Multimodal Biomarkers for Central Nervous System Disorders: Development, Validation, and Clinical Integration: Proceedings of a Workshop (2023)
Chapter: 3 Exploring the State of the Science of Multimodal Biomarkers for Central Nervous System Disorders
Developing multimodal biomarkers for central nervous system (CNS) disorders faces several key challenges, said Samantha Hutten, Director of Discovery and Translational Research at the Michael J. Fox Foundation for Parkinson’s Research. These include the diversity of biomarkers that will be needed; a lack of consensus on what should be measured and how; limited access to well-characterized clinical samples; lack of rigorous replication and validation; a need for collaboration between data scientists and those with disease-specific expertise; and a need to center the patient in these discussions. However, there are a few CNS diseases for which there has already been significant development of multimodal biomarkers, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD). Workshop participants explored lessons learned from the progress in these diseases about how to generate and use multimodal biomarkers and how to overcome potential challenges.
ALZHEIMER’S DISEASE
Clifford Jack, a professor of radiology and the Alexander Family Professor of Alzheimer’s Disease Research at the Mayo Clinic, provided a history of the diagnosis of AD and how our understanding of the disease has changed since the early 1980s. At that time, researchers believed that patients would either be normal (with no pathology in the brain at autopsy) or show dementia (and have pathology). He cited the 1984 National Institute of Neurological and Communicative Diseases and Stroke and Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria that stated that AD can only be reliably diagnosed at autopsy but said that AD was frequently diagnosed clinically based on non-specific cognitive impairment
or dementia (McKann et al., 1984). In the 1990s, it became clearer that there was a wide range of pathology related to aging and dementia and that AD symptoms were not specific to AD. He said, “AD is one of the pathologies that can be associated with dementia in the aging brain, but many others can as well . . . Co-occurrence is the rule, not the exception.”
More recently, the “biomarker era,” as Jack referred to it, has arisen. Biomarkers for AD include those that detect amyloid (Aβ42) and p-tau in cerebrospinal fluid (Andreasen et al., 1999); PiB-PET (Pittsburgh compound B positron emission tomography) brain imaging that detects beta-amyloid plaques (Klunk et al., 2004); and tau PET (positron emission tomography), brain imaging that can detect deposits of tau protein. These biomarkers have been used as diagnostic criteria and for selecting patients for research cohorts (Albert et al., 2011; McKann et al., 2011; Sperling et al., 2011). The National Institute on Aging (NIA) and Alzheimer’s Association (AA) developed the NIA-AA research framework in 2018 that integrated these biomarkers and held as a core principle that AD should be defined by its biology, not its symptoms (Jack et al., 2018). Following this framework, patients can now be sorted by their diagnostic profile based on an AT(N) biomarker grouping (Table 3-1). For example, a patient with a profile of A+/T-/N- is positive for amyloid plaques, negative for tau deposits, and negative for neuronal injury or neurodegeneration (Jack pointed out that the A and T biomarkers are specific to AD, while N is not). This framework, said Jack, also represents a loose disease staging as most patients show amyloid plaques, then tau deposits, then dementia.
Jack described an ongoing challenge in linking these biomarkers with the clinical progression of disease from unimpaired to mild cognitive impairment to dementia. There is disagreement in the field about whether it is more appropriate for a diagnosis of AD to follow the biology or the clinical presentation. He shared an example of a patient who presented with progressive amnestic dementia and was diagnosed with AD. However, her brain imaging was negative for amyloid plaques and tau deposits, and she was diagnosed with LATE disease.1 The reverse can also occur, Jack said, a second patient had a psychiatric disorder; however, she had an abnormal brain imaging for amyloid and tau. Therefore, this patient did not have a functional disorder but AD.
The NIA-AA framework could be updated, according to Jack.2 There are now plasma-based biomarkers for amyloid plaques (Ovod et al., 2017;
___________________
1 Limbic-predominant age-related TDP-43 encephalopathy or LATE disease is a form of dementia caused by misfolding of the protein, TDP-43. For more information, see here: https://www.nia.nih.gov/health/what-limbic-predominant-age-related-tdp-43-encephalopathy-late (accessed June 28, 2023).
2 An updated draft of the NIA-AA framework was released on July 15, 2023. For more information, see here: https://aaic.alz.org/nia-aa.asp (accessed July 16, 2023)
TABLE 3-1: Integrating Cognitive Staging with Biomarker Profiles
| Cognitive stage | ||||
|---|---|---|---|---|
| Cognitively Unimpaired | Mild Cognitive Impairment | Dementia | ||
| Biomarker Profile | A- T- (N)- | normal AD biomarkers. cognitively unimpaired | normal AD biomarkers with MCI | normal AD biomarkers with dementia |
| A+ T (N) | Preclinical Alzheimer’s pathologic change | Alzheimer’s pathologic change with MCI | Alzheimer’s pathologic change with dementia | |
| A+ T+ (N)- | Preclinical Alzheimer’s disease | Alzheimer’s disease with MCI(Prodromal AD) | Alzheimer’s disease with dementia | |
| A+ T+ (N)+ | ||||
| A+ T (N)+ | Alzheimer’s and concomitant suspected non Alzheimer’s pathologic change, cognitively unimpaired | Alzheimer’s and concomitant suspected non Alzheimer’s pathologic change with MCI | Alzheimer’s and concomitant suspected non Alzheimer’s pathologic change with dementia | |
| A- T+ (N)- | non-Alzheimer’s pathologic change, cognitively unimpaired | non-Alzheimer’s pathologic change with MCI | non-Alzheimer’s pathologic change with dementia | |
| A- T- (N)+ | ||||
| A- T+ (W+ | ||||
NOTES: Patients can be categorized based on biomarker profiles, including presence or absence (+/-) of amyloid plaques (A), tau protein (T) and markers for neurodegeneration (N). These biomarker profiles can be analyzed in combination with cognitive symptoms to assess Alzheimer’s disease (AD) staging from cognitively unimpaired to mild cognitive impairment (MCI) to dementia. There are three biomarker categories: normal AD biomarkers (no color), non-AD pathologic change (dark gray), and in the AD continuum (light gray).
SOURCE: Presented by Clifford Jack on March 13, 2023; Jack et al., 2018.
Nabers et al., 2018; Nakamura et al., 2018), and neurodegeneration (Lewczuk et al., 2018; Mattsson et al., 2017, 2019; Mielke et al., 2019; Zhou et al., 2017) that are cheaper, less invasive, and more accessible to patients. Jack argued for these to be integrated into the research framework, but with an understanding that fluid biomarkers are not necessarily equivalent to imaging. He also said that the field is entering a new era in which disease-modifying treatments are now available, so the framework will need to be applied in that new clinical context.
PARKINSON’S DISEASE
There are many ways that multimodal biomarkers for PD can be used in clinical drug development. Kirsten Taylor, the Group Leader of Neurocognitive and Digital Biomarkers in the Biomarkers and Translational Technologies section of the Neuroscience and Rare Disease Department at the F. Hoffmann-La Roche, Ltd. Pharma Research and Early Development Organization, provided an industry perspective on drug development for PD and
focused on four key types of biomarkers: target engagement biomarkers; diagnostic biomarkers; prognostic biomarkers; and disease progression or treatment response biomarkers. She said that it is critical that biomarkers be valid and robust so that companies can use the information to make decisions about whether and how to proceed through the phases of clinical trials. Taylor emphasized that a useful biomarker would measure a valid target (e.g., for PD, a key target is pathologic alpha-synuclein); show high test-retest reliability; be relatively sensitive to confounding factors (e.g., del Campo et al., 2012); have good standardization across clinical sites (e.g., for brain imaging); and be specific for the disease of interest. They would also be based on findings that can be replicated in independent datasets, that are shown in the target population of the intended clinical trial and are drawn from robust sample sizes. A company will consider all these factors and conduct a risk-benefit analysis about which biomarkers it might use in its clinical trials.
Taylor summarized recent work to identify additional biomarkers for PD. Some key biomarkers are not yet available, including PET tracers for brain imaging of pathological alpha-synuclein and robust assays for pathogenicity-relevant post-translational modifications of alpha-synuclein. However, there have been advances in potential read-outs of aggregated alpha-synuclein in cerebrospinal fluid (Shahnawaz et al., 2020), in prognostic tools that incorporate multiple types of data (Heinzel et al., 2019), and in brain imaging of dopamine transporter in the striatum (Schwartz et al., 2004). Still, there is a lack of biomarkers for disease progression or treatment response that show changes over time and are linked to clinical outcomes. A key challenge is that PD biomarkers show high variability and little change, said Taylor, so it is difficult to detect changes over time.
According to Taylor, multimodal biomarkers have the potential to provide a more holistic quantification of neurodegeneration, to identify biologically meaningful subgroups of PD patients, and to increase the signal to noise ratio. However, biomarkers are needed that are accessible to the patient population and that capture clinically meaningful outcomes. Taylor said that the most promising modalities are fluid/tissue tests, imaging using PET and single-photon emission computed tomography (SPECT), and magnetic resonance imaging (MRI) of the substantia nigra. There is also a potential role for digital biomarkers. For example, the Movement Disorders Society’s Unified Parkinson’s Disease Rating Scale provides a clinical framework, but it depends on infrequent evaluation of motor symptoms that are highly variable (Regnault et al., 2019; Zolfaghari et al., 2022). Taylor presented unpublished data showing that a simple finger-tapping test at home each day on a smartphone identified a change in treatment in an individual with PD (Figure 3-1).
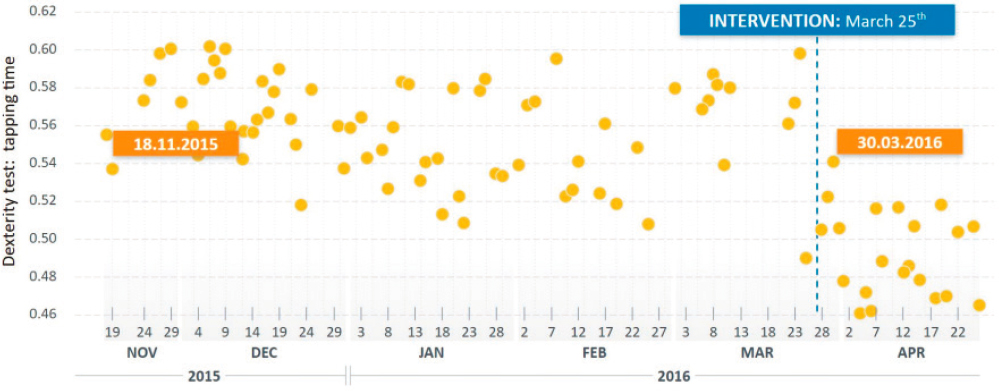
NOTES: An individual with PD was asked to perform a finger tapping test on a smartphone every day. Following a treatment intervention, finger tapping dexterity improved. Although these tests show high variability, the number of tests and their frequency yields a useful signal of improvement.
SOURCE: Presented by Kirsten Taylor, March 13, 2023 (unpublished data).
HUNTINGTON’S DISEASE
“Huntington’s disease is the prototypical autosomal dominant genetic disease,”3 said Jane Paulsen, a professor at the University of Wisconsin School of Medicine and Public Health. The gene responsible for the disease (huntingtin) was discovered in 1993 and has been found in many natural history studies of HD that have been conducted since the 1990’s, including the Cooperative Huntington Observational Research Trial (COHORT) beginning in 1995, Neurobiological Predictors of Huntington’s Disease (PREDICT-HD) in 2000, the European study REGISTRY in 2002, TRACK in 2008, and Enroll-HD, which began in 2012 worldwide and continues today (Biglan et al., 2013; Reilmann et al., 2014). HD has long been diagnosed using the Unified Huntington’s Disease Rating Scale, which is based on clinical evaluation of motor function, cognitive function, behavioral abnormalities, and functional capacity. However, this scale does not address the pre-symptomatic or prodromal phase of the disease in which a patient is known to have the gene variant associated with disease, but little or no clinical presentation (Ross et al., 2019). A working group of expert representatives from industry and academia recently developed an Integrated Staging System that integrates HD biomarkers, including presence of the gene, with clinical symptoms (Figure 3-2) (Tabrizi et al., 2022).
___________________
3 An autosomal dominant genetic condition occurs when a variant is present in only one allele or copy of a given gene and that one copy is enough to cause the disorder. For more information, see here: https://www.genome.gov/genetics-glossary/Autosomal-Dominant-Disorder (accessed June 28, 2023).
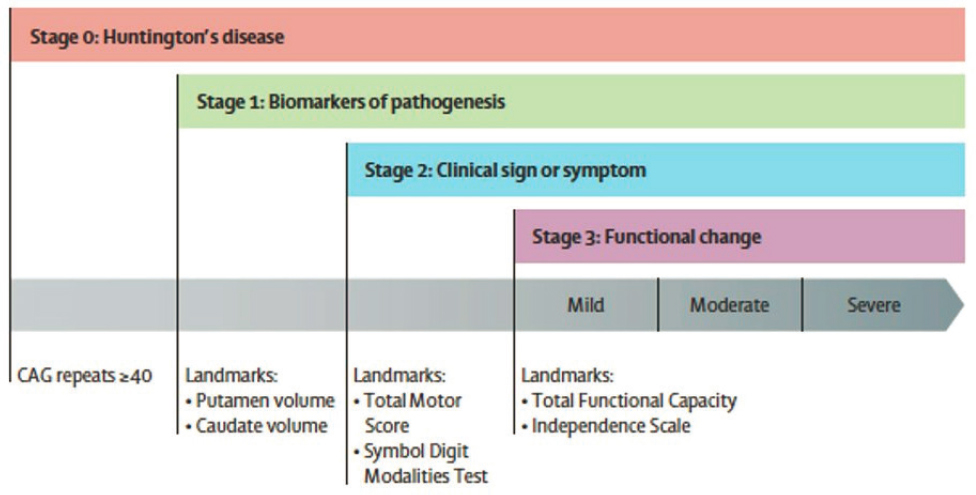
NOTE: Multiple biomarkers, including CAG repeats in the huntingtin gene and brain imaging to determine volume of putamen and caudate, are integrated with clinical symptoms and functional change into a staging system for HD.
SOURCE: Presented by Jane Paulsen on March 13, 2023; Tabrizi et al., 2022.
Paulsen described studies that showed the strong relationship between the number of CAG repeats in the HD gene and the age of onset of the disease (Langbehn et al., 2004, 2010; Penney et al., 1997; Zhang et al., 2011b). However, genetic and other factors also play a role (GeM-HD Consortium, 2015; Hong et al., 2021; Lee et al., 2022) and these have been integrated, through a collaborative process, into a multimodal prognostic biomarker to define subgroups for clinical trials (Tabrizi et al., 2022). There have been multiple biomarkers for HD that are based on blood or cerebrospinal fluid (Caron et al., 2022), Paulsen said. Although some have been integrated into useful measures (e.g., the presence of mutant huntingtin protein and neurofilament light chain in cerebrospinal fluid [Rodrigues et al., 2020]), they may fail to detect changes over time, limiting their utility for assessing disease progression and treatment. For brain imaging, there are currently no PET tracers that detect mutant huntingtin protein itself, but structural changes and abnormalities can be detected using other types of PET scans (Aylward et al., 2011a,b) and resting state MRI (Casella et al., 2020; Estevez-Fraga et al., 2020; Pini et al., 2020; Tan et al., 2021; Wijeratne et al., 2021). Paulsen emphasized that the scientific field has been prolific, but that it needs to work on its methodology, standardization, and transparency to make progress in developing these multimodal biomarkers and to make sure that researchers “compare apples to apples instead of apples to oranges.”
LESSONS LEARNED FOR DEVELOPING MULTIMODAL BIOMARKERS
Samantha Hutten shared that there has been progress in CNS multimodal biomarker development in AD, HD, and PD. For each of these disorders, there have been challenges in combining biomarkers into multimodal signatures that has needed to be addressed. Linda Brady highlighted that the lessons learned from AD, HD, and PD could help inform the development of biomarkers for other CNS disorders.
Equitable Access
Charisse Winston, an assistant project scientist at the University of California, San Diego, said that biomarkers that are non-invasive, accessible, and not time-consuming would best serve the patient population and would ultimately be used by those that need them. A workshop participant raised the issue of access as an ethical matter, particularly because some racial groups may not trust the medical establishment and so may be hesitant to participate in invasive procedures. Winston recounted the history of mistrust, particularly among African Americans, and said that the scientific and medical communities need to make intentional efforts toward engagement. Mary Thanh Hai mentioned that the Food and Drug Omnibus Reform Act of 20224 requires that sponsors develop a diversity plan, and currently the Food and Drug Administration (FDA) is assessing how to ensure that clinical trial patient populations are representative and that essential biomarkers are accessible.
Collaboration to Generate the Data Needed for Development and Validation of Multimodal Biomarkers
One topic that resonated among several workshop participants was the need for a range of data and collaborative studies to develop reliable multimodal biomarkers. Clinical trial data are not enough, said Thanh Hai; cohort studies, natural history studies, and epidemiological data will also be important. Natural history studies have been very important in the development of biomarkers for AD, said Jack, but he emphasized that AD has the advantage that it is very common, so researchers can follow relatively unbiased cohorts of people and track AD progression. This approach would not work for other less prevalent CNS disorders. When asked how researchers can avoid scientific biases in studies of psychiatric disorders, which often lead
___________________
4 To learn more about the Food and Drug Omnibus Reform Act of 2022, see here: https://www.congress.gov/117/bills/hr2617/BILLS-117hr2617enr.pdf (accessed May 22, 2023).
to results that are not replicable, Thanh Hai said one size will not fit all and that researchers could engage with the FDA on specific therapeutic areas, for example, through Prescription Drug User Fee Act (PDUFA) Formal Meetings (FDA, 2017). She acknowledged that this is a difficult question that could require years of engagement. Jack said, “Every study has biases. . . the more you ask a research volunteer to do, the greater the bias,” but he emphasized that natural history studies, even with biases, are important. Winston added that studies that draw on community clinic populations can be useful, particularly for diseases that are prevalent in those communities (e.g., diabetes).
Thanh Hai encouraged people to engage with the FDA if they have a potential biomarker and to work with the Biomarker Qualification Program; although it may take a long time to get a biomarker qualified, it is important to begin the process of sharing data, particularly for rare diseases. She added that different groups and companies could prospectively plan to collaborate and pool data to validate biomarkers. Paulsen also urged better collaboration and standardization of biomarker data acquisition and methodology, as well as the language that is used to describe clinical trials, so that findings can be better understood and applied.
Using Digital Tools for Multimodal Biomarkers
The need for more sensitive and reliable biomarkers was emphasized by Indu Navar, the founder of EverythingALS, who lost her husband to amyotrophic lateral sclerosis (ALS). She said that she and her husband were “shocked by how backward we are” when doctors asked them to report his walking and speech abilities on scales of zero to four instead of using continuous monitoring using sensors and digital tools to gather granular information. She believed that digital biomarkers would have enabled a faster diagnosis. She stressed the importance of establishing digital biomarkers for early diagnosis and more accurate clinical end points for drug trials.
Taylor discussed the promise of digital tools, including the use of smartphones, as a way to address test-retest variability. She acknowledged that indicators of PD, such as tremor, often have a high degree of variability, but said that smartphones can be used to take more frequent measurements that can be aggregated into a more stable signal. Thanh Hai cautioned that it is more accurate to refer to “digitally derived biomarkers” than to “digital biomarkers,” and said that the same principles and process for validation or qualification would apply to digitally derived biomarkers that apply to traditional biomarkers, but the evidence may be different. Jack said that digitally derived biomarkers are an extension of clinical assessment (e.g., for AD, they can be used to assess gait) and can be useful because they are less burdensome to the patient and can take measurements over longer time
frames than can be achieved in the clinic. Paulsen added that, for HD, the level of variability in these tests can itself indicate onset of disease.
Disease Specificity of Multimodal Biomarkers
Given the mixed and complex pathologies and heterogeneity of CNS disorders, several workshop participants highlighted the usefulness of both specific and non-specific biomarkers in clinical care. Taylor believes that the usefulness of a biomarker depends on its context of use, but that if a researcher or clinician hopes to target a specific process or disease for intervention, then they will need disease-specific biomarkers. Jack agreed and spoke about the use of biomarkers in AD, saying that the field uses both “core” and “non-core” biomarkers. Core biomarkers are specific to AD (e.g., amyloid plaques and tau) and non-core are not specific (e.g., neurofilament light chain, hippocampal shrinkage). But, he said, only core biomarkers are appropriate to use for diagnosis: “if you are going to treat a disease, if you’re going to modify the course of a disease, you must use disease-specific biomarkers.”









