From Molecular Insights to Patient Stratification for Neurological and Psychiatric Disorders: Proceedings of a Workshop (2022)
Chapter: 2 Leveraging New Genetic and Neuroscience Technologies to Advance Therapeutic Development: Opportunities and Challenges
“It is time for us as a collective field to capitalize on the promise of innovation to advance therapeutic development,” said Bill Martin, global therapeutic area head of neuroscience for Janssen Research & Development. Compared with other therapeutic areas, developing novel therapeutics for central nervous system indications takes longer, has one of the lowest clinical trials success rates, and is associated with a lower likelihood of approval, he said (Thomas, 2016) (see Figure 2-1). This translates not only to fewer medicines for neurological and psychiatric disorders, but has also resulted in the “withering commitment over time” from the pharmaceutical industry, he added.
On the positive side, B. Martin said that small and mid-sized pharmaceutical companies have stepped in to fill the void in neuroscience drug development, and even some larger companies are returning. In 2018, he said, venture capital invested $1.5 billion into neuroscience drug development, which was second only to their investments in oncology.
Learning from past mistakes will not be enough to advance the field, said B. Martin.1 Rather, the field will need to embrace new technologies that facilitate a shift from categorical descriptions of disease based solely on clinical features to biologically defined patient populations based on neuropathophysiological understanding, he said. This will require multimodal data from clinical, biological, and genetic studies, as well as new methodological frameworks and approaches to analyze and interpret these data to create novel insights. He added that at every stage of disease, biomarkers are instrumental in unraveling disease complexity and heterogeneity.
For example, to advance therapeutic development for Alzheimer’s disease, B. Martin cited five components of the diagnostic pathway—(1) clinical concept, (2) pathological hallmarks, (3) genetics, (4) biomarkers, and (5) new technologies—that need to evolve iteratively to yield greater biologically based insight into the disease. Each aspect is improved on by the other components, and each refinement brings the field closer to a deeper understanding of the disease, better frameworks for research and development, and improved treatment paradigms where the promise of the right medicine for the right patient at the right time can be realized, said B. Martin. Basing therapeutics on novel mechanisms closely linked to the underlying pathophysiology of the disease, he said, may provide remission or even prevention of disease.
B. Martin identified three interconnected and self-reinforcing components of a strategy to address these challenges:
___________________
1 Bill Martin and Alicia Martin were both speakers at the workshop. To denote who made which comments, their first initial is included wherever they are attributed throughout this proceedings.
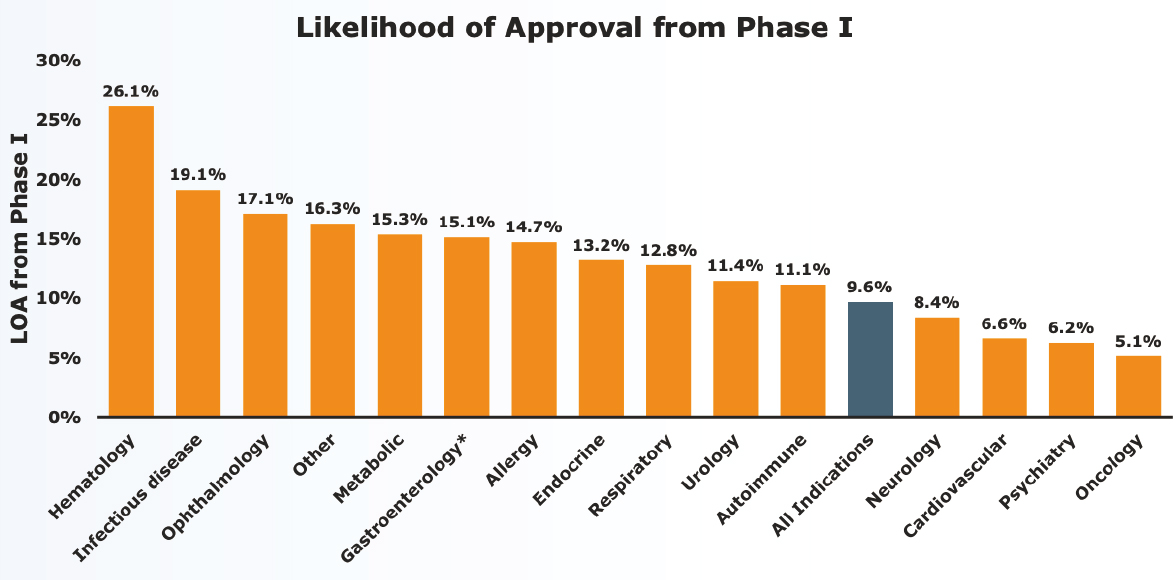
NOTES: LOA = likelihood of approval. The blue bar in the above graph denotes the LOA for all indications. *Gastroenterology does not include inflammatory bowel disease.
SOURCES: Presented by Bill Martin, October 5, 2021; Thomas, 2016.
- Advancing scientific understanding of disease. Advancing scientific understanding of diseases will require big data genomics, transcriptomics, proteomics) to gain a more comprehensive view of disease, as well as deep data—high-quality phenotypic data from large and diverse datasets, including electronic health records, medical literature, and trial databases. B. Martin noted that these data could become the basis for external control arms with real-world data, where clinical trials would permit real-world comparisons and insights into investigative new medicines.
- Building the conviction and commitment that the right trials with the right patients will bring success. Biomarkers are needed at every step along the drug development pipeline, said B. Martin, citing the 5R framework originally advanced by scientists at AstraZeneca to define the right target, right tissue, right safety parameters, right patients, and right commercial potential (Morgan et al., 2018). Many types of biomarkers are needed, he said (see Figure 2-2), noting that biomarkers that enable better patient stratification and measurement of response to treatment are especially important for translation. Also needed are digital biomarkers2 and blood-based biomarkers that can enable data capture on a much larger scale in many more cohorts of individuals. Digital biomarkers, for example, may accelerate decision making and clinical development by permitting remote monitoring of individuals in clinical studies, said B. Martin. He maintained that faster clinical development is essential to shift people from ruminating on the long history of clinical trial disappointments to believing in what is possible with the right focus and strategies.
- Improving collaboration and harmonization across stakeholders. To promote the conviction that investment will bring therapeutic advances and success for patients, B. Martin advocated for improved collaboration and harmonization. By building on the many public–private partnerships that have been formed, shared frameworks should be created so that research can be performed at scale, said B. Martin. Additional public–private partnerships and consortia are also needed to increase the ancestral and content diversity of genomic information and take advantage of recent advances, for example, in the use of polygenic risk scores for patient stratification. “We need to systematically and intentionally expand the diversity
___________________
2 “Digital biomarkers are defined as objective, quantifiable physiological and behavioral data that are collected and measured by means of digital devices such as portables, wearables, implantables, or digestibles.” Source: https://www.karger.com/Journal/guidelines/271954 (accessed January 31, 2022).
- of our large datasets if we want to create generalizable, actionable insights that can apply to all populations,” said B. Martin.
B. Martin also called for improved coordination between discovery and development through better alignment of the research efforts of industry, academia, and governmental bodies. Partnerships and data sharing among these entities can have a dramatic and positive impact on research advances and are essential in the crafting and implementation of regulatory policies and guidelines, he said.
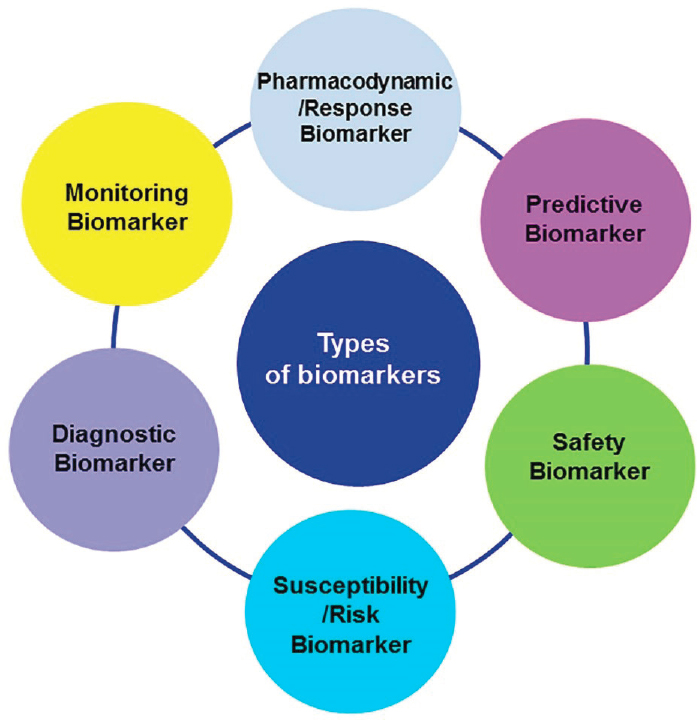
SOURCES: Presented by Bill Martin, October 5, 2021; García-Gutiérrez et al., 2020.
This page intentionally left blank.





