Biological Effectors of Social Determinants of Health in Cancer: Identification and Mitigation: Proceedings of a Workshop (2025)
Chapter: Proceedings of a Workshop
Proceedings of a Workshop
WORKSHOP OVERVIEW1
Social factors such as the conditions in which people are born, grow, work, live, and age; their education and income; and many other elements can influence their likelihood of developing cancer, the type of cancer, the cancer stage at diagnosis, the quality of care they receive, and their health outcomes. While the complex interactions among these factors, known as the social determinants of health (SDOH),2 can make it difficult to identify and quantify their biological consequences, researchers are beginning to pinpoint biological mechanisms through which social factors influence health and disease and elucidate how identifying and addressing social risk factors along the cancer care continuum could improve patient outcomes.
___________________
1 This workshop was organized by an independent planning committee whose role was limited to identification of topics and speakers. This Proceedings of a Workshop was prepared by the rapporteurs as a factual summary of the presentations and discussions that took place at the workshop. Statements, recommendations, and opinions expressed are those of individual presenters and participants and are not endorsed or verified by the National Academies of Sciences, Engineering, and Medicine, and they should not be construed as reflecting any group consensus.
2 According to the World Health Organization, SDOH are the nonmedical factors that influence health outcomes. They are the conditions in which people are born, grow, work, live, and age and the wider set of forces and systems shaping the conditions of daily life. These forces and systems include economic policies and systems, development agendas, social norms, social policies, and political systems. https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1 (accessed July 3, 2024).
To examine the complex interactions among biological variables and SDOH, and opportunities to mitigate the negative impacts of social factors on cancer-related health outcomes, the National Cancer Policy Forum of the National Academies of Sciences, Engineering, and Medicine hosted a workshop on March 20–21, 2024, that brought together participants with backgrounds in clinical care, cancer research, health care policy, patient advocacy, and related areas. This Proceedings of a Workshop summarizes the issues discussed and highlights observations and suggestions made. Those from individual participants are discussed throughout the proceedings, and highlights are presented in Boxes 1 and 2. (Box 1 lists observations on the relationships between SDOH, cancer, and health care biological effectors of SDOH, and Box 2 outlines potential strategies for integrating SDOH into cancer research and care.) Appendixes A, B, and C provide the workshop Statement of Task, agenda, and poster session participants, respectively. Speaker presentations, poster presentations, and the workshop webcast have been archived online.3
Chanita Hughes-Halbert, associate director for cancer equity and professor of public and population health sciences at the University of Southern California, provided context for the discussions by defining cancer health disparities, which she said are differences in both cancer risk and outcomes that are linked to social, economic, or environmental variables associated with disadvantage, such as race, ethnicity, socioeconomic status (SES), gender, and location. She noted that as the understanding of cancer health disparities expands, researchers are increasingly examining the role of these SDOH while also striving to create social, physical, and economic environments that enable everyone to attain their full potential for health and well-being, including through the objectives articulated in Healthy People 2030.4
Hughes-Halbert posited that a holistic, multilevel perspective can enable transdisciplinary teams to address the complexities of the impact of SDOH on health disparities (see Figure 1) (NIMHD, 2018). She noted that researchers are increasingly able to link social factors to health disparities, such as the higher rate of hormone receptor-negative breast cancer among Black women compared to White women (Linnenbringer et al., 2017). She suggested that it will be useful for researchers to continue examining this complex web of issues to create paradigm-shifting, transformational, multi-perspective approaches that both identify and mitigate cancer health disparities, from basic research to strategic implementation (Dankwa-Mullan et al., 2010).
___________________
3 See https://www.nationalacademies.org/event/41369_03-2024_biological-effectors-of-social-determinants-of-health-in-cancer-identification-and-mitigation-a-workshop (accessed June 24, 2024).
4 See https://health.gov/healthypeople (accessed May 22, 2024).
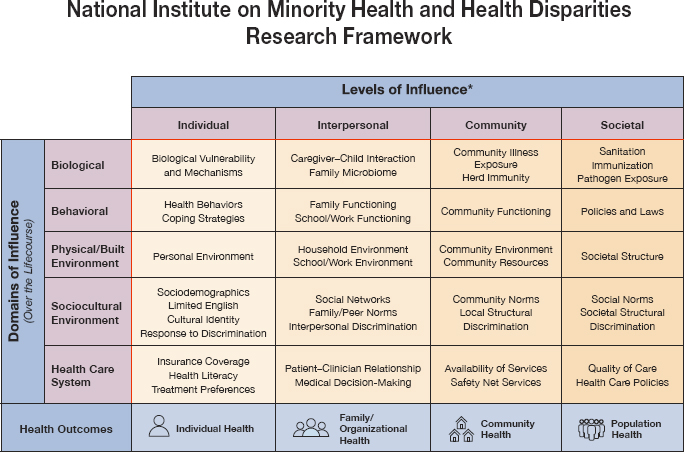
NOTE: *Health disparity populations: racial and ethnic minority groups (defined by OMB Directive 15),a people with lower socioeconomic status, underserved rural communities, sexual and gender minority groups, people with disabilities. Other fundamental characteristics: sex and gender, disability, geographic region.
SOURCES: Hughes-Halbert presentation, March 20, 2024; NIMHD, 2018.
_____________
a See https://spd15revision.gov/content/spd15revision/en/2024-spd15.html (accessed July 17, 2024).
“There is room for all of us and all disciplines to be involved in this research to really increase our understanding and ability to drive policy and the delivery of health care by understanding the contributions of racial and ethnic segregation, the neighborhood environment, psychosocial stressors, psychological distress, the things that we would typically include as part of our understanding and work around SDOH,” Hughes-Halbert emphasized.
Stanton Gerson, dean of the Case Western Reserve University School of Medicine, said that residents of distressed communities suffer devastating health effects because “poverty is a carcinogen,” as Samuel Broder famously said in 1989 (ACS, 2011). While an individual’s living conditions can contribute to cancer development, it is important to note that this depends on specific biological factors. Gerson stressed that it is critical to uncover connections
between the drivers of SDOH and the biological causes and consequences of cancer.
A range of biological mediators and pathways are associated with cancer, including genetics, epigenetics,5 ancestry, allostatic load, stress response, norepinephrine,6 cortisol,7 obesity, ingested and inhaled toxins, reactive oxygen,8 and inherited or acquired mutations, Gerson explained, while SDOH include a wide range of factors, such as poverty, racism, heat, noise, violence, disruption of sleep and circadian rhythms, environmental exposures, food access, and smoking. Hughes-Halbert noted that researchers are increasingly demonstrating connections between environmental exposures and health outcomes, such as finding microplastics in the hearts of patients with cardiovascular disease or linking chemical exposure to multiple negative health outcomes (Marfella et al., 2024; Woodruff, 2024).
HEALTH DISPARITIES AND SOCIAL DETERMINANTS OF HEALTH IN CANCER
Several speakers offered context on health disparities in cancer risk, onset, screening, treatment, and response to treatment, along with an overview of the relationships among SDOH, cancer, and health care.
Health Disparities in Cancer
Approximately 600,000 people die from cancer in the United States every year, making it the second leading cause of death, after heart disease (CDC, 2022). Yet, the disease burden is not experienced equally across the population. Otis Brawley, Bloomberg Distinguished Professor of Oncology
___________________
5 Epigenetics is the study of how age and exposure to environmental factors, such as diet, exercise, drugs, and chemicals, may change how genes are switched on and off without changing the actual DNA sequence. These changes can affect a person’s risk of disease and may be passed from parents to their children. See https://www.cancer.gov/publications/dictionaries/cancer-terms/def/epigenetics (accessed July 6, 2024).
6 Norepinephrine, or noradrenaline, is a neurotransmitter and hormone that is released in response to stress or low blood pressure. See https://www.cancer.gov/publications/dictionaries/cancer-terms/def/norepinephrine (accessed July 11, 2024).
7 Cortisol is a hormone that “helps your body respond to stress, regulate blood sugar, and fight infections.” See https://www.urmc.rochester.edu/encyclopedia/content.aspx?contenttypeid=167&contentid=cortisol_serum (accessed July 11, 2024).
8 Reactive oxygen is a free radical (an unstable molecule) that can accumulate in and cause damage to cells. See https://www.cancer.gov/publications/dictionaries/cancer-terms/def/reactive-oxygen-species (accessed July 11, 2024).
BOX 1
Observations from Individual Workshop Participants on the Relationships Among SDOH, Cancer, and Health Care
- Disparities in cancer screening, treatment, and outcomes are linked with social factors such as belonging to historically marginalized groups, lower socioeconomic status, and racial and economic discrimination and disadvantage, the impacts of which can go beyond the individual to span generations and communities. (Bae-Jump, Brawley, Evans, McCullough)
- Where patients live and receive care influences the quality of their care. (Frencher, Gerson, Watson)
- Research has linked social determinants of health (SDOH) with clinical outcomes, but an understanding of the specific pathways through which the upstream social context creates downstream health outcomes remains limited, in part because a dearth of data from diverse populations has limited researchers’ ability to deeply understand these complex linkages. (Bae-Jump, Carlos)
- Biomarkers such as epigenetic changes and extracellular vesicles could provide insights into the connections between SDOH and cancer. (Carlos, Evans, Llanos, Obeng-Gyasi)
- Access to biomarker testing is uneven, contributing to care disparities. (Ferris, Vidal)
- Electronic health records can enable collecting and acting upon SDOH data but are generally not consistently and effectively utilized for this purpose. (Fayanju, Ferris, Hughes-Halbert)
- Sufficient and sustainable funding models are important for incorporating interventions to address SDOH into clinical care and reducing health disparities. (Evans, Winn)
- Even as researchers continue to elucidate the downstream biological effectors of SDOH on health outcomes and develop ways to target those mechanisms to intervene, it remains important to address the structural root causes of the inequities that lead to health disparities. (Gottlieb, Hiatt, Llanos, Mbah, McCullough, Tucker-Seeley)
NOTE: This list is the rapporteurs’ synopsis of observations made by one or more individual speakers as identified. These statements have not been endorsed or verified by the National Academies of Sciences, Engineering, and Medicine. They are not intended to reflect a consensus among workshop participants.
BOX 2
Suggestions from Individual Workshop Participants on Integrating SDOH into Cancer Research and Care
Building Trust and Collaboration
- Repair bilateral trust in communities by enabling communities to lead initiatives to address health disparities and ensuring that community members benefit from the research and interventions. (Darien, McCormick, McCullough, Rogers, Winn)
- Improve collaboration and communication among researchers from different disciplines and among researchers, clinicians, and communities. (Fayanju, Frencher, McCullough, Tucker-Seeley, Winn)
- Support training and employment opportunities for community members to participate in navigation, patient advocacy, research, education, and policy. (Cogle, McDonald, Rogers, Vidal)
- When asking patients about social determinants of health (SDOH) in clinical settings, explain why the information is being collected and how it will be used; then, follow up to address needs identified during this process. (Fayanju, Hughes-Halbert, McCullough, Shulman, Smith, Tucker-Seeley)
- Discuss and address ethical concerns in biomarker testing to ensure trust and participation from vulnerable communities. (Cogle, McCullough)
Improving Data Collection and Analysis
- Improve metrics for holistically studying interconnected SDOH to accurately capture meaningful data. (Hughes-Halbert, McCullough, Shulman, Tucker-Seeley)
- Develop and implement effective methods, tools, and best practices to coordinate, collect, harmonize, link, and share SDOH data for research and care, and act upon SDOH in clinical care settings. (Bradley, Fayanju, Gomez, Gottlieb, Hughes-Halbert, McCullough, Rogers, Shulman, Tucker-Seeley)
and Epidemiology at Johns Hopkins University, defined health disparities research as the study of why some populations—defined by their gender, race, education level, area of geographic origin or residence, genetic ancestry, SES, or other factors—suffer unnecessarily worse health outcomes than others. The concepts of “health equity” and “health justice” relate to efforts focused on combating these health disparities, which affect disease incidence, outcome, mortality, and quality of life.
- Incentivize including diverse populations in clinical trials and studies. (Evans, Mbah, McCullough, Vidal, Yates)
Improving Care to Address Social Determinants of Health
- Focus on early-life exposures as a critical piece of cancer prevention. (Brawley, Fayanju, Llanos, Rebbeck, Seewaldt, Winn)
- Ensure equitable access to broadly validated biomarker tests. (Gerson, Vidal)
- Facilitate care coordination among primary care providers and specialists to address social needs and better support cancer prevention, screening, and treatment. (Bradley, Darien, Fayanju, Frencher)
- Implement interventions that reduce disparities in care access and quality now, even as research continues to elucidate the biological mechanisms linking SDOH with health outcomes. (Brawley, Shulman)
Incentivizing Strategies to Improve Health Equity
- Utilize existing reimbursement mechanisms for assessing and addressing SDOH and create new mechanisms. (Honig, Lofton, Tucker-Seeley)
- Reduce health disparities by implementing payment models that allow clinicians to spend more time with patients. (Brawley, Ferris, Hughes-Halbert, Llanos, Rogers, Seewaldt, Winn)
- Advocate for social policies that directly address socioeconomic needs, using biomarker data to highlight the severe impact of social conditions on health. (Cogle, McDonald)
NOTE: This list is the rapporteurs’ synopsis of suggestions made by one or more individual speakers as identified. These statements have not been endorsed or verified by the National Academies of Sciences, Engineering, and Medicine. They are not intended to reflect a consensus among workshop participants.
Health disparities researchers often aim to uncover the upstream factors that contribute to differences in disease development, detection, and treatment. Brawley emphasized the importance of early-life factors in cancer prevalence, stating that “cancer prevention and health promotion is a pediatric issue.” He noted, for example, that most people who smoke begin in their teens, and eating habits, which can lead to obesity, are learned in childhood.
Brawley emphasized that race—a common focus of health disparities research—is not a biological trait but rather a sociopolitical categorization, which is redefined every 10 years for the U.S. census. Anthropologists and the American Medical Association no longer accept biological definitions of race because they imply that certain traits are inherent or immutable and have been used to harm and dehumanize people in the past.9 However, Brawley pointed out that there are nevertheless areas of intersection between race and health. For example, he noted that a person’s area of geographic origin or genetic ancestry, categories that are different from but correlated with race, can influence health outcomes. In addition, certain racial groups are disproportionately represented among various SDOH, such as income level (Semega et al., 2019).
Methods to address health promotion and disease prevention are critical, Brawley explained, because this is where the spectrum of disease control begins, followed by screening, diagnosis, and treatment. He posited that the emphasis on diagnosis and treatment over prevention or risk reduction in the U.S. health care system has contributed to health disparities. For example, almost half of U.S. cancer mortality is attributable to known external risk factors, such as smoking or obesity, that are related to SDOH factors, such as race, education, and gender (Islami et al., 2018). In addition, extrinsic environmental factors can influence genetic markers and disease behavior, blurring the contributions of biology and health disparities to disease and making it difficult to determine why, for example in the United States, Black women are diagnosed with triple-negative breast cancer at double the rates of White women (Dietze et al., 2015; Millikan et al., 2008; Palmer et al., 2014).
Brawley characterized providing high-quality care, including preventive services, to populations that rarely receive it as today’s most pressing disease control challenge. For example, people who belong to historically disadvantaged groups or have lower SES are more likely to receive inadequate care (Crown et al., 2023). Brawley noted that while Black women have a consistently higher death rate from breast cancer than White women, this disparity emerged only after the implementation of screening, because some populations had less access to screening and follow-up care (Lund et al., 2008; Mandelblatt et al., 2016; van Ravesteyn et al., 2011). SES is particularly associated with care access and quality in the United States. For instance, people with lower SES are less likely to receive radiation treatment or have access to newer, higher-quality medical equipment (Mattes et al., 2021; Washington et al., 2022). Treatment disparities also stem from cultural differences, comorbidities, lack of insurance or transportation, and racial or economic discrimination
___________________
9 See the American Medical Association policy H-65.953, https://policysearch.ama-assn.org/policyfinder/detail/racism%20social%20construct?uri=%2FAMADoc%2FHOD.xml-H-65.953.xml (accessed June 27, 2024).
(Lannin et al., 1998). Overall, Brawley stated that equitable treatment does not exist in the United States (NASEM, 2024). He stressed that solutions do exist, citing research estimating that making evidence-based prevention and treatment programs accessible to all could save more than 100,000 U.S. lives every year (Siegel et al., 2018).
Defining Social Determinants of Health
Reginald Tucker-Seeley, principal and owner of Health Equity Strategies and Solutions, pointed out that in the United States, adverse SDOH are disproportionately distributed across race, ethnicity, SES, and other groupings. These maldistributions are shaped by interrelated social, economic, and environmental factors; affect health equity; and can be altered through informed action (Braveman and Gottlieb, 2014; Krieger, 2001a; NASEM, 2017).
The health care ecosystem is improving its understanding and conceptualization of SDOH and their links to poor health outcomes, social risks,10 and social needs,11 Tucker-Seeley explained. Addressing SDOH can reduce health care costs and improve efficiency, he stated, because while they may be beyond the traditional realm of health care, they substantially influence health, health behaviors, and health care access and navigation. Frameworks, such as Healthy People 2030, that organize SDOH into separate domains can inform efforts to measure and address them. Healthy People 2030 organizes SDOH according to five domains: economic stability, education access and quality, health care access and quality, neighborhood and built environment, and social and community context (ODPHP, 2020).
Research into the associations between adverse SDOH and cancer outcomes shows that people living in areas with fewer resources have lower screening rates, and those who live with financial insecurity and/or belong to historically marginalized groups experience substantial challenges in accessing health care (Islami et al., 2022). Adverse SDOH can manifest as food insecurity, lack of transportation, and social isolation. Individuals with these social needs fare worse across the cancer care continuum, from prevention to detection, diagnosis, survivorship, and end-of-life care (Tucker-Seeley, 2021), and research has shown that SDOH related to premature aging can have a particularly significant impact on mortality (Brady et al., 2023; Mode et al., 2016; NASEM, 2020).
___________________
10 Social risks are “specific adverse social conditions associated with poor health, such as social isolation or housing instability” (Alderwick and Gottlieb, 2019).
11 Social needs are “self-reported patient social care needs that are impacting the patient’s health, ability to participate in research, and how the patient is navigating cancer care” (Tucker-Seeley and Shastri, 2022).
Tucker-Seeley noted several recent efforts to address the links between SDOH and patients’ social needs, health, and health care-related outcomes, such as the consensus report Integrating Social Care into the Delivery of Health Care: Moving Upstream to Improve the Nation’s Health (NASEM, 2019). The report examined opportunities to integrate services to address social needs into health care delivery and highlighted potential actions to describe and assist with social needs, adjust clinical care to accommodate social needs, and align health care delivery with other community resources. Tucker-Seeley also pointed to another study that suggested methods to screen patients for social needs, navigate patients to appropriate services, and evaluate the impacts on patient outcomes (Taira et al., 2023).
Tucker-Seeley noted that programs to address social needs can be helpful if they include strategies to ensure that efforts are consistent and sustainable. However, he cautioned that they do not typically address systemic conditions that lie outside the realm of health care but are nevertheless relevant to disease risk (Castrucci and Auerbach, 2019). “Health care navigators and similar enhancements to health care can’t actually change the availability of resources in the community,” he stated. “They can’t raise the minimum wage, increase the availability of paid sick leave, or improve the quality of our education system. These are the systemic changes that are necessary to truly address the root causes of poor health.”
Financing efforts to address social needs presents another challenge. Tucker-Seeley suggested greater use of Z codes from the International Statistical Classification of Diseases and Related Health Problems (ICD-10)12 that are relevant for capturing SDOH. He noted that while Z codes can also enhance clinicians’ and health care systems’ quality improvement initiatives through improved data collection and analysis (CMS, 2023), they are underused, and payment pathways are unclear (Maksut et al., 2021).
Tucker-Seeley explained that more work is needed to implement standardized, well-resourced, sustainable interventions for the communities that need them most. To create fair and just opportunities for people to be as healthy as possible and access high-quality health care, he urged a focus on reimagining and implementing multi-sectoral collaborations that serve organizations, patients, and families and inviting input from patients, clinicians, payers, and social service and community-based organizations (Tucker-Seeley and Shastri, 2022). He challenged workshop participants to consider what tools an equitable cancer care delivery system needs so that patients and families come to expect—and receive—the best quality care.
___________________
12 See https://www.icd10data.com/ICD10CM/Codes/Z00-Z99 (accessed June 28, 2024).
Connecting Social Determinants of Health with Cancer Risk and Outcomes
Lauren McCullough, associate professor of epidemiology at the Rollins School of Public Health at Emory University, pointed out that the common throughline for all social inequities is the centuries of economic inequities that marginalized populations have suffered and that SDOH do not just happen but are caused by upstream social and institutional inequities.
The historical practice of mortgage and housing discrimination known as “redlining” provides one example. The downstream consequences include urban neighborhoods with high concentrations of poverty whose residents experience disparities in generational wealth, income, and education; inequities in health care access; and poorer health outcomes (Swope et al., 2022). Redlining has also contributed to residents’ increased exposure to environmental toxins in the air, soil, or water—which can cause molecular changes that have been linked to cancer and are known to affect children as early as in utero (Luo et al., 2017).
McCullough also highlighted how a person’s living environment can contribute to molecular changes linked with racial, ethnic, and gender disparities in the incidence and prognosis of obesity-related cancers. She noted that among women, 8 of the top 10 cancer mortality disparities are related to obesity. Obesity is especially prevalent among Black women (OMH, 2022), and they experience disparities in cancer incidence and prognosis compared with non-Black, non-obese people (ACS, 2022). McCullough noted that 95 percent of excess cancer deaths among Black women compared to White women are attributed to three obesity-related cancers (ACS, 2022). Obesity has other consequences that worsen health outcomes and increase cancer incidence, including metabolic dysfunction, insulin and glucose increases, changes in inflammation or immune function, and oxidative stress (Devericks et al., 2022; Lynch et al., 2010). McCullough added that among patients diagnosed with breast cancer, obesity is associated with diagnosis at a later stage; more aggressive subtypes; higher mortality rates; and poor treatment outcomes due to suboptimal dosing, comorbidities, and lower rates of treatment adherence (Matthews and Thompson, 2016; Pierobon and Frankenfeld, 2013; Ross et al., 2019).
Noting that the relationships between SDOH and health are complex, McCullough pointed to studies that show the health disparities gap between Black women and White women living in better-resourced neighborhoods is actually greater than the gap between Black women and White women living in less-resourced neighborhoods, highlighting the influence of structural racism and discrimination, which affect historically marginalized groups and create social isolation and stress, leading to inflammation, hormonal and epi-
genetic changes, and accelerated aging (Collin et al., 2019; Lord et al., 2023). She stressed that the effects of structural racism and discrimination are also transgenerational. “It didn’t just start with you,” McCullough said. “It was your parents and your grandparents and their ancestors […] this can be passed from generation to generation.”
McCullough said that the interactions between direct and indirect effects on biology can make the consequences of SDOH difficult to quantify. However, researchers have been able to pinpoint certain SDOH effects on biology, such as associations with deoxyribonucleic acid (DNA) methylome13 perturbations in women with breast cancer, development of late-stage disease, and development of cancer subtypes, with SDOH such as college graduation rates, job density,14 and contemporary mortgage discrimination (Do et al., 2020; Gohar et al., 2022; Miller-Kleinhenz et al., 2023, 2024). To inform successful interventions, she emphasized the importance of considering the scientific evidence and individual SDOH, such as health care access. While research into the relationship between SDOH and carcinogenic processes is growing, she added that more work is necessary to improve health equity, especially research that extends the focus beyond neighborhoods, epigenetics, and biological aging (Saini et al., 2019).
McCullough offered three suggestions for interventions to improve health equity. First, she said that it is important for the medical community to repair trust among marginalized communities and increase their engagement—and biological sample size in clinical studies—by acknowledging and validating systemic challenges that have prevented their inclusion in the past. Second, she suggested that researchers can work to improve SDOH metrics, noting that current metrics or indices are overlapping, do not clearly distinguish between SDOH elements, and may fail to capture positive elements, such as social connectedness or resilience. Finally, McCullough called for improved collaboration among researchers with expertise in basic science, population science, environmental science, and other disciplines to advance health equity.
EMERGING RESEARCH ON POTENTIAL BIOMARKERS RELEVANT TO SOCIAL DETERMINANTS OF HEALTH
Recent research has shed new light on the complex interaction of SDOH, cancer risks, and treatment outcomes. Several speakers described some of these
___________________
13 DNA methylation is an epigenetic process that regulates the transcription of DNA segments. The DNA methylome is a record of DNA methylation in an organism (Pelizzola and Ecker, 2011).
14 Job density is the number of jobs per square mile. See https://www.opportunityatlas.org/ (accessed July 11, 2023).
emerging findings and discussed how contextualizing cancer within a patient’s ancestry, cultural background, social and economic environment, and lived experiences can lead to better cancer prevention strategies, earlier detection, and personalized treatment decisions. They pointed to potential biomarkers that could be used to measure the biological impacts of SDOH, along with challenges and opportunities in incorporating these measures into care pathways through biomarker testing.
As the research community continues to identify and refine biological markers of SDOH, Lawrence Shulman, director of the Center for Global Cancer Medicine at the University of Pennsylvania, urged participants to keep in mind the interconnected nature of factors such as poverty, geography, obesity, and smoking. “They’re not individual, unconnected factors,” he said, “Much of the research that we do is very focused on one factor or another, but we can’t lose sight of the fact that they are, in fact, all interrelated.”
Examining the Interplay of Place, Space, and Ancestry
Clayton Yates, the John R. Lewis Professor of Pathology, Oncology, and Urology at Johns Hopkins University, discussed the Transatlantic Prostate Cancer Consortium,15 which was established to address the globally disproportionate burden of prostate cancer among Black men. Yates explained that the network of cohort participants and collection sites in Western Africa has enabled participation in international prostate research collaborations, such as the International Registry for Men with Advanced Prostate Cancer (Mucci et al., 2022). This registry is collecting information and blood samples to help understand what care strategies offer the best outcomes for men with advanced prostate cancer.
Yates and colleagues are working to identify molecular markers correlated with disparities in cancer outcomes. He said their research has identified a tumor-suppressive signature that was more common among men of African ancestry, who also have a lower survival rate compared to those with European ancestry (Minas et al., 2022). To gain more insight into the links between ancestry and cancer outcomes, Yates said, transcriptomic data analyses were conducted to identify which genes were expressed in samples from patients of European American, African American, or native African (Nigerian) ancestry (White et al., 2022). Yates noted that this study had the most comprehensive characterization of the prostate cancer exome16 among Nigerian men and found that they had a different transcriptional profile. This discovery guided
___________________
15 See https://thecaptc.org (accessed September 6, 2024).
16 The exome is the protein-coding portion of the genome. See https://www.genome.gov/genetics-glossary/Exome (accessed July 11, 2024).
more in-depth analyses comparing prostate cancer signatures among African American, Nigerian, and European men. At a cell-population level, they noted differences in immune cell populations between African American and European American men with prostate cancer. Additionally, Yates said they found that the tumors from African American men were enriched for interferon-expressing cells, making them more difficult to treat (Elhussin et al., 2023). Yates said additional analyses were conducted to identify potential gene targets responsible for the observed inflammatory signature, which provided a link between the expression of different genes and the resulting tumor-level differences in immune response in patients of different ancestries. Yates said that these research approaches identified potential biomarkers that might not have been obvious without a large African cohort and could inform future clinical therapies, including personalized treatment approaches for native African and African American men with prostate cancer.
Noting that White populations living in rural areas are three times more likely than any group living in urban areas to have poor outcomes from lung cancer, Robert A. Winn, director and Lipman Chair in Oncology at Virginia Commonwealth University Massey Comprehensive Cancer Center, pointed out that disparities between rural and urban populations illustrate another aspect of how place and space influence health.
Genomic Pathways Connecting Exposures with Health Outcomes
Articulating an overarching goal for “the right patient to receive the right test and the right treatment at the right time for the right price,” Ruth Carlos, professor of radiology at the University of Michigan, said that accomplishing this vision may require viewing SDOH in a more nuanced way that incorporates a holistic “society to cells to outcomes” approach.
Exogenous and endogenous stressors induce physiological reactions that affect cell function and disease development, she explained. The physiology of scarcity—whether of time, food, or other resources—also induces biological and psychological effects and can have a negative impact on downstream disparities and health, she explained.
Research has linked SDOH with clinical outcomes, Carlos stated, but the pathways through which the upstream social context creates downstream health outcomes are still largely unknown (Carlos et al., 2022). Potential mechanisms include genomic and epigenomic changes, peri-tumor17 environmental effects, and inflammation from stress responses. Evidence supporting these mechanisms includes a study that modeled how inequity in
___________________
17 The peri-tumor is the “microenvironment at the interface between healthy and malignant tissue” (Zhang et al., 2023).
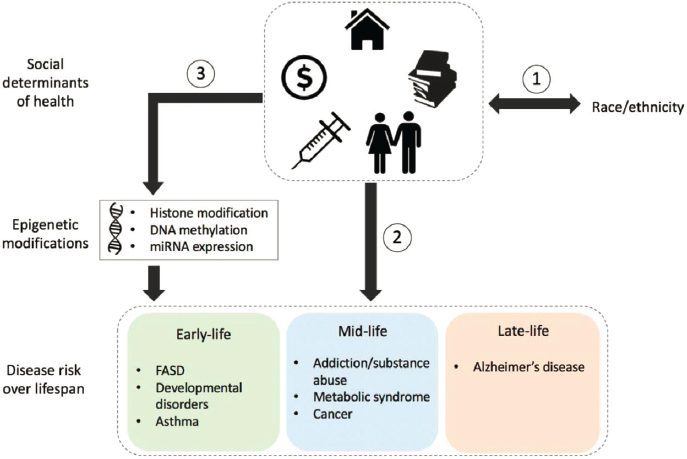
NOTE: DNA = deoxyribonucleic acid; FASD = fetal alcohol spectrum disorders; miRNA = microRNA.
SOURCES: Carlos presentation, March 20, 2024; Mancilla et al., 2020. CC BY 4.0.
the social environment promotes epigenetic changes that can last from early childhood into successive generations, pointing to the potential for the heritability of social trauma (see Figure 2) (Mancilla et al., 2020); a study that linked paternal SDOH and epigenetic changes with obesity rates of offspring (Milliken-Smith and Potter, 2021); and a study that found an association of noise and air pollution with epigenetic changes linked to cancer development and regulation (Eze et al., 2020).
Although it may be impossible to undo an environmental exposure, Carlos noted that some SDOH and the conserved transcriptional responses to adversity (CTRA)18 they induce are reversible. For example, the negative effects of social isolation, which is correlated with worse health outcomes, could be ameliorated through social, pharmacological, or behavioral interventions (Antoni and Dhabhar, 2019; Cacioppo et al., 2015; Knight et al., 2019). In addition, some CTRA are associated with improved well-being, presenting
___________________
18 Conserved transcriptional responses to adversity is a sustained change in immune cell gene expression profiles in response to environmental conditions (Cole, 2019).
an opportunity to look beyond social risks and focus on social resilience, which Carlos said has been an understudied part of the social context (Boyle et al., 2019; Fredrickson et al., 2015). She pointed out that it is also possible that CTRA-related gene expression could improve people’s ability to manage social stress through enhanced wariness or hypervigilance, she said (Cole, 2014).
Carlos noted the potential ethical dilemmas inherent in interventions that target the genetic effectors of SDOH to solve problems that have been collectively imposed on a specific group of people. However, she emphasized that social genetics, when appropriately aligned with policy and regulations that do not further burden populations, can offer a pathway to advance health equity.
Characterizing Cancer Subtypes and Social Determinants of Health with Population-Based Research
Victoria Bae-Jump, professor of gynecologic oncology at the University of North Carolina at Chapel Hill, discussed how population-based research approaches can help uncover the relationships between SDOH and patterns in cancer subtypes. Her research focuses on endometrial cancer, the fourth most common cancer among U.S. women (Siegel et al., 2024). Both the frequency and mortality rate of endometrial cancer are rising (Siegel et al., 2024; Ward et al., 2019), trends that researchers have linked with increasing rates of obesity, diabetes, and insulin resistance—known risk factors for this cancer type—as well as with an unexplained rise in more aggressive subtypes (Chia et al., 2007; Siegel et al., 2024).
Roy Jensen, director of The University of Kansas Cancer Center, agreed with Bae-Jump on the strong evidence for an association between endometrial cancer and obesity but noted that most of the biological research on cancer and obesity focuses on the tumor microenvironment and its adjacent adipose cells, which raises questions about the mechanism for this association because no adipose cells are near the endometrium. Bae-Jump stated that although the specific mechanisms are still being investigated, researchers hypothesize that obesity may create circulating signals that alter systemic factors in the uterus and lead to endometrial cancer.
Bae-Jump described significant racial disparities for endometrial cancer; Black and Hispanic women have higher mortality rates, increasing incidence rates, and lower 5-year survival rates than White women (Cote et al., 2015; Siegel et al., 2022). While the exact drivers of these racial disparities are unclear, Bae-Jump posited that they are likely influenced by multiple factors (see Figure 3), from historical structural and institutional inequities to SDOH, and known and unknown social and biological factors that culminate in inequities in cancer screening, detection, diagnosis, treatment, survivorship, and mortality (Warnecke et al., 2008).
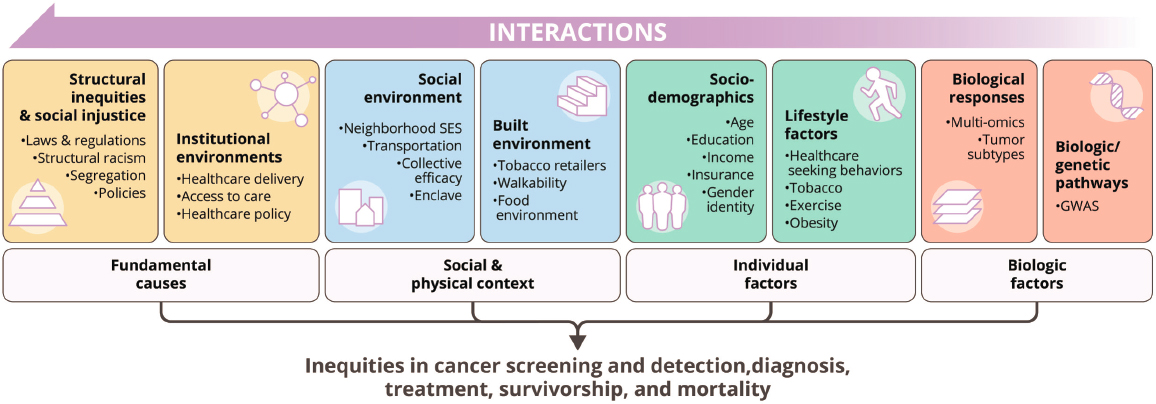
NOTE: GWAS = genome-wide association studies.
SOURCES: Bae-Jump presentation, March 20, 2024; The “Cell to Society” model created by the UNC Lineberger Cancer Center, adapted from Warnecke et al., 2008.
Historically, researchers focused on two broad subtypes of endometrial cancer. Type 1 was more common and treatable, and Type 2 was less common overall but more common in Black women, more aggressive, and associated with lower survival rates (Fowler and Mutch, 2008). With further genomic characterization of tumors through The Cancer Genome Atlas (TCGA) program, researchers identified four distinct molecular subtypes, each with different treatment pathways and survival rates but all with poorer health outcomes for Black women (who were not well represented in TCGA, Bae-Jump noted) (Dubil et al., 2018; Kandoth et al., 2013).
Determining which molecular subtype of endometrial cancer a patient has is critical to assigning the appropriate therapy. Recent research has shown that Black patients develop more aggressive subtypes and have worse progression and outcomes with all subtypes (Weigelt et al., 2023). The reason for these differences remains unclear. A significant challenge in endometrial cancer disparities research, Bae-Jump noted, is the lack of prospective population-based epidemiological studies that link endometrial cancer subtype with race, obesity, related comorbidities, SDOH, care access, and treatment offerings. Other challenges include a dearth of endometrial cancer samples from Black women to conduct large-scale molecular profiling studies and a limited understanding of how obesity and its related comorbidities affect endometrial cancer progression and treatment in Black women, she said.
Bae-Jump highlighted ways that the Carolina Endometrial Cancer Study (CECS) could help to answer some of these questions. CECS is a population-based prospective study of nearly 2,000 patients with endometrial cancer with the goal of uncovering how epidemiological factors, SDOH and upstream drivers, and tumor biology contribute to racial health disparities. “As we know, these factors have been looked at in their individual silos but really haven’t been brought together in one study,” Bae-Jump said, noting that the results could inform social, behavioral, and biological interventions.
Extracellular Vesicles and Epigenetic Aging
Michele Evans, deputy scientific director of the National Institute on Aging, discussed insights from investigations of the biologic pathways through which SDOH create health disparities. These investigations are based on data from Healthy Aging in Neighborhoods of Diversity across the Lifespan (HANDLS), an interdisciplinary, community-based, longitudinal epidemiologic study of race, SES, and age-associated health disparities among nearly 4,000 Black and White adults across Baltimore (Evans et al., 2010).19
___________________
19 See https://handls.nih.gov (accessed June 20, 2024).
Describing SDOH as agents that change molecular and biological pathways, Evans explained that they create biomarkers such as inflammatory proteins, extracellular vesicles (EVs),20 DNA methylation, and circulating cell-free mitochondrial DNA,21 which are associated with accelerated aging and health disparities. She described the measurement of these biomarkers as “liquid biopsies” that could potentially be used to aid early detection and inform treatment selection and prognosis. She noted that researchers are studying how SDOH influence these biomarkers (Mirza et al., 2023).
Studying EVs could also elucidate some of the molecular changes that may lead to cancer development. EVs mediate intracellular communication and contain various forms of ribonucleic acid, DNA, protein, and lipids (Noren Hooten and Evans, 2020). Mitochondrial DNA could be an important biomarker of health disparities, Evans noted, as it is released by cells under stresses associated with SDOH, cancer, and other chronic inflammatory diseases (Lazo et al., 2021). Her team’s research into EVs, inflammatory proteins, and poverty revealed that people in worse health, specifically men living below the poverty line, had higher levels of mitochondrial DNA and inflammatory proteins in their EVs (Byappanahalli et al., 2023). Surprisingly, the researchers did not find expected associations with race, which Evans said suggests that ancestry and genomic variation may be more important factors in health and biomarker differences than race (Byappanahalli et al., 2024; Morning, 2017; Wallace, 2012, 2013).
Another important factor in health and biomarker differences is epigenetic age acceleration, which is similar to weathering22 and can be studied via DNA methylation (Brown, 2015). A study of 19 biomarkers of organ system integrity in the HANDLS cohort found that White people living below the poverty level and Black people at all income levels showed accelerated epigenetic aging (Shen et al., 2023). This result, Evans said, “hammers home the effects of race-related stressors that lead to poor health and negative health outcomes.”
___________________
20 Extracellular vesicles are small, membranous fluid-filled sacs that help move substances into and out of cells and are involved in many pathological physiological processes (van Niel et al., 2018).
21 Circulating cell-free mitochondrial DNA are small segments of mitochondrial DNA that are released by stressed or damaged cells (Lazo et al., 2021). Mitochondrial DNA is the genetic material of the mitochondria, which produce energy for the cell. See https://www.genome.gov/genetics-glossary/Mitochondrial-DNA (accessed June 30, 2024).
22 The weathering hypothesis states that “the health of African American women may begin to deteriorate in early adulthood as a physical consequence of cumulative socioeconomic disadvantage” (Geronimus, 1992).
Stress and Allostatic Load
Several speakers highlighted opportunities to measure and understand the relationship between social stressors and cancer by focusing on allostatic load, which reflects the cumulative effects of chronic stress on the body.
Samilia Obeng-Gyasi, associate professor of surgery at The Ohio State University, investigates the mechanistic pathways that link socio-environmental factors, such as low SES or high rates of social isolation, with breast cancer initiation, progression, and metastasis (Abdel-Rahman et al., 2019; Bower et al., 2018; Ramsey et al., 2016). She discussed how the conceptual frameworks of ecosocial theory and weathering describe how these factors are internalized.
Ecosocial theory proposes that the world one lives in can become physically internalized. Its components include embodiment (how one’s body internalizes socio-environmental factors), embodiment pathways (internal biological or environmental mechanisms), and the interplay between structural and intermediary health determinants (such as governmental policy, the social construct of race or gender, living and working conditions, and the health care system) and disease exposure, susceptibility, and resistance (Krieger, 2001b, 2012).
Building on Evans’ discussion, Obeng-Gyasi defined weathering as the idea that adverse health outcomes and early health deterioration, for Black women in particular, stem from chronic exposure to racism, sexism, and classism (Geronimus, 1992). For example, African immigrant women have substantially better birth outcomes than U.S.-born women who are racialized as Black, despite being of similar genetic background (Agbemenu et al., 2019). Obeng-Gyasi explained that weathering becomes embodied through stress pathways, which are biological responses to stressful socio-environmental factors, such as social isolation or financial insecurity, and have multiple overlapping physiological and molecular impacts (Antoni and Dhabhar, 2019; Cole, 2013; Thames et al., 2019).
Allostatic load reflects the physiological and immune dysregulation that occurs with exposure to repeated stress events, failure to adapt to repeated stressors, failure to end a stress response, and inadequate release of stress hormones (IOM, 2001). Obeng-Gyasi described how allostatic load can be measured through biomarkers of primary mediators, such as cortisol or epinephrine, or their secondary and tertiary outcomes, such as high blood pressure or diabetes (see Figure 4) (Duong et al., 2017; Seeman et al., 1997).
High allostatic load can lead to permanent biological changes, is associated with poor health outcomes (Mathew et al., 2021; Rodriquez et al., 2019; Wiley et al., 2016), and appears to affect every part of the cancer continuum, from initiation to progression, treatment tolerability, metastasis, and mortality, Obeng-Gyasi said. Studies have shown that women with breast cancer and high allostatic loads—whether related to residential segregation, limited eco-
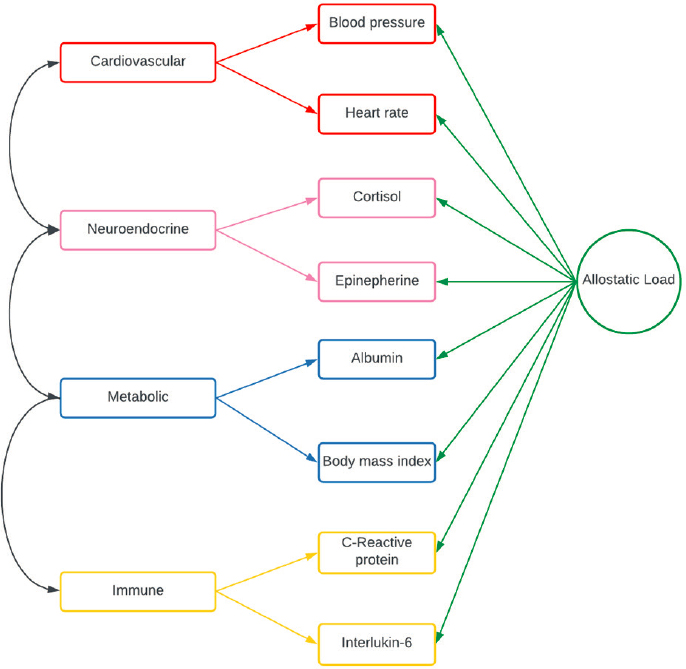
SOURCES: Obeng-Gyasi presentation, March 20, 2024; Wiley et al., 2016.
nomic opportunities or services, or lower SES—had more comorbidities, a higher incidence of triple-negative breast cancer, and worse all-cause mortality rate; they were also more likely to be Black, enrolled in Medicaid, and unmarried (Chen et al., 2024a, 2024b; Obeng-Gyasi et al., 2023).
Adana Llanos, associate professor of epidemiology at Columbia University, described how research is increasingly connecting higher levels of allostatic load with increased risk of more aggressive tumors and a lower quality of life (Guan et al., 2023; Wang et al., 2024; Xing et al., 2020a, 2020b). Her research team has demonstrated that living in areas of neighborhood divestment or redlining is associated with being underinsured and an increased risk of late-stage diagnosis, higher-grade tumors, greater incidence of triple-negative breast cancer, and lower breast cancer survival rates (Chen et al., 2024a; Plascak et
al., 2022). As a possible explanation, Llanos said that the weathering many Black women experience from chronic psychological stress results in high allostatic loads, measurable even in childhood and adolescence (Geronimus, 1992; Rainisch and Upchurch, 2013). Llanos noted that this could contribute to the higher breast cancer incidence among Black women compared with White women (Geronimus et al., 2006; Parente et al., 2013). Because both Black women and Black men are likely to have higher allostatic loads, Llanos urged a focus on developing early-life interventions that could help to reduce chronic stress before the cumulative impacts begin to build.
Researchers are investigating the feasibility of developing a standardized, validated method for calculating allostatic load scores to better understand cancer epidemiology and inequities, Llanos said. Studying the combined impact of allostatic load and neighborhood context on breast cancer could also shed light on biological mechanisms caused by persistent inequities, she explained, suggesting that incorporating SDOH could provide insights at multiple levels, from biology and disease etiology23 to improve public health (Kehm et al., 2022; McDade and Harris, 2022; Warnecke et al., 2008). “Given these multidimensional, multilevel factors, we need multilevel, multidimensional approaches,” Llanos stated.
Richard Schilsky, professor emeritus at the University of Chicago, cautioned that allostatic load is multidimensional and reflects many different processes and that its use as a biomarker for clinical practice should be contingent on appropriate validation and context. Obeng-Gyasi suggested that it could be incorporated into the clinical setting, noting that recent research into the feasibility of biological interventions to improve health outcomes by reducing a patient’s allostatic load has presented promising early results (OSUCCC, 2022). However, she cautioned that it is important to be aware that some treatment modalities, such as surgery or chemotherapy, can increase allostatic load. Shulman added that for those who already experience multiple stresses, a cancer diagnosis often leads to further stress.
Gerson stated that Black people are more likely to live with multiple overlapping social stressors and that separating ethnicity, race, or ancestry from these socioeconomic disease drivers is often challenging for researchers. He added that it is also a challenge to explain these drivers to the public. Highlighting how the embodiment of historical trauma—the biological effects of stress and weathering—becomes measurable through allostatic load, Scarlett Lin Gomez, professor of epidemiology and biostatistics at the University of California, San Francisco, underscored the importance of studies examining different social epigenetic pathways (Carlos et al., 2022).
___________________
23 Etiology is the “cause or causes of a disease.” See https://medlineplus.gov/ency/article/002356.htm (accessed June 30, 2024).
Suzanne Conzen, chief of the division of hematology and oncology at the University of Texas Southwestern Medical Center, discussed emerging laboratory research on the relationship between social stressors and breast cancer outcomes. Conzen noted that in vitro research demonstrated that glucocorticoid receptor tumor signaling activation can promote cancer cell survival and other pro-oncogenic functions (Moran et al., 2000). Additionally, a meta-analysis found that high glucocorticoid receptor expression in tumor samples from patients with early-stage breast cancer was correlated with worse outcomes for women with estrogen receptor-negative breast cancer (Pan et al., 2011). Conzen and colleagues conducted research to see if the physiological effects of social stressors in rodents play a role in cancer biology (Antoni et al., 2006; McClintock et al., 2005; Volden and Conzen, 2013) (see Figure 5).
The first model Conzen discussed involved assessing social stressors in genetically identical female mice with susceptibility to triple-negative breast cancer. Mice were maintained in either group housing or social isolation from weaning through 15 weeks of age (i.e., through puberty and young adulthood) and examined them for behavioral differences and endocrine responses when restrained (emulating a burrow collapse, a common stressful situation in the wild). Socially isolated mice had higher glucocorticoid levels in response to gentle restraint, consistent with a maladaptive stress response (Williams et al.,
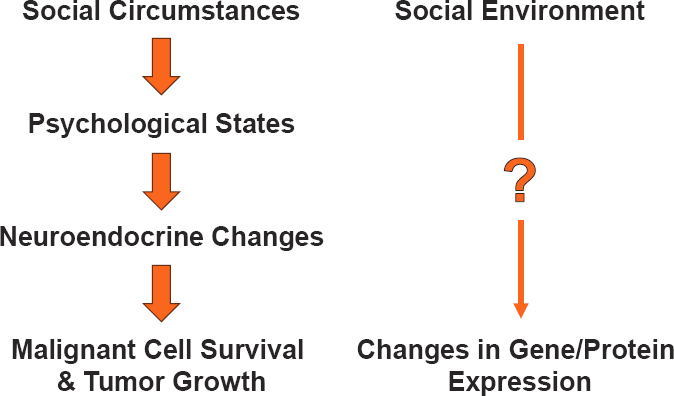
SOURCES: Conzen presentation, March 20, 2024; adapted from Gehlert et al., 2010. Reprinted by permission of the publisher, Taylor & Francis Ltd, http://www.tandfonline.com.
2009). Chronic isolation was also associated with increased triple-negative breast cancer tumor burden in this transgenic mouse model (Williams et al., 2009).
The second model Conzen discussed was designed to assess the development of spontaneous tumors in female rats experiencing social isolation versus grouped housing, again initiated from weaning through puberty and young adulthood. Social isolation was associated with earlier-onset and more aggressive breast cancer compared to rats who were in group housing from birth (Hermes et al., 2009). The researchers also found that social isolation through puberty and early adulthood correlated with impaired mammary gland development, corresponding to higher corticosterone (a glucocorticoid) reactivity (Johnson et al., 2018).
Conzen concluded that this research demonstrates that chronic social isolation versus social support can be modeled in animals to study the impact on cancer in a laboratory setting. The physiologic stress response to social isolation includes increased stress hormone production, accompanied by behavioral vigilance. Following chronic stress exposure (i.e., social isolation during puberty and young adulthood) in genetically predisposed mice and rats, breast tumors in these laboratory animal models appear earlier and are more aggressive. Additionally, Conzen said that the effects of chronic stress in late puberty/early adulthood need to be studied further to assess the impact of mammary gland development on breast cancer risk. She noted that ongoing research will assess if the biological effects of social isolation (as a model of early life stressors) can be mitigated by reintroducing group housing during puberty and young adulthood in female rats.
Equity Implications of Biomarker Testing
Defining biomarkers as measurement variables associated with disease outcomes, Gregory Vidal, medical oncologist at West Cancer Center, discussed how biomarker testing (or a lack of it) can influence cancer care and outcomes, with implications for health equity and health disparities. Certain biomarkers can be targeted with drugs, and many tumor types and mutations are being studied to facilitate the development of new targeted therapies (Hanjie Mo, 2021; Vogelstein et al., 2013). The cost of next-generation sequencing (NGS) of a patient’s DNA to find biomarkers has gone down dramatically,24 Vidal noted, and in many situations, NGS and hereditary germline testing are covered by the Centers for Medicare & Medicaid Services (CMS). This has made it more accessible and affordable for some patients to be tested and more
___________________
24 See https://www.genome.gov/about-genomics/fact-sheets/DNA-Sequencing-Costs-Data (accessed May 22, 2024).
likely for them to participate in clinical trials that require certain biomarkers (Sheinson et al., 2021a).
Biomarkers can only reduce health disparities if they are implemented equitably, noted Vidal. If certain groups receive less biomarker testing, then it follows that they may be less likely to participate in clinical trials or receive optimal treatment. Not all insurance companies cover biomarker testing, and not all health care organizations can afford to offer it. Despite an overall increase in biomarker testing, one study found that Black patients were being tested at lower rates than White patients for many cancers, which also affected their representation in clinical trials for new therapies (Bruno et al., 2022). Studies have also revealed testing disparities for those who lived in the South, enrolled in Medicaid, or older (Sheinson et al., 2021b).
Investigating these testing inequities further, Vidal and colleagues found that at the practice level, White patients with lung cancer received more timely testing than Black or Latinx patients (Vidal et al., 2023). Many clinicians who see mainly Black or Latinx patients were also less likely to offer testing (Vidal et al., 2024). The same racial disparities are seen in germline testing (Kurian et al., 2023). In response to these findings, Vidal’s clinic mandated that all patients undergoing first-line treatment for metastatic cancer receive NGS. They found that testing and clinical trial participation rates increased because this new policy reduced confusion over who met screening criteria and eliminated the impacts of any clinician bias. The clinic also established a tumor board to recommend clinical trials and treatment decisions to clinicians (VanderWalde et al., 2020). Vidal said that both steps reduced racial disparities, and the clinic now has a much higher percentage of patients who are Black, older, or female enrolled in clinical trials.
Based on these results, Vidal suggested that other institutions create systemic NGS and germline testing plans to take the decision out of the hands of clinicians and reduce the impact of bias (Subbiah and Kurzrock, 2016, 2023) and that continuing medical education focused on NGS could help clinicians become more educated about its usefulness. Gerson added that policies mandating full coverage for biomarker testing could also help to address inequities.
Karriem Watson, chief engagement officer at the National Institutes of Health (NIH) All of Us Research Program,25 highlighted the importance of access to care in influencing many aspects of a person’s cancer trajectory. Where patients live, where they receive their health care, and where clinical trials are undertaken all affect the care they receive. Watson noted that patients who receive care in community clinics are frequently excluded from clinical trials and suggested diversifying where they are conducted. For example, they
___________________
25 See https://allofus.nih.gov (accessed July 11, 2024).
could be expanded to include federally qualified health centers, which care for many underrepresented groups—who have the greatest burden of health disparities. Watson added that, to support equity, it is important for clinical research staff to reflect patient diversity, especially for those with the greatest burden of health disparities.
Andrea Ferris, president and chief executive officer of the LUNGevity Foundation, described how some of these issues are playing out in lung cancer. Lung cancer has been at the forefront of precision medicine, as researchers have pinpointed various disease drivers and developed targeted therapies and care pathways. However, despite the availability of tests to detect the known drivers, Ferris said that precision medicine care pathways are not being implemented effectively or equitably. Many patients are simply not receiving the tests that could inform targeted treatment decisions. This may be due to a number of factors, including reimbursement policy, overwhelmed clinicians, and lack of effective strategies for incorporating these tests into clinical workflows. Ferris expressed doubt that new knowledge generated through research will have a real impact on patients without better implementation strategies.
Building on these points in the context of research aimed at identifying biomarkers relevant to SDOH, Christopher Cogle, professor of medicine at the University of Florida and chief medical officer for Florida Medicaid, cautioned that laboratory tests are useful only if they inform interventions. Given the amount of evidence already available, he asked, “Why do we need a gene expression [test] to tell us that we need to focus on social needs?” Cogle noted that biomarker testing should not be used to validate socioeconomic issues, rather they should be used as tools to inform policy to address socioeconomic needs. He cautioned that it is essential to ensure investments in biomarker testing do not divert attention and resources from necessary social reforms. Cogle posited that while currently SDOH biomarkers are not meaningfully informing care, they may do so in the future. Cogle agreed with other speakers who noted that frequent surveys could be burdensome for patients, but he expressed skepticism that replacing in-person conversations about social needs with blood tests or cheek swabs—which may be seen as easier for many clinicians—would actually benefit patients. He suggested that an area with more promise may be public health surveillance. For example, he suggested that strategies such as testing sewage or air pollution levels could be leveraged to guide precision social health initiatives that are informed by biomedical evidence and targeted to areas with the greatest need.
OPPORTUNITIES FOR HEALTH CARE SYSTEMS TO ADDRESS SOCIAL RISK FACTORS
Many SDOH and health-related social needs extend beyond the areas that have traditionally been within the purview of health care systems. Several participants discussed the relevance of SDOH to care delivery, gaps in current practice that can lead to inadequate attention to social risk factors, and opportunities and considerations to better integrate SDOH into the cancer care continuum.
Recognizing the Influence of Upstream Factors on Health
Throughout the workshop, many participants highlighted the importance of upstream factors affecting a person’s health and the care they receive. Even as researchers elucidate the downstream biological mechanisms that connect SDOH with health outcomes—and potentially find ways to target those mechanisms to intervene—a number of speakers cautioned that it remains important to address the root causes of the inequities that lead to health disparities. Laura Gottlieb, professor of family community medicine at the University of California, San Francisco, pointed out that even if scientists invented a pill that blocks the biological effects of racism, including the allostatic loads that worsen cancer outcomes, it would not address the social needs patients and communities face. “This is an ethical dilemma that I think really challenges the entire research endeavor in this space or around biomedical research. […] All of us have to be grappling with it constantly, and as we do the downstream work, we also have to be doing the upstream work,” Gottlieb said.
Tucker-Seeley pointedly asked, “How much of the causal pathway do we need to explicate before we all believe that systemic racism is the driver that has sorted specific groups into these adverse circumstances, which have detrimental effects on their health?” He added that if racism is a key driver, it may be necessary to explore multilevel, multifactorial interventions—in health care and policy—that address historical harms, dismantle systemic racism, and improve SDOH.
Shulman reiterated that many of the factors that appear to be linked with health disparities (e.g., divested neighborhoods, challenges with health care access, epigenetic changes, and stress responses) are interrelated and stem from economic disparities. Llanos, Robert Hiatt, associate director of population sciences at the University of California, San Francisco, and Olive Mbah, senior health equity scientist at Flatiron Health, added that much of the research examines individual interventions—behavioral, dietary, therapeutic, and so on—that improve outcomes or prevent cancer development but suggested that focusing more on structural drivers at the population level could
yield greater impacts. Llanos suggested that changes to health care reimbursement structures could help to reduce care inequities, and Hiatt pointed to the importance of focusing on alleviating the impacts of persistent poverty. Although many SDOH connected with poverty, such as education, power, privilege, and social connections, cannot be changed quickly, Hiatt posited that income may be uniquely modifiable, noting that his group is studying the impact of policies such as earned income tax credits and basic guaranteed income on cancer rates and outcomes.
Stanley Frencher, medical director of surgical outcomes and quality at Martin Luther King, Jr. Community Hospital, added that there may be ancillary benefits from addressing patients’ social needs, even with little data to suggest it can impact cancer incidence or outcomes. “Even if we haven’t proven today that those things impact their cancer biology or even their cancer outcomes, I would argue they’re just the right thing to do,” he emphasized.
The Role of Trust and Communication
Several participants underscored the role of trust and communication in addressing SDOH and health disparities in research and health care. Beverly Rogers, chief executive officer of From Momma’s House, spoke about her experience as a breast cancer survivor and patient advocate. She described how her interactions with the health care system, as both a patient and a caregiver, have often left her feeling confused and frustrated. Citing patient–clinician communication as a key challenge, Rogers recalled often feeling that her clinicians were not communicating in a way that she was likely to understand. For example, it is well known that stress has a negative impact on health, but that information alone is not actionable. “Everybody tells you; your doctor tells you, your cardiologist tells you, your endocrinologist tells you, stress causes diabetes, stress causes heart problems, stress causes arthritis. I got all of those. So, what are you going to do for me?” Rogers asked. Noting that health care systems are challenging to navigate for many people, but especially older adults, she stressed the need to improve communication and coordination among the full spectrum of clinicians that patients see.
The importance of plain language and making information relevant to people’s lives also extends to researchers. Rogers suggested that researchers could go beyond describing best practices or hosting public listening sessions to engage with communities more meaningfully. She said building trust is essential and suggested that community members will be more willing to answer questions, about their neighborhood environment, for example, if they see a clear value in doing so and the results will apply to their lives and health concerns. Rogers emphasized that researchers and clinicians have a responsibility to use their training to improve people’s lives.
Tucker-Seeley pointed out that some of the health care delivery challenges that Rogers alluded to are solvable. Clinicians are not incentivized to view patients as humans struggling to navigate the complex, unfamiliar health care landscape, which contributes to health and health care disparities. “Ms. Rogers highlighted that she’s not just the arm that you’re using to take blood from. She’s not just this body part that’s being treated for this condition. She’s a whole person,” he stated.
To build better relationships and receive better care, Rogers pointed out that patients would benefit from more time with clinicians. Hughes-Halbert agreed that a lack of time creates missed opportunities to understand nuances in patients’ lives. Brawley noted that administrative realities prevent clinicians from spending more than 20 minutes with each patient and suggested that it could be valuable for people from all facets of the health care system, from clinicians to administrators to payers to CMS, to recognize and understand how their workflow may be contributing to health disparities.
In both health care and research, Winn said that organizations know it is important to build trust in communities, but he suggested that many have failed to examine their own lack of trustworthiness. Gaining trust takes time, and it is important to consistently show up and speak the right language, he said. Gwen Darien, executive vice president at the National Patient Advocate Foundation and a cancer survivor, agreed that access and trust are crucially important in the clinician–patient relationship. “We have to trust patients, patients’ voices, patients’ experiences, and what they think that they need, and not make any assumptions,” she said.
Noting that she regularly hears stories of bias, neglect, mistrust, and inequitable care from Black patients with cancer, Darien suggested that standardizing screening and testing processes, in addition to engaging meaningfully with patients to understand their perspectives, could help to increase access and equity. “This bias and this inequity are just persistent and intractable,” she said. “Making this [screening] the standard of practice for everybody—it would go such a long way towards addressing a number of inequities.”
Integrating Social Determinants of Health into the Care Continuum
Several participants spoke about opportunities for health care systems to systematically assess and attend to patients’ social needs, a goal that Gomez said can also benefit from community-based research and partnerships. Gottlieb described the 5A Framework, which outlines opportunities for health care systems to acknowledge patients’ SDOH and intervene when relevant (NASEM, 2019).
Conceptually, Gottlieb said it is important to recognize that SDOH are part of a broader spectrum of factors that shape individual and com-
munity health, neither good nor bad in and of themselves, and distinct from population health (Alderwick and Gottlieb, 2019). In addition to individual components, SDOH encompass upstream structural drivers, such as institutional policies, and their downstream effects on social risks, assets, and needs (Castrucci and Auerbach, 2019).
The 5A Framework organizes the different activities that fall under social care into five distinct categories. Each serves as an umbrella for many related components that affect health care access and delivery. Gottlieb detailed the three that focus on patients and health care delivery: awareness and identification of social risk factors, assistance to intervene on social risk factors, and adjustments to accommodate care for social risks. The other two categories, alignment of existing resources and advocacy to develop new resources, are more community focused.
Awareness relates to activities or strategies that health care systems employ to collect social information about their patients. These can be patient questionnaires, insurer-based health risk assessments, or analysis of consumer data. Clinicians also employ screening tools, but Gottlieb noted that available tools lack consistency and suggested that it may be helpful to undertake more validity testing and consensus building to determine the most promising tools (SIREN, 2019). Gottlieb noted that technology could help facilitate social risk screening; for example, many electronic health records (EHRs) include social risk dashboards. However, she said that these approaches are underused for SDOH applications and lack the data standardization needed to facilitate meaningful interventions.
Assistance refers to activities health care systems undertake to improve patients’ social context and thus their health and well-being, such as connecting patients with food resources or rent support. To guide and understand the impacts of such efforts, Gottlieb said that it is important to ask whether awareness of social factors contributes to assistance and then whether assistance, in whatever form it may take, leads to actual health improvements. For example, cancer care assistance has emphasized patient navigation but with little research on whether the interventions improve racial health equity outcomes (Korn et al., 2023). In addition, service referrals may not be effective if the services are not actually accessible. “Even if we provide assistance, not everyone gets their need met, in part because […] the effectiveness of referrals depends on the availability of services and decreasing the administrative burden of enrollment and sustaining enrollment,” Gottlieb said, noting the available resources to help researchers better understand these challenges.26,27
___________________
26 See https://www.pcori.org/research-results/2020/scoping-review-and-evidence-map-social-needs-interventions-improve-health-outcomes (accessed May 22, 2024).
27 See https://sirenetwork.ucsf.edu/tools/evidence-library (accessed May 22, 2024).
Adjustment strategies attempt to use social risk data to inform clinical care decisions. Gottlieb said that these strategies include offering less expensive medicine; reducing the complexity of treatment regimens; providing interpreting services; or offering mobile, evening, or weekend care (Korn et al., 2023). Clinicians often make these adjustments, but implementation has not been systematic or large scale, Gottlieb said, noting that one challenge is that clinicians often do not have access to a patient’s social risk data. Carlos added that downstream effects of SDOH, such as allostatic load, could be used as biomarkers that prompt health care systems to intervene with early-stage supportive potential actions to improve outcomes, such as treatment adherence support.
The final two framework categories, alignment and advocacy, can strengthen the social resources landscape at the community level. To advance these efforts, Gottlieb said that it would be helpful if health care systems collaborated with community advocates to improve social conditions by offering employment, investing resources, and supporting local services and activities.
DATA ISSUES AND GAPS RELEVANT TO SOCIAL DETERMINANTS OF HEALTH
Appropriately and accurately measuring SDOH is important if scientists and clinicians are to incorporate greater awareness and utility of these factors into cancer research and care. Reflecting on the workshop presentations and discussions, Shulman posited that a shared goal is to identify effective methods for collecting information about SDOH that are relevant to patient experiences and outcomes. Creating a standardized, unified approach across multiple, diverse care sites is a key challenge, Shulman said, and Gomez added that this challenge also extends into the research realm, where harmonizing SDOH measurement tools and factors is important for comparing results across studies. Cathy Bradley, dean of the Colorado School of Public Health, suggested that policies can help to support research into best practices to effectively collect, harmonize, link, and share data from all population groups.
Examples of Social Determinants of Health Screening Tools for Research and Clinical Support
Several speakers described examples of how health systems and research groups have sought to collect and use SDOH information to reduce disparities across the cancer care continuum.
Oluwadamilola Fayanju, chief of the division of breast surgery at the University of Pennsylvania Perelman School of Medicine, said that addressing
the unmet social needs of patients with cancer is the best way to reduce cancer treatment disparities, but there are a number of challenges to collecting the data necessary to understand these social needs. Using the National Comprehensive Cancer Network’s Distress Thermometer and Problem List,28 Fayanju surveyed 1,000 women with breast cancer and found that those whose distress increased over time were more likely to be unmarried or covered through Medicaid; in addition, Black respondents reported lower baseline distress scores, prompting fewer referrals for social services, but experienced less improvement in their distress over time (Fayanju et al., 2019), a disconnect that Fayanju suggested indicates a potential flaw in applying the distress assessment methods across racial or ethnic groups. Fayanju and her team also found that Black patients experienced longer times to their first postdiagnosis evaluation, more practical stressors, and delayed time to treatment, all of which are associated with worse survival rates (Fayanju et al., 2021; Richards et al., 1999).
Noting that oncologists are gatekeepers during key points of a patient’s cancer care journey, Fayanju underscored the importance of timing in terms of identifying and responding to social needs. “If we’re only collecting information about SDOH [or] unmet social needs at the time of first consult, we are already too late,” she said. “By the time someone arrives for her [treatment] visit, a die has been cast. And therefore, we have an opportunity, if not an obligation, to start collecting that data sooner.”
How SDOH information is collected is also very important, Fayanju stated. Electronic approaches have advantages and disadvantages, and it could be that people with the greatest needs are the least able to use patient portals or other technologies. In addition, Fayanju noted that EHRs typically capture only limited data on social needs, because it was never entered, collection was rushed or inconsistent, or status changes are unrecorded.
Fayanju is leading a clinical trial to assess strategies for collecting and addressing information on patients’ SDOH and social needs (ACC, 2024), with a goal of determining whether collecting this information as soon as possible after a breast cancer diagnosis could help alleviate modifiable SDOH and thereby improve care equity, effectiveness, and efficiency and reduce outcome disparities. The study will also assess time to intervention and enable a systematized, standardized comparison of three self-administered screening tools alongside the “usual care” of unstructured clinical collection. If the trial is successful for patients with breast cancer, Fayanju suggested that the approach could be expanded to other cancers and diseases.
Fayanju said that the greatest challenges have been the technical complexities of integrating the distress survey data into patient EHRs, leadership
___________________
28 See https://www.nccn.org/global/what-we-do/distress-thermometer-tool-translations (accessed July 22, 2024).
changes, and the lengthy review and approval processes from the many organizations involved. She also added that sufficient research funding will be critical to further elucidating best practices in this area and that successful research and interventions around SDOH will require strong and enduring partnerships among researchers, clinicians, institutions, and patients.
Cardinale Smith, chief quality officer for oncology for the Mount Sinai Health System, discussed how her institution implemented an EHR-integrated approach to assessing and addressing psychosocial distress. The psychological, social, spiritual, and financial burdens of cancer contribute to significant psychosocial distress among patients with cancer and cancer survivors (Liu et al., 2023), Smith said. The American College of Surgeons Commission on Cancer now mandates distress screening among its accredited institutions,29 and despite important progress toward reducing patients’ psychosocial distress, significant challenges remain.
To address these challenges, Mount Sinai staff created the Quality of Life and Support Survey, a customized electronic screening tool embedded in EHRs that asks patients about financial strain; mental and spiritual health; and access to transportation, food, and social support (Jones et al., 2024). The evidence-based questions underwent interdisciplinary review before being further refined by a community advisory board. Patients can access the survey in English or Spanish through a patient portal, and their responses are made available to their clinicians.
The survey is typically given before or during a patient’s second visit. An EHR mechanism to confirm communication helps to create a “closed loop” to ensure adequate response to the information patients provide, such as by prompting notifications to a social worker, chaplain, or child life coordinator. Smith explained that transportation has emerged as a common issue, and the system has also been instrumental in connecting patients with social workers, mental health specialists, or chaplains to address both practical and emotional needs.
Smith identified several lessons learned since the survey went live in 2022. First, she said that it is ideal to administer the surveys well in advance of appointments and via the patient portal rather than during in-person clinic visits. Noting that patients do not always feel comfortable discussing unmet needs, especially if they are not eventually addressed, Smith added that it is helpful for clinicians to clearly explain why the survey matters, what actions are being taken in response, and what patients can expect. Mount Sinai plans to update the survey to add more languages, expand its use to additional cancer center sites and patient groups, and create more mechanisms to ensure patients are connected to people and resources they need.
___________________
29 See https://www.facs.org/media/t5spw4jo/2016-coc-standards-manual_interactive-pdf.pdf (accessed June 18, 2024).
Wayne Lawrence, research fellow in the Division of Cancer Epidemiology & Genetics at the National Cancer Institute (NCI), said that he is encouraged by what he described as a change in focus among epidemiologists from identifying health risks to considering potential actions that could not only improve survivorship but also resolve health disparities. To continue forward progress, NCI has launched the Connect for Cancer Prevention Study,30 a large prospective cohort to study cancer causes and prevention. The study’s Structural and Social Determinants of Health Working Group is charged with describing measures that can capture both the cohort’s SDOH and the upstream structures that influence them, such as laws, policies, and cultural beliefs. Participants fill out questionnaires developed by researchers with expertise in cancer inequities, SDOH, and structural racism that are based on input from a participant advisory board about the difficulties, miscommunication, and mistrust people experience when navigating the health care system and their racial consciousness in the health care setting and beyond, Lawrence said.
Leveraging Real-World Data
Mbah discussed opportunities to leverage real-world data (RWD) to generate insights on SDOH, highlighting how analyzing EHR data, examining differential biomarkers, and investigating underlying social drivers of disease, care access, and treatment outcomes can help reveal the complex relationship between SDOH and cancer biomarkers and enable interventions that address cancer inequities.
Mbah explained that RWD is routinely collected from a variety of sources, such as wearable devices, insurance claims, clinical visits, and EHRs (FDA, 2024; Liu and Panagiotakos, 2022). She pointed out that data relevant to SDOH can be examined at the individual, care, or societal levels and described how integrating SDOH data with RWD from EHRs can help researchers study health equity. EHRs contain detailed clinical data not found in other sources, such as genomic biomarkers or mutations, and integrating these data can illuminate how SDOH contribute to racial and ethnic differences in predictive or prognostic biomarkers, including how that impacts treatment and outcomes. From a research perspective, Mbah noted that an important advantage is that accessing EHR data for a large study cohort is much easier and less expensive than conducting in-person longitudinal studies.
Flatiron, which is dedicated to generating real-world evidence from RWD analysis, has access to the EHR data of more than 3 million patients with cancer,31 and its researchers have published numerous studies linking area-level
___________________
30 See https://www.cancer.gov/connect-prevention-study (accessed June 18, 2024).
31 See https://flatiron.com/real-world-evidence (accessed June 30, 2024).
SDOH with RWD on a range of outcomes to advance cancer health equity in areas such as clinical trials participation, treatment initiation, and survival rates (Guadamuz et al., 2023a, 2023b, 2023c, 2023d). Flatiron researchers have found associations among societal advantage or deprivation, aggressive tumor characteristics, and negative breast cancer outcomes (Krieger et al., 2016; Pittell et al., 2023).
To build on this work, Mbah said that Flatiron is working on integrating a standardized SDOH screening into its EHR system to help cancer practices collect health-related social needs data at the point of care, an effort that she said supports CMS’ Enhancing Oncology Model (EOM)32 to improve health equity. She added that Flatiron is also assessing interactions among SDOH and clinical and genomic factors, such as biomarkers, treatment regiment, and outcomes.
Considerations for Using Electronic Health Records
Ferris suggested that enabling EHRs to collect more SDOH data in a more structured way could help advance research and the development of effective interventions to address SDOH. Fayanju emphasized the importance of EHR transparency and interoperability, noting that clinicians across specialties and health systems would have more information about their patients’ social needs—and know their patients better—if the data were easily viewable in an EHR. Gerson noted the opportunity to comprehensively assess the ability to record and reflect critical and intersecting SDOH and biomarkers within EHRs and make datasets available for studying these interactions as well as information sources for treatment decisions.
Hughes-Halbert posited that allostatic load is a factor that is particularly promising in terms of being potentially actionable clinically and via public health initiatives but said that inconsistencies in EHR data pose a challenge for using this metric effectively. She suggested that it may be useful to create a policy for structured, systematic EHR data collection to measure allostatic loads. Obeng-Gyasi agreed that more standardized and structured EHR data would be helpful, noting that implementing new methods for calculating allostatic load can be successful if they have clear, action-oriented workflows with assigned roles. However, she cautioned that calculating allostatic load is difficult because it is dynamic and relies on frequent clinical visits, adding that more clarity around its clinical utility is needed.
Darien emphasized the importance of eliminating persistent biases in EHRs and algorithms, which is particularly critical if the goal is to develop
___________________
32 See https://www.cms.gov/priorities/innovation/innovation-models/enhancing-oncology-model (accessed July 11, 2024).
strategies to better meet the social needs of diverse patients. In addition to funding, she said that multidisciplinary partnerships and collaborations that include the perspectives of patients and community-based organizations will be important in addressing these persistent biases.
Considerations for Using Questionnaires
Many participants discussed some common challenges associated with using questionnaires to assess SDOH. Rogers noted a need for improvement in the questionnaires she has encountered as a patient and family caregiver and stressed the importance of considering the challenges encountered by those with disabilities, limited literacy, or limited health knowledge when filling out screening tools or surveys. She also observed that creating a whole picture of one person’s SDOH across their lifespan and generational history is a large and time-consuming, but incredibly important, task. Carlos noted that in-depth collection of social risks and resilience factors is especially difficult, which is why a patient’s race is still used in prediction models as a proxy for SDOH. She suggested that it may be helpful to re-evaluate those methods to facilitate better collection of ancestry information along with SDOH and help clinicians see beyond a patient’s race.
Susan Schneider, past president of the Oncology Nursing Society, commented that it is also important to consider how and by whom screening forms are filled out. Based on waiting-room observations, Schneider said she has seen patients and their families take a variety of approaches to completing questionnaires, and that information could be important to interventions but is generally not being captured. Fayanju agreed and noted that her team is launching a qualitative research study of how forms are filled out in the waiting room to determine best methods for ensuring completion.
Cogle added that family support, and especially consent and consensus, is very important. For certain procedures, such as bone marrow transplants, many families appoint a spokesperson who communicates with clinicians and then reports back to the other family members. He suggested that this practice could also inform approaches to social needs assessments.
The Importance of Trust in Data Collection and Use
Several participants underscored how trust (or the lack of it) plays into the effectiveness of different approaches to SDOH data collection and use. Tucker-Seeley pointed out that SDOH-oriented questions in health care settings often focus on food, housing, and transportation (Moen et al., 2020). For patients who do not understand why they are being asked about those things or do not have a trusted relationship with the clinician or institution,
these questions can seem intrusive and suspicious. McCullough noted that even if patients share their food or housing insecurities, in many cases, little if anything is done to address them (Yan et al., 2022). Hughes-Halbert added that social risk screening is neither standardized nor typically acted upon and suggested that improved harmonization, consistency, and accountability could help health systems to effectively act upon needs, not merely identify them.
Darien emphasized the need for clinicians and health care systems to build trusted relationships with patients and understand the context of their daily lives. Jasmine McDonald, associate professor of epidemiology at the Columbia University Irving Medical Center and a self-identified Black female, shared that as a patient, she feels that lack of trust and often tells physicians that she too holds a doctorate to ensure the physician does not make false assumptions about her. First visits are especially anxiety inducing for patients, which offers an opportunity for clinicians to create a positive experience. Darien agreed, noting that asking questions is preferable to making assumptions, which are based on conscious and unconscious biases. While questions may feel intrusive, asking the right ones can be instrumental in directing care to where it will have the most impact. Cogle suggested that ensuring patients consistently see the same clinicians would build trust. “How can you build trust if you’re seeing a different person?” he asked.
Fayanju added that it is important, even for routine data collection, to explain to patients why they are being asked questions that may seem intrusive and clarify that all patients are being surveyed, not just one specific group. These clarifications can help people feel more comfortable revealing personal information, she said. Done correctly, Darien said, SDOH screening can build trust and trustworthiness. “People are afraid if they give some of this data out or talk about challenges, that they will be given lesser treatment,” Darien noted (Tucker-Seeley et al., 2024), “So, reframing this to actually build trust and build conversation is a really important goal.”
POTENTIAL AREAS OF FOCUS FOR FUTURE RESEARCH
Even as new insights and interventions emerge, much remains to learn about the social factors involved in cancer development and outcomes. Several participants pointed to opportunities to further elucidate how place, space, and ancestry interact to influence cancer risk and outcomes, as well as the structural root causes behind SDOH and possible interventions that could reduce health disparities. They discussed how different approaches to research practice and funding could help to make cancer research more relevant and applicable for diverse populations, particularly those who have been historically disadvantaged. Highlighting opportunities to advance community-driven research questions and approaches, many speakers also described strategies
to build evidence for multidimensional approaches targeting the individual, health system, environment, and policy levels.
Broadening Research Aims
Shulman observed that scientific grant programs may inadvertently encourage researchers to focus on overly narrow, measurable questions that, in their attempt to separate out one SDOH, such as smoking, fail to elucidate how SDOH are interrelated and part of a much larger picture. McCullough agreed that this poses a problem and suggested a need for improved collaboration among researchers, who she said are often more apt to work within their individual silos than together on large-scale projects that look beyond individual SDOH. For example, social scientists, computational biologists, and experts in artificial intelligence may be able to help researchers move beyond their niche specialties and create comprehensive care models that better reflect the patient experience and can be incorporated into pooled, coordinated, multidisciplinary efforts to improve it. “With coordinated efforts to be able to look at the breadth of social determinants and social factors, having data that can be pooled and merged, I think we make more than incremental steps,” McCullough said.
Tucker-Seeley noted the lack of an obvious infrastructure or overarching entity to support efforts that can truly move the needle. He said that community members are likely aware that researchers’ community visits or individual grant-funded projects are not going to be sufficient to truly solve these complex problems and suggested that funders emphasize new metrics that focus on real problems being solved.
Diversifying Clinical Studies and Trials
Many workshop participants considered how the diversity of patients who are included in research studies influences insights into SDOH and cancer and how this knowledge can be applied. A key issue Mbah noted is the lack of large and diverse datasets that cover tumor histology and subtypes, biomarkers, SDOH, treatment access, and treatment outcomes. Such datasets could assess the association of aggressive tumor subtypes with SDOH, treatment access, and outcomes, she said.
Evans emphasized that including diverse populations in clinical trials is important for eliminating disparities and improving cancer treatments and access to care (Oyer et al., 2022). Vidal added that diagnostics companies are developing risk stratification tools but fail to rigorously validate them for multiple populations based upon patient characteristics, such as race and other social factors. For example, he said that researchers have found that the
available risk stratification tools for breast cancer are inadequate for use with Black patients; one metric applied across all patients incorrectly labels Black patients as lower risk, resulting in worse outcomes (Fayanju et al., 2023). Evans suggested that policies could be developed to require that studies of cancer screening tools include diverse populations and prevention components, adding that NCI and NIH could also use funding structures to incentivize diversity in studies. Since drug developers are very responsive to the rules of the U.S. Food and Drug Administration (FDA), Evans and Vidal also suggested that FDA could mandate diverse representation in clinical trials as a condition for drug approvals.
While many people have encouraged diversifying clinical trials, Yates posited that this would only help improve efficacy in diverse populations if the drugs are developed for and tested on diverse biology in the first place. He noted that drug developers are increasingly aware of this mismatch, and recognition of a market for precision medicine therapies that target underserved populations is emerging.
Timothy Rebbeck, associate director for cancer equity and engagement at the Dana-Farber Cancer Institute and Harvard University, said that more diverse clinical trial data can help support clinical decisions that reduce disparities and improve care. However, he cautioned that such data should not be used to create race-based treatment pathways. Genomic differences exist among subgroups, but further research is needed to distinguish these differences from social drivers. Watson added that the All of Us study has data on a large and diverse population and takes a team science approach that is supported by a multidisciplinary slate of researchers and social scientists asking complex, intersectional questions.
Collaborating Across Disciplines and Communities
Winn noted that precision medicine is supposed to create individualized, whole-person care, but these approaches have been based on a patient’s genetics and do not account for SDOH. This exclusion creates an artificial conflict between “hard” and “soft sciences,” he said, and suggested that better alignment among researchers could facilitate more productive, multidisciplinary conversations about translational research and population health.
Winn and other participants highlighted how researchers could focus on learning to communicate better with various communities. Hughes-Halbert shared that her work on transdisciplinary, collaborative teams taught her that harmonizing terms and concepts can help scientists move forward. Tucker-Seeley suggested that community members could also be included in this harmonization process to ensure their perspective is heard and noted that researchers would benefit from a better understanding of the U.S. health care policy process.
Conzen and Victoria Seewaldt, the Ruth Ziegler Professor and Chair of the Department of Population Sciences at City of Hope, suggested that environmental health researchers could partner with social scientists to untangle the complex stressors different communities face. For example, rural communities may experience very different types of challenges than urban communities, and pollution exposures, access to healthy foods, access to resources, and access to health care may interact to create unique challenges (CDC, 2024; Losada-Rojas et al., 2021). Gerson suggested that policies could help to improve the feedback loop between researchers, individual patients, and at-risk communities to uncover knowledge gaps and identify what interventions people and communities want. He also suggested integrating the study of SDOH on cancer outcomes with targeting social needs to improve SDOH and therefore cancer outcomes.
McCullough posited that community members are more likely to support research if they are compensated as collaborators, similar to industry partnerships, and can see that the research is likely to produce tangible health impacts. “Research for the sake of research to end up in high-impact journals does not improve population health,” she noted. McCullough also emphasized the importance of studying the interconnectedness of not just SDOH and diseases but overall systems, such as the criminal justice system or environmental discrimination. “All of these things are connected,” she stated.
Advancing Meaningful Community Engagement
Several participants highlighted how community-engaged research methods can improve understanding of SDOH and discussed some of the nuances involved in effectively working with communities. McCullough said that it is important for communities to be included and engaged through the entire research process, pointing to focus groups she holds for Black women with cancer as one example. Rogers advised researchers to avoid entering communities by declaring they are “here to help.” Unless they can offer the specific help the community is asking for, she said, the visit usually will not bring value or yield helpful data. Brawley added that many communities are seeking action rather than more research.
Nicole Stout, assistant research professor of cancer prevention and control, health policy, management, and leadership at West Virginia University, emphasized how important it is for researchers to understand the cultural context in which they work and how it influences the stressors people experience. For example, rural communities often emphasize resilience and self-efficacy, and Yates shared that in his experience, rural residents were sometimes suspicious of outside intervention, at least until they had the opportunity to vet it themselves and perceive that it met their needs, not the needs identified
by the researchers. Winn and Stout added that rural communities are quite diverse in terms of age, race, and SDOH.
Pointing to HANDLS as an example of a study in which researchers were successful in gaining participants’ trust, Evans said that researchers first detailed their plan to key members of the neighborhood, including tenants’ associations, health ministries, state assemblymen, and city council members, who then formed a community advisory board. Next, they hosted a community event and invited residents to tour the mobile data collection unit and suggest improvements, which the researchers made, and started pilot visits to neighborhoods and local festivals, meeting more people along the way and sharing what they were learning as the study progressed.
Several participants emphasized the important role of language in facilitating effective communication and community partnerships. Rebbeck said that use of jargon is often a challenge when researchers speak with communities and attempt to collaborate with researchers in other disciplines. He suggested that a shared vocabulary that is refined, clear, and scientifically rigorous would help these relationships. Darien said that plain language and jargon-free communication can help but cautioned against overly simplifying or “dumbing down” medical language. She also pointed out that trust is a multidimensional relationship. Despite a lot of focus on why patients can trust their clinicians, she said that there has been less attention to the importance of clinicians’ trust in their patients (Grob et al., 2019).
OPPORTUNITIES TO INVEST IN IMPLEMENTATION
Throughout the workshop, many participants underscored the urgency of using existing knowledge to address health inequities and disparities and broadly implementing available solutions. Several speakers shared examples of programs and initiatives that seek to incorporate SDOH interventions into cancer prevention and clinical workflows. Building on these examples, many participants also discussed opportunities and challenges in translating evidence generated through research into practical solutions, intervening to prevent cancer and reduce disparities earlier in life, and creating patient- and community-driven interventions that effectively reach underserved communities. Some speakers also discussed ways to overcome technical, cultural, and institutional implementation challenges and build a community health workforce that is equipped to implement and sustain best practices to support equitable health systems.
Acting on Existing Knowledge
Highlighting a point raised throughout the workshop, Shulman stated that it is important to continue research into the biological effectors of SDOH, but
in the meantime, it is equally important to use existing knowledge to intervene and try to reduce health disparities. For example, if transportation is known to be a major factor that prevents patients from receiving the care that they need, he suggested that health care systems implement solutions to address that problem even while researchers continue to study other factors that also might impact care delivery or quality. Brawley added that while many workshop participants had emphasized the importance of conducting more research to better understand health disparities, especially racial disparities, and other differences among populations, they also underscored the importance of closing gaps in the implementation of effective interventions. For example, he said that new therapies are always welcome, but many patients cannot access or afford existing treatments, so the additional benefit of new therapies will be limited unless access and implementation challenges are addressed.
Assuming it is possible to effectively collect SDOH data, Bradley asked participants to suggest policy approaches that would put these data to use for addressing patients’ social needs. Shulman replied that it is important to determine patients’ social needs, intervene to address them, and measure the results to ensure they received optimal care. Schilsky noted that while many clinical trials ask quality-of-life questions, very little of those data end up on drug labels, but he suggested that making that information available could be meaningful to patients and clinicians. Rebbeck suggested creating a “go, no-go” guiding statement, akin to the FDA approval process, that indicates when data are sufficient to implement an intervention to address SDOH at the individual or public health level.
McDonald said that policies to facilitate communication and collaboration with community health workers will be especially important, and expressed her belief that broader SDOH screening can improve patient–clinician trust and reduce clinicians’ conscious and unconscious biases. She said that key challenges in assessing SDOH include the lack of standardized measures, uniformity in the frequency of delivering measures, choosing which SDOH measures to implement in the clinic, and the evaluation of the SDOH interventions implemented. Moreover, many SDOH scales have not been validated in different populations, suggesting that they may not be transferable to all groups.
McDonald described the Persistent Poverty Initiative,33 coordinated by NCI, which funds five centers to address cancer prevalence in areas of persistent poverty by building up local social capital through multigenerational initiatives that target both individuals and systems. Her institution, in collaboration with other New York-based institutions that are a part of the Social Capital
___________________
33 See https://cancercontrol.cancer.gov/hdhe/research-emphasis/underserved-areas/persistent-poverty (accessed August 7, 2024).
grant,34 seeks to focus on these issues through multiple cores. These include the Career Enhancement Core,35 which aims to equip the next generation of clinical research coordinators with the ability to identify and mitigate SDOH; and the Research and Methods Core,36 which creates standards for data collection, analysis, and harmonization across different studies and regions to enable assessment of associations and downstream impacts.
Highlighting prostate cancer as an example, Rebbeck discussed how clinicians can improve cancer care by acting on available evidence about the role of SDOH. As discussed throughout the workshop, Rebbeck said that disparities exist at all phases of the cancer continuum and are driven by complex, overlapping, and individual biological and social factors, such as genetics, SDOH, systemic racism, risk factors, health behaviors, and care quality. He posited that individually tailored interventions would help improve patient outcomes.
Rebbeck noted the significant disparities in prostate cancer incidence and mortality rates among people in different racial and ethnic groups (Siegel et al., 2024; Sung et al., 2021). Known risk factors for prostate cancer include age, family history, race and ethnicity, height, and obesity, most of which are not modifiable (Kensler and Rebbeck, 2020). Germline-inherited genetics could contribute to some disparities, Rebbeck noted, as prostate cancer is highly heritable (Conti et al., 2021); however, the relative impact of other factors, including SDOH, tumor size, or treatment pathway, does not appear to fully explain the higher mortality rates for non-Hispanic Black men (Ellis et al., 2018).
Cancer stage at the time of diagnosis is highly predictive for mortality. While prostate cancer screening can identify earlier-stage cases, Black men are screened at lower rates than other races (Bryant et al., 2022), suggesting that broader use of prostate cancer screening in this population could reduce disparities in mortality rates. Rebbeck added that standardizing care interventions can also reduce racial disparities, stating that when all the variables are controlled, as in a clinical trial, the disparities disappear (Dess et al., 2019). “We see this many times,” he said. “If we can completely control not for the selection of who gets in the trial but for what kind of care people get, we do not see disparities in the outcomes.”
Rebbeck suggested that it may be helpful to study how the molecular differences of the many prostate cancer subtypes differ by race (Kensler et al., 2022) and added that clinicians can also determine which patients’ genetic profiles increase their prostate cancer risk.
___________________
34 See https://reporter.nih.gov/project-details/10661344 (accessed August 5, 2024).
35 See https://www.socacenternyc.org/careerenhancementcore (accessed September 6, 2024).
36 See https://www.socacenternyc.org/reasearchandmethodscore (accessed September 6, 2024).
Seewaldt highlighted how applying existing knowledge about preventing metabolic disorders could help to address some of the drivers behind a range of health conditions. Noting that the age-adjusted mortality rate among U.S. women has risen over time (Kindig and Cheng, 2013), she said that factors such as lack of exercise and healthy food as well as increased incidence of Type 2 diabetes have played a particularly important role in women’s health in recent decades. As other speakers noted, Seewaldt underscored that many overlapping neighborhood SDOH contribute to poor health and lower life expectancies, from lack of access to healthy food to fewer trees, greater carcinogenic exposures, and increased air and noise pollution (Churchwell et al., 2020; Hoffman et al., 2020; Kind and Buckingham, 2018; Nardone et al., 2020; Sistrunk et al., 2022). As discussed, many of these overlapping SDOH can be traced back to historic mortgage discrimination and similar institutionally, structurally, and culturally racist policies that created segregated neighborhoods whose current residents have increased disease risk and burden and worse health outcomes (Hoffman et al., 2020; Nardone et al., 2020). “We’re looking at a very complex picture—a history of structural racism, discrimination, and ultimately, it’s not leading to one thing, not just breast cancer or prostate cancer, but it really leads to many diseases,” she said.
Seewaldt said that the diet of many Americans contains too many calories and too few nutritious elements, which, combined with a lack of other resources that encourage healthy living, is contributing to an increase in the incidence of insulin resistance, obesity, heart disease, hypertension, Type 2 diabetes, and cancer (USDA, 2020). Insulin resistance is a particularly serious health issue, as it stimulates hunger, turns carbohydrates into fat, and inhibits fat breakdown, creating conditions ripe for prediabetes (NIDDK, 2018). While research has consistently shown that Type 2 diabetes can be prevented through modest changes in diet and exercise and the drug metformin (Knowler et al., 2009), Seewaldt lamented that this knowledge has not led to significant reductions in diabetes rates. “We have the means to reverse prediabetes and prevent it from turning into diabetes. We have clinical trials, we have all the guidelines, and we are not implementing [them],” she said.
Seewaldt noted that no single variable, such as obesity or body mass index, can be used to predict breast cancer risk (Bandera et al., 2015; Chiu et al., 2011). Through her research into epigenetic changes in women with insulin resistance (but not diabetes), Seewaldt showed that the signaling pathways of certain genes linked to both insulin resistance and breast cancer accelerate cell aging that can increase cancer susceptibility (Shalabi et al., 2021; Vidal et al., 2024). To help reverse insulin resistance and prevent Type 2 diabetes through early interventions, she suggested further research and updates to public health guidelines. Conzen highlighted the value of upstream prevention strategies,
including low carbohydrate/high fiber nutrition education before insulin resistance begins (Foley, 2021).
Advancing Prevention and Early Life Interventions
Several participants emphasized the role of early-life exposures in contributing to cancer risk and highlighted potential opportunities to reduce that risk through early-life interventions. Winn suggested that policies to intervene at the elementary school age could cover physical education, nutrition, mental health, and overall wellness behaviors. Brawley also suggested integrating prevention and risk reduction programs in childhood education and targeting social settings rather than hospitals and clinics. Rebbeck agreed, noting that racial disparities exist well before diagnosis (Rebbeck and Haas, 2014). Seewaldt suggested increasing emphasis on physical education in schools and highlighted the importance of policies to reduce air pollution and other environmental exposures.
Fayanju added that interventions are likely to be more effective when they are introduced as early in life as possible. Evans suggested that cancer prevention specialists and other health workers could find ways to collaborate with labor unions to develop practical interventions, such as including health initiatives in their bargaining agreements or insisting on having a nurse on site in every school. She also noted that collaborating with teachers could offer another avenue to impact student health.
Focusing on Underserved Communities
Frencher urged attendees to focus on implementing SDOH-oriented interventions in communities facing the greatest health disparities. Because factors such as insurance status can impact a person’s access to care, including access to clinical trials, he stressed that health disparities do not come down to biology or SDOH but rather biology and SDOH, “and we have to do a better job of trying to combine those things.”
To deliver high-quality care in underserved communities, Frencher suggested focusing on increasing awareness of bias and finding opportunities to align goals and resources. He also suggested expanding the discussion beyond academics and nonprofit community clinics to include industry, entrepreneurs, and others, stressing that “if we don’t create business models that ultimately can deliver better care to our patients and communities of color in underserved areas, it’s not going to happen.” Ray Michael Bridgewater, president and chief executive officer of Assembly of Petworth, added that it is important to understand challenges vulnerable communities face, such as low literacy, really listen to them, and let them take the lead. “You’ll get more
cooperation, you’ll get more trust if you let the community drive the car,” he explained.
Alma McCormick, executive director of Messengers for Health37 and a member of the Crow Nation, shared how she put these approaches into practice in her community-based research to address cervical cancer among Crow women. The Crow community faces daunting socioeconomic circumstances, and most members lack access to quality health care. McCormick said that prior to her work, Crow women were not being screened for cervical cancer. In 2001, she helped create community-led cancer awareness and prevention measures that built upon Crow cultural strengths, values, and resources, such as spirituality, health, and community resilience.
McCormick explained that being a member of the community herself was critical to her ability to overcome community mistrust. She noted that her research partners also built trust with residents by demonstrating integrity, showing sincere compassion and concern, and engaging the community by making long drives to visit with them, listening to community voices, and allowing the community to take the lead. By focusing on the individual, woman-to-woman level, she said, the team was able to create an environment where women felt comfortable speaking openly about cancer. She added that it also helped that the project directly benefited Crow women and included services to address other pressing social issues, such as substance use disorders.
Medical mobile units are one strategy that some researchers and health systems have used to reach underserved communities. Shulman said that his institution uses mobile care units for screening, prevention, and risk reduction but only once they have established trust within the communities they serve. He said that mobile units are most effective if they are prepared to support the next steps. “If you find somebody who’s got an abnormal mammogram and she doesn’t have insurance, and she doesn’t have a primary care doctor, and she doesn’t have any connection with the health system, you need to be prepared to close those gaps and take care of that person without delay, without compromising her treatment and her outcome,” he said.
Many participants also discussed what resources are available to help community advocates and health workers. McDonald said that her institution, in collaboration with City University of New York, created a community science institute38 that trains community members to join institutional review boards, understand research details and clinical trial protocols, and become community advocates. She and Rogers suggested that it would be helpful if pathways could be created to connect such training programs with actual jobs. Cogle
___________________
37 See https://www.messengersforhealth.org/about-us (accessed June 20, 2024).
38 See https://news.columbia.edu/events/community-science-institute# (accessed June 20, 2024).
suggested that Medicaid provider enrollment policies can create opportunities to pay community health workers to offer peer support to patients, as is done in Florida and some other states. Yates commented that during his time at Tuskegee University, he hired community residents as patient navigators and community health educators and treated them as professional peers. However, in that case, their pay came from NIH grants, not the institution itself. “I think the model does work, but we have to find the funds,” he said.
Supporting Equitable Care Delivery
To accomplish the goals discussed throughout the workshop, Winn and others said that sufficient, sustained funding models will be critical. Evans suggested that policies could require that all patients with cancer be given state-of-the-art treatment, and Randall Oyer, executive medical director of cancer services at Penn Medicine Lancaster General Hospital, suggested this could be aided by a policy to eliminate “cancer deserts,” large areas without access to NCI-designated cancer centers. Evans also suggested that health care workers engage with policy proposals, such as House Resolution 40, a bill to study reparations proposals.39 Winn added that policies to address drug shortages could also help, because historically disadvantaged communities are significantly affected.
Batsheva Honig, health insurance specialist at CMS, described how emerging policies and reimbursement models, such as CMS’ EOM,40 can help to reduce health care disparities and advance health equity. EOM is a 5-year voluntary payment model for clinicians that aims to incentivize delivery of high-quality, person-centered, equitable care to Medicare fee-for-service beneficiaries who receive qualifying chemotherapy. In addition to traditional fee-for-service payments, Honig explained, EOM participants are eligible for two financial incentives to improve care quality and reduce costs: a Monthly Enhanced Oncology Services payment, which reimburses services such as patient navigation, social needs screening, and care planning, and a performance-based payment, which considers the total cost of a patient’s care and the quality of care after starting chemotherapy.
Honig said that CMS is continually testing and optimizing new ways to embed health equity into care, such as collecting beneficiary-level sociodemographic data, covering social needs screening, supporting patient naviga-
___________________
39 See https://www.congress.gov/bill/117th-congress/house-bill/40 (accessed July 1, 2024).
40 For information on EOM and Monthly Enhanced Oncology Services, see https://www.cms.gov/priorities/innovation/innovation-models/enhancing-oncology-model (accessed June 20, 2024).
tion, and developing health equity plans.41 EOM mandates that participants conduct social needs screening in transportation, food insecurity, and housing instability; additional optional screenings include social isolation, emotional distress, interpersonal safety, and financial toxicity. The idea is that by better understanding each beneficiary’s needs, individually tailored care plans can be developed that include referrals to community resources. “The key here is establishing and developing community linkages and partnerships as a way to address whole-person care needs,” Honig said.
EOM participants also collect electronic patient-reported outcomes (ePROs),42 without clinician amendment or interpretation, on topics such as symptoms, physical functioning, behavioral health, and social needs, Honig noted, adding that ePROs help clinicians focus on patient needs and improve patient communication, care management, satisfaction, and cancer outcomes.
Honig noted that the addition of new codes to the 2024 Physician Fee Schedule,43 including for SDOH risk assessment and community health integration, can improve health equity and outcomes. Under EOM, participants screen every beneficiary for health-related social needs monthly; the SDOH risk assessment, however, is included in the total cost-of-care model, not as a separate payment. The aims of the risk assessment requirement are to facilitate the process of connecting patients with resources to address their needs, helping to build patient trust; identify and address gaps in services; avoid service duplications; and establish community service partnerships.
Staci Lofton, senior director for health equity at Families USA,44 discussed these and other policies from her perspective as a consumer advocate. Families USA advocates for all people to have equitable access to high-quality health care to enable their best possible health, particularly those who have been historically marginalized. Lofton shared the story of a woman who recently passed away from misdiagnosed cervical cancer, an outcome that she argued was preventable. While clinicians want to help their patients, Lofton said that they are trapped inside a system that rewards clinicians who see a large volume of patients and provide many services, regardless of whether these services address patients’ health needs or reduce persistent health disparities.
To shift the incentives, Families USA advocates for a transition to a value-based payment system that would enable clinicians to conduct opti-
___________________
41 See https://www.cms.gov/files/document/mln9201074-health-equity-services-2024-physician-fee-schedule-final-rule.pdf-0 (accessed June 20, 2024).
42 See https://www.cms.gov/priorities/innovation/media/document/eom-epros-fs?domain=lnks.gd (accessed June 20, 2024).
43 See https://www.cms.gov/medicare/payment/fee-schedules/physician (accessed July 1, 2024).
44 See https://familiesusa.org/about (accessed June 20, 2024).
mal social needs assessments, connect patients with community resources, and support person-centered holistic care that minimizes preventable deaths. Lofton expressed enthusiasm for CMS’ EOM and the 2024 update to the Medicare Physician Fee Schedule. She said that the new codes for community health integration are particularly helpful because they enable patients to access services and resources in their own communities. She added that these changes can also create a demand for more community health workers, and she suggested focusing funding and policies on building a community health workforce that can leverage individuals’ shared backgrounds to build trusted patient–clinician relationships and thereby increase screening rates and other actions that improve public health.
Lofton also called attention to the Consolidated Appropriations Act of 2023,45 which provides funding mechanisms to build up community health workforce capacity, and the proposed Community Health Worker Access Act,46 which would improve access to community health services for Medicare and Medicaid enrollees to address preventable diseases, reduce cost inefficiencies, and improve health outcomes. Finally, Lofton noted that the process of collecting and assessing patient-reported data could be made easier if it were covered by a separate payment and clinicians were able to share those data with relevant caregivers, other clinicians, and health care institutions to create a coordinated and integrated care process.
Many speakers discussed strategies to leverage funding sources for implementing interventions to reduce disparities. Fayanju suggested that diversified partnerships can reduce funding uncertainties and added that the overall goal is not to collect data but to generate the evidence needed to convince health care systems of the importance of hiring and training staff to do the work that will improve health disparities and then building the infrastructure to support that work. To achieve sustainable funding for initiatives that address health disparities, Cogle suggested it will be important to understand new state laws such as HB 885 in Florida,47 which mandates insurance coverage of all biomarker tests for any health condition based not on medical necessity but clinical utility. “This [change from medical necessity to clinical utility] is a huge game changer,” he said.
___________________
45 See https://www.congress.gov/bill/117th-congress/house-bill/2617 (accessed June 20, 2024).
46 See https://www.congress.gov/bill/118th-congress/senate-bill/3892/text (accessed June 20, 2024).
47 See https://www.flsenate.gov/Session/Bill/2024/885 (accessed August 7, 2024).
Integrating Interventions for Social Determinants of Health into Clinical Workflows
Several participants suggested that important next steps are to increase investments in transdisciplinary research to translate laboratory-generated evidence on SDOH and cancer into concrete practices and then integrate those practices into clinical workflows. Gerson and Hughes-Halbert noted that policies to address care coordination, which is already fragmented, will be important as new screenings and interventions are added to care pathways and emphasized that all policies to address SDOH will be most effective if they are driven by patient and community input and engagement.
Several speakers highlighted opportunities to better equip primary care providers (PCPs) to assess and address SDOH and better support cancer prevention, screening, and treatment. Fayanju noted that the separation between primary and specialty care has led to situations where specialists are seeing patients whose health problems could have been addressed by PCPs, but she also acknowledged many PCPs are overburdened and struggle to screen and address social needs (Drake et al., 2021; Tikkanen et al., 2020). Bradley suggested that payment reforms to reimburse clinicians for patient-reported data collection and social needs interventions could also help by providing incentives for addressing social needs and connecting patients to resources through referrals. Frencher added that community PCPs may need more support to conduct complex and expensive genomic tests, perhaps through telemedicine-based mentoring, programs like Project Extension for Community Healthcare Outcomes,48 or overall enhanced collaboration among community health workers, specialists, and researchers.
Many participants also pointed out that adequately gathering and responding to SDOH information takes time. Winn, Ferris, and Seewaldt suggested that policies that allow clinicians to be reimbursed for taking the time to get to know their patients’ social needs could encourage them to spend more time with patients, develop lasting relationships, and offer holistic, whole-person care.
Darien highlighted the importance of building up the health care workforce in shortage areas so that patients can access care and build trusted relationships with clinicians. Bradley agreed that workforce development policies are important to create health care teams that can address social needs, and Tucker-Seeley suggested that health care systems build on current workflows, as opposed to adding new ones, to facilitate buy-in from busy clinicians. He also observed that having health care leaders and administrators experienced
___________________
48 See https://www.ahrq.gov/patient-safety/settings/multiple/project-echo/index.html (accessed June 20, 2024).
in social care delivery would help create effective pathways for implementation (Taira et al., 2023).
Moving from Ideas to Implementation to Advance Health Equity
Reflecting on the workshop discussions, Hughes-Halbert and Gerson stressed the importance of fostering clear and respectful communication and connections among clinicians, patients, communities, and researchers. “I think the main goal for this workshop was to really listen—to listen to each other, to listen to our communities, to listen to what the data are telling us, and to drive our path forward,” Hughes-Halbert said. To build upon the insights raised at the workshop, many participants also underscored the importance of action. Gerson emphasized that moving from ideas to implementation will require policy changes, funding, and collaboration. As Rogers put it, “Everybody is talking about disparities and equity, but who is going to take the ball and say, ‘I got this’? Let’s get some folks together and do what we need to do.”
REFERENCES
Abdel-Rahman, O., Y. Xu, S. Kong, J. Dort, M. L. Quan, S. Karim, A. Bouchard-Fortier, H. Cho, and W. Y. Cheung. 2019. Impact of baseline cardiovascular comorbidity on outcomes in women with breast cancer: A real-world, population-based study. Clinical Breast Cancer 19(2):e297–e305.
ACC (Abramson Cancer Center at Penn Medicine). 2024. Early point-of-service social and behavioral determinants of health (SBDOH) screening and enhanced navigation on care delivery for patients with breast cancer (BREAST_SBDOH). Bethesda, MD: National Library of Medicine. https://www.clinicaltrials.gov/study/NCT06019988?term=fayanju&rank=1 (accessed June 5, 2024).
ACS (American Cancer Society). 2011. Cancer Facts and Figures 2011. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2011.html (accessed July 11, 2024).
ACS. 2022. Cancer Facts & Figures for African American/Black People 2022–2024. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-facts-and-figures-for-african-americans/2022-2024-cff-aa.pdf (accessed May 22, 2024).
Agbemenu, K., S. Auerbach, N. S. Murshid, J. Shelton, and N. Amutah-Onukagha. 2019. Reproductive health outcomes in African refugee women: A comparative study. Journal of Women’s Health 28(6):785–793.
Alderwick, H., and L. M. Gottlieb. 2019. Meanings and misunderstandings: A social determinants of health lexicon for health care systems. The Milbank Quarterly 97(2):407–419.
Antoni, M. H., and F. S. Dhabhar. 2019. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer 125(9):1417–1431.
Antoni, M. H., S. K. Lutgendorf, S. W. Cole, F. S. Dhabhar, S. E. Sephton, P. G. McDonald, M. Stefanek, and A. K. Sood. 2006. The influence of bio-behavioural factors on tumour biology: Pathways and mechanisms. Nature Reviews Cancer 6(3):240–248.
Bandera, E. V., U. Chandran, C. C. Hong, M. A. Troester, T. N. Bethea, L. L. Adams-Campbell, C. A. Haiman, S. Y. Park, A. F. Olshan, C. B. Ambrosone, J. R. Palmer, and L. Rosenberg. 2015. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Research and Treatment 150(3):655–666.
Bower, J. E., S. L. Shiao, P. Sullivan, D. M. Lamkin, R. Atienza, F. Mercado, J. Arevalo, A. Asher, P. A. Ganz, and S. W. Cole. 2018. Prometastatic molecular profiles in breast tumors from socially isolated women. JNCI Cancer Spectrum 2(3):pky029.
Boyle, C. C., S. W. Cole, J. M. Dutcher, N. I. Eisenberger, and J. E. Bower. 2019. Changes in eudaimonic well-being and the conserved transcriptional response to adversity in younger breast cancer survivors. Psychoneuroendocrinology 103:173–179.
Brady, D., U. Kohler, and H. Zheng. 2023. Novel estimates of mortality associated with poverty in the U.S. JAMA Internal Medicine 183(6):618–619.
Braveman, P., and L. Gottlieb. 2014. The social determinants of health: It’s time to consider the causes of the causes. Public Health Reports 129(Suppl 2):19–31.
Brown, W. M. 2015. Exercise-associated DNA methylation change in skeletal muscle and the importance of imprinted genes: A bioinformatics meta-analysis. British Journal of Sports Medicine 49(24):1567–1578.
Bruno, D. S., L. M. Hess, X. Li, E. W. Su, and M. Patel. 2022. Disparities in biomarker testing and clinical trial enrollment among patients with lung, breast, or colorectal cancers in the United States. JCO Precision Oncology 6:e2100427.
Bryant, A. K., K. M. Lee, P. R. Alba, J. D. Murphy, M. E. Martinez, L. Natarajan, M. D. Green, R. T. Dess, T. R. Anglin-Foote, B. Robison, S. L. DuVall, J. A. Lynch, and B. S. Rose. 2022. Association of prostate-specific antigen screening rates with subsequent metastatic prostate cancer incidence at U.S. Veterans Health Administration facilities. JAMA Oncology 8(12):1747–1755.
Byappanahalli, A. M., N. Noren Hooten, M. Vannoy, N. A. Mode, N. Ezike, A. B. Zonderman, and M. K. Evans. 2023. Mitochondrial DNA and inflammatory proteins are higher in extracellular vesicles from frail individuals. Immunity & Ageing 20(1):6.
Byappanahalli, A. M., V. Omoniyi, N. Noren Hooten, J. T. Smith, N. A. Mode, N. Ezike, A. B. Zonderman, and M. K. Evans. 2024. Extracellular vesicle mitochondrial DNA levels are associated with race and mitochondrial DNA haplogroup. iScience 27(1):108724.
Cacioppo, J. T., S. Cacioppo, J. P. Capitanio, and S. W. Cole. 2015. The neuroendocrinology of social isolation. Annual Review of Psychology 66:733–767.
Carlos, R. C., S. Obeng-Gyasi, S. W. Cole, B. J. Zebrack, E. D. Pisano, M. A. Troester, L. Timsina, L. I. Wagner, J. A. Steingrimsson, I. Gareen, C. I. Lee, A. S. Adams, and C. H. Wilkins. 2022. Linking structural racism and discrimination and breast cancer outcomes: A social genomics approach. Journal of Clinical Oncology 40(13):1407–1413.
Castrucci, B. C., and J. Auerbach. 2019. Meeting individual social needs falls short of addressing social determinants of health. Health Affairs Blog. https://www.healthaffairs.org/content/forefront/meeting-individual-social-needs-falls-short-addressing-social-determinants-health (accessed May 22, 2024).
CDC (Centers for Disease Control and Prevention). 2022. Leading Causes of Death. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm (accessed May 22, 2024).
CDC. 2024. About Rural Health. https://www.cdc.gov/rural-health/php/about/index.html (accessed June 20, 2024).
Chen, J. C., M. I. Elsaid, D. Handley, J. J. Plascak, B. L. Andersen, W. E. Carson, T. M. Pawlik, N. Fareed, and S. Obeng-Gyasi. 2024a. Association between neighborhood opportunity, allostatic load, and all-cause mortality in patients with breast cancer. Journal of Clinical Oncology 42(15):1788–1798.
Chen, J. C., T. M. Pawlik, and S. Obeng-Gyasi. 2024b. ASO author reflections: Racialized economic segregation and allostatic load: The impact of internalizing our residential environments. Annals of Surgical Oncology 31(2):1005–1006.
Chia, V. M., P. A. Newcomb, A. Trentham-Dietz, and J. M. Hampton. 2007. Obesity, diabetes, and other factors in relation to survival after endometrial cancer diagnosis. International Journal of Gynecological Cancer 17(2):441–446.
Chiu, M., P. C. Austin, D. G. Manuel, B. R. Shah, and J. V. Tu. 2011. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care 34(8):1741–1748.
Churchwell, K., M. S. V. Elkind, R. M. Benjamin, A. P. Carson, E. K. Chang, W. Lawrence, A. Mills, T. M. Odom, C. J. Rodriguez, F. Rodriguez, E. Sanchez, A. Z. Sharrief, M. Sims, O. Williams, on behalf of the American Heart Association. 2020. Call to action: Structural racism as a fundamental driver of health disparities: A presidential advisory from the American Heart Association. Circulation 142(24):e454–e468.
CMS (Centers for Medicare & Medicaid Services). 2023. Using Z Codes: The Social Determinants of Health (SDOH) Data Journey to Better Outcomes. https://www.cms.gov/files/document/zcodes-infographic.pdf (accessed May 22, 2024).
Cole, S. W. 2013. Social regulation of human gene expression: Mechanisms and implications for public health. American Journal of Public Health 103(Suppl 1):S84–S92.
Cole, S. W. 2014. Human social genomics. PLOS Genetics 10(8):e1004601.
Cole, S. W. 2019. The conserved transcriptional response to adversity. Current Opinion in Behavioral Science 28:31–37.
Collin, L. J., R. Jiang, K. C. Ward, K. Gogineni, P. D. Subhedar, M. E. Sherman, M. M. Gaudet, C. R. Breitkopf, O. D’Angelo, S. Gabram-Mendola, R. Aneja, A. H. Gaglioti, and L. E. McCullough. 2019. Racial disparities in breast cancer outcomes in the metropolitan Atlanta area: New insights and approaches for health equity. JNCI Cancer Spectrum 3(3):pkz053.
Conti, D. V., B. F. Darst, L. C. Moss, E. J. Saunders, X. Sheng, A. Chou, F. R. Schumacher, A. A. A. Olama, S. Benlloch, T. Dadaev, M. N. Brook, A. Sahimi, T. J. Hoffmann, A. Takahashi, K. Matsuda, Y. Momozawa, M. Fujita, K. Muir, A. Lophatananon, P. Wan, L. Le Marchand, L. R. Wilkens, V. L. Stevens, S. M. Gapstur, B. D. Carter, J. Schleutker, T. L. J. Tammela, C. Sipeky, A. Auvinen, G. G. Giles, M. C. Southey, R. J. MacInnis, C. Cybulski, D. Wokołorczyk, J. Lubiński, D. E. Neal, J. L. Donovan, F. C. Hamdy, R. M. Martin, B. G. Nordestgaard, S. F. Nielsen, M. Weischer, S. E. Bojesen, M. A. Røder, P. Iversen, J. Batra, S. Chambers, L. Moya, L. Horvath, J. A. Clements, W. Tilley, G. P. Risbridger, H. Gronberg, M. Aly, R. Szulkin, M. Eklund, T. Nordström, N. Pashayan, A. M. Dunning, M. Ghoussaini, R. C. Travis, T. J. Key, E. Riboli, J. Y. Park, T. A. Sellers, H. Y. Lin, D. Albanes, S. J. Weinstein, L. A. Mucci, E. Giovannucci, S. Lindstrom, P. Kraft, D. J. Hunter, K. L. Penney, C. Turman, C. M.
Tangen, P. J. Goodman, I. M. Thompson, Jr., R. J. Hamilton, N. E. Fleshner, A. Finelli, M. Parent, J. L. Stanford, E. A. Ostrander, M. S. Geybels, S. Koutros, L. E. B. Freeman, M. Stampfer, A. Wolk, N. Håkansson, G. L. Andriole, R. N. Hoover, M. J. Machiela, K. D. Sørensen, M. Borre, W. J. Blot, W. Zheng, E. D. Yeboah, J. E. Mensah, Y. J. Lu, H. W. Zhang, N. Feng, X. Mao, Y. Wu, S. C. Zhao, Z. Sun, S. N. Thibodeau, S. K. McDonnell, D. J. Schaid, C. M. L. West, N. Burnet, G. Barnett, C. Maier, T. Schnoeller, M. Luedeke, A. S. Kibel, B. F. Drake, O. Cussenot, G. Cancel-Tassin, F. Menegaux, T. Truong, Y. A. Koudou, E. M. John, E. M. Grindedal, L. Maehle, K. T. Khaw, S. A. Ingles, M. C. Stern, A. Vega, A. Gómez-Caamaño, L. Fachal, B. S. Rosenstein, S. L. Kerns, H. Ostrer, M. R. Teixeira, P. Paulo, A. Brandão, S. Watya, A. Lubwama, J. T. Bensen, E. T. H. Fontham, J. Mohler, J. A. Taylor, M. Kogevinas, J. Llorca, G. Castaño-Vinyals, L. Cannon-Albright, C. C. Teerlink, C. D. Huff, S. S. Strom, L. Multigner, P. Blanchet, L. Brureau, R. Kaneva, C. Slavov, V. Mitev, R. J. Leach, B. Weaver, H. Brenner, K. Cuk, B. Holleczek, K. U. Saum, E. A. Klein, A. W. Hsing, R. A. Kittles, A. B. Murphy, C. J. Logothetis, J. Kim, S. L. Neuhausen, L. Steele, Y. C. Ding, W. B. Isaacs, B. Nemesure, A. J. M. Hennis, J. Carpten, H. Pandha, A. Michael, K. De Ruyck, G. De Meerleer, P. Ost, J. Xu, A. Razack, J. Lim, S. H. Teo, L. F. Newcomb, D. W. Lin, J. H. Fowke, C. Neslund-Dudas, B. A. Rybicki, M. Gamulin, D. Lessel, T. Kulis, N. Usmani, S. Singhal, M. Parliament, F. Claessens, S. Joniau, T. Van den Broeck, M. Gago-Dominguez, J. E. Castelao, M. E. Martinez, S. Larkin, P. A. Townsend, C. Aukim-Hastie, W. S. Bush, M. C. Aldrich, D. C. Crawford, S. Srivastava, J. C. Cullen, G. Petrovics, G. Casey, M. J. Roobol, G. Jenster, R. H. N. van Schaik, J. J. Hu, M. Sanderson, R. Varma, R. McKean-Cowdin, M. Torres, N. Mancuso, S. I. Berndt, S. K. Van Den Eeden, D. F. Easton, S. J. Chanock, M. B. Cook, F. Wiklund, H. Nakagawa, J. S. Witte, R. A. Eeles, Z. Kote-Jarai, and C. A. Haiman. 2021. Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction. Nature Genetics 53(1):65–75.
Cote, M. L., J. J. Ruterbusch, S. H. Olson, K. Lu, and R. Ali-Fehmi. 2015. The growing burden of endometrial cancer: A major racial disparity affecting Black women. Cancer Epidemiology, Biomarkers & Prevention 24(9):1407–1415.
Crown, A., S. Fazeli, A. W. Kurian, D. A. Ochoa, and K. A. Joseph. 2023. Disparity in breast cancer care: Current state of access to screening, genetic testing, oncofertility, and reconstruction. Journal of the American College of Surgeons 236(6):1233–1239.
Dankwa-Mullan, I., K. B. Rhee, D. M. Stoff, J. R. Pohlhaus, F. S. Sy, N. Stinson, Jr., and J. Ruffin. 2010. Moving toward paradigm-shifting research in health disparities through translational, transformational, and transdisciplinary approaches. American Journal of Public Health 100(Suppl 1):S19–S24.
Dess, R. T., H. E. Hartman, B. A. Mahal, P. D. Soni, W. C. Jackson, M. R. Cooperberg, C. L. Amling, W. J. Aronson, C. J. Kane, M. K. Terris, Z. S. Zumsteg, S. Butler, J. R. Osborne, T. M. Morgan, R. Mehra, S. S. Salami, A. U. Kishan, C. Wang, E. M. Schaeffer, M. Roach, 3rd, T. M. Pisansky, W. U. Shipley, S. J. Freedland, H. M. Sandler, S. Halabi, F. Y. Feng, J. J. Dignam, P. L. Nguyen, M. J. Schipper, and D. E. Spratt. 2019. Association of Black race with prostate cancer-specific and other-cause mortality. JAMA Oncology 5(7):975–983.
Devericks, E. N., M. S. Carson, L. E. McCullough, M. F. Coleman, and S. D. Hursting. 2022. The obesity–breast cancer link: A multidisciplinary perspective. Cancer and Metastasis Review 41(3):607–625.
Dietze, E. C., C. Sistrunk, G. Miranda-Carboni, R. O’Regan, and V. L. Seewaldt. 2015. Triple-negative breast cancer in African-American women: Disparities versus biology. Nature Reviews Cancer 15(4):248–254.
Do, W. L., K. Conneely, S. Gabram-Mendola, U. Krishnamurti, O. D’Angelo, J. Miller-Kleinhenz, K. Gogineni, M. Torres, and L. E. McCullough. 2020. Obesity-associated methylation in breast tumors: A possible link to disparate outcomes? Breast Cancer Research and Treatment 181(1):135–144.
Drake, C., H. Batchelder, T. Lian, M. Cannady, M. Weinberger, H. Eisenson, E. Esmaili, A. Lewinski, L. L. Zullig, A. Haley, D. Edelman, and C. M. Shea. 2021. Implementation of social needs screening in primary care: A qualitative study using the health equity implementation framework. BMC Health Services Research 21(1):975.
Dubil, E. A., C. Tian, G. Wang, C. M. Tarney, N. W. Bateman, D. A. Levine, T. P. Conrads, C. A. Hamilton, G. L. Maxwell, and K. M. Darcy. 2018. Racial disparities in molecular subtypes of endometrial cancer. Gynecologic Oncology 149(1):106–116.
Duong, M. T., B. A. Bingham, P. C. Aldana, S. T. Chung, and A. E. Sumner. 2017. Variation in the calculation of allostatic load score: 21 examples from NHANES. Journal of Racial and Ethnic Health Disparities 4(3):455–461.
Elhussin, I., E. Baraban, T. L. Lotan, C. H. Marshall, E. Antonarakis, M. J. Campbell, M. Davis, M. Dixon, I. Kim, S. Ambs, R. Kittles, A. B. Murphy, and C. Yates. 2023. Abstract c086: Prostate cancer: Integrative multi-omics profiling in patients of African descent. Cancer Epidemiology, Biomarkers & Prevention 32(Suppl 12):C086–C086.
Ellis, L., A. J. Canchola, D. Spiegel, U. Ladabaum, R. Haile, and S. L. Gomez. 2018. Racial and ethnic disparities in cancer survival: The contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. Journal of Clinical Oncology 36(1):25–33.
Evans, M. K., J. M. Lepkowski, N. R. Powe, T. LaVeist, M. F. Kuczmarski, and A. B. Zonderman. 2010. Healthy aging in neighborhoods of diversity across the life span (HANDLS): Overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethnicity & Disease 20(3):267–275.
Eze, I. C., A. Jeong, E. Schaffner, F. I. Rezwan, A. Ghantous, M. Foraster, D. Vienneau, F. Kronenberg, Z. Herceg, P. Vineis, M. Brink, J. M. Wunderli, C. Schindler, C. Cajochen, M. Röösli, J. W. Holloway, M. Imboden, and N. Probst-Hensch. 2020. Genome-wide DNA methylation in peripheral blood and long-term exposure to source-specific transportation noise and air pollution: The SAPALDIA study. Environmental Health Perspectives 128(6):67003.
Fayanju, O. M., K. Yenokyan, Y. Ren, B. A. Goldstein, I. Stashko, S. Power, M. J. Thornton, P. K. Marcom, and E. S. Hwang. 2019. The effect of treatment on patient-reported distress after breast cancer diagnosis. Cancer 125(17):3040–3049.
Fayanju, O. M., Y. Ren, I. Stashko, S. Power, M. J. Thornton, P. K. Marcom, T. Hyslop, and E. S. Hwang. 2021. Patient-reported causes of distress predict disparities in time to evaluation and time to treatment after breast cancer diagnosis. Cancer 127(5):757–768.
Fayanju, O. M., C. E. Edmonds, S. A. Reyes, C. Arciero, V. J. Bea, A. Crown, and K. A. Joseph. 2023. The landmark series—addressing disparities in breast cancer screening: New recommendations for Black women. Annals of Surgical Oncology 30(1):58–67.
FDA (U.S. Food and Drug Administration). 2024. Center for Biologics Evaluation and Research & Center for Drug Evaluation and Research Real-World Evidence. https://www.fda.gov/science-research/real-world-evidence/center-biologics-evaluation-and-research-center-drug-evaluation-and-research-real-world-evidence (accessed June 18, 2024).
Foley, P. J. 2021. Effect of low carbohydrate diets on insulin resistance and the metabolic syndrome. Current Opinion in Endocrinology, Diabetes and Obesity 28(5):463-468.
Fowler, W., and D. Mutch. 2008. Management of endometrial cancer. Women’s Health 4(5):479–489.
Fredrickson, B. L., K. M. Grewen, S. B. Algoe, A. M. Firestine, J. M. Arevalo, J. Ma, and S. W. Cole. 2015. Psychological well-being and the human conserved transcriptional response to adversity. PLoS One 10(3):e0121839.
Gehlert, S., A. Murray, D. Sohmer, M. McClintock, S. Conzen, and O. Olopade. 2010. The importance of transdisciplinary collaborations for understanding and resolving health disparities. Social Work in Public Health 25(3-4):408–422.
Geronimus, A. T. 1992. The weathering hypothesis and the health of African-American women and infants: Evidence and speculations. Ethnicity & Disease 2(3):207–221.
Geronimus, A. T., M. Hicken, D. Keene, and J. Bound. 2006. “Weathering” and age patterns of allostatic load scores among Blacks and Whites in the United States. American Journal of Public Health 96(5):826–833.
Gohar, J., W. L. Do, J. Miller-Kleinhenz, K. Conneely, U. Krishnamurti, O. D’Angelo, K. Gogineni, M. Torres, S. Gabram-Mendola, and L. E. McCullough. 2022. Neighborhood characteristics and breast tumor methylation: Using epigenomics to explore cancer outcome disparities. Breast Cancer Research and Treatment 191(3):653–663.
Grob, R., G. Darien, and D. Meyers. 2019. Why physicians should trust in patients. JAMA 321(14):1347–1348.
Guadamuz, J., R. Mamtani, X. Wang, C. A. Ryals, I. Altomare, A. A. Asfaw, W. C. Castillo, H. Pittell, G. S. Calip, and S. Sarkar. 2023a. Abstract a049: Racial/ethnic and socioeconomic inequities in clinical trial participation among U.S. community oncology patients, 2011–2021. Cancer Epidemiology, Biomarkers & Prevention 32(Suppl 1):A049.
Guadamuz, J., X. Wang, I. Altomare, and G. S. Calip. 2023b. Mediators of racial/ethnic inequities in clinical trial participation among U.S. patients with cancer, 2011–2022. Journal of Clinical Oncology 41(Suppl 16):6511.
Guadamuz, J. S., X. Wang, T. J. Royce, and G. S. Calip. 2023c. Sociodemographic inequities in telemedicine use among U.S. patients initiating treatment in community cancer centers during the ongoing COVID-19 pandemic, 2020–2022. JCO Oncology Practice 19(12):1206–1214.
Guadamuz, J. S., X. Wang, C. A. Ryals, R. A. Miksad, J. Snider, J. Walters, and G. S. Calip. 2023d. Socioeconomic status and inequities in treatment initiation and survival among patients with cancer, 2011–2022. JNCI Cancer Spectrum 7(5).
Guan, Y., J. Shen, J. Lu, B. F. Fuemmeler, L. S. Shock, and H. Zhao. 2023. Association between allostatic load and breast cancer risk: A cohort study. Breast Cancer Research 25(1):155.
Hanjie Mo, C. R. 2021. Biomarker-driven targeted therapies in solid tumor malignancies. Journal of Hematology Oncology Pharmacy 11:84–91.
Hermes, G. L., B. Delgado, M. Tretiakova, S. A. Cavigelli, T. Krausz, S. D. Conzen, and M. K. McClintock. 2009. Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proceedings of the National Academy of Sciences 106(52):22393–22398.
Hoffman, J. S., V. Shandas, and N. Pendleton. 2020. The effects of historical housing policies on resident exposure to intra-urban heat: A study of 108 U.S. urban areas. Climate 8(1):12.
IOM (Institute of Medicine). 2001. Health and behavior: The interplay of biological, behavioral, and societal influences. Washington, DC: The National Academies Press.
Islami, F., A. Goding Sauer, K. D. Miller, R. L. Siegel, S. A. Fedewa, E. J. Jacobs, M. L. McCullough, A. V. Patel, J. Ma, I. Soerjomataram, W. D. Flanders, O. W. Brawley, S. M. Gapstur, and A. Jemal. 2018. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA: A Cancer Journal for Clinicians 68(1):31–54.
Islami, F., C. E. Guerra, A. Minihan, K. R. Yabroff, S. A. Fedewa, K. Sloan, T. L. Wiedt, B. Thomson, R. L. Siegel, N. Nargis, R. A. Winn, L. Lacasse, L. Makaroff, E. C. Daniels, A. V. Patel, W. G. Cance, and A. Jemal. 2022. American Cancer Society’s report on the status of cancer disparities in the United States, 2021. CA: A Cancer Journal for Clinicians 72(2):112–143.
Johnson, M. B., J. N. Hoffmann, H. M. You, R. R. Lastra, S. Fernandez, J. W. Strober, A. B. Allaw, M. J. Brady, S. D. Conzen, and M. K. McClintock. 2018. Psychosocial stress exposure disrupts mammary gland development. Journal of Mammary Gland Biology and Neoplasia 23(1-2):59–73.
Jones, T., A. Snow, B. Aryeh, M. Liu, C. B. Smith, and M. Mazor. 2024. Integrating social drivers of health screening into ambulatory cancer care. Journal of Clinical Oncology 42(Suppl 16):e13692.
Kandoth, C., N. Schultz, A. D. Cherniack, R. Akbani, Y. Liu, H. Shen, A. G. Robertson, I. Pashtan, R. Shen, C. C. Benz, C. Yau, P. W. Laird, L. Ding, W. Zhang, G. B. Mills, R. Kucherlapati, E. R. Mardis, and D. A. Levine. 2013. Integrated genomic characterization of endometrial carcinoma. Nature 497(7447):67–73.
Kehm, R. D., A. A. M. Llanos, J. A. McDonald, P. Tehranifar, and M. B. Terry. 2022. Evidence-based interventions for reducing breast cancer disparities: What works and where the gaps are? Cancers 14(17).
Kensler, K. H., and T. R. Rebbeck. 2020. Cancer progress and priorities: Prostate cancer. Cancer Epidemiology, Biomarkers & Prevention 29(2):267–277.
Kensler, K. H., S. Awasthi, M. Alshalalfa, B. J. Trock, S. J. Freedland, M. R. Freeman, S. You, B. A. Mahal, R. B. Den, A. P. Dicker, R. J. Karnes, E. A. Klein, P. Lal, Y. Liu, E. Davicioni, W. Rayford, K. Yamoah, and T. R. Rebbeck. 2022. Variation in molecularly defined prostate tumor subtypes by self-identified race. European Urology Open Science 40:19–26.
Kind, A. J. H., and W. R. Buckingham. 2018. Making neighborhood-disadvantage metrics accessible—the neighborhood atlas. New England Journal of Medicine 378(26):2456–2458.
Kindig, D. A., and E. R. Cheng. 2013. Even as mortality fell in most U.S. counties, female mortality nonetheless rose in 42.8 percent of counties from 1992 to 2006. Health Affairs 32(3):451–458.
Knight, J. M., J. D. Rizzo, T. Wang, N. He, B. R. Logan, S. R. Spellman, S. J. Lee, M. R. Verneris, J. M. G. Arevalo, and S. W. Cole. 2019. Molecular correlates of socioeconomic status and clinical outcomes following hematopoietic cell transplantation for leukemia. JNCI Cancer Spectrum 3(4):pkz073.
Knowler, W. C., S. E. Fowler, R. F. Hamman, C. A. Christophi, H. J. Hoffman, A. T. Brenneman, J. O. Brown-Friday, R. Goldberg, E. Venditti, and D. M. Nathan. 2009. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes study. The Lancet 374(9702):1677–1686.
Korn, A. R., C. Walsh-Bailey, M. Correa-Mendez, P. DelNero, M. Pilar, B. Sandler, R. C. Brownson, K. M. Emmons, and A. Y. Oh. 2023. Social determinants of health and U.S. cancer screening interventions: A systematic review. CA: A Cancer Journal for Clinicians 73(5):461–479.
Krieger, N. 2001a. A glossary for social epidemiology. Journal of Epidemiology and Community Health 55(10):693–700.
Krieger, N. 2001b. Theories for social epidemiology in the 21st century: An ecosocial perspective. International Journal of Epidemiology 30(4):668–677.
Krieger, N. 2012. Methods for the scientific study of discrimination and health: An ecosocial approach. American Journal of Public Health 102(5):936–944.
Krieger, N., P. D. Waterman, J. Spasojevic, W. Li, G. Maduro, and G. Van Wye. 2016. Public health monitoring of privilege and deprivation with the index of concentration at the extremes. American Journal of Public Health 106(2):256–263.
Kurian, A. W., P. Abrahamse, A. Furgal, K. C. Ward, A. S. Hamilton, R. Hodan, R. Tocco, L. Liu, J. S. Berek, L. Hoang, A. Yussuf, L. Susswein, E. D. Esplin, T. P. Slavin, S. L. Gomez, T. P. Hofer, and S. J. Katz. 2023. Germline genetic testing after cancer diagnosis. JAMA 330(1):43–51.
Lannin, D. R., H. F. Mathews, J. Mitchell, M. S. Swanson, F. H. Swanson, and M. S. Edwards. 1998. Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA 279(22):1801–1807.
Lazo, S., N. Noren Hooten, J. Green, E. Eitan, N. A. Mode, Q. R. Liu, A. B. Zonderman, N. Ezike, M. P. Mattson, P. Ghosh, and M. K. Evans. 2021. Mitochondrial DNA in extracellular vesicles declines with age. Aging Cell 20(1):e13283.
Linnenbringer, E., S. Gehlert, and A. T. Geronimus. 2017. Black–White disparities in breast cancer subtype: The intersection of socially patterned stress and genetic expression. AIMS Public Health 4(5):526–556.
Liu, M., A. Snow, A. S. Bhardwaj, K. Terry, T. Bressler, J. Kreitman, J. Karpin, M. Mazor, and C. B. Smith. 2023. Enhancing cancer care: Leveraging technology for proactive psychosocial and social determinants of health screenings. JCO Oncology Practice 19(Suppl 11):583–583.
Liu, F., and D. Panagiotakos. 2022. Real-world data: A brief review of the methods, applications, challenges and opportunities. BMC Medical Research Methodology 22(1):287.
Lord, B. D., A. R. Harris, and S. Ambs. 2023. The impact of social and environmental factors on cancer biology in Black Americans. Cancer Causes & Control 34(3):191–203.
Losada-Rojas, L. L., Y. Ke, V. D. Pyrialakou, and G. Konstantina. 2021. Access to healthy food in urban and rural areas: An empirical analysis. Journal of Transport & Health 23:101245.
Lund, M. J., O. P. Brawley, K. C. Ward, J. L. Young, S. S. Gabram, and J. W. Eley. 2008. Parity and disparity in first course treatment of invasive breast cancer. Breast Cancer Research and Treatment 109(3):545–557.
Luo, Y., L. E. McCullough, J. Y. Tzeng, T. Darrah, A. Vengosh, R. L. Maguire, A. Maity, C. Samuel-Hodge, S. K. Murphy, M. A. Mendez, and C. Hoyo. 2017. Maternal blood cadmium, lead and arsenic levels, nutrient combinations, and offspring birthweight. BMC Public Health 17(1):354.
Lynch, B. M., D. W. Dunstan, G. N. Healy, E. Winkler, E. Eakin, and N. Owen. 2010. Objectively measured physical activity and sedentary time of breast cancer survivors, and associations with adiposity: Findings from NHANES (2003–2006). Cancer Causes & Control 21(2):283–288.
Maksut, J. L., C. Hodge, C. D. Van, A. Razmi, and M. T. Khau. 2021. Utilization of Z Codes for Social Determinants of Health Among Medicare Fee-for-Service Beneficiaries, 2019. https://www.cms.gov/files/document/z-codes-data-highlight.pdf (accessed May 22, 2024).
Mancilla, V. J., N. C. Peeri, T. Silzer, R. Basha, M. Felini, H. P. Jones, N. Phillips, M. H. Tao, S. Thyagarajan, and J. K. Vishwanatha. 2020. Understanding the interplay between health disparities and epigenomics. Frontiers in Genetics 11:903.
Mandelblatt, J. S., N. K. Stout, C. B. Schechter, J. J. van den Broek, D. L. Miglioretti, M. Krapcho, A. Trentham-Dietz, D. Munoz, S. J. Lee, D. A. Berry, N. T. van Ravesteyn, O. Alagoz, K. Kerlikowske, A. N. Tosteson, A. M. Near, A. Hoeffken, Y. Chang, E. A. Heijnsdijk, G. Chisholm, X. Huang, H. Huang, M. A. Ergun, R. Gangnon, B. L. Sprague, S. Plevritis, E. Feuer, H. J. de Koning, and K. A. Cronin. 2016. Collaborative modeling of the benefits and harms associated with different U.S. breast cancer screening strategies. Annals of Internal Medicine 164(4):215–225.
Marfella, R., F. Prattichizzo, C. Sardu, G. Fulgenzi, L. Graciotti, T. Spadoni, N. D’Onofrio, L. Scisciola, R. L. Grotta, C. Frigé, V. Pellegrini, M. Municinò, M. Siniscalchi, F. Spinetti, G. Vigliotti, C. Vecchione, A. Carrizzo, G. Accarino, A. Squillante, G. Spaziano, D. Mirra, R. Esposito, S. Altieri, G. Falco, A. Fenti, S. Galoppo, S. Canzano, F. C. Sasso, G. Matacchione, F. Olivieri, F. Ferraraccio, I. Panarese, P. Paolisso, E. Barbato, C. Lubritto, M. L. Balestrieri, C. Mauro, A. E. Caballero, S. Rajagopalan, A. Ceriello, B. D’Agostino, P. Iovino, and G. Paolisso. 2024. Microplastics and nanoplastics in atheromas and cardiovascular events. New England Journal of Medicine 390(10):900–910.
Mathew, A., A. Z. Doorenbos, H. Li, M. K. Jang, C. G. Park, and U. G. Bronas. 2021. Allostatic load in cancer: A systematic review and mini meta-analysis. Biological Research for Nursing 23(3):341–361.
Mattes, M. D., G. Suneja, B. G. Haffty, C. Takita, M. S. Katz, N. Ohri, C. Deville, Jr., M. L. Siker, and H. S. Park. 2021. Overcoming barriers to radiation oncology access in low-resource settings in the United States. Advances in Radiation Oncology 6(6):100802.
Matthews, S. B., and H. J. Thompson. 2016. The obesity–breast cancer conundrum: An analysis of the issues. International Journal of Molecular Sciences 17(6).
McClintock, M. K., S. D. Conzen, S. Gehlert, C. Masi, and F. Olopade. 2005. Mammary cancer and social interactions: Identifying multiple environments that regulate gene expression throughout the life span. The Journals of Gerontology: Series B 60(Special_ Issue_1):32–41.
McDade, T. W., and K. M. Harris. 2022. From society to cells and back again: New opportunities for discovery at the biosocial interface. Discover Social Science and Health 2(1):4.
Miller-Kleinhenz, J. M., L. Moubadder, K. M. Beyer, Y. Zhou, A. H. Gaglioti, L. J. Collin, J. Gohar, W. Do, K. Conneely, U. Krishnamurti, K. Gogineni, S. Gabram-Mendola, O. D’Angelo, K. Henry, M. Torres, and L. E. McCullough. 2023. Redlining-associated methylation in breast tumors: The impact of contemporary structural racism on the tumor epigenome. Frontiers in Oncology 13:1154554.
Miller-Kleinhenz, J. M., L. E. Barber, M. L. Maliniak, L. Moubadder, M. Bliss, M. J. Streiff, J. M. Switchenko, K. C. Ward, and L. E. McCullough. 2024. Historical redlining, persistent mortgage discrimination, and race in breast cancer outcomes. JAMA Network Open 7(2):e2356879.
Millikan, R. C., B. Newman, C. K. Tse, P. G. Moorman, K. Conway, L. G. Dressler, L. V. Smith, M. H. Labbok, J. Geradts, J. T. Bensen, S. Jackson, S. Nyante, C. Livasy, L. Carey, H. S. Earp, and C. M. Perou. 2008. Epidemiology of basal-like breast cancer. Breast Cancer Research and Treatment 109(1):123–139.
Milliken-Smith, S., and C. M. Potter. 2021. Paternal origins of obesity: Emerging evidence for incorporating epigenetic pathways into the social determinants of health framework. Social Science and Medicine 271:112066.
Minas, T. Z., J. Candia, T. H. Dorsey, F. Baker, W. Tang, M. Kiely, C. J. Smith, A. L. Zhang, S. V. Jordan, O. M. Obadi, A. Ajao, Y. Tettey, R. B. Biritwum, A. A. Adjei, J. E. Mensah, R. N. Hoover, F. J. Jenkins, R. Kittles, A. W. Hsing, X. W. Wang, C. A. Loffredo, C. Yates, M. B. Cook, and S. Ambs. 2022. Serum proteomics links suppression of tumor immunity to ancestry and lethal prostate cancer. Nature Communications 13(1):1759.
Mirza, M., A. Shrivastava, C. Matthews, N. Leighl, C. S. H. Ng, D. Planchard, S. Popat, J. Rotow, E. F. Smit, R. Soo, M. Tsuboi, F. Yang, B. Stiles, C. Grohe, and Y. L. Wu. 2023. Treatment decision for recurrences in non-small cell lung cancer during or after adjuvant osimertinib: An international Delphi consensus report. Frontiers in Oncology 13:1330468.
Mode, N. A., M. K. Evans, and A. B. Zonderman. 2016. Race, neighborhood economic status, income inequality and mortality. PLoS One 11(5):e0154535.
Moen, M., C. Storr, D. German, E. Friedmann, and M. Johantgen. 2020. A review of tools to screen for social determinants of health in the United States: A practice brief. Population Health Management 23(6):422–429.
Moran, T. J., S. Gray, C. A. Mikosz, and S. D. Conzen. 2000. The glucocorticoid receptor mediates a survival signal in human mammary epithelial cells. Cancer Research 60(4):867–872.
Morning, A. 2017. Kaleidoscope: Contested identities and new forms of race membership. Ethnic and Racial Studies 41(6):1055–1073.
Mucci, L. A., J. Vinson, T. Gold, T. Gerke, J. Filipenko, R. M. Green, S. G. Anderson, S. Badal, A. Bjartell, K. N. Chi, I. D. Davis, D. Enting, A. P. Fay, J. Lazarus, J. Mateo, R. McDermott, F. T. Odedina, D. Olmos, A. Omlin, A. A. Popoola, C. Ragin, R. Roberts, K. M. Russnes, C. Waihenya, K. H. Stopsack, T. Hyslop, P. Villanti, P. W. Kantoff, and D. J. George, on behalf of the IRONMAN Global Team. 2022. IRONMAN: A novel international registry of men with advanced prostate cancer. JCO Global Oncology (8):e2200154.
Nardone, A., J. A. Casey, R. Morello-Frosch, M. Mujahid, J. R. Balmes, and N. Thakur. 2020. Associations between historical residential redlining and current age-adjusted rates of emergency department visits due to asthma across eight cities in California: An ecological study. The Lancet Planetary Health 4(1):e24–e31.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2017. Communities in action: Pathways to health equity. Washington, DC: The National Academies Press.
NASEM. 2019. Integrating social care into the delivery of health care: Moving upstream to improve the nation’s health. Washington, DC: The National Academies Press.
NASEM. 2020. Social isolation and loneliness in older adults: Opportunities for the health care system. Washington, DC: The National Academies Press.
NASEM. 2024. Ending unequal treatment: Strategies to achieve equitable health care and optimal health for all. Washington, DC: The National Academies Press.
NIDDK (National Institute of Diabetes and Digestive and Kidney Diseases). 2018. Insulin Resistance & Prediabetes. https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes/prediabetes-insulin-resistance#insulinresistance (accessed June 20, 2024).
NIMHD (National Institute on Minority Health and Health Disparities). 2018. NIMHD Research Framework. https://www.nimhd.nih.gov/about/overview/research-framework/nimhd-framework.html (accessed May 22, 2024).
Noren Hooten, N., and M. K. Evans. 2020. Extracellular vesicles as signaling mediators in Type 2 diabetes mellitus. American Journal of Physiology-Cell Physiology 318(6):C1189–C1199.
Obeng-Gyasi, S., M. I. Elsaid, Y. Lu, J. C. Chen, W. E. Carson, T. J. Ballinger, and B. L. Andersen. 2023. Association of allostatic load with all-cause mortality in patients with breast cancer. JAMA Network Open 6(5):e2313989.
ODPHP (Office of Disease Prevention and Health Promotion). 2020. Healthy People 2030: Building a Healthier Future for All. https://health.gov/healthypeople (accessed July 24, 2024).
OMH (Office of Minority Health). 2022. Obesity and African Americans. https://minorityhealth.hhs.gov/obesity-and-african-americans (accessed August 5, 2024).
OSUCCC (Ohio State University Comprehensive Cancer Center). 2022. Feasibility study of biobehavioral stress reduction intervention in patients with triple negative breast cancer. Bethesda, MD: National Library of Medicine. https://clinicaltrials.gov/study/NCT05677802 (accessed June 5, 2024).
Oyer, R. A., P. Hurley, L. Boehmer, S. S. Bruinooge, K. Levit, N. Barrett, A. Benson, L. A. Bernick, L. Byatt, M. Charlot, J. Crews, K. DeLeon, L. Fashoyin-Aje, E. Garrett-Mayer, J. R. Gralow, S. Green, C. E. Guerra, L. Hamroun, C. M. Hardy, B. Hempstead, S. Jeames, M. Mann, K. Matin, W. McCaskill-Stevens, J. Merrill, G. S. Nowakowski, M. I. Patel, A. Pressman, A. G. Ramirez, B. Juanita Segura, B. Segarra-Vasquez, J. H. Williams, J. E. W. Jr, K. M. Winkfield, E. S. Yang, V. Zwicker, and L. J. Pierce. 2022. Increasing racial and ethnic diversity in cancer clinical trials: An American Society of Clinical Oncology and Association of Community Cancer Centers joint research statement. Journal of Clinical Oncology 40(19):2163–2171.
Palmer, J. R., E. Viscidi, M. A. Troester, C. C. Hong, P. Schedin, T. N. Bethea, E. V. Bandera, V. Borges, C. McKinnon, C. A. Haiman, K. Lunetta, L. N. Kolonel, L. Rosenberg, A. F. Olshan, and C. B. Ambrosone. 2014. Parity, lactation, and breast cancer subtypes in African American women: Results from the AMBER Consortium. Journal of the National Cancer Institute 106(10).
Pan, D., M. Kocherginsky, and S. D. Conzen. 2011. Activation of the glucocorticoid receptor is associated with poor prognosis in estrogen receptor-negative breast cancer. Cancer Research 71(20):6360–6370.
Parente, V., L. Hale, and T. Palermo. 2013. Association between breast cancer and allostatic load by race: National Health and Nutrition Examination Survey 1999–2008. Psycho-Oncology 22(3):621–628.
Pelizzola, M., and J. R. Ecker. 2011. The DNA methylome. FEBS Letters 585(13):1994–2000.
Pierobon, M., and C. L. Frankenfeld. 2013. Obesity as a risk factor for triple-negative breast cancers: A systematic review and meta-analysis. Breast Cancer Research and Treatment 137(1):307–314.
Pittell, H., G. Calip, A. Pierre, C. Ryals, and J. Guadamuz. 2023. P5 measures of neighborhood structural racism and overall survival among patients with metastatic breast cancer. Value in Health 26(Suppl 6):S2.
Plascak, J. J., K. Beyer, X. Xu, A. M. Stroup, G. Jacob, and A. A. M. Llanos. 2022. Association between residence in historically redlined districts indicative of structural racism and racial and ethnic disparities in breast cancer outcomes. JAMA Network Open 5(7):e2220908.
Rainisch, B. K., and D. M. Upchurch. 2013. Sociodemographic correlates of allostatic load among a national sample of adolescents: Findings from the National Health and Nutrition Examination Survey, 1999–2008. Journal of Adolescent Health 53(4):506–511.
Ramsey, S. D., A. Bansal, C. R. Fedorenko, D. K. Blough, K. A. Overstreet, V. Shankaran, and P. Newcomb. 2016. Financial insolvency as a risk factor for early mortality among patients with cancer. Journal of Clinical Oncology 34(9):980–986.
Rebbeck, T. R., and G. P. Haas. 2014. Temporal trends and racial disparities in global prostate cancer prevalence. Canadian Journal of Urology 21(5):7496–7506.
Richards, M. A., A. M. Westcombe, S. B. Love, P. Littlejohns, and A. J. Ramirez. 1999. Influence of delay on survival in patients with breast cancer: A systematic review. Lancet 353(9159):1119–1126.
Rodriquez, E. J., E. N. Kim, A. E. Sumner, A. M. Nápoles, and E. J. Pérez-Stable. 2019. Allostatic load: Importance, markers, and score determination in minority and disparity populations. Journal of Urban Health 96(Suppl 1):3–11.
Ross, K. H., K. Gogineni, P. D. Subhedar, J. Y. Lin, and L. E. McCullough. 2019. Obesity and cancer treatment efficacy: Existing challenges and opportunities. Cancer 125(10):1588–1592.
Saini, G., A. Ogden, L. E. McCullough, M. Torres, P. Rida, and R. Aneja. 2019. Disadvantaged neighborhoods and racial disparity in breast cancer outcomes: The biological link. Cancer Causes & Control 30(7):677–686.
Seeman, T. E., B. H. Singer, J. W. Rowe, R. I. Horwitz, and B. S. McEwen. 1997. Price of adaptation—allostatic load and its health consequences. MacArthur studies of successful aging. Archives of Internal Medicine 157(19):2259–2268.
Semega, J., M. Kollar, C. Creamer, and A. Mohanty. 2019. Income and Poverty in the United States: 2018. P60–P266. https://www.census.gov/library/publications/2019/demo/p60-266.html (accessed June 4, 2024).
Shalabi, S. F., M. Miyano, R. W. Sayaman, J. C. Lopez, T. A. Jokela, M. E. Todhunter, S. Hinz, J. C. Garbe, M. R. Stampfer, K. Kessenbrock, V. E. Seewaldt, and M. A. LaBarge. 2021. Evidence for accelerated aging in mammary epithelia of women carrying germline BRCA1 or BRCA2 mutations. Nature Aging 1(9):838–849.
Sheinson, D. M., W. B. Wong, C. Flores, S. Ogale, and C. P. Gross. 2021a. Association between Medicare’s national coverage determination and utilization of next-generation sequencing. JCO Oncology Practice 17(11):e1774–e1784.
Sheinson, D. M., W. B. Wong, C. S. Meyer, S. Stergiopoulos, K. T. Lofgren, C. Flores, D. V. Adams, and M. E. Fleury. 2021b. Trends in use of next-generation sequencing in patients with solid tumors by race and ethnicity after implementation of the Medicare national coverage determination. JAMA Network Open 4(12):e2138219.
Shen, B., N. A. Mode, N. Noren Hooten, N. L. Pacheco, N. Ezike, A. B. Zonderman, and M. K. Evans. 2023. Association of race and poverty status with DNA methylation-based age. JAMA Network Open 6(4):e236340.
Siegel, R. L., A. Jemal, R. C. Wender, T. Gansler, J. Ma, and O. W. Brawley. 2018. An assessment of progress in cancer control. CA: A Cancer Journal for Clinicians 68(5):329–339.
Siegel, R. L., K. D. Miller, H. E. Fuchs, and A. Jemal. 2022. Cancer statistics, 2022. CA: A Cancer Journal for Clinicians 72(1):7–33.
Siegel, R. L., A. N. Giaquinto, and A. Jemal. 2024. Cancer statistics, 2024. CA: A Cancer Journal for Clinicians 74(1):12–49.
SIREN (Social Interventions Research and Evaluation Network). 2019. Social Needs Screening Tool Comparison Table. https://sirenetwork.ucsf.edu/tools-resources/resources/screening-tools-comparison (accessed May 22, 2024).
Sistrunk, C., N. Tolbert, M. D. Sanchez-Pino, L. Erhunmwunsee, N. Wright, V. Jones, T. Hyslop, G. Miranda-Carboni, E. C. Dietze, E. Martinez, S. George, A. C. Ochoa, R. A. Winn, and V. L. Seewaldt. 2022. Impact of federal, state, and local housing policies on disparities in cardiovascular disease in Black/African American men and women: From policy to pathways to biology. Frontiers in Cardiovascular Medicine 9:756734.
Subbiah, V., and R. Kurzrock. 2016. Universal genomic testing needed to win the war against cancer: Genomics is the diagnosis. JAMA Oncology 2(6):719–720.
Subbiah, V., and R. Kurzrock. 2023. Universal germline and tumor genomic testing needed to win the war against cancer: Genomics is the diagnosis. Journal of Clinical Oncology 41(17):3100–3103.
Sung, H., J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal, and F. Bray. 2021. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 71(3):209–249.
Swope, C. B., D. Hernandez, and L. J. Cushing. 2022. The relationship of historical redlining with present-day neighborhood environmental and health outcomes: A scoping review and conceptual model. Journal of Urban Health 99(6):959–983.
Taira, B. R., K. Yadav, Y. Perez, A. Aleman, L. Steinberg, G. Tchakalian, and R. Tucker-Seeley. 2023. A formative evaluation of social care integration across a safety-net health system. NEJM Catalyst 4(4).
Thames, A. D., M. R. Irwin, E. C. Breen, and S. W. Cole. 2019. Experienced discrimination and racial differences in leukocyte gene expression. Psychoneuroendocrinology 106:277–283.
Tikkanen, R., A. Shah, and E. Schneider. 2020. The role of primary care practices in screening for patient social needs in the United States and other high-income countries. Health Services Research 55(S1):63–64.
Tucker-Seeley, R. D. 2021. Social determinants of health and disparities in cancer care for Black people in the United States. JCO Oncology Practice 17(5):261–263.
Tucker-Seeley, R. D., and S. S. Shastri. 2022. Integrating Social Care into Cancer Care Delivery: Are We Ready? https://dailynews.ascopubs.org/do/integrating-social-care-into-cancer-care-delivery-we-ready (accessed May 22, 2024).
Tucker-Seeley, R., M. Abu-Khalaf, K. Bona, S. Shastri, W. Johnson, J. Phillips, A. Masood, A. Moushey, and L. Hinyard. 2024. Social determinants of health and cancer care: An ASCO policy statement. JCO Oncology Practice 20(5):621–630.
USDA (U.S. Department of Agriculture). 2020. Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services. https://www.dietaryguidelines.gov/sites/default/files/2020-07/ScientificReport_of_the_2020DietaryGuidelinesAdvisoryCommittee_first-print.pdf (accessed June 20, 2024).
van Niel, G., G. D’Angelo, and G. Raposo. 2018. Shedding light on the cell biology of extracellular vesicles. Nature Reviews: Molecular Cell Biology 19(4):213–228.
van Ravesteyn, N. T., C. B. Schechter, A. M. Near, E. A. Heijnsdijk, M. A. Stoto, G. Draisma, H. J. de Koning, and J. S. Mandelblatt. 2011. Race-specific impact of natural history, mammography screening, and adjuvant treatment on breast cancer mortality rates in the United States. Cancer Epidemiology, Biomarkers & Prevention 20(1):112–122.
VanderWalde, A., A. Grothey, D. Vaena, G. Vidal, A. ElNaggar, G. Bufalino, and L. Schwartzberg. 2020. Establishment of a molecular tumor board (MTB) and uptake of recommendations in a community setting. Journal of Personalized Medicine 10(4):252.
Vidal, C. M., J. A. Alva-Ornelas, N. Z. Chen, P. Senapati, J. Tomsic, V. M. Robles, C. Resto, N. Sanchez, A. Sanchez, T. Hyslop, N. Emwas, D. Aljaber, N. Bachelder, E. Martinez, D. Ann, V. Jones, R. A. Winn, L. Miele, A. C. Ochoa, E. C. Dietze, R. Natarajan, D. Schones, and V. L. Seewaldt. 2024. Insulin resistance in women correlates with chromatin histone lysine acetylation, inflammatory signaling, and accelerated aging. Cancers 16(15):2735.
Vidal, G. A., N. Jain, A. Fisher, D. Sheinson, K. T. Lofgren, E. Ma, E. Yu, L. Comment, R. A. Miksad, and D. B. Daniel. 2023. Practice- and provider-level inequities in next-generation sequencing (NGS) testing by race/ethnicity for patients (pts) with advanced non-small cell lung cancer (aNSCLC) treated in the community setting. Journal of Clinical Oncology 41(Suppl 16):6508.
Vidal, G. A., N. Jain, A. Fisher, D. Sheinson, K. T. Lofgren, E. Ma, E. Yu, L. Comment, R. Miksad, M. Sincan, R. L. Martin, R. Zuniga, and D. Daniel. 2024. Racial and ethnic inequities at the practice and physician levels in timely next-generation sequencing for patients with advanced non-small-cell lung cancer treated in the U.S. community setting. JCO Oncology Practice 20(3):370–377.
Vogelstein, B., N. Papadopoulos, V. E. Velculescu, S. Zhou, L. A. Diaz, Jr., and K. W. Kinzler. 2013. Cancer genome landscapes. Science 339(6127):1546–1558.
Volden, P. A., and S. D. Conzen. 2013. The influence of glucocorticoid signaling on tumor progression. Brain, Behavior, and Immunity 30(Suppl 0):S26–S31.
Wallace, D. C. 2012. Mitochondria and cancer. Nature Reviews Cancer 12(10):685–698.
Wallace, D. C. 2013. A mitochondrial bioenergetic etiology of disease. Journal of Clinical Investigation 123(4):1405–1412.
Wang, F., M. B. Skiba, S. Follis, N. Liu, A. Bidulescu, A. K. Mitra, C. P. Mouton, L. Qi, and J. Luo. 2024. Allostatic load and risk of invasive breast cancer among postmenopausal women in the U.S. Preventive Medicine 178:107817.
Ward, E. M., R. L. Sherman, S. J. Henley, A. Jemal, D. A. Siegel, E. J. Feuer, A. U. Firth, B. A. Kohler, S. Scott, J. Ma, R. N. Anderson, V. Benard, and K. A. Cronin. 2019. Annual report to the nation on the status of cancer, featuring cancer in men and women age 20–49 years. Journal of the National Cancer Institute 111(12):1279–1297.
Warnecke, R. B., A. Oh, N. Breen, S. Gehlert, E. Paskett, K. L. Tucker, N. Lurie, T. Rebbeck, J. Goodwin, J. Flack, S. Srinivasan, J. Kerner, S. Heurtin-Roberts, R. Abeles, F. L. Tyson, G. Patmios, and R. A. Hiatt. 2008. Approaching health disparities from a population perspective: The National Institutes of Health Centers for Population Health and Health Disparities. American Journal of Public Health 98(9):1608–1615.
Washington, C., D. A. Goldstein, A. Moore, U. Gardner, Jr., and C. Deville, Jr. 2022. Health disparities in prostate cancer and approaches to advance equitable care. American Society of Clinical Oncology Educational Book 42:1–6.
Weigelt, B., A. Marra, P. Selenica, E. Rios-Doria, A. Momeni-Boroujeni, M. F. Berger, K. Arora, D. Nemirovsky, A. Iasonos, D. Chakravarty, N. R. Abu-Rustum, A. Da Cruz Paula, K. Dessources, L. H. Ellenson, Y. L. Liu, C. Aghajanian, and C. L. Brown. 2023. Molecular characterization of endometrial carcinomas in Black and White patients reveals disparate drivers with therapeutic implications. Cancer Discovery 13(11):2356–2369.
White, J. A., E. T. Kaninjing, K. A. Adeniji, P. Jibrin, J. O. Obafunwa, C. N. Ogo, F. Mohammed, A. Popoola, O. A. Fatiregun, O. P. Oluwole, B. Karanam, I. Elhussin, S. Ambs, W. Tang, M. Davis, P. Polak, M. J. Campbell, K. R. Brignole, S. O. Rotimi, W. Dean-Colomb, F. T. Odedina, D. N. Martin, and C. Yates. 2022. Whole-exome sequencing of Nigerian prostate tumors from the prostate cancer transatlantic consortium (CAPTC) reveals DNA repair genes associated with African ancestry. Cancer Research Communications 2(9):1005–1016.
Wiley, J. F., T. L. Gruenewald, A. S. Karlamangla, and T. E. Seeman. 2016. Modeling multisystem physiological dysregulation. Psychosomatic Medicine 78(3):290–301.
Williams, J. B., D. Pang, B. Delgado, M. Kocherginsky, M. Tretiakova, T. Krausz, D. Pan, J. He, M. K. McClintock, and S. D. Conzen. 2009. A model of gene-environment interaction reveals altered mammary gland gene expression and increased tumor growth following social isolation. Cancer Prevention Research 2(10):850–861.
Woodruff, T. J. 2024. Health effects of fossil fuel–derived endocrine disruptors. New England Journal of Medicine 390(10):922–933.
Xing, C. Y., M. Doose, B. Qin, Y. Lin, T. L. Carson, J. J. Plascak, K. Demissie, C. C. Hong, E. V. Bandera, and A. A. M. Llanos. 2020a. Pre-diagnostic allostatic load and health-related quality of life in a cohort of Black breast cancer survivors. Breast Cancer Research and Treatment 184(3):901–914.
Xing, C. Y., M. Doose, B. Qin, Y. Lin, J. J. Plascak, C. Omene, C. He, K. Demissie, C. C. Hong, E. V. Bandera, and A. A. M. Llanos. 2020b. Prediagnostic allostatic load as a predictor of poorly differentiated and larger sized breast cancers among Black women in the Women’s Circle of Health follow-up study. Cancer Epidemiology, Biomarkers & Prevention 29(1):216–224.
Yan, A. F., Z. Chen, Y. Wang, J. A. Campbell, Q. L. Xue, M. Y. Williams, L. S. Weinhardt, and L. E. Egede. 2022. Effectiveness of social needs screening and interventions in clinical settings on utilization, cost, and clinical outcomes: A systematic review. Health Equity 6(1):454–475.
Zhang, S., K. Regan, J. Najera, M. W. Grinstaff, M. Datta, and H. T. Nia. 2023. The peritumor microenvironment: Physics and immunity. Trends in Cancer 9(8):609–623.

































































