Review of Texas Commission on Environmental Quality's Ethylene Oxide Development Support Document (2025)
Chapter: 1 Introduction
1
Introduction
The Texas Clean Air Act (Chapter 382 of the Texas Health and Safety Code) authorizes the Texas Commission on Environmental Quality (TCEQ) to prevent and remedy conditions of air pollution (TCEQ 2015). Under this Act, TCEQ is mandated to conduct air permit reviews of all new and modified facilities to ensure that the operation of a proposed facility will not cause or contribute to a condition of air pollution.
Ethylene oxide has numerous industrial applications including use as a sterilant for medical devices. Human exposure to ethylene oxide remains an occupational and public health concern. Prior hazard assessments of ethylene oxide have characterized this chemical as a direct-acting mutagen. Prior hazard assessments have also classified ethylene oxide as carcinogenic to humans. Ethylene oxide is primarily produced in Texas and Louisiana, with sites in Texas accounting for nearly half of all emitted ethylene oxide in the United States (TCEQ 2020). In 2019, there were 26 facilities that either produced, processed, or used ethylene oxide in Texas (ATSDR 2022). Because ethylene oxide is emitted in Texas and has been determined by other agencies to be a carcinogen, TCEQ undertook a carcinogenic dose-response assessment for use in its remediation and air permitting programs.
TCEQ derives short- and long-term chemical-specific toxicity factors that are set to protect the health and welfare of the public, including sensitive subgroups (TCEQ 2015). Toxicity factors developed by TCEQ include acute and chronic inhalation effects screening levels (ESLs); acute and chronic inhalation reference values, generally for noncarcinogenic effects; chronic inhalation unit risk factor (URF) values for carcinogenic effects; and chronic oral reference dose and slope factor values (TCEQ 2015). ESLs are used in the air permitting process.1 Methods used for developing toxicity factors are described in TCEQ Guidelines to Develop Toxicity Factors (TCEQ 2015). TCEQ requested that the National Academies
___________________
1 TCEQ’s evaluation of ethylene oxide resulted in the derivation of an unadjusted URF of 2.5E-06 per ppb for lymphoid cancer and a chronic non-threshold ESL of 2.4 ppb.
of Sciences, Engineering, and Medicine (National Academies) review TCEQ’s Ethylene Oxide Carcinogenic Dose-Response Assessment: Development Support Document (hereafter referred to as the TCEQ DSD). This consensus report describes the result of the National Academies’ review.
ETHYLENE OXIDE
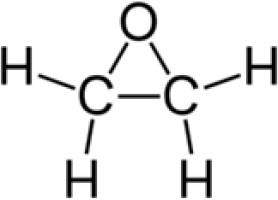
Ethylene oxide has the chemical structure shown in Figure 1-1. Ethylene oxide is a water soluble, flammable gas with a sweet odor. Ethylene oxide is used to make other compounds that are used in a wide array of products (ATSDR 2022; IARC 2012). The synthesis of ethylene glycols from ethylene oxide represents a major use of ethylene oxide (IARC 2012). Other important uses of ethylene oxide are for the sterilization of surgical equipment and medical devices, as a fumigant, and as a sterilant for certain spices and cosmetics (IARC 2012). Approximately 1,900 hospitals in the United States have ethylene oxide sterilization chambers (ATSDR 2022).
Ethylene oxide is one of the 188 hazardous air pollutants listed in the 1990 Clean Air Act Amendments. Industrial emissions, including releases from sterilization facilities, contribute to ambient air concentrations of ethylene oxide. Air samples collected at National Air Toxics Trends stations and Urban Air Toxics Monitoring Program stations reported the presence of ethylene oxide at concentrations of 0.1 to 0.2 ppb (U.S. EPA 2019), though the U.S. Environmental Protection Agency (EPA) and others have recently expressed concerns regarding the accuracy of the methods used to quantify ambient concentrations (Robinson et al. 2024; U.S. EPA 2021). Ethylene oxide air concentrations near ethylene oxide emitting sterilizer facilities in the Chicago area ranged up to 0.6 ppb (Ramboll 2019). Cigarette smoke can also result in ethylene oxide exposure to the general population. Occupational exposures to ethylene oxide concentrations that exceed ambient air levels can also occur (IARC 2012). In air, ethylene oxide undergoes oxidation via free-radical formation, with estimated half-lives of degradation of approximately one month to more than one year (ATSDR 2022).
Inhalation is the primary route of human exposure to ethylene oxide. Inhaled ethylene oxide is absorbed rapidly and distributed systemically via the blood. Lim-
ited (<10%) exhalation of ethylene oxide occurs after systemic uptake (Csanády et al. 2000). Ethylene oxide is readily metabolized via hydrolysis and glutathione conjugation, followed by urinary excretion of the glutathione conjugate—the predominant pathway in humans (Fennell and Brown 2001). Individuals with polymorphisms of the glutathione transferases, GSTT1 and GSTM1, may have increased sensitivity to ethylene oxide (Thier et al. 1999). For example, cigarette smokers with a nonfunctional GSTT1 allele have elevated internal doses of ethylene oxide (Fennell et al. 2000). In addition to inhaled exposure, trace amounts of ethylene oxide are formed endogenously from ethylene oxidation (Kirman and Hays 2017). Mice, rats, and humans exposed to similar ethylene oxide air concentrations develop tissue concentrations that are approximately equal. Tissue ethylene oxide concentrations are linearly related to relatively high inhalation concentrations of up to 100 ppm (Brown et al. 1996; Fennell and Brown 2001).
Ethylene oxide is a reactive epoxide that can directly alkylate macromolecules in the body. Ethylene oxide produces 2-hydroxyethyl adducts of DNA and proteins without metabolic activation (Hartwig et al. 2020; Jinot et al. 2018; Pottenger et al. 2019). Endogenous ethylene oxide also contributes to hemoglobin and DNA adduct formation (Jinot et al. 2018). Ethylene oxide hemoglobin adduct levels in occupationally exposed workers expressing a null GSTT1 genotype were higher than those expressing a nonnull GSTT1 genotype (Yong et al. 2001). Increased urinary excretion of 2-hydroxyethyl mercapturic acid, an ethylene oxide glutathione conjugate, was seen in hospital workers possessing a nonnull GSTT1 genotype (Haufroid et al. 2007). The mutagenic potential of the ethylene oxide–induced DNA adducts in vitro and in vivo in rodents is low (Hartwig et al. 2020). Genotoxicity of ethylene oxide occurs both in vitro and in vivo (Hartwig et al. 2020). Enhanced micronuclei formation, sister chromatid exchanges, and chromosomal aberrations have been seen in workers exposed to >0.5 ppm ethylene oxide (Ghosh and Godderis 2016; Karelová et al. 1987; Richmond et al. 1985; Sarto et al. 1984).
PRIOR CANCER ASSESSMENTS
Several studies of the carcinogenic potential of ethylene oxide have been published. Chronic inhalation cancer bioassays have been conducted in male and female B6C3F1 mice (NTP 1987), male F344 rats (Lynch et al. 1984), and male and female F344 rats (Snellings et al. 1984). Concentration-dependent increases in the incidence of malignant lymphomas, mammary gland and uterine adenocarcinomas, and lung neoplasms were reported in B6C3F1 mice (NTP 1987). Concentration-dependent increases in the incidence of splenic mononuclear cell leukemia, brain tumors, and peritoneal mesothelioma in the testes were observed in F344 rats (Garman et al. 1985; Lynch et al. 1984; Snellings et al. 1984).
Two cohort studies on cancer mortality in ethylene oxide–exposed workers were evaluated by TCEQ. These include the Union Carbide Corporation (UCC) (Teta et al. 1993) and the National Institute for Occupational Safety and Health (NIOSH) cohorts (Steenland et al. 1991). The UCC study followed
1,896 male workers exposed to ethylene oxide during chemical manufacturing, with an average cumulative exposure level of 67 ppm-years. The NIOSH study observed 18,235 male (45%) and female (55%) sterilization workers that had mean and median exposure levels of 27 and 5.6 ppm-years, respectively (see Stayner et al. 1993). Additional analyses of these study data have been published (Kirman et al. 2004; Stayner et al. 1993; Steenland et al. 2003, 2004; Valdez-Flores et al. 2010). The findings from these studies will be discussed in more detail in subsequent chapters of this report.
The International Agency for Research on Cancer (IARC) classified ethylene oxide in Group 1 (carcinogenic to humans) in 1994 (see IARC 1994). The Group 1 classification was upheld in subsequent IARC evaluations of ethylene oxide, most recently in 2012 (IARC 2012). The 2012 IARC evaluation found limited evidence in humans for a causal association with lymphatic and hematopoietic cancers and breast cancer. Even though the human evidence was not conclusive, ethylene oxide was classified as carcinogenic to humans (Group 1) because there was sufficient evidence that ethylene oxide causes cancer in experimental animals and strong evidence from exposed workers and in experimental systems that ethylene oxide, a direct-acting alkylating agent, operates by a genotoxic mechanism.
U.S. EPA also classified ethylene oxide as carcinogenic to humans (U.S. EPA 2016a,b). The U.S. EPA determination was based on less than conclusive epidemiological evidence of lymphohematopoietic cancers and breast cancer in exposed workers; evidence of lymphohematopoietic cancers in rats and mice and mammary carcinomas in mice following inhalation exposure; evidence that ethylene oxide is genotoxic and sufficient evidence supporting a mutagenic mode of action (MOA); and strong evidence that the key precursor events are anticipated to occur in humans and progress to tumors. Overall, U.S. EPA deemed its confidence in the hazard characterization of ethylene oxide as carcinogenic to humans as high. Some publications disagree with the recent hazard and mechanistic conclusions from IARC and U.S. EPA (e.g., Marsh et al. 2019; Vincent et al. 2019).
DEVELOPMENT OF TOXICITY VALUES
The procedures for developing chronic toxicity values are well established. Toxicity factors are derived from dose-response assessments of adverse health effects identified following a hazard assessment. The approach used by TCEQ to derive toxicity factors includes the following steps: review essential data and select key studies, conduct an analysis of the MOA, choose an appropriate dose metric, determine the point of departure (POD) for each key study, conduct appropriate dosimetric modeling, select a critical effect based on human equivalent exposure, and extrapolate from the adjusted POD to lower exposures based on MOA analysis (TCEQ 2015).
During the past decade, the National Academies have recommended the use of systematic review in the chemical risk assessment process. Systematic review provides a robust and transparent method to synthesize available scientific evi-
dence, especially when dealing with conflicting data or significant uncertainty, leading to more reliable and informed chemical risk assessments. A systematic review uses explicit, prespecified methods to identify, select, assess, and summarize findings of similar but separate studies to answer a focused research question. The systematic review process is undertaken to identify all relevant studies on the agent of interest; to evaluate the studies identified; and to provide a qualitative and, where possible, a quantitative synthesis of the identified studies. Figure 1-2 provides an overview of the systematic review process as it is applied to chemical hazard assessment.
THE COMMITTEE’S TASK
The committee that convened in response to TCEQ’s request included experts in environmental and occupational epidemiology, exposure assessment, leukemogenesis, mechanisms of carcinogenicity, inhalation toxicology, statistics, systematic review, and chemical risk assessment (see Appendix A for biographical information on the committee). The committee was asked to review the TCEQ DSD, plus Appendices 1–5. This document describes TCEQ’s derivation of a chronic inhalation URF for ethylene oxide. The committee was asked to review the methods, results, and conclusions of the TCEQ DSD and consider whether the conclusions are clearly presented, scientifically supported, and based on the best available scientific information. The verbatim statement of task is provided in Box 1-1.
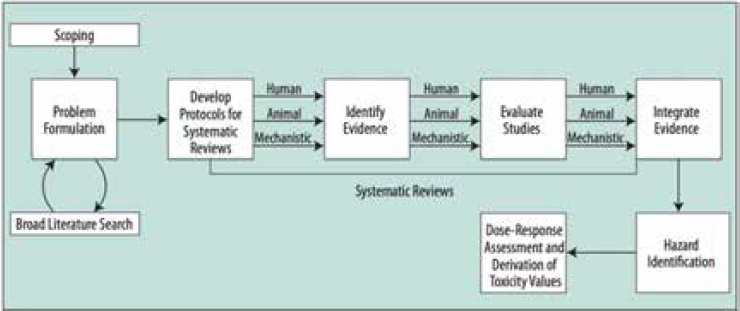
NOTE: Systematic review begins with scoping and extends through evidence integration used for hazard identification.
SOURCE: NRC (2014).
BOX 1-1
Statement of Task
An ad hoc committee of the National Academies of Sciences, Engineering, and Medicine will conduct a scientific review of the Texas Commission on Environmental Quality (TCEQ) 2020 Ethylene Oxide (EtO) Carcinogenic Dose-Response Assessment Development Support Document, plus Appendices 1-5. The committee will review the methods, results, and conclusions of TCEQ’s assessment document and consider whether the conclusions are clearly presented, scientifically supported, and based on the best available scientific information.
Aspects that will be considered during the peer review include:
- The overall weight of the evidence for a causal relationship between ethylene oxide and breast cancer risk in humans at occupational concentrations and at environmentally relevant concentrations of ethylene oxide in ambient air.
- The dose-response assessment for ethylene oxide, including:
- the appropriateness of the selected cancer endpoint(s), key data set for modeling, dose-response model, shape of the dose-response curve, and model fit criteria, in the context of relevant mode of action information;
- the model accuracy and validation analyses for lymphoid cancer and the conclusions based on those analyses, as well as the additional analyses that assumed a healthy worker effect for lymphoid cancer mortality; and
- any implications of the endogenous production of ethylene oxide, including from the perspective of biological significance, for risk-based air concentrations.
- Any additional relevant comments or issues about the TCEQ’s carcinogenic hazard and dose-response assessment of EtO.
Recommendations will be prioritized as follows:
- Tier 1: recommended revisions that are important for TCEQ to consider and address to improve critical scientific concepts, issues, or narrative in the assessment.
- Tier 2: suggested revisions that are encouraged to strengthen or clarify the scientific concepts, issues, or narrative in the assessment but are not critical. Other factors, such as agency practices and resources, might need to be considered by TCEQ before undertaking the revisions.
- Tier 3: considerations that might inform future evaluations of key science issues or inform development of future assessments.
THE COMMITTEE’S APPROACH TO THE TASK
To accomplish its task, the committee held ten meetings from March 2024 to January 2025. During the first public meeting, in April 2024, the committee heard from the sponsor in open session on the development of the TCEQ DSD and approaches used to derive the estimates presented in it. During the second public meeting, in June 2024, the committee heard a presentation from U.S. EPA regarding the development of its ethylene oxide Integrated Risk Information System (IRIS) assessment and conducted a question-and-answer session (see Appendix B) as part of its information-gathering activities. During each public session, interested parties were also provided the opportunity to address the committee. The committee also held eight closed meetings to discuss progress on this report. The committee reviewed the TCEQ DSD, numerous scientific publications, and all materials submitted to it by outside parties.
The committee was tasked with conducting a scientific review of the TCEQ DSD, not with conducting its own assessment. Therefore, the committee did not conduct its own literature search, review all relevant evidence, systematically formulate its own conclusions regarding causality, or derive or recommend values for the chronic inhalation URF. Likewise, the committee was not charged with reviewing either U.S. EPA’s IRIS assessment for ethylene oxide or TCEQ’s review of the U.S. EPA (2016a,b) assessment (Appendix 6 of the 2020 TCEQ DSD). The committee reviewed the TCEQ DSD and its methods and key literature, including relevant National Academies’ reports, and determined whether TCEQ’s conclusions were supported by that assessment and literature. Thus, the present report contains the committee’s findings and recommendations resulting from its review of the TCEQ DSD and Appendices 1-5.2 The committee notes that it does not provide a comprehensive discussion of all elements in the TCEQ DSD, although it does provide brief descriptions where necessary to give the reader some context for its recommendations.
ORGANIZATION OF THE REPORT
The committee organized its report to reflect the overarching elements of its charge. Specifically, Chapter 2 addresses TCEQ’s hazard assessment for breast cancer, and Chapter 3 reviews TCEQ’s dose-response assessment used to derive its chronic inhalation URF for ethylene oxide.
___________________
2 This sentence was changed after release of the report to clarify the scope of the committee’s review.






