Preventing and Treating Dementia: Research Priorities to Accelerate Progress (2025)
Chapter: Summary
Summary1
Dementia exacts a weighty emotional and financial toll on individuals, families, and communities. Every person will have a unique experience of dementia influenced by their individual context, and many find ways to adapt to cognitive changes and enjoy meaningful lives for many years. Over the long run, however, the effects of dementia can be devastating, with advanced stages often robbing individuals of their sense of self, their memories and independence, their emotional and financial well-being, and ultimately, their lives. Moreover, the societal impacts, including the effects on families and communities and the enormous health and long-term care costs, are likely to grow with an aging population in the United States and globally. Accelerating the development of effective strategies for preventing and treating Alzheimer’s disease and related dementias (AD/ADRD)—a collection of neurodegenerative conditions that can cause cognitive impairment and ultimately lead to clinical dementia—is therefore crucial to address the growing public health crisis posed by dementia and to provide hope to millions of people at risk and those afflicted worldwide.2 Offering a chance to
___________________
1 This summary does not include references. Citations for the discussion presented in this summary appear in the subsequent report chapters.
2 The committee uses the terms AD/ADRD and related dementias throughout the report for consistency with its Statement of Task. The term AD/ADRD refers to all causes of neurodegeneration that are included in the study scope and related dementias refer to all causes of neurodegeneration that are included in the study scope with the exception of Alzheimer’s disease. Additionally, for the purposes of this report and for consistency with commonly used terminology in the field, the term dementia refers to this group of neurogenerative diseases. The term clinical dementia will be used where referring to impairment that meets the clinical criteria for diagnosis of dementia. See Box S-1 and Chapter 1 for a discussion of terminology.
preserve cognitive function, reduce morbidity, and improve quality of life for individuals and their families is vital.
In the last decade, spurred by the National Alzheimer’s Project Act, the National Institutes of Health (NIH) has invested billions to support research on detecting, understanding, and developing interventions for AD/ADRD. These investments have led to many scientific advances, including the first pharmacological treatments to slow the progression of AD in some individuals, creating a foundation of knowledge from which much more can be learned. However, the pace of progress has not matched the growing urgency for interventions that can prevent or cure AD/ADRD and reduce the societal costs of these diseases.
At the direction of the U.S. Congress, the National Institute on Aging (NIA) and the National Institute of Neurological Disorders and Stroke (NINDS) asked the National Academies of Sciences, Engineering, and Medicine to convene an expert committee to examine and assess the current state of biomedical research and recommend research priorities to advance the prevention and treatment of AD/ADRD. The committee was charged with identifying specific near- and medium-term scientific questions that can be addressed through NIH funding in the next 3 to 10 years, as well as opportunities to overcome major barriers to progress on these scientific questions. Box S-1 describes the collection of neurodegenerative disorders encompassed by the term AD/ADRD for the purposes of this report.
Of note, the committee was not asked to comprehensively catalog and assess NIH’s programmatic activities related to AD/ADRD or to evaluate and make recommendations on the current strategic planning process used by NIH to set priorities. Rather this report is intended to
BOX S-1
Descriptions of Alzheimer’s Disease and Related Dementias
Alzheimer’s disease and related dementias (AD/ADRD): The committee’s task is broadly focused on a group of progressive cognitive disorders, which develop over the life course and are characterized by an acquired loss of cognitive function that influences memory, thinking, and behavior and eventually is severe enough to interfere with independence and daily tasks. For the purposes of this report and for consistency with the Statement of Task, the term AD/ADRD includes Alzheimer’s disease and the following related dementias: Lewy body dementia, frontotemporal dementia, limbic-predominant age-related TDP-43 encephalopathy, vascular dementia, and multiple etiology dementia. As understanding of
the biological basis for this group of diseases continues to evolve, the inclusion and distinction of different disorders that fall under AD/ADRD may change. Brief descriptions of each, including distinguishing features related to brain pathologies and cognitive and behavioral characteristics, are included below.
Alzheimer’s disease (AD) dementia: AD is defined by the specific presence and location of amyloid plaque and tau neurofibrillary tangle pathologies. The condition primarily affects individuals 65 and over. Individuals diagnosed prior to turning 65 are described as having early-onset AD and some of these will have genetic causes and be referred to as familial AD. Due to an extra copy of chromosome 21, which includes the APP gene, there is another form of early-onset AD called Down syndrome-related AD. Common symptoms of AD include memory loss; difficulty completing familiar tasks; impaired judgment; misplacing objects; changes in mood, personality, or behavior; and, eventually, difficulty walking, talking, and swallowing.
Lewy body dementia (LBD): LBD is associated with abnormal deposits of a protein called alpha-synuclein in certain regions of the brain (e.g., substantia nigra). These deposits, called Lewy bodies, may also be found in other types of dementia, including Alzheimer’s dementia and Parkinson’s disease dementia. Clinical symptoms of LBD typically begin to show at age 50 or older and can include visual or auditory hallucinations; changes in concentration, attention, alertness, and wakefulness; severe loss of other cognitive abilities that interfere with daily activities; REM sleep behavior disorder, impaired autonomic function, and impaired mobility with parkinsonian features (e.g., shuffling walk, stooped posture, balance problems and repeated falls, muscle rigidity, stiffness, tremors).
Frontotemporal dementia (FTD): FTD consists of a group of disorders caused by progressive nerve cell loss in the brain’s frontal or temporal lobes, leading to loss of function in these brain regions and deterioration in behavior, personality, and/or difficulty with producing or comprehending language. Some patients with FTD may also have motor neuron disease (also known as amyotrophic lateral sclerosis or Lou Gehrig’s disease) and vice versa. The two most prominent causes of FTD involve the proteins tau and TDP-43, although there are other types of FTD caused by specific genetic mutations and different protein inclusions. Unlike AD, FTD is more commonly diagnosed in midlife, among people between 40 and 60 years of age. The three major types of FTD include
behavioral variant frontotemporal dementia, which involves changes in personality, behavior, and judgment; primary progressive aphasia, which involves changes in the ability to use language to speak, read, write, name objects, and understand what others are saying; and movement disorders, which produce changes in muscle (motor neuron disease) or motor functions (parkinsonism). The latter can include symptoms associated with such atypical parkinsonian disorders as corticobasal syndrome and progressive supranuclear palsy. Mixed clinical presentations involving a combination of these symptoms are common in FTD.
Vascular dementia: Vascular dementia is caused by disruptions to vital blood and oxygen supply that also disrupt brain neurotoxin clearance, resulting in neuronal and glial injury and cell death culminating in cognitive decline and impairment. Vascular dementia is characterized by the presence of arteriolosclerosis and neuro-glio-vascular injuries to blood–brain barrier integrity. Cerebrovascular injuries include infarcts (micro or large vessel), hemorrhages (micro or lobar), myelin abnormalities due to small vessel disease, and cerebral amyloid angiopathy. Vascular dementia presents with similar symptoms to other types of dementia and can include confusion, challenges with organizing thoughts, difficulty with planning and communication, and physical symptoms such as reduced coordination and unsteady gait. Some but not all patients with vascular dementia may have an abrupt onset due to stroke or hemorrhage.
Limbic-predominant age-related TDP-43 encephalopathy (LATE): LATE was clinically recognized in 2019 as a type of dementia that is similar to AD in clinical presentation but involving a distinct pathology characterized by the accumulation of TDP-43 in the limbic system in the brain of older adults, typically among those over the age of 80 years. Symptoms of LATE can include memory loss and impaired cognition and decision making. Misdiagnosis as AD is believed to be widespread and co-occurrence with other types of dementia is also thought to be common; some patients diagnosed with AD may instead have LATE or a combination of both brain pathologies.
Multiple etiology dementia: Multiple etiology dementia occurs when two or more pathologies (mixed pathologies) co-occur in the brain of a person living with clinical dementia. The prevalence of such co-occurrence is widespread, and it is thought that most dementia cases among those over the age of 65 years are multiple etiology dementia. Symptoms reflect those associated with the distinct pathologies and may vary based on the type and extent of neuropathological changes present.
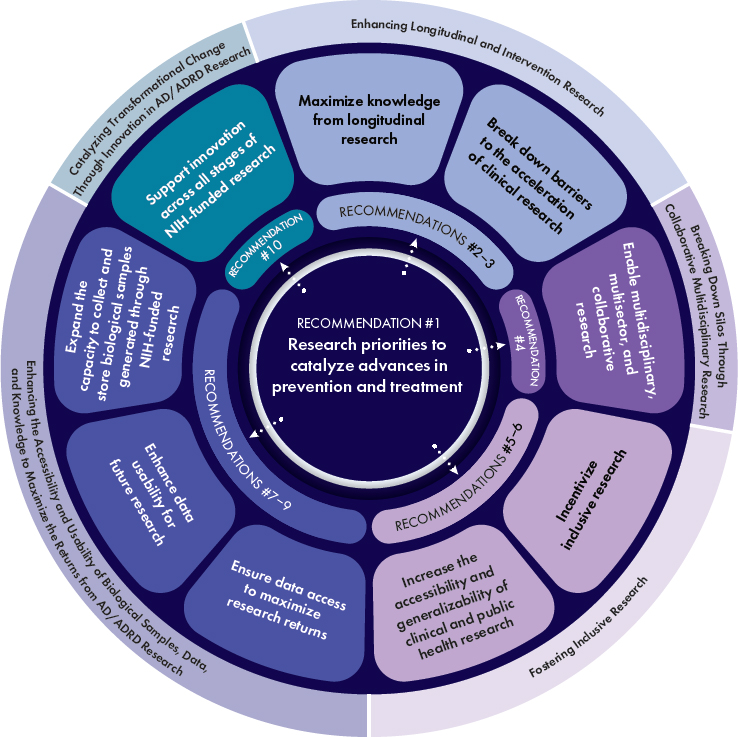
complement those efforts, highlighting opportunities to accelerate the translation of discoveries emerging from the vast and growing body of knowledge on AD/ADRD into effective strategies for prevention and treatment. The research priorities presented in this report represent the committee’s consensus views on the areas of scientific inquiry with the greatest promise to catalyze significant advances and maximize return on investment in the form of improved population health. These research priorities are summarized in Recommendation 1 and detailed in Table S-1. The committee’s Recommendations 2–10 are aimed at overcoming key, crosscutting barriers to progress on those research priorities (see Figure S-1). Although the report is focused on opportunities to advance the science, the ultimate objective is to ensure that research investments translate to societal benefit by improving the lives of those already living with cognitive and other forms of impairment from AD/ADRD and preventing many more from developing these conditions. Addressing both the research priorities and the recommendations will require sustained and dedicated resources and need to be guided at all stages by those with lived experience to ensure synergy between scientific priorities and the priorities of those directly affected by dementia.
STATE OF THE SCIENCE ON PREVENTING AND TREATING AD/ADRD
At present there are no interventions that prevent or cure AD/ADRD, and there is an urgent need for treatments that substantially improve the lives of people living with dementia and those of their families, care partners, and caregivers—a difficult reality that shaped much of the committee’s deliberations. However, the past few decades have brought significant advances in the understanding of AD/ADRD and in the development of tools and methods that can drive further progress. Noteworthy milestones include the ability to detect specific AD-related pathologies (amyloid and tau) years before symptoms emerge, the discovery of many new genes linked to AD/ADRD that shed light on pathogenic mechanisms, and the recognition that pathologies previously thought to distinguish different forms of dementia often co-occur. In addition, the recent approval of monoclonal anti-amyloid antibodies for early symptomatic stages of AD has given rise to optimism that AD and related neurodegenerative disorders can be treated to slow or halt disease progression and perhaps even prevented. Many researchers in the AD/ADRD field are encouraged by this momentum, hopeful that decades of inquiry and increased investment in research will soon lead to further breakthroughs in preventing and more effectively treating AD/ADRD, ultimately improving the lives of those affected by these disorders.
NOTE: AD/ADRD = Alzheimer’s disease and related dementias.
Yet, the contrast between this potential future and the current state is stark, and progress has not been even across the different types of dementia. Cutting-edge tools and technologies such as fluid and digital biomarkers, multiomics methods, and artificial intelligence are poised to radically change the research and clinical practice landscape. However, some current tools for cognitive and other clinical assessments are not able to detect early, more subtle changes in cognition and are, in some cases, biased. Furthermore, while digital assessments show promise, most still lack validation as reliable biomarkers. Advances enabling early detection and subtyping of AD have yet to be realized for related dementias. Although
the approval of anti-amyloid therapies for early symptomatic AD patients has generated optimism regarding the ability to slow cognitive decline, the clinical benefit of these therapies remains modest and has raised questions for many within the research community regarding their scope of application, long-term consequences, and the effect of their use in people living with mixed dementia. Further, people living with forms of dementia other than AD continue to have no treatments available that are approved by the U.S. Food and Drug Administration (FDA) beyond those for managing symptoms, crushing news for someone newly diagnosed with one of these forms today. Numerous studies on nonpharmacological strategies for preventing and mitigating AD/ADRD have also failed to provide definitive guidance on which approaches work and for whom, creating uncertainty for the public about how to protect cognitive health as they age. Despite considerable media coverage of health behaviors such as diet and exercise, definitive recommendations remain elusive. Consequently, the substantial scientific advances in AD/ADRD research have not translated into a widespread perception of progress among the public and policy makers.
The sense of stalled progress arises, in part, from a failure to effectively communicate the iterative and nonlinear nature of scientific advancement. Important achievements, such as declines in dementia prevalence and incidence rates, are not effectively communicated to audiences outside of the scientific community. The perception of stalled progress also underscores an urgent need—driven by compassion for those affected by these devastating disorders—for more rapid development of interventions to prevent or cure AD/ADRD, as well as treatments that substantially enhance the lives of those affected. Even if the accomplishments achieved to date were better recognized, the reality remains that there is a lack interventions to prevent or cure AD/ADRD and an urgent need for treatments that significantly improve the lives of individuals living with dementia and those close to them. The impact of dementia remains profound, despite the strides that have been made.
Based on its review of the research landscape, the committee identified the following scientific gaps that represent key bottlenecks that significantly impede progress toward preventing and treating AD/ADRD:
- There is a lack of rigorous evidence to support the identification of effective public health strategies for preventing AD/ADRD. Epidemiological research has yielded a large set of dementia risk factors (e.g., health behaviors, social isolation, socioeconomic disadvantage) based on statistical correlations. However, gaps in longitudinal data, data infrastructure, and study designs have resulted in limited understanding of causal relationships and the identification of exposures and system-level factors that, if mitigated, would have the greatest
- effect on the incidence of dementia. This impedes the development of effective public health approaches that could be implemented at a population scale to promote brain health across the life course and prevent AD/ADRD in diverse and especially disproportionately affected populations.
- There is an incomplete understanding of the biological basis and multiple etiologies underlying cognitive decline and dementia, as well as the mechanisms of resilience. There is still much that is not understood about AD/ADRD, conditions that may be characterized by decades of life-course health insults to brain and peripheral systems contributing to the development and detectable progression of neuropathology in the absence of clinical symptoms. Key knowledge gaps include how life-course exposures and other risk factors relate to pathobiology in diverse populations and the connections between molecular pathways that contribute to AD/ADRD and resilience (the maintenance of cognitive function despite the presence of brain pathology). This lack of understanding impedes drug discovery and the development of effective preventive and therapeutic intervention strategies. It also makes it difficult to predict the effect of risk factor reduction and drugs focused on single pathology targets on dementia incidence.
- There is a lack of effective, validated, and accessible tools and methods (e.g., novel biomarker tests, digital assessment technologies) for detecting early changes in brain health and accurately diagnosing, subtyping, and monitoring AD/ADRD in diverse populations. This foundational capability is essential to trace how the natural history of AD/ADRD differs from “healthy” brain aging. Progress made in the development of tools for AD needs to be expanded beyond amyloid and tau and extended to the multiple etiologies leading to dementia. The current capability gap impedes efforts to measure disease incidence and prevalence, to intervene early when chances of preventing and mitigating disease are greatest, to detect when treatments modify the trajectory of AD/ADRD, and to target specific interventions to the right populations (precision medicine).
Within each of these major scientific gaps of knowledge, there is also promising research that suggests opportunities to break current bottlenecks as new discoveries emerge. The discovery of imaging and fluid biomarkers for AD has catalyzed a shift in phenotyping procedures used in research and needs to be tested now in clinical practice. With the identification of risk genes/loci and the discovery of additional biomarkers, particularly for related dementias, current barriers to early detection, diagnosis, prognosis (e.g., the likelihood of progression to clinical dementia), and longitudinal
monitoring may be overcome, and it will be possible to quantify and better understand multiple etiology dementia. The combination of digital tools and computational methods such as artificial intelligence/machine learning that may be able to identify changes in traits (e.g., speech, gait, sleep behavior) that may precede current measures of cognitive decline similarly shows promise for enabling early detection of changes in brain health, early diagnosis, and prognosis. Digital tools have also opened new opportunities for passive and remote data collection and are changing the way investigators engage with study participants, particularly those from underresourced and underrepresented populations, and the public.
Investments in basic science and longitudinal cohort studies have led to a significant expansion of the therapeutic pipeline with novel promising interventions that are not specific to any single dementia type by uncovering shared molecular pathways contributing to AD/ADRD (e.g., autophagic and lysosomal, immune, metabolic, myelination), as well as resilience factors. Multiomics methods are creating new opportunities to (1) evaluate disease mechanisms in diverse populations and (2) to identify molecular disease subtypes and endophenotypes, thereby creating the foundation for precision medicine approaches to prevention and treatment in the future.3 Increased understanding of the links between AD/ADRD and chronic diseases such as hypertension and diabetes, along with encouraging evidence for multicomponent interventions focused on health behaviors, has highlighted the potential for public health strategies to reduce dementia risk.
SCIENTIFIC PRIORITIES FOR ADVANCING THE PREVENTION AND TREATMENT OF AD/ADRD
Building on the aforementioned examples of momentum and lines of promising research, the committee identified 11 research priorities and associated near- and medium-term scientific questions that it believes should be a focus of NIH-funded AD/ADRD biomedical research for the next 3 to 10 years. These research priorities, which are summarized in Recommendation 1 and detailed in Table S-1, fall into three broad areas:
- Quantify brain health across the life course and accurately predict risk of, screen for, diagnose, and monitor AD/ADRD.
- Build a more comprehensive and integrated understanding of the disease biology and mechanistic pathways that contribute to AD/ADRD development and resilience over the life course.
___________________
3 Multiomics methods involve the integrative analysis of multiple “-omics” datasets, such as those generated from genomic, proteomic, transcriptomic, epigenomic, and metabolomic methods.
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
| Research priorities to quantify brain health across the life course and accurately predict risk of, screen for, diagnose, and monitor AD/ADRD | ||
| 2-1: Develop better tools, including novel biomarker tests and digital assessment technologies, to monitor brain health across the life course and screen, predict, and diagnose AD/ADRD at scale. |
|
|
| 2-2: Implement advances in clinical research methods and tools to generate data from real-world clinical practice settings that can inform future research. |
|
|
|
||
| Research priorities to build a more comprehensive and integrated understanding of the disease biology and mechanistic pathways that contribute to AD/ADRD development and resilience over the life course | ||
| 3-1: Identify factors driving AD/ADRD risk in diverse populations, particularly understudied and disproportionately affected groups, to better understand disease heterogeneity—including molecular subtypes and disparities in environmental exposures—and to identify prevention opportunities and advance health research equity. |
|
|
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
| 3-2: Characterize the exposome and gene–environment interactions across the life course to gain insights into biological mechanisms and identify opportunities to reduce AD/ADRD risk and increase resilience. |
|
|
| 3-3: Elucidate the genetic and other biological mechanisms underlying resilience and resistance to identify novel targets and effective strategies for AD/ADRD prevention and treatment. |
|
|
| 3-4: Develop integrated molecular and cellular causal models to guide the identification of common mechanisms underlying AD/ADRD and their validation as novel targets for prevention and treatment. |
|
|
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
|
|
|
| Research priorities to catalyze advances in interventions for the prevention and treatment of AD/ADRD spanning from precision medicine to public health strategies | ||
| 4-1: Integrate innovative approaches and novel tools into the planning, design, and execution of studies to accelerate the identification of effective interventions. |
|
|
|
|
|
| 4-2: Advance the development and evaluation of combination therapies (including pharmacological and nonpharmacological approaches) to better address the multifactorial nature of AD/ADRD. |
|
|
| 4-3: Evaluate precision medicine approaches for the prevention and treatment of AD/ADRD to better identify interventions likely to benefit specific groups of individuals. |
|
|
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
|
|
|
| 4-4: Advance the adoption of standardized outcomes for assessing interventions that are sensitive, person-centered, clinically meaningful, and reflect the priorities of those at risk for or living with AD/ADRD. |
|
|
| 4-5: Evaluate the causal effects of public health approaches on overall dementia incidence and incidence in understudied and/or disproportionately affected populations. |
|
|
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
|
NOTE: The numbering of research priorities in this table reflects the numbering in the report chapters.
- Catalyze advances in interventions for the prevention and treatment of AD/ADRD spanning from precision medicine to public health strategies.
Importantly, the committee recommended research priorities that are not focused on gaps specific to individual dementia types. In an effort to move away from siloed thinking and toward more integrative research, the committee sought to emphasize opportunities that would be applicable across the spectrum of AD/ADRD and respond to the high prevalence of mixed pathologies in older individuals, as well as advance the committee’s broader goal of optimizing brain health and cognitive function across the life course. It remains essential, however, to continue to advance research focused on specific pathologies in parallel (e.g., discovery of biomarkers and development of outcome measures for specific pathologies) to improve the detection, diagnosis, and subtyping of AD/ADRD (see Chapter 2) and advance precision medicine approaches (see Chapter 4).
Intervention strategies to optimize brain health and cognitive function across the life course include population-scale public health approaches aimed at preventing dementia through risk factor reduction and increasing resilience. Although the mechanisms are not well understood, the decline in age-specific clinical dementia incidence in high-income countries such as the United States suggests such approaches have already yielded success. Improved understanding of the contributors to the observed decrease in clinical dementia incidence may open opportunities to accelerate prevention efforts and to better leverage public health investments not specifically targeted to AD/ADRD (e.g., initiatives aimed at improving cardiovascular health). Such efforts can be pursued now even in the absence of a complete understanding of the underlying biological mechanisms that mediate the effects of public health strategies.
Another approach to maximize the effect of research investments across multiple dementia types is to target interventions to common (shared) mechanisms for pathogenesis (e.g., neuroinflammation, lysosomal failure, vascular disease, dysmyelination, mitochondrial and metabolic dysfunction) and resilience. Such strategies will rely on an improved understanding of the multiple and likely intersecting molecular pathways that contribute to AD/ADRD. While NIH has been critiqued for an overemphasis in past decades on AD and amyloid-related research, the recognition in recent years of the multifactorial nature of AD/ADRD and the predominance of multiple pathologies has led to a greater appreciation of the need for efforts beyond amyloid and more focus on a diversity of pathways and their integration. By enabling the identification of initial molecular and environmental triggers, downstream pathophysiology, and biological mechanisms that promote resilience and healthy aging, an
integrated understanding of disease can guide combination approaches to AD/ADRD prevention and treatment that target multiple underlying pathways. Such an approach has the potential to achieve greater effect sizes and more meaningful patient outcomes.
Recommendation 1: Research priorities to catalyze advances in prevention and treatment
The National Institutes of Health (NIH) should focus on the research priorities and associated near- and medium-term scientific questions detailed in Table S-1 to advance a person-centered, multidisciplinary, and integrative research approach that will catalyze advances in the prevention and treatment of Alzheimer’s disease and related dementias (AD/ADRD). These research priorities cover the following areas:
- Develop better tools, including novel biomarker tests and digital assessment technologies, to monitor brain health across the life course and screen, predict, and diagnose AD/ADRD at scale (Research Priority 2-1)
- Implement advances in clinical research methods and tools to generate data from real-world clinical practice settings that can inform future research (Research Priority 2-2)
- Identify factors driving AD/ADRD risk in diverse populations, particularly understudied and disproportionately affected groups, to better understand disease heterogeneity—including molecular subtypes and disparities in environmental exposures—and to identify prevention opportunities and advance health research equity (Research Priority 3-1)
- Characterize the exposome and gene–environment interactions across the life course to gain insights into biological mechanisms and identify opportunities to reduce AD/ADRD risk and increase resilience (Research Priority 3-2)
- Elucidate the genetic and other biological mechanisms underlying resilience and resistance to identify novel targets and effective strategies for AD/ADRD prevention and treatment (Research Priority 3-3)
- Develop integrated molecular and cellular causal models to guide the identification of common mechanisms underlying AD/ADRD and their validation as novel targets for prevention and treatment (Research Priority 3-4)
- Integrate innovative approaches and novel tools into the planning, design, and execution of studies to accelerate the identification of effective interventions (Research Priority 4-1)
- Advance the development and evaluation of combination therapies (including pharmacological and nonpharmacological
- approaches) to better address the multifactorial nature of AD/ADRD (Research Priority 4-2)
- Evaluate precision medicine approaches for the prevention and treatment of AD/ADRD to better identify interventions likely to benefit specific groups of individuals (Research Priority 4-3)
- Advance the adoption of standardized outcomes for assessing interventions that are sensitive, person-centered, clinically meaningful, and reflect the priorities of those at risk for or living with AD/ADRD (Research Priority 4-4)
- Evaluate the causal effects of public health approaches on overall dementia incidence and incidence in understudied and/or disproportionately affected populations. (Research Priority 4-5)
The committee acknowledges that NIH has already made investments in each of these priority research areas to varying degrees. Given the breadth of the NIH AD/ADRD research portfolio, it is unsurprising that the committee did not identify any research priorities for which there had been no prior NIH investment. In some cases, research priorities identified by the committee, such as the development of biomarkers for monitoring brain health and the identification of factors driving risk in diverse populations, are already the focus of major NIH-funded research programs and initiatives, many of which are described in this report. Other identified research priorities, such as the characterization of the exposome and gene–environment interactions, the development of integrated molecular and cellular causal models, and the development of digital tools represent scientific areas of more recent or limited NIH investment. Relatedly, efforts to achieve associated near-term research opportunities, which are detailed in the right-hand column of Table S-1, may indeed be underway but have not yet been fully realized. Significant investment in the totality of the priority research areas is needed to address the knowledge gaps laid out in this report. Critically, beyond financial investment, success in tackling each of these research priorities will require an emphasis on the intentional expansion of research efforts beyond AD and the inclusion of diverse and understudied and/or disproportionately affected populations.
Importantly, Table S-1 is not intended as a prescribed research agenda. Nor is the identification of these priority areas meant to imply that lines of scientific inquiry outside of these areas are not of value or that work in all other areas should be suspended. There is a great deal of uncertainty in the process for scientific investigation regarding which discoveries from current research will lead to transformational advances in the future, and no guarantees can be offered regarding the ultimate fruitfulness of any specific line of inquiry. The committee’s intention, however, is that the priorities will be used as a guide in the rebalancing of NIH funding for AD/ADRD.
These are near- and medium-term priorities in the sense that investment and expansion in these priority areas should occur in the near and medium term. The timeline for realization of scientific advancements from these investments is inherently harder to predict. Closing the scientific knowledge gaps raised by these priorities can occur by working to answer the committee’s proposed scientific questions and acting on opportunities to overcome barriers to progress, as detailed in the recommendations that follow.
ENHANCING LONGITUDINAL AND INTERVENTION RESEARCH
Studies that follow individuals longitudinally and test interventions across time are needed to address the research priorities and associated scientific questions identified in Table S-1. Longitudinal cohort studies represent an important mechanism for identifying data that provide a comprehensive view of brain health and AD/ADRD development over the life course (including risk and resilience factors). Knowledge gained from such studies can be translated into protocols and sensitive tools (e.g., digital health technologies, biomarker assays) that can be deployed in research and practice for ongoing clinical monitoring and AD/ADRD prediction, detection, prognostication, and diagnosis. NIH has made significant investments to expand and leverage longitudinal research related to aging, resilience, and AD/ADRD.
Many datasets relevant to brain health and AD/ADRD have already been generated through longitudinal research focused on other health conditions, such as the Bogalusa Heart Study, which focuses on cardiovascular disease. A concerted effort to integrate those data with AD/ADRD outcomes could yield important insights into prevention and treatment strategies now while newer cohort studies remain ongoing. Progress to this end has been made through supplemental funding provided by NIH and other NIH funding mechanisms to incorporate an AD/ADRD focus into some existing cohorts, but the expansion of such efforts to other cohorts could help to fill current data gaps (e.g., data for younger age ranges and populations underrepresented in AD/ADRD research) and expand the set of measures that can be linked to brain health trajectories. Additionally, there remain opportunities to improve cohort representativeness, better capture data across the life course, bank samples for future analysis, and enable multicohort analyses through data harmonization.
Recommendation 2: Maximize knowledge from longitudinal research
To maximize knowledge from longitudinal research and enable future discoveries, the National Institutes of Health should prioritize investments in longitudinal research to address existing knowledge gaps regarding factors that influence brain health over the life course. These efforts should include the following:
- Invest in data infrastructure (see Recommendation 7), data harmonization (see Recommendation 8), and the cultivation of specialized expertise to enable the collection of data and conduct of analyses within and across existing cohorts, including those cohorts developed to characterize brain health and those created for examining other health outcomes.
- Create new, multidimensionally diverse (e.g., multilanguage, ethnoracial, geographic, socioeconomic) cohorts.
- Strategically add data points important to assessing brain health into existing cohorts constituted for research on other health conditions.
- Routinely collect early- and midlife exposure data (e.g., residential and work history, environmental toxicants, nutrition, education) from cohort study participants.
- Ensure that the data generated from shared biological samples are stored, searchable, and sharable.
Many entities (government, private, philanthropic, and academic) contribute to research for advancing interventions for AD/ADRD with complementary resources and expertise. While private industry is active in the development and evaluation of pharmacological agents and is often responsible for bringing therapeutics to market, other kinds of interventions and certain trial designs may be less appealing to industry owing to financial risk or the lack of financial incentives. This may be the case, for example, with many nonpharmacological interventions, repurposed drugs, and combination interventions, which are difficult to monetize. NIH plays a critical role in this complex research ecosystem by funding research on such interventions, incentivizing industry participation in collaborative efforts designed to develop and bring new and combination interventions to scale (see Recommendation 10), and supporting essential basic and translational research (e.g., target identification and validation) that feeds into the private-sector drug development pipeline. Increased collaboration and seamless transition between academia and industry throughout the research continuum could reduce the time to develop effective interventions. Likewise, fostering and incentivizing rapid and transparent data sharing from industry to nonindustry researchers would accelerate solutions.
NIH infrastructure investments for clinical research, such as the Alzheimer’s Clinical Trials Consortium, the Dominantly Inherited Alzheimer Network Trials Unit, and the Alzheimer’s Prevention Initiative, have facilitated increased collaboration with industry, philanthropy, and other partners (e.g., by using public–private partnerships) and innovation in AD/ADRD clinical trials (e.g., decentralization of trials using hub-and-spoke models, piloting platform trials, virtual engagement of participants, and
digital data collection). However, to accelerate the pace of discovery, these efforts to increase collaboration need to be expanded to a much greater scale as NIH continues to support clinical research to evaluate AD/ADRD interventions in the coming years.
As drug discovery and target validation efforts are scaled, phase 1b and phase 2 clinical trials in particular need to be expanded. Increasing the quantity and quality of small phase 1b and phase 2 proof-of-concept trials with a focus on mechanisms, informative biomarkers (e.g., target engagement, biomarkers for copathologies), and outcomes (e.g., pharmacokinetics and pharmacodynamics, surrogate outcomes) is needed to smooth the transition to and better guide decision making for larger, later-stage trials.
In anticipation of the increased demand for clinical trial investigators, attention is needed to address current gaps in the workforce (e.g., investigators with specialized expertise in pharmacology trials). Ensuring investigators new to conducting trials utilize existing training programs with best practices can help to improve the rigor of earlier-stage trials. NIH-funded clinical trial consortia, if adequately supported, could provide training for clinical trial sites to disseminate knowledge, standards, and best practices.
Recommendation 3: Break down barriers to the acceleration of clinical research
The National Institutes of Health (NIH) should continue to lead efforts across a multiplicity of relevant entities (e.g., pharmaceutical and biotechnology companies, academia, foundations) to accelerate the movement of promising interventions for Alzheimer’s disease and related dementias (AD/ADRD) into clinical trials and to expand the use of innovative approaches to improve the efficiency of clinical trials. These efforts should include the following:
- Organize NIH investments in basic and translational research related to potential molecular targets for intervention into a portfolio to create a pipeline of validated targets that can be transitioned into drug development.
- Expand the use of innovative trial designs (e.g., master protocols, platform, combination, adaptive trials) and increase investment in both early-phase (phase 1b and 2) proof-of-concept trials and later-stage pragmatic trials.
- Identify and promulgate best practices for decreasing the barriers to, and time for, the clinical trial startup phase (e.g., decentralized participant screening, creation and use of prescreened cohorts and screen-enroll mechanisms, use of electronic consenting procedures, centralized contracting, and institutional review board processes).
- Continue investing in innovative funding models, such as public–private partnerships, shared funding for global trials, and combined-phase funding, that support the progression of candidate interventions across the early-stage clinical research pipeline.
- Maximize coordination between NIH-funded AD/ADRD clinical trial programs and NIH-funded AD/ADRD centers (e.g., Alzheimer’s Disease Research Centers) and evaluate these centers for representative participant clinical trial enrollment.
STRATEGIES FOR ADDRESSING CROSSCUTTING BARRIERS THAT IMPEDE PROGRESS
The committee was asked to identify key barriers to advancing AD/ADRD prevention and treatment and to highlight opportunities to address these barriers to catalyze advances across the field. In its examination of the AD/ADRD research landscape, several impediments to progress were consistently identified across the continuum from basic to clinical research. These crosscutting barriers include
- siloing within the AD/ADRD field and across related domains of research (e.g., aging, neurodegenerative diseases more broadly, exposure science);
- insufficient population representativeness and generalizability of research;
- inadequate infrastructure and support for management and analysis of data, samples, and knowledge generated from AD/ADRD research; and
- inadequate support for innovative methods capable of realizing transformational progress.
The committee recognizes the significant NIH investment to address each of these key barriers. It should also be acknowledged that many barriers are not unique to dementia research, and other scientific fields are also working to overcome similar challenges. Accordingly, in considering the implementation of the recommendations below, NIH and other research funders should continuously monitor the broader research landscape for examples of how such challenges have been successfully tackled in other fields and consider opportunities to apply those strategies in AD/ADRD research.
Breaking Down Silos Through Collaborative, Multidisciplinary Research
The heterogeneity of AD/ADRD, the prevalence of mixed pathologies, and the multifactorial and intersecting nature of the diverse pathways that
lead to disease all suggest that the path to effective strategies for preventing and treating this group of neurodegenerative diseases lies in collaborative, multidisciplinary research. Yet, throughout its information-gathering process the committee encountered numerous silos, commonly reinforced by funding structures. Current funding strategies that target individual diseases, which have historically favored AD, fail to address the reality of overlapping and mixed pathologies that contribute to neurodegenerative disease, and they have contributed to the current dearth of effective therapies for related dementias. Research on pharmacological and nonpharmacological interventions are not well integrated, and as a result there have been few efforts to date to evaluate the effect of combination approaches despite a high likelihood that risk reduction and drug therapies will both be necessary elements of a strategy to reduce the incidence and effect of dementia. Moreover, the efforts of federal agencies supporting related areas of research are not adequately coordinated, resulting in missed opportunities to collaborate and effectively use existing investments in studies and infrastructure.
Innovative funding strategies and other incentives that encourage collaboration will be needed to address the current siloing of research and accelerate the development of interventions for preventing and treating AD/ADRD. Examples that have shown promise in AD/ADRD that could be expanded include
- multi-institute research consortia that facilitate harmonization, coordination, and data sharing;
- public–private partnerships that leverage the respective talents of investigators in academia and industry;
- challenge programs that encourage team science approaches and risk taking while bringing new talent into the field; and
- community-based participatory research approaches that include and engage research participants, people with lived experience, and the public.
Coordination and collaboration at the program and project levels are facilitated and may be incentivized by analogous efforts at the federal level. Recognizing the existing mechanisms already in place and the challenges of establishing new interagency bodies (e.g., time for agency personnel), the committee encourages NIA, NINDS, the National Institute of Mental Health, and other NIH funders of AD/ADRD research to identify further opportunities to maximally leverage the strengths, resources, and unique capacities of other agencies to advance shared focus areas. Examples of collaborations with other federal agencies might include collaborating with the Census Bureau to expand access to federal statistical research data
centers (FSRDCs) and facilitate the linkage of multiple data types relevant to AD/ADRD within the FSRDCs; working with the Centers for Medicare & Medicaid Services or FDA to tie expedited review processes for industry to data sharing policies; and working with the Centers for Disease Control and Prevention to generate a more robust evidence base for public health-level interventions. Building on the AMP model, collaboration with the Foundation for NIH can facilitate academic–industry research partnerships without creating financial conflicts of interest for academic researchers.
Recommendation 4: Enable multidisciplinary, multisector, and collaborative research
The National Institutes of Health (NIH) should expand mechanisms and leverage existing resources to break down silos and encourage multidisciplinary and integrative Alzheimer’s disease and related dementias (AD/ADRD) research efforts, including the following:
- Expand trans-NIH initiatives and cofunded projects focused on healthy aging and neurodegenerative diseases to reduce the siloing of research efforts by individual institutes and centers, better cross-link and use existing resources, and address inconsistencies in data sharing policies across NIH institutes and centers while prioritizing data access.
- Prioritize research funding for projects with multidisciplinary research teams (e.g., basic and clinical researchers, population scientists, data scientists and artificial intelligence specialists, and those with lived experience) that address community-informed research questions.
- Expand collaborations globally, including but not limited to low- and middle-income countries and other countries less often involved in such collaborations, for both longitudinal research and clinical trials to better understand the biology of AD/ADRD and enhance the generalizability of findings to diverse populations.
- The National Institute on Aging and the National Institute of Neurological Disorders and Stroke should collaborate with the National Center for Advancing Translational Sciences and others to speed up the translation of research advances to clinical and public health practice and, in turn, expand new research inquiries through the collection of real-world evidence.
Fostering Inclusive Research
A comprehensive understanding of disease heterogeneity and the role of population differences (e.g., in genetic/genomic risk and social factors such as poverty, stress, and education) is crucial to developing broadly
effective preventive and therapeutic intervention strategies for AD/ADRD. However, the populations that are disproportionately affected by dementia (e.g., certain ethnic/racial groups, people with low socioeconomic status or educational attainment) are persistently underrepresented in AD/ADRD research, both in observational studies and clinical trials. The result is limited generalizability of clinical research findings—including intervention safety and efficacy data—to the broader target population, impaired trust in the research enterprise, reduced understanding of disease biology (e.g., risk factors and causal mechanisms), clinical trial failures at later stages, and the compounding of existing health disparities.
Increasing the participation of underrepresented populations in dementia research has been a focus of past recommendations to NIH, and it is clear that NIA, NINDS, and other funders of dementia research are committed to and actively working on closing this gap. These efforts have included identifying best practices for engaging with and retaining diverse and underrepresented populations and connecting researchers to resources that can support more inclusive research. While there is some evidence to suggest that the efforts of NIH and those of the broader scientific community are starting to move the needle with regards to representation in AD/ADRD research, progress has been slow. Some measures of diversity in AD/ADRD-related studies are improving as compared to past decades, but this may not be consistent across all types of research or populations. It is imperative that NIH and AD/ADRD researchers continue to prioritize and incentivize inclusive research and increase accessibility for populations that are historically underrepresented despite being disproportionately affected by dementia.
Effective engagement with communities requires understanding and sensitivity to the different perspectives and cultures represented therein and this cannot be achieved without diverse and multidisciplinary research teams. Acknowledging the work NIH is already doing to foster a diverse and well-trained research workforce, continued efforts are needed to overcome barriers to entry (e.g., inadequate compensation for trainee and postdoctoral researchers on NIH awards) and ongoing career advancement. Such efforts not only help to address challenges related to underrepresentation in research but ensures the development of a skilled research workforce that benefits from the nation’s rich diversity of people and their broad range of perspectives and experiences.
Given the multiple, interrelated factors that are associated with chronic underrepresentation of certain populations, achieving greater inclusivity and accessibility in AD/ADRD research will require a multipronged approach. This should include (1) ensuring adequate resources are budgeted for community engagement, recruitment, and the development of culturally appropriate research tools; (2) consideration of ways to overcome or work around common factors that contribute to attrition at the screening stage
(exclusion criteria), particularly for members of underrepresented groups; (3) regular analysis of recruitment, enrollment, and retainment outcomes; and (4) building a diverse research workforce at all levels. Accountability—for NIH and NIH-funded investigators—will be a key determinant of future success in these endeavors.
Recommendation 5: Incentivize inclusive research
The National Institutes of Health should incentivize and guide the use of inclusive research practices to increase the accessibility of clinical and public health research and ensure that study populations are representative of populations at risk for and living with Alzheimer’s disease and related dementias (AD/ADRD). These efforts should include the following:
- Strengthen requirements for the recruitment of diverse populations as a condition for initiating data collection (e.g., use of sampling frames as a best practice for targeted and intentional outreach).
- Support research to further understand participant and institutional barriers to involvement in clinical research at all levels.
- Develop social determinants of health metrics to be used as measures of diversity.
- Incentivize the incorporation of standardized benchmark measurements that can be used to evaluate and correct selection bias into new and ongoing research studies.
- Work with the Centers for Medicare & Medicaid Services to explore Medicare and Medicaid enrollment as opportunities for data collection and for enrollees to receive information about participation in AD/ADRD research studies using an opt-in model.
- Support initiatives to identify and overcome barriers to entry and continued professional advancement for a diverse clinical research workforce.
Recommendation 6: Increase the accessibility and generalizability of clinical and public health research
Investigators supported by the National Institutes of Health (NIH) should adopt inclusive research practices to increase the accessibility of clinical and public health research and ensure that study populations are representative of populations at risk for and living with Alzheimer’s disease and related dementias (AD/ADRD). To increase research accessibility and generalizability, NIH-supported investigators should do the following:
- Reduce barriers to research participation (e.g., directing ineligible research volunteers to other studies, offering fair compensation,
- expanding opportunities for virtual participation and passive and/or remote data collection, using in-home testing kits).
- Eliminate unnecessarily restrictive exclusion criteria that screen out diversity in the study population.
- Invest in the development of long-term, mutually beneficial relationships between research institutions and communities, and embed trials sites in communities with underrepresented populations (decentralized trials).
- Meaningfully engage and incorporate the perspectives of research participants and their communities throughout the research design and execution process (e.g., through patient or community advisory councils, codesigning research, community-based participatory research methods, use of community members such as promotoras or health navigators to collect data).
Enhancing the Accessibility and Usability of Biological Samples, Data, and Knowledge to Maximize the Returns from AD/ADRD Research
The billions of dollars in funding from NIH and others that has supported scientific investigations in the dementia field and the development of a robust AD/ADRD research infrastructure represents a significant national investment. Careful stewardship of that investment requires ensuring that the products of research—including biological samples, raw data, and findings—are accessible to, and usable by, the broader scientific community for the purpose of knowledge generation. When data and samples are siloed and sequestered within individual research groups, the kind of collaborative and integrative research called for by the committee cannot be achieved. Importantly, an advantage of stored raw data collected through digital technologies (e.g., voice recordings, data from wearable devices and in-home sensors) over banked biosamples is that digital data are not a finite resource. If properly stored, these data can be used indefinitely and simultaneously by multiple users without losing value over time. Thus, the return on collection and storage can be exponential. Also critical is the compilation and synthesis of knowledge in such a manner that it can be easily accessed and used to draw insights to guide future research and inform clinical care.
AD/ADRD data infrastructure investments by NIH have included a number of different platforms and repositories to support storage and accessibility of diverse data types (e.g., fluid biomarker, neuroimaging, neuropathology, genomic and other omics data). A key challenge before NIH is to link its major data hubs (e.g., NIA Genetics of Alzheimer’s Disease Data Storage Site, National Alzheimer’s Coordinating Center,
AD Knowledge Portal) into an agile, integrated data ecosystem while preserving the autonomy of the individual platforms and their respective strengths and networks. This integrated system should enable researchers without deep data analytics expertise to locate, access, and query existing data from NIH-funded research and, when available, data submitted by other investigators. This is a formidable undertaking but critical to maximizing insights from AD/ADRD research and returns from NIH’s various investments.
Given the diversity of data types, data sources, and constraints such as privacy protection needs, there is no single solution to data management and accessibility. It will be important for NIH to work with investigators to identify solutions to data access challenges. While many datasets can and should be made publicly available, for others, such as clinical datasets with protected health information generated by private health systems, data accessibility may need to be achieved through other means.
Accessibility is necessary but not sufficient to ensure that data from past AD/ADRD research are usable to the fullest extent possible. Also critical is the expansion of data standardization and harmonization efforts to address the lack of interoperability and comparability of data from different studies, which impede data integration and cross-study analyses. Furthermore, increasingly complex tools for data integration and analysis are required to accommodate the growing volume and diversity of data being generated through AD/ADRD research. Artificial intelligence/machine learning and other computational methods (e.g., network analysis) hold great promise for enabling the linkage and subsequent extraction of insights from large and complex datasets, but there is a need for national-level resources that can support the development of such tools and analytic methods. The continued evolution of technology, tools, and analytic methods will create new opportunities to analyze data in ways that are unknown at present. Such future analyses may lead to the discovery and development of novel therapeutics or biomarkers.
Recommendation 7: Ensure data access to maximize research returns
Using the National Institutes of Health (NIH) Data Management and Sharing Policy as a foundation, NIH should convene and support an NIH workgroup to work with NIH-funded investigators to identify and implement solutions to barriers that impede access to data from Alzheimer’s disease and related dementias research. Specific issues that should be addressed by the NIH workgroup include but are not limited to the following:
- the need for a centralized and continuously updated NIH-managed system for locating and searching existing data sources across different (NIH and non-NIH) data platforms;
- provision of incentives and clear procedures for ensuring compliance with the Data Management and Sharing Policy;
- approaches to maximize access to data from initiatives funded by multiple NIH institutes and centers;
- incentivization of transparent reporting and the synthesis of findings from negative studies, including observational studies and clinical trials, ideally with accompanying data release;
- provision of project-specific supplemental funding, including additional administrative supplements, commensurate with the anticipated level of data and code sharing;
- formulation of guidance for subsets of data within any given dataset to be categorized into access levels based on the access controls needed to protect sensitive data (e.g., participant health information) such that the portion of data requiring no permission for use can be made publicly available;
- return of derived data and analysis code from data users, as well as the return of newly collected data from ancillary studies, to the parent study while respecting the need to protect intellectual property and innovation;
- approaches to facilitate access to data from international collaborations; and
- expansion of capacity for storing raw digital data (e.g., unstructured data such as images and high-velocity voice recordings and sensor data).
Recommendation 8: Enhance data usability for future research
To enable the usability of data generated by Alzheimer’s disease and related dementias research funded by the National Institutes of Health (NIH), NIH should do the following:
- Invest in data harmonization and interoperability efforts (e.g., use of common data elements) across data platforms and through collaborations across institutions and organizations, ensuring that levels of harmonization are aligned with the needs of different analytic approaches.
- Set requirements for user-intuitive data dictionaries.
- Explore new approaches, such as natural language processing, to automate the integration of different data types (e.g., clinical phenotype, multiomics data, exposure data).
- Fund the development and dissemination of novel open-source tools and analytic methods (e.g., large language models and other artificial intelligence/machine learning methods, statistical transport methods, data fusion approaches, synthetic data) to
- collect, link, explore, and query existing data and support efficient analyses when data privacy rules create barriers.
- Provide dedicated grants for investigators working in settings with proprietary data that are difficult to share (e.g., major clinical datasets) focused on data curation or supporting analyses by external researchers.
Consideration of the future value of biological samples collected from research participants or donated by other members of the public is also important when investing in infrastructure and plans for collection and storage. Furthermore, stored samples have little value if they are not accessible to the scientific community. Ensuring accessibility entails the development of transparent inventories of samples that are available to external investigators and clear processes for sample requests and decision making on sample sharing. NIH has made substantial investments in infrastructure for biobanking, but critical gaps remain. Initiatives and resources appear fragmented. Capacity to collect tissues through autopsy and to store biological samples—both cost-intensive undertakings—is limited. As a result, precious samples may be discarded at the completion of studies. Maximizing the use and value of participant samples will require a more structured and standardized system for collection, archiving, and access.
Recommendation 9: Expand the capacity to collect and store biological samples generated through National Institutes of Health (NIH)-funded research
The National Institute on Aging, along with the National Institute of Neurological Disorders and Stroke, should expand support for the collection and storage of valuable biological samples from NIH-funded Alzheimer’s disease and related dementias research in a manner that maximizes opportunities for future use. This should include the following:
- Provide supplements to researchers that meet the actual cost of storing and sharing samples following study completion.
- Expand support for the collection and storage of highly characterized biological samples (e.g., antemortem and postmortem blood and cerebrospinal fluid, donated brains) from participants of any longitudinal research studies and clinical trials, and from the public.
- Use standardized sample collection, assessment, and storage practices with careful consideration of the implications of different storage approaches for future value.
- Facilitate access to biological samples from international collaborations.
- Support digitized neuropathology to enable quantitative analysis using artificial intelligence and other computational methods.
Catalyzing Transformational Change Through Innovation in AD/ADRD Research
Accelerating progress in AD/ADRD prevention and treatment will require transformational change that can only be achieved through greater support for innovation in NIH-supported research. This may look different at different stages in the research continuum, as highlighted by the following examples:
- Basic research: developing and applying novel models and tools, seeking potential points of connection and commonalities with related fields (e.g., aging, other neurodegenerative diseases).
- Translational research: increasing the viability of innovative research targets and approaches.
- Clinical research: adopting innovative trial designs and participant recruitment and engagement mechanisms.
- Population research: identifying and integrating novel data sources that can be used to evaluate population-level strategies (e.g., policies or exposures that vary across larger geographic units) and effects on inequalities.
The current system for peer review at NIH favors investigators and projects for which there are strong track records and evidence for likely success based on existing preliminary data. Although the current process has many advantages, it is not ideally suited to promoting innovation and truly novel methods. Incentives are needed to promote more disruptive research approaches that may lead to significant steps forward. Agencies such as the Defense Advanced Research Projects Agency and the Advanced Research Projects Agency for Health are specifically focused on high-risk, high-reward research. Similarly, relatively new philanthropic funders have developed creative methods to identify and fund pilot-scale approaches to challenging scientific and medical problems. These groups may provide insight into how NIH can further enhance innovation, while working within its constraints. While an in-depth evaluation of funding structures and processes is beyond the scope of this report, the committee offers the following recommendation with suggestions for increasing innovation in AD/ADRD research.
Recommendation 10: Support innovation across all stages of National Institutes of Health (NIH)-funded research
NIH should use existing funding structures and other incentive mechanisms to stimulate innovation across all stages of Alzheimer’s disease and related dementias (AD/ADRD) research. This could include the following:
- Implement advances and tools generated by the Advanced Research Projects Agency for Health and others into NIH-funded AD/ADRD research, including advances that are specific to dementia and those that can be applied from other fields.
- Field a program-wide review of the opportunities and barriers to interdisciplinary and transformational research at NIH-funded AD/ADRD centers and infrastructure programs (e.g., the Alzheimer’s Disease Research Centers) and their capacity to prioritize the inclusion of diverse populations and foster innovative research with high potential for population impact.
- Capitalize on the best-in-class practices and technologies of other fields that are applicable to, and may address, current AD/ADRD research needs (e.g., data infrastructure and knowledge management, social engagement for recruitment).
- Prioritize support for research inquiries that have clear potential for future scalability and uptake.
- Build partnerships with foundations and other research funders to coordinate seamless funding pathways for fast-tracked phase 1–2 high-risk research opportunities.
- Identify and provide short-term funding for specific, highly innovative components of otherwise unsuccessful new and competing award applications.
- Identify past funded projects in the NIH portfolio that have progressed to real-world clinical implementation and adapt the grant review process to include criteria that promote real-world clinical implementation.
CONCLUDING REMARKS
The last decade of research has seen encouraging progress in the capability to detect early signals of changes in brain health, to understand the pathophysiologic mechanisms underlying AD/ADRD, and to develop and evaluate preventive and therapeutic interventions. Accelerating progress in AD/ADRD prevention and treatment will require transformation and new direction. With continued strategic research investments as outlined by the committee, there is good reason to hope that the coming years will see significant progress in the capability to prevent and treat AD/ADRD. Through the continued and collaborative efforts of NIH, academic researchers, private industry, health care professionals, funders, policy
makers, advocates, and people living with cognitive and other forms of impairment from AD/ADRD, it is possible to envision a future where dementia is not inevitable for millions of people across the globe but is preventable and treatable.



































