Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 17 (2014)
Chapter: 1 Acrylonitrile Acute Exposure Guideline Levels
1
Acrylonitrile1
Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could
_____________________________
1This document was prepared by the AEGL Development Team composed of Robert Young (Oak Ridge National Laboratory), Gary Diamond (SRC, Inc.), Julie Klotzbach (SRC, Inc.), Chemical Manager Susan Ripple (National Advisory Committee [NAC] on Acute Exposure Guideline Levels for Hazardous Substances), and Ernest V. Falke (U.S. Environmental Protection Agency). The NAC reviewed and revised the document and AEGLs as deemed necessary. Both the document and the AEGL values were then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC committee has concluded that the AEGLs developed in this document are scientifically valid conclusions based on the data reviewed by the NRC and are consistent with the NRC guidelines reports (NRC 1993, 2001).
experience notable discomfort, irritation, or certain asymptomatic, nonsensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure concentrations that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold concentrations for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Acrylonitrile is a monomer used in the manufacture of acrylic fibers, synthetic rubber, resins, plastics, adhesives, and acrylamide. Acrylonitrile has a sharp onion-garlic odor. Worldwide production is estimated at 4-4.5 million metric tons. The odor threshold for acrylonitrile ranges from 1.6 to 36.3 ppm. A level of distinct odor awareness of 145 ppm was calculated for acrylonitrile.
Nonlethal effects of occupational exposure to acrylonitrile include headache, nasal and ocular irritation, thoracic discomfort, nervousness, and irritability. Information from occupational studies indicates that these effects have occurred at exposures of 16-100 ppm for 20-45 min. Workers routinely exposed to acrylonitrile at 5 ppm experienced initial conjunctival irritation followed by some degree of accommodation, and routine exposure at 5-20 ppm resulted in complaints of headache, fatigue, nausea, and insomnia. No signs or symptoms were reported by informed male volunteers after exposure to acrylonitrile at up to 4.6 ppm for 8 h. Lethality following acute inhalation exposure to acrylonitrile has been reported, but exposures were not defined.
Acute exposure data are available for several laboratory species (monkey, rat, dog, rabbit, guinea pig, and cat) and demonstrate qualitatively similar responses between species, ranging from mild irritation (redness of exposed skin, lacrimation, and nasal discharge) and mild effects on ventilation and cardiovas-
cular responses to severe respiratory effects, convulsions, and death. A 4-h exposure to acrylonitrile at 30-100 ppm produced little or no effect in most species tested, but dogs appeared to be notably more sensitive, exhibiting severe effects at the 100 ppm. Developmental toxicity studies conducted in rats found nonlethal effects on fetal development that included decrements in fetal body weight without fetal malformations (25-100 ppm) (Saillenfait et al. 1993a) and nonlethal fetal malformations (40 and 80 ppm) (Murray et al. 1978). Murray et al. (1978) found three malformations in two of 33 liters from dams exposed at 40 ppm and 11 malformations in six of 35 litters from dams exposed at 80 ppm. The most serious malformation was one omphalocele at 40 and 80 ppm. These malformations were not confirmed in the Saillenfait et al. (1993a) study at exposures up to 100 ppm. A two-generation study found weight decrements in F1 offspring of the 90-ppm group, but no other evidence of exposure-related mortalities in adult animals, effects on reproduction or reproductive organs, or toxicity in developing offspring at exposures up to 90 ppm (Nemec et al. 2008). No effects on resorptions or live births were found in the single-generation or two-generation studies.
Lethality in rats appears to occur at cumulative exposure of 1,800-1,900 ppm-h for 30 min to 6 h, although for nose-only exposures it was notably higher (about 3,800 ppm-h). Analysis of exposure concentration-duration data suggest a near linear relationship (Cn × t = k, where n = 1.1; ten Berge et al. 1986). Results of studies in animals showed that lethality may be delayed especially at the lower limits of lethal exposures. One study provided evidence of teratogenic effects in rats following gestational exposure of dams to acrylonitrile at 80 ppm but not at 40 ppm. Another study showed an exposure-related decrease in fetal weight following gestational exposure of dams at 25, 50, or 100 ppm; no other reproductive or developmental effects were detected. Acrylonitrile toxicity appears to be directly related to its metabolism. Two major metabolic pathways have been described: conjugation with glutathione and epoxidation by microsomal cytochrome P450 2E1, which forms 2-cyanoethylene oxide (CEO). Metabolites from both pathways are subject to additional biotransformation. The glutathione conjugate may form a mercapturic acid which is excreted in urine. CEO is further metabolized via conjugation with glutathione (catalysis with cytosolic glutathione S-transferase [GST] or nonenzymatically) resulting in additional conjugates and via hydrolysis by microsomal epoxide hydrolase (EH). The secondary metabolites of CEO may also be further metabolized. Cyanide may be generated via the EH pathway and by one of the glutathione (GSH) conjugation products. Cyanide, in turn, is detoxified to thiocyanate via rhodanese-mediated reactions with thiosulfate.
Results of genotoxicity studies are mixed, but provide evidence that acrylonitrile is genotoxic, with positive results in in vitro (DNA strand breaks, sister chromatid exchange [SCE], chromosomal aberrations, and cell transformations) and in vivo (DNA damage, SCE, chromosomal aberrations, and micronuclei) models. The overall weight of evidence supports the conclusion that acrylonitrile is genotoxic. Results of long-term inhalation exposure cancer bioassays
have shown that acrylonitrile is carcinogenic in rats, with brain, spinal cord, Zymbal’s gland, tongue, small intestines and mammary glands identified as targets. Available data are sufficient for considering acrylonitrile to be carcinogenic in animals following chronic inhalation exposure.
The AEGL-1 values for acrylonitrile are based on the absence of effects in informed human volunteer (six males) exposed to acrylonitrile at 4.6 ppm for 8 h (Jakubowski et al. 1987), supported by observations of mild effects (initial conjunctival irritation, for which there was some accommodation) in workers routinely exposed at approximately 5 ppm (Sakurai et al. 1978). Therefore, the 8-h exposure at 4.6 ppm is considered a no-effect level for notable discomfort and a point-of-departure for deriving AEGL-1 values. That concentration is approximately 3-fold lower than concentrations reported by Wilson et al. (1948) to be associated with more severe effects in occupational settings (16-100 ppm for 20-45 min: headache, nasal and ocular irritation, discomfort of the chest, nervousness, and irritability). Pharmacokinetic variability is not likely to be significant for mild effects (ocular irritation) of low-level exposure. However, the point-of-departure is based on studies of healthy adults and, in the occupational studies, subjects who experienced repeated exposures to acrylonitrile, which may have resulted in some accommodation to the ocular irritation. Therefore, an intraspecies uncertainty factor of 3 was applied. No data are available on the relationship between exposure duration and severity of responses to acrylonitrile. Typically, in the absence of this information, AEGL-1 values based on an 8-h point-of-departure would be time scaled. However, in this case, the effect is ocular irritation, which would not be expected to have a response threshold that varies with exposure duration. Therefore, it is prudent to not time scale and the AEGL-1 values were held constant at 1.5 ppm for exposure durations of 10 and 30 min. However, 1.5 ppm exceeds AEGL-2 values for longer exposure durations; therefore, AEGL-1 values for 1 h, 4 h, and 8 h are not recommended.
The AEGL-2 values for acrylonitrile are based a developmental toxicity study conducted in rats, which showed that 12 ppm (6 h/day, gestation days 6-20) was a no-effect level for fetal toxicity, indicated by decrements in fetal body weight at higher concentrations (25-100 ppm). Support for the point-of-departure is provided from studies conducted in rats and monkeys. In monkeys, slight or modest reversible effects (transient skin flushing and elevation of respiration rates) were observed from 4-h exposures to acrylonitrile at 65 or 90 ppm (Dudley and Neal 1942). Slight transient effects were found in rats exposed to acrylonitrile at 305 ppm for 2 h (Dudley and Neal 1942). The effects resolved within 12 h postexposure. At higher concentrations or longer exposure durations, effects were more severe (rapid respiration, tremors, convulsions, and death). A threshold for these more severe effects in the rat appears to be above 305 ppm and below the threshold for lethality (the 2-h BMCL05 [benchmark concentration, 95% lower confidence limit at the 5% response rate] is 491 ppm) in the rat. An interspecies uncertainty factor of 6 (3 × 2) was applied; a factor of 3 accounts for possible species differences in toxicodynamics of acrylonitrile and a factor of 2 accounts for interspecies differences in toxicokinetics. On the
basis of BPK modeling, Sweeney et al. (2003) predicted a 2-fold difference the concentrations of acrylonitrile and its metabolite, cyanoethylene oxide (the metabolic precursor to cyanide), in blood and brain during 8-h exposures at 2 ppm. Higher cyanoethylene oxide concentrations were predicted in human blood and brain than in rats. A PBPK model developed by Takano et al. (2010) used data on in vitro metabolism of acrylonitrile in rat and human liver microsomes to estimate hepatic clearance of cyanoethylene oxide. The model predicted that repeated oral exposures to acrylonitrile at 30 mg/kg/day for 14 days would result in peak blood acrylonitrile concentrations that were approximately 2-fold higher in rats than humans. Although the Takano et al. (2010) model was evaluated using oral exposure data, experimental data for metabolism were obtained from in vitro microsome studies. Taken together, the Sweeney et al. (2003) and Takano et al. (2010) PBPK models support application of an interspecies uncertainty factor of 2 to account for differences in toxicokinetics. An intraspecies uncertainty factor of 6 (3 × 2) was applied; a factor of 3 for possible variation in toxicodynamics of acrylonitrile in the human population and a factor of 2 for variability in toxicokinetics. On the basis of PBPK modeling, Sweeney et al. (2003) predicted that human variability in toxicokinetics of acrylonitrile would result in the 95th percentile individual having acrylonitrile or cyanoethylene oxide concentrations in blood 1.8-fold higher than the average (mean) individual. This suggests that an intraspecies uncertainty factor of 2 would account for toxicokinetics variability in the human population. The total uncertainty factor was 36 (6 × 6). Time scaling from the 6-h experimental point-of-departure to AEGL-specific exposure durations was performed using the equation Cn × t = k, where n = 1.1 (ten Berge et al. 1986). Analysis of occupational exposures and effects indicated that routine exposure to acrylonitrile at 5-20 ppm resulted in complaints of headache, fatigue, nausea, and insomnia, which were neither irreversible nor escape-impairing effects. The concentrations range is approximately 20-to-80 fold higher than the 8-h AEGL-2, which suggests that 8-h AEGL-2 is sufficiently protective.
The AEGL-3 values were derived using 30-min, 1-h, 4-h, and 8-h BMCL05 estimates of lethality thresholds. Data for several AEGL-specific exposure periods were available from the reports by Appel et al. (1981a) and Dudley and Neal (1942). A 30-min BMCL05 of 1,748 ppm was calculated from the Appel et al. (1981a) data. The 1-, 2-, 4-, and 8-h BMCL05 values derived from rat lethality data published by Dudley and Neal (1942) are 1,024.4, 491.3, 179.5, and 185.8 ppm, respectively. With the exception of the 4-h value, the data show a consistent duration-dependent relationship; therefore, the 30-min, 1-h, and 8-h estimates were used to derive corresponding AEGL-3 values. Because the 4-h BMCL05 was essentially equivalent to the 8-h BMCL05, the 4-h AEGL-3 value was derived by time-scaling the 8-h BMCL05. The 10-min AEGL-3 value was derived by time-scaling from the 30-min rat BMCL05. Time scaling was performed using the equation Cn × t = k, where n = 1.1 (ten Berge et al. 1986). Although the dog appeared to be the most sensitive species, the overall database for
rats is more robust. The same uncertainty factors that were used to derive the AEGL-2 values were applied to the AEGL-3 values because the same toxicodynamic and toxicokinetic factors apply to both AEGl-2 and AEGL-3 dose-response relationships. An interspecies uncertainty factor of 6 (3 × 2) and an intraspecies uncertainty factor of 6 (3 × 2) were applied, for a total uncertainty factor of 36 (6 × 6).
The AEGL values for acrylonitrile are presented in Table 1-1.
1. INTRODUCTION
Acrylonitrile is a monomer used in the manufacture of acrylic fibers, synthetic rubber, resins, plastics, adhesives, and acrylamide. Acrylonitrile has a sharp onion-garlic odor. Worldwide production has been estimated at 4-4.5 million metric tons (Collins et al. 2003; NPI 2006). Production of acrylonitrile in the United States was 3.4 billion pounds in 1996 (NTP 2011). Chemical and physical data for acrylonitrile is presented in Table 1-2.
AIHA (1997) lists an odor threshold range of 1.6-21 ppm for acrylonitrile, and Ruth (1986) reported a range of 3.7-36.3 ppm. A level of distinct odor awareness of 145 ppm was calculated for acrylonitrile (see Appendix A).
TABLE 1-1 AEGL Values for Acrylonitrile
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) |
| AEGL-1 (nondisabling) | 1.5 ppm (3.3 mg/m3) | 1.5 ppm (3.3 mg/m3) | NRa | NRa | NRa | No-effect level for notable discomfort (ocular irritation) in human subjects, 4.6 ppm for 8 h (Sakurai et al. 1978; Jakubowski et al. 1987). |
| AEGL-2 (disabling) | 8.6 ppm (19 mg/m3) | 3.2 ppm (6.9 mg/m3) | 1.7 ppm (3.7 mg/m3) | 0.48 ppm (1.0 mg/m3) | 0.26 ppm (0.56 mg/m3) | No-effect level for fetal toxicity (fetal body weight) in rats, 12 ppm for 6 h (Saillenfait et al. 1993a). |
| AEGL-3 (lethal) | 130 ppm (280 mg/m3) | 50 ppm (110 mg/m3) | 28 ppm (61 mg/m3) | 9.7 ppm (21 mg/m3) | 5.2 ppm (11 mg/m3) | No-effect level for lethality (30-min, 1-h, and 8-h BMCL05) in rats (Dudley and Neal 1942; Appel et al. 1981a). |
aNot recommended. Absence of an AEGL-1 value does not imply that exposure at concentrations below the AEGL-2 value is without effect.
TABLE 1-2 Chemical and Physical Data for Acrylonitrile
| Parameter | Value | Reference |
| Synonyms | 2-propenenitrile; vinyl cyanide; acrylonitrile monomer; cyanoethylene | HSDB 2013 |
| CAS registry no. | 107-13-1 | HSDB 2013 |
| Chemical formula | C3H3N | HSDB 2013 |
| Molecular weight | 53.06 | HSDB 2013 |
| Physical state | Liquid | HSDB 2013 |
| Melting point | -82°C | HSDB 2013 |
| Boiling point | 77.3°C | HSDB 2013 |
| Density/specific gravity | 0.8 at 23°C/4°C | HSDB 2013 |
| Solubility in water | 74.5 g/L at 25°C | HSDB 2013 |
| Vapor density | 1.8 (air = 1) | HSDB 2013 |
| Vapor pressure | 109 mmHg at 25°C | HSDB 2013 |
| Conversion factors in air | 1 ppm = 2.17 mg/m3 1 mg/m3 = 0.46 ppm |
NIOSH 2011 |
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
A child exposed overnight in a room fumigated with acrylonitrile died. Vomiting, lacrimation, convulsions, respiratory difficulty, cyanosis, and tachycardia were present. Five adults also in the room experienced little or no effect (see Section 2.2.) (Grunske 1949). No exposure concentration-duration information was reported. Another case study involved the death of a 10-year-old girl who had a delousing agent containing acrylonitrile applied to her scalp (Lorz 1950). Following dermal application of the delousing agent, the girl’s head was wrapped in a cloth and she went to bed. Symptoms of nausea, headache, and dizziness were followed by repeated vomiting and coma. Cramps and increasing cyanosis were followed by death 4 h after application.
Loss of consciousness, convulsions, and respiratory arrest have been reported as outcomes of severe acute inhalation exposure to acrylonitrile (Buchter and Peter 1984). However, no exposure details were available.
The death of a worker cleaning an acrylonitrile-containing wagon at a train depot was attributed to exposure to the chemical (Bader and Wrbitzky 2006). No exposure data were available, although liquid acrylonitrile was present on the clothing of the individual. Cause of death was reportedly “blood circulation collapse”.
2.2. Nonlethal Toxicity
Wilson et al. (1948) reported that exposure of workers handling “polymerizers” at concentrations of 16-100 ppm for 20-45 min experienced dull head-
aches, nasal and ocular irritation, discomfort in the chest, nervousness, and irritability. Workers with notable poisoning (exposures not reported) experienced nausea, vomiting, and weakness. Some developed mild jaundice, low-grade anemia, and leukocytosis. No exposure details were provided for the workers with these more serious effects, but all recovered upon removal from exposure.
Five adults who spent the night in the room in which a child died of acrylonitrile poisoning (see Section 2.1.) had no signs of poisoning and complained only of ocular irritation (Grunske 1949). No exposure concentration-duration information was reported.
Lacrimation and visual disturbance were reported in some nonfatal exposures to acrylonitrile (Davis et al. 1973). Although exposure concentrations were not reported, these effects were likely associated with very high acrylonitrile concentrations.
In an analysis of 144 case reports of acute acrylonitrile poisoning, Chen et al. (1999) estimated that 60 cases were exposed to concentrations in the range of 18-258 ppm (40-560 mg/m3) and the remaining 84 cases were exposed at concentrations greater than 460 ppm (1000 mg/m3). Air measurements were not made at the time of the accident and were estimated from accident simulations and postaccident measurements (5 h after the accident). Subjective symptoms reported for 92-100% of the cases included dizziness, headache, chest tightness, feebleness, and hyperactive knee jerk. Sore throat, dyspnea, vomiting, abdominal pain, fainting, and congestion of the pharynx were reported in 60-87% of cases. Other less frequently reported symptoms or effects (5-32% of cases) included numbness of limbs, convulsion, rapid heart rate, cough, hoarseness, rough breathing sound, coma, and abnormal liver function (Chen et al. 1999).
Subchronic (about 3 years) occupational exposure to acrylonitrile at concentrations ranging from 0.6 to 6.0 mg/m3 (0.3 to 3 ppm) produced headaches, insomnia, general weakness, decreased working capacity, and irritability (Babanov et al. 1959).
In a report by Sakurai and Kusumoto (1972), the health records of 576 workers working in five acrylonitrile fiber plants over a 10-year period were examined. The report analyzed 4,439 examinations acquired over 10 years before 1970. Two cohorts, one exposed to concentrations of acrylonitrile below 11 mg/m3 (5 ppm) and the other exposed to less than 45 mg/m3 (20 ppm), were considered. Workers exposed to acrylonitrile at concentrations of 11 mg/m3 (5 ppm) complained of headache, fatigue, nausea, and insomnia. There was a positive correlation with exposure duration but not with the exposure concentration or age of workers. In a later report, however, Sakurai et al. (1978) stated that the study lacked adequate epidemiologic design, the findings were based on routine health examinations, and the “exposure levels were not reliably reported” and may have been much higher. In this later appraisal it was noted that many of the symptoms reported in Sakurai and Kusumoto (1972) were associated with exposures well in excess of 5 ppm. Sakurai et al. (1978) examined health records for 608 acrylonitrile fiber factory workers. Subjects were grouped into three cohorts that had median air concentrations (from spot samples) of approximately
<1 ppm, 1 ppm, and 5 ppm. They reported that “many workers” complained of initial conjunctival irritation and respiratory irritation and for which there was some accommodation; however, these effects were not attributed to specific exposure cohorts. Sakurai et al. (1978) stated that their findings were not contradictory to those of Wilson et al. (1948), because they reflected the older and less controlled workplace environment where concentrations could have been up to 20 ppm. Taken together, the Sakurai and Kusumoto (1972) and Sakurai et al. (1978) studies suggest mild and transient ocular irritation in association with exposures at 5 ppm (or less), with more severe outcomes (headache, fatigue, nausea, and insomnia) in association with higher exposures (5-20 ppm).
In cross-sectional studies of acrylonitrile-exposed workers, subjective symptoms reported with increased prevalence compared with unexposed workers included dizziness, headache, chest tightness, poor memory, irritation, and neurologic effects. Average workplace air concentrations associated with increased prevalence of these subjective symptoms were 1.13 ppm (Muto et al. 1992), 1.8 ppm (Kaneko and Omae 1992), and 0.48 ppm (Chen et al. 2000). Rongzhu et al. (2005) reported statistically significant deficits in several neurobehavioral tests measured in exposed workers in a Chinese acrylic fiber manufacturing plant with mean workplace air concentrations of 0.11 ppm (0-1.70 ppm) and 0.91 ppm (range 0-8.34 ppm) in two different process areas. Deficits in exposed workers compared with nonexposed workers were noted in a profile of mood states test (20-68% higher for negative moods such as anger and confusion), a simple reaction time test of attention and response speed (10-16% deficits), and the backward sequence of the digit span test of auditory memory (21-24% deficits).
Ocular irritation was a primary effect in a 24-year old man whose face, eyes, and body were sprayed by acrylonitrile (no concentration data) explosively released from a defective valve (Vogel and Kirkendall 1984). Mild conjunctivitis with no corneal clouding was reported. Results of fundascopic examination were normal.
A study was conducted to evaluate the metabolism and excretion of acrylonitrile in informed volunteer subjects (Jakubowski et al. 1987). The six volunteers (including the investigators) were all males aged 28-45 years. Being toxicologists, they were all aware of the toxic properties of acrylonitrile. The subjects were exposed for 8 h to acrylonitrile vapors generated by a saturator immersed in a thermostat-controlled water bath and diluted with carrier air to produce the desired acrylonitrile concentrations (5 or 10 mg/m3; equivalent to 2.3 and 4.6 ppm, respectively). Airflow in the 11.7-m3 chamber was approximately 200 m3/h. There were three 10-min breaks from the exposure at 2, 4, and 6 h. Gas chromatography was used to monitor the acrylonitrile concentration every 15 min. No symptoms were reported by any of the subjects. Limitations of the Jakubowski et al. (1987) study are that the objective of the study was to collect data on the toxicokinetics of acrylonitrile and not to evaluate health effects. All of the subjects were informed toxicologists who worked in the laboratory in
which the study was performed (stakeholders) and may have been more tolerant of mild irritant effects than less motivated individuals.
The World Health Organization (WHO 1983) summarized various workplace studies (Zotova 1975; Enikeeva et al. 1976; Delivanova et al.1978; Ivanov, State Medical Institute, Krasnoyarsk, USSR, personal commun. 1983). Blepharoconjunctivitis was reported following exposure to acrylonitrile at 5 ppm. Other nonocular symptoms were also reported.
Gincheva et al. (1977) reported no changes in the health status for a group of 23 men occupationally exposed to acrylonitrile at 1.9-3.3 ppm for 3-5 years.
2.3. Developmental and Reproductive Effects
Xu et al. (2003) reported that workers exposed to mean acrylonitrile concentration of 0.8 mg/m3 (0.37 ppm) had a significant decrease (46%) in sperm density when compared with unexposed controls. In addition, DNA strand breakage and sex chromosome aneuploidy were significantly increased in the sperm cells of exposed workers. Xu et al. (2003) stated that aneuploidy transmitted via germ cells is a major contributor to infertility, spontaneous abortion, stillbirths, and infant death.
Reproductive outcomes in workers exposed to acrylonitrile were evaluated by Dong and Pan (1995) and Dong et al. (2000). Several inconsistencies were noted in the reports. The following incidence values correct for inconsistencies between tables and text in the original study reports. Dong and Pan (1995) reported statistically significantly increased incidences of adverse reproductive outcomes in acrylic fiber workers exposed to an average acrylonitrile concentration of 3.7 ppm for 3.2-10.2 years when compared with unexposed controls. These adverse outcomes included premature delivery (10.7% vs. 3.5%) and sterility (5.0% vs. 1.8%) in exposed males compared with controls and stillbirths (4.5% vs. 0%) in exposed females compared with controls.
Dong et al. (2000) reported statistically significantly increased incidences of adverse reproductive outcomes in female acrylic fiber workers exposed to an average acrylonitrile concentration of 3.7 ppm for 10.4 years. Adverse outcomes included increased stillbirths (2.66% vs. 0.68%), birth defects (1.93% vs. 0.45%), and premature deliveries (8.23% vs. 3.87%) compared with controls.
A reported decreased in testosterone in acrylonitrile factory workers (Ivanescu et al. 1990) was confounded by concurrent exposure to other chemicals. No adverse effect was detected for gynecological health of 410 women occupationally exposed to acrylonitrile (no exposure details) compared with 436 unexposed women (Dorodnova 1976). Czeizel et al. (1999) reported on the rate and type of congenital abnormalities in 46,326 infants born to mothers living within a 25-km radius of an acrylonitrile factory in Hungary. Significant clusters of pectus excavatum (depressed sternum), undescended testes, and clubfoot were noted. The authors, however, reported that the overall results supported the null hypothesis of no effects of acrylonitrile in people living in the vicinity of the acrylonitrile factory.
2.4. Genotoxicity
2.4.1. In Vitro Studies
In experiments with human lymphocytes, Perocco et al. (1982) showed that exposure of human lymphocytes to acrylonitrile at 0.5 mM (26.5 μg/mL) resulted in a significant increase in sister chromatid exchange (SCE). Obe et al. (1985), however, was unable to demonstrate SCE-induction by acrylonitrile in human lymphocytes exposed for 24 h to acrylonitrile at concentrations of 1 or 10 μg/mL in the absence of S9 and for 1 h in the presence of S9 from Arochlor-induced rat livers.
Rizzi et al. (1984) examined the incorporation of [3H]TdR into DNA in HeLa cells. The test groups included a control and acrylonitrile-treated cells without hydroxyurea (-HU), and control and treated cells treated with hydroxyurea (+HU). The -HU/+HU relationship between treated and control cells and the value of +HU between treated and control cells were statistically significant at acrylonitrile concentrations of 0.18 (p < 0.01) and 0.036 mM (p < 0.09). It was concluded that acrylonitrile is mutagenic and genotoxic at very low concentrations. Contrary to this, Martin and Campbell (1985) failed to demonstrate unscheduled DNA repair in HeLa cells.
Acrylonitrile produced positive results in tests with human lymphoblasts (TK6, TK locus) both with and without metabolic activation (Crespi et al. 1985). Tests were conducted at acrylonitrile concentrations of 5-50 μg/mL for 3 h in the presence of S9 (from Arochlor-induced rat livers) or for 20 h without S9. There was a 3.5-fold increase in mutational frequency in the presence of S9 at 40 and 50 μg/mL. In the absence of S9, mutational frequency was increased 2-fold at 15 μg/mL and 1.3-fold at 20 μg/mL (compared with controls).
Crespi et al. (1985) also conducted tests using the AHH-1 cell line (HGPRT locus). Concentrations of acrylonitrile were 5-25 μg/mL for 28 h. Tests were conducted with metabolic activation and an expression period of 6 days. An approximate 4.5-fold increase in mutation frequency at 25 μg/mL was detected relative to controls which was similar to the response obtained with the benzo(a)pyrene (3.1 μg/mL, positive control).
The mutagenic potential of both acrylonitrile and its metabolite 2-cyanoethylene oxide (CEO) was examined using the TK human lymphoblast cell line (with and without S9) with heterozygous thymidine kinase (tk) locus as the marker (Recio et al. 1989). Cells were exposed for 2 h with an expression period of 6-8 days. Acrylonitrile was not mutagenic in the absence of S9 (less than a 2-fold increase in mutation frequency) over a concentration range of 0.4 to 1.5 mM (21 to 80 μg/mL). With S9, there was a statistically significant (p < 0.05) 4-fold mutagenic response at the highest concentration 1.5 mM (74 μg/mL). Survival was only 10% at 1.5 mM. The metabolite produced a 17-fold increase in mutation frequency without S9 at 100 μM. The results indicated acrylonitrile to be weakly mutagenic in mammalian cells, while the mutagenic response induced by CEO suggests that it may be the primary mutagenic metab-
olite of acrylonitrile. In a follow-up study (Recio et al. 1990), human TK6 lymphoblasts were treated with CEO (150 µM for 2 h). Base-pair substitution mutations and frameshift mutations were observed.
SCE and the induction of DNA single breaks were examined using adult human bronchial epithelial cells (Chang et al. 1990). The cultures were exposed for 20 h to acrylonitrile at 150, 300, 500, or 600 μg/mL and assessed for SCE and DNA strand breaks. Notable cytotoxicity was observed at 600 μg/mL, but not at the lower concentrations. SCEs were significantly increased (p < 0.01) at 150 and 300 μg/mL; incidence of SCE per cell was 6.6 and 10.7, respectively (3.7 in unexposed controls). The extent of DNA single strand breaks appeared to be positively correlated with acrylonitrile concentrations.
A human mammary epithelial cell (HMEC) DNA repair assay in secondary cultures of HMEC was reported by Butterworth et al. (1992). The cultures of normal HMEC were derived from mammoplasties of five healthy women. Although CEO was cytotoxic to HMEC at 1.0 mM, a positive unscheduled DNA synthesis response at 0.1 mM was produced thereby confirming its genotoxicity at subcytotoxic doses. Acrylonitrile exhibited considerable cytotoxicity but no genotoxicity was observed in the HMEC DNA repair assay.
2.4.2. In Vivo Studies
Beskid et al. (2006) noted moderate changes in chromosomal aberration patterns in chromosomes #1 and #4 as detected by the FISH assay in workers occupationally exposed to acrylonitrile compared with unexposed controls. In this study, smoking did not seem to have any effect on the pattern of aberrations detected.
Fan et al. (2006) detected increases in micronucleus formation in buccal mucosal cell and lymphocyte samples from both the low and intermediate exposure groups (concentrations not reported) of male workers in Shanghai, China when compared to matched unexposed males. They also noted a strong correlation between these findings and assays performed in the buccal mucosal cells and the circulating lymphocytes.
Xu et al. (2003) found that acrylonitrile had an effect on semen quality among exposed workers by inducing DNA strand breakage as detected by the Comet assay and sex chromosome nondisjunction in spermatogenesis as detected in the FISH assay. They also reported lower sperm counts in the exposed versus nonexposed subjects. The workers were employed by a recently opened plant (2.8 years exposure duration for all workers), which had a mean acrylonitrile concentration of 0.8 ± 0.25 mg/m3.
Chromosomal damage in peripheral lymphocytes of 18 workers exposed to acrylonitrile for an average of 15.4 years was studied by Thiess and Fleig (1978). The workers were also exposed to styrene, ethylbenzene, butadiene, and butylacrylate. The actual acrylonitrile exposure was not reported. Air concentrations of acrylonitrile over approximately 10 years averaged 5 ppm and were
reportedly representative of normal operating conditions. During the actual conduct of the study, workplace concentrations of acrylonitrile were about 1.5 ppm. The frequency of chromosomal aberrations in peripheral lymphocytes of the workers was not increased compared with the unexposed controls.
Borba et al. (1996) reported chromosomal aberrations and SCEs in 14 workers employed in the polymerization area and in 12 maintenance workers of an acrylic fiber plant. A control group consisted of 20 unexposed workers in administration jobs. No acrylonitrile exposure concentration or exposure duration terms were provided. No difference in SCEs was detected when the exposed groups and the controls were compared.
2.5. Carcinogenicity
Several occupational studies have evaluated the potential carcinogenicity of acrylonitrile, with mixed results. Many earlier studies reporting a positive association between acrylonitrile exposure and increased cancer risk were limited by inadequate exposure data, small study populations, insufficient length of follow-up, and other confounding factors (e.g., concomitant exposure to other chemicals, smoking). More recent occupational studies generally examined larger cohorts and had longer follow-up periods. Although results of more recent studies are also mixed, Blair et al. (1998) reported an increased risk of lung cancer mortality in large cohort of workers exposed to high concentrations of acrylonitrile (additional study details provided below).
EPA’s Integrated Risk Information System (IRIS) has an inhalation unit risk for acrylonitrile of 6.8 × 10-5 (μg/m3)-1, which is based on an excess incidence of respiratory cancer from an occupational study (O’Berg 1980). The inhalation unit risk was developed in 1983 (EPA 1984). However, a follow-up study (O’Berg et al. 1985) did not find an increased incidence of respiratory cancer in this cohort. The IRIS Program is currently reassessing this chemical. The availability of an inhalation unit risk requires that calculations of cancer risk from a single exposure to acrylonitrile be presented in an appendix to this document (NRC 2001). The calculations of cancer risk for a single exposure to acrylonitrile, based on the 1983 inhalation unit risk (EPA 1984), is presented in Appendix B. This calculation, however, may need to be revised following completion of the IRIS Program reevaluation.
Felter and Dollarhide (1997) concluded that the human weight of evidence for the carcinogenicity of acrylonitrile is insufficient. Their evaluation of the available human database showed no clear association between acrylonitrile exposure and human cancer; however, they stated that the studies did not have sufficient power to be able to rule out a small increase.
The International Agency for the Research on Cancer (IARC) modified their cancer classification for acrylonitrile from Group 2A (probably carcinogenic) to Group 2B (possibly carcinogenic to humans) (IARC 1999). This change was based on the lack of carcinogenic evidence from the more recent epidemio-
logic studies, with an overall conclusion that the potential carcinogenicity of acrylonitrile in humans is considered to be inadequate and no evidence of a causal association exists; however, they did note an increased risk of lung cancer was observed in individuals exposed at the highest concentrations of acrylonitrile in one of the largest studies conducted by the National Cancer Institute (Blair et al. 1998). They also found adequate evidence for carcinogenicity from studies with rats. Likewise, the National Toxicology Program (NTP 2011) concluded that acrylonitrile is “reasonably anticipated to be a human carcinogen” based on sufficient evidence of carcinogenicity in experimental animals.
Blair et al. (1998) evaluated the relationship between occupational exposure to acrylonitrile and cancer mortality in a cohort of over 25,000 workers employed in acrylonitrile production or use from the 1950s through 1983. An elevated risk of lung cancer mortality was observed in the highest quintile of cumulative exposure. The investigators concluded that the increased risk of lung cancer may indicate carcinogenic risk at high levels of exposure. Exposure to acrylonitrile was not associated with an increased risk of cancers of the stomach, brain, breast, prostate gland, or the lymphatic or hematopoietic systems. More recently, Cole et al. (2008) reviewed a retrospective-cohort study and case-control studies on acrylonitrile. It was concluded that the results of the epidemiologic studies did not support a causal relationship between acrylonitrile and all cancers or any specific type of cancer.
2.6. Summary
A concentration range of 1.6-6.3 ppm has been reported as the odor threshold for acrylonitrile in humans. A level of distinct odor awareness of 145 ppm was calculated for acrylonitrile. Nonlethal effects of occupational exposure to acrylonitrile include headache, nasal and ocular irritation, thoracic discomfort, nervousness, and irritability, but definitive exposure-response data are lacking. Available information indicates that such effects resolve following removal from exposure. No signs or symptoms were reported in male volunteer subjects following exposures up to 4.6 ppm for 8 h. Lethality following acute inhalation exposure to acrylonitrile has been reported.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
3.1.1. Monkey
Rhesus monkeys (two males and two females; 4.2-4.8 kg) were exposed to acrylonitrile at 65 or 90 ppm (two females) for 4 h (Dudley and Neal 1942). The test atmosphere was generated by bubbling air through acrylonitrile (purity determined through repeated fractional distillations free of cyanide and with a boil-
ing point of 76-77°C) and mixing the acrylonitrile-saturated air stream with a main air stream. Air flow through the exposure chamber was 260 L/min (± 2%). The concentration of acrylonitrile was varied by adjusting the volume of air passing through the bubbler. The concentration of acrylonitrile in the chamber was determined by the change in weight of the acrylonitrile in the bubbler, air flows, and start/stop times. Even at the highest concentration (90 ppm), all of the monkeys exhibited only slight redness of the face and genitals, and a slight increase in respiratory rate on initial exposure.
Dudley et al. (1942) exposed four rhesus monkeys to acrylonitrile at 56 ppm (average concentration) for 4 h/day, 5 days/week for 4 weeks. All four monkeys survived and showed no evidence of toxicity during the 4-week exposure period.
3.1.2. Dog
In their assessment of acrylonitrile lethality in multiple species, Dudley and Neal (1942) also exposed groups of two to four male and female dogs (5.5–12.0 kg; strain not specified) to various acrylonitrile concentrations for 4 h (see Table 1-3). The investigators found dogs to be more sensitive to acrylonitrile; exposures producing only minor effects in other species caused coma and death in the dogs.
Results of a 4-week repeat exposure experiment using two dogs exposed to an average concentration of acrylonitrile at 56 ppm for 4 h/day was reported by Dudley et al. (1942). After the first 4-h exposure, one dog died in convulsions while the second dog developed a transient paralysis of the hind legs after the fifth, thirteenth, and fourteenth exposure. Subsequent exposures were well tolerated.
3.1.3. Cat
In the study by Dudley and Neal (1942), groups of two to four cats (gender not specified; about 3.6 kg) were exposed to acrylonitrile for 4 h. Exposure at 100 ppm produced only salivation and slight transient effects (redness of the skin and mucosae) while exposure at 275 ppm resulted in more severe effects (marked salivation, signs of pain) but no deaths. At 600 ppm, 100% mortality (preceded by convulsions) occurred within 1.5 h of exposure.
Four cats were exposed to acrylonitrile at 56 ppm (average concentration) for 4 h/day, 5 days/week for 8 weeks (Dudley et al. 1942). The cats occasionally vomited, were lethargic, and lost weight. One cat developed a transitory weakness of the hind legs after the third exposure and died after the eleventh exposure. The remaining cats survived the entire exposure period with minimal effects.
TABLE 1-3 Toxicity of Acrylonitrile Vapor in Dogs Exposed for 4 Hours
| Concentration (ppm) | Gender | Effects |
| 30 | Female | Slight salivation by end of exposure period; no other effects. |
| Female | Slight salivation by end of exposure period; no other effects. | |
| Female | Slight salivation by end of exposure period; no other effects. | |
| Female | Slight salivation by end of exposure period; no other effects. | |
| 65 | Female | Severe salivation; weak by end of exposure. |
| Female | Coma by end of exposure; died at 8 h. | |
| 100 | Male | Severe salivation during exposure; full recovery within 24 h. |
| Female | Convulsions at 2.5 h; coma by end of exposure; partial paralysis of hind legs for 3 d. | |
| Female | Convulsions at 2.5 h; coma by end of exposure; full recovery within 48 h. | |
| 110 | Female | Coma at end of exposure; dead at 4.5 h. |
| Male | Coma at end of exposure; dead at 3 d. | |
| Female | Coma at end of exposure; food refusal for 10 d; slowly recovered. | |
| 165 | Female | Convulsions at 2 h; dead at 3 h. |
| Male | Coma from end of exposure to death at 4 h. |
Source: Adapted from Dudley and Neal 1942.
3.1.4. Rat
Dudley and Neal (1942) conducted single exposure experiments in which groups of 16 Osborne-Mendel rats (about 295 g, sex not specified) were exposed for 0.5, 1, 2, 4, or 8 h to various concentrations of acrylonitrile (see Table 1-4). Details regarding generation of the test atmospheres are provided in Section 3.1.1. Responses included initial stimulation of respiration followed by rapid shallow respiration. At concentrations above 300 ppm, rats started exhibiting signs of ocular and nasal irritation. Rats exposed to any concentration of acrylonitrile exhibited flushing (reddening) of the skin, nose, ears, and feet. Prior to death, the rats were gasping and convulsing. Gross pathology findings of dead rats revealed bright red lungs of “normal consistency” and dark red blood. Rats which survived any acute exposure to acrylonitrile exhibited no residual effects. Results of the experiments are summarized in Table 1-4.
In another phase of the study by Dudley and Neal (1942), rats (16/group) were exposed for 4 h to acrylonitrile at 635, 315, 130, or 100 ppm (see Table 1-5). Exposure at 130 ppm produced slight transient effects and no lethality. Effects were similar to those described in the preceding paragraph. Exposure at 315 ppm resulted in 31% mortality and exposure at 635 ppm produced 100% mortality.
In a lethality study conducted at Haskell Laboratory (1968), groups of adult male ChR-CD rats (248-268 g) were exposed to acrylonitrile for 4 h. The test chamber atmosphere was analyzed at least every half hour by gas chromatography. Test animals were observed for 14 days. During exposure the rats exhibited irregular respiration, hyperemia, lacrimation, tremors, and convulsions. Deaths during exposure occurred within 2-4 h after the start of the exposure. Deaths after exposure occurred between 7 min and 18 h. A 4-h LC50 of 333 ppm (275-405 ppm, 95% confidence interval) was reported. Rats surviving the exposure exhibited mild to severe, dose-related weight loss the first day of observation followed by normal weight gain.
Appel et al. (1981a) provided lethality data for groups of three to six male Wistar rats exposed to acrylonitrile for 30-180 min at exposure concentration varying with exposure duration (see Table 1-6). In this study (designed to assess potential antidotes for acute acrylonitrile toxicity), acrylonitrile vapor was generated by evaporating acrylonitrile (99.5% purity) in a halothane vaporator and adjusting the acrylonitrile vapor concentration with clean filtered air. Vapor concentration was determined by gas chromatography.
In a rat study reported by Vernon et al. (1990), a group of 10 adult Sprague-Dawley rats (five/sex) was exposed for 1 h to acrylonitrile at 1,008 ppm. None of the rats died. Clinical signs reported included rapid shallow breathing, decreased activity, nasal discharge, salivation, lacrimation, and coma (three of 10 animals). The extremities of all animals were red 37 min into the exposure. All rats recovered within 5 min after exposure ended.
TABLE 1-4 Toxicity of Acrylonitrile Vapor in Rats Exposed for 0.5 to 8 Hours
| Duration (h) | Concentration (ppm) | Mortality During Exposure(%) | Total Mortality(%) | Effectsa |
| 0.5 | 665 | 0 | 0 | Moderate transitory effects. |
| 1,270 | 0 | 0 | Marked; no residual effects in 24 h. | |
| 1,490 | 0 | 0 | Marked; no residual effects in 24 h. | |
| 2,445 | 0 | 0 | Marked; slight residual effects at 24 h. | |
| 1 | 665 | 0 | 0 | Marked transitory effects. |
| 1,270 | 0 | 0 | Marked effects; slight effects at 24 h; normal at 48 h. | |
| 1,490 | 0 | 25 | Deaths in 4 h; slight effects at 24 h in survivors. | |
| 2,445 | 0 | 81 | Deaths in 4 h; slight effects at 24 h in survivors. | |
| 2 | 305 | 0 | 0 | Slight transitory effects. |
| 595 | 0 | 6 | Marked transitory effects. | |
| 1,260 | 0 | 100 | Fatal; deaths within 4 h. | |
| 4 | 1,30 | 0 | 0 | Slight transitory effects. |
| 315 | 25 | 31 | Marked; no effects in survivors at 24 h. | |
| 635 | 50 | 100 | Fatal. | |
| 8 | 90 | 0 | 0 | Slight discomfort. |
| 135 | 0 | 0 | Moderate transitory effects. | |
| 210 | 6 | 6 | Marked transitory effects. | |
| 270 | 44 | 44 | Marked; no effects in survivors at 24 h. | |
| 320 | 94 | 94 | Fatal. |
aNonlethal effects included rapid respiration followed by rapid shallow breathing. Prior to death animals exhibited slow, gasping respiration, convulsions, and then coma.
Source: Adapted from Dudley and Neal 1942.
TABLE 1-5 Toxicity of Acrylonitrile Vapor in Rats Exposed for 4 Hours
| Concentration (ppm) | Mortality During Exposure (%) | Total Mortality (%) | Effects |
| 100 | 0 | 0 | Slight transitory effects. |
| 130 | 0 | 0 | Slight transitory effects. |
| 315 | 25 | 31 | Marked effects; no residual effects in survivors. |
| 635 | 50 | 100 | Death occurred in 2-6 h. |
Source: Adapted from Dudley and Neal 1942.
TABLE 1-6 Lethal Response of Rats Exposed to Acrylonitrile
| Concentration (ppm) | Duration (min) | Mortality Ratio |
| 650 | 180 | 1/3 |
| 950 | 120 | 1/3 |
| 1,100 | 120 | 3/3 |
| 1,600 | 30 | 0/3 |
| 2,600 | 30 | 1/3 |
| 3,000 | 30 | 6/6 |
| 2,400 | 10 | 0/3 |
Source: Adapted from Appel et al. 1981a.
A GLP-OECD guideline study sponsored by the Shanghai SECCO Petrochemical Company, Ltd., examined the acute toxicity of acrylonitrile in rats (WIL Research Laboratories 2005). In this study, groups of five male and five female Crl:CD/(SD) rats (8-12 weeks old; 242-297 g) were exposed to acrylonitrile (99.9% purity) for 4 h at 539, 775, 871, 1,006, or 1,181 ppm. The rats were acclimated for 7 days prior to exposure and observed for 14 days after exposure. Exposure was in a two-tiered conventional nose-only exposure system where exposure atmosphere conditions (temperature, oxygen, humidity) were monitored every 20-30 min. The acrylonitrile test atmosphere was generated by passing compressed nitrogen through the test material to create a vapor which was diluted with compressed air prior to being delivered to the exposure system. Actual acrylonitrile concentrations were determined by gas chromatography. Mortality data are summarized in Table 1-7. The report provided 4-h LC50 values of 964 ppm (857-1085 95% confidence interval) for males, 920 ppm (807-1050 95% confidence interval) for females, and 946 ppm (866-1,032 95% confidence interval) combined (determined by the method of Litchfield and Wilcoxon, 1949).
Clinical observations immediately following exposure included tremors, ataxia, labored respiration, hypoactivity, decreased defecation, and gasping, but there was no apparent exposure concentration-effect relationship. Necropsy findings in dead rats included the presence of a distended, gas-filled jejunum in one female of the 871-ppm group, distended gas-filled stomach in three females in the 871-ppm and 1,006-ppm groups, and dark, discoloration of the lungs in one male and one female in the 1,181-ppm group. No other findings were noted for rats that died. At scheduled sacrifice, the only finding was dark discoloration of the lungs in one male of the 871-ppm group.
3.1.5. Guinea Pig
Results of 4-h exposure experiments with guinea pigs (eight to 16 per group; about 695 g) are shown in Table 1-8 (Dudley and Neal 1942). Neither redness of the skin nor eyes was observed in guinea pigs, as it was in other species. Exposure
to acrylonitrile did cause watering of the eyes, nasal discharge, and coughing. As exposure increased, coughing was accompanied by moist breath sounds. Exposures that were lethal in dogs had very little effect on guinea pigs. Delayed death (3-6 days post exposure) was attributed to pulmonary edema.
3.1.6. Rabbit
In the Dudley and Neal (1942) report, groups of two to three albino rabbits (sex not specified; about 4.5 kg) were exposed to acrylonitrile for 4 h. Signs of exposure were similar to those observed for rats but the rabbits appeared to be more susceptible to acrylonitrile-induced lethality. Exposure at 100 or 135 ppm produced slight to marked transitory effects. Exposure at 260 ppm resulted in the mortality of one of two rabbits during exposure, and the second died within 4-5 h. Exposure at 580 ppm resulted in a similar response with the second rabbit dead within 3-4 h.
TABLE 1-7 Lethality in Rats Following Nose-only Inhalation Exposure to Acrylonitrile for 4 Hours
| Concentration (ppm) | Mortality During Exposure | Total Mortality | |||
| Male | Female | Male | Female | Comments | |
| 539 | 0 | 0 | 0 | 0 | |
| 775 | 0 | 0 | 0 | 0 | |
| 871 | 0 | 0 | 1 | 3 | Deaths at 0-1 d postexposure. |
| 1,006 | 1 | 1 | 3 | 4 | 2 males, 3 females at 0-1 d postexposure. |
| 1,181 | 4 | 3 | 5 | 4 | 1 male, 1 female at 0-1 d postexposure. |
Source: Adapted from WIL Research Laboratories 2005.
TABLE 1-8 Toxicity of Acrylonitrile Vapor in Guinea Pigs Exposed for 4 Hours
| Exposure Concentration (ppm) | Mortality (%) During Exposure | Total Mortality (%) | Effects |
| 100 | 0 | 0 | Slight to no effect. |
| 265 | 0 | 0 | Slight transitory effect; reduced feed consumption for 4 d. |
| 575 | 25 | 63 | Ocular and nasal irritation during exposure; delayed death (3-6 d) probably from pulmonary edema. |
| 1,160 | 13 | 100 | Five dead within 1.5 h postexposure; 2 dead at 18 h. |
Source: Dudley and Neal 1942.
In an 8-week repeat exposure study, three rabbits were exposed to acrylonitrile at 100 ppm (average concentration) for 4 h/day, 5 days/week (Dudley et al. 1942). The rabbits survived for the full exposure duration, but were drowsy and listless during exposure and gained no weight gain. No additional effects were observed.
3.2. Nonlethal Toxicity
3.2.1. Monkey
No evidence of toxicity was observed in rhesus monkeys (four per group; sex not specified) exposed to acrylonitrile at 56 ppm (126 mg/m3) for 4 h/day, 5 days/week for 4 weeks (Dudley et al. 1942). A slight increase in respiration on initial exposure was the only effect reported for two male and two female monkeys exposed for 4 h at 65 ppm (Dudley and Neal 1942). In the same study, two female monkeys exposed to acrylonitrile at 90 ppm for 4 h exhibited slight weakness, redness of the face and genitals, and a slight increase in respiratory rate. The effects resolved within 12-h postexposure. Details regarding generation of the test atmospheres are provided in Section 3.1.1.
3.2.2. Dog
In a preliminary investigation into the toxicity of acrylonitrile (Haskell Laboratory 1942), three dogs (strain, sex, age, and weight not specified) exposed to acrylonitrile a 25 ppm for 6 h had a rise in body temperature of at least 2°F. Exposure at 50 ppm resulted in a drop in body temperature of as much as 1.6°F. Three dogs were exposed for 1.75 h to acrylonitrile at 225 ppm. Two of the dogs exhibited an initial marked increase in pulse rate followed by a decrease. Blood pressure increased in two of three dogs and decreased in a third dog. Overt signs of exposure included ocular and nasal irritation, vomiting, incoordination, and “noisy” respiration. All dogs recovered within 24 h.
Four dogs exposed to acrylonitrile at 30 ppm for 4 h exhibited only slight salivation (Dudley and Neal 1942). Severity of effects increased with increasing concentration. Exposure at 65 ppm produced weakness in one dog and coma in another while exposure at 100 ppm resulted in convulsions in two of three dogs (see Section 3.1.2). All of the dogs in these exposure groups fully recovered within 48 h or less. Details regarding generation of the test atmospheres for these experiments are described in Section 3.1.1.
3.2.3. Cat
In the study by Dudley and Neal (1942), groups of two to four cats (sex not specified; about 3.6 kg) were exposed to acrylonitrile at 100 ppm for 4 h and exhibited only salivation and slight transient effects (redness of the skin and
mucosae) whereas exposure at 275 ppm resulted in more severe effects (marked salivation, signs of pain) but no deaths.
3.2.4. Rat
Dudley et al. (1942) exposed 16 rats to acrylonitrile at an average concentration of 100 ppm for 5 days/week for 8 weeks. Slight lethargy during exposure was the only adverse effect observed. During the test period, three of the seven females gave birth and raised normal litters.
Results of a study by Bhooma et al. (1992) demonstrated fibrin-network formation in the lungs of six male Wistar rats exposed to acrylonitrile at 100 ppm for 5 h/day for 5 days and observed for 28 days. Alveolar macrophage activity was elevated from postexposure day 1 to day 14 and returned to normal by day 28. Procoagulant activity in lavage fluid was unaltered for the first 5 days, but was elevated when assessed at days 14 and 28.
Quast et al. (1980) exposed rats to acrylonitrile at 20 and 80 ppm for 6 h/day, 5 days/week. The rats exhibited “minimal changes microscopically in the respiratory epithelium of the nasal turbinates of 80 ppm rats suggestive of slight degree of irritation” at the 6-month interim sacrifice interval. There was no mention of adverse effects associated with the 20-ppm exposure.
In the study by WIL Research Laboratories (2005), vocalization by rats when handled was reported in animals exposed (nose only) to acrylonitrile at 539 ppm for 4 h. Some rats exposed at 775 ppm exhibited ataxia, labored breathing, hyperactivity, and decreased urination and defecation during or after exposure. The rats in both groups were normal within 2 days (539-ppm group) or 8 days (775-ppm group) after exposure.
3.2.5. Rabbit
In the Dudley and Neal (1942) study, groups of two to three albino rabbits (sex not specified; about 4.5 kg) exposed to acrylonitrile at 100 or 135 ppm for 4 h had slight to marked transitory effects in respiratory pattern and signs of irritation.
3.2.6. Guinea Pig
Dudley et al. (1942) exposed 16 guinea pigs to an average concentration of acrylonitrile of 100 ppm for 4 h/day, 5 days/week for 8 weeks. The guinea pigs gained weight moderately and exhibited slight lethargy during the exposure but no other adverse signs were observed.
3.3. Developmental and Reproductive Effects
Acrylonitrile has been shown to produce fetal anomalies in rats following oral gavage dosing (Murray et al. 1976; Saillenfait and Sabate 2000) and hamsters following intraperitoneal injection (Willhite et al. 1981a,b). Dose-response
data for inhalation exposures is limited to two studies conducted in rats (Murray et al. 1978; Saillenfait et al. 1993a).
In a developmental toxicity study conducted by Murray et al. (1978), groups of 30 pregnant Sprague-Dawley rats were exposed to acrylonitrile (>99 purity) at 0, 40, or 80 ppm for 6 h/day on gestation days 6-15. The concentrations were selected on the basis of the threshold limit value of 20 ppm and preliminary results of a long-term inhalation toxicity study. Clinical signs (made daily), maternal body weight, and feed consumption were monitored and gross necropsies were performed. Standard developmental parameters were assessed. Sex, body weight, external abnormalities, and skeletal and soft-tissue anomalies of fetuses were evaluated. The rats were exposed in stainless steel and glass Rochester-type chambers (4.3 m3) with dynamic airflow conditions. Acrylonitrile vapor was generated by metering it into an airstream. The test atmosphere was analyzed by gas-liquid chromatography three times per day. Time-weighted mean concentrations of acrylonitrile were 40 ± 2 and 77 ± 8 ppm (mean ± standard deviation).
Results of the Murray et al. (1978) study are summarized in Tables 1-9, 1-10, and 1-11. Mean body weight and maternal body weight gain was significantly decreased during treatment in both dose groups. Relative to controls, food consumption was decreased during gestation days 15-17 but increased on days 18-20. Maternal liver weight was unaffected by acrylonitrile exposure. Pregnancy incidence, mean litter size, incidence of resorptions, and average fetal body measurements were unaffected by exposure to acrylonitrile. A significant (p < 0.06) increased incidence of total malformations was detected in litters of the 80-ppm group. Specific malformations included short tail, short trunk, missing ribs, delayed ossification of skull bones, omphalocele, and hemivertebrae, and were observed only in the 80-ppm treatment group. These high-dose effects were considered to be exposure related, because of similar findings in a gavage study by Murray et al. (1976). The investigators concluded that the data suggested a teratogenic effect of acrylonitrile at 80 ppm but that there was no evidence of teratogenicity or embryotoxicity in rats exposed at 40 ppm.
In contrast to the Murray et al. (1976) study, Saillenfait et al. (1993a) did not observe fetal malformations in rats exposed to acrylonitrile at concentrations up to 100 ppm. Groups of 20-23 pregnant Sprague-Dawley rats were exposed by inhalation to acrylonitrile (>99% purity) at 0, 12, 25, 50, or 100 ppm for 6 h/day on gestation days 6-20, and euthanized on day 21. Clinical signs of toxicity, maternal body weight, and feed consumption were monitored, and gross necropsies were performed. Fetal examinations included gender ratio, body weight, external abnormalities, and skeletal and soft-tissue anomalies. The rats were exposed in 200-L stainless steel chambers (23°C, 50% relative humidity) with dynamic and adjustable laminar air flow (10-20 m3/h). Acrylonitrile vapor was generated by bubbling air through a flask containing acrylonitrile, and the concentration in the chamber was calculated from the ratio of the amount of acrylonitrile vaporized to the total chamber air flow during the test period. Concentration of acrylonitrile was determined analytically by hourly sampling and gas-liquid chromatography.
TABLE 1-9 Maternal Toxicity in Rats Exposed by Inhalation to Acrylonitrilea
| Parameter | Exposure Concentration | ||
| 0 ppm | 40 ppm | 80 ppm | |
| No. deaths/no. females | 0/40 | 0/38 | 0/40 |
| Percentage pregnant (no.) | 88 (35) | 97 (37) | 90 (36) |
| Additional pregnancies (detected by stain) Body weight gain of dams | 0 | 0 | 3 |
| Gestation days 6-9 | 19 ± 5 | 1 ± 6b | -5 ± 10b |
| Gestation days 10-15 | 43 ± 8 | 32 ± 14b | 31 ± 17b |
| Gestation days 16-20 | 82 ± 12 | 84 ± 22 | 92 ± 15 |
| Liver weight (gestation day 21) | |||
| Absolute (g) | 16.0 ± 1.8 | 15.9 ± 1.8 | 15.3 ± 1.6 |
| Relative to body weight (g/kg) | 38.6 ± 2.9 | 41.3 ± 3.1 | 40.3 ± 4.3 |
aRats were exposed for 6 h/day on gestations days 6-15.
bp < 0.05
Source: Adapted from Murray et al. 1978.
TABLE 1-10 Litter Data for Pregnant Rats Exposed to Acrylonitrile Vapora
| Parameter | Exposure Concentration | ||
| 0 ppm | 40 ppm | 80 ppm | |
| No. of litters | 33 | 36 | 35 |
| Implantations/dam | 13 ± 2 | 13 ± 2 | 12 ± 3 |
| Live fetuses/litter | 13 ± 2 | 12 ± 2 | 12 ± 3 |
| Resorptions/litter | 0.6 ± 0.7 | 0.7 ± 1.1 | 0.5 ± 0.6 |
| Fetal body weight (g) | 5.79 ± 0.33 | 5.72 ± 0.42 | 5.90 ± 0.25 |
| Fetal crown-rump length (mm) | 43.9 ± 2.1 | 43.5 ± 2.2 | 43.7 ± 2.2 |
aRats were exposed for 6 h/day on gestation days 6-15.
Source: Adapted from Murray et al. 1978.
There were no maternal deaths, but a concentration-dependent decrease in absolute body weight gain was observed; the decrease was significant (p < 0.01) in the three highest exposure groups (-0.1, -7.8, and -24.3 g at 25, 50, and 100 ppm, respectively). No adverse effect on pregnancy rate, average number of implantations or number of live fetuses, incidences of nonsurviving implants and resorptions, or fetal sex ratio were found (see Table 1-12). A statistically significant (p < 0.01 to 0.005; see Table 1-12) exposure-related reduction in fetal weights was observed at 25 ppm and higher concentrations (13% to 15% decreases at 100 ppm). Evaluation of external, visceral, and skeletal variations in the fetuses revealed no acrylonitrile-related effects. The no-observed-adverse-effect level (NOAEL) for maternal and developmental toxicity was 12 ppm on the basis of fetal body weight.
TABLE 1-11 Incidence of Fetal Malformations in Litters of Rats Exposed to Acrylonitrile Vapor
| Parameter | Exposure Concentration | ||
| 0 ppm | 40 ppm | 80 ppm | |
| No. fetuses/no. litters examined | |||
|
External and skeletal malformations |
421/33 | 441/36 | 406/35 |
|
Visceral malformations |
140/33 | 148/36 | 136/35 |
| No. fetuses (litters) affected | |||
|
External malformations |
|||
|
Short tail |
0 (0) | 0 (0) | 2 (2) |
|
Short trunk |
0 (0) | 0 (0) | 1 (1) |
|
Imperforate anus |
0 (0) | 0 (0) | 0 (0) |
|
Omphalocele |
0 (0) | 1 (1) | 1 (1) |
|
Visceral malformations |
|||
|
Right-sided aortic arch |
0 (0) | 0 (0) | 0 (0) |
|
Missing kidney, unilateral |
0 (0) | 0 (0) | 0 (0) |
|
Anteriorly-displaced ovaries |
0 (0) | 0 (0) | 1 (1) |
| Skeletal malformations | |||
|
Missing vertebrae (associated with short tail) |
0 (0) | 2 (1) | 2 (2) |
|
Missing two vertebrae and a pair of ribs |
8 (1) | 2 (1) | 7 (2) |
|
Hemivertebra |
0 (0) | 0 (0) | 1 (1) |
|
Total malformed |
8 (1) | 3 (2) | 11 (6)a |
aRats were exposed for 6 h/day on gestation days 6-15.
bp < 0.05
Source: Adapted from Murray et al. 1978.
TABLE 1-12 Reproductive Parameters in Rats Exposed to Acrylonitrile Vapor on Gestation Days 6-20
| Parameter | 0 ppm | 12 pm | 25 ppm | 50 ppm | 100 ppm |
| No. deaths of treated females | 0/20 | 0/21 | 0/21 | 0/20 | 0/21 |
| Pregnant at euthanization (%) | 100.0 | 95.2 | 95.2 | 90.0 | 90.5 |
| No. examined litters | 20 | 20 | 20 | 18 | 19 |
| Implantations sitesa | 13.65 ± 2.81 | 14.80 ± 1.99 | 14.40 ± 3.38 | 15.11 ± 2.00 | 14.37 ± 2.17 |
| Live fetuses/littera | 12.30 ± 4.09 | 14.00 ± 2.18 | 13.85 ± 3.26 | 14.50 ± 1.89 | 13.63 ± 2.22 |
| Non-surviving implants/litter (%)a | 10.40 ± 22.75 | 5.44 ± 7.38 | 3.49 ± 6.10 | 3.89 ± 5.37 | 4.94 ± 8.33 |
| Resorption sites/litter (%)a | 10.40 ± 22.75 | 5.11 ± 6.46 | 3.49 ± 6.10 | 3.89 ± 5.37 | 4.94 ± 8.33 |
| Fetal sex ratio (male:female) (%) | 1.05 | 0.96 | 1.23 | 1.10 | 0.96 |
| Fetal body weight | |||||
| Male | 5.95 ± 0.28 | 5.79 ± 0.28 | 5.64 ± 0.36b | 5.54 ± 0.24b | 5.04 ± 0.36b |
| Female | 5.66 ± 0.36 | 5.51 ± 0.27 | 5.37 ± 0.28c | 5.18 ± 0.25b | 4.90 ± 0.49b |
aMean ± standard deviation.
bp < 0.05
cp < 0.01
Source: Adapted from Saillenfait et al. 1993a.
Nemec et al. (2008) conducted a two-generation reproductive toxicity study of acrylonitrile in Sprague-Dawley rats (25/sex/group) exposed (whole-body) at concentrations of 0, 5, 15, and 45 ppm (two offspring generations), and at 90 ppm (one offspring generation). Exposure were for 6 h/day, and were conducted on one litter per generation through F2 weanlings on postnatal day 28. After approximately 3 weeks of exposure following weaning, exposure of the 90-ppm F1 rats was terminated because of excessive systemic toxicity in the males. There were no exposure-related mortalities in adult animals, no functional effects on reproduction, no effects on reproductive organs, and no evidence of cumulative toxicity. There was no evidence of toxicity in pregnant and lactating dams or in developing animals. Adult systemic toxicity was limited to body weight and/or food consumption deficits in both sexes and generations (greater in males) at 45 and 90 ppm, and increased liver weights occurred in the 90-ppm F0 males and females and 45-ppm F1 males. Neonatal toxicity was limited to weight decrements in the 90-ppm F1 offspring. Signs of local irritation during and immediately following exposure were observed at 90 ppm. Microscopic lesions of the rostral nasal epithelium (site-of-contact irritation) were observed in some animals at 5-45 ppm. The NOAEL for reproductive toxicity over two generations and neonatal toxicity of acrylonitrile administered to rats via whole-body inhalation was 45 ppm. The NOAEL was 90 ppm for reproductive toxicity for the first generation, and 15 ppm for parental systemic toxicity.
3.4. Genotoxicity
Acrylonitrile has been extensively tested for genotoxic potential. Acrylonitrile has been shown to be mutagenic in Salmonella typhimurium, usually with metabolic activation (S9) (e.g., Milvy and Wolff 1977; de Meester et al. 1978; Lijinsky and Andrews 1980). Acrylonitrile produced both positive and negative outcomes in Escherichia coli and fungi (Saccharomyces cerevisiae); metabolic activation in these systems was not required for a positive response. Positive results for somatic cell mutation and aneuploidy were obtained in several studies with Drosophila melanogaster (reviewed by IARC 1999).
In in vitro assays with mammalian cells, acrylonitrile induced DNA strand breaks, gene mutations, sister-chromatid exchange and chromosomal aberrations; a positive genotoxic response was not obtained for aneuploidy or unscheduled DNA synthesis in rat hepatocytes. In several test systems, acrylonitrile induced cell transformations in mouse or Syrian hamster ovary cells (reviewed by IARC 1999).
Results from most in vivo mammalian cell assays (unscheduled DNA synthesis in rat hepatocytes or spermatocytes, chromosome aberrations in mouse and rat bone marrow or mouse spermatogonia, micronuclei in mouse bone marrow, and dominant lethal mutations in rat and mouse) were negative (reviewed by IARC 1999). Acrylonitrile induced sister-chromatid exchanges and chromosomal aberrations in mouse bone marrow (Fahmy 1999) and micronuclei in the bone marrow of rats (Wakata et al. 1998). Comet assays found DNA damage in
the forestomach, colon, bladder, lungs, and brain of mice following a single intraperitoneal injection of acrylonitrile, and in the forestomach, colon, kidneys, bladder, and lungs of rats injected with acrylonitrile (Sekihashi et al. 2002).
In studies with mammalian DNA, Solomon et al. (1984) identified and Yates et al. (1993) characterized the nature of adducts formed in interactions of mammalian DNA with CEO, the reactive metabolite of acrylonitrile.
In conclusion, results of in vitro and in vivo studies provide evidence that acrylonitrile is genotoxic. In in vitro models, acrylonitrile induced DNA strand breaks, sister-chromatid exchanges, chromosomal aberrations, and cell transformations. Following in vivo exposure, acrylonitrile induced DNA damage, sister-chromatid exchanges, chromosomal aberrations, and micronuclei. Although negative results have also been reported, the overall weight of evidence supports the conclusion that acrylonitrile has genotoxic activity.
3.5. Carcinogenicity
A cancer bioassay was conducted by Maltoni et al. (1977). In this study groups of 30 male and 30 female rats were exposed by inhalation to acrylonitrile at 5, 10, 20, or 40 ppm for 4 h/day, 5 days/week for 12 months. A group of rats exposed to clean air served as the control group. The rats were observed until death. Body weight was unaffected by the acrylonitrile exposure. There was a statistically significant increase in the percentage of animals with benign and malignant tumors (p < 0.01) and malignant tumors alone (p < 0.01). The total malignant tumors per 100 animals was noted for several treated groups, but lacked a definitive dose-response relationship. There was no increase in Zymbal’s gland tumors, extrahepatic angiosarcomas, or hepatomas. Encephalic glioma incidence was increased in rats exposed at 20 ppm (3.3%; 2/60) and 40 ppm (5%; 3/60). Although not statistically significant, the response was considered by the investigators to be of possible biologic relevance because the brain was shown to be a target organ in the oral administration part of the study.
Maltoni et al. (1988) also conducted experiments in which groups of 54 breeder female rats (Group I) were exposed to acrylonitrile at 60 ppm for 4 h/day, 5 days/week for 7 weeks followed by 7 h/day, 5 days/week for 97 weeks. A group of 60 female rats served as controls (Group II). Following transplacental exposure of the pregnant rats in Group I, inhalation exposure of offspring continued; exposures were for 4 h/day, 7 days/week for 7 weeks followed by 7 h/day, 5 days/week for 97 weeks (Group Ia), or 4 h/day, 5 days/week for 7 weeks followed by 7 h/day, 5 days/week for 8 weeks (Group Ib). Offspring group size was 67 males and 54 females in Group Ia and 60 of each gender in Group Ib. The control offspring group (Group IIa) included 158 males and 149 females. The percentage of animals with malignant tumors in the parental groups was 37% (20/54) in Group I and 16.7% (10/60) in the Group II (control). For the offspring in Group Ia, the percentage of animals (males and females) was 54.5% (66/121) and for Group Ib was 33.3% (40/120). For control offspring (Group IIa), the percentage of animals with malignant tumors was 17.9% (55/307).
In the long-term inhalation study by Quast et al. (1980), Sprague-Dawley (Spartan substrain) rats (100/sex/concentration) were exposed by inhalation to acrylonitrile at 0 (control), 20, and 80 ppm for 6 h/day, 5 days/week for 2 years (analytic concentrations were 20.1 ± 2.1 and 79.5 ± 7.3 ppm, respectively, at the 6-month sacrifice). A control group was exposed to clean air. The groups also included animals for interim sacrifices at 6 months (7/sex/concentration) and 12 months (13/sex/concentration). Hematology, urinalysis, and clinical chemistry assessments were performed at specific intervals. Clinical observations were made of body weight, mortality, clinical appearance, onset of tumors, and frequency of observed palpable tumors. All rats, regardless of time of death, were subjected to gross pathology examinations.
Alterations in the aforementioned clinical observations occurred earliest and with the highest frequency in the 80-ppm group. Mortality rate was significantly increased (p < 0.05) during the first year in both male and female rats of the 80-ppm group and for females of the 20-ppm group during the last 10 weeks of the study. Non-neoplastic effects for both exposure groups included concentration-related inflammation and degeneration of tissue in the nasal turbinates (mucosa suppurative rhinitis, hyperplasia, focal erosions, and squamous metaplasia of the respiratory epithelium, with hyperplasia of the mucous secreting cells). Although these tumors are known to occur spontaneously and at a high rate in Sprague-Dawley rats, they were observed earlier and at a higher frequency in acrylonitrile-exposed animals. Focal perivascular cuffing and gliosis were found in the brain of male rats at 20 ppm (2/99; p < 0.05) and 80 ppm (7/99; p < 0.05). They were also found in female rats at 20 ppm (2/100; p < 0.05) and 80 ppm (8/100; p < 0.05). There was an increased incidence of brain tumors (p < 0.05) in both sexes at 80 ppm compared with the controls, identified histopathologically as focal or multifocal glial-cell tumors (astrocytomas). Proliferative glial-cell lesion incidence was significantly increased in the 80-ppm males only.
Deaths of rats in the Quast et al. (1980) study were often attributable to severe ulceration of the Zymbal’s gland or mammary-tissue tumors, and suppurative pneumonia (80-ppm group only) resulting from acrylonitrile-induced pulmonary irritation. The frequency of Zymbal’s gland tumors was significantly increased in males (11/100; p < 0.05) and in females (10/100; p < 0.05) in the 80-ppm group; in females the highest incidence occurred during the 13- to 18-month interval. An incidence of 3/100 was observed in males exposed at 20 ppm (1/100 in controls). No Zymbal’s gland tumors were found in 20-ppm females. Tumor type and incidence data are presented in Table 1-13.
Felter and Dollarhide (1997) developed a concentration-response analysis of the astrocytoma incidence data reported by Quast et al. (1980). A polynomial dose-response model was applied to the data to estimate the EC10 and lower confidence limit on the EC10 (LEC10). The calculated unit risks for lifetime continuous exposure ranged from 8.2 × 10-6 per 1 µg/m3 (based on the EC10) to 1.1 × 10-5 per 1 µg/m3 (based on the LEC10). The unit risk based on the LEC10 corresponds to a lifetime 1 × 10-4 risk-specific exposure concentration of 9 µg/m3 (4.1 × 103 ppm).
TABLE 1-13 Tumor Type and Incidence Data for Rats Exposed to Acrylonitrile Vapor
| Concentration (ppm) | Zymbal’s Gland Carcinoma | Tongue Papilloma/ Carcinoma | Mammary Gland Fibroadenoma | Small Intestine Cystadenocarcinoma | Brain Astrocytoma |
| Males | |||||
| 0 | 1/100 | 1/96 | – | 2/99 | 0/100 |
| 20 | 3/100 | 0/14 | – | 2/20 | 4/99 |
| 80 | 11/100a | 7/89a | – | 14/98a | 15/99a |
| Females | |||||
| 0 | 0/100 | – | 79/100 | – | 0/100 |
| 20 | 0/100 | – | 95/100a | – | 4/100a |
| 80 | 10/100a | – | 75/100 | – | 17/100a |
aSignificantly different from control group (p < 0.05).
Source: Quast et al. 1980.
3.6. Summary
Acute exposure data from tests with various laboratory species (monkey, rat, dog, rabbit, guinea pig, and cat) revealed qualitatively similar responses ranging from mild irritation (redness of exposed skin, lacrimation, and nasal discharge) and mild effects on ventilation and cardiovascular responses to severe respiratory effects, convulsions, and death. Four-hour exposure to acrylonitrile at concentrations ranging from 30 to 100 ppm produced little or no effect in all species except dogs, which exhibited severe effects at 100 ppm. Results of a recent nose-only exposure study in rats showed that concentrations up to 50 ppm for 6 h or 225 ppm for 1.75 h produced only minor transient effects on blood pressure. Lethality in rats appears to occur at cumulative exposure of 1,800-1,900 ppm-h for 30-min to 6-h durations, although for nose-only exposures it is notably higher (about 3,800 ppm-h). Lethality data for various exposure durations and concentrations suggest a near linear relationship (Cn × t = k, where n = 1.1). Death may be delayed especially at the lower limits of lethal exposures. One study provided evidence for teratogenic effects in rats following gestational exposure of dams to acrylonitrile at 80 ppm but not at 40 ppm. Another study showed an exposure-related decrease in fetal weight following gestational exposure of dams to 25, 50, or 100 ppm acrylonitrile; no other reproductive or developmental effects were detected. Results of genotoxicity studies provide evidence that acrylonitrile is genotoxic, with positive results in in vitro (DNA strand breaks, sister-chromatid exchanges, chromosomal aberrations, and cell transformations) and in vivo (DNA damage, sister-chromatid exchanges, chromosomal aberrations, and micronuclei) models. The overall weight of evidence supports that acrylonitrile is genotoxic. Results of cancer bioassays have shown that acrylonitrile is carcinogenic in rats. The brain, spinal cord, Zymbal’s gland, tongue, small intestines, and mammary glands have all been identified as targets.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
Following inhalation exposure, acrylonitrile undergoes rapid absorption by passive diffusion. Data from six male volunteers exposed to acrylonitrile (5 or 22 ppm) for 8 h indicated that about 52% of the inhaled acrylonitrile was retained (Jakubowski et al. 1987). Approximately 91.5% retention was reported in rats exposed at 1,800 ppm (3,900 mg/m3) (Peter and Bolt 1984). These investigators also reported that rhesus monkeys absorbed nearly all acrylonitrile after 6 h.
Absorbed acrylonitrile is readily distributed throughout the body. Kedderis et al. (1996) reported detection of acrylonitrile and CEO in the blood, brain, and liver of Fisher F-344 rat 3 h after exposure at 186, 254, or 291 ppm. Concentrations of acrylonitrile and CEO tended to be greatest in the brain than in liver, and decreased rapidly following cessation of exposure. GSH depletion was shown to enhance tissue uptake of acrylonitrile into the brain, stomach, liver, kidneys, and blood of GSH-depleted (phorone/buthionine sulfoximine treatment) F-344 rats (Pilon et al. 1988). GSH depletion, however, resulted in a decrease in total radioactivity recovered in the brain, stomach, liver, kidneys, and blood and a decrease in the nondialyzable radioactivity (acrylonitrile-derived) in the same organs. Control rats showed an accumulation of radiolabel which was greatest in brain RNA; no radioactivity was detected in the DNA of any organ examined. In the GSH-depleted rats, radiolabel was greater in brain RNA than in that of the liver or stomach, but was only about half that observed in brain RNA of control rats.
Acrylonitrile is eliminated rapidly (half-time <1 h), primarily through metabolism and excretion of metabolites (Peter and Bolt, 1984; Kedderis et al. 1996). Excretion of acrylonitrile and its metabolites is primarily via the urine, with feces and exhaled air being minor routes of excretion. Acrylonitrile and its metabolites have been detected in the urine of exposed workers. Perbellini et al. (1998) reported that concentrations of acrylonitrile in urine of pre- and post-shift workers were greater than in nonexposed controls.
At 24 h after inhalation exposure of male Sprague-Dawley rats to acrylonitrile at 0, 4, 20, or 100 ppm for 6 h, 2-cyanoethylmercapturic acid, 2-hydroxyethylmercapturic acid, and thiocyanate were measured in the urine (Tardif et al. 1987). The relationship between total urinary metabolites and exposure appeared to be linear. A dose-dependent excretion profile was reported for male Wistar rats following inhalation exposure to acrylonitrile at 1, 5, 10, 50, or 100 ppm for 8 h (Müller et al. 1987). Cyanoethyl mercapturic acid, Scarboxymethyl cysteine, hydroxyethyl mercapturic acid, and thioglycolic acid were detected as urinary metabolites. The investigators concluded that urinary metabolite profiles may be useful for biologic monitoring of industrial exposure. Specifically, unmetabolized acrylonitrile and the metabolites, cyanoethyl mercapturic acid and thioglycolic acid, were considered important.
Acrylonitrile toxicity appears to be directly related to its metabolism. Two major metabolism pathways have been described (Dahl and Waruszewski 1989; Fennell et al. 1991; Kedderis et al. 1993; Burka et al. 1994; Gargas et al. 1995; Sumner et al. 1999). One pathway is conjugation with glutathione and the second is epoxidation by microsomal cytochrome P450 2E1 which forms CEO. Metabolites from both pathways are subject to additional biotransformation. The glutathione conjugate may form a mercapturic acid which is excreted in urine. CEO is further metabolized via conjugation with glutathione (catalysis with cytosolic GST or nonenzymatically) resulting in additional conjugates and via hydrolysis by microsomal epoxide hydrolase (EH). The secondary metabolites of CEO may also be further metabolized. Cyanide may be generated via the EH pathway and by one of the GSH conjugation products. Cyanide, in turn, is detoxified to thiocyanate via rhodanese-mediated reactions with thiosulfate. Thiocyanate has been detected in the blood and urine of volunteer subjects following exposure to acrylonitrile (21-51 ppm for 30 min) (Wilson and McCormick 1949).
Vodiĉka et al. (1990) provided data showing that rats exposed for 6 h to acrylonitrile at 75, 150, or 300 mg/m3 (equivalent to 35, 69, and 138 ppm, respectively) excreted thioethers at 35.0, 22.7, and 18.1%, respectively, of the dose within 24 h. About one-third to one-half of the excretion occurred during the 6-h exposure.
Benz and Nerland (2005) reported on the effect of cytochrome P450 inhibitors and anticonvulsants on the toxicity of acrylonitrile in male Sprague-Dawley rats. Treatment of rats with 1-benzylimidazole and ethanol effectively reduced blood cyanide concentrations and early seizures in rats given an LD90 subcutaneous dose of acrylonitrile but did not affect the clonic convulsions that precede death or acrylonitrile-induced mortality, thereby suggesting that acrylonitrile is acutely toxic even in the absence of cyanide.
4.2. Mechanism of Toxicity
The mechanism by which acrylonitrile causes irritation is unknown. Nasal tissue damage in rats may be related to metabolism of acrylonitrile by this tissue (Dahl and Waruszewski 1989). Hematologic effects may be due to acrylonitrile and CEO hemoglobin adducts (Bergmark 1997; Fennell et al. 2000), whereas GSH depletion in erythrocytes may result in the oxidation of hemoglobin to methemoglobin (Farooqui and Ahmed 1983).
Generally, the toxic effects following acute inhalation exposure to acrylonitrile appear to be irritation of the respiratory tract and the metabolism of acrylonitrile to cyanide. Acrylonitrile-induced neurologic effects in laboratory animals appear to involve the parent compound and the cyanide metabolite. The pivotal role cyanide in the acute toxicity of a series of aliphatic nitriles has been clearly demonstrated (Willhite and Smith 1981). Acrylonitrile-induced convulsions are likely the result of cyanide resulting from acrylonitrile metabolism (Nerland et al. 1989; Ghanayem et al. 1991), although results of metabolism studies by Benz and Nerland (2005) suggest that only the early seizures are cya-
nide-mediated and that severe clonic convulsions preceding death may be due to parent compound as previously described in Section 4.1. Other possible modes of action include inhibition of glyceraldehyde-3-phosphate dehydrogenase, by binding to critical cysteine residues (Campian et al. 2002), and ATP production by cyanide with respect to central nervous system effects. Additionally, it has been hypothesized that acrylonitrile-induced oxidative stress may be related to some neurologic effects (Fechter et al. 2003). Fechter et al. (2003) found that subcutaneously administered acrylonitrile depleted cochlear glutathione concentrations and potentiated noise-induced hearing loss in rats.
Cyanide formation by dams may be responsible, in part, for the developmental toxicity of acrylonitrile in animals. Saillenfait and Sabate (2000) reported that a series of aliphatic nitriles produced embryotoxicity similar to that observed for sodium cyanide. Saillenfait et al. (1993b) suggested that glutathione depletion may be involved in the embryotoxicity of inhaled acrylonitrile in rats.
4.3. Structure-Activity Relationships
Willhite and Smith (1981) demonstrated the importance of the acrylonitrile metabolite, cyanide, in the lethal response of CD-1 mice following intraperitoneal injections of acetonitrile, proprionitrile, acrylonitrile, n-butyronitrile, malonitrile, or succinonitrile. In studies on the effects of P450 inhibitors and anticonvulsants, Benz and Nerland (2005) reported that acrylonitrile appears to have inherent acute toxicity even in the absence of cyanide. With the data available for acrylonitrile and considering the apparent complexity of acrylonitrile acute toxicity compared with other nitriles, structure-activity relationships were not used in the derivation of AEGL values.
4.4. Species Variability
The effects of acute inhalation exposure to acrylonitrile are qualitatively similar among several animal species (monkey, dog, cat, rat, rabbit, and guinea pig). Nerland et al. (1989) categorized the clinical signs of acute inhalation exposure to acrylonitrile into four stages: (1) an excitatory phase characterized by lacrimation and agitation; (2) a tranquil phase in which cholinergic responses (salivation, lacrimation, urination, and defecation) occur; (3) a convulsive stage characterized by clonic seizures; and (4) a terminal stage characterized by paralysis and death. At least some of the variability in the toxic response to acrylonitrile may be a function of the cyanide metabolite and activity levels of rhodanese. Drawbaugh and Marrs (1987) reported that dogs have relatively lower concentrations of rhodanese and that rats had relatively high concentrations; overall species variability was about 3-fold. Results of experiments by Dudley and Neal (1942) also indicated that the dog was the most sensitive species.
Species differences in metabolism of acrylonitrile are notable. Both rats and mice appear to form CEO at a greater rate (1.5-fold and 4-fold, respectively)
than humans (Roberts et al. 1991). Although the rate of CEO formation was greater in mice, concentrations of CEO were only a third of that found in rats (Roberts et al. 1991) suggesting difference between these rodent species. The conjugation rate for CEO with GSH is reportedly faster in humans (1.5-fold) than in mice or rats (Kedderis et al. 1995). The hydrolysis of CEO by EH is notably higher in humans and virtually absent in mice and rats (Kedderis et al. 1995). On the basis of spectral analysis of acrylonitrile interaction with microsomal preparations from rats, mice, and humans, Appel et al. (1981b) conclude that rats resemble humans more closely than do mice.
4.5. Susceptible Populations
Due to the pivotal role of oxidative metabolism of acrylonitrile in the formation of cyanide, alterations in oxidative metabolism capacity (e.g., induction or inhibition of CYP2E1) may affect cyanide production rate (induction resulting in greater cyanide formation). Because cyanide detoxification may be affected by variances in sulfane sulfur as a source for thiocyanate formation via rodanese, individuals with lower circulating levels of sulfane sulfur (e.g., low cysteine content diets) may have lower capacity for cyanide detoxification. It is the net difference between the capacities of these processes that will ultimately determine the overall cyanide-induced toxicity.
Results of a study examining the relationship between cigarette smoking, acrylonitrile-derived hemoglobin adducts (N-(2-cyanoethyl)valine), and null genotypes for glutathione transferase (GSTM1 and GSTT1) were reported by Fennell et al. (2000). Analysis of the GST genotypes (by blood analysis) from 16 nonsmokers and 32 smokers (one to two packs/day) showed that hemoglobin adduct levels increased with increased cigarette smoking. Because the GSTM1 and GSTT1 genotypes had little effect on adduct concentrations, the results suggest that GST polymorphism may not be relevant to assessing susceptibility to acrylonitrile toxicity.
4.6. Concurrent Exposure Issues
Concurrent exposure to agents capable of altering CYP2E1 function or glutathione concentrations may affect the biotransformation of acrylonitrile and, therefore, its potential toxicity. Data are unavailable to allow for a quantitative adjustment of AEGL values due to potential concurrent exposure issues.
5. DATA ANALYSIS FOR AEGL-1
5.1. Human Data Relevant to AEGL-1
Occupational exposure to acrylonitrile at 16-100 ppm for 20-45 min produced headache, nasal and ocular irritation, discomfort of the chest, nervous-
ness, and irritability (Wilson et al. 1948). Occupational exposure at 0.3-3 ppm for approximately 3 years produced similar effects (Babanov et al. 1959). Sakurai et al. (1978) reported that workers routinely exposed to acrylonitrile at approximately 5 ppm in an acrylic fiber factory experienced initial conjunctival irritation, followed by some degree of accommodation. Occupational exposures to acrylonitrile at 5-20 ppm resulted in complaints of headache, fatigue, nausea, and insomnia (Sakurai and Kusumoto 1972; Sakurai et al. 1978). Six informed male volunteer subjects (including the investigators) exposed to acrylonitrile at 2.3 and 4.6 ppm for 8 h reported no symptoms of exposure (Jakubowski et al. 1987).
5.2. Animal Data Relevant to AEGL-1
Dudley et al. (1942) reported that rhesus monkeys exposed to acrylonitrile at 65 ppm for 4 h exhibited no adverse effects. Nonlethal responses in rats included slight to marked transitory effects from exposure to acrylonitrile at 665 ppm for 30 min or 1 h, 305 ppm for 2 h, 130 ppm for 4 h, and 90 ppm for 8 h. Four-hour exposure of dogs to acrylonitrile at 30 ppm, and guinea pigs, cats, and rabbits at 100 ppm resulted in slight to moderate transitory effects. WIL Research Laboratories (2005) reported only vocalization upon handling of rats exposed (nose-only) to acrylonitrile at 539 ppm for 4 h. Some rats exposed at 775 ppm exhibited ataxia, hyperactivity, and decreased urination and defecation. Other lethality bioassay reports simply indicated some exposures as nonlethal with no details regarding the presence or absence of nonlethal effects.
5.3. Derivation of AEGL-1 Values
The most relevant data for AEGL-1 derivation is the human response data reported by Jakubowski et al. (1987). No effects were observed in volunteer subjects exposed to acrylonitrile at 4.6 ppm for 8 h. Limitations of the study include that the objective of the study was to collect data on the toxicokinetics of acrylonitrile and not to evaluate health effects. All of the subjects were informed toxicologists who worked in the laboratory in which the study was performed (stakeholders) and may have been more tolerant of mild irritant effects than less motivated individuals. However, the outcome of the Jakubowski et al. (1987) study is supported by the report by Sakurai et al. (1978), in which workers routinely exposed to acrylonitrile at approximately 5 ppm experienced mild effects (initial conjunctival irritation, for which there was some accommodation). Therefore, the 8-h exposure at 4.6 ppm is considered a no-effect level for notable discomfort and a point-of-departure for deriving AEGL-1 values. That concentration is approximately 3-fold lower than concentrations reported by Wilson et al. (1948) to be associated with more severe effects in occupational settings (16-100 ppm for 20-45 min: headache, nasal and ocular irritation, discomfort of the chest, nervousness, and irritability). Therefore, 4.6 ppm was considered an
appropriate point-of-departure for AEGL-1 derivation. Pharmacokinetic variability is not likely to be significant for mild effects (ocular irritation) of low-level exposure. However, the point-of-departure is based on studies of healthy adults and, in the occupational studies, subjects who experienced repeated exposures to acrylonitrile, which may have resulted in some accommodation to the ocular irritation. Therefore, an intraspecies uncertainty factor of 3 was applied. No data are available on the relationship between exposure duration and severity of responses to acrylonitrile. Typically, in the absence of this information, AEGL-1 values based on an 8-h point-of-departure would be time scaled. However, in this case, the effect is ocular irritation, which would not be expected to have a response threshold that varies with exposure duration. Therefore, it is prudent to not time scale and the AEGL-1 values were held constant at 1.5 ppm for the 10- and 30-min durations. However, 1.5 ppm exceeds AEGL-2 values for longer exposure durations; therefore, AEGL-1 values for 1 h, 4 h, and 8 h are not recommended. AEGL-1 values for acrylonitrile are presented in Table 1-14, and their derivation is presented in Appendix C.
6. DATA ANALYSIS FOR AEGL-2
6.1. Human Data Relevant to AEGL-2
There are no quantitative acute exposure-response data regarding AEGL-2-type effects in humans. Numerous case reports of acute accidental exposure indicate that acrylonitrile produces symptoms consistent with neurotoxicity, including headache, dizziness, feebleness, hyperactive knee jerk reflex, numbness of extremities, and convulsions (Chen et al. 1999). However, exposure data are not adequate to provide a basis for AEGL-2 values (exposures were estimated from accident simulations and post-accident measurements and ranged from 18 to over 460 ppm). Studies of workers exposed for approximately 3 years also show effects of acrylonitrile-induced neurotoxicity, including headache, insomnia, general weakness, decreased working capacity, and irritability (Babanov et al. 1959). Due to the long exposure duration, the data are not suitable as the basis of AEGL-2 values.
6.2. Animal Data Relevant to AEGL-2
AEGL-2 type effects observed in laboratory animals include changes in respiratory patterns, tremors, and convulsions, the severity of which appear to increase immediately prior to death. The onset of the more severe effects was usually preceded by varying signs of irritation (salivation, redness of skin, and lacrimation). Post-exposure observation in multiple species showed qualitatively similar effects; effects, even severe ones, were often reversible when exposure ended.
TABLE 1-14 AEGL-1 Values for Acrylonitrile
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 1.5 ppm (3.3 mg/m3) |
1.5 ppm (3.3 mg/m3) |
NRa | NRa | NRa |
aNot recommended. Absence of an AEGL-1 value does not imply that exposure below the AEGL-2 valued is without adverse effect.
The report by Dudley and Neal (1942) provides data for six species (monkey, rat, dog, guinea pig, rabbit, and cat). For rats, 0.5-, 1-, 2-, 4-, or 8-h exposure to acrylonitrile at 2,445, 1,270, 305, or 135 ppm, respectively, produced reversible effects. Appel et al. (1981a) reported data for rats showing that 10-min exposure to acrylonitrile at 2,400 ppm or 30-min exposure at 1,600 ppm was not lethal. Dogs were more sensitive to the effects of acrylonitrile, as demonstrated by convulsions and coma at exposures as low as 65 ppm for 4 h (Dudley and Neal 1942). Results of a nose-only experiment with rats showed that 4-h exposure to acrylonitrile at 775 ppm was not lethal, but details were lacking regarding the attribution of observed effects (tremors, ataxia, labored breathing, hypoactivity, and gasping) to these exposures. For rabbits, 4-h exposure to acrylonitrile at up to 135 ppm produced slight to marked, but reversible, effects (Dudley and Neal 1942). Monkeys exposed to acrylonitrile at 65 or 90 ppm for 4 h exhibited transient skin flushing and transient elevation of respiration rate (Dudley and Neal 1942).
A developmental toxicity study conducted in rats found dose-related decrements in fetal body weight that became statistically significant at 25 ppm (6 h/day, gestation days 6-20) (Saillenfait et al. 1993a). The no-effect level was 12 ppm. Although evidence of fetal toxicity (e.g., decrements in fetal body weight or fetal crown-rump length) were not observed at 40 or 80 ppm (6 h/day, gestation days 6-15) (Murray et al. 1978), the Saillenfait et al. (1993a) study suggests that 12 ppm (6 h/day) is a no-effect level for nonlethal fetal toxicity.
6.3. Derivation of AEGL-2 Values
The AEGL-2 values are based a developmental toxicity study conducted in rats which showed that 12 ppm (6 h/day, gestation days 6-20) was a no-effect level for fetal toxicity, indicated by decrements in fetal body weight at higher concentrations (25-100 ppm). Support for the point-of-departure is provided from studies conducted in rats and monkeys. In monkeys, slight or modest reversible effects (transient skin flushing and elevation of respiration rates) were observed after 4-h exposures at 65 or 90 ppm (Dudley and Neal 1942). Slight transient effects (ocular and nasal irritation, redness of skin) were observed in rats following a 2-h exposure at 305 ppm (Dudley and Neal 1942). All effects resolved within 12 h postexposure. At higher concentrations or at longer exposure durations, effects were more severe (rapid respiration, tremors, convulsions, and death). A threshold for these more severe effects in the rat appears to be
above 305 ppm and below the threshold for lethality (the 2-h BMCL05 is 491 ppm in the rat [see Section 7, Data Analysis for AEGL-3]). An interspecies uncertainty factor of 6 (3 × 2) was applied; a factor of 3 to account for possible species differences in toxicodynamics of acrylonitrile and a factor of 2 to account for interspecies differences in toxicokinetics. On the basis of PBPK modeling, Sweeney et al. (2003) predicted a 2-fold difference in the concentrations of acrylonitrile and its metabolite, cyanoethylene oxide (the metabolic precursor to cyanide), in blood and brain during 8-h exposures to acrylonitrile at 2 ppm. Higher cyanoethylene oxide concentrations were predicted in human blood and brain than in rats. A PBPK model developed by Takano et al. (2010) used data on in vitro metabolism of acrylonitrile in rat and human liver microsomes to estimate hepatic clearance of cyanoethylene oxide. The model predicted that repeated oral exposures to acrylonitrile at 30 mg/kg/day for 14 days would result in peak blood acrylonitrile concentrations that were approximately 2-fold higher in rats than humans. Although the Takano et al. (2010) model was evaluated using oral exposure data, experimental data for metabolism were obtained from in vitro microsome studies. Taken together, the Sweeney et al. (2003) and Takano et al. (2010) PBPK models support application of an interspecies uncertainty factor of 2 to account for differences in toxicokinetics. An intraspecies uncertainty factor of 6 (3 × 2) was also applied; a factor of 3 to account for possible variation in toxicodynamics of acrylonitrile in the human population and a factor of 2 to account for variability in toxicokinetics. On the basis of PBPK modeling, Sweeney et al. (2003) predicted that human variability in toxicokinetics of acrylonitrile will result in the 95th percentile individual having acrylonitrile or cyanoethylene oxide concentrations in blood 1.8-fold higher than the average (mean) individual. That suggests that an intraspecies uncertainty factor of 2 would account for toxicokinetic variability in the human population. The total uncertainty factor was 36 (6 × 6). Time scaling for AEGL-2 specific durations from the 6-h experimental point-of-departure was performed using the equation Cn × t = k, where n = 1.1 (ten Berge et al. 1986). Data from occupational studies suggest that the AEGL-2 values are sufficiently protective. Occupational exposure data showed that routine exposure to acrylonitrile at 5-20 ppm (approximately 20-to-80-fold higher than the 8-h AEGL-2) resulted in complaints of headache, fatigue, nausea, and insomnia, which are neither irreversible nor escape-impairing effects (Sakurai and Kusumoto 1972; Sakurai et al. 1978). The 1-h and 4-h AEGL-2 values are also below the lower end of the range of exposures estimated for occupational accidents (over18 ppm) (Chen et al. 1999). The AEGL-2 values for acrylonitrile are presented in Table 1-15, and their derivation is summarized in Appendix C.
TABLE 1-15 AEGL-2 Values for Acrylonitrile
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 8.6 ppm (19 mg/m3) |
3.2 ppm (6.9 mg/m3) |
1.7 ppm (3.7 mg/m3) |
0.48 ppm (1.0 mg/m3) |
0.26 ppm (0.56 mg/m3) |
7. DATA ANALYSIS FOR AEGL-3
7.1. Human Data Relevant to AEGL-3
Quantitative exposure-response data in humans regarding the lethality of acrylonitrile were not available.
7.2. Animal Data Relevant to AEGL-3
Lethality data in multiple laboratory species (monkey, rat, dog, rabbit, guinea pig, and cat) are available. Lethality in rats appears to occur at cumulative exposures of 1,800-1,900 ppm-h for 30-min to 6-h exposure durations, although for nose-only exposures it is notably higher (about 3,800 ppm-h). Lethal response data for monkeys were not available. Dogs were the most sensitive species, with lethality in 1 of 2 dogs observed following a 4-h exposure to acrylonitrile at 65 ppm. However, a 4-h exposure of four dogs to acrylonitrile at 100 ppm resulted in no deaths, whereas exposure at 110 ppm killed two of three dogs. Data from studies of rats were the most extensive. Dudley and Neal (1942) provided response data in rats exposed for 0.5, 1, 2, 4, or 8 h. Thirty-minute exposure of rats to acrylonitrile concentrations as high as 2,445 ppm were without lethal effect. Exposure at 1,270 ppm for 1 h, 305 ppm for 2 h, 130 ppm for 4 h, or 135 ppm for 8 h did not result in deaths of any rats (16/group). A 4-h LC50 of 333 ppm was reported for rats (Haskell 1968). At higher concentrations, rats died within 2-4 h into the exposure period while deaths following exposure occurred between 7 min and 18 h; there was a 14-day observation period. There were no deaths among 10 rats exposed to acrylonitrile at 1,008 ppm for 1 h (Vernon et al. 1990). A mortality rate of 33% (1 of 3 rats) was reported in rats exposed at 650 ppm for 180 min, 950 ppm for 120 min, and 2,600 ppm for 30 min, but no deaths occurred at exposures of 1,600 ppm for 30 min or 2,400 ppm for 10 min (Appel et al. 1981a). Developmental toxicity studies conducted in rats found nonlethal effects on fetal development that included decrements in fetal body weight without fetal malformations (25-100 ppm) (Saillenfait et al. 1993a) and nonlethal fetal malformations (40 and 80 ppm) (Murray et al. 1978). Murray et al. (1978) found three malformations in two of 33 liters from dams exposed to acrylonitrile at 40 ppm and 11 malformations in six of 35 litters at 80 ppm, the most serious of which was one omphalocele at 40 and 80 ppm. These malformations were not confirmed in the Saillenfait et al. (1993a) study at concentrations up to 100 ppm. A two-generation study found weight decrements in the F1 offspring of the 90-ppm group, but no other evidence of exposure-related mortalities in adult animals, functional effects on reproduction or effects on reproductive organs, or toxicity in developing offspring at exposures up to 90 ppm (Nemec et al. 2008). No effects on resorptions or live births were found in the single-generation or two-generation studies.
7.3. Derivation of AEGL-3 Values
The AEGL-3 values were derived using BMCL05 as estimates of lethality thresholds. Data for 30-min, 1-h, 4-h, and 8-h AEGL-specific exposure periods are available from the reports by Appel et al. (1981a) and Dudley and Neal (1942). A 30-min BMCL05 of 1,784 ppm was calculated from the Appel et al. (1981a) data. The 1-, 2-, 4-, and 8-h BMCL05 values derived from lethality data published by Dudley and Neal (1942) were 1,024.4, 491.3, 179.5, and 185.8 ppm, respectively, for rats exposed to acrylonitrile at various concentrations for 1, 2, 4, or 8 h. With the exception of the 4-h value, the resulting BMCL05 values show a consistent duration-dependent relationship; therefore, the 30-min, 1-h, and 8-h estimates were used to derive corresponding AEGL-3 values. Because the 4-h BMCL05 was essentially equivalent to the 8-h BMCL05, the 4-h AEGL-3 was time-scaled using the 8-h BMCL05 of 185.9 ppm. The 10-min AEGL-3 value was derived by time-scaling from the 30-min rat BMCL05. Time scaling was performed using the equation Cn × t = k, where n = 1.1 (ten Berge et al. 1986). Although the dog appeared to be the most sensitive species, the overall database for rats is more robust. An interspecies uncertainty factor of 6 (3 × 2) was applied; a factor of 3 was applied to account for possible species differences in toxicodynamics of acrylonitrile and a factor of 2 to account for interspecies differences in toxicokinetics. On the basis of PBPK modeling, Sweeney et al. (2003) predicted a 2-fold difference the concentrations of acrylonitrile and the acrylonitrile metabolite, cyanoethylene oxide (the metabolic precursor to cyanide), in blood and brain during 8-h exposures to acrylonitrile at 2 ppm. Higher cyanoethylene oxide concentrations were predicted in human blood and brain than in rats. A PBPK model developed by Takano et al. (2010) used data on in vitro metabolism of acrylonitrile in rat and human liver microsomes to estimate hepatic clearance of cyanoethylene oxide in rats and humans. The model predicted that repeated oral exposures to acrylonitrile at 30 mg/kg/day for 14 days would result in peak blood acrylonitrile concentrations that were approximately 2-fold higher in rats than humans. Although the Takano et al. (2010) model was evaluated using oral exposure data, experimental data on metabolism were obtained from in vitro microsome studies. Taken together, the Sweeney et al. (2003) and Takano et al. (2010) PBPK models support application of an interspecies uncertainty factor of 2 to account for differences in toxicokinetics. An intraspecies uncertainty factor of 6 (3 × 2) was also applied; a factor of 3 was applied to account for possible variation in toxicodynamics of acrylonitrile in the human population and a factor of 2 to account for variability in toxicokinetics. On the basis of PBPK modeling, Sweeney et al. (2003) predicted that human variability in toxicokinetics of acrylonitrile would result in the 95th percentile individual having acrylonitrile or cyanoethylene oxide concentrations in blood 1.8-fold higher than the average (mean) individual. This suggests that an intraspecies uncertainty factor of 2 would account for toxicokinetic variability in the human population. The total uncertainty factor was 36 (6 × 6). The resulting AEGL-3 values are presented in Table 1-16, and their derivation is summarized in Appendix C.
TABLE 1-16 AEGL-3 Values for Acrylonitrile
| 10 min | 30 min | 1 h | 4 h | 8h |
| 130 ppm (280 mg/m3) |
50 ppm (110 mg/m3) |
28 ppm (61 mg/m3) |
9.7 ppm (21 mg/m3) |
5.2 ppm (11 mg/m3) |
8. SUMMARY OF AEGLs
8.1. AEGL Values and Toxicity End Points
The AEGL values for acrylonitrile are presented in Table 1-17. The AEGL-1 values are based on the absence of effects in male volunteer subjects exposed to acrylonitrile in a controlled-exposure study (Jakubowski et al. 1987) and occupational exposure data showing ocular irritation and headache at 16-20 ppm. The AEGL-2 values are based on a no-effect level for fetal toxicity (decreased fetal body weight) in rats exposed to acrylonitrile at 12 ppm for 6 h/day on gestation days 6-20 (Saillenfait et al. 1993a). The AEGL-3 values were derived on the basis of estimated lethality thresholds (BMCL05s) in rats (Dudley and Neal 1942; Appel et al. 1981a), the species for which the most lethality data are available.
8.2. Comparisons with Other Standards and Guidelines
The AEGL values and existing standards and guidelines for acrylonitrile are presented in Table 1-18. The 30-min AEGL-2 value is consistent with the immediately dangerous to life or health (IDLH) value and is approximately 26 times higher than the 30-min AEGL-2. The difference reflects different end points used to derive the values. The IDLH is based on human toxicity data and the 30-min AEGL-2 is based on fetal toxicity in rats. The emergency response planning guidline-2 (ERPG-2) is approximately 20 times higher than the 1-h AEGL-2 value. The ERPG-2 is based on reversible effects observed in dogs (salivation observed at 35 ppm for 4 h), whereas the 1-h AEGL-2 value is based on a no-effect level for fetal toxicity in rats (12 ppm, 6 h, gestation days 6-20). The ERPG-3 is approximately 3 times higher than the 1-h AEGL-3 value. The ERPG-3 is based on severe effects and lethality in dogs (65-200 ppm), whereas the 1-h AEGL-3 value is based on estimates of the duration-specific BMCL05 for lethality in rats.
8.3. Data Adequacy and Research Needs
Data were adequate for the development of AEGL values for acrylonitrile. Human data were used for deriving AEGL-1 values for 10 min and 30 min durations; however, values for 1 h, 4 h and 8 h are not recommended because they
would be higher than AEGL-2 values for the same durations. Data on developmental toxicity in rats, supported with more limited data in monkeys, were used for developing AEGL-2 values. A robust data set in rats allowed for derivation of AEGL-3 values.
TABLE 1-17 AEGL Values for Acrylonitrile
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h |
| AEGL-1 (nondisabling)a |
1.5 ppm l (3.3 mg/m3) |
1.5 ppm (3.3 mg/m3) |
NRa | NRa | NRa |
| AEGL-2 (disabling) |
8.6 ppm (19 mg/m3) |
3.2 ppm (6.9 mg/m3) |
1.7 ppm (3.7 mg/m3) |
0.48 ppm (1.0 mg/m3) |
0.26 ppm (0.56 mg/m3) |
| AEGL-3 (lethal) |
130 ppm (280 mg/m3) |
50 ppm (110 mg/m3) |
28 ppm (61 mg/m3) |
9.7 ppm (21 mg/m3) |
5.2 ppm (11 mg/m3) |
aNot recommended. Absence of an AEGL-1 value does not imply that exposure below the AEGL-2 value is without adverse effect.
TABLE 1-18 Standards and Guidelines for Acrylonitrile
| Guideline | Exposure Duration | ||||
| 1 min | 30 min | 1 h | 4 h | 8 h | |
| AEGL-1 | 1.5 ppm (3.3 mg/m3) |
1.5 ppm (3.3 mg/m3) |
NRa | NRa | NRa |
| AEGL-2 | 8.6 ppm 19 mg/m3 | 3.2 ppm 6.9 mg/m3 | 1.7 ppm 3.7 mg/m3 | 0.48 ppm 1.0 mg/m3 | 0.26 ppm 0.56 mg/m3 |
| AEGL-3 | 130 ppm (280 mg/m3) |
50 ppm (110 mg/m3) |
28 ppm (61 mg/m3) |
9.7 ppm (21 mg/m3) |
5.2 ppm (11 mg/m3) |
| ERPG-1 (AIHA)a | – | – | 10 ppm | – | – |
| ERPG-2 (AIHA) | – | – | 35 ppm | – | – |
| ERPG-3 (AIHA) | – | – | 75 ppm | – | – |
| IDLH (NIOSH)b | – | 85 ppm | – | – | – |
| TLV-TWA (ACGIH)c | – | – | – | – | 2 ppm (skin) |
| PEL-TWA (OSHA)d | – | – | – | – | 2 ppm |
| REL-TWA (NIOSH)e | – | – | – | – | 1 ppm |
| PEL-STEL/C (OSHA)f | 10 ppm (15 min) |
– | – | – | |
aNot recommended. Absence of an AEGL-1 value does not imply that exposure below the AEGL-2 value is without adverse effect.
aERPG (emergency response planning guidelines, American Industrial Hygiene Association) (AIHA 2013).
The ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor.
The ERPG-2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing irreversi-
ble or other serious health effects or symptoms that could impair an individual’s ability to take protective action.
The ERPG-3 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing life-threatening health effects.
b IDLH (immediately dangerous to life or health, National Institute for Occupational Safety and Health) (NIOSH 1994) represents the maximum concentration from which one could escape within 30 min without any escape-impairing symptoms, or any irreversible health effects.
cTLV-TWA (threshold limit value-time-weighted average, American Conference of Governmental Industrial Hygienists) (ACGIH 2012) is the time-weighted average concentration for a normal 8-h workday and a 40-h work week, to which nearly all workers may be repeatedly exposed, day after day, without adverse effect. Acrylonitrile is categorized as a confirmed animal carcinogen with unknown relevance to humans.
dPEL-TWA (permissible exposure limit – time-weighted average, Occupational Health and Safety Administration) (29CFR 1910.1045[2008]) is defined analogous to the ACGIH TLV-TWA, but is for exposures of no more than 10 h/day, 40 h/week.
eREL-TWA (recommended exposure limits – time-weighted average, National Institute for Occupational Safety and Health) (NIOSH 2011) is defined analogous to the ACGIH TLV-TWA.
fPEL-STEL/C (permissible exposure limit – short-term exposure limit and ceiling, Occupational Health and Safety Administration) (29CFR 1910.1045[2008]) is a 15-min time-weighted average that should not be exceeded at any time during the workday. A ceiling value should not be exceeded at any time.
9. REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 2012. TLVs and BEIs Based on the Documentation of the Threshold Limit values for Chemical Substances and Physical Agents and Biological Exposure Indices. American Conference of Governmental Industrial Hygienists, Cincinnati, OH.
AIHA (American Industrial Hygiene Association). 1997. Emergency Response Planning Guidelines: Acrylonitrile. American Industrial Hygiene Association, Fairfax, VA.
AIHA (American Industrial Hygiene Association). 2013. Current ERPG Values. 2013 ERPG/WEEL Handbook. American Industrial Hygiene Association Guideline Foundation, Fairfax, VA [online]. Available: https://www.aiha.org/get-involved/AIHAGuidelineFoundation/EmergencyResponsePlanningGuidelines/Documents/2013ERPGValues.pdf [accessed Mar. 25, 2014].
Appel, K.E., H. Peter, and H.M. Bolt. 1981a. Effect of potential antidotes on the acute toxicity of acrylonitrile. Int. Arch. Occup. Environ. Health 49(2):157-163.
Appel, K.E., H. Peter, M. Bolt, and H.M. Bolt. 1981b. Interaction of acrylonitrile with hepatic microsomes of rats and men. Toxicol. Lett. 7(4-5):335-340.
Babanov, G.P., V.N. Kljuchikov, N.I. Karajeva, and Z.V. Lileeva. 1959. Clinical symptoms of chronic poisoning by acrylonitrile [in Russian]. Vrach. Delo. 8:833-836 (as cited in WHO 1983).
Bader, M., and R. Wrbitzky. 2006. Follow-up biomonitoring after accidental exposure to acrylonitrile- Implications for protein adducts as a dose monitor for short-term exposure. Toxicol. Lett. 162(2-3):125-131.
Benz, F.W., and D.E. Nerland. 2005. Effect of cytochrome P450 inhibitors and anticonvulsants on the acute toxicity of acrylonitrile. Arch. Toxicol. 79(10):610-614.
Bergmark, E. 1997. Hemoglobin adducts of acrylamide and acrylonitrile in laboratory workers, smokers and nonsmokers. Chem. Res. Toxicol. 10(1):78-84.
Beskid, O., Z. Dusek, I. Solansky, and R.J. Sram. 2006. The effects of exposure to different clastogens on the pattern of chromosomal aberrations detected by FISH whole chromosome painting in occupationally exposed individuals. Mutat. Res. 594(1-2):20-29.
Bhooma, T., B. Padmavathi, and S.N. Devaraj. 1992. Effect of acrylonitrile on the procoagulant activity of rat lung. Bull. Environ. Contam. Toxicol. 48(2):321-326.
Blair, A., P.A. Stewart, D.D. Zaebst, L. Pottern, J.N. Zey, T.F. Bloom, B. Miller, E. Ward, and J. Lubin. 1998. Mortality of industrial workers exposed to acrylonitrile. Scand. J. Work Environ. Health 24(suppl. 2):25-41.
Borba, H., M. Monteiro, M.J. Proenca, T. Chaveca, V. Pereira, N. Lynce, and J. Rueff. 1996. Evaluation of some biomonitoring markers in occupationally exposed populations to acrylonitrile. Teratog. Carcinog. Mutagen. 16(4):205-218.
Buchter, A., and H. Peter. 1984. Clinical toxicology of acrylonitrile. G. Ital. Med. Lav. 6(3-4):83-86.
Burka, L.T., I.M. Sanchez, E.A. Ahmed, and B.I. Ghanayem. 1994. Comparative metabolism and deposition of acrylonitrile and methacrylonitrile in rats. Arch. Toxicol. 68(10):611-618.
Butterworth, B.E., S.R. Eldridge, C.S. Sprankle, P.K. Working, K.S. Bentley, and M.E. Hurtt. 1992. Tissue-specific genotoxic effects of acrylamide and acrylonitrile. Environ. Mol. Mutagen. 20(3): 148-155.
Campian, E.C., J. Cai, and F.W. Benz. 2002. Acrylonitrile irreversibly inactivates glyceraldehyde-3-phosphate dehydrogenase by alkylating the catalytically active cysteine 149. Chem. Biol. Interact. 140(3):279-291.
Chang, C.M., M.T. Hsia, G.D. Stoner, and I.C. Hsu. 1990. Acrylonitrile-induced sister-chromatid exchanges and DNA single-strand breaks in adult human bronchial epithelial cells. Mutat. Res. 241(4):355-360.
Chen, Y., C. Chen, S. Jin, and L. Zhou. 1999. The diagnosis and treatment of acute acrylonitrile poisoning: A clinical study of 144 cases. J. Occup. Health 41(3):172-176.
Chen, Y., C. Chen, and P. Zhu. 2000. Study on the effects of occupational exposure to acrylonitrile in workers. China Occup. Med. J. 18(3).
Cole, P., J.S. Mandel, and J.J. Collins. 2008. Acrylonitrile and cancer: A review of the epidemiology. Regul. Toxicol. Pharmacol. 52(3):342-351.
Collins, J.J., R. Cheng, G.M. Buck, J. Zhang, M. Klebanoff, E.F. Schisterman, T. Scheffers, H. Ohta, K. Takaya, H. Miyauchi, M. Markowitz, B. Divine, and S. Tsai. 2003. The feasibility of conducting a reproductive outcome study of Chinese acrylonitrile worker. J. Environ. Occup. Med. 1:29-32.
Crespi, C.L., C.G. Ryan, G.M. Seixas, T.R. Turner, and B.W. Penman. 1985. Tests for mutagenic activity using mutation assays at two loci in the human lymphoblast cell lines TK6 and AHH-1. Pp. 497-516 in Evaluation of Short-Term Tests for Carcinogens, J. Ashby, F.J. de Serres, M. Draper, M. Ishidate, Jr., B.H. Margolin, B.E. Matter, and M.D. Shelby, eds. Progress in Mutation Research Vol. 5. New York: Elsevier.
Crump, K.S., and R.B. Howe. 1984. The multistage model with a time-dependent dose pattern: Applications to carcinogenic risk assessment. Risk Anal. 4(3):163-176.
Czeizel, A.E., S. Hegedus, and L. Timar. 1999. Congenital abnormalities and indicators of germinal mutations in the vicinity of an acrylonitrile producing factory. Mutat. Res. 427(2):105-123.
Dahl, A.R., and B.A. Waruszewski. 1989. Metabolism of organonitriles and cyanide by rat nasal tissue enzymes. Xenobiotica 19(11):1201-1205.
Davis, J.H., J.E. Davies, A. Rafonnelli, and G. Reich. 1973. Investigation of fatal acrylonitrile intoxications. Pp. 547-556 in Pesticides and the Environment: A Continuing Controversy, W.B. Deichmann, ed. New York: Intercontinental Medical Book Corporation.
Delivanova, S., P. Popovski, and T. Orusev. 1978. Blepharoconjunctivitis in workers in the manufacture of synthetic polyacrylonitrile fibers [in Serbian]. God. Zb. Med. Fak. Skopje 24:279-282.
de Meester, C., F. Poncelet, M. Roberfroid, and M. Mercier. 1978. Mutagenicity of acrylonitrile. Toxicology 11(1):19-27.
Dong, D., and J. Pan. 1995. Acrylonitrile effect on worker’s reproductive system. Petrochem. Safe. Technol. Mag. 5:30-31.
Dong, D., D. Wang, X. Ai, and H. Zhang. 2000. Study of Acrylonitrile Hazardous Effects on Workers’ Reproductive System. Submitted to EPA by acrylonitrile Group, Inc., Washington, DC with cover letter dated September 15, 2000. EPA Document No. 89000000313. Microfiche No. OTS0559911.
Dorodnova, N.S. 1976. Gynecological morbidity and the specific functions of the female body in the chemical manufacture of “nitron” [in Russian]. Gig. Tr. Prof. Zabol. 8:45-46.
Drawbaugh, R.B., and T.C. Marrs. 1987. Interspecies differences in rhodanese (thiosulfate sulfurtransferase, EC 2.8.1.1) activity in liver, kidney and plasma. Comp. Biochem. Physiol. 86(2): 307-310.
Dudley, H.C., and P.A. Neal. 1942. Toxicology of acrylonitrile (vinyl cyanide). I. Study of the acute toxicity. J. Ind. Hyg. Toxicol. 24(2):27-36.
Dudley, H.C., T.R. Sweeney, and J.W. Miller. 1942. Toxicology of acrylonitrile. II. Studies of effects of daily inhalation. J. Ind. Hyg. Toxicol. 24(9):255-258.
Enikeeva, N.A., R.S. Ostrovskaja, L.V. Syso, Z.G. Podrez, L.L. Braginskaja, N.P. Gvozdev, N.E. Nesterova, A.N. Musserskaja, N.A. Suzdaltreva, and A.M. Efremov. 1976. Industrial hygiene and health status of workers involved in the manufacture of the synthetic fibre Nitron [in Russian]. Gig. Tr. Ohr. Zdor. Rab. Nef. Negtehim. Prom-sti. 9:22-25 (as cited in WHO 1983).
EPA (U.S. Environmental Protection Agency). 1984. Health Assessment Document for Acrylonitrile. EPA-600/8-82-007F. Office of Health and Environmental Assessment, Environmental Criteria and Assessment Office, U.S. Environmental Protection Agency, Research Triangle Park, NC.
EPA (U.S. Environmental. Protection Agency). 1991. Acrylonitrile (CAS No. 107-13-01). Integrated Risk information System, U.S. Environmental Protection Agency [online]. Available: http://www.epa.gov/ncea/iris/subst/0206.htm [accessed Mar. 24, 2014].
Fahmy, M.A. 1999. Evaluation of the genotoxicity of acrylonitrile in different tissues of male mice. Cytologia 64(1):1-9.
Fan, W., W.L. Wang, S. Ding, Y.L. Zhou, and F.S. Jin. 2006. Application of micronucleus test of buccal mucosal cells in assessing the genetic damage of workers exposed to acrylonitrile [in Chinese]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 24(2):106-108.
Farooqui, M.Y., and A.E. Ahmed. 1983. In vivo interactions of acrylonitrile with macromolecules in rats. Chem. Biol. Interact. 47(3):363-371.
Fechter, L.D., S.F. Klis, N.A. Shirwany, T.G. Moore, and D.B. Rao. 2003. Acrylonitrile produces transient cochlear function loss and potentiates permanent noise-induced hearing loss. Toxicol. Sci. 75(1):117-123.
Felter, S.P., and J.S. Dollarhide. 1997. Acrylonitrile: A reevaluation of the database to support an inhalation cancer risk assessment. Regul. Toxicol. Pharmacol. 26(3):281-287.
Fennell, T.R., G.L. Kedderis, and S.C. Sumner. 1991. Urinary metabolites of [1,2,3-13C]acrylonitrile in rats and mice detected by 13C nuclear magnetic resonance spectroscopy. Chem. Res. Toxicol. 4(6):678-687.
Fennell, T.R., J.P. MacNeela, R.W. Morris, M. Watson, C.L. Thompson, and D.A. Bell. 2000. Hemoglobin adducts from acrylonitrile and ethylene oxide in cigarette smokers: Effects of glutathione S-transferase T1-null and M1-null genotypes. Cancer Epidemiol. Biomarkers Prev. 9(7):705-712.
Gargas, M.L., M.E. Anderson, S.K. Teo, R. Batra, T.R. Fennell, and G.L. Kedderis. 1995. A physiologically-based dosimetry description of acrylonitrile and cyanoethylene oxide in the rat. Toxicol. Appl. Pharmacol. 134(2):185-194.
Ghanayem, B.I., M.Y. Farooqui, O. Elshabrawy, M.M. Mumtaz, and A.E. Ahmed. 1991. Assessment of the acute acrylonitrile-induced neurotoxicity in rats. Neurotoxicol. Teratol. 13(5):499-502.
Gincheva, N., N. Stamova, L. Hinkova, and S. Kyurktchiev, M. Spasovski, A. Bainova, M. Muhtarova, and V. Hristeva. 1977. Study of the health status of workers from the acrylonitrile department. P. 41 in Proceedings of the International Symposium on Occupational Health in the Production of Artificial Fibres-Abstracts, June 6-10, 1977, Finland (as cited in WHO 1983).
Grunske, F. 1949. Ventox and Ventox intoxication [in German]. Dtsch. Med. Wochenschr. 74(35/36):1081-1083.
Haber, F.R. 1924. On the history of the gas war. Pp. 76-92 in Five Lectures from the Years 1920-1923[in German]. Berlin, Germany: Verlag von Julius Springer.
Haskell Laboratory. 1942. Toxicity of Vinyl Cyanide. Medical Research Project No. M-97. Haskell Laboratory of Industrial Toxicology, Wilmington, DE. March 25, 1942.
Haskell Laboratory. 1968. Acute Inhalation Toxicity in Rats with Acrylonitrile (Inhibited): Methacrylonitrile (Inhibited); and Acetonitrile, October 21, 1968. Submitted to EPA by DuPont, Wilmington, DE, with cover letter dated October 15, 1992. EPA Document No. 88-920009947. Microfiche No. OTS0571605.
HSDB (Hazardous Substances Data Bank). 2013. Acrylonitrile. TOXNET Toxicology Data Network, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/ [accessed Jan. 13, 2014].
IARC (International Agency for the Research on Cancer). 1999. Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Vol. 71. Lyon, France: IARC Press [online]. Available: http://monographs.iarc.fr/ENG/Monographs/vol71/mono71.pdf [accessed March 20, 2014].
Ivanescu, M., M. Berinde, and L. Simionescu. 1990. Testosterone in sera of workers exposed to acrylonitrile. Endocrinologie 28(3-4):187-192.
Jakubowski, M., I. Linhart, G. Pielas, and J. Kopecky. 1987. 2-Cyanoethylmercapturic acid (CEMA) in the urine as a possible indicator of exposure to acrylonitrile. Br. J. Ind. Med. 44(12):834-840.
Kaneko, Y., and K. Omae. 1992. Effect of chronic exposure to acrylonitrile on subjective symptoms. Keio J. Med. 41(1):25-32.
Kedderis, G.L., R. Batra, and D.R. Koop. 1993. Epoxidation of acrylonitrile by rat and human cytochromes P450. Chem. Res. Toxicol. 6(6):866-871.
Kedderis, G.L., R. Batra, and M.J. Turner, Jr. 1995. Conjugation of acrylonitrile and 2-cyanoethylene oxide with hepatic glutathione. Toxicol. Appl. Pharmacol. 135(1): 9-17.
Kedderis, G.L., S.K. Teo, R. Batra, S.D. Held, and M.L. Gargas. 1996. Refinement and verification of the physiologically-based dosimetry description for acrylonitrile in rats. Toxicol. Appl. Pharmacol. 140(2):422-435.
Lijinsky, W., and A.W. Andrews. 1980. Mutagenicity of vinyl compounds in Salmonella typhimurium. Teratog. Carcinog. Mutagen. 1(3):259-267.
Litchfield, J.T., and F. Wilcoxon. 1949. Simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 96(2):99-113.
Lorz, H. 1950. Percutaneous poisoning with acrylonitrile [in German]. Dtsch. Med. Wochenschr. 75(33-34):1087-1088.
Maltoni, C., A. Cilberti, and V. Di Maio. 1977. Carcinogenicity bioassays on rats of acrylonitrile administered by inhalation and by ingestion. Med. Lav. 69(6):401-411.
Maltoni, C., A. Cilberti, G. Cotti, and G. Perino. 1988. Long-term carcinogenicity bioassay on acrylonitrile administered by inhalation and by ingestion to Sprague-Dawley rats. Ann. NY Acad. Sci. 534:179-202.
Martin, C.N., and J. Campbell. 1985. Tests for the induction of unscheduled DNA repair synthesis in HeLa cells. Pp. 375-379 in Evaluation of Short-Term Tests for Carcinogens, J. Ashby, F.J. de Serres, M. Draper, M. Ishidate, Jr., B.H. Margolin, B.E. Matter, and M.D. Shelby, eds. Progress in Mutation Research Vol. 5. New York: Elsevier.
Milvy, P., and M. Wolff. 1977. Mutagenic studies with acrylonitrile. Mutat. Res. 48(3-4):271-278.
Müller, G., C. Verkoyen, N. Soton, and K. Norpoth. 1987. Urinary excretion of acrylonitrile and its metabolites in rats. Arch. Toxicol. 60(6):464-466.
Murray, F.J., K.D. Nitschke, J.A. John, F.A. Smith, J.F. Quast, C.D. Blogg, and B.A. Schwetz. 1976. Teratologic Evaluation of Acrylonitrile Monomer given to Rats by Gavage. Toxicological Research Laboratory, Health and Environmental Research, Dow Chemical, USA, Midland, MI. October 22, 1976. Submitted to EPA by DuPont, Wilmington, DE with cover letter dated August 10, 1992. EPA Document No. 88920010410. Microfiche No. OTS0555785.
Murray, F.J., B.A. Schwetz, K.D. Nitsche, J.A. John, J.M. Norris, and P.J. Gehring. 1978. Teratogenicity of acrylonitrile given to rats by gavage or by inhalation. Food Cos-met. Toxicol. 16(6):547-551.
Muto, T., H. Sakurai, K. Omae, H. Minaguchi, and M. Tachi. 1992. Health profiles of workers exposed to acrylonitrile. Keio J. Med. 41(3):154-160.
Nagata, Y. 2003. Measurement of odor threshold by triangle odor bag method. Pp. 118-127 in Odor Measurement Review. Japan Ministry of the Environment, Tokyo [online]. Available: https://www.env.go.jp/en/air/odor/measure/02_3_2.pdf [accessed March 20, 2014].
Nemec, M.D., D.T. Kirkpatrick, J. Sherman, J.P. Van Miller, M.L. Pershing, and D.E. Strother. 2008. Two-generation reproductive toxicity study of inhaled acrylonitrile vapors in Crl:CD(SD) rats. Int. J. Toxicol. 27(1):11-29.
Nerland, E.E., F.W. Benz, and C. Babiuk. 1989. Effects of cysteine isomers and derivatives on acute acrylonitrile toxicity. Drug Metab. Rev. 20(2-4):233-246.
NIOSH (National Institute for Occupational Safety and Health). 1994. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHs): Acrylonitrile. National Institute for Occupational Safety and Health [online]. Available: http://www.cdc.gov/niosh/idlh/107131.html [accessed Mar. 24, 2014].
NIOSH (National Institute for Occupational Safety and Health). 2011. NIOSH Pocket Guide to Chemical Hazards: Acrylonitrile. National Institute for Occupational Safety and Health [online]. Available: http://www.cdc.gov/niosh/npg/npgd0014.html [accessed Mar. 24, 2014].
NPI (National Pollution Inventory). 2006. Acrylonitrile (2-Propenenitrile) Fact Sheet. Australian Government, Department of the Environment, Canberra [online]. Available: http://www.npi.gov.au/resource/acrylonitrile-2-propenenitrile [accessed Mar. 20, 2014].
NRC (National Research Council). 1985. Hydrazine. Pp. 5-21 in Emergency and Continuous Exposure Guidance Levels for Selected Airborne Contaminants, Vol. 5. Washington, DC: National Academy Press.
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
NTP (National Toxicology Program). 2011. Acrylonitrile. Pp. 28- 20 in Report on Carcinogens, 11th Ed. U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program [online]. Available: http://ntp.niehs.nih.gov/ntp/roc/twelfth/profiles/Acrylonitrile.pdf [accessed March 24, 2014].
Obe, G., A. Hille, R. Jonas, S. Schmidt, and U. Thenhaus. 1985. Tests for the induction of sister-chromatid exchanges in human peripheral lymphocytes in culture. Pp. 439-442 in Evaluation of Short-Term Tests for Carcinogens, J. Ashby, F.J. de Serres, M. Draper, M. Ishidate, Jr., B.H. Margolin, B.E. Matter, and M.D. Shelby, eds. Progress in Mutation Research Vol. 5. New York: Elsevier.
O’Berg, M.T. 1980. Epidemiologic study of workers exposed to acrylonitrile. J. Occup. Med. 22(4):245-252.
O’Berg, M.T., J.L. Chen, C.A. Burke, J. Walrath, and S. Pell. 1985. Epidemiologic study of workers exposed to acrylonitrile: An update. J. Occup. Med. 27(11):835-840.
Perbellini, L., A. Ganzi, G. Venturi, M. Cerpelloni, and F. Brugnone. 1998. Biological monitoring of acrylonitrile exposure. G. Ital. Med. Lav. Ergon. 20(1):10-14.
Perocco, P., G. Pane, S. Bolognesi, and M. Zannotti. 1982. Increase in sister chromatid exchange and unscheduled synthesis of deoxyribonucleic acid by acrylonitrile in human lymphocytes in vitro. Scand. J. Work Environ. Health 8(4):290-293.
Peter, H., and H.M. Bolt. 1984. Experimental pharmacokinetics and toxicology of acrylonitrile. G. Ital. Med. Lav. 6(3-4):77-81.
Pilon, D., A.E. Roberts, and D.E. Rickert. 1988. Effect of glutathione depletion on the uptake of acrylonitrile vapors and on its irreversible association with tissue macromolecules. Toxicol. Appl. Pharmacol. 95(2):265-278.
Quast, J.F., D.J. Schuetz, M.F. Balmer, T.S. Gushow, C.N. Park, and M.J. McKenna. 1980. A Two-year Toxicity and Oncogenicity Study with Acrylonitrile Following Inhalation Exposure of Rats. Dow Chemical Co., Toxicology Research Laboratory, Midland, MI. EPA Document No. 88920002471. Microfiche No. OTS0537281.
Recio, L., D. Simpson, J. Cocharane, H. Liber, and T.R. Skopek. 1989. Mutational specifity of 2-cyanoethylene oxide in human lymphoblastoid cells. Environ. Mol. Mutagen. 14(suppl. 15):162.
Recio, L., D. Simpson, J. Cochrane, H. Liber, and T.R. Skopek. 1990. Molecular analysis of hprt mutants induced by 2-cyanoethylene oxide in human lymphoblastoid cells. Mutat. Res. 242(3):195-208.
Rinehart, W.E., and T. Hatch. 1964. Concentration-time product (CT) as an expression of dose in sublethal exposures to phosgene. Am. Ind. Hyg. Assoc. J. 25(6):545-553.
Rizzi, R., E. Chiesara, D. Cova, M. Mattioli, and R. Di Lernia. 1984. Acrylonitrile: Mutagenicity in yeasts and genotoxicity in HeLa cells. Mutat. Res. 130:223.
Roberts, A.E., G.L. Kedderis, M.J. Turner, D.E. Rickert, and J.A. Swenberg. 1991. Species comparison of acrylonitrile expoxidation by microsomes from mice, rats and humans: Relationship to epoxide concentrations in mouse and rat blood. Carcinogenesis 12(3):401-404.
Rongzhu, L., C. Ziqiang, J. Fusheng, and J.J. Collins. 2005. Neurobehavioral effects of occupational exposure to acrylonitrile in Chinese workers. Environ. Toxicol. Pharmacol. 19(3):695-700.
Ruth, J.H. 1986. Odor thresholds and irritation levels of several chemical substances: A review. Am. Ind. Hyg. Assoc. J. 47(3):A142-A151.
Saillenfait, A.M., and J.P. Sabate. 2000. Comparative developmental toxicities of aliphatic nitriles: In vivo and in vitro observations. Toxicol. Appl. Pharmacol. 163(2):149-163.
Saillenfait, A.M., P. Bonnet, J.P. Guenier, and J. De Ceaurriz. 1993a. Relative developmental toxicities of inhaled aliphatic mononitriles in rats. Fundam. Appl. Toxicol. 20(3):365-375.
Saillenfait, A.M., J.P. Payan, I. Langonne, D. Beydon, M.C. Grandclaude, J.P. Sabate, and J. de Ceaurriz. 1993b. Modulation of acrylonitrile-induced embryotoxicity in vitro by glutathion depletion. Arch. Toxicol. 67(3):164-172.
Sakurai, H., and M. Kusumoto. 1972. Epidemiological study of health impairment among acrylonitrile workers [in Japanese]. J. Sci. Labour. 48(5):273-282.
Sakurai, H., M. Onodera, T. Utsunomiya, H. Minakuchi, H. Iwai, and H. Mutsumura. 1978. Health effects of acrylonitrile in acrylic fibre factories. Br. J. Ind. Med. 35(3):219-225.
Sekihashi, K., A. Yamamoto, Y. Matsumura, S. Ueno, M. Watanabe-Akanuma, F. Kassie, S. Knasmüller, S. Tsuda, and Y.F. Sasaki. 2002. Comparative investigation of multiple organs of mice and rats in the comet assay. Mutat. Res. 517(1-2):53-75.
Solomon, J.J., I.L. Cote, M. Wortman, K. Decker, and A. Segal. 1984. In vitro alkylation of calf thymus DNA by acrylonitrile. Isolation of cyanoethyl-adducts of guanine and thymine and carboxyethyl-adducts of adenine and cytosine. Chem. Biol. Interact. 51(2):167-190.
Sweeney, L.M., M.L. Gargas, D.E. Strother, and G.L. Kedderis. 2003. Physiologically based pharmacokinetic model parameter estimation and sensitivity and variability analyses for acrylonitrile disposition in humans. Toxicol. Sci. 71(1):27-40.
Sumner, S.C., T.R. Fennell, T.A. Moore, B. Chanas, F. Gonzalez, and B.I. Ghanayem. 1999. Role of cytochrome P450 2E1 in the metabolism of acrylamide and acrylonitrile in mice. Chem. Res. Toxicol. 12(11):1110-1116.
Takano, R., N. Murayama, K. Horiguchi, M. Kitajima, M. Kumamoto, F. Shono, and H. Yamazaki. 2010. Blood concentrations of acrylonitrile in humans after oral administration extrapolated from in vivo rat pharmacokinetics, in vitro human metabo-
lism, and physiologically based pharmacokinetic modeling. Regul. Toxicol. Pharmacol. 58(2):252-258.
Tardif, R., D. Talbot, M. Gerin, and J. Brodeur. 1987. Urinary excretion of mercapturic acids and thiocyanate in rats exposed to acrylonitrile: Influence of dose and route of administration. Toxicol. Lett. 39(2-3):255-261.
ten Berge, W.F., A. Zwart, and L.M. Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapours and gases. J. Hazard. Mater. 13(3):301-309.
Thiess, A.M., and I. Fleig. 1978. Analysis of chromosomes of workers exposed to acrylonitrile. Arch. Toxicol. 41(2):149-152.
Van Doorn, R., M. Ruijten, and T. van Harreveld. 2002. Guidance for the Application of Odor in Chemical Emergency Response. Presented at the NAC/AEGL-Meeting September 2002.
Vernon, P.A., L.H. Dulak, and R. Deskin. 1990. Acute toxicologic evaluation of acrylonitrile. J. Am. Coll. Toxicol. 1:114-115.
Vodiĉka, P., I. Gut, and E. Frantík. 1990. Effects of inhaled acrylic acid derivatives in rats. Toxicology 65(1-2):209-221.
Vogel, R.A., and W.M. Kirkendall. 1984. Acrylonitrile (vinyl cyanide) poisoning: A case report. Tex. Med. 80(5):48-51.
Wakata, A., Y. Miyamae, S. Sato, T. Suzuki, T. Morita, N. Asano, T. Awogi, K. Kondo, and M. Hayashi. 1998. Evaluation of the rat micronucleus test with bone marrow and peripheral blood: Summary of the 9th collaborative study by CSGMT/JEMS.MMS. Environ. Mol. Mutagen. 32(1):84-100.
WHO (World Health Organization). 1983. Acrylonitrile. Environonmental Health Criteria. 28. Geneva: World Health Organization [online]. Available: http://www.inchem.org/documents/ehc/ehc/ehc28.htm [accessed March 20, 2014].
WIL Research Laboratories. 2005. Acute Inhalation Toxicity Study of Acrylonitrile in Albino Rats. WIL-542001. WIL Research Laboratories, Ashland, OH.
Willhite, C.C., and R.P. Smith. 1981. The role of cyanide liberation in the acute toxicity of aliphatic nitriles. Toxicol. Appl. Pharmacol. 59(3):589-602.
Willhite, C.C., V.H. Ferm, and R.P. Smith. 1981a. Teratogenic effects of aliphatic nitriles. Teratology 23(3):317-323.
Willhite, C.C., M. Marin-Padilla, V.H. Ferm, and R.P. Smith. 1981b. Morphogenesis of axial skeletal (dysraphic) disorders induced by aliphatic nitriles. Teratology 23(3): 325-333.
Wilson, R.H., and W.E. McCormick. 1949. Acrylonitrile: Its physiology and toxicology. Ind. Med. Surg. 18(6):243-245.
Wilson, R.H., G.V. Hough, and W.E. McCormick. 1948. Medical problems encountered in the manufacture of American-made rubber. Ind. Med. Surg. 17(6):199-207.
Xu, D.X., Q.X. Zhu, L.K. Zheng, Q.N. Wang, H.M. Shen, L.X. Deng, and C.N. Ong. 2003. Exposure to acrylonitrile induced DNA strand breakage and sex chromosome aneuploidy in human spermatozoa. Mutat. Res. 537(1):93-100.
Yates, J.M., S.C. Sumner, M.J. Turner, L. Recio, and T.R. Fennell. 1993. Characterization of an addict and its degradation product produced by the reaction of cyanoethylene oxide with deoxythymidine and DNA. Carcinogenesis 14(7):1363-1369.
Zotova, L.A. 1975. Working conditions in the production of acrylonitrile and their effect on workers. Gig. Tr. Prof. Zabol. (8):8-11.
APPENDIX A
DERIVATION OF LEVEL OF DISTINCT ODOR AWARENESS FOR ACRYLONITRILE
The level of distinct odor awareness (LOA) represents the concentration above which it is predicted that more than half of the exposed population will experience at least a distinct odor intensity, and about 10% of the population will experience a strong odor intensity. The LOA should help chemical emergency responders in assessing the public awareness of the exposure due to odor perception. The LOA derivation follows the guidance of van Doorn et al. (2002). The odor detection threshold (OT50) for acrylonitrile was reported to be 8.8 ppm (Nagata 2003). Nagata (2003) also determined the odor threshold for the reference chemical n-butanol (OT50 = 0.038 ppm) for derivation of the corrected OT50, as shown below:
OT50 for acrylonitrile: 8.8 ppm
OT50 for n-butanol: 0.038 ppm
Corrected OT50 for acrylonitrile = 8.8 ppm × 0.04 ppm ÷ 0.038 ppm = 9.3 ppm
The concentration (C) leading to an odor intensity (I) of distinct odor detection (I = 3) is derived using the Fechner function:
I = kw × log (C ÷ OT50) + 0.5
For the Fechner coefficient, the default of kw = 2.33 will be used due to the lack of chemical-specific data:
3 = 2.33 × log (C ÷ 9.3) + 0.5, which can be rearranged to
Log (C ÷ 9.3) = (3 - 0.5) ÷ 2.33 = 1.07, and results in
C = (101.07) × 9.3 = 109.3 ppm
The resulting concentration is multiplied by an empirical field correction factor. It takes into account that in everyday life factors such as sex, age, sleep, smoking, upper airway infections, and allergies, as well as distraction, may increase the odor detection threshold by a factor of 4. In addition, it takes into account that odor perception is very fast (about 5 seconds), which leads to the perception of concentration peaks. On the basis of current knowledge, a factor of 1/3 is applied to adjust for peak exposure. Adjustment for distraction and peak exposure lead to a correction factor of 4 ÷ 3 = 1.33.
LOA = C × 1.33 = 110 ppm × 1.33 = 145.4 ppm
Therefore, the LOA for acrylonitrile is 145 ppm.
APPENDIX B
CARCINOGENICITY ASSESSMENT FOR ACRYLONITRILE
Carcinogenicity assessments for lifetime exposure to inhaled acrylonitrile have been conducted by EPA (1991) and Felter and Dollarhide (1997). On the basis of these assessments, two calculations for cancer risk are presented below.
Calculation A:
The EPA (1991) Integrated Risk Information System (IRIS) program derived an inhalation unit risk for acrylonitrile of 6.8 × 10-5 (μg/m3)-1 based on a statistically significant excess incidence of respiratory cancer from an occupational study (O’Berg 1980). In a cohort of 1,345 male textile workers exposed to acrylonitrile at 5-20 ppm (estimated) for at least 10 years, 25 cases of cancer, including eight cases of respiratory cancer, were reported. A positive trend was observed for increased cancer incidence with increased exposure duration and increased duration of followup time. However, a follow-up study of this cohort (O’Berg et al. 1985) did not find an increased incidence of respiratory cancer. The IRIS Program is currently reassessing this chemical.
To transform the unit risk for continuous lifetime exposure derived by EPA (1984) to a single 24-h exposure estimate, default procedures (linear transformation and correction by a factor of 6 to account for the relevance of sensitive stages in development) were applied, as recommended in the standing operating procedures for AEGL development (NRC 2001, see Appendix A).
On the basis of the inhalation unit risk of 6.8 × 10-5 (μg/m3)-1 derived by EPA (1991), an acrylonitrile concentration of 1.47 μg/m3 (equivalent to 1.47 × 10-3 mg/m3 or 6.78 × 10-4 ppm) is associated with a risk level of 1 in 10,000 for lifetime exposure.
To convert the 70-year exposure to a 24-h exposure, the concentration associated with a 1 in 10,000 risk level is multiplied by 25,600 (the number of days in 70 years):
24-h exposure = d × 25,600; where d = 6.78×10-4 ppm
= 6.78×10-4 ppm × 25,600 days
= 17.4 ppm
To account for uncertainty regarding variability in the stage of cancer process that acrylonitrile or its metabolites may act, a multistage factor of 6 is applied (Crump and Howe 1984):
= 17.4 ppm ÷ 6
= 2.9 ppm
Therefore, on the basis of the potential carcinogenicity of acrylonitrile, an acceptable 24-h exposure would be 2.9 ppm for a 10-4 risk.
If the exposure is limited to a fraction (f) of a 24-h period, the fractional exposure is 1/f × 24 h (NRC 1985). Extrapolation to 10 min was not calculated due to unacceptably large inherent uncertainty. For a 10-4 risk:
24-h exposure =2.9 ppm (5.6 mg/m3)
8-h exposure = 8.7 ppm (20 mg/m3)
4-h exposure = 17 ppm (38 mg/m3)
1-h exposure = 70 ppm (150 mg/m3)
30-min exposure = 140 ppm (300 mg/m3)
Exposures relating to 10-4, 10-5, and 10-6 risk levels are shown below in Table B-1.
TABLE B-1 Potential Cancer Riska Associated with Acute Inhalation to Acrylonitrile
| Risk Level | Exposure Duration | ||||
| 0.5 h | 1 h | 4 h | 8 h | 24 h | |
| 1 in 10,000 (10-4) |
140 ppm (300 mg/m3) |
69 ppm (150 mg/m3) |
17 ppm (38 mg/m3) |
8.7 ppm (20 mg/m3) |
2.9 ppm (6.3 mg/m3) |
| 1 in 100,000 (10-5) |
14 ppm (30 mg/m3) |
6.9 ppm (15 mg/m3) |
1.7 ppm (3.8 mg/m3) |
0.87 ppm (2.0 mg/m3) |
0.29 ppm (0.56 mg/m3) |
| 1 in 1,000,000 (10-6) |
1.4 ppm (3.0 mg/m3) |
0.69 ppm (1.5 mg/m3) |
0.17 ppm (0.38 mg/m3) |
0.087 ppm (0.20 mg/m3) |
0.029 ppm (0.056 mg/m3) |
aBased on the EPA (1984) carcinogenicity assessment.
A comparison of the AEGL-2 and AEGL-3 values with the estimated acrylonitrile concentration associated with a 10-4 cancer risk is shown in Table B-2. Estimated cancer risks for the AEGL-2 and AEGL-3 values are also provided, obtained by assuming a linear relationship between exposure concentration and cancer risk.
TABLE B-2 Comparison of AEGL Values and Potential Cancer Riska Associated with Acute Inhalation Exposure to Acrylonitrile
| Value | Exposure Duration | |||||
| 10 min | 30 min | 1 h | 4 h | 8 h | 24 h | |
| Cancer Risk (10-4) | – | 140 ppm | 70 ppm | 17 ppm | 8.7 ppm | 2.9 ppm |
| AEGL-1 value: | 1.5 ppm | 1.5 ppm | NRb | NRb | NRb | – |
| Estimated cancer risk: | – | 1.1 × 10-6 | – | – | – | – |
| AEGL-2 value: | 8.6 ppm | 3.2 ppm | 1.7 ppm | 0.48 ppm | 0.26 ppm | – |
| Estimated cancer risk: | – | 2.3 × 10-6 | 2.4 × 10-6 | 2.8 × 10-6 | 3.0 × 10-6 | – |
| AEGL-3 value: | 130 ppm | 50 ppm | 28 ppm | 9.7 ppm | 5.2 ppm | – |
| Estimated cancer risk: | – | 3.6 × 10-5 | 4.0 × 0-5 | 5.6 × 10-5 | 6.0 × 0-5 | – |
aBased on the EPA (1984) carcinogenicity assessment.
bNot recommended. Absence of an AEGL-1 value does not imply that exposure below the AEGL-2 value is without adverse effect.
Calculation B:
Felter and Dollarhide (1997) conducted a carcinogenicity assessment for acrylonitrile on the basis of rat tumor data from a 2-year inhalation study conducted by Quast et al. (1980). Briefly, Sprague-Dawley rats (100/sex/concentration) were exposed to acrylonitrile at 0 (control), 20, and 80 ppm for 6 h/day, 5 days/week for 2 years. The incidence of brain tumors, identified histopathologically as focal or multifocal glial cell tumors (astrocytomas), was significantly increased (p < 0.05) for both male and females at 80 ppm compared with the controls. Felter and Dollarhide (1997) developed a dose-response analysis of the astrocytoma incidence data reported by Quast et al. (1980). A polynomial dose-response model was applied to the data to estimate the EC10 and lower confidence limit on the EC10 (LEC10). The calculated unit risks for lifetime continuous exposure ranged from 8.2 × 10-6 per 1 µg/m3 (on the basis of the EC10) to 1.1 × 10-5 per 1 µg/m3 (on the basis of the LEC10). The unit risk based on the LEC10 corresponds to a lifetime 1 × 10-4 risk specific exposure concentration of 9 µg/m3 (4.1 × 103 ppm).
To transform the unit risk for continuous lifetime exposure derived by Felter and Dollarhide (1997) to a single 24-h exposure estimate, default procedures (linear transformation and correction by a factor of 6 to account for the relevance of sensitive stages in development) were applied, as recommended in the standing operating procedures on AEGL development (NRC 2001, see Appendix A).
To convert the 70-year exposure to a 24-h exposure, the concentration associated with a 1 in 10,000 risk level is multiplied by 25,600 (the number of days in 70 years):
24-h exposure = d × 25,600; where d = 4.1 × 10-3 ppm
= 4.1 × 10-3 ppm × 25,600 days
= 106 ppm
To account for uncertainty regarding variability in the stage of cancer process that acrylonitrile or its metabolites may act, a multistage factor of 6 is applied (Crump and Howe 1984):
= 106 ppm ÷ 6
= 18 ppm
Therefore, on the basis of the potential carcinogenicity of acrylonitrile, an acceptable 24-h exposure would be 18 ppm for a 10-4 risk.
If the exposure is limited to a fraction (f) of a 24-h period, the fractional exposure is 1/f × 24 h (NRC 1985). Extrapolation to 10 min was not calculated due to unacceptably large inherent uncertainty. For a 10-4 risk:
24-h exposure = 18 ppm (39 mg/m3)
8-h exposure = 54 ppm (120 mg/m3)
4-h exposure = 110 ppm (240 mg/m3)
1-h exposure = 430 ppm (940 mg/m3)
30-min exposure = 860 ppm (1,800 mg/m3)
Exposures relating to 10-4, 10-5, and 10-6 risk levels are shown in Table B-3.
TABLE B-3 Potential Cancer Risk Associated with Acute Inhalation to Acrylonitrile, Based on the Felter and Dollarhide (1997) Carcinogenicity Assessment
| Risk Level | Exposure Duration | ||||
| 0.5 h | 1 h | 4 h | 8 h | 24 h | |
| 1 in 10,000 (10-4) |
860 ppm (1900 mg/m3) |
430 ppm (940 mg/m3) |
110 ppm (240 mg/m3) |
54 ppm (120 mg/m3) |
18 ppm (39 mg/m3) |
| 1 in 100,000 (10-5) |
86 ppm (190 mg/m3) |
43 ppm (94 mg/m3) |
11 ppm (24 mg/m3) |
5.4 ppm (12 mg/m3) |
1.8 ppm (3.9 mg/m3) |
| 1 in 1,000,000 (10-6) |
8.6 ppm (19 mg/m3) |
4.3 ppm (9.4 mg/m3) |
1.1 ppm (2.4 mg/m3) |
0.54 ppm (1.2 mg/m3) |
0.018 ppm (0.39 mg/m3) |
A comparison of the AEGL-2 and AEGL-3 values with the estimated acrylonitrile concentration associated with a 10-4 cancer risk is shown in Table B-4. Estimated cancer risks for the AEGL-2 and AEGL-3 values are also provided, obtained by assuming a linear relationship between exposure concentration and cancer risk.
TABLE B-4 Comparison of AEGL-values and Potential Cancer Risk Associateda with Acute Inhalation Exposure to Acrylonitrile
| Value | Exposure Duration | |||||
| 10-min | 30-min | 1-h | 4-h | 8-h | 24-h | |
| Cancer Risk (10-4) | – | 860 ppm | 430 ppm | 110 ppm | 54 ppm | 18 ppm |
| AEGL-1 value: | 1.5 ppm | 1.5 ppm | NRb | NRb | NRb | – |
| Estimated cancer risk: | – | 1.7 × 10-7 | – | – | – | – |
| AEGL-2 value: | 8.6 ppm | 3.2 ppm | 1.7 ppm | 0.48 ppm | 0.26 ppm | – |
| Estimated cancer risk: | – | 3.7 × 10-7 | 4.0 × 10-7 | 4.5 × 10-7 | 4.8 × 10-7 | – |
| AEGL-3 value: | 130 ppm | 50 ppm | 28 ppm | 9.7 ppm | 5.2 ppm | – |
| Estimated cancer risk: | – | 5.8 × 10-6 | 6.5 × 10-6 | 9.0 × 10-6 | 9.7 × 10-6 | – |
aBased on the Felter and Dollarhide (1997) carcinogenicity assessment.
bNot recommended. Absence of an AEGL-1 value does not imply that exposure below the AEGL-2 value is without adverse effect.
APPENDIX C
DERIVATION OF AEGL VALUES
Derivation of AEGL-1 Values
| Key study: | Jakubowski, M., I. Linhart, G. Pielas, and J. Kopecky. 1987. 2-Cyanoethylmercapturic acid (CEMA) in the urine as a possible indicator of exposure to acrylonitrile. Br. J. Ind. Med. 44(12):834-840. Sakurai, H., M. Onodera, T. Utsunomiya, H. Minakuchi, H. Iwai, and H. Mutsumura. 1978. Health effects of acrylonitrile in acrylic fibre factories. Br. J. Ind. Med. 35(3):219-225. |
| Critical effect: | Absence of effects in volunteer subjects exposed for 8 h to acrylonitrile at 4.6 ppm (Jakubowski et al. 1987), supported by observations of mild effects (initial conjunctival irritation, for which there was some accommodation) in workers routinely exposed to acrylonitrile at approximately 5 ppm (Sakurai et al. 1978). That concentration is approximately 3-fold lower than concentrations reported by Wilson et al. (1948) to be associated with more severe effects in occupational settings (16-100 ppm for 20-45 min: headache, nasal and ocular irritation, discomfort of the chest, nervousness, and irritability). |
| Time scaling: | None applied. No data are available on the relationship between exposure duration and severity of responses to acrylonitrile. Typically, in the absence of this information, AEGL-1 values based on an 8-h point-of-departure would be time scaled. However, in this case, the effect is ocular irritation, which would not be expected to have a response threshold that varies with exposure duration. Therefore, it is prudent to not time scale and the AEGL-1 values were held constant at 1.5 ppm for the 10-min and 30-min values. That concentration exceeds AEGL-2 values for longer exposure durations; therefore, AEGL-1 values for the 1-h, 4-h, and 8-h durations are not recommended. |
| Uncertainty factors: | Total uncertainty factor: 3 Interspecies: 1, human subjects. Intraspecies: 3, pharmacokinetic variability is not likely to be significant for mild effects (ocular irritation) of low-level exposure. However, the point-of-departure is |
| based on studies of healthy adults and, in the occupational studies, subjects who experienced repeated exposures to acrylonitrile, which may have resulted in some accommodation to the ocular irritation. | |
| Modifying factor: | None |
| Calculations: | |
| 10-min AEGL-1: | 6 ppm ÷ 3 = 4. 1.5 ppm |
| 30-min AEGL-1: | 6 ppm ÷ 3 = 4. 1.5 ppm |
| 1-, 4-, and 8-h AEGL-1: | Not recommended |
| Derivation of AEGL-2 Values | |
| Key study: | Saillenfait, A.M., P. Bonnet, J.P. Guenier, and J. De Ceaurriz. 1993a. Relative developmental toxicities of inhaled aliphatic mononitriles in rats. Fundam. Appl. Toxicol. 20(3):365-375. |
| Critical effect: | No-effect level for fetal toxicity (no decrease fetal body weight and no effects on development or reproduction end points) in pregnant rats exposed to acrylonitrile at 12 ppm for 6 h/day on gestation days 6-20. |
| Support: | Sakurai et al. (1978) and Sakurai and Kusumoto (1972) noted that many of the symptoms (headache, fatigue, nausea, and insomnia) reported after initial occupational exposure were associated with exposures in excess of 5 ppm, and that the findings were not contradictory to those of Wilson et al. (1948), who reported that occupational exposure at 16-100 ppm for 20-45 min produced transient dull headaches, nasal and ocular irritation, discomfort in the chest, nervousness, and irritability. In monkeys, slight or modest reversible effects (transient skin flushing and elevation of respiration rats) were observed with 4-h exposures at 65 or 90 ppm (Dudley and Neal 1942). Slight transient effects (ocular and nasal irritation, redness of skin) were observed following a 2-h exposure at 305 ppm (Dudley and Neal 1942). |
| Time scaling: | Cn × t = k, where n = 1.1 (ten Berge et al. 1986) (12 ppm)1.1 × 360 min = 5,539 ppm-min |
| Uncertainty factors: | Total uncertainty factor: 36 Interspecies: 6, a factor of 3 was applied to account for possible species differences in toxicodynamics of acrylonitrile and a factor of 2 to account for interspecies differences in toxicokinetics. On the basis of PBPK modeling, Sweeney et al. (2003) predicted a 2-fold difference the concentrations of acrylonitrile and its metabolite, cyanoethylene oxide (the metabolic precursor to cyanide), in blood and brain during exposures to acrylonitrile at 2 ppm. Higher cyanoethylene oxide concentrations were predicted in human blood and brain than in rats. A PBPK model developed by Takano et al. (2010) used data on in vitro metabolism of acrylonitrile in rat and human liver microsomes to estimate hepatic clearance of cyanoethylene oxide. The model predicted that repeated oral exposures at 30 mg/kg/day for 14 days would result in peak blood acrylonitrile concentrations that were approximately 2-fold higher in rats than in humans. Although the Takano et al. (2010) model was evaluated using oral exposure data, experimental data for metabolism were obtained from in vitro microsome studies. Taken together, the Sweeney et al. (2003) and Takano et al. (2010) PBPK models support application of a factor of 2 to account for differences in toxicokinetics. |
| Intraspecies: | 6, a factor of 3 was applied to account for possible variation in toxicodynamics of acrylonitrile in the human population and a factor of 2 to account for variability in toxicokinetics. On the basis of PBPK modeling, Sweeney et al. (2003) predicted that human variability in toxicokinetics of acrylonitrile would result in the 95th percentile individual having acrylonitrile or cyanoethylene oxide concentrations in blood 1.8-fold higher than the average (mean) individual. This suggests that a factor of 2 would accommodate toxicokinetic variability in the human population. |
| Modifying factor: | None |
| Calculations: | |
| 10-min AEGL-2 | C1.1 × 10 min = 5,538 ppm-min 312 ppm ÷ 36 = 8.6 ppm |
| 30-min AEGL-2 | C1.1 × 30 min = 5,538 ppm-min 115 ppm ÷ 36 = 3.2 ppm |
| 1-h AEGL-2 | C1.1 × 60 min = 5,538 ppm-min 61 ppm ÷ 36 = 1.7 ppm |
| 4-h AEGL-2 | C1.1 × 240 min = 5,538 ppm-min 17.3 ppm ÷ 36 = 0.48 ppm |
| 8-h AEGL-2 | C1.1 × 480 min = 5,538 ppm-min 9.2 ppm ÷ 36 = 0.26 ppm |
| Derivation of AEGL-3 Values | |
| Key studies: | Dudley, H.C., and P.A. Neal. 1942. Toxicology of acrylonitrile (vinyl cyanide). I. Study of the acute toxicity. J. Ind. Hyg. Toxicol. 24(2):27-36. Appel, K.E., H. Peter, and H.M. Bolt. 1981a. Effect of potential antidotes on the acute toxicity of acrylonitrile. Int. Arch. Occup. Environ. Health 49(2):157-163. |
| Critical effect: | Estimated lethality threshold (30-min, 1-h, 2-h, 4-h, and 8-h BMCL05 values are 1,784.0, 1,024.4, 491.3, 179.5, and 185.8 ppm, respectively) for rats exposed at various concentrations of acrylonitrile for 30 min, 1, 2, 4, or 8 h. With the exception of the 4-h value, the resulting BMCL05 values show a consistent duration-dependent relationship; therefore, the 30-min, 1-h, and 8-h estimates were used to derive corresponding AEGL-3 values. Because the 4-h BMCL05 was essentially equivalent to the 8-h BMCL05, the 4-h AEGL-3 value was derived by time-scaling the 8-h BMCL05 of 185.9 ppm. |
| Time scaling: | Cn × t = k, where n = 1.1 (ten Berge et al. 1986); applied for derivation of 10-min and 4-h values only. |
| Uncertainty factors: | Total uncertainty factor: 36 Interspecies: 6, although the dog appears to be the most sensitive species, the overall database for rats is more robust thereby justifying use of the rat data. A factor of 3 was applied to account for possible species differences in toxicodynamics of acrylonitrile and a factor of 2 to account for interspecies differences in toxicokinetics. On the basis of PBPK modeling, Sweeney et al. (2003) predicted a 2-fold higher concentration of acrylonitrile and its metabolite, cyanoethylene oxide (the metabolic precursor to cyanide), in the blood and brain of humans than rats during exposures to acrylonitrile at 2 ppm. A |
| PBPK model developed by Takano et al. (2010) used data on in vitro metabolism of acrylonitrile in rat and human liver microsomes to estimate hepatic clearance of cyanoethylene oxide in rats and humans. The model predicted that repeated oral exposures at 30 mg/kg/day for 14 days would result in peak blood acrylonitrile concentrations that were approximately 2-fold higher in rats than humans. Although the Takano et al. (2010) model was evaluated using oral exposure data, experimental data for metabolism were obtained from in vitro microsome studies. Taken together, the Sweeney et al. (2003) and Takano et al. (2010) PBPK models support application of a factor of 2 to account for differences in toxicokinetics. | |
| Intraspecies: | 6, a factor of 3 was applied to account for possible variation in toxicodynamics of acrylonitrile in the human population and a factor of 2 was applied to account for variability in toxicokinetics. On the basis of PBPK models, Sweeney et al. (2003) predicted that human variability in toxicokinetics of acrylonitrile would result in the 95th percentile individual having acrylonitrile or cyanoethylene oxide concentrations in blood 1.8-fold higher than the average (mean) individual. That suggests that a factor of 2 would accommodate expected toxicokinetics variability in the human population. |
| Calculation: | For the 30-min, 1-h, and 8-h AEGL-3 values the 1-h and 8-h rat BMCL05 values were adjusted by the total uncertainty factor product of 36. |
| The 10-min value was derived by time-scaling from the 30-min rat BMCL05: | |
| (1,784 ppm)1.1 × 0.5 h = 1,885.8 ppm-h | |
| The 4-h value was derived by scaling from the 8-h rat BMCL05 (the 8-h BMCL05 was considered more appropriate that the 2-h value because it was derived from data for five dose groups rather than three): | |
| (185.8 ppm)1.1 × 8 h = 2,506.3 ppm-h | |
| 10-min AEGL-3: | C1.1 × 0.1667 h = 1,885.8 ppm-h 4,842.4 ppm ÷ 36 = 134 ppm (rounded to 130 ppm) |
| 30-min AEGL-3: | 30-min BMCL05 = 1,784 ppm 1,784 ppm ÷ 36 = 49.6 ppm (rounded to 50 ppm) |
| 1-h AEGL-3: | 1-h BMCL05 = 1,024.42 ppm 1,024.42 ppm ÷ 36 = 28.46 ppm (rounded to 28 ppm) |
| 4-h AEGL-3 | C1.1 × 4 h = 2,506.3 ppm-h 348.9 ppm ÷ 36 = 9.7 ppm |
| 8-h AEGL-3: | 8-h BMCL05 = 185.8 ppm 185.8 ppm ÷ 36 = 5.2 ppm |
APPENDIX D
TIME SCALING CALCULATIONS
The relationship between dose and time for any given chemical is a function of the physical and chemical properties of the substance and the unique toxicologic and pharmacologic properties of the individual substance. Historically, the relationship according to Haber (1924), commonly called Haber’s Law or Haber’s Rule (C × t = k, where C = exposure concentration, t = exposure duration, and k = a constant) has been used to relate exposure concentration and duration to effect (Rinehart and Hatch 1964). This concept states that exposure concentration and exposure duration may be reciprocally adjusted to maintain a cumulative exposure constant (k) and that this cumulative exposure constant will always reflect a specific quantitative and qualitative response. This inverse relationship of concentration and time may be valid when the toxic response to a chemical is equally dependent on the concentration and the exposure duration. However, an assessment by ten Berge et al. (1986) of LC50 data for certain chemicals revealed chemical-specific relationships between exposure concentration and exposure duration that were often exponential. This relationship can be expressed by the equation Cn × t = k, where n represents a chemical-specific, and even a toxic-end-point specific, exponent. The relationship described by this equation is basically the form of a linear regression analysis of the log-log transformation of a plot of C vs. t. ten Berge et al. (1986) examined the airborne concentration (C) and short-term exposure duration (t) relationship relative to death for approximately 20 chemicals and found that the empirically derived value of n ranged from 0.8 to 3.5 among this group of chemicals. Hence, the value of the exponent (n) in the equation Cn × t = k quantitatively defines the relationship between exposure concentration and exposure duration for a given chemical and for a specific health effect end point. Haber’s Rule is the special case where n = 1. As the value of n increases, the plot of concentration vs. time yields a progressive decrease in the slope of the curve.
For acrylonitrile, analysis of available data by ten Berge et al. (1986) showed that the relationship between exposure concentration and exposure duration was near linear, where n = 1.1 for the relationship Cn × t = k.
APPENDIX E
ACUTE EXPOSURE GUIDELINE LEVELS FOR ACRYLONITRILE
Derivation Summary
AEGL-1 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 1.5 ppm (3.3 mg/m3) |
1.5 ppm (3.3 mg/m3) |
NRa | NRa | NRa |
| Reference: Jakubowski, M., I. Linhart, G. Pielas, and J. Kopecky. 1987. 2-Cyanoethylmercapturic acid (CEMA) in the urine as a possible indicator of exposure to acrylonitrile. Br. J. Ind. Med. 44(12):834-840. Sakurai, H., M. Onodera, T. Utsunomiya, H. Minakuchi, H. Iwai, and H. Mutsumura. 1978. Health effects of acrylonitrile in acrylic fibre factories. Br. J. Ind. Med. 35(3): 219-225. | ||||
| Test species/Strain/Number: Six informed volunteer male human subjects (Jakubowski et al. 1987); occupational exposures (Sakurai et al. 1978). | ||||
| Exposure route/Concentrations/Durations: Inhalation; 2.3 or 4.6 ppm for 8 h. | ||||
| Effects: Absence of effects in volunteer subjects exposed for 8 h at 4.6 ppm (Jakubowski et al. 1987) supported by observations of mild effects (initial conjunctival irritation, for which there was some accommodation) in workers routinely exposed at approximately 5 ppm (Sakurai et al. 1978). | ||||
| End point/Concentration/Rationale: Ocular irritation, 4.6 ppm for 8 h, is considered a level at which mild effects may occur in some healthy adults. | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 3 Interspecies: 1, because study involved human subjects. Intraspecies: 3, pharmacokinetic variability is not likely to be significant for mild effects (ocular irritation) of low-level exposure. However, the point-of-departure is based on studies of healthy adults and, in the occupational studies, subjects experienced repeated exposures to acrylonitrile, which may have resulted in some accommodation to the ocular irritation. |
||||
| Modifying factor: None applied | ||||
| Animal-to-human dosimetric adjustment: No adjustments | ||||
| Time scaling: No data are available on the relationship between exposure duration and severity of responses to acrylonitrile. Typically, in the absence of this information, AEGL-1 values based on an 8-h point-of-departure would be scaled. However, in this case, the effect is ocular irritation, which would not be expected to have a response threshold that varies with exposure duration. Therefore, it is prudent to not time scale and the AEGL-1 values were held constant at 1.5 ppm for exposure durations of 10 and 30 min. However, 1.5 ppm exceeds the AEGL-2 values for longer exposure durations; therefore, AEGL-1 values for 1 h, 4 h and 8 h are not recommended. | ||||
| Data adequacy: AEGL-1 values for acrylonitrile are developed based on results from a controlled experiment with human volunteers, and also on occupational exposure data. The data effectively define a concentration at which mild effects (ocular irritation) may occur in some healthy adults for an AEGL-specific exposure duration (8 h). Because the AEGL-1 value (1.5 ppm) exceeds AEGL-2 values for longer exposure durations, AEGL-1 values for 1 h, 4 h and 8 h are not recommended. |
aNot recommended. Absence of an AEGL-1 value does not imply that exposure below the AEGL-2 value is without adverse effect.
AEGL-2 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 8.6 ppm (19 mg/m3) |
3.2 ppm (6.9 mg/m3) |
1.7 ppm (3.7 mg/m3) |
0.48 ppm (1.0 mg/m3) |
0.26 ppm (0.56 mg/m3) |
| Reference: Saillenfait, A.M., P. Bonnet, J.P. Guenier, and J. De Ceaurriz. 1993a. Relative developmental toxicities of inhaled aliphatic mononitriles in rats. Fundam. Appl. Toxicol. 20(3):365-375. | ||||
| Test Species/Strain/Sex/Number: Rat; Sprague-Dawley; 20-23/group | ||||
| Exposure route/Concentrations/Durations: Inhalation; 12, 25, 50, or 100 ppm for 6 h/day on gestation days 6-20. | ||||
| Effects: Dose-related decrease in fetal body weight at 25-100 ppm. | ||||
| End point/Concentration/Rationale: No decrease in fetal body weight or other developmental or reproductive effect in rats at 12 ppm, 6 h/day. | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 36 Interspecies: 6; 3 for toxicodynamics and 2 for toxicokinetics based on PBPK modeling (Sweeney et al. 2003; Takano et al. 2010). Intraspecies: 6; 3 for toxicodynamics and 2 for toxicokinetics based on PBPK modeling (Sweeney et al. 2003). |
||||
| Modifying factor: None | ||||
| Animal-to-human dosimetric adjustment: Not applicable | ||||
| Time scaling: Cn × t = k, where n = 1.1 as reported by ten Berge et al. (1986) | ||||
| Data adequacy: The AEGL-2 values are based on effects that are indicative of acrylonitrile exposure, but not yet demonstrating more severe toxicity (e.g., convulsions, extreme respiratory alterations) or irreversible effects. | ||||
AEGL-3 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 130 ppm (280 mg/m3) |
50 ppm (110 mg/m3) |
28 ppm (61 mg/m3) |
9.7 ppm (21 mg/m3) |
5.2 (11 mg/m3) |
| Reference: Dudley, H.C., and P.A. Neal. 1942. Toxicology of acrylonitrile (vinyl cyanide). I. Study of the acute toxicity. J. Ind. Hyg. Toxicol. 24(2):27-36. Appel, K.E., H. Peter, and H.M. Bolt. 1981a. Effect of potential antidotes on the acute toxicity of acrylonitrile. Int. Arch. Occup. Environ. Health 49(2):157-163. |
||||
| Test species/Strain/Sex/Number: Rats; Osborne-Mendel; sex not specified; 16/group (Dudley and Neal 1942). Rats; Wistar; male; 3-6/group (Appel et al. 1981a.) | ||
| Effects: Lethal response frequency (see Tables 1-3 and 1-5, Section 3.1.2 for details). | ||
| Exposure duration (h) | Concentration (ppm) | Mortality |
| 0.5 (Appel et al. 1981a) | 1,600 2,600 3,000 |
0/3 1/3 6/6 |
| 1 (Dudley and Neal 1942) | 665 1,270 1,490 2,445 |
0/16 0/16 4/16 13/16 |
| 2 (Dudley and Neal 1942) | 305 595 1,260 |
0/16 1/16 16/16 |
| 4 (Dudley and Neal 1942) | 130 315 635 90 |
0/16 2/16 16/16 |
| 8 (Dudley and Neal 1942) | 135 210 270 320 |
0/16 0/16 1/16 7/16 15/16 |
| End point/Concentration/Rationale: Estimated lethality threshold (30-min, 1-h, 2-h,4-h, and 8-h BMCL05 values are 1,784.0, 1,024.4, 491.3, 179.5, and 185.8 ppm, respectively) for rats exposed at various concentrations of acrylonitrile for 30 min, 1, 2, 4, or 8 h. With the exception of the 4-h value, the resulting BMCL05 values show a consistent duration-dependent relationship; therefore, the 30-min, 1-h, and 8-h estimates were used to derive corresponding AEGL-3 values. Because the 4-h BMCL05 was essentially equivalent to the 8-h BMCL05, the 4-h AEGL-3 value was derived by time-scaling the 8-h BMCL05. The 10-min AEGL-3 value was also derived by time-scaling from the 30-min rat BMCL05. | ||
| Uncertainty factors/Rationale: Total uncertainty factor: 36 Interspecies: 6; 3 for toxicodynamics and 2 for toxicokinetics based on PBPK modeling (Sweeney et al. 2003; Takano et al. 2010). Intraspecies: 6; 3 for toxicodynamics and 2 for toxicokinetics based on PBPK modeling (Sweeney et al. 2003). |
||
| Modifying factor: None applied | ||
| Animal-to-human dosimetric adjustment: Not applicable | ||
| Time scaling: Conducted for the 10-min and 4-h values using the equation Cn × t = k, with n = 1.1. The 4-h value was derived by scaling from the 8-h rat BMCL05 rather than the 2-h value because it was derived from data from five dose groups rather than three. |
||
| Data adequacy: Although definitive exposure-response data for lethality in humans are not available, data are available from acute and subchronic bioassays in multiple species. The animal data are sufficient for development of scientifically justified AEGL values. |
APPENDIX F
BENCHMARK-CONCENTRATION ANALYSIS FOR ACRYLONITRILE
BMCL01 30-minute Exposure of Rats (Appel et al. 1981a)
| Probit Model. (Version: 2.8; Date: 02/20/2007) |
| Input Data File: C:\BMDS\APPEL_30-MIN.(d) |
| Gnuplot Plotting File: C:\BMDS\APPEL_30-MIN.plt |
| Fri Jul 13 13:22:35 2007 |
| BMDS MODEL RUN |
The form of the probability function is:
P[response] = Background + (1-Background) *
CumNorm(Intercept+Slope*Log(Dose)),
where CumNorm(.) is the cumulative normal distribution function
Dependent variable = COLUMN3
Independent variable = COLUMN1
Slope parameter is not restricted
Total number of observations = 3
Total number of records with missing values = 0
Maximum number of iterations = 250
Relative Function Convergence has been set to: 1e-008
Parameter Convergence has been set to: 1e-008
User has chosen the log transformed model
Default Initial (and Specified) Parameter Values
Background = 0
Intercept = -30.2755
Slope = 3.91797
Asymptotic Correlation Matrix of Parameter Estimates
(*** The model parameter(s) -background -slope have been estimated at a boundary point, or have been specified by the user, and do not appear in the correlation matrix)
Intercept
Intercept 1
| Parameter Estimates (95.0% Wald Confidence Interval) | ||||
| Variable | Estimate | Standard Error | Lower Conf. Limit | Upper Conf. Limit |
| Background | 0 | NA | – | – |
| Intercept | -141.863 | 0.665192 | -143.167 | -140.559 |
| Slope | 18 | NA | – | – |
| NA, indicates that this parameter has hit a bound implied by some inequality constraint and thus has no standard error. | ||||
| Analysis of Deviance Table | |||||
| Model | Log (likelihood) | No. Parameters | Deviance | Test d.f. | P-value |
| Full model | -1.90954 | 3 | |||
| Fitted model | -1.99323 | 1 | 0.167371 | 2 | 0.9197 |
| Reduced mode | -8.15032 | 1 | 12.4816 | 2 | 0.001948 |
| AIC: 5.98646 | |||||
| Goodness of Fit Scaled | |||||
| Dose | Estimated Probability | Expected | Observed | Size | Residual |
| 1,600.0000 | 0.0000 | 0.000 | 0 | 3 | -0.000 |
| 2,600.0000 | 0.3729 | 1.119 | 1 | 3 | -0.142 |
| 3,000.0000 | 0.9878 | 5.927 | 6 | 6 | 0.272 |
| Chi-square = 0.09 d.f. = 2 P-value = 0.9541 | |||||
Benchmark dose computation
Specified effect = 0.05
Risk type = Extra risk
Confidence level = 0.95
BMC = 2,416.07
BMCL = 1,784.1
FIGURE F-1 Probit model with 0.95 confidence level.
BMCL05 1-h Exposure of Rats (Dudley and Neal 1942)
| Probit Model $Revision: 2.1 $ $Date: 2000/02/26 03:38:53 $ |
| Input Data File: C:\BMDS\UNSAVED1.(d) |
| Gnuplot Plotting File: C:\BMDS\UNSAVED1.plt |
| Thu Mar 01 08:34:09 2007 |
BMDS MODEL RUN
The form of the probability function is:
P[response] = Background + (1-Background) *
CumNorm(Intercept+Slope*Log(Dose)),
where CumNorm(.) is the cumulative normal distribution function
Dependent variable = COLUMN3
Independent variable = COLUMN1
Slope parameter is not restricted
Total number of observations = 4
Total number of records with missing values = 0
Maximum number of iterations = 250
Relative Function Convergence has been set to: 1e-008
Parameter Convergence has been set to: 1e-008
User has chosen the log transformed model
Default Initial (and Specified) Parameter Values
Background = 0
Intercept = -16.2084
Slope = 2.13067
Asymptotic Correlation Matrix of Parameter Estimates
(*** The model parameter(s) -background have been estimated at a boundary point, or have been specified by the user, and do not appear in the correlation matrix)
| Intercept | Slope | |
| Intercept | 1 | -1 |
| Slope | -1 | 1 |
Parameter Estimates
| Variable | Estimate | Standard Error |
| Background | 0 | NA |
| Intercept | -29.6647 | 6.43448 |
| Slope | 3.92636 | 0.860001 |
NA - Indicates that this parameter has hit a bound implied by some inequality constraint and thus has no standard error.
| Analysis of Deviance Table | ||||
| Model | Log (likelihood) | Deviance | Test d.f. | P-value |
| Full model | -16.7186 | |||
| Fitted model | -18.0178 | 2.5984 | 2 | 0.2728 |
| Reduced mode | -37.047 | 40.6567 | 3 | <0.0001 |
| AIC: 40.0356 | ||||
| Goodness of Fit Scaled | |||||
| Dose | Estimated Probability | Expected | Observed | Size | Residual |
| 665.0000 | 0.0000 | 0.000 | 0 | 16 | -0.01652 |
| 1,270.0000 | 0.0544 | 0.870 | 0 | 16 | -0.9591 |
| 1,490.0000 | 0.1644 | 2.630 | 4 | 16 | 0.9241 |
| 2,445.0000 | 0.8335 | 13.336 | 13 | 16 | -0.2251 |
| Chi-square = 1.82 d.f. = 2 P-value = 0.4015 | |||||
Benchmark dose computation
Specified effect = 0.05
Risk type = extra risk
Confidence level = 0.95
BMC = 256.83
BMCL = 1,024.42
FIGURE F-2 Probit model with 0.95 confidence level.
BMCL05 2-h Exposure of Rats (Dudley and Neal 1942)
| Probit Model $Revision: 2.1 $ $Date: 2000/02/26 03:38:53 $ |
| Input Data File: C:\BMDS\UNSAVED1.(d) |
| Gnuplot Plotting File: C:\BMDS\UNSAVED1.plt |
| Thu Mar 01 08:39:20 2007 |
BMDS MODEL RUN
The form of the probability function is:
P[response] = Background + (1-Background) *
CumNorm(Intercept+Slope*Log(Dose)),
where CumNorm(.) is the cumulative normal distribution function
Dependent variable = COLUMN3
Independent variable = COLUMN1
Slope parameter is not restricted
Total number of observations = 3
Total number of records with missing values = 0
Maximum number of iterations = 250
Relative Function Convergence has been set to: 1e-008
Parameter Convergence has been set to: 1e-008
User has chosen the log transformed model
Default Initial (and Specified) Parameter Values
Background = 0
Intercept = -17.8516
Slope = 2.70268
Asymptotic Correlation Matrix of Parameter Estimates
(*** The model parameter(s) -background have been estimated at a boundary point, or have been specified by the user, and do not appear in the correlation matrix)
| Intercept | Slope | |
| Intercept | 1 | -1 |
| Slope | -1 | 1 |
Parameter Estimates
| Variable | Estimate | Standard Error |
| Background | 0 | NA |
| Intercept | 64.9721 | 4558.92 |
| Slope | 9.92993 | 713.606 |
NA - Indicates that this parameter has hit a bound implied by some inequality constraint and thus has no standard error.
| Analysis of Deviance Table | ||||
| Model | Log (likelihood) | Deviance | Test d.f. | P-value |
| Full model | -3.74067 | |||
| Fitted model | -3.74067 | 5.37593e-008 | 1 | 0.9998 |
| Reduced mode | -31.199 | 54.9175 | 2 | <0.0001 |
| AIC: 11.4813 | ||||
| Goodness of Fit Scaled | |||||
| Dose | Estimated Probability | Expected | Observed | Size | Residual |
| 305.0000 | 0.0000 | 0.000 | 0 | 16 | -4.972e-008 |
| 595.0000 | 0.0625 | 1.000 | 1 | 16 | -3.32e-005 |
| 1260.0000 | 1.0000 | 16.000 | 16 | 16 | 0.0001623 |
| Chi-square = 0.00 d.f. = 1 P-value = 0.9999 | |||||
Benchmark dose computation
Specified effect = 0.05
Risk type = extra risk
Confidence level = 0.95
BMC = 588.401
BMCL = 491.304
FIGURE F-3 Probit model with 0.95 confidence level.
BMCL05 4-h exposure of rats (Dudley and Neal 1942)
Probit Model $Revision: 2.1 $ $Date: 2000/02/26 03:38:53 $
Input Data File: C:\BMDS\UNSAVED1.(d)
Gnuplot Plotting File: C:\BMDS\UNSAVED1.plt
Thu Mar 01 08:43:13 2007
BMDS MODEL RUN
The form of the probability function is:
P[response] = Background + (1-Background) *
CumNorm(Intercept+Slope*Log(Dose)), where CumNorm(.) is the cumulative normal distribution function
Dependent variable = COLUMN3
Independent variable = COLUMN1
Slope parameter is not restricted
Total number of observations = 3
Total number of records with missing values = 0
Maximum number of iterations = 250
Relative Function Convergence has been set to: 1e-008
Parameter Convergence has been set to: 1e-008
User has chosen the log transformed model
Default Initial (and Specified) Parameter Values
Background = 0
Intercept = -13.5273
Slope = 2.34824
Asymptotic Correlation Matrix of Parameter Estimates:
(*** The model parameter(s) -background have been estimated at a boundary point, or have been specified by the user, and do not appear in the correlation matrix)
| Intercept | Slope | |
| Intercept | 1 | -1 |
| Slope | -1 | 1 |
Parameter Estimates
| Variable | Estimate | Standard Error |
| Background | 0 | NA |
| Intercept | 50.8405 | 3148.13 |
| Slope | 8.75291 | 547.256 |
NA - Indicates that this parameter has hit a bound implied by some inequality constraint and thus has no standard error.
| Analysis of Deviance Table | ||||
| Model | Log (likelihood) | Deviance | Test d.f. | P-value |
| Full model | -9.93738 | |||
| Fitted model | -9.93738 | 2.60525e-007 | 1 | 0.9996 |
| Reduced mode | -32.8951 | 45.9154 | 2 | <0.0001 |
| AIC: 23.8748 | ||||
| Goodness of Fit Scaled | |||||
| Dose | Estimated Probability | Expected | Observed | Size | Residual |
| 130.0000 | 0.0000 | 0.000 | 0 | 16 | -3.783e-008 |
| 315.0000 | 0.3125 | 5.000 | 5 | 16 | -3.304e-006 |
| 635.0000 | 1.0000 | 16.000 | 16 | 16 | 0.0003609 |
| Chi-square = 0.00 d.f. = 1 P-value = 0.9999 | |||||
Benchmark dose computation
Specified effect = 0.05
Risk type = extra risk
Confidence level = 0.95
BMC = 276.026
BMCL = 179.532
FIGURE F-4 Probit model with 0.95 confidence level.
BMCL05 8-h Exposure of Rats (Dudley and Neal 1942)
| Probit Model $Revision: 2.1 $ $Date: 2000/02/26 03:38:53 $ |
| Input Data File: C:\BMDS\UNSAVED1.(d) |
| Gnuplot Plotting File: C:\BMDS\UNSAVED1.plt |
| Thu Mar 01 08:46:12 2007 |
BMDS MODEL RUN
The form of the probability function is:
P[response] = Background + (1-Background) *
CumNorm(Intercept+Slope*Log(Dose)), where CumNorm(.) is the cumulative
normal distribution function
Dependent variable = COLUMN3
Independent variable = COLUMN1
Slope parameter is not restricted
Total number of observations = 5
Total number of records with missing values = 0
Maximum number of iterations = 250
Relative Function Convergence has been set to: 1e-008
Parameter Convergence has been set to: 1e-008
User has chosen the log transformed model
Default Initial (and Specified) Parameter Values
Background = 0
Intercept = -13
Slope = 2.37276
Asymptotic Correlation Matrix of Parameter Estimates
(*** The model parameter(s) -background have been estimated at a boundary point, or have been specified by the user, and do not appear in the correlation matrix)
| Intercept | Slope | |
| Intercept | 1 | -1 |
| Slope | -1 | 1 |
Parameter Estimates
| Variable | Estimate | Standard Error |
| Background | 0 | NA |
| Intercept | 40.1969 | 9.34116 |
| Slope | 7.18845 | 1.66722 |
NA - Indicates that this parameter has hit a bound implied by some inequality constraint and thus has no standard error.
| Analysis of Deviance Table | ||||
| Model | Log (likelihood) | Deviance | Test d.f. | P-value |
| Full model | -18.4464 | |||
| Fitted model | -18.9141 | 0.935409 | 3 | 0.8169 |
| Reduced mode | -47.991 | 59.091 | 4 | <0.0001 |
| AIC: 41.8281 | ||||
| Goodness of Fit Scaled | |||||
| Dose | Estimated Probability | Expected | Observed | Size | Residual |
| 90.0000 | 0.0000 | 0.000 | 0 | 16 | -1.822e-007 |
| 135.0000 | 0.0000 | 0.000 | 0 | 16 | -0.002528 |
| 210.0000 | 0.0392 | 0.628 | 1 | 16 | 0.479 |
| 270.0000 | 0.5188 | 8.300 | 7 | 16 | -0.6506 |
| 320.0000 | 0.8977 | 14.363 | 15 | 16 | 0.5257 |
| Chi-square = 0.93 d.f. = 3 P-value = 0.8184 | |||||
Benchmark dose computation
Specified effect = 0.05
Risk type = extra risk
Confidence level = 0.95
BMC = 213.376
BMCL = 185.797
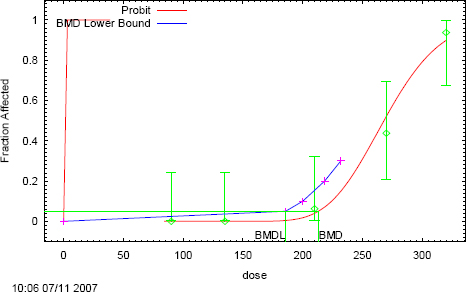
FIGURE F-5 Probit model with 0.95 confidence level.
APPENDIX G
LITCHFIELD AND WILCOXON LC50 CALCULATION
Dudley and Neal (1942): Lethality in Rats Exposed for 1 Hour to Acrylonitrile
| Dose | Mortality | Observed% | Expected% | Observed Expected | Chi-Square |
| 665.000 | 0/16 | 0 (0.30) | 0.28 | 0.02 | 0.0000 |
| 1,270.000 | 0/16 | 0 (3.80) | 9.95 | -6.15 | 0.0422 |
| 1,490.000 | 4/16 | 25.00 | 21.53 | 3.47 | 0.0071 |
| 2,445.000 | 13/16 | 81.25 | 82.13 | -0.88 | 0.0005 |
Values in parentheses are corrected for 0 or 100 percent Total = 0.0499
LC50 = 1870.153(1621.558-2156.859)*
Slope = 1.34(1.22-1.47)*
*These values are 95% confidence limits
Total animals = 64 Total doses = 4 Animals/dose = 16.00
Chi-square = total chi-square × animals/dose = 0.7986
Table value for Chi-square with 2 Degrees of Freedom = 5.9900
LC84 = 2502.530 LC16 = 1397.574 FED = 1.15 FS = 1.10 A = 1.07
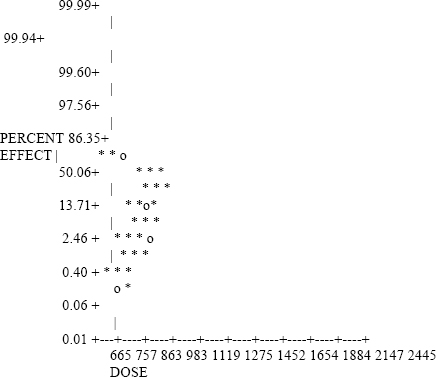
Expected Lethal Dose Values
| LC0.1 | 555.726 |
| LC1.0 | 834.159 |
| LC5.0 | 1,114.816 |
| LC10 | 1,271.215 |
| LC25 | 1,541.871 |
| LC50 | 1,870.153 |
| LC75 | 2,268.330 |
| LC90 | 2,751.283 |
| LC99 | 4,192.812 |
APPENDIX H
CATEGORY PLOT FOR ACRYLONITRILE
FIGURE H-1 Category plot of toxicity data and AEGL values for acrylonitrile.
TABLE H-1 Data Used in Category Plot for Acrylonitrile
| Source | Species | Sex | No. Exposures | ppm | Minutes | Category | Comments |
| AEGL-1 | 1.5 | 10 | AEGL | ||||
| AEGL-1 | 1.5 | 30 | AEGL | ||||
| AEGL-1 | NR | 60 | AEGL | ||||
| AEGL-1 | NR | 240 | AEGL | ||||
| AEGL-1 | NR | 480 | AEGL | ||||
| AEGL-2 | 8.6 | 10 | AEGL | ||||
| AEGL-2 | 3.2 | 30 | AEGL | ||||
| AEGL-2 | 1.7 | 60 | AEGL | ||||
| AEGL-2 | 0.48 | 240 | AEGL | ||||
| AEGL-2 | 0.26 | 480 | AEGL | ||||
| AEGL-3 | 130 | 10 | AEGL | ||||
| AEGL-3 | 50 | 30 | AEGL | ||||
| AEGL-3 | 28 | 60 | AEGL | ||||
| AEGL-3 | 9.7 | 240 | AEGL | ||||
| AEGL-3 | 5.2 | 480 | AEGL | ||||
| Appel et al. 1981a | Rat | m | 1 | 2400 | 10 | 2 | No mortality. |
| Dudley and Neal 1942 | Rat | 1 | 665 | 30 | 1 | Moderate transitory effects. | |
| Dudley and Neal 1942 | Rat | 1 | 1270 | 30 | 1 | Marked; no residual effects in 24 h. | |
| Dudley and Neal 1942 | Rat | 1 | 1490 | 30 | 1 | Marked; no residual effects in 24 h. | |
| Appel et al. 1981a | Rat | m | 1 | 1600 | 30 | 2 | No mortality. |
| Dudley and Neal 1942 | Rat | 1 | 2445 | 30 | 1 | Marked; slight residual effects to 24 h. | |
| Appel et al. 1981a | Rat | m | 1 | 2600 | 30 | SL | 33% mortality. |
| Source | Species | Sex | No. Exposures | ppm | Minutes | Category | Comments |
| Appel et al. 1981a | Rat | m | 1 | 3000 | 30 | 3 | 100% mortality. |
| Dudley and Neal 1942 | Rat | 1 | 665 | 60 | 2 | Marked transitory effects. | |
| Vernon et al. 1990 | Rat | b | 1 | 1008 | 60 | 2 | Rapid shallow breathing, decreased activity, nasal discharge, salivation, lacrimation, and coma (in 3 of 10 animals). |
| Dudley and Neal 1942 | Rat | 1 | 1270 | 60 | 2 | Marked effects; slight effects at 24 h; normal at 48 h. | |
| Dudley and Neal 1942 | Rat | 1 | 1490 | 60 | SL | 25% mortality; deaths in 4 h; slight effects at 24 h in survivors. | |
| Dudley and Neal 1942 | Rat | 1 | 2445 | 60 | SL | 81% mortality; deaths in 4 h; slight effects at 24 h in survivors. | |
| Dudley and Neal 1942 | Rat | 1 | 305 | 120 | 2 | Slight transitory effects. | |
| Dudley and Neal 1942 | Rat | 1 | 595 | 120 | SL | 6% mortality; marked transitory effects. | |
| Appel et al. 1981a | Rat | m | 1 | 950 | 120 | SL | 33% mortality. |
| Appel et al. 1981a | Rat | m | 1 | 1100 | 120 | 3 | 100% mortality. |
| Dudley and Neal 1942 | Rat | 1 | 1260 | 120 | 3 | 100% mortality; deaths within 4 h. | |
| Appel et al. 1981a | Rat | m | 1 | 650 | 180 | SL | 33% mortality. |
| Dudley and Neal 1942 | Dog | b | 1 | 30 | 240 | 1 | Slight salivation by end of exposure period; no other effects. |
| Dudley and Neal 1942 | Dog | 1 | 30 | 240 | 1 | ||
| Dudley and Neal 1942 | Monkey | 1 | 56 | 240 | 0 | ||
| Dudley and Neal 1942 | Cat | 1 | 56 | 240 | SL | ||
| Dudley and Neal 1942 | Monkey | 1 | 65 | 240 | 1 | ||
| Dudley and Neal 1942 | Dog | 1 | 65 | 240 | 2 |
| Dudley and Neal 1942 | Dog | b | 1 | 65 | 240 | SL | Mortality (1/2). |
| Dudley and Neal 1942 | Monkey | 1 | 90 | 240 | 1 | ||
| Dudley and Neal 1942 | Guinea Pig | 1 | 100 | 240 | 0 | Slight to no effect. | |
| Dudley and Neal 1942 | Cat | 1 | 100 | 240 | 1 | ||
| Dudley and Neal 1942 | Rabbit | 1 | 100 | 240 | 1 | ||
| Dudley and Neal 1942 | Rabbit | 1 | 100 | 240 | 1 | ||
| Dudley and Neal 1942 | Rat | m | 1 | 100 | 240 | 2 | Slight transitory effects. |
| Dudley and Neal 1942 | Dog | b | 1 | 100 | 240 | 2 | |
| Dudley and Neal 1942 | Dog | 1 | 100 | 240 | 2 | ||
| Dudley and Neal 1942 | Dog | b | 1 | 110 | 240 | SL | Mortality (2/3). |
| Dudley and Neal 1942 | Rat | 1 | 130 | 240 | 0 | Slight transitory effects. | |
| Dudley and Neal 1942 | Rat | 1 | 130 | 240 | 0 | Slight transitory effects. | |
| Dudley and Neal 1942 | Rabbit | 1 | 135 | 240 | 1 | ||
| Dudley and Neal 1942 | Dog | b | 1 | 165 | 240 | 3 | Mortality (2/2). |
| Dudley and Neal 1942 | Rabbit | 1 | 260 | 240 | SL | Mortality (1/2). | |
| Dudley and Neal 1942 | Guinea Pig | 1 | 265 | 240 | 1 | Slight transitory effect; reduced feed consumption for 4 d. | |
| Dudley and Neal 1942 | Cat | 1 | 275 | 240 | 2 | ||
| Dudley and Neal 1942 | Rat | 1 | 315 | 240 | SL | 31% mortality; marked; no effects in survivors at 24 h. | |
| Dudley and Neal 1942 | Rat | 1 | 315 | 240 | SL | 31% mortality; marked; no residual effects in survivors. | |
| Wil Research Laboratories, 2005 | Rat | b | 1 | 539 | 240 | 1 | No mortality. |
| Source | Species | Sex | No. Exposures | ppm | Minutes | Category | Comments |
| Dudley and Neal 1942 | Guinea Pig | 1 | 575 | 240 | SL | 63% mortality. | |
| Dudley and Neal 1942 | Rabbit | 1 | 580 | 240 | 3 | 100% mortality. | |
| Dudley and Neal 1942 | Cat | 1 | 600 | 240 | 3 | 100% mortality. | |
| Dudley and Neal 1942 | Rat | 1 | 635 | 240 | 3 | 100% mortality. | |
| Dudley and Neal 1942 | Rat | 1 | 635 | 240 | 3 | 100% mortality. | |
| Wil Research Laboratories 2005 | Rat | b | 1 | 775 | 240 | 1 | No mortality. |
| Wil Research Laboratories 2005 | Rat | b | 1 | 871 | 240 | SL | Mortality (4/10). |
| Wil Research Laboratories 2005 | Rat | b | 1 | 1006 | 240 | SL | Mortality (7/10). |
| Dudley and Neal 1942 | Guinea Pig | 1 | 1160 | 240 | 3 | 100% mortality. | |
| Wil Research Laboratories 2005 | Rat | b | 1 | 1181 | 240 | SL | Mortality (9/10). |
| Haskell Laboratory 1942 | Dog | 1 | 25 | 360 | 1 | ||
| Haskell Laboratory 1942 | Dog | 1 | 50 | 360 | 1 | ||
| Jakubowski et al. 1987 | Human | m | 1 | 2.3 | 480 | 0 | |
| Jakubowski et al. 1987 | Human | 4.6 | 480 | 0 | |||
| Dudley and Neal 1942 | Rat | 1 | 90 | 480 | 1 | Slight discomfort. | |
| Dudley and Neal 1942 | Rat | 1 | 135 | 480 | 1 | Moderate transitory effects. | |
| Dudley and Neal 1942 | Rat | 1 | 210 | 480 | SL | 6% mortality; marked transitory effects. | |
| Dudley and Neal 1942 | Rat | 1 | 270 | 480 | SL | 44% mortality; marked; no effects in survivors at 24 h. | |
| Dudley and Neal 1942 | Rat | 1 | 320 | 480 | SL | 94% mortality. | |
| Haskell Laboratory 1942 | Dog | 1 | 225 | 105 | 2 | Ocular and nasal irritation, vomiting, incoordination, and “noisy” respiration. |
| Saillenfait et al. 1993a | Rat | f | 15 | 12 | 6 | 0 | Fetal toxicity (fetal body weight). |
| Saillenfait et al. 1993a | Rat | f | 15 | 25 | 6 | 2 | Fetal toxicity (fetal body weight). |
| Murray et al. 1978 | Rat | f | 10 | 40 | 6 | 2 | Fetal malformations. |
For Category: 0 = no effect, 1 = discomfort, 2 = disabling, SL = some lethality, 3 = lethal


















































































