Personal Protective Equipment and Personal Protective Technology Product Standardization for a Resilient Public Health Supply Chain: Proceedings of a Workshop (2023)
Chapter: 9 Considering Approaches to Prioritizing Filling Gaps in Standards
9
Considering Approaches to Prioritizing Filling Gaps in Standards
Key Messages from Individual Speakers1
- Standards are required for creating and sustaining a resilient and trustworthy PPE supply chain that is complex but agile enough to deliver customized responses to a diverse population by leveraging processes, technologies, and people. (Jayaraman)
- Collaboration among federal agencies, instead of competition, could help ensure that when they have overlap, the agencies can agree which can best deal with a particular issue. (Rogers)
- Risk assessments can play an important role in helping increase the resilience of supply chains and the efficient use of PPE. (Shirley)
- It would be helpful for standards to account for innovation if they are to evolve as an innovation gets integrated into a product or process. (Jayaraman)
Building on the preceding sessions covering each of the components of the PPE supply chain, members of the next panel reflected on the standards gaps and issues raised and how they tie into the observations made
___________________
1 The following list of key messages is the rapporteurs’ summary of points made by the individual speakers identified, and the statements have not been endorsed or verified by the National Academies of Sciences, Engineering, and Medicine. They are not intended to reflect a consensus among workshop participants.
in the Product Standardization Task Force2 draft annual report. Before turning to the panelists, session moderator Tener Veenema recapped the gaps the Resilient Public Health Supply Chain Task Force identified (Figure 9-1), such as multiple gaps in conformity assessment criteria and procedures across most personal protective equipment (PPE) categories, except for respirators. Other standards gaps the task force identified included:
- Fit for respirators, gloves, and gowns for the diversity of the workforce, the public, and children.
- The interface of different types of PPE to evaluate how effective they are when put together as ensembles.
- Measuring a respirator’s effectiveness for source control; and
- Lack of guidance for selecting, using, and caring for PPE, including cleaning, disinfection, decontamination, storage, and reuse.
Veenema noted that a common theme throughout the workshop was improving collaboration across actors in establishing and communicating standards to help build more resilient supply chains that will be critical to future success in response efforts. The workshop’s speakers described additional gaps pertaining to:
- Harmony between standards in the United States and internationally to contribute to better designed PPE, rapidly scale PPE manufacturing in an emergency, and build confidence in the products recommended and distributed to the workforce and the public;
- The design of PPE for a wide range of users, body types, sizes, shapes, and different abilities.
- The design of PPE for children.
On a final note, before opening the panel discussion, Veenema recounted that the challenge is not exclusively to address gaps in standards but also in the complexity of the existing standards for manufacturers, distributors, purchasers, and end users. She also raised the importance of centering equity more intentionally in PPE regulation and education.
___________________
2 Additional information is available at https://aspr.hhs.gov/newsroom/Pages/SupplyChain-9Mar2022.aspx (accessed May 2, 2023).
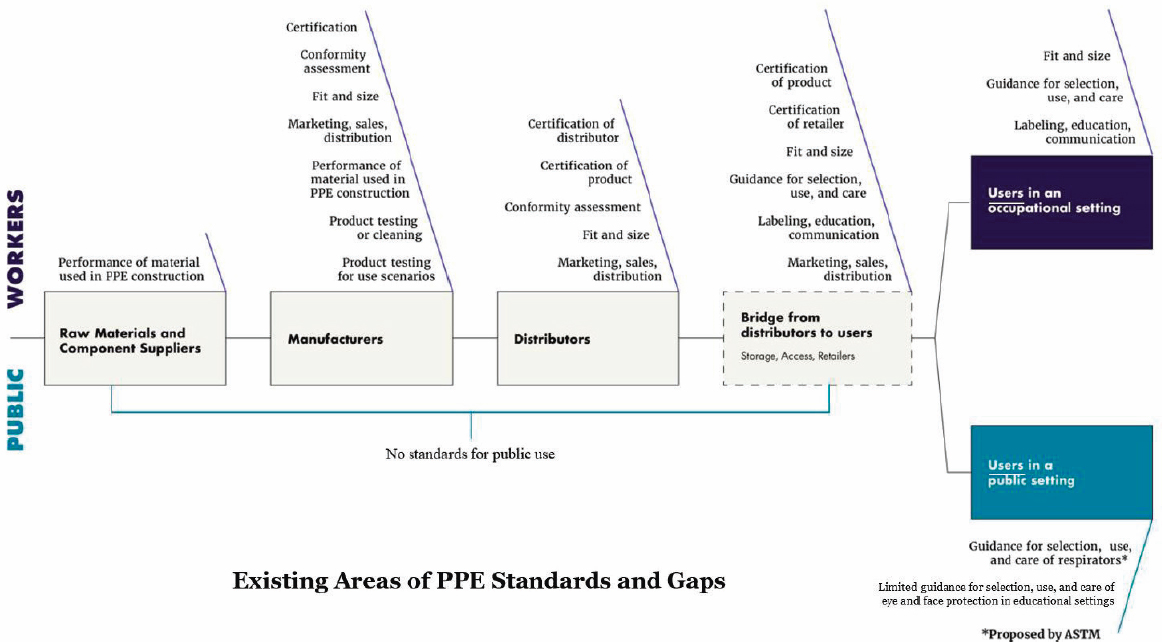
SOURCE: Presented by Tener Veenema on March 1 and 2, 2023, at the PPE/PPT Standardization for a Resilient Public Health Supply Chain Workshop. Figure created based on data provided in Joint Supply Chain Resilience Working Group. 2022 (unpublished).
CRITERIA FOR APPROACHES TO PRIORITIZING GAPS
Lisa Brosseau began the discussion by suggesting that efforts to address standards gaps take a more holistic view of PPE and consider what a universal standard might be and what the expectations would be for such a standard. She noted that the Occupational Safety and Health Administration (OSHA) has some general guidance on PPE that would serve as a good starting point as it outlines criteria for what users should expect from PPE. Brosseau also suggested that polling manufacturers for their input on what their users want and how hard it is for them to meet those criteria would be key to understanding what steps to take first.
Veenema added that a standard for standards would help streamline some of the complexity and variability across current domestic and international standards for federal agencies and other standards organizations within the United States. Brosseau added that such a standard would provide an outline for what should be considered and included in a consensus standard that addresses the performance and testing of PPE, making the process more uniform for manufacturers to follow.
Mark Shirley, director of integrated resiliency management at Sutter Health, said he agreed with the need for a standard for standards. He also said standards are living documents and thus require review after a certain defined period.
Sundaresan Jayaraman, professor of material sciences and engineering and founding director of the Kolon Center for Lifestyle Innovation at Georgia Institute of Technology, said he looks forward to when there will be interoperable standards for PPE. He then presented a list of lessons learned, both as a member of the task force and from this workshop. The first was that standards affect all actors in the PPE supply chain, and therefore all will benefit from standards. The other key message was that standards are required for creating and sustaining a resilient and trustworthy PPE supply chain that is complex but that is also agile enough to deliver customized responses to diverse populations by leveraging processes, technologies, and people.
Creating the standards to enable such a supply chain, said Jayaraman, will require a framework for prioritizing standards development based on the expected effect of a standard, the ease of realizing the standard, and a champion to shepherd the development of the standard. Two approaches Jayaraman noted for prioritizing standards development are (1) by type of PPE and (2) by attributes that cut across all types of PPE for a diversity of users and human factors (Figure 9-2). In his view, this is a good foundation for prioritization that reflects the guidance that users, employers, manufacturers, government, and certification organizations all need. In the end, said Jayaraman, the goal is to have standards that enable the
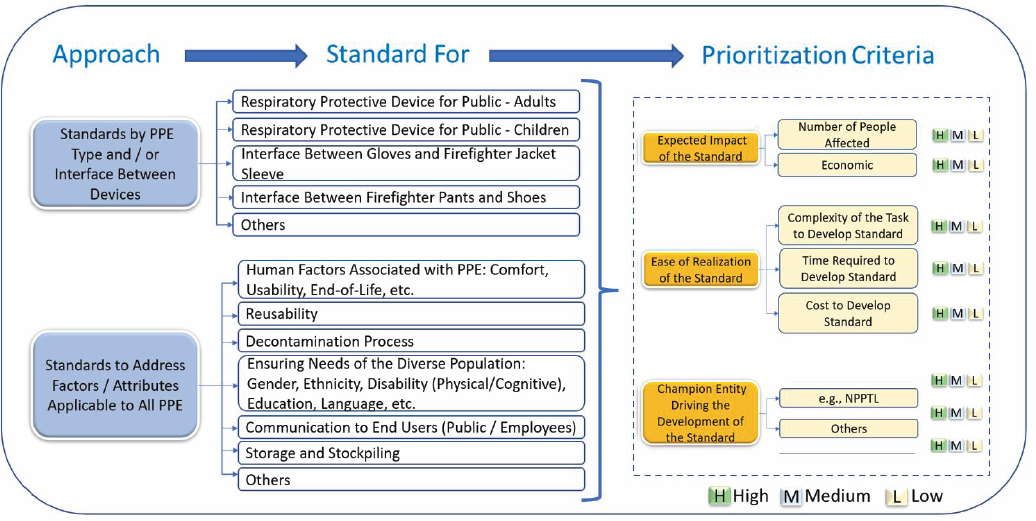
SOURCE: Adapted from figure created and presented by Sundaresan Jayaraman on March 2, 2023, at the PPE/PPT Standardization for a Resilient Public Health Supply Chain Workshop.
supply chain to provide PPE that satisfies what he called “the five Rs”: the right performance of the right quality, in the right quantity, at the right time, and at the right price.
Özlem Ergun said that if the goal is to achieve supply chain resilience and a PPE supply chain that works well with the five Rs, she would suggest analyzing how each developed standard affects various supply chain measures. For example, keeping inventory and stockpiling are resilience measures, as are flexible manufacturing processes that can ramp up and down quickly. She then noted that a standard developed in a certain way might affect stockpiling in a positive manner but make the manufacturing process less flexible. “These are complex systems, and we have to make a system-wide assessment of what it means for different types of supply chain resilience measures when we develop a standard,” said Ergun. Jayaraman agreed.
Bonnie Rogers, adjunct professor at the University of North Carolina-Chapel Hill and NIOSH consultant, agreed with Veenema and Brosseau about the need for a standard for the standards and added that end users have to be involved in discussions about creating standards. She noted that when she spoke with frontline workers, home health workers, and owners of home health agencies, she found that many did not know what fit testing was. Interviews with operating room nurses found there is a significant psychological discomfort that comes with wearing a respirator because they cannot speak to patients and patients cannot adequately hear them. These are the types of issues that it will be important for standards to address, said Rogers, and doing so requires a strategy to collect data from end users.
Rogers said that it is important to identify best practices in all settings, not just hospitals. She also said that it would be helpful for federal agencies to learn to collaborate better instead of competing so that when their activities overlap, they can agree which agency can best deal with a particular issue. “Thinking about the issues related to conformity assessment and the types of PPE that are available, it is not going to be the same for everybody, and that has to be taken into consideration,” said Rogers. Veenema pointed out that the third goal of the task force report states that although the capacities and capabilities of the supply chain enable resilience, it is the behavioral element of the actors within and across those systems that truly creates resilience.
Shirley commented on the importance of risk assessments for helping increase the resilience of supply chains and the efficient use of PPE. For example, it would be important to identify high-risk activities, such as those that may generate aerosols for an infectious agent that is transmitted via droplets, and at what point in asymptomatic community spread it would be best for everyone to have respirators if there is uncertainty whether aerosol transmission is involved. “These risk assessments are
critical, and we can do them proactively,” said Shirley. He suggested leaning toward following the precautionary principle and being conservative when uncertainty exists when conducting risk assessment and developing guidance.
Andrew Moy raised issues his manufacturing company encountered in trying to ramp up production of PPE. As a new business, for example, his company could not sell to the federal government because it takes 2 years of financial statements to get on the General Services Administration’s (GSA) schedule of approved vendors. His facility had the capacity to make more than 100 million masks, and of the product it did sell, 95 percent of its production went directly to consumers and health systems that wanted to buy U.S.-made products, though they wanted to purchase them at much lower costs.
He noted that because of the overwhelming number of applications that NIOSH received, it took 6 months for his company to get its application for certification approved, which is when vaccines came out. Sales plummeted, and if not for the anticounterfeiting technology the company developed, the company would have gone under. At the same time, Facebook and Google blocked all N95®3 respirator advertising, which penalized companies making legitimate, certified products but allowed counterfeiters to advertise with impunity. Today, as many as 90 percent of the legitimate manufacturers outside of the three biggest producers have either stopped production or are out of business. In short, a number of factors prevented his company, and others in the United States, from being integrated into the supply chain.
Jayaraman said it would be helpful for standards to account for innovation if they are to evolve as an innovation gets integrated into a product or process. “That is how the standard becomes living,” he said. At the same time, it is important to tie innovation back to quality assurance so that when a manufacturer comes up with a product, it still conforms to the relevant standards.
POTENTIAL OPTIONS TO ADDRESS THE GAPS IN STANDARDS DEVELOPMENT
When asked to propose ways to address the gaps in standards development, Jayaraman said the most important thing is to engage all partners in finding a solution to the challenge of developing standards, beginning with identifying how to remove any barriers. After that, the idea would be to use a prioritization methodology to identify which particular product and attributes would be the focus of efforts going forward.
___________________
3 N95 is a certification mark of the U.S. Department of Health and Human Services (HHS) and is registered in the United States and several international jurisdictions.
Moy, referring to matrix shown in Figure 9-2, said the first step is to identify the critical supply chains and manufacturers for each one. The next step would be to identify some way to work with both public and private purchasers and have each commit some percentage of their budget to U.S.-made products without being held to the same price points as overseas manufacturers. “We have to have resiliency that is based here in the United States, and we need to be able to use [Jayaraman’s] spreadsheet to help identify the gaps, strategic partners, and ways to allocate resources for the current situation and how to ramp up and flex during times when we need to,” said Moy.
Ergun agreed with involving all the actors in the system. She said that the nation’s leadership will and investment to build an incentive system could allow for manufacturing flexibility and the right levels of inventory. She also pointed to the need for governance and regulatory and policy leadership. Shirley said that there are federal agencies that are well positioned to lead other agencies in working together to ensure standards are aligned.







