Preventing and Treating Dementia: Research Priorities to Accelerate Progress (2025)
Chapter: 4 Development and Evaluation of Interventions for the Prevention and Treatment of AD/ADRD
4
Development and Evaluation of Interventions for the Prevention and Treatment of AD/ADRD
The committee was charged with reviewing and synthesizing the most promising areas of research into preventing and treating Alzheimer’s disease and related dementias (AD/ADRD), including nonpharmacological interventions (NPIs) and pharmacological interventions, and combinations thereof. To address this aspect of its task, the committee relied on input from a variety of sources. These included information and perspectives shared during a public workshop and findings from a scoping review of existing systematic reviews of the evidence on pharmacological agents and NPIs, supplemented with targeted literature searches of interventions not captured in the recent systematic reviews examined by the committee.
The committee was encouraged by the diversity of preventive and therapeutic interventions that are being evaluated for effectiveness against AD/ADRD, representing a notable expansion of intervention targets that reflects the growing understanding of the complex and multifactorial pathways that contribute to AD/ADRD (see Chapter 3). This expansion in candidate interventions demonstrates the value of the investments in basic and translational science that have been made over the last decade. Still, there is much additional work to be accomplished. There remains considerable uncertainty about steps that can be taken to prevent and slow the progression of AD/ADRD and about the optimal timing and strategy for intervening to maintain brain health. As a result, the substantial scientific advances in AD/ADRD research in recent years have not translated into a widespread perception of progress among the public and policy makers. This arises in part from a failure to effectively communicate important achievements to audiences outside of the scientific community. Communication efforts
aimed at sharing progress on dementia prevention and treatment with the public need to be programmatically enabled and use formats accessible to the public (e.g., social media). The perceptions of stalled progress also underscores an urgent need for more rapid development of interventions to prevent or cure AD/ADRD, as well as treatments that substantially enhance the lives of people living with these diseases and those of their families, care partners, and caregivers.
This chapter presents the committee’s assessment of the evidence on interventions to prevent and treat AD/ADRD and opportunities to accelerate progress. The chapter begins with a discussion of a framework that lays out the multiple dimensions for consideration in the pursuit of prevention and treatment strategies. It then highlights interventions that have promise for preventing, delaying, slowing, halting, or reversing AD/ADRD. This is followed by a discussion of strategies for improving clinical trials to accelerate the development of interventions that are safe and effective for the vastly heterogeneous populations at risk for and living with AD/ADRD and that have the potential to reduce the societal impact of these diseases. The chapter ends with a discussion of opportunities to advance a precision medicine approach to ensure people are getting the right combination of interventions at the right time based on their specific characteristics and stage in both the life-course and the brain health continuum.
A FRAMEWORK FOR CONSIDERING PREVENTION AND TREATMENT STRATEGIES
In considering opportunities and potential strategies for preventing and treating AD/ADRD, multiple dimensions need to be considered, including
- type of intervention (pharmacological versus nonpharmacological)
- target of intervention,
- life-course timing,
- balance of benefits and harms,
- level of intervention (population versus individual levels), and
- potential public health effects.
Type of Intervention
Interventions for preventing or treating AD/ADRD may be pharmacological, nonpharmacological, or a combination of both. NPIs represent a diverse collection of intervention strategies (Li et al., 2023), many of which target modifiable risk factors for dementia (Livingston et al., 2024). Included within this broad category are interventions focused on behavior (e.g., diet, use of vitamins and other supplements, exercise, stress management,
art-based therapies, mind–body–spirit connection approaches), cognitive stimulation and training, preserved and/or improved hearing, education, and social interactions.
Quite different in form but also falling within the NPI category are neuromodulation procedures—both invasive and noninvasive—including deep brain stimulation, transcranial pulse stimulation, repetitive transcranial magnetic stimulation, and ultrasound (Leinenga et al., 2024). Each type of NPI can be implemented alone (single-component interventions), or multiple NPIs may be combined as part of a multimodal intervention. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), which evaluated a combination of diet, exercise, cognitive training, and socialization, is an example of a multimodal NPI (Ngandu et al., 2015). In some studies, the same NPI will be used for all participants, while personalized risk-reduction trials may tailor interventions to the specific risks and preferences of each participant (Yaffe et al., 2024).
Pharmacological agents being evaluated for AD/ADRD include both novel therapeutics and repurposed drugs (i.e., drugs originally approved for an indication other than AD or related dementias) (Thunell et al., 2021). Therapeutics can be in the form of small-molecule drugs or biologics, such as monoclonal antibody infusions and vaccines. The Translational Research and Clinical Interventions category of the Common Alzheimer’s Disease Research Ontology (CADRO) provides a standardized mechanism for categorizing targets for therapeutics.1 Of note, many of the targets are not specific to AD or a related dementia but rather represent more conserved pathways or resilience mechanisms. Like NPIs, pharmacological agents can be used alone or in combination (e.g., anti-amyloid therapy plus a therapeutic targeting a different mechanistic pathway).
Target of Intervention
While interventions commonly target aspects of disease, in the case of AD/ADRD there are also opportunities for interventions to increase an individual’s resilience, by building cognitive reserve or enhancing the brain’s ability to adapt to neuropathological changes and thereby enhance and/or preserve its function. Resilience-focused strategies have received
___________________
1 CADRO lists the following potential targets for drug discovery and development: amyloid beta; tau; apolipoprotein E (APOE), lipids, and lipoprotein receptors; neurotransmitter receptors; or several pathways including neurogenesis; inflammation; oxidative stress; cell death; proteostasis/proteinopathies; metabolism and bioenergetics; vasculature; growth factors and hormones; synaptic plasticity/neuroprotection; gut–brain axis; circadian rhythm; epigenetic regulators; multitarget; unknown target; and other. CADRO is available at https://iadrp.nia.nih.gov/about/cadro (accessed April 16, 2024).
less attention in clinical intervention research but have the benefit of being agnostic to pathology classification, an important consideration given the predominance of mixed forms of dementia. Interventions that (1) promote early-life cognitive development, (2) promote brain health through improved vascular health, (3) reduce brain insults including head injury, stroke, and pathology based on proteins aggregates, or (4) enhance plasticity and reorganization after injury or within the context of disease (i.e., rehabilitation) may all reduce dementia burden. Within each of these categories there are many different intervention approaches. For example, stroke prevention would entail distinct interventions from head injury prevention.
Life-Course Timing
The question of life-course timing as it relates to the prevention and treatment of AD/ADRD is important because although cognitive development is most marked in early life, it continues across the life course, and brain injuries may occur at any age (WHO, 2022). Our understanding of brain plasticity and adaptability in adults is still unfolding, with important progress in recent decades in areas such as stroke recovery. Major opportunities for prevention of AD/ADRD are likely to present from early childhood through old age, but the timing of interventions can significantly shape who will potentially benefit (e.g., current cohorts of older adults will not benefit from childhood interventions) and the time delay before a payoff in terms of dementia prevention.
Much of the emphasis on opportunities for prevention, such as those based on the population attributable fraction popularized in the Lancet Commission report (Livingston et al., 2020, 2024), frames the fraction of cases preventable as a static number. However, the potential for prevention that might be achieved by targeting any specific preventive strategy will vary over time. It should also be acknowledged that preventive strategies that make people healthier means they will live longer, and even as more is understood regarding healthy aging and cognitive decline, age remains one of the strongest risk factors for AD/ADRD.
Balance of Benefits and Harms
The balancing of benefits and harms is integral to considering intervention strategies. While benefits and harms are often thought of in terms of health effects, a broader scope can include other considerations such as financial risk and exposure to stigma. Some interventions are desirable for benefits outside of their potential effect on AD/ADRD (e.g., hypertension management strategies or smoking reduction), or at least are unlikely to
have adverse consequences beyond opportunity costs (e.g., brain games). Others have uncertain or, in such cases as monoclonal antibody-based immunotherapies, known potential for harm. The potential for harm is especially important when considering scaling an intervention to a large number of individuals. If a serious adverse event occurs in only 1 percent of individuals, treating 6 million individuals with Alzheimer’s disease (AD) will lead to 60,000 such adverse events. The potential for harm is even greater if a treatment with the same rate of adverse events was applied to the nearly 50 million people estimated to be living with preclinical disease (i.e., people with AD pathology but who remain asymptomatic) (Brookmeyer and Abdalla, 2018; NASEM, 2021). Measuring the various benefits and costs (e.g., earnings, hope, medical costs, pain, risk of death) associated with a treatment will support decision making about its use by people living with AD/ADRD and their families.
These harms and benefits can be understood as components of the meaningful value provided by an intervention. Quantifying the value provided by these multiple considerations allows for a more comprehensive understanding of the meaningful benefit that can be offered by an intervention at a societal level (Neumann et al., 2022). For example, a disease modifying therapy that delays cognitive and functional decline at early stages of disease would likely also support greater workforce productivity from both persons living with the disease and their intended caregivers, increased social enjoyment, and reduced time requiring long-term support and services and would provide hope. However, the same therapy may also impose high out-of-pocket costs for patients and families, impose high costs on payers, lead to suffering via treatment administration or side effects, and, if the disease modifying therapy leads to increased risk of treatment-related death, shorten lifespan. Such approaches as dynamic microsimulation modeling can be used to assess the effect of these complex variables to estimate the meaningful benefit of an intervention.
Level of Intervention
Interventions for preventing and treating AD/ADRD can be categorized as targeting an individual or an entire population. Individual-level interventions require individuals to pursue specific behaviors (e.g., engaging in cognitive training), lifestyle changes, or medical treatments, whereas population-level interventions change the context in which individuals live to create a healthier environment or to reduce known determinants of AD/ADRD risk. Such categorization of interventions mirrors the individual- and system- or structural-level components that have a cumulative effect on AD/ADRD outcomes over the life course (see Figure 1-5). Personalized approaches can be used to adapt intervention strategies to reflect variations
across individuals and help to overcome some of the barriers that might otherwise hinder adoption and adherence. While pharmacological agents are inherently individual-level interventions, many NPIs can be implemented at the population level, such as through public health initiatives and policy changes.
Population-level interventions ultimately work via changes at an individual level, but they can be implemented at scale. For example, tobacco taxes or indoor smoking bans are population-level interventions geared to ultimately affect individual behaviors, such as those achieved with smoking cessation counseling. Population-level interventions focus on contextual changes, such as public health campaigns to raise awareness of hypertension management for AD/ADRD prevention. Research on health equity has emphasized the greater potential for population-level interventions to narrow equity gaps because individuals who are systematically disadvantaged are less able to take advantage of individual-level programs or behavior modification interventions.
Public Health Impacts
All proposed interventions should be evaluated against their potential to reduce the incidence and prevalence of AD/ADRD, as well as inequalities. This entails considering the feasibility of delivering proposed interventions to all individuals at risk and any differential effects of an intervention across diverse population groups. For many interventions, there may be an interaction with an individual’s level of risk, in which case it may be necessary to consider whether those at higher risk are likely to get more, the same, or less benefit. Understanding this interaction effect is important for targeting interventions most effectively—and cost-effectively.
CURRENT STATE OF EVIDENCE ON INTERVENTIONS FOR PREVENTING AND TREATING AD/ADRD
The committee’s assessment of the state of evidence on interventions for preventing and treating AD/ADRD was informed, in part, by an analysis of recent systematic reviews that evaluated pharmacological, nonpharmacological, and combination interventions for AD/ADRD. The methods used in the scoping review and descriptive summaries of included systematic reviews can be found in Appendix A. While this approach was useful for developing an overarching view of the landscape of intervention research, it was also subject to biases and limitations. These include the potential for a compounding of biases from individual systematic reviews (e.g., accumulation of publication and selection biases), variation in the quality of the included reviews, variation in the quality of primary
literature included in those systematic reviews, overlap in the primary studies included, and the inclusion of outdated information (Ballard and Montgomery, 2017). Additionally, because a limited number of systematic reviews were selected for the scoping review and given the potential lag between publication of primary studies and the conduct of systematic reviews synthesizing the body of evidence, the failure to identify a systematic review for a given intervention or population may not indicate a lack of primary evidence. This represents an important limitation when using a scoping review of existing systematic reviews for a gap analysis. To the extent possible, the review protocol sought to directly address some of these limitations. For these reasons, the committee chose to not make any conclusion on the effectiveness of a given intervention but rather to summarize major findings of relevance and indicate existing research gaps and future needs. Therefore, the committee used this analysis to identify gaps in the research landscape but relied on additional sources of information to guide its identification of promising interventions.
The scoping review captured research on a wide range of NPIs and pharmacological interventions, including some that are well-established as well as more cutting-edge approaches for which there is emerging clinical evidence, such as gene therapy. NPIs reviewed included lifestyle approaches but also more invasive modalities such as brain stimulation. The 65 articles reviewed illustrate a highly active research field that is focused on improving the lives of people living with AD/ADRD. However, this review revealed several critical research gaps in the landscape that impede the generation of the evidence required to develop effective and clinically meaningful interventions for people living with these conditions.
First, the scoping review found a paucity of evidence from studies evaluating population-level interventions. The absence of this evidence could be, in part, caused by limitations of the literature search methodology. However, further evaluation suggests that the effects of population-level interventions, which are often assessed using natural experiments, are rarely comprehensively evaluated, in part because of inadequate data infrastructure (see Chapter 5) (Kind, 2024). Concerted efforts to evaluate population-level interventions are needed to understand the observed decline in dementia prevalence and incidence in some high-income countries and to identify the key contributing factors so the same successes can be achieved in all populations.
Relatedly, very few systematic reviews were identified in the scoping review that assessed combination interventions (e.g., two or more interventions, including at least one pharmacological intervention) as the primary objective of the review. As described previously in this report, combination approaches may be able to target multiple mechanisms simultaneously or sequentially, potentially leading to more effective treatments. The combined
use of memantine and cholinesterase inhibitors represents the most commonly studied combination therapy identified in the scoping review, but many other combinations have been assessed (Kabir et al., 2020). The development of anti-amyloid monoclonal antibodies, as well as the demonstrated potential of multimodal approaches targeting modifiable risk factors, has invigorated interest in combination approaches that can be applied prior to or early in the disease trajectory. Several trials are underway to evaluate anti-amyloid antibodies in combination with other agents such as anti-tau agents and antisenescence agents (Cummings et al., 2024), and a trial evaluating the combination of a multidomain lifestyle intervention and metformin (an antidiabetes drug) was recently initiated (Barbera et al., 2023).
Clinical research on combination therapies is operationally complex. Carrying out this research requires access to appropriate clinical trial infrastructure that is capable of delivering multiple therapies acting on different biological pathways across multiple trial centers and conducting factorial design studies. Such research also necessitates the building of cooperative partnerships that enable collaboration between participating companies with candidates available for codevelopment, as well as the necessary philanthropic, federal, and academic partners. Agreements regarding study management, data sharing, investigational new drug possessorship, and new drug application filing responsibilities need to be worked out. Demonstrating the additive or synergistic efficacy and safety of components in combination therapies is more complicated than the evaluation of monotherapies and may require the use of factorial study designs that employ larger sample sizes, which results in increased costs. Additionally, the perceived costs and time requirements to comply with FDA regulatory requirements specific to combination therapies, such as long treatment exposure time and large number of participants, add an additional layer of difficulty (Salloway et al., 2020). The thorough examination of different combination approaches and the quantification of the relative effects of each intervention is essential to optimizing more personalized and effective intervention strategies.
Conclusion 4-1: Combinatorial interventions hold promise for addressing the multifactorial nature of dementia by simultaneously addressing multiple pathways and mechanisms.
Conclusion 4-2: The exploration of combination intervention approaches is limited by operational complexities that necessitate partnership building and access to appropriate clinical trial infrastructure, in addition to complicated and costly study designs. The evaluation of combinatorial interventions that include one or more nonpharmacological approaches is further limited by deficiencies in the rigor of study designs and the required time investment for both investigators and participants.
The most apparent gap identified in the scoping review was the paucity of evidence specific to related dementias. The majority of articles in the review included participant populations living with mild cognitive impairment (MCI) or AD or included a broad participant population iving with dementia. In the latter case, the inclusion and exclusion criteria for dementia varied by study and in many cases related dementias were specifically excluded, whereas in other cases no reference was made to the specific dementia types. Where multiple types of dementia were explicitly included in the study, often insightful subgroup analyses were not possible owing to a lack of individual data or because of significant methodological limitations of the included primary research. Just one systematic review dedicated to assessing pharmacological interventions for frontotemporal dementia (FTD) was identified and included in the scoping review.
Evidence for Lewy body dementia (LBD) and vascular dementia was similarly sparse. No reviews were found that specifically examined mixed etiology dementia. Predictably, exclusions of specific types of dementia were more prevalent in the literature for pharmacological interventions. The absence of evidence for related dementias was compounded by the limited and poor quality of the primary evidence, particularly for LBD and FTD. Juxtaposed against the many systematic reviews of well-designed randomized controlled trials (RCTs) of interventions for AD, the included systematic reviews for LBD and FTD primarily pulled from case reports and uncontrolled trial designs. There are only so many ways the authors can state that more well-designed trials are needed to advance therapies to treat these understudied conditions.
The scoping review was designed to capture a wide range of outcomes and was not restricted to outcomes associated with cognitive function. Cognitive and neuropsychiatric outcomes were most widely reported by the studies included in the scoping review. Functional outcomes and assessments of quality of life and overall well-being, which may be most important to people living with dementia, were rarely reported. In cases where these outcomes were reported, in nearly all cases they were included as secondary outcomes and the studies often lacked sufficient power to reliably detect an effect. Relatedly, in some cases, cognitive outcomes may be secondary outcomes of interest for interventions primarily focused on other health conditions, such as improving cardiovascular health, as was the case for the SPRINT MIND clinical trial.
It is important to note that this assessment represents a snapshot of the state of the evidence at the time of the review and does not take into account the most recently published primary studies, which would not have been included in systematic reviews. Additionally, the scoping review was not designed to capture information regarding the demographics (e.g., race, ethnicity, socioeconomic status) of the research participants included in the
primary studies for each of the systematic reviews. However, this does not imply the absence of a gap related to evidence for diverse populations and, as discussed later in this chapter and in Chapter 5, the committee notes that there is a vital need for greater inclusiveness and representativeness in AD/ADRD research.
Promising Interventions for Prevention and Treatment of AD/ADRD
Consideration of Different Endpoints
In considering which interventions under study for preventing and/or treating AD/ADRD show promise, the committee identified the following three categories of interventions based on different endpoints of relevance. Of note, these are not mutually exclusive, and some interventions will fall into more than one of the categories.
Interventions that show promise in improving quality of life and function in daily activities of living
This category includes interventions that are not specific to pathologic processes underlying AD/ADRD but may improve overall well-being, quality of life, and the ability to function more independently, which are important outcomes for people living with AD/ADRD. Examples of interventions in this category include art and music therapy, as well as some medications that treat neuropsychiatric symptoms of dementia. Some promising interventions, such as physical activity, may fit in this category and one or both categories that follow, and the evidence for such interventions is discussed in the sections below. Interventions that fit only in this category are generally considered care interventions, which are excluded from the committee’s charge and are therefore not a primary focus of this report. While not reviewed in depth in this report, interventions that specifically address neuropsychiatric symptoms may make a huge difference for people living with AD/ADRD and their care networks, even if they do not affect biological pathways thought to underlie the disease causing dementia. Such interventions merit priority and rigorous research alongside interventions targeting the biological mechanisms of AD/ADRD. Moreover, the links between treatment of neuropsychiatric symptoms and trajectories of cognitive decline remain inadequately explored and warrant further investigation. Treatment for depression, for example, is not only important to improving quality of life, but may also impact cognitive outcomes (Livingston et al., 2024), though evidence has been mixed and further studies are needed to draw firm conclusions (NASEM, 2017).
Interventions that may have some effect on the prevention of neurodegenerative diseases or the building of brain resilience
This category includes those
interventions that address known risk factors for the development of AD/ADRD and that may prevent the development of brain pathology or enhance the brain’s ability to adapt to neuropathological changes and maintain cognitive function (e.g., through cognitive reserve or neuroplasticity). In many cases, the mechanisms of action for such interventions are not well understood but are likely not specific to a single form of dementia. Examples of interventions that fit in this category (discussed further below) include those targeting social isolation, sensory impairment (e.g., hearing aids), and exposure to neurotoxicants. While it can be challenging to generate strong evidence demonstrating prevention or enhanced resilience, an example of evidence for such interventions is improvement in, or maintenance of, cognition independent of neuropathologic burden (Leng and Yaffe, 2024). Importantly, such interventions are often low cost, relatively safe, may have other health benefits, and could be combined with future therapies specifically targeting neurodegeneration.
Interventions that show promise in slowing neurodegeneration or represent an exciting research area for further exploration of its potential for slowing neurodegeneration
This category includes interventions aimed specifically at slowing or halting the accumulation of neuropathologies and the loss of neurons and synapses in individuals already experiencing neurodegeneration. Many of the promising interventions in this category are relatively new or underexplored and have emerged from recent basic and translational research on mechanistic pathways contributing to AD/ADRD (see Chapter 3). As a result, evidence from large phase 3 efficacy trials may not yet be available, but evidence of promise may come from preclinical research or mechanistic studies. While many emerging pharmacological agents fall into this category, some NPIs also show promise for slowing neurodegeneration.
The sections below discuss the pharmacological interventions and NPIs that the committee believes hold promise for preventing or treating AD/ADRD. For each intervention, the evidence related to each applicable category above is discussed.
Promising NPIs
NPIs can affect any of the three categories of endpoints discussed above. They generally are not targeted to a specific pathology but affect cognition and resilience through other mechanisms.
Cognitive interventions
Cognitive function interventions include several approaches—cognitive training, cognitive rehabilitation, and cognitive stimulation—that target different domains to promote cognitive
enhancement and maintenance. These approaches have been applied to a variety of psychiatric and neurodegenerative conditions with the goal of improving cognitive and functional outcomes in these contexts. Cognitive training is intended to strengthen specific cognitive functions through repetitive practice of a defined task or exercise with the goal to achieve generalizing effects beyond the trained task, while cognitive rehabilitation seeks to improve or maintain the cognitive ability to perform everyday tasks and can include compensatory strategies that can be modified at an individual level. Cognitive stimulation includes general engagement-oriented interventions that are intended to broadly enhance cognitive or social functions (e.g., reminiscence therapy) (He et al., 2019; Vemuri et al., 2016). Cognitive training and stimulation may provide a neuroprotective effect by promoting neuroplasticity and the building of cognitive reserve (Park and Bischof, 2013). Importantly, these interventions appear to carry few risks beyond opportunity costs, and can be easily combined with other interventions (see section on multimodal approaches). The effectiveness of these approaches in preventing or slowing cognitive and functional decline associated with AD/ADRD and whether these approaches have broader benefits to overall well-being among older adults have been subjects of extensive research; however, the findings are highly variable.
The ACTIVE study, the first large-scale, randomized trial to evaluate the effect of cognitive training interventions in community-dwelling older adults without significant cognitive impairment found evidence that these interventions may preserve the cognitive abilities necessary to maintain functional competence and to cope with functional impairments even after a long duration (up to 10 years for some intervention groups) (Rebok et al., 2014; Tennstedt and Unverzagt, 2013). Additionally, some evidence was found for reduced risk of dementia over 10 years among participants randomized to a speed-processing cognitive training intervention as compared to untreated controls (Edwards et al., 2017). However, limited high-quality experimental evidence is available to conclude that cognitive training interventions are effective in preventing or slowing cognitive decline in individuals living with MCI (Bahar‐Fuchs et al., 2019; Basak et al., 2020; Gates et al., 2019).
Beyond the ACTIVE study, computerized cognitive training approaches have been found to be associated with improvements in global cognition in older, cognitively healthy adults (Bonnechère and Klass, 2023; Gates et al., 2020; Hu et al., 2021; Zhang et al., 2019a) and with improvements to verbal, visual, and working memory in people living with MCI (Chan et al., 2024). Additionally, the use of such immersive modalities as virtual reality technologies benefit global cognition and subdomains of executive functions in people living with MCI (Kim et al., 2019; Papaioannou et al., 2022; Zhong et al., 2021). Cognitive stimulation activities have
demonstrated improvements in quality of life and well-being in several observational studies and experimental trials (Gomez-Soria et al., 2023; Tulliani et al., 2022; Vemuri et al., 2016), and cognitive rehabilitation interventions have been linked to improved daily function in people living with mild‐to‐moderate cognitive impairment due to dementia (Clare et al., 2019). Importantly, although some of the effects summarized here offer some promise, the evidence for commercially available cognitive interventions remains equivocal (Nguyen et al., 2022). Further research is needed to elucidate the mechanisms underlying the function of cognitive interventions and to evaluate their efficacy over time.
Interventions to promote social interaction and reduce social isolation and loneliness
Loneliness and social isolation are a growing public health concern owing to accumulating evidence linking these conditions to an increased risk of all-cause mortality (NASEM, 2020; Yu et al., 2023) and other health conditions. Social isolation—“the objective state of having few social relationships or infrequent social contact with others” (NASEM, 2020)—is distinct from loneliness, which is a subjective or perceived feeling of isolation. Of relevance to this report, both are associated with increased risk of dementia (Elovainio et al., 2022; Salinas et al., 2022; Sutin et al., 2020). The mechanisms by which loneliness and social isolation increase dementia risk are not well understood and remain a focus of ongoing study (Guarnera et al., 2023), but loneliness has been linked to several early cognitive and neuroanatomical markers of vulnerability (Salinas et al., 2022).
In observational studies, social activity and social support provide benefit for some measures of cognitive function for community-dwelling adults without AD/ADRD (Baptista et al., 2024), and social contact and engagement have been found to be protective for dementia (Joshi et al., 2024; Livingston et al., 2024). Social interactions may function in part to build cognitive reserve, which confers resilience even in the presence of brain pathologies (Xu et al., 2019). As a result, there is significant interest in interventions to promote social interaction and reduce social isolation and loneliness. Such interventions come in many forms, from in-person facilitator-led group discussions or activities (Kelly et al., 2017) to the use of web-based social networking sites (Baptista et al., 2024).
While some programs focus on creating opportunities for social engagement among older adults, others have been designed with the goal of creating opportunities for nonfamilial intergenerational engagement (Krzeczkowska et al., 2021; Petersen, 2023). The Experience Corp program, for example, trains older adults to volunteer as mentors for children in neighborhood elementary schools during the academic year, which not only addresses the social engagement needs of the adults but has the added potential benefit of improving the academic performance of children in underserved areas. An RCT of the
Baltimore Experience Corps program showed the program led to improved cognitive function, particularly for those with impaired executive function at baseline, as well as physical and social activity (Carlson, 2021; Carlson et al., 2008). Ongoing evaluations seek to determine whether these short-term effects translate to longer-term benefits in reducing risk for dementia. Innovative technologies, such as interactive social robots, are also providing new ways to increase social engagement and potentially reduce social isolation and loneliness in older adults (Baptista et al., 2024; Joshi et al., 2024).
Despite the promise suggested by observational studies and the Experience Corp program, recent reviews show that the results from intervention trials have generally been mixed with regards to the effects on cognitive function and social measures (e.g., loneliness, social identification, perceived social support) in people with and without AD/ADRD (Baptista et al., 2024; Joshi et al., 2024). This may stem, in part, from the heterogeneity of the interventions and outcome measures evaluated in the studies included in the reviews. There is, however, evidence suggesting that interventions to address social isolation and loneliness can improve quality of life in people living with AD/ADRD (Joshi et al., 2024).
While there is currently limited high-quality research on interventions to address social isolation and loneliness, this is an important area for future research and has the potential to offer benefit at the population level. The question of whether earlier and better interventions can not only improve quality of life but also maintain or improve cognition remains unanswered and should be a priority going forward. Given the heterogeneity of existing interventions, future trials would benefit from efforts to determine the specific aspects of social relationships that are needed to benefit cognitive function.
Physical activity interventions
Physical activity (e.g., aerobic exercise, mind–body exercise, strength and resistance training) practiced alone or in combination with other nonpharmacological and pharmacological approaches has been the subject of substantial research and public interest as a potentially effective strategy for preventing and treating cognitive decline associated with AD/ADRD, as well as for improving functional outcomes and the management of common neuropsychiatric symptoms. Physical activity has been hypothesized to reduce the risk of dementia and the development of neuropathologies through both direct (e.g., improved brain vasculature and blood flow and a reduction in inflammation and amyloid beta production) and indirect pathways (e.g., improved cardiovascular health) (De la Rosa et al., 2020; Iso-Markku et al., 2022). The effect of physical activity on the prevention of dementia, specifically AD and all-cause dementia, has been well demonstrated in the recent literature (De la Rosa et al., 2020; Guure et al., 2017; Iso-Markku et al., 2022; López-Ortiz et al., 2023; Zhang et al., 2023).
One umbrella review of published meta-analyses estimated a 30–40 percent reduction in risk of incident AD with regular physical activity as compared to inactivity (López-Ortiz et al., 2023). While a protective effect is relatively well documented, much less is known regarding the specific physical activity type, intensity, duration, and frequency that affords optimal protective benefits and how these factors may vary across individuals and dementia types. For example, there may be sex differences in optimal design of physical activity interventions. Several studies have shown that the cognitive gains from aerobic training are greater for older women as compared to older men (Mielke, 2024). Generally, though, moderate- and high-intensity exercise, as opposed to low-intensity exercise, has been demonstrated to be more effective in reducing risk for incident AD (Zhang et al., 2023).
Beyond promising evidence on the potential for prevention, evidence for the effect of physical activity on improvement of cognitive function in people already living with dementia remains less clear. Improvements in cognitive function associated with physical activity as measured by various cognitive assessments have been described in multiple studies across various types of dementia (De la Rosa et al., 2020; Groot et al., 2016; López-Ortiz et al., 2023). However, evidence for the beneficial effect of physical activity on improved cognitive function in people living with one or more types of dementia is not universally demonstrated (Brasure et al., 2017), and methodological limitations common to the study of nonpharmacological approaches limit the ability to make conclusions on causality and level of effect.
As with prevention, it is likely that the effect of physical activity on improvements in cognitive function is moderated by the type, intensity, and duration of the activity (Karamacoska et al., 2023) and more research is needed to systematically assess these components. Aerobic exercise has been linked to greater cognitive benefits in populations living with MCI, vascular dementia (Ahn and Kim, 2023; Zheng et al., 2016), AD (De la Rosa et al., 2020; Morris et al., 2017), and all-cause dementia (Groot et al., 2016) as compared to nonaerobic types of physical activity. The recently published EXERT trial, however, found that a stretching, balance, and range-of-motion exercise program was equally effective at slowing cognitive decline in participants with MCI as moderate-intensity aerobic training (Baker et al., 2022).
Physical activity may also be effective as a strategy for managing neuropsychiatric symptoms common to dementia, such as disturbed sleep and depression (Ahn and Kim, 2023; Cai et al., 2023; Wilfling et al., 2023), and for improving such physical functions as balance, gait function and speed, and muscular strength (Cai et al., 2023; Connors et al., 2018; López-Ortiz et al., 2023), which may translate to greater overall well-being and independence regardless of cognitive status. As one of many known,
modifiable risk factors, physical activity has been explored in combination with other NPIs (e.g., diet, cognitive training), as will be discussed in the next section.
These findings indicate that physical activity may be promising in the prevention of cognitive decline, and some evidence suggests that physical activity also provides a cognitive benefit for people living with related dementias. Critically, in both cases, insufficient evidence is available to confirm which activity types, intensities, and durations of activity are most efficacious to achieve a meaningful benefit and how these factors may change to achieve optimal benefit based on the characteristics of the intended user (e.g., age, comorbidities, health, and physical status).
Multicomponent lifestyle approaches
The highly complex and multifactorial nature of AD/ADRD and the high prevalence of mixed pathologies—which may result from common and pathology-specific mechanisms—suggest that targeting a single pathology or mechanism is unlikely to be sufficient for preventing or treating AD/ADRD on a large scale. There is increasing interest in understanding how multimodal interventions, which combine multiple therapeutic strategies, can simultaneously or sequentially target multiple modifiable risk factors and mechanisms underlying dementia (Barbera et al., 2023). Multimodal lifestyle interventions, which often include combinations of physical activity and exercise programs, diet and nutritional modifications, cognitive training approaches, social stimulation, and management of vascular and metabolic risk factors, have shown promise in both preventing and potentially slowing cognitive decline associated with various types of dementia (Thunborg et al., 2021), as well as contributing to improved physical and mental health, function, and well-being. The evidence remains unclear regarding how multimodal interventions may function to improve cognition or build cognitive reserve or resilience. However, it is likely that the targeting of multiple risk factors through multiple domains acts on various mechanisms and pathways, such as vascular pathways, inflammatory-immune mediated responses, insulin signaling, and mechanisms related to biological aging, both in isolation and synergistically (Barbera et al., 2023; Song et al., 2022).
Demonstrating the effect of individual lifestyle interventions (e.g., diet, cognitive training, exercise) has been challenging. In contrast, recent assessments of multimodal lifestyle interventions have expanded understanding of the potential of lifestyle approaches to slow or improve cognitive decline in cognitively healthy populations at risk for dementia (Ngandu et al., 2015; Yaffe et al., 2024) and to slow cognitive decline in individuals living with AD/ADRD (McMaster et al., 2020; Meng et al., 2022; Salzman et al., 2022), in addition to providing broader functional and quality-of-life benefits. The FINGER study, for example, demonstrated a 25 percent
greater improvement in global cognition and significantly decreased risk of cognitive decline in cognitively healthy, older participants as compared to controls (Ngandu et al., 2015). Cognitive benefits from lifestyle approaches have been observed among higher-risk APOE4 carriers (Solomon et al., 2018). Importantly, multimodal lifestyle interventions may reduce the risk of functional decline (Kulmala et al., 2019). Other research has demonstrated that the combination of computerized cognitive training and physical exercise interventions has more pronounced effects on cognition in both healthy older adults and those with MCI as compared to either intervention alone (Gavelin et al., 2021).
One of the most promising aspects of these multimodal approaches is the potential to tailor interventions to an individual’s personal risk factors and preferences, which may improve adherence and provide greater individual benefits. The Systematic Multi-Domain Alzheimer Risk Reduction Trial (SMARRT) applied this approach to its intervention group, allowing participants (cognitively healthy older adults at high risk for dementia) to select and set personalized risk-reduction goals based on their personal risk profile, preferences, and priorities for risk reduction. The findings of this 24-month trial indicated that this participant-driven, tailored multimodal approach has modest effects on improved cognitive function, risk composite scores, and quality of life and, importantly, was well received by participants (Yaffe et al., 2024).
Further interrogation of new combinations of interventions and deeper examination of specific individual characteristics (e.g., APOE genotype; comorbidities) may result in more precise and effective approaches for preventing disease and slowing cognitive decline. Efforts to evaluate the combined use of pharmacological interventions (disease modifying interventions for conditions that share risk factors with AD/ADRD) in conjunction with traditional lifestyle approaches to prevent dementia in high-risk populations are now underway. For example, the MET-FINGER study will apply the FINGER 2.0 multimodal intervention approach in combination with metformin in high-risk older adult participants with the APOE4 allele (Barbera et al., 2023).
Ultimately, multimodal interventions are likely feasible, tailorable to individual risk factors and preferences, and compatible with disease modifying therapies, suggesting that these approaches offer promise in the prevention and treatment of AD/ADRD. In future explorations, multimodal lifestyle interventions could be designed to be dynamic, allowing not only personalization but adjustment of thresholds, as in increasing physical activity intensity, over time.
Interventions to address sensory impairment
Hearing and vision problems are common conditions affecting older adults. Although the mechanisms
are not yet well understood, these sensory impairments are thought to not only co-occur with, but also contribute to, cognitive decline and dementia (Livingston et al., 2024) and may have some relationship with cardiovascular disease risk factors (Baiduc et al., 2023). As effective interventions to treat hearing and vision loss are widely available, there is great interest in understanding the effects those interventions may have on cognitive function, AD/ADRD risk, and quality of life for people living with MCI or dementia.
Hearing loss has been identified as a significant modifiable risk factor for developing dementia (Livingston et al., 2020, 2024). Associations between hearing loss and cognitive decline have been reported in numerous studies (Jayakody et al., 2018; Ray et al., 2018), although other studies have failed to find an association and questions remain regarding the causal nature of this association (Asakawa et al., 2024). Hearing loss is also linked to volume loss in specific brain regions (Armstrong et al., 2019; Llano et al., 2021). In contrast to many other AD/ADRD risk factors, a relatively simple and accessible intervention for hearing loss is available in the form of hearing aids, which have so far demonstrated very low risk levels for users. Observational studies have reported that use of hearing aids protects against dementia in people experiencing hearing loss and other risk factors (Livingston et al., 2024), suggesting this is a promising intervention for preventing AD/ADRD. Results from controlled intervention studies, however, have been mixed as to whether hearing aids maintain or improve cognitive function in people without preexistent dementia (Sanders et al., 2021). The signal was greatest for the executive-function cognitive domain, but study limitations precluded definitive conclusions. In older adults with cognitive impairment, there is some evidence that hearing aid use can improve quality of life and dementia-related behavioral symptoms but has little apparent effect on cognitive outcomes in this population (Dawes et al., 2019; Mamo et al., 2018).
This is an important area for additional research with the potential to help millions at risk for cognitive decline and dementia, and many questions remain that should be answerable with current science. For example, could better hearing aids have a greater effect? Is some form of cognitive or other training required to realize the benefits of improved hearing? Given the limited availability of high-quality evidence, it will be important for future research to address the methodological limitations (e.g., small study size, lack of control groups, problems with consistent hearing aid use) that have impeded efforts to assess the beneficial effects of hearing aid use on cognitive function (Asakawa et al., 2024; Dawes et al., 2019). Such research may inform future recommendations regarding screening for hearing loss in older adults (U.S. Preventive Services Task Force, 2021) and prompt further investment in the development
of more accessible and low-cost assessment methods to screen for hearing impairment (Lelo de Larrea-Mancera et al., 2022). Today, Medicare does not cover the cost of hearing aids. If they are or could be made to be effective in maintaining or improving cognition, and could be made affordable through better insurance coverage, these devices could make a significant difference in quality of life and could improve cognitive health on a population basis.
Vision impairment has also been identified as a risk factor for cognitive decline and dementia (Cao et al., 2023; Ehrlich et al., 2022; Livingston et al., 2024). As most cases of vision impairment are treatable with two cost-effective interventions—eyeglasses or contacts, and cataract surgery—the modifiable nature of this risk factor makes it an attractive target for intervention studies aimed at identifying strategies to slow cognitive decline and prevent dementia. While research is still ongoing, there is a small but growing evidence base suggesting the cognitive benefits of cataract surgery for older adults. Cataract surgery is associated with reduced risk for MCI (Miyata et al., 2018), and a number of studies (primarily observational) examining the effects of cataract surgery have observed beneficial effects on cognitive function in cognitively healthy adults (Pellegrini et al., 2020) and people living with MCI (Yoshida et al., 2024), although one RCT showed no improvement in performance on neuropsychological tests in cognitively healthy adults after surgery (Anstey et al., 2006).
While many studies evaluating cognitive effects have relatively short follow-up periods, one observational study with a control group found that cataract surgery slowed the rate of cognitive decline over a period of more than 10 years as measured using a test of episodic memory (Maharani et al., 2018). Additionally, surgery has been shown to reverse cataract-induced neuroanatomical changes (Lin et al., 2018). While the committee did not find evidence of cognitive benefits for people living with dementia (Yoshida et al., 2024), cataract surgery may improve quality of life and neuropsychiatric symptoms (Dawes et al., 2019).
The evidence base for the use of eyeglasses or contacts on AD/ADRD risk is extremely sparse, making it difficult to draw conclusions. One study found a correlation between wearing reading glasses and cognitive function, although the linkage was no longer significant after adjusting for education (Spierer et al., 2016).
As with hearing aids, the evidence base for interventions to address vision impairment is hampered by methodological limitations of existing studies. Future research should include adequate control groups and follow-up periods and address problems with cognitive tests, such as vision dependency and practice effects (Fukuoka et al., 2016).
Promising Pharmacological Interventions
The following promising pharmacological interventions are aimed at preventing or slowing neurodegeneration. With few exceptions (e.g., anti-amyloid antibodies), most are still in early phases of clinical research and efficacy data from large phase 3 or 4 clinical trials are not yet available.
Anti-amyloid treatments
The recent approval of two anti-amyloid monoclonal antibody therapies—lecanemab and donanemab—for early, symptomatic AD has generated much interest regarding the effectiveness of these therapies in slowing cognitive decline. While these approved anti-amyloid therapies are not a panacea, they represent two more tools than what previously existed for use in the treatment of AD. Much remains to be learned about how these anti-amyloid therapies can be implemented for maximum benefit and limited harm.
The clinical benefits and potential harms of anti-amyloid therapies have been well described in the literature. A phase 3 trial of lecanemab, which binds to amyloid beta-soluble protofibrils, demonstrated a reduction in brain amyloid levels and slowed clinical decline in select groups of participants living with early AD after 18 months. The study findings indicated modest improvements in cognitive and functional measures compared to placebo but were accompanied by risk of serious adverse events, particularly for carriers of two copies the APOE4 allele (van Dyck et al., 2023). Of note, a posthoc analysis suggested that the cognitive benefits of lecanemab may differ by gender with women potentially receiving less benefit (Mielke, 2024), but this requires further investigation in studies designed to detect such differences.
In clinical trials, donanemab, which removes amyloid plaques via microglial-mediated clearance, was also found to improve cognition and functional measures in participants with early AD after 76 weeks as compared to placebo (Mintun et al., 2021). As with lecanemab, adverse effects associated with amyloid-related imaging abnormalities were reported (Mintun et al., 2021; van Dyck et al., 2023). Top-line results from these trials indicated that the greatest clinical benefit was found in those participants presenting with the earliest levels of cognitive impairment. The donanemab trials, for example, found a 60 percent slowing of disease severity following treatment in those living with MCI as measured by the Integrated Alzheimer’s Disease Rating Scale (iADRS); a 40 percent decline in iADRS was observed across the total study population (AlzForum, 2024a).
These results and other questions raised from prior trials (e.g., differences across APOE4 status, sex, prevalence of adverse effects, long-term effects) open new opportunities to assess how these therapies can be used most effectively to slow and possibly prevent AD neuropathology and
cognitive decline. Two trials, the AHEAD Study (lecanemab) and TRAILBLAZER-Alz-3 (donanemab), are now in the process of evaluating the effect of these therapies on the prevention of disease progression in people at high risk of AD, as measured by time to clinical progression (AHEAD Study, 2024; Pugh, 2023). The phase 3 TRAILBLAZER-Alz-3 prevention trial enrolled cognitively unimpaired individuals who are considered to be at high risk for clinical AD, as determined by elevated plasma p-tau217 (AlzForum, 2024a). Similarly, the AHEAD Study enrolled cognitively unimpaired individuals with elevated brain amyloid (Rafii et al., 2023). The findings from these studies and others will provide critical evidence on the optimization of timing, dosing, and safety, as well as greater insights into target populations for maximum benefit in prevention and treatment of AD.
While initial evaluations of anti-amyloid therapies have occurred in people diagnosed with AD (with or without clinical symptoms), the safety and efficacy of these therapies in the context of mixed etiology dementia is of great interest given the prevalence of co-occurring pathologies. NIH posted a request for applications in 2024 to support the evaluation of anti-amyloid therapies in people with a clinical diagnosis of LBD who also exhibit evidence of AD brain pathology (NIH, 2024).
As discussed earlier in this chapter, there is also interest in combination interventions that involve the administration of other therapeutics, including anti-tau therapies, to patients alongside amyloid-lowering therapies (Cummings et al., 2024). Such studies may aid in understanding which molecular drivers are additive to amyloid lowering in terms of reducing or reversing symptoms. The combination of anti-amyloid antibodies with focused ultrasound to transiently open the blood–brain barrier is also being investigated as a means of enhancing delivery of the therapeutics to the brain (Rezai et al., 2024).
Importantly, the long-term effects of anti-amyloid therapies need to be evaluated and can be accomplished through the long-term follow-up of those patients who received treatment. Such assessments will help elucidate how the removal of amyloid from the brain affects clinical symptoms and disease progression over time and whether these represent meaningful therapeutic benefits. Additionally, long-term follow-up will help to understand the safety of these treatments (e.g., whether changes in brain volume have any negative effect) (Alves et al., 2023) and the effects of amyloid removal on the development of other neuropathologies, such as the progression of tau pathologies.
Immunomodulatory agents
There is a substantial body of evidence suggesting that neuroinflammation and immune dysfunction play key roles in the etiologic cascade that leads to neurodegeneration and AD/ADRD, as discussed in Chapter 3. Neuroinflammation may result from a number
of different mechanisms, such as the activation of microglia to a proinflammatory phenotype (Tejera et al., 2019; Wang et al., 2023) and the secretion of proinflammatory mediators by accumulating senescent cells, which may lead to tissue infiltration of immune cells, chronic inflammation, and tissue damage (Islam et al., 2023; Riessland and Orr, 2023). These processes are thought to be characteristics of immunosenescence, a gradual deterioration and dysfunction of immune function with aging that is also believed to contribute to neurodegenerative diseases, such as AD/ADRD (Bowirrat, 2022; Liu et al., 2023; Rommer et al., 2022; Zhao et al., 2020).
With the growing awareness of the role of inflammation in AD/ADRD has come interest in anti-inflammatory interventions as a means to prevent or slow neurodegeneration. The challenge for such therapies is to ameliorate detrimental inflammatory responses without impairing immune responses critical to the clearance of pathogenic protein aggregates. While data from clinical trials of nonsteroidal anti-inflammatory drugs such as aspirin have been disappointing in light of the epidemiological evidence suggesting a protective effect (ADAPT Research Group, 2013; Meyer et al., 2019), a multitude of other anti-inflammatory agents are under investigation and show promise in preclinical and early clinical research. For example, senolytics—compounds that selectively clear senescent cells that accumulate with age (Riessland and Orr, 2023)—may reduce inflammation associated with the senescence-associated secretory phenotype (see Chapter 3). Senolytic compounds under evaluation in phase 2 clinical trials include a combination of dasatinib (a tyrosine kinase inhibitor approved by FDA for the treatment of leukemia) and quercetin (a flavonoid with anti-inflammatory properties) (Cummings et al., 2024; Riessland and Orr, 2023). Data from a phase 1 feasibility trial showed that treatment of adults with early-stage AD with a combination of dasatinib and quercetin was well tolerated, and promising results from an exploratory analysis indicated the potential reduction of inflammatory markers (Gonzales et al., 2023) consistent with responses observed in preclinical studies (Zhang et al., 2019b).
Given the current absence of approved treatments for Lewy body dementia, there is considerable interest in neflamapimod, “an oral drug targeting the effects of neuroinflammation on the molecular mechanisms underlying degeneration of cholinergic degeneration in the basal forebrain” (Prins et al., 2024, p. 549). Results from a phase 2a trial were promising, with greater cognitive benefits observed for those without AD copathology, and the drug is now in phase 2b trials. Glucagon-like peptide-1 (GLP-1) agonists, discussed below, may also exert anti-inflammatory effects. Other novel therapies for AD that target inflammation and are currently being evaluated in clinical trials are described by Cummings and colleagues (2023a, 2024).
In addition to dysregulated inflammatory responses, other dysfunctions of the immune system accompany immunosenescence. While the myriad
changes that occur with immunosenescence are complex and not fully understood, in relation to AD/ADRD, a key dysfunction is impaired chemotaxis (cell movement in response to a chemical stimulus) and phagocytic function of microglia in the brain and peripheral macrophages (Liu et al., 2023; Rawji et al., 2016; Zhao et al., 2020). Such changes are thought to impair the clearance of abnormal protein aggregates. Thus, paradoxically, while some therapeutic strategies for AD/ADRD involve dampening elements of the immune system (inflammation), others are aimed at stimulating an immune response to enhance immune-mediated clearance of protein aggregates (Cummings et al., 2023b). One example of such immunostimulants under investigation in clinical trials for AD/ADRD is sargramostim, a recombinant form of a cytokine known to stimulate the development of phagocytosis by cells of the innate immune system (currently in phase 2 trials) (Cummings et al., 2023a,b; Van Eldik et al., 2016). Preclinical studies using AD mouse models showed that treatment with sargramostim activated microglia, reduced AD pathology by more than 50 percent, and rescued cognitive function (Kiyota et al., 2018). Exploratory analyses from the phase 1 trial were encouraging, showing evidence of cognitive benefit for the treated group and reduced markers of neurodegeneration (Potter et al., 2021a).
A different immunostimulatory approach being evaluated for AD/ADRD is the use of a vaccine adjuvant, protollin, which is a combination of bacterial outer membrane proteins and lipopolysaccharide. While vaccines usually contain a pathogen-specific component, the response to which is nonspecifically enhanced by the adjuvant, in this case the adjuvant is administered on its own to stimulate cells of the innate immune system, which will recognize the bacterial cell components. Researchers hope that the activated innate immune cells will move from the cervical lymph nodes to the brain and clear protein aggregates, such as amyloid beta (Valiukas et al., 2022). Following preclinical studies showing that protollin was effective in stimulating amyloid removal (Frenkel et al., 2008), a phase 1 clinical trial was initiated in 2021.
Importantly, both sargramostim and protollin are already approved by FDA for other uses and thus represent opportunities for drug repurposing. Moreover, because inflammation and immunosenescence are thought to be common underlying neurodegenerative mechanisms, the potential application of these immune-modulating therapies is not limited to AD and may extend to related dementias, offering promise of disease-agnostic approaches that can prevent or slow neurodegeneration. There is still much that is not well understood regarding the function of the immune systems and inflammation, however, and enhanced understanding of the timing and context of immune system modulation, as well as the contribution of specific mechanisms to disease states, will be critically important to knowing how and when to intervene safely using immunomodulatory agents.
Tau antisense oligonucleotides
Like anti-amyloid antibodies, antisense oligonucleotides (ASOs) are biologic therapeutics targeted to specific proteins implicated in the development of AD/ADRD. Whereas antibody-based therapeutics directly bind the protein of interest, ASOs target the protein by altering its gene expression. ASOs are synthetic oligonucleotides (or analogs of oligonucleotides) generally 12 to 30 nucleotides in length that bind to RNA (messenger RNAs that encode proteins or noncoding RNAs) (Bennett et al., 2019). RNA binding by ASOs can promote degradation of the bound RNA molecule, modulate the processing of the RNA through splicing and polyadenylation, or otherwise affect the translation of mRNA into protein, such as by disrupting RNA structures that block translation (Bennett et al., 2019; Silva et al., 2020). An advantageous feature of ASOs is the ability to directly translate genetic discoveries into drug discovery programs (Bennett et al., 2019).
ASOs are emerging as a class of therapeutics with great potential to treat neurodegenerative diseases. In 2016, an ASO was approved by FDA for the treatment of spinal muscular atrophy (FDA, 2016), and, in 2023, FDA granted accelerated approval to an ASO used to treat amyotrophic lateral sclerosis (FDA, 2023). The AD/ADRD clinical trials pipeline currently includes tau-targeted ASOs that are in phase 1 and 2 clinical trials (Cummings et al., 2024). Tauopathy is a feature of several neurodegenerative diseases, including AD and FTD. It is hoped that tau-targeted ASOs may slow or halt neurodegeneration by preventing the aggregation of hyperphosphorylated tau into neurofibrillary tangles—believed to be a key driver of neuronal loss—and the spreading of tau across neural networks (seeding). Encouraging data from a phase 1b trial showed that treatment of participants living with mild AD with a tau-targeted ASO was well tolerated and reduced cerebrospinal fluid (CSF) total tau concentration in a dose-dependent manner, achieving a mean reduction from baseline levels of more than 50 percent at 24 weeks after the last dose (Mummery et al., 2023). Brain tau levels were also reduced by the treatment as measured by tau-PET (Edwards et al., 2023).
Although data demonstrating the effects of anti-tau ASOs on cognitive outcomes in humans are not yet available, treatment of mice carrying a human tau gene rescued nest-building performance, a functional task that is deficient in mouse models of tauopathy and considered to be reflective of social behavior and cognitive function (DeVos et al., 2017). The prospect of a treatment to slow neurodegeneration caused by tauopathy associated with AD/ADRD is exciting; however, approval of such a treatment would require consideration of barriers to implementation, such as high cost of treatment and health care system delivery of a drug administered via an invasive procedure (recurring lumbar puncture).
Gene and cell therapy interventions
Gene and cell therapies represent increasingly active areas of intervention research for dementia, especially in AD. Gene therapy may show promise in the future based on recent advances in other neurodegenerative diseases, as in FDA approval of a gene therapy for spinal muscular atrophy (FDA, 2019a; Lennon et al., 2021; Sun and Roy, 2021). The development of gene therapies for dementia are exciting as these approaches are designed to prevent the development of neuropathologies and to slow or halt cognitive decline by precisely targeting the underlying mechanisms contributing to disease. Much of this research remains in preclinical stages, where the focus is on the development of vectors and well-validated targets. Potential targets being explored for AD gene therapies include amyloid pathway intermediates and the modulation of enzymes, tau protein downregulation, APOE4 downregulation and APOE2 upregulation, neurotrophin expression, and inflammatory cytokine alteration (Lennon et al., 2021). Gene therapies are also being pursued for related dementias. For example, one gene therapy target in FTD is the progranulin gene, mutation in which contributes to approximately 22 percent of familial FTD cases (Sevigny et al., 2024).
Preclinical studies using animal models have yielded some promising results from gene therapy candidates for improvements in the cognitive domains of memory and learning (Lennon et al., 2021; Tedeschi et al., 2021) and in the reduction of accumulated amyloid beta and tau (Loera-Valencia et al., 2018). However, the few early-stage clinical trials completed thus far, targeting the regulation of neurotrophic factors, have demonstrated mixed effects (Tedeschi et al., 2021) and suggest the need for further work on the development of effective delivery modalities (Lennon et al., 2021), along with continued identification of new vectors and therapeutic targets (Chen et al., 2020a). Preclinical studies and interim results from an early-phase clinical trial show that a gene therapy delivering the granulin gene using an adeno-associated virus vector was generally safe and well tolerated and could increase progranulin levels in vivo (Sevigny et al., 2024). Longer follow-up and additional studies are needed to assess the duration of effects and the clinical (e.g., cognitive, behavioral) benefits of treatment.
Cell therapies involve the use of living cells (e.g., stem cells, immune system cells) to prevent or treat diseases. At present, there are no FDA-approved cell therapies for the treatment of neurodegenerative diseases, but a variety of different cell therapies are under investigation in both preclinical and clinical studies (Chan et al., 2021; Cummings et al., 2023b). Cell types and anticipated mechanisms of action for investigational cell therapies vary (Kwak et al., 2018; Temple, 2023). Some stem cell therapies have been aimed at direct replacement of neuronal cells lost during neurodegeneration (Chen et al., 2023a). While this may be a viable therapeutic approach for
some neurodegenerative diseases, such as replacing dopaminergic neurons lost in Parkinson’s disease (Cha et al., 2023), a cell replacement strategy is more complex for diseases such as AD that feature a multiplicity of affected phenotypes and the loss of multiple distinct neuronal cell types (Goldman, 2016; Kwak et al., 2018; Loera-Valencia et al., 2018).
Alternative strategies to cell replacement that are of growing interest include modulating inflammation and stimulating neurogenesis and tissue regeneration, particularly in the hippocampal region of the brain (Kwak et al., 2018). Cell therapies using mesenchymal stem cells (MSCs, also known as medicinal signaling cells) have shown promise for these nonreplacement strategies (Chan et al., 2021). Most cell therapies being evaluated in clinical trials for treatment of AD use MSCs (Chan et al., 2021). While labeled as stem cells owing to their multipotential capacities, the use of MSCs in cell therapies is primarily focused on their ability to migrate to sites of injury or inflammation within the body and to modulate immune responses and stimulate tissue regeneration through their secretion of bioactive factors and via cell–cell interactions (Caplan, 2017; Chan et al., 2021; Jimenez-Puerta et al., 2020; Kwak et al., 2018).
One MSC-based cell therapy was given fast-track status by FDA in July 2024 for the treatment of mild AD following a successful phase 2a trial that achieved its primary safety and secondary efficacy endpoints, showing preliminary evidence of slowing cognitive and functional decline (Ciccone, 2024). While there has been some encouraging evidence for cell-based therapies, ongoing studies are needed to better elucidate mechanisms of action, to understand the duration of any beneficial effects, and to address such concerns as tumorigenesis and immune rejection of grafted cells (Chan et al., 2021; Chen et al., 2023a; Goldman, 2016). Scalability of cell-based therapies also needs to be considered.
While gene and cell therapies represent exciting avenues for research, evaluation of their safety and effectiveness for treating AD/ADRD is still underway, and it is unlikely that these therapies will be available for broad clinical use in the very near future.
Interventions targeting synaptic dysfunction
Synaptic dysfunction is a primary correlate of cognitive impairment in progressive neurodegenerative diseases. Synaptic impairment is thought to occur early in the neurodegenerative process across a range of pathologies and may result from a complex combination of pathologic mechanisms including toxic amyloid beta and tau oligomers (Gutierrez and Limon, 2022; Li and Selkoe, 2020); overactive glial cells (Yu et al., 2024); mitochondrial dysfunction (Morton et al., 2021); abnormal accumulation of alpha-synuclein (Trudler et al., 2021); and genetic mutations (Gelon et al., 2022). The development of therapeutics to enhance synaptic plasticity or confer synaptic neuroprotection represents an
increasingly active area of early-phase drug development for AD (Cummings et al., 2023b).
At present, there are multiple phase 2 and 3 trials of novel agents in progress for various unique drug targets related to synaptic dysfunction, some of which have demonstrated promise in enhancing synaptic function in early to moderate AD (Cummings et al., 2023b, 2024). Phase 2a clinical testing of a first-in-class small-molecule compound that targets a p75 neurotrophin receptor, for example, demonstrated potential efficacy in slowing AD pathology in participants with mild to moderate AD, as measured by CSF biomarkers amyloid beta40 and amyloid beta42; however, no change was observed in cognitive testing (Shanks et al., 2024). CT1812, a sigma-2 receptor antagonist that interferes with amyloid beta oligomer binding to neurons and thereby prevents synaptotoxicity, demonstrated promise in transgenic mouse models. A recent pilot study found no significant change from baseline as indicated by FDG-PET, clinical cognitive scales, or CSF biomarkers, but volumetric MRI illustrated “a trend towards tissue preservation” in the CT1812 treatment group (van Dyck et al., 2024). As of June 2023, CT1812 has advanced to a phase 2 trial that is being run by the Alzheimer’s Clinical Trials Consortium and will evaluate the safety and efficacy of different dosages as compared to placebo in participants with MCI or mild AD (AlzForum, 2024b).
Unlike anti-amyloid therapies currently on the market, these small-molecule drug therapies are administered orally and, thus, may be more accessible and require less costly clinical infrastructure. Additional, longer clinical trials for interventions targeting synaptic dysfunction will be required to monitor the safety of these compounds and to evaluate efficacy in larger study populations. In addition to further development and evaluation of agents targeting synaptic functions, parallel efforts are needed to identify and validate biomarkers and outcomes for synaptic function that can be used in clinical research to accurately measure target engagement and response (see later section on Identifying biomarkers for demonstrating target engagement and measuring treatment response).
Antidiabetic treatments
Shared pathophysiological mechanisms underlying both type 2 diabetes (T2D) and dementia, specifically AD, have led to growing interest in the use of antidiabetic treatments, such as glucagon-like peptide-1 receptor agonists (GLP-1RA) and metformin, to prevent AD in high-risk groups and to slow cognitive decline in those living with AD (Michailidis et al., 2022; Muñoz-Jiménez et al., 2020). Shared metabolic impairments, such as insulin resistance, impaired glucose metabolism, mitochondrial dysfunction, oxidative stress, and inflammation, mean that T2D and neurodegenerative diseases are closely linked, and individuals with T2D are at high risk of developing a neurodegenerative disease (Carvalho
and Moreira, 2023). The growing prevalence of chronic metabolic disorders in the United States indicates the need to evaluate antidiabetic treatments as strategies to both prevent incident dementia in people with T2D and to slow decline in people living with dementia with and without a T2D comorbidity.
Metformin, a commonly prescribed and extensively studied drug for treating high blood sugar associated with T2D, has shown some benefit in reducing the risk of incident cognitive decline and dementia in participants with T2D (Michailidis et al., 2022; Zhang et al., 2022). However, overall evidence for its protective effects on cognition remain mixed and necessitate further study (Luchsinger et al., 2017; Michailidis et al., 2022; Weinstein et al., 2019). GLP-1 receptor antagonists are newer T2D treatments that have shown early promise in improving cognitive function and memory and decreasing amyloid beta deposition and tau hyperphosphorylation in people living with dementia, although this research remains in preclinical and early clinical stages (Michailidis et al., 2022; Wang et al., 2022a). These drugs may also provide neuroprotective effects in cognitively healthy people living with diabetes (Hölscher, 2022; Nørgaard et al., 2022).
The approval of dual GLP-1/GIP receptor agonists for the treatment of T2D in 2022 has spurred even greater excitement about the potential benefits of these drugs for dementia and many other chronic diseases and health conditions, including prediabetes and obesity. These dual agonist treatments, which are more effective in crossing the blood–brain barrier, have demonstrated superior protective effects in preclinical and clinical studies as compared with single-receptor antagonists in study populations with diabetes as well as in study populations with AD and other neurodegenerative diseases such as Parkinson’s disease (Hölscher, 2022).
Promising Interventions That May Be Pharmacologic, Nonpharmacologic, or a Combination
Management of hypertension and other interventions that target vascular health
Risk factors associated with overall cardiovascular health, including hypertension, hypercholesterolemia, and metabolic disease, are also strongly associated with an increased risk of vascular dementia, AD, and mixed etiology dementia involving vascular pathologies. Risks of vascular dementia from these comorbid conditions may be higher for females than males (Mielke, 2024). Epidemiological research suggests that control of these overlapping risk factors may present opportunities to prevent the development of these highly prevalent neuropathologies in high-risk populations, while also simultaneously improving cardiovascular health.
Hypertension is a well-established risk factor for cerebrovascular disease, which is itself a major contributor to vascular dementia and AD. Hypertension in midlife has been associated with increased risk of dementia later in life
(Peters et al., 2019; Sierra, 2020). The damage associated with hypertension develops insidiously over the life course, beginning with arteriolar narrowing and microvascular changes that may develop into more significant cerebrovascular disease and an increased risk of cognitive impairment. The direct management of hypertension using antihypertensive agents (e.g., angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors, calcium channel blockers) in midlife and later has been extensively explored as a strategy for the prevention of dementia, and many observational studies have suggested that the use of antihypertensive agents has a beneficial effect on the prevention of dementia (Olmastroni et al., 2022; Petek et al., 2023; Sierra, 2020). However, results from large randomized trials have been inconsistent (Forette et al., 2002; Peters et al., 2008; SPRINT MIND Investigators, 2019), and the lack of conclusive evidence of effect from these trials may be in part attributable to the variation in blood pressure target levels and the assessment of cognitive outcomes (SPRINT MIND Investigators, 2019).
A 2021 Cochrane review concluded that there is insufficient evidence available from these trials to make a conclusion on the effect of hypertensive treatment on dementia or to inform clinical guidelines for the use of these agents to prevent dementia. Elucidating the efficacy of antihypertension interventions will likely require long-term follow-up of participants beginning in midlife and employing cognition as a primary rather than secondary outcome measure (Cunningham et al., 2021). Such efforts need to consider sex-specific differences in antihypertensive prescribing practices for males and females and potential effects on sex differences in cerebrovascular pathology and AD/ADRD (Mielke, 2024).
Chronically high levels of low-density lipoprotein (LDL) and total cholesterol in midlife, which are associated with poor cardiovascular health, have been linked to the occurrence of dementia in late life, specifically AD and vascular dementia. Hypercholesterolemia in late life does not appear to be associated with the development of AD. However, the underlying mechanisms associated with cholesterol and neurodegeneration are complex and likely involve multiple mediating factors (e.g., genetics; life stage; presence of comorbidities) (Loera-Valencia et al., 2018). As with antihypertensive agents, the protective effects of lipid-lowering medications (e.g., statins) described in observational trials (Chu et al., 2018; Olmastroni et al., 2022) have not been mirrored by results from recent RCTs. However, these trials primarily assessed the use of statins in late life in participants at risk of dementia (McGuinness et al., 2016). Understanding the control of hypercholesterolemia and dyslipidemia beginning in midlife, when its detrimental effects to brain health are thought to be most pervasive (Anstey et al., 2017), may provide new insights into the potential use of statins and or the identification of novel targets that could advance midlife preventive strategies for dementia in certain populations.
NPIs that can be broadly categorized as healthy lifestyle interventions (e.g., diet designed to reduce hypertension, physical exercise) have been assessed for their ability to prevent AD/ADRD through the targeting of modifiable risk factors that are shared with vascular health. Such interventions are discussed earlier in this chapter in the context of physical activity and multimodal NPIs.
Autophagy and mitophagy inducers
Analyses of AD/ADRD risk genes and drug targets have pointed to the key role played by impaired autophagy caused by dysfunction in the endosomal–lysosomal system in forms of dementia characterized by proteiopathies (Bellenguez et al., 2022; Caberlotto and Nguyen, 2014; Deng et al., 2017; Nixon and Rubinsztein, 2024). Autophagy is a highly conserved cellular process by which damaged proteins and organelles are delivered to lysosomes for degradation, thereby helping to maintain protein and overall cellular homeostasis (Klionsky and Emr, 2000). Mitophagy is a special case of autophagy involving the removal of excess or damaged mitochondria by encapsulation of the mitochondria in autophagosomes, which then fuse with lysosomes to create the phagocytic vacuoles in which the mitochondria are digested and degraded (Yang et al., 2024). Lysosomal dysfunction (e.g., caused by inadequate lysosomal acidification) and impaired autophagy are shared characteristics of aging and multiple neurodegenerative diseases (Nixon, 2020), as discussed in more detail in Chapter 3.
Neurons are unable to dilute cellular waste products through cell division and are therefore vulnerable to breakdowns in autophagy (Nixon, 2020), which leads to the buildup and aggregation of misfolded proteins and the accumulation of damaged mitochondria (Yang et al., 2024). There is consequently great interest in the induction of autophagy (including mitophagy) as a therapeutic strategy for AD/ADRD (Corasaniti et al., 2024; Djajadikerta et al., 2020; Eshraghi et al., 2022; Wang et al., 2021; Yang et al., 2024). Such strategies are supported by the observation that autophagy hyperactivation through genetic modification significantly decreased the accumulation of amyloid and prevented cognitive decline in AD mouse models (Rocchi et al., 2017). Importantly, autophagy induction strategies have the potential to affect multiple proteinopathies simultaneously, an advantage over single-target therapies such as monoclonal antibodies, given that mixed pathologies are found in most older people living with dementia.
Autophagy/mitophagy inducers being evaluated for effects on AD/ADRD include both small-molecule drugs and plant-derived phytochemicals, some of which are marketed and sold as dietary supplements or nutraceuticals. Several phytochemicals have shown encouraging effects on autophagy and mitophagy function in preclinical studies, both in vitro and in vivo, but with few exceptions, clinical data have yet to be generated
(Yang et al., 2024). One notable phytochemical autophagy inducer for which some clinical trial data are available is resveratrol, a polyphenolic compound found in a number of different plants but perhaps best known as a contributor to the health benefits of grapes and red wine. While effects on some biomarker trajectories were observed, larger trials are needed to evaluate changes in clinical outcomes (Jin et al., 2023; Moussa et al., 2017; Turner et al., 2015; Zhu et al., 2018). Rapamycin is another promising autophagy inducer being evaluated in phase 2 trials (Cummings et al., 2023a,b, 2024). A preclinical study showed that rapamycin induced autophagy and reduced amyloid beta levels and plaques (Chen et al., 2019).
Of note, autophagy inducers are often pleiotropic and drugs under investigation for other mechanisms of action may also exert effects on autophagy pathways, as is the case for metformin (discussed above) (Corasaniti et al., 2024; Yang et al., 2024). This yields opportunities for drug repurposing (Eshraghi et al., 2022). An important focus for future research is to elucidate the pathways by which these pleiotropic compounds exert their effects on AD/ADRD and the implications for such factors as dose and timing of treatment (Majumder et al., 2011).
Interventions to increase slow-wave sleep
Sleep has a complex, bidirectional relationship with AD/ADRD pathology and may be associated with the clearance of neurotoxic byproducts from the brain. Sleep architecture changes over the life course, with the time spent in slow-wave sleep (e.g., deep sleep) decreasing and becoming fragmented over time. For women, hormonal changes during the pre-, peri- and postmenopausal states have been shown to affect sleep (Mielke, 2024). Sleep–wake disorders are common in dementia and may play a role in worsening or accelerated cognitive and functional impairment through a variety of mechanisms, such as the accumulation of amyloid beta and tau (Wang and Holtzman, 2020). Increasing slow-wave sleep and sleep quality more broadly through the reduction of sleep disturbances has been of interest in interventions for AD/ADRD, but medications such as benzodiazepines and anticholinergics may not be effective or safe for longer-term use in older adults (Ferreira et al., 2021; Gerlach et al., 2018; Taylor-Rowan et al., 2021).
Orexin, a neuropeptide that plays a role in the regulation of multiple physiological processes, including the sleep–wake cycle (Sun et al., 2022), has been associated with increased nighttime wakefulness and sleep latency and decreased sleep efficiency in AD (Wang and Holtzman, 2020). Dysregulation of the orexinergic system may be associated with worsening cognitive decline (Liguori et al., 2014). Emerging research suggests treatments inhibiting orexin, such as dual orexin receptor antagonists, may have some effect on the improvement of sleep quality in preclinical studies (Duncan et al., 2019; Zhou and Tang, 2022) and in limited clinical studies in
older adults with insomnia (Zammit et al., 2020) and in people living with MCI or mild AD (Blackman et al., 2021). However, little is known regarding the cognitive or functional effects of orexin inhibitors. In one RCT involving people living with MCI, suvorexant, a dual orexin receptor antagonist, was found to significantly increase total sleep time and sleep efficiency as compared to placebo. However, these effects were not significant when assessed in participants living with mild AD (Blackman et al., 2021).
Bringing Interventions for Preventing AD/ADRD to Scale: Public Health and Policy Opportunities
The promising interventions discussed in the preceding sections, both pharmacological and nonpharmacological, are targeted to the individual level. Most NPIs and some pharmacologic interventions (e.g., medications for hypertension) focus on modifiable risk factors. A challenge, however, is that implementing such interventions often requires significant personal motivation, which has been a limiting factor in the success of such strategies as increasing physical activity. Moreover, some risk and protective factors for dementia originate from, and can only be addressed at, societal levels (Livingston et al., 2024). Examples include access to higher education (Xu et al., 2019)— as well as structural factors that perpetuate social disadvantage for some groups and exposure to such environmental contaminants as air pollution (Livingston et al., 2024). Often there are interactions among these factors that compound AD/ADRD risk. For example, socially disadvantaged groups are more likely to live in areas where there is greater exposure to environmental pollutants (Fairburn et al., 2019; Hauptman et al., 2023) and less opportunity for physical activity and social interaction (Finlay et al., 2022), which may contribute to increased risk for conditions that contribute to cardiovascular disease (e.g., hypertension, diabetes).
Targeting causative upstream social and environmental modifiers can thus have a broader and more significant effect on reducing AD burden than modifying proximal individual-level risk factors (Paul et al., 2019). This argues for increased attention to public health and policy population-level interventions to bring dementia prevention to scale. Examples of such interventions include public health campaigns to raise awareness of the importance of reducing cardiovascular disease risk for AD/ADRD prevention, programs to address nutritional deficiencies, neighborhood revitalization initiatives, programs such as Experience Corps that foster intergenerational exchange (AARP, 2024), and standard-setting policy actions to reduce environmental exposures. Additional important policies target social determinants of health, such as lifelong educational activities, food security, financial vulnerability, healthy housing access, workplace safety, retirement policies, and opportunities for older adults to continue
to meaningfully engage in community activities. Importantly, beyond the reduced risk of dementia and benefits to overall health, prevention strategies may delay dementia onset to later in life, resulting in an expansion in the number of healthy years of life and a compression in the amount of time living with disease (Livingston et al., 2024).
In some cases, efforts to implement such population-level strategies are already underway in the context of other public health threats or social needs and could be additionally framed as potential strategies preventing AD/ADRD. Although the mechanisms are not always well understood, the decline in age-specific dementia incidence in high-income countries such as the United States suggests that some approaches have already yielded success (Farina et al., 2022; Wolters et al., 2020). Improved understanding of the contributors to the observed decrease in dementia incidence, quantifying the role of changes in cardiovascular risk profiles and educational attainment for example, may open opportunities to accelerate prevention efforts and to better use public health investments not specifically targeted to AD/ADRD (e.g., initiatives aimed at improving cardiovascular health). Importantly, such efforts can be pursued now even in the absence of a complete understanding of the underlying biological mechanisms that mediate the effects of public health strategies. However, motivating the implementation of population-level interventions, particularly those with far-reaching consequences such as policy changes will require a robust evidence base. For example, compelling evidence causally linking specific environmental pollutants to increased incidence and/or earlier onset of AD/ADRD (and other neurodegenerative diseases) will likely be needed to persuade policy and law makers to change standards and to identify pollutant sources that can be controlled by regulation (Kilian and Kitazawa, 2018). There is generally a lack of evidence on the social and environmental exposures to target to enhance resilience and prevent the development and progression of AD/ADRD pathology.
As noted earlier, there is a paucity of data on the effectiveness of population-level strategies from intervention studies, likely caused, in part, by the challenges of rigorously studying these strategies. Going forward, there are new research opportunities to incorporate blood-based biomarkers into studies investigating population-level interventions. This will allow for the stratification of results based on the presence of pathological changes and permit the evaluation of an intervention’s effect on the risk of developing symptoms.
Conclusion 4-3: A holistic approach to dementia prevention, involving policies that foster quality education, health-promoting environments, and overall health improvement, is crucial and goes beyond the research-focused institutes. This is particularly true for low-income residents,
where prevention potential is high. These populations often grapple with resource, infrastructure, and funding challenges. Concurrently, research dedicated to public health initiatives aimed at monitoring success of dementia prevention is essential.
OPPORTUNITIES TO IMPROVE CLINICAL TRIALS FOR AD/ADRD
Improving Representativeness and External Validity Through Inclusive Clinical Trials
AD/ADRD is observed across all demographics. Therefore, any study aiming to better understand, prevent, or treat AD/ADRD should recruit and retain as diverse a population as possible. Limiting a study sample to a single demographic increases the likelihood that any effect observed represents an artifact of that sample instead of a feature of the disease; such design problems result in selection bias. From a scientific and clinical perspective, to fully understand and mitigate the complex and heterogeneous mechanisms underlying AD/ADRD pathology, it is necessary to design clinical trials that include diverse participant populations. For example, unexplained observations that could shed light on AD/ADRD etiology include the higher prevalence of AD/ADRD among African American and Hispanic individuals, and yet, these groups are consistently underrepresented in both AD/ADRD observational studies and randomized clinical trials (ADNI, 2012; Faison et al., 2007; Raman et al., 2022). In aducanumab trials, for example, only 3.2 percent of trial participants were of Hispanic/Latino background while a mere 0.6 percent of participants were African American (ICER, 2021). The lack of diversity across trials that are modeling disease progression or assessing the efficacy of interventions can result in biased or inaccurate findings that have low external validity, or generalizability, and can further exacerbate existing disparities in AD/ADRD health outcomes. Beyond race and ethnicity, other aspects of diversity that need to be better represented in clinical trial populations include sex and geography (e.g., rural, suburban, and urban), as well as socioeconomic status and social phenotypes.
One strategy to improve the applicability of AD/ADRD trial data to more heterogeneous populations is to modify the exclusion criteria used when recruiting trial participants. In many cases, researchers have relied on arbitrary exclusion criteria carried over from previous similar studies with very little to no justification for continued use (FDA, 2018; Indorewalla et al., 2021). Moreover, studies have shown that differential exclusion criteria based on race and ethnicity have been one significant barrier to entry for minority populations in AD/ADRD clinical trials (Raman et al., 2021). The screening criteria for trial participation, particularly among trials examining monoclonal antibodies, are also very restrictive when it comes to
prioritizing patients with high levels of amyloid deposits in their brain, and this requirement often results in the exclusion of people with less observable pathology (Reardon, 2023).
Although there are reasonable rationales for excluding people with comorbidities, such as diabetes and hypertension, and mixed pathologies from some trials, ultimately these are also critical elements of diversity needed to evaluate the potential of interventions for real-world effectiveness. Careful consideration will be needed to address such deficiencies and to incorporate participant populations that are multidimensionally diverse into future trial designs.
Trial participants are also often excluded from studies if they are already participating in other clinical trials (Myles et al., 2014). While such exclusions may be justified, strategies need to be explored to maximize the involvement of these (presumably highly engaged) participants once they become eligible. One such strategy may involve the creation of a database of eligible volunteers that can be used to track current involvement in clinical trials in real time so they can be notified of opportunities once their participation ends. Similar challenges arise with the exclusion of individuals who lack a care partner or caregiver or those who are excluded due to surpassing arbitrary age cutoffs (Mitchell et al., 2024). Thus, there is a need to rethink the exclusion criteria currently being employed in AD/ADRD clinical trials to ensure that researchers are not inadvertently excluding individuals who could both benefit from and contribute to the success of any given trial.
Efforts to increase recruitment of representative clinical trial participants would benefit from mechanisms that encourage a seamless transition from AD/ADRD diagnosis to trial recruitment. As such, trained primary care providers could play an important role in recruitment by informing patients of available study opportunities or by requesting their consent to be notified about future AD/ADRD research initiatives. Another approach could involve presenting an option to receive trial information and invitations to contribute to research at the time of Medicare or retirement benefits enrollment (Cummings, 2024). Mechanisms to enroll clinical trial participants from existing longitudinal observational studies may also help improve representativeness and external validity, particularly as cohorts become more diverse, although such practices need to be carefully orchestrated to ensure there is no risk to the integrity of the cohort study. A notable example comes from ALLFTD, a longitudinal study designed to help researchers better understand the natural history of frontotemporal lobar degeneration (both sporadic and autosomal-dominant forms) through the collection of clinical, neuropsychological, neuroimaging, and biofluid data (Boeve et al., 2020). Not only was the study designed to inform future clinical trials, but study participants may also be recruited into trials that evaluate potential disease modifying therapies or other interventions.
Providing research participants with increased ownership and decision making when it comes to choosing which studies they would like to participate in, instead of relying on study sites to make these decisions, can be another means to improve clinical trial recruitment and potentially the diversity of participants (Smith, 2024). Providing persons living with AD/ADRD and their caregivers and care partners with plenty of opportunities to ask questions, reiterating important trial details throughout the entirety of their involvement, as well as clearly communicating the risks and benefits of participation, are all strategies that can help people feel more empowered to make informed decisions and reduce some of the uncertainties and anxieties surrounding clinical trial enrollment (Connell et al., 2001; Indorewalla et al., 2021; Yancey et al., 2006).
To be successful in recruiting diverse study populations, it is important for AD/ADRD researchers to build long-term reciprocal relationships with communities, particularly those that have been traditionally excluded from research studies. Making use of community-based participatory research approaches can help with relationship building (NASEM, 2024). Researchers can also form working relationships with community-based primary care providers in order to receive referrals of patients who meet their study criteria (Heller et al., 2014; Indorewalla et al., 2021). Other opportunities to better engage with diverse communities include embedding trial sites within community settings (i.e., decentralized trials) and including people from those communities in the trial leadership. This could be done, for example, by partnering with federally qualified health centers and community health resource centers.
NIH has invested in developing and testing approaches to build community collaborations as a means of increasing participation in clinical research by diverse and underrepresented populations (NIA, n.d.). Funded efforts have included several research collaborations focused on testing approaches for, and building research partnerships with, priority populations. For example, the NIA-funded Engaging Communities of Hispanics/Latinos for Aging Research Network assesses barriers to research participation and builds collaborations between health centers, community-based organizations, residents, and researchers within Hispanic and Latinx populations in three large metropolitan areas (ECHAR, 2024).
Decentralized trials can use a hub-and-spoke model to allow the centralization of some core infrastructure while reducing barriers to trial participation by enabling data and sample collection at the embedded sites in diverse communities (Cummings, 2024). The Alzheimer’s Clinical Trials Consortium, for example, uses this model to encourage collaboration between the “hub,” which consists of leadership with extensive expertise in AD/ADRD research methods, and the “spokes,” which include site recruitment and engagement teams operating on the ground, with the ultimate
goal of enhancing community partnerships and increasing the participation of underserved populations in AD/ADRD clinical trials (Raman et al., 2022). Some aspects of participant screening (e.g., blood collection and testing), for example, could be conducted at community sites near residential areas and those who pass screening could then be referred on to trial sites.
Going one step further, trials are increasingly moving the interface with participants, including data and sample collection, to participants’ homes, which may address barriers to access for underrepresented participant populations. Digital health technologies are making such approaches more feasible. For example, a recent study demonstrated the feasibility of using a smartphone app to collect data on cognition, speech and language, and motor function in participants living with FTD (Taylor et al., 2023). However, such tools require careful evaluation for reliability and appropriate contexts of use given that people living with AD/ADRD may increasingly struggle with some data collection tasks as their disease progresses. Remote data collection also has the potential to reduce the level of effort required for caregivers or care partners who otherwise may have had to accompany the study participant to trial sites since the availability of a care giver or care partner is a common condition of participation for safety reasons.
In addition to collaborating with community members to advance AD/ADRD research goals, investigators can also encourage the sharing of participant experiences in past clinical trials through targeted education efforts in order to mitigate fears surrounding trial participation and promote the potential benefits (Clement et al., 2019; Indorewalla et al., 2021). By using these strategies and prioritizing inclusivity when designing clinical trials, researchers can better capture the heterogeneity of AD/ADRD disease mechanisms and develop interventions that can benefit more diverse populations.
Conclusion 4-4: The elimination of barriers to increasing clinical trial accessibility and reducing disparities in research participation decreases selection bias, improves the efficiency of clinical trials, and enhances the external validity and generalizability of trial results.
Improving the Evaluation of Interventions
Numerous factors contribute to the complexity related to assessing the effects of interventions and understanding the observed variability in intervention effects over time and in diverse populations. Population heterogeneity (e.g., genetic and epigenetic differences, comorbidities, sex and gender differences) needs to be considered and may be better understood by stratifying results by population group, such as by gender or genetic risk. The routine provision of sex/gender-disaggregated results from
clinical trials, for example, is important for understanding differences in the efficacy of interventions and may help to guide precision medicine approaches. Other contributing factors include the different measures and instruments currently used to assess outcomes and the challenges related to the complexity of interventions and the contexts in which they are implemented. Given these complexities, the ability to better integrate different data—genetic variants, biomarkers, and clinical endpoints, including both short- and long-term outcomes—will enable improvements in the evaluation of interventions.
As discussed earlier in this chapter, the evaluation of interventions needs to consider not just efficacy, but also other benefits (e.g., reduced costs of care, ability to continue working or living independently) and harms. Cost-effectiveness studies and the incorporation of pharmacoeconomic outcomes (e.g., episodes of hospitalization or other acute care) into late-stage clinical trials can help to generate data needed to understand the balance of benefits and harms for a given intervention (Fillit et al., 2010). However, novel frameworks are needed that move beyond traditional health economics measures of health care costs and quality of life. One such framework, the Value Flower, developed by the 2018 International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Special Task Force on US Value Assessments (Neumann et al., 2022), can guide new measures and data collection to quantify often overlooked elements of value, such as productivity, adherence-improving factors, reduction in uncertainty, real option value, value of hope, and scientific spillovers, among others.
Measuring Responses to Interventions
A notable gap related to assessing AD/ADRD interventions is the lack of consensus on what success looks like. Depending on the disease state of the population under study (e.g., preclinical, symptomatic, late-stage disease) and the objective of the intervention (prevention versus treatment), efficacy can be measured in different ways and commonly relies on the use of measures related to cognitive and functional outcomes, as well as changes in markers of pathobiology. For preventive strategies, risk-reduction outcomes as measured, for example, by risk scores that quantify risk and protective factors, provide a means of evaluating the intervention in the absence of clinical symptomology. Such approaches are particularly relevant for trials of NPIs targeting modifiable risk factors (Deckers et al., 2021; Hall et al., 2024; Yaffe et al., 2024).
A well-recognized challenge in evaluating AD/ADRD interventions is the variability in measures and instruments used to assess cognitive and functional status of study participants. Moreover, cognitive impairment exists on a spectrum and can be modifiable in real time, thus challenging
the ability to report or measure cognitive change, especially when study subjects may be slightly impaired compared to those who are at more advanced stages of cognitive decline. Neuropsychological assessments (e.g., Mini-Mental State Examination, Montreal Cognitive Assessment) are advantageous in that they do not require lengthy assessments by specialized clinicians and provide a numerical measure of cognitive function, but such tests have well-known limitations (Ritchie et al., 2015), as discussed in Chapter 2. Additionally, the multiplicity of test batteries that are used across AD/ADRD intervention studies complicates efforts to compare different study results in meta-analyses and impedes pooled analyses (NASEM, 2017).
Functional outcomes are often identified as measures of intervention effectiveness that are important to people living with AD/ADRD and their caregivers and care partners. However, similar to cognitive measures, there is variability in the ways that functional outcomes are assessed, and instruments such as the Instrumental Activities of Daily Living scale rely on reporting by study participants or their caregivers or care partners, which is subject to bias (Ritchie et al., 2015). Moreover, tools used to evaluate functional change in later dementia stages may not be sensitive enough to detect more subtle changes in function that may be observed in earlier stages of AD/ADRD (FDA, 2024a; NIA, 2024).
For related dementias, a notable challenge with the evaluation of interventions in clinical trials is the lack of validated, disease-specific outcome measures. For example, primary end points used in LBD trials are often AD-centric, using such scales as the AD Assessment Scale–Cognitive Subscale and the AD Cooperative Study–Clinical Global Impression of Change to measure cognitive function (Goldman et al., 2020). Moreover, the overemphasis on cognitive endpoints in clinical trials has contributed to the paucity of trials capturing other core or supportive features of LBD, such as visual hallucinations, REM sleep behavior disorder, autonomic symptoms, or motor signs of parkinsonism (Goldman et al., 2020). Improving clinical trials for related dementias will require the validation of outcomes in the target population.
For interventions that require regulatory approval (e.g., drugs and biologics), FDA historically has required demonstration of clinically meaningful effects. For interventions tested after the onset of dementia, this generally has entailed reporting on outcomes for both cognitive and functional measures, since small changes in cognitive outcomes measured with existing test batteries are not inherently considered to be clinically meaningful (FDA, 2024a). However, the shift in intervention strategies to initiate therapies at the earliest stages of disease, even prior to the development of any clinical symptoms, has necessitated reconsideration of approaches to evaluate interventions and to show clinically meaningful benefit. In early disease
stages, clinical changes may be subtle and challenging to capture using tools developed for evaluating interventions in later-stage patients (Boxer and Sperling, 2023). Recognizing this need, FDA has developed guidance on drug development for the treatment of early AD (prior to onset of clinical dementia) that builds on a staging system for the disease. The most recent version of the FDA’s draft guidance was released in March 2024 (FDA, 2024a). Similar guidelines are not available for other forms of dementia.
One evaluation approach for studies in early disease stages is to extend clinical trial durations to allow a time-to-event analysis for which time to a clinically meaningful event (cognition and/or function) can be compared between treatment groups. This could, for example, entail the evaluation of time required for conversion from preclinical to symptomatic disease or, for study populations that are already symptomatic, conversion from MCI to clinical dementia. It is important to keep in mind, however, that such clinical progression is not inevitable in the absence of intervention (Ritchie et al., 2015) and could require trials to have remarkably long follow-up periods in cases where onset of clinical symptoms may not be expected for years or even decades. For instance, even in studies enrolling only high-risk populations, evaluation of the preventive effects of anti-amyloid antibodies requires more than 4 years of follow-up owing to the variation in the rates at which individuals identified as having preclinical disease begin to show signs of cognitive decline. Long follow-up periods that extend the length of clinical trials can add substantially to the cost. Therefore, careful balancing of cost and length need to be considered during the design phase.
When clinically meaningful benefit of an intervention is demonstrated based on changes in cognitive measures relative to the comparison group, a stronger case can be made when there is evidence of benefit across multiple neuropsychological tests and when there are concomitant changes in characteristic pathophysiologic features (FDA, 2024a). FDA has indicated that it will consider surrogate endpoints that are “reasonably likely to predict clinical benefit” for the purposes of accelerated approval but emphasized the need for postapproval trials to “verify and describe clinical benefit” (FDA, 2024a, p. 6). This approach was used in the approval of two anti-amyloid antibody-based therapies for AD, aducanumab and lecanemab, with a biomarker—reduction in brain amyloid beta as measured by PET—serving as a surrogate endpoint for prediction of clinical benefit. This approach, while controversial (particularly as related to aducanumab approval), demonstrates the importance of the selection of appropriate surrogate endpoints, which will depend on the disease stage of study participants at the time of trial initiation and the mechanism of action for the intervention (FDA, 2024a). Such decisions by study sponsors are also likely to be influenced by current guidance from regulatory authorities (Yu et al., 2022).
Identifying biomarkers for demonstrating target engagement and measuring treatment response
As discussed in Chapter 2, biomarkers can serve multiple purposes in the context of AD/ADRD research. In addition to being used for clinical diagnosis (diagnostic biomarkers), determination of clinical trial eligibility (screening biomarkers), and guiding treatment decisions (prognostic biomarkers), biomarkers can also be used to demonstrate target engagement and to assess the response to an intervention (response biomarkers) (Cummings, 2019; Hampel et al., 2019). Given that large, long-duration studies may be needed to demonstrate that a disease-modifying therapy is engaging the intended target and modifying disease progression, biomarkers can play an important role in phase 2 clinical trials to demonstrate proof of concept prior to initiating trials intended to demonstrate clinical benefit (Cummings, 2019; Cummings et al., 2018). Without the ability to demonstrate target engagement, it can be challenging to know why a trial failed.
A notable impediment to drug development is the lack of biomarkers that could be used to demonstrate target engagement for many of the mechanistic pathways that might be targetable to prevent or treat AD/ADRD. Such biomarkers may need to be developed in conjunction with a candidate therapy to more readily establish clinical efficacy (Cummings, 2019). As noted in the preceding section, biomarkers may also be important intermediate or surrogate endpoints for phase 3 clinical trials testing the efficacy of an intervention in study populations that have not yet developed signs of AD/ADRD or who are in early stages (e.g., preclinical). Even when trials in academic settings are not intended to support regulatory approval of an intervention, the 5-year grant cycle may necessitate intermediate outcomes that can be measured before clinical endpoints may be reasonably expected.
While amyloid-PET is a frequently used response biomarker that has already been incorporated into regulatory approval processes for AD evaluation and treatments, the identification of additional response biomarkers is an active area of investigation. For AD, brain tau levels as measured by PET are also being evaluated as a response biomarker, but the evidence from past clinical trials of anti-amyloid antibody treatments regarding the ability of tau-PET to reflect clinically meaningful intervention effects has been mixed (Aisen et al., 2021; Boxer and Sperling, 2023). Also under development is a PET tracer for synaptic loss, which is not AD specific and may be more easily linked to meaningful cognitive and functional outcomes than biomarkers for other pathologic features such as plaques and tangles (Gregory et al., 2022; Márquez and Yassa, 2019). Disease-nonspecific biomarkers that closely correlate with clinical outcomes may be particularly beneficial for the study of mixed forms of dementia (Toledo et al., 2023).
Given the logistical difficulty and significant cost of PET scans, there is great interest in fluid-phase biomarkers of intervention response (Boxer
and Sperling, 2023). CSF biomarker levels are reflective of pathophysiologic processes in the central nervous system (Abdelmoaty et al., 2023), and CSF levels of amyloid beta and phosphorylated tau (p-tau)2 have been monitored in trials of anti-amyloid treatments (Cummings, 2019). However, collection of CSF poses its own logistical challenges. Blood-based biomarkers, which would be less invasive, have the potential to be useful tools for measuring intervention responses. Plasma levels of p-tau have been shown to be sensitive to the effects of anti-amyloid antibody treatments, although reductions in plasma p-tau181 and p-tau217 have been observed in clinical trials following treatment even in the absence of observed clinical benefit (Boxer and Sperling, 2023), indicating a need for further investigation of the ability of these biomarkers to predict clinically meaningful change during clinical trials. Such discrepancies also reinforce the need to study endpoints of disease over extended periods in ways that more realistically capture the clinical progression of study participants.
As is the case for diagnostic biomarkers (discussed in Chapter 2), the identification of response biomarkers for LBD, FTD, and other related dementias lags behind the progress made for AD, and this represents an area of unmet need (Abdelmoaty et al., 2023; Del Campo et al., 2022). While useful for LBD diagnostic purposes, alpha-synuclein seed amplification assays are not currently well suited for predicting or tracking disease progression (Vijiaratnam and Foltynie, 2023). It is therefore not clear whether they will be useful as measures of treatment response. In the absence of disease-specific response markers, biomarkers of neurodegeneration, which would be expected to change in response to disease-modifying therapies, may be of use in the evaluation of interventions for AD/ADRD. CSF total tau, atrophy on MRI, fluorodeoxyglucose (FDG) PET (which measures brain metabolic activity), neurofilament light chain (NfL), neurogranin, and visinin-like protein-1 (VILIP-1) are all potential biomarkers of neurodegeneration; as they are not specific to AD, they may be useful for evaluating interventions for related dementias (Abdelmoaty et al., 2023; Boxer et al., 2020; Cummings, 2019). Given that inflammation is a common shared feature of AD/ADRD (as discussed in Chapter 3), inflammatory markers are also being investigated as potential response biomarkers (Abdelmoaty et al., 2023). This approach could be broadened to consider other metrics of accelerated or premature aging (López-Otín et al., 2023).
While not required for use in clinical trials to evaluate the effects of interventions, qualification of response biomarkers through established FDA processes could help to accelerate the development of effective interventions
___________________
2 Tau, a microtubule-associated protein that plays an important role in intracellular transportation is thought to become hyperphosphorylated during the formation of neurofibrillary tangles, resulting in p-tau (Cummings, 2019).
for AD/ADRD (Cummings, 2019). A biomarker that has been qualified for a specific context of use can be relied upon to have a specific interpretation (FDA, 2020a,b), thus reducing the uncertainty that is currently involved in interpreting biomarker results from clinical trials.
While useful as intermediate outcomes and for early-response monitoring, a key issue with biomarkers is the lack of a direct link to functional or quality-of-life outcomes. The ideal measure would be one that can link interventions to cognitive changes. Further efforts are needed to better integrate molecular and clinical biomarkers.
Conclusion 4-5: The lack of validated mechanistic biomarkers, beyond amyloid and tau, that can confirm that a pathway has been engaged by an intervention is compounded by the lack of markers that can act as a surrogate endpoint for assessing the efficacy of an intervention and limit the capacity of clinical trials to rapidly evaluate interventions.
Importance of person-centered outcomes
The effectiveness of an intervention should be considered and defined in relation to what is important to those living with (or at risk for) the disease (DiBenedetti et al., 2020; Paulsen, 2024). While changes in biomarker levels thought to reflect changes in the underlying disease pathology have been used as a primary measure of successful treatment (e.g., as with anti-amyloid therapies), such outcomes may not necessarily correlate with the goal of actually helping people living with AD/ADRD along with their family members and care partners. Furthermore, many neuropsychological tests that are currently employed to assess disease severity may not accurately capture the priorities of people living with the disease, particularly regarding emotional and psychological effects (Hartry et al., 2018; NIA, 2024).
An analysis conducted by Harding and colleagues (2020) of over 350 outcome measurement instruments used in dementia research demonstrated that none of these tools had sufficient face validity to adequately capture the needs and priorities of people living with dementia and their care partners and caregivers. For this reason, having a thorough understanding of what matters most to people living with AD/ADRD can inform the development of interventions that better address their quality of life and well-being (Jessen et al., 2022).
The use of qualitative or ethnographic studies to obtain narratives from people living with AD/ADRD is one way to highlight individual experiences with these diseases and to uncover the treatment outcomes they value most (Saunders et al., 2021). According to one of the few qualitative studies that have examined patient-centered outcomes for AD, improving cognitive changes characterized by worsening memory and forgetfulness was the most important outcome for study participants, followed by stopping
or slowing the progression of the disease, and improving the ability to function on a day-to-day basis (DiBenedetti et al., 2020). Moreover, the emotional and social effects of AD, including increased feelings of frustration and stress, along with decreased outgoingness, was reported by most participants to be the most detrimental (DiBenedetti et al., 2020). Another study uncovered that, along with cognitive and functional outcomes, additional priorities for people living with AD include the desire to maintain their quality of life along with their identity and personality, recognizing the effect of mental health, and maintaining a healthy patient–caregiver relationship (Jessen et al., 2022; Tochel et al., 2019). In addition, the ability to remain at home or in community settings instead of receiving care at an in-patient institution could also improve disease trajectory and reduce economic costs at the individual or family levels and for society at large.
The push to have more representation from those with lived experience in determining dementia research priorities and outcomes has been rapidly growing in recent years, both from patient-advocacy groups and from the scientific community. Bechard and colleagues (2022), for example, found that almost all researchers surveyed advocated for the value of people with lived experience contributing to dementia research. However, the support for including the views of those living with AD/ADRD conceptually has not been translated adequately into practice because of a lack of prioritization along with a range of methodological challenges that accompany the engagement of this specific population in research (ASPE, 2017).
There have been active efforts internationally to overcome some of these challenges and better incorporate the voices of those with lived experience into AD/ADRD research. Alzheimer’s Europe, along with other government and charitable organizations, have emphasized the right of people living with AD/ADRD and caregivers and care partners to be included in dementia research (Alzheimer Society of Ireland, 2017; Gove et al., 2018; Miah et al., 2019). Moreover, the UK’s National Institute for Health and Care Research has provided guidance to researchers on engaging patients and the public in their research, including guidance related to coproducing a research project with members of the public along with payment for research participation (NIHR, 2019). Thus, codesign approaches and funding requirements are mechanisms that can better ensure the inclusion of outcomes that matter to those most affected.
Given the cognitive impairment resulting from AD/ADRD, people living with these diseases can struggle to articulate what really matters to them. For this reason, research tools used to engage individuals living with AD/ADRD need to be employed at different stages of disease and address the diversity of unique perspectives and experiences. The use of study partners could be one way to help people living with AD/ADRD monitor and report on various cognitive, functional, and emotional outcomes to ensure that
the wide breadth of the experiences of this population is fully captured. To enhance the benefits of using study partners in research, sponsors and investigators should work toward addressing the barriers for their participation and properly compensate them for their labor (Largent and Lynch, 2017). Caution is needed, however, when it comes to relying on subjective observations as primary data points; for example, men and women may report differently about a spouse suffering from cognitive decline (Stites et al., 2023). The efforts to prioritize person-centered outcomes should therefore have these considerations at the forefront to ensure that the voices of people living with AD/ADRD are incorporated in research that is both rigorous and representative of those most affected.
Evaluating longitudinal outcomes
Outcome measurement in dementia research can be challenging when conducting multiple or long-term assessments. Many neuropsychological tests measuring memory, for example, do not work well when used repeatedly, particularly over shorter time intervals (Lim et al., 2022). Using the same measurements numerous times over an extended period can result in unwanted noise and impair the assessment’s ability to accurately capture the desired construct. Moreover, the repeated use of the same instruments can result in improved performance owing to practice effects, thereby compromising the ability to effectively measure disease trajectory (Calamia et al., 2012; Duff et al., 2017; Lim et al., 2022)—although lack of a practice effect could be interpreted as an indicator of a cognitive issue (Öhman et al., 2021). The use of novel digital assessment tools can help overcome some of these challenges. Mobile devices, for example, can potentially provide more robust and reliable information over long-term application compared to traditional assessments (Öhman et al., 2021; Sliwinski et al., 2018). In addition, computerized or algorithm-based measures that can automatically generate alternative items or forms can help reduce the effect of practice or version effects (Miller and Barr, 2017; Öhman et al., 2021), while artificial intelligence methods can also be employed to better analyze cognitive data (Laske et al., 2015).
Given the long-term course of AD/ADRD and the numerous challenges associated with running clinical trials over an extended time period, researchers often have very little knowledge of what happens after a trial ends, including the degree to which participants continue to adhere to interventions. This knowledge gap is a persistent issue that extends to intervention approaches for many health conditions (NIH, 2021) and impedes efforts to understand how long-term maintenance affects outcomes such as the prevention or delay of disease onset. Generally, the more complex or demanding an intervention is, the harder it is for participants to consistently adhere to the regimen (Coley et al., 2019). Moreover, conducting research with people living with AD/ADRD presents an additional layer
of complexity given that cognitive status moderates adherence; thus, individuals with more advanced cognitive impairment will likely have more difficulty adhering to an intervention (Smith et al., 2017).
There have been numerous past efforts to characterize and improve patient adherence to AD/ADRD interventions. Tullo and colleagues (2023), for example, examined the effect of personal and demographic factors such as age, gender, cognitive ability, and personality, along with the influence of the spacing and consistency of training sessions, on adherence to a cognitive training regimen among adult participants. The study found that none of these variables reliably predicted compliance with cognitive training exercises, suggesting the limited and inconsistent role of individual difference factors in modulating adherence. Turunen and colleagues (2019) similarly investigated the long-term adherence of older adults to computerized cognitive training and found that familiarity with computers, having a spouse or roommate, better memory performance, and a positive outlook toward the study predicted an increased likelihood of beginning the training regimen; however, only previous computer use was associated with higher completion rates of the cognitive training exercises. Other studies have pointed toward building agency and encouraging participant engagement as critical components of intervention design (Yaffe et al., 2024). Furthermore, incorporating motivational features into the intervention to build self-efficacy (Jaeggi et al., 2023), increasing gamification (Koivisto and Malik, 2021), and capitalizing on a patient’s existing support system by having caregivers and care partners send personalized reminders and conduct regular check-ins can also be very beneficial for intervention adherence.
Given the limited follow-up period of many traditional clinical trials, it can be expected that additional data regarding long-term outcomes from interventions may be generated after the intervention has been implemented in real-world settings. Consequently, establishing systems to collect evidence on interventions that have been implemented in community and/or health care settings can help assess their real-world effectiveness in addition to their external validity. For example, the Alzheimer’s Network for Treatment and Diagnostics (ALZ-NET), a voluntary health care provider-enrolled network established in 2021 and led by the Alzheimer’s Association, is an initiative that will collect real-world data from clinical practice into a registry (Alzheimer’s Network, 2024; Carillo, 2024; NIA, 2022). This initiative will generate data to further evaluate the usage, effectiveness, and safety of FDA-approved therapies for AD. Registries and other real-world data collection platforms have the potential to significantly enhance the ability to evaluate interventions longitudinally and to elucidate the effects of such factors as social determinants of health and polypharmacy on intervention effectiveness. Partnership with advocacy organizations has the potential to improve such real-world data collection platforms developed in the future,
but realizing their potential will require overcoming numerous barriers to collecting and using real-world evidence, including siloed data sources, need for data harmonization, and privacy and data security concerns (NIA, 2022).
For decades, patient registries, which systematically collect and store uniform data on patients with a specific disease, condition, or procedure, have informed public health priorities, programs, and spending, as well as basic science and clinical and social science research (AHRQ, 2014). In the United States, cancer and kidney disease registries and, more recently, vaccination registries have been particularly effective along these dimensions. Despite the potential value of registries, it is only recently that registries focused on AD/ADRD were established. The dementia registries that do exist differ in their goals, the populations represented, the types of data collected and/or linked to, their geographical coverage, and how they are funded and sustained.
Recent and ongoing advances in AD/ADRD prevention, diagnosis, and treatment further underscore the importance of dementia registry data. Scientific advances are reshaping prevention strategies. Technological innovations are changing interactions with medical care. The health care system is exploring improved care delivery methods, and ongoing research is deepening insights into how social determinants of health affect dementia risk. New AD treatments are entering the market. With linkages to other datasets, a population-based dementia registry can support efforts to monitor these changes and assess, for example, who has access to new therapies and conduct postmarket surveillance to establish the effectiveness and safety of these treatments in real-world settings. The Swedish Dementia Registry (SveDem), which was initiated in 2007 and covers most of Sweden, is an example of a population-based registry that is informing researchers, policy makers, patients, and the public about current dementia treatment and care in Sweden. SveDem’s annual reports provide knowledge about diagnostics, medical treatment, and community and social support (Religa et al., 2015).
Monitoring Safety Outcomes
Adverse event monitoring is a critical part of the evaluation of novel and investigational therapies and extends beyond the clinical trial phase of research into postmarket surveillance. Adverse events can take myriad forms, both physical and psychological. Examples of adverse events associated with AD/ADRD treatments include gastrointestinal ulceration and bleeding, cardiovascular conditions such as severe sinus bradycardia, worsening neuropsychological symptoms, and in some extreme cases can lead to hospitalization and death (Khoury et al., 2018; Ruangritchankul et al., 2021). Current FDA-approved anti-amyloid antibody therapies for AD can result in adverse events that can be detected through magnetic resonance imaging (MRI) in the form of amyloid-related imaging abnormalities
(ARIA) (Withington and Turner, 2022). The imaging abnormalities have been linked to microhemorrhages (ARIA-H) and the leakage of fluid from blood vessels in the brain, which causes the collection of the fluid in the interstitial spaces and localized edema (ARIA-E). ARIA risk increases with drug dose, thus raising the potential for a tradeoff between safety and efficacy that needs to be considered in development of dosing regimens (Boxer and Sperling, 2023). In most cases ARIA is asymptomatic, but for some patients it can have serious health effects and in rare cases is fatal (Solopova et al., 2023; Sperling et al., 2011; Withington and Turner, 2022). As referenced later in the chapter, risk of ARIA is known to be increased among those with the APOE4 allele, a group that is also at higher risk for AD (Sperling et al., 2011). These combined factors complicate the assessment of risks and benefits in the use of current anti-amyloid therapies.
Mitigating the harms associated with potentially life-threatening adverse events requires effective mechanisms to carefully monitor and address their effects. Biomarkers such as liver function, muscle enzymes, and electrocardiograms can play a key role in indicating drug toxicity and have been used in the development of disease-modifying therapies for AD/ADRD (Cummings, 2019). The use of MRI to monitor the presence of ARIA has also been essential to monitoring safety outcomes for treatment with anti-amyloid therapies (Cummings, 2019). Scheduling multiple MRIs in regular intervals during the first month of drug exposure along with subsequent monitoring if ARIA symptoms are detected is one strategy employed to prioritize safety, both in clinical care and throughout the course of a trial (Cummings and Kinney, 2022). In addition, researchers and care providers can use blood tests and physical examination, along with future markers and direct reports from patients, to evaluate the safety and tolerability of AD/ADRD medication (Cummings et al., 2018).
Beyond the physiological adverse effects that can accompany pharmacologic treatments, there are additional safety considerations related to patient privacy and the stigma surrounding an AD/ADRD diagnosis (see Chapter 2). The need to maintain participant privacy and confidentiality in AD/ADRD research is especially prominent with the increased use of mobile applications in combination with artificial intelligence and machine learning approaches to report and monitor clinical symptoms and other pertinent health information. Without proper regulation, the use of these digital tools could lead to individuals having their data sold or misused and could even result in discrimination by insurance companies and employers (Anthes, 2020; Piendel et al., 2023). A review of 83 mobile health applications for neuropsychiatric conditions, including AD and dementia, found a significant lack of policies protecting patient privacy along with deficient HIPPA regulations regarding use of the data (Minen et al., 2021; Piendel et al., 2023). Thus, the continued use of these technologies to aid research
efforts needs to be accompanied by increased guardrails around their use to ensure that the privacy of patients and research participants is not compromised.
Understanding the Effects of Multicomponent Interventions
There is increasing interest in multimodal and combination interventions that have the ability to simultaneously or sequentially target multiple etiologies or risk factors and thereby prevent or delay the onset of dementia. In previous studies, a factorial study design has been used to elucidate the value of a multicomponent intervention over a single-component intervention for enhancing treatment outcomes (NASEM, 2017). However, there is an urgent need to also identify strategies that help determine which components or combinations of components contribute to notable benefits, whether that be maintaining overall brain health or slowing the progression of disease. A multiphase optimization strategy may be useful for this purpose when designing and implementing an intervention (Strayhorn et al., 2024).
When evaluating the effect of a multicomponent intervention using traditional methods, such as a two-arm RCT, the efficacy of each individual component is not estimated empirically (Strayhorn et al., 2024). Since the intervention components are combined as a package and evaluated together in these trials, this creates notable limitations when attempting to modify the interventions to make them more scalable, affordable, and efficient. A multiphase optimization strategy, however, enables researchers to assess the contributions of individual intervention components by allowing them to identify from a set of components the combination that best demonstrates effectiveness, affordability, scalability, and efficiency (Collins, 2018; Strayhorn et al., 2024). This strategy employs multiple phases of research, beginning with an optimization phase during which components are assessed individually and in combination and decisions are made about which combination to move forward. Then, in a subsequent evaluation stage, their combined efficacy is assessed, typically in a RCT. Thus, by using similar innovative decision-making strategies, AD/ADRD researchers can better understand the interactions between different components of a multimodal intervention, and be better equipped to tailor intervention approaches to meet the needs of a diverse population.
Accelerating Decision Making Within the Clinical Trial Pipeline
In the last 20 years, only three drugs—aducanumab, lecanemab, and donanemab—have been approved by FDA for the treatment of AD (Cummings et al., 2023a; FDA, 2024b), and the modest clinical benefits
of these drugs are accompanied by risk for serious adverse effects. There are currently no drugs specifically approved for the treatment of LBD (MacDonald et al., 2022), FTD (Khoury et al., 2021), limbic-predominant age-related TDP-43 encephalopathy (LATE) (Nag et al., 2020), or vascular dementia (Alzheimer’s Association, 2024), beyond those for managing symptoms. While there has been some promising evidence for nonpharmacological approaches to preventing dementia (NASEM, 2017), much of the evidence from clinical trials on NPIs has been negative or mixed, making it difficult to draw definitive conclusions. The prospect of waiting additional decades to develop safe, effective preventive and therapeutic interventions for AD/ADRD is untenable given the human and economic costs associated with these devastating diseases. To accelerate this timeline, mechanisms such as those discussed in the following sections are needed to facilitate the timely, successive progression of interventions through the clinical trial pipeline, maximally leveraging the results from earlier trials.
Prespecifying Go/No-Go Criteria
The incorporation of prespecified, go/no-go criteria into the clinical trials pipeline provides one means of accelerating decision making regarding interventions that should be advanced into subsequent trials. The incorporation of interim analyses into trial timelines for highest-priority outcomes, when appropriate, can improve efficiency. While safety and tolerability have traditionally been used as go/no-go criteria in early-phase clinical trials (Stallard et al., 2001), a growing repertoire of biomarkers that can be used to evaluate short-term intervention effects, such as target engagement, pharmacodynamics, and effects predictive of clinical benefit, may additionally be used to inform go/no-go decisions and over time begin to reduce the number of negative phase 3 clinical trials (Boxer and Sperling, 2023; Cummings, 2019). This further underscores the critical importance of continued biomarker discovery for improved clinical outcomes and the acceleration of progress in the prevention and treatment of AD/ADRD. However, a consideration in the use of go/no-go criteria to stop trials (particularly in early-stage phase 1b and 2 trials) early is the tradeoff between improving efficiency and loss of opportunities to learn about the biology of AD/ADRD, which is a fundamental element of NIH-funded AD/ADRD clinical research. Thus, use of go/no-go criteria, may not be appropriate in all trials.
Implementing Innovative Clinical Trial Designs
Although expansion of the portfolio of novel strategies for prevention and treatment of AD/ADRD is encouraging and should remain a priority,
evaluating the safety and efficacy of this growing number of interventions will require an increase in the efficiency and speed of clinical trials. The expense and time required for traditional clinical research approaches that entail successive testing of single interventions in a single study population serve as major impediments to the identification of effective interventions for AD/ADRD. Similar challenges experienced in the field of oncology led to the implementation of innovative clinical trial designs, such as platform trials (see Box 4-1) and the use of master protocols to increase efficiency (Boxer and Sperling, 2023). Such approaches may also be useful in the AD/ADRD field.
Master trial protocols enable the evaluation of multiple interventions in more than one target population within the same overall trial structure. Thus, a single protocol can be used to answer multiple research questions (Aisen et al., 2021; Woodcock and LaVange, 2017), thereby helping to achieve efficiencies in trial infrastructure, regulatory interactions, and data standardization (Boxer and Sperling, 2023). Master protocols are applicable to multiple trial types, including umbrella, basket, and platform trials (see Figures 4-1 and 4-2). These and other examples of innovative trial designs with the potential to accelerate the evaluation of interventions for preventing and treating AD/ADRD are described in Table 4-1. Importantly, basket trials have the potential to reduce siloing in AD/ADRD clinical research by incorporating multiple target populations into a single trial. A consideration related to the implementation of innovative trial designs for the evaluation of experimental pharmacological agents is the potential impact on processes and timelines for FDA approval (Brooks, 2024). Increased adoption of innovative trial designs by investigators will depend in part on the degree to which they are embraced by federal research funders and regulators.
In addition to novel clinical trial designs, enrichment strategies also have the potential to increase the efficiency of clinical trials. Enrichment involves the selection of a study population based on prospectively defined patient (or individual) characteristics to increase the likelihood of detecting an intervention effect (FDA, 2019b). Enrichment strategies also help to support precision medicine by targeting and tailoring interventions to those patients who would be expected to benefit from them based on results from subtyping (endotyping or phenotyping) using clinical or multiomics data, as discussed further later in this chapter. An important consideration for enrichment designs is whether the strategy could be used in practice to identify people to whom the intervention should be targeted and its potential usefulness in a broader population. FDA defines three categories of enrichment strategies:
- Strategies to decrease variability—The selection of study participants whose baseline measurements of a disease phenotype or a biomarker reflecting a disease fall within a narrow range can decrease variability and thereby increase study power.
BOX 4-1
Accelerated Decision Making in a Platform Trial: Lessons from I-SPY and Breast Cancer
The I-SPY 2 clinical trial was one of the first platform trials and employed an innovative research paradigm to enhance the development of neoadjuvant therapy for locally advanced breast cancer (QLHC, 2024). This novel paradigm differs from traditional clinical trial methods in its ability to test multiple therapeutic agents adaptively, thereby reducing the amount of time and the number of participants needed to carry out a study and improving trial efficiency (Esserman, 2024; QLHC, 2024). In addition, the adaptive trial design allows the use of early data from one group of patients to predict the response of subsequent patient groups and inform the assignment of participants to study arms, thus minimizing the exposure of study participants to therapies that do not work for them (FNIH, 2023; QLHC, 2024; Wang and Yee, 2019).
The I-SPY 2 trial model uses clinical biomarkers to classify a patient’s breast cancer into 1 of 10 molecular subtypes and then assigns that patient to a study arm using adaptive randomization. The primary endpoint is the pathologic complete response (pCR) or the complete elimination of the tumor in the breast and lymph nodes at the time of surgery, which follows treatment (Wang and Yee, 2019). Using statistical methods appropriate for a Bayesian adaptive design (Potter et al., 2021b), the predictive probabilities of each therapeutic agent are updated in real time based on the patient’s tumor subtype, the treatment received, and notable biomarker outcomes such pCR or MRI tumor volume (QLHC, 2024). A therapeutic agent under evaluation would be considered a success if it reaches a predetermined level of efficacy in one or more tumor subtypes; alternatively, its use may be discontinued if the predictive probability of success falls below a set threshold across subtypes (QLHC, 2024; Wang and Yee, 2019). This approach not only enables the assessment of multiple therapies simultaneously, but it also better mirrors how cancer patients receive clinical care in the real world (Esserman, 2024). Thus, researchers have an opportunity to use innovative clinical trials to inform care and, in turn, better use real-world data to optimize clinical trial design.
I-SPY 2 employs a master protocol that allows multiple agents to enter and leave the trial without having to stop enrollment or resubmit the entire study protocol for regulatory review (QLHC, 2024). Investigators are also aiming to receive accelerated approval for agents with optimal pCR rates, emphasizing the need for FDA collaboration (Esserman, 2024). In addition to unique regulatory considerations, the facilitation of
data sharing among researchers across all sectors is another hallmark of this study. Study investigators have made data from the trial publicly available to researchers under the condition that those who use the data in their studies put their own results back into the open-access platform (Esserman, 2024). In this way, researchers working in this space are not hindered unnecessarily by data availability concerns and can instead focus their efforts on developing effective therapies for breast cancer patients.
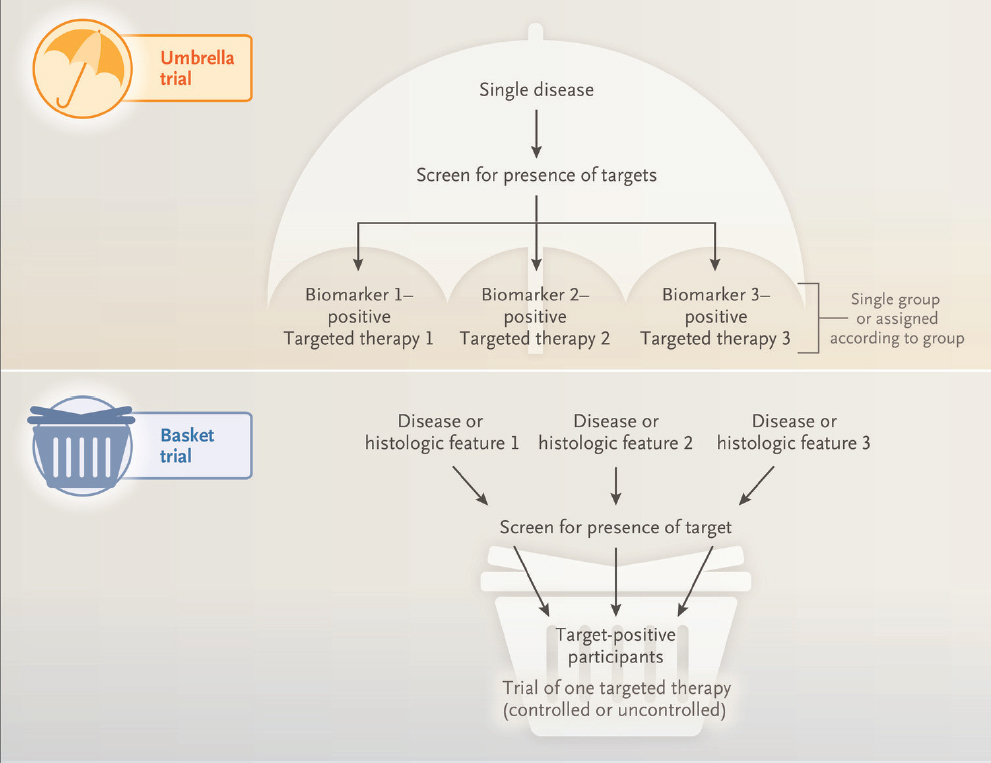
SOURCE: Woodcock and LaVange, 2017. Copyright © Reprinted with permission from Massachusetts Medical Society.
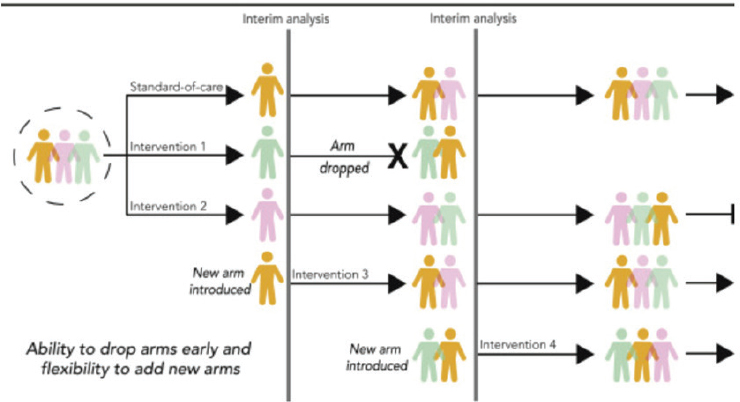
SOURCE: Park et al., 2020. CC BY-NC-ND 4.0. https://creativecommons.org/licenses/by-nc-nd/4.0/.
| Trial Design | Description | Illustrative Example of Application to AD/ADRD |
|---|---|---|
| Basket trials | Trials are designed for testing a single intervention in multiple populations (e.g., people with different disease types or subtypes) (Boxer and Sperling, 2023; Woodcock and LaVange, 2017). | A trial for a tau-targeted intervention in participants with AD or related dementia types characterized by tau pathology |
| Umbrella trials | Trials are designed to study multiple interventions (or combination interventions) in a single clinical population (e.g., people with the same disease). The use of a single placebo group increases the likelihood of participants receiving an intervention under study (Boxer and Sperling, 2023; Woodcock and LaVange, 2017). | A trial testing multiple different combinations of NPIs and anti-amyloid therapies in an AD population |
| Trial Design | Description | Illustrative Example of Application to AD/ADRD |
|---|---|---|
| Platform trials | Trials are designed to study multiple interventions for a single disease type in an open-ended manner with new interventions entering the trial as others leave it, based on a specified decision algorithm (Woodcock and LaVange, 2017). This design allows interventions to be tested in parallel instead of sequentially. Adaptive platform trials allow the addition of new study arms or individual arms to be halted based on an interim analysis indicating a lack of efficacy (Aisen et al., 2021). | A trial testing multiple anti-amyloid antibodies in an AD population with study arms halted as some antibodies are found to be ineffective and new arms started as new antibodies are ready for testing in clinical trials. |
| Seamless trials | Trials may facilitate expediency by consolidating clinical phases of drug development into a single, continuously amended protocol, thus eliminating gaps between phased trials (Hobbs et al., 2019). | Different doses of an investigational new drug for FTD are evaluated in a phase 2A trial that then transitions into a phase 2B trial with the selected dose without pausing or stopping the trial. |
| Phase 0 exploratory microdosing trials | Exploratory clinical trials evaluate subtherapeutic doses of novel drugs in first-in-human studies (Burt et al., 2020). | A new drug targeting a signaling pathway thought to be involved in AD is tested at subtherapeutic doses for safety, tolerability, and pharmacokinetics. |
| Pragmatic trials |
The primary purpose of these trials is providing information to decision makers on the balance of benefits, burdens, and risks of an intervention implemented at the individual or population level (Califf and Sugarman, 2015). Embedded pragmatic clinical trials are often conducted in care delivery settings, thus serving to bridge clinical care and research (NIH Collaboratory, n.d.). |
A trial of one or more NPIs implemented in a clinical practice setting and using electronic health records data to guide participant recruitment and for outcome monitoring. |
| N-of-1 trials | Personalized or single-subject clinical trials often use multiple-time-period, active-comparator crossover designs that allow randomization and masking and can provide answers about the optimal intervention for an individual (Davidson et al., 2021). | Evaluation of a treatment for sleep disorders in an individual living with LBD where the participant receives the treatment or placebo in random order over the specified treatment periods. |
NOTE: AD = Alzheimer’s disease; AD/ADRD = Alzheimer’s disease and related dementias; FTD = frontotemporal dementia; LBD = Lewy body dementia; NPI = nonpharmacological intervention.
- Prognostic enrichment strategies—The selection of study participants who are more likely to have a disease-related endpoint or significant worsening of a condition can result in greater between-group differences in absolute effect.
- Predictive enrichment strategies—The selection of study participants with a greater likelihood of responding to an intervention (based on physiologic/disease characteristics or a biomarker related to the mechanism of action) than other patients or people with the same condition can result in larger effect sizes and reduce the required size of the study population needed to detect an effect (FDA, 2019b).
Conclusion 4-6: The sequential and individual testing of interventions that characterizes the current clinical trial landscape impedes the rapid identification of effective interventions to prevent and treat AD/ADRD.
Leveraging Public–Private Partnerships
Government, private, philanthropic, and academic organizations all play major roles in accelerating the translation of scientific advances into clinical diagnostics and interventions for AD/ADRD and contribute complementary resources and expertise. While private industry is active in the development and evaluation of pharmacological agents, other kinds of interventions and certain trial designs may be less appealing to industry owing to financial risk or the lack of financial incentives. This may be the case, for example, with many NPIs, repurposed drugs, combination interventions, and even prevention trials, which are generally of long duration and require large study populations, evaluating novel pharmacological agents (Boxer and Sperling, 2023). Funding from public and philanthropic entities and other resources, such as academic consortia, can help to incentivize industry engagement by decreasing perceived financial risks, which is particularly important for smaller companies with more limited resources, and providing access to unique patient populations and valuable scientific expertise (Boxer and Sperling, 2023).
Notable examples of academic consortia that have partnered with industry to evaluate novel AD/ADRD interventions include the Dominantly Inherited Alzheimer’s Network Trials Unit, the Alzheimer’s Prevention Initiative, and the Alzheimer’s Clinical Trials Consortium. In addition to providing platforms for testing interventions, such consortia can help to ensure that data and samples from clinical trials are made available to other researchers (e.g., through data commons and biobanks), and that even negative trial results are published, all of which are recognized challenges when trials are solely industry sponsored (Boxer and Sperling, 2023). Academic researchers
involved with such consortia can also gain valuable experience with large trials, thus helping to train and expand the clinical research workforce. However, industry engagement may depend on how trials conducted by such consortia fit into the overall development program and timeline for drug candidates and whether the studies will be supportive for regulatory submission and approval (NASEM, 2024). Joint steering committees featuring leadership from academic and industry partners can be helpful for ensuring the needs of all partners are considered and met (Irizarry, 2024).
In addition to hosting clinical trial consortia, academic medical centers are also increasingly engaging with private partners in biomarker discovery. As noted earlier in this chapter, biomarkers are likely to play an increasingly important role in accelerating decision making within the clinical trial pipeline and reducing the number of failed efficacy trials. Much of current biomarker identification is being undertaken using discovery-based platforms, but ultimately biomarkers may form the basis for companion diagnostic assays that can help to identify which patients are likely to benefit from particular therapies or to be at increased risk of adverse side effects (Arafah et al., 2023). Academic researchers may be less familiar with FDA requirements for candidate biomarkers used in therapeutic decision making and may benefit from industry diagnostic partnerships, ideally early in the drug development process so that regulatory, legal, quality, and commercial considerations are factored into early clinical studies (Silva et al., 2018).
Reconfiguring Funding Models
Innovative funding models have the potential to overcome current barriers that impede timely progression of candidate interventions through the clinical trials pipeline. For example, in response to a gap/opportunity identified at the 2021 Alzheimer’s Research Summit, NIH initiated a new funding opportunity with the goal of streamlining and supporting seamless transitions in the early-stage evaluation of promising novel pharmacological interventions for AD/ADRD (NIH, 2023). The funding opportunity, released in 2023, bundles funding for early-stage clinical trials and thereby facilitates timely, successive progression from phase 1 to phase 1b/2a trials for drug candidates that meet prespecified, go/no-go safety and tolerability criteria.
Minimizing Barriers to Data Access and Usability
A final factor with the potential to accelerate decision making within the clinical trial pipeline and advance the prevention and treatment of AD/ADRD is the minimization of barriers to data access and usability so trials can evolve in real time and new insights can be gained from cross-study analyses. For example, data access is critical to conducting
rigorous meta-analyses, which can help to overcome the limitations of single clinical trials, including a lack of adequate power to detect intervention effects in the many subpopulations of interest (NASEM, 2024). However, inadequate budgeting within studies and a fragmented national data infrastructure creates significant challenges to data sharing efforts. Data are currently distributed across many different systems, which can impede the conduct of collaborative and integrative research.
In 2022, NIH held a workshop on real-world data infrastructure that supports research and clinical trials for AD/ADRD. Gaps and opportunities identified during the NIH workshop overlapped with opportunities shared with the committee during its public information-gathering workshop, including the need to (1) develop common data elements (CDEs) or harmonize data to improve interoperability and the ability to aggregate or pool data, (2) improve data access, and (3) protect privacy and confidentiality through data de-identification approaches. While harmonization and the development of CDEs has been a major focus area for NIA and NINDS, further efforts to standardize CDEs across AD/ADRD research resources are needed to maximize data sharing and interoperability (Hao et al., 2024), as discussed further in Chapter 5. As noted during the NIH workshop, data infrastructures also “must serve and include the communities who would most benefit from the treatments, innovations, and ideas that emerge from that infrastructure” (NIA, 2022, p. 1).
Recognizing the critical importance of data sharing, NIH issued a final data management and sharing policy that went into effect in January 2023.3 Under the policy, recipients are required to submit data management and sharing plans and to comply with those plans following NIH approval. While such requirements help to ensure the availability of data from NIH-funded trials, they do not address the underlying barriers (e.g., cost burden to investigators) at a study level, inadequacies of the existing data infrastructure, availability of tools (e.g., artificial intelligence/machine learning approaches) for enhancing data usability, or access to data from trials funded by other sponsors (see Chapter 5).
Learning from Clinical Trial Failures
Given the considerable investment of resources (monetary and other) required for clinical trials, a failure at phase 2 often results in the discontinuation of further evaluation of the intervention under study. However, this approach limits what can be learned from the study and the potential returns that can still be gained from the investment. Each clinical trial, even
___________________
3 https://grants.nih.gov/grants/guide/notice-files/NOT-OD-21-013.html (accessed May 21, 2024).
those that have not yielded hoped-for results, has the potential to provide valuable insights into disease mechanisms and avenues for future research. A negative clinical trial does not necessarily mean that the intervention under study was ineffective. For example, the follow-up period may have been insufficient to detect a difference between intervention and control arms, the trial may have failed to reach adequate therapeutic doses in affected regions of the central nervous system (Boxer and Sperling, 2023), or population heterogeneity may not have been adequately accounted for in the selection of the study population. The latter is a challenge for diseases such as AD/ADRD characterized by substantial heterogeneity despite similarity in clinical symptoms. Therefore, it is important to understand why a clinical trial might have failed and to use that knowledge to inform future trials; however, the publication of failed trial results is not standard practice.
Opportunities to learn from clinical trial failures include confirmatory trials, as well as subgroup analyses to understand if an intervention worked for some subpopulation of the study participants and if so, why. For example, a phase 2 RCT testing an inhibitor of 11-beta-hydroxysteroid dehydrogenase 1 (11β-HSD1)—an enzyme involved in the conversion of inert cortisone to active cortisol, which, when elevated in CSF, has been linked to cognitive decline—failed to find any cognitive benefit for AD patients with mild to moderate symptoms (Seckl, 2024). However, subgroup analysis suggested that the inhibitor may slow cognitive decline in patients with higher levels of p-tau181 who are at elevated risk of disease progression. The negative trial was unpublished, highlighting the challenges that arise when data from industry-sponsored trials are not accessible. Thus, there is a need to address disincentives that prevent companies from making data available that can enable learning from trial failures (Boxer and Sperling, 2023). It should be noted, however, that post hoc subgroup analyses can be misleading for various reasons (e.g., the analysis may not be adequately powered owing to smaller subgroup sizes, or there may be issues related to randomization). Consequently, it may be better to test new hypotheses based on subgroup analyses in phase 2 rather than phase 3 trials (Cummings, 2018).
Additionally, as described in Chapter 3, well-designed trials that employ specific and validated target engagement and measures can provide important information about a hypothesized mechanism of action, regardless of the success of the primary clinical endpoint in early drug discovery trials (Mohs and Greig, 2017). This means that failure of phase 2 candidates still provides valuable data about the targeted mechanism of action, which can then be used to refine research earlier in the therapeutic pipeline and future trials. However, the testing and measurement of specific mechanistic hypotheses in addition to the primary clinical endpoint is not routinely applied in drug discovery trials despite the potential return on investment.
Drug discovery trials, including those funded by NIH, would benefit from including measures for mechanistic hypothesis in addition to primary clinical endpoints in early-stage trials to accelerate future therapeutics development regardless of trial success.
In addition to informing future clinical trials, trial failures may present opportunities for reverse translation, whereby knowledge and experiences obtained through the study are used to guide new approaches to basic and preclinical research (Cummings et al., 2018). Observations of variability in intervention response can lead to new testable hypotheses regarding mechanisms of action, which may in turn help to identify new intervention targets that can be tested in future clinical trials. In this way, research is transformed from a linear to a continuous, cyclical process (Shakhnovich, 2018).
Conclusion 4-7: A deeper understanding of why clinical trials fail can provide critically important information that can inform the design of future clinical trials and present opportunities to guide new inquiries in basic and translational research.
ADVANCING A PRECISION MEDICINE APPROACH TO AD/ADRD PREVENTION AND TREATMENT
Despite similarity in clinical features, it is increasingly clear that the multifactorial nature of AD/ADRD means that the underlying disease processes vary widely across individuals, featuring differences at multiple levels, including genetics, transcriptomics, proteomics, lipidomics, epigenetics, metabolomics, and biochemistry, thus revealing perturbations in many biological pathways including but not limited to myelination, innate immunity, mitochondrial, vascular, and synaptic transmission (Allen et al., 2018; Barupal et al., 2019; Batra et al., 2024; Carrasquillo et al., 2017; Conway et al., 2018; Higginbotham et al., 2020; Johnson et al., 2022; McKenzie et al., 2017; Mostafavi et al., 2018; Mukherjee et al., 2020; Oatman et al., 2023; Strickland et al., 2020; Toledo et al., 2017; Yang et al., 2020). Further, studies focusing on cell-specific molecular perturbations demonstrated involvement in AD/ADRD of all brain cell types and identified many cell subtypes associated with risk or other endophenotypes of AD/ADRD, such as pathology or cognition (Cain et al., 2023; Green et al., 2024; İş et al., 2024; McKenzie et al., 2018; Min et al., 2023; Patel et al., 2022; Patrick et al., 2020; Wang et al., 2020). Given this complex and heterogeneous disease etiology, several groups have begun to propose molecular subtypes for AD/ADRD to facilitate discovery of treatments and biomarkers in a precision medicine framework (Chen et al., 2023b; Higginbotham et al., 2023; Hou et al., 2024; Iturria-Medina et al., 2022; Lian et al., 2023; Neff et al., 2021; Wan et al., 2020).
The multifactorial complexity, heterogeneity, and individuality in AD/ADRD pose a significant challenge to developing and selecting effective prevention and treatment strategies because responses to interventions can be highly variable. An intervention that is beneficial for one subset of the population may be ineffective or even harmful in other subgroups (Hampel et al., 2020; Neff et al., 2021; Sarkar et al., 2024). Precision medicine approaches, which often use genomic and other biomarker information to target and tailor interventions, are precisely suited to addressing the challenge of heterogeneity.
Accordingly, advancing precision medicine in AD/ADRD research has been a notable area of emphasis for NIH in recent years. The theme of the 2024 NIH Alzheimer’s Research Summit was “Building a Precision Medicine Research Enterprise.”4 Other recent meetings and workshops have focused on enabling precision medicine through open science5 and precision medicine approaches to combination therapies for preventing and treating AD/ADRD.6
Although precision medicine has been envisioned since the sequencing of the human genome, technological advances are providing the opportunity to bring the vision to fruition (Hampel et al., 2019). Multiomics methods, along with the data integration and predictive capabilities of artificial intelligence (Geifman et al., 2018; Vrahatis et al., 2023), are enabling the identification of disease subtypes and improved population risk stratification, while digital health technologies (e.g., wearables, sensors) allow individual-specific health information to guide precision intervention approaches in combination with more disease-specific data (Mohler et al., 2015). With these advances, it is possible to envision a not-so-distant future wherein individual-level profiling using multiomics (e.g., genomic, transcriptomics, proteomic, epigenetic, metabolomic), biomarkers, exposomes, digital health technology, and clinical data guides precision prevention and treatment approaches to optimize brain health over the life course (Hampel et al., 2022).
Despite their potential to better address complex, multifactorial diseases, precision medicine approaches have not yet been fully integrated into the development and evaluation (e.g., clinical trials) of interventions for AD/ADRD. This may in part stem from the incomplete understanding of the biological basis for AD/ADRD, as precision approaches are grounded
___________________
4 More information on the 2024 NIH Alzheimer’s Research Summit is available at https://www.nia.nih.gov/2024-alzheimers-summit (accessed October 20, 2024).
5 For more information see https://www.alz.org/alzheimers-precision-medicine/overview.asp (accessed October 20, 2024).
6 For more information see https://www.nia.nih.gov/research/dn/workshops/precision-medicine-approaches-developing-combination-therapies-treatment-and (accessed October 20, 2024).
in the disease biology. Continued investment in basic and translational science that leads to the identification of different disease subtypes and an understanding of differences across population groups (e.g., sex/gender, genetic ancestry, ethnoracial) will be pivotal to the rollout of precision medicine approaches. Indeed, research on intersectionality highlights that individuals live with multiple identities (e.g., gender, race, sexual orientation), each of which may be associated with distinct sources of disadvantage or resilience. These intersectional identities may synergistically influence response to interventions and treatments and thus should be considered as the field evolves in efforts to design inclusive studies and target and tailor interventions. Moreover, to prevent further exacerbation of disparities, precision medicine research needs to examine how social determinants of health may support or undermine precision health interventions (Hekler et al., 2020). Finally, molecular studies in AD/ADRD that are essential for a precision medicine approach are relatively rare in populations traditionally understudied in research despite having a higher AD risk, such as African Americans and Latin Americans with few exceptions (Hohman et al., 2016; Jin et al., 2015; Logue et al., 2014; N’Songo et al., 2017; Reddy et al., 2022, 2024; Reitz et al., 2013).
Precision medicine, which aims to diagnose and treat the right patient with the right therapy at the right time, is a broad and multifaceted field that is evolving at a rapid pace. In keeping with its charge, the committee focused its assessment of the application of precision medicine to AD/ADRD on research opportunities. While beyond the scope of this report, the committee recognizes that there are barriers that will need to be addressed in the translation of precision medicine approaches to AD/ADRD from research to real-world implementation. One example is the lack of clinician and public understanding of the scientific foundations of precision medicine. Training for primary care providers and education of the public will be important to advance precision medicine approaches to AD/ADRD prevention and treatment. Trained community health workers and patient navigators may be well positioned to bridge the gap between clinicians, patients, and research participants (Ramos et al., 2019a).
The sections below highlight opportunities to better use precision medicine approaches in developing and evaluating interventions for preventing and treating AD/ADRD.
Population Stratification
Too often clinical trial designs assume that diseases to which interventions are targeted are the same for all participants (Reitz, 2016). Observation of subpopulation responses indicates this is not the case (Yip et al., 2016) and repeatedly emphasizes the highly individualized character of many chronic
illnesses. AD/ADRD are a heterogeneous group of diseases caused by myriad pathophysiologic mechanisms (Allen et al., 2018; Barupal et al., 2019; Batra et al., 2024; Carrasquillo et al., 2017; Conway et al., 2018; Higginbotham et al., 2020; Oatman et al., 2023; Johnson et al., 2022; McKenzie et al., 2017; Mostafavi et al., 2018; Mukherjee et al., 2020; Strickland et al., 2020; Toledo et al., 2017; Yang et al., 2020). This heterogeneity suggests a need for stratification when evaluating interventions for prevention and treatment. Improving the success rate of AD/ADRD clinical trials is contingent on determining the right target population for each intervention study (Boxer and Sperling, 2023) and the use of tailored interventions that are provided at the appropriate time and using an optimized regimen.
Precision medicine approaches target interventions to specific populations or even individuals based on a set of characteristics that determine the likely effectiveness of a given approach for that person or subpopulation (Sarkar et al., 2024). This approach requires:
- the identification of criteria and factors that are appropriate for use in population stratification to optimally define differences across large and heterogenous populations, and
- the ability to screen individuals for those specific factors in order to target and tailor interventions in ways that optimize clinical outcomes.
This strategy has been effective in oncology (Lazar et al., 2010) (see Box 4-2) but is in its infancy for the prevention and treatment of AD/ADRD.
As shown in Figure 4-3, stratification to a subgroup level represents a first step in the transition from the one-size-fits-all model to a precision-based model. The stratification process can then be extended further to the personalization of intervention approaches based on an individual’s unique biological makeup and other factors such as lifestyle, family history, and preference. Importantly, a precision medicine approach does not imply the need to develop different therapies for each individual. Rather, therapies will be designed around subgroups of individuals who share certain characteristics, such as genetic risk or biomarkers, and are likely to benefit from the same intervention. Application of available interventions (e.g., treatment plans) can then be refined and tailored based on further stratification of patients into additional subgroup clusters, and in some cases, even to the individual level. As a simple, illustrative example, a cardiovascular exercise intervention could be modified from walking to swimming for individuals living with AD/ADRD who have balance issues.
Myriad characteristics can be used to stratify populations, including clinical disease stage and phenotype, genetics (e.g., APOE status, antioxidant
BOX 4-2
Precision Medicine Advances in Breast, Lung, and Other Cancers
Precision medicine for breast cancer involves testing to individualize interventions and treatment approaches. Testing often involves the evaluation of inherited risk, which has been estimated to be up to 10 percent of all breast cancer cases (Rizzolo et al., 2011). This type of evaluation is exemplified by testing for gene mutations in BRCA1 and BRCA2 and other genes, such as PALB2. Additional testing is also used to identify molecular targets for treatment or to predict drug responsiveness to treatment. In the first case, testing can help determine if drugs targeting specific targets, such as the HER2 protein, may be effective (Sun et al., 2021), and in the second case to determine if the patient can convert inactive drugs to their active forms, as seen with the popular agent tamoxifen (Dahabreh et al., 2010).
Transformational changes in the treatment of lung cancer have been realized with drugs targeting PD-1 receptor status for patients amenable to immune checkpoint therapy (Chen et al., 2020b; Jain et al., 2018). PD-1 is one of the best-characterized checkpoint proteins that when bound by its ligand PD-L1 or PD-L2 suppresses T-cell activation and allows cancer cells to escape the body’s intrinsic ability to fight the disease. In recent years, multiple lines of anti-PD-1 drugs have been developed. Patients with melanoma, renal cell carcinoma, non-small cell lung cancer, and some hematological cancers have all been found to respond positively to this type of treatment (Chen et al., 2020b).
genes)—including mitochondrial genes (e.g., TOMM22 and others)—other multiomics measures (e.g., transcriptome, proteome, metabolome), neuroimaging results (e.g., amyloid-PET), and other biological markers, as well as lifestyle factors, socioeconomic status, past exposures, and personality features (Sarkar et al., 2024). Advances in biomarkers enable stratification by copathology, which will be important as more therapies with specific pathological targets become available (Gibson et al., 2023; Toledo et al., 2023).
An ongoing problem with AD/ADRD clinical trials to date is the failure to adequately stratify populations. Studies may stratify participants based on a single genetic factor such as APOE status or neuropathology but not take into account other important contributing factors. An increasingly common precision medicine approach applied in AD/ADRD clinical trials is the use of enrichment designs (described earlier in this chapter)
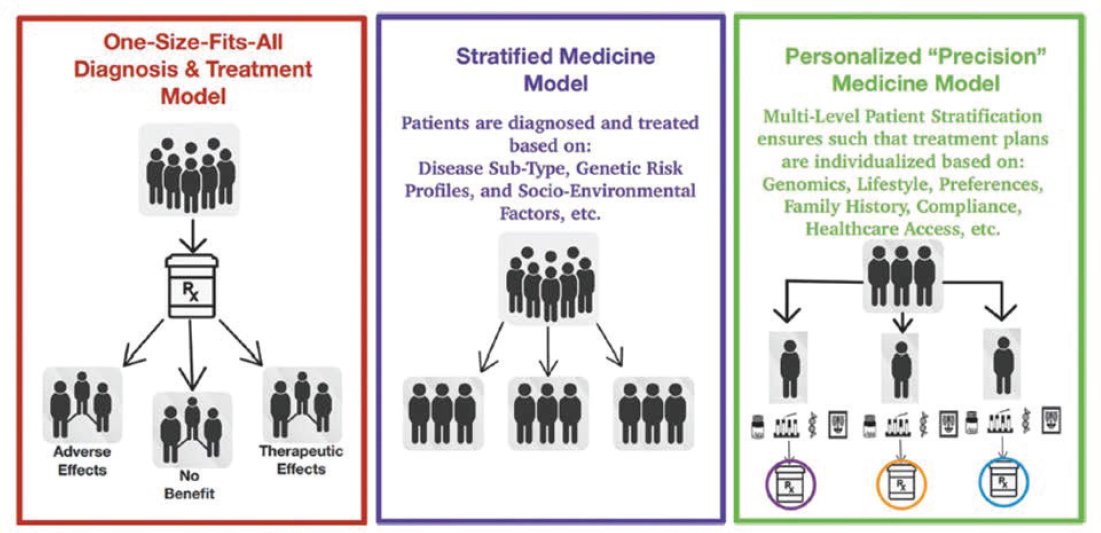
SOURCE: Sarkar et al., 2024. Copyright© and reprinted with permission from Elsevier.
that limit recruitment based on biomarker or other screening results to better target AD/ADRD interventions to at-risk populations. For example, the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease (A4) trial screened potential participants and selected at-risk individuals for the trial population based on evidence of brain amyloid accumulation on PET imaging and cognitive status (Sperling et al., 2014). Following a subgroup analysis for the FINGER prevention trial that showed that the beneficial cognitive effects of the multicomponent intervention were enhanced among APOE4 carriers (Solomon et al., 2018), a subsequent trial evaluating the FINGER multidomain lifestyle intervention in combination with metformin, a diabetes drug, was enriched for APOE4 carriers (Barbera et al., 2024). The Systematic Multi-Domain Alzheimer Risk Reduction Trial (SMARRT) included only participants with two or more dementia risk factors (Yaffe et al., 2024). While there are clear advantages to enrichment approaches to clinical trials, a tradeoff worth noting is the potential impact on the generalizability of the findings to a broader population (FDA, 2019b), which needs to be factored into decision making. Additionally, targeted trials utilizing stratification carry biases that need to be managed in the statistical analyses. Appropriate trial design is thus critical to successful execution of targeted clinical trials.
With recent advances in high-throughput multiomics approaches (e.g., genomics, proteomics, transcriptomics, metabolomics), progress is being made in the identification of different disease subtypes based on molecular markers that can be linked to specific underlying disease pathways and that may guide more effective stratification and intervention targeting strategies going forward (Arafah et al., 2023; Hampel et al., 2022; Harrison et al., 2020; Higginbotham et al., 2023; Iturria-Medina et al., 2022; Neff et al., 2021; Tijms et al., 2024; Wan et al., 2020). Bioinformatics approaches are enabling the identification of groups or modules of related molecular and biological functions (e.g., lipid metabolism, immune response, synaptic processes, myelination, mitochondrial, vascular) that may be altered (elevated or depressed) in AD/ADRD and their linkage to different subtypes (Higginbotham et al., 2023; Iturria-Medina et al., 2022; Johnson et al., 2022; Mostafavi et al., 2018; Neff et al., 2021; Tijms et al., 2024; Wan et al., 2020). These clusters of related molecular functions hint at specific pathophysiologic mechanisms that may give rise to different disease subtypes (as well as neuroprotective mechanisms in the case of resilience-associated subtypes).
While there has been some alignment in subtype identification across different omics approaches (e.g., proteomic versus transcriptomic versus metabolomic), differences have also been identified, emphasizing the value of complementary approaches that can yield different insights (Allen et al., 2018; Batra et al., 2024; Higginbotham et al., 2023; Johnson et al., 2022;
Wang et al., 2022b). Subtyping is also being pursued using data other than those generated with multiomics methods. For example, functional MRI has been used to identify subtypes displaying different patterns of impairment in functional connectivity within the brain (Chen et al, 2023b). As research advancing subtype discovery progresses, it will be important to integrate findings from different omic approaches and with other relevant data (e.g., clinical, cognitive, genetic risk, imaging, neuropathology) collected from research participants whose samples were used in subtyping (Allen et al., 2018; Campbell et al., 2022; Carrasquillo et al., 2015; Iturria-Medina et al., 2022; Reddy et al., 2021, 2024). These integrated findings may enhance understanding of subtypes and better guide stratification efforts.
Multiomic methods for subtyping are commonly carried out on brain tissue samples (Higginbotham et al., 2023; Iturria-Medina et al., 2022; Neff et al., 2021, Wan et al., 2020). Realizing the diagnostic and therapeutic potential of these methods will require understanding how subtypes identified in biofluid samples (e.g., CSF, plasma) reflect those identified in brain tissue (Higginbotham et al., 2023; Iturria-Medina et al., 2022; Tijms et al., 2024). Tijms and colleagues (2024) recently identified five distinct AD subtypes using mass spectrometry-based proteomic analysis of CSF. Each identified subtype featured a distinct genetic risk profile and was linked to a specific molecular process. Multiplexed blood-based biomarker panels based on the proteomics profiles are now being developed to increase the feasibility of subtyping individuals as part of a stratification and targeted treatment approach (Tijms et al., 2024).
As has been the case in oncology (see Box 4-1), the ability to subtype individuals based on distinct biological mechanisms will likely be key to effective prevention and treatment of AD/ADRD and requires continued investment in multiomics studies and advancing neuroimaging capabilities and the identification of diverse fluid-based biomarkers that can be developed into multiplexed panels. As with other areas of research, subtyping studies have primarily been conducted using samples from populations with limited diversity (Higginbotham et al., 2023) and have focused on AD. Thus, the expansion to diverse populations and related dementias should be a focus of future research (Reddy et al., 2024; Seifar et al., 2024), as should prospective studies that investigate whether an individual’s subtype changes with aging and disease progression from preclinical to MCI to clinical dementia (Iturria-Medina et al., 2022; Neff et al., 2021). The implications of mixed dementias for subtype-based population stratification and precision medicine approaches also need to be investigated (Boxer and Sperling, 2023).
Conclusion 4-8: Stratifying populations in a meaningful way using precision medicine approaches and applying this level of resolution to
the prevention and treatment of AD/ADRD will require investment in exploratory research, neuroimaging and multiomics approaches, and computational tools and analytics to elucidate determinants of AD/ADRD risk and variability of response to interventions in diverse populations.
Precision Approaches to AD/ADRD Prevention and Treatment
Precision prevention and treatment approaches are specifically designed to address the issue of molecular and clinical heterogeneity by identifying a person’s specific pattern of risk factors and/or underlying pathophysiologic processes and selecting a preventive or therapeutic intervention strategy that is likely to provide benefit. While much effort has gone into understanding genetic risk, precision approaches for AD/ADRD would benefit from a better understanding of underlying environmental factors and gene–environment interactions (discussed in Chapter 3) (Reitz, 2016), as well as integration of these data to guide clinical interventions. This will enable the consideration of potential interaction between an intervention and the individual level of risk—for instance, whether those at higher genetic risk for AD are likely to get more, the same, or less benefit from an intervention (Deckers et al., 2021; Hall et al., 2024; Lourida et al., 2019; Solomon et al., 2018). Different interventions may interact with an individual’s risk in different ways, and understanding interaction effects is critical for targeting interventions most effectively and cost-effectively.
Large and complex datasets are being generated using a combination of multiomics approaches, clinical assessments, and, increasingly, digital technologies. As such, the development of such computational tools and analytics as sophisticated multivariate methods and artificial intelligence/machine learning that can integrate datasets and extract patterns has become an important area for investment to advance precision medicine for AD/ADRD, as discussed further in Chapter 5. The application of such tools and methods to determine optimal approaches for stratifying populations, matching identified subgroups to interventions, and guiding the life-course timing of those interventions is a major research priority.
Precision Brain Health Approach to AD/ADRD Prevention
While many interventions for AD/ADRD have focused on midlife and late-life deficits, a precision brain health approach to AD/ADRD prevention should consider opportunities for personalized interventions implemented at earlier ages as part of a life-course approach to brain health optimization and resilience. Precision prevention approaches are predictive and rely on the ability to screen populations to identify those with increased
susceptibility (Ramos et al., 2019b). This enables a transition from generic risk-reduction strategies to personalized interventions focused on specific risk factors (Arafah et al., 2023).
Given the many factors that contribute to AD/ADRD risk (as discussed in Chapter 3) and the identification of distinct disease subtypes, as well as the limited success observed with single-component prevention trials, multifactorial prevention strategies designed based on risk profiles are increasingly being pursued (Gregory et al., 2022). While results from earlier trials of multicomponent risk-reduction interventions for AD/ADRD have been mixed (Rosenberg et al., 2020), the recent success of randomized trials such as SMARRT underscores the potential of a more tailored approach to simultaneously targeting multiple contributors to AD/ADRD (Yaffe et al., 2024). The design of SMARRT included enrichment for a higher-risk population and employing codesigned preventive strategies personalized to the participants’ specific risk factors (e.g., physical inactivity, poor sleep, social isolation, smoking behavior).
The integration of SMARRT with the health care delivery system was another important aspect of the trial that facilitated a personalized approach. The increased integration of clinical trials with health care delivery systems, which enables data collection on individual health profiles from electronic health records, was recommended previously as a priority for advancing dementia prevention (NASEM, 2017). Importantly, SMARRT included management of chronic conditions known to contribute to AD/ADRD risk (e.g., hypertension, diabetes), highlighting opportunities to better integrate personalized AD/ADRD, cardiovascular, and metabolic disease prevention. More collaborative approaches to intervention research are needed so prevention research for overlapping public health priorities is not hampered by existing—and often funding-related—silos.
Although personalized approaches to implementing interventions and shared decision making can help patients feel more empowered in their care and, in some cases, improve treatment adherence (Montori et al., 2023; Simmons et al., 2010; Umar et al., 2012), it is also important to recognize that individuals may at times be reluctant or unable to express their preferences and may not always choose the treatment strategy that is best supported by evidence-based guidelines (Say and Thomson, 2003). For this reason, the successful implementation of a multicomponent intervention not only requires an understanding of how different components perform, both individually and together, but also needs to factor in how patient preferences and attitudes can affect treatment outcomes.
The ecosystem in which an individual lives, studies, and works (i.e., social determinants of health) may enable or hamper their ability to take the actions necessary to prevent disease. This contextual component may contribute to the observed variation in effect when the same intervention
is implemented in diverse populations (e.g., low versus high education, low versus high socioeconomic status, those with or without a supportive family and/or social network). This variation can arise as a result of differences in uptake, adherence, and/or efficacy (Rebok et al., 2023). For instance, a person with very constrained financial resources living in a dangerous neighborhood may have limited ability to increase physical activity as part of a lifestyle-focused preventive intervention given the lack of safe places to walk or exercise in their surroundings. For this reason, the design of personalized prevention strategies needs to account for social determinants of health, including structural factors.
With the recent approval of pharmacological agents targeting underlying disease pathways for AD and the expectation that more may come to the market in the near future, it is likely that precision prevention approaches for AD/ADRD will increasingly feature multicomponent interventions that include both pharmacological agents and NPIs, particularly for people at heightened risk based on genetic markers and/or family history. As pharmacological agents may have more targeted mechanisms of action as compared to NPIs, precision approaches to combination interventions will need to be guided by an analysis of an individual’s biological makeup, including, optimally, genetic risk factors and multiomics perturbations that have been linked to specific disease pathways or susceptibility. A combination approach to precision prevention that follows a brain health optimization framework could involve sequential interventions such that relatively low-risk NPIs are implemented earlier in the life course. Monitoring of those at high risk (e.g., members of families in which AD or a related dementia is prevalent) could begin decades before typical age of onset of clinical symptoms to enable identification of early pathophysiologic changes that may trigger the use of pharmacological agents to complement NPIs. By monitoring for early signs of preclinical disease, initiation of pharmacological therapies could prevent overt and potentially irreversible cognitive change.
Precision Approaches to AD/ADRD Treatment
While prevention is the ultimate goal for AD/ADRD intervention research, effective treatments remain a priority given the millions of people living with cognitive impairment and clinical dementia in the United States and globally. Considering the vast heterogeneity of AD/ADRD, the predominance of mixed pathologies, and the co-occurrence with other diseases that may contribute to or exacerbate these neurodegenerative conditions, targeting and tailoring of treatment will likely be necessary (Devi, 2023). Precision treatment approaches can predict and guide the selection of therapies that maximize benefits to individuals while minimizing toxicity and
adverse events. Informed by genetics, molecular make-up derived from multiomics, biomarkers, and other individual-level information, precision treatment approaches targeting the specifics of the disease at a patient-level (drivers of disease and/or downstream consequences) can change the shape of the curve for mortality and/or severity of illness.
Given the multiple and interdependent disease pathways that contribute to AD/ADRD, advancing precision treatment approaches will require continued efforts to deconstruct AD/ADRD into distinct biological subtypes (or endophenotypes) so appropriate therapies can be selected (Hampel et al., 2017). An intervention that is effective for one subtype may not provide benefit for another (Devi, 2023). Moreover, different subtypes may progress at different rates, with implications for the timing of intervention approaches in a personalized management strategy (Geifman et al., 2018). Ongoing efforts employing multiomics approaches to identify subtypes (Higginbotham et al., 2023; Iturria-Medina et al., 2022; Neff et al., 2021), like the work by Tijms and colleagues (2024) described earlier, are helping to set the stage for precision treatment approaches. Each of the AD subtypes identified by their laboratory featured a distinct genetic risk profile and was linked to a specific molecular process (neuronal hyperplasticity, innate immune activation, RNA dysregulation, choroid plexus dysfunction, blood–brain barrier impairment). Different subtypes, which are continuing to be uncovered in this rapidly moving field, may benefit from treatments that target specific perturbed pathways in each individual as part of a precision medicine approach (Tijms et al., 2024). Where multiple pathways are engaged, potentially resulting in a mix of pathologic features, combination drug therapies may be needed to target each independently or in a coordinated manner. Combination therapies may be administered simultaneously or in sequence. In the case of sequential administration, optimum sequencing needs to be determined (Boxer and Sperling, 2023). Although there has been significant emphasis on the development of novel therapies for AD/ADRD, the ability to subtype individuals may alternatively enable an individualized drug repurposing approach whereby existing FDA-approved drugs are matched to an individual based on integrated multiomics data (Fang et al., 2020).
As noted earlier, there is increasing interest in leveraging artificial intelligence/machine learning methods to facilitate precision medicine approaches to treatment. Such methods use patient-level data (e.g., clinical and demographic information) and predictive models of disease progression under various treatment options to make personalized treatment recommendations (Hu et al., 2023; Liu et al., 2022). One recent study showed that a neural network was effective at predicting the most beneficial dementia treatment (acetylcholinesterase inhibitors and memantine) as measured by decline in cognitive test scores (Liu et al., 2022). While requiring further
validation and evaluation in real-world settings, such methods may also have utility in personalizing treatments with disease modifying therapies for AD/ADRD as more such treatments become available.
Precision Approaches to Addressing Safety Considerations
Although NPIs may generally have lower risk profiles, many drugs can have adverse effects. Such risks are common across diseases requiring drug treatment, but acceptability could differ for AD/ADRD depending on disease stage. For example, there may be less tolerance for risks during the preclinical stage when there is no apparent effect on cognitive function, and it is unclear whether the individual may go on to develop clinical symptoms.
For treatments that have known serious adverse effects (e.g., anti-amyloid antibody therapies), a personalized approach to treatment needs to include the consideration of the risk–benefit ratio, which may be affected by age, biomarker status, and other risk factors (Boxer and Sperling, 2023). It is also important to consider the timing of treatment initiation, balancing the risks from starting early versus waiting too long when the treatment may no longer be optimally effective.
In precision medicine approaches for cancer, companion diagnostic tests are used to match patients with specific therapies that are likely to provide benefit and can include components for identifying safety and tolerability concerns. Such testing may also be part of a precision medicine approach to AD/ADRD. Biomarkers that can predict who is at risk for developing more severe forms of ARIA or other adverse events that may be associated with future FDA-approved drugs could enable analogous testing to identify individuals in whom existing therapies are contraindicated, thus supporting a more personalized approach to treatment (Arafah et al., 2023). This is already being done to some degree with APOE4 testing (Cummings and Kinney, 2022). APOE4 carriers—and particularly people with two copies of the allele—are more likely to develop severe ARIA (Loomis et al., 2024), affecting the risk–benefit tradeoff for treatment with anti-amyloid therapies, although effects of pretreatment on amyloid burden and presence of vascular pathology (e.g., infarcts) need to be better understood, as do differences in risk among non-White populations. Appropriate use recommendations for anti-amyloid therapies include a recommendation for APOE genotyping (Blasco and Roberts, 2023). When such genotyping is conducted, genetic counseling is generally recommended. Given the paucity of skilled genetic counselors, this role may fall to treating clinicians (e.g., geriatricians, neurologists), highlighting the importance of equipping health care providers with resources needed to understand and counsel patients about safety considerations when implementing precision approaches to assessing and mitigating safety concerns.
RESEARCH PRIORITIES
Accelerating the evaluation of interventions for AD/ADRD and advancing precision approaches to ensure that individuals receive the right combination of interventions at the right times are critical to reducing the individual and societal impact of these diseases. NIH has made major research investments and created essential infrastructure to improve the clinical trial pipeline for AD/ADRD and advance precision medicine approaches. These investments have included the creation of programs and resources for improving the inclusivity of clinical research, collaborative research programs to identify and de-risk promising therapeutic targets, the creation of public–private partnerships to centralize essential resources and expertise for innovative clinical trial approaches (e.g., Alzheimer’s Prevention Initiative, Alzheimer’s Clinical Trials Consortium), and support for well-designed clinical trials of pharmacological and nonpharmacological approaches. The committee identified five research priorities that align with and build upon this broad foundation of NIH investments. These research priorities are listed in Table 4-2 and include key scientific questions and near-term research opportunities that would advance progress on the research priorities.
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
| 4-1: Integrate innovative approaches and novel tools into the planning, design, and execution of studies to accelerate the identification of effective interventions. |
|
|
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
| 4-2: Advance the development and evaluation of combination therapies (including pharmacological and nonpharmacological approaches) to better address the multifactorial nature of AD/ADRD. |
|
|
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
| 4-3: Evaluate precision medicine approaches for the prevention and treatment of AD/ADRD to better identify interventions likely to benefit specific groups of individuals. |
|
|
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
| 4-4: Advance the adoption of standardized outcomes for assessing interventions that are sensitive, person-centered, clinically meaningful, and reflect the priorities of those at risk for or living with AD/ADRD. |
|
|
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
| 4-5: Evaluate the causal effects of public health approaches on overall dementia incidence and incidence in understudied and/or disproportionately affected populations. |
|
|
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
|
REFERENCES
AARP. 2024. AARP Foundation Experience Corps. https://www.aarp.org/volunteer/programs/experience-corps/?intcmp=AE-VOL-PROG-C1R1 (accessed October 18, 2024).
Abdelmoaty, M. M., E. Lu, R. Kadry, E. G. Foster, S. Bhattarai, R. L. Mosley, and H. E. Gendelman. 2023. Clinical biomarkers for Lewy body diseases. Cell & Bioscience 13(1):209.
ADAPT Research Group. 2013. Results of a follow-up study to the randomized Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT). Alzheimer’s & Dementia 9(6):714-723.
ADNI (Alzheimer’s Disease Neuroimaging Initiative). 2012. Menu of demographics tables. https://adni.loni.usc.edu/wp-content/uploads/2012/08/ADNI_Enroll_Demographics.pdf (accessed August 19, 2024).
AHEAD Study. 2024. AHEAD Study. https://www.aheadstudy.org/ (accessed August 19, 2024).
Ahn, J., and M. Kim. 2023. Effects of exercise therapy on global cognitive function and, depression in older adults with mild cognitive impairment: A systematic review and meta-analysis. Archives of Gerontology and Geriatrics 106:104855.
AHRQ (Agency for Healthcare Research and Quality). 2014. AHRQ methods for effective health care. In Registries for evaluating patient outcomes: A user’s guide. Edited by R. E. Gliklich, N. A. Dreyer, and M. B. Leavy. Rockville, MD: Agency for Healthcare Research and Quality.
Aisen, P. S., R. J. Bateman, M. Carrillo, R. Doody, K. Johnson, J. R. Sims, R. Sperling, and B. Vellas. 2021. Platform trials to expedite drug development in Alzheimer’s disease: A report from the EU/US CTAD Task Force. Journal of the Prevention of Alzheimer’s Disease 8(3):306-312.
Allen, M., X. Wang, J. D. Burgess, J. Watzlawik, D. J. Serie, C. S. Younkin, T. Nguyen, K. G. Malphrus, S. Lincoln, M. M. Carrasquillo, C. Ho, P. Chakrabarty, S. Strickland, M. E. Murray, V. Swarup, D. H. Geschwind, N. T. Seyfried, E. B. Dammer, J. J. Lah, A. I. Levey, T. E. Golde, C. Funk, H. Li, N. D. Price, R. C. Petersen, N. R. Graff-Radford, S. G. Younkin, D. W. Dickson, J. R. Crook, Y. W. Asmann, and N. Ertekin-Taner. 2018. Conserved brain myelination networks are altered in Alzheimer’s and other neurodegenerative diseases. Alzheimer’s & Dementia 14(3):352-366.
Alves, F., P. Kalinowski, and S. Ayton. 2023. Accelerated brain volume loss caused by anti-β-amyloid drugs: A systematic review and meta-analysis. Neurology 100(20):e2114-e2124.
AlzForum. 2024a. Donanemab. https://www.alzforum.org/therapeutics/donanemab (accessed August 19, 2024).
AlzForum. 2024b. Ct1812. https://www.alzforum.org/therapeutics/ct1812 (accessed October 29, 2024).
Alzheimer Society of Ireland. 2017. Human rights and dementia. https://alzheimer.ie/creating-change/policy-on-dementia-in-ireland/human-rights-and-dementia/ (accessed August 20, 2024).
Alzheimer’s Association. 2024. Vascular dementia. https://www.alz.org/alzheimers-dementia/what-is-dementia/types-of-dementia/vascular-dementia (accessed August 20, 2024).
Alzheimer’s Network. 2024. Alzheimer’s network for treatment and diagnostics (ALZ-NET). https://www.alz-net.org/ (accessed August 20, 2024).
Anstey, K. J., S. R. Lord, M. Hennessy, P. Mitchell, K. Mill, and C. von Sanden. 2006. The effect of cataract surgery on neuropsychological test performance: A randomized controlled trial. Journal of the International Neuropsychological Society 12(5):632-639.
Anstey, K. J., K. Ashby-Mitchell, and R. Peters. 2017. Updating the evidence on the association between serum cholesterol and risk of late-life dementia: Review and meta-analysis. Journal of Alzheimer’s Disease 56(1):215-228.
Anthes, E. 2020. Alexa, do I have Covid-19? Nature 586(7827):22-25.
Arafah, A., S. Khatoon, I. Rasool, A. Khan, M. A. Rather, K. A. Abujabal, Y. A. H. Faqih, H. Rashid, S. M. Rashid, S. Bilal Ahmad, A. Alexiou, and M. U. Rehman. 2023. The future of precision medicine in the cure of Alzheimer’s disease. Biomedicines 11(2):335.
Armstrong, N. M., Y. An, J. Doshi, G. Erus, L. Ferrucci, C. Davatzikos, J. A. Deal, F. R. Lin, and S. M. Resnick. 2019. Association of midlife hearing impairment with late-life temporal lobe volume loss. JAMA Otolaryngology—Head & Neck Surgery 145(9):794-802.
Asakawa, T., Y. Yang, Z. Xiao, Y. Shi, W. Qin, Z. Hong, and D. Ding. 2024. Stumbling blocks in the investigation of the relationship between age-related hearing loss and cognitive impairment. Perspectives on Psychological Science 19(1):137-150.
ASPE (Office of Assistant Secretary for Planning and Evaluation Department of Health and Human Services). 2017. Challenges in involving people with dementia as study participants in research on care and services. https://aspe.hhs.gov/reports/challenges-involving-people-dementia-study-participants-research-care-services-0 (accessed August 20, 2024).
Bahar‐Fuchs, A., A. Martyr, A. M. Y. Goh, J. Sabates, and L. Clare. 2019. Cognitive training for people with mild to moderate dementia. Cochrane Database of Systematic Reviews 3(3):CD013069.
Baiduc, R. R., J. W. Sun, C. M. Berry, M. Anderson, and E. A. Vance. 2023. Relationship of cardiovascular disease risk and hearing loss in a clinical population. Scientific Reports 13(1):1642.
Baker, L. D., C. W. Cotman, R. Thomas, S. Jin, A. H. Shadyab, J. Pa, R. A. Rissman, J. B. Brewer, J. Zhang, Y. Jung, A. Z. LaCroix, K. Messer, and H. H. Feldman. 2022. Topline results of EXERT: Can exercise slow cognitive decline in MCI? Alzheimer’s & Dementia 18(S11):e069700.
Ballard, M., and P. Montgomery. 2017. Risk of bias in overviews of reviews: A scoping review of methodological guidance and four-item checklist. Research Synthesis Methods 8(1):92-108.
Baptista, C., A. R. Silva, M. P. Lima, and R. M. Afonso. 2024. Evaluating the cognitive effects of interventions to reduce social isolation and loneliness in older adults: A systematic review. Aging & Mental Health 28(12):1767-1776.
Barbera, M., D. Perera, A. Matton, F. Mangialasche, A. Rosenberg, L. Middleton, T. Ngandu, A. Solomon, and M. Kivipelto. 2023. Multimodal precision prevention—a new direction in Alzheimer’s disease. Journal of Prevention of Alzheimer’s Disease 10(4):718-728.
Barbera, M., J. Lehtisalo, D. Perera, M. Aspö, M. Cross, C. A. De Jager Loots, E. Falaschetti, N. Friel, J. A. Luchsinger, H. M. Gavelin, M. Peltonen, G. Price, A. S. Neely, C. Thunborg, J. Tuomilehto, F. Mangialasche, L. Middleton, T. Ngandu, A. Solomon, M. Kivipelto, S. A. Adeleke, C. Arvidsson, I. Barton, M. Bas, K. Cosby, J. Crispin, L. Dunn, M. Durkina, O. Elebring, J. Ford, P. Giannakopoulou, H. Gilkes, H. Graham, G. Hagman, R. Hall, H. Hallinder, A. Haqqee, M. Hartmanis, K. Hemiö, Z. Istvánfyová, D. Kafetsouli, K. Lakey, S. Lehtimäki, L. Lindström, P. MacDonald, A. Mäkelä, S. McGinn-Summers, C. Meius, A. Mirza, C. Oesterling, J. Ojala, A. Olawale, I. Ramanath, H.-M. Roitto, B. Sahib, S. Singh, M. Sundell, S. Taylor, D. Tharumaratnam, K. Uusimäki, J. Vaarala, H. Voutilainen, J. Åsander, the MET-FINGER study team. 2024. A multimodal precision-prevention approach combining lifestyle intervention with metformin repurposing to prevent cognitive impairment and disability: The MET-FINGER randomised controlled trial protocol. Alzheimer’s Research & Therapy 16(1):23.
Barupal, D. K., R. Baillie, S. Fan, A. J. Saykin, P. J. Meikle, M. Arnold, K. Nho, O. Fiehn, R. Kaddurah-Daouk, and C. Alzheimer Disease Metabolomics. 2019. Sets of coregulated serum lipids are associated with Alzheimer’s disease pathophysiology. Alzheimer’s Dementia (Amsterdam, Netherlands) 11:619-627.
Basak, C., S. Qin, and M. A. O’Connell. 2020. Differential effects of cognitive training modules in healthy aging and mild cognitive impairment: A comprehensive meta-analysis of randomized controlled trials. Psychology and Aging 35(2):220-249.
Batra, R., J. Krumsiek, X. Wang, M. Allen, C. Blach, G. Kastenmuller, M. Arnold, N. Ertekin-Taner, R. Kaddurah-Daouk, and Alzheimer’s Disease Metabolomics. Consortium 2024. Comparative brain metabolomics reveals shared and distinct metabolic alterations in Alzheimer’s disease and progressive supranuclear palsy. Alzheimer’s & Dementia 20(12):8294-8307.
Bechard, L. E., K. S. McGilton, L. E. Middleton, H. Chertkow, S. Sivananthan, and J. Bethell. 2022. Engaging people with lived experience of dementia in research: Perspectives from a multi-disciplinary research network. Canadian Geriatrics Journal 25(3):254-261.
Bellenguez, C., F. Kucukali, I. E. Jansen, L. Kleineidam, S. Moreno-Grau, N. Amin, A. C. Naj, R. Campos-Martin, B. Grenier-Boley, V. Andrade, P. A. Holmans, A. Boland, V. Damotte, S. J. van der Lee, M. R. Costa, T. Kuulasmaa, Q. Yang, I. de Rojas, J. C. Bis, A. Yaqub, I. Prokic, J. Chapuis, S. Ahmad, V. Giedraitis, D. Aarsland, P. Garcia-Gonzalez, C. Abdelnour, E. Alarcon-Martin, D. Alcolea, M. Alegret, I. Alvarez, V. Alvarez, N. J. Armstrong, A. Tsolaki, C. Antunez, I. Appollonio, M. Arcaro, S. Archetti, A. A. Pastor, B. Arosio, L. Athanasiu, H. Bailly, N. Banaj, M. Baquero, S. Barral, A. Beiser, A. B. Pastor, J. E. Below, P. Benchek, L. Benussi, C. Berr, C. Besse, V. Bessi, G. Binetti, A. Bizarro, R. Blesa, M. Boada, E. Boerwinkle, B. Borroni, S. Boschi, P. Bossu, G. Brathen, J. Bressler, C. Bresner, H. Brodaty, K. J. Brookes, L. I. Brusco, D. Buiza-Rueda, K. Burger, V. Burholt, W. S. Bush, M. Calero, L. B. Cantwell, G. Chene, J. Chung, M. L. Cuccaro, A. Carracedo, R. Cecchetti, L. Cervera-Carles, C. Charbonnier, H. H. Chen, C. Chillotti, S. Ciccone, J. Claassen, C. Clark, E. Conti, A. Corma-Gomez, E. Costantini, C. Custodero, D. Daian, M. C. Dalmasso, A. Daniele, E. Dardiotis, J. F. Dartigues, P. P. de Deyn, K. de Paiva Lopes, L. D. de Witte, S. Debette, J. Deckert, T. Del Ser, N. Denning, A. DeStefano, M. Dichgans, J. Diehl-Schmid, M. Diez-Fairen, P. D. Rossi, S. Djurovic, E. Duron, E. Duzel, C. Dufouil, G. Eiriksdottir, S. Engelborghs, V. Escott-Price, A. Espinosa, M. Ewers, K. M. Faber, T. Fabrizio, S. F. Nielsen, D. W. Fardo, L. Farotti, C. Fenoglio, M. Fernandez-Fuertes, R. Ferrari, C. B. Ferreira, E. Ferri, B. Fin, P. Fischer, T. Fladby, K. Fliessbach, B. Fongang, M. Fornage, J. Fortea, T. M. Foroud, S. Fostinelli, N. C. Fox, E. Franco-Macias, M. J. Bullido, A. Frank-Garcia, L. Froelich, B. Fulton-Howard, D. Galimberti, J. M. Garcia-Alberca, P. Garcia-Gonzalez, S. Garcia-Madrona, G. Garcia-Ribas, R. Ghidoni, I. Giegling, G. Giorgio, A. M. Goate, O. Goldhardt, D. Gomez-Fonseca, A. Gonzalez-Perez, C. Graff, G. Grande, E. Green, T. Grimmer, E. Grunblatt, M. Grunin, V. Gudnason, T. Guetta-Baranes, A. Haapasalo, G. Hadjigeorgiou, J. L. Haines, K. L. Hamilton-Nelson, H. Hampel, O. Hanon, J. Hardy, A. M. Hartmann, L. Hausner, J. Harwood, S. Heilmann-Heimbach, S. Helisalmi, M. T. Heneka, I. Hernandez, M. J. Herrmann, P. Hoffmann, C. Holmes, H. Holstege, R. H. Vilas, M. Hulsman, J. Humphrey, G. J. Biessels, X. Jian, C. Johansson, G. R. Jun, Y. Kastumata, J. Kauwe, P. G. Kehoe, L. Kilander, A. K. Stahlbom, M. Kivipelto, A. Koivisto, J. Kornhuber, M. H. Kosmidis, W. A. Kukull, P. P. Kuksa, B. W. Kunkle, A. B. Kuzma, C. Lage, E. J. Laukka, L. Launer, A. Lauria, C. Y. Lee, J. Lehtisalo, O. Lerch, A. Lleo, W. Longstreth, Jr., O. Lopez, A. L. de Munain, S. Love, M. Lowemark, L. Luckcuck, K. L. Lunetta, Y. Ma, J. Macias, C. A. MacLeod, W. Maier, F. Mangialasche, M. Spallazzi, M. Marquie, R. Marshall, E. R. Martin, A. M. Montes, C. M. Rodriguez, C. Masullo, R. Mayeux, S. Mead, P. Mecocci, M. Medina, A. Meggy, S. Mehrabian, S. Mendoza, M. Menendez-Gonzalez, P. Mir, S. Moebus, M. Mol, L. Molina-Porcel, L. Montrreal, L. Morelli, F. Moreno, K. Morgan, T. Mosley, M. M. Nothen, C. Muchnik, S. Mukherjee, B. Nacmias, T. Ngandu, G. Nicolas, B. G. Nordestgaard, R. Olaso, A. Orellana, M. Orsini, G. Ortega, A. Padovani, C. Paolo, G. Papenberg, L. Parnetti, F. Pasquier, P. Pastor, G. Peloso, A. Perez-Cordon, J. Perez-Tur, P. Pericard, O. Peters, Y. A. L. Pijnenburg, J. A.
Pineda, G. Pinol-Ripoll, C. Pisanu, T. Polak, J. Popp, D. Posthuma, J. Priller, R. Puerta, O. Quenez, I. Quintela, J. Q. Thomassen, A. Rabano, I. Rainero, F. Rajabli, I. Ramakers, L. M. Real, M. J. T. Reinders, C. Reitz, D. Reyes-Dumeyer, P. Ridge, S. Riedel-Heller, P. Riederer, N. Roberto, E. Rodriguez-Rodriguez, A. Rongve, I. R. Allende, M. Rosende-Roca, J. L. Royo, E. Rubino, D. Rujescu, M. E. Saez, P. Sakka, I. Saltvedt, A. Sanabria, M. B. Sanchez-Arjona, F. Sanchez-Garcia, P. S. Juan, R. Sanchez-Valle, S. B. Sando, C. Sarnowski, C. L. Satizabal, M. Scamosci, N. Scarmeas, E. Scarpini, P. Scheltens, N. Scherbaum, M. Scherer, M. Schmid, A. Schneider, J. M. Schott, G. Selbaek, D. Seripa, M. Serrano, J. Sha, A. A. Shadrin, O. Skrobot, S. Slifer, G. J. L. Snijders, H. Soininen, V. Solfrizzi, A. Solomon, Y. Song, S. Sorbi, O. Sotolongo-Grau, G. Spalletta, A. Spottke, A. Squassina, E. Stordal, J. P. Tartan, L. Tarraga, N. Tesi, A. Thalamuthu, T. Thomas, G. Tosto, L. Traykov, L. Tremolizzo, A. Tybjaerg-Hansen, A. Uitterlinden, A. Ullgren, I. Ulstein, S. Valero, O. Valladares, C. V. Broeckhoven, J. Vance, B. N. Vardarajan, A. van der Lugt, J. V. Dongen, J. van Rooij, J. van Swieten, R. Vandenberghe, F. Verhey, J. S. Vidal, J. Vogelgsang, M. Vyhnalek, M. Wagner, D. Wallon, L. S. Wang, R. Wang, L. Weinhold, J. Wiltfang, G. Windle, B. Woods, M. Yannakoulia, H. Zare, Y. Zhao, X. Zhang, C. Zhu, M. Zulaica, EADB, GR@ACE, DEGESCO, EADI, GERAD, Demgene, FinnGen, ADGC, CHARGE, L. A. Farrer, B. M. Psaty, M. Ghanbari, T. Raj, P. Sachdev, K. Mather, F. Jessen, M. A. Ikram, A. de Mendonca, J. Hort, M. Tsolaki, M. A. Pericak-Vance, P. Amouyel, J. Williams, R. Frikke-Schmidt, J. Clarimon, J. F. Deleuze, G. Rossi, S. Seshadri, O. A. Andreassen, M. Ingelsson, M. Hiltunen, K. Sleegers, G. D. Schellenberg, C. M. van Duijn, R. Sims, W. M. van der Flier, A. Ruiz, A. Ramirez, and J. C. Lambert. 2022. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nature Genetics 54(4):412-436.
Bennett, C. F., A. R. Krainer, and D. W. Cleveland. 2019. Antisense oligonucleotide therapies for neurodegenerative diseases. Annual Review of Neuroscience 42:385-406.
Blackman, J., M. Swirski, J. Clynes, S. Harding, Y. Leng, and E. Coulthard. 2021. Pharmacological and non-pharmacological interventions to enhance sleep in mild cognitive impairment and mild Alzheimer’s disease: A systematic review. Journal of Sleep Research 30(4):e13229.
Blasco, D., and J. S. Roberts. 2023. Implications of emerging uses of genetic testing for Alzheimer’s disease. Journal of Prevention of Alzheimer’s Disease 10(3):359-361.
Boeve, B., A. Boxer, H. Rosen, L. Forsberg, H. Heuer, D. Brushaber, B. Appleby, J. Biernacka, Y. Bordelon, H. Botha, P. Brannelly, B. Dickerson, S. Dickinson, D. Dickson, K. Domoto-Reilly, K. Faber, A. Fagan, J. Fields, A. Fishman, T. Foroud, D. Galasko, R. Gavrilova, T. Gendron, D. Geschwind, N. Ghoshal, J. Goldman, J. Graff-Radford, N. Graff-Radford, I. Grant, M. Grossman, G.-Y. Hsiung, E. Huang, E. Huey, D. Irwin, D. Jones, K. Kantarci, A. Karydas, D. Kaufer, D. Knopman, J. Kramer, W. Kremers, J. Kornak, W. Kukull, E. Lagone, I. Litvan, P. Ljubenkov, D. Lucente, I. R. A. Mackenzie, M. Manoochehri, J. Masdeu, S. McGinnis, M. Mendez, B. Miller, T. Miyagawa, K. Nelson, C. Onyike, A. Pantelyat, B. Pascual, R. Pearlman, L. Petrucelli, R. Rademakers, E. M. Ramos, K. Rankin, K. Rascovsky, J. Rexach, A. Ritter, E. Roberson, J. R. Martinez, M. Sabbagh, D. Salmon, R. Savica, W. Seeley, A. Staffaroni, J. Syrjanen, C. Tartaglia, N. Tatton, J. Taylor, A. Toga, S. Weintraub, D. Wheaton, B. Wong, and Z. Wszolek. 2020. The ARTFL LEFFTDS frontotemporal lobar degeneration (ALLFTD) protocol: Preliminary data and future plans (2081). Neurology 94(15Suppl).
Bonnechère, B., and M. Klass. 2023. Cognitive computerized training for older adults and patients with neurological disorders: Do the amount and training modality count? An umbrella meta-regression analysis. Games for Health Journal 12(2):100-117.
Bowirrat, A. 2022. Immunosenescence and aging: Neuroinflammation is a prominent feature of Alzheimer’s disease and is a likely contributor to neurodegenerative disease pathogenesis. Journal of Personalized Medicine 12(11):1817.
Boxer, A. L., and R. Sperling. 2023. Accelerating Alzheimer’s therapeutic development: The past and future of clinical trials. Cell 186(22):4757-4772.
Boxer, A. L., M. Gold, H. Feldman, B. F. Boeve, S. L.-J. Dickinson, H. Fillit, C. Ho, R. Paul, R. Pearlman, M. Sutherland, A. Verma, S. P. Arneric, B. M. Alexander, B. C. Dickerson, E. R. Dorsey, M. Grossman, E. D. Huey, M. C. Irizarry, W. J. Marks, M. Masellis, F. McFarland, D. Niehoff, C. U. Onyike, S. Paganoni, M. A. Panzara, K. Rockwood, J. D. Rohrer, H. Rosen, R. N. Schuck, H. D. Soares, and N. Tatton. 2020. New directions in clinical trials for frontotemporal lobar degeneration: Methods and outcome measures. Alzheimer’s & Dementia 16(1):131-143.
Brasure, M., P. Desai, H. Davila, V. A. Nelson, C. Calvert, E. Jutkowitz, M. Butler, H. A. Fink, E. Ratner, L. S. Hemmy, J. R. McCarten, T. R. Barclay, and R. L. Kane. 2017. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia. Annals of Internal Medicine 168(1):30-38.
Brookmeyer, R., and N. Abdalla. 2018. Estimation of lifetime risks of Alzheimer’s disease dementia using biomarkers for preclinical disease. Alzheimer’s & Dementia 14(8):981-988.
Brooks, M. 2024. FDA delays decision on Alzheimer’s hopeful donanemab. https://www.medscape.com/viewarticle/fda-delays-decision-alzheimers-hopeful-donanemab-2024a10004i7?ecd=WNL_trdalrt_pos1_240308_etid6362693&uac=345736CV&impID=6362693 (accessed August 20, 2024).
Burt, T., G. Young, W. Lee, H. Kusuhara, O. Langer, M. Rowland, and Y. Sugiyama. 2020. Phase 0/microdosing approaches: Time for mainstream application in drug development? Nature Reviews Drug Discovery 19(11):801-818.
Caberlotto, L., and T. P. Nguyen. 2014. A systems biology investigation of neurodegenerative dementia reveals a pivotal role of autophagy. BMC Systems Biology 8:65.
Cai, H., K. Zhang, M. Wang, X. Li, F. Ran, and Y. Han. 2023. Effects of mind-body exercise on cognitive performance in middle-aged and older adults with mild cognitive impairment: A meta-analysis study. Medicine (Baltimore) 102(34):e34905.
Cain, A., M. Taga, C. McCabe, G. S. Green, I. Hekselman, C. C. White, D. I. Lee, P. Gaur, O. Rozenblatt-Rosen, F. Zhang, E. Yeger-Lotem, D. A. Bennett, H. S. Yang, A. Regev, V. Menon, N. Habib, and P. L. De Jager. 2023. Multicellular communities are perturbed in the aging human brain and Alzheimer’s disease. Nature Neuroscience 26(7):1267-1280.
Calamia, M., K. Markon, and D. Tranel. 2012. Scoring higher the second time around: Meta-analyses of practice effects in neuropsychological assessment. Clinical Neuropsychologist 26(4):543-570.
Califf, R. M., and J. Sugarman. 2015. Exploring the ethical and regulatory issues in pragmatic clinical trials. Clinical Trials 12(5):436-441.
Campbell, A. S., C. C. G. Ho, M. Atık, M. Allen, S. Lincoln, K. Malphrus, T. Nguyen, S. R. Oatman, M. Corda, O. Conway, S. Strickland, R. C. Petersen, D. W. Dickson, N. R. Graff-Radford, and N. Ertekin-Taner. 2022. Clinical deep phenotyping of ABCA7 mutation carriers. Neurology Genetics 8(2):e655.
Cao, G.-Y., Z.-S. Chen, S.-S. Yao, K. Wang, Z.-T. Huang, H.-X. Su, Y. Luo, C. M. De Fries, Y.-H. Hu, and B. Xu. 2023. The association between vision impairment and cognitive outcomes in older adults: A systematic review and meta-analysis. Aging & Mental Health 27(2):350-356.
Caplan, A. I. 2017. Mesenchymal stem cells: Time to change the name! Stem Cells Translational Medicine 6(6):1445-1451.
Carillo, M. 2024. Envisioning the Future of AD/ADRD Research. Presentation at Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
Carlson, M. C. 2021. Productive social engagement as a vehicle to promote activity and neuro-cognitive health in later adulthood. Archives of Clinical Neuropsychology 36(7):1274-1278.
Carlson, M. C., J. S. Saczynski, G. W. Rebok, T. Seeman, T. A. Glass, S. McGill, J. Tielsch, K. D. Frick, J. Hill, and L. P. Fried. 2008. Exploring the effects of an “everyday” activity program on executive function and memory in older adults: Experience Corps®. Gerontologist 48(6):793-801.
Carrasquillo, M. M., J. E. Crook, O. Pedraza, C. S. Thomas, V. S. Pankratz, M. Allen, T. Nguyen, K. G. Malphrus, L. Ma, G. D. Bisceglio, R. O. Roberts, J. A. Lucas, G. E. Smith, R. J. Ivnik, M. M. Machulda, N. R. Graff-Radford, R. C. Petersen, S. G. Younkin, and N. Ertekin-Taner. 2015. Late-onset Alzheimer’s risk variants in memory decline, incident mild cognitive impairment, and Alzheimer’s disease. Neurobiology of Aging 36(1):60-67.
Carrasquillo, M. M., M. Allen, J. D. Burgess, X. Wang, S. L. Strickland, S. Aryal, J. Siuda, M. L. Kachadoorian, C. Medway, C. S. Younkin, A. Nair, C. Wang, P. Chanana, D. Serie, T. Nguyen, S. Lincoln, K. G. Malphrus, K. Morgan, T. E. Golde, N. D. Price, C. C. White, P. L. De Jager, D. A. Bennett, Y. W. Asmann, J. E. Crook, R. C. Petersen, N. R. Graff-Radford, D. W. Dickson, S. G. Younkin, and N. Ertekin-Taner. 2017. A candidate regulatory variant at the TREM gene cluster associates with decreased Alzheimer’s disease risk and increased TREMl1 and TREM2 brain gene expression. Alzheimer’s & Dementia 13(6):663-673.
Carvalho, C., and P. I. Moreira. 2023. Metabolic defects shared by Alzheimer’s disease and diabetes: A focus on mitochondria. Current Opinion in Neurobiology 79:102694.
Cha, Y., T. Y. Park, P. Leblanc, and K. S. Kim. 2023. Current status and future perspectives on stem cell-based therapies for Parkinson’s disease. Journal of Movement Disorders 16(1):22-41.
Chan, H. J., Yanshree, J. Roy, G. L. Tipoe, M. L. Fung, and L. W. Lim. 2021. Therapeutic potential of human stem cell implantation in Alzheimer’s disease. International Journal of Molecular Science 22(18):10151.
Chan, A. T. C., R. T. F. Ip, J. Y. S. Tran, J. Y. C. Chan, and K. K. F. Tsoi. 2024. Computerized cognitive training for memory functions in mild cognitive impairment or dementia: A systematic review and meta-analysis. NPJ Digital Medicine 7(1):1.
Chen, J., Z. Long, Y. Li, M. Luo, S. Luo, and G. He. 2019. Alteration of the Wnt/gsk3β/β-catenin signalling pathway by rapamycin ameliorates pathology in an Alzheimer’s disease model. International Journal of Molecular Medicine 44(1):313-323.
Chen, W., Y. Hu, and D. Ju. 2020a. Gene therapy for neurodegenerative disorders: Advances, insights and prospects. Acta Pharmaceutica Sinica B 10(8):1347-1359.
Chen, Y., Y. Pei, J. Luo, Z. Huang, J. Yu, and X. Meng. 2020b. Looking for the optimal PD-1/PD-L1 inhibitor in cancer treatment: A comparison in basic structure, function, and clinical practice. Frontiers in Immunology 11:1088.
Chen, X., S. Jiang, R. Wang, X. Bao, and Y. Li. 2023a. Neural stem cells in the treatment of Alzheimer’s disease: Current status, challenges, and future prospects. Journal of Alzheimer’s Disease 94(s1):S173-S186.
Chen, P., H. Yao, B. M. Tijms, P. Wang, D. Wang, C. Song, H. Yang, Z. Zhang, K. Zhao, Y. Qu, X. Kang, K. Du, L. Fan, T. Han, C. Yu, X. Zhang, T. Jiang, Y. Zhou, J. Lu, Y. Han, B. Liu, B. Zhou, Y. Liu, and Alzheimer’s Disease Neuroimaging Initiative. 2023b. Four distinct subtypes of Alzheimer’s disease based on resting-state connectivity biomarkers. Biological Psychiatry 93(9):759-769.
Chu, C.-S., P.-T. Tseng, B. Stubbs, T.-Y. Chen, C.-H. Tang, D.-J. Li, W.-C. Yang, Y.-W. Chen, C.-K. Wu, N. Veronese, A. F. Carvalho, B. S. Fernandes, N. Herrmann, and P.-Y. Lin. 2018. Use of statins and the risk of dementia and mild cognitive impairment: A systematic review and meta-analysis. Scientific Reports 8(1):5804.
Ciccone, I. 2024. FDA grants fast track designation for Longeveron’s Lomecel-B in mild Alzheimer’s disease. NeurologyLive. July 18. https://www.neurologylive.com/view/fda-grants-fast-track-designation-longeveron-lomecel-b-mild-ad.2024.
Clare, L., A. Kudlicka, J. R. Oyebode, R. W. Jones, A. Bayer, and I. Leroi. 2019. Individual goal-oriented cognitive rehabilitation to improve everyday functioning for people with early-stage dementia: A multi-centre randomised controlled trial (the GREAT trial). International Journal of Geriatric Psychiatry 34(5):709-721.
Clement, C., L. E. Selman, P. G. Kehoe, B. Howden, J. A. Lane, and J. Horwood. 2019. Challenges to and facilitators of recruitment to an Alzheimer’s disease clinical trial: A qualitative interview study. Journal of Alzheimer’s Disease 69(4):1067-1075.
Coley, N., T. Ngandu, J. Lehtisalo, H. Soininen, B. Vellas, E. Richard, M. Kivipelto, S. Andrieu, E. Richard, P. van Gool, E. M. van Charante, C. Beishuizen, S. Jongstra, T. van Middelaar, L. van Wanrooij, M. Hoevenaar-Blom, H. Soininen, T. Ngandu, M. Barbera, M. Kivipelto, F. Mangiasche, S. Andrieu, N. Coley, J. Guillemont, Y. Meiller, B. van de Groep, C. Brayne, M. Kivipelto, T. Ngandu, A. Solomon, T. Laatikainen, T. Strandberg, H. Soininen, J. Tuomilehto, R. Antikainen, J. Lindström, J. Lehtisalo, S. Havulinna, R. Rauramaa, T. Hänninen, T. Ngandu, L. Bäckman, A. Stigsdotter-Neely, T. Strandberg, R. Antikainen, J. Tuomilehto, A. Jula, M. Peltonen, E. Levälahti, M. Grönholm, J. Lehtisalo, K. Hemiö, B. Vellas, S. Guyonnet, I. Carrié, L. Brigitte, C. Faisant, F. Lala, J. Delrieu, H. Villars, E. Combrouze, C. Badufle, A. Zueras, S. Andrieu, C. Cantet, C. Morin, G. A. Van Kan, C. Dupuy, Y. Rolland, C. Caillaud, P.-J. Ousset, F. Lala, B. Fougère, S. Willis, S. Belleville, B. Gilbert, F. Fontaine, J.-F. Dartigues, I. Marcet, F. Delva, A. Foubert, S. Cerda, C. Marie Noëlle, C. Costes, O. Rouaud, P. Manckoundia, V. Quipourt, S. Marilier, E. Franon, L. Bories, M.-L. Pader, M.-F. Basset, B. Lapoujade, V. Faure, M. L. Yung Tong, C. Malick-Loiseau, E. Cazaban-Campistron, F. Desclaux, C. Blatge, T. Dantoine, C. Laubarie-Mouret, I. Saulnier, J.-P. Clément, M.-A. Picat, L. Bernard-Bourzeix, S. Willebois, I. Désormais, N. Cardinaud, M. Bonnefoy, P. Livet, P. Rebaudet, C. Gédéon, C. Burdet, F. Terracol, A. Pesce, S. Roth, S. Chaillou, S. Louchart, K. Sudres, N. Lebrun, N. Barro-Belaygues, J. Touchon, K. Bennys, A. Gabelle, A. Romano, L. Touati, C. Marelli, C. Pays, P. Robert, F. Le Duff, C. Gervais, S. Gonfrier, Y. Gasnier, S. Bordes, D. Begorre, C. Carpuat, K. Khales, J.-F. Lefebvre, S. M. El Idrissi, P. Skolil, J.-P. Salles, C. Dufouil, S. Lehéricy, M. Chupin, J.-F. Mangin, A. Bouhayia, M. Allard, F. Ricolfi, D. Dubois, M. P. Bonceour Martel, F. Cotton, A. Bonafé, S. Chanalet, F. Hugon, F. Bonneville, C. Cognard, F. Chollet, P. Payoux, T. Voisin, J. Delrieu, S. Peiffer, A. Hitzel, M. Allard, M. Zanca, J. Monteil, J. Darcourt, L. Molinier, H. Derumeaux, N. Costa, C. Vincent, B. Perret, C. Vinel, P. Olivier-Abbal, S. Andrieu, C. Cantet, and N. Coley. 2019. Adherence to multidomain interventions for dementia prevention: Data from the FINGER and MAPT trials. Alzheimer’s & Dementia 15(6):729-741.
Collins, L. M. (2018). Optimization of behavioral, biobehavioral, and biomedical Interventions: The Multiphase Optimization Strategy (MOST). Springer.
Connell, C. M., B. A. Shaw, S. B. Holmes, and N. L. Foster. 2001. Caregivers’ attitudes toward their family members’ participation in Alzheimer disease research: Implications for recruitment and retention. Alzheimer’s Disease and Associated Disorders 15(3):137-145.
Connors, M. H., L. Quinto, I. McKeith, H. Brodaty, L. Allan, C. Bamford, A. Thomas, J. P. Taylor, and J. T. O’Brien. 2018. Non-pharmacological interventions for Lewy body dementia: A systematic review. Psychological Medicine 48(11):1749-1758.
Conway, O. J., M. M. Carrasquillo, X. Wang, J. M. Bredenberg, J. S. Reddy, S. L. Strickland, C. S. Younkin, J. D. Burgess, M. Allen, S. J. Lincoln, T. Nguyen, K. G. Malphrus, A. I. Soto, R. L. Walton, B. F. Boeve, R. C. Petersen, J. A. Lucas, T. J. Ferman, W. P. Cheshire, J. A. van Gerpen, R. J. Uitti, Z. K. Wszolek, O. A. Ross, D. W. Dickson, N. R. Graff-Radford, and N. Ertekin-Taner. 2018. ABI3 and PLCG2 missense variants as risk factors for neurodegenerative diseases in Caucasians and African Americans. Molecular Neurodegeneration 13(1):53.
Corasaniti, M. T., G. Bagetta, P. Nicotera, S. Maione, P. Tonin, F. Guida, and D. Scuteri. 2024. Exploitation of autophagy inducers in the management of dementia: A systematic review. International Journal of Molecular Science 25(2):1264.
Cummings, J. 2018. Lessons learned from Alzheimer disease: Clinical trials with negative outcomes. Clinical and Translational Science 11(2):147-152.
Cummings, J. 2019. The role of biomarkers in Alzheimer’s disease drug development. Advances in Experimental Medical Biology 1118:29-61.
Cummings, J. 2024. Presentation at the Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
Cummings, J., and J. Kinney. 2022. Biomarkers for Alzheimer’s disease: Context of use, qualification, and roadmap for clinical implementation. Medicina (Kaunas) 58(7):952.
Cummings, J., A. Ritter, and K. Zhong. 2018. Clinical trials for disease-modifying therapies in Alzheimer’s disease: A primer, lessons learned, and a blueprint for the future. Journal of Alzheimer’s Disease 64(s1):S3-S22.
Cummings, J., Y. Zhou, G. Lee, K. Zhong, J. Fonseca, and F. Cheng. 2023a. Alzheimer’s disease drug development pipeline: 2023. Alzheimer’s & Dementia (NY) 9(2):e12385.
Cummings, J. L., A. M. L. Osse, and J. W. Kinney. 2023b. Alzheimer’s disease: Novel targets and investigational drugs for disease modification. Drugs 83(15):1387-1408.
Cummings, J., Y. Zhou, G. Lee, K. Zhong, J. Fonseca, and F. Cheng. 2024. Alzheimer’s disease drug development pipeline: 2024. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 10(2):e12465.
Cunningham, E. L., S. A. Todd, P. Passmore, R. Bullock, and B. McGuinness. 2021. Pharmacological treatment of hypertension in people without prior cerebrovascular disease for the prevention of cognitive impairment and dementia. Cochrane Database of Systematic Reviews 5(5):CD004034.
Dahabreh, I., T. Terasawa, P. Castaldi, and T. A. Trikalinos. 2010. CYP2D6 testing to predict response to tamoxifen in women with breast cancer: Pharmacogenomic. PLoS Currents 2:RRN1176.
Davidson, K. W., M. Silverstein, K. Cheung, R. A. Paluch, and L. H. Epstein. 2021. Experimental designs to optimize treatments for individuals: Personalized N-of-1 trials. JAMA Pediatrics 175(4):404-409.
Dawes, P., L. Wolski, I. Himmelsbach, J. Regan, and I. Leroi. 2019. Interventions for hearing and vision impairment to improve outcomes for people with dementia: A scoping review. International Psychogeriatrics 31(2):203-221.
De la Rosa, A., G. Olaso-Gonzalez, C. Arc-Chagnaud, F. Millan, A. Salvador-Pascual, C. García-Lucerga, C. Blasco-Lafarga, E. Garcia-Dominguez, A. Carretero, A. G. Correas, J. Viña, and M. C. Gomez-Cabrera. 2020. Physical exercise in the prevention and treatment of Alzheimer’s disease. Journal of Sport and Health Science 9(5):394-404.
Deckers, K., S. Köhler, T. Ngandu, R. Antikainen, T. Laatikainen, H. Soininen, T. Strandberg, F. Verhey, M. Kivipelto, and A. Solomon. 2021. Quantifying dementia prevention potential in the FINGER randomized controlled trial using the LIBRA prevention index. Alzheimer’s & Dementia 17(7):1205-1212.
Del Campo, M., H. Zetterberg, S. Gandy, C. U. Onyike, F. Oliveira, C. Udeh-Momoh, A. Lleó, C. E. Teunissen, and Y. Pijnenburg. 2022. New developments of biofluid-based biomarkers for routine diagnosis and disease trajectories in frontotemporal dementia. Alzheimer’s & Dementia 18(11):2292-2307.
Deng, Z., P. Sheehan, S. Chen, and Z. Yue. 2017. Is amyotrophic lateral sclerosis/frontotemporal dementia an autophagy disease? Molecular and Neurodegeneration 12(1):90.
Devi, G. 2023. A how-to guide for a precision medicine approach to the diagnosis and treatment of Alzheimer’s disease. Frontiers in Aging and Neuroscience 15:1213968.
DeVos, S. L., R. L. Miller, K. M. Schoch, B. B. Holmes, C. S. Kebodeaux, A. J. Wegener, G. Chen, T. Shen, H. Tran, B. Nichols, T. A. Zanardi, H. B. Kordasiewicz, E. E. Swayze, C. F. Bennett, M. I. Diamond, and T. M. Miller. 2017. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Science Translational Medicine 9(374):eaag0481.
DiBenedetti, D. B., C. Slota, S. L. Wronski, G. Vradenburg, M. Comer, L. F. Callahan, J. Winfield, I. Rubino, H. B. Krasa, A. Hartry, D. Wieberg, I. N. Kremer, D. Lappin, A. D. Martin, T. Frangiosa, V. Biggar, and B. Hauber. 2020. Assessing what matters most to patients with or at risk for Alzheimer’s and care partners: A qualitative study evaluating symptoms, impacts, and outcomes. Alzheimer’s Research & Therapy 12(1):90.
Djajadikerta, A., S. Keshri, M. Pavel, R. Prestil, L. Ryan, and D. C. Rubinsztein. 2020. Autophagy induction as a therapeutic strategy for neurodegenerative diseases. Journal of Molecular Biology 432(8):2799-2821.
Duff, K., T. J. Atkinson, K. R. Suhrie, B. C. A. Dalley, S. Y. Schaefer, and D. B. Hammers. 2017. Short-term practice effects in mild cognitive impairment: Evaluating different methods of change. Journal of Clinical and Experimental Neuropsychology 39(4):396-407.
Duncan, M. J., H. Farlow, C. Tirumalaraju, D.-H. Yun, C. Wang, J. A. Howard, M. N. Sanden, B. F. O’Hara, K. J. McQuerry, and A. D. Bachstetter. 2019. Effects of the dual orexin receptor antagonist DORA-22 on sleep in XFAD mice. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 5(1):70-80.
ECHAR (Engaging Communities of Hispanics/Latinos for Aging Research). 2024. Welcome to the ECHAR network. https://www.echarnetwork.com/ (accessed August 19, 2024).
Edwards, A. L., J. A. Collins, C. Junge, H. Kordasiewicz, L. Mignon, S. Wu, Y. Li, L. Lin, J. DuBois, R. M. Hutchison, N. Ziogas, M. Shulman, L. Martarello, D. Graham, R. Lane, S. Budd Haeberlein, and J. Beaver. 2023. Exploratory tau biomarker results from a multiple ascending-dose study of BIIB080 in Alzheimer disease: A randomized clinical trial. JAMA Neurology 80(12):1344-1352.
Edwards, J. D., H. Xu, D. O. Clark, L. T. Guey, L. A. Ross, and F. W. Unverzagt. 2017. Speed of processing training results in lower risk of dementia. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 3(4):603-611.
Ehrlich, J. R., J. Goldstein, B. K. Swenor, H. Whitson, K. M. Langa, and P. Veliz. 2022. Addition of vision impairment to a life-course model of potentially modifiable dementia risk factors in the U.S. JAMA Neurology 79(6):623-626.
Elovainio, M., J. Lahti, M. Pirinen, L. Pulkki-Råback, A. Malmberg, J. Lipsanen, M. Virtanen, M. Kivimäki, and C. Hakulinen. 2022. Association of social isolation, loneliness and genetic risk with incidence of dementia: UK Biobank Cohort Study. BMJ Open 12(2):e053936.
Eshraghi, M., M. Ahmadi, S. Afshar, S. Lorzadeh, A. Adlimoghaddam, N. Rezvani Jalal, R. West, S. Dastghaib, S. Igder, S. R. N. Torshizi, A. Mahmoodzadeh, P. Mokarram, T. Madrakian, B. C. Albensi, M. J. Łos, S. Ghavami, and S. Pecic. 2022. Enhancing autophagy in Alzheimer’s disease through drug repositioning. Pharmacology & Therapeutics 237:108171.
Esserman, L. 2024. Panelist remarks. Presentation at the Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
Fairburn, J., S. A. Schüle, S. Dreger, L. Karla Hilz, and G. Bolte. 2019. Social inequalities in exposure to ambient air pollution: A systematic review in the WHO European region. International Journal of Environmental Research and Public Health 16(17):3127.
Faison, W. E., S. K. Schultz, J. Aerssens, J. Alvidrez, R. Anand, L. A. Farrer, L. Jarvik, J. Manly, T. McRae, G. M. Murphy, Jr., J. T. Olin, D. Regier, M. Sano, and J. E. Mintzer. 2007. Potential ethnic modifiers in the assessment and treatment of Alzheimer’s disease: Challenges for the future. International Psychogeriatrics 19(3):539-558.
Fang, J., A. A. Pieper, R. Nussinov, G. Lee, L. Bekris, J. B. Leverenz, J. Cummings, and F. Cheng. 2020. Harnessing endophenotypes and network medicine for Alzheimer’s drug repurposing. Medical Research Reviews 40(6):2386-2426.
Farina, M. P., Y. S. Zhang, J. K. Kim, M. D. Hayward, and E. M. Crimmins. 2022. Trends in dementia prevalence, incidence, and mortality in the United States (2000-2016). Journal of Aging and Health 34(1):100-108.
FDA (U.S. Food and Drug Administration). 2016. FDA approves first drug for spinal muscular atrophy. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-spinal-muscular-atrophy (accessed August 19, 2024).
FDA. 2018. Evaluating inclusion and exclusion criteria in clinical trials: Workshop report. National Press Club: Washington, D.C.
FDA. 2019a. FDA approves innovative gene therapy to treat pediatric patients with spinal muscular atrophy, a rare disease and leading genetic cause of infant mortality. https://www.fda.gov/news-events/press-announcements/fda-approves-innovative-gene-therapy-treat-pediatric-patients-spinal-muscular-atrophy-rare-disease (accessed September 24, 2024).
FDA. 2019b. Enrichment strategies for clinical trials to support approval of human drugs and biological products. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enrichment-strategies-clinical-trials-support-approval-human-drugs-and-biological-products (accessed August 20, 2024).
FDA. 2020a. Qualification process for drug development tools guidance for industry and FDA staff. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/qualification-process-drug-development-tools-guidance-industry-and-fda-staff (accessed August 20, 2024).
FDA. 2020b. FDA approves first drug to image tau pathology in patients being evaluated for Alzheimer’s disease. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-image-tau-pathology-patients-being-evaluated-alzheimers-disease (accessed August 14, 2024).
FDA. 2023. FDA approves treatment of amyotrophic lateral sclerosis associated with a mutation in the SOD1 gene. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-amyotrophic-lateral-sclerosis-associated-mutation-sod1-gene (accessed August 19, 2024).
FDA. 2024a. Early Alzheimer’s disease: Developing drugs for treatment. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/early-alzheimers-disease-developing-drugs-treatment (accessed August 19, 2024).
FDA. 2024b. FDA approves treatment for adults with Alzheimer’s disease. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-adults-alzheimers-disease (accessed October 23, 2024).
Ferreira, P., A. R. Ferreira, and L. Fernandes. 2021. Use of benzodiazepines and related drugs and the risk of dementia: A review of reviews. European Psychiatry 64(S1):S423-S423.
Fillit, H., J. Cummings, P. Neumann, T. McLaughlin, P. Salavtore, and C. Leibman. 2010. Novel approaches to incorporating pharmacoeconomic studies into phase III clinical trials for Alzheimer’s disease. Journal of Nutrition, Health and Aging 14(8):640-647.
Finlay, J., M. Esposito, K. M. Langa, S. Judd, and P. Clarke. 2022. Cognability: An ecological theory of neighborhoods and cognitive aging. Social Science and Medicine 309:115220.
FNIH (Foundation for the National Institutes of Health). 2023. Biomarkers Consortium—I-SPY Trial-2 investigation of serial studies to predict your therapeutic response with imaging and molecular analysis: An adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. https://fnih.org/our-programs/biomarkers-consortium-i-spy-trial-2-investigation-of-serial-studies-to-predict-your-therapeutic-response-with-imaging-and-molecular-analysis-an-adaptive-breast-cancer-trial-design-in-the-setting/ (accessed August 20, 2024).
Forette, F., M.-L. Seux, J. A. Staessen, L. Thijs, M.-R. Babarskiene, S. Babeanu, A. Bossini, R. Fagard, B. Gil-Extremera, T. Laks, Z. Kobalava, C. Sarti, J. Tuomilehto, H. Vanhanen, J. Webster, Y. Yodfat, and W. H. Birkenhäger; Systolic Hypertension in Europe Investigators. 2002. The prevention of dementia with antihypertensive treatment: New evidence from the Systolic Hypertension in Europe (Syst-Eur) Study. Archives of Internal Medicine 162(18):2046-2052.
Frenkel, D., L. Puckett, S. Petrovic, W. Xia, G. Chen, J. Vega, A. Dembinsky-Vaknin, J. Shen, M. Plante, D. S. Burt, and H. L. Weiner. 2008. A nasal proteosome adjuvant activates microglia and prevents amyloid deposition. Annals of Neurology 63(5):591-601.
Fukuoka, H., C. Sutu, and N. A. Afshari. 2016. The impact of cataract surgery on cognitive function in an aging population. Current Opinions in Ophthalmology 27(1):3-8.
Gates, N. J., R. W. M. Vernooij, M. Di Nisio, S. Karim, E. March, G. Martínez, and A. W. S. Rutjes. 2019. Computerised cognitive training for preventing dementia in people with mild cognitive impairment. Cochrane Database of Systematic Reviews 3(3):CD012279.
Gates, N. J., A. W. S. Rutjes, M. Di Nisio, S. Karim, L. Y. Chong, E. March, G. Martínez, and R. W. M. Vernooij. 2020. Computerised cognitive training for 12 or more weeks for maintaining cognitive function in cognitively healthy people in late life. Cochrane Database of Systematic Reviews 2(2):CD012277.
Gavelin, H. M., C. Dong, R. Minkov, A. Bahar-Fuchs, K. A. Ellis, N. T. Lautenschlager, M. L. Mellow, A. T. Wade, A. E. Smith, C. Finke, S. Krohn, and A. Lampit. 2021. Combined physical and cognitive training for older adults with and without cognitive impairment: A systematic review and network meta-analysis of randomized controlled trials. Ageing Research Reviews 66:101232.
Geifman, N., R. E. Kennedy, L. S. Schneider, I. Buchan, and R. D. Brinton. 2018. Data-driven identification of endophenotypes of Alzheimer’s disease progression: Implications for clinical trials and therapeutic interventions. Alzheimer’s Research & Therapy 10(1):4.
Gelon, P. A., P. A. Dutchak, and C. F. Sephton. 2022. Synaptic dysfunction in ALS and FTD: Anatomical and molecular changes provide insights into mechanisms of disease. Frontiers in Molecular Neuroscience 15:1000183.
Gerlach, L. B., I. R. Wiechers, and D. T. Maust. 2018. Prescription benzodiazepine use among older adults: A critical review. Harvard Review of Psychiatry 26(5):264-273.
Gibson, L. L., C. Abdelnour, J. Chong, C. Ballard, and D. Aarsland. 2023. Clinical trials in dementia with Lewy bodies: The evolving concept of co-pathologies, patient selection and biomarkers. Current Opinion in Neurology 36(4):264-275.
Goldman, S. A. 2016. Stem and progenitor cell-based therapy of the central nervous system: Hopes, hype, and wishful thinking. Cell Stem Cell 18(2):174-188.
Goldman, J. G., L. K. Forsberg, B. F. Boeve, M. J. Armstrong, D. J. Irwin, T. J. Ferman, D. Galasko, J. E. Galvin, D. Kaufer, J. Leverenz, C. F. Lippa, K. Marder, V. Abler, K. Biglan, M. Irizarry, B. Keller, L. Munsie, M. Nakagawa, A. Taylor, and T. Graham. 2020. Challenges and opportunities for improving the landscape for Lewy body dementia clinical trials. Alzheimer’s Research & Therapy 12(1):137.
Gomez-Soria, I., I. Iguacel, J. N. Cuenca-Zaldivar, A. Aguilar-Latorre, P. Peralta-Marrupe, E. Latorre, and E. Calatayud. 2023. Cognitive stimulation and psychosocial results in older adults: A systematic review and meta-analysis. Archives of Gerontology and Geriatrics 115:105114.
Gonzales, M. M., V. R. Garbarino, T. F. Kautz, J. P. Palavicini, M. Lopez-Cruzan, S. K. Dehkordi, J. J. Mathews, H. Zare, P. Xu, B. Zhang, C. Franklin, M. Habes, S. Craft, R. C. Petersen, T. Tchkonia, J. L. Kirkland, A. Salardini, S. Seshadri, N. Musi, and M. E. Orr. 2023. Senolytic therapy in mild Alzheimer’s disease: A phase 1 feasibility trial. Nature Medicine 29(10):2481-2488.
Gove, D., A. Diaz-Ponce, J. Georges, E. Moniz-Cook, G. Mountain, R. Chattat, and L. Øksnebjerg. 2018. Alzheimer Europe’s position on involving people with dementia in research through PPI (patient and public involvement). Aging & Mental Health 22(6):723-729.
Green, G. S., M. Fujita, H. S. Yang, M. Taga, A. Cain, C. McCabe, N. Comandante-Lou, C. C. White, A. K. Schmidtner, L. Zeng, A. Sigalov, Y. Wang, A. Regev, H. U. Klein, V. Menon, D. A. Bennett, N. Habib, and P. L. De Jager. 2024. Cellular communities reveal trajectories of brain ageing and Alzheimer’s disease. Nature 633(8030):634-645.
Gregory, S., S. Saunders, and C. W. Ritchie. 2022. Science disconnected: The translational gap between basic science, clinical trials, and patient care in Alzheimer’s disease. Lancet Healthy Longevity 3(11):e797-e803.
Groot, C., A. M. Hooghiemstra, P. G. H. M. Raijmakers, B. N. M. van Berckel, P. Scheltens, E. J. A. Scherder, W. M. van der Flier, and R. Ossenkoppele. 2016. The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Research Reviews 25:13-23.
Guarnera, J., E. Yuen, and H. Macpherson. 2023. The impact of loneliness and social isolation on cognitive aging: A narrative review. Journal of Alzheimer’s Disease Reports 7(1):699-714.
Gutierrez, B. A., and A. Limon. 2022. Synaptic disruption by soluble oligomers in patients with Alzheimer’s and Parkinson’s disease. Biomedicines 10(7):1743.
Guure, C. B., N. A. Ibrahim, M. B. Adam, and S. M. Said. 2017. Impact of physical activity on cognitive decline, dementia, and its subtypes: Meta-analysis of prospective studies. BioMed Research International 2017(1):9016924.
Hall, A., M. Barbera, J. Lehtisalo, R. Antikainen, H. Huque, T. Laatikainen, T. Ngandu, H. Soininen, R. Stephen, T. Strandberg, M. Kivipelto, K. J. Anstey, and A. Solomon. 2024. The Australian National University Alzheimer’s Disease Risk Index (ANU-ADRI) score as a predictor for cognitive decline and potential surrogate outcome in the FINGER lifestyle randomized controlled trial. European Journal of Neurology 31(5):e16238.
Hampel, H., S. E. O’Bryant, S. Durrleman, E. Younesi, K. Rojkova, V. Escott-Price, J. C. Corvol, K. Broich, B. Dubois, and S. Lista. 2017. A precision medicine initiative for Alzheimer’s disease: The road ahead to biomarker-guided integrative disease modeling. Climacteric 20(2):107-118.
Hampel, H., A. Vergallo, G. Perry, and S. Lista; Alzheimer Precision Medicine Initiative. 2019. The Alzheimer Precision Medicine Initiative. Journal of Alzheimer’s Disease 68(1):1-24.
Hampel, H., F. Caraci, A. C. Cuello, G. Caruso, R. Nistico, M. Corbo, F. Baldacci, N. Toschi, F. Garaci, P. A. Chiesa, S. R. Verdooner, L. Akman-Anderson, F. Hernandez, J. Avila, E. Emanuele, P. L. Valenzuela, A. Lucia, M. Watling, B. P. Imbimbo, A. Vergallo, and S. Lista. 2020. A path toward precision medicine for neuroinflammatory mechanisms in Alzheimer’s disease. Frontiers in Immunology 11:456.
Hampel, H., R. Au, S. Mattke, W. M. van der Flier, P. Aisen, L. Apostolova, C. Chen, M. Cho, S. De Santi, P. Gao, A. Iwata, R. Kurzman, A. J. Saykin, S. Teipel, B. Vellas, A. Vergallo, H. Wang, and J. Cummings. 2022. Designing the next-generation clinical care pathway for Alzheimer’s disease. Nature Aging 2(8):692-703.
Hao, X., R. Abeysinghe, F. Zheng, P. E. Schulz, Alzheimer’s Disease Neuroimaging Initiative, and L. Cui. 2024. Mapping of Alzheimer’s disease related data elements and the NIH Common Data Elements. BMC Medical Informatics and Decision Making 24(Suppl 3):103.
Harding, A. J. E., H. Morbey, F. Ahmed, C. Opdebeeck, R. Elvish, I. Leroi, P. R. Williamson, J. Keady, and S. T. Reilly. 2020. A core outcome set for nonpharmacological community-based interventions for people living with dementia at home: A systematic review of outcome measurement instruments. The Gerontologist 61(8):e435-e448.
Harrison, J. R., S. Mistry, N. Muskett, and V. Escott-Price. 2020. From polygenic scores to precision medicine in Alzheimer’s disease: A systematic review. Journal of Alzheimer’s Disease 74:1271-1283.
Hartry, A., N. V. J. Aldhouse, T. Al-Zubeidi, M. Sanon, R. G. Stefanacci, and S. L. Knight. 2018. The conceptual relevance of assessment measures in patients with mild/mild-moderate Alzheimer’s disease. Alzheimers & Dementia (Amsterdam, Netherlands) 10:498-508.
Hauptman, M., M. L. Rogers, M. Scarpaci, B. Morin, and P. M. Vivier. 2023. Neighborhood disparities and the burden of lead poisoning. Pediatric Research 94(2):826-836.
He, W., M. Wang, L. Jiang, M. Li, and X. Han. 2019. Cognitive interventions for mild cognitive impairment and dementia: An overview of systematic reviews. Complementary Therapies in Medicine 47:102199.
Hekler, E., J. A. Tiro, C. M. Hunter, and C. Nebeker. 2020. Precision health: The role of the social and behavioral sciences in advancing the vision. Annals of Behavioral Medicine 54(11):805-826.
Heller, C., J. E. Balls-Berry, J. D. Nery, P. J. Erwin, D. Littleton, M. Kim, and W. P. Kuo. 2014. Strategies addressing barriers to clinical trial enrollment of underrepresented populations: A systematic review. Contemporary Clinical Trials 39(2):169-182.
Higginbotham, L., L. Ping, E. B. Dammer, D. M. Duong, M. Zhou, M. Gearing, C. Hurst, J. D. Glass, S. A. Factor, E. C. B. Johnson, I. Hajjar, J. J. Lah, A. I. Levey, N. T. Seyfried. 2020. Integrated proteomics reveals brain-based cerebrospinal fluid biomarkers in asymptomatic and symptomatic Alzheimer’s disease. Science Advances 6(43):eaaz9360.
Higginbotham, L., E. K. Carter, E. B. Dammer, R. U. Haque, E. C. B. Johnson, D. M. Duong, L. Yin, P. L. De Jayer, D. A. Bennett, D. Felsky, E. S. Tio, J. J. Lah, A. I. Levey, and N. T. Seyfried. 2023. Unbiased classification of the elderly human brain proteome resolves distinct clinical and pathophysiological subtypes of cognitive impairment. Neurobiology of Disease 186:106286.
Hobbs, B. P., P. C. Barata, Y. Kanjanapan, C. J. Paller, J. Perlmutter, G. R. Pond, T. M. Prowell, E. H. Rubin, L. K. Seymour, N. A. Wages, T. A. Yap, D. Feltquate, E. Garrett-Mayer, W. Grossman, D. S. Hong, S. P. Ivy, L. L. Siu, S. A. Reeves, and G. L. Rosner. 2019. Seamless designs: Current practice and considerations for early-phase drug development in oncology. Journal of the National Cancer Institute 111(2):118-128.
Hohman, T. J., J. N. Cooke-Bailey, C. Reitz, G. Jun, A. Naj, G. W. Beecham, Z. Liu, R. M. Carney, J. M. Vance, M. L. Cuccaro, R. Rajbhandary, B. N. Vardarajan, L. S. Wang, O. Valladares, C. F. Lin, E. B. Larson, N. R. Graff-Radford, D. Evans, P. L. De Jager, P. K. Crane, J. D. Buxbaum, J. R. Murrell, T. Raj, N. Ertekin-Taner, M. W. Logue, C. T. Baldwin, R. C. Green, L. L. Barnes, L. B. Cantwell, M. D. Fallin, R. C. Go, P. Griffith, T. O. Obisesan, J. J. Manly, K. L. Lunetta, M. I. Kamboh, O. L. Lopez, D. A. Bennett, J. Hardy, H. C. Hendrie, K. S. Hall, A. M. Goate, R. Lang, G. S. Byrd, W. A. Kukull, T. M. Foroud, L. A. Farrer, E. R. Martin, M. A. Pericak-Vance, G. D. Schellenberg, R. Mayeux, J. L. Haines, and T. A. Thornton-Wells; Alzheimer Disease Genetics Consortium. 2016. Global and local ancestry in African-Americans: Implications for Alzheimer’s disease risk. Alzheimer’s & Dementia 12(3):233-243.
Hölscher, C. 2022. Protective properties of GLP-1 and associated peptide hormones in neurodegenerative disorders. British Journal of Pharmacology 179(4):695-714.
Hou, B., Z. Wen, J. Bao, R. Zhang, B. Tong, S. Yang, J. Wen, Y. Cui, J. H. Moore, A. J. Saykin, H. Huang, P. M. Thompson, M. D. Ritchie, C. Davatzikos, and L. Shen; Alzheimer’s Disease Neuroimaging Initiative. 2024. Interpretable deep clustering survival machines for Alzheimer’s disease subtype discovery. Medical Image Analysis 97:103231.
Hu, M., X. Wu, X. Shu, H. Hu, Q. Chen, L. Peng, and H. Feng. 2021. Effects of computerised cognitive training on cognitive impairment: A meta-analysis. Journal of Neurology 268(5):1680-1688.
Hu, Y., J. Huerta, N. Cordella, R. G. Mishuris, and I. C. Paschalidis. 2023. Personalized hypertension treatment recommendations by a data-driven model. BMC Medical Informatics and Decision Making 23(1):44.
ICER (Institute for Clinical and Economic Review). 2021. Aducanumab for Alzheimer’s disease: Effectiveness and value. https://icer.org/wp-content/uploads/2020/10/ICER_ALZ_Final_Report_080521.pdf (accessed September 16, 2024).
Indorewalla, K. K., M. K. O’Connor, A. E. Budson, C. Guess DiTerlizzi, and J. Jackson. 2021. Modifiable barriers for recruitment and retention of older adults participants from underrepresented minorities in Alzheimer’s disease research. Journal of Alzheimer’s Disease 80(3):927-940.
Irizarry, M. 2024. Panelist Remarks. Presentation at the Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
Islam, M. T., E. Tuday, S. Allen, J. Kim, D. W. Trott, W. L. Holland, A. J. Donato, and L. A. Lesniewski. 2023. Senolytic drugs, dasatinib and quercetin, attenuate adipose tissue inflammation, and ameliorate metabolic function in old age. Aging Cell 22(2):e13767.
İş, Ö., X. Wang, J. S. Reddy, Y. Min, E. Yilmaz, P. Bhattarai, T. Patel, J. Bergman, Z. Quicksall, M. G. Heckman, F. Q. Tutor-New, B. Can Demirdogen, L. White, S. Koga, V. Krause, Y. Inoue, T. Kanekiyo, M. I. Cosacak, N. Nelson, A. J. Lee, B. Vardarajan, R. Mayeux, N. Kouri, K. Deniz, T. Carnwath, S. R. Oatman, L. J. Lewis-Tuffin, T. Nguyen, ADNI, M. M. Carrasquillo, J. Graff-Radford, R. C. Petersen, C. R. Jack, Jr., K. Kantarci, M. E. Murray, K. Nho, A. J. Saykin, D. W. Dickson, C. Kizil, M. Allen, and N. Ertekin-Taner. 2024. Gliovascular transcriptional perturbations in Alzheimer’s disease reveal molecular mechanisms of blood brain barrier dysfunction. Nature Communications 15(1):4758.
Iso-Markku, P., U. M. Kujala, K. Knittle, J. Polet, E. Vuoksimaa, and K. Waller. 2022. Physical activity as a protective factor for dementia and Alzheimer’s disease: Systematic review, meta-analysis and quality assessment of cohort and case-control studies. British Journal of Sports Medicine 56(12):701-709.
Iturria-Medina, Y., Q. Adewale, A. F. Khan, S. Ducharme, P. Rosa-Neto, K. O’Donnell, V. A. Petyuk, S. Gauthier, P. L. De Jager, J. Breitner, D. A. Bennett. 2022. Unified epigenomic, transcriptomic, proteomic, and metabolomic taxonomy of Alzheimer’s disease progression and heterogeneity. Science Advances 8(46):eabo6764.
Jaeggi, S. M., A. N. Weaver, E. Carbone, F. E. Trane, R. N. Smith-Peirce, M. Buschkuehl, C. Flueckiger, M. Carlson, J. Jonides, and E. Borella. 2023. EngAge—a metacognitive intervention to supplement working memory training: A feasibility study in older adults. Aging Brain 4:100083.
Jain, P., C. Jain, and V. Velcheti. 2018. Role of immune-checkpoint inhibitors in lung cancer. Therapeutic Advances in Respiratory Disease 12:1753465817750075.
Jayakody, D. M. P., P. L. Friedland, R. N. Martins, and H. R. Sohrabi. 2018. Impact of aging on the auditory system and related cognitive functions: A narrative review. Frontiers in Neuroscience 12:125.
Jessen, F., J. Georges, M. Wortmann, and S. Benham-Hermetz. 2022. What matters to patients with Alzheimer’s disease and their care partners? Implications for understanding the value of future interventions. Journal of Prevention of Alzheimer’s Disease 9(3):550-555.
Jimenez-Puerta, G. J., J. A. Marchal, E. López-Ruiz, and P. Gálvez-Martín. 2020. Role of mesenchymal stromal cells as therapeutic agents: Potential mechanisms of action and implications in their clinical use. Journal of Clinical Medicine 9(2):445.
Jin, S. C., M. M. Carrasquillo, B. A. Benitez, T. Skorupa, D. Carrell, D. Patel, S. Lincoln, S. Krishnan, M. Kachadoorian, C. Reitz, R. Mayeux, T. S. Wingo, J. J. Lah, A. I. Levey, J. Murrell, H. Hendrie, T. Foroud, N. R. Graff-Radford, A. M. Goate, C. Cruchaga, and N. Ertekin-Taner. 2015. TREM2 is associated with increased risk for Alzheimer’s disease in African Americans. Molecular Neurodegeneration 10:19.
Jin, S., X. Guan, and D. Min. 2023. Evidence of clinical efficacy and pharmacological mechanisms of resveratrol in the treatment of Alzheimer’s disease. Current Alzheimer’s Research 20(8):588-602.
Johnson, E. C. B., E. K. Carter, E. B. Dammer, D. M. Duong, E. S. Gerasimov, Y. Liu, J. Liu, R. Betarbet, L. Ping, L. Yin, G. E. Serrano, T. G. Beach, J. Peng, P. L. De Jager, V. Haroutunian, B. Zhang, C. Gaiteri, D. A. Bennett, M. Gearing, T. S. Wingo, A. P. Wingo, J. J. Lah, A. I. Levey, and N. T. Seyfried. 2022. Large-scale deep multi-layer analysis of Alzheimer’s disease brain reveals strong proteomic disease-related changes not observed at the RNA level. Nature Neuroscience 25(2):213-225.
Joshi, P., K. Hendrie, D. J. Jester, D. Dasarathy, H. Lavretsky, B. S. Ku, H. Leutwyler, J. Torous, D. V. Jeste, and R. R. Tampi. 2024. Social connections as determinants of cognitive health and as targets for social interventions in persons with or at risk of Alzheimer’s disease and related disorders: A scoping review. International Psychogeriatrics 36(2):92-118.
Kabir, M. T., M. S. Uddin, A. A. Mamun, P. Jeandet, L. Aleya, R. A. Mansouri, G. M. Ashraf, B. Mathew, M. N. Bin-Jumah, and M. M. Abdel-Daim. 2020. Combination drug therapy for the management of Alzheimer’s disease. International Journal of Molecular Science 21(9):3272.
Karamacoska, D., A. Butt, I. H. K. Leung, R. L. Childs, N. J. Metri, V. Uruthiran, T. Tan, A. Sabag, and G. Z. Steiner-Lim. 2023. Brain function effects of exercise interventions for cognitive decline: A systematic review and meta-analysis. Frontiers in Neuroscience 17:1127065.
Kelly, M. E., H. Duff, S. Kelly, J. E. McHugh Power, S. Brennan, B. A. Lawlor, and D. G. Loughrey. 2017. The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: A systematic review. Systematic Reviews 6(1):259.
Khoury, R., J. Rajamanickam, and G. T. Grossberg. 2018. An update on the safety of current therapies for Alzheimer’s disease: Focus on rivastigmine. Therapeutic Advances in Drug Safety 9(3):171-178.
Khoury, R., Y. Liu, Q. Sheheryar, and G. T. Grossberg. 2021. Pharmacotherapy for frontotemporal dementia. CNS Drugs 35(4):425-438.
Kilian, J., and M. Kitazawa. 2018. The emerging risk of exposure to air pollution on cognitive decline and Alzheimer’s disease—evidence from epidemiological and animal studies. Biomedical Journal 41(3):141-162.
Kim, O., Y. Pang, and J.-H. Kim. 2019. The effectiveness of virtual reality for people with mild cognitive impairment or dementia: A meta-analysis. BMC Psychiatry 19(1):219.
Kind, A. 2024. Panelist remarks. Presentation at the Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
Kiyota, T., J. Machhi, Y. Lu, B. Dyavarshetty, M. Nemati, I. Yokoyama, R. L. Mosley, and H. E. Gendelman. 2018. Granulocyte-macrophage colony-stimulating factor neuroprotective activities in Alzheimer’s disease mice. Journal of Neuroimmunology 319:80-92.
Klionsky, D. J., and S. D. Emr. 2000. Autophagy as a regulated pathway of cellular degradation. Science 290(5497):1717-1721.
Koivisto, J., and A. Malik. 2021. Gamification for older adults: A systematic literature review. Gerontologist 61(7):e360-e372.
Krzeczkowska, A., D. M. Spalding, W. J. McGeown, A. J. Gow, M. C. Carlson, and L. A. B. Nicholls. 2021. A systematic review of the impacts of intergenerational engagement on older adults’ cognitive, social, and health outcomes. Ageing Research Reviews 71:101400.
Kulmala, J., T. Ngandu, S. Havulinna, E. Levälahti, J. Lehtisalo, A. Solomon, R. Antikainen, T. Laatikainen, P. Pippola, M. Peltonen, R. Rauramaa, H. Soininen, T. Strandberg, J. Tuomilehto, and M. Kivipelto. 2019. The effect of multidomain lifestyle intervention on daily functioning in older people. Journal of the American Geriatric Society 67(6):1138-1144.
Kwak, K. A., S. P. Lee, J. Y. Yang, and Y. S. Park. 2018. Current perspectives regarding stem cell-based therapy for Alzheimer’s disease. Stem Cells International 2018:6392986.
Largent, E. A., and H. F. Lynch. 2017. Paying research participants: The outsized influence of “undue influence.” IRB 39(4):1-9.
Laske, C., H. R. Sohrabi, S. M. Frost, K. López-de-Ipiña, P. Garrard, M. Buscema, J. Dauwels, S. R. Soekadar, S. Mueller, C. Linnemann, S. A. Bridenbaugh, Y. Kanagasingam, R. N. Martins, and S. E. O’Bryant. 2015. Innovative diagnostic tools for early detection of Alzheimer’s disease. Alzheimer’s & Dementia 11(5):561-578.
Lazar, A. A., B. F. Cole, M. Bonetti, and R. D. Gelber. 2010. Evaluation of treatment-effect heterogeneity using biomarkers measured on a continuous scale: Subpopulation treatment effect pattern plot. Journal of Clinical Oncology 28(29):4539-4544.
Leinenga, G., X. V. To, L.-G. Bodea, J. Yousef, G. Richter-Stretton, T. Palliyaguru, A. Chicoteau, L. Dagley, F. Nasrallah, and J. Götz. 2024. Scanning ultrasound-mediated memory and functional improvements do not require amyloid-β reduction. Molecular Psychiatry 29(8):2408-2423.
Lelo de Larrea-Mancera, E. S., T. Stavropoulos, A. A. Carrillo, S. Cheung, Y. J. He, D. A. Eddins, M. R. Molis, F. J. Gallun, and A. R. Seitz. 2022. Remote auditory assessment using portable automated rapid testing (PART) and participant-owned devices). Journal of the Acoustical Society of America 152(2):807-819.
Leng, Y., and K. Yaffe. 2024. Harnessing brain pathology for dementia prevention. JAMA Neurology 81(3):229-231.
Lennon, M. J., G. Rigney, V. Raymont, and P. Sachdev. 2021. Genetic therapies for Alzheimer’s disease: A scoping review. Journal of Alzheimer’s Disease 84:491-504.
Li, S., and D. J. Selkoe. 2020. A mechanistic hypothesis for the impairment of synaptic plasticity by soluble Aβ oligomers from Alzheimer’s brain. Journal of Neurochemistry 154(6):583-597.
Li, X., M. Ji, H. Zhang, Z. Liu, Y. Chai, Q. Cheng, Y. Yang, D. Cordato, and J. Gao. 2023. Non-drug therapies for Alzheimer’s disease: A review. Neurology and Therapy 12(1):39-72.
Lian, P., X. Cai, C. Wang, K. Liu, X. Yang, Y. Wu, Z. Zhang, Z. Ma, X. Cao, and Y. Xu. 2023. Identification of metabolism-related subtypes and feature genes in Alzheimer’s disease. Journal of Translational Medicine 21(1):628.
Liguori, C., A. Romigi, M. Nuccetelli, S. Zannino, G. Sancesario, A. Martorana, M. Albanese, N. B. Mercuri, F. Izzi, S. Bernardini, A. Nitti, G. M. Sancesario, F. Sica, M. G. Marciani, and F. Placidi. 2014. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer’s disease. JAMA Neurology 71(12):1498-1505.
Lim, Y. Y., J. Kong, P. Maruff, J. Jaeger, E. Huang, and E. Ratti. 2022. Longitudinal cognitive decline in patients with mild cognitive impairment or dementia due to Alzheimer’s disease. Journal of Prevention of Alzheimer’s Disease 9(1):178-183.
Lin, H., L. Zhang, D. Lin, W. Chen, Y. Zhu, C. Chen, K. C. Chan, Y. Liu, and W. Chen. 2018. Visual restoration after cataract surgery promotes functional and structural brain recovery. EBioMedicine 30:52-61.
Liu, Q., N. Vaci, I. Koychev, A. Kormilitzin, Z. Li, A. Cipriani, and A. Nevado-Holgado. 2022. Personalised treatment for cognitive impairment in dementia: Development and validation of an artificial intelligence model. BMC Medicine 20(1):45.
Liu, Z., Q. Liang, Y. Ren, C. Guo, X. Ge, L. Wang, Q. Cheng, P. Luo, Y. Zhang, and X. Han. 2023. Immunosenescence: Molecular mechanisms and diseases. Signal Transduction and Targeted Therapy 8(1):200.
Livingston, G., J. Huntley, A. Sommerlad, D. Ames, C. Ballard, S. Banerjee, C. Brayne, A. Burns, J. Cohen-Mansfield, C. Cooper, S. G. Costafreda, A. Dias, N. Fox, L. N. Gitlin, R. Howard, H. C. Kales, M. Kivimäki, E. B. Larson, A. Ogunniyi, V. Orgeta, K. Ritchie, K. Rockwood, E. L. Sampson, Q. Samus, L. S. Schneider, G. Selbæk, L. Teri, and N.
Mukadam. 2020. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396(10248):413-446.
Livingston, G., J. Huntley, K. Y. Liu, S. G. Costafreda, G. Selbæk, S. Alladi, D. Ames, S. Banerjee, A. Burns, C. Brayne, N. C. Fox, C. P. Ferri, L. N. Gitlin, R. Howard, H. C. Kales, M. Kivimäki, E. B. Larson, N. Nakasujja, K. Rockwood, Q. Samus, K. Shirai, A. Singh-Manoux, L. S. Schneider, S. Walsh, Y. Yao, A. Sommerlad, and N. Mukadam. 2024. Dementia prevention, intervention, and care: 2024 report of the Lancet Standing Commission. Lancet 404(10452):572-628.
Llano, D. A., S. S. Kwok, and V. Devanarayan. 2021. Reported hearing loss in Alzheimer’s disease is associated with loss of brainstem and cerebellar volume. Frontiers in Human Neuroscience 15:739754.
Loera-Valencia, R., A. Piras, M. A. M. Ismail, S. Manchanda, H. Eyjolfsdottir, T. C. Saido, J. Johansson, M. Eriksdotter, B. Winblad, and P. Nilsson. 2018. Targeting Alzheimer’s disease with gene and cell therapies. Journal of Internal Medicine 284(1):2-36.
Logue, M. W., M. Schu, B. N. Vardarajan, J. Farrell, D. A. Bennett, J. D. Buxbaum, G. S. Byrd, N. Ertekin-Taner, D. Evans, T. Foroud, A. Goate, N. R. Graff-Radford, M. I. Kamboh, W. A. Kukull, J. J. Manly, and Alzheimer Disease Genetics Collaborative. 2014. Two rare AKAP9 variants are associated with Alzheimer’s disease in African Americans. Alzheimer’s & Dementia 10(6):609-618.e611.
Loomis, S. J., R. Miller, C. Castrillo-Viguera, K. Umans, W. Cheng, J. O’Gorman, R. Hughes, S. Budd Haeberlein, and C. D. Whelan. 2024. Genome-wide association studies of ARIA from the aducanumab phase 3 ENGAGE and EMERGE studies. Neurology 102(3):e207919.
López-Ortiz, S., S. Lista, P. L. Valenzuela, J. Pinto-Fraga, R. Carmona, F. Caraci, G. Caruso, N. Toschi, E. Emanuele, A. Gabelle, R. Nisticò, F. Garaci, A. Lucia, and A. Santos-Lozano. 2023. Effects of physical activity and exercise interventions on Alzheimer’s disease: An umbrella review of existing meta-analyses. Journal of Neurology 270(2):711-725.
López-Otín, C., M. A. Blasco, L. Partridge, M. Serrano, and G. Kroemer. 2023. Hallmarks of aging: An expanding universe. Cell 186(2):243-278.
Lourida, I., E. Hannon, T. J. Littlejohns, K. M. Langa, E. Hypponen, E. Kuzma, and D. J. Llewellyn. 2019. Association of lifestyle and genetic risk with incidence of dementia. JAMA 322(5):430-437.
Luchsinger, J. A., Y. Ma, C. A. Christophi, H. Florez, S. H. Golden, H. Hazuda, J. Crandall, E. Venditti, K. Watson, S. Jeffries, J. J. Manly, F. X. Pi-Sunyer, and Diabetes Prevention Program Research Group. 2017. Metformin, lifestyle intervention, and cognition in the diabetes prevention program outcomes study. Diabetes Care 40(7):958-965.
MacDonald, S., A. S. Shah, and B. Tousi. 2022. Current therapies and drug development pipeline in Lewy body dementia: An update. Drugs & Aging 39(7):505-522.
Maharani, A., P. Dawes, J. Nazroo, G. Tampubolon, and N. Pendleton. 2018. Cataract surgery and age-related cognitive decline: A 13-year follow-up of the English Longitudinal Study of Ageing. PLoS ONE 13(10):e0204833.
Majumder, S., A. Richardson, R. Strong, and S. Oddo. 2011. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS ONE 6(9):e25416.
Mamo, S. K., N. S. Reed, C. Price, D. Occhipinti, A. Pletnikova, F. R. Lin, and E. S. Oh. 2018. Hearing loss treatment in older adults with cognitive impairment: A systematic review. Journal of Speech, Language, and Hearing Research 61(10):2589-2603.
Márquez, F., and M. A. Yassa. 2019. Neuroimaging biomarkers for Alzheimer’s disease. Molecular Neurodegeneration 14(1):21.
McGuinness, B., D. Craig, R. Bullock, and P. Passmore. 2016. Statins for the prevention of dementia. Cochrane Database of Systematic Reviews 2016(1):CD003160.
McKenzie, A. T., S. Moyon, M. Wang, I. Katsyv, W. M. Song, X. Zhou, E. B. Dammer, D. M. Duong, J. Aaker, Y. Zhao, N. Beckmann, P. Wang, J. Zhu, J. J. Lah, N. T. Seyfried, A. I. Levey, P. Katsel, V. Haroutunian, E. E. Schadt, B. Popko, P. Casaccia, and B. Zhang. 2017. Multiscale network modeling of oligodendrocytes reveals molecular components of myelin dysregulation in Alzheimer’s disease. Molecular Neurodegeneration 12(1):82.
McKenzie, A. T., M. Wang, M. E. Hauberg, J. F. Fullard, A. Kozlenkov, A. Keenan, Y. L. Hurd, S. Dracheva, P. Casaccia, P. Roussos, and B. Zhang. 2018. Brain cell type specific gene expression and co-expression network architectures. Scientific Reports 8(1):8868.
McMaster, M., S. Kim, L. Clare, S. J. Torres, N. Cherbuin, C. D’Este, and K. J. Anstey. 2020. Lifestyle risk factors and cognitive outcomes from the multidomain dementia risk reduction randomized controlled trial, Body Brain Life for Cognitive Decline (BBL-CD). Journal of the American Geriatrics Society 68(11):2629-2637.
Meng, X., S. Fang, S. Zhang, H. Li, D. Ma, Y. Ye, J. Su, and J. Sun. 2022. Multidomain lifestyle interventions for cognition and the risk of dementia: A systematic review and meta-analysis. International Journal of Nursing Studies 130:104236.
Meyer, P. F., J. Tremblay-Mercier, J. Leoutsakos, C. Madjar, M. E. Lafaille-Magnan, M. Savard, P. Rosa-Neto, J. Poirier, P. Etienne, and J. Breitner. 2019. INTREPAD: A randomized trial of naproxen to slow progress of presymptomatic Alzheimer disease. Neurology 92(18):e2070-e2080.
Miah, J., P. Dawes, S. Edwards, I. Leroi, B. Starling, and S. Parsons. 2019. Patient and public involvement in dementia research in the European Union: A scoping review. BMC Geriatrics 19(1):220.
Michailidis, M., D. A. Tata, D. Moraitou, D. Kavvadas, S. Karachrysafi, T. Papamitsou, P. Vareltzis, and V. Papaliagkas. 2022. Antidiabetic drugs in the treatment of Alzheimer’s disease. International Journal of Molecular Sciences 23(9):4641.
Mielke, M. 2024. Sex and gender differences in Alzheimer’s disease and Alzheimer’s disease related dementias. Commissioned Paper for the Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias.
Miller, J. B., and W. B. Barr. 2017. The technology crisis in neuropsychology. Archives in Clinical Neuropsychology 32(5):541-554.
Min, Y., X. Wang, O. Is, T. A. Patel, J. Gao, J. S. Reddy, Z. S. Quicksall, T. Nguyen, S. Lin, F. Q. Tutor-New, J. L. Chalk, A. O. Mitchell, J. E. Crook, P. T. Nelson, L. J. Van Eldik, T. E. Golde, M. M. Carrasquillo, D. W. Dickson, K. Zhang, M. Allen, and N. Ertekin-Taner. 2023. Cross species systems biology discovers glial DDR2, STOM, and KANK2 as therapeutic targets in progressive supranuclear palsy. Nature Communications 14(1):6801.
Minen, M. T., A. Gopal, G. Sahyoun, E. Stieglitz, and J. Torous. 2021. The functionality, evidence, and privacy issues around smartphone apps for the top neuropsychiatric conditions. Journal of Neuropsychiatry and Clinical Neuroscience 33(1):72-79.
Mintun, M. A., A. C. Lo, C. Duggan Evans, A. M. Wessels, P. A. Ardayfio, S. W. Andersen, S. Shcherbinin, J. D. Sparks, J. R. Sims, M. Brys, L. G. Apostolova, and D. M. Skovronsky. 2021. Donanemab in early Alzheimer’s disease. New England Journal of Medicine 384(18):1691-1704.
Mitchell, A. K., R. Ehrenkranz, S. Franzen, S. H. Han, M. Shakur, M. McGowan, and H. A. Massett. 2024. Analysis of eligibility criteria in Alzheimer’s and related dementias clinical trials. Scientific Reports 14:15036.
Miyata, K., T. Yoshikawa, M. Morikawa, M. Mine, N. Okamoto, N. Kurumatani, and N. Ogata. 2018. Effect of cataract surgery on cognitive function in elderly: Results of Fujiwara-kyo Eye Study. PLoS ONE 13(2):e0192677.
Mohler, J., B. Najafi, M. Fain, and K. S. Ramos. 2015. Precision medicine: A wider definition. Journal of the American Geriatrics Society 63(9):1971-1972.
Mohs, R. C., and N. H. Greig. 2017. Drug discovery and development: Role of basic biological research. Alzheimer’s & Dementia (N Y) 3(4):651-657.
Montori, V. M., M. M. Ruissen, I. G. Hargraves, J. P. Brito, and M. Kunneman. 2023. Shared decision-making as a method of care. BMJ Evidence-Based Medicine 28(4):213-217.
Morris, J. K., E. D. Vidoni, D. K. Johnson, A. Van Sciver, J. D. Mahnken, R. A. Honea, H. M. Wilkins, W. M. Brooks, S. A. Billinger, R. H. Swerdlow, and J. M. Burns. 2017. Aerobic exercise for Alzheimer’s disease: A randomized controlled pilot trial. PLoS ONE 12(2):e0170547.
Morton, H., S. Kshirsagar, E. Orlov, L. E. Bunquin, N. Sawant, L. Boleng, M. George, T. Basu, B. Ramasubramanian, J. A. Pradeepkiran, S. Kumar, M. Vijayan, A. P. Reddy, and P. H. Reddy. 2021. Defective mitophagy and synaptic degeneration in Alzheimer’s disease: Focus on aging, mitochondria and synapse. Free Radical Biology and Medicine 172:652-667.
Mostafavi, S., C. Gaiteri, S. E. Sullivan, C. C. White, S. Tasaki, J. Xu, M. Taga, H. U. Klein, E. Patrick, V. Komashko, C. McCabe, R. Smith, E. M. Bradshaw, D. E. Root, A. Regev, L. Yu, L. B. Chibnik, J. A. Schneider, T. L. Young-Pearse, D. A. Bennett, and P. L. De Jager. 2018. A molecular network of the aging human brain provides insights into the pathology and cognitive decline of Alzheimer’s disease. Nature Neuroscience 21(6):811-819.
Moussa, C., M. Hebron, X. Huang, J. Ahn, R. A. Rissman, P. S. Aisen, and R. S. Turner. 2017. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. Journal of Neuroinflammation 14(1):1.
Mukherjee, S., L. Heath, C. Preuss, S. Jayadev, G. A. Garden, A. K. Greenwood, S. K. Sieberts, P. L. De Jager, N. Ertekin-Taner, G. W. Carter, L. M. Mangravite, and B. A. Logsdon. 2020. Molecular estimation of neurodegeneration pseudotime in older brains. Nature Communications 11(1):5781.
Mummery, C. J., A. Börjesson-Hanson, D. J. Blackburn, E. G. B. Vijverberg, P. P. De Deyn, S. Ducharme, M. Jonsson, A. Schneider, J. O. Rinne, A. C. Ludolph, R. Bodenschatz, H. Kordasiewicz, E. E. Swayze, B. Fitzsimmons, L. Mignon, K. M. Moore, C. Yun, T. Baumann, D. Li, D. A. Norris, R. Crean, D. L. Graham, E. Huang, E. Ratti, C. F. Bennett, C. Junge, and R. M. Lane. 2023. Tau-targeting antisense oligonucleotide MAPTRx in mild Alzheimer’s disease: A phase 1b, randomized, placebo-controlled trial. Nature Medicine 29(6):1437-1447.
Muñoz-Jiménez, M., A. Zaarkti, J. A. García-Arnés, and N. García-Casares. 2020. Antidiabetic drugs in Alzheimer’s disease and mild cognitive impairment: A systematic review. Dementia and Geriatric Cognitive Disorders 49(5):423-434.
Myles, P. S., E. Williamson, J. Oakley, and A. Forbes. 2014. Ethical and scientific considerations for patient enrollment into concurrent clinical trials. Trials 15:470.
N’Songo, A., M. M. Carrasquillo, X. Wang, J. D. Burgess, T. Nguyen, Y. W. Asmann, D. J. Serie, S. G. Younkin, M. Allen, O. Pedraza, R. Duara, M. T. Greig Custo, N. R. Graff-Radford, and N. Ertekin-Taner. 2017. African American exome sequencing identifies potential risk variants at Alzheimer disease loci. Neurology Genetics 3(2):e141.
Nag, S., L. L. Barnes, L. Yu, R. S. Wilson, D. A. Bennett, and J. A. Schneider. 2020. Limbic-predominant age-related TDP-43 encephalopathy in black and white decedents. Neurology 95(15):e2056-e2064.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2017. Preventing cognitive decline and dementia: A way forward. Edited by A. I. Leshner, S. Landis, C. Stroud and A. Downey. Washington, DC: The National Academies Press.
NASEM. 2020. Social isolation and loneliness in older adults: Opportunities for the health care system. Washington, DC: The National Academies Press.
NASEM. 2021. Implications for behavioral and social research of preclinical markers of Alzheimer’s disease and related dementias: Proceedings of a workshop—in brief. Edited by E. H. Forstag. Washington, DC: The National Academies Press.
NASEM. 2024. Preventing and treating Alzheimer’s disease and related dementias: Promising research and opportunities to accelerate progress: Proceedings of a workshop—in brief. Edited by O. Yost and A. Downey. Washington, DC: The National Academies Press.
Neff, R. A., M. Wang, S. Vatansever, L. Guo, C. Ming, Q. Wang, E. Wang, E. Horgusluoglu-Moloch, W. M. Song, A. Li, E. L. Castranio, J. Tcw, L. Ho, A. Goate, V. Fossati, S. Noggle, S. Gandy, M. E. Ehrlich, P. Katsel, E. Schadt, D. Cai, K. J. Brennand, V. Haroutunian, and B. Zhang. 2021. Molecular subtyping of Alzheimer’s disease using RNA sequencing data reveals novel mechanisms and targets. Science Advances 7(2):eabb5398.
Neumann, P. J., L. P. Garrison, and R. J. Willke. 2022. The history and future of the “ISPOR value flower”: Addressing limitations of conventional cost-effectiveness analysis. Value Health 25(4):558-565.
Ngandu, T., J. Lehtisalo, A. Solomon, E. Levälahti, S. Ahtiluoto, R. Antikainen, L. Bäckman, T. Hänninen, A. Jula, T. Laatikainen, J. Lindström, F. Mangialasche, T. Paajanen, S. Pajala, M. Peltonen, R. Rauramaa, A. Stigsdotter-Neely, T. Strandberg, J. Tuomilehto, H. Soininen, and M. Kivipelto. 2015. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 385(9984):2255-2263.
Nguyen, L., K. Murphy, and G. Andrews. 2022. A game a day keeps cognitive decline away? A systematic review and meta-analysis of commercially-available brain training programs in healthy and cognitively impaired older adults. Neuropsychology Review 32(3):601-630.
NIA (National Institute of Aging). n.d. Recruitment: Logistical barriers (milestone 12.C). https://www.nia.nih.gov/research/milestones/translational-clinical-research/trial-innovation/milestone-12-c (accessed August 19, 2024).
NIA. 2022. Gaps and opportunities for real-world data infrastructure. https://www.nia.nih.gov/sites/default/files/2022-09/workshop_report_nia-real-world-data-workshop.pdf (accessed September 25, 2024).
NIA. 2024. Clinically meaningful outcomes in AD/ADRD trials. https://www.nia.nih.gov/research/dbsr/workshops/clinically-meaningful-outcomes-ad-adrd-trials#summary (accessed August 22, 2024).
NIH (National Institutes of Health). 2021. Notice of special interest (NOSI): Improving patient adherence to treatment and prevention regimens to promote health. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-21-100.html (accessed August 20, 2024).
NIH. 2023. Seamless early-stage clinical drug development (phase 1 to 2a) for novel therapeutic agents for the spectrum of Alzheimer’s disease (AD) and AD-related dementias (ADRD) (UG3/UH3 clinical trial required). https://grants.nih.gov/grants/guide/pa-files/PAR-23-274.html?utm_source=OLPIA+Stakeholders&utm_campaign=589f1ebb3d-EMAIL_CAMPAIGN_2023_12_11_08_31&utm_medium=email&utm_term=0_589f1ebb3d-%5BLIST_EMAIL_ID%5D (accessed August 20, 2023).
NIH. 2024. Safety and efficacy of amyloid-beta directed antibody therapy in mild cognitive impairment and dementia with evidence of Lewy body dementia and amyloid-beta pathology (U01—Clinical trial required). https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-25-010.html (accesed October 28, 2024).
NIH Collaboratory. n.d. The embedded pragmatic clinical trial ecosystem. https://rethinkingclinicaltrials.org/chapters/design/what-is-a-pragmatic-clinical-trial/the-embedded-pct-ecosystem/ (accessed August 20, 2024).
NIHR (National Institute for Health and Care Research). 2019. PPI (patient and public involvement) resources for applicants to NIHR research programmes. https://www.nihr.ac.uk/ppi-patient-and-public-involvement-resources-applicants-nihr-research-programmes (accessed October 22, 2024).
Nixon, R. A. 2020. The aging lysosome: An essential catalyst for late-onset neurodegenerative diseases. Biochimica Biophysica Acta Proteins and Proteomics 1868(9):140443.
Nixon, R. A., and D. C. Rubinsztein. 2024. Mechanisms of autophagy–lysosome dysfunction in neurodegenerative diseases. Nature Reviews Molecular Cell Biology 25:926-946..
Nørgaard, C. H., S. Friedrich, C. T. Hansen, T. Gerds, C. Ballard, D. V. Møller, L. B. Knudsen, K. Kvist, B. Zinman, E. Holm, C. Torp-Pedersen, and L. S. Mørch. 2022. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: Data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 8(1):e12268.
Oatman, S. R., J. S. Reddy, Z. Quicksall, M. M. Carrasquillo, X. Wang, C. C. Liu, Y. Yamazaki, T. T. Nguyen, K. Malphrus, M. Heckman, K. Biswas, K. Nho, M. Baker, Y. A. Martens, N. Zhao, J. P. Kim, S. L. Risacher, R. Rademakers, A. J. Saykin, M. DeTure, M. E. Murray, T. Kanekiyo, Alzheimer’s Disease Neuroimaging Initiative, D. W. Dickson, G. Bu, M. Allen, and N. Ertekin-Taner. 2023. Genome-wide association study of brain biochemical phenotypes reveals distinct genetic architecture of Alzheimer’s disease related proteins. Molecular Neurodegeneration 18(1):2.
Öhman, F., J. Hassenstab, D. Berron, M. Scholl, and K. V. Papp. 2021. Current advances in digital cognitive assessment for preclinical Alzheimer’s disease. Alzheimer’s & Dementia (Amsterdam, Netherlands) 13(1):e12217.
Olmastroni, E., G. Molari, N. De Beni, O. Colpani, F. Galimberti, M. Gazzotti, A. Zambon, A. L. Catapano, and M. Casula. 2022. Statin use and risk of dementia or Alzheimer’s disease: A systematic review and meta-analysis of observational studies. European Journal of Preventive Cardiology 29(5):804-814.
Papaioannou, T., A. Voinescu, K. Petrini, and D. Stanton Fraser. 2022. Efficacy and moderators of virtual reality for cognitive training in people with dementia and mild cognitive impairment: A systematic review and meta-analysis. Journal of Alzheimer’s Disease 88(4):1341-1370.
Park, D. C., and G. N. Bischof. 2013. The aging mind: Neuroplasticity in response to cognitive training. Dialogues in Clinical Neuroscience 15(1):109-119.
Park, J. J. H., O. Harari, L. Dron, R. T. Lester, K. Thorlund, and E. J. Mills. 2020. An overview of platform trials with a checklist for clinical readers. Journal of Clinical Epidemiology 125:1-8.
Patel, T., T. P. Carnwath, X. Wang, M. Allen, S. J. Lincoln, L. J. Lewis-Tuffin, Z. S. Quicksall, S. Lin, F. Q. Tutor-New, C. C. G. Ho, Y. Min, K. G. Malphrus, T. T. Nguyen, E. Martin, C. A. Garcia, R. M. Alkharboosh, S. Grewal, K. Chaichana, R. Wharen, H. Guerrero-Cazares, A. Quinones-Hinojosa, and N. Ertekin-Taner. 2022. Transcriptional landscape of human microglia implicates age, sex, and APOE-related immunometabolic pathway perturbations. Aging Cell 21(5):e13606.
Patrick, E., M. Taga, A. Ergun, B. Ng, W. Casazza, M. Cimpean, C. Yung, J. A. Schneider, D. A. Bennett, C. Gaiteri, P. L. De Jager, E. M. Bradshaw, and S. Mostafavi. 2020. Deconvolving the contributions of cell-type heterogeneity on cortical gene expression. PLoS Computer Biology 16(8):e1008120.
Paul, K. C., M. Haan, E. R. Mayeda, and B. R. Ritz. 2019. Ambient air pollution, noise, and late-life cognitive decline and dementia risk. Annual Review of Public Health 40:203-220.
Paulsen, R. 2024. UsAgainstAlzheimer’s. Presentation at the Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
Pellegrini, M., F. Bernabei, C. Schiavi, and G. Giannaccare. 2020. Impact of cataract surgery on depression and cognitive function: Systematic review and meta-analysis. Clinical Experiments in Ophthalmology 48(5):593-601.
Petek, B., H. Häbel, H. Xu, M. Villa-Lopez, I. Kalar, M. T. Hoang, S. Maioli, J. B. Pereira, S. Mostafaei, B. Winblad, M. Gregoric Kramberger, M. Eriksdotter, and S. Garcia-Ptacek. 2023. Statins and cognitive decline in patients with Alzheimer’s and mixed dementia: A longitudinal registry-based cohort study. Alzheimer’s Research & Therapy 15(1):220.
Peters, R., N. Beckett, F. Forette, J. Tuomilehto, R. Clarke, C. Ritchie, A. Waldman, I. Walton, R. Poulter, S. Ma, M. Comsa, L. Burch, A. Fletcher, and C. Bulpitt. 2008. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial Cognitive Function Assessment (HYVET-COG): A double-blind, placebo controlled trial. Lancet Neurology 7(8):683-689.
Peters, R., J. Warwick, K. J. Anstey, and C. S. Anderson. 2019. Blood pressure and dementia. Neurology 92(21):1017-1018.
Petersen, J. 2023. A meta-analytic review of the effects of intergenerational programs for youth and older adults. Educational Gerontology 49(3):175-189.
Piendel, L., M. Vališ, and J. Hort. 2023. An update on mobile applications collecting data among subjects with or at risk of Alzheimer’s disease. Frontiers in Aging Neuroscience 15:1134096.
Potter, H., J. H. Woodcock, T. D. Boyd, C. M. Coughlan, J. R. O’Shaughnessy, M. T. Borges, A. A. Thaker, B. A. Raj, K. Adamszuk, D. Scott, V. Adame, P. Anton, H. J. Chial, H. Gray, J. Daniels, M. E. Stocker, and S. H. Sillau. 2021a. Safety and efficacy of sargramostim (GM-CSF) in the treatment of Alzheimer’s disease. Alzheimer’s & Dementia (N Y) 7(1):e12158.
Potter, D. A., C. A. Herrera-Ponzanelli, D. Hinojosa, R. Castillo, I. Hernandez-Cruz, V. A. Arrieta, M. J. Franklin, and D. Yee. 2021b. Recent advances in neoadjuvant therapy for breast cancer. Faculty Reviews 10:2.
Prins, N. D., W. de Haan, A. Gardner, K. Blackburn, H. M. Chu, J. E. Galvin, and J. J. Alam. 2024. Phase 2a learnings incorporated into RewinD-LB, a phase 2b clinical trial of neflamapimod in dementia with Lewy bodies. Journal of Prevention of Alzheimer’s Disease 11(3):549-557.
Pugh, M. A. M. 2023. TRAILBLAZER-ALZ 3—a deeper dive into time-to-event trials. Alzheimer’s & Dementia 19(S21):e070865.
QLHC (Quantum Leap Healthcare Collaborative). 2024. What is I-SPY and I-SPY Trials?. https://www.quantumleaphealth.org/for-investigators/i-spy-trials/ (accessed August 20, 2024).
Rafii, M. S., R. A. Sperling, M. C. Donohue, J. Zhou, C. Roberts, M. C. Irizarry, S. Dhadda, G. Sethuraman, L. D. Kramer, C. J. Swanson, D. Li, S. Krause, R. A. Rissman, S. Walter, R. Raman, K. A. Johnson, and P. S. Aisen. 2023. The AHEAD 3-45 Study: Design of a prevention trial for Alzheimer’s disease. Alzheimer’s & Dementia 19(4):1227-1233.
Raman, R., Y. T. Quiroz, O. Langford, J. Choi, M. Ritchie, M. Baumgartner, D. Rentz, N. T. Aggarwal, P. Aisen, R. Sperling, and J. D. Grill. 2021. Disparities by race and ethnicity among adults recruited for a preclinical Alzheimer’s disease trial. JAMA Network Open 4(7):e2114364.
Raman, R., P. S. Aisen, M. C. Carillo, M. Detke, J. D. Grill, O. C. Okonkwo, M. Rivera-Mindt, M. Sabbagh, B. Vellas, M. Weiner, and R. Sperling. 2022. Tackling a major deficiency of diversity in Alzheimer’s disease therapeutic trials: A CTAD Task Force report. Journal of Prevention of Alzheimer’s Disease 9(3):388-392.
Ramos, I. N., K. N. Ramos, and K. S. Ramos. 2019a. Driving the precision medicine highway: Community health workers and patient navigators. Journal of Translational Medicine 17(1):85.
Ramos, K. S., E. C. Bowers, M. A. Tavera-Garcia, and I. N. Ramos. 2019b. Precision prevention: A focused response to shifting paradigms in healthcare. Experiments in Biological Medicine (Maywood) 244(3):207-212.
Rawji, K. S., M. K. Mishra, N. J. Michaels, S. Rivest, P. K. Stys, and V. W. Yong. 2016. Immunosenescence of microglia and macrophages: Impact on the ageing central nervous system. Brain 139(Pt 3):653-661.
Ray, J., G. Popli, and G. Fell. 2018. Association of cognition and age-related hearing impairment in the English Longitudinal Study of Ageing. JAMA Otolaryngology and Head and Neck Surgery 144(10):876-882.
Reardon, S. 2023. Alzheimer’s drug trials plagued by lack of racial diversity. Nature 620(7973):256-257.
Rebok, G. W., K. Ball, L. T. Guey, R. N. Jones, H.-Y. Kim, J. W. King, M. Marsiske, J. N. Morris, S. L. Tennstedt, F. W. Unverzagt, and S. L. Willis. 2014. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatrics Society 62(1):16-24.
Rebok, G. W., A. Gellert, N. B. Coe, O. J. Clay, G. Wallace, J. M. Parisi, A. T. Aiken-Morgan, M. Crowe, K. Ball, R. J. Thorpe, M. Marsiske, L. B. Zahodne, C. Felix, and S. L. Willis. 2023. Effects of cognitive training on Alzheimer’s disease and related dementias: The moderating role of social determinants of health. Journal of Aging and Health 35(9 Suppl):40S-50S.
Reddy, J. S., M. Allen, C. C. G. Ho, S. R. Oatman, O. Is, Z. S. Quicksall, X. Wang, J. Jin, T. A. Patel, T. P. Carnwath, T. T. Nguyen, K. G. Malphrus, S. J. Lincoln, M. M. Carrasquillo, J. E. Crook, T. Kanekiyo, M. E. Murray, G. Bu, D. W. Dickson, and N. Ertekin-Taner. 2021. Genome-wide analysis identifies a novel LINC-PINT splice variant associated with vascular amyloid pathology in Alzheimer’s disease. Acta Neuropathologica Communications 9(1):93.
Reddy, J. S., J. Jin, S. J. Lincoln, C. C. G. Ho, J. E. Crook, X. Wang, K. G. Malphrus, T. Nguyen, N. Tamvaka, M. T. Greig-Custo, J. A. Lucas, N. R. Graff-Radford, N. Ertekin-Taner, and M. M. Carrasquillo. 2022. Transcript levels in plasma contribute substantial predictive value as potential Alzheimer’s disease biomarkers in African Americans. EBioMedicine, 78:103929.
Reddy, J. S., L. Heath, A. V. Linden, M. Allen, K. P. Lopes, F. Seifar, E. Wang, Y. Ma, W. L. Poehlman, Z. S. Quicksall, A. Runnels, Y. Wang, D. M. Duong, L. Yin, K. Xu, E. S. Modeste, A. Shantaraman, E. B. Dammer, L. Ping, S. R. Oatman, J. Scanlan, C. Ho, M. M. Carrasquillo, M. Atik, G. Yepez, A. O. Mitchell, T. T. Nguyen, X. Chen, D. X. Marquez, H. Reddy, H. Xiao, S. Seshadri, R. Mayeux, S. Prokop, E. B. Lee, G. E. Serrano, T. G. Beach, A. F. Teich, V. Haroutunian, E. J. Fox, M. Gearing, A. Wingo, T. Wingo, J. J. Lah, A. I. Levey, D. W. Dickson, L. L. Barnes, P. De Jager, B. Zhang, D. Bennett, N. T. Seyfried, A. K. Greenwood, and N. Ertekin-Taner. 2024. Bridging the gap: Multi-omics profiling of brain tissue in Alzheimer’s disease and older controls in multi-ethnic populations. Alzheimer’s Dementia 20(10):7174-7192.
Reitz, C. 2016. Toward precision medicine in Alzheimer’s disease. Annals of Translational Medicine 4(6):107.
Reitz, C., G. Jun, A. Naj, R. Rajbhandary, B. N. Vardarajan, L. S. Wang, O. Valladares, C. F. Lin, E. B. Larson, N. R. Graff-Radford, D. Evans, P. L. De Jager, P. K. Crane, J. D. Buxbaum, J. R. Murrell, T. Raj, N. Ertekin-Taner, M. Logue, C. T. Baldwin, R. C. Green, L. L. Barnes, L. B. Cantwell, M. D. Fallin, R. C. Go, P. Griffith, T. O. Obisesan, J. J. Manly, K. L.
Lunetta, M. I. Kamboh, O. L. Lopez, D. A. Bennett, H. Hendrie, K. S. Hall, A. M. Goate, G. S. Byrd, W. A. Kukull, T. M. Foroud, J. L. Haines, L. A. Farrer, M. A. Pericak-Vance, G. D. Schellenberg, R. Mayeux, and Alzheimer Disease Genetics Consortium. 2013. Variants in the ATP-binding cassette transporter (ABCA7), apolipoprotein E ϵ4, and the risk of late-onset Alzheimer disease in African Americans. JAMA 309(14):1483-1492.
Religa, D., S.-M. Fereshtehnejad, P. Cermakova, A.-K. Edlund, S. Garcia-Ptacek, N. Granqvist, A. Hallbäck, K. Kåwe, B. Farahmand, L. Kilander, U.-B. Mattsson, K. Nägga, P. Nordström, H. Wijk, A. Wimo, B. Winblad, and M. Eriksdotter. 2015. SveDem, the Swedish Dementia Registry—a tool for improving the quality of diagnostics, treatment and care of dementia patients in clinical practice. PLoS ONE 10(2):e0116538.
Rezai, A. R., P. F. D’Haese, V. Finomore, J. Carpenter, M. Ranjan, K. Wilhelmsen, R. I. Mehta, P. Wang, U. Najib, C. Vieira Ligo Teixeira, T. Arsiwala, A. Tarabishy, P. Tirumalai, D. O. Claassen, S. Hodder, and M. W. Haut. 2024. Ultrasound blood–brain barrier opening and aducanumab in Alzheimer’s disease. New England Journal of Medicine 390(1):55-62.
Riessland, M., and M. E. Orr. 2023. Translating the biology of aging into new therapeutics for Alzheimer’s disease: Senolytics. Journal of Prevention of Alzheimer’s Disease 10(4):633-646.
Ritchie, C. W., G. M. Terrera, and T. J. Quinn. 2015. Dementia trials and dementia tribulations: Methodological and analytical challenges in dementia research. Alzheimer’s Research & Therapy 7(1):31.
Rizzolo, P., V. Silvestri, M. Falchetti, and L. Ottini. 2011. Inherited and acquired alterations in development of breast cancer. Applied Clinical Genetics 4:145-158.
Rocchi, A., S. Yamamoto, T. Ting, Y. Fan, K. Sadleir, Y. Wang, W. Zhang, S. Huang, B. Levine, R. Vassar, and C. He. 2017. A Becn1 mutation mediates hyperactive autophagic sequestration of amyloid oligomers and improved cognition in Alzheimer’s disease. PLoS Genetics 13(8):e1006962.
Rommer, P. S., G. Bsteh, T. Zrzavy, R. Hoeftberger, and T. Berger. 2022. Immunosenescence in neurological diseases—Is there enough evidence? Biomedicines 10(11):2864.
Rosenberg, A., F. Mangialasche, T. Ngandu, A. Solomon, and M. Kivipelto. 2020. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: From FINGER to world-wide FINGERS. Journal of Prevention of Alzheimer’s Disease 7(1):29-36.
Ruangritchankul, S., P. Chantharit, S. Srisuma, and L. C. Gray. 2021. Adverse drug reactions of acetylcholinesterase inhibitors in older people living with dementia: A comprehensive literature review. Therapeutics and Clinical Risk Management 17:927-949.
Salinas, J., A. S. Beiser, J. K. Samra, A. O’Donnell, C. S. DeCarli, M. M. Gonzales, H. J. Aparicio, and S. Seshadri. 2022. Association of loneliness with 10-year dementia risk and early markers of vulnerability for neurocognitive decline. Neurology 98(13):e1337-e1348.
Salloway, S. P., J. Sevingy, K. Budur, J. T. Pederson, R. B. DeMattos, P. Von Rosenstiel, A. Paez, R. Evans, C. J. Weber, J. A. Hendrix, S. Worley, L. J. Bain, and M. C. Carrillo. 2020. Advancing combination therapy for Alzheimer’s disease. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 6(1):e12073.
Salzman, T., Y. Sarquis-Adamson, S. Son, M. Montero-Odasso, and S. Fraser. 2022. Associations of multidomain interventions with improvements in cognition in mild cognitive impairment: A systematic review and meta-analysis. JAMA Network Open 5(5):e226744.
Sanders, M. E., E. Kant, A. L. Smit, and I. Stegeman. 2021. The effect of hearing aids on cognitive function: A systematic review. PLoS ONE 16(12):e0261207.
Sarkar, S., N. Das, and K. Sambamurti. 2024. Development of early biomarkers of Alzheimer’s disease: A precision medicine perspective. In Comprehensive precision medicine, 1st ed., Vol. 2, edited by K. S. Ramos. Elsevier, pp. 511-525.
Saunders, S., G. Muniz-Terrera, S. Sheehan, C. W. Ritchie, and S. Luz. 2021. A UK-wide study employing natural language processing to determine what matters to people about brain health to improve drug development: The Electronic Person-Specific Outcome Measure (ePSOM) programme. Journal of Prevention of Alzheimer’s Disease 8(4):448-456.
Say, R. E., and R. Thomson. 2003. The importance of patient preferences in treatment decisions—challenges for doctors. BMJ 327(7414):542-545.
Seckl, J. 2024. 11β-hydroxysteroid dehydrogenase and the brain: Not (yet) lost in translation. Journal of Internal Medicine 295(1):20-37.
Seifar, F., E. J. Fox, A. Shantaraman, Y. Liu, E. B. Dammer, E. Modeste, D. M. Duong, L. Yin, A. N. Trautwig, Q. Guo, K. Xu, L. Ping, J. S. Reddy, M. Allen, Z. Quicksall, L. Heath, J. Scanlan, E. Wang, M. Wang, A. V. Linden, W. Poehlman, X. Chen, S. Baheti, C. Ho, T. Nguyen, G. Yepez, A. O. Mitchell, S. R. Oatman, X. Wang, M. M. Carrasquillo, A. Runnels, T. Beach, G. E. Serrano, D. W. Dickson, E. B. Lee, T. E. Golde, S. Prokop, L. L. Barnes, B. Zhang, V. Haroutunian, M. Gearing, J. J. Lah, P. Jager, D. A. Bennett, A. Greenwood, N. Ertekin-Taner, A. I. Levey, A. Wingo, T. Wingo, and N. T. Seyfried. 2024. Large-scale deep proteomic analysis in Alzheimer’s disease brain regions across race and ethnicity. Update in Alzheimer’s & Dementia 20(12):8878-8897
Sevigny, J., O. Uspenskaya, L. D. Heckman, L. C. Wong, D. A. Hatch, A. Tewari, R. Vandenberghe, D. J. Irwin, D. Saracino, I. Le Ber, R. Ahmed, J. D. Rohrer, A. L. Boxer, S. Boland, P. Sheehan, A. Brandes, S. R. Burstein, B. M. Shykind, S. Kamalakaran, C. W. Daniels, E. D. Litwack, E. Mahoney, J. Velaga, I. McNamara, P. Sondergaard, S. A. Sajjad, Y. M. Kobayashi, A. Abeliovich, and F. Hefti. 2024. Progranulin AAV gene therapy for frontotemporal dementia: Translational studies and phase 1/2 trial interim results. Nature Medicine 30(5):1406-1415.
Shakhnovich, V. 2018. It’s time to reverse our thinking: The reverse translation research paradigm. Clinical and Translational Science 11(2):98-99.
Shanks, H. R. C., K. Chen, E. M. Reiman, K. Blennow, J. L. Cummings, S. M. Massa, F. M. Longo, A. Börjesson-Hanson, M. Windisch, and T. W. Schmitz. 2024. p75 Neurotrophin receptor modulation in mild to moderate Alzheimer disease: A randomized, placebo-controlled phase 2a trial. Nature Medicine 30(6):1761-1770.
Sierra, C. 2020. Hypertension and the risk of dementia. Frontiers in Cardiovascular Medicine 7:5.
Silva, P. J., V. M. Schaibley, and K. S. Ramos. 2018. Academic medical centers as innovation ecosystems to address population -omics challenges in precision medicine. Journal of Translational Medicine 16(1):28.
Silva, A. C., D. D. Lobo, I. M. Martins, S. M. Lopes, C. Henriques, S. P. Duarte, J.-C. Dodart, R. J. Nobre, and L. Pereira de Almeida. 2020. Antisense oligonucleotide therapeutics in neurodegenerative diseases: The case of polyglutamine disorders. Brain 143(2):407-429.
Simmons, M., S. Hetrick, and A. Jorm. 2010. Shared decision-making: Benefits, barriers and current opportunities for application. Australasian Psychiatry 18(5):394-397.
Sliwinski, M. J., J. A. Mogle, J. Hyun, E. Munoz, J. M. Smyth, and R. B. Lipton. 2018. Reliability and validity of ambulatory cognitive assessments. Assessment 25(1):14-30.
Smith, M. 2024. Cure Alzheimer’s Fund. Presentation at the Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
Smith, D., J. Lovell, C. Weller, B. Kennedy, M. Winbolt, C. Young, and J. Ibrahim. 2017. A systematic review of medication non-adherence in persons with dementia or cognitive impairment. PLoS ONE 12(2):e0170651.
Solomon, A., H. Turunen, T. Ngandu, M. Peltonen, E. Levälahti, S. Helisalmi, R. Antikainen, L. Bäckman, T. Hänninen, A. Jula, T. Laatikainen, J. Lehtisalo, J. Lindström, T. Paajanen, S. Pajala, A. Stigsdotter-Neely, T. Strandberg, J. Tuomilehto, H. Soininen, and M. Kivipelto.
2018. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: A subgroup analysis of a randomized clinical trial. JAMA Neurology 75(4):462-470.
Solopova, E., W. Romero-Fernandez, H. Harmsen, L. Ventura-Antunes, E. Wang, A. Shostak, J. Maldonado, M. J. Donahue, D. Schultz, T. M. Coyne, A. Charidimou, and M. Schrag. 2023. Fatal iatrogenic cerebral β-amyloid-related arteritis in a woman treated with lecanemab for Alzheimer’s disease. Nature Communications 14(1):8220.
Song, S., Y. Stern, and Y. Gu. 2022. Modifiable lifestyle factors and cognitive reserve: A systematic review of current evidence. Ageing Research Reviews 74:101551.
Sperling, R. A., C. R. Jack, Jr., S. E. Black, M. P. Frosch, S. M. Greenberg, B. T. Hyman, P. Scheltens, M. C. Carrillo, W. Thies, M. M. Bednar, R. S. Black, H. R. Brashear, M. Grundman, E. R. Siemers, H. H. Feldman, and R. J. Schindler. 2011. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: Recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimer’s & Dementia 7(4):367-385.
Sperling, R., S. Salloway, D. J. Brooks, D. Tampieri, J. Barakos, N. C. Fox, M. Raskind, M. Sabbagh, L. S. Honig, A. P. Porsteinsson, I. Lieberburg, H. M. Arrighi, K. A. Morris, Y. Lu, E. Liu, K. M. Gregg, H. R. Brashear, G. G. Kinney, R. Black, M. Grundman. 2012. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: A retrospective analysis. Lancet Neurology 11(3):241-249.
Sperling, R. A., D. M. Rentz, K. A. Johnson, J. Karlawish, M. Donohue, D. P. Salmon, and P. Aisen. 2014. The A4 study: Stopping AD before symptoms begin? Science Translational Medicine 6(228):228fs213.
Spierer, O., N. Fischer, A. Barak, and M. Belkin. 2016. Correlation between vision and cognitive function in the elderly: A cross-sectional study. Medicine (Baltimore) 95(3):e2423.
SPRINT MIND Investigators. 2019. Effect of intensive vs standard blood pressure control on probable dementia: A randomized clinical trial. JAMA 321(6):553-561.
Stallard, N., J. Whitehead, S. Todd, and A. Whitehead. 2001. Stopping rules for phase II studies. British Journal of Clinical Pharmacology 51(6):523-529.
Stites, S. D., A. Gurian, C. Coykendall, E. A. Largent, K. Harkins, J. Karlawish, and N. B. Coe. 2023. Gender of study partners and research participants associated with differences in study partner ratings of cognition and activity level. Journals of Gerontology Series B: Psychological Sciences and Social Sciences 78(8):1318-1329.
Strayhorn, J. C., C. M. Cleland, D. J. Vanness, L. Wilton, M. Gwadz, and L. M. Collins. 2024. Using decision analysis for intervention value efficiency to select optimized interventions in the multiphase optimization strategy. Health and Psychology 43(2):89-100.
Strickland, S. L., H. Morel, C. Prusinski, M. Allen, T. A. Patel, M. M. Carrasquillo, O. J. Conway, S. J. Lincoln, J. S. Reddy, T. Nguyen, K. G. Malphrus, A. I. Soto, R. L. Walton, J. E. Crook, M. E. Murray, B. F. Boeve, R. C. Petersen, J. A. Lucas, T. J. Ferman, R. J. Uitti, Z. K. Wszolek, O. A. Ross, N. R. Graff-Radford, D. W. Dickson, and N. Ertekin-Taner. 2020. Association of ABI3 and PLCG2 missense variants with disease risk and neuropathology in Lewy body disease and progressive supranuclear palsy. Acta Neuropathologica Communications 8(1):172.
Sun, J., and S. Roy. 2021. Gene-based therapies for neurodegenerative diseases. Nature Neuroscience 24(3):297-311.
Sun, L., A. Wu, G. R. Bean, I. S. Hagemann, and C.-Y. Lin. 2021. Molecular testing in breast cancer: Current status and future directions. Journal of Molecular Diagnostics 23(11):1422-1432.
Sun, Y.-Y., Z. Wang, H.-Y. Zhou, and H.-C. Huang. 2022. Sleep–wake disorders in Alzheimer’s disease: A review. ACS Chemical Neuroscience 13(10):1467-1478.
Sutin, A. R., Y. Stephan, M. Luchetti, and A. Terracciano. 2020. Loneliness and risk of dementia. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences 75(7):1414-1422.
Taylor, J. C., H. W. Heuer, A. L. Clark, A. B. Wise, M. Manoochehri, L. Forsberg, C. Mester, M. Rao, D. Brushaber, J. Kramer, A. E. Welch, J. Kornak, W. Kremers, B. Appleby, B. C. Dickerson, K. Domoto-Reilly, J. A. Fields, N. Ghoshal, N. Graff-Radford, M. Grossman, M. G. Hall, E. D. Huey, D. Irwin, M. I. Lapid, I. Litvan, I. R. Mackenzie, J. C. Masdeu, M. F. Mendez, N. Nevler, C. U. Onyike, B. Pascual, P. Pressman, K. P. Rankin, B. Ratnasiri, J. C. Rojas, M. C. Tartaglia, B. Wong, M. L. Gorno-Tempini, B. F. Boeve, H. J. Rosen, A. L. Boxer, and A. M. Staffaroni. 2023. Feasibility and acceptability of remote smartphone cognitive testing in frontotemporal dementia research. Alzheimer’s & Dementia (Amsterdam, Netherlands) 15(2):e12423.
Taylor-Rowan, M., S. Edwards, A. H. Noel-Storr, J. McCleery, P. K. Myint, R. Soiza, C. Stewart, Y. K. Loke, and T. J. Quinn. 2021. Anticholinergic burden (prognostic factor) for prediction of dementia or cognitive decline in older adults with no known cognitive syndrome. Cochrane Database of Systematic Reviews 5(5):CD013540.
Tedeschi, D. V., A. F. da Cunha, M. R. Cominetti, and R. V. Pedroso. 2021. Efficacy of gene therapy to restore cognition in Alzheimer’s disease: A systematic review. Current Gene Therapy 21(3):246-257.
Tejera, D., D. Mercan, J. M. Sanchez-Caro, M. Hanan, D. Greenberg, H. Soreq, E. Latz, D. Golenbock, and M. T. Heneka. 2019. Systemic inflammation impairs microglial Aβ clearance through NLRP3 inflammasome. EMBO Journal 38(17):e101064.
Temple, S. 2023. Advancing cell therapy for neurodegenerative diseases. Cell Stem Cell 30(5):512-529.
Tennstedt, S. L., and F. W. Unverzagt. 2013. The ACTIVE study: Study overview and major findings. Journal of Aging Health 25(8 Suppl):3s-20s.
Thunborg, C., A. Rosenberg, M. Barbera, N. Coley, M. Ekblom, Ö. Ekblom, N. Levak, A. Solomon, and M. Kivipelto. 2021. Physical activity and sedentary time in a multimodal lifestyle intervention in prodromal Alzheimer’s disease: The MIND-ADMINI pilot trial. Alzheimer’s & Dementia 17(S10):e054890.
Thunell, J., Y. Chen, G. Joyce, D. Barthold, P. G. Shekelle, R. D. Brinton, and J. Zissimopoulos. 2021. Drug therapies for chronic conditions and risk of Alzheimer’s disease and related dementias: A scoping review. Alzheimer‘s & Dementia 17(1):41-48.
Tijms, B. M., E. M. Vromen, O. Mjaavatten, H. Holstege, L. M. Reus, S. van der Lee, K. E. J. Wesenhagen, L. Lorenzini, L. Vermunt, V. Venkatraghavan, N. Tesi, J. Tomassen, A. den Braber, J. Goossens, E. Vanmechelen, F. Barkhof, Y. A. L. Pijnenburg, W. M. van der Flier, C. E. Teunissen, F. S. Berven, and P. J. Visser. 2024. Cerebrospinal fluid proteomics in patients with Alzheimer’s disease reveals five molecular subtypes with distinct genetic risk profiles. Nature Aging 4(1):33-47.
Tochel, C., M. Smith, H. Baldwin, A. Gustavsson, A. Ly, C. Bexelius, M. Nelson, C. Bintener, E. Fantoni, J. Garre-Olmo, O. Janssen, C. Jindra, I. F. Jorgensen, A. McKeown, B. Ozturk, A. Ponjoan, M. H. Potashman, C. Reed, E. Roncancio-Diaz, S. Vos, and C. Sudlow. 2019. What outcomes are important to patients with mild cognitive impairment or Alzheimer’s disease, their caregivers, and health-care professionals? A systematic review. Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring 11:231-247.
Toledo, J. B., M. Arnold, G. Kastenmuller, R. Chang, R. A. Baillie, X. Han, M. Thambisetty, J. D. Tenenbaum, K. Suhre, J. W. Thompson, L. S. John-Williams, S. MahmoudianDehkordi, D. M. Rotroff, J. R. Jack, A. Motsinger-Reif, S. L. Risacher, C. Blach, J. E. Lucas, T. Massaro, G. Louie, H. Zhu, G. Dallmann, K. Klavins, T. Koal, S. Kim, K. Nho, L. Shen, R. Casanova, S. Varma, C. Legido-Quigley, M. A. Moseley, K. Zhu, M. Y. R.
Henrion, S. J. van der Lee, A. C. Harms, A. Demirkan, T. Hankemeier, C. M. van Duijn, J. Q. Trojanowski, L. M. Shaw, A. J. Saykin, M. W. Weiner, P. M. Doraiswamy, R. Kaddurah-Daouk, Alzheimer’s Disease Neuroimaging Initiative, and the Alzheimer Disease Metabolomics Collaborative. 2017. Metabolic network failures in Alzheimer’s disease: A biochemical road map. Alzheimer’s & Dementia 13(9):965-984.
Toledo, J. B., C. Abdelnour, R. S. Weil, D. Ferreira, F. Rodriguez-Porcel, A. Pilotto, K. A. Wyman-Chick, M. J. Grothe, J. P. M. Kane, A. Taylor, A. Rongve, S. Scholz, J. B. Leverenz, B. F. Boeve, D. Aarsland, I. G. McKeith, S. Lewis, I. Leroi, J. P. Taylor; and ISTAART Lewy body dementias Trial Methods Working Group. 2023. Dementia with Lewy bodies: Impact of co-pathologies and implications for clinical trial design. Alzheimer’s & Dementia 19(1):318-332.
Trudler, D., S. Sanz-Blasco, Y. S. Eisele, S. Ghatak, K. Bodhinathan, M. W. Akhtar, W. P. Lynch, J. C. Piña-Crespo, M. Talantova, J. W. Kelly, and S. A. Lipton. 2021. α-Synuclein oligomers induce glutamate release from astrocytes and excessive extrasynaptic NMDAR activity in neurons, thus contributing to synapse loss. Journal of Neuroscience 41(10):2264-2273.
Tulliani, N., M. Bissett, P. Fahey, R. Bye, and K. P. Y. Liu. 2022. Efficacy of cognitive remediation on activities of daily living in individuals with mild cognitive impairment or early-stage dementia: A systematic review and meta-analysis. Systematic Reviews 11(1):156.
Tullo, D., Y. Feng, A. Pahor, J. M. Cote, A. R. Seitz, and S. M. Jaeggi. 2023. Investigating the role of individual differences in adherence to cognitive training. Journal of Cognition 6(1):48.
Turner, R. S., R. G. Thomas, S. Craft, C. H. van Dyck, J. Mintzer, B. A. Reynolds, J. B. Brewer, R. A. Rissman, R. Raman, P. S. Aisen, Society for the Alzheimer’s Disease Cooperative, J. Mintzer, B. A. Reynolds, J. Karlawish, D. Galasko, J. Heidebrink, N. Aggarwal, N. Graff-Radford, M. Sano, R. Petersen, K. Bell, R. Doody, A. Smith, C. Bernick, A. Porteinsson, P. Tariot, R. Mulnard, A. Lerner, L. Schneider, J. Burns, M. Raskind, S. Ferris, G. Jicha, M. Quiceno, T. Obisesan, P. Rosenberg, D. Weintraub, K. Kieburtz, B. Miller, R. Kryscio, and G. Alexopoulis. 2015. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer’s disease. Neurology 85(16):1383-1391.
Turunen, M., L. Hokkanen, L. Bäckman, A. Stigsdotter-Neely, T. Hänninen, T. Paajanen, H. Soininen, M. Kivipelto, and T. Ngandu. 2019. Computer-based cognitive training for older adults: Determinants of adherence. PLoS ONE 14(7):e0219541.
Umar, N., D. Litaker, M.-L. Schaarschmidt, W. K. Peitsch, A. Schmieder, and D. D. Terris. 2012. Outcomes associated with matching patients’ treatment preferences to physicians’ recommendations: Study methodology. BMC Health Services Research 12(1):1.
U.S. Preventive Services Task Force. 2021. Screening for hearing loss in older adults: US Preventive Services Task Force recommendation statement. JAMA 325(12):1196-1201.
Valiukas, Z., R. Ephraim, K. Tangalakis, M. Davidson, V. Apostolopoulos, and J. Feehan. 2022. Immunotherapies for Alzheimer’s disease—a review. Vaccines (Basel) 10(9):1527. van Dyck, C. H., C. J. Swanson, P. Aisen, R. J. Bateman, C. Chen, M. Gee, M. Kanekiyo, D. Li, L. Reyderman, S. Cohen, L. Froelich, S. Katayama, M. Sabbagh, B. Vellas, D. Watson, S. Dhadda, M. Irizarry, L. D. Kramer, and T. Iwatsubo. 2023. Lecanemab in early Alzheimer’s disease. New England Journal of Medicine 388(1):9-21.
van Dyck, C. H., A. P. Mecca, R. S. O’Dell, H. H. Bartlett, N. G. Diepenbrock, Y. Huang, M. E. Hamby, M. Grundman, S. M. Catalano, A. O. Caggiano, and R. E. Carson. 2024. A pilot study to evaluate the effect of CT1812 treatment on synaptic density and other biomarkers in Alzheimer’s disease. Alzheimer’s Research & Therapy 16(1):20.
Van Eldik, L. J., M. C. Carrillo, P. E. Cole, D. Feuerbach, B. D. Greenberg, J. A. Hendrix, M. Kennedy, N. Kozauer, R. A. Margolin, J. L. Molinuevo, R. Mueller, R. M. Ransohoff, D. M. Wilcock, L. Bain, and K. Bales. 2016. The roles of inflammation and immune mechanisms in Alzheimer’s disease. Alzheimer’s & Dementia (N Y) 2(2):99-109.
Vemuri, P., J. Fields, J. Peter, and S. Klöppel. 2016. Cognitive interventions in Alzheimer’s and Parkinson’s diseases: Emerging mechanisms and role of imaging. Current Opinion in Neurology 29(4):405-411.
Vijiaratnam, N., and T. Foltynie. 2023. How should we be using biomarkers in trials of disease modification in Parkinson’s disease? Brain 146(12):4845-4869.
Vrahatis, A. G., K. Skolariki, M. G. Krokidis, K. Lazaros, T. P. Exarchos, and P. Vlamos. 2023. Revolutionizing the early detection of Alzheimer’s disease through non-invasive biomarkers: The role of artificial intelligence and deep learning. Sensors 23(9):4184.
Wan, Y. W., R. Al-Ouran, C. G. Mangleburg, T. M. Perumal, T. V. Lee, K. Allison, V. Swarup, C. C. Funk, C. Gaiteri, M. Allen, M. Wang, S. M. Neuner, C. C. Kaczorowski, V. M. Philip, G. R. Howell, H. Martini-Stoica, H. Zheng, H. Mei, X. Zhong, J. W. Kim, V. L. Dawson, T. M. Dawson, P. C. Pao, L. H. Tsai, J. V. Haure-Mirande, M. E. Ehrlich, P. Chakrabarty, Y. Levites, X. Wang, E. B. Dammer, G. Srivastava, S. Mukherjee, S. K. Sieberts, L. Omberg, K. D. Dang, J. A. Eddy, P. Snyder, Y. Chae, S. Amberkar, W. Wei, W. Hide, C. Preuss, A. Ergun, P. J. Ebert, D. C. Airey, S. Mostafavi, L. Yu, H. U. Klein, C. Accelerating Medicines Partnership-Alzheimer’s Disease, G. W. Carter, D. A. Collier, T. E. Golde, A. I. Levey, D. A. Bennett, K. Estrada, T. M. Townsend, B. Zhang, E. Schadt, P. L. De Jager, N. D. Price, N. Ertekin-Taner, Z. Liu, J. M. Shulman, L. M. Mangravite, and B. A. Logsdon. 2020. Meta-analysis of the Alzheimer’s disease human brain transcriptome and functional dissection in mouse models. Cell Reports 32(2):107908.
Wang, H., and D. Yee. 2019. I-SPY 2: A neoadjuvant adaptive clinical trial designed to improve outcomes in high-risk breast cancer. Current Breast Cancer Reports 11(4):303-310.
Wang, C., and D. M. Holtzman. 2020. Bidirectional relationship between sleep and Alzheimer’s disease: Role of amyloid, tau, and other factors. Neuropsychopharmacology 45(1):104-120.
Wang, X., M. Allen, S. Li, Z. S. Quicksall, T. A. Patel, T. P. Carnwath, J. S. Reddy, M. M. Carrasquillo, S. J. Lincoln, T. T. Nguyen, K. G. Malphrus, D. W. Dickson, J. E. Crook, Y. W. Asmann, and N. Ertekin-Taner. 2020. Deciphering cellular transcriptional alterations in Alzheimer’s disease brains. Molecular Neurodegeneration 15(1):38.
Wang, W.-W., R. Han, H.-J. He, Z. Wang, X.-Q. Luan, J. Li, L. Feng, S.-Y. Chen, Y. Aman, and C.-L. Xie. 2021. Delineating the role of mitophagy inducers for Alzheimer’s disease patients. Aging and Disease 12(3):852-867.
Wang, X. C., C. L. Chu, H. C. Li, K. Lu, C. J. Liu, Y. F. Cai, S. J. Quan, and S. J. Zhang. 2022a. Efficacy and safety of hypoglycemic drugs in improving cognitive function in patients with Alzheimer’s disease and mild cognitive impairment: A systematic review and network meta-analysis. Frontiers in Neurology 13:1018027.
Wang, X., M. Allen, O. Is, J. S. Reddy, F. Q. Tutor-New, M. Castanedes Casey, M. M. Carrasquillo, S. R. Oatman, Y. Min, Y. W. Asmann, C. Funk, T. Nguyen, C. C. Ho, K. G. Malphrus, N. T. Seyfried, A. I. Levey, S. G. Younkin, M. E. Murray, D. W. Dickson, N. D. Price, T. E. Golde, and N. Ertekin-Taner. 2022b. Alzheimer’s disease and progressive supranuclear palsy share similar transcriptomic changes in distinct brain regions. Journal of Clinical Investigation 132(2):e149904.
Wang, C., S. Zong, X. Cui, X. Wang, S. Wu, L. Wang, Y. Liu, and Z. Lu. 2023. The effects of microglia-associated neuroinflammation on Alzheimer’s disease. Frontiers in Immunology 14:1117172.
Weinstein, G., K. L. Davis-Plourde, S. Conner, J. J. Himali, A. S. Beiser, A. Lee, A. M. Rawlings, S. Sedaghat, J. Ding, E. Moshier, C. M. van Duijn, M. S. Beeri, E. Selvin, M. A. Ikram, L. J. Launer, M. N. Haan, and S. Seshadri. 2019. Association of metformin, sulfonylurea and insulin use with brain structure and function and risk of dementia and Alzheimer’s disease: Pooled analysis from 5 cohorts. PLoS ONE 14(2):e0212293.
WHO (World Health Organization). 2022. Optimizing brain health across the life course: WHO position paper. https://www.who.int/publications/i/item/9789240054561 (accessed August 13, 2024).
Wilfling, D., S. Calo, M. N. Dichter, G. Meyer, R. Möhler, and S. Köpke. 2023. Nonpharmacological interventions for sleep disturbances in people with dementia. Cochrane Database of Systematic Reviews 1(1):CD011881.
Withington, C. G., and R. S. Turner. 2022. Amyloid-related imaging abnormalities with anti-amyloid antibodies for the treatment of dementia due to Alzheimer’s disease. Frontiers in Neurology 13:862369.
Wolters, F. J., L. B. Chibnik, R. Waziry, R. Anderson, C. Berr, A. Beiser, J. C. Bis, D. Blacker, D. Bos, C. Brayne, J. F. Dartigues, S. K. L. Darweesh, K. L. Davis-Plourde, F. de Wolf, S. Debette, C. Dufouil, M. Fornage, J. Goudsmit, L. Grasset, V. Gudnason, C. Hadjichrysanthou, C. Helmer, M. A. Ikram, M. K. Ikram, E. Joas, S. Kern, L. H. Kuller, L. Launer, O. L. Lopez, F. E. Matthews, K. McRae-McKee, O. Meirelles, T. H. Mosley, Jr., M. P. Pase, B. M. Psaty, C. L. Satizabal, S. Seshadri, I. Skoog, B. C. M. Stephan, H. Wetterberg, M. M. Wong, A. Zettergren, and A. Hofman. 2020. Twenty-seven-year time trends in dementia incidence in Europe and the United States: The Alzheimer Cohorts Consortium. Neurology 95(5):e519-e531.
Woodcock, J., and L. M. LaVange. 2017. Master protocols to study multiple therapies, multiple diseases, or both. New England Journal of Medicine 377(1):62-70.
Xu, H., R. Yang, X. Qi, C. Dintica, R. Song, D. A. Bennett, and W. Xu. 2019. Association of lifespan cognitive reserve indicator with dementia risk in the presence of brain pathologies. JAMA Neurology 76(10):1184-1191.
Yaffe, K., E. Vittinghoff, S. Dublin, C. B. Peltz, L. E. Fleckenstein, D. E. Rosenberg, D. E. Barnes, B. H. Balderson, and E. B. Larson. 2024. Effect of personalized risk-reduction strategies on cognition and dementia risk profile among older adults: The SMARRT randomized clinical trial. JAMA Internal Medicine 184(1):54-62.
Yancey, A. K., A. N. Ortega, and S. K. Kumanyika. 2006. Effective recruitment and retention of minority research participants. Annual Review of Public Health 27:1-28.
Yang, H. S., C. C. White, H. U. Klein, L. Yu, C. Gaiteri, Y. Ma, D. Felsky, S. Mostafavi, V. A. Petyuk, R. A. Sperling, N. Ertekin-Taner, J. A. Schneider, D. A. Bennett, and P. L. De Jager. 2020. Genetics of gene expression in the aging human brain reveal TDP-43 proteinopathy pathophysiology. Neuron 107(3):496-508.e496.
Yang, J., H. Zhao, and S. Qu. 2024. Phytochemicals targeting mitophagy: Therapeutic opportunities and prospects for treating Alzheimer’s disease. Biomedicine & Pharmacotherapy 177:117144.
Yip, W.-K., M. Bonetti, B. F. Cole, W. Barcella, X. V. Wang, A. Lazar, and R. D. Gelber. 2016. Subpopulation treatment effect pattern plot (STEPP) analysis for continuous, binary, and count outcomes. Clinical Trials 13(4):382-390.
Yoshida, Y., K. Ono, S. Sekimoto, R. Umeya, and Y. Hiratsuka. 2024. Impact of cataract surgery on cognitive impairment in older people. Acta Ophthalmology 102(4):e602-e611.
Yu, J. C., J. P. Hlávka, E. Joe, F. J. Richmond, and D. N. Lakdawalla. 2022. Impact of nonbinding FDA guidances on primary endpoint selection in Alzheimer’s disease trials. Alzheimer’s & Dementia (N Y) 8(1):e12280.
Yu, X., T. C. Cho, A. C. Westrick, C. Chen, K. M. Langa, and L. C. Kobayashi. 2023. Association of cumulative loneliness with all-cause mortality among middle-aged and older adults in the United States, 1996 to 2019. Proceedings of the National Academy of Science USA 120(51):e2306819120.
Yu, Y., R. Chen, K. Mao, M. Deng, and Z. Li. 2024. The role of glial cells in synaptic dysfunction: Insights into Alzheimer’s disease mechanisms. Aging Disorders 15(2):459-479.
Zammit, G., Y. Dauvilliers, S. Pain, D. Sebök Kinter, Y. Mansour, and D. Kunz. 2020. Daridorexant, a new dual orexin receptor antagonist, in elderly subjects with insomnia disorder. Neurology 94(21):e2222-e2232.
Zhang, H., J. Huntley, R. Bhome, B. Holmes, J. Cahill, R. L. Gould, H. Wang, X. Yu, and R. Howard. 2019a. Effect of computerised cognitive training on cognitive outcomes in mild cognitive impairment: A systematic review and meta-analysis. BMJ Open 9(8):e027062.
Zhang, P., Y. Kishimoto, I. Grammatikakis, K. Gottimukkala, R. G. Cutler, S. Zhang, K. Abdelmohsen, V. A. Bohr, J. Misra Sen, M. Gorospe, and M. P. Mattson. 2019b. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nature Neuroscience 22(5):719-728.
Zhang, J. H., X. Y. Zhang, Y. Q. Sun, R. H. Lv, M. Chen, and M. Li. 2022. Metformin use is associated with a reduced risk of cognitive impairment in adults with diabetes mellitus: A systematic review and meta-analysis. Frontiers in Neuroscience 16:984559.
Zhang, X., Q. Li, W. Cong, S. Mu, R. Zhan, S. Zhong, M. Zhao, C. Zhao, K. Kang, and Z. Zhou. 2023. Effect of physical activity on risk of Alzheimer’s disease: A systematic review and meta-analysis of twenty-nine prospective cohort studies. Ageing Research and Reviews 92:102127.
Zhao, Y., J. K. Zhan, and Y. Liu. 2020. A perspective on roles played by immunosenescence in the pathobiology of Alzheimer’s disease. Aging and Disease 11(6):1594-1607.
Zheng, G., R. Xia, W. Zhou, J. Tao, and L. Chen. 2016. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: A systematic review and meta-analysis of randomised controlled trials. British Journal of Sports Medicine 50(23):1443-1450.
Zhong, D., L. Chen, Y. Feng, R. Song, L. Huang, J. Liu, and L. Zhang. 2021. Effects of virtual reality cognitive training in individuals with mild cognitive impairment: A systematic review and meta-analysis. International Journal of Geriatric Psychiatry 36(12):1829-1847.
Zhou, M., and S. Tang. 2022. Effect of a dual orexin receptor antagonist on Alzheimer’s disease: Sleep disorders and cognition. Frontiers in Medicine (Lausanne) 9:984227.
Zhu, C. W., H. Grossman, J. Neugroschl, S. Parker, A. Burden, X. Luo, and M. Sano. 2018. A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate (RGM) to slow the progression of Alzheimer’s disease: A pilot study. Alzheimer’s & Dementia (N Y) 4:609-616.













































































































