Protein Quality and Growth Monitoring Studies: Quality Factor Requirements for Infant Formula (2025)
Chapter: 2 Methodology
2
Methodology
INTRODUCTION
Two of the U.S. premarket regulatory requirements for infant formula involve specific “quality factors”: the biological quality of protein in the infant formula and the ability of the infant formula to support normal physical growth. As part of its task, the committee was asked to examine the state of the science regarding methodologies for assessing both factors. The purpose of the study was to explore alternate study designs and methods that could potentially be used to demonstrate that these two important quality factors have been met for any infant formula and are consistent with regulatory standards in global regions outside the United States to support international regulatory harmonization.
The requirements for the specific types of studies to be conducted and data and information to be submitted to the U.S. Food and Drug Administration (FDA), to assess quality factors of infant formula, are unique to the United States. Specific topics of interest to FDA include the following:
- The strengths and weaknesses of study designs that may be in common use in other global regions;
- The extent to which specific study designs have been validated; and
- The appropriateness of specific animal models.
The committee’s approach to gathering evidence related to these topics included conducting scoping reviews of the current published
literature; carrying out narrative reviews of unpublished (grey) literature; and assessing public documentation provided by the sponsor.
COMMITTEE’S INTERPRETATION OF THE TASK
The committee interpreted the statement of task to be a request for it to perform a scoping review of the available literature to identify the scientific evidence on the state of the science related to the biological quality of protein and the assessment of normal infant physical growth. The committee was guided by the statement of task (See Chapter 1, Box 1-1) and slide material provided during discussions with the study sponsor during an open meeting (see Boxes 2-1 and 2-2). The committee interpreted the task as a request to scan the available literature and provide an overview of the evidence, as opposed to performing a systematic review or meta-analysis. When the committee needed additional information outside of the articles identified by the scoping review, it undertook independent literature searches and gathered information from experts via open meetings (See Appendix C).
For the assigned task of providing FDA with recommendations for “alternative study designs” (see Boxes 2-1 and 2-2), the scope and defi-
BOX 2-1
Topics/Questions for Consideration by the Committee
Quality Factor of Sufficient Biological Quality of Protein
- Consider benefits and limitations of the protein efficiency ratio (PER) design as described in 21 CFR § 106.96(f).
- Identify alternative methods for determining protein quality and assess their suitability for providing quality factor assurances (21 CFR § 106.96(g)(3)).
- Identify gaps in research related to PER and alternative methods/study designs (e.g., collaborative validation of AOAC method with appropriate modifications for infant formula).
Quality Factor of Normal Physical Growth
- Consider benefits and limitations of the GMS design as described in 21 CFR § 106.96(b).
- Identify alternative methods and/or study designs and assess their suitability for providing quality factor assurances (21 CFR § 106.96(c)(2)(i)).
- Identify gaps in research related to GMS and alternative methods/study designs.
SOURCE: Open Meeting with FDA (Hansen, 2024).
BOX 2-2
Additional Information from FDA During Open Session
Alternatives to PER
Modern approaches for assessing protein quality, while showing significant promise, have yet to reach wide acceptability and standardization for use with infant formula:
- Indicator amino acid oxidation technique
- Net postprandial protein utilization
- Dual stable isotope approaches
- In vitro gastrointestinal models
Might some of these studies be used in combination or with supplemental data, in accordance with sound scientific principles, to meet our quality factor requirement?
Considerations to GMS Alternatives
Use of a concurrent versus historical control group
Infant age at enrollment
- No more than 2 weeks of age at time of study entry
Study duration
- No less than 15 weeks
Number and frequency of anthropometric measurements
- At least six total measurements with three measurements collected within the first 4 weeks of the study
SOURCE: Open Meeting with FDA (Hansen, 2024).
nition of “alternative” were ambiguous, given no formal specifications regarding (1) elements of FDA’s primary or standard study design requirements beyond those itemized in 21 CFR § 106.96(b) and § 106.121(a), which would serve as a benchmark or standard against which alternative designs could be compared; and (2) the extent to which FDA already defines or permits “alternative methods” used to conduct and report an adequate growth monitoring study (GMS). The committee therefore used expert judgment and interpreted the task as a request to review evidence on good clinical practices relevant to studies assessing infant growth.
COMMITTEE’S APPROACH AND METHODS
As described, the committee’s approach to gathering evidence included conducting scoping reviews of the published literature; holding public information-gathering sessions (see Appendix C); and assessing
public documentation provided by the sponsor. The committee provided conclusions and recommendations relevant to its task, but not all conclusions led to a recommendation.
Public Information-Gathering Methods
The committee used its expert judgment to identify experts to hear public comments during open sessions and the workshop (see Appendix C). In addition to public testimony, the committee identified methods to assess protein quality and normal physical growth used by other authoritative bodies in other countries. FDA provided information that was reviewed by the committee in its deliberations as well.
Scoping Review Methods
To understand the state of the science on the biological quality of protein in an infant formula and its ability to support normal physical growth, the committee carried out two scoping reviews to examine and report on (1) the methods or study design, including strengths and weaknesses; (2) whether they have been validated; and (3) whether methods vary globally. The committee commissioned the Academy of Nutrition and Dietetics (AND) to perform the evidence scans. Based on discussions between AND and the committee, the appropriate Population, Intervention/Exposure, Comparator, Outcome, and Study design framework was developed. Two protocols were developed to answer (1) growth monitoring measurement methods in infants up to 1 year of age and (2) protein quality methods in plant-based and animal-based infant formulas. Both protocols aimed to examine and report on the methods or study design being used, including reporting on their strengths and weaknesses and whether they have been validated, and examine whether methods vary globally. Both protocols are limited to primary articles published in English, in peer-reviewed journals between January 2000 and June 2024. The cut-off year of 2000 was used to limit the abundance of articles captured by the protocols, while ensuring that articles using the protein efficiency ratio (PER) method were also captured. Further, at the request of FDA, the Life Sciences Research Office (LSRO) in 1998 reviewed the literature on methods to determine protein quality in infant formula. The LSRO report “…recommended that the assessment of protein quality be based on an amino acid (AA) score with human milk as the reference protein, thus eliminating the PER as an index of protein quality for infant formulas” (Raiten et al., 1998). Thus, the committee considered a re-review of the literature prior to 2000 to be unnecessary. Appendix D presents further information about the search strategies.
Research Question 1: Biological Quality of Protein in Infant Formula
The first scoping review examined the state of the science regarding methodologies for assessing biological quality of protein in infant formula. The committee developed research questions pertaining to protein quality.
Research question 1 (RQ1) was
- In healthy animal studies, and in vitro digestion methodologies in combination with scoring methods, what is the state of the science regarding methodologies for assessing the biological quality of protein in infant formulas and the extent to which the methods have been validated?
- What study designs are used to assess protein quality?
- What methods and measures are used to assess protein quality?
- Do methods and measures vary globally?
Table 2-1 shows the eligibility criteria for RQ1. The scoping review was limited to studies enrolling healthy animal models or in vitro models, examining protein quality measures in animal-based, plant-based, or partially hydrolyzed infant formula compared to those with standard protein content or human milk amino acid pattern (HMAA) or infant AA requirements. The scoping review included only validity or reliability studies, experiments with and without randomization to treatment, in vitro digestibility studies, and meta-analyses and systematic reviews conducted worldwide.
| Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Study participants | Animal models; in vitro studies | Human studies |
| Health status of study participants | Studies with animal models or in vitro models representing healthy infants | Studies that exclusively enroll animals born prematurely or with metabolic conditions or disease or in vitro models of metabolic conditions or disease |
| Interventions/exposures | Cow’s milk–, plant- (e.g., soy or pea), or goat’s milk–based formula; partially hydrolyzed formula | Infant formulas deemed exempt by U.S. Food and Drug Administration (FDA), such as fully hydrolyzed AA formula or formulas for low birthweight infants or infants with metabolic diseases; toddler formulas |
| Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Comparators | Animals fed standard protein content or human milk AA pattern or infant AA requirements | Infant formulas deemed exempt by FDA, such as fully hydrolyzed amino acid (AA) formulas, and formulas for infants with metabolic diseases; toddler formulas |
| Outcomes | Protein or protein component amount, size, or structure; protein quality measures (e.g., protein efficiency ratio, net protein ratio, protein digestibility, bioavailability, Protein Digestibility–Corrected Amino Acid Score (PDCAAS), Digestible Indispensable Amino Acid Score (DIAAS), AA score); intake, growth, and body composition | Fecal microbiota, metabolomics, neural development, caco-2 cells/CaCO2, fatty acid profile, folate, niacin, minerals, zinc, prebiotics, flavonoids, oligosaccharides, phospholipids |
| Study design | Validity or reliability studies; experiments with randomization to treatments; nonrandomized experiments, including quasi-experimental, and controlled before-and-after studies; in vitro digestibility studies; meta-analyses; systematic reviews | Editorials; abstracts and conference abstracts; study protocols; narrative reviews |
| Publication status | Articles published in peer-reviewed journals | Articles that have not been peer reviewed and are not published in peer-reviewed journals, including unpublished data, manuscripts, preprints, reports, abstracts, and conference proceedings |
| Date of publication | January 2000 until the search date of July 22, 2024 | Articles published prior to January 2000 or after search date of July 22, 2024 |
| Language of publication | Articles published in English | Articles published in languages other than English |
| Country | Studies conducted in any country | None |
SOURCE: Generated by the Academy of Nutrition and Dietetics.
Research Question 2: Normal Physical Growth
The second scoping review examined the state of the science regarding methodologies for assessing the ability of an infant formula to meet quality factors with a focus on physical growth outcomes. The research question pertaining to normal physical growth.
Research question 2 (RQ2) was
- What study designs and methods, including benefits and limitations, are used to examine infant formula quality factors, including physical growth and body composition, in healthy term infants to 1 year of age?
- What are the strengths and weaknesses of study designs and methods used to measure quality of infant formula in human infants?
- Have the various study designs been validated?
- Do methods and measures to assess “quality of infant formula” vary globally?
Table 2-2 shows the eligibility criteria for RQ2. The scoping review was limited to studies enrolling full-term and healthy infants up to 12 months of age, examining physical growth, along with body composition and biomarkers of growth, in infants fed animal milk–based, plant-based, or partially hydrolyzed protein infant formula compared to those fed standard infant formula or human milk. Studies included in the protocol scan were limited to randomized and nonrandomized controlled trials, cohort and case-control studies, and meta-analyses and systematic reviews conducted in high-income countries, based on the United Nations (UN)/World Health Organization (WHO) index.
Screening
Two phases of screening were conducted; in each phase, all articles were screened independently by two reviewers. Screening occurred within a Web-based program (Rayyan) based on the inclusion and exclusion criteria determined a priori. Each article was reviewed to determine if it met the inclusion criteria; if any of the exclusion criteria were met, the article was excluded. Any discrepancies in reviewer decisions were decided through discussion and reaching consensus or by a third reviewer.
In the first phase of screening, for RQ1 and RQ2, 1,806 and 4,927 article titles and abstracts were screened by two independent reviewers (trained staff from the National Academies or AND) using the prespecified criteria in Tables 2-1 and 2-2, respectively.
| Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Study participants | Human infants ≤12 months of age | Animal or in vitro studies; children >1 year of age at baseline |
| Health status of study participants | Studies that enroll full-term, healthy infants | Studies that exclusively enroll preterm or premature infants; infants with high birth weight or low birth weight or infants diagnosed with a disease or condition |
| Interventions/exposures | Cow’s milk–based, plant-based (e.g., soy, pea), or goat’s milk–based formula and partially hydrolyzed formula with any compositional differences from standard formulas | All other specialized formulas (formulas deemed exempt by U.S. Food and Drug Administration (FDA) such as fully hydrolyzed amino acid (AA) formulas or formulas for infants with metabolic diseases); toddler formulas |
| Comparators | Infants fed standard infant formula or human milk or comparison to a national/international standard or reference, including z-scores | Specialized formulas (formulas deemed exempt by FDA such as fully hydrolyzed AA formulas or formulas for infants with metabolic diseases); toddler formulas |
| Outcomes | Primary outcomes: Physical growth (e.g., length, weight, body mass index (BMI) z-score, head circumference); length-forage z-scores, or weight-for-age z-scores; weight for length Secondary outcomes: body composition, biomarkers of growth Co-variables: sex, ethnicity, socioeconomic status |
Fecal microbiota, metabolomics, neurodevelopment, other general health outcomes |
| Publication status | Articles published in peer-reviewed journals | Articles that have not been peer reviewed and are not published in peer-reviewed journals, including unpublished data, manuscripts, preprints, reports, abstracts, and conference proceedings |
| Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Date of publication | January 2000 until the search date of June 23, 2024 | Articles published prior to January 2000 or after search date of June 23, 2024 |
| Language of publication | Articles published in English | Articles published in languages other than English |
| Study design | Randomized controlled trials; nonrandomized controlled trials, including quasi-experimental and controlled before-and-after studies; observational studies; prospective cohort studies; retrospective cohort studies; meta-analyses; systematic reviews; case-control studies | Editorials Narrative reviews Abstracts/conference abstracts Study protocols |
| Country | Studies conducted in high-income countries, based on the United Nations (UN) Human Development Index | Studies conducted outside of high-income countries, based on the UN Human Development Index |
SOURCE: Generated by the Academy of Nutrition and Dietetics.
In the second phase, for RQ1, the full text of 145 articles was screened by two independent reviewers (one committee member and one trained staff member from the National Academies or AND). Forty-one articles were included (see Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] diagram in Figure 2-1). For RQ2, the full text of 235 articles was screened by two independent reviewers (one committee member and one trained staff member from the National Academies or AND). One hundred and forty-three articles were included (16 systematic reviews, 4 pooled studies, and 125 articles that included 115 primary studies) (see PRISMA diagram in Figure 2-2). When an article was excluded during the second phase, the reason(s) for exclusion was documented.
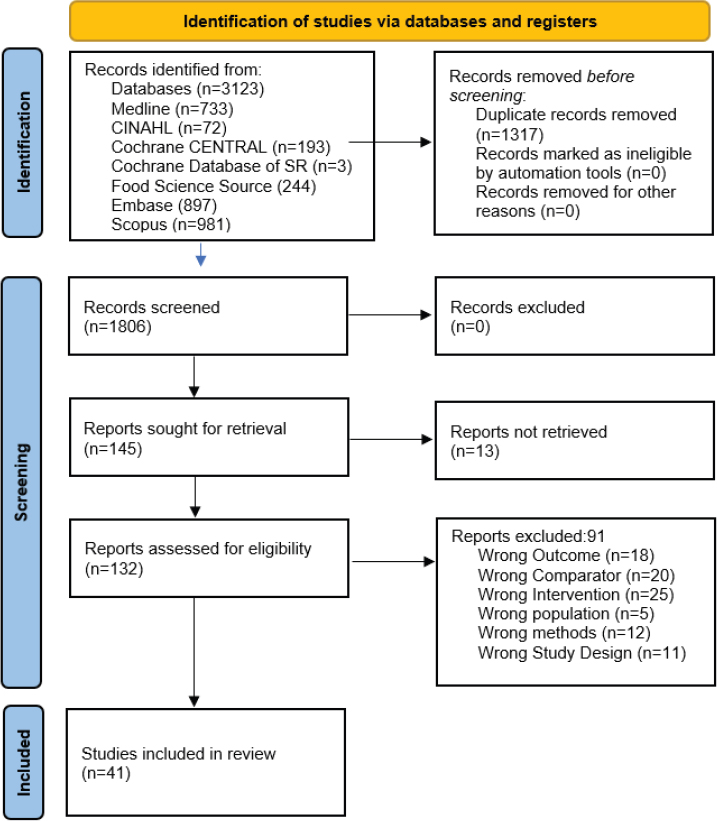
NOTES: CINAHL = Cumulative Index to Nursing and Allied Health Literature; n = number; SR = systematic review.
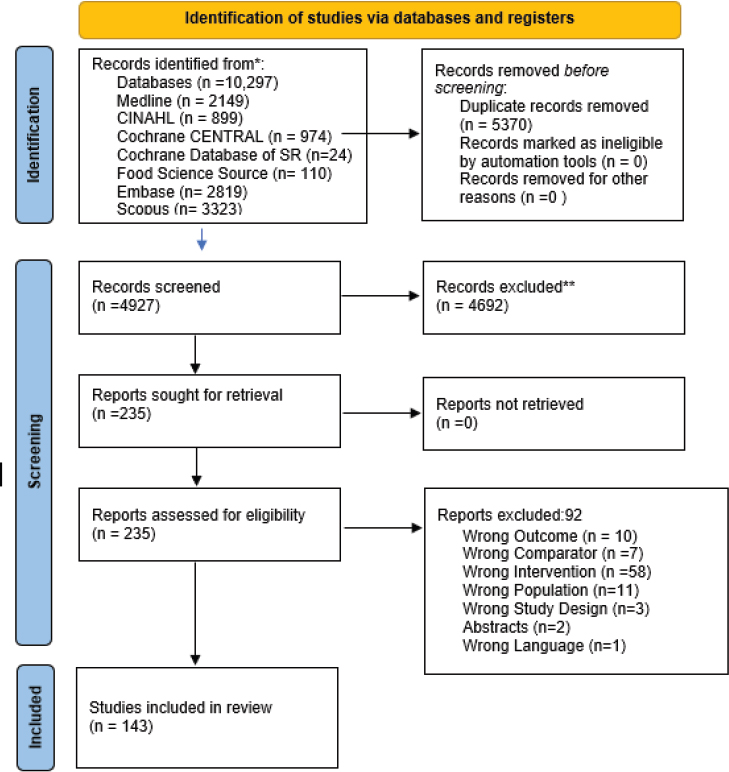
NOTES: CINAHL = Cumulative Index to Nursing and Allied Health Literature; n = number; SR = systematic review.
Data Extraction
AND-generated data extraction templates were designed to extract variables identified and approved by the committee. Trained National Academies staff members extracted data using an Excel spreadsheet and predetermined criteria. AND reviewed and confirmed all extracted data. The committee focused on collecting bibliographic information, study design, and methodologic details.
Protein Quality Scoping Review
The number of published studies per year was only one for 2001–2016, but for 2017–2024, it rose, reaching nine in 2022. Most studies were conducted in France (n = 9), with five or fewer from each of the Netherlands, China, Ireland, New Zealand, and the United States (see Appendix D). The included studies included animal, in vitro digestibility, and validity/reliability. Thirty-five of the 41 studies examined cow’s milk–based infant formula, and five studies investigated multiple types, including partially hydrolyzed formulas. Six studies examined plant-based formulas, and five examined goat’s milk–based infant formulas.
Normal Physical Growth Scoping Review
The greatest percentage of studies were conducted in the United States, followed by Italy, Spain, Germany, and China. Most studies were funded by the infant formula industry, followed by government. The study sample sizes were 100–500 participants, with an equal distribution of boys and girls in most studies that reported this information. The type of infant formula used was primarily cow’s milk–based, with a few using protein from plants, soy, or goat’s milk. The modifications for the experimental formulas included the amount of protein, hydrolysis of protein, AA modification, fat amount, fat type, presence of micronutrients, removal of lactose, and inclusion of prebiotics and/or probiotics. The outcome measure most frequently reported was infant weight, followed by length and head circumference. Weight-for-length z-scores were much less frequently reported in studies conducted inside (18 percent reported) than outside of (63 percent reported) the United States.
REFERENCES
Hansen, P. A. 2024. Alternatives to Protein Efficiency Ratio Rat Bioassay (PER) and Growth Monitoring Study (GMS) to Satisfy Quality Factor Requirements for Infant Formula—NASEM Consensus Study. PowerPoint Presentation presented at the April 3, 2024, Open Session National Academies of Sciences, Engineering, and Medicine Meeting, Washington, DC.
Raiten, D. J., J. M. Talbot, and J. H. Waters. 1998. “Assessment of Nutrient Requirements for Infant Formulas,” Journal of Nutrition, 128:2059S-2249S.











