The Role of Seafood Consumption in Child Growth and Development (2024)
Chapter: Appendix C: Commissioned Systematic Reviews
C
Commissioned Systematic Reviews
BACKGROUND
The Agriculture, Food, and Nutrition Evidence Center at Texas A&M University (Evidence Center) was contracted to perform three systematic reviews examining the associations between seafood nutrition and toxicant intake during pregnancy, lactation, and child growth and development. These reviews addressed three key questions:
- What are the associations between seafood consumption during pregnancy and lactation and child growth and development?
- What are the associations between seafood consumption during childhood and child growth and development?
- What are the associations between seafood toxicant exposure during pregnancy, lactation, and childhood and child growth and development?
The Evidence Center was asked to update two existing systematic reviews previously published by the USDA Nutrition Evidence Systematic Review Center conducted to inform the Dietary Guidelines for Americans 2020–2025 (DGA) that examined the relationship between seafood nutrition and health outcomes among pregnant and lactating women, as well as children (USDA/HHS, 2020). These two reviews are collectively referred to as the “nutrition reviews.” In addition, a third de novo systematic review was requested to examine associations of seafood-related contaminants (toxicological) with health outcomes during pregnancy, lactation, and childhood on child growth and development. This review is referred to as the “toxicology review.” For the toxicology systematic review, a scoping review was conducted to prioritize exposure–outcome associations with sufficient evidence to warrant a full systematic review. Relevant data and information for the systematic reviews was provided to the Evidence Center by the committee. The searches were run by the National Academies Resource Center librarian, and search results were provided to the Evidence Center. The Evidence Center drafted the review protocol, including relevant methodology, based on the provided information. Protocols for the three reviews were registered in PROSPERO (CRD42023432844).1 Supplemental online Appendix F provides the full search strategy for each systematic review. The full methodology report from the Evidence Center is available in online Appendix H. The
___________________
data extraction tables and lists of articles retrieved and evaluated by the Evidence Center are also described in online Appendix H.2
METHODOLOGY
PECOD Analytic Framework and Inclusion and Exclusion Criteria for Nutrition Reviews
Analytic Frameworks for Nutrition Reviews
The Evidence Center developed an analytic framework for the nutrition reviews. Figure C-1 shows the framework used for examining the associations of seafood consumption during pregnancy and lactation and neurocognitive development in the child. A similar framework was devised to examine associations of seafood consumption during childhood and adolescence and neurocognitive development in children. A complete description of the review methodology is provided in online Appendix H.
Inclusion and Exclusion Criteria
Inclusion and exclusion terms were provided by the committee and a PECOD (population, exposures, comparators, outcomes, designs [of studies]) table constructed for each nutrition review. Table C-1 shows the criteria
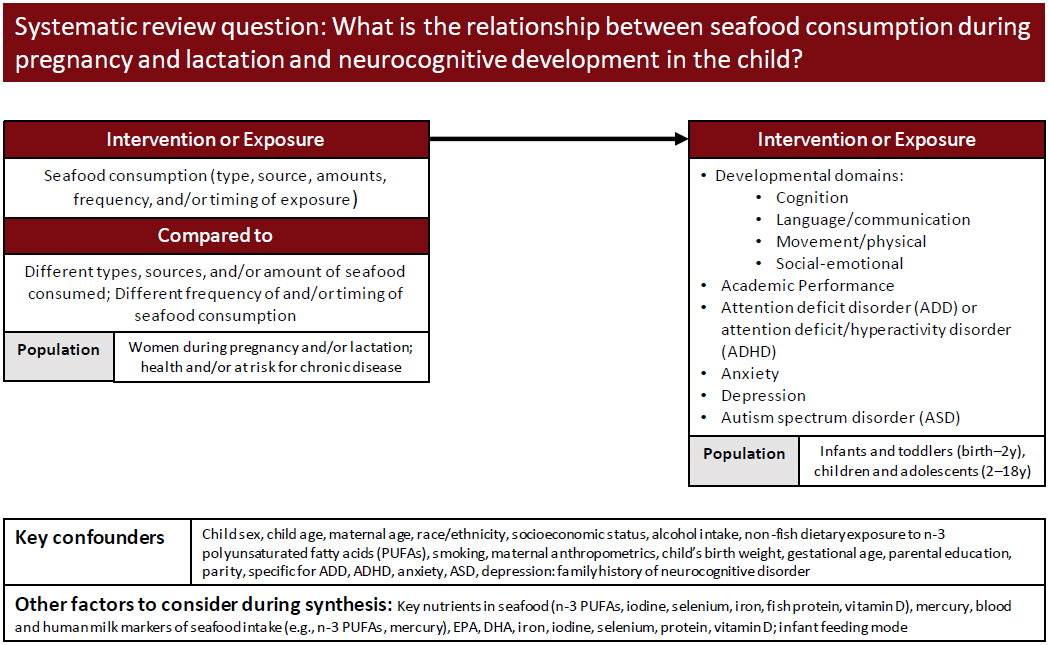
SOURCE: Texas A&M Agriculture, Food, and Nutrition Evidence Center, 2023.
___________________
2 Appendixes F through H can be found online at https://nap.nationalacademies.org/catalog/27623.
TABLE C-1 Inclusion and Exclusion Criteria for Associations of Seafood Consumption During Pregnancy and Lactation and Neurocognitive Development in the Child
| Component | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Populations |
Individuals living in countries ranked as high or very high on the human development index during the studya
|
|
| Exposures |
|
|
| Comparators: |
|
No comparator |
| Outcomes |
Neurodevelopment and neurodevelopmental disorders
|
|
| Study Designs |
|
|
a https://worldpopulationreview.com/country-rankings/hdi-by-country.
SOURCE: Texas A&M Agriculture, Food, and Nutrition Evidence Center, 2023.
developed for associations of seafood consumption during pregnancy and lactation and neurocognitive development in the child.
Screening
All records captured in the search were screened independently by two reviewers. Screening occurred within a web-based program (DistillerSR) using screening forms developed based on the inclusion and exclusion criteria determined a priori. Each article was reviewed to determine if it met the inclusion criteria, in which case the article was included, or if any of the exclusion criteria were met, in which case the article was excluded.
Screening was conducted in three stages or levels following the methodology of the original existing review. In level 1, the title of the article was reviewed. Title screening was used to exclude clearly irrelevant studies. Potential reasons for exclusion at the title level included wrong study population or country. If there was not a clear reason for exclusion, the article was included and moved to level 2, abstract screening. If there was no reason to exclude the article based on information in the abstract, it was included and moved to level 3, full-text screening. When an article was excluded at level 2 (abstract) or level 3 (full text) the screener indicated at least one reason for exclusion. Any disagreements on whether to include or exclude an article were discussed and resolved by the two screeners. If necessary, a third party was consulted to resolve differences.
Piloting was done to ensure the screening forms were adequate and that screeners interpreted the eligibility criteria similarly. For the pilot, screeners reviewed a common set of references at each screening level. The screeners discussed their responses, any questions or uncertainties they had when making their decision, and any concerns regarding the screening form. If necessary, this was repeated with another common set of references.
Manual searching was performed on all articles included after full-text screening. If a reference was found to be relevant to the present review that was not identified in the electronic search, it went through the screening process as detailed above. If an article identified through manual searching was included in the review, the librarian was notified to determine why the article was not found through the electronic search. If necessary, the search strategy would be updated and rerun, and newly identified articles would go through the screening process.
Data Extraction
Data from all included articles were extracted by a trained analyst using a systematic approach. Only data relevant to the review were extracted. To ensure data were extracted in a consistent manner for all articles, standard data extraction forms were used. Data fields for extraction were based on information outlined in the protocol and included important characteristics of the study design, methodology, results, and limitations. Data extraction was piloted on two or three articles (varying in study design, when appropriate) by all reviewers to ensure all relevant information was recorded and done so in a consistent manner. For the nutrition reviews, data extraction forms included similar fields as the existing reviews (see online Appendix H for data extraction fields for the nutrition review update). A second analyst reviewed the extracted data for accuracy and completeness. Any suggested changes were discussed between the reviewers. If necessary, a third analyst was consulted.
Risk-of-Bias Assessment
Risk of bias was assessed independently by two analysts using standardized tools specific to each study’s design for all included studies. If a study included multiple relevant results, the analysts assessed the risk of bias pertinent to each. If there were differences in risk of bias for the different results, more than one risk-of-bias assessment was reported for a paper. Cochrane risk-of-bias tools specific to the included study designs were used. These included ROB 2.0 for randomized controlled trials, Risk of Bias in Non-Randomized Studies–of Interventions (ROBINS-I), and Risk of Bias in Non-Randomized Studies–of Exposures (ROBINS-E) for nonrandomized studies of exposures. The analysts piloted the tools on two or three articles per study design to ensure a consistent approach and interpretation. Upon completion of the dual, independent risk-of-bias assessments, domain-level ratings and
the overall rating were compared between the two reviewers to assess interrater reliability. If there were differences, the reviewers discussed and determined the appropriate rating. If necessary, a third reviewer was consulted.
Data Synthesis
Synthesis of the evidence was conducted by the committee. To prepare for synthesis, a description of the evidence was drafted to provide details on the body of evidence including but not limited to the number of included articles, the number of included studies, study designs, country of origin, participant characteristics, description of the exposure across studies, outcomes, and outcome assessment tools.
PECOD Analytic Framework and Inclusion and Exclusion Criteria for Toxicology Review
Prior to conducting systematic reviews on toxicants from seafood consumed during pregnancy, lactation, childhood, or adolescence on child development and health outcomes, the Evidence Center conducted a scoping review to identify (1) toxicant exposures with sufficient evidence to warrant a systematic review, and (2) gaps in the evidence. This allowed the committee to prioritize exposure–outcome relationships that warranted systematic review. The initial scoping review and subsequent de novo systematic review for the toxicology review followed the same protocol as described above for the nutrition reviews.
Using terms provided by the committee, the Evidence Center developed an analytic framework for examining associations of seafood toxicant exposure during pregnancy, lactation, and childhood and child growth and development (Figure C-2).
Inclusion and exclusion terms were provided by the committee and a PECOD table constructed for the toxicology scoping review. Table C-2 shows the criteria developed for associations of seafood toxicant exposure during pregnancy, lactation, and childhood and child growth and development.
The committee also expressed an interest in capturing and evaluating the evidence related specifically to mercury exposure. The inclusion criteria applied in the scoping review required that studies report both fish and seafood intake as an exposure and a toxicant exposure, with demonstration of the associations between fish and/or seafood exposure to the toxin and/or the outcome. However, given that the primary source of mercury exposure is through fish and seafood intake, the committee was interested in examining the association between mercury and child health outcomes using studies that did not explicitly report fish or seafood intake.
Grading the Strength of the Evidence
A rating of the certainty of the evidence was determined for each conclusion using the GRADE3 approach. This includes an evaluation of the evidence by study design. For randomized controlled trials, the domains considered when determining the rating include risk of bias, indirectness, inconsistency, imprecision, and publication bias. Additional domains to be considered for other study designs included dose–response, magnitude of effect, and plausible confounding. Ratings were defined as high, moderate, low, or very low certainty.
RESULTS
Nutrition Systematic Reviews
The number of included articles from the combined nutrition updated systematic reviews are shown in Table C-3. Summary tables of the study characteristics, risk of bias, and outcomes for the nutrition reviews are provided in online Appendix H.
___________________
3 GRADE = Grading of Recommendations, Assessment, Development and Evaluation.
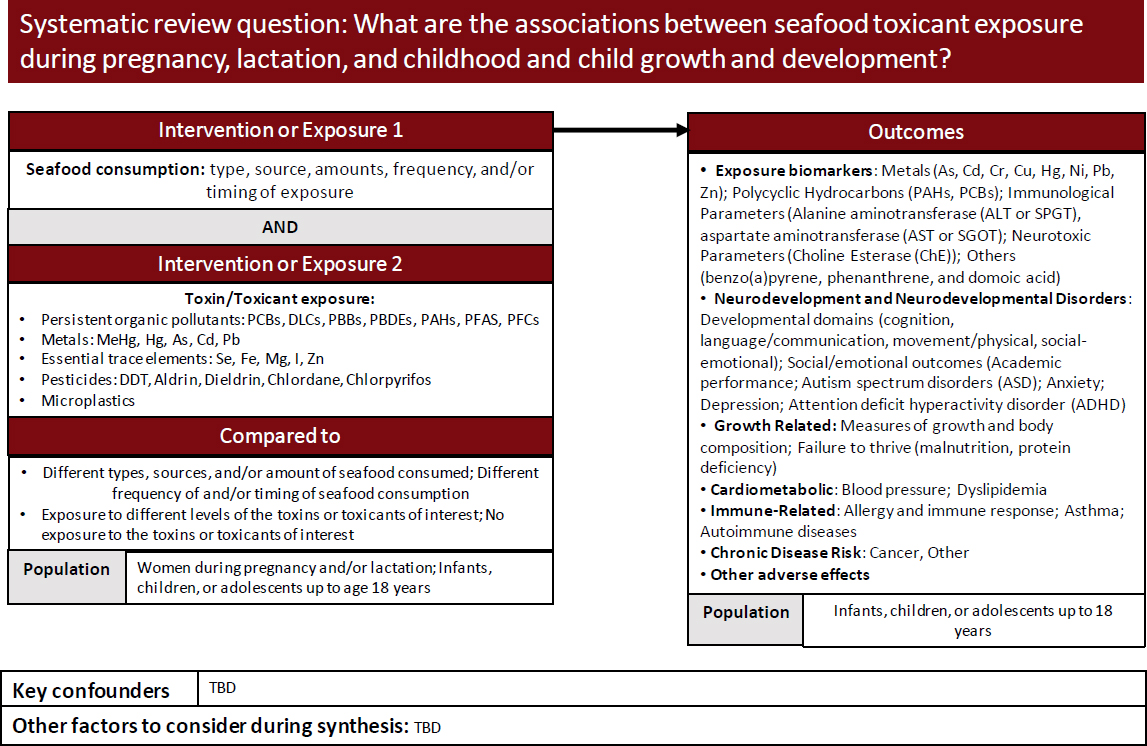
NOTE: As = arsenic; Cd = cadmium; Cr = chromium; Cu = copper; DDT = dichloro-diphenyl-trichloroethane; Fe = iron; Hg = mercury; I = iodine; MeHg = methylmercury; Mg = magnesium; Ni = nickel; PAHs = polycyclic aromatic hydrocarbons; Pb = lead; PBBs = polybrominated biphenyls; PBDEs = polybrominated diphenyl ethers; PCBs = polychlorinated biphenyls; PFAS = per- and polyfluoroalkyl substances; PFCs = perfluorochemicals; Se = selenium; Zn = zinc.
SOURCE: Texas A&M Agriculture, Food, and Nutrition Evidence Center, 2023.
TABLE C-2 PECOD Framework for Toxicology Review Inclusion and Exclusion Criteria
| Component | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Populations |
Human individuals living in countries ranked as high or very high on the human development index during the study.a
|
|
| Exposures: |
Exposure 1: Toxin or toxicants
Exposure 2: Seafood consumption
|
| Component | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Comparators |
|
No comparator |
| Outcomes |
Exposure biomarkers/indicators/levels (e.g., arsenobetaine) Neurodevelopment and Neurodevelopmental Disorders:
Growth-Related
Cardiometabolic
Immune-Related
Chronic Disease Risk
Other adverse effects |
|
| Study Designs |
|
|
a https://worldpopulationreview.com/country-rankings/hdi-by-country.
b A list of nonhuman primate and cross-sectional studies, as well as studies with algal toxin and microorganism exposures will be provided to the sponsor.
c Case reports will be excluded but will be reviewed on a case-by-case basis. If a case report meets the inclusion criteria, it will be included for further review.
SOURCE: Texas A&M Agriculture, Food, and Nutrition Evidence Center, 2023.
TABLE C-3 Nutrition Reviews: Number of Included Articles by Outcome Domains
| Outcome | Exposure | |
|---|---|---|
| Seafood Consumption During Childhood and Adolescence | Seafood Consumption During Pregnancy and Lactation | |
| Developmental domains | ||
| Cognition | 10 | 28 |
| Language/communication | 0 | 13 |
| Movement/physical | 3 | 16 |
| Social-emotional | 3 | 10 |
| Academic performance | 1 | 0 |
| Attention deficit disorder (ADD) or attention-deficit/hyperactivity disorder (ADHD) | 1 | 4 |
| Anxiety | 0 | 0 |
| Depression | 2 | 0 |
| Autism spectrum disorder (ASD) | 0 | 4 |
SOURCE: Texas A&M Agriculture, Food, and Nutrition Evidence Center, 2023.
Toxicology Systematic Review
Toxicology Scoping Review
A risk-of-bias assessment was not carried out for the toxicology scoping review. After extraction of study characteristics of eligible articles, two toxicant exposure–prioritized outcome pairs were identified to proceed with de novo reviews. These were polychlorinated biphenyls (PCBs) and growth and body composition (n = 4); and lead and developmental domains (n = 3). Summary tables of the study characteristics, risk of bias, and outcomes for lead and PCBs are provided in online Appendix H.
Toxicology Systematic Reviews
The articles included in the scoping review were reorganized by toxicant and prioritized outcome for each of the two exposure populations (women who are pregnant or lactating and children and adolescents). Any toxicant exposure–prioritized outcome pair with three or more articles was determined to have sufficient data for conducting a de novo systematic review. For the full systematic review, screening, data extraction, risk-of-bias assessment, and evidence synthesis were carried out as described above for the nutrition reviews.
EVALUATION OF EXISTING SYSTEMATIC REVIEWS FOR MERCURY
In addition to the updated nutrition reviews and the de novo toxicology review, the committee expressed an interest in capturing and evaluating the evidence related specifically to mercury exposure. The inclusion criteria applied in the scoping review required that studies report both fish and/or seafood intake as an exposure and a toxicant exposure, with demonstration of the associations between fish and/or seafood exposure to the toxin and/or the outcome. However, given that the primary source of mercury exposure is through fish or seafood consumption, the committee was interested in examining the association between mercury and child health outcomes using studies that did not explicitly report fish/seafood intake.
To determine whether an existing systematic review could be used the relevancy, timeliness, and quality was assessed as follows:
- Relevancy is assessed by comparing PICO4 elements of the existing review(s) to the desired review.
- Timeliness is based on the time of the literature search. What is considered “timely” will depend on the topic considering the volume of research being published and advancement in research methods.
- Quality of a systematic review is assessed using the AMSTAR 2 tool.
Given the large number of primary studies related to mercury exposure and child development, the committee’s request to expand the inclusion criteria to include studies without measures of fish/seafood intake related to mercury exposure, and the likelihood of an existing relevant, recent systematic review, the decision was made to search the literature for relevant, timely, and good quality systematic reviews on mercury exposure during pregnancy, lactation, childhood, or adolescence on child health and development outcomes.
Methodology
The Evidence Center’s information scientist conducted a search to identify existing recent relevant systematic reviews that examined the relationship between mercury exposure during pregnancy, lactation, childhood, or adolescence on child health and development outcomes including dates from 2020 to present. As described above, two reviewers screened all results from the search at the full-text level. Conflicts were resolved by a third reviewer.
The AMSTAR 2 quality assessment tool was used to assess the quality of included systematic reviews. The tool includes 16 items that were rated as “Yes,” “Partial Yes,” “No,” or “No meta-analysis conducted.” Some items were adapted for this review to account for the observational nature of the included studies. Two independent assessments were performed for each included review. Disagreements were discussed and resolved by the two reviewers. For the purposes of this review, an overall summary rating was determined for each systematic review by summing the item ratings (Yes = 1; Partial Yes = 0.5; No = 0; N/A = 1). Reviews that scored 8 or more (≥ 50 percent) were considered to be moderate-high quality; reviews that scored less than 8 (< 50 percent) were considered to be lower quality.
Results
A total of 53 articles were identified in the search for existing systematic reviews related to the association between mercury exposure during pregnancy, lactation, or childhood and child outcomes. After dual full-text screening, 12 articles were included. Existing systematic reviews were identified for all but two prioritized outcomes. No articles were identified in the search related to blood pressure; however, a review from 2019 was identified through manual searching and included. Table C-4 shows the identified systematic reviews by prioritized outcome for child health. The complete extracted data for mercury are provided in online Appendix H.
___________________
4 PICO = Population, Intervention, Comparator, Outcome.
TABLE C-4 Systematic Reviews for Mercury: Number of Included Articles by Outcome Categories
| Prioritized Outcome | Number of Systematic Reviews |
|---|---|
| Neurological disorders—ASD | 5 |
| Developmental domains | 3 |
| Growth—measures of growth or body composition | 3 |
| Biomarkers—gene expression | 1 |
| Neurological disorders—ADHD | 1 |
| Cardiometabolic—blood pressure | 1 |
| Immune-related—allergy, immune response | 1 |
| Academic performance | 0 |
| Growth—failure to thrive | 0 |
SOURCE: Texas A&M Agriculture, Food, and Nutrition Evidence Center, 2023.
REFERENCE
USDA/HHS (U.S. Department of Agriculture and U.S. Department of Health and Human Services). 2020. Dietary Guidelines for Americans, 2020–2025. 9th ed. dietaryguidelines.gov.
This page intentionally left blank.











