Genesis: The Scientific Quest for Life's Origin (2005)
Chapter: Part II The Emergence of Biomolecules -- 6 Stanley Miller’s Spark of Genius
Part II
The Emergence of Biomolecules
The top-down study of life’s origin—via the examination of ancient fossils—doesn’t tell us much about early biochemistry. Fossil cells, molecules, and isotopes are indistinguishable from those of contemporary life-forms; consequently, from them we can gain detailed knowledge only of the final state—modern cellular life.
Nevertheless, common sense points to the essential first step in life’s emergence—the synthesis and accumulation of abundant organic (that is, carbon-based) molecules, such as amino acids, sugars, and other vital molecules of which life would eventually be made. In the beginning, life’s raw materials consisted of water, rock, and the simplest and most basic volcanic gases—carbon dioxide, nitrogen, and maybe a little methane and ammonia. Add energy to the mix and organic molecules emerge.
The experimental pursuit of this ancient process, arguably the best understood aspect of life’s chemical origins, began in earnest just over a half-century ago, with the pioneering studies of University of Chicago graduate student Stanley Miller and his distinguished mentor, Harold Urey. Together they demonstrated the organic synthesis that occurred as Earth’s primitive atmosphere and ocean were subjected to bolts of lightning and the Sun’s intense radiation. Chemical processes in deep space, in the solar nebula, during asteroid and comet impacts, and even within the pressure cooker of Earth’s deep interior, also generated abundant organic molecules. By 4 billion years ago, Earth’s globe-spanning ocean must have become a complex, albeit dilute, soup of life’s building blocks. Though not alive, this chemical system was poised to undergo a sequence of increasingly complex stages of molecular organization and evolution.
6
Stanley Miller’s Spark of Genius
The idea that the organic compounds that serve as the basis of life were formed when the earth had an atmosphere of methane, ammonia, water, and hydrogen instead of carbon dioxide, oxygen, and water was suggested by Oparin and has been given emphasis recently by Urey and Bernal. In order to test this hypothesis an apparatus was built….
Stanley L. Miller, Science, May 15, 1953
The experimental investigation of life’s origin is a surprisingly recent game. Two centuries ago, many scientists accepted the intuitively reasonable idea, championed by Aristotle and a pantheon of other ancient scholars, that a life force permeates the cosmos. A central precept of this doctrine, known as vitalism, is that life arises spontaneously all around us, all the time. The question of life’s origin wasn’t asked—at least not in the modern experimental sense.
Think about your own experience and you’ll see why this idea of spontaneous generation isn’t such a strange notion. Mold seems to grow spontaneously on bread, maggots appear as if by magic in old meat, and every spring new plants sprout and grow in the season of renewal. It’s not surprising that so many people, for so long, accepted unquestioningly the spontaneous generation of life.
This view continued well into the nineteenth century, though not without a number of respected dissenters and considerable debate. The seventeenth-century invention of the microscope and the subsequent realization that microscopic life abounds complicated the story, but failed to resolve the controversy. Those who favored spontaneous generation saw microbes as just another manifestation of the life force;
those opposed saw microscopic life as an obvious source of experimental contamination.
The interpretation of experiments is rarely unambiguous, as highlighted by a delicious eighteenth-century exchange. The noted Italian physiologist Lazzaro Spallanzani explored spontaneous generation by comparing the behavior of boiled and unboiled sealed flasks, each filled with bacteria-infested, nutrient-rich water. He found that flasks boiled for an hour remained sterile indefinitely as long as they remained sealed, whereas unboiled flasks quickly became cloudy with microbes. Spallanzani concluded that ubiquitous microscopic life-forms contaminate any unsterilized experiment.
Englishman John Needham, an astute amateur experimenter who conducted his own fragrant experiments on hot mutton gravy, came to a different conclusion. Needham agreed that boiling kills microbes, but microbes soon reappeared in abundance when his gravy cooled. He argued that these cells arose by spontaneous generation. Spallanzani countered that Needham’s microbes came from airborne contamination and, to prove his point, he undertook a new series of experiments in which he demonstrated that no microbes appeared when he pumped air out of his flasks and then boiled the water. Needham disagreed: A property of the air, not the water, must carry the life force, he said.
We react to this historical incident with a biased worldview. Of course Spallanzani’s conclusions about microbial contamination were right and Needham’s support of spontaneous generation was wrong, we are tempted to say. But a naïve and impartial observer of the time, faced with these conflicting claims, would have had a difficult time choosing between invisible microbes and an invisible life force. Both arguments were internally consistent, so doubts remained.
The influential French chemist Louis Pasteur helped to abolish vitalism and the theory of spontaneous generation once and for all with his own brilliant series of experiments on sterilization. He prepared a nutrient-rich sugar solution and poured it into several beakers. One set of beakers was tightly sealed to prevent any contact with the ambient air. Other beakers were left open with a narrow twisting neck, so that the sugar solution was in contact with the ambient air, but unlikely to be contaminated by stray microbes, which were unable to traverse the long glass passage. As a control, he left other beakers wide open or deliberately contaminated them with ordinary dust. He showed that boiled water, if isolated from airborne microbes, remained
sterile indefinitely. Only microbial contamination causes new growth, not spontaneous generation—a result of tremendous practical as well as theoretical consequence. His discoveries proved of immense importance in reducing the incidence of infectious diseases, while his development of the pasteurization process transformed the production and preservation of food and, perhaps more important to French merchants, beer.
In the course of his elegant work, Pasteur also contributed directly to the study of life’s origin. By introducing the dictum that no life can occur without prior life, his findings pushed back life’s origin to an inconceivably remote time and place. How could anyone make observations or perform experiments to study an event so ancient and inaccessible?
SPECULATION
In 1871, Charles Darwin penned a speculative letter to his friend, the botanist Joseph Hooker. “If (and Oh! What a big if!),” he wrote, “we could conceive in some warm little pond, with all sorts of ammonia and phosphoric salts, light, heat, electricity, etc., present, that a protein compound was chemically formed ready to undergo still more complex changes….” Many contemporary chemists must have agreed that life’s origin, wherever and however it occurred, depended on three key resources.
First and foremost, terrestrial life requires liquid water, the essential growth medium for all living things. All living cells, even those that survive in the driest desert ecosystem, are formed largely of water. Our bodies are typically 70 percent water, while many foods are even more water-rich. Surely the first cells arose in a watery environment.
Life also needs a ready source of energy. The radiant energy of the Sun provides the most obvious supply for life today, but bolts of lightning, asteroid impacts, Earth’s inner heat, and the chemical energy of certain unstable minerals have also been invoked as life-triggering energy sources.
And, third, life depends on a variety of chemical elements. All known living organisms consume atoms of carbon, oxygen, hydrogen, and nitrogen, with a bit of sulfur and phosphorus and other elements as well. Atoms of these elements combine in graceful geometries to form essential biomolecules.
In spite of the intrinsic importance of the topic, Darwin’s private musings about a chemical origin of life (which he never published) flew in the face of conventional theology. “In the beginning, God created” was sufficient for most people, including a majority of the most distinguished scholars of the time. Not until the 1920s did such scientific speculation take a more formal guise.
Most notable among that pioneering modern school of origin theorists was the Russian biochemist Alexander Oparin. In 1922, while still in his twenties and under the scrutiny of the Soviet hierarchy, Oparin elaborated on the idea that life arose from a body of water that gradually became enriched in organic molecules—the so-called “Oparin Ocean” or the “primordial soup.” Somehow, he posited, life emerged as these molecules clustered together and self-organized into a chemical system that could duplicate itself. In many other cultures, where religious doctrine colored thinking on origins, these revolutionary ideas would have been seen as heretical, but Oparin’s ideas resonated with the materialist philosophical worldview of the Soviet leadership.
Many of Oparin’s postulates were echoed in 1929 in the independent ideas of British biochemist and geneticist J. B. S. Haldane. Haldane, himself a Marxist activist, contributed a brief, perceptive (many contemporaries would have said radical) article entitled “The Origin of Life” to the eclectic secularist British periodical The Rationalist Annual. He speculated on the production of large carbon-based molecules under the influence of the Sun’s ultraviolet radiation. Given such a chemical environment, Haldane envisioned the first living objects as self-replicating, specialized molecules.
Oparin and Haldane offered original and intriguing ideas. More important, their ideas were subject to experimental testing, but for some reason Oparin and his contemporaries didn’t try. It wasn’t until after World War II, a time of exuberant, can-do scientific optimism, that two chemists demonstrated a systematic experimental approach to prebiotic chemistry.
EXPERIMENTS
The apparently universal requirements of water, energy, and chemicals hint at a simple experimental approach for studying the origin of life. Such landmark experiments were devised early in the 1950s by Univer-
sity of Chicago chemist Harold Urey, a Nobel Prize winner of great renown, and his unknown second-year graduate student, the 23-year-old Stanley Miller.
Jeffrey Bada, a Miller student in the 1960s and an unswerving proponent of his teacher’s ideas in subsequent years, reveals some of the behind-the-scenes context of the historic Chicago work in his 2000 book (coauthored with Christopher Wills), The Spark of Life. Urey planted the seed for the now classic experiment in the fall of 1951, when he presented a seminar attended by Miller, who was a beginning graduate student. In that lecture and related publications, Urey proposed that life-triggering organic molecules might have been produced in abundance in a plausible primitive atmosphere of hydrogen, methane, and other relatively reactive gases.
A year later, after trying his hand at an unsuccessful project in nuclear theory, Miller asked Urey if he could attempt an experiment with the hopes of synthesizing amino acids, the building blocks of proteins. At first, so the story goes, Urey was reluctant and wanted Miller to try an easier, safer research project. But after continued pressure from Miller, Urey relented and gave his student a limit of one year to make headway.
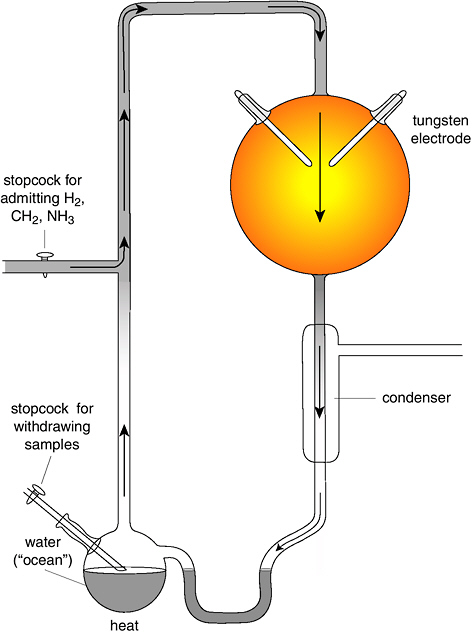
Scientists revere simple, elegant experiments, and that’s exactly what Miller and Urey devised—a primitive Earth sitting on a tabletop in a sealed glass vessel. [Plate 5] To the lower left, Miller positioned a 5-inch-diameter (300-milliliter) flask filled two-thirds with water—the primitive ocean. Glass tubing linked that flask to a 10-inch-diameter (5-liter) gas-filled flask, situated above and to the right. Miller’s atmosphere consisted of the relatively reactive gases methane (CH4, sold commercially as natural gas), ammonia (NH3, the strong-smelling component of ammonia-based cleaners), and hydrogen (H2, the explosive lighter-than-air gas of Hindenberg fame). Two pointed metal electrodes penetrated the upper flask to simulate “lightning” by sparking, and a flame gently heated the water-filled flask to mimic the ocean’s continuous evaporation. Sending sparks through a potentially explosive mix of hydrogen and methane is not for the faint of heart, nor would Miller’s unshielded glass apparatus have stood up to today’s government safety standards. But it was an elegant experimental design.
Following academic tradition, Miller the student performed the experiments, while his mentor waited in the wings. Miller began by
pumping out the system to remove any traces of atmospheric oxygen. Then he filled his apparatus with water and a 2:2:1 gas mixture of methane, ammonia, and hydrogen. He heated the water, set off tiny electric sparks in the gases to simulate lightning, and waited.
When the experiment began, the water was pure and clear, but within a couple of days the solution had turned yellowish and a black residue had begun to accumulate near the electrodes. It’s easy to imagine Stanley Miller’s excitement as he cut his first experiment short to analyze the tantalizing products. Had he produced amino acids?
Organic chemical analysis was no easy chore in the early 1950s. Miller resorted to paper chromatography, a classic and relatively quick technique that separates different molecules into discrete colored spots on absorbent paper. Miller opened the valve and removed the yellow solution. Concerned that microbes might begin to grow in the liquid and confuse his results, he added a lethal dose of mercury chloride to preclude any bacterial contamination. Then, after the tedious routine of drying down and concentrating his sample, he placed a small drop of concentrated run product near one corner of the chromatography paper. It dried as a small yellow dot.
Miller suspended the paper above a narrow trough filled with a freshly made alcohol–water solution. He carefully lowered the paper so that one edge close to the yellow spot just barely dipped into the clear solution, which gradually soaked into the paper and rose up the sheet by capillary action. As the alcohol solution moved, it carried the unknown molecules along with it. Within a few minutes the chemical spot had been smeared out into a narrow, 3-inch-long streak of unknown, mostly invisible chemicals.
Miller let his paper dry, rotated it 90 degrees, and repeated the process with a second solvent, this time a phenol solution. Molecules move differently through paper under the influence of different solvents, so this second step spread the 3-inch streak of chemicals into a diagnostic two-dimensional pattern of spots. After a second drying, Miller’s final step was to treat the paper with ninhydrin, a chemical that stains otherwise invisible amino acids into distinctive colors.
Almost immediately a discrete purple spot appeared exactly at the expected position for glycine (C2H5NO2), the simplest of life’s amino acids. Starting with nothing more than water and a few simple gases, Miller had made one of life’s essential biomolecules. The graduate stu-
dent and his advisor were elated, for the tabletop experiment had worked much faster than they expected.
Miller repeated the entire experiment for a week’s duration, cranking up the heat to a slow boil. The results were amazing. The water quickly yellowed, then gradually turned intriguing shades of pink and eventually to a deep red, while black gunk oozed down the sides of the larger flask. This time when Miller analyzed the contents of the flask, he found a complex mixture of organic molecules including at least a half-dozen different amino acids. Reactions of water and air had produced organic molecules in abundance.
Harold Urey encouraged the young investigator to write up his results immediately. Miller obliged and submitted a short manuscript to the high-profile journal Science in mid-February 1953, a scant five months since the project’s inception. Urey also generously withdrew his name from the paper so that the graduate student, not the Nobelist, would receive the lion’s share of credit.
Stanley Miller’s first publication was a bombshell. His two-page article in the May 15, 1953, issue of Science announced “A Production of Amino Acids Under Possible Primitive Earth Conditions.” The press had a field day. The New York Times printed a feature story, LIFE AND A GLASS EARTH, while tabloids speculated about synthetic life crawling out of test tubes.
THE COTTAGE INDUSTRY OF PREBIOTIC CHEMISTRY
The Miller–Urey experiment transformed the science of life’s chemical origins. For the first time, an experimental protocol mimicked plausible life-forming processes. For decades to come, Miller and his students would dominate the origin-of-life community.
Given such an exciting result, other groups jumped at the chance to duplicate the amino acid feat. Independent confirmation of Miller’s claims came within a few years from chemists at the University of Bristol in England and at the Carnegie Institution in Washington, D.C. (A careful search of the scientific literature also turned up a remarkably similar series of experiments that had been conducted decades earlier by the German chemist Walter Löb.) Thousands of subsequent experiments during the past half century have outlined the promise, as well as the limitations, of this idea that life arose as a chemical process at the surface of the Earth. Time and time again, variations of the
Miller–Urey process, including experiments using ultraviolet radiation, different gas mixtures, powdered minerals, and more, have demonstrated the synthesis of life’s most basic building blocks. Relatively easy to run, and now a relative cinch to analyze, these experiments continue to be a sort of cottage industry in origin-of-life research circles.
This genre of experiments yields amazing results. Dozens of amino acids have been synthesized from scratch, along with membrane-forming hydrocarbons, energy-rich sugars and other carbohydrates, and metabolic acids. The early inventories even included some of the building blocks of the genetic molecules DNA and RNA, though at first the 5-carbon sugar ribose (the “R” of RNA) and the two purines essential to both, adenine (C5H5N5) and guanine (C5H5ON5), were notably absent.
Much of this molecular diversity occurs because electric sparks and ultraviolet radiation trigger the formation of highly reactive chemical species, such as hydrogen cyanide (HCN) and formaldehyde (CH2O), which readily link to other molecules. Miller suspected, for example, that most of the amino acids produced in his experiments arose by a chemical process known as Strecker synthesis, in which hydrogen cyanide reacts with formaldehyde and ammonia.
Enthusiasm grew as other scientists discovered promising new chemical pathways. In 1960, John Oró of the University of Houston turned scientific heads when he discovered that a concentrated hydrogen–cyanide solution, when heated, produced lots of adenine, one of the missing purines and a crucial biomolecule that also plays a role in metabolism. Other chemists conducted similar experiments, starting with relatively concentrated solutions of formaldehyde (CH2O), a molecule thought to be common in some prebiotic environments. These experiments produced a rich, though random, variety of sugars, including a modest yield of ribose. Gradually, through such specialized experiments, gaps in the prebiotic inventory of life’s molecules were filled in.
The experiments of Oró and others, relying as they did on relatively concentrated solutions of reactive organic molecules, raised some eyebrows. The Miller-type spark experiments by themselves had never yielded hydrogen cyanide in sufficient concentrations to produce much adenine, or enough formaldehyde to make much ribose. But discoveries in the mid-1960s in the lab of Salk Institute pioneer Leslie Orgel pointed to a plausible fix. The early Earth was not uniformly hot; it
probably had ice caps and may have periodically experienced more extensive “ice ages,” as well. Orgel realized that such conditions might promote intriguing organic reactions. When an organic-rich water solution freezes, pure water ice crystals grow, while the dwindling reservoir of residual liquid becomes an increasingly concentrated organic brine (and recall that a higher concentration of “agents” may facilitate emergence).
Orgel and co-workers exploited this idea by slowly freezing flasks of dilute HCN solutions to −20°C. The procedure produced tiny volumes of extremely concentrated hydrogen cyanide, which reacted over weeks to months to produce small linkages of up to four HCN molecules. This curious phenomenon became the inspiration for one of the longest experiments in the history of origins research. Sometime in the mid-1970s, Miller, now a professor at the University of California, San Diego, and his co-workers repeated the Orgel protocol and stored their frozen flasks in the back of a freezer. In the late 1990s, more than two decades later, they removed the frozen solutions, which had developed curious dark concentrated clumps that were rich in organics. Analysis revealed an abundant production of adenine. It takes a lot of time for reactions to proceed at ultracold temperatures, but the primitive Earth had time to spare.
These remarkable results seem to defy convention: Heat, not cold, normally drives chemical reactions. You don’t make a cake by freezing batter. Nevertheless, additional freezing experiments have produced amino acids and other interesting biomolecules by this counterintuitive process of concentration. In this curious way, prebiotic cycles of freezing and thawing may have enhanced the emergence of biomolecules and thus provided a pathway to life.
DOUBTS
As exciting and important as the Miller–Urey results may be, seemingly intractable problems remain. Within a decade of Miller’s triumph, serious doubts began to arise about the true composition of Earth’s earliest atmosphere. Miller and Urey had exploited a highly reactive atmosphere of methane, ammonia, and hydrogen, which seemed a plausible early atmosphere to them. But by the 1960s, new geochemical calculations along with data from ancient rocks pointed to a much
less reactive early atmosphere of nitrogen and carbon dioxide, two gases that do almost nothing of interest in a Miller–Urey apparatus.
Miller and his supporters continue to counter with a pointed argument, difficult to dismiss. Life’s biomolecules match those of the original Miller–Urey experiment with great fidelity, they say. Doesn’t that fact alone argue for an atmosphere rich in reactive methane? Harold Urey is said to have often quipped, “If God did not do it this way, then He missed a good bet.” Nevertheless, most geochemists now discount the possibility of more than a trace of atmospheric methane or ammonia at the time of life’s emergence.
Added to this atmospheric concern is the fact that the molecular building blocks of life created by Miller and his colleagues represent only tiny steps on the long road to life. Living cells require that such small molecules be carefully selected and then linked together into vastly more complex structures—cell membranes, protein catalysts, DNA, RNA, and other so-called macromolecules. The prebiotic ocean was an extremely dilute solution of many thousands of different organic molecules, most of which play no known role in life. By what emergent processes were just the right molecules selected and organized?
The Miller–Urey scenario suffers from yet another nagging problem. Macromolecules tend to fragment, rather than form, when subjected to the energetic insults of lightning and the Sun’s ultraviolet light. These so-called ionizing forms of energy are great for making reactive molecular fragments that combine into modest-sized molecules like amino acids. Combining many amino acids into an orderly chainlike protein, however, is best accomplished in a less destructive energy domain. Emergent complexity relies on a flow of energy, to be sure, but not too much energy. Could life have emerged in the harsh glare of daylight, or was there perhaps a different, more benign origin environment?
Faced with such an impasse, a few maverick scientists began to look at other plausible venues for the cradle of life.