Preventing and Treating Dementia: Research Priorities to Accelerate Progress (2025)
Chapter: 2 Research Enabling the Longitudinal Evaluation of Brain Health and the Detection, Diagnosis, and Monitoring of AD/ADRD
2
Research Enabling the Longitudinal Evaluation of Brain Health and the Detection, Diagnosis, and Monitoring of AD/ADRD
The ability to precisely measure brain health over time and to accurately predict, detect, diagnose, and monitor changes in cognitive function is prerequisite to realizing significant advances in the prevention and treatment of Alzheimer’s disease and related dementias (AD/ADRD). In most cases, changes in the brain that lead to clinical dementia occur slowly over a period of decades. Knowing when and how best to intervene to prevent early triggers and perturbations or to change disease trajectory through treatment relies on an understanding of the unfolding of this longitudinal process. While research in recent years has led to significant progress in the capability to detect early changes in brain health and to identify specific brain pathologies associated with different forms of dementia, which often co-occur, there is still much that is not understood about the processes that give rise to AD/ADRD over the life course and how to prevent, delay, and halt these diseases. Additionally, research advances have not been adequately translated into clinical practice.
The number of people living with AD/ADRD continues to grow rapidly as the U.S. population ages. People experiencing changes in brain health struggle to get an accurate and timely diagnosis—a challenge exacerbated by the contribution of multiple chronic conditions and mixed brain pathologies to mild cognitive impairment (MCI) and clinical dementia—and to know what steps to take to maintain their cognitive and functional abilities. Thus, despite the progress that has been made, there is a critical need to advance the capability to precisely monitor brain health and identify when changes are indicative of a neuropathological change, and to determine causes and track progression of AD/ADRD.
This chapter describes priority areas of research that can address current scientific gaps. It begins with an overview of the benefits and challenges related to the detection and diagnosis of AD/ADRD and the ongoing monitoring of brain health. It goes on to examine the opportunities for data collection across the life course to enable brain health monitoring and early detection and accurate diagnosis of AD/ADRD. The chapter ends with a discussion of opportunities and tools to integrate knowledge from research into clinical care to advance a precision brain health approach.
ADDRESSING THE CHALLENGES RELATED TO THE DETECTION, DIAGNOSIS, AND MONITORING OF AD/ADRD TO ADVANCE PRECISION BRAIN HEALTH
As exemplified by the experiences of two members of this committee described in the prologue of this report, the journey to a diagnosis is too often a long and painful process characterized by uncertainty, frustration, emotional distress, and a sense of urgency for the individuals living with AD/ADRD, as well as their loved ones (Grunberg et al., 2022). Many people who consult with a physician regarding subjective cognitive impairments will not receive a diagnosis (Roth et al., 2023).
Little has changed about the clinical diagnostic process for AD/ADRD in the last decade. Complicated diagnostic journeys such as those faced by the two members of this committee and countless others raise questions regarding how the diagnostic process could have been improved if better data had been available earlier and knowledge gained from research was better integrated into clinical care. Recent biomedical advances, including in the areas of biomarker discovery and digital health technologies (discussed later in this chapter), are providing opportunities to significantly change the way brain health is monitored over time and to move the detection and diagnosis of AD/ADRD much earlier in the disease course.
Importance of Brain Health Monitoring, Early Detection, and Accurate Diagnosis
In the context of a life-course brain health optimization model (discussed in Chapter 1), a key function of brain health monitoring is to allow early intervention to maintain brain health, such as through lifestyle or health behavior modification, to preserve and improve brain structure and function well before the age of risk when changes transition from within the range of normal to the point of disease development. The identification of early risk factors that can be used to predict later-life cognitive impairment or clinical dementia enables targeted preventive approaches
that can be applied throughout the life course (e.g., mitigation of midlife risk factors) to optimize brain health in those most likely to benefit.
While maintaining brain health is the ideal goal, there are similarly benefits to early diagnosis, ideally before the onset of clinical symptoms (i.e., preclinical diagnosis; risks and benefits associated with preclinical diagnosis are discussed later in this chapter). The hope is that interventions (pharmacological and/or nonpharmacological) implemented at the preclinical disease stage,1 either by the individual or under the guidance of a clinician, may delay or slow progression of AD/ADRD to prevent cognitive impairment and the eventual loss of function and independence. In the absence of disease modifying therapies, distinguishing AD from related dementias may have had little effect on clinical management of patients presenting with MCI or clinical dementia. However, with the approval of anti-amyloid treatments (aducanumab, lecanemab, and donanemab) and the likelihood that some future drugs will target pathologies that are specific to AD or a related dementia, accurate diagnosis is needed to inform decisions regarding the appropriate treatments.
Current evidence suggests that recently approved anti-amyloid therapies may work best for people in the early stages of Alzheimer’s disease (AD) (see Chapter 4); the same may be true for future therapies for AD/ADRD. In the event that preclinical disease progresses to clinical symptoms, early diagnosis can enable the initiation of treatment early enough to maximize the chances of changing the disease trajectory. It can additionally provide people with the time and resources needed to plan for, and adapt to, eventual cognitive and behavioral changes associated with disease progression, including financial and decision-making implications (NASEM, 2021a).
Beyond the benefits to individuals, the ability to predict, detect, accurately diagnose, and phenotype AD/ADRD is critical to advancing research on preventive and therapeutic interventions and the development of precision medicine approaches. Accurate diagnosis and phenotyping allow for the identification of risk profiles and enable population stratification, which is a key factor in the success of precision medicine approaches, the aim of which is to ensure that the right people receive the right intervention(s) at the right time (discussed further in Chapter 4). Accurate diagnosis is also the basis for understanding trial results and designing future trials. The ability to longitudinally monitor disease allows the measurement of responses to interventions, which can help to identify effective interventions or inform changes to intervention strategies in the absence of evidence of benefit.
___________________
1 In the context of AD/ADRD, preclinical disease is characterized by brain pathology in the absence of cognitive impairment.
Challenges Impeding Brain Health Monitoring and AD/ADRD Detection and Diagnosis
There are numerous challenges that impede ongoing brain health monitoring and early and accurate diagnosis of AD/ADRD. Key scientific gaps remain in the understanding of how best to define and measure brain health and AD/ADRD and in the availability of tools with the capability to ascertain brain health status throughout the life course, including the presence of pathophysiological features of AD/ADRD.
Clinical symptoms of AD and related dementias often overlap, making it difficult to accurately diagnose an individual based solely on cognitive, behavioral, or personality changes. Additionally, presentations of disease may be atypical, such as nonamnestic presentations of AD where memory impairment is not the primary cognitive deficit (McKhann et al., 2011), and may differ across subpopulations and individuals depending on the brain region affected, among other factors, all of which adds to the diagnostic complexity (Devi, 2023). Cognitive impairment is not dichotomous but occurs on a continuum. Early changes in cognition may be subtle and difficult to detect with many initial neuropsychological screening tools. Moreover, as cognitive assessments are rarely conducted during routine clinical visits (a notable gap in a brain health optimization paradigm), a lack of baseline for cognitive function can make it difficult to detect a decline. All of these factors may contribute to underdiagnosis.
Research in recent years has led to a paradigm shift in the understanding of the development of cognitive impairment and clinical dementia. It is now clear that the etiology is in most cases multifactorial, and mixed pathologies are predominant, with numerous potential combinations of amyloid plaques, tau tangles, cerebrovascular disease, and other pathologies (Dubois et al., 2021). The role of different pathologies in driving clinical symptoms remains unclear. This complexity makes it challenging to accurately diagnose patients, characterize disease phenotypes, and differentiate the individual diseases that contribute to clinical dementia—raising questions about the current systems for distinguishing these neurodegenerative disorders (Ritchie et al., 2015). The distinction of AD/ADRD from other neurodegenerative diseases such as amyotrophic lateral sclerosis or Parkinson’s disease can also be difficult owing to disease co-occurrence and shared pathologic features, such as proteinopathies (Chu et al., 2023; Kawakami et al., 2019). Improved tools and methods are needed to better understand mixed pathologies, the connections between different pathologies, and clinical syndromes.
Given the diversity of potential pathologies that contribute to dementia, the challenges diagnosing AD/ADRD based on clinical symptoms, and the long period of silent pathophysiologic development prior to manifestation
of clinical symptoms, significant research investments are focused on the development of biomarkers for AD/ADRD that can enable earlier and more accurate diagnosis, as discussed in more detail later in this chapter. Advances in the capability to detect specific neuropathologies has led to proposals to redefine AD and other neurodegenerative diseases based on biomarkers (see Box 2-1). The development of a biological definition is most advanced for AD but similar efforts are being pursued for neuronal alpha-synuclein disease, a group of diseases that includes Lewy body dementia (LBD). Ultimately, biomarker panels may enable the precise determination of specific co-occurring pathologies present. However, there is a notable gap between biomarker-based definitions of AD/ADRD being employed in research settings and current clinical diagnostic processes used in the United States, which, as described earlier, are in most cases based solely on symptoms and cognitive testing without confirmation by imaging or other biomarkers.
Of note, the use of cerebrospinal fluid (CSF) amyloid beta and tau (e.g., total tau, phosphorylated tau) biomarkers in dementia diagnosis is common in European countries (Hort et al., 2010). However, even with the availability of more precise diagnostic tools, a lack of effective treatments may also discourage physicians from pursuing a definitive diagnosis (Dubois et al., 2016), although this may change as more target-specific treatments approved by the U.S. Food and Drug Administration (FDA) become available on the market.
There are also social and systemic issues (e.g., socioeconomic and cultural barriers) that impede AD/ADRD detection and early diagnosis and may contribute to underdiagnosis. These include the following:
- Lack of public awareness regarding signs and symptoms that could indicate an early change in brain health and that may warrant discussion with a health professional;
- Access and equity issues such as lack of access to care because of costs and lack of insurance, transportation difficulties, and absence of specialists in certain geographic areas with the knowledge and resources to detect and diagnose AD/ADRD; and
- Resistance to seeking a diagnosis owing to the fear of being stigmatized (see Box 2-2) and fear of the resulting family burden and costs (Dubois et al., 2016; Stites et al., 2022).
Framing public education efforts as brain health rather than brain disease might have better penetration and help to overcome some (albeit not all) of these social and systemic barriers.
BOX 2-1
Biological Definitions for Alzheimer’s Disease and Related Dementias (AD/ADRD)
Symptoms of AD/ADRD are often overlapping, creating challenges to accurate diagnosis and, as more pathology-specific drugs become available, the appropriate targeting of treatments to people with mild cognitive impairment (MCI) and clinical dementia. In response to these challenges, there has been an effort in recent years to define AD and other neurodegenerative diseases using a biological definition. While these efforts are most advanced for AD (Dubois et al., 2021; Hampel et al., 2021; Jack et al., 2018, 2024), similar efforts are underway for neuronal alpha-synuclein disease, a group of diseases that includes Lewy body dementia (LBD) (Höglinger et al., 2024; Simuni et al., 2024).
The Definition of Alzheimer’s Disease: The definition of AD and how it is diagnosed has evolved over the past century and has become a contentious topic. At the heart of the latest controversy is whether the definition of AD should be based on specific brain pathologies, in particular amyloid plaques and tau tangles, alone. Coupled with that are questions regarding the extent to which the clinical syndrome of cognitive decline and loss of functional independence should be considered. To illustrate this point, if a patient who had a clinical diagnosis of AD has an autopsy performed and is found to have TDP-43 pathology consistent with frontotemporal lobar dementia (FTLD-TDP) and a progranulin mutation, but no evidence of amyloid plaques or paired-helical filament tangles on neuropathological examination, did that patient really have AD?
In 2011 and 2018, the Alzheimer’s Association and the National Institute on Aging convened workgroups to develop frameworks to guide diagnosis of AD, initially for use in clinical research and more recently to translate into clinical practice. In 2024, the Alzheimer’s Association workgroup published an updated framework that endorsed a biological definition of AD and proposed guidelines for staging the disease with biomarkers and imaging (Jack et al., 2024). This framework differentiates the clinical syndrome of dementia from the currently proposed underlying etiologies and describes clinical staging for people with biological evidence of AD. The revised criteria in the 2024 framework define specific “Core 1” amyloid and tau biomarkers (e.g., amyloid-positron emissions tomography [PET], plasma phosphorylated (p)-tau217, and certain hybrid ratios such as CSF amyloid beta 42/40 and CSF p-tau 181/amyloid beta 42) that become abnormal early in the course of disease and can be used individually or in combination for the purposes of AD diagnosis (Jack et al., 2024). Other forms of tau reflecting deposits of aggregated tau in the brain that become abnormal later in the disease course (Core 2), along
with AD-nonspecific biomarkers of neurodegeneration and inflammation, can be used in staging, prognosis, and as an indicator of biological treatment effect. Importantly, under the revised criteria, amyloid-PET alone is sufficient for AD diagnosis, a notable change from the 2018 criteria that required pathologic amyloid and tau (Jack et al., 2024).
Confirming whether a patient with MCI or mild clinical dementia has AD pathology has become particularly relevant in the setting of Food and Drug Administration (FDA)-approved anti-amyloid antibodies. Multiple studies have demonstrated that up to 37 percent of patients with an MCI diagnosis and up to 25 percent of patients with mild dementia diagnosed with clinical AD do not show evidence of AD pathology on biomarker testing or autopsy studies (Bangen et al., 2016; Cummings, 2019; Landau et al., 2016; Sevigny et al., 2016). Recent advances in blood-based biomarkers should enable a more accurate diagnosis of AD and avoid the need for PET imaging or lumbar puncture to obtain CSF measures in most cases.
Defining AD by the pathologic process in the brain, specifically amyloid and tau pathology, is viewed by some as too reductionist, and not in keeping with the lay public conception of AD as the clinical dementia syndrome. Perhaps the most controversial issue is that a biological definition of AD allows for the detection of AD in people who do not yet have symptoms (the preclinical stage of AD). The workgroup recommended against testing biomarkers in asymptomatic people outside of research studies until there is evidence from ongoing prevention trials that treating at this stage of disease is beneficial.
Although convergent evidence suggests that cognitively unimpaired individuals with high levels of amyloid, and especially combined with abnormal tau biomarkers, are at increased risk of decline over time (Cody et al., 2024; Ossenkoppele et al., 2022; Sperling et al., 2024), it is important to acknowledge that many individuals with detectable amyloid neuropathology (particularly if not accompanied by tau pathology) will not progress to dementia within their lifetime. This raises concern for significant “overdiagnosis” and costly and potentially harmful treatment in an estimated 47 million U.S. adults (Brookmeyer and Abdalla, 2018) with biomarker-defined preclinical AD. Additional work is needed to understand the mechanisms of cognitive resilience that allow people to remain cognitively intact in the setting of brain pathology, as well as resistance mechanisms that allow some individuals with high amyloid to avoid neocortical tau spreading that is more closely associated with imminent cognitive decline.
An additional issue arises from the findings from longitudinal observational cohorts that have included diagnostic biomarkers and autopsy
studies. These studies have made clear that multiple other neurodegenerative processes contribute to cognitive decline, and that “pure” AD pathology—as defined by the presence of amyloid and tau—resulting in clinical dementia is relatively uncommon in older populations. Recent studies suggest that vascular pathology may exacerbate cognitive decline in the presence of amyloid pathology and potentially contributes to tau accumulation. This underscores the complexity of neurodegenerative disease, where multiple pathologies may coexist, leading to mixed pathology dementia. Currently, the lack of specific biomarkers for related dementias, such as vascular dementia, tauopathies such as frontotemporal dementia, and others makes it challenging to accurately diagnose these conditions. Consequently, individuals diagnosed with AD based solely on amyloid and tau positivity may actually have a different or mixed pathology dementia. In recognition that isolated AD is the exception in older populations with neuropathology, the revised criteria include biomarkers for copathologies that may commonly co-occur with AD, such as vascular brain injury as detected by magnetic resonance imaging and alpha-synuclein detected using seed amplification assays (Jack et al., 2024).
Beyond Alzheimer’s Disease: Diagnosis and identification of other dementia syndromes are less controversial, but investment and advances in biomarkers have lagged behind AD. As biomarkers become available for related dementias, there has been interest in similar efforts to develop a biological definition of disease. In 2024, Simuni and colleagues (2024) proposed that Parkinson’s disease and dementia with Lewy bodies be redefined as neuronal alpha-synuclein disease and suggested research criteria for a neuronal alpha-synuclein disease integrated staging system. Staging using the proposed system would be based on the presence of two biomarkers—neuronal alpha-synuclein and dopamine deficiency—the presence of pathogenic variants in the SNCA gene, and clinical signs and symptoms. Alternative research diagnostic criteria were proposed by Höglinger and colleagues (2024) that also included neuronal alpha-synuclein and genetic contributions (not limited to SNCA) but considered multiple forms of neurodegeneration beyond dopamine deficiency. As with the AD criteria, there remain many questions regarding the development of a biological definition for neurodegenerative diseases characterized by alpha-synuclein and the specific research criteria (Boeve et al., 2024). It is expected that criteria used in defining and staging AD/ADRD will continue to evolve as the development of biomarkers and disease modifying therapies advance.
BOX 2-2
Addressing the Stigma Associated with AD/ADRD
In surveys of middle-aged and older adults, dementia is commonly identified as one of the most feared health conditions of older age (Alzheimer Europe, 2011; Watson et al., 2023). Fear of dementia contributes to stigma associated with the disease, which can include patronization, stereotyping, social exclusion, and discrimination (ADI, 2012; Stites et al., 2022). Recent studies evaluating the social stigma associated with AD have illuminated its negative effects, which can include low self-esteem and isolation, along with poorer mental health outcomes and quality of life (Rosin et al., 2020; Stites et al., 2018). Stigma can also contribute to reduced health-seeking behaviors, resulting in later diagnosis, less use of health care services, and ultimately worse health outcomes (ADI, 2012; Rosin et al., 2020; Stites et al., 2018). For this reason, targeted efforts to reduce the stigma and harmful rhetoric surrounding this disease are of paramount importance to benefit those currently living with AD/ADRD along with individuals who may be at risk.
A LIFE-COURSE APPROACH TO BRAIN HEALTH AND DETECTION, DIAGNOSIS, AND MONITORING OF AD/ADRD: ADVANCING A NEW RESEARCH PARADIGM
AD/ADRD development, including its timeline and trajectory, need to be considered in the context of changes in brain health over time. Such knowledge may ultimately enable the linking of specific interventions for brain health optimization, disease prevention, and treatment to different stages across an individual’s entire life course. However, understanding of brain health over the life course is currently limited. Improving our understanding of brain health and disease development will require the identification of specific types of data that should be collected across different spans of the life course (see Figure 2-1).
There are myriad types of data that can be collected and evaluated over time that, if integrated, can provide a more comprehensive view of brain health and disease development. Collection of data that can help address current data biases are particularly important. While some forms of data relevant to AD/ADRD, such as those from blood-based biomarker testing and other molecular data derived from biosamples, are just coming to the forefront, many other types, including cognitive and functional data that make up clinical phenotypes as well as more routine health data (e.g., longitudinal measures of cardiovascular health), are currently collected in
SOURCE: Adapted from a figure provided by Rhoda Au.
the clinical setting. However, siloing in the data collection and assessment process impedes efforts to develop a full clinical picture. For example, blood lipid levels and blood pressure may be routinely considered in assessing risk for cardiovascular disease (CVD) (Reitz, 2016) but not vascular dementia despite evidence connecting CVD and dementia risk.
Clinical assessments conducted by care providers may yield data on cognitive and psychological status, motor function, affect, sensory issues, patient history (e.g., social factors, exposures, clinical history), and patient experiences, such as sleep difficulties. Some of these data may be patient reported while for others standardized tools, such as screening assessments, provide an objective means for measuring change over time.
The accessibility of these types of data and their application to the ongoing monitoring of brain health and disease diagnosis continue to evolve as existing tests and tools are improved and as the development of new tools enables the detection and measurement of signs and symptoms that are currently missed. For example, although cognitive changes are often viewed as being toward the end of the disease trajectory (and generally later in the life course), with more sensitive measures, it may be found that these
changes are not as downstream as once believed. By the time people notice symptoms of cognitive decline, often much has already changed in the brain. The ability to detect changes in an individual’s capacity to learn, for example, could provide an early indicator of a change in brain health. This underscores the importance of a life-course approach whereby brain health is tracked and optimized over time and detection of a negative trajectory of decline is possible within a person’s normal ranges of variability.
Digital tools are increasingly enabling some of these data (e.g., sleep disturbances, changes in motor function) to be collected outside of the clinic (e.g., via smartphone applications, wearables) and even in a passive manner (NIA, 2019), potentially providing a mechanism for alerting individuals and their clinicians when further assessment is warranted based on a change in status, and potentially facilitating earlier disease diagnosis. Sensitivity will need to be balanced with specificity and the implementation of such tools would need to consider the risk of alarm fatigue. This could be mitigated, for example, by alerting individuals based on trends involving multiple signals rather than single outlying data points, which would be a significant early detection advancement. By reducing reliance on subjective and self-reported measures, such tools may also help to address biases in clinical data.
While useful, these clinical and experiential data are not sufficient to elucidate the whole picture. It is also important to understand what is happening within the body, including biological signs of pathophysiology. At a gross level, this can be accomplished through imaging (e.g., positron emission topography [PET], magnetic resonance imaging [MRI]), which has been used to diagnose and monitor AD/ADRD. More recently, tests are being developed that can illuminate an individual’s molecular and cellular landscape. Testing of blood, tissue, and CSF can provide information on peripheral and central biomarkers,2 genetic risk, and epigenetic data that indicate how exposures have interacted with an individual’s genetic makeup (Jia et al., 2024). Such data not only play a role in disease detection and diagnosis but can also contribute to an understanding of the mechanistic underpinnings for AD/ADRD (discussed further in Chapter 3). As understanding of what specific types of data need to be collected and when these data should be collected across the life course, and as tools and technologies are developed and validated, these can be integrated into personal and clinical care practices as part of a brain health approach (see Table 2-1).
___________________
2 Peripheral biomarkers are measurable indicators of biological processes that may be measured less invasively from tissues outside the central nervous system, for example, using blood cells, skin fibroblasts, saliva, eyes (Htike et al., 2019). Central biomarkers, such as CSF samples and brain imaging (Hansson et al., 2018) are measurable indicators of biological processes occurring directly within the central nervous system.
| Example Data Types | Example Data Collection Frequency Across the Life Course | ||
|---|---|---|---|
| Biological measures | Blood | Genotype | Once |
| Proteome | Set intervals throughout life | ||
| Electrolytes | Set intervals throughout life | ||
| Skin | Skin biopsy | Set intervals from mid to late life | |
| Brain | MRI | Set intervals throughout life | |
| PET | Set intervals from mid to late life | ||
| CSF | Set intervals from mid to late life | ||
| Data on socioeconomic status | Parental income | Consistently throughout early life | |
| Developmental milestones | Consistently throughout early life | ||
| Family stressors | Consistently throughout early life | ||
| Personal income | Set intervals from mid to late life | ||
| Education | Consistently throughout early life and young adult life | ||
| Employment | Consistently throughout young adult to late life | ||
| Relationships | Set intervals throughout life | ||
| Digital data | Wearable | Steps | Continuously from young adult to late life |
| SpO2 and VO2 max | Continuously from young adult to late life | ||
| Sleep | Continuously from young adult to late life | ||
| 6-min walk | Continuously from young adult to late life | ||
| Smartphone | Video | Set intervals from mid to late life | |
| CLOX | Set intervals from mid to late life | ||
| Audio | Set intervals from mid to late life | ||
| Clinical data | EHR | Set intervals from mid to late life | |
| Neuropsychiatric assessments | Consistently in later life | ||
| Patient-reported outcome measures | Set intervals from mid to late life | ||
NOTES: This table provides select examples of the potential types of data that could be integrated into personal and clinical care practices across the life course as part of a precision brain health approach. MRI = magnetic resonance imaging; PET = positron emission tomography; CSF = cerebrospinal fluid; SpO2 VO2 Max = oxygen saturation and maximum volume of oxygen; CLOX = clock drawing test; EHR = electronic health record.
Research is needed to identify and describe the essential data elements necessary to inform AD/ADRD prediction, detection, diagnosis, treatment (selection, dose adjustment, and cessation), and ongoing monitoring (e.g., risk and resilience factors, molecular biomarkers, clinical signs and symptoms) and to understand how those essential data elements relate to the health of a person over the life course.
Conclusion 2-1: The current, incomplete understanding of brain health throughout the life course impedes the development of accessible and sensitive clinical tools that can predict, diagnose, and monitor changes in cognitive function and inform strategies to maximize brain health and prevent and treat AD/ADRD.
Improving and Expanding Tools for Assessing Cognition, Function, and Other Measures
Recent biomedical advances are paving the way for a major paradigm shift in the detection, diagnosis, and management of AD/ADRD. Current processes that rely on identification of clinical symptoms are inherently late-stage focused, but in the near future, new and improved tools such as blood-based biomarkers and digitally based assessments may provide earlier signals of changes in brain health, as compared to traditional cognitive assessments, and reduce reliance on invasive and expensive tests such as PET imaging and CSF biomarker tests (see Figure 2-2). Earlier detection and diagnosis, better prediction of cognitive outcomes, and enhanced monitoring of AD/ADRD through biomarkers and digital technologies can guide decision-making algorithms for risk stratification and early intervention, thereby advancing a precision brain health approach (Hampel et al., 2022a).
This paradigm shift is already underway in the research setting but has yet to transition to the clinical environment where such issues as reimbursement and electronic health record integration influence the adoption of new tools (Cutler, 2024), as does uncertainty regarding their clinical usefulness (Hampel et al., 2022a). Investment in refining and developing tools for data collection should emphasize, though not solely focus on, universally scalable tools that can ultimately be incorporated into clinical practice. Universally scalable tools are appropriate for all populations and can be scaled for use at a population level, ideally without requiring specialized expertise. The case for investment rests on the ability to reduce cost of treatment at a population level (e.g., through prevention and thereby reducing the number of people requiring treatment or by reducing the amount of time for which treatment is needed). The real-world applicability and effects of a tool should therefore be a consideration in investment decisions.
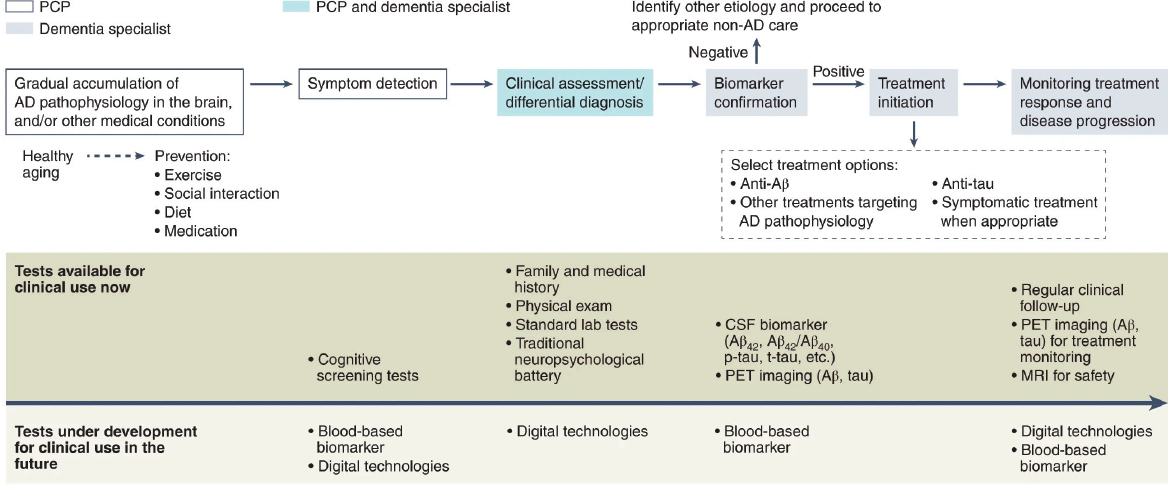
NOTE: AD = Alzheimer’s disease; CSF = cerebrospinal fluid, PET = positron emission tomography, PCP = primary care provider.
SOURCE: Hampel et al., 2022a.
As discussed in the preceding section, variation in measures and methods is a major barrier to data aggregation. It will therefore be important to give early consideration to the harmonization and standardization of measures to accelerate advancements of new tests that are promising. Research consortia are well positioned to play a role in this harmonization process.
Traditional Clinical Assessment Tools
Several validated clinical assessment tools are commonly used in both research and clinical settings to objectively identify alterations in cognition, behavior, and function. These tools include patient-reported outcome measures (PROMs) and other clinician-administered tests for assessing cognitive function include the Mini-Mental State Examination, Montreal Cognitive Assessment, and Mini Cognitive Assessment Instrument. Functional and behavioral status may be assessed using an instrumental activities of daily living scale and the Neuropsychiatric Inventory Questionnaire, respectively. Such tools may be used by clinicians as part of routine screening or a diagnostic workup for patients presenting with subtle changes in cognition or behavior (Hampel et al., 2022a). They are also used in research studies to guide the selection of clinical trial participants and for evaluating outcomes following an intervention (Ng et al., 2018). In a research context, the diversity of available tests and measures impedes efforts to compare findings across studies and to pool data for analysis across multiple studies (NASEM, 2017; Ritchie et al., 2015), although these challenges are being addressed through efforts to develop composite scores (Crane et al., 2012) and harmonize data (Mukherjee et al., 2023).
Despite their widespread use, these clinical assessment tools suffer from a number of limitations. The most obvious limitation of PROMs is a reliance on a patient’s ability to remember and report on their current state of function. Traditional modalities, such as clinician-administered pen- and-paper tests, are crude measures able to detect changes at later stages of cognitive impairment, but they are not able to detect early, more subtle changes in cognition. Such tests can also pose challenges for some individuals, including those with vision impairment, tremors, and similar physical impairments. Moreover, the data from such assessments may be biased; for example, such biases may be attributable to practice effects—although lack of a practice effect could be interpreted as an indicator of a cognitive issue (Öhman et al., 2021)—and the influences of cultural and language differences across populations that may skew test results and interpretations (Ng et al., 2018). The latter has been addressed through the development of visual-based cognitive screening tools that use culturally neutral pictures and figures and do not require language translation (Ng et al., 2018), but validation prior to clinical adoption is critical.
Furthermore, most clinical tests do not have gender-specific norms despite the fact that there are recognized gender differences in cognition throughout the lifespan. Women, for example, generally perform better on tests of verbal memory relative to men, which may reduce the likelihood of detecting amnestic MCI and result in the later diagnosis of women (Mielke, 2024). Recognizing these challenges, the National Institutes of Health (NIH) has invested in research infrastructure to develop and validate new tools that could be used in primary care and other similar care practice settings to detect cognitive impairment in diverse populations (see Box 2-3 for an example of such investments).
Digital Tools and Technologies
Digital tools and technologies have the potential to address some of the limitations of the traditional clinical assessment tools described above and are providing opportunities to answer questions that could not be addressed in the past. For example, a digital clock drawing test allows for real-time, highly sensitive assessment of neurocognitive behavior that would otherwise not be possible to obtain with the traditional pen-and-paper test currently used in clinical settings (Dion et al., 2020; Piers et al., 2017). Other examples include such technologies as tracking apps in smartphones and wearable fitness trackers, as well as home-based ambient sensing technologies (Cerino et al., 2021; NIA, 2019). The types of measures that can be collected using digital tools and technologies are broad and include sleep, gait, speech patterns, and typing behavior, changes in which may be indicators of early alterations in brain health or AD/ADRD development or progression. These technologies make it possible to collect data from environments in which people live (see Box 2-4), which allows that context to be captured and considered in analyses (Kaye, 2024). Moreover, by enabling frequent and even continuous data collection, data are less likely to be skewed by day-to-day variation in cognitive function (Cerino et al., 2021; Hampel et al., 2022a).
Digital tools and technologies enable passive data collection, which may reduce the cost and burden associated with data collection and help to reduce data bias. Passive data collection may also help overcome barriers to data collection arising from physical disabilities and sensory impairment, which may be common in some populations such as the oldest old (Corrada, 2024), as well as from those with advanced clinical dementia, for whom more active data collection may be challenging (Hampel et al., 2022a).
In the research setting, digital tools and technologies provide opportunities to increase inclusivity, allowing people interested in participating in research to collect and share data without the requirement (or with a
BOX 2-3
Consortium for Detecting Cognitive Impairment Including Dementia
The National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA)-funded Consortium for Detecting Cognitive Impairment, Including Dementia (DetectCID) is a collaborative research network established in 2017 and involves the collaboration of multidisciplinary research teams from the University of California San Francico, Albert Einstein College of Medicine, and Northwestern University (DetectCID, 2022). DetectCID is focused on improving the detection of cognitive impairment in primary care settings and in everyday clinical practice through the development, testing, and validation of novel paradigms, including new tools and protocols, to both increase the frequency and enhance the quality of patient evaluations. These efforts also prioritize addressing health disparities resulting from barriers to detection of cognitive impairment in diverse and underserved populations. The first phase of DetectCID focused on paradigm development and harmonization along with establishing interoperability among different evaluators and research sites.
The focus of the Consortium’s second phase was on cohort and population testing along with optimizing and validating the novel paradigms, particularly primary-care and other everyday clinical contexts (DetectCID, 2022). Ultimately, each of the three research teams developed and piloted a paradigm for detecting incident cognitive impairment that included a user-friendly, short (less than 10 minutes) cognitive assessment and an implementation protocol for use in primary care. Features of the developed paradigms vary. For example, some tests can be performed on tablets and some are picture-based to meet the needs of low-literacy populations. One is available in multiple languages and another is designed to be self-administered (Bernstein Sideman et al., 2022). The MyCog Cognitive Screener developed by Northwestern University is being used in an NIH-funded pragmatic trial and is available through the NIH Toolbox (2024). TabCat-BHA developed by UCSF is available via an online platform (TabCAT, 2024).
reduced requirement) for traveling to study sites and submitting biosamples. This not only reduces the burden and barriers for people living with AD/ADRD who are interested in participating in research (Nicosia et al., 2023), but also moves the research enterprise closer to being able to use citizen science (Öhman et al., 2021).
NIH has made recent investments in resources to expand the accessibility of digital tools and technologies for use in research. The NIA-funded Mobile
BOX 2-4
In-Home Monitoring: Advances from the Collaborative Aging Research Using Technology Initiative
The Collaborative Aging Research Using Technology (CART) Initiative is a multisite research initiative funded by National Institutes of Health (NIH) and the Department of Veterans Affairs (VA) that is using innovative new ways to study aging in place through the deployment of novel technologies and big data approaches to detect meaningful longitudinal changes in health across diverse populations of older adults. The four founding research sites for CART are Oregon Health and Science University (OHSU), Department of Veterans Affairs, Rush University, and University of Miami (OHSU, 2024a,b). The CART Initiative deploys a technology platform initially developed by OHSU’s Oregon Center for Aging and Technology Life Lab (OHSU, 2024c). The system of in-home sensors, installed in participant homes at each site, continuously collects data in real time. Sensors are designed to be sensitive to the presence of people and to not interfere with the daily lives of participants. Data collected and analyzed by CART includes data on mobility (e.g., walking speed and movement between rooms), socialization (e.g., phone and e-mail use), medication adherence, sleeping behaviors, and physiologic functions (e.g., body mass index, pulse). The vast quantities of data produced through monitoring are then analyzed by researchers to understand subtle changes in activity and function over time (OHSU, 2024c). The CART Initiative has developed several collaborations to advance research on healthy aging using this platform and the data it collects. For example, the Ecologically Valid, Longitudinal, and Unbiased Assessment of Treatment Efficacy in Alzheimer Disease (EVALUATE-AD) Trial supported by NIH and Merck is using the CART platform to determine the feasibility of using in-home monitoring systems for detecting changes in meaningful outcomes in participants with mild cognitive impairment (MCI) or early-stage AD. CART is also collaborating with Emory University and Georgia Institute of Technology to assess the use of the CART platform as a modality for delivering interventions in the homes of participants enrolled in the MCI Empowerment Program (OHSU, 2024d).
Toolbox, for example, provides researchers with validated, digital cognitive and other health measures, such as digital measures derived from the Patient-Reported Outcomes Measurement Information System (PROMIS®), that can be integrated into remote cognitive assessments for research (Mobile Toolbox, 2024). The Mobile Toolbox also provides a platform to develop new smartphone applications, as well as to collect and manage digital data from participants (King and Wagster, 2024).
In the clinical context, digital tools and technologies may help reduce barriers to early detection and monitoring of changes in brain health, as well as early disease diagnosis (see Box 2-5). Health systems and clinical teams are already overwhelmed by the need for diagnosis and treatment of AD/ADRD given the time-sensitive nature of this group of diseases. As noted above, the routine clinical assessment (in its best case) with PROMs is time intensive on the part of both the patient and provider and relies on a patient’s ability to remember and report on their current state of function.
BOX 2-5
Diagnostic Potential of Digital Data Combined with Artificial Intelligence
Digital data combined with artificial intelligence (AI) approaches may provide novel, scalable, and cost-effective tools for screening and diagnosing Alzheimer’s Disease and Related Dementias (AD/ADRD) in diverse populations regardless of language or sociocultural factors. Early efforts have included applying natural language processing methods to the assessment of digitized data from audio recordings of conventional neuropsychological exams. When the resulting data were combined with demographic data, models were able to classify participants into categories of normal cognition versus dementia, normal cognition or mild cognitive impairment (MCI) versus dementia, and normal cognition versus MCI (Amini et al., 2023; Paschalidis, 2024). Findings from this work suggest that this approach is effective in the identification of normal cognition from MCI and dementia and could be applicable as a remote tool that could be adapted to any language. This work did indicate less accuracy when differentiating normal cognition from MCI (Amini et al., 2023); however, other research demonstrated that a smartphone-based neuropsychological battery used to create a remote digital memory composite score could accurately and remotely distinguish cognitively healthy controls from participants living with MCI (Berron et al., 2024).
In addition to the described potential screening function, natural language processing techniques and machine learning methods are also being applied to digitized participant voice recordings from the Framingham Heart Study to predict progression from MCI to dementia—with an accuracy rate of 78.5 percent—within a 6-year span (Amini et al., 2024). This work demonstrates the potential use of digital data, such as digital voice data, in combination with AI methods to revolutionize the evaluation of brain health over time in ways that were not previously possible.
Digital tools that can offer continuous monitoring for alterations in brain health, such as changes identified in voice recordings or alterations in movement detected by smart watches and other wearables, may allow early intervention with nonpharmacological solutions when changes are still within the realm of normal, thereby potentially preventing disease development (Au et al., 2022; Öhman et al., 2021). Apps with digital versions of brief cognitive tests may help to identify early memory impairment associated with MCI (Berron et al., 2024; Cerino et al., 2021). By alerting individuals of a change in status that may indicate the need for a clinical assessment, such tools also offer opportunities to better support self-advocacy. They may also help to compensate for a lack of access to other diagnostic tools, such as some forms of imaging, that may not be available everywhere in the United States (e.g., rural areas), and they may improve the scalability and cost-effectiveness of regular screening for AD/ADRD (Öhman et al., 2021; Paschalidis, 2024). Despite the promising capabilities of digital tools and technologies, there remain several hurdles to their integration into clinical practice and mainstream use for ongoing brain health monitoring. With the notable exception of digital versions of existing clinical assessment tools, such as a digital Montreal Cognitive Assessment or Mini-Mental State Examination (Öhman et al., 2021), novel measures captured with these tools and technologies are not yet well accepted (Au et al., 2022). Validation efforts are needed to demonstrate the reliability of novel measures—that is, their accuracy relative to the outcome of interest. However, a challenge with the validation of digital tools and technologies is the lack of good reference data for benchmarking their performance (i.e., ground truthing). Given their own biases and other limitations, traditional clinical assessments may not be the best references against which to assess digital tools and technologies (Cook, 2024).
Another approach is to compare the digital data to fluid and/or imaging biomarker results (Öhman et al., 2021). In research contexts, digital tools and technologies are being used to collect data from people who often have not undergone biomarker or traditional clinical cognitive testing. Limiting data collection to those individuals who have undergone such testing would significantly limit the application and learning from digital tools and technologies. Another consideration is individuals’ level of comfort with digital tools (Tsuang, 2024). Engaging people living with or at risk for AD/ADRD in the development of digital tools and technologies may increase acceptability and ensure the measures being captured are meaningful to those from whom the data will be collected.
Because we are unable to foresee which data will be useful for AD/ADRD detection, diagnosis, and monitoring, it is important to consider how raw data can best be stored and archived in digital repositories for future analysis. An advantage of stored digital data over banked biosamples is that digital
data are not a finite resource. If properly stored, they can be used indefinitely and simultaneously by multiple data users without losing value over time. Thus, the return on collection and storage can be exponential.
There is also a need to consider and address data access, privacy, and confidentiality issues (Coravos et al., 2019) for different types of digital data. The use of commercial artificial intelligence (AI) and machine learning platforms for sharing and processing digital data, for example, may come with security and privacy concerns. De-identification, encryption, and the generation of synthetic data are potential approaches to data protection (Paschalidis, 2024). Developing the necessary data infrastructure that facilitates secure access to raw data in parallel to analytic methods is critical to realizing the full capabilities of these tools and technologies (Au et al., 2023).
Given the pace of technological advancements, investment in infrastructure and other resources may be needed to ensure academics can continue to push the cutting edge (Kaye, 2024; Paschalidis, 2024). For example, one needed resource to move digital tools forward is open-source digital data collection and processing tools for such functions as customizable applications, quality control, de-identification of data, and defining statistical summaries of the raw signal that capture some feature of clinical interest. Additional work is needed to rethink what data harmonization will need to look like given that (1) the technologies used to collect data will continue to evolve both for existing methods and still-to-emerge ones and (2) different analytic strategies have different definitions of what is analyzable (e.g., biostatistics versus automated AI analytics). Further, legacy methods of data sharing need to give way to new approaches that make data more easily and freely accessible and promote true democratization of data, without being hampered by outdated patriarchal governance and oversight policies or unnecessary data transfer fees.
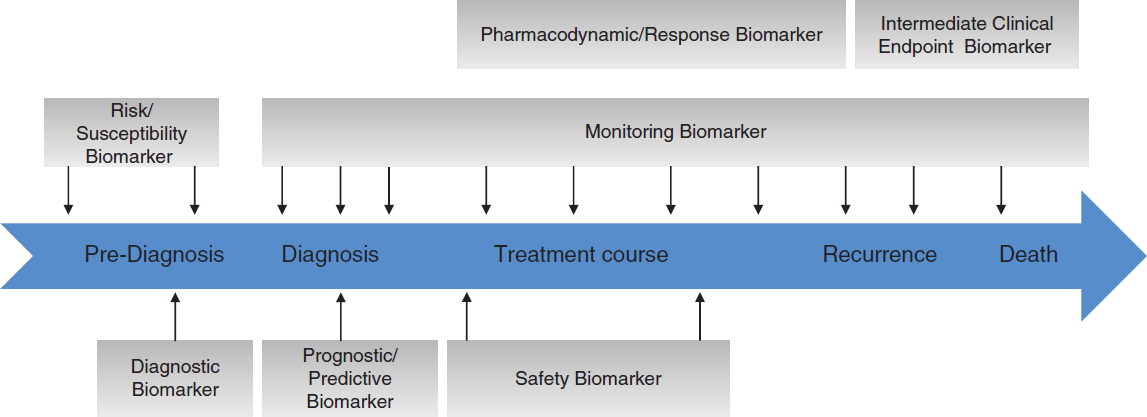
Biomarkers
Biomarker discovery for AD/ADRD is a rapidly expanding area of research and holds considerable promise for accelerating the prevention, diagnosis, and treatment of AD/ADRD. Biomarkers have different uses across the clinical continuum, including the characterization of risk; detection, diagnosis, and staging of disease (focused on both prodromal and symptomatic phases); prognosis; and measurement of intervention effect (Figure 2-3). The clinical significance of biomarkers arises from their potential to aid in the early and accurate diagnosis of AD/ADRD and to guide treatment decisions based on subtyping, such that patients are matched with therapies that are likely to work best for them.
There are myriad types of diagnostic and prognostic biomarkers under investigation, including fluid biomarkers, novel imaging biomarkers, digital
biomarkers, cognitive biomarkers, ocular biomarkers, and biomarkers of change in other areas (e.g., motor skills, sensory including vision and hearing). NIH has made significant investments in programs to support these biomarker discovery efforts (see Box 2-6).
It is important to keep in mind that many kinds of clinical data that may be collected are not biomarkers. Biomarkers need to be validated for their intended purpose (FDA-NIH Biomarker Working Group, 2020). A critical aspect of validation studies will be the evaluation of biomarker performance in diverse subpopulations (e.g., racial/ethnic, sex/gender, age) to understand any observed group-specific positivity differences. Variation in positivity across demographic groups has been observed for some biomarker types (e.g., PET imaging) but may be explained, for example, by inadequate stratification by disease status (MCI versus clinical dementia) or differences in the predominant etiology of cognitive impairment and dementia across groups, which may not be delineated by current diagnostic tools (Gao et al., 2023). The sections below describe promising advances and remaining gaps related to fluid, imaging, and digital biomarkers used to detect, diagnose, and monitor AD/ADRD. Biomarkers used in the context of clinical trials to demonstrate target engagement and evaluate responses to interventions are discussed in Chapter 4.
Fluid biomarkers
Tests to detect biomarkers of certain brain pathologies in AD using CSF or PET imaging are already in clinical use, but development of less invasive and more accessible and cost-effective blood-based biomarkers is an area of urgent need. Recent years have seen exciting progress in the discovery of peripheral, fluid-based biomarkers (Barthelemy et al., 2024; Gonzalez-Ortiz et al., 2023; Palmqvist et al., 2020; Zetterberg, 2022) that may help to address the limitations associated with imaging (e.g., cost, access in some regions of the country, coverage by insurance, need for highly trained analysts) and enable population-level screening. Although translation of these research advances to clinical practice has been slow, a blood test is now commercially available for AD based on blood amyloid levels, and the plasma phospho-tau biomarker p-tau217 has been demonstrated to be clinically equivalent to FDA-approved CSF biomarker tests used to detect AD pathology (Barthelemy et al., 2024). P-tau217 has been shown to detect AD neuropathological change in patients as many as 10 years before symptom onset and provides an opportunity to divide risks into AD versus non-AD risks. While more invasive than blood-based biomarkers, CSF biomarkers have also shown promise in detecting pathologic changes earlier than neuroimaging (Dubois et al., 2023)
At present, clinical recommendations indicate that blood-based biomarker tests and other AD diagnostics should not be used in clinical settings in asymptomatic patients; regardless, the potential to use simple finger stick
BOX 2-6
Examples of NIH Infrastructure Investments in Biomarker Discovery for Alzheimer’s Disease and Related Dementias (AD/ADRD)
Centrally Linked Longitudinal Peripheral Biomarkers of Alzheimer’s Disease in Multiethnic Populations (CLEAR-AD) Program: CLEAR-AD is an NIA-funded U19 program initiated in 2023 that focuses on discovering and validating centrally-linked peripheral molecular signatures (CLPMS) of Alzheimer’s disease (AD) in multiethnic populations (CLEAR-AD, 2024a; NIH RePORTER, 2024a). The $41 million program is led by Mayo Clinic Florida and the Indiana University School of Medicine (CLEAR-AD, 2024b) and has the following aims:
- “To discover CLPMS of the complex and heterogeneous AD pathophysiology and its copathologies.
- To identify longitudinal CLPMS that detect and predict dynamic neuroimaging, fluid biomarker, and clinical changes across AD spectrum.
- To characterize differences and similarities in CLPMS profiles across NHW [non-Hispanic White], African American (AA) and Latino American (LA) participants to uncover biomarker patterns in multi-ethnic groups.
- To make these vast resources available to the scientific community to amplify and accelerate its impact” (CLEAR-AD, 2024a).
Through these efforts the program will advance the identification of novel AD biomarkers with mechanistic insights, support a precision medicine approach to the discovery and validation of multiomics biomarkers, discover new potential therapeutic targets, and create a harmonized resource of endophenotype and multiomics data in NIH-supported cohorts for sharing with the scientific community (CLEAR-AD, 2024a).
Alzheimer’s Disease Neuroimaging Initiative (ADNI): ADNI was launched in 2004 as a longitudinal, multicenter study to validate biomarkers (e.g., imaging, genetic, biofluid) for AD (ADNI, 2024a). The ADNI study involves researchers across more than 60 sites in the United States and Canada who are collecting biomarker data to monitor the progression of AD in the brain across three disease stages: cognitively unimpaired, MCI, and clinical dementia (ADNI, 2024a). The primary goals of this initiative are to make this biomarker data, along with biospecimens, available to researchers and to improve how AD is diagnosed
and treated. Moreover, investigators from related AD and collaborating studies, such as the ADNI depression study, the Department of Defense ADNI and Worldwide ADNI, have partnered with ADNI, leveraging its research network and study model while providing ADNI researchers access to data from a larger pool of cohort participants (ADNI, 2024a,b). ADNI has been operating under a series of phases since its initial launch. Its current phase, ADNI4, was initiated in 2022 and will receive over $147 million in NIA funding over 5 years with a primary goal of expanding the inclusion of historically underrepresented groups in AD research (ADNI, 2024a). Data generated through ADNI have been shared with over 45,000 researchers globally and have contributed to more than 5,500 scientific publications (ADNI, 2024a).
Biomarkers for Vascular Contributions to Cognitive Impairment and Dementia (MarkVCID): MarkVCID is a multisite NIH-funded consortium focused on the discovery and validation of “promising predictive, diagnostic, target engagement and progression biomarkers of the brain small-vessel diseases involved in the vascular contribution to cognitive impairment and dementia” (MarkVCID, 2017). The program is overseen by NINDS and in its second phase, MarkVCID2, consists of 9 performance sites located across 15 U.S. medical centers, along with a Coordinating Center and an External Advisory Committee. The Coordinating Center, led by Massachusetts General Hospital, facilitates collaboration among participating research sites, ensures the use of a standardized set of study procedures and data collection methods, and manages data and analyses at the consortium level (MarkVCID, 2017). Research sites are tasked with validating a selection of biomarkers identified in the first phase of this program. In the first 2 years of the program, each project site worked to enroll over 200 research participants from diverse backgrounds who were experiencing cognitive decline or exhibiting early symptoms of cognitive impairment that may be linked to cerebrovascular small vessel disease. Over the course of the program, these individuals would be followed through annual clinic visits to monitor their symptom progression while utilizing harmonized data acquisition procedures for biomarker validation (MarkVCID, 2017). The consortium ultimately aims to foster the identification and availability of biomarkers that successfully identify disease pathways that should be targeted to prevent cognitive impairment due to small vessel disease and signal whether particular treatments are effective.
tests in at-home kits appears to be on the horizon (Huber et al., 2023). To address concerns regarding such direct-to-consumer commercialization, education of clinicians and the public regarding the clinical significance of results (e.g., that a positive biomarker result does not mean the individual will necessarily progress to having clinical dementia) will be an urgent priority, as will the communication of available information about interventions that may target associated risk factors. In the near term, blood-based biomarkers will likely be useful for screening and to guide more comprehensive and resource-intensive diagnostic workups, but with further validation it is feasible that they could serve as confirmatory biomarkers comparable to CSF and PET and in the future could be used to predict disease risk, monitor disease progression, and evaluate intervention effects (Hampel et al., 2022b, 2023).
A challenge, however, is that currently available biomarkers reflect only a limited number of pathologies, such as amyloid beta, tau, and neurodegeneration. There are other pathologies for which blood-based biomarkers desperately need to be identified. Indeed, biomarker discovery for most of the related dementias (e.g., LBD and frontotemporal dementia [FTD]) are at roughly the same point where the AD field was 25 years ago. As described further in Box 2-7, ongoing research is expanding the types of biomarkers that can be evaluated from blood and CSF samples, including those for alpha-synuclein (a hallmark of LBD but also found in some AD cases) (Scott et al., 2021) and TDP-43 pathology, which is common in but not limited to FTD (Cordts et al., 2023; Gifford et al., 2023; Irwin et al., 2024a,b). Proteomic analyses of plasma proteins are aiding the identification of other potential fluid biomarkers for AD/ADRD (Guo et al., 2024; Katzeff et al., 2022; Teunissen, 2024). Importantly, studies conducted in brain tissue reveal perturbations in many biological pathways, including perturbations that do not involve protein pathology (e.g., neuroinflammation, demyelination, energetics/mitochondrial perturbations, innate immunity, adaptive immunity, blood–brain barrier dysfunction, synaptic change). Blood-based biomarkers that reflect these complex and heterogeneous brain molecular changes can help investigators better understand disease pathways (described further in Chapter 3). In some cases, such biomarkers may be used solely for research purposes, while others may play an essential role in guiding precision medicine approaches to AD/ADRD.
Blood-based biomarkers that capture the complexity and heterogeneity of AD/ADRD, including brain copathologies and molecular changes, are necessary for molecular subtyping (Teunissen, 2024), as well as following these changes longitudinally even before clinical symptoms become apparent, as has been done using CSF biomarkers (Jia et al., 2024). To identify such molecular biomarkers, large-scale studies that assess multiomic (e.g., transcriptome, epigenome, proteome) changes in longitudinal cohorts with
BOX 2-7
Progress in the Discovery of Fluid Biomarkers for Related Dementias
Lewy Body Dementia (LBD)—At present, a definitive diagnosis of LBD—which includes dementia with Lewy bodies (DLB) and Parkinson’s disease dementia—can only be made postmortem. Alpha-synuclein seed amplification assays, such as the Real-Time Quaking-Induced Conversion (RT-QuIC) assay for the ultrasensitive detection of self-propagating misfolded alpha-synuclein aggregates in cerebrospinal fluid (CSF) and peripheral tissues (e.g., skin), have demonstrated promise as an accessible and accurate biomarker for DLB and Parkinson’s disease (Concha-Marambio et al., 2023; Gibson et al., 2023). A skin biopsy for the detection of phosphorylated alpha-synuclein deposition is also being evaluated for use in identifying synucleinopathies like DLB. As with the RT-QuIC assay, this approach for detecting the presence of alpha-synuclein shows promise in its sensitivity and specificity (Gibbons et al., 2024) as well as in its feasibility for use as a noninvasive test. The availability of a premortem diagnostic biomarker would advance opportunities in both research and in clinical care by providing a tool with which to understand LBD pathogenesis, support early and accurate diagnosis, and evaluate interventions (Bargar et al., 2021).
Frontotemporal Dementia (FTD)—Elevated levels of neurofilament light and phosphorylated neurofilament heavy in plasma and CSF are associated with the presence of neuronal injury and neurodegeneration for FTD and other neurodegenerative diseases such as amyotrophic lateral sclerosis and show promise as diagnostic and prognostic biomarkers (Gendron et al., 2022; Irwin et al., 2024b; Katzeff et al., 2022). While these potential markers are not specific to FTD, they could help to differentiate the disease from other types of dementia and neurodegenerative diseases, identify participants for clinical research, and provide earlier and more accurate diagnoses (Gendron et al., 2022; Katzeff et al., 2022).
validation in brain-based autopsy studies are necessary. Such studies will lay the foundation to detect peripheral molecular signatures that reflect brain molecular perturbations. Once validated in population-based and clinical studies, these signatures can become much-needed precision medicine biomarkers of AD/ADRD.
Although blood-based biomarkers are transforming early detection and diagnosis for AD/ADRD and are being assessed for use in AD risk screening (Palmqvist et al., 2019) and prognosis (Cullen et al., 2021; Palmqvist
et al., 2021), there remain many areas for innovation and research. Specifically, there is an urgent need for fluid biomarkers associated with diagnosis of non-AD tauopathies as well as other related dementias. In most cases, related dementias are still diagnosed very late and such diagnoses are very error prone owing to the heterogeneity and overlapping nature of the pathologies and clinical symptoms. There also is a need for novel biomarkers related to the specific cell types and mechanisms affected across the clinical stages of AD/ADRD.
Imaging biomarkers
Amyloid-PET is an imaging biomarker for AD that is used in both clinical and research settings for the detection of amyloid pathology and has played an important role in improving diagnostic accuracy (Hampel et al., 2022a). Tau PET tracers have also been approved by FDA (2020), and efforts are currently underway to evaluate their use for mapping the density and spatial distribution of tau pathology in the brain, which correlates with functional and cognitive outcomes in people living with AD (Fleisher et al., 2020; Hampel et al., 2022a). Tau imaging biomarkers may also be used to guide the selection of therapies and to monitor their effects. While Tau PET imaging is a valuable tool for diagnosing AD, it is costly and not widely accessible, and currently available tracers are insensitive to non-AD tauopathies. A study by Tsai et al. (2019) demonstrated that the Tau PET 18F-flortaucipir tracer has limited sensitivity and specificity in patients with FTD-related tauopathy. PET imaging biomarkers for other AD-related pathologic features, including inflammation, synaptic dysfunction, and neuronal injury, are being developed but are not yet ready for integration into clinical care.
Imaging biomarkers for related dementias are not as advanced as those for AD but represent an area of active research and development given the lack of tools available for confirmation of related dementias. Computed tomography (CT) and structural MRI are both used to examine structural brain markers in patients with suspected FTD and LBD (e.g., to look for signs of atrophy), while PET, single-photon emissions computed tomography (SPECT), and functional MRI are used to evaluate functional parameters such as metabolic activity, dopamine transporter uptake, regional blood flow, or hemodynamic changes (Peet et al., 2021), all of which may be useful in a differential diagnosis process (Ishii, 2020; Mavroudis et al., 2019). PET with fluorodeoxyglucose F-18 (18F-FDG-PET) has been used extensively in clinical and research settings to differentiate FTD from other pathologies through the examination spatial patterns of brain hypometabolism (Minoshima et al., 2022) and may also show promise in predicting near-term risk of developing clinical dementia (Heyer et al., 2024). Other functional neuroimaging tools such as electroencephalography (EEG) have been used alone or in combination with other approaches such
as polysomnography to examine sleep activity and aid in the supporting diagnosis of dementia with Lewy bodies (Law et al., 2020) and may have prognostic value more broadly (Law et al., 2020; van der Zande et al., 2020). Interestingly, 123I-meta-iodobenzylguanidine myocardial scintigraphy, which can be used to assess nerve damage in the peripheral nervous system, has shown promise for the diagnosis of LBD (Abdelmoaty et al., 2023; Blanc and Bousiges, 2022; Matsubara et al., 2022). Different imaging modalities may be more or less useful at prodromal versus clinical dementia stages of disease. Ongoing imaging biomarker discovery and validation is needed to address current challenges with a lack of specificity and sensitivity (Blanc and Bousiges, 2022).
The costs and capital requirements for the use of MRI reduce the accessibility of this neuroimaging modality. However, the advancement of emerging technologies such as low-field MRI may create more accessible options in certain use cases for extending neuroimaging in resource-limited settings because of the more portable nature and low power requirements of the equipment (Kimberly et al., 2023). In addition to the potential for clinical use, portable options such as low-field MRI could expand their feasibility in research settings and allow for use outside of traditional clinical settings, such as in a research participant’s home or in a dedicated research vehicle, and enhance engagement with diverse populations often underrepresented in clinical research (Deoni et al., 2023).
Digital biomarkers
The slow and subtle development of AD/ADRD creates an urgent need for diagnostic and prognostic biomarkers that can be detected early enough in the pathophysiological process to guide early interventions with the capability of preventing or delaying disease onset (Öhman et al., 2021). As discussed earlier in this chapter, digital tools and technologies are enabling the collection of streams of myriad physiological and behavioral data that can provide insights into an individual’s sensory, motor, and cognitive function. Importantly, digitally collected health-related data (e.g., gait, sleep, speech) does not necessarily translate to a digital biomarker, although this is a common mischaracterization (Au et al., 2022). The identification of digital biomarkers will require the same rigorous scientific investigation used to identify and validate fluid and imaging biomarkers.
At present, the regulatory framework for the validation and approval of biomarkers is not designed to accommodate the kinds of data being generated using digital tools and technologies, such as time-series data covering an extended period of time (Au et al., 2022). This regulatory misalignment may pose a barrier to the development and clinical translation of tools for evaluating digital biomarkers, such as by disincentivizing companies looking for assurance of a likely return on their investment. Even if it is
possible to simplify multiple streams of data into a single measure to better fit the existing regulatory framework, such a step would likely represent a loss of important information. The value in using digital modalities is in the multidimensional nature of the data. Rather than seeking to condense these data, there needs to be an effort to adjust the regulatory framework to accommodate “digital biomarker trajectories” that comprise a mix of signals that show an evolving pattern predictive of cognitive impairment. Such an adjustment may require FDA to develop new pathways for validation and approval of digital biomarkers (Au et al., 2022). It is possible to imagine that an updated regulatory framework would include different processes for different categories of digital biomarkers—those that correlate with traditional clinical, imaging, or fluid biomarkers, which could be used as reference standards for validation, and novel digital biomarkers that lack a biological correlate.
Addressing the regulatory framework is necessary but not sufficient to advance the widespread acceptance and use of digital biomarkers for AD/ADRD. Strategies to promote adoption include increasing comfort levels with digital tools and technologies in clinical settings such as by promoting the use of FDA-approved and reimbursable technologies (Au et al., 2022) and demonstrating the usefulness of digital biomarkers through clinical trials (Coravos et al., 2019; Kaye et al., 2023).
Conclusion 2-2: The current FDA regulatory framework for the validation and approval of biomarkers and the current Centers for Medicare & Medicaid Services reimbursement model is not designed to accommodate the types of data that are produced by digital tools and technologies. New regulatory pathways, such as those that support distinct processes for different categories of digital biomarkers, are needed to realize the potential of these emerging tools and technologies.
Conclusion 2-3: The last 10 years has seen a transformational advancement in the detection of AD-related pathologies in living people. Some pathologies can be detected many years before symptoms are detectable and have enabled the development of novel therapies for AD. There remain major gaps in available biomarkers for related dementias and the ability to quantify and longitudinally monitor mixed pathologies. In addition, there remain pathways not associated with protein pathologies (e.g., neuroinflammation, demyelination, energetics/mitochondrial perturbations, innate immunity, adaptive immunity, blood–brain barrier dysfunction, and synaptic function) that lack accessible and efficient measures.
Leveraging Longitudinal Cohort Studies
There is still much that is not understood about AD/ADRD, which feature decades of clinical but not pathologic dormancy. The molecular inflection points and the extent to which modifiable risk factors influence risk and progression of disease or the life-course time points during which risk factors are important (e.g., early life, midlife) need to be better understood. Illuminating this will require looking longitudinally at the exposome and molecular changes and how they interact across the lifespan, as discussed further in Chapter 3.
Longitudinal cohort studies represent an important mechanism for identifying essential data types that, when integrated, can provide a comprehensive view of brain health and AD/ADRD development over the life course (including risk and resilience factors). It is reasonable to imagine that, when viewed longitudinally, individuals will have trajectories that are not captured by cross-sectional studies or detectable on a group level but are related to treatments that will be relevant to that individual at a specific time point on an individual’s trajectory. Knowledge gained from such longitudinal studies can be translated into processes and tools, such as digital health technologies and biomarker assays, that can be used for ongoing monitoring and AD/ADRD prediction, detection, and diagnosis in clinical settings. Ultimately the goal of this research is to inform the development of more sensitive and accessible—across diverse social and cultural contexts—tools and methods that can be used in clinical settings to detect brain health changes at individual and population (aggregated data) levels. A notable example of such efforts is the ARTFL-LEFFTDS Longitudinal Frontotemporal Lobar Degeneration (ALLFTD). ALLFTD is a cohort of people with frontotemporal lobar degeneration from whom cognitive and behavioral data, imaging, and blood and CSF samples are being collected over time. This study aims to identify useful clinical measures and markers for predicting the onset of symptoms and use in future FTD treatment trials, in which cohort participants may be eligible to participate (ALLFTD, n.d.).
While some cohorts have been established specifically for the purpose of understanding brain health and the development of AD/ADRD, in other cases, measures of brain health and monitoring for AD/ADRD have been incorporated into cohorts designed for other purposes. For example, NIH provided funding for the addition of new cognitive and psychophysiological assessments and functional neuroimaging in the Midlife in the United States (MIDUS) study to improve understanding of the risk factors that lead to dementia and mechanisms to advance prevention (NIH RePORTER, 2024b). Box 2-8 provides descriptions of other examples of ongoing NIH-funded cohorts that were not established for the study of AD/ADRD but include a brain health component. Continuing to leverage these existing
BOX 2-8
Examples of NIH-Funded Cohort Studies Developed for Other Purposes that Provide Data on Brain Health and Alzheimer’s Disease and Related Dementias (AD/ADRD)
The Women’s Health Initiative (WHI): The WHI is a national health study that is funded by the National Heart, Lung, and Blood Institute (NHLBI) and was initiated in the 1990s (WHI, 2021a). Although the original study ended in 2005, WHI has since continued in the form of extension studies, which include annual health updates and outcomes for active participants. The WHI focuses on the prevention of major causes of death, disability, and frailty in older women, including cardiovascular disease, cancers, and osteoporotic fractures. With more than 161,000 women enrolled in the original study (WHI, 2021a), the WHI is one of the largest women’s health projects in the United States. The WHI Memory Study (WHIMS) was initiated in 1995 and enrolled 7,427 women age 65 years and older to investigated the effects of hormone therapy on risk of cognitive impairment and probable dementia, as well as changes in global cognition over time (WHI, 2021b). Over the course of the WHI study, several WHIMS-related ancillary studies were conducted, including the Supplemental Case Ascertainment Protocol, which aims to identify probable dementia and mild cognitive impairment cases in participants who are deceased or proxy dependent, the Magnetic Resonance Imaging study, the WHI Study of Cognitive Aging, the WHI Memory Study of Younger Women, and a study on the Epidemiology of Cognitive Health Outcomes (WHI, 2021b).
All of Us Research Program: The framework for the All of Us Research Program was developed by the National Insitutes of Health’s Precision Medicine Initiative Working Group of the Advisory Committee to the Director in 2015 (NIH, 2024b). The mission of the All of Us program is to “accelerate health research and medical breakthroughs, enabling individualized prevention, treatment, and care for all of us” (NIH, 2024a). The All of Us Research Program aimed to enroll at least one million U.S. participants as part of its efforts to build one of the largest and most diverse health databases of its kind (NIH, 2024c). The program collects different types of genetic and health data (e.g., data from surveys, electronic health records, and blood and urine tests). These data have been used to study various facets of brain health; for example, a recent study examined hypertension and type 2 diabetes as risk factors for dementia and underscored the value of this cohort for better understanding disease prevalence and risk factors (Nagar et al., 2022).
Framingham Heart Study (FHS): The FHS was launched in 1948 under the direction of what eventually became the National Heart, Lung,
and Blood Institute (NHLBI, n.d.). The original goal of the FHS was to identify common factors that contribute to cardiovascular disease (CVD). The study’s objectives are to understand the incidence and prevalence of and risk factors for CVD, to study trends in these rates and factors over time, and to examine familial patterns of CVD. The study initially recruited 5,209 men and women, ages 28–62 years, living in Framingham, MA from 1948 to 1952 (NHLBI, 2023) and has since become a multigenerational study that has gathered genetic information from more than 15,000 people across three generations (i.e., original participants, their children, and their grandchildren) (NHLBI, n.d.). The study continues to assess participants every 2 years under a contract to Boston University from the NHLBI, and receives grant support for specialized studies (NHLBI, 2023). The FHS Brain Aging Program (FHS-BAP) was established for the surveillance and evaluation of FHS participants for dementia using traditional and digital cognitive assessments and brain imaging (BU, n.d.a). The program has spurred the creation of three interrelated projects that are examining “vascular and inflammatory contributors to AD, identifying factors associated with AD risk and resilience, investigating the link between AD genetic vulnerabilities and chronic inflammation, and studying the impact of gene variants affecting immune function on AD-related changes” (BU, n.d.b). FHS-BAP is also establishing a robust platform to promote data sharing and collaboration for the purpose of accelerating AD research using FHS data. (BU, n.d.a). To accomplish this goal, the program offers tools that allow researchers to access and use FHS data; for example, a data dictionary is available to provide researchers with definitions and explanations for all variables and elements within datasets (BU, n.d.c).
Bogalusa Heart Study: The NHLBI-funded Bogalusa Heart Study is a longitudinal study focusing on cardiovascular risk factors among children and young adults living in a semirural parish of Bogalusa, Louisiana (NHLBI, 2018). The study collected data from approximately 14,000 people from birth to the age of 38 years in a population that is biracial (65 percent White and 35 percent Black) (American College of Cardiology, 2002). The study established that precursors of adult CVD begin as early as childhood. In 2019, researchers at Louisiana State University’s Pennington Biomedical Research Center and Mary Bird Perkins Cancer Center received a $14.5 million grant from NIA to examine the impact of high blood sugar levels in early life on brain health (Yawn, 2023).
Strong Heart Study (SHS): The NHLBI-supported SHS is the largest epidemiological study of CVD in American Indians. The SHS was launched in the early 1980s and has included numerous phases, including a pilot study and later expansion of a family study focused on genetic contributions to CVD and its risk factors. The most recent phase includes follow-up examinations for approximately 3,500 SHS participants (SHS, 2017a). Several in-depth ancillary studies have been incorporated into this most recent follow-up examination phase that are relevant to aging and brain health. These include studies on resilience in brain aging; psychological risk factors, quality of life, community, and brain aging; social determinants of health and neurodegeneration; and targets for precision prevention of AD/ADRD in American Indians, among others (SHS, 2017b).
Multi-Ethnic Study of Atherosclerosis (MESA): MESA is an NHLBI-funded cohort study that was developed to examine characteristics of subclinical CVD and the risk factors that predict progression to clinical CVD in diverse populations. The study involves more than 6,000 men and women, ages 45–84 without known CVD, from six different communities in the United States (MESA, 2020). The field sites involved in MESA include Columbia University, Johns Hopkins University, Northwestern University, University of California Los Angeles, University of Minnesota, and Wake Forest University. MESA-MIND was created as an ancillary study that specifically focuses on understanding how subclinical vascular disease may increase dementia risk. Participants of the MESA-MIND study undergo detailed cognitive testing and brain imaging (i.e., MRI and amyloid-PET), in addition to other clinical assessments (MESA-MIND, 2024).
Health and Retirement Study (HRS): The HRS, led by the University of Michigan, is a longitudinal study supported by the National Institute on Aging and the Social Security Administration (HRS, 2021). The study began in 1992 and at present includes a representative, active study population of about 20,000 people (HRS, 2024). While not the sole focus of the HRS, a goal of the study is to understand changes in cognitive health with aging and the impact of AD/ADRD on people within the United States (HRS, 2021). The HRS aims to continue expanding the number of minority participants to continue to serve as a major resource for studying racial and ethnic disparities within AD/ADRD. The Aging, Demographics, and Memory Study (ADAMS) is an HRS supplemental study that involves additional data collection through in-person clinical assessments to obtain detailed information on the participants’ cognitive status (HRS, 2024).
cohorts provides important opportunities for filling knowledge gaps, particularly regarding key transition points in the life course, and to prioritize the inclusion of data from underrepresented groups (e.g., data from the Black Women’s Health Study), which help address biases in existing data.
Care must be taken to mitigate potential spotlight effects resulting from basing conclusions and models on the data and data sources currently available while failing to adequately explore other potential explanations and paradigms. For example, there is increasing interest in the vascular dimensions of AD/ADRD based in part on the availability of data from established sources such as the Framingham Heart Study, but other equally important contributors to AD/ADRD may yet to be discovered.
Conclusion 2-4: Longitudinal cohort studies, including those that have been specifically designed for the purpose of understanding brain health and those created for other purposes, are essential mechanisms for distinguishing what types of data can be integrated to advance a life-course understanding of brain health and disease across diverse populations. There are immediate opportunities to accelerate advances in research by leveraging existing cohorts to address knowledge gaps and biases in data.
Opportunities to Optimize Data Collection and Analysis from Longitudinal Cohorts
Improving cohort representativeness
To ensure that data collection and evaluation strategies that emerge from cohort studies are not biased to certain subpopulations and have broad applicability, attention needs to be paid to ensuring cohort studies enroll diverse and representative populations. Some advances in cohort diversity have been achieved through NIH support for new cohorts (see Box 2-9) and increasing underrepresented populations in existing cohorts, such as the Health and Retirement Study (Nye et al., 2022), but these efforts need to be expanded. In doing so, representativeness should be considered across multiple dimensions including, but not limited to, race and ethnicity, occupation, geography (e.g., urban, rural), and socioeconomic status and social phenotypes.
While self-identified race and ethnicity data are often collected, use of genetic ancestry data can provide a more complete characterization of the diversity of cohorts and differences in risk across diverse groups (Reitz et al., 2023). Constructing cohorts solely of people from minoritized communities may better enable in-depth exploration of social factors that influence AD/ADRD risk and the understanding of heterogeneity in populations bearing increased risk of dementia (Weuve, 2024). For example, the Hispanic Community Health Study/Study of Latinos, the largest comprehensive study
BOX 2-9
The Health and Aging Brain Study: Health Disparities
The National Institute on Aging–funded Health and Aging Brain Study: Health Disparities (HABS-HD) was launched in 2022 and aims to understand the biological, social, and environmental factors that affect brain aging among diverse communities. This is the first large-scale, community-based project focusing on how biological, medical, environmental, and social factors, within a health disparities framework, contribute to AD risk within African Americans, Mexican Americans, and non-Hispanic Whites (NIH RePORTER, 2024c). One of the goals of the Health and Aging Brain Study is to identify racial/ethnic-specific risk profiles for cognitive loss among the diverse populations involved in the study (HCS Institute for Translational Research, 2022). The study will also collect life-course exposome and sociocultural data to examine how these factors affect biomarkers among diverse populations. Study data, including HABS-HD data, biofluid samples, and genomics data, will be available to the global scientific community (Toga et al., 2022).
of health and disease in people of Hispanic/Latino ancestry, assessed the risk and protective factors for chronic health conditions across more than 16,000 participants (NHLBI, 2024). Insights from this work have created opportunities for further research into health disparities and led to the dedicated investigation of aging and neurodegeneration within this population (González et al., 2019).
Another consideration for cohort diversity is the language spoken by participants. Cohorts have traditionally been restricted to a limited pool of spoken languages (e.g., English, Spanish, Chinese). As a result, tools developed and tested in existing cohorts may not translate well to a more diverse set of languages. Achieving more diverse cohorts will require investment in meaningful community engagement, which is discussed further in Chapter 5.
Capturing data from across the life course
While dementia is commonly thought of as a disease of old age based on the timing for the manifestation of the most severe symptoms, it is increasingly understood that dementia is often the culmination of a pathologic process that develops slowly over a period of decades and that AD/ADRD risk is a function of an individual’s genetics, which are set at conception, along with the physical and social exposures that occur throughout the life course, including
during gestational, childhood, adolescent, and adult life stages (Cadar, 2017; Whalley et al., 2006). To better understand the timing for key risk and protective factors related to AD/ADRD and to define data elements that should be included in a longitudinal data collection strategy, cohort studies need to enable the capture of data from across the entirety of the life course. While this may not be feasible for each individual cohort, multicohort analysis (discussed below) can allow a more complete picture to be developed from cohorts that include people of varying age ranges.
Early life is a critical period for the development of neuronal connections in the brain (WHO, 2022) and cognitive reserve (Livingston et al., 2020), which may lower the risk of dementia (Meng and D’Arcy, 2012). Although there has been some focus on the role of early-life education as a protective mechanism that functions through the development of cognitive reserve (Foverskov et al., 2020; McDowell et al., 2005; Meng and D’Arcy, 2012; Nguyen et al., 2016), less is known regarding the effect of other early-life exposures, both social (e.g., neglect and abuse) (Corney et al., 2022) and environmental (e.g., environmental neurotoxicants, such as lead) (Reuben, 2018). This stage of the life course has perhaps received the least attention, in part because of the large time lag between early life and AD/ADRD development. This gap may be addressed by ensuring routine data collection related to early-life exposures in cohort studies. Birth cohorts are of particular value for such endeavors. Such data would ideally be captured prospectively, as is being done in the NIH-funded National Longitudinal Study of Adolescent to Adult Health (Harris et al., 2019). However, it is also possible to fill data gaps retrospectively by collecting data from archival resources (Moceri et al., 2000).
The understanding of dementia as a disease that develops over many years has naturally shifted attention to the connection between events of midlife and late-life AD/ADRD development. Epidemiological studies of dementia risk have led to a particular focus on chronic diseases that manifest in midlife, such as hypertension and diabetes (Livingston et al., 2020; Whalley et al., 2006). Cohort studies focused on cardiovascular health are increasingly including outcomes related to brain health (see Box 2-8). However, there remain aspects of midlife, such as the effects of pregnancy and menopause on brain health and AD/ADRD in women, that are understudied (Barth and de Lange, 2020), and opportunities to better capture key data from this life period, including reproductive history, are being missed (Buckley, 2024).
Just as it is important to ensure cohort studies for AD/ADRD are inclusive of early life, much can be learned from prospective population-based studies of late life, when neuropathology, and particularly mixed pathologies, and cognitive impairment are far more prevalent (Corrada et al., 2012). Research focused on the oldest old, who are often excluded from
studies owing to such issues as functional disabilities, frailty, and sensory impairment (Corrada, 2024), can yield insights into resilience factors for AD/ADRD (Andersen, 2020), and, for studies including a brain donation component, links may be found between cognitive function and the accumulation of neuropathologic changes over time for this population (Beker et al., 2021; Corrada et al., 2012).
Banking samples for future analyses
Biobanking provides a mechanism to maximize opportunities to learn from and effectively use the investments in existing cohorts to facilitate AD/ADRD research (Francis et al., 2018). As technology and analytic methods continue to evolve, there may be future opportunities to glean insights from banked samples that investigators cannot necessarily anticipate at the time of sample collection, potentially leading to the development of future tools or therapeutics. Banked samples from longitudinal cohorts have advantages over ad hoc donated biosamples resulting from the ability to link data derived from the analysis of the banked samples to clinical and other data (e.g., exposure history) collected premortem from the individuals in the cohort (Kind, 2024). A tradeoff should be noted, however, that data linked to biological samples may function to limit the accessibility of those samples due to privacy concerns. While individual cohort studies may be limited in size based on resource constraints, analyzing banked samples collected from diverse cohorts can not only increase the population size for a given analysis but also the diversity represented in the analyzed samples (Miller, 2024).
The collection of deeply phenotyped and well-characterized brain donor tissue samples can add significant value to cohort studies by enabling the validation of clinical diagnoses and imaging surrogates through neuropathologic examination and the association of neuropathology with premortem data from cohort participants (Cairns et al., 2010; Franklin et al., 2015). If banked brain samples can thus be used to identify prognostic markers, such information could guide early intervention strategies for those determined to be at high risk (Robinson et al., 2018). Increasing the availability of banked brain samples from diverse populations requires public education and outreach to convey the importance of brain donation to advancing AD/ADRD research and to clarify that brain donation for research involves a separate consent process from that used for organ donation intended to save lives. Also needed are resources to collect, analyze, and store the donated samples. Reaching potential donors can be facilitated by engaging advocacy groups and community members who are themselves or have family members living with AD/ADRD (Francis et al., 2018). NIH has developed resources for the public and the Alzheimer’s Disease Research Centers (ADRCs) to support education efforts related to brain donation (NIA, n.d.a, 2022; NINDS, 2023), but inadequate resources have been a
noted challenge to brain banking efforts, contributing to lost opportunities for future research (Franklin et al., 2015).
Similarly, banked blood samples linked to clinical and other data from cohort participants represent an invaluable resource as increasingly high-throughput assays are enabling multiomic and other analyses such as mass spectrometry for signatures of past exposures, at scales previously unachievable (Buckley, 2024; Miller, 2024). The Framingham Heart Study exemplifies the scientific value of banked blood samples collected before disease onset and their use years later in new forms of testing (e.g., genomics, proteomics, metabolomics) for potential disease-predictive biomarkers as technology evolved, resulting in opportunities to maximally leverage the cohort study. The emergence of blood-based biomarkers for AD/ADRD has been enabled in part through the analysis of banked blood samples, which set the stage for prospective studies focused on testing biomarkers for accuracy in predicting clinical disease onset years later (Au et al., 2023).
Ensuring that banked samples will continue to be a highly valuable resource for future AD/ADRD research will require consideration regarding, and funding for, harmonized sample collection processes and optimal storage, as well as systems for researcher access to these precious samples, including inventories of samples available for external use (discussed further in Chapter 5). Lack of transparency regarding sample availability has been a barrier to fully realizing the value of biobanking investments. An unfortunate result of the high cost of biobanking is that many donated samples will be discarded before they can be used. This not only represents a lost opportunity to gain valuable knowledge from the samples but also does a disservice to those who donated the specimens.
Some biorepositories may be able to step in as resources and opportunity allow to preserve valuable specimens from closed studies that cannot maintain their samples; however, this currently ad hoc process could be facilitated by the use of standardized consent forms and collection procedures. For example, the National Centralized Repository for Alzheimer’s Disease and Related Dementias (NCRAD), an NIA-funded biorepository, was able to take in samples from the API Generation program, which was terminated prior to completing enrollment (NCRAD, 2024a), as well as samples that would have otherwise been lost from the Gingko Evaluation of Memory Study (NCRAD, 2024b). Further scaling of the capacity of this resource is needed to prevent further loss of precious biospecimens. See Box 2-10 for information on this and other NIH-funded biorepositories supporting AD/ADRD research.
BOX 2-10
Examples of National Institutes of Health (NIH)-Supported Alzheimer’s Disease and Related Dementias (AD/ADRD) Biorepositories
NIH-funded biorepositories are critical research resources that support the collection, storage, and accessibility of essential biological specimens collected from NIH-funded studies and other biomedical research.
National Centralized Repository for Alzheimer’s Disease and Related Dementias (NCRAD): NCRAD at the Indiana University School of Medicine is a National Institute on Aging (NIA)–funded biorepository established in 1990 for the purpose of supporting research on AD/ADRD etiology, detection, and development of therapeutics. NCRAD serves as a biorepository for a wide variety of biological sample types that have been generated by over 65 NIH-funded studies, such as the Alzheimer’s Disease Neuroimaging Initiative, the 90+ Study, and the Alzheimer’s Biomarker Consortium–Down Syndrome study, as well as clinic-based samples from Alzheimer’s Disease Research Centers, accounting for 70,000 samples from over 118,000 participants and providing cutting-edge fluid biomarker analysis to investigators (NCRAD, 2024c,d,e).
Biospecimen Exchange for Neurological Disorders (BioSEND): BioSEND, based at the Indiana University School of Medicine, is a National Institute of Neurological Disorders and Stroke (NINDS)-funded biomarker repository established in 2015. The repository stores biospecimens from studies, including phase 2 and 3 trials, funded by or conducted in collaboration with NINDS. BioSEND collects a broad range of specimen types (e.g., DNA, RNA, plasma, serum, CSF, and whole blood) from studies on a wide variety of neurological and neuropsychiatric diseases, including Lewy body dementia and frontotemporal dementia. Samples from over 30 different studies are available for access by investigators (BioSEND, 2024).
NeuroBioBank: The NeuroBioBank was established in September 2013 and is supported by NIA, NINDS, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Mental Health, and the National Institute on Drug Abuse. The NeuroBioBank serves as a resource for researchers studying many different neurological, neuropsychiatric, and neurodevelopmental diseases and disorders. It provides centralized access to six biorepositories that are housed at academic and research centers throughout the United States and also partners with research centers (ADRCs) and not-for-profit organizations (e.g., the Brain Donor Project). In addition to supporting
researchers’ access to well-characterized, postmortem brain tissue and related biospecimens from across its network, the NeuroBioBank offers a centralized resource of best practices to guide the acquisition, preparation, and sharing of stored tissues (NeuroBioBank, 2024).
AgingResearchBiobank: Established by NIA in 2018, the AgingResearchBiobank is an inventory system designed to hold and distribute biospecimens and phenotypic and clinical data from relevant NIA-funded clinical trials and observational studies. The AgingResearchBiobank includes (1) a biorepository that is designed to receive, store, and distribute biospecimens from different study collections; and (2) a data repository that serves as a data coordinating center to receive, archive, maintain, and distribute images and databases from various study collections (NIA, n.d.b). While only relevant NIA-funded studies can submit biospecimens and data to the AgingResearchBiobank (at no cost to investigators), qualified investigators from around the world are eligible to apply for access to biospecimens and data from the AgingResearchBiobank, which facilitates the sharing of these resources with the broader research community (NIA, n.d.b, 2024a).
Aging Cell Repository: The NIA-funded Aging Cell Repository is located at the Coriell Institute for Medical Research, which holds a number of other repositories funded by the National Institute of General Medical Sciences, the National Human Genome Research Institute, and NINDS, among others (Coriell Institute, 2024). The Aging Cell Repository stores and shares cells and other biological samples derived from both older animals and humans to researchers. The cells and DNA samples, which are collected using strict diagnostic criteria, are stored in accordance with high-quality standards of cell culture and DNA purification. Researchers from over 40 countries have used the cell cultures from the Aging Cell Repository for both cellular and molecular research focusing on aging and neurodegeneration. Each year the Coriell Institute ships approximately 1,200 cell cultures and over 400 DNA samples from the cell bank to investigators at academic, nonprofit, or government institutions (Moro, 2018).
Conclusion 2-5: The potential value of, and uses for, biobanked samples, specifically those from longitudinal cohorts, may not be clear at the time of collection but may emerge as new analytic approaches and tools are developed. Therefore, careful consideration is needed to plan for how these samples can be collected and stored to avoid limiting future use opportunities and barriers to access and analysis.
Scaling data and sample collection: Balancing scientific opportunities and resource constraints
Given the substantial costs of data and sample collection, storage, and analysis, data collection goals need to be balanced against resource restrictions and should consider the invasiveness of the testing procedure. Some data are easily collected with widely available tools and may form a minimal dataset for all cohorts while those of higher cost or burden to extract may need to be scaled down to smaller cohorts or subpopulations. As engineering and technological advances continue to increase the scale at which some data can be collected and analyzed cost-effectively, it may be possible to expand both cohort sizes and data collection. The shift to more virtual engagement with cohorts during the COVID-19 pandemic highlighted opportunities to reduce the burden and increase the scale of data collection (Buckley, 2024), providing a foundation for future cohort studies.
Enabling multicohort analysis through data access and harmonization
Another way to maximally use the data collected from individual cohorts is to conduct multicohort analyses, which have the potential to increase the diversity of the population included in an analysis. For example, data from four cohorts featuring populations in different life stages were used to study the natural history of age-related cognitive decline over the life course and the effect of social disparities on trajectories (Yang et al., 2024). Barriers to multicohort analyses include data access issues and variation in measures and methods used across different cohorts. Of note, making data accessible differs in practice from active data sharing and distribution; the former may raise fewer concerns regarding data security. For example, on some platforms, data can be made accessible for analysis while restricting the downloading of raw datasets. This could help address such concerns as patient privacy and the potential for the commercialization of patient data. As discussed further in Chapter 5, tiering data access is another strategy that can be used to address privacy and data security concerns. Data with the lowest level of sensitivity, such as some fully de-identified data, can be made publicly available, while increasing levels of restrictions on access can be applied as the sensitivity of the data increases.
Data harmonization provides a means of pooling data from multiple studies despite heterogeneity in measures and test methods (Hampton et al., 2023; Mukherjee et al., 2023). As an example, the Phenotype Harmonization Consortium, a component of the Alzheimer’s Disease Sequencing Project (ADSP) (see Chapter 3), was created to enable further genomic analyses through the intensive harmonization of rich endophenotypic data from 39 participating cohort studies on AD/ADRD and aging associated with the ADSP (ADSP, 2024; VMAC, 2024). However, given the complexity of post hoc harmonization and the need for specialized expertise and data
infrastructure, coordination among study investigators during the planning stage could facilitate multicohort analyses by proactively addressing potential impediments related to data access and harmonization, thereby accelerating progress in AD/ADRD research. NIA funded the Harmonized Cognitive Assessment Protocol to realize the development and validation of harmonized measures for longitudinal cohorts to enable these cross-study comparisons and improve the understanding of dementia risk in diverse populations in high- and low-income countries (see Box 2-11).
Considerations Regarding Real-World Implementation of Emerging and Novel Tools to Guide Clinical Action
The overarching goal of all clinical research is to improve what happens when a patient meets with a clinician. To maximize the benefits to society from research, discoveries such as advancements in biomarkers, data collection methods, and interventions need to be translated to real-world settings.
Research investments have generated significant advances in the ability to more accurately diagnose AD/ADRD earlier in the life course and to monitor disease progression using biomarkers and other tools, but this capability has not yet been realized in most clinical settings. As a result, clinical decision making largely continues to rely on methods that leave considerable uncertainty regarding the best course of action. In addition to direct benefits to patients resulting from better-informed clinical decision making, the implementation of new tools for data collection in clinical practice also provides opportunities to further advance knowledge through the collection of real-world evidence. For example, more precise diagnostic capabilities would enhance knowledge regarding the prevalence and incidence of AD and related dementias, a current knowledge gap discussed in Chapter 1. In addition to considering the potential benefits to patients, the potential risks associated with the adoption of new tools needs to be considered.
Overcoming Barriers to Real-World Implementation
The implementation of new tools in real-world settings necessitates the consideration of barriers and facilitators to adoption and, for clinical settings, integration into existing workflows. Implementation science provides an approach for building the evidence base for such considerations. Investment in implementation testing, for example, in the form of pilot programs, can identify pain points and guide strategies to facilitate tool uptake in a broader rollout phase (NASEM, 2024).
Clinical uptake of new tools for AD/ADRD detection, diagnosis, and monitoring (e.g., biomarkers and digital technologies) has been slow, in
BOX 2-11
Harmonized Cognitive Assessment Protocol
The Harmonized Cognitive Assessment Protocol (HCAP) Network represents a major NIA investment in the harmonization of methods to measure cognitive function of older individuals from around the world (HCAP Network, 2023). The design of HCAP was led by the Health and Retirement Study (HRS) investigators in collaboration with a number of its sister studies worldwide. The HCAP is a harmonized cognitive battery that includes neuropsychological test items from previously validated cognitive test batteries along with an informant interview to help detect and diagnose mild cognitive impairment and dementia (Kobayashi et al., 2024). Ultimately, this effort yielded a flexible instrument for measuring late-life cognitive function that is being used to generate harmonized data for cross-national comparison analyses that are sensitive to cultural, linguistic, and educational differences across countries (Kobayashi et al., 2024). The work of the HCAP Network involves harmonization activities encompassing the entirety of the longitudinal research process including sample design, protocol development and administration, statistical harmonization of collected data, and diagnostic algorithms, among others (NIH RePORTER, 2024d). Importantly, the HCAP Network, a group of researchers who collaborate to support the harmonization of international studies using HCAP (HCAP Network, 2023), facilitates the use of pilot projects to continuously improve harmonization of studies.
A notable challenge is maintaining test and measure harmonization despite cross-country variations in life-course factors, such as poverty, diet, and educational attainment, that influence cognitive function and AD/ADRD risk (NIH RePORTER, 2024d). The HCAP network has begun to publish initial findings on cross-national differences (Kobayashi et al., 2024).
NIA also supported the creation of the HRS International Partner Study network to oversee continued harmonization using HCAP across large nationally representative samples throughout the world. As of 2024, the HCAP Network members include 13 longitudinal studies of aging and cognition, including the Longitudinal Aging Study in India, the Survey of Health, Aging, and Retirement in Europe, and the China Health and Retirement Longitudinal Study, among others (HCAP Network, 2024). Existing and planned HCAP studies capture an estimated 75 percent of the global population age 65 years or older (Kobayashi et al., 2024).
part because of uncertainty in the health care community. Other barriers to the widespread use of new tools in clinical settings include considerations related to insurance coverage, electronic health record integration, and out-of-pocket costs for patients. See Box 2-12 for examples of the integration of new tools into clinical diagnostic criteria for AD and LBD. A multipronged approach is needed to support implementation, including the education of primary care providers on the capabilities and limitations of tools, grants for workforce training, and the development of collaborations between community physicians and researchers. Parallel efforts should be focused on educating the public to help people understand the importance of research, to help them critically analyze research findings communicated in the media, and to better advocate for themselves when meeting with their clinicians. Such efforts should be informed by best practices from the science of outreach and engagement.
Awareness of the importance of implementation science has grown in recent decades, and dedicated resources to support improvements in research translation have been established by NIH. Most notably, NIH’s National Center for Advancing Translational Sciences (NCATS) was established in 2011 and administers the Clinical and Translational Science Awards program, which is designed to provide funding support for research and workforce training to accelerate the translation of research discoveries into improved care (NCATS, 2024). To carry out its mission, NCATS collaborates with other NIH institutes and centers, including those involved in AD/ADRD research, as well as with other governmental agencies, private
BOX 2-12
Examples of the Integration of New Tools into Clinical Diagnostic Criteria
Alzheimer’s disease (AD): Initial criteria for the clinical diagnosis of AD were developed in 1984 (McKhann et al., 1984). A probable AD diagnosis could be made on the basis of clinical examination, including neuropsychological testing, and was supported by such factors as family history and the absence of other conditions that could explain the gradual and progressive deterioration of cognitive functions. The diagnostic criteria were updated in 2011 to reflect advances in tools and knowledge regarding AD (McKhann et al., 2011). The revised criteria retained but refined the categories of probable and possible AD dementia and allowed for an increased level of certainty when someone meeting the core clinical criteria were found to carry a causative AD genetic mutation (e.g.,
APP, PSEN1, PSEN2) or had imaging (PET) or fluid biomarker (CSF amyloid beta and tau) evidence of the AD pathophysiological process. The use of biomarker evidence in routine clinical diagnosis was not recommended given noted limitations in biomarker discovery and implementation at the time, but it was acknowledged as useful for clinical research and as an optional tool for clinicians (McKhann et al., 2011). Importantly, the 1984 and 2011 criteria are specific to clinical dementia caused by AD. Separate criteria address MCI caused by AD (Albert et al., 2011).
Dementia with Lewy bodies (DLB): The first consensus guidelines for the clinical and pathologic diagnosis for DLB were published in 1996 by the Consortium on Dementia with Lewy bodies and involved clinical assessment to confirm the presence of the central feature of progressive disabling cognitive impairment and the presence of at least one core clinical feature. The criteria included additional supportive and exclusion features that could make a possible or probable diagnosis more or less likely (McKeith et al., 1996). These consensus guidelines were updated in 2005 to reflect the addition of new knowledge of the clinical presentation of DLB and access to new biomarkers for clinical assessment (Yamada et al., 2020). The 2005 diagnostic criteria maintained a similar structure as the first edition with the central feature and three core clinical features to guide diagnosis, but added a category for suggestive features (e.g., REM sleep behavior; low dopamine transporter [DAT] uptake in basal ganglia, severe neuroleptic sensitivity). Importantly, for the first time, imaging biomarkers were included in categories for suggestive and supported features.
The most recent revised clinical criteria were released in 2017 and included specific criteria that distinguish diagnostic biomarkers from clinical features. The biomarkers were divided into two categories: indicative biomarkers (e.g., reduced DAT uptake in basal ganglia by SPECT/PET; abnormal MIBG myocardial scintigraphy and polysomnographic confirmation of REM sleep without atonia) and supportive biomarkers (e.g., preservation of the medial temporal lobe on CT/MRI, low uptake on SPECT/PET perfusion/metabolism scan with reduced occipital activity with or without cingulate island sign on FDG-PET, prominent posterior slow wave activity on EEG with periodic fluctuations in the pre-alpha/theta range). Looking forward, the development of novel imaging and skin biomarkers for alpha-synuclein and new fluid biomarkers (e.g., amplification assays for alpha-synuclein) may allow for the development of updated clinical criteria for the diagnosis of early stage/prodromal DLB (Yamada et al., 2020). Staging systems applying these new biomarkers are currently being explored for use in research (Simuni et al., 2024).
industry, academia, and patient-support organizations. Enhanced collaboration of NIA and NINDS with NCATS could yield opportunities to accelerate the translation of cutting-edge research tools for the diagnosis and monitoring of AD/ADRD into clinical practice.
Research to Understand the Risks of Overtreatment in the Context of Preclinical Diagnosis
As described earlier in this chapter, numerous challenges—including scientific, technologic, social, and systemic barriers—impede the early and accurate detection and diagnosis of AD/ADRD. These factors may contribute to underdiagnosis. As biomedical advancements and changes in clinical practice enable the detection and diagnosis of changes in brain health, it is also important to acknowledge the potential risks related to biomarker-based diagnosis of preclinical AD/ADRD (e.g., someone who has the hallmark neuropathologies of AD but no symptoms of cognitive decline; see Box 2-1). Beyond risks associated with any AD/ADRD diagnosis, such as psychological distress and stigma, one important risk associated with diagnosis in asymptomatic individuals is overtreatment, which is of concern given the availability of FDA-approved anti-amyloid therapies for AD that have rare but substantial side effects and significant out-of-pocket costs. The relative benefits and risks of anti-amyloid treatment in asymptomatic individuals with AD pathologies are unclear as current treatments are only approved for use in people with MCI and early Alzheimer’s dementia. In the case of AD, multiple studies have documented that not everyone with amyloid plaques in the brain will progress to MCI or clinical dementia within their lifetimes (Bennett et al., 2006; Knopman et al., 2003; Snowdon, 2003).
As discussed further in Chapter 3, the reasons for this are still under study but could include resilience factors or even death from another cause prior to clinical progression (Erickson et al., 2021; Langa and Burke, 2019; NASEM, 2021a,b). One modeling analysis estimated that the majority of the nearly 50 million Americans estimated to be living with elevated amyloid will not develop clinical dementia within their lifetimes (Brookmeyer and Abdalla, 2018). However, this analysis did not include the use of tau biomarkers, which more recent research indicates greatly improves prediction of cognitive decline. A recent large multicenter amyloid and tau PET study examined risk for future progression to MCI and clinical dementia in cognitively unimpaired cohort participants and found that participants with both amyloid and tau biomarkers had a much greater risk of progression to MCI and clinical dementia as compared to those who were negative for both biomarkers. Of those who were amyloid positive and positive for tau in the neocortical region, more than 50 percent progressed to MCI and 20 percent to clinical dementia within 6 years (Ossenkoppele et al., 2022). Several other
recent studies have similarly found an increased risk of decline over time in cognitively unimpaired individuals with high levels of amyloid and abnormal tau biomarkers (Cody et al., 2024; Sperling et al., 2024).
Since there remains uncertainty in the true likelihood of transition from normal cognition to MCI and clinical dementia for those with different biomarker profiles, research in representative populations is urgently needed to better define these transition probabilities (e.g., presence of elevated amyloid to MCI to clinical dementia). This information, along with evidence on the efficacy of new treatments at preclinical disease stages, should inform education efforts for both the public and clinicians (Chin and Erickson, 2024; Largent et al., 2020). Such education will be essential to help clinicians and the public understand the evolving status of biomarker testing, particularly as diagnostic tools are commercialized and potentially marketed directly to consumers, and the risks and benefits of interventions for people with different combinations of biomarkers and cognitive symptoms. Ultimately, it is important to ensure that individuals have the information they require to make informed decisions, especially for those who meet the criteria for preclinical AD.
Real-World Data Integration to Guide Clinical Monitoring, Diagnosis, and Action
As novel research tools and methods are incorporated into clinical practice, it will be important to collect data to understand their performance in real-world settings and how implementation is addressing current knowledge gaps, guiding clinical management, and affecting the experiences of people living with AD/ADRD.
Data collection is not useful by itself; it must be used in decision models to guide clinical decision making (Barron et al., 2021). However, not all data are actionable. Actionable data are those that have a bearing on a specific clinical decision within a larger decision model. Some data that are not actionable at present may be actionable in the future, an issue that can be accounted for in a decision model. Certain data only become relevant at certain stages (e.g., after symptom development) or life experiences (see Table 2-1). This informs the timing for collection of specific types of data. Actionable data, therefore, must be gathered at the right time in the context of resolving a specific clinical question; that is, data have value in this context only if they resolve uncertainty and guide clinical decisions. So framed, data do not have equal utility in all clinical decisions and, especially in the context of finite resources, should be gathered only to resolve uncertainty within a specific clinical decision model. Additionally, the frequency of data collection may vary across the life course; during specific times, it may be desirable to take measurements more frequently or even continuously,
which has been made feasible by technological advances. Within the context of a decision model, hypotheses regarding how different data interrelate may guide efforts to collect additional data as well as guiding decisions on how best to intervene across the life course.
Translating data into clinical action requires data integration and analysis, both longitudinal and cross-sectional. A notable gap is the absence of a framework or model for integrating disparate data sources (including structured and unstructured data) to generate knowledge regarding risk and to guide action. Given the diversity of potential data sources (e.g., clinical symptoms, molecular data, exposure data), addressing this gap will require data sharing and cross-disciplinary efforts.
In March 2023, NIH released a Request for Applications to fund an AD/ADRD Real-World Data Platform (HHS, 2023). The platform was intended to better capture and link data from multiple sources, thereby enhancing and accelerating the ability to answer scientific questions, particularly those not amenable to clinical trials, and increasing the engagement of and collection of data from diverse populations. However, in April 2024, NIH decided not to fund the platform, citing budgetary considerations and a desire to consider opportunities to leverage other federal large data initiatives (Wallin, 2024). The objectives of the original proposal remain important, but given the considerable investment and diversity of expertise that will be required to implement such a platform, consideration should be given to a precompetitive public–private partnership model. Existing public–private partnerships involving NIH, such as the Accelerating Medicines Partnership® Program for Alzheimer’s Disease (AMP-AD program) (NIA, 2023a) and the NIH Science and Technology Research Infrastructure for Discovery, Experimentation, and Sustainability (STRIDES) Initiative,3 which is providing NIH-supported investigators with affordable access to cloud services and environments, may serve as models.
Conclusion 2-6: To maximize the benefits to society from research, such discoveries as advancements in biomarkers, data collection methods, and interventions need to be translated to real-world settings. The implementation of such new tools into clinical practice in turn provides opportunities to further advance knowledge through the collection of real-world evidence.
RESEARCH PRIORITIES
The ability to detect and track changes in brain health will be key to the development of rational strategies for the prevention and treatment of AD/
___________________
3 https://datascience.nih.gov/strides (accessed June 12, 2024).
ADRD. Enabling the early and accurate identification of changes in brain health across diverse populations has been a major focus area for NIH. To this end, NIH has funded biomarker discovery and validation, as well as the development and implementation of digital tools and technologies to improve the capacity to diagnose and monitor AD/ADRD at its earliest stages and accurately distinguish the different forms of dementia (NIA, 2023b, 2024b). Infrastructure investments to support these efforts have included longitudinal cohort studies, as well as large, collaborative research programs (e.g., CLEAR-AD) and public–private partnerships. The committee identified two research priorities that align with and build upon this broad foundation of NIH investments. These research priorities are listed in Table 2-2 below and include key scientific questions and near-term research opportunities that would advance progress on the research priorities.
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
| 2-1: Develop better tools, including novel biomarker tests and digital assessment technologies, to monitor brain health across the life course and screen, predict, and diagnose AD/ADRD at scale. |
|
|
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
| 2-2: Implement advances in clinical research methods and tools to generate data from real-world clinical practice settings that can inform future research. |
|
|
REFERENCES
Abdelmoaty, M. M., E. Lu, R. Kadry, E. G. Foster, S. Bhattarai, R. L. Mosley, and H. E. Gendelman. 2023. Clinical biomarkers for Lewy body diseases. Cell & Bioscience 13(1):209.
ADI (Alzheimer’s Disease International). 2012. World Alzheimer report 2012. https://www.alzint.org/resource/world-alzheimer-report-2012/ (accessed August 20, 2024).
ADNI (Alzheimer’s Disease Neuroimaging Initiative). 2024a. About ADNI. https://adni.loni.usc.edu/about/ (accessed August 14, 2024).
ADNI. 2024b. Related and collaborative studies. https://adni.loni.usc.edu/about/related-collaborative-studies/ (accessed August 14, 2024).
ADSP (Alzheimer’s Disease Sequencing Project). 2024. Alzheimer’s Disease Sequencing Project Phenotype Harmonization Consortium (ADSP-PHC). https://adsp.niagads.org/funded-programs/phenotype-harmonization/ (accessed August 22, 2022).
Albert, M. S., S. T. DeKosky, D. Dickson, B. Dubois, H. H. Feldman, N. C. Fox, A. Gamst, D. M. Holtzman, W. J. Jagust, R. C. Petersen, P. J. Snyder, M. C. Carrillo, B. Thies, and C. H. Phelps. 2011. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia 7(3):270-279.
ALLFTD (the ARTFL-LEFFTDS Longitudinal Frontotemporal Lobar Degeneration study). n.d. Our Mission. https://www.allftd.org/mission (accessed November 11, 2024).
Alzheimer Europe. 2011. The value of knowing. https://www.alzheimereurope.org/resources/publications/valueknowing?language_content_entity=en#:~:text=The%20survey%20on%20%22Value%20of,Public%20Health%20and%2Alzheimer%20Europe (accessed August 22, 2024).
American College of Cardiology. 2002. Bogalusa Heart Study—Bogalusa. https://www.acc.org/Latest-in-Cardiology/Clinical-Trials/2010/02/23/18/57/Bogalusa (accessed August 14, 2024).
Amini, S., B. Hao, L. Zhang, M. Song, A. Gupta, C. Karjadi, V. B. Kolachalama, R. Au, and I. C. Paschalidis. 2023. Automated detection of mild cognitive impairment and dementia from voice recordings: A natural language processing approach. Alzheimer‘s & Dementia 19(3):946-955.
Amini, S., B. Hao, J. Yang, C. Karjadi, V. B. Kolachalama, R. Au, and I. C. Paschalidis. 2024. Prediction of Alzheimer’s disease progression within 6 years using speech: A novel approach leveraging language models. Alzheimer’s & Dementia 20(8):5262-5270.
Andersen, S. L. 2020. Centenarians as models of resistance and resilience to Alzheimer’s disease and related dementias. Advances in Geriatric Medicine & Research 2(3):e200018.
Au, R., V. B. Kolachalama, and I. C. Paschalidis. 2022. Redefining and validating digital biomarkers as fluid, dynamic multi-dimensional digital signal patterns. Frontiers of Digital Health 3:751629.
Au, R., Z. T. Popp, S. Low, P. H. Hwang, I. De Anda-Duran, S. Li, S. Rahman, H. Ding, A. Igwe, C. Karjadi, T. F. A. Ang, S. A. Devine, A. S. Gurnani, J. B. Mez, L. A. Farrer, P. Sunderaraman, H. Lin, and V. B. Kolachalama. 2023. Digital is the new blood: Enabling the present and the future. Alzheimer’s & Dementia 19(S21):e078220.
Bangen, K. J., A. L. Clark, M. Werhane, E. C. Edmonds, D. A. Nation, N. Evangelista, D. J. Libon, M. W. Bondi, and L. Delano-Wood. 2016. Cortical amyloid burden differences across empirically-derived mild cognitive impairment subtypes and interaction with APOE ɛ4 genotype. Journal of Alzheimer’s Disease 52(3):849-861.
Bargar, C., W. Wang, S. A. Gunzler, A. LeFevre, Z. Wang, A. J. Lerner, N. Singh, C. Tatsuoka, B. Appleby, X. Zhu, R. Xu, V. Haroutunian, W. Q. Zou, J. Ma, and S. G. Chen. 2021. Streamlined alpha-synuclein RT-QuIC assay for various biospecimens in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathologica Communications 9(1):62.
Barron, D. S., J. T. Baker, K. S. Budde, D. Bzdok, S. B. Eickhoff, K. J. Friston, P. T. Fox, P. Geha, S. Heisig, A. Holmes, J. P. Onnela, A. Powers, D. Silbersweig, and J. H. Krystal. 2021. Decision models and technology can help psychiatry develop biomarkers. Frontiers in Psychiatry 12:706655.
Barth, C., and A. G. de Lange. 2020. Towards an understanding of women’s brain aging: The immunology of pregnancy and menopause. Frontiers in Neuroendocrinology 58:100850.
Barthelemy, N. R., G. Salvado, S. E. Schindler, Y. He, S. Janelidze, L. E. Collij, B. Saef, R. L. Henson, C. D. Chen, B. A. Gordon, Y. Li, R. La Joie, T. L. S. Benzinger, J. C. Morris, N. Mattsson-Carlgren, S. Palmqvist, R. Ossenkoppele, G. D. Rabinovici, E. Stomrud, R. J. Bateman, and O. Hansson. 2024. Highly accurate blood test for Alzheimer’s disease is similar or superior to clinical cerebrospinal fluid tests. Nature Medicine 30(4):1085-1095.
Beker, N., A. Ganz, M. Hulsman, T. Klausch, B. A. Schmand, P. Scheltens, S. A. M. Sikkes, and H. Holstege. 2021. Association of cognitive function trajectories in centenarians with postmortem neuropathology, physical health, and other risk factors for cognitive decline. JAMA Network Open 4(1):e2031654.
Bennett, D. A., J. A. Schneider, Z. Arvanitakis, J. F. Kelly, N. T. Aggarwal, R. C. Shah, and R. S. Wilson. 2006. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66(12):1837-1844.
Bernstein Sideman, A., R. Chalmer, E. Ayers, R. Gershon, J. Verghese, M. Wolf, A. Ansari, M. Arvanitis, N. Bui, P. Chen, A. Chodos, R. Corriveau, L. Curtis, A. R. Ehrlich, S. E. Tomaszewski Farias, C. Goode, L. Hill-Sakurai, C. J. Nowinski, M. Premkumar, K. P. Rankin, C. S. Ritchie, E. Tsoy, E. Weiss, and K. L. Possin. 2022. Lessons from detecting cognitive impairment including dementia (DetectCID) in primary care. Journal of Alzheimer’s Disease 86(2):655-665.
Berron, D., W. Glanz, L. Clark, K. Basche, X. Grande, J. Gusten, O. V. Billette, I. Hempen, M. H. Naveed, N. Diersch, M. Butryn, A. Spottke, K. Buerger, R. Perneczky, A. Schneider, S. Teipel, J. Wiltfang, S. Johnson, M. Wagner, F. Jessen, and E. Duzel. 2024. A remote digital memory composite to detect cognitive impairment in memory clinic samples in unsupervised settings using mobile devices. NPJ Digital Medicine 7(1):79.
BioSEND. 2024. About BioSEND. https://biosend.org/about.html (accessed August 15, 2024).
Blanc, F., and O. Bousiges. 2022. Biomarkers and diagnosis of dementia with Lewy bodies including prodromal: Practical aspects. Revue Neurologique 178(5):472-483.
Boeve, B. F., A. A. Davis, Y. E. Ju, K. Kantarci, W. Singer, A. Videnovic, and NAPS coinvestigators. 2024. Concerns with the new biological research criteria for synucleinopathy. Lancet Neurology 23(7):659-660.
BU (Boston University). n.d.a. Mission and objectives: Framingham Heart Study Brain Aging Program (FHS-BAP). https://www.bumc.bu.edu/fhs-bap/about-us/mission-fhs-bap/ (accessed August 14, 2024).
BU. n.d.b. Evolution of the Framingham Heart Study and the birth of FHS-BAP. https://www.bumc.bu.edu/fhs-bap/about-us/history/ (accessed October 25, 2024).
BU. n.d.c. Access our data. https://www.bumc.bu.edu/fhs-bap/for-researchers/access-our-data/ (accessed August 14, 2024).
Brookmeyer, R., and N. Abdalla. 2018. Estimation of lifetime risks of Alzheimer’s disease dementia using biomarkers for preclinical disease. Alzheimer’s & Dementia 14(8):981-988.
Buckley, R. 2024. Panel discussion with committee. Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
Cadar, D. 2017. A life course approach to dementia prevention. Journal of Aging and Geriatric Medicine 1(2).
Cagney, D. N., J. Sul, R. Y. Huang, K. L. Ligon, P. Y. Wen, and B. M. Alexander. 2018. The FDA NIH Biomarkers, endpointS, and other Tools (BEST) resource in neuro-oncology. Neuro-Oncology 20(9):1162-1172.
Cairns, N. J., L. Taylor-Reinwald, J. C. Morris, and the Alzheimer’s Disease Neuroimaging Inititative. 2010. Autopsy consent, brain collection, and standardized neuropathologic assessment of ADNI participants: The essential role of the neuropathology core. Alzheimer’s & Dementia 6(3):274-279.
Cerino, E. S., M. J. Katz, C. Wang, J. Qin, Q. Gao, J. Hyun, J. G. Hakun, N. A. Roque, C. A. Derby, R. B. Lipton, and M. J. Sliwinski. 2021. Variability in cognitive performance on mobile devices is sensitive to mild cognitive impairment: Results from the Einstein Aging Study. Frontiers in Digital Health 3:78031.
Chin, N. A., and C. M. Erickson. 2024. Alzheimer’s disease, biomarkers, and mAbs — What does primary care need? New England Journal of Medicine 390(24):2229-2231.
Chu, Y., W. Hirst, and J. Kordower. 2023. Mixed pathology as a rule, not exception: Time to reconsider disease nosology. Handbook of Clinical Neurology 192:57-71.
CLEAR-AD (Centrally-linked Longitudinal pEripheral biomARkers of AD). 2024a. About. https://clear-ad.org/index.php/about/ (accessed August 14, 2024).
CLEAR-AD. 2024b. Home. https://clear-ad.org/ (accessed August 14, 2024).
Cody, K. A., R. E. Langhough, M. D. Zammit, L. Clark, N. Chin, B. T. Christian, T. J. Betthauser, and S. C. Johnson. 2024. Characterizing brain tau and cognitive decline along the amyloid timeline in Alzheimer’s disease. Brain 147(6):2144-2157.
Concha-Marambio, L., S. Pritzkow, M. Shahnawaz, C. M. Farris, and C. Soto. 2023. Seed amplification assay for the detection of pathologic alpha-synuclein aggregates in cerebrospinal fluid. Nature Protocol 18(4):1179-1196.
Cook, D. 2024. Digital health. Remarks at Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
Coravos, A., S. Khozin, and K. D. Mandl. 2019. Developing and adopting safe and effective digital biomarkers to improve patient outcomes. NPJ Digital Medicine 2(1).
Cordts, I., A. Wachinger, C. Scialo, P. Lingor, M. Polymenidou, E. Buratti, and E. Feneberg. 2023. TDP-43 proteinopathy specific biomarker development. Cells 12(4).
Coriell Institute. 2024. All biobanks. https://www.coriell.org/1/Browse/Biobanks (accessed October 23, 2024).
Corney, K. B., E. C. West, S. E. Quirk, J. A. Pasco, A. L. Stuart, B. A. Manavi, B. E. Kavanagh, and L. J. Williams. 2022. The relationship between adverse childhood experiences and Alzheimer’s disease: A systematic review. Frontiers in Aging Neuroscience 14:831378.
Corrada, M. 2024. Envisioning the future of AD/ADRD research. Presentation at Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
Corrada, M., D. Berlau, and C. Kawas. 2012. A population-based clinicopathological study in the oldest-old: The 90+ study. Current Alzheimer Research 9(6):709-717.
Crane, P. K., A. Carle, L. E. Gibbons, P. Insel, R. S. Mackin, A. Gross, R. N. Jones, S. Mukherjee, S. M. Curtis, D. Harvey, M. Weiner, D. Mungas, and the Alzheimer’s Disease Neuroimaging Initiative. 2012. Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging and Behavior 6(4):502-516.
Cullen, N., A. Leuzy, S. Palmqvist, S. Janelidze, E. Stomrud, P. Pesini, J. A. Allue, N. K. Procter, H. Zetterberg, K. Blennow, J. Dage, N. Mattsson-Carlgren, and O. Hansson. 2021. Individualized prognosis of cognitive decline and dementia in mild cognitive impairment based on plasma biomarker combinations. Nature Aging 1(1):114-123.
Cummings, J. 2019. The role of biomarkers in Alzheimer’s disease drug development. Advances in Experimental Medicine and Biology 1118:29-61.
Cutler, D. 2024. Enhancing the use of tools for ADRD detection, diagnosis, and monitoring. Presentation at Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
Deoni, S. C. L., P. Burton, J. Beauchemin, R. Cano-Lorente, M. D. De Both, M. Johnson, L. Ryan, and M. J. Huentelman. 2023. Neuroimaging and verbal memory assessment in healthy aging adults using a portable low-field MRI scanner and a web-based platform: Results from a proof-of-concept population-based cross-section study. Brain Structure and Function 228(2):493-509.
DetectCID. 2022. Advancing research towards the development of paradigms for detection of cognitive impairment and dementia that will benefit the public. https://www.detectcid.org/overview#mission (accessed September 6, 2024).
Devi, G. 2023. A how-to guide for a precision medicine approach to the diagnosis and treatment of Alzheimer’s disease. Frontiers in Aging Neuroscience 15:1213968.
Dion, C., F. Arias, S. Amini, R. Davis, D. Penney, D. J. Libon, and C. C. Price. 2020. Cognitive correlates of digital clock drawing metrics in older adults with and without mild cognitive impairment. Journal of Alzheimer’s Disease 75(1):73-83.
Dubois, B., A. Padovani, P. Scheltens, A. Rossi, and G. Dell’Agnello. 2016. Timely diagnosis for Alzheimer’s disease: A literature review on benefits and challenges. Journal of Alzheimer’s Disease 49(3):617-631.
Dubois, B., N. Villain, G. B. Frisoni, G. D. Rabinovici, M. Sabbagh, S. Cappa, A. Bejanin, S. Bombois, S. Epelbaum, M. Teichmann, M. O. Habert, A. Nordberg, K. Blennow, D. Galasko, Y. Stern, C. C. Rowe, S. Salloway, L. S. Schneider, J. L. Cummings, and H. H. Feldman. 2021. Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurology 20(6):484-496.
Dubois, B., C. A. F. von Arnim, N. Burnie, S. Bozeat, and J. Cummings. 2023. Biomarkers in Alzheimer’s disease: Role in early and differential diagnosis and recognition of atypical variants. Alzheimer’s Research & Therapy 15(1):175.
Erickson, C. M., N. A. Chin, S. C. Johnson, C. E. Gleason, and L. R. Clark. 2021. Disclosure of preclinical Alzheimer’s disease biomarker results in research and clinical settings: Why, how, and what we still need to know. Alzheimer’s & Dementia 13(1):e12150.
FDA (U.S. Food and Drug Administration). 2020. FDA approves first drug to image tau pathology in patients being evaluated for Alzheimer’s disease. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-image-tau-pathology-patients-being-evaluated-alzheimers-disease (accessed August 14, 2024).
FDA-NIH Biomarker Working Group. 2020. BEST (Biomarkers, EndpointS, and other Tools) resource. https://www.ncbi.nlm.nih.gov/books/NBK326791/ (accessed October 17, 2024).
Fleisher, A. S., M. J. Pontecorvo, M. D. Devous, Sr., M. Lu, A. K. Arora, S. P. Truocchio, P. Aldea, M. Flitter, T. Locascio, M. Devine, A. Siderowf, T. G. Beach, T. J. Montine, G. E. Serrano, C. Curtis, A. Perrin, S. Salloway, M. Daniel, C. Wellman, A. D. Joshi, D. J. Irwin, V. J. Lowe, W. W. Seeley, M. D. Ikonomovic, J. C. Masdeu, I. Kennedy, T. Harris, M. Navitsky, S. Southekal, M. A. Mintun, and A16 Study Investigators. 2020. Positron emission tomography imaging with [18F]Flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurology 77(7):829-839.
Foverskov, E., M. M. Glymour, E. L. Mortensen, M. Osler, G. T. Okholm, and R. Lund. 2020. Education and adolescent cognitive ability as predictors of dementia in a cohort of Danish men. PLoS ONE 15(8):e0235781.
Francis, P. T., H. Costello, and G. M. Hayes. 2018. Brains for dementia research: Evolution in a longitudinal brain donation cohort to maximize current and future value. Journal of Alzheimer’s Disease 66(4):1635-1644.
Franklin, E. E., R. J. Perrin, B. Vincent, M. Baxter, J. C. Morris, N. J. Cairns, and the Alzheimer’s Disease Neuroimaging Initiative. 2015. Brain collection, standardized neuropathologic assessment, and comorbidity in Alzheimer’s Disease Neuroimaging Initiative 2 participants. Alzheimer’s & Dementias 11(7):815-822.
Gao, Z., J. Li, L. Wang, and Y. Li. 2023. A systematic review of auricular therapy for poststroke cognitive impairment and dementia: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 102(7):E32933.
Gendron, T. F., M. G. Heckman, L. J. White, A. M. Veire, O. Pedraza, A. R. Burch, A. C. Bozoki, B. C. Dickerson, K. Domoto-Reilly, T. Foroud, L. K. Forsberg, D. R. Galasko, N. Ghoshal, N. R. Graff-Radford, M. Grossman, H. W. Heuer, E. D. Huey, G. R. Hsiung, D. J. Irwin, D. I. Kaufer, G. C. Leger, I. Litvan, J. C. Masdeu, M. F. Mendez, C. U. Onyike, B. Pascual, A. Ritter, E. D. Roberson, J. C. Rojas, M. C. Tartaglia, Z. K. Wszolek, H. Rosen, B. F. Boeve, A. L. Boxer, ALLFTD Consortium, and L. Petrucelli. 2022. Comprehensive cross-sectional and longitudinal analyses of plasma neurofilament light across FTD spectrum disorders. Cell Reports: Medicine 3(4):100607.
Gibbons, C. H., T. Levine, C. Adler, B. Bellaire, N. Wang, J. Stohl, P. Agarwal, G. M. Aldridge, A. Barboi, V. G. H. Evidente, D. Galasko, M. D. Geschwind, A. Gonzalez-Duarte, R. Gil, M. Gudesblatt, S. H. Isaacson, H. Kaufmann, P. Khemani, R. Kumar, G. Lamotte, A. J. Liu, N. R. McFarland, M. Miglis, A. Reynolds, G. A. Sahagian, M. H. Saint-Hillaire, J. B. Schwartzbard, W. Singer, M. J. Soileau, S. Vernino, O. Yerstein, and R. Freeman. 2024. Skin biopsy detection of phosphorylated alpha-synuclein in patients with synucleinopathies. JAMA 331(15):1298-1306.
Gibson, L. L., C. Abdelnour, J. Chong, C. Ballard, and D. Aarsland. 2023. Clinical trials in dementia with Lewy bodies: The evolving concept of co-pathologies, patient selection and biomarkers. Current Opinion in Neurology 36(4):264-275.
Gifford, A., N. Praschan, A. Newhouse, and Z. Chemali. 2023. Biomarkers in frontotemporal dementia: Current landscape and future directions. Biomarkers in Neuropsychiatry 8(4):100065.
Gonzalez-Ortiz, F., M. Turton, P. R. Kac, D. Smirnov, E. Premi, R. Ghidoni, L. Benussi, V. Cantoni, C. Saraceno, J. Rivolta, N. J. Ashton, B. Borroni, D. Galasko, P. Harrison, H. Zetterberg, K. Blennow, and T. K. Karikari. 2023. Brain-derived tau: A novel blood-based biomarker for Alzheimer’s disease-type neurodegeneration. Brain 146(3):1152-1165.
González, H. M., W. Tarraf, M. Fornage, K. A. González, A. Chai, M. Youngblood, M. L. A. Abreu, D. Zeng, S. Thomas, G. A. Talavera, L. C. Gallo, R. Kaplan, M. L. Daviglus, and N. Schneiderman. 2019. A research framework for cognitive aging and Alzheimer’s disease among diverse US latinos: Design and implementation of the Hispanic Community Health Study/Study of Latinos-Investigation of Neurocognitive Aging (SOL-INCA). Alzheimer’s & Dementia 15(12):1624-1632.
Grunberg, V. A., S. M. Bannon, P. Popok, M. Reichman, B. C. Dickerson, and A. M. Vranceanu. 2022. A race against time: Couples’ lived diagnostic journeys to young-onset dementia. Aging and Mental Health 26(11):2223-2232.
Guo, Y., J. You, Y. Zhang, W. S. Liu, Y. Y. Huang, Y. R. Zhang, W. Zhang, Q. Dong, J. F. Feng, W. Cheng, and J. T. Yu. 2024. Plasma proteomic profiles predict future dementia in healthy adults. Nature Aging 4(2):247-260.
Hampel, H., J. Cummings, K. Blennow, P. Gao, C. R. Jack, Jr., and A. Vergallo. 2021. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nature Reviews Neurology 17(9):580-589.
Hampel, H., R. Au, S. Mattke, W. M. van der Flier, P. Aisen, L. Apostolova, C. Chen, M. Cho, S. De Santi, P. Gao, A. Iwata, R. Kurzman, A. J. Saykin, S. Teipel, B. Vellas, A. Vergallo, H. Wang, and J. Cummings. 2022a. Designing the next-generation clinical care pathway for Alzheimer’s disease. Nature Aging 2(8):692-703.
Hampel, H., L. M. Shaw, P. Aisen, C. Chen, A. Lleó, T. Iwatsubo, A. Iwata, M. Yamada, T. Ikeuchi, J. Jia, H. Wang, C. E. Teunissen, E. Peskind, K. Blennow, J. Cummings, and A. Vergallo. 2022b. State-of-the-art of lumbar puncture and its place in the journey of patients with Alzheimer’s disease. Alzheimer’s & Dementia 18(1):159-177.
Hampel, H., Y. Hu, J. Cummings, S. Mattke, T. Iwatsubo, A. Nakamura, B. Vellas, S. O’Bryant, L. M. Shaw, M. Cho, R. Batrla, A. Vergallo, K. Blennow, J. Dage, and S. E. Schindler. 2023. Blood-based biomarkers for Alzheimer’s disease: Current state and future use in a transformed global healthcare landscape. Neuron 111(18):2781-2799.
Hampton, O. L., S. Mukherjee, M. J. Properzi, A. P. Schultz, P. K. Crane, L. E. Gibbons, T. J. Hohman, P. Maruff, Y. Y. Lim, R. E. Amariglio, K. V. Papp, K. A. Johnson, D. M. Rentz, R. A. Sperling, and R. F. Buckley. 2023. Harmonizing the preclinical Alzheimer cognitive composite for multicohort studies. Neuropsychology 37(4):436-449.
Hansson, O., A. Mikulskis, A. M. Fagan, C. Teunissen, H. Zetterberg, H. Vanderstichele, J. L. Molinuevo, L. M. Shaw, M. Vandijck, M. M. Verbeek, M. Savage, N. Mattsson, P. Lewczuk, R. Batrla, S. Rutz, R. A. Dean, and K. Blennow. 2018. The impact of pre-analytical variables on measuring cerebrospinal fluid biomarkers for Alzheimer’s disease diagnosis: A review. Alzheimer’s & Dementia 14(10):1313-1333.
Harris, K. M., C. T. Halpern, E. A. Whitsel, J. M. Hussey, L. A. Killeya-Jones, J. Tabor, and S. C. Dean. 2019. Cohort profile: The National Longitudinal Study of Adolescent to Adult Health (ADD Health). International Journal of Epidemiology 48(5):1415-1415k.
HCAP Network. 2023. About the HCAP network. https://hcap.isr.umich.edu/ (accessed August 15, 2024).
HCAP Network. 2024. HCAP network team. https://hcap.isr.umich.edu/people/ (accessed October 17, 2024).
HCS Institute for Translational Research. 2022. Health and Aging Brain Study—Health disparities. https://apps.unthsc.edu/itr/research/habs (accessed August 22, 2024).
Heyer, S., M. Simon, M. Doyen, A. Mortada, V. Roch, E. Jeanbert, N. Thilly, C. Malaplate, A. Kearney-Schwartz, T. Jonveaux, A. Bannay, and A. Verger. 2024. (18)F-FDG PET can effectively rule out conversion to dementia and the presence of CSF biomarker of neurodegeneration: A real-world data analysis. Alzheimer’s Research & Therapy 16(1):182.
HHS (U.S. Department of Health and Human Services). 2023. Alzheimer’s disease (AD) and AD-related dementias (ADRD) real-world data platform. https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-24-009.html (accessed August 15, 2024).
Höglinger, G. U., C. H. Adler, D. Berg, C. Klein, T. F. Outeiro, W. Poewe, R. Postuma, A. J. Stoessl, and A. E. Lang. 2024. A biological classification of Parkinson’s disease: The SynNeurGe research diagnostic criteria. Lancet Neurology 23(2):191-204.
Hort, J., A. Bartos, T. Pirttila, and P. Scheltens. 2010. Use of cerebrospinal fluid biomarkers in diagnosis of dementia across Europe. European Journal of Neurology 17(1):90-96.
HRS (Health and Retirement Study). 2021. The health and retirement study: Telling the story of aging in America. https://hrsonline.isr.umich.edu/sitedocs/databook-2021/HRS-Tellingthe-Story-of-Aging-in-America.pdf (accessed October 28, 2024).
HRS. 2024. Cognition data. https://hrs.isr.umich.edu/data-products/cognition-data#adams (accessed October 23, 2024).
Htike, T. T., S. Mishra, S. Kumar, P. Padmanabhan, and B. Gulyas. 2019. Peripheral biomarkers for early detection of Alzheimer’s and Parkinson’s diseases. Molecular Neurobiology 56(3):2256-2277.
Huber, H., L. Montoliu-Gaya, K. Blennow, H. Zetterberg, M. B. Rovira, A. Jeromin, X. M. Arús, and N. J. Ashton. 2023. A finger prick collection method for detecting blood biomarkers of neurodegeneration—a pilot study (DROP-AD). Alzheimer’s & Dementia 19(S14):e080275.
Irwin, K. E., P. Jasin, K. E. Braunstein, I. R. Sinha, M. A. Garret, K. D. Bowden, K. Chang, J. C. Troncoso, A. Moghekar, E. S. Oh, D. Raitcheva, D. Bartlett, T. Miller, J. D. Berry, B. J. Traynor, J. P. Ling, and P. C. Wong. 2024a. A fluid biomarker reveals loss of TDP-43 splicing repression in presymptomatic ALS-FTD. Nature Medicine 30(2):382-393.
Irwin, K. E., U. Sheth, P. C. Wong, and T. F. Gendron. 2024b. Fluid biomarkers for amyotrophic lateral sclerosis: A review. Molecular Neurodegeneration 19(1):9.
Ishii, K. 2020. Diagnostic imaging of dementia with Lewy bodies, frontotemporal lobar degeneration, and normal pressure hydrocephalus. Japanese Journal of Radiology 38(1):64-76.
Jack, C. R., Jr., D. A. Bennett, K. Blennow, M. C. Carrillo, B. Dunn, S. B. Haeberlein, D. M. Holtzman, W. Jagust, F. Jessen, J. Karlawish, E. Liu, J. L. Molinuevo, T. Montine, C. Phelps, K. P. Rankin, C. C. Rowe, P. Scheltens, E. Siemers, H. M. Snyder, and R. Sperling. 2018. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s & Dementia 14(4):535-562.
Jack, C. R., Jr., J. S. Andrews, T. G. Beach, T. Buracchio, B. Dunn, A. Graf, O. Hansson, C. Ho, W. Jagust, E. McDade, J. L. Molinuevo, O. C. Okonkwo, L. Pani, M. S. Rafii, P. Scheltens, E. Siemers, H. M. Snyder, R. Sperling, C. E. Teunissen, and M. C. Carrillo. 2024. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimer‘s & Dementia 20(8):5143-5169.
Jia, J., Y. Ning, M. Chen, S. Wang, H. Yang, F. Li, J. Ding, Y. Li, B. Zhao, J. Lyu, S. Yang, X. Yan, Y. Wang, W. Qin, Q. Wang, Y. Li, J. Zhang, F. Liang, Z. Liao, and S. Wang. 2024. Biomarker changes during 20 years preceding Alzheimer’s disease. New England Journal of Medicine 390(8):712-722.
Katzeff, J. S., F. Bright, K. Phan, J. J. Kril, L. M. Ittner, M. Kassiou, J. R. Hodges, O. Piguet, M. C. Kiernan, G. M. Halliday, and W. S. Kim. 2022. Biomarker discovery and development for frontotemporal dementia and amyotrophic lateral sclerosis. Brain 145(5):1598-1609.
Kawakami, I., T. Arai, and M. Hasegawa. 2019. The basis of clinicopathological heterogeneity in TDP-43 proteinopathy. Acta Neuropathologica 138(5):751-770.
Kaye, J. 2024 Development and use of digital tools to assess human health. Presentation at Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
Kaye, J. A., J. Marcoe, A. B. Mar, E. J. Hanna, A. Pierce, L. C. Silbert, Y. Shang, D. Schwartz, S. Gothard, N. Mattek, W. T. M. Au‐Yeung, J. S. Steele, and Z. T. Beattie. 2023. DETECT‐AD (Digital Evaluations and Technologies Enabling Clinical Translation for Alzheimer’s Disease): A simulated anti‐amyloid clinical trial using digital biomarkers as primary outcome measures. Alzheimer’s & Dementia 19(S11):e082057.
Kimberly, W. T., A. J. Sorby-Adams, A. G. Webb, E. X. Wu, R. Beekman, R. Bowry, S. J. Schiff, A. de Havenon, F. X. Shen, G. Sze, P. Schaefer, J. E. Iglesias, M. S. Rosen, and K. N. Sheth. 2023. Brain imaging with portable low-field MRI. Nature Reviews Bioengineering 1(9):617-630.
Kind, A. 2024. Discussant perspectives and committee discussion. Presentation at Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
King, J., and M. Wagster. 2024. Mobile toolbox: Expand your cognitive assessment reach! https://www.nia.nih.gov/research/blog/2024/06/mobile-toolbox-expand-your-cognitive-assessment-reach (accessed August 14, 2024).
Knopman, D. S., J. E. Parisi, A. Salviati, M. Floriach-Robert, B. F. Boeve, R. J. Ivnik, G. E. Smith, D. W. Dickson, K. A. Johnson, L. E. Petersen, W. C. McDonald, H. Braak, and R. C. Petersen. 2003. Neuropathology of cognitively normal elderly. Journal of Neuropathology and Experimental Neurology 62(11):1087-1095.
Kobayashi, L. C., R. N. Jones, E. M. Briceno, M. A. Renteria, Y. Zhang, E. Meijer, K. M. Langa, J. Lee, and A. L. Gross. 2024. Cross-national comparisons of later-life cognitive function using data from the Harmonized Cognitive Assessment Protocol (HCAP): Considerations and recommended best practices. Alzheimer’s & Dementia 20(3):2273-2281.
Landau, S. M., A. Horng, A. Fero, and W. J. Jagust. 2016. Amyloid negativity in patients with clinically diagnosed Alzheimer disease and MCI. Neurology 86(15):1377-1385.
Langa, K. M., and J. F. Burke. 2019. Preclinical Alzheimer disease—early diagnosis or overdiagnosis? JAMA Internal Medicine 179(9):1161-1162.
Largent, E. A., K. Harkins, C. H. van Dyck, S. Hachey, P. Sankar, and J. Karlawish. 2020. Cognitively unimpaired adults’ reactions to disclosure of amyloid PET scan results. PLoS ONE 15(2):e0229137.
Law, Z. K., C. Todd, R. Mehraram, J. Schumacher, M. R. Baker, F. E. N. LeBeau, A. Yarnall, M. Onofrj, L. Bonanni, A. Thomas, and J. P. Taylor. 2020. The role of EEG in the diagnosis, prognosis and clinical correlations of dementia with Lewy bodies—a systematic review. Diagnostics (Basel) 10(9):616.
Livingston, G., J. Huntley, A. Sommerlad, D. Ames, C. Ballard, S. Banerjee, C. Brayne, A. Burns, J. Cohen-Mansfield, C. Cooper, S. G. Costafreda, A. Dias, N. Fox, L. N. Gitlin, R. Howard, H. C. Kales, M. Kivimäki, E. B. Larson, A. Ogunniyi, V. Orgeta, K. Ritchie, K. Rockwood, E. L. Sampson, Q. Samus, L. S. Schneider, G. Selbæk, L. Teri, and N. Mukadam. 2020. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396(10248):413-446.
MarkVCID. 2017. MarkVCID consortium overview. https://markvcid.partners.org/about/m2-consortium-overview (accessed August 21, 2024).
Matsubara, T., M. Kameyama, N. Tanaka, R. Sengoku, M. Orita, K. Furuta, A. Iwata, T. Arai, H. Maruyama, Y. Saito, and S. Murayama. 2022. Autopsy validation of the diagnostic accuracy of 123I-metaiodobenzylguanidine myocardial scintigraphy for Lewy body disease. Neurology 98(16):e1648-e1659.
Mavroudis, I., F. Petridis, and D. Kazis. 2019. Cerebrospinal fluid, imaging, and physiological biomarkers in dementia with Lewy bodies. American Journal of Alzheimer’s Disease & Other Dementias 34(7-8):421-432.
McDowell, I., G. Xi, J. Lindsay, and M. Tierney. 2005. Mapping the connections between education and dementia. Journal of Clinical and Experimental Neuropsychology 29(2):127-141.
McKeith, I. G., D. Galasko, M. Kosaka, E. K. Perry, D. W. Dickson, L. A. Hansen, D. P. Salmon, J. Lowe, S. S. Mirra, E. J. Byrne, G. Lennox, N. P. Quinn, J. A. Edwardson, P. G. Ince, C. Bergeron, A. Burns, B. L. Miller, S. Lovestone, D. Collerton, E. N. H. Jansen, C. Ballard, R. A. I. de Vos, K. A. Jellinger, and R. H. Perry. 1996. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB). Neurology 47(5):1113-1124.
McKhann, G., D. Drachman, M. Folstein, R. Katzman, D. Price, and E. M. Stadlan. 1984. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS‐ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34(7):939-939.
McKhann, G. M., D. S. Knopman, H. Chertkow, B. T. Hyman, C. R. Jack, Jr., C. H. Kawas, W. E. Klunk, W. J. Koroshetz, J. J. Manly, R. Mayeux, R. C. Mohs, J. C. Morris, M. N. Rossor, P. Scheltens, M. C. Carrillo, B. Thies, S. Weintraub, and C. H. Phelps. 2011. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging—Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia 7(3):263-269.
Meng, X., and C. D’Arcy. 2012. Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS ONE 7(6):e38268.
MESA (Multi-Ethnic Study of Atherosclerosis). About. https://internal.mesa-nhlbi.org/about (accessed November 11, 2024).
MESA MIND. Study Overview. https://www.mesa-nhlbi.org/MESAMind/StudyOverview (accessed November 11, 2024).
Mielke, M. 2024. Sex and gender differences in Alzheimer’s disease and Alzheimer’s disease related dementias. Commissioned Paper for the Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias.
Miller, G. 2024. Using exposomics to uncover the environmental contributors to AD/ADRD. Presentation at Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
Minoshima, S., D. Cross, T. Thientunyakit, N. L. Foster, and A. Drzezga. 2022. 18F-FDG PET imaging in neurodegenerative dementing disorders: Insights into subtype classification, emerging disease categories, and mixed dementia with copathologies. Journal of Nuclear Medicine 63(Suppl 1):2S-12S.
Mobile Toolbox. 2024. Measuring cognitive change where it matters. https://mobiletoolbox.org/ (accessed October 15, 2024).
Moceri, V., W. Kukull, I. Emanual, G. van Belle, J. Starr, G. Schellenberg, W. McCormick, J. Bowen, L. Teri, and E. B. Larson. 2000. Using census data and birth certificates to reconstruct the early-life socioeconomic environment and the relation to the development of Alzheimer’s disease. Epidemiology 12(4)383-389.
Moro, M. 2018. The NIA aging cell repository: Facilitating research with aging cells. https://www.nia.nih.gov/research/blog/2018/05/nia-aging-cell-repository-facilitating-research-aging-cells (accessed August 9, 2024).
Mukherjee, S., S. E. Choi, M. L. Lee, P. Scollard, E. H. Trittschuh, J. Mez, A. J. Saykin, L. E. Gibbons, R. E. Sanders, A. F. Zaman, M. A. Teylan, W. A. Kukull, L. L. Barnes, D. A. Bennett, A. Z. Lacroix, E. B. Larson, M. Cuccaro, S. Mercado, L. Dumitrescu, T. J. Hohman, and P. K. Crane. 2023. Cognitive domain harmonization and cocalibration in studies of older adults. Neuropsychology 37(4):409-423.
Nagar, S. D., P. Pemu, J. Qian, E. Boerwinkle, M. Cicek, C. R. Clark, E. Cohn, K. Gebo, R. Loperena, K. Mayo, S. Mockrin, L. Ohno-Machado, A. H. Ramirez, S. Schully, A. Able, A. Green, S. Zuchner, S. Consortium, I. K. Jordan, and R. Meller. 2022. Investigation of hypertension and type 2 diabetes as risk factors for dementia in the all of US cohort. Scientific Reports 12(1):19797.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2017. Preventing cognitive decline and dementia: A way forward. Edited by A. I. Leshner, S. Landis, C. Stroud and A. Downey. Washington, DC: The National Academies Press.
NASEM. 2021a. Reducing the impact of dementia in America: A decadal survey of the behavioral and social sciences. Washington, DC: The National Academies Press.
NASEM. 2021b. Meeting the challenge of caring for persons living with dementia and their care partners and caregivers: A way forward. Edited by E. B. Larson and C. Stroud. Washington, DC: The National Academies Press.
NASEM. 2024. Preventing and treating Alzheimer’s disease and related dementias: Promising research and opportunities to accelerate progress: Proceedings of a workshop—in brief, edited by A. Downey and O. Yost. Washington, DC: The National Academies Press.
NCATS (National Center for Advancing Translational Sciences). 2024. About the CTSA program. https://ncats.nih.gov/research/research-activities/ctsa (accessed October 17, 2024).
NCRAD (National Centralized Repository for Alzheimer’s Disease and Related Dementias). 2024a. API generation program. https://ncrad.iu.edu/access-samples/available-samples/api (accessed August 15, 2024).
NCRAD. 2024b. GEMS. https://ncrad.iu.edu/access-samples/available-samples/gems (accessed August 15, 2024).
NCRAD. 2024c. Who we are. https://ncrad.iu.edu/about (accessed August 15, 2024).
NCRAD. 2024d. Explore a multitude of research samples at NCRAD. https://ncrad.iu.edu/access-samples/available-samples (accessed October 17, 2024).
NCRAD. 2024e. Enhance your study with biomarker analysis at ncrad. https://ncrad.iu.edu/biomarker-analysis (accessed October 17, 2024).
NeuroBioBank. 2024. About the NIH NeuroBioBank. https://neurobiobank.nih.gov/about/ (accessed August 15, 2024).
Ng, K. P., H. J. Chiew, L. Lim, P. Rosa-Neto, N. Kandiah, and S. Gauthier. 2018. The influence of language and culture on cognitive assessment tools in the diagnosis of early cognitive impairment and dementia. Expert Review of Neurotherapy 18(11):859-869.
Nguyen, T. T., E. J. Tchetgen Tchetgen, I. Kawachi, S. E. Gilman, S. Walter, S. Y. Liu, J. J. Manly, and M. M. Glymour. 2016. Instrumental variable approaches to identifying the causal effect of educational attainment on dementia risk. Annals of Epidemiology 26(1):71-76, e71-e73.
NHLBI (National Heart, Lung, and Blood Institute). n.d. Framingham Heart Study (FHS). https://www.nhlbi.nih.gov/science/framingham-heart-study-fhs (accessed August 14, 2024).
NHLBI. 2018. Bogalusa Heart Study (BHS) https://biolincc.nhlbi.nih.gov/studies/bhs/ (accessed October 25, 2024).
NHLBI. 2023. Framingham Heart Study—Cohort (FHS-Cohort). https://biolincc.nhlbi.nih.gov/studies/framcohort/ (accessed August 14, 2024).
NHLBI. 2024. Hispanic Community Health Study/Study of Latinos (HCHS/SOL). https://www.nhlbi.nih.gov/science/hispanic-community-health-studystudy-latinos-hchssol (accessed August 22, 2024).
NIA (National Institute on Aging). n.d.a. Brain donation. https://www.nia.nih.gov/health/brain-donation (accessed August 15, 2024).
NIA. n.d.b. AgingResearchBiobank. https://agingresearchbiobank.nia.nih.gov/about/ (accessed August 22, 2024).
NIA. 2019. National Institute on Aging Workshop on Applying Digital Technology for Early Diagnosis and Monitoring of Alzheimer’s Disease and Related Dementias.
NIA. 2022. Brain donation resources for ADRCS. https://www.nia.nih.gov/health/brain-donation/brain-donation-resources-adrcs (accessed October 17, 2024).
NIA. 2023a. Accelerating Medicines Partnership program for Alzheimer’s Disease (AMP AD). https://www.nia.nih.gov/research/amp-ad (accessed August 13, 2024).
NIA. 2023b. Fiscal year 2024 budget. https://www.nia.nih.gov/about/budget/fiscal-year-2024-budget (accessed August 22, 2024).
NIA. 2024a. Data sharing resources for researchers. https://www.nia.nih.gov/research/data-sharing-resources-researchers (accessed August 9, 2024).
NIA. 2024b. Fiscal year 2025 budget. https://www.nia.nih.gov/about/budget/fiscal-year-2025-budget (accessed August 22, 2024).
Nicosia, J., A. J. Aschenbrenner, D. A. Balota, M. J. Sliwinski, M. Tahan, S. Adams, S. S. Stout, H. Wilks, B. A. Gordon, T. L. S. Benzinger, A. M. Fagan, C. Xiong, R. J. Bateman, J. C. Morris, and J. Hassenstab. 2023. Unsupervised high-frequency smartphone-based cognitive assessments are reliable, valid, and feasible in older adults at risk for Alzheimer’s disease. Journal of the International Neuropsychological Society 29(5):459-471.
NIH (National Institutes of Health). 2024a. All of Us Research Program overview. https://allofus.nih.gov/about/program-overview (accessed August 14, 2024).
NIH. 2024b. All of Us—About. https://allofus.nih.gov/about (accessed October 25, 2024).
NIH. 2024c. FAQ. https://allofus.nih.gov/about/faq (accessed October 25, 2024).
NIH RePORTER. 2024a. Centrally-linked longitudinal peripheral biomarkers of AD in multiethnic populations. https://reporter.nih.gov/search/aPaS7obQX0SvtsI_0JMY0A/projectdetails/10555723 (accessed October 23, 2024.
NIH RePORTER. 2024b. Integrative pathways to cognitive, affective, and brain health. https://reporter.nih.gov/search/atFWblv290KNG-3jZzxCPA/project-details/10558956 (accessed August 22, 2024).
NIH RePORTER. 2024c. The Health and Aging Brain Study—Health Disparities. https://reporter.nih.gov/search/SLCPkzy1o0umXa2JsO8B0Q/project-details/10493844 (accessed August 15, 2024).
NIH RePORTER. 2024d. Research network for the harmonized cognitive assessment protocol. https://reporter.nih.gov/search/15EEC00B468BC3D77598B8961CAA4A01A2FFCEB861BF/project-details/10663818 (accessed August 15, 2024).
NIH Toolbox. 2024. MyCog: Overview of development. https://nihtoolbox.org/mycog/ (accessed August 22, 2024).
NINDS (National Institute of Neurological Disorders and Stroke). 2023. Consider donating your brain for neurological disease research. https://www.ninds.nih.gov/health-information/patient-caregiver-education/consider-donating-your-brain-neurological-disease-research (accessed August 15, 2024).
Nye, E., H. Lamont, and L. Anderson. 2022. Federal efforts to address racial and ethnic disparities in Alzheimer’s disease and related dementias. https://aspe.hhs.gov/sites/default/files/documents/5f3fc5aa6ae780f739265d40f20fc456/federal-racial-ethnic-disparities-adrd.pdf (accessed September 7, 2024).
Öhman, F., J. Hassenstab, D. Berron, M. Scholl, and K. V. Papp. 2021. Current advances in digital cognitive assessment for preclinical Alzheimer’s disease. Alzheimer’s & Dementia 13(1):e12217.
OHSU (Oregon Health and Science University). 2024a. About us. https://www.ohsu.edu/collaborative-aging-research-using-technology/about-us (accessed August 14, 2024).
OHSU. 2024b. CART mission. https://www.ohsu.edu/collaborative-aging-research-using-technology/cart-mission (accessed August 14, 2024).
OHSU. 2024c. The CART home. https://www.ohsu.edu/collaborative-aging-research-using-technology/cart-home (accessed October 15, 2024).
OHSU. 2024d. For industry and community partners. https://www.ohsu.edu/collaborative-aging-research-using-technology/industry-and-community-partners (accessed October 15, 2024).
Ossenkoppele, R., A. Pichet Binette, C. Groot, R. Smith, O. Strandberg, S. Palmqvist, E. Stomrud, P. Tideman, T. Ohlsson, J. Jogi, K. Johnson, R. Sperling, V. Dore, C. L. Masters, C. Rowe, D. Visser, B. N. M. van Berckel, W. M. van der Flier, S. Baker, W. J. Jagust, H. J. Wiste, R. C. Petersen, C. R. Jack, Jr., and O. Hansson. 2022. Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nature Medicine 28(11):2381-2387.
Palmqvist, S., S. Janelidze, E. Stomrud, H. Zetterberg, J. Karl, K. Zink, T. Bittner, N. Mattsson, U. Eichenlaub, K. Blennow, and O. Hansson. 2019. Performance of fully automated plasma assays as screening tests for Alzheimer disease-related beta-amyloid status. JAMA Neurology 76(9):1060-1069.
Palmqvist, S., S. Janelidze, Y. T. Quiroz, H. Zetterberg, F. Lopera, E. Stomrud, Y. Su, Y. Chen, G. E. Serrano, A. Leuzy, N. Mattsson-Carlgren, O. Strandberg, R. Smith, A. Villegas, D. Sepulveda-Falla, X. Chai, N. K. Proctor, T. G. Beach, K. Blennow, J. L. Dage, E. M. Reiman, and O. Hansson. 2020. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 324(8):772-781.
Palmqvist, S., P. Tideman, N. Cullen, H. Zetterberg, K. Blennow, Alzheimer’s Disease Neuroimaging Initiative, J. L. Dage, E. Stomrud, S. Janelidze, N. Mattsson-Carlgren, and O. Hansson. 2021. Prediction of future Alzheimer’s disease dementia using plasma phospho-tau combined with other accessible measures. Nature Medicine 27(6):1034-1042.
Paschalidis, I. C. 2024. Panelist Remarks. Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
Peet, B. T., S. Spina, N. Mundada, and R. La Joie. 2021. Neuroimaging in frontotemporal dementia: Heterogeneity and relationships with underlying neuropathology. Neurotherapeutics 18(2):728-752.
Piers, R. J., K. N. Devlin, B. Ning, Y. Liu, B. Wasserman, J. M. Massaro, M. Lamar, C. C. Price, R. Swenson, R. Davis, D. L. Penney, R. Au, and D. J. Libon. 2017. Age and graphomotor decision making assessed with the digital clock drawing test: The Framingham Heart Study. Journal of Alzheimer’s Disease 60(4):1611-1620.
Reitz, C. 2016. Toward precision medicine in Alzheimer’s disease. Annals of Translational Medicine 4(6):107.
Reitz, C., M. A. Pericak-Vance, T. Foroud, and R. Mayeux. 2023. A global view of the genetic basis of Alzheimer disease. Nature Reviews Neurology 19(5):261-277.
Reuben, A. 2018. Childhood lead exposure and adult neurodegenerative disease. Journal of Alzheimer’s Disease 64:17-42.
Ritchie, C. W., G. M. Terrera, and T. J. Quinn. 2015. Dementia trials and dementia tribulations: Methodological and analytical challenges in dementia research. Alzheimer’s Research & Therapy 7(1):31.
Robinson, A., R. McNamee, Y. S. Davidson, M. Horan, J. Snowden, L. McInnes, N. Pendleton, and D. Mann. 2018. Scores obtained from a simple cognitive test of visuospatial episodic memory performed decades before death are associated with the ultimate presence of Alzheimer disease pathology. Dementia and Geriatric Cognitive Disorders 45:79-90.
Rosin, E. R., D. Blasco, A. R. Pilozzi, L. H. Yang, and X. Huang. 2020. A narrative review of Alzheimer’s disease stigma. Journal of Alzheimer’s Disease 78(2):515-528.
Roth, S., N. Burnie, I. Suridjan, J. T. Yan, and M. Carboni. 2023. Current diagnostic pathways for Alzheimer’s disease: A cross-sectional real-world study across six countries. Journal of Alzheimer’s Disease Reports 7:659-674.
Scott, G. D., M. R. Arnold, T. G. Beach, C. H. Gibbons, A. G. Kanthasamy, R. M. Lebovitz, A. W. Lemstra, L. M. Shaw, C. E. Teunissen, H. Zetterberg, A. S. Taylor, T. C. Graham, B. F. Boeve, S. N. Gomperts, N. R. Graff-Radford, C. Moussa, K. L. Poston, L. S. Rosenthal, M. N. Sabbagh, R. R. Walsh, M. T. Weber, M. J. Armstrong, J. A. Bang, A. C. Bozoki, K. Domoto-Reilly, J. E. Duda, J. E. Fleisher, D. R. Galasko, J. E. Galvin, J. G. Goldman, S. K. Holden, L. S. Honig, D. E. Huddleston, J. B. Leverenz, I. Litvan, C. A. Manning, K. S. Marder, A. Y. Pantelyat, V. S. Pelak, D. W. Scharre, S. J. Sha, H. A. Shill, Z. Mari, J. F. Quinn, and D. J. Irwin. 2021. Fluid and tissue biomarkers of Lewy body dementia: Report of an LBDA symposium. Frontiers in Neurology 12:805135.
Sevigny, J., J. Suhy, P. Chiao, T. Chen, G. Klein, D. Purcell, J. Oh, A. Verma, M. Sampat, and J. Barakos. 2016. Amyloid PET screening for enrichment of early-stage Alzheimer disease clinical trials: Experience in a phase 1b clinical trial. Alzheimer Disease and Associated Disorders 30(1):1-7.
SHS (Strong Heart Study). 2017a. About Strong Heart Study. https://strongheartstudy.org/About (accessed August 23, 2024).
SHS. 2017b. Recently approved ancillary studies/collaborations by Strong Heart Study. https://strongheartstudy.org/Research/Ancillary-and-Sub-Studies/Approved-Ancillary-and-Sub-studies (accessed August 23, 2024).
Simuni, T., L. M. Chahine, K. Poston, M. Brumm, T. Buracchio, M. Campbell, S. Chowdhury, C. Coffey, L. Concha-Marambio, T. Dam, P. DiBiaso, T. Foroud, M. Frasier, C. Gochanour, D. Jennings, K. Kieburtz, C. M. Kopil, K. Merchant, B. Mollenhauer, T. Montine, K. Nudelman, G. Pagano, J. Seibyl, T. Sherer, A. Singleton, D. Stephenson, M. Stern, C. Soto, C. M. Tanner, E. Tolosa, D. Weintraub, Y. Xiao, A. Siderowf, B. Dunn, and K. Marek. 2024. A biological definition of neuronal alpha-synuclein disease: Towards an integrated staging system for research. Lancet Neurology 23(2):178-190.
Snowdon, D. A. 2003. Healthy aging and dementia: Findings from the Nun Study. Annals of Internal Medicine 139(5 Pt 2):450-454.
Sperling, R. A., M. C. Donohue, R. A. Rissman, K. A. Johnson, D. M. Rentz, J. D. Grill, J. L. Heidebrink, C. Jenkins, G. Jimenez-Maggiora, O. Langford, A. Liu, R. Raman, R. Yaari, K. C. Holdridge, J. R. Sims, and P. S. Aisen. 2024. Amyloid and tau prediction of cognitive and functional decline in unimpaired older individuals: Longitudinal data from the A4 and LEARN studies. Journal of Prevention of Alzheimer’s Disease 11(4):802-813.
Stites, S. D., R. Milne, and J. Karlawish. 2018. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia 10:285-300.
Stites, S. D., S. Midgett, D. Mechanic-Hamilton, M. Zuelsdorff, C. M. Glover, D. X. Marquez, J. E. Balls-Berry, M. L. Streitz, G. Babulal, J. F. Trani, J. N. Henderson, L. L. Barnes, J. Karlawish, and D. A. Wolk. 2022. Establishing a framework for gathering structural and social determinants of health in Alzheimer’s disease research centers. Gerontologist 62(5):694-703.
TabCAT. 2024. Detect cognitive changes earlier. https://tabcathealth.com/ (accessed August 22, 2024).
Teunissen, C. 2024. Biomarkers useful across the clinical continuum. Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
Toga, A., K. Yaffe, R. Rissman, L. Johnson, and S. O’Bryant. 2022. The Health & Aging Brain Study—Health Disparities (HABS-HD). https://experts.unthsc.edu/en/projects/the-health-aging-brain-study-health-disparities-habs-hd (accessed August 8, 2024).
Tsai, R. M., A. Bejanin, O. Lesman-Segev, R. LaJoie, A. Visani, V. Bourakova, J. P. O’Neil, M. Janabi, S. Baker, S. E. Lee, D. C. Perry, L. Bajorek, A. Karydas, S. Spina, L. T. Grinberg, W. W. Seeley, E. M. Ramos, G. Coppola, M. L. Gorno-Tempini, B. L. Miller, H. J. Rosen, W. Jagust, A. L. Boxer, and G. D. Rabinovici. 2019. 18F-flortaucipir (AV-1451) tau PET in frontotemporal dementia syndromes. Alzheimer’s Research & Therapy 11(1):13.
Tsuang, D. 2024. Panelist remarks. Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
van der Zande, J. J., A. A. Gouw, I. van Steenoven, M. van de Beek, P. Scheltens, C. J. Stam, and A. W. Lemstra. 2020. Diagnostic and prognostic value of EEG in prodromal dementia with Lewy bodies. Neurology 95(6):e662-e670.
VMAC (Vanderbilt Memory and Alzheimer’s Center). 2024. Alzheimer’s Disease Sequencing Project Phenotype Harmonization Consortium. https://vmacdata.org/adsp-phc (accessed August 22, 2024).
Wallin, C. 2024. Update from the NIA regarding Real-World Data Platform. Document provided to the Committee on the Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias on April 19, 2024. Available by request through the National Academies’ Public Access Records Office.
Watson, R., R. Sanson-Fisher, J. Bryant, and E. Mansfield. 2023. Dementia is the second most feared condition among Australian health service consumers: Results of a cross-sectional survey. BMC Public Health 23(1):876.
Weuve, J. 2024. Discussant perspectives and Committee Discussion. Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias Public Workshop, Hybrid. January 16–17, 2024.
Whalley, L. J., F. D. Dick, and G. McNeill. 2006. A life-course approach to the aetiology of late-onset dementias. Lancet Neurology 5(1):87-96.
WHO (World Health Organization). 2022. Optimizing brain health across the life course: WHO position paper. https://www.who.int/publications/i/item/9789240054561 (accessed August 13, 2024).
WHI (Women’s Health Initiative). 2021a. About WHI. https://www.whi.org/about-whi (accessed October 25, 2024).
WHI. 2021b. WHI Memory Study (WHIMS). https://www.whi.org/md/39/whi-memory-study-whims (accessed August 14, 2024).
Yamada, M., J. Komatsu, K. Nakamura, K. Sakai, M. Samuraki-Yokohama, K. Nakajima, and M. Yoshita. 2020. Diagnostic criteria for dementia with Lewy bodies: Updates and future directions. Journal of Movement Disorders 13(1):1-10.
Yang, Y. C., C. E. Walsh, K. Shartle, R. C. Stebbins, A. E. Aiello, D. W. Belsky, K. M. Harris, M. Chanti-Ketterl, and B. L. Plassman. 2024. An early and unequal decline: Life course trajectories of cognitive aging in the United States. Journal of Aging and Health 36(3-4):230-245.
Yawn, A. 2023. After 50 years of pioneering research in rural Louisiana, study pivots from heart to brain. https://sph.tulane.edu/after-50-years-pioneering-research-rural-louisiana-study-pivots-heart-brain#:~:text=The%20Bogalusa%20Heart%20Study%20%E2%80%93%20in,lead%20to%20declines%20in%20brain (accessed August 14, 2024).
Zetterberg, H. 2022. Biofluid-based biomarkers for Alzheimer’s disease-related pathologies: An update and synthesis of the literature. Alzheimer’s & Dementia 18(9):1687-1693.