Use of Supplementary Cementitious Materials for Concrete (2025)
Chapter: 2 Literature Review
CHAPTER 2
Literature Review
Introduction
This chapter contains information synthesized from the literature review on SCMs, including conventional SCMs such as coal ash (fly ash), slag cement, and silica fume. The issue of the shortage of fly ash is discussed. Also included in the discussion are harvested or beneficiated coal ash; NPs such as calcined clay, metakaolin, shale, and pumice; and other SCMs such as rice husk ash (RHA). Binary and ternary SCM mixtures are discussed, and the chapter includes a section on ASCMs.
Supplementary Cementitious Material
An SCM is defined by ASTM as an inorganic material that contributes to the properties of a cementitious mixture through hydraulic or pozzolanic activity or both (ASTM C125-15). By definition of ASTM C125-15, hydraulic cements and SCMs that have hydraulic properties react with water to harden, and that hardening process does not require drying. When Portland cement reacts with water in a concrete mixture, the hydration products from the reaction will include C-S-H and CH. The C-S-H is a desirable binder-gel material that contributes to the strength of concrete and has other useful properties. The CH is mostly undesirable. It elevates the alkalinity level in the paste pore solution and subsequently the concrete, but provides little strength, and, in fact, may contribute to material-related distress (MRD) mechanisms in concrete (Sutter 2015).
“Pozzolanic reactivity” refers to the property of a material that needs both water and CH as reactants in order to harden. The pozzolan consumes the CH and produces additional useful C-S-H binder gel in concrete. This pozzolanic reaction is the primary reason why SCMs contribute to concrete durability and mitigate many MRDs. The three conventional types of SCMs used in concrete, and discussed in this report, are coal ash (fly ash), slag cement, and silica fume. Other materials, which may be processed, rehabilitated, or manufactured to possess or generate pozzolanic or hydraulic/cementitious properties, include harvested coal ash, natural pozzolans, and ASCMs. The most commonly used pozzolan is Class F fly ash (Sutter 2016).
In 2023, ASTM updated ASTM C618-15 and adopted it as ASTM C618-23 with a revised title: Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. The updated specification includes several key changes, including a change in the definition of coal ash and some changes in chemical and physical requirements. The term “coal ash” now includes both fly ash and bottom ash produced from current power plants or harvested from landfills and impoundments of previously discarded ash. This specification change is in the process of being adopted in AASHTO M 295 as well. In the interim, this synthesis report will use the term “coal fly ash” (or simply “fly ash”) to distinguish it from “coal bottom ash” or “coal harvested ash.” The terms “coal fly ash” or “fly ash” will be used interchangeably when citing information in this report
from previous guides, research, case studies, specifications, technical briefs, and other references that refer to “fly ash” as the material that meets ASTM C618 in versions prior to the 2023 revision.
Coal Fly Ash
Coal fly ash (fly ash) is an airborne residue from the combustion processes of pulverizing coal; it is predominantly produced in power-generating plants. The fly ash is typically collected from flue gases by a variety of means, including venturi scrubbers, fabric filters, and electrostatic precipitators. Coal fly ash is classified as either fly ash Class F or Class C. Depending on its physical structure (i.e., crystalline or glassy) and chemical composition, fly ash may be pozzolanic, hydraulic, or exhibit both properties based on the principal oxides that affect the fly ash function in cement paste, mortar, or concrete. The majority of fly ash particles are glassy, solid, or hollow and are spherical in shape as singles or clusters. An example of fly ash particle shapes is shown in Figure 1. The ash varies in shape from round to slightly elongated. Individual particles in fly ash may range in size from less than 1 μm to greater than 0.04 in. (1 mm). Coal fly ash must comply with the fineness requirements of ASTM C618-23. This will maintain an efficient pozzolanic reaction of fly ash in concrete. Another important property is the loss on ignition (LOI), which represents the percent quantity of various forms of residual carbon, generated from the combustion of coal, present and intermixed with fly ash. The specified LOI limit is 6% in older versions of ASTM C618. This will minimize loss of entrained air in concrete mixtures. However, ASTM C618-23 allows higher limits with qualified laboratory tests, as explained in the following section. Fly ash has been the most commonly used SCM in concrete since the 1930s (Sutter 2016).
ASTM C618-23 Updates
In ASTM C618-23, Standard Specification for Coal Ash and Raw or Calcined Natural Pozzolans for Use in Concrete, the term “coal ash” includes both fly ash and bottom ash produced from current power plants or harvested from landfills and impoundments. Bottom ash is now clearly defined and specified for use in concrete, provided that it meets the requirements for either fly ash Class F or Class C. The chemical and physical requirements for Class N natural pozzolans are retained in ASTM C618-23. The minimum percentage of oxides (SiO2 + Al2O3 + Fe2O3) for Classes F and C

ash is now 50%, and for Class N it is 70%. Also, the maximum percentage of CaO for Class F is now specified at 18%, and for Class C is specified at greater than 18%. The LOI limit is 6% for Classes F and C. However, a provision has been added to approve the LOI for Class F at up to 12% on the condition of availability of acceptable performance records or laboratory testing. The LOI limit for Class N is 10%.
For physical requirements, ASTM C618-23 has maintained the maximum amount of ash material that can be retained on sieve No. 325 (45 μm) at 34% for all classes, but has also added a requirement of 10% maximum that can be retained on sieve No. 100 (150 m) for Classes F and C. It is assumed that this addition is a further recognition of the importance of the fineness of the ash in supporting more efficient reactions. The strength activity index (SAI) has been retained at a minimum of 75% of the control Portland cement mortar cubes at 7 and 28 days (ASTM C618-23).
Performance of Concrete with Coal Ash
The use of coal ash in concrete improves the properties of concrete in its fresh (plastic), hardening, and hardened phases. In fresh concrete, the use of coal ash improves mixture workability and reduces bleeding. In the hardening phase, coal ash reduces the heat of cement–water hydration and subsequently controls the rise in concrete temperature. In the hardened phase, coal ash improves concrete strength at later ages, reduces permeability to enhance durability, and mitigates shrinkage and shrinkage cracking.
As a result of enhanced durability, the concrete will have an increased resistance to sulfate attack and ASR, as well as more resistance to the ingress of corrosion-inducing chlorides from seawater. In cold regions, the use of fly ash with slag or silica fume in concrete mitigates the adverse chemical reaction of chloride-based deicing salts and increases resistance to the damaging effects of freeze–thaw cycles and surface scaling.
Table 1 shows a summary of changes in the properties of concrete when Class C or Class F coal ash partially replaces cement content in a concrete mixture (Kosmatka and Wilson 2011). It should be emphasized that the performance of concrete with fly ash may vary when the source of fly ash is changed because of possible differences in the chemical and physical properties of the ash. Variability can also occur in fly ash obtained from the same source over time due to changes in the energy production process, fly ash handling process, and other factors. A state DOT specification may require mixture design verification and concrete testing when the source of the fly ash is changed.
Concrete performance will also be affected by the rate of cement replacement with fly ash (Sutter 2016). The most effective method for evaluating the performance of fly ash in concrete
Table 1. Changes in the properties of concrete when Class C or Class F coal ash is used to replace Portland cement in a mixture (Kosmatka and Wilson 2011).
| Property | Class F Replacement | Class C Replacement |
|---|---|---|
| Initial set | Delayed | Delayed |
| Rate of strength gain | Slower | Same or higher |
| Heat of hydration | Significantly lower | Lower |
| Early strength (3–7 days) | Lower | Higher |
| Late strength (28–56 days) | Same or higher | Same or higher |
| ASR mitigation? | Significant mitigation | Only at high replacements |
and establishing proper mixture proportions for a specific application is preparing trial batches and performing concrete tests. The reason that trial batching and tests are necessary is that fly ash sources can have similar chemical and physical properties but have different pozzolanic or hydraulic reactivity (ACI 232.2R-18). Fly ash is normally used at the rate of 15% to 35% by mass of total cementitious material. Higher percentages of fly ash (high-volume fly ash) reaching 40%, 50%, and even 70% are used by some state DOTs to help reduce the rate of heat of hydration and control the rise in temperatures in mass concrete.
High-Volume Fly Ash
High-volume fly ash (HVFA) concrete is defined by ACI as having a large replacement by mass of cement with fly ash that exceeds 37% and can reach more than 50% in the concrete mixture. As shown in the survey findings in Chapter 3, several state DOTs are using HVFA in concrete construction. The main benefit of HVFA in concrete is the ability to achieve enhanced sustainability due to the large reduction in the Portland cement concrete in the mixture. Other important benefits include a significant reduction in the heat of hydration, lower concrete permeability and associated enhanced durability performance, and reduced potential for early cracking due to the slower strength development in concrete (ACI 232.3R-14). The low strength development of HVFA at an early age can be improved by adjusting the mixture ingredients such as through the use of a lower water to cementitious materials ratio (w/cm), adding water-reducing admixtures for efficient reactions, and including silica fume.
With its very low permeability and high durability, HVFA mixtures will resist sulfate attack, ASR, and steel corrosion in concrete (ACI 232.3R-14). In marine structures, the use of HVFA concrete will mitigate penetration of corrosion-inducing chlorides from sea water. The pozzolanic reaction of HVFA may create a more acidic environment in the concrete as CH is significantly depleted, which may promote a favorable environment for corrosion. However, the very low permeability of HVFA concrete in the marine structure will significantly delay penetration of chloride ions in reaching the reinforcement to start the corrosions process (Bentz et al. 2013).
Properties of Fresh Concrete with Coal Ash
Workability and Water Demand
Coal ash improves workability and cohesiveness and may reduce water demand for the same workability in fresh concrete. Since coal ash has a lower specific gravity (2.28–2.7) than that of Portland cement (typically 3.15), replacement of a portion of the cement mass with coal ash will increase the volume of the mixture paste for a given w/cm, thus increasing the plasticity and cohesiveness of the mixture (Lane 1983). Also, the spherical shape of the fly ash particles will increase the mobility of the mixture and may permit the mixture water to be reduced for a given workability (Brown 1980). Also, coal ash in air-entrained and non–air-entrained concrete mixtures reduces bleeding by providing a greater surface area of solid particles and requiring a lower water content for a given workability (Idorn and Henriksen 1984; Khayat et al. 2008).
Set Time
The use of coal ash as a replacement for cement can delay the set time of concrete. The extent of the delay is dependent on the rate of cement replacement. The set time can be managed by the use of accelerating admixtures and by adjusting mixture proportioning (ACI 232.2R-18). In pavements, the longer set time facilitates finishing and texturing of pavement slabs but may also promote plastic shrinkage cracks in conditions of high evaporation rate from the pavement surface due to hot or windy weather. Timely and effective surface curing will reduce the rate of evaporation and mitigate plastic shrinkage cracks.
Air Entrainment
When using coal ash in concrete mixtures, there may be a need to increase the dosage of air-entraining admixture (AEA) to achieve the required air content. This issue is especially critical in regions with freezing temperatures and the resulting need for adequate entrained air. Coal ash in concrete may reduce the entrained air content due to the adsorption of the air bubbles that are generated by the AEA and by the unburned carbon and other residue in the ash (ACI 232.2R-18; Sutter 2016).
ASTM C618-23 specifies the limit of LOI, which is a measure of the amount of unburned carbon in ash, to 6% for Class F (with conditional approval for up to 12% based on acceptable performance or laboratory test results) and 6% for Class C coal ash. The limitation on LOI is to better stabilize the air content in air-entrained concrete to resist the negative impact of freezing temperatures. However, unburned carbon is not the only contributor to the adsorption of air, as two types of ash may have the same LOI but produce different results with respect to air content. For example, in recent years, some power-generating plants have begun using powdered activated carbon (PAC) as an additive in the coal combustion process to adsorb mercury from flue gases. PAC is highly adsorptive, more so than the unburned carbon normally found in fly ash, and a small amount may not significantly affect the LOI value but can drastically affect the ash adsorption properties (Sutter 2016). Newly developed tests, such as the foam index test, iodine number test, and direct adsorption isotherm test, provide different approaches to measuring ash adsorption (Sutter et al. 2013). However, these tests have not yet been included in ASTM C618-23.
Heat of Hydration
As cement reacts chemically with water (hydration), the exothermic reaction generates heat that further energizes the hydration process and elevates the concrete temperature during the hardening process (ACI 232.2R-18). Replacing a portion of the cement mass in a concrete mixture with coal ash reduces the heat of hydration and the mixture temperature. The rate of reduction in the heat generated and the overall concrete temperature depend on the quantity and class of coal ash replacing the cement content. The pozzolanic reaction between Class F ash and CH does not add to the heat of cement hydration and, thus, will limit the rise in the overall concrete temperature, which is crucial in mass concrete. However, some Class C ashes with high CaO engage in some hydration processes, which may generate heat that contributes to the rise of concrete temperature. Measurement of the fresh concrete temperature and needed adjustments to the mixture ingredients and proportioning will ensure compatibility of the mixture with the type of application and the prevailing weather conditions under which the concrete is being placed and hardened.
Properties of Hardened Concrete with Coal Ash
Compressive Strength
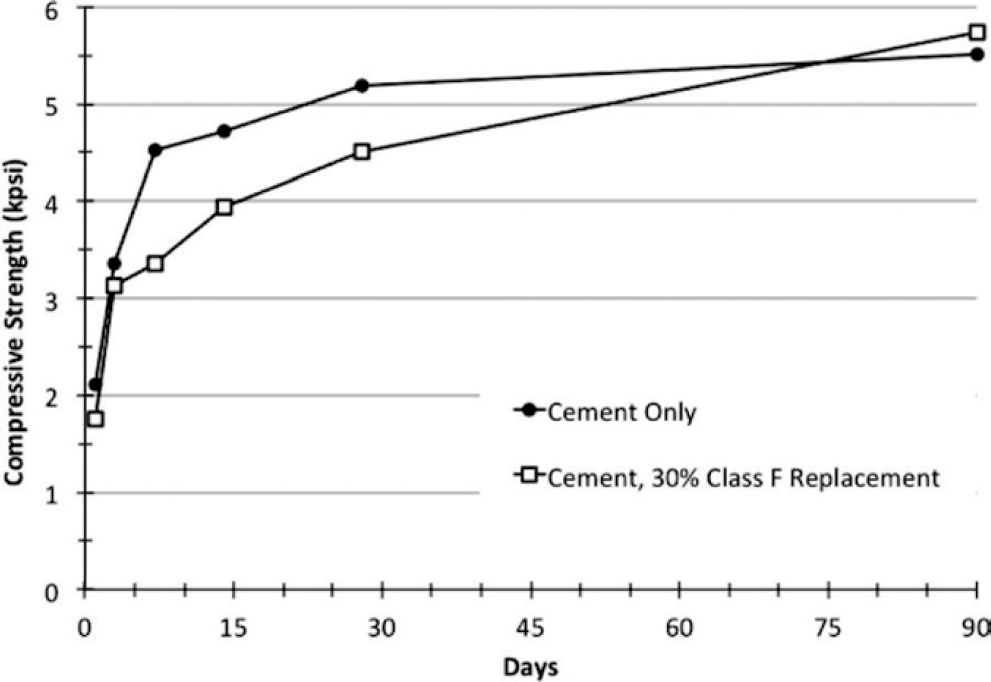
The compressive strength of concrete with coal ash is lower at early ages when compared to a control concrete mixture with no ash (ACI 232.2R-18). However, at later ages (beyond 28 days), the strength of concrete with fly ash starts to approach that of control concrete, and it exceeds the strength of control concrete at close to 91 days, as shown in Figure 2. Class C fly ash often exhibits a higher rate of reaction at early ages than Class F fly ash (Sutter et al. 2014). Also, using accelerating admixtures or water reducers, adjusting other mixture ingredients, or using ternary mixtures with silica fume will increase the early strength of concrete at 3 and 7 days, which is similar to the strength development for a control mixture with no coal ash.
Permeability and Durability
Use of fly ash in concrete mixtures reduces the permeability and improves the durability of concrete structures so that they better resist ASR, sulfate attack, and chloride penetration; it also
mitigates the impact of freeze–thaw action. Concrete permeability is reduced as a result of the pozzolanic reaction, where fly ash chemically reacts with CH and water to produce additional C-S-H binder gel that fills the pores in the hydrated paste, thus reducing the overall permeability of the concrete. Other important factors that contribute to lower concrete permeability are the use of low w/cm, better-graded aggregates, and water-reducing admixtures in the mixture, as well as effective curing of the concrete (ACI 232.2R-18). A study in Florida showed a significant reduction in water permeability (KH2O) with an increase in the percentage of fly ash replacement in cement in concrete mixtures (Figure 3) (Armaghani et al. 1992).
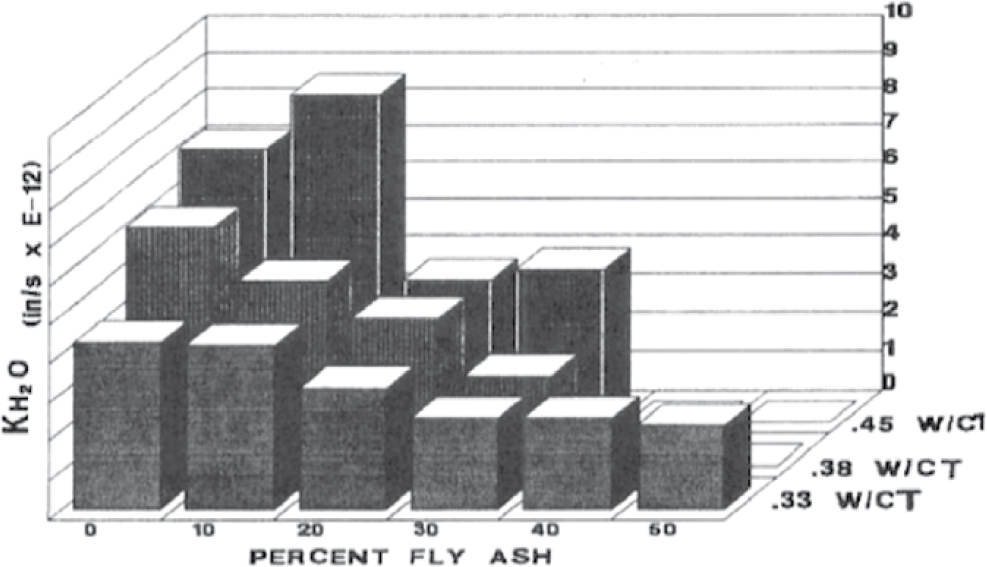
Figure 3. Effect of fly ash and w/cm on the water permeability of concrete (Armaghani et al. 1992).
Resistance to ASR
The use Class F fly ash with lower CaO and higher SiO2 content is effective in controlling the deleterious expansion of concrete due to ASR. The pozzolanic reaction of Class F fly ash depletes alkalis (specifically CH) in the paste. The reduction in hydroxyl ions associated with the consumption of CH leads to resistance to ASR and the accompanying expansion that causes cracking and deterioration of concrete. The amount of fly ash required in concrete to resist or limit ASR to an acceptable level is largely a function of the alkali content of the cement and the reactivity of the silica aggregate. Higher amounts of fly ash are required with higher alkalis in cement. Class C ash, with high CaO, is not as strongly pozzolanic as Class F ash; therefore, it consumes less CH and does not produce the same resistance to ASR as does Class F ash. If Class C ash with high CaO is used for ASR mitigation, relatively higher replacement levels are needed (e.g., 35% to 45%) (Sutter 2016).
Sulfate Resistance
Sulfate attack on concrete involves the reaction of sulfates from outside sources, such as soils in solution or sulfate-rich groundwater, with the paste portion of the concrete and, specifically tricalcium aluminate (C3A), to form an expansive product (calcium sulfoaluminate) inside the concrete. This adverse reaction results in expansion of the concrete, followed by cracking, which opens the concrete to more damaging reactions. This continuous reaction will ultimately lead to deterioration and disintegration of the concrete (ACI 232.2R-18). The damaging sulfate reaction can also be increased when the mixture aggregates contain a high sulfate content.
Class F fly ash can improve concrete’s resistance to the ingress of soluble sulfate solutions (Dhole et al. 2013). The increase in sulfate resistance is due in part to the continued reaction of fly ash with CH in concrete to form additional C-S-H binder gel, which fills in capillary pores in the cement paste, thus reducing the permeability of concrete and resisting the penetration of sulfate solutions to start the adverse reaction. Other mitigating factors are related to mixture ingredients and construction. These factors include low w/cm and effective concrete curing to further reduce concrete permeability (ACI 232.2R-18). However, the situation with Class C fly ash is somewhat less clear. Concrete mixtures that include Class C fly ash with a high percentage of CaO, especially when used at high rate of cement replacement (30%), are highly susceptible to sulfate attack and deterioration (Tikalsky and Carrasquillo 1993).
Resistance to Chloride Penetration
Reduction in concrete permeability will significantly slow the ingress of chloride ions from seawater and deicing salts on bridges in cold regions. Resistance to chloride penetration reduces the potential for early corrosion of the steel reinforcement in concrete (ACI 232.2R-18). One study demonstrated that even when small amounts of 8% to 12% of ultrafine fly ash were used as a cement replacement, a significant reduction in chloride diffusion was observed at early and later ages (Obla et al. 2000).
Resistance to Freeze–Thaw Damage
Research findings show that concrete with and without fly ash shows similar resistance to cycles of freezing and thawing (ACI 232.2R-18). The resistance to damage from freezing and thawing is dependent on mitigating factors related to the mixture ingredients and proportioning, primarily the adequacy of the air-void system, the quality of aggregates in the mixture, the maturity of the concrete, and the concrete’s moisture condition (Mindess et al. 2002).
Availability of Coal Fly Ash
Coal fly ash has historically been heavily relied upon by state DOTs to lower concrete permeability and provide durability benefits. However, since 2010, many North American coal-fired
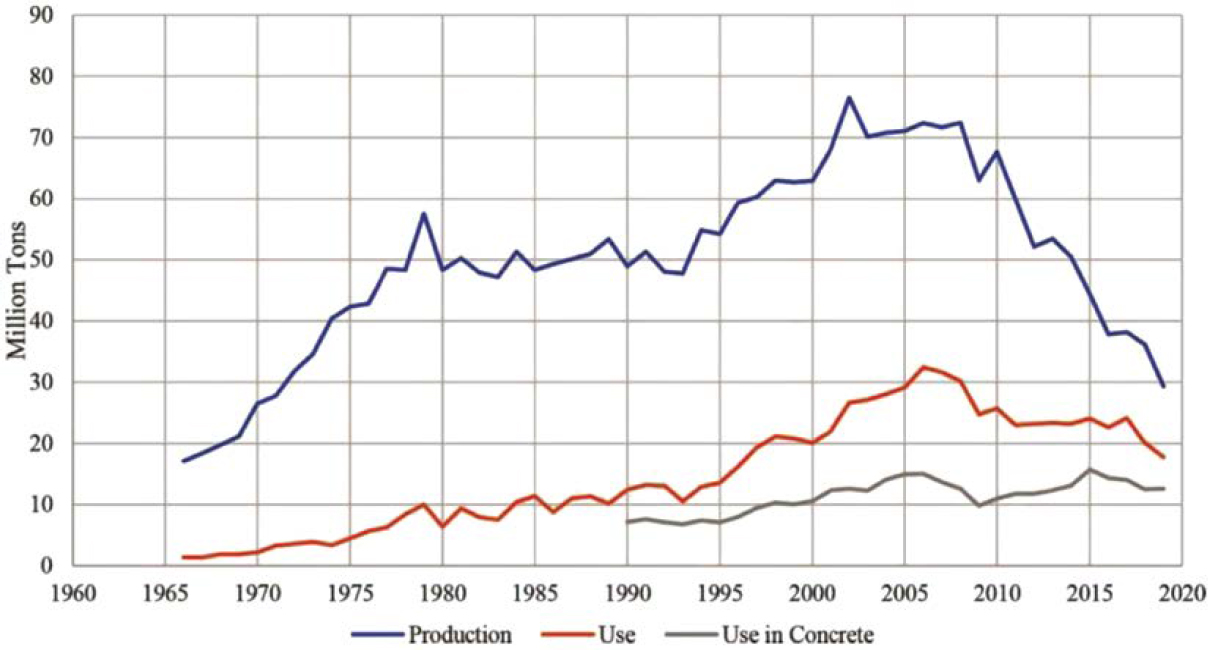
power plants have closed or been converted to natural gas, causing a reduction in ash production and availability in many regions of the United States. Between 2010 and 2018, total coal ash production declined by 57% (Figure 4). In 2018, 20 million tons of fly ash were produced, with 12.5 million tons used in concrete, which was a decline of 11% from 2017 [American Coal Ash Association (ACAA) 2020].
In 2022, more than 28 million short tons of coal fly ash (obtained mainly from combustion of powdered coal in power plants) were produced in the United States, including 11 million tons (39%) used in concrete and concrete products. The total coal fly ash quantity of 2022, when compared to the 16 million tons produced in 2015, is a reduction of 36%. Also, the coal fly ash used in concrete in 2022, when compared to the 16 million tons used in concrete in 2015, is a reduction of 31%. (ACAA n.d.a; ACAA n.d.b). Actions by the state DOTs to mitigate impact of the depletion of coal fly ash are presented in Chapter 3.
Harvested Coal Ash
Use of harvested coal ash is one of the solutions for the decline in the availability of coal fly ash. Over the years, a significant amount of ash that had been produced in coal-burning power plants was discarded in landfills due to noncompliance with the prevailing specifications governing beneficial reuse at the time, including previous versions of ASTM C618 for fly ash use in concrete. Much of the landfilled ash in its current form may be unsuitable for use in concrete without reprocessing and rehabilitation (ACI PRC-232.5-21). Harvesting ash from landfills or impoundments, when beneficiated, is currently feasible and will likely become an increasing source of coal ash supply over the next few years. However, prior to any harvesting of landfilled coal ash, it should be evaluated to determine (a) its heterogeneity, (b) its viability for use as concrete pozzolan, and (c) beneficiation measures that may be needed, such as carbon reduction and separation of deleterious substances (Sutter 2016).
Harvested ash needs to be improved to meet the requirements of ASTM C618-23. The improvement process involves drying, additional burning to remove excess carbon to lower the LOI, filtering adverse materials, and grinding to increase its pozzolanic reactivity. When processed, the ash needs to meet the chemical and physical specifications of ASTM C618-23 (similar
to coal ash Classes F and C) to be permitted for use in concrete. This improvement process will mitigate any negative impact of the ash on the air content of the fresh concrete and will achieve the rejuvenated pozzolanic properties that will ensure improved strength and durability of the hardened concrete (as with the use of coal ash Classes F and C).
Harvested coal ash varies in properties from source to source, similar to freshly produced fly ash. The harvested ash is typically pozzolanic, with high-silica oxide and low CaO content, and as such, it closely resembles Class F ash rather than Class C ash with its higher CaO content (Diaz-Loya et al. 2019; Al-Shmaisani et al. 2018). Some research attributes the low CaO in harvested ash to the ash’s consumption by hydraulic reaction during lengthy storage.
The LOI, which is generally considered a measure of unburned carbon in ash, may be elevated in harvested coal ash. Some harvested coal ash, depending on the CaO content, may contain chemically bound water in the form of hydrates. If hydrates are present, they will decompose during the LOI test, increasing the LOI value measured for the sample. However, it is important to note that an elevated LOI value due to the presence of hydrates in harvested ash does not imply that a concrete mixture with harvested ash will suffer a reduced air entrainment compared to a higher LOI from ash with high carbon content. Carbon analyzers based on infrared detection techniques and other technologies such as thermogravimetric analysis can help distinguish carbon content from hydrates in harvested coal ash (Wirth et al. 2019; Milla et al. 2019).
Strength activity index results for harvested coal ash may be slightly lower, higher, or comparable to those of conventional fly ash, depending in part on the particle size distribution of the material. The strength activity index of harvested coal ash can be enhanced by flash calcining, reduction of organic carbon (LOI), and particle size reduction through sieving and grinding (Diaz-Loya et al. 2019).
Slag Cement
Slag cement, representing ground and granulated blast-furnace slag, is a by-product of the manufacturing of iron. The iron manufacturing involves a blast furnace that is continuously charged from the top with iron oxide (ore, pellets, and sinter), fluxing stone (limestone or dolomite), and fuel (coke). Two products are formed inside the furnace: molten iron, which is collected in the bottom of the furnace, and blast-furnace slag, which floats on the pool of molten iron. Both are periodically tapped from the furnace at a temperature of approximately 2,700°F (1,500°C). The tapped blast-furnace slag is then quenched, almost instantaneously, with water to bring its temperature down below the boiling point of water, producing slag that has a high glassy content and is conducive to high cementitious properties after grinding.
The slag granules are dewatered, filtered from metallic iron residue, and ground to the proper fineness. The ground slag is referred to as “slag cement” (Figure 5). It is typically ground finer than Portland cement. The rate of reaction of slag in concrete increases with an increase in the slag’s fineness. Slag cement can be used as a separate material stored in a silo or bin at project sites or blended and ground with cement clinker to produce Portland blast-furnace slag cement (ACI 233R-17).
The main benefits of using slag cement as a replacement SCM for Portland cement in concrete are:
- Better concrete workability for easier placement, compaction, and surface finishability;
- Higher compressive and flexural strengths;
- Lower permeability for higher durability and resistance to chemical attack; and
- Lighter-colored cement for producing a lighter-colored concrete.

According to ASTM C898-18, the slag cement chemical composition should include oxides of silicon (silica), aluminum (alumina), calcium (lime) and magnesium. These oxides represent about 95% of slag cement (ACI 233R-17). The remaining 5% includes small amounts of sulfur and oxides of iron and manganese. Table 2 shows the range of chemical composition of slag cement (ACI 233R-17). With respect to fineness, ASTM C898-18 limits the amount of slag cement retained on a 45 μm (No. 325) sieve to 20%. Also, ASTM classifies slag cement in three strength grades: 80, 100, and 120. The strength grades are determined based on the SAI. In this context, SAI represents the ratio of the strength of slag cement mortar to the strength of cement mortar at 28 days. The minimum SAIs for slag cement grades 80, 100, and 120 are 75%, 95%, and 115%, respectively.
The specific gravity of slag cement ranges from 2.85 to 2.94, depending on the slag source, as compared to 3.15 for Portland cement [Slag Cement Association (SCA) n.d.b]. Therefore, a greater volume of slag cement may be used to replace the same mass of Portland cement and increase the volume of the paste, thus improving the workability of the mixture without increasing the w/cm ratio. Also, slag cement is light in color, which makes the color of concrete lighter and increases its reflectivity compared to concrete with only Portland cement (SCA n.d.c).
The silica content in slag cement is higher than that in Portland cement. Also, the CaO content is high, which makes slag a cementitious as well as a pozzolanic material (Table 3). However, when slag is mixed alone with water, the initial hydration is much slower than that of cement hydration.
Table 2. Range of chemical composition of slag cement in the United States and Canada (ACI 233R-17).
| Chemical Composition | Percent by Mass |
|---|---|
| SiO2 | 32 to 42 |
| AL2O3 | 7 to 16 |
| CaO | 32 to 45 |
| MgO | 5 to 15 |
| S | 0.7 to 2.2 |
| Fe2O3 | 0.1 to 1.5 |
| MnO | 0.2 to 1.0 |
Table 3. General chemical composition of slag cement versus cement (ACI 233R-17) 2022.
| Chemical Composition | Portland Cement Percentage | Slag Cement Percentage |
|---|---|---|
| CaO | 65 | 45 |
| SiO2 | 20 | 33 |
| Al2O3 | 4 | 10 |
| Fe2O3 | 3 | 1 |
| MgO | 3 | 6 |
The participation of Portland cement in the hydration process produces heat, C-S-H binder gel, and CH. The heat of Portland cement hydration energizes the hydration of the slag cement, including the pozzolanic reaction with CH, to produce additional C-S-H binder gel that fills the large pores in the cement paste, making it denser and the concrete less permeable (ACI 233R-17; Sutter 2016).
Performance of Slag Cement Concrete
In concrete, slag cement is used to replace cement by mass from 20% to 80%, depending on the application. The rate of replacement of slag cement in the concrete mixture is usually dictated by requirements for strength, permeability, workability, time of set and finishing, heat generation, and the level of required resistance to ASR, sulfate attack, and chloride penetration (SCA n.d.d). For maximum strength of concrete at 28 days, a replacement of 40% to 50% has been recommended, but this may vary depending on the slag grade. This is normally the optimum percentage range used in concrete to provide the lowest permeability to the ingress of chlorides and greatest resistance to ASR and sulfate attack (SCA n.d.d). For example, where high sulfate resistance is required, slag cement content of at least 50% by mass of the total cementitious material is used in the concrete mixture, unless previous testing has indicated that a lower percentage is adequate (Hooton and Emery 1990).
As a by-product of steel manufacturing, rather than being discarded in landfills, slag is recovered and processed for subsequent use in concrete to enhance the concrete’s fresh and hardened properties. In a typical binary concrete mixture, slag cement may replace 25% to 50% of the cement content in concrete mixtures to produce high-strength and high-performance pavements and structures. With such significant replacement of Portland cement, slag cement contributes to the reduction of greenhouse gas emissions and enhances environmental sustainability. Also, as an added environmental benefit, the light color of slag cement increases concrete reflectance and, thus, reduces the urban heat island effect (ACI 233R-17; SCA n.d.d).
The replacement rates used by state DOTs for pavements and structures are shown in Chapter 3. When using slag or any other SCM as a replacement for the Portland cement, trial batches and demonstration placements may be needed to arrive at a successful slag mixture for the type of ingredient used, type of application, and the climatic conditions in which the concrete is batched, transported, placed, consolidated, and finished (ACI 232.2R-18 and ACI 233R-17).
Properties of Fresh Concrete with Slag Cement
Workability and Water Demand
In general, slag cement improves the workability and cohesion of concrete mixtures due to the added paste volume from a high replacement rate for Portland cement and the lower specific gravity of slag (2.85–2.94). The use of trial batches and demonstration placements usually
results in successful slag concrete mixtures. Trial mixtures should include the specific mixture materials to be used for the specific application and the environmental conditions of a project. In lean mixtures, additional cementitious material, such as slag cement, will improve workability and cohesion to facilitate placement and consolidation and achieve better finishing characteristics. In concrete mixtures containing a high content of cementitious materials (cement plus slag cement), the concrete may be sticky and have poor finishability. The stickiness of the mixture is normally resolved by increasing the proportion of coarse aggregates to fine aggregates or adjusting the type or dosage of chemical admixtures (ACI 233R-17).
The water demand for a given slump may be 3% to 5% lower in concrete mixtures containing slag cement than in those without slag cement. The degree of water reduction will depend on the amount of slag cement used, properties of the aggregate, and other characteristics of the mixture. This variability, if evident, may be minimized by changes to the aggregate proportions or by changing the type or dosage of chemical admixtures (Meusel and Rose 1983).
Set Time
Delays in set time can be expected when more than 25% slag cement is used as a replacement for Portland cement in a concrete mixture. The degree to which the set time is affected depends on the temperature of the concrete, the percentage of replaced Portland cement, the admixture type and dosage used, the w/cm, and the characteristics of the Portland cement (ACI 233R-17). Higher temperatures (greater than 85°F) accelerate the set time even at 50% slag replacement of Portland cement (Hogan and Meusel 1981).
Temperature Control of Mass Concrete
Use of slag cement at appropriate replacement levels can be an effective means of controlling temperature rise in mass concrete (ACI 233R-17). This is illustrated in Figure 6, which shows the effect of slag cement on temperature rise in mass-concrete specimens cast in warm and cool weather (Soutsos et al. 2016). In this study, 4.9 ft (1.5 m) cube specimens were insulated with expanded polystyrene sheets on all but one of the surfaces used. As shown in Figure 6, concrete with 50% slag cement showed both a delay in reaching the maximum concrete temperature and a reduction in peak temperature compared to concrete without slag cement. These differences were more evident in cool weather than in warm weather.
Reducing concrete temperature is a major benefit of using of slag cement in concrete mixtures used in mass structural members. Some state DOTs, including the Florida Department of Transportation (FDOT), limit the allowable maximum temperature as well as the temperature differential between the surface and center of the mass member (FDOT 2024). Use of a high dosage of slag cement in mass concrete can contribute to achieving the temperature limitations required in the specifications. The specifications may also require implementing a cooling plan that can include the use of cooling pipes, staged placement of concrete, or cooling the batched concrete to ensure that the specified maximum allowable concrete temperature is not exceeded.
Properties of Hardened Slag Concrete
Compressive Strength
Slag cement concrete may lag in early-age strength compared to concrete without slag. However, at ages beyond 3 days, the strength development of concrete with slag achieves and then surpasses the strength development of no-slag concrete. One study showed that no-slag control concrete gains higher strength from 1 to 3 days (Hogan and Meusel 1981). Beyond 3 days, concrete with 40%, 50%, and 65% slag content reaches the strength of the control concrete and surpasses it after 7 days, as shown in Figure 7. At 28 days, concrete with 40% slag cement shows the
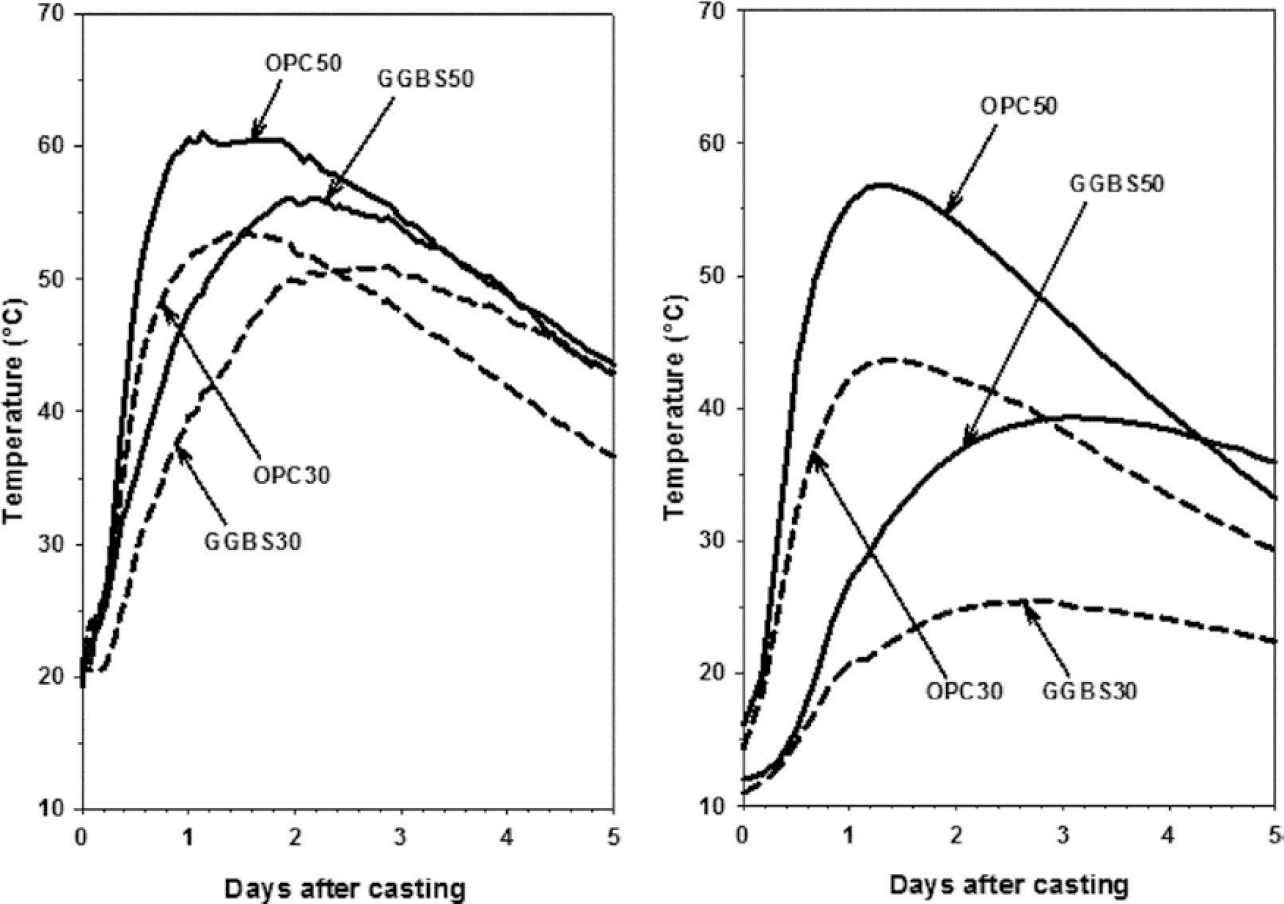
Figure 6. Temperature rise of 4.9 ft (1.5 m) concrete blocks cast in warm (left) and cool (right) weather with 0% and 50% slag cement (Soutsos et al. 2016).
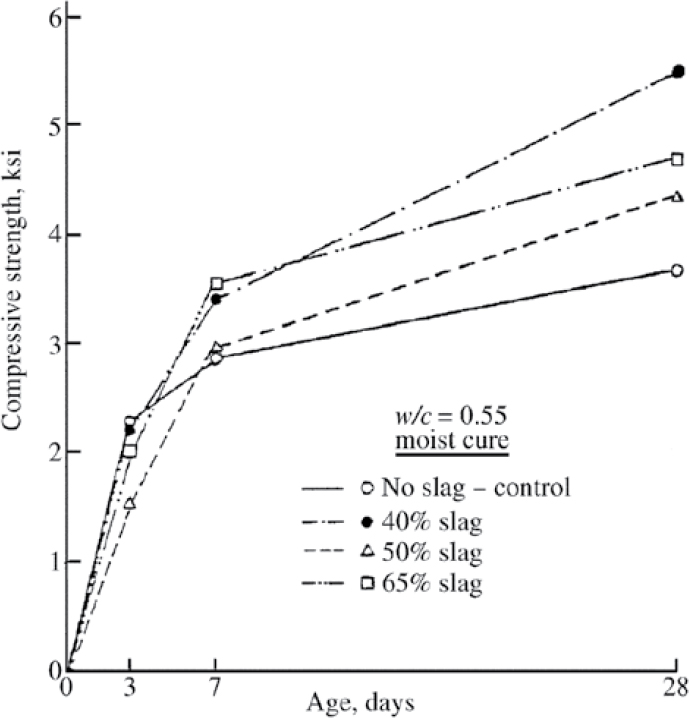
Figure 7. Compressive strength of concrete containing various percentages slag cement content compared with no-slag concrete (Hogan and Meusel 1981).
highest strength. Similar trends have been observed in the development of flexural strength and modulus of elasticity over time. Other factors that may influence the rate of strength development in slag cement concrete include slag grade (80, 100, and 120), w/cm, ambient temperature, the use of admixtures, and the effectiveness of curing (Hogan and Meusel 1981; ACI 233R-17).
Permeability and Durability of Concrete with Slag Cement
The permeability of concrete with slag cement at a mature age is much lower than that of concrete with no slag. The level of permeability decreases as the slag cement content increases (Hooton and Emery 1990). The reduction in concrete permeability is the result of densification of the cementitious paste due to reduction of the pore size from additional C-S-H binder gel produced from the pozzolanic reaction between the slag cement and the CH in the paste. Other major factors in reducing concrete permeability are low w/cm and exercising proper curing to maintain an efficient pozzolanic reaction (ACI 233R-17).
The use of low-permeable slag cement improves the durability and service life of concrete structures by increasing resistance to adverse chemical reactions, such as ASR and sulfate attack, and reduces chloride penetration that contributes to corrosion in concrete reinforcement. Also, by reducing the heat generated in mass-concrete elements, slag cement contributes to controlling temperature rise at early ages to prevent thermally induced cracking and loss of durability.
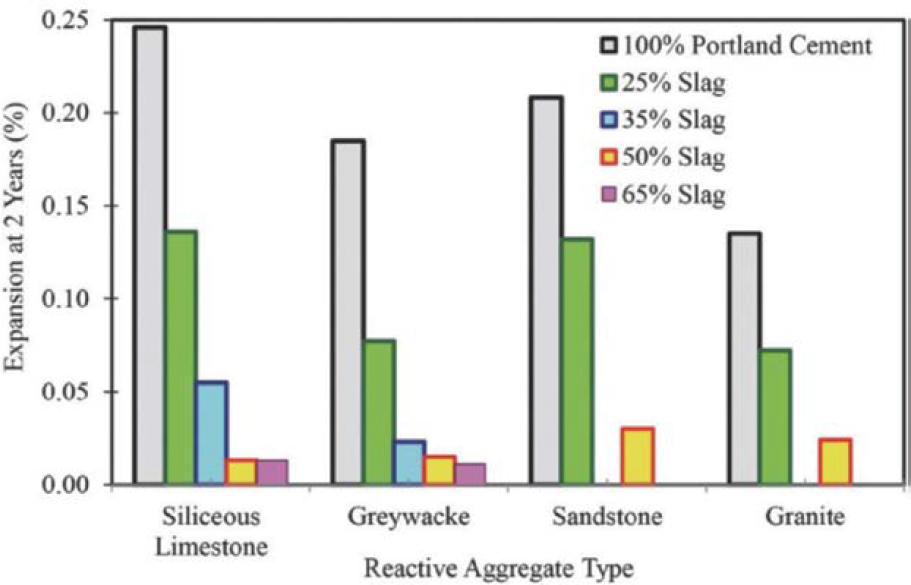
Resistance to ASR
Use of slag cement as a partial replacement for Portland cement is known to reduce ASR and its associated damaging expansion of concrete (Hogan and Meusel 1981). The resistance to ASR and concrete expansion increases with a higher percentage of slag cement, as shown in Figure 8 (SCA n.d.d). The benefits of slag cement in concrete are twofold. First, the pozzolanic reaction of alkalis and CH in the slag cement reduces much of alkalis needed for ASR, which in turn minimizes the formation of the expansive ASR product. Second, the slag’s ability to reduce the permeability of the concrete reduces the availability of moisture as a catalyst in the ASR reaction and the formation of expansive ASR products (ACI 233R-17).
Resistance to Sulfate Attack
Partial replacement of Portland cement with slag cement improves the sulfate resistance of concrete. The resistance increases with an increase in slag cement content in the mixture (ACI 233R-17). High resistance to sulfate attack has been demonstrated when the slag cement proportion exceeds 50% of the total cementitious material, as well as when Type II cements are used (Hogan and Meusel 1981). Three reasons have been cited for the resistance to sulfate attack. First, a reduction of C3A, which is the main cement constituent vulnerable to sulfate attack. Second, the depletion of CH by the pozzolanic reaction reduces the adverse reaction of CH and sulfates to form the destructive calcium sulfoaluminate. Third, the reduction in the permeability of concrete prevents the ingress of sulfates to begin the adverse reaction (ACI 233R-17). Ensuring the use of mixtures with low C3A cements and low w/cm, as well as applying proper curing, will significantly contribute to the resistance to sulfate attack. It should be noted that the alumina in the slag does not play a role in promoting sulfate attack in concrete with slag cement (SCA 2013).
Resistance to Chloride Penetration
Studies have shown that the reduced permeability of concrete containing slag cement significantly reduces the penetration and progression of chloride ions (ACI 233R-17). The reduction in permeability and resistance to chloride-ion intrusion increases as the level of slag cement increases in the mixture. A slight reduction in the alkalinity of concrete is expected due to the expected depletion of CH from the high pozzolanic reaction with slag. However, any negative impact of chloride-ion penetration as a result of increased alkalinity will be offset by the significant reduction in permeability, which will provide good protection against chloride ingress and steel corrosion. It should be noted that use of slag in concrete is not a substitute for producing quality concrete and following best practices in placement and curing.
With respect to resistance to deicing salts in cold regions, concrete with and without slag cement showed similar resistance to the penetration of deicing chloride salts and the prevention of surface scaling (ACI 233R-17). Therefore, other effective measures are needed, such as the use of appropriate admixtures in the concrete mixture suitable for cold regions, applying effective curing, and producing concrete with lower permeability. A combination of these factors will collectively improve the resistance of concrete to deicing salts and the prevention of surface scaling (ACI 233R-17).
Resistance to Freezing and Thawing
Many studies prior to and during the 1960s and 1970s have shown that the resistance to freezing and thawing of concrete was the same with and without slag cement (ACI 233R-17). Use of air entrainment in concrete has been demonstrated to be the main contributor to resistance to freeze–thaw action (ACI 233R-17). Air-entrained concrete containing 50% slag cement was found to be resistant to freezing and thawing, even though a measurable difference in mass loss was found when compared to the concrete made with Type II Portland cement and tested using ASTM C666/C666M (Procedure A) (Hogan and Meusel 1981).
Silica Fume
Silica fume is a very fine noncrystalline silica generated in electric arc furnaces as a by-product of the production of elemental silicon or alloys containing silicon [Silica Fume Association (SFA) 2022]. Silica fume is typically a gray powder with an appearance somewhat similar to Portland cement or some fly ashes, but is much finer in particle size (Figure 9). It is a highly pozzolanic material due to its very high amorphous silicon dioxide content (≥85%) and very fine spherical particles, which typically average between 0.1 and 0.5 μm. Figure 10 illustrates the difference in fineness between Portland cement (45 μm) and silica fume (0.5 μm) grains, and Table 4 shows a comparison

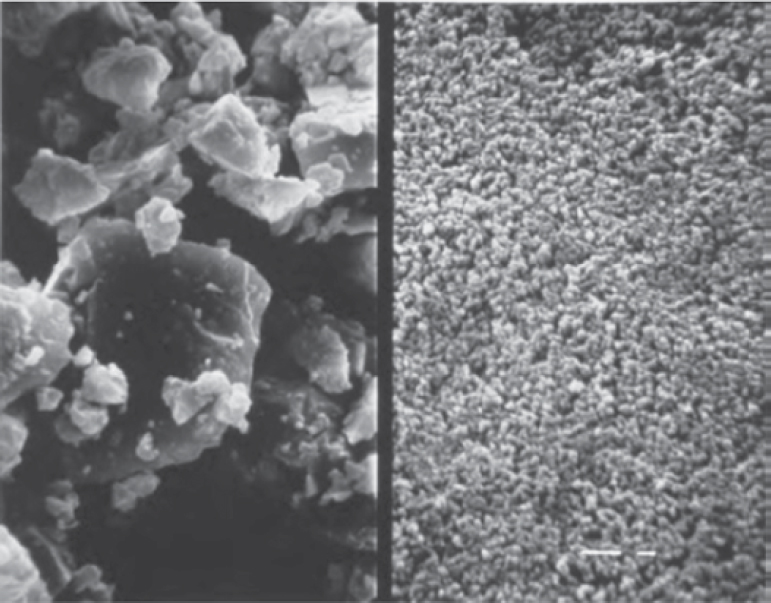
Table 4. Chemical compositions of cement, fly ash, slag, and silica fume (Kosmatka et al. 2016).
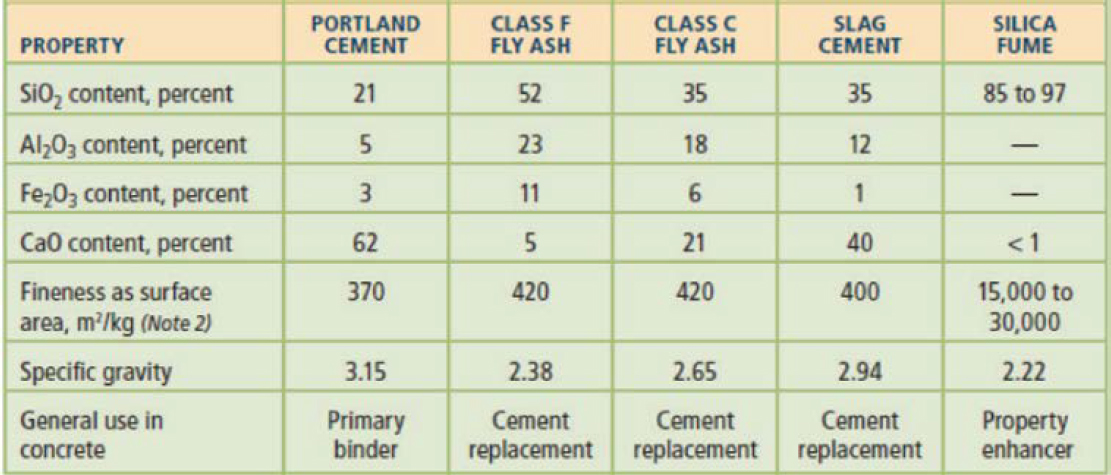
Note: Silica fume fineness was measured by the nitrogen adsorption method; others were measured by the air permeability method (Blaine method).
in chemical compositions among silica fume, Portland cement, and other conventional SCMs. The combination of the very high amorphous silicon dioxide content and extremely fine particles provides silica fume with high pozzolanic reactivity when used in concrete mixtures to enhance the strength and durability of concrete (ACI 234R-06).
Silica fume has been effective as an additive in binary and ternary mixtures to achieve the required strength and durability of self-consolidating concrete and high-strength concrete in high-performance bridges and in precast/prestressed plants (ACI 234R-06), and in ultra-high-strength concrete in accelerated bridge construction (Sutter 2016; Russell and Graybeal 2013).
Silica fume has historically been available in four basic forms: undensified, slurried, densified, and pelletized (SFA 2022). Densified (compacted) silica fume is the most common form used in construction. It is dense enough to be transported economically and can be handled like Portland cement or fly ash at a concrete plant. The densification process greatly reduces the dust associated with the extremely fine silica fume.
Fresh and hardened concrete members constructed using concrete containing silica fume are generally darker than those constructed using conventional concrete mixtures without silica fume. At higher silica fume dosage rates, the dark color of the mixture is more prominent. The color difference may lessen and, in some cases, virtually disappear after some time. Also, incorporating fly ash or slag in the mixture with silica fume will lessen the impact of silica fume’s darker color (SFA 2022; ACI 234R-06).
Silica fume may be added directly to concrete mixtures as a binary ingredient with Portland cement, in ternary mixtures with Portland cement and another SCM, or in quaternary mixtures with Portland cement, coal ash, and slag cement. According to ACI Committee 234 estimates, at least 120,000 metric tons (130,000 tons) of silica fume are used annually in concrete worldwide (ACI 234R-06).
Performance of Concrete with Silica Fume
Silica fume is used mostly as an additive in the concrete mixture and not necessarily as replacement of Portland cement. It is used to produce high-performance concrete with enhanced fresh and hardened properties. It improves the cohesiveness of the mixture for better placement and finishing quality, and it enhances concrete’s strength and durability. Silica fume is a highly reactive pozzolan. The hardened concrete benefits are twofold. First, the strength of the paste structure will increase, and its porosity will decrease. This leads to an increase in the strength of the paste and of the bond between the cement paste and aggregate particles, which both contribute to increasing the overall strength and other mechanical properties of the concrete. Second, by reducing the size of the pore structure with ultrafine particles and consuming the CH to form C-S-H, the concrete’s permeability and its alkalinity will substantially decrease, which will enhance durability and help resist chemical attacks (ACI 234R-06; Mindess et al. 2002).
Additional details regarding the impact of silica fume on concrete’s fresh and hardened properties are provided in the following.
Properties of Fresh Silica-Fume Concrete
Workability
Fresh concrete containing silica fume is more cohesive and less prone to segregation than concrete without silica fume. However, as the silica fume content is increased, the concrete becomes more cohesive (sticky) and loses some workability. It has been suggested that to overcome the
stickiness of silica-fume concrete mixtures and maintain the same workability, the slump of the of mixture needs to be increased by approximately 2 in. (50 mm) above the slump required for conventional concrete with no silica fume (Jahren 1983). Also, slump loss may be an issue in silicafume concrete mixtures. To control slump loss, adjustments are made in project-specific mixture ingredients, including the adjustment of dosage rates of high-range water reducer (HRWR) and WR (normal-range water reducer) admixtures.
The surface area of silica fume is extremely high due to its very fine particles, as shown in Table 4. As such, adding silica fume to concrete mixtures increases the water demand compared to ordinary concrete mixtures. However, adding more water to the mixture will not be a good option since this tends to negate the positive impact of silica fume on strength and durability. Therefore, HRWR admixtures or plasticizers are included in the mixture and, sometimes, in combination with ordinary water-reducing admixtures (WRAs) to increase fluidity and workability of the mixture without increasing the water content (ACI 234R-06).
Segregation and Set Time
Segregation is not common in silica-fume concrete due to the fineness of the material and increased cohesiveness of the mixture. However, segregation will occur in high-slump mixtures both with and without silica fume. Similarly, the set time is not affected as much by the presence of silica fume in the concrete mixture as it is by the type and dosage of admixtures HRWR and WR, which may also act as set retarders in the mixture (ACI 234R-06).
Air Entrainment
The dosage rate of air-entraining admixture to produce a required volume of air in concrete mixtures usually increases with an increased percentage of silica fume. Typically, the increase in the air-entraining admixture will be about 125% to 150% of that used in similar concrete without silica fume. This increase is attributed to the very high surface area of silica fume and possibly to the presence of carbon (Carette and Malhotra 1983).
Bleeding and Plastic Shrinkage
Bleeding in concrete with silica fume is significantly reduced compared to concrete with no silica fume. As the silica fume dosage is increased, bleeding will be reduced further. This effect is caused primarily by the high surface area of the silica fume that needs to be wetted. As a result, very little free water will be left in the mixture for bleeding (Grutzeck et al. 1982). Less bleeding facilitates earlier and efficient finishing of the concrete surface.
However, since silica-fume concrete exhibits significantly reduced bleeding, the potential for plastic shrinkage cracking is increased as the rate of evaporation may exceed the rate at which bleeding will replenish the surface moisture (Aïtcin et al. 1981). Therefore, care should be exercised to prevent early moisture loss from freshly placed silica-fume concrete, particularly in hot, dry, or windy weather. Among the effective measures to mitigate plastic shrinkage and cracking of freshly placed silica-fume concrete are fogging, misting, applications of evaporation retardants, windbreaks, and immediate curing with appropriate curing compounds (ACI 234R-06).
Heat of Hydration
The presence of silica fume in concrete increases the rate of heat evolution due to silica’s very fine particle size and high surface area. This accelerates the rate of cement hydration. The high rate of hydration produces more CH that is available to react with silica fume to produce a dense paste and contributes to high early-strength development in concrete (ACI 234R-06).
Properties of Hardened Silica-Fume Concrete
Strength Performance
Silica fume is a highly reactive pozzolan. In hydrating cement paste, silica fume will react with CH to form additional C-S-H binder, and it may consume the CH at 28 days. This vigorous reaction increases the strength of concrete by increasing the strength of the paste, the paste–aggregate bond, and the concrete-reinforcement bond. The contribution of silica fume to concrete strength development at normal curing temperatures is mainly at early ages up to 28 days. The 3-day strength may reach 50%, and at 7 days, strength will be about 70% of the 28-day strength. However, the strength gain after 90 days may be less than concrete without silica fume. Adding slag cement or coal ash will enhance the concrete strength at and beyond 90 days (SFA 2022; ACI 234R-06).
Permeability and Durability Performance
Including silica fume in concrete depletes the CH and adds to the C-S-H in the paste to reduce permeability and, thus, drastically improves the durability of the concrete. The contribution of silica fume to the reduction in concrete permeability is significant, with the reduction in the permeability coefficient being up to one order of magnitude or more depending on the mixture composition and dosage of the silica fume (ACI 234R-06). The low-permeability characteristics of silica-fume concrete are responsible for its improved durability in various aggressive environments to resist chemical attack, for reducing the impact of freeze–thaw cycles and deicer scaling, and for enhancing resistance to surface abrasion.
Resistance to ASR
Use of silica fume in sufficient quantities in concrete with or without other SCMs (slag cement or fly ash) can be effective in resisting ASR. The beneficial effect of silica fume is due to its significant pozzolanic reaction depleting the alkalis, which are considered important ingredients of ASR along with reactive silica in some mixture aggregates. A study by Uchikawa et al. showed that 10% by mass replacement of Portland cement with silica fume consumes almost three times more alkali than does Portland cement concrete without silica fume (Uchikawa et al. 1989).
Sulfate Resistance
Silica fume is very effective in reducing or preventing attacks from sodium sulfate. The primary mechanism by which silica fume improves resistance to sodium sulfate attack is the reduction of permeability augmented by reduced CH content due to pozzolanic reaction. However, when exposed to magnesium sulfate, the resistance of silica-fume concrete to sulfate ingress is less than that of concrete without silica fume. Apparently, the consumption of CH due to reaction with silica fume is detrimental to concrete durability in laboratory samples exposed to high concentrations of magnesium sulfate (ACI 234R-06).
Resistance to Chloride-Ion Penetration
Silica-fume concrete is commonly used in bridge decks and substructures and in other marine structures because of its ability to slow the ingress of chloride ions. By slowing the penetration of chlorides, the onset of corrosion is significantly delayed, and the rate of corrosion progression is reduced. Silica fume used in combination with appropriate mixture ingredients and proportions will further delay the onset of corrosion and significantly prolong the service life of structures. These approaches include use of low w/cm and corrosion-inhibiting admixtures, increasing the concrete cover of the steel reinforcement, and employing proper curing. It should be noted that despite the reduction in alkalinity and pH of concrete with depletion of CH, the potential for corrosion in the reinforcing steel is still considerably low because the permeability of high-performance

concrete with silica fume is very low, which prevents or drastically delays the ingress of chloride ions from reaching the reinforcing steel to affect the protective cover around the reinforcing bars and start the corrosion process (FDOT 2024; Armaghani et al. 1992; ACI 234R-06; Sutter 2016).
Abrasion Resistance
Abrasion resistance is an added advantage to using concrete with silica fume. For silica-fume concrete made with a particular aggregate, the higher the compressive strength, the higher the abrasion resistance. Additionally, paste containing silica fume develops an improved bond to aggregate particles, which further increases the abrasion resistance of the concrete (ACI 234R-06).
Performance of Silica Fume in Pavements
In cold regions, use of silica fume in concrete mixtures for pavement for highways and other flat surfaces improves resistance to the effects of freeze–thaw cycles and deicing salts. However, use of more than 5% silica fume in the mixture will reduce bleeding and increase cohesion. These challenging conditions may cause difficulties in finishing and texturing of the pavement surface. Other project-specific factors, such as dry and windy conditions, will further complicate such operations and may result in shrinkage cracking. It has been suggested that demonstration placements be constructed at the project site to establish appropriate windows for finishing and texturing of the pavement surface. Continuous early curing is critical to the success of pavements using silica-fume mixtures. This is due to the reduced bleeding from the freshly placed pavement, which increases the potential for rapid evaporation and formation of plastic shrinkage cracks (Figure 11) (SFA 2022). It has been suggested that, to mitigate evaporation shortly after the concrete is placed, the surface should be sprayed with very a fine mist of water or an evaporation retarder should be used to allow finishing and texturing of the surface with minimum loss of surface moisture. Conventional curing should commence (using moisture or a curing compound) as soon as surface texturing is complete and the concrete has attained sufficient strength (ACI 234R-06; SFA 2022).
Natural Pozzolans
The term “natural pozzolan” encompasses a broad range of earthen materials. A few of these materials are pozzolanic in their natural state. However, most NPs require some type of processing, such as drying, calcining, and grinding, to activate their pozzolanic characteristics (ACI 232.1R-12). Examples of NPs are calcined clays, including kaolinitic clays, shales, and
pumice, and volcanic ashes. NPs have been used to varying degrees since Roman times (Sutter 2016). In recent years, NPs have become more desirable for use as SCMs due to the decline in availability of fly ash in many regions of the United States, as will be discussed further in the survey results presented in Chapter 3.
The mineralogical constituents of NPs may vary substantially, ranging from amorphous materials to crystalline compounds. The presence and composition of amorphous compounds account for the pozzolanic reactivity of the NP material. Generally, amorphous silica reacts with CH and other alkalis more rapidly than does silica that is in the crystalline (inert sand) form (ACI 232.1R-12).
NPs are specified as Class N in ASTM C618-23 and AASHTO M 295. Two main differences can be noted in the chemical requirements of Class N NPs compared to coal ash Classes F and C. First, the minimum sum of SiO2, Al2O3 and Fe2O3 is 70% for Class N compared to 50% for fly ash Classes F and C. Second, the maximum loss on ignition is 10% compared to 6% for Classes F and C.
Types of Natural Pozzolans
Calcined Clay
Calcined clay is a naturally occurring raw material composed essentially of aluminosilicate minerals. Examples of clay are kaolinite, dickite, halloysite, and illite. The calcining of clay to remove bound water, or dehydroxylation, is typically done in rotary kilns or flash calciners where the clays are heated to a temperature adequate to decompose the clay’s crystalline structure and render it amorphous. For kaolinite clays, this temperature is approximately 1,020°F to 1,380°F (550°C to 750°C). Other clay types may require different thermal activation temperatures to optimize the material’s pozzolanic reactivity (ACI 232.1R-12).
Calcined clays have been used as pozzolans in concrete for many years. For example, calcined clays have been used since 1993 in the midwestern United States in construction of pavements, bridge decks, and parking structures (ACI 232.1R-12). Calcined kaolinite clays have been documented in the literature as containing a mixture of approximately 85% to 90% metakaolin, 5% to 10% quartz, and a residual mixture of illite and montmorillonite or smectite (Barger et al. 1997). Higher-purity calcined clays (greater than 95% metakaolin), specifically kaolinite, are classified as metakaolin. Calcined kaolinitic clay is used specifically to provide for resistance to ASR and to reduce concrete permeability. It has been used not only in binary concrete mixtures but also in ternary mixtures in combinations with either Class C or Class F fly ash or silica fume (ACI 232.1R-12).
Calcined Shale
Shale and slate consist largely of aluminosilicate clay minerals. In addition, there may be varying amounts of calcite (limestone), quartz, feldspar, and mica. The shale or slate is quarried by conventional means, crushed to a maximum size of 1.5 in. (38 mm), and then calcined (heat treated) in a rotary kiln at temperatures typically in the range of 1,800°F to 2,000°F (980°C to 1,090°C) for approximately 45 minutes. The clinker produced in the kiln ranges in size from 0.25 to 2 in. (6 to 51 mm). It is then air-cooled at the discharge of the kiln. This will result in a finely divided powder with a median particle size on the order of 5 microns, similar in fineness to a Type III Portland cement. Calcined shale has a typical elemental composition of 50% silica, 20% alumina, and 8% calcium (ACI 232.1R-12). This chemical composition mimics that of chemical composition of coal ash (ASTM C618-23).
The calcination process alters the crystalline structure of the aluminosilicate clay minerals, rendering them capable of engaging in a pozzolanic reaction. The amorphous (noncrystalline)
phases of the calcined shale will range from 50% to 75%, depending on the particular source and heat-treating process. The LOI of calcined shale may range from 1% to 5%. The LOI of calcined shale is due to the presence of both residual water molecules bound in the clay mineral structure and residual uncalcined calcite. Therefore, the LOI of calcined shale does not have any adverse effect on air entrainment of concrete (ACI 232.1R-12). According to Krisanda and the U.S. Geological Survey (USGS), production of common clay and shale in 2017 was 13.3 million metric tons, and they are produced mainly in the states of Alabama, North Carolina, New York, Ohio, and Texas (Krisanda and USGS 2017).
Metakaolin
Metakaolin (Al2O3 . 2SiO2) is a natural pozzolan produced by heat treating kaolinite clays. When it is heat treated in the temperature range of approximately 1,100°F to 1,650°F (600°C to 900°C), the chemically combined water is driven away, which forms an amorphous aluminosilicate called metakaolin. Metakaolin is a high-purity calcined clay. Kaolinite, which is a specific metakaolin material, contains greater than 95% metakaolin. It is typically white, has a mean particle size in the range of 2 to 10 μm, and has a bulk density in the range of 18 to 40 lb/ft3 (290 to 640 kg/m3) (ACI 232.1R-12). High-reactivity metakaolin (HRM), as described in AASHTO M 321-04, is derived from highly refined kaolinite clay that has been processed to remove impurities prior to calcination.
Although metakaolin has been used as a pozzolanic mineral admixture for many years, its use has grown rapidly since the mid-1980s. In the United States, metakaolin and HRM are manufactured from deposits of kaolin found mainly in Alabama, Arkansas, California, Georgia, and South Carolina, although kaolin is also available in other states. Production of kaolin in 2017 was 5.6 million metric tons (Krisanda and USGS 2017).
Pumice
Pumice is a natural pozzolan of volcanic origin. During an explosive eruption, volcanic gases dissolve in the liquid portion of magma and expand rapidly to create a foam or froth. Once above ground, the liquid part of the froth quickly solidifies to form glass. Pumice is actually a kind of glass and not a mixture of minerals. It is colorless or light gray and has the general appearance of rock froth. When ground, pumice can be a good pozzolanic material (Day and Shi 1995). Pumice has been available in the states of California, Idaho, Kansas, New Mexico, and Oregon. Production of pumice in the United States in 2017 was 383 million metric tons (Krisanda and USGS 2017).
Rice Husk Ash
RHA is not an NP since it is from a planted crop and does not exist naturally. Rice husks are the shells produced during the rice’s dehusking. Raw rice husks are composed of approximately 50% cellulose, 30% lignin, and 20% silica. After incinerating the rice husk to remove the lignin and cellulose, the ash left is 90% to 96% amorphous silica. Depending on the carbon content, RHA can have a range of colors, from nearly white to black (Mehta 1992).
RHA is a highly reactive pozzolan. In concrete, RHA improves compressive strength, decreases permeability, and improves durability. Use of RHA in concrete increases resistance to sulfate and acid attack as well as chloride-ion penetration. Also, concrete with RHA has shown excellent performance under freeze–thaw cycling (Zhang and Malhotra 1996). To achieve such benefits, RHA is included in the concrete mixture at 5% to 15% replacement by mass of Portland cement. In the United States, 9 million metric tons of rice were produced in 2021, mainly in the states of Arkansas, California, Louisiana, Mississippi, Missouri, and Texas [United States Department of Agriculture (USDA) 2022]. Assuming that rice husk is 10% of the weight of rice according to ACI 232.1R-12), approximately 1.9 million tons of rice husk may be available annually for processing into RHA.
Reaction Mechanism of Natural Pozzolans
The amorphous or poorly crystallized silica and alumina present in the NP will react with CH and other alkalis, derived from the hydration of Portland cement, to form additional C-S-H binder gel. During the pozzolanic reaction, there is a gradual decrease with time in the amount of free calcium hydroxide (Figure 12), and an increase in formation of C-S-H and calcium aluminosilicates that are similar to the hydration products of Portland cement. The result is that the hardened cement paste contains less CH and more C-S-H and other useful products, which reduces porosity and permeability of the cement paste and concrete.
Performance of Concrete with Natural Pozzolans
The most effective method for evaluating the performance of concrete containing a natural pozzolan and establishing proper mixture proportions for a specific application is the use of trial batches and a testing program. Any of the ACI conventional methods for proportioning a concrete mixture are also applicable for establishing trial mixture proportions for concrete containing a natural pozzolan (ACI 232.1R-12).
In most cases, NPs are used as a partial replacement of the Portland cement in a concrete mixture. Some NPs are used in a range of 15% to 35%, based on the mass of the total cementitious material in the concrete. More reactive NPs can be used in lower concentrations of 5% to 15% by mass of total cementitious material. Performance criteria, such as ASR mitigation, sulfate resistance, and temperature rise, could dictate the required minimum quantity of NP to be incorporated as a percentage of the total cementitious material.
The partial replacement of Portland cement by conventional and natural pozzolans with high levels of silica, alumina, and iron oxide increases the resistance of concrete to ASR, sulfate attack,

and chloride penetration. Also, an increase in NP fineness exposes more surface area of the material and accelerates the early pozzolanic reactions in concrete to achieve the strength and durability benefits (ACI 232.1R-12).
Properties of Fresh Concrete with NPs
Workability and Water Demand
Most natural pozzolans produce a cohesive concrete mixture that maintains a plastic consistency and has improved workability. The increased cohesiveness of the mixture allows the concrete to consolidate readily and flow freely under vibration; it also reduces segregation.
The influence of NPs on concrete mixture water demand is largely a function of the characteristics of the specific NP, such as its fineness, particle shape and structure (cellular or noncellular), and composition. The effect of a particular NP on the mixing water requirement is determined by laboratory tests or field experience (ACI 232.1R-12). A natural pozzolan with particles that are coarse, irregular shaped, or rough textured, or that retain a tubular interconnect pore structure could, however, require an increase in the water content for a given slump of the concrete and contribute to the increased bleeding and segregation of the fresh concrete.
Set Time
The use of an NP may not necessarily have any profound influence on the set time of concrete. Calcined clay and calcined shale have been reported to have little effect on concrete set time (Kosmatka et al. 2016). Other NPs may cause an increase in the set time. This is mostly due to the quantity of Portland cement they replace. The setting-time characteristics of concrete containing an NP are influenced by ambient and concrete temperature; cement type, source, content, and fineness; water content of the paste; water-soluble alkalis; and use and dosages of other admixtures. The actual effect of a given NP on set time may be determined by testing when a precise determination is needed, or by observation when a less precise determination is acceptable (ACI 232.1R-12).
Air Entrainment
The proportion of AEA required to achieve the desired air content in concrete may differ considerably for different sources and types of NPs. The influence on AEA dosage may be a function of both the fineness and carbon content of the natural pozzolan used. Most NPs do not contain carbon residue. Unlike in coal ash, the LOI in NPs may not be an issue in altering properties of fresh concrete. An exception would be RHA since carbon content is increased during incineration of rice husk.
Heat of Hydration
Because the total heat of hydration is reduced with a partial replacement of Portland cement with an NP, the heat rise in concrete may likewise be reduced. For these reasons, NPs have been used in high proportions as a partial replacement for Portland cement in mass concrete to reduce both the rate of rise in temperature and the maximum temperature (Saad et al. 1982). In low ambient temperatures, the quantity of NP incorporated in the mixture should be carefully evaluated as with the remaining mixture ingredients. In cold regions, the heat of hydration in concrete is expected to be lower than in concrete placed under normal temperature conditions. Lower heat of hydration can result in significant increase in set time and a decrease in the rate of early strength gain.
It should be noted that some of the more reactive NPs, such as HRM, may actually increase the heat of hydration and the rate of temperature rise in concrete. One study showed that the
rate of heat rise and maximum temperature of the concrete containing metakaolin were somewhat higher than that of the control concrete and concrete containing silica fume (Zhang and Malhotra 1996).
Properties of Hardened Concrete with NPs
Concrete containing an NP typically exhibits similar hardened properties as those using conventional SCMs. Improved properties include higher concrete strength, mostly at later ages, lower permeability, and enhanced durability. Concrete with NPs provide resistance to ASR, sulfate attack, and the penetration chlorides from seawater and deicing salts (Mather 1958).
Compressive Strength
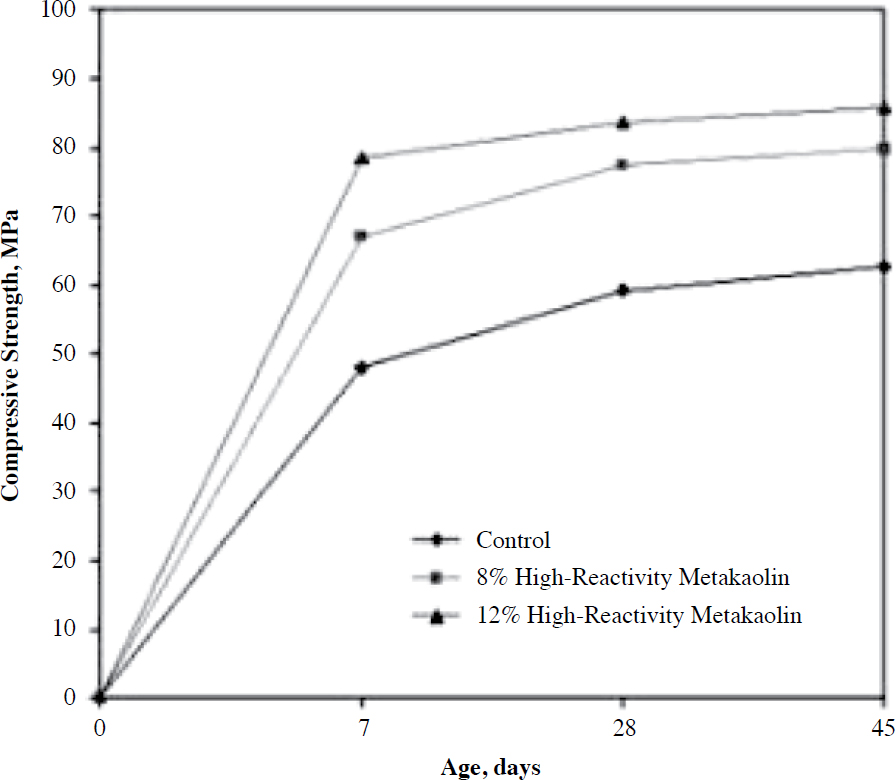
The effect of an NP on the compressive strength of concrete is directly related to its pozzolanic properties and other factors related to concrete mixture ingredients and proportion. An NP of high pozzolanic reactivity, such as metakaolin, may increase both early- and later-age strengths when used as replacement by mass or volume of the Portland cement in the mixture, as shown in Figure 13 (Hooton et al. 1997).
Reduced Permeability and Improved Durability
In most cases, the use of an NP will reduce the permeability of concrete and improve its durability. Although some NPs may slightly decrease permeability at early ages, the greatest reductions in permeability are generally observed at later ages (ACI 232.1R-12).
With improved durability, the use of NPs generally increases the resistance of concrete to ASR, sulfate attack, and chloride penetration. The relative improvement is greater for concrete
mixtures with low cement content and with Portland cement with higher C3A content. Under certain conditions, concrete with NPs may also reduce the impact of freeze–thaw action.
Resistance to Alkali-Silica Reaction
Many investigations have shown that NPs can be effective in controlling ASR and mitigating or eliminating map cracking and expansion resulting from ASR. The replacement rate of NP needed to effectively resist ASR depends on the NP type. The minimum content of a natural pozzolan (expressed as a percentage of the total cementitious material) necessary to control deleterious ASR should be determined by tests using ASTM C441-05, as described in ASTM C311-13 (ACI 232.1R-12).
Sulfate Resistance
Use of NPs with Portland cement in concrete generally increases resistance to aggressive attack by seawater and sulfate-bearing soil solution. One study showed that the sulfate resistance of mortar is highest when silica fume or a highly siliceous natural pozzolan is used. The relative improvement is greater for concrete with low cement content and with Portland cement of higher C3A content (Mather 1982).
Resistance to Chloride Penetration
Reduced permeability, as it relates to resistance to chloride-ion penetration, is important for corrosion protection of the reinforcing steel in concrete. One study showed that decreases in diffusion, permeability, and conductivity could be achieved by both increasing the concentration of an NP, such as HRM, and decreasing the mixture’s w/cm (Hooton et al. 1997).
Resistance to Freeze–Thaw Action
The effect of an NP on concrete’s resistance to freezing and thawing and to the action of deicing chemicals during freezing depends on the mixture proportioning, concrete maturity and compressive strength, degree of saturation of the concrete, and adequacy of the air-void system at the time of exposure (Lovewell 1971). For example, one study showed that concrete with 30% calcined shale had the same durability to freezing and thawing durability as control concrete without NP (Neal and Ramsburg 2002).
Drying Shrinkage
Tests should be conducted to determine the drying shrinkage characteristic of a concrete mixture with NPs, taking into consideration the particular project conditions. Also, it is assumed that, because concrete mixtures containing NPs typically have a lower modulus of elasticity compared to similar mixtures without pozzolans, the cracking tendency resulting from drying shrinkage in concrete containing NPs would be less than that of similar concrete without NPs (ACI 232.1R-12).
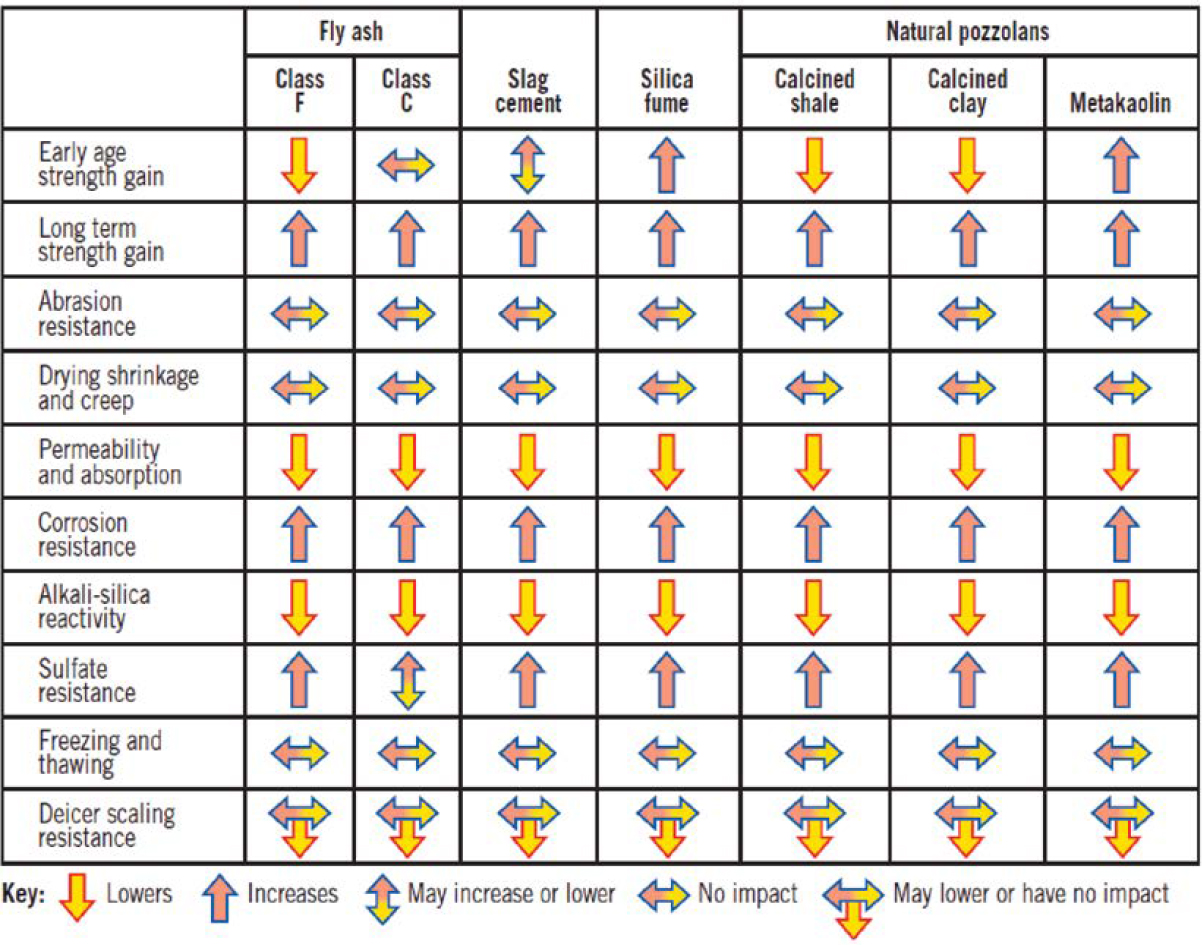
Summary Effects of SCMs on Properties of Fresh and Hardened Concrete
Tables 5 and 6 summarize the general impacts of SCMs on the properties of fresh and hardened concrete. These impacts may vary considerably between and often within classifications of the SCMs and by the SCM replacement rate of cement. Specified performance for the concrete application should be evaluated for SCM mixtures (Kosmatka et al. 2016). Also, other mixture ingredients and proportions, such as w/cm, admixtures, and cement content, may also affect properties of concrete with SCMs, as may weather conditions.
Table 5. Impact of SCMs on properties of fresh concrete (Kosmatka et al. 2016).
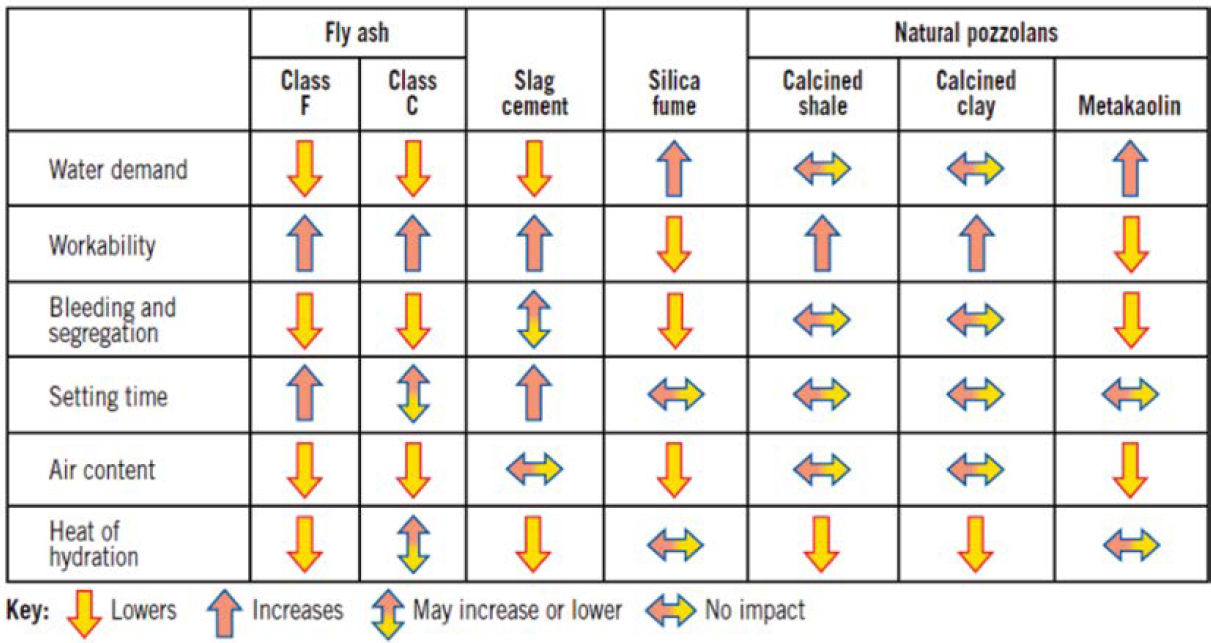
Table 6. Impact of SCMs on properties of hardened concrete (Kosmatka et al. 2016).
Ternary Mixtures
Ternary mixtures are concrete mixtures that contain Portland cement and two other SCMs in the binder fraction. The binder materials (SCMs and Portland cement) may be combined at the concrete batch plant or obtained as a preblended cementitious product that is added to the batch. Use of ternary mixtures improves the properties of fresh and hardened concrete through better workability/finishability, managed set time, and added strength and durability, while reducing the use of Portland cement for enhanced sustainability.
Another advantage of ternary mixtures is that the negative properties of one SCM on concrete can be offset by the positive properties of another (Sutter 2016). The amount of each SCM may affect concrete properties such as time of set, bleeding, slump loss, and strength development (ACI 233R-17). Combining two SCMs in the mixture and adjusting mixture proportioning (such as w/cm and admixture dosage rates) while ensuring effective curing will achieve the desired fresh and hardened concrete properties. The use of ternary mixtures incorporating Portland cement, silica fume, and either fly ash or slag cement has been on the increase in the last few years, as will be shown in discussion of the survey results of state DOTs in Chapter 3.
Ternary Mixture of Ordinary Portland Cement, Coal Ash, and Silica Fume
Ternary concrete mixtures containing coal ash and silica fume can produce concrete that has much improved strength and reduced permeability and resists chloride-ion penetration (Thomas et al. 1999). Silica fume is being used extensively in combination with coal ash. Research indicates that concrete with this SCM combination performs well. One report indicates that the combination works better than either material alone (ACI 234R-06). For example, silica fume added with HVFA in mass concrete will achieve a reduction in the generated heat of hydration, while the presence of silica fume will offset the loss of early-age strength resulting from using an HVFA. Earlier studies concluded that the combination of fly ash–silica fume produced much greater pozzolanic activity at 7 and 28 days than did concrete made with fly ash alone (Mehta and Gjørv 1982).
A study in Florida showed that silica fume reduces early-age water permeability and increases early strength. It also showed that fly ash contributes to a reduction in permeability at 91 days, as shown in Figure 14 (Armaghani et al. 1992). In the early 1990s, FDOT began to allow the use of ternary mixtures, including silica fume and fly ash or slag cement, in highly aggressive sea environments (FDOT 2024).
The state of New York DOT (NYSDOT) developed a ternary mixture for its concrete bridge decks to resist penetration of deicing salt. The mixture included 20% Class F fly ash and 6% silica fume by mass of the cementitious materials (Streeter 1996). In addition to an increase in ultimate strength, the resulting concrete showed a 20% to 30% reduction in chloride penetration compared to the control mixture with no SCMs. As a result, in 1998, NYSDOT began using this high-performance ternary mixture in all new bridge decks and deck rehabilitation projects.
Blends of silica fume and both Class F and C coal ash have been found to be effective in resisting ASR and controlling the damaging concrete expansion caused by ASR (Thomas et al. 1999). Similarly, coal ash silica fume ternary concrete mixtures have been found to be resistant to sulfate attack, and were even more effective than sulfate-resisting cement (Type V) (Fidjestøl and Frearson 1994).
Ternary Mixture of Ordinary Portland Cement, Slag Cement, and Silica Fume
Slag cement is also used in ternary concrete mixtures in combination with other SCMs such as coal ash and silica fume. This combination will bring about the desirable attributes of both SCMs

Figure 14. Effect of fly ash and silica fume ternary mixture on water permeability at 91 days (Armaghani et al. 1992).
to improve the properties of fresh and hardened concrete, while the characteristics of one SCM may offset the negative aspects of the other (ACI 233R-17). For example, when slag cement is used in concrete mixtures with silica fume, the amount of silica fume can be reduced compared to a mixture of equivalent durability with Portland cement and silica fume. Maintaining a low silica-fume percentage will improve the workability and reduce the stickiness of the mixture, while the presence of the small amount of silica fume will offset the reduction in early strength from the slag cement.
The combination of slag cement with relatively small amounts of silica fume also provides additional resistance to ASR and the damaging expansion caused by ASR. One study showed that both 50% slag cement and 25% slag cement in combination with 3.8% and 4% silica were effective in preventing deleterious ASR expansion and cracking of concrete (Hooton et al. 2013). Laboratory testing has demonstrated that silica fume and slag ternary mixtures exhibit reduced chloride penetration (Ozyildirim 1994) and have very low permeability to water (El-Dieb and Hooton 1995).
Alternative Supplementary Cementitious Materials
Introduction
ASCMs are increasingly of interest to agencies as the supply of conventional SCMs such as fly ash becomes more scarce and less reliable and as agencies aim to lower the carbon footprint of their transportation infrastructure (Armaghani and Cavalline 2020). ASCMs include a range of industrial by-products and waste materials, some of which exhibit characteristics suitable for use with limited processing. Other ASCMs require beneficiation, processing, or blending in order to achieve the desired performance (Snellings et al. 2023), and in recent years, some ASCMs have also been manufactured. Many of these materials are available in volumes that could support infrastructure projects, at least on a local or regional basis.
ASCMs are capable of providing pozzolanic or latent hydraulic reactions, contributing to the fresh and hardened properties of concrete, including durability performance. Pozzolanic
materials, which are often low-calcium aluminosilicates, react with CH and water to form additional cementitious hydrates. Latent hydraulic materials are able to react with water if activated by a soluble alkali, and they also consume CH in their reactions (Snellings et al. 2023). The reactive phases of these materials are often amorphous silicates, although some materials, such as calcium-rich steel slags, exhibit different mechanisms (Snellings et al. 2023). Figure 15 shows the chemical composition ranges of conventional SCMs and emerging and future ASCMs.
Although many waste and by-product materials include a significant amount of amorphous silica, they may not actually provide the physical properties, chemical composition, or reactivity required to support their use as an ASCM. The reactivity of an ASCM is of much interest when evaluating its use in fresh and hardened concrete. This reactivity is controlled by factors such as the amorphous phases and the material’s specific surface area (Snellings et al. 2023). It is generally understood, that as the specific surface area of material increases, its reactivity also increases (Ramanathan et al. 2022).
A number of reactivity tests have been developed that measure dissolution, heat release, consumption of CH, bound water, bulk resistivity, and strength (Snellings et al. 2023). Recent advancements in understanding the reactivity of an ASCM have been made, including the use of the R3 test, which is now ASTM C1897, Standard Test Methods for Measuring the Reactivity of Supplementary Cementitious Materials by Isothermal Calorimetry and Bound Water Measurements (Snellings et al. 2016; Wang et al. 2022), the modified R3 test (Suraneni 2021), and approaches being studied in ongoing projects. The R3 test includes two methods. In Method A, isothermal calorimetry is used to determine the heat of hydrating pastes composed of SCM, CH, calcium carbonate, potassium sulfate, and potassium hydroxide, with the heat of hydration value used to determine the chemical reactivity of the SCM. In Method B, the chemical reactivity of the

Figure 15. Chemical compositions of conventional SCMs and fillers (green and blue), ASCMs (yellow), and Portland cement (gray) (Snellings et al. 2023).
SCM is measured by determining the amount of chemically bound water of pastes composed of SCM, CH, calcium carbonate, potassium sulfate, and potassium hydroxide (ASTM C1897). The modified R3 test measures the heat release and CH consumption in a SCM-calcium hydroxide model system. It is similar to the R3 test, but without the additional sulfates and carbonates. This approach simplifies the system and provides results more representative of later-age properties than the R3 test (Suraneni 2021).
Since ASCMs are often by-product materials from industrial or construction processes, their beneficial reuse in concrete applications provides sustainability benefits associated with reducing the need for virgin materials, saving landfill space, and, in some cases, lowering embodied carbon due to reduced hauling distances (Cavalline et al. 2024; Cavalline and Sutter 2024; Snyder et al. 2018). Some ASCMs offer the potential for CO2 use/capture technologies such as mineral carbonation to produce ASCMs/fillers (Zajac et al. 2022b) or carbonation curing to support higher replacement rates (Nielsen et al. 2017). However, it is important to determine whether a material can be used in fresh concrete without adversely affecting the concrete’s properties. The impact of the material on a concrete mixture’s workability/finishability, strength gain, and durability must also be assessed. The potential of these materials to have adverse environmental effects must also be considered (Cavalline and Sutter 2024).
Despite the need for ASCMs to supplement the supply of conventional SCMs, challenges exist for agencies interested in evaluating their suitability for use, as shown in the responses to the survey questionnaire (Chapter 3). These include evaluation of their reactivity, their availability, their consistency, the performance of the material produced using the ASCM, environmental considerations, and the impacts and costs of beneficiation, handling, and transport. Concerns expressed by agencies regarding the use of some construction and industrial wastes include the variability of these materials, the availability of an adequate supply of a waste product to meet the needs of a specific project, performance of the waste product (or material produced using the waste product) when in service, and environmental impacts (Cackler 2018).
Recycled Materials from Waste and Construction Applications
Recycled Glass Pozzolan
Waste glass is produced across the United States in large volumes. The EPA has estimated that 12.3 million tons of waste container glass were produced in 2020 (EPA 2020). Container glass is typically fairly uniform in composition, but the individual units vary in color, which makes recycling them into new glass products difficult (Mirzahosseini and Riding 2014). Other types of glass waste are available in smaller quantities. These include plate glass and electrical glass (e-glass). Plate glass (also known as soda-lime glass) is typically used for windows and automobile windshields. This glass is typically clear or tinted. E-glass results from the production of reinforcement fiberglass. It is estimated that these three types of glass (waste container glass, plate glass, and e-glass) make up more than 90% of the glass produced each year in the United States (Wintour 2015).
Glass is an amorphous, high-silica material that exhibits pozzolanic behavior when ground into powder form. When used for concrete applications, the material is often called ground-glass pozzolan. Recycling centers and bottle redemption programs are suppliers of waste glass for these applications. Prior to use as a GGP, the glass must be cleaned using thermal, wet, and mechanical processes (Kaminsky et al. 2020). GGP has been found to be most effective when reduced in particle size to a fineness with a specific surface area greater than 210,000 in.2/lb (298 m2/kg) (Mirzahosseini and Riding 2014).
The chemical composition of GGP varies based on the type of glass used to produce it. Container glass and plate glass are both typically low in aluminum and high in sodium and potassium, while e-glass is typically lower in sodium and potassium. However, when the glass type included in GGP
production is controlled, the GGP produced can exhibit fairly uniform chemistry and reactivity (Kaminsky et al. 2020). Physical characteristics of GGP reported in the literature include a specific gravity ranging from 2.4 to 2.8 (Mohajerani et al. 2017), a Blaine’s fineness of 10,200 cm2/g, and an LOI of 1.0% (Rashidian-Dezfouli and Rangaraju 2018).
Most research on the performance of GGP in concrete applications has focused on soda-lime glass. As such, the term GGP used moving forward will refer to GGP produced from soda glass unless otherwise noted. When used in concrete, GGP exhibits a lower water demand than some other pozzolans (Mirzahosseini and Riding 2014). Studies have shown that mortar and concrete including GGP exhibit reduced absorption and permeability, which lowers susceptibility to sulfate attack, improves resistance to chloride penetration, and reduces carbonation potential (Shi et al. 2004; Zidol et al. 2020; Shayan and Xu 2006). Studies on GGP produced from liquid crystal display glass indicate that this material also shows similar performance benefits when used in concrete applications, with an additional advantage provided by its more uniform composition (You et al. 2019).
GGP is now specified by ASTM C1866, Standard Specification for Ground-Glass Pozzolan for Use in Concrete. ASTM C1866 designates two types of GGP: Type GS is soda-lime glass, and Type GE is e-glass. Both GS and GE GGP have been shown to provide benefits, including increased strength and reduced permeability, when used in concrete. Type GE glass can be used to effectively mitigate ASR. However, due to its high alkali content, Type GS glass is less effective at ASR mitigation, and its use is not recommended with ASR-susceptible aggregates unless its performance is assessed using ASTM C1293, Standard Test Method for Determination of Length Change of Concrete Due to Alkali-Silica Reaction.
Early field studies of GGP concrete have shown adequate performance (Shayan and Xu 2006). NYSDOT has been allowing GGP in mixtures for several years as a mineral filler (NYSDOT 2014), and field studies are planned (Kaminsky et al. 2020). Based on the availability of waste glass nationwide, GGP has the advantage of being a potential ASCM in most areas of the United States, although only a limited number of producers are processing the material to be used as an ASCM.
Fines from Concrete Recycling
It has been estimated that over 100 million tons of recycled concrete aggregate (RCA) fines are produced annually (EPA 2020; Cavalline et al. 2024). These fines are generated from the recycling of public and private concrete infrastructure at on-site, mobile, and stationary plants (Snyder et al. 2018). RCA fines include both the original aggregate used in the concrete as well as the bonded mortar. The particle sizes contained in the RCA fines are influenced by the quality of the source concrete and the crushing process. RCA fines are nonplastic and have been reported to have a specific gravity of 2.10 to 2.38 (Lim et al. 2003).
Recently, techniques have been used to improve crushing and sieving technologies to better separate the hardened cement binder and the aggregates contained in the RCA (Snellings et al. 2023). The chemical reaction between carbon dioxide in the air and CH and C-S-H in the concrete (carbonation) of the RCA components and the potential for carbonated RCA fines (cRCFs) to be used as an ASCM (or as an SCM in blended cement) have been the subject of several recent studies (Zajac et al. 2024; Zhang et al. 2023; Li and Wu 2022). Carbonation of the RCA fines can be performed as a wet process (Zajac et al. 2020a) or as a dry or semi-dry process (Zajac et al. 2022b).
Studies to date have focused on understanding the changes occurring during the carbonation process, factors that can be used to influence the resulting systems, and the reactivity of these systems (Zajac et al. 2020b; Lu et al. 2018; Zajac et al. 2024). Results have indicated that cRCF
used in blended cements can have positive effects on compressive strength at early hydration times compared to conventional blended cements due to the high pozzolanic activity of the alumina-silica gel combined with a filler effect of the CaCO3 from the cRCF.
Ceramic Waste
Waste ceramics from construction and demolition (C&D) exhibit pozzolanic behavior when ground finely. In modern C&D waste streams, ceramic waste can be classified as red-ware and white-ware (Snellings et al. 2023). Red-ware makes up the bulk of waste ceramics, and includes bricks, floor and roof tiles, and wall tile. These materials are often fired at low temperatures and are composed of the clay material local to the production site. White-ware ceramics include toilets, sinks, and tableware and are produced from high-grade kaolinitic clays typically fired above 1,200°C (Snellings et al. 2023). Both types of ceramic waste exhibit pozzolanic reactivity when ground to a sufficient fineness (Hoppe Filho et al. 2021; Zito et al. 2023). The pozzolanic reactivity of ceramic materials is supported by its amorphous and disordered aluminosilicate structure that results from the sintering process. The calcination of the source materials results in the formation of metakaolin from the source material’s kaolinite, which is responsible for reactivity (Hoppe Filho et al. 2021).
Studies have shown that the reactivity of waste ceramic ASCMs in mortar and concrete is similar to that of coal ash or natural pozzolans (Snellings et al. 2023). Although there is a slower early-age strength gain, later-age reactions ensure that strength gain continues, and lower permeability and related durability improvements are also a result (Hoppe Filho et al. 2021; Zito et al. 2023; Li et al. 2020). Ceramic waste appears to have a higher water demand than cement, and admixtures may be required to help provide the required workability (Carvalho et al. 2020; Zito et al. 2023). The color of the ceramic material may also influence the color of the mortar or concrete produced with it (Carvalho et al. 2020).
A practical challenge associated with use of ceramic waste, particularly from C&D sources, is the high instance of contamination of the waste stream with other materials. Selective demolition techniques and source separation technologies can assist with reducing contaminant materials (Cavalline and Weggel 2013; Vegas et al. 2015).
Ash from Combustion of Materials Other Than Coal
Municipal Solid Waste Incineration Ash
Municipal solid waste is produced across the United States, and incineration facilities are used in a number of locations, particularly in urban areas where landfill space is limited and hauling distances to landfills are high. Based on municipal solid waste incineration (MSWI) volumes, the amount of MSWI ash is estimated as approximately 7 million metric tons per year (Snellings et al. 2023).
MSWI ash is composed of particles that leave the furnace and are subsequently removed from the exhaust stream, typically prior to the point where sorbents are added to treat the gaseous effluent. MSWI is considered a separate material from air pollution control residues collected in devices such as scrubbers and electrostatic precipitators and can include sorbents, condensates, and reaction products along with the MSWI ash (Cho et al. 2020). MSWI ash typically makes up only 3% by weight of incineration waste and consists primarily of SiO2, CaO, and Al2O3, and also includes significant amounts of Cl, Na, and K and heavy metals such as Zn and Pb. MSWI ash typically has a specific gravity ranging from 1.7 to 2.4 and a relatively high LOI of approximately 13% (Cho et al. 2020).
Due to the soluble salts, chlorides, and heavy metals present in MSWI ash, it must typically be beneficiated prior to use in concrete. Beneficiation approaches used have included thermal
treatment, chemical stabilization, solidification, and extraction/separation using water or acids (Chandler et al. 1997; Snellings et al. 2023). Vitrification of MSWI ash at high temperatures has been found to reduce the presence of toxic metals and to immobilize other metals in a vitreous matrix (Ali et al. 2022). Studies of vitrified MSWI ash in concrete have shown that its performance is acceptable as an ASCM (Ali et al. 2022; Flesoura et al. 2021).
A key challenge to use of MSWI ash in the United States is that many MSWI plants combine residues produced (bottom ash, fly ash, and air pollution control residues) into a single waste stream. This approach is less common in Europe, where the three MSWI waste streams are kept separate, and beneficial reuse is more common (Crillesen et al. 2006).
Biomass Ash
Incineration of a wide range of biomass materials produces a great amount of ash worldwide. For common crops such as rice, maize, wheat, soybeans, and sugarcane, it has been estimated that 810 million metric tons per year are produced and are potentially available for use as ASCMs (Shah et al. 2022). Shah et al. (2022) have also estimated that if all forestry residues were collected and combusted, an additional 120 million tons would be available for use. It should be noted that many biomass sources are composted rather than combusted, so the availability of the waste in ash form varies greatly by material and location.
The type and therefore composition and characteristics of the biomass ash are influenced by the organic materials combusted, the part(s) of the plants combusted, and the technology used for combustion (Sigvardsen et al. 2019). It has also been found that the soil type also affects the composition of biomass ash. Therefore, the composition and potential use of biomass as a potential ASCM need to be evaluated on a local or regional basis. Biomass ash typically contains more alkalis (K2O and Na2O) and alkaline earth elements (CaO and MgO) than coal ash (Snellings et al. 2023).
Biomasses can be classified as woody (C-type biomass) or herbaceous (S-type biomass), with C-type biomass having a high CaO content and S-type biomass having a higher silica content. Other agricultural residues have a relatively high K2O content and are classified as K-type biomass. S-type biomass ash is typically more desirable for use in concrete due to its high silica content. However, the potential for higher alkali content should be considered, as should relatively high phosphate content, which may delay hydration reactions (Snellings et al. 2023).
Most biomass ash is composed of irregular, rough particles with a high internal porosity. It also has a high specific surface area that can negatively affect the water demand of concrete mixtures. Studies have found that relatively low replacement rates (on the order of 10% to 30%) are appropriate for most biomass ash types (Thomas et al. 2021; Fořt et al. 2021). Beneficiation of ash, including controlled or optimized burning and milling, could improve the quality of these materials (Medina et al. 2019; Gholizadeh-Vayghan et al. 2021).
Wastes from Mining and Industrial Process
Non-Ferrous Metallurgy Slag
Non-ferrous metallurgy (NFM) slag results from the production of non-ferrous metals such as copper, nickel, tin, and lead. It has been estimated that about 58 ± 8 million metric tons of NFM slag is generated yearly worldwide (Peys et al. 2022). NFM slag is typically composed of iron and silica, with calcium and aluminum also present to a significant extent along with minor or trace amounts of a range of other metals. NFM slag is typically hard and dense, with a range of iron-rich crystalline phases present. If NFM slag is quenched, it tends to be glassy (Snellings et al. 2023). NFM slag would be available for projects close to facilities producing NFM products, with
the composition of the NFM slag heavily dependent on the facility’s source material, products, and processes, as well as the slag’s post-processing.
Some NFM slags are inert, particularly those that are slowly cooled (Pan et al. 2019; Wang et al. 2021). However, rapidly cooled NFM slag has demonstrated reactivity due to its amorphous state (Hallet et al. 2020). Similarly to ferrous slag, NFM slag can slow cement hydration reactions, which can be notable if the NFM slag is ground more coarsely (Feng et al. 2019; Hallet et al. 2020). Using the R3 reactivity test, the reactivity of NFM slag has been shown to be similar to that of coal ash, with a similar later-age strength gain (Hallet et al. 2020; Feng et al. 2020).
Mining, Quarrying, and Dredging Wastes
Mining and quarrying operations produce waste materials in very large volumes. Worldwide, it is estimated that “extractive processes” generate waste volumes on the order of 5 × 109 to 10 × 109 tons annually (Martins et al. 2021). Mine tailings are typically considered finely crushed waste materials resulting from the separation of ore from the mined material. Due to the range of ores and the wide variety of materials present in the ores, mine tailings are highly variable, with a composition based on the source geology, the extraction and mineral processing methods, and other factors (Snellings et al. 2023). Materials deleterious to cement hydration and concrete performance can be present in significant amounts. These can include sulfide materials such as pyrite and pyrrhotite, which can cause internal sulfate attack (Duchesne et al. 2021). Many mine tailings have been found to be inert, but those containing clay minerals (particularly kaolinite) are of interest for potential use as ASCMs (Snellings et al. 2023).
Some aggregate washing residues (Escudero-Restrepo et al. 2020; Thapa et al. 2019) and dredging sediments (Snellings et al. 2016) have been found to contain promising levels of clay, which can be activated by calcination. Other processing steps, such as dewatering and grinding, may need to be considered, as well as the reactivity of the final product and its ability to not negatively affect the performance of the concrete being produced. Calcined dredging sediments have been found to show pozzolanic reactivity similar to that of coal ash but with enhanced reaction kinetics (Snellings et al. 2016). The reactivity was dependent on the source sediment, and increased water demand in the mixture was observed (Snellings et al. 2016).
Bauxite Residue
Bauxite residue is a waste material produced at aluminum refineries, particularly when the alumina is extracted by the Bayer process. The Bayer process includes removal of alumina from the bauxite ore by dissolving it in highly alkaline solutions in hydrothermal reactors (Snellings et al. 2023). It is estimated that approximately 170 million tons of bauxite residue are generated annually worldwide (Peys et al. 2022).
During the Bayer process, bauxite residue is removed as a caustic and alkaline sludge that must be dewatered. After dewatering, the material consists primarily of iron oxides with some alumina, silica, and titanium oxides. Some sodium alkalis are present, but the amount varies from source to source. Heavy metals are present in fairly low concentrations, and the silica is typically present as sodalite or cancrinite phases (Snellings et al. 2023). As removed during the dewatering process, the bauxite residue has a chemical composition that is largely inert and must receive additional processing in order to show potential as an ASCM. Its less favorable chemical composition shows both low reactivity and the potential to compromise the performance of concrete due to the presence of certain components. Studies describing approaches to improve the reactivity of the bauxite residue (through acid washing, hydrothermal washing, and high-temperature processing) and improve its performance are described in Snellings et al. (2023).
Evaluation of ASCMs for Use in Concrete Applications
To provide confidence in the use of ASCMs for concrete applications, the following considerations should be addressed (Cavalline and Sutter 2024):
- The characteristics of both the material (ASCM) and product (concrete) produced using the material
- The potential variability of the material’s characteristics
- The performance of the ASCM or system containing the ASCM
- Implementation of measures during design, construction, and use to mitigate potential environmental impacts
ASTM C1709, Standard Guide for Evaluation of Alternative Supplementary Cementitious Materials (ASCM) for Use in Concrete, provides guidance to agencies and other stakeholders to support the evaluation of a material for use in mortar and concrete. The general framework from ASTM C1709 is shown in Figure 16.
The feasibility of using an ASCM can generally be categorized by availability, consistency, and cost (Cavalline and Sutter 2024). The availability of an ASCM is often based on the proximity of a project or supplier to the source of a material and its processing, beneficiation, or distribution point. An adequate supply of a material to support the needs of a project or supplier must be considered, along with the uniformity and consistency of that supply. Project staging may also need to be considered as part of identification and selection of an ASCM. The consistency, or uniformity, of a material must also be evaluated, and this evaluation should take into account the processing and handling techniques to be used in its production (Cavalline and Sutter 2024).
Despite the economic and environmental benefits that may be offered by ASCMs, one key factor in the decision to use them is cost (Cavalline and Sutter 2024). Costs that need to be considered include material costs, production or beneficiation costs, transportation costs, and costs associated with the use of the material at a production facility (e.g., additional silos and plant equipment). If the use of the ASCM can be shown to provide cost savings compared to the use of a conventional material, the ASCM is more likely to be used, particularly if the decision to use the ASCM is driven by the contractor. Performance-based specifications also provide an opportunity for stakeholders to more readily use ASCMs (Cavalline and Sutter 2024).
To use an ASCM in concrete, stakeholders must demonstrate that the performance of the concrete using the ASCM is equivalent to that of conventional concrete or concrete containing an SCM, or that the ASCM concrete provides acceptable performance. The reactivity of an ASCM is a key consideration. ASTM C1709 provides guidance and technical information to support evaluation of ASCMs that are not covered by ASTM C618-23, ASTM C989, ASTM C1240, and ASTM C1866. ASTM C1709 indicates that the performance of the ASCM being evaluated should be compared to the performance of an existing (conventional) SCM, such as one within the scope of ASTM C618, ASTM C989, or ASTM C1240. ASTM C1709 then suggests a five-phase program for evaluating the ASCM, as follows:
- Stage I. Characterization of the material
- Stage II. Determination of suitable fineness
- Stage III. Testing to specifications such as ASTM C618, ASTM C989, ASTM C1240, or ASTM C1866
- Stage IV. Concrete performance tests
- Stage V. Field trials and long-term performance and durability assessment
Characterization of the material in Stage I should include a chemical analysis to identify the elements present and their quantities. If compounds may be present that could negatively affect the hydration of the cement or the properties of the concrete, tests should be performed to
![[Summary:] The flow begins with the candidate byproduct material identified, leads to initial screening and selection of targeted use, which leads to practical considerations, followed by cost analysis. Cost analysis leads to material characterization and application testing, which leads to environmental analysis, and ends at the decision to use byproduct material. [Full text version:] Candidate Byproduct Material Identified [down arrow] Initial Screening and Selection of Targeted Use •Known or unknown source and history •Materials characteristics •Review of agency specifications •Identification of candidate uses •Selection of targeted bound or unbound use(s) [down arrow] Practical Considerations •Availability in sufficient quantity and during appropriate timeframe(s) •Consistency of material and acceptable variability for targeted application •Off-site beneficiation and/or processing required for targeted use - Feasibility of byproduct to consistently meet specifications [down arrow] Cost Analysis •Cost of virgin material vs. cost of the industrial byproduct -Material costs -Hauling costs -Costs of on-site beneficiation and/or processing -Costs of storage and handling -Equipment required -Space requirements -Cost of blending material and hauling (if applicable) [down arrow] Material Characterization and Application Testing •Tests for qualification/preconstruction stage -Tests of byproduct material -Tests of application containing byproduct material •Tests upon delivery or construction -Tests of byproduct material -Tests of application containing byproduct material [down arrow] Environmental Analysis •Chemical analysis •Toxicity Characteristic Leaching Procedure (T C L P) •Other tests required by agency or regulations •Design considerations to protect the environment •Construction considerations to protect the environment •Environmental Product Declaration (E P D) in conformance with ISO 21930 and ISO 14025 [down arrow] Decision to Use Byproduct Material](https://www.nationalacademies.org/read/29140/assets/images/img-54-1.jpg)
evaluate the potential of these compounds to participate in such reactions. Stage II includes determination of the particle size distribution, fineness, and specific surface of the material. Evaluation of the material in mortar tests per ASTM C109/C109M is recommended, with the mortars containing ASCM compared to the performance of mortars produced with a control Portland cement. In Stage III, tests are conducted to compare the chemical, physical, and uniformity characteristics of the ASCM against the standard of the SCM to which the ASCM is being compared.
In Stage IV, concrete mixtures are prepared to facilitate evaluation of the ASCM in fresh and hardened concrete. The mixture(s) should be proportioned in a manner that reflects the anticipated or desired application of the material. ASTM C1709 recommends that the test program use admixtures that are commonly used, along with at least one conventional SCM and a control mixture for comparison. The ASTM C1709 standard indicates that mixtures should contain ASCM contents that bound the intended levels of use, with total cementitious contents varying from 337 to 674 lb/yd3 (200 to 400 kg/m3).
If a material shows promise for use as an ASCM in concrete through Stages I through IV, field trials should be performed (Stage V). Guidance for performing field trials is provided in ASTM C1709, along with suggestions regarding sampling and testing frequencies. During the first 6 months of production of the ASCM, the sampling and testing frequency should be increased from the recommended sampling and testing frequency required in the relevant standard. Variability of the ASCM should be determined periodically, and the agency can use these results to set limits on the uniformity of the ASCM (Cavalline and Sutter 2024).
The performance of some ASCMs has been demonstrated through research and field trials, and in some instances, guidance documents and standards exist. Practical challenges associated with use of many ASCMs include the processing and handling required to ensure appropriate composition and fineness, the volume produced, and the proximity to projects or ready-mixed concrete plants (Cavalline and Sutter 2024). If available locally in a sufficient quantity and appropriate uniformity, and limited processing and handling are required, ASCMs can offer an alternative to conventional SCMs (Snellings et al. 2023).
Approval for use by agencies and meeting specifications for projects are practical considerations faced by agencies and their stakeholders. Agencies approached by producers and supporters of these materials need to consider a number of factors associated with the feasibility of the use of each material (Cavalline and Sutter 2024). ASTM C1709 provides guidance to support agencies in evaluating materials for their potential use as ASCMs.





































