The Future Pediatric Subspecialty Physician Workforce: Meeting the Needs of Infants, Children, and Adolescents (2023)
Chapter: 6 Trends in the Pediatric PhysicianScientist Workforce
6
Trends in the Pediatric Physician–Scientist Workforce
Pediatric subspecialty physicians play a critical role in pursuing the research that leads to advances in child health. The robustness and endurance of the pediatric physician–scientist workforce pathway can have long-lasting effects on child and adolescent health through research to prevent, diagnose, and treat diseases that occur specifically in children or begin in childhood and affect the life course. As a result, advances in pediatric research have improved the lives of both children and adults. This chapter summarizes the pediatric research landscape in general, including examples of previous successes and unique challenges inherent to pediatric research. The chapter then delves into factors and barriers that affect the pediatric physician–scientist pathway and impact the ability of the physician subspecialty workforce to pursue a robust research portfolio that advances the care of all children and youth. There is limited available information specific to pediatric subspecialists, so much of this chapter is on the overall pediatric research workforce while considering the implications for research by physician–scientists in the pediatric medical subspecialties specifically. The challenges faced by the pediatric physician–scientist workforce are further compounded given the smaller numbers of pediatric subspecialists. Additionally, while other clinician scientists1 and non-clinician scientists (i.e., Ph.D. scientists who study pediatric subspecialty conditions and treatments) are discussed as relevant, the focus of this chapter is on the pediatric physician–scientist workforce.
___________________
1 For more information on the National Pediatric Nurse Scientists Collaborative, see https://npnsc.org/ (accessed May 3, 2023).
THE IMPORTANCE OF PEDIATRIC RESEARCH
In 1979, James B. Wyngaarden described “The Clinical Investigator as an Endangered Species” in his presidential address to the Association of American Physicians (Wyngaarden, 1979). Since then, discussions have continued about the plight of the physician–scientist, including pediatric physician–scientists specifically (Alvira et al., 2018; Daye et al., 2015; Milewicz et al., 2015; Rubenstein and Kreindler, 2014; Salata et al., 2018; Schafer, 2010; Zemlo et al., 2000). The scope of the pediatric research enterprise is transdisciplinary and has broadened to include the full spectrum of basic science, translational, community-based, population health, health services, health equity, and child health policy research (AAMC, 2023a; COPR, 2014; Williams et al., 2022). Furthermore, the development, recruitment, and retention of pediatric physician–scientists is critical to accelerate advances in wide-ranging fields such as molecular biology, genetics, genomics, precision medicine, health care delivery, health services research, and injury prevention (Alvira et al., 2018).
Pediatric Research Successes
Improving children’s health is essential to developing a productive and healthy population (NRC and IOM, 2004), and research is the foundation of evidence-based innovation in pediatric care. Recent developments in research and clinical care include the increasing application of genome sequencing to diagnosis, clinical monitoring, and treatment; progress in developing cell therapy for cancers that are resistant to treatment; advances in developing gene therapy for a growing number of single gene disorders; targeted research on surfactant therapies for pediatric and neonatal acute respiratory distress syndrome; immunotherapeutic modalities for the treatment of pediatric malignancies; and a clearer understanding of the relationship between the microbiome and specific diseases (Baruteau et al., 2017; Bick et al., 2019; De Luca et al., 2021; Gilbert et al., 2018; Holstein and Lunning, 2020; Hutzen et al., 2019; Janssens et al., 2018). Some examples of notable pediatric scientific achievements are listed in Box 6-1.
Success stories serve as testimony to the transformative impact of scientific discovery on clinical care, but such discoveries require ongoing efforts and partnerships among medical schools, hospitals, payers, advocates, clinicians, and researchers to address rising challenges and to optimize access of therapeutics to children as early as possible. Boxes 6-2 and 6-3 highlight two specific case studies of pediatric research success: spinal muscular atrophy (SMA) and the Children’s Oncology Group (COG). Challenges and controversy have unfolded as health systems navigate this era of precision health in pediatric disease, including access, handling, and delivery of
these drugs, particularly in the context of their high cost (zolgensma, used to treat SMA, costs $2.1 million per dose). These precision health opportunities also have motivated expansion of the newborn screening program and development of scalable assays to accelerate timely diagnosis of these now-curable conditions.
There has also been advancement in research, evaluation, and measurement infrastructure for child health services research, such as the increased representation of children in the National Patient-Centered Clinical Research Network (PCORnet) (Forrest et al., 2021) and the establishment of the National Cancer Institute Childhood Cancer Data Initiative (NCI, 2023a). Other examples of success can be seen in the substantial advancements in the care of prematurely born infants. Use of intrapulmonary surfactant and improvements in ventilation strategies, external environment
management, and neuromonitoring devices have improved viability (defined as the gestational age at which there is a 50 percent chance of survival with or without medical care) from 25 to 26 weeks’ gestation in the mid-1990s to 23–24 weeks gestation by the mid-2000s (Glass et al., 2015). Innovative research also has been applied to directly improving patient outcomes through the development of pediatric learning health systems (Forrest et al., 2021; Varnell et al., 2023) and uncovered insights related to implicit bias and racism on child and adolescent health (Goyal et al., 2020, 2015; Johnson et al., 2017a; Priest et al., 2013; Puumala et al., 2016; Trent et al., 2019). In addition to improved pediatric patient outcomes, pediatric research has far-reaching impacts on both children and adults’ life course health related to health equity, social and structural determinants of health, and other pressing issues that can improve health across the lifespan (Braveman et al., 2009; Cheng et al., 2022; Halfon et al., 2022; Woodward et al., 2022). (See Chapter 2 for more information on additional emerging health needs for pediatric patients that could be supported by pediatric research.)
UNIQUE CHALLENGES OF PEDIATRIC RESEARCH
Pediatric studies bring specific challenges, including ethical considerations, logistical and technical factors in administering interventions, smaller population size (especially for subspecialty care), developmental considerations in studying children across ages, reluctance to include pregnant women and their fetuses in clinical intervention research, and financial disincentives related to the limited commercial market potential for pediatric drugs compared with adult drugs (Blehar et al., 2013; Burckart, 2020; Caldwell et al., 2004; Kern, 2009; Rees et al., 2021; Shakhnovich et al., 2019; Steinbrook, 2002). As a result, despite the potential for lifelong benefit, fewer studies are conducted in children, even for diseases and conditions that are common in pediatrics, and children tend to be underrepresented in randomized clinical trials for diseases that affect both adults and children (Groff et al., 2020; Hwang et al., 2020; Thomson et al., 2010). Similarly, fewer clinical trials are performed in children compared with other patient populations (Bourgeois et al., 2014; Viergever and Rademaker, 2014), and “while as much as 65 percent of funding for studies in adult populations is provided by the pharmaceutical industry, nearly 60 percent of pediatric clinical trials are sponsored primarily by government and nonprofit organizations” (Rees et al., 2021, p.1237), showcasing the lack of financial incentives for robust industry participation in pediatric research.
Historical Limitations of Pediatric Research Compared with Adult Research
Historically there has been a mismatch between the number of pediatric randomized controlled trials (RCTs) and pediatric disease burden (Groff et al., 2020). Older studies have noted this paucity of pediatric RCTs in published literature (Cohen et al., 2007, 2010; Thomson et al., 2010). Bourgeois et al. (2012) reviewed the clinical trial landscape for conditions known to have pediatric involvement and found that nearly 60 percent of the disease burden was attributable to children, but only 12 percent of trials were pediatric. The authors concluded that the significant disparity between pediatric burden of disease and the level of clinical trial research devoted to pediatric populations may be due in part to having to rely on non-industry funding sources.
The evidence base for treatment and health research and development in children also lags behind that for adults (Viergever and Rademaker, 2014). A major contributor is the lack of pediatric natural history and clinical registry data due to the logistic, ethical, and legal challenges of performing clinical investigations in children (Brussee et al., 2016; Goulooze et al., 2020). Current data collection methods are based on experience with adult populations and do not sufficiently capture the effects of family, environment, and biological factors on children’s health and development. Specific aspects of pediatric RCTs that can undermine recruitment, retention, and trial success include ethical considerations around parental consent and child assent, scientific challenges in the paucity of well-validated clinical endpoints or biomarkers in children, and logistical issues such as the time and financial resources needed for participation. Overall consent rates in pediatric RCTs have improved, but are still not optimal (Groff et al., 2020; Lonhart et al., 2020), especially among minoritized groups.
A review of pediatric studies in ClinicalTrials.gov2 from 2008 to 2019 found a total of 36,136 clinical trials and 16,692 observational studies, with the number of pediatric clinical trials nearly doubling over this period (from 7,000 to almost 12,000), and with an overall decrease in drug trials, but an increase in behavioral trials (Zhong et al., 2021). Pediatric trials were mostly small scale, single site, and usually not funded by industry or the National Institutes of Health (NIH).
___________________
2 ClinicalTrials.gov is a database of clinical studies around the world and is provided by the U.S. National Library of Medicine.
Regulatory Landscape and Historical Efforts to Improve Pediatric Drug Development
Largely due to the challenges in pediatric research described previously, far more drugs have been approved for clinical use in adults than for pediatric populations, which leads to a significant proportion of drugs in children being an “off label” use for various medical conditions (Sachs et al., 2012). As a result, most drugs used to treat children are used without an adequate understanding of appropriate pharmacokinetics, dose, safety, or efficacy (NICHD, 2022a). Among other legislative attempts, two laws have aimed to address the study of drugs in pediatric populations—the Pediatric Research Equity Act (PREA)3 and the Best Pharmaceuticals for Children Act (BPCA), which provide requirements and incentives with the aim to expand the study of drugs in children (IOM, 2012).4 Specifically, PREA authorized the Food and Drug Administration (FDA) “to require pediatric studies in certain drugs and biological products. Studies must use appropriate formulations for each age group. The goal of the studies is to obtain pediatric labeling for the product” (FDA, 2019b). BPCA’s aim is to (1) “encourage the pharmaceutical industry to perform pediatric studies to improve labeling for patented drug products used in children by granting an additional 6 months of patent exclusivity,” and (2) “authorize NIH…to prioritize needs in various therapeutic areas and sponsor clinical trials of off-patent drug products that need further study in children, as well as training and other research that addresses knowledge gaps in pediatric therapeutics” (NICHD, 2022c). The two acts together contributed to the safe and effective use of more than 400 drugs within the first 5 years of implementation (Burckart, 2020), and today there are more than 1,050 small-molecule and biologic products with pediatric labeling from the results of these acts.5 However, Benning et al. (2021) found that pediatric label changes were not associated with subsequent changes in pediatric drug use, and although some drugs had increased pediatric use after gaining new pediatric indications, the pattern was not consistent.
Recent policy efforts to improve pediatric drug development also include the Research to Accelerate Cures and Equity for Children (RACE) Act, which requires evaluation of new drugs and biologics “substantially relevant to growth or progression” of pediatric cancer (Caruso, 2020), and the establishment of the Rare Pediatric Disease Priority Voucher Program,
___________________
3 Pediatric Research Equity Act of 2003, Public Law 108-155, 108th Congress.
4 Best Pharmaceuticals for Children Act of 2002, Public Law 107-109, 107th Congress.
5 Current as of June 27, 2023. See https://www.fda.gov/science-research/pediatrics/pediatric-labeling-changes (accessed June 27, 2023) to see access the Pediatric Labeling Changes Spreadsheet.
which awards companies with priority review for drugs targeting a list of rare diseases (Coppes et al., 2022; Hwang et al., 2019).
Diversity and Inclusion in Pediatric Research Populations
Representative and inclusive clinical trials include people’s heterogeneous lived experiences and living conditions, as well as demographic characteristics such as race and ethnicity, age, sex, and sexual orientation/gender identification (NIH, 2022a). The efficacy of treatments is best assessed when a diverse population is included in clinical trials (Masters et al., 2022). As with adult research, pediatric clinical trials have not always been appropriately inclusive of populations experiencing health disparities (Aristizabal et al., 2015; Faulk et al., 2020; Lund et al., 2009; Walsh and Ross, 2003; Winestone et al., 2019). Rees et al. (2022) found that most racial and ethnic groups were underrepresented in the trials conducted in the United States.
In recent years, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and NIH have made explicit efforts to increase inclusion of pregnant and lactating people, children, and people with intellectual disabilities in their research (Spong and Bianchi, 2018); however, there is still work to be done. For example, Chen et al. (2022) found that non-English-speaking communities were underrepresented in pediatric health research from 2012 to 2021, and only one in 10 pediatric research studies included patients with limited English proficiency. In addition, Watson et al. (2022) found that children in rural settings are underrepresented in clinical trials, potentially contributing to rural health disparities. As the pediatric population of the United States continues to become more diverse, including diverse populations in pediatric clinical trials is critical. Furthermore, Popkin et al. (2022) emphasized that meaningful inclusion in clinical research begins with training diverse medical and scientific workforces and enhancing the diversity of research and clinical teams. See later section on increasing the diversity of pediatric researchers, Chapter 4 for the current demographics of pediatric subspecialists, and Chapter 5 for influences on career choice for individuals who are underrepresented in medicine.
THE PEDIATRIC PHYSICIAN–SCIENTIST WORKFORCE
No single term defines a pediatric researcher, as individuals with a variety of professional backgrounds can engage in investigational activities. Physician–scientists possess a unique combination of clinical and research expertise based on their education and training that enables them to identify knowledge gaps in clinical care and research questions, gain
a comprehensive understanding of critical aspects of medical physiology through clinical epidemiology and disease-specific features, act as a bridge between basic science researchers and clinicians, and develop research strategies aimed at uncovering breakthroughs that can enhance clinical care (Singh et al., 2018; Williams et al., 2022; Yap, 2012). For the purposes of this report, the committee focused on the pediatric physician–scientists, namely those with M.D., D.O.,6 M.D./Ph.D., or M.D./MPH degrees who perform biomedical, behavioral science, health services research, or public health research of any type as their primary professional activity. This definition includes physician–scientists who are conducting “basic research” (fundamental investigations not specific to disease or patient population), “disease-oriented research” (investigations into the causes or treatments of diseases, with no patient involvement), or “patient-oriented research” (clinically oriented studies with direct patient contact) (Zemlo et al., 2000).
The Physician–Scientist Pathway
The decision to pursue a career as a physician–scientist can be made at many points in an individual’s training. The physician–scientist pathway has been described as “long and leaky” (Milewicz et al., 2015). Figure 6-1 highlights the physician–scientist workforce pathway and points of attrition, with best known estimates of losses, as well as the entry points for recruitment, though data are lacking across the continuum. There are various ways in which individuals may experience research career attrition, and each stage can have a cumulative effect. Researchers may choose not to return to research, pursue full-time clinical practice outside of academia, become assistant professors within academia but predominantly focus on patient care, obtain tenure but allocate little time to research, or substantially reduce the amount of research effort at varying points in their academic careers (Milewicz et al., 2015; NIH, 2014). Unfortunately, data on attrition rates specific to pediatric subspecialties are unknown. Across all disciplines, the numbers of physician–scientists have diminished, and the length of their productive scientific careers has decreased, with the average age of first independent funding at 46 years old (Daniels, 2015; Kerschner et al., 2018).
There are two major ways that pediatrician physician–scientists begin their career: directly following completion of an M.D. or D.O. program, and
___________________
6 The research literature primarily focuses on those with M.D. or M.D./Ph.D. and less on physician–scientists with D.O. degrees. However, there are increasing numbers of D.O. degrees among physician-scientists.
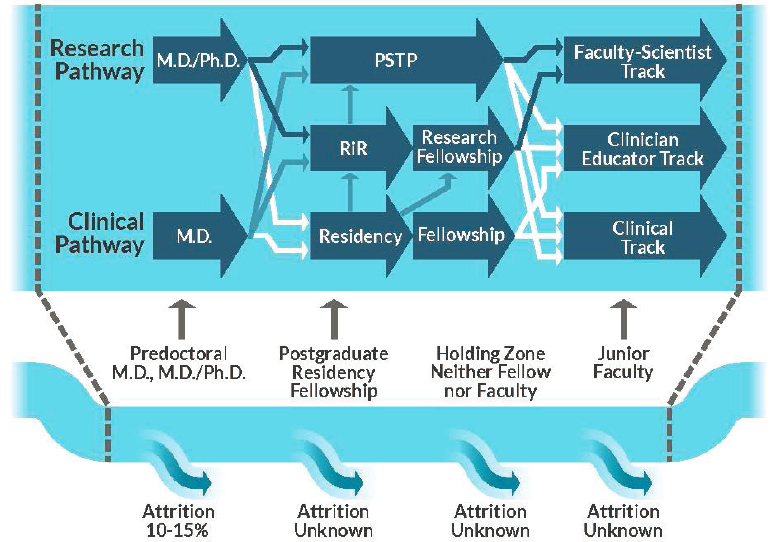
NOTES: Labeled block arrows represent training opportunities. Arrows indicate options for transitions between training programs, with solid navy arrows denoting the standard research-based physician–scientist training pathway, solid blue arrows indicating on-ramps, and solid white arrows showing points of physician–scientist loss, where trainees opt not to pursue research-based careers. PSTP: physician–scientist training program; RiR: research in residency.
SOURCES: Adapted from Williams et al., 2018; Milewicz et al., 2015. Used with permission of JCI Insight, permission conveyed through Copyright Clearance Center, Inc.
through M.D.-Ph.D. programs. Additionally, international medical graduates may be a resource for increasing the number of physician–scientists.
Following M.D./D.O. Programs
As mentioned in Chapters 4 and 5, the conventional pathway for a pediatric subspecialist involves completing a pediatric residency and then pursuing a subspecialty fellowship. During this fellowship, the trainee selects an academic focus that will serve as the foundation of their career development. Scholarly activity is a required component of most residency training programs as well as pediatric fellowship programs. In most pediatric fellowship programs, the initial year of training is largely clinical; trainees rarely have an opportunity to experience a research setting until their
second year of training. As a result, by the time they become fully integrated into a laboratory or clinical research program, establish a research focus, and begin to acquire the necessary tools to test hypotheses, they are often well into their second or third year of training. This can make it challenging to complete an independent research project—typically defined as a first author, peer-reviewed publication—by the end of a 3-year fellowship, let alone develop the skills and focus to be successful as a physician–scientist (Rubenstein and Kreindler, 2014).
Evidence shows that formal, structured research tracks have led to greater trainee research engagement (Ercan-Fang et al., 2017). As noted in Chapter 5, the American Board of Pediatrics (ABP) has developed several specialized physician–scientist training structures to speed up the time to become a pediatric physician–scientist, which reflect the frequent calls to reduce training time for physician–scientists (Blish, 2018; Milewicz et al., 2015). These integrated clinical and research pathways are also used by the American Board of Internal Medicine and the American Board of Family Medicine (Doubeni et al., 2017; Todd et al., 2013). There are also several institutional physician–scientist training initiatives (e.g., “umbrella” programs) that include seminars and research forums, along with providing research funding (Permar et al., 2020; Williams et al., 2022).
Following fellowship training, the most common pathway to an independent, academic career for pediatric subspecialty fellows is the transition from fellowship to junior faculty by way of a mentored physician–scientist award, usually in the NIH K series. The competition for these awards is high (see section on NIH funding later in this chapter), though the success rates between “M.D.-only” researchers and Ph.D.s or M.D.-Ph.D.s is comparable (Ley and Hamilton, 2008; NIH, 2014) (see section on M.D.-Ph.D.s).
International Medical Graduates
International medical graduates are individuals who received their primary medical degree from a medical school outside the United States and Canada. There have been proposals to better integrate international medical graduates into the research workforce to address the physician–scientist shortages (Muraro, 2002; Vidyasagar, 2007). However, while international medical graduates represent nearly one-fourth of the pediatric workforce, less than 1 percent designate research as their major professional activity (Duvivier et al., 2020).
M.D.-Ph.D. programs
M.D.-Ph.D. programs combine medical and graduate school within an integrated curriculum in order to train physicians for a career that combines clinical perspectives with research (Akabas et al., 2018). The
number of students entering M.D.‐Ph.D. programs has been slowly rising, and most graduates become academic physician–scientists (Garrison and Ley, 2022). A 2010 study by Brass and colleagues showed that attrition rates from M.D.-Ph.D. programs averaged 10 percent, and suggested the low rate might be because trainees typically receive tuition waivers for both medical school and graduate school plus a stipend. At that time, most of those who withdrew completed medical school or graduate school; approximately 80 percent of M.D.-Ph.D. program graduates worked in academia, industry, or research institutes (Brass et al., 2010). Over the past 50 years, the M.D.-Ph.D. training time in the United States has steadily increased, increasing from just over 6 years before 1975 to over 8 years in 2014, with no evidence that spending more time as an M.D.-Ph.D. student resulted in a greater research effort years later (Brass and Akabas, 2019).
M.D.-Ph.D. students represent approximately 3 percent of all medical students graduating each year (Akabas et al., 2018). In 2022, M.D.-Ph.D. programs matriculated 709 students and had an enrollment totaling 6,005 trainees in 155 medical schools across all disciplines (AAMC, 2022a). Roughly half of the programs are supported by NIH through a T327 mechanism from the National Institute of General Medical Sciences, which provides financial support and consistency in training activities (Williams et al., 2022). According to survey data from 2015, approximately 13 percent of M.D.-Ph.D. program graduates chose residency training in pediatrics (Akabas et al., 2018). Among those that chose to pursue a pediatrics residency, approximately 82 percent reported a subspecialty fellowship choice, with the largest percentages in hematology/oncology (21 percent), medical genetics (10 percent), endocrinology (7 percent), and infectious disease (7 percent) (Akabas et al., 2018). Table 6-1 includes the most recent data of M.D.-Ph.D. dual-degree program graduates from U.S. M.D.-granting medical schools in pediatric subspecialties.
Historically, M.D.-Ph.D. graduates have tended to cluster in certain fields, including pediatrics (Andriole et al., 2008; Paik et al., 2009). However, more recent data have indicated a trend away from the historical trends of internal medicine, pediatrics, neurology, and pathology (Akabas et al., 2018; Brass et al., 2010). This trend of decreasing M.D.-Ph.D. graduates in pediatrics may have implications for the overall pediatric research workforce.
___________________
7 An NIH T32 award is an Institutional Training Grant that is made to institutions to support groups of pre- and/or postdoctoral fellows, including trainees in basic, clinical, and behavioral research. Purpose: Ensures that a diverse and highly trained workforce is available to assume leadership roles in biomedical, behavioral, and clinical research. Issued to eligible institutions to support research training for groups of pre- and/or postdoctoral fellows. The number of positions or “slots” varies with each award (NICHD, 2023a).
TABLE 6-1 M.D.-Ph.D. Residents by Subspecialty, 2019–2021
| Subspecialty | 2019 | 2020 | 2021 |
|---|---|---|---|
| Adolescent Medicine | 1 | 2 | 1 |
| Child Abuse Pediatrics | 1 | 1 | 0 |
| Developmental-Behavioral Pediatrics | 4 | 4 | 2 |
| Neonatal-Perinatal Medicine | 15 | 18 | 15 |
| Pediatric Cardiology | 15 | 12 | 7 |
| Pediatric Critical Care Medicine | 8 | 6 | 9 |
| Pediatric Emergency Medicine | 4 | 4 | 1 |
| Pediatric Endocrinology | 5 | 5 | 6 |
| Pediatric Gastroenterology | 5 | 5 | 8 |
| Pediatric Hematology/Oncology | 36 | 27 | 25 |
| Pediatric Infectious Diseases | 21 | 18 | 17 |
| Pediatric Nephrology | 5 | 5 | 6 |
| Pediatric Pulmonology | 2 | 1 | 0 |
| Pediatric Rheumatology | 10 | 10 | 9 |
NOTE: *Hospital medicine excluded as there were no M.D.-Ph.D. graduates as active residents from 2019 through 2021.
SOURCE: AAMC, 2022b.
Data Limitations and Effect on Pediatric Physician–Scientist Workforce Projections
It is difficult to characterize the pediatric physician–scientist workforce overall (including numbers) due to data limitations and little to no coordination between the funders and other parties involved in the pediatric research workforce. For example, data from NIH on the number of physicians supported on T32 institutional training grants, receiving K01 Mentored Research Career Development Awards,8 and awarded R019Equivalent research grants are currently unavailable (Garrison and Ley,
___________________
8 “K awards provide support for senior postdoctoral fellows or faculty-level candidates. The objective of these programs is to bring candidates to the point where they are able to conduct their research independently and are competitive for major grant support.” A K01 award is the colloquial name for the Mentored Research Scientist Development Award, which “supports 3 to 5 years of mentored research training experience in the biomedical, behavioral, or clinical sciences. NICHD accepts K01 applications for only three research areas: Rehabilitation Research, Child Abuse and Neglect, and Population Research” (NICHD, 2023b).
9 The R01 is historically the oldest and most common grant mechanism used by NIH; R01s are for “mature research projects that are hypothesis-driven with strong preliminary data” and provide up to 5 years of support (NIH, 2023a).
2022; see section on NIH funding). The NIH Physician–Scientist Working Group last met in 2013 and there has not been an updated report since then, let alone one specific to the pediatric physician–scientist workforce. There is also a stark lack of communication and collaboration among funding agencies and other participants in the research enterprise to target important areas of pediatric research.
More information on the composition of career development awards and tracking of research careers by demographic factors such as sex, race and ethnicity, disability status, geography, type of science across the continuum (e.g., basic, clinical, implementation), topic/subspecialty, and professional background of the principal investigator (PI) is needed to truly quantify the pediatric physician–scientist workforce and to understand trends. There is also little to no tracking of outcomes from pediatric physician–scientist career development pathway programs. In addition to these quantitative data, qualitative data (e.g., on successful researchers as well as those researchers who leave the research track, including quality of relationship with mentors, satisfaction with career progression, reasons for attrition or retention) will be critical for retention of successful pediatric physician–scientists. During one of the committee’s public webinars, Ericka Boone, director for the Division of Biomedical Research Workforce, Office of Extramural Research, NIH, highlighted the need for data on the workforce and barriers to the pathway:
The only way we can have a clear understanding of what these barriers are is if we ask the individuals that are engaging in these research careers, especially those individuals who have exited out of the research career. What were those things? What were those barriers that just said, I can’t do this anymore?…Why are we losing postdocs? Why are we losing early career investigators? Why are they exiting out of these careers? And then doing something to keep them in.10
See Box 6-4 for clinician perspectives on the need for physician–scientists.
Trends in Research Work Profiles by Subspecialty
There are considerable disparities in the proportion of physicians who spend a significant amount of time in research activities across the pediatric subspecialties, and the data available are mostly self-reported (typically through ABP’s maintenance of certification process). As noted in Chapter 4, data from 2018–2022 reveal that among the ABP-certified pediatric
___________________
10 The webinar recording can be accessed at https://www.nationalacademies.org/event/11-02-2022/the-pediatric-subspecialty-workforce-and-its-impact-on-child-health-and-well-being-webinar-3.
subspecialists overall, less than 8 percent reported spending more than 50 percent of their time on research, and 48 percent reported not being involved in any research activities (ABP, 2023). Additionally, the amount of time dedicated to research varies across the subspecialties. ABP-certified subspecialties with the highest percentage of clinicians spending 50 percent or more of their time to research include hematology/oncology (21.6 percent), infectious disease (19.3 percent), rheumatology (14.2 percent), pulmonology (10.8 percent), and endocrinology (10.6 percent) (ABP, 2023). At the extremes, nearly 12 percent of hematology/oncology subspecialists spent 75 percent or more of their time on research while 69 percent of hospital medicine subspecialists reported spending no time on research. Macy et al. (2020) examined ABP’s maintenance of certification data from 2009–2016 and found that the number and proportion of pediatric subspecialists
engaged in research has not increased or decreased over the study time period, suggesting that previous efforts to bolster the pediatric physician–scientist workforce have not made a difference in increasing this segment of the workforce.
CHALLENGES TO THE PHYSICIAN–SCIENTIST WORKFORCE AND INTERVENTIONS TO SUPPORT PEDIATRIC RESEARCHERS
Pediatric physician–scientists continue to accelerate new basic science and medical insights, behavioral discoveries, and organizational effectiveness. However, the system for producing and nurturing physician–scientists has been inadequate, with limited funding, and heightened clinical and teaching demands (Salata et al., 2018; Williams et al., 2022). The shortage of resources available to support early career pediatric physician–scientists, combined with the multitude of influences on personal career decisions (see Chapter 5) and the demands of clinical practice, has resulted in a decreasing and aging workforce of pediatric researchers, with concerns for the viability of the workforce (Speer et al., 2023). Additionally, retention of physician–scientists in the mid-career space is also a major concern. While the committee has recommended increased flexibility in training curriculum including clinical-only tracks (see Chapter 5), it is critical to also continue to invest in pediatric researchers and to use strategies for improved recruitment and retention of pediatric physician–scientists. For example, flexible training curricula might allow for earlier exposure to research to help support the pediatric physician–scientist pathway.
Since 2017, Pediatric Research has published a series of commentaries that provide an opportunity for early career investigators to share experiences or inspirations that influenced their career path, thoughts on what contributed to their success or choices, and advice to others who are in early stages of their career (El-Khuffash, 2017; Guttmann, 2022; Harshman, 2021; Lovinsky-Desir, 2019; Menon, 2021; Salas, 2020; Sun, 2021). For many, early encounters with the medical or the health field, formative research experiences, patient encounters that informed research questions, and inspiring mentors helped fuel and define interest in medicine and/or research careers. Other key factors that influence early career physician–scientists include acquiring the needed academic and professional skills and training, resources, and protected time, and learning from failure (Christou et al., 2016; Flores et al., 2019; Ragsdale et al., 2014).
Threats to the sustainability and growth potential of the physician–scientist workforce and to pediatric research more broadly include: (1) lack of structured and sufficient mentorship and training, most significantly, but not exclusively, for fellows and early career investigators; (2) financial considerations (e.g., educational debt) that impact trainees’ decision to
pursue research; (3) protection of time for physician–scientists to engage in research; and (4) adequate funding (both extramural and institutional) for research (Alganabi and Pierro, 2021; Permar et al., 2020).
Specific efforts are needed to encourage and facilitate entry into research careers and foster the early phases of career development for pediatric physician–scientists. This is especially true for physician–scientists from populations underrepresented in the extramural scientific workforce (including the biomedical, clinical, behavioral, and social sciences workforces) (NIH, 2022b), which includes certain racial and ethnic groups, individuals with disabilities, individuals from economically disadvantaged backgrounds, and others (depending upon the discipline).
Academic physician–scientist retention is also distressingly low regardless of the mechanism of training (Williams et al., 2022), so efforts for retention of pediatric physician–scientists are also critical. The following sections provide an overview of several of the challenges faced by the pediatric research workforce, including mentorship financial considerations and protection of time, as well as interventions to increase representation in the research workforce. The adequacy and distribution of funding for research itself is discussed after this section.
Mentorship and Training
The importance of mentorship in career success and in advice to new researchers are highlighted in the Pediatric Research commentaries discussed above. The authors advise being intentional about seeking out skilled mentors to support the growth and development of the early career investigator, in addition to building a network of support that includes other investigators, peer mentors, and family and friends (El-Khuffash, 2017; Guttmann, 2022; Harshman, 2021; Lovinsky-Desir, 2019; Menon, 2021; Salas, 2020; Sun, 2021). Gaps in mentorship for physician–scientists exist across the entire professional timeline.
Medical School
In medical school, research often is introduced later in training, often too late for an individual to develop strong mentorship and pursue an area of scholarly interest in depth prior to the start of residency training. In 2020, medical school curriculum pathways from 145 schools were reviewed, and numerous examples of research opportunities for medical students were highlighted (McOwen et al., 2020). Some of the opportunities were explicitly research while others were a component of an optional or required scholarly concentration program rather than research in the traditional sense (McOwen et al., 2020; Thompson et al., 2020). Overall,
there has been a decline in the aspirations of graduating medical students to pursue research, which may be a result of curricular reforms that place increased emphasis on clinical decision making, and a decreased emphasis on foundations in basic science (Buja, 2019; Garrison and Ley, 2022). However, medical schools have also developed initiatives to bolster the number of physician–scientists. More than 30 medical schools have Physician–Scientist Training Programs (PSTPs) that integrate residency, fellowship, and postdoctoral training for trainees that commit to a physician–scientist career path (Garrison and Ley, 2022; Muslin et al., 2009), though only a small proportion of medical school graduates enter PSTPs (NRMP, 2023).
Residency and Fellowship Training
Not all residency training programs or specialty fellowships provide focused mentorship opportunities for research, which can limit the exposure of trainees to adequate career mentors. “In certain large non-university medical centers with expansive clinical outlays—which focus predominantly on the clinical mission as a driver (and determinant) of research activities—it is relatively rare to have physician–scientists on faculty in clinical departments, further limiting the role models for this career” (Williams et al., 2022). Residents are immersed in intense clinical training with relatively little time for or training in research, with a curriculum that emphasizes information relevant to clinical care and is largely defined by national medical board standards. Many residency programs lack a dedicated research track for physician–scientists, and there are insufficient guidelines, or dissemination of best practices, from the pediatric research community on research training and mentorship. The PSTP programs provide research training opportunities for both M.D. and M.D.-Ph.D. trainees both during or after the completion of clinical training (Williams et al., 2022). Some fellowship programs provide more comprehensive research experiences. While clinical competency must be ensured, greater exposure to research at this stage increases the chance of developing into a successful physician–scientist (Rubenstein and Kreindler, 2014). One possible solution is to encourage trainees to participate in extra years of fellowship; however, there are significant financial disincentives to remaining in training (at fellowship income levels) by comparison to opportunities for faculty or clinical positions (Rubenstein and Kreindler, 2014).
In 2022, the National Pediatric Scientist Collaborative Workgroup, a collaborative of leaders in pediatric research and medical education, surveyed pediatric residency program directors about barriers to developing physician–scientists. Three priority areas were identified: (1) institutional infrastructure, human resources, and financial resources to develop
physician–scientist training; (2) “dual professional identity formation” of the pediatric physician–scientist; and (3) input and pathway of candidates into this career path (Burns et al., 2022). This workgroup has begun to develop efforts to address these areas by creating guidelines for best practices in physician–scientist training. Other organizations, such as the Burroughs Wellcome Trust,11 also have invested resources into bolstering the M.D.-only researcher pathway through early career awards and career development workshops. There are other programs as well, such as the consortium funded National Clinician Scholars Program,12 which supports physicians and nurses through a two-year site-based research training fellowship (NCSP, 2023) and the Doris Duke Physician Scientists Fellowship program, which provides grants to physician–scientists at the subspecialty fellowship level who are seeking to conduct additional years of research beyond their subspecialty requirement (Doris Duke Foundation, 2023).
The relatively new NIH pilot program, “Stimulating Access to Research in Residency (StARR)” (R38) was initiated in 2017 to create new research opportunities for residents during their clinical training in efforts to recruit resident investigators and increase the body of clinician investigators. Hurst et al. (2019) describes a single institution’s pediatric physician–scientist development program, supported in part by an NIH R38 StARR award, that affords research-integrated training across the spectrum of research, for trainees interested in academic general pediatrics or a pediatrics subspecialty and includes support regarding mentor and mentorship teams, scholarly oversight committees, research productivity, educational enrichment, and professional development. The National R38 Consortium consists of principal investigators (PIs) and multiple principal investigators from the first round of R38 awards that were granted in 2018. In a report of early outcomes, PIs endorsed institutional commitment of new resources to support the program and viewed the program positively regarding enhancing research opportunities and recruitment, although there is a need to increase the pool and appointees from populations underrepresented in the extramural scientific workforce (Price Rapoza et al., 2022). After R38 appointment, resident investigator respondents reported a number of positive outcomes, including likelihood of pursuing a physician–scientist career, clarity of research direction, and expanded mentorship.
___________________
11 For more information about the Burroughs Wellcome Trust, see https://www.bwfund.org/ (accessed June 27, 2023).
12 This consortium was grown out of the previous Robert Wood Johnson Foundation Clinical Scholars program.
Early Career
Mentored time immediately after completion of fellowship during the first years of faculty appointment is critical. Mentored research training programs with a focus on fellows and/or early career faculty are offered in multiple settings, including individual institutions, national NIH or foundational programming, and pediatric specialty or subspecialty societies (Badawy et al., 2017; Chen et al., 2016; Cranmer et al., 2018; Kashiwagi et al., 2013; Vasylyeva et al., 2019). Of note, NIH has an embedded component of mentorship through the K-series awards with a requirement that applicants designate mentors and specify a formal mentoring plan (DeCastro et al., 2013; NIAID, 2021). Chen et al. (2016) described the multifaceted “Pediatric Mentoring Program” implemented for instructors and assistant professors in an academic pediatrics department with the goal of promoting retention and job satisfaction. The program consisted of mandatory annual/biannual mentor meetings as well as grant review assistance and peer-group mentoring and annual evaluations. The majority of participants described benefits related to understanding of criteria for advancement or progress toward career goals, but only a minority reported developing collaborations with peers or improved work–life balance. King et al. (2021) described self-reported benefits of participation in a one-year mentored clinical research training program for hematology/oncology fellows and early career faculty (pediatric and adult). Most participants endorsed the program as instrumental to retention in hematological research and facilitation of career development in research; those who endorsed a positive program impact performed better on conventional research metrics such as first author publications and percentage of effort in research when compared with the minority of participants who did not endorse positive program impact.
Mid-Career
While most formal mentoring programs are targeted towards early-career researchers and junior faculty, mentorship is also important in the mid-career space, particularly for female researchers and other populations underrepresented in the extramural scientific workforce (Bora, 2023; Lewiss et al., 2020; Sotirin and Goltz, 2023; Teshima et al., 2019). In academic medicine overall, high-quality mentorship is essential to faculty productivity, job satisfaction, and retention, as well as academic advancement (AAMC, 2023b; Bland et al., 2010; Mylona et al., 2016; Walensky et al., 2018). However, few universities have instituted formal mid-career mentoring programs, let alone mid-career programs specific to the pediatric scientific workforce, and mid-career physician–scientists have reported a lack of high-quality mentoring (Bora, 2023; Pololi et al., 2023). Specific
to pediatrics, a discussion-based workshop at the American Society of Pediatric Hematology/Oncology annual meeting found that mid-career participants frequently lacked mentors and thought that “mentors did not appreciate the complexity of the mid-career role” (Frugé et al., 2010).
Financial Considerations
Choosing a career as a physician–scientist is likely influenced by the loss of opportunities for higher salaries both because of extended periods of training (leading to deferred entry into faculty positions) and comparatively higher salaries for private practice (Donath et al., 2009; Pickering et al., 2014; Rosenberg, 1999; Somekh et al., 2019). For example, Zemlo et al. (2000) found that a rising level of student debt in the 1990s was correlated with a declining proportion of physicians choosing research careers. Physician–scientists can incur major debts because of the extra training time needed to gain expertise for both research and clinical care. As mentioned earlier, M.D.-Ph.D. programs may be more attractive for trainees due to the free tuition and avoidance of medical school debt. This incentive is not typically given for M.D.-only investigators, though several medical schools have begun to cover some or all of the cost of tuition through dedicated endowments. Additionally, salaries for pediatric physician–scientists can also be negatively affected by less clinical incentive income and low pay lines in awards. For example, career development awards (e.g., K-series awards) cover some salary support, but this often needs to be supplemented with cost sharing by the physician–scientists’ institutions (Daniels, 2015; Garrison and Deschamps, 2014; NIH, 2017a). Given the relatively lower clinical margin in pediatric departments, this issue may be particularly difficult for pediatric physician–scientists. (See Chapter 8 for more information.) During one of the committee’s public webinars, Mary Leonard, Arline and Pete Harman Professor and Chair of the Department of Pediatrics at Stanford University, director of the Stanford Maternal and Child Health Research Institute, and physician-in-chief of Lucile Packard Children’s Hospital, highlighted how financial considerations play a role in pursuing a pediatric research career: “Going the physician–scientist route defers compensation even further. You have longer trained intervals. In some places, you have lower salaries.”13 See Box 6-5 for clinician perspectives on the financial challenges for pediatric physician–scientists.
___________________
13 The webinar recording can be accessed at https://www.nationalacademies.org/event/07-19-2022/the-pediatric-subspecialty-workforce-and-its-impact-on-child-health-and-well-being-webinar-1.
NIH Loan Repayment Programs (LRPs) and Impact on Research Careers
Loan repayment for pediatric researchers is one approach to overcome financial barriers to entering or remaining in pediatric subspecialty research careers. During the committee’s public webinars, Stephanie Lovinsky-Desir, assistant professor and chief of the Pediatric Pulmonary Division at Columbia University Irving Medical Center, Morgan Stanley Children’s Hospital of New York Presbyterian discussed the role of loan repayment to help alleviate financial disincentives to pursuing a pediatric research career:
I applied for the NIH loan repayment program…it was really instrumental toward me staying in a field of pediatric research…a loan repayment that could help offset some of the burden that I have actually was helpful in making the decision to be able to remain in academia and remain in a research-intensive tract…loan repayment is super important for someone like me who didn’t benefit from generational wealth, and I have a ton of
debt, and so I’m not quite finished, I’m 10 years out of fellowship training, paying down all of those loans, even with the loan repayment.14
Since 1988, the NIH LRPs have supported early-stage investigator awardees in their pursuit of biomedical and behavioral research, including repaying up to $50,000 in educational loans per year in exchange for a commitment to research for at least 20 hours per week for at least 2 years (with possibility for renewal) (Lauer, 2019; NIH, 2022c). From FY2013 to FY2022, there were 3,105 LRP awards in pediatric research, with a 52 percent success rate overall (40 percent for new awards and 71 percent for renewal awards) and total funding of $177,264,184 (with over $105 million of that going to new awards) (NIH, 2023d). In FY2022, the mean award for new pediatrics applications was $88,669 while the mean for renewals was $47,720; the mean age of new awardees was 36 years (NIH, 2023d).
Lauer (2019) compared research-related outcomes between applicants who received and did not receive an LRP award (during fiscal years 2003–2009) with follow-up of research productivity through 2017 (see Figure 6-2). The author reported that receipt of an LRP award was associated with higher levels of “persistence in research” (composite measure including
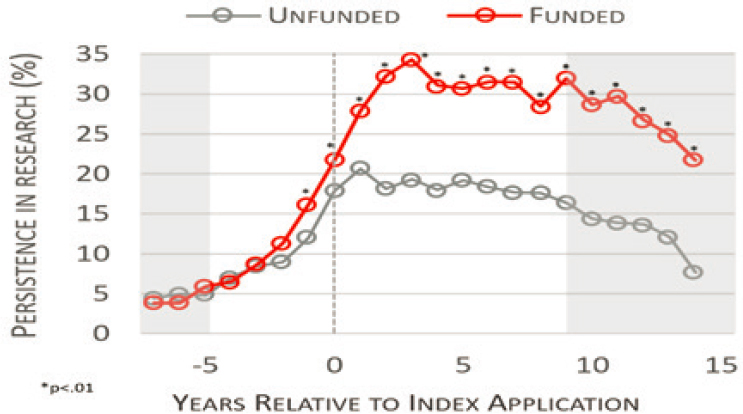
SOURCE: Adapted from Lauer, M. 2019. Reproduced with permission from the NIH Open Mike Blog.
___________________
14 The webinar recording can be accessed at https://www.nationalacademies.org/event/07-19-2022/the-pediatric-subspecialty-workforce-and-its-impact-on-child-health-and-well-being-webinar-1.
submission or receipt of grant or fellowship awards and publications) over a decade after initial application. Although confounding factors may contribute to findings, Lauer noted the NIH LRP Program is an important potential strategy for retention of early career investigators in the research workforce.
Across all NIH LRPs (including non-pediatric awards) in 2022, more than half of awardees had a total education-related debt of over $200,000 (NIH, 2023d). The NIH LRP is an attractive option to encourage the pursuit of a research career.
Protection of Research Time
The time required for pediatric subspecialists to be successful as physician–scientists can be substantial. Historically, a physician–scientist profile has required that 50 percent of one’s professional time be focused on research, but with increasing clinical demands and the need to fund this protected research time, a growing number of physicians are conducting research with less time allocated to the effort. NIH requires K-awardees to devote at least 75 percent FTE to research. However, only 3.4 percent of subspecialists report spending at least 75 percent of their time in research (ABP, 2023). Most individuals with R-level grants are devoting at least 50 percent of their time and often much more to research in order to be successful and competitive, yet only 7.6 percent of subspecialists report working at least 50 percent of their time on research (ABP, 2023).
Faculty physician–scientists balance many priorities, including teaching, clinical care, administration, and research. As the competition for securing extramural funding and meeting clinical productivity requirements continues to intensify, it has become increasingly difficult to maintain a career that adequately balances research and clinical care (COPR, 2014). Protection of research time allows physician–scientists to be freed from clinical duties to pursue research. Career development and training awards offer a safeguarded period for emerging scientists to establish a research program with protected research time in order to ultimately reach a point where they can independently conduct research with their own funding support (Garrison and Ley, 2022). Training awards, either institutional (e.g., T32/K12 grants) or individual (e.g., K series grants), require protection of at least 75 percent of their time.
T32 institutional training program grants are made to institutions to support groups of pre- and/or postdoctoral fellows and K12 institutional career development institutional grants (e.g., for PDSPs) aim to prepare newly trained physicians who have made a commitment to independent research careers by providing support to institutions that mentor clinical fellows and scientists (Garrison and Levy, 2022; NICHD, 2023a). Success of
a T32 program can be difficult to measure. A NICHD task force that conducted an in-depth review of its extramural training programs and mechanisms found that individuals supported by T32 programs had less-favorable outcomes when compared with individuals supported at the career level (either through individual K or institutional K12 grants) (NICHD, 2015; Steinbach et al., 2018). On the other hand, Abramson et al. (2021) found that having a T32 training grant doubled the probability that pediatric subspecialty fellows published during their fellowship. Historically, NICHD has “emphasized the institutional training and career development awards to a greater degree than many other NIH institutes and centers” and strongly invested in K12 development programs (Twombly et al., 2018).
However, the NICHD task force that reviewed its extramural training programs recommended placing more emphasis on individual awards compared with institutional training awards (NICHD, 2015; Steinbach et al., 2018), and NICHD announced their intention to allocate a greater proportion of its career development fund allocation to individual awards (Twombly et al., 2018). There are a variety of individual K programs targeting specific career stages and research areas. The largest programs are:
- K01 Mentored Research Scientist Career Development Award,
- K08 Mentored Clinical Scientist Research Career Development Award,
- K23 Mentored Patient‐Oriented Research Career Development Award, and
- K99 Pathway to Independence Award.
While M.D.s can apply for any of these awards, they are most often supported by the K08 and K23 mechanisms, which comprise approximately half of all K awards (Garrison and Ley, 2022). Estimates suggest that at least 3,000 physicians were supported on career development awards each year from 2011 through 2020, though there has not been an increase in career development awards targeted to physicians (Garrison and Ley, 2022). The number of pediatricians, let alone pediatric subspecialists, is unknown. (See the section on NIH in the funding section in this chapter for more information.)
Previous reports that have looked at bolstering the physician–scientist workforce have proposed increases in the number of career development awards so that early career physician–scientists have the necessary supports to initiate successful research programs (Jain et al., 2019; NIH, 2014; Salata et al., 2018). Multiple studies across different specialties have shown that NIH K awardee are more likely to receive subsequent, independent NIH awards than medical school graduates without them (Jeffe et al., 2018; King et al., 2013; Nikaj and Lund, 2019; Okeigwe et al., 2017). Funding
rates for K-series awards vary widely by NIH institute and federal agencies (e.g., the Agency for Healthcare Research and Quality [AHRQ]) and by year, with success rates typically ranging from 20 to 40 percent in a given cycle and many investigators requiring multiple submissions before receiving funding (AHRQ, 2016; Conte and Omary, 2018; NIH, 2023c). Those engaged in research without such funding sources may struggle to allocate sufficient time for investigation, leading to increased burden of professional responsibilities and risk for burnout. Most pediatric departments do not have the resources to support a sizeable amount of research time for more than a limited number of years. As careers progress, the cost of providing the “over the NIH cap” component of the salary can lead to increased pressure to perform clinical work instead of research, unless discretionary funds such as endowed professorships are provided to cover these costs, but these clinical requirements erode protected time.
Increasing the Diversity of Pediatric Researchers
As discussed in Chapter 5, to mirror the demographic make-up of the children and families it serves, the pediatric workforce will require special attention for the recruitment and retention of a diverse set of trainees; this is especially true for the pediatric research workforce. There is evidence that “scientific teams that are composed of diverse individuals with diverse perspectives, backgrounds, and mental models are better positioned to solve complex problems among children and their families” (Guevara et al., 2023), and are more likely to engage in research with diverse groups and communities and generate higher quality research (Ayedun et al., 2023; Campbell et al., 2013; Page, 2007). However, the number of pediatric physician–scientists from populations underrepresented in the extramural scientific workforce is small and growing at a slow rate (AAMC, 2019; Guevara et al., 2023; Lett et al., 2018), though exact statistics are not available. A principal recommendation of the NIH Physician–Scientist Workforce Working Group Report was to improve diversity among all researchers (NIH, 2014). However, structural, systemic, and cultural barriers exist for trainees and faculties from populations underrepresented in the extramural scientific workforce that limit entry or reduce retention in this career path (Behera et al., 2019), including limited opportunities for mentorship and mentorship training, bias and discrimination, and systemic factors at the institutional level (Kalet et al., 2022; Siebert et al., 2020). Minority academic pediatricians have identified a range of obstacles that impede the successful recruitment and retention of minority physicians, including insufficient financial resources, ineffective recruitment strategies, limited opportunities for career advancement, fewer research resources, and inadequate research support (Johnson et al., 2017b; Saboor et al., 2022). Other key
issues that threaten the retention of physician–scientists from populations underrepresented in the extramural scientific workforce include disparities in personal wealth, excessive service demands to provide diverse perspectives in committees and conferences, feelings of isolation arising from intersectionality, and apprehensions regarding reinforcing stereotypes (Kalet et al., 2022).
Strategies that have been suggested to promote representation in the research workforce include institutional antiracism policies; support for trainees and faculty from populations underrepresented in the extramural scientific workforce; encouraging diversity in public engagements and institutional leadership; providing child/elder care subsidies; tracking diversity outcome measures; and developing “diversity aware” training curricula (Williams et al., 2022). Interventions include faculty development programs like the Research in Pediatrics Initiative on Diversity, which is a national program of the Academic Pediatrics Association that provides mentoring, research training, and career development for URiM junior pediatrics faculty, and has resulted in increased grant productivity and promotion of participants (Flores et al., 2021). Pediatric departments and children’s hospitals, especially with national discussions of racism in society and medicine after the May 2020 murder of George Floyd, have recognized the need to address equity, diversity, and inclusion in all their activities, including the composition of faculty investigators (Pursley et al., 2020; Walker-Harding et al., 2020; Wright et al., 2020). Institutions are addressing diversity in a number of ways, all of which have the potential to be accelerated:
- Recruiting a much more diverse residents’ workforce, realizing that their own residency program is an important source of potential fellows.
- Recruiting more diverse fellows, recognizing that graduating fellows are an important source of future faculty.
- Creating fellowship opportunities specifically for populations underrepresented in the extramural scientific workforce.
- Creating internal K-12–like individual career development programs specifically for populations underrepresented in the extramural scientific workforce.
- Providing start-up packages for faculty that increase the chance of success in their research.
- Ensuring that appropriate and intensive mentoring is available for populations underrepresented in the extramural scientific workforce at each stage in their careers.
Given the importance of mentoring in the professional growth, development, and success of trainees and early career investigators—and the potential impact on racial disparities in R01 success (Ginther et al., 2011)—it is
important to support mentors in their efforts to gain the knowledge and skills needed to be effective mentors for trainees from diverse backgrounds, particularly for trainees from backgrounds underrepresented in the biomedical research workforce, as well as recognizing mentoring efforts and excellence (NASEM, 2019). Efforts such as the National Research Mentoring Network created/fostered opportunities for mentors and mentees to improve relationships through competency-based trainings offered in multiple types of settings and platforms, including Culturally Aware Mentoring (Byars-Winston et al., 2018; Sorkness et al., 2017).
Across all disciplines, there has been evidence that Black scientists are less likely to receive NIH funding when compared with White scientists (Ginther at al., 2011; Ginther, 2022). As discussed previously, career development awards are an important lever to support early career researchers. An analysis of the race-ethnicity of NIH K awardees from 2010 to 2022 found that while the numbers of Black and Hispanic applicants and awardees have steadily increased over time, the total number of Black and Hispanic applicants remains “quite low” (Lauer and Bernard, 2023). Notably, K award funding rates for Black applicants have increased over the past 3 years. NIH has taken steps to address racism in the scientific workforce and improve diversity, equity, and inclusion efforts (NIH, 2021). For example, the NIH UNITE Initiative was established to address structural racism and establish equity within the biomedical research enterprise (NIH, 2022d). The Extramural Research Ecosystem: Changing Policy, Culture and Structure to Promote Workforce Diversity Committee is charged with performing systematic reviews of NIH extramural policies and processes to identify areas for policy change to address the lack of diversity and inclusivity within the extramural research ecosystem. Priorities include supporting career pathways, research resources, and capacity at minority-serving institutions,15 promoting equity at extramural institutions in regard to environment and culture, and encouraging equity in policies and procedures at NIH.
Mid-Career Concerns and Retention
This chapter largely focuses on the early phases of the pediatric physician–scientist pathway, emphasizing that early career mentorship, protected research time, and funding are critical supports for entry into a research career, and these can lay a foundation to promote longevity over time. However, mid-career retention of physician–scientists and difficulties during the transition from career development (K series) awards to
___________________
15 MSIs are institutions of higher education that serve minority populations. See https://www.doi.gov/pmb/eeo/doi-minority-serving-institutions-program (accessed July 18, 2023).
independent research grants (R series) (the ‘K to R transition’) remain a major area of challenge (Daye et al., 2015; Good et al., 2018a; Yin et al., 2015), although exact data on attrition rates by pediatric subspecialists during this transition are lacking. Retention of pediatric physician–scientists is threatened by burnout, inadequate mentoring, an increasingly competitive funding environment, financial pressures related to loan repayment and salary, inadequate institutional support and protected time for scholarship, and difficulties balancing clinical and research demands, among other factors (Alvira et al., 2018; Bauserman et al., 2022; Christou et al., 2016; Rosenthal et al., 2020; Salata et al., 2018; Shafer, 2010). (See Chapter 5 for more information on clinician burnout.)
The K to R transition has been described as “tortuous and prolonged,” due to low success rates for R series awards and funding gaps (Yin et al., 2015). Women tend to be disproportionately affected at this transition point (Jagsi et al., 2009, 2011; Ghosh-Choudhary et al., 2022; NIH, 2014). Nguyen et al. (2023) found that women received 43 percent of all K awards from 1997–2011 and 34 percent of awards from 2012–2021. Funding rates were lower than faculty representation rates in 5 of the 13 departments assessed, including pediatrics. Regarding K to R transition, 37.7 percent of women who received mentored K awards between 1997 and 2011 successfully applied for R01-equivalent grants within 10 years, compared to 41.5 percent of men (Nguyen et al., 2023).
NIH has created a K99/R00 funding mechanism that incorporates the transition into the funding period (NCI, 2023c), but much of the burden of supporting this transition period falls on institutions, as the investigators require protection of time and financial resources to maintain a research team and program. Many institutions hold K to R transition workshops with programs such as mock grant review/study sections, structured mentoring for grant and manuscript preparation, and information about securing ancillary funding16 (Jones et al., 2011; Yin et al., 2015). While such guidance is helpful, physician–scientists often require protection of time from clinical activities and bridge funding to ensure that their research can continue if there is a lapse in funding. This is a substantial financial investment to which pediatric departments often cannot commit due to financial pressures, so pediatric physician–scientists may be particularly vulnerable during the K to R transition period. Given the current data limitations, it is important to collect more data on the rates of attrition across the pediatric
___________________
16 For examples, see https://ictr.johnshopkins.edu/education-training/seminars-courses/k-tor/ (accessed July 18, 2023), https://learn.partners.org/courseversion/922/ (accessed July 18, 2023), https://catalyst.harvard.edu/courses/grasp (accessed July 18, 2023), and https://tracs.unc.edu/index.php/services/education/r-writing-group (accessed July 18, 2023).
subspecialties, as well as evidence-based interventions for a successful K to R transition, in order to develop data-driven policies to address these issues.
THE PEDIATRIC RESEARCH FUNDING LANDSCAPE: ADEQUACY AND DISTRIBUTION
Funding is the greatest challenge to the physician–scientist workforce. The federal government—particularly NIH—is a significant funder of child health research in the United States. Other major sources of funding for pediatric research include other federal agencies, private foundations, state and local governments, pharmaceutical companies, device manufacturers, and biotechnology firms. Academic medical centers also bear many of the costs of training physician–scientists and supporting their research efforts. There is an increasing move to align efforts across various funding organizations to maximize resources and avoid redundancy, although currently there is little coordination or collaboration among funders. Additionally, as discussed in more detail below, physician–scientists rely on institutional support to build and maintain the basic infrastructure (e.g., personnel, space, equipment, and salary) for a sustainable research program.
NIH
NIH remains the largest government funding source for global biomedical research (Rees et al., 2021). Historically, pediatric research funding has been low compared with funding for adult diseases, though it has been increasing along with the requirements for NIH to report pediatric research spending annually (Gitterman et al., 2004, 2018a, 2023; Speer et al., 2023). Nearly all of NIH’s 27 institutes and centers fund child health research, with NICHD supporting the largest proportion of the pediatric portfolio (approximately 18 percent in 2021–202217) (Gitterman et al., 2018b; NICHD, 2022d; NIH, 2017b). Since 2013, annual NIH support for pediatric research has increased, with $5.7 billion allocated in 2022 (see Figure 6-3). NIH funding for child health has generally kept pace with overall NIH funding increases in absolute dollar amounts18 (Boat and Whitsett, 2021). While the total dollars spent on pediatric research has increased, the percentage of total NIH funding specific to pediatric research has remained steady at approximately 11 to 12 percent of the total NIH
___________________
17 Estimate provided to the committee by Rohan Hazra, NICHD, on May 11, 2023.
18 The overall NIH budget increased from $29 billion for FY2013 to $49 billion for FY2023. NIH funding adjusted for inflation (in projected constant FY2022 dollars) using the Biomedical Research and Development Price Index showed a smaller overall increase, from $36 billion in FY2015 to $47 billion in FY2023 (Sekar, 2023).
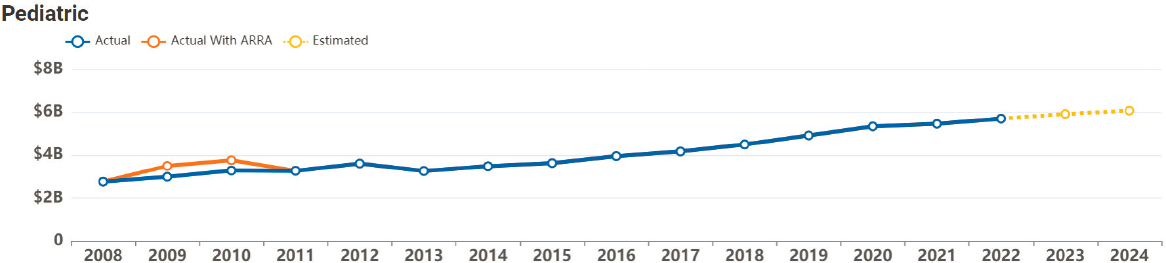
NOTES: 2023–2024 are estimates. ARRA = American Recovery and Reinvestment Act (American Recovery and Reinvestment Act of 2009, Public Law No. 111-5, 111th Congr., H.R. 1 (February 17, 2009)).
SOURCE: NIH, 2023b.
budget for the past decade (not adjusted for inflation).19 The number of applications for NIH awards by medical school pediatric departments has been steady from 2012 to 2019 (approximately 1,400 per year, with 16 to 21 percent of applications funded) (NIH, 2023c). There was a drop in applications in 2020 and 2021, with a rebound in the number of applications in 2022 to approximately 1,300 applications and a 20 percent success rate (NIH, 2023c).
NIH Research Funding by Subspecialty
NICHD funding for principal investigators with pediatric subspecialty fellowship training has risen steadily over time, with the largest increases coming in the past 4 years (see Figure 6-4).
Objective data on funding by pediatric subspecialty are not readily available, but self-reported survey data suggest that NIH research funding is not equally distributed across the different subspecialties in terms of total funding, proportion of R01-equivalent investigators, and other indicators (Good et al., 2018b). Good et al. (2018b) found that among the 907 R01-Equivalent Pediatric Physician–Scientist Awardees from 2012 to 2017, the highest percentages were in hematology/oncology, academic general pediatrics, and infectious disease (see Table 6-2). These differences across subspecialties warrant further exploration as they likely reflect a variety of factors, including the foundational tradition that these subspecialties have of doing research, uneven funding allocations for research in certain subspecialties, and the weakened research workforce pathway in certain subspecialties.
The critical role of the home institution in supporting research limits the scope of research activities in smaller, less academic institutions. Eligibility for some NIH awards requires institutions of higher education, often compelling freestanding children’s hospitals to apply through other institutions which, in turn, creates more administrative inefficiencies, costs, and hurdles. Furthermore, the lower reimbursement rates for pediatric clinical care (see Chapter 8) leaves limited resources for institutions to support research. These funding challenges have resulted in major disparities in the distribution of research activities across institutions, with the largest children’s hospitals with the strongest institutional infrastructure for research accounting for the majority of NIH-funded activities (see Table 6-3) (Good et al., 2018b). This disparity undermines the physician–scientist pathway in smaller institutions, especially those not associated with large academic centers, even though children in these locales would benefit equally (and
___________________
19 Estimate calculated using pediatric funding data from NIH (2023b) and overall NIH budget data from Sekar (2023); estimates not adjusted for inflation.
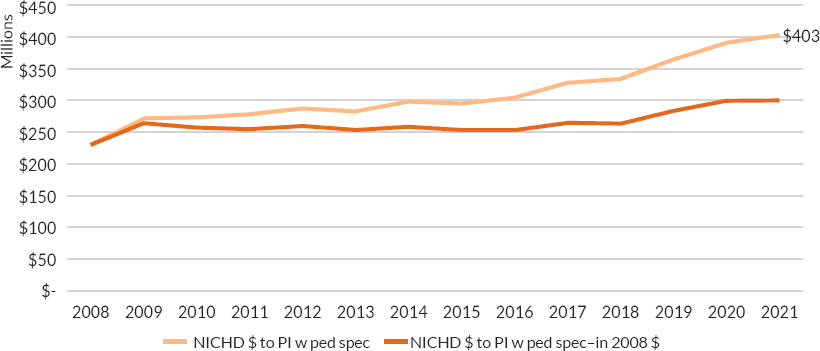
NOTES: Figure includes dollars from the American Recovery and Reinvestment Act. (American Recovery and Reinvestment Act of 2009, Public Law No. 111-5, 111th Congr., H.R. 1 (February 17, 2009)).
Funding levels were adjusted for inflation in research costs using the Biomedical Research and Development Price Index (BRDPI). For more information on BRDPI, see https://officeofbudget.od.nih.gov/gbiPriceIndexes.html.
SOURCE: Provided to the committee by Rohan Hazra, NICHD, on September 9, 2022. NICHD’s Child Health Information Retrieval Program (CHIRP): These are not official NIH-wide data.
TABLE 6-2 Pediatric Division Representation Among the 907 R01Equivalent Physician–Scientist Awardees, 2012–2017
| Pediatric Subspecialty | R01-Equivalent Awardees, No. (%) |
|---|---|
| Hematology/oncology | 146 (16.1) |
| Academic general | 109 (12.02) |
| Infectious disease | 93 (10.25) |
| Neonatology | 82 (9.04) |
| Endocrinology | 49 (5.4) |
| Neurology | 46 (5.07) |
| Pulmonology | 45 (4.85) |
| Gastroenterology | 44 (4.85) |
| Genetics | 39 (4.3) |
| Cardiology | 36 (3.97) |
| Nephrology | 32 (3.53) |
| Critical care | 31 (3.42) |
| Allergy and immunology | 31 (3.42) |
| Rheumatology | 15 (1.65) |
| Adolescent | 14 (1.54) |
| Behavioral and development | 10 (1.1) |
| Emergency medicine | 10 (1.1) |
| Non-pediatric primary training | 75 (8.27) |
NOTE: Physician–scientists who had not completed a residency in pediatrics were considered “non-pediatric primary training.”
SOURCE: Good et al., 2018b. Reproduced with permission from JAMA Pediatrics. Copyright ©2018 American Medical Association. All rights reserved.
perhaps more) from enrollment in investigational efforts, such as natural history studies, interventions, and clinical trials.
NIH funding to pediatric institutions and programs continues to be increasingly concentrated in a few sites. Boat and Whitsett (2021) analyzed the 2020 NIH Reporter data and found that 30 percent of NIH’s $1.96 billion funding to pediatric institutions and programs went to 3 children’s hospitals and 57 percent to the top 10 NIH grant recipients. Between 2013 and 2020, NIH funding of research to the top 10 grant recipients increased 93 percent while funding to those in the third and fourth deciles increased by about 10 percent (Boat and Whitsett, 2021). About one-third of pediatric departments have no NIH funding and more than half (57 percent) have five or fewer NIH grants. Factors differentiating the more and less well-funded programs include local institutional investments in research training, research leadership, and research faculty (Boat and Whitsett, 2021).
TABLE 6-3 Institutional Distribution of Pediatric R01-Equivalent Awards, 2012–2017
| Institutions with R0-1 Equivalent Awards | Number of Awards |
|---|---|
| Boston Children’s Hospital | 326 |
| Cincinnati Children’s Hospital | 289 |
| Children’s Hospital of Philadelphia | 184 |
| Seattle Children’s Hospital | 103 |
| Baylor College of Medicine | 78 |
| Nationwide Children’s Hospital | 73 |
| Emory University | 68 |
| Indiana University-Purdue University at Indianapolis | 65 |
| University of California, San Diego | 64 |
| University of Colorado, Denver | 61 |
| Stanford University | 58 |
| University of Pittsburgh | 57 |
| Vanderbilt University | 46 |
| University of Minnesota | 45 |
| Johns Hopkins University | 44 |
NOTE: The top 15 institutions that accounted for 1,561 (63 percent) of the 2,471 individual Pediatric R01 awards from January 2012 to May 17, 2017.
SOURCE: Good et al., 2018b. Reproduced with permission from JAMA Pediatrics. Copyright ©2018 American Medical Association. All rights reserved.
Trans-NIH Pediatric Research Consortium
In recognition of the need for a collaborative effort in cataloging and then supporting pediatric research across NIH, in 2018 the NIH Pediatric Research Consortium (N-PeRC) was developed. This trans-NIH initiative, led by NICHD, includes a representative from each of NIH’s 27 institutes and centers (NICHD, 2022d). The goal of N-PeRC is to “harmonize [pediatric research] activities across institutes, explore gaps in the overall pediatric research portfolio, and share best practices to advance science. The consortium meets several times a year to discuss scientific opportunities and potential new areas of collaboration, including efforts to enhance research training for the next generation of pediatricians [and pediatric surgeons]” (NICHD, 2022d). Other current priorities of the group include the COVID-19 pandemic and its impact on children and youth, pediatric medical devices, and aligning pediatric clinical trial and other research networks. As of the writing of this report, N-PeRC is still in the early stages of
development and has not released any publicly available reports or quantified the total amount of trans-NIH pediatric funding.
Other Federal Funders
Although NIH is the largest federal funder of child research, other federal funders also fund pediatric research, though the breadth and exact numbers are difficult to determine. For example, since 2015, AHRQ has given out $817 million in grants, with approximately $82.8 million to projects that include pediatrics20 (AHRQ, 2020). AHRQ has funded several projects related to pediatric telehealth implementation.21 The Centers for Disease Control and Prevention (CDC) funds various grants and cooperatives related to pediatrics through its various offices, though it is difficult to quantify exactly how much of the total CDC funding is for pediatric research. Programs of the Maternal and Child Health Bureau within the Health Resources and Services Administration improve the health and wellbeing of America’s mothers, children, and families (HRSA, 2023). These other federal funders are critical for pediatric research, but it is difficult to quantify the amounts specific to pediatric subspecialty research. There is also little coordination, transparency, or standardized metrics among federal pediatric research funders to prioritize specific areas for federal pediatric research support.
The Patient Centered Outcomes Research Institute
The Patient Centered Outcomes Research Institute (PCORI) is an independent nonprofit organization that funds comparative clinical effectiveness
___________________
20 The amount of pediatric funding was calculated by searching “pediatrics” in the AHRQ grants by state.
21 These include: Improving Recognition and Management of Hypertension in Youth: Comparing Approaches for Extending Effective [Clinical Decision Support] for use in a Large Rural Health System: This project is focused on using clinical decision support within the electronic health record to identify elevated blood pressure and hypertension in children and adolescents in primary care and subspecialty clinics at a large health system. Specifically, the grant notes that research on these tools is “crucial in rural areas where adolescent obesity is high and access to pediatric subspecialists is low” (AHRQ, 2023b); Feasibility Study of a Mobile Digital Personal Health Record for Family-Centered Care Coordination for Children and Youth with Special Healthcare Needs: This project aims to evaluate the feasibility of a digital personal health record mobile application to help coordinate care for children and youth with special health care needs (AHRQ, 2023a); and Telehealth Education for Asthma Connecting Hospital and Home (TEACHH): This project aims to determine the feasibility and acceptability, as well as impact on patient-reported outcomes, of implementing TEACHH for children with asthma throughout the hospital-to-home transition. If successful, the researchers hypothesize that TEACHH could also be used for other chronic childhood diseases, such as diabetes and sickle cell anemia.
research, meaning research in which two or more treatments or health practices are compared to help guide patient decision making. PCORI has funded more than 100 studies and projects focused on pediatric health (as of February 2022), with initiatives such as delivery of mental health services to children in underserved areas, evaluation of treatments for infantile spasms, improving access to hearing care for children in rural areas through telehealth visits, and psychotropic medication use in foster youth (PCORI, 2023).
Industry
In 2020, industry accounted for nearly two thirds of U.S. medical and health investment in research and development (Research America, 2022). However, as discussed previously, progress in pediatric pharmaceutical research has historically lagged that in adults due to the scarcity of available pediatric populations, physiologic differences between children and adults, ethical concerns about research involving children, highly competitive therapeutic domains, and lack of financial incentives with minimal financial returns on investment (given the lower market appeal for drugs for relatively rare pediatric conditions) (Benning et al., 2021; Bucci-Rechtweg, 2017; Cheng et al., 2022; Milne and Davis, 2014). Industry-sponsored trials involving children remain limited due to expected lower profitability (Speer et al., 2023). However, biopharmaceutical research companies are evaluating existing medicines that have already received approval for use in adults with the aim of ascertaining safe and effective dosages and methods of administration for children (PhRMA, 2020). In 2020, there were more than 2,100 industry-sponsored pediatric clinical trials underway,22 testing 580 investigational medicines23 in infants, children, and adolescents (PhRMA, 2020).
Foundations
Several foundations support pediatric research. For example, the Burroughs Wellcome Fund provides competitive peer-reviewed awards to both institutions and researchers, with an increasing focus on early career physician–scientists, physician-only scientists, and pediatric research. It has invested $250 million into support of physician–scientists
___________________
22 Clinicaltrials.gov with search terms Recruiting, Not Yet Recruiting, Active, Not Recruiting, Interventional Studies, Child and Industry (accessed June 7, 2019).
23 Number of medicines obtained through government and industry sources, and the Springer “AdisInsight” database. Current as of January 10, 2020.
since its inception in 1955.24 In collaboration with the Coalition for Medical Research, the Burroughs Wellcome Fund held a summit in 2022 and produced a policy brief on Developing the Next Generation of Diverse Pediatric Researchers (BWF, 2022). Some key themes that were discussed in the summit and policy brief include articulation of barriers to pediatric research, summary of legislative priority areas of focus for pediatric research, including the Pediatricians Accelerate Childhood Therapies (PACT) Act of 2021,25 and the Pediatric Subspecialty Loan Repayment Program. Other large foundations that support pediatric research include the Annie E. Casey Foundation, the Bill and Melinda Gates Foundation, the Cystic Fibrosis Foundation, the David & Lucile Packard Foundation, the Doris Duke Charitable Foundation,26 the Juvenile Diabetes Research Foundation, the Pritzker Traubert Foundation, the Robert Wood Johnson Foundation, and the Thrasher Fund, among others. The Damon Runyon Cancer Research Foundation also funds the Damon Runyon Clinical Investigator Award, which is a program that is exclusive to M.D.-only would-be physician–scientists.
The Role of Academic Medical Centers in Cultivating Physician–Scientists
While NIH and private foundations persist in investing in the development of the physician–scientist workforce, academic medical centers bear a significant responsibility for cultivating and recruiting the next generation of physician–scientists (Brown, 2018). Academic medical centers (AMCs) have historically emphasized the education of physicians, relying on the hospital’s inpatient and outpatient settings as primary training sites, and have made significant contributions to pediatric research (IOM, 2004; see Chapter 4 for more information on education and training). While research in AMCs brings in grant dollars, even with indirect cost funds, grants do not fully support the research conducted. Research funding has often relied
___________________
24 Data provide during a presentation at an information gathering session by Louis J. Muglia, President and CEO of the Burroughs Wellcome Fund. The webinar recording can be accessed at https://www.nationalacademies.org/event/11-02-2022/the-pediatric-subspecialty-workforce-and-its-impact-on-child-health-and-well-being-webinar-3.
25 PACT Act of 2021, S 1357, 117th Cong. (2021–2022).
26 Related to retention and prevention attrition, the Doris Duke Fund to Retain Clinical Scientists awards 5-year grants of $540,000 each to medical schools and affiliated hospitals to retain early-career physician–scientists who are facing extraprofessional demands of care-giving such as childcare and eldercare. Building on this program, the Doris Duke Foundation, the American Heart Association, the Burroughs Wellcome Fund, Rita Allen Foundation, and Walder Foundation collaborated in 2021 to offer a one-time COVID-19 Fund to Retain Clinical Scientists funding opportunity. See https://www.dorisduke.org/funding-areas/medical-research/fund-to-retain-clinical-scientists (accessed July 21, 2023).
on the cross-subsidization of clinical revenue, as well as philanthropic funding (Kerschner et al., 2018). However, with increasing costs of providing clinical care, along with the increasing proportion of patients insured by Medicaid (see Chapter 8), the ability for this cross-subsidization is decreasing (Lakshminrusimha et al., 2022). In smaller academic medical centers, it is likely impossible, resulting in even less ability to support early career researchers and compelling faculty to focus on generating more clinical revenue (Garrison and Ley, 2022). Children’s hospitals often prioritize clinical programs, with less investment in research, and training programs in freestanding children’s hospitals often have less opportunity for interaction with a broader array of both clinicians and researchers (as compared with programs in university settings) (Boat and Whitsett, 2021). During one of the committee’s public webinars, Sallie Permar (Nancy C. Paduano Professor and Chair of Pediatrics at Weill Cornell Medicine and pediatrician-in-chief at New York-Presbyterian/Weill Cornell Medical Center) highlighted the difficulties that AMCs and pediatric departments face in developing pediatric physician–scientists:
Our departments [have] a challenge in covering junior faculty years that are needed for that ongoing research training before a physician–scientist can become independently funded. There is not as much margin as adult departments and surgical departments to continue allowing for research, training, and development of that independence. What this leaves us with is a therapeutic pipeline for children that’s really threatened by not having enough bright new minds going into the field and really leaves out children when it comes to early adoption of novel therapies…we’re really limiting the therapeutic opportunities that we bring to children by not attracting more and speaking to not only the enthusiasm of these pediatric subspecialists, but their earning potential for the long term.27
KEY FINDINGS AND CONCLUSIONS
Key Findings
Finding #6-1: Unique aspects of pediatric clinical trials can affect recruitment, retention, and trial success, including ethical considerations, logistical and technical factors in administering interventions, smaller population size (especially for subspecialty care), and financial disincentives related to the limited commercial market potential for pediatric drugs compared with adult drugs. These challenges in the
___________________
27 The webinar recording can be accessed at https://www.nationalacademies.org/event/07-19-2022/the-pediatric-subspecialty-workforce-and-its-impact-on-child-health-and-well-being-webinar-1.
pediatric clinical trial enterprise present disadvantages to pursuing careers in pediatric research compared to adult research.
Finding #6-2: There is a limited number of pediatric researchers, with a variable and leaky pathway by subspecialty. The number and proportion of pediatric subspecialists engaged in research has remained steady over the past decade.
Finding #6-3: The paucity of objective data—including the exact number of researchers by subspecialty, percentage of time spent on research, and attrition across the research pathway (e.g., K to R transition)—makes it difficult to characterize the pediatric physician–scientist workforce.
Finding #6-4: The unequal distribution of funding across institutions—with funding for basic and clinical research concentrated in a small number of large U.S. pediatric medical centers and unequally distributed across the country—means that many institutions do not have the financial resources to provide adequate support to pediatric physician–scientists, particularly during the early phase of career development.
Finding #6-5: There is little coordination among pediatric research funders.
Finding #6-6: NIH funding for early career awards does not provide adequate support for investigator salary, mentorship nor research project expenses, thereby creating financial stress for institutions and departments, which are expected to cover the financial gaps.
Finding #6-7: Challenges to the growth of the pediatric physician–scientist workforce include the lack of a robust mentorship environment at different levels, particularly for fellows and early career investigators; financial considerations that impact trainees’ decisions to pursue research; protection of time for physician–scientists to engage in research; and adequate extramural and intramural funding for research.
Finding #6-8: Receipt of an NIH Loan Repayment Program award is associated with higher levels of persistence in research over a decade after initial application.
Conclusions
Conclusion #6-1: To improve the quantity and quality of pediatric health research, a robust pediatric physician–scientist pathway and workforce are critical.
Conclusion #6-2: More evidence is needed on funding trends, unmet needs, quality of research mentorship, and outcomes from pediatric physician–scientist career development pathway programs.
Conclusion #6-3: There is a need for improved communication and collaboration among funding agencies and other participants in the research enterprise to target important areas of research need and the development of metrics to quantify the impact of pediatric research, including long-term health outcomes.
Conclusion #6-4: Specific and intentional efforts are needed to encourage and facilitate entry into research careers and foster the early phases of career development for pediatric physician–scientists. This is especially true for physician–scientists from populations underrepresented in the extramural scientific workforce.
Conclusion #6-5: Partnerships between government, public, and industry organizations can play a key role in building career pathways for pediatric researchers to help push the frontiers in clinical trials, drug discovery, and health services research.
Conclusion #6-6: Mentorship is key for pediatric research career advancement. It is critical for pediatric departments to recognize, reward, and incentivize exceptional research mentorship at the individual level.
Conclusion #6-7: Salaries for pediatric physician–scientists are negatively affected by less clinical incentive income, in addition to already the low salaries of many of the pediatric subspecialties. The NIH LRP Program is an important policy lever for retention of early career investigators in the research workforce and may be especially important for pediatric subspecialist physician–scientists.
RECOMMENDATIONS
The continued advances in child health require continued scientific discovery from T0 to T4.28 This requires a highly skilled workforce in which the pediatric physician–scientist plays a crucial role. For the purposes of this report, the committee focused on the role of the pediatric subspecialty physician–scientists who are crucial in research to improve subspecialty care and related health and organizational outcomes. Training this workforce cannot be fully accomplished during the 12 months of scholarly activity required within a 3-year overall fellowship. Rather, physician–scientists need extended training accompanied by adequate research support at the beginning of their careers. Intentional efforts are needed to encourage and facilitate entry into research careers and foster the early phases of career development for pediatric physician–scientists, especially those from populations underrepresented in the extramural scientific workforce. The current system for producing and nurturing pediatric physician–scientists has been inadequate and needs to be improved. However, more evidence is needed on funding trends, unmet needs, quality of research mentorship, and outcomes from pediatric physician–scientist career development pathway programs to inform future efforts to support careers in research. Therefore, to achieve a goal of supporting the pediatric physician–scientist pathway, the committee makes the following recommendations.
RECOMMENDATION 6-1 The National Institutes of Health (NIH) Pediatric Research Consortium, with leadership from the National Institute of Child Health and Human Development and input from the NIH’s Scientific Workforce Diversity Office, and with appropriate additional funding, should engage with other government and nongovernment pediatric research funders to create and maintain a publicly
___________________
28 “The T0 pillar anchors basic science bench research, whereas T1 work extends basic science discovery to the first in human trials looking for safety and efficacy endpoints, proof-of-concept, and phase 1 clinic trials. T2 science includes the phase 2 and 3 clinical trials of diagnostics, therapeutics, devices, and other interventions for human health. The physician–scientist [needs] a different educational focus for this pillar than the T0/T1 physician–scientist. Education [needs to] cover clinical trials science, observational studies, meaningful endpoint detection, statistical methods focused on human populations, and human behavior. T3 science extends to phase 4 clinical trials and other observational studies such as health services and clinical outcomes research. Physician–scientists in this arena need education in community-based participatory research and cost-effectiveness and comparative effectiveness research methods. T4 science looks at population-level outcomes and how social determinants of health significantly influence health. Physician–scientists [need to] gain specialization in public policy and health disparities research to include population health guideline development and rigorous meta-analytic strategies” (Williams et al., 2022).
available central repository for qualitative and quantitative data on pediatric physician–scientists’ funding and success throughout their careers (e.g., tracking funding rates and attrition by pediatric subspecialty), including the development of new measures as needed to understand the initial success and retention of pediatric physician–scientists. The Association of Medical School Pediatric Department Chairs should provide supplemental data as needed.
Examples of data needed include the following:
- Quantitative data on the funding rates of career development and subsequent awards and the tracking of research careers by demographic factors such as sex/gender, race and ethnicity, disability status, geography, type of science across the continuum (e.g., basic, clinical, translational, implementation, health services research), topic/subspecialty, and professional background of the PI; and
- Qualitative data on successful researchers and those who leave the research track, including the quality of relationship with mentors; types of support received from their institutions; availability of statistical, epidemiologic, academic and grant writing training and support; availability of funding to present and publish research; satisfaction with career progression; and reasons for attrition or retention.
RECOMMENDATION 6-2 The National Institutes of Health and the Agency for Healthcare Research and Quality should increase the number of career development grants in pediatrics, particularly institutional training awards (e.g., the Pediatric Scientist Development Program), the Pediatric Loan Repayment Program, and K awards, with attention to providing such grants to physician–scientists from backgrounds that are underrepresented in the scientific workforce29 and for high-priority subspecialties in pediatric research. Funding for individual K awards should be increased to reflect current salaries and research project expenses and should include additional explicit funding for mentorship.
___________________
29 See Chapter 1 of this report for a discussion of underrepresentation in the scientific workforce.
REFERENCES
AAMC (Association of American Medical Colleges). 2019. Diversity in medicine: Facts and figures 2019. https://www.aamc.org/data-reports/workforce/report/diversity-medicine-facts-and-figures-2019 (accessed May 3, 2023).
AAMC. 2022a. 2022 FACTS: Enrollment, Graduates, and MD-PhD Data. Table B-11.2. https://www.aamc.org/data-reports/students-residents/data/2022-facts-enrollment-graduates-and-md-phd-data (accessed June 27, 2023).
AAMC. 2022b. Report on residents. Table B4. MD-PhD residents, by GME specialty. 2019–21 active residents. https://www.aamc.org/data-reports/students-residents/data/report-residents/2022/table-b4-md-phd-residents-gme-specialty (accessed June 27, 2023).
AAMC. 2023a. Physician-scientists. https://www.aamc.org/what-we-do/mission-areas/medical-research/physician-scientist (accessed March 8, 2023).
AAMC. 2023b. Mentoring. https://www.aamc.org/professional-development/affinity-groups/gfa/mentoring (accessed July 17, 2023).
ABP (American Board of Pediatrics). 2023. Survey results: 2018–2022 maintenance of certification enrollment surveys: Hours worked and work profiles. https://www.abp.org/dashboards/results-continuing-certification-moc-enrollment-surveys-2018-2022 (accessed April 27, 2023).
Abramson, E. L., P. Weiss, M. Naifeh, M. D. Stevenson, J. G. Duncan, J. A. Rama, E. Mauer, L. M. Gerber, and S.-T. T. Li. 2021. Scholarly activity during pediatric fellowship. Pediatrics 147(1).
AHRQ (Agency for Healthcare Research and Quality). 2016. Meeting minutes, November 2016. https://www.ahrq.gov/news/events/nac/2016-11-nac/nacmtg1116-minutes.html (accessed May 1, 2023).
AHRQ. 2020. AHRQ grants by state. https://www.ahrq.gov/funding/grant-mgmt/grants-by-state.html (accessed February 7, 2023).
AHRQ. 2023a. Feasibility study of a mobile digital personal health record for family-centered care coordination for children and youth with special healthcare needs. https://digital.ahrq.gov/ahrq-funded-projects/feasibility-study-mobile-digital-personal-health-record-family-centered-care-coordination-children (accessed April 3, 2023).
AHRQ. 2023b. Improving recognition and management of hypertension in youth: Comparing approaches for extending effective CDs for use in a large rural health system. https://digital.ahrq.gov/ahrq-funded-projects/improving-recognition-and-management-hypertension-youth-comparing-approaches (accessed April 3, 2023).
Akabas, M., I. Tartakovsky, and L. Brass. 2018. The national MD-PhD program outcomes study. American Association of Medical Colleges Reports. https://store.aamc.org/national-M.D.-Ph.D.-program-outcomes-study.html (acessed May 2, 2023).
Alganabi, M., and A. Pierro. 2021. Becoming an academic pediatric surgeon scientist in Canada. Seminars in Pediatric Surgery 30(1):151015.
Alvira, C. M., R. H. Steinhorn, W. F. Balistreri, J. R. Fineman, P. E. Oishi, J. F. Padbury, J. P. Kinsella, and S. H. Abman. 2018. Enhancing the development and retention of physician-scientists in academic pediatrics: Strategies for success. Journal of Pediatrics 200:277-284.
Andriole, D. A., A. J. Whelan, and D. B. Jeffe. 2008. Characteristics and career intentions of the emerging M.D./Ph.D. workforce. JAMA 300(10):1165-1173.
Aristizabal, P., J. Singer, R. Cooper, K. J. Wells, J. Nodora, M. Milburn, S. Gahagan, D. E. Schiff, and M. E. Martinez. 2015. Participation in pediatric oncology research protocols: Racial/ethnic, language and age‐based disparities. Pediatric Blood & Cancer 62(8):1337-1344.
Ayedun, A., V. Agbelese, L. Curry, R. Gotian, L. Castillo-Page, M. White, A. D. Antwi, M. Buchanan, M. Girma, D. Kline, C. Okeke, A. Raghu, H. Saleh, A. Schwartz, and D. Boatright. 2023. Perspectives on National Institutes of Health funding requirements for racial and ethnic diversity among medical scientist training program leadership. JAMA Network Open 6(5):e2310795-e2310795.
Badawy, S. M., V. Black, E. R. Meier, K. C. Myers, K. Pinkney, C. Hastings, J. M. Hilden, P. Zweidler‐McKay, L. C. Stork, and T. S. Johnson. 2017. Early career mentoring through the American Society of Pediatric Hematology/Oncology: Lessons learned from a pilot program. Pediatric Blood & Cancer 64(3):e26252.
Baruteau, J., S. N. Waddington, I. E. Alexander, and P. Gissen. 2017. Gene therapy for monogenic liver diseases: Clinical successes, current challenges and future prospects. Journal of Inherited Metabolic Disease 40(4):497-517.
Bauserman, M., M. Vasquez, P. R. Chess, M. Carbajal, H. French, K. Reber, E. Cicalese, K. Lawrence, B. Schwarz, A. Payne, R. Angert, M. Gillam-Krakauer, J. Sharma, E. Bonachea, J. Trzaski, L. Johnston, R. Dadiz, J. Enciso, A. Falck, M. Frost, M. Gray, S. Izatt, S. Kane, A. Kiefer, K. Leeman, S. Malik, P. Myers, J. Nair, D. O’Reilly, T. Sawyer, M. C. Smith, K. Stanley, J. Wambach, C. L. Wraight, M. Good, and O. F. D. W. Group. 2022. Essentials of neonatal-perinatal medicine fellowship: Scholarship perspective. Journal of Perinatology 42(4):528-533.
Behera, A., J. Tan, and H. Erickson. 2019. Diversity and the next-generation physician-scientist. Journal of Clinical and Translational Science 3(2-3):47-49.
Benning, T. J., N. D. Shah, J. W. Inselman, H. K. Van Houten, J. S. Ross, and K. D. Wyatt. 2021. Drug labeling changes and pediatric hematology/oncology prescribing: Measuring the impact of U.S. legislation. Clinical Trials 18(6):732-740.
Bick, D., M. Jones, S. L. Taylor, R. J. Taft, and J. Belmont. 2019. Case for genome sequencing in infants and children with rare, undiagnosed or genetic diseases. Journal of Medical Genetics 56(12):783-791.
Bland, C., Taylor, A., and S. Shollenberger. 2010. Mentoring systems: Benefits and challenges of diverse mentoring partnerships. https://www.aamc.org/professional-development/affinitygroups/gfa/faculty-vitae/mentoring-systems-benefits-challenges (accessed July 17, 2023).
Blehar, M. C., C. Spong, C. Grady, S. F. Goldkind, L. Sahin, and J. A. Clayton. 2013. Enrolling pregnant women: Issues in clinical research. Womens Health Issues 23(1):e39-e45.
Blish, C. A. 2018. Maintaining a robust pipeline of future physician-scientists. Journal of Infectious Diseases 218(Suppl 1):S40-S43.
Boat, T. F., and J. A. Whitsett. 2021. How can the pediatric community enhance funding for child health research? JAMA Pediatrics 175(12):1212-1214.
Bora, S. 2023. Strategic recommendations to promote justice, equity, diversity, and inclusion principles in the pediatric scientific workforce. https://www.societyforpediatricresearch.org/jedi-toolbox-faculty-mentoring/ (accessed July 17, 2023).
Bourgeois, F. T., S. Murthy, C. Pinto, K. L. Olson, J. P. Ioannidis, and K. D. Mandl. 2012. Pediatric versus adult drug trials for conditions with high pediatric disease burden. Pediatrics 130(2):285-292.
Bourgeois, F. T., K. L. Olson, J. P. Ioannidis, and K. D. Mandl. 2014. Association between pediatric clinical trials and global burden of disease. Pediatrics 133(1):78-87.
Branson, B. M., H. H. Handsfield, M. A. Lampe, R. S. Janssen, A. W. Taylor, S. B. Lyss, and J. E. Clark. 2006. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. Morbidity and Mortality Weekly Report: Recommendations and Reports 55(14):1-CE-4.
Brass, L. F., and M. H. Akabas. 2019. The national MD-PhD program outcomes study: Relationships between medical specialty, training duration, research effort, and career paths. JCI Insight 4(19).
Brass, L. F., M. H. Akabas, L. D. Burnley, D. M. Engman, C. A. Wiley, and O. S. Andersen. 2010. Are MD-PhD programs meeting their goals? An analysis of career choices made by graduates of 24 MD-PhD programs. Academic Medicine 85(4):692-701.
Braveman, P., and C. Barclay. 2009. Health disparities beginning in childhood: A life-course perspective. Pediatrics 124(Supplement_3):S163-S175.
Brown, N. J. 2018. Developing physician-scientists. Circulation Research 123(6):645-647.
Brussee, J. M., E. A. Calvier, E. H. Krekels, P. A. Välitalo, D. Tibboel, K. Allegaert, and C. A. Knibbe. 2016. Children in clinical trials: Towards evidence-based pediatric pharmacotherapy using pharmacokinetic-pharmacodynamic modeling. Expert Review of Clinical Pharmacology 9(9):1235-1244.
Bucci-Rechtweg, C. 2017. Enhancing the pediatric drug development framework to deliver better pediatric therapies tomorrow. Clinical Therapeutics 39(10):1920-1932.
Buja, L. M. 2019. Medical education today: All that glitters is not gold. BMC Medical Education 19(1):1-11.
Burckart, G. J. 2020. The revolution in pediatric drug development and drug use: Therapeutic orphans no more. Journal of Pediatric Pharmacology and Therapeutics 25(7):565.
Burns, A. M., D. J. Moore, C. S. Forster, W. Powell, S. Thammasitboon, M. K. Hostetter, P. Weiss, D. Boyer, M. A. Ward, and R. Blankenburg. 2022. Physician-scientist training and programming in pediatric residency programs: A national survey. Journal of Pediatrics 241:5-9. e3.
Burroughs Wellcome Fund (BWF). 2022. Developing the next generation of diverse pediatric researchers. https://www.dropbox.com/s/n07jtt0x0kcnq8k/May-5th-Convening-Program-FINAL.pdf?dl=0 (accessed 2023, April 28).
Byars-Winston, A., V. Y. Womack, A. R. Butz, R. McGee, S. C. Quinn, E. Utzerath, C. L. Saetermoe, and S. Thomas. 2018. Pilot study of an intervention to increase cultural awareness in research mentoring: Implications for diversifying the scientific workforce. Journal of Clinical and Translational Science 2(2):86-94.
Caldwell, P. H. Y., S. B. Murphy, P. N. Butow, and J. C. Craig. 2004. Clinical trials in children. Lancet 364(9436):803-811.
Campbell, L. G., S. Mehtani, M. E. Dozier, and J. Rinehart. 2013. Gender-heterogeneous working groups produce higher quality science. PLoS ONE 8(10):e79147.
Caruso, C.. 2020. RACE Act poised to advance pediatric cancer research. Cancer Discovery 10(10):1434.
CDC (Centers for Disease Control and Prevention). 2022. HIV and perinatal transmission. https://www.cdc.gov/hiv/group/pregnant-people/index.html (accessed December 19, 2022).
CFF (Cystic Fibrosis Foundation). 2022. Intro to CF. https://www.cff.org/intro-cf (accessed December 19, 2022).
Chen, A., S. Demaestri, K. Schweiberger, J. Sidani, R. Wolynn, D. Chaves-Gnecco, R. Hernandez, S. Rothenberger, E. Mickievicz, J. D. Cowden, and M. I. Ragavan. 2022. Inclusion of non–English-speaking participants in pediatric health research: A review. JAMA Pediatrics 177(1):81-88.
Chen, M. M., C. I. Sandborg, L. Hudgins, R. Sanford, and L. K. Bachrach. 2016. A multifaceted mentoring program for junior faculty in academic pediatrics. Teaching and Learning in Medicine 28(3):320-328.
Cheng, T. L., N. Monteiro, L. A. DiMeglio, A. T. Chien, E. S. Peeples, E. Raetz, B. Scheindlin, and S. C. Denne. 2016. Seven great achievements in pediatric research in the past 40 y. Pediatric Research 80(3):330-337.
Cheng, T. L., C. Russo, C. Cole, D. A. Williams, S. Shah, M. Patel, J. Raphael, J. Davis, D. Pursley, T. Cheng, S. U. Devaskar, J. Javier, L. Lee, and Council on Behalf of the Pediatric Policy. 2022. Advocacy for research starting early in the life course. Pediatric Research 91(6):1312-1314.
Christou, H., M. L. V. Dizon, K. N. Farrow, S. R. Jadcherla, K. T. Leeman, A. Maheshwari, L. P. Rubin, B. K. Stansfield, and D. H. Rowitch. 2016. Sustaining careers of physician-scientists in neonatology and pediatric critical care medicine: Formulating supportive departmental policies. Pediatric Research 80(5):635-640.
COG (Children’s Oncology Group). 2023. Children’s oncology group (COG): Who we are. https://childrensoncologygroup.org/childrens-oncology-group (accessed April 3, 2023).
Cohen, E., E. Uleryk, M. Jasuja, and P. C. Parkin. 2007. An absence of pediatric randomized controlled trials in general medical journals, 1985–2004. Journal of Clinical Epidemiology 60(2):118-123.
Cohen, E., R. D. Goldman, A. Ragone, E. Uleryk, E. G. Atenafu, U. Siddiqui, N. Mahmoud, and P. C. Parkin. 2010. Child vs adult randomized controlled trials in specialist journals: A citation analysis of trends, 1985–2005. Archives of Pediatric & Adolescent Medicine 164(3):283-288.
Conte, M. L., and M. B. Omary. 2018. NIH career development awards: Conversion to research grants and regional distribution. Journal of Clinical Investigation 128(12):5187-5190.
Coppes, M. J., C. Jackson, and E. M. Connor. 2022. I-act for children: Helping close the gap in drug approval for adults and children. Pediatric Research. https://doi.org/10.1038/s41390-022-02349-5.
COPR (Committee on Pediatric Research), M. D. Cabana, T. L. Cheng, A. J. Bauer, C. W. Bogue, A. T. Chien, J. M. Dean, B. Scheindlin, and A. Kelle. 2014. Promoting education, mentorship, and support for pediatric research. Pediatrics 133(5):943-949.
Cranmer, J. M., A. M. Scurlock, R. B. Hale, W. L. Ward, P. Prodhan, J. L. Weber, P. H. Casey, and R. F. Jacobs. 2018. An adaptable pediatrics faculty mentoring model. Pediatrics 141(5).
Daniels, R. J. 2015. A generation at risk: Young investigators and the future of the biomedical workforce. Proceedings of the National Academy of Sciences 112(2):313-318.
Daye, D., C. B. Patel, J. Ahn, and F. T. Nguyen. 2015. Challenges and opportunities for reinvigorating the physician-scientist pipeline. Journal of Clinical Investigation 125(3):883-887.
DeCastro, R., D. Sambuco, P. A. Ubel, A. Stewart, and R. Jagsi. 2013. Mentor networks in academic medicine: Moving beyond a dyadic conception of mentoring for junior faculty researchers. Academic Medicine 88(4):488-496.
De Luca, D., P. Cogo, M. C. Kneyber, P. Biban, M. G. Semple, J. Perez-Gil, G. Conti, P. Tissieres, and P. C. Rimensberger. 2021. Surfactant therapies for pediatric and neonatal ARDs: Espnic expert consensus opinion for future research steps. Critical Care 25(1):75.
Dermody, T. S., R. Hirsch, M. K. Hostetter, J. S. Orange, and J. W. St. Geme, III. 2019. Expanding the pipeline for pediatric physician-scientists. Journal of Pediatrics 207:3-7.e1.
Donath, E., K. B. Filion, and M. J. Eisenberg. 2009. Improving the clinician-scientist pathway: A survey of clinician-scientists. Archives of Internal Medicine 169(13):1241-1247.
Doris Duke Foundation. 2023. Physician Scientist Fellowship. https://www.dorisduke.org/funding-areas/medical-research/physician-scientist-fellowship/ (accessed June 27, 2023).
Doubeni, C. A., A. Davis, J. L. Benson, and B. Ewigman. 2017. From the Association of Departments of Family Medicine: A physician scientist pathway in family medicine residency training programs. Annals of Family Medicine 15(6):589.
Duvivier, R. J., M. E. Gusic, and J. R. Boulet. 2020. International medical graduates in the pediatric workforce in the United States. Pediatrics 146(6).
El-Khuffash, A. F. 2017. Early career investigator highlight–November. Pediatric Research 82(5):722.
Ercan-Fang, N. G., D. C. Rockey, C. J. Dine, S. Chaudhry, and T. Arayssi. 2017. Resident research experiences in internal medicine residency programs—a nationwide survey. American Journal of Medicine 130(12):1470-1476. e1473.
Farrar, M. A., and M. C. Kiernan. 2015. The genetics of spinal muscular atrophy: Progress and challenges. Neurotherapeutics 12(2):290-302.
Faulk, K. E., A. Anderson-Mellies, M. Cockburn, and A. L. Green. 2020. Assessment of enrollment characteristics for Children’s Oncology Group (COG) upfront therapeutic clinical trials 2004–2015. PLoS ONE 15(4):e0230824.
FDA. (U.S. Food and Drug Administration). 2019a. FDA approves innovative gene therapy to treat pediatric patients with spinal muscular atrophy, a rare disease and leading genetic cause of infant mortality. https://www.fda.gov/news-events/press-announcements/fda-approves-innovative-gene-therapy-treat-pediatric-patients-spinal-muscular-atrophy-rare-disease (accessed June 27, 2023).
FDA. 2019b. Pediatric research equity act: PREA. https://www.fda.gov/drugs/development-resources/pediatric-research-equity-act-prea (accessed May 8, 2023).
Finkel, R. S., E. Mercuri, B. T. Darras, A. M. Connolly, N. L. Kuntz, J. Kirschner, C. A. Chiriboga, K. Saito, L. Servais, E. Tizzano, H. Topaloglu, M. Tulinius, J. Montes, A. M. Glanzman, K. Bishop, Z. J. Zhong, S. Gheuens, C. F. Bennett, E. Schneider, W. Farwell, and D. C. De Vivo. 2017. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. New England Journal of Medicine 377(18):1723-1732.
Flores, G., F. S. Mendoza, M. R. DeBaun, E. Fuentes-Afflick, V. F. Jones, J. A. Mendoza, J. L. Raphael, and C. J. Wang. 2019. Keys to academic success for under-represented minority young investigators: Recommendations from the research in academic pediatrics initiative on diversity (RAPID) national advisory committee. International Journal for Equity in Health 18(1):93.
Flores, G., F. Mendoza, M. B. Brimacombe, and W. Frazier III. 2021. Program evaluation of the Research in Academic Pediatrics Initiative on Diversity (RAPID): Impact on career development and professional society diversity. Academic Medicine 96(4):549-556.
Forrest, C. B., K. M. McTigue, A. F. Hernandez, L. W. Cohen, H. Cruz, K. Haynes, R. Kaushal, A. N. Kho, K. A. Marsolo, V. P. Nair, R. Platt, J. E. Puro, R. L. Rothman, E. A. Shenkman, L. R. Waitman, N. A. Williams, and T. W. Carton. 2021. PCORnet 2020: Current state, accomplishments, and future directions. Journal of Clinical Epidemiology 129:60-67.
Frugé, E., J. Margolin, T. Horton, L. Venkateswaran, D. Lee, D. L. Yee, and D. Mahoney. 2010. Defining and managing career challenges for mid-career and senior stage pediatric hematologist/oncologists. Pediatric Blood & Cancer 55(6):1180-1184.
Garrison, H. H., and A. M. Deschamps. 2014. NIH research funding and early career physician scientists: Continuing challenges in the 21st century. Federation of American Societies for Experimental Biology Journal 28(3):1049-1058.
Garrison, H. H., and T. J. Ley. 2022. Physician-scientists in the United States at 2020: Trends and concerns. FASEB Journal 36(5):e22253.
Ghosh-Choudhary, S., N. Carleton, S. M. Nouraie, C. R. Kliment, and R. A. Steinman. 2022. Predoctoral MD-PhD grants as indicators of future NIH funding success. JCI Insight 7(6).
Gilbert, J. A., M. J. Blaser, J. G. Caporaso, J. K. Jansson, S. V. Lynch, and R. Knight. 2018. Current understanding of the human microbiome. Nature Medicine 24(4):392-400.
Ginther, D. K. 2022. Reflections on race, ethnicity, and NIH research awards. Molecular Biology of the Cell 33(1):ae1.
Ginther, D. K., W. T. Schaffer, J. Schnell, B. Masimore, F. Liu, L. L. Haak, and R. Kington. 2011. Race, ethnicity, and NIH research awards. Science 333(6045):1015-1019.
Gitterman, D. P., R. S. Greenwood, K. C. Kocis, B. R. Mayes, and A. N. McKethan. 2004. Did a rising tide lift all boats? The NIH budget and pediatric research portfolio. Health Affairs 23(5):113-124.
Gitterman, D. P., W. S. Langford, and W. W. Hay, Jr. 2018a. The uncertain fate of the National Institutes of Health (NIH) pediatric research portfolio. Pediatric Research 84(3):328-332.
Gitterman, D. P., W. S. Langford, and W. W. Hay, Jr. 2018b. The fragile state of the National Institutes of Health pediatric research portfolio, 1992-2015: Doing more with less? JAMA Pediatrics 172(3):287-293.
Gitterman, D. P., W. W. Hay, and W. S. Langford. 2023. Making the case for pediatric research: A life-cycle approach and the return on investment. Pediatric Research 93(4):797-800.
Glass, H. C., A. T. Costarino, S. A. Stayer, C. M. Brett, F. Cladis, and P. J. Davis. 2015. Outcomes for extremely premature infants. Anesthesia and Analgesia 120(6):1337-1351.
Good, M., S. J. McElroy, J. N. Berger, D. J. Moore, and J. L. Wynn. 2018a. Limited achievement of NIH research independence by pediatric K award recipients. Pediatric Research 84(4):479-480.
Good, M., S. J. McElroy, J. N. Berger, and J. L. Wynn. 2018b. Name and characteristics of National Institutes of Health R01-funded pediatric physician–scientists: Hope and challenges for the vanishing pediatric physician–scientists. JAMA Pediatrics 172(3):297-299.
Goulooze, S. C., L. B. Zwep, J. E. Vogt, E. H. Krekels, T. Hankemeier, J. N. van den Anker, and C. A. Knibbe. 2020. Beyond the randomized clinical trial: Innovative data science to close the pediatric evidence gap. Clinical Pharmacology & Therapeutics 107(4):786-795.
Goyal, M. K., T. J. Johnson, J. M. Chamberlain, L. Cook, M. Webb, A. L. Drendel, E. Alessandrini, L. Bajaj, S. Lorch, and R. W. Grundmeier. 2020. Racial and ethnic differences in emergency department pain management of children with fractures. Pediatrics 145(5).
Goyal, M. K., N. Kuppermann, S. D. Cleary, S. J. Teach, and J. M. Chamberlain. 2015. Racial disparities in pain management of children with appendicitis in emergency departments. JAMA Pediatrics 169(11):996-1002.
Groff, M. L., M. Offringa, A. Ein, Q. Mahood, P. C. Parkin, and E. Cohen. 2020. Publication trends of pediatric and adult randomized controlled trials in general medical journals, 2005-2018: A citation analysis. Children (Basel) 7(12).
Guevara, J. P., J. Aysola, R. Wade, B. Nfonoyim, M. Qiu, M. Reece, and K. N. Carroll. 2023. Diversity in the pediatric research workforce: A scoping review of the literature. Pediatric Research 94(3):904–914. https://doi.org/10.1038/s41390-023-02603-4.
Guttmann, K. F. 2022. Early career investigator biocommentary: Katherine Guttmann. Pediatric Research 92(4):916.
Halfon, N., S. A. Russ, and E. L. Schor. 2022. The emergence of life course intervention research: Optimizing health development and child well-being. Pediatrics 149(Supplement 5).
Harshman, L. A. 2021. Early career investigator highlight: Lyndsay A. Harshman. Pediatric Research 89(3):402.
Holstein, S. A., and M. A. Lunning. 2020. CAR T-cell therapy in hematologic malignancies: A voyage in progress. Clinical Pharmacology & Therapeutics 107(1):112-122.
HRSA (Health Resources and Services Administration). 2023. Maternal and Child Health Bureau (MCHB) awarded grants. https://data.hrsa.gov/topics/mchb/mchb-grants (accessed April 3, 2023).
Hunger, S. P., X. Lu, M. Devidas, B. M. Camitta, P. S. Gaynon, N. J. Winick, G. H. Reaman, and W. L. Carroll. 2012. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the Children’s Oncology Group. Journal of Clinical Oncology 30(14):1663-1669.
Hurst, J. H., K. J. Barrett, M. S. Kelly, B. B. Staples, K. A. McGann, C. K. Cunningham, A. M. Reed, R. A. Gbadegesin, and S. R. Permar. 2019. Cultivating research skills during clinical training to promote pediatric-scientist development. Pediatrics 144(2).
Hutzen, B., S. N. Paudel, M. Naeimi Kararoudi, K. A. Cassady, D. A. Lee, and T. P. Cripe. 2019. Immunotherapies for pediatric cancer: Current landscape and future perspectives. Cancer and Metastasis Reviews 38(4):573-594.
Hwang, T. J., F. T. Bourgeois, J. M. Franklin, and A. S. Kesselheim. 2019. Impact of the priority review voucher program on drug development for rare pediatric diseases. Health Affairs 38(2):313-319.
Hwang, T. J., A. G. Randolph, and F. T. Bourgeois. 2020. Inclusion of children in clinical trials of treatments for coronavirus disease 2019 (COVID-19). JAMA Pediatrics 174(9):825-826.
IOM (Institute of Medicine). 2004. Academic health centers: Leading change in the 21st century. Washington, DC: The National Academies Press. https://doi.org/10.17226/10734.
IOM. 2012. Safe and effective medicines for children: Pediatric studies conducted under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. Washington, DC: The National Academies Press. https://doi.org/10.17226/13311.
Jagsi, R., A. R. Motomura, K. A. Griffith, S. Rangarajan, and P. A. Ubel. 2009. Sex differences in attainment of independent funding by career development awardees. Annals of Internal Medicine 151(11):804-811.
Jagsi, R., R. DeCastro, K. A. Griffith, S. Rangarajan, C. Churchill, A. Stewart, and P. A. Ubel. 2011. Similarities and differences in the career trajectories of male and female career development award recipients. Academic Medicine 86(11):1415-1421.
Jain, M. K., V. G. Cheung, P. J. Utz, B. K. Kobilka, T. Yamada, and R. Lefkowitz. 2019. Saving the endangered physician-scientist—a plan for accelerating medical breakthroughs. New England Journal of Medicine 381(5):399-402.
Janssens, Y., J. Nielandt, A. Bronselaer, N. Debunne, F. Verbeke, E. Wynendaele, F. Van Immerseel, Y. P. Vandewynckel, G. De Tré, and B. De Spiegeleer. 2018. Disbiome database: Linking the microbiome to disease. BMC Microbiology 18(1):50.
Jędrzejowska, M. 2020. Advances in newborn screening and presymptomatic diagnosis of spinal muscular atrophy. Degenerative Neurological and Neuromuscular Disease 10:39-47.
Jeffe, D. B., and D. A. Andriole. 2018. Prevalence and predictors of US medical graduates’ federal F32, mentored-K, and R01 awards: A national cohort study. Journal of Investigative Medicine 66(2):340-350.
Johnson, T. J., A. M. Ellison, G. Dalembert, J. Fowler, M. Dhingra, K. Shaw, and S. Ibrahim. 2017a. Implicit bias in pediatric academic medicine. Journal of the National Medical Association 109(3):156-163.
Johnson, T. J., D. G. Winger, R. W. Hickey, G. E. Switzer, E. Miller, M. B. Nguyen, R. A. Saladino, and L. R. Hausmann. 2017b. Comparison of physician implicit racial bias toward adults versus children. Academic Pediatrics 17(2):120-126.
Jones, D. R., M. J. Mack, G. A. Patterson, and L. H. Cohn. 2011. A positive return on investment: Research funding by the thoracic surgery foundation for research and education (TSFRE). The Journal of Thoracic and Cardiovascular Surgery 141(5):1103-1106.
Kalet, A., A. M. Libby, R. Jagsi, K. Brady, D. Chavis-Keeling, M. H. Pillinger, G. L. Daumit, A. F. Drake, W. P. Drake, V. Fraser, D. Ford, J. S. Hochman, R. D. Jones, C. Mangurian, E. A. Meagher, G. McGuinness, J. G. Regensteiner, D. C. Rubin, K. Yaffe, and J. E. Ravenell. 2022. Mentoring underrepresented minority physician-scientists to success. Academic Medicine 97(4):497-502.
Kashiwagi, D. T., P. Varkey, and D. A. Cook. 2013. Mentoring programs for physicians in academic medicine: A systematic review. Academic Medicine 88(7):1029-1037.
Kern, S. E. 2009. Challenges in conducting clinical trials in children: Approaches for improving performance. Expert Review of Clinical Pharmacology 2(6):609-617.
Kerschner, J. E., J. R. Hedges, K. Antman, E. Abraham, E. Colón Negrón, and J. L. Jameson. 2018. Recommendations to sustain the academic mission ecosystem at U.S. medical schools. Academic Medicine 93(7):985-989.
King, A., I. Sharma-Crawford, A. F. Shaaban, T. H. Inge, T. M. Crombleholme, B. W. Warner, H. N. Lovvorn III, and S. G. Keswani. 2013. The pediatric surgeon’s road to research independence: Utility of mentor-based National Institutes of Health grants. Journal of Surgical Research 184(1):66-70.
King, A. A., S. K. Vesely, G. Dadzie, C. Calhoun, A. Cuker, W. Stock, A. Walker, J. Fritz, and L. Sung. 2021. Self-reported positive impact of mentored clinical research training is associated with academic success in hematology. Blood Advances 5(14):2919-2924.
Kliment, C. R., I. J. Barbash, J. S. Brenner, D. Chandra, K. Courtright, M. C. Gauthier, K. M. Robinson, L. P. Scheunemann, F. A. Shah, and J. D. Christie. 2021. COVID-19 and the early-career physician-scientist. Fostering resilience beyond the pandemic. ATS Scholar 2(1):19-28.
Lakshminrusimha, S., S. Murin, J. D. Kirk, Z. Mustafa, T. R. Maurice, N. Sousa, J. Lee, and D. A. Lubarsky. 2022. “Funds flow”: implementation at academic health centers: Unique challenges to pediatric departments. Journal of Pediatrics 249:6-10.e14.
Lampe, M. A., S. R. Nesheim, K. L. Oladapo, A. C. Ewing, J. Wiener, and A. P. Kourtis. 2023. Achieving elimination of perinatal HIV in the United States. Pediatrics 151(5):e2022059604.
Lauer, M. 2019. Outcomes for NIHloan repayment program awardees:A preliminary look. https://nexus.od.nih.gov/all/2019/05/21/outcomes-for-nih-loan-repayment-program-awardees-a-preliminary-look (accessed December 5, 2022).
Lauer, M., and M. A. Bernard. 2023. Mentored career development application (K) funding rates by race-ethnicity FY 2010-FY 2022. https://nexus.od.nih.gov/all/2023/03/16/mentored-career-development-application-k-funding-rates-by-race-ethnicity-fy-2010-fy-2022/ (accessed May 2, 2023).
Lett, E., W. U. Orji, and R. Sebro. 2018. Declining racial and ethnic representation in clinical academic medicine: A longitudinal study of 16 US medical specialties. PLoS ONE 13(11):e0207274.
Lewiss, R. E., J. K. Silver, A. A. Bernstein, A. M. Mills, B. Overholser, and N. D. Spector. 2020. Is academic medicine making mid-career women physicians invisible? Journal of Women’s Health 29(2):187-192.
Ley, T. J., and B. H. Hamilton. 2008. The gender gap in NIH grant applications. Science 322(5907):1472-1474.
Lonhart, J. A., A. R. Edwards, S. Agarwal, B. P. Lucas, and A. R. Schroeder. 2020. Consent rates reported in published pediatric randomized controlled trials. Journal of Pediatrics 227:281-287.
Lovinsky-Desir, S. 2019. Early career investigator biocommentary-pediatric research. Pediatric Research 85(1):6.
Lund, M. J., M. T. Eliason, A. E. Haight, K. C. Ward, J. L. Young, and R. D. Pentz. 2009. Racial/ethnic diversity in children’s oncology clinical trials: Ten years later. Cancer 115(16):3808-3816.
Macy, M. L., K. D. Van, L. K. Leslie, and G. L. Freed. 2020. Engagement in research among pediatric subspecialists at the time of enrollment in maintenance of certification, 2009− 2016. Pediatric Research 87(6):1128-1134.
Masters, J. C., J. A. Cook, G. Anderson, G. Nucci, A. Colzi, M.-P. Hellio, and B. Corrigan. 2022. Ensuring diversity in clinical trials: The role of clinical pharmacology. Contemporary Clinical Trials 118:106807.
McOwen, K. S., A. J. Whelan, and A. L. Farmakidis. 2020. Medical education in the United States and Canada, 2020. Academic Medicine 95(9S):S2-S4.
Mendell, J. R., S. Al-Zaidy, R. Shell, W. D. Arnold, L. R. Rodino-Klapac, T. W. Prior, L. Lowes, L. Alfano, K. Berry, K. Church, J. T. Kissel, S. Nagendran, J. L’Italien, D. M. Sproule, C. Wells, J. A. Cardenas, M. D. Heitzer, A. Kaspar, S. Corcoran, L. Braun, S. Likhite, C. Miranda, K. Meyer, K. D. Foust, A. H. M. Burghes, and B. K. Kaspar. 2017. Single-dose gene-replacement therapy for spinal muscular atrophy. New England Journal of Medicine 377(18):1713-1722.
Menon, S. 2021. Early career investigator: Biocommentary. Pediatric Research 89(5):1051.
Milewicz, D. M., R. G. Lorenz, T. S. Dermody, and L. F. Brass. 2015. Rescuing the physician-scientist workforce: The time for action is now. Journal of Clinical Investigation 125(10):3742-3747.
Milne, C.-P., and J. Davis. 2014. The pediatric studies initiative: After 15 years have we reached the limits of the law? Clinical Therapeutics 36(2):156-162.
Muraro, P. A. 2002. An international solution to recruiting physician-scientists? Nature Medicine 8(9):902-903.
Muslin, A. J., S. Kornfeld, and K. S. Polonsky. 2009. The physician scientist training program in internal medicine at Washington University School of Medicine. Academic Medicine 84(4):468-471.
Mylona, E., L. Brubaker, V. N. Williams, K. D. Novielli, J. M. Lyness, S. M. Pollart, V. Dandar, and S. A. Bunton. 2016. Does formal mentoring for faculty members matter? A survey of clinical faculty members. Medical Education 50(6):670-681.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2019. The science of effective mentorship in STEMM, edited by M. L. Dahlberg and A. Byars-Winston. Washington, DC: The National Academies Press.
NCI (National Cancer Institute). 2023a. Childhood Cancer Data Initiative (CCDI). https://www.cancer.gov/research/areas/childhood/childhood-cancer-data-initiative (accessed March 8, 2023).
NCI. 2023b. Children’s Oncology Group. https://datacatalog.ccdi.cancer.gov/resource/COG (accessed April 3, 2023).
NCI. 2023c. K99/R00—The pathway to independence award. https://www.cancer.gov/grants-training/training/funding/k99 (accessed July 18, 2023).
NCSP (National Clinician Scolars Program). About Us. https://nationalcsp.org/about-us (accessed June 27, 2023).
Nguyen, M., A. Panyadahundi, C. Olagun-Samuel, S. I. Chaudhry, M. M. Desai, A. Dardik, and D. Boatright. 2023. Transition from mentored to independent NIH funding by gender and department. JAMA 329(24):2189-2190.
NIAID (National Institute of Allergy and Infectious Diseases). 2021. How to find the right mentor for early-career awards. https://www.niaid.nih.gov/grants-contracts/find-early-career-awards-mentor (accessed July 17, 2023).
NICHD (National Institute of Child Health and Human Development). 2015. Review of NICHD training and career development programs. https://www.nichd.nih.gov/sites/default/files/2017-09/NICHD_training_review_091615_508.pdf (accessed July 18, 2023).
NICHD. 2017a.Congenitalhypothyroidism (CH). https://www.nichd.nih.gov/about/accomplishments/contributions/hypothyroidism#:~:text=Building%20on%20the%20discoveries%20that%20allowed%20mass%20screening,that%20results%20from%20problems%20with%20the%20thyroid%20gland (accessed November 29, 2022).
NICHD. 2017b. Hemophilus influenza type b (Hib) vaccine. https://www.nichd.nih.gov/about/accomplishments/contributions/hib-vaccine# (accessed November 29, 2022).
NICHD. 2017c. Phenylketonuria (PKU) and newborn screening. https://www.nichd.nih.gov/about/accomplishments/contributions/pku (accessed November 29, 2022).
NICHD. 2017d. Sudden infant death syndrome (SIDS). https://www.nichd.nih.gov/health/topics/sids (accessed December 19, 2022).
NICHD. 2022a. About BPCA. https://www.nichd.nih.gov/research/supported/bpca/about (accessed December 1, 2022).
NICHD. 2022b. Best Pharmaceuticals for Children Act (BPCA). https://www.nichd.nih.gov/research/supported/bpca (accessed December 1, 2022).
NICHD. 2022c. NIH Pediatric Research Consortium (N-PERC). https://www.nichd.nih.gov/research/supported/nperc (accessed December 2, 2022).
NICHD. 2022d. Safe to sleep. https://safetosleep.nichd.nih.gov/ (accessed December 19, 2022).
NICHD. 2023a. Institutional training grants (T32/K12). https://www.nichd.nih.gov/grants-contracts/training-careers/extramural/institutional (accessed April 28, 2023).
NICHD. 2023b. Career development (K) awards. https://www.nichd.nih.gov/grants-contracts/training-careers/extramural/career (accessed April 28, 2023).
NIH (National Institutes of Health). 2014. Physician-scientist workforce working group report. https://acd.od.nih.gov/documents/reports/PSW_Report_ACD_06042014.pdf (accessed April 27, 2023).
NIH. 2017a. Clarifying percent effort and support for career development (K) awardees. https://nexus.od.nih.gov/all/2017/10/11/clarifying-percent-effort-and-support-for-career-development-k-awardees/ (accessed July 21, 2023).
NIH. 2017b. Reporter. https://projectreporter.nih.gov/reporter.cfm (accessed December 13, 2022).
NIH. 2021. The Advisory Committee to the Director Working Group on Diversity Racism in Science Report. https://www.acd.od.nih.gov/documents/presentations/02262021DiversityReport.pdf (accessed May 8, 2023).
NIH. 2022a. Diversity and inclusion in clinical trials. https://www.nimhd.nih.gov/resources/understanding-health-disparities/diversity-and-inclusion-in-clinical-trials.html (accessed December 21, 2022).
NIH. 2022b. Populations underrepresented in the extramural scientific workforce. https://diversity.nih.gov/about-us/population-underrepresented (accessed July 10, 2023).
NIH. 2022c. Extramural loan repayment program for pediatric research (LRP-PR). https://grants.nih.gov/grants/guide/notice-files/NOT-OD-22-149.html (accessed May 8, 2023).
NIH. 2022d. UNITE. https://www.nih.gov/ending-structural-racism/unite (accessed December 5, 2022).
NIH. 2023a. Comparing popular research project grants—R01, R03, and R21. https://www.niaid.nih.gov/grants-contracts/research-project-grants (accessed February 7, 2023).
NIH. 2023b. Estimates of funding for various research, condition, and disease categories (RCDC). https://report.nih.gov/funding/categorical-spending#/ (accessed April 3, 2023).
NIH. 2023c. Success rates. https://report.nih.gov/funding/nih-budget-and-spending-data-past-fiscal-years/success-rates (accessed April 4, 2023).
NIH. 2023d. NIH LRP dashboard. https://dashboard.lrp.nih.gov/app/#/ (accessed April 25, 2023).
Nikaj, S., and P. K. Lund. 2019. The impact of individual mentored career development (K) awards on the research trajectories of early-career scientists. Academic Medicine 94(5):708-714.
NRC (National Research Council) and IOM. 2004. Children’s health, the nation’s wealth: Assessing and improving child health. Washington, DC: The National Academies Press.
NRMP (National Resident Matching Program). 2023. Results and data: 2023 main residency match. https://www.nrmp.org/wp-content/uploads/2023/05/2023-Main-Match-Results-and-Data-Book-FINAL.pdf (accessed July 18, 2023).
Okeigwe, I., C. Wang, J. A. Politch, L. J. Heffner, and W. Kuohung. 2017. Physician-scientists in obstetrics and gynecology: Predictors of success in obtaining independent research funding. American Journal of Obstetrics and Gynecology 217(1):84. e81-84, e88.
Page, S. 2007. The difference: How the power of diversity creates better groups, firms, schools, and societies. Princeton, NJ: Princeton University Press.
Paik, J. C., G. Howard, and R. G. Lorenz. 2009. Postgraduate choices of graduates from medical scientist training programs, 2004–2008. JAMA 302(12):1271-1273.
Patient Centered Outcomes Research Institute (PCORI). 2023. Children’s health. https://www.pcori.org/topics/childrens-health (accessed May 3, 2023).
Permar, S. R., R. A. Ward, K. J. Barrett, S. A. Freel, R. A. Gbadegesin, C. D. Kontos, P. J. Hu, K. E. Hartmann, C. S. Williams, and J. M. Vyas. 2020. Addressing the physician-scientist pipeline: Strategies to integrate research into clinical training programs. Journal of Clinical Investigation 130(3):1058-1061.
PhRMA (Pharmaceutical Research and Manufacturers of America). 2020. Medicines in development | 2020 report. https://phrma.org/-/media/Project/PhRMA/PhRMA-Org/PhRMAOrg/PDF/MID-Reports/MID-2020-Children_8_FINAL.pdf (accessed April 27, 2023).
Pickering, C. R., R. C. Bast, Jr., and K. Keyomarsi. 2015. How will we recruit, train, and retain physicians and scientists to conduct translational cancer research? Cancer 121(6): 806-816.
Pololi, L. H., A. T. Evans, J. T. Civian, L. A. Cooper, B. K. Gibbs, K. Ninteau, R. K. Dagher, K. Bloom-Feshbach, and R. T. Brennan. 2023. Are researchers in academic medicine flourishing? A survey of midcareer Ph.D. and physician investigators. Journal of Clinical and Translational Science 7(1):e105.
Popkin, R., P. Taylor-Zapata, and D. W. Bianchi. 2022. Physician bias and clinical trial participation in underrepresented populations. Pediatrics 149(2).
Price Rapoza, M., A. McElvaine, M. B. Conroy, K. Okuyemi, N. Rouphael, S. J. Teach, M. Widlansky, C. Williams, and S. R. Permar. 2022. Early outcomes of a new NIH program to support research in residency. Academic Medicine 97(9):1305-1310.
Priest, N., Y. Paradies, B. Trenerry, M. Truong, S. Karlsen, and Y. Kelly. 2013. A systematic review of studies examining the relationship between reported racism and health and wellbeing for children and young people. Social Science & Medicine 95:115-127.
Pursley, D. M., T. D. Coyne-Beasley, G. L. Freed, L. R. Walker-Harding, and J. L. Wright. 2020. “Organizational solutions: Calling the question” APS racism series: At the intersection of equity, science, and social justice. Pediatric Research 88(5):702-703.
Puumala, S. E., K. M. Burgess, A. B. Kharbanda, H. G. Zook, D. M. Castille, W. J. Pickner, and N. R. Payne. 2016. The role of bias by emergency department providers in care for American Indian children. Medical Care 54(6):562.
Ragsdale, J. R., L. M. Vaughn, and M. Klein. 2014. Characterizing the adequacy, effectiveness, and barriers related to research mentorship among junior pediatric hospitalists and general pediatricians at a large academic institution. Hospital Pediatrics 4(2):93-98.
Rees, C. A., M. C. Monuteaux, V. Herdell, E. W. Fleegler, and F. T. Bourgeois. 2021. Correlation between National Institutes of Health funding for pediatric research and pediatric disease burden in the US. JAMA Pediatrics 175(12):1236-1243.
Rees, C. A., A. Stewart, S. D. Mehta, E. Avakame, J. Jackson, J. McKay, E. N. Portillo, K. Michelson, C. P. Duggan, and E. W. Fleegler. 2022. Race and ethnicity in published pediatric clinical trial enrollment in the United States, 2011–2020. Pediatrics 149(1 Meeting Abstracts February 2022):629-629.
Research America. 2022. U.S. investments in medical and health research and development: 2016-2020. https://www.researchamerica.org/wp-content/uploads/2022/09/ResearchAmerica-Investment-Report.Final_.January-2022-1.pdf (accessed July 18, 2023).
Rosenberg, L. E. 1999. The physician-scientist: An essential—and fragile—link in the medical research chain. Journal of Clinical Investigation 103(12):1621-1626.
Rosenthal, S. L. 2020. Preventing burnout among academic medicine leaders: Experiencing leadership flow. JAMA Pediatrics 174(7):636-638.
Rubenstein, R., and J. Kreindler. 2014. On preventing the extinction of the physician-scientist in pediatric pulmonology. Frontiers in Pediatrics 2.
Saboor, S., S. Naveed, A. M. Chaudhary, M. Jamali, M. Hussain, J. Siddiqi, and F. Khosa. 2022. Gender and racial profile of the academic pediatric faculty workforce in the United States. Cureus 14(2):e22518.
Sachs, A. N., D. Avant, C. S. Lee, W. Rodriguez, and M. D. Murphy. 2012. Pediatric information in drug product labeling. JAMA 307(18):1914-1915.
Salas, A. A. 2020. Early career investigator highlight biocommentary. Pediatric Research 88(5):688.
Salata, R. A., M. W. Geraci, D. C. Rockey, M. Blanchard, N. J. Brown, L. J. Cardinal, M. Garcia, M. P. Madaio, J. D. Marsh, and R. F. Todd, III. 2018. US physician-scientist workforce in the 21st century: Recommendations to attract and sustain the pipeline. Academic Medicine 93(4):565.
SCDAA (Sickle Cell Disease Association of America). 2021. FAQs: Sickle cell disease. https://www.sicklecelldisease.org/sickle-cell-health-and-disease/faqs/ (accessed December 19, 2022).
Schafer, A. I. 2010. The vanishing physician-scientist? Translational Research 155(1):1-2.
Sekar, K. 2023. National Institutes of Health (NIH) funding: FY1996-FY2023. https://sgp.fas.org/crs/misc/R43341.pdf (accessed May 3, 2023).
Shakhnovich, V., C. P. Hornik, G. L. Kearns, J. Weigel, and S. M. Abdel-Rahman. 2019. How to conduct clinical trials in children: A tutorial. Clinical and Translational Science 12(3):218-230.
Siebert, A. L., S. Chou, O. Toubat, A. J. Adami, H. Kim, D. Daye, and J. M. Kwan. 2020. Factors associated with underrepresented minority physician scientist trainee career choices. BMC Medical Education 20(1):422.
Singh, U., J. Levy, W. Armstrong, R. Bedimo, C. B. Creech, E. Lautenbach, K. J. Popovich, J. Snowden, J. M. Vyas, H. M. A. Infectious Diseases Society of America, and P. I. D. Society. 2018. Policy recommendations for optimizing the infectious diseases physician-scientist workforce. The Journal of Infectious Diseases 218(suppl_1):S49-S54.
Somekh, I., E. Somekh, M. Pettoello-Mantovani, and R. Somech. 2019. The clinician scientist, a distinct and disappearing entity. Journal of Pediatrics 212:252-253.e252.
Sorkness, C. A., C. Pfund, E. O. Ofili, K. S. Okuyemi, J. K. Vishwanatha, and on behalf of the NRMN team. 2017. A new approach to mentoring for research careers: The national research mentoring network. BMC Proceedings 11(Suppl 12):22.
Sotirin, P., and S. M. Goltz. 2023. Mid-career faculty peer mentoring: Rationale and program design. New Directions for Higher Education 2023(201-202):5-19.
Speer, E. M., L. K. Lee, F. T. Bourgeois, D. Gitterman, W. W. Hay, Jr., J. M. Davis, and J. R. Javier. 2023. The state and future of pediatric research-an introductory overview: The state and future of pediatric research series. Pediatric Research:1-5.
Spong, C. Y., and D. W. Bianchi. 2018. Improving public health requires inclusion of underrepresented populations in research. JAMA 319(4):337-338.
Steinbach, W. J., D. K. Benjamin, Jr., and J. W. Sleasman. 2018. Funding pediatric subspecialty training: Are T32 grants the future? The Journal of Pediatrics 202:4-7.e1.
Steinbrook, R. 2002. Testing medications in children. New England Journal of Medicine 347(18):1462-1470.
Sun, L. R. 2021. Early career investigator highlight biocommentary. Pediatric Research 89(4):718.
Tejeda, H. A., S. B. Green, E. L. Trimble, L. Ford, J. L. High, R. S. Ungerleider, M. A. Friedman, and O. W. Brawley. 1996. Representation of African-Americans, Hispanics, and Whites in National Cancer Institute cancer treatment trials. Journal of the National Cancer Institute 88(12):812-816.
Teshima, J., A. J. S. McKean, M. T. Myint, S. Aminololama-Shakeri, S. V. Joshi, A. L. Seritan, and D. M. Hilty. 2019. Developmental approaches to faculty development. Psychiatric Clinics of North America 42(3):375-387.
Thompson, B. M., E. M. Moser, J. D. Gonzalo, D. R. Wolpaw, T. D. Dreibelbis, and T. M. Wolpaw. 2020. Penn State College of Medicine. Academic Medicine 95(9S):S434-S438.
Thomson, D., L. Hartling, E. Cohen, B. Vandermeer, L. Tjosvold, and T. P. Klassen. 2010. Controlled trials in children: Quantity, methodological quality and descriptive characteristics of pediatric controlled trials published 1948–2006. PLoS One 5(9).
Tisdale, S., and L. Pellizzoni. 2015. Disease mechanisms and therapeutic approaches in spinal muscular atrophy. Journal of Neuroscience 35(23):8691-8700.
Todd III, R. F., R. A. Salata, M. E. Klotman, M. L. Weisfeldt, J. T. Katz, S. X. Xian, D. P. Hearn, and R. S. Lipner. 2013. Career outcomes of the graduates of the American Board of Internal Medicine research pathway, 1995–2007. Academic Medicine 88(11):1747-1753.
Trachtenberg, F. L., E. A. Haas, H. C. Kinney, C. Stanley, and H. F. Krous. 2012. Risk factor changes for sudden infant death syndrome after initiation of Back-to-Sleep campaign. Pediatrics 129(4):630-638.
Trent, M., D. G. Dooley, J. Dougé, R. M. Cavanaugh, A. E. Lacroix, J. Fanburg, M. H. Rahmandar, L. L. Hornberger, M. B. Schneider, and S. Yen. 2019. The impact of racism on child and adolescent health. Pediatrics 144(2).
Twombly, D. A., S. L. Glavin, J. Guimond, S. Taymans, C. Y. Spong, and D. W. Bianchi. 2018. Association of National Institute of Child Health and Human Development career development awards with subsequent research project grant funding. JAMA Pediatrics 172(3):226-231.
Varnell, C. D., Jr., P. Margolis, J. Goebel, and D. K. Hooper. 2023. The learning health system for pediatric nephrology: Building better systems to improve health. Pediatric Nephrology 38(1):35-46.
Vasylyeva, T. L., M. E. Díaz-González de Ferris, D. S. Hains, J. Ho, L. A. Harshman, K. J. Reidy, T. M. Brady, D. M. Okamura, D. V. Samsonov, and S. E. Wenderfer. 2019. Developing a research mentorship program: The American Society of Pediatric Nephrology’s experience. Frontiers in Pediatrics 7:155.
Vidyasagar, D. 2007. Integrating international medical graduates into the physician-scientist pool: Solution to the problem of decreasing physician-scientists in the United States. Journal of Investigative Medicine 55(8):406-409.
Viergever, R. F., and C. M. Rademaker. 2014. Finding better ways to fill gaps in pediatric health research. Pediatrics 133(4):e824-e826.
Wade, C. 2021. Physician–scientists in the era of COVID-19: Gone but not forgotten. Academic Medicine 96(1):e5-e6.
Walensky, R. P., Y. Kim, Y. Chang, B. C. Porneala, M. N. Bristol, K. Armstrong, and E. G. Campbell. 2018. The impact of active mentorship: Results from a survey of faculty in the department of medicine at massachusetts general hospital. BMC Medical Education 18(1):108.
Walker-Harding, L. R., C. W. Bogue, K. D. Hendricks-Munoz, J. L. Raphael, and J. L. Wright. 2020. “Challenges and opportunities in academic medicine” APS racism series: At the intersection of equity, science, and social justice. Pediatric Research 88(5):699-701.
Walsh, C., and L. F. Ross. 2003. Are minority children under-or overrepresented in pediatric research? Pediatrics 112(4):890-895.
Watson, S. E., P. Smith, J. Snowden, V. Vaughn, L. Cottrell, C. A. Madden, A. S. Kong, R. McCulloh, C. Stack Lim, M. Bledsoe, K. Kowal, M. McNally, L. Knight, K. Cowan, and E. Yakes Jimenez. 2022. Facilitators and barriers to pediatric clinical trial recruitment and retention in rural and community settings: A scoping review of the literature. Clinical and Translational Science 15(4):838-853.
Williams, C. S., A. N. Iness, R. M. Baron, O. A. Ajijola, P. J. Hu, J. M. Vyas, R. Baiocchi, A. J. Adami, J. M. Lever, P. S. Klein, L. Demer, M. Madaio, M. Geraci, L. F. Brass, M. Blanchard, R. Salata, and M. Zaidi. 2018. Training the physician-scientist: Views from program directors and aspiring young investigators. JCI Insight 3(23).
Williams, C. S., W. K. Rathmell, J. M. Carethers, D. M. Harper, Y. M. D. Lo, P. J. Ratcliffe, and M. Zaidi. 2022. A global view of the aspiring physician-scientist. eLife 11:e79738.
Winestone, L. E., K. D. Getz, P. Rao, Y. Li, M. Hall, Y.-S. V. Huang, A. E. Seif, B. T. Fisher, and R. Aplenc. 2019. Disparities in pediatric acute myeloid leukemia (AML) clinical trial enrollment. Leukemia & Lymphoma 60(9):2190-2198.
Woodward, L. J., L. J. Horwood, B. A. Darlow, and S. Bora. 2022. Visuospatial working memory of children and adults born very preterm and/or very low birth weight. Pediatric Research 91(6):1436-1444.
Wright, J. L., J. N. Jarvis, L. M. Pachter, and L. R. Walker-Harding. 2020. “Racism as a public health issue” APS racism series: At the intersection of equity, science, and social justice. Pediatric Research 88(5):696-698.
Wyngaarden, J. B. 1979. The clinical investigator as an endangered species. New England Journal of Medicine 301(23):1254-1259.
Yap, K. K. 2012. The clinician-scientist: Uniquely poised to integrate science and medicine. Australian Medical Studies J 3:10-11.
Yin, H. L., J. Gabrilove, R. Jackson, C. Sweeney, A. M. Fair, and R. Toto. 2015. Sustaining the clinical and translational research workforce: Training and empowering the next generation of investigators. Academic Medicine 90(7):861-865.
Zemlo, T. R., H. H. Garrison, N. C. Partridge, and T. J. Ley. 2000. The physician‐scientist: Career issues and challenges at the year 2000. FASEB Journal 14(2):221-230.
Zhong, Y., X. Zhang, L. Zhou, L. Li, and T. Zhang. 2021. Updated analysis of pediatric clinical studies registered in clinicaltrials.gov, 2008–2019. BMC Pediatrics 21(1):1-14.
This page intentionally left blank.

























































