Carbon Utilization Infrastructure, Markets, and Research and Development: A Final Report (2024)
Chapter: 7 Chemical CO2 Conversion to Fuels, Chemicals, and Polymers
7
Chemical CO2 Conversion to Fuels, Chemicals, and Polymers
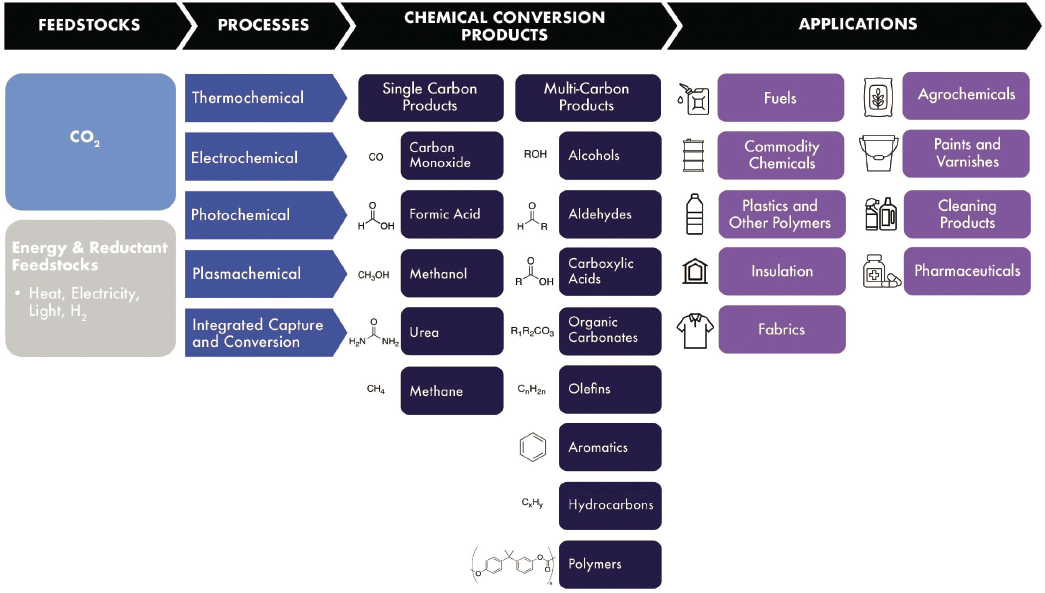
Carbon dioxide (CO2) is a potential feedstock for sustainable synthesis of carbon-based materials in a net-zero greenhouse gas (GHG) emissions future. As noted in previous chapters, most of the carbon in products that are manufactured and used today is derived from fossil feedstocks like natural gas and petroleum. Chapter 2 described markets for future products and intermediates derived from CO2, as well as the competitive alternatives of electrification and clean hydrogen to replace carbon-based fuels for energy and energy storage, biomass and recycled plastic or material waste as feedstocks for carbon-based products, and extensive cradle-to-grave carbon capture and storage with continued fossil production of chemical and material products. This chapter focuses on chemical transformations of CO2 into organic products where CO2 utilization has some competitive advantages in a net-zero future, at a scale and impact that warrants national U.S. research and development (R&D) investment (see Sections 2.2.5.2, 2.2.5.3, and 2.2.5.4). As shown in Figure 7-1, these products include fuels, chemical intermediates, commodity chemicals, and polymers and their precursors, and they can be produced by a variety of chemical processes. The remainder of the chapter describes the current status and R&D needs for chemical CO2 conversion processes and the resulting products, noting relevant applications where appropriate.
7.1 OVERVIEW OF CHEMICAL CONVERSION ROUTES FROM CO2 TO ORGANIC PRODUCTS
7.1.1 Organic Chemical Products That Can Be Derived from CO2
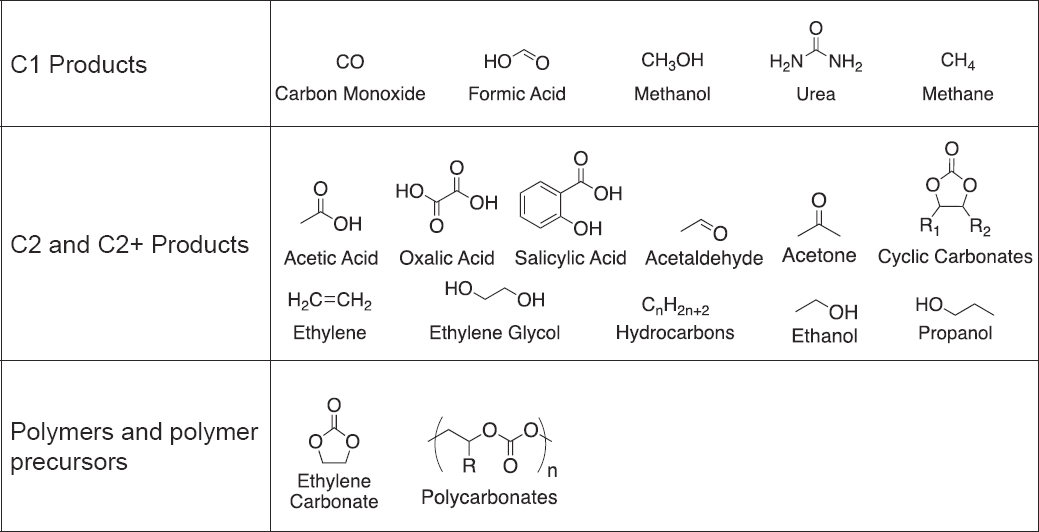
In principle, any carbon-based product can be formed chemically from CO2. This report focuses on conversion of CO2 to priority products in a net-zero future, including single carbon (C1) products, such as carbon monoxide, methanol, formic acid, urea, and methane, and multicarbon products, such as polycarbon oxygenates (alcohols, aldehydes, carboxylic acids, organic carbonates), olefins, aromatics, and hydrocarbons, including fuels (Figure 7-2). (See Chapter 2 for a detailed discussion of potential future market needs.) This chapter builds on Chapter 4 of the 2019 National Academies’ report Gaseous Carbon Waste Streams Utilization: Status and Research Needs (NASEM 2019).
Carbon-based organic chemicals include compounds of carbon and hydrogen, with or without additional elements such as oxygen and nitrogen. The modern chemical industry developed to use petroleum as a source of both carbon and energy that is inexpensive, easy to ship, and contains advantageous carbon-carbon bonds. Large-volume
SOURCE: Icons from the Noun Project, https://thenounproject.com. CC BY 3.0.
chemical intermediates such as methanol, ethylene, propylene, benzene, toluene, and xylenes (Ellis et al. 2023) underlie the production of most other final products and so are particularly important parts of the chemical market. Manufactured organic chemical products pervade modern life. Their applications include fuels; plastics and other polymers for pipes, insulation, and fabrics; agrochemicals including fertilizers; paints and varnishes; cleaning products; pharmaceuticals; and more. As the global economy transitions to one with net-zero emissions, the need for these products will remain, but they will have to be produced from a non-fossil-carbon feedstock.
Alternatives to chemical production from petroleum have been explored and developed when access to petroleum was constrained (such as during wars or trade embargoes), when other resources were abundant and inexpensive relative to petroleum, or when there was an interest to diversify potential carbon sources away from only petroleum, such as domestic biofuel (EPA 2018; Lamprecht 2007; NRC 2006). Technologies and processes to use alternative carbon feedstocks of coal, natural gas, and biomass and its derivatives were developed, including the production of hydrocarbon chemicals and fuels via “syngas” (carbon monoxide and hydrogen). In a net-zero future, petroleum use as a chemical feedstock likely will be highly constrained owing to costs or limits on the resulting CO2 emissions from the product life cycle. In this scenario, CO2 is one option of sustainable carbon feedstock to replace petroleum. In addition to a change in feedstocks for chemicals production, the routes to produce chemicals could proceed via different priority intermediates than those currently used in the chemical industry, owing to the different properties of CO2 as a feedstock as compared to petroleum. This chapter describes routes to potential future priority intermediates as well as final products.
Although not discussed in detail in this chapter, simply using CO2 as a feedstock does not eliminate net GHG emissions from the life cycle of organic chemical production. CO2 and other GHG emissions associated with the production of CO2 and other feedstocks, transformation of the feedstocks into the product, delivery of the product to the user, use of the product, and its eventual disposal or recycling also have to be eliminated. See Chapter 3 for more discussion of life cycle assessments for CO2 utilization.
7.1.2 Conversion Routes
There are several approaches to chemical conversion of CO2 to organic products, all of which are geared toward overcoming the main challenges of using CO2 as a chemical feedstock: its stability/nonreactivity, lack of carbon-carbon bonds, and presence as a dilute gas under ambient conditions. The ability of CO2 to serve as a sustainable carbon feedstock is tied to these properties, as it is the primary waste product of combustion and other organic-molecule decomposition processes. Formation of most organic products from CO2 requires energy input to overcome reaction barriers, some portion of which becomes energy stored in the product. Catalysts are often required to facilitate faster, more selective reactions. Thermochemical, electrochemical, photochemical, and plasmachemical reactions, as well as integrated CO2 capture and conversion, can incorporate both energy input and catalysis into CO2 conversion processes. Comparisons of “practical” energy requirements for different conversion pathways is challenging, as researchers often report different metrics for efficiency. Calculating the free energy of CO2 conversion to a given product is possible, but unproductive, as the actual amount of energy required will exceed the theoretical limit and vary by process. The committee’s first report quantified energy requirements for various carbon capture and hydrogen production processes, as well as the stoichiometric hydrogen requirements for several carbon-based products (see Figures 3-6, 3-7, and 3-8 in NASEM 2023). The following sections highlight status, challenges, and R&D opportunities for chemical CO2 conversion processes in a net-zero future.
7.2 EXISTING AND EMERGING PROCESSES, CHALLENGES, AND R&D OPPORTUNITIES
Chemical conversions of CO2 into organic chemicals span across technology readiness levels (TRLs). Figure 7-3 illustrates different pathways—thermochemical, electrochemical, photochemical, and plasmachemical—to produce organic chemicals from CO2 and describes the technical maturity of the most advanced design of each process type. The following sections discuss current technologies, challenges, and R&D opportunities for each of these conversion pathways, as well as for integrated capture and conversion and the production of polymers from CO2.
![Schematic of CO2 utilization processes to produce priority chemicals, fuels, and intermediates in a net-zero future, including maturity (approximate technology readiness level [TRL]) of the most advanced design of each process type: thermochemical, electrochemical, photochemical, or plasmachemical](https://www.nationalacademies.org/read/27732/assets/images/img-250.jpg)
NOTES: All paths begin with CO2 capture (point source capture or direct air capture), then conversion of the CO2 (blue) into intermediates of CO (pink) or methanol (green), or directly to products (black bold). Conversion of CO or methanol to products, or to olefin intermediates (yellow) is also shown, along with olefin conversion to plastics.
7.2.1 Thermochemical Conversion Pathways
This section describes pathways for thermochemical conversion of CO2 into the following products: carbon monoxide (CO) and synthesis gas (“syngas”: a mixture of CO and H2); methanol and its derivatives; formate/formic acid; C2+ hydrocarbons, oxygenates, and intermediates; C2 and C2+ carboxylic acids; fuels from Fischer-Tropsch synthesis using CO produced from CO2; and polymer precursors. It begins by describing current technologies and processes for these conversions, followed by discussions of the challenges with and R&D opportunities for thermochemical CO2 conversion. More emphasis is placed on the importance of CO2-derived intermediates than final products, as the steps to produce final products are well known once key intermediates are produced. Thermochemical conversion pathways and associated products are shown in Figure 7-4 and Table 7-1.

NOTE: CO2 source in gray, processes in purple, products in pink.
7.2.1.1 Current Technology
Carbon Monoxide and Syngas
CO2 conversion to CO is a key initial reaction for thermocatalytic pathways to hydrocarbon products. Processes for converting CO and its mixture with H2 (syngas) into various hydrocarbons have been subject to ongoing research for continuous improvement for more than a century. Once syngas is formed, a full set of proven commercial pathways are known for comprehensive chemical synthesis across all molecules comprising the current hydrocarbon chemicals economy (Cho et al. 2017; Xie and Olsbye 2023).
TABLE 7-1 Key Products from Thermochemical CO2 Conversion and Processes for Their Formation
| Product | Processes for Formation from CO2 |
|---|---|
| Carbon monoxide and syngas |
|
| Methanol and derivatives |
|
| Formate and formic acid |
|
| C2+ hydrocarbons, oxygenates, intermediates |
|
| Polymer precursors |
|
CO forms readily by hydrogenating CO2 in the reverse water-gas shift (RWGS) reaction (Reaction 7.1).1 Albeit typically in the presence of a catalyst. The RWGS (and forward water-gas shift [WGS]) reaction is equilibrium constrained, with conversion affected by temperature, which impacts the equilibrium and kinetics, and to a moderate degree by pressure (influencing reaction rates); RWGS reaction kinetics are well documented (Bustamante et al. 2004; Chen et al. 2020).
| RWGS reaction: CO2 + H2 ⇌ CO + H2O | (R7.1) |
The RWGS reaction typically uses copper or platinum, palladium, or rhodium catalysts supported on redox catalysts or supports such as ceria (CeO2) (Ye et al. 2019; Zhou et al. 2017), rather than the alumina-supported catalysts used for the forward WGS reaction, to avoid acidity that can lead to coking at the higher temperatures used for RWGS. While well-known catalysts for the forward reaction can also perform the reverse reaction, new formulations could offer improved performance at the temperatures and pressures required and with impurities present. For example, platinum-doped cerium oxide catalysts offer higher RWGS reaction rates and yields, at the expense of requiring a noble metal (Ampelli et al. 2015). For syngas production via RWGS to be economically viable, improvements in CO yield, CO productivity, and catalyst durability are also needed. As of yet, the RWGS reaction has not been fully developed because there has been no economic incentive to do so in the absence of a price on fossil carbon. However, several studies have examined potential catalysts for the reaction: Dimitriou et al. (2015) review the approach for liquid fuels production; Chen et al. (2020) describe formulation of metal versus metal-oxide catalyst to improve tolerance to poisons; Zhang et al. (2022a) present a molybdenum phosphide–based catalyst to avoid use of noble metals; and Daza and Kuhn (2016) review catalyst options for producing liquid fuels by CO2 hydrogenation. See Section 7.2.1.3 for more on catalyst development opportunities.
Another pathway for generating syngas is high-temperature solar thermochemical splitting of CO2 and water (Al-Shankiti et al. 2017; Pullar et al. 2019; Wenzel et al. 2016). Use of solar thermochemical technologies for hydrogen generation2 has lagged photovoltaic (PV)-electrolysis as a promising pathway for renewable hydrogen production, given the lower costs of PV electricity generation. However, solar thermochemical technologies for CO2 splitting may be cost-effective for hydrocarbon product synthesis because of the ability to integrate with energy/heat storage and recuperation to enable 24/7 industrial operations. Thermal cycling of the working redox materials and differential thermal expansion are issues for system design and durability.
Syngas also can be produced by catalytic dry reforming of methane, which uses CO2 as a soft oxidant and additional source of carbon: CO2 + CH4 ⇌ 2CO + 2H2, where half of the carbon and all of the oxygen come from CO2, and half of the carbon and all of the hydrogen come from methane (see, e.g., Shi et al. 2013). Dry reforming produces a syngas composition with CO/H2 ratio of 1, which is too rich in CO for methanol or other chemical synthesis, other than addition to olefins or epoxides via hydroformylation, which has limited market size and hence limited ability to uptake CO2 into products. Water-gas shift to remove some of the CO yields more H2 but results in additional CO2 formation, which via the subsequent reaction network of C1 chemistry (including the large endothermic heat of reaction for CO2 conversion) results in a net increase rather than consumption of CO2 (Sandoval-Diaz et al. 2022). CO separation via chemical looping or carbon rejection via solid nanofibers, or injection of additional clean H2 is needed to render dry reforming a viable pathway for chemical production (Challiwala et al. 2021), except for limited market volume products (e.g., dimethyl ether) where a 1:1 syngas ratio is directly consumed. Some process flue gas compositions also may benefit from a 1:1 syngas composition for some retrofit applications. Dry reforming can be used in conjunction with renewable methane3 to expand sequestration of carbon into products and synergistically produce solid carbon (see, e.g., Azara et al. 2019 and Chapter 6 of this report). However, the high temperatures required for CO2 conversion
___________________
1 RWGS is a stoichiometric reaction that converts CO2 into CO by consumption of H2. Typically, one adds an excess of H2 so that once a given amount of CO2 is “shifted” to CO, one has the desired ratio of H2/CO for subsequent reactions. Alternatively, one can add excess H2 after the RWGS reaction to obtain a desired ratio.
2 For more information on solar thermochemical technologies for hydrogen production, see Wexler et al. (2023).
3 Renewable methane is methane sourced from nonfossil feedstocks, like biomass, municipal solid waste, and other waste carbon-containing materials, like plastics.
by thermochemical dry reforming leads to substantial catalyst coking which, together with the need for carbon rejection to address the problem of incorrect (low) syngas ratio for most large-scale products, has limited commercial applications (Sandoval-Diaz et al. 2022).
Methanol and Derivatives
Pathways to convert CO2 to methanol include direct hydrogenation and a two-step process of RWGS followed by methanol production from syngas (Elsernagawy et al. 2020). The status of commercial pathways for methanol production from syngas has been reviewed by the National Energy Technology Laboratory (NETL n.d.(a)). In general, conversions and yields for direct CO2 hydrogenation to methanol are lower than for the two-step process via syngas under standard conditions owing to poorer activity and formation of additional water as a coproduct.
Research efforts have targeted improvements in yield and selectivity of direct CO2 conversion to methanol (Jiang et al. 2020; Ye et al. 2019). For example, increasing H2/CO2 ratios to 10 and operating at higher pressure (35 MPa) allows direct conversion to methanol above 95 percent yield with 98 percent selectivity for a conventional copper/zinc oxide/alumina (Cu/ZnO/Al2O3) catalyst (Bansode and Urakawa 2014). Use of dispersed copper nanoparticles encapsulated in metal organic frameworks via strong support interactions shows enhanced activity, near 100 percent selectivity to methanol, and reduced catalyst sintering while preventing agglomeration of the copper nanoparticles (Rungtaweevoranit et al. 2016). Zirconium dioxide acts as a promoter and support in copper-based catalysts for CO2 conversion to methanol (Lam et al. 2018). Catalysts incorporating indium (III) oxide on nickel or nickel-indium-aluminum/silica (Ni-In-Al/SiO2) enhance rates for low-pressure methanol synthesis (Richard and Fan 2017). Bifunctional catalysts are being developed that couple CO2 hydrogenation to methanol via copper, indium, or zinc-based catalysts with methanol dehydration or coupling using zeolites (Ye et al. 2019).
The CAMERE process provided an early pilot of two-step methanol production via RWGS and methanol synthesis (Joo et al. 1999). Samimi et al. (2018) examined addition of an in situ membrane for water removal during methanol synthesis using the CAMERE process, which showed improvements in methanol yields, as removal of water is expected to improve catalyst life. Subsequent analysis identified membrane options for enhancing RWGS in packed-bed membrane reactors (Dzuryk and Rezaei 2022).
From methanol, the subsequent steps to produce gasoline or olefins are fully developed and have initial commercial units in China (Gogate 2019). Methanol-to-olefins results in a ratio of C2=/C3= product from 0.7 to 1.1, whereas current technology from ethane (cracking) or propane (dehydrogenation) can give better than 90 percent yields of a specific olefin (Tian et al. 2015). Selective conversion of methanol to light olefins (ethylene, propylene) is essential for providing key intermediates for the chemical economy and can be achieved via catalyst and reactor optimization (Jiao et al. 2016; Tian et al. 2015). A process for converting methanol to gasoline has been demonstrated, and commercial operations are planned (NETL n.d.(b)). Methanol conversion to aromatics is also known and would allow coverage of a full spectrum of CO2 to polymer and chemical intermediates (Sibi et al. 2022). Methanol carbonylation is fully commercial at industrial scale. However, use of earth abundant metals in place of rhodium and iridium and avoidance of corrosive halogen promoters remain goals for practice of more sustainable, green chemistry (Kalck et al. 2020).
Formate and Formic Acid
Thermocatalytic routes to synthesize formate or formic acid from CO2 have been reported using molecular (homogeneous) catalysts, which also have relevance for direct methanol synthesis from CO2 (Wang et al. 2015a). Behr and Nowakowski (2014) reviewed both homogeneous and heterogeneous catalysts as well as attempts to develop commercial systems. Homogeneous catalysts based on ligand-modified platinum group metals are active for formate/formic acid production, with ruthenium, rhodium, and iridium showing highest activity, but are challenged by performance, cost, and low element abundance at commercial scale, which impedes industrial consideration. Significant activity is only achieved in the presence of base to produce formate salts instead of formic acid, which drives the endergonic reaction but inhibits product separation and adds cost. A wide variety of mono- or bidentate phosphine or amine ligands impart changes in steric effects and electron density that modify activity and selectivity, giving rise to a rich domain for experimentation. Recent developments include improved rates for
iron- or cobalt-based homogeneous catalysts promoted with phosphine ligands, but these systems again require expensive promoters for activity, either a base as in the precious metals catalysts, or a Lewis acid (Bernskoetter and Hazari 2017; Filonenko et al. 2018). Heterogeneous catalysts were known as early as 1932 (Raney nickel; Covert and Adkins 1932) but give poorer yields. Overall, direct thermocatalytic CO2 hydrogenation to formic acid or formate has progressed significantly because extensive exploration began in the 1970s, but the relatively low turnover frequencies, difficult separations, and expensive components have limited commercial deployment. Electrochemical approaches could be highly competitive in this space (see Section 7.2.2). Currently, formic acid is made on a 0.8 kiloton per annum global scale via thermocatalytic carbonylation of methanol to methyl formate, followed by base-catalyzed hydrolysis to formic acid and methanol (Hietala et al. 2016); it can potentially be made for small-scale markets via bioprocessing. The use of formic acid or formate at a larger scale—for instance, as a transport medium for syngas—would require process technology optimization and scale up, if this were found to be a competitive pathway versus methanol production.
One-Step C2+ Hydrocarbons, Oxygenates, and Intermediates
C-C bond coupling to form C2+ hydrocarbons and oxygenates is a challenge for thermochemical CO2 activation, although it is an area of active research (Fors and Malapit 2023; Pescarmona 2021; Zhang and Hou 2013). Coupling with epoxides is one means of activating CO2 (Kothandaraman and Heldebrant 2020).4 Multistep pathways to C2+ products via syngas formation followed by Fischer-Tropsch are described below, and multistep pathways to C2+ products via syngas formation followed by methanol synthesis and subsequent reactions to olefins or gasoline were described above.
“One-pot” synthesis of C−C bonded products can be attempted via either the methanol or Fischer-Tropsch synthesis routes, to save capital expenditure and simplify the number of process steps (Ye et al. 2019). The initial reaction of CO2 with H2 (RWGS) must overcome the high reaction activation energy of CO2 and equilibrium, and hence requires high temperature (950°C). To attempt a one-pot synthesis to yield methanol as an intermediate for coupling to olefins or dimethyl ether, the subsequent reaction(s) also must be able to take place selectively at high temperature. While multistep pathways from CO2 to methanol and derivatives or to Fischer-Tropsch products can exhibit high conversion at C−C yields of 80 percent or better, one-pot synthesis yields are restricted to 50 percent or lower because the subsequent conversion steps have to be conducted at the same high temperature as the RWGS reaction (Ye et al. 2019). Nonetheless, considerable research efforts continue for one-pot synthesis routes, given their potential lower costs.
C2 and C2+ Carboxylic Acids
CO2 has been widely explored as a carboxylation agent in the production of C2 and C2+ carboxylic acids, enabling more sustainable syntheses compared to current industrial methods, although catalytic approaches remain largely at the basic research stage (Cauwenbergh et al. 2022; Davies et al. 2021; Tortajada et al. 2018; Wang et al. 2017; Zhang et al. 2024). Unlike other CO2 conversions discussed in this chapter, these carboxylation reactions do not use CO2 as the source of all carbon atoms in the target compound, but rather as the source of a carboxyl group. Both heterogeneous (Zhang et al. 2024) and homogeneous (Cauwenbergh et al. 2022; Tortajada et al. 2018) catalytic systems have been studied, with palladium, rhodium, nickel, copper, and cobalt being among the most common metals for catalysis. A wide range of products are accessible through the various catalytic reaction pathways for carboxylation with CO2, which include nucleophilic addition of organometallic reagents, reductive coupling with organic (pseudo)halides, reaction with unsaturated hydrocarbons, and functionalization of sp, sp2, and sp3 hybridized C−H bonds (Davies et al. 2021; Tortajada et al. 2018). Synthesis of acrylic acid from CO2 and ethylene is of particular industrial interest given the widespread applications of these compounds in manufacturing and consumer products (Davies et al. 2021; Tortajada et al. 2018; Wang et al. 2017).
___________________
4 Epoxides are unstable molecules that require high energy for synthesis. For sustainable processing, the C2 epoxide co-reactant would have to be made from CO2 as well, via formation of syngas, synthetic methanol-to-olefins, and epoxidation of ethylene, for example. The other feedstocks and energy inputs for CO2-to-epoxide conversion would also need to have net-zero emissions on a life cycle basis.
Hydrocarbons from Fischer-Tropsch Synthesis
Fischer-Tropsch synthesis is a surface chain-growth polymerization reaction (Anderson-Schulz-Flory distribution) that converts syngas into a range of hydrocarbon products. Conversion of syngas to diesel and chemical products (waxes and lubricants) is fully commercial at the industrial refinery scale (see NETL n.d.(c)). Thus, generation of syngas from CO2, as described above, can enable production of fuels and commodity chemicals using already existing commercial methods, although this is not currently viable at scale (see Section 7.2.1.2).
The synthesis reactions between CO and H2 can be written as (Martín and Grossman 2011):
| nCO + (n+m/2)H2 → CnHm + nH2O, | (R7.2) |
which occurs by a chain growth mechanism to add –CH2– units:
| CO + 2H2 → –CH2– + H2O | ΔHr = −165 kJ/mol | (R7.3) |
Preferred industrial catalysts are cobalt and iron operating at temperatures between 200 and 350°C and pressures from 10–40 bar (Martín and Grossman 2011). Iron has higher WGS activity, leading to higher consumption of CO and of the produced water, which increases the H2/CO ratio, decreasing the probability for chain growth but reducing catalyst deactivation caused by water (Bukur et al. 2016). Iron catalysts also exhibit greater selectivity to unsaturated olefins because of additional surface intermediates formed. Product distributions can be modeled via a probability for chain growth, which depends on total pressure, H2:CO ratio (typically 1:1 to 2:1), the extent of WGS activity along the reactor, temperature, catalyst design, and pore structure of the support (Bukur et al. 2016).
Polymer Precursors
CO2 is used in the production of some monomers for polymerization reactions (Grignard et al. 2019). (Section 7.2.6 discusses direct polymerization of CO2.) For example, in 2012, Asahi Kasei Corporation industrialized a process to make bisphenol-A polycarbonate (BisA-PC) starting from ethylene oxide and CO2 (see Figure 7-5; Asahi Kasei n.d.;
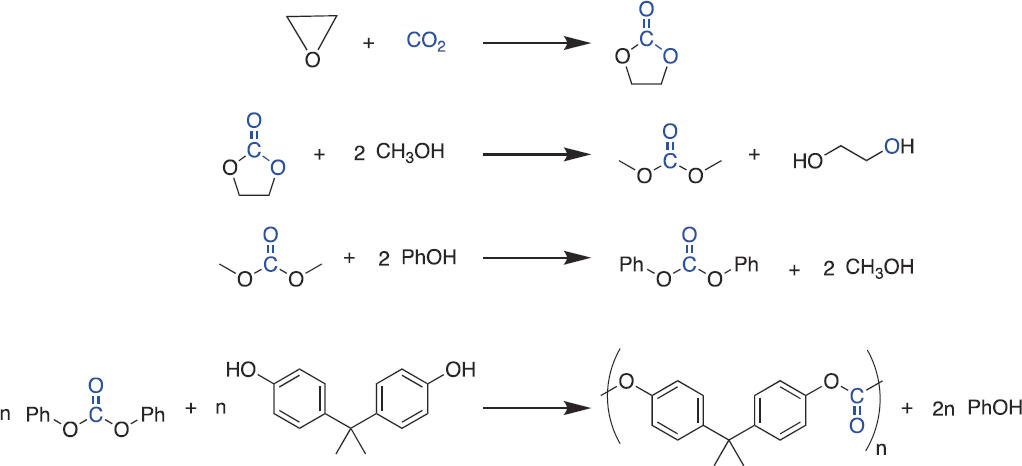
SOURCE: Reprinted with permission from S. Fukuoka, I. Fukawa, T. Adachi, H. Fujita, N. Sugiyama, and T. Sawa, 2019, “Industrialization and Expansion of Green Sustainable Chemical Process: A Review of Non-Phosgene Polycarbonate from CO2,” Organic Process Research & Development 23(2):145–169, https://doi.org/10.1021/acs.oprd.8b00391. Copyright (2019). American Chemical Society.
Fukuoka et al. 2019). The process first reacts ethylene oxide and CO2 to make ethylene carbonate, which then reacts with methanol to produce dimethyl carbonate. Dimethyl carbonate is converted to diphenyl carbonate via reactive distillation, and last, diphenyl carbonate and bisphenol-A are reacted to produce BisA-PC. In addition to being an opportunity for CO2 utilization, the route to BisA-PC via CO2 and ethylene oxide avoids the use of phosgene and the associated safety concerns of the traditional synthesis route.
7.2.1.2 Challenges
Thermochemical CO2 conversions, like all large-scale industrial catalytic processes, are subject to continuous improvement in product yield, catalyst durability, and reactor performance (conversion per unit mass of catalyst). The near-term industry focus for thermochemical CO2 conversion is improving the RWGS reaction because all subsequent process routes utilizing syngas are proven at scale, albeit not with sustainable energy inputs and circularity constraints. The RWGS reaction is thermodynamically favorable at high temperature, but under these conditions, catalyst deactivation owing to sintering, coke formation, reduction of active species, and/or CO poisoning can be a challenge (Chen et al. 2020; Goguet et al. 2004; Tang et al. 2021; Zhou et al. 2023). Industry is exploring the noncatalytic RWGS reaction as a potentially more cost-effective option, but this approach requires higher temperature, pressure, and metallurgy for high per-pass yields to minimize the need for CO2 recycling.
The difficulty of activating CO2 makes catalyst development for RWGS—and, indeed, for all thermochemical conversions of CO2—particularly challenging. Typical copper-based RWGS catalysts are not stable at the high temperatures required for reaction, and it is difficult to achieve high CO selectivity because of undesired methanation (Chen et al. 2017). Perovskite oxides can act as oxygen donor-acceptors to minimize methanation side reactions (Chen et al. 2020). Strong metal-support interactions, structure sensitivity to dispersed metal particle size, introduction of a second metal or metal oxide, and alkali promoters all provide opportunities for commercial improvement. Supported noble metals (e.g., platinum, rhodium, palladium, gold) and first-row transition metals (e.g., copper, iron) have been examined as catalysts, but the high cost for noble metals renders them impractical. For carboxylation reactions, challenges with catalyst (and catalytic system) development include poor stereo-, regio-, and enantio-selectivity; limited mechanistic understanding; and the requirement for stoichiometric reductant, alkylation agent, strong base, and/or toxic solvent (Cauwenbergh et al. 2022; Davies et al. 2021; Tortajada et al. 2018; Zhang et al. 2024).
Further catalyst development has to consider availability and sustainability of the elements chosen; thus iridium -and ruthenium-based catalysts are industrially or economically challenged. Future metal catalyst functionality from abundant materials is a goal, including use of transition-metal carbides. To this end, the use of transition-metal catalysts supported on metal oxides shows promise for RWGS, provided methane formation can be suppressed via techniques such as metal-support interactions for structure-sensitive hydrogen activation and CO2 hydrogenation reactions (Chen et al. 2020). An additional challenge will be developing catalysts that can tolerate specific CO2 feed stream compositions, including impurities. See Tables 4.3 and 4.4 in the committee’s first report (NASEM 2023) for an overview of impurities in CO2 streams from different sources (reproduced in Appendix H as Tables H-1 and H-2).
Other challenges involve reactor design and scaling of processes. The RWGS reaction exhibits a relatively low heat of reaction, and reactor design and scale up do not present significant challenges using fixed beds of catalysts with interstage cooling (Saw and Nandong 2016). Design of new gas-solid catalytic reactors with low volumetric heat transfer rates can be done from design principles without requiring demonstration. Producing fuels and other chemicals by coupling the RWGS reaction with Fischer-Tropsch or methanol synthesis requires that the high-temperature RWGS reactor outlet is cooled before sending the syngas to a Fischer-Tropsch or methanol synthesis reactor because catalysts for those reactions are not selective at high temperature. Because CO2 is difficult to activate, per pass conversions at the lower temperatures where “CO2 hydrogenation” (i.e., the forward direction of RWGS) can be coupled with Fischer-Tropsch or methanol synthesis are low, currently less than about 30 percent (Dang et al. 2019; Saeidi et al. 2021; Zhang et al. 2021). In such cases when CO2 conversion is below 95 percent, it has to be separated, recycled, and reheated, which reduces energy efficiency. Nonetheless, deployment to date is limited not by technology scale up, but by the lack of economic competitiveness of products derived from CO
via RWGS. Demonstrations have produced small amounts of product as a showcase (e.g., Dineen 2023). RWGS also can be performed at higher temperature via a noncatalytic thermal conversion reactor, where temperature is used to compensate for lack of catalyst. In this case, heat transfer can require larger-scale demonstration for reliable scale up. Overall heat transfer rates are not large, however, so this would be an optimization exercise and not a showstopper for industry.
Owing to the efficiency of large-scale chemical synthesis, the CO2-to-products industry of the future likely will entail large-scale plants in locations favorable to their deployment, and liquid or solid products will be shipped to market. Facilities that convert CO2 to CO and CO to products likely would need to be co-located, as it is impractical to build pipelines or other commercial transportation of CO, a toxic and reactive gas, beyond short commercial-unit trunklines. Where conditions do not favor large plants owing to water restrictions or land use or other limitations for CO2 capture and renewable power generation, distributed modular plants for integrated conversion of CO2 to CO and subsequent CO to liquid or solid products can be considered. The challenge for distributed modular processing, in competition with global mega-scale plants with low-cost shipping of products, is that capital costs and process scale do not increase at the same rate. Termed the “0.6 power rule,” capital costs for thermochemical reactions conducted in bulk equipment typically increase only at the 0.6 to 0.7 power of process scale, reflecting the fact that essential tasks for engineering design and fabrication must be performed regardless of scale, such that costs per unit of production decrease as production rates or annual capacities are increased (Timmerhaus and Peters 1991). Mini- and micro-channel reactors with improved heat transfer and membrane reactors are rare but scale closer to 1.0 power (i.e., capital costs increase proportionally to production volume), such that scale up requires “numbering up” smaller units rather than increasing the scale of a given process unit. To remain favorable despite their smaller production scale, distributed production plants have to be highly integrated, volumetrically efficient, and employ low capital expenditure approaches. Small-scale, stranded natural gas conversion facilities face similar constraints, and have generally chosen physical transport of the stranded gas as liquefied natural gas rather than reactive conversion to products on a distributed basis. Related considerations for CO2 transport versus small-scale conversion may come to the same conclusion.
Fischer-Tropsch synthesis, the demonstrated pathway for creating a chemical economy from CO, has never been economically competitive at small or intermediate scales despite numerous showcase demonstration projects (De Klerk 2014; Dieterich et al. 2020). Fischer-Tropsch is a C1 oligomerization process, which produces an Anderson-Schulz-Flory statistical distribution of hydrocarbons with a broad range of carbon numbers, including diesel through aviation (C9+) to heavy waxes for lubricants (C35+, which can also be cracked back to smaller molecular weight), as depicted in Figure 7-6. The multiple processing steps required result in high capital expenditure and poor ability to scale down. Catalytic studies seek to reduce or eliminate the heavy end wax (lubricants) formation but also to avoid using commercially nonviable metals such as ruthenium. Additionally, olefin yields for the Fischer-Tropsch pathway have been limited to around 50 percent, compared to around 80 percent for the methanol-pathway alternative (Ye et al. 2019)—that is, CO2 hydrogenation to methanol and subsequent conversion to olefins (He et al. 2019).
Thermochemical conversion of CO2 to chemicals and fuels has higher costs than current production from fossil hydrocarbons, even for the CO2 utilization processes that are well known. Under future conditions for sustainable synthesis of circular carbon chemicals and fuels, the large amount of capital and high energy required to capture CO2 from air and upgrade it from a thermodynamically degraded state into synthetic hydrocarbons will present significant hurdles for CO2 utilization. Production of sufficient clean hydrogen to meet demand for CO2 utilization and other applications could be particularly challenging, warranting additional R&D to facilitate scale up (NPC 2024). Delivery of the low-carbon-intensity, high-temperature process heat needed for thermochemical CO2 conversion could occur via electrification (see Section 7.2.1.3 for more on electrified reactors), redesign of furnaces to support use of clean hydrogen as a fuel, or implementation of carbon capture on existing fossil fuel furnaces (see Section 10.3.2.1 for more on these retrofitting options). Decisions about the optimal approach will require consideration of trade-offs in carbon intensity and reaction efficiency. Additionally, CO2 conversion pathways, such as RWGS, that require additional heat at high temperatures in the presence of hydrogen can present challenges in reactor design and metallurgy. Given high capital intensity of capture and conversion facilities, energy storage may be required for 24/7 access to renewable H2 for conversion of CO2. Two examinations of techno-economic
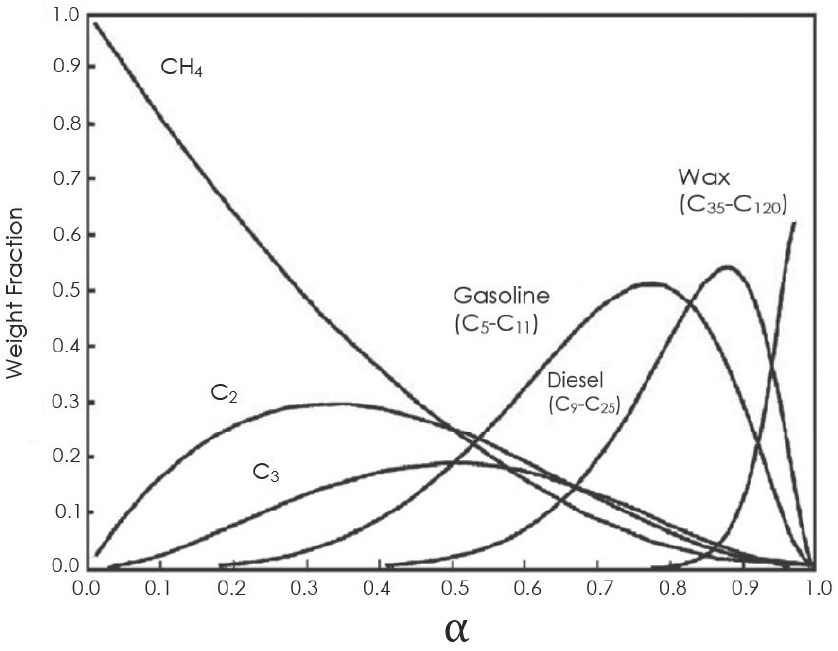
SOURCE: Reprinted with permission from M. Martín and I.E. Grossmann, 2011, “Process Optimization of FT-Diesel Production from Lignocellulosic Switchgrass,” Industrial & Engineering Chemistry Research 50(23):13485–13499, https://doi.org/10.1021/ie201261t. Copyright (2011) American Chemical Society.
potential for manufacture of CO2-derived aviation fuels found prices were about 2–7 times higher than current fuels, and there was little expectation that technology innovation could overcome that barrier, so strong policy support would be needed (Freire Ordóñez et al. 2022; Soler et al. 2022). Chapter 4 discusses policy options to support CO2 utilization in a net-zero emissions future.
7.2.1.3 R&D Opportunities
R&D opportunities for thermochemical CO2 conversion include integrated catalyst development and multiscale reactor optimization, process and systems integration, use of advanced characterization and discovery techniques, and development of electrified reactors. A related R&D opportunity, integrated capture and conversion (i.e., reactive capture), is discussed in Section 7.2.5. General descriptions of each R&D area are outlined below, as many are shared across conversion pathways and product targets. Examples are provided for conversion to specific products where relevant.
Integrated Catalyst Development and Multiscale Reactor Optimization
Catalyst discovery and development for RWGS is an active topic of research for CO2 utilization, given the bifunctional nature of RWGS catalysts (metal/metal oxide), observed structure sensitivity, and relevance of surface or lattice oxygen storage in controlling the reaction pathways. For CO2 hydrogenation reactions involving zeolite catalysts, synthesis of zeolites with desired structures is a key research area for integrated bifunctional catalyst
performance, including impacts of acidity, silicon/aluminum ratio, and pore structure. Furthermore, catalysts or catalytic systems should tolerate specific CO2 feed stream compositions, including impurities. These properties also affect the subsequent conversion of methanol to gasoline or dimethyl ether (with HZSM-5 zeolite) or olefins (with SAPO molecular sieves) (Ye et al. 2019). Computational design, including use of quantum mechanical simulations (e.g., density functional theory), can identify desired structures and compositions, but multiscale modeling to consider integrated reaction and transport properties is key for defining reactor-scale performance (Ye et al. 2019). Research on Fischer-Tropsch synthesis to enhance yields of lower molecular weight olefin products has long examined iron-based catalysts (Storch et al. 1961) with promotion by potassium, manganese, or copper as dopants (Dorner et al. 2010).
The switch from traditional Fischer-Tropsch synthesis with syngas as feed to “CO2 Hydrogenation” with CO2 and H2 as feed favors iron rather than cobalt-based catalysts owing to the former’s higher WGS potential (and hence RWGS potential). Olefin yields can be increased up to four-fold with reduced methane by-product (Saeidi et al. 2021). Lin et al. (2022) describe strategies for controlling Fischer-Tropsch product selectivity, including metal particle size, strong metal-support interactions, use of alkali and other cationic promoters, use of bifunctional catalysis to couple conventional carbon chain growth via cobalt or iron metal catalysts with carbide (Co2C or Fe2C) catalysts that enable nondissociated CO insertion for higher alcohol synthesis. Dual functional cobalt-manganese (Co-Mn), iron-manganese (Fe-Mn), and zinc/chromium-oxide (Zn/Cr-oxide) catalysts can achieve olefin selectivity greater than 40 percent, although CO2 and methane formation are problematic. Two key challenges to address are (1) developing a dual functional catalyst having comparable rates for CO formation from CO2 and subsequent hydrogenation of the CO-derived intermediate and (2) mechanistic understanding of possible formate or ketene intermediate species and their impact on observed product distributions that exceed limitations of the Anderson-Schulz-Flory mechanism.
As noted above, one challenge for thermochemical CO2 activation is C−C coupling to form C2+ products. The development of tandem catalysts and better understanding of C−C coupling mechanisms could improve selectivity for CO2 conversions to long-chain hydrocarbons (Gao et al. 2020). Tandem catalysis has been demonstrated for integrated ethylene, propylene, and aromatics production from CO2 (Gao et al. 2017, 2020; Saeidi et al. 2021; Zhang et al. 2019a). For example, Zhang et al. (2019a) prepared a zinc oxide/zirconium oxide (ZnO/ZrO2)-ZSM-5 tandem catalyst, with CO2 hydrogenation provided by ZnO/ZrO2 and C−C bond coupling and aromatization by H-ZSM-5. This system showed aromatic selectivity of 70 percent at 9 percent CO2 conversion and 613 K (340°C), indicating opportunities for further improvement. Detailed mechanistic modeling and catalyst characterization also can help catalyst development for production of C2+ chemicals. For example, Gao et al. (2017) developed a bifunctional In2O3/HZSM-5 catalyst with 4 nm pores that yielded 78.6 percent of C5+ liquid gasoline product from CO2 hydrogenation using a single integrated reactor, under conditions where Anderson-Schulz-Flory distribution would have limited C5+ production to less than 48 percent. More research efforts are needed to optimize catalyst compositions for tandem reactions in a single reactor and to improve reactor design for tandem processes involving multiple reactors.
Process and Systems Integration
The use of separate reaction steps—RWGS to form syngas, and then subsequent reactions of syngas to generate desired products—allows independent control of reaction conditions and catalyst formulation to achieve high yields for each step. Process intensification or integration of steps via coupling of endothermic and exothermic reactions could decrease capital costs, improve heat integration, and reduce energy use. System integration for CO formation will also be important, as CO is a stranded gas and further conversion is essential for rendering a commercial intermediate or product (González-Castaño et al. 2021). This integration will require new optimization of syngas catalysts for subsequent conversion steps, unlocking yet another era of interest in syngas catalyst optimization. For example, the endothermic RWGS reaction could be combined with exothermic methanol synthesis and further heat integrated into a methanol-to-product step (e.g., methanol to olefins). Traditional copper-zinc oxide (Cu-ZnO) catalysts for methanol synthesis from syngas are also active in WGS and hence could be considered for integrated CO2 hydrogenation to methanol (Ampelli et al. 2015). Use of small pore zeolites as supports allows a third-step integration of methanol to olefins (e.g., ethylene, propylene), although intermediate
dehydration can be a process challenge. Additional catalyst development and optimization are needed to improve kinetics and selectivity starting from CO2 rather than CO. Research is also needed on integrating catalyst design into the reaction and process system for optimization, as illustrated by González-Castaño et al. (2021) in their examination of the characteristics of copper-, cobalt-, iron-, and platinum-based catalysts for the RWGS reaction, including the impact of poisons.
Syngas generation from CO2 via RWGS could be integrated with the Fischer-Tropsch reaction pathways to provide an integrated route from CO2 to olefins or other C−C bonded products. One can use a coupled reaction sequence where the final reaction is not equilibrium constrained (e.g., methanol to olefins or fuels), to pull the reaction equilibrium constraint of RWGS and methanol synthesis to get a single reactor system that may operate at a lower temperature and obtain high conversion to fuels in a single pot. Such process intensification and integration would require integrated catalyst performance, as one cannot independently control reaction parameters for each elementary reaction step. Nonetheless, given the highly competitive nature of commercial industrial chemicals, this opportunity is driving innovative research in catalyst design and architecture for multifunctional syntheses, as well as reactor design and potential integrated separations. Research efforts are devoted to finding catalysts that improve the “CO2 hydrogenation” step and can be integrated with Fischer-Tropsch synthesis, methanol synthesis, and derivative conversion reactions to olefins or fuels so that the overall reactor can operate inexpensively at lower temperature and pressure, yet still give high per-pass yields (Gao et al. 2017; Saeidi et al. 2021). Research in catalyst and reactor design for the methanol-to-olefins process that targets incumbent distributions of olefin products would allow direct integration with existing petrochemical facilities and downstream processes, avoiding the need for extensive changes in equipment and operations.
Additional synergistic opportunities include integration of exothermic syngas reactions with high-temperature solid-oxide electrolyzer cells (SOECs) for hydrogen generation from water splitting, where thermal energy integration can improve electrical energy efficiency to near 100 percent (Hauch et al. 2020). A second synergy occurs with high-temperature solar thermochemical processes to split both CO2 and H2O to make syngas, integrated with thermal energy storage to allow 24/7 operation. More R&D is needed on thermal cycling of the working redox materials and differential thermal expansion to improve system design and durability. Given that production of net-zero fuels and chemicals requires use of atmospheric or biogenic (and not fossil point source) CO2, integrated capture and conversion of CO2 represents an essential opportunity to increase adsorption strength for low-concentration (420 ppm) atmospheric CO2, with synergistic use of chemical reactions to regenerate via conversion to preferred chemical products (see Section 7.2.5 for more detail).
Advanced Characterization and Discovery
Characterization techniques such as atomic force microscopy, transmission electron microscopy, and X-ray absorption spectroscopy provide in-depth analysis of supported nanoparticles. In addition, temporal analysis of reactors with gas chromatography-mass spectrometry analysis to obtain reaction rate data can complement the more established in situ Fourier-transform infrared spectroscopy approaches. Enhanced data acquisition combined with artificial intelligence/machine learning can provide important guidance for catalyst discovery.
Electrified Reactors
Given that low-cost, zero-emissions electricity can be produced directly from wind, solar, and other power sources (Lazard 2024), direct electrical heating of chemical reactors potentially can provide zero-carbon energy (heat) to drive the chemical conversions required for CO2 utilization. Chemicals and petroleum refining are responsible for approximately 50 percent of U.S. manufacturing CO2 emissions (EIA 2023), and the Department of Energy’s (DOE’s) industrial decarbonization roadmap highlights electrification (using zero-carbon electricity) as a key opportunity for decarbonizing these subsectors (DOE 2022). Simply replacing fossil-based electricity with clean electricity for currently electrified processes in the chemical industry could reduce the sector’s emissions by 35 percent, and further reductions are possible if additional processes (e.g., CO2 conversion) are electrified (Eryazici et al. 2021). Life cycle and techno-economic assessments of electrified reactors for syngas generation from CO2 will be critical for verifying GHG emissions reductions relative to conventional methods (Cao et al. 2022).
Catalyst options for electrically heated reactors have been investigated (Centi and Perathoner 2023). For example, Zheng et al. (2023) showed the efficacy of porous silicon carbide foams for Joule heating of RWGS catalysts at 650°C–700°C. Thor Wismann et al. (2022) examined electrically heated RWGS for methanol synthesis over a nickel-based catalyst, finding that routing the synthesis via methane formation reduced carbon deposition (coking). Dong et al. (2022) demonstrated that periodic pulsed heating can enhance selectivity to C2 hydrocarbons for methane reductive coupling, relative to steady-state operation. The “co-benefits” provided by the ability to rapidly pulse heat relative to conventional approaches with respect to concentration forcing are over and above the simpler replacement of fuel heat with low-carbon electrical energy to drive endothermic reactions. Similarly, microwave heating of catalyst particles, as demonstrated for methane conversion, may provide enhanced selectivity for endothermic CO2 reactions (i.e., RWGS) and reduce energy losses relative to bulk heating (Hunt et al. 2013). However, as opposed to direct heating via electric energy, microwave heating will suffer energy losses from conversion of electrical energy to electromagnetic radiation.
These examples show the potential for the emerging field of “electrified thermochemical” reactors (as opposed to the traditional “electrochemical” reactors) to provide new performance breakthroughs, especially for endothermic reactions such as CO2 reduction. The ability to rapidly change and control temporal and spatial heating to selectively heat catalyst surfaces versus bulk fluids, manipulate time constants for multistep reactions to improve selectivity, and reduce catalyst poisoning by operation under rapidly varying dynamic heating conditions (unlike traditional “concentration forcing” conditions), or use microwaves for selective catalyst heating provide new handles for catalyst and reaction control that are yielding promising results.
Solar thermochemical hydrogen production and solar thermochemical CO2 conversion can be readily integrated with thermal energy storage and improve the economic viability of high capital intensity processes (e.g., methanol and Fischer-Tropsch syntheses) that require 24/7 operation. R&D is needed to examine this synergy and consider its relative cost versus using solar photovoltaic energy plus battery storage to maintain 24/7 operability of Fischer-Tropsch or methanol synthesis.
7.2.2 Electrochemical Conversion Pathways
7.2.2.1 Current Technology
Significant progress has been made in developing electrocatalysts and electrochemical devices for the CO2 reduction reaction (CO2RR) to produce value-added chemicals, including C1 (carbon monoxide, methane, methanol, formic acid), C2 (ethylene, ethanol, acetic acid), and some C2+ (acetone, propanol, etc.) products. Low-temperature electrochemical conversion of CO2 to C1 products is occurring at the pilot scale (Grim et al. 2023; Masel et al. 2021; Xia et al. 2022), and high-temperature conversion of CO2 to CO is nearing commercialization (Hauch et al. 2020; Küngas 2020). Many studies have identified CO2RR electrocatalysts that are selective toward specific products. For example, noble metals such as silver and palladium are efficient in producing CO. First-row transition metals such as cobalt and nickel produce both CO and CH4. Main group metals such as tin, indium, and bismuth, as well as their oxides, are selective for formic acid production. A proton exchange membrane system using a lead/lead sulfate cathode was recently reported to produce formic acid with high selectivity and durability (Fang et al. 2024). At present, copper is the primary element identified that can catalyze CO2RR to C2 and C2+ products (Nitopi et al. 2019; Yan et al. 2023). Some studies have explored the possibility of enhancing the activity of copper using copper-based bimetallic alloys (Lee et al. 2018), and some have reported the production of long-chain hydrocarbons using non-copper-based catalysts (Zhou et al. 2022).
The status of theoretical simulations of electrochemical CO2RR was summarized by Xu and Carter (2019a). Modeling efforts are mostly based on density functional theory, which has difficulties describing key intermediates (CO) and electron-transfer reactions owing to errors in its electron exchange-correlation functionals. Recent work has shown that such errors can be corrected by including accurate wavefunction descriptions of exchange-correlation via embedding methods (e.g., Zhao et al. 2021). Multiscale modeling of bipolar membranes for electrochemical systems provides insights into structure–property–performance relationships that can help inform the design of CO2 electrolyzers (Bui et al. 2024).
TABLE 7-2 Electrochemical CO2RR Products with Equilibrium Potentials
| Reaction | E0 [V versus RHE] | Products |
|---|---|---|
| CO2 + 2H+ + 2e− → HCOOH(aq) | −0.12 | Formic acid |
| CO2 + 2H+ + 2e− → CO(g) + H2O | −0.10 | Carbon monoxide |
| CO2 + 6H+ + 6e− → CH3OH(aq) + H2O | 0.03 | Methanol |
| CO2 + 8H+ + 8e− → CH4(g) + 2H2O | 0.17 | Methane |
| CO2 + 4H+ + 4e− → C(s) + 2H2O | 0.21 | Graphite |
| 2CO2 + 2H+ + 2e− → (COOH)2(s) | −0.47 | Oxalic acid |
| 2CO2 + 8H+ + 8e− → CH3COOH(aq) + 2H2O | 0.11 | Acetic acid |
| 2CO2 + 10H+ + 10e− → CH3CHO(aq) + 3H2O | 0.06 | Acetaldehyde |
| 2CO2 + 12H+ + 12e− → C2H5OH(aq) + 3H2O | 0.09 | Ethanol |
| 2CO2 + 12H+ + 12e− → C2H4(g) + 4H2O | 0.08 | Ethylene |
| 2CO2 + 14H+ + 14e− → C2H6(g) + 4H2O | 0.14 | Ethane |
| 3CO2 + 16H+ + 16e− → C2H5CHO(aq) + 5H2O | 0.09 | Propionaldehyde |
| 3CO2 + 18H+ + 18e− → C3H7OH(aq) + 5H2O | 0.10 | Propanol |
NOTE: RHE = reversible hydrogen electrode.
SOURCE: Adapted from Nitopi et al. (2019).
Molecular electrocatalysts have also been studied extensively for CO2RR, with more than 100 different catalysts identified. Catalysts have been reported with 13 different transition metals, and a few examples exist of non-metal-containing catalysts (Francke et al. 2018). Although these catalysts operate under a wide variety of conditions, including organic and aqueous solvents, only a few different products have been reported. Under protic conditions, CO is the most common product, followed by formate or formic acid. Under nonprotic conditions, typical products are oxalate, CO, and carbonate (CO32−). A handful of systems have been reported that catalyze the six-electron reduction to methanol or the eight-electron reduction to methane, although some of these are not strictly homogeneous, but instead molecular catalysts immobilized onto electrode surfaces (Boutin and Robert 2021).
Among all the potential products from CO2RR, as shown in Table 7-2, CO and formic acid (HCOOH) are generally considered to be the most commercially viable molecules based on a recent review (Nitopi et al. 2019) and techno-economic assessment (Aresta et al. 2014). Conversions of CO2 to CO or HCOOH require only two electrons and are kinetically more facile than the multiple-electron and multiple bond formation-scission processes for products containing two or more carbons. Equally important, CO and HCOOH can be produced using catalysts that do not contain copper, therefore allowing the utilization and optimization of a wide range of electrocatalysts. HCOOH is a bulk chemical that can be used as a feedstock for the chemical industry and for energy storage (Aresta et al. 2014). One advantage of converting CO2 to CO is the higher efficiency of converting CO to value-added products, either through electrochemical (Jouny et al. 2019) or thermochemical (see Section 7.2.1) upgrading reactions. Converting CO2 to CO is also advantageous in terms of carbon utilization efficiency. The alkaline environment required to achieve high reaction rates for CO2RR results in large amounts of (bi)carbonate production, which has limited CO2RR selectivity for multiple carbon products (Nitopi et al. 2019). In contrast, CO2RR to CO can be carried out in a nonalkaline environment with high CO selectivity without producing (bi)carbonates. Furthermore, because CO is a gas, it is easier to separate from the electrolyte than liquid products (i.e., liquid-liquid separations are not required). The production of several C2 molecules, including ethylene (C2H4), ethanol (C2H5OH), and acetic acid (CH3COOH), has also been investigated extensively. It is widely accepted that these reactions proceed via the formation of a *CO-containing surface intermediate followed by its dimerization and subsequent reduction to form C2 products (Nitopi et al. 2019).
Although most current research on CO2RR focuses on low-temperature electrochemical devices, there are efforts in using intermediate-temperature (molten carbonate electrolysis) and high-temperature (solid oxide electrolysis) devices (Küngas 2020). For example, Figure 7-7 compares different electrochemical devices for CO2
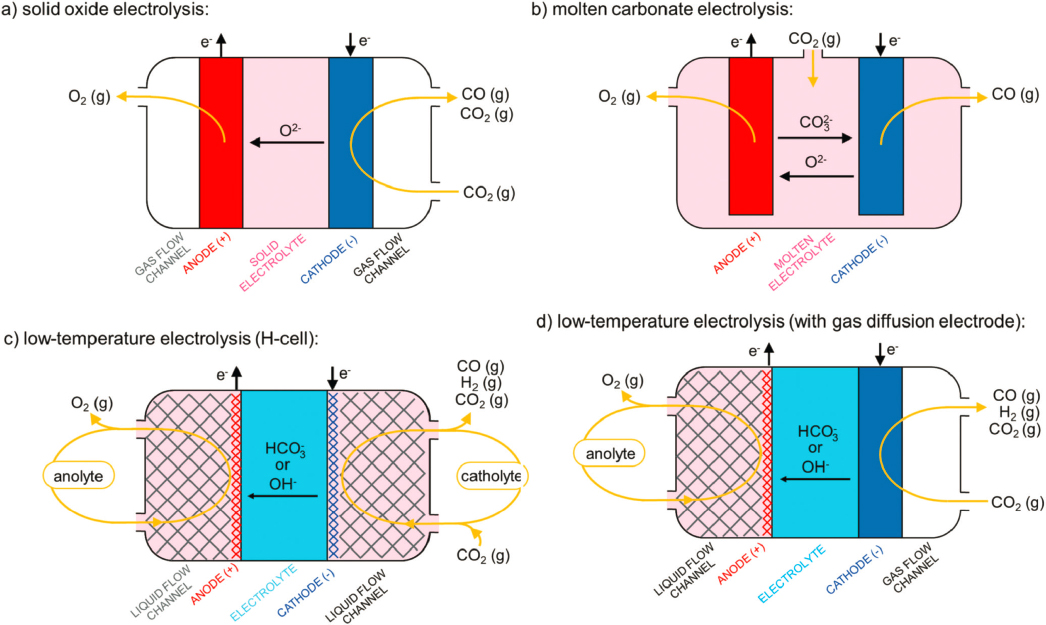
SOURCE: Küngas (2020), https://doi.org/10.1149/1945-7111/ab7099. CC BY-NC-ND 4.0.
reduction to CO. Based on an analysis by Hauch et al. (2020) high-temperature solid oxide electrolysis of CO2 to CO is considered to be approaching commercialization with promising catalytic rates and long-term durability (Hauch et al. 2020; Küngas 2020).
Electrochemical carboxylation reactions using CO2 are also of interest as a more environmentally friendly method of producing industrially relevant carboxylic acids (Ton et al. 2024; Vanhoof et al. 2024). These reactions can involve a variety of co-substrates, including alkenes, alkynes, benzyl, aryl, and alkyl halides, and aryl aldehydes and ketones, and thus are able to form a diverse range of products. The systems often require a sacrificial anode (commonly magnesium or zinc) to provide stabilizing metal ions for the radical anion species formed during electroreduction of substrate or CO2, but there are efforts to develop sacrificial-anode-free systems through electrolyte, substrate, and cell design to reduce cost and improve sustainability.
7.2.2.2 Challenges
Although electrocatalytic CO2RR can produce several C1 products (CO, HCOOH, methanol, and methane), one critical challenge is the selective production of >2e− reduced products, and C2+ products in particular, in high yield. The energy efficiency is further complicated by the competing hydrogen evolution reaction. As noted above, at present copper is the primary element identified that can catalyze CO2RR to C2+ products with appreciable Faradaic efficiency.5
___________________
5 Faradaic efficiency is a measure of selectivity of an electrochemical reaction, calculated as a ratio of the amount of product formed over the theoretical maximum amount based on the charge passed (Kempler and Nielander 2023).
As with heterogeneous systems, homogeneous CO2RR is also challenged by product selectivity for a single carbon-based product and the competing hydrogen evolution reaction, although there are examples of catalysts with high selectivity for CO and formate (Francke et al. 2018). However, few examples exist of homogeneous electrocatalysts that reduce CO2 beyond two electrons, and none that demonstrate C-C bond coupling to form C2+ products except for oxalate (Francke et al. 2018).
Most low-temperature CO2RR studies are at an early stage of development, primarily owing to issues with long-term stability and product selectivity (Grim et al. 2023; Küngas 2020). While some progress has been made in improving these metrics, in particular through developments in gas diffusion electrodes, challenges remain in reducing overpotential and improving stability at high current densities, as well as decreasing energy losses from carbonate formation (Wakerley et al. 2022). Although high-temperature CO2RR is considered to be at higher TRL, its feasibility only has been demonstrated for CO2 conversion to CO.
Electrochemical reactors exhibit a unit scaling factor (with capacity) of near 1.0, such that one must “number up” to achieve a large scale of production. This inability to reduce costs at increasing scale has been an issue for achieving cost-effective production of H2 via water electrolysis, and likely would be for CO2 electrolysis as well. Another consideration when scaling up electrochemical CO2 conversion systems is the energy requirement compared to that of alternative tandem electrocatalysis-thermocatalysis routes, as clean electricity availability may be limited by supply chain constraints. Where electrical efficiency for direct CO2 conversion is poor (i.e., high overpotential), it may be more efficient to generate clean H2 via water electrolysis and use that H2 to form syngas, which can then be converted thermochemically to fuels and chemicals using existing technologies, as described in Section 7.2.1.1 (Eryazici et al. 2021).
7.2.2.3 R&D Opportunities
A key R&D opportunity for electrochemical CO2 conversion is to expand the number of catalysts that can generate C2+ products with relatively high yields. One approach to enhance C2+ product generation is to modify copper with an element that is efficient for CO2 to CO conversion. The resulting bimetallic electrocatalysts could effectively convert CO2 to CO, which subsequently could be converted to C2+ products by copper. The CO-rich reaction environment also should inhibit the competing hydrogen evolution reaction that would otherwise reduce the selectivity for C2+ products on pure copper. Silver and gold are attractive options because of their high CO2RR selectivity to CO and their immiscibility with copper, which prevents changes in electrocatalytic properties owing to formation of bimetallic alloys. Utilization of multiple catalysts consisting of copper and a CO-producing electrocatalyst, in the form of either physical mixtures or segmented catalyst beds, has been demonstrated for CO2 conversion to multicarbon products (Yin et al. 2022). One common practice uses one metal as a catalytically active and conductive substrate onto which the second metal is deposited. Segmented electrodes also have been studied recently to control the separation between distinct catalysts. For example, two catalysts can be deposited adjacent to each other to produce a high concentration of CO that then flows over a C2-producing catalyst (Zhang et al. 2022b). As another approach, recent efforts have explored the utilization of electrocatalytic-thermocatalytic tandem processes to produce C2+ oxygenates and hydrocarbons (Biswas et al. 2022b; Lee et al. 2023), although more studies are needed to determine whether such tandem processes can be economically competitive. Computational modeling, specifically advanced quantum mechanics methods that go beyond density functional theory (see, e.g., Martirez et al. 2021) when needed for simulating electron-transfer reactions, combined with ab-initio molecular dynamics for solvent configurational sampling (see, e.g., Martirez and Carter 2023), along with machine-learned force-field molecular dynamics (Poltavsky and Tkatchenko 2021; Unke et al. 2021; Wu et al. 2023) to sample longer time and larger sample sizes, will be the methods of choice in the future. Additional research on multiscale modeling of mass transport effects is needed to improve understanding and optimization of electrochemical device design (Stephens et al. 2022).
In molecular systems, continued work on mechanisms, modifying the electronic properties of active sites, and understanding and modifying secondary coordination sphere interactions have provided some insight into inhibiting competitive hydrogen evolution and/or steering product selectivity (Barlow and Yang 2019). Trade-offs (e.g., scaling relationships) between activity and overpotential have been identified (see, e.g., Bernatis et al. 1994; Nie and McCrory 2022). However, these scaling relations can be broken with appropriate secondary sphere effects such as
proton-relays/hydrogen-bonding interactions (Costentin et al. 2012), charge (Azcarate et al. 2016; Margarit et al. 2020), or simultaneous changes in multiple reaction parameters (Klug et al. 2018; Martin et al. 2020). Redox-active ligands also have been used to delocalize charge to access catalytic intermediates at milder potentials (Queyriaux 2021). Some of these strategies are bio-inspired by mimicking either the electronic structure or local environment of enzymes that catalyze these reactions efficiently (Shafaat and Yang 2021). Immobilizing molecular catalysts onto certain types of electrodes also appears to result in different selectivity (Boutin et al. 2019). Ligand modifications can be applied to study local environmental effects that tune selectivity or inhibit hydrogen evolution. These strategies may be translatable to heterogeneous systems (Banerjee et al. 2019).
In addition to optimizing catalysts that are selective, stable, and scalable for CO2RR, it is important to develop scalable electrochemical devices. For example, stable and cost-effective anode catalysts, which are required to complete electrochemical systems for CO2RR, need to be identified. In particular, if the oxygen evolution reaction is used as the anodic reaction under acidic conditions, the costs of the iridium oxide (IrO2) catalysts need to be considered as a potential barrier for large-scale CO2RR. Use of inexpensive, alkaline-electrolyte-based anodes for the oxygen evolution reaction, enabled by dipolar membranes, may overcome this cost barrier, but high overpotential might still be a limiting issue (Nitopi et al. 2019). Pairing CO2RR with a different anodic reaction, which may produce a more valuable product than oxygen, could also be explored (Francke et al. 2018; van den Bosch et al. 2022). The reactor components (electrodes, catalysts, supports, membrane, electrolyte) and reaction conditions (pH values of electrolytes, flow rate, temperature, pressure) also need to be optimized (Sarswat et al. 2022; Stephens et al. 2022; Wakerley et al. 2022). Continued development of semi-empirical CO2 electrolyzers models could help inform scale up beyond lab- and pilot-scale systems (Edwards et al. 2023). Furthermore, because many CO2 sources contain various potential contaminants, it is also important to evaluate the tolerance of CO2RR electrocatalysts and membranes (Nitopi et al. 2019).
For electrocarboxylation reactions, primary R&D opportunities include further development of sacrificial-anode-free systems, experimental and theoretical studies to improve mechanistic understanding and facilitate catalyst design, and improvements to enantioselectivity (Ton et al. 2024; Vanhoof et al. 2024). Focusing research efforts on the most common industrial chemicals, developing flow systems, and designing more robust electrocatalysts could facilitate eventual scale up.
7.2.3 Photochemical/Photoelectrochemical Conversion Pathways
7.2.3.1 Current Technology
The use of light to directly drive CO2 reduction to fuels or other chemicals has been pursued via several different motifs. These include homogeneous systems that use molecular photosensitizers to absorb light (Figure 7-8) and systems that use a heterogeneous light absorber to generate the voltage required for CO2 reduction (Figure 7-9). In the latter, catalysis can occur directly at the semiconductor interface, with a heterogeneous or molecular catalyst appended to the semiconductor interface, or with molecular catalysts in solution (Kumar et al. 2012). These systems can be completely photo-driven, where no external voltage or energy source is needed, or photo-assisted, where light energy is used to provide a portion of the energy and reduce the applied voltage required to complete the chemical process.
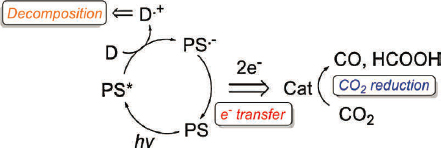
SOURCE: Reprinted from Y. Yamazaki, H. Takeda, and O. Ishitani, 2015, “Photocatalytic Reduction of CO2 Using Metal Complexes,” Journal of Photochemistry and Photobiology C: Photochemistry Reviews 25:109, Copyright (2015), with permission from Elsevier.

SOURCE: Used with permission of the Royal Society of Chemistry from X. Chang, T. Wang, and J. Gong, 2016, “CO2 Photo-Reduction: Insights into CO2 Activation and Reaction on Surfaces of Photocatalysts,” Energy & Environmental Science 9(7):2177–2196, https://doi.org/10.1039/C6EE00383D; permission conveyed through Copyright Clearance Center, Inc.
In addition to the metrics of Faradaic efficiency (product selectivity) and energetic efficiency (overpotential) used to evaluate electrochemical CO2 conversion, photochemical systems are also described by their photochemical quantum yield (Φ) that evaluates the efficiency in which absorbed photons generate product (Reaction 7.4; Kumar et al. 2012), where
| Φ = (moles product/absorbed photons) × (electrons needed for conversion) | (R7.4) |
Homogeneous photocatalytic systems typically have a photosensitizer, electron donor, and catalyst (Dalle et al. 2019). The photosensitizer absorbs light to generate the electron donor, which reduces the catalyst to initiate CO2 reduction. Most catalysts with activity toward electrochemical reduction (dark electrocatalysis) also have activity toward photocatalysis with an appropriate photosensitizer and donor. In some cases, the catalyst itself can serve as the photosensitizer (Das et al. 2022; Hawecker et al. 1986). The most common electron donors are aliphatic amines, NAD(P)H model compounds, ascorbate, and imidazole compounds. The choice of electron donor impacts the overall efficiency and stability of photocatalytic systems and can be involved in other reactivity (Sampaio et al. 2020). While the use of these sacrificial electron donors is common, they do not represent a sustainable method for photochemical reduction. Ideally, the electron donor would be water, but water is typically an insufficient reductant to drive CO2 reduction.
A number of strategies have been applied to improve the performance of heterogeneous photocatalytic (PC) systems for CO2 reduction. The semiconductor materials must have a suitable band gap (neither too large nor too small) to enable efficient visible light absorption while also being large enough to drive the reaction. The potential of the conductive and valence bands must be sufficient for CO2 reduction and water oxidation (Kalamaras et al. 2018; Liao and Carter 2013; Mayer 2023). Theoretical approaches to simulating (photo) electrochemical CO2RR and water splitting at the atomic scale with quantum mechanics modeling have helped elucidate the roles of the structure and composition of the electrochemical interface, absolute band edge positions relative to the redox potentials, charge carrier transport, and proton, electron, and hydride transfers (Govind Rajan et al. 2020; Liao and Carter 2013; Xu and Carter 2019b). Advancements have been made by focusing on materials architecture, which includes quantum dots, nanotubes and nanorods, two-dimensional materials, and more advanced nanostructures (Gui et al. 2021). Additionally, various dopants, sensitizers, and co-catalysts have been introduced to achieve the desired light-absorbing and catalytic properties. To prevent oxidation of the product by photogenerated holes on the photoabsorber, hole scavengers such as hydrogen peroxide (H2O2), sodium sulfite (Na2SO3), and alcohols are sometimes used (Chang et al. 2016). Several different photoreactors,
both batch and continuous types, also have been engineered to improve overall solar-to-product efficiencies. These reactor types are broadly categorized as slurry, fixed bed, and membrane (Khan and Tahir 2019). Key considerations include using geometry to maximize light absorption; using materials (photoabsorber/catalyst, reactor), heat exchange, mixing, and flow characteristics to maintain high contact between the reactants and catalyst; and product separation.
The most common configuration of photoelectrochemical (PEC) cells is composed of a semi-conductor photoelectrode and a counter electrode (White et al. 2015). Compared to PC systems, PEC systems may achieve higher efficiency, because electron-hole recombination is slowed by the external potential. Additionally, a greater variety of materials and configurations can be used. In most cases, CO2 reduction is accelerated using co-catalysts (Gui et al. 2021), which are often nanoparticles of metals or oxides. Molecular catalysts have also been attached onto surfaces to accelerate CO2 reduction (White et al. 2015). Other systems use solution-based co-catalysts to promote catalysis. P-type gallium phosphide (GaP) semiconductors have shown the direct photoelectrochemical reduction of CO2 to methanol with pyridinium additives (Barton Cole et al. 2010; Cohen et al. 2022; Sears and Morrison 1985; Xu and Carter 2019b; Xu et al. 2018). However, different optimal conditions, products, and yields have been reported (Costentin et al. 2018). Nanostructured electrodes have been used to enhance photocatalytic activity by engineering the band structure, increasing the surface area for catalysis, enhancing light absorption, and minimizing electron-hole recombination. Optimization of adsorbed cocatalysts may also help with selectivity and activity (Xu and Carter 2019a).
More recently, researchers have been exploring the use of localized surface plasmon resonance for light-driven CO2 reduction (Figure 7-10). This phenomenon is a result of the resonant photon-induced collective oscillation of valence electrons and is most commonly observed on nanostructured gold, silver, copper, and aluminum surfaces of nanoparticles (Robatjazi et al. 2021). Plasmonic photocatalysis can contribute to CO2 reduction by reducing the substrate (or catalyst if used) and providing local thermal heating (Verma et al. 2021; Wang et al. 2023a; Zhang

SOURCE: Hu et al. (2023), https://doi.org/10.1038/s41467-023-35860-2. CC BY 4.0.
et al. 2023). A few promising examples of plasmonic photocatalysis have been reported so far. For example, gold nanoparticles have been used to generate C1 and C2+ products from CO2 in water (Hu et al. 2023) and an ionic liquid solution (Yu and Jain 2019). Plasmonic photocatalytic systems have also been shown to accelerate the dry reforming of methane (CH4 + CO2) into syngas, although the formation of coke limits the lifetime of these catalytic systems (Cai and Hu 2019; Chen et al. 2019; Dieterich et al. 2020; Han et al. 2016; Jang et al. 2019; Zhou et al. 2020).
7.2.3.2 Challenges
The direct use of light to drive CO2 reduction in photochemical processes is at a basic research stage. Integration of photochemical processes has the potential to reduce the balance of systems costs but comes with other obstacles to be competitive with a photovoltaic and electrolyzer configuration (PV-EC), which already has demonstrated a solar-to-chemical-to-energy conversion efficiency of 21.3 percent for CO production (Liu et al. 2023). Several studies have described the benefits and drawbacks of these two configurations for hydrogen production, and many details from these analyses are also applicable for CO2 reduction (Ardo et al. 2018; Grimm et al. 2020; Rothschild and Dotan 2017; Shaner et al. 2016). In these analyses, PEC devices need significant improvements to both efficiency and stability. Additional advances in device architectures and operation schemes, such as the power management and light management scheme, are also critical to improve the competitiveness of using PEC versus PV-EC systems. Like electrochemical CO2 reduction, photochemical CO2 reduction also contends with product selectivity, particularly with respect to H2 co-generation and slow kinetics for CO2 reduction.
In homogeneous systems, most photosensitizers are composed of precious metals, although recent work has focused on the use of abundant components (Ho et al. 2023; Wang et al. 2023b; Xie et al. 2023; Zhang et al. 2019b). While turnover numbers are now reported in the tens of thousands (Dalle et al. 2019), systems with greater long-term stability are needed. Additionally, practical systems will need to demonstrate a catalytic cycle that does not require the use of sacrificial electron donors but instead uses water as the reductant.
Photochemical conversions traditionally have been limited by proximity and surface area contact requirements for photochemical energy. Reactors that combine high surface areas and/or deep penetration zone can overcome these limitations, as can use of high efficiency light-emitting diode arrays (essentially a new form of electrified reactor). These systems likely will have a scaling factor close to 1.0, thus requiring smaller units and numbering up to achieve large-scale production. Such designs tend to favor a modular approach that may make distributed production attractive, but which could limit the overall operating scale.
Additional challenges with photocatalytic systems include charge recombination, the requirement for hole scavengers, and product separations. High rates of charge recombination lead to lower overall quantum efficiency. The use of hole scavengers to prevent reoxidation of product at the photoabsorber adds to the overall cost. Because the cathodic and anodic products are co-generated and are often small molecules of similar sizes, product separation is also a challenge. Low-energy separation strategies, such as membranes, can reduce the CO2 footprint of the separation/purification process and require considerable future study. Fundamental transport properties of relevant solutes in these solutions are not widely available, which frustrates efforts to design improved membranes for such separations. Other separation methods, such as adsorption and distillation, considered alone or in combination, may play an important role in such separations (Sarswat et al. 2022). Careful consideration of process design and energy requirements would enable informed separation system design. As with membranes, fundamental studies of separation processes are needed using relevant, multicomponent systems.
Research on PEC cells has generated a greater fundamental understanding of semiconductor physics and electronic structure (Xu and Carter 2019b), and the architecture provides a variety of potential materials. However, most photoabsorbers that have been examined either spontaneously corrode (often under aqueous conditions) or experience photocorrosion during operation. Various protective layers, often metal oxides, have been used to improve stability (Lichterman et al. 2016). While plasmonic photocatalysis holds promise, the reported systems currently suffer from high energy input, low product yields, and high costs. In many cases, stability is also an issue. However, new bimetallic alloys have demonstrated resistance to coking for dry methane reforming (Zhou et al. 2020). Additionally, aside from a few reactions (Martirez et al. 2021; Zhou et al. 2020), the mechanisms of catalysis are not well understood, making rational improvements to activity challenging.
7.2.3.3 R&D Opportunities
There are many R&D opportunities for photochemically driven systems. Intrinsically, all motifs require light absorption, generation and separation of electron-hole pairs, and then catalytic reduction of CO2 paired with another oxidative half reaction. All three of these processes could be improved with further research. There are also specific challenges in the different motifs. While many possible architectures for light-driven CO2 reduction exist, critical analysis toward scalability, stability, and overall light-to-product efficiency is important, particularly with respect to other methods of carbon-neutral CO2 utilization.
In homogeneous systems, progress is needed in the use of abundant elements. While photocatalytic systems now operate with turnover frequencies in the thousands per hour, they require operation with longer-term stability for practical application (turnover numbers are typically around 104; Dalle et al. 2019). Additionally, systems need to demonstrate operation without the use of sacrificial electron donors, preferably with water and a closed catalytic cycle.
Computational modeling also needs further development, specifically faster, more accurate quantum methods for computing band gaps, absolute band edge positions in the presence of electrolyte, and charge carrier transport and reactions, combined with ab initio molecular dynamics for solvent configuration sampling, along with machine-learned force field molecular dynamics to sample longer time and larger sample sizes.
Photochemical systems continue to benefit from new materials architectures and formulation, which improve overall quantum efficiency and product selectivity. Continued research into reactor design can inform scalable design and performance metrics. Photoelectrochemical systems require improved methods to inhibit corrosion for greater stability.
As in electrochemical systems, catalyst selectivity, rates, and stability are also important. The dominant products in these systems tend to be C1. Obtaining C2+ products will require additional development; an improved understanding of mechanistic pathways for coupling C1 products could benefit this line of inquiry. Photochemical carboxylation to form more complex C2 and C2+ products has been demonstrated, but more research is needed to expand reactivity to unsaturated hydrocarbons and unactivated alkenes, in addition to addressing the other challenges for photocatalytic systems mentioned above (Cauwenbergh et al. 2022; Davies et al. 2021; Tortajada et al. 2018; Zhang et al. 2024). Integration of catalysts into photochemical systems also requires compatibility under operating conditions, as well as robust and stable methods of attachment that do not inhibit both light absorption and catalyst activity.
For plasmonic photocatalysis, the preparation of lower-cost and higher-efficiency noble metal nanoparticles is needed. At this point, the materials space has been minimally explored (Wang et al. 2023a; Zhang et al. 2023). The parameter space that includes the size, shape, and composition of the nanomaterials, as well as the reaction medium and absorption wavelengths, is not well mapped. The mechanisms of plasmonic photocatalysis are not well understood holistically from light absorption through chemistry (despite substantial analysis for individual components of the phenomenon), and advanced characterization may be required to understand the light-matter interaction at an atomic scale. These studies would also be used to inform more sophisticated computational models. Additionally, a better understanding of the molecule-metal interface in hybrid materials may open new routes for more selective or efficient catalysis (Verma et al. 2021; Wang 2023a; Zhang et al. 2023).
7.2.4 Plasmachemical Conversion Pathways
7.2.4.1 Current Technology
Among the possible means to replace fossil fuel–driven thermal conversion with carbon-emission-free electrically driven processes, the potential use of plasma—the phase of matter consisting of gaseous ions and free electrons, formed by passing electricity through a gas—is being explored. Plasmachemical pathways could provide the ability to tune separately reactive ion and electron properties, which may offer unique opportunities for CO2 conversion. Products of plasmachemical CO2 activation depend on the types of plasma used, reaction conditions (temperature, pressure, flow rate, and molar ratio of feeds), and the nature of co-reactants. In the absence of other co-reactants, plasma activation of CO2 generates a nonequilibrium ionized gas that enables the cleavage of the C=O bond in CO2 to produce CO and O2 (Snoeckx and Bogaerts 2017), which has been demonstrated using several types of plasma sources, including glow, radiofrequency, and microwave discharges (Ashford and Tu 2017; Xu et al. 2021).
When hydrogen-containing molecules (e.g., H2, H2O, CH4) are included as co-reactants, CO2 can be converted into a wide range of hydrocarbons and oxygenates (e.g., methanol, formaldehyde, and acetic acid) (Liu et al. 2020).
In principle, plasma activation has potential advantages over conventional processes. For example, high-temperature activation is required to react CO2 and CH4 owing to the inert nature of the C=O (Ediss = 5.5 eV) and C–H (Ediss = 4.5 eV) bonds in CO2 and CH4, respectively. This high-temperature condition limits the production of oxygenates thermodynamically. In contrast, CO2 and CH4 activation can be achieved at room temperature using a nonthermal plasma. The plasma-induced high-energy electrons in the nonequilibrium ionized gas can activate CO2 and CH4 molecules at low bulk gas temperatures to produce oxygenates. Nonthermal plasmachemical reactions of CO2 and CH4 have been used to produce both hydrocarbons and oxygenates (Liu et al. 2020). Plasmachemical reactions of CO2 and ethane also have been explored. A corona plasma was investigated for oxidative dehydrogenation of ethane with CO2 to produce CO, H2, and hydrocarbons. A dielectric barrier discharge plasma was used to convert ethane and CO2 to syngas (CO + H2) and formaldehyde. Reaction of ethane with CO2 activated by a dielectric barrier discharge plasma produced C1–C3 alcohols, aldehydes, and acids, in addition to hydrocarbons and CO (Biswas et al. 2022a).
7.2.4.2 Challenges
One of the main challenges in plasmachemical processes is controlling selectivity toward the desired products. Although plasma activation provides a promising route to achieve direct oxidation of light alkanes with CO2 to produce valuable oxygenated products, the involvement of and interactions between various reactive species results in a wide range of products, as illustrated in Figure 7-11 for plasmachemical reactions of CO2 and CH4. On the
SOURCE: A. Bogaerts, C. De Bie, R. Snoeckx, and T. Kozák, 2017, “Plasma Based CO2 and CH4 Conversion: A Modeling Perspective,” Plasma Processes and Polymers 14(6):1600070. Copyright (2017), with permission from Wiley.
other hand, for certain applications in which nonselective chemical mixtures are desired outcomes, such as for jet fuels, plasmachemical reaction of CO2 to hydrocarbon mixtures within the desired range of carbon numbers could be an attractive option. Beyond selectivity, challenges for plasmachemical CO2 conversion include process scale up (scaling factor of close to 1.0) and energy losses resulting from the conversion of electrical energy to plasma energy.
7.2.4.3 R&D Opportunities
At present, research on plasmachemical CO2 activation is primarily at the stage of lab-scale fundamental studies. Little to no work has been done on computational modeling of plasmachemical CO2 activation to date. The variety of gaseous and liquid products from plasmachemical CO2 activation necessitates post-reaction product separation and reduces the energy efficiency. Research focusing on low-energy separation strategies, such as membranes, would be helpful. Membranes today are not designed to separate such complex mixtures, so fundamental research on structure-property-processing of viable membrane candidates is needed. Catalysts may be employed with plasma to provide additional control of reaction selectivity, but significant challenges remain to achieve effective coupling of plasma and catalytic reactions. Understanding plasma-catalyst interactions will require characterization methods to accommodate the complexity of the reaction systems. For example, the chemical properties, surface area, porosity, and dielectric properties of catalyst materials can modify plasma properties. Conversely, the plasma can modify the nature of the catalyst as well. Furthermore, the size and form of the packing material in the catalyst bed can also affect plasma-catalytic activity and selectivity. From the perspective of reactor design, post-plasma-catalysis configurations need to be explored. Moving catalysts outside of the plasma discharge enables the differentiation of interactions with short-lived plasma species from catalytic reactions involving long-lived intermediates and products with the catalyst bed. Further understanding of the complex interactions within plasma-catalyst systems is critical for developing practical plasma-catalytic technologies for selective conversion of CO2 to desired products.
7.2.5 Integrated Capture and Conversion of CO2
Most work on CO2 utilization uses pure and concentrated CO2 streams as the substrate. However, CO2 is often found in a dilute stream, with concentrations that range from 0.04 percent in air, to 4–5 percent in natural gas-fired power generation, to greater than 95 percent in some industrial point sources (e.g., ethanol fermentation off-gas) (GAO 2022; NETL n.d.(d)). The composition of the balance of gases also depends on the CO2 stream, but they commonly contain water, oxygen, and inert gases such as N2. Industrial streams can also contain lower amounts of gases such as NOx and SOx (see Appendix H). Technologies at high readiness levels currently exist for both point source and direct air capture and concentration of CO2.
CO2 capture and utilization can be performed independently and in sequence. An example of a sequential commercial process exists in the George Olah Renewable Methanol Plant, which hydrogenates CO2 isolated from geothermal plant emissions to 4000 tons of methanol per year (Carbon Recycling International n.d.). Integration of capture and utilization provides advantages in process intensification, reducing capital and operational expenses. Integration of these two steps—capture and utilization—is often called reactive capture, defined here as the direct utilization of CO2 from dilute streams without going through a purified CO2 intermediate (Freyman et al. 2023). In most CO2 capture and concentration systems, CO2 capture is relatively passive except for air-handling, while regeneration of the sorbent to release and compress the CO2 requires most of the energy input. Direct use of dilute CO2 or sorbed CO2 reduces the need for the energy-intensive CO2 release/concentration step, as well as the need for CO2 transport or compression, as illustrated in Figure 7-12. The overall energetics of integrated capture and conversion will depend on the sorbent used and product formed (Heldebrant et al. 2022). For example, a technoeconomic assessment has shown that integration of CO2 capture with conversion to methyl formate can save up to 46 percent of the overall energy compared to the sequential process, and up to 8 and 7 percent of the cost and GHG emissions, respectively (Jens et al. 2019). Another analysis indicates that an integrated capture and conversion process can reduce the energy intensity for methanol production from CO2 by 50 percent compared to sequential capture and conversion approaches, with a 38 percent reduction in capital expenditure (Freyman et al. 2023).
SOURCE: Reprinted from M.C. Freyman, Z. Huang, D. Ravikumar, et al., 2023, “Reactive CO2 Capture: A Path Forward for Process Integration in Carbon Management,” Joule 7(4):631–651, https://doi.org/10.1016/j.joule.2023.03.013. Copyright (2023), with permission from Elsevier.
Various approaches have been made toward integrated capture and conversion of CO2, including reductions via electrochemical or thermal routes, synthesis of cyclic carbonates, and biological utilization.
7.2.5.1 Current Technology
An example of electrochemically driven integrated capture and conversion at a high TRL is the use of molten metal oxides to generate solid carbon, as described in Chapter 6. High-temperature (650–900°C) molten salts can capture CO2 from dilute streams, including flue gases and air, with high selectivity and form carbon materials upon reduction (Zhu et al. 2023). Because the carbon materials have to be removed from the electrode, this system is typically run in a batch mode with reuse of the molten salts (Carbon 2023).
Other examples of electrochemical integrated capture and conversion occur at lower temperatures. Aqueous approaches capitalize on the favorable reaction of hydroxide anions with CO2 to capture CO2 and form carbonate or bicarbonate, depending on the pH (Ghobadi et al. 2016). Bicarbonate electrolyzers use a cation exchange membrane or bipolar membrane to generate an acidic environment at the cathode/membrane interface, which reacts with bicarbonate to release CO2, which is then reduced at the cathode. The hydroxide formed as a product of CO2 reduction is regenerated as the sorbent. The major product depends on the electrocatalyst, with silver making CO and copper with a cationic surfactant giving CH4 (Lees et al. 2022; Zhang et al. 2022c). Another approach uses a CO2-binding organic liquid (ethylene glycol and choline hydroxide) to capture CO2 from simulated flue gas, which is released after transport through an anion exchange membrane. Electrolysis with a copper mesh cathode produces multiple carbon-based products, including CO, HCOOH, CH4, C2H4, C2H5OH, and C3H7OH. Under optimized conditions, a high of 64 percent Faradaic efficiency for carbon-based products is achieved (Prajapati et al. 2022).
In both configurations, the overall product selectivity depends on the local environment at the electrode (including water concentration) and optimizing the rate of substrate transport, both as captured CO2 and released CO2.
In the heterogeneous integrated capture and conversion systems that use hydroxide as the capture agent, CO2 is believed to be released at the electrode for reduction. There are, however, examples of homogeneous electrocatalysts that are believed to directly reduce bicarbonate. For example, [RuIII(edta)(H2O)]− directly reduces HCO3− to formate with Faradaic efficiencies as high as 90 percent electrochemically (Chatterjee et al. 2014) or photochemically (Mondal and Chatterjee 2016). A dinuclear copper complex that cooperatively binds carbonate has also shown photocatalytic activity toward the production of CO (Liu et al. 2012a).
Other CO2 capture solvents have been used for direct electrochemical reduction. Ionic liquids (ILs) with high solubility and selectivity for CO2 have been explored. ILs are characterized by their low vapor pressure, which minimizes evaporative losses during CO2 capture. They generally have large electrochemical windows (i.e., resistance to oxidation and reduction) and sufficient conductivity to not require the addition of external electrolytes. Early studies of ILs for electrosynthesis used CO2 with co-substrates to form more complex products (Alvarez-Guerra et al. 2015), where the choice of co-reductant guides the product. For example, CO2 is co-reduced with alcohols or alkyl iodides to form organic carbonates, most commonly dimethyl carbonate. Reduction of olefins with CO2 is a route to carboxylic acids, while the use of epoxides with CO2 forms cyclic organic carbonates. In studies with organic co-reductants, the most common heterogeneous electrode catalysts used are copper, platinum, and nickel. In contrast to the complex products produced with co-reductants, direct electrochemical reduction of CO2 in ILs by heterogeneous catalysts typically forms CO, a combination of CO and H2 (syngas), or more rarely, formate. The most common ILs for electrochemical reduction with no co-reductant are composed of imidazolium salts with fluorinated anions. Catalyst materials have varied but are most commonly metals supported by a carbon electrode (Alvarez-Guerra et al. 2015).
Homogeneous catalysts have been explored for CO2 reduction in ILs. Early studies focused on the highly selective catalyst Re(bpy)(CO)3Cl and an imidazolium cation-based IL with tetracyanoborate as the counteranion, demonstrating continued high selectivity for the product CO with a significantly reduced overpotential compared to operation in organic solvents (Grills et al. 2014). Further studies have demonstrated photocatalytic activity in supramolecular systems (Grills and Fujita 2010). The higher efficiency of these catalysts in ILs is attributed to the interaction between the imidazolium cation with the rhenium complexes through hydrogen-bonding interactions, leading to a milder reduction potential (Matsubara et al. 2015).
Thermochemical systems for integrated capture and conversion of CO2 use co-reductants to valorize the captured carbon. In heterogeneous systems, these are often called integrated carbon capture and utilization (ICCU). Most examples of heterogeneous integrated capture and conversion use “dual functional materials,” which capture CO2 in metal oxide materials (Omodolor et al. 2020; Shao et al. 2022). The input gas is then switched to a reductant. When hydrogen is used, methane is the predominant product, although some catalysts can promote the RWGS reaction (ICCU-RWGS). Alternatively, in ICCU-DRM (where DRM is dry reforming of methane), light alkanes such as methane can be used as the co-reductant to produce syngas (Kim et al. 2018; le Saché and Reina 2022) or reduce ethane to ethylene (Gambo et al. 2021). Both the capture and conversion steps are typically conducted at high temperatures, with the conversion step often requiring temperatures >500°C and/or high pressures (Sun et al. 2021).
There is precedent for using molecular catalysts for the hydrogenation of solubilized CO2. Multiple pincer-type catalysts have been tested for the hydrogenation of carbonate solution (hydroxide-captured CO2) to generate formate (Kar et al. 2018a). These hydrogenation reactions can be carried out at milder temperatures (80°C) and 50 bar H2, regenerate the hydroxide sorbent, and have turnover frequencies of 103 hr−1. Amine solutions, which capture CO2 to form ammonium carbamates, can also be hydrogenated to both ammonium formate (Kothandaraman et al. 2016a) and methanol (Kar et al. 2018b, 2019b; Kothandaraman et al. 2016b). To improve recyclability in these systems, work has been performed on immobilizing the amine sorbents and sorbent generation (Kar et al. 2019a).
A nonredox method of CO2 functionalization capitalizes on its reaction with epoxides to form cyclic carbonates. Various methods have been employed to perform this reaction, including the use of specially designed covalent organic frameworks (Talapaneni et al. 2015; Wang et al. 2015b), metal organic frameworks (Ding and Jiang 2018; Liu et al. 2016a; Zhang et al. 2016), and ILs that combine capture functionalities with catalytic active sites (Liu et al. 2016b).
Captured CO2 solutions have also been useful for other applications that do not involve conversion to chemicals or fuels. Several studies indicate that solvent-based capture can enhance CO2 mineralization to carbonates and related solids while regenerating the sorbent (Heldebrant et al. 2022). Another approach uses direct seawater electrolysis to promote the formation of mineral carbonates from oceanic dissolved inorganic carbon (La Plante et al. 2021, 2023), which is discussed further in Section 5.2.4 of Chapter 5.
An alternative form of integrated capture and conversion is sacrificial capture and utilization (Marocco Stuardi et al. 2019). In this case, the CO2–sorbent bond is retained in the final product, so the sorbent is not regenerated. This method has been used with amine-based capture agents to form alkylcarbamates, urethanes, and alkylureas, as well as reduced products such as formamides, formamidines, and methylamines. Sacrificial capture and utilization open the door for a greater variety of fine chemicals synthesized with CO2 as a C1 precursor.
7.2.5.2 Challenges
The challenges for electrochemical integrated capture and conversion mirror some of those in direct electrochemical reduction of CO2. Obtaining products with high selectivity while suppressing the hydrogen evolution reaction is an important goal. Several heterogeneous systems, particularly bicarbonate electrolyzers, use protons to release CO2 to the cathode. Thus, controlling the release of substrate and water to match arrival at the catalyst interface are important design aspects. Direct bicarbonate or carbonate reduction at heterogeneous electrodes may be challenging as the carbon-containing substrate is anionic, which lowers accessibility to a negatively charged electrode. Homogeneous systems have been proposed for direct electrochemical reduction of carbonate or bicarbonate, and may be better suited for such reactions, given that carbonate and bicarbonate are common ligands in transition-metal complexes (Krishnamurty et al. 1970). Unique to integrated systems is the challenge of matching the timescales of capture and conversion, which depends on the system architecture. There may also be a mismatch in the thermodynamics of the capture and conversion steps such that the integrated system has a larger energy requirement than the two processes separately (Appel and Yang 2024). Additionally, low conversion efficiencies can require additional downstream purification. Lastly, compared to pure CO2 reduction, fewer products are currently available in combined capture and conversion systems.
Thermochemical integrated capture and conversion has been explored using heterogeneous and homogeneous catalysts with promising results. Heterogeneous systems provide a diversity of potential reactions, including methanation, dry reforming of light alkanes, and RWGS, albeit at high temperatures. Homogeneous systems can produce formate and methanol at relatively high rates. The overall carbon footprint of these systems will depend on the operating temperature, as well as the source of the co-reductant. Most hydrogen is currently generated from fossil sources, so a major challenge in minimizing carbon emissions from CO2 utilization will be economical and abundant sources of clean hydrogen.
7.2.5.3 R&D Opportunities
All electrochemical integrated capture and conversion systems are still in the R&D stage except for the high-temperature molten carbonate systems that produce elemental carbon products. Research directions include aiming for a better understanding of CO2 speciation, concentration, and transport, including capture and release mechanisms. In homogeneous systems, there is an opportunity to use ligand design to capture CO2 in the secondary coordination sphere, or otherwise activate it for reduction (Sung et al. 2017). Additionally, some studies indicate that metal-ligand bonds can be used to capture CO2 for reduction, but systematic studies on this reactivity have not been performed (Sattler and Parkin 2014).
Ionic liquids are promising for electrochemical integrated capture and conversion because of their high solubility for CO2, large electrochemical windows, and general inertness to common contaminants in industrial flue gases. The exact speciation of CO2 in solution is still not entirely understood, as CO2 can exist in a soluble form or chemically interact with the imidazolium cations of the ILs. The mechanism of CO2 reduction—both directly and with co-reductants, is still not clear, and thus there are few catalyst design rules to guide development. However, there is significant room for improvement. In addition to developing new catalysts, ILs themselves are highly
tunable, as their cations and anions can be tailored for specific properties. For electrochemical reduction of CO2sorbing ILs, the viscosity of the solvent needs to be considered in electrolyzer design as it affects mass transport of substrate. Many of the electrolyzers have improved kinetics at lower temperatures, where CO2 is more soluble but ILs become even more viscous. ILs also tend to be hydroscopic, which can result in mixed solvents that have to be controlled under practical conditions. ILs are more expensive than most solvents and electrolyte combinations used in electrolysis, which could add scalability challenges. Very few studies have considered this cost, but in one case it was estimated to increase the total capital cost for solvent from less than 1 percent to 14 percent (Chang et al. 2021). More detailed studies are needed to determine the primary cost contributors more accurately.
Most integrated capture and conversion systems regenerate the sorbent, but, as introduced above, there are opportunities to use CO2 as a C1 precursor in sacrificial captures, where the sorbent–C bond is retained. A sacrificial capture system also can be used to synthesize valuable heteroatom-carbon bonds, as well as to introduce stereocenters. Further reduction expands the accessible functionalities; for example, syntheses of arylcarbamates, oxazolidine, urethanes, and alkylureas have been described (Bernoud et al. 2017; Feroci et al. 2005; Liu et al. 2012b; McGhee et al. 1995). Commercial amine reagents that capture CO2 to form carbamates can be reduced to form formamides, formamidines, methylamines, and aminals (Tlili et al. 2015). As the sorbent is a stoichiometric reagent, the reaction must provide significant added value to be cost-effective; thus, synthesis of fine chemicals typically has been targeted.
Because current methods of CO2 capture and concentration require significant inputs and infrastructure, the case of integrated capture and conversion is compelling. However, integrated systems will have to be evaluated holistically to determine whether they are advantageous over systems that perform sequential capture and concentration followed by utilization. While sorbents can kinetically activate nonpolar CO2 molecules, they also result in greater thermodynamic stability, requiring more energy for subsequent conversion. Integrated capture and conversion methods that use sorbents will have to consider their initial cost and recyclability (regeneration) in the overall techno-economic assessment of these processes. Incomplete capture and/or conversion of CO2 may also lead to downstream separations costs. High-performance, low-energy separation technologies, such as membranes, may play a key role in augmenting the electrochemical processes. These factors are important in evaluating the overall value propositions of integrated capture and conversion schemes.
7.2.6 Polymers
7.2.6.1 Current Technology
Direct polymerization of CO2 to make poly(CO2) is possible, but owing to the low reactivity of CO2, the synthesis conditions are exceptionally challenging, such as 1800 K (1527°C) and 40,000 MPa (Huang et al. 2020; Iota et al. 1999). There are no currently known efforts to further develop poly(CO2).
There is, however, commercial activity to synthesize polycarbonates from CO2, taking advantage of the specific chemistry of the CO2 molecule. As noted in Chapter 2, polycarbonate production occurs at a scale of about 1.5 Mt/year globally (Neelis et al. 2007), making it a promising opportunity for CO2 utilization. Inoue and coworkers first reported the polymerization of CO2 with oxiranes, such as propylene oxide, to form polycarbonates in 1969 (Inoue et al. 1969a, 1969b; see Figure 7-13). Numerous studies since have developed this and similar polymerization reactions further (Appaturi et al. 2021; Fukuoka 2012; Rehman et al. 2021; Tabanelli et al. 2019; Tan et al. 2021; Wołosz et al. 2022), motivated in part by the safety and toxicity benefits of using CO2 as a feedstock compared to traditional methods using phosgene.

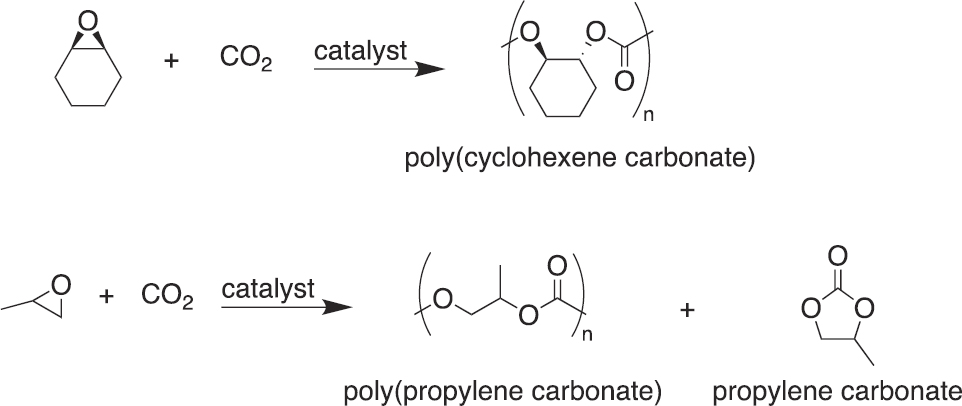
SOURCE: Used with permission of Angewandte Chemie, adapted from G.W. Coates and D.R. Moore, 2004, “Discrete Metal-Based Catalysts for the Copolymerization of CO2 and Epoxides: Discovery, Reactivity, Optimization, and Mechanism,” Angewandte Chemie International Edition 43(48):6618–6639; permission conveyed through Copyright Clearance Center, Inc.
Benefits to this approach include the industrial-scale availability of oxiranes such as ethylene oxide and propylene oxide, the lack of by-products produced in the reaction, the lack of need for stoichiometric co-reagents, and the fact that products can contain up to 50 percent CO2/O2 (Dabral and Schaub 2019). There has been extensive research on forming aliphatic polycarbonates using ring-strained monomers, such as cyclohexene oxide, ethylene oxide, propylene oxide, and others (Coates and Moore 2004; Darensbourg and Holtcamp 1996; Grignard et al. 2019; Huang et al. 2020; Kember et al. 2011; Liu and Lu 2023; Yeung et al. 2023) (Figure 7-14). However, the glass transition (i.e., softening) temperature of polypropylene carbonate is 35°C–40°C,6 and it decomposes at 250°C (Langanke et al. 2015), which limits its utility in conventional engineering thermoplastics applications (Coates and Moore 2004; von der Assen and Bardow 2014). Rather, aliphatic polycarbonates typically are used as binders in adhesives and ceramics (Langanke et al. 2015). This use case only provides extremely short-duration storage of CO2, as the binders are sacrificial and designed to decompose in the ceramic formation, rereleasing CO2. Polyetherol carbonates also can be produced via reaction of ring-strained monomers with CO2 (Dabral and Schaub 2019). Together, these aliphatic polycarbonates, prepared with hydroxyl end groups (i.e., polyols), are produced commercially (Grignard et al. 2019; Liu and Lu 2023). An important use of these polyols is to react them with isocyanates to produce polyurethanes (Liu and Lu 2023).
A substantial amount of research today is focused on developing catalysts to perform these reactions (Huang et al. 2020; Lidston et al. 2022). For example, the reaction of CO2 with ethylene oxide and propylene oxide occurs at high pressure7 using a variety of catalysts, such as organometallic compounds (e.g., ZnEt2), ammonium and phosphonium salts, alkali metal iodides, various aluminum and manganese catalysts, chromium catalysts, cobalt catalysts, lanthanide series catalysts, Lewis acids, and ion exchangers containing ammonium or phosphonium groups (Buysch 2011; Coates and Moore 2004; Darensbourg 2007; Darensbourg and Holtcamp 1996; Huang et al. 2020; Liu and Lu 2023). Bifunctional catalysts (e.g., alkali metal and zinc halide) are used under milder conditions. Most of these catalysts are metal based, including various catalysts involving aluminum and manganese, chromium or cobalt, as well as lanthanide series catalysts (Coates and Moore 2004; Darensbourg 2007; Darensbourg and Holtcamp 2007; Huang et al. 2020; Liu and Lu 2023; Yeung et al. 2023).
___________________
6 For comparison, the glass transition temperature of BisA-PC, a widely used engineering thermoplastic, is ~147°C.
7 For example, in their review of catalysts for copolymerization of CO2 and epoxides, Coates and Moore (2004) list pressure requirements of 7–135 atm for various catalytic systems.
Other families of polymers that have used CO2 directly in polymerization include polyureas synthesized from CO2 and diamines, polyesters from CO2 copolymerization with ethylene and other olefins, and poly(urethanes) from reacting CO2 with aziridines or with amino alcohols (Grignard et al. 2019). However, these polymers have not been commercialized and appear to be at the laboratory or bench scale at this time.
7.2.6.2 Challenges
Current challenges for deriving polymers from CO2 include the lack of routes to incorporate CO2 directly into polymerizations that involve aromatic compounds that yield the high glass-transition, tough, ductile engineering thermoplastics that are dominant in the polycarbonate field currently (e.g., BisA-PC). Much of the work to date has focused on polymerizing oxiranes and related compounds with CO2, and whether the portfolio of accessible monomers can be expanded to produce a wider variety of polymers with property profiles better matched to commercial needs is not well understood. Whether catalysts can be developed that permit rapid, economical polymerization of precision (i.e., stereochemistry-controlled) materials is unclear. More efficient catalysts are needed to expand the commercial opportunities for polymerizations that directly use CO2 (Huang et al. 2020). Many current catalysts do not exhibit the high productivity needed to drive production of CO2-based polymers to the same scale as, for example, polyolefins (Liu and Lu 2023). How the cost and property profile of polymers made with CO2 will compare to those of polymers made by conventional routes is not well-defined. While CO2-derived polycarbonates and polyols for polyurethane have been commercially available for more than a decade, they have seen limited market penetration; for example, CO2-derived aromatic polycarbonates are produced at about 0.90 Mt/year, representing only 16 percent of the total global annual production of this polymer (Nova-Institut 2023). The carbon cost of obtaining CO2 at sufficient purity and quantities to conduct such polymerizations is not well understood. Last, because one cannot rely on poly(CO2) to address the need for CO2-containing polymers, the question of where the co-monomers and reactants will come from (e.g., from fossil sources or carbon-neutral renewable resources) is not yet resolved at a commercial scale.
7.2.6.3 R&D Opportunities
The R&D opportunities for polymerizations involving CO2 stem from the challenges outlined above. Fundamental studies related to advanced catalyst discovery are needed to obtain further control over the stereochemistry of resulting polymers and increase the productivity of the catalysts to enable large-scale polymerization. Exploration of monomers beyond those that have been considered to date, coupled with catalyst development to permit their polymerization with CO2, could open new classes of materials to production directly from CO2 (Song et al. 2022). Combining electrochemical and organometallic catalysts may provide routes to additional materials than can be achieved by organometallic catalysts alone (Dodge et al. 2023). Advancing research to derive carbon-neutral, sustainable co-monomers for use in CO2 polymerizations is critical. Defining carbon-neutral routes to incorporation of aromatics in CO2-derived polycarbonates will broaden substantially their market opportunities. Most of the research on catalysts has focused on homogeneous catalytic approaches, so further exploration of heterogeneous catalysts may be fruitful (Huang et al. 2020).
7.3 CONCLUSIONS
Based on the discussions presented in Section 7.2, the committee highlights the following R&D needs and opportunities for chemical CO2 conversion:
- There are potential advantages using tandem catalysis combining two or more of the chemical conversion routes: thermochemical, electrochemical, photochemical, and plasmachemical processes. In some cases, the tandem strategy can lead to products that a single process cannot achieve, such as the conversion of CO2 to C3 oxygenates using tandem electrochemical-thermochemical reactions (Biswas et al. 2023; Garg et al. 2024).
- Thermochemical CO2 conversion typically requires high temperature, leading to challenges in controlling catalytic selectivity and catalyst stability. Alternative heating methods, such as (pulsed) electrical heating, could have potential advantages in better catalytic performance, energy savings from not heating the entire reactor, and reduced GHG emissions by using clean electricity (Zheng et al. 2023).
- Low-temperature electrochemical CO2 conversion faces challenges in terms of long-term catalyst stability and sensitivity to impurities. Copper remains the primary element that catalyzes the production of hydrocarbons and oxygenates containing two or more carbon atoms, often with low product selectivity and yield; more efforts need to be devoted to developing electrocatalysts that are selective, stable, and scalable to produce both C1 and multicarbon products, including some of the target molecules shown in Figure 7-2.
- Photochemical conversion of CO2 requires further fundamental understanding in developing materials and devices that can improve light absorption, generation and separation of electron-hole pairs, and subsequent reduction of CO2. Additional research into reactor design is also needed to optimize performance metrics and help inform scale up.
- Plasmachemical CO2 conversion leads to unselective production of multiple products. Although the introduction of catalysts will likely improve selectivity, more in-depth understanding of plasma-catalyst interactions is needed to enable scale up for practical applications.
- Integrated capture and conversion of CO2 offers advantages in improving overall energy efficiency and lowering capital requirements for separate steps of CO2 capture and subsequent catalytic conversion.
- The direct utilization of CO2 in polymerization reactions is currently limited to a narrow range of monomers. More research into catalyst design and development to enable rapid, stereoselective polymerization of a broader class of monomers with CO2, to access polymers with properties more like those of conventional thermoplastics, could markedly expand opportunities for polymers made directly from CO2.
- Capital costs for thermochemical reactions conducted in bulk equipment typically scale at 0.6 power with throughput, giving improved economics at larger scale. Electro-, photo(electro)-, and plasmachemical reactors, as well as endothermic thermochemical reactors using electrified modes of heating (e.g., electromagnetic radiation, some induction heating designs) often depend on surface area rather than volume, and capital costs increase to the 1.0 power of throughput or scale. For these cases, require of smaller units and numbering up can be required to achieve large-scale production. These processes are more amenable to distributed modular production but may be challenged to deliver low costs owing to the inability to scale individual units to obtain economies of scale.
As described above, the same product(s) in some cases can be produced by multiple conversion routes, each of which come with their own advantages and disadvantages. For example, for CO2 conversion to CO, the thermochemical conversion can be performed using existing technology, but it requires molecular H2; the electrochemical conversion avoids molecular H2 by using protons and electrons, but at present, the reaction rate and long-term stability cannot yet compete with the more mature thermochemical processes. When moving beyond R&D to demonstration and deployment, factors such as energy and infrastructure requirements, life cycle emissions, policy support, safety, and cost will need to be compared among different conversion routes to determine the best option for a given product or application. These considerations are discussed in more detail in Chapters 2, 3, 4, and 10.
7.3.1 Findings and Recommendations
The preceding overarching R&D needs for chemical CO2 conversion led the committee to the following findings and recommendations:
Finding 7-1: Challenges and opportunities for thermochemical CO2 conversion—Thermochemical CO2 conversion typically requires high temperatures, leading to challenges in controlling catalytic selectivity and catalyst stability (including tolerance to impurities). For thermochemical CO2 conversions to have net-zero or net-negative emissions, carbon-neutral energy, hydrogen, or other reductants are required. Alternative heating methods, such as (pulsed) electrical heating, could have potential advantages for better catalytic performance, energy savings from not needing to continually heat the entire reactor, and reduced greenhouse gas emissions by using clean electricity.
Integration of solar thermochemical hydrogen production and CO2 conversion with thermal energy storage could improve the economic viability of high capital intensity processes (e.g., reverse water gas shift reaction, methanol synthesis, and Fischer-Tropsch synthesis).
Recommendation 7-1: Support research on catalyst development, electrical heating, and carbon-neutral reductants for thermochemical CO2 conversion—Basic Energy Sciences within the Department of Energy’s Office of Science (DOE-BES), the National Science Foundation, and the Department of Defense (DoD) should increase support for experimental and theoretical discovery research into catalysts and processes that utilize carbon-neutral and efficient methods of electrical heating to convert CO2 to useful chemicals and chemical intermediates (e.g., targeted heating, microwave heating). DOE-BES, DoD, and DOE’s Office of Energy Efficiency and Renewable Energy, Office of Fossil Energy and Carbon Management, Office of Clean Energy Demonstrations, and Advanced Research Projects Agency–Energy should continue to support research and development (R&D) that facilitates scale up of thermochemical CO2 conversion to achieve net-zero CO2 utilization. This includes R&D on the production of low-carbon hydrogen and other carbon-neutral reductants and the integration of solar thermochemical hydrogen production and CO2 conversion with thermal energy storage.
Finding 7-2: Engineering and systems optimization needs for thermochemical CO2 conversion—Thermochemical and thermocatalytic conversion of CO2 to hydrocarbon products typically requires multiple reaction steps and is energy- and capital-intensive relative to current routes from fossil-based feedstocks. Incorporation and integration of low-carbon energy sources, such as variable renewable energy with low-cost storage, and the ability to deliver this low-carbon energy to high-temperature reaction systems, including options for dynamic operation, will require new engineering and systems optimization to provide plausible pathways for net-zero emissions chemical production.
Recommendation 7-2: Support research on integrated systems for thermochemical CO2 conversion—The Department of Energy’s Office of Fossil Energy and Carbon Management should fund applied research on integration of variable renewable energy and energy storage into efficient, heat-integrated process systems for CO2 conversion to hydrocarbon products.
Finding 7-3: Challenges for electrochemical CO2 conversion—Low-temperature electrochemical CO2 conversion faces challenges in long-term catalyst stability and robustness to impurities in the CO2 source. Copper remains the primary element that catalyzes the production of hydrocarbons and oxygenates containing two or more carbon atoms, often with low product selectivity. The cost and efficiency of electrochemical CO2 conversion is also impacted by the materials and performance metrics of the anodic reaction and the membrane.
Recommendation 7-3: Support research on developing electrocatalysts from abundant elements and membrane materials for electrochemical CO2 conversion technologies—Basic Energy Sciences within the Department of Energy’s Office of Science (DOE-BES) and DOE’s Office of Fossil Energy and Carbon Management (DOE-FECM) should devote more effort to experimental and theoretical research for discovering and developing electrocatalysts from abundant elements that are selective, stable, and scalable to produce both single- and multicarbon products for both low- and high-temperature electrochemical processes. DOE-BES and DOE-FECM should also invest in developing abundant-element electrocatalysts for water oxidation or alternative anodic reactions as well as cost-effective, scalable membrane materials that function over a wide pH range to lower the overall cost of electrochemical CO2 conversion. Long-term stability testing should be encouraged with new electrocatalyst development, along with testing for product selectivity and current density.
Finding 7-4: Fundamental research needs for photo(electro)chemical and plasmachemical CO2 conversion—Fundamental understanding of the sequence of processes involved in photochemical and photoelectrochemical conversion of CO2 is incomplete. Such understanding is required to improve light absorption, generation and
separation of electron-hole pairs, and subsequent reduction of CO2. Plasmachemical CO2 conversion also lacks in-depth understanding of plasma-catalyst interactions to improve product selectivity for practical applications. More research is needed to improve reactor design and reaction engineering for photochemical, photoelectrochemical, and plasmachemical CO2 conversions.
Recommendation 7-4: Support research on mechanisms, materials, and reactor design for photo(electro) chemical and plasmachemical CO2 conversion—Basic Energy Sciences within the Department of Energy’s (DOE’s) Office of Science should support more experimental and theoretical research into understanding fundamental mechanisms and materials discovery for photochemical, photoelectrochemical, and plasmachemical catalytic conversion of CO2. DOE’s Office of Fossil Energy and Carbon Management and Advanced Research Projects Agency–Energy should support research to enable development of improved materials, devices, and reactor design for such conversions.
Finding 7-5: Potential advantages of tandem catalysis for CO2 conversion—There are potential advantages in using tandem catalysis that combines two or more of the chemical conversion routes: thermochemical, electrochemical, photochemical, and plasmachemical processes. In some cases, the tandem strategy can lead to products that a single process cannot achieve.
Recommendation 7-5: Increase support for research on tandem catalysis for CO2 conversion—The Department of Energy’s Office of Fossil Energy and Carbon Management and Advanced Research Projects Agency–Energy should increase support for basic and applied research into tandem catalysis, including catalyst and membrane development, tandem reactor design, and process optimization.
Finding 7-6: Potential advantages of integrated capture and conversion of CO2—If the energy requirements and operational scales of the capture and conversion steps are matched, integrated capture and conversion of CO2 can offer advantages in improving overall energy efficiency and lowering capital requirements compared to separate steps of CO2 capture and subsequent catalytic conversion.
Recommendation 7-6: Increase support for research on integrated capture and conversion of CO2—Basic Energy Sciences within the Department of Energy’s (DOE’s) Office of Science, the National Science Foundation, and DOE’s Office of Fossil Energy and Carbon Management, Office of Energy Efficiency and Renewable Energy, and Advanced Research Projects Agency–Energy should increase support for basic and applied research into integrated CO2 capture and conversion, including discovery of molecules and materials, catalytic mechanisms, process optimization, CO2 stream purification, and reactor design.
Finding 7-7: Direct utilization of CO2 in polymerization is limited—The direct utilization of CO2 in polymerization reactions is currently limited to a narrow range of monomers.
Recommendation 7-7: Support research on catalyst development for CO2 polymerization with a broader class of monomers—Basic Energy Sciences within the Department of Energy’s (DOE’s) Office of Science and DOE’s Office of Fossil Energy and Carbon Management should support more experimental and theoretical research into catalyst design and development to enable rapid, stereoselective polymerization of a broader class of monomers with CO2. Such research could lead to polymers with properties more like those of conventional thermoplastics and/or thermosets, which could markedly expand opportunities for polymers made directly from CO2.
7.3.2 Research Agenda for Chemical CO2 Conversion to Organic Products
Table 7-3 presents the committee’s research agenda for chemical CO2 conversion to organic products, including research needs (numbered by chapter), and related research agenda recommendations (a subset of research-related
TABLE 7-3 Research Agenda for Chemical CO2 Conversion to Organic Products
| Research, Development, and Demonstration Need | Funding Agencies or Other Actors | Basic, Applied, Demonstration, Enabling | Research Area | Product Class | Long- or Short-Lived | Research Theme | Source |
|---|---|---|---|---|---|---|---|
| 7-A. Improvements in catalytic activity, selectivity, and stability (including tolerance to impurities) for thermochemical CO2 conversion. | DOE-BES NSF DoD |
Basic | Chemical—Thermochemical | Chemicals | Short-lived | Catalyst innovation and optimization | Fin. 7-1 Sec. 7.2.1.3 |
| Computational modeling and machine learning | |||||||
| 7-B. Discovery research into catalysts and processes that use alternative heating methods, such as (pulsed) electrical heating, with goals of improving catalyst performance, yielding energy savings, and reducing GHG emissions by using clean electricity. | DOE-BES NSF DoD |
Basic | Chemical—Thermochemical | Chemicals | Short-lived | Catalyst innovation and optimization | Fin. 7-1 Rec. 7-1 |
| Energy efficiency, electrification, and alternative heating | |||||||
| Computational modeling and machine learning | |||||||
| 7-C. Continued research and development into low-carbon hydrogen and other carbon-neutral reductants to facilitate scale up of thermochemical CO2 conversion that can achieve net-zero CO2 utilization. | DOE-BES DOE-EERE DOE-FECM DOE-ARPA-E DoD |
Enabling | Chemical—Thermochemical | Chemicals | Short-lived | Enabling technology and infrastructure needs | Rec. 7-1 |
| Recommendation 7-1: Support research on catalyst development, electrical heating, and carbon-neutral reductants for thermochemical CO2 conversion—Basic Energy Sciences within the Department of Energy’s Office of Science (DOE-BES), the National Science Foundation, and the Department of Defense (DoD) should increase support for experimental and theoretical discovery research into catalysts and processes that utilize carbon-neutral and efficient methods of electrical heating to convert CO2 to useful chemicals and chemical intermediates (e.g., targeted heating, microwave heating). DOE-BES, DoD, and DOE’s Office of Energy Efficiency and Renewable Energy, Office of Fossil Energy and Carbon Management, Office of Clean Energy Demonstrations, and Advanced Research Projects Agency–Energy should continue to support research and development (R&D) that facilitates scale up of thermochemical CO2 conversion to achieve net-zero CO2 utilization. This includes R&D on the production of low-carbon hydrogen and other carbon-neutral reductants and the integration of solar thermochemical hydrogen production and CO2 conversion with thermal energy storage. | |||||||
| 7-D. Engineering and systems optimization to integrate low-carbon energy sources with high-temperature reaction systems for CO2 conversion to hydrocarbon products. | DOE-FECM | Applied | Chemical—Thermochemical | Chemicals | Short-lived | Reactor design and reaction engineering | Fin. 7-2 Rec. 7-2 |
| Energy efficiency, electrification, and alternative heating | |||||||
| Recommendation 7-2: Support research on integrated systems for thermochemical CO2 conversion—The Department of Energy’s Office of Fossil Energy and Carbon Management should fund applied research on integration of variable renewable energy and energy storage into efficient, heat-integrated process systems for CO2 conversion to hydrocarbon products. | |||||||
| Research, Development, and Demonstration Need | Funding Agencies or Other Actors | Basic, Applied, Demonstration, Enabling | Research Area | Product Class | Long- or Short-Lived | Research Theme | Source |
|---|---|---|---|---|---|---|---|
| 7-E. Discovery and development of electrocatalysts from abundant elements that are selective, stable, robust to impurities in CO2 sources, and scalable, and that can produce single- and multicarbon products for both low- and high-temperature electrochemical processes. | DOE-BES DOE-FECM |
Basic Applied |
Chemical—Electrochemical | Chemicals | Short-lived | Catalyst innovation and optimization | Fin. 7-3 Rec. 7-3 |
| Computational modeling and machine learning | |||||||
| 7-F. Discovery and development of abundant-element electrocatalysts for water oxidation or alternative anodic reactions to improve the cost and efficiency of electrochemical CO2 conversion. | DOE-BES DOE-FECM |
Basic Applied |
Chemical—Electrochemical | Chemicals | Short-lived | Catalyst innovation and optimization | Fin. 7-3 Rec. 7-3 |
| Computational modeling and machine learning | |||||||
| 7-G. Development of economical membrane materials that function over a wide pH range to improve the cost, efficiency, and scalability of electrochemical CO2 conversion. | DOE-BES DOE-FECM |
Basic Applied |
Chemical—Electrochemical | Chemicals | Short-lived | Reactor design and reaction engineering | Fin. 7-3 Rec. 7-3 |
| Separations | |||||||
| Recommendation 7-3: Support research on developing electrocatalysts from abundant elements and membrane materials for electrochemical CO2 conversion technologies—Basic Energy Sciences within the Department of Energy’s Office of Science (DOE-BES) and DOE’s Office of Fossil Energy and Carbon Management (DOE-FECM) should devote more effort to experimental and theoretical research for discovering and developing electrocatalysts from abundant elements that are selective, stable, and scalable to produce both single- and multicarbon products for both low- and high-temperature electrochemical processes. DOE-BES and DOE-FECM should also invest in developing abundant-element electrocatalysts for water oxidation or alternative anodic reactions as well as cost-effective, scalable membrane materials that function over a wide pH range to lower the overall cost of electrochemical CO2 conversion. Long-term stability testing should be encouraged with new electrocatalyst development, along with testing for product selectivity and current density. | |||||||
| 7-H. Fundamental understanding of the sequence of processes involved in photochemical and photoelectrochemical conversion of CO2 for light absorption, generation and separation of electron-hole pairs, and subsequent reduction of CO2, across a variety of material types. | DOE-BES | Basic | Chemical—Photochemical Chemical—Photoelectrochemical |
Chemicals | Short-lived | Fundamental knowledge Computational modeling and machine learning | Fin. 7-4 Rec. 7-4 |
| 7-I. Discovery research into materials for photochemical, photoelectrochemical, and plasmachemical catalytic conversion of CO2. | DOE-BES | Basic | Chemical—Photochemical | Chemicals | Short-lived | Catalyst innovation and optimization | Rec. 7-4 |
| Chemical—Photoelectrochemical | Computational modeling and machine learning | ||||||
| Chemical—Plasmachemical | |||||||
| 7-J. In-depth understanding of plasma-catalyst interactions for product selectivity. | DOE-BES | Basic | Chemical—Plasmachemical | Chemicals | Short-lived | Fundamental knowledge Computational modeling and machine learning | Fin. 7-4 Rec. 7-4 |
| 7-K. Improved devices, reactor design, and reaction engineering for photochemical, photoelectrochemical, and plasmachemical CO2 conversions to optimize performance metrics and inform scale up. | DOE-FECM DOE-ARPA-E |
Applied | Chemical—Photochemical | Chemicals | Short-lived | Reactor design and reaction engineering | Fin. 7-4 Rec. 7-4 |
| Chemical—Photoelectrochemical | |||||||
| Chemical—Plasmachemical | |||||||
| Recommendation 7-4: Support research on mechanisms, materials, and reactor design for photo(electro)chemical and plasmachemical CO2 conversion—Basic Energy Sciences within the Department of Energy’s (DOE’s) Office of Science should support more experimental and theoretical research into understanding fundamental mechanisms and materials discovery for photochemical, photoelectrochemical, and plasmachemical catalytic conversion of CO2. DOE’s Office of Fossil Energy and Carbon Management and Advanced Research Projects Agency–Energy should support research to enable development of improved materials, devices, and reactor design for such conversions. | |||||||
| 7-L. Development of tandem catalysis processes that couple two or more thermochemical, electrochemical, photochemical, and plasmachemical processes, with a goal of accessing products that a single process alone cannot achieve. | DOE-FECM DOE-ARPA-E |
Basic Applied |
Chemical | Chemicals | Short-lived | Integrated systems Computational modeling and machine learning | Fin. 7-5 Rec. 7-5 |
| Recommendation 7-5: Increase support for research on tandem catalysis for CO2 conversion—The Department of Energy’s Office of Fossil Energy and Carbon Management and Advanced Research Projects Agency–Energy should increase support for basic and applied research into tandem catalysis, including catalyst and membrane development, tandem reactor design, and process optimization. | |||||||
| 7-M. Development of integrated CO2 capture and conversion, including discovery of molecules and materials, catalytic mechanisms, process optimization, CO2 stream purification, and reactor design. | DOE-BES NSF DOE-FECM DOE-ARPA-E DOE-EERE |
Basic Applied |
Chemical | Chemicals | Short-lived | Integrated systems Reactor design and reaction engineering | Fin. 7-6 Rec. 7-6 |
| Recommendation 7-6: Increase support for research on integrated capture and conversion of CO2—Basic Energy Sciences within the Department of Energy’s (DOE’s) Office of Science, the National Science Foundation, and DOE’s Office of Fossil Energy and Carbon Management, Office of Energy Efficiency and Renewable Energy, and Advanced Research Projects Agency–Energy should increase support for basic and applied research into integrated CO2 capture and conversion, including discovery of molecules and materials, catalytic mechanisms, process optimization, CO2 stream purification, and reactor design. | |||||||
| 7-N. Design and development of catalysts for rapid, stereoselective polymerization of a broader class of monomers with CO2, especially those that can lead to polymers with properties more like thermoplastics and/or thermosets. | DOE-BES DOE-FECM |
Basic Applied |
Chemical—Thermochemical | Polymers | Long-lived Short-lived |
Fundamental knowledge | Fin. 7-7 Rec. 7-7 |
| Catalyst innovation and optimization | |||||||
| Computational modeling and machine learning | |||||||
| Recommendation 7-7: Support research on catalyst development for CO2 polymerization with a broader class of monomers—Basic Energy Sciences within the Department of Energy’s (DOE’s) Office of Science and DOE’s Office of Fossil Energy and Carbon Management should support more experimental and theoretical research into catalyst design and development to enable rapid, stereoselective polymerization of a broader class of monomers with CO2. Such research could lead to polymers with properties more like those of conventional thermoplastics and/or thermosets, which could markedly expand opportunities for polymers made directly from CO2. | |||||||
NOTE: ARPA-E = Advanced Research Projects Agency–Energy; BES = Basic Energy Sciences; DoD = Department of Defense; DOE = Department of Energy; EERE = Office of Energy Efficiency and Renewable Energy; FECM = Office of Fossil Energy and Carbon Management; NSF = National Science Foundation.
recommendations from the chapter). The table includes the relevant funding agencies or other actors; whether the need is for basic research, applied research, technology demonstration, or enabling technologies and processes for CO2 utilization; the research theme(s) that the research need falls into; the relevant research area and product class covered by the research need; whether the relevant product(s) are long- or short-lived; and the source of the research need (chapter section, finding, or recommendation). The committee’s full research agenda can be found in Chapter 11.
7.4 REFERENCES
Al-Shankiti, I., B.D. Ehrhart, and A.W. Weimer. 2017. “Isothermal Redox for H2O and CO2 Splitting—A Review and Perspective.” Solar Energy 156(November):21–29. https://doi.org/10.1016/j.solener.2017.05.028.
Alvarez-Guerra, M., J. Albo, E. Alvarez-Guerra, and A. Irabien. 2015. “Ionic Liquids in the Electrochemical Valorisation of CO2.” Energy and Environmental Science 8(9):2574–2599. https://doi.org/10.1039/C5EE01486G.
Ampelli, C., S. Perathoner, and G. Centi. 2015. “CO2 Utilization: An Enabling Element to Move to a Resource- and Energy-Efficient Chemical and Fuel Production.” Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 373(2037):20140177. https://doi.org/10.1098/rsta.2014.0177.
Appaturi, J.N., R.J. Ramalingam, M.K. Gnanamani, G. Periyasami, P. Arunachalam, R. Adnan, F. Adam, M.D. Wasmiah, and H.A. Al-Lohedan. 2021. “Review on Carbon Dioxide Utilization for Cycloaddition of Epoxides by Ionic Liquid-Modified Hybrid Catalysts: Effect of Influential Parameters and Mechanisms Insight.” Catalysts 11(1). https://doi.org/10.3390/catal11010004.
Appel, A.M., and J.Y. Yang. 2024. “Maximum and Comparative Efficiency Calculations for Integrated Capture and Electrochemical Conversion of CO2.” ACS Energy Letters 9(2):768–770. https://doi.org/10.1021/acsenergylett.3c02489.
Ardo, S., D. Fernandez Rivas, M.A. Modestino, V. Schulze Greiving, F.F. Abdi, E. Alarcon Llado, V. Artero, et al. 2018. “Pathways to Electrochemical Solar-Hydrogen Technologies.” Energy and Environmental Science 11(10):2768–2783. https://doi.org/10.1039/C7EE03639F.
Aresta, M., A. Dibenedetto, and A. Angelini. 2014. “Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2.” Chemical Reviews 114(3):1709–1742. https://doi.org/10.1021/cr4002758.
Asahi Kasei. n.d. “Non-Phosgene Process for Producing Polycarbonate.” AsahiKASEI. https://www.asahi-kasei.com/r_and_d/innovation/#anc-10.
Ashford, B., and X. Tu. 2017. “Non-Thermal Plasma Technology for the Conversion of CO2.” CO2 Capture and Chemistry 2017 3(February 1):45–49. https://doi.org/10.1016/j.cogsc.2016.12.001.
Azara, A., E.-H. Benyoussef, F. Mohellebi, M. Chamoumi, F. Gitzhofer, and N. Abatzoglou. 2019. “Catalytic Dry Reforming and Cracking of Ethylene for Carbon Nanofilaments and Hydrogen Production Using a Catalyst Derived from a Mining Residue.” Catalysts 9(12):1069. https://doi.org/10.3390/catal9121069.
Azcarate, I., C. Costentin, M. Robert, and J.-M. Savéant. 2016. “Through-Space Charge Interaction Substituent Effects in Molecular Catalysis Leading to the Design of the Most Efficient Catalyst of CO2-to-CO Electrochemical Conversion.” Journal of the American Chemical Society 138(51):16639–16644. https://doi.org/10.1021/jacs.6b07014.
Banerjee, S., X. Han, and V. Sara Thoi. 2019. “Modulating the Electrode–Electrolyte Interface with Cationic Surfactants in Carbon Dioxide Reduction.” ACS Catalysis 9(6):5631–5637. https://doi.org/10.1021/acscatal.9b00449.
Bansode, A., and A. Urakawa. 2014. “Towards Full One-Pass Conversion of Carbon Dioxide to Methanol and Methanol-Derived Products.” Journal of Catalysis 309(January 1):66–70. https://doi.org/10.1016/j.jcat.2013.09.005.
Barlow, J.M., and J.Y. Yang. 2019. “Thermodynamic Considerations for Optimizing Selective CO2 Reduction by Molecular Catalysts.” ACS Central Science 5(4):580–588. https://doi.org/10.1021/acscentsci.9b00095.
Barton Cole, E., P.S. Lakkaraju, D.M. Rampulla, A.J. Morris, E. Abelev, and A.B. Bocarsly. 2010. “Using a One-Electron Shuttle for the Multielectron Reduction of CO2 to Methanol: Kinetic, Mechanistic, and Structural Insights.” Journal of the American Chemical Society 132(33):11539–11551. https://doi.org/10.1021/ja1023496.
Behr, A., and K. Nowakowski. 2014. “Chapter Seven—Catalytic Hydrogenation of Carbon Dioxide to Formic Acid.” Pp. 223–258 in Advances in Inorganic Chemistry 66, M. Aresta and R. van Eldik, eds. Academic Press. https://doi.org/10.1016/B978-0-12-420221-4.00007-X.
Bernatis, P.R., A. Miedaner, R.C. Haltiwanger, and D.L. DuBois. 1994. “Exclusion of Six-Coordinate Intermediates in the Electrochemical Reduction of CO2 Catalyzed by [Pd(Triphosphine)(CH3CN)](BF4)2 Complexes.” Organometallics 13(12):4835–4843. https://doi.org/10.1021/om00024a029.
Bernoud, E., A. Company, and X. Ribas. 2017. “Direct Use of CO2 for O-Arylcarbamate Synthesis via Mild Cu(II)-Catalyzed Aerobic C-H Functionalization in Pincer-Like Macrocyclic Systems.” Organometallic Chemistry of Pincer Complexes 845(September 15):44–48. https://doi.org/10.1016/j.jorganchem.2017.02.004.
Bernskoetter, W.H., and N. Hazari. 2017. “Reversible Hydrogenation of Carbon Dioxide to Formic Acid and Methanol: Lewis Acid Enhancement of Base Metal Catalysts.” Accounts of Chemical Research 50(4):1049–1058. https://doi.org/10.1021/acs.accounts.7b00039.
Biswas, A.N., L.R. Winter, B. Loenders, Z. Xie, A. Bogaerts and J.G. Chen. 2022a. “Oxygenate Production from Plasma-Activated Reaction of CO2 and Ethane.” ACS Energy Letters 7:236–241. https://pubs.acs.org/doi/10.1021/acsenergylett.1c02355.
Biswas, A.N., Z. Xie, R. Xia, S. Overa, F. Jiao, J.G. Chen. 2022b. “Tandem Electrocatalytic–Thermocatalytic Reaction Scheme for CO2 Conversion to C3 Oxygenates.” ACS Energy Letters 7(9):2904–2910. https://doi.org/10.1021/acsenergylett.2c01454.
Biswas, A.N., L.R. Winter, Z. Xie, and J.G. Chen. 2023. “Utilizing CO2 as a Reactant for C3 Oxygenate Production via Tandem Reactions.” JACS Au 3(2):293–305. https://doi.org/10.1021/jacsau.2c00533.
Bogaerts, A., C. De Bie, R. Snoeckx, and T. Kozák. 2017. “Plasma Based CO2 and CH4 Conversion: A Modeling Perspective.” Plasma Processes and Polymers 14(6):1600070. https://doi.org/10.1002/ppap.201600070.
Boutin, E., and M. Robert. 2021. “Molecular Electrochemical Reduction of CO2 Beyond Two Electrons.” Trends in Chemistry 3(5):359–372. https://doi.org/10.1016/j.trechm.2021.02.003.
Boutin, E., M. Wang, J.C. Lin, M. Mesnage, D. Mendoza, B. Lassalle-Kaiser, C. Hahn, T.F. Jaramillo, and M. Robert. 2019. “Aqueous Electrochemical Reduction of Carbon Dioxide and Carbon Monoxide into Methanol with Cobalt Phthalocyanine.” Angewandte Chemie International Edition 58(45):16172–16176. https://doi.org/10.1002/anie.201909257.
Bui, J.C., E.W. Lees, D.H. Marin, T.N. Stovall, L. Chen, A. Kusoglu, A.C. Nielander, et al. 2024. “Multi-Scale Physics of Bipolar Membranes in Electrochemical Processes.” Nature Chemical Engineering 1(1):45–60. https://doi.org/10.1038/s44286-023-00009-x.
Bukur, D.B., B. Todic, and N. Elbashir. 2016. “Role of Water-Gas-Shift Reaction in Fischer–Tropsch Synthesis on Iron Catalysts: A Review.” Catalysis Today 275(October):66–75. https://doi.org/10.1016/j.cattod.2015.11.005.
Bustamante, F., R.M. Enick, A.V. Cugini, R.P. Killmeyer, B.H. Howard, K.S. Rothenberger, M.V. Ciocco, B.D. Morreale, S. Chattopadhyay, and S. Shi. 2004. “High-Temperature Kinetics of the Homogeneous Reverse Water-Gas Shift Reaction.” AIChE Journal 50(5):1028–1041. https://doi.org/10.1002/aic.10099.
Buysch, H.-J. 2011. “Carbonic Esters.” In Ullmann’s Encyclopedia of Industrial Chemistry, 7th edition. https://doi.org/10.1002/14356007.a05_197.
Cai, X., and Y.H. Hu. 2019. “Advances in Catalytic Conversion of Methane and Carbon Dioxide to Highly Valuable Products.” Energy Science and Engineering 7(1):4–29. https://doi.org/10.1002/ese3.278.
Cao, G., R.M. Handler, W.L. Luyben, Y. Xiao, C.-H. Chen, and J. Baltrusaitis. 2022. “CO2 Conversion to Syngas via Electrification of Endothermal Reactors: Process Design and Environmental Impact Analysis.” Energy Conversion and Management 265(August 1):115763. https://doi.org/10.1016/j.enconman.2022.115763.
Carbon. 2023. “Climate Change Solution.” Carbon. https://carboncorp.org/climate-change-solution.html.
Carbon Recycling International. n.d. “George Olah Renewable Methanol Plant: First Production of Fuel from CO2 at Industrial Scale.” Carbon Recycling International. https://www.carbonrecycling.is/project-goplant.
Cauwenbergh, R., V. Goyal, R. Maiti, K. Natte, and S. Das. 2022. “Challenges and Recent Advancements in the Transformation of CO2 into Carboxylic Acids: Straightforward Assembly with Homogeneous 3d Metals.” Chemical Society Reviews 51(22):9371–9423. https://doi.org/10.1039/D1CS00921D.
Centi, G., and S. Perathoner. 2023. “Catalysis for an Electrified Chemical Production.” Catalysis Today 423(November 1): 113935. https://doi.org/10.1016/j.cattod.2022.10.017.
Challiwala, M.S., H.A. Choudhury, D. Wang, M.M. El-Halwagi, E. Weitz, and N.O. Elbashir. 2021. “A Novel CO2 Utilization Technology for the Synergistic Co-Production of Multi-Walled Carbon Nanotubes and Syngas.” Scientific Reports 11(1):1417. https://doi.org/10.1038/s41598-021-80986-2.
Chang, F., G. Zhan, Z. Wu, Y. Duan, S. Shi, S. Zeng, X. Zhang, and S. Zhang. 2021. “Technoeconomic Analysis and Process Design for CO2 Electroreduction to CO in Ionic Liquid Electrolyte.” ACS Sustainable Chemistry and Engineering 9(27):9045–9052. https://doi.org/10.1021/acssuschemeng.1c02065.
Chang, X., T. Wang, and J. Gong. 2016. “CO2 Photo-Reduction: Insights into CO2 Activation and Reaction on Surfaces of Photocatalysts.” Energy and Environmental Science 9(7):2177–2196. https://doi.org/10.1039/C6EE00383D.
Chatterjee, D., N. Jaiswal, and P. Banerjee. 2014. “Electrochemical Conversion of Bicarbonate to Formate Mediated by the Complex RuIII(edta) (edta4– = ethylenediaminetetraacetate).” European Journal of Inorganic Chemistry 2014(34):5856–5859. https://doi.org/10.1002/ejic.201402831.
Chen, G., G.I.N. Waterhouse, R. Shi, J. Zhao, Z. Li, L.-Z. Wu, C.-H. Tung, and T. Zhang. 2019. “From Solar Energy to Fuels: Recent Advances in Light-Driven C1 Chemistry.” Angewandte Chemie International Edition 58(49):17528–17551. https://doi.org/10.1002/anie.201814313.
Chen, X., Y. Chen, C. Song, P. Ji, N. Wang, W. Wang, and L. Cui. 2020. “Recent Advances in Supported Metal Catalysts and Oxide Catalysts for the Reverse Water-Gas Shift Reaction.” Frontiers in Chemistry 8(August):709. https://doi.org/10.3389/fchem.2020.00709.
Chen, X., X. Su, H.-Y. Su, X. Liu, S. Miao, Y. Zhao, K. Sun, Y. Huang, and T. Zhang. 2017. “Theoretical Insights and the Corresponding Construction of Supported Metal Catalysts for Highly Selective CO2 to CO Conversion.” ACS Catalysis 7(7):4613–4620. https://doi.org/10.1021/acscatal.7b00903.
Cho, W., H. Yu, and Y. Mo. 2017. “CO2 Conversion to Chemicals and Fuel for Carbon Utilization.” Chapter 9 in Recent Advances in Carbon Capture and Storage, Y. Yun, ed. Rijeka: IntechOpen. https://doi.org/10.5772/67316.
Coates, G.W., and D.R. Moore. 2004. “Discrete Metal-Based Catalysts for the Copolymerization of CO2 and Epoxides: Discovery, Reactivity, Optimization, and Mechanism.” Angewandte Chemie International Edition 43(48):6618–6639. https://doi.org/10.1002/anie.200460442.
Cohen, K.Y., R. Evans, S. Dulovic, and A.B. Bocarsly. 2022. “Using Light and Electrons to Bend Carbon Dioxide: Developing and Understanding Catalysts for CO2 Conversion to Fuels and Feedstocks.” Accounts of Chemical Research 55(7):944–954. https://doi.org/10.1021/acs.accounts.1c00643.
Costentin, C., S. Drouet, M. Robert, and J.-M. Savéant. 2012. “A Local Proton Source Enhances CO2 Electroreduction to CO by a Molecular Fe Catalyst.” Science 338(6103):90–94. https://doi.org/10.1126/science.1224581.
Costentin, C., J.-M. Savéant, and C. Tard. 2018. “Catalysis of CO2 Electrochemical Reduction by Protonated Pyridine and Similar Molecules. Useful Lessons from a Methodological Misadventure.” ACS Energy Letters 3(3):695–703. https://doi.org/10.1021/acsenergylett.8b00008.
Covert, L.W., and H. Adkins. 1932. “Nickel by the Raney Process as a Catalyst of Hydrogenation.” Journal of the American Chemical Society 54(10):4116–4117. https://doi.org/10.1021/ja01349a510.
Dabral, S., and T. Schaub. 2019. “The Use of Carbon Dioxide (CO2) as a Building Block in Organic Synthesis from an Industrial Perspective.” Advanced Synthesis and Catalysis 361(2):223–246. https://doi.org/10.1002/adsc.201801215.
Dalle, K.E., J. Warnan, J.J. Leung, B. Reuillard, I.S. Karmel, and E. Reisner. 2019. “Electro- and Solar-Driven Fuel Synthesis with First Row Transition Metal Complexes.” Chemical Reviews 119(4):2752–2875. https://doi.org/10.1021/acs.chemrev.8b00392.
Dang, S., H. Yang, P. Gao, H. Wang, X. Li, W. Wei, and Y. Sun. 2019. “A Review of Research Progress on Heterogeneous Catalysts for Methanol Synthesis from Carbon Dioxide Hydrogenation.” SI:18ncc_Energy 330(June 15):61–75. https://doi.org/10.1016/j.cattod.2018.04.021.
Darensbourg, D.J. 2007. “Making Plastics from Carbon Dioxide: Salen Metal Complexes as Catalysts for the Production of Polycarbonates from Epoxides and CO2.” Chemical Reviews 107(6):2388–2410. https://doi.org/10.1021/cr068363q.
Darensbourg, D.J., and M.W. Holtcamp. 1996. “Catalysts for the Reactions of Epoxides and Carbon Dioxide.” Coordination Chemistry Reviews 153(August 1):155–174. https://doi.org/10.1016/0010-8545(95)01232-X.
Das, S., D. Nugegoda, W. Yao, F. Qu, M.T. Figgins, R.W. Lamb, C.E. Webster, J.H. Delcamp, and E.T. Papish. 2022. “Sensitized and Self-Sensitized Photocatalytic Carbon Dioxide Reduction Under Visible Light with Ruthenium Catalysts Shows Enhancements with More Conjugated Pincer Ligands.” European Journal of Inorganic Chemistry 2022(8):e202101016. https://doi.org/10.1002/ejic.202101016.
Davies, J., J.R. Lyonnet, D.P. Zimin, and R. Martin. 2021. “The Road to Industrialization of Fine Chemical Carboxylation Reactions.” Chem 7(11):2927–2942. https://doi.org/10.1016/j.chempr.2021.10.016.
Daza, Y.A., and J.N. Kuhn. 2016. “CO2 Conversion by Reverse Water Gas Shift Catalysis: Comparison of Catalysts, Mechanisms and Their Consequences for CO2 Conversion to Liquid Fuels.” RSC Advances 6(55):49675–49691. https://doi.org/10.1039/C6RA05414E.
De Klerk, A. 2014. “Consider Technology Implications for Small-Scale Fischer-Tropsch GTL.” Gas Processing and LNG. http://gasprocessingnews.com/articles/2014/08/consider-technology-implications-for-small-scale-fischer-tropsch-gtl.
Dieterich, V., A. Buttler, A. Hanel, H. Spliethoff, and S. Fendt. 2020. “Power-to-Liquid via Synthesis of Methanol, DME or Fischer–Tropsch-Fuels: A Review.” Energy and Environmental Science 13(10):3207–3252. https://doi.org/10.1039/D0EE01187H.
Dimitriou, I., P. García-Gutiérrez, R.H. Elder, R.M. Cuéllar-Franca, A. Azapagic, and R.W.K. Allen. 2015. “Carbon Dioxide Utilisation for Production of Transport Fuels: Process and Economic Analysis.” Energy and Environmental Science 8(6):1775–1789. https://doi.org/10.1039/C4EE04117H.
Dineen, J. 2023. “Jet Fuel Made with Captured CO2 and Clean Electricity Set for Take-Off.” NewScientist September 28. https://www.newscientist.com/article/2394108-jet-fuel-made-with-captured-co2-and-clean-electricity-set-for-take-off.
Ding, M., and H.-L. Jiang. 2018. “Incorporation of Imidazolium-Based Poly(Ionic Liquid)s into a Metal–Organic Framework for CO2 Capture and Conversion.” ACS Catalysis 8(4):3194–3201. https://doi.org/10.1021/acscatal.7b03404.
Dodge, H.M., B.S. Natinsky, B.J. Jolly, H. Zhang, Y. Mu, S.M. Chapp, T.V. Tran, et al. 2023. “Polyketones from Carbon Dioxide and Ethylene by Integrating Electrochemical and Organometallic Catalysis.” ACS Catalysis 13(7):4053–4059. https://doi.org/10.1021/acscatal.3c00769.
DOE (Department of Energy). 2022. “Industrial Decarbonization Roadmap.” DOE/EE-2635. Washington, DC: Department of Energy. https://www.energy.gov/sites/default/files/2022-09/Industrial%20Decarbonization%20Roadmap.pdf.
Dong, Q., Y. Yao, S. Cheng, K. Alexopoulos, J. Gao, S. Srinivas, Y. Wang, et al. 2022. “Programmable Heating and Quenching for Efficient Thermochemical Synthesis.” Nature 605(7910):470–476. https://doi.org/10.1038/s41586-022-04568-6.
Dorner, R.W., D.R. Hardy, F.W. Williams, and H.D. Willauer. 2010. “Heterogeneous Catalytic CO2 Conversion to Value-Added Hydrocarbons.” Energy and Environmental Science 3(7):884–890. https://doi.org/10.1039/C001514H.
Dzuryk, S., and E. Rezaei. 2022. “Dimensionless Analysis of Reverse Water Gas Shift Packed-Bed Membrane Reactors.” Chemical Engineering Science 250(March 15):117377. https://doi.org/10.1016/j.ces.2021.117377.
Edwards, J.P., T. Alerte, C.P. O’Brien, C.M. Gabardo, S. Liu, J. Wicks, A. Gaona, et al. 2023. “Pilot-Scale CO2 Electrolysis Enables a Semi-Empirical Electrolyzer Model.” ACS Energy Letters 8(6):2576–2584. https://doi.org/10.1021/acsenergylett.3c00620.
EIA (U.S. Energy Information Administration). 2023. “Annual Energy Outlook 2023 Table 19. Energy-Related Carbon Dioxide Emissions by End Use.” https://www.eia.gov/outlooks/aeo/data/browser/#/?id=22-AEO2023&cases=ref2023&sourcekey=0.
Ellis, P.R., M.J. Hayes, N. Macleod, S.J. Schuyten, C.L. Tway, and C.M. Zalitis. 2023. “Chapter 10—Carbon Conversion: Opportunities in Chemical Productions.” Pp. 479–524 in Surface Process, Transportation, and Storage, Q. Wang, ed. Vol. 4. Gulf Professional Publishing. https://doi.org/10.1016/B978-0-12-823891-2.00006-5.
Elsernagawy, O.Y.H., A. Hoadley, J. Patel, T. Bhatelia, S. Lim, N. Haque, and C. Li. 2020. “Thermo-Economic Analysis of Reverse Water-Gas Shift Process with Different Temperatures for Green Methanol Production as a Hydrogen Carrier.” Journal of CO2 Utilization 41(October):101280. https://doi.org/10.1016/j.jcou.2020.101280.
EPA (U.S. Environmental Protection Agency). 2018. “Biofuels and the Environment: Second Triennial Report to Congress.” EPA/600/R-18/195. Washington, DC: U.S. Environmental Protection Agency. https://cfpub.epa.gov/si/si_public_record_Report.cfm?Lab=IO&dirEntryId=341491.
Eryazici, I., N. Ramesh, and C. Villa. 2021. “Electrification of the Chemical Industry—Materials Innovations for a Lower Carbon Future.” MRS Bulletin 46(12):1197–1204. https://doi.org/10.1557/s43577-021-00243-9.
Fang, W., W. Guo, R. Lu, Y. Yan, X. Liu, D. Wu, F.M. Li, et al. 2024. “Durable CO2 Conversion in the Proton-Exchange Membrane System.” Nature 626(7997):86–91. https://doi.org/10.1038/s41586-023-06917-5.
Feroci, M., M. Orsini, G. Sotgiu, L. Rossi, and A. Inesi. 2005. “Electrochemically Promoted C–N Bond Formation from Acetylenic Amines and CO2. Synthesis of 5-Methylene-1,3-Oxazolidin-2-Ones.” The Journal of Organic Chemistry 70(19):7795–7798. https://doi.org/10.1021/jo0511804.
Filonenko, G.A., R. van Putten, E.J.M. Hensen, E.A. Pidko. 2018. “Catalytic (de)hydrogenation Promoted by Non-precious Metals—Co, Fe and Mn: Recent Advances in an Emerging Field.” Chemical Society Reviews 47:1459–1483. https://doi.org/10.1039/C7CS00334J.
Fors, S.A., and C.A. Malapit. 2023. “Homogeneous Catalysis for the Conversion of CO2, CO, CH3OH, and CH4 to C2+ Chemicals via C–C Bond Formation.” ACS Catalysis 13(7):4231–4249. https://doi.org/10.1021/acscatal.2c05517.
Francke, R., B. Schille, and M. Roemelt. 2018. “Homogeneously Catalyzed Electroreduction of Carbon Dioxide—Methods, Mechanisms, and Catalysts.” Chemical Reviews 118(9):4631–4701. https://doi.org/10.1021/acs.chemrev.7b00459.
Freire Ordóñez, D., T. Halfdanarson, C. Ganzer, N. Shah, N. Mac Dowell, and G. Guillén-Gosálbez. 2022. “Evaluation of the Potential Use of E-Fuels in the European Aviation Sector: A Comprehensive Economic and Environmental Assessment Including Externalities.” Sustainable Energy and Fuels 6(20):4749–4764. https://doi.org/10.1039/D2SE00757F.
Freyman, M.C., Z. Huang, D. Ravikumar, E.B. Duoss, Y. Li, S.E. Baker, S.H. Pang, and J.A. Schaidle. 2023. “Reactive CO2 Capture: A Path Forward for Process Integration in Carbon Management.” Joule 7(4):631–651. https://doi.org/10.1016/j.joule.2023.03.013.
Fukuoka, S. 2012. “Non-Phosgene Polycarbonate from CO2: Industrialization of Green Chemical Process.” New York: Nova Science Publishers.
Fukuoka, S., I. Fukawa, T. Adachi, H. Fujita, N. Sugiyama, and T. Sawa. 2019. “Industrialization and Expansion of Green Sustainable Chemical Process: A Review of Non-Phosgene Polycarbonate from CO2.” Organic Process Research and Development 23(2):145–169. https://doi.org/10.1021/acs.oprd.8b00391.
Gambo, Y., S. Adamu, G. Tanimu, I.M. Abdullahi, R.A. Lucky, M.S. Ba-Shammakh, and M.M. Hossain. 2021. “CO2-Mediated Oxidative Dehydrogenation of Light Alkanes to Olefins: Advances and Perspectives in Catalyst Design and Process Improvement.” Applied Catalysis A: General 623(August 5):118273. https://doi.org/10.1016/j.apcata.2021.118273.
GAO (U.S. Government Accountability Office). 2022. “Decarbonization: Status, Challenges, and Policy Options for Carbon Capture, Utilization, and Storage.” GAO-22-105274. Technology Assessment. Washington, DC: U.S. Government Accountability Office. https://www.gao.gov/assets/730/723198.pdf.
Gao, P., S. Li, X. Bu, S. Dang, Z. Liu, H. Wang, L. Zhong, et al. 2017. “Direct Conversion of CO2 into Liquid Fuels with High Selectivity Over a Bifunctional Catalyst.” Nature Chemistry 9(10):1019–1024. https://doi.org/10.1038/nchem.2794.
Gao, X., T. Atchimarungsri, Q. Ma, T.-S. Zhao, and N. Tsubaki. 2020. “Realizing Efficient Carbon Dioxide Hydrogenation to Liquid Hydrocarbons by Tandem Catalysis Design.” EnergyChem 2(4):100038. https://doi.org/10.1016/j.enchem.2020.100038.
Garg, S., Z. Xie, and J.G. Chen. 2024. “Tandem Reactors and Reactions for CO2 Conversion.” Nature Chemical Engineering 1(2):139–148. https://doi.org/10.1038/s44286-023-00020-2.
Ghobadi, M.H., M. Firuzi, and E. Asghari-Kaljahi. 2016. “Relationships Between Geological Formations and Groundwater Chemistry and Their Effects on the Concrete Lining of Tunnels (Case Study: Tabriz Metro Line 2).” Environmental Earth Sciences 75(12):987. https://doi.org/10.1007/s12665-016-5785-0.
Gogate, M.R. 2019. “Methanol-to-Olefins Process Technology: Current Status and Future Prospects.” Petroleum Science and Technology 37(5):559–565. https://doi.org/10.1080/10916466.2018.1555589.
Goguet, A., F. Meunier, J.P. Breen, R. Burch, M.I. Petch, and A. Faur Ghenciu. 2004. “Study of the Origin of the Deactivation of a Pt/CeO2 Catalyst During Reverse Water Gas Shift (RWGS) Reaction.” Journal of Catalysis 226(2):382–392. https://doi.org/10.1016/j.jcat.2004.06.011.
González-Castaño, M., B. Dorneanu, and H. Arellano-García. 2021. “The Reverse Water Gas Shift Reaction: A Process Systems Engineering Perspective.” Reaction Chemistry and Engineering 6(6):954–976. https://doi.org/10.1039/D0RE00478B.
Govind Rajan, A., J.M.P. Martirez, and E.A. Carter. 2020. “Why Do We Use the Materials and Operating Conditions We Use for Heterogeneous (Photo)Electrochemical Water Splitting?” ACS Catalysis 10(19):11177–11234. https://doi.org/10.1021/acscatal.0c01862.
Grignard, B., S. Gennen, C. Jérôme, A.W. Kleij, and C. Detrembleur. 2019. “Advances in the Use of CO2 as a Renewable Feedstock for the Synthesis of Polymers.” Chemical Society Reviews 48(16):4466–4514. https://doi.org/10.1039/C9CS00047J.
Grills, D.C., and E. Fujita. 2010. “New Directions for the Photocatalytic Reduction of CO2: Supramolecular, scCO2 or Biphasic Ionic Liquid–scCO2 Systems.” The Journal of Physical Chemistry Letters 1(18):2709–2718. https://doi.org/10.1021/jz1010237.
Grills, D.C., Y. Matsubara, Y. Kuwahara, S.R. Golisz, D.A. Kurtz, and B.A. Mello. 2014. “Electrocatalytic CO2 Reduction with a Homogeneous Catalyst in Ionic Liquid: High Catalytic Activity at Low Overpotential.” The Journal of Physical Chemistry Letters 5(11):2033–2038. https://doi.org/10.1021/jz500759x.
Grim, R.G., J.R. Ferrell III, Z. Huang, L. Tao, and M.G. Resch. 2023. “The Feasibility of Direct CO2 Conversion Technologies on Impacting Mid-Century Climate Goals.” Joule 7(8):1684–1699. https://doi.org/10.1016/j.joule.2023.07.008.
Grimm, A., W.A. de Jong, and G.J. Kramer. 2020. “Renewable Hydrogen Production: A Techno-Economic Comparison of Photoelectrochemical Cells and Photovoltaic-Electrolysis.” International Journal of Hydrogen Energy 45(43):22545–22555. https://doi.org/10.1016/j.ijhydene.2020.06.092.
Gui, M.M., W.P. Cathie Lee, L.K. Putri, X.Y. Kong, L.L. Tan, and S.-P. Chai. 2021. “Photo-Driven Reduction of Carbon Dioxide: A Sustainable Approach Towards Achieving Carbon Neutrality Goal.” Frontiers in Chemical Engineering 3. https://www.frontiersin.org/articles/10.3389/fceng.2021.744911.
Han, B., W. Wei, L. Chang, P. Cheng, and Y.H. Hu. 2016. “Efficient Visible Light Photocatalytic CO2 Reforming of CH4.” ACS Catalysis 6(2):494–497. https://doi.org/10.1021/acscatal.5b02653.
Hauch, A., R. Küngas, P. Blennow, A.B. Hansen, J.B. Hansen, B.V. Mathiesen, and M.B. Mogensen. 2020. “Recent Advances in Solid Oxide Cell Technology for Electrolysis.” Science 370(6513):eaba6118. https://doi.org/10.1126/science.aba6118.
Hawecker, J., J.-M. Lehn, and R. Ziessel. 1986. “Photochemical and Electrochemical Reduction of Carbon Dioxide to Carbon Monoxide Mediated by (2,2'-Bipyridine)Tricarbonylchlororhenium(I) and Related Complexes as Homogeneous Catalysts.” Helvetica Chimica Acta 69(8):1990–2012. https://doi.org/10.1002/hlca.19860690824.
He, Z., M. Cui, Q. Qian, J. Zhang, H. Liu, and B. Han. 2019. “Synthesis of Liquid Fuel via Direct Hydrogenation of CO2.” Proceedings of the National Academy of Sciences 116(26):12654–12659. https://doi.org/10.1073/pnas.1821231116.
Heldebrant, D.J., J. Kothandaraman, N. Mac Dowell, and L. Brickett. 2022. “Next Steps for Solvent-Based CO2 Capture; Integration of Capture, Conversion, and Mineralisation.” Chemical Science 13(22):6445–6456. https://doi.org/10.1039/D2SC00220E.
Hietala, J., A. Vuori, P. Johnsson, I. Pollari, W. Reutemann, and H. Kieczka. 2016. “Formic Acid.” Pp. 1–22 in Ullmann’s Encyclopedia of Industrial Chemistry. https://doi.org/10.1002/14356007.a12_013.pub3.
Ho, P.-Y., S.-C. Cheng, F. Yu, Y.-Y. Yeung, W.-X. Ni, C.-C. Ko, C.-F. Leung, T.-C. Lau, and M. Robert. 2023. “Light-Driven Reduction of CO2 to CO in Water with a Cobalt Molecular Catalyst and an Organic Sensitizer.” ACS Catalysis 13(9):5979–5985. https://doi.org/10.1021/acscatal.3c00036.
Hu, C., X. Chen, J. Low, Y.-W. Yang, H. Li, D. Wu, S. Chen, et al. 2023. “Near-Infrared-Featured Broadband CO2 Reduction with Water to Hydrocarbons by Surface Plasmon.” Nature Communications 14(1):221. https://doi.org/10.1038/s41467-023-35860-2.
Huang, J., J.C. Worch, A.P. Dove, and O. Coulembier. 2020. “Update and Challenges in Carbon Dioxide-Based Polycarbonate Synthesis.” ChemSusChem 13(3):469–487. https://doi.org/10.1002/cssc.201902719.
Hunt, J., A. Ferrari, A. Lita, M. Crosswhite, B. Ashley, and A.E. Stiegman. 2013. “Microwave-Specific Enhancement of the Carbon–Carbon Dioxide (Boudouard) Reaction.” The Journal of Physical Chemistry C 117(51):26871–26880. https://doi.org/10.1021/jp4076965.
Inoue, S., H. Koinuma, and T. Tsuruta. 1969a. “Copolymerization of Carbon Dioxide and Epoxide.” Journal of Polymer Science Part B: Polymer Letters 7(4):287–292. https://doi.org/10.1002/pol.1969.110070408.
Inoue, S., H. Koinuma, and T. Tsuruta. 1969b. “Copolymerization of Carbon Dioxide and Epoxide with Organometallic Compounds.” Die Makromolekulare Chemie 130(1):210–220. https://doi.org/10.1002/macp.1969.021300112.
Iota, V., C.S. Yoo, and H. Cynn. 1999. “Quartzlike Carbon Dioxide: An Optically Nonlinear Extended Solid at High Pressures and Temperatures.” Science 283(5407):1510–1513. https://doi.org/10.1126/science.283.5407.1510.
Jang, W.-J., J.-O. Shim, H.-M. Kim, S.-Y. Yoo, and H.-S. Roh. 2019. “A Review on Dry Reforming of Methane in Aspect of Catalytic Properties.” SI: Green Catalysis 324(March 1):15–26. https://doi.org/10.1016/j.cattod.2018.07.032.
Jens, C.M., L. Müller, K. Leonhard, and A. Bardow. 2019. “To Integrate or Not to Integrate—Techno-Economic and Life Cycle Assessment of CO2 Capture and Conversion to Methyl Formate Using Methanol.” ACS Sustainable Chemistry and Engineering 7(14):12270–12280. https://doi.org/10.1021/acssuschemeng.9b01603.
Jiang, X., X. Nie, X. Guo, C. Song, and J.G. Chen. 2020. “Recent Advances in Carbon Dioxide Hydrogenation to Methanol via Heterogeneous Catalysis.” Chemical Reviews 120(15):7984–8034. https://doi.org/10.1021/acs.chemrev.9b00723.
Jiao, F., J. Li, X. Pan, J. Xiao, H. Li, H. Ma, M. Wei, et al. 2016. “Selective Conversion of Syngas to Light Olefins.” Science 351(6277):1065–1068. https://www.science.org/doi/10.1126/science.aaf1835.
Joo, O.-S., K.-D. Jung, I. Moon, A.Y. Rozovskii, G.I. Lin, S.-H. Han, and S.-J. Uhm. 1999. “Carbon Dioxide Hydrogenation to Form Methanol via a Reverse-Water-Gas-Shift Reaction (the CAMERE Process).” Industrial and Engineering Chemistry Research 38(5):1808–1812. https://doi.org/10.1021/ie9806848.
Jouny, M., G.S. Hutchings, and F. Jiao. 2019. “Carbon Monoxide Electroreduction as an Emerging Platform for Carbon Utilization.” Nature Catalysis 2(12):1062–1070. https://www.nature.com/articles/s41929-019-0388-2.
Kalamaras, E., M.M. Maroto-Valer, M. Shao, J. Xuan, and H. Wang. 2018. “Solar Carbon Fuel via Photoelectrochemistry.” SI: Decarbonising Fossil Fuel 317(November 1):56–75. https://doi.org/10.1016/j.cattod.2018.02.045.
Kalck, P., C. Le Berre, and P. Serp. 2020. “Recent Advances in the Methanol Carbonylation Reaction into Acetic Acid.” Coordination Chemistry Reviews 402(January):213078. https://doi.org/10.1016/j.ccr.2019.213078.
Kar, S., A. Goeppert, V. Galvan, R. Chowdhury, J. Olah, and G.K. Surya Prakash. 2018a. “A Carbon-Neutral CO2 Capture, Conversion, and Utilization Cycle with Low-Temperature Regeneration of Sodium Hydroxide.” Journal of the American Chemical Society 140(49):16873–16876. https://doi.org/10.1021/jacs.8b09325.
Kar, S., R. Sen, A. Goeppert, and G.K. Surya Prakash. 2018b. “Integrative CO2 Capture and Hydrogenation to Methanol with Reusable Catalyst and Amine: Toward a Carbon Neutral Methanol Economy.” Journal of the American Chemical Society 140(5):1580–1583. https://doi.org/10.1021/jacs.7b12183.
Kar, S., A. Goeppert, and G.K. Surya Prakash. 2019a. “Combined CO2 Capture and Hydrogenation to Methanol: Amine Immobilization Enables Easy Recycling of Active Elements.” ChemSusChem 12(13):3172–3177. https://doi.org/10.1002/cssc.201900324.
Kar, S., A. Goeppert, and G.K. Surya Prakash. 2019b. “Integrated CO2 Capture and Conversion to Formate and Methanol: Connecting Two Threads.” Accounts of Chemical Research 52(10):2892–2903. https://doi.org/10.1021/acs.accounts.9b00324.
Kember, M.R., A. Buchard, and C.K. Williams. 2011. “Catalysts for CO2/Epoxide Copolymerisation.” Chemical Communications 47(1):141–163. https://doi.org/10.1039/C0CC02207A.
Kempler, P.A., and A.C. Nielander. 2023. “Reliable Reporting of Faradaic Efficiencies for Electrocatalysis Research.” Nature Communications 14(1):1158. https://doi.org/10.1038/s41467-023-36880-8.
Khan, A.A., and M. Tahir. 2019. “Recent Advancements in Engineering Approach Towards Design of Photo-Reactors for Selective Photocatalytic CO2 Reduction to Renewable Fuels.” Journal of CO2 Utilization 29(January 1):205–239. https://doi.org/10.1016/j.jcou.2018.12.008.
Kim, S.M., P.M. Abdala, M. Broda, D. Hosseini, C. Copéret, and C. Müller. 2018. “Integrated CO2 Capture and Conversion as an Efficient Process for Fuels from Greenhouse Gases.” ACS Catalysis 8(4):2815–2823. https://doi.org/10.1021/acscatal.7b03063.
Klug, C.M., A.J.P. Cardenas, R.M. Bullock, M. O’Hagan, and E.S. Wiedner. 2018. “Reversing the Tradeoff Between Rate and Overpotential in Molecular Electrocatalysts for H2 Production.” ACS Catalysis 8(4):3286–3296. https://doi.org/10.1021/acscatal.7b04379.
Kothandaraman, J., and D.J. Heldebrant. 2020. “Catalytic Coproduction of Methanol and Glycol in One Pot from Epoxide, CO2, and H2.” RSC Advances 10(69):2557–42563. https://doi.org/10.1039/D0RA09459E.
Kothandaraman, J., A. Goeppert, M. Czaun, G.A. Olah, and G.K. Surya Prakash. 2016a. “CO2 Capture by Amines in Aqueous Media and Its Subsequent Conversion to Formate with Reusable Ruthenium and Iron Catalysts.” Green Chemistry 18(21):5831–5838. https://doi.org/10.1039/C6GC01165A.
Kothandaraman, J., A. Goeppert, M. Czaun, G.A. Olah, and G.K. Surya Prakash. 2016b. “Conversion of CO2 from Air into Methanol Using a Polyamine and a Homogeneous Ruthenium Catalyst.” Journal of the American Chemical Society 138(3):778–781. https://doi.org/10.1021/jacs.5b12354.
Krishnamurty, K.V., G. McLeod Harris, and V.S. Sastri. 1970. “Chemistry of the Metal Carbonato Complexes.” Chemical Reviews 70(2):171–197. https://doi.org/10.1021/cr60264a001.
Kumar, B., M. Llorente, J. Froehlich, T. Dang, A. Sathrum, and C.P. Kubiak. 2012. “Photochemical and Photoelectrochemical Reduction of CO2.” Annual Review of Physical Chemistry 63(May):541–569. https://doi.org/10.1146/annurev-physchem-032511-143759.
Küngas, R. 2020. “Review—Electrochemical CO2 Reduction for CO Production: Comparison of Low- and High-Temperature Electrolysis Technologies.” Journal of the Electrochemical Society 167(4):044508. https://doi.org/10.1149/1945-7111/ab7099.
La Plante, E.C., D.A. Simonetti, J. Wang, A. Al-Turki, X. Chen, D. Jassby, and G.N. Sant. 2021. “Saline Water-Based Mineralization Pathway for Gigatonne-Scale CO2 Management.” ACS Sustainable Chemistry and Engineering 9(3):1073–1089. https://doi.org/10.1021/acssuschemeng.0c08561.
La Plante, E.C., X. Chen, S. Bustillos, A. Bouissonnie, T. Traynor, D. Jassby, L. Corsini, D.A. Simonetti, and G.N. Sant. 2023. “Electrolytic Seawater Mineralization and the Mass Balances That Demonstrate Carbon Dioxide Removal.” ACS ES&T Engineering 3(7):955–968. https://doi.org/10.1021/acsestengg.3c00004.
Lam, E., K. Larmier, P. Wolf, S. Tada, O.V. Safonova, and C. Copéret. 2018. “Isolated Zr Surface Sites on Silica Promote Hydrogenation of CO2 to CH3OH in Supported Cu Catalysts.” Journal of the American Chemical Society 140(33):10530–10535. https://doi.org/10.1021/jacs.8b05595.
Lamprecht, D. 2007. “Fischer–Tropsch Fuel for Use by the U.S. Military as Battlefield-Use Fuel of the Future.” Energy and Fuels 21(3):1448–1453. https://doi.org/10.1021/ef060607m.
Langanke, J., A. Wolf, and M. Peters. 2015. “Chapter 5—Polymers from CO2—An Industrial Perspective.” Pp. 59–71 in Carbon Dioxide Utilisation, P. Styring, E.A. Quadrelli, and K. Armstrong, eds. Amsterdam: Elsevier. https://doi.org/10.1016/B978-0-444-62746-9.00005-0.
Lazard. 2024. “Lazard’s Levelized Cost of Energy+.” Lazard and Roland Berger. https://www.lazard.com/media/xemfey0k/lazards-lcoeplus-june-2024-_vf.pdf.
le Saché, E., and T.R. Reina. 2022. “Analysis of Dry Reforming as Direct Route for Gas Phase CO2 Conversion. The Past, the Present and Future of Catalytic DRM Technologies.” Progress in Energy and Combustion Science 89(March 1):100970. https://doi.org/10.1016/j.pecs.2021.100970.
Lee, C.W., K.D. Yang, D.-H. Nam, J.H. Jang, N.H. Cho, S.W. Im, and K.T. Nam. 2018. “Defining a Materials Database for the Design of Copper Binary Alloy Catalysts for Electrochemical CO2 Conversion.” Advanced Materials 30(42):1704717. https://doi.org/10.1002/adma.201704717.
Lee, M.G., X.-Y. Li, A. Ozden, J. Wicks, P. Ou, Y. Li, R. Dorakhan, et al. 2023. “Selective Synthesis of Butane from Carbon Monoxide Using Cascade Electrolysis and Thermocatalysis at Ambient Conditions.” Nature Catalysis 6(4):310–318. https://doi.org/10.1038/s41929-023-00937-0.
Lees, E.W., A. Liu, J.C. Bui, S. Ren, A.Z. Weber, and C.P. Berlinguette. 2022. “Electrolytic Methane Production from Reactive Carbon Solutions.” ACS Energy Letters 7(5):1712–1718. https://doi.org/10.1021/acsenergylett.2c00283.
Liao, P., and E.A. Carter. 2013. “New Concepts and Modeling Strategies to Design and Evaluate Photo-Electro-Catalysts Based on Transition Metal Oxides.” Chemical Society Reviews 42(6):2401–2422. https://doi.org/10.1039/C2CS35267B.
Lichterman, M.F., K. Sun, S. Hu, X. Zhou, M.T. McDowell, M.R. Shaner, M.H. Richter, et al. 2016. “Protection of Inorganic Semiconductors for Sustained, Efficient Photoelectrochemical Water Oxidation.” Electrocatalysis 262(March 15):11–23. https://doi.org/10.1016/j.cattod.2015.08.017.
Lidston, C.A.L., S.M. Severson, B.A. Abel, and G.W. Coates. 2022. “Multifunctional Catalysts for Ring-Opening Copolymerizations.” ACS Catalysis 12(18):11037–11070. https://doi.org/10.1021/acscatal.2c02524.
Lin, T., Y. An, F. Yu, K. Gong, H. Yu, C. Wang, Y. Sun, and L. Zhong. 2022. “Advances in Selectivity Control for Fischer–Tropsch Synthesis to Fuels and Chemicals with High Carbon Efficiency.” ACS Catalysis 12(19):12092–120112. https://doi.org/10.1021/acscatal.2c03404.
Liu, A.-H., R. Ma, C. Song, Z.-Z. Yang, A. Yu, Y. Cai, L.-N. He, Y.-N. Zhao, B. Yu, and Q.-W. Song. 2012b. “Equimolar CO2 Capture by N-Substituted Amino Acid Salts and Subsequent Conversion.” Angewandte Chemie International Edition 51(45):11306–11310. https://doi.org/10.1002/anie.201205362.
Liu, B., L. Ma, H. Feng, Y. Zhang, J. Duan, Y. Wang, D. Liu, and Q. Li. 2023. “Photovoltaic-Powered Electrochemical CO2 Reduction: Benchmarking Against the Theoretical Limit.” ACS Energy Letters 8(2):981–987. https://doi.org/10.1021/acsenergylett.2c02906.
Liu, L., S.-M. Wang, Z.-B. Han, M. Ding, D.-Q. Yuan, and H.-L. Jiang. 2016a. “Exceptionally Robust In-Based Metal–Organic Framework for Highly Efficient Carbon Dioxide Capture and Conversion.” Inorganic Chemistry 55(7):3558–3565. https://doi.org/10.1021/acs.inorgchem.6b00050.
Liu, M., L. Liang, X. Li, X. Gao, and J. Sun. 2016b. “Novel Urea Derivative-Based Ionic Liquids with Dual-Functions: CO2 Capture and Conversion Under Metal- and Solvent-Free Conditions.” Green Chemistry 18(9):2851–2863. https://doi.org/10.1039/C5GC02605A.
Liu, S., L.R. Winter and J.G. Chen, 2020. “Review of Plasma-Assisted Catalysis for Selective Generation of Oxygenates from CO2 and CH4,” ACS Catalysis 10:2855–2871. https://pubs.acs.org/doi/abs/10.1021/acscatal.9b04811.
Liu, X., S. Zhang, and Y. Ding. 2012a. “Synthesis, Characterization and Properties of a μ–η2: η2-Carbonato-Bridged Bis(Phosphinoferrocenyl) Copper(I) Complex from CO2 Fixation.” Inorganic Chemistry Communications 18(April 1): 83–86. https://doi.org/10.1016/j.inoche.2012.01.023.
Liu, Y., and X.-B. Lu. 2023. “Current Challenges and Perspectives in CO2-Based Polymers.” Macromolecules 56(5):1759–1777. https://doi.org/10.1021/acs.macromol.2c02483.
Margarit, C.G., N.G. Asimow, M.I. Gonzalez, and D.G. Nocera. 2020. “Double Hangman Iron Porphyrin and the Effect of Electrostatic Nonbonding Interactions on Carbon Dioxide Reduction.” The Journal of Physical Chemistry Letters 11(5):1890–1895. https://doi.org/10.1021/acs.jpclett.9b03897.
Marocco Stuardi, F., F. MacPherson, and J. Leclaire. 2019. “Integrated CO2 Capture and Utilization: A Priority Research Direction.” CO2 Capture and Chemistry 16(April 1):71–76. https://doi.org/10.1016/j.cogsc.2019.02.003.
Martin, D.J., C.F. Wise, M.L. Pegis, and J.M. Mayer. 2020. “Developing Scaling Relationships for Molecular Electrocatalysis Through Studies of Fe-Porphyrin-Catalyzed O2 Reduction.” Accounts of Chemical Research 53(5):1056–1065. https://doi.org/10.1021/acs.accounts.0c00044.
Martín, M., and I.E. Grossmann. 2011. “Process Optimization of FT-Diesel Production from Lignocellulosic Switchgrass.” Industrial and Engineering Chemistry Research 50(23):13485–13499. https://doi.org/10.1021/ie201261t.
Martirez, J.M.P., and E.A. Carter. 2023. “Solvent Dynamics Are Critical to Understanding Carbon Dioxide Dissolution and Hydration in Water.” Journal of the American Chemical Society 145(23):12561–12575. https://doi.org/10.1021/jacs.3c01283.
Martirez, J.M.P., J. L. Bao, and E.A. Carter. 2021. “First-Principles Insights into Plasmon-Induced Catalysis.” Annual Review of Physical Chemistry 72(1):99–119. https://doi.org/10.1146/annurev-physchem-061020-053501.
Masel, R.I., Z. Liu, H. Yang, J.J. Kaczur, D. Carrillo, S. Ren, D. Salvatore, and C.P. Berlinguette. 2021. “An Industrial Perspective on Catalysts for Low-Temperature CO2 Electrolysis.” Nature Nanotechnology 16(2):118–128. https://doi.org/10.1038/s41565-020-00823-x.
Matsubara, Y., D.C. Grills, and Y. Kuwahara. 2015. “Thermodynamic Aspects of Electrocatalytic CO2 Reduction in Acetonitrile and with an Ionic Liquid as Solvent or Electrolyte.” ACS Catalysis 5(11):6440–6452. https://doi.org/10.1021/acscatal.5b00656.
Mayer, J.M. 2023. “Bonds Over Electrons: Proton Coupled Electron Transfer at Solid–Solution Interfaces.” Journal of the American Chemical Society 145(13):7050–7064. https://doi.org/10.1021/jacs.2c10212.
McGhee, W., D. Riley, K. Christ, Y. Pan, and B. Parnas. 1995. “Carbon Dioxide as a Phosgene Replacement: Synthesis and Mechanistic Studies of Urethanes from Amines, CO2, and Alkyl Chlorides.” The Journal of Organic Chemistry 60(9):2820–2830. https://doi.org/10.1021/jo00114a035.
Mondal, T., and D. Chatterjee. 2016. “RuIII-edta (edta4− = ethylenediaminetetraacetate) Mediated Photocatalytic Conversion of Bicarbonate to Formate Over Visible Light Irradiated Non-Metal Doped TiO2 Semiconductor Photocatalysts.” RSC Advances 6(68):63488–63492. https://doi.org/10.1039/C6RA11464D.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2019. Gaseous Carbon Waste Streams Utilization: Status and Research Needs. Washington, DC: The National Academies Press. https://doi.org/10.17226/25232.
NASEM. 2023. Carbon Dioxide Utilization Markets and Infrastructure: Status and Opportunities: A First Report. Washington, DC: The National Academies Press. https://doi.org/10.17226/26703.
Neelis, M., M. Patel, K. Blok, W. Haije, and P. Bach. 2007. “Approximation of Theoretical Energy-Saving Potentials for the Petrochemical Industry Using Energy Balances for 68 Key Processes.” Energy 32(7):1104–1123. https://doi.org/10.1016/j.energy.2006.08.005.
NETL (National Energy Technology Laboratory). n.d.(a). “Syngas Conversion to Methanol.” National Energy Technology Laboratory. https://www.netl.doe.gov/research/carbon-management/energy-systems/gasification/gasifipedia/methanol.
NETL. n.d.(b). “Conversion of Methanol to Gasoline.” National Energy Technology Laboratory. https://www.netl.doe.gov/research/carbon-management/energy-systems/gasification/gasifipedia/methanol-to-gasoline.
NETL. n.d.(c). “Fischer-Tropsch Synthesis.” National Energy Technology Laboratory. https://www.netl.doe.gov/research/carbon-management/energy-systems/gasification/gasifipedia/ftsynthesis.
NETL. n.d.(d). “Point Source Carbon Capture from Power Generation Sources.” National Energy Technology Laboratory. https://netl.doe.gov/carbon-capture/power-generation.
Nie, W., and C.C.L. McCrory. 2022. “Strategies for Breaking Molecular Scaling Relationships for the Electrochemical CO2 Reduction Reaction.” Dalton Transactions 51(18):6993–7010. https://doi.org/10.1039/D2DT00333C.
Nitopi, S., E. Bertheussen, S.B. Scott, X. Liu, A.K. Engstfeld, S. Horch, B. Seger, et al. 2019. “Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte.” Chemical Reviews 119(12):7610–7672. https://doi.org/10.1021/acs.chemrev.8b00705.
Nova-Institut. 2023. “The Rise of Carbon Dioxide (CO2) as a Renewable Carbon Feedstock—More Than 1.3 Million Tonnes Capacity for CO2-Based Products Already Exist and Are Expected to at Least Quadruple by 2030.” https://nova-institute.eu/press/?id=428.
NPC (National Petroleum Council). 2024. “Chapter 2—Production at Scale.” In Harnessing Hydrogen: A Key Element of the U.S. Energy Future. https://harnessinghydrogen.npc.org/files/H2-CH_2-Production_at_scale-2024-04-23.pdf.
NRC (National Research Council). 2006. Sustainability in the Chemical Industry: Grand Challenges and Research Needs. Washington, DC: The National Academies Press. https://doi.org/10.17226/11437.
Omodolor, I.S., H.O. Otor, J.A. Andonegui, B.J. Allen, and A.C. Alba-Rubio. 2020. “Dual-Function Materials for CO2 Capture and Conversion: A Review.” Industrial and Engineering Chemistry Research 59(40):17612–17631. https://doi.org/10.1021/acs.iecr.0c02218.
Pescarmona, P.P. 2021. “Cyclic Carbonates Synthesised from CO2: Applications, Challenges and Recent Research Trends.” Current Opinion in Green and Sustainable Chemistry 29(June 1):100457. https://doi.org/10.1016/j.cogsc.2021.100457.
Poltavsky, I., and A. Tkatchenko. 2021. “Machine Learning Force Fields: Recent Advances and Remaining Challenges.” The Journal of Physical Chemistry Letters 12(28):6551–6564. https://doi.org/10.1021/acs.jpclett.1c01204.
Prajapati, A., R. Sartape, M.T. Galante, J. Xie, S.L. Leung, I. Bessa, M.H.S. Andrade, et al. 2022. “Fully-Integrated Electrochemical System That Captures CO2 from Flue Gas to Produce Value-Added Chemicals at Ambient Conditions.” Energy and Environmental Science 15(12):5105–5117. https://doi.org/10.1039/D2EE03396H.
Pullar, R.C., R.M. Novais, A.P.F. Caetano, M.A. Barreiros, S. Abanades, and F.A. Costa Oliveira. 2019. “A Review of Solar Thermochemical CO2 Splitting Using Ceria-Based Ceramics with Designed Morphologies and Microstructures.” Frontiers in Chemistry 7. https://www.frontiersin.org/articles/10.3389/fchem.2019.00601.
Queyriaux, N. 2021. “Redox-Active Ligands in Electroassisted Catalytic H+ and CO2 Reductions: Benefits and Risks.” ACS Catalysis 11(7):4024–4035. https://doi.org/10.1021/acscatal.1c00237.
Rehman, A., F. Saleem, F. Javed, A. Ikhlaq, S. Waqas Ahmad, and A. Harvey. 2021. “Recent Advances in the Synthesis of Cyclic Carbonates via CO2 Cycloaddition to Epoxides.” Journal of Environmental Chemical Engineering 9(2):105113. https://doi.org/10.1016/j.jece.2021.105113.
Richard, A.R., and M. Fan. 2017. “Low-Pressure Hydrogenation of CO2 to CH3OH Using Ni-In-Al/SiO2 Catalyst Synthesized via a Phyllosilicate Precursor.” ACS Catalysis 7(9):5679–5692. https://doi.org/10.1021/acscatal.7b00848.
Robatjazi, H., L. Yuan, Y. Yuan, and N.J. Halas. 2021. “Heterogeneous Plasmonic Photocatalysis: Light-Driven Chemical Reactions Introduce a New Approach to Industrially-Relevant Chemistry.” Pp. 363–387 in Emerging Trends in Chemical Applications of Lasers. ACS Symposium Series 1398. American Chemical Society. https://doi.org/10.1021/bk-2021-1398.ch016.
Rothschild, A., and H. Dotan. 2017. “Beating the Efficiency of Photovoltaics-Powered Electrolysis with Tandem Cell Photo-electrolysis.” ACS Energy Letters 2(1):45–51. https://doi.org/10.1021/acsenergylett.6b00610.
Rungtaweevoranit, B., J. Baek, J.R. Araujo, B.S. Archanjo, K.M. Choi, O.M. Yaghi, and G.A. Somorjai. 2016. “Copper Nano-crystals Encapsulated in Zr-Based Metal–Organic Frameworks for Highly Selective CO2 Hydrogenation to Methanol.” Nano Letters 16(12):7645–7649. https://doi.org/10.1021/acs.nanolett.6b03637.
Saeidi, S., S. Najari, V. Hessel, K. Wilson, F.J. Keil, P. Concepción, S.L. Suib, and A.E. Rodrigues. 2021. “Recent Advances in CO2 Hydrogenation to Value-Added Products—Current Challenges and Future Directions.” Progress in Energy and Combustion Science 85(July 1):100905. https://doi.org/10.1016/j.pecs.2021.100905.
Samimi, F., D. Karimipourfard, and M.R. Rahimpour. 2018. “Green Methanol Synthesis Process from Carbon Dioxide via Reverse Water Gas Shift Reaction in a Membrane Reactor.” Chemical Engineering Research and Design 140(December 1): 44–67. https://doi.org/10.1016/j.cherd.2018.10.001.
Sampaio, R.N., D.C. Grills, D.E. Polyansky, D.J. Szalda, and E. Fujita. 2020. “Unexpected Roles of Triethanolamine in the Photochemical Reduction of CO2 to Formate by Ruthenium Complexes.” Journal of the American Chemical Society 142(5):2413–2428. https://doi.org/10.1021/jacs.9b11897.
Sandoval-Diaz, L.E., R. Schlögl, and T. Lunkenbein. 2022. “Quo Vadis Dry Reforming of Methane?–A Review on Its Chemical, Environmental, and Industrial Prospects.” Catalysts 12(5). https://doi.org/10.3390/catal12050465.
Sarswat, A., D.S. Sholl, and R.P. Lively. 2022. “Achieving Order of Magnitude Increases in CO2 Reduction Reaction Efficiency by Product Separations and Recycling.” Sustainable Energy and Fuels 6(20):4598–4604. https://doi.org/10.1039/D2SE01156E.
Sattler, W., and G. Parkin. 2014. “Reduction of Bicarbonate and Carbonate to Formate in Molecular Zinc Complexes.” Catalysis Science and Technology 4(6):1578–1584. https://doi.org/10.1039/C3CY01065A.
Saw, S.Z., and J. Nandong. 2016. “Simulation and Control of Water-Gas Shift Packed Bed Reactor with Inter-Stage Cooling.” IOP Conference Series: Materials Science and Engineering 121(March):012022. https://doi.org/10.1088/1757-899X/121/1/012022.
Sears, W.M., and S.R. Morrison. 1985. “Carbon Dioxide Reduction on Gallium Arsenide Electrodes.” The Journal of Physical Chemistry 89(15):3295–3298. https://doi.org/10.1021/j100261a026.
Shafaat, H.S., and J.Y. Yang. 2021. “Uniting Biological and Chemical Strategies for Selective CO2 Reduction.” Nature Catalysis 4(11):928–933. https://doi.org/10.1038/s41929-021-00683-1.
Shaner, M.R., H.A. Atwater, N.S. Lewis, and E.W. McFarland. 2016. “A Comparative Technoeconomic Analysis of Renewable Hydrogen Production Using Solar Energy.” Energy and Environmental Science 9(7):2354–2371. https://doi.org/10.1039/C5EE02573G.
Shao, B., Y. Zhang, Z. Sun, J. Li, Z. Gao, Z. Xie, J. Hu, and H. Liu. 2022. “CO2 Capture and In-Situ Conversion: Recent Progresses and Perspectives.” Green Chemical Engineering 3(3):189–198. https://doi.org/10.1016/j.gce.2021.11.009.
Shi, L., G. Yang, K. Tao, Y. Yoneyama, Y. Tan, and N. Tsubaki. 2013. “An Introduction of CO2 Conversion by Dry Reforming with Methane and New Route of Low-Temperature Methanol Synthesis.” Accounts of Chemical Research 46(8):1838–1847. https://doi.org/10.1021/ar300217j.
Sibi, M.G., D. Verma and J. Kim. 2022. “Direct Conversion of CO2 into Aromatics Over Multifunctional Heterogeneous Catalysts.” Catalysis Reviews 1–60. https://www.tandfonline.com/doi/abs/10.1080/01614940.2022.2099058.
Snoeckx, R., and A. Bogaerts. 2017. “Plasma Technology—A Novel Solution for CO2 Conversion?” Chemical Society Reviews 46:5805–5863. https://doi.org/10.1039/C6CS00066E.
Soler, A., V. Gordillo, W. Lilley, P. Schmidt, W. Werner, T. Houghton, and S. Dell-Orco. 2022. “E-Fuels: A Technoeconomic Assessment of European Domestic Production and Imports Towards 2050.” Report no. 17/22. Brussels, Belgium: Concawe and Aramco. https://www.concawe.eu/wp-content/uploads/Rpt_22-17.pdf.
Song, B., A. Qin, and B.Z. Tang. 2022. “Syntheses, Properties, and Applications of CO2-Based Functional Polymers.” Cell Reports Physical Science 3(2):100719. https://doi.org/10.1016/j.xcrp.2021.100719.
Stephens, I.E.L., K. Chan, A. Bagger, S.W. Boettcher, J. Bonin, E. Boutin, A.K. Buckley, R. Buonsanti, E.R. Cave, and X. Chang. 2022. “2022 Roadmap on Low Temperature Electrochemical CO2 Reduction.” JPhys Energy 4(4):042003. https://doi.org/10.1088/2515-7655/ac7823.
Storch, H.H., N. Golumbic, and R.B. Anderson. 1961. The Fischer-Tropsch and Related Syntheses. 1st edition. New York: John Wiley and Sons.
Sun, S., H. Sun, P.T. Williams, and C. Wu. 2021. “Recent Advances in Integrated CO2 Capture and Utilization: A Review.” Sustainable Energy and Fuels 5(18):4546–4559. https://doi.org/10.1039/D1SE00797A.
Sung, S., D. Kumar, M. Gil-Sepulcre, and M. Nippe. 2017. “Electrocatalytic CO2 Reduction by Imidazolium-Functionalized Molecular Catalysts.” Journal of the American Chemical Society 139(40):13993–13996. https://doi.org/10.1021/jacs.7b07709.
Tabanelli, T., D. Bonincontro, S. Albonetti, and F. Cavani. 2019. “Chapter 7—Conversion of CO2 to Valuable Chemicals: Organic Carbonate as Green Candidates for the Replacement of Noxious Reactants.” Pp. 125–144 In Studies in Surface Science and Catalysis 178, S. Albonetti, S. Perathoner, and E.A. Quadrelli, eds. Elsevier. https://doi.org/10.1016/B978-0-444-64127-4.00007-0.
Talapaneni, S.N., O. Buyukcakir, S.H. Je, S. Srinivasan, Y. Seo, K. Polychronopoulou, and A. Coskun. 2015. “Nanoporous Polymers Incorporating Sterically Confined N-Heterocyclic Carbenes for Simultaneous CO2 Capture and Conversion at Ambient Pressure.” Chemistry of Materials 27(19):6818–6826. https://doi.org/10.1021/acs.chemmater.5b03104.
Tan, E.W.P., J.L. Hedrick, P.L. Arrechea, T. Erdmann, V. Kiyek, S. Lottier, Y.Y. Yang, and N.H. Park. 2021. “Overcoming Barriers in Polycarbonate Synthesis: A Streamlined Approach for the Synthesis of Cyclic Carbonate Monomers.” Macromolecules 54(4):1767–1774. https://doi.org/10.1021/acs.macromol.0c02880.
Tang, R., Z. Zhu, C. Li, M. Xiao, Z. Wu, D. Zhang, C. Zhang, et al. 2021. “Ru-Catalyzed Reverse Water Gas Shift Reaction with Near-Unity Selectivity and Superior Stability.” ACS Materials Letters 3(12):1652–1659. https://doi.org/10.1021/acsmaterialslett.1c00523.
Thor Wismann, S., K.-E. Larsen, and P. Mølgaard Mortensen. 2022. “Electrical Reverse Shift: Sustainable CO2 Valorization for Industrial Scale.” Angewandte Chemie International Edition 61(8):e202109696. https://doi.org/10.1002/anie.202109696.
Tian, P., Y. Wei, M. Ye, and Z. Liu. 2015. “Methanol to Olefins (MTO): From Fundamentals to Commercialization.” ACS Catalysis 5(3):1922–1938. https://doi.org/10.1021/acscatal.5b00007.
Timmerhaus, K.D., and M.S. Peters. 1991. Plant Design and Economics for Chemical Engineers. 4th edition. New York: McGraw-Hill.
Tlili, A., E. Blondiaux, X. Frogneux, and T. Cantat. 2015. “Reductive Functionalization of CO2 with Amines: An Entry to Formamide, Formamidine and Methylamine Derivatives.” Green Chemistry 17(1):157–168. https://doi.org/10.1039/C4GC01614A.
Ton, T.N., R.J. Baker, and K. Manthiram. 2024. “Recent Progress in the Development of Electrode Materials for Electrochemical Carboxylation with CO2.” Journal of Catalysis 432(April 1):115371. https://doi.org/10.1016/j.jcat.2024.115371.
Tortajada, A., F. Juliá-Hernández, M. Börjesson, T. Moragas, and R. Martin. 2018. “Transition-Metal-Catalyzed Carboxylation Reactions with Carbon Dioxide.” Angewandte Chemie International Edition 57(49):15948–15982. https://doi.org/10.1002/anie.201803186.
Unke, O.T., S. Chmiela, H.E. Sauceda, M. Gastegger, I. Poltavsky, K.T. Schütt, A. Tkatchenko, and K.-R. Müller. 2021. “Machine Learning Force Fields.” Chemical Reviews 121(16):10142–10186. https://doi.org/10.1021/acs.chemrev.0c01111.
van Den Bosch, B., J. Krasovic, B. Rawls, and A.L. Jongerius. 2022. “Research Targets for Upcycling of CO2 to Formate and Carbon Monoxide with Paired Electrolysis.” Current Opinion in Green and Sustainable Chemistry 34(April):100592. https://doi.org/10.1016/j.cogsc.2022.100592.
Vanhoof, J.R., S. Spittaels, and D.E. De Vos. 2024. “A Comparative Overview of the Electrochemical Valorization and Incorporation of CO2 in Industrially Relevant Compounds.” EES Catalysis 2(3):753–779. https://doi.org/10.1039/D4EY00005F.
Verma, R., R. Belgamwar, and V. Polshettiwar. 2021. “Plasmonic Photocatalysis for CO2 Conversion to Chemicals and Fuels.” ACS Materials Letters 3(5):574–598. https://doi.org/10.1021/acsmaterialslett.1c00081.
von der Assen, N., and A. Bardow. 2014. “Life Cycle Assessment of Polyols for Polyurethane Production Using CO2 as Feedstock: Insights from an Industrial Case Study.” Green Chemistry 16(6):3272–3280. https://doi.org/10.1039/C4GC00513A.
Wakerley, D., S. Lamaison, J. Wicks, A. Clemens, J. Feaster, D. Corral, S.A. Jaffer, et al. 2022. “Gas Diffusion Electrodes, Reactor Designs and Key Metrics of Low-Temperature CO2 Electrolysers.” Nature Energy 7(2):130–143. https://doi.org/10.1038/s41560-021-00973-9.
Wang, F., Z. Lu, H. Guo, G. Zhang, Y. Li, Y. Hu, W. Jiang, and G. Liu. 2023a. “Plasmonic Photocatalysis for CO2 Reduction: Advances, Understanding and Possibilities.” Chemistry—A European Journal 29(25):e202202716. https://doi.org/10.1002/chem.202202716.
Wang, J., W. Sng, G. Yi, and Y. Zhang. 2015b. “Imidazolium Salt-Modified Porous Hypercrosslinked Polymers for Synergistic CO2 Capture and Conversion.” Chemical Communications 51(60):12076–12079. https://doi.org/10.1039/C5CC04702A.
Wang, J.-W., X. Zhang, L. Velasco, M. Karnahl, Z. Li, Z.-M. Luo, Y. Huang, et al. 2023b. “Precious-Metal-Free CO2 Photoreduction Boosted by Dynamic Coordinative Interaction between Pyridine-Tethered Cu(I) Sensitizers and a Co(II) Catalyst.” JACS Au 3(7):1984–1997. https://doi.org/10.1021/jacsau.3c00218.
Wang, W.-H., Y. Himeda, J.T. Muckerman, G.F. Manbeck, and E. Fujita. 2015a. “CO2 Hydrogenation to Formate and Methanol as an Alternative to Photo- and Electrochemical CO2 Reduction.” Chemical Reviews 115(23):12936–12973. https://doi.org/10.1021/acs.chemrev.5b00197.
Wang, X., H. Wang, and Y. Sun. 2017. “Synthesis of Acrylic Acid Derivatives from CO2 and Ethylene.” Chem 3(2):211–228. https://doi.org/10.1016/j.chempr.2017.07.006.
Wenzel, M., L. Rihko-Struckmann, and K. Sundmacher. 2016. “Thermodynamic Analysis and Optimization of RWGS Processes for Solar Syngas Production from CO2.” AIChE Journal 63(1):15–22. https://doi.org/10.1002/aic.15445.
Wexler, R.B., E.B. Stechel, and E.A. Carter. 2023. “Materials Design Directions for Solar Thermochemical Water Splitting.” Pp. 1–63 in Solar Fuels. https://doi.org/10.1002/9781119752097.ch1.
White, J.L., M.F. Baruch, J.E. Pander III, Y. Hu, I.C. Fortmeyer, J.E. Park, T. Zhang, et al. 2015. “Light-Driven Heterogeneous Reduction of Carbon Dioxide: Photocatalysts and Photoelectrodes.” Chemical Reviews 115(23):12888–12935. https://doi.org/10.1021/acs.chemrev.5b00370.
Wołosz, D., P.G. Parzuchowski, and K. Rolińska. 2022. “Environmentally Friendly Synthesis of Urea-Free Poly(Carbonate-Urethane) Elastomers.” Macromolecules 55(12):4995–5008. https://doi.org/10.1021/acs.macromol.2c00706.
Wu, S., X. Yang, X. Zhao, Z. Li, M. Lu, X. Xie, and J. Yan. 2023. “Applications and Advances in Machine Learning Force Fields.” Journal of Chemical Information and Modeling 63(22):6972–6985. https://doi.org/10.1021/acs.jcim.3c00889.
Xia, R., S. Overa, and F. Jiao. 2022. “Emerging Electrochemical Processes to Decarbonize the Chemical Industry.” JACS Au 2(5):1054–1070. https://doi.org/10.1021/jacsau.2c00138.
Xie, J., and U. Olsbye. 2023. “The Oxygenate-Mediated Conversion of COx to Hydrocarbons–On the Role of Zeolites in Tandem Catalysis.” Chemical Reviews 123(20):11775–11816. https://doi.org/10.1021/acs.chemrev.3c00058.
Xie, W., J. Xu, U. Md Idros, J. Katsuhira, M. Fuki, M. Hayashi, M. Yamanaka, Y. Kobori, and R. Matsubara. 2023. “Metal-Free Reduction of CO2 to Formate Using a Photochemical Organohydride-Catalyst Recycling Strategy.” Nature Chemistry 15(6):794–802. https://doi.org/10.1038/s41557-023-01157-6.
Xu, S., and E.A. Carter. 2019a. “Optimal Functionalization of a Molecular Electrocatalyst for Hydride Transfer.” Proceedings of the National Academy of Sciences 116(46):22953–22958. https://doi.org/10.1073/pnas.1911948116.
Xu, S., and E.A. Carter. 2019b. “Theoretical Insights into Heterogeneous (Photo)Electrochemical CO2 Reduction.” Chemical Reviews 119(11):6631–6669. https://doi.org/10.1021/acs.chemrev.8b00481.
Xu, S., L. Li, and E.A. Carter. 2018. “Why and How Carbon Dioxide Conversion to Methanol Happens on Functionalized Semiconductor Photoelectrodes.” Journal of the American Chemical Society 140(48):16749–16757. https://doi.org/10.1021/jacs.8b09946.
Xu, S., H. Chen, C. Hardacre, and X. Fan. 2021. “Non-Thermal Plasma Catalysis for CO2 Conversion and Catalyst Design for the Process.” Journal of Physics D: Applied Physics 54(23):233001. https://doi.org/10.1088/1361-6463/abe9e1.
Yamazaki, Y., H. Takeda, and O. Ishitani. 2015. “Photocatalytic Reduction of CO2 Using Metal Complexes.” Journal of Photochemistry and Photobiology C: Photochemistry Reviews 25(December 1):106–137. https://doi.org/10.1016/j.jphotochemrev.2015.09.001.
Yan, T., X. Chen, L. Kumari, J. Lin, M. Li, Q. Fan, H. Chi, T.J. Meyer, S. Zhang, and X. Ma. 2023. “Multiscale CO2 Electrocatalysis to C2+ Products: Reaction Mechanisms, Catalyst Design, and Device Fabrication.” Chemical Reviews 123(17):10530–10583. https://doi.org/10.1021/acs.chemrev.2c00514.
Ye, R.-P., J. Ding, W. Gong, M.D. Argyle, Q. Zhong, Y. Wang, C.K. Russell, et al. 2019. “CO2 Hydrogenation to High-Value Products via Heterogeneous Catalysis.” Nature Communications 10(1):5698. https://doi.org/10.1038/s41467-019-13638-9.
Yeung, C.W.S., G.E.K.K. Seah, A.Y.X. Tan, S.Y. Tee, J.Y.C. Lim, and S.S. Goh. 2023. “Chapter 5—Functional Polymers from CO2 as Feedstock.” Pp. 129–171 in Circularity of Plastics, L. Zibiao, J.Y.C. Lim, and C.-G. Wang, eds. Elsevier. https://doi.org/10.1016/B978-0-323-91198-6.00005-X.
Yin, Z., J. Yu, Z. Xie, S.-W. Yu, L, Zhang, T. Akauola, J.G. Chen, W. Huang, L. Qi, and S. Zhang. 2022. “Hybrid Catalyst Coupling Single-Atom Ni and Nanoscale Cu for Efficient CO2 Electroreduction to Ethylene.” Journal of the American Chemical Society 144(45):20931–20938. https://doi.org/10.1021/jacs.2c09773.
Yu, S., and P.K. Jain. 2019. “Plasmonic Photosynthesis of C1–C3 Hydrocarbons from Carbon Dioxide Assisted by an Ionic Liquid.” Nature Communications 10(1):2022. https://doi.org/10.1038/s41467-019-10084-5.
Zhang, G., G. Wei, Z. Liu, S.R.J. Oliver, and H. Fei. 2016. “A Robust Sulfonate-Based Metal–Organic Framework with Permanent Porosity for Efficient CO2 Capture and Conversion.” Chemistry of Materials 28(17):6276–6281. https://doi.org/10.1021/acs.chemmater.6b02511.
Zhang, J., B. Guan, X. Wu, Y. Chen, J. Guo, Z. Ma, S. Bao, et al. 2023. “Research on Photocatalytic CO2 Conversion to Renewable Synthetic Fuels Based on Localized Surface Plasmon Resonance: Current Progress and Future Perspectives.” Catalysis Science and Technology 13(7):1932–1975. https://doi.org/10.1039/D2CY01967A.
Zhang, L., and Z. Hou. 2013. “Chapter 9—Transition-Metal-Catalyzed C-C Bond Forming Reactions with Carbon Dioxide.” Pp. 253–273 in New and Future Developments in Catalysis: Activation of Carbon Dioxide. Amsterdam: Elsevier Science and Technology.
Zhang, Q., M. Bown, L. Pastor-Pérez, M.S. Duyar, and T.R. Reina. 2022a. “CO2 Conversion via Reverse Water Gas Shift Reaction Using Fully Selective Mo–P Multicomponent Catalysts.” Industrial and Engineering Chemistry Research 61(34):12857–12865. https://doi.org/10.1021/acs.iecr.2c00305.
Zhang, T., J.C. Bui, Z. Li, A.T. Bell, A.Z. Weber, J. Wu. 2022b. “Highly Selective and Productive Reduction of Carbon Dioxide to Multicarbon Products via In Situ CO Management Using Segmented Tandem Electrodes.” Nature Catalysis 5(3):202–211. https://doi.org/10.1038/s41929-022-00751-0.
Zhang, X., A. Zhang, X. Jiang, J. Zhu, J. Liu, J. Li, G. Zhang, C. Song, and X. Guo. 2019a. “Utilization of CO2 for Aromatics Production Over ZnO/ZrO2-ZSM-5 Tandem Catalyst.” Journal of CO2Utilization 29(January 1):140–145. https://doi.org/10.1016/j.jcou.2018.12.002.
Zhang, X., M. Cibian, A. Call, K. Yamauchi, and K. Sakai. 2019b. “Photochemical CO2 Reduction Driven by Water-Soluble Copper(I) Photosensitizer with the Catalysis Accelerated by Multi-Electron Chargeable Cobalt Porphyrin.” ACS Catalysis 9(12):11263–11273. https://doi.org/10.1021/acscatal.9b04023.
Zhang, X., G. Zhang, C. Song, and X. Guo. 2021. “Catalytic Conversion of Carbon Dioxide to Methanol: Current Status and Future Perspective.” Frontiers in Energy Research 8. https://www.frontiersin.org/articles/10.3389/fenrg.2020.621119.
Zhang, X., W. Huang, L. Yu, M. García-Melchor, D. Wang, L. Zhi, and H. Zhang. 2024. “Enabling Heterogeneous Catalysis to Achieve Carbon Neutrality: Directional Catalytic Conversion of CO2 into Carboxylic Acids.” Carbon Energy 6(3): e362. https://doi.org/10.1002/cey2.362.
Zhang, Z., E.W. Lees, F. Habibzadeh, D.A. Salvatore, S. Ren, G.L. Simpson, D.G. Wheeler, A. Liu, and C.P. Berlinguette. 2022c. “Porous Metal Electrodes Enable Efficient Electrolysis of Carbon Capture Solutions.” Energy and Environmental Science 15(2):705–713. https://doi.org/10.1039/D1EE02608A.
Zhao, Q., J.M.P. Martirez, and E.A. Carter. 2021. “Revisiting Understanding of Electrochemical CO2 Reduction on Cu(111): Competing Proton-Coupled Electron Transfer Reaction Mechanisms Revealed by Embedded Correlated Wavefunction Theory.” Journal of the American Chemical Society 143(16):6152–6164. https://doi.org/10.1021/jacs.1c00880.
Zheng, L., M. Ambrosetti, A. Beretta, G. Groppi, and E. Tronconi. 2023. “Electrified CO2 Valorization Driven by Direct Joule Heating of Catalytic Cellular Substrates.” Chemical Engineering Journal 466(June 15):143154. https://doi.org/10.1016/j.cej.2023.143154.
Zhou, C., J. Zhang, Y. Fu, and H. Dai. 2023. “Recent Advances in the Reverse Water–Gas Conversion Reaction.” Molecules 28(22). https://doi.org/10.3390/molecules28227657.
Zhou, G., B. Dai, H. Xie, G. Zhang, K. Xiong, and X. Zheng. 2017. “CeCu Composite Catalyst for CO Synthesis by Reverse Water–Gas Shift Reaction: Effect of Ce/Cu Mole Ratio.” Journal of CO2 Utilization 21(October 1):292–301. https://doi.org/10.1016/j.jcou.2017.07.004.
Zhou, L., J.M.P. Martirez, J. Finzel, C. Zhang, D.F. Swearer, S. Tian, H. Robatjazi, et al. 2020. “Light-Driven Methane Dry Reforming with Single Atomic Site Antenna-Reactor Plasmonic Photocatalysts.” Nature Energy 5(1):61–70. https://doi.org/10.1038/s41560-019-0517-9.
Zhou, Y., A. José Martín, F. Dattila, S. Xi, N. López, J. Pérez-Ramírez, and B.S. Yeo. 2022. “Long-Chain Hydrocarbons by CO2 Electroreduction Using Polarized Nickel Catalysts.” Nature Catalysis 5(6):545–554. https://doi.org/10.1038/s41929-022-00803-5.
Zhu, Q., Y. Zeng, and Y. Zheng. 2023. “Overview of CO2 Capture and Electrolysis Technology in Molten Salts: Operational Parameters and Their Effects.” Industrial Chemistry and Materials 1(4):595–617. https://doi.org/10.1039/D3IM00011G.

















































