Reusable Health Care Textiles for Use in Personal Protective Equipment: Proceedings of a Workshop (2024)
Chapter: 2 Level Setting: The U.S. Ecosystem for PPE
2
Level Setting:
The U.S. Ecosystem for PPE
The first session laid the foundation for the workshop by defining key terms and concepts and by describing current complex networks and interconnected systems (i.e., the ecosystems) involved in the manufacturing, distribution, use, and disposal of reusable and disposable health care textiles (HCTs) for personal protective equipment (PPE) in the United States. These ecosystems were introduced by session moderator Elizabeth Easter, professor at the University of Kentucky, who began by referring participants to a set of key terms in the workshop briefing materials, which are included in Appendix B. These definitions and descriptions were compiled to ensure a shared understanding of the ecosystem. In some cases, workshop speakers further clarified or expanded on the definitions. However, every effort was made to be consistent with existing definitions and descriptions from standards organizations and federal agencies. The workshop planning committee established isolation and surgical gowns as the primary HCTs for PPE that were the focus for the workshop. Additional clarification was provided to note that HCTs that are not PPE (e.g., patient gowns, surgical drapes) were not within the scope of the workshop. While they are not HCTs, reusable elastomeric respirators, which are a form of reusable PPE, were also a focus of discussions for the workshop.
Easter emphasized that health care workers (HCWs) include not only medical professionals, but all individuals working within a health care setting who use PPE in their daily tasks or who come into direct contact with health care PPE. Thus, “HCW” encompasses employees who collect, clean, or otherwise care for soiled PPE.
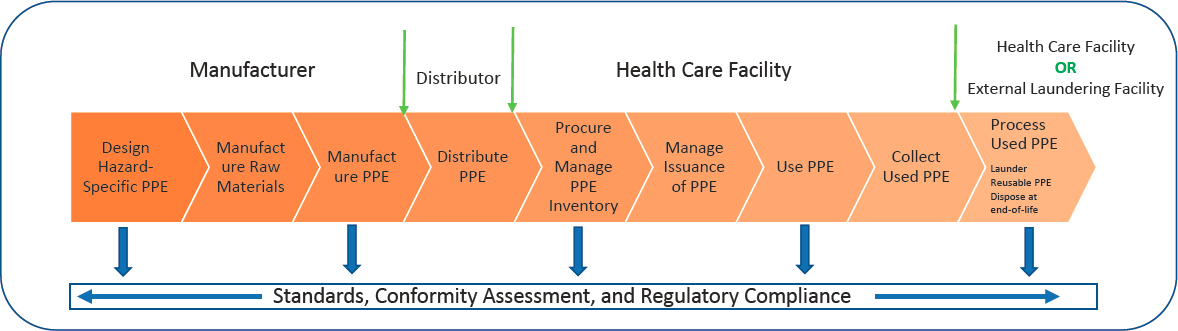
The PPE ecosystem is well established, robust, and entails major operations that begin with PPE design, manufacturing of raw materials, manufacturing of PPE, and distribution, Easter explained (see Figure 2-1). Next, the health care facility procures PPE, manages inventory and issuance, and utilizes PPE. Soiled PPE is collected and disposed of, laundered, or cleaned within the health care setting or at an external laundering facility. For reusable HCTs, laundering involves: (1) collecting and packing soiled items; (2) transporting the used HCTs to an in-house or external laundering department or facility; (3) receiving and laundering soiled HCTs; (4) testing laundered HCTs; (5) determining suitability for reuse; and (6) labeling, packing, and (where applicable) shipping laundered HCTs. The health care facility then receives, stores, and manages issuance of the laundered reusable HCTs. Standards, conformity assessment, and regulatory compliance apply to all operations of this ecosystem. Easter presented a video produced by the Textile Rental Services Association (TRSA) demonstrating the operations within a commercial laundry facility, including sorting soiled items, automated washing and drying, packing processes that ensure laundered HCTs remain clean, and sanitizing the carts that transported soiled HCTs.1 She introduced the life-cycle analysis of HCTs, which considers the cost, energy, equipment, people, and time used in creating, distributing, using, reusing, and ultimately recycling or disposing of a product. This analysis also determines the environmental effects of each of these stages of a product’s lifespan.
LIFE CYCLES OF TEXTILES USED FOR HEATH CARE PPE
John Wintz, group vice president at Standard Textile, provided an overview of reusable PPE manufacturing, processing, and end-user usage, focusing specifically on reusable medical gowns, including isolation and surgical gowns, that are used as PPE in health care settings. He also described regulatory requirements for the field, the evolution of reusable fabrics, and testing and tracking of reusable PPE. Charlie Merrow, chief executive officer at Merrow Manufacturing, compared and contrasted manufacturing processes for reusable and disposable HCTs.
Reusable HCT Life Cycle
The manufacturing of reusable medical gowns begins with development and extrusion of polyester filament yarns, said Wintz. The yarn is
___________________
1 The clip shown during the workshop can be found on the workshop recording and the full video may be accessed at https://www.youtube.com/watch?v=kauTYYj7Rc8 (accessed March 14, 2024).
SOURCE: Presented by Elizabeth Easter on March 4, 2024, at the Reusable HCTs for PPE Workshop.
shipped to a manufacturing plant where it is woven into fabric that is either 100 percent polyester or 99 percent polyester and 1 percent electrostatic dissipative yarn. Fabric formation entails wet finishing, dyeing, quality assurance testing of barrier performance, and other measures.2 Finished fabric is shipped to a cut-and-sew manufacturer that creates garments in a range of sizes with various closures. Isolation gowns typically use tape tie closures at the neck and waist, whereas surgical gowns feature a combination of snaps and tie closures. Additional quality assurance testing is conducted before products are delivered to health care settings or the laundry facilities that service them. In many cases, the reusable HCTs are purchased and owned by the laundry facility, not the hospitals using them, adding an additional level of oversight on the condition of the HCTs, as it is in the laundry facility’s best interest to provide their health care clients with high-quality HCTs. Wintz noted that Standard Textile trains end users on proper donning and doffing procedures, which resemble those for disposable products aside from the location for placing soiled linens.
Reusable isolation gowns are laundered before initial use, and then the method for tracking the number of reuses is implemented, Wintz explained. Tracking can be performed via barcodes, radio-frequency identification (RFID) chips, or grid labels affixed to gowns that are manually marked with indelible marker. Gowns are then distributed to health care settings, and carts of bagged gowns are directed to appropriate departments. After using the gowns, HCWs place soiled gowns in laundry bags. The laundry facility processes and inspects the gowns, marking the tracking grid or scanning the RFID chip or barcode on each item. Depending on the model, reusable gowns generally have a lifespan of 75–100 uses. Laundered gowns that have not reached this limit are redistributed into circulation, whether that means they are sent to health care facilities serviced by a laundry or added back into a facility’s supply by their on-site laundry. In cases where tracking indicates that a gown has reached the maximum number of uses specified, it is retired from service via donation, disposal in a landfill, repurposed in the health care system, or recycled. Wintz calculated that the average 500,000 isolation gowns that Standard Textile sells annually equate to 30–35 million uses if each gown is used 60–70 times.
___________________
2 Barrier performance represents the level of protection afforded by a medical gown. This is determined by the performance of each area of the gown “where direct contact with blood, body fluids, and other potentially infectious materials is most likely to occur.” Four levels of barrier performance are defined, with Level 1 being the lowest level of protection and Level 4 being the highest level of protection (ANSI/AAMI, 2022).
The ecosystem for surgical gowns is slightly more complicated, as it involves sterilization of surgical packs, said Wintz. Laundered surgical gowns are packed in a surgical packing area or facility and undergo 100 percent inspection (i.e., every item is individually inspected) over light tables, tracked, and sent to assembly, where they are folded using an aseptic technique. Wintz explained that these processes are conducted in adherence to the Association for the Advancement of Medical Instrumentation (AAMI) ST65 guidelines (ANSI/AAMI, 2018). Packs contain sterilization indicators that are read after the packs are steam sterilized and cooled. The sterilized surgical gowns are distributed to the operating room (OR), where they are used before undergoing the laundering and sterilization process once more.
Differences in Manufacturing of Reusable and Disposable HCTs
Following on Wintz’s description of the reusable HCT life cycle, Merrow discussed key differences in the manufacturing processes for reusable and disposable medical gowns. The manufacturing of disposable HCTs is highly automated, often involving sonic welding3 and other processes in lieu of sewing. Investment in the automation of disposable HCT manufacturing has been substantial. In contrast, reusable products are sewn, and the processes that craft them are similar to those used 20–30 years ago. The labor-intensive nature of reusable HCT manufacturing makes it more expensive than manufacturing disposable products. Additionally, reusable products are bulkier than disposable counterparts; therefore, the areas required to store and organize material before and after manufacturing vary from the layout of production facilities for disposables. Merrow highlighted an opportunity within the reusable HCT industry to update manufacturing processes and incorporate tracking technology to increase reusable HCT functionality and real-world application. While tracking is often performed by manually marking a tag with a permanent marker, incorporating RFID technologies into more reusable HCT products would improve tracking accuracy in the field. Furthermore, investment in automation could make it possible for products to be registered as they are manufactured, tracked throughout their lifespans, and measured again at disposal for a closed tracking loop. Innovation in PPE could enable reusable HCTs to be made from recycled materials, and the gowns themselves could then be recycled once reaching the end of the product’s lifespan. A similar path toward sustainability does not exist for disposable polyethylene products, Merrow contended.
___________________
3 Sonic Welding is a manufacturing process that can join textile parts by using high-frequency vibration to melt material at the junction of the parts (Micus et al., 2021).
Scaling of reusable HCTs is markedly different from that of disposable HCTs, said Merrow. Automated manufacturing of disposable medical gowns and masks scales with capital investment, whereas reusable HCTs scale with labor investment, which entails workforce training. Thus, an organization can scale manufacturing of a disposable product line more quickly than a reusable product line. Scaling requires investment, coordination, and demand. Increased automation of reusable PPE manufacturing would increase scalability, but the current demand for reusable PPE, particularly for reusable HCTs, is inadequate to motivate investment in automation, Merrow explained. He noted that the COVID-19 pandemic substantially increased demand for reusable PPE, spurring investment and scaling in this industry for the first time in two decades. Matching the scaling capacity of the disposable market within the reusable HCT industry would require replacing humans with robots. Developing the technology required for robots to sew HCTs is a 2- or 3-year process. Eventually, innovation could enable reusable PPE to be produced with processes that minimize environmental effects, lower prices, and enhance scaling capacity. Merrow stated that incorporation of existing technology could lower the cost of manufacturing, improve sustainability, and track reusable PPE throughout the life cycle within 3 years, given investment in implementation. Wintz remarked that robotics and highly efficient machinery are currently being used to increase the efficiency with which reusable HCTs are manufactured, and this innovation has resulted in decreased prices for reusable products.
Evolution of Fabric for Barriers
Over time, fabrics used as barriers have evolved to attain higher levels of performance, Wintz explained. The first generation of these fabrics were 100 percent cotton and featured low thread count, large pore sizes that increased with each processing, and lint generation. Furthermore, these fabrics were not resistant to liquids. The 1960s saw the introduction and growth of disposable fabrics with barrier resistance claims. In the 1970s, the industry began to shift toward the use of blended fabrics, incorporating 45–50 percent polyester with cotton to decrease the fabric’s pore size. However, pores continued to increase in size with each processing, lint generation remained high, and fabrics were not liquid resistant. Treating fabric with barrier chemicals originally developed for military uniforms achieved liquid resistance. A chemically treated, 100-percent-cotton iteration featured a higher thread count to decrease pore size. Yet, the cleaning and sterilization processes abraded the cotton, and liquid resistance decreased as the chemical barrier degraded. The 1980s ushered in the current generation of barrier fabrics, which contain 100 percent polyester
multifilament yarns that provide a high level of consistent and reliable liquid resistance. These fabrics are virtually lint free, easily processed, cost-efficient, and comfortable to wear. Meeting all flame-spread restrictions and requirements, this generation of fabrics is breathable, allowing body heat to evaporate while maintaining a barrier against liquids. Wintz shared his optimism about the future of reusable fabrics as barriers, given the technology fueling development of new synthetic yarns that will be hydrophobic, and compactable to tighten pore size and will feature a permanent liquid-resistant finish that is free of per- and polyfluoroalkyl substances (PFAS). Moreover, potential exists for the development of biodegradable 100 percent synthetic yarns.
Medical Gown Requirements
While policies and standards for medical gowns were the focus of a later session, high-level discussions were held to provide context regarding medical gown requirements. Wintz outlined the U.S. Food and Drug Administration (FDA) requirements for isolation gowns and surgical gowns, which are classified as Class I and Class II medical devices, respectively. Isolation gowns are typically AAMI Level 1 or 2, with Level 1 gowns constituting 95 percent of isolation gowns sold by Standard Textile. FDA requires that manufacturing of Class I devices takes place in a facility that adheres to the quality system regulation and is registered with the FDA.4 Surgical gowns range from AAMI Levels 1 through 4, and the majority of OR gowns are Level 3, said Wintz. In addition to meeting the requirements for isolation gowns, surgical gowns are subject to a higher level of scrutiny as Class II medical devices. Surgical gowns require 510(k) premarket submission to FDA to demonstrate that at the end of life (i.e., after a maximum number of cleaning and sterilizing cycles defined by the manufacturer) the device remains safe and effective in continuing to meet regulations regarding barrier performance, flammability standards, lint generation, and other metrics.5
Shipments of gowns are required to include instructions for use, which is a common requirement for health care industry supplies, Wintz noted. The instructions for reusable medical gowns specify indications for use, processing procedures, sterilization procedures, patching and mending criteria, application of fabric patches, guidelines for inspection, life use tracking, and recommendations for barrier performance
___________________
4 Quality System Regulation, 21 CFR Part 820 (October 7, 1996).
5 More information about Premarket Notification 510(k) is available at https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-notification-510k (accessed March 16, 2024).
testing throughout the life of the product. Required safety and efficacy tests assess fluid barrier performance, flammability, durability through 75 cleaning and sterilizing processes, strength, lint generation, toxicity, and compatibility with steam sterilization. Guidance from AAMI PB70 indicates specific standardized testing to determine performance level and assign ranked classification (ANSI/AAMI, 2022).
SUSTAINABILITY IN THE PPE INDUSTRY
Dan Glucksman, senior director for policy at the International Safety Equipment Association (ISEA), discussed market drivers of sustainability, sustainable practices in the PPE industry, and anticipated trends in the field. In addition to providing PPE manufacturers with a forum in which to develop standards, ISEA offers policy advocacy, insight into the PPE market, and education for customer-facing safety professionals that includes documentation of completed training via Occupational Safety and Health Administration (OSHA) 30 cards, proof of completing an OSHA-approved occupational health and safety training session. The U.S. PPE industry employs 130,000 people in manufacturing, distributing, and selling PPE, with PPE protecting 125 million workers, said Glucksman. The largest PPE needs are hand protection and safety apparel, used by 105 million and 102 million workers, respectively.
Market Drivers of Sustainability
Glucksman described growing market forces that drive sustainability and reflect growth in consumer products with environmental, social, and governance claims such as electric cars and energy-efficient household appliances. A consumer survey found that 60 percent of individuals are willing to pay more for a product with sustainable packaging (Feber et al., 2023). Sustainability efforts are increasing within the business sector, with 80 percent of surveyed business leaders viewing sustainability as helping to optimize and reduce costs and 86 percent seeing sustainability as an investment that protects against disruption (Gartner Inc., 2022). A survey of PPE suppliers found that 96 percent of respondents employ some sustainable business practices, and 70 percent of PPE manufacturers consider sustainability commitments important to their overall competitiveness (ISEA, 2023b). Furthermore, 68 percent of 370 hospitals surveyed have a sustainability procurement policy in place (Practice Greenhealth, 2023). Examples of sustainability efforts include PPE manufacturer Ansell’s substantial investment in sustainable packaging and provision of an environmental impact calculator to users. Thermal Compaction Group, a sustainable solutions company in the United Kingdom, converts dispos-
able face coverings into blocks of raw polypropylene that can be used to make products ranging from toolboxes to lawn chairs (Pigott, 2021). Glucksman noted that regulatory factors are also in play. For instance, in 2022 the U.S. Securities and Exchange Commission (SEC) proposed rules requiring listed companies to disclose greenhouse gas emissions for all material suppliers, transportation of materials, and manufacturing sites.6 Notwithstanding the high cost that compliance with this proposed regulation could carry, SEC recommended these rules in response to investor demand for useful, comparable, and reliable information. Additionally, a 2021 executive order from the Biden administration requires federal agencies to adopt various sustainability practices.7
Sustainability Trends in PPE Industry Practices
Glucksman highlighted a 2023 ISEA member survey that bench-marked current sustainability practices, assessed customer and stakeholder engagement, and predicted future trends (ISEA, 2023a). He noted that most ISEA members are mid-sized manufacturers. The survey found that 86 percent of respondents employ sustainable business practices related to product packaging. Furthermore, a majority of those surveyed use sustainable industry practices related to product materials and design (66 percent), operations and facilities (60 percent), and the supply chain (55 percent). Respondents cited regulatory compliance as the primary driver for sustainable practices, followed by new growth opportunities and alignment with company values and mission. Glucksman associated new growth opportunities with customer demand for sustainable products, citing one respondent’s feedback that “it’s critical to our mission and our customer base to be more sustainable than the industry.” Indeed, 87 percent of companies surveyed identified end-user pressure as an important driver of sustainability decisions. Even so, end users often do not want to pay a premium for sustainable products, and less than 10 percent of respondents indicated that end users are “very likely” to be willing to pay a premium for products that help meet environmental, social, and governance goals. “It’s all about trade-offs. Most everyone wants sustainability, but they don’t want the production cost increases required to pay for it,” a survey respondent commented. Nonetheless, 77 percent of companies surveyed consider sustainability an important
___________________
6 The SEC adopted these rules on March 6, 2024, after the conclusion of the workshop: The Enhancement and Standardization of Climate-Related Disclosures for Investors, 17 CFR Parts 210, 229, 230, 232, 239, and 249 (March 6, 2024).
7 Executive Order 14057: Catalyzing clean energy industries and jobs through federal sustainability (December 8, 2021).
driver of competitiveness, and 89 percent expect sustainability practices to become a more important purchase criterion in the next 3 years.
Currently, some companies are using sustainability as a differentia-tor, and Glucksman predicted that this trend would grow to the point that only companies with some level of sustainability in their operations have a chance of remaining competitive in the market. Customer preferences for inclusive and fashionable PPE are also driving business trends. The market is responding to increased demand for PPE that fits all body shapes, sizes, and genders. Moreover, users want PPE that is stylish and fits properly. Glucksman remarked that ISEA members strive to be responsive to these needs and are actively participating in developing solutions at the interface of manufacturing and customer preferences.
REUSABLE PPE CONSIDERATIONS IN HEALTH CARE
Pamela Falk, epidemiologist at the Association for Professionals in Infection Control and Epidemiology, outlined requirements for PPE in various medical contexts, current literature on reusable HCTs, and considerations regarding PPE inventory management, laundering, storage, and waste. She noted that use of PPE, such as gloves and gowns, became more widespread in health care in the 1980s during the acquired immunodeficiency syndrome epidemic to protect HCWs from exposure to blood and body fluids, and subsequently, potential exposure to infection from the human immunodeficiency virus and viral hepatitis. Use of PPE in health care has continued to expand, and gowns, gloves, and masks are routinely used by HCWs to protect themselves from infection and to diminish the transmission of infectious agents between patients, such as those placed in isolation.
PPE Considerations for Various Health Care Contexts
The health care setting informs PPE needs, as various types of PPE are required for the OR, endoscopy laboratory, patient rooms, isolation rooms for infectious diseases, and inpatient and outpatient areas. Falk asserted that the variability in PPE manufacturing and use across health care contexts precludes a one-step solution to PPE waste in health care. For example, PPE worn in trauma surgery ORs is required to be sterile and impermeable to body fluids. Surgeons often press their bodies against the surgical field, and they therefore need protection from exposure to blood and body fluid seepage. She noted that HCW exposure to the blood or body fluids from a patient with an infectious blood-borne disease may require post-exposure prophylaxis medication depending on the situation. Blood and body fluids passing through PPE (i.e., strikethrough) can
also harm the patient, as any blood transferred from the operative field to the surgeon’s skin could carry bacteria from the surgeon’s skin into the patient’s wound, creating risk for infection. Thus, surgical gown strikethrough is a breach in sterile technique, Falk explained.
All gowns worn in the OR must be sterile, but standards differ for various types of procedures and services in health care settings, said Falk. For instance, gowns worn for ophthalmology or other surgeries that involve low levels of blood or body fluid spillage don’t require the same level of impermeability as gowns used in surgeries with greater potential fluid transfer. Procedures performed outside of the OR, such as some endoscopy, urology, and ear, nose, and throat procedures, require gowns that guard against fluid transfer but do not always necessitate sterile gowns. The gowns worn by HCWs while giving patients baths must protect against soap, water, and body fluids, as baths may expose HCWs to feces, urine, and, in some cases, blood. In contrast, when dietary workers deliver meals to patients in isolation with multidrug-resistant microbial infections, no level of impermeability is required in gowns. However, the risk of transmitting infectious agents from one patient to another necessitates that gowns are worn as a barrier between the HCW and the environment. Before leaving a patient’s room, the dietary worker would need to remove and dispose of the gown to prevent germs from being carried into other areas of the hospital. Falk emphasized that the invisible nature of infectious agents makes it challenging to recognize the hazard they present on gowns that appear to be clean. The variety of needs stemming from diverse health care procedures and services requires an array of PPE.
Data Considerations
Given the variety of PPE purposes, Falk underscored the importance of stratifying PPE by use when generating data and conducting analyses on types of exposures in health care. An analysis that includes both PPE for bathing a patient and PPE for trauma surgery could give rise to confusing conclusions. Yet, much of the data currently available on PPE fail to make the distinction between sterile and non-sterile settings, she asserted. For example, a literature review examining PPE permeability might aggregate various gowns without stratifying data according to context, such as setting (e.g., OR) or procedure (e.g., endoscopy, meal delivery, or patient bathing). Therefore, conclusions based on non-stratified data may be confounding. Sterility and permeability requirements for given contexts should be considered and similar products compared in conducting PPE analyses, said Falk.
Logistics
Numerous factors must be considered in storing, laundering, and disposing of PPE, noted Falk. Chief among these factors is storage, as space is often a resource in short supply. Most hospitals do not have warehouses or material management areas, and on-unit space is prioritized for patient care. Given these space constraints, many hospitals use just-in-time inventory management, a strategy that involves keeping minimal inventory on hand and timing product delivery shortly before use. Packaged disposable gowns are more compact than their reusable counterparts, requiring less space for storage. Storage space for soiled linens and waste is also limited, and surgical gowns containing blood and body fluids may be considered biohazardous, thus requiring specialized, secure storage. Some large hospitals have autoclaves on-site that sterilize and shred biohazardous waste before sending it to a landfill.
An additional logistical consideration relates to product availability. The range of sizes, shapes, and styles of disposable gowns is currently wider than for reusables, although the reusable HCT industry is working to narrow this gap.
Laundering presents several logistical considerations highlighted by Falk. Reusable gown wash cycles must be tracked to ensure that the PPE continues to offer adequate protection. Reusable gowns contaminated with large amounts of blood can be difficult to wash. Workers in laundry facilities must wear PPE when sorting soiled reusable HCTs, generating additional laundry. Laundry facilities use chemicals and produce wastewater, and these environmental considerations should be considered. Most laundry facilities found in nursing homes and hospitals lack the capacity to sterilize gowns. Falk concluded that PPE waste can be reduced, but the complete elimination of disposable PPE is unlikely.
DISCUSSION
Tracking Reusable PPE Lifespan and Product Loss
In response to a question about the use of tracking to prevent product loss and ensure that PPE is not used beyond the established lifespan, Merrow stated that the technologies for improved tracking currently exist; manufacturers and hospital systems could organize an approach to using these technologies in a manner aligned with their goals. Wintz noted that RFID and barcode chips enable monitoring of both product usage and disappearance and that these tracking methods are currently used more often than manual marking. Each time a product is washed, a worker scans the RFID or barcode chip, and the electronic reader issues an alert
when a product reaches its maximum lifespan. Reusable PPE is clean at the time of tracking; thus, products sent to landfills, recycling, or donation programs are clean. In conducting cost analyses, Wintz reduces the number of uses from 75–100 to 55–65 to provide a conservative estimate that accounts for potential product loss or damage.
Reusable PPE and PFAS
A workshop participant asked if the elimination of PFAS chemicals—which demonstrate excellent water repellency and have therefore been used in PPE manufacturing to provide barrier resistance to liquids—affects the lifespan of reusable PPE. Wintz remarked that there are over 20,000 chemicals containing PFAS. Standard Textile does not manufacture products or have any surgical or isolation gowns containing PFAS or perfluorooctanoic acid and is working toward PFAS-free facilities within the next few years. Eliminating PFAS does not change the performance characteristics of HCTs in terms of lifespan, said Wintz. He added that Standard Textile is currently testing its PFAS-free products in accordance with FDA 510(k) filings. Easter stated that she has been involved in testing several varieties of PFAS-free gowns, and performance continued to meet the standards through 75 washes and, in some cases, up to 200 washes. Products free of PFAS are currently coming onto the market in the PPE arena. Wintz noted that for some types of reusable PPE, eliminating PFAS chemicals is not expected to increase manufacturing or consumer cost.
Demand for Reusables During the COVID-19 Pandemic
A question from the audience focused on buyer demand for reusable PPE during the COVID-19 pandemic. Merrow recounted that in situations where both disposable and reusable PPE were available, almost all hospitals chose disposable products, both for cash flow purposes and for standard practices within hospital systems. Hospitals tend to maintain as small an inventory of PPE as possible, and switching from disposable to reusable products requires a larger initial outlay. Supply shortages led 121 hospitals to purchase reusable PPE from Merrow Manufacturing, all of which returned to disposable products once they became available. In fall 2020, Merrow Manufacturing achieved price parity (the price level that sets two items equal in value to one another) between imported single-use disposable products and amortized reusable PPE, even when accounting for laundering costs, yet the preference for disposable PPE persisted, Merrow emphasized. This indicates that the hesitancy to adopt reusable products cannot solely be attributed to cost considerations, Merrow said. Wintz remarked that Standard Textile saw a similar trajectory. He linked
the preference for disposable PPE to familiarity and ease of use rather than an in-depth cost analysis, as costs are competitive. Notwithstanding this preference, Standard Textile—one of several U.S. manufacturers in the industry—sells approximately 500,000 reusable PPE products each year.
Innovation and User Engagement to Foster Adoption of Reusable PPE
In response to a question about design innovations that could increase HCW engagement in transitioning from disposable to reusable PPE, Merrow emphasized the value of soliciting and incorporating user feedback in designing, iterating, and improving PPE. He remarked that PPE is used in thousands of environments that feature subtle differences. Merrow Manufacturing incorporated input from health care providers in creating a reusable gown prototype crafted from 100 percent recycled product that could withstand 200 wash cycles. He highlighted a tremendous opportunity to improve fiber, fabric, and design to optimize sustainability and fitness to application. Wintz reiterated the opportunity for innovation within reusable PPE, including efforts to advance recyclable yarns to establish cradle-to-cradle, closed-loop systems in which HCT products at the end of their lifespan could be recycled into new products. He also expects further innovation in robotics for manufacturing reusable PPE. Efforts are needed to increase demand for reusable products and Wintz was optimistic that the current amplification of sustainability messaging generally taking place will foster demand for reusable PPE. He noted that increased use of reusable PPE will improve sustainability even if the use of disposables is not eliminated. Falk stated that many hospitals and nursing homes convene product evaluation committees to test technologies as they enter the market. Members of these committees use and evaluate newly available PPE in their work settings. This process is a mechanism for educating HCWs about products and generating buy-in. Falk added that an organization might engender resistance in pushing out an unfamiliar product to all employees simultaneously, particularly for products relied upon for safety.













