Genesis: The Scientific Quest for Life's Origin (2005)
Chapter: 12 Minerals to the Rescue
12
Minerals to the Rescue
But I happen to know exactly how life arose; it’s brand-new news, at least to the average layman like yourself. Clay. Clay is the answer. Crystal formation in fine clays provided the template, the scaffolding, for the organic compounds and the primitive forms of life. All life did, you see, was take over the phenotype that crystalline clays had evolved on their own.
John Updike, Roger’s Version, 1986
The first living entity emerged from interactions of air, water, and rock—the same raw materials that sustain life today. Of these three chemical ingredients, rocks—and the minerals of which they are made—have generally received little more than a footnote in theories of life’s emergence. The atmosphere and oceans have long enjoyed the starring roles in origin scenarios, while rocks and minerals sneak in and out as bit players—or simply as props—and then only when all other chemical tricks fail.
Some recent and fascinating experiments promise to change that misperception. Origin-of-life researchers have begun to realize that minerals must have played a sequence of crucial roles, beginning with the synthesis of biomolecules and during their subsequent assembly into growing and evolving structures.
The Miller–Urey chemical process works by ionizing gas molecules—blasting them with lightning or ultraviolet radiation and thereby stripping off electrons, so that small groups of atoms readily recombine into larger organic molecules. Interesting molecules inevitably emerge, but those energetic processes effectively prevent the formation of essential macromolecular structures, including the polymers and membranes required by all known life-forms. The Miller–Urey
scenario can’t have been the entire story. That’s why many researchers, especially those trained in geology, turn to rocks and minerals.
MINERALS AS PROTECTION
Rocky outcrops and overhangs—especially in tidal zones, where seawater evaporates and thus concentrates organic molecules—might have promoted macromolecular formation. Imagine a shaded cove where increasingly concentrated mixtures of organic molecules accumulated and reacted, protected by a rocky ledge from the Sun’s harmful radiation. Rocks might have served as Earth’s earliest sunblock. They may well have provided protection at a smaller scale as well. Many volcanic rocks of the early Earth were laced with countless air pockets left by expanding volcanic gases. Evaporating seawater might have deposited a rich mix of organic molecules in such tiny hollows, each like a small test tube where further reactions could proceed.
Mineralogist Joseph V. Smith, professor emeritus at the University of Chicago, envisions even smaller protected environments. He cites electron microscopy studies of weathered mineral surfaces, which often display myriad microscopic cracks and pores. Feldspar, the commonest of all rock-forming minerals, sometimes features millions of tiny weathered pockets, each the approximate size and shape of living cells, each providing a place for molecules to congregate, each pore and crack a separate experiment in molecular self-organization. [Plate 7]
POLYMERIZATION ON THE ROCKS
The production of macromolecules requires two concerted steps: The correct molecules must first be concentrated and then organized into the desired structure. In the case of lipid membranes, these two tasks occur virtually simultaneously and spontaneously; lipids in water separate and self-organize into a bilayer. But other key biological macromolecules, including proteins and carbohydrates, form from water-soluble units—amino acids and sugars. Consequently, they tend to break down, not form, in water.
One promising way to assemble such molecules from a dilute solution is to concentrate them on a surface. For decades, the prevailing paradigm has been that the molecules of life assembled at or near the ocean–atmosphere interface. The surface of a calm tidal pool, or per-
haps a primitive slick of water-insoluble molecules might have done the job. But then, as noted, these environments are open to lightning storms and ultraviolet radiation.
Origin scientists with a penchant for geology have long recognized that rocks might provide attractive alternative surfaces for concentration and assembly—a kind of scaffolding for the assembly of protolife. More than a half-century ago, the British biophysicist John Desmond Bernal advocated the special role of clays, which are ubiquitous minerals with regularly layered atomic structures.
Clays come in a wide range of compositional and structural variants, but all of them feature layers of strongly bonded silicon and aluminum atoms. The proclivity of clays to exhibit a surface electrostatic charge enhances their ability to adsorb organic molecules—a kind of molecular-scale static cling. What’s more, clays tend to occur as exceptionally fine-grained flat particles. Consequently, a palm-sized pile of ordinary clay can boast a reactive surface area greater than 1,000 square feet.
Subsequent experiments have supported Bernal’s speculations. In a 1978 study, Israeli biochemist Noam Lahav and colleagues discovered that amino acids concentrate and polymerize on clays to form small, protein-like molecules. Such reactions occur when a solution containing amino acids evaporates in the presence of clays—a situation not unlike the evaporation that dries up a shallow pond or tidal pool. Of special note is the fact that this process relies on cycles of heating and evaporation—and cycles, recall, are one of the key factors in the emergence of complexity. Patterns of daily and seasonal changes doubtless fostered the emergence of new molecular structures.
More recently, research by NASA-sponsored teams in California and New York has demonstrated that a variety of layered minerals can adsorb and assemble a variety of other organic molecules. In a tour de force series of experiments during the past two decades, chemist James Ferris and colleagues at Rensselaer Polytechnic Institute induced clays to act as scaffolds in the formation of RNA, the polymer that carries the genetic message enabling protein synthesis.
Ferris relied on the simplest of procedures. First, he prepared a solution of “activated” RNA nucleotides, each consisting of a ribose sugar bonded to a phosphate and a base, plus a reactive molecule called imidazole that promotes, or “activates,” bonding between nucleotides. Such a solution can sit on the lab bench for weeks with little change.
But sprinkle in a bit of a suitable clay mineral and the RNA pieces start to link up. In a matter of hours, lengths of 10 nucleotides form. By the end of 2-week experiments, the RPI team produced RNA strands of more than 50 nucleotides. The fine-grained clay particles had induced polymerization by a process not yet fully understood.
Buoyed by the Ferris team’s success, other origin-of-life researchers tried their hand at other biopolymers. Leslie Orgel, research professor at the Salk Institute for Biological Studies in San Diego, succeeded in forming a variety of proteinlike chains of amino acids up to several dozen molecules long. Orgel and his students discovered that different minerals preferentially select and polymerize different molecules from a water-based solution. By combining the right mineral with the right molecule, they could form polymers at will.
In conjunction with his experiments, Orgel also developed an elegant theory of “polymerization on the rocks,” in which he pointed out both the promise and problems with mineral surfaces. Minerals such as clays and hydroxides certainly can adsorb interesting biomolecules, he noted, including the amino acids and nucleotides essential to life. Furthermore, once two of these molecules are adsorbed close to each other, they have a tendency to bond. As more and more molecules are added to a lengthening polymer, however, the strand becomes more and more tightly bound to the mineral surface. How, he asks, can a polymer contribute to life if it’s stuck to the rocks?
One possible answer came from the Harvard University laboratory of geneticist Jack Szostak, who mixed together clays, RNA nucleotides, and lipids in the same experiment. Lo and behold, the clays not only adsorbed RNA, but also hastened the formation of lipid vesicles. In the process, RNA-decorated clay particles were incorporated into the vesicles. This spontaneous self-assembly of RNA-containing vesicles, though a long, long way from synthesizing life, is perhaps the closest anyone has come to forming a cell-like entity from scratch.
MORE MINERAL MAGIC
Every interaction between a mineral and a molecule requires knowledge of two different chemical entities—a crystalline solid and a tiny carbon-based cluster of atoms. Most origin-of-life experts come from the world of organic chemistry—the carbon-based biomolecule part. It’s easy to get the impression that minerals are brought into origin-of-
life experiments only when nothing else works, and then as a sort of magic powder. Atomic-scale details of the molecule–mineral interactions are usually fuzzy at best.
Gustaf Arrhenius, a senior NASA-supported researcher at the Scripps Institution of Oceanography, in La Jolla, California, has a somewhat different background. He knows organic chemistry, to be sure, but he was trained in inorganic chemistry and has a deep appreciation of crystalline solids and their structural idiosyncrasies. Consequently, he has zeroed in on the mineral group known as double-layer hydroxides. Like clays, the double-layer hydroxides are common in nature, and they come in almost limitless compositional variants, with magnesium, iron, chromium, nickel, calcium, aluminum, and many other elements mixing and matching in the double-layer structure. Additional complexity arises because small molecules—water, carbon dioxide, or a variety of other common species—occupy the spaces between the layers. By changing their chemical contents, Arrhenius has been able to fine-tune his double-layer hydroxides to perform specific chemical tasks.
Unlike Ferris and Orgel, Arrhenius did not try to form long polymers on the surfaces of his mineral powders; rather, he exploited the tendency of double-layer hydroxides to soak up small organic mol-

Gustaf Arrhenius demonstrated that double-layer hydroxide minerals have the ability to attract small molecules and catalyze their reactions into larger species of biological interest (after Arrhenius et al., 1993).
ecules in the rather large spaces between the layers. Once confined and concentrated, these small molecules tend to form larger molecular species that are not otherwise likely to emerge from the soup. His most interesting products so far have been sugar phosphates, in which a sugar molecule is linked to a phosphate group—a suggestive pairing, in that it forms the backbone of every DNA and RNA molecule.
Joseph Smith has explored yet another intriguing mineralogical wrinkle. Smith is an authority on zeolites, a diverse family of natural and synthetic crystals that feature latticelike frameworks of silicon, aluminum, and oxygen atoms. These open frameworks result in molecule-sized channels that run the length and width of the crystals. The tendency of some hydrocarbon molecules to enter these zeolite channels, while other molecules remain behind, lies at the heart of the multitrillion-dollar petroleum refining business.
Instead of looking at mineral surfaces, Smith imagines prebiotic molecules concentrating inside zeolite channels. Zeolites abound in the weathering products of volcanic rocks; organic molecules were certainly present in the same environments. Once packed with a long column of molecules, a zeolite might promote additional reactions, including polymerization. Smith even suggests that the first cell wall might have been “an internal mineral surface.” Experiments have yet to back up these ideas; it’s hard to study what’s going on inside a mineral. But the message is clear—minerals and molecules could have interacted in a lot of intriguing ways on the early Earth.
CLAY LIFE
Many researchers have looked to minerals to give life a jump-start, whether as protective containers, organizing surfaces, or catalytic engines of prebiotic chemistry. In a provocative 1966 article (and in numerous subsequent publications) the Scottish origin-of-life expert A. G. (Graham) Cairns-Smith carried these ideas even further by speculating that fine-grained crystals of clay might themselves have been the first life on Earth.
Virtually all other authorities assume that life emerged by a continuous chemical process of increasingly complex carbon chemistry. Today’s biochemistry is thus a direct, albeit highly evolved, successor of ancient carbon geochemistry. Cairns-Smith disagrees. “I believe the unity of biochemistry is of no direct help,” he says. “Evolution did not
start with the organic molecules that have now become universal to life: indeed I doubt whether the first organisms … had any organic molecules in them at all.” He points to five decades of failed attempts to synthesize key macromolecules, such as RNA and proteins, in any plausible prebiotic scenario. In his view, nucleic acids and proteins are too complicated to build without help from the inorganic world.
Cairns-Smith’s ideas at first seem fanciful, but he is a persuasive scientist, writer, and lecturer. He speaks in a soft Scottish accent, engaging his audience with a lucid, low-key delivery. He’s not pushy with his ideas, but he has the knack of drawing you into his way of thinking. His writings, both for academic and general audiences, eschew jargon and rely on simple, vivid illustrations. Seven Clues to the Origin of Life, his popular book on the clay-life hypothesis, reads like a mystery novel, complete with dialog, false leads, and quotations from Sherlock Holmes. Whether you’re persuaded or not, you have to admire Cairns-Smith’s style.
The crux of the argument rests on a simple analogy. Cairns-Smith likens the origin of life to the construction of a stone archway, with its carefully fitted blocks and crucial central keystone that locks the whole structure in place. But an arch cannot be built simply by piling one stone atop another. “The answer,” he says, “is with a scaffolding of some kind.” A simple support structure facilitates the construction and can then be removed. “I think this must have been the way our amazingly ‘arched’ biochemistry was built in the first place,” he wrote in a Scientific American article in 1985. “The parts that now lean together surely used to lean on something else—something low tech.” That something, he suggests, was a clay mineral.
Like Bernal before him, Cairns-Smith points to the ubiquity of clays, their negative surface charge, their affinity for organic molecules, and the immense reactive surface area their fine-grained layers provide. As he was quick to point out in a Geophysical Lab seminar in the summer of 2003, “I’m an organic chemist, not a clay mineralogist—though I often talk like one.” Then he embellishes the point with a surprising twist that reveals his mineralogical sophistication. Clay crystals grow, and their mutable layered structure can carry and pass on a kind of “genetic information,” in the form of a reproducible sequence of crystal defects or variable chemical compositions. Given their ability to grow and reproduce, might not clays be thought of as living?
Even the most chauvinistic of mineralogists tend to balk at Cairns-
Smith’s concept of “clay life” and “crystal genes,” but he does have an intriguing point. Genetic information in modern life is carried by DNA and RNA molecules, which, as noted, are each made up of sequences of only four different nucleotides—in effect, a four-letter genetic alphabet. Any conceivable message can be conveyed with such an alphabet and an appropriate code.
In much the same way, clay minerals commonly display periodic structural or compositional variations that might be used to convey information. For example, the clay structure features strong sheetlike layers of atoms that stack one on top of another. Each of these structural layers can adopt one of three different orientations relative to its neighbors—at 0, 120, or 240 degrees. The sequence of layer orientations is analogous to a three-letter alphabet. What’s more, layers of different thickness and chemical character sometimes interleave with each other, adding dramatically to the information-carrying potential of clays.
Periodic variations can also occur within any given layer; that is, each layer can display a feature called “twinning,” by which a given layer can incorporate small regions in all three different orientations. What’s more, many clays boast complex chemical compositions, with varying mixtures of aluminum, magnesium, iron, and other elements. These atoms reside in predictable repeating sites, each one surrounded by an octahedron of six oxygen atoms. Move along the surface of the clay particle and you’ll find an octahedral site every ten-billionths of an inch or so, as regular as clockwork. But these regimented sites in the clay structure often contain a more-or-less random sequence of aluminum, magnesium, and other elements, as well as vacant sites and other defects. Cairns-Smith argues that such a random distribution of atoms at the exposed mineral surface can also act as a kind of genetic sequence, each element representing a different letter in this peculiar alphabet. “In two-dimensional crystal genes, information would be held as a pattern on one face of the crystal,” he posits.
So what? How can a random sequence of layer orientations or a pattern of elements at the clay surface be regarded as a living entity? How can it possibly reproduce itself? Cairns-Smith proposes two intriguing, though unproved, possibilities. First, he speculates, clay crystals might grow in such a way as to copy any given layer, stacking sequence, or element pattern over and over again, and then flake apart in what amounts to an act of self-replication. In this scenario, clay par-
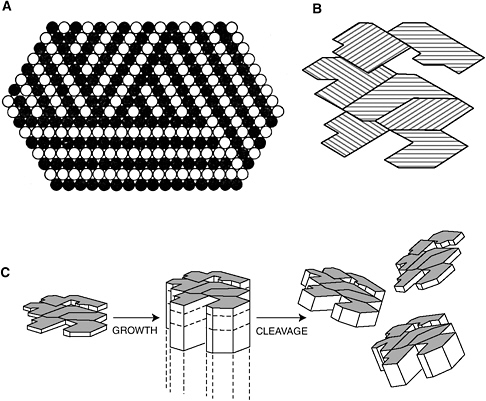
Graham Cairns-Smith suggests that clay minerals, which have a sheetlike layered structure, can carry genetic information in the form of three different layer orientations (A). Individual clay particles can possess a complex pattern of these orientations (B). If sequences of layers can be copied, then this genetic information might be passed on from one generation of clays to the next (C) (after Cairns-Smith, 1988).
ticles crystallize over and over again, and particularly stable sequences of layers or elements ultimately win out in a mineralogical version of evolution by natural selection.
Moreover, according to Cairns-Smith’s model, different exposed layer edges or elements at the clay surface have affinities for specific organic molecules. Not only do the negatively charged clay surfaces readily attract organic molecules, but clays also have the ability to catalyze reactions between surface-bound molecules. The self-replicating inorganic world of clays could have acted as the long-abandoned scaffolding for the organic world—a process he calls “genetic takeover.” The resulting macromolecule might then possess exactly the sort of
information-rich structure needed for the emergence of a carbon-based genetic mechanism.
TESTING
Any acceptable scientific theory must make testable predictions; otherwise, as Karl Popper consistently maintained, a theory is just idle speculation. Graham Cairns-Smith is well aware of this requirement, and he does not shy away from predictions.
A potentially testable feature of the clay-life hypothesis follows from the central requirement that clays evolve. In 1988, Cairns-Smith wrote a provocative essay entitled “The Chemistry of Materials for Artificial Darwinian Systems,” in which he elaborated on his idea that clays carry “genetic information” in the form of variable crystal patterns that can be passed from one generation of crystals to the next. Clays continuously form and dissolve in many geological environments. Perhaps, he posited, clays evolve by the selective replication of favorable patterns, coupled with the selective dissolution of unfavorable patterns. In this way, the evolution of clays is analogous to that of microorganisms. “The first step is to grow up a batch of the organisms,” Cairns-Smith explains. “Then a small sample of this is used to seed a new batch, which is in turn grown up—and so on. In such circumstances those types which reproduce fastest will be most likely to be carried on between the batches and will eventually become the only types present.”
By analogy, Cairns-Smith predicts, more successful (that is, more likely to be replicated) element arrangements will gradually dominate less successful variants. But can we test this idea in a laboratory experiment? Like a study of microorganism evolution, we would have to characterize the detailed state of numerous particles in the first generation of clays, and then monitor changes in subsequent generations. Cairns-Smith recognizes many experimental challenges. “Can the material be synthesized in the laboratory on a practical time scale? Can we find conditions that will allow crystals to grow? … And then the $64,000 one: Do sequences replicate accurately enough through growth?” Underlying all of these questions is the technical barrier: how to determine the exact elemental sequence of a clay particle.
For microbial populations, these problems have been solved. Microbes grow rapidly, they divide predictably, and they copy their DNA
with fidelity. And it’s relatively easy to conduct a broad survey of microbial DNA sequences—a task that has become automated by decades of genetic research and billions of dollars in capital investment. Molecular biologists have thus learned to document evolution in a microbial community.
But for clays, no such sequencing technology exists. To be sure, modern imaging instruments can provide some insights. Cairns-Smith advocates high-resolution transmission electron microscopy (HRTEM): “We should go for materials for which we can expect HRTEM to be applicable,” he writes. Nevertheless, characterization of the element arrangement in a single clay particle, much less the thousands of separate analyses necessary for an adequate clay-particle population survey, is beyond any current technique. This situation is nothing new—scientific progress has often had to wait for new technology.
![]()
Many earth scientists are drawn to the concept that clays, or some other mineral, played a crucial role in biogenesis. There’s a satisfying completeness in the integration of the solid, liquid, and gaseous parts of our planet to make life. Nevertheless, I find the reliance on clays a bit unsettling. It seems that all a chemist needs to do is sprinkle in a little clay powder and voilà: polymerization, self-assembly, synthesis, stabilization! Clays seem to do it all. The phenomenology is fascinating, but what’s actually going on at the atomic scale? Clays are inherently variable in their composition, ill-defined in their surface properties, and so fine-grained that it’s virtually impossible to be sure what molecule is binding to what surface. The “magic powder” aspects of clays leave me frustrated.
Given the choice between a poorly characterized fine-grained powder and an elegant faceted single crystal, I’ll take the crystal any day. And, as it turns out, that’s the only way you can tell left from right.