Veterans, Prescription Opioids and Benzodiazepines, and Mortality, 2007–2019: Three Target Trial Emulations (2025)
Chapter: Appendix E: Opioid Tapering and All-Cause Mortality, Including Suicide Mortality
E
Opioid Tapering and All-Cause Mortality, Including Suicide Mortality
INTRODUCTION
The committee was tasked with evaluating the relationship between prescribed opioid exposure and all-cause mortality among veterans. As explicitly stated in the statement of task, one of the studies conducted by the committee focused on the effect of opioid escalation on mortality. The statement of task did not direct the committee to examine the effect of opioid tapering on mortality. However, tapering among those taking opioids long term is one potential aspect that could impact mortality risk. Opioid tapering practices are almost always driven by clinicians’ individual-specific concerns, which are often not well documented (e.g., potential opioid misuse behaviors do not have an International Classification of Diseases code and may not be reflected in electronic health records). The committee notes that differences in opioid escalation practice are often due to clinical practice variation and adequate control of pain at the current dose, captured through time-varying pain intensity rating, which the committee does in study 2, Chapter 6 (effect of opioid escalation on all-cause mortality and suicide mortality).
In this report, the committee reviewed the work and opioid tapering protocol developed by the 2019 National Academies of Sciences, Engineering, and Medicine (the National Academies) committee. That committee determined that tapering was increasingly common in clinical practice, with clinical uncertainty around it. Specifically, it noted that it was unclear whether tapering to avoid known adverse events of opioid use, such as mortality, was safer than not doing so, given the increased risk of adverse events, such as suicide, that could be precipitated by stopping treatment, especially for individuals who are dependent on opioids. Anecdotal reports of individual harm resulting from discontinuation and tapering had emerged, but little empirical research had been conducted on the topic (Covington et al., 2020). Before attempting to implement the 2019 protocol or a revised one, the committee commissioned a review examining the effect of opioid tapering on mortality, the methodological limitations and study design issues, and currently funded randomized controlled trials examining opioid tapering. The appendix concludes with the committee’s observations on the potential utility of these studies in examining the causal effect of opioid tapering on all-cause mortality, including suicide mortality.
2019 NATIONAL ACADEMIES COMMITTEE’S OPIOID TARGET TRIAL TAPERING PROTOCOL
The National Academies (2019) proposed target trial for opioid tapering included eligibility criteria of individuals with long-term opioid treatment (LTOT) (3+ opioid prescriptions dispensed ≥21 days apart in an ≥84-day
period for ≥84-day supply) with an average opioid morphine milligram equivalent (MME)/day of ≥30 over the prior 84 days. Eligible individuals would all be receiving long-term benzodiazepine pharmacotherapy. Individuals would be randomized to one of four opioid tapering treatment strategies (a) no reduction (≤5 percent average decrease per month for 3 months), (b) slow reduction (>5 percent to ≤10 percent average decrease per month for 3 months), (c) moderate to fast reduction (>10 percent average decrease per month for 3 months), and (d) complete discontinuation within 3 months from baseline. The committee noted that “there is a lack of primary literature on the optimal rate of tapering speed.” Start of follow-up would begin at baseline, and individuals would be followed for 6 months. Outcomes of interest were all-cause and suicide mortality. Causal contrast is intent-to-treat and per protocol effects. In terms of statistical analysis, the 2019 committee noted that an individual’s baseline data may correspond to any of the opioid treatment strategies. One approach to address this is to create a clone of each strategy for each individual. The 2019 committee identified several challenges in developing the opioid tapering protocol, including “the appropriate classification of the intervention categories represents a specific challenge as it requires inference of the intended treatment strategy (e.g., continuation, slow taper, fast taper) from multiple consecutive prescription fills” and that guidelines and prescribing practices continue to evolve (see also Chapter 2 or Appendix D for more details regarding policy changes).
SUMMARY OF OPIOID TAPERING STUDIES
In the few years since the publication of the 2019 National Academies report, there have been a number of observational studies on opioid tapering. In evaluating the published literature, the committee commissioned a systematic review conducted by the University of Pennsylvania’s Penn Medicine Center for Evidence-Based Practice (CEP), which appears as a supplement at the end of this appendix. The review was conducted in November 2023, and identified 31 studies of opioid tapering in U.S. adults. The majority of these studies were published after the National Academies report (2019), with 22 during 2019 or later. The review applied standard methods to assess the scientific rigor and potential for bias of the studies. The committee noted that this approach is relatively conservative, in that the overall risk-of-bias rating can be no better than the lowest component rating. Many of the 31 observational studies were considered to have a “moderate” risk of bias, indicating that the designs were sound but not equivalent to a randomized controlled trial (RCT). Three studies reported all-cause mortality as an outcome (Binswanger et al., 2022; James et al., 2019; Krebs et al., unpublished), and only the Krebs study reported suicide mortality as an outcome (see Table E-1); all were evaluated as having a “moderate” risk of bias (see Annex). The unpublished study was presented and discussed with the committee in a public session; it was commissioned by the Department of Veterans Affairs (VA) to specifically carry out the 2019 National Academies opioid tapering trial protocol and appears in Appendix F of this report.
The studies suggest that opioid tapering was not associated with all-cause mortality (James et al., 2019; Binswanger et al., 2022; Krebs et al., unpublished) (see Table E-1). Both James and colleagues (2019) and Binswanger and colleagues (2022) were retrospective cohort designs. The Krebs study was an emulated opioid tapering trial using retrospective observational data from a cohort of individuals with LTOT for chronic pain and in VA primary care. The study examined two dose reduction strategies—small and large—and compared to those with no dose reduction over 12 months. Outcomes of interest were all-cause and suicide mortality. The results demonstrated no difference in all-cause mortality between a small versus no dose reduction (aHR1: 1.01; 95% CI2: 0.98–1.04). There was a statistically significant increase in all-cause mortality risk between those with a large versus no dose reduction (aHR: 1.23; 95% CI: 1.20–1.27). For suicide mortality, comparing either small or large dose reduction to those with no dose reduction resulted in no significant difference. It was noted that the study’s findings and conclusions were highly sensitive to different study design, statistical modeling, and measurement strategies. In addition, the treatment assignment was based on pharmacy records and as such may not accurately reflect whether a tapering was intended or actually took place.
___________________
1 aHR: adjusted hazards ratio.
2 CI: confidence interval.
TABLE E-1 Studies Examining the Effect Opioid Tapering On All-Cause and Suicide Mortality
| Study and Population | Results | Overall Risk of Bias |
|---|---|---|
| All-Cause Mortality | ||
|
Binswanger et al., 2022 N = 3,913 Non-veterans |
Decreasing dose trajectory, compared to stable trajectories, was not associated with all-cause mortality. aHR: 1.28; 95% CI: 0.87–1.86. | Moderate |
|
James et al., 2019 N = 572 Non-veterans |
Discontinuation was not associated with a significant risk for all-cause mortality. HR: 1.35; 95% CI: 0.92–1.98; p = 0.122. | Moderate |
|
Krebs et al., unpublished N = 207,204 Veterans |
No difference was reported in risk of all-cause mortality between small dose reduction group and control group. HR: 1.01; 95% CI: 0.98–1.04. Large dose reduction group had a small, statistically significant increase in all-cause mortality risk compared to the control group. HR: 1.23; 95% CI: 1.20–1.27. |
Moderate |
| Suicide Mortality | ||
|
Krebs et al., unpublished N = 207,204 Veterans |
No difference in risk of suicide mortality was reported for small dose reduction group versus control group. HR: 1.09; 95% CI: 0.89–1.35. No difference in risk of suicide mortality was reported for large dose reduction group versus control group. HR: 1.01; 95% CI: 0.77–1.33. Individuals who died during 1-year follow-up had no difference in risk of suicide mortality (as opposed to other cause of death) for either treatment comparison. |
Moderate |
NOTES: aHR = adjusted hazard ratio; CI = confidence interval; HR = hazard ratio.
Based on the presentation and discussion with Dr. Krebs and review of the two study results, the committee learned that the findings and conclusions of these studies are highly sensitive to study design, statistical modeling, and measurement choices. In the Krebs unpublished paper, additional limitations include that the treatment assignment of an individual was based on information at a point in time and may not reflect the dynamic nature of treatment strategies. Additional factors may also not be reflected, such as multiple and overlapping opioid prescriptions. Unmeasured confounding may still exist (such as discontinuation due to opioid misuse or clinical indications, which are not well reflected in clinical or administrative data). In other words, study results and conclusions vary substantially based on the choices made by researchers, even within a set of choices that are all reasonable. Because studies used different definitions of tapering and discontinuation and a wide range of designs, the committee felt that caution should be taken in directly comparing the effect estimates from the different studies.
In evaluating these opioid tapering studies, many on the committee raised concerns of entrenched problems that cannot be overcome when studying a clinical question so complex and ill defined, concerns that were also noted as limitations in the 2019 report. Most notable is a high potential for the presence of confounders that cannot be fully measured because the decision to taper or discontinue opioids is often driven by factors that are themselves causes of early death, such as developing opioid misuse or addiction. In addition, treatment selection bias can occur if the individuals who taper are intrinsically different from those who do not. That is very likely to be true of this research question, as the decision to taper or discontinue is often driven by individual behavior, such as risky opioid use, or adverse effects (Lovejoy et al., 2017).
Documentation of these confounding factors is likely incomplete. Significant challenges also arise with immortal time bias—which stems from using post-baseline treatment information for treatment group assignment. Specifically, aligning eligibility, treatment assignment, and the start of follow-up is essential to avoid immortal time and other key biases in observational research (Hernán et al., 2016). Tapering is measured through refills of decreasing dosage and, by definition, requires follow-up time to observe refills, making it impossible to align these
three-time anchors to the same day. Designs such as clone-censor-weight and parametric G-formula can address this misalignment, as in other studies in the report, but these were used in very few of the reviewed studies. These problems have become more evident as multiple independent research groups carried out work in this area. In the next section, the committee considers these problems in greater detail.
LIMITATIONS AND CHALLENGES OF OPIOID TAPERING TARGET TRIAL
The committee notes several limitations and/or challenges in designing the opioid tapering target trials using retrospective observational data to infer causality, including immortal time bias, treatment selection bias, and measurement bias (misclassification of the intervention and/or outcomes).
Immortal Time Bias
For any observational study, aligning the timing of the three crucial factors of eligibility, start of treatment, and start of follow-up is critical to avoiding immortal person time and other forms of bias. For opioid tapering, the exact time at which it starts is often ambiguous even when the decision making of the individual with evidence of LTOT and prescriber are known and even more so when ascertained by clinical or administrative records. For example, the prescriber and individual may decide to begin by increasing the time interval between doses, effectively increasing the number of days covered by an existing prescription. In clinical and administrative data, the decision would only become apparent over time when the prescription is refilled. In other words, defining opioid tapering from dose trajectories in administrative records requires post-eligibility follow-up time. If that is concurrent to the time that tapering is being measured, the study will be inherently flawed by immortal person time. This is because future information (refills or lack thereof) is used to retrospectively determine treatment group; the period before enough information is available to classify treatment group is “immortal” because an individual cannot die and be correctly classified. While alternative designs can avoid immortal person time, any misalignment of the three critical time anchors will result in bias. For example, if researchers use a 3-month period to define trajectories based on dispensed opioids (slow, fast, abrupt) and begin follow-up at the end of the 3 months, this avoids immortal person time. However, it conditions the analysis on surviving the 1-month treatment strategy identification period, which introduces selection bias. A particularly problematic form of selection bias occurs when immortal person time exists for some treatment groups but not others, such as when the days covered by dispensed opioids have lapsed with no immediate refill. Without future information on opioid refills, it can be impossible to distinguish whether this is the beginning of a discontinuation or a meaningless gap caused by non-consecutive pharmacy dispensed dates. However, using future refill information could result in immortal time for only those ultimately deemed to be continuing opioids, and treatment would appear safer than it is. The direction of the bias caused by the immortal time will vary depending on the specifics of the treatment group definitions but can be severe enough to “flip” the direction of the relationships.
When data at time zero are insufficient to assign a treatment strategy, a number of general approaches exist to handle this issue without inducing bias: randomly assign the individual to a strategy consistent with their treatment (slow, fast, or abrupt), create exact copies (“clones”) of the individual for each possible strategy that their data are consistent with at that time and censor observations when the prescription data are no longer consistent with a given treatment strategy, or use the parametric G-formula (as in study 2, Chapter 6) (Hernán and Robins, 2016). The committee notes that the clone approach, which was recommended by the 2019 report, was used in a small number of the studies included in the synthesis presented earlier in this appendix.
Treatment selection bias can occur if the individuals who taper are intrinsically different from those who do not. That is very likely to be true of this research question (Lovejoy et al., 2017). Documentation of these confounding factors is likely incomplete. For example, tapering could be more likely for individuals with uncontrolled psychiatric symptoms not noted in the medical records or less likely for individuals with pain and comorbid psychiatric symptoms that are being actively treated.
Misclassification of opioid tapering (intervention). The intervention strategy for tapering (slow, fast, abrupt) is inferred from dispensed prescription information. This is necessary to examine outcomes such as all-cause and suicide mortality, which require large sample sizes that would not be possible to achieve under study designs that
ascertain treatment choice through more direct means. However, consecutive dispensed prescription information may not coincide with the intended strategy, which could lead to misclassification bias of exposure.
In summary, given the challenges in the design of retrospective observational studies, and the risk of immortal time and selection biases, the committee finds that these challenges and limitations substantially limit how well such studies can infer causality between opioid tapering and mortality and are likely to contribute to inconclusive results. For this reason, the committee chose not to implement the tapering trial suggested in the 2019 report.
SUMMARY OF ONGOING RANDOMIZED CLINICAL TRIALS
Given the concerns about unmeasured confounding in observational designs, the committee reviewed databases (NIH RePORTER, PCORI Portfolio, and ClinicalTrials.gov) of currently funded studies and identified several ongoing and/or recently completed RCTs that directly or indirectly test opioid tapering protocols in June 2024, including 14 ongoing RCTs of various tapering interventions on long-term opioid use (see Table E-2). Three trials directly compare the effectiveness of different tapering protocols, and six trials will examine the effect of pharmacotherapies (buprenorphine, buspirone, tizanidine) on opioid reduction. Six trials will assess the effectiveness of cognitive behavior therapy interventions on opioid reduction. Several trials focus on outcomes of importance to individuals with evidence of LTOT, including pain and functioning, and most are not powered to examine mortality as an outcome, but as the trials are complete, it may be possible to combine their data via meta-analysis to examine mortality.
In reviewing the protocols for the ongoing trials, the committee noted that it avoided the limitations described and largely used pragmatic designs that are relevant to “real-world” practices to manage pain. However, recent studies suggest that opioid tapering may be unwelcome by many individuals on LTOT (Nevedal et al., 2022; Kosakowski et al., 2022; Lovejoy et al., 2017), so the trials may be subject to selection effects related to who is willing to be randomized for opioid tapering.
After reviewing the ongoing and recently completed RCTs, the committee notes that these studies will soon provide critical data relevant to understanding the potential harms and benefits of opioid tapering on a number of outcomes without the major methodological issues associated with retrospective observational studies. However, these trials are likely to be sufficiently powered to examine individual-centric outcomes associated with mortality but not mortality itself.
CONCLUSIONS
The committee did not implement the opioid tapering trial protocol suggested in the 2019 report, considering the following factors in its decision. First, the current statement of task did not direct the committee to examine the effect of opioid tapering on mortality. Second, the committee was aware of studies based on the 2019 report protocol that had been or were being completed and commissioned a review of these and other relevant studies conducted since the 2019 report. Based on the review, the committee determined that the risk of immortal time and selection biases is high and substantially limits the extent to which retrospective observational studies can infer causality between opioid tapering and mortality. Of note, Dr. Erin Krebs and her colleagues applied the 2019 protocol in an unpublished study (see Appendix F) included in the review. It was noted that the study’s findings and conclusions were highly sensitive to different study design, statistical modeling, and measurement strategies. In addition, the treatment assignment was based on pharmacy records and as such may not accurately reflect whether a tapering was intended or actually took place. Based on these observations, not available at the time of the 2019 report, the committee concludes that additional efforts to implement such a study is unlikely to yield more definitive results and, for these reasons, the committee chose not to implement the tapering trial suggested in the 2019 report. The committee focused the main report to reflect findings from the analyses addressing the statement of task.
The committee notes that several RCTs will soon produce results and add new knowledge for specific circumstances, including individual-initiated opioid tapering. These studies are subject to bias, most notably selection of who is willing to be randomized in clinical trials. They are not powered to provide information on tapering and all-cause or suicide mortality; however, they may offer an opportunity, once complete, to combine their data via meta-analysis to examine mortality as an outcome.
| No. | Project Period | Source | Title | Project Summary |
|---|---|---|---|---|
| 1 | 9/24/2019–5/31/2023 | NIH RePORTER | Pain Reduction and Opioid Medication Safety in ESRD (PROMISE) study |
Interventions: Randomization to two separate 9-month evidence-based interventions:
The control condition not assigned to either intervention will receive usual care and have the option to receive Acceptance and Commitment Therapy after 12 months. Outcomes: Reduction in opioid use (primary outcome) and improving pain severity (secondary outcome). |
| 2 | 9/24/2019–5/31/2024 | NIH RePORTER | Video-Telecare Collaborative Pain Management to Improve Function and Reduce Opioid Risk in Patients with End Stage Renal Disease Receiving Hemodialysis |
Interventions: Pragmatic randomized sequential, multiple assignment randomized trial. Individuals randomized to
Outcomes: 6-month composite outcome of LTOT dose reduction and pain response. |
| 3 | 8/1/2022–6/30/2027 | NIH RePORTER | Evaluating a Mechanistically Supported Pharmacotherapy to Treat Acute and Protracted Opioid Withdrawal |
Interventions: Placebo-controlled double-blind randomized controlled trials (RCT). Individuals randomized to
Outcomes: Opioid withdrawal severity, craving severity, and anxiety |
| 4 | 8/15/2023–7/31/2026 | NIH RePORTER | Optimizing Patient-Centered Opioid Tapering with Mindfulness-Oriented Recovery Enhancement (MORE) | Interventions: Hybrid 2 implementation-effectiveness RCT of MORE as delivered via an economically sustainable, insurance-reimbursable group medical visit (key implementation strategy) as an adjunct to an individual-centered opioid tapering protocol that leverages individual agency and therapeutic expectancy. Outcomes: reduction in opioid dosing and opioid-related harms. |
| 5 | 8/15/2023–7/31/2026 | NIH RePORTER | Sequential Trial of Adding Buprenorphine, Cognitive Behavioral Treatment, and Transcranial Magnetic Stimulation to Improve Outcomes of Long-Term Opioid Therapy for Chronic Pain |
Interventions: Individuals will be randomized to one of the following treatment arms:
|
| No. | Project Period | Source | Title | Project Summary |
|---|---|---|---|---|
| 6 | 12/30/2025 (Estimated) | CTG | Tapering From Long-Term Opioid Therapy in Chronic Pain Population. Randomized Controlled Trial With 12 Months Follow-Up |
Interventions: Individuals with long-term non-malignant pain were randomly allocated to
Outcomes: Opioid consumption (time frame: 12 months and 4 months). |
| 7 | 3/22/2018–8/31/2023 | CTG | Developing and Pilot Testing an Opioid Tapering Protocol |
Interventions: Individuals on moderate to high-dose chronic opioid pharmacotherapy for whom providers recommend tapering will be randomized to
Outcomes: Change in opioid dose over 6 months (time frame: up to 6 months). |
| 8 | 7/1/2022–2024-07 (Estimated) | CTG | Slow Opioid Tapering Pilot Study of Patients Using Chronic Opioid Therapy |
Interventions: Individuals randomized to the following:
Outcomes: Changes in measures of quality of life; change in measures of depression and anxiety. |
| 9 | 2016-05–2022-11 | CTG | Discontinuation From Chronic Opioid Therapy for Pain Using a Buprenorphin Taper |
Interventions:
Outcomes: Percentage of individuals who tolerate buprenorphine initiation (time frame: 8 hours post dose); number of participants achieving opioid cessation (time frame: 8 weeks after stabilization at Week 10); number of participants achieving opioid cessation post-taper: 1 month (time frame: 1 month post-taper). |
| No. | Project Period | Source | Title | Project Summary |
|---|---|---|---|---|
| 10 | 2024-02–2028-07 (Estimated) | CTG | Comprehensive Analgesic, Recovery, and Education Support for Spine Surgery Trial |
Interventions: Individuals with preoperative long term opioid use (LTOU) undergoing spine surgery randomized to:
Outcomes: Time to baseline opioid use (time frame: up to 1 year after surgery). |
| 11 | 2016–11/1/2023 | PCORI Portfolio | Comparing Ways to Help Veterans Manage Chronic Pain |
Interventions: Two types of chronic pain care for veterans in reducing pain and daily dose of opioids:
For those receiving high-dose opioids, the research team also compared offering versus not offering a switch to buprenorphine, a medicine that can treat pain or opioid use disorder. Key Findings: After 1 year, veterans who received the two types of care did not differ in overall pain or daily dose of opioids. For veterans receiving high doses, those who were or were not offered buprenorphine did not differ in overall pain or daily dose of opioids. Outcomes: Pain response (time frame: 12 months); 50 percent reduction in daily dose (time frame: 12 months). |
| 12 | 2016–3/1/2024 | PCORI Portfolio | Managing Long-Term Low Back Pain to Improve Health and Reduce Reliance on Opioid Medicines: Comparing Mindfulness Meditation and Cognitive Behavioral Therapy—Strategies to Assist with Management of Pain |
Interventions: Individuals with low back pain taking opioids received one of two interventions:
Outcomes: Opioid use, functioning, and pain at 1 year. |
| 13 | 2017–3/1/2024 | PCORI Portfolio | Comparing Cognitive Behavioral Therapy with Peer-Led Support Groups for Patients with Chronic Pain Who Want to Reduce Opioid Use |
Interventions: Individuals with chronic pain created a plan with their doctor to slowly reduce their opioid use and received one of the following:
Outcomes: Pain level and opioid use at 1 year. |
| No. | Project Period | Source | Title | Project Summary |
|---|---|---|---|---|
| 14 | 2017–3/1/2025 | PCORI Portfolio | Comparing Two Ways to Help Patients Manage Long-Term Non-Cancer Pain |
Setting: Three health systems in North Carolina and Tennessee. Interventions: Individuals with chronic pain taking opioids received one of the following:
Outcomes: Continued opioid use, functioning, pain, and other symptoms at 6, 12, and 18 months. |
REFERENCES
Binswanger, I. A., S. M. Shetterly, S. Xu, K. J. Narwaney, D. L. McClure, D. J. Rinehart, A. P. Nguyen, and J. M. Glanz. 2022. Opioid dose trajectories and associations with mortality, opioid use disorder, continued opioid therapy, and health plan disenrollment. JAMA Network Open 5(10):e2234671.
Covington, E. C., C. E. Argoff, J. C. Ballantyne, P. Cowan, H. M. Gazelka, W. M. Hooten, S. G. Kertesz, A. Manhapra, J. L. Murphy, S. P. Stanos, Jr., and M. D. Sullivan. 2020. Ensuring patient protections when tapering opioids: Consensus panel recommendations. Mayo Clinical Proceedings 95(10):2155-2171.
Hernán, M. A., and J. M. Robins. 2016. Using big data to emulate a target trial when a randomized trial is not available. American Journal of Epidemiology 183(8):758-764.
Hernán, M. A., B. C. Sauer, S. Hernandez-Diaz, R. Platt, and I. Shrier. 2016. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. Journal of Clinical Epidemiology 79:70-75.
James, J. R., J. M. Scott, J. W. Klein, S. Jackson, C. McKinney, M. Novack, L. Chew, and J. O. Merrill. 2019. Mortality after discontinuation of primary care-based chronic opioid therapy for pain: A retrospective cohort study. Journal of General Internal Medicine 34(12):2749-2755.
Kosakowski, S., A. Benintendi, P. Lagisetty, M. R. Larochelle, A. S. B. Bohnert, and A. R. Bazzi. 2022. Patient perspectives on improving patient-provider relationships and provider communication during opioid tapering. Journal of General Internal Medicine 37(7):1722-1728.
Krebs, E. E., B. Clothier, E. S. Goldsmith, A. S. Bohnert, B. C. Martinson, and S. Noorbaloochi. N. D. Report of preliminary results: Opioid dose reduction trial emulation.
Lovejoy, T. I., B. J. Morasco, M. I. Demidenko, T. H. A. Meath, J. W. Frank, and S. K. Dobscha. 2017. Reasons for discontinuation of long-term opioid therapy in patients with and without substance use disorders. Pain 158(3):526-534.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2019. An approach to evaluate the effects of concomitant prescribing of opioids and benzodiazepines on veteran deaths and suicides. Washington DC: National Academies Press.
Nevedal, A. L., C. Timko, M. C. Lor, and K. J. Hoggatt. 2022. Patient and provider perspectives on benefits and harms of continuing, tapering, and discontinuing long-term opioid therapy. Journal of General Internal Medicine 38(8):1802-1811.
ANNEX: EFFECT OF OPIOID TAPERING ON MORTALITY AND OTHER ADVERSE OUTCOMES: A RAPID SYSTEMATIC REVIEW
In November 2023, the National Academies of Sciences, Engineering, and Medicine (National Academies) contracted with the University of Pennsylvania’s Penn Medicine Center for Evidence-Based Practice (CEP) to conduct a rapid systematic review to inform the efforts of the Committee on Evaluating the Effects of Opioid and Benzodiazepine Co-Prescribing on All-Cause Mortality in Veterans. The review was completed in April 2024.
Methods
Literature Search
The initial search conducted by the National Academies identified 498 studies of potential interest, which were screened at title, abstract, and full-text levels. To identify additional studies, CEP performed snowball searches by manually reviewing the reference lists of all studies published in 2023 that were selected for inclusion, all systematic reviews published since 2020, and a Cochrane review published in 2017. This approach was selected to efficiently maximize output and limit redundancy. The snowball searches identified 16 additional studies for potential inclusion, which were screened.
Inclusion and Exclusion Criteria
The searches identified studies published in English between 2007 and 2023. Table E-3 lists the reasons for excluding studies.
Outcomes of Interest
The primary outcome of this review was mortality, including all cause, opioid overdose related, and fatal suicide. Secondary outcomes included the following:
- Behavioral health: suicidal ideation; suicide attempts; depression; anxiety.
- Opioid-related morbidity: opioid overdose; substance use disorder; functional impairment.
- Health care use: emergency department (ED) visits; readmissions; outpatient visits.
- Failure of tapering as indicated by opioid dose increase.
| Category | Exclusion |
|---|---|
| Publication type | Meeting abstracts Editorials, letters, errata Single case reports Guidelines or reviews |
| Population | Not U.S. Pediatric Not long-term opioid pharmacotherapy treatment |
| Intervention or comparison | Did not examine effect of tapering opioid treatment |
| Outcomes | Did not report adverse events Only reported peripheral outcomes (e.g., withdrawal symptoms, pain or heat sensitivity) |
NOTE: Tapering was defined to include the gradual, rapid, or abrupt dosage reduction or discontinuation of prescription opioids.
SOURCE: Penn Medicine CEP, consultants to the committee.
Peripheral outcomes of lesser interest were also identified, including measures of pain sensitivity and heat sensitivity. Withdrawal symptoms were not assessed except when included as a component of a composite measure (e.g., some studies evaluated the combined risk of experiencing an opioid overdose, withdrawal symptoms, or other adverse event).
Data Extraction
Table E-4 lists the elements of each study that were described in the summary table.
Risk of Bias Assessment
Each study was assessed for risk of bias using standardized tools developed by Cochrane. RCTs were evaluated with the revised Cochrane risk-of-bias tool for randomized trials (RoB 2). It was updated in 2019 and includes 28 criteria across five domains: (1) randomization, (2) deviation from the intended intervention, (3) missing outcome data, (4) outcome measurement, and (5) selection of results.
Non-randomized studies were evaluated with the Risk of Bias in Non-Randomized Studies—of Interventions (ROBINS-I) tool. Developed in 2016, it assesses 32 criteria across seven domains: (1) confounding, (2) participant selection, (3) classification of intervention, (4) deviation from the intended intervention, (5) missing outcome data, (6) outcome measurement, and (7) selection of results.
Additionally, at the suggestion of the committee, CEP evaluated the risk of immortal time bias for studies that reported mortality as an outcome. This was integrated as an additional domain into the Cochrane assessments.
Each domain was given a risk-of-bias assessment, and the individual domain ratings were then combined to yield an overall risk of bias for every study, categorized as low, moderate, serious, or critical. Cochrane characterizes these levels as follows:
- “Low” = is comparable to a well-performed randomized study.
- “Moderate” = provides sound evidence but cannot be considered comparable to a well-performed randomized study.
- “Serious” = has important problems.
- “Critical” = is too problematic to provide useful evidence.
The Appendix tables present detailed information on how to interpret the domain-specific and overall risk-of-bias assessments. The scoring algorithm indicates that if any single domain in a given study is assessed to be at moderate risk, then the overall risk for the study should not be better than moderate, even if all other domains are low. Furthermore, the risk-of-bias tools do not indicate or confirm that the results of a study are actually biased or
| Study characteristics | Title, author, year of publication. Study period/duration. |
| Population | Total N of included population. Veterans or non-veterans. Exclusion criteria. |
| Methodology | Study design. Data sources. Definition of long-term opioid pharmacotherapy. Definition of tapering. |
| Results | Description of outcome measures. Concise summary of results. |
SOURCE: Penn Medicine CEP, consultants to the committee.
that the results are, or are not, valid and reliable. Rather, the tools identify potential sources of bias and indicate the likelihood that various types of bias might have affected a study.
Results
The searches identified 514 studies for screening, and 31 were selected for inclusion. Figure E-1 summarizes the screening process.
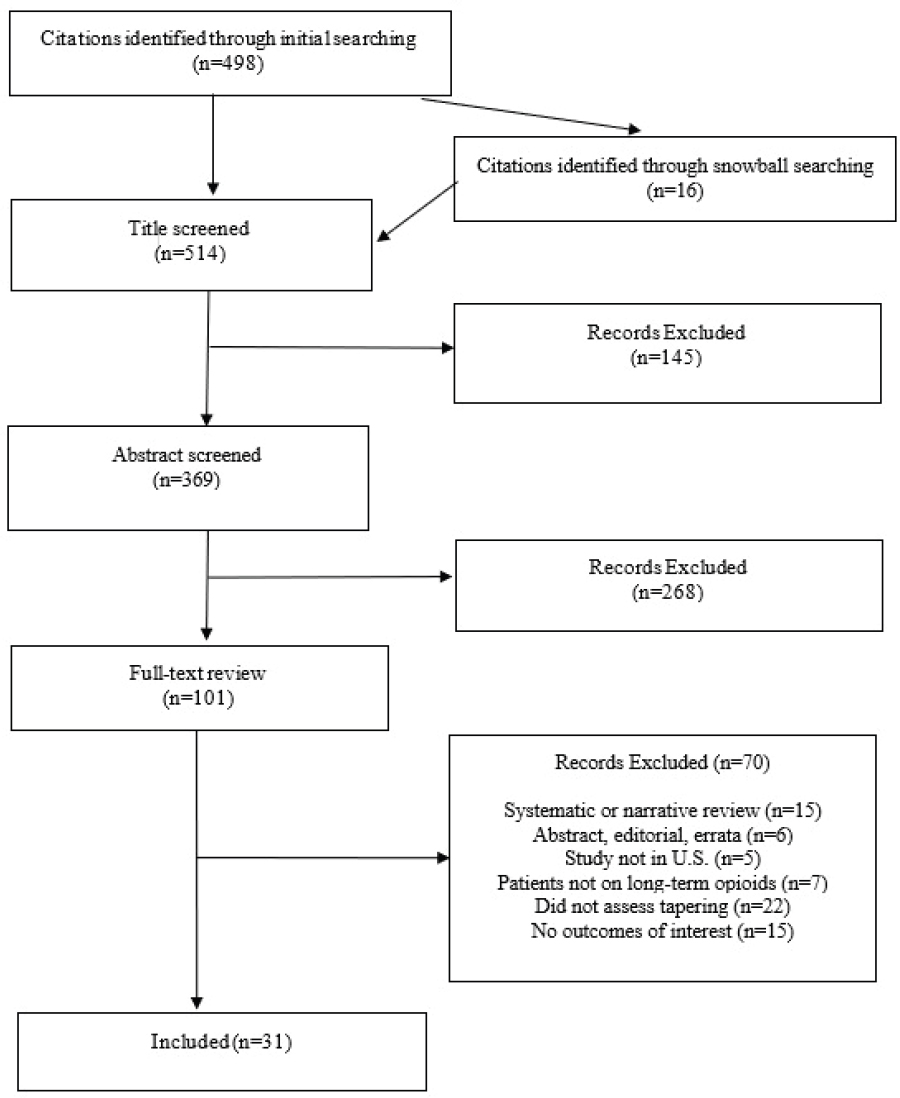
SOURCE: Penn Medicine CEP, consultants to the committee.
Study Characteristics
Thirty-one studies of opioid tapering in U.S. adults were included. Six studies focused exclusively on U.S. veterans; the others included a broader population. Eight studies reported data on mortality; four of those and four other studies reported episodes of nonfatal suicide and/or opioid overdose. Seven studies described ED visits or hospitalizations.
Nineteen studies focused on opioid dosage reduction; 12 studies examined the complete discontinuation of opioid treatment. In 17 studies, a minimum daily dose of opioids was used as an eligibility criterion. Eight of these studies used a threshold of at least 50 morphine milligram equivalents (MME)/per day. Two studies used lower thresholds (20 and 25); the others used higher baselines, 90–300.
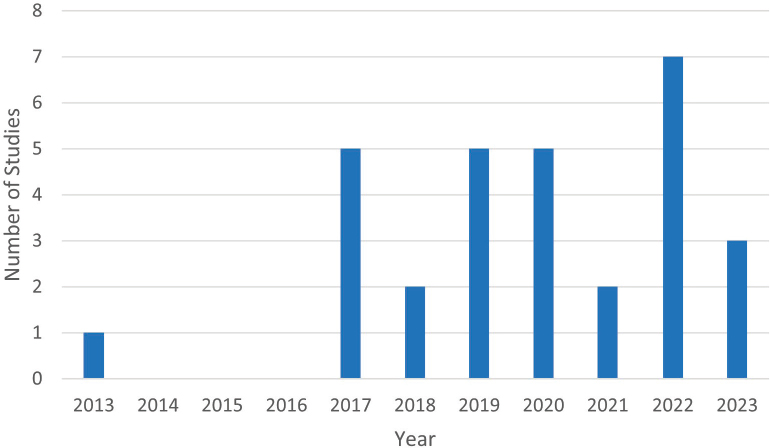
All but one study was published since 2017, and one-third of the studies are from 2022 and 2023. One study that used data collected during 2016 is unpublished. Figure E-2 summarizes the distribution by year.
Only one RCT was found, while two-thirds of the studies used retrospective cohort designs. Table E-5 summarizes the study designs.
SOURCE: Penn Medicine CEP, consultants to the committee.
| Study Design | N |
|---|---|
| Randomized Controlled Trial | 1 |
| Prospective cohort | 1 |
| Retrospective cohort | 22 |
| Chart review | 3 |
| Case control | 3 |
| Survey | 1 |
SOURCE: Penn Medicine CEP, consultants to the committee.
When risk of bias was assessed, 20 studies were rated as moderate, 10 studies as serious, and one as critical. For the 29 non-randomized studies, the primary concerns related to the potential for confounding of results, patients deviating from the intended tapering pathway, and missing data. Immortal time bias was evaluated for the 11 studies that reported mortality or associated outcomes (such as overdose or suicidal ideation). Five studies were assessed as low risk for immortal time bias, three as moderate, two as serious, and one (Oliva et al., 2020) as critical.
Outcomes
Mortality
The eight studies examining mortality presented heterogeneous results that may not be easily reconciled. Three studies reported all-cause mortality, and two found that tapering was not associated with an effect, while one study reported an increase in deaths in a population with a large rather than small dose reduction. Two studies reported suicide mortality, with one finding an increased risk associated with discontinuation and the other no effect. Three studies examined a combined metric for fatal and nonfatal overdoses; two reported that tapering was associated with increased risk of overdose, but the third study found no effect. Finally, two studies used a composite measure of suicide mortality and overdose, and both found that tapering was associated with higher risk. Only two of these studies focused on populations of veterans: the unpublished Krebs study and the large Oliva and colleagues (2020) study that was evaluated as having a critical risk of bias. Table E-6 presents key findings from these studies, excerpted from the full data summary tables.
Six studies were assessed as moderate risk of bias, due primarily to the possibility of confounding variables contributing to the outcomes and some concern about missing data or immortal time bias in several studies. One study was assessed at serious risk of bias because of substantial concerns about immortal time bias and patients who deviated from the intended tapering intervention. One study was rated as having a critical risk of bias because immortal time bias may have had a significant impact on the results; this study was also assessed as serious risk for how interventions were classified and outcomes measured.
Nonfatal Major Adverse Outcomes
Eight studies, including seven at moderate risk of bias due mainly to potential confounding, reported data on a variety of critical but nonfatal adverse events. Two studies found that tapering was associated with higher risk for overdose. One study, at serious risk of bias due to concerns about immortal time bias, reported that 9 percent of veterans experienced suicidal ideation following opioid discontinuation. Three studies found no significant association between tapering and subsequent opioid use or misuse, but two studies did find that patients who discontinued opioids were more likely to use heroin. Finally, two studies found an association between tapering and mental health crises. Table E-7 presents key findings from these studies.
Health Care Utilization
Several studies examined the effects of tapering on the use of ED, hospital, and primary care services. Two studies at moderate risk of bias reported that tapering was associated with a higher risk of ED use and hospitalization, while one small study at serious risk of bias found no difference in ED use after opioid discontinuation. Additionally, two studies found that tapering was associated with fewer primary care visits. The potential for confounding again accounts for almost all the concern related to risk of bias. Table E-8 summarizes these studies.
| Study and Population | Results | Overall Risk of Bias |
|---|---|---|
| All-Cause Mortality | ||
|
Binswanger et al., 2022 N = 3,913 Non-veterans |
Decreasing dose trajectory, compared to stable trajectories, was not associated with all-cause mortality. aHR: 1.28; 95% CI: 0.87–1.86. High-dose increasing trajectory was positively associated with mortality. aHR: 2.19; 95% CI: 1.44–3.32. |
Moderate |
|
James et al., 2019 N = 572 Non-veterans |
Discontinuation was not associated with a significant risk for all-cause mortality. HR: 1.35; 95% CI: 0.92–1.98; p = 0.122. | Moderate |
|
Krebs et al., unpublished N = 207,204 Veterans |
No difference was reported in risk of all-cause mortality between small dose reduction and control groups. HR: 1.01; 95% CI: 0.98–1.04. Large dose reduction group had a small, statistically significant increase in all-cause mortality risk compared to the control group. HR: 1.23; 95% CI: 1.20–1.27. |
Moderate |
| Suicide Mortality | ||
|
Hallvik et al., 2022 N = 14,596 Non-veterans |
Discontinuation was associated with increased risk of suicide mortality compared to stable or increasing dose: Abrupt discontinuation patients aHR: 3.63; 95% CI: 1.42–9.25. Reduction and discontinuation aHR: 4.47; 95% CI: 1.68–11.88. Dose reduction without discontinuation aHR: 1.29; 95% CI: 0.48–3.45. |
Moderate |
|
Krebs et al., unpublished N = 207,204 Veterans |
No difference in risk of suicide mortality was reported for small dose reduction group versus control group. HR: 1.09; 95% CI: 0.89–1.35. No difference in risk of suicide mortality was reported for large dose reduction group versus control group. HR: 1.01; 95% CI: 0.77–1.33. Among patients who died during 1-year follow-up, there was no difference in risk of suicide mortality (as opposed to other cause of death) for either treatment comparison. |
Moderate |
| Overdoses, Fatal and Nonfatal | ||
|
DiPrete et al., 2022 N = 19,443 Non-veterans |
Rapid reduction or discontinuation was associated with higher risk of fatal and nonfatal overdoses compared with gradual reduction after the first year. Year 1 HR: 1.43; 95% CI: 0.94–2.18. Years 2–4 HR: 1.95; 95% CI: 1.31–2.90. |
Moderate |
|
James et al., 2019 N = 572 Non-veterans |
Discontinuation was associated with risk for overdose death. HR: 2.94; 95% CI: 1.01–8.61; p = 0.049. |
Moderate |
|
Von Korff et al., 2019 N = 31,142 Non-veterans |
No difference was found in risk of overdose between tapering and control groups. RR: 0.76; 95% CI: 0.50–1.13. |
Serious |
| Overdose or Suicide | ||
|
Larochelle et al., 2022 N = 199,836 Non-veterans |
Tapering was associated with increased risk of mortality from opioid overdose or suicide compared to stable dosage. Taper RR: 1.15; 95% CI: 1.04–1.27. Abrupt discontinuation RR: 1.34; 95% CI: 0.97–1.79. |
Moderate |
|
Oliva et al., 2020 N = 1,394,102 Veterans |
Stopping opioid treatment was associated with increased risk of mortality from overdose or suicide. Stopping after <=30 days of opioid use HR: 1.67; 31–90 days of opioid use HR: 2.80; 91–400 days of opioid use HR: 3.95; >400 days of opioid use HR: 6.77. |
Critical |
NOTES: aHR = adjusted hazard ratio; CI = confidence interval; HR = hazard ratio; RR = risk ratio.
SOURCE: Penn Medicine CEP, consultants to the committee.
TABLE E-7 Nonfatal Adverse Outcomes
| Study and Population | Results | Overall Risk of Bias |
|---|---|---|
| Nonfatal Overdose | ||
|
Fenton et al., 2022 N = 19,377 Non-veterans |
Tapering was associated with increased risk of nonfatal overdose or withdrawal symptoms compared to the pre-taper period. Overdose or withdrawal aIRR: 1.57; 95% CI: 1.42–1.74. |
Moderate |
|
Agnoli et al., 2021 N = 113,618 Non-veterans |
Tapering was associated with increased risk of nonfatal overdose compared with non-tapering. Nonfatal overdose aIRR: 1.28; 95% CI: 1.15–1.43. |
Moderate |
| Suicidal Ideation | ||
|
Demidenko et al., 2017 N = 509 Veterans |
After discontinuation, 47 patients (9%) had suicidal ideation, and 12 patients (2%) had suicidal self-directed violence. | Serious |
| Opioid Use | ||
|
Sullivan et al., 2017 N = 35 Non-veterans |
Tapering was not associated with risk of opioid misuse. Opioid misuse (adjusted mean difference):0.06; 95% CI: -0.45–0.57; p = 0.81. |
Moderate |
|
Von Korff et al., 2017 N = 1,588 Non-veterans |
Tapering was not associated with risk of opioid use disorder (OUD). Prevalence of OUD in tapering group: 21.5%; 95% CI: 18.9–24.4. Prevalence in control group: 23.9%; 95% CI: 20.5–27.6. aRR: 1.08; 95% CI: 0.89–1.32. |
Moderate |
|
Huffman et al., 2013 N = 120 Non-veterans |
One year after discontinuation, 22.5% of patients had resumed opioid use. Patients who had previously been diagnosed with opioid abuse were no more likely than other patients to have resumed use. | Moderate |
| Heroin Use | ||
|
Binswanger et al., 2020 N = 22,962 Non-veterans |
Compared with no opioid discontinuations, one discontinuation was associated with heroin use. mOR: 2.54; 95% CI: 1.33–4.84. 2 discontinuations (mOR: 2.07; 95% CI: 0.93–4.58) and ≥3 discontinuations (mOR: 1.82; 95% CI: 0.79–4.20) were not significantly associated with heroin use. In a multivariable model, case patients were more than twice as likely to have been discontinued from opioids than control patients. mOR: 2.19; 95% CI: 1.27–3.78. |
Moderate |
|
Coffin et al., 2020 N = 602 Non-veterans |
Participants discontinued from opioids were more likely to use heroin. aOR: 1.57; 95% CI: 1.25–1.97. |
Moderate |
| Mental Health Crisis | ||
|
Fenton et al., 2022 N = 19,377 Non-veterans |
Tapering was associated with increased risk of mental health crisis. aIRR: 1.52; 95% CI: 1.35–1.71. |
Moderate |
|
Agnoli et al., 2021 N = 113,618 Non-veterans |
Tapering was associated with increased risk of mental health crisis. aIRR: 1.74; 95% CI: 1.50–2.01. |
Moderate |
NOTES: aIRR = adjusted incidence rate ratio; aOR = adjusted odds ratio; aRR = adjusted risk ratio; CI = confidence interval; mOR = matched odds ratio.
SOURCE: Penn Medicine CEP, consultants to the committee.
TABLE E-8 Health Care Utilization
| Study and Population | Results | Overall Risk of Bias |
|---|---|---|
| ED Visits | ||
|
Magnan et al., 2023 N = 113,604 Non-veterans |
Tapering was associated with increased risk of emergency department (ED) use. aIRR: 1.19; 95% CI: 1.16–1.21. |
Moderate |
|
Mazurenko et al., 2023 N = 9,633 Non-veterans |
Tapering was associated with increased risk of ED use. 50% tapering OR: 1.75; 95% CI: 1.48–2.06; p < 0.001. 30% tapering OR: 1.73; 95% CI: 1.50–1.98; p < 0.001. |
Moderate |
|
Husain et al., 2019 N = 125 Non-veterans |
Discontinuation was not associated with statistically significant change in ED use for pain: 15% in discontinued patients versus 5% in control group; p = 0.10. | Serious |
|
Mark et al., 2019 N = 494 Non-veterans |
45% of discontinued patients had an ED visit. | Moderate |
| Hospitalizations | ||
|
Magnan et al., 2023 N = 113,604 Non-veterans |
Tapering was associated with increased risk of hospitalization. aIRR: 1.16; 95% CI: 1.12–1.20. |
Moderate |
|
Mazurenko et al., 2023 N = 9, et al., 633 Non-veterans |
Tapering was associated with increased risk of hospitalization. 50% tapering OR: 1.67; 95% CI: 1.30–2.15; p < 0.001. 30% tapering OR: 1.39; 95% CI: 1.13–1.70; p < 0.001. |
Moderate |
|
Mark and Parish, 2019 N = 494 Non-veterans |
3% of discontinued patients had a hospitalization. | Moderate |
| Primary Care Visits | ||
|
Magnan et al., 2023 N = 113,604 Non-veterans |
Tapering was associated with fewer primary care visits. aIRR: 0.95; 95% CI: 0.94–0.96. |
Moderate |
|
Husain et al., 2019 N = 125 Non-veterans |
Discontinuation was associated with fewer primary care visits: 65% in discontinued patients versus 88% in control group; p < 0.01. | Serious |
NOTES: aIRR = adjusted incidence rate ratio; CI = confidence interval; ED = emergency department; OR = odds ratio.
SOURCE: Penn Medicine CEP, consultants to the committee.
Conclusions
The evidence base is complex and does not point to clear, consistent conclusions. Recent studies suggest that tapering may not be associated with all-cause mortality but might be associated with higher risk of death from overdose or suicide. Tapering was also associated with increased risk of nonfatal overdose, heroin use, or mental health crises in five studies, while three different studies found that it did not lead to opioid misuse. ED visits and hospitalizations appeared to increase when patients tapered opioid use, while primary care visits decreased.
In addition to the difficulty of reconciling these results, synthesizing the findings is challenging due to heterogeneous populations and limitations of the study designs. Only two of eight studies that reported mortality outcomes focused on populations of veterans, while just six of the overall set of 31 studies were specific to veterans. Tapering interventions varied widely, including 19 studies that reduced opioid dosage and 12 studies that entirely discontinued use, while the eight studies that reported mortality were evenly divided between dose reduction and discontinuation. Variability was also seen in how gradually or abruptly changes were initiated, with heterogeneity in how patients on LTOT were defined or identified.
The included studies also have inherent limitations. Most of them relied on large administrative and/or clinical databases that inherently have missing, inaccurate, or incomplete data. Additionally, opioid tapering can be clinically complex, and many patients may deviate from an intended regimen in various ways. Only one RCT was identified, while two-thirds of the studies used retrospective cohort designs. Many of these observational studies were assessed to be at moderate or higher risk of bias because, even after adjusting for multiple factors, it is unlikely that they could adequately account for all substantive sources of confounding, particularly for outcomes such as mortality, overdose, or ED use. Also, as noted, the risk-of-bias assessments indicate the broad likelihood that a given study might be biased in the specified domains but do not confirm that the results are in fact biased or invalid. For outcomes where the study findings are consistent and the risk of bias for the relevant studies is moderate to low, the results are likely to be more trustworthy. Where the findings are inconsistent and/or risk of bias is serious or critical, examining the specific risk of bias associated with each study may be one tool to address the challenges of interpreting the evidence base.
Finally, the evidence base reveals important evidence gaps. Only six studies since 2007 were identified that examined tapering in a population of veterans. Just three studies assessed all-cause mortality, including one that is unpublished. And only one RCT and one prospective cohort study were found, demonstrating a need for more robust research.
Risk of Bias Assessment
Tables E-9 and E-10 describe how to interpret the ROBINS-I risk-of-bias domains and overall assessment. These tables are excerpted from The Risk of Bias in Non-Randomized Studies—of Interventions (ROBINS-I) assessment tool, available at the Current version of ROBINS-I. The tool is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
TABLE E-9 Interpreting Risk of Bias Assessment by Domain for ROBINS-I
| Judgment | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result |
|---|---|---|---|---|---|---|---|
| Low risk of bias (the study is comparable to a well-performed randomized trial with regard to this domain) | No confounding expected. | (i) All participants who would have been eligible for the target trial were included in the study; and (ii) For each participant, start of follow-up and start of intervention coincided. | (i) Intervention status is well defined; and (ii) Intervention definition is based solely on information collected at the time of intervention. | The important co-interventions were balanced across intervention groups, and there were no deviations from the intended interventions (in terms of implementation or adherence) that were likely to impact on the outcome | (i) Data were reasonably complete; or (ii) Proportions of and reasons for missing participants were similar across intervention groups; or (iii) The analysis addressed missing data and is likely to have removed any risk of bias. | (i) The methods of outcome assessment were comparable across intervention groups; and (ii) The outcome measure was unlikely to be influenced by knowledge of the intervention received by study participants (i.e. is objective) or the outcome assessors were unaware of the intervention received by study participants; and (iii) Any error in measuring the outcome is unrelated to intervention status. | There is clear evidence (usually through examination of a pre-registered protocol or statistical analysis plan) that all reported results correspond to all intended outcomes, analyses and sub-cohorts. |
| Moderate risk of bias (the study is sound for a non-randomized study with regard to this domain but cannot be considered comparable to a well-performed randomized trial) | (i) Confounding expected, all known important confounding domains appropriately measured and controlled for; and (ii) Reliability and validity of measurement of important domains were sufficient, such that serious residual confounding is not expected. | (i) Selection into the study may have been related to intervention and outcome; and The authors used appropriate methods to adjust for the selection bias; or (ii) Start of follow-up and start of intervention do not coincide for all participants; and (a) the proportion of participants for which this was the case was too low to induce important bias; or (b) the authors used appropriate methods to adjust for the selection bias; or (c) the review | (i) Intervention status is well defined; and (ii) Some aspects of the assignments of intervention status were determined retrospectively. | (i) There were deviations from intended intervention, but their impact on the outcome is expected to be slight. or (ii) The important co-interventions were not balanced across intervention groups, or there were deviations from the intended interventions (in terms of implementation and/or adherence) that were likely to impact on the outcome; and The analysis was appropriate to estimate the effect of starting and adhering to intervention, allowing for deviations (in terms of implementation, adherence | (i) Proportions of and reasons for missing participants differ slightly across intervention groups; and (ii) The analysis is unlikely to have removed the risk of bias arising from the missing data. | (i) The methods of outcome assessment were comparable across intervention groups; and (ii) The outcome measure is only minimally influenced by knowledge of the intervention received by study participants; and (iii) Any error in measuring the outcome is only minimally related to intervention status. | (i) The outcome measurements and analyses are consistent with an a priori plan; or are clearly defined and both internally and externally consistent; and (ii) There is no indication of selection of the reported analysis from among multiple analyses; and (iii) There is no indication of selection of the cohort or subgroups for analysis and reporting on the basis of the results. continued |
| Judgment | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result |
|---|---|---|---|---|---|---|---|
| authors are confident that the rate (hazard) ratio for the effect of intervention remains constant over time. | and co-intervention) that were likely to impact on the outcome. | ||||||
| Serious risk of bias (the study has some important problems) | (i) At least one known important domain was not appropriately measured, or not controlled for; or (ii) Reliability or validity of measurement of an important domain was low enough that serious residual confounding is expected. | (i) Selection into the study was related (but not very strongly) to intervention and outcome; and This could not be adjusted for in analyses; or (ii) Start of follow-up and start of intervention do not coincide; and A potentially important amount of follow-up time is missing from analyses; and the rate ratio is not constant over time. | (i) Intervention status is not well defined; or (ii) Major aspects of the assignments of intervention status were determined in a way that could have been affected by knowledge of the outcome. | (i) The important co-interventions were not balanced across intervention groups, or there were deviations from the intended interventions (in terms of implementation and/or adherence) that were likely to impact on the outcome; and (ii) The analysis was not appropriate to estimate the effect of starting and adhering to intervention, allowing for deviations (in terms of implementation, adherence and co-intervention) that were likely to impact on the outcome. | (i) Proportions of missing participants differ substantially across interventions; or Reasons for missingness differ substantially across interventions; and (ii) The analysis is unlikely to have removed the risk of bias arising from the missing data; or Missing data were addressed inappropriately in the analysis; or the nature of the missing data means that the risk of bias cannot be removed through appropriate analysis. | (i) The methods of outcome assessment were not comparable across intervention groups; or (ii) The outcome measure was subjective (i.e., vulnerable to influence by knowledge of the intervention received by study participants); and the outcome was assessed by assessors aware of the intervention received by study participants; or (iii) Error in measuring the outcome was related to intervention status. | (i) Outcomes are defined in different ways in the methods and results sections, or in different publications of the study; or (ii) There is a high risk of selective reporting from among multiple analyses; or (iii) The cohort or subgroup is selected from a larger study for analysis and appears to be reported on the basis of the results. |
| Critical risk of bias (the study is too problematic to provide any useful evidence on the effects of intervention) | (i) Confounding inherently not controllable or (ii) The use of negative controls strongly suggests unmeasured confounding. | (i) Selection into the study was very strongly related to intervention and outcome; and This could not be adjusted for in analyses; or (ii) A substantial amount of follow-up time is likely to be missing from analyses; and the rate ratio is not constant over time. | (Unusual) An extremely high amount of misclassification of intervention status (e.g., because of unusually strong recall biases). | (i) There were substantial imbalances in important co-interventions across intervention groups, or there were substantial deviations from the intended interventions (in terms of implementation and/or adherence) that were likely to impact on the outcome; and (ii) The analysis was not appropriate to estimate the effect of starting and adhering to intervention, allowing for deviations (in terms of implementation, adherence and co-intervention) that were likely to impact on the outcome. | (i) (Unusual) There were critical differences between interventions in participants with missing data; and (ii) Missing data were not, or could not, be addressed through appropriate analysis. | The methods of outcome assessment were so different that they cannot reasonably be compared across intervention groups. | (i) There is evidence or strong suspicion of selective reporting of results; and (ii) The unreported results are likely to be substantially different from the reported results. |
| No information on which to base a judgment about risk of bias for this domain. | No information on whether confounding might be present. | No information is reported about selection of participants into the study or whether start of follow-up and start of intervention coincide. | No definition of the intervention or no explanation of the source of information about intervention status is reported. | No information is reported on whether there is deviation from the intended intervention. | No information is reported about missing data or the potential for data to be missing. | No information is reported about the methods of outcome assessment. | There is too little information to make a judgment (for example, if only an abstract is available for the study). |
SOURCE: Penn Medicine CEP, consultants to the committee.
TABLE E-10 Overall Risk-of-Bias Assessment for ROBINS-I
| Judgment | Within Each Domain | Across Domains | Criterion |
|---|---|---|---|
| Low risk of bias | The study is comparable to a well-performed randomized trial with regard to this domain | The study is comparable to a well-performed randomized trial | The study is judged to be at low risk of bias for all domains |
| Moderate risk of bias | The study is sound for a nonrandomized study with regard to this domain but cannot be considered comparable to a well-performed randomized trial | The study provides sound evidence for a non-randomized study but cannot be considered comparable to a well-performed randomized trial | The study is judged to be at low or moderate risk of bias for all domains |
| Serious risk of bias | The study has some important problems in this domain | The study has some important problems | The study is judged to be at serious risk of bias in at least one domain, but not at critical risk of bias in any domain |
| Critical risk of bias | The study is too problematic in this domain to provide any useful evidence on the effects of intervention | The study is too problematic to provide any useful evidence and should not be included in any synthesis | The study is judged to be at critical risk of bias in at least one domain. |
| No information | No information on which to base a judgment about risk of bias for this domain | No information on which to base a judgment about risk of bias | There is no clear indication that the study is at serious or critical risk of bias and there is a lack of information in one or more key domains of bias |
SOURCE: Penn Medicine CEP, consultants to the committee.
REFERENCES
Agnoli, A., G. Xing, D. J. Tancredi, E. Magnan, A. Jerant, and J. J. Fenton. 2021. Association of dose tapering with overdose or mental health crisis among patients prescribed long-term opioids. JAMA 326(5):411-419.
Binswanger, I. A., J. M. Glanz, M. Faul, J. A. Shoup, L. M. Quintana, J. Lyden, S. Xu, and K. J. Narwaney. 2020. The association between opioid discontinuation and heroin use: A nested case-control study. Drug and Alcohol Dependence 217:108248.
Binswanger, I. A., S. M. Shetterly, S. Xu, K. J. Narwaney, D. L. McClure, D. J. Rinehart, A. P. Nguyen, and J. M. Glanz. 2022. Opioid dose trajectories and associations with mortality, opioid use disorder, continued opioid therapy, and health plan disenrollment. JAMA Network Open 5(10):e2234671.
Coffin, P. O., C. Rowe, N. Oman, K. Sinchek, G.-M. Santos, M. Faul, R. Bagnulo, D. Mohamed, and E. Vittinghoff. 2020. Illicit opioid use following changes in opioids prescribed for chronic non-cancer pain. PLoS One 15(5):e0232538.
Demidenko, M. I., S. K. Dobscha, B. J. Morasco, T. H. Meath, M. A. Ilgen, and T. I. Lovejoy. 2017. Suicidal ideation and suicidal self-directed violence following clinician-initiated prescription opioid discontinuation among long-term opioid users. General Hospital Psychiatry 47:29-35.
DiPrete, B. L., S. I. Ranapurwala, C. N. Maierhofer, N. Fulcher, P. R. Chelminski, C. L. Ringwalt, T. J. Ives, N. Dasgupta, V. F. Go, and B. W. Pence. 2022. Association of opioid dose reduction with opioid overdose and opioid use disorder among patients receiving high-dose, long-term opioid therapy in North Carolina. JAMA Network Open 5(4):e229191.
Krebs, E. E., B. Clothier, E. S. Goldsmith, A. S. Bohnert, B. C. Martinson, S. Noorbaloochi. n.d. Report of preliminary results: Opioid dose reduction trial emulation.
Fenton, J. J., E. Magnan, I. E. Tseregounis, G. Xing, A. L. Agnoli, and D. J. Tancredi. 2022. Long-term risk of overdose or mental health crisis after opioid dose tapering. JAMA Network Open 5(6):e2216726.
Fenton, J. J., E. M. Magnan, A. L. Agnoli, S. G. Henry, G. Xing, and D. J. Tancredi. 2021. Longitudinal dose trajectory among patients tapering long-term opioids. Pain Medicine 22(7):1660-1668.
Glanz, J. M., I. A. Binswanger, S. M. Shetterly, K. J. Narwaney, and S. Xu. 2019. Association between opioid dose variability and opioid overdose among adults prescribed long-term opioid therapy. JAMA Network Open 2(4):e192613.
Hallvik, S. E., S. El Ibrahimi, K. Johnston, J. Geddes, G. Leichtling, P. T. Korthuis, and D. M. Hartung. 2022. Patient outcomes after opioid dose reduction among patients with chronic opioid therapy. Pain 163(1):83-90.
Huffman, K. L., G. W. Sweis, A. Gase, J. Scheman, and E. C. Covington. 2013. Opioid use 12 months following interdisciplinary pain rehabilitation with weaning. Pain Medicine 14(12):1908-1917.
Husain, J. M., M. LaRochelle, J. Keosaian, Z. Xuan, K. E. Lasser, and J. M. Liebschutz. 2019. Reasons for opioid discontinuation and unintended consequences following opioid discontinuation within the topcare trial. Pain Medicine 20(7):1330-1337.
James, J. R., J. M. Scott, J. W. Klein, S. Jackson, C. McKinney, M. Novack, L. Chew, and J. O. Merrill. 2019. Mortality after discontinuation of primary care-based chronic opioid therapy for pain: A retrospective cohort study. Journal of General Internal Medicine 34(12):2749-2755.
Krebs, E. E., B. Clothier, E. S. Goldsmith, A. S. Bohnert, B. C. Martinson, and S. Noorbaloochi. n.d. Report of preliminary results: Opioid dose reduction trial emulation.
Larochelle, M. R., S. Lodi, S. Yan, B. A. Clothier, E. S. Goldsmith, and A. S. B. Bohnert. 2022. Comparative effectiveness of opioid tapering or abrupt discontinuation vs no dosage change for opioid overdose or suicide for patients receiving stable long-term opioid therapy. JAMA Network Open 5(8):e2226523.
Magnan, E. M., D. J. Tancredi, G. Xing, A. Agnoli, A. Jerant, and J. J. Fenton. 2023. Association between opioid tapering and subsequent health care use, medication adherence, and chronic condition control. JAMA Network Open 6(2): e2255101-e2255101.
Mark, T. L., and W. Parish. 2019. Opioid medication discontinuation and risk of adverse opioid-related health care events. Journal of Substance Abuse Treatment 103:58-63.
Mazurenko, O., J. Blackburn, P. Zhang, S. Gupta, C. A. Harle, K. Kroenke, and K. Simon. 2023. Recent tapering from long-term opioid therapy and odds of opioid-related hospital use. Pharmacoepidemiology and Drug Safety 32(5):526-534.
Oliva, E. M., T. Bowe, A. Manhapra, S. Kertesz, J. M. Hah, P. Henderson, A. Robinson, M. Paik, F. Sandbrink, A. J. Gordon, and J. A. Trafton. 2020. Associations between stopping prescriptions for opioids, length of opioid treatment, and overdose or suicide deaths in US Veterans: Observational evaluation. BMJ 368:m283.
Sullivan, M. D., J. A. Turner, C. DiLodovico, A. D’Appollonio, K. Stephens, and Y.-F. Chan. 2017. Prescription opioid taper support for outpatients with chronic pain: A randomized controlled trial. The Journal of Pain 18(3):308-318.
Von Korff, M., K. Saunders, S. Dublin, R. L. Walker, M. Thakral, K. J. Sherman, E. J. Ludman, R. N. Hansen, M. Parchman, and S. M. Shortreed. 2019. Impact of chronic opioid therapy risk reduction initiatives on opioid overdose. The Journal of Pain 20(1):108-117.
Von Korff, M., R. L. Walker, K. Saunders, S. M. Shortreed, M. Thakral, M. Parchman, R. N. Hansen, E. Ludman, K. J. Sherman, and S. Dublin. 2017. Prevalence of prescription opioid use disorder among chronic opioid therapy patients after health plan opioid dose and risk reduction initiatives. International Journal of Drug Policy 46:90-98.
This page intentionally left blank.























