Examining Glucagon-Like Peptide-1 Receptor (GLP-1R) Agonists for Central Nervous System Disorders: Proceedings of a Workshop (2025)
Chapter: 2 GLP-1 Mechanisms in the Brain
2
GLP-1 Mechanisms in the Brain
HIGHLIGHTS
- GLP-1 receptors are present throughout the brain and can contribute to a wide range of behaviors and conditions. (Drucker, Rinaman, Secher)
- Research is ongoing into which parts of the brain are directly and indirectly affected by GLP-1R agonists, but a complete understanding has yet to be achieved. (Rinaman)
- Experiments in rodents suggest that the endogenous central GLP-1 system contributes to metabolic state-dependent modulation of motivated behavior. (Rinaman)
- Although GLP-1R agonists can gain access to certain regions of the brain, this access seems to be through specialized uptake around the circumventricular organs, not through the blood–brain barrier. The precise mechanism by which this happens has not yet been explained. (Secher)
NOTE: This list is the rapporteurs’ summary of points made by the individual speakers identified, and the statements have not been endorsed or verified by the National Academies of Sciences, Engineering, and Medicine. They are not intended to reflect a consensus among workshop participants.
OVERVIEW OF GLP-1 CIRCUITRY IN THE CENTRAL NERVOUS SYSTEM
Linda Rinaman, a distinguished research professor in the Department of Psychology and the R. Bruce Masterton Professor of Neuroscience at Florida State University, offered an overview of the central endogenous GLP-1 neuronal projection system. In addition, she described the distribution of the GLP-1 receptors (GLP-1R) and which ones may be accessible to systemic GLP-1R agonists.
To illustrate the central GLP-1 projection system, Rinaman showed a simplified version of the system in a rodent (Figure 2-1). The cell bodies of the GLP-1 neurons, which express glucagon, are located in the caudal hindbrain. They are split about 60–40 percent between the nucleus of the solitary tract and the intermediate reticular nucleus. The cells in the caudal nucleus of the solitary tract receive direct vagal sensory afferent input, and their axons branch widely to reach a large number of subcortical targets. Those targets include the midbrain, pons, hypothalamus, and limbic forebrain, including the amygdala and the bed nucleus of the stria terminalis.
If the GLP-1 neurons in the hindbrain are activated by physiological or pharmacological methods, or if the central GLP-1 receptors are activated, Rinaman said, this suppresses motivated behaviors such as food intake, drug self-administration, operant responding for drugs, and exploratory behaviors (Maniscalco and Rinaman, 2018). In rodent models, activation of the GLP-1 neurons also results in activation of stress responses, including anxiety-like behavior (Maniscalco and Rinaman, 2018).
Rinaman said that her laboratory discovered that the GLP-1 neurons in the rodent hindbrain are suppressed—that is, their ability to be activated is reduced—during states of negative energy balance, for example, after 18 hours of fasting in rats or mice (Maniscalco and Rinaman, 2018). The suppression of the GLP-1 neurons is accompanied by increased food intake, drug self-administration, operant responding, and exploratory behavior. “This is well recognized in people that [train] animals for operant responding for drugs,” Rinaman said. “If you have them in a food-restricted state, they’ll learn the task and perform the task much better.” In sum, she said, “our working hypothesis is that the central endogenous GLP-1 system modulates motivated behavior, and it does so in a metabolic-state-dependent manner.”
The axons of the hindbrain GLP-1 neurons innervate many subcortical brain regions, Rinaman said. GLP-1 neurons that target one region, such as the hypothalamus, thalamus, or bed nucleus of the stria terminalis, have axon collaterals that branch widely and reach every central target that is known to receive GLP-1 input, including the spinal cord. “So there doesn’t
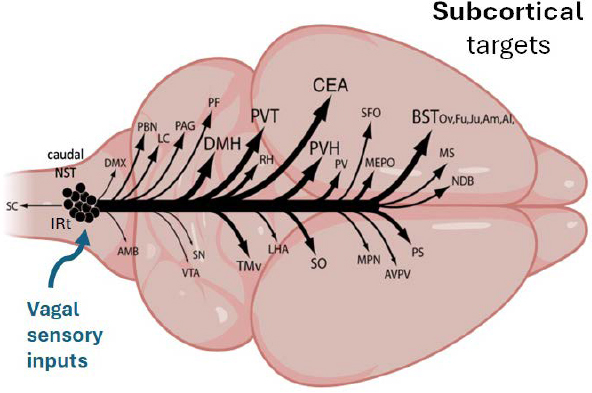
NOTES: The caudal hindbrain is located on the left. AMB = ambiguus nucleus; AVPV = anteroventral periventricular nucleus; BSTov,fu,ju,am,al =oval, fusiform, juxtacapsular nuclei and anteromedial, anterolateral areas of the bed nuclei of the stria terminalis; CEA = central nucleus of the amygdala; DMH = dorsomedial nucleus of the hypothalamus; DMX = dorsal motor nucleus of the vagus nerve; LC = locus ceruleus; LHA = lateral hypothalamic area; MEPO = median preoptic nucleus; MPN = medial preoptic nucleus; MS = medial septum; NDB = nucleus of the diagonal band; PAG = periaqueductal gray; PBN = parabrachial nucleus; PF = parafascicular nucleus; PS = parastrial nucleus; PV = periventricular nucleus; PVH = paraventricular nucleus of the hypothalamus; PVT = paraventricular nucleus of the thalamus; RH = rhomboid nucleus of the thalamus; SC = spinal cord; SFO = subfornical organ; SN = substantia nigra; SO = supraoptic nucleus; TMv = ventral part of the tuberomammillary nucleus; VTA = ventral tegmental area.
SOURCE: Presented by Linda Rinaman on September 10, 2024. Adapted from Gu et al. (2013).
seem to be a point-to-point, one-to-one subpopulation of cells that target different areas,” she said.
The GLP-1 receptor is expressed in all these subcortical target regions and also in cortical and hippocampal regions that do not get axonal input from the GLP-1 neurons in the hindbrain (Randolph et al., 2024). Since the GLP-1 receptor is often present in the membrane of neural processes, including the axon terminals (Farkas et al., 2021), Rinaman said, it is possible that the GLP-1 receptor made by those cortical and hippocampal neurons is trafficked to their axon terminals in subcortical regions that do receive GLP-1 input.
Interestingly, she said, since the hindbrain GLP-1 neurons do not express GLP-1 receptors, they cannot be accessed directly by systemically
administered GLP-1R agonists. They also, Rinaman added, get little or no synaptic input from neurons that express GLP-1 receptor in the nodose ganglion or area postrema. Thus, the vagal sensory neurons that express the receptor and respond to the systemic administration of GLP-1R agonists do not synaptically target the hindbrain GLP-1 neurons. Additionally, hindbrain GLP-1 neurons are not necessary for GLP-1R agonists applied systemically to suppress food intake. Nor are these GLP-1 neurons activated to express c-Fos when GLP-1R agonists are applied systemically (Brierley et al., 2021; Card et al., 2018; Secher et al., 2014).
However, she continued, a recent paper showed that ablating the GLP1R-expressing neurons in the hindbrain dorsal vagal complex, including the nucleus of the solitary tract, blocks the ability of the systemic receptor agonist to suppress intake (Huang et al., 2024). Blocking the receptors in the arcuate nucleus or in the nodose ganglion does not block the effect, she said, “so we really need to focus on these hindbrain NST [nucleus of the solitary tract] neurons.”
To address the issue of whether GLP-1R agonists applied systemically directly access central GLP-1 receptors, Rinaman showed some images of brain regions with GLP-1 receptors and the axons of GLP-1 neurons labeled in different colors (Farkas et al., 2021). The circumventricular organs, including the area postrema, are key areas where systemically administered GLP-1R agonists—and perhaps endogenous circulating GLP-1—have ready access to GLP-1 receptors, she said. There may also be access to some periventricular regions near the circumventricular organs.
A number of published studies have put a fluorescent tag onto GLP-1R agonists, including exendin, liraglutide, and semaglutide, and then used the fluorescent labeling to visualize the distribution of the agonist in the brain (see, e.g., Gabery et al., 2020). Rinaman said, “It could be that we’re underestimating the amount to which these drugs penetrate just by virtue of the way those experiments are done and the non-enhanced visualization of the receptors.” There are various reasons that the experiments using the fluorescent tags may underestimate the extent to which the GLP-1R agonists reach targets in the brain, she added. For instance, when a GLP-1R agonist is bound to a ligand, it promotes receptor internalization. Thus, there may be competition—if endogenous GLP-1 is already occupying a receptor, then the fluorescently tagged GLP-1R agonist may not attach and label it. Furthermore, since the GLP-1 receptors are trafficked and inserted into the axon terminal membranes, that may make it difficult to visualize binding, because one is looking at diffuse scattered axon terminals rather than concentrated neuronal cell body labeling.
Rinaman offered four key takeaways. First, the endogenous central GLP-1 system appears to contribute to metabolic state-dependent modulation of motivated behavior. Second, the hindbrain GLP-1 neurons are nei-
ther directly nor indirectly engaged by systemically administered GLP-1R agonists. Third, systemic GLP-1R agonists may access only a subset of the central GLP-1 receptors, including those in circumventricular organs and adjacent brain regions. However, current fluorescent imaging techniques may underestimate brain penetrance, and better techniques will be needed to get a fuller picture of which GLP-1 receptors are reached by systemically administered GLP-1R agonists. Fourth, GLP-1R protein is more prevalent in axon terminals than in neuronal cell bodies, and it is possible that endogenous GLP-1 and GLP-1R agonists may bind to GLP-1 receptors in regions beyond those in which GLP-1R mRNA is expressed.
Concerning research gaps and opportunities, Rinaman pointed to whether perinatal development of the GLP-1/GLP-1R system may help explain individual differences in responsiveness to GLP-1R agonists, why there are sex differences in the endogenous GLP-1/GLP-1R system and why GLP-1R agonists are more effective for weight loss in women than in men, and whether chronic exposure to GLP-1R agonists affects central GLP-1 receptors.
MECHANISMS OF CNS PENETRANCE FOR GLP-1R AGONISTS
Anna Secher, a scientific director within Obesity and MASH (metabolic dysfunction-associated steatohepatitis) at Novo Nordisk, discussed what is known about how GLP-1R agonists gain access into the brain. She began by reiterating something the previous two speakers had said—that interactions with GLP-1 receptors can play a role in various behaviors and conditions, such as appetite regulation and, potentially, neurodegenerative diseases, and that there are many different GLP-1 receptors scattered throughout the brain. Indeed, researchers have identified more than 50 brain regions with GLP-1 receptors, she said.
That raises the question of whether and how GLP-1R agonists, which were initially developed for use in the rest of the body, make their way into the brain. Molecules in the blood can get into the brain in three basic ways, Secher said. The first is by crossing the blood–brain barrier, which is composed of tightly connected epithelial cells. The barrier allows some small molecules to pass via diffusion and more active methods that transport desired molecules such as glucose or amino acids into the brain, but the barrier prevents most molecules and other substances from crossing into the brain. Second, cerebrospinal fluid has its own way of passing into the brain. Cerebrospinal fluid is produced by the choroid plexus and then secreted into the ventricular space of the brain; the choroid plexus contains a blood–cerebrospinal fluid barrier to protect the brain from potentially damaging substances in the blood. The third type of access is via the circumventricular organs, which include but are not limited to the median eminence in the hypothalamus and the area postrema in the hindbrain,
and the pituitary gland. These organs have fenestrated capillaries, that is, capillaries with small pores that allow proteins and other large molecules to leave the bloodstream and enter adjacent organs.
To explore how well GLP-1R agonists access various parts of the brain, Secher’s team labeled various agonists with fluorescent tags so that when they administered the agonists to mice, they could see which areas of their brains lit up. They found that although many regions of the brain have GLP-1 receptors, when the mice were dosed with the GLP-1R agonist semaglutide, the semaglutide ended up on only a fraction of the mouse brain’s GLP-1 receptors, mainly in the hypothalamus, brainstem, and septum.
“So,” Secher asked, “does that mean that these are the only regions that are activated by GLP-1 receptor agonists?” She and her team do not believe that this is the case, she said, “because when you have interaction with one neuron, this can communicate to many other neurons in deeper layers of the brain.” To test whether that was happening, her team used c-Fos, a protein that can be used as a marker for neuronal activation. When they administered semaglutide to lab animals, the c-Fos distribution indicated that a number of brain regions were being indirectly activated by the GLP-1R agonist that had not shown up as being directly activated (Gabery et al., 2020). And, Secher added, since c-Fos is not a global marker, it is possible that many other brain regions were also indirectly activated by semaglutide but did not show up with the c-Fos signal.
Secher suggested that there may be limitations to the evaluation of the distribution and access of the GLP-1R agonists by the labeling method. GLP-1R agonists are internalized upon binding and are transported away from the region where they are binding. With this background, Secher addressed the issue of how GLP-1R agonists—and also antagonists—gain access into the brain. “We actually don’t know yet,” she said, “but we do have hypotheses.” One of the hypotheses is that GLP-1R agonists gain access through interactions around the circumventricular organs. In addition to their fenestrated capillaries, the circumventricular organs also contain specialized cell structures called tanycytes, which send projections into the surrounding blood vessels and also into the brain parenchyma, that is, the brain’s functional tissue, which comprises neurons, glial cells, and collagen proteins. These tanycytes have been shown to play a role in the access that leptin, a hormone that regulates the balance of food intake and energy expenditure, has into the brain through an interaction with leptin receptors. “We wondered whether that was the same mechanism with our GLP-1 receptor agonist,” she said. To investigate this, they carried out an electron microscopy analysis with Csaba Fekete of the Institute of Experimental Medicine at the Hungarian Academy of Sciences and found that the tanycytes did have GLP-1 receptors. Other cells related to the blood–brain barrier did not have these receptors, Secher said. “This indicates that it’s not a general access across the blood–brain barrier we have here with our
GLP-1 receptor agonist but rather a specialized uptake, likely around circumventricular organs.”
She concluded with the following key takeaways: Both GLP-1 and acylated GLP-1 analogs have access to discrete brain regions. The access seems to be not through the blood–brain barrier but rather through a specialized uptake around the circumventricular organs. And the mechanism of how these molecules are taken up remains to be fully explained.
DISCUSSION
Cheryl Lohman, an independent researcher, asked whether GLP-1R agonists may be effective against long COVID given that inflammation is known to play a role in that condition. Daniel Drucker answered that it is a good question, but he knows of no rigorous scientific data that address the issue.
Endogenous Inducers of GLP-1 Release
Frances Jensen, a professor of neurology and the chair of neurology at the Perelman School of Medicine, University of Pennsylvania, and codirector of Penn Translational Neuroscience Center, noted that the focus in the field so far has been on administering GLP-1R agonists, but there are other ways that one could trigger the release of GLP-1. “What are other endogenous inducers of endogenous GLP-1 release,” she asked, “and why has that not been a pathway for pharmaceutical development?”
Drucker answered that this is still an area of investigation, but it has received less attention given the effectiveness of GLP-1R agonists. “I think the bump in the road was really the development of oral semaglutide, which showed that you could give oral GLP-1 and have pretty impressive pharmaceutical activity,” he said. “Novo Nordisk has demonstrated that you can get 50 milligrams of oral semaglutide in new formulation and have 15 percent body weight loss.” It is not easy to achieve the same level of GLP-1 secretion with other approaches, he said, but some companies are still working to develop effective GLP-1 secretagogues, or substances that cause GLP-1 to be secreted, he said. “It’s just much harder for them to be competitive in the new era of small-molecule GLP-1 receptor agonists, oral peptide therapeutics.” Secher added that there may be a physiological limit to the amount of GLP-1 that can be produced with endogenous inducers of endogenous GLP-1 release compared with pharmacological inducers.
Brian Fiske asked Rinaman about the role of brain-derived GLP-1. Rinaman explained that when GLP-1R agonist drugs are given systemically, they are not recruiting the central GLP-1 endogenous neurons but are accessing a subset of the receptors. “Under the circumstances where the GLP-1 neurons in the hindbrain are activated through physiological
mechanisms—or they can be activated through chemogenetics, optogenetics, things like that—you do get decrease in food intake, you get increase in avoidance behavior, you get stress responses,” she said. “The drugs are activating that system differently. They’re jumping over the neurons themselves and then directly accessing just a subset of the receptors.”
Hayes followed up by asking Rinaman whether, if one could activate the endogenous GLP-1 system through a different therapeutic modality in addition to treating the system with a GLP-1R agonist, there would be a further enhancement of weight loss. Rinaman answered yes, citing research that demonstrated additional suppression of food intake when semaglutide was combined with chemogenetic activation of the GLP-1 neurons (Brierley et al., 2021). That might offer a good target for future pharmacotherapies, she suggested.
Penetrance of the Blood–Brain Barrier
Christian Hölscher, cofounder and chief scientific officer of Kariya Pharmaceuticals and a professor of neuroscience at the Henan University of Chinese Medicine, asked Drucker about the data that showed that tirzepatide and semaglutide have no effect in the mouse model of Alzheimer’s disease. Perhaps the reason is that those drugs do not cross the blood–brain barrier, Hölscher suggested. Earlier GLP-1R agonists could cross into the brain and do have effects on the mouse model of Alzheimer’s disease, but the newer drugs have been designed to stay in the blood for a very long time and do not really cross into the brain, said Hölscher.
Drucker agreed that these newer drugs do not meaningfully penetrate the brain, but Secher, Rinaman, and others have shown they do meaningfully signal to many regions deep within the brain. Furthermore, he continued, they produce 15 to 20 percent weight loss, which is mediated by many circuits in the brain. Given that, Drucker said, the key questions are, “Where do we need to go to activate the critical regions to achieve meaningful neuroprotection? And how might that differ from what we can activate peripherally with these medicines to produce powerful weight loss?” Perhaps, he suggested, GLP-1R agonists that get into the brain more efficiently might produce more neuroprotection, but would they also lead to more adverse events? A key question that remains to be answered is which drugs and which brain targets will maximize the neuroprotective benefits while minimizing adverse events.
Another workshop participant inquired about data that speak to differences in the nonlipidated versus lipidated GLP-1R agonists and their differential effects on blood–brain barrier penetrance. Secher said that to their surprise, her team has found that nonlipidated molecules penetrate the same way or show up in the same areas as lipidated molecules. “It’s probably something more around this GLP-1 receptor recognition,” she said.
Sex and Age Differences
Alexandra Sinclair, a professor of neurology at the University of Birmingham, asked whether there are sex or age differences in the distribution of the GLP-1 receptors around the brain or in the access of the GLP-1 receptor agonists into the brain. Secher answered that she has not systematically looked at sex differences in her mouse model but added that she should do that. Concerning aging, she said, there may well be some differences in the blood–brain barrier between young mice and very old mice, but she is not sure they could be detected with the methods she currently uses.
Rinaman said that unpublished data from her lab show that diet exposure during the perinatal period of development in rats can profoundly affect the axonal projections of the GLP-1 neurons, and it also seems to affect the expression of the receptors. Her lab is now studying whether those effects persist into adulthood. “I’m really excited about that because it suggests the potential for individual differences in the central GLP-1 system that could be attributed to dietary effects or perhaps stress exposures early in development,” she said. This could help explain individual differences among humans in responsiveness to these drugs, including some well-known sex differences. For example, previous research has shown that females appear to be more sensitive to the ability of GLP-1R agonists to suppress food intake and body weight (e.g., Richard et al., 2016).
Hayes added that his lab has a paper under review looking at sex differences in response to GLP-1R agonists and how the estrous cycle affects the expression of the GCG gene, which codes for preproglucagon, and the GLP-1 receptor gene. When the GLP-1R agonists are administered during the estrous cycle affects how effective the drugs are in terms of weight loss. This could point to differing effects in human patients, for instance, between pre- and postmenopausal women, said Hayes.
GLP-1R Antagonists
Iván Montoya, director of the Division of Therapeutics and Medical Consequences at the National Institute on Drug Abuse (NIDA), asked about the effects of GLP-1R antagonists. Drucker said that there has been a series of attempts over time to develop these antagonists for the treatment of postbariatric hypoglycemia and other orphan conditions as well. “We don’t have a lot of human data with prolonged exposure, other than in the context of hypoglycemia, that people have looked at,” he said, so it is not clear what other effects, either beneficial or adverse, the antagonists might have. However, Drucker added, in the bariatric surgery hypoglycemia trials, there do not seem to be any adverse effects. “It’s a question that would merit further study,” he said.
Heterogeneity of Response
Fatima Cody Stanford, an associate professor of medicine and pediatrics and obesity medicine physician-scientist at the Massachusetts General Hospital and Harvard Medical School, said that as someone who has treated more than 3,000 patients with obesity, she does have strong data supporting the heterogeneity of response to GLP-1R agonists in practice. Some patients are nonresponders to the drugs, while others have a high response, and these distinct responses arise even within individual families. Ultimately, the only way she knows how well a patient will respond to the drug is to wait and see.
Hayes asked whether the GLP-1 receptors develop a tolerance similar to what is seen with opioid receptors in the brain. Drucker answered that it does happen sometimes that a person responds initially but then stops responding and does not achieve the desired weight loss. “Sometimes we switch them from one medicine to another, and sometimes people respond better, and other times they don’t,” he said. However, Drucker added, we just don’t have enough data to know with any certainty what is going on at the receptor level or postreceptor level that is behind this phenomenon.
In sum, much current research is aimed at understanding the endogenous GLP-1 system in the brain, particularly how GLP-1R agonists gain access to certain regions of the brain and their effects once they are in the brain, but the complexity of GLP-1 pathways has so far kept scientists from developing a complete understanding of the system.









