Myopia: Causes, Prevention, and Treatment of an Increasingly Common Disease (2024)
Chapter: 4 Assessment and Diagnostic Technologies
4
Assessment and Diagnostic Technologies
CURRENT STANDARD CLINICAL ASSESSMENTS AND DIAGNOSTIC TECHNOLOGIES
This chapter reviews the current assessments and diagnostic technologies used in screenings and clinical evaluations of myopia. The assessment and diagnostic measurements used in these evaluations shape researchers’ understanding of myopia because they are the recorded, and therefore studied, characteristics of myopia.
Screening efforts are used to identify vision abnormalities, including refractive errors like myopia, in nonspecialist settings. These nonspecialist settings are usually settings where many target populations congregate (e.g., schools for children), public locales in the community at large (e.g., shopping centers), and primary or general medical clinics. Eye assessments in these settings include basic optotypes (e.g., presentation of letters at specified distances), autorefractor devices to directly measure an eye’s refractive error, and photoscreeners. The original photoscreeners photographed the red reflex (red color within the eye’s pupil during a flash photograph) from both eyes of a subject. Based on characteristics of the photographed red reflex, the image obtained could be used to identify refractive errors, obstructions in the visual axis (e.g., cataracts, tumors), and ocular misalignments associated with abnormal visual development.
Modern photoscreeners use infrared illumination and digital imaging, have autorefraction capabilities, and typically include some decision support (e.g., image quality assessment, screen pass/fail and need for referral). Modern photoscreeners include stand-alone devices such as the Spot and PlusoptiX and, more recently, smartphone-based variants such as the GoCheck Kids (Nallasamy et al., 2024). (For more details regarding vision screening including performance, accessibility, and barriers, see Chapter 8.)
Clinical History and Standard Eye Exam
Upon reaching the clinic, whether via screening or for an eye examination, the following describes the current assessments and diagnostic technologies someone with myopia would encounter in a clinical evaluation (Jacobs et al., 2022). As part of a comprehensive eye examination, the clinical assessment first includes obtaining the medical history. This includes (but is not limited to) recording the duration and progression of visual symptoms, past medical or surgical diagnoses, medication use, and family history. The duration and progression of visual symptoms provides information about the potential course of the myopia, with earlier onset and rapid progression increasing the likelihood of becoming highly myopic. Past medical or surgical diagnoses provide information about associated diseases or pathological forms of myopia. As an example of associated disease, primary congenital glaucoma can cause an increase in the size of the eye leading to axial myopia, due to globe wall distensibility with rapid expansion; as an
example of myopia from prior other treatments, a scleral reinforcement buckle surgically placed to repair a retinal detachment can also increase the length of the eye, resulting in myopia. Medication use is also important, as some medications can cause myopia as a side effect (e.g., topiramate used for migraines or seizure control). A review of family history helps in identifying possible genetic or syndromic risks for myopia, given the strong heritability of myopia (Tedja et al., 2019).
Next, a physical examination is performed to assess the state of the eye. The physical examination includes measurements of visual acuity at distance (typically 20 ft or 6 m) both without and with correction. A refraction will be performed, which may consist of an autorefractor measurement of the refractive state of the eye, an interactive subjective manifest refraction in which lenses are replaced (typically with a phoropter) based on the patient’s perception of improvement or deterioration of vision, and/or a retinoscopy, in which the examiner replaces lenses based on assessing a streak of light reflected from the examined eye.
Children and young adults can dynamically adjust their lens power to focus on near objects by contracting the ciliary muscle, which changes the crystalline lens shape, a process called accommodation. For pediatric and young adult patients, pharmacologic cycloplegia (a temporary condition that paralyzes the eye’s ciliary muscle, which controls accommodation) should be performed to determine the refractive state of the eye without the influence of accommodation.
Regarding accommodation, studies have noted accommodative lag differences in myopes compared to emmetropes (those with no refractive error). Specifically, there is an insufficiency of accommodation for near targets in myopes compared to emmetropes (Gwiazda et al., 1993). Accommodative lag is measured by having the individual look at targets varying from far to near distances, either physically or optically via lenses, and plotting the refraction measurements against the target distances. The slope of the accommodative response to the change in target distance is farther from 1:1 for myopes than it is for emmetropes. Typically, an autorefractor is used for these refraction measurements, and the target is placed along the line of sight. Performing this measurement requires specialized equipment, such as an open-field autorefractor (e.g., Grand Seiko WAM-5500), and a technician who must move the target either manually or by a motor and record the multiple measurements. Because accommodative lag has not been shown to predate the development of myopia (Mutti et al., 2006), the diagnostic value of this measurement appears to be limited compared to other, easier-to-obtain diagnostic measurements such as current refractive error (Zadnik et al., 2015). Accommodative measurements hence are not in widespread clinical use as an influence on myopia progression management.
After refraction, the remainder of the physical examination will include measurements of intraocular pressure, pupillary examination, an examination of the front of the eye, and a dilated exam of the back of the eye. Intraocular pressure is measured using tonometry. There are a variety of tonometer mechanisms and techniques, such as the air puff, thin filament rebound (which does not require anesthetic drops) and contact variants such as Goldmann tonometry (does require topical anesthesia). Increased intraocular pressure is a major risk factor for the development of glaucoma, which, as alluded to earlier, is associated with myopia.
The pupillary examination measures the response of the pupil to a light stimulus, both within the eye receiving the stimulus and in the other eye. The reflexive constrictive pupillary response to a light stimulus provides information on optic nerve and cortical visual pathway health. As detailed in the research diagnostics section later, the pupillary response may also provide information about specific neurons that can affect myopia development. The
examination of the front of the eye is usually performed with a slit lamp biomicroscope, and the examination of the back of the eye is performed with direct or indirect ophthalmoscopy (a medical exam that allows a doctor to see the inside of the eye using a magnifying lens and light) after dilation with dilating drops.
These examination techniques provide magnified views of the anatomy of the eye, so that the practitioner can look for physical evidence that may be associated with or be a consequence of myopia.
Other diagnostic technologies are used to supplement this standard clinical assessment. They include fundus imaging, ocular biometry, optical coherence tomography (OCT), electroretinograms (ERGs), contrast sensitivity testing, and automated visual field testing.
Fundus Imaging
Fundus imaging provides a recorded, portable, and reviewable assessment of the posterior eye. Fundus imaging is typically performed with fundus photography or videography (Figure 4-1) or with scanning laser ophthalmoscopy. In fundus photography/videography—which is now mostly digital—one takes color images of the retina through a dilated pupil with approximately a 30° to 50° field of view through lenses specifically designed for imaging the posterior eye. Multiple images can be montaged together to provide a wider field of view. Fundus cameras range from purpose-specific models made by major ophthalmic device companies to add-ons for consumer cameras, including smartphone cameras.
Angiography, the mapping of blood vessels, is carried out to assess the retinal and deeper vasculature and can be obtained using injected dyes such as fluorescein and indocyanine green to provide contrast as the dyes circulate through the vasculature. Scanning laser ophthalmoscopy is another way to image the fundus and uses a confocal and scanning laser to obtain images. Depending on the laser wavelengths used, the retinal image obtained and reconstructed by this method may appear in pseudo-color. Modern commercial versions of this technique can have fields of view larger than regular fundus photography and can image through non-dilated pupils with proper alignment.
Overall, fundus imaging provides a recorded color view of the retina which should provide similar information to the clinical fundus examination to assess for posterior eye pathologies associated with myopia, including maculopathy, glaucoma, and retinal detachment.
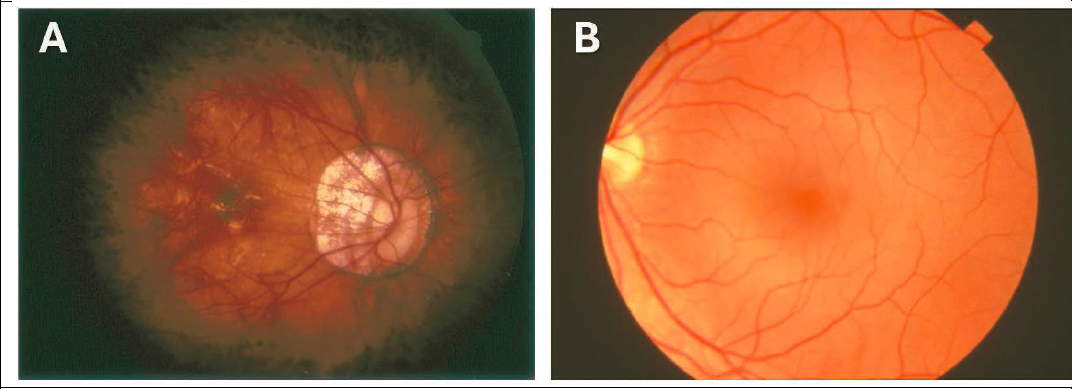
SOURCE: (A) American Academy of Ophthalmology, 2020; (B) National Eye Institute, 2020.
Ocular Biometry
Ocular biometry is used to measure the curvature of the optical elements within the eye and the length of the eye. The major application for ocular biometry devices is for cataract surgery, so most of the measurements from ocular biometry are those needed to calculate the optical power of intraocular lenses in the context of cataract surgery. These include curvature measurements of the cornea, which provide information about the power and astigmatism of the cornea, and central length measurements through the eye, such as central cornea thickness, anterior chamber depth, lens thickness, and axial length. Originally, the curvature and axial length information were captured using separate devices—a keratometer and ultrasound A-scan, respectively—but most modern ocular biometers combine those modalities into a single device. Modern ocular biometers include a keratometer and/or cross-sectional corneal imaging device such as OCT (see discussion below) or Scheimpflug photography to measure the corneal curvature. For the axial length measurements, optical interferometry is used either via partial coherence interferometry or OCT.
While many studies have investigated the relationships between myopia and these various ocular biometric measurements, the major measurement of concern for myopia is axial length (Jones et al., 2005; Mutti et al., 2007; Tideman et al., 2016; Wong et al., 2010). For fixed anterior segment optics, an axially longer eye results in the image being focused in front of the retina, a condition that defines myopia. Multiple studies have shown the relationship between increasing axial length, myopia, and pathologic myopia risk. Longitudinal axial length measurements are also used to assess the effects of myopia interventions and treatments aimed at slowing or stabilizing axial lengthening.
Optical Coherence Tomography
OCT is a micrometer-scale cross-sectional imaging technology that is a major advancement in clinical diagnostic information, particularly for retinal diseases (Figure 4-2). Current clinical OCT systems can image either the front of the eye (anterior segment OCT systems) or the back of the eye (retinal OCT systems), and their micrometer-scale cross-sectional imaging allows either corneal or retinal layers to be visualized in detail. In the context of myopia, retinal OCT is currently used to assess microstructural changes in the retina, particularly as they relate to myopic maculopathies.
More specifically, OCT imaging can show clinicians retinal layer thinning (associated with degeneration or glaucoma), disruptions in retinal layers (outer retinal degenerations), and accumulations of pathologic fluid (as might occur with neovascularization). A technique called OCT angiography is also available, making it possible to view microvasculature without the use of injected dyes as an alternative to standard (dye-based) angiography when examining vascular disruptions (Jia et al., 2015; Mariampillai, 2008) as might be seen in pathologic myopia (Wong et al., 2019; Zheng et al., 2022). OCT has also been used in research applications for morphometric and choroidal analysis (those research applications will be covered in a later section of this chapter).
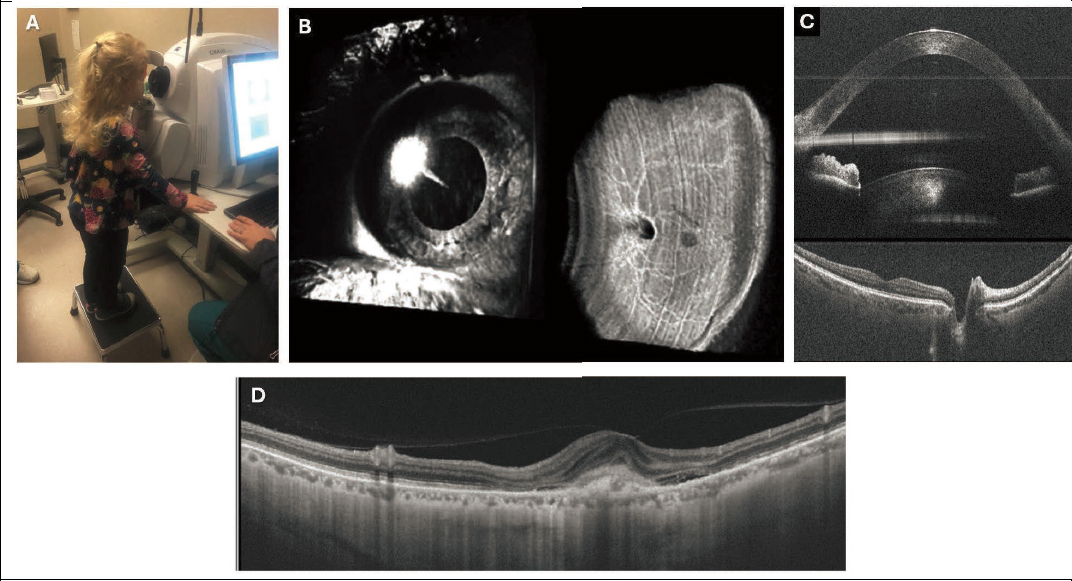
NOTES: (A) Though standard clinical OCT systems are not designed specifically for children, cooperative children can be imaged using them. (B) OCT is a three-dimensional imaging technique capable of imaging both the front and the back of the eye, both shown here, though standard clinical systems usually use only one image or the other. (C) The three-dimensional OCT volume is composed of two-dimensional slices termed B-scans as shown here. B-scans offer high-resolution cross-sectional views of ocular anatomy. They are typically viewed vertically as shown here. Anterior eye shown in top image and retina in bottom image. (D). An example of OCT to visualize pathologic myopia, specifically neovascularization (the “bump” just right of the middle of the image).
SOURCES: (A) Courtesy Katherine Weise, OD, University of Alabama at Birmingham; (B) and (C) reprinted with permission from McNabb et al., 2018; © Optica Publishing Group; (D) from Gupta et al., 2024.
Electroretinograms
ERGs are noninvasive clinical recordings of the electrical response of the retina in response to light (Ramkumar et al., 2024). Electrodes are placed on the anesthetized cornea or on the skin below the eye and record the electrical responses from the eye, similar to an electrocardiogram (EKG) for the heart or an electroencephalogram (EEG) for the brain. These responses are generally characterized as waveforms, and varying the visual stimulus allows different waveforms to be extracted. Aspects of these waveforms are thought to correspond largely or entirely to different cell types within the retina. More specifically, these electrical responses represent responses to light by the retinal photoreceptors and bipolar cells, with different parts of the waveform corresponding to those cell layers. Using different ambient conditions (dark/light) and light stimuli (full-field, multi-focal, pattern), different parts of the retinal circuitry and areas can be interrogated. The full-field ERG consists of two major waves: a negative a-wave generated by the photoreceptors and a positive b-wave generated by activity of the retinal ON bipolar cells. The ON and OFF neural pathways contain parallel retinal circuitry that signals when the retina responds to the onset or the offset of light stimuli, respectively
(Ichinose & Habib, 2022; Schiller, 1992; see Chapter 5, Stimulation of ON vs. OFF Visual Pathways). Certain inherited retinal diseases, like retinitis pigmentosa and congenital stationary night blindness, can result in development of myopia. Because the first of these two diseases causes photoreceptor loss and the second results in diminished or absent b-wave amplitudes, ERG can be used to assess the loss of photoreceptor or ON bipolar function (Frishman, 2013; Pardue & Peachey 2014). More directly related to myopia, there have been limited studies showing associations between myopia and changes in the nerve signals observed by ERG (Chan, 2022; Gupta et al., 2022; Poudel et al., 2024; Zahra, 2023). The underlying hypothesis is that local retinal signaling, such as through ON pathways, is involved in myopia development. The associations between ERG and myopia are thought to be related to changes in signaling, though the literature is not conclusive at this point, and ERG is not an ubiquitous myopia diagnostic for this purpose.
Contrast Sensitivity
Contrast sensitivity tests the ability of the eye to discriminate between differences in luminance or color. This is different from standard optotype testing such as the Snellen acuity test, which presents a high contrast black target on a white background. Contrast sensitivity can provide a better indication of subtle disturbances in visual function that high-contrast testing cannot provide. There are a variety of ways to carry out contrast sensitivity, ranging from specialized eye charts (e.g., Pelli-Robson contrast chart, Hamilton Veale chart) to machine-generated stimuli or gratings (e.g., ColorDome by Diagnosys, Functional Vision Analyzer by Stereo Optical; Liou & Chiu, 2001; Poudel et al., 2024). More recently, a computerized program displaying targets with combinations of spatial frequency and contrast combined with eye tracking was used to obtain contrast sensitivity testing in children and nonverbal individuals (Mooney et al., 2021). As discussed previously in the ERG section, myopia is associated with differences in ON-OFF retinal pathways, and given that ON pathways have higher contrast sensitivity, the disturbances in those pathways could be identified via contrast sensitivity testing. Clinically, contrast sensitivity is used in clinical trials of optical devices such as contact lenses, but its role as a myopia diagnostic tool is not widespread.
Visual Field Tests
Visual field tests are used clinically to systematically assess deficits in the patient’s field of vision. Clinically, visual fields are currently obtained with automated perimeters using sophisticated algorithms to present lights of appropriate size and intensity at different locations in the patient’s field of vision in a repeatable and reproducible fashion. The requirement for patient interaction nevertheless introduces a subjective component into the test. In the context of current myopia care, visual fields are used to assess vision loss related to the increased risk for glaucoma with myopia or directly from pathologic myopia changes.
Summary
A variety of assessments and diagnostic technologies are currently clinically available to identify and characterize the myopic eye. The mainstay of the current clinical assessment includes the history and physical—particularly a measurement of the refraction—and is supplemented by diagnostic technologies, particularly fundus imaging and ocular biometry.
Those assessments and diagnostic tests provide a snapshot of the myopic eye at the time of the exam. This in turn provides a baseline to track longitudinal trajectories and changes in refraction and axial length in response to therapies (see Box 4-1).
DIAGNOSTICS AND ASSESSMENTS IN CLINICAL RESEARCH
Additional diagnostic technologies have been used in clinical research to assess myopia. These are not currently used in standard clinical practice for myopia in the United States and are instead being developed and explored to better understand the myopic eye.
Ocular (Eye) Shape
Eye shape, also referred to as ocular shape, is known to be different in myopic eyes compared to non-myopic eyes. Measurements of eye shape are also important as physical data that can be used to create models of the eye, particularly when combined with refraction data. A variety of methods are used to measure eye shape, including neuroimaging (typically with magnetic resonance imaging [MRI]), ultrasound B-scans, multi-axis refraction and/or biometry, and OCT.
As described earlier, axial myopia results from an elongation of the eye’s axial length. This is only a one-dimensional measurement, though, and researchers have examined the myopic eye in two and three dimensions (i.e., eye shape) to identify other differences in a myopic eye that has grown “too long” for the anterior segment optics. One could hypothesize that an axially elongating eye might produce a football-shaped eye (termed prolate) with the long axis in the anterior-posterior direction; an oblate eye would instead have the long axis in the lateral direction (Figure 4-3). The earliest studies used MRI and ultrasound to explore this and, in some cases, showed that myopic eyes were generally less oblate than non-myopic eyes (Atchison et al., 2005; Cheng et al., 1992).
A less oblate eye in myopia also dovetails well with the peripheral refraction concept of myopia, where in an aspheric posterior eye, peripheral hyperopic defocus can drive eye growth and elongation (Smith, 2011). Performing refractions on-axis and off-axis (central and peripheral) provides information about the position of the retinal image plane relative to the anterior segment optics (Mutti et al., 2000). Ocular biometry can similarly be performed on- and off-axis to provide a physical mapping and measurement of the shape of the posterior eye (Schmid, 2003; Verkicharla et al., 2015). Retinal OCT functions as an automated interferometric sweeping of the posterior eye and hence also provides a physical mapping and measurement of the shape of the posterior eye with better depth and sampling resolution. With the optical techniques, artifacts introduced by refraction and display rendering need to be removed to recover the actual morphometry. Additionally, the direct measurement from these optical techniques is optical path length, which requires assumed or nominal refractive indices to convert to physical distances. Once the artifacts have been removed, OCT recapitulates the findings of the MRI studies using a more readily available ophthalmic technology (Kuo et al., 2016).
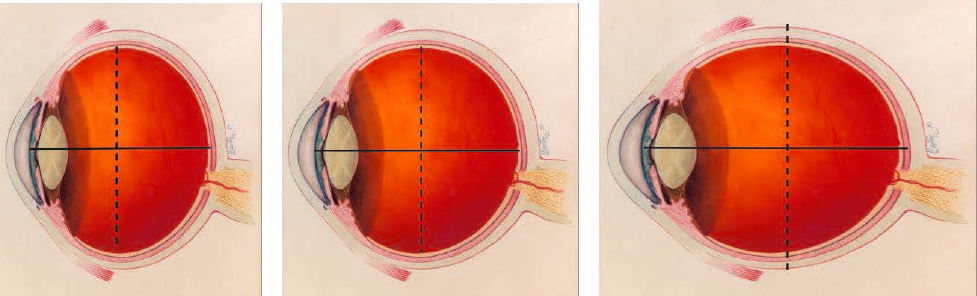
NOTE: The center image represents a nominally circular eye (or in three-dimensions, spherical). The left image represents an oblate eye shape where the equatorial axis (dashed line) is longer than the anterior-posterior axis (solid line, or axial length). The right image represents a prolate eye shape where the equatorial axis is shorter than the anterior-posterior axis (axial length). Again, these are exaggerated to illustrate the concept. In actuality, the differences in axes will usually require measurement rather than mere visual inspection, and few eyes will be at the extremes illustrated here.
SOURCE: Adapted from the eye illustration found at https://medialibrary.nei.nih.gov/sites/default/files/media-images/NEI-medialibrary-2817499.png.
Figure 4-3 describes eye shape in global terms such as prolate, oblate, or spherical. However, global shape descriptors may not account for changes that only occur locally. As an example of a local shape change, a staphyloma is an outpouching of the eye due to a thinned sclera from extensive eye elongation in high myopia. More recently, using higher Tesla magnetic field MRI systems with more resolution, local differences have been shown in shape, particularly in pathologically myopic eyes (Moriyama et al., 2011). The MRIs showed the staphylomatous outpouchings as well as other localized disturbances even when a staphyloma was not identified on clinical exam by the fundus examination. Ultrasound is a more clinically accessible imaging technology in the eye care setting and can also be used to identify local changes such as staphylomas; despite its clinically accessibility, ultrasound has been used in few studies to assess these changes (Ito et al., 2022).
OCT represents resolution improved by an order of magnitude over both ultrasound and even the newer MRI systems used clinically, and in the context of eye shape OCT also shows local posterior eye shape changes in pathologically myopic eyes. OCT has been used to identify and report newly described conditions such as dome-shaped maculopathy (Gaucher et al., 2008), to create new cross-sectional anatomic definitions of staphylomas based on inflections in the profile of the posterior eye (Shinohara et al., 2017), and to enable methods to measure and describe local posterior eye shape variability (McNabb et al., 2021; Tan et al., 2021). The primary limitation of OCT relative to MRI and ultrasound is OCT’s limited imaging range in width and depth, which is due to OCT light needing to reach the target tissue to form the image. Advances using contact techniques hold promise to increase light access to the extreme ocular periphery (Ni et al., 2023), and in myopic eyes with a thinned choroid OCT light is sometimes able to image the sclera of the posterior eye.
The morphology of the posterior eye differs in myopes from what it is in emmetropes, particularly with increasing degrees of myopia, in both global shape descriptors and local shape variability. While some of these morphologic changes can be qualitatively visualized from the image output of the diagnostic devices, quantitative measures have to date relied on research-specific software and image analysis that are not yet broadly available for general clinical use.
Choroidal Imaging
The choroid, situated between the retina and the sclera, is a vital layer of tissue in the eye responsible for supplying oxygen and nutrients to the retina, aiding in its health and function. It also regulates the amount of light entering the eye by absorbing excess stray light, thus preventing glare. Conversely, the sclera, the tough outer layer of the eye, encases and safeguards the eyeball while providing structural support to maintain its shape. Serving as an attachment site for the muscles controlling eye movement, the sclera ensures proper functionality and protection of the eye.
Measuring Choroidal Thickening and Thinning
Choroidal thickness is a compelling area to study in myopia because the choroid is adjacent to the sclera, which remodels in myopia. The vascular choroid can also thicken and thin on short time scales, resulting in anterior and posterior displacement of the retina relative to the anterior segment optics of the eye. The normal choroid in the macula is approximately 200 µm thick. The advent of optical interferometric imaging techniques with sufficient µm scale resolution have allowed the in vivo measurement of choroidal thickness; these techniques include one-dimensional optical biometers using partial-coherence interferometry and two- and three-dimensional OCT (Chiang et al., 2015; Read et al., 2010; Wang et al., 2016). These optical interferometric imaging techniques produce peaks at interface changes, such as between the vitreous and retinal surface, the retinal pigment epithelium at the outer retina-anterior choroidal interface, and the posterior interface between the choroid and the sclera.
The one-dimensional partial-coherence interferometric optical biometers do not automatically find the signal at the posterior choroidal boundary, so it is necessary to manually analyze and mark the waveform it generates to identify that interface and generate measures of choroidal thickness. Due to loss of light reflecting back to the detector in the case of deeper structures and/or noise in the measurements, the ability to identify the more posterior interfaces relies heavily on manual interpretation of the waveform. Similarly, two- and three-dimensional OCT both generate intensity peaks at interface changes, and due to the techniques’ dimensional nature they produce histology-like cross-sectional images, which can be used to identify the anterior and posterior choroidal boundaries for thickness measurements (Figure 4-4).
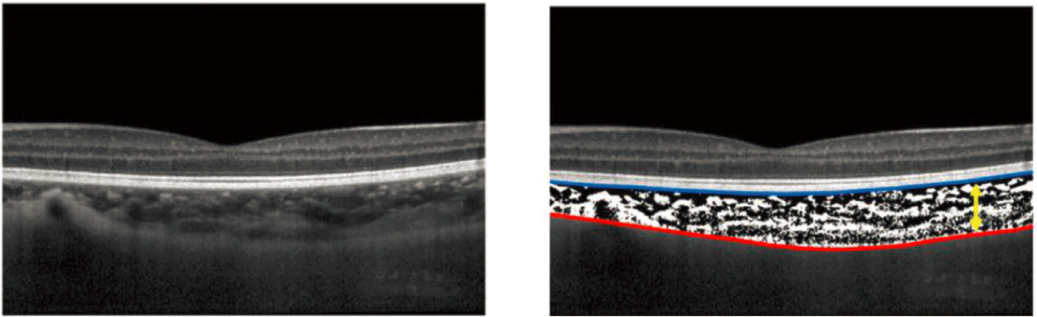
NOTE: The image on the left is an averaged B-scan, and the image on the right shows the anterior (blue) and posterior (red) boundaries labeled to then calculate a thickness value between the two boundaries.
SOURCE: Reprinted from Ostrin et al., 2023, under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International License (https://creativecommons.org/licenses/by-nc-nd/4.0/).
The ability to identify choroidal boundaries is not typically provided in the software packaged with commercial OCT devices to date, so it requires manual, custom-automated, or semi-automated marking and labeling of the boundaries. Again, as an optical technique, OCT is subject to signal loss with tissue depth, a problem affected by such factors as the pigmentation of the imaged eye, the thickness of the choroid itself, and the characteristics and power of the light source of the particular OCT system.
Altogether, studies of human choroidal thickness have generally shown that myopes have a thinner choroid than non-myopes (Read et al., 2019). Measurements of short time-scale dynamic choroidal responses using partial-coherence interferometry and OCT have in some studies shown the expected changes, with transient thickening of the choroid in response to myopic defocus and thinning with hyperopic defocus (Chiang et al., 2015; Read et al., 2010). However, the scale of the changes has been on the order of the resolution of the imaging systems, and other studies have not detected the expected dynamic choroidal responses to defocus (Wang et al., 2016). Technical choices associated with choroidal image analysis, including those discussed earlier--such as manual vs. semi-automated vs. automated segmentation, masked vs. unmasked analysis, area vs. volume reporting--can contribute to the variability of current measurements. Additionally, other physiologic and logistical factors can confound measurements of choroidal thickness, such as time of day and previous fluid consumption (Ostrin et al., 2023).
Overall, though it is compelling and promising, dynamic choroidal imaging in humans remains an active area of study and development.
Measuring Choroidal Vasculature
In addition to choroidal thickness, the vasculature of the choroid itself can be studied. Dye-based angiographic techniques, such as indocyanine green angiography, have been used clinically for examination of the choroidal vasculature in myopia (e.g., Ohno-Matsui et al., 1996). OCT angiography is a more recent dye-free alternative that uses the motion of blood cells within the vessels as the contrast. OCT angiography has been used to image the choroidal vasculature and has shown flow deficits in pathologic myopia that are qualitatively visible on the en face projection, as noted earlier in the standard clinical assessments and diagnostics section.
Metrics have been developed to quantify vessel morphology, such as through measurements of length, branching, and density. However, it should be noted that technical constraints affect the information within a choroidal OCT angiography scan. These can include projection artifacts (“shadows” from more superficial vessels in the scan) and flow detection limits related to the OCT source and scan parameters (blood velocity above and below certain levels does not generate a signal; Corvi et al., 2021; Ferrara et al., 2016). Source image quality, noise, and motion artifacts also affect the derived measures. Resolving these artifacts is an active area of research in the effort to produce accurate depth-resolved angiograms (Wang et al., 2016).
The above-mentioned limits could affect the accuracy and reliability of indices and metrics describing choroidal vasculature morphology in OCT angiography. Nevertheless, setting those limits aside, most cross-sectional studies have generally reflected decreases in vessel density that appear to be consistent with the already noted decrease in choroidal thickness seen with higher myopia (Al-Sheikh et al., 2017; Devarajan et al., 2020; Liu et al., 2022; Wu et al., 2021; Xu et al., 2021), although other cross-sectional studies have not found differences in the vascular parameters (Chang et al., 2022; Scherm et al., 2019).
Scleral Imaging
Scleral imaging is an area of interest in studying myopia, given that elongation of the eye necessarily involves changes to the sclera, particularly the posterior sclera wall. Though ultrasound could be used to image the posterior sclera, there is sparse literature describing this. Optical techniques such as OCT are normally limited by signal loss through the retinal pigment epithelium and choroid, but the thinning of posterior eye layers with high myopia allows deeper OCT imaging and visualization of the full thickness of the posterior sclera (Imamura et al., 2011; Ohno-Matsui, 2012). Cross-sectional studies to date confirm the known clinical observations regarding thinning of posterior eye layers with higher myopia.
Newer directions include investigating in vivo differences in the collagenous organization within the posterior sclera and using the easier-to-access anterior sclera as a proxy for the posterior sclera. Recent attempts to investigate the collagenous organization within the posterior sclera in research participants have used polarization-sensitive OCT (Liu et al., 2023; Ohno-Matsui et al., 2024). Polarization-sensitive OCT can be used to detect scleral birefringence. Birefringence, or double refraction, occurs when a material is not uniformly organized at an atomic or molecular level which will cause light to travel differently through it depending on the polarization of the light and/or the direction light enters the material. In the case of the sclera, the bi-refringence is related to the organization and structure of the collagen within the sclera. Polarization sensitive OCT studies show cross-sectional differences in collagenous organization and structure within the sclera among myopes with and without pathologic features.
Turning to the anterior sclera, its anterior location makes it more clinically accessible and hence an area of interest in research. For instance, there are diagnostic tests for biomechanical properties, but they require direct access to the tissue. This makes them difficult to perform on the posterior sclera, but if there were a relationship between the anterior and posterior sclera properties, then measuring the anterior sclera could serve as a proxy for posterior sclera events. Study results to date using OCT to measure anterior scleral thickness have been mixed, with some studies showing a correlation between refractive errors and anterior scleral thickness (Dhakal et al., 2020; Vurgese et al., 2012; Zhou et al., 2023) and others not showing a correlation (Buckhurst et al., 2015; Pekel et al., 2015).
Pupillometry to Assess Rods, Cones, and Intrinsically Photosensitive Retinal Ganglion Cells (ipRGCs)
Retinal illumination triggers the pupillary light reflex (PLR), a reflexive constriction of the pupillary sphincter muscle. This response has long been exploited by clinicians to probe the integrity of the retina and optic nerve, the parasympathetic innervation of ocular structures (cranial nerve), and the brainstem regions where the two are linked. An example of a pupillometer used clinically is shown in Figure 4-5. The retinal circuitry that signals the pupillary light reflex overlaps heavily with those linked to effects of luminance on axial elongation of the eye. They carry a steady-state irradiance signal driven by the retinal ON channel.
In mice, selective ablation (removal or destruction of a body part or tissue or its function) of ipRGCs eliminates retinal input to the obligatory brainstem relay center and blocks the pupillary light reflex (Guler et al., 2008; Hatori et al., 2008; Hattar et al., 2006). In mouse models lacking rod and cone function, this reflex persists due to the intrinsic photosensitivity of the M1 subtype of ipRGCs. In wild-type mice, however, the pupillary light reflex reflects contributions
from rods and cones as well as from melanopsin within, and this appears to be true in humans as well. The pupillary light reflex thus provides a practical way to assess the integrity of neuronal types and networks that encode luminance at the retinal level. These are ostensibly the same as networks implicated in dopaminergic mechanisms that suppress axial elongation during eye development, as discussed elsewhere in this report (see Chapter 6, Dopaminergic Amacrine Cells and ipRGCs—Irradiance-coding Cells and Circuits).
Methods are available for assessing the specific contributions of rod, cone, and melanopsin to the pupillary light reflex in clinical as well as research settings for animal and human subjects. These methods have been documented in a report from members of the International Pupil Colloquium establishing formal standards for various kinds of pupillometry (Kelbsch et al., 2019). For example, the shutoff of melanopsin phototransduction is slower than in rods and cones, so the post-illumination pupil response, measured well after the light is turned off, provides an index of melanopsin response with minimal rod or cone contribution.
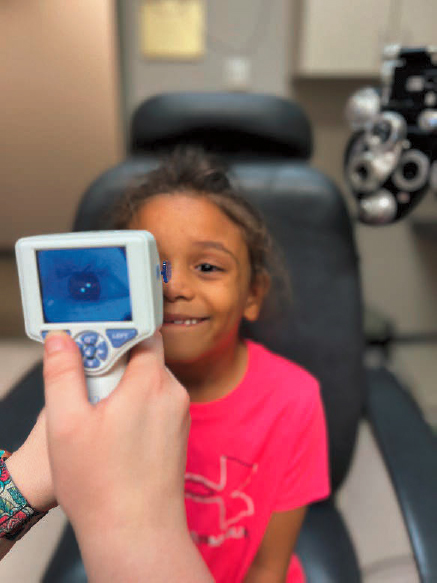
SOURCE: Courtesy of Katherine Weise, OD, University of Alabama at Birmingham.
Adaptive Optics
Adaptive optics is a technique used to improve the resolution of an imaging system by measuring and correcting for aberrations (optical imperfections or distortions) in the imaging path, typically by precise dynamic adjustment of deformable mirrors to compensate for the light distortions in the system. The technique was developed in astronomy to improve the imaging of celestial bodies by accounting for dynamic aberrations introduced by the earth’s atmosphere. Adaptive optics was subsequently used in ophthalmology to correct for the aberrations introduced by the cornea and crystalline lens and obtain high resolution images of cells in the retina that would otherwise be blurred by the eye’s optics in vivo. In addition, adaptive optics can be used to create visual stimuli on the retina that are limited only by diffraction due to the eye’s pupil alone, or to create artificial optical conditions by superimposing new aberrations for
vision simulation. Overall, adaptive optics has not only transformed the study of retinal structure and function in vivo in humans and animal models but provided new avenues for testing the impact of the eye’s native and modified optics (via lenses, refractive surgery, intraocular lenses from cataract surgery, etc.) on visual function.
In the context of myopia, the adaptive optics scanning laser ophthalmoscope has revealed the structure of the foveal cone photoreceptor mosaic and how it varies with axial length (Dabir et al., 2015; Li et al., 2010 Wang et al., 2019). These findings have consequences for models of the expansion of the eye that determine its final shape and the factors that limit visual performance in myopia. Nevertheless, the effect of myopic retinal stretching on inner retinal anatomy and connectivity remains unknown. The use of OCT enhanced by adaptive optics and precise 3D image registration has shown the feasibility of imaging the topography of the ganglion cells in humans in vivo (Liu et al., 2017).
Aberrometry
Aberrometry—or measurement, analysis, and reporting of “optical imperfections” in an optical system—provides a holistic view of the optical quality of the eye and includes the sum of contributions from all the optical elements. This view, while not sufficient to pinpoint the specific optical quality of the crystalline lens or cornea, is very useful for describing the retinal image quality and refraction. Moreover, aberrometry accounts for changes in retinal shape that accompany axial elongation. Aberrometry has advantages over other methods for quantifying refractive error and optical quality in general in the context of myopia. For example, traditional autorefractor and retinoscopic measures of refraction often use smaller pupils compared to the wavefront sensor. The interaction between different aberrations significantly impacts the best image plane (Applegate et al., 2003). The higher-order aberrations are further exaggerated for larger pupils and peripheral eccentricities. Peripheral wavefront sensors are available that allow measuring aberrations across a wide visual field to characterize image quality (Jaeken & Artal, 2012; Jaeken et al., 2011; Pusti et al., 2023). Importantly, the measured aberrations can be converted to metrics of retinal image quality to assess how the image varies with factors such as accommodation and pupil size (Marsack et al., 2004). The application of aberrometry to evaluate the myopic eye is described in Chapter 6 (Eye Models).
Overall, aberrometry, in conjunction with morphometry of the cornea and crystalline lens, lays the foundation for an ideal eye model that would form the basis for testing new treatments and detailing the optics of the myopic eye. To this end, longitudinal studies of aberrometry and morphometry of the cornea and crystalline lens are needed, both early in childhood and prior to myopia onset, so that the causality and progression of the optical changes in normal and myopic eye growth can be investigated.
BOX 4-1
Diagnostic Technologies for Children
While the diagnostic technologies discussed in this chapter (specifically fundus photography, biometry, OCT, ERGs, contrast testing, visual fields, pupillometry, and aberrometry) are primarily used in adults and were not designed specifically for children, they can also be performed in children—especially school-aged and older depending on the degree of cooperation from the child and the patience of the person performing the test.
Some younger children may also be able to participate in the testing. Large-scale, randomized clinical trials of children with myopia often include several of the tests listed. However, there will be older children who will not have the attention or motivation to complete testing on a given day. In such cases, returning for retesting alone may be helpful to maximize attention and motivation and minimize apprehension.
To make diagnostic technology explicitly child-friendly, manufacturers should consider testing that:
- does not require direct contact with the eye: the further from the child, the better
- takes no more than a few seconds to minutes to obtain the measurement
- can be adjusted for varying sizes of children
- is portable or handheld so it can be applied in various settings
- avoids needing long instructions
- avoids using very bright lights
- has pictures for matching response or requested task
- has normative data for children if it is expected to differ from that of an adult.
Regarding the last bullet, some diagnostic devices will assist in interpretation of the measurement results by comparing them to normative databases of the measurement. If the measurement was not designed specifically for children, the normative database is typically based on adult measurements (e.g., OCT nerve fiber layer thicknesses for glaucoma). Hence, to be useful in children, normative databases for specific diagnostic measurements should also include data for children.
DIAGNOSTICS FOR ASSESSING THE ENVIRONMENT
The visual environment has been associated with the development of myopia in many studies. This includes associations with increased myopia and near work (Guo et al., 2016; Pärssinen & Kauppinen, 2019) and decreased myopia with more outdoor activity (He et al., 2015; Jones et al., 2007; Rose et al., 2008; Wu et al., 2020). Near work and outdoor time have predominantly been assessed using surveys and questionnaires or indirectly, such as by using educational attainment or occupation as a proxy for the amount of near work. To further study these environmental conditions, methods to objectively measure specific environmental variables have been developed. These include wearable light meters (because light is one of the principal aspects of the outdoors) and depth-sensing cameras to measure the distance of objects in the environment.
Wearable Light Meters
Wearable light meters are used to detect and record the wearer’s environmental exposure to light. Reported wearable light meters to date have included dedicated, single-function light sensors worn as a pendant or affixed to clothing (Read et al., 2018), dedicated light sensors that can be attached to worn eyeglasses (Stampfi et al., 2023; Wen et al., 2021), and multi-function wristwatches with an incorporated light sensor (Read et al., 2015; Verkicharla et al. 2017; Ye et al., 2019). Depending on the configurations, the multi-function wristwatches could also measure other parameters (date/time, geographic position) that in turn could be used to obtain other associated data (e.g., weather) used to supplement the light measurement data. The light sensors on these wristwatches typically measure visible light intensity, though some have ultraviolet or near infrared measurement capabilities dependent on the included sensor. The published wristwatch studies have used either commercially available software and hardware (Philips Actiwatch 2), custom-developed software for a commercially available smartwatch (FitSight: custom app on a Sony SmartWatch), or custom-developed hardware and software (MuMu). As an aside, contemporary iOS and Android smartwatches do include light sensors in their suite of sensors. Though there have not yet been reports of their use in myopia studies, these in-built sensors appear to present an opportunity to obtain the desired light measurement from a relatively general-purpose device.
Depth-Sensing Cameras
Depth-sensing cameras are used for detecting the position of objects in the environment relative to the camera. A variety of techniques can be used for depth sensing, including structured illumination, by which a pattern is used to illuminate a scene and changes in the pattern are used to estimate the 3D position of elements in the scene; time-of-flight, which like radar or ultrasound uses the time it takes emitted energy to reflect from the scene back to the detector and is used to calculate the position of that element in the scene relative to the camera; and stereo cameras, where two or more cameras with a fixed relationship are used to image the scene and differences between the two imaged perspectives are used to calculate the position of objects in the scene.
In the study of myopia, wearable depth-sensing cameras can record the position of objects in the workspace of the wearer to provide direct measurements of the distance of objects during near work or outdoor activities. Consumer-grade (i.e., readily commercially attainable) depth-sensing cameras have only become available in the last decade, and the application of this technology in myopia is still new. Recent studies attaching these cameras to spectacle-like frames or helmets have demonstrated their potential use not only in relatively stationary indoor environments (García et al., 2017; Read et al., 2023) but even in dynamic, open outdoor environments (Banks, 2024). Alternatively, some current smartphones also have integrated depth-sensing cameras. Smartphone cameras designed and intended for face-scanning of the user can measure spatial relationships between the phone and its user. In the context of myopia, this information is used by on-device apps (e.g., Apple’s Screen Distance) and has been used by researchers to objectively record viewing distance for children using smartphones (Richards et al., 2024).
Altogether, these increasingly accessible environment-sensing diagnostics and technologies are likely to constitute critical technologies for measuring and understanding the world that the myopic eye experiences, which this report refers to as the “visual diet.”
OTHER ASSESSMENT AND DIAGNOSTIC TECHNOLOGIES
Artificial intelligence (including machine learning and deep learning) is a rapidly advancing area in general and specifically in ophthalmic research. While the concept of artificial intelligence is not new, within the last decade deep learning approaches such as convolutional neural networks have made rapid gains in tasks such as image classification that rival human performance (e.g., ImageNet challenge). The widespread use of diagnostic imaging in ophthalmology and optometry have benefited from these recent advances in artificial intelligence-based image classification. This research has progressed to the point that there are now U.S. Federal Drug Administration approved artificial intelligence–based diabetic retinopathy diagnostics for fundus photographs (U.S. Food & Drug Administration, 2018).
Artificial intelligence approaches are particularly suitable for complex entities with multiparameter and/or nonlinear associations and typically depend on large, labeled datasets for training. Because myopia has complex genetic/hereditary and environmental underpinnings and exists in a field where diagnostic imaging is common, myopia seems particularly amenable to artificial intelligence approaches. Efforts to date in the myopia space have focused on diagnostic classification of images (e.g., presence of myopia, degree of myopia), identification of pathologic myopia features within images, prediction of targeted parameters (e.g., refractive errors, related parameters from other imaging modalities) from diagnostic imaging, and prediction of progression from biometric data, refraction data, or other recorded medical data (Du & Ohno-Matsui, 2022).
Artificial intelligence for myopia is still nascent and growing, and there is not yet broad usage or consensus as to its role in current clinical practice for myopia. Even for research, the ability to investigate associations, perform classifications, and create predictions is linked to the availability of the underlying data. Investigations are limited to recorded data; in other words, if one wants to predict the future axial-length elongation of an eye from refraction measurements, at some point a dataset with refraction and longitudinal axial-length data will need to be used for training. Clinical datasets will inherently be limited to the available diagnostic measurements. Also, the set of training data required for deep learning approaches is typically large, though if a similar model already exists, transfer learning approaches can be used that decrease the size of new training data.
Electronic health records hold the promise of large pools of medical data. However, because one clinician may qualitatively describe the same entity differently than another clinician or some data may simply not be recorded, the variability and consistency of the recording of granular data require an extensive investment in data preprocessing, cleaning, and harmonization before its use for applications such as artificial intelligence. There are current efforts underway to perform these tasks in the ophthalmic space, such as that of the Sight Outcomes Research Collaborative, 1 to pool ophthalmic data in electronic health records across multiple large academic medical centers. For both artificial intelligence and electronic health records, there can be limitations in representation and hence resultant bias. For instance, the underrepresentation of populations—whether they be underrepresented by ethnicity, by age, by lack of clinical usage, or by other characteristics—will be perpetuated in subsequent models or analyses whether in a training dataset for artificial intelligence or in an electronic health record
___________________
1 For more information, see https://www.sourcecollaborative.org/about
dataset (Evans et al. 2022). The potential of artificial intelligence approaches to assist in myopia diagnostics has certainly been recognized, and the field is currently actively developing the data sources and defining the desired associations to realize that potential.
CONCLUSIONS
The landscape of myopia assessment and diagnosis remains marked by a notable absence of consensus regarding mandatory components within clinical examinations. While routine or comprehensive examination standards exist, specific guidelines for patients with myopia lack uniformity, leading to a spectrum of variability in clinical practices. This lack of standardization not only affects the consistency of clinical care but also hampers the availability of cohesive data types crucial for subsequent analyses, including population studies and advancements in artificial intelligence. Despite this challenge, the horizon is bright with the emergence of promising diagnostic biomarkers poised to revolutionize myopia diagnostics, management, and our understanding of the disease. However, their definitive role in both research and clinical care necessitates further exploration through rigorous clinical studies.
Furthermore, given that children represent the demographic most susceptible to myopia progression, there is a pressing need to develop assessment and diagnostic technologies tailored specifically for this vulnerable population. Such initiatives hold the potential to significantly enhance accessibility and efficacy of myopia management strategies targeted toward children, thus addressing a critical aspect of public health concern.
Conclusion 4-1: There is no consensus as to the mandatory assessment and diagnostic components of a clinical examination of the myopic patient aside from clinical standards for routine/comprehensive examinations in general. This lack of standardization creates variability in clinical care and affects the availability of data for downstream analyses such as for population studies and artificial intelligence efforts. When available and feasible, the following clinical tools may enhance the assessment of the myopic eye.
- In addition to a dilated fundus exam, fundus imaging is valuable to assessing posterior eye pathologies associated with myopia, as it records color views of the retina.
- Optical coherence tomography provides high-resolution cross-sections for more detailed assessment of structural retinal changes.
- Devices that measure axial length allow for more detailed assessment of eye growth, which may help assess risk to eye health.
Conclusion 4-2: There are promising diagnostic biomarkers (e.g., axial elongation, changes in choroidal thickness and ocular shape, optical aberrations) and technologies (electroretinograms based on the hypothesis that outer-retinal neuron signaling affects myopia development) that may be useful for myopia diagnostics, management, and understanding of the disease. Further work in clinical studies is needed to definitively establish their role in research and clinical care of myopia.
Conclusion 4-3: Children are most at risk of myopia progression, and designing assessment and diagnostic technologies for children enhances the description of their myopic eyes, which may allow for more precise treatment in this critical population.
RECOMMENDATIONS
Recommendation 4-1: Ophthalmologists and optometrists should always use cycloplegic eye drops in children to obtain a consistent retinoscopy/refractive error reading along with pupillary dilation for visualizing the health of the middle and back of the eye, particularly for younger patients who have large accommodative and pupil constriction ability. If possible and available, other objective structural measurements of the eye should be obtained, such as axial length and optical coherence tomography. Payors (e.g., health/vision insurances) should reimburse for these examinations and tests to ensure their performance longitudinally.
Recommendation 4-2: Researchers and developers of assessment and diagnostic technologies should design assessments and tests to better understand the myopic eye, its development, and its environment (the visual diet). In addition to identifying eyes that are already myopic, there is also a need to identify eyes at risk for myopia in childhood and to identify other key events (e.g., pre-pathologic myopia state in adulthood). Other diagnostic technologies to support these goals include, for example, biometric and functional measurements to develop individualized eye modeling, improved choroidal imaging, and methods to sense and measure the visual environment.
Recommendation 4-3: Professionals examining the eye and organizations representing them (such as the American Academy of Pediatrics, American Academy of Pediatric Ophthalmology and Strabismus, American Academy of Optometry, and American Academy of Ophthalmology), together with researchers and other stakeholders in the field of myopia, should discuss and develop consensus standards for the assessments and diagnostics they deem most important for population-level studies. Development of a consortium/network repository for myopia-related clinical data by international or national entities (e.g., International Myopia Institute, National Eye Institute) would further these efforts. This would benefit standardization not only for clinical care but also for research, particularly with artificial intelligence efforts.
Recommendation 4-4: Developers of assessment and diagnostic technologies should consider the ability to use the technology in multiple age groups and settings as major design criteria. This includes making the technology time-efficient and “child-friendly.” To ensure the broadest adoption, such technologies should also be made as portable and cost-effective for the end user as is feasible.
REFERENCES
Al-Sheikh, M., Phasukkijwatana, N., Dolz-Marco, R., Rahimi, M., Iafe, N. A., Freund, K. B., Sadda, S. R., & Sarraf, D. (2017). Quantitative OCT angiography of the retinal microvasculature and the choriocapillaris in myopic eyes. Investigative Ophthalmology & Visual Science, 58(4), 2063–2069. https://doi.org/10.1167/iovs.16-21289
American Academy of Ophthalmology. (2020). Pathologic myopia with tilted disc and peripapillary atrophy of RPE and choroid [Image]. Eyewiki. https://eyewiki.org/File:AA0_13422.jpg
Applegate, R. A., Hilmantel, G., Thibos, L. N., & Hong, X. (2003). Interaction between aberrations to improve or reduce visual performance. Journal of Cataract & Refractive Surgery, 29(8), 1487–1495. http://dx.doi.org/10.1016/S0886-3350(03)00334-1
Atchison, D. A., Pritchard, N., Schmid, K. L., Scott, D. H., Jones, C. E., & Pope, J. M. (2005). Shape of the retinal surface in emmetropia and myopia. Investigative Ophthalmology & Visual Science, 46(8), 2698–2707. https://doi.org/10.1167/iovs.04-1506
Banks, M. (2023). Depth statistics of indoor and outdoor light [Workshop presentation]. Workshop on the Rise in Myopia: Exploring Possible Contributors and Investigating Screening Practices, Policies, and Programs. National Academies of Sciences, Engineering, and Medicine. Washington, DC, United States. https://www.nationalacademies.org/event/41360_12-2023_workshop-on-the-rise-in-myopia-exploring-possible-contributors-and-investigating-screening-practices-policies-and-programs
Buckhurst, H. D., Gilmartin, B., Cubbidge, R. P., & Logan, N. S. (2015). Measurement of scleral thickness in humans using anterior segment optical coherent tomography. PLOS One, 10(7), e0132902. https://doi.org/10.1371/journal.pone.0132902
Chan, H. H. L., Choi, K. Y., Ng, A. L. K., Choy, B. N. K., Chan, J. C. H., Chan, S. S. H., Li, S. Z. C., & Yu, W. Y. (2022). Efficacy of 0.01% atropine for myopia control in a randomized, placebo-controlled trial depends on baseline electroretinal response. Scientific Reports, 12(1), 11588. https://doi.org/10.1038/s41598-022-15686-6
Chang, X., Li, M., Lv, L., Yan, X., Liu, Y., Zhu, M., Wang, J., Wang, P., & Xiang, Y. (2022). Assessment of choroidal vascularity and choriocapillaris blood perfusion after accommodation in myopia, emmetropia, and hyperopia groups among children. Frontiers in Physiology, 13, 854240. https://doi.org/10.3389/fphys.2022.854240
Cheng, H. M., Singh, O. S., Kwong, K. K., Xiong, J., Woods, B. T., & Brady, T. J. (1992). Shape of the myopic eye as seen with high-resolution magnetic resonance imaging. Optometry and Vision Science, 69(9), 698–701. https://doi.org/10.1097/00006324-199209000-00005
Chiang, S. T., Phillips, J. R., & Backhouse, S. (2015). Effect of retinal image defocus on the thickness of the human choroid. Ophthalmic & Physiological Optics, 35(4), 405–413. https://doi.org/10.1111/opo.12218
Corvi, F., Su, L., & Sadda, S. R. (2021). Evaluation of the inner choroid using OCT angiography. Eye, 35(1), 110–120. https://doi.org/10.1038%2Fs41433-020-01217-y
Dabir, S., Mangalesh, S., Schouten, J. S., Berendschot, T. T., Kurian, M. K., Kumar, A. K., Yadav, N. K., & Shetty, R. (2015). Axial length and cone density as assessed with adaptive optics in myopia. Indian Journal of Ophthalmology, 63(5), 423–426. https://doi.org/10.4103/0301-4738.159876
Devarajan, K., Sim, R., Chua, J., Wong, C. W., Matsumura, S., Htoon, H. M., Schmetterer, L., Saw, S. M., & Ang, M. (2020). Optical coherence tomography angiography for the assessment of choroidal vasculature in high myopia. The British Journal of Ophthalmology, 104(7), 917–923. https://doi.org/10.1136/bjophthalmol-2019-314769
Dhakal, R., Vupparaboina, K. K., & Verkicharla, P. K. (2020). Anterior sclera undergoes thinning with increasing degree of myopia. Investigative Ophthalmology & Visual Science, 61(4), 6. https://doi.org/10.1167/iovs.61.4.6
Du, R., & Ohno-Matsui, K. (2022). Novel uses and challenges of artificial intelligence in diagnosing and managing eyes with high myopia and pathologic myopia. Diagnostics (Basel), 12(5), 1210. https://doi.org/10.3390%2Fdiagnostics12051210
Evans, N. G., Wenner, D. M., Cohen, I. G., Purves, D., Chiang, M. F., Ting, D. S. W., & Lee, A. Y. (2022). Emerging ethical considerations for the use of artificial intelligence in ophthalmology. Ophthalmology Science, 2(2), 100141. https://doi.org/10.1016/j.xops.2022.100141
Ferrara, D., Waheed, N. K., & Duker, J. S. (2016). Investigating the choriocapillaris and choroidal vasculature with new optical coherence tomography technologies. Progress in Retinal and Eye Research, 52, 130–155. https://doi.org/10.1016/j.preteyeres.2015.10.002
Frishman, L. J. (2013). Electrogenesis of the electroretinogram. In S. R. Sadda (Ed.), Ryan’s Retinal Imaging and Diagnostics (pp. e178–e202). https://doi.org/10.1016/b978-0-323-26254-5.00007-7
García, M. G., Ohlendorf, A., Schaeffel, F., & Wahl, S. (2017). Dioptric defocus maps across the visual field for different indoor environments. Biomedical Optics Express, 9(1), 347–359. https://doi.org/10.1364/BOE.9.000347
Gaucher, D., Erginay, A., Lecleire-Collet, A., Haouchine, B., Puech, M., Cohen, S. Y., Massin, P., & Gaudric, A. (2008). Dome-shaped macula in eyes with myopic posterior staphyloma. American Journal of Ophthalmology, 145(5), 909–914. https://doi.org/10.1016/j.ajo.2008.01.012
Guo, L., Yang, J., Mai, J., Du, X., Guo, Y., Li, P., & Zhang, W. H. (2016). Prevalence and associated factors of myopia among primary and middle school-aged students: A school-based study in Guangzhou. Eye, 30(6), 796–804. https://doi.org/10.1038/eye.2016.39
Gupta, S. K., Chakraborty, R., & Verkicharla, P. K. (2022). Electroretinogram responses in myopia: A review. Documenta Ophthalmologica, 145, 77–95. https://doi.org/10.1007/s10633-021-09857-5
Gupta, Y., Weng, C. Y., & Lim, J. I. (2024). Myopic CNVM. EyeWiki. American Academy of Ophthalmology. https://eyewiki.org/Myopic_CNVM
Gwiazda, J., Thorn, F., Bauer, J., & Held, R. (1993). Myopic children show insufficient accommodative response to blur. Investigative Ophthalmology & Visual Science, 34(3), 690–694. https://pubmed.ncbi.nlm.nih.gov/8449687/
Hattar, S., Kumar, M., Park, A., Tong, P., Tung, J., Yau, K. W., & Berson, D. M. (2006). Central projections of melanopsin-expressing retinal ganglion cells in the mouse. Journal of Comparative Neurology, 497(3), 326–349. https://doi.org/10.1002/cne.20970
He, M., Xiang, F., Zeng, Y., Mai, J., Chen, Q., Zhang, J., Smith, W., Rose, K., & Morgan, I. G. (2015). Effect of time spent outdoors at school on the development of myopia among children in China: A randomized clinical trial. JAMA, 314(11), 1142–1148. https://doi.org/10.1001/jama.2015.10803
Ichinose, T., & Habib, S. (2022). ON and OFF Signaling Pathways in the Retina and the Visual System. Frontiers in Ophthalmology, 2, 989002. https://doi.org/10.3389/fopht.2022.989002
Imamura, Y., Iida, T., Maruko, I., Zweifel, S. A., & Spaide, R. F. (2011). Enhanced depth imaging optical coherence tomography of the sclera in dome-shaped macula. American Journal of Ophthalmology, 151(2), 297–302. https://doi.org/10.1016/j.ajo.2010.08.014
Ito, K., Lye, T. H., Dan, Y. S., Yu, J. D. G., Silverman, R. H., Mamou, J., & Hoang, Q. V. (2022). Automated classification and detection of staphyloma with ultrasound images in pathologic myopia eyes. Ultrasound in Medicine & Biology, 48(12), 2430–2441. https://doi.org/10.1016/j.ultrasmedbio.2022.06.010
Jacobs, D. S., Afshari, N. A., Bishop, R. J., Keenan, J. D., Lee, J. K., Shen, T. T., & Vitale, S. (2022). Refractive errors Preferred Practice Pattern guideline 2022. American Academy of Ophthalmology. https://www.aao.org/education/preferred-practice-pattern/refractive-errors-ppp-2022
Jaeken, B., & Artal, P. (2012). Optical quality of emmetropic and myopic eyes in the periphery measured with high-angular resolution. Investigative Ophthalmology & Visual Science, 53(7), 3405–3413. https://doi.org/10.1167/iovs.11-8993
Jaeken, B., Lundström, L., & Artal, P. (2011). Fast scanning peripheral wave-front sensor for the human eye. Optics Express, 19(8), 73–82. https://doi.org/10.1364/oe.19.007903
Jia, Y., Bailey, S. T., Hwang, T. S., McClintic, S. M., Gao, S. S., Pennesi, M. E., Flaxel, C. J., Lauer, A. K., Wilson, D. J., Hornegger, J., Fujimoto, J. G., & Huang, D. (2015). Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proceedings of the National Academy of Sciences of the United States of America, 112(18), E2395–E2402. https://doi.org/10.1073/pnas.1500185112
Jones, L. A., Mitchell, G. L., Mutti, D. O., Hayes, J. R., Moeschberger, M. L., & Zadnik, K. (2005). Comparison of ocular component growth curves among refractive error groups in children. Investigative Ophthalmology & Visual Science, 46(7), 2317–2327. https://doi.org/10.1167/iovs.04-0945
Jones, L. A., Sinnott, L. T., Mutti, D. O., Mitchell, G. L., Moeschberger, M. L., & Zadnik, K. (2007). Parental history of myopia, sports and outdoor activities, and future myopia. Investigative Ophthalmology & Visual Science, 48(8), 3524–3532. https://doi.org/10.1167/iovs.06-1118
Kelbsch, C., Strasser, T., Chen, Y., Feigl, B., Gamlin, P. D., Kardon, R., Peters, T., Roecklein, K. A., Steinhauer, S. R., Szabadi, E., Zele, A. J., Wilhelm, H., & Wilhelm, B. J. (2019). Standards in Pupillography. Frontiers in Neurology, 10, 129. https://doi.org/10.3389/fneur.2019.00129
Kuo, A. N., Verkicharla, P. K., McNabb, R. P., Cheung, C. Y., Hilal, S., Farsiu, S., Chen, C., Wong, T. Y., Ikram, M. K., Cheng, C. Y., Young, T. L., Saw, S. M., & Izatt, J. A. (2016). Posterior eye shape measurement with retinal OCT compared to MRI. Investigative Ophthalmology & Visual Science, 57(9), OCT196–OCT203. https://doi.org/10.1167/iovs.15-18886
Li, K. Y., Tiruveedhula, P., & Roorda, A. (2010). Intersubject variability of foveal cone photoreceptor density in relation to eye length. Investigative Ophthalmology & Visual Science, 51(12), 6858–6867. https://doi.org/10.1167/iovs.10-5499
Liou, S. W., & Chiu, C. J. (2001). Myopia and contrast sensitivity function. Current Eye Research, 22(2), 81–84. https://doi.org/10.1076/ceyr.22.2.81.5530
Liu, L., Zhu, C., Yuan, Y., Hu, X., Chen, C., Zhu, H., & Ke, B. (2022). Three-dimensional choroidal vascularity index in high myopia using swept-source optical coherence tomography. Current Eye Research, 47(3), 484–492. https://doi.org/10.1080/02713683.2021.2006236
Liu, X., Jiang, L., Ke, M., Sigal, I. A., Chua, J., Hoang, Q. V., Chia, A. W., Najjar, R. P., Tan, B., Cheong, J., Bellemo, V., Chong, R. S., Girard, M. J. A., Ang, M., Liu, M., Garhöfer, G., Barathi, V. A., Saw, S. M., Villiger, M., & Schmetterer, L. (2023). Posterior scleral birefringence measured by triple-input polarization-sensitive imaging as a biomarker of myopia progression. Nature Biomedical Engineering, 7(8), 986–1000. https://doi.org/10.1038/s41551-023-01062-w
Liu, Z., Kurokawa, K., Zhang, F., Lee, J. J., & Miller, D. T. (2017). Imaging and quantifying ganglion cells and other transparent neurons in the living human retina. Proceedings of the National Academy of Sciences of the United States of America, 114(48), 12803–12808. https://doi.org/10.1073/pnas.1711734114
Mariampillai, A., Standish, B. A., Moriyama, E. H., Khurana, M., Munce, N. R., Leung, M. K., Jiang, J., Cable, A., Wilson, B. C., Vitkin, I. A., & Yang, V. X. (2008). Speckle variance detection of microvasculature using swept-source optical coherence tomography. Optics Letters, 33(13), 1530–1532. https://doi.org/10.1364/ol.33.001530
Marsack, J. D., Thibos, L. N., & Applegate, R. A. (2004). Metrics of optical quality derived from wave aberrations predict visual performance. Journal of Vision, 4(4). https://doi.org/10.1167/4.4.8
McNabb, R. P., Polans, J., Keller, B., Jackson-Atogi, M., James, C. L., Vann, R. R., Izatt, J. A., & Kuo, A. N. (2018). Wide-field whole eye OCT system with demonstration of quantitative retinal curvature estimation. Biomedical Optics Express, 10(1), 338–355. https://doi.org/10.1364/BOE.10.000338
McNabb, R. P., Liu, A. S., Gospe, S. M., 3rd, El-Dairi, M., Meekins, L. C., James, C., Vann, R. R., Izatt, J. A., & Kuo, A. N. (2021). Quantitative topographic curvature maps of the posterior eye utilizing optical coherence tomography. Retina, 41(4), 804–811. https://doi.org/10.1097/IAE.0000000000002897
Mooney, S. W. J., Alam, N. M., & Prusky, G. T. (2021). Tracking-based interactive assessment of saccades, pursuits, visual field, and contrast sensitivity in children with brain injury. Frontiers in Human Neuroscience, 15, 737409. https://doi.org/10.3389/fnhum.2021.737409
Moriyama, M., Ohno-Matsui, K., Hayashi, K., Shimada, N., Yoshida, T., Tokoro, T., & Morita, I. (2011). Topographic analyses of shape of eyes with pathologic myopia by high-resolution three-dimensional magnetic resonance imaging. Ophthalmology, 118(8), 1626–1637. https://doi.org/10.1016/j.ophtha.2011.01.032
Mutti, D. O., Hayes, J. R., Mitchell, G. L., Jones, L. A., Moeschberger, M. L., Cotter, S. A., Kleinstein, R. N., Manny, R. E., Twelker, J. D., Zadnik, K., & CLEERE Study Group. (2007). Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Investigative Ophthalmology & Visual Science, 48(6), 2510–2519. https://doi.org/10.1167/iovs.06-0562
Mutti, D. O., Mitchell, G. L., Hayes, J. R., Jones, L. A., Moeschberger, M. L., Cotter, S. A., Kleinstein, R. N., Manny, R. E., Twelker, J. D., Zadnik, K., & CLEERE Study Group. (2006). Accommodative lag before and after the onset of myopia. Investigative Ophthalmology & Visual Science, 47(3), 837–846. https://doi.org/10.1167/iovs.05-0888
Mutti, D. O., Sholtz, R. I., Friedman, N. E., & Zadnik, K. (2000). Peripheral refraction and ocular shape in children. Investigative Ophthalmology & Visual Science, 41(5), 1022–1030. https://pubmed.ncbi.nlm.nih.gov/10752937/
Nallasamy, S., Silbert, D. I., & Chang, L. (2024). EyeWiki: Photoscreening. EyeWiki. American Academy of Ophthalmology. https://eyewiki.aao.org/Photoscreening
National Eye Institute. (n.d.). Illustrations of different eye shapes [Image]. https://medialibrary.nei.nih.gov/media/3551
___. (2020). Normal retina: Fundus photograph-normal retina [Image]. https://medialibrary.nei.nih.gov/media/3825
Ni, S., Nguyen, T. P., Ng, R., Woodward, M., Ostmo, S., Jia, Y., Chiang, M. F., Huang, D., Skalet, A. H., Campbell, J. P., & Jian, Y. (2023). Panretinal optical coherence tomography. IEEE Transactions on Medical Imaging, 42(11), 3219–3228. https://doi.org/10.1109/TMI.2023.3278269
Ohno-Matsui, K., Akiba, M., Moriyama, M., Shimada, N., Ishibashi, T., Tokoro, T., & Spaide, R. F. (2012). Acquired optic nerve and peripapillary pits in pathologic myopia. Ophthalmology, 119(8), 1685–1692. https://doi.org/10.1016/j.ophtha.2012.01.047
Ohno-Matsui, K., Igarashi-Yokoi, T., Azuma, T., Sugisawa, K., Xiong, J., Takahashi, T., Uramoto, K., Kamoi, K., Okamoto, M., Banerjee, S., & Yamanari, M. (2024). Polarization-sensitive OCT imaging of scleral abnormalities in eyes with high myopia and dome-shaped macula. JAMA Ophthalmology, 142(4), 310–319. https://doi.org/10.1001/jamaophthalmol.2024.0002
Ohno-Matsui, K., & Tokoro, T. (1996). The progression of lacquer cracks in pathologic myopia. Retina, 16(1), 29–37. https://doi.org/10.1097/00006982-199616010-00006
Ostrin, L. A., Harb, E., Nickla, D. L., Read, S. A., Alonso-Caneiro, D., Schroedl, F., Kaser-Eichberger, A., Zhou, X., & Wildsoet, C. F. (2023). IMI—The dynamic choroid: New insights, challenges, and potential significance for human myopia. Investigative Ophthalmology & Visual Science, 64(6), 4. https://doi.org/10.1167/iovs.64.6.4
Pardue, M. T., & Peachey, N. S. (2014). Mouse b-wave mutants. Documenta Ophthalmologica. Advances in Ophthalmology, 128(2), 77–89. https://doi.org/10.1007/s10633-013-9424-8
Park, H., Tan, C. C., Faulkner, A., Jabbar, S. B., Schmid, G., Abey, J., Iuvone, P. M., & Pardue, M. T. (2013). Retinal degeneration increases susceptibility to myopia in mice. Molecular Vision, 19, 2068–2079. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3786452/
Pärssinen, O., & Kauppinen, M. (2019). Risk factors for high myopia: A 22-year follow-up study from childhood to adulthood. Acta Ophthalmologica, 97(5), 510–518. https://doi.org/10.1111/aos.13964
Pekel, G., Yağcı, R., Acer, S., Ongun, G. T., Çetin, E. N., & Simavlı, H. (2015). Comparison of corneal layers and anterior sclera in emmetropic and myopic eyes. Cornea, 34(7), 786–790. https://doi.org/10.1097/ICO.0000000000000422
Poudel, S., Jin, J., Rahimi-Nasrabadi, H., Dellostritto, S., Dul, M. W., Viswanathan, S., & Alonso, J. M. (2024). Contrast sensitivity of ON and OFF human retinal pathways in myopia. The Journal of Neuroscience, 44(3), e1487232023. https://doi.org/10.1523/jneurosci.1487-23.2023
Pusti, D., Kendrick, C. D., Wu, Y., Ji, Q., Jung, H. W., & Yoon, G. (2023). Widefield wavefront sensor for multidirectional peripheral retinal scanning. Biomedical Optics Express, 14(9), 4190–4204. https://doi.org/10.1364%2FBOE.491412
Ramkumar, H. L., Epley, D., Shah, V. A., Kumar, U. R., Tripathy, K., Prakalapakorn, S. G., Hyde, R. A., Lim, J. I., Griffiths, D., Karth, P. A., & Rodriguez, S. (2024). Electroretinogram. EyeWiki. American Academy of Ophthalmology. https://eyewiki.aao.org/Electroretinogram
Read, S. A., Collins, M. J., & Sander, B. P. (2010). Human optical axial length and defocus. Investigative Ophthalmology & Visual Science, 51(12), 6262–6269. https://doi.org/10.1167/iovs.10-5457
Read, S. A., Collins, M. J., & Vincent, S. J. (2015). Light exposure and eye growth in childhood. Investigative Ophthalmology & Visual Science, 56(11), 6779–6787. https://doi.org/10.1167/iovs.14-15978
Read, S. A., Fuss, J. A., Vincent, S. J., Collins, M. J., & Alonso-Caneiro, D. (2019). Choroidal changes in human myopia: Insights from optical coherence tomography imaging. Clinical & Experimental Optometry, 102(3), 270–285. https://doi.org/10.1111/cxo.12862
Read, S. A., Vincent, S. J., Tan, C. S., Ngo, C., Collins, M. J., & Saw, S. M. (2018). Patterns of daily outdoor light exposure in Australian and Singaporean children. Translational Vision Science & Technology, 7(3), 8. https://doi.org/10.1167/tvst.7.3.8
Richards, J., Jakulski, M., Rickert, M., & Kollbaum, P. (2024). Digital device viewing behavior in children. Ophthalmic and Physiological Optics, 44(3), 546–553. https://doi.org/10.1111/opo.13288
Rose, K. A., Morgan, I. G., Ip, J., Kifley, A., Huynh, S., Smith, W., & Mitchell, P. (2008). Outdoor activity reduces the prevalence of myopia in children. Ophthalmology, 115(8), 1279–1285. https://doi.org/10.1016/j.ophtha.2007.12.019
Scherm, P., Pettenkofer, M., Maier, M., Lohmann, C. P., & Feucht, N. (2019). Choriocapillary blood flow in myopic subjects measured with OCT angiography. Ophthalmic Surgery, Lasers & Imaging Retina, 50(5), e133–e139. https://doi.org/10.3928/23258160-20190503-13
Schiller, P. H. (1992). The ON and OFF channels of the visual system. Trends in Neurosciences, 15(3), 86–92. https://doi.org/10.1016/0166-2236(92)90017-3
Schmid, G. F. (2003). Axial and peripheral eye length measured with optical low coherence reflectometry. Journal of Biomedical Optics, 8(4), 655–662. https://doi.org/10.1117/1.1606461
Shinohara, K., Shimada, N., Moriyama, M., Yoshida, T., Jonas, J. B., Yoshimura, N., & Ohno-Matsui, K. (2017). Posterior staphylomas in pathologic myopia imaged by widefield optical coherence tomography. Investigative Ophthalmology & Visual Science, 58(9), 3750–3758. https://doi.org/10.1167/iovs.17-22319
Smith, E. L. III. (2011). Prentice Award Lecture 2010: A case for peripheral optical treatment strategies for myopia. Optometry and Vision Science, 88(9), 1029–1044. https://doi.org/10.1097%2FOPX.0b013e3182279cfa
Stampfli, J. R., Schrader, B., di Battista, C., Häfliger, R., Schälli, O., Wichmann, G., Zumbühl, C., Blattner, P., Cajochen, C., Lazar, R., & Spitschan, M. (2023). The light-dosimeter: A new device to help advance research on the non-visual responses to light. Lighting Research & Technology, 55(4-5), 474–486. https://doi.org/10.1177/14771535221147140
Tan, B., McNabb, R. P., Zheng, F., Sim, Y. C., Yao, X., Chua, J., Ang, M., Hoang, Q. V., Kuo, A. N., & Schmetterer, L. (2021). Ultrawide field, distortion-corrected ocular shape estimation with MHz optical coherence tomography (OCT). Biomedical Optics Express, 12(9), 5770–5781. https://doi.org/10.1364/BOE.428430
Tideman, J. W., Snabel, M. C., Tedja, M. S., van Rijn, G. A., Wong, K. T., Kuijpers, R. W., Vingerling, J. R., Hofman, A., Buitendijk, G. H., Keunen, J. E., Boon, C. J., Geerards, A. J., Luyten, G. P., Verhoeven, V. J., & Klaver, C. C. (2016). Association of axial length with risk of uncorrectable visual impairment for Europeans with myopia. JAMA Ophthalmology, 134(12), 1355–1363. https://doi.org/10.1001/jamaophthalmol.2016.4009
U.S. Food & Drug Administration. (2018, April 11). FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems [Press release]. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye
Verkicharla, P. K., Ramamurthy, D., Nguyen, Q. D., Zhang, X., Pu, S. H., Malhotra, R., Ostbye, T., Lamoureux, E. L., & Saw, S. M. (2017). Development of the FitSight Fitness Tracker to increase time outdoors to prevent myopia. Translational Vision Science & Technology, 6(3), 20. https://doi.org/10.1167/tvst.6.3.20
Verkicharla, P. K., Suheimat, M., Pope, J. M., Sepehrband, F., Mathur, A., Schmid, K. L., & Atchison, D. A. (2015). Validation of a partial coherence interferometry method for estimating retinal shape. Biomedical Optics Express, 6(9), 3235–3247. https://doi.org/10.1364%2FBOE.6.003235
Vurgese, S., Panda-Jonas, S., & Jonas, J. B. (2012). Scleral thickness in human eyes. PLOS One, 7(1), e29692. https://doi.org/10.1371/journal.pone.0029692
Wang, Y., Bensaid, N., Tiruveedhula, P., Ma, J., Ravikumar, S., & Roorda, A. (2019). Human foveal cone photoreceptor topography and its dependence on eye length. eLife, 8, e47148. https://doi.org/10.7554/eLife.47148
Wang, D., Chun, R. K., Liu, M., Lee, R. P., Sun, Y., Zhang, T., Lam, C., Liu, Q., & To, C. H. (2016). Optical defocus rapidly changes choroidal thickness in schoolchildren. PloS One, 11(8), e0161535. https://doi.org/10.1371/journal.pone.0161535
Wen, L., Cheng, Q., Cao, Y., Li, X., Pan, L., Li, L., Zhu, H., Mogran, I., Lan, W., & Yang, Z. (2021). The Clouclip, a wearable device for measuring near-work and outdoor time: Validation and comparison of objective measures with questionnaire estimates. Acta Ophthalmologica, 99(7), e1222–e1235. https://doi.org/10.1111/aos.14785
Wolffsohn, J. S., Flitcroft, D. I., Gifford, K. L., Jong, M., Jones, L., Klaver, C. C. W., Logan, N. S., Naidoo, K., Resnikoff, S., Sankaridurg, P., Smith, E. L., 3rd, Troilo, D., & Wildsoet, C. F. (2019). IMI—Myopia control reports overview and introduction. Investigative Ophthalmology & Visual Science, 60(3), M1–M19. https://doi.org/10.1167/iovs.18-25980
Wong, H. B., Machin, D., Tan, S. B., Wong, T. Y., & Saw, S. M. (2010). Ocular component growth curves among Singaporean children with different refractive error status. Investigative Ophthalmology & Visual Science, 51(3), 1341–1347. https://doi.org/10.1167/iovs.09-3431
Wong, C. W., Teo, Y. C. K., Tsai, S. T. A., Ting, S. W. D., Yeo, Y. S. I., Wong, W. K. D., Lee, S. Y., Wong, T. Y., & Cheung, C. M. G. (2019). Characterization of the choroidal vasculature in myopic maculopathy with optical coherence tomographic angiography. Retina, 39(9), 1742–1750. https://doi.org/10.1097/iae.0000000000002233
Wu, P. C., Chen, C. T., Chang, L. C., Niu, Y. Z., Chen, M. L., Liao, L. L., Rose, K., & Morgan, I. G. (2020). Increased time outdoors is followed by reversal of the long-term trend to reduced visual acuity in Taiwan primary school students. Ophthalmology, 127(11), 1462–1469. https://doi.org/10.1016/j.ophtha.2020.01.054
Wu, H., Xie, Z., Wang, P., Liu, M., Wang, Y., Zhu, J., Chen, X., Xu, Z., Mao, X., & Zhou, X. (2021). Differences in retinal and choroidal vasculature and perfusion related to axial length in pediatric anisomyopes. Investigative Ophthalmology & Visual Science, 62(9), 40. https://doi.org/10.1167/iovs.62.9.40
Xu, A., Sun, G., Duan, C., Chen, Z., & Chen, C. (2021). Quantitative assessment of three-dimensional choroidal vascularity and choriocapillaris flow signal voids in myopic patients using SS-OCTA. Diagnostics, 11(11), 1948. https://doi.org/10.3390/diagnostics11111948
Ye, B., Liu, K., Cao, S., Sankaridurg, P., Li, W., Luan, M., Zhang, B., Zhu, J., Zou, H., Xu, X., & He, X. (2019). Discrimination of indoor versus outdoor environmental state with machine learning algorithms in myopia observational studies. Journal of Translational Medicine, 17(1), 314. https://doi.org/10.1186/s12967-019-2057-2
Zadnik, K., Sinnott, L. T., Cotter, S. A., Jones-Jordan, L. A., Kleinstein, R. N., Manny, R. E., Twelker, J. D., Mutti, D. O., & Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study Group (2015). Prediction of juvenile-onset myopia. JAMA Ophthalmology, 133(6), 683–689. https://doi.org/10.1001/jamaophthalmol.2015.0471
Zahra, S., Murphy, M. J., Crewther, S. G., & Riddell, N. (2023). Flash electroretinography as a measure of retinal function in myopia and hyperopia: A systematic review. Vision, 7(1), 15. https://doi.org/10.3390%2Fvision7010015
Zheng, F., Chua, J., Ke, M., Tan, B., Yu, M., Hu, Q., Cheung, C. M. G., Ang, M., Lee, S. Y., Wong, T. Y., SNEC Retina Group, Schmetterer, L., Wong, C. W., & Hoang, Q. V. (2022). Quantitative OCT angiography of the retinal microvasculature and choriocapillaris in highly myopic eyes with myopic macular degeneration. The British Journal of Ophthalmology, 106(5), 681–688. https://doi.org/10.1136/bjophthalmol-2020-317632
Zhou, J., He, H., Yang, Q., Wang, J.-Y., You, Z.-P., & Liu, L.-L. (2023). Comparison of anterior sclera thickness in emmetropes and myopes. BMC Ophthalmology, 23(1), 67. https://doi.org/10.1186/s12886-023-02775-x
























