Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11 (2012)
Chapter: 3 Selected Chlorosilanes: Acute Exposure Guideline Levels
Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
1This document was prepared by the AEGL Development Team composed of Chery Bast (Oak Ridge National Laboratory), Julie M. Klotzbach (Syracuse Research Corporation), and Chemical Manager Ernest V. Falke (National Advisory Committee [NAC] on Acute Exposure Guideline Levels for Hazardous Substances). The NAC reviewed and revised the document and AEGLs as deemed necessary. Both the document and the AEGL values were then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC committee has concluded that the AEGLs developed in this document are scientifically valid conclusions based on the data reviewed by the NRC and are consistent with the NRC guidelines reports (NRC 1993, 2001).
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure concentrations that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold levels for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Chlorosilanes contain one or more chlorine atoms covalently bonded to a silicon atom; the maximum chlorine-to-silicon ratio is four. Chlorosilanes are chemical intermediates used in the production of silicone and silicone-containing materials, and are often produced in bulk and transported to manufacturing sites for use. Chlorosilanes are corrosive, and inhalation exposure might cause nasal, throat, or lung irritation, coughing, wheezing, and shortness of breath. Chlorosilanes react rapidly with water, steam, or moisture; hydrolysis yields hydrogen chloride (HCl) gas along with silanols and other condensation products.
The 26 chlorosilanes considered in this chapter are:
Allyl trichlorosilane
Amyl trichlorosilane
Butyl trichlorosilane
Chloromethyl trichlorosilane
Dichlorosilane
Diethyl dichlorosilane
Dimethyl chlorosilane
Dimethyl dichlorosilane
Diphenyl dichlorosilane
Dodecyl trichlorosilane
Ethyl trichlorosilane
Hexyl trichlorosilane
Methyl chlorosilane
Methyl dichlorosilane
Methyl trichlorosilane
Methylvinyl dichlorosilane
Nonyl trichlorosilane
Octadecyl trichlorosilane
Octyl trichlorosilane
Propyl trichlorosilane
Tetrachlorosilane
Trichloro(dichlorophenyl)silane
Trichlorophenylsilane
Trichlorosilane
Trimethyl chlorosilane
Vinyl trichlorosilane
Although chemical-specific toxicity data are not available for many of these chlorosilanes, acute inhalation data from rat studies are available for structurally-similar chlorosilanes (propyl trichlorosilane, methyl trichlorosilane, vinyl trichlorosilane, ethyl trichlorosilane, methylvinyl dichlorosilane, methyl dichlorosilane, dimethyl dichlorosilane, dimethyl chlorosilane, trimethylchlorosilane, and tetrachlorosilane). These data suggest that the acute toxicity of chlorosilanes is largely explained by the HCl hydrolysis product; acute toxicity of these chlorosilanes is qualitatively (based on clinical signs) and quantitatively (based on molar equivalents of HCl) similar to that of HCl (Jean et al. 2006).
On the basis of these data, and in the absence of appropriate chemical-specific data for the chlorosilanes considered in this document, the AEGLs for HCl were used to derive AEGLs for the chlorosilanes. For each class of chloro-silanes (mono-, di-, tri-, and tetra-chlorosilanes), the molar ratio (moles of HCl released per mole of chlorosilane, assuming complete hydrolysis) was used to adjust the AEGL values for HCl to the equivalent concentration of chlorosilane. Detailed information on the derivation of AEGLs for HCl is available in NRC (2004). The calculated values are listed in the Table 3-1.
1. INTRODUCTION
Chlorosilanes contain one or more chlorine atoms covalently bonded to a silicon atom; the maximum chlorine-to-silicon ratio is four. Chlorosilanes are chemical intermediates used in the production of silicone and silicone-containing materials, and are often produced in bulk and transported to manufacturing sites for use.
Chlorosilanes react very rapidly with water, steam, or moisture, releasing HCl gas (AIHA 1998, 1999, 2001a,b,c, 2006). The primary vapor detected in air when chlorosilanes are released is HCl; much less of the parent chlorosilane is detectable (Nakashima et al. 1996; Jean et al. 2006). In an experiment using 11 different chlorosilanes, Jean et al. (2006) reported that the percentage of parent chlorosilane in the test atmosphere ranged from <10% to 58%; other constituents of the atmosphere (in addition to HCl) included silanols and other condensation products. When x-ray microanalysis was performed on air filtered from a dichlorosilane exposure chamber, small (<1 μM in diameter), unidentified particles containing silicon and chloride were detected (Nakashima et al. 1996).
Numerous reports of chlorosilane spills and releases have been received by the U.S. Coast Guard National Response Center. For example, between January 1990 and July 2007, there were 23 reports of dichlorosilane releases ranging from 6 to 2,596 pounds; 32 reports of trichlorosilane releases ranging from 2.6 to 343 pounds; and 14 reports of tetrachlorosilane releases ranging from 2 to 330 pounds (USCG 2007). Releases were from both fixed and mobile sources and were the result of equipment failure and operator error.
TABLE 3-1 Summary of AEGL Values for Selected Chlorosilanesa
| Compound | Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) |
| MONOCHLOROSILANES | |||||||
| Dimethyl chlorosilane | AEGL-1 | 1.8 ppm | 1.8 ppm | 1.8 ppm | 1.8 ppm | 1.8 ppm | AEGLs for HCl |
| Methyl chlorosilane | AEGL-2 | 100 ppm | 43 ppm | 22 ppm | 11 ppm | 11 ppm | (NRC 2004) |
| Trimethylchlorosilane | AEGL-3 | 620 ppm | 210 ppm | 100 ppm | 26 ppm | 26 ppm | |
| DICHLOROSILANES | |||||||
| Dichlorosilane | AEGL-1 | 0.90 ppm | 0.90 ppm | 0.90 ppm | 0.90 ppm | 0.90 ppm | AEGLs for HCl |
| Diethyl dichlorosilane | AEGL-2 | 50 ppm | 22 ppm | 11 ppm | 5.5 ppm | 5.5 ppm | divided by a molar |
| Dimethyl dichlorosilane | AEGL-3 | 310 ppm | 110 ppm | 50 ppm | 13 ppm | 13 ppm | adjustment factor of 2 |
| Diphenyl dichlorosilane | (NRC 2004) | ||||||
| Methyl dichlorosilane | |||||||
| Methylvinyl dichlorosilane | |||||||
| TRICHLOROSILANES | |||||||
| Allyl trichlorosilane | AEGL-1 | 0.60 ppm | 0.60 ppm | 0.60 ppm | 0.60 ppm | 0.60 ppm | AEGL values for HCl |
| Amyl trichlorosilane | AEGL-2 | 33 ppm | 14 ppm | 7.3 ppm | 3.7 ppm | 3.7 ppm | divided by a molar |
| Butyl trichlorosilane Chloromethyl trichlorosilane | AEGL-3 | 210 ppm | 70 ppm | 33 ppm | 8.7 ppm | 8.7 ppm | adjustment factor of 3 (NRC 2004) |
| Dodecyl trichlorosilane | |||||||
| Ethyl trichlorosilane | |||||||
| Hexyl trichlorosilane | |||||||
| Methyl trichlorosilane | |||||||
| Nonyl trichlorosilane | |||||||
| Octadecyl trichlorosilane | |||||||
| Octyl trichlorosilane | |||||||
| Propyl trichlorosilane |
TABLE 3-1
| Compound | Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) |
| TRICHLOROSILANES (continued) | |||||||
| Trichloro(dichlorophenyl)silane Trichlorophenylsilane Trichlorosilane |
|||||||
| Vinyl trichlorosilane | |||||||
| TETRACHLOROSILANE | |||||||
| AEGL-1 AEGL-2 AEGL-3 |
0.45 ppm 25 ppm 160 ppm |
0.45 ppm 11 ppm 53 ppm |
0.45 ppm 5.5 ppm 25 ppm |
0.45 ppm 2.8 ppm 6.5 ppm |
0.45 ppm 2.8 ppm 6.5 ppm |
AEGL values for HCl divided by a molar adjustment factor of 4 (NRC 2004) |
aValues given in ppm. To convert ppm to mg/m3: (ppm × molecular weight) ÷ 24.5. See Appendix A for the appropriate molecular weight. For mono-, di-, and tri-chlorosilanes not listed, use of HCl equivalents may be considered for AEGL-value derivation.
The chlorosilanes have pungent irritating odors, are corrosive, and inhalation exposure might cause nasal, throat, or lung irritation, coughing, wheezing, and shortness of breath. Although chemical-specific toxicity data are not available for many of the chlorosilanes, acute inhalation data from rat studies are available for structurally-similar chlorosilanes (propyl trichlorosilane, methyl trichlorosilane, vinyl trichlorosilane, ethyl trichlorosilane, methylvinyl dichlorosilane, methyl dichlorosilane, dimethyl dichlorosilane, dimethyl chlorosilane, trimethylchlorosilane, and tetrachlorosilane). These data suggest that the acute toxicity of chlorosilanes is from the HCl hydrolysis product; acute toxicity of the chlorosilanes is qualitatively (based on clinical signs) and quantitatively (based on molar equivalents of HCl) similar to that of HCl (Jean et al. 2006) (see Section 4.3).
On the basis, and in the absence of adequate chemical-specific data for the chlorosilanes considered in this document, the AEGL values for HCl (NRC 2004) were used to obtain AEGL values for the chlorosilanes. The molar ratio (moles HCl released per mole of chlorosilane, assuming complete hydrolysis) was used to adjust the AEGL values for HCl to the equivalent concentration of chlorosilane. Available physicochemical data for the 26 chlorosilanes covered in this chapter are presented in Appendix A.
2. HUMAN TOXICITY DATA
An accidental release of tetrachlorosilane at a chemical plant in a south San Francisco industrial park provided some human exposure data (Kizer et al. 1984). A delivery truck taking a short-cut through a chemical plant hit the tank-coupling unit of a tetrachlorosilane storage tank. The pipeline ruptured and the tetrachlorosilane liquid spilled onto the moist ground; it hydrolyzed rapidly and formed a large gray-white cloud that quickly spread. Workers were unable to stop the leak because the valve was behind a wire enclosure, and approximately 1,200 gallons of tetrachlorosilane was released before the leak was stopped several hours later. By that time, the cloud had risen 500 feet and had spread more than a mile over the industrial park. Five- to ten-thousand employees from 600 businesses over 3 square miles were evacuated. Twenty-eight people reported to local hospitals for treatment of eye or airway irritation. Seven of the patients were employees at the chemical plant, and six of them were smokers. The remaining 21 patients were firemen, policemen, passersby, and employees of other companies in the area. There were no deaths, and no one was hospitalized. Six of the chemical plant employees were referred for further evaluation; these employees were all male, ranged in age from 25 to 56, and were all smokers. Their exposures ranged from 10 to 20 min in duration. Symptoms generally resolved within 24 h, and included lacrimation, rhinorrhea, burning of the mouth and throat, headache, coughing, and wheezing. Pulmonary function tests were normal except that mild obstructive airway disease was noted in four patients. However, it was unclear if the disease was from exposure to tetrachlorosilane or
related to smoking status. Two patients also complained of pedal dysesthesias after the accident. No air concentrations of tetrachlorosilane or HCl were reported.
Reactive airways dysfunction syndrome is an asthma-like condition that develops after a single exposure to high concentrations of a chemical irritant, and has been described after exposure to HCl. Symptoms occur within minutes to hours after the initial exposure and can persist as nonspecific bronchial hyper-responsiveness for months to years (Bernstein 1993). Promisloff et al. (1990) reported reactive airways dysfunction syndrome in three male police officers (36-45 years of age) who responded to a roadside chemical spill. The subjects were exposed to unquantified amounts of sodium hydroxide, tetrachlorosilane, and HCl as a byproduct of trichlorosilane hydrolysis. Because of the mixture of irritants involved in the release, it is probable that all of the compounds contributed to the syndrome observed after this accident.
3. ANIMAL TOXICITY DATA
3.1. Acute Toxicity
One-hour LC50 (lethal concentration, 50% lethality) studies were conducted for 10 chlorosilanes: tetrachlorosilane, propyl trichlorosilane, vinyl trichlorosilane, methyl trichlorosilane, ethyl trichlorosilane, methylvinyl dichlorosilane, dimethyl dichlorosilane, methyl dichlorosilane, trimethyl chlorosilane, and dimethyl chlorosilane (Jean et al. 2006). In each study, groups of five male and five female Fischer 344 rats were exposed to varying concentrations of a chlorosilane for 1 h and observed for up to 14 days. The studies appeared to conform to Good Laboratory Practices and were well-described. The authors used nominal concentrations to calculate LC50 values because chlorosilanes react rapidly with moisture to produce HCl and other hydrolysis products. Using actual chamber concentrations of chlorosilanes would only reflect toxicity of the parent compound, not the toxicity of the parent compound and hydrolysis products. There was agreement between the electrolytic conductivity detector and the nominal concentrations, indicating efficient vaporization of the test material.
Clinical signs were consistent with HCl exposure and included lacrimation, salivation, dried material around the eyes or nose, green staining around the nose and mouth, and perineal urine staining. Labored breathing, rales, hypoactivity, closed or partially closed eyes, prostration, corneal opacity or opaqueness, and swollen or necrotic paws also were observed. Hemorrhage, congestion, and consolidation of the lungs; ectasia of the lungs; gaseous distension of the gastrointestinal tract; absence of body fat; obstruction of nostrils; dried or firm nares; alopecia around the eyes; and discoloration of hair were observed at necropsy. Mortality data and LC50 values from 1-h exposure studies with rats are summarized in Table 3-2.
TABLE 3-2 Mortality Data and LC50 Values from 1-Hour Exposure Studies
| Exposure Concentration |
Mortalit | LC50, ppm (95% confidence | |||
| Compound | (ppm) | Male | Female | Total | limits) |
| Tetrachlorosilane | 1,209 | 1/5 | 2/5 | 3/10 | 1,312 (1,006-1,529) a |
| 1,497 | 5/5 | 3/5 | 8/10 | ||
| 3,051 | 5/5 | 5/5 | 10/10 | ||
| Propyl trichlorosilane | 1,123 | 0/5 | 0/5 | 0/10 | 1,352 (1,254-1,455) a |
| 1,317 | 2/5 | 2/5 | 4/10 | ||
| 1,414 | 3/5 | 4/5 | 7/10 | ||
| Vinyl trichlorosilane | 1,186 | 0/5 | 0/5 | 0/10 | 1,611 (1,505-1,724) b |
| 1,605 | 4/5 | 2/5 | 6/10 | ||
| 1,681 | 2/5 | 1/5 | 3/10 | ||
| 1,989 | 5/5 | 5/5 | 10/10 | ||
| Methyl trichlorosilane | 622 | 0/5 | 0/5 | 0/10 | 1,365 (1,174-2,104) a |
| 1,047 | 0/5 | 1/5 | 1/10 | ||
| 1,439 | 4/5 | 2/5 | 6/10 | ||
| 3,075 | 5/5 | 5/5 | 10/10 | ||
| Ethyl trichlorosilane | 1,156 | 1/5 | 1/5 | 2/10 | 1,257 (1,175-1,320) a |
| 1,326 | 4/5 | 2/5 | 6/10 | ||
| 1,415 | 5/5 | 5/5 | 10/10 | ||
| Methylvinyl dichlorosilane | 1,597 2,005 | 1/5 3/5 | 0/5 2/5 | 1/10 5/10 | 2,021 (1,806-2,257) a |
| 2,119 | 3/5 | 3/5 | 6/10 | ||
| 2,242 | 4/5 | 3/5 | 7/10 | ||
| Dimethyl dichlorosilane | 1,309 2,077 | 0/5 4/5 | 0/5 1/5 | 0/10 5/10 | 2,092 (1,492-2,240) a |
| 2,353 | 5/5 | 3/5 | 8/10 | ||
| 2,762 | 5/5 | 5/5 | 10/10 | ||
| Methyl dichlorosilane | 1,431 1,678 | 0/5 1/5 | 0/5 2/5 | 0/10 3/10 | 1,785 (1,671-1,963) a |
| 1,889 | 4/5 | 3/5 | 7/10 | ||
| Trimethyl chlorosilane | 3,171 4,139 | 0/5 2/5 | 0/5 0/5 | 0/10 2/10 | 4,257 (4,039-4,488) b |
| 4,268 | 3/5 | 3/5 | 6/10 | ||
| 5,121 | 5/5 | 5/5 | 10/10 | ||
| Dimethyl chlorosilane | 4,108 4,179 | 1/5 1/5 | 1/5 1/5 | 2/10 2/10 | 4,478 (4,281-6,327) a |
| 4,409 | 3/5 | 3/5 | 6/10 | ||
| 4,589 | 3/5 | 2/5 | 5/10 |
aProbit analysis.
bSpearman-Karber analysis.
Source: Jean et al. 2006. Reprinted with permission; copyright 2006, Inhalation Toxicology.
In another study, groups of 10 male ICR mice were exposed for 4 h to nominal concentrations of dichlorosilane at 49-259 ppm, followed by a 14-day observation period (Nakashima et al. 1996). Mortality was 0/10, 0/10, 1/10, 6/10, 4/10, 10/10, 10/10, 9/10, and 10/10 for groups exposed at 0, 49, 100, 131, 141, 199, 216, 218, and 259 ppm, respectively. An LC50 of 144 ppm was calculated.
3.2. Developmental and Reproductive Toxicity
No data on developmental or reproductive toxicity were found.
3.3. Genotoxicity
The only genotoxicity data found were for tetrachlorosilane. Tetrachlorosilane was not mutagenic in Salmonella typhimurium strains TA98, TA100, TA 1535, TA1537, or TA1538; Saccharomyces cerevisiae strain D-4; or Escherischia coli strains W3110/polA+ and P3478/polA- either with or without metabolic activation. It was also negative in a L5178Y mouse lymphoma assay (AIHA 1999).
3.4. Chronic Toxicity and Carcinogenicity
No data on chronic toxicity or carcinogenicity were found.
3.5. Summary
Although toxicity data are sparse for individual chlorosilanes, well-conducted 1-h inhalation toxicity studies in rats are available for a series of chlorosilanes (Jean et al. 2006). In general, LC50 values for monochlorosilanes were approximately twice the LC50 values for dichlorosilanes and three times the LC50 values for trichlorosilanes. Tetrachlorosilane had an LC50 value similar to the trichlorosilanes; however, there were experimental difficulties at the lowest concentration tested. Clinical signs were indicative of severe irritation or corrosion. The evidence suggests that the acute toxicity of chlorosilanes is largely attributable to the release of HCl; however, no information on the identity or potential toxicity of other decomposition products was found. No data concerning developmental or reproductive toxicity, genotoxicity, or carcinogenicity for exposure to the chlorosilanes were found in the literature.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
No information was found concerning the metabolism and disposition of chlorosilanes.
4.2. Mechanism of Toxicity
Chlorosilanes react violently with water to produce HCl gas (AIHA 1998, 1999, 2001a,b,c, 2006). In an experiment using 11 different chlorosilanes, Jean et al. (2006) reported that the percentage of parent chlorosilane in the test atmosphere range from <10 to 58%; other constituents of the atmosphere (in addition to HCl) included silanols and other condensation products. Nakashima et al. (1996) reported that small particles containing silicon and chlorine were detected in an inhalation exposure chamber into which dichlorosilane was introduced; the identity and quantity of particles were not reported. IPCS (2002a) reported that, when heated, trimethylchlorosilane decomposition could release HCl and phosgene. No other information on potential decomposition products of chlorosilanes was found. Available data suggest that the acute toxicity of chlorosilanes is largely from the HCl hydrolysis product; acute toxicity of the chlorosi-lanes is qualitatively (based on clinical signs) and quantitatively (based on molar equivalents of HCl) similar to that of HCl.
4.3 Structure Activity Relationships
A 1-h LC50 study with HCl was performed in rats and used for comparison with the chlorosilane 1-h LC50 values (Jean et al. 2006). According to the authors, the study with HCl was unpublished, but was performed in the same laboratory and was conducted using the same protocol as that used in the chlorosilane study (1-h whole-body exposure with a 14-day recovery period). Five rats per sex were exposed to HCl at 0, 2,456, 3,236, or 4,210 ppm for 1 h and observed for up to 14 days. Chamber concentrations were determined by a Fourier transform infrared spectrophotometer analyzer. Clinical signs included labored breathing; gasping; emaciation; rough coat; lethargy; corneal opacity; crusting, necrotic, discolored, and blocked nares or nasal opening; paws with missing, necrotic, or swollen digits; and weight loss. Gross pathology of animals dying during the study included irritation and necrosis of most extremities, severe respiratory-tract injuries, and corneal opacity. A 1-h LC50 of 3,627 ppm was calculated for HCl.
The LC50 data obtained for the chlorosilanes showed a strong association with chlorine content for the mono-, di-, and tri-chlorosilanes. In general, LC50 values for monochlorosilanes were approximately twice the LC50 values for dichlorosilanes and three times the LC50 values for trichlorosilanes. Tetrachlorosilane exhibited an LC50 value similar to the trichlorosilanes.
The predicted 1-h LC50 values for the chlorosilanes, based on HCl equivalents, are presented in Table 3-3. The predicted values for the chlorosilanes are comparable to the experimentally-derived 1-h LC50 values (log * log regression analysis of chlorosilane LC50 values vs. the number of chlorine groups yielded an r2 value of 0.97). The data suggest that the acute toxicity of the chlorosilanes is similar to or slightly less than what would be expected based on HCl molar
equivalents. The within-class LC50 values were not significantly influenced by the number or type of hydrocarbon R-group(s) present (methyl, ethyl, propyl, or vinyl). Cases where the predicted value is less might be attributed to incomplete hydrolysis in the test atmosphere; however, continued hydrolysis and generation of HCl would be expected for any remaining chlorosilane when in contact with moist tissues (mucous membranes, lung) (Jean et al. 2006). This information taken in conjunction with the observed clinical signs suggests that the acute toxicity of the chlorosilanes is quantitatively and qualitatively similar to HCl and that the HCl hydrolysis product is responsible for the acute toxicity of the chlorosilanes.
TABLE 3-3 Measured and Predicted 1-Hour LC50 Values for Selected
| Compound |
Measured LC50 (ppm) (95% confidence limits) |
Predicted LC50 (ppm) |
Predicted Ratio of LC50 Values |
Measured Ratio of LC50 Values |
| Hydrogen chloride | 3,627 | |||
| Tetrachlorosilane | 1,312 (1,006-1,529) | 3,627 ÷ 4 = 907 | 4:1 | 2.8:1 |
| Propyl trichlorosilane | 1,352 (1,254-1,455) | 3,627 ÷ 3 = 1,209 | 3:1 | 2.7:1 |
| Vinyl trichlorosilane | 1,611 (1,505-1,724) | 3,627 ÷ 3 = 1,209 | 3:1 | 2.3:1 |
| Methyl trichlorosilane | 1,365 (1,174-2,104) | 3,627 ÷ 3 = 1,209 | 3:1 | 2.7:1 |
| Ethyl trichlorosilane | 1,257 (1,175-1,320) | 3,627 ÷ 3 = 1,209 | 3:1 | 2.9:1 |
| Methylvinyl | 2,021 (1,806-2,257) | 3,627 ÷ 2 = 1,814 | 2:1 | 1.8:1 |
| dichlorosilane | ||||
| Dimethyl dichlorosilane | 2,092 (1,492-2,240) | 3,627 ÷ 2 = 1,814 | 2:1 | 1.7:1 |
| Methyl dichlorosilane | 1,785 (1,671-1,963) | 3,627 ÷ 2 = 1,814 | 2:1 | 2:1 |
| Trimethyl chlorosilane | 4,257 (4,039-4,488) | 3,627 ÷ 1 = 3,627 | 1:1 | 0.9:1 |
| Dimethyl chlorosilane | 4,478 (4,281-6,327) | 3,627 ÷ 1 = 3,627 | 1:1 | 0.8:1 |
| Source: Adapted from Jean et al. 2006. | ||||
The 4-h mouse LC50 of 144 ppm for dichlorosilane (Nakashima et al. 1996) also supports the conclusion that the acute inhalation toxicity of chlorosilanes is from the HCl hydrolysis product. The reported 1-h mouse LC50 for HCl is 1,108 ppm (NRC 2004). Scaling across time for HCl may be accomplished using the equation Cn × t = k, where n = 1 based on regression analysis of combined rat and mouse LC50 data (1-100 min) (NRC 2004). Scaling the 1-h LC50 value for HCl of 1,108 ppm to a 4-h period yields an approximate 4-h LC50 value of 277 ppm. Dividing this 4-h LC50 by a molar adjustment factor of 2, yields a predicted LC50 of 139 ppm for dichlorosilane, which is similar to the experimentally-derived value of 144 ppm.
The 26 chlorosilanes addressed in this chapter include those with alkane, alkene, aromatic, and chlorinated substituents. Although the evidence from Jean et al. (2006) suggests that the acute toxicity is from HCl formed as a hydrolysis product, the data were generated using 11 of the 26 chlorosilanes, including primarily alkane-subsituted compounds and two of the three compounds with alkene substituent groups. Of the 26, two have aromatic substituents and two (including one of the aromatics) have chlorinated substituents; none of those was among the tested compounds.
4.4. Other Relevant Information
4.4.1. Species Variability
Data were not available regarding species variability in lethal and nonlethal toxicity from chlorosilane exposure. Differences in response to HCl have been observed between primates and rodents. Rodents exhibit sensory and respiratory irritation after exposure to high concentrations of HCl. Concentration-dependent decreases in respiratory frequency indicative of a protective mechanism are observed in rodents, whereas baboons exposed at 500, 5,000, or 10,000 ppm exhibited concentration-dependent increases in respiratory frequency indicative of a compensatory response to hypoxia and a possible increase in the total dose delivered to the lung (NRC 1991). Kaplan et al. (1988) found that five of six mice died when exposed to HCl at 2,550 ppm for 15 min, but no baboons died when exposed at 10,000 ppm for 15 min. The LC50 values reported by Darmer et al. (1974), Wohlslagel et al. (1976), and Higgins et al. (1972) indicate that mice are approximately three times more sensitive than rats to HCl. Guinea pigs also appear to be more sensitive than rats to HCl; however, the guinea pig studies have provided conflicting results. For respiratory irritants such as HCl, the mouse “may not be a good model for extrapolation to humans,” because “mice appear to be much more susceptible to the lethal effects of HCl than other rodents or baboons. To some extent, this increased susceptibility may be due to less effective scrubbing of HCl in the upper respiratory tract” (NRC 1991).
Because most rodents are obligatory nose breathers whereas humans may be mouth breathers, especially during exercise, Stavert et al. (1991) studied the effects of inhaling HCl through the nose and mouth in rats. HCl was delivered directly to the trachea by cannulation. Higher mortality rates occurred with orally-cannulated rats compared with rats exposed by nose. Tracheal necrosis and inflammatory-cell accumulation were found in cannulated rats, whereas effects in nose-breathing rats were confined to the nasal passages. These results indicate that the site of injury and resultant toxicologic effects differ with oral or nasal breathing, with the former mode resulting in more severe responses under similar exposure situations.
4.4.2. Susceptible Populations
No information was available on populations that might be especially sensitive to chlorosilane or HCl. However, clinical signs of chlorosilane and HCl exposure are consistent with contact irritation. In general, contact-irritant effects are not expected to vary widely among individuals. However, as noted by NRC (2004), asthmatic persons and others with sensitive airways might be more susceptible to the effects of HCl inhalation.
On the basis of the study by Stavert et al. (1991), which showed more severe respiratory responses to HCl in orally-cannulated rats compared with nose-breathing rats, it is possible that persons who habitually breathe orally might experience more pronounced or different health effects than those who primarily breathe nasally. Likewise, physical exertion might intensify the respiratory effects of HCl or chlorosilane exposure as individuals shift from nasal to oral breathing during exertion.
5. DATA ANALYSIS FOR AEGL-1
5.1. Summary of Human Data Relevant to AEGL-1
No human data relevant to development of AEGL-1 values were found.
5.2. Summary of Animal Data Relevant to AEGL-1
No animal data relevant to development of AEGL-1 values were found.
5.3. Derivation of AEGL-1
AEGL-1 values for the chlorosilanes were determined by modifying the AEGL-1 values that were established for HCl. The use of HCl as a surrogate for chlorosilanes was deemed appropriate because adverse effects from exposure to chlorosilanes have been attributed to their hydrolysis product, HCl. The AEGL-1 values for HCl were based on a no-observed-adverse-effect level in exercising adult with asthma (NRC 2004). The same AEGL-1 value was applied across all
specified exposure periods, because mild irritation generally does not vary greatly over time and because prolonged exposure is not expected to result in an enhanced effect (NRC 2004). The key study and calculations used to determine the AEGL-1 values for HCl are summarized in Appendixes C and E (more detail is available in the technical support document for HCl published in NRC [2004]). The molar ratio (moles of HCl released per mole of chlorosilane, assuming complete hydrolysis) was used to adjust the AEGLs for HCl to the equivalent concentration of chlorosilane. Although the 1-h rat LC50 value for tetrachlorosilane suggests that only 3 moles of HCl were produced, the use of a molar adjustment factor of 4 is considered appropriate because of experimental difficulties that occurred at lower exposure concentrations in this study. The use of the molar adjustment factor of 4 will yield protective AEGL values and is consistent with the approach taken for the overall chlorosilane database. The AEGL-1 values for the chlorosilanes are presented in Table 3-4, and their calculations are presented in Appendix B.
TABLE 3-4 AEGL-1 Values for Selected Chlorosilanesa
| Compound | 10 min | 30 min | 1 h | 4 h | 8 h |
| MONOCHLOROSILANES | |||||
| Dimethyl chlorosilane | 1.8 ppm | 1.8 ppm | 1.8 ppm | 1.8 ppm | 1.8 ppm |
| Methyl chlorosilane | |||||
| Trimethyl chlorosilane | |||||
| DICHLOROSILANES | |||||
| Dichlorosilane | 0.90 ppm | 0.90 ppm | 0.90 ppm | 0.90 ppm | 0.90 ppm |
| Diethyl dichlorosilane | |||||
| Dimethyl dichlorosilane | |||||
| Diphenyl dichlorosilane | |||||
| Methyl dichlorosilane | |||||
| Methylvinyl dichlorosilane | |||||
| TRICHLOROSILANES | |||||
| Allyl trichlorosilane | 0.60 ppm | 0.60 ppm | 0.60 ppm | 0.60 ppm | 0.60 ppm |
| Amyl trichlorosilane | |||||
| Butyl trichlorosilane | |||||
| Chloromethyl trichlorosilane | |||||
| Dodecyl trichlorosilane | |||||
| Ethyl trichlorosilane | |||||
| Hexyl trichlorosilane | |||||
| Methyl trichlorosilane | |||||
| Nonyl trichlorosilane | |||||
| Octadecyl trichlorosilane | |||||
| Octyl trichlorosilane | |||||
| Propyl trichlorosilane | |||||
| Trichloro(dichlorophenyl)silane | |||||
| Trichlorophenylsilane | |||||
| Trichlorosilane | |||||
| Vinyl trichlorosilane | |||||
| TETRACHLOROSILANE | 0.45 ppm | 0.45 ppm | 0.45 ppm | 0.45 ppm | 0.45 ppm |
aValues given in ppm. To convert ppm to mg/m3: (ppm × molecular weight) ÷ 24.5. See Appendix A for the appropriate molecular weight.
6.3. Derivation of AEGL-2
AEGL-2 values for the chlorosilanes were determined by modifying the AEGL-2 values that were established for HCl. The use of HCl as a surrogate for chlorosilanes was deemed appropriate because adverse effects from exposure to chlorosilanes have been attributed to their hydrolysis product, HCl. AEGL-2 values for HCl were based on severe nasal or pulmonary histopathologic changes in rats (exposed for 30 min to 8 h) or a modification of the mouse 50% respiratory rate decrease (RD50) (exposed for 10 min) (NRC 2004). The key study and calculations used to determine the AEGL-2 values for HCl are summarized in Appendixes C and E (more detail is available in the technical support document for HCl published in NRC [2004]). The molar ratio (moles of HCl released per mole of chlorosilane, assuming complete hydrolysis) was used to adjust the AEGLs for HCl to the equivalent concentration of chlorosilane. The AEGL-2 values for the chlorosilanes are presented in Table 3-5, and their calculations are presented in Appendix B.
7. DATA ANALYSIS FOR AEGL-3
7.1. Summary of Human Data Relevant to AEGL-3
No human data relevant to development of AEGL-3 values were found.
7.2. Summary of Animal Data Relevant to AEGL-3
One-hour rat LC50 values were reported by Jean et al. (2006) to be 4,478 ppm for dimethyl dichlorosilane and 2,021 ppm for methylvinyl dichlorosilane. One-hour rat LC50 values for trichlorsilanes were 1,257, 1,352, and 1,611 ppm for ethyl trichlorosilane, propyl trichlorosilane, and vinyl trichlorosilane, respectively (Jean et al. 2006). A 1-h rat LC50 value of 1,312 ppm was reported for tetrachlorosilane (Jean et al. 2006). A 4-h mouse LC50 value of 144 ppm was reported for dichlorosilane (Nakashima et al. 1996), but the mouse is considered to be an unreliable model for the acute toxicity of HCl in humans (NRC 1991, 2004). No animal data relevant to development of AEGL-3 values were found for the other chlorosilanes.
7.3. Derivation of AEGL-3
AEGL-3 values for the chlorosilanes were determined by modifying the AEGL-3 values that were established for HCl. The use of HCl as a surrogate for chlorosilanes was deemed appropriate because adverse effects from exposure to chlorosilanes have been attributed to their hydrolysis product, HCl. The AEGL-3 values for HCl were based on a 1-h rat LC50 value divided by 3 to estimate a lethality threshold (NRC 2004). The key study and calculations used to determine the AEGL-3 values for HCl are summarized in Appendixes C and E (more detail
is available in the technical support document for HCl published in NRC [2004]). The molar ratio (moles of HCl released per mole of chlorosilane, assuming complete hydrolysis) was used to adjust the AEGLs for HCl to the equivalent concentration of chlorosilane. The AEGL-2 values for the chlorosilanes are presented in Table 3-6, and their calculations are presented in Appendix B.
8. SUMMARY OF AEGLS
8.1. AEGL Values and Toxicity End Points
AEGL values for selected chlorosilanes are summarized in Table 3-7. Derivation summary tables appear in Appendix E, and category plots for the selected chlorosilanes are in Appendix F. AEGL values were based on molar adjustments of the AEGL values for HCl. For mono-, di-, and tri- chlorosilanes not listed, use of HCl equivalents may be considered for AEGL-value derivation.
TABLE 3-5 AEGL-2 Values for Selected Chlorosilanesa
| Compound | 10 min | 30 min | 1 h | 4 h | 8 h |
| MONOCHLOROSILANES | |||||
| Dimethyl chlorosilane | 100 ppm | 43 ppm | 22 ppm | 11 ppm | 11 ppm |
| Methyl chlorosilane | |||||
| Trimethyl chlorosilane | |||||
| DICHLOROSILANES | |||||
| Dichlorosilane | 50 ppm | 22 ppm | 11 ppm | 5.5 pm | 5.5 ppm |
| Diethyl dichlorosilane | |||||
| Dimethyl dichlorosilane | |||||
| Diphenyl dichlorosilane | |||||
| Methyl dichlorosilane | |||||
| Methylvinyl dichlorosilane | |||||
| TRICHLOROSILANES | |||||
| Allyl trichlorosilane | 33 ppm | 14 ppm | 7.3 ppm | 3.7 pm | 3.7 ppm |
| Amyl trichlorosilane | |||||
| Butyl trichlorosilane | |||||
| Chloromethyl trichlorosilane | |||||
| Dodecyl trichlorosilane | |||||
| Ethyl trichlorosilane | |||||
| Hexyl trichlorosilane | |||||
| Methyl trichlorosilane | |||||
| Nonyl trichlorosilane | |||||
| Octadecyl trichlorosilane | |||||
| Octyl trichlorosilane | |||||
| Propyl trichlorosilane | |||||
| Trichloro(dichlorophenyl)silane | |||||
| Trichlorophenylsilane | |||||
| Trichlorosilane | |||||
| Vinyl trichlorosilane | |||||
| TETRACHLOROSILANE | 25 ppm | 11 ppm | 5.5 ppm | 2.8 ppm | 2.8 ppm |
aValues given in ppm. To convert ppm to mg/m3: (ppm × molecular weight) ÷ 24.5. See Appendix A for the appropriate molecular weight.
TABLE 3-6 AEGL-3 Values for Selected Chlorosilanes
| Compound | 10 min | 30 min | 1 h | 4 h | 8 h |
| MONOCHLOROSILANES | |||||
| Dimethyl chlorosilane | 620 ppm | 210 ppm | 100 ppm | 26 ppm | 26 ppm |
| Methyl chlorosilane | |||||
| Trimethyl chlorosilane | |||||
| DICHLOROSILANES | |||||
| Dichlorosilane | 310 ppm | 110 ppm | 50 ppm | 13 ppm | 13 ppm |
| Diethyl dichlorosilane | |||||
| Dimethyl dichlorosilane | |||||
| Diphenyl dichlorosilane | |||||
| Methyl dichlorosilane | |||||
| Methylvinyl dichlorosilane | |||||
| TRICHLOROSILANES | |||||
| Allyl trichlorosilane | 210 ppm | 70 ppm | 33 ppm | 8.7 ppm | 8.7 ppm |
| Amyl trichlorosilane | |||||
| Butyl trichlorosilane | |||||
| Chloromethyl trichlorosilane | |||||
| Dodecyl trichlorosilane | |||||
| Ethyl trichlorosilane | |||||
| Hexyl trichlorosilane | |||||
| Methyl trichlorosilane | |||||
| Nonyl trichlorosilane | |||||
| Octadecyl trichlorosilane | |||||
| Octyl trichlorosilane | |||||
| Propyl trichlorosilane | |||||
| Trichloro(dichlorophenyl)silane | |||||
| Trichlorophenylsilane | |||||
| Trichlorosilane | |||||
| Vinyl trichlorosilane | |||||
| TETRACHLOROSILANE | 160 ppm | 53 ppm | 25 ppm | 6.5 ppm | 6.5 ppm |
aValues given in ppm. To convert ppm to mg/m3: (ppm × molecular weight) ÷ 24.5. See Appendix A for the appropriate molecular weight.
8.2. Comparison with Other Standards and Guidelines
There are no standards or guidelines for most of the chlorosilanes considered in this chapter. The few guidelines available are Emergency Response Planning Guidelines (ERPGs) and Workplace Environmental Exposure Level (WEEL) ceiling levels for trimethylchlorosilane, dimethyl dichlorosilane, trichlorosilane, methyl trichlorosilane, vinyl trichlorosilane, and tetrachlorosilane. Available standards and guidelines are presented in Tables 3-8. The available ERPG values are comparable to the AEGLs derived herein.
TABLE 3-7 Summary of AEGL Values for Selected Chlorosilanesa
| Compound | Classification | 10 min | 30 min | 1 h | 4 h | 8 h |
| MONOCHLOROSILANES | ||||||
| Dimethyl chlorosilane | AEGL-1 | 1.8 ppm | 1.8 ppm | 1.8 ppm | 1.8 ppm | 1.8 ppm |
| Methyl chlorosilane | AEGL-2 | 100 ppm | 43 ppm | 22 ppm | 11 ppm | 11 ppm |
| Trimethyl chlorosilane | AEGL-3 | 620 ppm | 210 ppm | 100 ppm | 26 ppm | 26 ppm |
| DICHLOROSILANES | ||||||
| Dichlorosilane | AEGL-1 | 0.90 ppm | 0.90 ppm | 0.90 ppm | 0.90 ppm | 0.90 ppm |
| Diethyl dichlorosilane | AEGL-2 | 50 ppm | 22 ppm | 11 ppm | 5.5 pm | 5.5 ppm |
| Dimethyl dichlorosilane | AEGL-3 | 310 ppm | 110 ppm | 50 ppm | 13 ppm | 13 ppm |
| Diphenyl dichlorosilane | ||||||
| Methyl dichlorosilane | ||||||
| Methylvinyl dichlorosilane | ||||||
| TRICHLOROSILANES | ||||||
| Allyl trichlorosilane | AEGL-1 | 0.60 ppm | 0.60 ppm | 0.60 ppm | 0.60 ppm | 0.60 ppm |
| Amyl trichlorosilane | AEGL-2 | 33 ppm | 14 ppm | 7.3 ppm | 3.7 pm | 3.7 ppm |
| Butyl trichlorosilane | AEGL-3 | 210 ppm | 70 ppm | 33 ppm | 8.7 ppm | 8.7 ppm |
| Chloromethyl trichlorosilane | ||||||
| Dodecyl trichlorosilane | ||||||
| Ethyl trichlorosilane | ||||||
| Hexyl trichlorosilane | ||||||
| Methyl trichlorosilane | ||||||
| Nonyl trichlor osilane | ||||||
| Octadecyl trichlorosilane | ||||||
| Octyl trichlorosilane | ||||||
| Propyl trichlorosilane | ||||||
| Trichloro(dichlorophenyl)silane | ||||||
| Trichlorophenylsilane | ||||||
| Trichlorosilane | ||||||
| Vinyl trichlorosilane |
TABLE 3-7
| Compound | Classification | 10 min | 30 min | 1 h | 4 h | 8 h |
| TETRACHLOROSILANE | ||||||
| AEGL-1 | 0.45 ppm | 0.45 ppm | 0.45 ppm | 0.45 ppm | 0.45 ppm | |
| AEGL-2 | 25 ppm | 11 ppm | 5.5 ppm | 2.8 ppm | 2.8 ppm | |
| AEGL-3 | 160 ppm | 53 ppm | 25 ppm | 6.5 ppm | 6.5 ppm |
aValues given in ppm. To convert ppm to mg/m3: (ppm × molecular weight) ÷ 24.5. See Appendix A for the appropriate molecular weight.
TABLE 3-8 Extant Standards and Guidelines for Selected Chlorosilanes
| Exposure Duration | |||||
| Guideline | 10 min | 30 min | 1 h | 4 h | 8 h |
| MONOCHLOROSILANES | |||||
| AEGL-1 | 1.8 ppm | 1.8 ppm | 1.8 ppm | 1.8 ppm | 1.8 ppm |
| AEGL-2 | 100 ppm | 43 ppm | 22 ppm | 11 ppm | 11 ppm |
| AEGL-3 | 620 ppm | 210 ppm | 100 ppm | 26 ppm | 26 ppm |
| Trimethylchlorosilane | |||||
| ERPG-1 (AIHA)a | 3 ppm | ||||
| ERPG-2 (AIHA)a | 20 ppm | ||||
| ERPG-3 (AIHA)a | 150 ppm | ||||
| WEEL (AIHA)b | 5 ppm (ceiling) | ||||
| DICHLOROSILANES | |||||
| AEGL-1 | 0.90 ppm | 0.90 ppm | 0.90 ppm | 0.90 ppm | 0.90 ppm |
| AEGL-2 | 50 ppm | 22 ppm | 11 ppm | 5.5 ppm | 5.5 ppm |
| AEGL-3 | 310 ppm | 110 ppm | 50 ppm | 13 ppm | 13 ppm |
| Dimethyl dichlorosilane | |||||
| ERPG-1 (AIHA)a | 2 ppm | ||||
| ERPG-2 (AIHA)a | 10 ppm | ||||
| ERPG-3 (AIHA)a | 75 ppm | ||||
| WEEL (AIHA)b | 2 ppm (ceiling) | ||||
| TRICHLOROSILANES | |||||
| AEGL-1 | 0.60 ppm | 0.60 ppm | 0.60 ppm | 0.60 ppm | 0.60 ppm |
| AEGL-2 | 33 ppm | 14 ppm | 7.3 ppm | 3.7 ppm | 3.7 ppm |
| AEGL-3 | 210 ppm | 70 ppm | 33 ppm | 8.7 ppm | 8.7 ppm |
| Trichlorosilane | |||||
| ERPG-1 (AIHA)a | 1 ppm | ||||
| ERPG-2 (AIHA)a | 3 ppm | ||||
| ERPG-3 (AIHA)a | 25 ppm | ||||
| WEEL (AIHA)b | 0.5 ppm (ceiling) | ||||
| Methyl trichlorosilane | |||||
| ERPG-1 (AIHA)a | 0.5 ppm | ||||
| ERPG-2 (AIHA)a | 3 ppm | ||||
| ERPG-3 (AIHA)a | 15 ppm | ||||
| WEEL (AIHA)b | 1 ppm (ceiling) | ||||
| Methyl trichlorosilane | |||||
| ERPG-1 (AIHA)a | 0.5 ppm | ||||
| ERPG-2 (AIHA)a | 5 ppm | ||||
TABLE 3-8
| Exposure Duration | |||||
| Guideline | 10 min | 30 min | 1 h | 4 h | 8 h |
| ERPG-3 (AIHA)a | 50 ppm | ||||
| WEEL (AIHA)b | 1 ppm (ceiling) | ||||
| TETRACHLOROSILANE | |||||
| AEGL-1 | 0.45 ppm | 0.45 ppm | 0.45 ppm | 0.45 ppm | 0.45 ppm |
| AEGL-2 | 25 ppm | 11 ppm | 5.5 ppm | 2.8 ppm | 2.8 ppm |
| AEGL-3 | 160 ppm | 53 ppm | 25 ppm | 6.5 ppm | 6.5 ppm |
| ERPG-1 (AIHA)a | 0.75 ppm | ||||
| ERPG-2 (AIHA)a | 5 ppm | ||||
| ERPG-3 (AIHA)a | 37 ppm | ||||
| WEEL (AIHA)b | 1 ppm (ceiling) | ||||
aERPG (Emergency Response Planning Guidelines, American Industrial Hygiene Association) (AIHA 2010).
ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing effects other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor. An ERPG-1 was not derived because of insufficient data. ERPG-2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing irreversible or other serious health effects or symptoms that could impair an individual’s ability to take protective action. The ERPG-2 for BCME is based on animal data, and was intended to be below 0.21 ppm, which was calculated to have a 1 × 10-4 excess carcinogenicity risk, and 0.7 ppm, which caused serious respiratory lesions in animals. ERPG-3 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing life-threatening health effects. The ERPG-3 for BCME is based on animal lethality data. bWEEL (Workplace Environmental Exposure Level, American Industrial Hygiene Association) (AIHA 2010).
WEELs are health-based values, expressed as either time-weighted average (TWA) concentrations or ceiling values believed to provide guidance for protection of most workers exposed as a result of their occupations. A WEEL ceiling value is the instantaneous concentration that should not be exceeded at any time during the workday to prevent acute adverse health effects or discomfort.
8.3. Data Adequacy and Research Needs
There are no human or animal data on chlorosilanes relevant to AEGL-1 health end points. Likewise, there are no appropriate human data and few animal data relevant to AEGL-2 end points. A single study (Jean et al. 2006) that estimated LC50 values for 11 of the 26 chlorosilanes considered in this chapter provided data on lethality (an AEGL-3 end point). This study also supports the inference that the hydrolysis product, HCl, is largely responsible for the acute
inhalation toxicity of the chlorosilanes. There is anecdotal information on other hydrolysis and decomposition products (Nakashima et al. 1996). However, no information on the chemical form, physiological disposition, or potential toxicity of these decomposition products was found. Additional research on the identity and potential toxicity of decomposition products would enhance confidence in the database.
The available data on chlorosilane toxicity is limited to 11 of the 26 compounds addressed herein, and there were no data on chlorosilanes with aromatic or chlorinated substituents. The lack of data on the contribution of aromatic or chlorinated substituents to the toxicity of the chlorosilanes introduces uncertainty with respect to the protection afforded by using the molar equivalent of AEGL values for HCl as a surrogate for the AEGLs estimated for diphenyl dichlorosilane, trichloro(dichlorophenyl)silane, and trichlorophenylsilane. Additional research would enhance confidence in the AEGLs for these compounds.
The database on HCl was described by NRC (2004, pp. 107-109) as follows:
Human data are limited to one study showing no significant effects in asthmatic subjects and to dated anecdotal information. Furthermore, the involvement of [reactive airway dysfunction syndrome] in HCl toxicity is unclear. Many more data are available for animal exposures; however, many of those studies used compromised animals or very small experimental groups, resulting in limited data for many species but no in-depth database for a given species. Also, some studies involve very short exposures to high concentrations of HCl. Thus, confidence in the AEGL values is at best moderate.
One important area of uncertainty is the role of ambient humidity on the release of HCl and the toxicity of chlorosilanes. The LC50 values reported by Jean et al. (2006), and used as the basis for concluding that the toxicity of chlorosilanes is well-predicted by HCl content, were obtained at a relative humidity of 35%. Higher humidity would probably have increased the degree of hydrolysis, resulting in higher HCl concentrations and lower concentrations of parent compound; whether this would affect the lethal concentrations is unknown and merits additional research.
9. REFERENCES
AIHA (American Industrial Hygiene Association). 1998. Trichlorosilane (CAS No. 10025-78-2). Emergency Response Planning Guidelines. American Industrial Hygiene Association, Fairfax, VA [online]. Available: http://www.aiha.org/insideaiha/GuidelineDevelopment/ERPG/Pages/default.aspx [accessed Sept. 2010].
AIHA (American Industrial Hygiene Association). 1999. Tetrachlorosilane (CAS No. 10026-04-7). Emergency Response Planning Guidelines. American Industrial Hy-
giene Association, Fairfax, VA [online]. Available: http://www.aiha.org/insideaiha/GuidelineDevelopment/ERPG/Pages/default.aspx [accessed Sept.r 2010].
AIHA (American Industrial Hygiene Association). 2001a. Dimethyl dichlorosilane (CAS No. 75-78-5). Emergency Response Planning Guidelines. American Industrial Hygiene Association, Fairfax, VA [online]. Available: http://www.aiha.org/insideaiha/GuidelineDevelopment/ERPG/Pages/default.aspx [accessed Sept. 2010.
AIHA (American Industrial Hygiene Association). 2001b. Methyl trichlorosilane (CAS No. 75-79-6). Emergency Response Planning Guidelines. American Industrial Hygiene Association, Fairfax, VA [online]. Available: http://www.aiha.org/insideaiha/GuidelineDevelopment/ERPG/Pages/default.aspx accessed September 2010.
AIHA (American Industrial Hygiene Association). 2001c. Trimethyl chlorosilane (CAS No. 75-77-4). Emergency Response Planning Guidelines. American Industrial Hygiene Association, Fairfax, VA [online]. Available: http://www.aiha.org/insideaiha/GuidelineDevelopment/ERPG/Pages/default.aspx [accessed Sept. 2010].
AIHA (American Industrial Hygiene Association). 2006. Vinyl trichlorosilane (CAS No. 75-94-5). Emergency Response Planning Guidelines. American Industrial Hygiene Association, Fairfax, VA [online]. Available: http://www.aiha.org/insideaiha/GuidelineDevelopment/ERPG/Pages/default.aspx [accessed Sept. 2010].
AIHA (American Industrial Hygiene Association). 2010. Emergency Response Planning Guidelines and Workplace Environmental Exposure Levels. American Industrial Hygiene Association, Fairfax, VA [online]. Available: http://www.aiha.org/insideaiha/GuidelineDevelopment/Pages/default.aspx [accessed Sept. 2010].
Alarie, Y. 1981. Dose-response analysis in animal studies: Prediction of human responses. Environ. Health Perspect. 42:9-13.
ASTM (American Society for Testing and Materials). 1991. Standard Test Method for Estimating Sensory Irritancy of Airborne Chemicals. Method E981. Pp. 610-619 in Book of Standards, Volume 11.04. Philadelphia, PA: American Society for Testing and Materials.
Barrow, C.S., Y. Alarie, J.C. Warrick, and M.F. Stock. 1977. Comparison of the sensory irritation response in mice to chlorine and hydrogen chloride. Arch. Environ. Health 32(2):68-76.
Bernstein, J.A. 1993. Reactive Airways Dysfunction Syndrome (RADS). DPICtions publication of the Drug & Poison Information Center, University of Cincinnati, Volume 12(2), April-June.
Bisesi, M.S. 1994. Organic silicon esters. Pp. 3096-3101 in Patty’s Industrial Hygiene and Toxicology, 4th Ed., Vol. II, Part D., G.D. Clayton, and F.E. Clayton, eds. New York: John Wiley & Sons.
ChemFinder. 2007a. Dimethyl chlorosilane (CAS No. 1006-35-9). Cambridge Scientific [online]. Available: http://chemfinder.cambridgesoft.com [accessed Sept. 2010].
ChemFinder. 2007b. Methylvinyl dichlorosilane (CAS No. 124-70-9). Cambridge Scientific. Available: http://chemfinder.cambridgesoft.com [accessed Sept. 2010].
Darmer, K.I., E.R. Kinkead, and L.C. DiPasquale. 1974. Acute toxicity in rats and mice exposed to hydrogen chloride gas and aerosols. Am. Ind. Hyg. Assoc. J. 35(10): 623-631.
EPA (U.S. Environmental Protection Agency). 1987. P. C-15 in Technical Guidance for Hazards Analysis. Emergency Planning for Extremely Hazardous Substances. EPA-OSWER-8-0001. U.S. Environmental Protection Agency, Federal Emergency Management Agency, U.S. Department of Transportation, Washington, DC. December 1987 [online]. Available: http://www.epa.gov/osweroe1/docs/chem/tech.pdf [accessed Nov. 14, 2011].
ESIS (European Chemical Substances Information System). 2011. Methyl chlorosilane (CAS Reg. No. 993-00-0). EC No. 213-600-4. European Commission, Joint Research Center, Institute for Health and Consumer Protection, Ispra, Italy [online]. Available: http://esis.jrc.ec.europa.eu/ [accessed Nov. 11, 2011].
Higgins, E.A., V. Fiorca, A.A. Thomas, and H.V. Davis. 1972. Acute toxicity of brief exposures to HF, HCL, NO2, and HCN with and without CO. Fire Technol. 8(2): 120-130.
HSDB (Hazardous Substances Data Bank). 2002a. Trichloro(chloromethyl) silane (CASRN 1558-25-4). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010].
HSDB (Hazardous Substances Data Bank). 2002b. Silicon Tetrachloride (CAS RN 10026-04-7). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010].
HSDB (Hazardous Substances Data Bank). 2007a. Allyltrichlorosilane (CASRN 107-37-9). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010].
HSDB (Hazardous Substances Data Bank). 2007b. Trichloropentylsilane (CASRN 107-72-2). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010].
HSDB (Hazardous Substances Data Bank). 2007c. Butyltrichlorosilane (CASRN 7521-80-4). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010.
HSDB (Hazardous Substances Data Bank). 2007d. Diethyldichlorosilane (CASRN. 1719-53-5). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010].
HSDB (Hazardous Substances Data Bank). 2007e. Dichlorodiphenylsilane (CASRN. 80-10-4). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010].
HSDB (Hazardous Substances Data Bank). 2007f. Dodecyltrichlorosilane (CASRN 4484-72-4). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010].
HSDB (Hazardous Substances Data Bank). 2007g. Trichloroethylsilane (CASRN. 115-21-9). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB accessed September 2010.
HSDB (Hazardous Substances Data Bank). 2007h. Hexyltrichlorosilane (CASRN 928-65-4). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010].
HSDB (Hazardous Substances Data Bank). 2007i. Methyltirchlorosilane (CASRN 75-79-6). TOXNET, Specialized Information Services, U.S. National Library of Med-
icine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010].
HSDB (Hazardous Substances Data Bank). 2007j. Nonyltrichlorosilane (CASRN 5283-67-0). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010].
HSDB (Hazardous Substances Data Bank). 2007k. Octyltrichlorosilane (CASRN 5283-66-9). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010].
HSDB (Hazardous Substances Data Bank). 2007l. Trichloropropylsilane (CASRN 141-57-1). TOXNET, Specialized Information Services, U.S. National Library of Medicine: Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010].
HSDB (Hazardous Substances Data Bank). 2007m. Trichloro(dichlorophenyl)silanes (CASRN. 27137-85-5). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010].
HSDB (Hazardous Substances Data Bank). 2007n. Phenyltrichlorosilane (CASRN. 98-13-5). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010].
HSDB (Hazardous Substances Data Bank). 2007o. Trichlorosilane (CASRN 10025-78-2). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010].
HSDB (Hazardous Substances Data Bank). 2007p. Trimethylchloro silane (CASRN 75-77-4). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010].
HSDB (Hazardous Substances Data Bank). 2007q. Trichlorovinyl silane (CASRN 75-94-5). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 2010].
HSDB (Hazardous Substances Data Bank). 2010a. Dimethyldichlorosilane (CASRN 75-78-5). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Nov. 16, 2011].
HSDB (Hazardous Substances Data Bank). 2010b. Octadecyltrichlorosilane (CASRN 112-04-9). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Nov. 16, 2011].
IPCS (International Programme on Chemical Safety). 1997. Dichlorosilane (CASRN 4109-96-0). International Chemical Safety Card ICSC 0442. International Programme on Chemical Safety and the Commission of the European Communities, Geneva, Switzerland [online]. Available: http://www.inchem.org/documents/icsc/icsc/eics0442.htm [accessed Nov. 14, 2011].
IPCS (International Programme on Chemical Safety). 2002a. Trimethyl chlorosilane (CASRN 75-77-4). International Chemical Safety Card ICSC 0966. International Programme on Chemical Safety and the Commission of the European Communi-
ties, Geneva, Switzerland [online]. Available: http://www.inchem.org/documents/icsc/icsc/eics0966.htm [accessed Nov. 14, 2011].
IPCS (International Programme on Chemical Safety). 2002b. Methyldichlorosilane (CASRN 75-54-7). International Chemical Safety Card ICSC 0297. International Programme on Chemical Safety and the Commission of the European Communities, Geneva, Switzerland [online]. Available: http://www.inchem.org/documents/icsc/icsc/eics0297.htm [accessed Nov. 14, 2011].
Jean, P.A., R.H. Gallavan, G.B. Kolesar, W.H. Siddiqui, J.A. Oxley, and R.G. Meeks. 2006. Chlorosilane acute inhalation toxicity and development of an LC50 prediction model. Inhal. Toxicol. 18(8):515-522.
Kaplan, H.L., A. Anzueto, W.G. Switzer, and R.K. Hinderer. 1988. Effects of hydrogen chloride on respiratory response and pulmonary function of the baboon. J. Toxicol. Environ. Health 23(4):473-493.
Kizer, K.W., L.G. Garb, and C.H. Hine. 1984. Health effects of silicon tetrachloride: Report of an urban accident. J. Occup. Med. 26(1):33-36.
Nakashima, H., K. Omae, T. Takebayshi, C. Ishizuka, H. Sakurai, K. Yamazaki, M. Na-kaza, T. Shibata, M. Kudo, and S. Koshi. 1996. Acute and subacute inhalation toxicity of dichlorosilane in male ICR mice. Arch. Toxicol. 70(3-4):218-223.
NJ DHSS (New Jersey Department of Health and Senior Services). 2009. Methyl Chloro-silane (CAS Reg. No. 993-00-0). Hazardous Substance Fact Sheet. New Jersey Department of Health and Senior Services: Trenton, NJ [online]. Available: http://nj.gov/health/eoh/rtkweb/documents/fs/1240.pdf [accessed Nov. 14, 2011].
NRC (National Research Council). 1991. Hydrogen chloride. Pp. 37-52 in Permissible Exposure Levels and Emergency Exposure Guidance Levels for Selected Airborne Contaminants. Washington, DC: National Academy Press.
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
NRC (National Research Council). 2004. Hydrogen chloride. Pp. 77-122 in Acute Exposure Guideline Levels for Selected Airborne Chemicals. Volume 4. Committee on Toxicology, Washington, DC: National Academies Press.
Promisloff, R.A., G.S. Lenchner, A. Phan, and A.V. Cichelli. 1990. Reactive airway dysfunction syndrome in three police officers following a roadside chemical spill. Chest 98(4):928-929.
Schaper, M. 1993. Development of a database for sensory irritants and its use in establishing occupational exposure limits. Am. Ind. Hyg. Assoc. J. 54(9):488-544.
Sellakumar, A.R., C.A. Snyder, J.J. Solomon, and R.E. Albert. 1985. Carcinogenicity of formaldehyde and hydrogen chloride in rats. Toxicol. Appl. Pharmacol. 81(3 Pt. 1):401-406.
SRC (Syracuse Research Corporation). 2011. Methyl chlorosilane (CAS Reg. No. 993-00-0). PhysProp Database. Syracuse Research Corporation: Syracuse, NY [online]. Available: http://www.syrres.com/what-we-do/databaseforms.aspx?id=386 [accessed Nov. 14, 2011].
Stavert, D.M., D.C. Archuleta, M.J. Behr, and B.E. Lehnert. 1991. Relative acute toxici-ties of hydrogen fluoride, hydrogen chloride, and hydrogen bromide in nose- and pseudo-mouth- breathing rats. Fundam. Appl. Toxicol. 16(4):636-655.
Stevens, B., J.Q. Koenig, V. Rebolledo, Q.S. Hanley, and D.S. Covert. 1992. Respiratory effects from the inhalation of hydrogen chloride in young adult asthmatics. J. Occup. Med. 34(9):923-929.
ten Berge, W.F., A. Zwart, and L.M. Appleman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapours and gases. J. Hazard. Mater. 13(3):301-309.
Toxigenics, Inc. 1984. 90-day Inhalation Toxicity Study of Hydrogen Chloride Gas in B6C3F1 Mice, Sprague-Dawley Rats and Fischer-344 Rats, Revised. Toxigenics, Inc., Decatur, IL. 68 pp.
USCG (U.S. Coast Guard). 2007. National Response Center Standard Data Report. U.S. Coast Guard: Washington, DC [online]. Available: www.nrc.uscg.mil [accessed Sept. 2010].
Vernot, E.H., J.D. MacEwen, C.C. Haun, and E.R. Kinkead. 1977. Acute toxicity and skin corrosion data for some organic and inorganic compounds and aqueous solutions. Toxicol. Appl. Pharmacol. 42(2):417-423.
Wohlslagel, J., L.C. DiPasquale, and E.H. Vernot. 1976. Toxicity of solid rocket motor exhaust: Effects of HCl, HF, and alumina on rodents. J. Combust. Toxicol. 3:6169.
APPENDIX A
PHYSICAL AND CHEMICAL PROPERTIES OF SELECTED CHLOROSILANES
TABLE A-1 Chemical and Physical Properties for Allyl Trichlorosilane
| Parameter | Value | References |
| Synonyms | Propen-3-yltrichlorosilane; trichloroallylsilane; trichloro- 2-propenyl-silane | HSDB 2007a |
| CAS registry no. | 107-37-9 | HSDB 2007a |
| Chemical formula | C3H5Cl3Si | HSDB 2007a |
| Molecular weight | 175.52 | HSDB 2007a |
| Physical state | Colorless liquid | HSDB 2007a |
| Melting point | 35°C | HSDB 2007a |
| Boiling point | 117.5°C | HSDB 2007a |
| Vapor density (air = 1) | 6.05 | HSDB 2007a |
| Liquid density/specific gravity | 1.20 g/cm3 at 20°C | HSDB 2007a |
| Solubility in water | Hydrolyzes to form HCl | HSDB 2007a |
| Vapor pressure | 53 mm Hg at 47.5°C | HSDB 2007a |
| Conversion factors | 1 ppm = 7.2 mg/m3 1 mg/m3 = 0.14 ppm |
TABLE A-2 Chemical and Physical Properties for Amyl Trichlorosilane
| Parameter | Value | References |
| Synonyms | Pentylsilicon trichloride; pentyltrichlorosilane; trichloropentylsilane; trichloroamylsilane; trichloropentylsilane | HSDB 2007b |
| CAS registry no. | 107-72-2 | HSDB 2007b |
| Chemical formula | C5H11Cl3Si | HSDB 2007b |
| Molecular weight | 205.59 | HSDB 2007b |
| Physical state | Colorless to yellow liquid | HSDB 2007b |
| Boiling point | 172°C | HSDB 2007b |
| Liquid density/specific gravity | 1.1330 g/cm3 at 20°C | HSDB 2007b |
| Solubility in water | Hydrolyzes to form HCl | HSDB 2007b |
| Conversion factors | 1 ppm = 8.4 mg/m3 1 mg/m3 = 0.12 ppm |
TABLE A-3 Chemical and Physical Properties for Butyl Trichlorosilane
| Parameter | Value | References |
| Synonyms | Trichlorobutyl silane; butylsilicon trichloride | HSDB 2007c |
| CAS registry no. | 7521-80-4 | HSDB 2007c |
| Chemical formula | C4H9Cl3Si | HSDB 2007c |
| Molecular weight | 191.56 | HSDB 2007c |
| Physical state | Colorless liquid | HSDB 2007c |
| Boiling point | 148.5°C | HSDB 2007c |
| Vapor density (air = 1) | 6.4 | HSDB 2007c |
| Liquid density/specific gravity | 1.160 g/cm3 at 20°C | HSDB 2007c |
| Solubility in water | Hydrolyzes to form HCl | HSDB 2007c |
| Conversion factors | 1 ppm = 7.8 mg/m3 1 mg/m3 = 0.13 ppm |
TABLE A-4 Chemical and Physical Properties for Chloromethyl
| Parameter | Value | References |
| Synonyms | Silane, trichloro(chloromethyl)-; Chloromethyl(trichloro)-silane | HSDB 2002a |
| CAS registry no. | 1558-25-4 | HSDB 2002a |
| Chemical formula | CH2Cl4Si | HSDB 2002a |
| Molecular weight | 183.93 | HSDB 2002a |
| Physical state | Liquid | HSDB 2002a |
| Boiling point | 118°C | EPA 1987 |
| Liquid density/specific gravity | 1.476 g/cm3 | HSDB 2002a |
| Vapor pressure | 30 mm Hg at 25°C | EPA 1987 |
| Conversion factors | 1 ppm = 7.5 mg/m3 1 mg/m3 = 0.13 ppm |
TABLE A-5 Chemical and Physical Properties for Dichlorosilane
| Parameter | Value | References |
| Synonyms | Chlorosilane; silicon chloride hydride | IPCS 1997 |
| CAS registry no. | 4109-96-0 | IPCS 1997 |
| Chemical formula | H2Cl2Si | IPCS 1997 |
| Molecular weight | 101.01 | IPCS 1997 |
| Physical state | Colorless gas | IPCS 1997 |
| Melting point | -122°C | IPCS 1997 |
| Boiling point | 8°C | IPCS 1997 |
| Vapor density (air = 1) | 3.48 | IPCS 1997 |
| Solubility in water | Hydrolyzes to form HCl | IPCS 1997 |
| Vapor pressure Conversion factors | 163.6 kPa at 20°C 1 ppm = 4.1 mg/m3 1 mg/m3 = 0.24 ppm | IPCS 1997 |
TABLE A-6 Chemical and Physical Properties for Diethyl Dichlorosilane
| Parameter | Value | References |
| Synonyms | Dichloroethylsilane | HSDB 2007d |
| CAS registry no. | 1719-53-5 | HSDB 2007d |
| Chemical formula | C4H10Cl2Si | HSDB 2007d |
| Molecular weight | 157.11 | HSDB 2007d |
| Physical state | Colorless liquid | HSDB 2007d |
| Melting point | -96.5°C | HSDB 2007d |
| Boiling point | 129°C | HSDB 2007d |
| Vapor density (air = 1) | 5.14 | HSDB 2007d |
| Liquid density/specific gravity | 1.0504 at 20°C | HSDB 2007d |
| Solubility in water | Hydrolyzes to form HCl | HSDB 2007d |
| Vapor pressure | 11.9 mm Hg at 25°C | HSDB 2007d |
| Conversion factors | 1 ppm = 6.4 mg/m3 1 mg/m3 = 0.16 ppm |
TABLE A-7 Chemical and Physical Properties for Dimethyl Chlorosilane
| Parameter | Value | References |
| Synonyms | Chlorodimethylsilane | ChemFinder 2007a |
| CAS registry no. | 1066-35-9 | ChemFinder 2007a |
| Chemical formula | C2H7ClSi | ChemFinder 2007a |
| Molecular weight | 94.62 | ChemFinder 2007a |
| Melting point | -111°C | ChemFinder 2007a |
| Boiling point | 36.4°C | ChemFinder 2007a |
| Conversion factors | 1 ppm = 3.9 mg/m3 1 mg/m3 = 0.26 ppm |
TABLE A-8 Chemical and Physical Properties for Diethyl Dichlorosilane
| Parameter | Values | Reference |
| Synonyms | Dichlorodimethylsilane | AIHA 2001a |
| CAS registry no. | 75-78-5 | HSDB 2010a |
| Chemical formula | C2H6Cl2Si | HSDB 2010a |
| Molecular weight | 129.06 | HSDB 2010a |
| Physical state | Colorless liquid | HSDB 2010a |
| Melting point | <-70°C | AIHA 2001a |
| Boiling point | 70.3°C | HSDB 2010a |
| Flash point | -9°C | AIHA 2001a |
| Density | 1.07 g/cm3 at 25°C | HSDB 2010a |
| Solubility in water | Reacts and decomposes | AIHA 2001a |
| Vapor pressure | 115 mm Hg at 20°C | AIHA 2001a |
| Conversion factors | 1 mg/m3 = 0.19 ppm 1 ppm = 5.3 mg/m3 |
TABLE A-9 Chemical and Physical Properties for Diphenyl Dichlorosilane
| Parameter | Value | References |
| Synonyms | Dichlorodiphenyl silane; diphenylsilicon dichloride; diphenylsilyl dichloride | HSDB 2007e |
| CAS registry no. | 80-10-4 | HSDB 2007e |
| Chemical formula | C12H10Cl2Si | HSDB 2007e |
| Molecular weight | 253.2 | HSDB 2007e |
| Physical state | Colorless liquid | HSDB 2007e |
| Melting point | -22°C | HSDB 2007e |
| Boiling point | 305°C | HSDB 2007e |
| Vapor density (air = 1) | 8.45 | HSDB 2007e |
| Liquid density/specific gravity | 1.204 at 25°C | HSDB 2007e |
| Solubility in water | Hydrolyzes to form HCl | HSDB 2007e |
| Vapor pressure | 4.986 kPa at 192°C | HSDB 2007e |
| Conversion factors | 1 ppm = 10.3 mg/m3 1 mg/m3 = 0.097 ppm |
TABLE A-10 Chemical and Physical Properties for Dodecyl Trichlorosilane
| Parameter | Value | References |
| Synonyms | Trichlorododecyl silane | HSDB 2007f |
| CAS registry no. | 4484-72-4 | HSDB 2007f |
| Chemical formula | C12H25Cl3Si | HSDB 2007f |
| Molecular weight | 303.77 | HSDB 2007f |
| Physical state | Colorless to yellow liquid | HSDB 2007f |
| Boiling point | 288°C | HSDB 2007f |
| Liquid density/specific gravity | 1.026 at 25°C | HSDB 2007f |
| Solubility in water | Hydrolyzes to form HCl | HSDB 2007f |
| Conversion factors | 1 ppm = 12 mg/m3 1 mg/m3 = 0.081 ppm |
TABLE A-11 Chemical and Physical Properties for Ethyl Trichlorosilane
| Parameter | Value | References |
| Synonyms | Ethyl silicon trichloride; trichloro ethylsilane; trichloroethylsilicane; trichloroethyl silicon | HSDB 2007g |
| CAS registry no. | 115-21-9 | HSDB 2007g |
| Chemical formula | C2H5Cl3Si | HSDB 2007g |
| Molecular weight | 163.51 | HSDB 2007g |
| Physical state | Colorless liquid | HSDB 2007g |
| Melting point | -105.6°C | HSDB 2007g |
| Boiling point | 100.5°C | HSDB 2007g |
| Vapor density (air = 1) | 5.6 | HSDB 2007g |
| Liquid density/specific gravity | 1.238 at 20°C | HSDB 2007g |
| Solubility in water | Hydrolyzes to form HCl | HSDB 2007g |
| Vapor pressure | 47.18 mm Hg at 25°C | HSDB 2007g |
| Conversion factors | 1 ppm = 6.7 mg/m3 1 mg/m3 = 0.15 ppm |
TABLE A-12 Chemical and Physical Properties for Hexyl Trichlorosilane
| Parameter | Value | References |
| Synonyms | Trichlorohexylsilane | HSDB 2007h |
| CAS registry no. | 928-65-4 | HSDB 2007h |
| Chemical formula | C6H13Cl3Si | HSDB 2007h |
| Molecular weight | 219.61 | HSDB 2007h |
| Physical state | Colorless liquid | HSDB 2007h |
| Boiling point | 190°C | HSDB 2007h |
| Liquid density/specific gravity | 1.1100 g/cm3 at 20°C | HSDB 2007h |
| Solubility in water | Hydrolyzes to form HCl | HSDB 2007h |
| Conversion factors | 1 ppm = 8.9 mg/m3 1 mg/m3 = 0.11 ppm |
TABLE A-13 Chemical and Physical Properties for Methyl Chlorosilane
| Parameter | Values | Reference |
| Synonyms | Chloromethylsilane | ESIS 2011 |
| CAS registry no. | 993-00-0 | SRC 2011 |
| Chemical formula | CH5ClSi | ESIS 2011 |
| Molecular weight | 77.57 | SRC 2011 |
| Physical state | Liquid | SRC 2011 |
| Melting point | -135°C | SRC 2011 |
| Boiling point | 7°C | SRC 20114 |
| Solubility in water | Reacts and decomposes in water | NJ DHSS 2009 |
| Log P (octanol-water partition coefficient) | 1.33 | SRC 2011 |
| Conversion factors | 1 mg/m3 = 0.32 ppm 1 ppm = 3.2 mg/m3 |
TABLE A-14 Chemical and Physical Properties for Methyl Dichlorosilane
| Parameter | Values | Reference |
| Synonyms | Dichloromethylsilane; monomethyl dichlorosilane | IPCS 2002b |
| CAS registry no. | 75-54-7 | IPCS 2002b |
| Chemical formula | CH4Cl2Si | IPCS 2002b |
| Molecular weight | 115.0 | IPCS 2002b |
| Physical state | Colorless liquid | IPCS 2002b |
| Melting point | -92°C | IPCS 2002b |
| Boiling point | 41°C | IPCS 2002b |
| Vapor Density (air = 1) | 3.97 | IPCS 2002b |
| Solubility in water | Reacts and decomposes in water; soluble in benzene, ether, and heptane | IPCS 2002b |
| Vapor pressure | 47.1 kPa at 20°C | IPCS 2002b |
| Flash point | -22°C | IPCS 2002b |
| Auto-ignition temperature | 290°C | IPCS 2002b |
| Conversion factors | 1 mg/m3 = 0.21 ppm 1 ppm = 4.7 mg/m3 |
TABLE A-15 Chemical and Physical Properties for Methyl Trichlorosilane
| Parameter | Value | Reference |
| Synonyms | Trichloromethylsilane | AIHA 2001b |
| CAS registry no. | 75-79-6 | HSDB 2007i |
| Chemical formula | CH3Cl3Si | HSDB 2007i |
| Molecular weight | 149.48 | HSDB 2007i |
| Physical state | Liquid | AIHA 2001b |
| Melting point | -90°C | HSDB 2007i |
| Boiling point | 65.6°C | HSDB 2007i |
| Density | 5.17 g/cm3 | Bisesi 1994 |
| Solubility in water | Reacts and decomposes | AIHA 2001b |
| Vapor pressure | 134 mm Hg at 20°C | AIHA 2001b |
| Flash point | 3°C | Bisesi 1994 |
| Conversion factors | 1 mg/m3 = 0.16 ppm 1 ppm = 6.1 mg/m3 |
TABLE A-16 Chemical and Physical Properties for Methylvinyl Dichlorosilane
| Parameter | Value | References |
| Synonyms | Dicloro methylvinylsilane; Vinyl methyl dichlorosilane | ChemFinder 2007b |
| CAS registry no. | 124-70-9 | ChemFinder 2007b |
| Chemical formula | C3H6Cl2Si | ChemFinder 2007b |
| Molecular weight | 141.1 | ChemFinder 2007b |
| Boiling point | 92°C | ChemFinder 2007b |
| Liquid density/specific gravity | 1.08 at 20°C | ChemFinder 2007b |
| Conversion factors | 1 ppm = 5.8 mg/m3 1 mg/m3 = 0.17 ppm |
TABLE A-17 Chemical and Physical Properties for Nonyl Trichlorosilane
| Parameter | Value | References |
| Synonyms | Trichlorononylsilane | HSDB 2007j |
| CAS registry no. | 5283-67-0 | HSDB 2007j |
| Chemical formula | C9H19Cl3Si | HSDB 2007j |
| Molecular weight | 261.72 | HSDB 2007j |
| Physical state | Water-white liquid | HSDB 2007j |
| Liquid density/specific gravity | 1.072 g/cm3 at 25°C | HSDB 2007j |
| Solubility in water | Hydrolyzes to form HCl | HSDB 2007j |
| Conversion factors | 1 ppm = 10.7 mg/m3 1 mg/m3 = 0.094 ppm |
TABLE A-18 Chemical and Physical Properties for Octadecyl Trichlorosilane
| Parameter | Value | References |
| Synonyms | Silane, trichlorooctadecyl, trichlorooctadecylsilane | HSDB 2010b |
| CAS registry no. | 112-04-9 | HSDB 2010b |
| Chemical formula | C18H37Cl3Si | HSDB 2010b |
| Molecular weight | 387.93 | HSDB 2010b |
| Physical state | Water-white liquid | HSDB 2010b |
| Melting point | About 20°C | HSDB 2010b |
| Boiling point | 380°C | HSDB 2010b |
| Liquid density/specific gravity | 0.984 g/cm3 at 25°C | HSDB 2010b |
| Conversion factors | 1 ppm = 16 mg/m3 1 mg/m3 = 0.063 ppm |
TABLE A-19 Chemical and Physical Properties for Octyl Trichlorosilane
| Parameter | Value | References |
| Synonyms | Trichlorooctylsilane | HSDB 2007k |
| CAS registry no. | 5283-66-9 | HSDB 2007k |
| Chemical formula | C8H17Cl3Si | HSDB 2007k |
| Molecular weight | 247.67 | HSDB 2007k |
| Physical state | Fuming liquid | HSDB 2007k |
| Boiling point | 232°C | HSDB 2007k |
| Liquid density/specific gravity | 1.073 g/mL | HSDB 2007k |
| Solubility in water | Hydrolyzes to form HCl | HSDB 2007k |
| Conversion factors | 1 ppm = 10 mg/m3 1 mg/m3 = 0.099 ppm |
TABLE A-20 Chemical and Physical Properties for Propyl Trichlorosilane
| Parameter | Value | References |
| Synonyms | Trichloropropylsilane; | HSDB 2007b |
| n-propyl trichlorosilane | ||
| CAS registry no. | 141-57-1 | HSDB 2007b |
| Chemical formula | C3H7Cl3Si | HSDB 2007b |
| Molecular weight | 177.53 | HSDB 2007b |
| Physical state | Colorless liquid | HSDB 2007b |
| Boiling point | 123.5°C | HSDB 2007b |
| Vapor density (air = 1) | 6.1215 | HSDB 2007b |
| Liquid density/specific gravity | 1.195 g/cm3 at 20°C | HSDB 2007b |
| Solubility in water | Hydrolyzes to form HCl | HSDB 2007b |
| Vapor pressure | 28.8 mm Hg at 20°C | HSDB 2007b |
| Conversion factors | 1 ppm = 7.2 mg/m3 1 mg/m3 = 0.14 ppm |
TABLE A-21 Chemical and Physical Properties for Tetrachlorosilane
| Parameter | Value | References |
| Synonyms | Trichloropropylsilane; | HSDB 2007b |
| n-propyl trichlorosilane | ||
| CAS registry no. | 141-57-1 | HSDB 2007b |
| Chemical formula | C3H7Cl3Si | HSDB 2007b |
| Molecular weight | 177.53 | HSDB 2007b |
| Physical state | Colorless liquid | HSDB 2007b |
| Boiling point | 123.5°C | HSDB 2007b |
| Vapor density (air = 1) | 6.1215 | HSDB 2007b |
| Liquid density/specific gravity | 1.195 g/cm3 at 20°C | HSDB 2007b |
| Solubility in water | Hydrolyzes to form HCl | HSDB 2007b |
| Vapor pressure | 28.8 mm Hg at 20°C | HSDB 2007b |
| Conversion factors | 1 ppm = 7.2 mg/m3 1 mg/m3 = 0.14 ppm |
TABLE A-22 Chemical and Physical Properties for Trichloro(dichlorophenyl)silane
| Parameter | Value | References |
| Synonyms | Dichlorophenyltrichlorosilane | HSDB 2007m |
| CAS registry no. | 27137-85-5 | HSDB 2007m |
| Chemical formula | C6H3Cl5Si | HSDB 2007m |
| Molecular weight | 280.43 | HSDB 2007m |
| Physical state | Straw-colored liquid | HSDB 2007m |
| Boiling point | 260°C | HSDB 2007m |
| Liquid density/specific gravity | 1.562 g/cm3 | HSDB 2007m |
| Conversion factors | 1 ppm = 11.4 mg/m3 1 mg/m3 = 0.087 ppm |
TABLE A-23 Chemical and Physical Properties for Trichlorophenylsilane
| Parameter | Value | References |
| Synonyms | Phenyltrichlorosilane; phenylsilicon trichloride | HSDB 2007n |
| CAS registry no. | 98-13-5 | HSDB 2007n |
| Chemical formula | C6H5Cl3Si | HSDB 2007n |
| Molecular weight | 211.55 | HSDB 2007n |
| Physical state | Colorless liquid | HSDB 2007n |
| Boiling point | 201°C | HSDB 2007n |
| Vapor density (air = 1) | 7.36 | HSDB 2007n |
| Liquid density/specific gravity | 1.321 g/cm3 at 20°C | HSDB 2007n |
| Solubility in water | Hydrolyzes to form HCl | HSDB 2007n |
| Vapor pressure | 0.426 mm Hg at 25°C | HSDB 2007n |
| Conversion factors | 1 ppm = 8.6 mg/m3 1 mg/m3 = 0.12 ppm |
TABLE A-24 Chemical and Physical Properties for Trichlorosilane
| Parameter | Value | References |
| Synonyms | Silicochloroform | HSDB 2007o |
| CAS registry no. | 10025-78-2 | HSDB 2007o |
| Chemical formula | Cl3HSi | HSDB 2007o |
| Molecular weight | 135.47 | HSDB 2007o |
| Physical state | Colorless liquid | HSDB 2007o |
| Melting point | -126.5°C | HSDB 2007o |
| Boiling point | 31.8°C | HSDB 2007o |
| Vapor density (air = 1) | 4.67 | HSDB 2007o |
| Liquid density/specific gravity | 1.3417 g/cm3 at 20°C | HSDB 2007o |
| Solubility in water | Hydrolyzes to form HCl | HSDB 2007o |
| Vapor pressure | 594 mm Hg at 25°C | HSDB 2007o |
| Conversion factors | 1 ppm = 5.3 mg/m3 1 mg/m3 = 0.18 ppm |
TABLE A-25 Chemical and Physical Properties for Trimethyl Chlorosilane
| Parameter | Value | References |
| Synonyms | Chlorotrimethylsilane; monochlorotrimethylsilicon | HSDB 2007p |
| CAS registry no. | 75-77-4 | HSDB 2007p |
| Chemical formula | C3H9ClSi | HSDB 2007p |
| Molecular weight | 108.642 | HSDB 2007p |
| Physical state | Colorless liquid | HSDB 2007p |
| Melting point | -40°C | HSDB 2007p |
| Boiling point | 57°C | HSDB 2007p |
| Vapor density (air = 1) | 3.75 | HSDB 2007p |
| Liquid density/specific gravity | 0.854 g/cm3 at 25°C | HSDB 2007p |
| Solubility in water | Hydrolyzes rapidly | HSDB 2007p |
| Flash point | 0°F (open cup) | HSDB 2007p |
| Conversion factors in air | 1 mg/m3 = 0.23 ppm 1 ppm = 4.4 mg/m3 |
TABLE A-26 Chemical and Physical Properties for Vinyl Trichlorosilane
| Parameter | Value | References |
| Synonyms | Trichlorovinylsilane; vinylsilicon tetrachloride; trichlorovinyl silicon | HSDB 2007q |
| CAS registry no. | 75-94-5 | HSDB 2007q |
| Chemical formula | C2H3Cl3Si | HSDB 2007q |
| Molecular weight | 161.49 | HSDB 2007q |
| Physical state | Fuming liquid | HSDB 2007q |
| Melting point | -95°C | HSDB 2007q |
| Boiling point | 91.5°C | HSDB 2007q |
| Vapor density (air = 1) | 5.61 | HSDB 2007q |
| Liquid density/specific gravity | 1.2426 g/cm3 at 20°C | HSDB 2007q |
| Solubility in water | Hydrolyzes to form HCl | HSDB 2007q |
| Vapor pressure | 65.9 mm Hg at 25°C | HSDB 2007q |
| Conversion factors | 1 ppm = 6.6 mg/m3 1 mg/m3 = 0.15 ppm |
APPENDIX B
DERIVATION OF AEGL VALUES FOR SELECTED CHLOROSILANES
Derivation of AEGL-1
| Monochlorosilanes | |
| Key study: | AEGL-1 values for HCl (Stevens et al. 1992; NRC 2004) |
| 10-min AEGL-1: 30-min AEGL-1: 1-h AEGL-1: 4-h AEGL-1: 8-h AEGL-1: |
1.8 ppm 1.8 ppm 1.8 ppm 1.8 ppm 1.8 ppm |
| Dichlorosilanes | |
| Key study: | AEGL-1 values for HCl (Stevens et al. 1992; NRC 2004) divided by a molar adjustment factor of 2 |
| 10-min AEGL-1: 30-min AEGL-1: 1-h AEGL-1: 4-h AEGL-1: 8-h AEGL-1: |
1.8 ppm ÷ 2 = 0.90 ppm 1.8 ppm ÷ 2 = 0.90 ppm 1.8 ppm ÷ 2 = 0.90 ppm 1.8 ppm ÷ 2 = 0.90 ppm 1.8 ppm ÷ 2 = 0.90 ppm |
| Trichlorosilanes | |
| Key study: | AEGL-1 values for HCl (Stevens et al. 1992; NRC 2004) divided by a molar adjustment factor of 3 |
| 10-min AEGL-1: 30-min AEGL-1: 1-h AEGL-1: 4-h AEGL-1: 8-h AEGL-1: |
1.8 ppm ÷ 3 = 0.60 ppm 1.8 ppm ÷ 3 = 0.60 ppm 1.8 ppm ÷ 3 = 0.60 ppm 1.8 ppm ÷ 3 = 0.60 ppm 1.8 ppm ÷ 3 = 0.60 ppm |
| Tetrachlorosilane | |
| Key study: | AEGL-1 values for HCl (Stevens et al. 1992; NRC 2004) divided by a molar adjustment factor of 4 |
| 10-min AEGL-1: 30-min AEGL-1: 1-h AEGL-1: 4-h AEGL-1: 8-h AEGL-1: |
1.8 ppm ÷ 4 = 0.45 ppm 1.8 ppm ÷ 4 = 0.45 ppm 1.8 ppm ÷ 4 = 0.45 ppm 1.8 ppm ÷ 4 = 0.45 ppm 1.8 ppm ÷ 4 = 0.45 ppm |
| Derivation of AEGL-2 | |
| Monochlorosilanes | |
| Key study: | AEGL-2 values for HCl (Barrow et al. 1977; Stavert et al. 1991; NRC 2004). |
| 10-min AEGL-1: 30-min AEGL-1: 1-h AEGL-1: 4-h AEGL-1: 8-h AEGL-1: |
100 ppm 43 ppm 22 ppm 11 ppm 11 ppm |
| Dichlorosilanes | |
| Key study: | AEGL-2 values for HCl (Barrow et al. 1977; Stavert et al. 1991; NRC 2004) divided by a molar adjustment factor of 2 |
| 10-min AEGL-2: 30-min AEGL-2: 1-h AEGL-2: 4-h AEGL-2: 8-h AEGL-2: |
100 ppm ÷ 2 = 50 ppm 43 ppm ÷ 2 = 22 ppm 22 ppm ÷ 2 = 11 ppm 11 ppm ÷ 2 = 5.5 ppm 11 ppm ÷ 2 = 5.5 ppm |
| Trichlorosilanes | |
| Key study: | AEGL-2 values for HCl (Barrow et al. 1977; Stavert et al. 1991; NRC 2004) divided by a molar adjustment factor of 3 |
| 10-min AEGL-2: 30-min AEGL-2: 1-h AEGL-2: 4-h AEGL-2: 8-h AEGL-2: |
100 ppm ÷ 3 = 33 ppm 43 ppm ÷ 3 = 14 ppm 22 ppm ÷ 3 = 7.3 ppm 11 ppm ÷ 3 = 3.7 ppm 11 ppm ÷ 3 = 3.7 ppm |
| Tetrachlorosilane | |
| Key study: | AEGL-2 values for HCl (Barrow et al. 1977; Stavert et al. 1991; NRC 2004) divided by a molar adjustment factor of 4 |
| 10-min AEGL-2: 30-min AEGL-2: 1-h AEGL-2: 4-h AEGL-2: 8-h AEGL-2: |
100 ppm ÷ 4 = 25 ppm 43 ppm ÷ 4 = 11 ppm 22 ppm ÷ 4 = 5.5 ppm 11 ppm ÷ 4 = 2.8 ppm 11 ppm ÷ 4 = 2.8 ppm |
| Derivation of AEGL-3 | |
| Monochlorosilanes | |
| Key study: | AEGL-3 values for HCl (Wohlslagel et al. 1976; Vernot et al. 1977; NRC 2004) |
| 10-min AEGL-1: 30-min AEGL-1: 1-h AEGL-1: 4-h AEGL-1: 8-h AEGL-1: |
620 ppm 210 ppm 100 ppm 26 ppm 26 ppm |
| Dichlorosilanes | |
| Key study: | AEGL-3 values for HCl (Wohlslagel et al. 1976; Vernot et al. 1977; NRC 2004) divided by a molar adjustment factor of 2 |
| 10-min AEGL-3: 30-min AEGL-3: 1-h AEGL-3: 4-h AEGL-3: 8-h AEGL-3: |
620 ppm ÷ 2 = 310 ppm 210 ppm ÷ 2 = 105 ppm (rounded to 110) 100 ppm ÷ 2 = 50 ppm 26 ppm ÷ 2 = 13 ppm 26 ppm ÷ 2 = 13 ppm |
| Trichlorosilanes | |
| Key study: | AEGL-3 values for HCl (Wohlslagel et al. 1976; Vernot et al. 1977; NRC 2004) divided by a molar adjustment factor of 3 |
| 10-min AEGL-3: 30-min AEGL-3: 1-h AEGL-3: 4-h AEGL-3: 8-h AEGL-3: |
620 ppm ÷ 3 = 210 ppm 210 ppm ÷ 3 = 70 ppm 100 ppm ÷ 3 = 33 ppm 26 ppm ÷ 3 = 8.7 ppm 26 ppm ÷ 3 = 8.7 ppm |
| Tetrachlorosilane | |
| Key study: | AEGL-3 values for HCl (Wohlslagel et al. 1976; Vernot et al. 1977; NRC 2004) divided by a molar adjustment factor of 4 |
| 10-min AEGL-3: 30-min AEGL-3: 1-h AEGL-3: 4-h AEGL-3: 8-h AEGL-3: |
620 ppm ÷ 4 = 160 ppm 210 ppm ÷ 4 = 53 ppm 100 ppm ÷ 4 = 25 ppm 26 ppm ÷ 4 = 6.5 ppm 26 ppm ÷ 4 = 6.5 ppm |
APPENDIX C
DERIVATION OF AEGL VALUES FOR HYDROGEN CHLORIDE (NRC 2004)
Derivation of AEGL-1 Values
| Derivation of AEGL-1 Values | |||
| Key study: | Stevens et al. 1992 | ||
| Toxicity end point: | No-observed-adverse-effect level in exercising asthmatic subjects | ||
| Time-scaling: | Cn × t = k (default of n = 1 for shorter to longer exposure period) (1.8 ppm)1 × 0.75 h = 1.35 ppm-h |
||
| Uncertainty factors: | None | ||
| 10-min AEGL-1: | 1.8 ppm | ||
| 30-min AEGL-1: | 1.8 ppm | ||
| 1-h AEGL-1: | 1.8 ppm | ||
| 4-h AEGL-1: | 1.8 ppm | ||
| 8-h AEGL-1: | 1.8 ppm | ||
| Derivation of AEGL-2 Values | |||
| 10-min AEGL-2 | |||
| Key study: | Barrow et al. (1977) | ||
| Toxicity end point: | Mouse RD50 of 309 ppm | ||
| 10-min AEGL-2: | 309 ppm ÷ 3 = 100 ppm One-third of the RD50 corresponds to an approximate decrease in respiratory rate of 30%, and decreases in the range of 20-50% correspond to moderate irritation (ASTM 1991). | ||
| 30-min, 1-, 4-, and 8-h AEGL-2 | ||
| Key study: | Stavert et al. 1991 | |
| Toxicity end point: | Severe nasal (nose breathers) or pulmonary (mouth breathers) effects in rats exposed at 1,300 ppm for 30 min | |
| Time-scaling: | C1 × t = k (n = 1 for shorter to longer exposure periods) (1,300 ppm)1 × 0.5 h = 650 ppm-h |
|
| Uncertainty factors: | 3 for intraspecies variability 3 for interspecies variability Combined uncertainty factor of 10 | |
| Modifying factor: | 3 for sparse database | |
| 30-min AEGL-2: | C1 × 0.5 h = 650 ppm-h C = 1,300 ppm 1,300 ppm ÷ 30 = 43 ppm |
|
| 1-h AEGL-2: | C1 × 1 h = 650 ppm-h C = 650 ppm 650 ppm ÷ 30 = 22 ppm |
|
| 4-h AEGL-2: | 1-h AEGL-2 ÷ 2 = 11 ppm | |
| 8-h AEGL-2: | 1-h AEGL-2 ÷ 2 = 11 ppm | |
| Derivation of AEGL-3 Values | ||
| Key studies: | Wohlslagel et al. (1976); Vernot et al. (1977) | |
| Toxicity end point: | One-third of the rat 1-h LC50 (an estimated no-effect level for death) LC50 = 3,124 ppm ÷ 3 = 1,041 ppm |
|
| Time-scaling: | C1 × t = k (n = 1 for shorter to longer exposure periods) (1,041 ppm)1 × 1 h = 1,041 ppm-h |
|
| Uncertainty factors: | 3 for intraspecies variability 3 for interspecies variability Combined uncertainty factor of 10 | |
| 10-min AEGL-3: | C1 × 0.167 h = 1,041 ppm-h C = 6,234 ppm 6,234 ppm ÷ 10 = 623.4 ppm |
|
| 30-min AEGL-3: | C1 × 0.5 h = 1,041 ppm-h C = 2,082 ppm 2,082 ppm ÷ 10 = 208 ppm |
|
| 1-h AEGL-3: | C1 × 1 h = 1,041 ppm-h C = 1,041 ppm 1,041 ppm ÷ 10 = 104.1 ppm |
|
| 4-h AEGL-3: | C1 × 4 h = 1,041 ppm-h C = 260.25 ppm 260.25 ppm ÷ 10 = 26 ppm |
|
| 8-h AEGL-3: | Set equal to 4-h AEGL-3 = 26 ppm |
SUMMARY OF KEY STUDY AND RATIONALE USED TO DERIVE AEGL VALUES FOR HYDROGEN CHLORIDE (Excerpted from NRC 2004)
AEGL-1 Values
Because appropriate human data exist for exposure to HCl, they were used to identify AEGL-1 values. Exposure to HCl at 1.8 ppm for 45 min resulted in a no-observed-adverse-effect level in 10 exercising young adult asthmatic subjects (Stevens et al. 1992). Because exercise will increase HCl uptake and exacerbate irritation, those asthmatic subjects are considered a sensitive subpopulation. Therefore, because the test subjects were a sensitive subpopulation and the end point was essentially a no-effect level, no uncertainty factor was applied to account for sensitive human subpopulations. Adequate human data were available, so no uncertainty factor was applied for animal to human extrapolation. The no-effect level was held constant across the 10- and 30-min and 1-, 4-, and 8-h exposure time periods. That approach was considered appropriate because mild irritant effects generally do not vary greatly over time, and the end point of a no-effect level in a sensitive population is inherently conservative.
AEGL-2 Values
The AEGL-2 for the 30-min and 1-, 4-, and 8-h time points was based on severe nasal or pulmonary histopathology in rats exposed to HCl at 1,300 ppm for 30 min (Stavert et al. 1991). A modifying factor of 3 was applied to account for the relatively sparse database describing effects defined by AEGL-2. The AEGL-2 values were further adjusted by a total uncertainty factor of 10—3 for intraspecies variability, supported by the steep concentration-response curve, which implies little individual variability; and 3 for interspecies variability. Using the default value of 10 for interspecies variability would bring the total adjustment to 100 instead of 30. That would generate AEGL-2 values that are not supported by data on exercising asthmatic subjects, an especially sensitive subpopulation. Exercise increases HCl uptake and exacerbates irritation; no effects were noted in exercising young adult asthmatic subjects exposed to HCl at 1.8 ppm for 45 min (Stevens et al. 1992). Using a total uncertainty factor of 30 would yield 4- and 8-h values of 3.6 ppm (instead of 11 ppm). The prediction that humans would be disabled by exposure for 4 or 8 h to 3.6 ppm cannot be supported when exercising asthmatic subjects exposed to one-half that concentration for 45 min exhibited no effects. The shorter time points would yield values 4 to 7 times the 1.8-ppm value; however, confidence in the time-scaling for HCl is good for times up to 100 min, because the value of n was derived from a regression analysis of rat and mouse mortality data with exposure durations ranging from 1 min to 100 min. The 30-min value of 43 ppm derived with a total uncertainty factor of 10 is reasonable in light of the fact that baboons exposed at
500 ppm for 15 min experienced only a slightly increased respiratory rate. Therefore, a total uncertainty factor of 10, accompanied by the modifying factor of 3, is most consistent with the database. Thus, the total factor is 30. Time-scaling for the 1-h AEGL exposure period used the Cn × t = k relationship, where n = 1 based on regression analysis of combined rat and mouse LC50 data (1 to 100 min) as reported by ten Berge et al. (1986). The 4- and 8-h AEGL-2 values were derived by applying a modifying factor of 2 to the 1-h AEGL-2 value, because time-scaling would yield a 4-h AEGL-2 of 5.4 ppm and an 8-h AEGL-2 of 2.7 ppm, close to the 1.8 ppm tolerated by exercising asthmatic subjects without observed adverse health effects. Repeated-exposure rat data suggest that the 4- and 8-h values of 11 ppm are protective. Rats exposed to HCl at 10 ppm for 6 h/day, 5 days/week for life exhibited only tracheal and laryngeal hyperplasia, and rats exposed to HCl at 50 ppm for 6 h/day, 5 days/week for 90 days exhibited only mild rhinitis.
The 10-min AEGL-2 was derived by dividing the mouse RD50 of 309 ppm by a factor of 3 to obtain a concentration causing irritation (Barrow et al. 1977). It has been determined that human response to sensory irritants can be predicted on the basis of the mouse RD50. For example, Schaper (1993) has validated the correlation of 0.03 × RD50 = TLV (threshold limit value) as a value that will prevent sensory irritation in humans. The 0.03 represents the half-way point between 0.1 and 0.01 on a logarithmic scale, and Alarie (1981) has shown that the RD50 multiplied by 0.1 corresponds to “some sensory irritation,” whereas the RD50 value itself is considered “intolerable to humans.” Thus, it is reasonable that one-third of the RD50, a value half-way between 0.1 and 1 on a logarithmic scale, might cause significant irritation to humans. Furthermore, one-third of the mouse RD50 for HCl corresponds to an approximate decrease in respiratory rate of 30%, and decreases in the range of 20-50% correspond to moderate irritation (ASTM 1991).
AEGL-3 Values
The AEGL-3 was based on a 1-h rat LC50 study (Wohlslagel et al. 1976; Vernot et al. 1977). One-third of the 1-h LC50 value of 3,124 ppm was used as an estimated concentration causing no deaths. That estimate is inherently conservative (no deaths observed in the same study at 1,813 ppm). A total uncertainty factor of 10 will be applied—3 for intraspecies variation, because the steep concentration-response curve implies limited individual variability; and 3 to protect susceptible individuals. Using a full value of 10 for interspecies variability (total uncertainty factor of 30) would yield AEGL-3 values that are inconsistent with the overall data set.
A number of factors argue for the use of an uncertainty factor of 10 instead of 30, they are: (1) the steep concentration-response curve for lethality observed in the Wohlslagel et al. (1976) study in which the estimated LC0 (one-third of the LC50 of 3,124 ppm) is lower than the experimental LC0 of 1,813
ppm. The LC0 selection is conservative, and the steep concentration-response curve argues for little interindividual variability; (2) AEGL-3 values generated from a total uncertainty factor of 30 would be close (within a factor of 2) to the AEGL-2 values generated from data on exercising asthmatic subjects; (3) Sella-kumar et al. (1985) exposed rats to HCl at 10 ppm for 6 h/day, 5 days/week for life and only observed increased trachael and laryngeal hyperplasia. The estimated 6-h AEGL-3 using an intraspecies uncertainty factor of 3 is 17 ppm, close to the concentration inhaled in the lifetime study in which only mild effects were induced; and (4) rats exposed to HCl at 50 ppm for 6 h/day, 5 days/week for 90 days exhibited mild rhinitis (Toxigenics Inc. 1984). This level is already twice the AEGL-3 value, which is intended to protect against death.
Thus, the total uncertainty factor was set at 10. It was then time-scaled to the specified 10- and 30-min and 4-h AEGL exposure periods using the Cn × t = k relationship, where n = 1 based on regression analysis of combined rat and mouse LC50 data (1 min to 100 min) as reported by ten Berge et al. (1986). The 4-h AEGL-3 also was adopted as the 8-h AEGL-3 because of the uncertainty of time-scaling to 8 h with an n value derived from exposure durations of up to 100 min.
The 5-min rat LC0 of 30,000 ppm (Higgins et al. 1972) supports the 10-min AEGL-3 value. Extrapolating that value across time (n = 1) to 10 min and applying an uncertainty factor of 10 yields a value of 1,500 ppm, suggesting that the proposed AEGL-3 value is protective. Also, if the 5-min rat LC50 of 41,000 ppm for HCl vapor (Darmer et al. 1974) is divided by 3 to estimate a no-effect level for death, extrapolated to 10 min, and an uncertainty factor of 10 is applied, a supporting value of 683 ppm is obtained.
APPENDIX D
ACUTE EXPOSURE GUIDELINE LEVELS FOR SELECTED CHLOROSILANES
Derivation Summary
AEGL-1 VALUES FOR MONOCHLOROSILANES
| 10 min | 30 min | 1 h | 4 h | 8 r |
| 1.8 ppm | 1.8 ppm | 1.8 ppm | 1.8 ppm | 1.8 ppm |
| Key reference: NRC (National Research Council). 2004. Hydrogen chloride. Pp. 22-122 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 4. Washington, DC: National Academies Press. | ||||
| End point/Concentration/Rationale: AEGL-1 values for HCl were adopted as AEGL-1 values for monochlorosilanes. This approach is considered reasonable because qualitative and quantitative data on chlorosilanes suggest that the HCl hydrolysis product is largely responsible for the acute toxicity of the chlorosilanes. | ||||
| Data adequacy: Mechanism-of-action data were considered adequate for the derivation of AEGL-1 values for chlorosilanes based on analogy to HCl. Confidence in the AEGL-1 values for chlorosilanes is low, reflecting the lack of data on AEGL-1 end points after chlorosilane exposure and reliance on HCl data. Additional research on AEGL-1 effects of chlorosilanes would reduce uncertainty. | ||||
AEGL-1 VALUES FOR TRICHLOROSILANES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 0.90 ppm | 0.90 ppm | 0.90 ppm | 0.90 ppm | 0.90 ppm |
| Key reference: NRC (National Research Council). 2004. Hydrogen chloride. Pp. 77-122 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 4. Washington, DC: National Academies Press. | ||||
| End point/Concentration/Rationale: AEGL-1 values for dichlorosilanes were derived by adjusting the AEGL-1 values for HCl by the molar ratio of HCl to trichlorosilanes. This approach is considered reasonable because qualitative and quantitative data on chlorosilanes suggest that the HCl hydrolysis product is largely responsible for the acute toxicity of the chlorosilanes. | ||||
| Molar Adjustment Factor: 2 | ||||
| Data adequacy: Mechanism-of-action data were considered adequate for the derivation of AEGL-1 values for chlorosilanes based on analogy to HCl. Confidence in the AEGL-1 values for chlorosilanes is low, reflecting the lack of data on AEGL-1 end points after chlorosilane exposure and reliance on HCl data. Additional research on AEGL-1 effects of chlorosilanes would reduce uncertainty. | ||||
AEGL-1 VALUES FOR TRICHLOROSILANES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 0.60 ppm | 0.60 ppm | 0.60 ppm | 0.60 ppm | 0.60 ppm |
Key reference: NRC (National Research Council). 2004. Hydrogen chloride. Pp. 77-122 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 4. Washington, DC: National Academies Press.
End point/Concentration/Rationale: AEGL-1 values for trichlorosilanes were derived by adjusting the AEGL-1 values for HCl by the molar ratio of HCl to trichlorosilanes. This approach is considered reasonable because qualitative and quantitative data on chlorosilanes suggest that the HCl hydrolysis product is largely responsible for the acute toxicity of the chlorosilanes.
Molar Adjustment Factor: 3
Data adequacy: Mechanism-of-action data were considered adequate for the derivation of AEGL-1 values for chlorosilanes based on analogy to HCl. Confidence in the AEGL-1 values for chlorosilanes is low, reflecting the lack of data on AEGL-1 end points after chlorosilane exposure and reliance on HCl data. Additional research on AEGL-1 effects of chlorosilanes would reduce uncertainty.
AEGL-1 VALUES FOR TETRACHLOROSILANE
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 0.45 ppm | 0.45 ppm | 0.45 ppm | 0.45 ppm | 0.45 ppm |
Key reference: NRC (National Research Council). 2004. Hydrogen chloride. Pp. 77-122 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 4. Washington, DC: National Academies Press.
End point/Concentration/Rationale: AEGL-1 values for tetrachlorosilane were derived by adjusting the AEGL-1 values for HCl by the molar ratio of HCl to tetrachlorosilane. This approach is considered reasonable because qualitative and quantitative data on chlorosilanes suggest that the HCl hydrolysis product is largely responsible for the acute toxicity of the chlorosilanes.
Molar adjustment factor: 4
Data adequacy: Mechanism-of-action data were considered adequate for the derivation of AEGL-1 values for chlorosilanes based on analogy to HCl. Confidence in the AEGL-1 values for chlorosilanes is low, reflecting the lack of data on AEGL-1 end points after chlorosilane exposure and reliance on HCl data. Additional research on AEGL-1 effects of chlorosilanes would reduce uncertainty.
AEGL-2 VALUES FOR MONOCHLOROSILANES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 100 ppm | 43 ppm | 22 ppm | 11 ppm | 11 ppm |
Key reference: NRC (National Research Council). 2004. Hydrogen chloride. Pp. 77-122 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 4. Washington, DC: National Academies Press.
End point/Concentration/Rationale: AEGL-2 values for HCl were adopted as AEGL-2 values for monochlorosilanes. This approach is considered reasonable because qualitative and quantitative data on chlorosilanes suggest that the HCl hydrolysis product is largely responsible for the acute toxicity of the chlorosilanes.
Data adequacy: Mechanism-of-action data were considered adequate for the derivation of AEGL-2 values for chlorosilanes based on analogy to HCl. Confidence in the AEGL-2 values for chlorosilanes is moderate, reflecting the limited data on AEGL-2 end points after chlorosilane exposure and reliance on HCl data. Additional research on AEGL-2 effects of chlorosilanes would reduce uncertainty.
AEGL-2 VALUES FOR DICHLOROSILANES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 50 ppm | 22 ppm | 11 ppm | 5.5 ppm | 5.5 ppm |
Key reference: NRC (National Research Council). 2004. Hydrogen chloride. Pp. 77-122 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 4. Washington, DC: National Academies Press.
End point/Concentration/Rationale: AEGL-2 values for dichlorosilanes were derived by adjusting the AEGL-2 values for HCl by the molar ratio of HCl to dichlorosilane. This approach is considered reasonable because qualitative and quantitative data on chlorosilanes suggest that the HCl hydrolysis product is largely responsible for the acute toxicity of the chlorosilanes.
Molar adjustment factor: 2
Data adequacy: Mechanism-of-action data were considered adequate for the derivation of AEGL-2 values for chlorosilanes based on analogy to HCl. Confidence in the AEGL-2 values for chlorosilanes is moderate, reflecting the limited data on AEGL-2 end points after chlorosilane exposure and reliance on HCl data. Additional research on AEGL-2 effects of chlorosilanes would reduce uncertainty.
AEGL-2 VALUES FOR TRICHLOROSILANES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 33 ppm | 14 ppm | 7.3 ppm | 3.7 ppm | 3.7 ppm |
Key reference: NRC (National Research Council). 2004. Hydrogen chloride. Pp. 77-122 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 4. Washington, DC: National Academies Press.
End point/Concentration/Rationale: AEGL-2 values for trichlorosilanes were derived by adjusting the AEGL-2 values for HCl by the molar ratio of HCl to trichlorosilane. This approach is considered reasonable because qualitative and quantitative data on chlorosilanes suggest that the HCl hydrolysis product is largely responsible for the acute toxicity of the chlorosilanes.
Molar adjustment factor: 3
Data adequacy: Mechanism-of-action data were considered adequate for the derivation of AEGL-2 values for chlorosilanes based on analogy to HCl. Confidence in the AEGL-2 values for chlorosilanes is moderate, reflecting the limited data on AEGL-2 end points after chlorosilane exposure and reliance on HCl data. Additional research on AEGL-2 effects of chlorosilanes would reduce uncertainty.
AEGL-2 VALUES FOR TETRACHLOROSILANE
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 25 ppm | 11 ppm | 5.5 ppm | 2.8 ppm | 2.8 ppm |
Key reference: NRC (National Research Council). 2004. Hydrogen chloride. Pp. 77-122 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 4. Washington, DC: National Academies Press.
End point/Concentration/Rationale: AEGL-2 values for tetrachlorosilane were derived by adjusting the AEGL-2 values for HCl by the molar ratio of HCl to tetrachlorosilane. This approach is considered reasonable because qualitative and quantitative data on chlorosilanes suggest that the HCl hydrolysis product is largely responsible for the acute toxicity of the chlorosilanes.
Molar adjustment factor: 4
Data adequacy: Mechanism-of-action data were considered adequate for the derivation of AEGL-2 values for chlorosilanes based on analogy to HCl. Confidence in the AEGL-2 values for chlorosilanes is moderate, reflecting the limited data on AEGL-2 end points after chlorosilane exposure and reliance on HCl data. Additional research on AEGL-2 effects of chlorosilanes would reduce uncertainty.
AEGL-3 VALUES FOR MONOCHLOROSILANES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 620 ppm | 210 ppm | 100 ppm | 26 ppm | 26 ppm |
Key reference: NRC (National Research Council). 2004. Hydrogen chloride. Pp. 77-122 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 4. Washington, DC: National Academies Press..
End point/Concentration/Rationale: AEGL-3 values for HCl were adopted as AEGL-3 values for monochlorosilanes. This approach is considered reasonable because qualitative and quantitative data on chlorosilanes suggest that the HCl hydrolysis product is largely responsible for the acute toxicity of the chlorosilanes.
Data adequacy: Data were considered adequate for the derivation of AEGL-3 values for chlorosilanes based on analogy to HCl. Confidence in the AEGL-3 values for chlorosilanes is high, reflecting the availability of lethality data on 11 of the 26 chlorosilanes considered and evidence for the role of HCl as the proximate toxicant. No additional research is needed on AEGL-3 end points.
AEGL-3 VALUES FOR DICHLOROSILANES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 310 ppm | 110 ppm | 50 ppm | 13 ppm | 13 ppm |
Key reference: NRC (National Research Council). 2004. Hydrogen chloride. Pp. 77-122 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 4. Washington, DC: National Academies Press.
End point/Concentration/Rationale: AEGL-3 values for dichlorosilanes were derived by adjusting the AEGL-3 values for HCl by the molar ratio of HCl to dichlorosilane. This approach is considered reasonable because qualitative and quantitative data on chlorosilanes suggest that the HCl hydrolysis product is largely responsible for the acute toxicity of the chlorosilanes.
Molar adjustment factor: 2
Data adequacy: Data were considered adequate for the derivation of AEGL-3 values for chlorosilanes based on analogy to HCl. Confidence in the AEGL-3 values for chlorosilanes is high, reflecting the availability of lethality data on 11 of the 26 chlorosilanes considered and evidence for the role of HCl as the proximate toxicant. No additional research is needed on AEGL-3 end points.
AEGL-3 VALUES FOR TRICHLOROSILANES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 210 ppm | 70 ppm | 33 ppm | 8.7 ppm | 8.7 ppm |
Key reference: NRC (National Research Council). 2004. Hydrogen chloride. Pp. 77-122 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 4. Washington, DC: National Academies Press.
End point/Concentration/Rationale: AEGL-3 values for trichlorosilanes were derived by adjusting the AEGL-3 values for HCl by the molar ratio of HCl to trichlorosilane. This approach is considered reasonable because qualitative and quantitative data on chlorosilanes suggest that the HCl hydrolysis product is largely responsible for the acute toxicity of the chlorosilanes.
Molar adjustment factor: 3
Data adequacy: Data were considered adequate for the derivation of AEGL-3 values for chlorosilanes based on analogy to HCl. Confidence in the AEGL-3 values for chlorosilanes is high, reflecting the availability of lethality data on 11 of the 26 chlorosilanes considered and evidence for the role of HCl as the proximate toxicant. No additional research is needed on AEGL-3 end points.
AEGL-3 VALUES FOR TETRACHLOROSILANE
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 160 ppm | 53 ppm | 25 ppm | 6.5 ppm | 6.5 ppm |
Key reference: NRC (National Research Council). 2004. Hydrogen chloride. Pp. 77-122 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 4. Washington, DC: National Academies Press.
End point/Concentration/Rationale: AEGL-3 values for tetrachlorosilane were derived by adjusting the AEGL-3 values for HCl by the molar ratio of HCl to tetrachlorosilane. This approach is considered reasonable because qualitative and quantitative data on chlorosilanes suggest that the HCl hydrolysis product is largely responsible for the acute toxicity of the chlorosilanes.
Molar adjustment factor: 4
Data adequacy: Data were considered adequate for the derivation of AEGL-3 values for chlorosilanes based on analogy to HCl. Confidence in the AEGL-3 values for chlorosilanes is high, reflecting the availability of lethality data on 11 of the 26 chlorosilanes considered and evidence for the role of HCl as the proximate toxicant. No additional research is needed on AEGL-3 end points.
APPENDIX E
DERIVATION SUMMARY TABLES FOR HYDROGEN CHLORIDE (Excerpted from NRC 2004)
Derivation Summary
AEGL-1 VALUES FOR HYDROGEN CHLORIDE
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 1.8 ppm | 1.8 ppm | 1.8 ppm | 1.8 ppm | 1.8 ppm |
| Key reference: Stevens, B., J.Q. Koenig, V. Rebolledo, Q.S. Hanley, and D.S. Covert, D.S. 1992. Respiratory effects from the inhalation of hydrogen chloride in young adults with asthma. J. Occup. Med. 34(9): 923-929. | ||||
| Test species/Strain/Number: Human adults with asthma, 10 | ||||
| Exposure route/Concentrations/Durations: Inhalation at 0, 0.8, or 1.8 ppm for 45 min while exercising (1.8 ppm was determinant for AEGL-1) | ||||
| Effects: No treatment-related effects were observed in any of the individuals tested | ||||
| End point/Concentration/Rationale: The highest concentration tested was a no-effect level for irritation in a sensitive human population (10 asthmatic individuals tested) and was selected as the basis of AEGL-1. Effects assessed included sore throat, nasal discharge, cough, chest pain or burning, dyspnea, wheezing, fatigue, headache, unusual taste or smell, total respiratory resistance, thoracic gas volume at functional residual capacity, forced expiratory volume, and forced vital capacity. All subjects continued the requisite exercise routine for the duration of the test period. | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: Interspecies: 1, test subjects were human Intraspecies: 1, test subjects were sensitive population (exercising asthmatic subjects) | ||||
| Modifying factor: Not applicable | ||||
| Animal-to-human dosimetric adjustment: Insufficient data | ||||
| Time-scaling: The AEGL-1 values for a sensory irritant were held constant across time because it is a threshold effect and prolonged exposure will not result in an enhanced effect. In fact one might become desensitized to the respiratory-tract irritant over time. Also, this approach was considered valid since the end point (no treatment-related effects at the highest concentration tested in exercising asthmatic subjects) is inherently conservative. | ||||
| Data quality and research needs: The key study was well-conducted in a sensitive human population and is based on no treatment-related effects. Additionally, the direct-acting irritation response is not expected to vary greatly among individuals. Therefore, confidence in the AEGL values is high. | ||||
AEGL-2 VALUES FOR HYDROGEN CHLORIDE
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 100 ppm | 43 ppm | 22 ppm | 11 ppm | 11 ppm |
| Key references: Stavert, D.M., D.C. Archuleta, M.F. Behr, and B.E. Lehnert. 1991. Relative acute toxicities of hydrogen chloride, hydrogen fluoride, and hydrogen bromide in nose- and pseudo-mouth-breathing rats. Fundam. Appl. Toxicol. 16(4):636-655. (30-, 1-, 4-min and 8-h AEGLs) Barrow, C.S., Y. Alarie, M. Warrick, and M.F. Stock. 1977. Comparison of the sensory irritation response in mice to chlorine and hydrogen chloride. Arch. Environ. Health 32(2):68-76. (10-min AEGL) | ||||
| Test species/Strain/Number: F344 rats, 8 males/concentration (30-min, 1-, 4-, and 8-h); Male Swiss Webster mice (10-min) | ||||
| Exposure route/Concentrations/Durations: inhalation at 0 or 1,300 ppm for 30 min (1,300 ppm was determinant for 30-min, 1-, 4-, and 8-h AEGL-2) | ||||
| Effects (30-min, 1-, 4-, and 8-h): 0 ppm, no effects; 1,300 ppm, severe necrotizing rhinitis, turbinate necrosis, thrombosis of nasal submucosa vessels in nose-breathers; 1,300 ppm, severe ulcerative tracheitis accompanied by necrosis and luminal ulceration in mouth-breathers (determinant for AEGL-2); RD50 = 309 ppm (determinant for 10-min AEGL-2) | ||||
| End point/Concentration/Rationale: 1,300 ppm for 30 min; severe lung effects (ulcerative tracheitis accompanied by necrosis and luminal ulceration) or nasal effects (necrotizing rhinitis, turbinate necrosis, thrombosis of nasal submucosa vessels histopathology) in pseudo-mouth breathing male F344 rats (30-min, 1-, 4-, and 8-h); RD50 of 309 ppm ÷ 3 to estimate irritation (10-min) | ||||
| Uncertainty Factors/Rationale (30-min, 1-, 4-, and 8-h): Total uncertainty factor: 10 Intraspecies: 3, steep concentration-response curve implies limited individual variability. Interspecies: 3, use of an intraspecies uncertainty factor of 10 would bring the total uncertainty/modifying factor to 100 instead of 30. That would generate AEGL-2 values that are not supported by data on exercising asthmatic subjects, an especially sensitive subpopulation because exercise increases HCl uptake and exacerbates irritation. No effects were noted in exercising young adult with asthma exposed to HCl at 1.8 ppm for 45 min (Stevens et al. 1992). Using a total uncertainty factor of 30 would yield 4- and 8-h values of 3.6 ppm (instead of 11 ppm). It is not supportable to predict that humans would be disabled by exposure at 3.6 ppm for 4- or 8-h when exercising asthmatic subjects exposed to one-half this level for 45 min had no effects. The shorter time points would yield values 4- to 7 times above 1.8 ppm; however, the confidence in the time scaling for HCl is good for times up to 100 min because the value of n value was derived from a regression analysis of rat and mouse mortality data with exposure durations ranging from 1 min to 100 min. The 30-min value of 43 ppm derived with the total uncertainty factor of 10 is reasonable in light of the fact that baboons exposed to 500 ppm for 15 min experienced only a slightly increased respiratory rate. | ||||
AEGL-2 VALUES FOR HYDROGEN CHLORIDE
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 100 ppm | 43 ppm | 22 ppm | 11 ppm | 11 ppm |
| Modifying factor: 30-min, 1-, 4-, and 8-h AEGLs: 3 based on sparse database for AEGL-2 effects and that the effects observed at the concentration used as the basis for AEGL-2 values were somewhat severe. 10-min AEGL-2: the 10-min AEGL-2 value was derived by dividing the mouse RD50 of 309 ppm by a factor of 3 to obtain a concentration causing irritation (Barrow et al. 1977). One-third of the mouse RD50 for HCl corresponds to an approximate decrease in respiratory rate of 30%, and decreases in the range of 20% to 50% correspond to moderate irritation (ASTM 1991). |
||||
| Animal-to-human dosimetric adjustment: Insufficient data | ||||
| Time-scaling: Cn × t = k, where n=1, based on regression analysis of combined rat and mouse LC50 data (1 min to 100 min) reported by ten Berge et al. (1986). Data point used to derive AEGL-2 was 30 min. AEGL-2 values for 1-h exposure period was based on extrapolation from the 30-min value. The 4- and 8-h AEGL-2 values were derived by applying a modifying factor of 2 to the 1-h AEGL-2 value because time scaling would yield a 4-h AEGL-2 value of 5.4 ppm and an 8-h AEGL-2 of 2.7 ppm, close to the 1.8 ppm tolerated by exercising asthmatic subjects without adverse health effects. | ||||
| Data quality and research needs: Confidence is moderate since the species used is more sensitive than primates to the effects of HCl, the chemical is a direct-acting irritant, and a modifying factor was included to account for the relative severity of effects and sparse data base. |
AEGL-3 VALUES FOR HYDROGEN CHLORIDE
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 620 ppm | 210 ppm | 100 ppm | 26 ppm | 26 ppm |
| Key reference: Vernot, E.H., J.D. MacEwen, C.C. Haun, and E.R. Kinkead. 1977. Acute toxicity and skin corrosion data for some organic and inorganic compounds and aqueous solutions. Toxicol. Appl. Pharmacol. 42(2):417-423. Wohlslagel, J., L.C. DiPasquale, and E.H. Vernot. 1976. Toxicity of solid rocket motor exhaust: Effects of HCl, HF, and alumina on rodents. J. Combust. Toxicol. 3:61-69. |
||||
| Test species/Strain/Sex/Number: Sprague-Dawley rats, 10 males per concentration | ||||
| Exposure route/Concentrations/Durations: inhalation at 0, 1,813, 2,585, 3,274, 3,941, or 4,455 ppm for 1 h | ||||
AEGL-3 VALUES FOR HYDROGEN CHLORIDE
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 620 ppm | 210 ppm | 100 ppm | 26 ppm | 26 ppm |
| Effects: | Concentration | Mortality | |||
| 0 ppm | 0/10 | ||||
| 1,813 ppm | 0/10 | ||||
| 2,585 ppm | 2/10 | ||||
| 3,274 ppm | 6/10 | ||||
| 3,941 ppm | 8/10 | ||||
| 4,455 ppm | 10/10 | ||||
| LC50: reported as 3,124 ppm (determinant for AEGL-3) | |||||
| End point/Concentration/Rationale: One-third of the 1-h LC50 (3,124 × 1/3 = 1,041 ppm) to estimate a concentration causing no deaths. | |||||
| Uncertainty factors/Rationale: | |||||
| Total uncertainty factor: 10 | |||||
| Intraspecies: 3, a steep concentration-response curve implies limited individual variability. | |||||
| Interspecies: 3, (1) the steep concentration-response curve for lethality observed in the Wohlslagel et al. (1976) study in which 1,041 ppm (one-third of the LC50 of 3,124 ppm) was lower than the LC0 of 1,813 ppm. This is a conservative selection of the LC0 and the steep concentration-response curve argues for little interindividual variability; (2)AEGL-3 values generated from a total uncertainty factor of 30 would be close to the AEGL-2 values (within a factor of 2) generated above which are reasonable when compared with data on exercising asthmatic subjects; (3) Sellakumar et al. (1985) exposed rats to 10 ppm of HCl for 6 h/day, 5 days/week for life and only observed increased trachael and laryngeal hyperplasia. The estimated 6-h AEGL-3 using an intraspecies uncertainty factor of 3 is 17 ppm, close to the level used in the lifetime study in which only mild effects were induced; and (4) rats exposed at 50 ppm for 6 h/day, 5 days/week for 90 days exhibited mild rhinitis (Toxigenics Inc. 1984). This level is already 2 times that of the AEGL-3 value for death. Thus, the total uncertainty factor is 10. | |||||
| Modifying factor: Not applicable | |||||
| Animal-to-human dosimetric adjustment: Insufficient data | |||||
| Time-scaling: Cn × t = k, where n = 1, based on regression analysis of rat and mouse mortality data (1 min to 100 min) reported by ten Berge et al. (1986). | |||||
| Reported 1-h data point was used to derive AEGL-3 values. AEGL-3 values for 10-min, 30-min, and 4-h were based on extrapolation from the 1-h value. The 4-h value was adopted as the 8-h value. | |||||
| Data quality and research needs: Study is considered appropriate for AEGL-3 derivation because exposures are over a wide range of HCl concentrations and utilize a sufficient number of animals. Data were insufficient to derive a no-effect level for death. One-third of the LC50 has been utilized previously for chemicals with steep concentration-response curves. Also, in the key study, no deaths were observed in rats exposed at 1,813 ppm. | |||||
APPENDIX F
CATEGORY PLOTS FOR SELECTED CHLOROSILANES
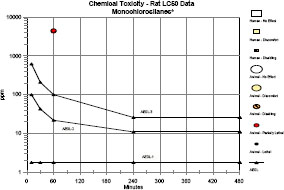
FIGURE F-1 Category plot for monochlorosilanes. *Data plotted are for trimethyl chlo-rosilane and dimethyl chlorosilane.

FIGURE F-2 Category plat of dichlorosilanes. *Data plotted are for methylvinyl dichlo-rosilane, dimethyl dichlorosilane, and methyl dichlorosilane.

FIGURE F-3 Category plot for trichlorosilanes. *Data plotted are for propyl trichlorosi-lane, vinyl trichlorosilane, methyl trichlorosilane, and ethyl trichlorosilane.
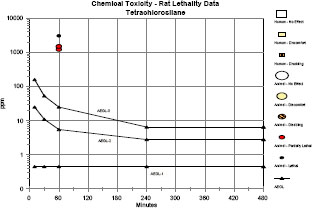
FIGURE F-4 Category plot for tetrachlorosilane.




























































