Protein Quality and Growth Monitoring Studies: Quality Factor Requirements for Infant Formula (2025)
Chapter: Appendix E: Modification of Infant Formula and Growth: A Scoping Review
Appendix E
Modification of Infant Formula and Growth:
A Scoping Review
ABSTRACT
Introduction
The aim of this scoping review is to report on the state of the science regarding methodologies for assessing the ability of infant formula to support normal human physical growth.
The research question examined in this scoping review is the following: In healthy full-term human infants <1 year of age, what is the availability of evidence assessing the impact of alternative formulas compared to standard formulas or human milk on physical infant growth outcomes? Sub-questions include the following:
- What study designs are used to examine this relationship?
- What types of formula alterations are being evaluated?
- What growth outcomes are reported?
- Do study designs and measures vary globally?
Methods
This scoping review was registered at Open Science Framework (Rozga et al., n.d.). It was conducted using the framework introduced by Arskey and O’Malley (2005) and developed by Levac et al. (2010), the Joanna Briggs Institute (Peters et al., 2020), and the Cochrane Collaboration (Higgins et al., 2024). The findings are reported according to the Preferred
Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist for scoping reviews (Moher et al., 2009).
Eligibility Criteria
Table E-1 lists the eligibility criteria. Studies were included if they included full-term healthy infants to investigate intake of formula with any compositional differences compared to standard formula or human milk. Studies were required to be published in 2000 or later to represent contemporary laboratory methods and infant formulas. Articles were required to be published in the English language due to resource constraints.
Information Sources
An information specialist designed the search strategies and searched MEDLINE, Cochrane CENTRAL, CINAHL, Cochrane Database of Systematic Reviews, Food Science Source, Embase, and Scopus on July 22, 2024. Table E-2 shows a sample search strategy for MEDLINE. Search results were managed and deduplicated by the information specialist using EndNote software (EndNote, 2013).
Article Selection
Article screening for eligibility criteria was conducted in two phases. In the first stage, titles and abstracts were uploaded using Rayyan software (Moher et al., 2009) and screened independently by two reviewers. In the second phase, the full texts were reviewed and screened against eligibility criteria by two independent reviewers. Any discrepancies in reviewer decisions were decided through discussion and reaching consensus or by a third reviewer.
Data Extraction
A template was designed to extract study characteristics and approved by an expert panel. Data were extracted to the standardized Excel sheet by one analyst and reviewed by a second. Data extracted from each article include bibliographic information, study design, details on the infant population, details on the composition of intervention and comparison infant formulas, and outcomes of physical growth that are reported.
| Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Study participants | Human infants ≤12 months of age | Animal or in vitro studies; children >1 year of age at baseline |
| Health status of study participants | Studies that enroll full-term, healthy infants | Studies that exclusively enroll preterm or premature infants; infants with high birth weight or low birth weight or infants diagnosed with a disease or condition |
| Interventions/exposures | Cow’s milk–based, plant-based (e.g., soy, pea), or goat’s milk–based formula and partially hydrolyzed formula with any compositional differences from standard formulas | All other specialized formulas (formulas deemed exempt by U.S. Food and Drug Administration (FDA) such as fully hydrolyzed amino acid (AA) formulas or formulas for infants with metabolic diseases); toddler formulas. |
| Comparators | Infants fed standard infant formula or human milk or comparison to a national/international standard or reference, including z-scores | Specialized formulas (formulas deemed exempt by FDA such as fully hydrolyzed AA formulas or formulas for infants with metabolic diseases); toddler formulas |
| Outcomes | Primary outcomes: physical growth (e.g., length, weight, body mass index (BMI) z-score, head circumference); length-forage z-scores, or weight-for-age z-scores; weight for length Secondary outcomes: body composition, biomarkers of growth Co-variables: sex, ethnicity, socioeconomic status |
Fecal microbiota, metabolomics, neurodevelopment, other general health outcomes |
| Publication status | Articles published in peer-reviewed journals | Articles that have not been peer reviewed and are not published in peer-reviewed journals, including unpublished data, manuscripts, preprints, reports, abstracts, and conference proceedings |
| Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Date of publication | January 2000 until the search date of June 23, 2024 | Articles published prior to January 2000 or after search date of June 23, 2024 |
| Language of publication | Articles published in English | Articles published in languages other than English |
| Study design | Randomized controlled trials; nonrandomized controlled trials, including quasi-experimental and controlled before-and-after studies; observational studies; prospective cohort studies; retrospective cohort studies; meta-analyses; systematic reviews; case-control studies | Editorials Narrative reviews Abstracts/conference abstracts Study protocols |
| Country | Studies conducted in high-income countries, based on the United Nations (UN) Human Development Index | Studies conducted outside of high-income countries, based on the UN Human Development Index |
| # | Query |
|---|---|
| S22 | S21 AND DT 20000101-20250101 |
| S21 | S20 AND LA English |
| S20 | S19 NOT PT (comment or editorial or news or newspaper article) |
| S19 | S18 NOT ((MH “Animals”) NOT ((MH “Humans”) AND (MH “Animals”))) |
| S18 | S9 AND S14 AND S17 |
| S17 | S15 OR S16 |
| S16 | TI (infant* OR newborn* OR neonat*) OR AB (infant* OR newborn* OR neonat*) OR CI (infant* OR newborn* OR neonat*) |
| S15 | MH (Infant OR “Infant, Newborn”) |
| S14 | S10 OR S11 OR S12 OR S13 |
| S13 | TI (length OR “head circumference” OR “body circumference” OR height OR anthropometric*) OR AB (length OR “length-for-age” OR LAZ OR “weight-for-length” OR “head circumference” OR “body circumference” OR height OR anthropometric*) OR CI (length OR “head circumference” OR “body circumference” OR height OR anthropometric*) |
| S12 | TI (“body mass” OR BMI OR bodyweight OR body weight OR “weight gain” OR weighed OR “weight-for-age” OR “z-score” OR WAZ) OR AB (“body mass” OR BMI OR bodyweight OR body weight OR “weight gain” OR weighed OR “weight-for-age” OR “z-score” OR WAZ) OR CI (“body mass” OR BMI OR bodyweight OR body weight OR “weight gain” OR weighed OR “weight-for-age” OR “z-score” OR WAZ) |
| S11 | TI (“body composition” OR “air displacement plethysmography” OR “dual-energy x-ray absorptiometry” OR “bioelectrical impedance” OR “skinfold thickness”) OR AB (“body composition” OR “air displacement plethysmography” OR “dual-energy x-ray absorptiometry” OR “bioelectrical impedance” OR “skinfold thickness”) OR CI (“body composition” OR “air displacement plethysmography” OR “dual-energy x-ray absorptiometry” OR “bioelectrical impedance” OR “skinfold thickness”) |
| S10 | MH (“infant, newborn/gd”) |
| S9 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 |
| S8 | TI (“human-identical milk” OR “human milk based protein” OR “human milk oligosaccharides” OR “manufactured human milk”) OR AB (“human-identical milk” OR “human milk based protein” OR “human milk oligosaccharides” OR “manufactured human milk”) OR CI (“human-identical milk” OR “human milk based protein” OR “human milk oligosaccharides” OR “manufactured human milk”) |
| # | Query |
|---|---|
| S7 | TI (((plant OR soy OR pea OR oat OR oats) N1 (formula OR formulas)) AND infant) OR AB (((plant OR soy OR pea OR oat OR oats) N1 (formula OR formulas)) AND infant) OR CI (((plant OR soy OR pea OR oat OR oats) N1 (formula OR formulas)) AND infant) |
| S6 | TI (((goat OR cow OR bovine) N1 (formula OR formulas or milk)) AND infant) OR AB (((goat OR cow OR bovine) N1 (formula OR formulas or milk)) AND infant) OR CI (((goat OR cow OR bovine) N1 (formula OR formulas or milk)) AND infant) |
| S5 | TI ((milk OR whey OR casein OR dairy OR “partially hydrolyzed” OR “partially hydrolysed” OR “amino acid*”) N1 (formula OR formulas)) OR AB ((milk OR whey OR casein OR dairy OR “partially hydrolyzed” OR “partially hydrolysed” OR “amino acid*”) N1 (formula OR formulas)) OR CI ((milk OR whey OR casein OR dairy OR “partially hydrolyzed” OR “partially hydrolysed” OR “amino acid*”) N1 (formula OR formulas)) |
| S4 | TI ((regular OR standard*) N1 (formula OR formulas)) OR AB ((regular OR standard*) N1 (formula OR formulas)) OR CI ((regular OR standard*) N1 (formula OR formulas)) |
| S3 | TI (“formula-fed” OR “fed formula” OR “fed formulas” OR “formula feeding” OR “feeding formula” OR “feeding formulas” OR “formula diet” OR “based formula” OR “based formulas” OR “formula milk”) OR AB (“formula-fed” OR “fed formula” OR “fed formulas” OR “formula feeding” OR “feeding formula” OR “feeding formulas” OR “formula diet” OR “based formula” OR “based formulas” OR “formula milk”) OR CI (“formula-fed” OR “fed formula” OR “fed formulas” OR “formula feeding” OR “feeding formula” OR “feeding formulas” OR “formula diet” OR “based formula” OR “based formulas” OR “formula milk”) |
| S2 | TI (infant N1 (formula OR formulas)) OR AB (infant N1 (formula OR formulas)) OR CI (infant N1 (formula OR formulas)) |
| S1 | MH (“Infant Formula”) |
Synthesis of Results
The study selection process is documented in a PRISMA flowchart (EndNote, 2013). Characteristics and results of included studies are narratively described, and results are also represented in charts, figures, and a heat map. As is customary with scoping reviews, critical appraisal of study quality was not conducted.
Results
The database searches identified 4,927 unique articles; 235 full texts were reviewed against eligibility criteria, and 143 articles were included (see Figure E-1) (Abrahamse-Berkeveld et al., 2024; Abrams et al., 2015; Ahrens et al., 2018; Alexander et al., 2016; Alliet et al., 2022; Andres et al., 2012; Ashley et al., 2012; Ben et al., 2004; Billeaud et al., 2014, 2022; Breij et al., 2019; Bruzzese et al., 2009; Camier et al., 2021; Cekola et al., 2015; Cesare Marincola et al., 2016; Chouraqui et al., 2008; Civardi et al., 2017; Collell et al., 2016; Costalos et al., 2008; Czerkies et al, 2018; Davis et al., 2008; Demmelmair et al., 2022; Escribano et al., 2012; Estorninos et al., 2022; Fanaro et al., 2005; Fleddermann et al., 2014, 2017, 2018; Fusch et al., 2001; Gale et al., 2012; Gianni et al., 2018; Gibson et al., 2009; Gil-Campos et al., 2012; Giovannini et al., 2013; Haschke et al., 2014; He et al., 2022; Hedrick et al., 2021; Hegar et al., 2008; Hernell and Lönnerdal,, 2002; Heubi et al., 2000; Hoffman et al., 2008, 2019; Holscher et al., 2012; Inostroza et al., 2014; Jankiewicz et al., 2023; Jaramillo-Ospina et al., 2022; Jasani et al., 2017; Jiang et al., 2022; Jinno et al., 2020; Jochum et al., 2023; Johnston et al., 2015; Kantaras et al., 2024; Karaglani et al., 2020; Koletzko et al., 2009; Koo et al., 2003; Kouwenhoven et al. 2020, 2021a,b; Kuehn et al., 2022; Lambidou et al., 2021; Lasekan et al., 2006, 2011, 2014, 2022; Li, F., et al., 2019; Li, X., et al. 2019; Liang et al., 2024; Lien et al., 2004; Liotto et al., 2018; Litmanovitz et al., 2013; Lonnerdal et al., 2016; López-Velázquez et al., 2013; Mackey et al., 2013; Makrides et al., 2000, 2005; Maldonado et al., 2012, 2019; Marriage et al., 2015; Martin et al., 2014; Meli et al., 2014; Milani et al., 2023; Morris et al., 2000; Mugambi et al., 2012; Neumer et al., 2021; Nieto-Ruiz et al., 2019; Oropeza-Ceja et al., 2021; Parschat et al., 2021; Pastor et al., 2006; Patro-Gołąb et al., 2016; Petersen et al., 2020; Picaud et al., 2020; Piemontese et al., 2011; Plaza-Diaz et al., 2023; Puccio et al., 2007, 2017; Putet et al., 2016; Radke et al., 2017; Räihä et al., 2002; Rao et al., 2009; Ren et al., 2022; Rigo et al., 2019; Rodriguez-Herrera et al., 2019; Román et al., 2020; Rozé et al., 2012; Rzehak et al., 2009; Sandström et al., 2008; Scalabrin et al., 2012; Schmelzle et al., 2003; Sepúlveda-Valbuena et al., 2021; Shahramian et al., 2018; Shen et al., 2021; Singhal et al., 2010; Szajewska and Chmielewska, 2013; Szajewska et al., 2015, 2017; Takahashi et al., 2023; Teoh et al., 2022; Timby et al., 2014; Tinghäll Nilsson et al., 2023, 2024; Tonon et al., 2021; Totzauer et al., 2018; Trabulsi et al., 2011; Troesch et al., 2019; Turck et al., 2006; Udell et al., 2005; Vandenplas et al., 2020; Veereman-Wauters et al., 2011; Vendt et al., 2006; Vlieger et al., 2009; Wang, L., et al., 2019; Wang, Y., et al., 2021; Weizman and Alsheikh, 2006; Wu et al., 2017; Xia et al., 2021; Xu et al., 2015; Yang et al., 2022; Yeiser et al., 2016; Yin et al., 2023; Zhang et al., 2023; Zhou et al., 2014; Ziegler et al., 2007, 2015). The 143 articles had 16 sys-
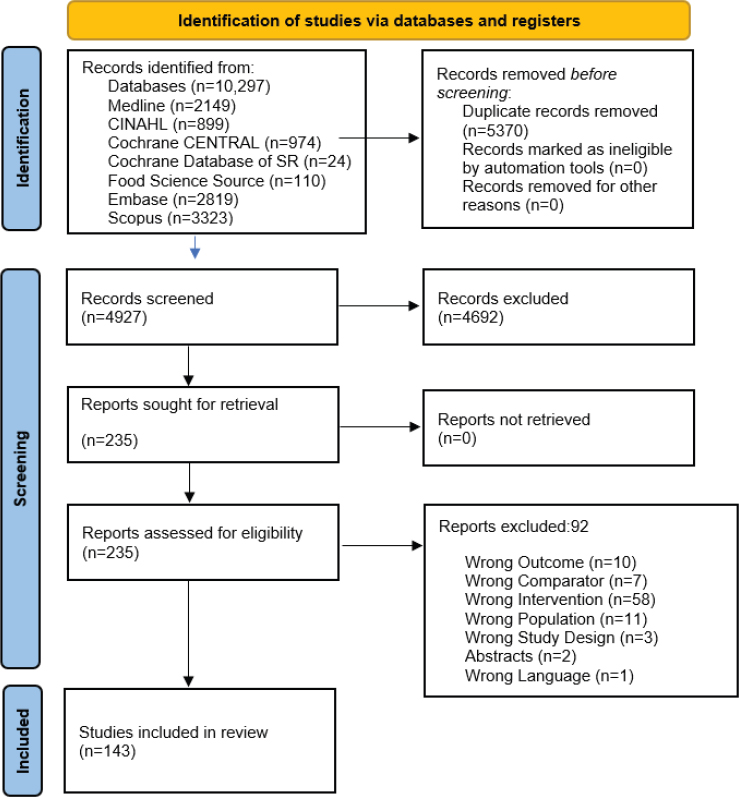
NOTES: CINAHL = Cumulative Index to Nursing and Allied Health Literature; n = number; SR = systematic reviews.
tematic reviews (Abrams et al., 2015; Gale et al., 2012; Jankiewicz et al., 2023; Jasani et al., 2017; Liang et al., 2024; Makrides et al., 2005; Milani et al., 2023; Mugambi et al., 2012; Patro-Gołąb et al., 2016; Rao et al., 2009; Ren et al., 2022; Szajewska et al., 2015, 2017; Udell et al., 2005; Wang, L., et al., 2019; Zhang et al., 2023), four pooled studies (Alexander et al., 2016; Camier et al., 2021; Czerkies et al., 2018; Haschke et al., 2014), and 115
primary studies published in 125 articles (see Figure E-2). Three articles reported results of the EU Childhood Obesity Study (Collell et al., 2016; Escribano et al., 2012; Koletzko et al., 2009), four articles reported results of the BeMIM study (Demmelmair et al., 2022; Fleddermann et al., 2014, 2017, 2018), Kouwenhoven et al. (2020, 2021a,b) reported results of one study in three articles, and Tinghäll Nilsson et al. (2023, 2024) reported results of one study in two articles. Among the 115 primary studies (123 articles), 108 studies (116 articles) were randomized controlled trials (RCTs) (Abrahamse-Berkeveld et al., 2024; Ahrens et al., 2018; Alliet et al., 2022; Ashley et al., 2012; Ben et al., 2004; Billeaud et al., 2014; Breij et al., 2019; Bruzzese et al., 2009; Cekola et al., 2015; Cesare Marincola et al., 2016; Chouraqui et al., 2008; Civardi et al., 2017; Collell et al., 2016; Costalos et al., 2008; Davis et al., 2008; Demmelmair et al., 2022; Escribano et al., 2012; Estorninos et al., 2022; Fanaro et al., 2005; Fleddermann et al., 2014, 2018; Fusch et al., 2001; Gianni et al., 2018; Gibson et al., 2009; Gil-
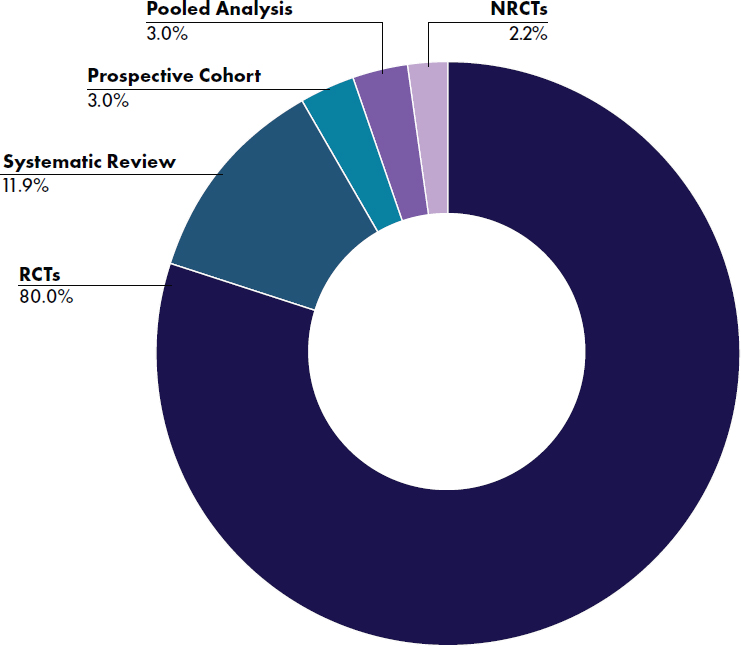
NOTES: NRCTs = nonrandomized controlled trials; RCTs = randomized controlled trials.
Campos et al., 2022; Giovannini et al., 2013; Haschke et al., 2014; He et al., 2022; Hedrick et al., 2021; Hegar et al., 2008; Heubi et al., 2000; Hoffman et al., 2008, 2019; Holscher et al., 2012; Inostroza et al., 2014; Jaramillo-Ospina et al., 2022; Jiang et al., 2022; Johnston et al., 2015; Kantaras et al., 2024; Karaglani et al., 2020; Koletzko et al., 2009; Koo et al., 2003; Kouwenhoven et al. 2020, 2021a,b; Kuehn et al., 2022; Lambidou et al., 2021; Lasekan et al., 2006, 2011, 2014, 2022; Li, F., et al., 2019; Li, X., et al. 2019; Lien et al., 2004; Liotto et al., 2018; Litmanovitz et al., 2013; Lonnerdal et al., 2016; López-Velázquez et al., 2013; Mackey et al., 2013; Makrides et al., 2000; Maldonado et al., 2012, 2019; Marriage et al., 2015; Martin et al., 2014; Meli et al., 2014; Morris et al., 2000; Neumer et al., 2021; Nieto-Ruiz et al., 2019; Oropeza-Ceja et al., 2021; Parschat et al., 2021; Petersen et al., 2020; Picaud et al., 2020; Rigo et al., 2019; Rodriguez-Herrera et al., 2019; Román et al., 2020; Rozé et al., 2012; Rzehak et al., 2009; Sandström et al., 2008; Scalabrin et al., 2012; Schmelzle et al., 2003; Sepúlveda-Valbuena et al., 2021; Shahramian et al., 2018; Shen et al., 2021; Singhal et al., 2010; Szajewska et al., 2017; Takahashi et al., 2023; Teoh et al., 2022; Timby et al., 2014; Tinghäll Nilsson et al., 2023, 2024; Tonon et al., 2021; Totzauer et al., 2018; Trabulsi et al., 2011; Troesch et al., 2019; Turck et al., 2006; Vandenplas et al., 2020; Veereman-Wauters et al., 2011; Vendt et al., 2006; Vlieger et al., 2009; Wang, Y., et al., 2021; Wu et al., 2017; Xia et al., 2021; Xu et al., 2015; Yang et al., 2022; Yeiser et al., 2016; Yin et al., 2023; Zhou et al., 2014; Ziegler et al., 2015); of these, 62 studies (70 articles) included a nonrandomized human milk–fed arm (Abrahamse-Berkeveld et al., 2024; Ahrens et al., 2018; Alliet et al., 2022; Ben et al., 2004; Breij et al., 2019; Cesare Marincola et al., 2016; Collell et al., 2016; Davis et al., 2008; Demmelmair et al., 2022; Escribano et al., 2012; Fleddermann et al., 2014, 2018; Gianni et al., 2018; Giovannini et al., 2013; He et al., 2022; Holscher et al., 2012; Inostroza et al., 2014; Jaramillo-Ospina et al., 2022; Koletzko et al., 2009; Kuehn et al., 2022; Lambidou et al., 2021; Lasekan et al., 2022; Li, X., et al. 2019; Liang et al., 2024; Liotto et al., 2013; Litmanovitz et al., 2013; López-Velázquez et al., 2013; Mackey et al., 2013; Makrides et al., 2000; Marriage et al., 2015; Martin et al., 2014; Meli et al., 2014; Nieto-Ruiz et al., 2019; Oropeza-Ceja et al., 2021; Parschat et al., 2021; Petersen et al., 2020; Picaud et al., 2020; Piemontese et al., 2011; Plaza-Diaz et al., 2023; Putet et al., 2016; Radke et al., 2017; Räihä et al., 2002; Rodriguez-Herrera et al., 2019; Román et al., 2020; Rzehak et al., 2009; Sandström et al., 2008; Scalabrin et al., 2012; Sepúlveda-Valbuena et al., 2021; Shahramian et al., 2018; Shen et al., 2021; Singhal et al., 2010; Takahashi et al., 2023; Teoh et al., 2022; Timby et al., 2014; Tinghäll Nilsson et al., 2023, 2024; Totzauer et al., 2018; Trabulsi et al., 2011; Troesch et al., 2019; Vandenplas et al., 2020; Veereman-Wauters et al., 2011; Wang, Y., et al., 2021; Wu et al., 2017; Xia et al., 2021; Yin et al., 2023; Zhou et al., 2014; Ziegler et al., 2015), three were non-
randomized controlled trials (Billeaud et al., 2022; Hernell and Lönnerdal, 2002; Jochum et al., 2023), and four were prospective cohort studies (Andres et al., 2012; Jinno et al., 2020; Pastor et al., 2006; Tonon et al., 2021).
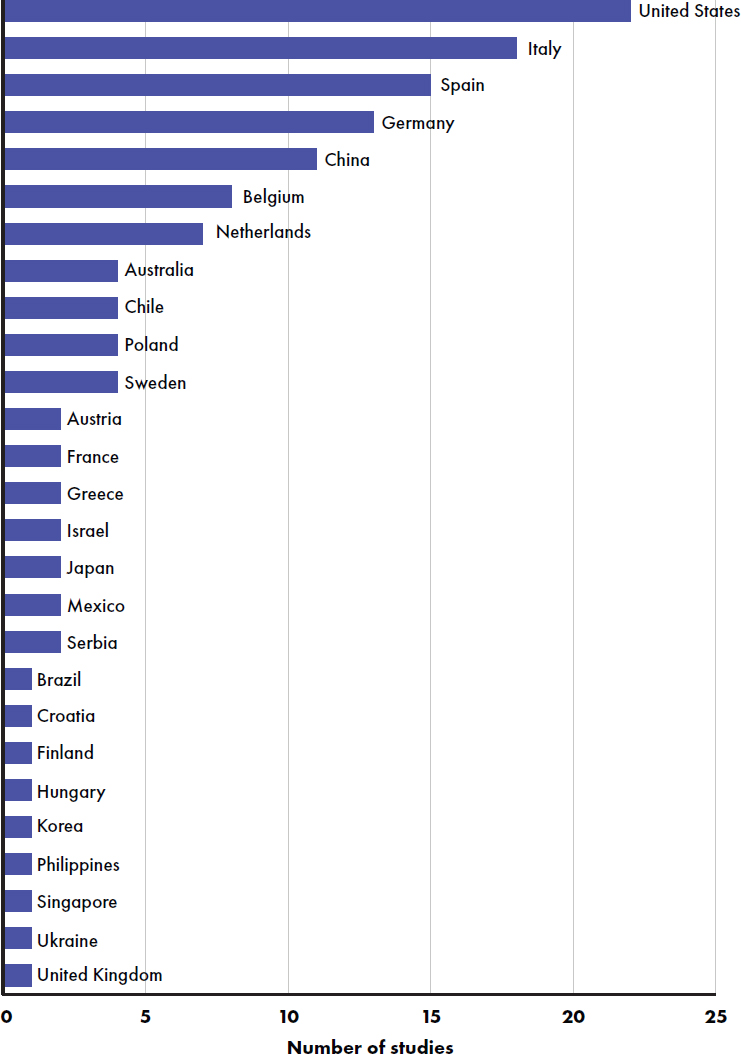
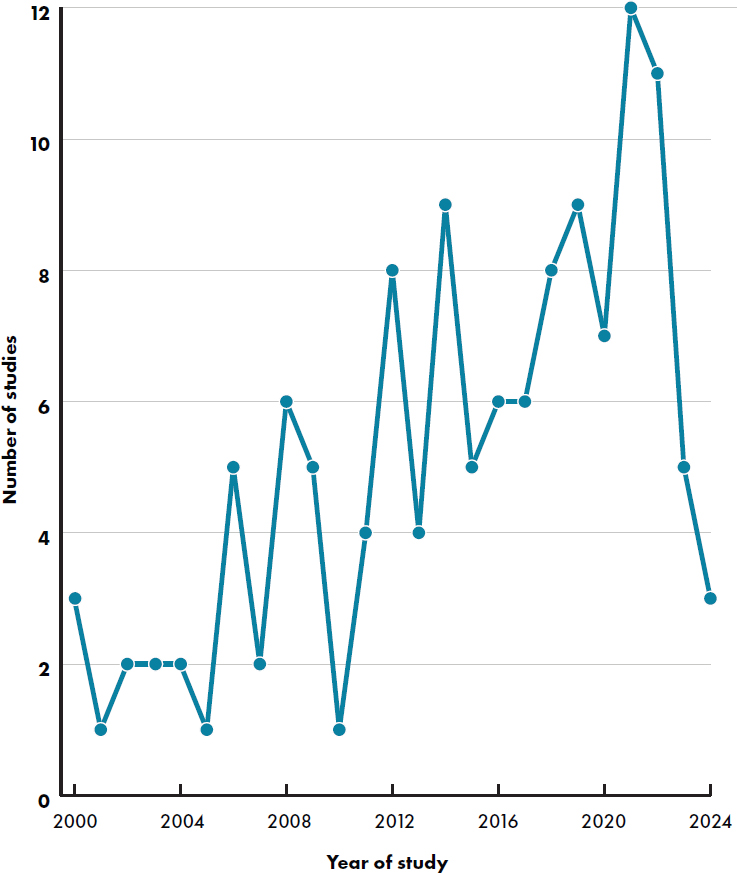
Articles were published 2000–2024 with a stable increase in publications over time (see Figure E-3). The greatest number of studies were conducted in the United States (Andres et al., 2012; Ashley et al., 2012; Cekola et al., 2015; Davis et al., 2008; Hedrick et al., 2021; Heubi et al., 2000; Hoffman et al., 2008, 2019; Holscher et al., 2012; Johnston et al.,
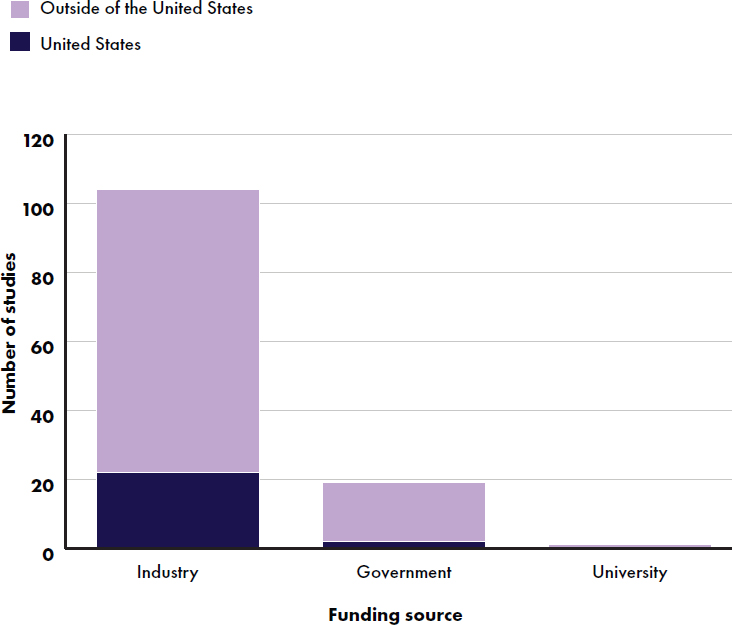
2015; Koo et al., 2003; Kuehn et al., 2022; Lasekan et al., 2006, 2011, 2014, 2022; Lien et al., 2004; Mackey et al., 2013; Marriage et al., 2015; Yeiser et al., 2016; Ziegler et al., 2007, 2015), followed by Italy (Ben et al., 2004; Bruzzese et al., 2009; Cesare Marincola et al., 2016; Civardi et al., 2017; Fanaro et al., 2005; Gianni et al., 2018; Giovannini et al., 2013; Kantaras et al., 2024; Liotto et al., 2018; Meli et al., 2014; Puccio et al., 2007, 2017; Räihä et al., 2002; Rodriguez-Herrera et al., 2019), Spain (Collell et al., 2016; Gil-Campos et al., 2012; Maldonado et al., 2012, 2019; Nieto-Ruiz et al., 2019; Pastor et al., 2006; Plaza-Diaz et al., 2023; Rodriguez-Herrera et al., 2019; Román et al., 2020; Sepúlveda-Valbuena et al., 2021), Germany (Escribano et al., 2012; Fusch et al., 2001; He et al., 2022; Jochum et al., 2023; Koletzko et al., 2009; Kouwenhoven et al. 2020, 2021a,b; Lambidou et al., 2021; Parschat et al., 2021; Petersen et al., 2020; Picaud et al., 2020; Radke et al., 2017; Rzehak et al., 2009; Schmelzle et al., 2003), and China (Ben et al., 2004; Jiang et al., 2022; Li, F., et al., 2019; Li, X., et al. 2019; Lonnerdal et al., 2016; Shen et al., 2021; Wang, Y., et al., 2021; Wu et al., 2017; Xia et al., 2021; Xu et al., 2015) (see Figure E-4). Studies were funded by industry (100 studies) (Abrahamse-Berkeveld et al., 2024; Ahrens et al., 2018; Alliet et al., 2022; Andres et al., 2012; Ashley et al., 2012; Ben et al., 2004; Billeaud et al., 2014, 2022; Breij et al., 2019; Bruzzese et al., 2009; Cekola et al., 2015; Cesare Marincola et al., 2016; Civardi et al., 2017; Costalos et al., 2008; Davis et al., 2008; Demmelmair et al., 2022; Fleddermann et al., 2014, 2018; Gianni et al., 2018; Gibson et al., 2009; Gil-Campos et al., 2012; Giovannini et al., 2013; He et al., 2022; Hedrick et al., 2021; Hegar et al., 2008; Heubi et al., 2000; Hoffman et al., 2008, 2019; Holscher et al., 2012; Inostroza et al., 2014; Jaramillo-Ospina et al., 2022; Jochum et al., 2023; Johnston et al., 2015; Kantaras et al., 2024; Karaglani et al., 2020; Koo et al., 2003; Kouwenhoven et al. 2020, 2021a,b; Kuehn et al., 2022; Lambidou et al., 2021; Lasekan et al., 2006, 2011, 2014, 2022; Li, F., et al., 2019; Li, X., et al. 2019; Lien et al., 2004; Liotto et al., 2018; Lonnerdal et al., 2016; Makrides et al., 2000; Maldonado et al., 2012, 2019; Marriage et al., 2015; Meli et al., 2014; Morris et al., 2000; Neumer et al., 2021; Nieto-Ruiz et al., 2019; Oropeza-Ceja et al., 2021; Parschat et al., 2021; Pastor et al., 2006; Peters et al., 2020; Picaud et al., 2020; Piemontese et al., 2011; Plaza-Diaz et al., 2023; Puccio et al., 2017; Putet et al., 2016; Räihä et al., 2002; Rodriguez-Herrera et al., 2019; Román et al., 2020; Rozé et al., 2012; Rzehak et al., 2009; Sandström et al., 2008), the government (18 studies) (Andres et al., 2012; Escribano et al., 2012; Hernell and Lönnerdal, 2002; Jiang et al., 2022; Koletzko et al., 2009; Kouwenhoven et al. 2020, 2021a,b; Makrides et al., 2000; Neumer et al., 2021; Nieto-Ruiz et al., 2019; Oropeza-Ceja et al., 2021; Pastor et al., 2006; Rzehak et al., 2009; Singhal et al., 2010; Totzauer et al., 2018; Wu et al., 2017; Xu et al., 2015), and a university (one
study) (Sepúlveda-Valbuena et al., 2021), and some received funding from multiple sources (see Figure E-5).
Study Populations in Primary Studies
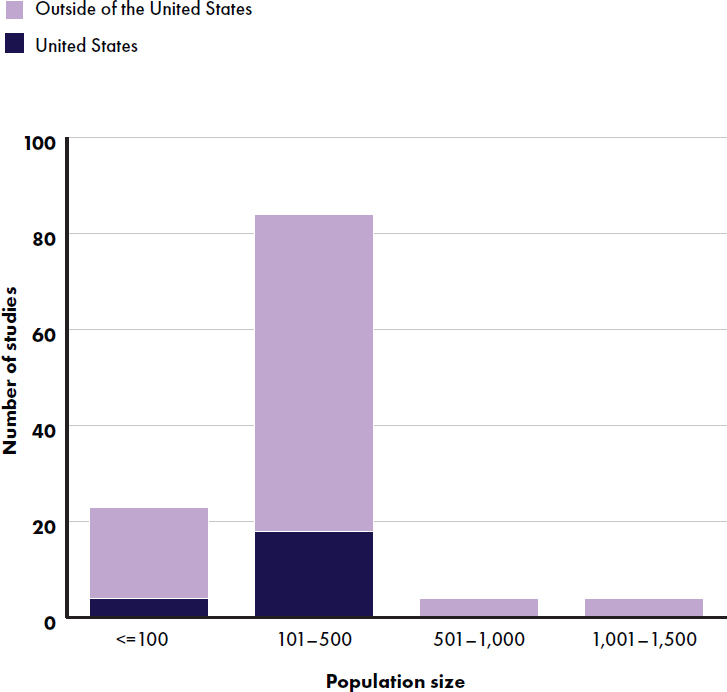
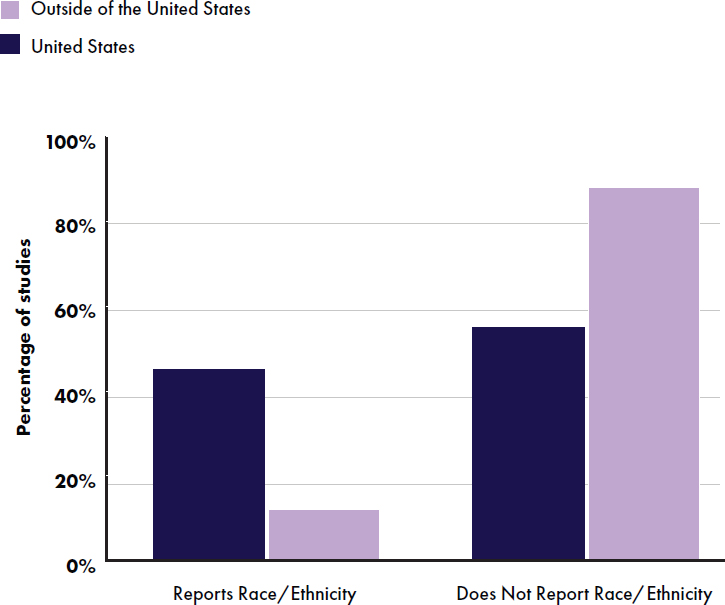
Most studies had a population size of 100–500 participants (84 studies) (see Figure E-6). All studies except one reported an equal distribution of boys and girls, and 25 studies did not report the proportion of girls and boys. Twenty-one studies reported race/ethnicity; 10 were conducted in the United States and 11 (14 articles) were conducted outside of the United States (Alliet et al., 2022; He et al., 2022; Jochum et al., 2023; Kouwenhoven et al. 2020, 2021a,b; Piemontese et al., 2011; Puccio et al., 2007; Putet et al., 2016; Román et al., 2020; Scalabrin et al., 2012; Teoh et al., 2022; Tinghäll Nilsson et al., 2023; Zhou et al., 2014) (see Figure E-7).
Food and Drug Administration (FDA) Requirements Reported in Primary Studies
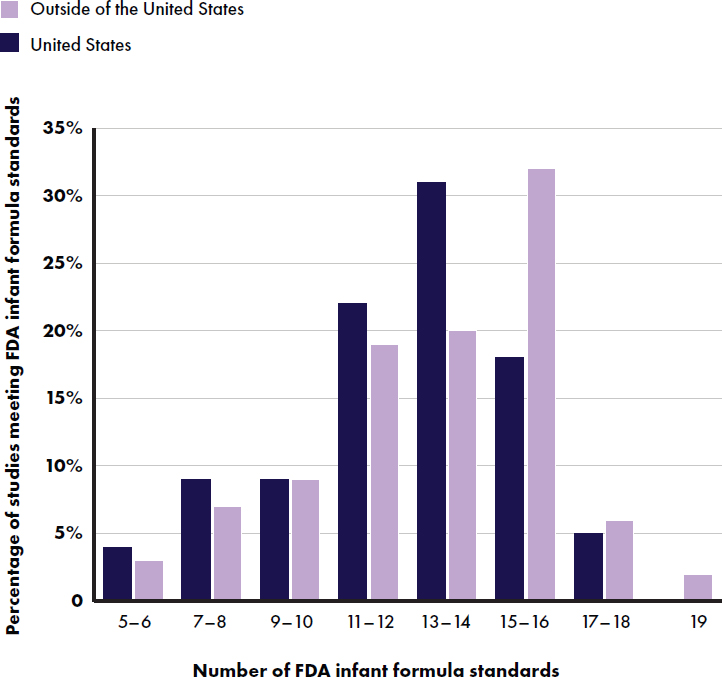
FDA’s Code of Federal Regulations (Title 21) report quality factors for infant formulas (FDA and HHS, n.d.). Table E-3 lists 19 quality standards and the number of studies reporting them in and outside of the United States. Only two studies reported all 19 quality standards (Rigo et al., 2019; Zhou et al., 2014). Of the 22 studies conducted in the United States, 55 percent (12 studies) (Cekola et al., 2015; Haschke et al., 2014; Johnston et al., 2015; Koo et al., 2003; Kuehn et al., 2022; Lasekan et al., 2006, 2011, 2014, 2022; Marriage et al., 2015; Yeiser et al., 2016; Ziegler et al., 2015) met at least 13 of the 19 standards; 61 percent (57 out of 93 studies) of those conducted outside the United States met this criterion (see Figure E-8). The least frequently reported standards among studies in the United States were head circumference z-score (3 studies (14 percent)) (Cekola et al., 2015; Kuehn et al., 2022; Lasekan et al., 2006) and weight-
| Variables | Number of Studies in U.S. | Number of Studies Conducted Outside of the U.S. |
|---|---|---|
| Number of Articles; Number of Studies | 22; 22 | 101; 93 |
| Infants Enrolled by 15 Days of Life | 17 (77%) | 44 (47%) |
| Exclusive Formula Feeding (for intervention and control/standard groups) | 22 (100%) | 82 (88%); 6 NR |
| Equal to or More Than 15 Weeks of Intervention (not an age, a duration) | 18 (82%) | 72 (77%); 1 NR |
| Standard Formula Comparator | 21 (95%) | 78 (84%) |
| Time of Growth Measurement Performance (3 or more within 1 month = 1) | 6 (27%) | 5 (5%) |
| Number of Time Points (equal or more than 6 = 2) | 6 (27%) | 27 (29%) |
| Weight Reported | 21 (95%) | 93 (100%) |
| Weight Statistical Significance Reported | 16 (73%) | 83 (89%) |
| Weight Z-Score Reported | 4 (18%) | 59 (63%) |
| Length Reported | 20 (91%) | 84 (90%) |
| Length Statistical Significance Reported | 16 (73%) | 74 (79%) |
| Length Z-Score Reported | 6 (27%) | 57 (61%) |
| Head Circumference Reported | 19 (86%) | 78 (85%) |
| Head Circumference Statistical Significance Reported | 14 (64%) | 70 (76%) |
| Head Circumference Z-Score Reported | 3 (14%) | 50 (54%) |
| Weight/Length Z-Score Reported | 3 (14%) | 30 (33%) |
| Formula Intake Reported | 18 (82%) | 65 (70%) |
| AEs/Tolerance Variables Reported | 21 (95%) | 80 (86%) |
| Attrition of Subjects Reported | 20 (91%) | 84 (90%) |
| Not FDA Requirement—Biomarkers Reported | 14 (64%) | 46 (50%) |
NOTES: AEs = adverse events, FDA = U.S. Food and Drug Administration; NR = not reported; U.S. = United States.
NOTE: FDA = U.S. Food and Drug Administration.
for-length (3 studies (14 percent)) (Cekola et al., 2015; Davis et al., 2008; Kuehn et al., 2022). Among studies outside of the United States, the least frequently reported standard was three reported growth measurements with 1 month (5 studies (5 percent)) (He et al., 2022; Li, X., et al., 2019; Rigo et al., 2019; Totzauer et al., 2018; Zhou et al., 2014).
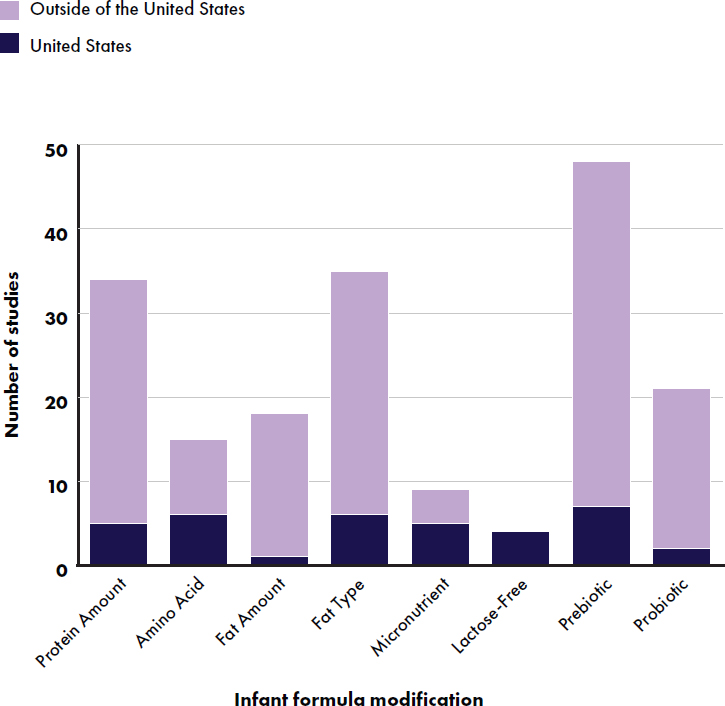
Infant Formula Modifications Evaluated in Primary Studies
Types of infant formula included cow (111 studies), plant/other (2 studies) (Gianni et al., 2018; Gibson et al., 2009), soy (2 studies) (Andres et al., 2012; Hoffman et al., 2008), and goat (4 studies) (He et al., 2022; Xu et al., 2015; Yin et al., 2023; Zhou et al., 2014). All goat-based studies
were conducted outside of the United States. Eighteen studies evaluated partially hydrolyzed formulas (Ahrens et al., 2018; Ben et al., 2004; Billeaud et al., 2022; Cekola et al., 2015; Fusch et al., 2001; Holscher et al., 2012; Jochum et al., 2023; Kantaras et al., 2024; Kuehn et al., 2022; Lasekan et al., 2006; Picaud et al., 2020; Rigo et al., 2019; Román et al., 2020; Rzehak et al., 2009; Schmelzle et al., 2003; Wang, Y., et al., 2021; Yang et al., 2022). The formula modifications included protein amount (Ahrens et al., 2018; Billeaud et al., 2022; Collell et al., 2016; Davis et al., 2008; Demmelmair et al., 2022; Escribano et al., 2012; Fleddermann et al., 2014, 2017, 2018; He et al., 2022; Hedrick et al., 2021; Inostroza et al., 2014; Jinno et al., 2020; Koletzko et al., 2009; Kouwenhoven et al. 2020, 2021a,b; Lasekan et al., 2006; Li, F., et al., 2019; Lien et al., 2004; Liotto et al., 2018; Lonnerdal et al., 2016; Martin et al., 2014; Neumer et al., 2021; Oropeza-Ceja et al., 2021; Petersen et al., 2020; Picaud et al., 2020; Plaza-Diaz et al., 2023; Putet et al., 2016; Räihä et al., 2002; Rigo et al., 2019; Román et al., 2020; Timby et al., 2014; Tinghäll Nilsson et al., 2023, 2024a; Totzauer et al., 2018; Trabulsi et al., 2011; Turck et al., 2006; Wu et al., 2017; Xu et al., 2015; Zhou et al., 2014), amino acid (AA) modification (Davis et al., 2008; Demmelmair et al., 2022; Fleddermann et al., 2014, 2017, 2018; Jinno et al., 2020; Johnston et al., 2015; Kouwenhoven et al. 2020, 2021a,b; Kuehn et al., 2022; Lasekan et al., 2022; Lien et al., 2004; Petersen et al., 2020; Räihä et al., 2002; Sandström et al., 2008; Sepúlveda-Valbuena et al., 2021; Tonon et al., 2021; Xu et al., 2015), fat amount (Ashley et al., 2012; Ben et al., 2004; Cesare Marincola et al., 2016; Demmelmair et al., 2022; Fleddermann et al., 2014, 2017, 2018; Gianni et al., 2018; Hegar et al., 2008; Jinno et al., 2020; Lambidou et al., 2021; Liotto et al., 2018; Meli et al., 2014; Morris et al., 2000; Neumer et al., 2021; Nieto-Ruiz et al., 2019; Pastor et al., 2006; Schmelzle et al., 2003; Wang, Y., et al., 2021; Xu et al., 2015; Zhou et al., 2014), fat type (Abrahamse-Berkeveld et al., 2024; Andres et al., 2012; Ben et al., 2004; Billeaud et al., 2014, 2022; Breij et al., 2019; Cesare Marincola et al., 2016; Civardi et al., 2017; Demmelmair et al., 2022; Fleddermann et al., 2014, 2017, 2018; Fusch et al., 2001; Gianni et al., 2018; Gibson et al., 2009; Hedrick et al., 2021; Hoffman et al., 2008, 2019; Jaramillo-Ospina et al., 2022; Jiang et al., 2022; Jinno et al., 2020; Johnston et al., 2015; Koo et al., 2003; Li, X., et al. 2019; Litmanovitz et al., 2013; Makrides et al., 2000; Meli et al., 2014; Morris et al., 2000; Nieto-Ruiz et al., 2019; Petersen et al., 2020; Schmelzle et al., 2003; Sepúlveda-Valbuena et al., 2021; Shen et al., 2021; Teoh et al., 2022; Timby et al., 2014; Xia et al., 2021; Xu et al., 2015; Yeiser et al., 2016; Zhou et al., 2014), micronutrients (Ashley et al., 2012; Davis et al., 2008; Hedrick et al., 2021; Hoffman et al., 2008; Mackey et al., 2013; Xu et al., 2015; Zhou et al., 2014), lactose free (Heubi et al., 2000; Lasekan 2006, 2011, 2014), prebiotics (Ahrens et al., 2018; Alliet et al., 2022; Ashley et al., 2012; Bruzzese et al., 2009; Cesare Marincola et al., 2016; Chouraqui
et al., 2008; Civardi et al., 2017; Costalos et al., 2008; Estorninos et al., 2022; Fanaro et al., 2005; Fusch et al., 2001; Giovannini et al., 2013; Hoffman et al., 2019; Jochum et al., 2023; Kuehn et al., 2022; Lambidou et al., 2021; Lasekan et al., 2022; Li, X., et al. 2019; López-Velázquez et al., 2013; Marriage et al., 2015; Meli et al., 2014; Neumer et al., 2021; Nieto-Ruiz et al., 2019; Parschat et al., 2021; Picaud et al., 2020; Piemontese et al., 2011; Puccio et al., 2007, 2017; Radke et al., 2017; Rigo et al., 2019; Rodriguez-Herrera et al., 2019; Román et al., 2020; Rozé et al., 2012; Scalabrin et al., 2012; Schmelzle et al., 2003; Sepúlveda-Valbuena et al., 2021; Shahramian et al., 2018; Szajewska et al., 2017; Teoh et al., 2022; Tonon et al., 2021; Veereman-Wauters et al., 2011; Vlieger et al., 2009; Wang, Y., et al., 2021; Xu et al., 2015; Yang et al., 2022; Yeiser et al., 2016; Ziegler et al., 2007), and probiotics (Cekola et al., 2015; Gibson et al., 2009; Gil-Campos et al., 2012; Holscher et al., 2012; Inostroza et al., 2014; López-Velázquez et al., 2013; Maldonado et al., 2012, 2019; Martin et al., 2014; Meli et al., 2014; Nieto-Ruiz et al., 2019; Plaza-Diaz et al., 2023; Puccio et al., 2007; Radke et al., 2017; Rozé et al., 2012; Sepúlveda-Valbuena et al., 2021; Szajewska et al., 2017; Vendt et al., 2006;Vlieger et al., 2009; Wang, L., et al., 2019; Weizman and Alsheikh, 2006) (see Figure E-9).
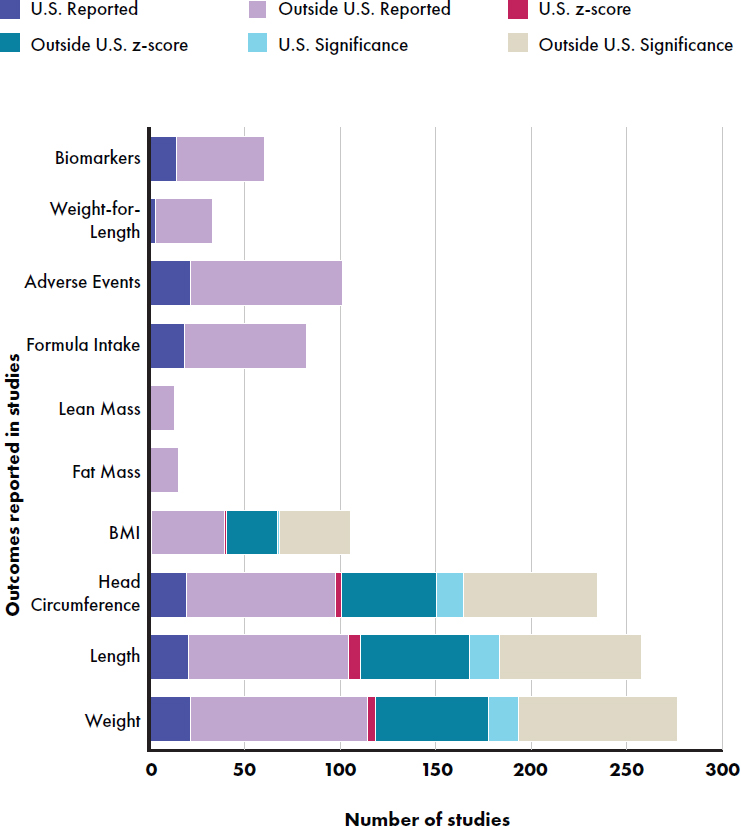
Outcomes Reported in Primary Studies
The most frequently reported outcome was weight, followed by length and head circumference (see Figure E-10 and Table E-4). The least frequently reported outcomes were body composition (lean and fat mass) and body mass index. Z-scores were far less frequently reported (e.g., weight 18 percent) for studies inside than outside of the United States (e.g., weight 63 percent). A wide variety of biomarkers were reported, including bone mineral, gut microbiome, and developmental values. The most frequently reported biomarkers with nutrition related (AAs, iron, and carotenoids), and the least frequently reported were bone mineral status values.
Pooled Studies
Four pooled analyses studies met criteria and were included in this scoping review (Alexander et al., 2016; Camier et al., 2021; Czerkies et al., 2018; Haschke et al., 2014). Three evaluated protein amount modification (Alexander et al., 2016; Camier et al., 2021; Haschke et al., 2014) in infant formula. Haschke et al. (2014) conducted a pooled analysis of three studies; one study was included in this scoping review (Inostroza et al., 2014), and two studies were excluded (conference abstracts). Alexander et al. (2016) pooled individual data from 11 RCTs. Five of the 11 RCTs
are included in this scoping review (Chouraqui et al., 2008; Gibson et al., 2009; Meli et al., 2014; Puccio et al., 2007; Putet et al., 2016), and six were excluded (publication date, population, and reported outcomes). Camier et al. (2021) pooled results from two cohorts with multiple publications. The investigative purposes of the articles included in the in this pooled analysis were outside the scope of this scoping review and therefore not included. Czerkies et al. (2018) conducted a pooled analysis that included seven clinical trials to evaluate the effectiveness of partially hydrolyzed formulas compared to standard formulas on growth and stools (Czerkies et al., 2018). Four studies from pooled analysis were included in this scoping review; three studies were excluded because they were conference
NOTES: BMI = body mass index; U.S. = United States.
| Number of Studies in U.S. Reporting Significance | Number of Studies in U.S. Reporting Statistically Significant Results | Number of Studies Conducted Outside of the U.S. Reporting Significance | Number of Studies Conducted Outside of the U.S. Reporting Statistically Significant Results | |
|---|---|---|---|---|
| Weight z-score at 4 months | 8 | 0 | 39 | 4 |
| Weight at 4 months | 13 | 0 | 45 | 3 |
| Weight z-score at the end of the study | 8 | 1 | 57 | 5 |
| Length at 4 months | 12 | 0 | 39 | 3 |
| Head circumference at 4 months | 11 | 0 | 39 | 1 |
| Weight z-score by sex/gender at the end of the study | 7 | 2 | 9 | 3 |
| Weight z-score by race/ethnicity at the end of the study | 1 | 0 | 1 | 0 |
NOTES: U.S. = United States.
proceedings (Cekola et al., 2015; Chouraqui et al., 2008; Meli et al., 2014; Puccio et al., 2007).
Systematic Reviews
Sixteen systematic reviews met criteria and were included in this scoping review (see Table E-5) (Abrams et al., 2015; Ahrens et al., 2018; Alexander et al., 2016; Alliet et al., 2022; Andres et al., 2012; Bruzzese et al., 2009; Gale et al., 2012; Jankiewicz et al., 2023; Jasani et al., 2017; Jiang et al., 2022; Liang et al., 2024; Makrides et al., 2005; Milani et al., 2023; Mugambi et al., 2012; Patro-Gołab et al., 2016; Rao et al., 2009; Ren et al., 2022; Szajewska et al., 2015, 2017; Udell et al., 2005; Wang, L., et al., 2019; Zhang et al., 2023). The majority of the systematic reviews searched at least two databases (with the exception of two: Gale et al., 2012; Makrides et al., 2005), conducted risk of bias assessments of included articles (with the exception of two: Makrides et al., 2005; Udell et al., 2005), and conducted meta-analyses (with the exception of two: Abrams et al., 2015; Szajewska et al., 2015). Only three systematic reviews evaluated the certainty of evidence for each reported outcome (Jasani et al., 2017; Liang et al., 2024; Mugambi et al., 2012). Years of publication were 2005–2024. Topics included amount and type of fat (Jasani et al., 2017; Makrides et al., 2005; Udell et al., 2005; Zhang et al., 2023), pre-, pro-, and postbiotics (Liang et al., 2024; Mugambi et al., 2012; Rao et al., 2009; Szajewska et al., 2015), protein (Abrams et al., 2015; Milani et al., 2023; Patro-Gołab et al., 2016; Ren et al., 2022), fermentation (Szajewska et al., 2015), nucleotides (Wang, L., et al., 2019), goat milk (Jankiewicz et al., 2023), and formula compared to human milk (Gale et al., 2012).
| Author | Year | Number of Articles | 2 or More Databases | ROB | COE | Meta-Analysis |
|---|---|---|---|---|---|---|
| Fat | ||||||
| Makrides et al. (2005) | 2005 | 14 | 0 | 0 | 0 | 1 |
| Udell et al. (2005) | 2005 | 5 | 1 | 0 | 0 | 1 |
| Jasani et al. (2017) | 2017 | 15 | 1 | 1 | 1 | 1 |
| Zhang et al. (2023) | 2023 | 16 | 1 | 1 | 0 | 1 |
| Pre- or Probiotics | ||||||
| Rao et al. (2009) | 2009 | 11 | 1 | 1 | 0 | 1 |
| Mugambi et al. (2012) | 2012 | 23 | 1 | 1 | 1 | 1 |
| Szajewska et al. (2013) | 2013 | 9 | 1 | 1 | 0 | 1 |
| Liang et al. (2024) | 2024 | 9 | 1 | 1 | 1 | 1 |
| Formula vs. Human Milk | ||||||
| Gale et al. (2012) | 2012 | 15 | 0 | 1 | 0 | 1 |
| Protein | ||||||
| Abrams et al. (2015) | 2015 | 6 | 1 | 1 | 0 | 0 |
| Patro-Gołab et al. (2016) | 2016 | 12 | 1 | 1 | 0 | 1 |
| Ren et al. (2022) | 2022 | 19 | 1 | 1 | 0 | 1 |
| Milani et al. (2023) | 2023 | 5 | 1 | 1 | 0 | 1 |
| Fermentation | ||||||
| Szajewska et al. (2017) | 2015 | 5 | 1 | 1 | 0 | 0 |
| Nucleotides | ||||||
| Wang et al. (2019) | 2019 | 8 | 1 | 1 | 0 | 1 |
| Goat Milk | ||||||
| Jankiewicz et al. (2023) | 2023 | 4 | 1 | 1 | 0 | 1 |
NOTE: COE = certainty of evidence; ROB = risk of bias.
REFERENCES
Abrahamse-Berkeveld, M., S. N. Jespers, P. C. Khoo, V. Rigo, S. M. Peeters, R. H. van Beek, O. F. Norbruis, S. Schoen, M. Marintcheva-Petrova, and E. M. van der Beek. 2024. Infant milk formula with large, milk phospholipid-coated lipid droplets enriched in dairy lipids affects body mass index trajectories and blood pressure at school age: Followup of a randomized controlled trial. American Journal of Clinical Nutrition 119(1):87–99.
Abrams, S. A., K. M. Hawthorne, and M. Pammi. 2015. A systematic review of controlled trials of lower-protein or energy-containing infant formulas for use by healthy full-term infants. Advances in Nutrition 6(2):178–188.
Ahrens, B., C. Hellmuth, N. Haiden, D. Olbertz, E. Hamelmann, M. Vusurovic, M. Fleddermann, R. Roehle, A. Knoll, B. Koletzko, U. Wahn, and K. Beyer. 2018. Hydrolyzed formula with reduced protein content supports adequate growth: A randomized controlled noninferiority trial. Journal of Pediatric Gastroenterology and Nutrition 66(5):822–830.
Alexander, D. D., J. Yan, L. C. Bylsma, R. S. Northington, D. Grathwohl, P. Steenhout, P. Erdmann, E. Spivey-Krobath, and F. Haschke. 2016. Growth of infants consuming whey-predominant term infant formulas with a protein content of 1.8 g/100 kcal: A multicenter pooled analysis of individual participant data. American Journal of Clinical Nutrition 104(4):1083–1092.
Alliet, P., Y. Vandenplas, P. Roggero, S. N. Jespers, S. Peeters, J.-P. Stalens, G. A. Kortman, M. Amico, B. Berger, and N. Sprenger. 2022. Safety and efficacy of a probiotic-containing infant formula supplemented with 2’-fucosyllactose: A double-blind randomized controlled trial. Nutrition Journal 21(1):11.
Andres, A., M. A. Cleves, J. B. Bellando, R. T. Pivik, P. H. Casey, and T. M. Badger. 2012. Developmental status of 1-year-old infants fed breast milk, cow’s milk formula, or soy formula. Pediatrics 129(6):1134–1140.
Arskey, H., and L. O’Malley. 2005. Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology 8(1):19–32.
Ashley, C., W. H. Johnston, C. L. Harris, S. I. Stolz, J. L. Wampler, and C. L. Berseth. 2012. Growth and tolerance of infants fed formula supplemented with polydextrose (PDX) and/or galactooligosaccharides (GOS): Double-blind, randomized, controlled trial. Nutrition Journal 11:38.
Ben, X.-m., X.-y. Zhou, W.-h. Zhao, W.-l. Yu, W. Pan, W.-l. Zhang, S.-m. Wu, C. M. V. Beusekom, and A. Schaafsma. 2004. Growth and development of term infants fed with milk with long-chain polyunsaturated fatty acid supplementation. Chinese Medical Journal 117(8):1268–1270.
Billeaud, C., G. Puccio, E. Saliba, B. Guillois, C. Vaysse, S. Pecquet, and P. Steenhout. 2014. Safety and tolerance evaluation of milk fat globule membrane-enriched infant formulas: A randomized controlled multicenter non-inferiority trial in healthy term infants. Clinical Medicine Insights Pediatrics 8:51–60.
Billeaud, C., L. Adamon, H. Piloquet, N. P. Hays, L. Dupuis, I. Metreau, and A. Léké. 2022. A new partially hydrolyzed whey-based follow-on formula with age-adapted protein content supports healthy growth during the first year of life. Frontiers in Pediatrics 10:937882.
Breij, L. M., M. Abrahamse-Berkeveld, Y. Vandenplas, S. N. Jespers, A. C. de Mol, P. C. Khoo, M. Kalenga, S. Peeters, R. H. van Beek, and O. F. Norbruis. 2019. An infant formula with large, milk phospholipid-coated lipid droplets containing a mixture of dairy and vegetable lipids supports adequate growth and is well tolerated in healthy, term infants. American Journal of Clinical Nutrition 109(3):586–596.
Bruzzese, E., M. Volpicelli, V. Squeglia, D. Bruzzese, F. Salvini, M. Bisceglia, P. Lionetti, M. Cinquetti, G. Iacono, and S. Amarri. 2009. A formula containing galacto- and fructo-oligosaccharides prevents intestinal and extra-intestinal infections: An observational study. Clinical Nutrition 28(2):156–161.
Camier, A., C. Davisse-Paturet, P. Scherdel, S. Lioret, B. Heude, M. A. Charles, and B. de Lauzon-Guillain. 2021. Early growth according to protein content of infant formula: Results from the EDEN and ELFE birth cohorts. Pediatric Obesity 16(11):e12803.
Cekola, P. L., L. A. Czerkies, H. M. Storm, M. H. Wang, J. Roberts, and J. M. Saavedra. 2015. Growth and tolerance of term infants fed formula with probiotic Lactobacillus reuteri. Clinical Pediatricsics 54(12):1175–1184.
Cesare Marincola, F., S. Corbu, M. Lussu, A. Noto, A. Dessì, S. Longo, E. Civardi, F. Garofoli, B. Grenci, and E. Mongini. 2016. Impact of early postnatal nutrition on the NMR urinary metabolic profile of infant. Journal of Proteome Research 15(10):3712–3723.
Chouraqui, J. P., D. Grathwohl, J. M. Labaune, J. M. Hascoet, I. de Montgolfier, M. Leclaire, M. Giarre, and P. Steenhout. 2008. Assessment of the safety, tolerance, and protective effect against diarrhea of infant formulas containing mixtures of probiotics or probiotics and prebiotics in a randomized controlled trial. American Journal of Clinical Nutrition 87(5):1365–1373.
Civardi, E., F. Garofoli, S. Longo, M. E. Mongini, B. Grenci, I. Mazzucchelli, M. Angelini, A. Castellazzi, F. Fasano, and A. Grinzato. 2017. Safety, growth, and support to healthy gut microbiota by an infant formula enriched with functional compounds. Clinical Nutrition 36(1):238–245.
Collell, R., R. Closa-Monasterolo, N. Ferré, V. Luque, B. Koletzko, V. Grote, R. Janas, E. Verduci, and J. Escribano. 2016. Higher protein intake increases cardiac function parameters in healthy children: Metabolic programming by infant nutrition—Secondary analysis from a clinical trial. Pediatric Research 79(6):880–888.
Costalos, C., A. Kapiki, M. Apostolou, and E. Papathoma. 2008. The effect of a prebiotic supplemented formula on growth and stool microbiology of term infants. Early Human Development 84(1):45–49.
Czerkies, L. A., B. D. Kineman, S. S. Cohen, H. Reichert, and R. S. Carvalho. 2018. A pooled analysis of growth and tolerance of infants exclusively fed partially hydrolyzed whey or intact protein-based infant formulas. International Journal of Pediatrics 2018:4969576.
Davis, A. M., B. J. Harris, E. L. Lien, K. Pramuk, and J. Trabulsi. 2008. Alpha-lactalbuminrich infant formula fed to healthy term infants in a multicenter study: Plasma essential amino acids and gastrointestinal tolerance. European Journal of Clinical Nutrition 62(11):1294–1301.
Demmelmair, H., M. Fleddermann, and B. Koletzko. 2022. Infant feeding choices during the first post-natal months and anthropometry at age seven years: Follow-up of a randomized clinical trial. Nutrients 14(19).
EndNote. 2013. Version X9. Version X9. Philadelphia, PA: Clarivate.
Escribano, J., V. Luque, N. Ferre, G. Mendez-Riera, B. Koletzko, V. Grote, H. Demmelmair, L. Bluck, A. Wright, and R. Closa-Monasterolo. 2012. Effect of protein intake and weight gain velocity on body fat mass at 6 months of age: The EU Childhood Obesity Programme. International Journal of Obesity 36(4):548–553.
Estorninos, E., R. B. Lawenko, E. Palestroque, J. Lebumfacil, M. Marko, and C. I. Cercamondi. 2022. Infant formula containing bovine milk-derived oligosaccharides supports age-appropriate growth and improves stooling pattern. Pediatric Research 91(6):1485–1492.
Fanaro, S., J. Jelinek, B. Stahl, G. Boehm, R. Kock, and V. Vigi. 2005. Acidic oligosaccharides from pectin hydrolysate as new component for infant formulae: Effect on intestinal flora, stool characteristics, and pH. Journal of Pediatric Gastroenterology and Nutrition 41(2):186–190.
FDA (Food and Drug Administration) and HHS (Department of Health and Human Services). n.d. 106.96 requirements for quality factors for infant formulas https://www.access-data.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=106.96 (accessed Janurary 16, 2024).
Fleddermann, M., H. Demmelmair, V. Grote, T. Nikolic, B. Trisic, and B. Koletzko. 2014. Infant formula composition affects energetic efficiency for growth: The BeMIM study, a randomized controlled trial. Clinical Nutrition 33(4):588–595.
Fleddermann, M., H. Demmelmair, V. Grote, M. Bidlingmaier, P. Grimminger, M. Bielohuby, and B. Koletzko. 2017. Role of selected amino acids on plasma IGF-I concentration in infants. European Journal of Nutrition 56(2):613–620.
Fleddermann, M., H. Demmelmair, C. Hellmuth, V. Grote, B. Trisic, T. Nikolic, and B. Koletzko. 2018. Association of infant formula composition and anthropometry at 4 years: Follow-up of a randomized controlled trial (BeMIM study). PLoS ONE. 13(7).
Fusch, C., H. Skopnik, S. Wirth, and M. Radke. 2001. Double-blind, randomised, placebo-controlled study to evaluate the nutritional efficacy of Omneo 1. Final study results. Pediatrika. 21:49–54.
Gale, C., K. M. Logan, S. Santhakumaran, J. R. C. Parkinson, M. J. Hyde, and N. Modi. 2012. Effect of breastfeeding compared with formula feeding on infant body composition: A systematic review and meta-analysis. American Journal of Clinical Nutrition 95(3):656–669.
Gianni, M. L., P. Roggero, C. Baudry, C. Fressange-Mazda, P. le Ruyet, and F. Mosca. 2018. No effect of adding dairy lipids or long chain polyunsaturated fatty acids on formula tolerance and growth in full term infants: A randomized controlled trial. BMC Pediatrics 18(1):10.
Gibson, R. A., D. Barclay, H. Marshall, J. Moulin, J. C. Maire, and M. Makrides. 2009. Safety of supplementing infant formula with long-chain polyunsaturated fatty acids and Bifidobacterium lactis in term infants: A randomised controlled trial. British Journal of Nutrition 101(11):1706–1713.
Gil-Campos, M., M. Á. López, M. V. Rodriguez-Benítez, J. Romero, I. Roncero, M. D. Linares, J. Maldonado, E. López-Huertas, R. Berwind, and K. L. Ritzenthaler, et al. 2012. Lactobacillus fermentum CECT 5716 is safe and well tolerated in infants of 1–6 months of age: A randomized controlled trial. Pharmacology Research 65(2):231–238.
Giovannini, M., E. Verduci, G. Zuccotti, G. Biasucci, A. Podestà, A. Rottoli, D. Gregori, S. Ballali, G. Banderali, and E. Riva. 2013. Safety of a formula supplemented with galactooligosaccharides in term infants. International Journal of Probiotics and Prebiotics. 8:67–74.
Haschke, F., E. E. Ziegler, and D. Grathwohl. 2014. Fast growth of infants of overweight mothers: Can it be slowed down? Annals of Nutrition and Metabolism 64:19–24.
He, T., F. Woudstra, F. Panzer, A. Haandrikman, H. J. Verkade, and L. van Lee. 2022. Goat milk based infant formula in newborns: A double-blind randomized controlled trial on growth and safety. Journal of Pediatric Gastroenterology and Nutrition 75(2):215–220.
Hedrick, J., M. Yeiser, C. L. Harris, J. L. Wampler, H. E. London, A. C. Patterson, and S. S. Wu. 2021. Infant formula with added bovine milk fat globule membrane and modified iron supports growth and normal iron status at one year of age: A randomized controlled trial. Nutrients 13(12):4541.
Hegar, B., R. Rantos, A. Firmansyah, J. De Schepper, and Y. Vandenplas. 2008. Natural evolution of infantile regurgitation versus the efficacy of thickened formula. Journal of Pediatric Gastroenterology and Nutrition 47(1):26–30.
Hernell, O., and B. Lönnerdal. 2002. Iron status of infants fed low-iron formula: No effect of added bovine lactoferrin or nucleotides. American Journal of Clinical Nutrition 76(4):858–864.
Heubi, J., R. Karasov, K. Reisinger, M. Blatter, L. Rosenberg, J. Vanderhoof, P. M. Darden, J. Safier, T. Martin, and A. R. Euler. 2000. Randomized multicenter trial documenting the efficacy and safety of a lactose-free and a lactose-containing formula for term infants. Journal of the American Dietetic Association 100(2):212–217.
Higgins, J. P. T., J. Thomas, J. Chandler, M. Cumpston, T. P. M. Li, and V. A. Welch (editors). 2024. Cochrane Handbook for Systematic Reviews of Interventions. www.training.cochrane.org/handbook (accessed January 15, 2025).
Hoffman, D., E. Ziegler, S. H. Mitmesser, C. L. Harris, and D. A. Diersen-Schade. 2008. Soy-based infant formula supplemented with DHA and ARA supports growth and increases circulating levels of these fatty acids in infants. Lipids 43(1):29–35.
Hoffman, D. R., C. L. Harris, J. L. Wampler, A. C. Patterson, and C. L. Berseth. 2019. Growth, tolerance, and DHA and ARA status of healthy term infants receiving formula with two different ARA concentrations: Double-blind, randomized, controlled trial. Prostaglandins, Leukotrienes, and Essential Fatty Acids 146:19–27.
Holscher, H. D., L. A. Czerkies, P. Cekola, R. Litov, M. Benbow, S. Santema, D. D. Alexander, V. Perez, S. Sun, and J. M. Saavedra. 2012. Bifidobacterium lactis Bb12 enhances intestinal antibody response in formula-fed infants: A randomized, double-blind, controlled trial. Journal of Parenteral and Enteral Nutrition 36(1 Suppl):106s–117s.
Inostroza, J., F. Haschke, P. Steenhout, D. Grathwohl, S. E. Nelson, and E. E. Ziegler. 2014. Low-protein formula slows weight gain in infants of overweight mothers. Journal of Pediatric Gastroenterology and Nutrition 59(1):70–77.
Jankiewicz, M., L. van Lee, M. Biesheuvel, E. M. Brouwer-Brolsma, L. van der Zee, and H. Szajewska. 2023. The effect of goat-milk-based infant formulas on growth and safety parameters: A systematic review and meta-analysis. Nutrients 15(9).
Jaramillo-Ospina, A. M., R. Toro-Campos, T. Murguía-Peniche, J. L. Wampler, S. S. Wu, C. L. Berseth, and R. Uauy. 2022. Added bovine milk fat globule membrane in formula: Growth, body composition, and safety through age 2: An RCT. Nutrition 97:111599.
Jasani, B., K. Simmer, S. K. Patole, and S. C. Rao. 2017. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database of Systematic Reviews 2017(3).
Jiang, B., Y. Xia, L. Zhou, X. Liang, X. Chen, M. Chen, X. Li, S. Lin, N. Zhang, and L. Zheng. 2022. Safety and tolerance assessment of milk fat globule membrane-enriched infant formulas in healthy term Chinese infants: A randomised multicenter controlled trial. BMC Pediatrics 22(1):465.
Jinno, S., K. Yamazaki, Y. Nakamura, and T. Kinouchi. 2020. Growth of term infants fed a commercial infant formula with a protein content of 2.2 g/100 kcal: An observational follow-up study. Bioscience, Biotechnology, and Biochemistry 84(3):633–639.
Jochum, F., M. Meyer-Krott, T. Hübler, M. Lorenz, R. Bedikian, J. Zakarian, A. Litzka, G. Judex, H. Hertzberg, and D. Klee. 2023. Real-world evidence study on tolerance and growth in infants fed an infant formula with two human milk oligosaccharides vs mixed fed and exclusively breastfed infants. Molecular and Cellular Pediatrics 10(1):7.
Johnston, W. H., C. Ashley, M. Yeiser, C. L. Harris, S. I. Stolz, J. L. Wampler, A. Wittke, and T. R. Cooper. 2015. Growth and tolerance of formula with lactoferrin in infants through one year of age: Double-blind, randomized, controlled trial. BMC Pediatrics 15:173.
Kantaras, P., A. Kokkinopoulou, J. H. Hageman, M. Hassapidou, O. Androutsos, M. Kanaki, I. Bovee-Oudenhoven, E. Karaglani, A.-M. Kontochristopoulou, and R. Bos. 2024. Growth and gut comfort of healthy term infants exclusively fed with a partially hydrolysed protein-based infant formula: A randomized controlled double-blind trial. Frontiers in Pediatrics 12:1328709.
Karaglani, E., I. Thijs-Verhoeven, M. Gros, C. Chairistanidou, G. Zervas, C. Filoilia, T.-M. Kampani, V. Miligkos, M. Matiatou, and S. Valaveri. 2020. A partially hydrolyzed whey infant formula supports appropriate growth: A randomized controlled non-inferiority trial. Nutrients 12(10):3056.
Koletzko, B., R. Von Kries, R. Closa, J. Escribano, S. Scaglioni, M. Giovannini, J. Beyer, H. Demmelmair, D. Gruszfeld, and A. Dobrzanska. 2009. Lower protein in infant formula is associated with lower weight up to age 2 y: A randomized clinical trial. American Journal of Clinical Nutrition 89(6):1836–1845.
Koo, W. W., M. Hammami, D. P. Margeson, C. Nwaesei, M. B. Montalto, and J. B. Lasekan. 2003. Reduced bone mineralization in infants fed palm olein-containing formula: A randomized, double-blinded, prospective trial. Pediatrics 111(5 Pt 1):1017–1023.
Kouwenhoven, S. M., N. Antl, M. J. Finken, J. W. Twisk, E. M. van der Beek, M. Abrahamse-Berkeveld, B. J. van de Heijning, H. Schierbeek, L. M. Holdt, and J. B. van Goudoever. 2020. A modified low-protein infant formula supports adequate growth in healthy, term infants: A randomized, double-blind, equivalence trial. American Journal of Clinical Nutrition 111(5):962–974.
Kouwenhoven, S. M., N. Antl, M. J. Finken, J. W. Twisk, E. M. van der Beek, M. Abrahamse-Berkeveld, B. J. van de Heijning, J. B. van Goudoever, and B. V. Koletzko. 2021a. Long-term effects of a modified, low-protein infant formula on growth and body composition: Follow-up of a randomized, double-blind, equivalence trial. Clinical Nutrition 40(6):3914–3921.
Kouwenhoven, S. M., M. Fleddermann, M. J. Finken, J. W. Twisk, E. M. van der Beek, M. Abrahamse-Berkeveld, B. J. van De Heijning, D. van Harskamp, J. B. van Goudoever, and B. V. Koletzko. 2021b. Early-life metabolic and hormonal markers in blood and growth until age 2 years: Results from a randomized controlled trial in healthy infants fed a modified low-protein infant formula. Nutrients 13(4):1159.
Kuehn, D., S. H. Zeisel, D. F. Orenstein, J. B. German, C. J. Field, S. Teerdhala, A. Knezevic, S. Patil, S. M. Donovan, and B. Lönnerdal. 2022. Effects of a novel high-quality protein infant formula on energetic efficiency and tolerance: A randomized trial. Journal of Pediatric Gastroenterology and Nutrition 75(4):521–528.
Lambidou, M., B. Alteheld, R. Fimmers, F. Jochum, A. Nomayo, and P. Stehle. 2021. Impact of an infant formula containing a novel fat blend (cow’s milk fat, fish and vegetable oil) and prebiotics on stool fatty acid soaps and erythrocyte fatty acid profiles in full-term healthy newborns. Annals of Nutrition & Metabolism 77(3):138–145.
Lasekan, J. B., M. Neylan, S. Luebbers, W. W. K. Koo, and J. Walters. 2006. Growth, tolerance and biochemical measures in healthy infants fed a partially hydrolyzed rice protein-based formula: A randomized, blinded, prospective trial. Journal of the American College of Nutrition 25(1):12–19.
Lasekan, J. B., J. Jacobs, K. S. Reisinger, M. B. Montalto, M. P. Frantz, and M. M. Blatter. 2011. Lactose-free milk protein-based infant formula: Impact on growth and gastrointestinal tolerance in infants. Clinical Pediatrics 50(4):330–337.
Lasekan, J. B., H. K. Linke, J. S. Oliver, J. D. Carver, M. M. Blatter, M. J. Kuchan, J. M. Cramer, and P. F. Pollack. 2014. Milk protein-based infant formula containing rice starch and low lactose reduces common regurgitation in healthy term infants: A randomized, blinded, and prospective trial. Journal of the American College of Nutrition 33(2):136–146.
Lasekan, J., Y. Choe, S. Dvoretskiy, A. Devitt, S. Zhang, A. Mackey, K. Wulf, R. Buck, C. Steele, and M. Johnson. 2022. Growth and gastrointestinal tolerance in healthy term infants fed milk-based infant formula supplemented with five human milk oligosaccharides (HMOs): A randomized multicenter trial. Nutrients 14(13):2625.
Levac, D., H. O. Colquhoun, and K. K. Brien. 2010. Scoping studies: Advancing the methodology. Implementation Science 5(69).
Li, F., S. S. Wu, C. L. Berseth, C. L. Harris, J. D. Richards, J. L. Wampler, W. Zhuang, G. Cleghorn, C. D. Rudolph, and B. Liu. 2019. Improved neurodevelopmental outcomes associated with bovine milk fat globule membrane and lactoferrin in infant formula: A randomized, controlled trial. Journal of Pediatrics 215:24–31.e28.
Li, X., Y. Peng, Z. Li, B. Christensen, A. B. Heckmann, H. Stenlund, B. Lönnerdal, and O. Hernell. 2019. Feeding infants formula with probiotics or milk fat globule membrane: A double-blind, randomized controlled trial. Frontiers in Pediatrics 7:347.
Liang, X., Y. Li, Z. Zhao, R. Ding, J. Sun, and C. Chi. 2024. Safety and efficacy of adding postbiotics in infant formula: A systematic review and meta-analysis. Pediatric Research 95(1):43–51.
Lien, E. L., A. M. Davis, and A. R. Euler. 2004. Growth and safety in term infants fed reduced-protein formula with added bovine alpha-lactalbumin. Journal of Pediatric Gastroenterology and Nutrition 38(2):170–176.
Liotto, N., A. Orsi, C. Menis, P. Piemontese, L. Morlacchi, C. C. Condello, M. L. Giannì, P. Roggero, and F. Mosca. 2018. Clinical evaluation of two different protein content formulas fed to full-term healthy infants: A randomized controlled trial. BMC Pediatrics 18:1–7.
Litmanovitz, I., K. Davidson, A. Eliakim, R. H. Regev, T. Dolfin, S. Arnon, F. Bar-Yoseph, A. Goren, Y. Lifshitz, and D. Nemet. 2013. High beta-palmitate formula and bone strength in term infants: A randomized, double-blind, controlled trial. Calcified Tissue International 92(1):35–41.
Lönnerdal, B., A. S. Kvistgaard, J. M. Peerson, S. M. Donovan, and Y. M. Peng. 2016. Growth, nutrition, and cytokine response of breast-fed infants and infants fed formula with added bovine osteopontin. Journal of Pediatric Gastroenterology and Nutrition 62(4):650–657.
López-Velázquez, G., L. Díaz-García, A. Anzo, M. Parra-Ortiz, B. Llamosas-Gallardo, A. A. Ortiz-Hernández, J. Mancilla-Ramírez, J. M. Cruz-Rubio, and P. Gutiérrez-Castrellón. 2013. Safety of a dual potential prebiotic system from Mexican agave “Metlin® and Metlos®,” incorporated to an infant formula for term newborn babies: A randomized controlled trial. Revista de Investigacion Clinica 65(6):483–490.
Mackey, A. D., D. Albrecht, J. Oliver, T. Williams, A. C. Long, and P. T. Price. 2013. Plasma carotenoid concentrations of infants are increased by feeding a milk-based infant formula supplemented with carotenoids. Journal of the Science of Food and Agriculture 93(8):1945–1952.
Makrides, M., M. A. Neumann, B. Jeffrey, E. L. Lien, and R. A. Gibson. 2000. A randomized trial of different ratios of linoleic to α-linolenic acid in the diet of term infants: Effects on visual function and growth. American Journal of Clinical Nutrition 71(1):120–129.
Makrides, M., R. A. Gibson, T. Udell, K. Ried, and International LCPUFA Investigators. 2005. Supplementation of infant formula with long-chain polyunsaturated fatty acids does not influence the growth of term infants. American Journal of Clinical Nutrition 81(5):1094–1101.
Maldonado, J., F. Cañabate, L. Sempere, F. Vela, A. R. Sánchez, E. Narbona, E. López-Huertas, A. Geerlings, A. D. Valero, and M. Olivares. 2012. Human milk probiotic lactobacillus fermentum CECT5716 reduces the incidence of gastrointestinal and upper respiratory tract infections in infants. Journal of Pediatric Gastroenterology and Nutrition 54(1):55–61.
Maldonado, J., M. Gil-Campos, J. Maldonado-Lobón, M. Benavides, K. Flores-Rojas, R. Jaldo, I. Jiménez Del Barco, V. Bolívar, A. Valero, and E. Prados. 2019. Evaluation of the safety, tolerance and efficacy of 1-year consumption of infant formula supplemented with Lactobacillus fermentum CECT5716 Lc40 or Bifidobacterium breve CECT7263: A randomized controlled trial. BMC Pediatrics 19(1).
Marriage, B. J., R. H. Buck, K. C. Goehring, J. S. Oliver, and J. A. Williams. 2015. Infants fed a lower calorie formula with 2 ′ FL show growth and 2 ′ FL uptake like breast-fed infants. Journal of Pediatric Gastroenterology and Nutrition 61(6):649–658.
Martin, F.-P. J., S. Moco, I. Montoliu, S. Collino, L. Da Silva, S. Rezzi, R. Prieto, M. Kussmann, J. Inostroza, and P. Steenhout. 2014. Impact of breast-feeding and high- and low-protein formula on the metabolism and growth of infants from overweight and obese mothers. Pediatric Research 75(4):535–543.
Meli, F., G. Puccio, C. Cajozzo, G. L. Ricottone, S. Pecquet, N. Sprenger, and P. Steenhout. 2014. Growth and safety evaluation of infant formulae containing oligosaccharides derived from bovine milk: A randomized, double-blind, noninferiority trial. BMC Pediatrics 14:1-11.
Milani, G. P., V. Edefonti, V. De Cosmi, S. Bettocchi, A. Mazzocchi, M. Silano, A. Pietrobelli, and C. Agostoni. 2023. Protein and growth during the first year of life: A systematic review and meta-analysis. Pediatric Research. 94(3):878–891.
Moher, D., A. Liberati, J. Tetzlaff, D. G. Altman, and PRISMA Group. 2009. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine 6(7):e1000097.
Morris, G., J. Moorcraft, A. Mountjoy, and J. C. K. Wells. 2000. A novel infant formula milk with added long-chain polyunsaturated fatty acids from single-cell sources: A study of growth, satisfaction and health. European Journal of Clinical Nutrition 54(12):883–886.
Mugambi, M. N., A. Musekiwa, M. Lombard, Y. Young, and R. Blaauw. 2012. Synbiotics, probiotics or prebiotics in infant formula for full term infants: A systematic review. Nutrition Journal 11(1).
Neumer, F., O. Urraca, J. Alonso, J. Palencia, V. Varea, S. Theis, M. Rodriguez-Palmero, J. A. Moreno-Muñoz, F. Guarner, and G. Veereman. 2021. Long-term safety and efficacy of prebiotic enriched infant formula—A randomized controlled trial. Nutrients 13(4):1276.
Nieto-Ruiz, A., J. A. García-Santos, M. G. Bermúdez, F. Herrmann, E. Diéguez, N. Sepúlveda-Valbuena, S. García, M. T. Miranda, R. De-Castellar, and M. Rodríguez-Palmero. 2019. Cortical visual evoked potentials and growth in infants fed with bioactive compounds-enriched infant formula: Results from COGNIS randomized clinical trial. Nutrients 11(10):2456.
Oropeza-Ceja, L. G., J. L. Rosado, D. Ronquillo, O. P. García, M. d. C. Caamaño, C. García-Ugalde, R. Viveros-Contreras, and M. Á. Duarte-Vázquez. 2018. Lower protein intake supports normal growth of full-term infants fed formula: A randomized controlled trial. Nutrients 10(7):886.
Parschat, K., C. Melsaether, K. R. Jäpelt, and S. Jennewein. 2021. Clinical evaluation of 16-week supplementation with 5HMO-mix in healthy-term human infants to determine tolerability, safety, and effect on growth. Nutrients 13(8).
Pastor, N., B. Soler, S. H. Mitmesser, P. Ferguson, and C. Lifschitz. 2006. Infants fed docosahexaenoic acid- and arachidonic acid-supplemented formula have decreased incidence of bronchiolitis/bronchitis the first year of life. Clinical Pediatrics 45(9):850–855.
Patro-Gołąb, B., B. M. Zalewski, S. M. Kouwenhoven, J. Karaś, B. Koletzko, J. B. van Goudoever, and H. Szajewska. 2016. Protein concentration in milk formula, growth, and later risk of obesity: A systematic review. Journal of Nutrition 146(3):551–564.
Peters, M. D., C. Godfrey, P. McInerney, Z. Munn, A. C. Tricco, and H. Khalil. 2020. Chapter 11: Scoping reviews (2020 version). In Manual for Evidence Synthesis, edited by E. Aromataris and Z. Munn. Adelaide, Australia: JBI.
Petersen, H., A. Nomayo, R. Zelenka, J. Foster, J. Tvrdík, and F. Jochum. 2020. Adequacy and safety of α-lactalbumin–enriched low-protein infant formula: A randomized controlled trial. Nutrition 74.
Picaud, J.-C., B. Pajek, M. Arciszewska, I. Tarczón, J. Escribano, R. Porcel, T. Adelt, E. Hassink, A. Rijnierse, and M. Abrahamse-Berkeveld. 2020. An infant formula with partially hydrolyzed whey protein supports adequate growth and is safe and well-tolerated in healthy, term infants: A randomized, double-blind, equivalence trial. Nutrients 12(7):1–16.
Piemontese, P., M. L. Giannì, C. P. Braegger, G. Chirico, C. Grüber, J. Riedler, S. Arslanoglu, M. van Stuijvenberg, G. Boehm, and J. Jelinek. 2011. Tolerance and safety evaluation in a large cohort of healthy infants fed an innovative prebiotic formula: A randomized controlled trial. PLoS ONE 6(11).
Plaza-Diaz, J., F. J. Ruiz-Ojeda, J. Morales, A. I. C. de la Torre, A. García-García, C. N. de Prado, C. Coronel-Rodríguez, C. Crespo, E. Ortega, and E. Martín-Pérez. 2023. Effects of a novel infant formula on weight gain, body composition, safety and tolerability to infants: The INNOVA 2020 Study. Nutrients 15(1):147.
Puccio, G., C. Cajozzo, F. Meli, F. Rochat, D. Grathwohl, and P. Steenhout. 2007. Clinical evaluation of a new starter formula for infants containing live Bifidobacterium longum BL999 and prebiotics. Nutrition 23(1):1–8.
Puccio, G., P. Alliet, C. Cajozzo, E. Janssens, G. Corsello, N. Sprenger, S. Wernimont, D. Egli, L. Gosoniu, and P. Steenhout. 2017. Effects of infant formula with human milk oligosaccharides on growth and morbidity: A randomized multicenter trial. Journal of Pediatric Gastroenterology and Nutrition 64(4):624–631.
Putet, G., J.-M. Labaune, K. Mace, P. Steenhout, D. Grathwohl, V. Raverot, Y. Morel, and J.-C. Picaud. 2016. Effect of dietary protein on plasma insulin-like growth factor-1, growth, and body composition in healthy term infants: A randomised, double-blind, controlled trial (Early Protein and Obesity in Childhood (EPOCH) study). British Journal of Nutrition 115(2):271–284.
Radke, M., J.-C. Picaud, A. Loui, G. Cambonie, D. Faas, H. N. Lafeber, N. de Groot, S. S. Pecquet, P. G. Steenhout, and J.-M. Hascoet. 2017. Starter formula enriched in prebiotics and probiotics ensures normal growth of infants and promotes gut health: A randomized clinical trial. Pediatric Research 81(4):622–631.
Räihä, N. C., A. Fazzolari-Nesci, C. Cajozzo, G. Puccio, A. Monestier, G. Moro, I. Minoli, E. Haschke-Becher, C. Bachmann, and M. Van’t Hof. 2002. Whey predominant, whey modified infant formula with protein/energy ratio of 1.8 g/100 kcal: Adequate and safe for term infants from birth to four months. Journal of Pediatric Gastroenterology and Nutrition 35(3):275–281.
Rao, S., R. Srinivasjois, and S. Patole. 2009. Prebiotic supplementation in full-term neonates: A systematic review of randomized controlled trials. Archives of Pediatrics and Adolescent Medicine 163(8):755–764.
Ren, Q., K. Li, H. Sun, C. Zheng, Y. Zhou, Y. Lyu, W. Ye, H. Shi, W. Zhang, and Y. Xu. 2022. The association of formula protein content and growth in early infancy: A systematic review and meta-analysis. Nutrients 14(11):2255.
Rigo, J., S. Schoen, M. Verghote, B. van Overmeire, W. Marion, M. Abrahamse-Berkeveld, and P. Alliet. 2019. Partially hydrolysed whey-based formulae with reduced protein content support adequate infant growth and are well tolerated: Results of a randomised controlled trial in healthy term infants. Nutrients 11(7):1654.
Rodriguez-Herrera, A., K. Mulder, H. Bouritius, R. Rubio, A. Muñoz, M. Agosti, G. Lista, L. Corvaglia, T. Ludwig, and M. Abrahamse-Berkeveld. 2019. Gastrointestinal tolerance, growth and safety of a partly fermented formula with specific prebiotics in healthy infants: A double-blind, randomized, controlled trial. Nutrients 11(7):1530.
Román, E., J. M. M. Villares, F. D. Ortega, A. C. Martínez, L. P. Sirvent, L. Santana, J. C. Rivero, A. Alshweki, C. Cercamondi, and S. Dahbane. 2020. Real-world study in infants fed with an infant formula with two human milk oligosaccharydes. Nutrición hospitalaria: Órgano oficial de la Sociedad Española de Nutrición Clínica y Metabolismo (SENPE) 37(4):698-706.
Rozé, J.-C., S. Barbarot, M.-J. Butel, N. Kapel, A.-J. Waligora-Dupriet, I. De Montgolfier, M. Leblanc, N. Godon, P. Soulaines, and D. Darmaun. 2012. An α-lactalbumin-enriched and symbiotic-supplemented v. a standard infant formula: A multicentre, double-blind, randomised trial. British Journal of Nutrition 107(11):1616–1622.
Rozga, M., D. Handu, and L. Moloney. n.d. Infant formulas and human physical growth: A scoping review. osf.io/3nd5t (accessed September 13, 2024).
Rzehak, P., S. Sausenthaler, S. Koletzko, D. Reinhardt, A. von Berg, U. Krämer, D. Berdel, C. Bollrath, A. Grübl, and C. P. Bauer. 2009. Short- and long-term effects of feeding hydrolyzed protein infant formulas on growth at ≤6 y of age: Results from the German Infant Nutritional Intervention Study. American Journal of Clinical Nutrition 89(6):1846–1856.
Sandström, O., B. Lönnerdal, G. Graverholt, and O. Hernell. 2008. Effects of α-lactalbumin-enriched formula containing different concentrations of glycomacropeptide on infant nutrition. American Journal of Clinical Nutrition 87(4):921–928.
Scalabrin, D. M., S. H. Mitmesser, G. W. Welling, C. L. Harris, J. D. Marunycz, D. C. Walker, N. A. Bos, S. Tölkkö, S. Salminen, and J. A. Vanderhoof. 2012. New prebiotic blend of polydextrose and galacto-oligosaccharides has a bifidogenic effect in young infants. Journal of Pediatric Gastroenterology and Nutrition 54(3):343–352.
Schmelzle, H., S. Wirth, H. Skopnik, M. Radke, J. Knol, H.-M. Böckler, A. Brönstrup, J. Wells, and C. Fusch. 2003. Randomized double-blind study of the nutritional efficacy and bifidogenicity of a new infant formula containing partially hydrolyzed protein, a high β-palmitic acid level, and nondigestible oligosaccharides. Journal of Pediatric Gastroenterology and Nutrition 36(3):343–351.
Sepúlveda-Valbuena, N., A. Nieto-Ruiz, E. Diéguez, F. Herrmann, M. Escudero-Marín, R. De-Castellar, M. Rodríguez-Palmero, M. T. Miranda, J. A. García-Santos, and M. G. Bermúdez. 2021. Growth patterns and breast milk/infant formula energetic efficiency in healthy infants up to 18 months of life: The COGNIS study. British Journal of Nutrition 126(12):1809–1822.
Shahramian, I., G. Kalvandi, H. Javaherizadeh, M. Khalili, N. M. Noori, M. Delaramnasab, and A. Bazi. 2018. The effects of prebiotic supplementation on weight gain, diarrhoea, constipation, fever and respiratory tract infections in the first year of life. Journal of Paediatrics and Child Health. 54(8):875–880.
Shen, L., W. Huang, X. Xu, L. Wang, Q. Wang, S. Li, and X. Yuan. 2021. Stool saponified fatty acid, behavior, growth, and stool characteristics in infants fed a high-OPO formula: A randomized, double-blind clinical trial. Frontiers in Pediatrics 9:712201.
Singhal, A., K. Kennedy, J. Lanigan, H. Clough, W. Jenkins, A. Elias-Jones, T. Stephenson, P. Dudek, and A. Lucas. 2010. Dietary nucleotides and early growth in formula-fed infants: A randomized controlled trial. Pediatrics 126(4):e946–e953.
Szajewska, H., and A. Chmielewska. 2013. Growth of infants fed formula supplemented with Bifidobacterium lactis Bb12 or Lactobacillus GG: A systematic review of randomized controlled trials. BMC Pediatrics 13(1).
Szajewska, H., A. Skórka, and M. Pieścik-Lech. 2015. Fermented infant formulas without live bacteria: A systematic review. European Journal of Pediatrics 174(11):1413–1420.
Szajewska, H., M. Ruszczyński, H. Szymański, I. Sadowska-Krawczenko, A. Piwowarczyk, P. B. Rasmussen, M. B. Kristensen, C. E. West, and O. Hernell. 2017. Effects of infant formula supplemented with prebiotics compared with synbiotics on growth up to the age of 12 mo: A randomized controlled trial. Pediatric Research. 81(5):752–758.
Takahashi, N., H. Shoji, H. Arai, K. Tanaka, S. Kakiuchi, H. Yoda, and T. Shimizu. 2023. Effect of biotin supplementation in infant formula: A multi-center study in Japan. Pediatrics International 65(1):e15359.
Teoh, O. H., T. P. Lin, M. Abrahamse-Berkeveld, A. Winokan, Y. S. Chong, F. Yap, M. Marintcheva-Petrova, E. M. van der Beek, and L. P. Shek. 2022. An infant formula with large, milk phospholipid-coated lipid droplets supports adequate growth and is well-tolerated in healthy, term Asian infants: A randomized, controlled double-blind clinical trial. Nutrients 14(3):634.
Timby, N., E. Domellöf, O. Hernell, B. Lönnerdal, and M. Domellöf. 2014. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: A randomized controlled trial. American Journal of Clinical Nutrition 99(4):860–868.
Tinghäll Nilsson, U., O. Hernell, B. Lönnerdal, M. L. Hartvigsen, L. N. Jacobsen, A. S. Kvistgaard, and P. Karlsland Åkeson. 2023. Low-protein formulas with alpha-lactalbumin-enriched or glycomacropeptide-reduced whey: Effects on growth, nutrient intake and protein metabolism during early infancy: A randomized, double-blinded controlled trial. Nutrients 15(4).
Tinghäll Nilsson, U., B. Lönnerdal, O. Hernell, A. S. Kvistgaard, L. N. Jacobsen, and P. Karlsland Åkeson. 2024. Low-protein infant formula enriched with alpha-lactalbumin during early infancy may reduce insulin resistance at 12 months: A follow-up of a randomized controlled trial. Nutrients 16(7).
Tonon, K. M., T. M. Tomé, E. M. B. Mosquera, N. P. Perina, and T. Lazarini. 2021. The effect of infant formulas with 4 or 8 g/L GOS/FOS on growth, gastrointestinal symptoms, and behavioral patterns: A prospective cohort study. Global Pediatric Health 8.
Totzauer, M., V. Luque, J. Escribano, R. Closa-Monasterolo, E. Verduci, A. ReDionigi, J. Hoyos, J. P. Langhendries, D. Gruszfeld, and P. Socha. 2018. Effect of lower versus higher protein content in infant formula through the first year on body composition from 1 to 6 years: Follow-up of a randomized clinical trial. Obesity. 26(7):1203–1210.
Trabulsi, J., R. Capeding, J. Lebumfacil, K. Ramanujam, P. Feng, S. McSweeney, B. Harris, and P. DeRusso. 2011. Effect of an α-lactalbumin-enriched infant formula with lower protein on growth. European Journal of Clinical Nutrition 65(2):167–174.
Troesch, B., J. Demmelmair, M. Gimpfl, C. Hecht, G. Lakovic, R. Roehle, L. Sipka, B. Trisic, M. Vusurovic, and R. Schoop. 2019. Suitability and safety of L-5-methyltetrahydrofolate as a folate source in infant formula: A randomized-controlled trial. PLoS ONE 14(8):e0216790..
Turck, D., C. Grillon, E. Lachambre, P. Robiliard, L. Beck, J.-L. Maurin, C. Kempf, J.-P. Bernet, J. Marx, and F. Lebrun. 2006. Adequacy and safety of an infant formula with a protein/energy ratio of 1.8 g/100 kcal and enhanced protein efficiency for term infants during the first 4 months of life. Journal of Pediatric Gastroenterology and Nutrition 43(3):364–371.
Udell, T., R. A. Gibson, and M. Makrides. 2005. The effect of α-linolenic acid and linoleic acid on the growth and development of formula-fed infants: A systematic review and meta-analysis of randomized controlled trials. Lipids 40(1):1–11.
Vandenplas, Y., V. de Halleux, M. Arciszewska, P. Lach, V. Pokhylko, V. Klymenko, S. Schoen, M. Abrahamse-Berkeveld, K. A. Mulder, and R. Porcel Rubio. 2020. A partly fermented infant formula with postbiotics including 3′-GL, specific oligosaccharides, 2′-FL, and milk fat supports adequate growth, is safe and well-tolerated in healthy term infants: A double-blind, randomised, controlled, multi-country trial. Nutrients 12(11):1–17.
Veereman-Wauters, G., S. Staelens, H. Van de Broek, K. Plaskie, F. Wesling, L. Roger, A. McCartney, and P. Assam. 2011. Physiological and bifidogenic effects of prebiotic supplements in infant formulae. Journal of Pediatric Gastroenterology and Nutrition 52(6):763–771.
Vendt, N., H. Grünberg, T. Tuure, O. Malminiemi, E. Wuolijoki, V. Tillmann, E. Sepp, and R. Korpela. 2006. Growth during the first 6 months of life in infants using formula enriched with Lactobacillus rhamnosus GG: Double-blind, randomized trial. Journal of Human Nutrition and Dietetics 19(1):51–58.
Vlieger, A. M., A. Robroch, S. van Buuren, J. Kiers, G. Rijkers, M. A. Benninga, and R. te Biesebeke. 2009. Tolerance and safety of Lactobacillus paracasei ssp. paracasei in combination with Bifidobacterium animalis ssp. lactis in a prebiotic-containing infant formula: A randomised controlled trial. British Journal of Nutrition 102(6):869–875.
Wang, L., S. Mu, X. Xu, Z. Shi, and L. Shen. 2019. Effects of dietary nucleotide supplementation on growth in infants: A meta-analysis of randomized controlled trials. European Journal of Nutrition 58(3):1213–1221.
Wang, Y., Z. Li, J.-l. Wu, L. Zhang, M. Liu, M. Tan, A. Botma, M. Liu, K. A. Mulder, and M. Abrahamse-Berkeveld. 2021. A partially hydrolyzed formula with synbiotics supports adequate growth and is well tolerated in healthy, Chinese term infants: A double-blind, randomized controlled trial. Nutrition 91:111472.
Weizman, Z., and A. Alsheikh. 2006. Safety and tolerance of a probiotic formula in early infancy comparing two probiotic agents: A pilot study. Journal of the American College of Nutrition 25(5):415–419.
Wu, S.-L., D. Ding, A.-P. Fang, P.-Y. Chen, S. Chen, L.-P. Jing, Y.-M. Chen, and H.-L. Zhu. 2017. Growth, gastrointestinal tolerance and stool characteristics of healthy term infants fed an infant formula containing hydrolyzed whey protein (63%) and intact casein (37%): A randomized clinical trial. Nutrients 9(11):1254.
Xia, Y., B. Jiang, L. Zhou, J. Ma, L. Yang, F. Wang, H. Liu, N. Zhang, X. Li, and P. Petocz. 2021. Neurodevelopmental outcomes of healthy Chinese term infants fed infant formula enriched in bovine milk fat globule membrane for 12 months—A randomized controlled trial. Asia Pacific Journal of Clinical Nutrition 30(3):401–414.
Xu, M., Y. Wang, Z. Dai, Y. Zhang, Y. Li, and J. Wang. 2015. Comparison of growth and nutritional status in infants receiving goat milk-based formula and cow milk-based formula: A randomized, double-blind study. Food and Nutrition Research 59.
Yang, J., S. I. Yang, K. Jeong, K. W. Kim, Y. H. Kim, T. K. Min, B. Y. Pyun, J. Lee, J. A. Jung, and J. H. Kim. 2022. A partially hydrolyzed whey formula provides adequate nutrition in high-risk infants for allergy. Nutrition Research and Practice 16(3):344–353.
Yeiser, M., C. L. Harris, A. L. Kirchoff, A. C. Patterson, J. L. Wampler, E. N. Zissman, and C. L. Berseth. 2016. Growth and tolerance of infants fed formula with a new algal source of docosahexaenoic acid: Double-blind, randomized, controlled trial. Prostaglandins Leukotrienes and Essential Fatty Acids 115:89–96.
Yin, N., X. Liu, X. Zhang, J. Wen, H. Ma, X. Yin, C. K. Xie, Y. Hou, and J. Wang. 2023. Comparison of the effects of different infant formulas on the growth and development and intestinal flora of infants. Food Science and Nutrition 11(2):1113–1126.
Zhang, Z., Y. Wang, Y. Li, X. Lin, Z. Hong, J. Huang, X. Zhang, Y. Yang, and Y. Su. 2023. Effects of Sn-2-palmitate-enriched formula feeding on infants’ growth, stool characteristics, stool fatty acid soap contents and bone mineral content: A systematic review and meta-analysis of randomized controlled trials. Critical Reviews in Food Science and Nutrition 63(30):10256–10266.
Zhou, S. J., T. Sullivan, R. A. Gibson, B. Lönnerdal, C. G. Prosser, D. J. Lowry, and M. Makrides. 2014. Nutritional adequacy of goat milk infant formulas for term infants: A double-blind randomised controlled trial. British Journal of Nutrition 111(9):1641–1651.
Ziegler, E., J. A. Vanderhoof, B. Petschow, S. H. Mitmesser, S. I. Stolz, C. L. Harris, and C. L. Berseth. 2007. Term infants fed formula supplemented with selected blends of prebiotics grow normally and have soft stools similar to those reported for breast-fed infants. Journal of Pediatric Gastroenterology and Nutrition 44(3):359–364.
Ziegler, E. E., D. A. Fields, S. D. Chernausek, P. Steenhout, D. Grathwohl, J. M. Jeter, S. E. Nelson, and F. Haschke. 2015. Adequacy of infant formula with protein content of 1.6 g/100 kcal for infants between 3 and 12 months. Journal of Pediatric Gastroenterology and Nutrition 61(5):596–603.