Multimodal Biomarkers for Central Nervous System Disorders: Development, Validation, and Clinical Integration: Proceedings of a Workshop (2023)
Chapter: 2 Multimodal Biomarkers for Central Nervous System Disorders
Central nervous system (CNS) disorders are complex and heterogenous, both in the ways that they are experienced by patients and in their scientific underpinnings. Multimodal biomarkers may capture some of that complexity to enable better understanding and treatment of these disorders. As the scientific community works to develop, standardize, validate, and ultimately use multimodal biomarkers in the clinic, Linda Brady emphasized that it will be important to consider patient perspectives alongside scientific progress and regulatory requirements.
UNDERSTANDING THE IMPORTANCE OF MULTIMODAL BIOMARKERS TO PATIENTS
When Jillian Papa, Board President of Emotions Matter, was first diagnosed with borderline personality disorder (BPD) ten years ago, she was relieved to know that there were other people like her, that there was a name for the condition, and that there were treatment options. Her diagnosis was framed as a mood disorder or emotional dysregulation without information about the biological basis of her condition. Papa said that at first, she did not want to try medication because she was scared and perceived a stigma around taking it; she opted instead for dialectical behavioral therapy,1 but it was not enough. She eventually took medication and now
___________________
1 Dialectical behavior therapy is a type of talk therapy that is based on cognitive behavioral therapy.
no longer meets the diagnostic criteria for borderline personality disorder. Papa describes herself as “in recovery.”
Since becoming a patient advocate and learning more about BPD, Papa now believes that it would have been helpful to have more information, particularly from brain imaging. This type of information could have helped her understand that her condition had a biological basis, such as changes or abnormalities in her amygdala, and would have made it easier for her to choose medication sooner rather than dialectical behavioral therapy. She said, “I think about how informative it could have been” while choosing treatments if she could have seen her brain scan. In addition to helping with treatment decision making and recovery, Papa said that a broader range of biological information could help generate trust between patients and doctors, which can be a particular challenge for patients with BPD.
As a neurologist, movement disorder specialist, and someone living with Parkinson’s disease (PD), María De León, Patient Council member at the Michael J. Fox Foundation for Parkinson’s Research, shared her perspective on the importance of biomarkers. She said that biomarkers are critical for diagnosis, prognosis, and staging of PD as well as monitoring responses to treatment. Standardized biomarkers would be particularly helpful for patients that are atypical and more likely to be misdiagnosed due to unusual clinical presentations or to demographic characteristics that do not match a clinician’s expectations. De León herself was an atypical patient as a young woman with more non-motor symptoms than motor symptoms. She emphasized the importance of correlating biomarkers (such as brain imaging data or biomarkers in cerebrospinal fluid) with clinical outcomes, pointing out that it is not helpful to develop a drug that only improves how a patient’s brain scan looks but not their symptoms or disease progression. Standardization and rigorous evaluation of biomarkers would enable them to move from research to clinical application, especially in underserved communities. De León ended by saying that objective and reliable biomarkers can help to equalize the care of patients.
Brady moderated a short discussion with Papa and De León, beginning with a question about whether and how biomarkers could help combat stigma surrounding CNS disorders. Papa believed they could and said that BPD was highly stigmatized; as an example, she pointed to part of the penal code in California that excludes BPD from a pre-trial program that diverts people from jail to mental health programs.2 She said that biomarkers could reduce this stigma by bringing focus to the biological basis of the disease rather than framing it as a behavior and mood disorder. De León agreed and emphasized that the stigma surrounding mental health issues often dispropor-
___________________
2 To learn more about California Penal Code 1001.36. (SB 215), which went into effect on June 27, 2018, see here: https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201720180SB215 (accessed on April 28, 2023).
tionately impacts women and those in Latino communities. An objective biomarker of disease can help empower these patients to get the help they need.
In response to a question about the possibility of digital biomarkers that might provide data that are generated at home and in real-time, both Papa and De León said they believed those would be helpful. De León pointed out that it is important for a clinician to get a more holistic view of a patient’s experience rather than only seeing what is presented in the doctor’s office. She said that it could better enable patients to personalize or modify their treatment and to know when to ask for help. However, she cautioned, clinicians cannot be expected to go through the many hours’ worth of the data that could be generated.
On the question of use, privacy, and protection of biological data, Papa said that data about her brain seems more sensitive than other types of information and that she would like to see strong protections. To her, the biggest question about data sharing is whether sharing her data will help others with BPD, which she considers to be a top priority. De León added that there can also be data concerns related to insurance (e.g., whether insurance companies choose to cover certain medications), what family members would choose to know about genetic risk, and what to do with data when a patient dies.
UNDERSTANDING HOW MULTIMODAL BIOMARKERS FIT INTO FDA’S REGULATORY OVERSIGHT
For a new drug to be approved for marketing in the United States, the Food and Drug Administration (FDA) must determine that the drug is safe and effective for use under the conditions prescribed, recommended, or suggested in the product’s labeling (21 U.S.C. 355(d)). Mary Thanh Hai, the Deputy Director for Clinical in the Office of New Drugs Immediate Office at the FDA, provided an overview of FDA’s regulatory framework for drug approval and how biomarkers can contribute to this framework. For drug approval, the FDA requires that a sponsor demonstrate “substantial evidence of effectiveness” (FDA, 2019). Efficacy of a drug for the treatment of condition can be a measure of how a patient functions, feels, or survives. Alternatively, a drug may show efficacy through a validated surrogate endpoint shown to predict a specific clinical benefit. There is also an expedited drug approval process, known as the Accelerated Approval Program,3 in which a drug can show efficacy through a validated surrogate endpoint or intermediate clinical endpoint that is reasonably likely to predict a clinical benefit that would require a post-approval trial to verify this clinical
___________________
3 For more information on the FDA’s Accelerated Approval Program, see here: https://www.fda.gov/drugs/nda-and-bla-approvals/accelerated-approval-program (accessed June 28, 2023).
benefit.4 It is possible that a biomarker could act as a surrogate endpoint, but there are other roles that a biomarker could play in the drug approval process.
Thanh Hai described the BEST (Biomarkers, EndpointS, and other Tools) resource, which was developed in 2016 by an FDA–National Institutes of Health (NIH) working group to harmonize the terms that are used when discussing biomarkers and how they are used (FDA-NIH Working Group, 2016) (see Figure 2-1). The BEST resource defines a biomarker as
a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathologic processes, or biological responses to a therapeutic intervention, and includes a surrogate endpoint.
When using a biomarker during the drug approval process, Thanh Hai said, the sponsor needs to be very specific about its context of use and provide evidence to support its use (Margolis, 2016). A biomarker can become qualified for that context of use by going through the rigorous Biomarkers Qualification Program,5 which demonstrates its validity and reliability.
There are several reasons to develop multimodal biomarkers rather than those that are unimodal, Thanh Hai said. First, many diseases (e.g.,
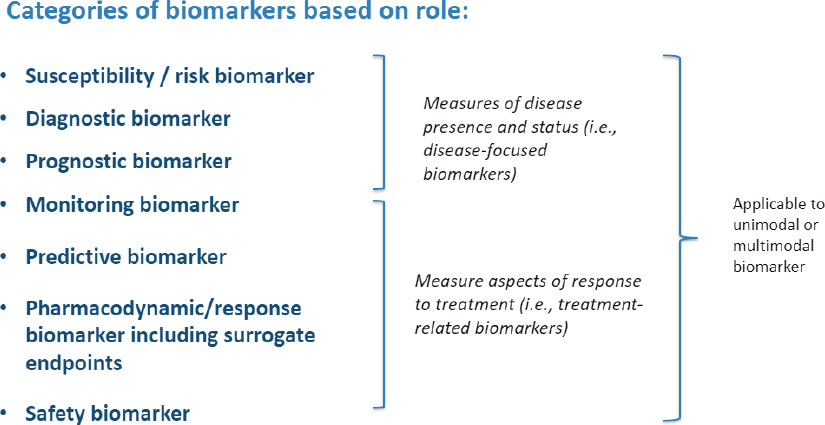
SOURCE: Presented by Mary Thanh Hai, March 13, 2023; FDA-NIH Working Group, 2016.
___________________
4 For more information about the FDA’s Demonstrating Substantial Evidence of Effectiveness for Human Drug and Biological Products Guidance for Industry, see here: https://www.fda.gov/media/133660/download (accessed June 28, 2023).
5 To learn more about the Biomarker Qualification Program, see here: https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/biomarker-qualification-program (accessed on May 22, 2023).
cardiovascular disease) are complex with a wide range of genetic, environmental, and lifestyle factors that can yield a variety of potential biomarkers as well as therapeutic approaches. Second, diseases present on a continuum and multimodal biomarkers can be used to better describe the stages of disease. For example, natural history studies of type 1 diabetes over the past 30 years have shown that autoantibodies to pancreatic islet cells can act as an effective biomarker because they predict diabetes years before clinical symptoms arise. Third, diseases may have a known biological pathway, but have different clinical presentations. For example, Fabry disease is due to a mutation in the gene that encodes alpha galactosidase A, an enzyme that breaks down lipids. It is a rare disease that affects multiple organs, making drug development and clinical trials difficult; better biomarkers are needed that are based on defects in the enzyme itself in different types of patients.
In March 2022, the FDA held a workshop to develop consensus definitions for multimodal biomarkers (FDA, 2022). Thanh Hai said that no consensus has yet been reached, but provided a proposed definition for multimodal biomarker:
a defined characteristic or characteristics that includes features based on two or more measurements evaluated through an algorithm as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions and environmental exposures.
She noted that multimodal biomarkers will be subject to the same evaluation criteria as unimodal biomarkers for analytical validation; clinical validation; clinical utility for diagnosis, treatment, or prevention of disease; and qualification to be deemed reliable in a given context of use. However, they will face additional challenges in meeting the criteria and raise questions that unimodal biomarkers do not, including: Does each component have to be independently validated? What if the different components change in different directions? Which component biomarker is the most critical? Given such, the amount of data required to validate a multimodal biomarker will be more than what is required for unimodal biomarkers. Thanh Hai concluded by saying that there are important opportunities for multimodal biomarkers, but that there will be no “one-size-fits-all” approach to validating them. Moreover, collaboration among leaders across sectors (e.g., patient groups, academia, industry, and regulators) will be necessary, she added.





