Exploring Military Exposures and Mental, Behavioral, and Neurologic Health Outcomes Among Post-9/11 Veterans (2025)
Chapter: 7 Results for Neurologic Outcomes
7
Results for Neurologic Outcomes
This chapter describes the neurologic outcomes of interest and presents the results of the case-control studies and from the structured literature review. These outcomes include amyotrophic lateral sclerosis (ALS), dementia, multiple sclerosis (MS), and Parkinson’s disease. Criteria to prioritize results of the statistical analyses presented in this chapter are an adjusted odds ratio (OR) of at least 1.10 and the exclusion of 1.0 in the 95% confidence interval (CI). The chapter also includes conclusions on possible relationships between neurologic disorders and the military exposures and presents results of stratified analyses and a cumulative exposure analysis. Although traumatic brain injury (TBI) was specified as an outcome in the Statement of Task, the committee instead treated it as a covariate and stratified each exposure–outcome pair by TBI status, as TBI is not caused by military exposures. The outcomes in this chapter were also stratified by age to investigate whether patterns differed in older and younger groups. For these results, the committee prioritized reporting those in which the adjusted ORs between strata were different, at least one stratum’s OR was above 1.0 and the lower bound of the 95% CI was also above 1.0, and the 95% CIs did not overlap between strata. Full results of all data analyses are in Appendix G.
Due to time constraints, the committee began its structured literature search before obtaining data on exposures from the Individual Longitudinal Exposure Record (ILER); therefore, it is not directly based on each of the exposure categories the committee had available for its analyses (see Chapter 4 for more information). For example, ILER data contained
exposures for dust and particulate matter (PM), exhaust, and incinerator emissions, while the literature search was conducted for PM because it is a component of these exposures and has a robust literature base. Additionally, burn pits have been known to be associated with other component pollutants, such as PM, dust, metals, volatile organic compounds, and fuels (IOM, 2011). The committee has detailed literature search results in sections for some of these specific pollutant components, even if no specific studies on burn pits were identified in the search. Chapter 4 describes how the committee integrated evidence from its statistical analyses with the literature search results to draw its conclusions. The committee cautions again that the results presented are based on a sample of post-9/11 veterans who served in Southwest Asia or Afghanistan and received care at Veterans Health Administration (VHA), so the sample used for its analyses has limited generalizability. All conclusions should be interpreted as specific to this population.
AMYOTROPHIC LATERAL SCLEROSIS
ALS is a neurodegenerative disease that affects muscle movement and breathing. Muscle mass reduces as ALS progresses and patients become unable to control voluntary movement. Symptoms most often develop between ages 55 and 75 (NINDS, 2024a). The most common cause of death is breathing failure caused by motor neuron degeneration, usually occurring within 3–5 years from symptom onset (NINDS, 2024a). In 2018, the U.S. National ALS Registry had 21,655 confirmed or likely ALS cases, or 6.6 cases per 100,000 persons (Mehta et al., 2023).
A case-control study of post-9/11 veterans receiving care in VHA between 2002 and 2015 calculated a rate of 19.7 ALS cases per 100,000 persons (Sagiraju et al., 2020). According to VHA, 4,540 veterans received care for ALS in 2020 (VHA, 2021).
Analysis Results
There were 296 cases (0.0%) of ALS. In Figure 7-1, exposure to exhaust is associated with a risk-conferring relationship on ALS, with an adjusted OR of 1.43 (95% CI: 1.003–2.04), though the committee notes that both exposure to exhaust and ALS are rare. Given the low number of ALS cases with exposure to radiation (less than 10), the committee does not present results to preserve Department of Veterans Affairs (VA) data privacy standards. All exposures had no differences in the magnitude of association when stratifying by TBI, which suggests no interaction between these exposures and TBI on odds of ALS. Stratified analyses were underpowered
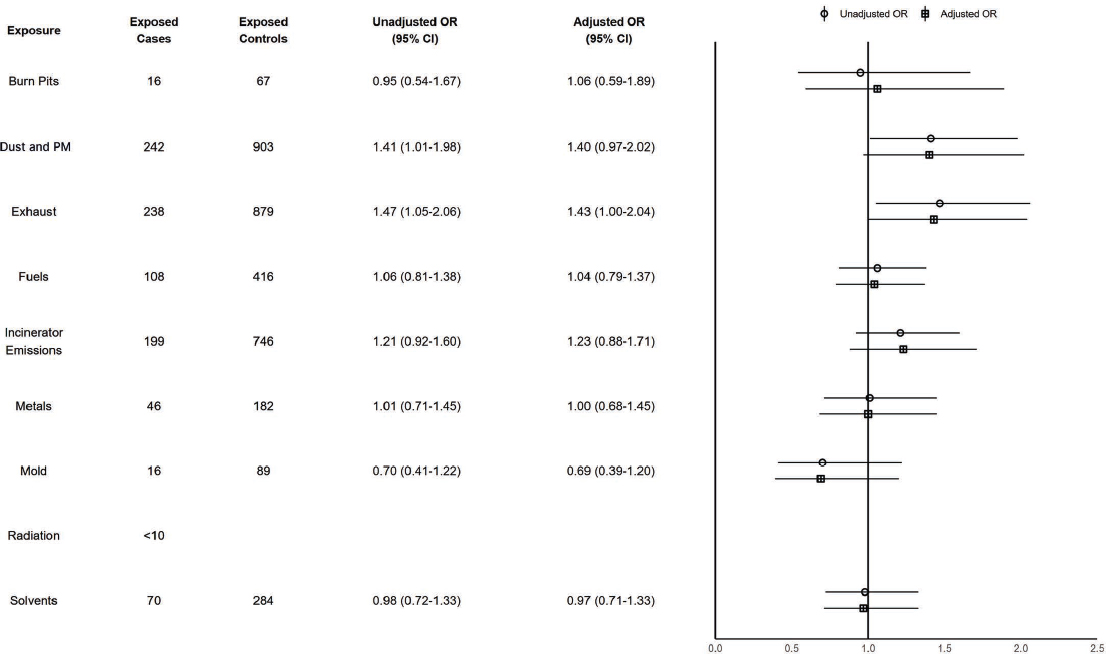
NOTES: Exposed cases n = 296; exposed controls n = 1,181. ALS = amyotrophic lateral sclerosis; CI = confidence interval; ILER = Individual Longitudinal Exposure Record; OR = odds ratio; PM = particulate matter.
for this analysis due to the small number of veterans with ALS and TBI; CIs within the TBI strata were very wide.
Literature Search Results
Burn Pits and ALS
The search produced no studies on the relationship between burn pits and ALS. However, one study examining military-related exposures and ALS survival among veterans reported that self-reported exposure to burning trash, feces, or manure during deployment was not significantly associated with ALS survival (Beard et al., 2017).
PM and ALS
The literature search yielded nine studies, including one meta-analysis (Gong et al., 2023) and two systematic reviews (Oliveira et al., 2020; Saucier et al., 2023), on PM and ALS. One study examined military-related factors and ALS in veterans (Beard et al., 2017). These studies investigated PM2.5 and PM10 as well as specific air pollutants, diesel exhaust, and dust. This section first presents findings by particulate size, followed by specific pollutants and sources of exposure.
Four studies on PM2.5 and ALS are mixed. The meta-analysis reported no significant association between long-term PM2.5 and ALS (Gong et al., 2023). By contrast, the systematic review with narrative synthesis by Saucier and colleagues (2023) reported that two of three studies identified reported a risk-conferring relationship between PM2.5 and ALS. In addition, one large cohort study reported that PM2.5 was associated with increased risk of ALS mortality (Peters et al., 2024). This effect did not reach statistical significance but was robust in two-pollutant models. A large case-control study using Danish administrative data found a null association between PM2.5 and ALS diagnosis (Parks et al., 2022).
Three studies, a meta-analysis (Gong et al., 2023), systematic review (Saucier et al., 2023), and large Dutch cohort (Peters et al., 2024), assessed the relationship between PM10 and ALS outcomes. All reported that PM10 was not significantly associated with ALS.
Two case-control studies of occupational exposure to PM and ALS outcomes by Goutman and colleagues (2022, 2023) reported mixed results. Specifically, they reported that such exposure was not associated with having ALS or with ALS survival. However, occupational PM exposure was significantly associated with cervical onset of ALS compared to lumbar onset; it was not significantly associated with bulbar onset.
Four studies reported findings on specific pollutants and ALS. The systematic review by Oliveira and colleagues (2020) identified one study that found a risk-conferring relationship between nitrogen oxides and ALS, a protective association between ozone and ALS, and no correlation between benzopyrene and ALS. The large Danish case-control study reported protective, but not significant, effects of nitrogen oxides and carbon monoxide and ALS, while elemental carbon significantly increased odds of ALS (Parks et al., 2022). Lastly, the Dutch cohort study reported that elemental carbon and nitrogen dioxide were associated with increased risk of ALS mortality, though these associations did not reach statistical significance (Peters et al., 2024); the review by Saucier and colleagues (2023) found a consistent, significant risk-conferring relationship between nitrogen dioxide and ALS.
Five studies assessed the relationship between diesel exhaust and ALS, of which one was among veteran populations. This veterans’ study found that self-reported exposure to exhaust from heaters or generators during deployment was not significantly associated with ALS survival (Beard et al., 2017). One systematic review identified four studies on occupational exposure to diesel exhaust, of which three found a risk-conferring relationship with ALS (Saucier et al., 2023). The review also identified one study that found that truck driving, which involves occupational exposure to diesel exhaust, was also significantly associated with increased odds of ALS. Dickerson and colleagues (2018) found significantly increased odds of ALS among men who worked in occupations associated with diesel exhaust exposure (agriculture, hunting, forestry, and fishing; construction). In comparison, two studies by Goutman and colleagues (2022, 2023) reported no association between occupational exposure to combustion/diesel exhaust and ALS diagnosis or survival; however, occupational diesel exhaust exposure was significantly associated with cervical (but not bulbar) onset compared to lumbar onset of ALS.
Two studies investigated dust exposure and ALS. The study of military-related exposures found that self-reported exposure to high levels of dust and sand during the first Gulf War was not significantly associated with ALS survival (Beard et al., 2017). However, the systematic review of environmental risk factors for ALS identified one study of occupational exposure to organic dust and silica that found that these were significantly associated with ALS on a continuous scale (Saucier et al., 2023).
Fuels and ALS
Two studies examined fuel exposure and ALS survival. The study by Beard and colleagues (2017) on military-related exposures reported that exposure to diesel and/or other petrochemical fumes during military service was not significantly associated with ALS survival. Similarly, a cohort study
that investigated the association between occupational exposure to toxicants in a petroleum company (e.g., from refining) and all-cause and specific-cause mortality among nearly 30,000 Canadian petroleum workers reported a moderate but non-significant excess of ALS cases (15 observed, 8.8 expected) for a standardized mortality ratio of 1.70 (Schnatter et al., 2019).
Metals and ALS
The search yielded 16 studies, including an umbrella review of umbrella reviews of systematic review and meta-analyses (Mentis et al., 2021), three additional meta-analyses (Capozzella et al., 2014; Duan et al., 2023; Kamalian et al., 2023), and one systematic review (Oliveira et al., 2020). One study examined military-related factors and ALS in veterans (Beard et al., 2017). The studies investigated environmental and occupational metal exposure, as well as metal concentrations in biologic samples. Studies investigated both unspecified metals or groups of metals as well as specific metals, including lead, aluminum, cadmium, chromium, copper, manganese, mercury, nickel, selenium, zinc, and others.
Eight studies assessed aggregate and/or unspecified metals and ALS. Although several (but not all) meta-analyses showed a consistent risk-conferring relationship between unspecified metals or metals as a class and ALS, additional environmental and occupational studies reported more mixed findings. With respect to the reviews, an umbrella review reported that pooled analyses based on four studies showed more than double the odds of ALS among those exposed to heavy metals excluding lead compared to those not exposed (Mentis et al., 2021). The effect was significant, and the authors assessed the weight of the evidence as “suggestive” (Mentis et al., 2021). Similarly, the meta-analysis by Duan and colleagues (2023) reported that exposure to metals significantly increased odds of ALS, with a relatively large effect size (OR=1.79 [95% CI: 1.43–2.23]) for any metals. In contrast to the reviews, two studies examined exposure to metals in ambient air using data from various waves of the Environmental Protection Agency (EPA) National Air Toxics Assessment (NATA) and did not find a significant association with ALS (Malek et al., 2015; Wu et al., 2024). Studies of occupational metal exposure were mixed. A meta-analysis by Capozzella and colleagues (2014) reported no association between metal exposure and ALS, based on pooled analyses of two case-control studies. A pair of case-control studies reported significant associations with ALS prevalence and onset segment, but not ALS survival (Goutman et al., 2022, 2023), and a study of occupation and ALS using Danish administrative data reported no significant association between occupation in metal products industries (metal foundry; metal goods and pipe factory; machine manufacturing) and ALS (Dickerson et al., 2018).
With respect to specific metals and ALS, lead was the most studied metal, though again none of the studies were among veteran populations; findings were inconsistent. An umbrella review reported that lead significantly increased odds of ALS and the authors assessed the evidence based on nine included studies as “convincing” (Mentis et al., 2021). Findings from studies of environmental and occupational lead exposure were inconsistent. One meta-analysis reported one included study with mixed results for occupational lead exposure and odds of ALS (Capozzella et al., 2014). The Dickerson and colleagues (2018) study on occupation and ALS found that occupations associated with lead exposure (agriculture, hunting, forestry, and fishing occupations and construction work) were associated with significantly increased odds of ALS. However, use of occupation as proxy for lead exposure cannot distinguish unique effects of lead from other neurotoxicants associated with these occupations, such as diesel exhaust or pesticides. Regarding environmental lead exposure, a meta-analysis reported that environmental lead exposure significantly increased odds of ALS based on nine studies of moderate quality (Duan et al., 2023). Among three additional studies of environmental lead exposure, one found that lead levels in topsoil, pediatric blood, and sewage sludge were significantly associated with prevalence of a composite neurodegenerative disease outcome including ALS (Newell et al., 2024); one study reported that lead concentrations in moss and lichens were not associated with the spatial density of ALS cases based on residence (Antonioni et al., 2023); and a case-control study using EPA NATA data at the residential zip code level did not find a significant association between lead concentrations in ambient air and ALS (Wu et al., 2024). Finally, two studies including a meta-analysis (Kamalian et al., 2023) focused on lead concentrations in biologic samples and reported ALS patients had higher lead concentrations in blood and cerebrospinal fluid (Bocca et al., 2015; Kamalian et al., 2023) but not in hair or urine (Bocca et al., 2015).
Several studies investigated ALS and cadmium from environmental exposures and concentrations in biologic samples. None focused on veteran populations and findings were inconsistent. Four studies on environmental exposures included a systematic review that identified one study, which found that environmental cadmium exposure was positively associated with increased ALS risk (Oliveira et al., 2020). Similarly, one study found that residential exposure to cadmium in ambient air significantly increased odds of ALS (Wu et al., 2024), and another study found that cadmium in sewer sludge was significantly associated with prevalence of neurodegenerative disease including ALS (Newell et al., 2024). However, an ecological study reported no association between cadmium in moss and lichen and residential density of ALS cases (Antonioni et al., 2023). Two case-control studies of biologic samples reported inconsistent results. Whereas a nested
case-control study reported that the highest tertile of blood cadmium concentration was significantly associated with ALS compared to the lowest tertile (Peters et al., 2021), another reported no significant differences in blood, hair, or urinary cadmium concentrations between ALS cases and controls (Bocca et al., 2015).
Three studies assessed chromium and ALS (Andrew et al., 2022; Antonioni et al., 2023; Capozzella et al., 2014), with mixed results. Whereas a study of residential exposure to industrial chemicals released in air using EPA Toxics Release Inventory data found that higher chromium exposure was associated with increased ALS odds (Andrew et al., 2022), a meta-analysis reported one study finding no significant association between chromium and ALS odds (Capozzella et al., 2014). The ecological study by Antonioni and colleagues (2023) found no association between chromium in moss and lichens and residential ALS density.
Two studies examined copper and ALS with inconsistent results. A meta-analysis reported no associations between ALS and copper concentrations in biologic samples (Kamalian et al., 2023). One ecological study found that copper concentrations from moss and lichens were strongly correlated with density of ALS cases by residency (Antonioni et al., 2023).
Among four studies identified on manganese and ALS, two assessed concentrations in biologic samples (Bocca et al., 2015; Kamalian et al., 2023) and two focused on environmental exposures (Antonioni et al., 2023; Wu et al., 2024); results were mixed. The two studies that assessed biologic samples included a meta-analysis (Kamalian et al., 2023), which reported no association with ALS, and the Bocca and colleagues (2015) study that reported that hair but not serum or urine manganese concentrations were lower in ALS patients compared with controls. The two environmental studies included the ecological study of manganese in moss and lichens and residential ALS density (Antonioni et al., 2023) and a study on residential exposure to manganese in ambient air (Wu et al., 2024), and neither reported a significant association between these exposures and ALS.
In three studies on mercury and ALS, of which one examined residential exposure to mercury in ambient air (Wu et al., 2024) and two assessed concentrations in blood, serum, plasma, hair, or urine (Bocca et al., 2015; Kamalian et al., 2023), all reported no association with ALS. Two studies investigated residential exposure to nickel in ambient air, of which one reported that higher concentrations were associated with increased odds of ALS (Andrew et al., 2022) and the other reported no significant association (Wu et al., 2024).
Three studies on selenium and ALS reported mixed results. The meta-analysis found elevated selenium in serum and plasma among ALS participants compared to controls (Kamalian et al., 2023). Two studies of environmental exposure, including an ecological study of selenium
concentration in moss and lichen and spatial density of ALS cases based on residence (Antonioni et al., 2023) and a case-control study of selenium in ambient air linked to residential zip code (Wu et al., 2024), reported no association with ALS.
Two studies assessed the relationship between zinc concentrations in blood and cerebrospinal fluid, including one meta-analysis (Kamalian et al., 2023). A case-control study found that higher blood zinc concentrations were associated with significantly decreased odds of ALS (Peters et al., 2021), while the meta-analysis reported no significant association between zinc concentrations from cerebrospinal fluid and ALS (Kamalian et al., 2023). Several studies investigated other metals, including aluminum, arsenic, iron, magnesium, and silver, and reported no association with ALS outcomes (Antonioni et al., 2023; Kamalian et al., 2023; Wu et al., 2024).
Finally, the study of military-related exposures among veterans found that self-reported exposure to depleted uranium in munitions or armor during the Gulf War was not significantly associated with ALS survival (Beard et al., 2017).
Radiation and ALS
The committee identified four studies, including one meta-analysis (Lopes et al., 2022), on radiation exposure and ALS outcomes; one study was among veterans (Beard et al., 2017). The study of military-related exposures among veterans found that self-reported exposure to ionizing radiation from nuclear weapons tests or occupation from Hiroshima or Nagasaki during the use of the atomic bomb during World War II was not significantly associated with ALS survival (Beard et al., 2017). The meta-analysis investigated the relationship between occupational exposure to ionizing radiation and a composite measure of diseases of the nervous system, which included ALS (among other conditions) (Lopes et al., 2022). Using 16 studies, the authors found that workers exposed to radiation had significantly lower mortality from nervous system disorders compared to the general population; the authors note this result may be explained by the healthy worker effect. In another analysis, however, the authors calculated a pooled relative risk from three studies on medical radiation workers and did not find significantly increased risk of mortality from nervous system diseases between exposed people and unexposed controls. These conflicting results likely reflect the heterogeneity of the conditions included in the outcome measures. One additional study assessed self-reported occupational exposures and ALS onset segment (i.e., the region of the brainstem with affected neurons—bulbar, cervical, or lumbar) and ALS survival (Goutman et al., 2023). It found that radiation was associated with both bulbar onset and cervical onset compared to lumbar onset of ALS but not with ALS
survival. In a case-control study by the same authors, participants with ALS did not report significantly higher occupational exposure to radiation compared to controls (Goutman et al., 2022).
Solvents and ALS
The literature search yielded seven studies on solvent exposure and ALS, including one study of veterans. This study of military-related exposures and ALS survival found that exposure to paint, solvents, or petrochemical substances was not significantly associated with ALS survival (Beard et al., 2017). Three meta-analyses examining environmental and occupational exposures including solvents and ALS reported mixed results from pooled analyses. Two studies reported that solvent exposure was associated with increased odds of ALS (Duan et al., 2023; Zhang et al., 2023), while a third did not find a significant association, though it did only include two case-control studies (Capozzella et al., 2014). Two case-control studies examined residential exposure to solvents using exposure data from NATA and ALS and found that residential aromatic organic/chlorinated solvent exposure significantly increased likelihood of developing ALS (Malek et al., 2015; Wu et al., 2024). Additionally, a study of residential exposure to industrial chemicals released in air using EPA Toxics Release Inventory data found that higher exposures to the solvents styrene and dichloromethane were both associated with increased ALS odds (Andrew et al., 2022).
Mold and ALS
The committee’s search yielded zero results on the possible relationship between exposure to mold and the risk of ALS.
Conclusion
Conclusion 7-1: Based on its analysis of the available data, the committee finds there is a possible risk-conferring relationship between exposure to exhaust and amyotrophic lateral sclerosis (ALS). The committee does not find a possible risk-conferring relationship between exposure to burn pits, dust and particulate matter (PM), fuels, incinerator emissions, metals, mold, radiation, or solvents and ALS.
Based on the literature review, there is suggestive evidence of a possible risk-conferring relationship between exposure to solvents and ALS. There is mixed evidence of a possible risk-conferring relation
ship between exposure to PM and ALS. There is limited evidence of a possible risk-conferring relationship between exposure to metals and ALS. There is insufficient evidence of a possible risk-conferring relationship between exposure to burn pits, fuels, or radiation and ALS. There is no identified literature on the relationship between exposure to mold and ALS.
Synthesizing the committee’s data analysis and literature review, the committee concludes there is a possible risk-conferring relationship between exposure to exhaust or solvents and ALS. The committee further concludes there is inadequate or insufficient evidence of a possible risk-conferring relationship between exposure to burn pits, dust and PM, fuels, incinerator emissions, metals, mold, or radiation and ALS.
DEMENTIA
Dementia is a syndrome characterized by progressive loss of cognitive functions that affects the ability to participate in usual daily activities. It is distinct from normal age-related cognitive decline (NINDS, 2024b), which happens commonly to people after age 65 and is not considered a disorder. Multiple forms of dementia exist; Alzheimer’s disease is the most common (Sheppard and Coleman, 2020)—a progressive neurodegenerative disorder that results from accumulation of amyloid and tau proteins in the brain and is characterized by progressive memory loss (Scheltens et al., 2021). It is the most common neurodegenerative disease (Sheppard and Coleman, 2020). Other forms of dementia include frontotemporal dementia, vascular dementia, and dementia with Lewy bodies.
Among the U.S. population generally, projections using the U.S. Census and Chicago Health and Aging Project, a longitudinal population-based study of chronic health conditions in older people, suggest a prevalence of Alzheimer’s disease for those age 65+ of 10.9% in 2024 (Alzheimer’s Association, 2024). Similarly, a screened subset of respondents to the Health and Retirement Study, a nationally representative longitudinal survey and interview study researching aging, found the prevalence of dementia in the U.S. population aged 65+ in 2012 to be 10.5% (Hudomiet et al., 2018).
Estimates of dementia and Alzheimer’s disease are similar for veterans and the U.S. general population. A cross-sectional analysis of VHA electronic health records of veterans aged 50+ receiving VHA care from 2000 to 2019 calculated a prevalence of 1.50% and 0.41% for all types of dementia and Alzheimer’s disease, respectively, in 2019 (Dinesh et al., 2023); this relatively low rate may be due to including younger individuals. A 2005 cross-sectional study of VA patients aged 65+ found a dementia prevalence
of 7.3% (Krishnan et al., 2005). A meta-analysis of 11 studies of U.S. veterans published between 2000 and 2016 with a mean age of 65+ reported a pooled dementia prevalence of 10.1% (Williamson et al., 2018).
Analysis Results
There were 3,890 cases (0.3%) of dementia. In Figure 7-2, none of the adjusted ORs and associated CIs for these exposures and dementia met the prioritization criteria for additional discussion. Though none of the age-stratified associations met the committee’s priority criteria, there was some evidence of higher odds of dementia in populations younger than 65 associated with exposure to dust and PM (ORs of 1.07 versus 0.84), exhaust (ORs of 1.07 versus 0.88), and incinerator emissions (ORs of 1.10 versus 0.87). The average dementia diagnosis in the population is older than 65; therefore, the elevated estimates among the younger-than-65 subgroup could indicate that these exposures may be risk factors for early dementia. All exposures had no differences in the magnitude of association when stratified by TBI, which suggests no interaction between these exposures and TBI on odds of dementia.
Literature Search Results
PM and Dementia
The search yielded one umbrella review (Tan et al., 2022), eight additional meta-analyses (Abolhasani et al., 2023; Cheng et al., 2022; Dhiman et al., 2022; Fu et al., 2019; Gong et al., 2023; Tang et al., 2023; Wilker et al., 2023; Zhao et al., 2021), and three systematic reviews with narrative synthesis (Chandra et al., 2022; Cristaldi et al., 2022; Oliveira et al., 2020). These studies generally focused on environmental exposure to PM10 and PM2.5 (such as from air pollution), and none were specific to military populations. Given the relative abundance of meta-analyses on the relationship between PM and the risk of dementia, the committee chose not to further consider individual original analyses on PM and dementia.
The majority of studies consistently showed a risk-conferring relationship between PM2.5 exposure and an increased risk of dementia or Alzheimer’s disease (Abolhasani et al., 2023; Chandra et al., 2022; Cheng et al., 2022; Cristaldi et al., 2022; Dhiman et al., 2022; Fu et al., 2019; Gong et al., 2023; Tan et al., 2022; Tang et al., 2023; Zhao et al., 2021). These relationships were consistently risk-conferring despite using different literature search strategies and different exposure and outcome measures. One meta-analysis did not find a statistically significant relationship between PM2.5 and dementia (Wilker et al., 2023).

NOTES: Exposed cases n = 3,890; exposed controls n = 15,459. CI = confidence interval; ILER = Individual Longitudinal Exposure Record; OR = odds ratio; PM = particulate matter.
The relationship between PM10 and dementia is more mixed. The umbrella review reported that, based on one meta-analysis, PM10 was associated with reduced risk of Alzheimer’s disease (Tan et al., 2022). One additional meta-analysis of four studies found a significant risk-conferring association between PM10 and vascular dementia (Gong et al., 2023). However, another reported that PM10 was not significantly associated with dementia, and the quality of evidence was low (Zhao et al., 2021). A systematic review identified two studies, which both found some evidence that PM10 was positively correlated with increased dementia (Oliveira et al., 2020).
Fuels and Dementia
One small (n = 112) study examined exposure to fuels and dementia (Adani et al., 2020). The study was not specific to veteran populations and focused on early-onset dementia, including early-onset Alzheimer’s disease and early-onset frontotemporal dementia. It reported that self-reported diesel/gasoline exposure was not associated with significantly increased odds of any early-onset dementia outcome.
Metals and Dementia
The literature search yielded nine studies on metal exposure and dementia, of which three were meta-analyses (Du et al., 2017; Xu et al., 2018; Zhao et al., 2021). The studies assessed different metals and metalloids and none were in military or veteran populations. Results were mixed.
Four studies, including a meta-analysis (Xu et al., 2018), examined the relationship between lead, mercury, and cadmium and dementia. Findings were inconsistent. With respect to lead, the meta-analysis found significantly lower levels of lead in Alzheimer’s patients compared to controls (Xu et al., 2018). An ecological study on environmental exposures and prevalence rates of a composite measure of neurodegenerative diseases, including Alzheimer’s disease, found concentrations of lead in topsoil, lead and cadmium in sewage sludge, and lead in blood from pediatric populations in the United States were significantly associated with the composite measure of neurodegenerative disease prevalence (Newell et al., 2024). Two case-control studies did not find a significant relationship between lead concentrations and dementia (Adani et al., 2020; Yang et al., 2018). Regarding mercury, whereas the meta-analysis found a significant relationship, the ecological study and two case-control studies did not (Adani et al., 2020; Newell et al., 2024; Xu et al., 2018; Yang et al., 2018). With regard to cadmium, the meta-analysis reported a risk-conferring relationship (Xu et al., 2018) and the ecological study found that cadmium concentrations in sewer sludge were significantly associated with
prevalence of neurodegenerative disease including Alzheimer’s (Newell et al., 2024), while the case-control study by Yang and colleagues (2018) did not find a significant relationship.
Four studies including two meta-analyses (Xu et al., 2018; Zhao et al., 2021) investigated the relationship between aluminum and dementia. One meta-analysis reported significantly higher circulatory levels of aluminum in Alzheimer’s disease patients than controls (Xu et al., 2018). In the other meta-analysis, aluminum exposure was associated with increased dementia risk (Zhao et al., 2021). However, in analyses stratified by exposure source, aluminum in drinking water was associated with increased odds of dementia, while aluminum from occupational exposure was not, which suggests that the overall association was likely driven by drinking water exposure. In addition to the meta-analyses, one small Italian case-control study reported that self-reported occupational exposure to aluminum was associated with increased but not significant odds of overall early-onset dementia, early-onset frontotemporal dementia, and early-onset Alzheimer’s dementia (Adani et al., 2020), and another study in Pakistan found higher levels of hair aluminum concentration in Alzheimer’s disease and dementia patients than controls (Arain et al., 2015).
Several studies also assessed other metals and dementia. Among two studies on manganese and dementia, a meta-analysis found significantly lower levels among Alzheimer’s disease patients than controls (Du et al., 2017), whereas an additional cross-sectional study found significantly higher levels of hair manganese concentrations in Alzheimer’s and dementia patients compared to controls (Arain et al., 2015). One cross-sectional study reported that Alzheimer’s disease patients had higher levels of urinary arsenic and blood chromium and lower blood selenium concentrations than controls (Strumylaite et al., 2022), while another found similar results for arsenic and no association for selenium alone but increased risk among those with high arsenic and low selenium (Yang et al., 2018). Finally, a cohort study that pooled data from six European countries found long-term exposure to nickel and vanadium as components of PM2.5 was associated with decreased risk of dementia mortality but no association with five other metal and metalloid components of PM2.5 (copper, iron, potassium, silicon, and zinc) (Andersen et al., 2022).
Radiation and Dementia
The committee identified two meta-analyses, neither of which was specific to veterans. One meta-analysis showed that chronic exposure to ionizing radiation significantly increased risk of dementia incidence and mortality (Srivastava et al., 2023). The other investigated the relationship between occupational exposure to ionizing radiation and a composite measure of
mental and behavioral disorders including dementia and Alzheimer’s disease, among others, and between ionizing radiation exposure and a composite measure of diseases of the nervous system, which again included Alzheimer’s disease and others (e.g., Parkinson’s disease, ALS) (Lopes et al., 2022). This study found that radiation workers had significantly lower mortality from both nervous system and mental and behavioral disorders compared to the general population; the authors note this result may be explained by the healthy worker effect. In another analysis, however, the authors calculated a pooled relative risk from three studies on medical radiation workers and did not find significantly increased risk of mortality from nervous system diseases between exposed people and unexposed controls (Lopes et al., 2022). These conflicting results likely reflect the heterogeneity of the conditions included in the outcome measures.
Solvents and Dementia
Two studies investigating solvent exposure and dementia were identified. A meta-analysis reported a statistically significant risk-conferring relationship between solvents and dementia risk (Zhao et al., 2021). In addition, a small case-control study found that self-reported exposures to solvents such as degreasing agents, paint removers, thinners, and specific chemicals (e.g., toluene, xylene), among others, was not significantly associated with overall early-onset dementia, early-onset Alzheimer’s, or early-onset frontotemporal dementia (Adani et al., 2020).
Burn Pits or Mold and Dementia
The committee’s search yielded zero results on the possible relationships between exposure to burn pits or mold and the risk of dementia.
Conclusion
Conclusion 7-2: Based on its analysis of the available data, the committee does not find a possible risk-conferring relationship between exposure to burn pits, dust and particulate matter (PM), exhaust, fuels, incinerator emissions, metals, mold, radiation, or solvents and dementia.
Based on the literature review, there is suggestive evidence of a possible risk-conferring relationship between exposure to PM and dementia. There is mixed evidence of a possible risk-conferring relationship between exposure to metals or radiation and dementia. There is limited evidence of a possible risk-conferring relationship
between exposure to solvents and dementia. There is insufficient evidence of a possible risk-conferring relationship between exposure to fuels and dementia. There is no identified literature on the relationship between exposure to burn pits or mold and dementia.
Synthesizing the committee’s data analysis and literature review, the committee concludes there is a possible risk-conferring relationship between exposure to PM and dementia. The committee further concludes there is inadequate or insufficient evidence of a possible risk-conferring relationship between exposure to burn pits, dust, exhaust, fuels, incinerator emissions, metals, mold, radiation, or solvents and dementia.
MULTIPLE SCLEROSIS
MS is a chronic neurologic disorder that usually begins in young adults (ages 20–40). It is caused by autoimmune attack of the myelin sheath around neurons, resulting in inflammation, demyelination, and neuronal dysfunction. MS symptoms can include problems with vision, muscle weakness, bladder control, fatigue, mood and cognitive changes, and tingling, numbness, or pain in the arms, legs, trunk, or face. The course of MS can vary substantially by individual, with some people experiencing mild, relatively short-term symptoms (flare-ups) and others having sustained problems with progressive disability (NINDS, 2025). The estimated 2010 national cumulative MS prevalence over 10 years was 309.2 cases per 100,000 adults (Wallin et al., 2019). The 2020 global prevalence was estimated to be 35.9 per 100,000 people (Walton et al., 2020). The prevalence for VHA users was estimated to be 262 per 100,000 in 2014 (VA, 2023).
Analysis Results
There were 3,208 cases (0.3%) of MS. In Figure 7-3, none of the adjusted ORs and associated CIs for these exposures and MS met the prioritization criteria for additional discussion. All exposures had no differences in the magnitude of association when stratifying by TBI, which suggests no interaction between these exposures and TBI on odds of MS.
Literature Search Results
PM and MS
Six studies, including two meta-analyses (Lotfi et al., 2022; Tang et al., 2021) and three systematic reviews (Cristaldi et al., 2022; Farahmandfard
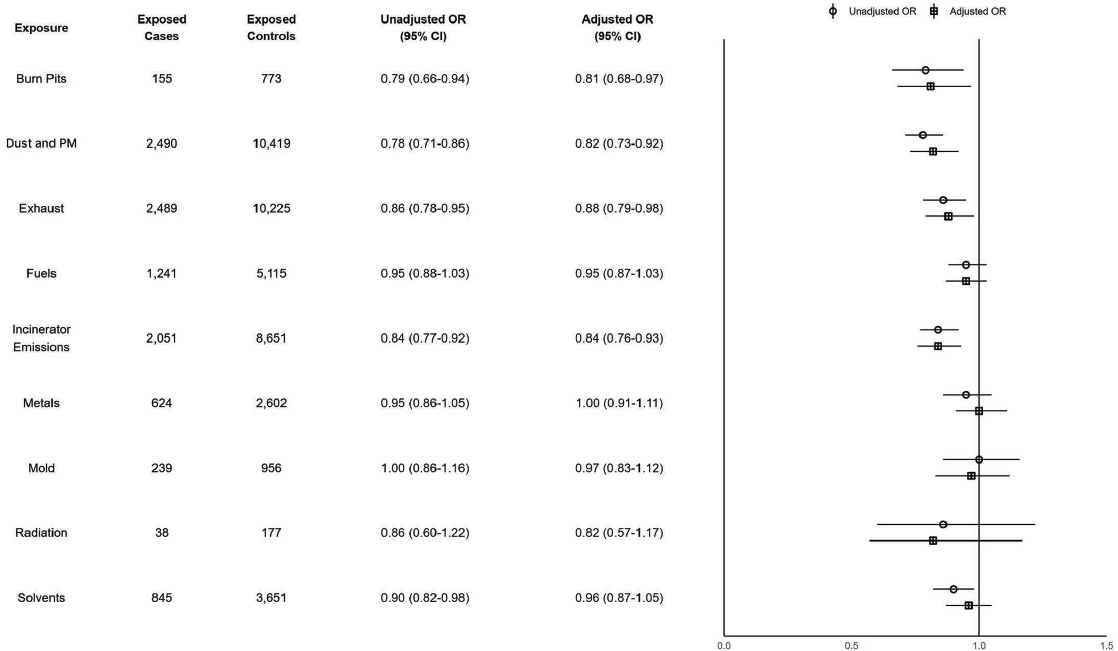
NOTES: Exposed cases n = 3,208; exposed controls n = 12,827. CI = confidence interval; ILER = Individual Longitudinal Exposure Record; MS = multiple sclerosis; OR = odds ratio; PM = particulate matter.
et al., 2021; Oliveira et al., 2020), investigated the relationship between PM and MS. These studies investigated overall PM (including both PM2.5 and PM10), PM10 alone, PM2.5 alone, and specific air pollutants.
Studies of PM overall reported inconsistent findings. One meta-analysis that included 10 studies of PM2.5 and/or PM10 and MS incidence and/or relapse reported a significant risk-conferring relationship between PM and MS in pooled analyses using data from all studies (Lotfi et al., 2022). Subgroup analyses by study design, PM diameter, and MS outcome (incidence versus relapse) consistently showed a risk-conferring relationship. However, those restricted to case-control studies and to MS relapse were not statistically significant. In comparison, a meta-analysis did not find a correlation between PM2.5-10 and MS in pooled analyses from two included studies (Tang et al., 2021). Two systematic reviews similarly reported inconsistent findings (Cristaldi et al., 2022; Farahmandfard et al., 2021).
Four studies examined the association between PM2.5 and MS; findings are inconsistent. In pooled analyses, one meta-analysis reported a significant association between PM2.5 and MS incidence or relapse (Lotfi et al., 2022), while another meta-analysis reported no association (Tang et al., 2021). Similarly, a systematic review identified two studies on PM2.5 and MS and found that they were associated in one study but not the other (Oliveira et al., 2020). One additional study, a large Dutch cohort study, did not find a positive association between PM2.5 and MS (Peters et al., 2024). Three of these same studies also investigated PM10 and MS (Lotfi et al., 2022; Oliveira et al., 2020; Tang et al., 2021). Both meta-analyses reported a statistically significant risk-conferring relationship between PM10 and MS. The systematic review identified two studies with differing PM10 results (Oliveira et al., 2020).
Three studies assessed specific pollutants and MS. A meta-analysis found no statistically significant effect between any of the pollutants studied (benzene, carbon monoxide, nitrogen dioxide, ozone) and MS (Tang et al., 2021). One additional study, the large Dutch cohort study, similarly did not find an association between specific pollutants (elemental carbon and nitrogen dioxide) and MS (Peters et al., 2024). By contrast, a systematic review identified three studies, all of which showed an association between nitrogen oxides and MS prevalence or relapse (Farahmandfard et al., 2021).
Fuels and MS
The literature search produced one meta-analysis that investigated occupational exposures, including offshore petroleum workers and workers exposed to oil well fumes, and MS (Vitturi et al., 2023). It reported, based on one study each, that offshore petroleum workers had increased odds
of MS diagnosis, and workers exposed to toxic fumes from oil wells had increased risk of having MS.
Metals and MS
Ten studies, including three meta-analyses (Nirooei et al., 2022; Sarihi et al., 2021; Vitturi et al., 2023) and one systematic review (Oliveira et al., 2020), examined metal exposure and MS. The studies primarily investigated metal concentrations in biologic samples (e.g., blood, fingernails, hair, urine). No studies were among veteran populations, and none looked at unspecified metals or metals as a class. Studies were of varying quality, including many small case-control studies, and findings were inconsistent.
Five or more studies, including at least one meta-analysis, assessed the relationships between lead, cadmium, or mercury and MS. Results on the relationships between each metal and MS were mixed. Of six studies on lead and MS, most, including a meta-analysis (Sarihi et al., 2021), assessed lead concentrations in blood, urine, and fingernails. Among these studies, blood and urinary lead concentrations were not significantly different in MS patients compared to controls (Armon-Omer et al., 2024; Sarihi et al., 2021). By contrast, a small case-control study reported that lead concentrations in fingernails were significantly higher in MS patients than controls (Moradi et al., 2023). Among MS patients, one cross-sectional study reported that higher blood lead concentrations were associated with worse functional status, but the association did not reach significance (Knyszyńska et al., 2022). Similarly, there were no significant differences in blood lead concentrations among relapsing-remitting compared to progressive MS patients (Yılmaz and Gönen, 2023). One case-control study investigated self-reported environmental exposure to lead and found that MS cases were significantly more likely to report such exposure (Napier et al., 2016). With respect to cadmium, a meta-analysis found that blood cadmium concentrations were significantly higher in MS patients than controls (Sarihi et al., 2021). Additionally, two small case-control studies reported that cadmium concentrations in fingernails and urine were significantly higher in MS patients than controls, and that higher urinary cadmium was correlated with disease severity (Armon-Omer et al., 2024; Moradi et al., 2023), while a third cross-sectional study reported no association between blood cadmium concentrations and MS functional status (Knyszyńska et al., 2022). Among MS patients, blood cadmium concentrations were not significantly different by type of MS (Yılmaz and Gönen, 2023). Studies on mercury and MS also reported mixed findings. Two meta-analyses, including one focused on occupational exposures (Vitturi et al., 2023) and another on blood concentrations (Sarihi et al., 2021), both reported no association between mercury and MS. By contrast, two additional case-control studies, of which one assessed self-reported mercury
exposure (Napier et al., 2016) and the other examined urinary mercury concentrations (Armon-Omer et al., 2024), reported significant associations with MS. Among MS patients, one study reported no difference in mercury concentrations in hair between those with relapsing-remitting and progressive MS (Yılmaz and Gönen, 2023).
Only a few studies (three or fewer) examined relationships between aluminum, arsenic, magnesium, manganese, nickel, or zinc and MS. These studies were generally small and findings were generally inconsistent. Specifically, for aluminum, two small case-control studies reported that concentrations of aluminum in hair and urine were higher in MS patients than controls, but the effect was only significant for hair (Arain et al., 2015; Armon-Omer et al., 2024). Aluminum concentrations in hair were significantly higher among those with progressive MS compared to those with relapsing-remitting MS (Yılmaz and Gönen, 2023). Regarding arsenic, a meta-analysis (Sarihi et al., 2021) and an additional case-control study (Armon-Omer et al., 2024) found higher concentrations of arsenic in MS patients compared to controls, though the association in the case-control study was not significant; among MS patients, arsenic concentrations did not differ significantly between those with relapsing-remitting and progressive MS (Yılmaz and Gönen, 2023). Similarly, for magnesium, a meta-analysis reported that blood magnesium concentrations were not significantly associated with MS (Nirooei et al., 2022), while a systematic review identified one study, which found that magnesium exposure was associated with decreased odds of MS (Oliveira et al., 2020) and magnesium was higher in hair samples of those with relapsing-remitting MS compared to progressive MS among MS patients (Yılmaz and Gönen, 2023). Relatedly, among three studies on nickel, one small case-control study reported significantly higher nickel in fingernails of MS patients compared to controls (Moradi et al., 2023); another small case-control study reported that urinary nickel was higher in MS patients than controls, but the association did not reach statistical significance (Armon-Omer et al., 2024), and there were no differences in blood or hair nickel concentrations among those with relapsing-remitting and progressive MS (Yılmaz and Gönen, 2023). Of two studies that assessed cobalt and MS, one reported significantly higher cobalt concentrations in fingernails of MS patients compared to controls (Moradi et al., 2023). Among MS patients, one study found no significant differences in blood cobalt concentrations by type of MS (Yılmaz and Gönen, 2023).
For manganese and zinc, findings from a few studies were generally in the same direction. However, owing to the limited available evidence, similar findings among only a few studies were insufficient to establish a consistent risk-conferring association. Three studies of manganese and MS, including the Nirooei and colleagues (2022) meta-analysis, reported consistently and significantly higher manganese concentrations from blood, hair, and
fingernails among MS patients than controls (Arain et al., 2015; Moradi et al., 2023). Despite the inclusion of a meta-analysis, which is generally higher quality than the case-control studies with small sample sizes, because these manganese concentrations were assessed after MS diagnoses, these studies cannot preclude reverse causation. Among MS patients, manganese concentrations did not differ significantly by type (Yılmaz and Gönen, 2023). The same meta-analysis also investigated blood zinc concentrations. In pooled analyses, blood zinc concentrations were significantly lower in MS patients than controls (Nirooei et al., 2022). Among MS patients, zinc concentrations were higher in relapsing-remitting MS patients’ hair samples compared to those of progressive MS patients (Yılmaz and Gönen, 2023).
Several studies reported no association between chromium, copper, or selenium and MS outcomes (Armon-Omer et al., 2024; Nirooei et al., 2022; Yılmaz and Gönen, 2023).
Radiation and MS
The search yielded one meta-analysis that assessed occupational radiation exposure and MS (Vitturi et al., 2023). Based on one included study, it reported that occupational radiation exposure was not significantly associated with MS.
Solvents and MS
The literature search produced four studies, including one umbrella review of umbrella reviews (Mentis et al., 2021) and two meta-analyses (Gerhardsson et al., 2021; Vitturi et al., 2023). The umbrella review reported that organic solvent exposure significantly increased odds of MS, but this was based on weak evidence (Mentis et al., 2021). Of the two meta-analyses, one that included 14 case-control studies reported that exposure to organic solvents was associated with significantly increased risk of developing MS in pooled analyses (Gerhardsson et al., 2021). However, the other meta-analysis of six studies did not find a significant relationship between occupational solvent exposure and MS (Vitturi et al., 2023). In addition to the reviews, a small (n = 713) case-control study by Napier and colleagues (2016) reported that MS cases were less likely to report solvent exposure compared to controls, but this relationship was not statistically significant.
Burn Pits or Mold and MS
The committee’s search yielded zero results on the possible relationships between exposure to burn pits or mold and the risk of MS.
Conclusion
Conclusion 7-3: Based on its analysis of the available data, the committee does not find a possible risk-conferring relationship between exposure to burn pits, dust and particulate matter (PM), exhaust, fuels, incinerator emissions, metals, mold, radiation, or solvents and multiple sclerosis (MS).
Based on the literature review, there is suggestive evidence of a possible risk-conferring relationship between exposure to solvents and MS. There is limited evidence of a possible risk-conferring relationship between exposure to PM, fuels, or metals and MS. There is insufficient evidence of a possible risk-conferring relationship between exposure to radiation and MS. There is no identified literature on the relationship between exposure to burn pits or mold and MS.
Synthesizing the committee’s data analysis and literature review, the committee concludes there is a possible risk-conferring relationship between exposure to solvents and MS. The committee further concludes there is inadequate or insufficient evidence of a possible risk-conferring relationship between exposure to burn pits, dust and PM, exhaust, fuels, incinerator emissions, metals, mold, or radiation and MS.
PARKINSON’S DISEASE
Parkinson’s disease is a degenerative disorder of the nervous system that weakens, damages, or kills nerve cells by accumulating abnormal forms of the protein alpha-synuclein in areas responsible for motor control (NINDS, 2024c). It is defined by four main symptoms: tremors, rigidity, bradykinesia (slowing of spontaneous and automatic movements), and postural instability. As the disease progresses, daily tasks become increasingly difficult to accomplish without assistance, and nonmotor symptoms may develop, including autonomic changes, cognitive impairment, mood disorders, and hallucinations (NINDS, 2024c). Most adults are diagnosed around age 70, though a small proportion of cases have early-onset disease (NINDS, 2024c). The Parkinson’s Foundation estimates that approximately 1 million U.S. adults aged 45+ have Parkinson’s disease (Marras et al., 2018). VA estimates that 110,000 veterans have Parkinson’s disease (VA, 2021).
Analysis Results
There were 1,155 cases (0.1%) of Parkinson’s disease. Figure 7-4 shows that exposure to dust and PM and exhaust are associated with a risk-conferring relationship with Parkinson’s disease with adjusted ORs of 1.22 (95% CI: 1.03–1.44) and 1.24 (95% CI: 1.05–1.46), respectively. Analyses stratified by TBI status also indicated elevated odds of Parkinson’s disease associated with fuel exposure among people with TBI with an adjusted OR of 2.88 (95% CI: 1.11–7.49); people without TBI had an OR below 1.00. TBI stratified analyses were underpowered to detect differences in association due to the small number of people with Parkinson’s disease and TBI in the sample. All exposures had no differences in the magnitude of association when stratifying by age (younger than 50 years old versus 50+), which suggests no interaction between these exposures and age on odds of Parkinson’s disease.
Literature Search Results
PM and Parkinson’s Disease
The literature search identified 10 studies on PM and Parkinson’s disease, of which one was an umbrella review of meta-analyses (Tan et al., 2022), five were additional meta-analyses (Dhiman et al., 2023; Fu et al., 2019; Gong et al., 2023; Hu et al., 2019; Wang et al., 2020), two were systematic reviews with narrative synthesis (Cristaldi et al., 2022; Oliveira et al., 2020), and two were large European cohort studies (Cole-Hunter et al., 2023; Peters et al., 2024). These studies investigated PM overall, PM2.5, PM10, and specific pollutants.
Two meta-analyses that examined overall PM (including studies that focused on both PM2.5 and PM10) did not find a significant association with Parkinson’s disease (Hu et al., 2019; Wang et al., 2020). When restricting to PM by diameter size, none of six studies, including the umbrella review (Tan et al., 2022), four meta-analyses (Dhiman et al., 2023; Gong et al., 2023; Hu et al., 2019; Wang et al., 2020), and a systematic review (Oliveira et al., 2020), found a correlation between PM10 and Parkinson’s disease. The relationship between PM2.5 and Parkinson’s disease was more inconsistent. Two meta-analyses reported that PM2.5 had a significant risk-conferring relationship with Parkinson’s disease (Dhiman et al., 2023; Fu et al., 2019), though one found this effect only for long-term PM2.5 exposure (Fu et al., 2019); one other meta-analysis found no significant association (Gong et al., 2023). Similarly, a systematic review reported that included studies generally found a risk-conferring relationship between PM2.5 and Parkinson’s disease, though not all reached statistical significance (Cristaldi
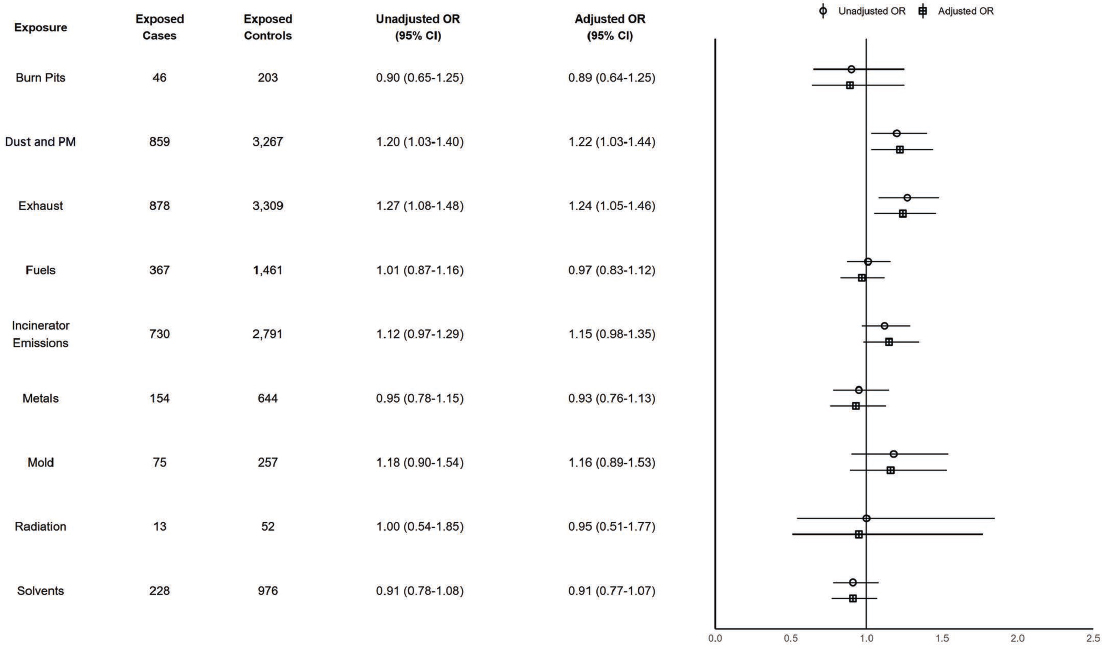
NOTES: Exposed cases n = 1,155; exposed controls n = 4,596. CI = confidence interval; ILER = Individual Longitudinal Exposure Record; OR = odds ratio; PM = particulate matter.
et al., 2022). In addition, a large cohort study that pooled data from seven cohort studies across six European countries reported a significant linear association between PM2.5 and Parkinson’s disease (Cole-Hunter et al., 2023). However, the umbrella review of meta-analyses, two meta-analyses, and one cohort study did not find that PM2.5 was significantly associated with Parkinson’s disease (Hu et al., 2019; Peters et al., 2024; Tan et al., 2022; Wang et al., 2020).
Six studies investigated the relationship between specific pollutants and Parkinson’s disease. A meta-analysis reported that carbon monoxide showed a risk-conferring relationship with Parkinson’s disease (Hu et al., 2019) but the umbrella review and another meta-analysis did not find an association (Dhiman et al., 2023; Tan et al., 2022). Studies on ozone and nitrogen oxides were mixed. Ozone was found to be protective in one cohort study (Cole-Hunter et al., 2023), but another cohort study, the umbrella review, and two meta-analyses found no association with Parkinson’s disease (Dhiman et al., 2023; Hu et al., 2019; Peters et al., 2024; Tan et al., 2022). One meta-analysis (Hu et al., 2019) found a positive association with nitrogen oxides, but the umbrella review, a meta-analysis, and a cohort study found no association (Dhiman et al., 2023; Peters et al., 2024; Tan et al., 2022). A systematic review (Oliveira et al., 2020) reported inconsistent findings for nitrogen dioxide and five other studies found no association (Cole-Hunter et al., 2023; Dhiman et al., 2023; Hu et al., 2019; Peters et al., 2024; Tan et al., 2022). One meta-analysis did not find an association between sulfur dioxide and Parkinson’s disease (Hu et al., 2019). Lastly, the two cohort studies also reported no association between black carbon (Cole-Hunter et al., 2023) or elemental carbon (Peters et al., 2024) and Parkinson’s disease mortality.
Fuels and Parkinson’s Disease
The search yielded one umbrella review of umbrella reviews of systematic reviews and meta-analyses that investigated a wide range of risk factors and neurologic disorders, including Parkinson’s disease (Mentis et al., 2021). The study found that exposure to hydrocarbons, the main component of fuels like petroleum and natural gas, was associated with significantly increased odds of developing Parkinson’s, but this association was based on weak evidence.
Metals and Parkinson’s Disease
The search produced five studies on metals and Parkinson’s disease, including two meta-analyses (Du et al., 2018; Zhao et al., 2023) and one systematic review (Oliveira et al., 2020). None were in veteran populations
and findings were mixed. Exposure to manganese has been of particular concern as a potential risk factor for Parkinson’s disease, especially when occupationally mediated. Therefore, this section presents those results first, followed by evidence on unspecified metals, and then other specific metals—lead, copper, and others.
The two meta-analyses and the systematic review investigated the relationship between manganese and Parkinson’s disease. All reported that manganese was associated with Parkinson’s disease, but not all correlations reached statistical significance. In one meta-analysis, occupational or environmental exposure to manganese increased odds of Parkinson’s disease with borderline significance in pooled analyses combining four studies (Zhao et al., 2023). This same study did not identify a significant relationship between manganese from dietary intake and Parkinson’s disease. The other meta-analyses reported that, in pooled analyses from 22 studies, patients with Parkinson’s disease had significantly increased levels of manganese in blood (serum, plasma, and whole blood) compared to controls, but there were no significant differences in cerebrospinal fluid (Du et al., 2018). Finally, the systematic review identified one study that assessed exposure to manganese from traffic-related air pollution and found that manganese was positively spatially associated with Parkinson’s disease (Oliveira et al., 2020).
Two studies reported a correlation between occupational exposure to unspecified metals and Parkinson’s disease. The Zhao and colleagues (2023) meta-analysis reported occupational and environmental exposure to unspecified metals associated with increased but not significant odds of Parkinson’s disease in pooled analyses based on eight included studies. In addition, an ecological study using French administrative data found higher Parkinson’s disease incidence in cantons with a higher proportion of workers in metallurgy and metal fabrication occupations (Vlaar et al., 2018).
Few studies (three or fewer) investigated lead, copper, or magnesium and Parkinson’s disease, with inconsistent results. Regarding lead, a meta-analysis found that bone lead concentration was significantly higher in Parkinson’s disease cases than controls and that occupational lead exposure was associated with increased but not significant odds of Parkinson’s disease (Zhao et al., 2023). The Oliveira and colleagues (2020) systematic review on environmental exposures and neurodegenerative diseases identified two ecological studies on airborne lead exposure, both of which found lead was spatially related to Parkinson’s disease. In contrast to these reviews, a small (n = 80) case-control study conducted at an outpatient clinic in India found that blood lead concentrations were significantly lower in Parkinson’s disease patients than controls (Gupta et al., 2017). As for copper, a meta-analysis found that serum copper concentrations were significantly lower in Parkinson’s disease patients compared to controls (Zhao et al., 2023).
However, the systematic review identified one study, which found that copper was spatially related to Parkinson’s disease (Oliveira et al., 2020). With respect to magnesium, the meta-analysis by Zhao and colleagues (2023) reported magnesium in cerebrospinal fluid was significantly higher in cases with Parkinson’s disease but found no association between dietary intake of magnesium and Parkinson’s disease, while the systematic review identified one study that reported that ambient magnesium was spatially related to Parkinson’s disease cases (Oliveira et al., 2020). The meta-analysis by Zhao and colleagues (2023) also investigated a range of other metals. In pooled analyses, it found blood cadmium and plasma or serum zinc concentrations were significantly lower in cases with Parkinson’s disease than in controls. It also reported that calcium and selenium from dietary sources; mercury from occupational or environmental exposure; and copper, iron, and zinc from both dietary and occupational/environmental sources were not significantly associated with Parkinson’s disease.
Radiation and Parkinson’s Disease
The committee identified one meta-analysis by Lopes and colleagues (2022) on occupational exposure to ionizing radiation on mortality from a composite measure of diseases of the nervous system, which included Parkinson’s disease. As described previously, this study found that radiation workers had significantly lower mortality from nervous system disorders compared to the general population but a nonsignificant increase in risk of nervous system disease mortality among exposed medical radiation workers compared to internal controls. These conflicting results likely reflect the heterogeneity of the conditions included in the outcome measures. When restricted to three studies investigating Parkinson’s disease as an outcome, pooled standardized mortality ratio analyses showed no significant difference in mortality between those exposed to radiation and the general population. However, in pooled excess relative risk analysis, the authors did find significant evidence of a dose-response relationship between exposure and Parkinson’s disease mortality and incidence—that is, increased exposure to ionizing radiation was significantly associated with increased risk.
Solvents and Parkinson’s Disease
The literature search yielded three studies, including an umbrella review of umbrella reviews of systematic review and meta-analyses (Mentis et al., 2021), a case-control study (Sallmén et al., 2024), and an ecological study (Vlaar et al., 2018). The umbrella review found that organic solvent exposure was associated with significantly increased odds of Parkinson’s disease, but this association was based on weak evidence (Mentis et al., 2021).
The Finnish case-control study found no association between occupational exposure to solvents and Parkinson’s disease (Sallmén et al., 2024), and an ecological study using French administrative data to evaluate the association of Parkinson’s disease with industry sectors found that most occupations in the study with expected exposure to solvents (e.g., construction, chemical or paper manufacturing) were not associated with Parkinson’s disease incidence, except for metal manufacture (Vlaar et al., 2018).
Burn Pits or Mold and Parkinson’s Disease
The committee’s search yielded zero results on the possible relationships between exposure to burn pits or mold and the risk of Parkinson’s disease.
Conclusion
Conclusion 7-4: Based on its analysis of the available data, the committee finds there is a possible risk-conferring relationship between exposure to dust and particulate matter (PM) or exhaust and Parkinson’s disease. The committee does not find a possible risk-conferring relationship between exposure to burn pits, fuels, incinerator emissions, metals, mold, radiation, or solvents and Parkinson’s disease.
Based on the literature review, there is mixed evidence of a possible risk-conferring relationship between exposure to PM, metals, or solvents and Parkinson’s disease. There is limited evidence of a possible risk-conferring relationship between exposure to fuels and Parkinson’s disease. There is insufficient evidence of a possible risk-conferring relationship between exposure to radiation and Parkinson’s disease. There is no identified literature on the relationship between exposure to burn pits or mold and Parkinson’s disease.
Synthesizing the committee’s data analysis and literature review, the committee concludes there is a possible risk-conferring relationship between exposure to dust and PM or exhaust and Parkinson’s disease. The committee further concludes there is inadequate or insufficient evidence of a possible risk-conferring relationship between exposure to burn pits, fuels, incinerator emissions, metals, mold, radiation, or solvents and Parkinson’s disease.
SUMMARY
Through its data analysis and literature review, the committee examined possible relationships between neurologic outcomes (ALS, dementia, MS, and Parkinson’s disease) and its exposures of interest: burn pits, dust and PM, exhaust, fuels, incinerator emissions, metals, mold, radiation, and solvents. Synthesizing evidence from data analyses and a structured literature review, the committee concluded that six possible risk-conferring relationships exist between any exposure of interest and any neurologic outcome—between exhaust and ALS, solvents and ALS, PM and dementia, solvents and MS, dust and PM and Parkinson’s disease, and exhaust and Parkinson’s disease. Dust and PM, exhaust, and solvents were associated with possible risk-conferring relationships; evidence was inadequate or insufficient of a possible risk-conferring relationship between burn pits, fuels, incinerator emissions, metals, mold, or radiation and any neurologic outcome. All four outcomes were associated with possible risk-conferring relationships. The committee notes that, based on its sample population, conclusions are specific to post-9/11 veterans who served in Southwest Asia or Afghanistan and received care at VHA.
REFERENCES
Abolhasani, E., V. Hachinski, N. Ghazaleh, M. R. Azarpazhooh, N. Mokhber, and J. Martin. 2023. Air pollution and incidence of dementia: A systematic review and meta-analysis. Neurology 100(2):E242–E254.
Adani, G., T. Filippini, C. Garuti, M. Malavolti, G. Vinceti, G. Zamboni, M. Tondelli, C. Galli, M. Costa, M. Vinceti, and A. Chiari. 2020. Environmental risk factors for early-onset Alzheimer’s dementia and frontotemporal dementia: A case-control study in northern Italy. International Journal of Environmental Research and Public Health 17(21):1–18.
Alzheimer’s Association. 2024. 2024 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia 20(5):3708–3821.
Andersen, Z. J., J. Zhang, J. T. Jørgensen, E. Samoli, S. Liu, J. Chen, M. Strak, K. Wolf, G. Weinmayr, S. Rodopolou, E. Remfry, K. de Hoogh, T. Bellander, J. Brandt, H. Concin, E. Zitt, D. Fecht, F. Forastiere, J. Gulliver, B. Hoffmann, U. A. Hvidtfeldt, W. M. Monique Verschuren, K. H. Jöckel, R. So, T. Cole-Hunter, A. J. Mehta, L. H. Mortensen, M. Ketzel, A. Lager, K. Leander, P. Ljungman, G. Severi, M. C. Boutron-Ruault, P. K. E. Magnusson, G. Nagel, G. Pershagen, A. Peters, D. Rizzuto, Y. T. van der Schouw, S. Schramm, M. Stafoggia, K. Katsouyanni, B. Brunekreef, G. Hoek, and Y. H. Lim. 2022. Long-term exposure to air pollution and mortality from dementia, psychiatric disorders, and suicide in a large pooled European cohort: ELAPSE study. Environment International 170:107581.
Andrew, A., J. Zhou, J. Gui, X. Shi, M. Li, A. Harrison, B. Guetti, R. Nathan, T. Butt, D. Peipert, M. Tischbein, E. P. Pioro, E. Stommel, and W. Bradley. 2022. ALS risk factors: Industrial airborne chemical releases. Environmental Pollution 295:118658.
Antonioni, A., V. Govoni, L. Brancaleoni, A. Donà, E. Granieri, M. Bergamini, R. Gerdol, and M. Pugliatti. 2023. Amyotrophic lateral sclerosis and air pollutants in the province of Ferrara, northern Italy: An ecological study. International Journal of Environmental Research and Public Health 20(8):5591.
Arain, M. S., H. I. Afridi, T. G. Kazi, F. N. Talpur, M. B. Arain, A. Kazi, S. A. Arain, and J. Ali. 2015. Correlation of aluminum and manganese concentration in scalp hair samples of patients having neurological disorders. Environmental Monitoring and Assessment 187(2):10.
Armon-Omer, A., T. Mansor, M. Edelstein, E. Bukovetzky, L. Groisman, E. Rorman, A. S. Nov, and R. Shahien. 2024. Association between multiple sclerosis and urinary levels of toxic metals and organophosphates: A cross-sectional study in Israel. Multiple Sclerosis and Related Disorders 83:105445.
Beard, J. D., L. S. Engel, D. B. Richardson, M. D. Gammon, C. Baird, D. M. Umbach, K. D. Allen, C. L. Stanwyck, J. Keller, D. P. Sandler, S. Schmidt, and F. Kamel. 2017. Military service, deployments, and exposures in relation to amyotrophic lateral sclerosis survival. PLoS ONE 12(10):e0185751.
Bocca, B., G. Forte, R. Oggiano, S. Clemente, Y. Asara, A. Peruzzu, C. Farace, S. Pala, A. G. Fois, P. Pirina, and R. Madeddu. 2015. Level of neurotoxic metals in amyotrophic lateral sclerosis: A population-based case-control study. Journal of the Neurological Sciences 359(1–2):11–17.
Capozzella, A., C. Sacco, A. Chighine, B. Loreti, B. Scala, T. Casale, F. Sinibaldi, G. Tomei, R. Giubilati, F. Tomei, and M. V. Rosati. 2014. Work related etiology of amyotrophic lateral sclerosis (ALS): A meta-analysis. Annali di Igiene 26(5):456–472.
Chandra, M., C. B. Rai, N. Kumari, V. K. Sandhu, K. Chandra, M. Krishna, S. H. Kota, K. S. Anand, and A. Oudin. 2022. Air pollution and cognitive impairment across the life course in humans: A systematic review with specific focus on income level of study area. International Journal of Environmental Research and Public Health 19(3):1405.
Cheng, S., Y. Jin, Y. Dou, Y. Zhao, Y. Duan, H. Pei, and P. Lyu. 2022. Long-term particulate matter 2.5 exposure and dementia: A systematic review and meta-analysis. Public Health 212:33–41.
Cole-Hunter, T., J. Zhang, R. So, E. Samoli, S. Liu, J. Chen, M. Strak, K. Wolf, G. Weinmayr, S. Rodopolou, E. Remfry, K. de Hoogh, T. Bellander, J. Brandt, H. Concin, E. Zitt, D. Fecht, F. Forastiere, J. Gulliver, B. Hoffmann, U. A. Hvidtfeldt, K. H. Jöckel, L. H. Mortensen, M. Ketzel, D. Yacamán Méndez, K. Leander, P. Ljungman, E. Faure, P. C. Lee, A. Elbaz, P. K. E. Magnusson, G. Nagel, G. Pershagen, A. Peters, D. Rizzuto, R. C. H. Vermeulen, S. Schramm, M. Stafoggia, K. Katsouyanni, B. Brunekreef, G. Hoek, Y. H. Lim, and Z. J. Andersen. 2023. Long-term air pollution exposure and Parkinson’s disease mortality in a large pooled European cohort: An ELAPSE study. Environment International 171:107667.
Cristaldi, A., M. Fiore, G. Oliveri Conti, E. Pulvirenti, C. Favara, A. Grasso, C. Copat, and M. Ferrante. 2022. Possible association between PM2.5 and neurodegenerative diseases: A systematic review. Environmental Research 208:112581.
Dhiman, V., T. Trushna, D. Raj, and R. R. Tiwari. 2022. Is air pollution associated with increased risk of dementia? A meta-analysis of epidemiological research. Neurology India 70(3):1004–1019.
Dhiman, V., T. Trushna, D. Raj, and R. R. Tiwari. 2023. Is ambient air pollution a risk factor for Parkinson’s disease? A meta-analysis of epidemiological evidence. International Journal of Environmental Health Research 33(8):733–750.
Dickerson, A. S., J. Hansen, M. A. Kioumourtzoglou, A. J. Specht, O. Gredal, and M. G. Weisskopf. 2018. Study of occupation and amyotrophic lateral sclerosis in a Danish cohort. Occupational and Environmental Medicine 75(9):630–638.
Dinesh, D., Q. Shao, M. Palnati, S. McDannold, Q. Zhang, A. A. T. Monfared, G. K. Jasuja, H. Davila, W. Xia, L. R. Moo, D. R. Miller, and N. Palacios. 2023. The epidemiology of mild cognitive impairment, Alzheimer’s disease and related dementia in U.S. veterans. Alzheimer’s and Dementia Journal 19(9):3977–3984.
Du, K., M. Liu, Y. Pan, X. Zhong, and M. Wei. 2017. Association of serum manganese levels with Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. Nutrients 9(3):231.
Du, K., M. Y. Liu, Y. Z. Pan, X. Zhong, and M. J. Wei. 2018. Association of circulating manganese levels with Parkinson’s disease: A meta-analysis. Neuroscience Letters 665:92–98.
Duan, Q. Q., Z. Jiang, W. M. Su, X. J. Gu, H. Wang, Y. F. Cheng, B. Cao, X. Gao, Y. Wang, and Y. P. Chen. 2023. Risk factors of amyotrophic lateral sclerosis: A global meta-summary. Frontiers in Neuroscience 17:1177431.
Farahmandfard, M. A., A. Naghibzadeh-Tahami, and N. Khanjani. 2021. Ambient air pollution and multiple sclerosis: A systematic review. Reviews on Environmental Health 36(4):535–544.
Fu, P., X. Guo, F. M. H. Cheung, and K. K. L. Yung. 2019. The association between PM 2.5 exposure and neurological disorders: A systematic review and meta-analysis. Science of the Total Environment 655:1240–1248.
Gerhardsson, L., L. Hou, and K. Pettersson. 2021. Work-related exposure to organic solvents and the risk for multiple sclerosis—A systematic review. International Archives of Occupational and Environmental Health 94(2):221–229.
Gong, Y., X. Zhang, X. Zhao, H. Chang, J. Zhang, Z. Gao, Y. Mi, Y. Chen, H. Zhang, C. Huang, and Z. Yu. 2023. Global ambient particulate matter pollution and neurodegenerative disorders: A systematic review of literature and meta-analysis. Environmental Science and Pollution Research 30(14):39418–39430.
Goutman, S. A., J. Boss, C. Godwin, B. Mukherjee, E. L. Feldman, and S. A. Batterman. 2022. Associations of self-reported occupational exposures and settings to ALS: A case–control study. International Archives of Occupational and Environmental Health 95:1567–1586.
Goutman, S. A., J. Boss, C. Godwin, B. Mukherjee, E. L. Feldman, and S. A. Batterman. 2023. Occupational history associates with ALS survival and onset segment. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 24(3-4):219–229.
Gupta, V., N. G. Ansari, R. K. Garg, and S. Khattri. 2017. Determination of CD, CR, PB and NI contents among Parkinson’s disease individuals: A case-control study. International Journal of Neuroscience 127(9):770–775.
Hu, C. Y., Y. Fang, F. L. Li, B. Dong, X. G. Hua, W. Jiang, H. Zhang, Y. Lyu, and X. J. Zhang. 2019. Association between ambient air pollution and Parkinson’s disease: Systematic review and meta-analysis. Environmental Research 168:448–459.
Hudomiet, P., M. D. Hurd, and S. Rohwedder. 2018. Dementia prevalence in the United States in 2000 and 2012: Estimates based on a nationally representative study. Journal of Gerontology Series B 73(Suppl_1):S10–S19.
IOM (Institute of Medicine). 2011. Long-term health consequences of exposure to burn pits in Iraq and Afghanistan. Washington, DC: The National Academies Press.
Kamalian, A., I. Foroughmand, L. Koski, M. Darvish, A. Saghazadeh, A. Kamalian, S. Z. E. Razavi, S. Abdi, S. R. Dehgolan, A. Fotouhi, and P. M. Roos. 2023. Metal concentrations in cerebrospinal fluid, blood, serum, plasma, hair, and nails in amyotrophic lateral sclerosis: A systematic review and meta-analysis. Journal of Trace Elements in Medicine and Biology 78:127165.
Knyszyńska, A., K. Skonieczna-Żydecka, D. Koziarska, L. Stachowska, A. Kotwas, M. Kulaszyńska, A. Lubkowska, and B. Karakiewicz. 2022. Searching for the relationship between the concentration of heavy metals in the blood and the clinical course of multiple sclerosis: A cross-sectional study in Poland. International Journal of Environmental Research and Public Health 19(11):6548.
Krishnan, L. L., N. J. Petersen, A. L. Snow, J. A. Cully, P. E. Schulz, D. P. Graham, R. O. Morgan, U. Braun, M. L. Moffett, H.-J. Yu, and M. E. Kunik. 2005. Prevalence of dementia among Veterans Affairs medical care system users. Dementia and Geriatric Cognitive Disorders 20(4):245–253.
Lopes, J., K. Leuraud, D. Klokov, C. Durand, M. O. Bernier, and C. Baudin. 2022. Risk of developing non-cancerous central nervous system diseases due to ionizing radiation exposure during adulthood: Systematic review and meta-analyses. Brain Sciences 12(8):984.
Lotfi, F., M. Mansourian, O. Mirmoayyeb, S. Najdaghi, V. Shaygannejad, and N. Esmaeil. 2022. Association of exposure to particulate matters and multiple sclerosis: A systematic review and meta-analysis. NeuroImmunoModulation 29(1):21–27.
Malek, A. M., A. Barchowsky, R. Bowser, T. Heiman-Patterson, D. Lacomis, S. Rana, Y. Ada, and E. O. Talbott. 2015. Exposure to hazardous air pollutants and the risk of amyotrophic lateral sclerosis. Environmental Pollution 197:181–186.
Marras, C., J. C. Beck, J. H. Bower, E. Roberts, B. Ritz, G. W. Ross, R. D. Abbott, R. Savica, S. K. Van Den Eeden, A. W. Willis, and C. M. Tanner, on behalf of the Parkinson’s Foundation P4 Group. 2018. Prevalence of Parkinson’s disease across North America. npj Parkinson’s Disease 4:21.
Mehta, P., J. Raymond, Y. Zhang, R. Punjani, M. Han, T. Larson, O. Muravov, R. H. Lyles, and D. K. Horton. 2023. Prevalence of amyotrophic lateral sclerosis in the United States, 2018. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 24(7–8):702–708.
Mentis, A. F. A., E. Dardiotis, V. Efthymiou, and G. P. Chrousos. 2021. Non-genetic risk and protective factors and biomarkers for neurological disorders: A meta-umbrella systematic review of umbrella reviews. BMC Medicine 19(1):6.
Moradi, A., N. Honarjoo, A. A. Besalatpour, and M. Etemadifar. 2023. Human exposure to dust and heavy metals in industrial regions and its relationship with the prevalence of multiple sclerosis disease. Environmental Monitoring and Assessment 195(4):471.
Napier, M. D., C. Poole, G. A. Satten, A. Ashley-Koch, R. A. Marrie, and D. M. Williamson. 2016. Heavy metals, organic solvents, and multiple sclerosis: An exploratory look at gene-environment interactions. Archives of Environmental and Occupational Health 71(1):26–34.
Newell, M. E., A. Babbrah, A. Aravindan, R. Rathnam, R. Kiernan, E. M. Driver, D. A. Bowes, and R. U. Halden. 2024. Prevalence rates of neurodegenerative diseases versus human exposures to heavy metals across the United States. Science of the Total Environment 928:172260.
NINDS (National Institute of Neurological Disorders and Stroke). 2024a. Amyotrophic Lateral Sclerosis (ALS). https://www.ninds.nih.gov/health-information/disorders/amyotrophic-lateral-sclerosis-als (accessed May 14, 2025).
NINDS. 2024b. Dementias. https://www.ninds.nih.gov/health-information/disorders/dementias# (accessed July 19, 2024).
NINDS. 2024c. Parkinson’s Disease. https://www.ninds.nih.gov/health-information/disorders/parkinsons-disease (accessed July 22, 2024).
NINDS. 2025. Multiple Sclerosis. https://www.ninds.nih.gov/health-information/disorders/multiple-sclerosis (accessed May 14, 2025).
Nirooei, E., S. M. A. Kashani, S. Owrangi, F. Malekpour, M. Niknam, F. Moazzen, P. Nowrouzi-Sohrabi, S. Farzinmehr, and H. Akbari. 2022. Blood trace element status in multiple sclerosis: A systematic review and meta-analysis. Biological Trace Element Research 200(1):13–26.
Oliveira, M., A. Padrão, A. Ramalho, M. Lobo, A. C. Teodoro, H. Gonçalves, and A. Freitas. 2020. Geospatial analysis of environmental atmospheric risk factors in neurodegenerative diseases: A systematic review. International Journal of Environmental Research and Public Health 17(22):1–25.
Parks, R. M., Y. Nunez, A. A. Balalian, E. A. Gibson, J. Hansen, O. Raaschou-Nielsen, M. Ketzel, J. Khan, J. Brandt, R. Vermeulen, S. Peters, J. Goldsmith, D. B. Re, M. G. Weisskopf, and M. A. Kioumourtzoglou. 2022. Long-term traffic-related air pollutant exposure and amyotrophic lateral sclerosis diagnosis in Denmark: A Bayesian hierarchical analysis. Epidemiology 33(6):757–766.
Peters, S., K. Broberg, V. Gallo, M. Levi, M. Kippler, P. Vineis, J. Veldink, L. van den Berg, L. Middleton, R. C. Travis, M. M. Bergmann, D. Palli, S. Grioni, R. Tumino, A. Elbaz, T. Vlaar, F. Mancini, T. Kühn, V. Katzke, A. Agudo, F. Goñi, J. H. Gómez, M. Rodríguez-Barranco, S. Merino, A. Barricarte, A. Trichopoulou, M. Jenab, E. Weiderpass, and R. Vermeulen. 2021. Blood metal levels and amyotrophic lateral sclerosis risk: A prospective cohort. Annals of Neurology 89(1):125–133.
Peters, S., F. Bouma, G. Hoek, N. Janssen, and R. Vermeulen. 2024. Air pollution exposure and mortality from neurodegenerative diseases in the Netherlands: A population-based cohort study. Environmental Research 259:119552.
Sagiraju, H. K. R., S. Zivkovic, A. C. VanCott, H. Patwa, D. Gimeno Ruiz de Porras, M. E. Amuan, and M. J. V. Pugh. 2020. Amyotrophic lateral sclerosis among veterans deployed in support of post-9/11 U.S. conflicts. Military Medicine 185(3–4):e501–e509.
Sallmén, M., I. Burstyn, S. Uuksulainen, A. Koskinen, C. Hublinm, and M. Sainio. 2024. Parkinson’s disease and occupational exposure to organic solvents in Finland: A nationwide case-control study. Scandinavian Journal of Work, Environment and Health 50(1):39–48.
Sarihi, S., M. Niknam, S. Mahjour, M. Hosseini-Bensenjan, F. Moazzen, S. Soltanabadi, and H. Akbari. 2021. Toxic heavy metal concentrations in multiple sclerosis patients: A systematic review and meta-analysis. EXCLI Journal 20:1571–1584.
Saucier, D., P. P. W. Registe, M. Bélanger, and C. O’Connell. 2023. Urbanization, air pollution, and water pollution: Identification of potential environmental risk factors associated with amyotrophic lateral sclerosis using systematic reviews. Frontiers in Neurology 14:1108383.
Scheltens, P., B. De Strooper, M. Kivipelto, H. Holstege, G. Chetelat, C. E. Teunissen, J. Cummings, and W. M. van der Flier. 2021. Alzheimer’s disease. Lancet 397(10284):1577–1590.
Schnatter, A. R., N. C. Wojcik, and G. Jorgensen. 2019. Mortality update of a cohort of Canadian petroleum workers. Journal of Occupational and Environmental Medicine 61(3):225–238.
Sheppard, O., and M. Coleman. 2020. Alzheimer’s disease: Etiology, neuropathology and pathogenesis. In Alzheimer’s disease: Drug discovery, edited by X. Huang. Brisbane, Australia: Exon Publications.
Srivastava, T., E. Chirikova, S. Birk, F. Xiong, T. Benzouak, J. Y. Liu, P. J. Villeneuve, and L. B. Zablotska. 2023. Exposure to ionizing radiation and risk of dementia: A systematic review and meta-analysis. Radiation Research 199(5):490–505.
Strumylaite, L., R. Kregzdyte, O. Kucikiene, D. Baranauskiene, V. Simakauskiene, R. Naginiene, G. Damuleviciene, V. Lesauskaite, and R. Zemaitiene. 2022. Alzheimer’s disease association with metals and metalloids concentration in blood and urine. International Journal of Environmental Research and Public Health 19(12):7309.
Tan, L., E. Nakanishi, and M. Lee. 2022. Association between exposure to air pollution and late-life neurodegenerative disorders: An umbrella review. Environment International 158:106956.
Tang, C., Q. R. Li, Y. M. Mao, Y. R. Xia, H. S. Guo, J. P. Wang, Z. W. Shuai, and D. Q. Ye. 2021. Association between ambient air pollution and multiple sclerosis: A systemic review and meta-analysis. Environmental Science and Pollution Research 28(41):58142–58153.
Tang, J., A. Chen, F. He, M. Shipley, A. Nevill, H. Coe, Z. Hu, T. Zhang, H. Kan, E. Brunner, X. Tao, and R. Chen. 2023. Association of air pollution with dementia: A systematic review with meta-analysis including new cohort data from China. Environmental Research 223:115048.
VA (Department of Veterans Affairs). 2021. VA Research on Parkinson’s Disease. https://www.research.va.gov/topics/parkinsons.cfm (accessed September 5, 2024).
VA. 2023. Frequently Asked Questions Relating to Military Service. https://www.va.gov/MS/RESOURCES/FAQ_index.asp (accessed May 14, 2025).
VHA (Veterans Health Administration). 2021. VHA directive 1101.07: Amyotrophic lateral sclerosis system of care. Washington, DC: Department of Veterans Affairs.
Vitturi, B. K., A. Montecucco, A. Rahmani, G. Dini, and P. Durando. 2023. Occupational risk factors for multiple sclerosis: A systematic review with meta-analysis. Frontiers in Public Health 11:1285103.
Vlaar, T., S. Kab, Y. Schwaab, N. Frery, A. Elbaz, and F. Moisan. 2018. Association of Parkinson’s disease with industry sectors: A French nationwide incidence study. European Journal of Epidemiology 33(11):1101–1111.
Wallin, M. T., W. J. Culpepper, J. D. Campbell, L. M. Nelson, A. Langer-Gould, R. A. Marrie, G. R. Cutter, W. E. Kaye, L. Wagner, H. Tremlett, S. L. Buka, P. Dilokthornsakul, B. Topol, L. H. Chen, and N. G. LaRocca, on behalf of the U.S. Multiple Sclerosis Prevalence Workgroup. 2019. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology 92(10):e1029–e1040.
Walton, C., R. King, L. Rechtman, W. Kaye, E. Leray, R. A. Marrie, N. Robertson, N. La Rocca, B. Uitdehaag, I. van der Mei, M. Wallin, A. Helme, C. Angood Napier, N. Rijke, and P. Baneke. 2020. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Multiple Sclerosis 26(14):1816–1821.
Wang, Y., Y. Liu, and H. Yan. 2020. Effect of long-term particulate matter exposure on Parkinson’s risk. Environmental Geochemistry and Health 42(7):2265–2275.
Wilker, E. H., M. Osman, and M. G. Weisskopf. 2023. Ambient air pollution and clinical dementia: Systematic review and meta-analysis. BMJ 381:e071620.
Williamson, V., S. A. M. Stevelink, K. Greenberg, and N. Greenberg. 2018. Prevalence of mental health disorders in elderly U.S. military veterans: A meta-analysis and systematic review. American Journal of Geriatric Psychiatry 26(5):534–545.
Wu, F., A. M. Malek, J. M. Buchanich, V. C. Arena, J. R. Rager, R. K. Sharma, J. E. Vena, T. Bear, and E. O. Talbott. 2024. Exposure to ambient air toxicants and the risk of amyotrophic lateral sclerosis (ALS): A matched case control study. Environmental Research 242:117719.
Xu, L., W. Zhang, X. Liu, C. Zhang, P. Wang, and X. Zhao. 2018. Circulatory levels of toxic metals (aluminum, cadmium, mercury, lead) in patients with Alzheimer’s disease: A quantitative meta-analysis and systematic review. Journal of Alzheimer’s Disease 62(1):361-372.
Yang, Y. W., S. H. Liou, Y. M. Hsueh, W. S. Lyu, C. S. Liu, H. J. Liu, M. C. Chung, P. H. Hung, and C. J. Chung. 2018. Risk of Alzheimer’s disease with metal concentrations in whole blood and urine: A case–control study using propensity score matching. Toxicology and Applied Pharmacology 356:8-14.
Yılmaz, M. Z., and M. Gönen. 2023. Investigation of the presence of heavy metals in the progression of multiple sclerosis. Turk Noroloji Dergisi 29(3):209-215.
Zhang, G., M. E, and X. Zhou. 2023. Environmental and occupational solvents exposure and amyotrophic lateral sclerosis: A systematic review and meta-analysis. Neurological Sciences 44(8):2803-2809.
Zhao, Y., A. Ray, L. Portengen, R. Vermeulen, and S. Peters. 2023. Metal exposure and risk of Parkinson disease: A systematic review and meta-analysis. American Journal of Epidemiology 192(7):1207-1223.
Zhao, Y. L., Y. Qu, Y. N. Ou, Y. R. Zhang, L. Tan, and J. T. Yu. 2021. Environmental factors and risks of cognitive impairment and dementia: A systematic review and meta-analysis. Ageing Research Reviews 72:101504.
This page intentionally left blank.





































