Rethinking Race and Ethnicity in Biomedical Research (2025)
Chapter: 4 Existing Guidance on Using Race and Ethnicity in Biomedical Research
4
Existing Guidance on Using Race and Ethnicity in Biomedical Research
Many partners in the biomedical research enterprise, including federal governmental agencies, professional societies, journals, and other institutions have published guidelines outlining changes they want to see in the use of race and ethnicity for clinical practice guidelines, clinical algorithms, clinical trial enrollment procedures, data reporting, and more. This chapter reviews some of these current guidelines and identifies gaps that remain. This existing guidance served the committee both in its deliberations and in developing its recommendations and their rationale, which are presented in Chapters 5 and 6. Before discussing specific guidance, it is worth noting some of the large gaps in guidance that exist. One area where the committee struggled to find guidance for the use of race and ethnicity is in earlier translational stages (T0 and T1) of biomedical research. As discussed in Chapter 3, race and ethnicity may be less relevant to the research questions at these early translational stages compared with later translational stages (T2–T4). However, there may be instances, particularly in T1, where human participants may be involved in research and guidance for how to collect and use race and ethnicity data would be beneficial. The committee also struggled to find specific guidance for the use of race and ethnicity for biobanks, which may hold health samples and health information for thousands of human samples.
GUIDANCE FOR USING POPULATION DESCRIPTORS IN GENETICS AND GENOMICS RESEARCH
The 2023 National Academies report Using Population Descriptors in Genetics and Genomics Research: A New Framework for an Evolving Field (“population descriptors” report), provided detailed guidance on the use of descriptors such as race, ethnicity, and ancestry for investigators conducting research using genetics or genomics data. Recommendations relevant to the broader biomedical community provided in that report are summarized in Box 4-1. Readers conducting genetics and genomics
BOX 4-1
Using Population Descriptors in Genetics and Genomics Research
Requisites for Sustained Change
The population descriptors report included recommendations addressing long standing issues in genomics research: the use of typological thinking, analysis of environmental factors into examinations of genetic effects, and community engagement (NASEM, 2023).
- Researchers should not use race as a proxy for human genetic variation. To eliminate typological thinking and establish new models that better reflect the complex reality of human genetic ancestry, researchers should avoid typological thinking when grouping people in genetics and genomics studies, refrain from using race as a proxy for human genetic variation, and be sensitive to the connotations and impacts of the labels they do choose to use.
- Researchers should directly evaluate the environmental factors or exposures that are of potential relevance to their study, rather than rely on descriptors like race and ethnicity as proxies. A lack of information about environmental factors can cause descriptors to be used as proxies for environmental factors and result in the attribution of unexplained phenotypic variance between populations to unmeasured genetic differences. When measuring environmental factors is not feasible and population descriptors need to be used as proxies, then researchers should explain why these descriptors were chosen, how they are relevant, and how they are being used.
- Researchers should work in ongoing partnerships with study participants and community experts to integrate community perspectives into the research and to inform the selection and use of population descriptors. The legacy of community engagement by researchers in genetics is fraught with failure to respect communities and avoid harm. Successful community engagement takes effort and often requires creating a multidisciplinary research team, but these efforts are essential for success.
Best Practices for Researchers
The population descriptors report provided detailed practical guidance and tools for researchers who use descent-associated population descriptors in their genetics or genomics research while acknowledging that there is no single solution or approach that will work for all researchers in all situations.
- Researchers should first determine thoughtfully whether descriptors are needed at all for their study.
- Researchers should tailor their use of population descriptors to the type and purpose of the study, in alignment with scientific and ethical guiding principles, and explain how and why they used these descriptors.
- Researchers should disclose the process by which they selected and assigned group labels and the rationale for any grouping of samples. Where new labels are developed for legacy samples, researchers should provide descriptions of new labels relative to old ones.
Implementation and Accountability
Genetics and genomics research (and all research, truthfully) occurs within a complex research ecosystem. Institutions provide infrastructure and workforce, granting agencies provide essential funding, and professional societies and journals offer outlets for dissemination of results. All of these actors share responsibility for making lasting and meaningful change. Researchers cannot do this alone.
- Funding agencies, research institutions, journals, and professional societies should offer tools widely to their communities to facilitate the implementation of the report’s recommendations. These tools should be publicly available, especially when they are supported by public funds.
- Funding agencies and research institutions should incentivize and reward investigators for fostering interdisciplinary collaborations among researchers with different areas of expertise to facilitate the inclusion of environmental measures and the engagement of diverse communities.
- Multidisciplinary advisory groups should be established to periodically evaluate the report’s recommendations, best practices, and the implementation strategies established by funders, research institutions, professional societies, and journals.
research are encouraged, though, to read the population descriptors report for more detailed guidance.
As in this current report, the population descriptors report acknowledged that other parties in the broader genomics research ecosystem have critical roles in effecting change. Thus, the report provided specific guidance and recommendations for funding agencies, research institutions, research journals, and professional societies to assist in implementing the recommendations, establishing mechanisms of accountability, and creating an environment that will foster the recommended changes.
GUIDANCE FOR COLLECTING RACE AND ETHNICITY DATA IN CLINICAL TRIALS
Clinical trials are vital for testing new treatments aimed at reducing disease morbidity and mortality. Historically, trial populations did not represent the diversity of the general U.S. population, nor the diversity of the populations affected by the disease or condition under study. Clinical trials may encounter difficulties enrolling minoritized groups because trial recruitment may not be tailored to fit the needs of diverse groups. The underrepresentation of racial and ethnic minority populations has consequences, including limiting opportunities for equitable access to new therapies, threatening the efficiency of research, and limiting the generalizability of the results (NASEM, 2022).
Many populations remain underrepresented in trials for drugs approved by the U.S. Food and Drug Administration (FDA) (Martei et al., 2024), including Black, Hispanic, American Indian, and Alaska Native populations. As an example, Chen et al. (2021) conducted a review of clinical trials that led to the approval of 24 cardiometabolic treatments from 2006 to 2024 and found that only 2.9 percent of the 187,294 participants enrolled in these trials were Black (Chen et al., 2024; Martei et al., 2024). However, it is difficult to track the demographics of clinical trial participants in the United States. Although FDA guidance has encouraged the collection and reporting of participant age, sex, race, and ethnicity in clinical trials for many years (FDA, 2016), progress has been slow (Martei et al., 2024). For example, in a study that looked at more than 20,000 U.S.-based clinical trials, only 43 percent reported any race and ethnicity data (Turner et al., 2022). FDA “Drug Trial Snapshots” provides a helpful overview of the demographics of clinical trial participants in Phase III trials of approved drugs each year. However, this does not account for the many trials that never receive FDA approval nor earlier Phase I or Phase II trials. FDA Snapshots also does not track clinical trials for medical devices. Further, inconsistencies in reporting trial data to ClinicalTrials.gov make it difficult to examine race and ethnicity across a large number of clinical trials (NASEM, 2022).
Given the importance of enrolling diverse populations in clinical trials and research, a recent National Academies report examined strategies to improve the representation of underrepresented populations in clinical research and provided a series of recommendations to advance research with diverse populations (Box 4-2). Since the report was released, some of these recommendations have been implemented, including a
BOX 4-2
Improving Representation in Clinical Trials and Research
To improve research quality and to support greater health equity, it is important for all populations to have access and opportunities to participate in clinical research. In 2022, the National Academies released a report titled Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups. The actions outlined in that report are critical for ensuring that diverse populations have the opportunity and access to participate in biomedical research and that the ethical principles outlined in Chapter 2 of this report are realized. This 2022 report concluded that improving representation in clinical research is urgent, requires investment, and is the responsibility of everyone involved in the clinical research enterprise. The report’s epilogue describes a more equitable future emerging as a result of a paradigm shift that transfers power from investigators and institutions toward community members.
The 2022 report included 17 recommendations, which divide into several themes:
Reporting: Current data tracking and monitoring of the demographics of clinical trial participants are insufficient and do not provide a clear picture of who is participating in clinical research in the United States. Therefore, the committee recommended that the U.S. Department of Health and Human Services (HHS) establish an intradepartmental taskforce on research equity charged with coordinating data collection and developing better accrual tracking systems across federal agencies. The report also recommended that the National Institutes of Health (NIH) standardize the submission of demographic characteristics to Clinicaltrials.gov beyond existing guidelines so that trial characteristics are labeled uniformly across the database and can be easily disaggregated, exported, and analyzed by the public.
Increased Accountability: For new investigational drugs and devices, industry sponsors have a responsibility to ensure that the populations enrolled in those studies are representative of the population affected by the disease or condition under study. The report recommended that the FDA use their existing authorities to ensure that studies are designed with representation in mind and that study teams are appropriately planning for enrolling appropriate populations in their studies. The report also recommended that the Office of Human Research Protections (OHRP) and the FDA direct institutional review boards (IRBs) to assess and report representativeness as a measure of sound research design.
Federal Incentives: Improving representation of underrepresented and excluded populations in clinical research requires an investment of money, time, and effort. To offset some of these costs and to incentivize industry to make
these investments, the report recommended that Congress should establish a taskforce to study new incentives such as extended market exclusivity, tax credits, or fast-track criteria for new drug and device applications for trials that achieve representative enrollment. The committee also recommended that the Centers for Medicare & Medicaid Services (CMS) incentivize community providers to enroll and retain participants in clinical trials by reimbursing for the time and infrastructure that is required and that CMS expedite coverage decisions for drugs and devices that have been approved based on clinical development programs that are representative of the populations most affected by the treatable condition.
Remuneration: Reported barriers for diverse population participation in clinical research includes the lack of adequate reimbursement for expenses such as lost wages, transportation cost, housing and lodging costs, and more. Therefore, the report recommended that federal regulatory agencies, including the OHRP, NIH, and FDA, develop guidance to direct local IRBs on equitable compensation to research participants and their caregivers. This guidance should allow for differential compensation to research participants and their caregivers according to the time and financial burdens of their participation.
Education, Workforce, and Partnerships: Research suggested that engaging with community members can lead to more equitable study designs and increase participation of these community members in clinical research. Therefore, the report recommended that HHS should invest in building a community research infrastructure intended to improve representation in research. This funding should go to agencies such as the Health Resources Service Administration, NIH, Agency for Healthcare Research and Quality (AHRQ), Centers for Disease and Control and Prevention (CDC), and Indian Health Service to expand the capacity of community health centers and safety net hospitals to participate in and initiate clinical research focused on conditions that disproportionately affect the patient populations they serve. The committee also recommended that leaders of academic medical centers and large health systems should recognize research to advance community engaged scholarship as an area of excellence for promotion or tenure.
Congressional requirement that drug and device manufacturers must submit a diversity action plan to the FDA that details their plans to enroll a diverse population in the clinical trials. Although specific strategies to improve representation in clinical trials are outside of the scope for this committee, the committee wanted to highlight some of the findings of this report, which are critical for ensuring health equity and that all populations have the opportunity to contribute to clinical research.
In an effort to increase the diversity of clinical trial populations and broaden the eligibility criteria for clinical trial enrollment, the FDA has advised sponsors “to consider patients with co-morbid illnesses such as chronic kidney, heart, and liver disease, prior or concurrent malignancy, and extremes of weight” (Martei et al., 2024, p. 387; see also FDA, 2020). In another strategy to increase diversity, adaptive designs would start with a more narrowly defined population and then expand trial eligibility to a broader population, based on interim safety data (FDA, 2020). This guidance has important implications for the inclusion of participants in trials since many underrepresented minority populations have higher rates of multiple overlapping comorbidities, e.g., heart disease and chronic kidney disease.
FDA Diversity Action Plans
To improve representation in clinical trials, the FDA introduced guidance in 2022 for inclusion of diversity action plans in study design (FDA, 2022). In December 2022, an omnibus appropriations bill, the Consolidated Appropriations Act 2023 (P.L. 117-328), was passed, including the Food and Drug Omnibus Reform Act of 2022 (FDORA). This act includes “provisions intended to promote diversity in clinical trial enrollment, encourage the growth of decentralized clinical trials, and streamline clinical trials” (Peloquin, 2023). Under this act, the FDA has the legal authority to mandate sponsors of Phase III or other major drug trials to specify enrollment goals by age, sex, race, and ethnicity (Martei et al., 2024). This requirement includes a “rationale for these goals, informed by disease prevalence or incidence among various demographic groups, and an action plan for meeting these goals including demographic-specific outreach and enrollment strategies, inclusion and exclusion practices, and diversity training for study personnel” (Martei et al., 2024, p. 387).
However, there are many challenges to operationalizing FDORA. One shortcoming is the lack of an enforcement mechanism if sponsors fail to meet the intended plan for recruitment. Although the FDA could technically deny approval for a drug that does not meet recruitment targets, it is highly unlikely to delay approval and access for a safe and effective medication for this reason. Further, even after a drug is on the market, race and ethnicity data are not required for post-marketing studies and are rarely reported in postmarket databases, such as the Food and Drug Administration Event Reporting System (FAERS) (Muñoz et al., 2024).
Another set of challenges is the availability and source of the data used to inform the diversity action plans, limitations of the datasets used, timeliness of the data, and lack of data for rare diseases. Examples of currently available data that can inform diversity action plans include:
- Registries such as SEER (the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program) or state cancer registries. The strengths are that these often have outcome data, but one limitation is that they lag and are not available in real time.
- Real-world data, such as data based on health care administrative (insurance) and pharmacy claims as well as data from electronic health records or electronic
- medical records. The large sample sizes available from these sources is a strength. However, these data also have many limitations—they are protected and limited to being collected at point of service, and they may be unstructured. Claims data often lack clinical outcomes/endpoints, and often people are lost to follow-up.
- Databases from companies such as Flatiron1 have combined real-world data from multiple sources.
Sponsors of clinical trials are still seeking clarity to unanswered questions about diversity action plans. For example, if a sponsor recruits predominantly Black populations globally for a trial, how does that affect diversity action plans for drug approvals in the United States? Another challenge to reducing the burden of trials as stipulated in the diversity action plans is the absence of a national policy that provides safe harbor to the sponsors for the financial reimbursement of out-of-pocket expenses associated with participating in clinical trials (travel, lodging, lost wages, childcare/eldercare costs, etc.). There is also a tension between sponsors’ time pressures to bring drugs to market and operationalizing diversity action plans.
GUIDANCE FOR COMMUNITY AND PARTICIPANT ENGAGEMENT IN BIOMEDICAL RESEARCH
In recent years, there has been a growing recognition of the importance of collaborating with communities throughout the research process. Community engagement can be defined as “the process of working collaboratively with and through groups of people affiliated by geographic proximity, special interest, or similar situations to address issues affecting the well-being of those people” (CDC, 1997, p. 9). Moreover, as noted by Bergstrom and colleagues, community engagement is not simply a set of methods confined to a particular study but rather “a way of communication, decision making, and governance that gives community members the power to own the change they want to see, leading to equitable outcomes” (Bergstrom et al., 2012, p. 4).
Although an extensive review of best practice in community engagement is outside the scope of this report and merits further consideration, collaborative decision making throughout the research process is critical for improving the use of race and ethnicity in research studies, given how these concepts are interwoven with identity, social context, environmental exposures, and health. The health of individuals and communities is affected by socioeconomic, lifestyle, and behavioral factors as well as by the physical environment and the political and legal landscape (Hanson, 1988; IOM, 2003a,b). Community engagement has been an important strategy for achieving racial and ethnic representation in research (e.g., Beech and Heitman, 2024); the goal is to engage community members in the process of designing protocols, including procedures for collection and use of race and ethnicity data, that meet their needs and that account for the barriers they face to participation. Inclusive community involvement throughout the research process can also increase the relevance, quality, generalizability, and
___________________
1 https://flatiron.com/ (accessed October 16, 2024).
dissemination of health research (Hood et al., 2010). Community representatives and patient advocates can play a role in identifying and prioritizing research questions that are important to specific populations, provide insight and networks to aid dissemination of research results, and contribute to translating research findings into practice and policy (Hood et al., 2010). Racial and ethnic populations are not a cultural monolith, so including perspectives from a range of community members can increase research relevance to the broader community.
As part of their work, the committee sought to hear community perspectives on the use of race and ethnicity in biomedical research and hosted a moderated discussion with invited panelists. (See Appendix A for the agenda and session objectives.) Panelists discussed their experiences with the collection and use of race and ethnicity data during different phases of biomedical research and how to build trust and stronger partnerships between scientists and communities. The session emphasized the importance of partnering with communities to better address race- and ethnicity-related issues in biomedical research and highlighted several important themes. First, panelists highlighted that race and ethnicity are social constructs that are challenging to define but have meaning for people’s lives. The panelists emphasized the role of race and ethnicity for their own identities and the importance of seeing people like them and their communities in biomedical studies, further underscoring the importance of diverse representation in research. Moreover, when asked about their motivations for participating in studies, panelists named contributing data that could improve health among their communities as well as sharing information about their racial and ethnic identities for research purposes. For example, one panelist said, “[I was] following the footsteps of my mom and knowing that all of the different things that she had been involved [in] . . . was helping others in our community. My getting involved again [in clinical trials] was mainly because of the limited amount of participation from African American males particularly getting involved in research or practices in which the community as a whole could be better served” (Donald Adams, Jr., in remarks to the committee on March 14, 2024).
Second, panelists emphasized the importance of balancing qualitative methods with standardized measures of race and ethnicity to capture more granular information about an individual’s identity and about the heterogeneity of populations. Racial identity may be fluid and may shift as a result of life experiences and the social context individuals are in. Although most people use categorical descriptors to identify their race or ethnicity, these classifications are more complex and fluid for some individuals (Croll and Gerteis, 2019). Collecting additional information about racial and ethnic identities allows researchers to more accurately reflect the complexity of these classifications (Saperstein, 2012). However, panelists did note that we still need standardized approaches to asking about race and ethnicity in medical research settings to minimize misclassification, which can further exacerbate problems.
Lastly, understanding the narrative of what makes people who they are and what is important to communities can improve biomedical research, increase the quality of data about race and ethnicity, and illuminate unexpected and rewarding lines of inquiry. However, doing this work requires deep engagement with communities and research participants that cannot be episodic or transactional. As panelists noted, partnerships that are equitable support long-term, longitudinal relationships that are built on trust, and
future collaboration is predicated on continuous engagement and follow-through after the study. Building relationships with communities requires consistent engagement and an understanding of a community’s culture, viewpoints, and history (NASEM, 2022). These relationships take time to build but can be broken in an instant. Building and maintaining these relationships therefore requires an investment of resources and time that many academic researchers find challenging, given the limitations of grant budgets and timelines. Industry-sponsored research faces similar challenges of maintaining funding for community sites in the time between one study ending and another study beginning. Another challenge is that in order to get IRB approval, every party engaged in research, including staff from a community organization, must undergo the required human subjects protection training (Anderson et al., 2012). This may be challenging for those in community organizations to complete in a timely manner. Although outside of the scope of this report, these are real challenges for conducting and funding community engaged research that require attention.
Despite these challenges, the committee identified community partnership as a key strategy for implementing the report’s recommendations, especially in later stages of translational research (T2–T4), and for improving the use of race and ethnicity in future biomedical research. For those engaging in this work, how to conduct this engagement and where to begin will depend on the context of the study, the experience of the researcher and their institution working with the surrounding community, and what resources have already been built within the community. Although most academic medical centers have a long history of community service, a more comprehensive approach to community engagement in research would lead to more discoveries and healthier communities (Wilkins and Alberti, 2019). The following section outlines some strategies for doing this work and some examples of successful community engagement. (See Chapter 6 for more information on the role of community engagement in the committee’s recommendations.)
Operationalizing Community Engagement
From a pragmatic perspective, community engagement exists on an engagement continuum, ranging from outreach to shared leadership (CDC, 2011; see also Table 4-1). On one end of the engagement spectrum, communication is primarily unidirectional, with communities only informed about current research and results (Hood et al., 2010). In the middle of the spectrum, engagement transitions toward bidirectional communication, and communities are engaged in important, but limited, research processes, such as recruiting research participants (Hood et al., 2010). On the other end is community-based participatory research (CBPR), which consists of shared decision-making governance and leadership to facilitate equal involvement of community partners and researchers (Hood et al., 2010; Paskett et al., 2003). CBPR not only increases community understanding of important research topics but also enables researchers to understand and address community priorities and to implement culturally sensitive research approaches (NIMHD, 2024). Moving from left to right along this spectrum of engagement increases transparency and dialogue between community members and researchers and requires greater time, effort, and financial investment from study teams.
TABLE 4-1 Community Engagement Continuum: A Comparison of Levels of Engagement
| Increasing Levels of Community Engagement | |||||
|---|---|---|---|---|---|
| Level of Engagement | Outreach | Consult | Involve | Collaborate | Shared Leadership |
| Definition | Interaction with the community where information flows from a researcher or research entity to the community | An information-seeking practice that incorporates community input on research design, implementation, or dissemination in the decision-making process | The involvement of multiple interested parties by researchers toward achieving goals established by the researcher | The formation of partnerships between researchers and other interested parties to achieve a common goal | Consensus-driven research process based on a strong system of relationships, reciprocity, and trust; final decision making is at the community level |
| Goal or Objective | Provide the community with information about research studies or results | Inform communities about research proposals or results; community feedback informs researchers’ decision-making | Obtain more participation from communities on research issues via community-led organizations | Form partnerships and work together on each aspect of research study from development to solution | Work together as equals on accomplishing a mutually agreed-upon research goal |
| Methods | Information or materials distributed via email, phone calls, mailing lists, community visits, presentations at community events, announcements, listening sessions, or media interviews | Surveys, questionnaires, facilitated discussions, focus groups, interviews, social media engagement, email blasts, newsletters, websites, SMS mobile, community input sessions, advisory boards | Involve partners and interested parties to obtain feedback or input on research | Partners and other interested parties share responsibility with researchers for achieving the study goals | Researchers and community partners create a common agenda and research aims together Shared framework for performance measurement and continuous communication |
| Level of Engagement | Outreach | Consult | Involve | Collaborate | Shared Leadership |
|---|---|---|---|---|---|
| Examples for Use of Race and Ethnicity | Researcher provides community participants with information about why race and ethnicity are being collected and how the data will be used | A researcher seeks input from community advisory board on a research proposal to design the protocol for collecting race- and ethnicity-related data | A researcher seeks the help of community-led organizations to reach diverse populations for input on a research study of racial or ethnic health disparities | A researcher partners with a community organization to help identify and screen participants for a study about a health issue important to the community | A research team and community work together to identify a health problem important to the population; the community and study team co-design the research aims, protocol, and procedures |
| Examples of Specific Research Studies or Programs | Recruitment of Three Generations of African American Women into Genetics Research (Taylor, 2009): Participants were recruited using methods such as advertisements, presenting to churches, and outreach to HBCU sororities |
NIH All of Usa: Research program that supports and recommends that awardees establish community or participant advisory boards to provide input on issues like recruitment methods and design of promotional messaging and engagement materials |
NIH Community Engagement Alliance (CEAL)b: A research network designed to work with community-based organizations to strengthen partnership through community-engaged research to address racial, ethnic, and socioeconomic health disparities |
Jackson Heart Studyc: A 20-year prospective community-based cohort that successfully enrolled 5,306 African American participants by mobilizing community support and engagement through partnerships |
Wisconsin Alzheimer’s Instituted: Organization committed to health equity for people living with dementia; primary goal was to support the community; community members initiated their participation in research |
| Pros | Expedient, less costly Quick, broad dissemination of research results Potential for subsequent relationship building |
Develops connections Increases transparency and trust between researchers and community |
Community involvement increases the potential for change Increases cultural sensitivity and humility among researchers |
Builds more lasting and collaborative relationships, given increased commitment from community partners—in the form of time or other resources | Builds strong trust and long-lasting relationships Organizational structures may facilitate the sustainability of the initiative beyond the initial project |
| Cons | Limited public/community input or influence Could be perceived as impersonal or intrusive Risk of failing to reach deeper levels of engagement |
Additional time and resources required to collect, analyze, and disseminate community input Not incorporating community feedback exposes researchers to criticism and could perpetuate problems |
Requires more time and effort from community members | More labor intensive for researchers Relinquishing power to community partners can be difficult; researchers have less control of the process and may have to accept unanticipated outcomes |
Takes time, consistency, flexibility, and focus from both research and community partners Better suited for broader issues that call for significant investment and participants of varying skills |
a See https://allofus.nih.gov/about/who-we-are/all-us-community-and-participant-advisory-boards (accessed November 20, 2024).
b See https://ceal.nih.gov/ (accessed November 20, 2024).
c See https://www.jacksonheartstudy.org/Community/Ways-to-Participate (accessed November 20, 2024).
d See https://wai.wisc.edu/ (accessed November 20, 2024).
NOTES: See Appendix B for an extended version of this table. This table is a general guide, and there may be other considerations for specific populations, such as American Indians. See text for more information about best practices. HBCU = historically black colleges and universities.
SOURCE: Some content adapted from the CDC Principles of Community Engagement and New York City Department of Health and Mental Hygiene Community Engagement Framework and associated resources2 (CDC, 2011; NYC Dept of Health and Mental Hygiene, 2017).
___________________
2 https://www.nyc.gov/site/doh/health/health-topics/race-to-justice.page (accessed July 10, 2024).
Successful Examples of Community Engagement
Developing relationships with communities means engaging with community members in meaningful and sustained ways throughout the research process. The Patient-Centered Outcomes Research Institute (PCORI) articulates this approach as its foundational expectations for research partnerships, which it requires from researchers who apply for funding.3 The expectations include team diversity and representation to mirror the community, early and ongoing engagement with partners (including decision making), dedicated funds for engagement and partner compensation, training and capacity building for the team, shared decision making with partners, and ongoing review and assessment of engagement. PCORI sees these as “building blocks for meaningful, effective, and sustainable engagement with patients, communities, and other partners in research.” The strategies are intentionally general because the elements of community engagement should be tailored to the specific context based on goals of the community, researchers, and project, which may mean taking into account community preferences, cultural beliefs, values, and historical background. PCORI offers a number of illustrative examples of its expectations in action.4
PCORI-funded studies are more likely to directly engage participants and include contributions from this engagement in research design decisions (Forsythe et al., 2019). Further, a review of PCORI-funded studies found that this community engagement is “important to patients and clinicians, recruitment and retention of study participants, data collection processes, interpretation of results, and dissemination.” PCORI also continues to monitor and evaluate their progress in advancing the National Priorities for Health, which will help researchers evaluate which aspects of PCORI’s approach are most effective for engaging community members in biomedical research.
A second example is the Jackson Heart Study of cardiovascular disease among African Americans in the Jackson, Mississippi, metropolitan area. It is a 20-year prospective community-based cohort study that successfully enrolled 5,306 African American participants by mobilizing community support and engagement through partnerships with over 100 churches, government agencies, and community-based and nonprofit organizations (Addison et al., 2021). The Community Outreach Center (CORC), which was created to maintain trust and community engagement throughout the study, utilized community health education activities, health promotion, and health prevention messages within the Jackson community. Due to the efforts of the CORC, the Jackson Heart Study recruited the largest cohort of African Americans to participate in a study on cardiovascular disease and maintained high retention, with 85 percent of participants returning from the first exam to the second exam. The study also developed an extensive media campaign that was channeled through trusted community leaders, which began at least one year prior to the inception of the study (Addison et al., 2021; Martei et al.,
___________________
3 https://www.pcori.org/engagement/engagement-resources/engagement-research-pcoris-foundational-expectations-partnerships (accessed May 10, 2024).
4 https://www.pcori.org/engagement/foundational-expectations/in-action (accessed July 10, 2024).
2024). This large community-based cohort study now includes a biospecimen and data repository as well as a registry for the recruitment of Black participants into ancillary clinical trials (Martei et al., 2024). Furthermore, the approach could potentially be adapted to build cancer survivorship cohorts for management of long-term cardiovascular outcomes (Martei et al., 2024).
In another example, a pivotal cluster-randomized trial of blood pressure reduction used a nontraditional health care setting to develop and test a blood pressure control program for Black men. The study enrolled a total of 319 Black male participants with hypertension across 52 Black-owned barbershops (Victor et al., 2018). The intervention group saw a 95 percent retention rate, achieved by using “multiple blood pressure screenings, ID cards, follow-up calls at 3 months, culturally specific health sessions, and payments to offset the costs of generic drugs and transportation to pharmacies” (Martei et al., 2024, p. 388; Victor et al., 2018).5
Collaborating with Indian Tribes on Biomedical Research Studies
Working with American Indians and Alaska Natives (AIANs) on research studies requires additional considerations that are absent when working with other communities. Some of these are the result of the longstanding sovereignty of Indian Tribes within the boundaries of what is now the United States and their resulting governmental structures. This sovereign status is a defining feature of American Indian Tribes. Other reasons stem from the sharply different frames of reference of Western science and Indigenous knowledge.
Differences in Viewpoint
It is critical that researchers become aware and sensitive to the culture, belief systems, history, and priorities of the AIAN group with which they would like to work (Hiratsuka et al., 2012). Few non-native researchers possess such an awareness, especially a deep understanding of the continuing effect of American colonialism on the people they seek to include in their study. Emphasizing this point, a speaker shared with the committee, “If you want to study a native community, you will have to actually come to our world. You have to assimilate to us instead of the other way around. You have to leave your perspectives and beliefs at the door and be open to our perspectives and beliefs” (Audie Atole in remarks to the committee on March 14, 2024).
Biomedical researchers may find it challenging to understand non-Western scientific paradigms that undergird Indigenous knowledge and how to integrate that knowledge into a study. There is an entrenched Western mindset that Indigenous worldviews of studying natural phenomena and human health, which have developed over centuries (even millennia in some cases), are deficient and primitive (Beauchamp and Childress,
___________________
5 https://www.cedars-sinai.org/newsroom/update-cedars-sinais-la-barbershop-study/ (accessed July 10, 2024).
2001; Deloria Jr., 1999; Jonsen et al., 1998). “Native peoples tend to see their cultures as encompassing systems of knowledge and understanding that are fundamental to the continuation of the Tribe itself. Any harm to culture is perceived as a direct harm to the ability of the Tribe to continue into the future” (Tsosie, 2007, p. 402). Traditional knowledge and stories are intellectual property and sources of a Tribe’s collective wealth passed down across generations (Cruikshank, 1992; Harding et al., 2012; Tsosie, 2002). As with any sovereign nation, a Tribal government has a responsibility to safeguard its Tribe’s cultural, intellectual, and spiritual inheritance along with the rights and resources of its people and place.
Consent from the Tribal Government
Biomedical researchers must understand that each Indian Tribal government is the only entity authorized to represent and make decisions on behalf of that Tribe. There are 574 distinct and federally recognized Tribes or Tribal entities afforded sovereignty within the United States today.6
Many Tribes have legally enforceable codes that set forth whether and how research will be done in their Nation, on their land, and with members of their Tribe (Carroll et al., 2022). For example, the Ho-Chunk Nation’s Tribal research code establishes an application and permitting process that is overseen by the Tribal IRB and is “designed to protect individuals, communities and the Nation itself from improper research procedures” (Ho-Chunk Nation, 2005, p. 2). The Ho-Chunk Nation’s code also protects the rights of individuals who are members of the Tribe; secures ownership of and provides protections for the Nation’s data; and ensures “appropriate Nation and community participation in the design and evaluation of research, and appropriate local opportunities in employment in all research projects permitted . . . within the Ho-Chunk community” (Ho-Chunk Nation, 2005, p. 2). Researchers who want to work with the Ho-Chunk Nation—or with many other Tribes—must apply for a permit either through the Tribe’s IRB or through the U.S. Indian Health Service (in addition to any academic or institutional IRB). A Tribal IRB considers potential adverse impacts to the Tribe, the Tribe’s government, and Tribal individuals and does so with knowledge and a viewpoint that an academic IRB may lack (Around Him et al., 2019); thus, the two review processes are complementary.
When Tribes do not have IRBs, there are still cultural and formal protocols that must be followed by researchers (Garba et al., 2023). Often it is a matter of getting in front of the Tribal council and head of the Tribe, who function as the de facto IRB—something that can take months. Sometimes an approval is also needed from a cultural head in addition to the governmental body. Therefore, before approaching Tribes about
___________________
6 https://www.federalregister.gov/documents/2021/01/29/2021-01606/indian-entities-recognized-by-and-eligible-to-receive-services-from-the-united-states-bureau-of (accessed July 10, 2024).
permission to carry out reservation-based research, researchers should learn about the Tribe and its protocols, laws, codes, beliefs, and structures.
“[T]ribal needs may not accommodate research timelines, which are often too short (short-term or one-time federally funded initiatives) or too long (publication-heavy research without actual remedies for the community). It is rare that a federally funded initiative is timely and sustained, that the grant is received when needed by the Tribe, and that the Tribe is ready with adequate staff and processes in place” (Harding et al., 2012, p. 7).
With 78 percent of AIANs living off reservation, urban-based AIAN research has its own set of “protocols” that are distinct from reservation- or Nation-based research. There are several urban centers around the country that have some institutional body—whether formal or informal—where researchers can find out about procedures and protocols for seeking to carry out research, including what to avoid. These bodies may be able to assist in or have a mechanism for granting community assent and access (e.g., Metropolitan Urban Indian Directors,7 National Urban Indian Family Coalition,8 Seattle Indian Health Board9).
Data Sovereignty and Intellectual Property Rights
Researchers may face challenges centering on trust, data ownership, and sovereign rights. Although research is trending toward more open-source approaches, this may be at odds with the values and rights of Indigenous populations (Garrison and Carroll, 2024). Differences may include conceptions of how knowledge may be generated, used, and shared as well as who owns both the material and information collected for a study and the data, analyses, and disseminating documents generated from those collected materials. This necessitates that a material and data sharing agreement (MDSA) be crafted collaboratively and then agreed to and signed by all parties (Harding et al., 2012; see also Hayward et al., 2021). All parties will benefit, and trust will be built when material and data-sharing issues are discussed and resolved early in the study’s life cycle.
Here the complementary perspectives of academic and Tribal IRBs can be useful. An awareness of and sensitivity to past and ongoing abuses of Tribal material and information underscore why an MDSA is needed (Chadwick et al., 2019; Claw et al., 2021; Garrison and Carroll, 2023). Tribes may want the MDSA to include language that defines how Tribal information can be used in publications. They may want it to provide clarity about intellectual property rights in the context of studies involving participants who are members of sovereign nations. The MDSA will likely confirm that all materials and information remain the property of the Tribe and that they are not to be shared with a third party without Tribal permission or used for anything
___________________
7 https://muidmn.org/committees (accessed October 16, 2024).
8 https://www.nuifc.org/ (accessed October 16, 2024).
9 https://www.sihb.org/ (accessed October 16, 2024).
other than permitted research. Furthermore, all materials will be returned to the Tribe at the conclusion of the study. Developed by the Indigenous Data Alliance,10 the CARE Principles for Indigenous Data Governance11 can provide guidance for researchers engaging in this work. With a foundation of Indigenous worldviews, the CARE principles are collective benefit, authority to control, responsibility, and ethics (Carroll et al., 2022).
Informed Consent
Entry into a Tribal nation or an Indigenous community requires time on site and introduction to key members relevant to the envisioned study well before the research is set to begin. Once a relationship with the Tribe or community is established, researchers should consider partnering with or have on the research team one or two trusted members of the Tribe or community. Someone who “speaks the language” of both Indigenous and Western science can facilitate collaborations that build mutual trust and respect (Hatch et al., 2023). It is important for researchers to communicate the risks, consequences, and benefits of consenting to participating in a research study, by offering participants clear and concise descriptions of the purpose of the research, the use and storage of the information collected, and any potential issues of anonymity (Harding et al., 2012). There will likely need to be confidentiality agreements “signed by university research personnel who have access to project material and data. These forms are held by Tribal researchers under secured conditions so that they know who has access to the data and for what purpose” (Harding et al., 2012, p. 9).
Strategies for Success
Partnering with Tribal nations entails time and commitment to overcome unexpected barriers or roadblocks, including potential geographic and logistical challenges that are unique to each Tribe (Jones et al., 2019; Laveaux and Christopher, 2009). Each Tribe has distinct protocols for research approvals, and researchers often underestimate the time required to meet them. While perhaps time-consuming, Tribal approval processes allow time for cultural immersion, demonstrating trustworthiness and commitment, and developing relationships (Jones et al., 2019). Essential to productive collaborations with Tribal nations, relationship building benefits from cultural sensitivity, humility, and openness to personal growth (Jones et al., 2019). Taken a step further, understanding Tribes’ priorities could also result in changes to the research questions or study design.
___________________
10 https://www.gida-global.org/whoweare (accessed October 16, 2024).
11 https://www.gida-global.org/care (accessed October 16, 2024).
Some research studies have used a version of CBPR (Laveaux and Christopher, 2009) or coproduction, and the outcomes benefited equitably both the Indian Tribe and the academic researchers (Woodbury et al., 2019). Some examples are:
- The Center for Braiding Indigenous Knowledges and Science,12 which is not biomedical research, but has been funded by the National Science Foundation at $30 million for 5 years.13
- The Center for Indigenous Health Research and Policy,14 which focuses on cardiovascular and related confounding diseases, like diabetes and obesity, and behavioral and environmental changes to reduce their prevalence, especially by expanding access to healthy foods and tapping into traditional cultural practices.
- The Confederated Tribes of the Umatilla Indian Reservation and Oregon State University collaborated to address Tribal exposures to polycyclic aromatic hydrocarbons and assess ways to improve community health. As part of the process, they collaborated on the formation of a model material and data sharing agreement and a confidentiality agreement (Harding et al., 2012).
Other resources, including extramural grant opportunities, can be found through parts of the federal government that focus on biomedical research with AIAN populations, such as the NIH Tribal Health Research Office, NIH Native American Research Centers for Health, HHS Indian Health Service, HHS Office of Minority Health, and Environmental Protection Agency’s Science to Achieve Results (STAR) tribal environmental health research program.
GUIDANCE ON THE COLLECTION OF RACE AND ETHNICITY INFORMATION IN ELECTRONIC HEALTH RECORDS
As noted in Chapter 3, electronic health records (EHRs) are now the standard method for collecting and retrieving patient and patient-related information. EHR systems have become extremely sophisticated, and multiple commercial systems are in use by health care systems across the United States and the globe. EHR data are now used in biomedical research studies and in the training and validation of AI algorithms. Yet despite repeated calls for standardizing EHR data collection on race and ethnicity, such standardization requirements do not exist (IOM, 2003b; NASEM, 2024). A 2018 report by AHRQ said, “Given variations in locally relevant populations, no single national set of additional ethnicity categories is best for all entities that collect these data” and recommended tailoring the more granular ethnicity groups based on locally relevant categories chosen from a national standard set of options (AHRQ, 2018). Since then, some have explored the use of
___________________
12 https://www.umass.edu/gateway/research/indigenous-knowledges (accessed October 16, 2024).
13 https://www.umass.edu/news/article/umass-amherst-partnering-indigenous-communities-launch-30m-nsf-center-braiding (accessed October 16, 2024).
14 https://medicine.okstate.edu/cihrp/ (accessed October 16, 2024).
imputation models to expand the granularity in ethnic and racial categories for Hispanic, Asian, Native Hawaiian, and Pacific Islander populations (NASEM, 2024). It should be noted that the revised U.S. Office of Management and Budget (OMB) standards now recommend collecting more granular data as well (OMB, 2024).
The U.S. Office of the National Coordinator for Health Information Technology is building standardized data elements that are being evaluated ahead of becoming required elements in EHR systems. EHR systems are currently required to support not only the OMB categories for race and ethnicity but also the more granular CDC Race and Ethnicity Code Set representations of race, ethnicity, and Tribal affiliation (NASEM, 2024).15
GUIDANCE FOR RACE AND ETHNICITY IN CLINICAL PRACTICE GUIDELINES
Several organizations, such as the U.S. Preventive Services Task Force (USPSTF), AHRQ, and the American Academy of Pediatrics (AAP), have issued guidance on the use of race and ethnicity in clinical practice guidelines. The USPSTF creates evidence-based clinical practice guidelines to guide the delivery of clinical preventive services. In 2021, the USPSTF acknowledged that systemic racism is a root cause of health disparities in preventive care and made a commitment to promote antiracism and health equity in its preventative care recommendations (Davidson et al., 2021). Its recommendations and next steps include the following:
- “Consider race as a social, not a biological construct
- Promote racial and ethnic diversity in membership and leadership and foster a culture of inclusivity
- Commission a report to understand how systemic racism undermines the benefits of evidence-based clinical preventive services
- Iteratively update methods to overcome health inequities experienced by populations affected by systemic racism
- Communicate gaps created by systemic racism in all dissemination efforts
- Collaborate with its partners and experts to reduce the influence of systemic racism on health” (Davidson et al., 2021, p. 2406; Doubeni et al., 2021).
In December 2023, AHRQ developed a health equity framework for incorporating a health equity lens that spans the entirety of the USPSTF recommendation-making process (J. Lin et al., 2023, 2024). The report provides an equity framework for each phase of the USPSTF guideline development: “(1) topic nomination, selection, and prioritization, (2) development of the work plan, (3) evidence review, (4) evidence deliberation, (5) development of the recommendation statement, and (6) dissemination of recommendation(s)” (J. Lin et al., 2023, p. 5).
The AAP released a policy statement in June 2022 to tackle the eradication of race-based medicine as a component of a larger effort to deconstruct the structural and
___________________
15 https://www.healthit.gov/isa/representing-patient-race-and-ethnicity (accessed May 12, 2024).
systemic injustices responsible for racial health disparities (Wright et al., 2022). The following recommendations were adopted:
- “The AAP will critically examine all policies and practice guidelines for the presence of race-based approaches in their development and deconstruct, revise, and retire, if necessary, all policies and practice guidelines that include race assignment as a part of clinical decision-making.
- The AAP will critically examine all policies and guidelines currently under development as well as consideration of all such future documents to ensure the exclusion of race assignment as part of clinical decision-making.
- The AAP will leverage the ‘Words Matter’ document to ensure that all authors, editors, presenters, media spokespersons, and other content contributors recognize race as a social construct only and desist from any use, or its reference, as a biological proxy” (p. 5-6).
Application of the AAP framework to a clinical pathways library at Boston Children’s Hospital established several recommended practices, including: (1) “define race, ethnicity, and ancestry rigorously; (2) assess the most likely mechanisms underlying epidemiologic associations; (3) consider whether inclusion of the term is likely to mitigate or exacerbate existing inequities; and (4) exercise caution when applying population-level data to individual patient encounters” (Rosen et al., 2023, p. 1-2). This process, and those framed by the USPSTF and AHRQ, could serve as a useful model for other organizations looking to reevaluate how race and ethnicity are incorporated into clinical pathways.
GUIDANCE FOR RACE AND ETHNICITY IN CLINICAL ALGORITHMS
Despite the potential of clinical algorithms to exacerbate existing health disparities, there are very few studies that evaluate the disparity effects of using clinical algorithms. In addition, some algorithms are proprietary, or created by private companies such as EHR vendors or payers, and even less is known about their development and impact on racial and ethnic disparities. One of the few existing resources that tackles this subject in a comprehensive manner is a recent report by AHRQ that reviewed the literature to examine the effect of 18 statistical algorithms on racial and ethnic differences in access to care, quality of care, and health outcomes (Tipton et al., 2023). The review found that algorithms sometimes perpetuate or exacerbate disparities and at other times reduce racial and ethnic disparities. Rarely do the algorithms have no discernable effect on disparities. An important conclusion from the AHRQ report is that the quality of the evidence was found to be weak in terms of rigorous study designs (Powe, 2024). Most studies suggesting deleterious effects were designed as simulations, modeling what might happen when an algorithm is used in care. Such studies are susceptible to biases. Few used randomized clinical trials or pre-post implementation designs to examine outcomes.
Tipton and coauthors (2023) also reviewed the literature for mitigation strategies used to address potential race-based harms from algorithms and identified six types of mitigation strategies, which are summarized in the AHRQ report:
- removing a race or ethnicity input variable from the algorithm
- replacing race or another input variable with a different measure
- adding an input variable
- recalibrating the algorithm with a more representative patient population
- stratifying algorithms to assess patients of different racial and ethnic groups separately
- using different statistical techniques within algorithms
The authors concluded, “It is unclear from the current evidence base if certain types of strategies are generally more effective than others” (Tipton et al., 2023, p. 30). Employing mitigation strategies to influence health outcomes can have mixed results, improving one outcome while worsening another. For example, most studies showed that omitting race from the estimated kidney function test (eGFR) led to an increase in diagnoses of chronic and severe renal disease in Black patients (Delgado et al., 2022). Thus, the removal of race and ethnicity values from this algorithm can result in earlier interventions to treat chronic kidney disease and increase the referral of Black patients for kidney transplants. However, omitting race from eGFR may also exacerbate disparities related to clinical trial eligibility or cancer therapy access because Black patients are classified as having more severe kidney disease (Tipton et al., 2023; see also Figure 3 in Siddique et al., 2024).
In a report summarizing a meeting sponsored by the Doris Duke Foundation and others in June 2023, the Council of Medical Specialty Societies also observed that the consequences of using race as a variable in research studies can vary considerably and can be beneficial (e.g., if race is included in an intentional, well-considered effort to reduce inequities); neutral/have no impact; or harmful (e.g., if including race perpetuates disparities or the misconception of innate biological differences between racial groups) (CMSS, 2023). According to the report, the “path to improving health equity will likely differ for each algorithm” (CMSS, 2023, p. 4). The best option for a particular algorithm may be to exclude race altogether; to replace race with an alternative variable, such as measures of social determinants of health; to stop using the algorithm and replace it with one that promotes equity; or to continue to include race as it is currently being used (CMSS, 2023). For this reason, there is likely not a one-size-fits-all solution to the persistent problems surrounding the use of clinical algorithms that incorporate race and ethnicity data.
GUIDANCE FOR RACE AND ETHNICITY IN CLINICAL AI ALGORITHMS
Bias in AI Algorithms
Sources of artificial intelligence (AI) bias can appear throughout the developmental life cycle of AI algorithms (Cary Jr. et al., 2024; see also Box 4-3). To address racial and ethnic bias in the development and use of health care algorithms, AHRQ and the
BOX 4-3
Sources of Bias in Artificial Intelligence (AI) Algorithms
Problem Formulation/Specification: The development of a predictive model necessitates specifying the overall goals, actions available for decision making, and, in the case of predicting an outcome of interest, clearly defining an outcome that is likely to be complex and ambiguous. For example, Obermeyer et al. demonstrated that a health care model used to identify patients with complex health needs was racially biased because the outcome was specified using health care costs as a proxy for illness burden. Because of unequal access to health care, the model unfairly predicted that members of groups with less health care access were healthier than they were (Obermeyer et al., 2019).
Data: Following problem specification, model derivation typically entails applying a statistical or AI method to a dataset of historical cases in order to discover useful patterns. Biases in the data will be captured in the model because model derivation methods aim to capture statistical features of the input dataset. For example, if there is systemic racism in health care access and delivery, historical EHR data will encode these biases and lead to discriminative predictive models (Gijsberts et al., 2015). Moreover, historical data may not reflect the current context, potentially missing key information.
Modeling: Given a dataset, derivation and validation of a model is done to optimize model performance relative to some success criteria that are technically encoded as an objective function. Typically, the objective function focuses on pure overall predictive performance by maximizing accuracy. Since fairness is not explicitly accounted for in the objective function, such optimization may lead to models that have lower accuracy in a specific group or groups.
Implementation: Biases can emerge during the implementation, often called deployment, of a model. This can occur, for example, when the implementation context differs significantly from the historical context of the training data, resulting in poor performance in groups that were underrepresented in the training data. Furthermore, biases can arise from a failure to recognize the limitations of a purely predictive model, whose predictions may be insufficient for interventions that require causal information.
Monitoring: Biases can appear at any point after model deployment, even if the model was fair during initial deployment. Vigilance is thus essential on an ongoing basis. New bias may develop because the data used to train the model may no longer be representative of the real-world population as time passes. For example, new racial groups may emerge on which the model was not trained and on which it performs poorly. Another reason is changing societal norms and legislation—that is, a model that was considered fair during initial deployment may become unfair as social and ethical standards evolve.
Source: Adapted from Anderson et al. (2024).
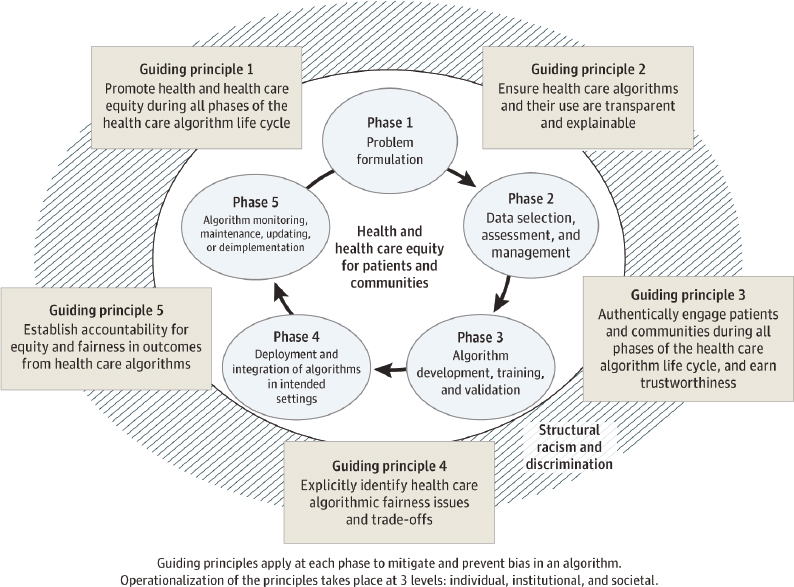
SOURCES: Chin et al. (2023), CC-BY-ND; Matheny et al. (2020).
National Institute on Minority Health and Health Disparities convened an expert panel that developed guiding principles to mitigate or eliminate the impact of bias at each phase of the algorithm life cycle (Figure 4-1; Chin et al., 2023).
The AI Program in the FDA’s Center for Devices and Radiological Health has identified major regulatory science gaps and challenges in clinical AI algorithms. One of the focus areas of program activity is “to develop methods to measure and quantify algorithmic bias, reduce performance difference among subpopulations, and ensure generalizability” (FDA, 2023). Presumably, this will include consideration of the use of race and ethnicity as input variables and assessment of performance on racial and ethnic subpopulations.
Reporting Guidelines and Repositories for AI Algorithms
As research in health AI algorithms has progressed, reporting guidelines have been developed to assure scientific validity, clarity of presented results, reproducibility, and adherence to ethical principles. Some of these guidelines (Box 4-4), such as SPIRIT-AI and CONSORT-AI, are extensions for AI of existing guidelines that were developed for biomedical research studies. More are under development, such as STARD-AI,
BOX 4-4
Reporting Guidelines for Biomedical Artificial Intelligence (AI) Algorithms
SPIRIT-AI is the AI-specific version of the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) and provides guidelines for reporting clinical trial protocols for AI interventions (Cruz Rivera et al., 2020).
CONSORT-AI is the AI-specific version of the Consolidated Standards of Reporting Trials (CONSORT) and provides guidelines for reporting randomized trials of AI interventions (Liu et al., 2020).
FUTURE-AI provides guidelines based on six guiding principles for AI research: fairness, universality, traceability, usability, robustness and explainability (FUTURE). The fairness guideline calls for maintaining the same performance across subgroups of individuals of different ethnicities (though not across different races) (Lekadira et al., 2021).
HUMANE (Harmonious Understanding of Machine Learning Analytics Network) provides a checklist of 60 questions with binary, multiple choice, or free-text responses to assess the reporting quality of medical AI studies (du Toit et al., 2023).
MI-CLAIM (Minimum Information about Clinical Artificial Intelligence Modeling) provides reporting guidelines for clinical AI models (Norgeot et al., 2020).
MINIMAR (Minimum Information for Medical AI Reporting) provides minimum reporting standards for medical AI applications (Hernandez-Boussard et al., 2020).
CLAIM (Checklist for Artificial Intelligence in Medical Imaging) provides reporting standards for medical-imaging AI studies (Mongan et al., 2020).
RQS (Radiomics Quality Score) is an assessment tool for evaluating the methodological quality of radiomics—quantitative approach to medical imaging—studies, including those that use AI (Lambin et al., 2017).
STARD-AI is the AI-specific version of the Standards for Reporting of Diagnostic Accuracy Studies and provides guidelines for reporting diagnostic accuracy of AI models. It is under development (Sounderajah et al., 2021).
TRIPOD-AI is the AI-specific version of the Transparent Reporting of a Multivariable Prediction Model of Individual Prognosis or Diagnosis and provides reporting guidelines for studies developing, validating, or updating diagnostic and prognostic AI models. It is under development (Collins et al., 2021).
PROBAST-AI is the AI-specific version of the Prediction Model Risk Of Bias Assessment Tool and provides guidelines for assessing the risk of bias and applicability of diagnostic and prognostic AI models. It is under development (Collins et al., 2021).
TRIPOD-AI, and PROBAST-AI. Of the currently published guidelines, commentary on the use of race and ethnicity is limited. Only FUTURE-AI calls for the evaluation of AI across ethnic categories, though not across racial categories.
Two repositories for clinical practice guidelines are an extensive free repository maintained by the ECRI Guidelines Trust16 and the repository maintained by Guideline Central.17 An online database of clinical statistical algorithms with race-based guidelines, medications with race-based guidelines, and medical devices with differential racial performance is available (Clinical Algorithms with Race, 2023). The FDA also maintains a database of AI and machine learning-enabled medical devices,18 including approved AI algorithms.19
GUIDANCE ON THE REPORTING OF RACE AND ETHNICITY DATA IN PUBLICATIONS
Publication in medical and science journals is an important avenue through which the use of race and ethnicity in research can be improved. The language used and the ways race and ethnicity are discussed reflect the care with which the concepts were considered throughout the research process. Clear descriptions of how and why race and ethnicity were used in a study promote transparency, openness, and replicability—principles of sound science introduced in Chapter 2. While the inclusion of race and ethnicity in health research has increased over time, these terms are almost never clearly defined (Martinez, 2023a). Less than 50 percent of articles across disciplines and only approximately 30 percent of medical and epidemiological publications had any justification regarding their use of race or ethnicity (Martinez, 2023a, 2023b). There is a lack of standardized practice around the use of race as a study variable and inconsistency in how researchers conceptualize and report on race in biomedical publications (Boyd et al., 2020).
There have been many attempts to establish guidelines directed toward researchers, journal editors, and reviewers to standardize practices for discussing the use of race and ethnicity data. In a recent article, nine biomedical journal editors provided guidance for using race, ethnicity, and geographic origin as proxies for genetic ancestry groups when publishing study results (Feero et al., 2024). Although focused on genetics, the authors encouraged researchers throughout the biomedical community to embrace these best practices, which would help to ensure scientific accuracy and the interpretability of published research across biomedical research disciplines. Among existing reporting guidelines, a few common themes have emerged, including:
- Explain why race and ethnicity are being used as study variables.
- Define race and ethnicity as they are used in the study.
___________________
16 https://guidelines.ecri.org/ (accessed August 27, 2024).
17 https://www.guidelinecentral.com/guidelines/ (accessed August 27, 2024).
18 https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-aiml-enabled-medical-devices (accessed August 27, 2024).
19 https://medicalfuturist.com/fda-approved-ai-based-algorithms/ (accessed August 27, 2024).
- Describe how race and ethnicity data were collected.
- Avoid using race or ethnicity as explanatory variables for observed health disparities.
- Use clear, consistent, and inclusive language in reporting on race and ethnicity.
It is important that researchers have a clear understanding of the objectives of the study they are developing, select the most appropriate variables needed to carry out these objectives, and use them consistently (Lu et al., 2022). According to the International Committee of Medical Journal Editors (ICMJE) recommendations, “Authors should define how they determined race or ethnicity and justify their relevance” and if they choose to not collect these data, they should explain their reasonings behind this decision (ICMJE, 2023, p. 7). The Journal of the American Medical Association (JAMA) states that if “race and ethnicity categories were collected for a study, the reasons that these were assessed should be described in the Methods section” (Flanagin et al., 2021, p. 623). Moreover, researchers should note if the collection of these data was a requirement by a funding agency (Flanagin et al., 2021). Employing race and ethnicity as study variables without any clear reasoning for doing so implies that these categories are a “primary, natural, and neutral means of grouping humans” (Bhopal, 1997, p. 1751; see also Kaplan and Bennett, 2003), a practice that should be avoided.
When race and ethnicity are used as study variables, researchers will need to define these terms as they are used in their study (Boyd et al., 2020). In their guidance on race and ethnicity reporting, JAMA offers some discussion of definitions that could be of use to researchers as a starting point (Flanagin et al., 2021). To avoid conflating race with ethnicity, JAMA suggests that researchers use “race and ethnicity” as opposed to “race/ethnicity” in their publications (Flanagin et al., 2021). Journals and funding agencies can encourage researchers to carefully appraise their use of these terms by setting requirements for the inclusion of precise definitions of these constructs in their submissions (Lu et al., 2022) and ensuring clear descriptions in the methods sections of articles (Feero et al., 2024).
Researchers will need to describe in the methods section how race and ethnicity data were collected. If self-report was used to assign participants into racial or ethnic categories, researchers should specify if they were given a fixed set of options to choose from or if the questions were open-ended (Kaplan and Bennett, 2003). Some journals consider self-identification with an option to write-in the desired response or select multiple categories to be the gold standard for collecting this type of data. When assignments to racial or ethnic categories were made by research or health care personnel, it should be specified how these categories were attributed (AHA, 2021). Ultimately, researchers should do their best to collect data that capture as much of the multidimensional nature of an individual’s identity as possible with the tools at their disposal (NHGRI, 2016; Roth, 2016). In doing so, they are better equipped to develop and execute studies that are more rigorous and transparent.
Other guidelines advise against treating race and ethnicity as explanatory variables for health disparities found between different groups. Rather, their use in health disparities research is appropriate “as [a way] . . . of examining the underlying sociocultural reasons for these disparities” (Rivara and Finberg, 2001, p. 119). An association
between membership in a racial or ethnic group and a higher incidence of a disease does not in itself establish causality (Kaplan and Bennett, 2003). Instead of using race and ethnicity as explanations for the existence of disparities in health, investigating other relevant factors, including racism and discrimination, socioeconomic status, social class, environmental exposures, and educational attainment, will likely provide more meaningful results (Bonham et al., 2018; Kaplan and Bennett, 2003; NHGRI, 2016). Several journals have advised that the collection of race and ethnicity data is best done in conjunction with all relevant social and structural factors that influence the research question (AHA/ASA, 2021; Flanagin et al., 2021). Explicitly using terms like “structural racism” and “racial equity” in the study could be another important way to highlight the impact of racism on health outcomes and avoid misusing race and ethnicity data (Flanagin et al., 2021). Boyd and colleagues (2020) suggest that researchers name racism as a key determinant of health, identify what type of racism (interpersonal, institutional, internalized) is relevant, consider the mechanism by which it is operating, and examine additional forms of oppression that may exacerbate racism’s effects (Boyd et al., 2020). By considering the multitude of social and environmental forces that impact the health of individuals and populations, researchers can avoid reinforcing the existence of a biological basis of race and get a clearer understanding of what drives differences in health outcomes between groups.
A number of journals provide style-based guidance on terminology, capitalization, and the use of inclusive language when describing racial and ethnic groups in biomedical publications. A few examples follow. Authors are advised to use language that is “neutral, precise, and respectful” when describing research participants and discouraged from using terminology that may be stigmatizing (ICMJE, 2023, p. 17). The term “White” is acceptable while “Caucasian” is not, because the latter is rooted in “racist taxonomies used to justify slavery” (Popejoy, 2021, p. 463). Descriptors like “Non-White” and “Other” homogenize populations or groups of study participants, thus obscuring meaningful data and reducing the power and validity of a research study. An exception to using “Non-White” or “Other” may be when it was an explicit preselected category in an existing database or research instrument (Flanagin et al., 2021). Grouping together different populations that are unrelated except for their being “non-White” can contribute to the assumption that whiteness is the reference category. It is preferable to identify the smaller population groups, whenever possible (AHA, 2021). Capitalizing the names of racial and ethnic groups and avoiding the use of pejorative terms like brown and yellow are recommended (Flanagin et al., 2021).
Remaining Challenges with Race and Ethnicity Reporting Guidelines
Despite the concordance among journals on many aspects of reporting on race and ethnicity in biomedical research studies, several challenges remain. One obstacle is the difficulty of maintaining and tracking adherence to these guidelines. Since the majority of the policies on race and ethnicity have been published in recent years, there is not yet much information on adherence to updated guidance documents (Martinez, 2023a). Notably, guidelines developed by the ICMJE and JAMA in 2004 have largely not been
met (Martinez, 2023a). An examination of reporting of race and ethnicity in three JAMA network journals before and after the development of the 2021 AMA Manual of Style found improvement in the reporting of measures, coding, and style-based policies but did not explore the impact of the guidance on how race and ethnicity are defined in publications and the justifications for their use (Flanagin et al., 2023). It was also unclear in their analysis whether the noted improvements in reporting occurred prior to submission, during peer review, or during the editing process following acceptance of the manuscript (Flanagin et al., 2023). Thus, in order for the biomedical research community to benefit from these guidelines and publish studies that employ race and ethnicity categories in a thoughtful manner, there need to be mechanisms in place to enforce these guidelines and a formalized way of measuring their impact.
A second challenge is the lack of standardization across journals and disciplines concerning the reporting of race and ethnicity in biomedical research studies (Lu et al., 2022). The distinctions between race and ethnicity as descriptors along with recommendations on when to use each term have not yet been consistently agreed upon (Lu et al., 2022). A scoping review of publications that offer guidance on the use of population descriptors like race and ethnicity in genomics research found that many of the guidance documents had definitions of “race” that differed from one another along the social–biological axis and offered conflicting opinions on how strictly these descriptors should be defined (Mauro et al., 2022). The extent of this widespread disagreement demonstrates that a lack of standardized definitions for these terms results in considerable confusion on their use (Mauro et al., 2022). The biomedical research community could benefit from ensuring that reporting guidelines for race and ethnicity in publications are clearly defined and enforced in a systematic manner (see Chapter 6 for recommendations).
Another limitation of publishing guidelines focused on race and ethnicity is the potential to inadvertently obfuscate the impact of racism on health disparities (see Chapter 3, section “Health Disparities and the Study of Racism”). One way that this can be observed is in how frequently terms like race and ethnicity appear in publications, but not terms like structural or systemic racism. A PubMed database search conducted in 2020 turned up a mere 86 articles that included both race as a term and structural or institutional racism (Boyd et al., 2020). Furthermore, research on health disparities often posits the role of “environmental” or “social” factors without explicitly mentioning how racism is the underlying mechanism by which these factors have biological consequences (Boyd et al., 2020). This framing devalues racism as a social and political force that has a resounding influence on health and health care. In addition, without careful consideration of racism as a relevant contributor to health outcomes, researchers run the risk of incorrectly attributing health disparities to innate biological or genetic characteristics, thereby stigmatizing and alienating populations that are already marginalized within the biomedical research enterprise.
This section makes clear that useful guidance on reporting race and ethnicity data exists; however, gaps in implementation remain. Moreover, the time of publication is late in the research process, and reporting guidelines often focus on using the “right” language or terms. Although those are important, many issues related to the conceptualization and use of race and ethnicity occur earlier in a study.
CHAPTER SUMMARY AND CONCLUSIONS
The committee set out to use the research process cycle introduced in Chapter 3 as a framework for analyzing existing guidance on the use of race and ethnicity in biomedical research. It quickly became apparent that there is a host of best practice information on reporting standards. There have also been efforts to provide guidance to improve representation in clinical trials, promote community engagement, standardize EHR data collection, and reexamine the use of race and ethnicity in clinical tools. But guidance is lacking for other stages in the research process, notably for earlier steps such as conception, leaving open the possibility of misuse.
Conclusion 4-1: There are gaps in guidance on the use of race and ethnicity in research studies, especially early in the research process. Some helpful guidance for recruitment and data collection exists, yet improvements and accountability are needed. Existing publishing guidelines are useful, but the biomedical research community could benefit from further standardization for reporting race and ethnicity in publications and enforcement in a systematic manner. In addition, guidance is needed on study design, engagement and partnership with communities and other invested parties, use of legacy data, data analysis, and interpretation of results.
Some existing recommendations attempt to provide direction for considering race and ethnicity earlier in study design, suggesting, for example, the need to provide clear rationale for the use of race and ethnicity. However, these guidelines tend to lack detailed standards illustrating the kind of rationale required, allowing researchers to justify their approach without strong scientific reasoning. Furthermore, these guidelines can be difficult to enforce at the time of publication because publishing and disseminating results are downstream of many key points of decision making in the research process. These earlier junctures in study design and execution can be pivotal intervention points for improving the use of race and ethnicity in biomedical research.
Also based on their analysis of the existing guidance and the gaps identified, the committee provides the following conclusions:
Conclusion 4-2: Conducting biomedical research with American Indian or Alaska Native Tribes is distinct from working with other populations. Enrolled Tribal members have both a racial identity and citizenship within one of the 574 federally recognized Tribes. As Tribes are sovereign nations, soliciting entrance to the community may require demonstrated understanding of their history as survivors of relocation, reservation, assimilation, and termination federal policy. Members may be guarded toward participating in research. It is critical to recognize requirements for working with Tribal nations, including distinct IRB processes, data sovereignty, and approval for dissemination of results.
Conclusion 4-3: There is a critical gap in guidance regarding the use of race and ethnicity in all phases of the development of clinical algorithms and biomedical
AI models, including in problem formulation, data selection, algorithm development, deployment, and monitoring. There are inadequate reporting, regulatory, and legal standards for the use of race and ethnicity in clinical prediction models and other clinical decision tools. Additional investment in creating repositories and specific standards for assessment across racial and ethnic groups as appropriate could help close these gaps and promote transparency in clinical algorithms and biomedical AI models.
REFERENCES
Addison, C., B. W. Campbell Jenkins, M. White, D. Thigpen Odom, M. Fortenberry, G. Wilson, P. McCoy, L. Young, C. Woodberry, K. Herron, J. Clark, M. Payton, and D. A. LaVigne. 2021. Twenty years of leading the way among cohort studies in community-driven outreach and engagement: Jackson State University/Jackson Heart Study. International Journal of Environmental Research and Public Health 18(2):696.
AHA/ASA (American Heart Association/American Stroke Association). 2021. Disparities research guidelines. https://www.ahajournals.org/disparities-research-guidelines (accessed September 5, 2024).
AHRQ (Agency for Healthcare Research and Quality). 2018. Race, ethnicity, and language data: Standardization for health care quality improvement. https://www.ahrq.gov/research/findings/final-reports/iomracereport/index.html (accessed July 3, 2024).
Anderson, E. E., S. Solomon, E. Heitman, J. M. DuBois, C. B. Fisher, R. G. Kost, M. E. Lawless, C. Ramsey, B. Jones, A. Ammerman, and L. F. Ross. 2012. Research ethics education for community-engaged research: A review and research agenda. Journal of Empirical Research on Human Research Ethics 7(2):3–19.
Anderson, J. W., N. Shaikh, and S. Visweswaran. 2024. Measuring and reducing racial bias in a pediatric urinary tract infection model. AMIA Joint Summits on Translational Science Proceedings 2024:488–497.
Around Him, D., T. A. Aguilar, A. Frederick, H. Larsen, M. Seiber, and J. Angal. 2019. Tribal IRBs: A framework for understanding research oversight in American Indian and Alaska Native communities. American Indian Alaska Native Mental Health Resources 26(2):71–95.
Beauchamp, T. L., and J. F. Childress. 2001. Principles of biomedical ethics. New York: Oxford University Press.
Beech, B. M., and E. Heitman. 2024. Race and research: Perspectives on minority participation in health studies. Washington, DC: American Public Health Association.
Bergstrom, D., K. Rose, J. Olinger, and K. Holly. 2012. The community engagement guide for sustainable communities. PolicyLink. Available at https://www.policylink.org/resources-tools/community-engagement-guide-for-sustainable-communities (accessed August 4, 2024).
Bhopal, R. 1997. Is research into ethnicity and health racist, unsound, or important science? BMJ 314(7096):1751.
Bonham, V. L., E. D. Green, and E. J. Pérez-Stable. 2018. Examining how race, ethnicity, and ancestry data are used in biomedical research. JAMA 320(15):1533–1534.
Boyd, R. W., E. G. Lindo, L. D. Weeks, and M. R. McLemore. 2020. On racism: A new standard for publishing on racial health inequities. Health Affairs Forefront, July 2. https://www.healthaffairs.org/content/forefront/racism-new-standard-publishing-racial-health-inequities (accessed August 4, 2024).
Byeon, Y. J. J., R. Islamaj, L. Yeganova, W. J. Wilbur, Z. Lu, L. C. Brody, and V. L. Bonham. 2021. Evolving use of ancestry, ethnicity, and race in genetics research—A survey spanning seven decades. American Journal of Human Genetics 108(12):2215–2223.
Carroll, S. R., I. Garba, R. Plevel, D. Small-Rodriguez, V. Y. Hiratsuka, M. Hudson, and N. A. Garrison. 2022. Using Indigenous standards to implement the CARE principles: Setting expectations through tribal research codes. Frontiers in Genetics 13:823309.
Cary Jr., M. P., S. Bessias, J. McCall, M. J. Pencina, S. D. Grady, K. Lytle, and N. J. Economou-Zavlanos. 2024. Empowering nurses to champion health equity & BE FAIR: Bias elimination for fair and responsible AI in healthcare. Journal of Nursing Scholarship 00:1–10.
CDC (Centers for Disease Control and Prevention). 1997. Principles of community engagement, 1st ed. Atlanta, GA: Centers for Disease Control and Prevention.
CDC. 2011. Principles of community engagement, 2nd ed. Atlanta, GA: Centers for Disease Control and Prevention. https://www.atsdr.cdc.gov/community-engagement/php/about/index.html (accessed January 9, 2025).
Chadwick, J. Q., K. C. Copeland, D. E. Branam, J. A. Erb-Alvarez, S. I. Khan, M. T. Peercy, M. E. Rogers, B. R. Saunkeah, J. B. Tryggestad, and D. F. Wharton. 2019. Genomic research and American Indian Tribal communities in Oklahoma: Learning from past research misconduct and building future trusting partnerships. American Journal of Epidemiology 188(7):1206–1212.
Chen, S., and J. Li. 2021. Participation of Black US residents in clinical trials of 24 cardiovascular drugs granted FDA approval, 2006–2020. JAMA Network Open 4(3):e212640.
Chin, M. H., N. Afsar-Manesh, A. S. Bierman, C. Chang, C. J. Colón-Rodríguez, P. Dullabh, D. G. Duran, M. Fair, T. Hernandez-Boussard, M. Hightower, A. Jain, W. B. Jordan, S. Konya, R. H. Moore, T. T. Moore, R. Rodriguez, G. Shaheen, L. P. Snyder, M. Srinivasan, C. A. Umscheid, and L. Ohno-Machado. 2023. Guiding principles to address the impact of algorithm bias on racial and ethnic disparities in health and health care. JAMA Network Open 6(12):e2345050.
Claw, K. G., N. Dundas, M. S. Parrish, R. L. Begay, T. L. Teller, N. A. Garrison, and F. Sage. 2021. Perspectives on genetic research: Results from a survey of Navajo community members. Frontiers in Genetics 12:734529.
Clinical Algorithms with Race. 2023. Risk calculators, laboratory tests, and therapy recommendations with race-based guidelines. https://clinical-algorithms-with-race.org/risk-calculators-laboratory-tests-therapy-recommendations (accessed March 1, 2024).
CMSS (Council of Medical Specialty Societies). 2023. Reconsidering race in clinical algorithms: Driving equity through new models in research and implementation. https://cmss.org/reconsidering-race-in-clinical-algorithms-driving-equity-through-new-models-in-research-and-implementation/ (accessed August 4, 2024).
Collins, G. S., P. Dhiman, C. L. Andaur Navarro, J. Ma, L. Hooft, J. B. Reitsma, P. Logullo, A. L. Beam, L. Peng, B. Van Calster, M. van Smeden, R. D. Riley, and K. G. Moons. 2021. Protocol for development of a reporting guideline (TRIPOD-AI) and risk of bias tool (PROBAST-AI) for diagnostic and prognostic prediction model studies based on artificial intelligence. BMJ Open 11(7):e048008.
Croll, P. R., and J. Gerteis. 2019. Race as an open field: Exploring identity beyond fixed choices. Sociology of Race and Ethnicity 5(1):55–69.
Cruikshank, J. 1992. Life lived like a story. Lincoln, NE: University of Nebraska Press.
Cruz Rivera, S., X. Liu, A.-W. Chan, A. K. Denniston, M. J. Calvert, A. Darzi, C. Holmes, C. Yau, D. Moher, H. Ashrafian, J. J. Deeks, L. Ferrante di Ruffano, L. Faes, P. A. Keane, S. J. Vollmer, A. Y. Lee, A. Jonas, A. Esteva, A. L. Beam, M. B. Panico, C. S. Lee, C. Haug, C. J. Kelly, C. Yau, C. Mulrow, C. Espinoza, J. Fletcher, D. Moher, D. Paltoo, E. Manna, G. Price, G. S. Collins, H. Harvey, J. Matcham, J. Monteiro, M. K. ElZarrad, L. Ferrante di Ruffano, L. Oakden-Rayner, M. McCradden, P. A. Keane, R. Savage, R. Golub, R. Sarkar, S. Rowley, S.-A. The, C.-A. W. Group, A. I. Spirit, C.-A. S. Group, A. I. Spirit, and C.-A. C. Group. 2020. Guidelines for clinical trial protocols for interventions involving artificial intelligence: The SPIRIT-AI extension. Nature Medicine 26(9):1351–1363.
Davidson, K. W., C. M. Mangione, M. J. Barry, M. D. Cabana, A. B. Caughey, E. M. Davis, K. E. Donahue, C. A. Doubeni, A. H. Krist, M. Kubik, L. Li, G. Ogedegbe, L. Pbert, M. Silver-stein, M. Simon, J. Stevermer, C. W. Tseng, and J. B. Wong. 2021. Actions to transform U.S. Preventive Services Task Force methods to mitigate systemic racism in clinical preventive services. JAMA 326(23):2405–2411.
Delgado, C., M. Baweja, D. C. Crews, N. D. Eneanya, C. A. Gadegbeku, L. A. Inker, M. L. Mendu, W. G. Miller, M. M. Moxey-Mims, G. V. Roberts, W. L. St. Peter, C. Warfield, and N. R. Powe. 2022. A unifying approach for GFR estimation: Recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. American Journal of Kidney Diseases 79(2):268–288.e261.
Deloria Jr., V. 1999. Spirit and reason: The Vine Deloria, Jr., Reader. Wheat Ridge, CO: Fulcrum.
Doubeni, C. A., M. Simon, and A. H. Krist. 2021. Addressing systemic racism through clinical preventive service recommendations from the U.S. Preventive Services Task Force. JAMA 325(7):627–628.
du Toit, C., T. Q. B. Tran, N. Deo, S. Aryal, S. Lip, R. Sykes, I. Manandhar, A. Sionakidis, L. Stevenson, H. Pattnaik, S. Alsanosi, M. Kassi, N. Le, M. Rostron, S. Nichol, A. Aman, F. Nawaz, D. Mehta, R. Tummala, L. McCallum, S. Reddy, S. Visweswaran, R. Kashyap, B. Joe, and S. Padmanabhan. 2023. Survey and evaluation of hypertension machine learning research. Journal of the American Heart Association 12(9):e027896.
FDA (U.S. Food and Drug Administration). 2016. Guidance for industry and Food and Drug Administration staff. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/collection-race-and-ethnicity-data-clinical-trials (accessed July 3, 2024).
FDA. 2020. Enhancing the diversity of clinical trial populations—Eligibility criteria, enrollment practices, and trial designs: Guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enhancing-diversity-clinical-trial-populations-eligibility-criteria-enrollment-practices-and-trial (accessed July 9, 2024).
FDA. 2022. Diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials: Guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/diversity-plans-improve-enrollment-participants-underrepresented-racial-and-ethnic-populations (accessed July 3, 2024).
FDA. 2023. Artificial intelligence program: Research on AI/ML-based medical devices. https://www.fda.gov/medical-devices/medical-device-regulatory-science-research-programs-conducted-osel/artificial-intelligence-program-research-aiml-based-medical-devices (accessed April 15, 2024).
Feero, W. G., R. D. Steiner, A. Slavotinek, T. Faial, M. J. Bamshad, J. Austin, B. R. Korf, A. Flanagin, and K. Bibbins-Domingo. 2024. Guidance on use of race, ethnicity, and geographic origin as proxies for genetic ancestry groups in biomedical publications. JAMA 331(15):1276–1278.
Flanagin, A., T. Frey, S. L. Christiansen, and AMA Manual of Style Committee. 2021. Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA 326(7):621–627.
Forsythe, L. P., K. L. Carman, V. Szydlowski, L. Fayish, L. Davidson, D. H. Hickam, C. Hall, G. Bhat, D. Neu, L. Stewart, M. Jalowsky, N. Aronson, and C. U. Anyanwu. 2019. Patient engagement in research: Early findings from the patient-centered outcomes research institute. Health Affairs 38(3):359–367.
Garba, I., R. Sterling, R. Plevel, W. Carson, F. M. Cordova-Marks, J. Cummins, C. Curley, D. David-Chavez, A. Fernandez, D. Hiraldo, V. Hiratsuka, M. Hudson, M. B. Jäger, L. L. Jennings, A. Martinez, J. Yracheta, N. A. Garrison, and S. R. Carroll. 2023. Indigenous peoples and research: Self-determination in research governance. Frontiers in Research Metrics and Analytics 8.
Garrison, N. A., and S. R. Carroll. 2023. Genetic research with Indigenous peoples: Perspectives on governance and oversight in the U.S. Frontiers in Research Metrics and Analytics 8. https://doi.org/10.3389/frma.2023.1286948.
Garrison, N. A., and S. R. Carroll. 2024. Enhancing genomic research through the lens of indigenous data sovereignty. In B. M. Beech and E. Heitman (eds.), Race and research: Perspectives on minority participation in health studies, 2nd ed. Washington, DC: APHA Press. Pp. 23–36.
Gijsberts, C. M., K. A. Groenewegen, I. E. Hoefer, M. J. Eijkemans, F. W. Asselbergs, T. J. Anderson, A. R. Britton, J. M. Dekker, G. Engström, G. W. Evans, J. de Graaf, D. E. Grobbee, B. Hedblad, S. Holewijn, A. Ikeda, K. Kitagawa, A. Kitamura, D. P. de Kleijn, E. M. Lonn, M. W. Lorenz, E. B. Mathiesen, G. Nijpels, S. Okazaki, D. H. O’Leary, G. Pasterkamp, S. A. Peters, J. F. Polak, J. F. Price, C. Robertson, C. M. Rembold, M. Rosvall, T. Rundek, J. T. Salonen, M. Sitzer, C. D. Stehouwer, M. L. Bots, and H. M. den Ruijter. 2015. Race/ethnic differences in the associations of the Framingham risk factors with carotid IMT and cardiovascular events. PLOS One 10(7):e0132321.
Hanson, P. 1988. Citizen involvement in community health promotion: A role application of CDC’s PATCH model. International Quarterly of Community Health Education 9(3):177–186.
Harding, A., B. Harper, D. Stone, C. O’Neill, P. Berger, S. Harris, and J. Donatuto. 2012. Conducting research with tribal communities: Sovereignty, ethics, and data-sharing issues. Environmental Health Perspectives 120(1):6–10.
Hatch, M., J. Parrish, S. Heppell, S. Augustine, L. Campbell, L. Divine, J. Donatuto, A. Groesbeck, and N. Smith. 2023. Boundary spanners: A critical role for enduring collaborations between indigenous communities and mainstream scientists. Ecology and Society 28(1).
Hayward, A., E. Sjoblom, S. Sinclair, and J. Cidro. 2021. A new era of indigenous research: Community-based indigenous research ethics protocols in Canada. Journal of Empirical Research on Human Research Ethics 16(4):403–417.
Hernandez-Boussard, T., S. Bozkurt, J. P. A. Ioannidis, and N. H. Shah. 2020. Minimar (minimum information for medical AI reporting): Developing reporting standards for artificial intelligence in health care. Journal of the American Medical Informatics Association 27(12):2011–2015.
Hiratsuka, V. Y., J. K. Brown, T. J. Hoeft, and D. A. Dillard. 2012. Alaska native people’s perceptions, understandings, and expectations for research involving biological specimens. International Journal of Circumpolar Health 71:18642.
Ho-Chunk Nation. 2005. Tribal research code. https://tribalinformationexchange.org/wp-content/uploads/2019/03/Ho-Chunk-Nation-Tribal-Research-Code.pdf (accessed August 4, 2024).
Hood, N. E., T. Brewer, R. Jackson, and M. E. Wewers. 2010. Survey of community engagement in NIH-funded research. Clinical Translational Science 3(1):19–22.
ICMJE (International Committee of Medical Journal Editors). 2023. Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals. https://www.icmje.org/recommendations/ (accessed July 3, 2024).
IOM (Institute of Medicine). 2003a. The future of the public’s health in the 21st century. Washington, DC: National Academy Press. https://doi.org/10.17226/10548.
IOM. 2003b. Unequal treatment: Confronting racial and ethnic disparities in health care. Washington, DC: The National Academies Press.
Jones, E. J., E. Haozous, L. S. Larsson, and M. P. Moss. 2019. Perspectives on conducting research in Indian country. Nursing Research 68(6):488–493.
Jonsen, A., M. Siegler, and W. Winslade. 1998. Clinical ethics, 4th ed. New York: McGraw-Hill.
Kaplan, J. B., and T. Bennett. 2003. Use of race and ethnicity in biomedical publication. JAMA 289(20):2709–2716.
Lambin, P., R. T. H. Leijenaar, T. M. Deist, J. Peerlings, E. E. C. de Jong, J. van Timmeren, S. Sanduleanu, R. Larue, A. J. G. Even, A. Jochems, Y. van Wijk, H. Woodruff, J. van Soest, T. Lustberg, E. Roelofs, W. van Elmpt, A. Dekker, F. M. Mottaghy, J. E. Wildberger, and S. Walsh. 2017. Radiomics: The bridge between medical imaging and personalized medicine. Nature Reviews Clinical Oncology 14(12):749–762.
Laveaux, D., and S. Christopher. 2009. Contextualizing CBPR: Key principles of CBPR meet the Indigenous research context. Pimatisiwin 7(1):1.
Lekadira, K., R. Osuala, C. S. Gallin, N. Lazrak, K. Kushibar, G. Tsakou, S. Auss’o, L. C. a. Alberich, K. Marias, M. Tskinakis, S. Colantonio, N. Papanikolaou, Z. Salahuddin, H. Woodruff, P. Lambin, and L. Mart’i-Bonmat’i. 2021. Future-AI: Guiding principles and consensus recommendations for trustworthy artificial intelligence in medical imaging. arXiv:2109.09658.
Lin, J., E. Webber, and S. Bean. 2023. Health equity framework for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality. https://www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/2023-12/health-equity-framework-report.pdf (accessed August 4, 2024).
Lin, J. S., E. M. Webber, S. I. Bean, and C. V. Evans. 2024. Development of a health equity framework for the U.S. Preventive Services Task Force. JAMA Network Open 7(3):e241875.
Liu, X., S. Cruz Rivera, D. Moher, M. J. Calvert, A. K. Denniston, A.-W. Chan, A. Darzi, C. Holmes, C. Yau, H. Ashrafian, J. J. Deeks, L. Ferrante di Ruffano, L. Faes, P. A. Keane, S. J. Vollmer, A. Y. Lee, A. Jonas, A. Esteva, A. L. Beam, A.-W. Chan, M. B. Panico, C. S. Lee, C. Haug, C. J. Kelly, C. Yau, C. Mulrow, C. Espinoza, J. Fletcher, D. Paltoo, E. Manna, G. Price, G. S. Collins, H. Harvey, J. Matcham, J. Monteiro, M. K. ElZarrad, L. Ferrante di Ruffano, L. Oakden-Rayner, M. McCradden, P. A. Keane, R. Savage, R. Golub, R. Sarkar, S. Rowley, the SPIRIT-AI and CONSORT-AI Working Group. 2020. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: The CONSORT-AI extension. Nature Medicine 26(9):1364–1374.
Lu, C., R. Ahmed, A. Lamri, and S. S. Anand. 2022. Use of race, ethnicity, and ancestry data in health research. PLOS Global Public Health 2(9):e0001060.
Martei, Y. M., H. A. Afari, and C. E. Guerra. 2024. Diversity in cardio-oncology clinical trials. JACC: CardioOncology 6(3):386–389.
Martinez, R. A. 2023a. Extant race and ethnicity communication guidelines in health research. Presentation to the Committee on the Use of Race and Ethnicity in Biomedical Research.
Martinez, R. A. M., N. Andrabi, A. N. Goodwin, R. E. Wilbur, N. R. Smith, and P. N. Zivich. 2023b. Conceptualization, operationalization, and utilization of race and ethnicity in major epidemiology journals, 1995–2018: A systematic review. American Journal of Epidemiology 192(3):483–496.
Matheny, M. E., D. Whicher, and S. Thadaney Israni. 2020. Artificial intelligence in health care: A report from the National Academy of Medicine. JAMA 323(6):509–510.
Mauro, M., D. S. Allen, B. Dauda, S. J. Molina, B. M. Neale, and A. C. F. Lewis. 2022. A scoping review of guidelines for the use of race, ethnicity, and ancestry reveals widespread consensus but also points of ongoing disagreement. American Journal of Human Genetics 109(12):2110–2125.
Mongan, J., L. Moy, and C. E. Kahn, Jr. 2020. Checklist for Artificial Intelligence in Medical Imaging (CLAIM): A guide for authors and reviewers. Radiology Artificial Intelligence 2(2): e200029.
Muñoz, M. A., G. J. Dal Pan, Y. J. Wei, H. Xiao, C. Delcher, A. Giffin, N. Sadiq, and A. G. Winterstein. 2024. Sociodemographic characteristics of adverse event reporting in the USA: An ecologic study. Drug Safety 47(4):377–387.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2022. Improving representation in clinical trials and research: Building research equity for women and underrepresented groups. Washington, DC: The National Academies Press.
NASEM. 2023. Using population descriptors in genetics and genomics research: A new framework for an evolving field. Washington, DC: The National Academies Press.
NASEM. 2024. Unequal treatment revisited: The current state of racial and ethnic disparities in health care: Proceedings of a workshop. Washington, DC: The National Academies Press.
NHGRI (National Human Genome Research Institute). 2016. Workshop on the use of race and ethnicity in genomics and biomedical research. https://www.genome.gov/event-calendar/Workshop-on-Use-of-Race-Ethnicity-in-Genomics-Biomedical-Research (accessed April 15, 2024).
NIMHD (National Institute on Minority Health and Health Disparities). 2024. Community-based participatory research program (CBPR). https://www.nimhd.nih.gov/programs/extramural/community-based-participatory.html (accessed September 5, 2024).
Norgeot, B., G. Quer, B. K. Beaulieu-Jones, A. Torkamani, R. Dias, M. Gianfrancesco, R. Arnaout, I. S. Kohane, S. Saria, E. Topol, Z. Obermeyer, B. Yu, and A. J. Butte. 2020. Minimum Information about Clinical Artificial Intelligence Modeling: The MI-CLAIM checklist. Nature Medicine 26(9):1320–1324.
NYC Dept of Health (New York City Department of Health and Mental Hygiene). 2017. Community engagement framework. https://www.nyc.gov/assets/doh/downloads/pdf/che/community-engagement-framework.pdf (accessed September 4, 2024).
Obermeyer, Z., B. Powers, C. Vogeli, and S. Mullainathan. 2019. Dissecting racial bias in an algorithm used to manage the health of populations. Science 366(6464):447–453.
OMB (U.S. Office of Management and Budget). 2024. Revisions to OMB’s statistical policy directive no. 15: Standards for maintaining, collecting, and presenting federal data on race and ethnicity. Federal Register. https://www.federalregister.gov/documents/2024/03/29/2024-06469/revisions-to-ombs-statistical-policy-directive-no-15-standards-for-maintaining-collecting-and (accessed August 4, 2024).
Paskett, E. D., M. L. Katz, C. R. DeGraffinreid, and C. M. Tatum. 2003. Participation in cancer trials: Recruitment of underserved populations. Clinical Advances in Hematology and Oncology 1(10):607–613.
Peloquin, D., Barnes, M., Lam, C. 2023. Congress enacts legislation requiring guidance on clinical research diversity and modernization. https://www.ropesgray.com/en/insights/alerts/2023/01/congress-enacts-legislation-requiring-guidance-on-clinical-research-diversity-and-modernization (accessed July 3, 2024).
Popejoy, A. B. 2021. Too many scientists still say Caucasian. Nature 596(7873):463.
Powe, N. R. 2024. Race, health care algorithms, and precision health equity. Annals of Internal Medicine 177(4):537–538.
Rivara, F. P., and L. Finberg. 2001. Use of the terms race and ethnicity. Archives of Pediatrics & Adolescent Medicine 155(2):119.
Rosen, R. H., A. Epee-Bounya, D. Curran, S. Chung, R. Hoffmann, L. K. Lee, C. Marcus, C. M. Mateo, J. E. Miller, C. Nereim, E. Silberholz, S. N. Shah, C. V. Theodoris, H. Wardell, A. S. Winn, S. Toomey, J. A. Finkelstein, V. L. Ward, and A. Starmer. 2023. Race, ethnicity, and ancestry in clinical pathways: A framework for evaluation. Pediatrics 152(6):e2022060730.
Roth, W. D. 2016. The multiple dimensions of race. Ethnic and Racial Studies 39(8):1310–1338.
Saperstein, A. 2012. Capturing complexity in the United States: Which aspects of race matter and when? Ethnic and Racial Studies 35(8):1484–1502.
Siddique, S. M., K. Tipton, B. Leas, C. Jepson, J. Aysola, J. B. Cohen, E. Flores, M. O. Harhay, H. Schmidt, G. E. Weissman, J. Fricke, J. R. Treadwell, and N. K. Mull. 2024. The impact of health care algorithms on racial and ethnic disparities: A systematic review. Annals of Internal Medicine 177(4):484–496.
Sounderajah, V., H. Ashrafian, R. M. Golub, S. Shetty, J. De Fauw, L. Hooft, K. G. M. Moons, G. S. Collins, D. Moher, P. M. M. Bossuyt, A. Darzi, A. Karthikesalingam, A. K. O. Denniston, B. A. Mateen, D. S. W. Ting, D. E. Treanor, D. King, F. Greaves, J. Godwin, J. Pearson-Stuttard, L. Harling, M. D. F. McInnes, N. Rifai, N. Tomašev, P. Normahani, P. F. Whiting, R. Aggarwal, S. J. Vollmer, S. R. Markar, T. Panch, and X. Liu. 2021. Developing a reporting guideline for artificial intelligence-centred diagnostic test accuracy studies: The STARD-AI protocol. BMJ Open 11.
Taylor, J. Y. 2009. Recruitment of three generations of African American women into genetics research. Journal of Transcultural Nursing 20(2):219–226.
Tipton, K., B. F. Leas, E. Flores, C. Jepson, J. Aysola, J. Cohen, M. Harhay, H. Schmidt, G. Weissman, J. Treadwell, N. K. Mull, and S. M. Siddique. 2023. Impact of healthcare algorithms on racial and ethnic disparities in health and healthcare. Comparative Effectiveness Review, no. 268. Rockville, MD: Agency for Healthcare Research and Quality.
Tsosie, R. 2002. Reclaiming native stories: An essay on cultural appropriation and cultural rights. Arizona State Law Journal 34:299–358.
Tsosie, R. 2007. Cultural challenges to biotechnology: Native American genetic resources and the concept of cultural harm. Journal of Law, Medicine & Ethics 35(3):396–411.
Turner, B. E., J. R. Steinberg, B. T. Weeks, F. Rodriguez, and M. R. Cullen. 2022. Race/ethnicity reporting and representation in U.S. clinical trials: A cohort study. The Lancet Regional Health–Americas 11:100252.
Victor, R. G., K. Lynch, N. Li, C. Blyler, E. Muhammad, J. Handler, J. Brettler, M. Rashid, B. Hsu, D. Foxx-Drew, N. Moy, A. E. Reid, and R. M. Elashoff. 2018. A cluster-randomized trial of blood-pressure reduction in Black barbershops. New England Journal of Medicine 378(14):1291–1301.
Wilkins, C. H., and P. M. Alberti. 2019. Shifting academic health centers from a culture of community service to community engagement and integration. Academic Medicine 94(6):763–767.
Woodbury, R. B., S. Ketchum, V. Y. Hiratsuka, and P. Spicer. 2019. Health-related participatory research in American Indian and Alaska Native communities: A scoping review. International Journal of Environmental Research and Public Health 16(16).
Wright, J. L., W. S. Davis, M. M. Joseph, A. M. Ellison, N. J. Heard-Garris, T. L. Johnson, and the American Academy of Pediatrics Board Committee on Equity. 2022. Eliminating race-based medicine. Pediatrics 150(1):e2022057998.
This page intentionally left blank.





































