Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 18 (2014)
Chapter: 1 Bromine Chloride
1
Bromine Chloride1
Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could
____________________
1This document was prepared by the AEGL Development Team composed of Sylvia Talmage (Oak Ridge National Laboratory), Heather Carlson-Lynch (SRC, Inc.), Chemical Manager Marquea King (National Advisory Committee [NAC] on Acute Exposure Guideline Levels for Hazardous Substances), and Ernest V. Falke (U.S. Environmental Protection Agency). The NAC reviewed and revised the document and AEGLs as deemed necessary. Both the document and the AEGL values were then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC committee has concluded that the AEGLs developed in this document are scientifically valid conclusions based on the data reviewed by the NRC and are consistent with the NRC guidelines reports (NRC 1993, 2001).
experience notable discomfort, irritation, or certain asymptomatic, nonsensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure concentrations that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold concentrations for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Bromine chloride is a red-brown liquid. It is formed when bromine and chlorine react reversibly in the liquid and vapor phases. When equimolar amounts of the halogens are reacted, about 60% of the mixed halogens are present as bromine chloride (about 40% is dissociated). The interhalogen compounds are very reactive and hydrolyze readily.
Bromine chloride is used as a water-treatment biocide and in organic synthesis involving addition across olefinic double bonds to produce bromochloro compounds and for aromatic brominations, where an aromatic bromide and hydrogen chloride are produced. Bromine chloride also has application as a brominating agent in the preparation of fire-retardant chemicals, pharmaceuticals, high-density brominated liquids, agricultural chemicals, dyes, and bleaching agents.
No data relevant to deriving AEGL-1 values for bromine chloride were found. Thus, AEGL-1 values are not recommended.
Relevant data for deriving AEGL-2 values for bromine chloride were also not found. However, in accordance with the standing operating procedures for developing AEGL values (NRC 2001), AEGL-2 values were determined by dividing the AEGL-3 values by 3, because the dose-response curve for bromine chloride is steep (0% lethality at 40 ppm and almost 100% lethality at 120 ppm).
For AEGL-3 values, the point-of-departure was the threshold for lethality estimated from a study by Dow Chemical (1977). In that study, the mortality rate in rats exposed to bromine chloride at 20, 40, 80, or 120 ppm for 7 h was 0/6, 0/6, 1/6, and 5/6, respectively. Benchmark concentration analysis was used to estimate the no-observed-adverse-effect level (NOAEL) for lethality (NRC 2001). The 7-h BMCL05 (benchmark concentration, 95% lower confidence limit with 5% response) was 39.4 ppm. A total uncertainty factor of 10 was applied; a factor of 3 for interspecies differences and a factor of 3 for intraspecies variability. The effects of direct-acting irritants like bromine chloride are not expected to differ significantly between species or among individuals (NRC 2001). In addition, a modifying factor of 3 was applied to account for the sparse data on bromine chloride and the uncertainty in the exposure concentrations in the Dow Chemical study. Time scaling was performed using the equation Cn × t = k. Data on bromine chloride were inadequate to derive an empirical value for n, so default values of n = 3 for extrapolating to shorter durations and n = 1 for extrapolating to longer durations were used (NRC 2001). Because of the uncertainty associated with time scaling a 7-h point-of-departure to a 10-min value, the 10-min AEGL-3 value was set equal to the 30-min value.
The AEGL values for bromine chloride are presented in Table 1-1.
1. INTRODUCTION
Bromine chloride is a red-brown liquid (Lang 2006). It is formed when bromine and chlorine react reversibly in the liquid and vapor phases. When equimolar amounts of the halogens are reacted at room temperature, about 60% of the mixed halogens are present as bromine chloride (about 40% is dissociated) (Dagani et al. 2000).
TABLE 1-1 AEGL Values for Bromine Chloride
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) |
| AEGL-1 nondisabling) |
NRa | NRa | NRa | NRa | NRa | Insufficient data. |
| AEGL-2 (disabling) |
1.1 ppm (5.2 mg/m3) |
1.1 ppm (5.2 mg/m3) |
0.83 ppm (3.9 mg/m3) |
0.53 ppm (2.5 mg/m3) |
0.37 ppm (1.7 mg/m3) |
One-third of the AEGL-3 values. |
| AEGL-3 (lethal) |
3.2 ppm (15 mg/m3) |
3.2 ppm (15 mg/m3) |
2.5 ppm (12 mg/m3) |
1.6 ppm (7.6 mg/m3) |
1.1 ppm (5.2 mg/m3) |
Threshold for lethality in the rat (Dow Chemical Co. 1977). |
aNot recommended. Absence of an AEGL-1 value does not imply that exposures below the AEGL-2 value are without adverse effects.
The physical properties of mixed halogens are generally intermediate between those of the component halogens (Lang 2006; Frim and Ukeles 2011); however, mixed halogens are polar while single halogen molecules are not (Cotton and Wilkinson 1980). Bromine chloride is a strong oxidizing agent (Dagani et al. 2000). In general, interhalogen compounds are more chemically reactive than elemental halogens due to the weakness of the interhalogen bond (Cotton and Wilkinson 1980; Barrie et al. 2012). Among the diatomic interhalogens, bromine chloride is the least stable, dissociating reversibly to its elemental components (Cotton and Wilkinson 1980; Lang 2006).
The mixed halogen compounds readily hydrolyze (Cotton and Wilkinson 1980). Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids, including hydrochloric, hypochloric, hydrobromic, and hypobromous acids. The relative proportions of the products depend on pH, but have little dependence on temperature (Liu and Margerum 2001). The following equations show some of the primary reactions (Liu and Margerum 2001; Frim and Ukeles 2011):
2BrCl ↔ Br2 + Cl2
BrCl + H2O ↔ HCl + HOBr
Cl2 + H2O ↔ HOCl + HCl
Br2 + H2O ↔ HOBr + HBr
Ions (e.g., Br-, Cl-) may also exist in equilibrium with the molecules presented above (Liu and Margerum 2001). As a result of the numerous chemical species that may be formed on contact with water, a release of bromine chloride into the atmosphere may result in human exposure to mixtures of varying composition, depending on the environmental humidity and its pH; physiologic sources of moisture (e.g., sweat, moisture in the upper respiratory tract) may also create localized exposures to mixtures including hydrolysis products.
The vapor density of bromine chloride has not been determined; however, on the basis of molecular weight (115.36 g/mol), bromine chloride vapor is approximately four times heavier than dry air (average molecular weight of 28.96 g/mol at standard temperature and pressure). The chemical and physical properties of bromine chloride are presented in Table 1-2.
Bromine chloride is used as a water-treatment biocide. Its advantages over chlorine include activity over a wider pH range, more rapid disinfection, effectiveness at lower residual concentrations, and lower aquatic toxicity (Frim and Ukeles 2011). Bromine chloride is used in organic synthesis involving addition across olefinic double bonds to produce bromochloro compounds, and for aromatic brominations, where an aromatic bromide and hydrogen chloride are produced. Bromine chloride is also used as a brominating agent in the preparation of fire-retardant chemicals, pharmaceuticals, high density brominated liquids, agricultural chemicals, dyes, and bleaching agents (Frim and Ukeles 2011).
TABLE 1-2 Chemical and Physical Properties of Bromine Chloride
|
|
||
| Parameter | Value | References |
|
|
||
| Synonyms | Bromochloride | HSDB 2011 |
| CAS registry no. | 13863-41-7 | HSDB 2011 |
| Chemical formula | BrCl | HSDB 2011 |
| Molecular weight | 115.36 | HSDB 2011 |
| Physical state | Red-brown liquid at ≤5ºC | HSDB 2011 |
| Melting point | -66ºC | HSDB 2011 |
| Boiling point | 5ºC (decomposes) | HSDB 2011 |
| Solubility in water | Reacts with water | HSDB 2011 |
| Density (water =1) | 2.32 g/L at 25ºC | IPCS 2009 |
| Vapor pressure | 2.368 kPa (17.8 mm Hg) at 25ºC | IPCS 2009 |
| Conversion factors | 1 ppm = 4.72 mg/m3 1 mg/m3 = 0.212 ppm |
|
|
|
||
2. HUMAN TOXICITY DATA
No human data on the odor threshold, lethal concentrations, developmental toxicity, reproductive toxicity, genotoxicity, or carcinogenicity of bromine chloride were found.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
A single, unpublished study of the acute lethality of bromine chloride was found (Dow Chemical Co. 1977). Groups of six male Sprague-Dawley rats were exposed in a 19-L glass cylinder to bromine chloride at nominal concentrations of 550, 960, 2,110, or 2,925 ppm for 7 h. The vapor was metered from a cylinder containing liquid bromine chloride and mixed with clean air before entering the chamber. Flow rates for the vapor and clean air were used to estimate the nominal concentrations. The investigators reported that the vapor had been analyzed and showed 70% chloride and 30% bromine (molar fraction); it is unclear where the sample was taken or how it was analyzed. Relative humidity in the exposure chamber was not reported, but a diagram of the exposure chamber showed that the air supply passed through a desiccant (Drierite scrubber) before entering the chamber, suggesting that the humidity was probably low.
A separate experiment was conducted to measure the actual chamber concentrations, because the rats appeared to have survived exposure at concentrations far above “working tolerance levels.” Six rats were exposed to bromine
chloride at a nominal concentration of 1,100 ppm (estimated on the basis of the mass of bromine chloride liquid lost from the cylinder and air flow rate) for 5 h. Air samples were taken from the gas inlet and from the top, middle, and bottom of the chamber, once per hour; the heights of the three chamber sample inlets were not reported. The air samples were scrubbed through a solution of potassium iodide (1 g/50 mL) and a known amount of 0.025 N sodium thiosulfate (quantity not reported) until the scrubbing solution exhibited a yellow color indicating free iodine; subsequently, the samples were titrated iodometrically to a starch-iodide end point. Total halogen concentration in ppm was reported; the investigators indicated that the halogen concentration was calculated using an assumption of 70% Cl and 30% Br. The halogen concentration estimates presented in Table 1-3 show that the concentration in the bottom of the chamber was roughly twice the concentrations of the middle and top of the chamber.
The investigators estimated the actual exposure concentrations of bromine chloride in the acute lethality study as 4% of the nominal values. That estimate appears to be based on the average concentration in the top and middle chambers (approximately 42-45 ppm) divided by the nominal concentration (1,100 ppm). The actual concentrations were estimated to be 20, 40, 80, and 120 ppm (nominal concentrations of 550, 960, 2,110, and 2,925 ppm, respectively).
In the lethality study, the behavior of the rats was consistent with the observed vapor stratification, as rats tried to breathe the air in the top of the chamber. The report did not indicate the frequency or duration of rearing behavior, nor the dimensions of the inhalation chamber; thus, it is unclear whether the rats were exposed primarily to vapor concentrations corresponding to the bottom, middle, or top of the chamber. However, the estimated concentrations may be conservative, as only the concentrations in the top and middle of the chamber, which were lower than those in the bottom of the chamber, were used in the calculations. Furthermore, because chlorine gas is less dense (vapor density of 1.4 [NRC 2004a]) than bromine (vapor density of 3.5 [NRC 2010]) or bromine chloride (estimated vapor density of approximately 4), the upper portions of the chamber may have contained more chlorine gas than other constituents.
All rats exhibited respiratory problems during and after exposure. At all concentrations, rats lost considerable body weight and recovery to normal was slow. The death of a single rat exposed to bromine chloride at 80 ppm occurred 3 days after exposure; deaths at 120 ppm occurred during the exposure. The primary cause of death was severe upper- and lower-respiratory tract irritation. Mortality and observations over a 14-day period after exposure are presented in Table 1-4.
3.2. Developmental and Reproductive Toxicity
No data on the developmental or reproductive toxicity of bromine chloride were found.
TABLE 1-3 Analytic Measurements of Bromine Chloride in the Test Chamber
| Time of Sample (h) | Concentration (ppm) in Chamber Where Sample Was Takena | |||
| Gas inlet | Top | Middle | Bottom | |
| 1 | 529 | 78a | 58 | 96 |
| 2 | 527 | 53 | 44 | 86 |
| 3 | 544 | 40 | 32 | 98 |
| 4 | 507 | 37 | 40 | 86 |
| 5 | 502 | 32 | 40 | 88 |
| 6 | 530 | – | – | – |
| Average | 523 | 48 (41, excluding sample 1) | 43 | 91 |
aNominal concentration was 1,100 ppm.
bStudy authors believed that this sample was potentially contaminated by the initial inlet sample.
Source: Adapted from Dow Chemical Co. 1977.
TABLE 1-4 Mortality Data and Observations from a Study of Rats Exposed to Bromine Chloride
| Nominal concentration (ppm) | Estimated actual concentration (ppm) | Exposure Duration | Mortality | Observations |
| 550 | 20 | 7 h | 0/6 | Respiratory distress, bloody eyes and noses, yellow fur, and weight loss with slow recovery. |
| 960 | 40 | 5 h | 0/6 | Extreme respiratory irritation, bloody eyes and noses, and yellow fur. |
| 960 | 40 | 7 h | 0/6 | Respiratory distress, bloody eyes and noses, yellow fur, and weight loss with slow recovery. |
| 2,110 | 80 | 7 h | 1/6 | Death on day 3 after exposure; severe respiratory-tract irritation, yellow fur, and considerable weight loss with slow recovery in remaining rats. |
| 2,925 | 120 | 7 h | 5/6 | Deaths during exposure; severe upper- and lower-respiratory tract irritation and subsequent mouth breathing. Yellow fur and extreme weight loss with slow recovery in surviving rat. |
Source: Adapted from Dow Chemical Co. 1977.
3.3. Genotoxicity
No data on the genotoxicity of bromine chloride were found.
3.4. Chronic Toxicity and Carcinogenicity
No data on the chronic toxicity or carcinogenicity of bromine chloride were found.
3.5. Summary
A single study on the lethality of bromine chloride was found. Groups of six male Sprague-Dawley rats were exposed at concentrations of 20, 40, 80, or 120 ppm for 7 h (Dow Chemical Co. 1977). Mortality rates at those concentrations were 0/6, 0/6, 1/6, and 5/6, respectively. All rats experienced respiratory problems during and after the exposure. No data on developmental toxicity, reproductive toxicity, genotoxicity, and chronic toxicity or carcinogenicity of bromine chloride were found.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
No information on the metabolism or disposition of bromine chloride in humans or animals is available.
4.2. Mechanism of Toxicity
Halogens are contact irritants. Death in the single study of bromine chloride was due to severe irritation of the upper- and lower-respiratory tract (Dow Chemical Co. 1977), providing evidence for the direct contact mode of action.
4.3. Structure-Activity Relationships
In the atmosphere, bromine chloride is expected to exist in equilibrium with its dissociation and hydrolysis products, including chlorine, bromine, hydrogen chloride, and hydrogen bromide. Although the data on bromine chloride is sparse, information is available on the toxicity of its dissociation and hydrolysis products, all of which exhibit similar direct-contact irritation modes of action. Table 1-5 shows LC50 (lethal concentration, 50% lethality) values for the four compounds in the mouse and rat, along with the 7-h rat LC50 for bromine chloride. The LC50 values suggest that chlorine and bromine are more toxic than the hydrogenated forms, and that chlorine may be somewhat more toxic than
bromine. In addition, time-scaling the 1-h rat LC50 values for chlorine using the equation Cn × t = k (n =2 [NRC 2004a]) results in estimated 7-h LC50 values of 110-170 ppm, compared with the LC50 of 98 ppm for bromine chloride estimated from the study by Dow Chemical Co. (1977). Thus, on the basis of sparse (and uncertain) data, the lethality of bromine chloride appears to be comparable to that of chlorine.
4.4. Other Relevant Information
4.4.1. Species Variability
No data on species variability in response to bromine chloride were found. For other halogens, the mouse appeared to be slightly more sensitive than the rat (see Table 1-5).
4.4.2. Susceptible Populations
No data on populations susceptible to the effects of bromine chloride were found. Individuals with respiratory diseases or individuals under stress may be more susceptible to the effects of bromine chloride.
TABLE 1-5 Comparison of LC50 Values for Bromine Chloride and Its Dissociation and Hydrolysis Products
| Chemical | 30 min | 1 h | 2 h | 3 h | 6 h | 7 h |
| Mouse | ||||||
| Chlorinea | 127 | 137 | <170 | <10 | - | ~250 |
| Bromineb | 174 | – | 240 | >40 | <22 | >750 |
| Hydrogen chloridec | 2,600 | 1,108 | – | – | – | – |
| Hydrogen bromide | – | 814d | – | – | – | – |
| Rat | ||||||
| Bromine chloride | – | – | – | – | – | 98d |
| Chlorinea | 700 | 293-455 | – | – | – | – |
| Bromineb | – | – | – | – | – | – |
| Hydrogen chloridec | 4,700 | 3,124 | – | – | – | – |
| Hydrogen bromide | >1,300e | 2,858f | – | – | – | – |
aNRC 2004a.
bNRC 2010.
cNRC 2004b.
dDow Chemical Co. 1977; based on estimated actual exposure concentrations.
eStavert et al. 1991.
fMacEwen and Vernot 1972.
4.4.3. Concentration-Exposure Duration Relationship
No data on concentration-exposure duration relationships for bromine chloride were found.
4.4.4. Concurrent Exposure Issues
No data on concurrent exposure issues for bromine chloride were found.
5. DATA ANALYSIS FOR AEGL-1
5.1. Human Data Relevant to AEGL-1
No data on human exposure to bromine chloride were found.
5.2. Animal Data Relevant to AEGL-1
No animal data on bromine chloride relevant to developing AEGL-1 values were found.
5.3. Derivation of AEGL-1 Values
No data relevant to deriving AEGL-1 values for bromine chloride were available. Therefore, AEGL-1 values are not recommended.
6. DATA ANALYSIS FOR AEGL-2
6.1. Human Data Relevant to AEGL-2
No data on human exposure to bromine chloride were found.
6.2. Animal Data Relevant to AEGL-2
Seven-hour exposures of rats to analytically-determined concentrations of bromine chloride at 20, 40, 80, or 120 ppm resulted in mortality rates of 0/6, 0/6, 1/6, and 5/6, respectively (Dow Chemical Co. 1977). Severe clinical signs and respiratory problems were observed at all concentrations. Those effects are more severe than those defined by AEGL-2 values.
6.3. Derivation of AEGL-2 Values
No data relevant to deriving AEGL-2 values for bromine chloride were available. The dose-response curve for bromine chloride is steep, with 0, 17, and
83% mortality at 40, 80, and 120 ppm, respectively (Dow Chemical Company 1977). In accordance with NRC (2001) guidelines for chemicals with steep dose-response curves, the AEGL-2 values were derived by dividing the AEGL-3 values by 3 (see Section 7.3). AEGL-2 values for bromine chloride are presented in Table 1-6; the calculations are presented in Appendix A and a category graph of AEGL values and toxicity data is presented in Appendix B.
7. DATA ANALYSIS FOR AEGL-3
7.1. Human Data Relevant to AEGL-3
No data on human exposure to bromine chloride were found.
7.2. Animal Data Relevant to AEGL-3
Seven-hour exposures of rats to estimated concentrations of bromine chloride at 20, 40, 80, or 120 ppm resulted in mortality rates of 0/6, 0/6, 1/6, and 5/6, respectively (Dow Chemical Co. 1977). Severe clinical signs and respiratory problems were observed at all concentrations. The death at 80 ppm occurred 3 days after exposure.
7.3. Derivation of AEGL-3 Values
Benchmark concentration analysis was applied to the Dow Chemical Co. (1977) data to estimate the NOAEL for lethality (NRC 2001). The data yielded a 7-h BMCL05 of 39.4 ppm and BMC01 of 60.2 ppm (see Appendix C). The BMCL05 of 39.4 ppm was selected as the point-of-departure. A total uncertainty factor of 10 was applied; a factor of 3 for interspecies differences and a factor of 3 for intraspecies variability. The effects of direct-acting irritants like bromine chloride are not expected to differ significantly between species or among individuals (NRC 2001). A modifying factor of 3 was applied to account for the sparse data on bromine chloride and the uncertainty in the exposure concentrations in the Dow Chemical study. Time scaling was performed using the equation Cn × t = k. Data on bromine chloride were inadequate to derive an empirical value for n, so default values of n = 3 for extrapolating to shorter durations and n = 1 for extrapolating to longer durations were used (NRC 2001). Because of the uncertainty associated with time scaling a 7-h point-of-departure to a 10-min value, the 10-min AEGL-3 value was set equal to the 30-min value. AEGL-3 values for bromine chloride are presented in Table 1-7; the calculations are presented in Appendix A and a category graph of AEGL values and toxicity data is presented in Appendix B.
8. SUMMARY OF AEGLS
8.1. AEGL Values and Toxicity End Points
AEGL values for bromine chloride are presented in Table 1-8, and a summary of the derivations is provided in Appendix D.
8.2. Other Standards and Guidelines
There are no other standards or guidelines for bromine chloride. AEGL values for the dissociation and hydrolysis products of bromine chloride (including chlorine, bromine, hydrogen chloride, and hydrogen bromide) are presented in Table 1-9 for comparison with the values derived for bromine chloride. The comparison suggests that the AEGLs for bromine chloride, which are lower than those of chlorine, should be protective. Although bromine appears to be somewhat less toxic than chlorine (see Table 1-5), the AEGL-3 values for bromine are lower than those for chlorine as a consequence of the less robust database on bromine.
8.3. Data Adequacy and Research
The database on bromine chloride is sparse. Only a single, unpublished acute lethality study is available (Dow Chemical Co. 1977). The exposure concentrations in the study are uncertain as a result of vapor stratification in the chamber and lack of concentration measurements during the study. The AEGL values derived for bromine chloride are supported by comparison to AEGL values for its dissociation and hydrolysis products. However, additional studies of the acute toxicity of bromine chloride, with analysis of actual exposure concentrations and speciation of the compounds in the exposure chamber, should be conducted to refine the AEGL-3 values and provide data relevant to AEGL-2 and AEGL-1 end points. Additional studies comparing the acute toxicity of bromine chloride with that of its dissociation and hydrolysis products would also be beneficial.
TABLE 1-6 AEGL-2 Values for Bromine Chloride
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 1.1 ppm (5.2 mg/m3) |
1.1 ppm (5.2 mg/m3) |
0.83 ppm (3.9 mg/m3) |
0.53 ppm (2.5 mg/m3) |
0.37 ppm (1.7 mg/m3) |
TABLE 1-7 AEGL-3 Values for Bromine Chloride
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 3.2 ppm (15 mg/m3) |
3.2 ppm (15 mg/m3) |
2.5 ppm (12 mg/m3) |
1.6 ppm (7.6 mg/m3) |
1.1 ppm (5.2 mg/m3) |
TABLE 1-8 AEGL Values for Bromine Chloride
| Classification | Exposure Duration | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h | |
| AEGL-1 (nondisabling) |
NRa | NRa | NRa | NRa | NRa |
| AEGL-2 (disabling) |
1.1 ppm (5.2 mg/m3) |
1.1 ppm (5.2 mg/m3) |
0.83 ppm (3.9 mg/m3) |
0.53 ppm (2.5 mg/m3) |
0.37 ppm (1.7 mg/m3) |
| AEGL-3 (lethal) |
3.2 ppm (15 mg/m3) |
3.2 ppm (15 mg/m3) |
2.5 ppm (12 mg/m3) |
1.6 ppm (7.6 mg/m3) |
1.1 ppm (5.2 mg/m3) |
aNot recommended. Absence of an AEGL-1 value does not imply that exposures below the AEGL-2 value are without adverse effects.
TABLE 1-9 AEGL Values for Bromine Chloride and Its Dissociation and Hydrolysis Products
| Classification | Exposure Duration | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h | |
| Bromine Chloride | |||||
| AEGL-1 | NRa | NRa | NRa | NRa | NRa |
| AEGL-2 | 1.1 ppm | 1.1 ppm | 0.83 ppm | 0.53 ppm | 0.37 ppm |
| AEGL-3 | 3.2 ppm | 3.2 ppm | 2.5 ppm | 1.6 ppm | 1.1 ppm |
| Chlorine (NRC 2004a) | |||||
| AEGL-1 | 0.50 ppm | 0.50 ppm | 0.50 ppm | 0.50 ppm | 0.50 ppm |
| AEGL-2 | 2.8 ppm | 2.8 ppm | 2.0 ppm | 1.0 ppm | 0.70 ppm |
| AEGL-3 | 50 ppm | 28 ppm | 20 ppm | 10 ppm | 7.1 ppm |
| Bromine (NRC 2010) | |||||
| AEGL-1 | 0.033 ppm | 0.033 ppm | 0.033 ppm | 0.033 ppm | 0.033 ppm |
| AEGL-2 | 0.55 ppm | 0.33 ppm | 0.24 ppm | 0.13 ppm | 0.095 ppm |
| AEGL-3 | 19 ppm | 12 ppm | 8.5 ppm | 4.5 ppm | 3.3 ppm |
| Hydrogen Chloride (NRC 2004b) | |||||
| AEGL-1 | 1.8 ppm | 1.8 ppm | 1.8 ppm | 1.8 ppm | 1.8 ppm |
| AEGL-2 | 100 ppm | 43 ppm | 22 ppm | 11 ppm | 11 ppm |
| AEGL-3 | 620 ppm | 210 ppm | 100 ppm | 26 ppm | 26 ppm |
| Hydrogen Bromide (NRC 2014) | |||||
| AEGL-1 | 1.0 ppm | 1.0 ppm | 1.0 ppm | 1.0 ppm | 1.0 ppm |
| AEGL-2 | 250 ppm | 83 ppm | 40 ppm | 10 ppm | 5 ppm |
| AEGL-3 | 740 ppm | 250 ppm | 120 ppm | 31 ppm | 15 ppm |
aNot recommended. Absence of an AEGL-1 value does not imply that exposures below the AEGL-2 value are without adverse effects.
9. REFERENCES
Barrie, M.D., D.L. Dahlstrom, E. Goswami, and R. Kaetzel. 2012. The halogens. Pp. 1033-1108 in Patty’s Industrial Hygiene and Toxicology, 6th Ed., E. Bingham, and B. Cohrssen, eds New York: Wiley.
Cotton, F.A., and G. Wilkinson. 1980. Advanced Inorganic Chemistry: A Comprehensive Text, 4th Ed. New York: John Wiley and Sons.
Dagani, M.J., H.J. Barda, T.J. Benya, and D. C. Sanders. 2000. Bromine compounds. In Ullmann’s Encyclopedia of Industrial Chemistry, New York: John Wiley and Sons.
Dow Chemical Co. 1977. Evaluation of Acute Inhalation Toxicity of Bromine Chloride in Rats. Dow Report No. 77 2993. Submitted to EPA by Dow Chemical Company, Midland, MI, with Cover Letter Dated 05/28/92. EPA Document No. 88-920002267.
Frim, R., and S.D. Ukeles. 2011. Bromine, inorganic compounds. In Kirk-Othmer Encyclopedia of Chemical Technology. New York: John Wiley and Sons.
IPCS (International Programme on Chemical Safety). 2009. Bromine chloride (CAS Reg. No. 13863-41-7). International Chemical Safety Card 1713. International Programme on Chemical Safety and the Commission of the European Communities [online]. Available: http://www.ilo.org/dyn/icsc/showcard.display?p_lang=en&p_card_id=1713 [accessed June 20, 2014].
HSDB (Hazardous Substances Data Bank). 2011. Bromine Chloride. TOXNET Toxicology Data Network, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/ [accessed June 20, 2014].
Lang, J.P. 2006. Chlorine, bromine, iodine, and astatine: Inorganic chemistry. In Encyclopedia of Inorganic Chemistry. New York: John Wiley and Sons.
Liu, Q., and D.W. Margerum. 2001. Equilibrium and kinetics of bromine chloride hydrolysis. Environ. Sci. Technol. 35(6):1127-1133.
MacEwen, J.D., and E.H. Vernot. 1972. Toxic Hazards Research Unit Annual Technical Report: 1972. AMRL-TR-72-62, AD 755 358, Aerospace Medical Research Laboratory, Wright-Patterson Air Force Base, OH [online]. Available: http://www.dtic.mil/dtic/tr/fulltext/u2/755358.pdf [accessed June 20, 2014].
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
NRC (National Research Council). 2004a. Chlorine. Pp. 11-76 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 4. Washington, DC: The National Academies Press.
NRC (National Research Council). 2004b. Hydrogen chloride. Pp. 77-122 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 4. Washington, DC: The National Academies Press.
NRC (National Research Council). 2010. Bromine. Pp. 13-45 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 9. Washington, DC: The National Academies Press.
NRC (National Research Council). 2014. Hydrogen bromide. Pp. 429-457 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 17. Washington, DC: The National Academies Press.
Stavert, D.M., D.C. Archuleta, M.J. Behr, and B.E. Lehnert. 1991. Relative acute toxicities of hydrogen fluoride, hydrogen chloride, and hydrogen bromide in nose- and pseudo-mouth-breathing rats. Fundam. Appl. Toxicol. 16(4):636-655.
APPENDIX A
DERIVATION OF AEGL VALUES
Derivation of AEGL-1 Values
Data on bromine chloride were insufficient to derive AEGL-1 values; therefore, AEGL-1 values are not recommended.
Derivation of AEGL-2 Values
The AEGL-2 values for bromine chloride were derived by dividing the AEGL-3 values by 3.
| 10-min AEGL-2: | 3.2 ppm ÷ 3 = 1.1 ppm |
| 30-min AEGL-2: | 3.2 ppm ÷ 3 = 1.1 ppm |
| 1-h AEGL-2: | 2.5 ppm ÷ 3 = 0.83 ppm |
| 4-h AEGL-2: | 1.6 ppm ÷ 3 = 0.53 ppm |
| 8-h AEGL-2: | 1.1 ppm ÷ 3 = 0.37 ppm |
Derivation of AEGL-3 Values
| Key study: | Dow Chemical Co. 1977. Evaluation of Acute Inhalation Toxicity of Bromine Chloride in Rats. Dow Report No. 77 2993. Submitted to EPA by Dow Chemical Company, Midland, MI, with Cover Letter Dated 05/28/92. EPA Document No. 88-920002267. |
| Toxicity end point: | Lethality threshold, BMCL05 of 39.4 ppm for a 7-h exposure (see Appendix C) |
| Time scaling: | Cn × t = k; default values of n = 3 for extrapolating to shorter durations and n = 1 for extrapolating to longer durations (NRC 2001) (39.4 ppm ÷ 30)3 × 7 h = 15.85707 ppm-h (39.4 ppm/30)1 × 7 h = 9.19333 ppm-h |
| Uncertainty factors: | Total uncertainty factor: 10 Interspecies: 3, because the mechanism of action of direct-acting irritants is not expected to differ greatly among species. |
| Intraspecies: 3, because the mechanism of action of direct-acting irritants is not expected to differ greatly among individuals. | |
| Modifying factor: | 3, to account for sparse database and uncertainty associated with the exposure concentrations in the key study. |
| Calculations: | |
| 10-min AEGL-3: | Set equal to the 30-min AEGL-3 value of 3.2 ppm, because of the uncertainty associate with time-scaling a 7-h point-of-departure to a 10-min value. |
| 30-min AEGL-3: | (15.85707 ppm-h ÷ 0.5 h)1/3 C = 3.2 ppm |
| 1-h AEGL-3: | (15.85707 ppm-h ÷ 1 h)1/3 C = 2.5 ppm |
| 4-h AEGL-3: | (15.85707 ppm-h ÷ 4 h)1/3 C = 1.6 ppm |
| 8-h AEGL-3: | (9.19333 ppm-h ÷ 8 h) C = 1.1 ppm |
APPENDIX B
CATEGORY PLOT FOR BROMINE CHLORIDE

FIGURE B-1 Category plot of toxicity data and AEGL values for bromine chloride.
TABLE B-1 Data Used in Category Plot for Bromine Chloride
| Source | Species | ppm | Minutes | Category |
| AEGL-2 | 1.1 | 10 | AEGL | |
| AEGL-2 | 1.1 | 30 | AEGL | |
| AEGL-2 | 0.83 | 60 | AEGL | |
| AEGL-2 | 0.53 | 240 | AEGL | |
| AEGL-2 | 0.37 | 480 | AEGL | |
| AEGL-3 | 3.2 | 10 | AEGL | |
| AEGL-3 | 3.2 | 30 | AEGL | |
| AEGL-3 | 2.5 | 60 | AEGL | |
| AEGL-3 | 1.6 | 240 | AEGL | |
| AEGL-3 | 1.1 | 480 | AEGL | |
| Dow Chemical Co. 1977 | Rat | 20 | 420 | 2, respiratory distress |
| Rat | 40 | 420 | 2, respiratory distress | |
| Rat | 40 | 300 | 2, extreme respiratory irritation | |
| Rat | 80 | 420 | SL (1/6) | |
| _Rat | 120 | 420 | _SL (5/6) | |
For category: 0 = no effect, 1 = discomfort, 2 = disabling, SL = some lethality, 3 = lethality.
APPENDIX C
DERIVATION OF BENCHMARK
CONCENTRATION FOR BROMINE CHLORIDE
Probit Model. (Version: 3.2; Date: 10/28/2009)
Input Data File: C:/Users/hclynch.ESC1/Documents/BMDS
220/Data/lnp_Dax_Setting.(d)
Gnuplot Plotting File: C:/Users/hclynch.ESC1/Documents/BMDS
220/Data/lnp_Dax_Setting.plt
Wed Sep 11 12:38:40 2013
BMDS_Model_Run
The form of the probability function is:
P[response] = Background
+ (1-Background) * CumNorm(Intercept+Slope*Log(Dose)), where CumNorm(.) is the cumulative normal distribution function
Dependent variable = Effect
Independent variable = Dose
Slope parameter is not restricted
Total number of observations = 3
Total number of records with missing values = 0
Maximum number of iterations = 250
Relative Function Convergence has been set to: 1e-008
Parameter Convergence has been set to: 1e-008
User has chosen the log transformed model
Default Initial (and Specified) Parameter Values
Background = 0
Intercept = -9.28868
Slope = 2.05319
Asymptotic Correlation Matrix of Parameter Estimates
(***The model parameter(s) -background have been estimated at a boundary point, or have been specified by the user, and do not appear in the correlation matrix)
| intercept | slope | |
| intercept | 1 | -1 |
| slope | -1 | 1 |
Parameter Estimates
| 95.0% Wald Confidence Interval | ||||
| Variable | Estimate | Standard Error | Lower Conf. Limit | Upper Conf. Limit |
| Background | 0 | NA | ||
| Intercept | -21.8829 | 9.72809 | -40.9496 | -2.81617 |
| slope | 4.77295 | 2.11983 | 0.61815 | 8.92775 |
NA - Indicates that this parameter has hit a bound implied by some inequality constraint and thus has no standard error.
Analysis of Deviance Table
| Model | Log (likelihood) | No. Parameters | Deviance | Test d.f. | P-value |
| Full model | -5.40673 | 3 | |||
| Fitted model | -5.40679 | 2 | 0.000114402 | 1 | 0.9915 |
| Reduced model | -11.4573 | 1 | 12.101 | 2 | 0.002357 |
AIC: 14.8136
Goodness of Fit
| Dose | Estimated Probability | Expected | Observed | Size | Scaled Residual |
| 40.0000 | 0.0000 | 0.000 | 0.000 | 6 | -0.008 |
| 80.0000 | 0.1666 | 1.000 | 1.000 | 6 | 0.000 |
| 120.0000 | 0.8334 | 5.000 | 5.000 | 6 | -0.000 |
Chi-square = 0.00 d.f. = 1 P-value = 0.9940
Benchmark Dose Computation
Specified effect = 0.05
Risk Type = Extra risk
Confidence level = 0.95
BMD = 69.4182
BMDL = 39.372
Probit Model. (Version: 3.2; Date: 10/28/2009)
Input Data File: C:/Users/hclynch.ESC1/Documents/BMDS
220/Data/lnp_Dax_Setting.(d)
Gnuplot Plotting File: C:/Users/hclynch.ESC1/Documents/BMDS
220/Data/lnp_Dax_Setting.plt
Wed Sep 11 12:39:15 2013
BMDS_Model_Run
The form of the probability function is:
P[response] = Background + (1-Background) *
CumNorm(Intercept+Slope*Log(Dose)),
where CumNorm(.) is the cumulative normal distribution function
Dependent variable = Effect
Independent variable = Dose
Slope parameter is not restricted
Total number of observations = 3
Total number of records with missing values = 0
Maximum number of iterations = 250
Relative Function Convergence has been set to: 1e-008
Parameter Convergence has been set to: 1e-008
User has chosen the log transformed model
Default Initial (and Specified) Parameter Values
background = 0
intercept = -9.28868
slope = 2.05319
Asymptotic Correlation Matrix of Parameter Estimates
(***The model parameter(s) -background have been estimated at a boundary point, or have been specified by the user, and do not appear in the correlation matrix)
| intercept | slope | |
| intercept | 1 | -1 |
| slope | -1 | 1 |
Parameter Estimates
| 95.0% Wald Confidence Interval | |||||
| Variable | Estimate | Standard Error | Lower Conf. Limit | Upper Conf. Limit | |
| Background | 0 | NA | |||
| Intercept | -21.8829 | 9.72809 | -40.9496 | -2.81617 | |
| slope | 4.77295 | 2.11983 | 0.61815 | 8.92775 | |
NA - Indicates that this parameter has hit a bound implied by some inequality constraint and thus has no standard error.
Analysis of Deviance Table
| Model | Log (likelihood) | No. Parameters | Deviance | Test d.f. | P-value |
| Full model | -5.40673 | 3 | |||
| Fitted model | -5.40679 | 2 | 0.000114402 | 1 | 0.9915 |
| Reduced model | -11.4573 | 1 | 12.101 | 2 | 0.002357 |
AIC: 14.8136
Goodness of Fit
| Dose | Estimated Probability | Expected | Observed | Size | Scaled Residual |
| 40.0000 | 0.0000 | 0.000 | 0.000 | 6 | -0.008 |
| 80.0000 | 0.1666 | 1.000 | 1.000 | 6 | 0.000 |
| 120.0000 | 0.8334 | 5.000 | 5.000 | 6 | -0.000 |
Chi-square = 0.00 d.f. = 1 P-value = 0.9940
Benchmark Dose Computation
Specified effect = 0.01
Risk Type = Extra risk
Confidence level = 0.95
BMD = 60.1816
BMDL = 27.4878
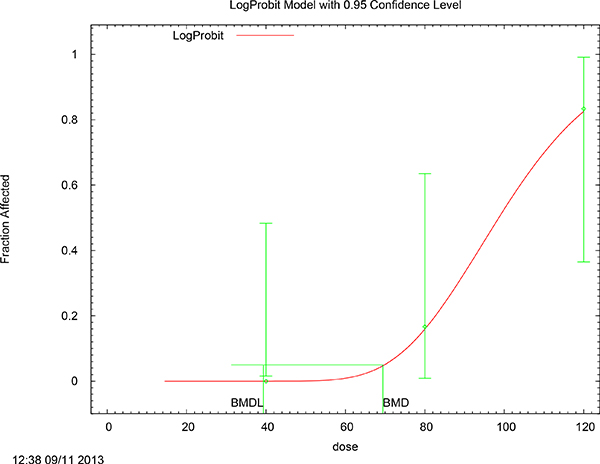
FIGURE C-1 LogProbit model with 0.95 confidence level.
APPENDIX D
ACUTE EXPOSURE GUIDELINE LEVELS FOR BROMINE CHLORIDE
Derivation Summary
AEGL-1 VALUES
Data on bromine chloride were insufficient to derive AEGL-1 values; therefore, AEGL-1 values are not recommended.
AEGL-2 VALUES
|
|
||||
| 10 min | 30 min | 1 h | 4 h | 8 h |
|
|
||||
| 1.1 ppm | 1.1 ppm | 0.83 ppm | 0.53 ppm | 0.37 ppm |
|
|
||||
| Data adequacy: The database on bromine chloride was inadequate for deriving AEGL-2 values. However, because bromine chloride has a steep dose-response curve (0% mortality at 40 ppm and almost 100% mortality at 120 ppm), the AEGL-2 values were derived by dividing the AEGL-3 values by 3 (NRC 2001). | ||||
|
|
||||
AEGL-3 VALUES
|
|
||||
| 10 min | 30 min | 1 h | 4 h | 8 h |
|
|
||||
| 3.2 ppm | 3.2 ppm | 2.5 ppm | 1.6 ppm | 1.1 ppm |
|
|
||||
| Key reference: Dow Chemical Co. 1977. Evaluation of Acute Inhalation Toxicity of Bromine Chloride in Rats. Dow Report No. 77 2993 EPA Document No.: 88-920002267. | ||||
|
|
||||
| Test species/Strain/Number: Rat; Sprague-Dawley; 6 males/group | ||||
|
|
||||
| Exposure route/Concentrations/Durations: Inhalation; 0, 40, 80, or 120 ppm for 7 h | ||||
|
|
||||
| Effects: Mortality | ||||
| 20 ppm: 0/6 | ||||
| 40 ppm: 0/6 | ||||
| 80 ppm: 1/6 (death 3 days after exposure) | ||||
| 120 ppm: 5/6 (deaths during exposure) | ||||
|
|
||||
| End point/Concentration/Rationale: Approximate threshold for death, BMCL05 of 39.4 ppm for a 7-h exposure | ||||
|
|
||||
| Uncertainty factors/Rationale: | ||||
| Total uncertainty factor: 10 | ||||
| Interspecies: 3, because the mechanism of action of direct-acting irritants is not expected to differ greatly among species. | ||||
| Intraspecies: 3, because the mechanism of action of direct-acting irritants in not expected to differ greatly among individuals. | ||||
|
|
||||
| Modifying factor: 3, to account for the sparse database and uncertainty in the exposure concentrations in the key study. | ||||
|
|
||||
| Animal-to-human dosimetric adjustment: Not applied | ||||
|
|
||||
| Time scaling: Cn × t = k; default values of n = 3 for extrapolating to shorter durations and n = 1 for extrapolating to longer durations (NRC 2001). The 30-min value was adopted as the 10-min value. | ||||
|
|
||||
| Data adequacy: The database on lethality from exposure to bromine chloride was considered adequate. The values are supported by the rich database on lethality for the related chemical chlorine. | ||||
|
|
||||























