Developing a Guide for On-Bridge Stormwater Treatment Practices (2024)
Chapter: Appendix D: Task 3 Laboratory Report 2: Water Quality Column Study
List of Figures
Figure 14 Ortho-Phosphate Concentrations Measured at the Effluent (A3) for Experiment 63-1
List of Tables
Table 2 Summary of Four Column Experiments Conducted with Selected Media
Table 3 Influent Water Quality for the Column Experiments
Table 4 LCRA Analysis of Final Column Effluent Samples
Table 5 Summary of Final Effluent Water Quality Data for Experiment 14-1
Table 8 Summary of Dissolved Cu, Zn and O-Phosphate in the Column Experiments
Table 9 Selected Summary of 6PPD-Quinone Concentrations in Stormwater and Urban Roadways
1. Introduction
The water quality testing study employed a series of column experiments to evaluate the water quality treatment performance of the recommended non-proprietary media blend described in Interim Report I and in the Laboratory Permeability and Clogging Study (Laboratory Report 1). Since the main goal of the research project is to develop a best management practice (BMP) with a footprint small enough to install on a bridge, which requires a relatively high treatment rate, this study evaluated pollutant removal performance in a system with a loading rate of 50 in/hr and compared removals at this higher rate to removal at more typical loadings of 14 in/hr. A media similar to the selected blend has been tested extensively at approximately 14 in/hr. Therefore, the comparison in this study between performance at 14 in/hr and 50 in/hr is intended to determine how increased loading affects treatment performance compared to a baseline that has been more thoroughly tested in field conditions.
Performance of the media blend for removal of dissolved copper and dissolved zinc is of primary interest with dissolved phosphorus of secondary interest. The metrics for removal of these contaminants were based on benchmarks set by the Washington State Department of Ecology.
Performance for zinc and copper was be compared to Washington State Technology Assessment Protocol Ecology (TAPE) benchmarks (Washington State Department of Ecology, 2018):
- 30 percent removal of dissolved copper for the influent concentration range of 0.005 mg/L to 0.02 mg/L, and
- 60 percent removal of dissolved zinc for the influent concentration range of 0.02 mg/L to 0.30 mg/L
In addition, the research team identified 6PPD-quinone as an emerging constituent of concern for inclusion as another secondary objective, because it is associated with tire wear debris and has been demonstrated to be toxic to a variety of fish species (Varshney et al., 2022, Brinkman et al., 2022). In contrast to the permeability and clogging study, this water quality study focused on removal of dissolved solutes. While both dissolved and total metal and phosphorus concentrations were analyzed from effluent samples, the influent water was settled to avoid clogging of the columns during the testing period, therefore the total concentrations reported herein are not intended to representative of highway runoff.
2. Filtration Media Description
The water quality testing employed the same batch of filtration media described in the permeability and clogging study. Media components were obtained and mixed in coordination with Walrath Landscape Supply in Tacoma, Washington. The mix consisted of the following components by volume:
- Sand (60%) – Lane Mountain 20/30 Silica Sand. This sand product has a tight particle size distribution, with particulates mostly retained on the #20 (0.85 mm) and #30 (0.6 mm) sieves. It is substantially coarser than typical bioretention sand but has little capacity for metal ion adsorption.
- Coconut Coir Pith (20%) – Botanicare Cocogro (owned by ScottsMiracle-Gro, Inc.). Coconut Coir Pith is a high surface area material derived from coconut husk and contains hydroxyl, alkyl and carboxyl functional groups. Previous studies have shown this to be a promising adsorbent for divalent metal ions such as copper and zinc (e.g., Tirpack et al., 2021; Swarnalatha and Ayoob, 2014).
- Granular Activated Carbon (15%) – Carbon Activated Corporation COL-L 60 Coal Base Reagglomorated Activated Carbon. This product is designed for water purification. This is the primary GAC stocked by Walrath for stormwater treatment applications with an 8x30 gradation, meaning it primarily contains particles passing the #8 (2.36 mm) sieve and retained on the #30 (0.6 mm) sieve. Coal Based Activated carbons have a high surface area with significant potential for adsorption of hydrophobic contaminants and potential for removing copper and zinc (e.g., Shahrokhi-Shahraki et al., 2021)
- Activated Alumina (5%) - Axens Solutions ActiGuard AAFS50. This product is an iron enhanced activated alumina. It has a gradation of 0.5 to 2.5 mm (similar to 8x30, but not identical). It has potential for removal of phosphorus, copper and lead (Scott et al., 2020).
3. Highway Runoff Collection
Stormwater was collected from a hazardous material trap (HMT) located in a BMP exclusively receiving highway runoff from Loop 360 in Austin, TX on two occasions. The first was on 1/25/23 when 1.16 inches of rainfall was reported. The second sample was collected on 4/21/23 after a storm event of 0.94 inches. Both of these events occurred during periods of normal rainfall and prior to the drought conditions Austin experienced during the summer of 2023.
The stormwater was placed in a series of 5-gallon buckets and stored at 4 C prior to use. Stormwater from the first sampling event was used for the first three experiments and stormwater from the second sampling event was used for the final experiment. In experiment one individual buckets were used sequentially to operate the column. In subsequent experiments, the buckets required for the entire experiment were mixed in a large plastic container and pumped to the columns from the container. The HMT is an 8,000-gallon basin that captures the first flush of runoff and retains it until maintenance staff drains it. Its purpose is to capture spills (when not raining), but to the research team’s knowledge, there has not been a spill at that location. The highway runoff was collected at two different time frames. Most of the column studies conducted in this work (14 and 50 in/hr) utilized water from the first collection period. The final column study (50 in/hr) utilized water from the second collection period. Water quality composition was determined for both waters.
This site was previously used to compare the quality of stormwater from the highway with that being discharged directly from the bridge (Malina et al., 2005). Table 1 compares the flow weighted concentrations from the bridge and highway approach. A statistical analysis comparing runoff quality at the two sites shows that the highway runoff had either higher concentrations or was not significantly different from the bridge discharge.
Table 1 Mean and Median Concentrations of Constituents in Runoff from the Bridge and Approach Highway from Malina et al., 2005
| Constituent | Units | Bridge | Highway Approach | ||
|---|---|---|---|---|---|
| Average | Median | Average | Median | ||
| Copper, Total | µg/L* | 16.4 | 12.9 | 23.5 | 21.9 |
| Copper, Dissolved | µg/L* | 4.24 | 3.60 | 6.46 | 5.69 |
| Lead, Total | µg/L* | 9.93 | 8.90 | 13.1 | 13.7 |
| Lead, Dissolved | µg/L* | n/a | n/a | n/a | n/a |
| Zinc, Total | µg/L* | 167 | 168 | 135 | 130 |
| Zinc, Dissolved | µg/L* | 28.8 | 28.0 | 30.7 | 29.1 |
| Nitrogen, Nitrate (As N) | mg/L | 0.345 | 0.290 | 0.399 | 0.361 |
| Nitrogen, Kjeldahl, Total | mg/L | 0.970 | 1.03 | 1.54 | 1.29 |
| Chemical Oxygen Demand | mg/L | 33.3 | 24.0 | 56.2 | 50.5 |
| Phosphorus, Total (As P) | mg/L | 0.107 | 0.090 | 0.142 | 0.125 |
| Phosphorus, Dissolved (As P) | mg/L | 0.071 | 0.050 | 0.076 | 0.060 |
| Suspended Solids - Total | mg/L | 112 | 91.0 | 119 | 123 |
| Suspended Solids - Volatile | mg/L | 21.3 | 19.0 | 25.0 | 26.0 |
| Fecal Coliform | cfu/100 mL | 5550 | 5500 | 4925 | 4650 |
| Oil & Grease, Total Recoverable | mg/L | 4.79 | 4.76 | 6.24 | 5.64 |
* to convert to mg/L, divide μg/L by 1000 μg/mg
n/a : Indicates that there were insufficient detections of this constituent to allow for statistics to be calculated
4. Experimental Approach and Setup
The experimental approach involved operating three parallel column trains with two intermediate sampling points and one final effluent sampling point in each column train as shown in Figure 1. Four separate experiments were conducted. A preliminary test at a loading rate of 14 in/hr was conducted with three replicate PVC columns packed with media. Previously, this loading rate has been used to test similar media blends1. A second confirmatory test was conducted at 14 in/hr to validate the results from the first experiment. All of the columns were packed with media in this experiment as well. A third experiment was conducted at 50 in/hr loading using the PVC column setup. In this third experiment, two of the column trains contained the selected media and one column was packed with silica to serve as a control because sand does not adsorb significant amounts of dissolved metals or phosphorus. In the fourth and final experiment, only one column train was operated as the stormwater supply was nearly exhausted and there was a persistent drought affecting Austin, TX. In this final column experiment, we used a Teflon column train and appurtenances and monitored both the 6PPD-quinone as well as copper, zinc and phosphorus. A summary of the experiment conditions is shown in Table 2. The alphabetic
___________________
1 Per Interim Report 1, media similar to the selected blend has been tested with a loading rate of 14 in/hr in research funded by the State of Washington, and in similar studies by members of the project team.
identifiers indicate the different parallel columns. The numeric identifiers indicate the intermediate and final effluent from each column. For example, A3 is the final effluent from the A column. A single influent sample location provides the representative influent for the overall system.
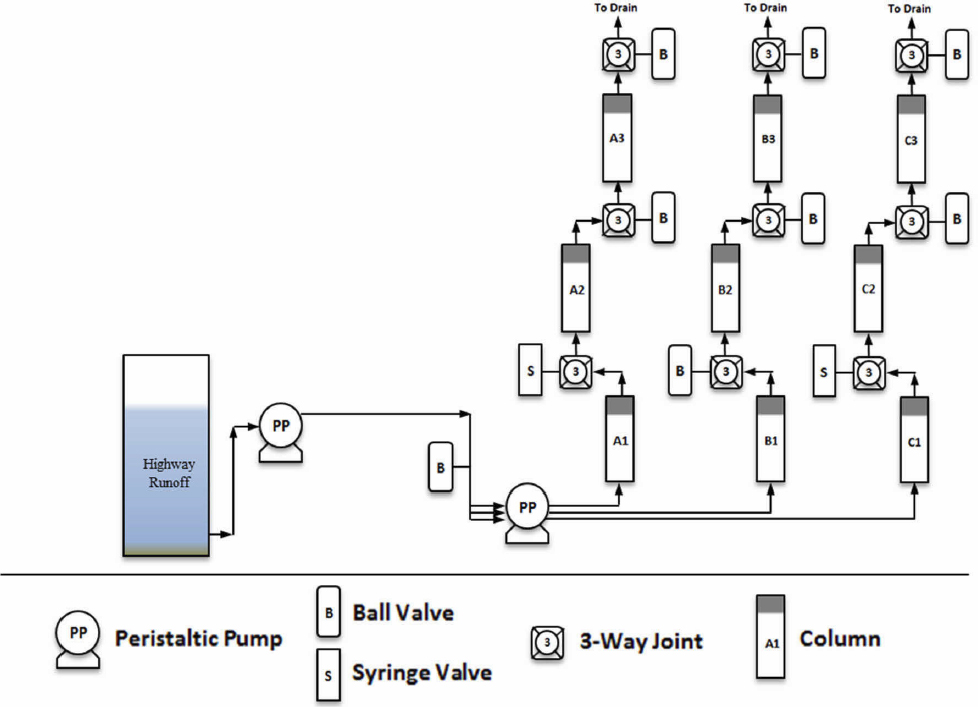
Table 2 Summary of Four Column Experiments Conducted with Selected Media
| Experiment No. | Column Materials | Loading Rate (in/hr) | Design Flowrate (ml/min) | Runtime (hrs) | Total Volume per Column (L) | Primary Analyte |
|---|---|---|---|---|---|---|
| 14-1 | PVC | 14 | 3 | 100 | 18 | Cu |
| 14-2 | PVC | 14 | 3 | 80 | 14.3 | Cu, Zn |
| 50-1 | PVC | 50 | 10.7 | 80 | 51.7 | Cu, Zn, P |
| 63-1 | Teflon* | 63 | 10.7 | 65.5 | 41 | Cu, Zn, P, 6PPD-quinone |
* Note, the Teflon column was 7/8” inside diameter compared to 1” inside diameter for PVC columns, so this resulted in a slightly higher loading rate for the same design flowrate.
The goal of the column studies was to evaluate representative performance over a normal operating cycle. It is likely that the systems will clog well before treatment processes are exhausted (Pitt and Clark, 2010, Pitt and Colyar, 2021). Therefore, this study is not intended to evaluate the
sorption/treatment capacity of the columns but intended to represent typical performance that is expected as media conditions and ages. A normal maintenance cycle is expected to be one year and may be extended to some degree by scraping the surface of the media. Performance of the media up to and through about one year of loading is considered to be representative of normal operations; if significant decline is observed during this period, then testing may be discontinued.
For columns operating at 50 in/hr, we dosed the equivalent of approximately 1 year of runoff to the columns, which is about 4,000 inches treated through the column footprint (i.e., equivalent to a column of water 4,000 inches tall over the footprint of each column). This is based on a representative sizing factor of 300 sq-ft of media bed per acre treated with an average precipitation depth of 30 inches per year. For the columns operating at 14 in/hr, we treated the equivalent of approximately 4 months of runoff, which is equivalent to about 1,400 in of runoff dosed in Experiment 14-1 and approximately 3.4 months of runoff in Experiment 14-2.
Samples for the targeted pollutants (dissolved forms of copper, zinc, and phosphorus, and 6PPD-quinone) were collected at the influent to the columns and effluent from the columns. At least 12 samples for each of the columns were collected for the 50 in/hr experiments. Dissolved metals and 6PPD-quinone samples from both the final effluent and intermediate sampling points were collected and analyzed at UT, but the quality control on the zinc samples was not sufficient for experiments 14-1 and 50-1. Additionally, multiple samples from the final column train effluents were split and analyzed at both the UT lab and a certified external lab for confirmation for metals, total phosphorus and ortho-phosphate. All analysis for 6PPD-quinone was conducted at UT. This sampling frequency applies to the dissolved forms of copper, zinc, phosphorus, and 6PPD-quinone.
Columns were packed wet (distilled-deionized water) with the selected media and the mass within each column was recorded. Each column section (e.g, A1, A2 and A3 as shown in Figure 1) consisted of 6 in of PVC tubing connected to the next section via a three-way tee (to allow sampling at the end of each section). The total column length was 18 inches with a column inside diameter of 1 inch. The last experiment utilized Teflon columns, tubing and appurtenances. The column inner diameter was only 7/8 inch and the column was 18 inches long. A fiberglass mesh screen was placed at the bottom and top of each column sub-section to prevent loss of media from each section. The columns were operated in upflow mode for all experiments. Distilled-deionized water was initially pumped through the system prior to switching to stormwater after the column effluent was clear of residual media particles. Once the influent was switched to stormwater the experiment began.
This report provides a summary of select data from these column experiments. Data for experimental results not presented in the main body of the report are provided in Appendix D.1 and B.
5. Water Quality Analysis
Raw water samples and selected final effluent samples were analyzed at a certified lab (LCRA Environmental Laboratory) prior to each experimental run. Laboratory data reports are available upon request. Due to delays in the project primarily related to COVID-19 (including laboratory
staffing, procurement of supplies and instrument access), there were some deviations from our original analytical plan (we omitted turbidity measurements and measured more of our dissolved metal samples at LCRA). Given that our preliminary tests found no issues with clogging and the influent water was pre-settled, there was no need to measure turbidity as a surrogate for TSS. In addition, we sent a larger number of samples to LCRA for analysis than originally planned and focused efforts at UT on dissolved metals and pH in the first three experiments and dissolved metals, pH and 6PPD-quinone in the final experiment.
Four separate analyses were conducted of the raw water. Table 3 shows the parameter values observed. Variations in the concentrations are attributed to deviations among the buckets of water stored. As noted above, these analyses were performed on settled water with relatively little suspended material remaining, so it is expected that dissolved and total metals and phosphorus values will be similar. The increase in pH and ammonia and decrease in nitrate suggest that some biological activity occurred during storage of the water in the 4°C constant temperature room.
Table 3 Influent Water Quality for the Column Experiments
| Parameter | MRL | Raw Water 1 | Raw Water 1 | Raw Water 1 | Raw Water 2 |
|---|---|---|---|---|---|
| pH | 7.33 | 7.96 | 7.99 | ||
| Alkalinity (mg/L CaCO3) | 20 | 60.8 | 51.2 | 52.9 | 57.7 |
| Ammonia Nitrogen (mg/L as N) | 0.02 | 0.0319 | 0.0655 | 0.256 | |
| Calcium Total (mg/L) | 0.2 | 35.5 | 29.2 | 29 | 31.3 |
| Calcium Dissolved (mg/L) | 0.2 | 25.9 | 28.3 | ||
| Magnesium Total (mg/L) | 0.2 | 1.87 | 1.91 | 1.75 | 1.87 |
| Magnesium Dissolved (mg/L) | 0.2 | 1.68 | 1.84 | ||
| Iron Total (mg/L) | 0.05 | ND | 0.185 | 0.0586 | 0.136 |
| Iron Dissolved (mg/L) | 0.05 | ND | ND | ||
| Copper Total (mg/L) | 1 | 0.012 | 0.00932 | 0.0086 | 0.017 |
| Copper Dissolved (mg/L) | 1 | 0.00748 | 0.0093 | ||
| Zinc Total (mg/L) | 1 | ND | 0.0514 | 0.0274 | 0.0651 |
| Zinc Dissolved (mg/L) | 1 | 0.0411 | 0.0302 | ||
| Total Phosphorus (mg/L as P) | 0.02 | 0.236 | 0.103 | 0.0796 | 0.0605 |
| Ortho-Phosphate (mg/L as P) | 0.01 | 0.2 | 0.0441 | 0.0439 | ND |
| Nitrite/Nitrate (mg/L as N) | 0.02 | 6.12 | 1.18 | 0.788 | |
| Total Organic Carbon (mg/L) | 0.5 | 7.13 | 8.7 | 8.85 | 8.51 |
| TOC, Dissolved (mg/L) | 0.5 | 7.48 | 8.47 | 8.6 |
6. Column Experiments at 14 in/hr loading rate
Experiments one and two (14-1 and 14-2) focused on baseline testing of the column system. Both dissolved copper and zinc were added to the column; however, there appeared to be an analytical issue with the zinc concentrations, so the experiment focused on removal of copper and verification of the column operation.
Preliminary Experiment 14-1.
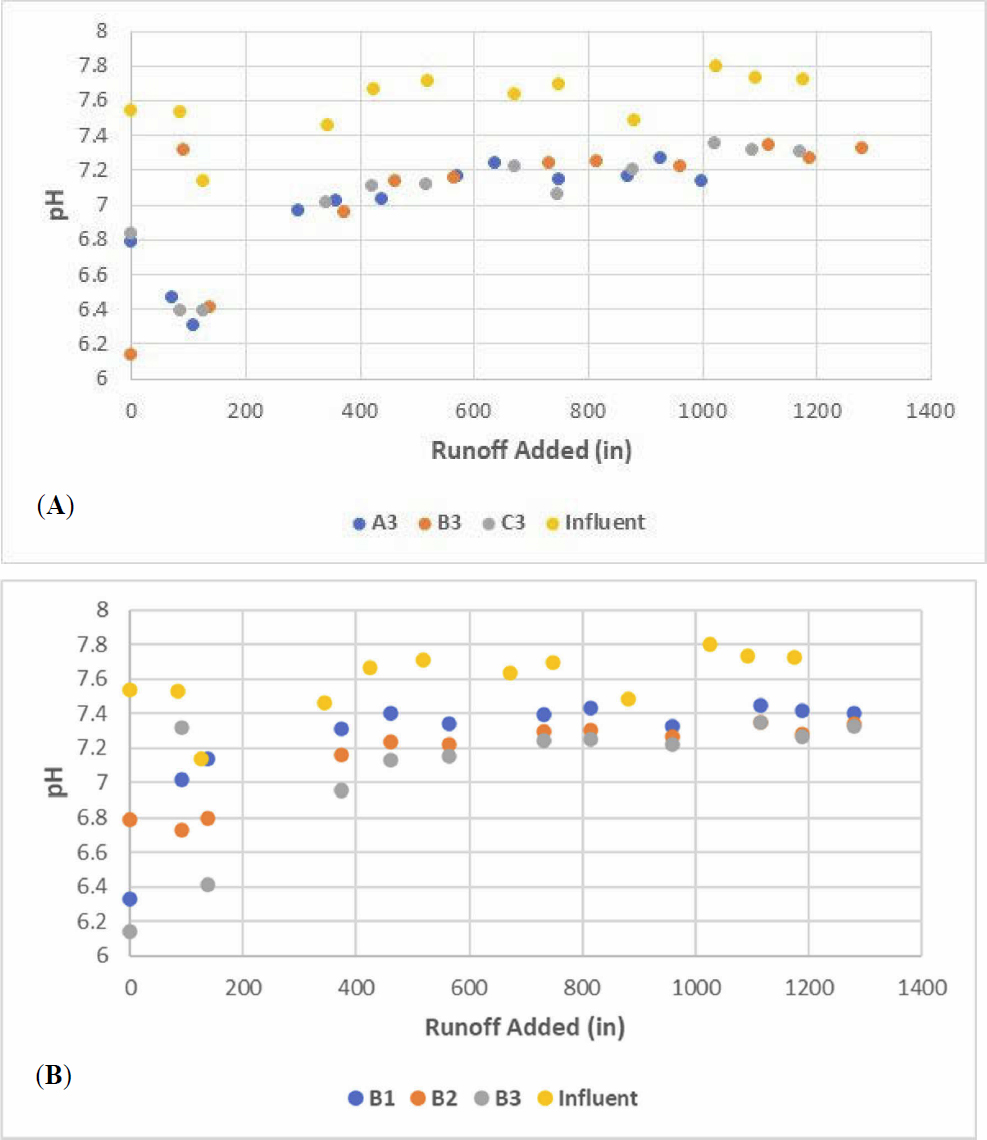
In the preliminary experiment, 14-1, only samples of effluent from A3, B3 and C3 that were taken at the end of the experiment were analyzed by LCRA; pH and dissolved copper data were analyzed at UT for twelve samples. Figure 2 shows that the pH data are consistent among the three parallel column trains and that there is an initial increase in pH as the stormwater passes through the column, displacing the distilled-deionized water used to pack the columns. However, even after 1400 inches of runoff passed through the column, the effluent pH was still slightly lower than the influent pH. The data for dissolved copper measured at the two intermediate sampling points, shown in Figure 3 Intermediate and Final Effluent Fractions of Dissolved Copper Remaining from a Single Column Train (Train A) in Experiment 14-1 for an influent concentration of 10 μ/Land the final effluent point show an expected trend in which concentrations increase over time with higher concentrations at the sampling points closer to the influent as shown in Figure 3 (for example, A1 is the closest sampling point to the influent feed and A3 is the final effluent from the column).

The data in Figure 4 also show that the final effluent concentrations from Columns A, B and C meet the TAPE benchmark removal of 30 percent for dissolved copper. Moreover, the data appear to plateau after about 1000 inches of stormwater runoff has passed; removals were at least 65 percent for all three columns. Differences among the parallel columns are likely due to heterogeneity in packing of the material or variability in the flowrates.

In addition to the dissolved metals and phosphorus analysis performed at UT, water quality analysis was performed at LCRA for on the influent and final effluent based on samples taken at the end of experiment 14-1 (taken after the samples shown in Figure 4). The LCRA analysis results
in Table 4 confirm the ability to remove dissolved copper to TAPE benchmarks and highlight the ability to remove dissolved phosphate using the media.
Table 4 LCRA Analysis of Final Column Effluent Samples
| Dissolved Concentrations of: | Influent Dissolved Conc. |
Column A Effluent Dissolved Conc and (% removal) | Column B Effluent | Column C Effluent |
|---|---|---|---|---|
| Copper (ug/L) | 11 | 3.03 (72%) | 5.10 (54%) | 3.79 (66%) |
| Ortho-Phosphate as P (ug/L) | 200 | 84.6 (48.8%) | 97.6 (51.2%) | 88.0 (56%) |
| Total Phosphorus as P (ug/L) | 236 | 109 (53.8%) | 113 (52.1%) | 98.7(58.2%) |
The results of the preliminary baseline experiment at 14 in/hr also suggested that the experimental setup was capable of performing the testing of dissolved metals removal. Measurements of these copper removals from the final sample sent to LCRA was lower than obtained from the removal reported for the final sample analyzed at UT for Column B, but still met the TAPE benchmarks for dissolved copper. These differences may have been related to the rapid increase in concentration observed in Column A in the UT data at 1400 hours.
Data for phosphorus removal is also shown in Table 4 for the final sample from the column. The data show that at influent dissolved ortho-phosphate concentrations of 200 μg/L as P, removals ranged from 48.8 to 56% across the three columns (based on one final effluent sample). The small differences between dissolved ortho-phosphate and total phosphate are likely due to pre-settling of the stormwater and other forms of dissolved phosphorus besides ortho-phosphate. Therefore, they are likely not reflective of total phosphorus removals from un-settled runoff, which would be higher than the reported value.
In addition to the dissolved metals and phosphorus analysis, a more complete water quality analysis was performed on the influent and final effluent of experiment 14-1 as shown in Table 5. These results suggest that most of the major water quality parameters do not change significantly because of the treatment. However, there is a significant reduction in organic carbon concentrations and a reduction in Mg concentrations after treatment. Two of the three columns show a significant reduction in nitrate/nitrite. The value of nitrate/nitrite from column A3 is inconsistent with the samples from the other two columns as well as the influent data (which only had 1.18 mg/L as N for nitrate/nitrite)
Table 5 Summary of Final Effluent Water Quality Data for Experiment 14-1
| Parameter | MRL | Influent | Effluent A-3 | Effluent B-3 | Effluent C-3 |
|---|---|---|---|---|---|
| Alkalinity (mg/L CaCO3) | 20 | 60.8 | NA | 56.4 | NA |
| Ammonia Nitrogen (mg/L as N) | 0.02 | 0.0319 | 0.214 | 0.202 | 0.231 |
| Calcium Total (mg/L) | 0.2 | 35.5 | 29.1 | 29.8 | 29.9 |
| Calcium Dissolved (mg/L) | 0.2 | 35.6 | 27.6 | 28.6 | 29 |
| Magnesium Total (mg/L) | 0.2 | 1.87 | 1.49 | 1.55 | 1.53 |
| Magnesium Dissolved (mg/L) | 0.2 | 1.9 | 1.46 | 1.49 | 1.5 |
| Iron Total (mg/L) | 0.05 | ND | ND | ND | ND |
| Iron Dissolved (mg/L) | 0.05 | ND | ND | ND | ND |
| Nitrite/Nitrate (mg/L as N) | 0.02 | 6.12 | 12.7 | 0.845 | 0.791 |
| Total Organic Carbon (mg/L) | 0.5 | 7.13 | 2.71 | 2.92 | 4.15 |
| TOC, Dissolved (mg/L) | 0.5 | 7.48 | 3.08 | 3.01 | 4.26 |
Preliminary Experiment 14-2.
For the second experiment conducted at a loading rate of 14 in/hr, pH, dissolved copper and dissolved zinc were analyzed at UT. Triplicate column trains were operated for over 1200 inches of rainfall. Influent flowrates from the column trains A, B and C were 2.54, 3.27 and 2.99 ml/min, respectively. As a result, the number of inches per hour of stormwater added to each column at each sampling point varied slightly.
The trends in pH mimicked those from Experiment 14-1 as shown in Figure 5A. A lower pH was measured in the effluent of all three columns that gradually increased and plateaued to a value that was still lower than the influent pH. Thus, the media reduced the pH of the water at the beginning of the run followed by a difference of approximately 0.4 pH units at the plateau. A decrease in pH is expected when dissolved metals adsorb to oxide media. Moreover, within a single column train, the pH increased more rapidly in the first column stage (e.g., B1) compared to the later stages (see Figure 5B). This result is consistent with expected trends in adsorption to mineral oxides present in the selected media.
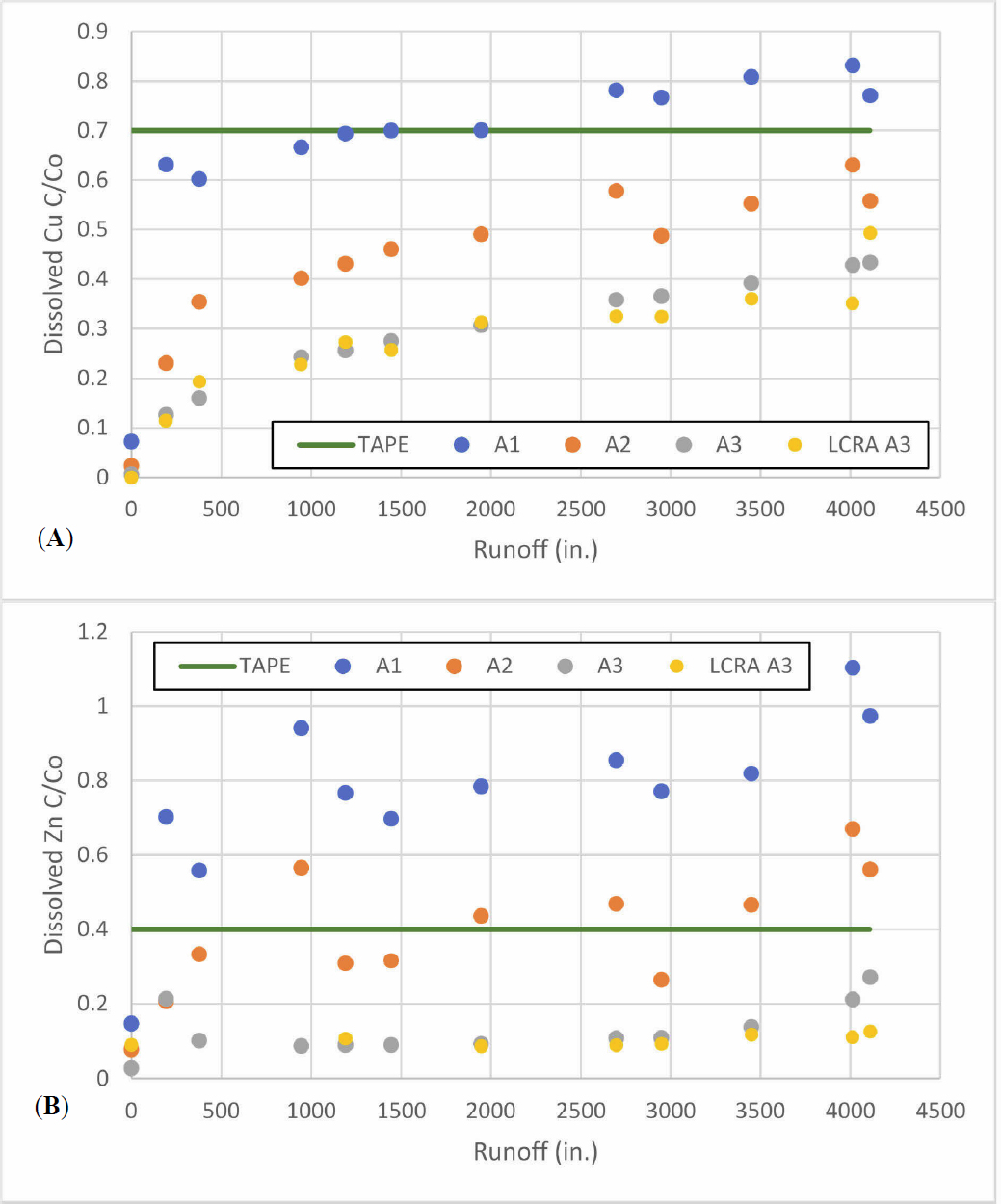
Data for copper removal for the 14-2 experiment are also consistent with the results from experiment 14-1. Dissolved copper and dissolved zinc removal, shown in Figure 6, met the TAPE benchmarks of 30 and 60 percent removal, respectively, but the performance was slightly better than seen in experiment 14-1. Comparison of zinc and copper performance (plotting as the fraction of dissolved metal ion remaining, (Ceffluent/Cinfluent = C/Co) for the parallel column trains shows that copper removal meets the TAPE benchmarks throughout the experimental run
for all three treatment trains, but zinc removal was unstable at the beginning of the run. After a period of acclimation, dissolved zinc data met the TAPE benchmark of 60 percent removal after a start-up period. For dissolved copper, all three column sections met the TAPE benchmarks as shown in Figure 7. In contrast, for dissolved zinc, there was greater variability in the data as shown in Figure 8 in which only the final effluent (A3 and B3) consistently meets the TAPE benchmarks after the first 350 inches of stormwater has been added. Column train B exhibits significantly more variability than column train A. Column train C showed results more similar to column A but fewer data points were available for the intermediate sampling locations.
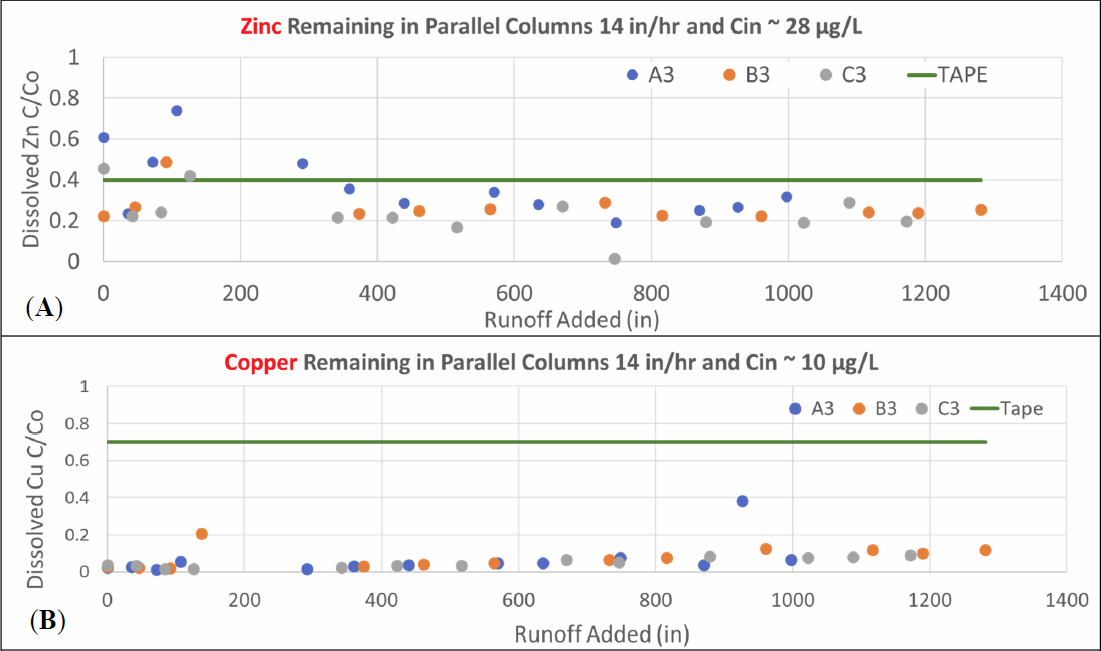


Comparison of the data obtained from the UT labs and LCRA for dissolved copper and dissolved zinc as well as data for ortho phosphate removal is provided in Table 6. The data show small differences in the influent concentrations of the dissolved iron but similar removals for copper. The removal of zinc calculated using the LCRA data is slightly lower than values obtained from UT.
| Influent Feed | Media A | Media B | Media C | Column Average | Removal (%) | |||||||
| LCRA | UT | LCRA | UT | LCRA | UT | LCRA | UT | LCRA | UT | LCRA | UT | |
| Diss. Cu | 12.25 | 8.9 | 2.11 | 1.25 | 1.79 | 1.03 | 1.2 | 0.73 | 1.7 | 1.00 | 86% | 89% |
| Diss. Zn | 33.05 | 26.16 | 13.65 | 6.73 | 9.685 | 6.28 | 9.43 | 5.7 | 10.92 | 6.24 | 67% | 76% |
| O- Phos | 0.066 | 0.140 | 0.152 | 0.165 | 0.152 | |||||||
Of particular note, is that the initial ortho-phosphate value measured as P is lower than the dissolved phosphate value determined from experiment 14-1. Again, this is likely due to variability within the containers. One change made between the two experiments was to mix all of the stormwater needed for an experiment and use a single container for storing the influent throughout the run. Nevertheless, while the data from experiment 14-1 with substantially higher phosphorus (ortho-P = 0.2 mg/L as P) achieved over 50 percent reduction in ortho phosphate from the influent to the final effluent. The data from experiment 14-2 shown in Table 6 shows an increase in ortho-P across all of the treatment trains. This data suggests that dissolved phosphorus is being exported from the media to a certain extent at low phosphorus influent concentrations; in other words the removal of phosphorus by certain components of the media is less than that being leached by other components. The source of ortho phosphate has not been investigated, though low levels of ortho phosphate could be associated with coconut coir, GAC, or sand. Notably, minor increase in ortho phosphate concentration would likely be more than offset by effective removal of particulate bound phosphorus if particulate removal is considered.
7. Column Experiments at High Loading Rates
Two experiments were conducted at higher loading rates. Experiment 50-1 employed a 50 in/hr loading rate to the column. In experiment 50-1, three PVC column trains were operated for 80 hours. Two of the column trains were packed with the selected media and one of the column trains was packed with silica sand. The silica sand was not expected to sorb either the metal ions or phosphate and thus served as a control for experiment. In experiment 63-1, only one teflon column was operated. While both dissolved copper and zinc were also analyzed, a secondary focus of the experiment was to assess whether the media was effective at removing 6PPD-quinone. Unfortunately, it was not possible to find one-inch teflon fittings for 1-inch inner diameter tubing so we employed a 7/8 inner diameter column but maintained a similar flowrate of 10.4 mL/min as the previous column study. This resulted in a loading rate of 63 in/hr (higher than the design test rate). However, given the performance of the 50 in/hr experiment, this was deemed a reasonable challenge to the media. Additionally, to prevent photodegradation of 6PPD-quinone in the
translucent Teflon column, aluminum foil was wrapped around the column and the feed water container was covered.
Experiment 50-1.
Results from experiment 50-1 utilized the same protocol as experiment 14-2 in terms of column preparation, media packing and conditioning with distilled-deionized water for two of the columns. However, column train C was packed with silica sand to serve as a nonsorbing control for dissolved copper and zinc. The data shown in Figure 9 highlight the ability of the media to remove dissolved copper at these higher loading rates. Moreover, the results from UT and the results from LCRA are comparable. In contrast to the two columns trains A and B which are packed with the selected media, column C shows initially high breakthrough that increases to complete (100 percent) breakthrough of copper from the final effluent.
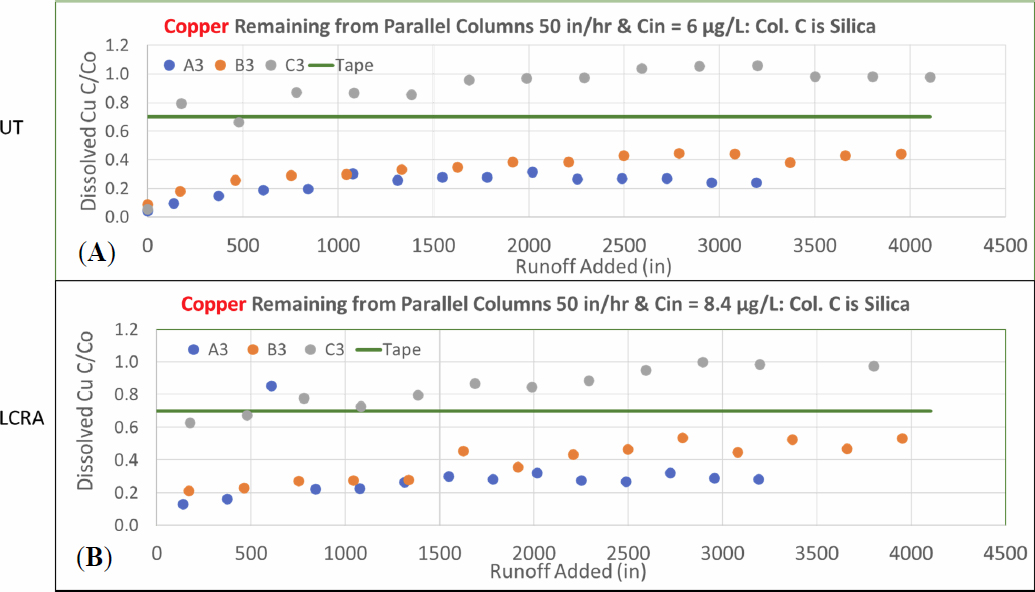
Comparison of dissolved copper removal in Figure 10 from the 14 and 50 in/hr shows slightly less removal for the 50 in/hr column train which also has a lower influent concentration. However, final effluent dissolved copper concentrations from sampling port A3 easily meet the TAPE benchmark of 30 percent reduction and support the hypothesis that this media is capable of meeting TAPE benchmark removal rates of dissolved copper even at these higher treatment rates.

Dissolved zinc removal also met the TAPE benchmarks for the 50 in/hr loading rate in the column trains packed with media for Column B, but the last data point for Column A exceeded the TAPE benchmark. Comparison of total and dissolved zinc values for this last data point is much more consistent with the previous data points. The total zinc concentration reported by LCRA was 6.18 ug/L for the final sample is more consistent with the preceding data and corresponds to over 84% removal. Given that the influent total zinc value (39.4) is similar to the dissolved influent concentration (35.7 ug/L), the final dissolved zinc value appears to be an error. It is also important to note that the dissolved zinc concentrations did not achieve the TAPE benchmark for the column trains packed with silica as shown in Figure 11. The greater percent removal of dissolved zinc compared to dissolved copper is also evident in Figure 11.
Dissolved ortho-phosphate concentrations decreased with increasing time in the 50 in/hr experiment for columns A and B. The initial concentrations of ortho-phosphate in the final effluent from the column trains containing media (A and B) are significantly higher than the influent concentrations and decrease over the course of the experiment. However, even after 4,000 hours, the effluent concentrations remain higher than the influent concentrations, suggesting that the media are exporting phosphorous as previously seen in experiment 14-2. In contrast, the data shown in Figure 12 for the control silica column (column C) display significantly lower concentrations of ortho-phosphate which increase slightly during the experiment with removals plateauing at approximately 20 percent. These results are consistent with results reported for experiment 14-2 but in contrast to experiment 14-1 in which approximately 50% of phosphate removal was observed. These data support the previous conjecture that the selected media do
export phosphorus; however, results suggest that the net release of P is primarily observed when the influent ortho-phosphate concentrations are low.


Comparison of the dissolved ortho-phosphate, copper and zinc concentrations and removals are presented in Table 7 for data analyzed by both UT (copper only) and LCRA. The table highlights the relatively similar removal values obtained from the two labs for copper. Data provided for total zinc removal (analyzed by LCRA) also suggests that removal of total zinc is higher than the dissolved zinc. However, it should be noted that the stormwater had been settled prior to use in the columns and therefore, most of the zinc is in the dissolved form.
| Influent Feed | Media A | Media B | Silica C | Column Average | Removal (%) | |||||||
| Dissolved: | LCRA | UT/LCRA-T | LCRA | UT/LCRA-T | LCRA | UT/LCRA-T | LCRA | UT/LCRA-T | LCRA | UT/LCRA-T | LCRA | UT/LCRA-T |
| Cu(ug/L) | 8.37 | 6 | 2.46 | 1.53 | 4.01 | 2.52 | 11.31 | 5.96 | 3.24 | 2.025 | 61% | 66% |
| Zn (ug/L) | 35.7 | 39.4 | 13.2 | 13 | 8.02 | ND | 41.7 | 24.45 | 10.61 | 13 | 70% | 67% |
| P (mg/L) | 0.044 | 0.108 | 0.075 | 0.035 | 0.092 | |||||||
| NOTE: LCRA-T samples are total zinc values from LCRA | ||||||||||||
Experiment 63-1 Cu, Zn and P Removal.
The second collection of stormwater was used in this experiment which again contained a low concentration of total phosphorus of 0.06 mg/L as P and less than 0.01 mg/L of ortho-phosphate as P. Experiment 63-1 utilized only one column due to a lack of stormwater. The columns and appurtenances were comprised of Teflon which is recommended by an Interagency Report from the State of Washington in a statement for studies with 6PPD-quinone. It is also the material of choice for reducing losses of metal ions (Washington State Department of Ecology). In experiment 63-1, a smaller diameter (2.22 cm) column was employed, but the flow rate was maintained close to that used in experiment 50-1. The resulting column loading rate for stormwater was 63 in/hr. In addition, additional dissolved copper and zinc were added to the column to increase the influent concentrations to 14 and 55 ug/L, respectively. Thus, this final experiment allowed comparison of even higher loading rates with a lower mass of media (consistent with the reduction in column volume compared to column 14-1).
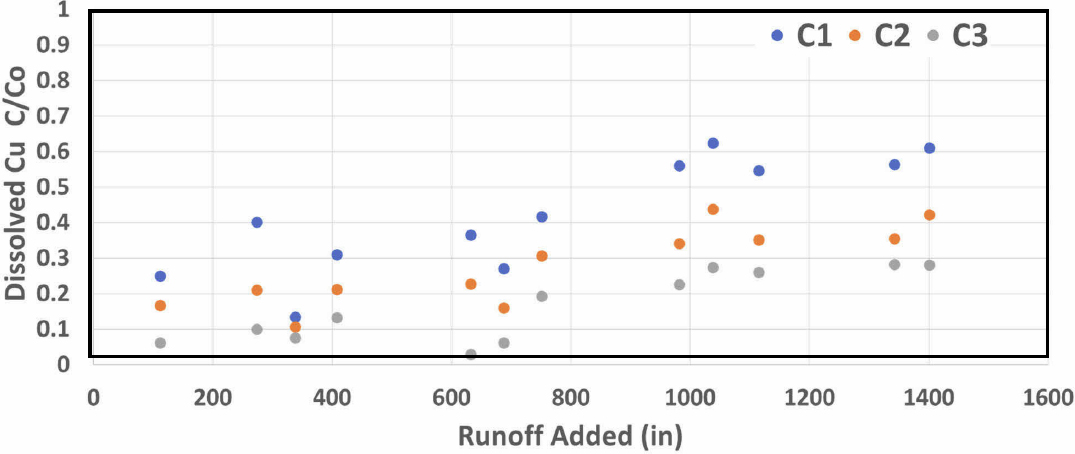
The results from the column test were consistent with the previous experiment at 50 in/hr as shown in Figure 14. With the lower mass and higher loading rate, the copper removal from the effluent was approximately 58 percent at the end of the experiment, a value slightly lower than the 66 percent removal (UT data) observed in experiment 50-1. For zinc, the removals appear to be comparable or even improved slightly compared to the 75 percent removal determined in experiment 50-1. Thus, it appears that the media is capable of meeting the TAPE benchmarks even at these higher loading rates.
The phosphorus data are also consistent with our previous reports. The low concentration of phosphorus led to a net export of phosphorus from the column as shown in Figure 15. The initial high concentration of the first sample is reflective of the media in distilled-deionized water prior to switching to the stormwater. There was a rapid, immediate decrease in the concentration of ortho-phosphate followed by a relatively constant concentration of phosphate throughout the remainder of the experiment at concentrations that ranged from 58 to 84 ug/L.

Preliminary Analytical Method Development and Assessment of Removal of 6PPD-quinone.
6PPD-quinone is introduced to the environment as an oxidation product of 6PPD, which is ubiquitous in rubber products, such as tires, and subsequently deposited on roadways (Benis et al., 2023). Rainwater ultimately carries 6PPD and its degradation products from roadways into nearby streams, with salmon mortality directly linked to traffic activity (Tian et al. 2022).
The analytical method employed for determination of 6PPD-quinone involves solid phase extraction of the aqueous samples followed by liquid-chromatography/mass spectrometry. Method development at UT was conducted in cooperation with Ian Riddington in the Chemistry Department at UT. Standards for 6PPD-Quinone (10 mg, solid) and D5-6PPD-Quinone (100 mg/L, in acetonitrile) were purchased from HPC (Atlanta, GA). A 1000 mg/L stock solution of 6PPD-Quinone was made using 1 mg in 1mL of acetonitrile. Similarly, a 1000 ug/L stock solution of the D5-6PPD-Quinone internal standard used during the analysis was made by diluting the standard in acetonitrile. Methanol (ACS grade) and Acetonitrile (ACS grade) were purchased from Fisher Scientific. Distilled-deionized water (18 MΩ-cm) was generated by a Milli-Q Water System.
Prior to analysis, the stormwater samples were concentrated using solid phase extraction (SPE) of 6PPD-Quinone. Modifications of the method used by Tian, et al. (2022) to reduce the sample volume. SPE cartridges were preconditioned using 5 mL of methanol, followed by 10 mL of DI water. After loading 20 mL of stormwater water, the cartridge was washed with 10 mL of Milli-Q water. The analyte was eluted with 5 mL of methanol and captured in a 5 mL glass concentration vial. The eluate was concentrated to 1 mL using nitrogen gas before being capped in a 2 mL amber vial with teflon septa and stored at 4℃. Extractions were performed in triplicate on a stormwater sample spiked to 40 ug/L resulting in an average of 52 % ± 2.5 % recovery of the analyte.
Quantification of 6PPD-Quinone in the stormwater water was performed using a Shimadzu 8060 LC MS\MS Triple Quadrupole fitted with an Agilent ZORBAX RRHD Eclipse Plus C18 column. The injection volume was 1μL, and a gradient of 0.1% formic acid in water (A) and
acetonitrile (B) was used: 0-6 min 75%(A)/25%(B), 6-7.5 min 5%(A)/95%(B), 7.5-10 min 75%(A)/25%(B). Electrospray ionization (ESI) and multi-reaction monitoring (MRM) were used for detection. A five-point calibration curve from 0.01-100 μg/L was used to determine stormwater concentrations (R2 > 0.999). All stormwaters were spiked with 10 ug/L of the internal standard stock solution to account for instrument variability. An MDL of 0.011 ug/L was determined.
The influent stormwater had an average concentration of 0.13 ug/L ± 0.027 ug/L. To ensure quantifiable concentrations at the effluent points of the column, the influent was spiked to an expected final concentration of 0.53 ug/L. After adjusting for losses due to the SPE method, the measured concentration was 0.52 ug/L, a 1.9 % difference from the target value.
To assess the potential capacity of the media for 6PPD-quinone, a preliminary isotherm was conducted. A six-point isotherm was conducted by filling teflon vials with stormwater water and spiking them with the 6PPD-Quinone stock solution at varying concentrations. The initial concentrations ranged from 1 to 1000 ug/L to yield equilibrium solution concentrations over 2.5 orders of magnitude (0.1 to 50 ug/L). A 1 g dose of the adsorbent was added to each vial to provide this range of values for the equilibrated samples. The samples were placed on a shaker table for 24 hours before extracting. While this time frame may not have achieved equilibrium, the goal of the experiment was to provide a preliminary assessment of capacity. The results shown in Figure 15 followed a linear isotherm with the linear constant (Kd) equal to 16.67 L/g and an R2 = 0.979. The adsorption capacity for a concentration of 0.53 ug/L would be 8.84 μg/g.
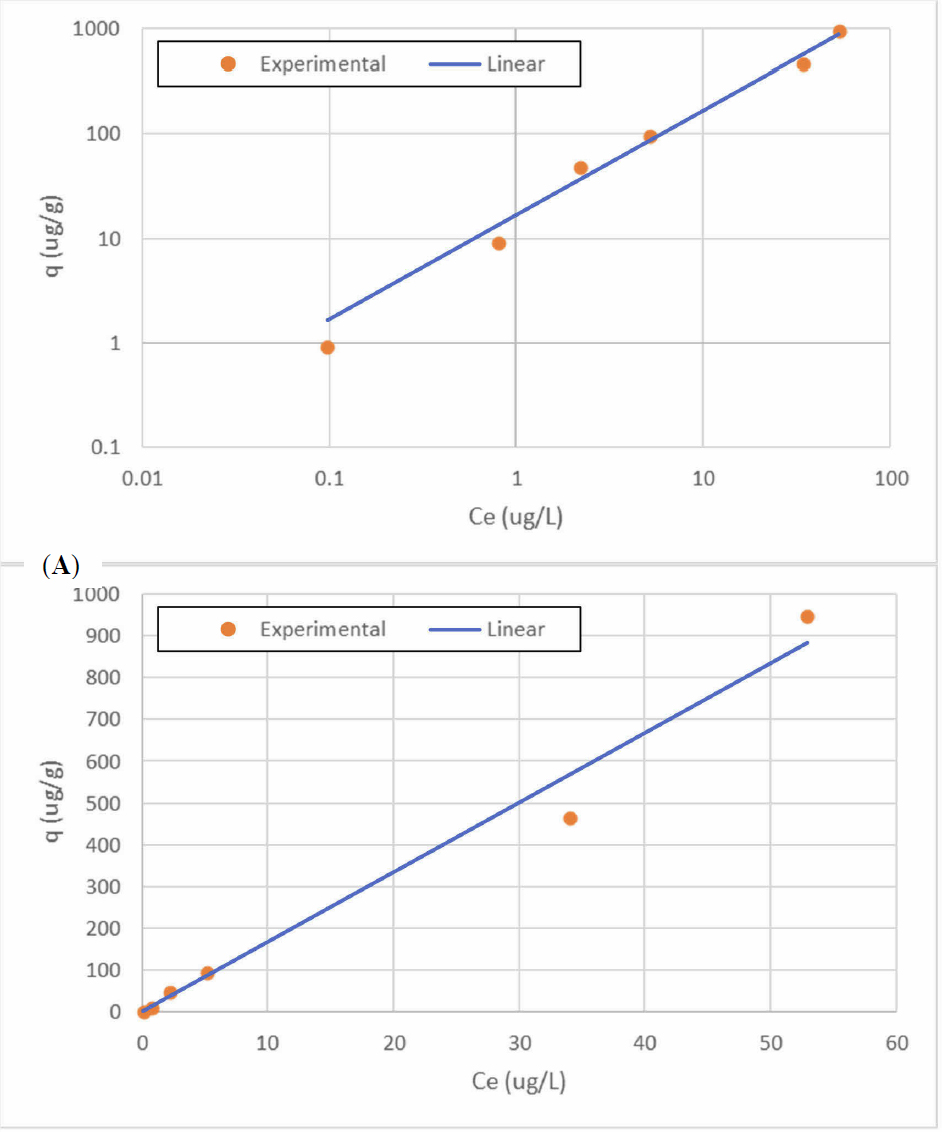
Assessment of Removal of 6PPD-Quinone in Column Experiment 63-1.
As indicated in Figure 16, the influent concentration of 6PPD-Quinone decreased during the experiment even though the container was opaque, there may have been some loss to the container and/or degradation in the water. As a result, the fractional removals were calculated for each data point using the measured influent. For the first 2500 inches of runoff, no samples were taken from the intermediate sampling points, A1 and A2. The media was able to achieve more than 90 percent removal of the 6-PPD-Quinone with increasing removal for each column section from A1 to A3.
8. Discussion
The results of the laboratory testing confirmed that the selected media was capable of removing dissolved copper and zinc to TAPE benchmarks under the conditions of high loading (greater than or equal to 50 in/hr of runoff) from highway runoff collected from a site in Austin, TX. For the high loading rate columns, only three data points for dissolved zinc and one data point for dissolved copper exceeded the TAPE benchmarks over the entire course of the experiment based on the data collected by LCRA. While one of these high zinc data points appeared at the end of the experiment, that data point was significantly higher than the total zinc concentration measured by LCRA for the same sample; if the total zinc concentration of that sample is used to calculate removal, then the effluent easily meets the TAPE benchmark. Moreover, both of the other two columns operated at greater than 50 in/hr met the TAPE benchmark. Thus, the preponderance of data suggests that the media has the capacity for dissolved zinc and copper removal for at least one year of runoff. In some cases, there was a trend of declining removal toward the end of the trials, suggesting that performance will gradually decline over time.
Average removals for the four experiments conducted are shown in Table 8. Copper removal was more susceptible to reduced removal at the higher loading than zinc. In fact, in the final experiment when the zinc concentration was spiked to almost double the previous runs, the zinc removal was significantly higher. While this result is expected, it does point out the importance of considering the initial concentrations when developing treatment media design criteria.
With respect to dissolved phosphate, it is important to note that this study employed pre-settled stormwater and it is likely that much of the particulate phosphate had been removed from the influent source prior to the experiments. The relatively low concentrations of dissolved ortho-phosphate revealed the potential for leaching from of phosphorus from the media. However, for the first experiment where ortho-phosphate concentrations were high (200 ug/L), the removal was also high (55 percent). As a result, it is evident that there are components of the media that are capable of removing significant amounts of phosphorus which other components are prone to leaching minor amounts of phosphorus when influent concentrations are low.
Table 8 Summary of Dissolved Cu, Zn and O-Phosphate in the Column Experiments
| Loading Rate (in/hr) | Initial Concentration (ug/L) | Final Average Concentration (ug/L) | End of Run Average Removal* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu | Zn | Ortho-P | Cu | Zn | Ortho-P | Cu | Zn | Ortho-P | |
| 14 | 11 | - | 200 | 3.95 | - | 90.1 | 64.1% | - | 55.0% |
| 14 | 12.3 | 33.1 | 66.5 | 1.7 | 10.92 | 152 | 86.2% | 67.0% | Increase |
| 50 | 8.4 | 35.7 | 44 | 3.3 | 10.6 | 71.2 | 60.7% | 70.3% | Increase |
| 63 | 14.5 | 59.7 | <10 | 7.1 | 7.5 | 84.3 | 51.0% | 87.4% | Increase |
| * Data was averaged over the final four samples and removals were averaged for multiple runs at equivalent loading rates | |||||||||
The quantification and removal of 6PPD-quinone in urban runoff has become of increasing importance due to its negative impact on aquatic life. Recent studies reported a median lethal dose (LD50) for Brook and Rainbow trout populations at 590 and 1000 ng/L and as low as 95 ng/L for Coho Salmon (Brinkmann et al., 2022, Tian et al., 2022). Although the 6PPD-quinone concentration in the stormwater used in our study was measured at 130 ng/L, another study
reported a peak concentration of 230 ng/L during a storm event in Ontario, Canada, thus urban runoff can pose a significant hazard to aquatic life if left unchecked (Johannessen et al., 2022). Indeed, in a recent report data for relevant concentrations of 6PPD-quinone for streams and runoff were summarized and Table 9 provides a summary of the data specifically for stormwater, highway and roadway runoff. There is still considerable uncertainty and scientific disagreement regarding a relevant concentration for a standard that is related to fish toxicity, but this report found an LC50 value of 95 ng/L.
Table 9 Selected Summary of 6PPD-Quinone Concentrations in Stormwater and Urban Roadways
| Location | Water Type | Grab/Composite Duration | 6PPD-quinone Concentrations (ng/L) | Land Use | Reference No. from Source Report |
|---|---|---|---|---|---|
| Seattle, WA | Roadway Runoff | Grab (24 h) | 50-1270 | Urban Highway | 1,2 |
| Los Angeles, CA | Roadway Runoff | Grab unspecified | 270-400 | Urban Highway | 1,2 |
| Saskatoon, SK, Canada | Stormwater Runoff | Grab unspecified or composite (4h) | 86-1400 | Urban | 1,2 |
| Nanaimo, BC, Canada | Stormwater | Grab unspecified | 48-5580 | Urban | 3 |
| Michigan | Road Puddles | Grab (unique) | 54-660 | Various | 8 |
| Hong Kong | Urban runoff | Grab unspecified | 21-243 | Dense, traffic | 9 |
Source: https://apps.ecology.wa.gov/publications/documents/2203020.pdf
The experiments conducted in this work spiked the influent with a concentration of 524 ng/L of 6PPD-quinone. While the influent concentration dropped over time, the effluent concentration at the end of the experiment conducted at a 63 in/hr highway runoff loading rate was 11 ng/L, suggesting that the selected media has significant potential for removal of this contaminant within concentration ranges found in highway runoff. Moreover, the effluent concentration falls below toxicity values reported in recent literature.
9. Conclusions
The results of the water quality column studies can be summarized as follows:
- Column studies with the selected media at 14 inches/hour confirmed the removal of dissolved copper and zinc documented in previous studies.
- Column studies at 50 and 63 inches/hour demonstrated removal of dissolved copper and zinc that exceeds the Washington Department of Ecology TAPE benchmarks, although the concentration reduction was somewhat less for copper at the higher flow rates than was observed at 14 in/hr.
- Dissolved phosphorus reduction was largely a function of influent concentrations, with more than 50 percent reduction when the influent dissolved concentration was at least 0.2 mg/L, but export occurred when influent concentrations were as low as about 0.05 mg/L. TAPE benchmarks for phosphorus are expressed as total phosphorus, therefore an assessment media performance should consider both the removal of particulate-bound phosphorus and the minor increases in ortho phosphorus that may occur at lower influent concentrations.
- The media was able to achieve more than 90 percent removal of the 6-PPD-Quinone at 63 inches/hour loading rate through the study duration, which resulted in effluent concentrations well below the LC50.
- No breakthrough in dissolved copper and zinc was observed when 4000 inches of runoff was applied to the columns, which indicates that the adsorptive capacity of 18 inches of media is sufficient to treat the equivalent of at least 30 inches of rainfall at a sizing rate of 300 sq-ft media per acre without replacement or renewal based on the proposed sizing guidelines. Similarly, no breakthrough was observed for 6-PPD-Quinone.
- In order to consistently meet TAPE benchmarks for dissolved copper and zinc at 50 in/hr, a media depth of 18 inches appears to be necessary.
10. References
Abad, M., et al., 2002, Physico-chemical and chemical properties of some coconut coir dusts for use as a peat substitute for containerised ornamental plants, Bioresource Technology, Vol 82, No. 3, 241-245, https://doi.org/10.1016/S0960-8524(01)00189-4
Brinkman, M. et al., (2022) Acute toxicity of the tire rubber-derived chemical 6PPD-quinone to four fishes of commercial, cultural, and ecological importance. Environmental Science & Technology Letters, 9(4), 333-338. DOI: 10.1021/acs.estlett.2c00050
Environmental Assessment and Water Quality Programs, Washington State Department of Ecology. (2022) 6PPD in Road Runoff Assessment and Mitigation Strategies, apps.ecology.wa.gov/publications/documents/2203020.pdf
Fohet, L., et al. “Time-Concentration Profiles of Tire Particle Additives and Transformation Products Under Natural and Artificial Aging.” The Science of the Total Environment., vol. 859, 2023, https://doi.org/10.1016/j.scitotenv.2022.160150.
Johannessen, C. et al. The Tire Wear Compounds 6PPD-Quinone and 1,3-Diphenylguanidine in an Urban Watershed. Arch Environ Contam Toxicol 82, 171–179 (2022). https://doi.org/10.1007/s00244-021-00878-4
Scott, I. et al., (2020). Development of a regeneration technique for aluminum-rich and iron-rich phosphorus sorption materials. Water, 12(6), 1784, https://doi.org/10.3390/w12061784
Shahrokhi-Shahraki, R., Benally, C., El-Din, M. G., & Park, J. (2021). High efficiency removal of heavy metals using tire-derived activated carbon vs commercial activated carbon: Insights into the adsorption mechanisms. Chemosphere, 264, 128455, https://doi.org/10.1016/j.chemosphere.2020.128455
Swarnalatha, K., & Ayoob, S. (2016). Adsorption studies on coir pith for heavy metal removal. International Journal of Sustainable Engineering, 9(4), 259-265, https://doi.org/10.1016/j.watres.2020.116648
Tian, Z., et al., (2022). 6PPD-quinone: Revised toxicity assessment and quantification with a commercial standard. Environmental Science & Technology Letters, 9(2), 140-146. https://doi:10.1021/acs.estlett.1c00910
Tirpak, R. A., et al., (2021). Conventional and amended bioretention soil media for targeted pollutant treatment: A critical review to guide the state of the practice. Water Research, 189, 116648, https://www.tandfonline.com/doi/full/10.1080/19397038.2016.1152323
Washington State Department of Ecology, 6PPD Washington State Interagency Webinar followup, https://www.ezview.wa.gov/Portals/_1962/Documents/6ppd/6PPD%20Webinar%20Follow-Up%20Document%20-%20June%202023.pdf
Zoroufchi Benis, K., et al., (2023). Environmental Occurrence and Toxicity of 6PPD Quinone, an Emerging Tire Rubber-Derived Chemical: A Review. Environmental Science & Technology Letters. DOI:10.1021/acs.estlett.3c00521
APPENDIX D.1
Raw Data for Experimental Results
This appendix contains raw laboratory analysis results for samples analyzed by UT Austin. Laboratory data reports for samples analyzed by LCRA are available upon request.
Experiment 14-1 Column Sampling Port Data
UT Dissolved Copper Data
| Time (hrs) | Dissolved Cu (ug/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inf | A1 | A2 | A3 | B1 | B2 | B3 | C1 | C2 | C3 | |
| 8 | - | 3.78 | 1.08 | 0.18 | 2.11 | 0.57 | 0.53 | 2.44 | 1.63 | 0.61 |
| 20 | - | 4.35 | 1.74 | 0.49 | 3.13 | 1.05 | 0.37 | 3.94 | 2.06 | 0.97 |
| 24 | - | 4.70 | 1.34 | 0.32 | 3.00 | 1.24 | 0.84 | 1.31 | 1.04 | 0.75 |
| 29 | 10.68 | 6.69 | 2.12 | 1.61 | 1.72 | 0.60 | 0.15 | 3.03 | 2.08 | 1.29 |
| 45 | - | 5.06 | 2.25 | 1.18 | 3.61 | 1.11 | 0.90 | 3.57 | 2.23 | 0.28 |
| 49 | 9.16 | 6.98 | 2.89 | 1.14 | 2.54 | 1.41 | 0.68 | 2.66 | 1.57 | 0.61 |
| 54 | 9.75 | 2.28 | 0.45 | 0.33 | 3.10 | 1.68 | 0.79 | 4.09 | 3.00 | 1.89 |
| 70 | 9.74 | 6.01 | 1.77 | 0.77 | 4.76 | 1.63 | 0.99 | 5.50 | 3.35 | 2.22 |
| 74 | 9.82 | 7.05 | 1.83 | 0.39 | 4.24 | 1.18 | 0.60 | 6.11 | 4.28 | 2.68 |
| 80 | 11.68 | 4.91 | 2.13 | 0.57 | 4.26 | 0.65 | 0.71 | 5.36 | 3.44 | 2.54 |
| 96 | 9.58 | 9.37 | 2.08 | 0.70 | 4.40 | 1.39 | 1.16 | 5.53 | 3.49 | 2.77 |
| 100 | 9.51 | 2.96 | 0.52 | 3.41 | 5.49 | 2.97 | 2.48 | 5.99 | 4.13 | 2.75 |
UT pH Data
| pH | |||||||||
| Time(hrs) | A1 | A2 | A3 | B1 | B2 | B3 | C1 | C2 | C3 |
| 7.94 | 7.16 | 6.93 | 6.62 | 7.04 | 6.80 | 6.39 | 7.22 | 7.03 | 6.81 |
| 19.55 | 7.68 | 7.47 | 7.49 | 7.64 | 7.36 | 7.36 | 7.71 | 7.54 | 7.48 |
| 24.10 | 7.49 | 7.29 | 7.19 | 7.12 | 7.34 | 7.20 | 7.04 | 7.29 | 7.06 |
| 29.10 | 7.14 | 6.94 | 7.37 | 7.09 | 6.84 | 7.26 | 7.19 | 7.23 | 7.18 |
| 45.09 | 7.29 | 7.19 | 7.12 | 7.34 | 7.20 | 7.04 | 7.29 | 7.06 | 7.32 |
| 49.09 | 7.46 | 7.31 | 7.20 | 7.42 | 7.28 | 7.11 | 7.38 | 7.25 | 7.17 |
| 53.59 | 7.40 | 7.27 | 7.14 | 7.14 | 7.26 | 7.15 | 7.32 | 7.47 | 7.34 |
| 70.09 | 7.43 | 7.30 | 7.20 | 7.36 | 7.42 | 7.27 | 7.40 | 7.40 | 7.28 |
| 74.08 | 7.56 | 7.32 | 7.21 | 7.11 | 7.23 | 6.99 | 7.30 | 7.40 | 7.50 |
| 79.58 | 7.46 | 7.26 | 7.10 | 7.47 | 7.27 | 7.17 | 7.58 | 7.46 | 7.42 |
| 95.83 | 7.45 | 7.30 | 7.22 | 7.47 | 7.33 | 7.25 | 7.71 | 7.59 | 7.40 |
| 99.96 | 7.27 | 7.45 | 7.14 | 7.55 | 7.30 | 7.24 | 7.53 | 7.43 | 7.33 |
Experiment 14-2 Column Sampling Port Data
UT Dissolved Copper Data
| Time (hrs) | Dissolved Cu (ug/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inf | A1 | A2 | A3 | B1 | B2 | B3 | C1 | C2 | C3 | |
| 0.00 | 9.45 | 0.96 | 0.75 | 0.23 | 0.59 | 1.72 | 0.25 | 1.24 | 0.47 | 0.35 |
| 3.00 | 9.61 | 1.46 | 0.45 | 0.26 | 2.37 | 7.30 | 0.24 | 1.17 | 2.59 | 0.33 |
| 6.00 | 9.41 | 2.81 | 1.24 | 0.12 | 2.19 | 0.93 | 0.19 | 1.53 | 4.68 | 0.14 |
| 9.00 | 9.52 | 2.57 | 1.10 | 0.51 | 3.65 | 0.95 | 1.98 | 4.07 | 2.73 | 0.16 |
| 24.50 | 9.14 | 3.19 | 0.98 | 0.16 | 2.63 | 1.19 | 0.28 | 4.48 | 1.67 | 0.22 |
| 30.25 | 9.28 | 3.00 | 1.32 | 0.27 | 3.52 | 1.90 | 0.37 | 5.63 | 6.54 | 0.32 |
| 37.00 | 8.92 | 3.27 | 1.34 | 0.33 | 3.48 | 2.79 | 0.43 | 5.58 | 3.35 | 0.32 |
| 48.00 | 8.90 | 3.45 | 1.43 | 0.43 | 4.47 | 2.40 | 0.58 | 6.83 | 8.25 | 0.59 |
| 53.50 | 8.88 | 3.32 | 1.73 | 0.42 | 4.40 | 3.27 | 0.69 | 5.67 | 5.52 | 0.46 |
| 63.00 | 8.97 | 3.66 | 2.32 | 0.68 | 4.81 | 4.51 | 1.11 | 6.86 | 6.05 | 0.75 |
| 73.25 | 8.94 | 2.97 | 1.19 | 0.32 | 4.80 | 7.65 | 1.06 | 1.56 | 7.11 | 0.68 |
| 78.00 | 8.85 | 0.40 | 1.46 | 3.39 | 4.72 | 13.22 | 0.89 | 6.82 | 1.93 | 0.71 |
| 84.00 | 8.85 | 3.57 | 1.61 | 0.59 | 4.71 | 11.18 | 1.06 | 3.33 | 2.30 | 0.79 |
UT Zinc Data
| Time (hrs) | Dissolved Zn (ug/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inf | A1 | A2 | A3 | B1 | B2 | B3 | C1 | C2 | C3 | |
| 0.00 | 30.46 | 13.45 | 12.85 | 18.54 | 0.62 | 44.31 | 6.82 | 2.41 | 5.84 | 13.88 |
| 3.00 | 31.17 | 19.00 | 13.98 | 7.37 | 12.86 | 443.05 | 8.36 | 3.87 | 23.73 | 6.97 |
| 6.00 | 31.83 | 22.50 | 22.31 | 15.52 | 20.80 | 51.70 | 15.58 | 5.91 | 52.17 | 7.74 |
| 9.00 | 31.42 | 52.42 | 50.83 | 23.23 | 20.22 | 30.22 | 39.61 | 8.54 | 43.06 | 13.22 |
| 24.50 | 28.62 | 25.40 | 15.08 | 13.77 | 5.44 | 16.01 | 6.71 | 14.42 | 29.48 | 6.19 |
| 30.25 | 29.46 | 12.37 | 10.38 | 10.49 | 17.96 | 16.45 | 7.34 | 50.59 | 31.87 | 6.39 |
| 37.00 | 29.55 | 10.74 | 6.43 | 8.45 | 18.43 | 25.26 | 7.66 | 95.27 | 44.18 | 5.02 |
| 48.00 | 27.25 | 8.44 | 0.93 | 9.26 | 27.15 | 4.31 | 7.90 | 68.20 | 102.23 | 7.36 |
| 53.50 | 26.59 | 8.20 | 8.17 | 7.48 | 18.30 | 23.29 | 6.02 | 35.66 | 43.41 | 0.42 |
| 63.00 | 26.66 | 42.81 | 15.77 | 5.12 | 21.28 | 53.92 | 5.98 | 48.71 | 65.48 | 5.22 |
| 73.25 | 25.84 | 9.77 | 9.24 | 6.51 | 23.24 | 10.53 | 6.23 | 4.70 | 53.39 | 4.92 |
| 78.00 | 26.03 | 8.46 | 5.30 | 7.00 | 13.53 | 26.65 | 6.22 | 40.08 | 7.96 | 7.53 |
| 84.00 | 26.12 | 23.45 | 3.98 | 8.31 | 13.10 | 29.96 | 6.68 | 4.73 | 8.63 | 5.13 |
UT pH Data
| pH | ||||||||||
| Influent | A1 | A2 | A3 | B1 | B2 | B3 | C1 | C2 | C3 | |
| 0.00 | 7.54 | 6.36 | 6.59 | 6.79 | 6.33 | 6.79 | 6.14 | 6.55 | 6.55 | 6.84 |
| 6.00 | 7.53 | 6.93 | 6.53 | 6.47 | 7.02 | 6.73 | 7.32 | 7.04 | 6.71 | 6.39 |
| 9.00 | 7.14 | 6.98 | 6.61 | 6.31 | 7.14 | 6.80 | 6.41 | 7.13 | 6.98 | 6.39 |
| 24.50 | 7.46 | 7.31 | 7.04 | 6.96 | 7.31 | 7.16 | 6.96 | 7.33 | 7.14 | 7.01 |
| 30.25 | 7.66 | 7.36 | 7.11 | 7.02 | 7.40 | 7.23 | 7.13 | 7.42 | 7.20 | 7.11 |
| 37.00 | 7.71 | 7.31 | 7.10 | 7.04 | 7.34 | 7.22 | 7.15 | 7.40 | 7.20 | 7.12 |
| 48.00 | 7.64 | 7.40 | 7.25 | 7.17 | 7.40 | 7.29 | 7.24 | 7.40 | 7.32 | 7.22 |
| 53.50 | 7.70 | 7.34 | 7.25 | 7.24 | 7.43 | 7.31 | 7.25 | 7.51 | 7.40 | 7.06 |
| 63.00 | 7.48 | 7.35 | 7.18 | 7.15 | 7.32 | 7.27 | 7.22 | 7.40 | 7.35 | 7.20 |
| 73.25 | 7.80 | 7.32 | 7.15 | 7.16 | 7.45 | 7.35 | 7.35 | 7.33 | 7.52 | 7.35 |
| 78.00 | 7.73 | 7.13 | 7.15 | 7.27 | 7.42 | 7.28 | 7.27 | 7.45 | 7.31 | 7.32 |
| 84.00 | 7.72 | 7.34 | 7.16 | 7.14 | 7.40 | 7.34 | 7.33 | 7.42 | 7.32 | 7.31 |
Experiment 50-1 Column Sampling Port Data
UT Dissolved Copper Data
| Time (hrs) | Dissolved Cu (ug/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inf | A1 | A2 | A3 | B1 | B2 | B3 | C1 | C2 | C3 | |
| 0 | 6.00 | 3.40 | 0.45 | 0.26 | 13.70 | 6.06 | 0.54 | 8.47 | 5.50 | 0.33 |
| 4 | 6.06 | 4.81 | 1.40 | 0.57 | 11.16 | 4.99 | 1.10 | 6.64 | 6.43 | 4.74 |
| 10 | 5.97 | 6.33 | 2.53 | 0.90 | 6.20 | 4.78 | 1.55 | 7.30 | 6.31 | 3.96 |
| 16 | 5.48 | 4.93 | 2.82 | 1.13 | 5.40 | 4.62 | 1.75 | 8.05 | 6.75 | 5.19 |
| 22 | 5.42 | 4.57 | 2.92 | 1.17 | 5.11 | 3.86 | 1.80 | 7.07 | 6.90 | 5.19 |
| 28 | 5.33 | 4.15 | 3.10 | 1.81 | 4.98 | 4.00 | 1.99 | 7.32 | 7.28 | 5.11 |
| 34 | 6.03 | 4.45 | 3.34 | 1.55 | 4.98 | 4.14 | 2.08 | 7.63 | 7.53 | 5.71 |
| 40 | 6.02 | 4.73 | 3.75 | 1.67 | 4.77 | 3.24 | 2.30 | 7.17 | 7.41 | 5.78 |
| 46 | 5.96 | 4.53 | 3.12 | 1.67 | 4.77 | 4.01 | 2.30 | 4.83 | 7.59 | 5.81 |
| 52 | 6.59 | 4.22 | 3.46 | 1.87 | 4.80 | 4.24 | 2.57 | 7.53 | 8.14 | 6.20 |
| 58 | 6.37 | 4.49 | 3.17 | 1.60 | 4.77 | 4.30 | 2.65 | 5.56 | 8.01 | 6.29 |
| 64 | 6.27 | 4.45 | 3.09 | 1.62 | 4.98 | 6.59 | 2.63 | 7.57 | 8.10 | 6.32 |
| 69.5 | 5.93 | 4.51 | 3.71 | 1.61 | 4.59 | 6.38 | 2.26 | 7.54 | 13.37 | 5.85 |
| 75.5 | 6.06 | 2.46 | 4.65 | 1.44 | 4.49 | 10.86 | 2.58 | 7.42 | 8.27 | 5.85 |
| 81.5 | 5.96 | 3.84 | 2.94 | 1.46 | 4.02 | 5.46 | 2.63 | 6.60 | 7.64 | 5.83 |
UT pH Data
| Time (hrs) | Influent | A1 | A2 | A3 | B1 | B2 | B3 | C1 | C2 | C3 |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 7.40 | 7.00 | 7.21 | 7.35 | 6.87 | 6.96 | 7.20 | 8.75 | 9.08 | 9.58 |
| 3.5 | 7.45 | 6.97 | 6.74 | 6.53 | 7.15 | 6.96 | 6.79 | 7.94 | 8.95 | 8.99 |
| 9.5 | 7.57 | 7.17 | 7.00 | 7.03 | 7.27 | 7.18 | 7.20 | 7.67 | 8.05 | 8.67 |
| 15.5 | 7.41 | 7.21 | 7.09 | 7.10 | 7.31 | 7.26 | 7.29 | 7.51 | 7.63 | 7.85 |
| 21.5 | 7.50 | 7.22 | 7.17 | 7.23 | 7.32 | 7.30 | 7.35 | 7.53 | 7.60 | 7.71 |
| 27.5 | 7.37 | 7.23 | 7.11 | 7.14 | 7.26 | 7.25 | 7.27 | 7.48 | 7.51 | 7.48 |
| 33.5 | 7.40 | 7.26 | 7.17 | 7.19 | 7.29 | 7.24 | 7.34 | 7.46 | 7.44 | 7.59 |
| 39.5 | 7.49 | 7.31 | 7.30 | 7.26 | 7.36 | 7.31 | 7.28 | 7.47 | 7.51 | 7.60 |
| 45.5 | 7.38 | 7.22 | 7.14 | 7.24 | 7.27 | 7.28 | 7.25 | 7.49 | 7.47 | 7.58 |
| 51.5 | 7.42 | 7.30 | 7.21 | 7.28 | 7.34 | 7.30 | 7.37 | 7.49 | 7.48 | 7.49 |
| 57.5 | 7.41 | 7.23 | 7.13 | 7.24 | 7.35 | 7.28 | 7.33 | 7.46 | 7.49 | 7.54 |
| 63.5 | 7.41 | 7.25 | 7.16 | 7.24 | 7.38 | 7.30 | 7.34 | 7.44 | 7.43 | 7.52 |
| 69.5 | 7.54 | 7.25 | 7.18 | 7.27 | 7.43 | 7.34 | 7.39 | 7.60 | 7.55 | 7.59 |
| 75.5 | 7.63 | 7.22 | 7.19 | 7.26 | 7.46 | 7.34 | 7.37 | 7.59 | 7.60 | 7.66 |
| 81.5 | 7.78 | 7.34 | 7.26 | 7.30 | 7.55 | 7.44 | 7.42 | 7.76 | 7.71 | 7.73 |
Experiment 63-1 Column Sampling Port Data
UT Dissolved Copper Data
| Time (hrs) | Dissolved Cu (ug/L) | |||
|---|---|---|---|---|
| Inf | A1 | A2 | A3 | |
| 0 | 14.17 | 1.03 | 0.34 | 0.10 |
| 3 | 13.59 | 8.58 | 3.14 | 1.72 |
| 6 | 14.12 | 8.50 | 5.01 | 2.27 |
| 15 | 13.70 | 9.13 | 5.51 | 3.32 |
| 19 | 13.63 | 9.47 | 5.89 | 3.50 |
| 23 | 13.62 | 9.54 | 6.27 | 3.75 |
| 31 | 13.82 | 9.69 | 6.78 | 4.25 |
| 43 | 12.81 | 10.02 | 7.41 | 4.60 |
| 47 | 12.85 | 9.86 | 6.27 | 4.70 |
| 55 | 12.50 | 10.11 | 6.91 | 4.90 |
| 64 | 12.23 | 10.17 | 7.71 | 5.25 |
| 66 | 12.09 | 9.33 | 6.75 | 5.25 |
UT Dissolved Zinc Data
| Time (hrs) | Dissolved Zn (ug/L) | |||
|---|---|---|---|---|
| Inf | A1 | A2 | A3 | |
| 0 | 55.65 | 8.20 | 4.40 | 1.57 |
| 3 | 52.99 | 37.27 | 10.94 | 11.28 |
| 6 | 54.97 | 30.74 | 18.29 | 5.58 |
| 15 | 53.66 | 50.51 | 30.37 | 4.70 |
| 19 | 52.06 | 39.92 | 16.03 | 4.68 |
| 23 | 53.24 | 37.16 | 16.77 | 4.82 |
| 31 | 53.80 | 42.23 | 23.47 | 4.99 |
| 43 | 51.08 | 43.69 | 23.94 | 5.53 |
| 47 | 49.76 | 38.39 | 13.14 | 5.43 |
| 55 | 45.09 | 36.95 | 21.03 | 6.25 |
| 64 | 29.49 | 32.57 | 19.77 | 6.22 |
| 66 | 23.74 | 23.14 | 13.33 | 6.43 |
Experiment 63-1
UT 6-PPD-quinone Data
| Time (hrs) | 6PPD-Q (ug/L) | |||
|---|---|---|---|---|
| Inf | A1 | A2 | A3 | |
| 0 | 0.524 | - | - | 0.062 |
| 3 | - | - | - | - |
| 6 | 0.528 | - | - | 0.012 |
| 15 | 0.402 | - | - | 0.036 |
| 19 | 0.432 | - | - | 0.007 |
| 23 | 0.323 | - | - | 0.008 |
| 31 | 0.404 | - | - | 0.030 |
| 43 | 0.295 | - | - | 0.004 |
| 47 | 0.356 | 0.078 | 0.014 | 0.012 |
| 55 | 0.370 | 0.097 | 0.019 | 0.014 |
| 64 | 0.292 | 0.068 | 0.014 | 0.008 |
| 66 | 0.244 | 0.073 | 0.022 | 0.011 |
UT pH Data
| pH | ||||
|---|---|---|---|---|
| Time (hrs) | Influent | A1 | A2 | A3 |
| 0.0 | 7.45 | 5.92 | 6.61 | 7.31 |
| 3.0 | 7.44 | 7.16 | 5.92 | 6.81 |
| 6.0 | 7.38 | 7.25 | 7.09 | 7.02 |
| 15.0 | 7.41 | 7.35 | 7.25 | 7.25 |
| 19.0 | 7.55 | 7.48 | 7.40 | 7.40 |
| 23.0 | 7.55 | 7.45 | 7.37 | 7.35 |
| 31.0 | 7.55 | 7.47 | 7.43 | 7.40 |
| 43.0 | 7.64 | 7.45 | 7.44 | 7.41 |
| 47.0 | 7.55 | 7.45 | 7.39 | 7.35 |
| 55.0 | 7.73 | 7.61 | 7.55 | 7.49 |
| 64.0 | 7.94 | 7.76 | 7.65 | 7.59 |
| 65.5 | 7.93 | 7.71 | 7.59 | 7.59 |