Summary
Sunscreens have been available since the 1930s to help mitigate harms to human skin from the sun. UV (ultraviolet) filters are the active ingredients in sunscreens that reduce the level of UV radiation reaching the skin by absorbing, reflecting, and/or scattering the sun’s UV rays, thereby reducing their ability to reach the skin. UV filters are ubiquitous in their use in sunscreens, are used in other consumer products for the purposes of UV stabilization, and have been detected in aquatic environments (water, soil, and biota). Their presence in the environment, while itself not indicative of environmental harm, has led to a rapid increase in research in recent years on their potential environmental impact.
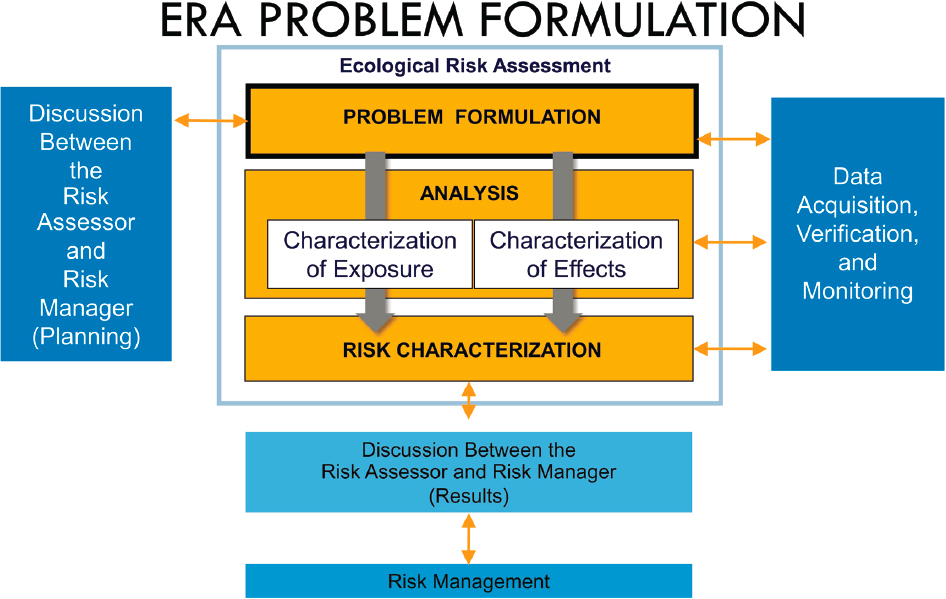
The challenge for understanding the risks from UV filters to aquatic environments is in determining whether and under what conditions individual or mixtures of UV filters are a risk to organisms and ecosystems—either alone or in combination with other environmental stressors—and where these conditions might occur. To that end, an ecological risk assessment (ERA) is a process to identify the particular exposure setting(s) in which a stressor (in this case, UV filters) could be the cause of ecological impacts. ERAs involve integrating information about exposures in the environment with information about adverse effects (Figure S.1). While ERAs can be conducted with limited information, increasing amounts of information about exposure and effects can lead to higher “tier” ERAs that have lesser degrees of uncertainty. Screening can occur as part of the tiers and serves to differentiate among chemicals that may pose a risk, clearly do not pose a risk, or require additional information in order to conduct a more thorough evaluation.
STUDY TASK AND APPROACH
In accordance with the statement of task (detailed in Chapter 1), this report reviews the state of science on the sources and inputs, fate, exposure, and effects of UV filters in aquatic environments and the availability and applicability of data for conducting ERAs. It also reviews the epidemiological and clinical literature on the efficacy of sunscreen in preventing UV damage to human skin, the state of knowledge on potential human behavior changes, and the resulting health impacts related to skin cancer prevention from changes in sunscreen usage (e.g., reducing sunscreen use or switching to sunscreens with different active ingredients). It is not within the committee’s scope to conduct the final step of an ERA: the risk characterization.
The scope of the study is limited to the United States. There are currently 16 UV filters allowed by the U.S. Food and Drug Administration (FDA) for use in any sunscreen sold in the United States, plus an additional proprietary UV filter, ecamsule, approved for use in limited products (Box S.1). In addition, the scope of the report
is specifically on UV filters and not sunscreen inactive ingredients. While UV filters are used in a broad range of products, the committee’s scope was to focus on their use in sunscreens.
This summary describes the findings (as bolded statements), conclusions, and recommendations of the study committee along with some of the supporting information upon which they are derived. This information is described in further detail in the report with supporting references.
USES OF UV FILTERS IN SUNSCREENS
The 17 UV filters described in this report vary in their physical and chemical properties, which influence both their environmental fate and potential toxicity as well as their contributions to skin protection. Two of the UV filters are inorganic particulates (titanium dioxide [TiO2] and zinc oxide [ZnO]), while the others are organic compounds.
UV filters are part of sunscreen formulations that consist of mixtures of active and inactive ingredients, which influences their effectiveness as sunscreens and may influence their environmental input rate, fate, and toxicity. Usually, an individual UV filter does not provide protection against the entire UV wavelength range, resulting in the use of mixtures of UV filters in sunscreen formulations to achieve broad-spectrum protection (i.e., coverage in UVA and UVB).
The compounds used as UV filters are not used exclusively in products marketed as sunscreens. Many different products may contribute to the release and detection of UV filters in the environment. Total volumes of production, sales, or usage give an indication of the potential level of a chemical in the environment. However, disentangling tonnage estimates between sunscreens and other product uses, especially other personal care products, is challenging. From a review of multiple data sources, the committee found that the UV filters in most common use in personal care products marketed in the United States are avobenzone, homosalate, octinoxate, octocrylene, octisalate, oxybenzone, titanium dioxide, and zinc oxide.
SOURCES AND INPUTS OF UV FILTERS INTO THE ENVIRONMENT
Direct Release of UV Filters During Surface Water Contact Activities
Direct release of UV filters can potentially occur during aquatic recreation (via rinse off into the water) and sunscreen application (particularly for aerosols/sprays). Highly variable concentrations of some UV filters have been correlated with the time, location, and intensity of recreational activity. Additionally, some studies have used estimates of rinse-off rates, in combination with the number of swimmers and sunscreen application and reapplication rates, to estimate the amounts of UV filters entering an aquatic system.
Direct Release of UV Filters from Stormwater and Wastewater
Information to trace UV filters that enter wastewater systems specifically from sunscreens and determine their percentage contribution to the total loading was not available. Instead, the committee describes the transport and abatement of UV filters in these systems, with the presumption that sunscreens will be a contributor. However, no studies have systematically measured stormwater concentrations of organic UV filters, and there are limited studies measuring stormwater concentrations of inorganic UV filters.
In regards to decentralized wastewater treatment, inorganic UV filters are likely to be retained within on-site biological treatment systems (e.g., cesspools, septic systems) or removed from the effluent as the water subsequently infiltrates leaching fields or permeates soil. There is insufficient research available to make similar assessments regarding organic UV filters. The presumed mechanisms for removal (i.e., aerobic or anaerobic degradation) of the UV filters vary by chemical structure. Septic systems experience a failure rate of about 10 to 20 percent, potentially resulting in subsurface groundwater transport and discharge of contaminants into nearby surface waters.
The extent of UV filter removal at centralized wastewater treatment plants (WWTPs) depends on the chemical’s affinity for association (e.g., biosorption) to bacterial sewage solids and susceptibility to biodegradation. In WWTP conditions, organic UV filters vary from ready biodegradable (aminobenzoic acid, octinoxate, octisalate,
padimate O) to inherently biodegradable (homosalate, oxybenzone, sulisobenzone) to nonbiodegradable (avobenzone, dioxybenzone, ecamsule, ensulizole, octocrylene). Titanium dioxide and zinc oxide are inorganic and hence will not biodegrade. Organic UV filters with higher log Kows such as homosalate, meradimate, octocrylene, octinoxate, octisalate, and padimate O, as well as the inorganic UV filters titanium dioxide and zinc oxide, are most likely to be highly removed from the effluent. Studies have shown their presence in sewage solids that are collected and disposed of off-site (landfills, land applied, incinerated). Wastewater systems are not currently designed to remove UV filters specifically, though the effectiveness of advanced treatment options are under study. In the case of combined sewer systems, wherein occasionally during larger rainfall events untreated sewage and rainfall runoff bypass the wastewater treatment plant facility entirely, UV filters and other contaminants can be discharged with no or limited treatment into surface waters.
FATE CHARACTERISTICS OF UV FILTERS
Once released to the environment, UV filters can partition into different environmental compartments (e.g., air, water, sediment, organisms). Organic UV filters are expected to display an array of environmental behaviors based on their range of measured physico-chemical properties. With the notable exceptions of ensulizole, aminobenzoic acid, trolamine salicylate, and sulisobenzone, the organic UV filters are generally hydrophobic and thus would be expected to partition to organic fractions, including particles and sediments. Oxybenzone is moderately water soluble, with less distinct partitioning between aqueous and organic sediment compartments. However, testing conditions for solubility and other physico-chemical properties are not standardized for freshwater or marine/saltwater.
There is a wide range of UV filter behaviors with respect to biodegradation and photostability. Avobenzone, dioxybenzone, ensulizole, and octocrylene have been shown to have low biodegradability, while oxybenzone and octocrylene appear to be relatively photostable in laboratory settings. However, the laboratory settings may not accurately recreate environmental conditions. In particular, the complex local molecular environment for UV filters can dramatically impact UV filter photostability.
The existing fate and transport data suggest that aggregation of TiO2 and ZnO with other particles in the water column leads to formation of larger sized particles that settle out of the water column and accumulate in river, lake, or estuary sediments. Increasing salinity leads to more rapid aggregation of particles. Whereas dissolution of TiO2 has been reported, it is likely to be a very slow process, and water quality drivers for this process remain ill defined. ZnO does dissolve to zinc ions in water, after which the fate of zinc ions is influenced by pH, redox, solids, and anions present in the water. The lifetime of inorganic UV filters in the water column is determined by their size, density, surface charge, and tendency to aggregate with other particles in water, which then promotes settling into sediments.
ESTIMATED AND MEASURED CONCENTRATIONS IN WATER AND SEDIMENTS
The committee compiled available data on UV filter concentrations in water and sediments and investigated their potential relationship with site-specific characteristics related to (1) source strength, (2) proximity to potential sources, and (3) residence time of receiving waters. Because many studies were not designed to characterize the spatial and temporal characteristics of the UV filters at sampling locations, they provide snapshots in time and/or space and are not robust statistical representations of the presence of UV filters, though some studies do include temporal and spatial variations in the concentrations of UV filters.
The committee used oxybenzone as an example UV filter for analysis due to the relatively high number of aquatic measurements available compared to other UV filters. The analysis showed that occurrence measurements of UV filters reflect the spatial and temporal variability in the local setting. The highest measured concentrations of most UV filters occur in shallow waters, within or near recreational areas (e.g., swimming beaches), and during the day.
For several organic UV filters—oxybenzone, octocrylene, homosalate, avobenzone, and octinoxate—the available data show that the highest measured environmental concentrations in water are in the range of 1 to 10 µg/L, though most measurements for these and all measurements for other organic UV filters are below 1 µg/L.
Except for octocrylene and octinoxate, which have maximum recorded concentration values between 0.1 and 2.4 µg/g dry wt, all other UV filters exhibit maximum recorded concentrations in sediments below 0.1 µg/g dry wt. There are fewer observations for organic UV filters in sediments than in water.
The elements used in inorganic UV filters (titanium, zinc) occur naturally in water and sediments at concentrations that vary regionally, complicating measurements of the incremental contribution from sunscreens.
A few publications model inputs, transformations, and/or exposure concentrations of UV filters in the environment based on inputs and environmental conditions. Although predicted estimates are typically above concentrations of UV filters measured in surface waters, modeled and measured aquatic and sediment concentration data provide ranges, including potential upper bounds, on exposure levels of UV filters to organisms.
BIOACCUMULATION AND MEASURED CONCENTRATIONS IN BIOTA
Bioaccumulation refers to the accumulation of a chemical into an organism via all routes of exposure. UV filters exhibit a range of bioaccumulation potentials, driven primarily by the lipophilicity of the compound and the metabolism of the parent compound by biota. Bioaccumulation can be predicted by hydrophobicity (the tendency to partition out of the water, which is related to lipophilicity), though this does not take metabolism within an organism into account.
High-quality, laboratory-based bioaccumulation factors (BAFs) or bioconcentration factors (BCFs) available for avobenzone, octocrylene, octinoxate, oxybenzone, homosalate, padimate O, and titanium dioxide reveal a low to moderate bioaccumulation potential. For all other UV filters, reliable laboratory-based BAFs or BCFs are not available.
Since chemicals can be integrated into the tissue or intestinal tracts of organisms, and predators of those organisms may consume the contaminants secondarily, chemicals may persist in the food web and ecosystem. BAF or BCF studies for the most lipophilic UV filters indicate a low likelihood of trophic magnification, although some have measurable BCFs. Additional research, for example determination of critical body burdens, may clarify if accumulations of UV filters contribute to long-term stress of biota.
CONCLUSIONS ON UV FILTER FATE, EXPOSURE, AND BIOACCUMULATION
Fate Characteristics: Based on their physical and chemical properties, some UV filters have the potential to persist and accumulate in aquatic ecosystems. Specifically, avobenzone, dioxybenzone, ecamsule, ensulizole, and octocrylene have been shown to have low rates of biodegradation in laboratory settings. However, empirical evidence of rates of degradation and dissipation in the environment is limited. Inorganic UV filters (zinc oxide, titanium dioxide) are expected to aggregate in the water column and deposit into sediments. Environmental characteristics of receiving waters will also influence the fate of UV filters including physical mixing, advection, light intensity, and spectral range, and dissolved organic matter. Likewise, depending on compound-specific lipophilicity, environmental compartmentalization and species-specific location and metabolism, some UV filters (avobenzone, octocrylene, oxybenzone, homosalate, padimate O, titanium dioxide, zinc oxide) and/or their metabolites were found to be present in tissues of aquatic animals and plants; however, bioaccumulation studies suggest that bioaccumulative transfer to higher levels of the food web are limited.
Environmental Occurrence: Measurements of UV filters in surface waters and sediments along with modeling efforts reveal spatially and temporally variable patterns, some of which have been shown to reflect the degree of human activity locally present (i.e., recreation) in addition to the physico-chemical characteristics of the aquatic
systems and UV filters. This pattern of exposure is based on analysis of the UV filters with the most environmental measurements (oxybenzone, followed by octocrylene and octinoxate), though even these measurements are typically snapshots in space and time. Current sampling programs provide limited data, especially in regard to identifying sources, a particular problem for the inorganic UV filters. Maximum observed concentrations of these UV filters can exceed 1 μg/L (ppb) with the highest concentrations found in surface waters immediately off beaches at semi-enclosed embayments. UV filters also enter aquatic systems through treated or untreated wastewater. Treatment can differentially degrade and/or remove a portion of the UV filters present in influents (most likely to be removed are homosalate, meradimate, octocrylene, octinoxate, octisalate, padimate O, titanium dioxide, and zinc oxide based on log Kow and laboratory testing), with some of these being translocated to sewage solids. The measured concentrations for water bodies receiving wastewater effluent have been generally lower than for waters with recreational activities, though the environmental setting is expected to be a factor. Measured concentrations do not necessarily reflect UV filters solely from sunscreen since they are present in a range of products, with other personal care products being the category of product most likely to have similar fates as sunscreens.
REVIEW OF STUDIES ON THE EFFECTS OF UV FILTERS IN AQUATIC ENVIRONMENTS
Laboratory toxicity tests are most widely used to provide the effects data required for ERAs. However, not all studies are designed for use in ERAs, and may instead be informative to understanding modes of action or otherwise illuminating areas of concern for further study. For a subset of UV filters, the committee identified data potentially suitable for use in an ERA in order to inform the committee’s assessment of progress and research needs toward higher-tiered ERAs. Acute and chronic toxicity studies vary significantly in their relevance and the reliability of methods, particularly due to the importance of studying nonstandard test species for which there are not standardized methods, thus limiting identification of accurate toxicity thresholds.
More acute (shorter [hours to days] duration, usually higher test concentrations) toxicity studies are available than chronic (substantial portions of a lifetime or critical life stage) toxicity studies. For the purposes of categorizing acute toxicity thresholds, EPA considers values under 1,000 μg/L to be “highly toxic.” This metric does not represent a determination of hazard for risk assessment and was used by the committee instead as a means of comparing laboratory toxicity results across UV filters within a few orders of magnitude of concentrations that have been observed in aquatic environments. Acute toxicity has been observed under 1,000 µg/L for dioxybenzone, octinoxate, oxybenzone, padimate O, titanium dioxide (in the presence of UV radiation), and zinc oxide and in a few studies for avobenzone and octocrylene. In addition, QSAR (quantitative structure-activity relationships) estimates for aminobenzoic acid, cinoxate, meradimate, homosalate, and octisalate suggest possible toxicity below 1,000 µg/L. Toxicity values typically exceed solubility for the poorly soluble UV filters (solubility under 100 µg/L). Figure S.2 depicts the range of variability in acute toxicity results for the UV filters for which the committee assessed the applicability of the available data for ERAs.
Chronic toxicity studies are critically important, especially given the number of UV filters with low solubilities (many under 100 μg/L in pure water) that would be expected to be found at relatively low dissolved concentrations in the environment. While many UV filters do have chronic toxicity data, availability is highly limited especially for nonstandard species.
Ideally, for ERAs, sufficient information about a compound is available to produce a species sensitivity distribution (SSD). SSDs are used to predict a concentration at a low level of probability where it is unlikely for organisms to be harmed. Sufficient data are available for the development of acute SSDs for oxybenzone, octinoxate, titanium dioxide, and zinc oxide and chronic SSDs for oxybenzone and zinc oxide. Otherwise, significant gaps remain related to the availability of studies on nonstandard organisms (especially marine organisms), chronic studies, and sediment toxicity studies (critical for the hydrophobic organic UV filters and the inorganic UV filters). Filling knowledge gaps in toxicity data will inform higher tiered risk assessments as well as information about potential effects on threatened and endangered species via data for surrogate species.
Genomic, biochemical/biomarker, physiology, and cell-/receptor-based assay systems reveal a diverse array of information is also available from acute and chronic studies of UV filters. There are not yet sufficient studies to

describe the pathway to population responses. However, evidence is accumulating that oxidative stress, genotoxicity, neurotoxic, or endocrine modulation modes of action may be present for some of the UV filters.
Few studies have investigated effects of UV filters on the ecosystem processes of nutrient cycling, organic matter decomposition, primary production, and species interactions.
Multiple UV Filters and Stressors Context
Aquatic ecosystems experience myriad co-occurring stressors that can both (1) complicate attribution of observed ecosystem degradation and (2) lead to cumulative or interacting effects with UV filters. These co-occurring stressors can include multiple UV filters due to the mixtures found in sunscreen formulations, other chemicals, and environmental stressors (e.g., increasing temperatures, salinity, UV radiation). Limited data indicate that interacting effects are possible from the combination of multiple UV filters or UV filters and other environment stressors. Increasing temperatures are of particular concern due to the potential for increasing cumulative and interacting impacts from climate change. However, results of studies on multiple stressors are variable in whether they may result in additive, synergistic, and/or antagonistic effects.
CONCLUSIONS ON ENVIRONMENTAL EFFECTS
Environmental Effects: For most UV filters, acute toxicity information is available for standard algal, invertebrate, and fish species, though in some cases data from only one to two studies per taxa are applicable for use in an ERA (chronic data is limited across UV filters). Data are limited for nonstandard but important ecological receptors, particularly marine species, often challenged by the lack of standard toxicity test methods and the use of important but nonstandard endpoints (e.g., bleaching and/or algal loss in corals). Many UV filters have either laboratory observations or QSAR estimates of acute toxicity under 1,000 μg/L. However, toxicity results range widely for most UV filters and in some cases, only a few studies support toxicity below 1,000 μg/L. For the UV filters with low solubility, results are typically above solubility, indicating a need for chronic studies of lower exposure concentrations. Supporting toxicological information has been developed using cell-line tests and other studies that can help elucidate toxic modes of action (e.g., narcosis, endocrine disruption, enzyme inhibition), which may be useful for informing assessments if they can be linked through adverse outcome pathways to population endpoints used for ERA. Studies on effects on community interactions and ecosystem processes are largely absent and, at this time, are mostly presumed based on effects on taxa involved in key community and ecosystem functions. Spatial overlap is expected between habitats occupied by threatened and endangered species and UV filter exposure zones, and effects will depend on magnitudes of exposure and the sensitivity of the species (for which little is known).
Multiple UV Filter and Stressor Context: Risks that UV filters may pose to aquatic ecosystems will occur within the context of other global (e.g., climate change variables) and local stressors (e.g., pollution, physical damage). Ecological risk assessments commonly consider chemicals individually, which is appropriate. However, there are two aspects associated with multiple stressors and their potential for antagonistic, additive or synergistic effects that are important to consider when planning an ecological risk assessment for UV filters: (1) the possibility of mixture effects of multiple UV filters and (2) possible interactions of UV filters with other predominant environmental stressors (e.g., temperature, UV light, salinity, other chemicals). UV filters that co-occur in products or in the environment are candidates for considering possible mixture-related effects and risks. Among the global predominant stressors on aquatic ecosystems, increasing temperature has been shown to be a major stressor on its own, but it is also known to exacerbate the effects of toxicants and is important to consider when assessing effects and risks from UV filters in aquatic environments.
SUNSCREEN EFFICACY AND USE FOR HUMAN HEALTH
Exposure to UVR causes sunburn and photoaging in human skin and is a risk factor for the development of skin cancers, both keratinocyte carcinomas and melanomas. Large randomized controlled trials and longitudinal observational studies have demonstrated that consistent use of broad-spectrum, SPF 30 sunscreen when outdoors reduces the risk of developing skin cancer, photoaging, and sunburn, though research has been focused on populations with fair skin. Sunscreen use is part of a recommended regimen of photoprotection that also includes the use of protective clothing, hats, sunglasses, sun avoidance, and shade-seeking behaviors.
Only about a third of the U.S. population uses sunscreen regularly, though use is higher during outdoor activities and at the beach (between 70 and 80 percent). Even when sunscreen is used, dosage (i.e., amount applied and rate of reapplication) usually does not meet recommendations for optimal effectiveness. Women are nearly twice as likely to use sunscreen as men. Routine sunscreen use is highest among non-Hispanic whites.
Correlates of Consumers’ Preferences and Choice of Sunscreen
Sunscreen preferences are primarily driven by perceived effectiveness (e.g., SPF) and cosmetic preferences (e.g., skin feel, scents, and appearance on skin). These features can be influenced by UV filters and other ingredients in sunscreen. However, in general, consumers are not knowledgeable about the ingredients in their sunscreens. Some consumers can also be influenced in their choice of sunscreens based on perceived impacts on the environment. Sunscreen use research and consumer online reviews of sunscreen products indicate
that sunscreens with organic UV filters are on average most highly rated, and that sunscreens with inorganic UV filters are on average more expensive and sometimes considered less cosmetically acceptable than their organic UV filter counterparts. However, products of both types can be found among highly rated purchases. There are only a few empirical publications that report on consumer attitudes and knowledge about potential effects on aquatic environments, including coral reefs.
POTENTIAL CHANGES TO SUNSCREEN USE AND THE HUMAN HEALTH CONSEQUENCES
Restrictions on certain UV filters may have negative impacts on the use of sunscreen to prevent skin cancer, sunburn, and photoaging if they lead to reduced sunscreen usage. Potential alternative scenarios to current use and choice of sunscreens were identified by the committee. The likelihood of each scenario—and therefore the resulting human health outcomes related to preventing skin cancer—is in part dependent on which UV filters are available, which may vary by location. The ability to purchase broad-spectrum, SPF 30+ sunscreen that people will actually use is a key determinant of health outcomes.
- Scenarios likely to lead to negative effects on health:
- Decreased use of sunscreen with no change to other sun protective behaviors
- Decreased use of sunscreen with suboptimal increases in other sun protective behaviors
- Use of alternative sun protection products with UV filters that do not meet FDA standards
- Scenarios likely to lead to no or minimal effects on health:
- Decreased use of sunscreen with optimal practice of other sun protective behaviors
- Obtaining sunscreens with restricted ingredients from elsewhere
- Switching to alternate formulations
- Scenario likely to lead to positive effects on health:
- Increased use of sunscreen
CONCLUSIONS ON IMPLICATIONS FOR HUMAN HEALTH
UV Radiation and Sunscreen Benefits: Exposure to UVA and UVB radiation increases the risk of both acute and chronic injury to humans. Acute effects are inflammation, usually referred to as sunburn. Chronic effects include malignant melanoma, basal cell and squamous cell cancers, photoaging, and a host of precancerous changes to the skin. The burden of skin cancers in terms of disease, death, and health care costs is high and melanoma rates are increasing. Also, there are specific photosensitive conditions for which exposure to UV radiation is more harmful. These relationships have been established by extensive epidemiologic studies, largely conducted in fair-skinned populations. Consistent use of SPF 30 broad-spectrum sunscreens has been shown experimentally and in observational studies to reduce risk for melanoma, squamous cell skin cancer, sunburn, and photoaging. The overall level of certainty regarding these benefits is high.
Sunscreen Usage and Preferences: Behavioral studies have found that the use of sunscreen for photoprotection is inadequate. It is not used by all who should use it, too little is used, and/or it is not reapplied frequently enough. It has been found from usage data, internet product reviews, and surveys that individuals prefer sunscreens with high SPF and various cosmetic features. Of particular relevance with regard to cosmetic aspects is that some consumers will have a lower preference for preparations containing inorganic filters (studies on preferences do not distinguish particulate sizes) in the concentrations needed when these agents are used alone without organic filters. However, studies have also found that many consumers lack knowledge of the active ingredients in their preferred sunscreens.
Implications of Changing Sunscreen Use: Reduced availability of consumer-preferred sunscreen formulations may lead to reduced use of sunscreens. Human health outcomes will depend on the available UV filters, which will drive both consumer use and sunscreen efficacy. Educational and motivational campaigns that encourage the use of broad-spectrum SPF 30+ sunscreens at recommended levels along with other photoprotective behaviors
where feasible, as well as implementation of environmental supports such as public shade structures, may mitigate these harms.
RECOMMENDATIONS
Recommendation 1: The U.S. Environmental Protection Agency should conduct an ecological risk assessment (ERA) for all currently marketed UV filters and any new ones that become available. There is an urgent need to conduct such an assessment, driven by the evidence of local exposures of aquatic organisms in U.S. aquatic ecosystems to UV filters, potentially including endangered species, and experimentally demonstrated potential for environmental impact, either alone or in context of other system stressors. The results of the ERA should be shared with the U.S. Food and Drug Administration for their consideration of the environment in their oversight of UV filters. The following points are critical for conducting an ERA for UV filters:
- The ERA is expected to include information from acute and chronic toxicity studies with standard test species and life stages, methodologies, and biological endpoints. However, nonstandard species and additional biological endpoints should also be considered given the diversity of important ecological species potentially exposed to UV filters and the potential for adverse effects not captured in standard test protocols (e.g., corals and their unique endpoints related to bleaching). While not currently used for regulatory ecological risk assessments in the United States, cell-line tests and other New Approach Methods such as molecular/biochemical changes may be useful for elucidating toxic modes of action (e.g., narcosis, endocrine disruption) and potential for effects.
- Because UV filters often occur in mixtures within products and in varying compositions across products, ERAs should not only consider UV filters individually, but also evaluate the potential for risks from co-occurring UV filters. Mixture considerations could be based on co-occurrence in the environment, as well as common exposure pathways and modes of action.
- ERAs should consider the environmental settings or exposure scenarios, specifically the potential for localized (in space and time) elevated UV filter concentrations in the water column and/or sediment that provide habitat for a diverse or unique biological community. Settings to give particular attention to are (1) coral reefs in shallow near-shore environments with heavy recreational use and limited transport/flow of seawater and/or colocated near communities where wastewater and urban runoff may enter the marine environment, and (2) lentic (slow-moving) freshwater systems with heavy recreational activity or wastewater effluent and high water residence time, especially the habitats of sensitive species such as amphibians and mussels.
While conducting an ERA in the near term is imperative, future assessments will be improved by increased data collection. Knowledge gaps have been outlined in each chapter.
Recommendation 2: The U.S. Environmental Protection Agency, partner agencies (e.g., Centers for Disease Control and Prevention, U.S. Department of the Interior, U.S. Food and Drug Administration, National Institutes of Health, National Oceanic and Atmospheric Administration, National Science Foundation), and sunscreen formulators and UV filter manufacturers should conduct, fund or support, and share research and data on sources, fate processes, environmental concentrations, bioaccumulation studies, modes of action, and ecological and toxicity testing for UV filters alone and as part of sunscreen formulations. Additionally, epidemiological risk modeling and behavioral studies related to sunscreen usage should be conducted to better understand human health outcomes from changing availability and usage. Coordination among these organizations would improve collection of the various types of relevant data needed for an ecological risk assessment. Future research should adhere to international or national standards where applicable for analytical chemistry and toxicological studies, and follow accepted principles for ensuring good quality information from testing and measurement protocols, report their methodologies, and undergo scientific peer review of both protocols and findings for quality assurance. This may include new national/international standards for toxicity testing on relevant species and the addition of nontraditional biological endpoints for acute and chronic toxicity. Public access and transparency in all data and research outcomes is critical for assurance of public and environmental health.









