The State of the U.S. Biomedical and Health Research Enterprise: Strategies for Achieving a Healthier America (2024)
Chapter: 3 Streamlined, Coordinated, and Increasingly Impactful Funding
3
STREAMLINED, COORDINATED, AND INCREASINGLY IMPACTFUL FUNDING
According to the National Science Board’s Science & Engineering Indicators, global research and development (R&D) expenditures have grown from $726 billion in 2000 to $2.4 trillion in 2019 (Burke et al., 2022). The United States is the global leader in gross domestic expenditure on R&D with $656 billion in 2019, accounting for 27% of the global total (Burke et al., 2022). In the same year, China accounted for 22% of the global total with $526 billion; therefore, China and the United States alone account for half of all global R&D. Other countries with significant gross expenditures on R&D include Japan, Germany, and South Korea (Burke et al., 2022).
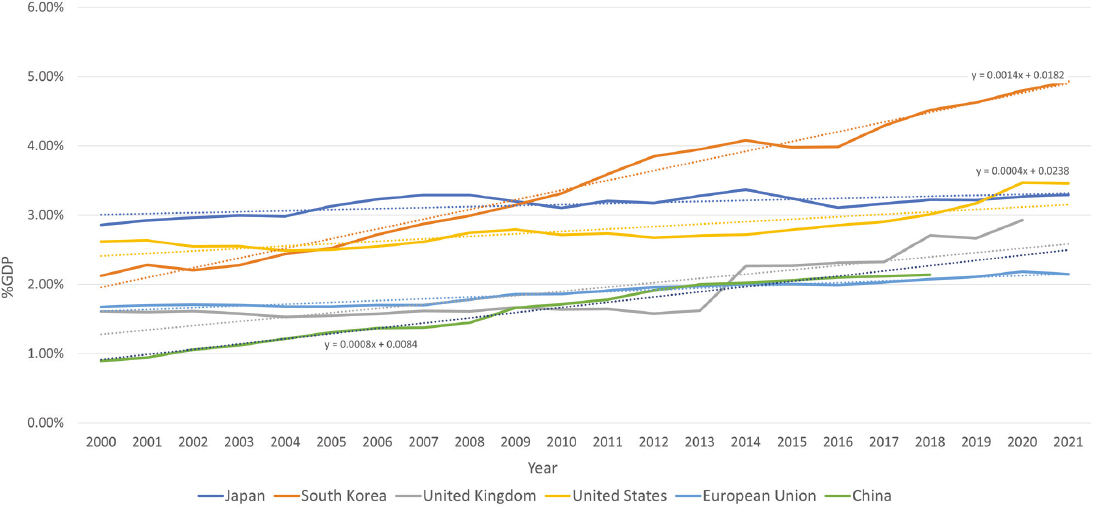
In 2019, the United States spent more than 3% of its $21.4 trillion gross domestic product (GDP) on R&D and absolute spending on R&D has increased from $268 billion in 2000 to $656 billion in 2019 (Burke et al., 2022). While the United States spends the most globally in absolute dollars on R&D, other countries have started to invest a greater percentage of their GDPs in R&D than the United States does (see Figure 3-1). China has increased its percentage of GDP spending at nearly two times the rate of the United States (see Figure 3-1) and South Korea is spending 5% of its GDP on R&D—second only to Israel at 5.56% (Investopedia, 2024; World Bank, 2023). Basic research expenditures as a percentage of GDP mirror patterns of total research expenditures, with South Korea showing the greatest increase between 2000 and 2021, followed by China and the United States, while Japan has seen much less growth (see Figure 3-2).
Funding is not everything when it comes to biomedical research, but it does enable continuous, focused, and innovative research. The United States has long been a global leader in biomedical research—partially due to large financial investments—but the trends described above show that the rest of the globe is
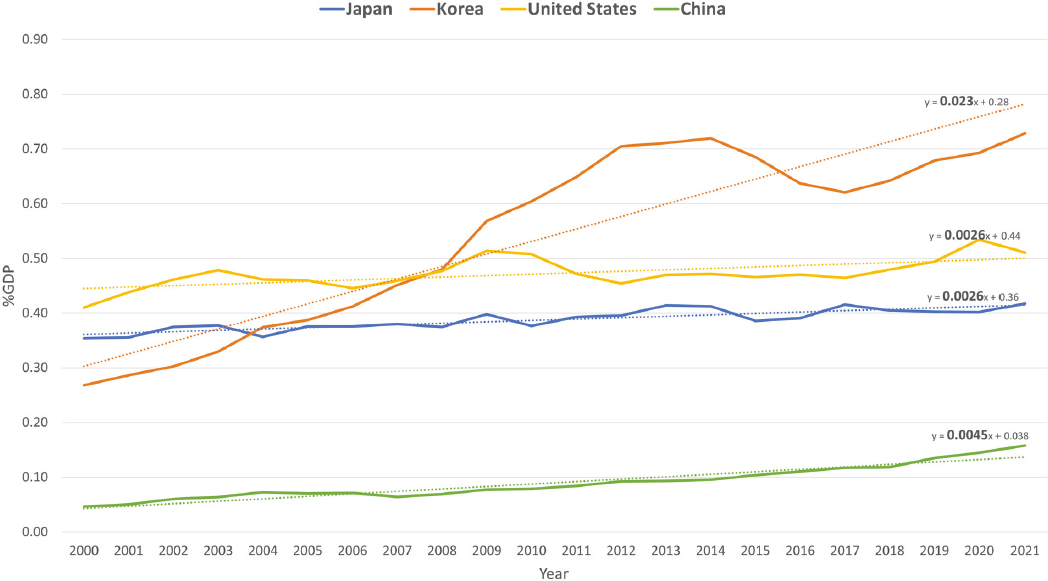
NOTES: Dotted lines indicate linear trends over time, with corresponding equations shown above each trend line. Research expenditures include all research and are not limited to biomedical research. China’s basic science research expenditures are increasing at a rate of 0.0045% gross domestic product (GDP) per year, while the United States and Japan’s basic science research expenditures are increasing at a rate of 0.0026% GDP per year and South Korea’s are increasing at a rate of 0.023% GDP per year. Parameters for the data shown in Figure 3-2 within the Main Science and Technology Indicators database are as follows: time period = 2000–2021; reference areas = South Korea, United States, China, and Japan; measure = basic science research expenditure; and unit of measure = percentage GDP.
SOURCE: OECD, n.d.b.
catching up. To continue to enable American breakthroughs and maintain global leadership, the sources, coordination, and disbursement of funding across the U.S. biomedical research enterprise should be examined.
FUNDING ALWAYS COMES WITH AN AGENDA
Many, if not all, of America’s scientific breakthroughs were enabled by large financial investments. The U.S. biomedical research enterprise is driven by federal funding, mostly through the National Institutes of Health (NIH), but is also supported by funding from industry, venture capital, and philanthropy. Each of these funding sources arrives with an agenda and is very rarely “no strings attached.” The somewhat piecemeal funding apparatus of the U.S. biomedical research enterprise and these competing agendas are barriers to a truly coordinated system, where initiatives to solve complex problems are launched in concert instead of in parallel and funding is focused on where it will have the largest impact.
“Venture capital funding drives the biotechnology industry, but money is the main driver of venture capital,” James Flynn, managing partner at Deerfield Management Company, said when he spoke to the authors of the Special Publication. Deerfield is an investment firm focused on life science research, medical device development, diagnostics, digital health, and health services (Deerfield, n.d.). To Flynn’s point, organizations such as Deerfield are driven by one main obligation—providing returns to their investors (Zider, 1998). This focus requires venture capital firms to seek clear value propositions and greatly limit their risk, which narrows the aperture of the number and type of projects and science they are comfortable supporting. Investor expectations of receiving returns on investment also preclude full incorporation of venture capital endeavors into existing R&D funding models (Zider, 1998).
Like venture capital, industry spending is also driven by expectations of profit. While the U.S. government is the largest single investor in biomedical research, private industry as a sector is the largest overall investor, spending more than double the NIH budget in 2019 on development and bringing products to market (Research!America and Teconomy Partners, LLC, 2022). Despite industry’s significant investments in biomedical research, its focus is mostly on products rather than discovery research (Research!America and Teconomy Partners, LLC, 2022). According to a recent report published by the Deloitte Centre for Health Solutions, the 20 pharmaceutical companies that spend the most on R&D spent a total of $139 billion in 2022, a slight decrease from the high of $141 billion in 2021 (Deloitte Centre for Health Solutions, 2023). The average cost of bringing a
drug to market was $1.9 billion in 2021 and $2.2 billion in 2022 (Deloitte Centre for Health Solutions, 2023).
Deloitte reports that internal rates of return from R&D investment have been declining for the past decade, and several factors contribute to these decreased margins—most notably the increasing duration of clinical trials (Deloitte Centre for Health Solutions, 2023). The average time span of a clinical trial from the start of Phase I to the end of Phase III has grown longer in the last decade, from approximately 6.2 to 7.1 years (Deloitte Centre for Health Solutions, 2023). The average clinical trial cycle is longest for cancer drugs, at more than 11 years, and shortest for infectious diseases, at 4.3 years (Deloitte Centre for Health Solutions, 2023). Although pharmaceutical companies do invest in their own R&D, industry also relies on federally funded discovery research as starting points for asset development—underscoring the need for robust public funding for the U.S. biomedical research enterprise.
Philanthropic gifts are a small but growing aspect of the U.S. biomedical research enterprise’s funding portfolio. According to one report, although federal funding for university research grew less than 1% annually between 2005 and 2010, science philanthropy grew and has continued to grow at 5% annually (Murray, 2013).
Philanthropic investments and gifts—when compared to public or private funding—can come with fewer or different restrictions on how the donations are spent. Philanthropic dollars from grateful patients often go to individual researchers rather than their institution, providing individuals with more leeway on what projects they would like to focus on, but providing little benefit to the institution writ large—until the individual researcher achieves a breakthrough product or discovery, which is not guaranteed to happen. Larger gifts and endowments, however, can provide more flexibility and allow institutions and individual researchers to support early-stage research that much federal funding cannot. Philanthropy can be an important source of funding for research focused on innovation and discovery and of support for early-career researchers as they launch their careers (Conn et al., 2023).
Due to venture capital and industry’s focus on returns on investment and philanthropy’s often narrow and personalized guardrails, many promising biomedical discoveries languish due to lack of funding until their utility is appreciated, which may not happen for years, decades, or ever. The agendas that accompany funding type are preventing the U.S. biomedical research enterprise from producing maximum returns on investment, because products that spent years in development may sit unused until they are translated into diagnostics or treatments by someone willing to risk the time and money on them.
OVERCOMING THE FUNDING VALLEY OF DEATH
Not all ideas can be translated successfully, and even promising discoveries are not guaranteed a path to the patient. To move promising theories into actionable therapeutics, researchers must find funding from venture capital, angel investors, or private equity, or cobble together available government grants to continue their work. Many efforts to find funding fail. This translational support gap is called the “funding valley of death” and prevents the advancement of potential breakthrough therapies from idea to action.
One area of great success in the translation of basic research into marketable therapies was the passage of the 1983 Orphan Drug Act (Swann, 2018). The Act provided financial incentives for companies to pursue drug approvals for rare diseases—conditions affecting so few people that the typical financial calculus precluded industry investment. In the 20 years after its passing, the Orphan Drug Act led to 232 new drug approvals that helped about 11 million patients (Swann, 2018).
Other programs and efforts are attempting to address the funding valley of death, including:
- The Small Business Innovation Research program, which promotes innovation and entrepreneurship and increases private-sector commercialization of publicly funded research discoveries (SBIR STTR, n.d.);
- The Small Business Technology Transfer program, which aims to foster technology transfer between small businesses and research institutions (SBIR STTR, n.d.);
- The Biomedical Advanced Research and Development Authority, which establishes public–private partnerships to bridge translational gaps and has supported the U.S. Food and Drug Administration (FDA) approval of 89 vaccines, drugs, therapies, and diagnostic tools for public health emergencies (medicalcountermeasures.gov, 2024);
- NIH’s Clinical Translational Science Awards program, which enhances training in translational sciences and provides academic scientists and small companies with hard-to-access and expensive technologies via direct expert contract support (NIH NCATS, n.d.);
- The Advanced Research Projects Agency for Health, which supports transformative biomedical and health research ranging from molecular to societal innovations for health solutions (ARPA-H, n.d.);
- Efforts from NIH to leverage philanthropic and industry resources for translational discovery by establishing successful public–private partnerships
- Efforts from NIH to facilitate clinical trials for meritorious early and mid-stage projects by establishing standing clinical trial networks that help advance early-stage research to proof-of-concept. At that point, industry could pick up and carry projects to approval—if that does not happen, NIH itself undertakes the task of carrying the trials forward (NIH SEED, n.d.).
through the Foundation for the National Institutes of Health (FNIH, n.d.); and
Even with these programs in place, additional opportunities for facilitating the translation of discoveries into tangible patient benefits exist and should be utilized. The Defense Innovation Unit of the Department of Defense “accelerates the adoption of commercial technology throughout the military,” partnering with external entities to “rapidly prototype and field dual-use capabilities that solve operational challenges at speed and scale” (DIU, n.d.). In-Q-Tel, a venture capital firm funded by the Central Intelligence Agency, explores, identifies, and funds emerging technologies and startups that can benefit the U.S. government (IQT, n.d.). The Department of Energy Loan Programs Office “provides attractive debt financial for high-impact, large-scale energy infrastructure projects,” helped launch the Tesla Model S, and supported the upgrade of Ford manufacturing facilities to build vehicles with improved fuel efficiency (energy.gov, 2023). Sustained investment in basic, translational, clinical, and manufacturing R&D by all participants in the ecosystem—even when policies and market conditions are adequate to support at-risk investments—will help facilitate translation and more effectively deliver discoveries to market.
COORDINATED AND FOCUSED FUNDING IS NECESSARY TO ADDRESS AMERICA’S HEALTH CHALLENGES
The fragmented and disparate economic models that undergird the U.S. biomedical research enterprise are not producing sufficient investment to develop treatments for several key public health needs. R&D into antimicrobial resistance, for example, is severely underfunded and leaves the American people vulnerable to persistent infections because market realities have forced companies doing this work to exit the field (Bayer Global, 2023). Another area of need is the renewed production of older drugs that have gone through the entire translational process and are now being sold as generics but are in short supply or no longer produced (Noguchi, 2023b). An exclusive focus on profit undervalues the critical nature of generic drugs, which compose 90% of prescriptions in the United States, and ignoring these shortages may leave
people who depend on these drugs without access to their more affordable versions (Noguchi, 2023b).
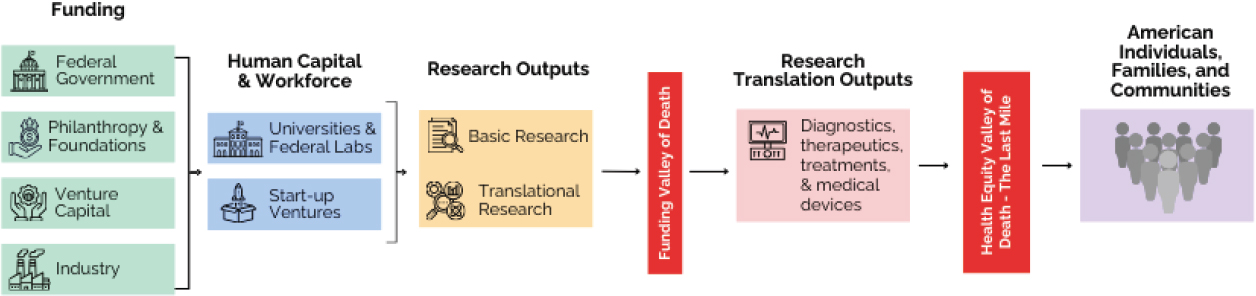
The current U.S. biomedical research enterprise, illustrated in Figure 3-3, despite a wealth of breakthroughs and positive inputs, falls short of its immense potential. The siloed nature of funding sources, as well as their competing agendas, is concerning for the future of the emerging, complex health conditions that threaten American well-being.
The United States needs to break down these silos and develop an overarching vision for biomedical research funding, much in the same vein as the national strategic vision. Although the United States spends more on biomedical R&D than any other nation, federal investment is fragmented across agencies, institutes, and centers, with little cohesion or overarching strategy to address population needs or the social determinants of health. Research expenditures across sectors are driven not by a national mission to improve health, but by motivations such as marketability and profit margins that do not always benefit patients.
CALL TO ACTION
Bringing together federal funders, venture capital, industry, and philanthropy to form a proactive funding body and mechanism will enable the pooling of resources to collaboratively address national biomedical research priorities. The COVID-19 pandemic illustrated the success and power of such partnerships, which should be leveraged more strategically for long-term progress and not only in times of crisis. This funding body should use all available models and best practices to inform its composition and work, but should especially investigate the successes and mechanisms of the Foundation for the National Institutes of Health Accelerating Medicines Partnership and the Advanced Research Projects Agency for Health Investor Catalyst Hub.
The U.S. government is the largest single investor in biomedical research and should take the lead in bringing other investors to the table to co-fund research that addresses the nation’s health priorities. The priorities established in the national strategic vision (see Chapter 2) will help direct investment, clearly identify and minimize investment risks, and bring returns on investment in the long term. To better realize the potential of basic science, the funding valley of death that prevents worthy discoveries from being translated into therapeutics and products must also be addressed and eliminated.
Establishing a national strategic vision for coordinated biomedical and health research to improve the lives of Americans will catalyze funders to invest in a focused and synergistic manner. With the federal government bringing venture
capital, industry, and philanthropy to the table, their pooled resources could be leveraged to tackle the most serious diseases facing the American people. Relying solely on the government to improve population health is short sighted—much greater gains can be achieved with pooled resources and a shared strategy, and improving the health of all Americans will benefit all Americans.
To realize this vision, the authors of this Special Publication propose the following:
Priority 2-1: A federally established national biomedical research funding collaborative, guided by best practices from existing international models, and federal determinations of how best to organize and allocate shared investments from the government, private sector, and philanthropy. The funding collaborative could be empowered to:
- Analyze successful existing models to develop best practices for the implementation of new methods for financing and accelerating biomedical research;
- Create a large-scale funding model to address the health challenges identified in the national strategic vision; and
- Develop new philanthropic collectives to encourage pooled, strategic gifts that can make a large impact.
Priority 2-2: Federally developed initiatives and funding strategies to specifically address the issue of the “funding valley of death” to translate promising basic research into breakthrough therapies, diagnostics, and treatments—helping to ensure that the full value of the U.S. biomedical research enterprise reaches all patients equitably.