Realizing the Potential of Genomics across the Continuum of Precision Health Care: Proceedings of a Workshop (2023)
Chapter: 5 Exploring Logistical Barriers to Genetic Testing
In this session, speakers discussed logistical challenges to genetic testing that will need to be addressed for the full implementation of genomics in precision health care. The session was moderated by Victoria Pratt, vice president of Molecular Diagnostic Quality Assessments at Optum Genomics.
THE EFFECT OF CODING AND REIMBURSEMENT ON ACCESS
Genetic testing is complex, and the science is advancing “faster than any other area in laboratory medicine,” said Lee Hilborne, senior medical director at Quest Diagnostics and professor of pathology and laboratory medicine at the David Geffen School of Medicine at the University of California, Los Angeles (UCLA). However, the processes for reporting results and reimbursing providers for services have not kept pace with the technological advances. As discussed earlier, genetic testing is a tool and obtaining a result is only the first step in the process. The tool provides value when appropriate actions can be taken based on the results. As testing technology advances and the cost of testing becomes much less of a barrier, he said, the greater challenge will be what happens after the test results become available.
System Barriers to Genetic Testing
Hilborne reviewed some of the main system barriers to access to genetic testing before discussing coding and reimbursement in depth.
Knowledge. Although there is general awareness of genetic testing, greater understanding of genetics and its role in precision medicine is needed by many providers (physicians, genetic counselors, laboratory professionals, and others), patients, payers and employers, and the public, Hilborne said.
Language. Dealing with the nomenclature of clinical laboratory testing is challenging in general, and the language of genes and genetic testing is
very complicated, especially for those who do not encounter it regularly, Hilborne said. Providers and patients rely on geneticists and genetic counselors to interpret genetic testing results.
Complexity. There are multiple indications for genetic testing, including diagnostic, prognostic, or predictive testing; screening to determine carrier status; and pharmacogenetic testing for therapeutic response, Hilborne said. Genetic panels for a given condition can vary across laboratories. In addition to indication, patient history, ethnicity, known genetics, and patient preferences also come into play when choosing which test to order.
Value. The ACCE model has been used to evaluate the value of genetic tests according to analytic validity, clinical validity, clinical utility, and associated ethical, legal, and social implications.1 Laboratories routinely assess analytic and clinical validity; however, establishing the clinical utility of new genetic tests has often been challenging. There may be areas in which having the ability to do genetic testing does not necessarily mean you should, Hilborne said. He added that meeting payers’ criteria for clinical utility has been an impediment to coverage for some tests.
Cost. The cost of sequencing a genome has decreased significantly as the technology for sequencing has advanced, yet patient costs and reimbursement do not always reflect this. “Genetic testing remains disproportionately costly compared to other laboratory diagnostics,” Hilborne said, adding that deciding whether to incur the cost of a test requires consideration of medical appropriateness (e.g., the extent to which benefit outweighs risk) and medical necessity.
Coding and Reimbursement. Early Current Procedural Terminology (CPT®) coding for molecular diagnostics required selecting a code for each of the individual laboratory procedures performed for the testing and “stacking” them. Later, gene-specific codes were created, each of which incorporated the analytical laboratory services that were previously coded separately. There are still some nonspecific codes for groups of procedures, Hilborne said, including a catchall code for molecular pathology procedures that do not have a code.
The pricing of genetic tests initially reflected that most of the testing was for individual genes and was done using the labor-intensive Sanger sequencing method. As technology advanced, multianalyte assays with algorithmic analysis became more common, and new CPT codes were created in 2015 to address the emerging multianalyte genomic sequencing procedures (GSPs). Currently, there are about 50 GSP codes, Hilborne said; however, the list is not comprehensive, and while the GSP codes identify the indication, ambiguity persists because of the range of procedures available.
___________________
1 For historical information on the ACCE model see https://www.cdc.gov/genomics/gtesting/acce/index.htm (accessed December 15, 2022).
In 2017, codes were created for proprietary laboratory analyses (PLAs) that are generally specific to a particular laboratory (or manufacturer). There are currently about 350 PLA codes, and he noted that there are some payment challenges associated with these codes.
Specificity versus simplicity is on ongoing challenge for the coding of genetic testing. Complex surgical procedures (e.g., liver transplantation) generally have a single CPT code, while there are numerous codes for molecular services. Although payers and other groups have sought streamlined solutions, he said, specificity regarding which services were performed is needed to adjudicate claims.
The reporting of genetic testing services is complex, Hilborne concluded. CPT is the code set recognized by the Health Insurance Portability and Accountability Act of 1996, but the coding struggles to keep pace with the evolution of the technology, he said. In addition, there are a number of ongoing efforts to provide new solutions for the challenges of cataloging and coding the ever-increasing number of genetic tests (e.g., NIH Genetic Test Registry, Palmetto MolDx DEX Registry, Concert Genetics Coding Engine).
Alluding to earlier remarks in the day, one participant asked what will happen to the ecosystem for genetic testing when a genome costs $100. Hilborne replied that the way genetic testing is considered, from the perspective of CPT, will change as the cost shifts more toward the professional work rather than the technical work. He added that the value added by the work of genetic counselors and other genetics professionals (e.g., Ph.D. medical geneticist, physician medical geneticist) will also need to be considered when discussing coding and reimbursement to ensure equitable access.
Addressing Coding and Reimbursement Challenges
Hilborne highlighted several opportunities to address these coding and reimbursement challenges and to realize the potential of genomics in precision health care:
- Developing clear evidence of clinical validity and clinical utility.
- Coming to general agreement on what constitutes an appropriate genetic evaluation for a patient with a specific condition to curb fraud, waste, and abuse that emerges from uncertainty.
- Reexamining what constitutes a genetic test. Hilborne suggested that separate codes might be needed for the technical component (the sequencing) and for the analysis of the data by the laboratory professionals as well as by geneticists and others.
- Reducing the complexity of ordering genetic tests and of the results reports. Ordering could be restructured around the clinical prob-
- lem rather than the genetic test (e.g., clinicians could order testing for hereditary breast and ovarian cancer rather than needing to know which specific gene tests to order). He noted that reimbursement issues regarding reflex testing would need to be addressed. For example, diagnosis codes entered to support the original test might not support downstream reflex testing.
ADDING GENOMICS TO THE PRIMARY CARE TOOLBOX
Mylynda Massart, assistant professor of family medicine at the University of Pittsburgh and founder and director of the University of Pittsburgh Medical Center (UPMC) Primary Care Precision Medicine Center, highlighted some of the many uses of genetic testing in primary care. These uses included validating the results patients receive from direct-to-consumer genetic testing; screening for hereditary cancer syndromes; pharmacogenomics for medication prescribing; prenatal carrier risk assessment; newborn screening, which is increasingly incorporating genomic sequencing; chronic disease management; polygenic risk scores; and the management of common adult genetic disorders. She shared her perspective on how to make genomics more readily accessible to primary care clinicians and others.
Barriers to the Implementation of Genomics as a Tool in Primary Care
For most individuals, primary care is the main access point in the health care system, covering urban and rural areas as well as underserved communities. There are many uses for genetic testing in primary care, Massart said, and primary care providers should be able to use genetic testing as a tool just as they use radiology as a tool (e.g., ordering and interpreting x-rays and magnetic resonance imaging without needing to consult a radiologist). If the findings indicate the need for specialized care, patients can then be referred to a specialist. She noted that medical geneticists will always be needed for expert consultation and for management of patients with rare genetic diseases or classic genetic syndromes.
There are many barriers to the implementation of genomics as a tool in primary care, Massart said. Regarding the testing itself, the availability, cost, and reimbursement of genetic testing are barriers, as is the laboratory turnaround time, which can be slow. Clinical barriers include the lack of patient access to clinical genetic testing services in general and particularly among underserved populations, the limited availability of algorithms, and a lack of randomized controlled trial data and real-world data to understand outcomes and value. There are informatics challenges, including how best to manage the huge volumes of data and how best to provide genetic testing results and point-of-care clinical decision support in the
EHR. Research-related challenges include complicated participant consent processes and an inability to integrate research and clinical care and share research information for the benefit of the patient. From an educational perspective, current and future health care professionals are not receiving training in genomics and precision medicine. Finally, she said, there are ethical, legal, and social implications of genetic testing that need to be better addressed. A question was asked about managing data security and Massart said, “The reality is that true data security is nearly impossible.” The solution is to enact policies and approaches that prevent the use of an individual’s genomic information against them. She added that patients should also be able to track how their data are being used and shared (e.g., for their clinical care, for research).
Addressing the Challenges in Primary Care
Massart highlighted five main areas of opportunity to enable the use of genomics as a tool in precision care:
- The EHR. Orderable genetic tests need to be included in the EHR, Massart said. The EHR should also include discrete reporting of results that then triggers clinical decision support to aid in data interpretation.
- Cost and reimbursement. Massart highlighted the need for transparency around the cost and reimbursement of genetic testing as well as sensible reimbursement models and clear pathways for obtaining reimbursement. Furthermore, she said, out-of-pocket costs for patients should be affordable.
- Guidelines and education. Massart suggested that “genomic clinicians” are needed. These providers could integrate genomics in routine care but not necessarily be formally trained in medical genomics. She also highlighted the need for clear algorithms for use in determining who needs testing and when to test; minimal competencies for ordering genetic tests, providing pre- and posttest counseling and interpretation of test results; and increased coverage of genomics in medical education.
- Scalability of tools. To provide genomics services at scale there is a need for more genetic counselors and increased diversity among genetic counselors. Other needs Massart identified included reimbursement for services provided by genetic counselors, and new models for both synchronous and asynchronous contributions from genetic counselors.
- Testing. Genomic testing needs to be streamlined, Massart said, noting that the cost of sequential gene-by-gene testing is approach-
- ing the cost of whole exome2 or whole genome sequencing. She also highlighted the need for indication-driven interpretation of findings, the reporting of all actionable secondary findings to patients, and the ability to reanalyze prior sequencing data for reinterpretation as knowledge evolves.
A Stepwise Approach to Integrating Genomics into Primary Care
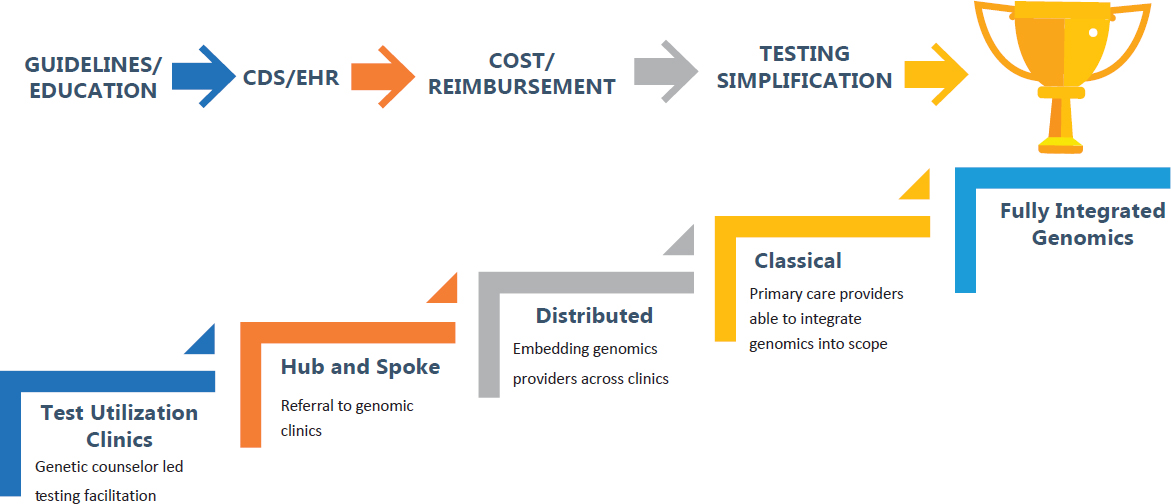
Achieving full integration of genomics into primary care requires improved guidelines and education, enhanced clinical decision support and EHR connectivity, better transparency of costs and reimbursement, and simplification of testing, Massart said. She outlined four models that together make up a “coordinated stepwise approach to the future” (see Figure 5-1).
Genetic Testing Utilization Clinic Model
One system-wide solution that has already proven effective in some places is genetic testing utilization clinics. These clinics, overseen by genetic counselors, work in partnership with laboratories to support clinicians and patients in accessing appropriate genetic testing. This model helps to reduce the risks of medical mistakes associated with inappropriate test selection, inadequate pre- and posttest counseling, poor utilization management, and inaccurate interpretation of test results, Massart explained. It can also help address clinicians’ avoidance of ordering testing because of how overwhelming the process can be.
Hub-and-Spoke Model of Centralized Genomic Clinics Serving Primary Care
The second model, which Massart noted is currently in use at University of Pittsburgh Medical Center, establishes centralized hubs of primary care genomics expertise to serve the primary care clinics in a health system or geographic region. A strength of this model is that fewer genomic experts are needed, and she described it as a stopgap measure until the education of new primary care genomics experts can catch up with the demand. In this way, she said, there can be support for clinicians to ensure that patients get the best test, coverage, informed consent, and interpretation of results. Another strength is that each expert consultation provides opportunities to educate referring clinicians. There are weaknesses, however, including
___________________
2 An exome is the sequence of all protein-coding portions, or exons, in a genome (Green, 2023).
SOURCE: Mylynda Massart, National Academies of Sciences, Engineering, and Medicine workshop presentation, October 12, 2022.
the potential for delays in accessing services and the need to see providers who are outside the patient’s medical home, which can present barriers to access, especially for underserved communities. Massart added that this model can still feel more like a specialist consultation than an embedded part of one’s primary care.
Distributive Model of Embedding Genomics Providers in Primary Care
The next step would be a distributed model in which genetic counselors are embedded within primary care clinics to provide genomics expertise (similar to pharmacists, social workers, and nutritionists who work within primary care practices). Strengths of this model, Massart said, are the ability to rapidly scale, and the opportunity to relieve the primary care provider of the need to manage the nuances of genetic testing and interpretation of results. A key barrier to implementing this model, she noted, is the fact that genetic counselors are not uniformly recognized as health care providers for the purposes of reimbursement.
Integration of Genetics into the Classical Family Medicine Model
The last step discussed by Massart is the integration of genetics into the classical model of family medicine such that it becomes part of the scope of practice. Family medicine physicians are broadly trained and already manage the majority of their patients’ needs, referring them to specialty care when needed. Expanding the scope of primary care and family medicine practice to include genomics will require the education of trainees and support for practicing physicians.
MEDICARE COVERAGE DECISIONS RELATED TO GENOMIC TESTING
Bruce Quinn, an independent consultant at Bruce Quinn Associates, LLC, provided an overview of evolving national and local Medicare coverage decisions for genomic testing. One issue, he said, is that Medicare policies and rules do not always match up with Medicare data. As background he pointed out that there are no copayments for laboratory tests, including genomic tests, for those covered by Medicare, and fee schedules for laboratory tests are publicly available. Prices for new tests are initially set by the Centers for Medicare & Medicaid Services and are reset every 3 years based on a national survey of commercial insurance prices.
Data on Medicare Part B payments for laboratory testing are also made publicly available, and Quinn shared some observations based on recent data. The top 15 genomic testing codes paid by Medicare in calendar year
2020 accounted for $2.2 billion, 88 percent of genomic test payments. The most frequent test code paid by Medicare, COVID-19 polymerase chain reaction (PCR) testing, accounted for nearly $1 billion of that total. Of the remaining $1.5 billion paid for the other 14 codes, 42 percent was attributable to proprietary single tests (e.g., Cologuard®, Oncotype DX®). Another 32 percent of the remaining $1.5 billion was paid out for what Quinn said are “heavily abused codes.” He described how he used publicly available information from the Department of Justice (e.g., code patterns used by laboratories that were indicted) and Medicare laboratory claims data for his analysis. These laboratories often have nondescript names (e.g., ABC Lab, Best Lab) and primarily bill Tier 2 CPT codes (especially 81408 and 81407 for sequence analysis of genes associated with very rare disorders), and code 87798 (a nonspecific code for detection of pathogens by PCR), Quinn said. He noted that the six codes most frequently used by these laboratories for fraudulent Medicare billing are used infrequently by well-known laboratories. If payments for codes that are “heavily abused” are excluded, proprietary tests now comprise 60 percent of non-COVID-19-testing Medicare payments, indicating that they have done very well in the current Medicare system, he said.
National and Local Coverage Decisions for Genomics Tests
National Coverage Decisions
Quinn discussed three main Medicare national coverage decisions for genomics tests. In 2014, CMS issued a decision to cover Cologuard®, a DNA-based screening test for colon cancer that is done on a self-collected and submitted fecal sample. The price for Cologuard® is about $500, and the product is advertised heavily to the public. Currently, he said, it is one of the highest paid codes in Medicare (about $300 million).
In 2018, CMS issued a decision for next-generation sequencing in cancer. This decision guarantees national coverage for next-generation sequencing-based companion diagnostics for cancer if they are U.S. Food and Drug Administration (FDA) approved, Quinn said, adding that the intent is to incentivize developers to seek FDA approval. Currently, the decision blocks the use of the same test more than once, which presents a barrier to the use of liquid biopsy tests to detect minimal residual disease or recurrence.
In 2021, CMS issued a national coverage decision for liquid biopsy for colorectal cancer screening. Per the decision, CMS will cover any FDA-approved blood-based colorectal cancer screening test that achieves 74 percent sensitivity and 90 percent specificity. At the time of the workshop, no test meeting this standard had been approved by FDA. Quinn pointed out
how this decision again uses policy to incentivize development and approval of a test with important public health implications.
Local Coverage Decisions
There are three Medicare Administrative Contractors (MACs) who issue local coverage decisions for genomic tests for their designated geographic areas, and they behave in different ways, Quinn said.
The National Government Services (NGS) MAC system covers Minnesota, Wisconsin, Illinois, New York, and the states of New England. Quinn said NGS MAC has strict policies and makes far fewer genomic test payments than other MACs (per capita or per million Medicare beneficiaries). As a result, fraudulent laboratories tend to avoid these states and NGS MAC has essentially “zero fraudulent payments.”
The Novitas system covers Texas and the surrounding states, as well as Pennsylvania and Florida. After being “battered by high abnormal payments,” which Quinn said likely reached $1 billion, Novitas is drafting very strict new local coverage decisions. For example, they will not cover any cardiology gene testing, and will only cover gene tests for cancer that are endorsed by guidelines.
The MolDx system covers the rest of the country and was established to make local coverage decisions specifically for molecular diagnostic tests. Quinn described MolDx as a “complex body of policies, rules, tech assessments, websites, [and] databases.” He noted that MolDx has comparatively liberal policies for the coverage of minimal residual disease testing, pharmacogenetics, and hereditary testing. For pharmacogenetics, for example, MolDx covers all testing recommended by CPIC guidelines.
Reform and Regulation
The Medicare Coverage for Innovative Technology (MCIT) program, proposed by the Trump administration in 2020, called for Medicare to cover any device, including tests, designated by FDA as a breakthrough device, Quinn said. Coverage would be automatic and would initially be for 4 years. MCIT was repealed by the Biden administration in 2021, in favor of a new program called Transitional Coverage for Emerging Technology. Quinn noted there have been several town hall meetings and reports, but no proposal has been released (Fleisher and Blum, 2022; Zeitler et al., 2022).
Coverage decisions, coding, and reimbursement are “very complex and multidimensional,” Quinn concluded. They are influenced by evidence but exist primarily in a policy world driven by rules and regulations, as well as unspoken rules and traditions.
DISCUSSION
Developing Guidelines to Support Clinical Decision Making and Coverage of Testing
Irons and Massart discussed that the practice guidelines that inform clinical decision support are generally written by professional societies, and that the writing of guidelines for presymptomatic patients is not keeping pace with practitioners’ needs. Many payers will only reimburse guideline-endorsed tests, Irons said, suggesting that professional societies should work with practitioners to develop guidelines. Family medicine and primary care doctors should be involved in generating guidelines, Massart said, adding that her clinic is currently developing a guideline for the evaluation and management of connective tissue disorders in the primary care setting, including identifying patients for genetic testing. The lack of reimbursement by Medicare and commercial insurance companies is a barrier for patient access to emerging precision medicine applications, Massart said. Even patients with insurance will incur out-of-pocket costs if services are not covered, she added, creating a situation where “only those with means get access.” An additional challenge is that guidelines on competencies in genetics and genomics that have been long endorsed by the American Academy of Family Physicians are not being integrated into family medicine training, Massart said.
Closing the Policy and Knowledge Gaps that Affect Equitable Access
Much of the discussion focused on addressing the issues that affect equitable access to genetic testing. One participant noted that “policy always lags far behind the evidence” and that this gap mostly affects those who are already underserved by the health system. Because the United States does not have a national health care system, Massart said, addressing this gap will require “a multipronged commitment across the country to solve these challenges and a [prioritization of] policy, protection, access, and equity.” She said that lack of coverage for services is a barrier to access, and many patients will not benefit from precision medicine services until those services have uniform coverage by payers. Further, when only those with means can access new services, the data collected are not representative of the larger population and these data often inform implementation, algorithm development, and coverage decisions.
One participant raised the issue of coverage under Medicaid and access to genetic services for those who receive their health care at safety net clinics, pointing out that women now have better access to cancer germline testing at safety net clinics, but they still face challenges getting genetic
counseling and follow-up care if they test positive. Hilborne said that Medicaid programs vary by state, and it is important to start having discussions about what policies and processes need to change to ensure access under Medicaid.
Feero said that rurality also presents access challenges that lead to disparities. “In rural states that are less well off, budgets are extremely tight [and] chronic disease is extremely prevalent,” and he said that genetics and genomics become low priority. In these areas the gap in access to new technologies continues to widen. Massart praised the work of those in the National Health Service Corps and others serving patients in the nation’s Federally Qualified Health Centers and suggested that advances in telemedicine can be used to help close gaps in access to care if rural areas can get equitable access to the necessary digital technology and services.
There are knowledge gaps among providers, Hilborne said, especially regarding new technologies and approaches. Patients can receive care that is technically appropriate, but opportunities for accessing more advanced care can be missed. It is essential to find ways to get information to providers and patients, especially in underserved communities, so they can make informed decisions about their care.
Advocating for Action on Systemic Change
Henley emphasized that the care those in underserved communities receive is not only a result of the level of knowledge or skill of providers. “Our health care system was built on racism,” she said, and racism and disparities in health care persist today. The health care system has been mired in bureaucratic “red tape” for decades and decades, which has sowed divisiveness. As a result, patients get “caught up in the system,” Henley said, and she described it as “tragic” and “embarrassing” that people of color are still treated differently when seeking care in the United States. This will continue, she said, until more individuals in the medical community say enough is enough.
“Everyone, in every position, needs to advocate for change,” Henley said. She urged participants to use their voices to advocate for action by those who are in positions to make change. This includes policy makers, members of Congress, and those responsible for creating the red tape. Valuable time is being wasted in meeting after meeting, year after year, as the same issues are discussed and nothing changes. Instead, she said, it is time to change the conversation and focus on “changing the health care system and pushing our policy makers to do better.”
This page intentionally left blank.













