Examining Glucagon-Like Peptide-1 Receptor (GLP-1R) Agonists for Central Nervous System Disorders: Proceedings of a Workshop (2025)
Chapter: 6 Neurodegenerative Disorders and Other Emerging Areas
6
Neurodegenerative Disorders and Other Emerging Areas
HIGHLIGHTS
- GLP-1R agonists have been evaluated across multiple preclinical neurodegenerative and neuropsychiatric disorder models and have been found highly promising. Clinical trials of GLP-1R agonists for the treatment of Parkinson’s disease have had mixed results, but their results, combined with the results of preclinical research, indicate that the drugs have enough promise for research to continue. The agonists are also being tested in clinical trials of Alzheimer’s disease. (Athauda, Greig, Hölscher)
- For GLP-1R agonists being considered for treatment of brain disorders and injuries, an important consideration is how well they cross the blood–brain barrier. (Athauda, Hölscher)
- Some recently developed dual agonists can cross the blood–brain better than previous GLP-1R agonists and, in animal studies, are better at protecting the brain than the older single agonists. (Hölscher)
- GLP-1R agonists show promise in treating the raised intracranial pressure associated with idiopathic intracranial hypertension and traumatic brain injury, headache and migraine pain, neuropathic pain, and the raised intraocular pressure that can lead to glaucoma. (Sinclair)
- Biomarkers can play a valuable role in the development of GLP-1R agonists, particularly in serving as surrogate endpoints to evaluate their efficacy in treating various neurodegenerative disorders. (Jawidzik)
NOTE: This list is the rapporteurs’ summary of points made by the individual speakers identified, and the statements have not been endorsed or verified by the National Academies of Sciences, Engineering, and Medicine. They are not intended to reflect a consensus among workshop participants.
The action of glucagon-like peptide-1 receptor (GLP-1R) drugs in treating neurodegenerative disorders and other disorders is currently receiving a great deal of attention, said Edwin George, a clinical reviewer in the Office of New Drugs in the Food and Drug Administration’s (FDA) Center for Drug Evaluation and Research. He listed three objectives for the session focused on those uses: (1) to review the current state of knowledge regarding the mechanisms of action of GLP-1R agonists and their use in treating neurodegenerative disorders, pain syndromes, and other central nervous system (CNS) disorders; (2) to discuss the available scientific evidence on the clinical efficacy of GLP-1R agonists for treating various CNS disorders; and (3) to discuss the challenges relating to these issues, including knowledge gaps, clinical trial design, and biomarker development.
GLP-1 RECEPTOR ACTIVITY IN NEURODEGENERATIVE DISORDERS
Explaining why researchers studying neurodegenerative diseases are interested in GLP-1R agonists, Nigel Greig, who leads the Drug Design and Development Section within the Translational Gerontology Branch at the National Institute on Aging at the National Institutes of Health (NIH), said that there are many commonalities between type 2 diabetes and neurodegenerative disorders, particularly related to cell death mechanisms. And, indeed, a large study of patients being treated for type 2 diabetes found that those treated with a GLP-1R agonist were 60 percent less likely to develop Parkinson’s disease than those given glitazones, a standard treatment for insulin resistance (Brauer et al., 2020). Thus, researchers have been examining the role of GLP-1 receptor activity in neurodegenerative disorders.
GLP-1 receptors are distributed throughout the brain, Greig said, including in the amygdala, hippocampus, and hypothalamus (Lu et al., 2014), and they are found on neurons, epithelial cells of the choroid plexus
(Botfield et al., 2017), and microglia and astrocytes (Jia et al., 2015). Two decades of research in cellular and animal models have found that GLP-1R agonists have neurotrophic, neuroprotective, and anti-inflammatory actions and mitigate brain insulin resistance. Thus, they have potential to treat a wide variety of neurological disorders and injuries.
The key question, Greig continued, is whether these actions of GLP-1R agonists seen in cellular and animal models translate to humans. Answering this question will require addressing other questions, such as which agonists should be evaluated and when in the disease process should they be evaluated. For an overview of the field, he recommended reading a recent publication by Kopp and colleagues (2024).
Quickly reviewing a number of studies, Greig said that research conducted in lab animals indicates that GLP-1 receptors are expressed across the lifespan and in disease states, such as a rodent model for Parkinson’s disease. Corresponding studies in humans found that GLP-1 receptors were found in the substantia nigra in people with Parkinson’s disease. In short, the receptors are expressed across age and disease state.
When cells with GLP-1 receptors are put in culture and exposed to GLP-1R agonists, the result is a stronger phenotypic expression, said Greig; so if, for example, the cell expresses tyrosine hydroxylase, administration of the agonist results in more tyrosine hydroxylase being expressed. The GLP1R agonists also provide a protective effect, keeping cells alive when they are treated with chemicals that would otherwise kill them (Li et al., 2009).
Turning briefly to the pathways through which GLP-1R activation has its effects, Greig said that they are well known (see, e.g., Kopp et al., 2022). To find out which pathways are involved in neurotrophic and neuroprotective actions, one can put selective inhibitors of the various pathways into a cell culture and observe which ones prevent the effects of the GLP-1R activation. And once these pathways have been identified, their markers can be used in human studies as biomarkers of target engagement by assaying brain-derived exosomes from plasma (Athauda et al., 2019).
Switching to research done on GLP-1R agonists in preclinical models of Parkinson’s disease, Greig first described work done on the MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of Parkinson’s disease, which is created by exposing a mouse to the dopamine cell poison MPTP. When MPTP mice are treated with the GLP-1R agonist exendin-44, the drug protects the tyrosine hydroxylase–positive neurons in the mouse’s brain and preserves the levels of dopamine and metabolites in those neurons (Li et al., 2009).
A second model is the MitoPark mouse, a progressive Parkinson’s disease model created by deleting TFAM (transcription factor A, mitochondrial) in midbrain dopamine neurons. A careful study of the effects of a GLP-1R agonist in that mouse model found that the drug improved the
mouse’s motor abilities and increased motivated behavior, increased dopamine levels, and protected neurons. The authors of the study (Wang et al., 2021) concluded that early administration of this GLP-1R agonist (PT320, a sustained-release version of exenatide) could be an important neuroprotective therapeutic strategy against Parkinson’s disease.
Summarizing, Greig said that GLP-1R agonists have been evaluated across multiple preclinical neurodegenerative and neuropsychiatric disorder models and, largely, have been found highly promising. Combining two or more agonists together, as in unimolecular multiagonists that target the receptors for GLP-1 and glucose-dependent insulinotropic polypeptide (GIP), is more effective than using a single GLP-1R agonist, although only if they are taken up by the brain. Furthermore, in human clinical trials of Parkinson’s disease, GLP-1R agonists are demonstrating promise, and the agonists are also being tested in clinical trials of Alzheimer’s disease. In the future, Greig said, it would be valuable to test GLP-1R agonists against other neurodegenerative diseases and brain injury with a focus on finding the best agents and best times in the disease process to initiate treatment.
GLP-1R AGONISTS IN TREATING PARKINSON’S DISEASE
Building on Greig’s discussion of preclinical data on GLP-1R agonists and Parkinson’s disease, Dilan Athauda, a consultant neurologist and neuroscientist at University College London, spoke about the clinical data concerning the efficacy of using the agonists in treating Parkinson’s disease. He began by noting, as Greig had, that cohort studies have found that patients taking GLP-1R agonists for type 2 diabetes have a significantly reduced risk of developing Parkinson’s disease (Brauer et al., 2020; Svenningsson et al., 2016; Tang et al., 2024).
In light of those findings, a clinical pilot study was carried out on 44 patients with Parkinson’s disease. The patients were an average of 60 years old, had had Parkinson’s for an average of 10 years, and were on an average of 980 mg of the dopamine precursor levodopa daily. In the study they were given either the GLP-1R agonist exenatide or a control treatment for 12 months. They were tested at baseline, 6 months, 12 months, and 14 months on a variety of motor and cognitive tests, and the group given the exenatide scored significantly better on both types of tests than the control group. The drug was well tolerated, and when the patients were retested at 96 weeks, or about 22 months, the improvement in the treatment group versus the controls had been maintained (Aviles-Olmos et al., 2013).
Athauda and his group followed that up with their own study examining the effects of a different form of exenatide on Parkinson’s disease. Their 60 patients had experienced somewhat less advanced Parkinson’s, on average, but saw a similar effect on motor control, with the treatment
group seeing improved motor control and the placebo group continuing to get worse over the course of the study (see Figure 6-1). In this case, however, there were no significant differences in cognitive measures (Athauda et al., 2017). Looking more closely, the researchers found reduced dopamine terminal loss in the brains of the patients, improved neuropsychiatric symptoms such as mood and emotional well-being, and evidence that the drug (when injected) crossed the blood–brain barrier and enhanced insulin signaling in the brain (Athauda et al., 2018, 2019).
Other studies have not had such positive results. A 2022 study by the biotech company Peptron Inc. examined the effects of a sustained-release form of exenatide, PT320, over 48 weeks and found no significant effect on motor control, although there was at least one significant improvement in a quality-of-life measure. The results have not been published, Athauda said, though they were described in a press release (Chang, 2022). The company said it believes there was enough of a signal to continue its studies.
A small study of liraglutide, another GLP-1R agonist, found no significant improvement in motor control, although it did see improvement in some nonmotor measures as well as in some quality-of-life scores (Malatt et al., 2022). A study of the effects of NLY01, a PEGylated1 form of exenatide, on a group of 225 people who had Parkinson’s disease for an average of only 1 year found that 36 weeks of treatment had no effect on motor function or nonmotor measures (McGarry et al., 2024). Another
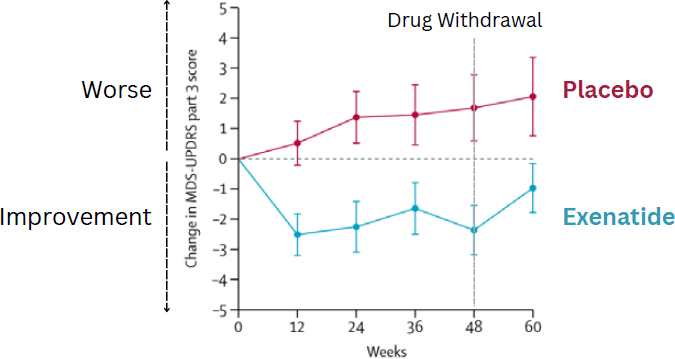
NOTE: MDS-UPSDRS part 3 = Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III.
SOURCE: Presented by Dilan Athauda on September 10, 2024. Adapted from Athauda et al., 2017. Reprinted from The Lancet, with permission from Elsevier.
___________________
1 PEGylation is the act of attaching polyethylene glycol (PEG) chains to a molecule. It is often used with drugs to increase the amount of time they remain in the bloodstream.
trial of a GLP-1R agonist, lixisenatide, for treatment of early Parkinson’s disease did find an effect on motor function, with the treatment group maintaining their score and the placebo group getting worse, although there were no significant changes on secondary outcomes (Meissner et al., 2024).
Given the varying results of these trials, Athauda asked, should researchers continue to explore this class of drugs for use to treat Parkinson’s disease? In light of the strong preclinical evidence, he said, he believes that GLP-1R agonists should have an effect on Parkinson’s disease, and the fact that the clinical trials have used different methods and different drugs and doses justifies additional research. A major question is which of these drugs cross the blood–brain barrier; while exenatide, for instance, does seem to cross the barrier and get into cerebrospinal fluid (Athauda et al., 2017) liraglutide does not (Christensen et al., 2015).
Looking forward, Athauda recommended collaboration and data sharing across GLP-1 clinical trials to identify gaps in knowledge and biomarkers of target engagement. He also said that the newer double and triple agonists (such as GLP-1 combined with GIP and glucagon) show greater promise and may prove to be more effective against Parkinson’s disease.
PENETRATION OF THE BLOOD–BRAIN BARRIER BY GLP-1 CLASS DRUGS AND NEUROPROTECTION
Christian Hölscher discussed the importance of GLP-1 class drugs being able to pass through the blood–brain barrier if they are to help treat neurodegenerative diseases.
He began by describing the potential value of these drugs. GLP-1 is a growth factor that normalizes energy use in the brain, reduces chronic inflammation, and greatly enhances neuronal survival and synaptic activity. Preclinical studies have shown GLP-1R agonists to have good effects on both Parkinson’s disease and Alzheimer’s disease; for instance, more than a decade ago the diabetes drug liraglutide had positive effects in a mouse model of Alzheimer’s disease (McClean et al., 2011). And a 12-month, double-blind, placebo-controlled phase 2 clinical study that Hölscher was involved with found improved cognition and reduced brain shrinkage in Alzheimer’s patients treated with liraglutide (Femminella et al., 2019). It offered a proof of concept that GLP-1 analogs are effective in the clinic, Hölscher said, but the effect was limited, and better drugs are needed. And indeed, he added, two phase 3 trials are now examining the effects of semaglutide in Alzheimer’s patients, so more information should be available soon.
To illustrate the importance of drugs being able to get past the blood–brain barrier, Hölscher referred to two of the same trials that Athauda had discussed. Athauda and colleagues (2017) had found that a once-weekly form of exendin-4 given over 7 months significantly improved motor function in patients with Parkinson’s disease, while McGarry and
colleagues (2024) reported that NYL01, a PEGylated form of exendin-4, given over 36 weeks had no effect on Parkinson’s patients. The difference could be due to NYL01, which is created by adding polyethylene glycol (PEG) polymer chains to the exendin-4 molecule, being too large to cross the blood–brain barrier, Hölscher explained. An experiment by Yun and colleagues (2018) demonstrated this explicitly.
This is a problem for the current generation of GLP-1R agonists, Hölscher said, given that they are designed to treat type 2 diabetes and to stay in the bloodstream as long as possible so that they do not enter the brain well. For that reason, his group has designed some new peptide drugs that are designed to cross the blood–brain barrier more quickly; they are dual agonists, activating both the GLP-1 receptor and the glucose-dependent insulinotropic polypeptide receptor. GIP is a peptide hormone that acts a growth factor, much like GLP-1, and GIP analogs have been shown to have neuroprotective effects in both Alzheimer’s and Parkinson’s patients.
Recent work has shown that the dual agonists developed by his group enter the brain effectively and, in many cases, more quickly than the traditional GLP-1R agonists such as exenatide and lixisenatide (Rhea et al., 2023; Salameh et al., 2020). (Both liraglutide and semaglutide are particularly poor at crossing the blood–brain barrier and did not enter the brain in significant level in these studies.) Tests in the MPTP mouse model of Parkinson’s disease showed that two of the dual agonists developed by Hölscher’s group, DA-JC4 and DA-CH5, were neuroprotective (Feng et al., 2018).
In conclusion, Hölscher said, the fact that these dual agonists can cross the blood–brain better than previous GLP-1R agonists and are better at protecting the brain than single agonists, as demonstrated by animal studies, supports the idea that the new dual agonists “will be much more effective in the clinic.” The group’s most promising dual agonist, DA-CH5, has now entered phase 1 clinical trials.
TREATING IDIOPATHIC INTRACRANIAL HYPERTENSION AND OTHER PRESSURE-RELATED DISORDERS
Alexandra Sinclair discussed the potential of GLP-1R agonists in treating disorders related to increased pressure in the brain and the eyes. In addition to their better-known anti-obesity and neuroprotective effects, GLP-1R agonists also reduce intracranial pressure, interocular pressure, and pain from migraines and neuropathy. This makes it possible that these agonists could be used in the treatment of such things as idiopathic intracranial hypertension, traumatic brain injury (TBI), space-flight-associated neuroocular syndrome, and glaucoma, as well as migraines and neuropathy. Furthermore, she added, the fact that these agonists can act against multiple issues at once broadens their potential clinical utility (see Figure 6-2).
Preclinical research in her lab has determined where GLP-1R agonists are expressed in the brain, traced the neurological pathways by which they work, and shown that one such agonist, exenatide, does a better job of reducing intracranial pressure in lab animals than other known drugs (Botfield et al., 2017). Importantly, she added, the way GLP-1R agonists reduce intracranial pressure is independent of what has caused the pressure, meaning that the agonists can be effective for any condition alleviated by reducing intracranial pressure, including idiopathic intracranial hypertension, TBI, and stroke with raised intracranial pressure.
Focusing on idiopathic intracranial hypertension, she said it is a disease that affects almost exclusively women between puberty and menopause who have obesity. The disease is thought to be driven by metabolic syndrome and excess androgens. People with it have increased intracranial pressure, and the resulting pressure on the optical nerve can lead to blindness as well as headaches. It is a rare disease, occurring in about 2 per 100,000 people, and so far, there are no effective drugs to treat it. In a phase 2 clinical trial, Sinclair’s group found that exenatide produced a significant reduction in intercranial pressure in adults with idiopathic intracranial hypertension; the pressure reduction was independent of weight loss, and the treatment also significantly reduced the frequency of headaches in the subjects (Krajnc et al., 2023: Mitchell et al., 2023).
Turning to the treatment of migraine and headache pain, Sinclair said that GLP-1R expression has been found in the trigeminocervial complex, which is known to play a key role in migraines, and that in a migraine mouse model, a GLP-1R agonist reduced pain by stimulating interleukin 10 (Halloum et al., 2024; Jing et al., 2021, 2023). A small human trial, whose results have not been published, found that a GLP-1R agonist
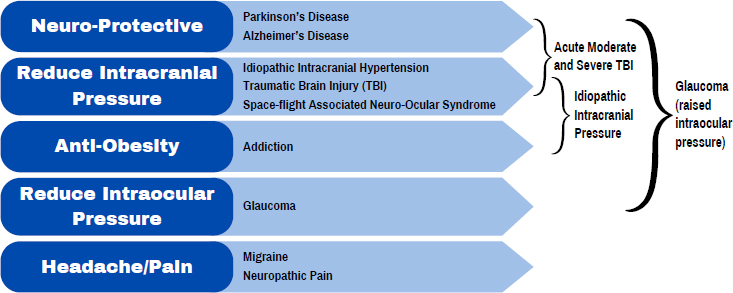
NOTE: GLP-1R = glucagon-like peptide-1 receptor; TBI = traumatic brain injury.
SOURCE: Presented by Alexandra Sinclair on September 10, 2024.
reduced headache frequency but not body mass index (BMI) in a group of 26 migraine patients with obesity.
On the topic of treating neuropathic pain with GLP-1R agonists, Sinclair said that there has been no clinical research, but some preclinical research indicates the agonists could be effective. A typical way that patients develop neuropathic pain is through diabetic or metabolic causes or injury leading to inflammation, and preclinical data indicate that GLP-1R agonists activate the GLP-1 receptors on microglia in the spinal dorsal horn, increasing interleukin 10 levels and reducing markers of inflammation. In mice models, a GLP-1R agonist reduced neuropathic pain and pain signaling.
Concerning TBI, Sinclair continued, the primary way it manifests its effects is through raised intracranial pressure, with leads to an inflammatory cascade and secondary injury, causing brain cell death and other poor outcomes. Preclinical data have shown that GLP-1R agonists have neuroprotective effects against moderate TBI. They attenuate inflammation and improve functional outcomes, including cognitive function (Zhang et al., 2020). However, no clinical trials have examined the use of GLP-1R agonists in treating TBI, nor have any studies examined epidemiological data.
By contrast, there is evidence in humans—from retrospective case reviews—that GLP-1R agonists can reduce intraocular pressure and lower the risk of glaucoma (Hallaj et al., 2025; Niazi et al., 2024; Sterling et al., 2023). This fits with preclinical data, which have found that GLP-1R agonists act in the retinal ganglion cells of the optic nerve to reduce inflammation, oxidative stress, and neuron death (Lawrence et al., 2023; Sterling et al., 2020).
In summary, Sinclair said, GLP-1R agonists may someday be used to treat more than just type 2 diabetes and obesity. They show promise in treating the raised intracranial pressure associated with idiopathic intracranial hypertension and TBI, headache and migraine pain, neuropathic pain, and the raised intraocular pressure that can lead to glaucoma. These wider uses, in turn, may create opportunities for more personalized medicine, as many patients present with more than one of these conditions, such as patients with obesity, diabetes, and migraines or glaucoma. The goal will be to tailor the drugs’ effects to target specific patient subgroups depending on their comorbidities. This will require clever clinical trial designs and trials that are suitably powered.
BIOMARKERS IN DRUG DEVELOPMENT
Laura Jawidzik, the deputy director of the Division of Neurology 1 at the FDA’s Center for Drug Evaluation and Research, provided an overview of the use of biomarkers in drug development. She began by stating that a biomarker is “a defined characteristic that is measured as an indicator
of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions.” The types of biomarkers include molecular (such as a genetic test), histologic (such as a pathological assessment), radiographic (such as magnetic resonance image), or physiologic (such as blood pressure or an electroencephalogram). Biomarkers generally measure disease presence or status or aspects of response to treatment. However, a biomarker is not a measure of how a patient feels, functions, or survives (FDA-NIH Biomarker Working Group, n.d.).
The purposes for which biomarkers are used include making diagnoses, monitoring conditions, monitoring responses, predicting future states, making prognoses, assessing safety, and assessing susceptibility or risk, Jawidzik said. Then she offered a number of examples of biomarkers, including the SMN1 gene, which is used to diagnose spinal muscular atrophy; magnetic resonance imaging, which is used to monitor the status of multiple sclerosis; the ApoE4 gene, which serves as a marker for susceptibility to Alzheimer’s disease; and the safety biomarker alanine transaminase, high levels of which indicate issues with the liver.
FDA urges drug developers to use biomarkers at all stages of drug development, Jawidzik said, from discovery through preclinical work and clinical trials and, finally, post marketing. Specifically, biomarkers can be used in proof-of-concept studies, such as to show that a drug is performing as expected; in selecting subjects for a trial, such as those who are more likely to respond to the treatment; in monitoring, as in looking for patients who may have adverse responses in the trial; and determining efficacy by, for instance, providing a surrogate endpoint for the primary outcome of interest. A surrogate endpoint, she explained, is an endpoint used in clinical trials as a substitute for a direct measure of how a patient feels, functions, or survives. There are two types: a validated surrogate endpoint, which has enough evidence supporting its predictive value that the FDA can use it directly in its approval decision; and a reasonably likely surrogate endpoint, which is supported by a mechanistic rationale or epidemiological data but not by enough clinical evidence to validate it, so it can be used only for accelerated approval of drugs or expedited access to medical devices. An example of a validated endpoint is blood pressure, as there is clear clinical evidence that lowering blood pressure will have health benefits, such as reducing the risk of stroke, so a drug can be approved merely on the basis of its ability to lower blood pressure.
Currently there are no validated surrogate endpoints for use as primary endpoints in clinical trials for drugs with neurological actions to treat neurological diseases, she said, but there are several reasonably likely surrogate endpoints. For example, a reduction in amyloid beta plaque was used for accelerated approval of lecanemab for Alzheimer’s disease, and neurofilament light chain was used as a reasonably likely surrogate for the
accelerated approval of tofersen for treatment of ALS (amyotrophic lateral sclerosis) in patients with the SOD1 mutation. In cases where a reasonably likely surrogate endpoint has been used for accelerated approval, the FDA still requires additional clinical data to show that there is a clinically meaningful outcome.
Biomarkers do not need to be qualified to be used in a drug development program, Jawidzik said, and the type and level of evidence needed to support a biomarker’s use depends on the specific context. The FDA does have a Biomarker Qualification Program,2 but it is generally used to prequalify biomarkers for use in drug development programs so that they can be used without further later qualification.
The FDA requires good scientific evidence for a biomarker to be used in drug development, and that evidence can take several forms. There should be a biological rationale for the use of the biomarker, if it is known. Assays should be analytically validated, and there should be a good understanding of potential sources of variability in the measurements. The relationship among the biomarker, outcome of interest, and treatment (where applicable) should be characterized for the particular proposed use. The types of data used to assess the strength of the association between the biomarker and its proposed outcome can include retrospective or prospective studies, registry data, and randomized controlled trial data.
In summary, Jawidzik said, the FDA encourages the use of biomarkers throughout the drug development life cycle, and it believes that biomarkers can help lead to the identification of more safe and effective therapies. George agreed, saying that a large focus in neurodegenerative diseases is early intervention and preventing neuronal death which reveals a need for biomarkers that can provide information on inflammation and changes in neuroprotective proteins.
DISCUSSION
In response to a question from George, Greig said that insulin resistance in the brain is linked with both Alzheimer’s disease and Parkinson’s disease, but the roles insulin resistance plays in these diseases are not clear. GLP-1R agonists trigger a whole series of cascades that relate to insulin resistance, neurotrophic actions, neuroprotective actions, anti-inflammatory actions, and many other actions, leading to an extremely complex system.
Matthew Coghlan, vice president and head of incretin portfolio science at Eli Lilly and Company, asked about the relative value of GIP analogs
___________________
2 For more information about the FDA’s Biomarker Qualification Program, see https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/biomarker-qualification-program (accessed November 9, 2024).
versus GLP-1R agonists. Hölscher responded that GIP analogs alone do have protective effects, but the effects are much greater when GIP is used in conjunction with GLP-1R agonists. Greig added that GIP can be very effective by itself with the right treatment regimen.
Mahin Khatami, retired from the National Cancer Institute, asked if long-term use of GLP-1R agonists might affect immune-responsive tissue, leading to cancer. Athauda answered that these drugs have been used for a couple of decades in humans, and epidemiological data show no evidence of an increased risk for various cancers, such as pancreatic cancer, thyroid cancer, or renal cancer. Sinclair agreed that long-term data have shown no indication of an increased cancer risk associated with these drugs.
Linda Rinaman asked how GLP-1R agonists exert their protective effect of sparing dopamine neurons from cell death given that these neurons do not have GLP-1 receptors. Greig responded that these neurons do have GLP-1 receptors, as has been shown in multiple ways. Hölscher said that the question of whether the dopaminergic neurons have GLP-1 receptors may not be particularly important anyway because these neurons are part of an entire system, with other types of cells that do have GLP-1 receptors. So if a GLP-1R agonist triggers anti-inflammatory responses in the surrounding cells, the dopaminergic cells would benefit from the agonist even if they themselves did not have the receptors. Rinaman responded that it does matter in the sense that it is important to understand the precise mechanisms by which the GLP-1R agonists exert their effects. Matt Hayes agreed that it is important to understand the mechanisms and said that there is good evidence that there are no GLP-1 receptors on dopamine neurons, at least in the ventral tegmental area.
Next, Hayes commented that as researchers design GLP-1 drugs that can cross the blood–brain barrier, they must take into account adverse effects that would accompany enhanced blood–brain barrier penetrance. So, he asked, what considerations should go into the design of these drugs in terms of this penetrance? Hölscher answered that it will depend on the desired purpose of the drug. Drugs to treat diabetes, for instance, should stay in the blood as long as possible and not cross the blood–brain barrier, whereas drugs for treating diseases of the central nervous system must be able to get into the brain. As for balancing the positive effects with adverse effects, he said that there are relatively few adverse effects to worry about; the drugs have proved to be safe and well tolerated, with minimal side effects. Hayes replied that nausea and vomiting are two well-known side effects of some GLP-1R agonists and that they should not be thought of as “minimal” because they are a leading reason why people stop taking prescribed drugs.
In summary, preclinical work indicates that GLP-1R agonists have potential for treating a number of neurodegenerative and neuropsychiatric
disorders. Clinical research on the use of these agonists for Parkinson’s disease has shown mixed results, while there have been promising results for the treatment of raised intracranial pressure, and clinical testing is ongoing for Alzheimer’s disease. According to several participants, a key factor in the effectiveness of GLP-1R agonists may be how well they cross the blood–brain barrier.
This page intentionally left blank.













