The Clinical Utility of Compounded Bioidentical Hormone Therapy: A Review of Safety, Effectiveness, and Use (2020)
Chapter: Appendix B: Study Methods
Appendix B
Study Methods
LITERATURE REVIEW
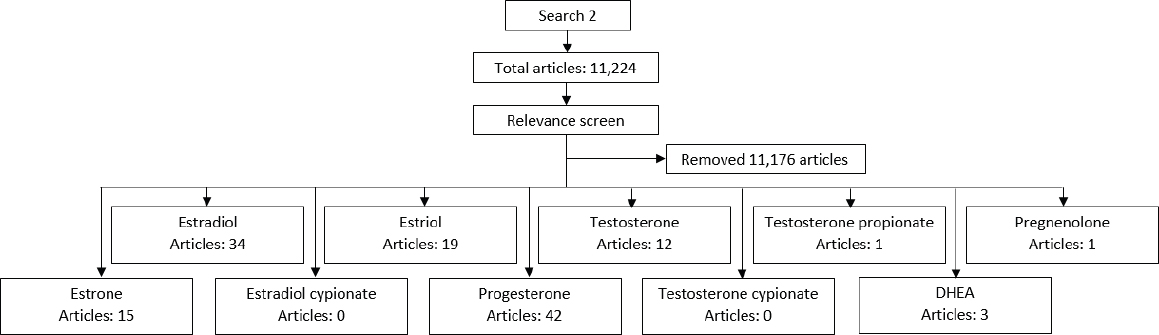
In coordination with one of the National Academies’ senior research librarians, the committee constructed a literature search strategy (see Figure B-1) that would produce an evidence-based body of research that could inform its work. A preliminary search (Search 1) queried six databases (Embase, Medline, PubMed, Scopus, Toxnet, and ClinicalTrials.gov) for content related to the safety, effectiveness, and clinical use of compounded bioidentical hormone therapy (cBHT). Results from Search 1 were limited to peer-reviewed articles published in the English language without any date restrictions, including human, animal, and in vitro studies. Editorials, commentaries, letters, and notes were excluded. This search resulted in 16,874 articles. Given the lack of specificity of the first search, in a second search (Search 2), the committee decided to expand and restrict certain search terms in order to produce a more relevant literature base. With all other search parameters remaining the same, this second search provided 11,224 articles with potential relevance to the committee’s charge.
Of these 11,224 articles, those that included the terms compounding, compounded, bioidentical, or bio-identical and one of the 10 evaluated hormones in the title, keywords, or abstract were considered by the committee. Applying these criteria provided the committee with less than 50 articles to review. Of note, there are a number of articles with relevance to multiple hormones.
In order to complement the committee’s search for literature related to the clinical utility of cBHT, the committee commissioned three additional
literature searches by the National Academies Research Center. Search 3 probed Embase, Medline, PubMed, Scopus, and Google for position statements on hormone therapy. Search 4 queried the LexisNexis database for federal and state cases, federal bills, the Federal Register, and law reviews for content related to cBHT. Search 5 explored Open Access Theses and Dissertations, ProQuest Research Library, and WorldCat Dissertations and Theses to identify theses and dissertations that could inform the committee’s understanding of the clinical use of cBHT. A further search of ClinicalTrials.gov, the European Union Clinical Trials Register, and the World Health Organization International Clinical Trials Registry Platform was performed, but no additional studies were identified from this query.1
Assessment of the Literature
Search Strategies
Preliminary Search
Date performed: February 6, 2019
Articles obtained: 16,874
Databases: Embase, Medline, PubMed, Scopus, Toxnet, ClinicalTrials.gov
Search Parameters:
1900 to present
Peer-reviewed articles
English language
International
Search Terms:
- “Bioidentical Hormone Replacement Therapy”
- Biosimilar Pharmaceuticals
- biosimilar pharmaceuticals/administration and dosage
- Hormone Replacement Therapy
- dehydroepiandrosterone
- estradiol
- estradiol cypionate
___________________
1 In addition to the formal literature searches, study stakeholders, including the U.S. Food and Drug Administration, Professional Compounding Centers of America, representatives of select 503B outsourcing facilities, nonprofit professional organizations, and practicing medical prescribers of cBHT also submitted suggested articles and other references for the committee’s review. Furthermore, during the National Academies’ external review process, additional articles were suggested by reviewers of the report. Based on the criteria described above, all relevant articles were added to the total body of collected peer-reviewed evidence.
-
- estriol
- estrogens
- estrone
- pregnenolone
- progesterone
- testosterone
- testosterone cypionate
- testosterone propionate
- Outcomes
- drug-related side effects and adverse reactions
- effectiveness, efficacy
- safety
- Drug Compounding
- Complex Mixtures
- Biological Medicines
- Specific Groups or Procedures
- adolescents (13–19 years old)
- pre and/or postmenopause
- sex reassignment
- antigens
Refined Search
Date performed: March 19, 2019
Articles obtained: 11,224
Databases: Embase, Medline, PubMed, Scopus, Toxnet, ClinicalTrials.gov
Search Parameters:
1900 to present
Peer-reviewed articles
English language
International
Search Terms:
- Hormone Replacement Therapy
- dehydroepiandrosterone
- estradiol
- estradiol cypionate
- estriol
- estrone
- pregnenolone
- progesterone
- testosterone
-
- testosterone cypionate
- testosterone propionate
- Outcomes
- drug-related side effects and adverse reactions
- effectiveness, efficacy
- safety
- Drug Compounding
- Bioidentical
- Specific Groups or Procedures
- adolescents (13–19 years old)
- pre and/or postmenopause
- sex reassignment
- autoimmune disease
- cardiovascular risk
- breast cancer
- hypoactive sexual desire
Position Statement Search
Date performed: March 19, 2019
Articles obtained: 410
Databases: Embase, Medline, PubMed, Scopus, Google
Search Terms:
- Hormone
- Position Statement
- Organization (only included in Google searches)
Legal Document Search
Date performed: April 24, 2019
Documents obtained: 5,983
Database: LexisNexis
Search Parameters:
No date restrictions
Federal and state cases, combined
Federal Register, all
U.S. law reviews and journals, combined
Congressional Record, 1985–current
Federal full text of bills
State full text of bills
Federal bill tracking
State bill tracking
Search Terms:
- Dehydroepiandrosterone
- Estradiol
- Estradiol Cypionate
- Estriol
- Estrone
- Pregnenolone
- Progesterone
- Testosterone
- Testosterone Cypionate
- Testosterone Propionate
Dissertation and Thesis Search
Date performed: July 24, 2019
Articles obtained: 62
Databases: Open Access Theses and Dissertations, ProQuest Research Library, WorldCat Dissertations and Theses
Search Parameters:
No date restrictions
Dissertations
English language
International
Human subjects
Search Terms:
- Hormone Replacement Therapy
- Hormones
- dehydroepiandrosterone
- estradiol
- estradiol cypionate
- estriol
- estrone
- pregnenolone
- progesterone
- testosterone
- testosterone cypionate
- testosterone propionate
- Drug Compounding OR Bioidentical
- Physicians’ Practice Patterns
- Attitude to Health
- Side Effects OR Effectiveness OR Safety
Clinical Trials Search
Date performed: May 29, 2019
Trials obtained: 77
Databases: ClinicalTrials.gov,
European Union Clinical Trials Register,
World Health Organization International Clinical Trials Registry Platform
Search Parameters:
No date restrictions
International
All study phases
Search Terms:
- Hormones
- dehydroepiandrosterone
- estradiol
- estradiol cypionate
- estriol
- estrone
- pregnenolone
- progesterone
- testosterone
- testosterone cypionate
- testosterone propionate
- Drug Compounding OR Bioidentical
This page intentionally left blank.