Preventing and Treating Dementia: Research Priorities to Accelerate Progress (2025)
Chapter: 5 Advancing Research Priorities for Preventing and Treating Alzheimer's Disease and Related Dementias
5
Advancing Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias
Accelerating the development of effective preventive and treatment interventions for Alzheimer’s disease and related dementias (AD/ADRD) that can preserve cognitive function and improve quality of life is a global public health priority. In the last decade, spurred by the National Alzheimer’s Project Act, the National Institutes of Health (NIH) has invested billions of dollars to support research on detecting, understanding, and developing interventions for AD/ADRD. The committee reviewed this broad research landscape, working to identify areas of promise and priorities for future investments, and found that the investments by NIH and others have led to many scientific advances and created a foundation of knowledge from which much more can be learned. However, the pace of progress has not matched the growing urgency for interventions that can prevent, slow, or cure AD/ADRD and reduce the societal impact of these diseases.
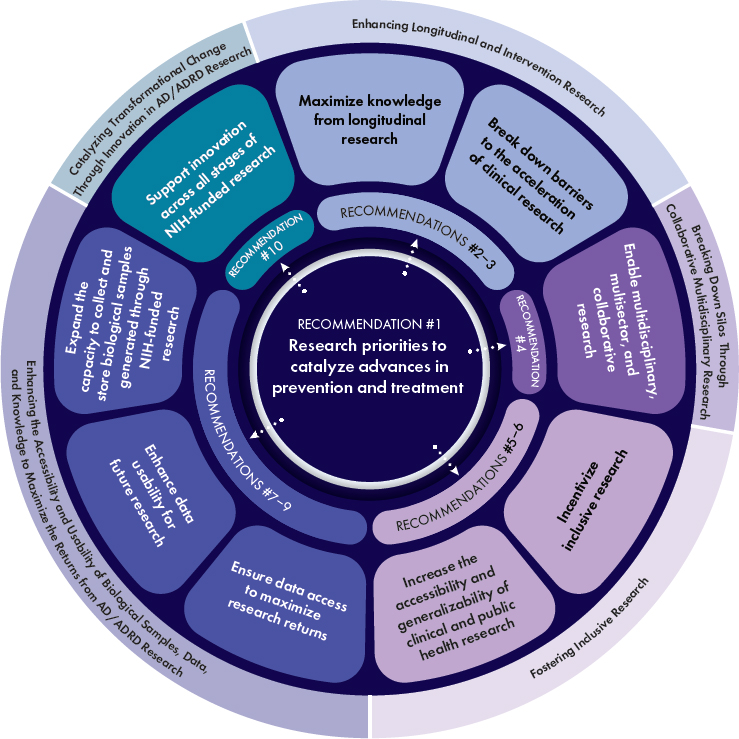
This final chapter presents the committee’s recommendations for accelerating progress on the research priorities identified in Chapters 2, 3, and 4 with the goal of advancing the science needed to develop effective preventive and therapeutic strategies. The identified research priorities represent areas of scientific inquiry with the greatest promise to catalyze significant advances and maximize return on investment. These research priorities are summarized in Recommendation 1 and detailed in Table 5-1. The committee’s Recommendations 2–10 are aimed at overcoming key barriers to progress on those research priorities (see Figure 5-1). Addressing both the research priorities and the recommendations will require sustained and dedicated resources and need to be guided at all stages by those with lived
experience to ensure synergy between scientific priorities and the priorities of those directly affected by dementia.
ADVANCING KEY SCIENTIFIC PRIORITIES RELATED TO PREVENTING AND TREATING AD/ADRD
The past few decades have brought significant advances in the understanding of AD/ADRD and in the development of tools and methods that can drive further progress. Notable milestones include the ability to detect specific AD-related pathologies (amyloid and tau) years before symptoms emerge, the discovery of many new genes linked to AD/ADRD that shed
light on pathogenic mechanisms, and the recognition that pathologies previously thought to distinguish different forms of dementia often co-occur (NIH, 2023a). While encouraged, the committee also found notable gaps in scientific knowledge and research capabilities during its review of the AD/ADRD research landscape. The following scientific gaps represent key bottlenecks that significantly impede progress toward preventing and treating AD/ADRD.
- There is a lack of rigorous evidence to support the identification of effective public health strategies for preventing AD/ADRD. Epidemiological research has yielded a large set of dementia risk factors (e.g., health behaviors, social isolation, socioeconomic disadvantage) based on statistical correlations. However, gaps in longitudinal data, data infrastructure, and study designs have resulted in limited understanding of causal relationships and the identification of exposures and system-level factors that, if mitigated, would have the greatest effect on the incidence of dementia. This impedes the development of effective public health approaches that could be implemented at a population scale to promote brain health across the life course and prevent AD/ADRD in diverse and especially disproportionately affected populations.
- There is an incomplete understanding of the biological basis and multiple etiologies underlying cognitive decline and dementia, as well as the mechanisms of resilience. There is still much that is not understood about AD/ADRD, conditions that may be characterized by decades of life-course health insults to brain and peripheral systems contributing to the development and detectable progression of neuropathology in the absence of clinical symptoms. Key knowledge gaps include how life-course exposures and other risk factors relate to pathobiology in diverse populations and the connections between molecular pathways that contribute to AD/ADRD and resilience (the maintenance of cognitive function despite the presence of brain pathology). This lack of understanding impedes drug discovery and the development of effective preventive and therapeutic intervention strategies. It also makes it difficult to predict the effect of risk factor reduction and drugs focused on single-pathology targets on dementia incidence.
- There is a lack of effective, validated, and accessible tools and methods (e.g., novel biomarker tests, digital assessment technologies) for detecting early changes in brain health and accurately diagnosing, subtyping, and monitoring AD/ADRD in diverse populations, This foundational capability is essential to trace how the natural history of AD/ADRD differs from “healthy” brain aging. Progress made
- in the development of tools for AD needs to be expanded beyond amyloid and tau and extended to the multiple etiologies leading to dementia. The current capability gap impedes efforts to measure disease incidence and prevalence, to intervene early when chances of preventing and mitigating disease are greatest, to detect when treatments modify the trajectory of AD/ADRD, and to target specific interventions to the right populations (precision medicine).
Within each of these major scientific gaps of knowledge, there is also promising research that suggests opportunities to break current bottlenecks as new discoveries emerge. The discovery of imaging and fluid biomarkers for AD has catalyzed a shift in phenotyping procedures used in research and needs to be tested now in clinical practice. With the identification of risk genes/loci and the discovery of additional biomarkers, particularly for related dementias, current barriers to early detection, diagnosis, prognosis (e.g., the likelihood of progression to clinical dementia), and longitudinal monitoring may be overcome, and it will be possible to quantify and better understand multiple etiology dementia. The combination of digital tools and computational methods such as artificial intelligence/machine learning (AI/ML) that may be able to identify changes in traits (e.g., speech, gait, sleep behavior) that may precede current measures of cognitive decline similarly shows promise for enabling early detection of changes in brain health, early diagnosis, and prognosis (Amini et al., 2024). Digital tools have also opened new opportunities for passive and remote data collection and are changing the way investigators engage with study participants, particularly those from underresourced and underrepresented populations, and the public (Kaye et al., 2021).
Investments in basic science and longitudinal cohort studies have led to a significant expansion of the therapeutic pipeline with novel promising interventions that are not specific to any single dementia type by uncovering shared molecular pathways contributing to AD/ADRD (e.g., autophagic and lysosomal, immune, metabolic, mylelination), as well as resilience factors (Cummings et al., 2024). Multiomics methods1 are creating new opportunities to evaluate disease mechanisms in diverse populations (Reddy et al., 2024) and to identify molecular disease subtypes and endophenotypes (Fang et al., 2020), thereby creating the foundation for precision medicine approaches to prevention and treatment in the future. Increased understanding of the links between AD/ADRD and chronic diseases such as hypertension and diabetes (Nagar et al., 2022), along with encouraging
___________________
1 Multiomics methods involve the integrative analysis of multiple “omics” datasets, such as those generated from genomic, proteomic, transcriptomic, epigenomic, and metabolomic methods.
evidence for multicomponent interventions focused on health behaviors, has highlighted the potential for public health strategies to reduce dementia risk.
Scientific Priorities for Advancing the Prevention and Treatment of AD/ADRD
Building on the aforementioned examples of momentum and lines of promising research, the committee identified 11 research priorities and associated near- and medium-term scientific questions that it believes should be a focus of NIH-funded AD/ADRD biomedical research for the next 3 to 10 years. These research priorities, which are summarized in Recommendation 1 and detailed in Table 5-1, fall into three broad areas:
- Quantify brain health across the life course and accurately predict risk of, screen for, diagnose, and monitor AD/ADRD.
- Build a more comprehensive and integrated understanding of the disease biology and mechanistic pathways that contribute to AD/ADRD development and resilience over the life course.
- Catalyze advances in interventions for the prevention and treatment of AD/ADRD spanning from precision medicine to public health strategies.
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
| Research priorities to quantify brain health across the life course and accurately predict risk of, screen for, diagnose, and monitor AD/ADRD | ||
| 2-1: Develop better tools, including novel biomarker tests and digital assessment technologies, to monitor brain health across the life course and screen, predict, and diagnose AD/ADRD at scale. |
|
|
| 2-2: Implement advances in clinical research methods and tools to generate data from real-world clinical practice settings that can inform future research. |
|
|
|
||
| Research priorities to build a more comprehensive and integrated understanding of the disease biology and mechanistic pathways that contribute to AD/ADRD development and resilience over the life course | ||
| 3-1: Identify factors driving AD/ADRD risk in diverse populations, particularly understudied and disproportionately affected groups, to better understand disease heterogeneity—including molecular subtypes and disparities in environmental exposures—and to identify prevention opportunities and advance health research equity. |
|
|
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
| 3-2: Characterize the exposome and gene–environment interactions across the life course to gain insights into biological mechanisms and identify opportunities to reduce AD/ADRD risk and increase resilience. |
|
|
| 3-3: Elucidate the genetic and other biological mechanisms underlying resilience and resistance to identify novel targets and effective strategies for AD/ADRD prevention and treatment. |
|
|
| 3-4: Develop integrated molecular and cellular causal models to guide the identification of common mechanisms underlying AD/ADRD and their validation as novel targets for prevention and treatment. |
|
|
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
|
|
|
| Research priorities to catalyze advances in interventions for the prevention and treatment of AD/ADRD spanning from precision medicine to public health strategies | ||
| 4-1: Integrate innovative approaches and novel tools into the planning, design, and execution of studies to accelerate the identification of effective interventions. |
|
|
|
|
|
| 4-2: Advance the development and evaluation of combination therapies (including pharmacologic and nonpharmacologic approaches) to better address the multifactorial nature of AD/ADRD. |
|
|
| 4-3: Evaluate precision medicine approaches for the prevention and treatment of AD/ADRD to better identify interventions likely to benefit specific groups of individuals. |
|
|
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
|
|
|
| 4-4: Advance the adoption of standardized outcomes for assessing interventions that are sensitive, person-centered, clinically meaningful, and reflect the priorities of those at risk for or living with AD/ADRD. |
|
|
| 4-5: Evaluate the causal effects of public health approaches on overall dementia incidence and incidence in understudied and/or disproportionately affected populations. |
|
|
| Research Priority | Key Scientific Questions | Near-Term Research Opportunities to Address Key Scientific Questions |
|---|---|---|
|
NOTE: The numbering of research priorities in this table reflects the numbering in the report chapters.
Recommendation 1: Research priorities to catalyze advances in prevention and treatment
The National Institutes of Health (NIH) should focus on the research priorities and associated near- and medium-term scientific questions detailed in Table 5-1 to advance a person-centered, multidisciplinary, and integrative research approach that will catalyze advances in the prevention and treatment of Alzheimer’s disease and related dementias (AD/ADRD). These research priorities cover the following areas:
- Develop better tools, including novel biomarker tests and digital assessment technologies, to monitor brain health across the life course and screen, predict, and diagnose AD/ADRD at scale (Research Priority 2-1)
- Implement advances in clinical research methods and tools to generate data from real-world clinical practice settings that can inform future research (Research Priority 2-2)
- Identify factors driving AD/ADRD risk in diverse populations, particularly understudied and disproportionately affected groups, to better understand disease heterogeneity—including molecular subtypes and disparities in environmental exposures—and to identify prevention opportunities and advance health research equity (Research Priority 3-1)
- Characterize the exposome and gene–environment interactions across the life course to gain insights into biological mechanisms and identify opportunities to reduce AD/ADRD risk and increase resilience (Research Priority 3-2)
- Elucidate the genetic and other biological mechanisms underlying resilience and resistance to identify novel targets and effective strategies for AD/ADRD prevention and treatment (Research Priority 3-3)
- Develop integrated molecular and cellular causal models to guide the identification of common mechanisms underlying AD/ADRD and their validation as novel targets for prevention and treatment (Research Priority 3-4)
- Integrate innovative approaches and novel tools into the planning, design, and execution of studies to accelerate the identification of effective interventions (Research Priority 4-1)
- Advance the development and evaluation of combination therapies (including pharmacological and nonpharmacological approaches) to better address the multifactorial nature of AD/ADRD (Research Priority 4-2)
- Evaluate precision medicine approaches for the prevention and treatment of AD/ADRD to better identify interventions likely to benefit specific groups of individuals (Research Priority 4-3)
- Advance the adoption of standardized outcomes for assessing interventions that are sensitive, person-centered, clinically meaningful, and reflect the priorities of those at risk for or living with AD/ADRD (Research Priority 4-4)
- Evaluate the causal effects of public health approaches on overall dementia incidence and incidence in understudied and/or disproportionately affected populations. (Research Priority 4-5)
The committee acknowledges that NIH has already made investments in each of these priority research areas to varying degrees. Given the breadth of the NIH AD/ADRD research portfolio, it is unsurprising that the committee did not identify any research priorities for which there had been no prior NIH investment. In some cases, research priorities identified by the committee, such as the development of biomarkers for monitoring brain health and the identification of factors driving risk in diverse populations, are already the focus of major NIH-funded research programs and initiatives, many of which are described in this report. Other identified research priorities, such as the characterization of the exposome and gene–environment interactions, the development of integrated molecular and cellular causal models, and the development of digital tools, represent scientific areas of more recent or limited NIH investment. Relatedly, efforts to achieve associated near-term research opportunities, which are detailed in the right-hand column of Table 5-1, may indeed be underway but have not yet been fully realized. Significant investment in the totality of the priority research areas is needed to address the knowledge gaps laid out in this report. Critically, beyond financial investment, success in tackling each of these research priorities will require an emphasis on the intentional expansion of research efforts beyond AD and the inclusion of diverse and understudied and/or disproportionately affected populations.
Importantly, Table 5-1 is not intended as a prescribed research agenda. Nor is the identification of these priority areas meant to imply that lines of scientific inquiry outside of these areas are not of value or that work in all other areas should be suspended. There is a great deal of uncertainty in the process for scientific investigation regarding which discoveries from current research will lead to transformational advances in the future and no guarantees can be offered regarding the ultimate fruitfulness of any specific line of inquiry. The committee’s intention, however, is that the priorities will be used as a guide in the rebalancing of NIH funding for AD/ADRD. Closing the scientific knowledge gaps raised by these priorities can occur by working to answer the committee’s proposed scientific questions and acting on opportunities to overcome barriers to progress, as detailed in the recommendations that follow.
Opportunities to Enhance Longitudinal and Intervention Research to Advance Key Scientific Priorities for Preventing and Treating AD/ADRD
Studies that follow individuals longitudinally and test interventions across time are needed to address the research priorities and associated scientific questions identified in Table 5-1. This section highlights opportunities to ensure such research has the greatest possible impact by maximizing the insights that can be gleaned and accelerating the pace of discovery.
Leveraging Longitudinal Research
Longitudinal cohort studies represent an important mechanism for identifying data that provide a comprehensive view of brain health and AD/ADRD development over the life course (including risk and resilience factors). Knowledge gained from such studies can be translated into protocols and sensitive tools (e.g., digital health technologies, biomarker assays) that can be deployed in research and practice for ongoing clinical monitoring and AD/ADRD prediction, detection, prognostication, and diagnosis.
Numerous cohorts have been established specifically for the study of cognitive impairment and dementia. The constituents of such cohorts often are limited to upper age ranges, and such studies will generally collect measures (e.g., exposures, health data) determined a priori to be specific to AD/ADRD. With the growing understanding of AD/ADRD as conditions that develop over the life course, there has been recognition of opportunities to use data relevant to brain health and AD/ADRD generated through longitudinal research focused on other health conditions (e.g., the Bogalusa Heart Study, which focuses on cardiovascular disease), as discussed in Chapter 2. Integrating those data with AD/ADRD outcomes can help to fill current data gaps (e.g., data for younger age ranges and populations underrepresented in AD/ADRD research), expand the set of measures that can be linked to brain health trajectories, and yield important insights on prevention and treatment strategies while newer AD/ADRD-focused cohort studies remain ongoing.
NIH has made significant investments in recent years to expand and better use its existing support for longitudinal research related to aging, resilience, and AD/ADRD. Such investments have included
- the creation of new cohorts (e.g., the ARTFL-LEFFTDS Longitudinal Frontotemporal Lobar Degeneration [ALLFTD] study) and the augmentation of existing cohorts (e.g., the Longevity Consortium) to better understand AD/ADRD risk and resilience across diverse populations;
- the integration of AD/ADRD research into existing cohorts established for examining other health outcomes (e.g., funding the
- Aging, Demographics, and Memory Study as a supplement to the Health and Retirement Study); and
- collaborative efforts to harmonize outcomes (e.g., the Harmonized Cognitive Assessment Protocol) to facilitate cross-study comparisons and multicohort analyses.
Recognizing these prior investments, the committee encourages NIH to continue and expand support for longitudinal AD/ADRD research that can fill data gaps and address the scientific questions included in Table 5-1. In addition to establishing new cohorts that can meet the need for diverse and representative populations in dementia research, this should include a concerted effort to identify other existing cohorts—including those funded by agencies other than the National Institute on Aging (NIA) and the National Institute of Neurological Disorders and Stroke (NINDS), as well as international studies—to which a focus on AD/ADRD could be added through supplemental funding and other appropriate NIH funding mechanisms. Such efforts may provide an opportunity to pilot novel tools such as biomarker tests and digital technologies. Additionally, NIH should ensure funding opportunities are designed to maximize the insights from longitudinal research through attention to data accessibility and harmonization and the collection and storage of data (e.g., digital, exposure) and biosamples (see Recommendations 7, 8, and 9). Proactive coordination and planning through the convening of investigators from different cohort studies, before study initiation whenever possible, can help to identify approaches that would enable data access, interoperability, and harmonization.
Recommendation 2: Maximize knowledge from longitudinal research
To maximize knowledge from longitudinal research and enable future discoveries, the National Institutes of Health should prioritize investments in longitudinal research to address existing knowledge gaps regarding factors that influence brain health over the life course. These efforts should include the following:
- Invest in data infrastructure (see Recommendation 7), data harmonization (see Recommendation 8), and the cultivation of specialized expertise to enable the collection of data and conduct of analyses within and across existing cohorts, including those cohorts developed to characterize brain health and those created for examining other health outcomes.
- Create new, multidimensionally diverse (e.g., multilanguage, ethnoracial, geographic, socioeconomic) cohorts.
- Strategically add data points important to assessing brain health into existing cohorts constituted for research on other health conditions.
- Routinely collect early- and midlife exposure data (e.g., residential and work history, environmental toxicants, nutrition, education) from cohort study participants.
- Ensure that the data generated from shared biological samples are stored, searchable, and sharable.
Accelerating Translational and Clinical Intervention Research
Decades of research and hundreds of clinical trials have yielded only a limited number of treatments for Alzheimer’s disease (AD) that offer modest clinical benefits (Boxer and Sperling, 2023; Cummings et al., 2024; Kim et al., 2022), and no treatments have been approved by the U.S. Food and Drug Administration (FDA) for related dementias (Liu et al., 2019; MacDonald et al., 2022; Nag et al., 2020), beyond those for managing symptoms. Evidence for some nonpharmacological approaches to preventing cognitive decline and dementia (physical activity, cognitive training), while encouraging, has been inconclusive (NASEM, 2017), leaving the public with much uncertainty about steps they should take to protect their cognitive function as they age. This lack of progress toward effective strategies for preventing and treating AD/ADRD reflects the complex and multifactorial pathobiology of this group of diseases, but it also underscores the need to accelerate the translational research yielding novel targets for interventions and to expand and improve the efficiency of clinical trials.
Many entities (government, private, philanthropic, and academic) contribute to research for advancing interventions for AD/ADRD with complementary resources and expertise. NIH plays a critical role in this complex research ecosystem by funding research on interventions and certain trial designs that may be less appealing to industry owing to financial risk or the lack of financial incentives, incentivizing industry participation in collaborative efforts designed to develop and bring new and combination interventions to scale (see Recommendation 10), and supporting basic and translational research (e.g., target identification and validation) that feeds into the private-sector drug development pipeline. As discussed in Chapter 3, NIH has made significant infrastructure investments (e.g., Accelerating Medicines Partnership® Program for Alzheimer’s Disease [AMP-AD], Target Enablement to Accelerate Therapy Development for Alzheimer’s Disease [TREAT-AD], Model Organism Development and Evaluation for Late-onset Alzheimer’s Disease [MODEL-AD]) to generate a pipeline that can translate discoveries from basic research into candidates that can be evaluated in clinical trials. With the necessary infrastructure in place, there is now an opportunity to scale and diversify these efforts to a broader set of risk factors and pathways (e.g., neuroinflammation, cellular senescence, lysosomal dysfunction, mitochondrial dysfunction).
There has been notable growth in innovation in clinical trials in the last couple of decades, particularly outside of AD/ADRD, highlighting opportunities to learn from the successes and lessons from other fields. As discussed in Chapter 4, NIH infrastructure investments, such as the Alzheimer’s Clinical Trials Consortium, the Alzheimer’s Prevention Initiative, and the Dominantly Inherited Alzheimer Network Trials Unit, have facilitated increased collaboration with industry, philanthropy, and other partners (e.g., by using public–private partnerships) and innovation in AD/ADRD clinical trials (e.g., decentralization of trials, piloting platform trials, virtual engagement of participants, and digital data collection). However, to accelerate the pace of discovery, these efforts need to be expanded to a much greater scale as NIH continues to support clinical research to evaluate AD/ADRD interventions in the coming years.
Drug discovery can take 15–20 years, and a long-term focus on the mechanisms involved in AD/ADRD to allow multiple targets to be tested through clinical proof-of-concept (phase 1b and 2) trials is critically important. Expanded support (e.g., via R61/R33 mechanisms) for the validation of novel targets or effective strategies for the prevention and treatment of AD/ADRD is needed to ensure that an adequate number of validated targets (roughly 50 per year) can serve as input to the pipeline with the goal of yielding promising candidates that could be transitioned to clinical trials (within academia or industry).
As drug discovery and target validation efforts are scaled, phase 1b and phase 2 clinical trials in particular need to be expanded. Increasing the quantity and quality of small phase 1b and phase 2 proof-of-concept trials with a focus on mechanisms, informative biomarkers (e.g., target engagement, biomarkers for copathologies), and outcomes (e.g., pharmacokinetics and pharmacodynamics, surrogate outcomes) is needed to smooth the transition to and better guide decision making for larger, later-stage trials. Importantly, as the incorporation of additional biomarkers and imaging for pathologies associated with related dementias (e.g., TDP-43, alpha-synuclein) becomes more feasible, including them within each early-phase study, even those clinical trials of single therapeutic agents targeting a different pathology, would be highly informative of potential links among copathologies. In the meantime, banking of biological samples can enable future measurement of these pathologies.
In anticipation of the increased demand for clinical trial investigators, attention is needed to address current gaps in the workforce (e.g., investigators with specialized expertise in pharmacology trials). Ensuring investigators new to conducting trials use existing training programs with best practices can help to improve the rigor of earlier-stage trials. The NIA and Alzheimer’s Association-funded Institute on Methods and Protocols for Advancement of Clinical Trials in ADRD (IMPACT-AD), for example,
provides multidisciplinary training for current and future principal investigators on the design, conduct, and analysis of clinical trials. IMPACT-AD also works to strengthen the broader clinical trial workforce through dedicated training opportunities for clinician-researchers and clinical trial support staff (Berkness et al., 2021).
The expanded use of innovative clinical trial designs, described in Chapter 4, has the potential to significantly accelerate clinical research. Master protocols—trial protocols for use with multiple substudies (FDA, 2022)—and platform, combination, and adaptive trial designs create efficiencies and enable a shift away from a clinical research paradigm focused on testing individual interventions in single populations sequentially.
Additional opportunities to improve trial efficiency can be realized by ensuring a trial-ready pool of research participants and a ready clinical trial infrastructure. Recruitment and enrollment represent major bottlenecks in the clinical trial pipeline (Langbaum et al., 2023). As has been done in the cancer field, NIH-funded Alzheimer’s Disease Research Centers (ADRCs) and other AD/ADRD-focused centers should be evaluated and held accountable for enrolling participants in clinical trials.
A key strategy to improve trial efficiency at the recruitment phase is to reduce screen failure.2 With master protocols, a single recruitment pool can be used to populate multiple substudies. Volunteers that may be ineligible for one substudy could potentially be enrolled in a different substudy under the same protocol. Highly phenotyped and increasingly diverse cohorts represent invaluable pools of prescreened participants (Gregory et al., 2022). Examples include ALLFTD (discussed in Chapter 2) and the North American Prodromal Synucleinopathy (NAPS) study, which enrolls people with rapid eye movement (REM) sleep behavior disorders who are at risk of developing LBD, Parkinson’s, or other neurological disorders (NAPS Consortium, 2024). Similarly, the Trial-Ready Cohort-Down Syndrome collects longitudinal data (e.g., blood and cognitive tests, imaging) on people with Down syndrome to fast-track the enrollment process as soon as they are eligible and matched with a qualifying clinical study (TRC-DS, 2024). While biases in the cohort populations need to be considered, drawing from such pools could accelerate startup.
Finally, there needs to be consideration of opportunities to use technology to move from screen fail to screen enroll. While registries3 are one mechanism to track volunteers who did not meet screening criteria but
___________________
2 Screen failure occurs when a potential participant is screened for but is not able to enroll in the trial (Parekh et al., 2022).
3 NIA maintains a list of registries and matching services for AD/ADRD clinical trials at https://www.nia.nih.gov/health/clinical-trials-and-studies/registries-and-matching-services-clinical-trials (accessed October 19, 2024).
may be eligible for other studies (e.g., people who exhibit signs of cognitive impairment but fail the screen for AD pathology), other technologies, such as social engagement platforms, should be explored. There is also a need for studies on best practices for community screening and referral.4
A ready clinical trial infrastructure can help to speed up other aspects of the startup phase. These include centralized support functions, such as centralized institutional review boards (now required by NIH for most multisite trials) and contracting; systems for decentralized screening (online or at local community centers); and systems for electronic and staged consent processes. The development of clinical trial networks that use a hub-and-spoke model to centralize some core infrastructure can reduce the pragmatic challenges and burdens for investigators at trial sites embedded in communities (e.g., federally qualified health centers). To support the development of such networks, ADRCs could play a role in creating registries of regional clinical trial sites, and NIH-funded clinical trial consortia, if adequately supported, could provide training for clinical trial sites to disseminate knowledge, standards, and best practices.
Recommendation 3: Break down barriers to the acceleration of clinical research
The National Institutes of Health (NIH) should continue to lead efforts across a multiplicity of relevant entities (e.g., pharmaceutical and biotechnology companies, academia, foundations) to accelerate the movement of promising interventions for Alzheimer’s disease and related dementias (AD/ADRD) into clinical trials and to expand the use of innovative approaches to improve the efficiency of clinical trials. These efforts should include the following:
- Organize NIH investments in basic and translational research related to potential molecular targets for intervention into a portfolio to create a pipeline of validated targets that can be transitioned into drug development.
- Expand the use of innovative trial designs (e.g., master protocols, platform, combination, adaptive trials) and increase investment in both early-phase (phase 1b and 2) proof-of-concept trials and later-stage pragmatic trials.
- Identify and promulgate best practices for decreasing the barriers to, and time for, the clinical trial startup phase (e.g., decentralized participant screening, creation and use of prescreened cohorts and screen-enroll mechanisms, use of electronic
___________________
4 While beyond the scope of this report, it should be noted that an important issue that arises with regards to screening of individuals for participation in clinical research is the return of screening results to the individuals (NASEM, 2018).
- consenting procedures, centralized contracting, and institutional review board processes).
- Continue investing in innovative funding models, such as public–private partnerships, shared funding for global trials, and combined-phase funding, that support the progression of candidate interventions across the early-stage clinical research pipeline.
- Maximize coordination between NIH-funded AD/ADRD clinical trial programs and NIH-funded AD/ADRD centers (e.g., Alzheimer’s Disease Research Centers) and evaluate these centers for representative participant clinical trial enrollment.
STRATEGIES FOR ADDRESSING CROSSCUTTING BARRIERS THAT IMPEDE PROGRESS ON AD/ADRD SCIENTIFIC PRIORITIES
The committee was asked to identify key barriers to advancing AD/ADRD prevention and treatment and to highlight opportunities to address these barriers to catalyze advances across the field. In its examination of the AD/ADRD research landscape, several impediments to progress were consistently identified across the continuum from basic to clinical research. These crosscutting barriers include
- siloing within the AD/ADRD field and across related domains of research (e.g., aging, neurodegenerative diseases more broadly, exposure science);
- insufficient population representativeness and generalizability of research;
- inadequate infrastructure and support for management and analysis of data, samples, and knowledge generated from AD/ADRD research; and
- inadequate support for innovative methods capable of realizing transformational progress.
The sections below discuss opportunities and strategies for addressing these barriers. As detailed in each of the sections, the committee recognizes the significant NIH investment to address each of these key barriers. Examples of NIH activities highlighted in the sections below are meant to be illustrative and do not represent a comprehensive cataloging of such efforts as the committee was not charged with a review of NIH’s programs. It should also be acknowledged that many barriers are not unique to dementia research, and other scientific fields are also working to overcome similar challenges. Accordingly, in considering the implementation of the recommendations below, NIH and other research funders should
continuously monitor the broader research landscape for examples of how such challenges have been successfully tackled in other fields and consider opportunities to apply those strategies in AD/ADRD research.
Breaking Down Silos Through Collaborative, Multidisciplinary Research
The heterogeneity of AD/ADRD, the prevalence of mixed pathologies, and the multifactorial and intersecting nature of the diverse pathways that lead to disease all suggest that the path to effective strategies for preventing and treating this group of neurodegenerative diseases lies in collaborative, multidisciplinary research. Yet, throughout its information-gathering process the committee encountered numerous silos, commonly reinforced by funding structures, that impede efforts to elucidate the biological basis of AD/ADRD and advance prevention and treatment. Current funding strategies that target individual diseases, which have historically favored AD, fail to address the reality of overlapping and mixed pathologies that contribute to neurodegenerative disease, and they have contributed to the current dearth of effective therapies for related dementias. There have been few efforts to develop a larger integrated model of aging and neurodegenerative disease despite clear overlap in research endeavors and shared mechanisms (see Chapter 3). As discussed in Chapter 4, research on pharmacological and nonpharmacological interventions are not well integrated, and as a result there have been few efforts to date to evaluate the effect of combination approaches despite a high likelihood that risk reduction and drug therapies will both be necessary elements of a strategy to reduce the incidence and impact of dementia. Moreover, the efforts of federal agencies supporting related areas of research are not adequately coordinated, resulting in missed opportunities to leverage synergies and effectively use existing investments in studies and infrastructure. Innovative funding strategies, such as multi-institute research consortia and public–private partnerships, and other incentives, as well as the application of collaborative research mechanisms, and greater coordination and integration of research and infrastructure are needed to address the current siloing of research and accelerate the development of interventions for preventing and treating AD/ADRD. Disincentives to team approaches, such as academic promotion structures, also need to be addressed.
Funding opportunities that encourage collaboration across disease areas by bringing together multidisciplinary teams have the potential to accelerate the development of not only interventions that target common underlying mechanisms but also disease-agnostic resilience mechanisms, as discussed in Chapter 3. Supporting collaboration across disciplines also facilitates the application of approaches and technologies to address research questions and technical challenges in novel ways. Multi-institute research consortia represent one mechanism for fostering collaborative research and bridging
the divide between basic and clinical research (Gladman et al., 2019). For example, the Biomarkers for Vascular Contributions to Cognitive Impairment and Dementia Consortium (MarkVCID) was established to advance the discovery and validation of biomarkers for small vessel diseases of the brain involved in cognitive impairment and dementia, as cerebrovascular small vessel disease is a commonly identified pathology in mixed dementia (Greenberg, 2017; MarkVCID, 2017). Ultimately, the consortium seeks to deliver biomarker kits that can be used in intervention trials, thereby translating basic science findings into clinical research (Gladman et al., 2019). Diverse VCID is another example of a multi-institute research program focused on understanding the role that cerebrovascular disease plays in AD/ADRD for diverse populations with the goal of improving diagnosis and treatment (Diverse VCID, 2024). Multi-institute consortia often have the benefit of effectively using existing, mature research infrastructure to scale up research efforts. Coordinating centers play a key role in such consortia to centralize resources and facilitate harmonization, coordination, and data sharing across the multiple participating research institutions. When supported by multiple funders, research consortia also provide opportunities to align and better use existing resources and future investments across funding organizations.
Multi-institute research collaborations should not be limited to U.S. institutions. Dementia is a global challenge—the burden of which is borne in large part by people living in low- and middle-income countries (LMICs)—and it will not be overcome by the siloed efforts of individual countries (Nature Medicine, 2023). From a health equity perspective, international collaborations can help to address the problems of overrepresentation of some populations in AD/ADRD research and the underrepresentation of many global populations living outside North America and Western Europe. Underrepresented populations include those within LMICs but also in some high-income countries (e.g., within Asia and the Middle East). Such global collaborations can also help to uncover rare genetic variants and answer important scientific questions regarding the relative contributions of genetic ancestry and sociocultural factors (e.g., social determinants of health) to dementia risk and resilience. It should be acknowledged, however, that current international laws and regulations, including but not limited to the General Data Protection Regulation of the European Union, pose a formidable impediment to the reciprocal exchange of data and biological samples with researchers from other countries. While not insurmountable (and not limited to AD/ADRD), these issues are complex and require careful legal analysis. Investigator-level relationships can lead to some workarounds, but ultimately these barriers need to be addressed at the level of national governmental leaders. As a major global funder of biomedical (including AD/ADRD) research and intellectual leader, NIH can spearhead
efforts to overcome these barriers by engaging its counterparts in other countries and the broader research community to understand their needs and jointly develop practical mid- to long-term solutions. Such solutions will need to address mechanisms for data access (e.g., cloud-based mechanisms, federated learning platforms) and security (e.g., encryption, use of synthetic data), and the more challenging issues of biosample sharing.
Public–private partnerships create unique opportunities for cross-discipline collaboration (within the United States and globally) and are established specifically to more effectively leverage the respective talents of investigators in academia and industry toward a shared goal. A notable example in the AD/ADRD field is AMP-AD (see Box 3-4 in Chapter 3). Government and philanthropic funding for such partnerships can incentivize industry engagement in activities that might otherwise be considered too financially risky, as well as encouraging innovation (e.g., innovative trial designs) (Boxer and Sperling, 2023). While there are multiple mechanisms for establishing public–private partnerships, the Foundation for the National Institutes of Health (FNIH)—a nonprofit organization—specifically focuses on convening partners, including NIH, academic institutions, industry, philanthropy, and advocacy organizations (FNIH, 2023) and may be positioned to facilitate collaboration without creating financial conflicts of interest for academic researchers. Current gap areas that may benefit from public–private partnerships include efforts to develop combination interventions that include pharmacological and nonpharmacological components (see Chapter 4), and the development of a platform for real-world data collection (see Chapter 2).
Challenge programs are another powerful mechanism for facilitating interdisciplinary approaches while simultaneously pushing boundaries and driving further innovation in AD/ADRD research. Such programs often set ambitious goals and use prize money to incentivize investigators to tackle complex problems from novel perspectives. By encouraging rapid iteration and establishing feedback loops, challenge programs can push researchers to develop and refine ideas and strategies in relatively short periods of time. A recent example is the Pioneering Research for Early Prediction of Alzheimer’s and Related Dementias Eureka (PREPARE) Challenge, a multiphase competition that was launched by NIA in fiscal year 2023 with support from the NASA Tournament Lab (Driven Data, 2024).5 The objective of the challenge is to spur innovation in data science to advance solutions for accurate prediction of AD/ADRD in diverse and historically underrepresented populations. PREPARE is designed to bring teams together to explore how AI/ML and other computing approaches can be used to collect and analyze data
___________________
5 More information on the PREPARE challenge is available at https://www.drivendata.org/competitions/253/competition-nih-alzheimers-adrd-1/ (accessed July 2, 2024).
in ways that could advance the development of tools and technologies for clinical and research use in predicting disease. By encouraging team science approaches and risk taking, challenge programs such as PREPARE stimulate creativity within the scientific community and accelerate the translation of discoveries into tangible solutions. They can also serve as a means of bringing new talent into a field. Moreover, these initiatives often cultivate a culture of innovation within the research workforce by establishing a supportive ecosystem where participants can exchange ideas, bring to bear diverse expertise, and forge partnerships that transcend institutional and geographic boundaries. While challenge programs are not the answer to every knowledge gap, NIH should continue to employ this model where applicable.
Collaborative research mechanisms that engage people living with AD/ADRD and members of the community in which the study is being conducted are critical to ensuring that research on prevention and treatment strategies is conducted in alignment with what is important to those living with, or who are at risk for, the diseases. Such mechanisms come in many forms with the level of engagement varying across a continuum, from advisory bodies (e.g., patient and community advisory boards, focus groups) to community-based participatory research and coproduction models featuring shared decision making between researchers and participants at all phases (Reyes et al., 2023; UK Research and Innovation, 2024). The mechanism employed should be informed by the nature of the study. Advocacy and other organizations that serve people with AD/ADRD and their care partners are also key partners and can facilitate opportunities for the engagement of people with lived experience in research. The benefits are myriad and include enhancing understanding of the experiences, needs, and values of those being asked to participate in research; improving equity in AD/ADRD research; facilitating recruitment and retention of hard-to-reach populations; and informing researchers as to the acceptability and feasibility of both the intervention strategies and the research methods (Kowe et al., 2022; Reyes et al., 2023).
Coordination and collaboration at the program and project level is facilitated and may be incentivized by analogous efforts at the federal level. Collaboration among NIH institutes, centers, and offices and with other federal agencies, such as the Centers for Disease Control and Prevention (CDC), the Centers for Medicare & Medicaid Services (CMS), and the Department of Veteran’s Affairs, occurs through a variety of mechanisms with variable levels of formality (e.g., National Alzheimer’s Project Act Federal SubGroup, NIH-Wide Microphysiological Systems working group) (NIH, n.d, 2024a).
Greater coordination is needed to reduce siloing across the existing major NIH research investments to leverage the knowledge that has
been generated by these individual efforts to advance the AD/ADRD field more broadly. For example, AMP-AD functions in parallel to the AMP for Parkinson’s Disease and Related Disorders (AMP-PDRD) and the AMP for Amyotrophic Lateral Sclerosis (AMP-ALS). Similarly, other major AD/ADRD-related programs for genetics research, biobanking, and data infrastructure funded by NIA, NINDS, and other NIH institutes are not well integrated. Breaking down these silos to maximize return on these research investments will require action beyond that which can be driven at the investigator level and will instead require NIH and others leading these programs to actively incentivize opportunities for coordination and integration and the breakdown of any logistical and technical barriers.
Recognizing the existing mechanisms already in place and the challenges of establishing new interagency bodies (e.g., time for agency personnel), the committee encourages NIA, NINDS, and other NIH funders of AD/ADRD research to identify further opportunities to maximally leverage the strengths, resources, and unique capacities of other agencies to advance shared focus areas. For example, existing federal-level collaboration and cofunding among NIA, NINDS, and the National Institute of Environmental Health Sciences (NIEHS) related to exposome research and precision environmental health approaches to AD/ADRD risk reduction and disease prevention (Stetler, 2023) could create new opportunities and incentives for environmental scientists at NIEHS-funded centers to work with ADRCs to advance exposome research related to AD/ADRD. Expanded collaboration across NIA, NINDS, and the National Institute of Mental Health (e.g., building on the joint investments in the Psych-AD program6 [NIH, 2023b] and other cofunding opportunities [NIH, 2023c]) can accelerate efforts to elucidate interactions between mechanistic pathways contributing to the development of neuropsychiatric symptoms and those underlying the development of AD/ADRD neuropathology. Such efforts may uncover novel targets or strategies for intervention.
Examples of collaborations with other federal agencies might include (1) collaborating with the U.S. Census Bureau to expand access to federal statistical research data centers (FSRDCs) and facilitate the linkage of multiple data types relevant to AD/ADRD within the FSRDCs, (2) working with CMS or FDA to tie expedited review processes for industry to data sharing policies, and (3) working with the CDC to generate a more robust evidence base for public health-level interventions.
___________________
6 More information about Psych-AD is available on the AD-Knowledge Portal at https://adknowledgeportal.synapse.org/Explore/Programs/DetailsPage?Program=Psych-AD (accessed October 19, 2024).
Recommendation 4: Enable multidisciplinary, multisector, and collaborative research
The National Institutes of Health (NIH) should expand mechanisms and leverage existing resources to break down silos and encourage multidisciplinary and integrative Alzheimer’s disease and related dementias (AD/ADRD) research efforts, including the following:
- Expand trans-NIH initiatives and cofunded projects focused on healthy aging and neurodegenerative diseases to reduce the siloing of research efforts by individual institutes and centers, better cross-link and use existing resources, and address inconsistencies in data sharing policies across NIH institutes and centers while prioritizing data access.
- Prioritize research funding for projects with multidisciplinary research teams (e.g., basic and clinical researchers, population scientists, data scientists and artificial intelligence specialists, and those with lived experience) that address community-informed research questions.
- Expand collaborations globally, including but not limited to low- and middle-income countries and other countries less often involved in such collaborations, for both longitudinal research and clinical trials to better understand the biology of AD/ADRD and enhance the generalizability of findings to diverse populations.
- The National Institute on Aging and the National Institute of Neurological Disorders and Stroke should collaborate with the National Center for Advancing Translational Sciences and others to speed up the translation of research advances to clinical and public health practice and, in turn, expand new research inquiries through the collection of real-world evidence.
Fostering Inclusive Research
A recurring challenge noted throughout the previous chapters of this report is the inadequate diversity of participants included in AD/ADRD research. Lack of representation of specific subpopulations in clinical research is not a problem unique to dementia. Across numerous domains of biomedical research, there are large population groups that are less able to benefit from investments in clinical research and the resulting discoveries because they were not adequately represented in the studies that yielded those discoveries (NASEM, 2022). The result is limited generalizability of clinical research findings to the broader target population, impaired trust in the research enterprise, reduced understanding of the biological phenomena under study, clinical trial failures at later stages, and the
compounding of existing health disparities. As noted in a recent National Academies report, “An equitable clinical research enterprise would include trials and studies that match the demographics of the disease burden under study” (NASEM, 2022, p. 1).
As discussed in Chapter 1, the impacts of dementia are not experienced uniformly across the U.S. population (Brewster et al., 2019) or globally. In the United States, racial and ethnic disparities have continued to persist despite overall decreases in clinical dementia prevalence. Black and Hispanic people are more likely to develop clinical dementia compared to non-Hispanic White people (Chen and Zissimopoulos, 2018), and the lifetime risk for women is about twice that of men (Mielke, 2024). The prevalence of AD/ADRD is also increased in rural and lower-income areas (Powell et al., 2020; Wing et al., 2020). And yet, the populations that are disproportionately affected by dementia are persistently underrepresented in AD/ADRD research, both in observational studies and clinical trials (Gilmore-Bykovskyi et al., 2019; Godbole et al., 2023; Lim et al., 2023). The cascading effects on dementia research are myriad and include
- the limited understanding of the contributing factors (e.g., rare genetic variants) and pathways that increase risk and causally contribute to AD/ADRD in diverse populations, which may result in a focus on risk-reduction strategies and intervention targets that are not likely to be effective for some groups who are at higher risk of dementia;
- effects on the establishment of biomarker cutoffs for diagnosis and normative comparisons; and
- the limited generalizability of research findings (i.e., external validity) (Gianattasio et al., 2021), including safety and efficacy data from clinical trials (Canevelli et al., 2019; Franzen et al., 2022), which raises concerns regarding the potential to implement interventions that are ineffective or even unsafe for certain population groups.
Representative studies that incorporate measures of social determinants of health along with multiomics, cardiometabolic, AD and other dementia biomarkers, and cognition are urgently needed. Intensifying investment in understanding factors that contribute to cognitive decline and impairment in underserved populations will enable the implementation of comprehensive, innovative, accessible, and affordable therapies that will mitigate multiple mechanisms driving AD/ADRD.
Increasing the participation of underrepresented populations in dementia research has been a focus of past recommendations to NIH (NASEM, 2017, 2021), and it is clear that NIA, NINDS, and other funders of dementia research are committed to and actively working on closing this gap (Hodes,
2023). Several of the research implementation milestones established by NIH to support the goals of the National Plan to Address Alzheimer’s Disease (see Chapter 1) specifically address increasing the inclusion of diverse and underrepresented populations in AD/ADRD research.7 Many other milestones include a focus on diverse and higher-risk special populations (e.g., individuals with Down Syndrome). Additionally, NIA has indicated that investigator requests to submit large grant applications (i.e., grant application with direct costs totaling $500,000 or more for a single year of support) will receive priority review if they
(1) include proposed planned enrollment tables representative of the population affected by the disease/condition, and (2) are appropriately inclusive of racial and ethnic minority groups; participants across the lifespan; as well as other populations experiencing health disparities (Santora, 2023).
Given the multiple, interrelated factors that are associated with chronic underrepresentation of certain populations (e.g., some ethnic/racial, people with low socioeconomic or educational attainment), achieving greater inclusivity and accessibility in AD/ADRD research will require a multipronged approach. While many of the barriers are well known and NIH appropriately continues to support research to further elucidate these factors (Ashford et al., 2022, 2023; Mindt et al., 2023), such an approach will benefit from the regular analysis of recruitment, enrollment, and retainment outcomes. For example, the identification of factors that result in studies falling short of recruitment goals can help to identify strategies (e.g., oversampling, use of sampling frames) that can be used to overcome persisting impediments while avoiding the introduction of bias from the use of different recruitment practices for different subpopulations (Raman et al., 2021). Such efforts will ultimately help to build the science of recruitment. The implementation of standardized common data elements for recruitment- and enrollment-based factors could enable better ascertainment of biases at specific stages in the recruitment and screening processes (Manly et al., 2021).
Selection processes—resulting from targeted outreach, selective enrollment, and highly patterned attrition—make it difficult to understand how observations in a selected sample (i.e., study population) relate to the general
___________________
7 Implementation milestones specifically focused on inclusion of diverse and underrepresented populations in AD/ADRD research include Milestone 1.C: Population Studies: Diverse cohorts; Milestone 12.A: Recruitment: Diverse community partnerships; Milestone 12.K: Health Equity: Inclusion and retention of underrepresented populations in clinical research; and Milestone 12.L: Health Equity: Inclusion of diverse communities in AD/ADRD research. The AD/ADRD research implementation milestone database is available at https://www.nia.nih.gov/research/milestones (accessed July 1, 2024).
population of older adults (Gibbons et al., 2024). Statistical methods can enable generalization from a highly selected sample to more general target populations based on shared variables measured in the selected sample and the target population. Key to these approaches is having measurements that are identical in the highly selected sample and the target population. Many key demographics (e.g., race, ethnicity, age, gender) are measured with standardized questions, but some important characteristics, such as education and measures of health or cognition, may be measured differently across data sources. There has been little attention to using the same measures as are available in a surveillance-type study of the target population (e.g., the U.S. Census or the National Health and Nutrition Examination Survey), which could help generalize estimates from the highly selected sample to a more representative group. Thus, there is a need for standardized benchmark measurements to be incorporated into new and ongoing studies to evaluate and correct for selection bias.
One novel mechanism that may enable broad reach to a diverse population and mitigate some biases introduced by current recruitment practices is the establishment of an opt-in option for newly age-eligible individuals at the time of Medicare or Medicaid enrollment to receive information on AD/ADRD studies and invitations to contribute to research. While mailings to Medicare beneficiaries are already used in clinical trial recruitment strategies (Grill and Galvin, 2014), such an initiative could better target trial information to enrollees based on areas of interest and baseline data. Enrollees who opt in could be asked for a blood sample, a baseline digital cognitive test, and to fill out a basic life history questionnaire, all of which could be included in a repository. Screening and other data could be provided back to those who opt in as an incentive. Importantly, Medicare and Medicaid already cover routine costs that accompany clinical trial participation, a step that improved equitable access to clinical trials (Takvorian et al., 2021). A collaborative effort by NIH and CMS to establish the infrastructure for such an initiative would further advance health research equity and help to scale up efforts to improve the representativeness of AD/ADRD research.
In 2018, NIA released a National Strategy for Recruitment and Participation in Alzheimer’s and Related Dementias Clinical Research8 that aims to meet the goal of engaging “broad segments of the public in the Alzheimer’s and related dementias research enterprise, with a particular focus on underrepresented communities, so studies with an aim to better understand and eventually cure these disorders can successfully and more quickly enroll and retain individuals” (NIA, 2024a). This strategy and its
___________________
8 The National Strategy, along with the associated planning guide and online toolbox, is available at https://www.nia.nih.gov/research/recruitment-strategy (accessed July 1, 2024).
related planning guide outline best practices and potential resources for study sites and researchers in overcoming barriers to engaging with and retaining diverse and underrepresented populations in clinical research (NIA, 2024a). Practices highlighted in the strategy align well with those that have been emphasized in the published literature (Brewster et al., 2019; Davis and Bekker, 2022; Mindt et al., 2023) and were discussed with the committee during its public workshop (NASEM, 2024). Examples include the following:
- Establish relationships with communities, employing culturally and linguistically appropriate modalities and content during outreach efforts, and build trust.
- Incorporate community voices into all phases of the study (e.g., through such mechanisms as community advisory boards, community-science partnership boards, and community-based participatory methods).
- Embed trials in communities (particularly those with populations at increased risk), for example by working with federally qualified health centers.
- Take trial activities to the participants by expanding opportunities for virtual engagement, use of in-home testing kits (e.g., for blood sample collection), and digital/remote data collection to increase accessibility.
- Give back to communities, and compensate participants for their time.
To support such efforts, NIA also created an online toolbox of resources—the Alzheimer’s and Dementia Outreach, Recruitment, and Engagement Resources (ADORE)—that is based on the recommendations and practices outlined in the planning guide. Resources from this searchable repository include videos specific to diverse and underrepresented communities, outreach materials that can be adapted for use in local communities, and resources from other centers and organizations that can be used to improve recruitment and retention. Knowledge regarding the engagement of diverse populations in research can continue to be evaluated and practices optimized through the use of community-based participatory research projects and other approaches.
While these NIH efforts connect researchers to resources that can support more inclusive research, costs associated with employing these best practices may remain a barrier to adoption. The committee found limited examples of supplemental funding from NIH to expand the participation of underrepresented groups (NINDS, 2021, 2024). Ensuring adequate resources are budgeted for community engagement, recruitment, and the development
of culturally appropriate research tools is critical to furthering inclusive AD/ADRD research. Additional funding, including through supplemental funding, could be provided by NIH for recruitment of diverse populations and specific clinical trial financial needs not well covered by project budgets (e.g., transportation for participants, additional medical supplies needed by participants). Strategies for using technological advances to bring these best practices to scale in a cost-effective manner also need to be considered.
Building a diverse research workforce at all levels is another critical factor that was emphasized in the national strategy and planning guide (NIA, 2018, 2019a). Effectively engaging with communities requires insights into, and sensitivity to, the different perspectives and cultures represented therein. This cannot be achieved without a diverse and multidisciplinary study team (NASEM, 2021). Recognizing that inadequate diversity of the scientific workforce is a broader issue that transcends individual studies, NIH established and recently expanded the Resource Centers for Minority Aging Research program, which aims to provide career development (e.g., training, mentorship) to early-career scientists from diverse backgrounds who conduct research related to aging, AD/ADRD, and health disparities in older adults in the areas of social, behavioral, psychological, and economic research (NIA, 2024b). Acknowledging the work NIH is already doing to foster a diverse research workforce, continued efforts are needed to understand and mitigate the barriers to entry and ongoing career advancement into leadership positions. This will include system-level research on barriers, metrics for evaluation, and the expansion of such existing support mechanisms as paid internships and mentorships, collaborative research partnerships, competitive stipends, and salary support to retain researchers in academia).
The development of a diverse research workforce requires early-career professionals to choose to enter and advance their careers in the field. Inadequate compensation for trainees and postdoctoral researchers under NIH-funded awards, however, represents a significant financial barrier to entry into the biomedical research field that may deter pursuit of academic research careers and limit diversity within the research workforce (NIH ACD Working Group, 2023; Sainburg, 2023). Ph.D. training represents a financial burden, even when participating in a fully funded program or prestigious institutional or individual NIH-funded awards (e.g., T32, F31 or F99). Financial stressors extend into postdoctoral training with the rising costs of housing, childcare, and the desire to maintain a reasonable quality of life frequently cited as major career concerns. Between 2020 and 2022, there was a nearly 10 percent decrease in the number of NIH-funded postdoctoral researchers in the health and science fields in the United States (Gewin, 2023). The current NIH structure for postdoctoral stipends results in wages that are below the cost of living in major metropolitan areas and current rules for supplementing stipends from NIH training awards to bring
compensation up to a living wage requires the use of non-federal funds, which represents an additional barrier (NIH Office of Extramural Research, 2024; Sainburg, 2023). Relatedly, compensation following training years, in comparison to compensation that could be received in private industry with similar expertise, may not entice early-career researchers to initiate or continue to pursue a career in academic research (Sainburg, 2023). These early financial pressures, in combination with other factors (e.g., uncertain career prospects), disproportionately affect marginalized groups and act as impediments at all career stages to the development of a strong and diverse biomedical research workforce. These factors need to be better understood and swiftly addressed in the context of the AD/ADRD field.
Importantly, endeavors to foster a diverse research workforce need to be focused on overcoming systemic barriers, such as ethnic and racial disparities in the awarding of federal research funding (Ginther et al., 2011; Nguyen et al., 2023) and the lack of research infrastructure and protected faculty time for research at such institutions as historically Black colleges and universities and tribal colleges and universities. Such efforts not only help to address challenges related to underrepresentation in research but ensure that the scientific workforce benefits from the nation’s rich diversity of people and their broad range of perspectives and experiences.
Efforts to increase inclusive AD/ADRD research have largely focused on recruitment practices, with less attention given to other factors such as eligibility (Franzen et al., 2022) and retention (Gilmore-Bykovskyi et al., 2019). Exclusion based on eligibility requirements can have the undesirable effect of disengaging people who otherwise would have been interested and willing to participate in research and potentially limiting generalizability to the broader target population, as might be the case when comorbidities such as active depression and diabetes are used as exclusion criteria (Mitchell et al., 2024; Ritchie et al., 2015). Understanding that some eligibility criteria may be instituted to protect the safety of research participants, particularly in the context of clinical trials, consideration should be given to ways to overcome or work around common factors that contribute to attrition at the screening stage (e.g., waiving caregiver/care partner requirements if adequate cognitive function can be demonstrated through ongoing evaluation), particularly for members of underrepresented groups. For example, recent FDA guidance on enhancing the diversity of clinical trial populations suggests considering whether criteria used in earlier-phase trials can be eliminated or modified in later-stage studies (FDA, 2020). There also needs to be consideration as to how gaps in knowledge regarding AD/ADRD in diverse populations may exacerbate underrepresentation caused by eligibility requirements. In developing inclusion and exclusion criteria, study investigators also need to be cognizant of sex and gender differences and how those might influence recruitment and retention.
Accountability—for NIH and NIH-funded investigators—will be a key determinant of future success in these endeavors. On the front end, funding requirements can be used as policy levers to ensure inclusivity is a priority for investigators and considered from the outset of the study. For instance, in some cases restrictions on initiating data collection could be imposed until recruitment goals are met. On the back end, there also needs to be routine tracking of sociodemographic features such as gender, race/ethnicity, socioeconomic status, and geographic region of participants enrolled in NIH-funded AD/ADRD studies.
NIA’s Clinical Research Operations & Management System, a system for reporting, tracking, and management of enrollment data and study documents from NIA-funded clinical trials (NIA, 2023a), can aid in monitoring progress toward recruitment goals for the inclusion of underrepresented groups. Such data can inform the implementation and enforcement of policies to address the enrollment of diverse populations and help to identify best practices from successful studies. However, reporting requirements need to be broadened beyond clinical trials to include all study types involving human participants.
While there is some evidence to suggest that the efforts of NIH and those of the broader scientific community are starting to move the needle with regards to representation in AD/ADRD research, progress has been slow. Some measures of diversity in AD/ADRD-related studies are improving as compared to past decades (Lim et al., 2023), but this may not be consistent across all types of research (Franzen et al., 2022) or populations. It is imperative that NIH and AD/ADRD researchers continue to prioritize and incentivize inclusive research and increase accessibility for populations that are historically underrepresented despite being disproportionately affected by dementia.
Recommendation 5: Incentivize inclusive research
The National Institutes of Health should incentivize and guide the use of inclusive research practices to increase the accessibility of clinical and public health research and ensure that study populations are representative of populations at risk for and living with Alzheimer’s disease and related dementias (AD/ADRD). These efforts should include the following:
- Strengthen requirements for the recruitment of diverse populations as a condition for initiating data collection (e.g., use of sampling frames as a best practice for targeted and intentional outreach).
- Support research to further understand participant and institutional barriers to involvement in clinical research at all levels.
- Develop social determinants of health metrics to be used as measures of diversity.
- Incentivize the incorporation of standardized benchmark measurements that can be used to evaluate and correct selection bias into new and ongoing research studies.
- Work with the Centers for Medicare & Medicaid Services to explore Medicare and Medicaid enrollment as opportunities for data collection and for enrollees to receive information about participation in AD/ADRD research studies using an opt-in model.
- Support initiatives to identify and overcome barriers to entry and continued professional advancement for a diverse clinical research workforce.
Recommendation 6: Increase the accessibility and generalizability of clinical and public health research
Investigators supported by the National Institutes of Health (NIH) should adopt inclusive research practices to increase the accessibility of clinical and public health research and ensure that study populations are representative of populations at risk for and living with Alzheimer’s disease and related dementias (AD/ADRD). To increase research accessibility and generalizability, NIH-supported investigators should do the following:
- Reduce barriers to research participation (e.g., directing ineligible research volunteers to other studies, offering fair compensation, expanding opportunities for virtual participation and passive and/or remote data collection, using in-home testing kits).
- Eliminate unnecessarily restrictive exclusion criteria that screen out diversity in the study population.
- Invest in the development of long-term, mutually beneficial relationships between research institutions and communities, and embed trials sites in communities with underrepresented populations (decentralized trials).
- Meaningfully engage and incorporate the perspectives of research participants and their communities throughout the research design and execution process (e.g., through patient or community advisory councils, codesigning research, community-based participatory research methods, use of community members such as promotoras or health navigators to collect data).
Enhancing the Accessibility and Usability of Biological Samples, Data, and Knowledge to Maximize the Returns from AD/ADRD Research
The billions of dollars in funding from NIH and others that has supported scientific investigations in the dementia field and the development of a robust AD/ADRD research infrastructure represents a significant national investment. Careful stewardship of that investment requires attention to opportunities to maximize returns in the form of knowledge, scientific progress, and, ultimately, benefit to society, including those at risk for or living with AD/ADRD. This involves ensuring that the products of research—including biological samples, raw data, and findings—are accessible to, and usable by, the broader scientific community for the purpose of knowledge generation. When data and samples are siloed and sequestered within individual research groups, the kind of collaborative and integrative research called for by the committee cannot be achieved. Sharing processes are likely to differ for finite biological samples as compared to raw data collected through digital technologies (e.g., voice recordings, data from wearable devices and in-home sensors) that can be used indefinitely and simultaneously by multiple data users without losing value over time. Also critical is the compilation and synthesis of knowledge in such a manner that it can be easily accessed and used to draw insights to guide future research and inform clinical care.
Data Access and Usability
The usability of data for future research generally depends on their accessibility, ability to be linked to or compared with other data, and the availability of tools that enable analysis. Small but meaningful signals may only be detectable when data are aggregated into large datasets. Thus, the ability to aggregate data and analyze large datasets with powerful computational tools will enable the generation of new scientific insights (NIA, 2023b). Such capabilities have been significant focus areas for NIH. NIH has affirmed its commitment to open science and open-source principles (Jorgenson, 2023) and is working to apply the FAIR (findable, accessible, interoperable, and reusable) principles in its efforts to create a more integrated data ecosystem to support AD/ADRD research (NIA, 2023b; Wilkinson et al., 2016).
In 2020, NIH released an updated policy on data management and sharing, which went into effect in 2023 (NIH, 2020). The data management and sharing policy requires investigators to submit a data management and sharing plan as a component of an award application and to comply with the plan following NIH approval. The plan is required to specify how scientific data and associated metadata will be managed and shared, with consideration to potential restrictions or limitations on data sharing (NIH, 2020).
In recognition of the gap between the requirements for data sharing and real-world practices, NIH is also investing in research to better understand the factors that pose barriers to or facilitate data sharing (NIH RePORTER, 2023a). Such barriers may include concerns regarding the inability to control how data will be used, potential effects on the ability of the original investigators to publish their analyses of the data, the need to protect participant privacy and confidentiality, and the substantial resources and effort required to share data (Alzheimer Europe, n.d.; Arias et al., 2024). Such barriers point to the importance of data infrastructure and adequate financial support to cover costs, as well as culture, for aligning data sharing practices with current policy.
Cultural barriers to data sharing are a broader issue within the scientific community and beyond the scope of this report. Still, it is worth emphasizing the need for incentives to drive meaningful compliance with existing policies and ensuring consistency across data sharing policies (e.g., across different NIH institutes and centers). Academic reward systems in particular need to place greater value on data sharing. For instance, reuse of datasets could be given similar status as publication citations, but this necessitates a system to ensure original data generators are credited when their data are reused (Pierce et al., 2019).
Reflecting the substantial breadth of NIH-funded AD/ADRD research initiatives, AD/ADRD data infrastructure investments by NIH have included a number of different platforms and repositories to support data storage and accessibility (see Box 5-1). For example,
- All ADRC data (e.g., fluid biomarker, neuroimaging, neuropathology, and clinical data) are stored in the National Alzheimer’s Coordinating Center (NACC). Such data, including those derived from NIH-supported longitudinal cohort studies, have been critical to fluid and imaging biomarker discovery efforts.
- Data generated by the Alzheimer’s Disease Sequencing Project (ADSP) consortia (the AI/ML Consortium, the Phenotype Harmonization Consortium, and the Functional Genomics Consortium) and projects are deposited in NIA data repositories, including the NIA Genetics of Alzheimer’s Disease Data Storage Site (NIAGADS).
- Multiomics data collected through such programs as AMP-AD and MODEL-AD are stored in the AD Knowledge Portal and are informing drug development, beginning with target identification and validation and continuing through all phases of clinical trials (Hodes and Buckholtz, 2016; NIA, 2023c).
A key challenge before NIH is to link its major data hubs (e.g., NIAGADS, NACC, Laboratory of Neuro Imaging [LONI]/ADNI, AD Knowledge Portal)
BOX 5-1
Select NIH-Supported Efforts to Strengthen Data Infrastructure and Promote Data Access and Usability for Alzheimer’s Disease and Related Dementias (AD/ADRD) Research
AD Knowledge Portal: The National Institute on Aging (NIA)supported AD Knowledge Portal is a centralized platform maintained by Sage Bionetworks that connects investigators to data, resources, and tools generated from the programs that make up NIA’s Alzheimer’s Disease Translational Research Program (see Chapter 3) (AD Knowledge Portal, 2024a). Some of these programs include the AMP-AD, TREAT-AD, M2OVE-AD, MODEL-AD, and Resilience-AD. The portal provides information on available data from studies associated with these programs, as well as computational tools and target-enabling resources, among other resources (AD Knowledge Portal, 2024b). The portal also supports the integration of a number of external data analysis platforms to better facilitate the usability and interoperability of data from the portal (AD Knowledge Portal, 2024c). Additionally, the portal provides information about, and navigation to, additional resources and tools that can be found on other platforms (e.g., the Alzheimer’s Disease Preclinical Efficiency Database, Agora, AD Atlas, NIAGADS) (AD Knowledge Portal, 2024a).
Agora: Agora is an NIA-funded, interactive web platform managed by Sage Bionetworks that hosts high-dimensional transcriptomic, proteomic, and metabolomic data and novel drug targets for AD. The over 900 novel drug targets included on the platform were nominated by researchers working in the AD/ADRD field, including researchers involved with AMP-AD (see Box 3-4) and TREAT-AD (see Box 3-5) (Agora, 2024a). The web application provides several different interfaces that can be used by investigators to explore genes and targets, such as a gene comparison tool through which detailed expression information can be viewed and compared (Agora, 2024b).
National Institute on Aging Genetics of Alzheimer’s Disease Data Storage Site (NIAGADS): NIAGADS is supported by a cooperative agreement between NIA and the University of Pennsylvania and serves as a national repository for genotypic data (e.g., data from genome-wide association studies, next-generation sequencing, targeted genome sequencing) to facilitate research on including the discovery of new therapeutic approaches (NIAGADS, 2024a). All NIA-funded studies of genetics of late-onset AD are required to deposit data at NIAGADS and/or a different site approved by NIA (2023d). As of October 2024, the continuously updated repository includes 128 datasets and over 23 types of data (NIAGADS, 2024b). In addition to storing and facilitating access to and the exploration of these data, NIAGADS connects investigators
to other datasets stored on other platforms (e.g., imaging data from the Alzheimer’s Disease Neuroimaging Initiative) (NIAGADS, 2024a).
National Alzheimer’s Coordinating Center (NACC): NACC is an NIA-funded resource and hub for research coordination housed at the University of Washington that serves as a centralized data repository for ADRCs (NACC, 2024a), which contribute data from enrolled participants to the NACC uniform dataset (UDS) (NACC, 2024b). The UDS contains data from all participants enrolled by the ADRCs since 2005; importantly, these data do not represent a representative sample of the U.S. population. In 2012, NACC launched a voluntary Frontotemporal Lobar Degeneration module, followed by similar modules for Lewy body disease and Down syndrome in 2017 and 2020, respectively (NACC, 2024b). The NACC data platform is undergoing significant modernization to enable greater integration of data streams and data linking across different repositories (e.g., National Centralized Repository for Alzheimer’s Disease and Related Dementias, NIAGADS) with ADRC data, as well as providing tools to facilitate data searching, visualization, and access by investigators (NACC, 2024c).
into an agile, integrated data ecosystem while preserving the autonomy of the individual platforms and their respective strengths and networks (NIA, 2023b), with an emphasis on the inclusion of repositories with data on related dementias. This integrated system should enable researchers without deep data analytics expertise to locate, access, and query existing data from NIH-funded research and, when available, data submitted by other investigators. This is a formidable undertaking but critical to maximizing insights from AD/ADRD research and returns from NIH’s various investments. Insights captured during recent NIH-hosted workshops on the topics of data infrastructure and interoperability (NIA, 2023b, 2024c) can guide these efforts.
Given the diversity of data types, data sources, and constraints such as privacy protection needs, there is no single solution to data management and accessibility. For example, although AI/ML models may facilitate the sharing and processing of digital data, commercially available platforms lack transparency and have associated privacy and security concerns. It will be important for NIH to work with investigators to identify solutions to data access challenges. While many datasets can and should be made publicly available, for others, such as clinical datasets with protected health information generated by private health systems, data accessibility may
need to be achieved through other means (e.g., federated data analyses). One solution to data that cannot be shared is to enable code generated by external investigators (generated, for example, using a synthetic dataset) to be run using a secure system that outputs the results from the executed program without allowing the investigator to see the data being analyzed. Consideration could also be given to categorizing data into access levels. At the lowest level would be those data that can be made freely available without any permission process to access them. Higher levels would involve increasingly more stringent access permission processes based on the sensitivity of the data and privacy/confidentiality concerns. For datasets that include both sensitive and nonsensitive data, such an approach may improve access to those data that are not sensitive by reducing hurdles associated with permission processes.
Accessibility is necessary but not sufficient to ensure that data from past AD/ADRD research are usable to the fullest extent possible. A key challenge has been the lack of interoperability and comparability of data from different studies, which impedes data integration and cross-study analyses. Investments in basic and preclinical research and longitudinal cohort studies have resulted in the generation of large and diverse (e.g., genetic, exposome, functional omics) datasets that can guide the development of effective interventions for AD/ADRD. A notable challenge, however, is the harmonization and integration of data to make them available and ready for analysis so that the many potential targets can be comparatively evaluated and prioritized for intervention development, with consideration of the needs of diverse populations.
One way NIH has sought to make data more comparable is through the standardization and harmonization of data collection tools and outcome measures (e.g., common data elements [CDEs]). The NINDS Common Data Elements project, for instance, had resulted in the creation of data standards for neuroscientific clinical research, including both general and disease-specific CDEs (NINDS, n.d.). Another example of such efforts is the Harmonized Cognitive Assessment Protocol (HCAP) (see Box 2-11 in Chapter 2), the design of which was led by the investigators of the Health and Retirement Study with the goal of creating a flexible instrument for detailed cognitive assessment of older adults that would be comparable across populations in diverse contexts (e.g., cultural, educational, social, economic, political) (Gross et al., 2023). In the digital data space, NIH is seeking to develop standards for wearable/sensor data and automated scripts that can convert raw data into a standardized format to facilitate integration with other patient data types (e.g., clinical, molecular, digital) (NIA, 2019b, 2024d). The status of these efforts is unclear, however, and expansion of data standardization and harmonization remains a priority. In February 2023, NIA convened an expert meeting to explore the use of
CDEs to harmonize data from real-world-data (RWD) sources, such as health care claims and electronic health records, with the goal of accelerating AD/ADRD research (Lieberthal et al., 2023). Looking to the future, new harmonization methods will be required to meet the needs of a broad range of analytic approaches (e.g., biostatistics/epidemiology, data science/AI, and other emerging methods such as quantum computing) used for both structured and unstructured data (e.g., digital images and raw digital data streams such as voice recordings).
With the growing volume and diversity of data being generated through AD/ADRD research, data integration and analysis are requiring increasingly complex tools and analytic methods. Such capabilities will be needed to advance precision medicine approaches, for example, by enabling the extraction of patterns that could guide population stratification, matching of subgroups to interventions, and life-course timing for those interventions. AI/ML and other computational methods (e.g., network analysis) hold great promise for enabling the linkage and subsequent extraction of insights from large and complex datasets to inform intervention strategies. These methods need to be biologically informed and relevant to clinical phenotypes, which will require close collaborations between AI/data scientists and biomedical researchers (Recommendation 4).
Academia is well suited to the experimentation and small-scale, proof-of-concept development that can lead to novel analytic tools but may lack access to the computational resources available in private industry (NASEM, 2024). Thus, there is a need for national-level resources that can support the development of novel, open-source data acquisition, ingestion, cleaning, processing, and harmonization tools to deliver data in a sufficient form for different analytic methods. Some initiatives that could meet this need are already underway. The National Artificial Intelligence Research Resource (NAIRR) pilot, an NSF-led, multipartner effort—including federal agencies and private-sector, nonprofit, and philanthropic partners—to develop a proof of concept for shared national AI research resources, represents a first step in in this direction. As part of the NAIRR pilot, NIH will co-lead with the DOE an operational focus area on data privacy and security and “provide open and controlled-access to NIH computing and data platforms and biomedical datasets to support health care-focused AI research (NSF, 2024).
Additionally, in 2021, NIA awarded funds for the creation of Artificial Intelligence and Technology Collaboratories (AITCs), which serve as national resources to “promote the development and implementation of artificial intelligence approaches and technology through demonstration projects to improve care and health outcomes for older Americans, including persons living with dementia and their caregivers” (NIA, 2023e). The AITCs are developing approaches for the analysis of raw digital data; creating policies
and best practices for the incorporation of AI in partnership with industry, venture capitalists, and health care systems; and piloting new tools and technologies in collaboration with these partners and the NIA Small Business Programs (NIA, 2023e). The AITCs operate through pilot awards and are intended to create an ecosystem that fosters innovation and transdisciplinary collaboration spanning academia and industry (Abadir et al., 2024; NIH RePORTER, 2023b). In the longer term, NIH will need to evaluate the success of such innovative models and their potential role in broader efforts to use AI/ML and other technologies to accelerate data integration and knowledge generation.
Recommendation 7: Ensure data access to maximize research returns
Using the National Institutes of Health (NIH) Data Management and Sharing Policy as a foundation, NIH should convene and support an NIH workgroup to work with NIH-funded investigators to identify and implement solutions to barriers that impede access to data from Alzheimer’s disease and related dementias research. Specific issues that should be addressed by the NIH workgroup include but are not limited to the following:
- the need for a centralized and continuously updated NIH-managed system for locating and searching existing data sources across different (NIH and non-NIH) data platforms;
- provision of incentives and clear procedures for ensuring compliance with the Data Management and Sharing Policy;
- approaches to maximize access to data from initiatives funded by multiple NIH institutes and centers;
- incentivization of transparent reporting and the synthesis of findings from negative studies, including observational studies and clinical trials, ideally with accompanying data release;
- provision of project-specific supplemental funding, including additional administrative supplements, commensurate with the anticipated level of data and code sharing;
- formulation of guidance for subsets of data within any given dataset to be categorized into access levels based on the access controls needed to protect sensitive data (e.g., participant health information) such that the portion of data requiring no permission for use can be made publicly available;
- return of derived data and analysis code from data users, as well as the return of newly collected data from ancillary studies, to the parent study while respecting the need to protect intellectual property and innovation;
- approaches to facilitate access to data from international collaborations; and
- expansion of capacity for storing raw digital data (e.g., unstructured data such as images and high-velocity voice recordings and sensor data).
Recommendation 8: Enhance data usability for future research
To enable the usability of data generated by Alzheimer’s disease and related dementias research funded by the National Institutes of Health (NIH), NIH should do the following:
- Invest in data harmonization and interoperability efforts (e.g., use of common data elements) across data platforms and through collaborations across institutions and organizations, ensuring that levels of harmonization are aligned with the needs of different analytic approaches.
- Set requirements for user-intuitive data dictionaries.
- Explore new approaches, such as natural language processing, to automate the integration of different data types (e.g., clinical phenotype, multiomics data, exposure data).
- Fund the development and dissemination of novel open-source tools and analytic methods (e.g., large language models and other artificial intelligence/machine learning methods, statistical transport methods, data fusion approaches, synthetic data) to collect, link, explore, and query existing data and support efficient analyses when data privacy rules create barriers.
- Provide dedicated grants for investigators working in settings with proprietary data that are difficult to share (e.g., major clinical datasets) focused on data curation or supporting analyses by external researchers.
Biobanking
The continued evolution of technology, tools, and analytic methods will create new opportunities to analyze previously stored biological samples in ways that may not be possible to predict at present. Such future analyses may lead to the development of novel therapeutics or biomarkers. Consideration of this future value of biological samples collected from research participants or donated by other members of the public is important when investing in infrastructure and plans for collection and storage. For example, attention needs to be given to the harmonized collection and storage of pre- and postmortem samples from across cohorts so that variation in collection and storage procedures does not introduce undesired variability that compromises the comparability of data from the later analyses of those samples. Furthermore, stored samples have little value if they are not accessible to the scientific community. Ensuring accessibility entails the
development of transparent inventories of samples that are available to external investigators and clear processes for sample requests and decision making on sample sharing. As described earlier, access to biological samples from international collaborations remains a priority barrier to be addressed.
NIH has made substantial investments in infrastructure for biobanking, as described in Chapter 2. However, critical gaps remain, and initiatives and resources appear fragmented. Research institutions outside of the ADRCs may lack the pathology laboratory infrastructure to collect certain valuable samples (e.g., well-characterized brain tissue) from decedents enrolled in longitudinal studies. With adequate support, ADRCs and other large NIH-funded programs such as the Alzheimer’s Clinical Trials Consortium could expand autopsy and brain collection services to participants enrolled in studies at other institutions. Such a system may help to address the current dearth of samples from diverse participants but would need to ensure that costs for autopsy and transportation to and from the donation site do not fall on the research participants or their families. Additionally, capacity to store biosamples, also a cost-intensive undertaking, is limited. As a result, precious samples may be discarded at the completion of studies. Repositories may be sent sample collections with little advance notice and through processes that are ad hoc.
Maximizing the use and value of biological samples will require a more structured and standardized system for collection, archiving, and access. Biosample sharing systems should also require the return of results from analyses of shared samples back to the parent study so that these new data are made available along with any previously generated phenotypic and other data related to the shared sample (see Recommendation 2). This will ensure future requests for biosamples are made in the context of knowing what has already been measured and will avoid duplicate measurements on precious samples.
Technologies that have enabled the digitization and computational analysis of histopathological samples are transforming the practice of neuropathology. The portability and rapid transferability of digital image files in comparison to traditional slides enhances the accessibility of these valuable data sources (Scalco et al., 2023). The capability to use AI/ML for analysis of whole slide images opens opportunities to shift to quantitative measurement of the neuroanatomic distribution of pathologies (Shakir and Dugger, 2022). This kind of scalable quantitative method has the potential to allow deeper phenotyping of AD/ADRD and the correlation of specific clinical phenotypes to patterns of neuropathology. Incorporation of digital pathology methods into cohort studies is just getting underway, and digitization of existing slide inventories at ADRCs has been slow. ADRC personnel have reported a lack of resource support for digital pathology and ML (Scalco et al., 2023). NIH can advance AD/ADRD
disease phenotyping and the current understanding of the neuropathologic landscape by ensuring adequate support for the implementation and refinement of digital pathology methods. Importantly, infrastructure support needs to go beyond equipment (e.g., slide scanners, data storage/servers) and include the full range of expertise needed for implementation (e.g., pathologists, ML engineers, information technology personnel, statisticians, and database managers) (Shakir and Dugger, 2022). These efforts should be guided by the existing ADRC Digital Pathology Working Group (Scalco et al., 2023).
Recommendation 9: Expand the capacity to collect and store biological samples generated through National Institutes of Health (NIH)-funded research
The National Institute on Aging, along with the National Institute of Neurological Disorders and Stroke, should expand support for the collection and storage of valuable biological samples from NIH-funded Alzheimer’s disease and related dementias research in a manner that maximizes opportunities for future use. This should include the following:
- Provide supplements to researchers that meet the actual cost of storing and sharing samples following study completion.
- Expand support for the collection and storage of highly characterized biological samples (e.g., antemortem and postmortem blood and cerebrospinal fluid, donated brains) from participants of any longitudinal research studies and clinical trials, and from the public.
- Use standardized sample collection, assessment, and storage practices with careful consideration of the implications of different storage approaches for future value.
- Facilitate access to biological samples from international collaborations.
- Support digitized neuropathology to enable quantitative analysis using artificial intelligence and other computational methods.
Catalyze Transformational Change Through Innovation in AD/ADRD Research
Accelerating progress in AD/ADRD prevention and treatment will require transformational change that can only be achieved through greater support for innovation in NIH-supported research. Such efforts may look different at different stages in the research continuum and would include the following:
- Basic research: developing and applying novel models and tools, seeking potential points of connection and commonalities with related fields (e.g., aging, other neurodegenerative diseases).
- Translational research: increasing the viability of innovative research targets and approaches.
- Clinical research: adopting innovative trial designs and participant recruitment and engagement mechanisms.
- Population research: identifying and integrating novel data sources that can be used to evaluate population-level strategies (e.g., policies or exposures that vary across larger geographic units) and effects on inequalities.
The current system for peer review at NIH favors investigators and projects for which there are strong track records and evidence for likely success based on existing preliminary data. Although the current process has many advantages, it is not ideally suited to promoting innovation and truly novel methods. Incentives and novel strategies are needed to promote more disruptive research approaches that may lead to significant steps forward.
Agencies such as the Defense Advanced Research Projects Agency and the Advanced Research Projects Agency for Health (ARPA-H) are specifically focused on high-risk, high-reward research to generate innovative solutions to complex problems. While the committee’s charge is to recommend research priorities for NIH, ARPA-H, with its focus on accelerating better health outcomes, holds promise for advancing the kind of transformational research that is needed to accelerate the prevention and treatment of AD/ADRD. Indeed, the Advisory Council on Alzheimer’s Research, Care, and Services recommended that ARPA-H “implement a dementia portfolio that enables the translation and demonstration of scientific breakthroughs in the diagnosis, treatment, and management of dementias and facilitates efficient translation of evidence to patient care” (Advisory Council on Alzheimer’s Research, Care, and Services, 2024, p. 21).
As an example of its potential for breakthroughs in dementia treatment, in April 2024 ARPA-H forayed into the field of neurodegenerative diseases with its funding of a new project on Cell Therapies for Neuroinflammation and Neurodegeneration (CT-NEURO) (ARPA-H, 2024a). The CT-NEURO program aims to develop an immune cell-based, disease-agnostic platform that can deliver therapeutics to the brain and central nervous system to treat diverse neurological conditions, including neurodegeneration. As projects such as CT-NEURO come to completion, NIH should be prepared to transition relevant innovations into its portfolio of ongoing AD/ADRD research. For example, another potential ARPA-H project of relevance is the advancement of digital histopathology capabilities including automated analysis using AI/ML (NASEM, 2024). While envisioned as supporting the
Cancer Moonshot initiative, such a capability also has clear relevance to neurodegenerative disease research and practice (see Recommendation 9).
Of note, because ARPA-H-funded research is time delimited by design, transition of the resulting scientific and technological breakthroughs into real-world application is an integral part of the life cycle of ARPA-H projects and programs. The Project Accelerator Transition Innovation Office (PATIO) helps ARPA-H awardees identify investors and customers but also works with CMS and FDA to develop strategies for regulatory approval (e.g., for technologies that may not fit into traditional FDA pathways) and reimbursement (ARPA-H, 2024b). This approach creates the necessary demand–pull conditions to ensure innovations reach the market (Pannu et al., 2023). The absence of a similar transition model for innovative technologies (e.g., digital technologies for AD/ADRD diagnosis) arising from NIH-funded research serves as a barrier to the financial sustainability of the innovative product and future product development.
The NIH Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) funding mechanisms and the NIH Blueprint for Neuroscience Research program provide some support for converting research ideas into commercially viable products. For example, in addition to providing funding for drug discovery and development, the Blueprint Neurotherapeutics Network offers access to consultants with extensive industry drug development experience (NIH, 2024b). Such mechanisms, however, fall short of addressing the needs around nontraditional regulatory pathways and reimbursement, gaps that warrant NIH attention.
Funding mechanisms such as SBIR/STTR and NIH Blueprint function to derisk investment in innovation. The phased funding in SBIR, for example, enables awardees to establish the scientific merit, feasibility, and commercialization of the proposed research in phase 1 (approximately 0.5–2 years) prior to receipt of a larger and longer-term (1–3 years) funding award for phase 2 (NIH, 2024c). A fast-track path allows a single application to cover both phases. Some philanthropic funders also seek to derisk novel approaches by funding proof-of-concept research and pilot-scale projects to generate the initial data needed to secure longer-term funding, including follow-on awards from NIH (FNIH, 2023).
These kinds of phased funding approaches, which can involve a review of a priori-specified milestones prior to transition to phase 2 (NASEM, 2015), could be adapted to further promote innovative and high-risk AD/ADRD research. For example, partnerships between NIH and philanthropic groups could create a seamless transition from philanthropic proof-of-concept funding to NIH grant funding. Additionally, NIH and the FNIH could collaborate to build private–public partnerships for fast-tracked phase 1–2 high-risk research opportunities. In such a model, NIH could fund phase 1 with the goal of lowering the risk for the phase 2 component, which would
be focused on scalability and could largely be funded through industry. Public–private partnerships enable the leveraging of the respective talents of industry and academia and best-in-class practices (e.g., data infrastructure and knowledge management, social engagement) from other sectors.
Other funding mechanisms to support innovation could include supplements focused on critical scientific areas or technologies and R56 awards (or similar) focused on high-risk, high-reward research. The R56 mechanism would allow discrete pieces of an R01 application that include novel ideas to be funded in cases where the full application did not meet criteria for an R01 award.
Recommendation 10: Support innovation across all stages of National Institutes of Health (NIH)-funded research
NIH should use existing funding structures and other incentive mechanisms to stimulate innovation across all stages of Alzheimer’s disease and related dementias (AD/ADRD) research. This could include the following:
- Implement advances and tools generated by the Advanced Research Projects Agency for Health and others into NIH-funded AD/ADRD research, including advances that are specific to dementia and those that can be applied from other fields.
- Field a program-wide review of the opportunities and barriers to interdisciplinary and transformational research at NIH-funded AD/ADRD centers and infrastructure programs (e.g., the Alzheimer’s Disease Research Centers) and their capacity to prioritize the inclusion of diverse populations and foster innovative research with high potential for population impact.
- Capitalize on the best-in-class practices and technologies of other fields that are applicable to, and may address, current AD/ADRD research needs (e.g., data infrastructure and knowledge management, social engagement for recruitment).
- Prioritize support for research inquiries that have clear potential for future scalability and uptake.
- Build partnerships with foundations and other research funders to coordinate seamless funding pathways for fast-tracked phase 1–2 high-risk research opportunities.
- Identify and provide short-term funding for specific, highly innovative components of otherwise unsuccessful new and competing award applications.
- Identify past funded projects in the NIH portfolio that have progressed to real-world clinical implementation and adapt the grant review process to include criteria that promotes real-world clinical implementation.
CONCLUDING REMARKS
The last decade of research has seen encouraging progress in the capability to detect early signals of changes in brain health, to understand the pathophysiologic mechanisms underlying AD/ADRD, and to develop and evaluate preventive and therapeutic interventions. Accelerating progress in AD/ADRD prevention and treatment will require transformation and new direction. With continued strategic research investments as outlined by the committee, there is good reason to hope that the coming years will see significant progress in the capability to prevent and treat AD/ADRD. Through the continued and collaborative efforts of NIH, academic researchers, private industry, health care professionals, funders, policy makers, advocates, and people living with cognitive and other forms of impairment from AD/ADRD, it is possible to envision a future where dementia is not inevitable for millions of people across the globe but is preventable and treatable.
REFERENCES
Abadir, P., E. Oh, R. Chellappa, N. Choudhry, G. Demiris, D. Ganesan, J. Karlawish, B. Marlin, R. M. Li, N. Dehak, A. Arbaje, M. Unberath, T. Cudjoe, C. Chute, J. H. Moore, P. Phan, Q. Samus, N. L. Schoenborn, A. Battle, and J. D. Walston. 2024. Artificial intelligence and technology collaboratories: Innovating aging research and Alzheimer’s care. Alzheimer’s & Dementia 20(4):3074-3079.
AD Knowledge Portal. 2024a. Welcome to the AD Knowledge Portal. https://adknowledgeportal.synapse.org/ (accessed August 22, 2024).
AD Knowledge Portal. 2024b. Explore. https://adknowledgeportal.synapse.org/Explore/Studies (accessed October 29, 2024).
AD Knowledge Portal. 2024c. Analysis platforms. https://adknowledgeportal.synapse.org/Analysis%20Platforms (accessed August 22, 2024).
Advisory Council on Alzheimer’s Research, Care, and Services. 2024. Public members of the Advisory Council on Alzheimer’s Research, Care, and Services: 2024 recommendations. https://aspe.hhs.gov/collaborations-committees-advisory-groups/napa/napa-documents/napa-recommendations (Accessed on November 11, 2024).
Agora. 2024a. About. https://agora.adknowledgeportal.org/about (accessed August 22, 2024).
Agora. 2024b. Gene comparison tool. https://agora.adknowledgeportal.org/genes/comparison (accessed August 22, 2024).
Alzheimer Europe. n.d. Data sharing in dementia research—the EU landscape. https://www.alzheimer-europe.org/policy/positions/data-sharing-dementia-research-eu-landscape?language_content_entity=en (accessed September 19, 2024).
Amini, S., B. Hao, J. Yang, C. Karjadi, V. B. Kolachalama, R. Au, and I. C. Paschalidis. 2024. Prediction of Alzheimer’s disease progression within 6 years using speech: A novel approach leveraging language models. Alzheimer’s & Dementia 20(8):5262-5270.
Arias, J. J., A. M. Tyler, L. M. Beskow, M. C. Carillo, S. Dickinson, J. Goldman, M. A. Majumder, M. M. Mello, H. M. Snyder, and J. S. Yokoyama. 2024. Data stewardship in FTLD research: Investigator and research participant views. Alzheimer’s & Dementia 20(4):2886-2893.
ARPA-H (Advanced Research Projects Agency for Health). 2024a. ARPA-H funds project to develop treatments for neurodegenerative diseases. https://arpa-h.gov/news-and-events/arpa-h-funds-project-develop-treatments-neurodegenerative-diseases (accessed August 21, 2024).
ARPA-H. 2024b. Project Accelerator Transition Innovation Office (PATIO). https://arpa-h.gov/engage-and-transition/patio (accessed August 21, 2024).
Ashford, M. T., R. Raman, G. Miller, M. C. Donohue, O. C. Okonkwo, M. R. Mindt, R. L. Nosheny, G. A. Coker, R. C. Petersen, P. S. Aisen, M. W. Weiner, and the Alzheimer’s Disease Neuroimaging Initiative. 2022. Screening and enrollment of underrepresented ethnocultural and educational populations in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimer’s & Dementia 18(12):2603-2613.
Ashford, M. T., D. Zhu, J. Bride, E. McLean, A. Aaronson, C. Conti, C. Cypress, P. Griffin, R. Ross, T. Duncan, X. Deng, A. Ulbricht, J. Fockler, M. R. Camacho, D. Flenniken, D. Truran, S. R. Mackin, C. Hill, M. W. Weiner, D. Byrd, R. W. Turner II, H. Cham, M. Rivera Mindt, and R. L. Nosheny. 2023. Understanding online registry facilitators and barriers experienced by Black brain health registry participants: The Community Engaged Digital Alzheimer’s Research (CEDAR) Study. Journal of Prevention of Alzheimer’s Disease 10(3):551-561.
Berkness, T., M. C. Carrillo, R. Sperling, R. Petersen, P. Aisen, C. Flournoy, H. Snyder, R. Raman, and J. D. Grill. 2021. The Institute on Methods and Protocols for Advancement of Clinical Trials in ADRD (IMPACT-AD): A novel clinical trials training program. Journal of Prevention of Alzheimer’s Disease 8(3):286-291.
Boxer, A. L., and R. Sperling. 2023. Accelerating Alzheimer’s therapeutic development: The past and future of clinical trials. Cell 186(22):4757-4772.
Brewster, P., L. Barnes, M. Haan, J. K. Johnson, J. J. Manly, A. M. Napoles, R. A. Whitmer, L. Carvajal-Carmona, D. Early, S. Farias, E. R. Mayeda, R. Melrose, O. L. Meyer, A. Zeki Al Hazzouri, L. Hinton, and D. Mungas. 2019. Progress and future challenges in aging and diversity research in the United States. Alzheimer’s & Dementia 15(7):995-1003.
Canevelli, M., G. Bruno, G. Grande, F. Quarata, R. Raganato, F. Remiddi, M. Valletta, V. Zaccaria, N. Vanacore, and M. Cesari. 2019. Race reporting and disparities in clinical trials on Alzheimer’s disease: A systematic review. Neuroscience & Biobehavioral Reviews 101:122-128.
Chen, C., and J. M. Zissimopoulos. 2018. Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimer’s & Dementia (N Y) 4:510-520.
Cummings, J., Y. Zhou, G. Lee, K. Zhong, J. Fonseca, and F. Cheng. 2024. Alzheimer’s disease drug development pipeline: 2024. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 10(2):e12465.
Davis, R., and P. Bekker. 2022. Recruitment of older adults with dementia for research: An integrative review. Research into Gerontological Nursing 15(5):255-264.
Diverse VCID. 2024. Who we are. https://diversevcid.ucdavis.edu/our-goal (accessed August 22, 2024).
Driven Data. 2024. PREPARE: Pioneering Research for Early Prediction of Alzheimer’s and Related Dementias EUREKA Challenge. https://www.drivendata.org/competitions/253/competition-nih-alzheimers-adrd-1/ (accessed November 9, 2024).
Fang, J., A. A. Pieper, R. Nussinov, G. Lee, L. Bekris, J. B. Leverenz, J. Cummings, and F. Cheng. 2020. Harnessing endophenotypes and network medicine for Alzheimer’s drug repurposing. Medical Research Review 40(6):2386-2426.
FDA (Food and Drug Administration). 2020. Enhancing the diversity of clinical trial populations—eligibility criteria, enrollment practices, and trial designs guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enhancing-diversity-clinical-trial-populations-eligibility-criteria-enrollment-practices-and-trial?utm_medium=email&utm_source=govdelivery (accessed August 21, 2024).
FDA. 2022. Master protocols: Efficient clinical trial design strategies to expedite development of oncology drugs and biologics guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/master-protocols-efficient-clinical-trial-design-strategies-expedite-development-oncology-drugs-and (accessed August 21, 2024).
FNIH (Foundation for the National Institutes of Health). 2023. Our mission. https://fnih.org/who-we-are/ (accessed August 21, 2024).
Franzen, S., J. E. Smith, E. van den Berg, M. Rivera Mindt, R. L. van Bruchem-Visser, E. L. Abner, L. S. Schneider, N. D. Prins, G. M. Babulal, and J. M. Papma. 2022. Diversity in Alzheimer’s disease drug trials: The importance of eligibility criteria. Alzheimer’s & Dementia 18(4):810-823.
Gewin, V. 2023. Postdoctoral researchers warn NIH that cost-of-living pressures are gutting the workforce. Nature, June 29.
Gianattasio, K. Z., E. E. Bennett, J. Wei, M. L. Mehrotra, T. Mosley, R. F. Gottesman, D. F. Wong, E. A. Stuart, M. E. Griswold, D. Couper, M. M. Glymour, M. C. Power, and the Alzheimer’s Disease Neuroimaging Initiative. 2021. Generalizability of findings from a clinical sample to a community-based sample: A comparison of ADNI and ARIC. Alzheimer’s & Dementia 17(8):1265-1276.
Gibbons, L., T. Mobley, E. Mayeda, C. Lee, N. Gatto, A. LaCroix, L. McEvoy, P. Crane, and E. Hayes-Larson. 2024. How generalizable are findings from a community-based prospective cohort study? Extending estimates from the Adult Changes in Thought Study to its source population. Journal of Alzheimer’s Disease 100(1):163-174.
Gilmore-Bykovskyi, A. L., Y. Jin, C. Gleason, S. Flowers-Benton, L. M. Block, P. Dilworth-Anderson, L. L. Barnes, M. N. Shah, and M. Zuelsdorff. 2019. Recruitment and retention of underrepresented populations in Alzheimer’s disease research: A systematic review. Alzheimer’s & Dementia (N Y) 5:751-770.
Ginther, D., S. W., J. Schnell, B. Masimore, F. Liu, L. Haak, and R. Kington. 2011. Race, ethnicity, and NIH reserach awards. Science 333(6045):1015-1019.
Gladman, J. T., R. A. Corriveau, S. Debette, M. Dichgans, S. M. Greenberg, P. S. Sachdev, J. M. Wardlaw, and G. J. Biessels. 2019. Vascular contributions to cognitive impairment and dementia: Research consortia that focus on etiology and treatable targets to lessen the burden of dementia worldwide. Alzheimer’s & Dementia (N Y) 5:789-796.
Godbole, N., S. C. Kwon, J. M. Beasley, T. Roberts, J. Kranick, J. Smilowitz, A. Park, S. E. Sherman, C. Trinh-Shevrin, and J. Chodosh. 2023. Assessing equitable inclusion of underrepresented older adults in Alzheimer’s disease, related cognitive disorders, and aging-related research: A scoping review. Gerontologist 63(6):1067-1077.
Greenberg, S. M. 2017. William M. Feinberg award for excellence in clinical stroke: Big pictures and small vessels. Stroke 48(9):2628-2631.
Gregory, S., S. Saunders, and C. W. Ritchie. 2022. Science disconnected: The translational gap between basic science, clinical trials, and patient care in Alzheimer’s disease. Lancet Healthy Longevity 3(11):e797-e803.
Grill, J. D., and J. E. Galvin. 2014. Facilitating Alzheimer disease research recruitment. Alzheimer’s Disease & Associated Disorders 28(1):1-8.
Gross, A. L., C. Li, E. M. Briceno, M. Arce Renteria, R. N. Jones, K. M. Langa, J. J. Manly, E. Nichols, D. Weir, R. Wong, L. Berkman, J. Lee, and L. C. Kobayashi. 2023. Harmonisation of later-life cognitive function across national contexts: Results from the harmonized cognitive assessment protocols. Lancet Healthy Longevity 4(10):e573-e583.
Hodes, R. J. 2023. NIA’s commitment to inclusion. https://www.nia.nih.gov/research/blog/2023/11/nias-commitment-inclusion (accessed August 21, 2024, 2024).
Hodes, R. J., and N. Buckholtz. 2016. Accelerating Medicines Partnership: Alzheimer’s Disease (AMP-AD) knowledge portal aids Alzheimer’s drug discovery through open data sharing. Expert Opinions on Therapeutic Targets 20(4):389-391.
Jorgenson, L. 2023. Advancing the promise of open science: We want to hear from you! https://osp.od.nih.gov/advancing-the-promise-of-open-science/ (accessed August 22, 2024).
Kaye, J., P. Aisen, R. Amariglio, R. Au, C. Ballard, M. Carrillo, H. Fillit, T. Iwatsubo, G. Jimenez-Maggiora, S. Lovestone, F. Natanegara, K. Papp, M. E. Soto, M. Weiner, B. Vellas, and the EU/US CTAD Task Force. 2021. Using digital tools to advance Alzheimer’s drug trials during a pandemic: The EU/U.S. CTAD Task Force. Journal of Prevention of Alzheimer’s Disease 8(4):513-519.
Kim, C. K., Y. R. Lee, L. Ong, M. Gold, A. Kalali, and J. Sarkar. 2022. Alzheimer’s disease: Key insights from two decades of clinical trial failures. Journal of Alzheimer’s Disease 87(1):83-100.
Kowe, A., H. Panjaitan, O. A. Klein, M. Boccardi, M. Roes, S. Teupen, and S. Teipel. 2022. The impact of participatory dementia research on researchers: A systematic review. Dementia (London) 21(3):1012-1031.
Langbaum, J. B., J. Zissimopoulos, R. Au, N. Bose, C. J. Edgar, E. Ehrenberg, H. Fillit, C. V. Hill, L. Hughes, M. Irizarry, S. Kremen, D. Lakdawalla, N. Lynn, K. Malzbender, T. Maruyama, H. A. Massett, D. Patel, D. Peneva, E. M. Reiman, K. Romero, C. Routledge, M. W. Weiner, S. Weninger, and P. S. Aisen. 2023. Recommendations to address key recruitment challenges of Alzheimer’s disease clinical trials. Alzheimer’s & Dementia 19(2):696-707.
Lieberthal, R., N. Vakil, and M. Wilson. 2023. Deriving common data elements from real-word data for Alzheimer’s disease related dementias. McLean, VA: MITRE Corp.
Lim, A. C., L. L. Barnes, G. H. Weissberger, M. Lamar, A. Nguyen, L. Fenton, J. Herrera, and D. S. Han. 2023. Quantification of race/ethnicity representation in Alzheimer’s disease neuroimaging research in the USA: A systematic review. Communications Medicine 3:101.
Liu, M. N., C. I. Lau, and C. P. Lin. 2019. Precision medicine for frontotemporal dementia. Frontiers in Psychiatry 10:75.
MacDonald, S., A. S. Shah, and B. Tousi. 2022. Current therapies and drug development pipeline in Lewy body dementia: An update. Drugs & Aging 39(7):505-522.
Manly, J. J., A. Gilmore-Bykovskyi, and K. D. Deters. 2021. Inclusion of underrepresented groups in preclinical Alzheimer’s disease trials—opportunities abound. JAMA Network Open 4(7):e2114606.
MarkVCID. 2017. MarkVCID consortium overview. https://markvcid.partners.org/about/m2-consortium-overview (accessed August 21, 2024).
Mielke, M. 2024. Sex and gender differences in Alzheimer’s disease and Alzheimer’s disease related dementias. Commissioned Paper for the Committee on Research Priorities for Preventing and Treating Alzheimer’s Disease and Related Dementias.
Mindt, M. R., O. Okonkwo, M. W. Weiner, D. P. Veitch, P. Aisen, M. Ashford, G. Coker, M. C. Donohue, K. M. Langa, G. Miller, R. Petersen, R. Raman, and R. Nosheny. 2023. Improving generalizability and study design of Alzheimer’s disease cohort studies in the United States by including under-represented populations. Alzheimer‘s & Dementia 19(4):1549-1557.
Mitchell, A. K., R. Ehrenkranz, S. Franzen, S. H. Han, M. Shakur, M. McGowan, and H. A. Massett. 2024. Analysis of eligibility criteria in Alzheimer’s and related dementias clinical trials. Scientific Reports 14(1):15036.
NACC (National Alzheimer’s Coordinating Center). 2024a. National Alzheimer’s Coordinating Center. https://naccdata.org/ (accessed October 25, 2024).
NACC. 2024b. About NACC Data. https://naccdata.org/requesting-data/nacc-data (accessed October 25, 2024).
NACC. 2024c. NACC’s Data Platform. https://naccdata.org/adrc-resources/nacc-data-platform (accessed October 25, 2024).
Nag, S., L. L. Barnes, L. Yu, R. S. Wilson, D. A. Bennett, and J. A. Schneider. 2020. Limbic-predominant age-related TDP-43 encephalopathy in black and white decedents. Neurology 95(15):e2056-e2064.
Nagar, S. D., P. Pemu, J. Qian, E. Boerwinkle, M. Cicek, C. R. Clark, E. Cohn, K. Gebo, R. Loperena, K. Mayo, S. Mockrin, L. Ohno-Machado, A. H. Ramirez, S. Schully, A. Able, A. Green, S. Zuchner, S. Consortium, I. K. Jordan, and R. Meller. 2022. Investigation of hypertension and type 2 diabetes as risk factors for dementia in the All of Us cohort. Scientific Reports 12(1):19797.
NAPS Consortium. 2024. About NAPS. https://www.naps-rbd.org/about-the-consortium (accessed October 29, 2024).
NASEM (National Academies of Sciences, Engineering, and Medicine). 2015. SBIR/STTR at the National Institutes of Health. Washington, DC: The National Academies Press.
NASEM. 2017. Preventing cognitive decline and dementia: A way forward. Edited by A. I. Leshner, S. Landis, C. Stroud and A. Downey. Washington, DC: The National Academies Press.
NASEM. 2018. Returning individual research results to participants: Guidance for a new research paradigm. Washington, DC: The National Academies Press.
NASEM. 2021. Reducing the impact of dementia in America: A decadal survey of the behavioral and social sciences. Washington, DC: The National Academies Press.
NASEM. 2022. Improving representation in clinical trials and research: Building research equity for women and underrepresented groups, edited by K. Bibbins-Domingo and A. Helman. Washington, DC: The National Academies Press.
NASEM. 2024. Preventing and treating Alzheimer’s disease and related dementias: Promising research and opportunities to accelerate progress: Proceedings of a workshop—in brief, edited by O. Yost and A. Downey. Washington, DC: The National Academies Press.
Nature Medicine. 2023. Dementia research needs a global approach. Nature Medicine 29:279. https://doi.org/10.1038/s41591-023-02249-z.
Nguyen, M., S. I. Chaudhry, M. M. Desai, K. Dzirasa, J. E. Cavazos, and D. Boatright. 2023. Gender, racial, and ethnic and inequities in receipt of multiple National Institutes of Health research project grants. JAMA Network Open 6(2):e230855.
NIA (National Institute on Aging). 2018. Together we make a difference: National strategy for recruitment and participation in Alzheimer’s and related dementias clinical research. NIA. www.nia.nih.gov/sites/default/files/2018-10/alzheimers-disease-recruitment-strategy-final.pdf (accessed on October 25, 2024).
NIA. 2019a. Alzheimer’s disease and related dementias: Clinical studies recruitment planning guide. NIA.
NIA. 2019b. National Institute on Aging workshop on applying digital technology for early diagnosis and monitoring of Alzheimer’s disease and related dementias. https://www.nia.nih.gov/research/dn/workshops/workshop-applying-digital-technology-early-diagnosis-and-monitoring (accessed September 19, 2024).
NIA. 2023a. NIA’s clinical research operations & management system (CROMS). https://www.nia.nih.gov/research/grants-funding/nias-clinical-research-operations-management-system-croms (accessed August 21, 2024).
NIA. 2023b. NIH AD/ADRD platforms workshop: FAIRness within and across infrastructures. Workshop summary. June 20-21, 2023.
NIA. 2023c. Research enterprise. https://www.nia.nih.gov/report-2019-2020-scientific-advances-prevention-treatment-and-care-dementia/research-enterprise (accessed August 22, 2024).
NIA. 2023d. Alzheimer’s disease genomics sharing plan. https://www.nia.nih.gov/research/dn/alzheimers-disease-genomics-sharing-plan (accessed October 25, 2024).
NIA. 2023e. Artificial intelligence and technology collaboratories for aging research. https://www.nia.nih.gov/research/dbsr/artificial-intelligence-and-technology-collaboratories-aging-research (accessed August 21, 2024).
NIA, 2024a. National strategy for recruitment and participation in Alzheimer’s and related dementias clinical research. https://www.nia.nih.gov/research/recruitment-strategy (accessed October 29, 2024).
NIA. 2024b. Resource centers for minority aging research (2023-2028). https://www.nia.nih.gov/research/dbsr/resource-centers-minority-aging-research-rcmar (accessed August 21, 2024, 2024).
NIA. 2024c. The NIH aging and AD/ADRD omics data resources: A path to interoperability. https://www.nia.nih.gov/research/dn/workshops/nih-aging-and-ad-adrd-omics-data-resources-path-interoperability (accessed August 22, 2024).
NIA. 2024d. Enabling tech: Wearable technologies (milestone 11.C). https://www.nia.nih.gov/research/milestones/diagnosis-assessment-and-disease-monitoring/enabling-tech-wearable-technologies (accessed August 21, 2024, 2024).
NIAGADS (National Institute on Aging Genetics of Alzheimer’s Disease Data Storage Site). 2024a. About. https://www.niagads.org/about/ (accessed November 11, 2024).
NIAGADS. 2024b. Qualified access data. https://www.niagads.org/qualified-access-data/ (accessed November 11, 2024).
NIH (National Institutes of Health). n.d. Collaboration details. https://crs.od.nih.gov/CRSPublic/View.aspx?Id=8704 (accessed August 21, 2024).
NIH. 2020. Final NIH policy for data management and sharing. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-21-013.html (accessed August 22, 2024).
NIH. 2023a. NIH professional judgment budget for Alzheimer’s disease and related dementias for fiscal year 2023. https://www.nia.nih.gov/sites/default/files/2021-08/nih_ad-adrd_bypass_budget_fy23.pdf (accessed September 19, 2024).
NIH. 2023b. Advancements build momentum: 10 years of Alzheimer’s disease and related dementias research. NIH Scientific Progress Report.
NIH. 2023c. Novel mechanism research on neuropsychiatric symptoms (NPS) in Alzheimer’s dementia (R01 clinical trial optional). https://grants.nih.gov/grants/guide/pa-files/PAR-23-207.html (accessed October 29, 2024).
NIH. 2024a. Tissue chip program collaborations and partnerships. https://ncats.nih.gov/research/research-activities/tissue-chip/collaborations (accessed August 21, 2024).
NIH. 2024b. Blueprint neurotherapeutics network (BPN) for small molecules. https://neuroscienceblueprint.nih.gov/neurotherapeutics/bpn-small-molecules (accessed August 21, 2024).
NIH. 2024c. Understanding SBIR and STTR. https://seed.nih.gov/small-business-funding/small-business-program-basics/understanding-sbir-sttr (accessed October 29, 2024).
NIH ACD Working Group (NIH Advisory Committee to the Director Working Group on Re-Envisioning NIH-Supported Postdoctoral Training). 2023. Report to the NIH Advisory Committee to the Director.
NIH Office of Extramural Research, 2024. NIH grants policy statement. https://grants.nih.gov/grants/policy/nihgps/html5/section_11/11.3.10_stipend_supplementation__compensation__and_other_income.htm (accessed on October 25, 2024).
NIH RePORTER. 2023a. Identifying barriers to optimizing data sharing and accelerate discovery in Alzheimer’s disease and related dementia research. https://reporter.nih.gov/search/Yx1EJNc3W0-s33aUvMdY3A/project-details/10568214 (accessed August 22, 2024).
NIH RePORTER. 2023b. Utilizing technology and AI approaches to facilitate independence and resilience in older adults. https://reporter.nih.gov/search/9mSA_KiHAk-InwQPU18rSg/project-details/10274370 (accessed August 21, 2024, 2024).
NINDS (National Institute of Neurological Disorders and Stroke). n.d. Project overview. https://www.commondataelements.ninds.nih.gov/ProjReview (accessed October 29, 2024).
NINDS. 2021. Notice of special interest (NOSI): Administrative supplements to promote diversity for NINDS Alzheimer’s disease and Alzheimer’s disease-related dementias (AD/ADRD) awardees. https://grants.nih.gov/grants/guide/notice-files/NOT-NS-21-047.html (accessed August 21, 2024).
NINDS. 2024. Notice of special interest (NOSI): Administrative supplements to promote diversity for NINDS ADRD awardees. https://grants.nih.gov/grants/guide/notice-files/NOT-NS-24-071.html (accessed August 21, 2024).
NSF (National Science Foundation). 2024. National artificial intelligence research resource pilot. https://new.nsf.gov/focus-areas/artificial-intelligence/nairr#nairr-pilot-partners-and-contributors-890 (accessed October 29, 2024).
Pannu, J., J. Schmitt, and J. Swett. 2023. ARPA-H should zero in on pandemic prevention. https://issues.org/arpa-h-pandemic-prevention-pannu-schmitt-swett/ (accessed August 21, 2024, 2024).
Parekh, D., V. M. Patil, K. Nawale, V. Noronha, N. Menon, S. More, S. Goud, S. Jain, V. Mathrudev, Z. Peelay, S. Dhumal, S. Jogdhankar, and K. Prabhash. 2022. Audit of screen failure in 15 randomised studies from a low and middle-income country. Ecancermedicalscience 16:1476.
Pierce, H., A. Dev, E. Statham, and B. E. Bierer. 2019. Credit data generators for data reuse. Nature 570(7759):30-32.
Powell, W. R., W. R. Buckingham, J. L. Larson, L. Vilen, M. Yu, M. S. Salamat, B. B. Bendlin, R. A. Rissman, and A. J. H. Kind. 2020. Association of neighborhood-level disadvantage with Alzheimer disease neuropathology. JAMA Network Open 3(6):e207559.
Raman, R., Y. T. Quiroz, O. Langford, J. Choi, M. Ritchie, M. Baumgartner, D. Rentz, N. T. Aggarwal, P. Aisen, R. Sperling, and J. D. Grill. 2021. Disparities by race and ethnicity among adults recruited for a preclinical Alzheimer disease trial. JAMA Network Open 4(7):e2114364.
Reddy, J. S., L. Heath, A. V. Linden, M. Allen, K. P. Lopes, F. Seifar, E. Wang, Y. Ma, W. L. Poehlman, Z. S. Quicksall, A. Runnels, Y. Wang, D. M. Duong, L. Yin, K. Xu, E. S. Modeste, A. Shantaraman, E. B. Dammer, L. Ping, S. R. Oatman, J. Scanlan, C. Ho, M. M. Carrasquillo, M. Atik, G. Yepez, A. O. Mitchell, T. T. Nguyen, X. Chen, D. X. Marquez, H. Reddy, H. Xiao, S. Seshadri, R. Mayeux, S. Prokop, E. B. Lee, G. E. Serrano, T. G. Beach, A. F. Teich, V. Haroutunian, E. J. Fox, M. Gearing, A. Wingo, T. Wingo, J. J. Lah, A. I. Levey, D. W. Dickson, L. L. Barnes, P. De Jager, B. Zhang, D. Bennett, N. T. Seyfried, A. K. Greenwood, and N. Ertekin-Taner. 2024. Bridging the gap: Multi-omics profiling of brain tissue in Alzheimer’s disease and older controls in multi-ethnic populations. Alzheimer’s & Dementia 20(10):7174-7192.
Reyes, L., C. J. Scher, and E. A. Greenfield. 2023. Participatory research approaches in Alzheimer’s disease and related dementias literature: A scoping review. Innovation in Aging 7(7):igad091.
Ritchie, C. W., G. M. Terrera, and T. J. Quinn. 2015. Dementia trials and dementia tribulations: Methodological and analytical challenges in dementia research. Alzheimer’s Research & Therapy 7(1):31.
Sainburg, T. 2023. American postdoctoral salaries do not account for growing disparities in cost of living. Research Policy 52(3):104714.
Santora, K. 2023. NIA new deadlines: Awaiting receipt of application (ARA) for large budget grant applications. https://www.nia.nih.gov/research/blog/2023/11/nia-new-deadlines-awaiting-receipt-application-ara-large-budget-grant (accessed August 21, 2024).
Scalco, R., Y. Hamsafar, C. L. White, J. A. Schneider, R. R. Reichard, S. Prokop, R. J. Perrin, P. T. Nelson, S. Mooney, A. P. Lieberman, W. A. Kukull, J. Kofler, C. D. Keene, A. Kapasi, D. J. Irwin, D. A. Gutman, M. E. Flanagan, J. F. Crary, K. C. Chan, M. E. Murray, and B. N. Dugger. 2023. The status of digital pathology and associated infrastructure within Alzheimer’s disease centers. Journal of Neuropathology & Experimental Neurology 82(3):202-211.
Shakir, M. N., and B. N. Dugger. 2022. Advances in deep neuropathological phenotyping of Alzheimer disease: Past, present, and future. Journal of Neuropathology & Experimental Neurology 81(1):2-15.
Stetler, C. 2023. NIH to fund exposome research coordinating center. https://factor.niehs.nih.gov/2023/10/science-highlights/nih-funding-to-support-exposome-research-coordinating-center (accessed August 21, 2024, 2024).
Takvorian, S., C. Guerra, and W. Schpero. 2021. A hidden opportunity — Medicaid’s role in supporting equitable access to clinical trials. New England Journal of Medicine 384(21):1975-1978.
TRC-DS. 2024. About TRC-DS. https://www.trcds.org/about/ (accessed October 29, 2024).
UK Research and Innovation. 2024. Co-production in research. https://www.ukri.org/manage-your-award/good-research-resource-hub/research-co-production/ (accessed August 21, 2024, 2024).
Wilkinson, M. D., M. Dumontier, I. J. Aalbersberg, G. Appleton, M. Axton, A. Baak, N. Blomberg, J. W. Boiten, L. B. da Silva Santos, P. E. Bourne, J. Bouwman, A. J. Brookes, T. Clark, M. Crosas, I. Dillo, O. Dumon, S. Edmunds, C. T. Evelo, R. Finkers, A. Gonzalez-Beltran, A. J. Gray, P. Groth, C. Goble, J. S. Grethe, J. Heringa, P. A. C. ‘t Hoen, R. Hooft, T. Kuhn, R. Kok, J. Kok, S. J. Lusher, M. E. Martone, A. Mons, A. L. Packer, B. Persson, P. Rocca-Serra, M. Roos, R. van Schaik, S. A. Sansone, E. Schultes, T. Sengstag, T. Slater, G. Strawn, M. A. Swertz, M. Thompson, J. van der Lei, E. van Mulligen, J. Velterop, A. Waagmeester, P. Wittenburg, K. Wolstencroft, J. Zhao, and B. Mons. 2016. The FAIR guiding principles for scientific data management and stewardship. Scientific Data 3:160018.
Wing, J., D. A. Levine, A. Ramamurthy, and C. Reider. 2020. Alzheimer’s disease and related disorders prevalence differs by Appalachian residence in Ohio. Journal of Alzheimer’s Disease 76(4):1309-1316.

























































