Advancing Research on Understanding Environmental Effects of UV Filters from Sunscreens: Proceedings of a Workshop (2023)
Chapter: Standardizing Approaches for Toxicity Testing
Standardizing Approaches for Toxicity Testing
The workshop’s second day focused on standardizing approaches for toxicity testing to generate reliable, reproducible data to inform EPA’s ERAs on UV filters. To set the stage, Sandy Raimondo (EPA) gave a presentation on the importance of standardized toxicological methods and how EPA uses toxicity test data, which was followed by a series of three short talks on methods for coral ecotoxicology. Participants then delved deeper into challenges and opportunities in toxicity testing for UV filters in a panel discussion and structured breakout group discussions.
EPA PERSPECTIVE: THE IMPORTANCE OF STANDARDIZED TOXICOLOGICAL METHODS FOR AQUATIC ORGANISMS
Raimondo, a research ecologist at EPA’s Gulf Ecosystem Measurement and Modeling Division, discussed how EPA uses toxicology testing data, the importance of standardized test methods, and how new approach methodologies can help to generate environmentally and ecologically relevant data that are usable for policy making. “This [NASEM 2022] report is going to spark a flurry of research,” she said. “We know this, and we want to make sure that the data that are collected provide information that we can use in a consistent, defensible, and reproducible manner.”
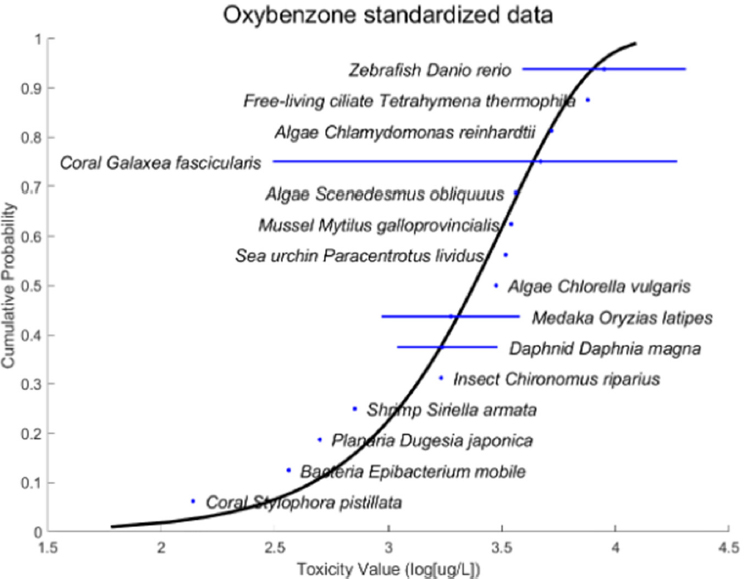
In conducting ERAs, EPA typically relies upon data generated from replicable, controlled laboratory studies of a single stressor, but there are scant data of this sort for UV filters. To fill this gap, Raimondo said that researchers could adapt widely accepted standardized test methods to generate conclusive, statistically significant concentration response curves for UV filters for both standard and nonstandard aquatic species. They discussed some particular considerations researchers should be aware of if they want their findings to be useful for EPA and other decision-making bodies (example shown in Figure 3). She stressed that test methods could standardize exposure duration, biological response, age, and life stage for each organism tested. It is also critical, she pointed out, to ensure consistency in terms of water temperature, active ingredient level, salinity, pH level, analytical methodologies, and endpoints in order to generate data that will be comparable across species and laboratories and usable for ERAs. Standardized test methods are different for chronic and acute testing, but in either case, she said it is important to avoid open-ended toxicity values and establish species sensitivity distributions as a quantitative metric for understanding effects.
Raimondo noted that standardized data, the “gold standard” for study defensibility, inform ERAs and improve the understanding of how chemicals affect organisms, which organisms or taxa are most at risk, where EPA should prioritize its resources, and what mitigation effects could reduce the environmental impacts. EPA and others can also utilize such data to establish a chemical’s de minimis environmental impacts, create water quality standards for officials who grant local permits and conduct environmental restoration, and assess the impacts of accidental chemical releases into the environment. Standardized data also reduce variation when comparing effects across different chemicals and species, helping to guide EPA in prioritizing environmental mitigation and management efforts.
Even standardized methods and data have limitations. Since it is not possible or necessarily ethical to test every species that may possibly be affected by a contaminant, efforts to extrapolate effects across species can help to focus testing efforts on species that are easier to work with. It is useful to examine a wide variety of endpoints, but there are also limitations to the interpretation
and extrapolation across endpoints. Finally, Raimondo underscored the persistent challenge of translating between laboratory studies, field studies, and the exceedingly complex physical and biological environments of real-world aquatic and marine ecosystems.
In addition to long-established toxicity testing methods, Raimondo said that new approach methodologies can help to obtain reproducible, defensible, and environmentally and ecologically relevant endpoints for UV filter toxicity testing. The most important element of any testing approach, she continued, is to have a consistent, transferable test design with quantitative endpoints that show how organisms respond to a stressor, which can then be used to create higher-tier assessments that improve environmental realism and lead to ecologically relevant conclusions. New approach methodologies can inform toxicity tests that are sensitive to marine environments, account for abiotic variability, and support interspecies extrapolations. In addition, Raimondo said that screening sensitivity assays can lead to more applicable toxicity knowledge in order to prioritize certain chemicals. To move forward, she reiterated the need for interlaboratory consensus on effects and endpoints and stressed the importance of publishing quantitative data and repeating studies.
LIGHTNING TALKS: METHODS FOR CORAL ECOTOXICOLOGY
In a series of three lightning talks, researchers shared specific approaches being explored to refine toxicology studies, particularly in the context of coral.
Considerations for Valid Exposure Designs to Generate Data That Is Relevant for an Era
Craig Downs (Haereticus Environmental Laboratory) discussed four considerations when testing exposure to UV filters for ERA-relevant data: contaminant concentration, exposure medium, exposure timing, and light. Typically, the assumed concentration of UV filters in the water column is measured after a UV filter is added to the water vessel. However, Downs said it is important to recognize that some UV filters move out of the water column to the meniscus layer, vessel walls, or organisms’ structural supports and could also be significantly affected by volatilization, all of which can lead to inaccurate measurements of the actual concentration.1 To combat this problem, he suggested using Teflon liners for beakers and conducting vigorous prescreening tests before introducing an organism in order to understand what confounding leachates may be present in exposure media.
Using the right exposure medium is crucial, Downs emphasized. Noting that filtered seawater is not consistent across sources and often has unknown amounts of xenobiotics, total organic carbon, and other confounding organic matter, he said that a better alternative is for researchers to create their own artificial seawater, for which many established recipes are available. He added that in order to use artificial seawater to approximate the real-world conditions in which UV filters interact with living organisms, it is important to not only approximate the chemical composition of seawater but include known biological and organic components, which themselves affect and are affected by UV filters. Exposure timing also has implications for the handling of exposure media. Noting that tests can be designed with static or semi-static exposures, Downs said that changing the exposure media at set intervals can help to mitigate the issue of loss that can occur over longer exposure periods.
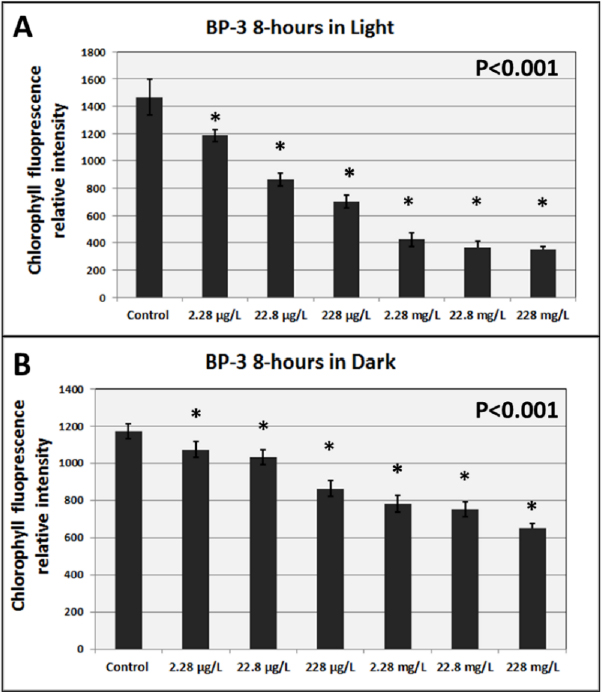
Finally, Downs underscored the important role of light as both a driver of UV filter toxicity and a necessary requirement for culturing and exposure. Standard laboratory lighting does not replicate the natural light organisms experience (light experiment shown in Figure 4).2, 3, 4 It is possible to use natural light, though this presents added logistical challenges and protocols necessarily will vary depending on a laboratory’s location and environment. Downs suggested moving toward establishing a replicable LED array with justified simulated solar spectrum at model species depth and including true solar incidence angles attenuated with neutral density filters to provide a justified photosynthetic intensity and UV spectrum relevant to the natural environment of the species being studied.
___________________
1 Chen, T. H., Wu, Y. T., & Ding, W. H. (2016). UV-filter benzophenone-3 inhibits agonistic behavior in male Siamese fighting fish (Betta splendens). Ecotoxicology (London, England), 25(2), 302–309. https://doi.org/10.1007/s10646-015-1588-4.
2 Collins, P., & Ferguson, J. (1994). Photoallergic contact dermatitis to oxybenzone. The British Journal of Dermatology, 131(1), 124–129. https://doi.org/10.1111/j.1365-2133.1994.tb08469.x.
3 Downs, C. A., Kramarsky-Winter, E., Segal, R., Fauth, J., Knutson, S., Bronstein, O., Ciner, F. R., Jeger, R., Lichtenfeld, Y., Woodley, C. M., Pennington, P., Cadenas, K., Kushmaro, A., & Loya, Y. (2016). Toxicopathological effects of the sunscreen UV filter, Oxybenzone (Benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the U.S. Virgin Islands. Archives of Environmental Contamination and Toxicology 70(2), 265–288. https://doi.org/10.1007/s00244-015-0227-7.
4 Zhong, X., Downs, C. A., Li, Y., Zhang, Z., Li, Y., Liu, B., Gao, H., & Li, Q. (2020). Comparison of toxicological effects of oxybenzone, avobenzone, octocrylene, and octinoxate sunscreen ingredients on cucumber plants (Cucumis sativus L.). The Science of the Total Environment, 714, 136879. https://doi.org/10.1016/j.scitotenv.2020.136879.
Standardization of Toxicity Tests on Corals to Meet Regulatory Requirements
Sascha Pawlowski (BASF) highlighted BASF research relevant to protecting corals from potentially toxic UV filters and discussed how the U.S. context relates to the regulatory context in Europe. He noted that there are nearly 30 UV filters registered in Europe, for which hazard risk assessments are required under the European Union’s Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation. Within this framework, if a chemical is deemed hazardous, predicted no-effect concentration (PNEC) levels must be derived, typically using standard laboratory organisms. Until standardized toxicity tests are available, these PNEC levels can be used as surrogates to protect coral, freshwater sediment, and all nonstandard organisms.5, 6
___________________
5 Pawlowski, S., Moeller, M., Miller, I. B., Kellermann, M. Y., Schupp, P. J., & Petersen-Thiery, M. (2021). UV filters used in sunscreens-A lack in current coral protection? Integrated Environmental Assessment and Management, 17(5), 926–939. https://doi.org/10.1002/ieam.4454.
6 Pawlowski, S., Lütjens, L. H., Preibisch, A., Acker, S., & Petersen-Thiery, M. (2023, submitted). Cosmetic UV filters in the environment–State of the art in EU regulations, science and possible knowledge gaps. Journal of Cosmetic Science, Special Series: Sun Protection.
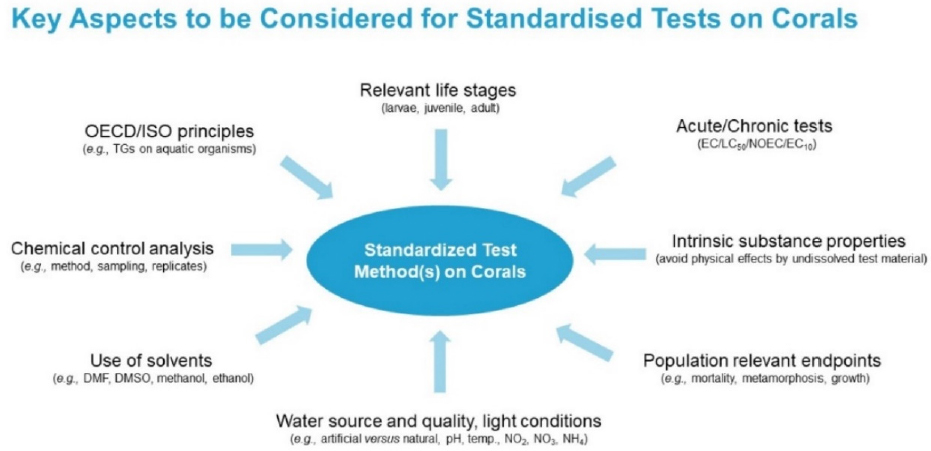
To work toward standardized toxicity tests for future regulatory requirements, key aspects to be considered are shown in Figure 5. BASF is adapting existing guidelines, including guidelines from the Organisation for Economic Co-operation and Development (OECD) and the International Organization for Standardization (ISO) for new test organisms. In designing these tests, he said it is important to consider all life stages, from larvae to adulthood; to account for both acute and chronic toxicity; to discriminate between intrinsic toxicity and physical effects; and to use population-relevant endpoints such as mortality, metamorphosis, and growth. In terms of methodology, he added that it is important to ensure water quality and light conditions are suitable for coral survival, to avoid unnatural and potentially confounding solvents, to use chemical control analysis to refer the results to nominal concentrations, and to measure the stability and the recovery of the substance at the end of the exposure regime.
In collaboration with the University of Oldenburg, Germany, BASF has conducted short-term acute toxicity tests on lab-bred adult corals and longer tests on more sensitive larvae, which Pawlowski said are ready for prevalidation under ISO/OECD. Researchers have also studied longer-term effects of certain solvents, such as dimethylformamide, dimethyl sulfoxide, ethanol, and methanol. Chronic toxicity tests for coral fragments are in progress, and the team next plans to focus on coral bioaccumulation tests, with the goal of developing a standardized coral toxicity testing method that generates high-quality data in the next 5–10 years, Pawlowski said.
Applied Development of Standardized Coral Toxicity Tests
Abigail Renegar (Nova Southeastern University) provided additional context on the challenges of developing standardized coral toxicity tests. Corals are highly sensitive to factors such as temperature, light, water flow, salinity, pH, and alkalinity and also exhibit a wide range of chemical responses, within species and during certain life stages, all of which makes them quite difficult to study. As a further complication, corals have a symbiotic relationship with zooxanthellae, making it necessary for study endpoints to consider impacts on both organisms, she said.
Despite the challenges, researchers have generated highly reproducible toxicity thresholds in corals based on histological changes and growth rate. However, Renegar cautioned that generating reproducible, low-variability, high-quality data require significant attention to detail, minimization of coral handling, and further optimization of methods.
Regardless of the particular chemical or coral species being tested, toxicity assessments generally follow the same essential structure. First, pre-exposure observations are used to establish a baseline of growth rate and photosynthetic efficiency. Then, measurements during exposure or immediately post-exposure are used to assess mortality, coral condition, polyp behavior, mucus production, coloration changes, tissue swelling or attenuation, growth rate, and photosynthetic ability. Finally, post-exposure measurements are used to assess bioaccumulation, gene expression, and histological changes such as tissue architecture, cellular integrity, and zooxanthellae condition. Within this overall structure there are a number of testing approaches, each of which has its own set of strengths and weaknesses. For example, Renegar said that static-renewal tests are useful for finding and assessing the range of responses to new chemicals or working with a new species but tend to underestimate toxicity. Continuous recirculating systems are closed, use passive dosing, and are useful for short, acute exposures to volatile and semivolatile compounds. Flow-through methodology is useful for 96-hour acute and 21-day chronic exposures, maintains high water quality, and results in reliable and consistent exposure concentrations for chemicals that are difficult to work with, like UV filters.
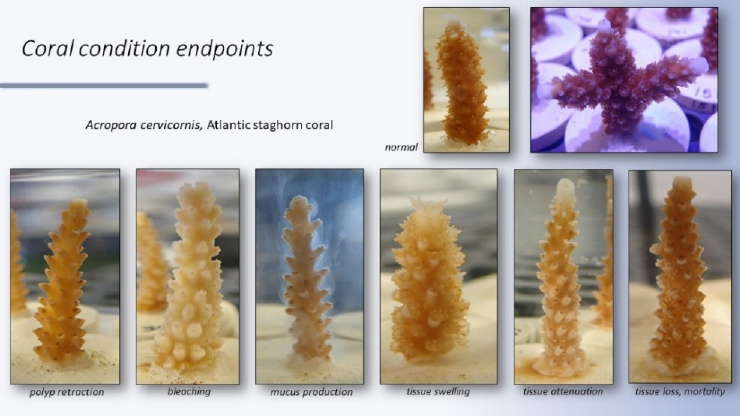
To continue to refine and standardize testing approaches, Renegar said that it will be vital to overcome the challenges of analytical chemistry and balance testing standards and consistency with existing regulatory guidelines and metrics that are scalable for environmentally relevant exposures. Atlantic staghorn coral (Acropora cervicornis) is one of Renegar’s test species to show coral condition endpoints from environmentally relevant exposures of UV filters (Figure 6). She suggested prioritization of corals and other nonstandard organisms of significant ecological importance in the context of UV filters and also suggested that EPA and OECD should develop standard coral toxicity test methods for chronic exposure with population-relevant endpoints. She added that the scientific community as a whole should focus on producing reliable, actionable data with appropriate quality assurance/quality control reporting standards and noted that molecular methods may also be applicable.
PANEL DISCUSSION
Transitioning to a panel discussion, moderator Carys Mitchelmore (University of Maryland) reviewed the challenges of analytical testing of UV filters that were brought up during the workshop’s first day and emphasized the need for multidisciplinary, interlaboratory collaboration to make progress. She then invited an additional five panelists to join Raimondo, Downs, Pawlowski, and Renegar for a discussion of progress, opportunities, and outstanding challenges relevant to aquatic toxicology of UV filters. The additional panelists were Iain Davies (Personal Care Products Council), Marc Leonard (L’Oréal), Mandy Annis (U.S. Fish and Wildlife Service), Jeffrey Steevens (U.S. Geological Survey), and Dan Villeneuve (EPA). Together with Mitchelmore, panelists shared opening remarks and then engaged in an open discussion to bring together perspectives from a wide range of organizations and sectors.
The environmental safety program of the Personal Care Products Council relies on regulatory guidance to assess the environmental risks of UV filters. Davies said that more testing is needed to generate relevant, reliable data for many UV filters, especially for understanding chronic exposures and understanding effects on underrepresented taxa. In Europe, he noted that dynamic predicted no-effect concentrations are required for acute and chronic exposure and for the most sensitive and environmentally relevant species. In the United States, EPA’s Office of Prevention, Pesticides, and Toxic Substances has helpful risk assessment frameworks and problem formulations for identifying, testing, and generating reliable data on standard and nonstandard species. He added that other frameworks, such as the Criteria for Reporting and Evaluating Ecotoxicity Data and Canada’s Ecoquest, could also be helpful to consult.
L’Oréal has developed a variety of methodologies for chronic toxicity tests of chemical compounds. To develop approaches relevant to the highly complex context of coral reefs, Leonard stressed that multiple challenges should be overcome. In particular, he pointed to the need to take into account the nuances of particular species and the influence of environmental factors such as light length, intensity, distribution, and positioning poses special challenges. Leonard also underscored that new test methodologies for coral should be biologically relevant to the taxonomically diverse assemblages of organisms that comprise coral reefs and stressed the importance of using species sensitivity distributions to identify and test the most sensitive species.
Resource managers with the U.S. Fish and Wildlife Service draw upon the best available data to conserve, protect, and enhance fish and wildlife resources and their habitats. Annis highlighted her team’s work with freshwater mussels, a species often considered to be the backbone of many freshwater aquatic ecosystems. Mussels, which improve water quality and stabilize cycling streams with their filtering activity, are similar to corals in that they are extremely sensitive to environmental perturbations and have a complex life cycle that makes them challenging to study. To stave off mussel population declines, researchers are working to create a baseline characterization of mussel health across life stages, establish suitable surrogate species, and develop standardized procedures to study lethal and sublethal exposure levels and durations.
Building on these points, Jeffrey Steevens made a case for broadening the focus of toxicity testing for UV filters to better account for the full diversity of species that make up aquatic ecosystems. The taxa that may be affected by these chemicals live in extremely complex ecosystems, with flowing sediment, terrestrial interfaces, and both static and moving water. He pointed to freshwater mussels (which burrow into sediment and filter water at a high rate) and mayflies (which emerge through the surface microlayer in their early life stages) as examples of species with unique
considerations in the context of their potential interactions with UV filters and related chemicals. He noted that impacts on such species could potentially be missed with toxicity testing methodologies that are more narrowly focused on the water column.
Villeneuve noted that it will take time and resources to fill the many data gaps with regard to UV filters and their environmental impacts. He said that new approach methodologies can potentially generate data more rapidly and cost effectively, can be combined with modeling to make predictions about other species that cannot be tested, and can potentially generate accessible data for use in ERAs. However, he cautioned that existing new approach methodologies include many uncertainties, especially around lab-to-field extrapolation, making them perhaps best suited to lower-tier assessments. He suggested combining these approaches with more traditional approaches and placing high priority on appropriate problem formulations, key UV filter data gaps, coral sensitivity, and species sensitivity distributions.
Raimondo, Downs, Pawlowski, and Renegar briefly expanded upon their previous remarks to transition into the panel discussion. To develop the most defensible ERAs, Raimondo stressed that EPA needs the best science available, rooted in minimal variability and strong reproducibility, consensus on endpoints, and an emphasis on ecological and environmental realism. Downs built on his remarks about the importance of standardizing the use of light—a major driver of coral growth and UV filter activity—in toxicity testing. He pointed to a need for EPA or OECD standards on light, which he said should be designed to emulate the light composition and intensity test species encounter at different times of day and at different geographical locations.
Pawlowski emphasized that compromises will be necessary to create EPA/OECD testing protocols that create reproducible results, include appropriate endpoints, and achieve consistent test conditions and realistic species sensitivity distributions. Recognizing that most laboratories around the world do not have dedicated expertise in working with corals, he said it is especially important to establish a standardized framework for testing that can be implemented broadly by people with varying levels of expertise and experience. While progress has been made and some standard guidelines exist, Renegar reiterated that standardized EPA and OECD compatible methods for nonstandard species like corals remain a key need. She said that advancing this work will require multiple partners, significant investments of time and resources, a consensus approach to endpoint selection, data reliability requirements, consistent methods for analytical chemistry, chronic assays, and an understanding of mixed or multiple stressors.
Closing out the panelists’ introductory remarks, Mitchelmore stressed the critical importance of advancing analytical methods for toxicological evaluation that are appropriate for the test chemical and exposure design and considerate of factors such as UV filter solubility, solvents, and photosensitivity. She said that protocols should include steps to minimize opportunities for mistakes, contamination, mismeasurements, and misinterpretation. These methods can be adapted from existing EPA and OECD standards and guidelines, with modifications for nonstandard species and adequate quality assurance/quality control in order to enable reporting of reliable, relevant data for ERAs.
Mitchelmore moderated an open discussion between panelists and workshop attendees that further explored toxicity testing challenges, the role of new approach methodologies and species sensitivity distributions, the importance of creating standards, and considerations specific to working with corals and mussels.
Testing Challenges
Developing appropriate study designs and exposure regimes for toxicology testing poses significant challenges. Steevens noted that flow-through testing establishes constant-exposure concentrations, which are important for studies of longer duration, although for those studies, researchers should also add food, which influences exposure. Mitchelmore agreed, adding that organisms also excrete fluids, representing another variable, although the water movement in flow-through systems keeps the exposure more homogenous.
Pawlowski highlighted the importance of the materials that are used, as certain plastic vessels could be problematic. Mitchelmore said that flow-through system standards require glass labware to maintain water quality, keep exposure homogenous, and minimize loss and contamination. Downs reiterated that artificial seawater is better than filtered seawater, especially for acute tests, to eliminate the presence of confounding chemicals. Renegar agreed that using artificial seawater eliminates a source of variability, and Pawlowski noted that he is aware of several labs that use artificial seawater to culture corals and for chronic testing.
Renegar noted that creating realistic flow-through tests with optimized dosage rates requires intensive sampling, time to reach equilibrium, and appropriate consideration of loss. Avoiding loss of control organisms is critical, Raimondo said, because nonoptimized conditions can affect toxicity understanding and lab-to-field extrapolation. Mitchelmore agreed and suggested that coral researchers should adapt conditions for standard organisms, where many of these variables are prescribed to control survival.
Another challenge is addressing contributions from multiple stressors. Renegar stated that scientists learn by understanding one thing at a time, so each stressor should be studied individually first, then combined with others in stepwise fashion.
New Approach Methodologies and Species Sensitivity Distributions
Once relevant, reliable data have been generated, next steps for UV filters can be determined, but Davies noted that this process will take time. To speed progress, problem formulation is an important consideration. Since it is not possible to test all chemicals on all organisms every day to create ERAs or hazard assessments, Davies suggested that temporal and spatial exposure assessments and monitoring can provide more data to home in on problem formulations that define which organisms should be tested, where they live, and which data points are needed.
Villeneuve noted that new approach methodologies go beyond the conventional expose- and-observe technique to more predictive approaches, such as studying an organism’s molecular biochemistry and interpolating hazards or population-level or ecosystem-level modeling for outcome prediction. Raimondo reiterated that EPA is working to develop new approach methodologies to overcome many of the challenges identified in this workshop, both for UV filters and for other chemicals under EPA’s purview. These approaches are promising for filling data gaps, especially for understudied species, but she underscored the need for more testing in order to reduce uncertainty to a level appropriate for an ERA. “Any approach is only as good as the data that you put into it,” Raimondo stated.
Participants explored the benefits and downsides of focusing on species sensitivity distributions. Steevens pointed out that one inherent flaw of this approach is that, by definition, it aims to protect 95 percent of species and requires readjustment if more sensitive species are identified, as has happened for mussels. Many difficult-to-culture species are never tested, and without that data the species sensitivity distribution may underestimate the effects. Raimondo added that the wide
taxonomic diversity within aquatic ecosystems makes it difficult for species sensitivity distributions to account for what is unknown, but she said this is an area in which new approach methodologies may help to fill gaps. Pawlowski stated that while species sensitivity distribution data are widely available and commonly used in Europe to protect nontested species, the huge range of variability can reduce confidence in species sensitivity distribution assumptions. Davies suggested European and U.S. safety assessment factors could be merged by replacing organisms with no observed effect concentration (NOEC) with organisms with 10 percent effect concentration (EC10) to improve the species sensitivity distribution and reduce uncertainty.
Creating Standards
To work toward standardization in toxicity testing for UV filters, Raimondo stated that EPA is focused on reducing sources of variability or uncertainty in terms of factors like pH level, light, salinity, test chambers, water quality, and temperature, all of which can affect analyses. Mitchelmore agreed that water quality standards should include baseline exposure media to keep water quality stable. Pawlowski said that reducing variability provides data on intrinsic control, but he cautioned that in many cases ecological relevance is still uncertain and data on corals, especially for longer exposure periods, is still lacking. He added that creating universal toxicity test standards will require collaboration and guidance from relevant authorities.
Raimondo said organizations like EPA and OECD look to “round-robin” testing, in which multiple institutions apply the same methods and get similar results, to ensure standards are transferable and reproducible. However, Raimondo and Mitchelmore noted that the time required for this “gold standard” approach poses a challenge as it can take 5–20 years to fully refine and standardize methodology for existing and new taxa. While Raimondo said that it is important to continue to share protocols and lay the groundwork for round-robin testing in the long run, Mitchelmore suggested that convening a working group to develop informal standard protocols could be a helpful near-term step to establish a set of minimum standards and facilitate comparison across studies. Pawlowski and Mitchelmore added repeating studies and publishing the methods and results in detail is critical to increasing transparency and working toward standard protocols.
Special Considerations for Corals and Mussels
Participants discussed several particular considerations for working with corals and mussels. Whether a substance is toxic or not depends on how toxicity is defined. Asked whether settlement should be considered a key part of coral’s life cycle, Pawlowski replied that for short-term mortality studies, settlement is a key endpoint that is probably affected at any concentration. Renegar reiterated that corals are complex organisms and suggested that the microbiome that develops around them should be studied extensively to understand the cumulative effects on the whole ecosystem. For corals, polyp retraction is a common response to contaminate exposure, but its ecological relevance and long-term health impacts are unclear. Mitchelmore agreed that such responses could either be an early sensitivity indicator or a change that does not impact health, and Pawlowski noted that every substance that enters an organism causes a physiological response. Davies suggested that the debate over appropriate toxicological endpoints for corals underscores the need for greater collaboration.7
___________________
7 Burns, E. E., & Davies, I. A. (2021). Coral ecotoxicological data evaluation for the environmental safety assessment of Ultraviolet Filters. Environmental Toxicology and Chemistry, 40(12), 3441–3464. https://doi.org/10.1002/etc.5229.
Asked about current progress in toxicity testing of UV filters in mussels, Steevens said that researchers have identified a promising test species and determined sensitive endpoints. The next step, he said, is to determine how to prepare test solutions, observe how the mussels respond, and perform bioassays to establish standard thresholds. Annis added that because mussels are challenging to study, much of the available data reflect single snapshots in time. It is important to continue to learn about multiple life stages through chronic assessments to determine biologically relevant, longer-term exposure effects from chemical stressors, she said.
BREAKOUT DISCUSSIONS
In the workshop’s final session, participants divided into small groups for focused discussions around the key challenges of working with nonstandard organisms and endpoints and opportunities to make progress in addressing these challenges. Representatives from each group summarized the outcomes of these discussions, which are combined and summarized in the sections below.
What Are the Main Challenges Encountered When Working with Nonstandard Organisms or Endpoints?
Several participants pointed out that the standard species used in toxicity testing were selected as standards based on a number of factors, including the ease with which they can be accessed and used. The challenges of identifying nonstandard species to target, reliably accessing those organisms, and resolving practical considerations for culturing them in a laboratory are not trivial. For many nonstandard species, the lack of a baseline understanding of behavior, genotype, characteristics, life cycles, and sensitivity hampers the ability to design culture conditions that mimic the natural environment and conduct toxicity tests that are appropriate and relevant. It is also important to consider complex interactions such as symbiosis, the role of the microbiome, and other indirect effects on organisms’ biology and community interactions, as well as the potential for seasonal variability, both in an organism’s natural environment and in potential UV filter exposure patterns, some participants noted.
In selecting endpoints, a few participants suggested that researchers would benefit from defining which responses are considered to be adverse, what sublethal endpoints should be considered, and how laboratory measurements relate to population, growth, or other indicators of viability. The timescale of both exposures and effects is also important. Many participants noted that studying modes of action, while not always essential to toxicity testing, can help to inform the design of future UV filters to avoid causing toxicity via the same mechanisms.
Additional challenges may include developing standardized new approach methodologies, eliminating technician bias, ensuring results are comparable across experiments and laboratories, establishing minimum reporting standards, and developing and validating methods for extrapolating for species that remain difficult to study.
Are Challenges Magnified When Testing under Certain Conditions for Both Standard and Nonstandard Tests for These Chemicals?
Several participants said that these challenges are magnified by a dearth of baseline knowledge about UV filters, the ecosystems that they interact with, and the best ways to develop
laboratory tests with ecological relevance. The limited understanding of the unique chemical properties of UV filters, how they interact with seawater, and how they act in mixtures makes it particularly difficult to design tests that will extrapolate to real-world environments. Some participants pointed to for the importance of more research to elucidate the dynamics of partitioning, non-monotonic chemical responses, and solvents’ fates and interactions with organisms. Finally, a better understanding of the effects of timing (and related considerations, such as moon phases) and how the medium affects exposure can help to inform study design and reduce the likelihood of loss or contamination.
What Progress Is Being Made in Addressing These Challenges?
Some participants said that the very existence of the 2022 report and this workshop represent important steps toward creating more reliable and reproducible data collection and analysis standards. In addition, several participants said that toxicokinetic and toxicodynamic modeling approaches have improved opportunities to estimate internal tissue exposures and suggested that the tiered modeling exposure frameworks from the International Cooperation of Cosmetic Safety (ICCS) can help to translate direct releases into exposures. The Society of Environmental Toxicology and Chemistry’s (SETAC) aquatic testing subgroups, ICCS’ coral working group, the Sanger Institute’s coral genome sequencing studies, and others have also made important strides in the field, some participants noted.
A few participants also said the development of and increased access to mathematical tools and technologies as well as diagnostic multi-omics literature have helped address some of these challenges, along with improvements in fact-finding testing to guide nonstandard methods, improved coral husbandry techniques, and data sharing.
What Standardizations, Innovations, and/or Other Focused Efforts Are Needed to Move Forward on Addressing These Challenges?
Many participants pointed to the importance of a centralized, global, appropriately funded interagency collaboration involving industry, regulators, and academic researchers to determine what test methods are available, identify the most defensible, and avoid repeating mistakes. Several participants discussed roles for a variety of stakeholders, suggesting that a champion may be needed to help propel a broad collaboration; working groups could be useful for creating action steps and disseminating findings; sponsors could help to support new facilities, produce standardized specimens, and establish research incentives, scholarships, and internships; and data repositories could help increase data accessibility with proper attribution, ownership, and reliability protocols.
From a scientific perspective, some participants underscored the importance of improving problem formulation. For this, it would likely be helpful to identify areas where exposure is highest, discover other exposure pathways beyond sunscreens, and generate robust, statistical endpoint data that would be useful in EPA ERAs. In terms of specific suggestions for future research, some participants mentioned investing in more studies of benthic organisms, sediment, and freshwater species; refining methods to culture nonstandard organisms for laboratory testing; studying multiple stressors separately; and working to further enhance, validate, and increase the use of new approach methodologies. A few participants also suggested expanding into in vitro assays and ground truthing, experimenting with cryopreservation, generating toxicokinetic evidence, updat-
ing water resistance testing, and standardizing animal husbandry techniques. Pilot studies and validation studies can help to clarify endpoints, refine test protocols, and ensure reliability. Finally, many participants pointed to the value of including absorption, distribution, metabolism, elimination, and reference compounds in study designs and reiterated the importance of carefully considering species selection to ensure species are geographically appropriate and including consideration of endangered species.
To move toward standardized tests and inform ERAs, a few participants suggested learning from biologists, especially coral biologists, but also suggested looking to other countries’ standards, studies of toxicology, existing tools, and exposure models in other domains. Some participants suggested broadening ERAs to incorporate a wider range of evidence types and many participants reiterated the desire to find the right exposure media; identify the most important variables; use mesocosms to enable dose dependent responses, transportation, and location in the water column; use field exposures to give environmental context to laboratory tests; estimate biological responses for nonstandard organisms via modeling and nonstandard metrics for sublethal effects; and ask experts to translate toxicology work into real-world scenarios.
From a broader perspective, some participants mentioned the usefulness of deep reflection on industry responsibility and ethics, a better understanding of consumer behavior, a holistic approach to ecosystem stability, an acknowledgment that animals should not be sacrificed unnecessarily, and a push for more open science practices.
What Are Existing Research Programs, Capabilities, and Infrastructure That Can Contribute to Addressing Challenges in Gaps in Research on UV Filter Toxicity?
Many relevant organizations have the resources and expertise to advance collaborative efforts in this space. In particular, a few participants pointed to EPA, OECD, FDA, SETAC, ICCS, ISO, the U.S. Fish and Wildlife Service, and ASTM International as potential key players. In addition to regulations in other countries, some participants said the field can find useful models in EPA’s Science to Achieve Results (STAR) program, the Great Lakes Restoration Initiative (GLRI), the “reef safe” designation, the oil industry’s experience with spills, and eco-epidemiology approaches that are used to characterize stressors and determine causes of decline to understand multi-stressor impacts on corals. Finally, many participants emphasized that the public is a stakeholder whose interest can spur action, prioritization, and funding to create a better understanding of the risks associated with UV filters and inform the path forward.












