-1). PSI continues to advocate for and support pharmaceutical EPR programs, creating resources such as briefing documents,
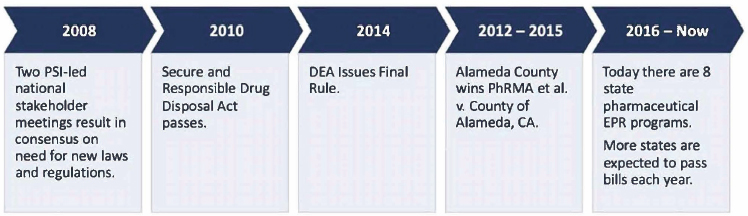
NOTE: DEA = Drug Enforcement Administration; EPR = extended producer responsibility; PhRMA = Pharmaceutical Research and Manufacturers of America; PSI = Product Stewardship Institute.
SOURCES: Presented by Hanz Atia, June 26, 2023. © Product Stewardship Institute, 2023. Used with permission.
drug take-back pilot demonstrations, a how-to guide for pharmacists,1 and public and provider educational materials. PSI has also developed a list of “16 elements of an effective EPR law” and helps states to draft model legislation that incorporates EPR best practices, Atia said.
Pharmaceutical EPR laws were first passed by Vermont and Massachusetts in 2016, followed by California, New York, and Washington in 2018, Oregon in 2019, Maine in 2021, and Illinois in 2022. Atia noted that the EPR programs in these eight states are funded by drug manufacturers. They highlighted select elements of the EPR laws in Maine, Illinois, Oregon, and New York that bar, discourage, or present barriers to in-home disposal methods.
Maine
Maine’s law specifically excludes in-home disposal methods from stewardship programs, Atia said. Proposed disposal plans
must ensure that collection methods used under the program include mail-back envelopes and collection receptacles and do not include home disposal methods involving packets, bottles, or other containers that a person may use to render non-retrievable or destroy a covered drug that is household pharmaceutical waste by means of chemical process [Public Law 2021, c. 94, § 2 (NEW)].2
___________________
1 See https://productstewardship.us/wp-content/uploads/2022/11/160920_PSI_Pharmacy_Guide_vS.pdf (accessed November 18, 2023).
2 See https://legislature.maine.gov/statutes/38/title38sec1612-1.html (accessed November 18, 2023).
This exclusion appears again later in the law. Atia said this exclusion stems from Maine’s experience with active pharmaceutical ingredients in the environment. For example, some organic farms could no longer label products as organic due to the presence of active pharmaceutical ingredients in the water system.
Illinois
The Illinois EPR law has similarities to Maine with regard to in-home disposal. Atia drew attention to a section on promotional materials for a drug take-back program. These materials “may not be used to promote in-home disposal products of any kind including, but not limited to, in-home disposal products of authorized collectors participating in a drug take-back program.”3
Oregon
Per Oregon law, the operator of the take-back program must “discourage the disposal of covered drugs in the garbage or sewer system.”4
New York
New York law requires that proposals include “how covered drugs will be safely and securely tracked and handled from collection through final disposal and destruction, [and] policies to ensure security and compliance with all applicable laws and regulations including disposal and destruction at a permitted waste disposal facility meeting federal requirements.”5 Atia said this presents a barrier for in-home disposal systems that are disposed of by patients and are not tracked.
International Approaches
To further inform the discussions, Atia said the European Union EPR program for pharmaceuticals includes drop-off kiosks in pharmacies. In Canada, prescription drugs, OTC medicines, and natural health products are returned to collection sites as part of the Medications Return Program managed and paid for by the Health Product Stewardship Association.
___________________
3 See https://www.ilga.gov/legislation/ilcs/ilcs3.asp?ActID=4321&ChapterID=35 (accessed November 18, 2023).
4 See https://www.oregonlegislature.gov/bills_laws/lawsstatutes/2019orlaw0659.pdf (accessed November 18, 2023).
5 See https://www.nysenate.gov/legislation/bills/2017/S9100 (accessed November 18, 2023).
In-Home Disposal Compared to Take-Back Programs
In closing, Atia said each of the different drug disposal models has benefits and drawbacks. As discussed, an advantage of in-home systems such as disposal pouches is that they are more accessible for certain populations (e.g., home-bound individuals). However, complexity, cost, environmental impact, and lack of trackability can present challenges.
Regardless of approach, a key element of pharmaceutical EPR programs is a requirement for education and outreach by the manufacturer or program administrator, Atia said. They shared results from a recent survey of 2,000 Americans that found that more than half did not know what to do with unused pharmaceuticals, and more than two-thirds “would be willing to change disposal habits if they knew how.”6
PERSPECTIVES ON REGULATORY AUTHORITY
Following the presentation, Atia was joined by four panelists who shared their perspectives on the current laws and regulations relevant to in-home drug disposal systems. Panelists included Uttam Dhillon, partner at Michael Best & Friedrich LLP; Kristin Fitzgerald, an environmental protection specialist at EPA; Mary Kellington, Safe Medicine Return program manager for the Washington State Department of Health; and Patrick Raulerson, senior regulatory counsel for CDER at FDA. The discussion was moderated by Lewis Grossman, Ann Loeb Bronfman Professor of Law at American University Washington College of Law.
EPA
Two federal laws implemented by EPA are applicable to the disposal of medication. The Clean Air Act governs incineration, including monitoring and emissions controls, and the Resource Conservation and Recovery Act (RCRA) governs land disposal of hazardous and solid waste, K. Fitzgerald said. RCRA gives EPA regulatory authority over the “cradle-to-grave management of hazardous waste” but excludes household disposal, she explained. States and localities may regulate the disposal of household hazardous waste (e.g., the state pharmaceutical EPR laws discussed by Atia above).
K. Fitzgerald noted that EPA also administers the Clean Water Act and the Safe Drinking Water Act, but neither of these provide authority applicable to in-home drug disposal. EPA can recommend that medica-
___________________
6 See https://swnsdigital.com/us/2021/04/more-than-two-thirds-of-americans-dont-know-how-to-properly-dispose-medications-new-research-reveals/ (accessed November 18, 2023).
tions not be flushed, she said, but does not have authority under these laws to prohibit this action. EPA can prohibit flushing of medication by health care facilities under RCRA but has no authority to regulate flushing of medications by households.
EPA is interested in what happens when a product leaves the home––specifically, where it goes and who is exposed to it, K. Fitzgerald said. Again, EPA cannot regulate household disposal, but the agency recommends that people take their hazardous waste (e.g., used motor oil, antifreeze) to household hazardous waste collections and avoid disposal methods that lead to local landfills.
For disposal of unused medication, the consensus recommendation from EPA and all federal agencies is to use pharmaceutical take-back programs (e.g., DEA periodic take-back days or mail-back envelopes, permanent take-back kiosks in the community). “EPA fully supports FDA’s recent decision to require manufacturers of opioids to make … prepaid mail-back envelopes available to customers filling opioid prescriptions,” K. Fitzgerald said, and EPA looks forward to wider availability of free mail-back envelopes in 2024 (when it is anticipated that the modified REMS will be approved). She explained that mail-back envelopes are destroyed by a “DEA reverse distributor” in a permitted, licensed, controlled incinerator because incineration is currently the only destruction technology that meets the DEA requirement that a disposed controlled substance be non-retrievable. EPA supports this approach, she said, because incineration of mail-back envelopes provides a “dual public health benefit,” removing opioids from the home and protecting the environment by reducing the flushing of opioids (which can lead to contamination of sources of drinking water).
The removal of the non-retrievable standard from the SUPPORT Act opens up the possibility for development of in-home disposal kits that could be discarded in household trash. K. Fitzgerald said this approach could mean that pharmaceutical products are still accessible to children, pets, or those seeking drugs until trash pickup day. Furthermore, she said that about 80 percent of trash in the United States goes to landfills (versus incineration), which can lead to pharmaceutical ingredients in the environment. She noted that some in-home disposal kits work mechanistically via adsorption to activated charcoal. However, acidic conditions in landfills can cause desorption and leaching of pharmaceutical ingredients, which are not removed by routine wastewater processing of landfill leachate. Incineration is preferable to landfill disposal because incineration completely destroys the pharmaceutical ingredients, she continued, and leads to a “better environmental outcome” as a result of effective monitoring and emission controls under the Clean Air Act.
FDA
“The opioid analgesic REMS is primarily focused on education of patients and health care practitioners on the safe use of opioids,” Raulerson said. FDA has required a REMS for all opioid analgesics dispensed in outpatient settings.
As mentioned, the SUPPORT Act gave FDA the authority to require, as part of a REMS, that certain patients be provided a safe disposal system or disposal packaging with a drug, if it is determined that doing so “could be expected to mitigate the risk of abuse of that drug or overdose associated with that drug, including accidental exposure,” Raulerson said. In considering when and how to apply this authority, the agency will consider the potential implications for the health care delivery system. For example, Raulerson said it is important to consider how a REMS modification mandating the provision of disposal systems might impact existing voluntary initiatives (e.g., retail pharmacy disposal programs) and state- and local-level disposal policies and product stewardship programs.
As discussed by Marta Sokolowska, under this new authority FDA is modifying the opioid analgesic REMS to require manufacturers of opioids to make prepaid mail-back envelopes and educational materials available to outpatient dispensers to give patients with their prescribed opioid. The next step will be for the manufacturers to collectively submit a proposal with modifications to the REMS that meet these requirements. FDA will then determine if the proposal is acceptable and approve it. Manufacturers would then begin to implement the changes. He noted that there can be some back and forth on elements of the proposal, and there is a dispute resolution process if needed.
Raulerson said that mail-back envelopes were the first opioid REMS modification mandated because all the necessary systems were already in place. This included the availability of federally regulated incinerators that could be used to meet the DEA requirement that disposed systems be non-retrievable. In addition, he said rules have been in place for a decade that are designed to ensure that mail-back envelopes are fit for purpose.
The removal of the non-retrievable standard from the SUPPORT Act in 2023 “opened the door for [FDA] to consider a wider range of things that might be dispensed to patients,” Raulerson said, including the in-home disposal systems that are the subject of this workshop. Raulerson said that, to his knowledge, there is no regulation of in-home drug disposal systems by any federal, state, or local agency to ensure they are fit for purpose. He suggested that minimum specifications and standards for in-home disposal systems would need to be established before FDA would mandate them under a REMS. In this regard, Raulerson said that “this workshop is one of the first places we are having robust discussion of what those speci-
fications might be and how we would create them.” There is a range of options for standards development, and the agency is open to discussion. He emphasized that this is new territory, and FDA wants to be sure that rules and standards based on the knowledge and systems of today do not inadvertently stifle innovation or limit the development of better or easier-to-use systems. As discussed throughout the workshop, multiple solutions might be needed to meet the needs of different patients, and he said FDA’s primary goal is “removing the risk from the home.”
Federal Drug Enforcement
Dhillon shared his perspective as former acting administrator of DEA and a former federal prosecutor. When he joined DEA in 2018, the weight of contributions at each National Prescription Drug Take Back Day that year was nearly 1 million pounds (drugs and packaging). Although this is an important program that gives people an opportunity to return unused drugs, the events only take place twice each year. In addition, he said contributions have declined since 2018 and currently hover around 600,000 pounds for the most recent take-back days. Permanent drop-off kiosks are also available, but use is variable.
A lot of attention is focused on the number of deaths from illicit drug use (e.g., fentanyl, heroin), but the number of people who die from prescription drug overdose is often not discussed, Dhillon said. CDC estimated there were 16,000 overdose deaths involving prescription opioids in 2020, or 44 people each day, he said.7 Addiction to prescription opioids may lead to fentanyl or heroin misuse and overdose. A significant contributor to this problem is that “prescriptions that are languishing in people’s medicine cabinets … are being diverted” and are then used inappropriately. “From a law enforcement perspective, the goal is always to reduce supply,” he said.
Dhillon said the simplest way to reduce the supply of diverted prescription drugs (and associated addiction and overdose deaths) is to make it easy for people to eliminate those drugs from their homes. He suggested that in-home disposal is perhaps the easiest method to accomplish that goal. In-home disposal systems provide an option for people who cannot or do not want to bring their unused drugs to a take-back event or community kiosk. Dhillon observed that people can be hesitant to walk into a police station to dispose of drugs.
Participant David Schiller, chief executive officer of NarcX and formerly of DEA, agreed and said the national take-back initiative has been
___________________
7 See https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates (accessed November 18, 2023).
very successful, but some people still perceive an association with criminal activity, and some fear they will be investigated for dropping off unused drugs. Schiller noted that diversion of drugs from the take-back stockpiles prior to destruction is an ongoing concern. Every year there are arrests of law enforcement officers who have stolen drugs from the national take-back or collection kiosks. Schiller emphasized the importance of educating the public about the diversion of unused opioids from homes and the need to dispose.
Washington State: A Case Example of State Regulation of Drug Disposal
Kellington said 26 states now have laws authorizing some kind of drug take-back program. Washington’s statewide Safe Medication Return program was launched in 2020 following passage of the state’s drug take-back law in 2018. Kellington explained that the state law was modeled after successful local ordinances, and that existing local take-back programs were incorporated into the state program.
“Safe Medication Return accepts prescription and over-the-counter medications in any dose and in any form,” Kellington said, including pet medications. Medical devices containing drugs are accepted, but empty medical devices, exposed needles, and sharps are not. Also excluded are “vitamins, homeopathic remedies, schedule 1 drugs, and drugs administered in a clinical setting.”
Products can be returned using free prepaid mailers (ordered online or by phone or picked up at one of 442 mailer distribution locations in the community); deposited at one of 800 widely distributed secure collection kiosks (e.g., pharmacies, law enforcement agencies, long-term care facilities, and substance use disorder treatment programs); or brought to community take-back events. Kellington noted that only 2 of the state’s 281 population centers do not have return kiosks or mailer distribution sites, and mailers are distributed in these areas via direct mail campaigns. All products collected are incinerated, and the program costs are covered by drug manufacturers, she said. More than 175 tons of product have been collected by Safe Medication Return since inception. Kellington said that although less than 2 percent of returned medication is given back via mail, the mailers are an important option and provide access for residents who are not served by or do not want to use kiosks and/or collection events.
Kellington said nothing in Washington state law prohibits the use of other in-home disposal methods, but she believed it was “unlikely” that methods other than mailers could be incorporated into the state-regulated program. She also pointed out that some local ordinances prohibit the disposal of medication at solid waste facilities (e.g., King County’s Waste
Acceptance Rule8). Safe Medication Return program operators are also required by the take-back law to discourage people from flushing medication or placing it in the trash.
___________________
8 See https://kingcounty.gov/en/legacy/about/policies/rules/utilities/put716pr (accessed November 18, 2023).









