Myopia: Causes, Prevention, and Treatment of an Increasingly Common Disease (2024)
Chapter: 2 Myopia and the Human Eye: A Primer
2
Myopia and the Human Eye: A Primer
This chapter lays the groundwork for the remainder of the report by providing fundamental information about myopia and the eye. Descriptions and definitions of components of the eye are included here (and also in the Glossary) to assist readers unfamiliar with the eye and/or myopia in understanding the remaining chapters.
The concept of myopia was first described in 350 BCE by Aristotle, who noted that myopic individuals narrowed their eyelids to decrease the aperture of light (de Jong, 2018). Many decades later, squeezing of the eyelids was associated with causing a pinhole effect (stenopeic slit) to elicit a sharper image (de Jong, 2018). The smaller pupillary aperture allows for a narrower beam of light rays to reach the central macular retina rather than stimulating the extra-macular retina with indirect, aberrant rays. The term myopia originates from the Greek word μφϖψ, derived from μωειν (muein, to close) and ϖψ (ops, the eye), which together means short-sighted (de Jong, 2018). By the 1700s, it was shown that nearsighted eyes are longer in length from front to back (axial length) relative to non-myopic eyes (Glauder, 1751).
Today, much is known about eye growth from birth. The length of the eye can have a strong influence on what is known as refractive error of the eye, with farsighted eyes being too short (hyperopia) and nearsighted eyes being too long (myopia) to allow for all optical component contributions to focus an object of regard on the retinal plane. (The component contributions include corneal curvature and thickness, lens curvature and thickness, and the optical indices of the cornea, lens, and vitreous humor). It is known that while newborns have a wide range of refractive error, including hyperopia and myopia, in general they are hyperopic (Cook & Glasscock, 1951; Mutti et al., 2005; see Figure 2-1). Refractive error due to optical component mismatch causes blurred vision. The infant eye grows to focus light more clearly on the retinal plane, generally starting from a smaller axial length consistent with hyperopia, a refinement process in response to visual blur that is called emmetropization. Myopia develops when the combined optical component powers are too strong for the eye’s axial length, focusing the image in front of the retinal plane with a concomitant defocused image on the retina (see Figure 2-2). In common childhood myopia, an excessive increase in axial length appears to be the cause.
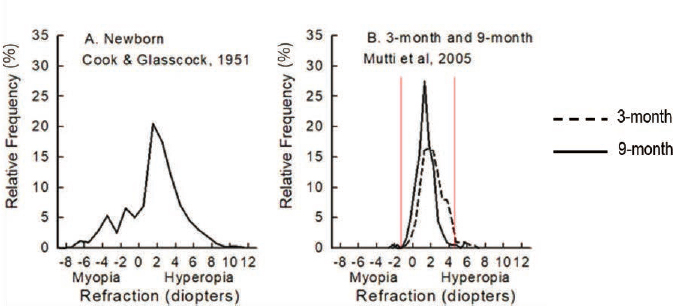
SOURCE: Committee-generated from data in Cook & Glasscock, 1951, Mutti et al., 2005.
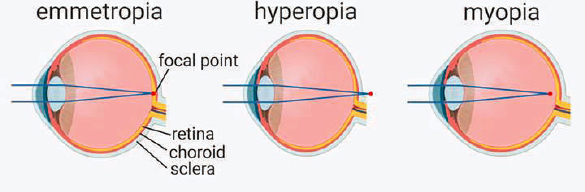
NOTE: This figure illustrates how axial length of the eye affects whether what is being viewed is focused on the retina (emmotropia [normal vision]), behind the retina (hyperopia [farsightedness]), or in front of the retina (myopia [nearsightedness]).
SOURCE: Reprinted from Tkatchenko & Tkatchenko, 2019, with permission from Elsevier.
Myopia occurs when the homeostasis between the length and shape of the eye versus its focusing elements fails to occur. Major lines of evidence pinpoint the role of the retina and early visual encoding in regulating eye growth (Rohrer & Stell, 1994). The inability of the optics of the eye to match the elongating eye in myopia suggests that whereas in an emmetrope a coordinated and intricate relationship between the eye’s optics and its length/shape is tightly maintained, this coordination is not the case in a myope. However, both the mechanism(s) responsible for this coordinated growth (emmetropization) and those leading to myopia development have remained elusive. (Also, see Box 5-2: Are mechanisms signaling normal eye growth and myopia the same?) What is known is explained in Chapters 5 and 6.
The eyes in most children grow to near-emmetropia or to be slightly farsighted within the first year of life (Mayer et al., 2005; Mutti, 2007; Mutti et al., 2005). However, even after infancy it is theorized that eyes receive “stop” and “go” signals that regulate growth (Rohrer & Stell, 1994). If these refractive growth signals are disrupted or abnormal, the eye can elongate too much. Once an eye is nearsighted, typically it will continue to grow and become progressively more nearsighted (Gwiazda et al., 1993; Norton, 1999; Wildsoet, 1997); wearing a regular pair of glasses or contact lenses does not prevent this further progression (Hou et al., 2018; Walline et al., 2020). Chapter 7 summarizes what is known about the effects of treatment.
The average age of onset of myopia in U.S. children is roughly 11 years old, and onset generally ranges from age 7 to age 16 (Kleinstein, 2012). After the onset, in about half of myopic
U.S. children there is a slow increase in the amount of myopia, a process that stabilizes around age 15. About 75% of myopic eyes are stable by age 18, but 4% may continue to lengthen to age 24 (COMET Group, 2013).
BOX 2-1
What Is a Diopter?
A diopter (D) is the unit of measurement for any focusing lens, such as for a refractive error prescription (spectacles or contact lenses), a telescope or microscope, or the natural lens inside of one’s eye. Thus, a higher (stronger) prescription will be indicated by larger D values, whereas a milder prescription will have smaller D values.
There is a reciprocal relationship between refractive error in diopters and the focal length of the spectacle lens (measured in meters) needed to achieve clear vision. For example, a lens with 1 diopter (1 D) strength will bring an object into focus at 1 meter. A lens with a 2 diopter (2 D) strength will have a focal length of 1/2 meter. A lens with a 3 diopter strength (3 D) will have a focal length of 1/3 meter. Thus, a shorter focal length means a stronger focusing power for the lens.
Convex-shaped lenses, such as the natural lens inside the eye, converge the light passing through them, bringing the light to a focal point. By contrast, concave-shaped lenses diverge the light passing through them, spreading the light into a wider optical path (see bottom panel of Figure 2-3). To signify their converging or diverging effect, convex lenses, which are used to correct farsightedness, have a positive D value, whereas concave lenses, which are used to correct nearsightedness, have a minus D value.
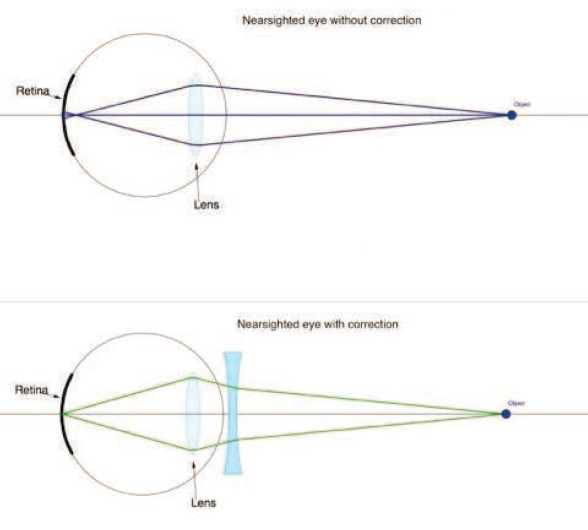
SOURCE: Reprinted from Keramati, 2019, under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License (https://creativecommons.org/licenses/by-nc-sa/4.0).
MYOPIC BLUR
Light from distant objects enters the eye in parallel rays. The eye has two refractive elements, the cornea and the lens, that transmit the parallel rays through the eye and attempt to converge the light to a perfect point on the retina. When this happens, the eye is in perfect focus and the individual sees clearly.
If the light rays entering the eye focus behind the back of the eye or the retina, the eye is too short compared to its optical power. This condition is known as hyperopia or farsightedness. In children and younger adults, the eye can compensate for mild and moderate amounts of hyperopia by making the crystalline lens more convex, thus converging the light more strongly on the plane of the retina of the short eye. This dynamic focusing process is called accommodation and usually occurs quickly and without conscious effort (although from mid-adulthood, the crystalline lens gradually loses its elasticity and accommodation becomes increasingly difficult).
If the light rays entering the eye focus in front of the retina, the eye is too long for its optical power. This represents the myopic or nearsighted eye. The eye itself cannot do anything to compensate for even the smallest amounts of myopia. The eyelids can squeeze together or squint, which creates a pinhole effect. Such a pinhole momentarily reduces the blurriness of vision when the blur is from refractive error, improving central vision, but peripheral vision is then dramatically reduced, making a pinhole an infeasible management option for myopia in daily life. The options for managing the side effect of blur from the myopic eye are therefore generally centered on optical devices such as glasses or surgical procedures.
ANATOMY OF THE EYE
The human eye is a complex organ with many components that contribute to its functioning. Figure 2-4 shows the components that are mentioned in this report. Each of these components is then briefly described.
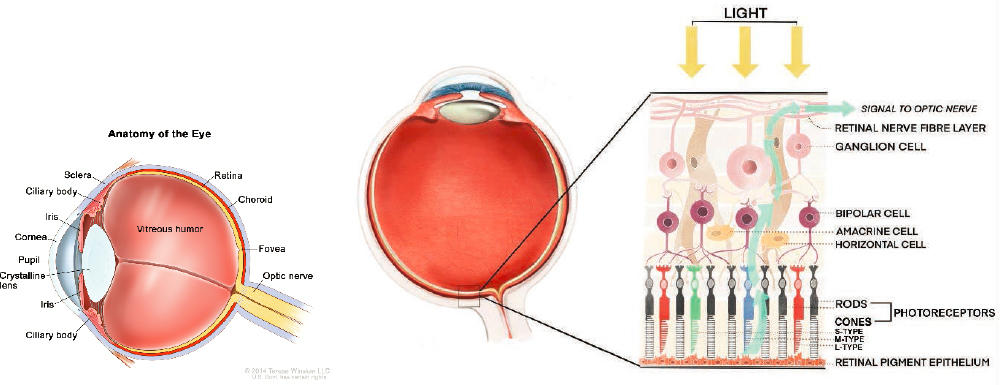
SOURCE: (A) © 2014 Terese Winslow LLC, U.S. Govt. has certain rights (updated from figure appearing in National Cancer Institute, 2008); (B) adapted from Kilduff, 2020, with permission from Gene Vision.
Amacrine cells—A class of retinal neurons that interact with ganglion cells and bipolar cells. Amacrine cells are diverse in their structural and biochemical features, suggesting they perform a variety of functions. For example, the starburst class of amacrine cells plays a role in detecting the direction of a moving image on the retina.
Choroid—A layer of tissue lying beneath the retina that has a very rich blood supply. A key role of the choroid is to provide nutrients and oxygen for the retina.
Ciliary body—The tissue that produces nutrient fluid for the eye and that contains the ciliary muscle, the involuntary smooth muscle that controls the optical power of the crystalline lens by slightly altering its shape.
Cornea—A transparent tissue about half a millimeter thick that allows light to enter the eye. The curved shape of the cornea is responsible for focusing light rays toward the crystalline lens and retina. The cornea is part of the tough, outer shell of the eye.
Crystalline lens—A transparent, biconvex structure situated inside the eye. After passing through the cornea and pupil, light passes through the 3-to-4-millimeter-thick crystalline lens before reaching the retina. The curvature of this lens is altered without conscious effort by the ciliary muscle (see above) to help precisely focus the retinal image, for example steepening in curvature to help focus light from nearby objects, in a process called accommodation. Beyond age 40 years, the crystalline lens gradually loses the flexibility needed to change shape, which leads to difficulty focusing on near objects such as smaller fonts or print—a condition termed presbyopia.
Fovea—The area of the central retina with the highest density of cone photoreceptors, which provides maximum visual acuity.
Iris—A highly pigmented tissue that serves the same function in the eye as an aperture stop in a camera, namely, to control the amount of light that enters the eye. In bright conditions the iris constricts, while in dim conditions the iris dilates. The iris also determines a person’s eye color, e.g., blue or brown eyes.
Macula—A round area at the center of the retina, or the back of the eye, that is responsible for central vision, color vision, and fine details. It is about 5 millimeters across and 250 microns thick and contains blood vessels and nerve fibers. The fovea constitutes the central part of the macula.
Optic nerve—A bundle of approximately 1 million nerve fibers that travels from the eye to the brain. The nerve fibers are the axons of retinal ganglion cells; they convey information about the visual image, including its color, whether it is moving, and its level of contrast.
Pupil—A circular opening formed by the iris that controls the level of light entering the eye. The size of the pupil changes reflexively in response to the intensity of incident light. When the pupil constricts, it has an optical effect like a pinhole; this pinhole effect reduces retinal image blurriness in an eye with refractive error.
Retina—A multilayered tissue lining the inner surface of the back half of the eye. The retina is responsible for detecting light, performing the first steps in processing the visual signal and sending neural signals about the visual image to the brain. The neural cells of the retina (see rods and cones, below) perform a diverse range of computations to sense not only color and brightness but also movement, speed/direction, and other image features.
Retinal ganglion cells—The terminal neurons for processing the retinal signal. The axons of the retinal ganglion cells form the optic nerve, which sends visual information to the brain. More than 18 different types of retinal ganglion cells have been identified, depending on the species. Some retinal ganglion cells contain photosensitive pigments and can be directly
stimulated by light or through rod and cone pathways, such as intrinsically photosensitive retinal ganglion cells (ipRGCs) that express Opn4 or melanopsin. Other photopigments found in retinal ganglion cells include neuropsin (Opn5) and encephalopsin (Opn3).
Retinal pigment epithelium—A single layer of highly specialized cells, situated between the retinal photoreceptors and the choroid. These cells have many functions that are essential to healthy functioning of the retina.
Rods and cones—Light-sensitive cells in the retina, also known as photoreceptors. Rods are specialized for detecting images under dim illumination and are more abundant in the periphery of the retina than in the central, foveal region. Cones are specialized for detecting light under bright conditions and are much more abundant in the central, foveal region. The human retina contains three types of cones, each most sensitive to either blue (short wavelength), green (medium wavelength), or red (long wavelength) light and therefore known as S-, M- and L-cones, for short.
Sclera—An opaque, tough layer of tissue that forms the outer shell of the middle and back of the eye. The tissue of the sclera is continuous with the cornea. The sclera largely determines the size and shape of the eye.
Vitreous—A transparent gel that fills the cavity between the crystalline lens and the retina. By occupying much of the eye’s volume, the vitreous acts in concert with the sclera to maintain the shape of the eye. Also known as the vitreous humor.
MYOPIA—MOVING FORWARD
Although refractive error and myopia have been studied for hundreds of years, limited evidence is available to fully understand the progression of myopia and its rising incidence. While lifestyle-related risk factors in people’s daily lives may have changed over that time, their effect on eyesight must still operate through basic anatomical and physiological development. This report seeks to use the most current knowledge on the biological regulation of eye growth to understand how changes in modern lifestyle may have made humans more susceptible to the development of myopia.
Having set the foundation for understanding the structural features of the myopic eye and its etiology, the key then is to understand what we know about managing the risk of incidence and the risk of progression, and how these risks play out not just over childhood and adolescence but over a lifetime of risks of eye conditions associated with myopia. To pull all of this together as a societal public health issue rather than simply an individual medical decision-making exercise, this report also explores what is known about disparities in the screening for, diagnosis of, and effective management of myopia over the life course.
REFERENCES
COMET Group. (2013). Myopia stabilization and associated factors among participants in the Correction of Myopia Evaluation Trial (COMET). Investigative Ophthalmology & Visual Science, 54(13), 7871–7884. https://doi.org/10.1167/iovs.13-12403
Cook, R. C., & Glasscock, R. E. (1951). Refractive and ocular findings in the newborn. American Journal of Ophthalmology, 34(10), 1407–1413. https://doi.org/10.1016/0002-9394(51)90481-3
de Jong P. T. V. M. (2018). Myopia: Its historical contexts. The British Journal of Ophthalmology, 102(8), 1021–1027. https://doi.org/10.1136/bjophthalmol-2017-311625
Glauder, G. F. (1751). Hermann Boerhavens Abhandlung von Augenkrankheiten und deroselben Kur. Herman Boerhaaven’s treatise on eye diseases and their cure.
Gwiazda, J., Thorn, F., Bauer, J., & Held, R. (1993). Myopic children show insufficient accommodative response to blur. Investigative Ophthalmology & Visual Science, 34(3), 690–694. https://pubmed.ncbi.nlm.nih.gov/8449687/
Hou, W., Norton, T. T., Hyman, L., Gwiazda, J., & COMET Group. (2018). Axial elongation in myopic children and its association with myopia progression in the Correction of Myopia Evaluation Trial. Eye & Contact Lens, 44(4), 248–259. https://doi.org/10.1097/ICL.0000000000000505
Keramati, B. (2019). Introduction to physics: An outline of selected topics. Pressbooks. https://pressbooks.pub/introphys1/
Kilduff, C. (2020). Microscopic view of the cells in the retina. Figure in retina. Gene Vision. https://gene.vision/retina/
Kleinstein, R. N., Sinnott, L. T., Jones-Jordan, L. A., Sims, J., Zadnik, K., & Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error Study Group. (2012). New cases of myopia in children. Archives of Ophthalmology, 130(10), 1274–1279. https://doi.org/10.1001/archophthalmol.2012.1449
Mayer, D. L., Beiser, A. S., Warner, A. F., Pratt, E. M., Raye, K. N., & Lang, J. M. (1995). Monocular acuity norms for the Teller Acuity Cards between ages one month and four years. Investigative Ophthalmology & Visual Science, 36(3), 671–685.
Mutti D. O. (2007). To emmetropize or not to emmetropize? The question for hyperopic development. Optometry and Vision Science, 84(2), 97–102. https://doi.org/10.1097/OPX.0b013e318031b079
Mutti, D. O., Mitchell, G. L., Jones, L. A., Friedman, N. E., Frane, S. L., Lin, W. K., Moeschberger, M. L., & Zadnik, K. (2005). Axial growth and changes in lenticular and corneal power during emmetropization in infants. Investigative Ophthalmology & Visual Science, 46(9), 3074–3080. https://doi.org/10.1167/iovs.04-1040
National Cancer Institute. (2008). Eye anatomy [Image]. https://visualsonline.cancer.gov/details.cfm?imageid=7161
Norton T. T. (1999). Animal models of myopia: Learning how vision controls the size of the eye. ILAR Journal, 40(2), 59–77. https://doi.org/10.1093/ilar.40.2.59
Rohrer, B., & Stell, W. K. (1994). Basic fibroblast growth factor (bFGF) and transforming growth factor beta (TGF-beta) act as stop and go signals to modulate postnatal ocular growth in the chick. Experimental Eye Research, 58(5), 553–561. https://doi.org/10.1006/exer.1994.1049
Tkatchenko, T. V., & Tkatchenko, A. V. (2019). Pharmacogenomic approach to antimyopia drug development: Pathways lead the way. Trends in Pharmacological Sciences, 40(11), 833–852. https://doi.org/10.1016/j.tips.2019.09.009
Walline, J. J., Walker, M. K., Mutti, D. O., Jones-Jordan, L. A., Sinnott, L. T., Giannoni, A. G., Bickle, K. M., Schulle, K. L., Nixon, A., Pierce, G. E., Berntsen, D. A., & BLINK Study Group. (2020). Effect of high add power, medium add power, or single-vision contact lenses on myopia progression in children: The BLINK randomized clinical trial. JAMA, 324(6), 571–580. https://doi.org/10.1001/jama.2020.10834
Wildsoet C. F. (1997). Active emmetropization—Evidence for its existence and ramifications for clinical practice. Ophthalmic & Physiological Optics, 17(4), 279–290.






