Myopia: Causes, Prevention, and Treatment of an Increasingly Common Disease (2024)
Chapter: 7 Current and Emerging Treatment Options for Myopia
7
Current and Emerging Treatment Options for Myopia
This chapter focuses on clinical aspects of myopia: the treatments that are currently used and those that are being developed. The early parts of the chapter describe how the uncorrected blur associated with myopia can be treated. The chapter then transitions to the treatment of myopia progression. The foundations of the natural history of myopia are also described, so the reader may evaluate the efficacy of myopia progression treatments compared to myopic eye growth without intervention. Details from large-scale, randomized controlled clinical trials are included to provide examples of how myopia research is built, including cornerstone studies that laid the foundation for our current understandings in optical, pharmaceutical, and environmental interventions.
After touching on key perspectives of current treatment options from the International Myopia Institute1 and the 2023 Cochrane review on myopia, the chapter transitions to emerging treatment options. The chapter does not attempt to be exhaustive, and instead aims to identify those treatments that hold the most promise (for more detail see Khanal et al., 2024). While treatment options are available for myopia onset and progression, the effect sizes remain small. This chapter includes theories on why current treatments are not more effective in order to stimulate new research areas. The goal of this chapter is to describe myopia and its progression in a manner that provides support for intervening, from considerations for funding and research directions to conversations about clinical care.
Over the last 100 years and especially in the last two decades, much work has been done to determine the natural growth of the eye, its excessive growth in nearsightedness (myopia), and subsequently how to slow down this natural myopia progression. Treatment of the myopic eye has historically centered on correcting the blurry distance vision associated with it. By the 1600s, it was discovered that concave lenses could help focus light onto the retina of a long (myopic) eye by diverting the light rays entering the eye (Frangenberg, 1991). Despite having clearer vision with glasses, however, the nearsighted eye continued to elongate, worsening the condition (Hou et al., 2018). Therefore, in the late 1900s researchers began to turn their focus toward slowing down this growth or myopia progression, at first in animals (Norton et al., 1977; Wallman et al., 1978; Wiesel & Raviola, 1977). In 2003, a multi-center clinical trial funded by the National Institutes of Health was the first to show that the myopic growth of the human eye could be slowed with an optical intervention in U.S. school-aged children (Gwiazda et al., 2003). Since then, in addition to correcting myopic blur, attempts at slowing the growth of the myopic eye have been explored using a variety of treatment options.
While being less myopic is beneficial, the preferred outcome would instead be the complete cessation of myopia progression (or better, the prevention of it altogether). If the length
___________________
of the eye becomes excessive, the treatment may only partially reduce the risks of retinal detachment, myopic maculopathy, and glaucoma.
CURRENT TREATMENT OPTIONS FOR MYOPIA
Optical Treatments for Myopia
Glasses have been the mainstay for alleviating the side effect of blurry distance vision associated with myopia for hundreds of years and include a spectacle frame and lenses. The lens shape is concave, with the thinnest part of the lens in the middle, which helps the parallel rays of light entering the long eye diverge so they focus farther into the eye, preferably on the plane of the retina. The lens material was originally glass, which provides superior optics. Plastic lenses are used more often today due to their lighter weight. For children, plastic lenses are generally made of polycarbonate, a shatter-resistant material that protects the eyes of the child. Children may also have lenses made out of trivex, which makes lenses even thinner and lighter.
The most traditional lens design for treating the side effect of blur in myopia is single-vision, meaning there is one focusing power across the lens and not two, as in bifocals, and not a gradual increase in near power, as in progressive-addition lenses. Photochromic lenses, which change from light to dark and vice versa with changing ultraviolet (UV) light, do not significantly affect myopic blur in any way. Glasses can generally be worn by children of any age and require a proper fit so that the center and thinnest part of the lens sits directly in front of the eye’s pupil.
Contact lenses began to be used in the United States in the 1930s. Today’s contact lenses are plastic and offer more oxygen permeability than early glass versions (Moreddu, 2019). Contact lenses made of hard plastic are called rigid gas permeable (RGP) lenses. These lenses correct myopic blur in similar ways to glasses except that they are worn on the front surface of the eye, the cornea. RGP lenses also laid the foundation for orthokeratology, a system where RGP lenses are worn overnight and change the shape of the cornea enough to focus the light on the myopic retina so that vision is clear during the day without wearing the lenses. Soft contact lenses are made of a hydrogel material and were first FDA-approved in 1971. Unlike RGP contact lenses, which are custom fit to the shape of the cornea, soft contact lenses are generally 14 mm in diameter and drape over the approximately 12 mm diameter cornea. While contact lenses may be worn at any age, their use in myopic children generally starts at age 7 or 8 years, based on literature showing the safety of contact lenses in this age group (Chalmers et al., 2021; Sankaridurg et al., 2013; Walline et al., 2007, 2008, 2013).
Surgical Treatments for Myopia
Refractive Surgery
Refractive surgery is a term that describes any procedure that corrects the refractive error of the eye. The goal is to improve uncorrected visual acuity, thereby reducing dependency on glasses or contact lenses. One popular early refractive surgery was radial keratotomy. First introduced in the United States in 1978 (Bores, 1981), this procedure used deep radial incisions in the cornea to cause its flattening, thereby reducing its refractive power and moving the focus posteriorly to better match the longer myopic eye. In 1994, data were published showing the
safety and efficacy of radial keratotomy, with 70% of participants reporting no need to wear vision correction 10 years after surgery (Waring et al., 1994). However, subsequent longer-term experience with radial keratotomy showed continued corneal flattening with progressive hyperopia and irregular astigmatisms, among other changes (Koosha et al., 2024). The procedure was gradually abandoned with the advent of more predictable excimer-based laser refractive surgery and the FDA approval of this laser refractive surgery in 1995.
Corneal surface ablations like photorefractive keratectomy were originally performed, followed by laser in situ keratomileusis (LASIK). For treatment of myopia, both procedures are used to flatten the cornea to reduce its refractive power. In 2002, the American Academy of Ophthalmology reported that LASIK was generally safe, effective, and predictable, especially in low to moderate myopia (Sugar et al., 2002). Since then, other refractive surgeries have been studied and developed, including variants of excimer surface ablation such as laser-assisted subepithelial keratectomy (LASEK) and femtosecond laser-based small-incision lenticule extraction (SMILE), which uses the femtosecond laser to precisely dissect a portion of the corneal stroma, which is then physically removed and causes a similar net flattening of the cornea. See Box 7-1 for more information about how optical corrections and refractive surgery do not alter risk for myopia complications.
BOX 7-1
Optical Corrections and Refractive Surgery Do Not Alter Risk for Myopia Complications
Despite refractive surgery’s positive impact on minimizing dependency on glasses and contacts, it must be noted that refractive surgery corrects only the optical blur from the refractive error. Refractive surgery does not directly address presbyopia (loss of accommodation), and for those individuals who have lost accommodation due to age, reading glasses may still be needed after successful refractive surgery.
Perhaps most important to note is that the axially myopic eye remains physically long even after refractive surgery. So, while uncorrected vision is often much better following refractive surgery, the retina has still stretched from axial elongation of the eye and is still, as in any myopic eye, at increased risk for thinning, holes, and detachments (Haarman et al., 2020). This means that despite better uncorrected vision after refractive surgery, dilated eye exams are still important in addressing the potential ocular health risks associated with myopia.
Clear Lens Extraction
Clear lens extraction, also known as refractive lensectomy, is another surgical option to modify how light focuses on the retina. Clear lens extraction may be performed when corneal surgery is not possible. It is similar to cataract surgery whereby the human lens is removed; however, in clear lens extraction, the lens is removed in the general absence of cataract. Typically, a lens implant follows to correct distance vision. As with refractive surgery, the eye retains its original length. Reports in 1999 showed that individuals with high myopia who had clear lens extraction had nearly double the incidence of retinal detachments after seven years compared to those who did not have the surgery (Colin et al., 1999); however, later studies show better safety (Fernández-Vega et al., 2003; Srinivasan et al., 2016).
An important point is that clear lens extraction and corneal refractive surgery do not mitigate presbyopia. Because the crystalline lens is responsible for providing accommodation
and the ability to see ranges from far to near, when the crystalline lens is removed in clear lens extraction and replaced with a distance-vision-correcting intraocular lens implant, reading glasses are required following the procedure to see near. Currently available intraocular lenses cannot accurately accommodate in response to demand like the natural human lens, though there are multi-focal, extended depth of focus, and intraocular lenses with hinged haptics to provide various degrees of vision (from distance to near).
Phakic Intraocular Lens Implantation
Phakic intraocular lens implantation may be a refractive surgery option in high myopia when the cornea is not well suited for a laser or surgical procedure. In this procedure, the human lens is left in the eye and an additional lens is placed inside the eye. The lens may be inserted into the front (anterior chamber) of the eye and supported by the iris or iris angle. It may also be placed in the space behind the iris and in front of the natural lens. There are risks associated with phakic intraocular lenses, primarily related to their proximity to structures in the eye, though in 2009 the American Academy of Ophthalmology reported that the short-term safety and efficacy are acceptable. Continued evaluation is required to determine long-term risk of complications and safety (Huang et al., 2009).
TREATMENT OPTIONS TO MITIGATE SIDE EFFECTS OF AN AXIALLY ELONGATED EYE
Because myopia is associated with increased risk of retinal pathology (Haarman et al., 2020), there may be occasions when it is important to consider preventive measures that maximize retinal health. In contrast to interventions that aim to slow down the growth of the eye, these approaches are designed to minimize risk to the eye once it has already become too long and myopic (see Table 7-1).
Surgical Treatments for Retinal Effects of Myopia
Prophylactic retinal procedures have been considered in patients with high myopia. However, there is no clear answer on the value of these preventative procedures. Pneumatic retinopexy involves a surgeon injecting an expanding gas bubble into the back of the eye in an effort to press the retina closer to the back of the eye. Laser retinopexy aims to avoid detached retina by more firmly attaching the retina using laser photocoagulation. Scleral buckle attempts to hold the retina in place, while a cryotherapy or laser is used to “tack” down the retina. The American Academy of Ophthalmology suggests that prophylactic retinal procedures be based on many factors including symptoms, extent of the condition including thinning, holes, or tears, and post-operative complication risk (Silva & Blumenkranz, 2013). It should also be noted that prophylactic retinal procedures do not alter the side effect of blur related to myopia. These procedures are intended to prevent retinal detachments, which can leave an individual blind if left untreated.
Pharmaceutical/Nutraceutical Treatments for Retinal Effects of Myopia
The macula is the area in the retina that is most involved in clear central vision. Lutein is a naturally occurring antioxidant found in the macular region that is thought to act as a filter of
light, perhaps protecting the eye from sunlight damage. Lutein has been shown to provide benefit to the health of the macula since the Age-Related Eye-Disease Study 2 (AREDS2) was published in 2013 (Age-Related Eye Disease Study 2 Research Group, 2013). In age-related macular degeneration, the macula becomes fragile and functions more poorly as a result of aging-related pathological processes, such as chronic inflammation and lipid deposition, especially in those with a family history of this disease. In myopia, the macula can become fragile and function more poorly as a result of the retina stretching to accommodate the growth of the eye. In 2023, Yoshida et al. (2023) reported benefits of lutein supplements related to macular pigment optical density in highly myopic individuals in a randomized clinical trial. Because visual acuity, contrast sensitivity, and electroretinogram values were similar at 6 months between those who took lutein and those who took a placebo, further study in long-term and practical benefits is needed. However, lutein remains an emerging prophylactic management option for high myopia and its associated myopic maculopathy.
TABLE 7-1 Treatment Options for Myopia: Optical, Surgical, and Pharmaceutical/Nutraceutical
| Treatment Options for Myopia | Optical | Surgical | Pharmaceutical/Nutraceutical |
|---|---|---|---|
| For alleviating optical blur | Glasses Contact lenses Pinhole |
Laser refractive surgery (e.g., photorefractive keratectomy/LASIK/SMILE) Clear lens extraction Phakic intraocular lens implantation |
None available |
| To mitigate the risks associated with an axially elongated eye |
Prophylactic retinal procedures:
|
Lutein |
SOURCE: Committee generated.
HISTORY OF MYOPIA PROGRESSION WITHOUT INTERVENTION
Longitudinal Studies on Growth of the Human Eye and Myopia Progression
While myopic eyes were being studied in animals in the mid to late 20th century, little information was available on the typical growth of myopia (without intervention) in U.S. children. However, two large-scale longitudinal observational studies in diverse groups of U.S. children have shaped what is known about the natural growth of the human eye and myopia progression. Longitudinal studies on myopia progression show that without intervention myopic eyes continue to elongate throughout childhood. These studies thus provide the rationale for other longitudinal studies that evaluate how significantly myopia progression can be slowed with intervention.
The CLEERE Study
In 1989, the Orinda Longitudinal Study of Myopia was launched, the first of its kind, aiming to investigate normal eye growth and the development of myopia. In 1997, the study added three clinical centers to assess the influence of ethnicity on normal ocular and refractive error development, and thenceforward became known as the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE; National Library of Medicine, 2005).
In 1997, the Correction of Myopia Evaluation Trial (COMET) was funded by the National Eye Institute as a large-scale, randomized controlled trial of myopic children. The 3-year randomized COMET study was extended to year five. After the fifth year, the COMET study participants were no longer asked to remain in their randomized lens assignment. The COMET study became a natural history study (typical growth of myopia without intervention) of myopia when it became known as COSMICC (Collaborative Observational Study of Myopia in COMET Children; National Library of Medicine, 2016).
CLEERE was a seminal myopia study conducted between 1989 and 2010 at five clinical sites in the United States. Designed as an observational cohort study of ocular development and myopia onset, it collected data on an ethnically diverse group of over 4,500 non-myopic children ages 6 through 11 years at baseline. The CLEERE study was funded by the National Eye Institute and provided a foundation for natural history elements of myopia incidence and predictive factors of myopia progression in U.S. children (Jones-Jordan et al., 2021; Kleinstein et al., 2012; Zadnik et al., 2015).
Key Findings from the CLEERE Study:
- The CLEERE study defined myopia as −0.75 D or more of myopia in each of the principal meridians.
- The age at which a child became myopic ranged from 7 to 16 years, with the largest number of children diagnosed at age 11.
- The incidence rate of new cases increased yearly until age 11, then decreased.
- Among all non-myopic children at baseline, 16.4% (749/4,556) became myopic during the school-aged years.
- The proportion of new cases of myopia differed by race in U.S. school-aged children:
- 27.3% of Asian American children had new myopia during the time of the study, as did:
- 21.4% of Hispanic children,
- 14.5% of Native American children,
- 13.9% of Black children, and
- 11% of White children.
Predictors of myopia, when studied in 414 children with complete biometric and accommodative data who became myopic during the school-aged years, were elucidated. Factors associated with risk of myopia onset included having myopic parents, low amounts of time outdoors, high accommodative convergence-to-accommodation (AC/A) ratio (i.e., how much the eyes turn in when focusing power is changed), ocular components that resemble myopic eyes (long axial length, low lens power, relatively hyperopic retinal periphery), low amounts of hyperopia, and astigmatism. Despite these many risk factors, future myopia could be predicted best in non-myopic children by their current spherical equivalent refractive error alone. Optimal
cut-points for predicting future myopia decreased in hyperopia with age. Six-year-old children who were at less than +0.75 D of hyperopia were at increased risk for developing myopia, followed by +0.50 D for children ages 7–8, +0.25 D for those ages 9–10, and plano at age 11. Children who remained farsighted by age 8 or 9 (and had no parents who were near-sighted) tended to avoid near-sightedness altogether.
The CLEERE study also found that myopia progression is a function of age as well as of race and ethnicity. Myopia progressed faster in younger children. Asian American children experienced statistically significantly faster myopia progression compared with Hispanic children (estimated 3-year difference of −0.46 D), Black children (−0.88 D), and Native American children (−0.48 D), but with a similar progression to that of White children (−0.19 D). Parental history of myopia, time spent reading, and time spent in outdoor/sports activities were not statistically significant factors in multivariate models.
The COSMICC Study
The Collaborative Observational Study of Myopia in COMET Children (COSMICC) was funded as a longitudinal, subsequent, observational cohort study by the National Eye Institute and provided a foundation for natural history elements of myopia progression in U.S. school-aged children. The COMET study had preceded the COSMICC study using the same participants. Nearly all COMET participants switched from either progressive-addition spectacle lenses or single-vision spectacle lenses to single-vision glasses or contact lenses. Of the original ethnically diverse group of 469 children, 362 (77%) were studied longitudinally to year 14, when the average age was 24.1 years (Scheiman et al., 2016).
Key Findings from the COMET/COSMICC Study:
- School-aged children living in the United States in the study progressed 0.50 D per year on average wearing single-vision glasses.
- Younger children progressed faster and developed a higher level of myopia despite myopia being similar to the older cohort at baseline.
- A higher level of education in the parent of a COMET child was associated with a higher level of myopia in the COMET child.
- The average age when myopia progression stopped was 15 years; 48% of children were stable at age 15 and 77% at age 18, but 4% were still experiencing myopia progression at age 24.
- The average amount of myopia at the end of myopia progression was −4.87 D.
CURRENT TREATMENT OPTIONS FOR SLOWING MYOPIA PROGRESSION
How Myopia Progression Occurs: A Recap
In U.S. population-based studies, most preschool children are farsighted (Borchert et al., 2011; Multi-Ethnic Pediatric Eye Disease Study Group, 2010; Wen et al., 2013). As discussed in Chapter 2, farsighted or hyperopic eyes have axial lengths that are too short for the optical power of the eye. As the child develops, the eye will become less hyperopic due primarily to growth in axial length that outpaces the decreases in optical power of the cornea and crystalline lens (Gordon & Donzis, 1985; Mutti et al., 2018). As the eye grows, the refractive error moves closer
to zero diopters or “emmetropia,” so this growth process is referred to as emmetropization. The eyes of most children never grow to reach a point of perfect emmetropia, leaving them slightly farsighted.
The mechanisms of emmetropization are not completely known, as discussed in Chapters 5 and 6. One theory is that retinal blur signals the eye to grow axially (front to back) and the system is fine-tuned by accommodation, the process by which the lens changes shape to focus near objects on the retina. However, if the eye of the preschool child fails to receive the internal signals to stop eye growth, it will continue to grow and axial length will become too long for the optics to compensate, creating blur on the retina and stimulating yet more axial growth. It is known that once a child’s eye is nearsighted, the eye continues to grow, becoming more and more myopic throughout the school-age years, even when corrected (Gwiazda et al., 1993; Hou et al., 2018; Norton, 1999; Walline et al., 2020a; Wildsoet, 1997). This continuous process is called “myopia progression.”
Early animal studies using chicks (Wallman et al., 1978), tree shrews, (Norton et al., 1977), and monkeys (Wiesel & Raviola, 1977) helped researchers discover that eye growth could be modulated. Eyelid closure or light scattering lenses applied to deprive the eye of form vision (a procedure known as form deprivation), were found to cause the eye to grow excessively and become highly myopic. Negative-powered lenses worn by an animal were shown to induce compensatory eye growth, leaving the animal myopic when the lenses were removed (Hung et al., 1995). Later research in monkeys showed that form-deprivation was also reversible (Qiao-Grider et al., 2004; Smith et al., 2002). If eye growth could be manipulated in animals, researchers wondered if eye growth could be slowed in humans. Thus, scientists began to investigate methods to slow myopic progression in children using a range of treatments, including optical methods (progressive-addition lenses, soft multifocal contact lenses, and orthokeratology rigid gas-permeable lenses) and pharmaceutical methods (atropine eye drops, pirenzepine eye drops).
OPTICAL TREATMENT TO SLOW HUMAN EYE GROWTH
Early Theories on Optical Mechanisms
Typically, children have very strong and accurate focusing systems for near work (Hofstetter, 1944). However, in the 1990s, Gwiazda et al. (1993) and Abbott et al. (1998) reported that some myopes were not very accurate at near visual. Animal research suggested that blurry vision may cause eye growth (Norton, 1999; Wildsoet, 1997). Researchers hypothesized that if vision could become clearer by using a lens that improves near visual focus, less blur may cause less eye growth. As mentioned earlier in this chapter, progressive-plus powered lenses used in a large-scale, national, multicenter, randomized clinical trial funded by the National Eye Institute did work to slow the growth of the eye (Gwiazda et al., 2003). Despite statistical significance, however, the effect was small and did not support widespread use of multifocal spectacle lenses. However, the study did show proof of concept that human eye growth could be slowed with optical interventions.
Clinical researchers went on to study other lens modalities based on experimental myopia studies in animals. Perhaps it wasn’t near visual focus (accommodation), or lack thereof, driving eye growth. Two important concepts helped researchers refine this line of thinking. First, research suggested that blur on the retina could induce eye growth; however, it is now
understood that the direction of the blur is important. When the direction of the blur is known (i.e., whether the focal point is in front of or behind the retina, myopic or hyperopic), this is known as defocus. Natural eye growth typically starts with an eye that is too short, where the optics land behind the back of the eye. The eye can alter its lens to focus the image on the retina, or it can grow. Based on animal experiments, it is theorized that blur caused by hyperopic defocus (image focused behind the retina) could be a driver of eye growth for emmetropization (Troilo et al., 2019). Second, Smith et al. (2017, 2009a) showed that the central retina may not be very important in eye growth. When the macula or central retina is compromised in animals, the remaining healthy peripheral retina can still slow and drive eye growth with various lens powers (Huang et al., 2011; Smith et al., 2007, 2009a).
Questions arose: How important is the peripheral retina to focus? And, if hyperopic defocus, in which the optics of the eye focus behind the retina, was a driver for eye growth, could myopic defocus, in which the optics of the eye focus in front of the retina, be a signal to slow down the growth of the eye? Further, if this relative myopic defocus occurred in the periphery, would the growing eye have a stronger stop signal? And how would fovea-driven accommodation change the peripheral refractive status? Glasses to treat myopia have historically ensured that light is focused on the back central part of the eye. This is accomplished with a single divergent lens power and allows the wearer to have clear central vision. However, because of the increasingly prolate (i.e., oval) shape of the growing myopic eye, the peripheral retina remains blurry. Progressive addition lenses that have increasingly strong near power may have worked in the COMET study by creating relative myopic defocus in the peripheral retina rather than the hypothesized impact on near focus. That said, the relative myopic defocus would have occurred only on the superior (top part of the) retina since progressive addition lenses are placed in the inferior (bottom part of the) view of the patient.
The Bifocal Lenses in Nearsighted Kids (BLINK) study attempted to answer the question: If putting the focal plane in front of the retina could be accomplished in all meridians of the peripheral retina, would eye growth slow down using multifocal soft contact lenses? These lenses were typically used for individuals over age 40 for near visual focus, making them readily commercially available. The optics of the soft contact lens include a center zone for clear central distance vision, while the periphery of the lens adds medium- and high-powered optics to help individuals see more clearly up close (Figure 7-1). However, in children who could see close-up just fine, it was reasoned that the lenses could create relative myopic defocus, thus creating a system where more of the retina is receiving a hypothetical stop signal. The results supported the theory underlying the study: there was a dose-response relationship between the level of peripheral myopic defocus and treatment efficacy (Walline et al., 2020b). Medium additional power slowed myopia progression relative to single-vision lenses, but not to a statistically significant degree. High additional power slowed down eye growth by 0.38 D over 3 years, which did differ statistically from the single vision lens option.
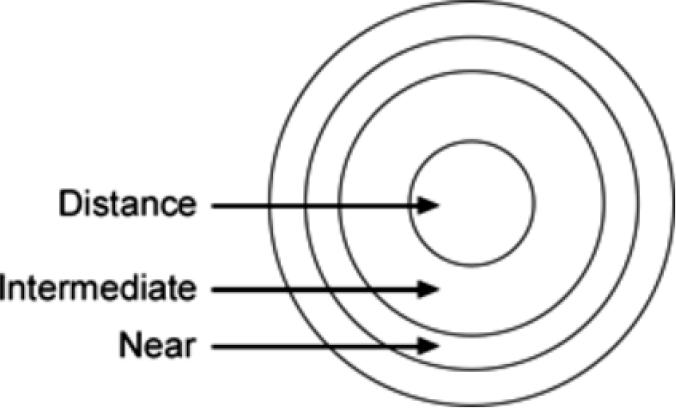
SOURCE: Adapted from Walline et al., 2017.
CORNERSTONE HUMAN STUDIES ON SLOWING THE GROWTH OF THE HUMAN EYE
The Correction of Myopia Evaluation Trial (COMET)
COMET was the first large-scale, randomized clinical trial to show proof of concept that growth of the human eye could be slowed with an optical intervention (progressive-addition lenses in glasses). The researchers who created COMET, which was funded by the National Eye Institute and conducted in the United States, reasoned that blur on the retina causes growth of the eye (Norton, 1999; Wildsoet, 1997) and that some myopes had poor near-focusing skills (Abbott et al., 1998; Gwiazda et al., 1993). If near focus could be made more accurate, in this case by using a progressive-addition lens to provide gradually increasing reading power in the bottom of the child’s spectacle lenses, it was hypothesized that near vision would become clearer, thus reducing retinal blur, and thus reducing human eye growth.
COMET investigators at four U.S. sites recruited a group of ethnically diverse children ages 6 to less than 12 years old who had low to moderate spherical-equivalent myopia, ranging from −1.25 D to −4.50 D by cycloplegic autorefraction. The average baseline age of the 469 children randomized was 9.3 years (+/− 1.3 years). Participants were randomized to either single-vision lenses or progressive-addition lenses at a 1:1 ratio. At year three, 462 of the 469 (98.5%) randomized participants were retained and evaluated. The COMET study group went on to publish 36 peer-reviewed journal articles between 2001 and 2018 (COMET Group, 2013; Gwiazda et al., 2002, 2011; Hou et al., 2018; Hyman et al., 2001).
The COMET study showed that the children who wore single-vision glasses had progressed in their myopia on average by −1.48 (±0.06) D (more nearsighted), while those wearing progressive-addition lenses had progressed by only -1.28 (±0.06) D. This difference of 0.20 (+0.08) D between the two groups was statistically significant (P = 0.004). Results were similar concerning change in axial length. Children wearing single-vision glasses had eye growth or axial elongation of 0.75 (+0.02) mm, and those wearing progressive-addition lenses had eye
growth of 0.64 (+0.02) mm (See Figure 7-2.) This 0.11 (+0.03) mm difference between the two groups was also statistically significant (P = 0.0002). Importantly, this treatment effect was seen in the first year only; no additional treatment effect was seen in years two and three. COMET investigators deemed these treatment differences statistically different but not clinically meaningful. However, this was the first large-scale, prospective, multi-center, federally funded randomized clinical trial to show slowing of human eye growth by an optical intervention.
Key Findings from the COMET Study:
- Progressive-plus powered lenses slowed myopia progression, but only by 0.20 D over three years.
- Axial length growth was also slowed by 0.11 mm using the progressive-addition lens.
- These findings were deemed statistically significant but not enough to warrant a change in clinical practice.
- The treatment effect was seen only in the first year. No additional treatment effect was seen in years two and three.
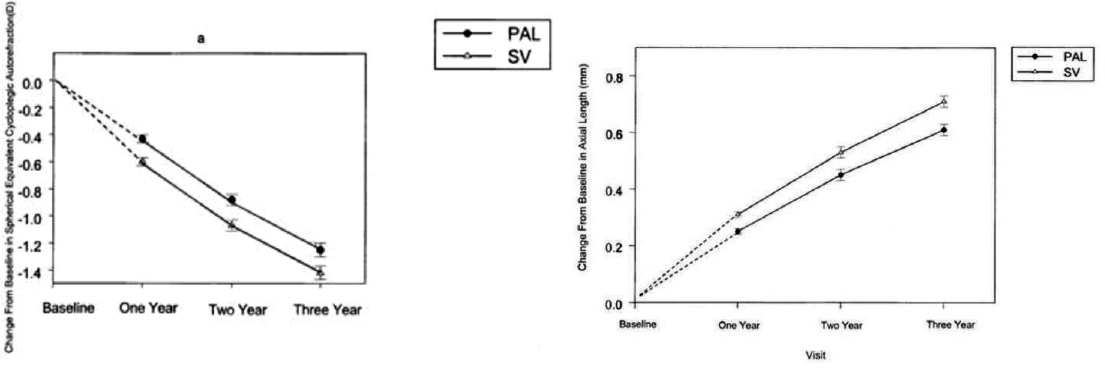
NOTES: Progressive-Addition Lenses (PAL) had a treatment effect compared to single-vision lenses (SV). The treatment effect on both spherical equivalent refractive error and axial length was largely seen only in the first year of the study. Mean change in (A) spherical equivalent refractive error. (B) Mean increases in the axial length of eyes of children in the PAL and SVL groups at each annual visit. Dashed lines are included for illustrative purposes, to show the similarity of the two treatment groups at baseline. Error bars, SE.
SOURCE: Gwiazda et al., 2003.
The Bifocal Lenses in Nearsighted Kids (BLINK) Study
BLINK was a large-scale, multicenter, randomized control trial funded by the National Eye Institute to evaluate myopia progression in 294 children wearing single-vision, soft contact lenses vs. commercially available multifocal contact lenses. Children were ages 7 to 11 years at baseline with spherical equivalent myopia between −0.75 and −5.00 D and less than one diopter of astigmatism when recruited between 2014 and 2016 (Walline et al., 2017).
By 2010, peripheral positive lens defocus, when the focus of light is behind the peripheral retina, had been shown to induce central axial myopia in monkeys (Smith et al., 2009b, 2010).
Prior to this time, blur-inducing myopic growth was thought to be concentrated on the central retina. These studies were the first of their kind suggesting that the peripheral retina could mediate the axial length of the eye. If peripheral minus lens defocus could induce myopia, could peripheral myopic defocus (where the light is focused in front of the peripheral retina) minimize myopic progression? Animal studies suggested that this was true (Huang et al., 2012; Smith et al. 2013).
Human studies followed with a variety of optical interventions. Orthokeratology, a technique in which children wear rigid gas-permeable plastic (hard) contact lenses overnight to flatten the cornea, may work in this mechanism to minimize peripheral hyperopic defocus. MiSight contact lenses, the first soft contact lenses FDA-approved for treating myopia progression, were also suggested to put the focus of the light in front of the retina. These daily-disposable contact lenses use alternating concentric rings within the optics of the contact lens and may also work by taking advantage of relative myopic defocus.
BLINK investigators wondered if commercially available, standard, soft multifocal contact lenses could be used in U.S. children to create relative myopic defocus and reduce myopia progression over three years. Three groups were compared, each wearing a different type of contact lenses: single-vision, medium-add power, and high-add-power. As shown in Figure 7-3, the contact lenses choice altered the position of focused light on the peripheral retina. At year three, 292 of 294 (99%) randomized participants were retained and evaluated (Berntsen et al., 2023; Chandler et al., 2023; Gaume et al., 2022; Walline et al., 2013, 2020b).
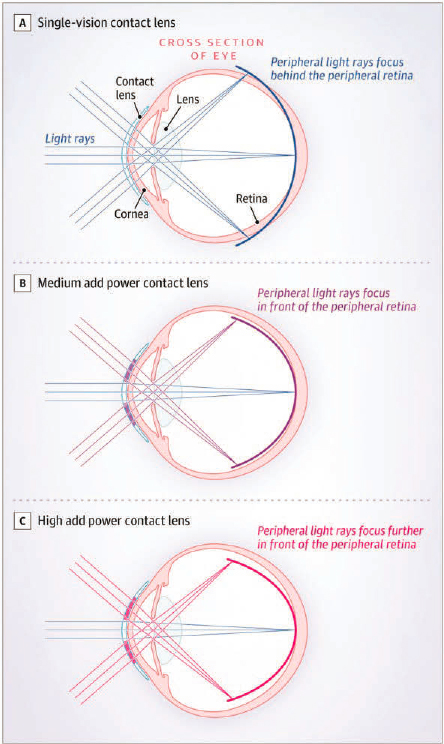
NOTE: In the BLINK study, three groups of children wore three different contact lenses that altered the peripheral focus to be either (A) behind the retina using single-vision lenses; (B) slightly in front of the peripheral retina using medium-add-power contact lenses; or (C) further in front of the peripheral retina using high-add-power contact lenses.
SOURCE: Walline et al., 2020b.
Key Findings from the BLINK Study:
- School-aged children living in the United States progressed −1.05 D over 3 years (average of −0.35 D per year) wearing single-vision contact lenses. Commercially available, soft multifocal contact lenses with a high add were able to slow myopia progression by 0.46 D over three years.
- There was no statistically significant difference between single-vision contact lenses and lenses with medium add powers.
- The number of adverse events were minimal, supporting the safety of soft contact lenses in children ages 7 to 11 years old.
- Nearly 5 years of multifocal contacts with an add power did not significantly affect the participants’ ability to focus at near without the lens.
- Despite the theory of myopic peripheral defocus slowing myopia and the potential to induce peripheral defocus in a multifocal contact lens, most peripheral defocus metrics and defocus at most peripheral retinal loci accounted for little to no variance in the treatment effect of the +2.50 D addition lens. Thus, the mechanism of the treatment effect did not appear to be peripheral defocus or pupil size (Berntsen et al., 2023). (Also see Figure 7-4.)
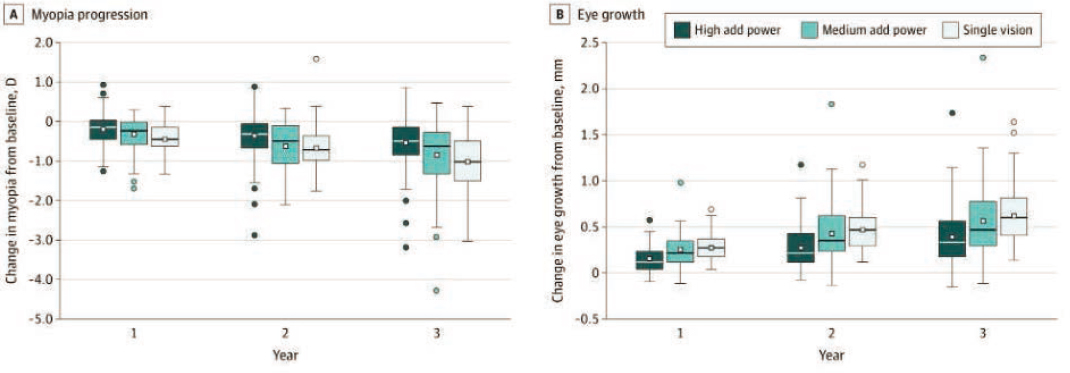
NOTE: Results of the BLINK study for refractive error change (A) and change in axial length; (B) High-add powers were able to slow myopia progression by 0.46 D and axial elongation by 0.23 mm over three years. There were no statistically significant differences between single-vision contact lenses and medium-add powers. See Figure 7-3 for description of the treatment groups.
SOURCE: Walline et al., 2020b.
CURRENT OPTICAL TREATMENTS FOR MYOPIA PROGRESSION AVAILABLE IN THE UNITED STATES
Currently, there are no spectacle options available in the United States for treatment of myopia progression (see treatment section below). However, in addition to the CooperVision Biofinity Multifocal, other contact lens options have been used to slow the growth of the eye (see Figure 7-5) and are based on the theory of providing relative peripheral myopic defocus.
The MiSight® contact lens by CooperVision, Inc., is the only soft contact lens option that is FDA-approved specifically for slowing the growth of the myopic eye. Long-term study results from large-scale, multicenter, national, longitudinal clinical research suggest that (a) myopia slows in children wearing these daily disposable contact lenses compared to children wearing single-vision contact lenses; (b) the benefit occurs even in later childhood; (c) the treatment effect continues to accrue beyond the initial year into subsequent years; and (d) although there is a rebound effect after use of the contact lenses is discontinued, it is not statistically significant (Chamberlain et al., 2022 Lumb et al., 2023).
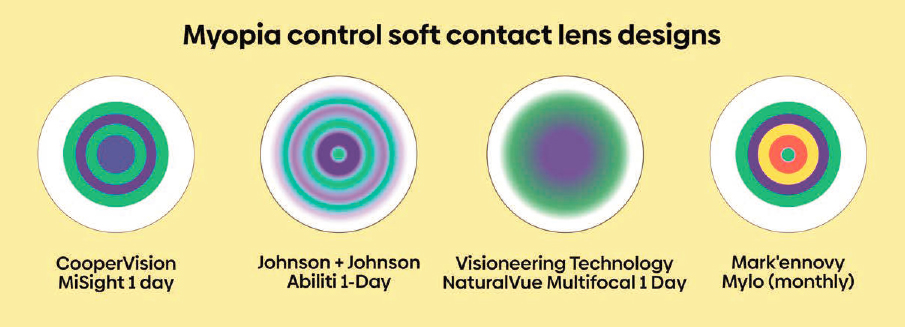
NOTE: The MiSight 1 day contact lens by CooperVision is the only FDA-approved treatment option for myopia control currently available in the United States. Other contact lens companies are working toward such labeling. These soft contact lenses use concentric rings of variable optics to try and direct some of the light entering the eye to focus in front of the retina, rather than in focus with the retina, in hopes of serving as a partial stop signal for eye growth.
SOURCE: Figure reproduced from “How do myopia control soft contact lenses work?” Published on MyKidsVision.org with the permission of MyopiaProfile.com.
Additionally, orthokeratology rigid contact lenses also seem to take advantage of minimizing peripheral hyperopic defocus. As mentioned earlier, these lenses are worn at night to reshape the cornea by morning to minimize the need for glasses or contacts during the day. Because the cornea takes on the shape of the lens and is flattened, the optics of the eye are pulled in front of the retina. In a meta-analysis, orthokeratology was reported to slow down the axial-length growth of the eye on average by 0.25 mm over the course of 2 years (Wen et al., 2015). Ranking just below the use of high- and mid-concentration atropines, orthokeratology seems to provide the most effective treatment for slowing growth in axial length, according to the Cochrane Review from 2023 (Lawrenson et al., 2023).
BOX 7-2
What Is the Role of Astigmatism in Myopia Development and Treatment Options?
In a population-based study of U.S. preschoolers living in and around Los Angeles, California, the prevalence of astigmatism was 6% in non-Hispanic White children and 8% in Asian children (Wen et al., 2013); in Hispanic and African American children in the same area, the prevalence was higher at 17% and 13% respectively (Fozailoff et al., 2011). In a population-based study in and around Baltimore in 2013, myopic preschool children were 4.6 times more likely to have astigmatism than children without refractive error (McKean-Cowdin et al., 2011).
Astigmatism in children is often corneal or lenticular in origin. Infantile astigmatism is associated with myopia during the school-aged years (Gwiazda et al., 2000). From Gwiazda et al. (2000) through to CLEERE (Zadnik et al., 2015), against-the-rule astigmatism (astigmatism where the steepest curve lies near the 180-degree meridian) was associated with a higher risk of myopia onset. The mechanism for why against-the-rule astigmatism is more associated with myopia onset than with-the-rule astigmatism (a more common form of astigmatism, where the steepest curve lies near the 90-degree meridian) is unclear; however, neither is as predictive for myopia onset as spherical equivalent refractive error (Zadnik et al., 2015).
The role of astigmatism in myopia onset and progression is made more unclear as most randomized controlled treatment trials (the gold standard in research) often exclude participation by children with high astigmatism, in order to study a more homogenous eye type. Because this may limit well-studied treatment options and disproportionately affect racial and ethnic groups due to differences in astigmatism prevalence, further study of the role of astigmatism in myopia onset and progression treatments may be beneficial. That said, astigmatism is not emphasized in this report, since its influence on myopia development and progression appears to be limited.
PHARMACOLOGICAL TREATMENTS FOR MYOPIA PROGRESSION
Myopia has been associated with education, implicating a role for near work (see Chapter 5, Onset and Progression of Myopia). Thus, paralyzing the eye’s focusing power even in one of the two eyes might reduce myopia progression (Luedde, 1932). In the mid-1960s and 1970s, atropine 1% was being studied, given its ability to paralyze the eye’s focusing ability for near work. Atropine eye drops showed a treatment effect in the treated eye, but only while using the drop (Bedrossian, 1966, 1979; Brodstein et al., 1984). Later, studies using eye drop alternatives to atropine like cyclopentolate (Yen et al., 1989) and tropicamide (Shih et al., 1999) showed no treatment benefit, despite the ability of these alternatives to at least moderately paralyze the eye’s ability to focus near objects (or increase pupil size). Taken together, these results suggested that atropine’s beneficial effects on myopia were potentially due to another mechanism and not to the blocking of accommodation. Despite evidence that atropine eye drops could slow down the growth of the eye, side effects remained. Light sensitivity from the dilated pupil and near blur that accompanies the use of atropine makes daily use challenging and has the potential to increase the dropout rate in atropine studies. However, as the prevalence of myopia increased in
the late 1900s, especially in Asia, the need for a treatment that effectively slowed myopia progression became urgent.
Early Theories on Pharmaceutical Mechanisms
Atropine Treatment
The mechanism to explain how atropine works on the eye remains unclear. Atropine is a nonselective muscarinic cholinergic antagonist. Thus, atropine’s protective effects on myopia progression were expected to be due to inhibiting the effects of acetylcholine on muscarinic acethylcholine receptors, which are found on the smooth muscle fibers in the ciliary muscle that adjusts the lens shape during focusing. However, both historical and recent literature suggests that atropine’s action on myopia is not related to action on muscarinic receptors, as atropine can also inhibit myopic eye growth in chicks, animals whose vision accommodation is mediated by nicotinic and not muscarinic receptors (McBrien et al., 2013; Stone et al., 1991). Also, ablating cholinergic retinal neurons did not alter the response to atropine (Fischer et al., 1998; Thomson et al., 2021; see review McBrien et al., 2013).
Atropine treatment has been associated with changes in choroidal thickness. However, evidence for a causal role is missing. Choroidal thickness has been shown to increase with atropine 1.0% in children (Jiang et al., 2021b; Zhang et al., 2016), with atropine 0.01% after 3 months (Wu et al., 2023), and in the LAMP study using various concentrations of low-dose atropine that were dose-dependent (Yam et al., 2022a). However, a recent meta-analysis found no significant effect with atropine 0.01% (Meng et al., 2023). Choroidal thickness has also been shown to be increased in myopic children treated with orthokeratology (Xiao et al., 2024), repeated low-level red therapy (Liu et al., 2024), multi-focal soft contact lenses (Peng & Jiang, 2023), and defocus-incorporated multiple-segment (DIMS) lenses (Chun, 2023). In addition, choroidal thickness changes have been observed in many animal studies in response to experimental myopia (Che, 2024; Chen et al., 2022; Jordan-Yu, 2021; Wallman, 1995) and when directly increasing choroidal thickness from intravitreal injections of atropine in chickens (Mathis et al., 2021). Collectively, this evidence does not support a causal relationship of atropine on choroidal thickness.
Another way atropine may have its anti-myopic effect is through dopamine, a neurotransmitter found in the retina and reported as a potential “stop” signal for myopic eye growth (Feldkaemper & Schaeffel, 2013; Stone et al., 1989; Zhou et al., 2017). Atropine has been shown to increase dopamine in the eye (Mathis et al., 2021; Schwahn et al., 2000; Zhu et al., 2022), particularly at high doses (Thomson et al., 2021). Studies investigating a potential causal role of atropine on dopamine release have been mixed, with dopamine receptor antagonists reducing atropine-induced choroidal thickening in response to flickering light in chickens (Mathis et al., 2023), while dopamine receptor antagonists did not block atropine’s inhibition on myopia in chickens (Thomson et al., 2021). In addition, atropine reduced axial elongation in Lrp2-/- mice but did not alter dopamine or 3,4-dihydroxyphenylacetic acid (DOPAC) levels (van der Sande et al., 2023).
Other potential sites of action for atropine include vasoactive intestinal polypeptide (Wang et al., 2024), nitric oxide (Carr & Stell, 2016), and gamma-aminobutyric acid (GABA) (Barathi et al., 2014). More research is needed to evaluate the causal mechanisms of atropine, which may provide opportunities to optimize the treatment and reduce side effects.
Pirenzepine Ophthalmic Solution
The development of pirenzepine ophthalmic solution was an attempt to slow down the growth of the myopic eye using an alternative nightly eye drop. Atropine is a nonselective muscarinic antagonist that has affinity for all five acetylcholine receptor subtypes found in the retina. While this allowed the concentration of atropine to be effective in more diluted concentrations, side effects remain pervasive given its nonselective nature, affecting many parts of the eye. Pirenzepine is a more selective muscarinic receptor 1 (MR1) antagonist (Leech et al., 1995; Rickers & Schaeffel, 1995). In a 2003 study, pirenzepine was shown to be well tolerated in children (Bartlett et al., 2003). A 2008 report suggested that pirenzepine was effective for slowing refractive error progression but did not have a statistically significant effect on axial length (Siatkowski et al., 2008). Interestingly, animal experiments suggest a potential influence of pirenzepine on dopamine by increasing tyrosine hydroxylase expression in the retina (Qian et al., 2015).
An effective drug-based treatment for myopia would have the advantage of convenience, especially if the drug could be taken by mouth. Therefore, further research is needed to identify pharmacological agents that are more effective and have fewer adverse effects than current options.
CORNERSTONE ATROPINE STUDIES FOR MYOPIA PROGRESSION
Atropine Treatment of Myopia (ATOM) Study
ATOM was a cornerstone study that evaluated the effect of atropine 1% on myopia progression in a double-masked, randomized clinical trial. Children were from a single center in Singapore, ranging in age from 6 to 12 years old and with myopia ranging from −1.00 D to −6.00 D. Children were randomly assigned to two groups: the active treatment participants received atropine 1% eye drops in one eye and placebo drops in the fellow eye, while the control participants received placebo eye drops in each eye. At the end of the 2-year study, 346 of the 400 (86.5%) randomized participants were evaluated. The results of ATOM were published by Chua in 2006 and showed a very good treatment effect of 0.92 D in myopic refractive error at the end of year two (−1.20 +0.69 D in placebo-treated control eyes vs. −0.28 ± 0.92 D in atropine-treated eyes; P <0.001). Axial length was also nearly halted using atropine 1% with only 0.02 +0.35 mm growth in 2 years compared to 0.38 ±0.38 mm in the placebo-treated control eyes (Chua et al., 2006). Despite the treatment effect while using atropine, stopping the treatment caused a greater increase in myopia in atropine-treated eyes compared to placebo-treated eyes (−1.14 ± 0.80 D vs. −0.38 ± 0.39 D respectively, (P < 0.0001), with the atropine group almost catching up with the placebo group after stopping the atropine drops (Tong et al., 2009).
In children that were treated for two years and then untreated for one year, the treatment difference at year three between the atropine untreated group and the placebo untreated group diminished from 0.92 D to 0.28 D, almost negating the benefits of atropine’s use. Additionally, although the overall treatment effect of atropine 1% remained better than placebo, the side effects of poor near visual acuity and light sensitivity remained. Further, it is unclear if accommodation skills and near visual acuity returned to normal after stopping atropine. As such, a need for an eye drop with fewer side effects was emerging.
Atropine Treatment of Myopia 2 (ATOM 2) Study
ATOM 2 was a second seminal study, evaluating the effect of atropine 0.5%, 0.1%, and 0.01% on myopia progression in a double-masked, randomized clinical trial. Investigators proposed that reducing the concentration of atropine 1% would result in less pupil dilation, better near vision, and less light sensitivity, while still slowing myopia progression. In 2012, ATOM 2 revealed the safety and efficacy of 0.5%, 0.1%, and 0.01% atropine applied over two years in 400 children from Singapore 6 to 12 years of age who had at least 2.00 D of myopia. The mean progression of myopia was not significantly different between the 0.5% and 0.1% groups (−0.30 ± 0.60D and −0.38 ± 0.60D). Both concentrations were more effective than the group treated with 0.01% (−0.49 ± 0.63D). However, the two stronger concentrations had statistically more side effects than the 0.01% atropine, which showed negligible changes in accommodative ability, near visual acuity, and pupillary size. Further, after stopping the treatment for one year, subjects on 0.5% atropine had significantly more rebound progression than those who had been on 0.1% and 0.01% atropine (Chia et al., 2014).
The ATOM 2 study was the first of its kind and limited by the lack of a control group, since 0.01% was initially chosen by investigators to serve as a possible placebo treatment. Regardless, given the lack of side effects and similar results, the atropine 0.01% became the new atropine of choice for the treatment of myopia progression for children living in East Asia (Chen & Yao, 2021; see Figure 7-6).
Key Clinical Findings from the ATOM and ATOM 2 studies:
- School-aged children living in Singapore experienced only 0.28 D of myopia progression in 2 years while treated with atropine 1% eye drops every evening, a treatment effect of 0.92 D. However, side effects of near blur, light sensitivity, and pupil dilation accompanied the atropine 1% use.
- After stopping the atropine 1% eye drop, myopia progressed more quickly than in children placed on placebo eye drops. This is called the rebound effect, and it reduced the treatment effect from 0.92 D on the eye drops at year two to 0.28 D off the eye drops at year 3.
- Lower concentrations of atropine have reasonably good treatment effects with less rebound. In 2016, atropine 0.01% showed a reasonably good treatment effect without the strong rebound effect.
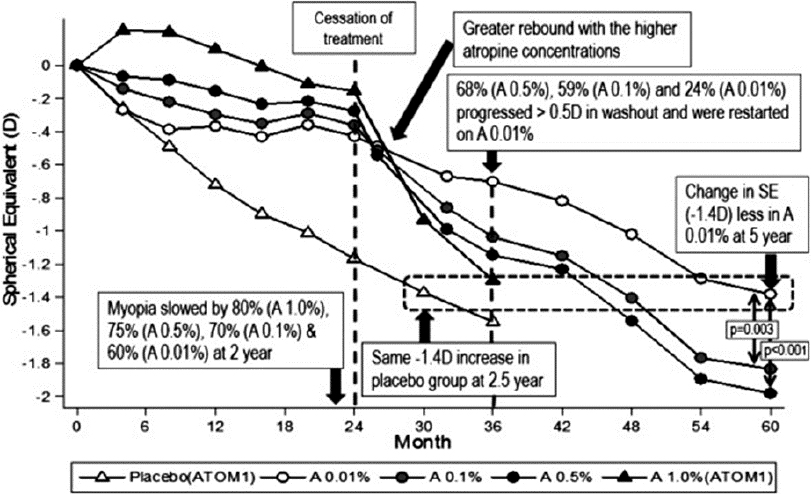
NOTE: The spherical equivalent refractive error plotted across time for the three atropine treatment groups (0.01%, 0.1%, and 0.5%) and placebo control.
SOURCE: Chia et al., 2016.
Low-Concentration Atropine for Myopia Progression (LAMP) Study
LAMP is a five-year, single-center, randomized clinical trial of atropine 0.05%, 0.025%, and 0.01% in children ages 4 to 12 years old living in Hong Kong. In year one, the authors found that the 0.05% atropine group had the largest reduction in myopic progression. While side effects were dose-dependent, they found that atropine was relatively well tolerated in all the groups. The Phase 2 report describes the second year of the study in which the atropine groups remained at the same concentrations, and the placebo group stopped using the placebo drop and started using 0.05% atropine. The authors suggested that 0.05% atropine is the most effective dose of atropine and side effects were well tolerated. Over three years, those in continued treatment continued to show dose-dependent effects (see Figure 7-7). Washout rebound was also concentration-dependent, but the differences in rebound myopia between 0.01%, 0.025%, and 0.05% were not significant (Yam et al., 2019, 2020, 2022b).
Key Findings from the LAMP Study:
- Children ages 4 to 12 years at baseline experienced the best treatment effect using 0.05% atropine compared to those using atropine 0.025% and 0.01%.
- Treatment benefit for myopic axial elongation was small at 0.05 mm at year one using atropine 0.01% in 2019.
- The success of low-dose atropine in children living in Asia prompted large-scale randomized clinical trials in non-Asian countries.
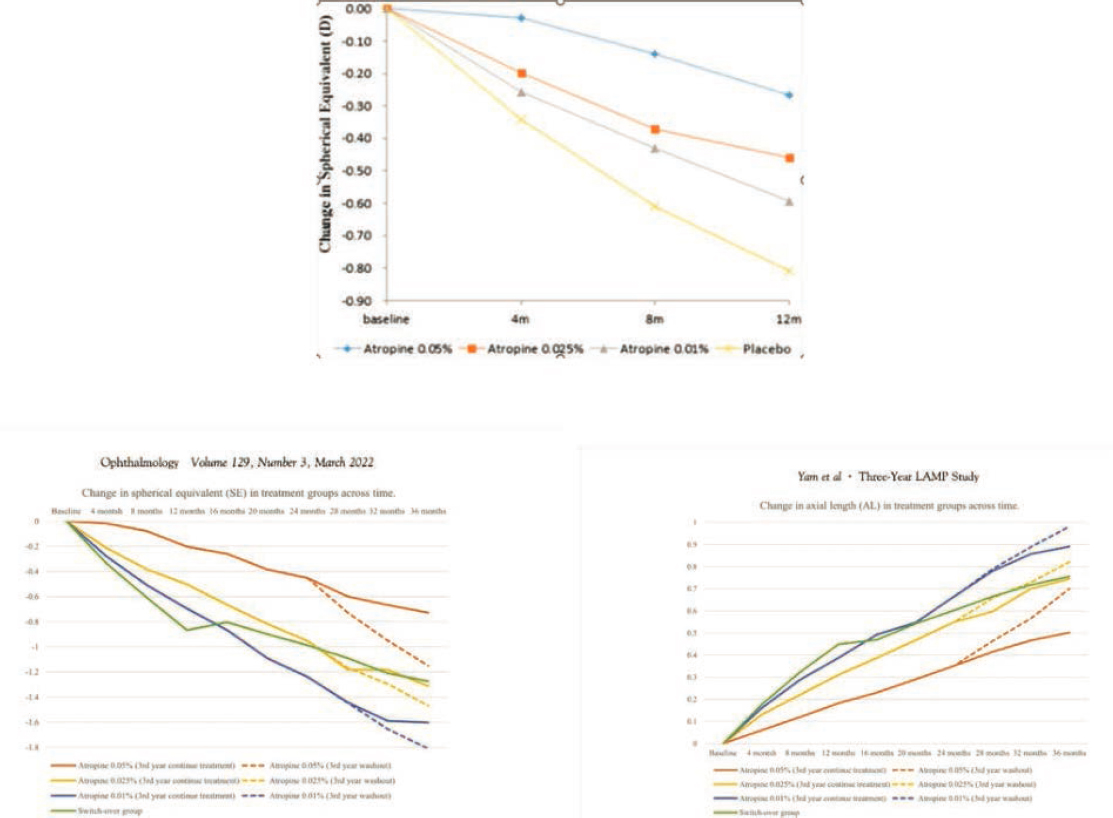
SOURCE: Yam et al., 2019.
CURRENT PHARMACEUTICAL TREATMENTS AVAILABLE IN THE UNITED STATES
The ATOM study results in 2006 (Chua et al., 2006) may be the impetus for atropine’s widespread use for myopia control. A report of a recent survey completed by pediatric ophthalmologists worldwide suggests that over half the respondents treat myopia progression and that 70% prescribe atropine as their go-to treatment for myopia control (Zloto et al., 2018). While atropine 0.01% was the most popular concentration (Zloto et al., 2018), two large-scale, randomized clinical trials conducted in the United States reported marginal to no treatment effect using either atropine 0.01% or atropine 0.02% (Repka et al., 2023; Zadnik et al., 2023). The LAMP study of children with myopia in Hong Kong suggests that 0.05% may be the optimal concentration in Chinese children, balancing side effects and treatment effect (Yam et al., 2022b).
Studies suggest that the higher the concentration of atropine is, the better the treatment effect (Chia et al., 2012; Yam et al., 2022b)—often, but not always (Gong et al., 2017b; Zadnik et al., 2023). However, the side effects do seem to be consistently dose-dependent, with higher
concentrations causing more light sensitivity, near blurred vision, and rebound growth after stopping use of the drops (Gong et al., 2017b; Yam et al. 2022b).
In summary, atropine is the only widely used pharmacological treatment for myopia progression. Yet, there remain many unanswered questions related to atropine. While long-term efficacy is not well studied, recently published data attempt to provide insight. Participants in the ATOM study were contacted 10 and 20 years after the conclusion of the original clinical trial. Despite atropine concentrations ranging from 0.01% to 1.0% demonstrating short-term efficacy during the clinical trial, these treatments did not have a long-term effect 10 and 20 years after treatment ended, at least in the sample of participants who could be re-contacted (Li et al., 2024). These results underscore a need for research that addresses how long atropine should be used, the ideal age for stopping treatment, and how to stop treatment. Research should also look at the long-term effect of prolonged accommodative paralysis on near visual acuity and accommodative amplitudes. Additionally, as atropine is studied, new delivery systems may be discovered along with ideal dosing cadences and/or day/night timing of the dose. Research in combination therapy, as described later in this chapter, such as atropine-plus orthokeratology or multifocal contact lenses, for example, would also be beneficial, especially in higher than 0.01% concentrations of atropine.
A better understanding of the causal mechanisms of atropine is needed. Using an intentionally integrated, multi-disciplinary approach could provide new therapeutic targets that increase efficacy while minimizing side effects for children. More studies are needed to identify the ideal dosing characteristics, including the concentration and cadence of more specific pharmaceuticals and potentially new delivery systems. In addition, longer studies are needed to determine how and when to end the intervention to provide the optimal effect with minimal rebound.
CONTEMPORARY SYSTEMATIC REVIEWS AND GLOBAL PERSPECTIVES
In the National Academies’ (NRC, 1989) review on myopia, no treatment options were confirmed to slow down the naturally occurring growth of the human eye. Decades later, much evidence points to the value of attempting to control myopia progression. Two recent reports provide a rigorous summary of the current literature on myopia treatments and control, one authored by the Cochrane Living Systematic Review and Meta-Analysis author committee and the other by the International Myopia Institute. Both the International Myopia Institute and the Cochrane review and meta-analysis are internationally recognized, and together they provide a convergence of expertise on myopia that can provide a foundation for current and emerging treatment strategies. Figure 7-8 below highlights the results of a database search of the term “myopia” within research titles between 1842–2024.
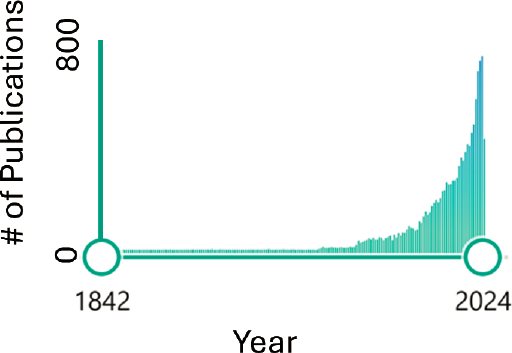
SOURCE: Committee generated based on PubMed database search performed July 2024.
Cochrane Living Systematic Review and Meta-Analysis
The Cochrane Living Systematic Review and Meta-analysis is the third Cochrane publication led by Jeff Walline since 2011 (Lawrenson et al., 2023; Walline et al., 2011, 2020a). The 2023 version was last updated in 2021. Stated objectives include the following:
- To assess the comparative efficacy of optical, pharmacological, and environmental interventions for slowing myopia progression in children using network meta-analysis;
- To generate a relative ranking of myopia control interventions according to their efficacy;
- To produce a brief economic commentary, summarizing the economic evaluations assessing myopia control interventions in children;
- To maintain the currency of the evidence using a living systematic review approach.
While the 2023 Cochrane review of myopia literature (Lawrenson et al., 2023) spans 265 pages, an important graphic from the review, representing a novel overview of myopia treatment options available today, is reproduced in Figure 7-9. The Cochrane review included 64 studies that randomized 11,617 children, aged 4 to 18 years. Studies were mostly conducted in China or other Asian countries (39 studies, 60.9%) and North America (13 studies, 20.3%). Searches included CENTRAL (which contains the Cochrane Eyes and Vision Trials Register); MEDLINE; Embase; and three trials registers. The search date was 26 February 2022. Randomized controlled trials (RCTs) of optical, pharmacological, and environmental interventions for slowing myopia progression in children aged 18 years or younger were included (Lawrenson et al., 2023).
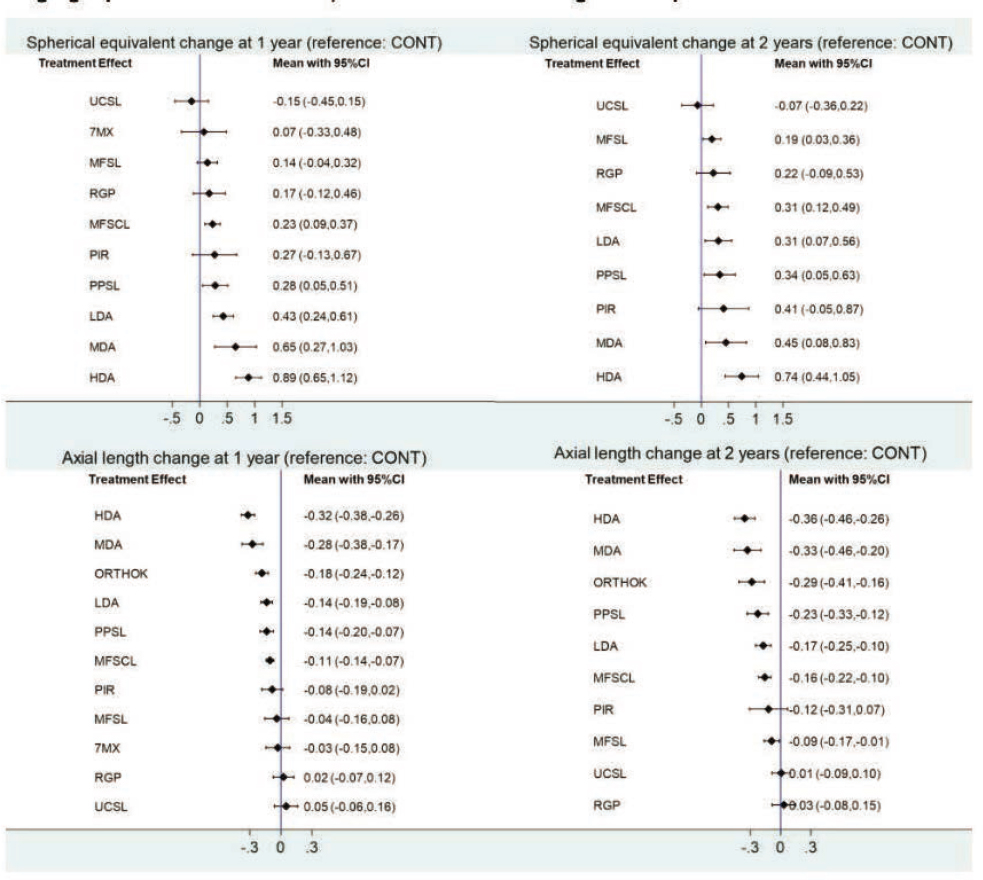
NOTES: Estimates of effect from network meta-analyses for all treatments versus control for progression of myopia (based on spherical equivalent and axial length) at 1 and 2 years. Comparisons with control are less precise than direct meta-analyses due to the lack of directly comparative evidence. 7MX = 7-methylxanthing; HDA = high-dose atropine; LDA = low-dose atropine; MDA = moderate-dose atropine; MFSCL = multifocal soft contact lenses; MFSL = multifocal spectacle lenses; ORTHOK = orthokeratology; PIR = pirenzipine; PPSL = peripheral plus spectacle lenses; RGP = rigid gas-permeable contact lenses; UCSVL = undercorrected single vision spectacles.
SOURCE: Lawrenson et al., 2023.
Key Findings from the 2023 Cochrane Review of Myopia Treatment Effect on Myopia Progression:
- No treatment provides more than a 0.75 D treatment effect, on average, of less myopic progression of spherical equivalent refractive error over two years.
- No treatment on average provides more than a 0.37 mm treatment effect of reduced axial elongation over two years.
- After high- and mid-dose atropine pharmaceuticals, peripheral-plus spectacle lenses and orthokeratology contact lenses may be the most effective optical treatments.
- The treatment effect is not sustained in the second year for the pharmaceuticals even while on treatment, suggesting that the treatment effect is not as cumulative as would be ideal.
- Orthokeratology has the biggest treatment effect in the second year.
- Treatment effect for spherical equivalent refractive error and axial length are highly correlated.
- Under-correction or no correction may promote axial elongation and myopia progression.
The International Myopia Institute (IMI)
The IMI was created in 2015 when the World Health Organization and Brien Holden Vision Institute met to address increasing levels of myopia and the associated risk to sight. Experts from around the world have since provided a convergence of expertise in the formulation of evidence-based recommendations for “classification, patient management, and future research” (International Myopia Institute, 2024). IMI white papers and clinical summaries serve to broadly disseminate its perspectives to the general public as well as to “scientists, clinicians, policy makers, government and educators” in an effort to foster collaboration and shared knowledge.
The most recent citation of the IMI is the 2023 IMI Digest (Sankaridurg et al., 2023). Salient points from the 2023 abstract are quoted below. The committee believes this abstract provides a strong reflection of the state of myopia today and potential strategic future directions.
Key Findings from the 2023 IMI Digest:
Studies in animal models have continued to explore how wavelength and intensity of light influence eye growth and have examined new pharmacologic agents and scleral cross-linking as potential strategies for slowing myopia. In children, the term ‘premyopia’ is gaining interest with increased attention to early implementation of myopia control. Most studies use the IMI definitions of ≤ −0.5 diopters (D) for myopia and ≤ −6.0 D for high myopia, although categorization and definitions for structural consequences of high myopia remain an issue. Clinical trials have demonstrated that newer spectacle lens designs incorporating multiple segments, lenslets, or diffusion optics exhibit efficacy comparable to contact lens-based optical approaches. Clinical considerations and factors influencing efficacy for soft multifocal contact lenses and orthokeratology are discussed. Topical atropine remains the only widely accessible pharmacologic treatment. Rebound observed with higher concentration of atropine is less evident with lower concentrations or optical interventions. Overall, myopia control treatments show little adverse effect on visual function and appear generally safe, with longer wear times and combination therapies maximizing outcomes. An emerging category of light-based therapies for children requires comprehensive safety data to enable risk versus benefit analysis. Given the success of myopia control strategies, the ethics of including a control arm in clinical trials is heavily debated. (Sankaridurg et al., 2023, p. 1).
Global Trends in prescribing patterns: Despite the increasing levels of clinical activity in myopia control, single vision spectacles (32%) and contact lenses (7.5%) were still the most commonly prescribed methods of correction (although this is slowly decreasing), but myopic controlling spectacles are now being prescribed (15.2%) along with myopia controlling soft contact lenses (8.7%), orthokeratology (11.6%) and atropine therapy (7.2%; Wolffsohn, 2022).
EMERGING CLINICAL TREATMENT OPTIONS FOR MYOPIA PROGRESSION
There is still much to be learned about myopia and its progression. The COMET study, discussed earlier in the chapter, was the first large-scale, randomized clinical trial to show proof of concept that the growth of the human eye could be slowed by an optical intervention. Its results, published in 2003, showed a 0.20 D treatment benefit in spherical equivalent refractive error over 3 years, which was deemed statistically significant but not clinically meaningful. Since COMET results came out, over 20,000 new research articles have been published on myopia. Still, the treatment effects may be viewed as marginal at best despite two more decades of research.
Novel treatment options are required to advance the field of myopia control in more meaningful ways. Emerging treatment options, including new perspectives on time outdoors as well as optical, pharmaceutical, chromatic, and surgical strategies, are all reviewed in turn next.
Environmental Strategies
Time Outdoors Versus Near Work
Because the increasing prevalence of myopia is likely due, in large part, to the effects of environment, clinicians will be recommending modification of their pediatric patients’ visual experience more and more as part of their care. Increased time outdoors will probably become a common recommendation. Restricting time spent engaged in near work seems more problematic and may have minimal benefit given the limited effect of near work on the rate of myopia progression (discussed in Chapter 5). The following reviews evidence on the effects of increased time outdoors on the risk of myopia onset and rate of progression.
In contrast to the controversy that surrounds the effects of near work on myopia, there is a broader consensus regarding the protective effects of more time outdoors (McBrien et al., 2009). Unlike studies of near work, both cross-sectional and longitudinal data show effects. In the cross-sectional perspective, children without myopia spend more time outdoors (Dirani et al., 2009; Jones-Jordan et al., 2011; Rose et al., 2008). More importantly, in the longitudinal perspective, emmetropic children who spend more time outdoors have a lower probability of becoming myopic (French et al., 2013b; Guggenheim et al., 2012; Jones et al., 2007; Zadnik et al., 2015).
The data on near work and time outdoors come from surveys of parents, so they are subject to criticism for recall bias and lack of detail. The failure to find effects for near work bring the utility of these surveys into question, yet these same parental surveys repeatedly detect the effects of time outdoors. Two important questions in this area of myopia research arise: (a) What is the mechanism by which time outdoors has this protective effect? and (b) Does this protective effect apply to the progressing myope in addition to the emmetropic child at risk for onset?
Effect of Time Outdoors on Onset Versus Progression
A meta-analysis conducted in 2017 concluded that the protective effects of time outdoors only apply to delaying or preventing myopia onset and not to slowing the rate of myopia progression (reviewed in Xiong et al., 2017). Xiong et al. (2017) analysis of six longitudinal studies showed a dose-response reduction in the probability of myopia onset with increased time outdoors. The asymptote of protection was reached at about 2 hours per day (Xiong et al., 2017). This amount of outdoor time parallels the recommendation from the International Myopia Institute (Jonas et al., 2021). The value of this meta-analysis is that it differentiated the incident myope from the prevalent myope and analyzed the effect of time outdoors in each group. It found no evidence for a dose-response relationship between time outdoors and myopia progression (Xiong et al., 2017).
Results from the Xiong et al. (2017) meta-analysis and the Orinda Longitudinal Study of Myopia suggest that 10 to 14 hours of outdoor time per week can substantially reduce the risk of myopia onset (Jones et al., 2007). In the analysis of CLEERE data, every additional hour of time outdoors reduced the odds of onset by 2–4% (Zadnik et al., 2015). CLEERE results also parallel the findings of Xiong’s meta-analysis of progression. The rate of myopia progression showed no differences across quartiles of time outdoors (Jones-Jordan et al., 2012). There are exceptions, however. Wu et al. (2018b) found positive effects on both incidence and progression as the result of a program increasing time outdoors at school. Interestingly, the same investigators found effects only on incidence and no significant effects on progression in their earlier, 2013 report (Wu et al., 2013). Another exception is the findings from India in a study by Saxena et al. that spending more than 14 hours outdoors per week reduced the odds of showing some degree of myopic progression compared to having a stable myopic refractive error (Saxena et al., 2017).
At present, the evidence suggests that time outdoors is more effective in preventing or delaying myopia onset than in slowing its progression. Is the lack of effect on progression the result of myopic children spending less time outdoors? This behavior of spending lower amounts of time outdoors seems characteristic of myopic children but not to the extent that insufficient variation in behavior would invalidate the conclusion from this analysis. Jones-Jordan pointed out that even if time outdoors were restricted to the lower three of the four quartiles of time spent outdoors reported for emmetropic children, those representing the majority of the lowest amounts of outdoor time, there would still be detectable protective effects of more time outdoors against onset (Jones-Jordan et al., 2012). The lack of effect in slowing progression of myopia is therefore unlikely to be due to the tendency of myopic children to spend less time outdoors. That amount of time would still be sufficient to delay or prevent the onset of myopia in an emmetropic child.
Time Outdoors: What Is the Protective Mechanism?
Understanding the mechanism by which time outdoors exerts its protective effect would have tremendous benefit. Basic mechanisms underlying the physiology of eye growth would be revealed along with the possibility of controlling that growth (see Chapters 5 and 6 for discussion). Having children spend more time outdoors is an inexpensive intervention, and physical activity can have the collateral benefit of affecting rates of obesity, cardiovascular disease, and diabetes (Colberg et al., 2016). On the other hand, outdoor time can also have harmful effects on the health of the skin and eye with increased rates of cancer, cataract, and macular degeneration (Chawda & Shinde, 2022). Activation of the protective effects of time outdoors without exposure to higher energy short-wavelength light could preserve the benefits of
time outdoors while avoiding harm. There are several candidate mechanisms for the protective effects of time outdoors (also see Chapter 5 on Onset and Progression). These are discussed next.
Ultraviolet Light
Time outdoors increases ultraviolet light exposure and cutaneous production of vitamin D (Barger-Lux & Heaney, 2002; Holick, 1995). Lower plasma levels of vitamin D show a linear relationship with more myopic refractive errors in European, East Asian, and American study samples (Choi et al., 2014; Guggenheim et al., 2014; Mutti & Marks, 2011; Tideman et al., 2016; Wolf et al., 2023; Yazar et al., 2014). The effect size is small, however, and the amount of variability is quite large by comparison. For example, the Western Australian Pregnancy Cohort (Raine) Study reported that a 100-nanomolar increase in circulating 25(OH)D3, an increase equal to nearly the entire range of serum values, would only be associated with a small difference in refractive error of 0.60 D (Yazar et al., 2014). Likewise, a Mendelian randomization analysis found that no significant effect on refractive error could be attributed to differences in circulating 25(OH)D (Cuellar-Pardita et al., 2017). An analysis of data from the Avon Longitudinal Study of Parents and Children (ALSPAC) performed by Guggenheim et al. (2014) concluded what most investigators in the field now see as the role of vitamin D in myopia: any associations between vitamin D and myopia are likely due to associations between time outdoors and myopia.
Flatter Dioptric Space
The outdoor environment presents the eye with a different set of viewing distances compared to indoors. Objects outdoors are more uniformly distant while objects indoors are at various and closer distances from the eye. Diopters of optical demand for clear vision are the inverse of the number of meters an object is from the eye. This optical environment may be thought of as the eye’s dioptric space. Flitcroft presented one of the first analyses of indoor vs. outdoor dioptric space (Flitcroft, 2012). He characterized indoors as more varied in terms of dioptric stimuli in comparison to the relatively more flat, distant dioptric space outdoors. There are several challenges with attributing the protective effect of outdoors to its characteristically flat dioptric space. One is that peripheral myopia would seem to dominate the indoor scene during most near-work taking place indoors. A second is that the effects of defocus may be substantial in animal models, but these effects have not translated into substantial influences of refractive error development in either human infants or children.
Lastly, there is an implication of inferior-superior asymmetry along the vertical meridian in both defocus and therefore eye length in this analysis, one that is not found in human data. Nasal-temporal asymmetries are commonly found, but not meaningful inferior-superior asymmetry (Atchison et al., 2004, 2005, 2006; Mutti et al., 2019; Verkicharla et al., 2016). For example, vertical peripheral eye lengths at 30 degrees eccentricity in BLINK children only differed by 0.15 mm. Greater levels of myopia show the same differences in shape along the vertical meridian of the eye as well as along the horizontal meridian of the eye (i.e., lateral, or left to right); both become less oblate by the same amount as a function of increasing refractive error (Mutti et al., 2019; Verkicharla et al., 2016). Indoor and outdoor spaces may be different in many ways, dioptric space included, but any differences must also be relevant to the development of refractive error. The flatter dioptric space outdoors seems unlikely to be the source of protective effects. The accommodative system, however, needs to function differently in the flatter (outdoor) and steeper (indoor) dioptric spaces to optimize retinal image quality. For
an account of the relative role of the periphery and fovea in accommodative effort, and its consequences for outdoor and indoor dioptric spaces, please see Chapter 6, Role of the Non-foveal Retina in Accommodation.
Absence of Near Work
Near work is seldom performed when outdoors, at least by children. The protective effects of time outdoors might be thought of as the protective effects of not engaging in near work. This view would depend on near work and time outdoors having a negative correlation, where more of one means less of the other. That assumption seems reasonable but, interestingly, most studies find that these are two independent factors with no negative correlation. For instance, Guggenheim et al. (2012) reported no significant correlation between these two variables in ALSPAC and they were also uncorrelated in the Orinda Longitudinal Study of Myopia (OLSM), CLEERE, and the Sydney Myopia Study (Jones et al., 2007; Rose et al., 2008; Zadnik et al., 2015).
Higher-Irradiance Sunlight
The more widely accepted hypothesis for the protective effects of time outdoors is that exposure to higher irradiance sunlight stimulates the release of dopamine from the retina, which, in turn, has an inhibitory effect on axial elongation (Norton et al., 2013; Rose et al., 2008; Stone et al., 1989). As discussed in detail in Chapter 6, retinal dopamine is secreted by amacrine cells stimulated through their connections with ON-bipolar cells. Intrinsically photosensitive retinal ganglion cells (ipRGCs) containing the photopigment melanopsin also provide excitatory input to sustained-firing dopaminergic amacrine cells through their own light-evoked responses (Zhang et al., 2008). Therefore, bright visible light increases retinal dopamine through stimulation of both traditional photoreceptors and nontraditional photosensitive ipRGCs. The release of dopamine may enhance and prolong the firing of melanopsin-driven responses in ipRGCs through the actions of cyclic adenosine monophosphate (cyclic AMP; Beaulieu et al., 2015; Sodhi & Hartwick, 2014).
Because ipRGCs project to the olivary pretectal nucleus, an area of the midbrain in the central nervous system that controls the reaction of the pupil of the eye to light, it has been hypothesized that evaluation of adaptive changes in pupillary responses to repeated light stimuli may represent an assay of this release of retinal dopamine. Results from this type of pupillometry support the hypothesis that non-myopic individuals produce more retinal dopamine in response to light stimulation. Less myopic refractive error in adults showed a linear correlation with greater pupillary constriction in response to repeated pulses of short-wavelength blue light and slower redilation following offset of the blue light stimuli (Mutti et al., 2020). Shorter axial lengths in children were correlated with slower redilation following offset of the blue light stimuli and faster pupillary escape in response to repeated pulses of long-wavelength red light. Interestingly, these responses related to axial length were only found in the summer months, a time when environmental light is more available, and not during winter months (Reidy et al., 2024). These findings suggest that more hyperopic/less myopic individuals may have a greater ability to take advantage of light exposure and its accompanying protective effect.
An important question for future research is whether any deficiencies in retinal responses to light exposure in children at risk for myopia onset are present early in life or develop along with the onset of myopia. A related question is this: If more and more children become myopic who could have avoided it by spending more time outdoors, does increasing their time outdoors
now slow myopia progression in that subpopulation? Studies may begin to see effects of time outdoors on progression for this reason. Another issue is whether any deficiencies are intrinsic and unchanging or whether early behaviors may alter the ability of the retina to respond in a protective manner to environmental light. An analysis of ALSPAC data by Shah et al. showed positive effects in lowering the odds of future myopia onset between 10 and 15 years of age that were attributable to increased time outdoors as early as 3 years of age (Shah et al., 2017). Clearly, whatever the capabilities of the retina might be to respond in a beneficial manner to environmental light, that capability needs to be coupled with actual time outdoors in order produce protective effects.
International Examples of the Effects of Outdoor Time
Regardless of whether early exposure to more time outdoors changes retinal responses, early exposure may be a reasonable strategy simply on the basis of applying inhibitory influences on axial elongation over more years. Scandinavia and its uncharacteristic lower prevalence of myopia compared to other parts of Europe provides an interesting example (Hagen et al., 2018). Countries at northern latitudes may have a difference of as much as 13 hours in length of day between summer and winter. Extended darkness in winter does not sound consistent with obtaining protective effects from light exposure that would lower a national prevalence of myopia. Yet, Scandinavians seem to value whatever length of daylight is available. There is a cultural awareness of the need for light exposure in these countries, perhaps from the need to maintain adequate levels of vitamin D. This ethos is encapsulated in the word “friluftsliv” or “open-air living,” a dedication to spending as much time outdoors as possible whatever the conditions. Babies are routinely left outdoors in strollers year-round as their parents shop or eat at cafes.
The Scandinavian example perhaps shows that even if sunlight is limited, following the recommendation of at least two hours per day, especially when experienced early in life, may be sufficient to have a positive effect on the prevalence of myopia. Taiwan also provides an encouraging example. Decades of increasing prevalence of myopia began to decline two years after schools in that country implemented a program encouraging 120 minutes per day of time outdoors (Wu et al., 2018a). Australia shares a similarly low prevalence of myopia, has an abundance of available sunlight, and a culture that values extended time spent in outdoor recreation. Australian children were reported to spend an average of 105 ± 42 min/day outdoors compared to 61 ± 40 min/day for children in Singapore (Read et al., 2018). These differences may in part account for the differences in myopia prevalence. The prevalence of myopia in teenagers in Singapore is on the order of 74% (Quek et al., 2004) while far fewer Australian teens are myopic (30%; French et al., 2013).
While studies support the hypothesis that myopia onset is prevented or delayed by spending time outdoors, many questions remain. For instance, determining the causal mechanisms for the protective effect of time outdoors could provide the opportunity to apply those key factors of the outdoor space while children are indoors. Numerous strategies are being considered: increased indoor illumination from larger windows, painting outdoor scenes on school walls, or having children look into devices delivering light to the eye with wavelengths anywhere from red to violet (Dolgin, 2024). If the protective mechanisms of time outdoors involve retinal dopamine release, investigations into other visual conditions that enhance retinal dopamine release, like retinal ON pathways, would be beneficial. Regardless of the mechanisms, encouraging children to spend more time outdoors, particularly early in life, could provide
protective effects against myopia onset. However, due to the lack of evidence for time outdoors to slow myopia progression, it seems unlikely to serve as an effective adjunct to treatments aimed at slowing progression once children become myopic.
Optical Strategies for Mitigating Myopia Progression
Glasses for Slowing Myopia Progression
In addition to contact lenses for altering peripheral focus, emerging technology with “peripheral-plus spectacle lenses” shows promise in slowing axial growth of the eye. Unlike other treatment options that are used off-label in the United States (orthokeratology lenses, atropine, soft multi-focal lenses), these peripheral-plus spectacle lenses have been developed for the purpose of myopia control, although they are not yet FDA-approved and thus not commercially available in the country at this time. However, the lenses are available in Canada and in other parts of the world. While no studies have confirmed a treatment benefit in U.S. children, meta-analysis suggests that the treatment effect is fairly equal to if not more effective than that of contact lenses that attempt to control myopia (Lawrenson et al., 2023).
Three novel myopia-control spectacle options use peripheral myopic defocus as their mechanism. Each uses a lens as its base that provides a clear image centrally on the back of the eye. Additionally, surrounding a clear central zone of the lens, there is an overlay of “lenslets” that intend to create myopic defocus over much of the retina. The Hoya MiyoSmart® lens uses a zone of lenslets to provide myopic defocus over the mid-peripheral retina (Lam et al., 2020) (Figure 7-10). The Essilor Stellest® lens adopts a volume approach with a greater number of lenslets designed to create more dimensions of myopic defocus (Li et al., 2023a; Figure 7-11). Neither of these two lens designs seems to alter the focusing plane of the eye, likely because—unlike multifocal contact lenses—each convex lenslet forms a discrete image on the retina that cannot be fused to form a clear, continuous image.
For the ZEISS MyoCare® lens there is less longitudinal data, but the lens operates somewhat similarly in its attempt to create myopic defocus in the peripheral retina (Liu et al., 2023). Instead of using lenslets, this ZEISS lens is reported to create higher-order aberrations in the periphery as a signal to slow growth. SightGlass Vision DOT® is another spectacle lens design used to slow myopia; however, it uses reduced contrast, not myopic defocus, as its hypothesized mechanism is related to cone opsin photopigment defects (Rappon et al., 2022; see Chapter 5, Syndromic Myopia for more details). Similar to the dual focus spectacle lenses above, there is a clear zone in the spectacle lens at the center for maximum clarity in central vision. This requires careful measurement of the distance between the pupils and from the center of the pupil to the bottom of the frame.
There are several practical benefits to spectacles that may be able to slow the growth of the eye. First, glasses are the standard treatment option for children with myopia. Since the lenses seem to be well-tolerated (Gao et al., 2021; Lam et al., 2020; Rappon et al., 2022), the treatment is minimally different compared to wearing standard, vision-correcting spectacles. Additionally, these spectacles allow for greater powers of astigmatism to be corrected than some previous optical treatment options, which allows for more diversity in children who may benefit from wearing them. However, further study is required to determine the effect of peripheral refractive errors on road driving, as at least one early report suggests that the driving performance of young drivers may be impaired with the addition of myopia defocus (Ortiz-Peregrina et al., 2022).
In addition to the functional aspects of these lenses and their treatment effects, it may also be helpful to better understand how these lenses truly affect the peripheral retinal image. For example, it may be helpful to know if eccentricity from the macula toward the periphery of the retina is an important factor, in regard to the placement of the zone of defocus or reduced contrast. One study in rhesus monkeys suggests that myopia defocus in the near periphery can slow axial myopia, and that defocus beyond 20 degrees from the fovea does not (Smith et al., 2020). However, further studies are needed to confirm this result. Additionally, the effect of the corrective multifocal lens options on the amount of peripheral image quality—myopic defocus or contrast reduction—should be determined using a combination approaches: in situ optical bench measurements, on-eye evaluation with objective assays of optical quality (such as wavefront sensing and double-pass point spread function measurement), and wide-angle eye models. This may allow researchers to subsequently determine if these treatment options show an association that is consistent with their purported mechanism of action. At least one study showing treatment benefit using multifocal contact lenses with a high-add power suggested that peripheral defocus was not associated in a substantial manner with treatment benefit (Berntsen et al., 2023). (For more on evaluating the optical quality of these treatments and their mechanisms, also refer to the section below, “Mechanisms of Optical Treatments and their Limitations”.)
SOURCE: Lam et al., 2020.
Visual stimuli are composed of luminance and chromatic information. The potential influence of luminance on myopia development was covered in the section on environment earlier in this chapter. Below, the committee considers the chromatic information in visual stimuli.
Longitudinal Chromatic Aberrations (LCA)
When light enters the eye, chromatic aberration causes the various wavelengths that make up the light to focus at different locations in the back of the eye. For light focused on the retina, long wavelengths (reds) are actually focused a little behind the retina, while short wavelengths (blues) are focused in front of the retina (Figure 7-11). There are several possible ways that such chromatic stimuli could be processed by the retina to provide signals about eye growth. While there are inter-species differences in the cone spectral types, the short-wavelength cones (Scones) and their pathways are similar among mammals, implicating their role in the processing of LCA (Calkins, 2001). One hypothesis is that this LCA provides a cue for the eye to grow to emmetropia (Rucker & Wallman, 2009; see review in Troilo et al., 2019). If red light is in
sharper focus than blue light, that could signal that the eye is too short and provide “grow” signals to elongate the eye. If blue light is focused more sharply than red, that could signal slowing of eye growth. A study with human participants reported that watching movies filtered to be in focus in the red plane for 45 minutes produced longer axial lengths in emmetropic participants, while young participants with myopia did not respond to the stimuli (Swiatczak & Schaeffel, 2022).
Many animal studies have investigated these effects by housing animals in monochromatic, narrowband lighting and have found significant effects on refractive development. Such experiments have been done using fish, chickens, guinea pigs, tree shrews, and rhesus monkeys. The studies conducted with fish (Kröger & Wagner, 1996), chickens (Foulds et al., 2013; Rohrer et al., 1992; Seidemann & Schaeffel, 2002; Shaeffel & Howland, 1991; Torii et al., 2017), and guinea pigs (Jiang et al., 2014; Liu et al., 2011; Long et al., 2009; Wang et al., 2011) have tended to support the LCA hypothesis, with short-wavelength light producing relative hyperopic refractive shifts and long-wavelength light producing relative myopia refractive shifts. Studies in other animals have shown the opposite effects, specifically in tree shrews (Gawne et al., 2017; She et al., 2023), and rhesus monkeys (Hung et al., 2018; Liu et al., 2014; Smith et al., 2015). Recently, a quantitative model has been developed to explain how LCA detection by the short- and long-wavelength sensitive cone photoreceptor types present in the tree shrew retina can decode the sign of defocus (Gawne et al., 2021). Notably, this LCA model of emmetropization provided predictions that were validated by experimental results (Khanal et al., 2023). Further study is necessary to clarify the role of LCA as a mechanism to drive refractive eye growth, especially due to the potentially conflicting differences in species, even among mammalian species.
Atypical Opsins: Opn3, Opn4, and Opn5
A second mechanistic hypothesis for short-wavelength light is detection through atypical opsins: Opn3, Opn4, and Opn5. These atypical opsins are all found in the retinal ganglion cells and have wavelength sensitivities ranging from 470–485, 480–485, and 350–450 nm, respectively (Guido et al., 2022; although see Emanuel & Do, 2023, for controversy about melanopsin wavelength sensitivity). Studies using mice with mutations in Opn3, Opn4, and Opn5 have demonstrated an influence on refractive development and/or myopia susceptibility (Chakraborty et al., 2022; Jiang et al., 2021a; Linne et al., 2023; Liu et al., 2022). Furthermore, the protective effect of violet light (360–400 nm) in wild-type mice was eliminated in a mouse without Opn5, and this effect was most prominent two hours prior to the end of the diurnal phase of the light cycle (Jiang et al., 2021a), demonstrating a causal effect of Opn5 and a diurnal effect of violet light exposure.
Emerging data in tree shrews also shows that short-wavelength light (420 nm) that activates OPN5 completely suppresses the expected myopia shift induced by experimental lens defocus (Grytz & Lang, 2023). Interestingly, the same wavelength that was found to be protective in mice (365 nm) was not protective in tree shrews, presumably due to lens absorbance. Modeling of lens transmittance indicated that wavelengths of 420 nm would be transmitted effectively through the tree shrew ocular lens and this wavelength inhibited myopia susceptibility.
Finally, violet light exposure in children using violet-light-emitting frames (360–400 nm at 310 µW/cm2) has also been shown to provide some benefit (change of 0.5 D in 24 weeks) (Torii et al., 2022).
NOTE: Rays of longer wavelength (red) are focused behind the retina and shorter wavelength rays (blue) are focused in front of the retina. As a consequence, the image of a point in the green focus plane is a focused on green, with halos in red and blue.
SOURCE: Vinas-Pena (2015). Reprinted with permission from the author.
Repeated Low-Level Red Light (RLRL) Therapy
RLRL was first reported as a treatment for slowing myopia progression in 2022 and has quickly become commercially available in several countries, but not the United States (Khanal et al., 2024). RLRL treatment generally consists of exposure to laser light (650 +/-10 nm; 1600 lux with a power of 0.29 mW for a 4-mm pupil) for 3 minutes, twice a day, 5 days per week (Jiang et al., 2022). Several randomized clinical trials have reported efficacy of RLRL in controlling myopia (Chen et al., 2022, 2023; Dong et al., 2023; Jiang et al., 2022; Lin et al., 2023; Tian et al., 2022; Xiong et al., 2021, 2022, 2023; Zhou et al., 2023). These studies have reported an impressive slowing of axial elongation and myopia refractive errors. For instance, one study found a 54% reduction in myopia over 12 months (He et al., 2023) and a decrease in myopia progression by –0.59 D in refractive error and 0.26 mm in axial elongation (Jiang et al., 2022). Some of the reported benefits of RLRL treatment include axial shrinkage of the globe in a minority of patients (Wang et al., 2023), which may involve effects of scleral myofibroblasts, as observed in an animal model (Phillips & McBrien, 2004).
While most reports of RLRL efficacy and safety have been positive, side effects include photosensitivity during treatment and temporary after-images with treatment (Deng et al., 2023; Khanal et al., 2024; Tang et al., 2023; Wang et al., 2023). In addition, there appears to be a strong rebound effect when RLRL treatment is stopped (Chen et al., 2023; Xiong et al., 2022). A single case report of a 12-year-old with retinal damage after 5 months of RLRL therapy has raised concerns about the long-term safety of this treatment (Liu et al., 2024). Furthermore, bench testing of two RLRL therapy devices (Sky-n1201a and a Future Vision) found that the devices reached or exceeded the maximum permissible exposure after three minutes of continuous viewing (Ostrin & Schill, 2014). The mechanisms of RLRL treatment effects are not known and may include improved choroidal blood flow, nitric oxide signaling, mitochondrial effects via photobiomodulation, and/or reduction of scleral hypoxia (Zhou et al., 2023). Researchers agree that more research is needed to determine the mechanisms and evaluate the safety of RLRL for children (Salzano et al., 2023; Schaeffel & Wildsoet, 2024).
Structural and Surgical Strategies for Mitigating Myopia Progression
Scleral Cross Linking
The ultimate outcome of myopic eye growth is elongation of the ocular globe caused by remodeling of the sclera. This axial elongation involves changes in scleral biomechanics. Patients with high myopia can develop a posterior staphyloma in which the sclera thins and then develops an outpouching. Thus, one potential approach to treating progressive myopia has been to prevent the sclera from expanding using scleral cross-linking. Scleral cross-linking causes increased stiffness of the scleral tissue by forming new covalent bonds between collagen fibers and decreasing the space between fibers (Yasir et al., 2023).
There are multiple approaches to scleral cross-linking, and thus far the majority of studies on scleral cross-linking have been in animal models to demonstrate safety and feasibility. Studies in form-deprived rabbits and lens-defocus guinea pigs (Chen et al. 2023; Ding et al., 2021; Li et al., 2017; Liu et al., 2016) have shown that UV-A light and the photosensitizer riboflavin have the potential to increase the biomechanical strength of the sclera and slow the development of myopia. This approach requires the application of riboflavin to the targeted tissue and then exposure to UV-A light to create the chemical reaction. When feasibility studies were performed in human eyes with existing blindness (Li et al., 2023b), signs of inflammation or toxicity were observed, suggesting safety concerns. A limitation of this approach is reaching the most posterior globe, where changes in myopia are most prominent (Yasir et al., 2023).
Other cross-linking approaches have used Rose Bengal with green light, methylene blue with red light, and the non-photoactivated cross-linker genipin (Yasir et al., 2023). The approach with the least cytotoxicity appears to be low-level laser therapy using red or near-infrared light to penetrate deeper into the tissue (Yasir et al., 2023). Studies using RLRL therapy in myopic children found a benefit in controlling axial elongation (see section on RLRL above); however, it is not known currently whether the mechanism of action of RLRL is through scleral crosslinking. Importantly, some animal studies have reported potential risks associated with scleral cross-linking, such as increased intraocular pressure in genipin-treated guinea pigs (Guo et al., 2024). Repeated genipin injections in tree shrews caused degeneration of photoreceptors and retinal pigment epithelium (Hamdaoui et al., 2022). Therefore, additional studies are needed to develop scleral cross-linking methods that are both safe and effective.
Pharmacological and Combination Strategies
The only widely used pharmacological treatment for myopia progression is atropine, as described above. Its effectiveness in low doses in U.S. children necessitates further study. There are only a few emerging pharmacological strategies that have been tested in animal models and translated into the clinic. For instance, 7-MX, which blocks adenosine receptors and is a caffeine metabolite, has shown some benefit in rhesus monkeys with lens-induced myopia (Smith et al., 2021), but limited efficacy in clinical trials (Trier et al., 2023). (A full review of this treatment can be found in Khanal et al., 2024.)
When monotherapies provide incremental benefit to slow myopia progression, it makes sense to consider combination therapies. Any combination of optical (e.g., special contact lenses), pharmaceutical (typically low-dose atropine), environmental (more time outdoors), and structural (scleral cross-linking) could be considered, even up to a quadruple combination attempt. However, the typical combination studied thus far has been limited to dual therapies, including low-dose atropine with an optical intervention including soft contact lenses,
orthokeratology, or special spectacle lenses. A review by Pucker (2023) and another by Cochrane (Lawrenson et al., 2023) suggest that some dual therapies may slow myopia progression more than monotherapies and that orthokeratology in combination with atropine 0.01% seems to have the largest treatment effect; however, the largest treatment benefit with dual therapy compared to monotherapy is 0.15 D per year. Some studies show a statistically significant difference between monotherapy and additive dual therapy; however, no combination shows a doubling effect between monotherapy and dual therapy (Pucker, 2023).
Research on combination therapies for myopia progression thus far has focused on using low-dose atropine (0.01%) with Asian children living in Asia. Concerning children in the United States, there is a study of atropine 0.01% in combination with multifocal contact lenses using a +2.50 add; however, that study showed no benefit in this combination (Jones et al., 2022). Future research is necessary to determine the effectiveness of combining treatments. It should be noted that if a monotherapy is not effective in treating myopia progression, a study of its combination with another effective (or ineffective) treatment is likely to suggest no benefit as well. As such, the effectiveness of the monotherapy must be proven first, especially in U.S. studies.
DECISIONS IN TREATMENT
For the optometrist and ophthalmologist considering the treatment of myopia progression, a thorough and ongoing review of myopia literature will be required. Despite modest advances in how much eye growth can be slowed, the preponderance of evidence suggests that treatment for myopia progression should at least be considered for all myopic children.
As the field of myopia progresses, it may be that treatment strategies will become specific to an individual patient. Myopia control methods do seem to slow the progression of myopia and its axial elongation. However, many questions remain. Is it standard of care? Are the treatment effects worth the cost of the treatment strategies? Do the treatment strategies require use throughout the school-aged years? Or beyond? And does the safety of the treatment outweigh the risk to the eye? It is questions like these that make treating for myopia progression sometimes difficult.
Care should be taken by the eye care professional to avoid suggesting to patients that a cure for myopia exists or that treatment effects will be more substantial than warranted by the evidence. The practitioner should offer an evidence-based approach to the treatment of myopia progression when discussing with patients and their families. Factors that identify who may benefit most from treatment or in whom myopia is more likely to progress are considered next.
Key Elements in the Decision to Pursue Myopia Control Treatment Options
- Once nearsighted, the eye tends to grow, especially between ages 7 and 16 years old. The average rate of progression in U.S. children is approximately 0.50 D per year; it may be faster in younger children.
- The average age of cessation in myopia progression is between 15 and 16 years. 75% of children with low to moderate myopia in the early school-aged years stop becoming more nearsighted at age 18; 4% are still growing more nearsighted at age 24.
- The preponderance of evidence suggests that myopia progression can be treated. While studies typically show 2- and 3-year results, clinical treatment is likely required to be much longer and may persist into early adulthood.
- Current treatment options have the following implications:
- Atropine 0.01% may not be effective in U.S. children; higher doses may be required, but side effects and rebound effects should be considered.
- Orthokeratology requires a larger pupil than the optical center for treatment to be effective.
- Multifocal contact lenses for use in the treatment of myopia progression must have a center for distance and near power in the surround; the add power must be 2.50 D or higher.
- The only FDA-approved drug or device in the United States is the MiSight daily disposable contact lens.
- Special spectacles that use peripheral myopic defocus or contrast to attempt to slow down the progression of myopia are available in some parts of North America but are not FDA approved at this time and therefore not available in the United States.
- The largest treatment effect of any current treatment option is less than 0.75 D over two years. There is currently no cure for myopia progression.
- Past progression does not predict future progression. Delaying treatment until a criterion rate of progression is reached may not be ideal.
- Outdoor play seems to have a protective effect on myopia onset. Two hours per day or 14 hours per week may be enough to delay the onset of myopia.
- Under-correction is not ideal and may cause further progression, and overcorrection is likely to cause faster progression as well. The aim is to optimize acuity by providing an accurate refractive correction.
- Nearsighted eyes are at risk for retinal detachment, especially greater than - 6.00 D of myopia. Myopia greater than –5.00 D would benefit from annual dilated eye exams.
- Refractive error: +0.75 D or less hyperopia at age 6 is likely to progress. Consider low, non-myopic refractive error a risk factor in the younger ages.
- Age of patient at onset of myopia is important: The younger the child, the faster the nearsightedness seems to progress. Additionally, the more years a patient has myopia, the more myopia they are likely to have as an adult.
Ideal Characteristics of Treatments
As researchers continue to review evidence and attempt to push the field forward, the ideal characteristics of methods to slow the growth of the eye are starting to emerge. The following list of characteristics reflects the committee’s view.
Accessibility and Inclusion
- Ideal solutions would show a meaningful effect on diverse groups of individuals on both spherical equivalent refractive error and axial length.
- Treatments should be done prior to onset to delay or prevent myopia.
- Their administration should be feasible for a child, working toward noninvasive and nonpainful delivery.
- The cadence of treatment should be timely and convenient for children and families.
- Cost-effectiveness must be prioritized to minimize health disparities.
Durability and Reliability of Effect
- New treatments should work toward having no rebound effect, prioritizing long-term data that show lasting effects into adulthood when myopic eyes may take on more cumulative risk.
- When monotherapies show effectiveness alone, it would be ideal if they showed added benefit when combined.
- Treatments with the most potential for success would likely affect multiple parts of the eye to increase the effect.
- They would also have been proven by robust bench or animal work with known and plausible mechanisms that support the translational research efficiently and frugally.
- Ideal treatments would also be FDA-approved to underscore the goal of systemic and ocular health and safety of the child.
WHY DON’T MYOPIA TREATMENTS WORK BETTER FOR CONTROLLING MYOPIA PROGRESSION?
Alternative Theories for Mechanisms
An unexpected feature of optical myopia control is that its effects seem limited in magnitude, limited in duration, and similar in magnitude across the underlying range of myopia progression and elongation. These issues were noted in an extensive recent review by Brennan et al. (2021). In clinical trials lasting at least 2–3 years, children in the intervention group typically experience only 0.50 D to 0.75 D less myopia progression, or 0.2 mm to 0.3 mm less axial elongation, than control subjects. A second characteristic is that treatment benefit is often greatest in the first year and may not continue to accrue past the second or third year of myopia control (Huang et al., 2016 Lawrenson et al., 2023).
The third characteristic is more than curious. It poses a potential challenge to the assumed underlying mechanism for optical myopia control: imposing a peripheral “STOP” signal of myopic defocus. An inhibitory signal, such as myopic defocus, might be expected to slow myopic changes in proportion to the underlying rate of change, the rate that would have occurred without treatment. A 50% treatment effect for a child with fast myopia progression should yield a greater treatment effect than a 50% treatment effect for a child changing more slowly. Proportional inhibition, one that is relative to the underlying untreated rate of change, should create skew in the distribution of refractive error and axial length for treated children. Notably, the distribution of refractive errors or axial lengths is strikingly similar between treated and control children (Charman & Radhakrishan, 2021). This overlap of treated and control distributions led Brennan et al. (2021) to conclude that this form of myopia control produces more of an offset between groups, an absolute treatment effect rather than a relative one.
Reconsidering the Role of Accommodation in Myopia Progression
The mechanism for optical myopia control should therefore be one that results in a discrete rather than a proportional treatment effect, that is randomly distributed, and that only
accrues over a limited period of time. One such potential mechanism might be chronic relaxation of accommodation. The additional peripheral positive power in optical myopia control reduces the accommodative response of children (Cheng et al., 2019; Gong et al., 2017b). Chronic relaxation of the ciliary muscle may reduce the net force on the anterior segment of the eye, resulting in a less prolate/more oblate shape and a reduced axial length. The eye would essentially be redistributed into a relatively wider equatorial diameter with an accompanying shorter axial length. This is essentially the reverse of the proposed forces underlying the effect of accommodation on emmetropization (discussed in Chapter 5).
Chronically inhibiting accommodation might also be viewed as the reverse of the forces that created myopia in the first place, a reversal of the increase in tension in the anterior segment of the eye from a failure of crystalline lens stretch (discussed in Chapter 6). The near work habits of close working distance and uninterrupted periods of near work hypothesized to affect the rate of myopia progression represent prolonged accommodation. An increase in working distance and more frequent breaks from near work might be analogous to chronic reduction in accommodation. Future longitudinal studies investigating these factors should evaluate whether their effects are similar to or exceed those for myopia control. Modifying these behaviors may influence progression, but an interesting question is whether these strategies affect progression by a meaningful amount over a meaningful period of time or do they face the same limitations as current myopia control.
The pattern of peripheral eye growth during optical myopia control provides some support for this redistribution hypothesis. The pattern of eye expansion during myopic progression is characterized by axial elongation exceeding peripheral expansion. The eye becomes more prolate or less oblate. Optical myopia control results in more symmetric expansion between the central and peripheral retina. Peripheral eye length data from the BLINK study indicated that the treatment effect was greatest at the fovea and not in the periphery, as might be predicted with a center-distance contact lens. This pattern of inhibition of elongation meant multifocal contact lenses either neutralized or reversed the increase in retinal steepness seen with single-vision lenses.
Elongation during orthokeratology for myopia control also resulted in more symmetric expansion, expansion that was actually greater for the nasal retina than with single-vision spectacles by up to 0.21 mm (Huang et al., 2022). Peripheral refractive error, and indirect measure of eye shape, also showed more symmetric expansion during optical myopia control with DIMS specialty spectacles compared to single-vision correction (Zhang et al., 2020). Measurements of peripheral eye length beyond the ±30° obtained from optical biometers would be useful for evaluating this hypothesis by determining how the shape of the eye is affected by accommodation during optical myopia control.
Furthermore, as reviewed in the section below on “Mechanisms of Optical Treatments and their Limitations,” it remains unclear how existing and emerging treatments interact with each individual eye and modify the retinal image distribution as a function of eccentricity. Thus, this remains an important avenue for future research that will require not only individualized models of the eye’s optics but also in situ characterization of the optical properties across a large field of view for the treatments.
Mechanisms of Optical Treatments and Their Limitations
To understand the purported mechanisms of treatment effects of optical corrections—spectacles or contact lenses—at least three parallel lines of inquiry are needed, along the lines of
those conducted recently (Arias et al., 2023; Jaskulski et al., 2020; Papadogiannis et al., 2023; Sah et al., 2022). These are (a) in situ characterization of the optical performance of the corrective lens (Arias et al., 2023; Jaskulaki et al., 2020), (b) on-eye evaluation of visual performance with the corrective lenses (Papadogiannis et al., 2023), and (c) integration of the correction with eye models to evaluate retinal image quality (Sah et al., 2022). These need to be carried out with imposed variations in (at least) pupil size, retinal eccentricity, wavelength, and object distance (i.e., through focus characterization), since these variables capture the essential elements that vary in the visual diet through these corrective optics.
The optical characterization of the lenses in situ has been carried out using several techniques, including high-resolution aberrometry and high-dynamic range double-pass point-spread-function measurement. The utility of the point-spread-function measurement is to overcome the limitation imposed by the relatively sparse sampling imposed by the Shack Hartmann wavefront sensor with a finite number of lenslets. To effectively quantify optical quality at high spatial frequencies, as might be expected from multifocal optics such as the MiSight® and the spectacle lenses with repeating diffuse or defocus microstructures (DOT® and MiyoSmart® DIMS respectively), a point-spread-function, double-pass image in vivo and scatter quantification are required.
The differences in methods notwithstanding, the manipulation of optical contrast is uniformly evident in the periphery of a majority of these corrective lenses beyond the purported and intended change in the relative peripheral refraction. An open question that follows is the relative impact of optical defocus (magnitude, sign, dependence on eccentricity) vs. contrast as the driving mechanism in arresting eye growth with these treatments.
A few observations from prior studies are of note in this regard. When tested on an optical bench in situ, the DIMS lens exhibited increased sharpness and contrast in the periphery as compared to DOT and single-vision lenses (Arias et al., 2023). Under photopic conditions, the single-vision DIMS and DOT lenses all reduced contrast in the periphery, with the DOT lenses leading to the largest reduction. The DOT lens reduced contrast the most under mesopic lighting in the presence of glare sources in the Arias et al. (2023) study. The mechanism of contrast reduction is attributed to a pupil-size-dependent alteration in light scattering properties of the lenses, which have a clear zone in the center and periodic diffusing microstructures around the clear zone. The amount of scattered light at visual angles greater than 3 degrees was higher than that for standard elderly observers for these lenses.
Besides scattering, the DOT lenses were similar in their focusing properties to single-vision lenses, except for a diffraction pattern induced by the periodic microstructures. All lenses led to a hyperopic shift in the periphery when typical optical aberrations present in a myopic eye were incorporated into the measurement. This emphasizes the interaction inherent between these optical corrections and the native aberrations present in an eye, especially in the periphery. It also indicates the need for (c) above, i.e. integrating the corrections into realistic and individualized eye models to predict retinal image quality in vivo as a function of retinal eccentricity. In addition to the hyperopic shift, the DIMS lens led to a larger depth of focus compared to the other two, leading overall to sharper and higher contrast images through focus.
The lack of a defined peripheral myopic defocus was noted in the on-eye evaluation of other optical corrections: aspherical lenslets (Stellest®), defocusing lenslets (MiyoSmart®), and multifocal contact lenses (MiSight®; Papadogiannis et al., 2023). The fact that these devices have varying and insufficient efficacy indicates a mechanism besides peripheral myopic defocus mediating their treatment effects. Furthermore, relative peripheral refraction used to quantify
optical quality is ill-defined because of the large depth of focus in the periphery attributed to the increased magnitude of higher-order aberrations and astigmatism. The horizontal progressive-addition (Perifocal®) was the only lens in this study noted to create a more myopic focus in the periphery (by ~1 D), but also to interact with the eye’s aberrations, leading to an overall larger variability in its effect on peripheral image quality (Papadogiannis et al., 2023).
All four of these lenses were intended to create a myopic relative peripheral refraction with positive powered zones. However, the Stellest®, MiyoSmart®, and MiSight® treatments did not induce the intended myopic relative peripheral refraction. This seeming discrepancy can be attributed to the inability of classical paraxial eye models to capture the optical performance at greater retinal eccentricities that are important for myopic eye growth. For example, the effect of the large variation of optical power profiles across the pupil and at peripheral visual fields, as may be expected for the multifocal optical lens designs of these treatments, is not adequately captured by traditional foveocentric paradigms for measuring refraction (see Chapter 6 for an elaboration of foveocentric vs. retinocentric views of refractive error). Again, all lenses exhibited reductions in contrast in the peripheral retina, as evaluated from double-pass point-spread-function and peripheral acuity measurements. In the Papadogiannis et al. (2023) study, the MiSight contact lens led to the largest reduction in contrast. Reductions in contrast and shifts in image focus are two suggested mechanisms of action of these corrections, but it has been argued that changes in accommodative capacity altered by these corrections might be yet another plausible mechanism. Overall, the mechanisms by which optical corrections function in arresting abnormal eye growth remain incompletely understood.
The importance of native optical quality and its interaction with the corrective optics emerges as a unifying finding from the bench and on-eye evaluations. This suggests that treatments such as the Perifocal and MiSight lens, which are intended to function through changes in peripheral refraction, led to larger inter-subject variability and are in general more sensitive to the native optical quality. On the other hand, the repeating diffuse or defocusing lenslets seem to be more resilient to the native optical quality and tend to function through a general reduction in peripheral contrast. Overall, this further emphasizes the need for establishing eye models to fully evaluate the optical correction as a function of retinal eccentricity, pupil size, wavelength, and object distance.
Theories on Contributions of ON/OFF Pathway Dysfunction to Myopia Progression
Increasing evidence suggests that retinal ON and OFF pathways have different influences on refractive eye growth. Retinal ON and OFF pathways are parallel neural circuits in the retina that are activated when light stimulation increases or decreases, respectively. For instance, bright light stimulates the retinal ON pathway and stimulates retinal dopamine release, which is thought to be a “stop” signal for axial growth (see Chapter 6; reviewed in Hendriks et al., 2017; Mazade et al., 2024; Zeitz et al., 2023). Dopamine is a retinal neuromodulator that alters the neural connectivity of the retina under different lighting conditions by modulating the gap junction between retinal neurons. In addition, participants exposed to white text on a black background for 60 minutes, which disproportionally stimulates retinal ON pathways, exhibited increased choroidal thickness, while participants exposed to black text on a white background, stimulating retinal OFF pathways, had thinner choroids (Aleman et al., 2018). Finally, mutations in retinal ON pathways, such as in patients with congenital stationary blindness, result in myopia (reviewed in Hendriks et al., 2017; Mazade et al., 2024; Zeitz et al., 2023).
These results suggest that visual stimuli activating retinal ON pathways could provide a signaling cue that slows refractive eye growth and thus protects against myopia, while visual stimuli that activate retinal OFF pathways may be myopigenic. Furthermore, the myopic retina may develop an imbalance in the ON and OFF pathways. Functional measurements of the retina using the electroretinogram indicate a decreased b-wave amplitude in individuals with myopia, indicating some dysfunction at an early stage of the retinal ON pathways (Poudel et al., 2024). In addition, emerging data from mouse retinas exposed to lens-induced myopia have an imbalance of ON and OFF pathway activation in their inner retinal neurons, with ON pathway activation decreasing (Mazade & Pardue, 2023, 2024). These results suggest that imbalance of ON and OFF pathway stimulation in the visual diet could contribute to myopic eye growth and that these stimuli could alter the development of ON and OFF pathways in the myopic retina, which may further decrease the ability of the retina to process low contrast and regulate retinal illuminance in bright environments (Poudel et al., 2024).
As noted above, changes in contrast may be an integral component of defocus and optical corrections, as described in the section above, “Mechanisms of Optical Treatments and their Limitations.” Contrast is detected jointly by the retinal ON and OFF pathways (see Chapter 6). Thus, if optical corrections for myopia control create a “rebalancing” of the ON and OFF retinal pathways, myopic growth signals in the eye may be diminished. However, if the eye is still experiencing myopigenic visual stimuli during treatment, then the drive for myopic eye growth may still be present and produce continued signaling for axial elongation and continued development of a retinal ON/OFF pathway imbalance in the retina, and thus a positive-feedback loop for continued myopia progression.
COST-EFFECTIVENESS OF TREATMENTS
Economic assessments of healthcare interventions aid evidence-based advocacy, policymaking, and patient care (Sorenson et al., 2008) by helping decision-makers to understand the quantity of resources required for treatment or prevention, to understand the value of treating or preventing the condition, and to weigh these considerations against other uses of the resources. The economic burden associated with myopia grows with its prevalence. In 2015, globally, uncorrected myopia cost $244 billion, and myopic macular degeneration accounted for $6 billion in potential productivity loss2 (Khanal et al., 2024; Smith et al., 2009c). This may seem like a small amount given the size of the economy and the enormous impact of other conditions, but it becomes important to compare the burden with the cost of reducing it. Also, it is important to recognize that the data used in the estimate are from before 2015 and 2009, respectively. Since that time, the opportunities to be productive without vision correction have changed, the cost of vision correction has changed in ways other than just inflationary pressures, and the knowledge of long-term consequences and cost of treating them has also changed. (Note: This section draws heavily on the commissioned paper prepared by Khanal and colleagues [2024] for this study.)
Direct Costs
Due to the increasing prevalence of myopia and the strain on healthcare resources, a reliable economic evaluation of myopia treatments is necessary to maximize benefits that can be obtained using the budget that is available. Data are sparse on the economic benefits of myopia
___________________
2 All $ figures are in USD, unless otherwise indicated.
treatments, such as the benefits of improved classroom behavior because of a child not losing attention, increased educational attainment or other skills that lead to higher incomes, and the monetary value of decreased incidence of later complications. In Singapore, the annual direct cost of treating myopia for teens before 2010 was $25 million (Lim et al., 2009) and $755 million for adults (Zheng et al., 2013); part of the reason for the disparate cost is that there are many more myopic adults than teens. The cost estimate for Singapore includes refractive surgery, glasses, contact lenses, solutions, and associated myopia visual impairment issues.
Lack of economic evaluation evidence may hamper decision-makers’ abilities to make rapid decisions about allocating resources to myopia interventions (Fricke et al., 2023). The cost of myopia treatment varies significantly worldwide, but direct costs are the major contributor. A review of the literature review was published in 2021 with data from 2002 (Spain) to 2018 (Iran) and other studies in 2006, 2009, and 2013; the previously published cost figures do not appear to have been inflation-adjusted for the reviewer (Foo et al., 2021). In the United States, the annual direct costs range from $14 to $26 per capita over the entire population, with contact lenses being the most expensive item on a per-patient basis (Foo et al., 2021). In Singapore, the mean annual direct cost of myopia is approximately SGD$900 ($709) per patient. The major drivers of this cost are spectacles, contact lenses, and optometry services (Zheng et al., 2013). For Singaporean school children, the mean annual direct cost of myopia per student with myopia is SGD$221.68 ($148), with higher costs associated with higher family income and parental education levels (Lim et al., 2009).
Financial Burden and Cost-Effectiveness of Interventions
The cost of myopia treatment is a significant financial burden for patients and their families, and effective control methods are needed to alleviate these costs (Tang et al., 2021). There are now evidence-based standards of care for myopia globally (Gifford et al., 2019; Hendicott & Block, 2022; Németh et al., 2021; Saxena et al., 2023; Tapasztó et al., 2023). The 2023 Myopia Consensus Statement released by The World Society of Pediatric Ophthalmology and Strabismus states, “There is sufficient evidence to warrant the adoption of myopia prevention and control measures in clinical practice in children with progressive myopia of childhood” (World Society of Pediatric Ophthalmology & Strabismus, 2023). Despite the increasing recognition of myopia as a high-priority problem and the establishment of a task force by the American Academy of Ophthalmology (Modjtahedi et al., 2021), there are no formalized insurance or vision plan services that cover the costs of myopia treatments in the United States.
The cost-effectiveness of various myopia treatment options varies. In earlier years, Gwiazda (2009) and Chang & Joo (2012) pointed out limitations in available treatments and information on the treatments that were available; this included short-term benefits and side effects (Chang & Joo, 2012; Gwiazda, 2009). However, in 2021, Foo et al. suggested that myopia treatment and prevention costs are justified based on the opportunity to reduce the ongoing expenditures related to myopia. Discussions of the cost-effectiveness of myopia treatments are drawing more attention (Bullimore & Brennan, 2023; Vutipongsatorn et al., 2019). One systematic review noted that the annualized value of lifetime direct expenditures per patient for contact lenses in Iran ranged from $198.30 to $378.10 per patient, while the costs for spectacles and refractive procedures were $342.50 and $19.10, respectively (Foo et al., 2021). Lifetime direct costs for the three options are $9374, $5203, and $568. The authors of the original study from Iran (Mohammadi et al., 2018) note that a U.S. study (Javitt & Chiang, 1994)
had reached similar conclusions about substantially lower lifetime costs for surgery in comparison with soft contact lenses.
As noted, the annual prevalence-based direct expenditures for myopia across the entire population were between $14 and $26 per capita in the United States, in comparison with $56 per capita in Iran and $199 per capita in Singapore. Hong et al. (2022) examined the cost-effectiveness of photorefractive myopia screening at age 11, administering atropine 0.01% eye drops for positive cases. They found that screening plus atropine eye drops saved seven lifelong blindness cases per 100,000 children and had an incremental cost-effectiveness ratio of NZ$1,590 (95% CI 1390, 1791) per quality-adjusted life-years gained, which is generally considered a good value.
In a recent systematic review, Agyekum et al. (2023a) evaluated the cost-effectiveness of interventions for myopia and its complications, including those for preventing myopia progression, correcting refractive error, and treating pathologic myopia using costs, quality-adjusted life-years, and incremental cost-effectiveness ratio as outcome measures. They found that low-concentration atropine (0.01%) and corneal refractive surgery were the most cost-effective treatments for myopia, and ranibizumab and conbercept were the most affordable treatments for pathological myopia, that is, for the more severe levels of myopia that have an adverse effect on the health of the eye.
Preventing myopia progression was reported to be more cost-effective than treating pathological myopia. For instance, the use of 0.01% atropine for myopia progression produced an incremental cost-effectiveness ratio of $1,001 per quality-adjusted life-year, as compared with $12,852 to $246,486 per quality-adjusted life-year for treating pathologic myopia. Although the cost-effectiveness of refractive surgery was low, 0.01% atropine was found to be more cost-effective due to lower treatment cost and the additional benefits of preventing myopia complications and related visual impairment.
More recently, a Markov model was used to perform an economic evaluation on the cost-effectiveness of 13 interventions for preventing myopia progression in children (Agyekum et al., 2023a). A comparison of the economic implications of 13 different treatments would have a great deal of value if it was understood how much society should be willing to spend to avoid a change in spherical equivalent or to shorten the axial length; unfortunately, that is not known. So, while the results are interesting, they are challenging to interpret and fail to rule out choices that are dominated (less effective and more expensive). In the future, this type of analysis should be replicated with a focus on a longer time period (over which the long-term complications for the myopic eye can be included) and the results should be converted to changes in quality-adjusted life years, as has been done in other studies. The cost of children spending time outdoors should include the opportunity cost of time, the potential risk to eye health, the risk of skin cancer, and risks to personal safety.
Apart from the evidence of effectiveness, evidence regarding the value proposition of interventions seems critical. To address this gap, Fricke et al. (2023) developed and modeled a system for assessing and comparing the lifetime financial expenses of active myopia management (i.e., use of treatments) against traditional myopia management (i.e. use of corrective lenses). In contrast to Agyekum et al. (2023b), who modeled cost-effectiveness options for a 10-year-old myope with varying levels of myopia, Fricke et al. (2023) presented data using an 8-year-old who presented with symptomatic –0.75D correction in both eyes in urban areas of Australia and China and was given the choice of active or traditional myopia management. Options for these managements were available in both nations, and the costs of all
product kinds, essential appointment fees, and other relevant expenditures were calculated using data provided by important sources in both countries. The authors found that the lowest lifetime cost options were anti-myopia spectacles in Australia and low-dose atropine in China. The lifetime cost for traditional myopia management, with a 3% discount rate, was $7,437 (95% CI: $4,953 to $10,740) in Australia and $8,006 (95% confidence interval: $3,026 to $13,707) in China. The final level of myopia had the greatest impact on the lifetime costs of myopia in both countries. This type of work should be replicated for other countries with varying health care financing systems.
In summary, lifetime savings from reduced myopia often offset the upfront costs of myopia treatments in childhood. The use of low concentrations of atropine is cost-effective, but it requires the additional cost of correction with spectacles or contact lenses; 0.05% atropine can reduce myopia progression in children with acceptable side effects and can minimize adult myopia treatment costs (Agyekum et al., 2023b). Nevertheless, comprehensive economic reviews of myopia treatments and evidence of their cost-effectiveness are lacking. As childhood myopia is increasing in prevalence and severity worldwide, and multiple myopia treatments are now available, a robust and comprehensive analysis of the cost-effectiveness of myopia treatments will provide critical data for health policy decisions, thereby maximizing health outcomes with limited resources.
CONCLUSIONS
Conclusion 7-1: Treatment options for myopia progression have increased in the last 20 years, with clinical trials showing that axial growth of the human eye can be slowed down with optical and pharmaceutical intervention. Current treatments for myopia progression include multifocal optical corrections (orthokeratology, soft multi- and dual-focal contact lenses, peripheral refractive error spectacles) and atropine eye drops.
Conclusion 7-2: Atropine is the only pharmacological treatment for myopia progression widely available and used across the globe. Stronger concentrations of atropine produce better treatment effects but more side effects, including rebound effect. Atropine 0.01% is the mostly widely used; however, the treatment effects appear limited. The mechanism of action for atropine remains elusive, and long-term effects require more study.
Conclusion 7-3: While still an emerging treatment strategy, time outdoors is consistently reported to have a protective effect on myopia prevention, especially in the younger years. Studies indicate 2 hours per day may provide the needed amount of outdoor exposure, but exact recommendations for exposure time, time of day, luminance levels, and chromatic contributions have not been determined.
Conclusion 7-4: Current treatments for myopia progression have limited effects. The largest treatment effect of any published treatment option remains under 0.75 diopters over 2 years, based on recent systematic reviews. Some treatment options are effective in the first year and less effective in subsequent years. The reasons for this limited efficacy are
unknown, but potential reasons include a role for accommodation, incomplete data on how optical corrections affect the retinal image, and factors that influence ON and OFF pathway balance. Further work is also needed to understand the mechanisms of action underlying the efficacy of optical treatments.
Conclusion 7-5: Current treatments stop working after cessation and can have a rebound effect with subsequent rapid eye growth. It is therefore unclear how and when treatment should be stopped, and if rapid eye growth during rebound is more detrimental to the eye than slow and steady growth without treatment. Ideal treatment options would show similar or accruing treatment effects with each year of use, without rebound effects.
Conclusion 7-6: Current literature suggests that combination therapy shows minimal to no additive effectiveness. However, studies have only used the minimally effective 0.01% concentration of atropine in combination with other treatments. Combination therapy should be studied using higher concentrations.
Conclusion 7-7: Clinical trials for myopia treatments have provided important insights into the progression of myopia. Predictors of myopia progression include a child’s existing refractive error, age, sex, and ethnic identity. Past myopia progression does not predict future progression. Myopia tends to progress faster when onset is at younger ages, which may suggest early treatment of myopia in the preschool years. It is unclear whether treatments known to be effective for low to moderate myopia in school-aged children are effective in preschool-aged children, and also unclear whether treatments for low to moderate myopia in school-aged children are effective in high myopia or myopia associated with genetic and systemic disorders.
Conclusion 7-8: Treatment of myopia may be needed daily and to be undertaken for a decade or more. Therefore, safety of the treatment is paramount and new treatment options and interventions need to be carefully evaluated to determine any negative side effects.
Conclusion 7-9: The current state of knowledge of treatment options reflects our limited understanding of the fundamental mechanisms of eye length regulation and how treatments act to alter the progression—and perhaps even the onset—of the disease.
RECOMMENDATIONS
Recommendation 7-1: Funding agencies, foundations, and NGOs, including the National Institutes of Health, should support research to develop new treatment strategies for myopia (age at initiation and cessation, optimal treatment for a given population, efficacy of combination treatments, optimal timing for treatment combinations such as alternating vs. simultaneous, longer-term outcomes), as well as to determine the mechanisms that underlie current treatments. Progress in this area
needs intentionally integrated, multi-disciplinary research in basic and clinical vision science to understand the mechanisms by which therapies can control eye growth. Areas deserving urgent focus are listed here.
- Develop fundamental studies—potentially including animal models—of the mechanisms by which existing and new therapies affect eye growth.
- Perform research on the mechanisms of atropine action to determine the causes of treatment effects and side effects, thereby providing opportunities to optimize treatment efficacy in children and to develop novel pharmaceuticals with fewer side effects.
- Design studies to identify the ideal dosing characteristics of current and novel pharmaceuticals, including concentration and cadence, to slow eye growth.
- Develop new pharmaceutical options to provide structural scleral reinforcement, without creating dose-dependent side effects. Further research is needed to identify pharmacological agents that are more effective and have fewer adverse effects than current options.
- Determine optimal parameters for time outdoors, including duration per day, spectral distribution, time of day, and needed safety measures, to prevent or delay myopia onset. Such studies may create the opportunity to develop treatments for myopia that can be used indoors, independently of time outdoors.
- Combine bench and eye model studies of visual optics including the spectral composition of light, peripheral refractive characterization, and contrast to develop optical corrections for best visual performance and optical quality.
- Develop rigorous investigations of combination therapies.
- Conduct longer-term studies or assessments in adulthood to weigh the costs of myopia treatment against its benefits in terms of the ultimate amount of myopia and effects on ocular health.
Recommendation 7-2: Treatment safety is paramount, given that myopia control treatments are likely to be used throughout childhood and perhaps through young adulthood. Scientists should develop strategies to minimize short-term and long-term side effects.
Recommendation 7-3: Funding for multicenter randomized clinical trials should be directed toward longer-term human studies, starting at earlier ages on treatment and off treatment, to determine long-term benefit with respect to ultimate refractive error and ocular health.
REFERENCES
Abbott, M., Schmid, K. L., & Strang, N. C. (1998). Differences in the accommodation stimulus response curves of adult myopes and emmetropes. Ophthalmic and Physiological Optics, 18(1), 13–20. https://doi.org/10.1016/S0275-5408(97)00072-0
Age-Related Eye Disease Study 2 Research Group. (2013). Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA, 309(19), 2005–2015. https://doi.org/10.1001/jama.2013.4997
Agyekum, S., Chan, P. P., Adjei, P. E., Zhang, Y., Huo, Z., Yip, B. H. K., Ip, P., Wong, I. C. K., Zhang, W., Tham, C. C., Chen, L. J., Zhang, X. J., Pang, C. P., & Yam, J. C. (2023a). Cost-effectiveness analysis of myopia progression interventions in children. JAMA Network Open, 6(11), e2340986. https://doi.org/10.1001/jamanetworkopen.2023.40986
Agyekum, S., Chan, P. P., Zhang, Y., Huo, Z., Yip, B. H. K., Ip, P., Tham, C. C., Chen, L. J., Zhang, X. J., Pang, C. P., & Yam, J. (2023b). Cost-effectiveness analysis of myopia management: A systematic review. Frontiers in Public Health, 11, 1093836. https://doi.org/10.3389/fpubh.2023.1093836
Aleman, A. C., Wang, M., & Schaeffel, F. (2018). Reading and myopia: Contrast polarity matters. Scientific Reports, 8(1), 10840. https://doi.org/10.1038/s41598-018-28904-x
Arias, A., Ohlendorf, S., Artal, P., & Wahl, S. (2023). In-depth optical characterization of spectacle lenses for myopia progression management. Optica, 10, 594–603. https://doi.org/10.1364/OPTICA.486389
Ashby, R., Ohlendorf, A., & Schaeffel, F. (2009). The effect of ambient illuminance on the development of deprivation myopia in chicks. Investigative Ophthalmology & Visual Science, 50(11), 5348–5354. https://doi.org/10.1167/iovs.09-3419
Ashby, R. S., & Schaeffel, F. (2010). The effect of bright light on lens compensation in chicks. Investigative Ophthalmology & Visual Science, 51(10), 5247–5253. https://doi.org/10.1167/iovs.09-4689
Atchison, D. A., Jones, C. E., Schmid, K. L., Pritchard, N., Pope, J. M., Strugnell, W. E., & Riley, R. A. (2004). Eye shape in emmetropia and myopia. Investigative Ophthalmology & Visual Science, 45(10), 3380–3386. https://doi.org/10.1167/iovs.04-0292
Atchison, D. A., Pritchard, N., Schmid, K. L., Scott, D. H., Jones, C. E., & Pope, J. M. (2005). Shape of the retinal surface in emmetropia and myopia. Investigative Ophthalmology & Visual Science, 46(8), 2698–2707. https://doi.org/10.1167/iovs.04-1506
Atchison, D. A., Pritchard, N., & Schmid, K. L. (2006). Peripheral refraction along the horizontal and vertical visual fields in myopia. Vision Research, 46(9), 1450–1458. https://doi.org/10.1016/j.visres.2005.10.023
Atchison, D. A., & Smith, G. (2000). Chromatic aberrations. In Elsevier eBooks (pp. 180–193). https://doi.org/10.1016/b978-0-7506-3775-6.50021-3
Barathi, V. A., Chaurasia, S. S., Poidinger, M., Koh, S. K., Tian, D., Ho, C., Iuvone, P. M., Beuerman, R. W., & Zhou, L. (2014). Involvement of GABA transporters in atropine-treated myopic retina as revealed by iTRAQ quantitative proteomics. Journal of Proteome Research, 13(11), 4647–4658. https://doi.org/10.1021/pr500558y
Barger-Lux, M. J., & Heaney, R. P. (2002). Effects of above average summer sun exposure on serum 25-hydroxyvitamin D and calcium absorption. The Journal of Clinical Endocrinology & Metabolism, 87(11), 4952–4956. https://doi.org/10.1210/jc.2002-020636
Bartlett, J. D., Niemann, K., Houde, B., Allred, T., Edmondson, M. J., & Crockett, R. S. (2003). A tolerability study of pirenzepine ophthalmic gel in myopic children. Journal of Ocular Pharmacology and Therapeutics, 19(3), 271–279. https://doi.org/10.1089/108076803321908392
Beaulieu, J. M., Espinoza, S., & Gainetdinov, R. R. (2015). Dopamine receptors—IUPHAR review 13. British Journal of Pharmacology, 172(1), 1–23. https://doi.org/10.1111/bph.12906
Bedrossian, R. H. (1966). Treatment of progressive myopia with atropine. In Proceedings of the XX International Congress of Ophthalmology. Munich.
Bedrossian, R. H. (1979). The effect of atropine on myopia. Ophthalmology, 86(5), 713–719. https://doi.org/10.1016/s0161-6420(79)35455-0
Berntsen, D. A., Ticak, A., Sinnott, L. T., Chandler, M. A., Jones, J. H., Morrison, A., Jones-Jordan, L. A., Walline, J. J., Mutti, D. O., & BLINK Study Group. (2023). Peripheral defocus, pupil size, and axial eye growth in children wearing soft multifocal contact lenses in the BLINK Study. Investigative Ophthalmology & Visual Science, 64(14), 3. https://doi.org/10.1167/iovs.64.14.3
Borchert, M. S., Varma, R., Cotter, S. A., Tarczy-Hornoch, K., McKean-Cowdin, R., Lin, J. H., Wen, G., Azen, S. P., Torres, M., Tielsch, J. M., Friedman, D. S., Repka, M. X., Katz, J., Ibironke, J., Giordano, L., & Joint Writing Committee for the Multi-Ethnic Pediatric Eye Disease Study and the Balitmore Pediatric Eye Disease Study Groups. (2011). Risk factors for hyperopia and myopia in preschool children: The Multi-Ethnic Pediatric Eye Disease and Baltimore Pediatric Eye Disease studies. Ophthalmology, 118(10), 1966–1973. https://doi.org/10.1016/j.ophtha.2011.06.030
Bores, L. D., Myers, W., & Cowden, J. (1981). Radial keratotomy: An analysis of the American experience. Annals of Ophthalmology, 13(8), 941–948. https://pubmed.ncbi.nlm.nih.gov/7294635/
Brennan, N., Toubouti, Y., Cheng, X., & Bullimore, M. (2021). Efficacy in myopia control. Progress in Retinal and Eye Research, 83, 100923. https://doi.org/10.1016/j.preteyeres.2020.100923
Brodstein, R. S., Brodstein, D. E., Olson, R. J., Hunt, S. C., & Williams, R. R. (1984). The treatment of myopia with atropine and bifocals. A long-term prospective study. Ophthalmology, 91(11), 1373–1379. https://doi.org/10.1016/s0161-6420(84)34138-0
Bullimore, M. A., & Brennan, N. A. (2023). Juvenile-onset myopia-who to treat and how to evaluate success. Eye, 38(3), 450–454. https://doi.org/10.1038/s41433-023-02722-6
Calkins, D. J. (2001). Seeing with S cones. Progress in Retinal Eye Research, 20(3), 255–287. https://doi.org/10.1016/s1350-9462(00)00026-4
Carr, B. J., & Stell, W. K. (2016). Nitric oxide (NO) mediates the inhibition of form-deprivation myopia by atropine in chicks. Scientific Reports, 6(1), 9. https://doi.org/10.1038/s41598-016-0002-7
Chakraborty, R., Landis, E. G., Mazade, R., Yang, V., Strickland, R., Hattar, S., Stone, R. A., Iuvone, P. M., & Pardue, M. T. (2022). Melanopsin modulates refractive development and myopia. Experimental Eye Research, 214, 108866. https://doi.org/10.1016/j.exer.2021.108866
Chalmers, R. L., McNally, J. J., Chamberlain, P., & Keay, L. (2021). Adverse event rates in the retrospective cohort study of safety of pediatric soft contact lens wear: The ReCSS study. Ophthalmic & Physiological Optics, 41(1), 84–92. https://doi.org/10.1111/opo.12753
Chamberlain, P., Bradley, A., Arumugam, B., Hammond, D., McNally, J., Logan, N. S., Jones, D., Ngo, Cheryl, Peixoto-de-Matos, S., Hunt, C., & Young, G. (2022). Long-term effect of dual-focus contact lenses on myopia progression in children: A 6-year multicenter clinical trial. Optometry and Vision Science, 99(3), 204–212. https://doi.org/10.1097/OPX.0000000000001873
Chandler, M. A., Robich, M. L., Jordan, L. A., Mutti, D. O., Berntsen, D. A., Fenton, R., Day, E., & Walline, J. J. (2023). Accommodation in children after 4.7 years of multifocal contact lens wear in the BLINK study randomized clinical trial. Optometry and Vision Science, 100(7), 425–431. https://doi.org/10.1097/OPX.0000000000002040
Chang, D. J., & Joo, C. K. (2012). Current and future options for myopia treatment. Journal of the Korean Medical Association, 55(4). https://doi.org/10.5124/jkma.2012.55.4.362
Charman, W. N., & Radhakrishnan, H. (2021). Do optical treatments for the control of myopia progression produce proportional or absolute reductions in progression rates? Ophthalmic & Physiological Optics, 41, 192–197. https://doi.org/10.1111/opo.12750
Chawda, D., & Shinde, P. (2022). Effects of solar radiation on the eyes. Cureus, 14(1), e30857. https://doi.org/10.7759%2Fcureus.30857
Che, D., Qiao, D., Cao, Y., Zhang, Y., Zhou, Q., Tong, S., Miao, P., & Zhou, J. (2024). Changes in choroidal hemodynamics of form-deprivation myopia in Guinea pigs. Biochemical and Biophysical Research Communications, 692, 149348. https://doi.org/10.1016/j.bbrc.2023.149348
Chen, Z., Lv, X., Lai, L., Xu, Y., & Zhang, F. (2023). Effects of riboflavin/Ultraviolet-A(UVA) scleral crosslinking on the mechanical behavior of the scleral fibroblasts of lens-induced myopia guinea pigs. Experimental Eye Research, 235, 109618. https://doi.org/10.1016/j.exer.2023.109618.
Chen, C., & Yao, J. (2021). Efficacy and adverse effects of atropine for myopia control in children: A meta-analysis of randomized controlled trials. Journal of Ophthalmology, 2021. https://doi.org/10.1155/2021/4274572
Chen, Y., Xiong, R., Chen, X., Zhang, J., Bulloch, G., Lin, X., Wu, X., & Li, J. (2022). Efficacy comparison of repeated low-level red light and low-dose atropine for myopia control: A randomized controlled trial. Translational Vision Science & Technology, 11(10), 33. https://doi.org/10.1167/tvst.11.10.33
Cheng, X., Xu, J., & Brennan, N. A. (2019). Accommodation and its role in myopia progression and control with soft contact lenses. Ophthalmic & Physiological Optics, 39, 162–171. https://doi.org/10.1111/opo.12614
Chia, A., Chua, W. H., Cheung, Y. B., Wong, W. L., Lingham, A., Fong, A., & Tan, D. (2012). Atropine for the treatment of childhood myopia: Safety and efficacy of 0.5%, 0.1%, and 0.01% doses (atropine for the treatment of myopia 2). Ophthalmology, 119(2), 347–354. https://doi.org/10.1016/j.ophtha.2011.07.031
Chia, A., Chua, W. H., Wen, L., Fong, A., Goon, Y. Y., & Tan, D. (2014). Atropine for the treatment of childhood myopia: Changes after stopping atropine 0.01%, 0.1% and 0.5%. American Journal of Ophthalmology, 157(2), 451–457.e1. https://doi.org/10.1016/j.ajo.2013.09.020
Choi, J. A., Han, K., Park, Y. M., & La, T. Y. (2014). Low serum 25-hydroxyvitamin D is associated with myopia in Korean adolescents. Investigative Ophthalmology & Visual Science, 55, 2041–2047. https://doi.org/10.1167/iovs.13-12853
Chua, W. H., Balakrishnan, V., Chan, Y. H., Tong, L., Ling, Y., Quah, B. L., & Tan, D. (2006). Atropine for the treatment of childhood myopia. Ophthalmology, 113(12), 2285–2291. https://doi.org/10.1016/j.ophtha.2006.05.062
Chun, R. K. M., Zhang, H., Liu, Z., Tse, D. Y. Y., Zhou, Y., Lam, C. S. Y., & To, C. H. (2023). Defocus incorporated multiple segments (DIMS) spectacle lenses increase the choroidal thickness: a two-year randomized clinical trial. Eye and Vision (London, England), 10(1), 39. https://doi.org/10.1186/s40662-023-00356-z
Colberg, S. R., Sigal, R. J., Yardley, J. E., Riddell, M. C., Dunstan, D. W., Dempsey, P. C., Horton, E. S., Castorino, K., & Tate, D. F. (2016). Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care, 39(11), 2065–2079. https://doi.org/10.2337/dc16-1728
Colin, J., Robinet, A., & Cochener, B. (1999). Retinal detachment after clear lens extraction for high myopia: Seven-year follow-up. Ophthalmology, 106(12), 2281–2285. https://doi.org/10.1016/S0161-6420(99)90526-2
COMET Group. (2013). Myopia stabilization and associated factors among participants in the Correction of Myopia Evaluation Trial (COMET). Investigative Ophthalmology & Visual Science, 54(13), 7871–7884. https://doi.org/10.1167/iovs.13-12403
Cuellar-Partida, G., Williams, K. M., Yazar, S., Guggenheim, J. A., Hewitt, A. W., Williams, C., Wang, J. J., Kho, P. F., Saw, S. M., Cheng, C. Y., Wong, T. Y., Aung, T., Young, T. L., Tideman, J. W. L., Jonas, J. B., Consortium for Refractive Error and Myopia (CREAM), Mitchell, P., Wojciechowski, R., Stambolian, D., Hysi, P., … MacGregor, S. (2017). Genetically low vitamin D concentrations and myopic refractive error: A Mendelian randomization study. International Journal of Epidemiology, 46(6), 1882–1890. https://doi.org/10.1093/ije/dyx068
Deng, B., Li, W., Chen, Z., Zeng, J., & Zhao, F. (2023). Temporal bright light at low frequency retards lens-induced myopia in guinea pigs. PeerJ, 11, e16425. https://doi.org/10.7717/peerj.16425
Ding, H., He, M.-N., & Han, D. (2021). Protective effects of riboflavin-UVA-mediated posterior sclera collagen cross-linking in a guinea pig model of form-deprived myopia. International Journal of Ophthalmology, 14(3), 333–340. https://doi.org/10.18240/ijo.2021.03.01.
Dirani, M., Tong, L., Gazzard, G., Zhang, X., Chia, A., Young, T. L., Rose, K. A., Mitchell, P., & Saw, S. M. (2009). Outdoor activity and myopia in Singapore teenage children. British Journal of Ophthalmology, 93(8), 997–1000. https://doi.org/10.1136/bjo.2008.150979
Dolgin, E. (2024). A myopia epidemic is sweeping the globe. Here’s how to stop it. Nature, 629(8014), 989–991. https://doi.org/10.1038/d41586-024-01518-2
Dong, J., Zhu, Z., Xu, H., & He, M. (2023). Myopia control effect of repeated low-level red-light therapy in Chinese children: A randomized, double-blind, controlled clinical trial. Ophthalmology, 130(2), 198–204. https://doi.org/10.1016/j.ophtha.2022.08.024
Emanuel, A. J., & Do, H. M. T. (2023). The multistable melanopsins of mammals. Frontiers in Ophthalmology, 3. https://doi.org/10.3389/fopht.2023.1174255
Feldkaemper, M., & Schaeffel, F. (2013). An updated view on the role of dopamine in myopia. Experimental Eye Research, 114, 106–119. https://doi.org/10.1016/j.exer.2013.02.007
Fernández-Vega, L., Alfonso, J. F., & Villacampa, T. (2003). Clear lens extraction for the correction of high myopia. Ophthalmology, 110(12), 2349–2354. https://doi.org/10.1016/S0161-6420(03)00794-2
Fischer, A. J., Miethke, P., Morgan, I. G., & Stell, W. K. (1998). Cholinergic amacrine cells are not required for the progression and atropine-mediated suppression of form-deprivation myopia. Brain Research, 794(1), 48–60. https://doi.org/10.1016/s0006-8993(98)00188-7
Flitcroft, D. I. (2012). The complex interactions of retinal, optical and environmental factors in myopia aetiology. Progress in Retinal and Eye Research, 31(6), 622–660. https://doi.org/10.1016/j.preteyeres.2012.06.004
Foo, L. L., Lanca, C., Wong, C. W., Ting, D., Lamoureux, E., Saw, S. M., & Ang, M. (2021). Cost of myopia correction: A systematic review. Frontiers in Medicine, 8, 718724. https://doi.org/10.3389/fmed.2021.718724
Foulds, W. S., Barathi, V. A., & Luu, C. D. (2013). Progressive myopia or hyperopia can be induced in chicks and reversed by manipulation of the chromaticity of ambient light. Investigative Ophthalmology & Visual Science, 54(12), 8004–8012. https://doi.org/10.1167/iovs.13-12476
Fozailoff, A., Tarczy-Hornoch, K., Cotter, S., Wen, G., Lin, J., Borchert, M., Azen, S., Varma, R., & Writing Committee for the MEPEDS Study Group. (2011). Prevalence of astigmatism in 6- to 72-month-old African American and Hispanic children: The Multi-ethnic Pediatric Eye Disease Study. Ophthalmology, 118(2), 284–293. https://doi.org/10.1016/j.ophtha.2010.06.038
Frangenberg, T. (1991). Perspectivist aristotelianism: Three case-studies of Cinquecento Visual Theory. Journal of the Warburg and Courtauld Institutes, 54, 137–158. https://doi.org/10.2307/751485
French, A. N., Morgan, I. G., Burlutsky, G., Mitchell, P., & Rose, K. A. (2013a). Prevalence and 5- to 6-Year Incidence and progression of Myopia and Hyperopia in Australian schoolchildren. Ophthalmology, 120(7), 1482–1491. https://doi.org/10.1016/j.ophtha.2012.12.018
French, A. N., Morgan, I. G., Mitchell, P., & Rose, K. A. (2013b). Risk factors for incident myopia in Australian schoolchildren: The Sydney adolescent vascular and eye study. Ophthalmology, 120(10), 2100–2108. https://doi.org/10.1016/j.ophtha.2013.02.035
Fricke, T. R., Sankaridurg, P., Naduvilath, T., Resnikoff, S., Tahhan, N., He, M., & Frick, K. D. (2023). Establishing a method to estimate the effect of antimyopia management options on lifetime cost of myopia. The British Journal of Ophthalmology, 107(8), 1043–1050. https://doi.org/10.1136/bjophthalmol-2021-320318
Gao, Y., Lim, E. W., Yang, A., Drobe, B., & Bullimore, M. A. (2021). The impact of spectacle lenses for myopia control on visual functions. Ophthalmic & Physiological Optics, 41(6), 1320–1331. https://doi.org/10.1111/opo.12878
Gaume Giannoni, A., Robich, M., Berntsen, D. A., Jones-Jordan, L. A., Mutti, D. O., Myers, J., Shaw, K., Walker, M. K., Walline, J. J., & BLINK Study Group. (2022). Ocular and nonocular adverse events during 3 years of soft contact lens wear in children. Optometry and Vision Science, 99(6), 505–512. https://doi.org/10.1097/OPX.0000000000001902
Gawne, T. J., Grytz, R., & Norton, T. T. (2021). How chromatic cues can guide human eye growth to achieve good focus. Journal of Vision, 21(5), 11. https://doi.org/10.1167/jov.21.5.11
Gawne, T. J., Ward, A. H., & Norton, T. T. (2017). Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Research, 140, 55–65.
Gifford, K. L., Richdale, K., Kang, P., Aller, T. A., Lam, C. S., Liu, Y. M., Michaud, L., Orr, J. B., Rose, K. A., Saunders, K. A., Saunders, K. J., Seidel, D., Tideman, J. W. L., & Sankaridurg, P. (2019). IMI—Clinical management guidelines report. Investigative Ophthalmology & Visual Science, 60(3), M184–M203. https://doi.org/10.1167/iovs.18-25977
Gong, Q., Janowski, M., Luo, M., Wei, H., Chen, B., Yang, G., & Liu, L. (2017a). Efficacy and adverse effects of atropine in childhood myopia: A meta-analysis. JAMA Ophthalmology, 135(6), 624–630. https://doi.org/10.1001/jamaophthalmol.2017.1091
Gong, C. R., Troilo, D., & Richdale, K. (2017b). Accommodation and phoria in children wearing multifocal contact lenses. Optometry and Vision Science, 94, 353–360. https://doi.org/10.1097/OPX.0000000000001044
Gordon, R. A., & Donzis, P. B. (1985). Refractive development of the human eye. Archives of Ophthalmology, 103, 785–789. https://doi.org/10.1001/archopht.1985.01050060045020
Grytz, R. & Lang, R. (2023, December 5). Workshop on the Rise in Myopia: Exploring Possible Contributors and Investigating Screening Practices, Policies, and Programs. National Academies of Sciences, Engineering, and Medicine. Washington, DC, USA. https://www.nationalacademies.org/event/41360_12-2023_workshop-on-the-rise-in-myopia-exploring-possible-contributors-and-investigating-screening-practices-policies-and-programs
Guggenheim, J. A., Northstone, K., McMahon, G., Ness, A. R., Deere, K., Mattocks, C., Pourcain, B. S., & Williams, C. (2012). Time outdoors and physical activity as predictors of incident myopia in childhood: A prospective cohort study. Investigative Ophthalmology & Visual Science, 53(6), 2856–2865. https://doi.org/10.1167/iovs.11-9091
Guggenheim, J. A., Williams, C., Northstone, K., Howe, L. D., Tilling, K., St Pourcain, B., McMahon, G., & Lawlor, D. A. (2014). Does vitamin D mediate the protective effects of time outdoors on myopia? Findings from a prospective birth cohort. Investigative Ophthalmology & Visual Science, 55(12), 8550–8558. https://doi.org/10.1167/iovs.14-15839
Guido, M. E., Marchese, N. A., Rios, M. N., Morera, L. P., Diaz, N. M., Garbarino-Pico, E., & Contin, M. A. (2022). Non-visual opsins and novel photo-detectors in the vertebrate inner retina mediate light responses within the blue spectrum region. Cellular and Molecular Neurobiology, 42(1), 59–83. https://doi.org/10.1007/s10571-020-00997-x
Guo, L., Tao, J., Guo, Z., Tong, Y., Chen, S., Zhao, X., & Hua, R. (2024). Morphological and vascular evidence of glaucomatous damage in myopic guinea pigs with scleral crosslinking. Scientific Reports, 14(1), 298. https://doi.org/10.1038/s41598-023-48461-2
Gwiazda, J. (2009). Treatment options for myopia. Optometry and Vision Science, 86(6), 624–628. https://doi.org/10.1097/OPX.0b013e3181a6a225
Gwiazda, J., Deng, L., Dias, L., Marsh-Tootle, W., & COMET Study Group. (2011). Association of education and occupation with myopia in COMET parents. Optometry and Vision Science, 88(9), 1045–1053. https://doi.org/10.1097/OPX.0b013e31822171ad
Gwiazda, J., Hyman, L., Hussein, M., Everett, D., Norton, T. T., Kurtz, D., Leske, M. C., Manny, R., Marsh-Tootle, W., & Scheiman, M. (2003). A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Investigative Ophthalmology & Visual Science, 44(4), 1492–1500. https://doi.org/10.1167/iovs.02-0816
Gwiazda, J., Grice, K., Held, R., McLellan, J., & Thorn, F. (2000). Astigmatism and the development of myopia in children. Vision Research, 40(8), 1019–1026. https://doi.org/10.1016/s0042-6989(99)00237-0
Gwiazda, J., Marsh-Tootle, W. L., Hyman, L., Hussein, M., Norton, T. T., & COMET Study Group. (2002). Baseline refractive and ocular component measures of children enrolled in the correction of myopia evaluation trial (COMET). Investigative Ophthalmology & Visual Science, 43(2), 314–321. https://pubmed.ncbi.nlm.nih.gov/11818372/
Gwiazda, J., Thorn, F., Bauer, J., & Held, R. (1993). Myopic children show insufficient accommodative response to blur. Investigative Ophthalmology & Visual Science, 34, 690–694. https://pubmed.ncbi.nlm.nih.gov/8449687/
Haarman, A. E. G., Enthoven, C. A., Tideman, J. W. L., Tedja, M. S., Verhoeven, V. J. M., & Klaver, C. C. W. (2020). The complications of myopia: A review and meta-analysis. Investigative Ophthalmology & Visual Science, 61(4), 49. https://doi.org/10.1167/iovs.61.4.49
Hagen, L. A., Gjelle, J. V. B., Arnegard, S., Pedersen, H. R., Gilson, S. J., & Baraas, R. C. (2018). Prevalence and possible factors of myopia in Norwegian adolescents. Scientific Reports, 8(1), 13479. https://doi.org/10.1038/s41598-018-31790-y
Hamdaoui, M. E., Levy, A. M., Stuber, A. B., Girkin, C. A., Kraft, T. W., Samuels, B. C., & Grytz, R. (2022). Scleral crosslinking using genipin can compromise retinal structure and function in tree shrews. Experimental Eye Research, 219, 109039. https://doi.org/10.1016/j.exer.2022.109039
He, X., Wang, J., Zhu, Z., Xiang, K., Zhang, X., Zhang, B., Chen, J., Yang, J., Du, J., Niu, C., Leng, M., Huang, J., Liu, K., Zou, H., He, M., & Xu, X. (2023). Effect of repeated low-level red light on myopia prevention among children in China with premyopia: A randomized clinical trial. JAMA Network Open, 6(4), e239612. https://doi.org/10.1001/jamanetworkopen.2023.9612
Hendicott, P., & Block, S. S. (2022). How the World Council of Optometry produced new guidelines for myopia management. Community Eye Health, 35(117), 21–22. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10061246/
Hendriks, M., Verhoeven, V. J. M., Buitendijk, G. H. S., Polling, J. R., Meester-Smoor, M. A., Hofman, A., RD5000 Consortium, Kamermans, M., Ingeborgh van den Born, L., & Klaver, C. C. W. (2017). Development of refractive errors—What can we learn from inherited retinal dystrophies? American Journal of Ophthalmology, 182, 81–89. https://pubmed.ncbi.nlm.nih.gov/28751151/
Hofstetter, H. W. (1944). A comparison of Duane’s and Donders tables of the amplitude of accommodation. American Journal of Optometry and Archives of American Academy of Optometry, 21(7), 345–363. https://journals.lww.com/optvissci/citation/1944/09000/a_comparison_of_duane_s_and_donders__tables_of_the.1.aspx
Holick, M. F. (1995). Environmental factors that influence the cutaneous production of vitamin D. The American Journal of Clinical Nutrition, 61(3), 638S–645S. https://doi.org/10.1093/ajcn/61.3.638s
Hong, C. Y., Boyd, M., Wilson, G., & Hong, S. C. (2022). Photorefraction screening plus atropine treatment for myopia is cost-effective: A proof-of-concept Markov analysis. Clinical Ophthalmology, 16, 1941–1952. https://doi.org/10.2147/OPTH.S362342
Hou, W., Norton, T. T., Hyman, L., Gwiazda, J., & COMET Group (2018). Axial elongation in myopic children and its association with myopia progression in the Correction of Myopia Evaluation Trial. Eye & Contact Lens, 44(4), 248–259. https://doi.org/10.1097/ICL.0000000000000505
Huang, D., Schallhorn, S. C., Sugar, A., Farjo, A. A., Majmudar, P. A., Trattler, W. B., & Tanzer, D. J. (2009). Phakic intraocular lens implantation for the correction of myopia: A report by the American Academy of Ophthalmology. Ophthalmology, 116(11), 2244–2258. https://doi.org/10.1016/j.ophtha.2009.08.018
Huang, J., Hung, L. F., & Smith, E. L., 3rd (2011). Effects of foveal ablation on the pattern of peripheral refractive errors in normal and form-deprived infant rhesus monkeys (Macaca mulatta). Investigative Ophthalmology & Visual Science, 52(9), 6428–6434. https://doi.org/10.1167/iovs.10-6757
Huang, J., Hung, L. F., & Smith, E. L., 3rd (2012). Recovery of peripheral refractive errors and ocular shape in rhesus monkeys (Macaca mulatta) with experimentally induced myopia. Vision Research, 73, 30–39. https://doi.org/10.1016/j.visres.2012.09.002
Huang, J., Wen, D., Wang, Q., McAlinden, C., Flitcroft, I., Chen, H., Saw, S. M., Chen, H., Bao, F., Zhao, Y., Hu, L., Li, X., Gao, R., Lu, W., Du, Y., Jinag, Z., Yu, A., Lian, H., Jiang, Q., Yu, Y., … Qu, J. (2016). Efficacy Comparison of 16 Interventions for Myopia Control in Children: A Network Meta-analysis. Ophthalmology, 123(4), 697–708. https://doi.org/10.1016/j.ophtha.2015.11.010
Huang, X. L., Ding, C., Chen, Y., Chen, H., & Bao, J. (2022). Orthokeratology reshapes eyes to be less prolate and more symmetric. Contact Lens & Anterior Eye, 45(4), 101532. https://doi.org/10.1016/j.clae.2021.101532
Hung, L. F., Arumugam, B., She, Z., Ostrin, L., & Smith, E. L., 3rd. (2018). Narrow-band, long-wavelength lighting promotes hyperopia and retards vision-induced myopia in infant rhesus monkeys. Experimental Eye Research, 176, 147–160. https://doi.org/10.1016/j.exer.2018.07.004
Hung, L. F., Crawford, M. L., & Smith, E. L. (1995). Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature Medicine, 1(8), 761–765. https://doi.org/10.1038/nm0895-761
Hyman, L., Gwiazda, J., Marsh-Tootle, W. L., Norton, T. T., Hussein, M., & COMET Group (2001). The Correction of Myopia Evaluation Trial (COMET): Design and general baseline characteristics.
Jaskulski, M., Singh, N. K., Bradley, A., & Kollbaum, P. S. (2020). Optical and imaging properties of a novel multi-segment spectacle lens designed to slow myopia progression. Ophthalmic & Physiological Optics, 40(5), 549–556. https://doi.org/10.1111/opo.12725
Javitt, J. C., & Chiang, Y. P. (1994). The socioeconomic aspects of laser refractive surgery. Archives of Ophthalmology, 112(12), 1526–1530. https://doi.org/10.1001/archopht.1994.01090240032022
Jiang, X., Pardue, M. T., Mori, K., Ikeda, S. I., Torii, H., D’Souza, S., Lang, R. A., Kurihara, T., & Tsubota, K. (2021a). Violet light suppresses lens-induced myopia via neuropsin (OPN5) in mice. Proceedings of the National Academy of Sciences of the United States of America, 118(1). https://doi.org/10.1073/pnas.2018840118
Jiang, L., Zhang, S., Schaeffel, F., Xiong, S., Zheng, Y., Zhou, X., Lu, F., & Qu, J. (2014). Interactions of chromatic and lens-induced defocus during visual control of eye growth in guinea pigs (Cavia porcellus). Vision Research, 94, 24–32. https://doi.org/10.1016/j.visres.2013.10.020
Jiang, Y., Zhang, Z., Wu, Z., Sun, S., Fu, Y., & Ke, B. (2021b). Change and recovery of choroid thickness after short-term application of 1% atropine gel and its influencing factors in 6-7-year-old children. Current Eye Research, 46(8), 1171–1177. https://doi.org/10.1080/02713683.2020.1863431
Jiang, Y., Zhu, Z., Tan, X., Kong, X., Zhong, H., Zhang, J., Xiong, R., Yuan, Y., Zeng, J., Morgan, I. G., & He, M. (2022). Effect of repeated low-level red-light therapy for myopia control in children: A multicenter randomized controlled trial. Ophthalmology, 129(5), 509–519. https://doi.org/10.1016/j.ophtha.2021.11.023
Jonas, J. B., Ang, M., Cho, P., Guggenheim, J. A., He, M. G., Jong, M., Logan, N. S., Liu, M., Morgan, I., Ohno-Matsui, K., Pärssinen, O., Resnikoff, S., Sankaridurg, P., Saw, S. M., Smith, E. L., 3rd, Tan, D. T. H., Walline, J. J., Wildsoet, C. F., Wu, P. C., Zhu, X., … Wolffsohn, J. S. (2021). IMI prevention of myopia and its progression. Investigative ophthalmology & visual science, 62(5), 6. https://doi.org/10.1167/iovs.62.5.6
Jones, J. H., Mutti, D. O., Jones-Jordan, L. A., & Walline, J. J. (2022). Effect of combining 0.01% atropine with soft multifocal contact lenses on myopia progression in children. Optometry and Vision Science, 99(5), 434–442. https://doi.org/10.1097/OPX.0000000000001884
Jones, L. A., Sinnott, L. T., Mutti, D. O., Mitchell, G. L., Moeschberger, M. L., & Zadnik, K. (2007). Parental history of myopia, sports and outdoor activities, and future myopia. Investigative Ophthalmology & Visual Science, 48(8), 3524–3532. https://doi.org/10.1167/iovs.06-1118
Jones-Jordan, L. A., Mitchell, G. L., Cotter, S. A., Kleinstein, R. N., Manny, R. E., Mutti, D. O., Twelker, J. D., Sims, J. R., Zadnik, K., & CLEERE Study Group. (2011). Visual activity before and after the onset of juvenile myopia. Investigative Ophthalmology & Visual Science, 52(3), 1841–1850. https://doi.org/10.1167/iovs.09-4997
Jones-Jordan, L. A., Sinnott, L. T., Chu, R. H., Cotter, S. A., Kleinstein, R. N., Manny, R. E., Mutti, D. O., Twelker, D. J., & Zadnik, K. (2021). Myopia progression as a function of sex, age, and ethnicity. Investigative Ophthalmology & Visual Science, 62(10), 36. https://doi.org/10.1167/iovs.62.10.36
Jones-Jordan, L. A., Sinnott, L. T., Cotter, S. A., Kleinstein, R. N., Manny, R. E., Mutti, D. O., Twelker, J. D., Zadnik, K., & CLEERE Study Group (2012). Time outdoors, visual activity, and myopia progression in juvenile-onset myopes. Investigative Ophthalmology & Visual Science, 53(11), 7169–7175. https://doi.org/10.1167%2Fiovs.11-8336
Jordan-Yu, J. M., Teo, K. Y. C., Chakravarthy, U., Gan, A., Tan, A. C. S., Cheong, K. X., Wong, T. Y., & Cheung, C. M. G. (2021). Polypoidal choroidal vasculopathy features vary according to subfoveal choroidal thickness. Retina, 41(5), 1084–1093. https://doi.org/10.1097/iae.0000000000002966
Khanal, S., Harrington, S., & Tomiyama, E. (2024). [Treatment of childhood myopia]. Commissioned Paper for the Committee on Focus on Myopia: Pathogenesis and Rising Incidence.
Khanal, S., Norton, T. T., & Gawne, T. J. (2023). Limited bandwidth short-wavelength light produces slowly-developing myopia in tree shrews similar to human juvenile-onset myopia. Vision Research, 204, 108161. https://doi.org/10.1016/j.visres.2022.108161
Kinoshita, N., Konno, Y., Hamada, N., Kanda, Y., Shimmura-Tomita, M., Kaburaki, T., & Kakehashi, A. (2020). Efficacy of combined orthokeratology and 0.01% atropine solution for slowing axial elongation in children with myopia: A 2-year randomised trial. Scientific Reports, 10(1), 12750. https://doi.org/10.1038/s41598-020-69710-8
Kleinstein, R. N., Sinnott, L. T., Jones-Jordan, L. A., Sims, J., & Zadnik, K. (2012). New cases of myopia in children. Archives of Ophthalmology, 130(10), 1274–1279. https://doi.org/10.1001/archophthalmol.2012.1449
Koosha, N., Riazi, M. S., Janfaza, P., Mohammadbeigy, I., Rahimi, A., Mehri, K., Mohsen, P., & Peyman, A. (2024). Laser vision correction after radial keratotomy: A systematic review and meta-analysis. Journal of Cataract & Refractive Surgery, 50(7), 767–776. https://doi.org/10.1097/j.jcrs.0000000000001426
Kröger, R. H. H., & Wagner, H. J. (1996). The eye of the blue acara (Aequidens pulcher, Cichlidae) grows to compensate for defocus due to chromatic aberration. Journal of Comparative Physiology A, 179(6), 837–842. https://doi.org/10.1007/bf00207362
Lam, C. S. Y., Tang, W. C., Tse, D. Y., Lee, R. P. K., Chun, R. K. M., Hasegawa, K., Qi, H., Hatanaka, T., & To, C. H. (2020). Defocus incorporated multiple segments (DIMS) spectacle lenses slow myopia progression: A 2-year randomized clinical trial. British Journal of Ophthalmology, 104(3), 363–368. https://doi.org/10.1136/bjophthalmol-2018-313739
Lawrenson, J. G., Shah, R., Huntjens, B., Downie, L. E., Virgili, G., Dhakal, R., Verkicharla, P. K., Li, D., Mavi, S., Kernohan, A., Li, T., Walline, J. J. (2023). Interventions for myopia control in children: A living systematic review and network meta-analysis. The Cochrane Database of Systematic Reviews, 2(2), CD014758. https://doi.org/10.1002/14651858.CD014758.pub2
Leech, E. M., Cottriall, C. L., & McBrien, N. A. (1995). Pirenzepine prevents form deprivation myopia in a dose-dependent manner. Ophthalmic & Physiological Optics, 15(5), 351–356. https://pubmed.ncbi.nlm.nih.gov/8524553/
Li, X., Huang, Y., Yin, Z., Liu, C., Zhang, S., Yang, A., Drobe, B., Chen, H., & Bao, J. (2023a). Myopia control efficacy of spectacle lenses with aspherical lenslets: Results of a 3-year follow-up study. American Journal of Ophthalmology, 253, 160–168. https://doi.org/10.1016/j.ajo.2023.03.030
Li, Yu, Qi, Y., Sun, M., Zhai, C., Wei, W., & Zhang, F. (2023b). Clinical feasibility and safety of scleral collagen cross-linking by riboflavin and ultraviolet A in pathological myopia blindness: A pilot study. Ophthalmology and Therapy, 12(2), 853–866. https://doi.org/10.1007/s40123-022-00633-5
Li, Y., Yip, M., Ning, Y., Chung, J., Toh, A., Leow, C., Liu, N., Ting, D., Schmetterer, L., Saw, S. M., Jonas, J. B., Chia, A., & Ang, M. (2024). Topical atropine for childhood myopia control: The atropine treatment long-term assessment study. JAMA Ophthalmology, 142(1), 15–23. https://doi.org/10.1001/jamaophthalmol.2023.5467
Li, T., Zhou, X., Li, B., & Jiang, B. (2017). Effect of MT3 on retinal and choroidal TGF-β2 and HAS2 expressions in form deprivation myopia of guinea pig. Journal of Ophthalmology. https://doi.org/10.1155/2017/5028019
Lim, M. C., Gazzard, G., Sim, E. L., Tong, L., & Saw, S. M. (2009). Direct costs of myopia in Singapore. Eye, 23(5), 1086–1089. https://doi.org/10.1038/eye.2008.225
Linne, C., Mon, K. Y., D’Souza, S., Jeong, H., Jiang, X., Brown, D. M., Zhang, K., Vemaraju, S., Tsubota, K., Kurihara, T., Pardue, M. T., & Lang, R. A. (2023). Encephalopsin (OPN3) is required for normal refractive development and the GO/GROW response to induced myopia. Molecular Vision, 29, 39–57. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10243678/
Liu, R., Hu, M., He, J. C., Zhou, X. T., Dai, J. H., Qu, X. M., Liu, H., & Chu, R. Y. (2014). The effects of monochromatic illumination on early eye development in rhesus monkeys. Investigative Ophthalmology & Visual Science, 55(3), 1901–1909. https://doi.org/10.1167/iovs.13-12276
Liu, S., Li, S., Wang, B., Lin, X., Wu, Y., Liu, H., Qu, X., Dai, J., Zhou, X., & Zhou, H. (2016). Scleral cross-linking using riboflavin UVA irradiation for the prevention of myopia progression in a guinea pig model: Blocked axial extension and altered scleral microstructure. PloS One, 11(11), e0165792. https://doi.org/10.1371/journal.pone.0165792
Liu, A. L., Liu, Y. F., Wang, G., Shao, Y. Q., Yu, C. X., Yang, Z., Zhou, Z. R., Han, X., Gong, X., Qian, K. W., Wang, L. Q., Ma, Y. Y., Zhong, Y. M., Weng, S. J., & Yang, X. L. (2022). The role of ipRGCs in ocular growth and myopia development. Science Advances, 8(19), eabm9027. https://doi.org/10.1126/sciadv.abm9027
Liu, R., Qian, Y. F., He, J. C., Hu, M., Zhou, X. T., Dai, J. H., Qu, X. M., & Chu, R. Y. (2011). Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Experimental Eye Research, 92(6), 447–453. https://doi.org/10.1016/j.exer.2011.03.003
Liu, Z., Sun, Z., Du, B., Gou, H., Wang, B., Lin, Z., Ren, N., Pazo, E. E., Liu, L., & Wei, R. (2024). The effects of repeated low-level red-light therapy on the structure and vasculature of the choroid and retina in children with premeyopia. Ophthalmology and Therapy, 13, 729–759. https://doi.org/10.1007/s40123-023-00875-x
Liu, X., Wang, P., Xie, Z., Sun, M., Chen, M., Wang, J., Huang, J., Chen, S., Chen, Z., Wang, Y., Li, Y., Qu, J., & Mao, X. (2023). One-year myopia control efficacy of cylindrical annular refractive element spectacle lenses. Acta Ophthalmologica, 101(6), 651–657. https://doi.org/10.1111/aos.15649
Long, Q., Chen, D. H., & Chu, R. Y. (2009). Illumination with monochromatic long-wavelength light promotes myopic shift and ocular elongation in newborn pigmented guinea pigs. Cutaneous and Ocular Toxicology, 28(4), 176–180. https://doi.org/10.3109/15569520903178364
Luedde, W. H. (1932). Monocular cycloplegia for the control of myopia. American Journal of Ophthalmology, 15, 603–610. https://doi.org/10.1016/S0002-9394(32)90282-7
Lumb, E., Sulley, A., Logan, N. S., Jones, D., & Chamberlain, P. (2023). Six years of wearer experience in children participating in a myopia control study of MiSight® 1 day. Contact lens & anterior eye, 46(4), 101849. https://doi.org/10.1016/j.clae.2023.101849
Mathis, U., Feldkaemper, M., Liu, H., & Schaeffel, F. (2023). Studies on the interactions of retinal dopamine with choroidal thickness in the chicken. Graefes Archive for Clinical and Experimental Ophthalmology, 261(2), 409–425. https://doi.org/10.1007/s00417-022-05837-w
Mathis, U., Feldkaemper, M. P., & Schaeffel, F. (2021). Effects of single and repeated intravitreal applications of atropine on choroidal thickness in alert chickens. Ophthalmic Research, 64(4), 664–674. https://doi.org/10.1159/000515755
Mazade, R., & Pardue, M. T. (2023). Rod pathway electrical activity is modulated in the myopic mouse. Investigative Ophthalmology & Visual Science, 65(8). https://iovs.arvojournals.org/article.aspx?articleid=2786113
Mazade, R., & Pardue, M. T. (2024). Inhibition to the rod pathway is modulated in lens-induced myopic mice. Investigative Ophthalmology & Visual Science, 65(7), https://iovs.arvojournals.org/article.aspx?articleid=2796960&resultClick=1
Mazade, R., Palumaa, T., & Pardue, M. T. (2024). Insights Into Myopia from Mouse Models. Annual review of vision science, 10.1146/annurev-vision-102122-102059. Advance online publication. https://doi.org/10.1146/annurev-vision-102122-102059
McBrien, N. A., Morgan, I. G., & Mutti, D. O. (2009). What’s hot in myopia research-the 12th international myopia conference, Australia, 2008. Optometry and Vision Science, 86(1), 2–3. https://doi.org/10.1097/opx.0b013e3181940364
McBrien, N. A., Stell, W. K., & Carr, B. (2013). How does atropine exert its anti-myopia effects? Ophthalmic & Physiological Optics, 33(3), 373–378. https://doi.org/10.1111/opo.12052
McKean-Cowdin, R., Varma, R., Cotter, S. A., Tarczy-Hornoch, K., Borchert, M. S., Lin, J. H., Wen, G., Azen, S. P., Torres, M., Tielsch, J. M., Friedman, D. S., Repka, M. X., Katz, J., Ibironke, J., Giordano, L., & Multi-Ethnic Pediatric Eye Disease Study and the Baltimore Pediatric Eye Disease Study Groups. (2011). Risk factors for astigmatism in preschool children: The multi-ethnic pediatric eye disease and Baltimore pediatric eye disease studies. Ophthalmology, 118(10), 1974–1981. https://doi.org/10.1016/j.ophtha.2011.06.031
Meng, Q. Y., Miao, Z. Q., Liang, S. T., Wu, X., Wang, L. J., Zhao, M. W., & Guo, L. L. (2023). Choroidal thickness, myopia, and myopia control interventions in children: a Meta-analysis and systematic review. International Journal of Ophthalmology, 16(3), 453–464. https://doi.org/10.18240/ijo.2023.03.17
Modjtahedi, B. S., Abbott, R. L., Fong, D. S., Lum, F., Tan, D., & Task Force on Myopia. (2021). Reducing the global burden of myopia by delaying the onset of myopia and reducing myopic progression in children: The Academy’s Task Force on Myopia. Ophthalmology, 128(6), 816–826. https://doi.org/10.1016/j.ophtha.2020.10.040
Mohammadi, S. F., Alinia, C., Tavakkoli, M., Lashay, A., & Chams, H. (2018). Refractive surgery: The most cost-saving technique in refractive errors correction. International Journal of Ophthalmology, 11(6), 1013–1019. https://doi.org/10.18240/ijo.2018.06.20
Moreddu, R., Vigolo, D., & Yetisen, A. K. (2019). Contact Lens Technology: From fundamentals to applications. Advanced Healthcare Materials, 8(15). https://doi.org/10.1002/adhm.201900368
Multi-Ethnic Pediatric Eye Disease Study Group. (2010). Prevalence of myopia and hyperopia in 6- to 72-month-old African American and Hispanic children: the multi-ethnic pediatric eye disease study. Ophthalmology, 117(1), 140–147. https://doi.org/10.1016/j.ophtha.2009.06.009
Mutti, D. O., & Marks, A. R. (2011). Blood levels of vitamin D in teens and young adults with myopia. Optometry and Vision Science, 88(3), 377–382. https://doi.org/10.1097%2FOPX.0b013e31820b0385
Mutti, D. O., Mulvihill, S. P., Orr, D. J., Shorter, P. D., & Hartwick, A. T. E. (2020). The effect of refractive error on melanopsin-driven pupillary responses. Investigative Ophthalmology & Visual Science, 61(8), 22. https://doi.org/10.1167%2Fiovs.61.12.22
Mutti, D. O., Sinnott, L. T., Lynn Mitchell, G., Jordan, L. A., Friedman, N. E., Frane, S. L., & Lin, W. K. (2018). Ocular component development during infancy and early childhood. Optometry and Vision Science, 95(10), 976–985. https://doi.org/10.1097%2FOPX.0000000000001296
Mutti, D. O., Sinnott, L. T., Reuter, K. S., Walker, M. K., Berntsen, D. A., Jones-Jordan, L. A., Walline, J. J., & Bifocal Lenses In Nearsighted Kids (BLINK) Study Group. (2019). Peripheral refraction and eye lengths in myopic children in the Bifocal Lenses in Nearsighted Kids (BLINK) study. Translational Vision Science & Technology, 8(5), 17. https://doi.org/10.1167%2Ftvst.8.2.17
MyKidsVision. (n.d.). How do myopia control soft contact lenses work? https://www.mykidsvision.org/knowledge-centre/how-do-myopia-control-soft-contact-lenses-work
Myopia Profile. (n.d.). Biofinity® multifocal. https://www.myopiaprofile.com/product/biofinity
National Library of Medicine. (2005). The Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study. https://clinicaltrials.gov/study/NCT00000169#study-overview
National Library of Medicine. (2016). Correction of Myopia Evaluation Trial (COMET). https://clinicaltrials.gov/study/NCT00000113?term=COMET%20myopia&rank=1
National Research Council (1989). Myopia: Prevalence and Progression. Washington, DC: The National Academies Press. https://doi.org/10.17226/1420.
Németh, J., Tapasztó, B., Aclimandos, W. A., Kestelyn, P., Jonas, J. B., De Faber, J. H. N., Januleviciene, I., Grzybowski, A., Nagy, Z. Z., Pärssinen, O., Guggenheim, J. A., Allen, P. M., Baraas, R. C., Saunders, K. J., Flitcroft, D. I., Gray, L. S., Polling, J. R., Haarman, A. E., Tideman, J. W. L., Wolffsohn, J. S., … Resnikoff, S. (2021). Update and guidance on management of myopia. European Society of Ophthalmology in cooperation with International Myopia Institute. European Journal of Ophthalmology, 31(3), 853–883. https://doi.org/10.1177/1120672121998960
Nickla, D. L., & Totonelly, K. (2011). Dopamine antagonists and brief vision distinguish lens-induced- and form-deprivation-induced myopia. Experimental Eye Research, 93(6), 782–785. https://doi.org/10.1016/j.exer.2011.08.001
Norton, T. (1999) Animal models of myopia: learning how vision controls the size of the eye. ILAR Journal, 40(2), 59–77. https://doi.org/10.1093/ilar.40.2.59
Norton, T. T., Casagrande, V. A., & Sherman, S. M. (1977). Loss of Y-cells in the lateral geniculate nucleus of monocularly deprived tree shrews. Science, 197(4305), 784–786. https://doi.org/10.1126/science.887922
Norton, T. T., & Siegwart, J. T., Jr. (2013). Light levels, refractive development, and myopia–A speculative review. Experimental Eye Research, 114, 48–57. https://doi.org/10.1016/j.exer.2013.05.004
Ortiz-Peregrina, S., Casares-López, M., Castro-Torres, J. J., Anera, R. G., & Artal, P. (2022). Effect of peripheral refractive errors on driving performance. Biomedical Optics Express, 13(10), 5533–5550. https://doi.org/10.1364/BOE.468032
Ostrin, L., & Schill, A. (2014) Red light instruments for myopia exceed safety limits. Ophthalmic and Physiological Optics, 44(2), 241–248. https://doi.org/10.1111/opo.13272
Papadogiannis, P., Börjeson, C., & Lundström, L. (2023). Comparison of optical myopia control interventions: effect on peripheral image quality and vision. Biomedical Optics Express, 14(7), 3125–3137. https://doi.org/10.1364/BOE.486555
Peng, T., & Jiang, J. (2023). Efficiency and related factors of multifocal soft contact lenses in controlling myopia. Eye & Contact Lens, 49(12), 535–541. https://doi.org/10.1097/ICL.0000000000001043
Phillips, J. R., & McBrien, N. A. (2004). Pressure-induced changes in axial eye length of chick and tree shrew: significance of myofibroblasts in the sclera. Investigative Ophthalmology & Visual Science, 45(3), 758–763. https://doi.org/10.1167/iovs.03-0732
Poudel, S., Jin, J., Rahimi-Nasrabadi, H., Dellostritto, S., Dul, M. W., Viswanathan, S., & Alonso, J. M. (2024). Contrast sensitivity of ON and OFF human retinal pathways in myopia. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 44(3), e1487232023. https://doi.org/10.1523/JNEUROSCI.1487-23.2023
Pucker, A. D. (2023) Understanding options for combination myopia management. Ophthalmology Times, 48(5). https://www.ophthalmologytimes.com/view/understanding-options-for-combination-myopia-management
Qian, L., Zhao, H., Li, X., Yin, J., Tang, W., Chen, P., Wang, Q., & Zhang, J. (2015). Pirenzepine inhibits myopia in guinea pig model by regulating the balance of MMP-2 and TIMP-2 expression and increased tyrosine hydroxylase levels. Cell Biochemistry and Biophysics, 71(3), 1373–1378. https://doi.org/10.1007/s12013-014-0359-9
Qiao-Grider, Y., Hung, L. F., Kee, C.-s., Ramamirtham, R., & Smith, E. L., 3rd. (2004). Recovery from form-deprivation myopia in rhesus monkeys. Investigative Ophthalmology & Visual Science, 45(10), 3361–3372. https://doi.org/10.1167/iovs.04-0080
Quek, T. P. L., Chua, C. G., Chong, C. S., Chong, J. H., Hey, H. W., Lee, J., Lim, Y. F., & Saw, S. (2003). Prevalence of refractive errors in teenage high school students in Singapore. Ophthalmic and Physiological Optics/Ophthalmic & Physiological Optics, 24(1), 47–55. https://doi.org/10.1046/j.1475-1313.2003.00166.
Rappon, J., Neitz, J., Neitz, M., Chung, C., & Chalberg, T. W. (2022). Two-year effectiveness of a novel myopia management spectacle lens with full-time wearers. Investigative Ophthalmology & Visual Science, 63(7), 408. https://iovs.arvojournals.org/article.aspx?articleid=2779016
Read, S. A., Vincent, S. J., Tan, C., Ngo, C., Collins, M. J., & Saw, S. (2018). Patterns of daily outdoor light exposure in Australian and Singaporean children. Translational Vision Science & Technology, 7(3), 8. https://doi.org/10.1167/tvst.7.3.8
Reidy, M. G., Hartwick, A. T. E., & Mutti, D. O. (2024). The association between pupillary responses and axial length in children differs as a function of season. Scientific Reports, 14(1), 598. https://doi.org/10.1038/s41598-024-51199-0
Repka, M. X., Weise, K. K., Chandler, D. L., Wu, R., Melia, B. M., Manny, R. E., Kehler, L. A. F., Jordan, C. O., Raghuram, A., Summers, A. I., Lee, K. A., Petersen, D. B., Erzurum, S. A., Pang, Y., Lenhart, P. D., Ticho, B. H., Beck, R. W., Kraker, R. T., Holmes, J. M., Cotter, S. A., … Pediatric Eye Disease Investigator Group. (2023). Low-dose 0.01% atropine eye drops vs placebo for myopia control: A randomized clinical Trial. JAMA Ophthalmology, 141(8), 756–765. https://doi.org/10.1001/jamaophthalmol.2023.2855
Rickers, M., & Schaeffel, F. (1995). Dose-dependent effects of intravitreal pirenzepine on deprivation myopia and lens-induced refractive errors in chickens. Experimental Eye Research, 61(4), 509–516. https://doi.org/10.1016/s0014-4835(05)80147-2
Rohrer, B., Schaeffel, F., & Zrenner, E. (1992). Longitudinal chromatic aberration and emmetropization: Results from the chicken eye. Journal of Physiology, 449(1), 363–376. https://doi.org/10.1113/jphysiol.1992.sp019090
Rose, K. A., Morgan, I. G., Ip, J., Kifley, A., Huynh, S., Smith, W., & Mitchell, P. (2008). Outdoor activity reduces the prevalence of myopia in children. Ophthalmology, 115(8), 1279–1285. https://doi.org/10.1016/j.ophtha.2007.12.019
Rucker, F. J., & Wallman, J. (2009). Chick eyes compensate for chromatic simulations of hyperopic and myopic defocus: Evidence that the eye uses longitudinal chromatic aberration to guide eye-growth. Vision Research, 49, 1775–1783. https://doi.org/10.1016/j.visres.2009.04.014
Sah, R. P., Jaskulski, M., & Kollbaum, P. S. (2022). Modelling the refractive and imaging impact of multi-zone lenses utilised for myopia control in children’s eyes. Ophthalmic & Physiological Optics, 42(3), 571–585. https://doi.org/10.1111/opo.12959
Salzano, A. D., Khanal, S., Cheung, N. L., Weise, K. K., Jenewein, E. C., Horn, D. M., Mutti, D. O., & Gawne, T. J. (2023). Repeated low-level red-light therapy: The next wave in myopia management? Optometry and Vision Science, 100(12), 812–822. https://doi.org/10.1097/OPX.0000000000002083
Sankaridurg, P., Chen, X., Naduvilath, T., Lazon de la Jara, P., Lin, Z., Li, L., Smith, E. L., 3rd, Ge, J., & Holden, B. A. (2013). Adverse events during 2 years of daily wear of silicone hydrogels in children. Optometry and Vision Science, 90(9), 961–969. https://doi.org/10.1097/OPX.0000000000000017
Sankaridurg, P., Berntsen, D., Bullimore, M., Cho, P., Flitcroft I., Gawne, T.J., Gifford, K. L., Jong, M., Kang, P., Ostrin, L.A., Santodomingo-Rubido, J., Wildsoet, J., Wolffsohn, J.S. (2023) IMI 2023 Digest. Investigative Ophthalmology & Visual Science, 64(6), 7. https://doi.org/10.1167/iovs.64.6.7
Saxena, R., Vashist, P., Tandon, R., Pandey, R. M., Bhardawaj, A., Gupta, V., & Menon, V. (2017). Incidence and progression of myopia and associated factors in urban school children in Delhi: The North India Myopia Study (NIM Study). PloS One, 12(12), e0189774. https://doi.org/10.1371/journal.pone.0189774
Saxena, R., Sharma, P., & Pediatric Ophthalmology Expert Group. (2020). National consensus statement regarding pediatric eye examination, refraction, and amblyopia management. Indian journal of ophthalmology, 68(2), 325–332. https://doi.org/10.4103/ijo.IJO_471_19
Schaeffel, F., & Howland, H. C. (1991). Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Research, 31(4), 717–734. https://doi.org/10.1016/0042-6989(91)90011-s
Schaeffel, F., & Wildsoet, C. F. (2024). Red light therapy for myopia: Merits, risks and questions. Ophthalmic & Physiological Optics, 10.1111/opo.13306. Advance online publication.
Scheiman, M., Gwiazda, J., Zhang, Q., Deng, L., Fern, K., Manny, R. E., Weissberg, E., & Hyman, L. (2016) Longitudinal changes in corneal curvature and its relationship to axial length in the Correction of Myopia Evaluation Trial (COMET) cohort, Journal of Optometry, 9(1), 13–21., https://doi.org/10.1016/j.optom.2015.10.003
Schwahn, H. N., Kaymak, H., & Schaeffel, F. (2000). Effects of atropine on refractive development, dopamine release, and slow retinal potentials in the chick. Visual Neuroscience, 17(2), 165–176. https://doi.org/10.1017/s0952523800171184
Seidemann, A., & Schaeffel, F. (2002). Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Research, 42(20), 2409–2417. https://doi.org/10.1016/s0042-6989(02)00262-6
Shah, R. L., Huang, Y., Guggenheim, J. A., & Williams, C. (2017). Time outdoors at specific ages during early childhood and the risk of incident myopia. Investigative Ophthalmology & Visual Science, 58(3), 1158–1166. https://doi.org/10.1167%2Fiovs.16-20894
She, Z., Ward, A. H., & Gawne, T. J. (2023). The effects of ambient narrowband long-wavelength light on lens-induced myopia and form-deprivation myopia in tree shrews. Experimental eye research, 234, 109593. https://doi.org/10.1016/j.exer.2023.109593.
Shih, Y. F., Chen, C. H., Chou, A. C., Ho, T. C., Lin, L. L., & Hung, P. T. (1999). Effects of different concentrations of atropine on controlling myopia in myopic children. Journal of Ocular Pharmacology and Therapeutics, 15(1), 85–90. https://doi.org/10.1089/jop.1999.15.85
Siatkowski, R. M., Cotter, S. A., Crockett, R. S., Miller, J. M., Novack, G. D., Zadnik, K., & U.S. Pirenzepine Study Group. (2008). Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Journal of AAPOS, 12(4), 332–339. https://doi.org/10.1016/j.jaapos.2007.10.014
Silva, R. A., & Blumenkranz, M. S. (2013). Prophylaxis for retinal detachment. American Academy of Ophthalmology, The Ophthalmic News and Education Network. https://www.aao.org/education/current-insight/prophylaxis-retinal-detachments
Smith, E. L., 3rd, Arumugam, B., Hung, L. F., She, Z., Beach, K., & Sankaridurg, P. (2020). Eccentricity-dependent effects of simultaneous competing defocus on emmetropization in infant rhesus monkeys. Vision Research, 177, 32–40. https://doi.org/10.1016/j.visres.2020.08.003
Smith, E. L., 3rd, Huang, J., Hung, L. F., Blasdel, T. L., Humbird, T. L., & Bockhorst, K. H. (2009a). Hemiretinal form deprivation: Evidence for local control of eye growth and refractive development in infant monkeys. Investigative Ophthalmology & Visual Science, 50(11), 5057–5069. https://doi.org/10.1167/iovs.08-3232
Smith, E. L., 3rd, Hung, L. F., & Huang, J. (2009b). Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Research, 49(19), 2386–2392. https://doi.org/10.1016/j.visres.2009.07.011
Smith, E. L., 3rd, Hung, L. F., Arumugam, B., Holden, B. A., Neitz, M., & Neitz, J. (2015). Effects of long-wavelength lighting on refractive development in infant rhesus monkeys. Investigative Ophthalmology & Visual Science, 56(11), 6490–6500. https://doi.org/10.1167%2Fiovs.15-17025
Smith, E. L., 3rd, Hung, L. F., Huang, J., & Arumugam, B. (2013). Effects of local myopic defocus on refractive development in monkeys. Optometry and Vision Science, 90(11), 1176–1186. https://doi.org/10.1097/OPX.0000000000000038
Smith, E. L., 3rd, Hung, L. F., Huang, J., Blasdel, T. L., Humbird, T. L., & Bockhorst, K. H. (2010). Effects of optical defocus on refractive development in monkeys: Evidence for local, regionally selective mechanisms. Investigative Ophthalmology & Visual Science, 51(8), 3864–3873. https://doi.org/10.1167/iovs.09-4969
Smith, E. L., 3rd, Hung, L. F., Kee, C. S., & Qiao, Y. (2002). Effects of brief periods of unrestricted vision on the development of form-deprivation myopia in monkeys. Investigative Ophthalmology & Visual Science, 43(2), 291–299. https://pubmed.ncbi.nlm.nih.gov/11818369/
Smith, E. L., 3rd, Hung, L. F., She, Z., Beach, K., Ostrin, L. A., & Jong, M. (2021). Topically instilled caffeine selectively alters emmetropizing responses in infant rhesus monkeys. Experimental Eye Research, 203, 108438. https://doi.org/10.1016/j.exer.2021.108438
Smith, E. L., 3rd, Ramamirtham, R., Qiao-Grider, Y., Hung, L. F., Huang, J., Kee, C. S., Coats, D., & Paysse, E. (2007). Effects of foveal ablation on emmetropization and form-deprivation myopia. Investigative Ophthalmology & Visual Science, 48(9), 3914–3922. https://doi.org/10.1167/iovs.06-1264
Smith, T. S., Frick, K. D., Holden, B. A., Fricke, T. R., & Naidoo, K. S. (2009c). Potential lost productivity resulting from the global burden of uncorrected refractive error. Bulletin of the World Health Organization, 87(6), 431–437. https://doi.org/10.2471/blt.08.055673
Sodhi, P., & Hartwick, A. T. (2014). Adenosine modulates light responses of rat retinal ganglion cell photoreceptors through a cAMP-mediated pathway. Journal of Physiology, 592(18), 4201–4220. https://doi.org/10.1113%2Fjphysiol.2014.276220
Sorensen, L., Gyrd-Hansen, D., Kristiansen, I. S., Nexøe, J., & Nielsen, J. B. (2008). Laypersons’ understanding of relative risk reductions: Randomised cross-sectional study. BMC Medical Informatics and Decision Making, 8(1). https://doi.org/10.1186/1472-6947-8-31
Srinivasan, B., Leung, H.Y., Cao, H., Liu, S., Chen, L., & Fan, A.H. (2016). Modern phacoemulsification and intraocular lens implantation (refractive lens exchange) is safe and effective in treating high myopia. Asia-Pacific Journal of Ophthalmology, 5(6), 438–444. https://doi.org/10.1097/APO.0000000000000241
Stone, R. A., Lin, T., & Laties, A. M. (1991). Muscarinic antagonist effects on experimental chick myopia. Experimental Eye Research, 52(6), 755–758. https://doi.org/10.1016/0014-4835(91)90027-c.
Stone, R. A., Lin, T., Laties, A. M., & Iuvone, P. M. (1989). Retinal dopamine and form-deprivation myopia. Proceedings of the National Academy of Sciences, 86(18), 704–706. https://doi.org/10.1073/pnas.86.2.704
Sugar, A., Rapuano, C. J., Culbertson, W. W., Huang, D., Varley, G. A., Agapitos, P. J., de Luise, V. P., & Koch, D. D. (2002). Laser in situ keratomileusis for myopia and astigmatism: safety and efficacy: A report by the American Academy of Ophthalmology. Ophthalmology, 109(1), 175–187. https://doi.org/10.1016/s0161-6420(01)00966-6
Swiatczak, B., & Schaeffel, F. (2022). Myopia: why the retina stops inhibiting eye growth. Scientific Reports, 12(1), 21704. https://doi.org/10.1038/s41598-022-26323-7
Tang, J., Liao, Y., Yan, N., Dereje, S., Wang, J., Luo, Y., Wang, Y., Zhou, W., Wang, X., & Wang, W. (2023). Efficacy of repeated low-level red-light therapy for slowing the progression of childhood myopia: A systematic review and meta-analysis. American Journal of Ophthalmology, 252. https://doi.org/10.1016/j.ajo.2023.03.036
Tang, N., Zhao, X., Chen, J., Liu, B., & Lu, L. (2021). Changes in the choroidal thickness after macular buckling in highly myopic eyes. Retina, 41(9), 1858. https://doi.org/10.1097/IAE.0000000000003125
Tapasztó, B., Flitcroft, D. I., Aclimandos, W. A., Jonas, J. B., De Faber, J. H. N., Nagy, Z. Z., Kestelyn, P. G., Januleviciene, I., Grzybowski, A., Vidinova, C. N., Guggenheim, J. A., Polling, J. R., Wolffsohn, J. S., Tideman, J. W. L., Allen, P. M., Baraas, R. C., Saunders, K. J., McCullough, S. J., Gray, L. S., Wahl, S., … SOE Myopia Consensus Group. (2023). Myopia management algorithm. Annexe to the article titled Update and guidance on management of myopia. European Society of Ophthalmology in cooperation with International Myopia Institute. European Journal of Ophthalmology. https://doi.org/10.1177/11206721231219532
Thomson, K., Kelly, T., Karouta, C., Morgan, I., & Ashby, R. (2021). Insights into the mechanism by which atropine inhibits myopia: Evidence against cholinergic hyperactivity and modulation of dopamine release. British Journal of Pharmacology, 178(22), 4501–4517. https://doi.org/10.1111/bph.15629
Tian, L., Cao, K., Ma, D. L., Zhao, S. Q., Lu, L. X., Li, A., Chen, C. X., Ma, C. R., Ma, Z. F., & Jie, Y. (2022). Investigation of the efficacy and safety of 650 nm low-level red light for myopia control in children: A randomized controlled trial. Ophthalmology and Therapy, 11(6), 2259–2270. https://doi.org/10.1007/s40123-022-00585-w
Tideman, J. W., Polling, J. R., Voortman, T., Jaddoe, V. W., Uitterlinden, A. G., Hofman, A., Vingerling, J. R., Franco, O. H., & Klaver, C. C. (2016). Low serum vitamin D is associated with axial length and risk of myopia in young children. European Journal of Epidemiology, 31(5), 491–499. https://doi.org/10.1007/s10654-016-0128-8
Tong, L., Huang, X. L., Koh, A. L., Zhang, X., Tan, D. T., & Chua, W. H. (2009). Atropine for the treatment of childhood myopia: Effect on myopia progression after cessation of atropine. Ophthalmology, 116(3), 572–579. https://doi.org/10.1016/j.ophtha.2008.10.020
Torii, H., Kurihara, T., Seko, Y., Negishi, K., Ohnuma, K., Inaba, T., Kawashima, M., Jiang, X., Kondo, S., Miyauchi, M., Miwa, Y., Katada, Y., Mori, K., Kato, K., Tsubota, K., Goto, H., Oda, M., Hatori, M., & Tsubota, K. (2017). Violet Light Exposure Can Be a Preventive Strategy Against Myopia Progression. EBioMedicine, 15, 210–219. https://doi.org/10.1016/j.ebiom.2016.12.007
Torii, H., Mori, K., Okano, T., Kondo, S., Yang, H. Y., Yotsukura, E., Hanyuda, A., Ogawa, M., Negishi, K., Kurihara, T., & Tsubota, K. (2022). Short-term exposure to violet light emitted from eyeglass frames in myopic children: A randomized pilot clinical trial. Journal of Clinical Medicine, 11(20), 6000. https://doi.org/10.3390/jcm11206000
Trier, K., Cui, D., Ribel-Madsen, S., & Guggenheim, J. (2023). Oral administration of caffeine metabolite 7-methylxanthine is associated with slowed myopia progression in Danish children. British Journal of Ophthalmology, 107(10), 1538–1544. https://doi.org/10.1136/bjo-2021-320920
Troilo, D., Smith, E. L., 3rd, Nickla, D. L., Ashby, R., Tkatchenko, A. V., Ostrin, L. A., Gawne, T. J., Pardue, M. T., Summers, J. A., Kee, C. S., Schroedl, F., Wahl, S., & Jones, L. (2019). IMI - Report on experimental models of emmetropization and myopia. Investigative Ophthalmology & Visual Science, 60(3), M31–M88. https://doi.org/10.1167/iovs.18-25967
van der Sande, E., Polling, J. R., Tideman, J. W. L., Meester-Smoor, M. A., Thiadens, A. A. H. J., Tan, E., De Zeeuw, C. I., Hamelink, R., Willuhn, I., Verhoeven, V. J. M., Winkelman, B. H. J., & Klaver, C. C. W. (2023). Myopia control in Mendelian forms of myopia. Ophthalmic & Physiological Optics: The Journal of the British College of Ophthalmic Opticians (Optometrists), 43(3), 494–504. https://doi.org/10.1111/opo.13115
Verkicharla, P. K., Suheimat, M., Schmid, K. L., & Atchison, D. A. (2016). Peripheral refraction, peripheral eye length, and retinal shape in myopia. Optometry and Vision Science, 93(9), 1072–1078. https://doi.org/10.1097/opx.0000000000000905
Vutipongsatorn, K., Yokoi, T., & Ohno-Matsui, K. (2019). Current and emerging pharmaceutical interventions for myopia. British Journal of Ophthalmology, 103, 1539–1548. https://doi.org/10.1136/bjophthalmol-2018-313798
Walline, J. J., Gaume Giannoni, A., Sinnott, L. T., Chandler, M. A., Huang, J., Mutti, D. O., Jones-Jordan, L. A., Berntsen, D. A., & BLINK Study Group. (2017). A randomized trial of soft multifocal contact lenses for myopia control: Baseline data and methods. Optometry and Vision Science, 94(9), 856–866. https://doi.org/10.1097%2FOPX.0000000000001106
Walline, J. J., Greiner, K. L., McVey, M. E., & Jones-Jordan, L. A. (2013). Multifocal contact lens myopia control. Optometry and Vision Science, 90(11), 1207–1214. https://doi.org/10.1097/opx.0000000000000036
Walline, J. J., Jones, L. A., Rah, M. J., Manny, R. E., Berntsen, D. A., Chitkara, M., Gaume, A., Kim, A., & Quinn, N. (2007). Contact Lenses in Pediatrics (CLIP) study: Chair time and ocular health. Optometry and Vision Science, 84(9), 896–902. https://doi.org/10.1097/opx.0b013e3181559c3c
Walline, J. J., Jones, L. A., Sinnott, L., Manny, R. E., Gaume, A., Rah, M. J., Chitkara, M., & Lyons, S. (2008). A randomized trial of the effect of soft contact lenses on myopia progression in children. Investigative Ophthalmology & Visual Science, 49(11), 4702. https://doi.org/10.1167/iovs.08-2067
Walline, J. J., Lindsley, K., Vedula, S. S., Cotter, S. A., Mutti, D. O., & Twelker, J. D. (2011). Interventions to slow progression of myopia in children. The Cochrane Database of Systematic Reviews, (12), CD004916. https://doi.org/10.1002/14651858.cd004916.pub3
Walline, J. J., Lindsley, K. B., Vedula, S. S., Cotter, S. A., Mutti, D. O., Ng, S. M., & Twelker, J. D. (2020a). Interventions to slow progression of myopia in children. The Cochrane Database of Systematic Reviews, 1(1), CD004916. https://doi.org/10.1002/14651858.cd004916.pub4
Walline, J. J., Walker, M. K., Mutti, D. O., Jones-Jordan, L. A., Sinnott, L. T., Giannoni, A. G., Bickle, K. M., Schulle, K. L., Nixon, A., Pierce, G. E., Berntsen, D. A., & BLINK Study Group (2020b). Effect of high add power, medium add power, or single-vision contact lenses on myopia progression in children: The BLINK randomized clinical trial. JAMA, 324(6), 571–580. https://doi.org/10.1001/jama.2020.10834
Wallman, J., Turkel, J., & Trachtman, J. (1978). Extreme myopia produced by modest change in early visual experience. Science, 201(4362), 1249–1251. https://doi.org/10.1126/science.694514
Wallman, J., Wildsoet, C., Xu, A., Gottlieb, M. D., Nickla, D. L., Marran, L., Krebs, W., & Christensen, A. M. (1995). Moving the retina: choroidal modulation of refractive state. Vision Research, 35(1), 37–50. https://doi.org/10.1016/0042-6989(94)e0049-q
Wang, W., Jiang, Y., Zhu, Z., Zhang, S., Xuan, M., Chen, Y., Xiong, R., Bulloch, G., Zeng, J., Morgan, I. G., & He, M. (2023). Clinically significant axial shortening in myopic children after repeated low-level red light therapy: A retrospective multicenter analysis. Ophthalmology and Therapy, 12(2), 999–1011. https://doi.org/10.1007/s40123-022-00644-2
Wang, Y., Li, L., Tang, X., Fan, H., Song, W., Xie, J., Tang, Y., Jiang, Y., & Zou, Y. (2024). The role of vasoactive intestinal peptide (VIP) in atropine-related inhibition of the progression of myopia. BMC Ophthalmology, 24(1), 41. https://doi.org/10.1186/s12886-024-03309-9
Wang, F., Zhou, J., Lu, Y., & Chu, R. (2011). Effects of 530 nm green light on refractive status, melatonin, MT1 receptor, and melanopsin in the guinea pig. Current Eye Research, 36(2), 103–111. https://doi.org/10.3109/02713683.2010.526750
Waring, G. O., 3rd, Lynn, M. J., & McDonnell, P. J. (1994). Results of the prospective evaluation of radial keratotomy (PERK) study 10 years after surgery. Archives of Ophthalmology, 112(10), 1298–1308. https://doi.org/10.1001/archopht.1994.01090220048022
Wen, D., Huang, J., Chen, H., Bao, F., Savini, G., Calossi, A., Chen, H., Li, X., & Wang, Q. (2015). Efficacy and acceptability of orthokeratology for slowing myopic progression in children: A systematic review and meta-analysis. Journal of Ophthalmology, 2015, 360806. https://doi.org/10.1155/2015/360806
Wen, G., Tarczy-Hornoch, K., McKean-Cowdin, R., Cotter, S. A., Borchert, M., Lin, J., Kim, J., Varma, R., & Multi-Ethnic Pediatric Eye Disease Study Group. (2013). Prevalence of myopia, hyperopia, and astigmatism in non-Hispanic white and Asian children: Multi-ethnic pediatric eye disease study. Ophthalmology, 120(10), 2109–2116. https://doi.org/10.1016/j.ophtha.2013.06.039
Wiesel, T. N., & Raviola, E. (1977). Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature, 266(5597), 66–68. https://doi.org/10.1038/266066a0
Wildsoet, C. F. (1997). Active emmetropization: Evidence for its existence and ramifications for clinical practice. Ophthalmic & Physiological Optics, 17, 279–290. https://pubmed.ncbi.nlm.nih.gov/9390372/
Wolf, A. T., Klawe, J., Liu, B., & Ahmad, S. (2023). Association between serum vitamin D levels and myopia in the National Health and Nutrition Examination Survey (2001-2006). Ophthalmic Epidemiology, 31(3), 229–239. https://doi.org/10.1080/09286586.2023.2232460
World Society of Pediatric Ophthalmology & Strabismus. (2023). Myopia consensus statement 2023. https://www.wspos.org/swdcore/uploads/WSPOS-Myopia-Consensus-Statement-2023-1.pdf
Wolffsohn, J. S., Whayeb, Y., Logan, N. S., & Weng, R. (2023). IMI—Global Trends in Myopia Management Attitudes and Strategies in Clinical Practice—2022 Update. Investigative Ophthalmology & Visual Science, 64(6), 6. https://doi.org/10.1167/iovs.64.6.6
Wu, J., Gong, H., Li, H., Liang, J., Zhang, X., Yang, H., Liu, X., Zhang, G., Cheng, G., Bai, G., & Zhang, H. (2023). Changes in choroidal thickness in myopic children with 0.01% atropine: Evidence from a 12-month follow-up. Photodiagnosis and Photodynamic Therapy, 42, 103528. https://doi.org/10.1016/j.pdpdt.2023.103528
Wu, P. C., Chang, L. C., Niu, Y. Z., Chen, M. L., Liao, L. L., & Chen, C. T. (2018a). Myopia prevention in Taiwan. Annals of Eye Science, 3, 12. https://aes.amegroups.org/article/view/4010/4715
Wu, P. C., Chen, C. T., Lin, K. K., Sun, C. C., Kuo, C. N., Huang, H. M., Poon, Y. C., Yang, M. L., Chen, C. Y., Huang, J. C., Wu, P. C., Yang, I. H., Yu, H. J., Fang, P. C., Tsai, C. L., Chiou, S. T., & Yang, Y. H. (2018b). Myopia Prevention and Outdoor Light Intensity in a School-Based Cluster Randomized Trial. Ophthalmology, 125(8), 1239–1250. https://doi.org/10.1016/j.ophtha.2017.12.011
Wu, P. C., Tsai, C. L., Wu, H. L., Yang, Y. H., & Kuo, H. K. (2013). Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology, 120(5), 1080–1085. https://doi.org/10.1016/j.ophtha.2012.11.009
Xiang, F., He, M., & Morgan, I. G. (2012). Annual changes in refractive errors and ocular components before and after the onset of myopia in Chinese children. Ophthalmology, 119(7), 1478–1484. https://doi.org/10.1016/j.ophtha.2012.01.017
Xiao, J., Pan, X., Hou, C., & Wang, Q. (2024). Changes in subfoveal choroidal thickness after orthokeratology in myopic children: A systematic review and meta-analysis. Current Eye Research. https://doi.org/10.1080/02713683.2024.2310618
Xiong, F., Mao, T., Liao, H., Hu, X., Shang, L., Yu, L., Lin, N., Huang, L., Yi, Y., Zhou, R., Zhou, X., & Yi, J. (2021). Orthokeratology and low-intensity laser therapy for slowing the progression of myopia in children. BioMed Research International. https://doi.org/10.1155/2021/8915867.
Xiong, S., Sankaridurg, P., Naduvilath, T., Zang, J., Zou, H., Zhu, J., Lv, M., He, X., & Xu, X. (2017). Time spent in outdoor activities in relation to myopia prevention and control: A meta-analysis and systematic review. Acta Ophthalmologica, 95(6), 551–566. https://doi.org/10.1111/aos.13403
Xiong, R., Zhu, Z., Jiang, Y., Wang, W., Zhang, J., Chen, Y., Bulloch, G., Yuan, Y., Zhang, S., Xuan, M., Zeng, J., Morgan, I. G., & He, M. (2022). Sustained and rebound effect of repeated low-level red-light therapy on myopia control: A 2-year post-trial follow-up study. Clinical & Experimental Ophthalmology, 50(9), 1013–1024. https://doi.org/10.1111/ceo.14149
Xiong, R., Zhuoting, Z., Jiang, Y., Wang, W., Zhang, J., Chen, Y., Bulloch, G., Yuan, Y., Zhang, S., Xuan, M., Zeng, J., & Morgan, I. G. (2023). Longitudinal changes and predictive value of choroidal thickness for myopia control after repeated low-level red-light therapy. Ophthalmology, 130(3), 286–296. https://doi.org/10.1016/j.ophtha.2022.10.002.
Yam, J. C., Jiang, Y., Lee, J., Li, S., Zhang, Y., Sun, W., Yuan, N., Wang, Y. M., Yip, B. H. K., Kam, K. W., Chan, H. N., Zhang, X. J., Young, A. L., Tham, C. C., Cheung, C. Y., Chu, W. K., Pang, C. P., & Chen, L. J. (2022a). The association of choroidal thickening by atropine with treatment effects for myopia: Two-year clinical trial of the Low-Concentration Atropine For Myopia Progression (LAMP) Study. American Journal of Ophthalmology, 237, 130–138. https://doi.org/10.1016/j.ajo.2021.12.014
Yam, J. C., Jiang, Y., Tang, S. M., Law, A. K. P., Chan, J. J., Wong, E., Ko, S. T., Young, A. L., Tham, C. C., Chen, L. J., & Pang, C. P. (2019). Low-Concentration Atropine for Myopia Progression (LAMP) study: A randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology, 126(1), 113–124. https://doi.org/10.1016/j.ophtha.2018.05.029
Yam, J. C., Li, F. F., Zhang, X., Tang, S. M., Yip, B. H. K., Kam, K. W., Ko, S. T., Young, A. L., Tham, C. C., Chen, L. J., & Pang, C. P. (2020). Two-year clinical trial of the Low-Concentration Atropine for Myopia Progression (LAMP) study: Phase 2 report. Ophthalmology, 127(7), 910–919. https://doi.org/10.1016/j.ophtha.2019.12.011
Yam, J. C., Zhang, X. J., Zhang, Y., Wang, Y. M., Tang, S. M., Li, F. F., Kam, K. W., Ko, S. T., Yip, B. H. K., Young, A. L., Tham, C. C., Chen, L. J., & Pang, C. P. (2022b). Three-year clinical trial of Low-Concentration Atropine for Myopia Progression (LAMP) study: Continued versus washout: Phase 3 report. Ophthalmology, 129(3), 308–321. https://doi.org/10.1016/j.ophtha.2021.10.002
Yasir, Z. H., Sharma, R., & Zakir, S. M. (2023). Scleral collagen cross linkage in progressive myopia. Indian Journal of Ophthalmology. https://doi.org/10.4103/IJO.IJO_1392_23.
Yazar, S., Hewitt, A. W., Black, L. J., McKnight, C. M., Mountain, J. A., Sherwin, J. C., Oddy, W. H., Coroneo, M. T., Lucas, R. M., & Mackey, D. A. (2014). Myopia is associated with lower vitamin D status in young adults. Investigative Ophthalmology & Visual Science, 55(7), 4552–4559. https://doi.org/10.1167/iovs.14-14589
Yen, M. Y., Liu, J. H., Kao, S. C., & Shiao, C. H. (1989). Comparison of the effect of atropine and cyclopentolate on myopia. Annals of Ophthalmology, 21(5), 180–187. https://pubmed.ncbi.nlm.nih.gov/2742290/
Yoshida, T., Takagi, Y., Igarashi-Yokoi, T., & Ohno-Matsui, K. (2023). Efficacy of lutein supplements on macular pigment optical density in highly myopic individuals: A randomized controlled trial. Medicine, 102(12), e33280. https://doi.org/10.1097/MD.0000000000033280
Zadnik, K., Schulman, E., Flitcroft, I., Fogt, J. S., Blumenfeld, L. C., Fong, T. M., Lang, E., Hemmati, H. D., Chandler, S. P., & CHAMP Trial Group Investigators. (2023). Efficacy and safety of 0.01% and 0.02% atropine for the treatment of pediatric myopia progression over 3 years: A randomized clinical trial. JAMA Ophthalmology, 141(10), 990–999. https://doi.org/10.1001/jamaophthalmol.2023.2097
Zadnik, K., Sinnott, L. T., Cotter, S. A., Jones-Jordan, L. A., Kleinstein, R. N., Manny, R. E., Twelker, J. D., Mutti, D. O., & Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study Group. (2015). Prediction of juvenile-onset myopia. JAMA Ophthalmology, 133(6), 683–689. https://doi.org/10.1001/jamaophthalmol.2015.0471
Zeitz, C., Roger, J. E., Audo, I., Michiels, C., Sánchez-Farías, N., Varin, J., Frederiksen, H., Wilmet, B., Callebert, J., Gimenez, M., Bouzidi, N., Blond, F., Guilllonneau, X., Fouquet, S., Léveillard, T., Smirnov, V. M., Vincent, A., Héon, E., Sahel, J., . . . Picaud, S. (2023). Shedding light on myopia by studying complete congenital stationary night blindness. Progress in Retinal and Eye Research, 93, 101155. https://doi.org/10.1016/j.preteyeres.2022.101155
Zhang, H. Y., Lam, C. S. Y., Tang, W. C., Leung, M., & To, C. H. (2020). Defocus incorporated multiple segments spectacle lenses changed the relative peripheral refraction: A 2-Year randomized clinical trial. Investigative Ophthalmology & Visual Science, 61(5), 53. https://doi.org/10.1167/iovs.61.5.53
Zhang, D. Q., Wong, K. Y., Sollars, P. J., Berson, D. M., Pickard, G. E., & McMahon, D. G. (2008). Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proceedings of the National Academy of Sciences, 105(37), 14181–14186. https://doi.org/10.1073/pnas.0803893105
Zhang, X. J., Zhang, Y., Kam, K. W., Tang, F., Li, Y., Ng, M. P. H., Young, A. L., Ip, P., Tham, C. C., Chen, L. J., Pang, C. P., & Yam, J. C. (2023). Prevalence of Myopia in Children Before, During, and After COVID-19 Restrictions in Hong Kong. JAMA network open, 6(3), e234080. https://doi.org/10.1001/jamanetworkopen.2023.4080
Zhang, Z., Zhou, Y., Xie, Z., Chen, T., Gu, Y., Lu, S., & Wu, Z. (2016). The effect of topical atropine on the choroidal thickness of healthy children. Scientific Reports, 6, 34936. https://doi.org/10.1038/srep34936
Zheng, Y. F., Pan, C. W., Chay, J., Wong, T. Y., Finkelstein, E., & Saw, S. M. (2013). The economic cost of myopia in adults aged over 40 years in Singapore. Investigative Ophthalmology & Visual Science, 54(12), 7532–7537. https://doi.org/10.1167/iovs.13-12795
Zhou, L., Tong, L., Li, Y., Williams, B. T., & Qiu, K. (2023). Photobiomodulation therapy retarded axial length growth in children with myopia: Evidence from a 12-month randomized controlled trial evidence. Scientific Reports, 13(1), 3321. https://doi.org/10.1038/s41598-023-30500-7
Zhou, X., Pardue, M. T., Iuvone, P. M., & Qu, J. (2017). Dopamine signaling and myopia development: What are the key challenges. Progress in Retinal and Eye Research, 61, 60–71. https://doi.org/10.1016/j.preteyeres.2017.06.003
Zloto, O., Wygananski-Jaffe, T., Farzavandi, S., Gomez-de-Liaño, Sprunger, D., Mezer, E. (2018). Current trends among pediatric ophthalmologists to decrease myopia progression—An international perspective. Graefe’s Archive for Clinical and Experimental Ophthalmology, 256(12), 2457–2466. https://doi.org/10.1007/s00417-018-4078-6
Zhu, Q., Goto, S., Singh, S., Torres, J. A., & Wildsoet, C. F. (2022). Daily or Less Frequent Topical 1% Atropine Slows Defocus-Induced Myopia Progression in Contact Lens-Wearing Guinea Pigs. Translational Vision Science & Technology, 11(3), 26. https://doi.org/10.1167/tvst.11.3.26
This page intentionally left blank.

































































