Submarine Exposure Guidance Levels for Selected Hydrofluorocarbons: HFC-236fa, HFC-23,and HFC-404a (2000)
Chapter: Toxicokinetics
HFC-143a
Chemical and Physical Properties
|
Common name: |
HFC-143a |
|
Chemical name: |
1,1,1-Trifluoroethane |
|
Synonyms: |
FC-143a; hydrofluorocarbon 143a |
|
CAS number: |
420-46-6 |
|
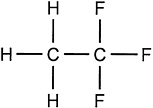
Structural formula: |
CH3CF3 |
|
Description: |
Colorless gas |
|
Molecular weight: |
84.04 |
|
Boiling point: |
-47.3°C at 760 mm Hg |
|
Melting point: |
-111.3°C |
|
Flash point and flammability: |
-90°C |
|
Solubility: |
Soluble in chloroform and ether |
|
Auto-ignition: |
750°C |
|
Conversion factors: |
1 ppm = 3.40 mg/m3; 1 mg/m3 = 0.29 ppm |
Toxicokinetics
In a study of rats exposed by inhalation to HFC-143a at 4,800 ppm for 4-5 hr, 2,2,2-trifluoroethanol was the only metabolite detected. Nuclear magnetic resonance showed that HFC-143a was metabolized slowly, primarily through an oxidative route (DuPont 1994, as cited in AIHA 1996).
