Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 18 (2014)
Chapter: 2 Carbonyl Fluoride
2
Carbonyl Fluoride1
Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
_____________________
1This document was prepared by the AEGL Development Team composed of Jennifer Rayner (Oak Ridge National Laboratory), Julie Klotzbach (SRC, Inc.), Chemical Manager Iris Camacho (National Advisory Committee [NAC] on Acute Exposure Guideline Levels for Hazardous Substances), and Ernest V. Falke (U.S. Environmental Protection Agency). The NAC reviewed and revised the document and AEGLs as deemed necessary. Both the document and the AEGL values were then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC committee has concluded that the AEGLs developed in this document are scientifically valid conclusions based on the data reviewed by the NRC and are consistent with the NRC guidelines reports (NRC 1993, 2001).
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure concentrations that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold concentrations for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Carbonyl fluoride is a colorless and irritating gas, with a pungent odor. It is hygroscopic, and is hydrolyzed into carbon dioxide and hydrogen fluoride by water. It is used as an intermediate in the synthesis of organic compounds. The thermal decomposition of fluoropolymers, such as polytetrafluoroethylene and polyfluoroethylenepropylene, is a major source of exposure because carbonyl fluoride is the major reaction product from the rapid destruction of plastic materials at temperatures above 500°C. Pyrolysis products are composed of a large number of compounds, can be of variable composition, and pose significant analytic challenges. Pyrolysis products of polytetrafluoroethylene include a number of highly toxic compounds in addition to carbonyl fluoride, including perfluoroisobutylene, which is approximately 10-fold more toxic than phosgene (Patocka and Bajgar 1998; IPCS 2004).
Carbonyl fluoride is a strong irritant of the eyes and respiratory tract. Its irritancy is hypothesized to be due to hydrogen fluoride, a known sensory irritant. However, the toxicity of carbonyl fluoride is greater than that of hydrogen fluoride, and may be the result of the chemical’s deep penetration into the lungs as well as the production of hydrogen fluoride. No data on exposure of humans to carbonyl fluoride were found.
No AEGL-1 values for carbonyl fluoride were derived because of insufficient data. Data were also inadequate to derive AEGL-2 values. According to the standing operating procedures for deriving AEGL values (NRC 2001), AEGL-3 values may be divided by 3 to estimate AEGL-2 values. That approach is justified because carbonyl fluoride appears to have a steep concentration-
response curve. Rats exposed to carbonyl fluoride at 5 or 10 ppm for 4 h experienced dyspnea and rapid, shallow respiration (DuPont 1956, 1959). At concentrations of 26.7 ppm or higher for 4 h death occurred (DuPont 1976).
The AEGL-3 values for carbonyl fluoride were derived by using the BMCL05 (benchmark concentration, 95% lower confidence limit with 5% response) of 5.2 ppm from a study in rats (DuPont 1976) as the point-of-departure. Rapid to convulsive respiration and pulmonary edema were observed in rats exposed to carbonyl fluoride for 4 h. Death occurred at all concentrations tested. An interspecies uncertainty factor of 3 was applied, because the toxicity of a direct-acting irritant in not expected to differ greatly among species. A study by Scheel et al. (1968a) provides some support for a factor of 3. However, carbonyl fluoride was generated via polytetrafluoroethylene pyrolysis in that study; therefore, exposure included other pyrolysis products. Exposure of rats to carbonyl fluoride at 310 ppm resulted in focal hemorrhage of the lungs and pulmonary edema, observed 24 h after exposure. The investigators stated that those effects were produced at the same concentration in other species, including the dog, rabbit, guinea pig, and mouse, although individual data and photomicrographs of the lungs were not provided for those species. As noted earlier, carbonyl fluoride also has a steep concentration-response curve. An intraspecies uncertainty factor of 3 was applied because effects from a direct-acting irritant of the lungs and respiratory tract are not expected to differ greatly among individuals. Time scaling was performed using the equation Cn × t = k, where the exponent n ranges from 0.8 to 3.5 (ten Berge et al. 1986). Data on carbonyl fluoride were inadequate to derive an empirical value for n, so default values of n = 3 for extrapolating to shorter durations and n = 1 when extrapolating to longer durations were used (NRC 2001). The 30-min AEGL-3 value was adopted for the 10-min value in accordance with the standing operating procedures for developing AEGL values (NRC 2001).
The AEGL values for carbonyl fluoride are presented in Table 2-1.
TABLE 2-1 AEGL Values for Carbonyl Fluoride
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) |
| AEGL-1 (nondisabling) |
NRa | NRa | NRa | NRa | NRa | Insufficient data. |
| AEGL-2 (disabling) |
0.35 ppm (0.95 mg/m3) |
0.35 ppm (0.95 mg/m3) |
0.28 ppm (0.76 mg/m3) |
0.17 ppm (0.46 mg/m3) |
0.087 ppm (0.23 mg/m3) |
One-third of the AEGL-3 values (NRC 2001) |
| AEGL-3 (lethal) |
1.0 ppm (2.7 mg/m3) |
1.0 ppm (2.7 mg/m3) |
0.83 ppm (2.2 mg/m3) |
0.52 ppm (1.4 mg/m3) |
0.26 ppm (0.70 mg/m3) |
4-h rat BMCL05 (DuPont 1976) |
aNot recommended. Absence of an AEGL-1 value does not imply that exposures below the AEGL-2 value are without adverse effects.
1. INTRODUCTION
Carbonyl fluoride is a colorless, pungent, and irritating gas. It is hygroscopic, and is hydrolyzed by water (HSDB 2009). Chemical and physical properties of carbonyl fluoride are presented in Table 2-2. Carbonyl fluoride can be prepared from fluorine or bromine trifluoride and carbon monoxide. Alternately, it can be prepared by the action of silver fluoride on carbon monoxide or through the reaction of phosgene with sodium fluoride and hydrogen cyanide. It is a thermal decomposition product of fluoropolymers, such as polytetrafluoroethylene and polyfluoroethylenepropylene, heated at temperatures above 500°C. Pyrolysis products are composed of a large number of compounds, can be of variable composition, and pose significant analytic challenges. For polytetrafluoroethylene, pyrolysis products include a number of highly toxic compounds in addition to carbonyl fluoride, including perfluoroisobutylene, which is approximately 10-fold more toxic than phosgene (Patocka and Bajgar 1998; IPCS 2004). Carbonyl fluoride is used as a chemical intermediate in the synthesis of organic compounds, such as fluorinated alkyl isocyanates (HSDB 2009). Recent production data were not found. Carbonyl fluoride is shipped as a liquefied compressed gas (NIOSH 2011a).
TABLE 2-2 Chemical and Physical Properties of Carbonyl Fluoride
|
|
||
| Parameter | Value | References |
|
|
||
|
Synonyms |
Carbon difluoride oxide; carbon fluoride oxide; carbonic difluoride; carbon oxyfluoride; carbonyl fluoride, difluoroformaldehyde; fluophosgene; fluoroformyl fluoride; fluorophosgene |
HSDB 2009 |
|
CAS registry no. |
353-50-4 |
HSDB 2009 |
|
Chemical formula |
COF2 |
NIOSH 2011a |
|
Molecular weight |
66.007 |
HSDB 2009 |
|
Physical state |
Colorless gas |
HSDB 2009 |
|
Melting point |
-111.26°C |
HSDB 2009 |
|
Boiling point |
-84.57°C |
HSDB 2009 |
|
Solubility in water |
Unstable in presence of water, very hygroscopic |
HSDB 2009 |
|
Vapor density (air = 1) |
2.29 |
NIOSH 2011a |
|
Vapor pressure |
4.45 × 104 mm Hg at 25°C |
HSDB 2009 |
|
Flammability limits |
Nonflammable |
NIOSH 2011a |
|
Conversion factors |
1 ppm = 2.7 mg/m3 |
NIOSH 2011a |
|
|
||
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
No reports of human lethality following exposure to carbonyl fluoride were found.
2.2. Nonlethal Toxicity
The odor of carbonyl fluoride is described as pungent and irritating (NIOSH 2011a), but no information on the odor threshold was available. No case reports or epidemiologic studies of exposure to carbonyl fluoride were found.
2.3. Developmental and Reproductive Toxicity
No data regarding the developmental or reproductive toxicity of carbonyl fluoride in humans were found.
2.4. Genotoxicity
No data regarding the genotoxicity of carbonyl fluoride in humans were found.
2.5. Carcinogenicity
No data regarding the carcinogenicity of carbonyl fluoride in humans were found.
2.6. Summary
No information on human exposure to carbonyl fluoride was available. Carbonyl fluoride is a strong irritant to the skin, eyes, mucous membranes, and respiratory tract; direct contact with the skin may cause frostbite (HSDB 2009).
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
3.1.1. Rats
Groups of two male ChR-CD rats were exposed to carbonyl fluoride by inhalation at nominal concentrations of 5 or 10 ppm and a group of six rats was exposed at 100 ppm for 4 h. Three rats exposed at 100 ppm died, and pathologic examination showed they had acute tracheobronchitis and pulmonary congestion. The surviving rats exhibited no pathologic changes. The 4-h LC50 (lethal
Scheel et al. (1968a) evaluated the acute inhalation toxicity of carbonyl fluoride in groups of five male and five female Greenacres Controlled Flora rats (8 and 24 weeks old). Carbonyl fluoride was generated by polytetrafluoroethylene pyrolysis at 550ºC. The authors referenced work by Coleman et al. (1968), which identified carbonyl fluoride as a principal toxic component of the pyrolysis gases, as their rationale for using polytetrafluoroethylene pyrolysis to produce carbonyl fluoride. Atmospheres were generated with a metered air stream into the exposure chamber, and concentrations were measured by the hydrolysable fluoride method. Rats were exposed at various concentrations with the lowest being 310 ppm for 1 h, followed by a 14-day observation period. (With the exception of the 310-ppm value, actual concentrations were not provided). Deaths usually occurred within 24 h with few latent deaths. The LC50 values for the 8- and 24-week-old rats were 360 and 460 ppm, respectively. Although an age difference in mortality was apparent, no difference between the sexes was found. Exposure at 310 ppm resulted in focal hemorrhage of the lungs and pulmonary edema, observed 24 h after exposure. The authors stated that those effects were produced at the same concentration in other species, including the dog, rabbit, guinea pig, and mouse, although individual data and photomicrographs of the lungs were not provided for those species. The lungs showed rapid cellular reorganization and clearing of edema 48 h after exposure, but alveolar damage was still present. Extravasation of red cells from damaged capillaries continued for up to 7 days; the effect was accompanied by mild interstitial fibrosis. Although data were not provided, Scheel et al. (1968a) reported that a 4-h exposure at 90 ppm also resulted in approximately 50% mortality.
DuPont (1976) exposed male ChR-CD rats (10/group) to carbonyl fluoride (>97% pure) at 26.7, 30.8, 32.7, 41.3, 44.7, 47.2 (48.8 by infra-red analysis), or 47.6 ppm for 4 h. Test atmospheres were analyzed with a fluoride-specific electrode and confirmed by infra-red analysis. Deaths occurred at every concentration. Mortalities in the respective groups were 5/10, 3/10, 3/10, 6/10, 8/10, 9/10, and 6/10. The calculated LC50 was 34.3 ppm. The calculated BMC01 was 10.4 ppm and the BMCL05 was 5.2 ppm. Respiration in the rats varied directly with exposure concentration and ranged from rapid shallow breathing to convulsive respiration. Pathologic examination revealed white plaques, red focal spots, and consolidation and edema of the lungs. Liver congestion and bright red spleens were also found.
3.2. Nonlethal Toxicity
3.2.1. Rats
Groups of four male albino rats were exposed to carbonyl fluoride at nominal concentrations of 2.5 or 5 ppm for 2 and 2.5 h in a preliminary investigation
of toxicity (DuPont 1956). The rats were then observed for 24 h or 8 days. The low concentration of 2.5 ppm was not lethal to the rats and no clinical signs developed. At 5 ppm, the rats developed slight dyspnea and cyanosis. No other information was reported.
In other studies conducted by DuPont (1959), groups of two male ChR-CD rats were exposed to carbonyl fluoride at nominal concentrations of 5 or 10 ppm and a group of six rats was exposed at 100 ppm for 4 h. Clinical signs included rapid, shallow respiration and loss of weight in the 5- and 10-ppm groups, but no pathologic changes were found. The data were presented in a table of a one-page preliminary report.
TABLE 2-3 Summary of Acute Inhalation Data in Laboratory Animals
| Species (age) | Concentration (ppm) | Exposure Duration | Effect | Reference |
| Rat | 2.5a | 2, 2.5 h | None0 | DuPont 1956 |
| 5.0a | 2, 2.5 h | Slight dyspnea and cyanosis | ||
| Rat | 5a | 4 h | Rapid, shallow respiration | DuPont 1959 |
| 10a | 4 h | Rapid, shallow respiration | ||
| 100a | 4 h | LC50, pulmonary congestion | ||
| Rat (8 wk) | 360 | 1 h | LC50 | Scheel et al. 1968ab |
| Rat (24 wk) | 460 | 1 h | LC50 | |
| Rat (8 wk) | 90 | 4 h | LC50 | |
| Rat | 26.7 | 4 h | 50% mortality | DuPont 1976 |
| 30.8 | 4 h | 30% mortality | ||
| 32.7 | 4 h | 30% mortality | ||
| 34.3 | 4 h | LC50 (calculated) | ||
| 41.3 | 4 h | 60% mortality | ||
| 44.7 | 4 h | 80% mortality | ||
| 47.2 (48.8 IRc) | 4 h | 90% mortality | ||
| 47.6 | 4 h | 60% mortality | ||
aNominal concentrations.
bExposed rats to polytetrafluoroethylene pyrolysis products (550ºC) and reported concentrations of measured fluoride.
cMeasurement by infrared analysis.
3.3. Repeated Dose Toxicity
Scheel et al. (1968b) examined whether the toxic action of carbonyl fluoride is due to the toxicity of hydrogen fluoride. Twenty male and 20 female rats (Greenacres Controlled Flora) were exposed to polytetrafluoroethylene pyrolysis products (temperature not reported) for 1 h per day for 5 days. Although the authors state that the exposures were to carbonyl fluoride at 50 ppm, the total exposure reported of 158 ppm-h and graphic data presented in the report indicate that successive daily exposures were at concentrations of 52, 43, 29, 25, and 9 ppm. Urine was collected and analyzed for fluoride, tissues were collected and analyzed for inhibition of succinic dehydrogenase, and urine was analyzed for protein, glucose, ketones, and occult blood. After 5 days of exposure (158 ppm-h plus 18 g of particulates), mortality was 22%; rats died during or shortly after exposure, and rats that died exhibited extreme malaise and weakness. No deaths occurred until the third day. Urinary fluoride increased from 3 to 42 mg/L in 5 days. Eighteen days after the last exposure, the urinary fluoride concentration was four times that of controls. Protein, glucose, ketones, and occult blood were detected in the urine. A 30% weight loss occurred subsequent to exposure. The succinic dehydrogenase activity in the kidneys was inhibited to less than 5% of its normal value. Increased levels of succinic dehydrogenase activity were produced in the lungs. The liver showed enlarged nuclei and fatty infiltration. The metabolic inhibition was reversible, as were the pathologic changes in the lungs (with the exception of a small amount of emphysematous change), liver, and kidneys (examined 18 days after exposure). The authors concluded that the toxic syndrome described in this study is compatible with the descriptions of hydrogen fluoride toxicity.
3.4. Developmental and Reproductive Toxicity
No data on the developmental or reproductive toxicity of carbonyl fluoride were found.
3.5. Genotoxicity
No data on the genotoxicity of carbonyl fluoride were found.
3.6. Chronic Toxicity and Carcinogenicity
No data on the chronic toxicity or carcinogenicity of carbonyl fluoride were found.
3.7. Summary
Acute inhalation of carbonyl fluoride causes rapid or labored respiration and respiratory irritation, pulmonary congestion and edema, increases in urinary
fluoride excretion, proteinuria, and can cause death in rats. Varying LC50 values for rats have been reported: 4-h LC50 of 34.3 ppm (DuPont 1976), 90 ppm (Scheel et al. 1968a), and 100 ppm (DuPont 1959), and 1-h LC50s of 360 ppm and 460 ppm for 8-week-old and 24-week-old rats, respectively. The 1-h LC50 for hydrogen fluoride in rats is 1,278 ppm (MacEwen and Vernot 1970). Converting the carbonyl fluoride concentration of 460 ppm to an equivalent concentration of hydrogen fluoride yields a concentration of 867 ppm, which suggests that carbonyl fluoride produces toxicity greater than that caused by hydrogen fluoride released by hydrolysis. Converting the LC50 for hydrogen fluoride to an equivalent concentration of carbonyl fluoride gives a predicted value of 680 ppm, nearly 50% greater than that observed. No data on the developmental toxicity, reproductive toxicity, genotoxicity, chronic toxicity, or carcinogenicity of carbonyl fluoride were found.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
Carbonyl fluoride is hygroscopic and hydrolyzed in the moist respiratory tract to carbon dioxide and two moles of hydrogen fluoride (Arito and Soda 1977). Hydrogen fluoride is soluble in water and is absorbed by the respiratory tract (HSDB 2012). It has a relatively low dissociation constant (3.5 × 10-4), which allows the non-ionized compound to penetrate the skin, respiratory system, or gastrointestinal tract. The fluoride ion is readily absorbed into the bloodstream and is distributed to all organs. Equilibrium is rapidly reached (Perry et al. 1994). Elimination is primarily through the kidneys.
4.2. Mechanism of Toxicity
Carbonyl fluoride is a contact irritant that hydrolyzes in the presence of water to hydrogen fluoride. Hydrogen fluoride is irritating to the skin, eyes, and respiratory tract. Exposure via inhalation produces pulmonary hemorrhage, congestion, and death in laboratory animals (HSDB 2012). Carbon dioxide is also produced when carbonyl fluoride is hydrolyzed. However, carbon dioxide toxicity occurs at very high concentrations, and the 10-h time-weighted average is currently set at 5,000 ppm (NIOSH 2011b).
4.3. Structure-Activity Relationships
Carbonyl fluoride is the fluorine analogue of phosgene (carbonyl chloride). However, only a small amount of phosgene hydrolyzes when it comes into contact with moisture (NRC 2002), whereas carbonyl fluoride is “instantly hydrolyzed by water” (HSDB 2009). The primary mechanism of action of phosgene is acylation resulting in lipid and protein denaturation, irreversible membrane changes, and disruption of enzymatic function. Death is caused by
pulmonary edema following a latency period of 24 h or longer (NRC 2002). The mechanism of action for carbonyl fluoride is unknown. A latency period was not reported by DuPont (1976), but Scheel et al. (1968a) reported that deaths usually occurred within 24 h of exposure with few latent deaths. In the DuPont (1976) study, a 4-h exposure to carbonyl fluoride in rats led to pulmonary consolidation and edema. Scheel et al. (1968a) reported deep lung focal hemorrhage and edema in rats exposed for 1 h to carbonyl fluoride produced from polytetrafluoroethylene pyrolysis.
4.4. Other Relevant Information
No additional relevant information on carbonyl fluoride was found.
4.4.1. Species Variability
According to Scheel et al. (1968a), pathologic changes in the respiratory tract and liver following exposure to carbonyl fluoride at 310 ppm for 1 h were similar in the dog, rat, mouse, rabbit, and guinea pig.
4.4.2. Susceptible Populations
No information was available on populations especially sensitive to carbonyl fluoride toxicity.
4.4.3. Concentration-Exposure Duration Relationship
The concentration-exposure duration relationship for many irritant and systemically acting vapors and gases may be described by the equation Cn × t = k, where the exponent ranges from 0.8 to 3.5 (ten Berge et al. 1986). In the absence of chemical-specific data from which to derive an empirical value for the exponent n, default values of n = 3 when extrapolating to shorter durations and n = 1 when extrapolating to longer durations were used (NRC 2001).
4.4.4. Concurrent Exposure Issues
No concurrent exposure issues for carbonyl fluoride were identified.
5. DATA ANALYSIS FOR AEGL-1
5.1. Human Data Relevant to AEGL-1
No human data relevant to developing AEGL-1 values for carbonyl fluoride were found.
5.2. Animal Data Relevant to AEGL-1
Male albino rats were exposed to carbonyl fluoride at nominal concentrations of 2.5 or 5 ppm for 2 and 2.5 h (DuPont 1956). The low concentration of 2.5 ppm was not lethal to the rats and no clinical signs developed.
5.3. Derivation of AEGL-1 Values
Details of the animal study of carbonyl fluoride (DuPont 1956) were insufficient to consider using it as a basis to derive AEGL-1 values. Therefore, AEGL-1 values are not recommended.
6. DATA ANALYSIS FOR AEGL-2
6.1. Human Data Relevant to AEGL-2
No human data relevant to developing AEGL-2 values for carbonyl fluoride were found.
6.2. Animal Data Relevant to AEGL-2
In a study of rats exposed to carbonyl fluoride at nominal concentrations of 2.5 or 5 ppm for 2 or 2.5 h, the no-effect level was 2.5 ppm for both durations. Rats exposed at 5 ppm exhibited dyspnea and cyanosis (DuPont 1956). Rats exposed to carbonyl fluoride at nominal concentrations of 5 or 10 ppm for 4 h (DuPont 1956, 1959) also experienced dyspnea and rapid shallow respiration; a no-effect level was not identified in the study. However, almost no information on the experimental details or assessments of the animals was reported. Thus, the DuPont (1956, 1959) studies do not provide adequate information to accurately define a no-effect level for AEGL-2 effects.
6.3. Derivation of AEGL-2 Values
The available studies on carbonyl fluoride are inadequate for deriving AEGL-2 values. Results of the DuPont (1956, 1959, 1976) studies indicate a steep exposure-response curve. The highest no-effect level for lethality was 10 ppm for 4 h (DuPont 1959) and the lowest lethal concentration was 26.7 ppm for 4 h, with 50% lethality (DuPont 1976). Thus, AEGL-2 values for carbonyl fluoride were set at one-third of the AEGL-3 values, in accordance with the standing operating procedures for developing AEGL values (NRC 2001).
TABLE 2-4 AEGL-2 Values for Carbonyl Fluoride
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 0.35 ppm | 0.35 ppm | 0.28 ppm | 0.17 ppm | 0.087 ppm |
| (0.95 mg/m3) | (0.95 mg/m3) | (0.76 mg/m3) | (0.46 mg/m3) | (0.23 mg/m3) |
7. DATA ANALYSIS FOR AEGL-3
7.1. Human Data Relevant to AEGL-3
No human data relevant to developing AEGL-3 values for carbonyl fluoride were found.
7.2. Animal Data Relevant to AEGL-3
DuPont (1976) exposed male ChR-CD rats to carbonyl fluoride at 26.7, 30.8, 32.7, 41.3, 44.7, 47.2, or 47.6 ppm for 4 h. Deaths occurred at every concentration. The calculated LC50 was 34.3 ppm. The calculated BMC01 was 10.4 ppm and the BMCL05 was 5.2 ppm (DuPont 1976). The study by Scheel et al. (1968a) was not considered relevant because animals were exposed to a mixture of pyrolysis products of polytetrafluoroethylene, including carbonyl fluoride (Arito and Soda 1977).
7.3. Derivation of AEGL-3 Values
The AEGL-3 values for carbonyl fluoride were determined by using the BMCL05 of 5.2 ppm derived from the study by DuPont (1976) as the point-of-departure. The BMCL05 was more conservative than the BMC01 calculated from the same study. Rapid to convulsive respiration and pulmonary edema were observed in rats exposed for 4 h. Death occurred at all concentrations. An interspecies uncertainty factor of 3 was applied because the toxicity of a direct-acting irritant is not expected to differ substantially among species. The Scheel et al. (1968a) study provides some support for the interspecies uncertainty factor of 3. However exposure to carbonyl fluoride was generated via polytetrafluoroethylene pyrolysis; therefore, exposure included other pyrolysis products. Exposure of rats to carbonyl fluoride at 310 ppm resulted in focal hemorrhage of the lungs and pulmonary edema, observed 24 h after exposure. The investigators stated that the same effects were produced at the same concentration in other species, including the dog, rabbit, guinea pig, and mouse, but individual data and photomicrographs of the lungs were not provided for those species. An intraspecies uncertainty factor of 3 was applied because carbonyl fluoride is a direct-acting irritant of the lungs and its effects are not expected to differ greatly among individuals.
The concentration-exposure duration relationship for many irritant and systemically-acting vapors and gases can be described by the equation Cn × t = k, where the exponent n ranges from 0.8 to 3.5 (ten Berge et al. 1986). In the absence of chemical-specific data from which to derive an empirical value for the exponent n, default values of n = 3 for extrapolating to shorter durations and n = 1 for extrapolating to longer durations were used (NRC 2001). The AEGL-3 values for carbonyl fluoride are presented in Table 2-5.
8. SUMMARY OF AEGLS
8.1. AEGLS Values and Toxicity End Points
AEGL-1 values for carbonyl fluoride are not recommended because of insufficient data. AEGL-2 values are based on a three-fold reduction of the AEGL-3 values because experimental data were not available to empirically derive AEGL-2 values. AEGL-3 values for carbonyl fluoride are based on a BMCL05 derived from lethality data in rats. AEGL values for carbonyl fluoride are presented in Table 2-6. For comparison, Table 2-7 provides the AEGL values for phosgene and hydrogen fluoride, because those chemicals are also pyrolysis products of polytetrafluorethylene (see Sections 3.7, 4.1, and 4.2).
8.2. Other Standards and Guidelines
Standards and guidelines for carbonyl fluoride are presented in Table 2-8. No other emergency standards such as emergency response planning guidelines or immediately dangerous to life and health values are available for carbonyl fluoride. The threshold limit value–time-weighted average for carbonyl fluoride was established by the American Conference of Governmental Industrial Hygienists (ACGIH) on the basis of data from Scheel et al. (1968a). The 8-h AEGL values are much lower than the industrial standards and guidelines for carbonyl fluoride. The values of ACGIH and the National Institute for Occupational Safety and Health were determined by analogy with fluorides and hydrogen fluoride and are intended to minimize the potential for pulmonary irritation and disabling bone changes.
TABLE 2-5 AEGL-3 Values for Carbonyl Fluoride
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 1.0 ppm (2.7 mg/m3) | 1.0 ppm (2.7 mg/m3) | 0.83 ppm (2.2 mg/m3) | 0.52 ppm (1.4 mg/m3) | 0.26 ppm (0.70 mg/m3) |
TABLE 2-6 AEGL Values for Carbonyl Fluoride
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h |
| AEGL-1 (nondisabling) |
NR a | NR a | NR a | NR a | NR a |
| AEGL-2 (disabling) |
0.35 ppm (0.95 mg/m3) |
0.35 ppm (0.95 mg/m3) |
0.28 ppm (0.76 mg/m3) |
0.17 ppm (0.46 mg/m3) |
0.087 ppm (0.23 mg/m3) |
| AEGL-3 (lethal) |
1.0 ppm (2.7 mg/m3) |
1.0 ppm (2.7 mg/m3) |
0.83 ppm (2.2 mg/m3) |
0.52 ppm (1.4 mg/m3) |
0.26 ppm (0.70 mg/m3) |
aNot recommended. Absence of an AEGL-1 value does not imply that exposures below the AEGL-2 value are without adverse effects.
TABLE 2-7 AEGL Values for Phosgene and Hydrogen Fluoride
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h |
| Phosgene (NRC 2002) | |||||
| AEGL-1 (nondisabling) | NRa | NRa | NRa | NRa | NRa |
| AEGL-2 (disabling) | 0.60 ppm | 0.60 ppm | 0.30 ppm | 0.08 ppm | 0.04 ppm |
| AEGL-3 (lethal) | 3.6 ppm | 1.5 ppm | 0.75 ppm | 0.20 ppm | 0.09 ppm |
| Hydrogen fluoride (NRC 2004) | |||||
| AEGL-1 (nondisabling) | 1.0 ppm | 1.0 ppm | 1.0 ppm | 1.0 ppm | 1.0 ppm |
| AEGL-2 (disabling) | 95 ppm | 34 ppm | 24 ppm | 12 ppm | 12 ppm |
| AEGL-3 (lethal) | 170 ppm | 62 ppm | 44 ppm | 22 ppm | 22 ppm |
aNot recommended. Absence of an AEGL-1 value does not imply that exposures below the AEGL-2 value are without adverse effects.
TABLE 2-8 Standards and Guidelines for Carbonyl Fluoride
| Guideline | Exposure Duration | |||||
| 10 min | 15 min | 30 min | 1 h | 4 h | 8 h | |
| AEGL-1 | NR | – | NR | NR | NR | NR |
| AEGL-2 | 0.35 ppm (0.95 mg/m3) | – | 0.35 ppm (0.95 mg/m3) | 0.28 ppm (0.76 mg/m3) | 0.17 ppm (0.46 mg/m3) | 0.087 ppm (0.23 mg/m3) |
| AEGL-3 | 1.0 ppm (2.7 mg/m3) | – | 1.0 ppm (2.7 mg/m3) | 0.83 ppm (2.2 mg/m3) | 0.52 ppm (1.4 mg/m3) | 0.26 ppm (0.70 mg/m3) |
| TLV-TWA (ACGIH)a | – | – | – | – | – | 2 ppm (5.4 mg/m3) |
| REL-TWA (NIOSH)b | – | – | – | – | – | 2 ppm (5.4 mg/m3) |
| TLV-STEL (ACGIH)c | – | 5 ppm (13 mg/m3) | – | – | – | – |
| REL-STEL (NIOSH)d | – | 5 ppm (15 mg/m3) | – | – | – | – |
| MAC (The Netherlands)e | – | 0.38 ppm (1 mg/m3) | – | – | – | – |
aTLV-TWA (threshold limit value – time-weighted average, American Conference of Governmental Industrial Hygienists) (ACGIH 2012) is the time-weighted average concentration for a normal 8-h workday and a 40-h workweek to which nearly all workers may be repeatedly exposed, day after day, without adverse effect.
bREL-TWA (recommended exposure limit – time-weighted average, National Institute for Occupational Safety and Health) (NIOSH 2011a) is defined as the time-weighted average concentration for up to a 10-h workday during a 40-h workweek.
cTLV-STEL (threshold limit value – short-term exposure limit, American Conference of Governmental Industrial Hygienists) (ACGIH 2012) is defined as a 15-min time-weighted average exposure which should not be exceeded at any time during the workday even if the 8-h time-weighted average is within the TLV-TWA. Exposures above the TLV-TWA up to the STEL should not be longer than 15 min and should not occur more than four times per day. There should be at least 60 min between successive exposures in that range.
dREL-STEL (recommended exposure limit – short-term exposure limit, National Institute for Occupational Safety and Health) (NIOSH 2011a) is defined as a 15-min time-weighted average exposure that should not be exceeded at any time during the workday.
eMAC (maximaal aanvaarde concentratie [maximal accepted concentration], Dutch Expert Committee for Occupational Standards, The Netherlands (MSZW 2004) is defined analogous to the ACGIH TLV-TWA.
8.3. Data Adequacy and Research Needs
There are no human data available on carbonyl fluoride. DuPont (1956, 1959, and 1976) conducted studies of rats exposed to carbonyl fluoride for 2-4 h, but two of the studies reported only nominal concentrations, used relatively few animals, and reported few study details. Scheel et al. (1968a, 1968b) exposed rats to carbonyl fluoride produced by burning polytetrafluoroethylene. While carbonyl fluoride is a major pyrolysis product, it is not the only pyrolysis product produced (Arito and Soda 1977) and the rats were probably exposed to other compounds that contain fluoride. The animals were also exposed to the particulate matter produced from polytetrafluoroethylene pyrolysis, which may have increased observed effects. Additional acute animal studies in other species and with a greater range of concentrations would be helpful in deriving AEGL values. No studies on genotoxicity or reproductive and developmental toxicity were found; additional studies evaluating those outcomes would also help to strengthen the basis of the AEGL values for carbonyl fluoride.
9. REFERENCES
ACGIH (American Conference of Government and Industrial Hygienists). 2012. Carbonyl Fluoride (CAS Reg. No. 353-50-4). Documentation of the Threshold Limit Values and Biological Exposure Indices. American Conference of Government and Industrial Hygienists, Cincinnati, OH.
Arito, H., and R. Soda. 1977. Pyrolysis products of polytetrafluoroethylene and polyfluoroethylenepropylene with reference to inhalation toxicity. Ann. Occup. Hyg. 20(3): 247-255.
Coleman, E., L.D. Scheel, R.E. Kupel, and R.L. Larkin. 1968. The identification of toxic compounds in the pyrolysis products of polytetrafluoroethylene. Am. Ind. Hyg. Assoc. J. 29(1):33-40.
DuPont (E.I. DuPont de Nemours and Company, Inc.). 1956. Toxicity Studies of Pyrolysis Products of Fluorinated Polymers (Teflon Polytetrafluoroethylene). Haskell Laboratory Report No. 18-56. Submitted to EPA by DuPont, Haskell Laboratory, Newark, DE, with Cover Letter Dated 10/15/92. EPA Document No. 8EHQ-1092-11415. Microfiche No. OTS0571353.
DuPont (E.I. DuPont de Nemours and Company, Inc.). 1959. Toxicity Studies of Carbonyl Fluoride. Haskell Laboratory Report No. 32-59. DuPont, Haskell Laboratory, Newark, DE.
DuPont (E.I. DuPont de Nemours and Company, Inc.). 1976. Acute Inhalation Toxicity Studies of Hydrogen Fluoride and Carbonyl Fluoride. Haskell Laboratory Report No. 485-76. DuPont, Haskell Laboratory, Newark, DE.
HSDB (Hazardous Substances Data Bank). 2009. Carbonyl Fluoride (CAS Reg. No. 353-50-4). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed August 2013].
HSDB (Hazardous Substances Data Bank). 2012. Hydrogen fluoride (CAS Reg. No. 7664-39-3). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed August 2013].
IPCS (International Programme on Chemical Safety). 2004. Perfluoroisobutylene. IPCS Card No. 1216. International Programme on Chemical Safety and the Commission of the European Communities [online]. Available: http://www.inchem.org/documents/icsc/icsc/eics1216.htm [accessed May 2013].
MacEwen, J.D., and E.H. Vernot. 1970. Toxic Hazards Research Unit Annual Technical Report: 1970. AMRL-TR-70-77. AD 714694. Aerospace Medical Research Laboratory, Wright-Patterson Air Force Base, OH [online]. Available: http://www.dtic.mil/dtic/tr/fulltext/u2/714694.pdf [accessed june 23, 2014].
MSZW (Ministerie van Sociale Zaken en Werkgelegenheid). 2004. Nationale MAC-lijst 2004: Carbonyfluoride en PTFE-pyrolyseproducten. Den Haag: SDU Uitgevers [online]. Available: http://www.lasrook.net/lasrookNL/maclijst2004.htm [accessed June 24, 2014].
NIOSH (National Institute for Occupational Safety and Health). 2011a. NIOSH Pocket Guide to Chemical Hazards: Carbonyl Fluoride [online]. Available: http://www.cdc.gov/niosh/npg/npgd0108.html [accessed August 2013].
NIOSH (National Institute for Occupational Safety and Health). 2011b. NIOSH Pocket Guide to Chemical Hazards: Carbon Dioxide [online]. Available: http://www.cdc.gov/niosh/npg/npgd0103.html [accessed August 2013].
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
NRC (National Research Council). 2002. Phosgene. Pp. 15-70 in Acute Exposure Guideline Levels for Selected Airborne Chemicals. Washington, DC: The National Academies Press.
NRC (National Research Council). 2004. Hydrogen fluoride. Pp 123-197 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 4. Washington, DC: The National Academies Press.
Patocka, J., and J. Bajgar. 1998. Toxicology of Perfluoroisobutene. ASA Newsletter [online]. Available: http://www.asanltr.com/ASANews-98/pfib.html [accessed August 2013].
Perry, W.G., F.A. Smith, and M.B. Kent. 1994. The halogens. Pp. 4449-4522 in Patty’s Industrial Hygiene and Toxicology, 4th Ed., Vol. II, Part F, G.F. Clayton, and F.E. Clayton, eds. New York: John Wiley & Sons.
Scheel, L.D., W.C. Lane, and W.E. Coleman. 1968a. The toxicity of polytetrafluoroethylene pyrolysis products – including carbonyl fluoride and a reaction product, silicon tetrafluoride. Am. Ind. Hyg. Assoc. J. 29(1):41-48.
Scheel L.D., L. McMillan, and F.C. Phipps. 1968b. Biochemical changes associated with toxic exposures to polytetrafluoroethylene pyrolysis products. Am. Ind. Hyg. Assoc. J. 29(1):49-53.
ten Berge, W.F., A. Zwart, and L.M. Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapours and gases. J. Hazard. Mater. 13(3):301-309.
APPENDIX A
DERIVATION OF AEGL VALUES FOR CARBONYL FLUORIDE
Derivation of AEGL-1 Values
AEGL-1 values are not recommended because of insufficient data on carbonyl fluoride. Absence of AEGL-1 values does not imply that exposures below the AEGL-2 values are without adverse effects.
Derivation of AEGL-2 Values
In the absence of empirical data on carbonyl fluoride, the AEGL-2 values for carbonyl fluoride were set at one-third of the AEGL-3 values. That approach is in in accordance with the standing operating procedures for developing AEGL values for chemicals with steep concentration-response curves (NRC 2001). Rats exposed to carbonyl fluoride at 5 or 10 ppm for 4 h experienced dyspnea and rapid shallow respiration (DuPont 1956, 1959). At higher concentrations (26.7 ppm or higher for 4 h), death occurred, indicating a steep exposure-response curve (DuPont 1976).
Calculations:
|
10-min AEGL-2: |
1.04 ppm ÷ 3 = 0.35 ppm |
|
30-min AEGL-2: |
1.04 ppm ÷ 3 = 0.35 ppm |
|
1-h AEGL-2: |
0.83 ppm ÷ 3 = 0.28 ppm |
|
4-h AEGL-2: |
0.52 ppm ÷ 3 = 0.17 ppm |
|
8-h AEGL-2: |
0.26 ppm ÷ 3 = 0.087 ppm |
Derivation of AEGL-3 Values
|
Key study: |
DuPont (E.I. DuPont de Nemours and Company, Inc.). 1976. Acute Inhalation Toxicity Studies of Hydrogen Fluoride and Carbonyl Fluoride. Haskell Laboratory Report No. 485-76. DuPont, Haskell Laboratory, Newark, DE |
|
Toxicity end point: |
Threshold for lethality; BMCL05 of 5.2 ppm |
|
Time scaling: |
The concentration-exposure duration relationship for many irritant and systemically-acting vapors and gases may be described by |
|
the relationship Cn × t = k, where the exponent n ranges from 0.8 to 3.5 (ten Berge et al. 1986). In the absence of chemical-specific data to derive an empirical value for n, default values of n = 3 for extrapolating to shorter durations and n = 1 for extrapolating to longer durations were used (NRC 2001). The 30-min AEGL-3 value was adopted for the 10-min value in accordance with the standing operating procedures for developing AEGL values (NRC 2001). |
|
|
Uncertainty factors: |
Interspecies: 3, effects of a direct-acting irritant of the lungs and respiratory tract are not expected to differ greatly among species. The study by Scheel et al. (1968a) provides some support for a factor of 3. However, exposure to carbonyl fluoride was generated via polytetrafluoroethylene pyrolysis; therefore, exposure included other pyrolysis products. Exposure of rats to carbonyl fluoride at 310 ppm resulted in focal hemorrhage of the lungs and pulmonary edema, observed 24 h after exposure. The investigators stated that those effects were produced at the same concentration in other species, including the dog, rabbit, guinea pig, and mouse. However, individual data and photomicrographs of the lungs were not provided for those species. Intraspecies: 3, effects of a direct-acting irritant of the lungs and respiratory tract are not expected to differ greatly among individuals. |
|
Modifying factor: |
None applied |
|
Calculations: |
|
|
10-min AEGL-3: |
C3 × 30 min = 33.74592 ppm-min |
|
30-min AEGL-3: |
C3 × 30 min = 33.74592 ppm-min |
|
1-h AEGL-3: |
C3 × 60 min = 33.74592 ppm-min |
|
4-h AEGL-3: |
C × 240 min = 124.8 ppm-min |
|
8-h AEGL-3: |
C × 480 min = 124.8 ppm-min |
APPENDIX C
ACUTE EXPOSURE GUIDELINE LEVELS FOR CARBONYL FLUORIDE
Derivation Summary
AEGL-1 VALUES
No AEGL-1 values were derived for carbonyl fluoride because of insufficient data.
AEGL-2 VALUES
|
|
||||
| 10 min | 30 min | 1 h | 4 h | 8 h |
|
|
||||
| 0.35 ppm | 0.35 ppm | 0.28 ppm | 0.17 ppm | 0.087 ppm |
|
|
||||
| Data adequacy: Data on carbonyl fluoride were inadequate for deriving AEGL-2 values, so values were estimated by dividing the AEGL-3 values by 3. That procedure, based on guidance in NRC (2001), is applicable for chemicals with steep concentration-response curves. Rats exposed to carbonyl fluoride at 5 or 10 ppm for 4 h experienced dyspnea and rapid shallow respiration (DuPont 1956, 1959). At higher concentrations (26.7 ppm and higher for 4 h), death occurred indicating a steep exposure-response curve (DuPont 1976). | ||||
|
|
||||
AEGL-3 VALUES
|
|
||||
| 10 min | 30 min | 1 h | 4 h | 8 h |
|
|
||||
| 1.0 ppm | 1.0 ppm | 0.83 ppm | 0.52 ppm | 0.26 ppm |
|
|
||||
| Key reference: DuPont (E.I. DuPont de Nemours and Company, Inc.) 1976. Acute Inhalation Toxicity Studies of Hydrogen Fluoride and Carbonyl Fluoride. Haskell Laboratory Report No. 485-76. DuPont, Haskell Laboratory, Newark, DE. | ||||
|
|
||||
| Test species/Strain/Number: Rat; ChR-CD; 10/group | ||||
|
|
||||
| Exposure route/Concentrations/Durations: Inhalation; 26.7, 30.8, 32.7, 41.3, 44.7, 47.2, 47.6 ppm for 4 h | ||||
|
|
||||
| Effects: Rapid shallow respiration and pulmonary edema. 26.7 ppm: 50% mortality 30.8 ppm: 30% mortality 32.7 ppm: 30% mortality 41.3 ppm: 60% mortality 44.7 ppm: 80% mortality 47.2 ppm: 90% mortality 47.6 ppm: 60% mortality |
||||
|
|
||||
| End point/Concentration/Rationale: Threshold for lethality (BMCL05 of 5.2 ppm) | ||||
|
|
||||
| Uncertainty factors/Rationale: Interspecies: 3, effects of a direct-acting irritant of the lungs and respiratory tract are not expected to differ greatly among species. The study by Scheel et al. (1968a) provides some support for a factor of 3. However exposure to carbonyl fluoride was generated |
||||
|
|
||||
|
|
||||
| via polytetrafluoroethylene pyrolysis; therefore, exposure included other pyrolysis products. Exposure of rats to carbonyl fluoride at 310 ppm resulted in focal hemorrhage of the lungs and pulmonary edema, observed 24 h after exposure. The investigators stated that this effect was produced at the same concentration in other species, including the dog, rabbit, guinea pig, and mouse. However, individual data and photomicrographs of the lungs were not provided for those species. Intraspecies: 3, effects of a direct-acting irritant of the lungs and respiratory tract are not expected to differ greatly among individuals. |
||||
|
|
||||
| Modifying factor: None | ||||
|
|
||||
| Animal-to-human dosimetric adjustment: Not applied | ||||
|
|
||||
| Time scaling: The concentration-exposure duration relationship for many irritant and systemically-acting vapors and gases has been described by the relationship Cn × t = k, where the exponent n ranges from 0.8 to 3.5 (ten Berge et al. 1986). In the absence of chemical-specific to derive an empirical value for n, default values of n = 3 for extrapolating to shorter durations and n = 1 for extrapolating to longer durations were used (NRC 2001). The 30-min AEGL-3 value was adopted for the 10-min value in accordance with the standing operating procedures for developing AEGL values (NRC 2001). | ||||
|
|
||||
| Data adequacy: The study was well done with an appropriate number of animals. Analytic concentrations were measured and an end point consistent with the AEGL-3 definition was observed. | ||||
|
|
||||
APPENDIX D
BENCHMARK DOSE CALCULATIONS
BMDS MODEL RUN BMCL05
The form of the probability function is:
P[response] = Background + (1-Background)
CumNorm(Intercept+Slope*Log(Dose)),
where CumNorm(.) is the cumulative normal distribution function
Dependent variable = COLUMN3
Independent variable = COLUMN1
Slope parameter is restricted as slope >= 1
Total number of observations = 8
Total number of records with missing values = 0
Maximum number of iterations = 250
Relative Function Convergence has been set to: 1e-008
Parameter Convergence has been set to: 1e-008
User has chosen the log transformed model
Default Initial (and Specified) Parameter Values
Background = 0
Intercept = -7.52665
Slope = 2.13336
Asymptotic Correlation Matrix of Parameter Estimates
(***The model parameter(s) -background have been estimated at a boundary point, or have been specified by the user, and do not appear in the correlation matrix)
| intercept | slope | |
| intercept | 1 | -1 |
| slope | -1 | 1 |
Parameter Estimates
| 95.0% Wald Confidence Interval | ||||
| Variable | Estimate | Standard Error | Lower Conf. Limit | Upper Conf. Limit |
| Background | 0 | NA | ||
| Intercept | -6.88857 | 2.62927 | -12.0418 | -1.7353 |
| Slope | 1.94933 | 0.724479 | 0.529374 | 3.36928 |
NA - Indicates that this parameter has hit a bound implied by some inequality constraint and thus has no standard error.
Analysis of Deviance Table
| Model | Log (likelihood) | # Param’s | Deviance | Test d.f. | P-value |
| Full model | -40.8638 | 8 | |||
| Fitted model | -44.0906 | 2 | 6.45349 | 6 | 0.3744 |
| Reduced model | -55.4518 | 1 | 29.1759 | 7 | 0.0001344 |
AIC: 92.1812
Goodness of Fit
| Dose | Estimated Probability | Expected | Observed | Size | Scaled Residual |
| 0.0000 | 0.0000 | 0.000 | 0 | 10 | 0.000 |
| 26.7000 | 0.3136 | 3.136 | 5 | 10 | 1.271 |
| 30.8000 | 0.4179 | 4.179 | 3 | 10 | -0.756 |
| 32.7000 | 0.4639 | 4.639 | 3 | 10 | -1.039 |
| 41.3000 | 0.6423 | 6.423 | 6 | 10 | -0.279 |
| 44.7000 | 0.6981 | 6.981 | 8 | 10 | 0.702 |
| 47.2000 | 0.7340 | 7.340 | 9 | 10 | 1.188 |
| 47.6000 | 0.7394 | 7.394 | 6 | 10 | -1.004 |
Chi-square = 6.26 d.f. = 6 P-value = 0.3951
Benchmark Dose Computation
Specified effect = 0.05
Risk Type = Extra risk
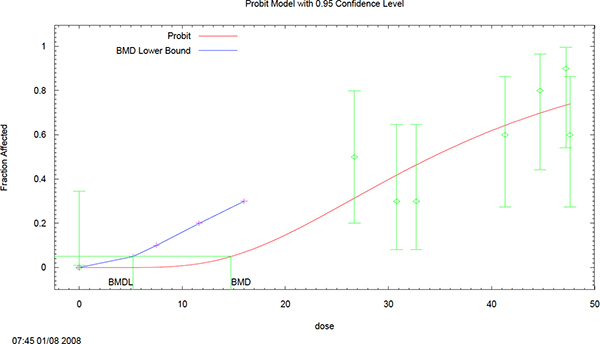
Confidence level = 0.95
BMD = 14.7319
BMDL = 5.21965
BMDS MODEL RUN BMC01
The form of the probability function is:
P[response] = Background + (1-Background) *
CumNorm(Intercept+Slope*Log(Dose)), where CumNorm(.) is the cumulative normal distribution function
Dependent variable = COLUMN3
Independent variable = COLUMN1
Slope parameter is restricted as slope >= 1
Total number of observations = 8
Total number of records with missing values = 0
Maximum number of iterations = 250
Relative Function Convergence has been set to: 1e-008
Parameter Convergence has been set to: 1e-008
User has chosen the log transformed model
Default Initial (and Specified) Parameter Values
Background = 0
Intercept = -7.52665
Slope = 2.13336
Asymptotic Correlation Matrix of Parameter Estimates
(***The model parameter(s) -background have been estimated at a boundary point, or have been specified by the user, and do not appear in the correlation matrix)
| intercept | slope | |
| intercept | 1 | -1 |
| slope | -1 | 1 |
Parameter Estimates
| 95.0% Wald Confidence Interval | ||||
| Variable | Estimate | Standard Error | Lower Conf. Limit | Upper Conf. Limit |
| Background | 0 | NA | ||
| Intercept | -6.88857 | 2.62918 | -12.0417 | -1.73548 |
| Slope | 1.94933 | 0.724454 | 0.529424 | 3.36923 |
NA - Indicates that this parameter has hit a bound implied by some inequality constraint and thus has no standard error.
Analysis of Deviance Table
| Model | Log (likelihood) | No. Parameters | Deviance | Test d.f. | P-value |
| Full model | -40.8638 | 8 | |||
| Fitted model | -44.0906 | 2 | 6.45349 | 6 | 0.3744 |
| Reduced model | -55.4518 | 1 | 29.1759 | 7 | 0.0001344 |
AIC: 92.1812
Goodness of Fit
| Dose | Estimated Probability | Expected | Observed | Size | Scaled Residual |
| 26.7000 | 0.3136 | 3.136 | 5 | 10 | 1.271 |
| 30.8000 | 0.4179 | 4.179 | 3 | 10 | -0.756 |
| 32.7000 | 0.4639 | 4.639 | 3 | 10 | -1.039 |
| 41.3000 | 0.6423 | 6.423 | 6 | 10 | -0.279 |
| 44.7000 | 0.6981 | 6.981 | 8 | 10 | 0.702 |
| 47.2000 | 0.7340 | 7.340 | 9 | 10 | 1.188 |
| 47.6000 | 0.7394 | 7.394 | 6 | 10 | -1.004 |
| 0.0000 | 0.0000 | 0.000 | 0 | 10 | 0.000 |
Chi-square = 6.2 d.f. = 6 P-value = 0.3951
Benchmark Dose Computation
Specified effect = 0.01
Risk Type = Extra risk
Confidence level = 0.95
BMD = 10.3855
BMDL = 2.64042

APPENDIX E
CATEGORY PLOT FOR CARBONYL FLUORIDE

FIGURE E-1 Category plot of toxicity data and AEGL values for carbonyl fluoride.
TABLE E-1 Data Used in the Category Plot for Carbonyl Fluoride
| Source | Species | Sex | No. of Exposures | ppm | Time (min) Category | Comments | |
| AEGL-2 | 0.35 | 10 | AEGL | ||||
| AEGL-2 | 0.35 | 30 | AEGL | ||||
| AEGL-2 | 0.28 | 60 | AEGL | ||||
| AEGL-2 | 0.17 | 240 | AEGL | ||||
| AEGL-2 | 0.087 | 480 | AEGL | ||||
| AEGL-3 | 1 | 10 | AEGL | ||||
| AEGL-3 | 1 | 30 | AEGL | ||||
| AEGL-3 | 0.83 | 60 | AEGL | ||||
| AEGL-3 | 0.52 | 240 | AEGL | ||||
| AEGL-3 | 0.26 | 480 | AEGL | ||||
| DuPont 1956 | Rat | M | 1 | 2.5 | 120 | 0 | No effect |
| DuPont 1956 | Rat | M | 1 | 2.5 | 150 | 0 | No effect |
| DuPont 1956 | Rat | M | 1 | 5 | 120 | 1 | Slight dyspnea; cyanosis |
| DuPont 1956 | Rat | M | 1 | 5 | 150 | 1 | Slight dyspnea; cyanosis |
| DuPont 1959 | Rat | M | 1 | 5 | 240 | 1 | Rapid, shallow respiration |
| DuPont 1959 | Rat | M | 1 | 10 | 240 | 1 | Rapid, shallow respiration |
| DuPont 1959 | Rat | M | 1 | 100 | 240 | SL | Pulmonary congestion |
| Scheel et al. 1968a | Rat | B | 1 | 360 | 60 | SL | 50% mortality |
| Scheel et al. 1968a | Rat | B | 1 | 460 | 60 | SL | 50% mortality |
| Scheel et al. 1968a | Rat | B | 1 | 90 | 240 | SL | 50% mortality |
| DuPont 1976 | Rat | M | 1 | 26.7 | 240 | SL | 50% mortality |
| DuPont 1976 | Rat | M | 1 | 30.8 | 240 | SL | 30% mortality |
| DuPont 1976 | Rat | M | 1 | 32.7 | 240 | SL | 30% mortality |
| DuPont 1976 | Rat | M | 1 | 41.3 | 240 | SL | 60% mortality |
| DuPont 1976 | Rat | M | 1 | 44.7 | 240 | SL | 80% mortality |
| DuPont 1976 | Rat | M | 1 | 47.2 | 240 | SL | 90% mortality |
| DuPont 1976 | Rat | M | 1 | 47.6 | 240 | SL | 60% mortality |
For category: 0 = no effect, 1 = discomfort, 2 = disabling, SL = some lethality, 3 = lethality.




























