Health Risk Considerations for the Use of Unencapsulated Steel Slag (2023)
Chapter: 2 Electric Arc Furnace Steelmaking and Slag Formation, Composition, and Distribution
2
Electric Arc Furnace Steelmaking and Slag Formation, Composition, and Distribution
STEEL PRODUCTION AND SLAG FORMATION IN AN ELECTRIC ARC FURNACE
The growth of electric arc furnace (EAF) steelmaking in North America has occurred mainly in the past 40 years due to low electricity prices, an abundance of steel scrap (especially near large population centers), and the development of mini and macro steel mills based on metal recycling. Scrap availability and price has made steel produced by remelting in these new highly efficient steel plants very competitive in the world steel economy. During the melting of steel a multicomponent liquid oxide called slag is formed and floats on top of the liquid steel. The slag is separated from the liquid steel, cooled, solidified, and processed into a material suitable for an application. It is this processed EAF slag that is the subject of this study. Slags from other processes, either ferrous or non-ferrous, are not the focus of this report.
A schematic of the EAF is given in Figure 2-1 where the input and output materials per ton of production are highlighted. The furnace is refractory lined, and scrap and iron reduced from iron ore are charged into the furnace. (Refractory material is heat-resistant and infusible at the highest temperature of the steelmaking process.) Carbon electrodes pass current through the scrap to the furnace lining which creates an arc that supplies heat. Additional heat to aid in melting is supplied by blowing oxygen and natural gas. Alloys such as silicon and aluminum can be added to supply heat by reaction with oxygen, and their oxides, silica and alumina, become part of the slag. Lime, dolomite, and carbon are also added to the furnace during melting to control the slag chemistry. In addition, gases (mainly carbon monoxide, carbon dioxide, and nitrogen) and dusts from materials with a high vapor pressure such as zinc, tin, lead, and cadmium are vented from the furnace.
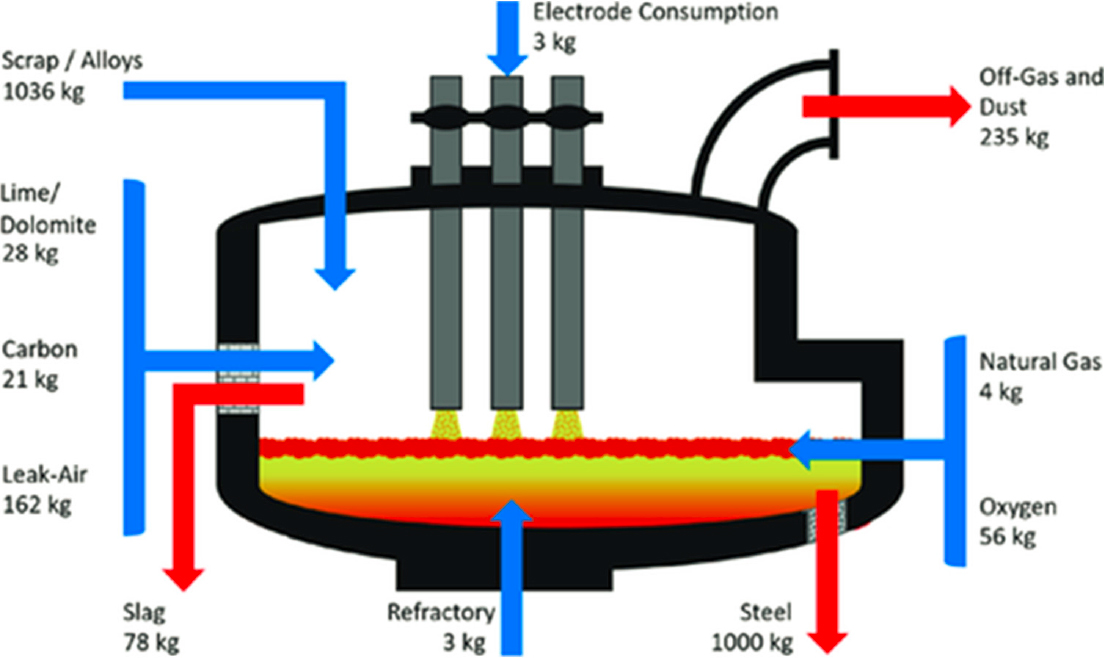
SOURCE: Hay et al. (2021).
Scrap can be of various qualities, depending upon the grade to be cast. In addition, other iron units, which are purer than scrap—for example, iron produced from iron ore that contains carbon (a pig iron) or a product called hot briquetted iron (HBI) from direct reduction of iron ore—can be added, if very high-quality steels are to be produced that require strict chemistry control.
Slag forms on top of liquid steel because liquid iron is not the stable form of iron in contact with air resulting in oxidation of iron. At higher temperatures, when the iron is liquid, slag (a liquid oxide) forms that initially is predominantly FeO as the majority of the liquid steel is iron. However, as liquid steel contains significant quantities of other elements, such as silicon and manganese, which are also not stable in air, these other elements become present in the slag that forms on top of the liquid steel bath.
The slag formed from oxidation is highly corrosive to refractory materials; in the EAF process, this slag chemistry is tailored by adding lime and magnesia (from dolomitic lime) to form a liquid slag that has sufficient area to cover the bath at a depth that reduces oxidation by acting as a barrier to contact between the air or injected oxygen and the bath. Silicon and aluminum can also be added to reduce FeO and MnO levels in the slag, to increase silica and alumina levels in the slag, and to control its chemistry.
This process leads to a liquid slag on top of the liquid steel that contains predominantly CaO, FeO, MnO, SiO2, and MgO. MgO contents are set to reduce refractory dissolution and increase furnace life; however, as almost all alloying elements present in the liquid steel can be oxidized, one also finds trace amounts of most of these alloying elements in the liquid slag at levels often less than 2 weight percent.
The EAF slag predominantly contains CaO, SiO2, MgO, MnO, and FeO; however, there is a significant range in the slag chemistry values. In addition to the alloying elements found in steels, the liquid slag reacts with the steel, and one finds phosphorus contents in the slag when it contains high levels of FeO and lime.
Liquid slag is controlled to ensure it has sufficient volume to cover the bath and a chemistry that ensures it is liquid. Steel plants have standard operating practices to ensure that the slag chemistry and volume fall within a range that ensures it is liquid and not corrosive to the refractory materials and that it is deep enough to ensure that oxidation of the liquid steel is controlled. The variation measured in the slag chemistry is related to the chemistry of the scrap that is melted, the amount of oxidation, the amount of slag forming materials added to the furnace, and the specifics of furnace operation. The liquid slag chemistry can be controlled within close tolerances when the scrap composition can be closely controlled, especially for the production of high-purity low carbon steels. For other EAF-produced steels for which scrap is not fully sorted, there can be significant quantities of residual elements (such as copper or nickel) that are not easily oxidized. In general, EAF steels can be readily recognized by their residual copper contents, as typical scrap recycling does not separate copper (such as in wires and other parts) during shredding. In addition, it is common for EAF steels to have higher nitrogen contents than steels produced from iron ore due to nitrogen pick-up during oxidation. Thus “clean” iron units from pig iron or HBI are added as a method of achieving higher purities.
The liquid slag from the EAF is separated from the liquid metal by pouring the liquid steel from the EAF into a receiving vessel referred to as a ladle. The liquid slag that remains in the furnace (the EAF slag) is then poured into another container (a slag pot) and transported (as a liquid) to an area in the plant where it is dumped into a pile and allowed to cool and solidify. Once solid and at a temperature where it can be safely handled, a slag processing company collects, grinds, and removes solid steel for resale and separates the slag particles by size before application. The EAF slag is the majority of the slag produced in an EAF plant (up to 75 percent). There are two other slag sources in an EAF operation: (1) the ladle slag that is formed by adding slag formers to the ladle, and (2) a pit slag that is formed by spillage and other uncontrolled processes. This ladle slag is generally a calcium aluminate with very low FeO and MnO contents, is often handled separately by the slag processor, and is the majority of the remaining slag weight. These three slags can be processed and sold separately or blended to form different products depending on desired end use. Products from blended slag will have different physical and chemical properties.
The amount of slag produced is a function of the amount of steel produced by an EAF. Thus, the amount of slag produced annually can be predicted from the amount of steel produced annually. Mexico, Canada, and the United States produce steel for the U.S. steel market. The numbers of EAFs and approximate steelmaking capacity in each country for steel production are given in Table 2-1.
TABLE 2-1 EAF Annual Steel Production Capacity in North America
| Country | Number of EAFs | EAF Potential Capacity (million tons) |
|---|---|---|
| Mexico | 22 | 14 |
| Canada | 15a | 8 |
| United States | 152b | 82 |
| Total | 189c | 104 |
a Includes 1 furnace currently not producing steel.
b Includes 19 furnaces not producing.
c Includes 20 furnaces currently not producing, with 1.5 million tons of idled capacity.
SOURCE: AIST (2023).
Approximately 60.9 million tons of steel were produced in the United States in 2021 by recycling using the EAF process to melt steel scrap. In general, between 68 to 72 percent of annual total steel production comes from steel recycling.
The potential maximum capacity of production by the EAF route in the United States is approximately 82 million tons per year; however, the actual production is significantly less than those values, and it varies annually with demand, steel price, availability of electricity at a competitive price, the number of active furnaces, and the level of imported steel. The Association for Iron & Steel Technology (AIST) annually publishes the production rates of steel mills in the United States. Production data only for EAF steel plants are shown in Table 2-2. The amount of EAF slag produced annually is estimated to be between 10 and 15 percent of the amount of steel produced. Thus, the weight of EAF slag produced in the United States in 2022 ranged between 5.9 and 8.9 million tons.
TABLE 2-2 EAF Actual Steel Production in the United States
| Year | EAF Production (million tons) | % of Total Steel Production by the EAF |
|---|---|---|
| 2018 | 58.8 | 68 |
| 2019 | 61.5 | 70 |
| 2020 | 51.6 | 71 |
| 2021 | 60.9 | 71 |
| 2022 | 59.0 | 72 |
SOURCE: USGS (2023a).
An EAF consolidates the scrap by melting to form a liquid pool. All materials in the scrap that are not oxidized or vaporized during the process become part of the overall chemistry of the resulting steel bath. The oxidized materials become part of the liquid slag that floats on top of the steel bath. Vapors and dusts are collected by an air pollution control device that uses a filter (usually fabric) to collect dust particles from the hot off-gases from the furnace. The particles or dust collected in the process are often recycled through the EAF to increase and recover their zinc content. EAF dust is designated by the U.S. Environmental Protection Agency as a hazardous waste; in addition to zinc and tin, it contains elements such as cadmium and lead and is regulated accordingly.
There are four general types of scrap that are fed into an EAF: carbon steel scrap, alloy steel scrap, stainless steel scrap, and tool steel scrap. There are subcategories within each general category. For example, carbon steel scrap is segregated by carbon content and generally contains manganese, silicon, and aluminum in addition to carbon. Alloy steels contain a variety of elements depending upon the details of specific alloy compositions. It is common to find many different elements in alloy steels, including aluminum, boron, chromium, copper, manganese, nickel, niobium, silicon, and titanium, as well as trace
elements such as phosphorus and sulfur. Stainless steel scrap contains high quantities of chromium and nickel, while tool steels can contain cobalt, molybdenum, tungsten, and vanadium. All of the elements mentioned in these alloy chemistries can and will become part of the slag chemistry as a minor constituent. The only elements that are not significantly oxidized are nitrogen, copper, and nickel; the EAF process causes increases in these residual elements, and, for certain grades, clean iron units from pig iron or iron carbide are added to the furnace to reduce copper and nickel contents by dissolution.
When stainless steels are melted there will also be significant quantities of chromium oxide (Cr2O3) present in the slag due to the high level of chromium in stainless steels.
EAF slags are an ionic solution and can be developed to have a desired constant composition that is repeatable when similar grades are cast. In many operations the addition of the slag forming materials is tailored to ensure that the slag chemistry is consistent from heat to heat; however, due to the operational difference between EAF operations, slag chemistries can vary between plants. As carbon steels, especially low carbon steels, are the majority of grades cast, and the chemistry of these grades is very consistent, with very tight compositional ranges that necessitate close control of steel chemistry, scrap sorting and control is necessary to ensure that these chemistry specifications are met. It is expected under these conditions that the EAF slag chemistries of the majority components will also be very consistent during the production of low carbon steels.
SLAG COMPOSITION
Due to the high production quantities of carbon steels compared to stainless steel, the majority of slags formed (more than 95 percent) are from the melting of carbon steels. The major difference in chemistries between these two slags is related to the chromium oxide content found during the melting of chromium-containing stainless steels; however, some chromium oxide content can be found in slags during the melting of carbon steels if the refractories contain chromium oxide, or stainless or specialty steel scrap is part of the scrap addition to the EAF.
An example of slag chemistries during stainless steel production from Holappa et al. (2021) is given in Table 2-3 where significant quantities of chromium oxide are found in the EAF slag; however, due to the high reactivity of chromium with FeO and the addition of slag reducing agents such as silicon and aluminum in the EAF, there are low levels of FeO and MnO in the EAF slag from stainless steel production.
While most chemical analysis of EAF slags is focused on the majority components, other elements in melted steel alloys can be present at low levels. Piatak et al. (2021) provided data on the detailed chemistries of EAF slags. Ranges of the concentrations of major slag constituents are provided in Table 2-4a, and minor constituents are provided in Table 2-4b. There is wide variation in slag composition sampled from different EAF plants in the United States when one also measures the minor components found in slags. This is due to the variation in the steel chemistries that are recycled and the detailed operational practices used in the EAF. The ranges noted in Table 2-4a suggest there was co-mingling of the EAF slag samples with ladle slag samples, as low values of FeO and MnO are not normal in an EAF slag but are representative of the ladle slag chemistry. However, the average values for the majority of the samples were EAF slag alone.
TABLE 2-3 Constituents of Slag from Steps in EAF Stainless Steelmaking
| Approximate Weight Percent | |
| EAF | |
| CaO | 40–45 |
| SiO2 | 25–30 |
| Al2O3 | 5–10 |
| MgO | 5–12 |
| Cr203 | 3–7 |
SOURCE: Holappa et al. (2021).
TABLE 2-4a Selected Major Components of EAF Slags
| Weight Percent | ||||||||
| CaO | Al2O3 | MgO | SiO2 | FeO | MnO | Na2O | TiO2 | |
| Min to Max | 2.3–60 | 2–22.6 | 3–15 | 5–32 | 1–50.9 | 0.4–15.6 | 0–0.84 | 0.33–1.32 |
| Average | 31 | 6.8 | 7.6 | 15.9 | 27.8 | 4.4 | 0.1 | 0.6 |
SOURCE: Piatak et al. (2021).
TABLE 2-4b Selected Minor Components of EAF Slags
| Cr | As | Ba | Cd | P | Pb | Sb | Sn | V | Zn | |
| Min to Max | 320–200,000 | 0.5–7 | 160–3,600 | 0.1–19 | 100–5,400 | 3–3,000 | 1–18 | 3–34 | 170–1,710 | 31–6,800 |
| Average | 16,873 | 2.3 | 1195 | 4 | 2,161 | 533 | 5.2 | 17.5 | 873 | 648 |
SOURCE: Piatak et al. (2021).
The National Slag Association (NSA) defines several types of steel slags that are produced during the EAF steel manufacturing process. In addition to the furnace slag and the ladle slag discussed previously, NSA notes there are pit slags and clean-up slags, which are formed from material spilled or removed from the ladle after tapping the melt-in furnace.
These additional slag materials are related to the furnace and ladle slags but can also contain materials not usually found in either the furnace or ladle slag. There is an opportunity for interaction with other materials used in the steelmaking enterprise as material is often dropped into the area below the furnace for disposal. Contamination by coal, coke, or oil is possible in these waste slags due to disposal or leakage. Contamination by carbon-bearing materials also occurs by intentional additions to the furnace. Natural gas and coal or coke can be added as a fuel or as a foaming agent to protect furnace refractories from excessive heat. Thus, some slag samples can contain carbon.
Proctor et al. (2000) reported the composition of EAF slags and found 27 elements in more than 10 percent of the samples that were obtained from 45 EAF plants in the United States and Canada. While many of these elements were at low levels and similar to those found by Piatak, it is indicative of the fact that exact chemistries of the slags are necessary to fully understand the slag and its interaction with the environment.
Suh et al. (2014) reported the following slag composition from a plant in Seattle, Washington (Table 2-5). In addition to the expected elements, this slag contains detectable sodium oxide, titanium oxide, chromium oxide, and zinc oxide. It also contained trace amounts of antimony, barium, cadmium, cobalt, copper, lead, molybdenum, nickel, potassium, silver, thallium, titanium, vanadium, and zinc, again suggesting that the slag is a repository for all oxidizable elements in the steel melted in an EAF.
Most steel melted in the furnace (more than 98 percent) is continuously cast in slab, bloom, or billet machines. Slab casters are used in flat rolled strip or plate production. Bloom casters are used for rail and special bar quality steels, and billet casters are used when the products are for construction or special bar qualities. For example, the rebar found in construction products is produced by billet casting, while automobile bodies are produced from slab casting. In all of these different casting machines, it is rare for there to be different types of casters in the same plant; however, all steel plants produce many grades, and the grade cast can change from heat to heat, especially in billet and bloom casting. Thus, the chemistry of the slag produced in an EAF plant can differ significantly from heat to heat depending upon the grade cast. Generally, the ladle slag chemistries are more consistent from heat to heat as these are added and tailored to allow sulfur removal and the adsorption of alumina.
TABLE 2-5 Composition of EAF Slag from a Steel Production Facility in Seattle, WA
| Compound | Percent Composition |
|---|---|
| Al2O3 | 7.27 |
| CaO | 29.38 |
| Cr2O3 | 1.43 |
| FeO | 29.02 |
| MgO | 12.7 |
| MnO | 6.22 |
| Na2O | 0.08 |
| P2O5 | 0.45 |
| SO3 | 0.43 |
| SiO2 | 14.16 |
| TiO2 | 0.27 |
| ZnO | 0.02 |
SOURCE: Suh et al. (2014).
Large tonnage carbon steel producers that melt a majority of similar grades of scrap and have control of the scrap supply produce EAF slag with consistent chemistry. However, steel producers that sell many grades would be expected to have much more variable slag chemistries.
Overall, it is important to note that actual slag chemistries can vary depending upon the actual process utilized in a steel plant, the source of scrap, type of furnace and its tapping mechanism, the actual chemistry of the recycled steel scrap, and the type of steel grade. Thus, there is no general slag chemistry that can be defined for all steel grades, other than to state that it predominantly contains CaO, SiO2, MgO, MnO, and FeO.
As scrap and furnace operation can vary, slag samples that are routinely collected from the furnace and ladle allow the extent of variation to be documented. These slag chemistries are shared with slag processors.
SLAG PROCESSING, DISTRIBUTION, AND USE
At the end of the EAF steelmaking process, molten slag goes through several processes prior to storage and distribution. The main slag processing steps are cooling, crushing, metal recovery, sizing/screening, grinding, and stockpiling.
In the EAF and on the ladle, the slag is a liquid solution of the oxides. Slag temperatures on tapping can be as high as 1,600 °C (2,912 °F). After a heat is tapped, the slag is either poured into a slag pot and then transferred to a slag pit or poured into a slag pit below the furnace where it will cool. The liquid slag must be cooled from 1,600 °C to ambient temperatures and will form either a glass, a crystalline solid, or a combination of a glass and solid, depending upon the cooling rate and chemistry of the slag. Thus, the solidified slag can be a combination of glass and precipitated crystalline phases. The solidified slag will also react with the atmosphere absorbing both water vapor and carbon dioxide as the slag cools.
When slag is removed from the furnace or the ladle, generally it is not possible to accomplish this task without also removing a portion of the steel that was melted. For example, when draining the furnace, it is very difficult to avoid draining some liquid steel. When emptying a ladle, one always empties some liquid steel, as it is not a good practice to drain a ladle fully during casting, and liquid steel is always left in the bottom of the ladle for product quality reasons. Thus, the slag contains steel that can be in the form of droplets or solidified pieces and is more valuable than the slag.
Cooling is an essential step in the processing of all types of slag. It is important as the cooling method and rate of cooling affect the physical and mineralogical properties of the material. After the steel
slag is cooled, the material is stockpiled for the metal recovery and sizing processes. The metal recovery process aims to separate the metallic portion of the unprocessed slag and return it back to the mill for use.
The sizing process separates the steel slag into two or three different size fractions by screening processes. In some steel plants, slag goes through crushers before screening based on desired end use. The slag processing plants have similar facilities to those of aggregate plants, and standard aggregate tests (specific gravity, absorption, gradation, etc.) are also performed on steel slag aggregates. After completion of the processing, steel slags are typically separated into open-air stockpiles based on size and left to age until they are sold or transferred to slag disposal areas. The term “aging” of steel slag refers to the open-air stockpiling of steel slag to provide adequate exposure to moisture. The slag literature also refers to this process as “weathering”; however, to avoid confusion, weathering will be reserved for breakdown of geological materials in environmental conditions.
Slag volume can change through time due to free MgO and CaO in the slag, which when hydrated can result in swelling detrimental to many civil engineering applications (Yildirim and Prezzi, 2009, pp. 41–45). Due to this instability, some slag processing facilities treat slag to decrease the volume instability caused by the expansive components of steel slag by changing its chemical and/or mineralogical properties. In the literature, there are examples of special steel slag treatment techniques that are used in some steel plants to minimize the undesirable volumetric instability of slag. These techniques include using additives, steam treatment, and aging.
Because the treatment methods require the use of special equipment and additional processing, they incur additional costs. Therefore, these treatments may not be used, and cooled steel slag is stockpiled and kept in open air for aging under atmospheric conditions.
As previously discussed, during steel production there are several types of slags generated at the mill. Typically, these slags are transitioned to the processor and kept segregated. This allows for them to be used separately for specific applications or blended as needed to meet physical/chemical product specifications by the end user.
GEOGRAPHIC DISTRIBUTION OF ELECTRIC ARC FURNACE FACILITIES AND SLAG PROCESSORS
About 140 EAFs operate in 119 steel plants that currently produce steel in 31 states as shown in Table 2-6. Production of specialty and stainless alloys is the focus of about 80 of the EAFs, which are in approximately 40 percent of the steel plants. The other steel plants are focused on carbon steel production, which also produce the majority of the slag that is available for processing due to the very high production tonnage of carbon steels compared to specialty and stainless steels.
The United States Geological Survey (USGS) reports estimated slag sales tonnages in the United States annually. According to its latest report in 2022, total domestic slag sales were estimated to be 17 million tons, of which 51 percent was from steelmaking (8.67 million tons) and 49 percent from ironmaking (USGS, 2022). Thus, the amount of slag from EAFs that was sold is approximately 6.2 million tons in 2022 (assuming that 72 percent of the steelmaking slag was from EAF). The amount of slag sold annually will vary with the level of demand for the product as there is significant slag inventory that has been built up over the years. Slag processing companies distribute the slag that they gather from the steelmaker after it is aged and processed to remove metallics that have recycling value and crushed to the sizes required for a product to be sold to a customer.
The committee identified 91 slag processing facilities in the United States that service the 119 currently operating EAF steel plants. Six of these facilities are near steelmaking facilities that are currently closed, but these facilities can continue to process existing slag reserves. The total capacity of many of the specialty and stainless plants is small, with only 26 specialty and stainless steel facilities having a capacity more than 50,000 tons per year. Thus, a total of 79 facilities are responsible for the production of the slag that is managed by processors.
The EAF steel production capacity by state is shown in Table 2-6. As a number of plants have more than one EAF, the slag processing facilities in a state can be less than the number of furnaces in a state;
however, there are slag processing facilities in all states that have operating EAF plantss, except for Kansas. The majority of steel produced by the EAF route occurs in Alabama, Arkansas, Illinois, Iowa, Indiana, Kentucky, Ohio, Pennsylvania, Mississippi, North Carolina, South Carolina, and Texas.
In addition, there are at least 12 EAF-based steel plants that have been either closed or have ceased to melt steel. It is not known, at this time, if these plants will be reopened in the future.
TABLE 2-6 Number of EAF Plants and Slag Processing Facilities by State in the United States
| State | Number of Operating EAF Plants (and Furnaces) | Number of Slag Processing Facilities | EAF Capacity (tons/yr) |
|---|---|---|---|
| Alabama | 7 (11) | 5 | 7,723,000 |
| Arizona | 2 (2) | 1 | 334,000 |
| Arkansas | 5 (5) | 5 | 8,845,000 |
| California | 0 | 1 | 0 |
| Colorado | 1 (1) | 1 | 1,100,000 |
| Florida | 2 (2) | 2 | 1,067,000 |
| Georgia | 1 (1) | 1 | 925,000 |
| Illinois | 7 (7) | 4 | 3,970,000 |
| Indiana | 8 (15) | 5 | 8,796,000 |
| Iowa | 6 (4) | 3 | 2,822,144 |
| Kansas | 1 (4) | 0 | 24,000 |
| Kentucky | 2 (2) | 2 | 3,052,000 |
| Louisiana | 0 | 1 | 0 |
| Michigan | 2 (2) | 2 | 900,907 |
| Minnesota | 1 (1) | 1 | 502,000 |
| Mississippi | 3 (5) | 2 | 3,563,000 |
| Missouri | 3 (4) | 1 | 450,000 |
| Nebraska | 1 (1) | 1 | 1,134,000 |
| New Jersey | 1 (1) | 1 | 653,000 |
| New York | 2 (2) | 1 | 544,000 |
| North Carolina | 2 (2) | 2 | 2,010,000 |
| Ohio | 9 (10) | 9 | 7,261,000 |
| Oklahoma | 1 (1) | 1 | 360,000 |
| Oregon | 3 (3) | 1 | 902,359 |
| Pennsylvania | 20 (20) | 11 | 4,735,000 |
| South Carolina | 4 (6) | 3 | 7,598,000 |
| Tennessee | 4 (4) | 4 | 1,735,000 |
| Texas | 10 (12) | 8 | 6,811,381 |
| Utah | 1 (1) | 1 | 908,000 |
| Virginia | 2 (2) | 2 | 1,555,000 |
| Washington | 4 (3) | 2 | 1,126,954 |
| West Virginia | 1 (2) | 1 | 265,000 |
| Wisconsin | 3 (5) | 1 | 606,181 |
| TOTAL | 119 (141) | 86 | 82,300,000 |
SOURCE: See Appendixes C and D for data used for this table.
The locations of EAF steelmaking plants and slag processing facilities in the United States are shown in Figure 2-2. As can be seen in the figure, the distribution and number of slag processing facilities is generally aligned with the number and distribution of steelmaking plants. States with greater numbers of steel plants (AL, IN, OH, and PA) also tend to have greater numbers of processing facilities (see Table 2-6). The committee assumes that slag processing facilities may have a service area up to about 50 km from the processor because the higher weight relative to stone aggregate increases the cost of hauling the slag from the processor to the site of application.
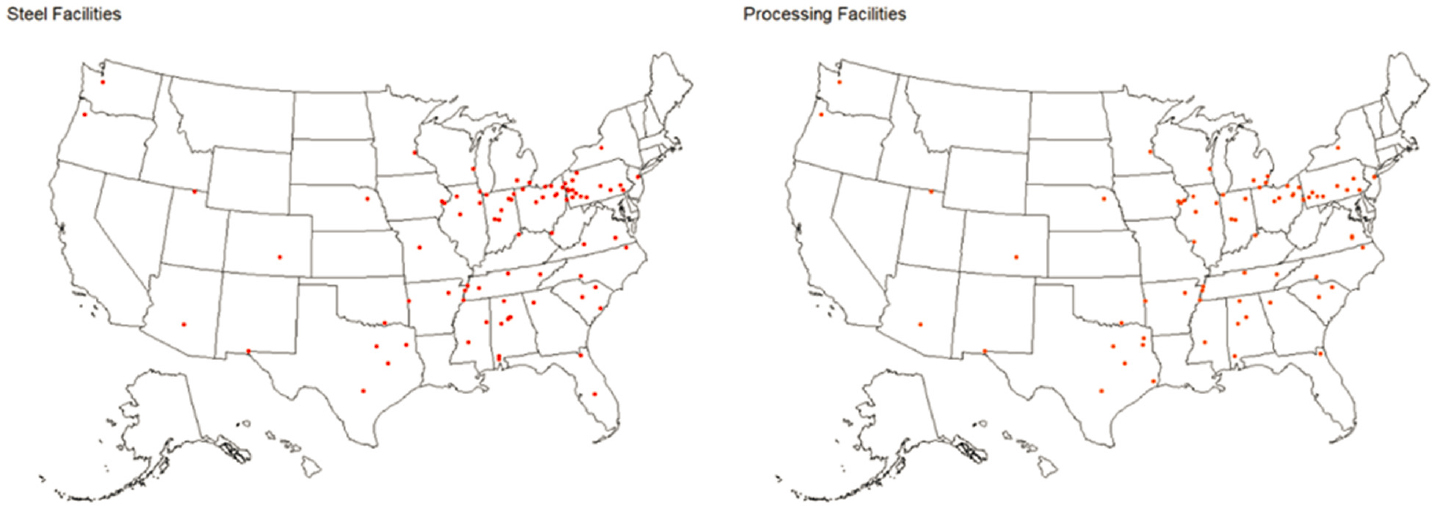
NOTE: See Appendixes C and D for the data used for this figure.
As mentioned above, the amount of slag produced is related to the weight of steel produced, and estimates are that the weight of EAF slag produced is between 10 percent and 15 percent of the weight of steel produced. Thus, the total amount of EAF slag that could be potentially produced at full steel production capacity would be between 8 and 12 million tons per year. Actual annual slag production will follow the steel production quantities in a given year; however, the amount of slag processed can be either higher or lower, depending upon the use of historical slag piles or market conditions, where the demand for processed slag will determine the amount of slag sold.
Typically processing, storage, and distribution of slag are performed by slag processing companies that work closely with the steel producers, and typically the work is done in relative proximity to the steel plant. Most of the slag processing companies receive steel slag for free, and after processing it, they sell it in the open market (Yildirim and Prezzi, 2009). The iron and steel producers may receive a percentage of the revenue from the slag sales. However, depending on the slag processing and steel companies involved, financial agreements may differ. In some plants, substantial amounts of steel slag are fed back to the furnaces as a source of flux and iron.
According to the USGS, “Data are unavailable on actual U.S. ferrous slag production, but domestic slag sales in 2021 were estimated to be 17 million tons valued at about $460 million. Blast furnace slag from iron making accounted for 49% of the tonnage sold with 87 percent of the value ($400 million). BOF [basic oxygen furnace] and EAF slags from steel making account for 51 percent of tonnage sold with 13 percent of the value ($60 million). Owing to low unit values, most slag types can be shipped only short distances by truck, but rail and waterborne transportation allow for greater travel distances.”1
___________________
1 See https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-iron-steel-slag.pdf.
In 2020, the selling price of BOF and EAF slag in the United States ranged from $1 to $25 per metric ton, with an average of $7.05 per metric ton.2 Because the unit price of slag is low and its dry unit weight is typically higher than that of natural aggregates, long-distance transport of slag in large quantities is not economical. However, use of EAF slag instead of stone aggregate becomes more competitive with stone aggregate if there is a slag processing facility nearby where the slag will be used. The supply and availability of slag in the United States is expected to increase because the number of EAF plants continues to grow. Ongoing research is focused on mitigating slag’s potential for volumetric expansion and determining new applications for slag in the construction industry.
Determining how and where unencapsulated EAF slag is used can be challenging. According to the NSA website, steel slag can be used as an aggregate substitute for encapsulated and unencapsulated applications such as construction project entrances, road base, parking lots, embankment fill, culverts, road shoulders, gabions, railroad beds, erosion control, driveways, asphalt aggregate, unpaved trails, trench backfill, septic fields, soil remediation, rip rap, road anti-skid, pH neutralizer, agricultural lime, environmental applications, permeable reactive barriers, and hot mix asphalt.
Considering the broader category of steel slag, the material can be used by homeowners for landscaping around pools and patios.3
The 2021 estimated sales breakdown of all steel slag by use according to a USGS canvas of slag processors is provided in Table 2-7. The USGS states, “A number of respondents provided breakouts that represent only the dominant use(s) of their slag; accordingly, the minor use categories are likely underreported. The data also incorporate some estimates; precision is probably no more than two significant digits.” Beyond the estimated quantities of slag sold for different general beneficial uses, more exact quantities for different beneficial uses were not available.
TABLE 2-7 Estimated Sales Breakdown of Steel Slag by Use, 2021
| Steel Furnace Slag Use | Percentage of Total Tons Slag Sold | Estimated Million Tons of Slag, by Use |
|---|---|---|
| Asphaltic concrete | 12.4 | 1 |
| Road bases and surfaces | 44.8 | 3.6 |
| Fill | 12.9 | 1 |
| Clinker (solid material for cement making) | 2.9 | 0.23 |
| Miscellaneous—railroad ballast, roofing, mineral wool, soil conditioner | 6.3 | 0.51 |
| Other or unspecified—including return to furnaces and other uses | 20.7 | 1.7 |
SOURCE: USGS (2023b); see https://www.usgs.gov/centers/national-minerals-information-center/iron-and-steel-slag-statistics-and-information.
STATE GOVERNMENT OVERSIGHT OF SLAG USE
Industrial sectors generate hundreds of millions of tons of nonhazardous secondary materials each year. As indicated in Chapter 1, the goal of beneficial use of those materials is to use them as substitutes for virgin materials in a way that provides a functional benefit, meets product specification, reduces waste stream needing disposal, and does not pose concerns to human health or the environment.
When a material is defined as a solid waste, states have varying degrees of regulatory requirements, which may include by-product testing to determine the chemical and physical characteristics of the product, placement restrictions, and allowed uses. Of the 31 states with EAF plants, 13 specifically exempt slag from
___________________
2 See https://www.usgs.gov/centers/national-minerals-information-center/iron-and-steel-slag-statistics-and-information.
3 See http://www.harscocrushedrock.com/materials/steel-slag-construction-projects.php.
the definition of solid waste in their state statutes; some also exempt slag in their solid waste regulations.4 These 13 states represent 70 of the 108 operating facilities or 65 percent of the operating EAF plants in the United States. Based on Table 2-6, potential capacity from operating steel facilities in exempt states is 45,871,111 tons out of a total U.S. capacity of 81,317,470 tons, or 56 percent.
States that have chosen not to exempt slag from the state’s definition of solid waste typically include a condition that to be considered exempt from being regulated as a solid waste, the slag is to be managed as an item of commercial value and not discarded to prevent unauthorized dumping of the material. Many states use language similar to the North Carolina state statute: “Steel slag that is a product of the electric arc furnace steelmaking process; provided, that such steel slag is sold and distributed in the stream of commerce for consumption, use, or further processing into another desired commodity and is managed as an item of commercial value in a controlled manner and not as a discarded material or in a manner constituting disposal” [NC G.S. § 130A-290(g)].5
The state of Ohio excludes slag from the definition of solid waste but includes conditions for use of slag in the state’s water pollution control regulations, stating that the slag cannot pose a risk to public health or the environment or that it does not exceed an environmental standard (such as a maximum contaminant level in groundwater).
In terms of regulations applying to use of slag in the proximity of residential properties, it is difficult to determine with certainty which states allow it and which states do not; however, two states appear to have explicit restrictions. In Oregon the Columbia Steel Casting Company was approved to beneficially use slag for “non-residential construction fill, utility fill, or road base applications.”
The state of Wisconsin has a statute indicating that “no person may use unencapsulated slag on private property within 100 feet of a residential dwelling or a building intended to be used in whole or in part as a school or daycare facility without prior approval of the department.”6 A 2015 human health risk assessment performed by the Wisconsin Department of Health Services concluded that “unhealthy exposures to children via direct contact may result from the use of slag on residential or daycare properties” and therefore recommended that the use of unconfined slag and mixtures of slag and ladle slag in residential settings be restricted (Streiffer and Thiboldeaux, 2015).
The Colorado Department of Public Health & Environment has a preapproved beneficial use for “steel slag as an aggregate substitute” with a condition for use stating, “The beneficial use of steel slag must meet engineering specification or other appropriate specifications for the end use.”7
SUMMARY
The majority of EAF slag that is processed is from the EAF, and as carbon steel production predominates in the United States, the majority of EAF slag that is processed comes from carbon steel producers. The chemical composition of EAF slag varies according to the steel alloy melted, the source of the scrap used, and EAF operational practices. The majority of the slag constituents are formed from oxidation of the liquid steel and the addition of CaO and MgO during melting. The EAF slag predominantly contains FeO, lime, silica, magnesia, and MnO. Stainless steel EAF slags can contain significant quantities of chromium oxide due to oxidation of the high chromium content of stainless steels, in addition to lime, silica, and magnesia.
A number of additional chemical elements (as oxides) can be found in small concentrations (less than 2 weight percent) in an EAF slag due to the presence in scrap of materials that are difficult to separate during scrap processing or due to the chemistry of the steel that is recycled. Such elements include antimony, arsenic, barium, molybdenum, nickel, tin, titanium, vanadium, and zinc.
___________________
4 C. Hodes, EPA, personal communication, February 25, 2022.
5 See https://www.ncleg.net/enactedlegislation/statutes/html/bysection/chapter_130a/gs_130a-290.html.
6 Wisconsin Solid Waste Reduction, Recovery and Recycling Statute 287.29[2]; see https://docs.legis.wisconsin.gov/statutes/statutes/287/ii/29/2.
7 See https://cdphe.colorado.gov/hm/recycling. See beneficial use Table 3.
A total of 140 EAFs operate in 117 steel plants that currently produce steel in 33 states. The committee identified 91 slag processing facilities in the United States that service the 117 currently operating EAF steel plants.
The amount of slag produced annually is approximately 10 to 15 percent of the amount of steel produced by EAF plants. In 2022 an estimated 6.34 million tons of slag from EAF plants were sold. The total amount of EAF slag that could potentially be produced at full production capacity in the United States is 8 to 12 million tons per year.
Owing to low unit value, slag is generally transported to projects in close proximity to the processing facility by truck. Quantitative information on how and where unencapsulated EAF slag is used is limited. The majority of steel furnace slag use appears to be road bases and surfaces. However, the annual amount of EAF slag used for residential applications at the national level is unknown.
Regulations on the approval and use of steel slag vary by state. More than half of the operating steel facilities in the United States are in states that exempt slag from the definition of waste and consider it a consumer product. When slag is exempted for regulation as a solid waste by a state environmental agency, it is not clear how adherence to any conditional requirements for that exemption are evaluated or enforced.











