Protein Quality and Growth Monitoring Studies: Quality Factor Requirements for Infant Formula (2025)
Chapter: Appendix C: Open Meeting Agendas
Appendix C
Open Meeting Agendas
This appendix contains the following:
- C-1: April 3, 2024, public session agenda
- C-2: April 3, 2024, public session presentation slides
- C-3: July 9–10, 2024, public session workshop agenda
- C-4: September 4, 2024, public session agenda
- C-5: October 22, 2024, public session agenda
The presentation slides from the Meeting 7 public workshop are in the Public Access File, available by request via the National Academies. https://www.nationalacademies.org/our-work/protein-quality-and-growth-monitoring-studies-to-satisfy-quality-factor-requirements-for-in-fant-formula
C-1: Meeting Agenda
Virtual
April 3, 2024
11:30 am–12:30 pm ET
Open Session
| 11:30 am | Review of Committee’s Task Patricia Hansen, U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition |
| 11:50 am | Q & A with Sponsor Stephanie Atkinson |
| 12:15 pm | Closing Remarks Stephanie Atkinson |
| 12:30 pm | Adjourn Open Session |
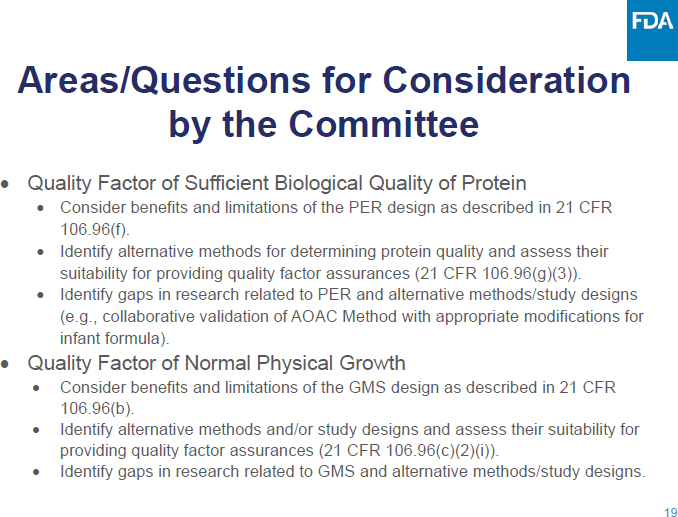
C-2: April 3, 2024
Open Session Presentation Slides






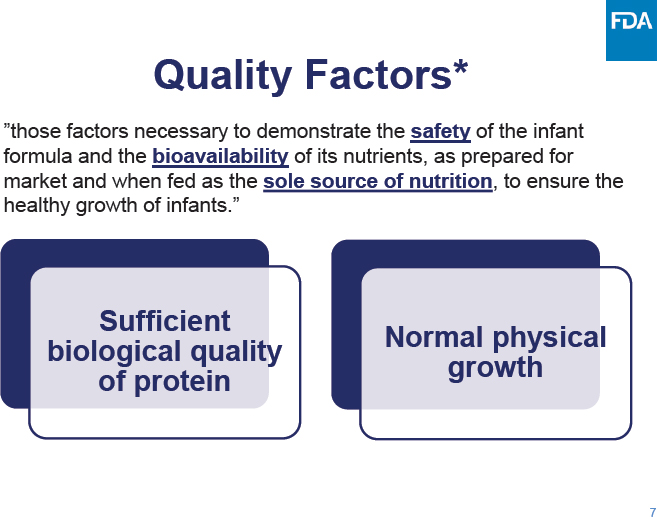


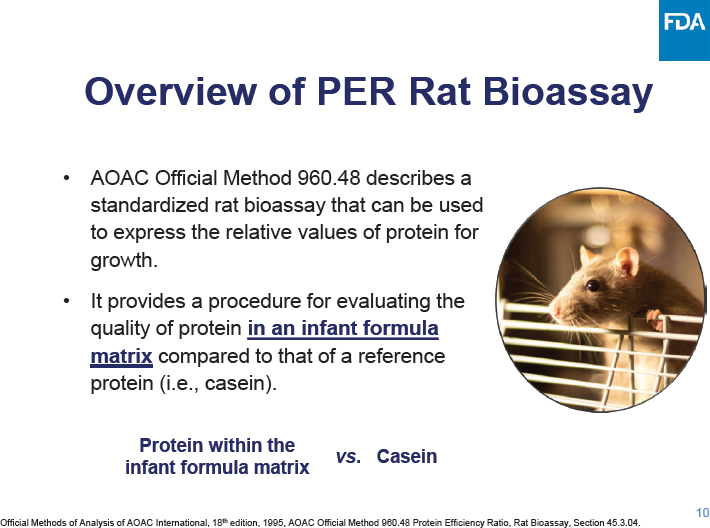
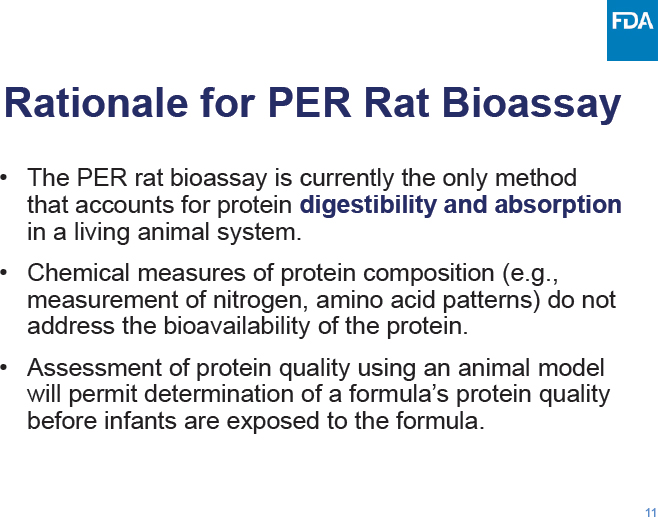




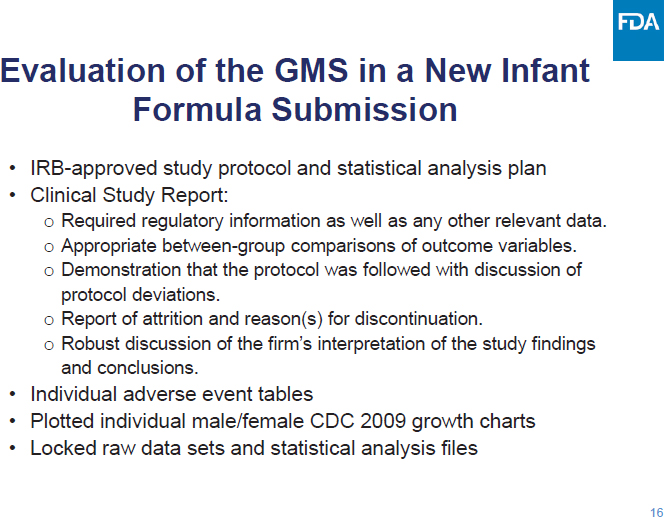




C-3: Meeting Agenda
Advances in the Science to Assess Quality Factors for Infant Formulas in United States
July 9–10, 2024
The National Academies’ Keck Center
Washington, DC
The goal of this workshop is to gather information on current global perspectives on the rationale for use of protein quality assessment measures, the applicability of animal model research, and challenges of assessing protein quality using infant growth in clinical studies for the purpose of approving new infant formulas for market.
Objectives:
- Provide a critical review of the state of the science on current methods for assessment of protein quality and their use/limitations as measures of protein quality of infant formula.
- Explore the approaches considered by other countries in establishing regulations for protein quality in infant formula and growth in infants fed a new infant formula.
- Obtain a global perspective of the variation in requirements for the conduct of clinical trials in infants for the purpose of regulatory approval locally/internationally and the challenges experienced.
Tuesday, July 9, 2024
Open Session
| 8:45 am |
Welcome and Chair’s Statement
Stephanie Atkinson, Committee Chair Session 1: Models for Assessment of Protein Quality in Infant Formula Moderated by: Rajavel Elango |
| 9:00 |
Critical Analysis of PER Method to Assess Protein Quality in Infant Formula Robert Burns, Ph.D., Indiana University Bloomington |
| 9:20 |
PDCAAS and DIAAS as an Assessment of Amino Acid Score and Use of Human Milk Pattern as a Reference Paul Moughan, Ph.D., D.Sc., Hon D.Sc., FRSNZ, FRSC, Massey University, New Zealand |
| 9:40 | Q&A |
| 10:00 |
Models of Ileal Digestion and Amino Acid Composition of Breast Milk in Protein Quality Studies Daniel Tomé, Ph.D., AgroParisTech, Université Paris-Saclay, France |
| 10:30 | Q&A |
| 10:45 | Break |
| 11:00 |
Session 2: Global Perspectives on Guidance for Measures and Regulation of Quality Factors in Assessing Infant Formula—Panel Discussion Moderated by: John C. Wallingford |
| 11:00 |
Perspectives from the European Union Brian Kineman, Ph.D., Regulatory Affairs, Danone Perspectives from Multinational Corporations Robert DiGregorio, D.O., Harmony Baby Nutrition Perspectives from the United States Russell Merritt, M.D., Ph.D., Division of Gastroenterology, Hepatology and Nutrition, University of Southern California, Los Angeles |
| 11:30 | Questions and Discussion |
| 12:00 pm | Adjourn Open Session |
| 1:00 pm | Closed Session |
| 4:30 pm | Adjourn |
Wednesday, July 10, 2024
Open Session
| 8:30 am |
Welcome and Chair’s Statement Stephanie Atkinson, Committee Chair Session 3: Challenges in the Conduct and Interpretation of Clinical Trials for Regulatory Approval for a New Infant Formula Moderated by: Steven Abrams |
| 8:40 |
Body Composition as a Measure of Quality of Growth in Infants Shane Norris, Ph.D., MASSAF, FAAS, University of Southampton, U.K. |
| 9:00 |
Challenges in Conduct of Clinical Trials Assessing Quality Factors for Infant Formula: Methods, Cost, and Options Michael B Montalto, Ph.D., Principal, Montalto Consulting Services in Health Sciences |
| 9:20 |
Optimizing Clinical Trial Design for Studies in Infants Deborah O’Connor, Ph.D., RD, Department of Nutritional Sciences, University of Toronto |
| 9:40 | Q&A |
| 10:10 |
Closing Remarks
Stephanie Atkinson, Committee Chair |
| 10:30 | Adjourn Open Session |
| 11:00 am | Closed Session |
| 1:00 pm | Adjourn Meeting |
C-4: Meeting Agenda
September 4, 20241
C-5: Meeting Agenda
October 22, 2024
11:00 am–12:00 pm Eastern
Open Session
| 10:00 am |
Welcome and Introductions
Stephanie Atkinson, Chair |
| 10:35 am |
Panel Discussion
Moderated by Stephanie Atkinson, Chair
|
| 12:00 pm | Adjourn Meeting |
___________________
1 There was no printed agenda for this open public meeting with sponsors. The video recording is available at: https://www.nationalacademies.org/event/43605_09-2024_protein-quality-and-growth-monitoring-studies-to-satisfy-quality-factor-requirements-for-in-fant-formula-meeting-with-sponsor (accessed January 20, 2025).















