Exploring the Science on Measures of Body Composition, Body Fat Distribution, and Obesity: Proceedings of a Workshop Series (2024)
Chapter: 6 Communicating How Obesity Is Defined and Diagnosed
6
Communicating How Obesity Is Defined and Diagnosed
The first session of the June 2023 workshop featured three presentations highlighting the work of the Lancet Commission on the definition and diagnosis of clinical obesity, communicating about it using a disease staging system, and specifically the Edmonton Obesity Staging System (EOSS) in the adult and pediatric populations. A panel and audience discussion followed. Ihuoma Eneli welcomed participants to the second workshop.
Eneli provided background and informed the audience of the two-part series, exploring the science and measuring body composition, body fat distribution, and obesity. The first workshop (April 2023), she explained, reviewed the World Health Organization (WHO) definition of obesity, examined it as a condition of excess body fat that impairs health, and outlined the strengths and limitations of body mass index (BMI) as a surrogate measure of body fat. Eneli shared that the second workshop builds on the first, focusing on how to communicate with diverse groups and sectors about body composition, BMI, adiposity, and health. She outlined the session topics, including strategies for providers to communicate with patients on the diagnosis and definition of obesity, influence and effects of weight bias and stigma, ethics and challenges in communicating about the intersection of weight and health, and long-term strategies to inspire a cultural shift on perceptions of weight.
THE LANCET COMMISSION ON THE DEFINITION AND DIAGNOSIS OF CLINICAL OBESITY
Francesco Rubino, chair of metabolic and bariatric surgery at the King’s College London, was the first presenter. He began by underscoring
that obesity is not universally accepted as a disease and is widely considered a condition of excess adiposity that indicates an increased risk for future disease. The concept of it as a disease is not well developed, despite various clinical guidelines for treatment and management and national public health targets.
In medicine, Rubino continued, conditions that increase a person’s risk to develop a chronic disease also present an opportunity to define strategic and efficient targets for treatment. The clinical signs and symptoms for a disease can range from activities of daily living to organ dysfunction. He said that BMI is not a clinical parameter and does not measure excess adiposity or indicate tissue or organ function.
Consequently, Rubino said, the clinical signs and symptoms for obesity and treatment targets are poorly defined. The lack of a clear causal sequence becomes apparent when providers apply prevention strategies that are unsuitable for severe obesity, complications, or comorbidities. Rubino stated that treatment plans that do not work can lead patients to become vulnerable to predatory advertisements for weight loss fixes in popular media. He summarized that the loose definition for obesity as a health condition (or disease) reinforces misinformation and disinformation and promotes weight stigma.
To illustrate this point, Rubino shared his research that surveyed people about their beliefs on the cause of obesity across four countries (O’Keeffe et al., 2020). One question asked if it could be cured through lifestyle changes, such as diet and exercise; respondents who agreed also scored higher for fatphobia, meaning they held more stigmatizing views. According to Rubino, the most significant contributor to stigma is a lack of understanding of obesity as a disease, which is invariably reversible without pharmacotherapy or surgical intervention.
Rubino affirmed that the concept of obesity as a disease is one of the most controversial and polarizing issues in medicine. Physician opponents point to patients with excess adiposity who do not show clinical manifestations (e.g., limitations of daily activities), although it is a marginal segment of patients, he said.
Rubino emphasized that BMI was not designed or intended as a clinical measure of excess adiposity. In its original form, it was a population-level measure for the increased risk of mortality. He pointed to football players and boxers as an outlier group with a high BMI because of their muscle or lean body mass.
Rubino added that BMI does not measure clinical manifestations of obesity in tissue or organs. Furthermore, the BMI-related risk for diabetes or mortality varies across groups segmented by ethnicity, age, and gender. Consequently, BMI cannot accurately reflect the risk for complications or mortality across population segments.
To address these issues, Rubino introduced the Lancet Commission on Clinical Obesity, which is tasked with generating a consensus among a group of 60 experts on the mechanisms underlying clinical manifestations of obesity as a disease. According to Rubino, it is developing a consensus report to determine the definition and diagnostic criteria for clinical obesity.
Rubino noted the substantial barriers to gain consensus among commission experts. Historically, he said, obesity has been described as excess adiposity that presents a risk to health. The commission is grappling with the etiology of the disease, distinct pathophysiology, and clinical manifestations or illness. A condition of risk is not per se a disease, he said.
Rubino shared several questions the commission is considering. Does obesity have a clinical phenotype? What is an accurate measure of excess adiposity, and is it necessary to diagnose obesity? With additional anthropometric measures, will patients present with an illness or clinical manifestation? Although experts will continue to deliberate on these topics, Rubino claimed agreement that BMI is an insufficient measure for obesity.
Rubino summarized that, in obesity, anthropometrics and BMI do not measure organ dysfunction. In some diseases, such as diabetes, biomarkers are strongly associated with clinical manifestations. He affirmed that the only way to determine the expression of obesity is to focus on clinical manifestations and that the consensus report will have profound implications for clinical practice, public health, and weight stigma.
ADIPOSITY-BASED CHRONIC DISEASE AND THE COMPLICATIONS-CENTRIC APPROACH TO DISEASE STAGING AND MANAGEMENT
W. Timothy Garvey, professor of medicine at the Department of Nutrition Sciences at the University of Alabama at Birmingham, gave the second presentation, which focused on obesity or adiposity-based chronic disease (ABCD) and its management using a complications-centric approach.
Garvey began by providing a time line and progression for ABCD. He stated that obesity was first regarded as a disease in 2012 by the American Association of Clinical Endocrinology (AACE) in a position statement that outlined the three criteria required for a disease diagnosis from the American Medical Association (AMA). Garvey maintained that obesity is a disease because of (1) characteristic signs or symptoms (excess adiposity measured by BMI) that (2) results in cardiometabolic and biomechanical complications (harm or morbidity), and (3) impairs the normal function of some aspect of the body (dysregulation of body functions).
To further define obesity, Garvey said, the AACE held a Consensus Conference on Obesity in 2014 with multidisciplinary stakeholders from different sectors, including biomedical, government and regulatory, health
care, research, and education. It aimed to discuss and agree on the definition of obesity, determine available options to manage it, identify therapeutic modalities and their cost, and pinpoint knowledge gaps and decide how to approach them.
Garvey recounted the primary issue that emerged was that a diagnosis exclusively based on anthropometric measures (BMI) was unreliable. The consensus was that these did not measure excess adiposity or indicate an impact on health, particularly across racial and ethnic groups. Consequently, reliable treatment and health care benefits could not be determined, and no actionable policy steps were identified. However, conference stakeholders agreed on two components for an accurate diagnosis: (1) a measure for excess adiposity; although BMI is imperfect, it is ingrained worldwide; and (2) a manifestation of severe complications, confirming morbidity and mortality (Garvey et al., 2014).
Two years later, the AACE Obesity Guidelines were released for obesity management using a complications-centric approach to care (Garvey et al., 2016). The goal was to treat the cardiometabolic, biomechanical, and quality of life complications that impaired health. He emphasized that the endpoint was not weight loss but preventing and treating complications.
Garvey detailed the three stages outlined in the guidelines. The algorithm included two diagnostic components: anthropometrics and clinical manifestation to guide treatment. Stage 0 represented an absence of complications. Stage 1 was characterized by mild to moderate complications and aimed to treat and prevent or limit complications with weight loss. Stage 2 included severe complications and involved pharmacotherapy and/or surgery.
Garvey shared that in the 2016 AACE guidelines, risk was stratified using clinical stages, which are simple and intuitive for clinicians to use and cost effective because evidence-based therapy is expensive. Weight-loss therapy can prevent the progression from the clinical stages to end-stage manifestations, leading to risk for other diseases, and also treat end-stage manifestations, prevent the onset of other diseases, and limit polypharmacy.
Garvey moved on by explaining the three types of health complications from obesity (see Figure 6-1). The first is quality of life, which negatively impacts mental health (e.g., anxiety) and physical health (e.g., pain). The second is cardiometabolic, leading to poor metabolic and vascular outcomes, such as insulin resistance and prediabetes, metabolic syndrome, dyslipidemia, prehypertension, and hepatic steatosis. The third type is biochemical and a function of excess body fat over time.
Garvey recalled that shortly after the release of the guidelines, AACE and the American College of Endocrinology introduced a more precise and medically actionable diagnostic term to convey obesity as a disease and not just an elevated BMI (Mechanick et al., 2017):
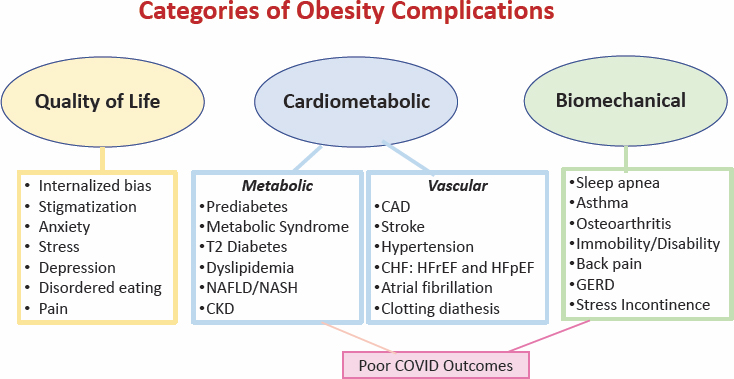
NOTE: T2 = type 2; CAD = coronary artery disease; CHF = congestive heart failure; CKD = chronic kidney disease; GERD = gastroesophageal reflux disease; HFrEF = heart failure with reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; NAFLD/NASH = nonalcoholic fatty liver disease/nonalcoholic steato hepatitis.
SOURCE: Presented by W. Timothy Garvey, June 26, 2023. Reprinted with permission.
Adiposity-Based Chronic Disease (ABCD) is a new diagnostic term for obesity that explicitly identifies a chronic disease, alludes to a precise pathophysiologic basis, and avoids the stigmata and confusion related to the differential use and multiple meanings of the term “obesity.”
Abnormalities in the mass, distribution, and function of adipose tissue, a lifelong chronic disease, may lead to health complications, morbidity, and mortality.
Garvey discussed the Cardiometabolic Disease Staging System, for which he tested the efficacy by stratifying adults in the national CARDIA Cohort (Guo et al., 2014). Stage 0, he said, represents a metabolically healthy individual without any metabolic syndrome traits. Stage 1 denotes one or two metabolic syndrome risk factors. Stage 2 is characterized by impaired fasting glucose, impaired glucose tolerance, or metabolic syndrome. Stage 3 denotes two of three conditions or impaired fasting glucose, impaired glucose tolerance, or metabolic syndrome. Stage 4 indicates type 2 diabetes and/or cardiovascular disease. Garvey highlighted that people who met the criteria for prediabetes and metabolic syndrome (Stage 3) had a 40-fold increased risk for diabetes and all-cause and cardiovascular disease mortality compared to Stage 0.
According to Garvey, although these issues were paramount in the progress of diagnosing and treating obesity, the foremost issue is the International
Classification of Diseases, Tenth Revision (ICD-10)—it charts disease prevalence over time. Its code E66 is used for health care–related billing for treatment in the United States and several other countries. He pointed out that E66 indicates that the conditions are the result of excess calorie consumption, which is scientifically incorrect and medically unactionable. Garvey stressed that calorie counting would not track obesity treatment; it reinforces an inaccurate medical concept, promotes stigmatization, and limits public health.
Garvey then introduced an alternative ICD-10 coding that he and his colleague, Jeffrey I. Mechanick, proposed, which is scientifically accurate and medically actionable. The distinction between it and the current ICD-10 is that obesity is designated as a pathophysiological category without overt cause, caused by a genetic abnormality, or aggravated by other causes (e.g., endocrine, iatrogenic, disability) (Garvey and Mechanick, 2020).
Garvey explained that the B code signifies the BMI classification or a measure of the degree of adiposity. A C code identifies obesity-related complications bucketed into five categories with unique codes. A D code classifies the severity of the complication as mild, moderate, or severe. One example, Garvey offered, is sleep apnea, which would have a unique code. Severity criteria would use the apnea/hypopnea index: a score of <5 would be normal, a score of 5–30 would be mild to moderate, and a score of >30 would be severe.
Garvey concluded that the principles underlying the alternative ICD-10 coding and staging systems for ABCD are based on the presence, severity, and risk of related complications, and he urged their adoption to assist with decisions related to disease management.
EOSS FOR ADULTS AND PEDIATRICS
Geoff Ball, professor and associate chair of research in the Department of Pediatrics and the Alberta Health Services Chair in Obesity Research in the Faculty of Medicine and Dentistry at the University of Alberta, gave the third presentation. Ball continued the discussion on the value of staging in obesity by focusing on the EOSS and its application to the pediatric population (Sharma and Kushner, 2009).
Ball began by providing the background and overview of the EOSS, a clinical and functional staging system (Sharma and Kushner, 2009). It identifies the consequences of obesity with increasing severity by stage (0–4) and outlines relevant strategies for prevention and management (Sharma and Kushner, 2009).
Ball informed the audience that the EOSS was published in 2009 and aimed to be an accurate measure for obesity. A nationally representative sample of adults from the National Health and Nutrition Examination
Survey (NHANES) dataset were stratified by EOSS score, which demonstrated that the severity of health consequences increased with it but not with BMI as a stand-alone measure (Kuk et al., 2011; Padwal et al., 2011). The survival curves diverged when stratified by EOSS score but not by BMI class (see Figure 6-2). EOSS also differentiated between those with low and greater health risks, revealing the specificity of EOSS, he said.
Ball moved on to the Edmonton Obesity Staging System for Pediatrics (EOSS-P) that he and his colleagues developed (Hadjiyannakis et al., 2016).
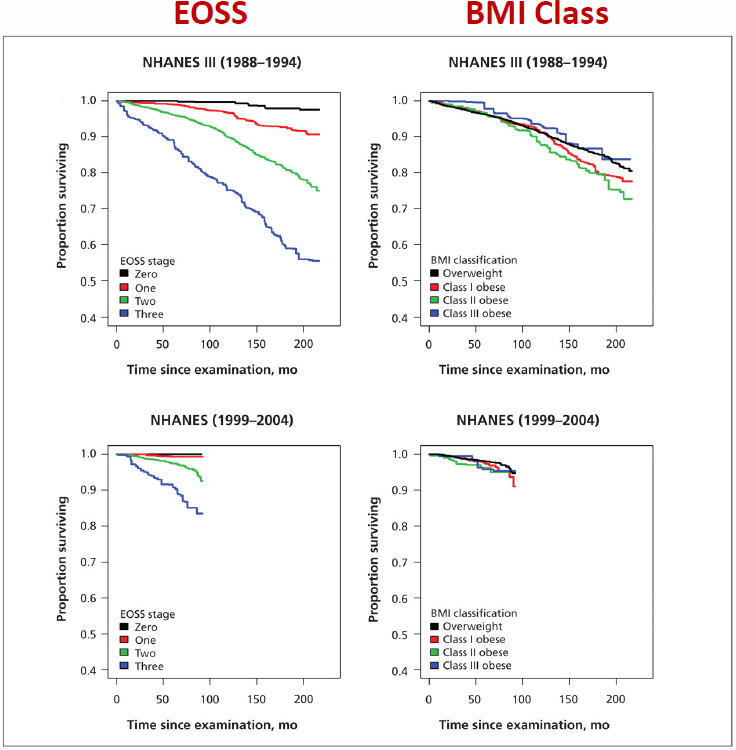
NOTE: BMI = body mass index; EOSS= Edmonton Obesity Staging System; NHANES = National Health and Nutrition Examination Survey.
SOURCES: Presented by Geoff Ball, June 26, 2023. Padwal et al. (2011). Reprinted with permission.
He shared four domains of health risk factors in the EOSS-P: mental, metabolic, mechanical, and social milieu. Ball explained that each of the domains is scored from 0 to 3, and the highest score in any domain is the EOSS-P score (also 0–3) (see Figure 6-3). He noted that in the pediatric population, anthropometrics reliably identified health risk using the EOSS-P across weight categories (Hadjiyannakis et al., 2019).
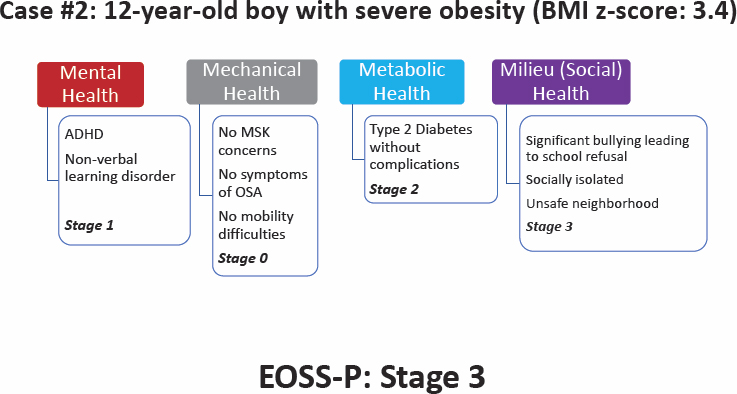
Ball elaborated that the EOSS-P provides a comprehensive health assessment of children’s health. To illustrate how it works, he presented two case studies.
The first was a 12-year-old boy with severe obesity (see Figure 6-4). In each of the four domains, the boy scored 0, for an overall EOSS-P score of 0. Ball commented that it may be surprising for a boy with severe obesity to be in Stage 0. However, research has shown that approximately 10–15 percent of children who have severe obesity are in this category; they are typically younger and have less visceral or central adiposity. Ball pointed out that using an anthropometric measure, such as BMI, would tell a different, abbreviated story.
Ball introduced a second case study to demonstrate how the EOSS-P can differentiate health risk for children with the same BMI z-score (see Figure 6-5). Reviewing the scores in each of the four domains, mental
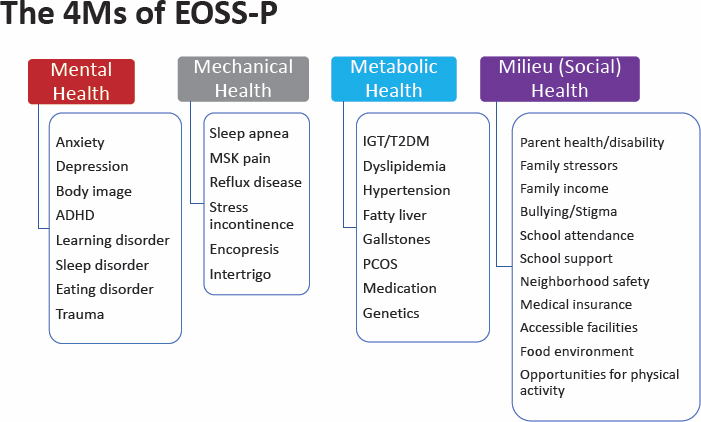
NOTE: ADHD = attention-deficit/hyperactivity disorder; BMI = body mass index; EOSS-P = Edmonton Obesity Staging System for Pediatrics; IGT/T2DM = impaired glucose tolerance/type 2 diabetes mellitus; PCOS = polycystic ovary syndrome.
SOURCE: Presented by Geoff Ball, June 26, 2023. Reprinted with permission.
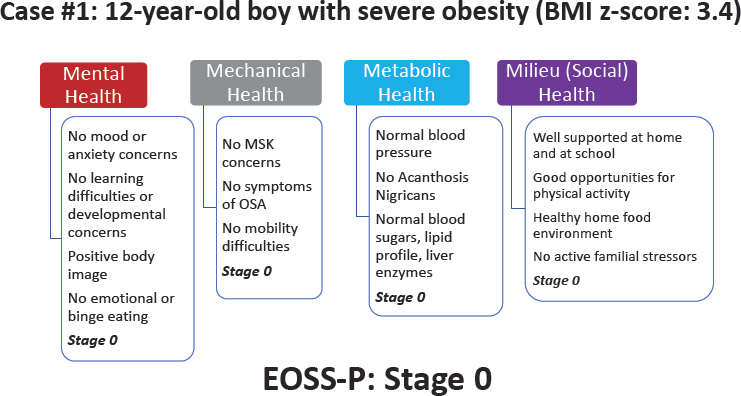
NOTE: BMI = body mass index; EOSS-P = Edmonton Obesity Staging System for Pediatrics; MSK = musculoskeletal system; OSA = obstructive sleep apnea.
SOURCE: Presented by Geoff Ball, June 26, 2023. Reprinted with permission.
NOTE: ADHD = attention-deficit/hyperactivity disorder; BMI = body mass index; EOSS-P = Edmonton Obesity Staging System for Pediatrics; MSK = musculoskeletal system.
SOURCE: Presented by Geoff Ball, June 26, 2023. Reprinted with permission.
health (1), mechanical (0), metabolic (2), and social milieu (3), generates an EOSS-P score of 3. The boy has ADHD and a nonverbal learning disorder (mental health), has type 2 diabetes without complications (metabolic), and is socially isolated in an unsafe neighborhood where he has experienced severe bullying (milieu). Ball reminded the audience that an anthropometric measure would be the same as in the first case study.
Ball highlighted an observational cohort study that examined the health risks of 850 boys in Canada and tested the association between the EOSS-P and the WHO BMI classification (Hadjiyannakis et al., 2019). The results showed that mental health concerns were most common of the four EOSS-P domains. Metabolic and mental health were equally distributed across BMI classes, and the social milieu and mechanical health challenges were more common in children with class III obesity. He also shared that BMI class underestimated disease burden in some children, overestimated it in others, and did not identify health risks. Ball ended by emphasizing that in the same study, the EOSS-P revealed health risks across weight categories.
PANEL AND AUDIENCE DISCUSSION
Scott Butsch led the audience and panel in a moderated discussion. Questions from the audience focused on the differences between the EOSS and EOSS-P; differences between the terms “obesity” and “ABCD”; communicating with patients about internalized weight bias; and the Lancet Commission’s engagement with researchers in nutrition and social science.
Differences Between the EOSS and EOSS-P
Butsch asked the first question to Ball, who responded that the EOSS-P has fewer categories because obesity and its process do not manifest in children as they do in adults, such as a relatively low prevalence of type 2 diabetes, hypertension, and obesity-related conditions.
He also mentioned some subjectivity in the social milieu domain of the EOSS-P. The results depend on the instrument used, the person reporting symptoms or experiences, and whether the respondent is a parent or a child.
Developing tools for clinicians requires thinking about the end user and the ability to integrate into electronic health records (EHRs). Novel approaches must be easy, evidence based, and reliable.
Communicating with Patients About Internalized Weight Bias and the Biology of Weight Regulation
Butsch turned to Rubino’s presentation on internalized weight bias among patients with overweight or obesity. How can clinicians communicate
and bring this bias into a discussion with patients in a surgical clinic? Rubino said that internalized weight bias is clear in his practice and that patients are typically apologetic and feel they need to justify their bariatric surgery.
Patients seeking surgery for other problems, such as for the gallbladder, expect to be treated with respect and recognized as needing medical attention for a medical problem. They do not feel the need to justify their surgery. Medical professionals are partly responsible for internalized weight bias because of their focus on fixing obesity through lifestyle changes.
Rubino shared that when talking with bariatric surgery patients, he starts by explaining the biology of weight regulation and that patients cannot undo obesity at will, which normalizes the patient–provider relationship.
Garvey added that a recent AACE policy statement calls out internalized weight bias as a parameter for clinicians to assess in all patients. It must be identified like other health complications because it affects quality of life and the success of medical treatment, and it is an area for future study.
Differences Between Obesity-Related Terminology: ABCD Versus Cardiometabolic Disease
An audience member asked Garvey if ABCD is also a cardiometabolic disease. Furthermore, what is the difference between “complication” and “comorbidity in ABCD?” Garvey reiterated that obesity is not a cardiometabolic disease but that it has a complex interrelationship. Furthermore, he said, obesity is not required for cardiometabolic disease. Someone with ample lean body mass may have cardiometabolic disease if fat is distributed in their intra-abdominal area and they have inflammation and structural changes in their fat tissue. The reason that most patients who are diagnosed with cardiometabolic disease also have excess adiposity or obesity is that excess adiposity exacerbates it, and it can improve with weight loss.
Garvey noted that the Lancet Commission is addressing the term “complication.” Rubino added that commission members have different opinions about comorbidities versus complications. According to Garvey, a comorbidity of obesity is a related complication, disease, or condition that occurs because of it. However, the commission report will distinguish between comorbidities and conditions, disorders, or diseases based on the pathophysiology of obesity.
EOSS for Adults Versus Children
The audience posed two more questions for Ball about the differences between EOSS-P and EOSS. Do the four domains apply only to the pediatric population (EOSS-P)? How is BMI incorporated into the adult EOSS? Ball reiterated that the four domains can stand alone and were
designed to group multiple health indicators into one measure; some measures may be missing from the EOSS-P.
Garvey noted evidence that childhood obesity shortens the life span by 10 years and emphasized the seriousness of it. He asked how the staging in pediatrics relates to adult health?
Ball considered Garvey’s concern as the reason for tracking EOSS-P scores over time. The scores have some predictive use but vary based on the person administering the EOSS-P. If obesity is a dysfunction of the weight regulatory system early in childhood, he explained, an adolescent may not have any complications of obesity, yet still have it.
Rubino added that regardless of the approach or staging, all three panelists agree that BMI cannot be a clinical parameter. The Lancet Commission is addressing the pediatric area of research in terms of how to define the illness of obesity.
Lancet Commission Engagement with Nutrition and Social Science Researchers
An audience member commented on the social and environmental impacts of obesity, asking whether the Lancet Commission is discussing it with nutrition and sociology researchers. According to Garvey, because obesity is linked to several other chronic conditions, such as diabetes, cardiovascular disease, and nonalcoholic fatty liver disease, the commission is consistently interacting and engaging with researchers at professional meetings, such as the American Diabetes Association.











