Myopia: Causes, Prevention, and Treatment of an Increasingly Common Disease (2024)
Chapter: 6 Myopia Pathogenesis: From Retinal Image to Scleral Growth
6
Myopia Pathogenesis: From Retinal Image to Scleral Growth
This chapter considers how the diverse tissues of the eye interact with the visual environment in ways that could regulate refractive eye growth. First, the committee reviews how animal models have been used to study both emmetropization1 and myopia. Then, the chapter covers how optical structures (cornea and crystalline lens) contribute to the retinal image; what evidence there is for retinal mechanisms of eye growth; the involvement of the retina, retinal pigment epithelium (RPE), choroid, and sclera; key signaling molecules in retino-scleral signaling (dopamine, retinoic acid, nitric oxide); and finally, the role of circadian rhythms.
KEY FINDINGS FROM ANIMAL MODELS OF EMMETROPIZATION AND MYOPIA
Animal models are instrumental in exploring the mechanisms of both healthy and diseased processes in the human body. The myopia field has used several different animal models to investigate environmental, cellular, and genetic factors that influence refractive eye growth. There is remarkable similarity in the response to experimental myopia across a diverse range of species, from fish to nonhuman primates, suggesting the presence of evolutionary conserved pathways for refractive eye growth. Regarding animal models that have been shown to respond to experimental myopia, Figure 6-1 shows that fish evolved the earliest, followed by birds, rodents, and nonhuman primates. This figure supports the theory that functional vision is important for survival and thus, it seems plausible that a fundamental pathway(s) could have evolved to carefully modulate the growth of the eye to match its optical power; thus, providing in-focus vision for survival. At the same time, it is feasible that differences have evolved across species. The myopia literature has many examples of species differences with respect to experimental myopia (see reviews in Bullimore, 2024; Chakraborty et al., 2020; Troilo et al., 2019). In this report, the committee focused on similarities among experimental myopia studies to find clues about the fundamentally conserved mechanisms that may underlie myopia across many species, including humans. At this time, there is not enough evidence to indicate whether some of the differences between species, like the response to monochromatic stimuli (see Table 5-4), reveal important modifications to myopia mechanisms or are due to artifacts or other experimental factors that have not yet been identified. From an evolutionary perspective, some animal models are closer to humans and may be more similar in mechanisms (see Figure 6-1). However, as with research on other physiological systems, different animal models are expected to provide essential insights into causal mechanisms of myopia at different levels (tissue, cells, genes, optics) that are needed to advance our understanding of emmetropia and myopia.
___________________
1 As a reminder, emmetropization is the natural (ideal) development in the young eye that responds to the visual environment by steadily reshaping the ocular globe so that axial length allows image focus to land squarely on the retina.
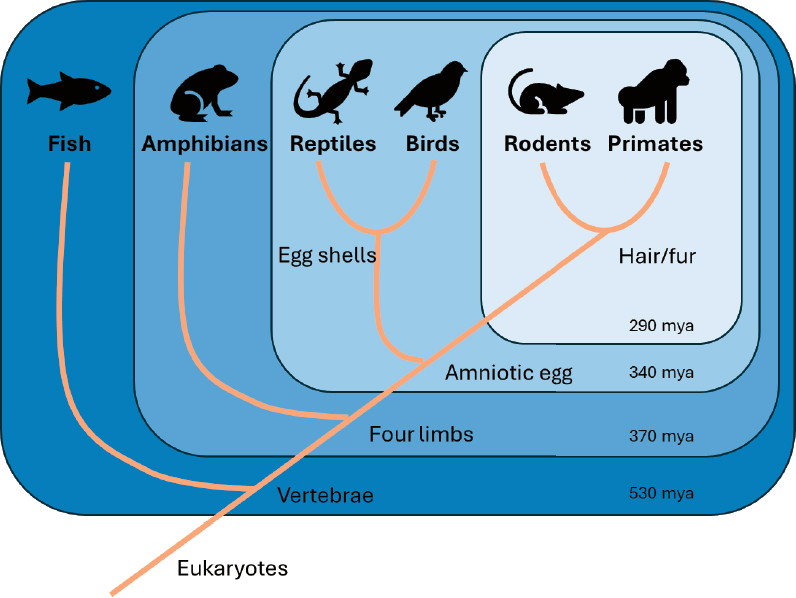
NOTE: MYA = million years ago.
SOURCE: Cornell, 2016.
Animal models have provided evidence that visually driven eye growth during development is an active process that can be disrupted (reviewed in Troilo et al., 2019; Wallman & Winawer, 2004). The first experiments to disrupt the normal growth of the eye to reach an emmetropic state used “form deprivation”—an experimental manipulation in which form vision is disrupted either by suturing the eyelid shut or by placing a diffuser lens in front of the eye—and showed that the eye became more myopic (reviewed in Gollender et al., 1979; Hodos & Kuenzel 1984; McKanna & Casagrande, 1978; Raviola & Wiesel, 1985; Sherman et al., 1977; Smith et al., 1980; Troilo et al., 2019; Wallman et al., 1978; Wiesel & Raviola, 1977; Yinon et al., 1980, 1983). Importantly, these studies in animals appear to model clinical conditions in which children with obstructed vision (due to congenital cataracts, corneal opacity, congenital optic neuropathy, etc.) or with low vision develop myopia (Bullimore, 2024; Rabin et al., 1981; Zadnik & Mutti, 1995), although myopia does not always develop in these cases (see Fledelius et al., 2014).
Additionally, removing the form-deprivation manipulation (referred to as “recovery”) would reverse the myopia as the eye recovered back to emmetropia (Wallman & Adams, 1987). Further studies revealed that this regulation of refractive eye growth could be fine-tuned, as demonstrated by lens-induced myopia (reviewed in Troilo et al., 2019). Remarkably, chicks could quickly compensate for plus or minus lenses to the exact diopter to place the focal point back on the retina (Irving et al., 1991; Schaeffel et al., 1988, 1990).
As noted above, the ability to undergo emmetropization and respond to form deprivation or lens-induced myopia is conserved across a large range of species, including fish (Shen et al., 2005), chickens (Gottlieb et al., 1987; Irving et al., 1991; Schaeffel et al., 1990), mice (Barathi et al., 2008; Schaeffel et al., 2004), guinea pigs (Howlett & McFadden 2006; Lu et al., 2006), tree
shrews (Siegwart & Norton, 1998), and nonhuman primates (Raviola & Wiesel, 1990; Smith et al., 1987; Troilo & Judge, 1993; von Noorden & Crawford, 1978). Considering the wide range of ocular features across these species—which differ in ocular anatomy, accommodative abilities, whether foveal or afoveal, degree of eye movement, binocularity, retinal circuitry, etc.—these results suggest a common fundamental pathway that exists across all species that controls refractive eye growth and can be disrupted by similar visual stimuli (see review by Troilo et al., 2019).
Additionally, these experiments have revealed a critical period for emmetropization and the response to form deprivation and lens defocus in mammals and chickens, which starts after eye opening and diminishes with age (see Bullimore, 2024). In contrast, fish, which grow continuously throughout life, are also responsive to experimental myopia beyond juvenile ages (Shen et al., 2005). These data suggest that myopia is most effectively induced in the actively growing eyes of juvenile animals. However, several studies have demonstrated that myopia can also be induced in adult chickens (Harman et al., 1999; Papastergiou et al., 1998; Saltarelli et al., 2004) and monkeys (Troilo et al., 2000b).
Researchers have employed animal models to investigate the origin of the refractive eye growth signals. Studies using partial occluders to deliver myopigenic stimuli to limited quadrants of the eye (Smith et al., 2009; Wallman et al., 1987), surgical methods to sever the optic nerve (Raviola & Wiesel, 1985; Troilo et al., 1987), or pharmacological inhibitors to block retinal ganglion cell activation of higher order visual circuits (Norton et al., 1994) have demonstrated that local retinal signals can control eye growth.
Animal models have also been instrumental in providing insights into the influence of ambient visual stimuli. Numerous animal models have shown that bright light is protective for experimental myopia, supporting the epidemiological findings in children (Muralidharan et al., 2021; Troilo et al., 2019). In addition, the use of animal models has revealed that dopamine levels are increased after bright light exposure (Cameron et al., 2009; French et al., 2013; Landis et al., 2021) and that blocking dopamine receptors blocks the beneficial effects of bright light in chicks (Ashby & Schaeffel, 2010), suggesting a potential mechanism of action. In the context of circadian rhythms, animal models have shown diurnal rhythms associated with refractive error, axial length and choroidal thickness (Campbell et al., 2012; Nickla et al., 1998; Nickla et al., 2017; Stone et al., 2024; Weiss & Schaeffel, 1993). In addition, deletion of melanopsin or clock genes have shown a modulatory effect on refractive development (Chakraborty et al., 2021; Stone et al., 2019). Spectral factors modulating refractive eye growth have also been extensively examined in animal models (reviewed in Gawne & Norton, 2020; Strickland et al., 2020; Troilo et al., 2019; Yoon et al., 2021).
The signaling pathways that modulate visually driven eye growth have remained elusive. The current understanding is that a signaling pathway is initiated with visual stimuli in the retina. Signaling molecule(s) then trigger other targets in the RPE and choroid or traverse these layers to ultimately induce scleral remodeling and ocular elongation. Each of these structures is covered below to consider how the structure and function of the retina, RPE, choroid, and sclera could align with a role in the signaling cascade for refractive eye growth. Additionally, identifying the components of the signaling pathway would provide potential pharmacological targets for novel myopia treatments. Many different signaling molecules have been implicated in the retina, including dopamine, nitric oxide, retinoic acid, and melanopsin (reviewed in Brown et al., 2022), which are discussed below.
OPTICAL MECHANISMS OF MYOPIA
Open and Closed-loop Control of Eye Growth
Broadly, an external stimulus can evoke an open or closed loop response from a biological system. The reflex of moving one’s hand away from a hot object is an open loop response whereby the input (temperature) directly drives the response or output of the system (moving hand away). In contrast, the ability of the body to regulate its body temperature continuously over time in the face of changing external environmental conditions or internal physiology works in a closed-loop manner. The body can maintain temperature homeostasis because it has this ability to continuously drive itself toward a stable equilibrium in a closed loop. To implement a closed loop, the system consists of sensors that continuously measure the variable(s) of interest, actuators that effect a change in the system’s response, and a comparator or control system that compares the output of the system against the input sensed variable(s) and finely tunes the actuator’s response based on the differential, in case the output is offset from a set point.
Myopia is a case where the closed-loop homeostasis in the length and shape of the eye goes astray. Following the closed-loop control system model, the sensed variable of interest is the refractive error, while the actuators correspond to the changes in eye anatomy—eye length, choroid thickness, scleral growth and remodeling, properties of the cornea and crystalline lens—which all serve to alter the effective refractive state of the eye. The comparator or control loop’s task is to deduce information about the eye’s refractive state based on the retinal image and visual processing and instruct the actuators accordingly, to minimize the resultant refractive error. For this system to function appropriately during normal emmetropization requires an intricate closed-loop machinery that can precisely measure and continually adjust the refractive state of the eye.
These mechanisms by which the closed-loop system measures and fine-tunes the refractive state remain incompletely understood, but they undoubtedly utilize key retinal image features such as defocus (i.e., location of the optimal image plane in front of or behind the retina), higher-order optical blur (shape, size, and orientation), and contrast (spatial, spectral, and temporal). On the other hand, luminance is an aspect of the retinal image physically unrelated to the refractive state of the eye which cannot provide a differential feedback signal; thus, it cannot actively contribute to closed-loop emmetropization. Rather, the effect of luminance on myopic eye growth operates through an open loop (see also Chapter 5, under Effects of Luminance). In comparison to the closed-loop system tuning the refractive state, much is known about the retinal mechanisms that encode luminance and the molecular pathways by which this signal offers a protective effect for myopia.
The Role of the Eye’s Optics in Emmetropization
The eye’s optics—primarily the cornea and the crystalline lens—constitute the focusing elements of the eye, akin to the objective of a microscope. Rarely in any other part of the body are the rules of physics so elegantly applicable to biology as in the eye’s optical elements. The similarities with a conventional optical lens, from the point of view of both its virtues and its limitations, have piqued the curiosity of astronomers, vision scientists, and physicists such as Helmholtz and Newton. Peering through telescopes and using an indirect ophthalmoscope to
inspect a person’s fundus led Helmholtz to the speculation that the eye is ridden with optical aberrations that are far more complex than those found in a traditional optical lens.
Many decades later, the application of sophisticated technologies to accurately measure the optical imperfections confirmed Helmholtz’s prediction. Not only was it shown that the eye’s optics are afflicted with aberrations beyond those that can be simply corrected with a spherocylindrical lens, but it was found that those aberrations vary with several factors—pupil size, wavelength, visual field, and eye shape. That the ensuing visual system can support a rich experience of the external world in a healthy eye, despite these fluid imperfections, is credit to the sophisticated and adaptable neural processing that takes place in the retina and the brain.
A recent review of published literature on ocular component development highlights the interplay between expansion of the globe and optical changes from the cornea and crystalline lens that together are both necessary to produce and then maintain emmetropia (Figure 6-2). The axial anterior-posterior length of the eye may increase by 5 mm between birth and maturity at age 20 years. Substantial amounts of myopia would be the result were it not for coordinated optical changes in other parts of the eye. Surface flattening of the refractive components and loss of optical power must occur during elongation to maintain balance between the eye’s focal length and its physical length. These power losses come from flattening of the cornea early in infancy and then primarily from flattening, thinning, and power loss of the crystalline lens in childhood. This coordination between global expansion and optical compensation from the crystalline lens is due in large part to their anatomical connection by way of the ciliary body and zonules. The crystalline lens is continually adding new fibers throughout life, but its thinning from infancy through childhood indicates that it is being stretched into a thinner, flatter shape until the majority of global expansion is complete by age 10 years. At that point, the crystalline lens displays the net thickening seen throughout adulthood. Evidence from animal models suggests that the vitreous chamber depth drives the main compensation for changes in optical power in refractive development (Smith & Hung, 2000; Smith et al., 2013).
SOURCE: Reprinted from Rozema, 2023, under a Creative Commons CC BY-NC 4.0 Attribution NonCommercial International License (https://creativecommons.org/licenses/by-nc/4.0).
Ocular Component Characteristics Before, During, and After Myopia Onset
The criterion for the refractive error that defines myopia varies. The diagnosis may be made when myopic refractive error begins to affect distance visual acuity, for example at −0.50 diopters (D) spherical equivalent. Another definition might include the time when acceleration of axial elongation begins, several years before the appearance of negative diopters of refractive error. This process of elongation is depicted in Figure 6-3A, showing axial length at annual visits 5 years before onset (negative visit numbers), at myopia onset during visit 0 (refractive error reaching −0.75 D in all prescription meridians), and then during myopia progression following onset (positive visit numbers; Mutti et al., 2007).
The CLEERE study found that the axial lengths of age-, sex-, and ethnicity-matched children who remained emmetropic (open circles) were no different on average from the axial lengths of those who went on to become myopic (filled squares) 4–5 years before myopia onset. Axial elongation became significantly faster in children who eventually became myopic at visit 3, three years before myopia onset, and then during every subsequent year (Mutti et al., 2007). Axial elongation reached its maximum rate in the year of onset. Accelerated axial elongation in pre-myopic and myopic children compared to emmetropic children can also be seen in data from the Singapore Cohort Study of the Risk Factors for Myopia (SCORM) study 2–3 years before the age of myopia onset (more myopic than −0.50 D), where onset is marked in Figure 6-3B by a different colored dot for each age group (Rozema et al., 2019). Interestingly, both the SCORM
and the CLEERE studies found that myopic diopters appeared when the average axial length reached roughly 23.8 mm, this despite being conducted in Singapore and the United States, respectively (Mutti et al., 2007; Rozema et al., 2019).
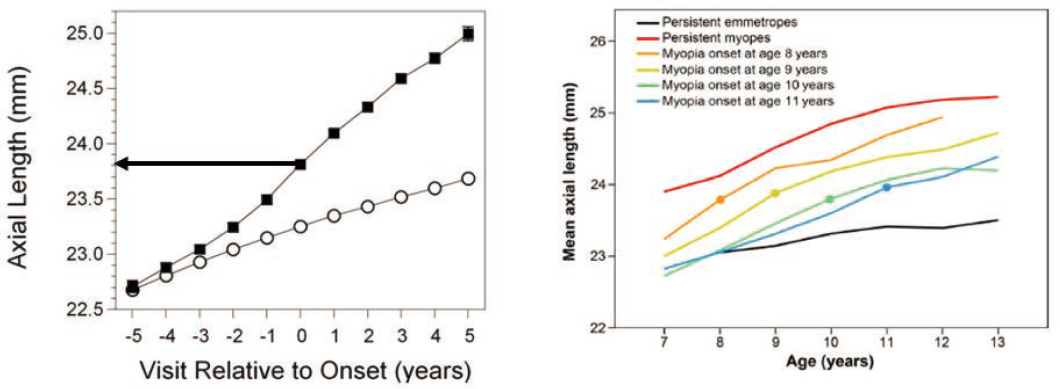
NOTES: (A) Axial lengths from Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) before myopia onset (negative visit numbers), in the year of onset (visit 0), and after myopia onset (positive visit numbers). Data from children who remained always-emmetropic are shown as open circles, while data from those who eventually became myopic are shown as filled squares. Pre-myopic axial lengths −5 and −4 years before the onset of myopia are similar between the two groups, but acceleration occurred in the children who went on to develop myopia starting −3 years before myopia onset. The arrow indicates that the average axial length at onset was 23.8 mm. (B) Mean spherical equivalent refraction and axial length data in right eyes from the Singapore Cohort Study of the Risk Factors for Myopia (SCORM) according to age and split by ages of onset. The dots represent the value of axial length at the first myopic visit. The average axial length at onset was roughly 23.8 mm regardless of age at onset.
SOURCES: Mutti et al., 2007; Rozema et al., 2019.
The onset of myopia appears to be a discrete event. Accelerated axial elongation may occur for several years while distance acuity remains good. However, the onset of significant negative diopters of myopic refractive error typically takes place within one year. The shape of this relationship between refractive error and axial length is depicted in Figure 6-4A (Tideman et al., 2018) using data from the Generation R study (Dutch children measured at 6 and 9 years old; n = 6,934) and the Rotterdam Study III (Dutch adults 57 years old; n = 2957). The relationship is linear for moderate to low amounts of hyperopia, then flattens across a narrow range of axial lengths while emmetropia is maintained, then becomes linear again when refractive error becomes myopic (Tideman et al., 2018). This inflection point is something of a “cliff” for myopia onset. Onset is the time when axial elongation is rapid (Figures 6-4A and 6-4B) and when the crystalline lens no longer loses power in amounts adequate to compensate for axial elongation (Figure 6-4B; Mutti et al., 2007, 2012). Crystalline lens power changes are equal between children who remain emmetropic and those who become myopic prior to onset (visits −5 to −1). During the year of myopia onset (visit 0) and in each subsequent year (visits 1 to 5), myopic negative diopters of refractive error appear, because the crystalline lens no longer loses enough power to compensate for axial elongation (significant deficits in power loss are marked with asterisks in Figure 6-4B; Mutti et al., 2012).
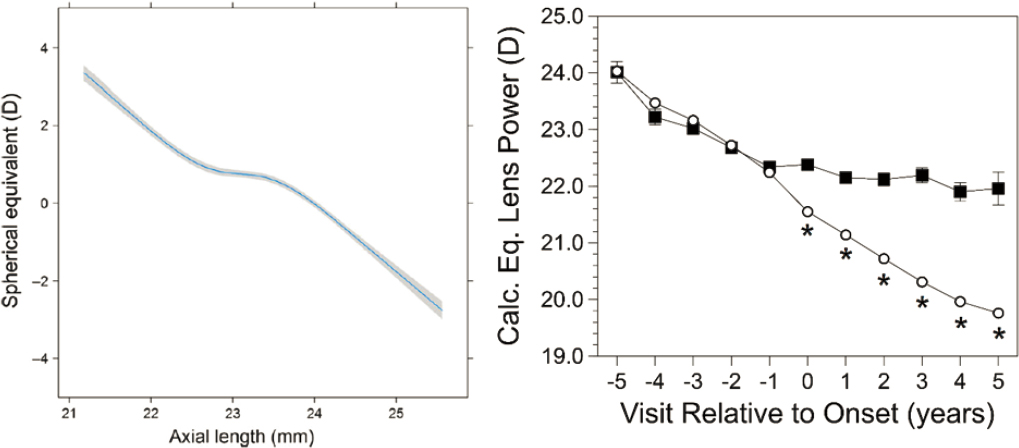
NOTES: (A) Association between spherical equivalent and axial length at 9 years of age from the Generation R study and the Rotterdam Study III. The mean and 95% CI were adjusted for age, gender and height. Note the inflection points on the curve on either side of low levels of hyperopia, perhaps the first inflection indicating the start of acceleration of elongation and the second the point of failure of crystalline lens optical compensation. (B) Calculated lens equivalent powers from (CLEERE) before myopia onset (negative visit numbers), in the year of onset (visit 0), and after myopia onset (positive visit numbers). Always emmetropic data are shown as open circles and those who eventually became myopic are shown as filled squares. Asterisks mark significant differences (inadequate losses of lens power) between became-myopic and emmetropic children occurring at myopia onset and every year following onset.
SOURCES: (A) Reprinted from Tideman et al., 2018, under a Creative Commons CC BY 4.0 Attribution International License (https://creativecommons.org/licenses/by/4.0/); (B) Reprinted with permission from Mutti et al., 2012.
Importance of Optical Contributions to the Retinal Image
This section addresses the optical contributions to the retinal image and their implications for myopic eye growth. Many reviews have covered the research done in this area, and the purpose here is not to provide an exhaustive summary of them but to consider the key findings and open questions in this area.
The properties of the retinal image are fundamentally governed by the optics of the eye. The facets of this retinal image that provide the primary cues for normal emmetropization are currently unknown. While a lot of research has been dedicated to deciphering the critical properties of the retinal image that govern eye growth—blur, contrast, chromatic aberration, peripheral defocus among them—there remains a lack of consensus concerning what are the most potent cue(s). As the gatekeeper of the retinal image, the eye’s optics have a critical role to play in shaping the cues that ultimately govern eye growth.
Specific to the properties of the retinal image, accumulating evidence suggests a potent role for time outdoors in delaying the onset of myopia progression as well as slowing eye growth once myopiogenesis is under way, though its underlying mechanisms remain unknown (see Chapter 5, Onset and Progression of Myopia). The role of light intensity outdoors is hypothesized to play a key role, and other factors—such as spectrum, chromaticity, as well as the
spatial frequency and dioptric structure—may also play a role. The optics of the anterior eye encode these features of the visual environment, the so-called “visual diet,” into the retinal image. Consequently, the ocular optics inform what critical feature(s) of the visual diet are most critical for eye length regulation.
A major unresolved question is whether the myopic axial elongation is a cause or consequence of the myopic eye’s optics. There are both major changes in the myopic eye’s optics (reviewed below) and large inter-subject variability. It remains unknown whether the changes in eye optics create an impairment in myopia early on to detect blur precisely to regulate eye length, leading to an open-loop eye elongation similar to the effect of form deprivation (Troilo et al., 2019). The converse possibility is that myopic eye growth is caused by the failure of another mechanism besides the optics, and that the changes in the eye’s optics are all but a result of the changes the eye undergoes in its shape and anatomy as a result of normal eye growth.
Many optical treatments exist for slowing myopic eye growth (reviewed in Chapter 7), yet the mechanisms of their action remain inadequately characterized. This is a critical unknown that perhaps partly accounts for their variable efficacy. For any optical correction to work effectively and to devise new and better strategies for treatment requires understanding the interplay between the optics of the corrective device and the eye’s own optics, and how the combined optical system (correction + native eye) interacts with the visual environment. Specifically, what is the interplay between the properties of the combined retinal image constructed by the eye’s native and growing shape and its optics, coupled with the correction change, on the one hand, and retinal eccentricity, light wavelength, dioptric distance (near vs. far), accommodative demand, and spatial frequency content of the visual environment, on the other? Ultimately, individualized and average eye models are required to test-drive the treatments in order to deduce this interplay and devise the best corrective strategies.
Thus, to understand the mechanisms of both tightly regulated and uncontrolled eye growth, be equipped to suggest preventative strategies (time outdoors, for example), and devise new treatments, it is important to understand the optical factors that govern the retinal image.
AN IMPROVED FRAMEWORK FOR STUDYING THE ROLE OF THE RETINAL IMAGE IN REGULATING EYE GROWTH
In the context of understanding eye growth a single sphero-cylindrical definition of foveal refraction is insufficient. Instead refractive error must be considered across the curved surface of the retina. This carries the consequence that local retinal image defocus can only be determined once the 3D structure of the viewed scene, off axis performance of the eye and eye shape has been accurately defined. This, in turn, introduces an under-appreciated level of complexity and interaction between the environment, ocular optics and eye shape that needs to be considered when planning and interpreting the results of clinical trials on myopia prevention. (Flitcroft, 2012, p. 622).
This text from Flitcroft indicates the need for a fresh framework to treat the retinal image in the context of the development of refractive error. Prior literature has emphasized the “foveocentric” framework—one in which defocus and the retinal image are defined at the fovea using paraxial optics, ocular shape is defined by axial length, and the spatial structure of the
visual world is not relevant. While this framework has led to important findings as they relate to factors in the retinal image that drive visual acuity and accommodation, it is inadequate to describe properties of refractive error and image quality across the retina, and thus falls short in providing information on potential cues for emmetropization. Key results in animal models also indicate that the fovea isn’t necessary for inducing myopia (Bullimore, 2024; Smith et al., 2005, 2009). With continued advancements in technologies to detail the visual environment (e.g., stereo scene cameras), the eye’s optics (e.g., wavefront sensors, autorefractors), and the eye’s shape (e.g., wide-field OCT), a more complete picture is beginning to emerge of the complex interaction between the three factors.
The need for such a framework arises based on the observation that the key mechanisms involving emmetropization involve the retinal periphery and the spatial structure of the visual stimulus (indoors vs. outdoors, for example). A paraxial, spherically symmetric, on-axis treatment of image formation cannot account for these observations. Furthermore, experimental evidence shows that local manipulations in the retinal image, based on an asymmetrically blurred visual field for example, can create similarly localized eye growth (Diether et al., 1997; Smith et al., 2009, 2010; Wallman et al., 1987). To make such deductions from the local defocus with the precision required for emmetropization requires having information about the dioptric and spatio-chromatic-temporal structure of the environment, the image-forming characteristics of the eye’s optics, and the detection of the retinal image.
A Triangle of Interacting Factors
Figure 6-5 represents these interacting factors as a triangle, as opposed to a linear transformation, with the intention of denoting the strict interdependency between any two of them. That is, holding any two of the factors constant but allowing the third to vary can substantially alter the characteristics of the retinal image (Figure 6-4). For example, given the visual environment and image formation/detection specific to an individual’s eye, the accommodative state, pupil size, and chromatic aberration all attributed to that eye’s optics can drastically change the retinal image and hence the cues that are available to sense the focus error. A similar case can be made for the differences in the spatio-chromatic-temporal structure and dioptric content of the visual scene that reaches the retina. Holding the eye’s optics and image formation machinery fixed, reading indoors versus riding a bike outdoors will result in very different retinal image distribution. Table 6-1, reproduced from Flitcroft (2012), lists the key differences between the foveocentric view and this retinocentric view of refraction.
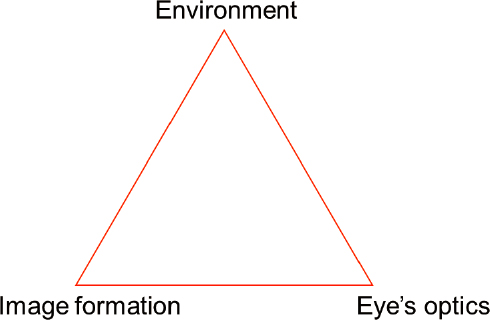
NOTE: The three facets—features of the environment, the eye’s optics and the factors relevant for early visual encoding that lead to retinal image formation—interact together to govern the visual diet.
SOURCE: Committee generated.
TABLE 6-1 Differences Between the Foveocentric and Retinocentric Views of Refraction
| Foveocentric view | Retinocentric view |
|---|---|
|
An eye has a single refraction |
An eye has a graded, complex pattern of refractions across the retinal surface |
|
Refraction and retinal image blur are defined at a single point (the fovea) |
Refraction and retinal image blur are defined across a 3-dimensional curved plane (the retina) |
|
Spatial structure of the visual environment irrelevant |
Spatial structure of the environment contributes to the defocus of the image at each point in the retinal image |
|
Ocular shape unimportant apart from axial length |
Three dimensional ocular shape is fundamentally important |
|
Paraxial optics provides an adequate description of the eye’s optics |
Wide-angle ray tracing needed to fully define the eye’s optics |
|
Near work with a bifocal add is optically equivalent to far work |
Near work with a bifocal add is not optically equivalent to far work |
|
Relevant for visual acuity and accommodation |
Relevant for understanding optical regulation of eye growth |
SOURCE: Flitcroft, 2012, p. 654.
The following details the key characteristics of the three governing factors relevant to myopia in this retinocentric framework.
Environment
Chapter 5 (Onset and Progression) addresses the various factors in the environment pertinent to the onset and progression of myopia. Technologies that effectively quantify these environmental factors in children are rare and an important area of research and development where future resources may be devoted. Briefly, the environmental factors are light intensity, chromatic and spatial frequency spectra, dioptric variation, and temporal dynamics (due to eye, head, and body motion). These physical attributes are substantially different based on a person’s location (indoors vs. outdoors, earth’s latitude, etc.), their activity (doing near work vs. riding a bike), and time of day (dawn vs. dusk). The unknowns in this area concern characterizing the visual environment specific to children both prior to myopia onset and longitudinally, as children engage in their everyday activities.
Eye’s Optics
The optical properties of the cornea and crystalline lens govern how light is channeled to the retina. Their shape (curvature, asphericity), refractive index distribution, effective optical zone diameter, and optical performance on- and off-axis, as well as their relative placement in space within the anterior chamber of the eye are all important parameters that together dictate the
light distribution on the retina. Relevant in the context of axial elongation is how these parameters change longitudinally, especially in early childhood, with accommodation and with varying pupil diameters.
Image Formation
The early visual encoding of the retinal image involves sampling in space, time, and spectrum according to the shape of the eye, spatial and spectral topography of the photoreceptor mosaic, light adaptation in cone and rod phototransduction, and eye movements. Together these factors provide the signals from the external world that are available for detecting blur for regulating eye growth.
Computational Models of Retinal Image Formation and Visual Encoding
The framework described above allows any sequence of three-dimensional hyperspectral visual scenes (x, y, z, t, λ) to be transformed into the spatial (x, y, z), spectral (λ), and temporal (t) variations in the retinal image. These facets of the retinal image form the basis of downstream retinal circuits dedicated to signaling luminance, contrast, color, and form. The same facets of the image are critical as cues for eye growth. Naturally, computational models linking the environment, the eye’s optics, and image formation would be instrumental in understanding mechanisms of normal eye growth, myopia pathogenesis, and treatments.
Eye Models
Many eye models exist in the literature that are based on anatomical parameters derived from biometry and imaging (reviewed in Atchison & Thibos, 2016). However, the classical eye models fall short in many ways. Most eye models are based on paraxial optics and are functional only for small fields of view (± 5 degrees), similar in spirit to the above noted foveocentric view. Thus, they do not fully reproduce the image quality and aberrations across the visual field relevant for myopia. In addition, they are based on population averages and cannot easily be generalized across variations in refractive error, accommodative state, or chromatic dispersion due to variations in ocular components, age, and eye shape.
Aberrometry provides a comprehensive account of the eye’s optics (see Chapter 4) and can, in principle, account for inter-individual variations and the complex interactions between retinal shape, ocular surfaces, refractive index, and accommodation. It has long been appreciated that ray tracing through classical eye models falls short in precisely estimating the measured wavefront from aberrometry. Wide-angle eye models that best mimic the ocular anatomy and aberrations across a wide visual field are needed. While examples of such models exist (Polans et al., 2015), larger datasets of imaging and aberrometry will allow for better generalization of these models so they can be applied across the population and include the varying optical conditions relevant for myopia. Individualized eye models based on a retinocentric view are essential to further understand the mechanisms underlying the onset and progression of myopia, in particular how the visual environment interacts with the eye’s optics, and to customize treatments specific to each eye.
Early Visual Encoding
Early visual encoding refers to the initial formation of an image on the retina by the eye’s optics, the transduction of that image into electrical signals by the photoreceptors, and the
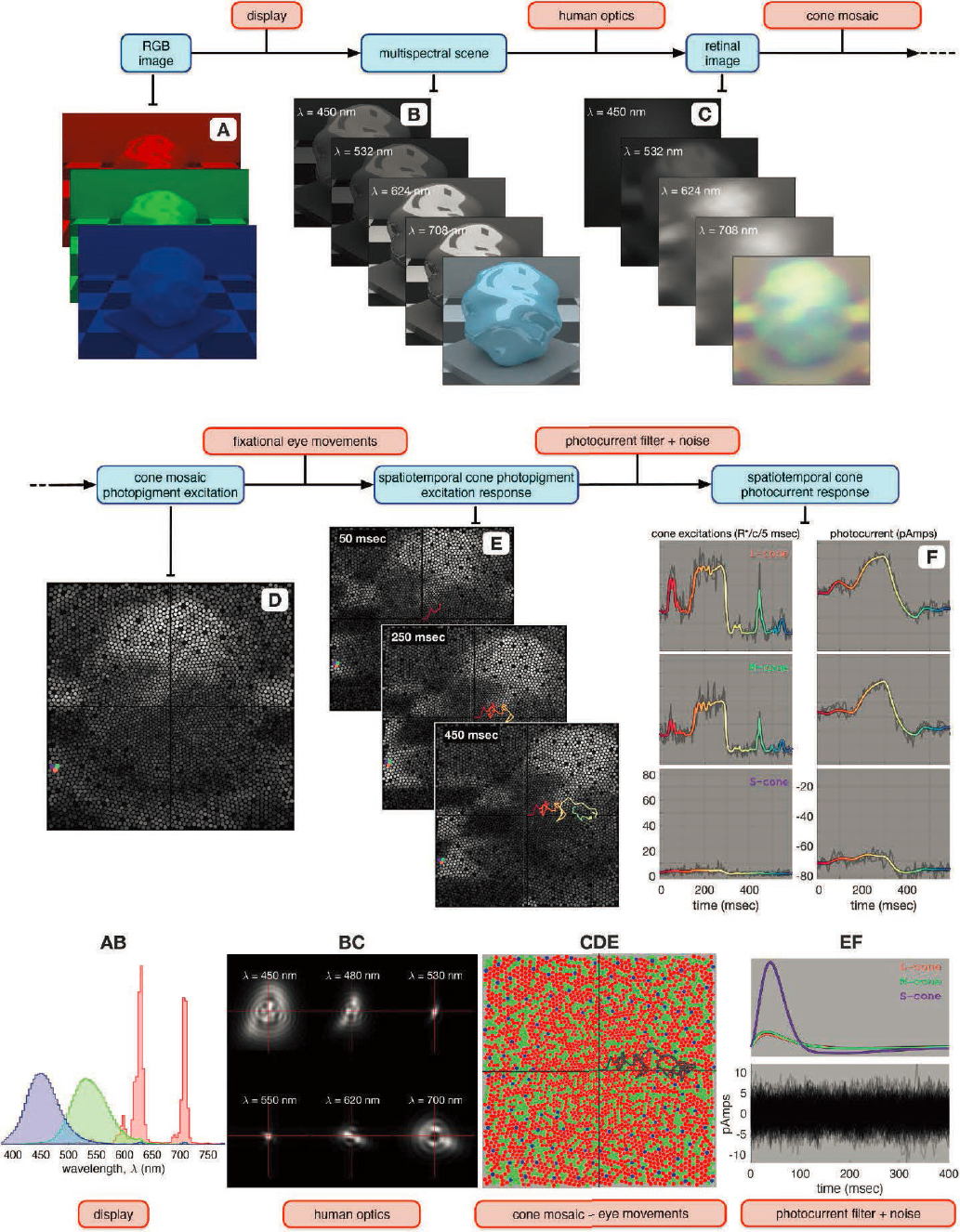
subsequent transformations of the encoded image by retinal circuitry. The Image Systems and Engineering Toolbox for Biology (ISETBio; see Figure 6-6) is an example of a computational modelling platform for early vision that allows one to incorporate aspects of the environment/visual diet, the eye’s optics, and image formation properties and yields cone photoreceptor signal outputs in response to any 3D hyperspectral visual scene (x, y, z, t, λ; Wandell et al., 2022; Zhang et al., 2022). It has found application in predicting spatial contrast sensitivity and color perception, among other visual tasks. Importantly, the ISETBio model allows probing the system at intermediate stages to quantify losses and encoding at various steps—for example, the physiological optics, the cone photoreceptor lattice, eye movements—and visualize the intermediate product of each processing unit. Refinements to existing parameters and later stages of retinal processing can be (and already are being) continually added into the software as more data from experiments become available. This is mentioned here to highlight an example of an existing framework for determining the retinal image from a visual scene after initial visual encoding and subject to the optical manipulations of the eye’s optics. Moreover, it is constructed with the flexibility of taking as input personalized visual scenes, eye parameters, cone mosaics, eye movements and imposed treatments, and provides an avenue to ask mechanistic questions about the signals that the eye uses to sense blur and its sign.
Ocular Optics
The longitudinal changes in the focusing elements of the eye are shown in Figure 6-2. With respect to the corneal curvature, no specific trend—steep or flat—is characteristic of myopes or axial elongation, but rather curvature is associated with eye size (Guggenheim et al., 2013). Further, longer axial lengths and flatter corneas can confound the degree of myopia, so both parameters need to be measured simultaneously. Llorente et al. (2004) observed that the steeper myopic corneas had a higher negative asphericity (flatter in the periphery than the center), and the combined effect of curvature and asphericity led to an overall lower corneal spherical aberration in myopes.
SOURCE: Reprinted from Cottaris et al., 2020, under a Creative Commons CC BY-NC-ND 4.0 License (https://creativecommons.org/licenses/by-nc-nd/4.0).
People with high myopia have been found to have a significantly thinner crystalline lens than emmetropes, specifically by a mean 0.046 mm. A decrease in −0.12 mm per mm of axial length (Muralidharan et al., 2019; Zhang et al., 2023) and an overall lower lens power of −1.2 D per mm of axial length is observed in the crystalline lens in myopic adults. Although this morphology is known, it remains unknown whether the gradient index properties of the crystalline lens are different in myopes, if at all. Differences in gradient index between emmetropes and myopes are important to consider in developing accurate eye models. Pupil size differences in myopes are also inconclusive—with studies indicating no differences (Orr et al., 2015) or else larger pupil size in myopes (Cakmak et al., 2010; Charman & Radhakrishnan, 2009; Guillon et al., 2016; Poudel et al., 2024).
With respect to aberrations besides spherical and cylindrical, in myopes one consistently observes one lower magnitude of primary-4th-order spherical aberration, consistent with a prolate shaped eye, its axial length longer than its width and height (Carkeet et al., 2002; Collins et al., 1995; Llorente et al., 2004). Chromatic aberrations do not seem to be affected by refractive error (Wildsoet et al., 1993), but in infants a larger amount of longitudinal chromatic aberration (LCA) is noted on account of the higher optical focusing power (Wang et al., 2018). In the periphery, myopes experience a positive refraction (image plane behind the retina) compared to emmetropes for whom the periphery is myopic (image plane in front of the retina; reviewed in Romashchenko et al., 2020). Besides the relatively less oblate shape in myopes compared to non-myopes, no other differences between eye shape are noted between refractive groups. These differences in eye shape have consequences for aberrations, including refraction and astigmatism. Changes in peripheral refraction may be more a consequence rather than a cause of myopia, given that peripheral refraction at baseline did not predict an onset of myopia in the future (Mutti et al., 2011; Sng et al., 2011). However, it is not clear to what degree these changes in peripheral shape contribute to myopia’s progression, owing to the visual system’s inability to detect blur and regulate eye growth.
Both young and adult myopes consistently show increased accommodative lag, and the accommodative response function decreases as myopia progresses (Abbott et al., 1998; Gwiazda et al., 1993; McBrien et al., 1986). This established observation is the basis of the near work hypothesis for myopia onset and progression discussed in detail in Chapter 5. Myopes showed different structural changes in response to an accommodative effort. A larger change in lens shape per diopter of change in accommodative focus is needed in myopes (Gwiazda et al., 1999; Mutti et al., 2017), accompanied with smaller reductions in ciliary muscle thickness but larger muscle movements (Bolz et al., 2007; Wagner et al., 2019; Wang et al., 2022).
The impact of accommodation on peripheral refraction deserves more study, since peripheral refraction is affected not only by eye shape but also by the peripheral focusing properties of the crystalline lens, such as its field curvature. Results are mixed on this issue. Whatham et al. (2009) showed hyperopic shifts in the near periphery with accommodation but the farther periphery either remained the same or demonstrated a myopic shift. A hyperopic shift is also noted by Walker & Mutti (2002). On the other hand, Davies & Mallen (2009), and Calver et al. (2007) found no associations due to accommodation between peripheral refractions (and their sign) and the refractive status. Overall, this large variability may be attributed to methodological differences as well as to the other lower and higher order aberrations, such as coma and astigmatism, which are large in magnitude and highly variable in the periphery and impact the best focus of the retinal image quality.
Photoreceptors: Retinal Density and Ratios
A few models have been proposed for the shape of eye growth that would predict different impacts of the linear and angular cone density in myopes in the foveal center (see Figure 3 in Strang et al., 1998). The model of global expansion suggests a proportional stretching of the retina with increasing eye length, such that the number of cones in each square millimeter area of the retina will decrease with increasing axial length, but the angular density will remain constant. The equatorial stretching model posits a simple posterior movement of the retina without expansion, such that the linear density remains the same, while the angular density increases with axial length. The over-development model suggests that the photoreceptors continue to migrate toward the fovea with eye elongation, leading to an increase in linear density and a still steeper increasing angular density with axial length. In marmosets, an increased linear cone density was observed with lens-induced eye growth (Troilo, 1998), and the overdevelopment model is inspired by this observation. Increasing linear cone spacing is also reported in chicks with eye growth that is unaccompanied by significant changes in angular cone density (Kisilak et al., 2012). An increased angular density would indicate better visual acuity in myopes compared to emmetropes.
Both histology and, more recently, in vivo adaptive optics imaging (see Chapter 4) have revealed the structure of the human photoreceptor mosaic (Curcio et al., 1990; Wang et al., 2019; Wells-Gray et al., 2016). Cone density peaks at the foveal center and decreases with eccentricity (Provis et al., 2013). The fovea is defined as the region of the highest cone density, ~1 mm in diameter, and with an absence of S-cones and rods. Rods begin to appear at ~1–2° from the foveal center, reaching high densities rivaling that of the cones at ~20° eccentricity. Rods outnumber cones by 10- to 20-fold between 10° and 30° eccentricity. Figure 6-7 shows the rod:cone ratio as a function of retinal eccentricity, imaged with an adaptive optics scanning laser ophthalmoscope (AOSLO), reproduced from Wells-Gray et al. (2016).
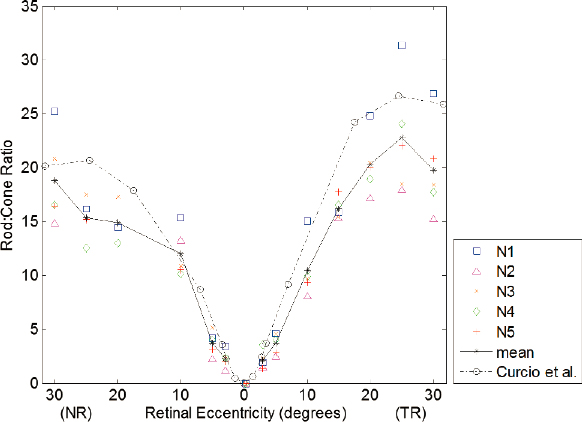
NOTE: Solid line is the mean of 5 subjects (N1–N5), and the OT dashed line is obtained from histology for comparison. NR = nasal retina; TR = temporal retina.
SOURCE: Wells-Gray et al., 2016.
Using a state-of-the-art adaptive optics scanning laser ophthalmoscope, Wang et al. (2019) revealed the structure of the foveal cone mosaic in emmetropes and myopes and found
that, in general, shorter eyes have higher peak cone densities in linear units while longer eyes have lower linear peak cone density. Similar studies of rod topography in myopes are lacking. A lower linear cone density was also observed with an adaptive optics imaging study undertaken in children (ages 5.8 to 15.8 years) at eccentricities of 0.2 mm from the foveal center (Mirhajianmoghadam et al., 2020). These findings would be consistent with the global expansion model, in that longer eyes undergo an expansion at the fovea. However, when Wang et al. (2019) plotted the angular density in the fovea vs. axial length, they found an increasing trend.
Taken together, it becomes apparent that a combination of global expansion and equatorial stretching are needed to explain the foveal cone density in myopes, and that the foveal expansion does not occur in proportion to the length of the eye. The higher angular density in myopes would lead to a higher cone sampling density and predict better visual acuity when best corrected. However, this does not seem to be the case. Myopes routinely tend to have poorer acuity compared to emmetropes, even when the effect of the eye’s optics is bypassed with the use of interference fringes (Atchison et al., 2006; Coletta & Watson, 2006) or with adaptive optics (Rossi et al., 2007). Prior to the adaptive optics imaging of the foveal cone mosaic by Wang et al. (2019), this would be attributed to the retinal stretching leading to reduced foveal cone density, but the reasons for these deficits must be post-receptoral. For example, it has been suggested that abnormal eye growth can lead to a loss of ganglion cells (Atchison et al., 2006). More work is needed to detail the inner retinal anatomy and wiring connectivity in myopia and assess whether the poorer visual performance is a cause or consequence of myopia.
Photoreceptors: Wavelength Sensitivity
The retinas of Old World primates, including humans, contain three different types of cones whose photopigments differ slightly in their sensitivity to different wavelengths of light. Each photopigment consists of a chromophore, 11-cis retinal, which is the same in all mammalian cones and undergoes photoisomerization when it absorbs a photon. This chromophore is covalently bound to an “opsin,” a protein found in the visual system that “tunes” the spectral sensitivity of the photopigment. Each cone expresses only one of the three opsin genes, producing three unique types of cones that are relatively more sensitive to either short(i.e., bluer), medium- or long- (i.e., redder) wavelength light, referred to as S-, M-, and L-cones for short. The signals from the three cone classes form the basis for color vision (among other visual capacities), and mutations in the opsin genes result in well-known inherited deficits in color vision, popularly but incorrectly referred to as “color blindness.”
The distribution of cones varies across the retina, being most dense at the fovea and dropping off steeply with distance from the fovea (i.e., with eccentricity). In comparison with the cone mosaic’s spatial topography, its spectral composition is relatively uncharacterized by its eccentricity, apart from the layout of the S-cones. This is because of the close similarity in the protein structure of L- and M-cones precluding their separation via histochemical markers. Nevertheless, S-cones are typically absent in the foveal center, peaking in their density at ~1–2° from the fovea and increasing in their proportions to reach ~10% of all cones by 10° (Curcio et al., 1991). Adaptive optics imaging has made it possible to detail the cone spectral types in humans (Roorda & Williams, 1999), yet the cone spectral type variation vs. eccentricity, especially in the foveal center, remains unknown. mRNA analysis of donor eyes has revealed that the retina becomes increasingly L-cone dominated in the far periphery (Neitz et al., 2006). Within individuals, variations in L:M cone ratios have been observed.
Related to this, an association has been suggested between the L:M cone ratio, cone opsin gene polymorphisms, and myopia. In a study conducted in a Norwegian population with a relatively low prevalence of myopia, it was suggested that the L:M cone ratio, combined with milder versions of L opsin gene polymorphisms, has a role to play in myopia (Hagen et al., 2019). High L:M cone ratios seemed protective in females, leading to lower degree of myopia in the Norwegian cohort, while lower L:M ratios, close to 1:1, were observed in East Asians (without concomitant measures of refractive error; Kuchenbecker et al., 2014) as compared to a 2:1 ratio observed in people of European ancestry (Carroll et al., 2002).
Cross-sectional studies reported conflicting results on the relationship between myopia and color vision deficiency (CVD). In Chinese high school students, ages 15–18 years (Qian et al., 2009), and in Iranian primary school students, ages 7–12 years (Ostadimoghaddam et al., 2014), a lower prevalence of myopia was noted in students with red-green CVD compared to color-normal individuals. However, no relationship was observed between red-green CVD and refractive error among Iranian children ages 7 to 12 years (Rajavi et al., 2015). A recent 5-year longitudinal study in China found lower cumulative incidence and change in spherical equivalent refraction in people with CVD: 35.4% (17/48) vs. 56.7% (1017/1794), and −1.81 D vs. −2.41 D respectively between CVD and the color-normal group (Gan et al., 2022). One limitation was the highly unbalanced number of children in both groups. The causal link between cone spectral composition, CVD, and myopia remains an open question. It has been suggested that chromatic cues, including transverse chromatic aberration and LCA, play a role in eye length regulation, and that there might be differences in the mechanisms by which these cues are encoded and utilized as cues in CVD, for example differences in cone contrast and their impact on accommodation.
While the recent myopia boom cannot naturally be attributed to the genetic differences inherent to people with CVD, these studies suggest a potential protective effect for myopigenic environmental factors in CVD via previously unknown mechanisms. LCA provides an important cue for accommodation and has been implicated in emmetropization using a model that compares the outputs of S-cones vs. LM-cone signals (Gawne & Norton, 2020). Thresholds for detecting S-cone increments and decrements of 3 cycles per degree grating patterns are shown to be worse in myopes; however, whether this reduced S-cone sensitivity constitutes one of the underlying causes for the impaired accommodative system in myopes is unknown. Again, as in the case of increased accommodative lag, longitudinal studies starting at an early age are required to establish whether the decrease in S-cone sensitivity in myopes is a cause or consequence of abnormal eye growth.
Retinal Periphery
Seminal work done in rhesus monkeys has implicated the essential role of the retinal periphery in both emmetropization and myopia pathogenesis, motivating treatments that focus on manipulating the quality of the peripheral retinal image (Smith et al., 2005, 2009). Few observations are of note, however. Peripheral form deprivation and lens-induced refractive error disrupted normal emmetropization and led to foveal myopia despite clear central vision (Smith et al., 2009). Foveal ablation of the central 5–6° did not impact the normal emmetropization of animals reared with unrestricted vision, nor the recovery of the animals from form deprivation myopia once the diffusers were removed, suggesting that the periphery beyond the central 5- to 6-degree region is sufficient for emmetropization (Huang et al., 2011; Smith et al., 2007). Taken together, these studies lead to the conclusion that the retinal periphery alone can regulate visually
guided emmetropization, while the fovea and perifovea are not essential. The dominant role of the periphery in this may be attributed to its proportionally larger retinal area in comparison to the fovea, which occupies only a small fraction of the retina (Wallman & Winawer, 2004).
An alternative interpretation of these experiments is that cone photoreceptor circuits may not be the prominent retinal circuit guiding refractive eye growth. This hypothesis is further supported by studies in transgenic mouse models in which the rod or cone photoreceptor pathways were dysfunctional due to genetic mutations; the loss of rod function resulted in the eye not responding to experimentally induced myopia (Park et al., 2014) while the loss of cone function increased myopia susceptibility (Chakraborty et al., 2019). Hyperopic peripheral defocus as a risk factor and myopic peripheral defocus as treatments have their origins in these findings. However, such treatments have had limited success (as elaborated in Chapter 7).
Furthermore, studies on associations with near work, an activity purported to create peripheral hyperopic defocus, have led to inconsistent associations with myopia onset and progression. As indicated in Chapter 5, the negative impact of near work may be most potent during early age.
Role of the Non-foveal Retina in Accommodation
Near work is recognized as a risk factor for myopia (see Chapter 5). Given the importance of the retinal periphery in eye growth, it is worth considering if or whether the non-foveal retinal image makes a contribution to accommodative effort. There is evidence that even in the absence of a foveal stimulus, accommodative stimulation at 5–15° eccentricity creates a refractive change (Hartwig et al., 2011). An accommodative effort was observed when subjects fixated a foveal stimulus with no accommodative drive, while the retina was stimulated up to 30° with an accommodative demand (Gu & Legge, 1987). Patients with loss of foveal function due to juvenile macular degeneration also show a refractive change in response to an accommodative stimulus (White & Wick, 1995). In sum, the non-foveal retina can drive accommodation in the absence of a foveal near target.
Outside of these laboratory experiments, except in the case of diseases like macular degeneration, the fovea and the periphery are always stimulated together. To test how this real-life scenario affects peripheral contributions to accommodation, annular stimuli were used wherein the accommodative demand in the different parts of the fovea and periphery could be independently manipulated (Labhishetty et al., 2019). It was demonstrated that stimulating the retina in the perifovea with an annulus whose inner diameter was 8 degrees reaching up to 14 degrees eccentricity led to an accommodative effort, even though the fovea and parafovea were stimulated with the reverse accommodative demand.
Thus, peripheral hyperopia can drive an accommodative effort even when the foveal image is focused. This has consequences for how the accommodation system functions in the flatter vs. steeper dioptric environment found outdoors vs. indoors, respectively. In a flatter dioptric environment, accommodative demand is low and fairly uniform across the retina, exerting a proportionally lower impact on the periphery, compared to a steeper dioptric environment that has wider variability in accommodative stimuli (in diopters) and across the visual field. This larger variability indoors—both in dioptric distance and in its distribution across the retina—may lead to a conflict between the fovea and periphery, resulting in an ambiguous response of the accommodative system, in contrast to the outdoor environment where such a conflict is comparatively lower.
Peripheral Retinal Image Quality
The visual periphery has been the focus of many studies on aberrometry, including deducing the peripheral retinal shape and refraction from the experimental data (reviewed in Romashchenko et al., 2020). As stated earlier, myopes have peripheral hyperopia compared to emmetropes, for whom the periphery is myopic. Relative peripheral hyperopia seems to be a consequence rather than cause of myopic eye growth (Atchison et al., 2015; Mutti et al., 2011). That is, relative peripheral refraction depends on the magnitude of myopia, although these differences between the degree of myopia begin to appear only at 20° eccentric or greater. Relative refraction, or defocus, as a measure of the best image plane relative to the retina is challenging to determine precisely in the periphery. Image quality in the peripheral retina is affected by large magnitudes of coma, astigmatism, and other aberrations. Together, these expand the depth of focus significantly, and interactions between different aberrations lead to a shift in the defocus (compared to the relative refraction) where image quality is most optimal.
In contrast, aberrometry-derived metrics of retinal image quality, like modulation transfer function, better characterize images in the retinal periphery (Marsack et al., 2004). With distance foveal refraction, myopes have worse modulation transfer functions (poorer retinal image quality) along the horizontal visual field (calculated from aberrometry) than emmetropes do. This is the case from the fovea up until 20 degrees, after which the modulation transfer functions become similar between the two groups. These observations come from an analysis performed on wavefront aberration data of the horizontal visual field collected from 2,492 eyes (60% emmetropes, 20% each myopes and hyperopes) in Europe, Australia, and North America (Romashchenko et al., 2020). The same dataset was used to estimate the shape and orientation of blur (point-spread function) in the periphery in different refractive groups (Figure 6-8) (Zheleznyak, 2023). The blur study showed that along the horizontal nasal visual field from 0 to 30 degrees, the myopic retina experiences vertically elongated blur (circumferential or tangential), while emmetropic and hyperopic retinas experience horizontal or radially elongated blur when the fovea is refracted for distance vision. This radial to tangential anisotropy in blur orientation is attributed to the interaction of peripheral optics and retinal shape. It is known that in the periphery, the visual system has greater sensitivity for radially oriented targets compared to tangential ones. This preference is consistent with the peripheral optical blur orientation and resultant retinal images experienced habitually, given that even after optical corrections, the same bias holds in orientation preference, with some differences observed between refractive error groups (Leung et al., 2021; Zheleznyak et al., 2016).
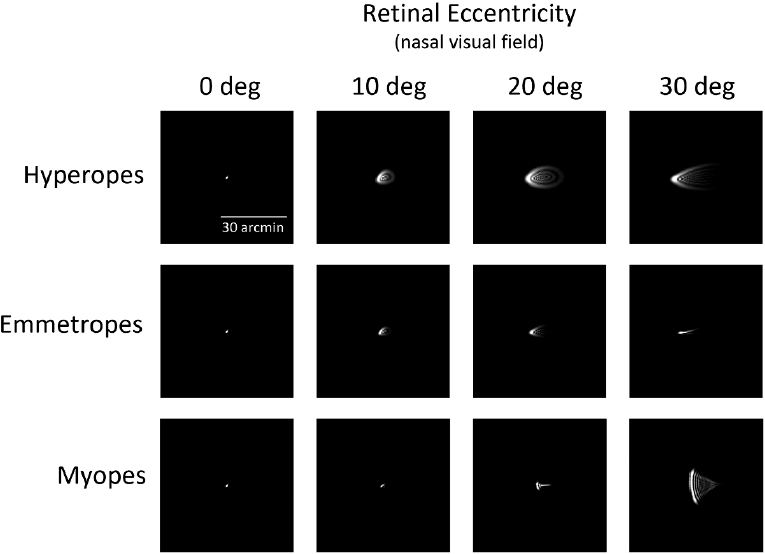
NOTE: Optical blur, shown as the point-spread function, for different refractive groups as a function of eccentricity. Pupil size was 4mm for the simulation, and monochromatic light was used.
SOURCE: Zheleznyak, 2023.
When published values of eccentricity-dependent chromatic aberrations—longitudinal and transverse—were incorporated, the retinal image (as estimated by the modulation transfer function) in the myopic peripheral retina was optimal for short wavelengths, while longer wavelengths were more optimal for hyperopes (Figure 6-9; Zheleznyak et al., 2024). This is denoted by the size of the point-spread function in the periphery; at wavelengths of 405 nm the point-spread function is relatively smaller in the myopes than at wavelengths of 695 nm, while the hyperopes show the opposite trend. Emmetropes and hyperopes exhibited more tangential blur at greater eccentricities (20° and 30°) for all wavelengths, while the blur shape for myopes depended on wavelength and eccentricity; at 30°, wavelengths greater than 505 nm (bluish-green) had a more vertical orientation bias. This optical blur anisotropy in the periphery, including the effects due to chromatic aberration, is suggested by the study as a potential cue for emmetropization that could be sensed by orientation-selective mechanisms in the retina.
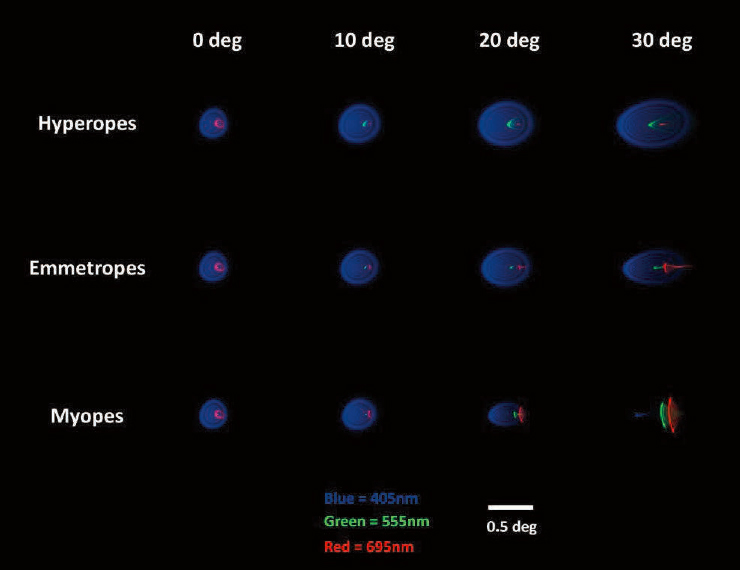
NOTE: The three colors, red, green and blue, represent the blur on the retina created by 405 nm, 555 nm, and 695 nm wavelength light, respectively, considering the effects of monochromatic and chromatic aberrations as a function of eccentricity.
SOURCE: Reprinted from Zheleznyak et al., 2024, under a Creative Commons CC BY-NC-ND 4.0 License (https://creativecommons.org/licenses/by-nc-nd/4.0).
Summary
This section covered the characteristics of ocular optics and image formation that together determine the facets of the retinal image that may be pertinent as cues for emmetropization. These facets all vary in specific ways with defocus, eye shape, and retinal eccentricity. Deducing the direction of eye growth, therefore, is a problem of finding the most potent image facet(s) amenable to be detected by retinal cells and circuits to initiate the retinal-scleral signaling cascade (see next section). Here is listed a summary of these retinal image properties:
- Wavelength-dependent defocus or chromatic aberration: longitudinal and transverse
- Optical blur: shape, size, and orientation
- Contrast: spatial, spectral, and temporal contrast.
RETINAL CELLS AND CIRCUITS REGULATING EYE GROWTH
Earlier sections of this report implicate at least three sorts of retinal signals that could link specific features of the visual environment to dysregulation of eye growth in myopia. These are luminance (irradiance or light intensity), defocus or blur, and wavelength (color). This section surveys what is known about the specific retinal neurons and synaptic circuits that encode these stimulus features and their possible involvement in myopia pathogenesis.
The Retina’s Central Role in Myopia Pathogenesis
The neural retina appears to be the key link between the properties of retinal images and the regulation of eye growth at the sclera. The retina has been shown to encode critical image features that have been linked to eye-growth regulation (including luminance, wavelength, and spatial contrast). Blurred or defocused images continue to affect eye growth even when the optic nerve is crushed, severing the link between eye and brain (McFadden & Wildsoet, 2020; Norton et al., 1994; Troilo & Wallman, 1991; Troilo et al., 1987; Wildsoet, 2003; Wildsoet & Pettigrew, 1988; Wildsoet & Wallman, 1995). Thus, post-retinal processing is not required for retinal images to affect eye growth. Though the brain and integrative visual behaviors may play some role, as discussed later, there is broad consensus that the retina is both necessary and sufficient as the neural link between retinal images and eye growth.
Diverse lines of evidence explored in this section reinforce the prevailing view that the retina is essential for encoding retinal image features that affect eye growth. For example, mutations in diverse genes disrupting retinal phototransduction or synaptic signaling in mice result in myopia or in the perturbation of dopamine levels (e.g., Nob [nyx gene], as reported in Pardue et al., 2008); mGluR6 (Grm6 gene), reported in Chakraborty et al., 2015; and Lrit3 and GPR179, reported in Zeitz et al., 2023; see review by Mazade et al., 2024). Retinal degenerative diseases are frequently associated with high myopia (Hendriks et al., 2017; Park et al., 2013; see Chapter 5 for additional information about genetics). Both dopamine and melanopsin have been implicated in myopigenesis, as discussed below, and their ocular expression is largely limited to the neural retina.
Retinal Cells and Circuits Encoding Light Intensity
The epidemiological and experimental animal studies considered in Chapter 5 suggest that environmental light intensity affects the propensity to develop myopia. Retinal irradiance or photon flux is thus among the best-established dimensions of the retinal image implicated in refractive development. This is significant because most retinal neurons are poorly suited for encoding luminance. The retina has evolved adaptation mechanisms that filter out responses to continuous background illumination in order to enhance spatio-temporal contrast. Due to lateral inhibition (Hartline & Ratliff, 1958; Kuffler 1953) and other mechanisms, most retinal neurons are unreliable reporters of environmental illumination (Barlow & Levick, 1969).
However, there is a specialized retinal network that does encode steady-state light intensity (Figure 6-10, panel A). This system was first probed as early as the 1960s (Barlow & Levick, 1969), but has been studied particularly intensively since the discovery of the intrinsically photosensitive retinal ganglion cells (ipRGCs; Aranda & Schmidt, 2020; Do et al., 2019). ipRGCs are unique among retinal ganglion cells (RGCs) in their capacity to respond directly to light, much like rod and cone photoreceptors, using melanopsin as their photopigment. The ipRGCs are also highly unusual among RGCs in their capacity to signal how much total visible light is in the environment. They encode light intensity stably over many hours and distribute this nerve signal to diverse brain regions. Their outputs to the brain drive constriction of the pupil, phase shifts of circadian rhythms, and reductions in melatonin levels in the bloodstream (Aranda & Schmidt, 2020; Do, 2019), among many other functions.
The ‘luminance network’ also includes the dopaminergic amacrine cells (DACs; Figure 6-10A). DACs, like ipRGCs, encode light intensity. Brighter ambient light triggers more DAC nerve-impulse spiking (Raviola, 2002; Zhang et al., 2008, 2017). This also increases dopamine
release, which may act as a retino-scleral stop signal in refractive development (Norton & Siegwart, 2013; Schaeffel & Feldkaemper, 2013).
Both DAC and ipRGC signals are driven by light through a specialized component of the ON pathway (Figure 6-10A), that appears optimized for luminance coding and is highly conserved among mammals, including humans. This network encompasses a remarkable number of the retinal components implicated in eye-growth regulation in mammals, including dopamine, the ON pathway, and melanopsin. It also establishes a conceptual bridge to a much broader and burgeoning field of academic, clinical, design, and policy work informed by the effects of light on health. Examples in this field include sleep and circadian health, lighting and architectural design, human factors and shift work, and phototherapy for depression (Lucas et al., 2014). It is becoming increasingly clear that inadequate (or ill-timed) activation of this retinal luminance network, a hazard of contemporary lifestyles in urbanized environments, threatens physical and mental well-being in diverse ways.

NOTES: (A) Elements of the luminance network. Key synaptic connections are confined to the two gray ‘luminance’ sublayers, both driven by the ON pathway. The bottom (innermost) sublayer is the conventional ON sublayer. It is supplied with excitatory input from the main axon terminal field of certain types of ON bipolar cells. For simplicity, only a single type (“6”) is shown here, in reference to mouse bipolar Type 6 and its probable primate equivalent, DB6. The upper luminance stratum—the “accessory ON sublayer”—lies (surprisingly) at the top margin of the inner plexiform layer (IPL), within a part of the OFF sublayer. There, descending ON bipolar axons that also supply the lower luminance band make en passant synapses in the accessory ON sublayer on their way by. Targets of this accessory ON channel drive include the dopaminergic amacrine cell (DAC; red) and some intrinsically photosensitive retinal ganglion cells (ipRGCs), including the M1 type (blue). Like all ipRGCs, M1 cells can respond directly to light through melanopsin phototransduction. Some DACs receive glutamatergic input from intra-retinal axons of M1 cells (not shown). The M2 ipRGC type (lavender), like most ipRGCs other than M1s, receives its synaptic input in the conventional ON sublayer. This network encodes environmental luminance much more faithfully than other retinal networks do. (B) Elements of the visual motion network. Key synaptic connections are largely confined to the two pink “motion” sublayers. The lower one sits in the middle of the ON sublayer and receives input from a set of ON bipolar cells (yellow; “5”) distinct from those serving the luminance network. The upper ‘motion’ stratum is served by the OFF pathway, with input from axon terminals of OFF bipolar cells (purple; “3”). Starburst amacrine cells (SACs; green, with ON and OFF varieties) make their synaptic connections mainly within these two bands. Direction-selective retinal ganglion cells (“DS RGCs”; burgundy) are bistratified, with dendrites in both bands. They receive input from ON and OFF bipolar cells there, but also from the SACs. GABA inhibition from SACs to DS RGCs exhibits a highly ordered spatial asymmetry that tunes DS RGCs to
SOURCE: Committee generated.
Dopamine as a Stop Signal for Refractive Eye Growth
A growing body of work indicates that dopamine signaling in the retina is necessary for emmetropization and that the release of dopamine by DACs is protective for myopia development (Feldkaemper & Schaeffel, 2013; Mazade et al., 2024; Stone et al. 1989; Zhou et al. 2017). Experiments from multiple vertebrate species suggest that decreased retinal concentrations of dopamine are associated with increased ocular growth and myopia development, while increased retinal dopamine concentrations are associated with a slowing of ocular growth (Feldkaemper & Schaeffel, 2013). This is based on high-performance liquid chromatography results of retinal or vitreal dopaminergic amacrine (DA) and/or DOPAC (DA metabolite) that show reduced levels in response to form deprivation in primates (Iuvone et al., 1989), chickens (McBrien et al., 2001; Papastergiou et al., 1998; Stone et al., 1989), and guinea pigs (Dong et al., 2011), and negative lens defocus in chickens (Guo et al., 1995; Ohngemach et al., 1997). Moreover, it is well-established that retinal dopamine synthesis is stimulated by light, and a number of studies have shown that outdoor activity and/or bright light inhibits myopia, potentially through dopamine-mediated mechanisms (Ashby & Schaeffel, 2010; Chen et al., 2017; Cohen et al., 2012; Feldkaemper et al., 1999; Landis et al., 2021).
If dopamine levels and/or signaling are decreased during myopic eye growth, then increasing DA levels or DA receptor activity would be predicted to prevent myopia. This prediction is supported by experiments in which dopamine or dopamine agonists have shown protective effects on myopia development (Ashby et al., 2007; Brown et al., 2022; Dong et al., 2011; Iuvone et al., 1991; McCarthy et al., 2007; Rohrer et al., 1993; Schmid & Wildsoet, 2004; Stone et al., 1989; Yan et al., 2015). Additionally, dopamine antagonists (Wu et al., 2016) or mice genetically manipulated to eliminate tyrosine hydroxylase, a dopamine precursor (Bergen et al., 2016) have shown increased myopia susceptibility when dopamine levels are low. Together these studies suggest that DA receptor activation is needed for normal refractive eye growth under challenging/abnormal visual conditions (form deprivation or lens defocus) and that increasing DA levels in the eye can prevent myopic growth signals.
However, the role of dopamine in the control of postnatal ocular growth is likely complicated, as not all experiments to activate or inhibit dopamine signaling have similar results. For instance, treatment with the dopamine receptor antagonist 6-hydroxydopamine and the catecholamine depleting agent, reserpine, prevented (rather than facilitated) development of form-deprivation myopia in chickens by reducing axial eye growth (Schaeffel et al., 1995). Interestingly, dopamine agonists are not as effective for lens-induced myopia across multiple species (Ashby et al., 2007; Dong et al., 2011; Iuvone et al., 1991; McCarthy et al., 2007; Rohrer et al., 1993; Schmid & Wildsoet, 2004; Stone et al., 1989; Yan et al., 2015). Additionally, dopamine does not seem to affect eye growth under normal conditions, but only under myopigenic conditions as in the case of form-deprivation and lens-induced myopia (Dong et al., 2011; Junfeng et al., 2010; Landis et al., 2020; Rohrer et al., 1993; Yan et al., 2015). These findings suggest that modulation of visually driven eye growth is not simply due to the level of retinal dopamine.
Dopaminergic Amacrine Cells and ipRGCs—Irradiance-coding Cells and Circuits
DA cells are a type of retinal interneuron found in all vertebrate retinas. These widefield polyaxonal spiking amacrine cells are the sole source of dopamine within the eye (Witkovsky, 2004). Ample evidence from animal studies supports a role for dopamine as a retino-scleral signal in emmetropization (see review from Brown et al., 2022; Figure 6-10A). Mice lacking dopamine through tyrosine hydroxylase knockout (Bergen et al., 2016) or toxin administration (Wu et al., 2016) develop myopia. Both form-deprivation myopia and lens-induced myopia lower dopamine and DOPAC levels in most species tested (Dong et al., 2011; Guo et al., 1995; Iuvone et al., 1989; Ohngemach et al., 1997; Papastergiou et al., 1998; Stone et al., 1989; Sun et al., 2018). Dopamine receptor agonists or the dopamine precursor, L-DOPA, prevent form-deprivation myopia and to a smaller extent lens-induced myopia (Gao et al., 2006; Junfeng et al., 2010; Landis et al., 2020; Mao et al., 2016; Mao & Liu, 2017). The protective effect of high luminance on chick lens-induced myopia was abolished by a dopamine antagonist (spiperone; Ashby & Schaeffel, 2010). It is noteworthy that in some studies, manipulations of dopamine signaling only appear to affect axial elongation when visual input is disrupted (Landis et al., 2020; Mao et al., 2010).
Taken together, the evidence strongly backs the conclusion that dopamine acts as a ‘stop’ signal for axial elongation (Feldkaemper & Schaeffel, 2013) with roles in diverse myopia models. Dopamine is thus a key neural regulator of growth, though probably not the only one.
How does dopamine put the brakes on eye growth? DA diffuses throughout the retina, and although it may not actually reach the sclera, it does affect choroidal thickness (Mathis et al., 2023), which in turn is altered in many myopia models. This seems to be an indirect effect, since the choroid doesn’t express DA receptors. The RPE may be the link between DACs and the choroid; RPE cells do express DA receptors (Dearry et al., 1990; Gallemore & Steinberg, 1990; Mathis et al., 2023).
Overall, the findings suggest that DA signaling is important for regulation of eye growth, but that its role is complex and best understood in the context of visually driven myopia and a broad retinal and ocular network of cellular intercommunication (see the section on the role of RPE below; Brown et al., 2022; Feldkaemper & Schaeffel, 2013; Mazade, Palumaa, & Pardue, 2024; Zhou et al., 2017). These findings implicate DACs in mechanisms of myopia pathogenesis, but these amacrine cells also play important roles in intrinsic circadian regulation of ocular tissues. Circadian mechanisms in turn have been suggested as playing some role in emmetropization (Chakraborty et al., 2018; Stone et al., 2020), so DACl may be implicated in this context as well. DACs are also key players during light adaptation and in regulating intercellular gap-junctional coupling among retinal cells (Goel & Mangel, 2021; McMahon & Dowling, 2023; Witkovsky, 2004).
DACs are ON-type retinal neurons: they respond to light increments by depolarizing and increasing their spike rate. There are many varieties of ON-type amacrine and ganglion cells, but DACs are unusual among them in having very sustained responses to steady illumination. Their firing rate is proportional to light intensity, so they can be said to encode luminance (Zhang et al., 2008). Increased spiking in DACs results in increased release of dopamine from their varicosities. This released dopamine acts in paracrine fashion at sites widely distributed within the retina, serving as the main signal for the transition of retinal circuitry from scotopic (dim light stimuli that activate only rod photoreceptors) to photopic (bright light stimuli that activate mainly cone and ipRGCs photoreceptors) vision (Jackson et al., 2012; see review in Bloomfield
& Volgyi, 2009). Paracrine signaling suits dopamine well for affecting other layers of the ocular globe, perhaps very far from the site of release (Popova, 1995).
The ON cone bipolar drive to DACs is unusual. Bipolar inputs to DACs, as to all RGCs and amacrine cells, are made in the inner plexiform layer (IPL), a synaptic layer in the inner retina interconnecting bipolar, amacrine and ganglion cells (Figure 6-10). OFF bipolar cells make their outputs in the outer (sclerad) part of the IPL (the “OFF sublayer”) and ON bipolar cells do so in the inner (vitread) part of the IPL (the “ON sublayer”). Surprisingly, though both DACs and M1 ipRGCs are ON cells, their dendrites are found in the outermost margin of the IPL, abutting the cells of the inner nuclear layer, in what should be the OFF sublayer. The resolution of this paradox is that these two ON cell types get their excitatory ribbon synaptic input from en passant synapses from the axonal shafts of ON cone bipolar cells passing through the OFF sublayer on their way to the ON sublayer of the IPL (Dumitrescu et al., 2009; Hoshi et al., 2009). This thin isolated extra layer of ON-channel output has been called the ‘accessory ON sublamina.’
The en passant output synapses of ON cone bipolar cells have a highly unusual ultrastructure, with multiple synaptic ribbons and only a single postsynaptic partner, unlike the dyad synapses found elsewhere in the IPL. These specializations may optimize the circuit’s ability to stably encode irradiance. Segregation of this part of the ON channel in this way almost certainly means that this bipolar input is subject to a very different sort of pre- and post-synaptic amacrine-cell inhibition. This may help to explain why M1 cells have among the weakest receptive-field surrounds of any RGCs (Zhao et al., 2017). Bipolar drive to the DAC/ipRGC system comes from a subset of ON cone bipolar types. These appear to be reliable encoders of retinal irradiance (Sabbah et al.). They are also among those receiving the strongest input from the primary rod system (Demb & Singer 2012). This may relate to the outsized role of rods in driving dopamine release (Cameron et al., 2009; Pérez-Fernández et al., 2019; Zhou et al., 2017).
DACs contribute significantly to light-adaptation mechanisms in the retina. Dopamine levels are high in the presence of light, but they are also higher during the biological day regardless of lighting due to autonomous intraretinal circadian rhythmicity (see review in Ko, 2020). Retinal melatonin exhibits the inverse relationship to light exposure and circadian phase (Ko, 2020). Dopamine is a master regulator of retinal sensitivity, helping to shift the retina into a light-adapted state optimized for daylight conditions by influencing many specific neuronal types and their intercommunications with each other. The capacity of the DAC to link light intensity to diverse actions and physiological effects and to do so at remote locations through paracrine signaling seems nicely matched to the requirements of retino-scleral signaling for refractive eye growth. Nevertheless, mechanistic details have not been identified at this point.
One way dopamine affects retinal sensitivity is by regulating gap junctions throughout the neural retina. One key circuit regulated in this way is the “primary rod pathway,” which is crucial for conveying the most sensitive rod signals to ganglion cells. The gap junction in question is known as connection no. 36, encoded by the Gjd2 gene, which is significantly linked to myopia in GWAS studies (Chen et al. 2012; Solouki et al. 2010; van der Sande et al., 2022).
In mice, a subset of DACs receives a second source of glutamatergic excitatory ON input from intraretinal axons of ipRGCs, branching off their main axon en route to the optic nerve. This input can sustain light responses in these DACs even in the absence of functional rod and cone input, through melanopsin phototransduction by M1 ipRGCs and their glutamatergic synapses onto DACs (Munteanu et al., 2018). M1 cells themselves receive this unusual ON bipolar drive in the accessory ON sublayer. Thus, mouse DACs may receive the specialized ON
drive not only directly from the bipolar cells themselves but also indirectly through their inputs from M1 cells. Mouse models indicate that ipRGCs play a role in emmetropization. Eliminating ipRGC photosensitivity (through melanopsin knockout) or killing ipRGCs (using diphtheria toxin) impacts myopigenesis in mice, at least partly through DACs (Chakraborty et al., 2022; Liu et al., 2022; Mazade et al., 2024). Surprisingly, dopamine release in the retina is apparently neither driven by nor dependent on melanopsin (Cameron et al., 2009). In fact, rods contribute much more than cones to the light-evoked release of dopamine (Pérez-Fernández et al., 2019).
The similarities in structure and function between M1 ipRGCs and DACs are remarkable. As already noted, both receive specialized ON channel input and are unusual among inner retinal neurons in encoding irradiance (luminance). They deploy their dendrites in the same narrow sublamina of the IPL—the accessory ON sublayer. Genetic manipulations that alter the stratification of DACs produce similar alterations in M1 ipRGCs (Matsuoka et al., 2011). Both are unusual among amacrine and ganglion cells in sometimes extending processes into the outer plexiform layer. Further, M1s and DACs reciprocally influence one another, through dopamine receptors on the M1 cells (Van Hook et al., 2012) and glutamatergic synapses from M1 axons onto the DACs (Zhang et al., 2008). Though some DACs exhibit transient light responses, sustained DACs encode light intensity just as ipRGCs do, through a mix of contributions from rods, cones, and melanopsin.
Diverse Roles of the ON Pathway in Luminance Coding and Myopia Development
Complete congenital stationary night blindness (cCSNB) is a genetic disorder that disrupts dim light vision, among other abnormalities. It is linked to mutations in genes required for signaling at the photoreceptor-to-ON-bipolar synapse. This specialized synapse inverts the sign of the voltage response to light, changing it from hyperpolarizing (OFF) in the rod and cone photoreceptors to depolarizing (ON) in the postsynaptic bipolar cells. The ON bipolar cell response (as reflected in the electroretinogram [ERG] b-wave) is perturbed in diverse mutations that disrupt this signaling process in ON bipolar cells, from photoreceptor synapse, with their metabotropic glutamate receptors through their depolarizing light response, reflecting an excitatory cation conductance through transient receptor potential M1 channels (Pardue & Peachey, 2014). All these mutations share a myopic phenotype and a severely disrupted b-wave (an ON-bipolar-cell-mediated ERG component). The consistent appearance of myopia in cCSNB across diverse genetic contexts provides strong evidence in favor of the ON pathway itself playing an integral role in the development of myopia (reviewed in Hendriks et al., 2017; Mazade et al., 2024; Zeitz et al., 2023).
A recent analysis suggests that the ON pathway is relatively understimulated in reading as compared to walking, perhaps a link to the protective effects of time spent outdoors on myopia prevalence (Poudel et al., 2023). Additionally, another study suggests ON pathways are less responsive in myopic eyes based on ERG recordings (Poudel et al., 2024), and this is supported by patch clamp recordings from myopic mouse eyes (Mazade & Pardue, 2023).
The ON pathway is of special relevance to both scotopic and short-wavelength sensitivity. In mammals, two distinct photoreceptor channels in the retina—for rods and for short-wavelength cones—share a unique dependence on the ON pathway. The most sensitive ‘primary’ rod pathway is entirely dependent on a dedicated bipolar type for scotopic vision—the rod bipolar cell (Demb & Singer, 2012). This is an ON type bipolar cell type, sharing the same ‘sign-inverting’ metabotropic glutamate receptors as cone bipolar cells of the ON type. Though rods have other ways of signaling, disruption of the rod bipolar signal will elevate the threshold
for detecting light in dim conditions. cCSNB mutations that disrupt ON pathway signaling silence the rod bipolar cell’s light response and have particularly severe impacts on dim-light vision. Likewise, there is a specific type of bipolar cell that appears to be the sole conduit of pure signals derived from the short-wavelength cones. This type appears conserved across mammalian retinas (Haverkamp et al., 2005). It too is an ON type bipolar cell. Thus, the “ON-channel defect” in congenital stationary night blindness should also be viewed as differentially impacting not only the sensitive rod vision but also the short-wavelength chromatic channel.
Several genes linked to high myopia are associated with the first synapse in the ON pathway linking rods and cones to ON bipolar cells. This involves a ‘sign-inverting’ metabotropic glutamate receptor (mGluR6), which regulates current flow through a transient receptor potential channel (Mazade et al., 2024; Zeitz et al., 2023). As mentioned above, another gene linked to myopigenesis is Gjd2, which codes for the gap junctional protein connexin 36. Connexin 36 gap junctions are required for signal transmission of the most sensitive (‘primary’) retinal rod pathways. They mediate the transmission of sensitive rod signals from AII amacrine cells to cone bipolar axon terminals in the IPL. Knockouts of connexin 36 are thus one mouse model of cCSNB (Demb & Singer, 2012; van der Sande et al., 2022). However, connexin 36 is expressed in diverse types of retinal neurons (Massey et al. 2003; van der Sande et al., 2022) and also in the RPE (Fadjukov et al., 2022), so the contribution of rod signaling defects to the myopigenic effects of Gjd2 mutations remains to be determined.
Though the available data strongly implicate the ON channel, the OFF channel might also carry beneficial effects of time outdoors. There are diverse ways of selectively silencing the ON channel, but not so for the OFF. Is the present focus on the ON-channel contribution simply a reflection of this, or a real asymmetry? If the OFF channel were selectively silenced, would there be the same sort of effects as with ON defects because it would similarly disrupt ON/OFF balance? Mice whose OFF pathway was partially silenced through a Vsx1 mutation had normal ocular development and blunted responses to form deprivation (Chakraborty et al., 2014). It would be very informative to know if the ON channel is necessary and sufficient for the effects of image properties on the sclera.
Evidence for Neural and Ocular Cell Populations in Eye-growth Regulation
The Brain
As noted above, numerous studies using optic-nerve crush to block retinal output indicate that higher-order visual processing in the brain is not required for the eye’s response to myopigenic alterations in visual input (Choh et al. 2006; Gastinger et al., 2006; Troilo & Wallman, 1991; Troilo et al., 1987; Wildsoet & Wallman, 1995). Further, form-deprivation myopia can be induced in both chicks (McBrien et al., 1995) and tree shrews (Norton et al., 1994) after intravitreal injections of the sodium-channel blocker tetrodotoxin. Although subtle impacts of nerve crush on eye-growth regulation have been reported in mice, guinea pigs, and some species of non-human primates (Gong et al., 2020; McFadden & Wildsoet, 2020; Raviola & Wiesel, 1990), even these subtle impacts may ultimately be traceable to the retina. For example, severing the optic nerve triggers degeneration in most retinal ganglion cells, and likely compromises the central retinal artery, which supplies the inner retina (Flitcroft, 2012). In a more targeted manipulation, ganglion-cell spiking has been silenced with tetrodotoxin. This did not block the effects of form deprivation on refractive growth in chicks (McBrien et al., 1995; Wildsoet & Wallman, 1995) or tree shrews (Norton et al., 1994). Whether the link between eye and brain is disrupted by cutting optic axons or blocking their spiking, this will affect both eye
movements and accommodation and thus the pattern of spatio-temporal contrast experienced by the retina.
In a related finding, Bitzer & Schaeffel (2006) found that visual stimulation that normally triggered myopia in chicks no longer did so if they were anesthetized by either of two methods. Glucagon-expressing amacrine cells that report the sign of defocus in their expression of an immediate-early-gene in intact alert animals failed to do so in anesthetized animals or in an in vitro preparation (Bitzer & Schaeffel, 2006). There are many possible explanations for these results, but they suggest that active, dynamic vision in a complex world is important for linking retinal images to eye growth, even if the key biological mechanisms lie within the eye. Together, the data strongly suggest that although some influence of central vision cannot be definitively ruled out, the main links between visual experience and eye growth lie within the eye itself and depend on retinal mechanisms.
There are a variety of possible mechanisms through which the brain may contribute to the regulation of ocular growth. First, the brain can modulate the retina by direct neural connectivity. Although the optic nerve carries signals almost exclusively from eye to brain in mammals, there are a few ‘centrifugal’ optic nerve fibers carrying signals from brain to retina. In primates, these comprise only a few hundred axons, arising mainly from small populations of hypothalamic neurons (Gastinger et al., 2006). This sparse, diffuse retinal innervation seems poorly suited to encoding spatial contrast or retinal location, but it could contribute to the representation of other stimulus features controlling eye growth such as luminance, for which detailed information about spatial structure is not essential.
A second opportunity for contributions from central visual mechanisms comes in the context of accommodation, through which the brain dynamically adjusts the optical power of the eye’s crystalline lens under both reflexive and voluntary control, for example, by ‘active vision’ through gaze shifts to locations at different depths in extrapersonal space and by vergence-accommodation coupling in the ‘near reflex.’
The brain’s control over accommodation is exerted through the autonomic nervous system, which may contribute in other ways to the regulation of eye growth. For example, brainstem autonomic centers control choroidal blood flow through a nitric oxide signaling mechanism (Li et al., 2016; Reiner et al., 2018). Surgical interruption of the autonomic innervation of the chick eye alters normal circadian rhythms in eye growth and choroidal thickness, both of which have been linked to myopia mechanisms (Li et al., 2016; Nickla & Schroedl, 2019).
There are still more factors to consider. The brain’s oculomotor system is responsible for making diverse patterns of voluntary and reflexive eye movements, both conjugate and vergence, and either fast or slow through the actions of the extraocular striated muscles. Accommodation and pupil diameter are dynamically regulated through intraocular smooth muscles. The patterns of these movements may differ between indoor and outdoor environments (see previous chapter).
Finally, whole-body movements through the world alter the structure and dynamics of the retinal image—for example the ‘optic flow’ that occurs during walking or driving. In this sense, the brain actively shapes the visual diet that the retina is exposed to. Beyond this, complex higher-level brain mechanisms govern decision-making, including whether to opt for an outdoor or indoor environment over the course of the day.
Still, the weight of evidence suggests that the effects of retinal images on eye growth are mostly an intrinsic regulatory function of the eye itself. If their outputs to the brain are not essential, are the RGCs themselves required for the process in some other way? ipRGCs make
glutamatergic synapses onto DACs through intraretinal axons. Both ipRGCs and conventional RGCs are also linked to many diverse amacrine cell types through gap junctions. Blocking RGC spiking would presumably affect “centrifugal” (intraretinal) influences to some extent, but this had no effect on refractive development. Some role for ipRGCs in the effects of luminance on refractive development seems likely, as discussed above. On balance, though, there seems little compelling evidence that RGCs play a critical role in linking retinal images to scleral growth-control mechanisms. Certainly, RGCs are not required for other retinal cell types to encode luminance, spatial contrast, or wavelength.
Ganglion and Amacrine Cells
As discussed above, a luminance network involving both amacrine-cell (DACs) and ganglion-cell (ipRGC) components has been linked to the protective effects of daylight outdoors (Chakraborty et al., 2018, 2022; Feldkaemper & Schaeffel, 2013; Mutti et al. 2020; Zhou et al. 2017). New evidence implicating neuropsin (Opn5) in the protective effects of violet light appears to implicate RGCs as well, because within the retina this atypical opsin is apparently selectively expressed in ganglion cells (D’Souza et al., 2022). More generally, while the brain clearly relies on RGCs for information about the retinal image, it might not be the case that the brain’s activity is involved in the mechanisms linking retinal images to eye growth.
For example, blocking nerve impulses in RGCs with the sodium-channel blocker tetrodotoxin stops communication between retina and brain but leaves the effects of image perturbation on eye growth intact (McBrien et al. 1995; Norton et al. 1994). In other words, while brain visual mechanisms are entirely dependent on RGCs for information about the retinal image, linkage with the brain is not necessary for the effects of visual experience on eye growth. Experimentally ablating various collections of chick retinal neurons by various means triggers changes in eye growth and alters the ocular response to defocus or blur (Bitzer & Schaeffel, 2004; Ehrlich et al., 1990; Fischer et al., 1997, 1998). Further, in chicks, specific retinal interneurons exhibit differential molecular responses to hyperopic and myopic defocus imposed in vivo (Fischer et al., 1999). These are a subtype of amacrine cell that expresses the insulin-related hormone glucagon.
Photoreceptors and Bipolar Cells
As mentioned earlier, mutations in genes disrupting retinal signaling in mice result have been found to result in myopia and/or effect dopamine levels (Nob [nyx]; Pardue et al., 2008; mGluR6 [Grm6]; Chakraborty et al., 2015); and Lrit3 and GPR179 (Mazade et al., 2024; Zeitz et al., 2023). Similarly, retinal degenerative diseases are frequently associated with high myopia in humans and mice (Hendriks et al., 2017; Park et al., 2013; see Chapter 5). Mutations disrupting signaling between photoreceptors and ON bipolar cells are also associated with myopia (Zeitz et al., 2023). The loss of rod function in transgenic mice prevents experimental myopia (Park et al., 2014), while the loss of cone function (Chakraborty et al., 2019) or melanopsin function (Chakraborty et al., 2022) caused increased myopic shifts.
Why In Vitro Models Are Poised to Advance This Field
Whatever the brain’s role, if it were hypothesized that the eye can autonomously link retinal images to local changes in eye growth, the key biological processes would be narrowed to a small and accessible piece of tissue. The question from a neurobiological perspective is then, Which cells are critically involved in linking image data to scleral output? Current technology
makes it relatively easy to monitor the excitability of many types of retinal neurons and how visual stimulus patterns alter retinal activity. In the context of myopigenesis, data from the chick model indicate that the scleral response to image manipulation can be remarkably fast—on the order of hours or less. Surprisingly, the one study that attempted to measure this found that lens defocus effects did not occur in vitro, nor in intact animals anesthetized by either of two different methods (Bitzer & Schaeffel, 2006). It seems important to try to develop a stronger empirical grounding for the idea that there is an autonomous ocular process of refractive growth. If myopigenesis could be captured in a dish, a wealth of imaging, electrophysiological, and pharmacological tricks could be used to corner the key biological processes.
Retinal Mechanisms for Encoding Defocus
Most retinal neurons are sensitive to spatial and temporal contrast. Tuning differs from one neuronal type to the next and is typically modulated by visual context. Blur reduces spatiotemporal contrast, which will reduce the responsiveness of most retinal cells. Thus, at least in this very general sense, the retina can encode blur. Hyperopic and myopic defocus of the retinal image similarly blur the image in the photoreceptor plane, but they exert opposite effects on eye growth. The eye detects and homeostatically minimizes defocus through differential eye growth (emmetropization). Thus, though defocus and blur share some features, they are not the same. Since the connection between eye and brain is not required for the differing effects of hyperopic and myopic defocus, the retina must encode the sign of defocus, but how?
There are cues in the visual image that might permit direct and real-time neural discrimination between myopic and hyperopic defocus, but such retinal neurons that can discriminate the sign of defocus have not yet been identified. The closest “defocus detector” neuron to be identified so far has been the finding that glucagonergic amacrine cells (GA cells) in chicks encode the sign of defocus in the levels of expression of ZENK (a.k.a. zif268 or Egr-1), an immediate early gene (ZENK [a.k.a. zif268; Egr-1]; Bitzer & Schaeffel, 2006; Brand et al., 2007). GA cells are killed by colchicine, which enhances axial eye growth, but not by other agents, such as quisqualate and ethylcholine mustard, that fail to have this effect despite killing many other amacrine cells (Fischer et al. 1999). This evidence implicates GA cells as key players in regulating chick eye growth.
However, these effects have not been reproduced in any in vitro experiments, nor when the chicks were anesthetized (Bitzer & Schaeffel, 2006). Thus, much remains uncertain about how these amacrine cells encode the sign of defocus and how these signals might engage scleral growth mechanisms. The relevance of this avian glucagon story for human emmetropization is therefore unclear. Mammals appear to lack a glucagon-expressing amacrine cell type, though a cell of similar function but lacking glucagon expression might exist (Mathis & Schaeffel, 2007).
The effects of defocus on eye growth may not require the presence of neurons carrying a real-time sign-of-defocus signal. Dynamics in accommodation and/or circadian rhythms in eye growth could also provide cues by modulating the responsiveness of contrast-sensitive retinal neurons on different time scales.
It seems important to know whether the key effects of defocus on eye growth can be recapitulated in an in vitro context where these other dynamic factors would play no role. In other words, a key unresolved question is whether the retina autonomously computes some internal representation of the sign of defocus from the retinal image (Schaeffel & Wildsoet, 2013).
In relation to evidence that nitric oxide (NO) may be a key retino-scleral signal (see below), it may also be relevant that the retina is one source of ocular nitric oxide synthase (NOS) (Lee et al., 2023). Very specific sets of amacrine cells express neuronal nitric oxide synthase (nNOS; Jacoby et al., 2018; Park et al., 2020).
Chromatic Mechanisms
As is mentioned above, LCA is a property of the retinal image that could be used to compute the sign of defocus (see Effects of Chromacity in Chapter 5). These experiments have shown that the spectral influence on myopia susceptibility is species-dependent. For instance, short-wavelength (blue) light slows eye growth and is protective for experimental myopia in chickens and guinea pigs (Foulds et al., 2013; Jiang et al., 2014; Liu et al., 2011; Long et al., 2009; Rohrer et al., 1992; Schaeffel et al., 1991; Seidemann & Schaeffel, 2002; Torii et al., 2017; Wang et al., 2011), but long wavelength (red) light has this effect in Rhesus monkeys and tree shrews (Gawne et al., 2017; Hung et al., 2018; Liu et al., 2014; She et al., 2023; Smith et al., 2015). Gawne & Norton (2020) have developed a model, based on opponent long-and short-wavelength-sensitive contrast channels, to explain how LCA could be read out by the nervous system to control eye growth. However, emmetropization is observed in chicks reared in quasi-monochromatic environments, so chromatic cues are apparently not necessary (Smith et al., 2015). In addition, the differences in chromatic sensitivity for eye growth between close mammalian species do not lend support for this to be an evolutionarily conserved pathway for refractive eye growth.
Short-wavelength light is abundant in daylight and is particularly effective in activating unconventional retinal opsins, namely melanopsin (Opn4) and neuropsin (Opn5), both of which have been linked to mechanisms of myopia (Chakraborty et al., 2022; D’Souza et al., 2021; Jiang et al., 2021; Linne et al., 2023). These experiments have implicated Opn5 in the protective effects of violet light (Jiang et al., 2021) and shown that violet light had a diurnal effect, with evening hours being the most protective in the mouse model. The protective effects of blue light in a mouse model were eliminated in a mouse with dysfunctional cones (Strickland et al., 2020).
Chromatic encoding in the retina is a cone-based process and operates best in daylight conditions. There are numerous types of wavelength-sensitive RGCs, with patterns that differ dramatically across myopia model organisms. All mammals share a blue-yellow opponent mechanism in the inner retina which seems well suited to some aspects of encoding LCA. Short-wavelength-cone-specific bipolar cells appear to be a conserved feature of mammalian retinal organization (Haverkamp et al., 2005), and they are a type of ON bipolar cell. Some of these contribute to conscious color vision (Dacey & Lee, 1994)
RETINA-TO-SCLERA SIGNALING CASCADE: THE ROLES OF THE RETINAL PIGMENT EPITHELIUM (RPE), CHOROID, AND SCLERA IN POSTNATAL OCULAR GROWTH AND MYOPIA DEVELOPMENT
Convincing evidence over the past 40 years indicates that postnatal eye growth is largely controlled by an intraocular retina-to-sclera chemical cascade (see Figure 6-10). This cascade is initiated by the quality of visual images on the retina, leading to molecular changes in the retina, the RPE and choroid, ultimately effecting changes in the sclera through scleral extracellular matrix synthesis and scleral biomechanics, resulting finally in eye shape. While many aspects of
this cascade remain to be determined, discussed below are key findings that have elucidated some elements of this process.
RPE
The RPE has been implicated in refractive eye development, as it is located immediately adjacent to the retina, where it can relay any retina-derived growth regulatory signals to the choroid and sclera. The structure of the RPE consists of a single layer of pigmented cells that are interdigitated with the photoreceptor outer segments. The RPE has important functions for the regeneration of visual pigments and ionic transport (Strauss, 2005). Moreover, the RPE is known to be a major source of cytokines and growth factors (Strauss, 2005) and several studies have demonstrated differential expression of several genes in the RPE during changes in visually guided eye growth (Zhang et al., 2012, 2013, 2019).
Morphological changes have been reported in the RPE of experimental animals with induced myopia (Fleming et al., 1997; Harman et al., 1999; Lin et al., 1993). In chick and mammalian models of myopia, increases in the total area of the RPE layer were coupled with an increase in the surface area of individual RPE cells without increases in RPE cell number, as a mechanism of maintaining the coverage of the expanded globe (Fleming et al., 1997; Harman et al., 1999; Lin et al., 1993). Furthermore, in chick eyes allowed to recover from form-deprivation-induced myopia, significant edema and altered basal infoldings in the RPE along with thickening of Bruch’s membrane were noted (Liang et al., 1996). Involvement of the RPE in refractive eye growth has also been implicated in co-culture experiments, in which the presence of the RPE stimulated a proliferation of scleral fibroblasts (Seko et al., 1994, 1997). Moreover, the presence of the RPE was required in eye cup preparations to mediate the effects of insulin on choroid thickening and scleral glycosaminoglycan (GAG) synthesis in eyes recovering from myopia (Sheng et al., 2013). Taken together, these animal studies indicate that the RPE may, at least in part, participate in the retina-to-sclera signaling cascade in vivo to regulate ocular growth postnatally.
Evidence for Choroidal Involvement
The choroid is a complex tissue, consisting of a rich blood supply, lymphatic vessels, stromal cells, intrinsic choroidal neurons, extravascular smooth muscle, and axons of sympathetic, parasympathetic, and sensory neurons (Nickla & Wallman, 2010). Of much interest are the choroidal changes associated with emmetropization and the control of postnatal ocular growth, as any retinal-derived scleral growth regulator must pass through the choroid, or act on the choroid to synthesize additional molecular signals that can subsequently act on the sclera to stimulate scleral extracellular matrix remodeling.
Several studies have characterized the choroidal changes associated with recovery and compensation for myopic defocus. In chickens, choroidal thickening (Wallman et al., 1995), increased choroidal permeability (Pendrak et al., 2000; Rada & Palmer, 2007), and increased choroidal blood flow (Fitzgerald et al., 2002; Jin & Stjernschantz, 2000) have been well documented during recovery from induced myopia. In guinea pigs, choroidal thinning is associated with lens-induced myopia (Yu et al. 2021) and form deprivation (FD; Chen et al. 2022, 2024). Choroidal thinning is accompanied by decreased blood vessel density and reduced choroidal blood flow (Che et al., 2024). Similar to chicks, recovery from form deprivation myopia is associated with choroidal thickening and increased choroidal vessel diameter (Chen et
al., 2022). Additionally, during recovery or compensation to positive lenses, increases have been observed in the choroidal synthesis of interleukin 6 (IL-6) retinoic acid (Mertz & Wallman, 2000; Summers & Martinez, 2021; Troilo et al., 2006), the retinoic-acid synthesizing enzyme ALDH1a2 (Harper et al., 2016), ovotransferrin (Rada et al., 2001), and apolipoprotein A-I (Summers et al., 2016). Of these four, retinoic acid is the most promising candidate as a direct scleral growth regulator (discussed below).
In addition to the choroidal vascular and chemical changes associated with changes in ocular growth, the overall thickness of the choroid has been shown to be modulated in response to visual stimuli. Wallman et al. (1995) were the first to show that choroidal thickness is modulated in response to optical defocus in chickens, becoming thicker with myopic defocus (image in front of the retina), thereby pushing the retina forward toward the image plane, and thinner with hyperopic defocus (image behind the retina). Choroidal thickness changes have subsequently been demonstrated in mammalian models of myopia and in humans, although choroidal changes are small (< 50 micrometers) in primates, contributing to less than a 1 D change in refractive error (Troilo et al., 2000a). It has been suggested that the modulation of choroidal thickness is one mechanism to rapidly adjust the position of the retina closer to the focal plane, moving the retina proximally under conditions of myopic defocus, and distally with hyperopic defocus. It has also been suggested that choroidal thickness can mediate the scleral response. For example, if a thicker choroid provides a greater diffusional barrier to a stimulatory growth factor secreted by the retina or retinal pigment epithelium (Nickla & Wallman, 2010), or if it affords greater protection from stretching of the sclera by the intraocular pressure (van Alphen, 1961, 1986), then scleral growth might decrease after the choroid becomes thicker in myopic eyes. Choroidal thickening has been attributed to changes in the tonus of extravascular smooth muscle, possibly via inputs from both parasympathetic nitrergic and sympathetic adrenergic systems (Poukens et al., 1988), changes in choroidal vascular permeability (Pendrak et al., 2000; Rada & Palmer, 2007), altered transport of fluid from the retina across the RPE (Crewther et al., 2006), and increased synthesis and accumulation of Thyaluronic acid (Rada et al. 2010).
Changes in the Sclera
The elongation of the eye is closely related to the biomechanical properties of the sclera, which in turn are largely dependent on the composition of the scleral extracellular matrix. Therefore, an understanding of the cellular and extracellular events involved in the regulation of scleral growth and remodeling during childhood and young adulthood will provide future avenues for the treatment of myopia and its associated ocular complications.
The Highly Myopic Human Sclera
In highly myopic human eyes, the sclera undergoes significant thinning and gradually expands under the force of normal intraocular pressure. This thinner sclera and elongated globe put individuals at increased risk of serious disorders that can lead to blindness, such as retinal detachment, glaucoma, and macular degeneration (Pan et al., 2013; Qiu et al., 2013). Additionally, in myopia the tensile strength of the sclera is reduced, and the elasticity of the sclera is increased, especially at the posterior pole (Avetisov et al., 1983). The scleral stroma exhibits a more layered, lamellar structure, like that of the cornea (Curtin & Teng, 1958; Funata & Tokoro, 1990; McBrien et al., 1991). Scleral collagen fibril bundles are thinner as compared
with those of emmetropic human eyes, and a preponderance of unusually small-diameter fibrils averaging below 60–70 nm can be found at the posterior pole of the sclera (Curtin & Teng, 1958; Curtin et al., 1979).
Researchers have also observed in the highly myopic eye abnormal collagen fibrils associated with an amorphous cementing substance and the presence of fissured or star-shaped fibrils (Curtin & Teng, 1958; Curtin et al., 1979, 1985). Together, these observations suggest a derangement of the growth and organization of the collagen fibrils in the highly myopic sclera, due to abnormal fibril formation, the accumulation of abnormal non-collagenous extracellular matrix material, and/or the presence of accentuated breakdown or catabolism of the sclera. As proof of concept, mice that lack the glycosylated proteins (proteoglycans) lumican and fibromodulin, or that have a mutation in the lumican gene (L199P) exhibit abnormal scleral collagen fibrils, overall scleral thinning, and increased axial length (Chakravarti et al., 2003; Song et al., 2016). It has been found that sulfated proteoglycans continue to be synthesized and accumulate in the human sclera throughout young adulthood (Rada et al., 2000a). This fact, together with the known functions of sulfated proteoglycans in collagen fibril assembly and organization, suggests that abnormal scleral proteoglycan biosynthesis in childhood and adolescent years may lead to disruption of the normal scleral extracellular matrix and abnormalities in ocular globe size and refraction, either as a result of an intrinsic defect or in response to the visual environment.
Given the important role of the sclera in controlling eye size and refraction, the association of genetic mutations in several scleral extracellular matrix components with the development of high myopia is not surprising. Genes that have been found to be responsible for myopia in association with other genetic syndromes include COL2A1 and COL11A1 for Stickler syndromes type 1 and 2 respectively (Annunen et al., 1999), lysyl-protocollagen hydroxylase for type VI Ehlers-Danlos syndrome (Heikkinen et al., 1997), COL18/A1 for Knobloch syndrome (Mahajan et al., 2010), and fibrillin for Marfan syndrome (Kainulainen et al., 1994; Paluru et al., 2003). Additionally, mutations in the gene, LEPREL1, encoding prolyl 3-hydroxylase 2, a gene responsible for collagen crosslinking, is associated with high myopia in Bedouin Israeli consanguineous kindred (Mordechai et al., 2011). Together, these conditions underscore the importance of extracellular matrix in maintaining scleral integrity, eye size, and refraction.
Scleral Changes in Experimental Myopia
Animal models of myopia have demonstrated an association between the development of induced myopia and recovery and significant changes in scleral collagen and proteoglycan synthesis, accumulation, and turnover (Norton & Rada, 1995; Rada et al., 1991, 2000b). In most vertebrates, an inner layer of cartilage and an outer fibrous layer comprise the sclera. However, in placental mammals (including humans and other primates such as the marmoset, macaque monkey, tree shrew, and mice), the entire sclera consists of the fibrous, type I collagen-dominated extracellular matrix, and the inner layer of cartilage that other vertebrates have is absent. Interestingly, the extracellular matrix molecules that were previously believed to be unique to cartilage, such as aggrecan, PRELP, and cartilage olimeric matrix protein (COMP), have been shown to be present in the mammalian sclera (Young et al., 2003), suggesting that cartilaginous components have been retained in the sclera through evolution and serve important biochemical and biomechanical functions (Coster et al., 1987; Johnson et al., 2006; Rada et al., 1997). In both mammals (Norton & Rada, 1995; Rada et al., 2000b) and birds (Gottlieb et al., 1990; Marzani & Wallman, 1997), the fibrous sclera thins and loses material as ocular elongation
accelerates. In birds, increased growth of the cartilaginous layer of the sclera as the eye elongates is accompanied by an increase in dry weight and in the synthesis and accumulation of proteoglycans (Christensen & Wallman, 1991; Rada et al. 1991). At some level, all vertebrates probably use similar signaling mechanisms to control the sclera, but do so by controlling growth in the cartilage, where it is present, and by controlling remodeling in the fibrous sclera.
Similar to the scleral changes of the highly myopic human eye, described above, myopia development in nonhuman primates and tree shrews is associated with scleral thinning and changes in collagen fiber diameter and organization (Phillips et al., 2000; Rada et al., 2000b), and changes in biomechanical properties of the sclera associated with myopia development have been reported in tree shrews, guinea pigs, mice and chickens (Brown et al., 2022; Grytz & Siegwart, 2015; Hoerig et al., 2022; Lewis et al., 2014; McBrien et al., 2009). In tree shrews, the visco-elasticity in the sclera, as measured as the “creep rate” (continued elongation under a constant tension similar to that produced by intraocular pressure), was shown to increase significantly in form-deprived eyes relative to the contralateral control eye (Siegwart & Norton, 1999; Figure 6-11).
NOTES: Creep rate from deprived eyes (filled circles) and control eyes (open circles), and from eyes recovering from form deprivation myopia (filled triangles) and controls (open triangles) under 1 gram of tension. The data are plotted at the day of treatment when the creep rate was measured (visual experience). The dashed lines are the average creep rate of normal, untreated tree shrew sclera. Single asterisks indicate significant differences (p < 0.05) between the treated and control eye values. Double asterisks indicate significant differences between recovering and control eyes (p < 0.05).
SOURCE: Siegwart & Norton, 1999.
The increase in visco-elasticity would be expected to render the sclera more extensible, so that normal intraocular pressure may produce an enlargement of the vitreous chamber. Remarkably, when unrestricted vision in these animal subjects was restored (recovery), the scleral creep rate decreased rapidly and fell significantly below control levels within 2 days of removal of the diffuser, contributing to a recovery from myopia.
Nevertheless, the macromolecular changes in the sclera, directly responsible for mediating the increase in scleral creep rate during myopia development, have not been identified. Based on the minimal amount of time required to effect changes in scleral visco-elasticity in response to visual stimuli, it is reasonable to speculate that the synthesis and accumulation of
smaller non-collagenous proteins may be altered in the sclera during visually guided ocular growth that may influence collagen fibril interactions and scleral biomechanics.
For example, changes in scleral cross-linking have been suggested to play a role controlling scleral visco-elasticity and ocular elongation. As mentioned above, mutations in prolyl-3-hydroxylase 2, an enzyme involved in collagen cross-linking, are associated with high-axial-grade myopia in an Israeli kindred (Mordechai et al., 2011). Using the tree shrew animal model of form deprivation myopia, McBrien and Norton (1994) demonstrated that prevention of collagen cross-linking, through the systemic administration of β-aminoproprionitrile (β-APN), resulted in markedly exaggerated elongation in myopic eyes as compared to myopic eyes treated with vehicle only. Interestingly, β-APN had no effect on contralateral control eyes, suggesting that additional scleral constituents are involved in restraining ocular elongation under normal visual conditions, even when scleral cross-linking is reduced. These results exemplify the dynamic nature of the sclera and compel investigation into the molecular basis of the visually driven changes in scleral biomechanics.
In the chick model, the outer fibrous layer of the sclera also undergoes remodeling during the development of myopia, as evidenced by an increased expression of matrix metalloproteinase-2 (MMP-2), decreased expression of tissue inhibitor of metalloproteinase (TIMP)-2, an endogenous inhibitor of MMP-2 (Rada & Brenza, 1995; Rada et al., 1999), decreased rate of proteoglycan synthesis (Marzani & Wallman, 1997; Rada et al., 1994), and overall thinning (Gottlieb et al., 1990). In contrast to the fibrous layer of chick sclera, the cartilaginous layer demonstrates increased synthesis and accumulation of DNA and of proteoglycans (Figure 6-12A; particularly of aggrecan) and overall thickening during the development of myopia (Christensen & Wallman, 1991; Rada et al., 1991, 1994).
In all species examined, the changes in scleral extracellular matrix synthesis and degradation are greatest at the posterior pole of the globe (Norton & Rada, 1995; Rada et al., 1994, 2000b), suggesting that these animal models of myopia accurately model the scleral changes associated with high myopia in humans. The localized response in the posterior sclera may be related to regional differences in the growth states of the scleral fibroblasts in this region or may be a reflection of a concentration of visually induced changes in the retina, choroid, and sclera along the visual axis. In chicks, tree shrews, and marmosets, scleral changes associated with myopia development are rapidly reversed when eyes are allowed to experience unrestricted vision from a prior period of form vision deprivation or when negative lens-induced defocus is discontinued (recovery). The slowed elongation of the vitreous chamber in the recovering eyes is associated with decreases in MMP-2 activity, increases in TIMP-2 activity, and increased proteoglycan synthesis in the fibrous sclera of marmosets and tree shrews (McBrien & Gentle, 2003; Rada et al., 2000b). In addition, GAG levels, which are reduced during myopia development by negative lenses, return to normal (Moring et al., 2007). In the posterior cartilaginous sclera of chicks, there is a rapid decrease in proteoglycan synthesis within hours following restoration of unrestricted vision (Figure 6-12 B; Summers Rada & Hollaway, 2011). In both chicks and tree shrews, changes in scleral GAG synthesis and levels during recovery occur prior to, or at least as early as, the most rapid deceleration in vitreous chamber elongation (Moring et al., 2007; Summers Rada & Hollaway, 2011), suggesting that changes in scleral extracellular matrix remodeling are responsible for changes in ocular elongation and refraction.
NOTES: (A) Changes in 35S04-scleral proteoglycan synthesis during the development of form-deprivation myopia and (B) during recovery from induced myopia. Scleral proteoglycan synthesis rates are rapidly increased in response to form deprivation and rapidly decreased following removal of the occluder in response to the induced myopic defocus.
SOURCE: (A) Adapted from Rada et al., 1992; (B) Summers Rada & Hollaway, 2011.
Taken together, these animal studies demonstrate that scleral extracellular remodeling occurs rapidly in response to visual stimuli to adjust eye size and refraction. Identification of the visually driven signals within the eye responsible for the regulation of scleral remodeling would greatly aid in the understanding of the pathogenesis of myopia and likely lead to a viable antimyopia therapy. While more work is needed to confirm these results in human sclera, a few studies indicate that alterations in scleral stiffness and extracellular matrix metabolism are present in the human sclera with increased axial length (reviewed in Boote et al., 2020; Harper & Summers, 2015; Katayama et al., 2021).
Chemical Mediators in the Retina-to-Sclera Signaling Cascade
As mentioned above, it is theorized that visually induced changes in ocular length are the result of a retina-to-choroid-to-scleral signaling cascade (or retino-scleral signaling pathways) that ultimately results in extracellular matrix remodeling of the scleral shell (Norton & Rada, 1995; Rada et al., 1991, 2000b). The exact members of this signaling cascade and how they interact have not yet been determined. Figure 6-12 summarizes the results of multiple investigations by many researchers that have identified key signaling molecules and pathways involved in the retina, RPE, choroid, and sclera.
Retinoic Acid
The vitamin A derivative, all-trans-retinoic acid (atRA) may be an important component for the control of postnatal ocular growth (McFadden et al., 2004; Mertz & Wallman, 2000; Seko et al., 1998; Troilo et al., 2006). Experimental treatment with atRA in guinea pigs, chickens, and mice resulted in larger eye size (Brown et al., 2023; Li et al., 2010; McFadden et al., 2004, 2006), but not always myopic refractive errors (McFadden et al., 2004). Molecular biological approaches have revealed that ocular atRA synthesis is regulated in response to visual stimuli
exclusively though choroidal expression of the atRA synthesizing enzyme, retinaldehyde dehydrogenase 2 (RALDH2; Harper et al., 2016; Rada et al., 2012). While the source of atRA production was unknown, it was demonstrated in chicks and humans that RALDH2 is synthesized by a population of stromal cells, some of which are closely associated with blood vessels (Harper et al., 2015, 2016; Rada et al., 2012; Summers et al., 2020). In chicks, RALDH2 positive cells increased with recovery (Harper et al., 2016). Furthermore, RALDH2+ cells have been shown to co-localize with the intermediate filament vimentin (human) and collagen type I (chick). The presence of vimentin and collagen type I in RALDH2 positive cells suggests that RALDH2+ cells may resemble perivascular fibroblasts and suggests a potential role for retinoic acid in mediating some aspects of the choroidal response during recovery from induced myopia (Harper et al., 2016).
NOTES: The diagram summarizes the key tissue-specific signaling pathways found to be involved in visually guided eye development. Visual form deprivation and hyperopic optical defocus stimulate eye growth, whereas myopic defocus inhibits it. The retina processes information about optical defocus and converts this information into molecular signals, which are transmitted across the retina, RPE and choroid to the sclera via a multilayered signaling cascade. The signals generated by optical defocus cause remodeling of the sclera and adjust the growth rate of the posterior segment of the eye to match the optical power of the eye with its axial length.
SOURCE: Adapted from Summers et al., 2021.
Adenosine
Adenosine is one of the four nucleosides of DNA and RNA and is found abundantly in organic compounds. A role for adenosine in myopia was found with evidence that 7-methylxanthine (7-MX), a nonselective inhibitor of adenosine receptors and a metabolite of caffeine, influenced scleral collagen and proteoglycan content in rabbits (Trier et al., 1999). Many other studies have also shown that endogenous, exogenous, or genetic manipulation or adenosine can alter axial length (Beach et al., 2018; Liu et al., 2020; Smith et al., 2021; Srinivasalu et al., 2018). Treatment with 7-MX has been shown to reduce myopia progression in chicken, rabbits, guinea pigs, macaques, and children (Cui et al., 2011; Hung et al., 2018; Nie et al., 2012; Trier et al., 2008; Wang et al., 2014). However, oral 7-MX treatment in chickens and
tree shrews showed minimal effects on lens defocus and no effect on form deprivation (Khanal et al. 2020; Liu et al., 2020; Wang et al., 2014).
Nitric Oxide
Evidence is now emerging that nitric oxide (NO) may play a significant role in the postnatal control of ocular growth and myopia development. In vivo administration of the nonspecific nitric oxide synthase inhibitor, L-NAME, inhibits the choroidal and scleral responses associated with recovery from induced myopia. Specifically, blockade of NO synthesis prevents choroidal thickening and choroidal IL6 synthesis in recovering chick eyes; it also disinhibits scleral proteoglycan synthesis, resulting in an increase in the rate of axial elongation in recovering eyes (Nickla & Wildsoet, 2004; Nickla et al., 2006; Summers & Martinez, 2021). Furthermore, administration of NO donors (PAPA-NONOate and sodium nitroprusside; SNP) as well as the NO precursor, l-arginine, slows the rate of ocular elongation and myopia in chicks and guinea pigs (Carr & Stell, 2016; McFadden, 2021). Additionally, NO has been shown to mediate the ocular growth-inhibiting properties of both the muscarinic receptor antagonist, atropine (Abdel-Messeih et al., 2017), and the dopamine agonist, quinpirole (Nickla et al., 2013).
Therefore, experts predict that NO is one of the earliest signaling events in the process of emmetropization, since administration of L-NAME immediately prior to recovery blocks the recovery-induced increase in IL6 observed following 6 hours of recovery (Summers & Martinez, 2021) as well as the change in choroidal thickening and axial elongation observed 7 hours after recovery (Nickla & Wildsoet, 2004). Taken together, these results suggest that NO acts to slow myopic eye growth, and inhibition of NO synthesis prevents recovery from induced myopia. While neither the cellular source of NO nor the target of NO mediating these effects on eye growth have been identified, it is possible that one or more cell populations in the choroid is a potential target of NO. This prediction is based on the observation that administration of L-NAME reduces choroidal concentrations of nitrate (Nickla et al., 2006) and that choroidal expression of interleukin 6 (IL6) is mediated by NO (Summers & Martinez, 2021).
CIRCADIAN RHYTHMS AND THE REGULATION OF POSTNATAL OCULAR GROWTH
Rhythms in Ocular Growth
Many studies over the past 50 years have suggested that circadian rhythms may play a role in the control of ocular growth (Jensen & Matson, 1957; Lauber & Kinnear, 1979; Lauber et al., 1961; Lauber & McGinnis, 1966; Lauber & Kivett, 1981). Initial work in chickens demonstrated that exposure to constant light or constant darkness resulted in excessive eye growth and corneal flattening, leading to the idea that emmetropization requires a normal light/dark cycle to synchronize ocular rhythms. Moreover, the growing eyes of chickens and monkeys demonstrated a diurnal rhythm in axial length: eyes grew faster during the day than during the night, while eyes that were deprived of form vision by translucent diffusers grew rapidly during both day and night (Nickla & Wildsoet, 1998a,b; Papastergiou et al., 1998; Weiss & Schaeffel, 1993) resulting in excessive overall elongation.
The rate of growth of the chicken eye is largely determined by the rate of synthesis of the extracellular matrix proteoglycan aggrecan by scleral chondrocytes (Rada et al., 1991, 1992, 1994). Because ocular growth shows diurnal fluctuations, it was predicted that scleral
proteoglycan synthesis should also fluctuate in a diurnal pattern, and their phases should be correlated. To test this hypothesis, proteoglycan synthesis was measured in punches of sclera, dissected from normal or form-deprived chicken eyes in the morning, afternoon, or midnight, and cultured for 2 hours in radiolabeled sulfur. Results from these studies showed that proteoglycan synthesis was highest during the morning and lowest at midnight (Nickla et al., 1999). Moreover, the rhythm in scleral proteoglycan synthesis appears to be endogenous, as opposed to being light-driven, as it persists even when sclera are isolated and cultured over several days (Nickla et al., 1999, 2001). These results strongly support the observation that diurnal rhythms in scleral extracellular matrix synthesis underlie the diurnal rhythms in axial length in chicks. They demonstrate the existence of an endogenous clock in scleral chondrocytes, the first report of clocks in non-neuronal tissues in vertebrates.
There is also a rhythm in the thickness of the chicken choroid, with a peak at around midnight, coincident with the shortest axial length, and the relative phases of the rhythms in choroidal thickness and axial length change during experimentally induced changes in ocular growth rate, suggesting an influence of ocular rhythms on normal ocular growth and refraction (Nickla & Wildsoet, 1998a,b; Papastergiou et al., 1998). The human choroid exhibits a similar rhythm in thickness, with the choroid being significantly thicker at night and thinner in the daytime. These thickness changes, of about 20 µm, were significantly negatively correlated with systolic blood pressure (Usui et al., 2012).
Molecular Regulation of Ocular Circadian Rhythms
Genetic studies in humans and in animal models have implicated many genes and pathways involved in circadian rhythms (Hysi et al., 2020; Stone et al., 2024). Retinal levels of dopamine, a diurnally oscillating transmitter (discussed above), are decreased in form-deprived eyes, but only during the daytime when levels are normally highest. This suggests that form deprivation might constitute a type of “constant lighting condition” similar to constant light or darkness, leading to excessive axial elongation (Stone et al., 1989). Altered retinal expression of clock and circadian rhythm-related genes have been identified through experimental myopia in chickens and mice (Karouta et al., 2021; Riddel et al., 2016; Stone et al., 2011; Tkatchenko & Tkatchenko, 2021).
Specific visual alterations that experimentally induce refractive errors in chicks each alters the diurnal expression of clock and circadian rhythm genes (Stone et al., 2020). In mice, retinal-specific knockout of the clock gene Bmal1 induces myopia (Stone et al., 2019); knockout of the melanopsin gene in the retina, a key modulator of circadian rhythms, alters normal eye development and augments experimental myopia (Chakroborty et al., 2022); and ablating intrinsically photosensitive retinal ganglion cells (ipRGCs), which contain melanopsin, suppresses myopia (Liu et al., 2022). In addition, findings from human genome-wide association studies have identified hundreds of specific genes and genetic loci associated with myopia and/or refractive error, including genes that point to genetic networks involving light sensitivity and circadian control (Fleming et al., 1997; Tedja et al., 2018).
Taken together, these experimental and clinical observations provide compelling evidence for the role of circadian rhythms in postnatal ocular growth regulation and myopia pathogenesis.
CONCLUSIONS
Conclusion 6-1: Retinal images regulate eye growth mainly through intrinsic regulatory functions of the eye itself. The entire retina—not only, or even primarily, the fovea—plays a critical role in this process.
Conclusion 6-2: The ‘luminance network’ of the retina provides a mechanistic link between the reduced time today’s child spends outdoors and the increased incidence of myopia. This network, which encodes light intensity, includes dopaminergic amacrine cells and melanopsin-expressing intrinsically photosensitive retinal ganglion cells. It is uniquely dependent on some subset of channels within the retinal ON pathways, which have been implicated in myopia pathogenesis in diverse ways.
Conclusion 6-3: A closed feedback loop is essential for achieving precise homeostasis of eye growth. The specific contributions from candidate retinal image properties responsible for fine-tuning this process—such as defocus, blur, contrast (spatial, spectral, and temporal) and chromaticity—are currently unknown. The specific mechanisms through which these features are encoded by the retino-scleral signaling cascade are also unknown. More research is needed to determine how the retina encodes these image features and links them to refractive growth signals.
Conclusion 6-4: Animal models have provided important insights into potentially conserved processes controlling postnatal eye growth, such as visually driven signaling events in the retina, retinal pigment epithelium, and choroid that regulate the remodeling of the scleral extracellular matrix as well as eye size and refraction.
Conclusion 6-5: In infancy through adulthood, the sclera responds rapidly to visual stimuli by remodeling the extracellular matrix, altering biomechanical properties and changing eye shape and refraction.
Conclusion 6-6: Animal studies show that the quality of the peripheral retinal image—whether signed defocus, blur, contrast, or some other feature—is critical for emmetropization. For children, predicting the peripheral retinal image with optical interventions or environmental risk factors is more complicated than previously appreciated, due to individual and eccentricity-specific differences in optical aberrations and how they vary with eye shape. This problem arises from the lack of adequate eye models that link visual diet to image formation across the entire retina.
RECOMMENDATIONS
Recommendation 6-1: Funding agencies, including the National Institutes of Health, the National Science Foundation, the Department of Defense, and private
foundations, as well as industry, should seek to fund proposals across disciplines for both human and animal studies to investigate the mechanisms of emmetropization and myopia, including candidates for retino-scleral signaling, retinal neurons that detect the sign of defocus, the role of the choroid in regulating eye growth, the changes in the sclera that lead to axial elongation, gene–environment interactions, and the development of in vitro experimental models.
Recommendation 6-2: Funding agencies should target audacious proposals to foster the innovative, multidisciplinary research that is needed to fully harmonize our understanding of the visual information processing by the retina that leads to changes in scleral remodeling. Particular gaps in knowledge include the visual environment, ocular optics, retinal circuits, and signaling proteins involved in retino-scleral signaling.
Recommendation 6-3: The field of myopia research should adopt a retinocentric—in contrast to a foveocentric—approach.
- For basic research, this means funding the development of eye models that can be readily tailored to individual variation (“personalized models”) to link the visual diet to image formation across the entire retina.
- For industry, this means developing better technologies to measure 3D eye shape and assess the refractive state across the entire retina.
- For clinical researchers, this means guiding proposed optical treatments with a full understanding of the consequences for the peripheral retinal image.
REFERENCES
Abbott, M. L., Schmid, K. L., & Strang, N. C. (1998). Differences in the accommodation stimulus response curves of adult myopes and emmetropes. Ophthalmic Physiology Optics, 18(1), 13–20. https://doi.org/10.1046/j.1475-1313.1998.00294.x
Abdel-Messeih, P. L., Nosseir, N. M., & Bakhe, O. H. (2017). Evaluation of inflammatory cytokines and oxidative stress markers in prostate cancer patients undergoing curative radiotherapy. Central European Journal of Immunology, 42, 68–72.
Aleman, A. C., Wang, M., & Schaeffel, F. (2018). Reading and Myopia: Contrast Polarity Matters. Scientific Reports, 8(1), 10840. https://doi.org/10.1038/s41598-018-28904-x
Annunen, S., Korkko, J., Czarny, M., Warman, M. L., Brunner, H. G., Kaariainen, H., … Ala-Kokko, L. (1999). Splicing mutations of 54-bp exons in the COL11A1 gene cause Marshall syndrome, but other mutations cause overlapping Marshall/Stickler phenotypes. American Journal of Human Genetics, 65, 974–983.
Aranda, M. L., & Schmidt, T. M. (2021). Diversity of intrinsically photosensitive retinal ganglion cells: circuits and functions. Cellular and Molecular Life Sciences, 78(3), 889–907. https://doi.org/10.1007/s00018-020-03641-5
Ashby, R., McCarthy, C. S., Maleszka, R., Megaw, P., & Morgan, I. G. (2007). A muscarinic cholinergic antagonist and a dopamine agonist rapidly increase ZENK mRNA expression in the form-deprived chicken retina. Experimental Eye Research, 85, 15–22.
Ashby, R. S., & Schaeffel, F. (2010). The effect of bright light on lens compensation in chicks. Investigative Ophthalmology & Visual Science, 51(10), 5247–5253. https://doi.org/10.1167/iovs.09-4689
Atchison, D. A., Li, S. M., Li, H., Li, S. Y., Liu, L. R., Kang, M. T., … Wang, N. (2015). Relative peripheral hyperopia does not predict development and progression of myopia in children. Investigative Ophthalmology & Visual Science, 56(10), 6162–6170. https://doi.org/10.1167/iovs.15-17200
Atchison, D. A., Schmid, K. L., & Pritchard, N. (2006). Neural and optical limits to visual performance in myopia. Vision Research, 46(21), 3707–3722.
Avetisov, E. S., Savitskaya, N. F., Vinetskaya, M. I., & Iomdina, E. N. (1983). A study of biochemical and biomechanical qualities of normal and myopic eye sclera in humans of different age groups. Metabolic Pediatric System Ophthalmology, 7, 183–188.
Barlow, H. B., & Levick, W. R. (1969). Changes in the maintained discharge with adaptation level in the cat retina. Journal of Physiology, 202(3), 699–718. https://doi.org/10.1113/jphysiol.1969.sp008836
Bitzer, M., Kovacs, B., Feldkaemper, M., Schaeffel F. (2006). Effects of muscarinic antagonists on ZENK expression in the chicken retina. Experimental Eye Research, 82(3), 379–388. http://doi.org/10.1016/j.exer.2005.07.010
Bloomfield, S. A., & Völgyi, B. (2009). The diverse functional roles and regulation of neuronal gap junctions in the retina. Nature Reviews. Neuroscience, 10(7), 495–506. https://doi.org/10.1038/nrn2636
Bolz, M., Prinz, A., Drexler, W., & Findl, O. (2007). Linear relationship of refractive and biometric lenticular changes during accommodation in emmetropic and myopic eyes. British Journal of Ophthalmology, 91(3), 360–365. https://doi.org/10.1136/bjo.2006.099879
Boote, C., Sigal, I. A., Grytz, R., Hua, Y., Nguyen, T. D., & Girard, M. J. A. (2020). Scleral structure and biomechanics. Progress in Retinal and Eye Research, 74, 100773. https://doi.org/10.1016/j.preteyeres.2019.100773
Brand, C., Schaeffel, F., & Feldkaemper, M. P. (2007). A microarray analysis of retinal transcripts that are controlled by image contrast in mice. Molecular Vision, 13, 920–932.
Brown, D. M., Kowalski, M. A., Paulus, Q. M., Yu, J., Kumar, P., Kane, M. A., … Pardue, M. T. (2022). Altered structure and function of murine sclera in form-deprivation myopia. Investigative Ophthalmology & Visual Science, 63(13), 13. https://doi.org/10.1167/iovs.63.13.13
Brown, D. M., Yu, J., Kumar, P., Paulus, Q. M., Kowalski, M. A., Patel, J. M., … Pardue, M. T. (2023). Exogenous all-trans retinoic acid induces myopia and alters scleral biomechanics in mice. Investigative Ophthalmology & Visual Science, 64(5), 22. https://doi.org/10.1167/iovs.64.5.22
Bullimore, M. (2024). [Animal models of myopia: Lessons for the understanding of human myopia]. Commissioned Paper for the Committee on Focus on Myopia: Pathogenesis and Rising Incidence.
Cakmak, H. B., Cagil, N., Simavli, H., Duzen, B., & Simsek, S. (2010). Refractive error may influence mesopic pupil size. Current Eye Research, 35(2), 130–136.
Calver, R., Radhakrishnan, H., Osuobeni, E., & O’Leary, D. (2007). Peripheral refraction for distance and near vision in emmetropes and myopes. Ophthalmic Physiology Optics, 27, 584–593.
Campbell, M. C., Bunghardt, K., Kisilak, M. L., & Irving, E. L. (2012). Diurnal rhythms of spherical refractive error, optical axial length, and power in the chick. Investigative Ophthalmology & Visual Science, 53(10), 6245–6253. https://doi.org/10.1167/iovs.11-8844
Carkeet, A., Saw, S. M., Gazzard, G., Tang, W., & Tan, D. T. (2004). Repeatability of IOLMaster biometry in children. Optometry and Vision Science, 81(11), 829–834. https://doi.org/10.1097/00006324-200411000-00014
Carroll, J., Neitz, J., & Neitz, M. (2002). Estimates of L: M cone ratio from ERG flicker photometry and genetics. Journal of Vision, 2(8), 1–1. https://doi.org/10.1167/2.8.1
Chakraborty, R., Landis, E. G., Mazade, R., Yang, V., Strickland, R., Hattar, S., … Pardue, M. T. (2022). Melanopsin modulates refractive development and myopia. Experimental Eye Research, 214, 108866. https://doi.org/10.1016/j.exer.2021.108866
Christensen, A. M., & Wallman, J. (1991). Evidence that increased scleral growth underlies visual deprivation myopia in chicks. Investigative Ophthalmology & Visual Science, 32, 2143–2150.
Cohen, Y., Peleg, E., Belkin, M., Polat, U., & Solomon, A. S. (2012). Ambient illuminance, retinal dopamine release and refractive development in chicks. Experimental Eye Research, 103, 33–40. https://doi.org/10.1016/j.exer.2012.08.005
Coletta, N. J., & Watson, T. (2006). Effect of myopia on visual acuity measured with laser interference fringes. Vision Research, 46(5), 636–651. https://doi.org/10.1016/j.visres.2005.05.025
Collins, M. J., Wildsoet, C. F., & Atchison, D. A. (1995). Monochromatic aberrations and myopia. Vision Research, 35(9), 1157–1163.
Cornell, B. (2016). Cladograms. https://old-ib.bioninja.com.au/standard-level/topic-5-evolution-and-biodi/54-cladistics/cladograms.html
Coster, L., Rosenberg, L. C., van der Rest, M., & Poole, A. R. (1987). The dermatan sulfate proteoglycans of bovine sclera and their relationship to those of articular cartilage. An immunological and biochemical study. Journal of Biological Chemistry, 262, 3809–3812.
Cottaris, N. P., Wandell, B. A., Rieke, F., & Brainard, D. H. (2020). A computational observer model of spatial contrast sensitivity: Effects of photocurrent encoding, fixational eye movements, and inference engine. Journal of Vision, 20(7), 17. https://doi.org/10.1167/jov.20.7.17
Curcio, C. A., Allen, K. A., Sloan, K. R., Lerea, C. L., Hurley, J. B., Klock, I. B., & Milam, A. H. (1991). Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. Journal of Comparative Neurology, 312(4), 610–624. https://doi.org/10.1002/cne.903120411
Curcio, C. A., Sloan, K. R., Kalina, R. E., & Hendrickson, A. E. (1990). Human photoreceptor topography. Journal of Comparative Neurology, 292(4), 497–523.
Curtin, B. J. (1985). The Myopias: Basic Science and Clinical Management. Harper & Row.
Curtin, B. J., Iwamoto, T., & Renaldo, D. P. (1979). Normal and staphylomatous sclera of high myopia. An electron microscopic study. Archives of Ophthalmology, 97, 912–915.
Curtin, B. J., & Teng, C. C. (1958). Scleral changes in pathological myopia. Transactions of the American Academy of Ophthalmology and Otolaryngology, 62, 777–788.
Davies, L. N., & Mallen, E. A. H. (2009). Influence of accommodation and refractive status on the peripheral refractive profile. British Journal of Ophthalmology, 93, 1186–1190. https://doi.org/10.1136/bjo.2008.153130
Diether, S., & Schaeffel, F. (1997). Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Research, 37(6), 659–668. https://doi.org/10.1016/s0042-6989(96)00224-6
Do, M. T. H. (2019). Melanopsin and the intrinsically photosensitive retinal ganglion cells: Biophysics to behavior. Neuron, 104(2), 205–226. https://doi.org/10.1016/j.neuron.2019.07.016
Dong, F., Zhi, Z., Pan, M., Xie, R., Qin, X., Lu, R., … Zhou, X. (2011). Inhibition of experimental myopia by a dopamine agonist: Different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Molecular Vision, 17, 2824–2834.
Feldkaemper, M., & Schaeffel, F. (2013). An updated view on the role of dopamine in myopia. Experimental Eye Research, 114, 106–119. https://doi.org/10.1016/j.exer.2013.03.003
Fischer, A. J., Morgan, I. G., & Stell, W. K. (1999). Colchicine causes excessive ocular growth and myopia in chicks. Vision Research, 39(4), 685–697. https://doi.org/10.1016/s0042-6989(98)00178-3
Fledelius, H. C., Goldschmidt, E., Haargaard, B., & Jensen, H. (2014). Human parallels to experimental myopia? A literature review on visual deprivation. Acta Ophthalmologica, 92(8), 724–729. https://doi.org/10.1111/aos.12412
Fleming, P. A., Harman, A. M., & Beazley, L. D. (1997). Changing topography of the RPE resulting from experimentally induced rapid eye growth. Visual Neuroscience, 14, 449–461.
Flitcroft, D. I. (2012). The complex interactions of retinal, optical and environmental factors in myopia aetiology. Progress in Retinal and Eye Research, 31(6), 622–660. https://doi.org/10.1016/j.preteyeres.2012.06.004
Funata, M., & Tokoro, T. (1990). Scleral change in experimentally myopic monkeys. Graefe’s Archive for Clinical and Experimental Ophthalmology, 228, 174–179.
Gan, J., Li, S.-M., Atchison, D. A., Kang, M.-T., Wei, S., He, X., … Wang, N. (2022). Association between color vision deficiency and myopia in Chinese children over a five-year period. Investigative Ophthalmology & Visual Science, 63(2), 2. https://doi.org/10.1167/iovs.63.2.2
Gastinger, M. J., Tian, N., Horvath, T., & Marshak, D. W. (2006). Retinopetal axons in mammals: Emphasis on histamine and serotonin. Current Eye Research, 31(7–8), 655–667. https://doi.org/10.1080/02713680600776119
Gawne, T. J., & Norton, T. T. (2020). An opponent dual-detector spectral drive model of emmetropization. Vision Research, 173, 7–20.
Gawne, T. J., Ward, A. H., & Norton, T. T. (2017). Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Research, 140, 55–65.
Gottlieb, M. D., Joshi, H. B., & Nickla, D. L. (1990). Scleral changes in chicks with form-deprivation myopia. Current Eye Research, 9, 1157–1165. https://doi.org/10.3109/02713689009022297
Guillon, M., Dumbleton, K., Theodoratos, P., Gobbe, M., Wooley, C. B., & Moody, K. (2016). The effects of age, refractive status, and luminance on pupil size. Optometry and Vision Science, 93(9), 1093–1100.
Guo, S. S., Sivak, J. G., Callender, M. G., & Diehl-Jones, B. (1995). Retinal dopamine and lens-induced refractive errors in chicks. Current Eye Research, 14, 385–389. https://doi.org/10.3109/02713689508995475
Gwiazda, J., Grice, K., & Thorn, F. (1999). Response AC/A ratios are elevated in myopic children. Ophthalmic & Physiological Optics: The Journal of the British College of Ophthalmic Opticians (Optometrists), 19(2), 173–179. https://doi.org/10.1046/j.1475-1313.1999.00437.x
Hagen, L. A., Arnegard, S., Kuchenbecker, J. A., Gilson, S. J., Neitz, M., Neitz, J., & Baraas, R. C. (2019). The association between L:M cone ratio, cone opsin genes and myopia susceptibility. Vision Research, 162, 20–28. https://doi.org/10.1016/j.visres.2019.06.006
Harman, A. M., Hoskins, R., & Beazley, L. D. (1999). Experimental eye enlargement in mature animals changes the retinal pigment epithelium. Visual Neuroscience, 16(4), 619–628. https://doi.org/10.1017/s0952523899164022
Harper, A. R., Summers, J. A., Wang, X., Moiseyev, G., & Ma, J. X. (2016). Postnatal chick choroids exhibit increased retinaldehyde dehydrogenase activity during recovery from form deprivation induced myopia. Investigative Ophthalmology & Visual Science, 57, 4886–4897. https://doi.org/10.1167/iovs.15-18635
Harper, A. R., Wiechmann, A. F., Moiseyev, G., Ma, J. X., & Summers, J. A. (2015). Identification of active retinaldehyde dehydrogenase isoforms in the postnatal human eye. PLoS One, 10(4), e0122008. https://doi.org/10.1371/journal.pone.0122008
Hartline, H. K., & Ratliff, F. (1958). Spatial summation of inhibitory influences in the eye of Limulus, and the mutual interaction of receptor units. Journal of General Physiology, 41(5), 1049–1066. https://doi.org/10.1085/jgp.41.5.1049
Hartwig, A., Charman, W. N., & Radhakrishnan, H. (2011). Accommodative response to peripheral stimuli in myopes and emmetropes. Ophthalmic & Physiological Optics, 31(1), 91–99. https://doi.org/10.1111/j.1475-1313.2010.00783.x
Haverkamp, S., Wässle, H., Duebel, J., Kuner, T., Augustine, G. J., Feng, G., & Euler, T. (2005). The primordial, blue-cone color system of the mouse retina. Journal of Neuroscience, 25(22), 5438–5445. https://doi.org/10.1523/jneurosci.1117-05.2005
Heikkinen, J., Toppinen, T., Yeowell, H., Krieg, T., Steinmann, B., Kivirikko, K. I., & Myllyla, R. (1997). Duplication of seven exons in the lysyl hydroxylase gene is associated with longer forms of a repetitive sequence within the gene and is a common cause for the type VI variant of Ehlers-Danlos syndrome. American Journal of Human Genetics, 60, 48–56.
Hung, L.-F., Arumugam, B., She, Z., Ostrin, L., & Smith, E. L. III. (2018). Narrow-band, long-wavelength lighting promotes hyperopia and retards vision-induced myopia in infant rhesus monkeys. Experimental Eye Research, 176, 147–160.
Hysi, P. G., Choquet, H., Khawaja, A. P., Wojciechowski, R., Tedja, M. S., Yin, J., Simcoe, M. J., Patasova, K., Mahroo, O. A., Thai, K. K., Cumberland, P. M., Melles, R. B., Verhoeven, V. J. M., Vitart, V., Segre, A., Stone, R. A., Wareham, N., Hewitt, A. W., Mackey, D. A., Klaver, C. C. W., … Hammond, C. J. (2020). Meta-analysis of 542,934 subjects of European ancestry identifies new genes and mechanisms predisposing to refractive error and myopia. Nature Genetics, 52(4), 401–407. https://doi.org/10.1038/s41588-020-0599-0
Iuvone, P. M., Tigges, M., Fernandes, A., & Tigges, J. (1989). Dopamine synthesis and metabolism in rhesus monkey retina: Development, aging, and the effects of monocular visual deprivation. Visual Neuroscience, 2(6), 465–471. https://doi.org/10.1017/S0952523800006870
Iuvone, P. M., Tigges, M., Stone, R. A., Lambert, S., & Laties, A. M. (1991). Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Investigative Ophthalmology & Visual Science, 32(6), 1674–1677. https://doi.org/10.1167/iovs.12-10126
Jackson, C. R., Ruan, G. X., Aseem, F., Abey, J., Gamble, K., Stanwood, G., Palmiter, R. D., Iuvone, P. M., & McMahon, D. G. (2012). Retinal dopamine mediates multiple dimensions of light-adapted vision. Journal of Neuroscience, 32(27), 9359–9368. https://doi.org/10.1523/JNEUROSCI.1770-12.2012
Jensen, L. S., & Matson, W. E. (1957). Enlargement of avian eye by subjecting chicks to continuous incandescent illumination. Science, 125(3248), 741. https://doi.org/10.1126/science.125.3248.741
Jiang, L., Zhang, S., Schaeffel, F., Xiong, S., Zheng, Y., Zhou, X., Lu, F., & Qu, J. (2014). Interactions of chromatic and lens-induced defocus during visual control of eye growth in guinea pigs (Cavia porcellus). Vision Research, 94, 24–32. https://doi.org/10.1016/j.visres.2013.10.020
Johnson, J. M., Young, T. L., & Rada, J. A. (2006). Small leucine rich repeat proteoglycans (SLRPs) in the human sclera: Identification of abundant levels of PRELP. Molecular Vision, 12, 1057–1066.
Kainulainen, K., Karttunen, L., Puhakka, L., Sakai, L., & Peltonen, L. (1994). Mutations in the fibrillin gene responsible for dominant ectopia lentis and neonatal Marfan syndrome. Nature Genetics, 6(1), 64–69. https://doi.org/10.1038/ng0194-64
Karouta, C., Kucharski, R., Hardy, K., Thomson, K., Maleszka, R., Morgan, I., & Ashby, R. (2021). Transcriptome-based insights into gene networks controlling myopia prevention. The FASEB Journal, 35(12), e21846. https://doi.org/10.1096/fj.202100376R
Katayama, H., Furuhashi, M., Umetsu, A., Hikage, F., Watanabe, M., Ohguro, H., & Ida, Y. (2021). Modulation of the physical properties of 3d spheroids derived from human scleral stroma fibroblasts (HSSFs) with different axial lengths obtained from surgical patients. Current Issues in Molecular Biology, 43(3), 1715–1725. https://doi.org/10.3390/cimb43030121
Kisilak, M. L., Bunghardt, K., Hunter, J. J., Irving, E. L., & Campbell, M. C. (2012). Longitudinal in vivo imaging of cones in the alert chicken. Optometry and Vision Science: Official Publication of the American Academy of Optometry, 89(5), 644–651. https://doi.org/10.1097/OPX.0b013e31825489df
Ko, G. Y. (2020). Circadian regulation in the retina: From molecules to network. European Journal of Neuroscience/EJN. European Journal of Neuroscience, 51(1), 194–216. https://doi.org/10.1111/ejn.14185
Kroger, R. H., Hirt, B., & Wagner, H. J. (1999). Effects of retinal dopamine depletion on the growth of the fish eye. Journal of Comparative Physiology A, 184(4), 403–412. https://doi.org/10.1007/s003590050334
Kuchenbecker, J. A., Neitz, J., & Neitz, M. (2014). Ethnic variation in the ratio of long- to middle-wavelength sensitive cones. Investigative Ophthalmology & Visual Science, 55(13), 4539. https://doi.org/10.1167/iovs.14-15726
Kuffler, S. W. (1953). Discharge patterns and functional organization of mammalian retina. Journal of Neurophysiology, 16(1), 37–68. https://doi.org/10.1152/jn.1953.16.1.37
Labhishetty, V., Cholewiak, S. A., & Banks, M. S. (2019). Contributions of foveal and non-foveal retina to the human eye’s focusing response. Journal of Vision, 19(12), 18. https://doi.org/10.1167/19.12.18
Landis, E., Chrenek, M., Chakraborty, R., Strickland, R., Bergen, M., Yang, V., Iuvone, P., & Pardue, M. (2020). Increased endogenous dopamine prevents myopia in mice. Experimental Eye Research, 193, 107956. https://doi.org/10.1016/j.exer.2020.107956
Lauber, J. K., & Kinnear, A. (1979). Eye enlargement in birds induced by dim light. Canadian Journal of Ophthalmology, 14(3), 265–269.
Lauber, J. K., & Kivett, V. K. (1981). Environmental control of the rearing conditions and early preglaucomatous lesions in chicks. Experimental Eye Research, 32(5), 501–509. https://doi.org/10.1016/s0014-4835(81)80038-4
Lauber, J. K., & McGinnis, J. (1966). Eye lesions in domestic fowl reared under continuous light. Vision Research, 6(11-12), 619–626. https://doi.org/10.1016/0042-6989(66)90127-2
Lauber, J. K., Shutze, J. V., & McGinnis, J. (1961). Effects of exposure to continuous light on the eye of the growing chick. Proceedings of the Society for Experimental Biology and Medicine, 106(4), 871–872. https://doi.org/10.3181/00379727-106-26505
Leung, T.-W., Li, R. W., & Kee, C. S. (2021) Meridional anisotropy of foveal and peripheral resolution acuity in adults with emmetropia, myopia, and astigmatism. Investigative Ophthalmology & Visual Science, 62(10), 11. https://doi.org/10.1167/iovs.62.10.11
Li, C., Fitzgerald, M. E., Del Mar, N., & Reiner, A. (2016). Stimulation of Baroresponsive Parts of the Nucleus of the Solitary Tract Produces Nitric Oxide-mediated Choroidal Vasodilation in Rat Eye. Frontiers in Neuroanatomy, 10, 94. https://doi.org/10.3389/fnana.2016.00094
Li, C., McFadden, S. A., Morgan, I., Cui, D., Hu, J., Wan, W., & Zeng, J. (2010). All-trans retinoic acid regulates the expression of the extracellular matrix protein fibulin-1 in the guinea pig sclera and human scleral fibroblasts. Molecular Vision, 16, 689–697. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2855729/
Liang, H., Crewther, S. G., Crewther, D. P., & Pirie, B. (1996). Morphology of the recovery from form deprivation myopia in the chick. Australian and New Zealand Journal of Ophthalmology, 24(1), 41–44. https://doi.org/10.1111/j.1442-9071.1996.tb01486.x
Lin, T., Grimes, P. A., & Stone, R. A. (1993). Expansion of the retinal pigment epithelium in experimental myopia. Vision Research, 33(13), 1881–1885. https://doi.org/10.1016/0042-6989(93)90173-w
Liu, R., Hu, M., He, J. C., Zhou, X. T., Dai, J. H., Qu, X. M., Liu, H., & Chu, R. Y. (2014). The effects of monochromatic illumination on early eye development in rhesus monkeys. Investigative Ophthalmology & Visual Science, 55(3), 1901–1909. https://doi.org/10.1167/iovs.13-12276
Liu, A. L., Liu, Y. F., Wang, G., Shao, Y. Q., Yu, C. X., Yang, Z., Zhou, Z. R., Han, X., Gong, X., Qian, K. W., Wang, L. Q., Ma, Y. Y., Zhong, Y. M., Weng, S. J., & Yang, X. L. (2022). The role of ipRGCs in ocular growth and myopia development. Science Advances, 8(5), eabm9027. https://doi.org/10.1126/sciadv.abm9027
Liu, R., Qian, Y. F., He, J. C., Hu, M., Zhou, X. T., Dai, J. H., Qu, X. M., & Chu, R. Y. (2011). Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Experimental Eye Research, 92(6), 447–453. https://doi.org/10.1016/j.exer.2011.03.003
Llorente, L., Barbero, S., Cano, D., Dorronsoro, C., & Marcos, S. (2004). Myopic versus hyperopic eyes: axial length, corneal shape and optical aberrations. Journal of Vision, 4(4), 288–298. https://doi.org/10.1167/4.4.288
Long, Q., Chen, D. H., & Chu, R. Y. (2009). Illumination with monochromatic long-wavelength light promotes myopic shift and ocular elongation in newborn pigmented guinea pigs. Cutaneous and Ocular Toxicology, 28(4), 176–180. https://doi.org/10.1080/15569520902987094
Mahajan, V. B., Olney, A. H., Garrett, P., Chary, A., Dragan, E., Lerner, G., Murray, J., & Bassuk, A. G. (2010). Collagen XVIII mutation in Knobloch syndrome with acute lymphoblastic leukemia. American Journal of Medical Genetics Part A, 152A, 2875–2879. https://doi.org/10.1002/ajmg.a.33620
Mao, J. F., Liu, S. Z., Qin, W. J., & Xiang, Q. (2011). Modulation of TGFβ(2) and dopamine by PKC in retinal Müller cells of guinea pig myopic eye. International Journal of Ophthalmology, 4(4), 357–360. https://doi.org/10.3980/j.issn.2222-3959.2011.04.06
Marsack, J. D., Thibos, L. N., & Applegate, R. A. (2004). Metrics of optical quality derived from wave aberrations predict visual performance. Journal of Vision, 4(4), 322–328. https://doi.org/10.1167/4.4.8
Marzani, D., & Wallman, J. (1997). Growth of the two layers of the chick sclera is modulated reciprocally by visual conditions. Investigative Ophthalmology & Visual Science, 38, 1726–1739. https://doi.org/10.1007/s00417-022-05837-w
Mathis, U., Feldkaemper, M., Liu, H., & Schaeffel, F. (2023). Studies on the interactions of retinal dopamine with choroidal thickness in the chicken. Graefe’s Archive for Clinical and Experimental Ophthalmology, 261(2), 409–425. https://doi.org/10.1007/s00417-022-05837-w
Mathis, U., & Schaeffel, F. (2007). Glucagon-related peptides in the mouse retina and the effects of deprivation of form vision. Graefe’s Archive for Clinical and Experimental Ophthalmology, 245(2), 267-275. https://doi.org/10.1007/s00417-006-0282-x
Mazade, R., & Pardue, M. T. (2023). Rod pathway electrical activity is modulated in the myopic mouse. Investigative Ophthalmology & Visual Science, 64(8), 843.
McBrien, N. A., Cottriall, C. L., & Annies, R. (2001). Retinal acetylcholine content in normal and myopic eyes: a role in ocular growth control? Visual Neuroscience, 18, 571–580. https://doi.org/10.1017/S0952523801184035
McBrien, N. A., & Gentle, A. (2003). Role of the sclera in the development and pathological complications of myopia. Progress in Retinal and Eye Research, 22, 307–338. https://doi.org/10.1016/S1350-9462(02)00063-0
McBrien, N. A., & Millodot, M. (1986). The effect of refractive error on the accommodative response gradient. Ophthalmic and Physiological Optics, 6(2), 145–149. https://doi.org/10.1111/j.1475-1313.1986.tb01056.x
McBrien, N. A., Moghaddam, H. O., Reeder, A. P., & Moules, S. (1991). Structural and biochemical changes in the sclera of experimentally myopic eyes. Biochemical Society Transactions, 19, 861–865. https://doi.org/10.1042/bst0190861
McBrien, N. A., & Norton, T. T. (1994). Prevention of collagen crosslinking increases form-deprivation myopia in tree shrew. Experimental Eye Research, 59, 475–486. https://doi.org/10.1006/exer.1994.1134
McCarthy, C. S., Megaw, P., Devadas, M., & Morgan, I. G. (2007). Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Experimental Eye Research, 84, 100–107. https://doi.org/10.1016/j.exer.2006.09.008
McFadden, S. A., Howlett, M. H., & Mertz, J. R. (2004). Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Research, 44, 643–653. https://doi.org/10.1016/j.visres.2003.10.027
Mertz, J. R., & Wallman, J. (2000). Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Experimental Eye Research, 70, 519–527. https://doi.org/10.1006/exer.1999.0807
Mirhajianmoghadam, H., Jnawali, A., Musial, G., Queener, H. M., Patel, N. B., Ostrin, L. A., & Porter, J. (2020). In vivo assessment of foveal geometry and cone photoreceptor density and spacing in children. Scientific Reports, 10, 8942. https://doi.org/10.1038/s41598-020-65645-2
Moring, A. G., Baker, J. R., & Norton, T. T. (2007). Modulation of glycosaminoglycan levels in tree shrew sclera during lens-induced myopia development and recovery. Investigative Ophthalmology & Visual Science, 48, 2947–2956. https://doi.org/10.1167/iovs.07-0083
Munteanu, T., Noronha, K. J., Leung, A. C., Pan, S., Lucas, J. A., & Schmidt, T. M. (2018). Light-dependent pathways for dopaminergic amacrine cell development and function. eLife, 7, e39866. https://doi.org/10.7554/eLife.39866
Muralidharan, G., Martínez-Enríquez, E., Birkenfeld, J., Velasco-Ocana, M., Pérez-Merino, P., & Marcos, S. (2019). Morphological changes of human crystalline lens in myopia. Biomedical Optics Express, 10(12), 6084–6095. https://doi.org/10.1364/BOE.10.006084
Mutti, D. O., Hayes, J. R., Mitchell, G. L., Jones, L. A., Moeschberger, M. L., Cotter, S. A., Kleinstein, R. N., Manny, R. E., Twelker, J. D., & Zadnik, K. (2007). Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Investigative Ophthalmology & Visual Science, 48, 2510–2519. https://doi.org/10.1167/iovs.06-0562
Mutti, D. O., Mitchell, G. L., Jones-Jordan, L. A., Cotter, S. A., Kleinstein, R. N., Manny, R. E., Twelker, J. D., Zadnik, K., & CLEERE Study Group. (2017). The response AC/A ratio before and after the onset of myopia. Investigative Ophthalmology & Visual Science, 58(3), 1594–1602. https://doi.org/10.1167/iovs.16-19093
Mutti, D. O., Mitchell, G. L., Sinnott, L. T., Jones-Jordan, L. A., Moeschberger, M. L., Cotter, S. A., Kleinstein, R. N., Manny, R. E., Twelker, J. D., Zadnik, K., & CLEERE Study Group. (2012). Corneal and crystalline lens dimensions before and after myopia onset. Optometry and Vision Science: Official Publication of the American Academy of Optometry, 89(3), 251–262. https://doi.org/10.1097/OPX.0b013e3182418213
Mutti, D. O., Sinnott, L. T., Mitchell, G. L., Jones-Jordan, L. A., Moeschberger, M. L., Cotter, S. A., Kleinstein, R. N., Manny, R. E., Twelker, J. D., & Zadnik, K. (2011). Relative peripheral refractive error and the risk of onset and progression of myopia in children. Investigative Ophthalmology & Visual Science, 52(1), 199-205. https://doi.org/10.1167/iovs.09-4826
Neitz, M., Balding, S. D., McMahon, C., Sjoberg, S. A., & Neitz, J. (2006). Topography of long- and middle-wavelength sensitive cone opsin gene expression in human and Old World monkey retina. Visual Neuroscience, 23(3-4), 379-385. https://doi.org/10.1017/S095252380623325X
Nickla, D. L., Jordan, K., Yang, J., & Totonelly, K. (2017). Brief hyperopic defocus or form deprivation have varying effects on eye growth and ocular rhythms depending on the time-of-day of exposure. Experimental Eye Research, 161, 132–142. https://doi.org/10.1016/j.exer.2017.06.003
Nickla, D. L., Lee, L., & Totonelly, K. (2013). Nitric oxide synthase inhibitors prevent the growth-inhibiting effects of quinpirole. Optometry and Vision Science, 90, 1167-1175. https://doi.org/10.1097/OPX.0000000000000046
Nickla, D. L., Rada, J. A., & Wallman, J. (1999). Isolated chick sclera shows a circadian rhythm in proteoglycan synthesis perhaps associated with the rhythm in ocular elongation. Journal of Comparative Physiology A: Sensory, Neural, and Behavioral Physiology, 185, 81-90. https://doi.org/10.1007/s003590050366
Nickla, D. L., & Schroedl, F. (2019). Effects of autonomic denervations on the rhythms in axial length and choroidal thickness in chicks. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 205(1), 139-149. https://doi.org/10.1007/s00359-018-01310-4
Nickla, D. L., & Wallman, J. (2010). The multifunctional choroid. Progress in Retinal and Eye Research, 29, 144-168. https://doi.org/10.1016/j.preteyeres.2009.12.002
Nickla, D. L., & Wildsoet, C. F. (2004). The effect of the nonspecific nitric oxide synthase inhibitor NGnitro-L-arginine methyl ester on the choroidal compensatory response to myopic defocus in chickens. Optometry and Vision Science, 81, 111-118. https://doi.org/10.1097/00006324-200402000-00008
Nickla, D. L., Wildsoet, C., & Troilo, D. (2001). Endogenous rhythms in axial length and choroidal thickness in chicks: implications for ocular growth regulation. Investigative Ophthalmology & Visual Science, 42, 584-588. https://iovs.arvojournals.org/article.aspx?doi=10.1167/iovs.01-0185
Nickla, D. L., Wildsoet, C., & Wallman, J. (1998a). The circadian rhythm in intraocular pressure and its relation to diurnal ocular growth changes in chicks. Experimental Eye Research, 66, 183-193. https://doi.org/10.1006/exer.1997.0435
Nickla, D. L., Wildsoet, C., & Wallman, J. (1998b). Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Experimental Eye Research, 66, 163-181. https://doi.org/10.1006/exer.1997.0426
Nickla, D. L., Wilken, E., Lytle, G., Yom, S., & Mertz, J. (2006). Inhibiting the transient choroidal thickening response using the nitric oxide synthase inhibitor l-NAME prevents the ameliorative effects of visual experience on ocular growth in two different visual paradigms. Experimental Eye Research, 83, 456-464. https://doi.org/10.1016/j.exer.2006.01.017
Norton, T. T., & Rada, J. A. (1995). Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Research, 35, 1271-1281. https://doi.org/10.1016/0042-6989(94)00220-E
Norton, T. T., & Siegwart, J. T. Jr. (2013). Light levels, refractive development, and myopia—speculative review. Experimental Eye Research, 114, 48-57. https://doi.org/10.1016/j.exer.2013.05.004
Ohngemach, S., Hagel, G., & Schaeffel, F. (1997). Concentrations of biogenic amines in fundal layers in chickens with normal visual experience, deprivation, and after reserpine application. Visual Neuroscience, 14, 493-505. https://doi.org/10.1017/S0952523800010909
Orr, J. B., Seidel, D., Day, M., & Gray, L. S. (2015). Is Pupil Diameter Influenced by Refractive Error? Optometry and Vision Science, 92(4), e106-e114. https://doi.org/10.1097/OPX.0000000000000481
Ostadimoghaddam, H., Yekta, A. A., Heravian, J., Azimi, A., Hosseini, S. M., Vatandoust, S., Sharifi, F., & Abolbashari, F. (2014). Prevalence of refractive errors in students with and without color vision deficiency. Journal of Ophthalmic & Vision Research, 9(4), 484–486. https://doi.org/10.4103/2008-322X.150828
Paluru, P., Ronan, S. M., Heon, E., Devoto, M., Wildenberg, S. C., Scavello, G., Holleschau, A., Mäkitie, O., Cole, W. G., King, R. A., & Young, T. L. (2003). New locus for autosomal dominant high myopia maps to the long arm of chromosome 17. Investigative Ophthalmology & Visual Science, 44(5), 1830–1836. https://doi.org/10.1167/iovs.02-0697
Pan, C. W., Cheung, C. Y., Aung, T., Cheung, C. M., Zheng, Y. F., Wu, R. Y., Mitchell, P., Lavanya, R., Baskaran, M., Wang, J. J., Wong, T. Y., & Saw, S. M. (2013). Differential associations of myopia with major age-related eye diseases: the Singapore Indian Eye Study. Ophthalmology, 120(2), 284–291. https://doi.org/10.1016/j.ophtha.2012.07.065
Papastergiou, G. I., Schmid, G. F., Laties, A. M., Pendrak, K., Lin, T., & Stone, R. A. (1998). Induction of axial eye elongation and myopic refractive shift in one-year-old chickens. Vision Research, 38, 1883-1888. https://doi.org/10.1016/S0042-6989(97)00326-5
Phillips, J. R., Khalaj, M., & McBrien, N. A. (2000). Induced myopia associated with increased scleral creep in chick and tree shrew eyes. Investigative Ophthalmology & Visual Science, 41, 2028-2034. https://iovs.arvojournals.org/article.aspx?doi=10.1016/S0042-6989(97)00326-5
Polans, J., Jaeken, B., McNabb, R. P., Artal, P., & Izatt, J. A. (2015). Wide-field optical model of the human eye with asymmetrically tilted and decentered lens that reproduces measured ocular aberrations. Optica, 2(2), 124–134. https://opg.optica.org/optica/fulltext.cfm?uri=optica-2-2124&id=310933
Poudel, S., Jin, J., Rahimi-Nasrabadi, H., Dellostritto, S., Dul, M. W., Viswanathan, S., & Alonso, J. M. (2024). Contrast sensitivity of ON and OFF human retinal pathways in myopia. Journal of Neuroscience, 44(3). https://doi.org/10.1523/JNEUROSCI.0904-21.2021
Provis, J. M., Dubis, A. M., Maddess, T., & Carroll, J. (2013). Adaptation of the central retina for high acuity vision: cones, the fovea and the avascular zone. Progress in Retinal and Eye Research, 35, 63–81. https://doi.org/10.1016/j.preteyeres.2013.01.005
Qian, Y. S., Chu, R. Y., He, J. C., Sun, X. H., Zhou, X. T., Zhao, N. Q., Hu, D. N., Hoffman, M. R., Dai, J. H., Qu, X. M., & Pao, K. E. (2009). Incidence of myopia in high school students with and without red-green color vision deficiency. Investigative Ophthalmology & Visual Science, 50(4), 1598–1605. https://doi.org/10.1167/iovs.07-1362
Qiu, M., Wang, S. Y., Singh, K., & Lin, S. C. (2013). Association between myopia and glaucoma in the United States population. Investigative Ophthalmology & Visual Science, 54, 830-835. https://doi.org/10.1167/iovs.12-10251
Rada, J. A., Achen, V. R., Penugonda, S., Schmidt, R. W., & Mount, B. A. (2000). Proteoglycan composition in the human sclera during growth and aging. Investigative Ophthalmology & Visual Science, 41(7), 1639-1648. https://pubmed.ncbi.nlm.nih.gov/10845580/
Rada, J. A., Achen, V. R., Perry, C. A., & Fox, P. W. (1997). Proteoglycans in the human sclera: Evidence for the presence of aggrecan. Investigative Ophthalmology & Visual Science, 38(9), 1740-1751.
Rada, J. A., & Brenza, H. L. (1995). Increased latent gelatinase activity in the sclera of visually deprived chicks. Investigative Ophthalmology & Visual Science, 36(8), 1555-1565.
Rada, J. A., Hollaway, L. R., Lam, W., Li, N., & Napoli, J. L. (2012). Identification of RALDH2 as a visually regulated retinoic acid synthesizing enzyme in the chick choroid. Investigative Ophthalmology & Visual Science, 53(3), 1649-1662. https://doi.org/10.1167/iovs.08-2366
Rada, J. A., Matthews, A. L., & Brenza, H. (1994). Regional proteoglycan synthesis in the sclera of experimentally myopic chicks. Experimental Eye Research, 59(6), 747-760.
Rada, J. A., McFarland, A. L., Cornuet, P. K., & Hassell, J. R. (1992). Proteoglycan synthesis by scleral chondrocytes is modulated by a vision-dependent mechanism. Current Eye Research, 11(8), 767-782.
Rada, J. A., Nickla, D. L., & Troilo, D. (2000). Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Investigative Ophthalmology & Visual Science, 41(7), 2050-2058.
Rada, J. A., Perry, C. A., Slover, M. L., & Achen, V. R. (1999). Gelatinase A and TIMP-2 expression in the fibrous sclera of myopic and recovering chick eyes. Investigative Ophthalmology & Visual Science, 40(13), 3091-3099.
Rada, J. A., Thoft, R. A., & Hassell, J. R. (1991). Increased aggrecan (cartilage proteoglycan) production in the sclera of myopic chicks. Developmental Biology, 147(2), 303-312.
Rajavi, Z., Sabbaghi, H., Baghini, A., Yaseri, M., Sheibani, K., & Norouzi, G. (2015). Prevalence of color vision deficiency and its correlation with amblyopia and refractive errors among primary school children. Journal of Ophthalmic & Vision Research, 10(2), 130-138.
Reiner, A., Fitzgerald, M. E. C., Del Mar, N., & Li, C. (2018). Neural control of choroidal blood flow. Progress in Retinal and Eye Research, 64, 96-130. https://doi.org/10.1016/j.preteyeres.2017.12.001
Riddell, N., Giummarra, L., Hall, N. E., & Crewther, S. G. (2016). Bidirectional expression of metabolic, structural, and immune pathways in early myopia and hyperopia. Frontiers in Neuroscience, 10, 390.
Rohrer, B., Schaeffel, F., & Zrenner, E. (1992). Longitudinal chromatic aberration and emmetropization: Results from the chicken eye. Journal of Physiology, 449(1), 363-376.
Rohrer, B., Spira, A. W., & Stell, W. K. (1993). Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium. Visual Neuroscience, 10(3), 447-453.
Romashchenko, D., Rosén, R., & Lundström, L. (2020). Peripheral refraction and higher order aberrations. Clinical and Experimental Optometry, 103(1), 86-94. https://doi.org/10.1111/cxo.12943
Roorda, A., & Williams, D. R. (1999). The arrangement of the three cone classes in the living human eye. Nature, 397(6719), 520-522. https://doi.org/10.1038/17383
Rossi, E. A., Roorda, A., & Do Cooper, R. (2007). Visual performance in emmetropia and low myopia after correction of high-order aberrations. Journal of Vision, 7(8), 14-14.
Rozema J. J. (2023). Refractive development I: Biometric changes during emmetropisation. Ophthalmic & Physiological Optics: The Journal of the British College of Ophthalmic Opticians (Optometrists), 43(3), 347–367. https://doi.org/10.1111/opo.13094
Rozema, J., Dankert, S., Iribarren, R., Lanca, C., & Saw, S. M. (2019). Axial growth and lens power loss at myopia onset in Singaporean children. Investigative Ophthalmology & Visual Science, 60(9), 3091-3099.
Saltarelli, D., Wildsoet, C., Nickla, D., & Troilo, D. (2004). Susceptibility to form-deprivation myopia in chicks is not altered by an early experience of axial myopia. Optometry and Vision Science, 81(2), 119-126. https://doi.org/10.1097/00006324-200402000-00010
Sampaio, L. de O., Bayliss, M. T., Hardingham, T. E., & Muir, H. (1988). Dermatan sulphate proteoglycan from human articular cartilage. Variation in its content with age and its structural comparison with a small chondroitin sulphate proteoglycan from pig laryngeal cartilage. The Biochemical Journal, 254(3), 757–764. https://doi.org/10.1042/bj2540757
Schaeffel, F., Bartmann, M., Hagel, G., & Zrenner, E. (1995). Studies on the role of the retinal dopamine/melatonin system in experimental refractive errors in chickens. Vision Research, 35(9), 1247-1264.
Schaeffel, F., Hagel, G., Bartmann, M., Kohler, K., & Zrenner, E. (1994). 6-Hydroxy dopamine does not affect lens-induced refractive errors but suppresses deprivation myopia. Vision Research, 34(2), 143-149.
Schaeffel, F., & Howland, H. C. (1991). Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Research, 31(4), 717-734.
Schmid, K. L., & Wildsoet, C. F. (2004). Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optometry and Vision Science, 81(2), 137-147.
Seidemann, A., & Schaeffel, F. (2002). Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Research, 42(20), 2409-2417.
Seko, Y., Shimizu, M., & Tokoro, T. (1998). Retinoic acid increases in the retina of the chick with form deprivation myopia. Ophthalmic Research, 30(6), 361-367.
Seko, Y., Tanaka, Y., & Tokoro, T. (1994). Scleral cell growth is influenced by retinal pigment epithelium in vitro. Graefe’s Archive for Clinical and Experimental Ophthalmology, 232(9), 545-552.
___. (1997). Apomorphine inhibits the growth-stimulating effect of retinal pigment epithelium on scleral cells in vitro. Cell Biochemistry and Function, 15(3), 191-196.
She, Z., Ward, A. H., & Gawne, T. J. (2023). The effects of ambient narrowband long-wavelength light on lens-induced myopia and form-deprivation myopia in tree shrews. Experimental Eye Research, 234, 109593. https://doi.org/10.1016/j.exer.2023.109593
Sheng, C., Zhu, X., & Wallman, J. (2013). In vitro effects of insulin and RPE on choroidal and scleral components of eye growth in chicks. Experimental Eye Research, 116, 439-448.
Siegwart, J. T., Jr., & Norton, T. T. (1999). Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Research, 39(3), 387-407.
Smith, E. L., III, Huang, J., Hung, L. F., Blasdel, T. L., Humbird, T. L., & Bockhorst, K. H. (2009). Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Investigative Ophthalmology & Visual Science, 50(11), 5057–5069. https://doi.org/10.1167/iovs.08-3232
Smith, E. L., III, & Hung, L. F. (2000). Form-deprivation myopia in monkeys is a graded phenomenon. Vision Research, 40(4), 371–381. https://doi.org/10.1016/s0042-6989(99)00184-4
Smith, E. L., III, Hung, L. F., Arumugam, B., Holden, B. A., Neitz, M., & Neitz, J. (2015). Effects of long-wavelength lighting on refractive development in infant rhesus monkeys. Investigative Ophthalmology & Visual Science, 56(11), 6490-6500.
Smith, E. L., III, Hung, L. F., & Huang, J. (2009). Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Research, 49(19), 2386-2392.
Smith, E. L., III, Hung, L. F., Huang, J., & Arumugam, B. (2013). Effects of local myopic defocus on refractive development in monkeys. Optometry and Vision Science: Official Publication of the American Academy of Optometry, 90(11), 1176–1186. https://doi.org/10.1097/OPX.0000000000000038
Smith, E. L., III, Hung, L. F., Huang, J., Blasdel, T. L., Humbird, T. L., & Bockhorst, K. H. (2010). Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Investigative Ophthalmology & Visual Science, 51(8), 3864–3873. https://doi.org/10.1167/iovs.09-4969
Smith, E. L., III, Kee, C. S., Ramamirtham, R., Qiao-Grider, Y., & Hung, L. F. (2005). Peripheral vision can influence eye growth and refractive development in infant monkeys. Investigative Ophthalmology & Visual Science, 46(11), 3965-3972. https://doi.org/10.1167/iovs.05-0292
Sng, C. C., Lin, X. Y., Gazzard, G., Chang, B., Dirani, M., Lim, L., Selvaraj, P., Ian, K., Drobe, B., Wong, T. Y., & Saw, S. M. (2011). Change in peripheral refraction over time in Singapore Chinese children. Investigative Ophthalmology & Visual Science, 52(11), 7880-7887. https://doi.org/10.1167/iovs.11-7290
Stone, R. A., Lin, T., Laties, A. M., & Iuvone, P. M. (1989). Retinal dopamine and form-deprivation myopia. Proceedings of the National Academy of Sciences, USA, 86(2), 704-706.
Stone, R. A., McGlinn, A. M., Baldwin, D. A., Tobias, J. W., Iuvone, P. M., & Khurana, T. S. (2011). Image defocus and altered retinal gene expression in chick: Clues to the pathogenesis of ametropia. Investigative Ophthalmology & Visual Science, 52(8), 5765-5777. https://doi.org/10.1167/iovs.10-7131
Stone, R. A., McGlinn, A. M., Chakraborty, R., Lee, D. C., Yang, V., Elmasri, A., Landis, E., Shaffer, J., Iuvone, P. M., Zheng, X., Sehgal, A., & Pardue, M. T. (2019). Altered ocular parameters from circadian clock gene disruptions. PLoS ONE, 14(12), e0217111. https://doi.org/10.1371/journal.pone.0217111
Stone, R. A., Tobias, J. W., Wei, W., Schug, J., Wang, X., Zhang, L., Iuvone, P. M., & Nickla, D. L. (2024). Diurnal retinal and choroidal gene expression patterns support a role for circadian biology in myopia pathogenesis. Scientific Reports, 14, 533. https://doi.org/10.1038/s41598-023-07185-1
Strang, N. C., Winn, B., & Bradley, A. (1998). The role of neural and optical factors in limiting visual resolution in myopia. Vision Research, 38(12), 1713-1721. https://doi.org/10.1016/S0042-6989(97)00363-4
Summers, J. A., & Martinez, E. (2021). Visually induced changes in cytokine production in the chick choroid. eLife, 10, e59916. https://doi.org/10.7554/eLife.59916
Summers, J. A., Cano, E. M., Kaser-Eichberger, A., & Schroedl, F. (2020). Retinoic acid synthesis by a population of choroidal stromal cells. Experimental Eye Research, 201, 108252. https://doi.org/10.1016/j.exer.2020.108252
Summers, J. A., Schaeffel, F., Marcos, S., Wu, H., & Tkatchenko, A. V. (2021). Functional integration of eye tissues and refractive eye development: Mechanisms and pathways. Experimental Eye Research, 209, 108693. https://doi.org/10.1016/j.exer.2021.108693
Summers Rada, J. A., & Hollaway, L. R. (2011). Regulation of the biphasic decline in scleral proteoglycan synthesis during the recovery from induced myopia. Experimental Eye Research, 92(5), 394-400. https://doi.org/10.1016/j.exer.2011.03.004
Tedja, M. S., Wojciechowski, R., Hysi, P. G., Eriksson, N., Furlotte, N. A., Verhoeven, V. J. M., Iglesias, A. I., Meester-Smoor, M. A., Tompson, S. W., Fan, Q., Khawaja, A. P., Cheng, C. Y., Höhn, R., Yamashiro, K., Wenocur, A., Grazal, C., Haller, T., Metspalu, A., Wedenoja, J., Jonas, J. B., … Klaver, C. C. W. (2018). Genome-wide association meta-analysis highlights light-induced signaling as a driver for refractive error. Nature Genetics, 50(6), 834–848. https://doi.org/10.1038/s41588-018-0127-7
Tideman, J. W. L., Polling, J. R., Vingerling, J. R., Jaddoe, V. W. V., Williams, C., Guggenheim, J. A., & Klaver, C. C. W. (2018). Axial length growth and the risk of developing myopia in European children. Acta ophthalmologica, 96(3), 301–309. https://doi.org/10.1111/aos.13603
Tkatchenko, T. V., & Tkatchenko, A. V. (2021). Genome-wide analysis of retinal transcriptome reveals common genetic network underlying perception of contrast and optical defocus detection. BMC Medical Genomics, 14, 153. https://doi.org/10.1186/s12920-021-01022-w
Torii, H., Kurihara, T., Seko, Y., Negishi, K., Ohnuma, K., Inaba, T., Kawashima, M., Jiang, X., Kondo, S., Miyauchi, M., Miwa, Y., Katada, Y., Mori, K., Kato, K., Tsubota, K., Goto, H., Oda, M., Hatori, M., & Tsubota, K. (2017). Violet light exposure can be a preventive strategy against myopia progression. EBioMedicine, 15, 210–219. https://doi.org/10.1016/j.ebiom.2016.12.007
Trier, K., Olsen, E. B., Kobayashi, T., & Ribel-Madsen, S. M. (1999). Biochemical and ultrastructural changes in rabbit sclera after treatment with. British Journal of Ophthalmology, 83, 1370–1375. https://doi.org/10.1136/bjo.83.12.1370
Troilo, D. (1998). Changes in Retinal Morphology following Experimentally Induced Myopia. In Vision Science and Its Applications, Technical Digest Series (pp. SuC.4). Optica Publishing Group.
Troilo, D., Nickla, D. L., Mertz, J. R., & Summers Rada, J. A. (2006). Change in the synthesis rates of ocular retinoic acid and scleral glycosaminoglycan during experimentally altered eye growth in marmosets. Investigative Ophthalmology & Visual Science, 47(4), 1768-1777. https://doi.org/10.1167/iovs.05-1229
Troilo, D., Nickla, D. L., & Wildsoet, C. F. (2000a). Choroidal thickness changes during altered eye growth and refractive state in a primate. Investigative Ophthalmology & Visual Science, 41(5), 1249-1258. https://doi.org/10.1016/j.ebiom.2016.11.021
Troilo, D., Nickla, D. L., & Wildsoet, C. F. (2000b). Form deprivation myopia in mature common marmosets (callithrix jacchus). Investigative Ophthalmology & Visual Science, 41(8), 2043–2049. https://pubmed.ncbi.nlm.nih.gov/10892841/
Usui et al., (2012) Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Investigative Ophthalmology & Visual Science, Vol. 53, 2300
van Alphen, G. W. (1961). On emmetropia and ametropia. Optica Acta, 142(Suppl), 1-92.
___. (1986). Choroidal stress and emmetropization. Vision Research, 26, 723-734. https://doi.org/10.1016/0042-6989(86)90068-3
Wagner, S., Zrenner, E., & Strasser, T. (2019). Emmetropes and myopes differ little in their accommodation dynamics but strongly in their ciliary muscle morphology. Vision Research, 163, 42-51. https://doi.org/10.1016/j.visres.2019.08.002
Walker, T. W., & Mutti, D. O. (2002). The effect of accommodation on ocular shape. Optometry and Vision Science, 79, 424–430.
Wallman, J., Gottlieb, M. D., Rajaram, V., & Fugate-Wentzek, L. A. (1987). Local retinal regions control local eye growth and myopia. Science, 237(4810), 73–77. https://doi.org/10.1126/science.3603011
Wallman, J., Wildsoet, C., Xu, A., Gottlieb, M. D., Nickla, D. L., Marran, L., Krebs, W., & Christensen, A. M. (1995). Moving the retina: choroidal modulation of refractive state. Vision Research, 35, 37-50. https://doi.org/10.1016/0042-6989(94)e0049-q
Wallman, J., & Winawer, J. (2004). Homeostasis of eye growth and the question of myopia. Neuron, 43(4), 447-468. https://doi.org/10.1016/j.neuron.2004.08.008
Walls, G. (1942). The Vertebrate Eye and Its Adaptive Radiations. The Cranbrook Press.
Wandell, B. A., Brainard, D. H., & Cottaris, N. P. (2022). Visual encoding: Principles and software. Progress in Brain Research, 273(1), 199–229. https://doi.org/10.1016/bs.pbr.2022.04.006
Wang, J., Candy, T. R., Teel, D. F., & Jacobs, R. J. (2008). Longitudinal chromatic aberration of the human infant eye. Journal of the Optical Society of America A, 25(9), 2263-2270. https://doi.org/10.1364/JOSAA.25.002263
Wang, F., Zhou, J., Lu, Y., & Chu, R. (2011). Effects of 530 nm green light on refractive status, melatonin, MT1 receptor, and melanopsin in the guinea pig. Current Eye Research, 36, 103–111.
Wang, X., Zhu, C., Hu, X., Liu, L., Liu, M., Yuan, Y., & Ke, B. (2022). Changes in Dimensions and Functions of Crystalline Lens in High Myopia Using CASIA2 Optical Coherence Tomography. Ophthalmic Research, 65(6), 712-721. https://doi.org/10.1159/000526246
Wang, Y., Bensaid, N., Tiruveedhula, P., Ma, J., Ravikumar, S., & Roorda, A. (2019). Human foveal cone photoreceptor topography and its dependence on eye length. eLife, 8, e47148. https://doi.org/10.7554/eLife.47148
Weiss, S., & Schaeffel, F. (1993). Diurnal growth rhythms in the chicken eye: Relation to myopia development and retinal dopamine levels. Journal of Comparative Physiology A, 172, 263-270. https://doi.org/10.1007/BF00216471
Wells-Gray, E., Choi, S., & Bries, A. (2016). Variation in rod and cone density from the fovea to the mid-periphery in healthy human retinas using adaptive optics scanning laser ophthalmoscopy. Eye, 30, 1135–1143. https://doi.org/10.1038/eye.2016.107
Whatham, A., Zimmermann, F., Martinez, A., Delgado, S., de la Jara, P. L., Sankaridurg, P., & Ho, A. (2009). Influence of accommodation on off-axis refractive errors in myopic eyes. Journal of Vision, 9(3), 1–13. https://doi.org/10.1167/9.3.14
White, J. M., & Wick, B. (1995). Accommodation in humans with juvenile macular degeneration. Vision Research, 35(6), 873–880.
Wildsoet, C. F., Atchison, D. A., & Collins, M. J. (1993). Longitudinal chromatic aberration as a function of refractive error. Clinical and Experimental Optometry, 76(4).
Witkovsky, P. (2004). Dopamine and retinal function. Documenta Ophthalmologica: Advances in Ophthalmology, 108(1), 17–40. https://doi.org/10.1023/b:doop.0000019487.88486.0a
Young, T. L., Guo, X. D., King, R. A., Johnson, J. M., & Rada, J. A. (2003). Identification of genes expressed in a human scleral cDNA library. Molecular Vision, 9, 508-514.
Zadnik, K., & Mutti, D. O. (1995). How applicable are animal myopia models to human juvenile onset myopia? Vision Research, 35, 1283-1288. https://doi.org/10.1016/0042-6989(94)00238-F
Zhang, L. Q., Cottaris, N. P., & Brainard, D. H. (2022). An image reconstruction framework for characterizing initial visual encoding. eLife, 11, e71132. https://doi.org/10.7554/eLife.71132
Zhang, Y., Liu, Y., Ho, C., & Wildsoet, C. F. (2013). Effects of imposed defocus of opposite sign on temporal gene expression patterns of BMP4 and BMP7 in chick RPE. Experimental Eye Research, 109, 98-106. https://doi.org/10.1016/j.exer.2013.01.004
Zhang, Y., Liu, Y., & Wildsoet, C. F. (2012). Bidirectional, optical sign-dependent regulation of BMP2 gene expression in chick retinal pigment epithelium. Investigative Ophthalmology & Visual Science, 53, 6072-6080. https://doi.org/10.1167/iovs.12-9864
Zhang, Z., Mu, J., Wei, J., Geng, H., Liu, C., Yi, W., Sun, Y., & Duan, J. (2023). Correlation between refractive errors and ocular biometric parameters in children and adolescents: A systematic review and meta-analysis. BMC Ophthalmology, 23(1), 472. https://doi.org/10.1186/s12886-023-03222-7
Zhang, Y., Phan, E., & Wildsoet, C. F. (2019). Retinal defocus and form-deprivation exposure duration affects RPE BMP gene expression. Scientific Reports, 9, 7332. https://doi.org/10.1038/s41598-019-43785-4
Zhao, X., Reifler, A. N., Schroeder, M. M., Jaeckel, E. R., Chervenak, A. P., & Wong, K. Y. (2017). Mechanisms creating transient and sustained photoresponses in mammalian retinal ganglion cells. Journal of General Physiology, 149(3), 335-353. https://doi.org/10.1085/jgp.201611720
Zheleznyak, L. (2023). Peripheral optical anisotropy in refractive error groups. Ophthalmic and Physiological Optics, 43(3), 435-444. https://doi.org/10.1111/opo.13104
Zheleznyak, L., Barbot, A., Ghosh, A., & Yoon, G. (2016). Optical and neural anisotropy in peripheral vision. Journal of Vision, 16(5), 1. https://doi.org/10.1167/16.5.1
Zheleznyak, L., Liu, C., & Winter, S. (2024). Differentiating positive from negative retinal defocus by peripheral chromatic optical cues. https://opg.optica.org/boe/fulltext.cfm?uri=boe-15-95098&id=554451
























































