Measuring Meaningful Outcomes for Adult Hearing Health Interventions (2025)
Chapter: 3 General Principles for Core Outcome Sets and Outcome Measurement
3
General Principles for Core Outcome Sets and Outcome Measurement
As noted in Chapter 1, outcome measurement is important in health care, as the resulting data can help clinicians, researchers, and individuals understand which interventions work best for which populations and thereby improve the patient experience (ICHOM, n.d.). Understanding which outcomes are most important to measure, as well as how to measure those outcomes, is the key to assessing the efficacy and effectiveness of various interventions. Furthermore, creating consistency in a field of health care through the use of a core outcome set (and corresponding standardized measures)1 contributes to strengthening the evidence base and allowing for comparison across time, samples, and interventions. This chapter provides an overview of best practices for the development of core outcome sets, the assessment of outcome measures (in general), and the state of core outcome set development (and corresponding measurement) for hearing health interventions. The meaningfulness of specific outcomes are discussed in Chapter 4 and then more fully described and assessed in Chapter 5. Chapter 6 evaluates current measures for core outcomes.
___________________
1 The word standardized is used here in a broad sense to indicate that the same measures are being used for specific outcomes, and that there are prescribed materials and procedures for the use of these measures. The committee does not imply that the measures are part of national or international standards.
OVERVIEW OF OUTCOMES AND OUTCOME MEASURES
The Institute of Medicine proposed a framework where health care quality is described by the six aims of health care (i.e., safe, timely, effective, equitable, efficient, and patient-centered) (IOM, 2001). Quality of health care is assessed by structural, process, and outcome measures. Structural measures generally consider the provider’s capacity and infrastructure while process measures generally consider whether the clinician or provider follows clinical practice guidelines (AHRQ, 2015). Outcome measures, the focus of this study, seek to quantify the effect of an intervention or treatment on health status in specific outcomes (Williamson et al., 2017).
Patient-reported outcome measures gather data directly from patients about their perceived health status and outcomes. Patient-reported outcomes have been defined as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else” (NQF, 2013). Typical patient-reported outcomes in health care include health-related quality of life, symptoms and symptom burden, experience with care, functional status, and health behaviors (Churruca et al., 2021; NQF, 2013). As noted by Tysome and colleagues:
Outcome measures are often clinician-[centered], measuring aspects that are deemed important primarily to health care professionals. However, it is our patients’ well-being and the impact of our interventions on their lives that are most important in our practice. It follows that patient-[centered] outcomes should be [prioritized] in assessing both individual practice and reporting results of clinical trials. (Tysome et al., 2015, p. 512)
Patient-reported outcome measures are ideally standardized, psychometrically validated questionnaires that collect data directly from patients about their health-related priorities and outcomes (Churruca et al., 2021).
CORE OUTCOME SETS
When assessing the effect of an intervention on an individual’s health, researchers and clinicians can consider measuring a multitude of outcomes. A core outcome set recommends what specific outcomes, at a minimum, researchers and clinicians should measure and report when assessing an intervention’s effectiveness (Clarke and Williamson, 2016). Failure to report consistently defined outcomes, failure to use a common set of measures
to evaluate those outcomes, or failure to evaluate outcomes prioritized by key partners (such as patients and clinicians) leads to research waste (Chalmers and Glasziou, 2009; Kirkham and Williamson, 2022; O’Connor and Brinker, 2013).2
The failure to measure a common set of outcomes has been noted across many areas in health care research. For example:
- An examination of 8,942 oncology clinical trials on ClinicalTrials.gov led to the identification of “more than 25,000 outcomes across oncology trials that occurred only once or twice” (Hirsch et al., 2013, p. 977).
- A review of 99 studies of bariatric surgery outcomes found that 85 percent of the 1,088 outcomes reported were only reported in one study (Hopkins et al., 2015).
- A review of 278 papers on the treatment of conductive and mixed hearing loss found 837 different outcomes were reported (Hill-Feltham et al., 2021).
Furthermore, researchers may define or measure commonly used outcomes differently (Williamson et al., 2017). For example, in the review of 99 studies of bariatric surgery outcomes, even common outcomes such as weight loss were either not defined or had contradictory definitions (Hopkins et al., 2015).
Core outcome sets and their corresponding measures have typically been defined for use in research (and clinical trials in particular) to enhance the consistency and quality of research, allow for meta-analysis of multiple smaller studies, and facilitate comparison of effectiveness among different interventions (to inform health care decision making) (Chiarotto et al., 2017; Clarke and Williamson, 2016; Kirkham et al., 2017; Kirkham and Williamson, 2022; Tysome et al., 2015; Williamson et al., 2017). As noted by Clarke and Williamson (2016):
[T]he [standardization] which is achieved with a core outcome set is not intended to stifle innovation. The outcomes in the set should represent the minimum to be collected in all trials, and researchers should continue to measure and report additional outcomes of particular relevance to their topic. (p. 2)
Creating a comprehensive yet feasible core outcome set is challenging but not new to health care. Over the last few decades, hundreds of
___________________
2 “Research waste refers to poor-quality research output that is often perceived as of minimal use to health policy makers and clinicians” (Sogi, 2023, p. 179).
core outcome sets have been developed for specific health conditions or specific populations in either research or clinical settings (or both) (Gargon et al., 2021; Kirkham et al., 2017; Prinsen et al., 2016a). The number of core outcome sets has been increasing, as has the number of sets intended for use in both research and clinical settings. However, Gargon et al. (2021) note that “further research is needed to understand and optimize the methods used to develop [core outcome sets] for multiple settings” (p. 9).
Best Practices for Developing a Core Outcome Set
The Core Outcome Measures in Effectiveness Trials (COMET) Initiative strives to raise awareness of problems with outcome measurement, encourage the development and implementation of evidence-based core outcome sets, promote patient engagement in the process, provide resources, and reduce duplication of efforts (Williamson et al., 2017). The COMET Handbook notes that developing a core outcome set requires engaging multiple partners to achieve consensus. This is typically done through an iterative Delphi process. However, “research to identify optimal methods of developing [core outcome sets] is ongoing and there is currently wide variation in the approaches used” to build consensus (Williamson et al., 2017, p. 6).
The Core Outcome Set-STAndards for Development (COS-STAD) project used a consensus-based process to identify methodological guidelines for the development of core outcome sets, noting that “no gold standard method for the development of a [core outcome set] currently exists” (Kirkham et al., 2017, p. 2). The COS-STAD project identified 11 minimum standards for the development of core outcome sets, “regardless of the specific consensus method chosen,” but it did not address how to define or measure those outcomes (Kirkham et al., 2017). These standards fall into three main areas: scope specification, involvement of key partners, and consensus process.
Overall, core outcome set developers first need to determine what to measure (known as the scope) (Kirkham et al., 2017; Williamson et al., 2017). This includes specifying the health condition, the target population, specific interventions, and specific settings (i.e., research, routine care, or both). Next, the developers need to review the current evidence base in order to identify all potential core outcomes as well as to determine which outcomes are most important to measure (Williamson et al., 2017). Evidence may come from sources such as systematic reviews, published individual qualitative and quantitative studies, national datasets, and importantly, through direct input from key partners (including researchers, affected individuals, care partners, and clinicians) on what outcomes
are the most meaningful to them. As noted by Clarke and Williamson (2016):
If core outcome sets are to contribute to improvements in health and social care, by helping patients and the public, practitioners and policy makers to make better decisions about interventions, they need to contain the outcomes that really matter to these stakeholders. (p. 2)
Once the range of potential core outcomes has been identified, developers need to define outcomes with agreed-upon, unambiguous definitions of the outcome (Williamson et al., 2017). The process for coming to consensus on the set needs to be transparent. Criteria for determining the individual outcomes to be included in the core set need to be defined in advance, as well as how outcomes will be dropped or added during the process (Kirkham et al., 2017).
MEASUREMENT OF CORE OUTCOMES
A variety of outcome measures often exist that could be used to evaluate a specific outcome; this complicates the ability to compare interventions (Williamson et al., 2017). Once a core outcome set is established, the next challenge is to determine how to measure the individual outcomes (Clarke and Williamson, 2016). Two examples of published principles and methods to inform the process of identifying a core set of outcomes and their corresponding measures are described below.
Core Quality Measures Collaborative
The Core Quality Measures Collaborative (CQMC) published a series of principles for core set measure selection (CQMC, 2021). For creating core measure sets, it emphasizes that the set must be comprehensive and holistic to assess if care meets the full definition of high-quality care as defined by the Institute of Medicine (IOM, 2001). According to CQMC, measures should produce meaningful and useful information for patients, consumers, and clinicians. A core measure set needs to have a balance between specialty-specific measures and measures that are relevant across specialties and settings. The set should be as concise and efficient as possible to reduce the burden and include a mix of types of measures. CQMC recommends including patient-reported measures and standardized digital measures. It encourages the innovation of novel measures, particularly ones addressing the social determinants of health.
Additionally, CQMC has outlined a series of necessary measure characteristics, including that the measures should promote health and
health care improvement, not have harmful unintended consequences, measure social needs, be scientifically sound, and balance innovation with burden.
COnsensus-based Standards for the selection of health Measurement INstruments
COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) is an international initiative focused on developing best practices for selecting appropriate outcome measures for both research and clinical practice (COSMIN, n.d.). COMET and COSMIN partnered to develop guidelines for the selection of measures for a core outcome set (Prinsen et al., 2016a,b). These guidelines were developed through a consensus-based Delphi process.
The best practices identified by COSMIN for selecting core measures mirrors the process for determining a core outcome set (see Figure 3-1). First, the outcome, domain, target population, and context of use need to be identified. The guideline further notes that separate measures may be needed for subgroups of the target population or by setting. Next, all existing measures need to be identified through the examination of systematic reviews, literature reviews, and other sources. Many different outcome measures need to be considered including “assessments by health professionals, biomarkers, clinical rating scales, imaging tests, laboratory tests, patient questionnaires, and performance-based tests” (Prinsen et al., 2016b, p. 2). Third, the outcome measures need to be evaluated for their quality. (See later in this section for a discussion of quality assessment of outcome measures.)
Finally, one measure needs to be selected for each core outcome, with recognition, as noted earlier, that a different or additional measure may be needed based on specific target population subgroups or settings. The COSMIN guideline notes:
Ideally, an [outcome measure] included in a [core outcome set] has high-quality evidence for all measurement properties. However, in practice, there is often unknown or (very) low evidence for some measurement properties. (Prinsen et al., 2016a, p. 17)
The guideline suggests that some measures may be recommended based on a baseline of evidence for its quality, but that for others, further validation studies or the development of a new measure may be needed. Similar to the identification of the initial core outcome set, the guideline suggests using a consensus process to select the final outcome measures.
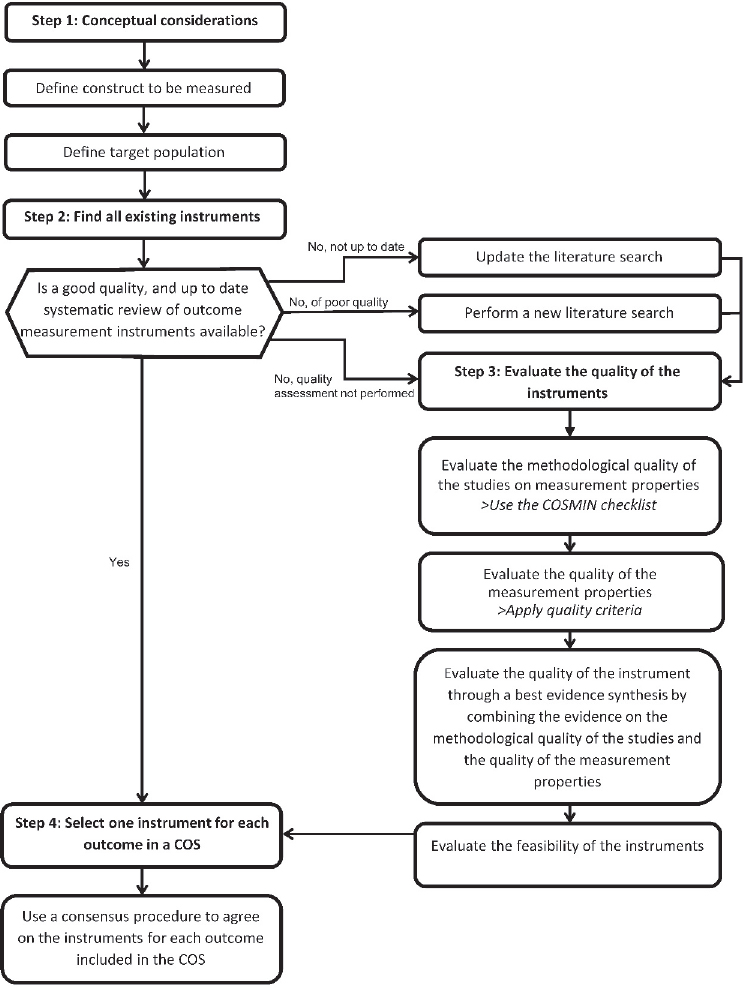
NOTE: COS = core outcome set; COSMIN = COnsensus-based Standards for the selection of health Measurement INstruments.
SOURCE: COSMIN, 2024b. CC BY 4.0.
ASSESSMENT OF OUTCOME MEASURES
Different groups use slightly different methodologies for assessing the quality of individual outcome measures. Some key initiatives are highlighted below.
Partnership for Quality Measurement
In 1999, the National Quality Forum (NQF) was established to promote consensus-based practices for quality measurement and public reporting (NQF, n.d. a). NQF, with support from the Centers for Medicare & Medicaid Services (CMS), developed a process for evaluating and endorsing measures, which also included the consideration of measure harmonization (consideration of other related or competing measures). As of March 2023, CMS now contracts with Battelle (under its Partnership for Quality Measurement [PQM]) as the consensus-based entity to review and endorse quality measures (NQF, n.d. b).3
Criteria for Endorsement
PQM uses five criteria to determine endorsement: importance, feasibility, scientific acceptability, use and usability, and equity (PQM, 2023). Importance reflects whether use of the measure can lead to improved outcomes and whether the outcome is meaningful to target populations. Feasibility considers the “people, tools, tasks, and technologies necessary to implement [the] measure” (PQM, 2024a). Scientific acceptability comprises the measure’s reliability (whether the measure can be implemented consistently and the results are repeatable) and validity (whether the measure has been risk adjusted, and the score can identify good versus poor quality). Use and usability reflects whether the information gathered from the measure can be used by various partners (e.g., consumers, clinicians, policy makers) to improve care. Finally, the optional domain of equity reflects “the extent to which the measure can identify differences in care for certain patient populations” (PQM, 2024b).
COSMIN Guideline
As noted previously, COSMIN provides guidance on suggested methods for assessing both the quality of the studies of an outcome measure as well as the quality of the measure itself (Prinsen et al., 2016a). Similar to the PQM approach, COSMIN’s criteria for high-quality measurement
___________________
3 This responsibility was previously held by the National Quality Forum.
properties consider reliability (including internal consistency and measurement error), validity (including content validity, structural validity, and cross-cultural validity), and responsiveness (i.e., the ability of the measure to detect change over time). COSMIN specifically suggests first evaluating the measure for its content validity (i.e., does the content of the measure adequately reflect the outcome), including face validity. COSMIN suggests examining internal structure of the measure next, focusing on structural validity (i.e., can the scores of the measure adequately reflect the dimensionality of the outcome) and internal consistency (i.e., the interrelatedness of the measure items). Finally, it suggests other criteria be used as appropriate to the specific measure and context. The COSMIN guideline (Prinsen et al., 2016b) also recognizes the importance of considering various aspects of the feasibility of implementing each measure:
- Patient’s comprehensibility
- Interpretability
- Ease of administration
- Length of the outcome measure
- Completion time
- Patient’s mental ability level
- Ease of standardization
- Clinician’s comprehensibility
- Type of outcome measure
- Cost of an outcome measure
- Required equipment
- Type of administration
- Availability in different settings
- Copyright
- Patient’s physical ability level
- Regulatory agency’s requirement for approval
- Ease of score calculation
CORE OUTCOME SETS AND MEASUREMENT FOR HEARING HEALTH INTERVENTIONS
No core outcome set has been broadly accepted by the hearing health community (Allen et al., 2022; Barker et al., 2015; Cox et al., 2000; Danermark et al., 2013; Granberg et al., 2014; Gurgel et al., 2012). In addition to the lack of consensus for the most meaningful outcomes, a variety of measures are being used to assess the same outcomes. For example, Granberg and colleagues (2014) performed a systematic review of outcome measures for hearing health interventions among studies separated into two pools. Pool 1 contained articles from large databases (i.e., MEDLINE,
CINAHL, EMBASE, and PsycInfo) and pool 2 contained articles from smaller databases (i.e., AMED, ERIC, Sociological Abstracts, PsycArticles, and CENTRAL). A total of 246 different outcome measures were identified among the 87 studies in pool 1, and 122 measures were identified among the 35 studies included in pool 2. Thirty-five percent of the outcome measures in pool 1 and 20 percent of the outcome measures in pool 2 involved audiometric measures of speech understanding under controlled test conditions. Furthermore, a study of the outcome measures used in the use of telehealth for both cochlear implant and hearing aid users showed that its “systematic literature review of 49 articles revealed over 250 discrete outcomes” (Laird et al., 2024, p. 1).
The use of multiple different measures further contributes to the inability to compare studies or pool data. As noted by Barker and colleagues, “A recommended core outcome set would not preclude researchers from including other measures relevant to an individual study but would at least provide a central dataset for meta-analysis” (Barker et al., 2015, p. 571). Moreover, the outcomes and corresponding measures currently being used may not adequately reflect the “real-world experiences and needs” of individuals with hearing difficulties (Moberly et al., 2023). The following sections highlight several distinct efforts to develop core outcome sets or define baseline outcome measures for hearing health interventions. These encompass both broad and more narrow aspects of hearing health.
International Outcome Inventory
In 1999, the Eriksholm Workshop on Measuring Outcomes in Audiological Rehabilitation Using Hearing Aids convened 15 experts to determine the state-of-the-art in self-report outcome measurement, to identify areas of disagreement or controversy, to outline approaches to resolving problems, and, in general, to work toward a coherent framework for outcome measurement that will encompass all of the goals and requirements for these kinds of data (Cox, 2000).
The group noted that outcome measurement serves different purposes, including to assess rehabilitative outcomes for an individual with hearing difficulties (in the clinical setting), to assess the effectiveness of a particular provider, to assess the effectiveness of a new intervention (e.g., clinical trials), and to evaluate the effect of the intervention on quality of life (Cox et al., 2000). The group agreed that “the characteristics of an optimal outcome measure vary as a function of the purpose of the measurement,” and it endorsed “the concept of generating a brief universally applicable outcome measure” (Cox et al., 2000). The work of this group led to the development of the International Outcome Inventory for Hearing Aids (IOI-HA)—a seven-item questionnaire to evaluate the effectiveness of hearing aids (Cox and
Alexander, 2002). The group envisioned this measure as a supplement to outcome measures used for specific settings, interventions, or other narrower purposes as a means to pool research data across studies (Cox et al., 2000).
A version of the questionnaire exists both for hearing aids (IOI-HA) (Cox et al., 2000; Cox and Alexander, 2002) and for alternative interventions (IOI-AI), such as auditory training (Noble, 2002). The International Outcome Inventory measures are composed of seven separate items, each assessing one of the following areas:
- hours of use,
- benefit,
- residual activity limitations,
- satisfaction,
- residual participation restrictions,
- impact on others, and
- quality of life.
The International Outcome Inventory measures assume that each of the seven domains assessed is important and independent from the others such that results are typically displayed as an array of seven scores compared to norms for each of the seven domains. However, factor analyses of responses to the seven items have typically identified either one or two factors reflecting the correlation among the responses to the seven items (Wong and Hickson, 2012).
International Classification of Functioning, Disability, and Health
The World Health Organization’s International Classification of Functioning, Disability, and Health (ICF), endorsed in 2001, is considered the “international standard to describe and measure health and disability” (WHO, 2025). The framework is based on an integrated model that considers an individual’s functioning and disability as well as their individual contextual factors, such as environmental factors (e.g., family attitudes, organizational policies) and personal factors (e.g., age, gender) (see Figure 3-2; Meyer et al., 2016).
Both comprehensive and brief core sets have been developed for a variety of health conditions based on the ICF framework. Danermark et al. found:
A [comprehensive core set] includes as many categories as needed for describing the whole spectrum of problems for a certain condition. The [brief core set] is derived from the [comprehensive core set] and serves as a brief assessment of the functioning of a person with a certain condition. (Danermark et al., 2013, p. 324)
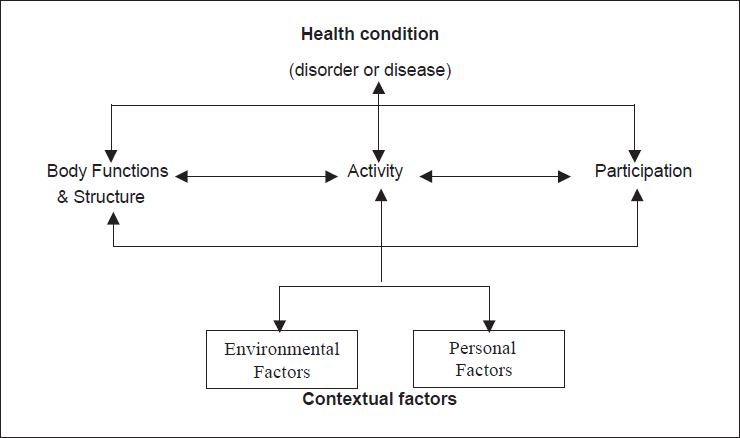
NOTE: ICF = International Classification of Functioning, Disability, and Health.
SOURCE: Geneva, Switzerland: World Health Organization; 2002. CC BY-NC-SA 3.0 IGO.
Both ICF core sets for hearing loss include outcomes in the domains of body function (e.g., hearing functions), body structure (e.g., structure of external, middle, and inner ear), participation (e.g., handling stress and other psychological demands, conversation, community life), and environment (e.g., products and technology for communication, immediate family, health services and systems). However, it is important to note that unlike the focus of this report, these core sets are intended for a comprehensive description of the individual’s hearing-related function, and not as a baseline for reporting outcomes.
Standardized Format for Reporting Hearing Outcomes in Clinical Trials
In 2012, with input from a range of hearing experts and organizations, the Hearing Committee of the American Academy of Otolaryngology-Head and Neck Surgery endorsed a standard for reporting hearing outcomes in clinical trials; it noted that “the lack of an adequate standardized method for reporting level of hearing function in clinical trials has hampered the ability of investigators to draw comparisons across studies” (Gurgel et al., 2012, p. 803). Over a 2-year period, the group developed a new hearing outcomes scale to reflect the function of patients across a wide range of audiometric loss. This scale consists of a scattergram that relates average pure-tone threshold to word
recognition score. The group emphasized that “the new standards represent minimal reporting standards. Investigators are encouraged to consider and report additional hearing data if relevant and create innovative ways to assess and report hearing outcomes” (Gurgel et al., 2012, p. 806).
It is important to note that in response to this study, the American Academy of Audiology voiced concern about the applicability of these reporting standards to all clinical trials (Carlson, 2013). It argued that the use of the average pure-tone threshold would not be sensitive to noise-induced hearing loss or drug-induced ototoxicity and that word recognition tests would be problematic for “non-English speakers, pediatric populations, ill patients, and in situations when time constraints are necessarily imposed” (Carlson, 2013, p. 349).
In a subsequent response, Jackler and colleagues (2013) noted that “while we acknowledge that there is no such thing as an ‘ideal’ standard, we do believe that the formula adopted will substantially improve the value of our literature” (p. 350). They further noted that
The standard merely defines a minimum dataset for comparison purposes and is not expected to be sufficient, unto itself, for many research purposes. Authors are encouraged to include additional data as needed to fully describe their study population.
Auditory Rehabilitation Outcomes Network
The Auditory Rehabilitation Outcomes Network (AURONET) is an international group established in 2014 to develop core sets of patient-centered hearing outcome measures for use in both clinical trials and individual practice settings based on the type of hearing loss (Tysome et al., 2015). The initiative focuses on four core areas: hearing (e.g., auditory perception, speech discrimination, sound localization), physical (e.g., pain, ear discharge), economic (e.g., cost to individual, society, and health care system), and psychosocial (e.g., relationships, quality of life, perception of self) (Tysome et al., 2015).
The group’s first effort examined the treatment of conductive or mixed hearing loss (Hill-Feltham et al., 2021; Johansson et al., 2018; Tysome et al., 2015). As noted earlier in this chapter, a scoping review of the literature on the treatment of conductive and mixed hearing loss found 837 different outcomes were reported among the 278 papers reviewed (Hill-Feltham et al., 2021). Hill-Feltham and colleagues (2021) grouped these outcomes into nine domains of types of measures:
- hearing threshold,
- speech testing,
- questionnaires,
- immittance,4
- binaural,
- electrophysiology,
- tinnitus, and
- device output.
Hearing threshold measures (e.g., air–bone gap, pure-tone average, bone conduction thresholds, air conduction thresholds) accounted for about two-thirds (64.5 percent) of the reported outcomes. Speech testing accounted for about 20 percent of the reported outcomes, and the authors noted that the number of different measures used were too numerous to list them all in the study. Questionnaires (i.e., patient-reported outcome measures) accounted for only 8.8 percent of the measures reported. The researchers noted that “no single outcome measurement was reported in all studies” (Hill-Feltham et al., 2021, p. 244). They also noted that:
In summary, the predominance of audiometry-based outcome measurements may have led to a comparative paucity of other outcome measurement being used. A [core outcome set] containing a broader range of outcome measurements with greater face validity to real-world situations would enhance the present evidence base, as well as moving away from a predominately clinician-[centered] approach, to a more patient-[centered] one. (p. 244)
Core Outcome Measures in the Tinnitus International Delphi Study
The TINnitus research NETwork (TINNET) is a European collaboration that focuses, in part, on developing “standards for tinnitus clinical trials and outcome measurements in clinical trials and everyday practice” (Hall et al., 2015, p. 4). Drawing on best practices described by COMET and COSMIN (earlier in this chapter), the Core Outcome Measures in Tinnitus International Delphi (COMiT’ID) initiative used a Delphi study process to propose core outcome sets for sound-based, psychology-based, and drug-based clinical trials of chronic subjective tinnitus in adults (see Table 3-1; Hall et al., 2018). The three core outcome sets share one common outcome: tinnitus intrusiveness. The initiative’s next steps include determination of the best measure for each outcome.
Self-Report Core Outcome Domain Set
An Australian initiative noted that while there are a variety of outcome domains for hearing rehabilitation, “there is no consensus about which
___________________
4 The domain of immittance contains objective outcome measurements of middle ear function (Hill-Feltham et al., 2021).
TABLE 3-1 COMiT’ID Core Outcome Sets by Intervention Type
| Sound-Based Trials Core Set | Psychology-Based Trials Core Set | Drug-Based Trials Core Outcome Set |
|---|---|---|
| Tinnitus intrusiveness | Tinnitus intrusiveness | Tinnitus intrusiveness |
| Ability to ignore | Tinnitus acceptance | Tinnitus loudness |
| Concentration | Mood | |
| Quality of sleep | Negative thoughts and beliefs | |
| Sense of control | Sense of control | |
NOTE: COMiT’ID = Core Outcome Measures in Tinnitus International Delphi.
SOURCE: Hall et al., 2018. CC BY 4.0.
outcome domains should be measured, when they should be measured, and how they should be measured” (Allen et al., 2022, p. 1). The consensus-based process engaged 79 hearing health professionals and 64 adults with hearing difficulties in a three-round Delphi process to identify the “most important” domains. This process resulted in seven potential domains, which were narrowed to four top domains by a group of 11 experts (including clinicians and patient advocates). These four domains (in rank order) were:
- communication ability,
- personal relationships,
- well-being, and
- participation restrictions.
The study reported that there was a dearth of measures for a wide variety of the domains deemed important.
FINDINGS
This section lists the committee’s findings under their appropriate headings.
Core Outcome Sets
Finding 3-1: Core outcome sets represent the minimum set of data to be collected.
Finding 3-2: A lack of standard outcomes leads to multiple different outcomes being measured and reported for the same condition.
Finding 3-3: The use of core outcome sets can help enhance the consistency and quality of research, allow for meta-analysis of multiple
smaller studies, and facilitate the comparison of effectiveness among different interventions.
Finding 3-4: While there is no single best method for developing a core outcome set, using a consensus process is important. Other key steps and best practices include:
- Define scope;
- Gather evidence to identify all potential outcomes;
- Group outcomes into domains;
- Determine which outcomes are most important to measure; and
- Provide transparency of the consensus process, including inclusion criteria and the steps for adding and dropping outcomes during the process.
Measuring Core Outcomes
Finding 3-5: Multiple measures often exist to evaluate a single, specific outcome.
Finding 3-6: The use of different measures for the same outcome contributes to the challenges in comparing interventions.
Finding 3-7: The COSMIN process for selecting measures mirrors the process for determining a core outcome set, including:
- Use of a consensus-based process;
- Definition of scope;
- Gathering of evidence to identify all potential measures;
- Grouping of outcomes into domains; and
- Selection of one measure for each core outcome.
Finding 3-8: Different measures may be needed by context, such as for specific subpopulations or settings.
Assessment of Outcome Measures
Finding 3-9: In practice, there is often a lack of evidence for the quality of measures.
Finding 3-10: Different methodologies exist to assess the quality of individual measures.
Finding 3-11: PQM uses five criteria to determine endorsement: importance, feasibility, scientific acceptability (including reliability and validity), use and usability, and equity.
Finding 3-12: Similar to the PQM approach, COSMIN’s criteria for high-quality measurement properties consider reliability, validity, and responsiveness (i.e., the ability of the measure to detect change over time).
Core Outcome Sets for Hearing Health Interventions
Finding 3-13: No core outcome set has been broadly accepted by the hearing health community.
Finding 3-14: In addition to the lack of consensus for the most important outcomes, a variety of measures are being used to assess the same outcomes, which further contributes to the inability to compare studies or pool data.
Finding 3-15: Several efforts have sought to develop a core outcome set or define baseline outcome measures for hearing rehabilitation, either broadly or for more narrow aspects of hearing health.
REFERENCES
AHRQ (Agency for Healthcare Research and Quality). 2015. Types of health care quality measures. https://www.ahrq.gov/talkingquality/measures/types.html (accessed May 22, 2024).
Allen, D., L. Hickson, and M. Ferguson. 2022. Defining a patient-centred core outcome domain set for the assessment of hearing rehabilitation with clients and professionals. Frontiers in Neuroscience 16:787607.
Barker, F., E. MacKenzie, L. Elliott, and S. de Lusignan. 2015. Outcome measurement in adult auditory rehabilitation: A scoping review of measures used in randomized controlled trials. Ear and Hearing 36(5):567–573.
Carlson, D. 2013. American Academy of Audiology response to Gurgel et al. Otolaryngology-Head and Neck Surgery 149(2):349–350.
Chalmers, S., and P. Glasziou. 2009. Avoidable waste in the production and reporting of research evidence. Lancet 374(9683):86–89.
Chiarotto, A., R. W. Ostelo, D. C. Turk, R. Buchbinder, and M. Boers. 2017. Core outcome sets for research and clinical practice. Brazilian Journal of Physical Therapy 21(2):77–84.
Churruca, K., C. Pomare, L. A. Ellis, J. C. Long, S. B. Henderson, L. E. D. Murphy, C. J. Leahy, and J. Braithwaite. 2021. Patient-reported outcome measures (PROMs): A review of generic and condition-specific measures and a discussion of trends and issues. Health Expectations 24(4):1015–1024.
Clarke, M., and P. R. Williamson. 2016. Core outcome sets and systematic reviews. Systematic Reviews 5:11.
COSMIN (COnsensus-based Standards for the selection of health Measurement INstruments). n.d. About COSMIN. https://www.cosmin.nl/about (accessed June 25, 2024).
COSMIN. 2024b. Guideline for selecting instruments for a core outcome set. https://www.cosmin.nl/tools/guideline-selecting-proms-cos/ (accessed June 26, 2024).
Cox, R. 2000. Editorial. Ear and Hearing 21(4):1S.
Cox, R. M., and G. C. Alexander. 2002. The International Outcome Inventory for Hearing Aids (IOI-HA): Psychometric properties of the English version. International Journal of Audiology 41(1):30–35.
Cox, R., M. Hyde, S. Gatehouse, W. Noble, H. Dillon, R. Bentler, D. Stephens, S. Arlinger, L. Beck, D. Wilkerson, S. Kramer, P. Kricos, J. P. Gagné, F. Bess, and L. Hallberg. 2000. Optimal outcome measures, research priorities, and international cooperation. Ear and Hearing 21(4 Suppl):106s–115s.
CQMC (Core Quality Measures Collaborative). 2021. CQMC core sets: Guiding principles and documents: Principles for core set measure selection. https://www.qualityforum.org/CQMC_Core_Sets.aspx (accessed June 26, 2024).
Danermark, B., S. Granberg, S. E. Kramer, M. Selb, and C. Möller. 2013. The creation of a comprehensive and a brief core set for hearing loss using the International Classification of Functioning, Disability and Health. American Journal of Audiology 22(2):323–328.
Gargon, E., S. L. Gorst, K. Matvienko-Sikar, and P. R. Williamson. 2021. Choosing important health outcomes for comparative effectiveness research: 6th annual update to a systematic review of core outcome sets for research. PLOS ONE 16(1):E0244878.
Granberg, S., J. Dahlström, C. Möller, K. Kähäri, and B. Danermark. 2014. The ICF core sets for hearing loss—Researcher perspective. Part I: Systematic review of outcome measures identified in audiological research. International Journal of Audiology 53(2):65–76.
Gurgel, R. K., R. K. Jackler, R. A. Dobie, and G. R. Popelka. 2012. A new standardized format for reporting hearing outcome in clinical trials. Otolaryngology—Head and Neck Surgery 147(5):803–807.
Hall, D. A., H. Haider, D. Kikidis, M. Mielczarek, B. Mazurek, A. J. Szczepek, and C. R. Cederroth. 2015. Toward a global consensus on outcome measures for clinical trials in tinnitus: Report from the first international meeting of the COMIT initiative, November 14, 2014, Amsterdam, The Netherlands. Trends in Hearing 19:2331216515580272.
Hall, D. A., H. Smith, A. Hibbert, V. Colley, H. F. Haider, A. Horobin, A. Londero, B. Mazurek, B. Thacker, and K. Fackrell. 2018. The COMiT’ID study: Developing core outcome domains sets for clinical trials of sound-, psychology-, and pharmacology-based interventions for chronic subjective tinnitus in adults. Trends in Hearing 22:2331216518814384.
Hill-Feltham, P. R., M. L. Johansson, W. E. Hodgetts, A. V. Ostevik, B. J. McKinnon, P. Monksfield, R. Sockalingam, T. Wright, and J. R. Tysome. 2021. Hearing outcome measures for conductive and mixed hearing loss treatment in adults: A scoping review. International Journal of Audiology 60(4):239–245.
Hirsch, B. R., R. M. Califf, S. K. Cheng, A. Tasneem, J. Horton, K. Chiswell, K. A. Schulman, D. M. Dilts, and A. P. Abernethy. 2013. Characteristics of oncology clinical trials: Insights from a systematic analysis of ClinicalTrials.gov. JAMA Internal Medicine 173(11): 972–979.
Hopkins, J. C., N. Howes, K. Chalmers, J. Savovic, K. Whale, K. D. Coulman, R. Welbourn, R. N. Whistance, R. C. Andrews, J. P. Byrne, D. Mahon, and J. M. Blazeby. 2015. Outcome reporting in bariatric surgery: An in-depth analysis to inform the development of a core outcome set, the BARIACT study. Obesity Reviews 16(1):88–106.
ICHOM (International Consortium for Health Outcomes Measurement). n.d. Why measure outcomes? https://www.ichom.org/why-measure-outcomes (accessed November 12, 2024).
IOM (Institute of Medicine). 2001. Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academy Press.
Jackler, R., R. Gurgel, R. Dobie, and G. Popelka. 2013. Reply to Dr. Carlson’s letter: A new standardized format for reporting hearing outcome in clinical trials. Otolaryngology-Head and Neck Surgery 149(2):350.
Johansson, M. L., J. R. Tysome, P. Hill-Feltham, W. E. Hodgetts, A. Ostevik, B. J. McKinnon, P. Monksfield, R. Sockalingam, and T. Wright. 2018. Physical outcome measures for conductive and mixed hearing loss treatment: A systematic review. Clinical Otolaryngology 43(5):1226–1234.
Kirkham, J. J., and P. Williamson. 2022. Core outcome sets in medical research. BMJ Medicine 1(1):e000284.
Kirkham, J. J., K. Davis, D. G. Altman, J. M. Blazeby, M. Clarke, S. Tunis, and P. R. Williamson. 2017. Core Outcome Set-STAndards for Development: The COS-STAD recommendations. PLoS Medicine 14(11):e1002447.
Laird, E., C. Sucher, K. Nakano, and M. Ferguson. 2024. Systematic review of patient and service outcome measures of remote digital technologies for cochlear implant and hearing aid users. Frontiers in Audiology and Otology 2:1403814.
Meyer, C., C. Grenness, N. Scarinci, and L. Hickson. 2016. What is the International Classification of Functioning, Disability, and Health and why is it relevant to audiology? Seminars in Hearing 37(3):163–186.
Moberly, A. C., T. McRackan, and T. N. Tatami. 2023. Expanding real-world outcomes in adults with hearing loss. https://bulletin.entnet.org/clinical-patient-care/article/22873582/expanding-realworld-outcomes-in-adults-with-hearing-loss (accessed December 19, 2024).
Noble, W. 2002. Extending the IOI to significant others and to non-hearing-aid-based interventions. International Journal of Audiology 41(1):27–29.
NQF (National Quality Forum). 2013. Patient reported outcomes (PROs) in performance measurement. https://www.qualityforum.org/Publications/2012/12/Patient-Reported_Outcomes_in_Performance_Measurement.aspx (accessed June 26, 2024).
NQF. n.d. a. NQF’s history. https://www.qualityforum.org/about_nqf/history/ (accessed June 26, 2024).
NQF. n.d. b. Measure applications partnership. https://www.qualityforum.org/setting_priorities/partnership/measure_applications_partnership.aspx (accessed June 26, 2024).
O’Connor, D. P., and M. R. Brinker. 2013. Challenges in outcome measurement: Clinical research perspective. Clinical Orthopaedics and Related Research 471(11):3496–3503.
PQM (Partnership for Quality Measurement). 2023. Endorsement and maintenance (E&M) guidebook. https://p4qm.org/sites/default/files/2023-10/Del-3-6-Endorsement-and-Maintenance-Guidebook-Final_0.pdf#page=40 (accessed June 26, 2024).
PQM. 2024a. Endorsement & maintenance (E&M). https://p4qm.org/EM (accessed June 26, 2024).
PQM. 2024b. PQM measure evaluation rubric worksheet. https://p4qm.org/media/3161 (accessed December 20, 2024).
Prinsen, C. A. C., S. Vohra, M. R. Rose, M. Boers, P. Tugwell, M. Clarke, P. R. Williamson, and C. B. Terwee. 2016a. Guideline for selecting outcome measurement instruments for outcomes included in a core outcome set. https://cosmin.nl/wp-content/uploads/COSMIN-guideline-selecting-outcome-measurement-COS.pdf (accessed June 25, 2024).
Prinsen, C. A. C., S. Vohra, M. R. Rose, M. Boers, P. Tugwell, M. Clarke, P. R. Williamson, and C. B. Terwee. 2016b. How to select outcome measurement instruments for outcomes included in a “core outcome set”—A practical guideline. Trials 17(1):449.
Sogi, G. M. 2023. Research waste. Contemporary Clinical Dentistry 14(3):179.
Tysome, J. R., P. Hill-Feltham, W. E. Hodgetts, B. J. McKinnon, P. Monksfield, R. Sockalingham, M. L. Johansson, and A. F. Snik. 2015. The Auditory Rehabilitation Outcomes Network: An international initiative to develop core sets of patient-centred outcome measures to assess interventions for hearing loss. Clinical Otolaryngology 40(6):512–515.
WHO (World Health Organization). 2002. Towards a common language for functioning, disability and health (ICF). Geneva, Switzerland: World Health Organization.
WHO. 2025. International Classification of Functioning, Disability, and Health (ICF). https://www.who.int/standards/classifications/international-classification-of-functioning-disability-and-health (accessed January 11, 2025).
Williamson, P. R., D. G. Altman, H. Bagley, K. L. Barnes, J. M. Blazeby, S. T. Brookes, M. Clarke, E. Gargon, S. Gorst, N. Harman, J. J. Kirkham, A. McNair, C. A. C. Prinsen, J. Schmitt, C. B. Terwee, and B. Young. 2017. The COMET handbook: Version 1.0. Trials 18:1–50.
Wong, L., and L. Hickson. 2012. Evidence-based practice in audiology: Evaluating interventions for children and adults with hearing impairment. San Diego, CA: Plural Publishing.
This page intentionally left blank.



















