Nonhuman Primate Models in Biomedical Research: State of the Science and Future Needs (2023)
Chapter: 5 Future Needs and Opportunities for Nonhuman Primate Models in Biomedical Research
5
Future Needs and Opportunities for Nonhuman Primate Models in Biomedical Research
The committee was charged with examining the current and future roles of nonhuman primates (NHPs) in research supported by the National Institutes of Health (NIH). As described in Chapter 2, NHPs play an essential role in advancing scientific research that continues to improve and preserve countless lives by, for example, enabling the development of safe and effective vaccines against infectious agents, the development of methods that are improving the success of organ transplantation, and the introduction of new treatments for diseases such as Parkinson’s and sickle cell disease. While great progress has been made toward developing in vitro and in silico technologies and approaches for answering questions of biomedical relevance (see Chapter 4), these new approach methodologies are as yet unable to recapitulate the complexity of many human biological systems and diseases, and have not yet been validated for many applications. Consequently, the committee concluded that NHPs will remain critical models in multiple areas of biomedical research for years to come. Scientific progress in future years will determine the extent to which reliance on NHPs can be reduced. For now, however, given the need for continued use of NHPs in NIH-supported biomedical research, it is critical to ensure that these animal models are used as effectively as possible, particularly given the ongoing shortage of NHPs available for research, as highlighted in Chapter 3. Research should be conducted humanely with respect to the well-being of the animals under study and at the highest level of scientific integrity and rigor.
Building on its assessment of the current landscape (Chapters 2, 3, and 4), this chapter describes the committee’s perspective on the future role of NHPs in NIH-supported biomedical research. The chapter begins with an overview of the factors that will influence the future need for NHPs in biomedical research, which is followed by a discussion of research domains in which the need for NHPs is likely to grow. After touching briefly on future needs for specific NHP species, the chapter explores opportunities to enhance the efficiency and translational relevance of NIH-supported research with NHPs, before ending with the committee’s conclusions regarding the future of NHP research.
FACTORS INFLUENCING THE FUTURE NEED FOR NHPs IN BIOMEDICAL RESEARCH
The future need for NHP models in NIH-supported biomedical research will be driven by many of the same factors that have shaped the current landscape, including pressing current public health needs and preparedness for future threats to public health. Other factors include scientific advances, national policies, and the availability of NHP research resources and infrastructure. Each of these factors is explored below.
Pressing Public Health Needs
The use of NHPs in biomedical research is driven by the imperative to reduce human suffering and improve the quality of human life (Friedman et al., 2017; Harding 2017). Public health threats that currently contribute to significant morbidity and mortality in the United States are likely to continue to guide priority areas for NIH-supported NHP research. These threats to public health include chronic diseases and aging-related conditions that impose a tremendous and growing societal and economic burden, but for which few effective interventions for prevention and treatment are presently available. Some diseases, such as amyotrophic lateral sclerosis (ALS), can be fatal within a few years of diagnosis, underscoring the importance of advancing biomedical research as quickly as possible using the best available models, which in many cases are NHPs (Uchida et al., 2012). As emphasized by one participant in a public meeting held for this study, patients are waiting (Murry, 2022).
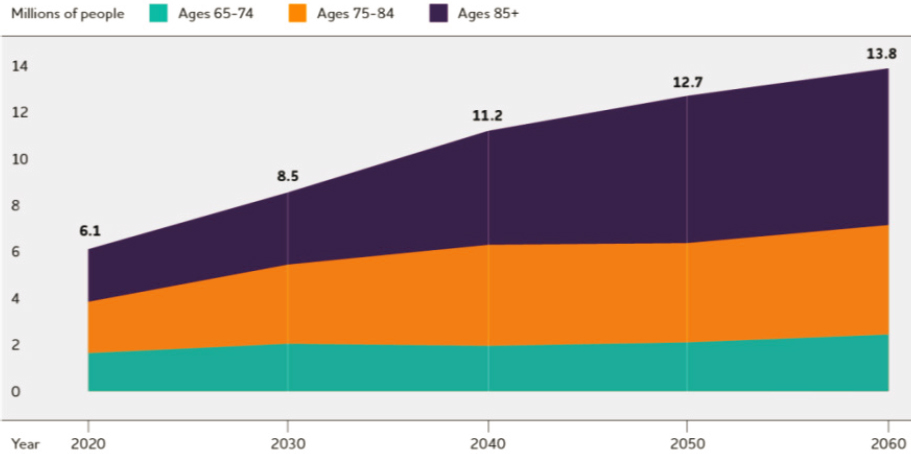
The lack of progress with clinical translation of research on these public health threats often reflects a lack of foundational knowledge regarding underlying mechanisms or disease processes. This knowledge gap underscores the importance of continued investment in fundamental basic and early translational research to enhance understanding of human biology and pathophysiological processes and to identify targets for intervention. As an illustrative example, the number of U.S. adults with Alzheimer’s disease is projected to more than double in the next 40 years, reaching nearly 14 million (AA, 2022) (see Figure 5-1). The enormous public health and economic impacts of Alzheimer’s disease are influenced not only by its increasing prevalence but also by its long duration. In 2022, the health care and long-term care costs for Alzheimer’s disease and related dementias were estimated at $321 billion (Skaria, 2022). Yet while scientists’ knowledge of the pathophysiology underlying Alzheimer’s disease has advanced tremendously over the last three decades, the translation of those insights into clinical gains has been limited (King, 2018).
Clinical progress on Alzheimer’s disease has been hampered in part by the absence of a naturally occurring animal model for this disease and an overreliance on rodent models (Drummond and Wisniewski, 2017; Van Dam and De Deyn, 2011). Transgenic mice that developed hallmark plaques and signs of cognitive deficits associated with the disease were engineered starting in the 1990s, contributing to a better understanding of disease mechanisms. However, treatments shown to clear plaques in mouse models failed to benefit human patients in clinical trials, indicating that these animal models were not fully recapitulating the human condition. Therefore, NHP models in which Alzheimer’s disease phenotypes are induced and three-dimensional human brain organoids are being pursued as complementary models for nonclinical drug testing (Arnsten et al., 2019; Beckman et al., 2019, 2021; King, 2018). In vitro models are well suited for high-throughput drug screening and continued investigation of pathogenesis at the cellular and molecular levels but cannot be used to evaluate the cognitive outcomes of treatments. NHP models, by contrast, enable evaluation of putative therapeutics in the context of complete biological systems that closely mimic those of humans in such areas as lifespan and cognitive function (Lear et al., 2022; Mattison and Vaughn 2017).
SOURCE: Image from the 2022 Alzheimer’s Disease Facts and Figures report was reproduced with permission from the Alzheimer’s Association.
Preparedness for Future Threats to Public Health
Investments in biomedical research are generally based on the most prevalent diseases imposing the greatest health burden. However, this investment must be balanced with preparedness for unknown future threats. Chapter 4 explores ways in which the use of NHPs could be reduced or complemented by new approach methodologies without a substantial negative effect on critical research areas in the United States, but it is important as well to consider the opportunity cost of not having access to NHPs. A tool commonly used in risk assessment is the Johari Window, which quantifies awareness of the state of knowledge about the situation at hand (Beach, 1982; Ha, 2019; Luft and Ingham, 1955). The most difficult challenges to prepare for are those described as “unknown unknowns.” In 2020, the world faced an unanticipated challenge in the form of an infectious disease—the emergence of the SARS-CoV-2 virus and the COVID-19 pandemic. As documented in this report, having access to NHPs was essential for the development of vaccines and therapies for this new threat, and, as noted during the committee’s August 2022 public workshop, shortages of NHP resources hindered the development of some products (Bailey, 2022). In the context of the unanticipated need for NHP models for COVID-19 research, NHP resources were conserved for COVID-19 research at a cost to progress in other important research domains (NIH, 2022d). It may not be possible to meet future unexpected public health needs even with similar resource reallocation strategies if action is not taken to address the ongoing shortage in NHP supply. This possibility highlights the importance of maintaining an adequate reserve of NHPs to respond to the challenges of new infectious diseases (Carlson et al., 2022; Howard and Fletcher, 2012); chemical, biological, or radiological warfare threats (DiCarlo et al., 2011; Singh and Olabisi, 2017); or other unanticipated threats as an essential component of U.S. national security and competitiveness.
The disruption to the biomedical research enterprise and ongoing NHP research that occurred during the COVID-19 pandemic demonstrated the importance of planning and preparing for public health emergencies, a process that needs to include the development of a framework for prioritization of NHP resources and biocontainment facilities in emergency scenarios. As demand for NHPs surged early in the pandemic, NIH assembled an expert panel of subject matter experts to provide input to the Office of Research Infrastructure Programs (ORIP) on the urgency of each of the funded studies and its potential public health impact. The panel’s recommendations were then conveyed to the National Primate Research Centers (NPRCs) and other ORIP-supported NHP facilities (HHS, 2021), and used by NIH and the NPRCs to guide the development of mechanisms for the prioritization of scarce NHP resources during the pandemic. This experience provides an opportunity to learn about what did and did not work well to inform the development of a framework for future public health emergencies.
Scientific Advances
Scientific knowledge is constantly advancing, and as a result, short- and long-term needs for NHP research will differ. The latter can be challenging to predict because science does not advance in a linear fashion (NRC, 2007). Rather, scientific progress can be characterized by turning points and sharp departures in lines of inquiry, and a great deal of uncertainty surrounds which of numerous discoveries emerging at a given point in time will be informative for future research and how findings may ultimately be used.
The maturity of new approach methodologies continues to advance at a rapid pace, and accordingly, in vitro and in silico models are being applied to answer an increasingly diverse set of biomedical research questions. As described in Chapter 4, the data provided by new approach methodologies are expanding scientists’ knowledge of disease pathways, helping to contextualize evidence from other sources, and enabling cost-effective screening, among other applications. In combination with other approaches, in vitro and in silico models make valuable contributions to the overall weight of scientific evidence. Given the range of possible interactions within even a single organ—much less within the entire human body—and the physiologic and structural complexity required of new approach methodologies to fully recapitulate an in vivo system, it is unlikely that the prediction of all in vivo outcomes is an imminent reality. The committee is optimistic, however, about the potential for new approach methodologies to reduce reliance on NHPs in the future as technology and science advance. Given that NHPs are likely to remain a limited and high-cost resource—even with further investments in domestic breeding to address current shortages—advances in new approach methodologies offer the potential benefit of helping to reduce costs and mitigate future NHP shortages. In anticipation of this, attention is needed to the development of a strategy for the advancement of new approach methodologies that can be used in conjunction with NHP models to optimize the application of NHP research and to answer pressing biomedical research questions in the future. One consideration for such a strategy is the inclusion of new approach methodology experts in the review process for NIH research proposals involving NHP models.
While some areas of advancing science, such as new approach methodologies, may reduce the future need for NHPs, others, such as genetic engineering, may create new demands for NHP models that can better replicate the human condition and address biomedical and societal priorities (NASEM, 2019; Sato and Sasaki, 2018). As with new approach methodologies, whether research using transgenic NHPs will achieve the antici-
pated improvements in translation to human health benefits relative to traditional models is not yet clear (Prescott, 2020). The use of genetically engineered NHPs is relatively limited in scope at the present time, and the technology has yet to mature to the point of readiness to assess its value and impact. As the efficiency of genome editing technologies improves and current challenges are addressed (Aida and Feng, 2020; Park and Silva, 2019), and as more laboratories develop the capabilities to establish these model systems, the committee anticipates acceleration of transgenic NHP models in the future.
While neither engineered (e.g., transgenic, transplant) non-NHP animal models nor human studies were included in the committee’s definition of new approach methodologies and consequently not focus areas for this report, it is important to acknowledge that scientific advances in both areas may play a role in reducing reliance on NHPs going forward. Transgenic animal models (e.g., rodents, pigs) are increasingly being used to understand the function of human genes and genetic disease–related pathology. Relevant examples include the ACE2 (angiotensin-converting enzyme 2) expressing transgenic mice used for COVID-19 research (Winkler et al., 2020) and mice expressing human genes linked to Alzheimer’s disease (Baglietto-Vargas et al., 2021). Transplant of human cells and tissues (for example, bone marrow or thymus) into mice has enabled the creation of mouse models with elements of human immune systems that have been useful in understanding some aspects of human immunodeficiency virus (HIV) pathogenesis and evaluation of the efficacy of antiretroviral therapies (Hassounah et al., 2016), as well as study of cancer immunology and immunotherapy (Tian et al., 2020).
Nonclinical NHP studies may be precursors to human studies when there are concerns regarding human safety risks associated with research protocols. For example, nonclinical studies using NHPs may be undertaken prior to Phase 1 clinical trials. Advances in technologies, such as those used for imaging (Prescott and Poirier, 2021; SCHEER, 2017), and evolving approaches to clinical trials, including human challenge studies and Phase 0 microdose studies (Burt et al., 2017, 2018; Palacios and Shah, 2019; SCHEER, 2017), are opening new opportunities to conduct ethical biomedical research in human volunteers. With respect to the opportunities to reduce reliance on NHPs through human studies, however, attention needs to be focused on widely recognized challenges in achieving adequate clinical trial enrollments (Briel et al., 2021; IOM, 2012; Unger et al., 2016) and ensuring that risks are not borne disproportionately by groups that may be more likely to enroll in “first-in-human” trials, particularly disadvantaged populations (Kalbaugh et al., 2021).
National Policy
Policy decisions have enormous potential to influence the future of NIH-supported NHP research. The committee understands the continued emphasis of policy makers on the development and implementation of new approach methodologies with the potential to reduce reliance on NHPs and other animal models. Critically, however, policy needs to be informed by the state of the science. Legislation that prohibits or imposes insurmountable barriers to NHP research in the absence of validated alternative models can potentially put human lives at risk, increase economic costs, spur relocation of U.S. research programs to other countries, and compromise U.S. global leadership in public health and biomedical research.
The use of NHPs in federally funded research has been the focus of ongoing congressional attention. In the Further Consolidated Appropriations Act of 2020, Congress placed restrictions on the use of NHPs (as well as dogs and cats) in research funded by the U.S.
Department of Veterans Affairs (VA).1 The Act directed the VA to develop plans for reducing or eliminating NHP research (Grimm, 2019). This legislation, along with another bill introduced but not enacted,2 demonstrates the potential for future legislation to place constraints on NIH-supported NHP research.
Policy actions may also catalyze the continued development of new approach methodologies with the potential to reduce reliance on NHPs. In response to a congressional directive to accelerate efforts to identify and implement nonanimal alternatives for biomedical research,3 NIH launched a new working group on Novel Alternative Methods under the auspices of the NIH Advisory Committee to the Director (ACD) in November 2022 (Chang and Jorgenson, 2022). The charge to the new ACD working group is to identify high-priority areas for NIH investment in the development and use of novel alternative methods that can (1) advance understanding of biologic processes or states, and (2) complement and/or potentially replace traditional models used in biomedical research (NIH, 2022a). As discussed in Chapter 4, the Food and Drug Administration (FDA) has also established an Alternative Methods Working Group and undertaken other initiatives focused on identifying and advancing opportunities to incorporate new approach methodologies into the regulatory pathway (FDA, 2021a,b), a process that is further encouraged by the FDA Modernization Act 2.04 enacted in December 2022 (see Chapter 4). The FDA may also continue to use regulatory guidance, such as the 2022 guidance on mitigating NHP supply constraints arising from the COVID-19 pandemic (FDA, 2022), to identify opportunities to reduce reliance on NHPs. The 2022 guidance encourages sponsors to use NHPs only when there is no other relevant model.
Availability of NHP Research Resources and Infrastructure
Resources necessary for the continued study of NHPs in the United States have declined in the face of growing needs for the use of NHPs to advance scientific knowledge and protect human health, as described in Chapter 3. Shortages have resulted from overreliance on foreign sources for NHPs and the failure to develop adequate resources for domestic breeding (NASEM, 2021; O’Grady, 2022; Subbaraman, 2021). The challenges associated with availability of NHPs will likely continue to influence their use in the future as efforts to increase supply fall short of the needs created by surges in demand, such as was experienced during the COVID-19 pandemic. Ensuring a supply of NHPs that can meet the needs of the nation’s biomedical research enterprise will require a commitment to supplying NIH-supported investigators from domestic resources.
The development and implementation of a national plan for NHP research resources would help ensure the availability of NHPs to meet the nation’s public health needs. Operational and programmatic elements that need to be considered for such a plan include but are not limited to the
- allocation of scarce NHP resources to priority research areas;
- coordination of domestic breeding plans and the welfare of the animals;
- monitoring and evaluation of changing resource needs across the biomedical research landscape;
___________________
1 Further Consolidated Appropriations Act, 2020 (P.L. 116-94, 116th Congress) (December 20, 2019).
2 The Primate Protection and Research Modernization Act was introduced in December 2018 but was not enacted. See https://www.congress.gov/bill/115th-congress/senate-bill/3773/all-info (accessed November 7, 2022).
3 Further Consolidated Appropriations Act, 2022, Committee Print of the Committee on Appropriations U.S. House of Representatives: Legislative Text and Explanatory Statement, P.L. 117-103, 117th Congress, (April 2022).
4 FDA Modernization Act 2.0 (S. 5002 — 117th Congress [2021–2022]).
- implementation of centralized tracking systems for NHP resources,
- formalization of collaborative research and training opportunities for the study of NHP models and new approach methodologies; and
- implementation of other data sharing opportunities.
Investigative teams in the private sector—major users of NHPs in the United States—have the financial means to acquire and study NHPs. But research conducted under private-sector sponsorship is generally not shared unless such sharing is advantageous to the sponsor. For example, data generated in the FDA drug approval process are not disclosed unless the sponsor elects to do so.5 There have been instances of public–private data sharing, such as the Early Detection Research Network (Srivastava and Wagner, 2020), but in general, data that impact the intellectual property rights or future development plans of private companies are kept private, even after major findings have been published (Hopkins et al., 2018; Modi et al., 2022). In contrast, the findings of NIH-sponsored research must be disclosed openly (NIH, 2022e), and a data sharing plan is a required part of grant applications (NIH, 2023b). Without increased NIH support for domestic production of NHPs, NHP research would likely move increasingly into the private sector, where funding is more abundant, priorities are market driven, and disclosure is limited. Another likely outcome of limitations on NHP research infrastructure in the United States is for research programs to move to countries such as China, where government investment in NHP research is substantial (Einhorn and Lew, 2022; Normile, 2022).
RESEARCH DOMAINS IN WHICH THE NEED FOR NHPs IS LIKELY TO GROW
There are specific domains of scientific inquiry for which research using NHPs is currently indispensable, and the committee anticipates that the need for NHPs in these domains will continue to grow. These domains and the committee’s rationale for anticipating this continued growth are described in Table 5-1. The committee identified these domains after considering the nation’s most pressing public health needs and the evolving state of the science, discussed earlier in this chapter, as well as the findings presented in previous chapters of this report regarding research domains in which NHPs are contributing to advances in human health (Chapter 2), current and future anticipated demand for NHPs (Chapter 3), and the current and future potential of new approach methodologies to reduce reliance on NHPs (Chapter 4).
Neuroscience and infectious disease are research domains likely to grow in the future, as reflected in NIH priorities for NHP research (Eisinger, 2022). The complexity of the primate brain is not adequately modeled by any in silico or in vitro system or other animal species (Feng et al., 2020; Hutchison and Everling, 2012; Roelfsema and Treue, 2014), and the burden of neurologic disease will continue to rise as the population ages (Riggs, 1998). Certain infectious diseases have a pathogenic mechanism seen only in primate species, and the COVID-19, Zika, Ebola, and other recent epidemics demonstrate the importance of NHP models to understanding novel diseases and testing the safety and efficacy of vaccines and therapeutics. Immunotherapy using cellular-, protein-, or nucleic acid–based therapeutics has emerged as another vital area of NHP research that is likely to continue to grow (Lisa et al., 2017; Tanaka et al., 2014; Voge and Alvarez, 2019), and the degree of molecular similarity between humans and NHPs is key to understanding the effectiveness of these novel thera-
___________________
5 Investigational New Drug Application (21 C.F.R. § 312.130).
TABLE 5-1 Research Domains in Which the Need for Nonhuman Primates (NHPs) Is Likely to Grow
| Research Domain | Rationale |
|---|---|
| Basic and applied research in behavior and neuroscience | Many approaches for monitoring and manipulating structures and processes within the brain are invasive and rarely can be carried out in healthy human volunteers. Functional imaging in humans has allowed analysis of the normal and diseased brain in ways not previously possible. The insights gained from imaging in humans are complemented and expanded by imaging in NHPs, which also provides the opportunity to explore the actual neural activity that generates the imaging signals. In addition, movement, cognition, and affect are emergent properties that are a consequence of the activity of large networks of neurons with precise interconnections. The complex interconnections between neurons in these networks are a result of detailed genetic programs and experience. These factors are not easily recreated by tissue culture and organoids. Key brain regions and connections important to normal motor, cognitive, and affective function in humans are absent in rodents but present in NHPs. Genetic engineering of NHPs is creating new opportunities to study neurological disorders. |
| Infectious diseases, including those that are emerging or feature primate-specific pathogenesis or susceptibility | Emerging infectious diseases—both novel agents, such as SARS-CoV-2, and reemerging threats, such as multidrug-resistant tuberculosis and polio—have the potential to abruptly increase needs for NHP research. Pandemics and epidemics create urgent requirements for model systems that can recapitulate the course and outcomes of human disease and support the safety and efficacy testing of vaccines and therapeutics. NHPs are invaluable as models for emerging infectious diseases because of their anatomical, physiological, and immunological similarities to humans and their susceptibility to many human pathogens. |
| Genetics | The close genetic similarities between humans and NHPs provide an opportunity to understand the evolution and function of gene sequences involved in health and disease. Increasing computational capacity to study gene sequence data will allow the study of not only single-mutation diseases but also complex polygenic disorders and epigenetic regulation of gene expression. Gene therapies, including both somatic cell modification in individual patients and heritable germline modification, hold enormous promise for treatment of currently untreatable genetic disorders with severe consequences for affected humans. |
| Development of therapies requiring a primate-specific target | Many biologic therapeutics are designed to engage human targets with high specificity that may manifest their intended pharmacological activity only in other primates. Examples include protease-activated receptors that mediate platelet activation and monoclonal antibodies. As a result of their specificity, therapeutics developed using mice or other nonprimate models may miss their intended target in humans, necessitating studies in NHPs. Stem cell–based therapies, like gene therapies, have generated a great deal of excitement, but safety and feasibility studies in NHPs continue to be critical. In the case of countermeasures that cannot be evaluated for efficacy through human challenge studies, animal studies, including NHP studies when scientifically justified, provide a pathway for regulatory approval under the Food and Drug Administration’s Animal Rule. |
| Reproductive biology | Getting pregnant and carrying a pregnancy to term are complicated processes during which something can go wrong, leading to challenges with fertility. Implications of factors that can contribute to the inability of a woman to conceive, such as aging, failure to ovulate, oocyte maturity, implantation failure, and gynecological diseases and infections, can be modeled in NHPs to address questions directly relevant to human female reproduction. |
| Aging, obesity, and inflammatory disease | Chronic inflammation is a major factor in age-related diseases. The adaptive immune system deteriorates with advancing age, but the impact of aging on specific immune cells and their role in inflammation remain incompletely understood. NHPs are invaluable as models for these processes because of their anatomical, physiological, and immunological similarities to humans, as well as their susceptibility to spontaneous obesity, insulin resistance, and related cardiometabolic comorbidities. Some cancers also arise spontaneously in aging NHPs and are remarkably similar to those seen in humans with regard to incidence, risk factors, and progression to metastasis. Effects of environment, activity, diet, and exposure to medications can be strictly controlled for NHPs and evaluated longitudinally, something that cannot be achieved in humans in an uncontrolled setting. |
| Example Research Topics |
|---|
|
|
|
|
|
|
pies. Likewise, some aspects of reproductive biology are primate specific and, in the case of reproductive health in women, understudied (Metz, 2022; Slawson, 2019); the public health, social, and economic burdens associated with infertility and diseases of the reproductive system will continue to drive needs for NHPs in research in this area. Finally, NHP research in aging and chronic inflammatory diseases is likely to increase given the enormous public health burden associated with such diseases in the United States and the opportunities to study them in the context of the long-term care increasingly being provided to aging NHP research subjects (Verdier et al., 2015). Of note, increased numbers of long-term NHP studies undertaken in the context of aging research also provide new opportunities to study naturally occurring cancers that arise spontaneously in aging NHPs (Deycmar et al., 2023). Treatment of such cancers may inform new tumor-targeting strategies, an advance that not only has high relevance to human disease but also may benefit the NHPs themselves.
FUTURE NEEDS FOR SPECIFIC NHP SPECIES
While multiple macaque species continue to be used in and contribute knowledge to different domains of biomedical research (e.g., neuroscience, infectious disease), rhesus macaques (Macaca mulatta) are likely to remain the predominant species under study based on the abundance of data characterizing this species—including the first and most-studied NHP genome (Gibbs et al., 2007; Rogers, 2022)—and the increasing availability of immunologic reagents, as well as their current widespread use in research funded by NIH (NHP Investigators Survey, 2022; NPRC Information Request, 20226; ORIP, 2018) and globally. These NHPs play a key role in both HIV research (Cooper et al., 2022; Rogers, 2022) and the study of outbreaks, such as Zika virus infection (Dudley et al., 2019; Haese et al., 2021), Ebola (Geisbert, 2017), and COVID-19 (Albrecht et al., 2021; Hild et al., 2021). These animals develop type 2 diabetes mellitus (Havel et al., 2017) and naturally occurring cancers of the colon (Ozirmak Lermi et al., 2022), breast (Wood et al., 2006), and cervix (Wood et al., 2007), with mechanisms of disease pathogenesis nearly identical to those of humans. They are abundant and are not endangered, or even threatened, in the wild (CITES, 2023; Singh et al., 2020).
Use of the common marmoset (Callithrix jacchus) is likely to increase because this small species is the best characterized and most widely used of the New World NHP species (Colman et al., 2020; Han et al., 2022a; Malukiewicz et al., 2020). These NHPs are increasingly used for studies of neurodevelopment and neurodegenerative diseases. Their shorter lifespan relative to other NHPs allows for lifetime studies in a relatively short period of time. They are a tractable species, and they reproduce well under laboratory housing conditions, typically producing twins or triplets and thus allowing for studies of gene–environment interactions. The first representative marmoset genome was sequenced in 2014 (Worley et al., 2014), and transgenic marmosets have been generated (Sasaki et al., 2009).
Some other species will likely continue to be studied to answer specific research questions. For example, both vervet monkeys (Chlorocebus aethiops) and squirrel monkeys (Saimiri sciureus) provide unique insights into neurodegenerative diseases such as Alzheimer’s disease (Frye et al., 2021; Patel et al., 2021), and owl monkeys (Aotus spp.) continue to be used in malaria research (Sharma et al., 2022).
___________________
6 This reference refers to written responses to a committee information request from each of the seven NPRCs (Washington NPRC, Oregon NPRC, California NPRC, Tulane NPRC, Emory NPRC, Wisconsin NPRC, and Southwest NPRC). Individual responses to the committee’s information request can be found in the committee’s public access file.
OPPORTUNITIES FOR ENHANCING NIH-SUPPORTED RESEARCH WITH NHPs
NHPs are precious resources demanding robust stewardship and the conduct of rigorous research that maximizes the knowledge and actionable insight obtained from each individual animal and study. Opportunities to use NHP resources more effectively include using thoughtful approaches for sample collection and sharing, fostering openness and data sharing, learning from natural disease in NHPs, and incentivizing collaboration among NHP research groups. Tools and technologies, including noninvasive monitoring and imaging approaches, artificial intelligence/machine learning (AI/ML), extended reality (XR), and laparoscopy, can be applied to increase the impact and rigor of NHP research, dissect natural disease behavior, reduce or refine sample collection, and improve alignment with processes being implemented in clinical settings. Each of these interrelated approaches to enhancing the use of NHPs in NIH-supported biomedical research, many of which have been noted previously by other expert groups (Bliss-Moreau et al., 2021a; NIH, 2020; ORIP, 2018), is discussed below.
Centralized Tracking and Stewardship of NIH NHP Resources
No clear or complete accounting of domestic NHP populations maintained through NIH funding was provided to the committee. NIH institutes, centers, and offices contract for NHPs as needed with contract research organizations and universities. However, the process used for allocating available NHP resources to researchers in need of NHP models did not appear fully transparent to the committee or consistent across resources, making it difficult to assess equitability for different researchers and disciplines or the research community at large. Given the unique value of NHPs to the research community, there is an opportunity to enhance NIH-supported NHP research through more formalized, centralized, digitized and digitalized, and comprehensive national management and tracking of NHPs maintained and used by NIH’s intramural and extramural programs. The NHP animal locator service based at the Washington NPRC is an invaluable resource for investigators nationwide (NHPRC, 2023; WaNPRC, 2023); expansion of this service and designation of NIH staff to support that effort would improve transparency regarding the availability of scarce NHP resources and allow their more efficient utilization.
Increasing Use of Data-Driven Noninvasive or Minimally Invasive Technologies
This section describes noninvasive or minimally invasive technologies with the potential to enhance biomedical research conducted using NHP models. Examples of these technologies include digital biomarkers, XR, laparoscopy, and imaging; also discussed is the use of computational methods, including AI/ML and computer vision. Some of these technologies, such as laparoscopy and imaging, are already used in NHP studies to some degree (Association of Primate Veterinarians, 2019; Basso et al., 2021; Milham et al., 2018; Prescott and Poirier, 2021). Digital biomarkers and XR technologies have been used with NHPs on a limited basis (Davis et al., 2021; Usher et al., 2018) but are used more commonly with rodent species (Baran et al., 2020, 2021; Venkatesan et al., 2021). These technologies have been implemented within clinical phase drug development and even health care (Crawshaw et al., 2015; FDA, 2020; Mori et al., 2022). Leveraging technologies and approaches that
are non- or minimally invasive offers many potential benefits for NHP research, including enabling
- longitudinal assessment of disease progression that allows each animal to serve as its own baseline control, leading to increased sensitivity and reducing variability and numbers of animals needed for the research; and
- improved measurements of drug safety, tolerability, and efficacy through the incorporation of drug distribution measurements in real time.
The sections that follow provide further descriptions of the potential uses and benefits of non- or minimally invasive technologies in the context of NHP research. Note that the benefits described under each technology-specific subsection are intended to be illustrative and do not represent a comprehensive cataloging. It should be noted as well that current grant mechanisms offer little support for the development of novel, less invasive procedures for NHP research, despite the potential of these approaches and technologies to benefit animal welfare and data quality and reduce the numbers of animals used.
Digital Biomarkers
A digital biomarker refers to a characteristic or set of characteristics, collected from digital technologies, that are measured as an indicator of normal biological processes; pathogenic processes; or responses to an exposure or intervention, including therapeutic interventions (Vasudevan et al., 2022). Digital biomarker data can be collected continuously from freely moving NHPs in their home environment (Prescott et al., 2021) using a variety of digital monitoring technologies, including internal (e.g., injectable, implantable, or ingestible) and external (e.g., wearable, camera, or electromagnetic field detector) devices (Baran et al., 2022; Brattain et al., 2016; Koizumi et al., 2021; Ma et al., 2020; Prescott et al., 2021). Examples of the use of digital biomarkers in NHP research include detection and tracking of experimentally induced or naturally occurring diseases (NIH and HHS, 2020), including subclinical (mild) disease that may cause NHPs to be distressed without showing observable clinical signs (Davis et al., 2021). Other applications include the study of social behavior and cognition using micro-electrocorticogram recordings (Xu et al., 2022). Approaches using digital biomarkers have also been assessed for their utility in managing Alzheimer’s disease in human patients (Harms et al., 2022) and, more commonly, in research using rodent models across a range of diseases (Baran et al., 2020, 2021; Defensor et al., 2019; Do et al., 2020; Geuther et al., 2022; Golini et al., 2020; Hobson et al., 2020; Wotton et al., 2020).
Use of digital biomarkers enables the collection of objective, quantifiable, and clinically relevant measures of physiological and behavioral response to disease progression or therapeutic intervention (Motahari-Nezhad et al., 2021; Traverso et al., 2015). Increased accuracy of experimental observations may be achieved by eliminating the subjectivity that results from inconsistency in human observations, as well as the influences of human intervention when recordings are made (Baran et al., 2022). Collection of digital biomarker data may also improve the planning, design, and execution of NHP studies by identifying outliers before a study begins.
NHP data collection and study outcomes can be influenced by routine handling or in-room observations that impact NHPs’ physiology and behavior (Clarke et al., 1988, 1994; Pomerantz et al., 2022). Digital biomarker technologies have the potential to enhance the quality of research by decreasing the data variability that results from stress and alteration of the basal physiology and behavior of NHPs associated with in-person checks or handling.
Similar effects are observed in the clinical setting when outcomes of patients’ physiological and behavioral assessments are impacted by stress and drive for higher performance in a clinic versus at home (Drawz et al., 2012; Mallick et al., 2009). Thus, another potential benefit of biomarker collection is increased precision and sensitivity for detecting significant pharmacologic or toxicologic changes, as well as enhanced study reproducibility.
Digital biomarker technologies have the potential to provide a more holistic assessment and view of NHPs because they offer continuous monitoring instead of the snapshot derived from traditional assessments. Continuous collection of digital biomarkers has the potential to enhance current NHP approaches by providing unbiased longitudinal monitoring of behavioral and physiological function for various research domains, from aging to neurological diseases to safety testing. In NHP research, standard techniques for quantifying biological age and the progression of aging are challenging to implement reproducibly and longitudinally in cohorts within and/or between research laboratories (Baran et al., 2021; Shively et al., 2021). The ability to conduct longitudinal disease characterization and tracking may be especially useful in diseases with variable onset and rates of progression, as well as when the health of NHPs may decline rapidly without obvious warning signs or so slowly that change is difficult to assess (Baran et al., 2022). Use of digital biomarker technologies not only provides an opportunity to align with clinical approaches and provide a higher-resolution picture of NHP health or disease state, but also has the potential, noted earlier, to reduce the number of NHPs required as NHPs on longitudinal studies can serve as their own controls.
Digital biomarkers may also reduce reliance on traditional measurements, such as blood collection under sedation or some types of neurobehavioral recordings collected from restrained NHPs, which require handling the animals (Prescott et al., 2021) and may necessitate removing them from family groups. In addition, the potential replacement of histopathology endpoints, which require euthanasia, with digital biomarkers in specific contexts of use may reduce the number of NHPs needed in a study. In some cases, moreover, digital biomarkers may be able to show modest therapeutic improvement that is clinically relevant but that traditional measures are not sensitive enough to detect (Baran et al., 2022).
Overall, continued development and broader implementation of digital biomarker approaches for NHP studies provides an important opportunity to enhance NHP research through assessments that are objective (not impacted by perceptual biases), have high resolution (collected continuously), and are realistic (collected within the home environment of NHPs) (Baran et al., 2022).
Extended Reality, Including Virtual and Augmented Reality
XR technology, including virtual and augmented reality, can be used for NHP management, staff training, and clinical assessments (AALAS, 2020). Use of this technology is just now emerging within the life sciences. While it is not yet widely applied to the handling and care of NHPs, experience in other veterinary and research settings (McCool et al., 2019; Tang et al., 2020) suggests that XR technology has the potential to
- increase the efficiency of NHP clinical assessments and veterinary training, such as by enabling staff who are assessing an NHP to share observations with the veterinary team in real time while accessing health and study records;
- reduce NHP stress and potentially morbidity and mortality by allowing staff to make decisions in real time;
- provide access to NHP subject matter experts and researchers on- and offsite;
- reduce the number of personnel entering the facility, thereby reducing the risk of contamination;
- standardize the collection and reporting of clinical and pathology outcomes; and
- augment training of personnel and researchers by allowing access to immersive training prior to in-person training (McCool et al., 2019), making the latter more effective with less disruption of and impact on NHPs and reducing the number of animals required.
Minimally Invasive Surgical Techniques
Laparoscopic surgery, also referred to as minimally invasive surgery of the abdomen, has become the gold standard in human medicine for many abdominal surgical procedures, and multiple publications have described its benefits in NHP research (Chai, 2015; Hutz et al., 1988; Kumar et al., 2011; Liao et al., 2004; Rippy et al., 1996), indicating the value of expanding future use of this technique when possible. As in the clinical context, laparoscopic surgery results in less postoperative pain, inflammation, and infection, and NHPs therefore require less time to recover as compared with laparotomy (open surgery). Additionally, the laparoscopic approach allows for repeated visualization of the site, biopsy collection, and intervention within the same NHP. This capability minimizes interanimal variability and facilitates longitudinal studies of disease progression within the same animal in lieu of relying on postmortem samples collected from replicates (Baran et al., 2011). Wider implementation of laparoscopy thus has the potential to enhance animal well-being and reduce the variability of research data while increasing precision and sensitivity.
Imaging
Imaging technologies, such as optical imaging, magnetic resonance imaging (MRI), ultrasonography, computed tomography (CT), positron emission tomography (PET), and single-photon emission computed tomography (SPECT), can be used to facilitate longitudinal monitoring of disease progression and biological responses over time (Bercovich and Javitt, 2018). CT, including contrast-enhanced CT, can be used for quantitative assessment of body composition (using, for example, tissue density to distinguish healthy from diseased tissue) (Troschel et al., 2020). In a recent example, a combination of CT and fluorine-18 fluorodesoxyglucose (18F-FDG) PET imaging was used to collect additional data on COVID-19 pathogenesis in the lung and to evaluate therapies in NHPs (Naninck et al., 2022). MRI has been particularly useful in neuroscience, enabling study of NHPs’ affective states, brain evolution, and face processing, among other topics (Prescott and Poirier, 2021). Insights regarding normal and diseased brain states gained from functional imaging using PET and MRI in humans are complemented and expanded by imaging in NHPs, in which exploration of the neural activity that generates the imaging signals is possible. For some studies, the potential decrease in variability achieved by using as the control values the baseline measurements obtained with imaging of the same animal (as opposed to separate control animals) may also reduce the number of animals required for detecting a change in response to a physiological perturbation.
Computational Methods, Including AI/ML and Computer Vision
AI/ML methods and applications are described in Chapter 4 in the context of approaches for complementing or reducing reliance on NHP models. AI/ML can also assist with the analysis of NHP experimental data to obtain information on and insight into the systems being studied. Through AI/ML analyses, one can obtain both real-time and accelerated access
to insights that can expedite decision making, which in turn may reduce the length of a study or enable faster intervention if needed.
Computer vision, a subcategory of AI, has been used in the analysis of video data collected during studies of NHP behavior and physiology (Bala et al., 2020; Brattain et al., 2016). Deep learning, another AI subcategory, has been used to count NHP retinal ganglion cell axons in optic nerve tissue images, outperforming manual counts (Ritch et al., 2020). In the analysis of histological imaging data, AI/ML methods can be trained to recognize specific features in imaging data, and once validated, can be used to examine other images to identify relevant features; such methods are already having an impact in radiology and tumor histology. AI/ML methods can also be used to analyze complex patterns in imaging or video data and, given appropriate training data, draw inferences about NHPs’ behavior and psychology (Bala et al., 2020; Berger et al., 2020; Yao et al., 2023) in the same way that AI/ML systems are being trained to recognize changes in human psychology (e.g., cognitive decline). Such systems could also be used to identify neurodegenerative disorders or symptoms and to monitor their progression or potential response to therapies.
The behavioral and neuroscience fields offer additional examples of AI/ML applied in NHP research to increase data processing efficiency, extract additional knowledge and insights from available data, and provide predictions. These examples include behavioral evaluations (Lauer et al., 2022), identification of predictors of neurodegeneration, imaging genetics and genomics, classification of electrocorticograms, and preprocessing and automating MRI analysis (Bogdan et al., 2017; Choi et al., 2021; Hadj-Bouziane et al., 2003; Neff, 2020; Pilkiw and Takehara-Nishiuchi, 2018; Teil et al., 2021).
Outside of neuroscience, AI/ML methods have been used to better understand the humoral immune responses of vaccine candidates by identifying the features of humoral immunity that distinguish protective from nonprotective responses (Ackerman et al., 2017). Within cardiovascular pathology, these methods have been used for histopathology diagnosis (Glass et al., 2022). These computational tools can also expedite NHP biomarker discovery and indicators of disease outcomes (Marino et al., 2016). Hybrid approaches may involve integrating AI/ML with system biology (Antontsev et al., 2021) and can allow for translation of computational models across species (NHPs and humans) (Brubaker and Lauffenburger, 2020).
Ultimately, the successful deployment of any computational methods and their effective use in NHP studies will require sharing the data, computational models, and software code. Such sharing will help ensure that the data are used to maximum effect and that the results from each study are reproducible (or falsifiable).
Increasing Impact by Combining Technologies
While each of the above technologies may have a positive impact on NHP research, combining them holds the potential for greater impact (EY, 2018). For example, combining digital biomarker data collected continuously from freely moving animals in their home environment with AI/ML extraction (such as computer vision) could enable the development of digital NHP twins for specific applications, as seen in the behavioral sciences (Baran et al., 2022), given sufficient and relevant training data for the task at hand. Such a system could also facilitate the identification of subtle behavioral changes that would enable earlier detection of the effects of experimental treatment (Davis et al., 2021), which in turn might allow investigators to intervene sooner, thereby improving NHP care. Such predictive models would require substantial validation before they could be widely used or the results from such studies could be fully trusted. Still, the noninvasive nature of digital biomarkers means
that such approaches could be developed at relatively low cost and without undue stress or risk to NHPs.
Combining NHP training (Graham et al., 2012) (see the section on Data-Driven Advances in NHP Care and Management that follows) with digital biomarker technologies may achieve a combinatorial effect that goes beyond what a single technology can provide, as both approaches reduce stress on NHPs and decrease the variability of research data. Incorporating laparoscopic, imaging, and digital biomarker technologies for longitudinal assessment of disease progression, when appropriate, also has the potential for achieving a combinatorial effect.
Data-Driven Advances in NHP Care and Management
In NHP research, the iterative scientific process is often focused on questions related to human health, but the process also applies to other aspects of NHP research, including standard management practices for NHP care. The data generated from NHP research are inexorably linked to the conditions in which the animals exist in the laboratory, even if this link is not always explicitly recognized. For example, social conditions in which infant monkeys are born and reared have lasting impacts on their psychosocial development (Harlow et al., 1965), neurobiology (Martin et al., 1991; Sánchez et al., 1998; Struble and Riesen, 1978), and programing of the immune system (Capitanio, 1998; Hawkley et al., 2012; Lubach et al., 1995). Furthermore, social housing in adulthood prevents the development of and reduces existing deleterious behaviors (Baker et al., 2012; Gottlieb et al., 2015; Schapiro et al., 1996). Accordingly, a federal mandate requires housing NHPs in social contexts,7 although exemptions to this rule are allowed for scientific reasons.
The impact of social context is likely greater than was previously thought. A growing body of literature demonstrates that the adult monkey’s social context (even when the animal has been reared to adulthood in social groups) impacts the data generated by translational NHP models for the study of human health. Such impacts have been observed, for example, in studies related to mood disorders—influenced by variability in access to social partners (Charbonneau et al., 2022), and variability in the number and strength of close relationships (Bliss-Moreau et al., 2021b)—and immune function (Capitanio and Cole, 2015; Castell et al., 2022; Guerrero-Martin et al., 2021).
Little funding is available with which to test specific hypotheses related to social context, leaving many open questions. Nonetheless, these findings suggest that the best NHP models for studying human health are NHPs living in social contexts—and preferably specific types of social contexts that include full access to partners in stable relationships. Similarly, research has shown that providing NHPs with control over their environment by training them to participate actively in research studies, such that they are cooperative during drug administration or blood sampling reduces stress, decreases the variability of research data, and more accurately models diabetes in a macaque model (Graham et al., 2012). This finding is perhaps not surprising, as it mirrors the voluntary provision of samples by human patients. Additionally, both providing social housing and training NHPs to cooperate in studies have been found to reduce the variability of the data produced by macaque models for the study of infectious and metabolic diseases (Graham et al., 2012; Guerrero-Martin et al., 2021), a finding with important implications for the number of animals necessary to conduct a meaningful study (less-variable data requires fewer animals to draw conclusions), as well as for reproducibility. As with social housing, however, acclimating or training animals to par-
___________________
7 Housing Facilities, General (9 C.F.R. § 3.75).
ticipate actively in studies requires significant resources, including personnel with expertise and time.
Although it is becoming increasingly apparent that the methods used with NHPs influence the data they generate, various factors impede the ability to fully comprehend that relationship. Peer-reviewed articles often do not report important methodological details (e.g., housing conditions, including whether the animals were pair-housed and what environmental enhancements were provided; handling techniques, such as acclimation and training strategies). Best practices intended to guide researchers in including such details in the methods sections of their manuscripts (Bliss-Moreau et al., 2021a; Percie du Sert et al., 2020; Pomerantz et al., 2022) are often neglected because of lack of awareness, or the desire of the author or the journal to adhere to word count limits. In fact, the authorship guidelines of many top journals actively encourage researchers to truncate their methods sections. This practice presents a challenge not only to researchers who wish to replicate published studies but also to the research community’s efforts to assess how widespread different practices are.
It is also important to note that NIH specifically does not fund research focused on the effect of housing conditions or handling techniques on animal models unless the research is framed in a directly translational fashion (e.g., the effect of social stability on the immune response), and non-NIH sources of funding for such studies are few and limited with respect to the amount of funds available. Details about care conditions that could dramatically impact data outcomes are often very minimal or not included in grant applications, mirroring their lack of inclusion in publications and making it difficult to evaluate whether future research will address these issues. As a result, researchers and grant application evaluators face challenges in determining how changes in housing or handling will impact the data generated. This lack of information on care conditions also presents a challenge for leveraging current scientific insights to speed both scientific discovery and future improvements in NHP science. This gap needs to be addressed through explicit recognition of the importance of these factors and matched by strategic investments in determining their effect on research outcomes. Such investments would ensure that these factors are addressed in scientific practice, detailed in the reporting of scientific discoveries, and ultimately leveraged to ensure the well-being of NHPs and maximum benefit from future NHP research.
Facilitation of Intergroup Collaboration and Information Sharing
Facilitating Collaboration among NHP Researchers
While examples of collaborative NHP research can be found within and among research institutions, including NPRCs (Messinger et al., 2021; Yee et al., 2022), increasing collaboration across NHP research groups would have several potential benefits, including more efficient use of individual animals, facilitation of data and knowledge sharing, and improvements in the rigor of research. Collaborative approaches could provide an opportunity for, and reduce barriers to, incorporating novel technologies, including new approach methodologies, into research using NHPs.
Regardless of whether collaborations would be established within a single research institution or across multiple facilities where NHPs are being used, plans for each upcoming NHP study would be shared among participating researchers so additional samples could be taken or data collected from the same NHP cohort for use by multiple research groups. For example, one uninfected (control) cohort could serve as a control for multiple unrelated research questions, as long as appropriate samples and data were collected from those animals. Such efforts would require extensive preplanning, harmonization of protocols, and
ongoing communication, as well as the resources for collection and storage of samples and the digitization and digitalization of data. Also important would be cultural climates that foster collaboration and mechanisms and regulatory flexibility for multiple researchers to be mapped to a single group of animals.
Promoting intergroup collaborations will require (1) investigator-driven efforts—an example of which is ManyPrimates, which employs a “crowd-sourced” method of cooperation and data sharing dedicated to accelerating research on primate cognition (Altschul et al., 2019); and (2) explicit NIH funding opportunities (see Box 5-1). Collaborative opportunities could further be realized through screening for common diseases enzootic in captive NHP breeding colonies/populations, such as genital papillomavirus infections; screening for early cancer detection; and multicenter studies of common morbidities, such as osteoarthritis and metabolic disease, that involve banking of samples and data from affected animals in a central resource for community analysis.
Effective interinstitutional collaborations require tools and strategies that allow for acquiring and storing data in harmonized and digital formats, sharing records, and integrating clinical and research data. The Zika research group at the Wisconsin NPRC (Dudley et al., 2019), for example, made its data public early in the Zika virus pandemic via LabKey—an electronic tool that allows data to be anonymized and published in a public forum (Butler, 2016)—which led to collaborations across NPRCs. These collaborations allowed researchers at the centers to pool data and generate discoveries that would have taken significantly more time and resources had the centers’ teams been isolated from one another (Dudley et al., 2018; Raasch et al., 2022).
Efforts to promote collaboration among NHP research groups are not without challenges. One of the most persistent barriers to large-scale collaboration has been funding limitations. NIH funding mechanisms are often not designed for intergroup collaboration, contributing
to the creation of silos in research (ORIP, 2018). Other challenges include the potential loss of intellectual property or misattribution of credit for academic career advancement (NIH, 2020) and, in cases of international collaboration, differences in the regulations guiding the use of NHPs in research across countries (Mitchell et al., 2021). Funders and other relevant parties will need to address these barriers if the potential of collaboration for the advancement of NHP research is to be realized.
Promoting Quality, Openness, and Data Sharing in NHP Research
The past decade has witnessed a surge of interest in improving the quality of science, born out of notable failures to replicate scientific findings across studies, laboratories, or species (Errington et al., 2021; Open Science Collaboration, 2015; Owens, 2018). The result has been increased focus on developing methods and reporting structures that will allow for the evaluation and improvement of scientific rigor and reproducibility (Bliss-Moreau et al., 2021a; Hewitt et al., 2017). The expectation is that increasing the transparency of experimental design and data analysis, along with data sharing, will enhance the ability of scientists to evaluate the quality of published scientific studies and replicate their findings, although it should be noted that, while replication of findings is often viewed as a gold standard for high-quality research (Collins and Tabak, 2014), a study can be repeatable without being rigorous or translationally relevant.
Replication is rare in NHP science because of the scarcity and critical importance of NHP resources. Only 26.5 percent of the 264 NIH-supported investigators who responded to the questions on this topic in a committee-generated survey reported carrying out any kind of replication of existing studies (NHP Investigators Survey, 2022). Still, practices that allow for evaluation and improvement of the rigor and scientific impact of existing research offer a means of promoting quality in NHP studies. They include the adoption of good practices for documentation, digitization and digitalization, and data reporting and the sharing of experimental materials and data, which are discussed in more detail below. Wider adoption of such practices, however, will require systems of accountability and mechanisms for addressing the real and perceived barriers to their implementation.
Facilitating Data Sharing
Data sharing is a key means of assessing and promoting rigor in NHP research and can also help reduce redundant research by enabling investigators to access existing data sets instead of having to replicate existing ones using additional animals. As discussed previously, moreover, large, curated data sets are required for training and validation of computational tools (Alharbi and Rashid, 2022; Yao et al., 2023). For these reasons, it is important to promote data sharing opportunities by developing and maintaining a robust infrastructure that can accommodate the large quantities and types of data generated within the diverse NHP research ecosystem, including all relevant metadata (e.g., genetic characteristics and origins of the animals, clinical data, how the animals were housed and handled [Pomerantz et al., 2022]; other specifics of the study design), without which the value of shared data can be limited, potentially leading to biased interpretations.
The genomics field has been a leader in data sharing, providing an example of the potential benefits of open access. It is common practice for researchers to deposit raw genomic data before or upon publication (NIH, 2020). At present, two databases developed by the NPRCs in collaboration with the University of California, Santa Cruz, enable sharing of NHP genomic and annotated phenotypic data (NIH, 2020). In addition, the Gene Expres-
sion Omnibus database, which includes gene expression data from NHPs, serves as a public transcriptomics data repository (Han et al., 2022b; NCBI, 2021).
Other examples of NHP data sharing include the National Institute on Aging (NIA)–supported Primate Aging Database8 (Kemnitz, 2019) at the Wisconsin NPRC, which captures biological variables relevant to aging, and an effort led by the California NPRC to facilitate access to longitudinal behavioral, clinical, and other data generated as part of its Biobehavioral Assessment Program. In the latter example, which was recently described in a review by Capitanio (2021), infant monkeys at the California NPRC underwent comprehensive behavioral and immunophenotyping shown to have significant predictive capacity for a variety of behavioral and disease-related processes (Baxter et al., 2021; Kinnally et al., 2019; Myers et al., 2021). These data are made available via the California NPRC colony records, allowing researchers to select animals based on early temperament and physiology, and also serving as a valuable resource for the identification and characterization of naturally occurring animal models, most recently yielding a naturally occurring macaque model for the study of autism (Myers et al., 2021).
The cultural shift toward increased data sharing is far broader than the NHP research enterprise and continues to evolve (NIH, 2023a). The need remains, however, to encourage data sharing among qualified investigators and to provide the resources necessary to sustain large data sharing efforts. In the committee’s survey of NIH-funded NHP researchers, many researchers self-reported sharing their data via trusted repositories (80 percent of 264 respondents), with fewer sharing data analytic methods (code) in such repositories (50 percent) (NHP Investigators Survey, 2022). Beyond the concerns that have impeded data sharing in science more generally, including those related to intellectual property and data security (Alter and Vardigan, 2015), additional concerns and barriers related to the sharing of NHP data in particular were identified. In responses to the committee’s survey, lack of sufficient resources was a recurring theme, with additional concerns including a lack of “trusted repositories,” storage space for data, and personnel to manage shared data, along with limited funding to support these ventures. Some respondents stated that NHP data are precious, expensive, nuanced resources often used for many projects in any given laboratory and supporting multiple trainees. Respondents indicated that sharing in this context reduces the laboratories’ ability to use data for their own purposes, opens up opportunities to be “scooped,” and creates intellectual property issues. The sensitivity and complexity of NHP research were also cited as barriers to sharing, with concerns in this regard including how shared data would be interpreted and limited quality control regarding which data would be shared. Expressed as well was the possibility that animal rights groups would use the data and expose the laboratory to information requests under the Freedom of Information Act and state public records laws, which vary from state to state and to which public universities are subject (NHP Investigators Survey, 2022). It is important to note here that different kinds of data have different risk levels; for example, the risk is greater with video data than with omics data.
Improving the Consistency of Health and Research Record Documentation for NHPs
For various reasons, information on the life, health, and experimental history of NHPs is often not communicated across laboratories or in publications. Clinical records are in different formats in different laboratories, while experimental data are usually separate from medical records and may be unavailable because of blinded study designs or intellectual
___________________
8 See https://primatedatabase.org/ (accessed November 19, 2022).
property concerns. Currently, some of these health and experimental records are required by funders, but there are no consistent criteria for their collection, digitization, and digitalization. Furthermore, NIH typically funds projects for 5 years at a time, and the maintenance of experimental data may be discontinued when funding ends—a particular concern for long-lived species such as NHPs.
Even among those tracking these data, the types of information captured vary (Bliss-Moreau et al., 2021a; NIH, 2020). Inconsistent tracking of lifelong health and experimental data for NHPs has the potential to impact both research outcomes and animal welfare. Previous housing or husbandry conditions, social interactions or isolation, health conditions (including infection status for non–specific pathogen free [SPF] NHPs), and experimental and clinical histories may affect experimental outcomes in unpredictable ways, potentially obscuring the impact of experimental manipulations (ARRIVE, n.d.). Thus, a mechanism is needed for collecting and sharing in a consistent format across research groups a minimum set of experimental, medical, and life history data for NHPs used in NIH-supported biomedical research.
Consistent assessments and health screening criteria would not only improve scientific rigor and guide NHP health management but also facilitate more consistent reporting across investigative teams and provide a means of better matching NHPs with future studies, particularly if information on available NHPs at NPRCs were accessible to the broader research community (as discussed earlier in the context of the California NPRC’s Biobehavioral Assessment Program). Lessons from the advancement of personalized medicine may be applicable to NHP characterization and research, particularly in the context of population stratification and diagnostics. However, this level of characterization is most likely to occur in domestically bred NHPs, providing further rationale for investment in domestic resources to reduce reliance on imported animals. In the long term, standardized record systems could be pursued, but given the burden associated with the development and maintenance of such systems, mechanisms that foster the harmonization and adoption of good documentation practices provide a path to more immediate benefit.
A number of examples of useful record systems from the clinical realm could be adapted for NHP research. They include, for example, electronic health records; clinical trial–like case report forms; and standard data models, such as Cancer mCODE (Minimal Common Oncology Data Elements) (MITRE, 2022) and the Standard for Exchange of Nonclinical Data (SEND), which is used for submission of nonclinical study data to the FDA (Choudhary et al., 2018). At a minimum, record systems need to be digital, searchable, and interoperable while also complying with the FAIR (Findability, Accessibility, Interoperability, and Reuse) Guiding Principles (Wilkinson et al., 2016).9 The development and implementation of such systems will require resources including but not limited to standardized information systems and the personnel needed to manage the records and facilitate their use by investigators.
Development of Genetic and Genotype–Phenotype Resources
Progress has been made in the genomic sequencing of select NHP species commonly used in research, including rhesus macaques (Gibbs et al., 2007), vervets (Warren et al., 2015), and marmosets (Worley et al., 2014). The macaque Genotype and Phenotype (mGAP) resource10 is notable in that it is the first public website providing for macaques searchable, annotated gene variant data, along with disease phenotype data, enabling the linking
___________________
9 See https://www.go-fair.org/fair-principles/ (accessed November 19, 2022).
10 See https://mgap.ohsu.edu/ (accessed November 19, 2022).
of variants to specific disorders (Bimber et al., 2019). Gene–disease associations based on this unique resource have been reported for such serious diseases as retinitis pigmentosa (Peterson et al., 2019), epidermolysis bullosa (Johnson et al., 2020), and the hypomyelination disorder Pelizaeus-Merzbacher disease (Sherman et al., 2021). Understanding of the substantial genetic variability within and across NHP species is increasing and can be leveraged for discovery and interpretation (Cheng et al., 2022).
Major histocompatibility complex (MHC) typing in acquired immunodeficiency syndrome (AIDS) research is another example of progress in model characterization. Specific MHC haplotypes have been linked to specific outcomes in simian immunodeficiency virus (SIV)–infected macaques, most notably natural suppression of viral replication and protection against progression to a neuro-AIDS phenotype (Sauermann et al., 2008). Characterization of the MHC haplotypes of macaques has become commonplace, and attempts to review the haplotypes before individual macaques are selected for a study have proven valuable in allowing researchers to select animals best suited to the research questions at hand.
Much work remains, however, before the characterization of NHP models is as extensive as that of murine models, which benefit from an International Mouse Phenotyping Consortium (IMPC).11 The IMPC is focused on systematically characterizing mouse strains and models using a standardized battery of methods aimed at revealing disease manifestations across multiple body systems (Muñoz-Fuentes et al., 2018). The results of these tests are then made widely available through the IMPC website, with clear reference to their potential translational value, as are the mice themselves. A similar system could be established to ensure optimal characterization of NHPs at NPRCs and facilitate the sharing of tissues, animals, and data across institutions.
A key complement to the development of NHP genomic resources is the development and expansion of NHP phenotyping resources. Pathology resources are a required component of all NRPCs, but they are constrained by limited funding and the variability of necropsy procedures and reporting protocols across institutions. While all NRPCs and National Resources queried by the committee reported performing necropsies (ranging from 75 percent to 100 percent of decedent NHPs), the types of examination performed vary, ranging from the conduct of both gross and histological necropsy examinations on all decedents to gross examinations only, depending on the study protocol (National Resources Information Request, 202212; NPRC Information Request, 2022). Tissue storage and sharing practices also vary with respect to how long samples are stored and with whom they are shared. Examples of focused collaborative efforts to standardize the collection and reporting of pathology outcomes include studies of hypertrophic cardiomyopathy (Reader et al., 2016), the development of standards for examination of the brain (Pardo et al., 2012), and the International Harmonization of Nomenclature and Diagnostic Criteria project (Colman et al., 2021). Notably, the latter project was a grassroots collaboration among pathologists in the private sector (pharmaceutical companies and contract research organizations), academia, and NIH, accomplished largely on a volunteer basis. NIH support for disease phenotyping of NHPs could have a transformative impact on the research landscape.
As NHP models are further characterized, it will be important to have agreed-upon standardized terminology for communicating such information as genetic background and phenotype. An example of the development of such standardized nomenclature is the work
___________________
11 See https://www.mousephenotype.org/ (accessed November 19, 2022).
12 This reference refers to written responses to a committee information request from each of the four ORIP-supported National Resources. Individual responses to the committee’s information request can be found in the committee’s public access file.
of the International Committee on Standardized Genetic Nomenclature for Mice (MGI, 2022); that nomenclature is now required by major journal publishers that adhere to the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines (Percie du Sert et al., 2020). The strain, source, and genetic modifications of different mouse models have profound effects on the data produced by these animals. Thus, it is essential to have a clear way to communicate these types of details for NHP data so the data can be interpreted appropriately, and the utility of prior research can be realized. Subdisciplines within the NHP research enterprise currently suffer from a lack of such standardized nomenclature to facilitate communication and could benefit from the use of an approach similar to that used for mice. Given the broad scope of professionals who work with NHPs across the conservation, zoological, anthropology, primatology, and biomedical fields, such an effort would need to be cross-disciplinary to have maximum impact and would benefit from federal sponsorship.
Improvement of NHP Research Infrastructure
Resources for Biobanking
Biobanking of samples collected from individual animals used in research studies represents an important opportunity to optimize and share NHP resources going forward, and potentially to reduce the number of NHPs needed to address scientific questions. Biobanking, an area of growing interest and support within NIH, requires appropriate structure and funding, and a thoughtful approach to sample collection for biobanking requires anticipating potential future data needs and considering costs. Additionally, biobanked samples have little value if they are sequestered and unavailable to researchers, but the opportunities available for NHP researchers to access biobanks are currently limited.
Compared with biobanks for other types of animals, primate biobanks contain more diverse and less-standardized samples, with frequent changes in availability (Witham and Wells, 2022). In addition, samples are often taken sporadically over long periods of time by different people (Witham and Wells, 2022). The development of active processes for maintaining and sharing biobanked samples will be critical to maximizing the utilization of NHP resources.
There are several useful examples of successful biobanking both within (NIA, 2022) and outside of the NHP research ecosystem (MAF, 2022; NNTC, 2022). The Golden Retriever Lifetime Study, conducted by the Morris Animal Foundation, is an example outside of the NHP research world that involves the crowdsourced collection of genetic and other samples throughout the life of individual dogs, submitted voluntarily by owners using standardized collection protocols (MAF, 2022). NIA’s Nonhuman Primate Tissue Bank serves as a repository of tissue for researchers conducting aging studies funded by NIH or other government institutions at no cost to the researchers (NIA, 2022). And digital pathology resources, such as the Primate Pathology Image Database at the Oregon NPRC, make it possible to share experimental and natural disease findings without repeating work and wasting resources (NIH, 2010).
Physical Infrastructure
Expansion of the physical infrastructure supporting NHP programs offers opportunities to ensure that housing and husbandry conditions align with best practices based on the evolving science of animal welfare. Experience with shortages of laboratory space during the COVID-19 pandemic demonstrated the importance of building adequate facilities for
NHP research, including biocontainment spaces (Animal Biosafety Level 3 or 4) adjacent to facilities housing NHPs (Hild et al., 2021; NASEM, 2021). Physical infrastructure to support expanded breeding colonies may include animal support facilities such as veterinary hospital space, quarantine areas, transition facilities for moving animals into and out of breeding colonies, and staff support facilities. The recent infrastructure funding opportunities offered by the Coronavirus Aid, Relief, and Economic Security (CARES) Act in 202113 and ORIP in 2019, 2020, and 2022 have provided much-needed support in this domain.
Information Technology Infrastructure
Investment in data infrastructure is necessary to improve the tracking of NHP demand and use to better support planning and coordination, both of which are necessary to guide effective and appropriate use of NHP resources going forward. As described in Chapter 3, past tracking efforts in the United States have been ad hoc, and existing data systems have been fragmented and largely inaccessible to the scientific community. Systems for tracking animal use for scientific purposes in the European Union, such as the ALURES Statistical EU Database,14,15 provide useful models for investment in data infrastructure and support openness in animal research. Importantly, such investments will also be integral to future efforts to reduce reliance on NHPs by enabling accurate measurement of the impact of such measures as changes in policy and implementation of nonanimal models. Improved information technology infrastructure is also critical for storing data and meeting new NIH data sharing requirements (NIH, 2023a).
Training the Next Generation of NHP Research and Care Professionals
NHP research has been affected by shortfalls in critical human resources (Hild et al., 2021), as detailed in Chapter 3. The future of NHP research will depend on training and ongoing support for investigators, care staff, and veterinary professionals so they have the knowledge and skills required to work productively and appropriately with NHP research subjects. Furthermore, progress will be facilitated by enhanced attention to workforce diversity, broadly defined not only in terms of the sociopolitical features of identity already recognized as important by NIH (2019), but also with respect to training backgrounds and trajectories (including but not limited to conscious integration of trainees with expertise in new approach methodologies). Diversity in the scientific workforce is critical for many reasons, not the least of which is that it improves scientific outcomes and speeds discovery (Freeman and Huang, 2014; Medin and Lee, 2012; Nielsen et al., 2017). Initiatives to train the next-generation workforce and to support current professionals from diverse backgrounds rely in turn on the availability of training support for early-career scientists and for people in staff positions who may or may not be in formalized training programs (e.g., animal care technicians). Existing opportunities to support the professional development of NHP researchers include the ORIP T35 and T32 pipeline for veterinary researchers and public–private partnerships for training (such as the Boehringer Ingelheim program for veterinary
___________________
13 Coronavirus Aid, Relief, and Economic Security Act (P.L. 116-136, 116th Congress), (March 27, 2020).
14 Regulation (EU) 2019/1010 of the European Parliament and of the Council (OJ L 170/115, 25.6.2019).
15 Available at https://ec.europa.eu/environment/chemicals/lab_animals/alures_en.htm (accessed November 7, 2022).
scholars) (BIVSP, 2022; NIH, 2022b, 2022c). However, there are impediments to ensuring the human resources needed to support NHP research, including
- a limited number of funded positions at institutions with the ability to train PhD, DVM, and MD researchers for work with NHPs;
- the lack of programs for recruiting and training non-PhD support professionals, including research technologists, animal care technicians, veterinary technicians, and behaviorists, deficits that exacerbate the current shortage of workers at NHP facilities and limit the ability of well-trained researchers to complete their studies; and
- societal misperceptions of and stigma directed at NHP research, which can be a barrier to recruiting scientists and support staff to the field and, at times, their retention.
Ensuring a diverse scientific workforce with the skills at all levels needed to conduct rigorous NHP research will require coordinated outreach at earlier stages of education. It will also require more opportunities for the public and scientists to gain an appreciation of the actuality of the work conducted at NHP facilities and the rationale for and benefits of the research.
CONCLUSIONS
This chapter has described the results of a forward-looking evaluation undertaken by the committee to address the aspects of its charge related to exploring the future role of NHPs in biomedical research supported by NIH. The committee began this effort by acknowledging that any activity involving future predictions should be undertaken with caution given the numerous scientific and policy uncertainties that shape the future landscape. With this caution in mind, the committee provides the following conclusions regarding the NHP research and resources that will be vital to supporting the mission and priorities of NIH going forward.
Conclusion 5-1: Given the nation’s most pressing public health needs and the evolving state of the science, specific domains of research—including neuroscience and neurodegenerative disorders, preparedness for unanticipated communicable infectious threats, immunotherapy, reproduction, aging, and chronic inflammatory diseases—are likely to require increased use of nonhuman primates in the future. The species distribution of future need for such research is likely to remain weighted toward macaques (particularly rhesus and cynomolgus), with increased use of marmosets.
Conclusion 5-2: The 2018 report of the National Institutes of Health (NIH) Office of Research Infrastructure Programs, Nonhuman Primate Evaluation and Analysis, included recommendations for improving communication and collaboration within the nonhuman primate (NHP) research community, increasing domestic NHP supply capabilities, addressing limitations in NIH funding mechanisms, promoting training in NHP care and research, and enhancing the utility and value of existing NHP resources. These solutions and recommendations have not yet been fully implemented and remain critically important.
Conclusion 5-3: Addressing the challenges posed for the national research infrastructure by a persistent lack of nonhuman primates (NHPs) will require a commitment and comprehensive national effort focused on expanding domestic NHP resources.
Conclusion 5-4: The creation of a national plan for allocation and expansion of nonhuman primate resources is necessary to optimize the use of this critical scientific resource. Such a plan will require adequate monetary, physical, and personnel resources, as well as a centralized tracking system to match need to investment in a data-driven fashion.
Conclusion 5-5: Continued development and validation of new approach methodologies (in vitro and in silico model systems) is critically important to support further advances in biomedical research. This may reduce the need for nonhuman primate (NHP) models in the future, and/or enhance their utility. Additionally, this may help to mitigate shortages in NHP supply and the high cost of NHP research.
Conclusion 5-6: Given the limited numbers of nonhuman primates (NHPs) available for research, it is incumbent upon investigators and the National Institutes of Health (NIH) to make the best use of each animal through cooperative efforts, data sharing, purposeful planning, and use of data-driven care and management methods for the long-term care and use of NHPs in research. Examples of successful cooperative efforts from the community of NIH-funded NHP researchers—including collaborative working groups; data-sharing resources for clinical and clinical pathology data, gene expression profiling, and genotype data; and biospecimen repositories—can serve as models for broader adoption.
Conclusion 5-7: A system for consistent reporting is needed to adequately capture the life, scientific, and medical history, including experimental treatments and procedures, of individual nonhuman primates (NHPs). The need for complete NHP life histories further supports the development of increased domestic breeding capacity in the United States to maximize the accurate and complete sharing of clinical and experimental data. Currently, the incentives, mandates, and infrastructure within the National Institutes of Health research enterprise are insufficient to support uniform data management and reporting across all NHP research programs.
Conclusion 5-8: Recent advances in genomics, bioinformatics, imaging, digital biomarkers (e.g., noninvasive home enclosure neural and behavioral recordings), extended reality, and artificial intelligence/machine learning have revealed opportunities to understand normal biology and the mechanisms of disease. Such technologies and approaches have the potential to augment the scientific knowledge that can be gained from individual nonhuman primate (NHP) studies and, in some cases, enable less invasive use of NHPs. Leveraging these opportunities for NHP research will require tracking the genotype, phenotype, and history of each animal used, as well as transdisciplinary interactions.
Conclusion 5-9: Additional investments will be needed to implement, maintain, train, and use current and emerging technologies (such as digital biomarkers, artificial intelligence/machine learning, imaging, extended reality, and laparoscopy), as well as data-driven husbandry practices, with the potential to enhance nonhuman primate research funded by the National Institutes of Health.
REFERENCES
AA. 2022. 2022 Alzheimer’s disease facts and figures. https://www.alz.org/alzheimers-dementia/facts-figures#:~:text=More%20than%206%20million%20Americans%20of%20all%20ages%20have%20Alzheimer’s,older%20(10.7%25)%20has%20Alzheimer’s (accessed February 24, 2023).
AALAS. 2020. Reimagining training and 3Rs through extended reality. https://www.ingentaconnect.com/contentone/aalas/jaalas/2020/00000059/00000005/art00017?crawler=true&mimetype=application/pdf (accessed February 24, 2023).
Ackerman, M. E., D. H. Barouch, and G. Alter. 2017. Systems serology for evaluation of HIV vaccine trials. Immunological Reviews 275(1):262-270.
Aida, T., and G. Feng. 2020. The dawn of non-human primate models for neurodevelopmental disorders. Current Opinion in Genetics and Development 65:160-168.
Albrecht, L., E. Bishop, B. Jay, B. Lafoux, M. Minoves, and C. Passaes. 2021. COVID-19 research: Lessons from non-human primate models. Vaccines (Basel) 9(8).
Alharbi, W. S., and M. Rashid. 2022. A review of deep learning applications in human genomics using next-generation sequencing data. Human Genomics 16(1):26.
Alter, G. C., and M. Vardigan. 2015. Addressing global data sharing challenges. Journal of Empirical Research on Human Research Ethics 10(3):317-323.
Altschul, D. M., M. J. Beran, M. Bohn, J. Call, S. DeTroy, S. J. Duguid, C. L. Egelkamp, C. Fichtel, J. Fischer, M. Flessert, D. Hanus, D. B. M. Haun, L. M. Haux, R. A. Hernandez-Aguilar, E. Herrmann, L. M. Hopper, M. Joly, F. Kano, S. Keupp, A. P. Melis, A. Motes Rodrigo, S. R. Ross, A. Sánchez-Amaro, Y. Sato, V. Schmitt, M. K. Schweinfurth, A. M. Seed, D. Taylor, C. J. Völter, E. Warren, and J. Watzek. 2019. Establishing an infrastructure for collaboration in primate cognition research. PLoS One 14(10):e0223675.
Antontsev, V., A. Jagarapu, Y. Bundey, H. Hou, M. Khotimchenko, J. Walsh, and J. Varshney. 2021. A hybrid modeling approach for assessing mechanistic models of small molecule partitioning in vivo using a machine learning-integrated modeling platform. Scientific Reports 11(1):11143.
Arnsten, A. F. T., D. Datta, S. Leslie, S.-T. Yang, M. Wang, and A. C. Nairn. 2019. Alzheimer’s-like pathology in aging rhesus macaques: Unique opportunity to study the etiology and treatment of Alzheimer’s disease. Proceedings of the National Academy of Sciences 116(52):26230-26238.
ARRIVE (Animal Research: Reporting of In Vivo Experiments). n.d. Housing and husbandry. https://arriveguidelines.org/arrive-guidelines/housing-and-husbandry (accessed February 16, 2023).
Association of Primate Veterinarians. 2019. Association of primate veterinarians’ guidelines for laparoscopic reproductive manipulation of female nonhuman primates in biomedical research. Journal of the American Association for Laboratory Animal Science 58(6):750-752.
Baglietto-Vargas, D., S. Forner, L. Cai, A. Martini, L. Trujillo-Estrada, V. Swarup, M. Nguyen, K. Huynh, D. Javonillo, K. Tran, J. Phan, S. Jiang, E. Kramár, C. Nuñez-Diaz, G. Balderrama-Gutierrez, F. Garcia, J. Childs, C. Rodriguez-Ortiz, J. Garcia-Leon, M. Kitazawa, M. Shahnawaz, D. Matheos, X. Ma, C. Da Cunha, K. Walls, R. Ager, C. Soto, A. Gutierrez, I. Moreno0Gonzalez, A. Mortazavi, A. Tenner, G. MacGregor, M. Wood, K. Green, and F. LaFerla. 2021. Generation of a humanized Ab expressing mouse demonsrating aspects of Alzheimer’s disease-like pathology. Nature Communications 12:2421.
Bailey, M. 2022. Nonhuman primate model systems: State of the science and future needs. Presentation at Committee on the State of the Science and Future Needs for Nonhuman Primate Model Systems Meeting #3: Public Workshop, Washington, DC, August 25.
Baker, K. C., M. A. Bloomsmith, B. Oettinger, K. Neu, C. Griffis, V. Schoof, and M. Maloney. 2012. Benefits of pair housing are consistent across a diverse population of rhesus macaques. Applied Animal Behaviour Science 137(3-4):148-156.
Bala, P. C., B. R. Eisenreich, S. B. M. Yoo, B. Y. Hayden, H. S. Park, and J. Zimmermann. 2020. Automated markerless pose estimation in freely moving macaques with OpenMonkeyStudio. Nature Communications 11(1):4560.
Baran, S. W., M. I. Perret-Gentil, E. J. Johnson, E. L. Miedel, and J. Kehler. 2011. Rodent laparoscopy: Refinement for rodent drug studies and model development, and monitoring of neoplastic, inflammatory and metabolic diseases. Laboratory Animals 45(4):231-239.
Baran, S. W., A. D. Gupta, M. A. Lim, A. Mathur, D. J. Rowlands, L. R. Schaevitz, S. K. Shanmukhappa, and D. B. Walker. 2020. Continuous, automated breathing rate and body motion monitoring of rats with paraquat-induced progressive lung injury. Frontiers in Physiology 11:569001.
Baran, S. W., M. A. Lim, J. P. Do, P. Stolyar, M. D. Rabe, L. R. Schaevitz, and S. M. Cadena. 2021. Digital biomarkers enable automated, longitudinal monitoring in a mouse model of aging. Journals of Gerontology: Series A, Biological Sciences and Medical Sciences 76(7):1206-1213.
Baran, S. W., N. Bratcher, J. Dennis, S. Gaburro, E. M. Karlsson, S. Maguire, P. Makidon, L. Noldus, Y. Potier, G. Rosati, M. Ruiter, L. Schaevitz, P. Sweeney, and M. R. LaFollette. 2022. Emerging role of translational digital biomarkers within home cage monitoring technologies in preclinical drug discovery and development. Frontiers in Behavioral Neuroscience 15:758274.
Basso, M. A., S. Frey, K. A. Guerriero, B. Jarraya, S. Kastner, K. W. Koyano, D. A. Leopold, K. Murphy, C. Poirier, W. Pope, A. C. Silva, G. Tansey, and L. Uhrig. 2021. Using non-invasive neuroimaging to enhance the care, well-being and experimental outcomes of laboratory non-human primates (monkeys). Neuroimage 228:117667-117667.
Baxter, A., J. P. Capitanio, K. L. Bales, and E. L. Kinnally. 2021. Biobehavioral organization shapes the immune epigenome in infant rhesus macaques (Macaca mulatta). Brain, Behavior, and Immunity 96:256-270.
Beach, E. K. 1982. Johari’s window as a framework for needs assessment. Journal of Continuing Education in Nursing 13(3):28-32.
Beckman, D., S. Ott, K. Donis-Cox, W. G. Janssen, E. Bliss-Moreau, P. H. Rudebeck, M. G. Baxter, and J. H. Morrison. 2019. Oligomeric Aβ in the monkey brain impacts synaptic integrity and induces accelerated cortical aging. Proceedings of the National Academy of Sciences 116(52):26239-26246.
Beckman, D., P. Chakrabarty, S. Ott, A. Dao, E. Zhou, W. G. Janssen, K. Donis-Cox, S. Muller, J. H. Kordower, and J. H. Morrison. 2021. A novel tau-based rhesus monkey model of Alzheimer’s pathogenesis. Alzheimer’s & Dementia 17(6):933-945.
Bercovich, E., and M. C. Javitt. 2018. Medical imaging: From roentgen to the digital revolution, and beyond. Rambam Maimonides Medical Journal 9(4).
Berger, M., N. S. Agha, and A. Gail. 2020. Wireless recording from unrestrained monkeys reveals motor goal encoding beyond immediate reach in frontoparietal cortex. eLife 9.
Bimber, B. N., M. Y. Yan, S. M. Peterson, and B. Ferguson. 2019. MGAP: The macaque genotype and phenotype resource, a framework for accessing and interpreting macaque variant data, and identifying new models of human disease. BMC Genomics 20(1):176.
BIVSP (Boehringer Ingelheim Veterinary Scholars Program). 2022. The Boehringer Ingelheim Veterinary Scholars Program: Supporting future veterinarians. https://www.boehringer-ingelheim.com/science-innovation/animal-health-innovation/boehringer-ingelheim-veterinary-scholars-program (accessed April 3, 2023).
Bliss-Moreau, E., R. R. Amara, E. A. Buffalo, R. J. Colman, M. E. Embers, J. H. Morrison, E. E. Quillen, J. B. Sacha, and C. T. Roberts. 2021a. Improving rigor and reproducibility in nonhuman primate research. American Journal of Primatology 83(12):e23331.
Bliss-Moreau, E., A. C. Santistevan, B. Beisner, G. Moadab, J. Vandeleest, and B. McCowan. 2021b. Monkey’s social roles predict their affective reactivity. Affective Science 2(3):230-240.
Bogdan, R., B. J. Salmeron, C. E. Carey, A. Agrawal, V. D. Calhoun, H. Garavan, A. R. Hariri, A. Heinz, M. N. Hill, A. Holmes, N. H. Kalin, and D. Goldman. 2017. Imaging genetics and genomics in psychiatry: A critical review of progress and potential. Biological Psychiatry 82(3):165-175.
Brattain, L. J., R. Landman, K. A. Johnson, P. Chwalek, J. Hyman, J. Sharma, C. Jennings, R. Desimone, G. Feng, and T. F. Quatieri. 2016. A multimodal sensor system for automated marmoset behavioral analysis. Paper read at 2016 IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN), June 14-17, 2016.
Briel, M., B. S. Elger, S. McLennan, S. Schandelmaier, E. von Elm, and P. Satalkar. 2021. Exploring reasons for recruitment failure in clinical trials: A qualitative study with clinical trial stakeholders in Switzerland, Germany, and Canada. Trials 22(1):844.
Brubaker, D. K., and D. A. Lauffenburger. 2020. Translating preclinical models to humans. Science 367(6479):742-743.
Burt, T., C. S. John, J. L. Ruckle, and L. T. Vuong. 2017. Phase-0/microdosing studies using PET, AMS, and LC-MS/MS: A range of study methodologies and conduct considerations. Accelerating development of novel pharmaceuticals through safe testing in humans—A practical guide. Expert Opinion on Drug Delivery 14(5):657-672.
Burt, T., L. T. Vuong, E. Baker, G. C. Young, A. D. McCartt, M. Bergstrom, Y. Sugiyama, and R. Combes. 2018. Phase 0, including microdosing approaches: Applying the Three Rs and increasing the efficiency of human drug development. Alternatives to Laboratory Animals 46(6):335-346.
Butler, D. 2016. Zika researchers release real-time data on viral infection study in monkeys. Nature, February 23. https://www.nature.com/articles/nature.2016.19438 (accessed April 3, 2023).
Capitanio, J. P. 1998. Social experience and immune system measures in laboratory-housed macaques: Implications for management and research. Institute for Laboratory Animal Research 39(1):12-20.
Capitanio, J. P. 2021. Knowledge of biobehavioral organization can facilitate better science: A review of the biobehavioral assessment program at the California National Primate Research Center. Animals 11(8).
Capitanio, J. P., and S. W. Cole. 2015. Social instability and immunity in rhesus monkeys: The role of the sympathetic nervous system. Philosophical Transactions of the Royal Society B 370(1669).
Carlson, C. J., G. F. Albery, C. Merow, C. H. Trisos, C. M. Zipfel, E. A. Eskew, K. J. Olival, N. Ross, and S. Bansal. 2022. Climate change increases cross-species viral transmission risk. Nature 607(7919):555-562.
Castell, N., S. M. Guerrero-Martin, L. H. Rubin, E. N. Shirk, J. K. Brockhurst, C. E. Lyons, K. M. Najarro, S. E. Queen, B. W. Carlson, R. J. Adams, C. N. Morrell, L. Gama, D. R. Graham, C. Zink, J. L. Mankowski, J. E. Clements, and K. A. Metcalf Pate. 2022. Effect of single housing on innate immune activation in immunodeficiency virus-infected pigtail macaques (Macaca nemestrina) as a model of psychosocial stress in acute HIV infection. Psychosomatic Medicine 84(8):966-975.
Chai, N. 2015. Endoscopy and endosurgery in nonhuman primates. Veterinary Clinics of North America: Exotic Animal Practice 18(3):447-461.
Chang, H., and L. Jorgenson. 2022. Catalyzing the development and use of alternative methods to advance biomedical research. NIH Advisory Committee to the Director. https://acd.od.nih.gov/documents/presentations/11032022_Biomedical_.Research.pdf (accessed February 24, 2023).
Charbonneau, J. A., D. G. Amaral, and E. Bliss-Moreau. 2022. Social housing status impacts rhesus monkeys’ affective responding in classic threat processing tasks. Scientific Reports 12(1):4140.
Cheng, K. C., R. D. Burdine, M. E. Dickinson, S. C. Ekker, A. Y. Lin, K. C. K. Lloyd, C. M. Lutz, C. A. MacRae, J. H. Morrison, D. H. O’Connor, J. H. Postlethwait, C. D. Rogers, S. Sanchez, J. H. Simpson, W. S. Talbot, D. C. Wallace, J. M. Weimer, and H. J. Bellen. 2022. Promoting validation and cross-phylogenetic integration in model organism research. Disease Models & Mechanisms 15(9).
Choi, H., S. Lim, K. Min, K. H. Ahn, K. M. Lee, and D. P. Jang. 2021. Non-human primate epidural ECoG analysis using explainable deep learning technology. Journal of Neural Engineering 18(6).
Choudhary, S., A. Walker, K. Funk, C. Keenan, I. Khan, and K. Maratea. 2018. The Standard for the Exchange of Nonclinical Data (SEND): Challenges and promises. Toxicologic Pathology 46(8):1006-1012.
CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora). 2023. Macaca mulatta. https://cites.org/eng/taxonomy/term/1137 (accessed January 13, 2023).
Clarke, A. S., W. A. Mason, and G. P. Moberg. 1988. Differential behavioral and adrenocortical responses to stress among three macaque species. American Journal of Primatology 14(1):37-52.
Clarke, A. S., W. A. Mason, and S. P. Mendoza. 1994. Heart rate patterns under stress in three species of macaques. American Journal of Primatology 33(2):133-148.
Collins, F. S., and L. A. Tabak. 2014. Policy: NIH plans to enhance reproducibility. Nature 505(7485):612-613.
Colman, R. J., S. Capuano, J. Bakker, J. Keeley, K. Nakamura, and C. Ross. 2020. Marmosets: Welfare, ethical use, and IACUC/regulatory considerations. Institute on Laboratory Animal Research Journal 61(2-3):167-178.
Colman, K., R. N. Andrews, H. Atkins, T. Boulineau, A. Bradley, A. Braendli-Baiocco, R. Capobianco, D. Caudell, M. Cline, T. Doi, R. Ernst, E. van Esch, J. Everitt, P. Fant, M. M. Gruebbel, L. Mecklenburg, A. D. Miller, K. J. Nikula, S. Satake, J. Schwartz, A. Sharma, A. Shimoi, C. Sobry, I. Taylor, V. Vemireddi, J. Vidal, C. Wood, and J. L. Vahle. 2021. International Harmonization of Nomenclature and Diagnostic Criteria (INHAND): Non-proliferative and proliferative lesions of the non-human primate (M. fascicularis). Journal of Toxicologic Pathology 34(3 Suppl):1s-182s.
Cooper, E. B., L. J. N. Brent, N. Snyder-Mackler, M. Singh, A. Sengupta, S. Khatiwada, S. Malaivijitnond, Z. Qi Hai, and J. P. Higham. 2022. The rhesus macaque as a success story of the anthropocene. eLife 11:e78169.
Crawshaw, B. P., H.-L. Chien, K. M. Augestad, and C. P. Delaney. 2015. Effect of laparoscopic surgery on health care utilization and costs in patients who undergo colectomy. JAMA Surgery 150(5):410-415.
Davis, S., L. Milechin, T. Patel, M. Hernandez, G. Ciccarelli, S. Samsi, L. Hensley, A. Goff, J. Trefry, S. Johnston, B. Purcell, C. Cabrera, J. Fleischman, A. Reuther, K. Claypool, F. Rossi, A. Honko, W. Pratt, and A. Swiston. 2021. Detecting pathogen exposure during the non-symptomatic incubation period using physiological data: Proof of concept in non-human primates. Frontiers in Physiology 12:691074.
Defensor, E., J. Villarreal, and L. Schaevitz. 2019. Obtaining non-invasive readouts and early safety signals in rodents. Journal of Pharmacological and Toxicological Methods 99:106595.
Deycmar, S., B. Gomes, J. Charo, M. Ceppi, and J. M. Cline. 2023. Spontaneous, naturally occurring cancers in non-human primates as a translational model for cancer immunotherapy. Journal for ImmunoTherapy of Cancer 11(1).
DiCarlo, A. L., C. Maher, J. L. Hick, D. Hanfling, N. Dainiak, N. Chao, J. L. Bader, C. N. Coleman, and D. M. Weinstock. 2011. Radiation injury after a nuclear detonation: Medical consequences and the need for scarce resources allocation. Disaster Medicine and Public Health Preparedness 5(S1):S32-S44.
Do, J. P., E. B. Defensor, C. V. Ichim, M. A. Lim, J. A. Mechanic, M. D. Rabe, and L. R. Schaevitz. 2020. Automated and continuous monitoring of animal welfare through digital alerting. Comparative Medicine 70(4):313-327.
Drawz, P. E., M. Abdalla, and M. Rahman. 2012. Blood pressure measurement: Clinic, home, ambulatory, and beyond. American Journal of Kidney Diseases 60(3):449-462.
Drummond, E., and T. Wisniewski. 2017. Alzheimer’s disease: Experimental models and reality. Acta Neuropathologica 133(2):155-175.
Dudley, D. M., K. K. Van Rompay, L. L. Coffey, A. Ardeshir, R. I. Keesler, E. Bliss-Moreau, P. L. Grigsby, R. J. Steinbach, A. J. Hirsch, R. P. MacAllister, H. L. Pecoraro, L. M. Colgin, T. Hodge, D. N. Streblow, S. Tardif, J. L. Patterson, M. Tamhankar, M. Seferovic, K. M. Aagaard, C. S. Martín, C. Y. Chiu, A. T. Panganiban, R. S. Veazey, X. Wang, N. J. Maness, M. H. Gilbert, R. P. Bohm, K. M. Adams Waldorf, M. Gale, Jr., L. Rajagopal, C. E. Hotchkiss, E. L. Mohr, S. V. Capuano, 3rd, H. A. Simmons, A. Mejia, T. C. Friedrich, T. G. Golos, and D. H. O’Connor. 2018. Miscarriage and stillbirth following maternal Zika virus infection in nonhuman primates. Nature Medicine 24(8):1104-1107.
Dudley, D. M., M. T. Aliota, E. L. Mohr, C. M. Newman, T. G. Golos, T. C. Friedrich, and D. H. O’Connor. 2019. Using macaques to address critical questions in Zika virus research. Annual Review of Virology 6(1):481-500.
Einhorn, B., and L. Lew. 2022. Lab monkeys are the latest Covid shortage. Bloomberg, September 28. https://www.bloomberg.com/news/articles/2022-09-28/research-monkey-shortage-boosts-china-s-vaccine-development?leadSource=uverify%20wall (accessed January 11, 2023).
Eisinger, R. 2022. Nonhuman primate model systems: State of the science and future needs. Presentation at Committee on the State of the Science and Future Needs for Nonhuman Primate Model Systems Meeting #3: Public Workshop, Washington, DC, August 25.
Errington, T. M., A. Denis, N. Perfito, E. Iorns, and B. A. Nosek. 2021. Challenges for assessing replicability in preclinical cancer biology. eLife 10:e67995.
EY (Ernst and Young). 2018. The combinatorial effect of emerging technologies. https://assets.ey.com/content/dam/ey-sites/ey-com/en_gl/topics/advisory/ey-the-combinatorial-effect-of-emerging-technologies.pdf (accessed March 20, 2023).
FDA (U.S. Food and Drug Administration). 2020. Medical imaging and drug development. https://www.fda.gov/drugs/development-resources/medical-imaging-and-drug-development (accessed March 17, 2023).
FDA. 2021a. Advancing new alternative methodologies at FDA. https://www.fda.gov/media/144891/download (accessed February 24, 2023).
FDA. 2021b. Innovative Science and Technology Approaches for New Drugs (ISTAND) pilot program. https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/innovative-science-and-technology-approaches-new-drugs-istand-pilot-program (accessed February 24, 2023).
FDA. 2022. Nonclinical considerations for mitigating nonhuman primate supply constraints arising from the COVID-19 pandemic. https://www.fda.gov/media/155950/download (accessed February 24, 2023).
Feng, G., F. E. Jensen, H. T. Greely, H. Okano, S. Treue, A. C. Roberts, J. G. Fox, S. Caddick, M.-m. Poo, W. T. Newsome, and J. H. Morrison. 2020. Opportunities and limitations of genetically modified nonhuman primate models for neuroscience research. Proceedings of the National Academy of Sciences 117(39):24022-24031.
Freeman, R. B., and W. Huang. 2014. Collaboration: Strength in diversity. Nature 513(7518):305.
Friedman, H., N. Ator, N. Haigwood, W. Newsome, J. S. Allan, T. G. Golos, J. H. Kordower, R. E. Shade, M. E. Goldberg, M. R. Bailey, and P. Bianchi. 2017. The critical role of nonhuman primates in medical research. Pathogens & Immunity 2(3):352-365.
Frye, B. M., S. Craft, C. S. Latimer, C. D. Keene, T. J. Montine, T. C. Register, M. E. Orr, K. Kavanagh, S. L. Macauley, and C. A. Shively. 2021. Aging-related Alzheimer’s disease-like neuropathology and functional decline in captive vervet monkeys (Chlorocebus aethiops sabaeus). American Journal of Primatology 83(11):e23260.
Geisbert, T. W. 2017. First Ebola virus vaccine to protect human beings? The Lancet 389(10068):479-480.
Geuther, B., M. Chen, R. J. Galante, O. Han, J. Lian, J. George, A. I. Pack, and V. Kumar. 2022. High-throughput visual assessment of sleep stages in mice using machine learning. Sleep 45(2).
Gibbs, R. A., J. Rogers, M. G. Katze, R. Bumgarner, G. M. Weinstock, E. R. Mardis, K. A. Remington, R. L. Strausberg, J. C. Venter, R. K. Wilson, M. A. Batzer, C. D. Bustamante, E. E. Eichler, M. W. Hahn, R. C. Hardison, K. D. Makova, W. Miller, A. Milosavljevic, R. E. Palermo, A. Siepel, J. M. Sikela, T. Attaway, S. Bell, K. E. Bernard, C. J. Buhay, M. N. Chandrabose, M. Dao, C. Davis, K. D. Delehaunty, Y. Ding, H. H. Dinh, S. Dugan-Rocha, L. A. Fulton, R. A. Gabisi, T. T. Garner, J. Godfrey, A. C. Hawes, J. Hernandez, S. Hines, M. Holder, J. Hume, S. N. Jhangiani, V. Joshi, Z. M. Khan, E. F. Kirkness, A. Cree, R. G. Fowler, S. Lee, L. R. Lewis, Z. Li, Y. S. Liu, S. M. Moore, D. Muzny, L. V. Nazareth, D. N. Ngo, G. O. Okwuonu, G. Pai, D. Parker, H. A. Paul, C. Pfannkoch, C. S. Pohl, Y. H. Rogers, S. J. Ruiz, A. Sabo, J. Santibanez, B. W. Schneider, S. M. Smith, E. Sodergren, A. F. Svatek, T. R. Utterback, S. Vattathil, W. Warren, C. S. White, A. T. Chinwalla, Y. Feng, A. L. Halpern, L. W. Hillier, X. Huang, P. Minx, J. O. Nelson, K. H. Pepin, X. Qin, G. G. Sutton, E. Venter, B. P. Walenz, J. W. Wallis, K. C. Worley, S. P. Yang, S. M. Jones, M. A. Marra, M. Rocchi, J. E. Schein, R. Baertsch, L. Clarke, M. Csürös, J. Glasscock, R. A. Harris, P. Havlak, A. R. Jackson, H. Jiang, Y. Liu, D. N. Messina, Y. Shen, H. X. Song, T. Wylie, L. Zhang, E. Birney, K. Han, M. K. Konkel, J. Lee, A. F. Smit, B. Ullmer, H. Wang, J. Xing, R. Burhans, Z. Cheng, J. E. Karro, J. Ma, B. Raney, X. She, M. J. Cox, J. P. Demuth, L. J. Dumas, S. G. Han, J. Hopkins, A. Karimpour-Fard, Y. H. Kim, J. R. Pollack, T. Vinar, C. Addo-Quaye, J. Degenhardt, A. Denby, M. J. Hubisz, A. Indap, C. Kosiol, B. T. Lahn, H. A. Lawson, A. Marklein, R. Nielsen, E. J. Vallender, A. G. Clark, B. Ferguson, R. D. Hernandez, K. Hirani, H. Kehrer-Sawatzki, J. Kolb, S. Patil, L. L. Pu, Y. Ren, D. G. Smith, D. A. Wheeler, I.
Schenck, E. V. Ball, R. Chen, D. N. Cooper, B. Giardine, F. Hsu, W. J. Kent, A. Lesk, D. L. Nelson, E. O’Brien W, K. Prüfer, P. D. Stenson, J. C. Wallace, H. Ke, X. M. Liu, P. Wang, A. P. Xiang, F. Yang, G. P. Barber, D. Haussler, D. Karolchik, A. D. Kern, R. M. Kuhn, K. E. Smith, and A. S. Zwieg. 2007. Evolutionary and biomedical insights from the rhesus macaque genome. Science 316(5822):222-234.
Glass, C., K. J. Lafata, W. Jeck, R. Horstmeyer, C. Cooke, J. Everitt, M. Glass, D. Dov, and M. A. Seidman. 2022. The role of machine learning in cardiovascular pathology. Canadian Journal of Cardiology 38(2):234-245.
Golini, E., M. Rigamonti, F. Iannello, C. De Rosa, F. Scavizzi, M. Raspa, and S. Mandillo. 2020. A non-invasive digital biomarker for the detection of rest disturbances in the SOD1G93A mouse model of ALS. Frontiers in Neuroscience 14:896.
Gottlieb, D. H., A. Maier, and K. Coleman. 2015. Evaluation of environmental and intrinsic factors that contribute to stereotypic behavior in captive rhesus macaques (Macaca mulatta). Applied Animal Behaviour Science 171:184-191.
Graham, M. L., E. F. Rieke, L. A. Mutch, E. K. Zolondek, A. W. Faig, T. A. Dufour, J. W. Munson, J. A. Kittredge, and H. J. Schuurman. 2012. Successful implementation of cooperative handling eliminates the need for restraint in a complex non-human primate disease model. Journal of Medical Primatology 41(2):89-106.
Grimm, D. 2019. 2020 U.S. spending bill restricts some animal research, pushes for lab animal retirement. https://www.science.org/content/article/2020-us-spending-bill-restricts-some-animal-research-pushes-lab-animal-retirement (accessed February 24, 2023).
Guerrero-Martin, S. M., L. H. Rubin, K. M. McGee, E. N. Shirk, S. E. Queen, M. Li, B. Bullock, B. W. Carlson, R. J. Adams, L. Gama, D. R. Graham, C. Zink, J. E. Clements, J. L. Mankowski, and K. A. Metcalf Pate. 2021. Psychosocial stress alters the immune response and results in higher viral load during acute simian immunodeficiency virus infection in a pigtailed macaque model of human immunodeficiency virus. Journal of Infectious Diseases 224(12):2113-2121.
Ha, K.-M. 2019. Integrating the resources of Korean disaster management research via the Johari Window. Evaluation and Program Planning 77:101724.
Hadj-Bouziane, F., M. Meunier, and D. Boussaoud. 2003. Conditional visuo-motor learning in primates: A key role for the basal ganglia. Journal of Physiology, Paris 97(4-6):567-579.
Haese, N. N., V. H. J. Roberts, A. Chen, D. N. Streblow, T. K. Morgan, and A. J. Hirsch. 2021. Nonhuman primate models of Zika virus infection and disease during pregnancy. Viruses 13(10).
Han, H. J., S. J. Powers, and K. L. Gabrielson. 2022a. The common marmoset—Biomedical research animal model applications and common spontaneous diseases. Toxicologic Pathology 50(5):628-637.
Han, L., X. Wei, C. Liu, G. Volpe, Z. Zhuang, X. Zou, Z. Wang, T. Pan, Y. Yuan, X. Zhang, P. Fan, P. Guo, Y. Lai, Y. Lei, X. Liu, F. Yu, S. Shangguan, G. Lai, Q. Deng, Y. Liu, L. Wu, Q. Shi, H. Yu, Y. Huang, M. Cheng, J. Xu, Y. Liu, M. Wang, C. Wang, Y. Zhang, D. Xie, Y. Yang, Y. Yu, H. Zheng, Y. Wei, F. Huang, J. Lei, W. Huang, Z. Zhu, H. Lu, B. Wang, X. Wei, F. Chen, T. Yang, W. Du, J. Chen, S. Xu, J. An, C. Ward, Z. Wang, Z. Pei, C.-W. Wong, X. Liu, H. Zhang, M. Liu, B. Qin, A. Schambach, J. Isern, L. Feng, Y. Liu, X. Guo, Z. Liu, Q. Sun, P. H. Maxwell, N. Barker, P. Muñoz-Cánoves, Y. Gu, J. Mulder, M. Uhlen, T. Tan, S. Liu, H. Yang, J. Wang, Y. Hou, X. Xu, M. A. Esteban, and L. Liu. 2022b. Cell transcriptomic atlas of the non-human primate Macaca fascicularis. Nature 604(7907):723-731.
Harding, J. D. 2017. Nonhuman primates and translational research: Progress, opportunities, and challenges. Institute on Laboratory Animal Research Journal 58(2):141-150.
Harlow, H. F., R. O. Dodsworth, and M. K. Harlow. 1965. Total social isolation in monkeys. Proceedings of the National Academy of Sciences 54(1):90-97.
Harms, R. L., A. Ferrari, I. B. Meier, J. Martinkova, E. Santus, N. Marino, D. Cirillo, S. Mellino, S. Catuara Solarz, I. Tarnanas, C. Szoeke, J. Hort, A. Valencia, M. T. Ferretti, A. Seixas, and A. Santuccione Chadha. 2022. Digital biomarkers and sex impacts in Alzheimer’s disease management—Potential utility for innovative 3P medicine approach. European Association for Predictive, Preventive, and Personalized Medicine Journal 13(2):299-313.
Hassounah, S., T. Mesplède, and M. Wainberg. 2016. Nonhuman primates and humanized mice for studies of HIV-1 integrase inhibitors: A review. Pathogens and Immunity 1(1):41-67.
Havel, P. J., P. Kievit, A. G. Comuzzie, and A. A. Bremer. 2017. Use and importance of nonhuman primates in metabolic disease research: Current state of the field. Institute on Laboratory Animal Research Journal 58(2):251-268.
Hawkley, L. C., S. W. Cole, J. P. Capitanio, G. J. Norman, and J. T. Cacioppo. 2012. Effects of social isolation on glucocorticoid regulation in social mammals. Hormones and Behavior 62(3):314-323.
Hewitt, J. A., L. L. Brown, S. J. Murphy, F. Grieder, and S. D. Silberberg. 2017. Accelerating biomedical discoveries through rigor and transparency. Institute on Laboratory Animal Research Journal 58(1):115-128.
HHS (U.S. Department of Health and Human Services). 2021. Updated notice of limited availability of research non-human primates. https://grants.nih.gov/grants/guide/notice-files/not-od-21-080.html (accessed February 24, 2023).
Hild, S. A., M. C. Chang, S. J. Murphy, and F. B. Grieder. 2021. Nonhuman primate models for SARS-CoV-2 research: Infrastructure needs for pandemic preparedness. Lab Animal 50(6):140-141.
Hobson, L., R. S. Bains, S. Greenaway, S. Wells, and P. M. Nolan. 2020. Phenotyping in mice using continuous home cage monitoring and ultrasonic vocalization recordings. Current Protocols in Mouse Biology 10(3):e80.
Hopkins, A. M., A. Rowland, and M. J. Sorich. 2018. Data sharing from pharmaceutical industry sponsored clinical studies: Audit of data availability. BMC Medicine 16(1):165.
Howard, C. R., and N. F. Fletcher. 2012. Emerging virus diseases: Can we ever expect the unexpected? Emerging Microbes and Infections 1(12):e46.
Hutchison, R. M., and S. Everling. 2012. Monkey in the middle: Why non-human primates are needed to bridge the gap in resting-state investigations. Frontiers in Neuroanatomy 6:29. https://doi/org/10.3389/fnana.2012.00029
Hutz, R. J., D. J. Dierschke, and R. C. Wolf. 1988. Menstrual cycles in rhesus monkeys (Macaca mulatta) are unaffected by a single dose of the anesthetics ketamine and xylazine administered during the midfollicular phase at laparoscopy. American Journal of Primatology 15(1):79-84.
IOM (Institute of Medicine). 2012. Public engagement and clinical trials: New models and disruptive technologies: Workshop summary. Edited by V. Weisfeld, R. A. English and A. B. Claiborne. Washington, DC: The National Academies Press.
Johnson, A. L., S. M. Peterson, M. M. L. Terry, B. Ferguson, L. M. Colgin, and A. D. Lewis. 2020. Spontaneous KRT5 gene mutation in rhesus macaques (Macaca mulatta): A novel nonhuman primate model of epidermolysis bullosa simplex. Veterinary Pathology 57(2):344-348.
Kalbaugh, C. A., J. M. Kalbaugh, L. McManus, and J. A. Fisher. 2021. Healthy volunteers in US phase I clinical trials: Sociodemographic characteristics and participation over time. PLoS One 16(9):e0256994.
Kemnitz, J. W. 2019. Database for indices of aging in nonhuman primates. Innovation in Aging 3(Suppl 1):S957.
King, A. 2018. The search for better animal models of Alzheimer’s disease. Nature, July 25. https://www.nature.com/articles/d41586-018-05722-9 (accessed February 24, 2023).
Kinnally, E. L., S. J. Martinez, K. Chun, J. P. Capitanio, and L. C. Ceniceros. 2019. Early social stress promotes inflammation and disease risk in rhesus monkeys. Scientific Reports 9(1):7609.
Koizumi, M., N. Nogami, K. Owari, A. Kawanobe, T. Nakatani, and K. Seki. 2021. Motility profile of captive-bred marmosets revealed by a long-term in-cage monitoring system. Frontiers in Systems Neuroscience 15:645308.
Kumar, V., A. Raj, and P. Kumar. 2011. Pregnancy diagnosis by laparoscopy in free range rhesus macaques (Macaca mulatta). Open Veterinary Journal 1(1):32-34.
Lauer, J., M. Zhou, S. Ye, W. Menegas, S. Schneider, T. Nath, M. M. Rahman, V. Di Santo, D. Soberanes, G. Feng, V. N. Murthy, G. Lauder, C. Dulac, M. W. Mathis, and A. Mathis. 2022. Multi-animal pose estimation, identification and tracking with DeepLabCut. Nature Methods 19(4):496-504.
Lear, A., S. N. Baker, H. F. Clarke, A. C. Roberts, M. C. Schmid, and W. Jarrett. 2022. Understanding them to understand ourselves: The importance of NHP research for translational neuroscience. Current Research in Neurobiology 3:100049.
Liao, P., N. Bodkin, E. Cho, T. Alexander, and H. Ortmeyer. 2004. Minimally invasive surgery via laparoscopy for intra-abdominal biopsy in obese rhesus macaques (Macaca mulatta). Comparative Medicine 54(2):159-164.
Lisa, M. S., R. Charani, F. M. Michael, and A. Parisa. 2017. Therapeutic antibody-based drugs in the treatment of human inflammatory disorders. In Immunotherapy, edited by M. Krassimir. Rijeka: IntechOpen.
Lubach, G. R., C. L. Coe, and W. B. Ershler. 1995. Effects of early rearing environment on immune-responses of infant rhesus monkeys. Brain, Behavior, and Immunity 9(1):31-46.
Luft, J., and H. Ingham. 1955. The Johari Window, a graphic model of interpersonal awareness. Proceedings of the Western Training Laboratory in Group Development.
Ma, H., J. D. Lundy, E. L. Cottle, K. J. O’Malley, A. M. Trichel, W. B. Klimstra, A. L. Hartman, D. S. Reed, and T. Teichert. 2020. Applications of minimally invasive multimodal telemetry for continuous monitoring of brain function and intracranial pressure in macaques with acute viral encephalitis. PLoS One 15(6):e0232381.
MAF (Morris Animal Foundation). 2022. Advancing canine health. https://www.morrisanimalfoundation.org/golden-retriever-lifetime-study-rfp (accessed February 24, 2023).
Mallick, S., R. Kanthety, and M. Rahman. 2009. Home blood pressure monitoring in clinical practice: A review. The American Journal of Medicine 122(9):803-810.
Malukiewicz, J., V. Boere, M. A. B. de Oliveira, M. D’Arc, J. V. A. Ferreira, J. French, G. Housman, C. I. de Souza, L. Jerusalinsky, R. d. M. F, M. V.-M. M, S. B. Moreira, E. S. I. de Oliveira, F. S. Pacheco, J. Rogers, A. Pissinatti, R. C. H. Del Rosario, C. Ross, C. R. Ruiz-Miranda, L. C. M. Pereira, N. Schiel, F. de Fátima Rodrigues da Silva, A. Souto, V. Šlipogor, and S. Tardif. 2020. An introduction to the Callithrix genus and overview of recent advances in marmoset research. Institute on Laboratory Animal Research Journal 61(2-3):110-138.
Marino, S., H. P. Gideon, C. Gong, S. Mankad, J. T. McCrone, P. L. Lin, J. J. Linderman, J. L. Flynn, and D. E. Kirschner. 2016. Computational and empirical studies predict Mycobacterium tuberculosis-specific T cells as a biomarker for infection outcome. PLOS Computational Biology 12(4):e1004804.
Martin, L. J., D. M. Spicer, M. H. Lewis, J. P. Gluck, and L. C. Cork. 1991. Social deprivation of infant rhesus monkeys alters the chemoarchitecture of the brain: I. Subcortical regions. The Journal of Neuroscience 11(11):3344.
Mattison, J. A., and K. L. Vaughan. 2017. An overview of nonhuman primates in aging research. Experimental Gerontology 94:41-45.
McCool, K. E., S. A. Bissett, T. L. Hill, L. A. Degernes, and E. C. Hawkins. 2019. Evaluation of a human virtual-reality endoscopy trainer for teaching early endoscopy skills to veterinarians. Journal of Veterinary Medical Education 47(1):106-116.
Medin, D. L., and C. D. Lee. 2012. Diversity makes better science. https://www.psychologicalscience.org/observer/diversity-makes-better-science (accessed April 25, 2023).
Messinger, A., N. Sirmpilatze, K. Heuer, K. K. Loh, R. B. Mars, J. Sein, T. Xu, D. Glen, B. Jung, J. Seidlitz, P. Taylor, R. Toro, E. A. Garza-Villarreal, C. Sponheim, X. Wang, R. A. Benn, B. Cagna, R. Dadarwal, H. C. Evrard, P. Garcia-Saldivar, S. Giavasis, R. Hartig, C. Lepage, C. Liu, P. Majka, H. Merchant, M. P. Milham, M. G. P. Rosa, J. Tasserie, L. Uhrig, D. S. Margulies, and P. C. Klink. 2021. A collaborative resource platform for non-human primate neuroimaging. Neuroimage 226:117519.
Metz, C. N. 2022. To better understand women’s health, we need to destigmatize menstrual blood. Scientific American, May 13. https://www.scientificamerican.com/article/to-better-understand-women-rsquo-s-health-we-need-to-destigmatize-menstrual-blood/ (accessed February 15, 2023).
MGI. 2022. Mouse genome informatics. http://www.informatics.jax.org/ (accessed February 24, 2023)
Milham, M. P., L. Ai, B. Koo, T. Xu, C. Amiez, F. Balezeau, M. G. Baxter, E. L. A. Blezer, T. Brochier, A. Chen, P. L. Croxson, C. G. Damatac, S. Dehaene, S. Everling, D. A. Fair, L. Fleysher, W. Freiwald, S. Froudist-Walsh, T. D. Griffiths, C. Guedj, F. Hadj-Bouziane, S. Ben Hamed, N. Harel, B. Hiba, B. Jarraya, B. Jung, S. Kastner, P. C. Klink, S. C. Kwok, K. N. Laland, D. A. Leopold, P. Lindenfors, R. B. Mars, R. S. Menon, A. Messinger, M. Meunier, K. Mok, J. H. Morrison, J. Nacef, J. Nagy, M. O. Rios, C. I. Petkov, M. Pinsk, C. Poirier, E. Procyk, R. Rajimehr, S. M. Reader, P. R. Roelfsema, D. A. Rudko, M. F. S. Rushworth, B. E. Russ, J. Sallet, M. C. Schmid, C. M. Schwiedrzik, J. Seidlitz, J. Sein, A. Shmuel, E. L. Sullivan, L. Ungerleider, A. Thiele, O. S. Todorov, D. Tsao, Z. Wang, C. R. E. Wilson, E. Yacoub, F. Q. Ye, W. Zarco, Y. D. Zhou, D. S. Margulies, and C. E. Schroeder. 2018. An open resource for non-human primate imaging. Neuron 100(1):61-74.e62.
Mitchell, A. S., R. Hartig, M. A. Basso, W. Jarrett, S. Kastner, and C. Poirier. 2021. International primate neuroscience research regulation, public engagement and transparency opportunities. Neuroimage 229:117700.
MITRE. 2022. MITRE Health. https://health.mitre.org/mcode/ (accessed February 24, 2023).
Modi, N. D., A. Y. Abuhelwa, R. A. McKinnon, A. V. Boddy, M. Haseloff, M. D. Wiese, T. C. Hoffmann, E. D. Perakslis, A. Rowland, M. J. Sorich, and A. M. Hopkins. 2022. Audit of data sharing by pharmaceutical companies for anticancer medicines approved by the US Food and Drug Administration. JAMA Oncology 8(9):1310-1316.
Mori, H., S. J. Wiklund, and J. Y. Zhang. 2022. Quantifying the benefits of digital biomarkers and technology-based study endpoints in clinical trials: Project moneyball. Digital Biomarkers 6(2):36-46.
Motahari-Nezhad, H., M. Péntek, L. Gulácsi, and Z. Zrubka. 2021. Outcomes of digital biomarker-based interventions: Protocol for a systematic review of systematic reviews. JMIR Research Protocols 10(11):e28204.
Muñoz-Fuentes, V., P. Cacheiro, T. F. Meehan, J. A. Aguilar-Pimentel, S. D. M. Brown, A. M. Flenniken, P. Flicek, A. Galli, H. H. Mashhadi, M. Hraběde Angelis, J. K. Kim, K. C. K. Lloyd, C. McKerlie, H. Morgan, S. A. Murray, L. M. J. Nutter, P. T. Reilly, J. R. Seavitt, J. K. Seong, M. Simon, H. Wardle-Jone, A.-M. Mallon, D. Smedley, H. E. Parkinson, and IMPC Consortium. 2018. The International Mouse Phenotyping Consortium (IMPC): A functional catalogue of the mammalian genome that informs conservation. Conservation Genetics 19(4): 995-1005.
Murry, C. 2022. Nonhuman primate model systems: State of the science and future needs. Presentation at Committee on the State of the Science and Future Needs for Nonhuman Primate Model Systems—Public meeting, virtual, November 21.
Myers, A. K., C. F. Talbot, L. A. Del Rosso, A. C. Maness, S. M. V. Simmons, J. P. Garner, J. P. Capitanio, and K. J. Parker. 2021. Assessment of medical morbidities in a rhesus monkey model of naturally occurring low sociality. Autism Research 14(7):1332-1346.
Naninck, T., N. Kahlaoui, J. Lemaitre, P. Maisonnasse, A. De Mori, Q. Pascal, V. Contreras, R. Marlin, F. Relouzat, B. Delache, C. Hérate, Y. Aldon, M. van Gils, N. Zabaleta, R. Ho Tsong Fang, N. Bosquet, R. W. Sanders, L. H. Vandenberghe, C. Chapon, and R. Le Grand. 2022. Computed tomography and [18F]-FDG PET imaging provide additional readouts for COVID-19 pathogenesis and therapies evaluation in non-human primates. iScience 25(4):104101.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2019. Care, use, and welfare of marmosets as animal models for gene editing-based biomedical research: Proceedings of a workshop. Edited by A. F. Johnson and L. Anestidou. Washington, DC: The National Academies Press.
NASEM. 2021. Rapid response by laboratory animal research institutions during the COVID-19 pandemic: Lessons learned: Proceedings of a workshop–in brief. Washington, DC: The National Academies Press.
National Resources Information Request. 2022. Information request responses. Document provided to the Committee on the State of the Science and Future Needs for Nonhuman Primate Model Systems on August 10, 2022. Available by request through the National Academies’ Public Access Records Office.
NCBI (National Center for Biotechnology Information). 2021. Geo overview. https://www.ncbi.nlm.nih.gov/geo/info/overview.html (accessed February 24, 2023).
Neff, E. P. 2020. Nonhuman primate neuroimaging opens up. Lab Animal 49(5):139-143.
NHP (Nonhuman Primates) Investigators Survey. 2022. See Appendix E.
NHPRC (Nonhuman Primate Research Centers Consortium). 2023. Login. https://locator.nhprc.org/Access/Login?ReturnUrl=%2FLocator%2FAnimalListing%2FIndex (accessed January 16, 2023).
NIA (National Institute on Aging). 2022. Nonhuman primate tissue bank handbook. https://www.nia.nih.gov/research/dab/nonhuman-primate-tissue-bank-handbook (accessed April 3, 2023).
Nielsen, M. W., S. Alegria, L. Börjeson, H. Etzkowitz, H. J. Falk-Krzesinski, A. Joshi, E. Leahey, L. Smith-Doerr, A. W. Woolley, and L. Schiebinger. 2017. Gender diversity leads to better science. Proceedings of the National Academy of Sciences 114(8):1740-1742.
NIH (National Institutes of Health). 2010. Primate pathology database collaborative. https://grantome.com/grant/NIH/P51-RR000164-49-8880 (accessed April 3, 2023).
NIH. 2016. Nonhuman Primate Transplantation Tolerance Cooperative Study Group (U19), edited by NIAID. https://grants.nih.gov/grants/guide/rfa-files/RFA-AI-22-002.html (accessed April 3, 2023).
NIH. 2019. Notice of NIH’s interest in diversity. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-20-031.html (accessed February 13, 2023).
NIH. 2020. Fostering rigorous research: Lessons learned from nonhuman primate models and charting the path forward. https://osp.od.nih.gov/wp-content/uploads/NHP_Workshop_Report_FINAL_20200218.pdf (accessed February 24, 2023).
NIH. 2022a. ACD Working Group on Catalyzing the Development and Use of Novel Alternative Methods to Advance Biomedical Research. https://acd.od.nih.gov/working-groups/novel-alternatives.html (accessed March 24, 2023).
NIH. 2022b. Individual training grants. https://orip.nih.gov/comparative-medicine/programs/training-and-career-development/individual-training-grants (accessed February 24, 2023).
NIH. 2022c. Institutional research training grants. https://orip.nih.gov/comparative-medicine/programs/training-and-career-development/institutional-training-grants (accessed February 24, 2023).
NIH. 2022d. NHP study—NIH data call for priorities, availability, and investment. Document provided to the Committee on the State of the Science and Future Needs for Nonhuman Primate Model Systems on November 15, 2022. Available by request through the National Academies’ Public Access Records Office.
NIH. 2022e. When and how to comply. https://publicaccess.nih.gov/ (accessed February 24, 2023).
NIH. 2023a. 2023 NIH data management and sharing policy. https://oir.nih.gov/sourcebook/intramural-program-oversight/intramural-data-sharing/2023-nih-data-management-sharing-policy (accessed January 13, 2023).
NIH. 2023b. Scientific data sharing. https://sharing.nih.gov/data-management-and-sharing-policy/about-data-management-and-sharing-policies/data-management-and-sharing-policy-overview (accessed January 13, 2023).
NIH and HHS (U.S. Department of Health and Human Services). 2020. Validation of animal models and tools for biomedical research: Session VI—Validation of nonhuman primate models for preclinical research. https://orip.nih.gov/sites/default/files/Validation_Session_VI_Meeting_Report_Final_508.pdf (accessed April 3, 2023).
NNTC (National NeuroAIDS Tissue Consortium). 2022. National NeuroAIDS Tissue Consortium. https://nntc.org/ (accessed February 24, 2023).
Normile, D. 2022. China bets big on brain research with massive cash infusion and openness to monkey studies. Science. https://www.science.org/content/article/china-bets-big-brain-research-massive-cash-infusion-and-openness-monkey-studies (accessed January 11, 2023).
NPRC (National Primate Research Center) Information Request. 2022. Information request responses. Document provided to the Committee on the State of the Science and Future Needs for Nonhuman Primate Model Systems on August 10, 2022. Available by request through the National Academies’ Public Access Records Office.
NRC (National Research Council). 2007. A strategy for assessing science: Behavioral and social research on aging. Edited by I. Feller and P. C. Stern. Washington, DC: The National Academies Press.
O’Grady, C. 2022. Airline’s decision to end monkey transports will worsen shortage in research. Science. https://www.science.org/content/article/airline-s-decision-end-monkey-transports-will-worsen-shortage-research (accessed March 9, 2023).
Open Science Collaboration. 2015. Estimating the reproducibility of psychological science. Science 349(6251):aac4716.
ORIP (Office of Research Infrastructure Programs). 2018. Nonhuman primate evaluation and analysis part 2: Report of the expert panel forum on challenges in assessing nonhuman primate needs and resources for biomedical research. https://orip.nih.gov/about-orip/research-highlights/nonhuman-primate-evaluation-and-analysis-part-2-report-expert-panel (accessed February 24, 2023).
Owens, B. 2018. Replication failures in psychology not due to differences in study populations. Nature, November 29. https://www.nature.com/articles/d41586-018-07474-y (accessed January 13, 2023).
Ozirmak Lermi, N., S. B. Gray, C. M. Bowen, L. Reyes-Uribe, B. K. Dray, N. Deng, R. A. Harris, M. Raveendran, F. Benavides, C. L. Hodo, M. W. Taggart, K. Colbert Maresso, K. M. Sinha, J. Rogers, and E. Vilar. 2022. Comparative molecular genomic analyses of a spontaneous rhesus macaque model of mismatch repair-deficient colorectal cancer. PLoS Genetics 18(4):e1010163.
Palacios, R., and S. K. Shah. 2019. When could human challenge trials be deployed to combat emerging infectious diseases? Lessons from the case of a Zika virus human challenge trial. Trials 20(2):702.
Pardo, I. D., R. H. Garman, K. Weber, W. F. Bobrowski, J. F. Hardisty, and D. Morton. 2012. Technical guide for nervous system sampling of the cynomolgus monkey for general toxicity studies. Toxicologic Pathology 40(4):624-636.
Park, J. E., and A. Silva. 2019. Generation of genetically engineered non-human primate models of brain function and neurological disorders. American Journal of Primatology 81(2):e22931.
Patel, A. G., P. N. Nehete, S. R. Krivoshik, X. Pei, E. L. Cho, B. P. Nehete, M. D. Ramani, Y. Shao, L. E. Williams, T. Wisniewski, and H. Scholtzova. 2021. Innate immunity stimulation via CPG oligodeoxynucleotides ameliorates Alzheimer’s disease pathology in aged squirrel monkeys. Brain 144(7):2146-2165.
Percie du Sert, N., V. Hurst, A. Ahluwalia, S. Alam, M. T. Avey, M. Baker, W. J. Browne, A. Clark, I. C. Cuthill, U. Dirnagl, M. Emerson, P. Garner, S. T. Holgate, D. W. Howells, N. A. Karp, S. E. Lazic, K. Lidster, C. J. MacCallum, M. Macleod, E. J. Pearl, O. H. Petersen, F. Rawle, P. Reynolds, K. Rooney, E. S. Sena, S. D. Silberberg, T. Steckler, and H. Würbel. 2020. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMJ Open Science 4(1):e100115.
Peterson, S. M., T. J. McGill, T. Puthussery, J. Stoddard, L. Renner, A. D. Lewis, L. M. A. Colgin, J. Gayet, X. Wang, K. Prongay, C. Cullin, B. L. Dozier, B. Ferguson, and M. Neuringer. 2019. Bardet-Biedl syndrome in rhesus macaques: A nonhuman primate model of retinitis pigmentosa. Experimental Eye Research 189:107825.
Pilkiw, M., and K. Takehara-Nishiuchi. 2018. Neural representations of time-linked memory. Neurobiology of Learning and Memory 153(Pt A):57-70.
Pomerantz, O., K. C. Baker, R. U. Bellanca, M. A. Bloomsmith, K. Coleman, E. K. Hutchinson, P. J. Pierre, J. L. Weed, and National Primate Research Centers’ Behavioral Management Consortium. 2022. Improving transparency—A call to include social housing information in biomedical research articles involving nonhuman primates. American Journal of Primatology 84(6):e23378.
Prescott, M. 2020. Ethical and welfare implications of genetically altered non-human primates for biomedical research. Journal of Applied Animal Ethics Research 2(2):151-176.
Prescott, M. J., and C. Poirier. 2021. The role of MRI in applying the 3Rs to non-human primate neuroscience. Neuroimage 225:117521.
Prescott, M. J., C. Clark, W. E. Dowling, and A. C. Shurtleff. 2021. Opportunities for refinement of non-human primate vaccine studies. Vaccines (Basel) 9(3).
Raasch, L. E., K. Yamamoto, C. M. Newman, J. R. Rosinski, P. M. Shepherd, E. Razo, C. M. Crooks, M. I. Bliss, M. E. Breitbach, E. L. Sneed, A. M. Weiler, X. Zeng, K. K. Noguchi, T. K. Morgan, N. A. Fuhler, E. K. Bohm, A. J. Alberts, S. J. Havlicek, S. Kabakov, A. M. Mitzey, K. M. Antony, K. K. Ausderau, A. Mejia, P. Basu, H. A. Simmons, J. C. Eickhoff, M. T. Aliota, E. L. Mohr, T. C. Friedrich, T. G. Golos, D. H. O’Connor, and D. M. Dudley. 2022. Fetal loss in pregnant rhesus macaques infected with high-dose African-lineage Zika virus. PLOS Neglected Tropical Diseases 16(8):e0010623.
Reader, J. R., D. R. Canfield, J. F. Lane, S. Kanthaswamy, A. Ardeshir, A. M. Allen, and R. P. Tarara. 2016. Left ventricular hypertrophy in rhesus macaques (Macaca mulatta) at the California National Primate Research Center (1992-2014). Comparative Medicine 66(2):162-169.
Riggs, J. E. 1998. The aging population: Implications for the burden of neurologic disease. Neurologic Clinics 16(3):555-560.
Rippy, M. K., D. R. Lee, S. L. Pearson, J. C. Bernal, and T. J. Kuehl. 1996. Identification of rhesus macaques with spontaneous endometriosis. Journal of Medical Primatology 25(5):346-355.
Ritch, M. D., B. G. Hannon, A. T. Read, A. J. Feola, G. A. Cull, J. Reynaud, J. C. Morrison, C. F. Burgoyne, M. T. Pardue, and C. R. Ethier. 2020. AxoNet: A deep learning-based tool to count retinal ganglion cell axons. Scientific Reports 10(1):8034.
Roelfsema, P. R., and S. Treue. 2014. Basic neuroscience research with nonhuman primates: A small but indispensable component of biomedical research. Neuron 82(6):1200-1204.
Rogers, J. 2022. Genomic resources for rhesus macaques (Macaca mulatta). Mammalian Genome 33(1):91-99.
Skaria, A. P. 2022. The economic and societal burden of Alzheimer disease: Managed care considerations. American Journal of Managed Care 28(10):S188-S196.
Sánchez, M. M., E. F. Hearn, D. Do, J. K. Rilling, and J. G. Herndon. 1998. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Research 812(1):38-49.
Sasaki, E., H. Suemizu, A. Shimada, K. Hanazawa, R. Oiwa, M. Kamioka, I. Tomioka, Y. Sotomaru, R. Hirakawa, T. Eto, S. Shiozawa, T. Maeda, M. Ito, R. Ito, C. Kito, C. Yagihashi, K. Kawai, H. Miyoshi, Y. Tanioka, N. Tamaoki, S. Habu, H. Okano, and T. Nomura. 2009. Generation of transgenic non-human primates with germline transmission. Nature 459(7246):523-527.
Sato, K., and E. Sasaki. 2018. Genetic engineering in nonhuman primates for human disease modeling. Journal of Human Genetics 63(2):125-131.
Sauermann, U., R. Siddiqui, Y. S. Suh, M. Platzer, N. Leuchte, H. Meyer, K. Mätz-Rensing, H. Stoiber, P. Nürnberg, G. Hunsmann, C. Stahl-Hennig, and M. Krawczak. 2008. MHC class I haplotypes associated with survival time in simian immunodeficiency virus (SIV)-infected rhesus macaques. Genes & Immunity 9(1):69-80.
Schapiro, S. J., M. A. Bloomsmith, L. M. Porter, and S. A. Suarez. 1996. Enrichment effects on rhesus monkeys successively housed singly, in pairs, and in groups. Applied Animal Behaviour Science 48(3):159-171.
SCHEER (Scientific Committee on Health Environmental and Emerging Risks). 2017. Final opinion on the need for non-human primates in biomedical research, production and testing of products and devices. https://ec.europa.eu/environment/chemicals/lab_animals/pdf/Scheer_may2017.pdf (accessed April 3, 2023).
Sharma, A., B. Jenkins, A. Akue, L. E. Lambert, S. Orr-Gonzalez, M. L. Thomas, A. Mahamar, B. S. Diarra, A. Dicko, M. Fried, and P. E. Duffy. 2022. Plasmodium falciparum in Aotus nancymaae: A new model for placental malaria. Journal of Infectious Diseases 226(3):521-527.
Sherman, L. S., W. Su, A. L. Johnson, S. M. Peterson, C. Cullin, T. Lavinder, B. Ferguson, and A. D. Lewis. 2021. A novel non-human primate model of Pelizaeus-Merzbacher disease. Neurobiology of Disease 158:105465.
Shively, C. A., A. Lacreuse, B. M. Frye, E. S. Rothwell, and M. Moro. 2021. Nonhuman primates at the intersection of aging biology, chronic disease, and health: An introduction to the American Journal of Primatology special issue on aging, cognitive decline, and neuropathology in nonhuman primates. American Journal of Primatology 83(11):e23309.
Singh, V. K., and A. O. Olabisi. 2017. Nonhuman primates as models for the discovery and development of radiation countermeasures. Expert Opinion on Drug Discovery 12(7):695-709.
Singh, M., A. Kumar, and H. N. Kumara. 2020. Rhesus monkey: Macaca mulatta. International Union for Conservation of Nature. https://www.iucnredlist.org/species/12554/17950825 (accessed January 13, 2023).
Slawson, N. 2019. “Women have been woefully neglected”: Does medical science have a gender problem? The Guardian, December 18. https://www.theguardian.com/education/2019/dec/18/women-have-been-woefully-neglected-does-medical-science-have-a-gender-problem (accessed February 15, 2023).
Srivastava, S., and P. D. Wagner. 2020. The early detection research network: A national infrastructure to support the discovery, development, and validation of cancer biomarkers. Cancer Epidemiology, Biomarkers & Prevention 29(12):2401-2410.
Struble, R. G., and A. H. Riesen. 1978. Changes in cortical dendritic branching subsequent to partial social isolation in stumptailed monkeys. Developmental Psychobiology 11(5):479-486.
Subbaraman, N. 2021. The US is boosting funding for research monkeys in the wake of COVID. Nature, July 15. https://www.nature.com/articles/d41586-021-01894-z (accessed March 9, 2023).
Tanaka, T., Y. Hishitani, and A. Ogata. 2014. Monoclonal antibodies in rheumatoid arthritis: Comparative effectiveness of tocilizumab with tumor necrosis factor inhibitors. Biologics 8:141-153.
Tang, F. M. K., R. M. F. Lee, R. H. L. Szeto, J. C. T. Cheung, and O. M. Y. Ngan. 2020. Experiential learning with virtual reality: Animal handling training. Innovation and Education 2(1):2.
Tate, L. 2021. Tulane University to lead national research partnership to speed up COVID-19 vaccines and drug discoveries. https://news.tulane.edu/pr/tulane-university-lead-national-research-partnership-speed-covid-19-vaccines-and-drug-discoveries (accessed January 12, 2022).
Teil, M., M. L. Arotcarena, and B. Dehay. 2021. A new rise of non-human primate models of synucleinopathies. Biomedicines 9(3).
Tian, H., Y. Lyu, Y. Yang, and Z. Hu. 2020. Humanized rodent models for cancer research. Frontiers in Oncology 10.
Traverso, G., G. Ciccarelli, S. Schwartz, T. Hughes, T. Boettcher, R. Barman, R. Langer, and A. Swiston. 2015. Physiologic status monitoring via the gastrointestinal tract. PLoS One 10(11):e0141666.
Troschel, A. S., F. M. Troschel, T. D. Best, H. A. Gaissert, M. Torriani, A. Muniappan, E. E. Van Seventer, R. D. Nipp, E. J. Roeland, J. S. Temel, and F. J. Fintelmann. 2020. Computed tomography–based body composition analysis and its role in lung cancer care. Journal of Thoracic Imaging 35(2).
Uchida, A., H. Sasaguri, N. Kimura, M. Tajiri, T. Ohkubo, F. Ono, F. Sakaue, K. Kanai, T. Hirai, T. Sano, K. Shibuya, M. Kobayashi, M. Yamamoto, S. Yokota, T. Kubodera, M. Tomori, K. Sakaki, M. Enomoto, Y. Hirai, J. Kumagai, Y. Yasutomi, H. Mochizuki, S. Kuwabara, T. Uchihara, H. Mizusawa, and T. Yokota. 2012. Non-human primate model of amyotrophic lateral sclerosis with cytoplasmic mislocalization of TDP-43. Brain 135(Pt 3):833-846.
Unger, J. M., E. Cook, E. Tai, and A. Bleyer. 2016. The role of clinical trial participation in cancer research: Barriers, evidence, and strategies. American Society of Clinical Oncology Educational Book 35:185-198.
Usher, W., P. Klacansky, F. Federer, P. T. Bremer, A. Knoll, J. Yarch, A. Angelucci, and V. Pascucci. 2018. A virtual reality visualization tool for neuron tracing. IEEE Transactions on Visualization and Computer Graphics 24(1):994-1003.
Van Dam, D., and P. P. De Deyn. 2011. Animal models in the drug discovery pipeline for Alzheimer’s disease: Animal models for AD. British Journal of Pharmacology 164(4):1285-1300.
Vasudevan, S., A. Saha, M. E. Tarver, and B. Patel. 2022. Digital biomarkers: Convergence of digital health technologies and biomarkers. NPJ Digital Medicine 5(1):36.
Venkatesan, M., H. Mohan, J. R. Ryan, C. M. Schürch, G. P. Nolan, D. H. Frakes, and A. F. Coskun. 2021. Virtual and augmented reality for biomedical applications. Cell Reports Medicine 2(7):100348.
Verdier, J. M., I. Acquatella, C. Lautier, G. Devau, S. Trouche, C. Lasbleiz, and N. Mestre-Francés. 2015. Lessons from the analysis of nonhuman primates for understanding human aging and neurodegenerative diseases. Frontiers in Neuroscience 9:64.
Voge, N. V., and E. Alvarez. 2019. Monoclonal antibodies in multiple sclerosis: Present and future. Biomedicines 7(1).
WaNPRC (Washington National Primate Research Center). 2023. Project review process. https://wanprc.uw.edu/animal-resources/project-review-process/ (accessed January 12, 2023).
Warren, W. C., A. J. Jasinska, R. García-Pérez, H. Svardal, C. Tomlinson, M. Rocchi, N. Archidiacono, O. Capozzi, P. Minx, M. J. Montague, K. Kyung, L. W. Hillier, M. Kremitzki, T. Graves, C. Chiang, J. Hughes, N. Tran, Y. Huang, V. Ramensky, O. W. Choi, Y. J. Jung, C. A. Schmitt, N. Juretic, J. Wasserscheid, T. R. Turner, R. W. Wise-man, J. J. Tuscher, J. A. Karl, J. E. Schmitz, R. Zahn, D. H. O’Connor, E. Redmond, A. Nisbett, B. Jacquelin, M. C. Müller-Trutwin, J. M. Brenchley, M. Dione, M. Antonio, G. P. Schroth, J. R. Kaplan, M. J. Jorgensen, G. W. Thomas, M. W. Hahn, B. J. Raney, B. Aken, R. Nag, J. Schmitz, G. Churakov, A. Noll, R. Stanyon, D. Webb, F. Thibaud-Nissen, M. Nordborg, T. Marques-Bonet, K. Dewar, G. M. Weinstock, R. K. Wilson, and N. B. Freimer. 2015. The genome of the vervet (Chlorocebus aethiops sabaeus). Genome Research 25(12):1921-1933.
Wilkinson, M. D., M. Dumontier, I. J. Aalbersberg, G. Appleton, M. Axton, A. Baak, N. Blomberg, J.-W. Boiten, L. B. da Silva Santos, P. E. Bourne, J. Bouwman, A. J. Brookes, T. Clark, M. Crosas, I. Dillo, O. Dumon, S. Edmunds, C. T. Evelo, R. Finkers, A. Gonzalez-Beltran, A. J. G. Gray, P. Groth, C. Goble, J. S. Grethe, J. Heringa, P. A. C. ’t Hoen, R. Hooft, T. Kuhn, R. Kok, J. Kok, S. J. Lusher, M. E. Martone, A. Mons, A. L. Packer, B. Persson, P. Rocca-Serra, M. Roos, R. van Schaik, S.-A. Sansone, E. Schultes, T. Sengstag, T. Slater, G. Strawn, M. A. Swertz, M. Thompson, J. van der Lei, E. van Mulligen, J. Velterop, A. Waagmeester, P. Wittenburg, K. Wolstencroft, J. Zhao, and B. Mons. 2016. The FAIR guiding principles for scientific data management and stewardship. Scientific Data 3(1):160018.
Winkler, E., A. Bailey, N. Kafai, S. Nair, B. McCune, J. Yu, J. Fox, R. Chen, J. Earnest, S. Keeler, J. Ritter, L. Kang, S. Dort, A. Robichaud, R. Head, M. Holtzman, and M. Diamond. 2020. SARS-CoV-2 infection of human ACE2transgenic mice causes severe lung inflammation and impaired function. Nature Immunology 31:1327-1335.
Witham, C., and S. Wells. 2022. Nonhuman primates’ tissue banks: Resources for all model organism research. Mammalian Genome 33(1):241-243.
Wood, C. E., A. L. Usborne, M. F. Starost, R. P. Tarara, L. R. Hill, L. M. Wilkinson, K. R. Geisinger, E. A. Feiste, and J. M. Cline. 2006. Hyperplastic and neoplastic lesions of the mammary gland in macaques. Veterinary Pathology 43(4):471-483.
Wood, C. E., Z. Chen, J. M. Cline, B. E. Miller, and R. D. Burk. 2007. Characterization and experimental transmission of an oncogenic papillomavirus in female macaques. Journal of Virology 81(12):6339-6345.
Worley, K. C., W. C. Warren, J. Rogers, D. Locke, D. M. Muzny, E. R. Mardis, G. M. Weinstock, S. D. Tardif, K. M. Aagaard, N. Archidiacono, N. A. Rayan, M. A. Batzer, K. Beal, B. Brejova, O. Capozzi, S. B. Capuano, C. Casola, M. M. Chandrabose, A. Cree, M. D. Dao, P. J. de Jong, R. C.-H. del Rosario, K. D. Delehaunty, H. H. Dinh, E. E. Eichler, S. Fitzgerald, P. Flicek, C. C. Fontenot, R. G. Fowler, C. Fronick, L. A. Fulton, R. S. Fulton, R. A. Gabisi, D. Gerlach, T. A. Graves, P. H. Gunaratne, M. W. Hahn, D. Haig, Y. Han, R. A. Harris, J. Herrero, L. W. Hillier, R. Hubley, J. F. Hughes, J. Hume, S. N. Jhangiani, L. B. Jorde, V. Joshi, E. Karakor, M. K. Konkel, C. Kosiol, C. L. Kovar, E. V. Kriventseva, S. L. Lee, L. R. Lewis, Y.-s. Liu, J. Lopez, C. Lopez-Otin, B. Lorente-Galdos, K. G. Mansfield, T. Marques-Bonet, P. Minx, D. Misceo, J. S. Moncrieff, M. B. Morgan, L. V. Nazareth, I. Newsham, N. B. Nguyen, G. O. Okwuonu, S. Prabhakar, L. Perales, L.-L. Pu, X. S. Puente, V. Quesada, M. C. Ranck, B. J. Raney, M. Raveendran, D. R. Deiros, M. Rocchi, D. Rodriguez, C. Ross, M. Ruffier, S. J. Ruiz, S. Sajjadian, J. Santibanez, D. R. Schrider, S. Searle, H. Skaletsky, B. Soibam, A. F. A. Smit, J. B. Tennakoon, L. Tomaska, B. Ullmer, C. E. Vejnar, M. Ventura, A. J. Vilella, T. Vinar, J.-H. Vogel, J. A. Walker, Q. Wang, C. M. Warner, D. E. Wildman, D. J. Witherspoon, R. A. Wright, Y. Wu, W. Xiao, J. Xing, E. M. Zdobnov, B. Zhu, R. A. Gibbs, R. K. Wilson, and the Marmoset Genome Sequencing and Analysis Consortium. 2014. The common marmoset genome provides insight into primate biology and evolution. Nature Genetics 46(8):850-857.
Wotton, J. M., E. Peterson, L. Anderson, S. A. Murray, R. E. Braun, E. J. Chesler, J. K. White, and V. Kumar. 2020. Machine learning-based automated phenotyping of inflammatory nocifensive behavior in mice. Molecular Pain 16:1744806920958596.
Xu, H., Y. Su, J. Sharma, W. Menegas, C. Trimmer, F. Liang, R. Landman, G. Zhang, M. Sur, R. Saxe, G. Feng, R. Desimone. 2022. Large scale ECoG recording during social scene watching and social behaviors in marmosets. Conference. Neuroscience 2022. Sesson 179. November 13, 2022.
Yao, Y., P. Bala, A. Mohan, E. Bliss-Moreau, K. Coleman, S. M. Freeman, C. J. Machado, J. Raper, J. Zimmermann, B. Y. Hayden, and H. S. Park. 2023. OpenMonkeyChallenge: Dataset and benchmark challenges for pose estimation of non-human primates. International Journal of Computer Vision 131(1):243-258.
Yee, J. L., R. Grant, A. J. Haertel, C. Allers, A. B. Carpenter, K. K. A. Van Rompay, and J. A. Roberts. 2022. Multi-site proficiency testing for validation and standardization of assays to detect specific pathogen-free viruses, coronaviruses, and other agents in nonhuman primates. Journal of Medical Primatology 51(4):234-245.





































